Commission, April 21, 2017, No M.8362
EUROPEAN COMMISSION
Judgment
LONZA GROUP / CAPSUGEL
Subject: Case M.8362 - Lonza Group / Capsugel Commission decision pursuant to Article 6(1)(b) of Council Regulation No 139/2004 (1) and Article 57 of the Agreement on the European Economic Area (2)
Dear Sir/Madam,
(1) On 17 March 2017, the European Commission received a notification of a proposed concentration pursuant to Article 4 of Council Regulation (EC) No 139/2004 (3) by which Lonza Group AG ("Lonza", Switzerland) acquires within the meaning of Article 3(1)(b) of the Merger Regulation control of the whole of Capsugel SA ("Capsugel", Luxembourg) by way of purchase of shares (the Transaction) (4). Lonza and Capsugel are collectively referred to as "the Parties".
I. THE PARTIES AND THE OPERATION
(2) Lonza is a company active in the supply of various services from research to final product manufacturing in the pharmaceutical, health care and life science industries worldwide. It is also active in the manufacture and development of active ingredients for use in the agricultural sector, surface protection in various industries, and water treatment.
(3) Capsugel designs, develops and manufactures a range of dosage forms for the pharmaceutical and nutrition industries. Capsugel’s products include oral dosage delivery products, including hard gelatine, soft gelatine, liquid and alternative polymer capsules.
(4) On 14 December 2016, the Parties concluded a stock purchase agreement for Lonza to acquire 100% of Capsugel's shares.
(5) In the light of the above, the Transaction constitutes a concentration within the meaning of Article 3(1)(b) of the Merger Regulation.
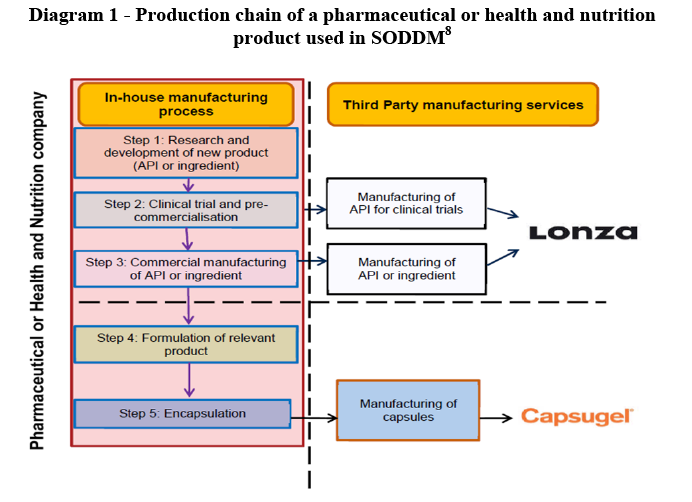
II. EU DIMENSION
(6) The undertakings concerned have a combined aggregate worldwide turnover of more than EUR 2 500 million (5) (Lonza: EUR 3 562 million, Capsugel: EUR 907 million) and a combined aggregate turnover of more than EUR 100 million in each of at least three Member States (France (Lonza: EUR […] million, Capsugel: EUR […] million), Germany (Lonza: EUR […] million, Capsugel: EUR […] million), United Kingdom (Lonza: EUR […] million, Capsugel: EUR […] million). Each of them has an aggregate turnover of more than EUR 25 million in each of at least three of the Member States included above (France, Germany, United Kingdom) and has an EU-wide turnover in excess of EUR 100 million (Lonza: EUR […] million, Capsugel: EUR […] million), but they do not achieve more than two-thirds of their aggregate EU-wide turnover within one and the same Member State.
(7) The notified operation therefore has an EU dimension under Article 1(3) of the Merger Regulation.
III. COMPETITIVE ASSESSMENT
A. INTRODUCTION
(8) Through the proposed acquisition, Lonza's objective is to expand its offering and open up new market opportunities in the pharmaceutical and health and nutrition industries.
(9) The Parties' products are largely complementary.
(10) Lonza is active in contract manufacturing of active pharmaceutical ingredients (APIs) for pharmaceutical customers, and manufactures and sells a few APIs on a proprietary basis. (6) Lonza also manufactures and sells health and nutrition ingredients which are sold as raw materials for health and nutrition customers. (7)
(11) Capsugel is primarily active in the production and supply of various forms of oral dosages in which a drug can be delivered, also called solid oral dosage delivery mechanisms (SODDMs). More specifically, Capsugel provides empty and liquid filled hard capsules as well as soft gelatin and alternative polymer capsules. Capsugel also offers ancillary technology solutions services such as dosage formulation services and development services for solid oral dosage products.
(12) The activities of the Parties are located at different stages of the production process of finished drugs, as illustrated by the graphic overview of the production chain of a pharmaceutical or health and nutrition product used in SODDMs.
(13) As a result, the activities of the Parties do not give rise to any horizontal overlap. The transaction leads to de minimis vertical links in the limited instances where Capsugel purchases ingredients to be directly integrated into the production of its liquid filled capsules. Since the Parties nevertheless have activities in neighbouring markets, the decision will assess the possible conglomerate effects of the Transaction.
B. Market definition
(14) This section defines the relevant markets where Lonza or Capsugel are active, and which are vertically related or neighbouring.
(1) Markets where Lonza is active
i. API contract manufacturing services to pharmaceutical companies
Product market
(15) In previous decisions, the Commission analysed the market for contract manufacturing of finished dose pharmaceuticals, considering possible segmentation by technology and know-how needed to produce different forms of pharmaceuticals (e.g. cytotoxic products) or by type of API used, but ultimately left the exact definition open. The Commission has not previously assessed the market for contract manufacturing of APIs.
(16) The Notifying Party submits that the relevant market for Lonza is contract manufacturing of APIs.
(17) Lonza submits that for the production of APIs, it generally uses basic chemistry production methods. Whilst the manufacturing method will depend on the specific API, in general the process involves the purchase of basic chemicals and reagents (e.g., acids, bases, reducing agents) and industrial solvents (e.g., acetone, methanol, water) which are then chemically processed, isolated and purified (e.g., centrifuged, crystalized, filtered) to produce advance intermediates and the final API. Lonza adds that it also occasionally uses microbial fermentation and enzymatic production during the manufacturing processes, although this is less common and normally specific to a particular process.
(18) The Notifying Party argues that it is not necessary to consider a possible segmentation by manufacturing method because all technologies to manufacture APIs are standard and readily available. The Notifying Party however considers that the precise market definition can be left open.
(19) For the purpose of the present case, the product market definition can be left open, since irrespective of whether the market is defined as encompassing the contract manufacturing of all APIs or is segmented by manufacturing method, the Transaction does not raise serious doubts as to its compatibility with the internal market.
Geographic market
(20) The Notifying Party considers that the relevant geographic market of contract manufacturing of APIs is worldwide.
(21) In its previous decisional practice, (9) the Commission considered that the geographic market for contract manufacturing of finished dose pharmaceuticals is at least EEA-wide and likely to be global but ultimately left the exact geographic scope open. The market investigation in this case confirmed that contract manufacturing of APIs would be at least EEA-wide in scope and possibly worldwide. (10)
(22) For the purpose of the present case, the geographic market definition can be left open, since irrespective of whether the market for contract manufacturing of APIs and its possible segmentations are EEA-wide or global, the Transaction does not raise serious doubts as to its compatibility with the internal market.
ii. API manufacturing on a proprietary basis
Product market
(23) The Commission has previously considered that the manufacturing of APIs forms a distinct relevant product market which is upstream to the market for finished dose pharmaceuticals, and that each individual API may potentially constitute a relevant product market, whereas certain APIs may be substitutable with each other for all, or for a range of applications, but ultimately left the product market definition open.(11)
(24) The Notifying Party considers that the relevant product market is the market for the manufacture of APIs.
(25) For the purpose of the present case, the product market definition can be left open, since irrespective of whether the market is defined as encompassing the manufacturing of all APIs or of individual API, the Transaction does not raise serious doubts as to its compatibility with the internal market.
Geographic market
(26) The Commission has previously considered that the markets for the manufacture and supply of APIs are wider than the market for finished dose pharmaceuticals and possibly worldwide. Nevertheless, the exact scope of the geographic market was left open. (12)
(27) The Notifying Party submits that the market for the manufacture of APIs is global in nature, and if not then at least EEA-wide.
(28) For the purpose of the present case, the geographic market definition can be left open, since irrespective of whether the markets for manufacturing of proprietary APIs and its possible segmentations are EEA-wide or global, the Transaction does not raise serious doubts as to its compatibility with the internal market.
iii. Manufacturing and commercialisation of human health and nutrition ingredients
Product market
(29) The Notifying Party submits that human health and nutrition ingredients belong to a single relevant product market, and that there is no need to segment by production method or by ingredient.
(30) In a previous case,(13) the Commission considered the human health and nutrition market distinguishing between cultures, colour and flavour ingredients for food production and envisaging various sub-delineation within these categories, but ultimately left the precise market definition open. In another case,(14) the Commission considered a market for synthetic emulsifiers, with a possible distinction by type of emulsifiers (15) but ultimately left the market definition open.
(31) In this case, Lonza is manufacturing a small range of nutrition and health ingredients, including among others L-Carnitine, Nicotinates and sorbitan ester products.
(32) For the purpose of the present case, the product market definition can be left open, since irrespective of whether the market for the manufacturing of health and nutrition ingredients is defined as encompassing the manufacturing of all nutrition and health ingredients or is segmented by categories (e.g. by type of synthetic emulsifiers), by production method or by individual ingredient, the Transaction does not raise serious doubts as to its compatibility with the internal market.
Geographic market
(33) According to the Notifying Parties' view, the relevant geographic market definition for manufacturing of human health and nutrition ingredients is global in nature or at least EEA-wide.
(34) As regards the geographic market definition, in previous decisions, the Commission considered the geographic scope of markets for the manufacturing of human health and nutrition ingredients would be at least EEA-wide and possibly worldwide (16), but left the exact definition open.
(35) For the purpose of the present case, the geographic market definition can be left open, since irrespective of whether the market for manufacturing of nutrition and health ingredients and its possible segmentations are EEA-wide or global, the Transaction does not raise serious doubts as to its compatibility with the internal market.
(2) Markets where Capsugel is active
i. Solid oral dosage delivery mechanisms
Product market
(36) In previous decisions (17), the Commission considered the production of solid oral dosage delivery mechanisms (SODDM) as a distinct market that is upstream to the pharmaceutical, over-the-counter (OTC) and nutrition industries. The Commission has also considered both a wider market for the production of SODDM to the pharmaceutical, OTC and nutrition industries and the narrower segments: (i) hard gelatine capsules; (ii) soft gelatine capsules; (iii) liquid filled capsules and (iv) alternative polymer capsules. The Commission ultimately left the product market definition open.
(37) The Notifying Party indicated that the relevant product market is the market for SODDM and there is no need to further define sub-segments according to the different types, i.e. empty hard gelatine capsules, soft gelatine capsules, liquid filled hard capsules and alternative polymer capsules.
(38) The Notifying Party specified that empty hard gelatine capsules have been used in the pharmaceutical industry for over 100 years and submitted that with some limited exceptions, there are no drugs or nutritionals for which hard gelatine capsules are the sole or preferred method of oral dosage, or for which hard gelatine capsules offer significant performance advantages over alternative dosage forms.
(39) The Notifying Party added that other oral dosage mechanisms on the market include soft gelatine capsules, liquid filled hard capsules, alternative polymer capsules, tablets, powders and liquids. Soft gelatine capsules are primarily used to deliver oils and active ingredients that are dissolved or suspended in oil. Traditional hard and soft gelatine capsules are derived from pork or beef products. To meet the dietary restrictions and religious requirements of certain consumers, capsule suppliers have developed alternative polymer capsules, which do not contain any gelatine or other animal products.
(40) In an internal document, another type of capsules is identified, namely inhalation capsules.(18) These capsules would be designed more for respiratory and systemic drug delivery. (19) The Notifying Party considers that it is not a separate segment and that inhalation capsules can be either hard gelatine or alternative polymer capsules.
(41) For the purpose of the present case, the product market definition can be left open, since irrespective of whether the market for the manufacturing of SODDM is defined as encompassing the manufacturing of all SODDM or is segmented by type of capsules (hard gelatine, soft gelatine, alternative polymer and liquid filled), the Transaction does not raise serious doubts as to its compatibility with the internal market.
Geographic market
(42) As regards the geographic market definition, in a previous decision (20) the Commission suggested that it is at least EEA-wide.
(43) For the purpose of the present case, the geographic market definition can be left open, since irrespective of whether the market for manufacturing of SODDM and its possible segmentations are EEA-wide or global, the Transaction does not raise serious doubts as to its compatibility with the internal market.
ii. Dosage formulation and development services
Product market
(44) The Commission did not analyse the market of dosage formulation and development services in its previous decisions.
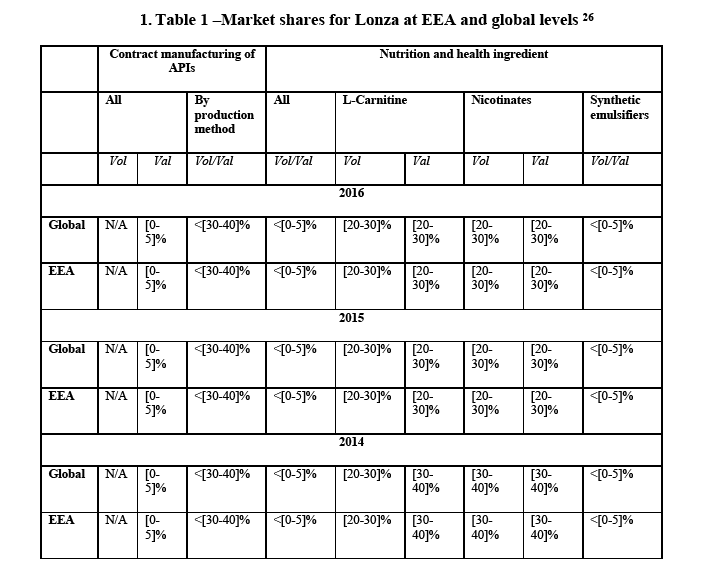
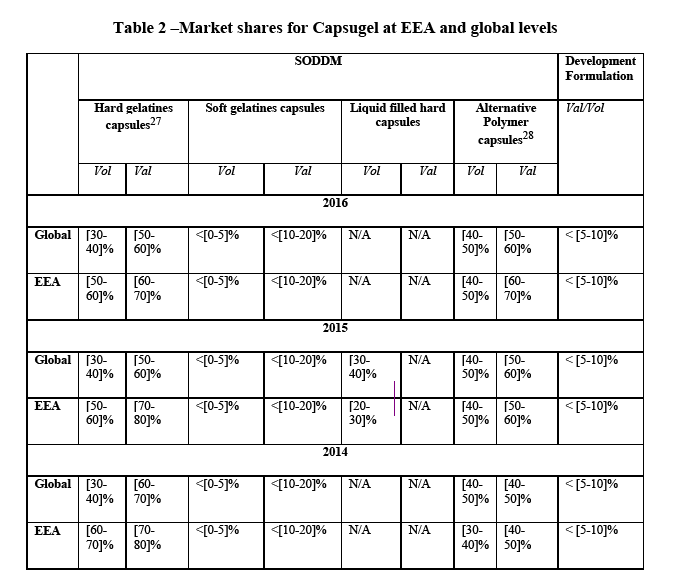
(45) The Notifying Party submits that the relevant product market to be the provision of contract development and manufacturing organisation (CDMO) services which include dosage-related technology and development solutions services for solid oral dosage products to biopharmaceutical and consumer health and nutrition companies. According to the Notifying Party while not all providers offer all the same or identical services, such dosage-related technology and development solutions services for solid oral dosage products can include design of the dosage formulation, technology selection consulting (i.e. identifying the appropriate dosage form for a product), development of targeted delivery technologies (i.e. modified and targeted release solutions), bioavailability enhancement solutions such as the development of spray-dried dispersions and nanoparticle technologies, formulation optimization consulting, etc.
(46) The Notifying party does not consider it appropriate to further segment development services (dosage related technology and development solutions services for solid oral dose products) by technology. Although some variation exists, reportedly the majority of competitors providing these services have a broad menu of offerings similar to Capsugel and customers for solid oral dosage development services generally do not view the sector in any sub-categories by technology, but consider development services to be one sector.
(47) For the purpose of the present case, the product market definition can be left open, since irrespective of whether the market for the dosage formulation and development services is defined as encompassing all CDMO services or is segmented by technology, the Transaction does not raise serious doubts as to its compatibility with the internal market.
Geographic market
(48) According to the Notifying Party's view, the geographic market of dosage formulation and development services is worldwide.
(49) For the purpose of the present case, the geographic market definition can be left open, since irrespective of whether the market for dosage formulation and development services and its possible segmentations are EEA-wide or global, the Transaction does not raise serious doubts as to its compatibility with the internal market.
C. Competitive assessment
(1) Vertical relationships
(50) The Transaction leads to vertical links between the manufacture of nutrition and health ingredients by Lonza, and more specifically the manufacturing of sorbitan ester and L-Carnitine products (Lonza, upstream) and the manufacturing of liquid- filled hard capsules (Capsugel, downstream).
i. Sorbitan ester (Lonza, upstream) and liquid-filled hard capsules (Capsugel, downstream)
(51) Capsugel purchases Lonza's sorbitan ester products (sold under Lonza's trademark Glycomul) and uses them as synthetic emulsifiers in the encapsulation process of its liquid-filled hard capsules for certain health and nutrition customers. More specifically, in 2016, Capsugel purchased EUR […] worth of Glycomul globally ([50-60]% of Lonza's Glycomul sales), all of which outside of the EEA.
(52) The Notifying Party submits that Lonza's market share in the upstream market for the manufacturing and supply of all human health and nutrition ingredients, of synthetic emulsifiers or any possible narrower segmentation including a market limited to sorbitan esters or sorbitan monolaurate is de minimis, less than [0-5]% at global and EEA level.
(53) The Notifying Party submits that Capsugel's market share in the downstream market for all SODDM is [10-20]% at global level and [10-20]% at EEA level, of soft gelatine and liquid-filled hard capsules together is less than [10-20]% at global and EEA level, and for liquid-filled hard capsules is only slightly higher at around [30-40]%, [30-40]% at global level and [20-30]% at EEA-level.
(54) The Commission did not identify any risk of foreclosure post-Transaction.
(55) As to input foreclosure, the market investigation indicated that sufficient alternative sources of supply will remain post-Transaction. (21) Capsugel identified for instance [name of supplier] as an alternative supplier, from whom it used to purchase sorbitan ester. The existence of alternative sources of supply is also confirmed by [name of supplier], the only other current customer of Lonza for Glycomul, which specified that it is a "very small buyer of Glycomul for industrial use coating application".(22)
(56) As to customer foreclosure, in addition to Capsugel having limited purchases of Glycomul, this product has a variety of applications, not limited to food emulsifier, but also including cosmetics, plastics, inks and coatings. Lonza's other customer [name of supplier] is not active like Capsugel in SODDM. Suppliers will therefore continue having sufficient output post-Transaction.
(57) In view of the above, the Transaction does not give rise to serious doubts as to its compatibility with the internal market stemming from the vertical relationship between Lonza sorbitan ester Glycomul product and Capsugel liquid filled hard capsules activities. The same conclusion holds if the markets are defined as broader than sorbitan ester products or liquid filled of hard capsules.
ii. L-Carnitine (Lonza, upstream) and liquid-filled hard capsules (Capsugel, downstream)
(58) Capsugel purchases Lonza's L-Carnitine (Carnipure) product, which is a derivative of amino-acids, for the formulation of certain liquid filled hard capsules. Carnipure is used as raw ingredient in a variety of human and animal health and nutrition products, in order to aid weight management or to supplement a consumer's intake of L-Carnitine through their diet. In 2016, Capsugel purchased EUR […] of Carnipure from Lonza, of which EUR […] were purchased at EEA level.
(59) The Notifying Party submits that Lonza's market share in the upstream market for the manufacturing and supply of nutrition and health ingredients is less than [0-5]% at global or EEA-level. More specifically in the segment for L-Carnitine, its market share decreased from [30-40]% in value and [20-30]% in volume in 2014 to [20- 30]% in value and [20-30]% in volume in 2016 at global level and from [30-40]% in value and [20-30]% in volume in 2014 to [20-30]% in value and [20-30]% in volume in 2016 at EEA level.
(60) As mentioned above in paragraph (53) above, the Notifying Party submits that Capsugel's market share in the downstream market for the manufacture of all SODDM is [10-20]% at global level and [10-20]% at EEA level, of soft gelatine and for liquid-filled hard capsules is only slightly higher at around [10-20]% at global and EEA level, and for liquid-filled hard capsules only slightly around [30- 40]%, [30-40]% at global level and [20-30]% at EEA-level.
(61) The Commission did not identify any risk of foreclosure post-Transaction.
(62) As to input foreclosure, post-Transaction, there will remain several competitors holding market shares above 5% at global and EEA levels, such as Biosint, Liaoning, Northeast pharmaceuticals, Kaiyuan Hangtai Fine Chemicals and Zhejiang Jiashan Chengda. Capsugel's current main supplier of L-Carnitine is [name of supplier]. In addition, Lonza sales of Carnipure to Capsugel represent less than [0-5]% of its total sales of Carnipure. The market investigation confirmed that customers, and in particular Lonza's other customers such as [name of customer] and [name of customer], could find alternative sources of supply and no risk of foreclosure were identified. (23)
(63) As to customer foreclosure, in addition to Capsugel's limited amount of purchases of L-Carnitine, the latter can be sold not only to Capsugel's competitors but also directly to nutrition and health companies. Suppliers will therefore continue having sufficient output post-Transaction.
(64) In view of the above, the Transaction does not give rise to serious doubts as to its compatibility with the internal market stemming from the vertical relationship between Lonza L-Carnitine Carnipure product and Capsugel liquid filled hard capsules activities. The same conclusion holds if the markets are defined as broader than L-Carnitine products or liquid filled of hard capsules.
(2) Conglomerate effects
Introduction
(65) The Commission investigated whether the Transaction would lead to conglomerate effects within the meaning of its Guidelines on the assessment of non-horizontal mergers (non-horizontal merger guidelines), (24) since the Parties have to a certain extent complementary portfolios.
(66) According to the non-horizontal merger guidelines, while non-horizontal mergers are usually not anti-competitive, the combination of products in closely related markets may confer upon the merged entity the ability and incentive to leverage a strong market position in one market to another, by means of tying or bundling. Tying and bundling, as such, often have no anti-competitive effects, as companies engage in tying and bundling in order to provide their customers with better products or offerings in cost-effective ways. In certain circumstances, however, these practices may lead to a reduction in rivals' actual or potential ability or incentive to compete. This could, in turn, reduce the competitive pressure on the merged entity, thus allowing it to increase prices. (25)
Products and markets concerned
(67) The Commission assesses the extent to which the combination of Lonza's activities in contract manufacturing of APIs, manufacturing of proprietary APIs and manufacturing of human health and nutrition ingredients and Capsugel's position in the manufacturing of SODDM and dosage formulation and development services could have anti-competitive conglomerate effects.
(68) Indeed, post-Transaction, the merged entity will be able to provide combined offers, including its pharmaceutical and nutrition and health ingredients as well as formulation and encapsulation services.
(69) The activities of Lonza and Capsugel ultimately address the needs of the same customer base, of the nutrition and health industry and of the pharmaceutical industry. The Parties can be considered as being active in closely related neighbouring product markets.
(70) In the EEA, Capsugel manufactures more specifically a) hard capsules (80-90% of sales), b) soft gelatines capsules and liquid filled hard capsules (<1% of sales) and c) alternative polymer capsules (approx. 10% of sales). Capsugel also performs formulation and development services. The Commission considers that an assessment of potential conglomerate effects is necessary in view of the strong position of Capsugel on the market for SODDM, in particular in relation to the potential sub-segments for hard gelatine capsules, liquid filled capsules and alternative polymer capsules where the market shares are close to or above [30- 40]%.
(71) The Parties' market shares at global and EEA levels in the different markets are summarized in Tables 1 and 2 here below.
(72) Tables 1 and 2 show Capsugel’s strong presence in the EEA in hard capsules, alternative polymer capsules and to a lesser extent, liquid filled hard capsules. The respondents to the market investigation indeed generally considered that Capsugel has a very strong market position in hard capsules especially in Europe. (29)
(73) The Notifying Party submits that the combination of a contract manufacturer and a capsule supplier does not provide the merged entity with any material or insurmountable competitive advantage vis-à-vis customers or competitors. According to the Notifying Party customers have significant and sufficient negotiating strength on a bilateral basis to either continue to buy individual products from the merged entity or, in the alternative, to easily and quickly switch to one of the many rivals of Lonza and Capsugel, for each of the necessary individual inputs, thus defeating any tying or bundling strategy.
(74) The Commission assesses below whether the merged entity could and is likely to engage into tying or bundling strategies and whether such strategies could lead to the exclusion of competitors from the market and ultimately to possible price increases.
(75) Given the nature of the products, there is no technical bundling possible, i.e. – capsules cannot be technically tied so that they could only be used for APIs produced in-house. The merged entity could however offer commercial bundling, which is foreseen in the Transaction synergies through cross-selling of products. (30)
(76) As regards the possibility for commercial bundling, the market investigation showed that the Transaction is not likely to give rise to effects through commercial bundling either, since alternative suppliers for manufacturing of ingredients, such as Catalent, Fareva, Patheon and Evonik for contract manufacturing of APIs to pharmaceutical companies, and for manufacturing of capsules, such as ACG, Qualicaps, Suhueng, and Pharmacapsulas for the manufacture of hard gelatine capsules, would remain on the markets.
(77) Two of the respondents to the market investigation (31) indicated a concern that the merged entity will impose its capsules in the context of its offers for contract manufacturing of APIs, leading to the exclusion of competing capsules providers that would not be able to provide such combined offers. However, other respondents and in particular all the customers considered such scenario unlikely, since they would keep the possibility of buying the products separately from other alternative sources of supply. One respondent replied that “Lonza could try to implement such a practice [imposing to all customers a bundle] but it would likely not be attractive to customers who could then go to other suppliers".(32) Many respondents consider that alternative suppliers both on the market for capsules and pharmaceutical ingredients will remain.(33)
(78) In general, respondents considered that the merged entity “may propose combination solutions comprising multiple products and services” (34) and “customers may wish to consider purchasing such combined solution”.(35) However, as a range of alternative sources of supply will continue to be available, the combined entity “will not be in a position to force customers to purchase a combined package of goods and/or services” (36). Moreover, according to a common customer, if the merged entity will be better to address supply chain needs, it will not engage into imposing commercial bundles as "it would likely alienate customers and drive business away overall".(37) Thus, the allegation by a competitor of Capsugel that customers will likely be locked in (38) is not supported by the overall results of the market investigation. What is more, the market investigation suggests that any anti-competitive bundling strategy would have the effect to make the customers look for alternative suppliers.
(79) In particular, even if Capsugel's market position is strong in hard gelatine and alternative polymer capsules, it faces competitive constraints from existing players such as ACG, Qualicaps, Suhueng, and Pharmacapsulas mentioned above. In addition, several respondents considered that there would not be high barriers to entry for these products.(39) Market participants also indicated that pharmaceutical companies could relatively easily switch supplier of capsules, since this variation does not require an agreement from the regulatory authorities which only need to be informed.(40)
(80) The market investigation showed that the combination of Lonza and Capsugel's products could enhance a new market trend to provide combined offers to customers. One of the market participants outlines the positive effects post- transaction considering that there will be a possibility for "higher level of service and quality through suppliers consolidation and better alignment to pharma industry" with "possible end-to-end, integrated offering for customers with positive business case (i.e. leaner supply chain, easier planning of supply)".(41) Other market participants highlighted that this merger follows a trend in the market to offer the full supply chain services and that other competitors, such as Patheon, started to also implement such strategy.(42) Another respondent considered however that the combined offer could change the competitive landscape as competing companies may not be able to house the expertise of the merged entity and could find it difficult to compete in the development of novel therapeutics. A respondent to the market investigation (43) pointed out that combining the expertise is worth noting because it has the capacity to change the therapeutic development business related to the delivery of novel modality. The synergies of the Transaction anticipated by the Parties confirmed that the merger may create new combination products or development projects, although such projects are not "intrinsic to the deal" and would require substantial investments. (44) However, the market investigation did not bring elements as to why such innovation, which may better address customers' needs, would be ultimately harmful to competition and in particular why competitors would not be able to replicate such strategy.
(81) In light of the above considerations, the Commission did not identify serious doubts as to the compatibility of the Transaction with the internal market stemming from the combination of Lonza's and Capsugel's portfolio of products and services.
IV. CONCLUSION
(82) For the above reasons, the European Commission has decided not to oppose the notified operation and to declare it compatible with the internal market and with the EEA Agreement. This decision is adopted in application of Article 6(1)(b) of the Merger Regulation and Article 57 of the EEA Agreement.
1 OJ L 24, 29.1.2004, p. 1 (the 'Merger Regulation'). With effect from 1 December 2009, the Treaty on the Functioning of the European Union ('TFEU') has introduced certain changes, such as the replacement of 'Community' by 'Union' and 'common market' by 'internal market'. The terminology of the TFEU will be used throughout this decision.
2 OJ L 1, 3.1.1994, p. 3 (the 'EEA Agreement').
3 OJ L 24, 29.1.2004, p. 1 (the "Merger Regulation").
4 Publication in the Official Journal of the European Union No C 96, 28.3.2017, p. 6.
5 Turnover calculated in accordance with Article 5 of the Merger Regulation and the Commission Consolidated Jurisdictional Notice (OJ C 95, 16.4.2008, p. 1).
6 Lonza's sales of proprietary APIs relate mainly to [name of drug] and [name of drug].
7 Lonza's main proprietary nutrition and health ingredients are: (i) L-Carnitine (Carnipure®); (ii) Nicotinates; (iii) a larch bark derived compound consisting of soluble fibre arabinogalactan and bioactive flavonoids (ResistAid®); (iv) Collagen (UC II®); and (v) a combination of zinc monomethionine aspartate, magnesium aspartate and vitamin B6 (ZMA®).
8 Step 5 Encapsulation is a process that only applies to capsules, which are just one type of solid oral dosage form.
9 See e.g. COMP/M.6278 Takeda/Nycomed, paragraph 23; COMP/M.5555 – Novartis / Ebewe, paragraph 19; COMP/M.5530 – Glaxo Smith Kline / Stiefel Laboratories, paragraph 43; COMP/M.5253 - Sanofi-Aventis/Zentiva, paragraph 191-192.
10 See replies to question 2.1.4 of Questionnaire Market Investigation.
11 See e.g. COMP/M.6278 Takeda/Nycomed, paragraphs 17-18; M.5555 – Novartis / Ebewe, paragraph 16; Case COMP/M.5295 - Teva/Barr, paragraph 189; COMP/M.5253 - Sanofi- Aventis/Zentiva, paragraphs 179-181.
12 See e.g. COMP/M.6278 Takeda/Nycomed, paragraphs 19; COMP/M.5555 – Novartis / Ebewe, paragraph 20; COMP/M.5295 - Teva/Barr, paragraph 190; COMP/M.5253 - Sanofi- Aventis/Zentiva, paragraphs 186.
13 See COMP/M.3845 - PAI/Chr. Hansen, paragraphs 10-12.
14 See COMP/M.5109 - Danisco/Abitec, paragraphs 10-25.
15 Undistilled Monoglycerides ("MONO-DI"), Distilled Monoglycerides ("DISMO"), Diacetyl Tartaric Esters of Monoglycerides ("DATEM"), Sodium or Calcium Stearoyl Lactylates ("SSL/CSL") and other emulsifiers.
16 COMP/M.3845 - PAI/Chr. Hansen, paragraphs 13-14; COMP/M.5109 - Danisco/Abitec, paragraphs 27-30.
17 See Case COMP/M.6231 - KKR/Capsugel, paragraphs 10-15. See also Case COMP/M.2922 -Pfizer/Pharmacia
18 Annex 5.4.2 of the Form CO, page 42.
19 http://www.capsugel.com/biopharmaceuticals/capsule-encapsulation-technologies/inhalation- capsules/dpi-technology/
20 See COMP/M.6231 - KKR/Capsugel, paragraph 14
21 Replies to question 4.1 – Questionnaire market investigation.
22 Reply of [name of supplier] to question 4.1 – Questionnaire market investigation.
23 Replies to question 4.1 – Questionnaire market investigation.
24 Commission's Guidelines on the assessment of non-horizontal mergers under the Council Regulation on the control of concentrations between undertakings (OJ C 265, 18.10.2008, p.6).
25 Non-horizontal merger guidelines, paragraph 91.
26 Only nutrition ingredients where market shares of Lonza are close to [30-40]% are added. As to proprietary APIs, Lonza market shares are less than 5% for [name of drug] and less than 25% for [name of drug] both at EEA and global levels (see footnote 6).
27 The market shares in the table reflect Capsugel's market position for hard gelatine capsules overall and a possible segment for inhalation hard gelatine capsules only.
28 The market shares in the table reflect Capsugel's market position for alternative polymer capsules overall and a possible segment for inhalation alternative polymer capsules only.
29 Replies to question 6.2 – Questionnaire market investigation
30 See Form CO, paragraphs 307 and followings.
31 Replies to e.g. question 5.1 – Questionnaire market investigation.
32 Replies to question 7.1 – Questionnaire market investigation.
33 Replies to question 7.2 – Questionnaire market investigation.
34 Reply of a customer to question 7.2 – Questionnaire market investigation.
35 Reply of a customer to question 7.1 – Questionnaire market investigation.
36 Replies to question 7.2 – Questionnaire market investigation
37 Reply of a customer to question 7.3 – Questionnaire market investigation.
38 A reply to question 7.3 – Questionnaire market investigation.
39 See e.g. Minutes of the conference call with a customer, 13 March 2017; Minutes of the conference call with a competitor, 7 March 2017.
40 See e.g. Minutes of the conference call with a customer, 13 March 2017.
41 A reply to question 8 – Questionnaire market investigation.
42 See e.g. Minutes of the conference call with a customer, 13 March 2017; Minutes of the conference call with a competitor, 7 March 2017.
43 Replies to question 7.3 – Questionnaire market investigation.
44 See Annex 5.4.5 of the Form CO, "Project Ama Dablam – Commercial synergies DD", Lonza, 24 November 2016.