Commission, July 17, 2015, No M.7583
EUROPEAN COMMISSION
Judgment
CSL/ NOVARTIS INFLUENZA VACCINES BUSINESS
Dear Sir/Madam,
Subject:Case M.7583 - CSL/ NOVARTIS INFLUENZA VACCINES BUSINESS
Commission decision pursuant to Article 6(1)(b) of Council Regulation No 139/20041 and Article 57 of the Agreement on the European Economic Area2
(1) On 12 June 2015, the European Commission received notification of a proposed concentration pursuant to Article 4 of the Merger Regulation by which CSL Limited ("CSL" or the "Notifying Party", Australia) will acquire sole control of Novartis AG's ("Novartis", Switzerland) human influenza vaccines business ("the Target"), by way of purchase of shares and assets (the "Transaction").3
(2) CSL and the Target are designated hereinafter as the "Parties".
I. THE PARTIES
(3) CSL is an Australia-based pharmaceutical company active worldwide in research, development, manufacturing and marketing of biotherapies, vaccines (via its subsidiary bioCSL) and other pharmaceuticals.
(4) Novartis is a Switzerland-based global pharmaceutical company active in research, development, manufacturing and marketing of originator pharmaceuticals and generic pharmaceuticals. In Europe, the Novartis business includes: Novartis Pharmaceuticals, Alcon (eye care pharmaceuticals) and Sandoz (generic pharmaceuticals).
(5) The Transaction follows Novartis' sale of its vaccines business (excluding influenza) to GlaxoSmithKline plc ("GSK"), which was approved by the Commission in January 2015.4 The Target consists of Novartis' influenza vaccines business, which is held by Novartis Vaccines Holdings Limited and a subsidiary thereof, and comprises in particular of three manufacturing sites (Liverpool, United Kingdom, Holly Springs, United States, and […] production lines in Marburg, Germany).
II. THE OPERATION AND THE CONCENTRATION
(6) On 26 October 2014, CSL and Novartis entered into a Share and Business Sale Agreement ("SAPA"), pursuant to which the Target will be indirectly solely controlled by CSL.
(7) The acquisition will be effected by way of the purchase of shares and assets within the conditions of the SAPA. CSL will acquire all of the shares in Novartis Vaccines Holdings Limited and its subsidiary and any new entity, should such be created, which is incorporated to hold Novartis' Human Influenza Vaccines Business and the assets of the Flu Group Businesses.
(8) The Transaction therefore constitutes a concentration within the meaning of Article 3(1)(b) of the Merger Regulation.
III. UNION DIMENSION
(9) The operation does not have Union dimension within the meaning of Article 1 of the Merger Regulation. Since the Parties' combined worldwide turnover is below EUR 5,000 million (CSL: EUR 4,088 million, the Target: EUR […] million), the thresholds of Article 1(2) of the Merger Regulation are not met. Similarly, the transaction does not have Union dimension pursuant to the threshold set out in Article 1(3) of the Merger Regulation as the aggregate Community-wide turnover of the Target is below EUR 100 million (EUR […] million).
(10) On 9 April 2015 the Commission received, by means of a reasoned submission, a referral request pursuant to Article 4(5) of the Merger Regulation whereby CSL requested the Commission to take jurisdiction. According to CSL, the transaction would be reviewable under the national merger control laws of three Member States, namely Austria, Germany and the UK. A copy of this submission was promptly transmitted to Member States on the same day.
(11) The Commission took a view in favour of the referral request so that the Parties could benefit from the one-stop-shop procedure and the costs and burdens associated of multiple filings be reduced. Moreover, the Commission's recent experience in reviewing cases related to human vaccines5 was considered to be an additional argument in favour of the referral request.6
(12 None of the Member States that would have jurisdiction to review the transaction objected to the request within the deadline of 15 working days. Therefore, the transaction is deemed to have Union dimension in terms of Article 4(5) of the Merger Regulation. This was communicated to the Parties on 4 May 2015.
IV. COMMISSION'S ASSESSMENT
IV.1. Product market definition
(13) Influenza is an acute illness mainly affecting the respiratory system and caused by an infection with an influenza virus, of which there are three broad groups: A, B and C. All marketed influenza vaccines are intended to protect against A and B group virus infections, which present more serious health concerns.
(14) Influenza viruses mutate regularly, rendering the body's immune system ineffective against the mutated form. Influenza outbreaks caused by such mutations can be seasonal epidemics (caused by a slow, gradual genetic drift in the genetic makeup of the virus) or pandemic (caused by a sudden, drastic genetic shift in the genetic makeup of the virus).
(15) Influenza vaccines may be segmented depending in particular on their end-use, age indication, composition, way of preparation, route of administration and viral coverage.
(16) First, depending on the end-use (which relates to the type of the virus), influenza vaccines can be divided into three types (seasonal, pre-pandemic, and pandemic):
a. Seasonal flu vaccines are sold and administered every year. Because circulating influenza viruses evolve continuously, the strains included in the vaccine have to be updated frequently (annually) based on a recommendation from the World Health Organisation ("WHO").
b. Pre-pandemic flu vaccines are monovalent vaccines intended to protect against a potential drift in an existing influenza strain that could cause a future pandemic. The objective of a pre-pandemic vaccine is to provide some background level of immunity in the event a pandemic virus emerges, even if the immunity is not fully effective.
c. Pandemic flu vaccines can only be prepared once a pandemic virus has been identified by means of an official declaration of an influenza pandemic emergency by the WHO. To ensure swift supply of a pandemic vaccine, there are specific procedures in place in Europe based on a "mock-up" (prototype) vaccine, which has to be authorised by the Commission on the basis of the scientific assessment by the European Medicines Agency ("EMA") in advance of the pandemics.
(17) Second, regarding influenza vaccines, the marketing authorisation issued by the Commission should, when appropriate, define as part of the Summary of Product Characteristics for which age group the product is indicated. It may also contain specific information, conditions or restrictions for use in special patient populations such as the paediatric (below a certain age threshold) and elderly (above a certain age threshold) population.
(18) Third, the difference between egg-based and cell-based flu vaccines refers to the production process of these vaccines. Egg-based flu vaccines are grown and incubated in fertilized chicken eggs, whereas cell-based flu vaccines are produced using mammalian cells instead of eggs.
(19) Fourth, certain vaccines contain a substance, separate from the underlying virus antigen, which has been added to increase the body's immune response to the vaccine. This substance is referred to as the adjuvant, and vaccines containing such substance are referred to as adjuvanted vaccines.
(20) Fifth, seasonal flu vaccines are administered via different delivery systems: needle (intramuscular and intradermal), nasal spray or needle-free (using a needle-free injection device). There is currently also ongoing research of a possibility to use micro-needle patches as an alternative route of administration.
(21) Finally, most seasonal flu vaccines currently marketed are trivalent (“TIV”): they contain three strains of the influenza virus, two sub-type A strains, and one type B strain. Recently, some manufacturers started offering quadrivalent flu vaccines (“QIV”) which immunize recipients against four strains of influenza virus, the three listed above plus an additional type-B strain.
Notifying Party's views
(22) The Notifying Party submits that flu vaccines should be grouped into seasonal, pandemic and pre-pandemic flu vaccines, and argues that in this case overlaps occur only with respect to seasonal flu vaccines.
(23) Indeed, the Notifying Party is of the view that any further segmentation7 of the seasonal flu vaccine market would be inappropriate given the high degree of demand-side substitutability between seasonal influenza vaccines. Therefore, the Notifying Party submits that the relevant product market for the purpose of this decision is the one for the supply of seasonal flu vaccines, in relation to which actual overlaps would occur in Germany and the United Kingdom (and potential overlaps in Italy and Spain due to CSL being a future competitor).
Past Commission decisions
ATC classification for pharmaceuticals
(24) In its past merger decisions in the pharmaceutical sector, the Commission referred to the third level ("ATC3") of the European Pharmaceutical Market Research Association ("EphMRA") classification as the starting point for defining the relevant product market. However, in a number of cases, the Commission found that the ATC3 level classification did not yield the appropriate market definition. As a result, where appropriate and based on the factual evidence collected during the market investigation, the Commission defined the relevant product market at the ATC4 level or at a level of molecule or a group of molecules that are considered interchangeable so as to exercise competitive pressure on one another. The overlap in therapeutic uses does not necessarily imply any particular economic substitution patterns between products.8
(25) In relation to vaccines, the Commission recently assessed mergers in markets for human vaccines where it took as a starting point the disease for which the vaccines were intended, but did not specifically analyse influenza vaccines.9 Similarly, in a past decisions dealing with flu vaccines, namely, Novartis/Chiron, the Commission noted that "vaccines intended for other diseases are clearly not therapeutically substitutable to flu vaccines", while ultimately leaving the market for flu vaccines open.10
The Commission's assessment
Distinction between seasonal, pandemic and pre-pandemic influenza vaccines
(26) The market investigation confirmed that the seasonal influenza vaccines have a number of characteristics distinguishing them from pandemic and pre-pandemic influenza vaccines.
(27) On the supply-side, respondents to the market investigation indicate that a manufacturer currently only active in seasonal influenza vaccines would incur high costs and face regulatory barriers to enter the (pre-)pandemic flu vaccines business.11 Pandemic vaccines have a particular authorization process (for which, for example, development of a mock-up vaccine may be required). They are only produced when a pandemics is officially declared by the WHO and aim at addressing the specific (pandemic) strain of virus.
(28) Similarly, demand-side substitution is limited as only suppliers who have an approved mock-up vaccine in the EEA can enter into a contract with governments whereby they reserve production capacity in case of a pandemic outbreak.12 Therefore, vaccines approved and marketed as seasonal flu vaccines cannot be used as pandemic or pre-pandemic flu vaccines in a straightforward way.
(29) In light of the above, the Commission concludes that seasonal influenza vaccines constitute a separate market from pre-pandemic and pandemic influenza vaccines.
Sub-segmentation of the seasonal influenza vaccines market
(30) As to the question of whether the seasonal influenza vaccines should be further sub- divided, results of the market investigation broadly confirm that the customers perceive different seasonal flu vaccines available on the market as substitutable.
a) Age indication
(31) The majority of respondents from the demand-side indicate that the most important criterion in vaccine selection is the age indication and most of the influenza vaccines currently marketed in Europe have broad age indications.13 There are a few exceptions including vaccines with age indication only for children or only for elderly (above 65 years old), which are not suitable for use in the general population.14
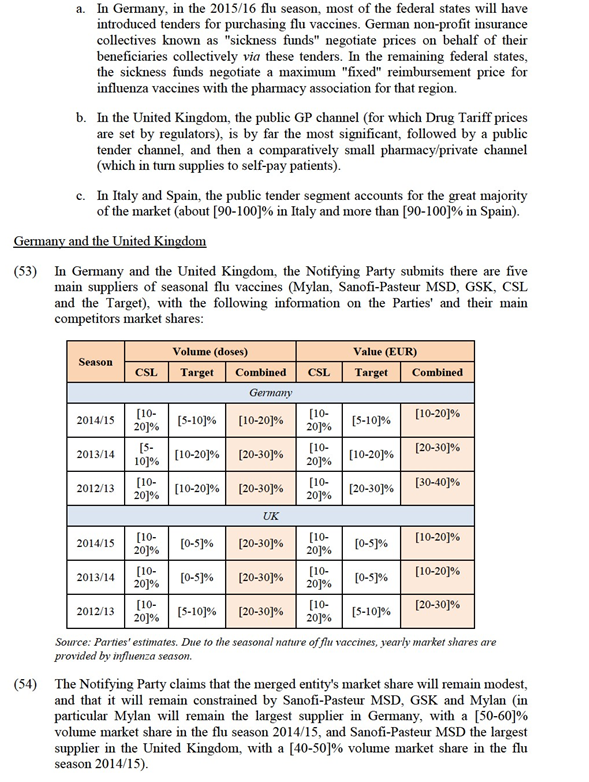
b) Egg- and cell- based vaccines
(32) As to the potential sub-segmentation between egg-based and cell-based vaccines, only the minority of respondents confirm that they regularly purchase cell-based flu vaccines and the majority of those customers indicate that it is a niche product purchased in very small quantities and used only for patients suffering from egg allergy.15 Only one respondent indicated purchasing cell-based vaccines for the general population. Opinions of Key Opinion Leaders are also mixed. While some confirm that cell-based vaccines slightly increase safety for patients with egg allergies,16 others indicate that there is no general recommendation to use cell-based vaccines for patients with egg-allergies and that the decision is mostly taken on a case-by-case basis, and therefore that egg-based and cell-based vaccines are substitutable.17
c) Adjuvanted and non-adjuvanted vaccines
(33) As to the potential sub-segmentation between adjuvanted and non-adjuvanted vaccines, the market investigation yielded mixed results. Adjuvanted vaccines are not currently marketed in the UK. In Germany, Italy and Spain, the majority of respondents purchased adjuvanted vaccines in the last flu season to administer them to the elderly population and partly to adult risk patients.18 There seems to be a preference to purchase adjuvanted vaccines in Spain and Italy, whereas in Germany there appear to be no clear recommendations and no practice to purchase adjuvanted vaccines for any specific groups of population.19
d) Mode of administration
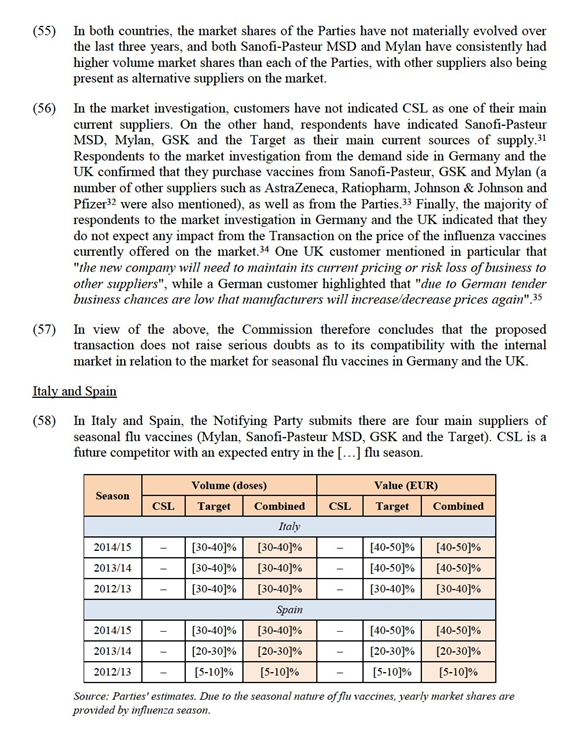
(34) As to the potential sub-segmentation according to the mode of administration, the majority of respondents do not qualify it as an important criterion.20
e) TIV and QIV vaccines
(35) Finally, as to the potential sub-segmentation between TIV and QIV, only a minority of respondents use QIV vaccines, mostly for use in specific risk groups. Only a few customers use QIV vaccines for the general population.21 With respect to the future of the QIV vaccines, roughly half of the respondents expect that QIV will replace TIV in the future.22 Key Opinion Leaders expect a shift from TIV to QIV, even though opinions about QIV's effectiveness differ. One Key Opinion Leader stated for instance that "there is currently no sufficiently robust scientific evidence that would point to a significantly higher medical efficacy".23
Conclusion
(36) The market investigation confirmed furthermore that seasonal flu vaccines are to a large extent commoditised products, with the majority of respondents to the market investigation confirming that price is the most important factor when selecting seasonal flu vaccines. Most customers further indicate that brand is the least important factor in the selection of seasonal influenza vaccines.24
(37) In light of the above, the Commission considers that the question whether seasonal influenza vaccines should be further segmented by different criteria can be left open for the purpose of this decision, as no serious doubts arise in relation to seasonal influenza vaccines irrespective of the market definition.
IV.2. Geographic market definition
(38) The Commission has previously analysed the vaccine markets at national level. More specifically, the Commission considered that, while vaccine manufacturers typically produce vaccines for the whole EEA, the supply of vaccines to wholesalers is affected by different national regulations and reimbursement systems in each Member State, which confer to those markets a national dimension. Furthermore, it noted that market characteristics, distribution channels, sales patterns, vaccines schedules and price setting for flu vaccines vary significantly among countries.25
(39) Responses to the market investigation in this case also point to the national scope of the seasonal influenza vaccine markets, in particular in light of national regulatory frameworks, prices and reimbursement. All of the competitors confirm that their commercial relations with customers typically take place at least at the national level (a small proportion indicate to have commercial relations at a regional (infra- national) level).26
(40) In light of the above, the seasonal influenza vaccine markets are analysed at the national level.
Pipeline products
(41) In its previous practice, the Commission assessed the potential for products in research & development to enter into competition with other products which are either on the market or at the development stage by reference to their characteristics, intended therapeutic use, and expected therapeutic and economic substitutability.27
(42) As regards the geographic dimension of pipeline pharmaceuticals, in line with its previous practice, the Commission considers that since pipeline products need to be assessed with reference to the R&D in a given area and to the extent that R&D for the relevant products is normally global, the geographic scope of the market should be global or at least be EEA-wide.28
IV.3. Competitive assessment – seasonal influenza vaccines
(43) The Parties' activities give rise to affected markets regarding seasonal influenza vaccines, where the Parties supply the following products in the EEA:
a. CSL markets Afluria (age 5+ years, egg-based, TIV, non-adjuvanted) and a non-branded vaccine (age 5+ years, egg-based, TIV, non-adjuvanted);
b. the Target markets Agrippal/Begripal (age 6+ months, egg-based, TIV, non- adjuvanted), Fluad (age 65+ years, egg-based, TIV, adjuvanted), Optaflu (age 18+ years, cell-based, TIV, non-adjuvanted) and Fluvirin (age 4+ years, egg-based, TIV, non-adjuvanted).
(44) For the purposes of this decision, the effects of the proposed transaction are assessed on an overall market for seasonal influenza vaccines. The assessment is not materially affected if one were to consider each of the segmentations contemplated above.
a) Age indication
(45) Since CSL's vaccine is a multi-purpose vaccine covering all ages above 5 years, there is no overlap below this age group. As regards age groups above 5 years, the Parties' main competitors cover all age groups and the structure of the market is not materially different to that of the overall market.
b)Egg- and cell-based vaccines
(46) Only the Target supplies cell-based vaccines and therefore there is no overlap in this category. Regarding egg-based vaccines, the Parties' main competitors supply such vaccines and the structure of the market is not materially different to that of the overall market.
c) Adjuvanted and non-adjuvanted vaccines
(47) Only the Target supplies the former category (therefore there is no overlap regarding adjuvanted vaccines) and the Parties' main competitors supply non-adjuvanted vaccines and the structure of the market is not materially different to that of the overall market.
d) Route of administration
(48) Whilst all the Parties' vaccines marketed in the EEA are intramusucular vaccines,29 the structure of this particular segment is not materially different to that of the overall market.
e) TIV and QIV vaccines
(49) While the Parties only market TIV, they both have QIV in development stage. This pipeline to pipeline overlap is analysed below at paragraphs (62) through (65).
(50) Regarding the overall market for seasonal flu vaccines, the Parties' activities overlap in two Member States: Germany and the United Kingdom (both affected markets). For two additional Member States, Italy and Spain, the Target has a market share greater than 20% and CSL intends to start selling its flu vaccine as of the […] flu season.
(51) The Transaction follows a trend of consolidation in the market for seasonal flu vaccines over the last few years. Out of the seven main suppliers in the EEA (Abbott respectively Mylan,30 Baxter, Crucell, CSL, GSK, Novartis, Sanofi-Pasteur MSD), two (Baxter and Crucell) have exited the market in Germany, the United Kingdom, Italy and Spain in the 2012/13 and 2013/14 flu seasons.
(52) While the structure of demand for seasonal flu vaccines varies across the four abovementioned countries, these markets are typically markets within which purchasing is done via tenders or where ther is degree or regulatory oversight on pricing:
(59) The Notifying Party submits that CSL obtained national approval for its seasonal flu vaccine in 2014 in Italy and February 2015 in Spain, and expects to be able to launch it in both countries in the […] flu season. It further claims that, even assuming that CSL were not to enter the seasonal flu vaccines markets in Spain and Italy, four suppliers would remain after the Transaction.
(60) Respondents to the market investigation from the demand side in Italy and Spain confirmed that they purchase vaccines from Sanofi-Pasteur, GSK and Mylan (Johnson & Johnson is also mentioned), as well as from the Target.36 In particular, Sanofi-Pasteur MSD is the largest supplier in Italy and Spain with a volume market share of [50-60]% (Italy) and [40-50]% (Spain), in the flu season 2014/15, and in both countries, one additional strong competitor – GSK and Mylan, respectively – is also present. Finally, the majority of respondents to the market investigation in Italy and Spain indicated that they do not expect any impact from the Transaction on the price of the influenza vaccines currently offered on the market.37
(61) In view of the above, the Commission concludes that the proposed transaction does not raise serious doubts as to its compatibility with the internal market in relation to the market for seasonal flu vaccines in Italy and Spain.
Quadrivalent influenza vaccines (QIV)
(62) The proposed Transaction gives rise to a pipeline overlap regarding the hypothetical narrower market for QIV. Indeed, CSL has a QIV currently undergoing clinical trials,38 while the Target has three QIV pipeline products.39
(63) The Notifying Party submits that, to the extent that QIV vaccines are considered in isolation, CSL and the Target will be late comers in the supply of QIV vaccines in Europe compared to some of their key competitors. It further notes that there will likely be as many competitors as are currently supplying TIV influenza vaccines in Europe given that the other main TIV suppliers market QIV or have it in their pipeline.
(64) In addition the market investigation confirmed that GSK is currently marketing a QIV vaccine in Europe since the 2013/14 flu season (Fluarix/Influsplit Tetra),40 while Sanofi-Pasteur MSD has a pipeline QIV in phase III (Vaxigrip Tetra), which has already been launched in the US,41 and Mylan has a pipeline QIV in phase III.42 Furthermore, the majority of respondents to the market investigation from the demand side indicated that they do not expect any impact from the Transaction on the prices of QIV,43 as well as on innovation regarding influenza vaccines.44
(65) In view of the above, the Commission therefore concludes that the proposed Transaction does not raise serious doubts as to its compatibility with the common market in relation to the pipeline overlap in quadrivalent seasonal flu vaccines.
V. CONCLUSION
(66) For the above reasons, the European Commission has decided not to oppose the notified operation and to declare it compatible with the internal market and with the EEA Agreement. This decision is adopted in application of Article 6(1)(b) of the Merger Regulation and Article 57 of the EEA Agreement.
1 OJ L 24, 29.1.2004, p. 1 ('the Merger Regulation'). With effect from 1 December 2009, the Treaty on
the Functioning of the European Union ("TFEU") has introduced certain changes, such as the
replacement of "Community" by "Union" and "common market" by "internal market". The
terminology of the TFEU will be used throughout this decision.
2 OJ L 1, 3.1.1994, p.3 ("the EEA Agreement").
3 Publication in the Official Journal of the European Union No C 206, 23.06.2015, p. 11.
4 See case M.7276 – GlaxoSmithKline / Novartis vaccines business (excl. influenza) / Novartis
Consumer Health business.
5 In particular GlaxoSmithKline/Novartis Vaccines Business (excl. influenza), which covered the sale of
the rest of Novartis' vaccines business and is therefore closely related to the case at hand.
6 Commission Notice on Case Referral in respect of concentrations OJ 2005/C 56/02, paragraph 31.
7 E.g. distinguishing by age indication, between egg-based and cell-based vaccines, adjuvanted and
non-adjuvanted, TIV and QIV, or by route of administration.
8 See e.g. cases M.7480 – Actavis/Allergan; M.7279 – Mylan/Abbott EPD-DM; M.7276 –
GlaxoSmithKline/Novartis vaccines business (excl. influenza)/Novartis Consumer Health business;
M.7275 –Novartis/GlaxoSmithKline Oncology Business and M.5253 –Sanofi-Aventis/Zentiva.
9 See e.g. cases M.7276 – GlaxoSmithKline/Novartis vaccines business (excl. influenza)/Novartis
Consumer Health business.
10 See case M.4049 –Novartis/Chiron.
11 See replies to question 24 – Phase I questionnaire to competitors.
12 See agreed minutes of a call with a customer dated 19 May 2015.
13 See replies to question 8 – Phase I questionnaire to customers.
14 Those vaccines include among others MedImmune's Fluenz and Fluenz Tetra recommended for
children below 18 years and Novartis' Fluad recommended for the elderly above 65 years. See replies
to question 8 – Phase I questionnaire to customers.
15 See replies to question 11 – Phase I questionnaire to customers.
16 See agreed minutes of a call with a Key Opinion Leader dated 22 May 2015.
17 See agreed minutes of a call with Key Opinion Leaders dated 19 May 2015 and 22 May 2015.
18 See replies to question 12 – Phase I questionnaire to customers.
19 See agreed minutes of a call with a competitor dated 22 May 2015.
20 See replies to question 9 – Phase I questionnaire to customers.
21 See replies to question 13 – Phase I questionnaire to customers.
22 See replies to question 14 – Phase I questionnaire to customers.
23 See agreed minutes of a call with a competitor dated 22 May 2015.
24 See replies to question 9 – Phase I questionnaire to customers.
25 See e.g. cases M.4049 – Novartis / Chiron and M.7276 – GlaxoSmithKline / Novartis vaccines
business (excl. influenza) / Novartis Consumer Health business.
26 See replies to question 4 – Phase I questionnaire to competitors.
27 See M.7275 – Novartis/GlaxoSmithKline Oncology Business, Commission decision of 28 January
2015; M.6969 – Valeant Pharmaceuticals International / Bausch & Lomb Holdings, Commission
decision of 5 August 2013; M.6278 – Takeda/Nycomed, Commission decision of 29 July 2011;
M.5778 – Novartis/Alcon, Commission decision of 9 August 2010; M.5476 – Pfizer/Wyeth,
Commission decision of 17 July 2009; M.1846 – Glaxo Wellcome/Smithkline Beecham, Commission
decision of 8 May 2000, M.1878 – Pfizer/Warner Lambert, Commission decision of 22 May 2000;
M.1846 – Glaxo Wellcome/Smithkline Beecham, Commission decision of 8 May 2000; and M.737 –
Ciba-Geigy/Sandoz, Commission decision of 04 February 1998..
28 See M.7275 – Novartis/GlaxoSmithKline Oncology Business, Commission decision of 28 January
2015; and M.737 –Ciba-Geigy/Sandoz, Commission decision of 17 July 1996.
29 With the exception of CSL, but not the Target, developing a needle-free injection device which has
been approved in the US (but not in the EEA).
30 On 27 April 2015, Mylan completed its acquisition of Abbott Laboratories' non-U.S. developed
markets speciality and branded generics business, which includes Abbott's trivalent influenza vaccine
(Influvac). See: http://newsroom.mylan.com/index.php?s=2429&item=123282.
31 See replies to question 12 - Phase I questionnaire to competitors
32 Pfizer distributes CSL's flu vaccines in the UK but this is not a flu vaccine manufacturer in the EEA
33 See replies to questions 5 and 8 - phase I questionnaire to customers (Germany, the UK)
34 See replies to question 25 - phase I to questionnaire to competitors and to questions 23 - phase I questionnaire to customers (Germany, the UK)
35 See replies to questions 23 - phase I questionnaire to customers (Germany, the UK)
36 See replies to questions 5 and 8 – Phase I questionnaire to customers (Italy, Spain).
37 See replies to question 25 – Phase I questionnaire to competitors and to question 23 – Phase I
questionnaire to customers (Italy, Spain).
38 CSL expects to submit its QIV pipeline product for Marketing Authorisation in Europe in […], using
[…]. Assuming CSL receives this Marketing Authorization, CSL will introduce its QIV vaccine in
Europe with an indication of 5 years and over for the […] flu season.
39 The Target's QIV pipeline products are (i) an adjuvanted QIV for the paediatric segment 6 months – 6
years which, in the EU, is expected to be filed in […] and approved / launched in […] (or potentially
one year later); (ii) FLUCELVAX QIV, for 4 years and older, with an expected US launch in […]
(although whether to bring this vaccine into the EU remains under consideration); and (iii) an
adjuvanted QIV for 65y+, with an expected file in […] and launch in the EU in […].
40 See replies to question 6 – Phase I questionnaire to competitors.
41 See agreed minutes of a call with a competitor dated 22 May 2015.
42 See e.g. https://www.clinicaltrialsregister.eu/ctr-search/trial/2014-001042-24/DE
43 See replies to question 24 – Phase I questionnaire to customers.
44 See replies to question 25 – Phase I questionnaire to customers