Commission, March 13, 2015, No M.7459
EUROPEAN COMMISSION
Judgment
BECTON DICKINSON AND COMPANY/ CAREFUSION
Dear Sir/Madam,
Subject:Case M.7459 - BECTON DICKINSON AND COMPANY / CAREFUSION
Commission decision pursuant to Article 6(1)(b) of Council Regulation No 139/20041 and Article 57 of the Agreement on the European Economic Area2
(1) On 6 February 2015, the European Commission received notification of a proposed concentration pursuant to Article 4 of the Merger Regulation by which the undertakings Becton Dickinson and Company ("Becton Dickinson", "BD" or "the Notifying Party") acquires within the meaning of Article 3(1)(b) of the Merger Regulation control of the whole of the undertaking CareFusion Corporation ("CareFusion" or "CF"), both of the US, by way of purchase of shares.3 Becton Dickinson and CareFusion are collectively referred to as "the Parties".
1.THE PARTIES AND THE OPERATION
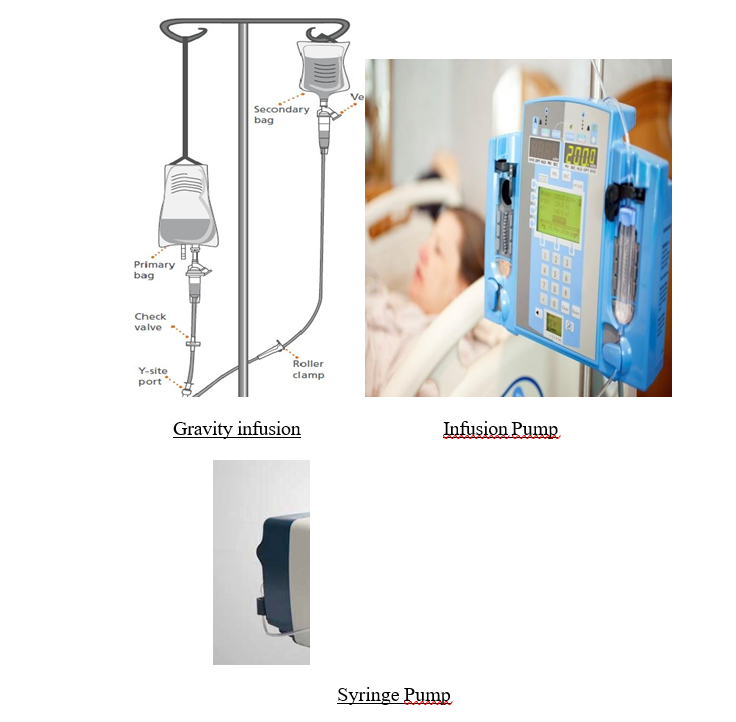
(2) Becton Dickinson is a global medical technology company that develops, manufactures and sells medical devices, instrument systems and reagents. It is focused on improving drug delivery, enhancing the quality and speed of diagnosing infectious diseases and cancers, and advancing research, discovery and production of new drugs and vaccines.
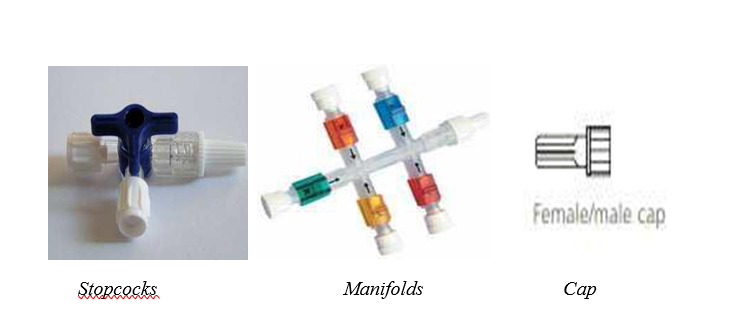
(3) CareFusion is a company active in the health care industry, providing products and services that help hospitals improve the safety and quality of care. It develops technologies including infusion pumps and intravenous ("IV") sets, automated dispensing and patient identification systems, ventilation and respiratory products, surgical instruments, etc.
(4) Becton Dickinson is taking over the entirety of CareFusion (the "Transaction"). Therefore, the Transaction constitutes a concentration within the meaning of Article 3(1)(b) of the Merger Regulation.
2.EU DIMENSION
(5) The undertakings concerned have a combined aggregate world-wide turnover of more than EUR 5 000 million (Becton Dickinson: EUR 6 226 million, CareFusion EUR 2 832 million). Each of them has an EU-wide turnover in excess of EUR 250 million (Becton Dickinson: EUR […], CareFusion EUR […]), and they do not achieve more than two-thirds of their aggregate EU-wide turnover within one and the same Member State. The notified operation therefore has an EU dimension within the meaning of Article 1(2) of the Merger Regulation.
3.COMPETITIVE ASSESSMENT
(6) According to the Notifying Party, the two companies' activities overlap horizontally in a limited number of affected markets. Those include (a) non-dedicated intravenous sets (IV sets); (b) closed system drug-transfer devices ("closed systems"); (c) antimicrobial scrubs; and (d) biopsy needles.
(7) The Transaction also gives rise to a vertical link between the supply of syringes by Becton Dickinson and the supply of syringe-based pumps by CareFusion and a vertical link for the sales of catheters under original equipment manufacturing ("OEM") agreement.4
3.1.Horizontal effects
3.1.1.IV products
3.1.1.1.Relevant product market
(8) IV sets and accessories are consumables used in infusion therapy. Infusion therapy refers to the delivery of medication or other fluids directly into a patient's vein. The pressure required to deliver the medication into the patient's veins can be created through gravity (by placing the medication bag in an elevated position) or through the use of an electric infusion pump or syringe-based pump.
(9) IV sets contain a length of tubing with basic connectors (referred to as "luer" connectors). IV sets are used to connect the IV bag or pump to the patient. They are mainly composed of primary sets, extension sets and secondary sets. A primary set is the main set of tubing used during infusion that is connected to the medication source. An extension set is an IV set that connects to the primary set on one end (using a "female" luer connector), and to the patient on the other (using a "male" luer connector). Secondary sets are sets used either simply to provide additional length to the already existing tubing, or to administer additional fluids which need to be infused but cannot be mixed with the solutions infused through the primary set.
(10) There are a number of accessories that are often used in combination with the IV sets to regulate flow and access to the IV lines, such as needle free connectors, stopcocks, manifolds or caps
(11) Needle-free connectors provide a safe way to access intravenous catheters, which are commonly used to administer fluids, blood, and medications. They greatly reduce the risk of needle stick injuries by allowing IV sets to be connected to catheters without the use of a needle. There are three main types of needle free connectors: (i) split septum; (ii) mechanical valve and (iii) integrated extension lines.
(12) Stopcocks are basic plastic taps that control the flow of fluid into the IV set. Manifolds are simply a series of 3 to 5 stopcocks connected to each other in a single product. Finally, caps are simply basic plastic covers designed to close off an access point to an IV line when not in use.
3.1.1.1.1.Dedicated IV sets vs non-dedicated IV sets
A.Notifying Party's view
(13) According to the Notifying Party, there are two types of IV sets that are used in infusion therapies: dedicated sets and non-dedicated sets. The Notifying Party submits that while the dedicated sets are mainly used with infusion pumps and are customised to be used with a certain manufacturer's pump, the non-dedicated sets are overwhelmingly used with gravity based IV therapy. Technically, non-dedicated IV sets could also be used with IV pumps but this practice is very rare, since it neutralizes the benefit of IV pumps. Indeed, the added value of an IV pump is to precisely measure and control the pressure and flow of fluid in the tubing, which is why they require the use of dedicated IV sets, whose size and tubing diameter is specially tailored for the infusion pump’s microprocessor. Therefore, using a non-dedicated set with an infusion pump, could lead to incorrect calculations and control of the flow of fluid.
(14) The Notifying Party submits that IV dedicated sets represent a different market than the market for non-dedicated IV sets for the following main reasons.
(15) First, dedicated IV sets are designed to operate only with the relevant manufacturer's infusion pumps and cannot be used for gravity-based IV therapy, while the non-dedicate sets are mainly used for the gravity-based infusion. Some producers, when they register an infusion pump for CE marking, specify which dedicated sets are compatible with it and vice-versa.5
(16) Second, dedicated sets are typically more expensive that the non-dedicated sets.
(17) Third, dedicated IV sets typically require a specific component or section of the set to enable recognition of the dedicated set by the pump. A specific mould is required to produce this component. Therefore dedicated and non-dedicated IV sets are normally produced on separate production lines. The production lines are not normally interchangeable and would require modification and specific moulds due to the different components of the sets and switching from non-dedicated IV sets to dedicated and vice versa would require an important investment in terms of tooling, production line, and engineering resources. According to the Parties, switching from dedicated sets to non- dedicated sets would require a EUR […] million expenses, while switching from non- dedicated sets to dedicated sets would be even more onerous in particular because of the intellectual property protecting dedicated sets (CareFusion's internal assumption is EUR […] million investment).
B.Commission's assessment
(18) The Commission has never defined any of these markets before. In a recent case concerning dialysis consumables (comprising mainly bloodlines, dialyser and fluids), the Commission left open whether the market should be defined as an overall product market or per each component.6 More recently, the Spanish Competition Authority (SCA) analysed the IV markets in 20137, when CareFusion acquired Grupo Sendal, a Spanish manufacturer of non-dedicated IV components. The SCA took the view that dedicated IV systems and non-dedicated IV systems belong to different product markets. The SCA also analysed the effect of that transaction on each of the different components of the non- dedicated market on which the companies concerned overlapped.
(19) Respondents to the market investigation indicated that indeed a distinction between dedicated sets and the non-dedicated sets should be made from both demand and supply side.8 Moreover, customers responding to the market investigation have also confirmed that the dedicated IV sets are more expensive than the non-dedicated IV sets.9
(20) In addition, the market investigation responses revealed that different purchasing patterns apply for dedicated IV sets and non-dedicated IV sets. Due to the lack of compatibility between different dedicated IV sets (primary, secondary and extension sets), they are often purchased all together, from the same manufacturer. On the contrary, non-dedicated IV sets from different manufacturers are all interchangeable. They are therefore seldom purchased within a single contract, concluded with the same supplier, but customers usually purchase them as different items separately.10 This has also been confirmed by competitors answering to the market investigation, who mentioned that they usually supply each of these components separately, within separate contracts.11
(21) The Parties' activities do not overlap in dedicated IV sets (Becton Dickinson is not active in this segment).Therefore, this market will not be further analysed.
(22) Regarding a possible further segmentation of non-dedicated IV sets into the three different components (primary, secondary and extension sets), the product market definition can be left open, as the proposed concentration does not raise serious doubts as to its compatibility with the internal market irrespective of any plausible market definition.
3.1.1.1.2.IV accessories (needle free connectors, caps, manifolds and stopcocks)
A.Notifying Party's view
(23) The Notifying Party takes the view that the relevant market should be defined at the level of each non-dedicated IV accessory: needle free connectors, stopcocks/manifolds and caps, as there is no demand side substitutability between these products. As regards supply side considerations, the Notifying Party submits that some degree of supply-side substitutability in the non-dedicated IV set and accessories industry might exist, but not all manufacturers of needle-free connectors necessarily manufacture non-dedicated primary IV sets for example.
(24) The Notifying Party further underlines that, although customers can purchase the different components as stand-alone items and then assemble them themselves, they also purchase IV non-dedicated sets and accessories as a bundle. This notwithstanding, the Notifying Party states that it is rare for hospitals to buy all components together and that they would usually mix and match the components from different suppliers. Therefore, the Parties have also provided market shares for each component separately.
(25) As regards manifolds and stopcocks, the Notifying Party submits that as manifolds represent several stopcocks assembled together, they should be considered as being part of the same segment and not separately considered.
(26) In addition, although the Notifying Party admits that there are several types of needle free connectors, the needle free connectors market should not be further segmented as customers consider them entirely substitutable and the choice of a specific type is a matter of user preference. In addition, most competitors offer all three types of needle free connectors.
B.Commission's assessment
(27) The responses to the market investigation indicated that non-dedicated accessories like needle free connectors, caps, manifolds or stopcocks can be used both with dedicated and with non-dedicated IV sets.12 Furthermore, the majority of customers responding to the market investigation buy each of the different non-dedicated sets or accessories separately and in most instances from different suppliers. Only some of them buy them in a bundle as part of the same contract.13 The reason indicated for such a choice was customers' desire to have the possibility of procuring these products at the best prices. Suppliers have also confirmed this purchasing pattern.14
(28) As regards the difference between manifolds and stopcocks, the market investigation results were mixed. Although there are customers that buy manifolds as a ready-made product, others buy stopcocks and assemble them in order to create as many manifolds as needed.15 On the supply side, competitors have indicated that switching production from stopcocks to manifolds would take approximately 1-2 years and important investments. In addition to that, the know-how associated to the production of manifolds has also been indicated as a barrier to switching production from stopcocks to manifolds.16
(29) In the present case, the product market definition as regards stopcocks and manifolds can be left open, as the proposed concentration does not raise serious doubts as to its compatibility with the internal market with respect to any plausible market definition.
(30) Finally, as to the different types of needle free connectors, the competitors answering to the market investigation mentioned that, although the majority of them produce all three types of needle free connectors, it is not easy to switch production from one type to another due to the different production process, the different moulds that are being used, different design and know-how associated. However, customers have largely confirmed Parties' views as regards demand side substitutability, mentioning that they can use the three main types of needle free connectors interchangeably for a given procedure17. One customer mentioned that "The needle free connectors are overall relatively simple and similar products."18
(31) In the present case, the Commission concludes that the product market definition of each of the non-dedicated accessories (needle free connectors, caps, stopcocks and manifolds) can be left open, as the proposed concentration does not raise serious doubts as to its compatibility with the internal market with respect to any plausible market definition. In addition, the Commission also considers that the exact product market definition for manifolds and stopcocks can be left open as the proposed concentration does not raise serious doubts as to its compatibility with the internal market under any plausible market definition.
3.1.1.2.Geographic market
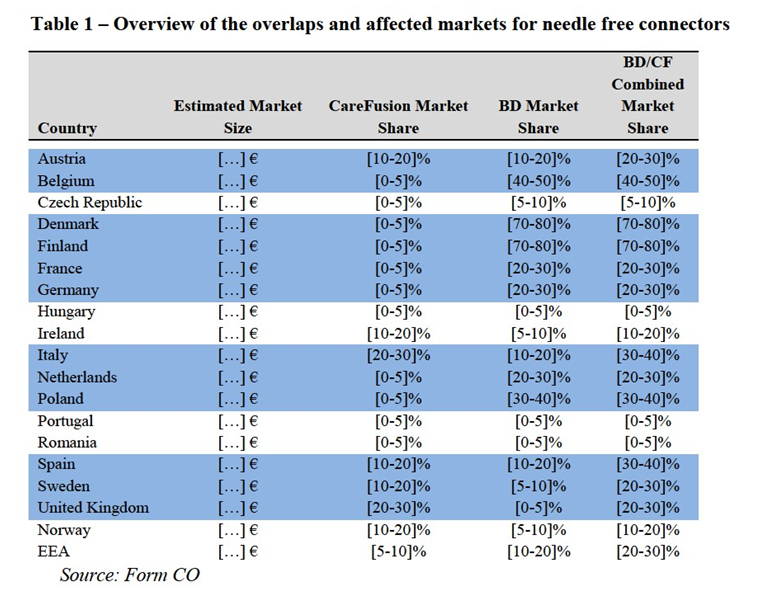
(32) The Notifying Party submits that there is significant evidence suggesting that the relevant geographic market for all the non-dedicated IV sets and accessories are EEA- wide such as low regulatory barriers (single marketing requirement being to obtain the CE mark) or low transport costs.
(33) The market investigation replies reveal that local presence of suppliers of non- dedicated sets and accessories is a requisite in this market. Customers mentioned reasons like security of supply, trainings needed for the use of these products, the need of monitoring of accidents, fast deliveries and communication with the representatives of the suppliers.19
(34) Almost all suppliers have stated during the market investigation that there is a need to have local representatives in order to be able to participate in tenders in a given country in order to be able to provide sales and after sales support, promotion and keeping the contact with clinics.20
(35) In the present case, the geographic market for non-dedicated sets and accessories can be left open, as the proposed concentration does not raise serious doubts as to its compatibility with the internal market with respect to any plausible geographic market definition.
3.1.1.3.Competitive assessment
(36) The Parties are both active in the supply of non-dedicated IV sets and accessories. As regards the IV dedicated sets, there is no overlap as Becton Dickinson is not active in this segment. At component level, the Transaction gives rise to several affected markets in non-dedicated extension sets, needle-free connectors, stopcocks, manifolds and caps.
(37) In the EEA, BD markets non-dedicated primary IV sets under its "R 87" brand and extension sets under its "Connecta" brand. As it does not market non-dedicated IV secondary sets in the EEA, this segment will not be further analysed. BD markets three different needle-free connectors: Q-Syte, a cannula designed to be used with Baxter International Inc.'s Interlink needle-free access device and Posiflow. In the EEA, BD markets stopcocks under its "Connecta Plus" brand and manifolds under its "Connecta Multiflow" brand. BD also markets caps under its "BD Luer-Lok" and simple "BD" brand.
(38) CareFusion markets non-dedicated primary, secondary and extension sets under its SmartSite and MaxPlus brands. CareFusion also markets needle-free connectors under three brand names: SmartSite, MaxPlus and MaxZero. On the contrary, CareFusion markets stand-alone stopcocks and caps without a specific brand name. CareFusion also markets extension sets with integrated stopcocks, including sets marketed under its SmartSite, and MaxPlus brands.
(39) In the absence of reliable public sources for calculating market shares, the Parties have used public reports in the IV field21 and adjusted the shares following a bottom-up approach principally based on tender data and internal best estimates.
3.1.1.3.1.General characteristics of the non-dedicated sets and accessories
(40) The Notifying Party considers that the markets for IV non-dedicated sets and accessories are all mature markets, with an annual estimated growth rate of only [0-5]%. Moreover, the Notifying Party submits that products are heavily commoditised and that there is no brand loyalty. Differentiation is mainly based on price and availability of a wide product range.
(41) As regards switching costs, it further submits that costs are low and mainly correspond to the cost of launching a new tender. Customers are mainly hospitals, which sometimes join in group buying schemes, usually at regional level.
(42) During the market investigation, customers have mentioned that when choosing their suppliers of non-dedicated sets or accessories, the most important criteria they take into account are price, quality and ease of use. The brand does not play an important role in this market, according to customers.22
(43) Especially in the Nordic countries, customers are often represented by central purchasing departments which purchase non-dedicated IV sets and accessories on behalf of several hospitals and clinics.23 Customers use both tendering process and bilateral negotiations when purchasing non-dedicated IV sets or accessories. However, when analysed by country, the Commission observed that Nordic countries like Finland or Denmark use tendering procedure more often and for the majority of their purchases, whereas Spanish or Italians customers use more frequently bilateral negotiations24 Competitors have also confirmed this purchasing pattern of their customers.25
(44) As regards the period of the contracts resulting from tenders, customers have submitted that most often they run from 2-4 years; in several cases an initial period of 2 years is provided and the possibility of prolonging it with 1+1 years.26
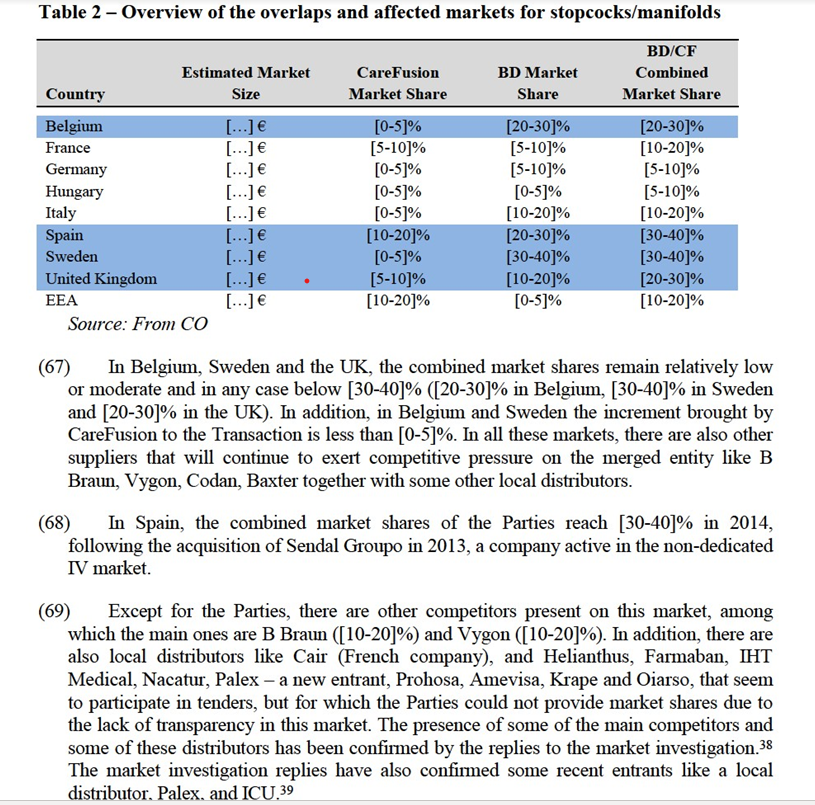
(45) Switching to a different supplier at the end of a contract is considered relatively easy. For example, referring to needle free connectors, one customer said that "switching suppliers is therefore not particularly complicated if the quality is the same."27 Although some training is needed for the new products, this is not perceived as a high barrier for customers to switch to a different supplier.28 Moreover, around 35-40% of the respondent customers have changed suppliers in the last five years.29
3.1.1.3.2.Non-dedicated extension sets
(46) In the non-dedicated extension sets, the Parties' activities give rise to affected markets in Spain ([20-30]% combined market shares) and the UK ([30-40]% combined market share). In both countries the increment is minimal and less than [0-5]%. In addition, there are several other competitors active in these markets that will continue to compete with the merged entity. According to the Notifying Party, in Spain, Hospira (a distributor) with a market share of [30-40]% represents the main challenger of the Parties. Also B Braun ([10- 20]%), Fresenius ([10-20]%) and Vygon ([5-10]%) are offering similar products in this market. In UK, the main challenger is Vygon, with an estimated market share of [40-50]%. According to the Parties, there are also other competitors present like ICU ([10-20]%), Codan, B Braun and Baxter, all three with a comparable market share of [5-10]%.
(47) The Parties argue that especially in UK, the significant pressure to cut costs and the particular set up of the purchasing of these products (above a certain thresholds of £113,057 , it is the National Health Service – NHS – who organises the tenders on behalf of the hospitals), makes the UK market very competitive.
(48) Taking into account the relatively low market shares of the Parties, the minimal increment and the existence of other important competitors that will continue to compete with the merged entity, the Commission takes the view that no serious doubts are likely to arise for non-dedicated extension sets as to the compatibility of the proposed Transaction with the internal market.
3.1.1.3.3.Needle free connectors
(49) In the needle-free connectors, at EEA level the market shares would reach [20-30]% (with an increment of [10-20]%). Taking into account the relatively low market share of the Parties, the fact that there are other strong competitors like B Braun, Vygon, ICU, Codan and several other distributors that will continue to exert competitive pressure on the merged entity, the Commission concludes that no serious doubts are likely to arise for needle free connectors as to the compatibility of the proposed Transaction with the internal market on an EEA level.
(50) At national level, the combined market shares reach more than [20-30]% in Austria, Belgium, Denmark, Finland, France, Germany, Italy, the Netherlands, Poland, Spain, Sweden and the UK.
(51) Table 1 below sets out the Parties' position on the needle free connectors in these countries.
 20]%). These alternative suppliers have been confirmed by the market investigation replies.30
20]%). These alternative suppliers have been confirmed by the market investigation replies.30
(54) In Poland, the combined market shares of the Parties reach [30-40]%, with an increment brought by CareFusion of [0-5]%. According to the Parties, the other main competitors active in this country are Vygon ([20-30]%), B Braun ([10-20]%), ICU ([10-20]%), Codan ([5-10]%) and Baxter ([0-5]%).
(55) In both Italy and Poland, although the combined market shares of the Parties exceed [30-40]%, the Commission takes the view that no serious doubts are likely to arise for needle free connectors as to the compatibility of the proposed Transaction with the internal market for the following reasons: (i) the existence of other important suppliers that will continue to represent a competitive constraint for the merged entity in these markets; (ii) the confirmed new entry, (iii) the absence of any particular concern raised during the market investigation and (iv) the general characteristic of this markets as explained above.
C.Denmark, Finland
(56) In Denmark, the combined market shares of the Parties are high, reaching [70-80]%. According to the Parties, this is due to the position of Becton Dickinson, who has a manufacturing facility in Sweden. However, CareFusion has limited presence here with a market share of only [0-5]%. According to the Notifying Party, there are only two other competitors, namely B Braun ([5-10]%) and Codan ([10-20]%), together with an independent distributor, Mediplast, distributing ICU's MicroClave needle free connector. The Parties consider that the small number of players is due to the small size of the market which makes it less commercially attractive31, while competitors prefer to focus on the main European countries that are commercially more attractive. The estimated size of the Danish market in 2014 was less than EUR [0.8-1] million.
(57) The Parties argue that the position of Becton Dickinson is due to its products which are particularly well received in this country since it entered the market in 1990s. They also argue that the Danish market is mainly a bidding market (approximately 80% of the market). Therefore needle free connectors are often purchased through tender procedures..32
(58) The Commission investigated whether there are enough alternative suppliers to the merged entity, taking into account that switching suppliers in this market appears to be relatively easy. The replies received during the market investigation have indicated indeed Vygon, Codan as alternative suppliers, but also some others as B Braun and Mediplast (distributor).
(59) As regards recent entry, the market investigation confirmed that B Braun has entered the Danish market in the last five years, gaining a market share of [5-10]%.33
(60) Considering the high market shares of Becton Dickinson and the reduced presence of CareFusion, the Commission has also investigated whether CareFusion is a particularly strong innovator in this market. The market investigation replies did not support that thesis.34
(61) Taking into account (i) the small increment of less than [0-5]%, (ii) the existence of other competitors that will continue to compete with the merged entity, (iii) the fact that CareFusion does not seem to be a particularly strong innovator in this field nor to pursue any targeted strategy in this market, (iv) the recent entry and (v) the relative ease for customers to switch suppliers, the Commission takes the view that no serious doubts are likely to arise for needle free connectors in Denmark as to the compatibility of the proposed Transaction with the internal market.
(62) In Finland, the overall competitive situation is similar with Denmark. The combined market share reaches [70-80]%, with an increment of [0-5]%. B Braun is also present with a market share of [5-10]%. According to the Parties, in recent tenders they also met the distributor Mediplast and Codan. The Parties mention that they have no visibility on the rest of the market. During the market investigation, several customers have indicated that there are alternative suppliers such as B Braun or Mediplast. One customer indicated that "there are also other suppliers in the market like B Braun, Steripolar (a distributor), Medic (a distributor), Mediplast, Fresenius or Argon that could supply the same products. This is also true for the particular market of needle free connectors where several suppliers are available."35
(63) B Braun and ICU have indicated that they have recently entered this market, together with Baxter and Fresenius. However, the latter two have not confirmed their entry.36
(64) One customer mentioned that the products of CareFusion and those of Becton Dickinson are more complementary than similar. Moreover, when testing the CareFusion's products, it considered that they "were not as good as Becton Dickinson's products".37
(65) Taking into account (i) the small increment of [0-5]%, (ii) the existence of other competitors that will continue to compete with the merged entity, (iii) the fact that CareFusion neither seems to be an innovator in this field nor to pursue any targeted strategy in this market, (iv) the recent entry and (v) the ease of switching suppliers, the Commission takes the view that no serious doubts are likely to arise for needle free connectors in Finland as to the compatibility of the proposed Transaction with the internal market.
3.1.1.3.4.Stopcocks/manifolds
A.Stopcocks/manifolds
(66) In an overall market for stopcocks/manifolds, the combined market shares reach more than [20-30]% in Belgium, Spain, Sweden and the UK. The EEA market for stopcocks/manifolds would not be an affected market.
 2015 and (iv) the absence of any particular concern expressed during the market investigation, the Commission concludes that no serious doubts are likely to arise for stopcocks/manifolds in Belgium, Spain, Sweden and the UK as to the compatibility of the proposed Transaction with the internal market.
2015 and (iv) the absence of any particular concern expressed during the market investigation, the Commission concludes that no serious doubts are likely to arise for stopcocks/manifolds in Belgium, Spain, Sweden and the UK as to the compatibility of the proposed Transaction with the internal market.
B.Manifolds segment
(72) Should the market of stopcocks/manifolds be further segmented, there will be only two affected markets for the manifold segment, namely Belgium and Spain. However, the combined market shares will remain moderate ([20-30]% in Belgium and [20-30]% in Spain), with very low increments brought by CareFusion ([0-5]% in Belgium and [0-5]% in Spain). The EEA market for stopcocks/manifolds would not be affected.
(73) Moreover, according to the Parties, Becton Dickinson has decided to discontinue its sales of manifolds in the EEA because the market seems no longer economically profitable. The Parties state that, in 2015, they expect to have only few units sold in Poland and that the market shares of Becton Dickinson will be minimal.
(74) Taking into account (i) the general characteristics of these markets presented above, (ii) the moderate market shares of the Parties and small increments, (iii) the existence of other competitors that will continue to exert competitive pressure on the merged entity, (iv) Becton Dickinson's discontinuation of manifolds sales in the course of 2015 and (iv) the absence of any particular concern expressed during the market investigation, the Commission concludes that no serious doubts are likely to arise for manifolds in Belgium and Spain as to the compatibility of the proposed Transaction with the internal market.
C.Stopcocks segment
(75) In the stopcocks segment only, the combined activities of the Parties give rise to five affected markets in Belgium, France, Spain, Sweden and the UK. The EEA market for stopcocks/manifolds would not be an affected market.
(76) In Belgium, France and the UK, the combined market shares remain moderate ([20- 30]% in Belgium, [20-30]% in France and [20-30]% in the UK). In addition, in Belgium and Sweden the increment brought by CareFusion to the Transaction is less than [0-5]%. In all these markets, there are also other suppliers that will continue to exert competitive pressure on the merged entity like B Braun, Codan, Baxter, Fresenius or Vygon, together with some other local distributors.
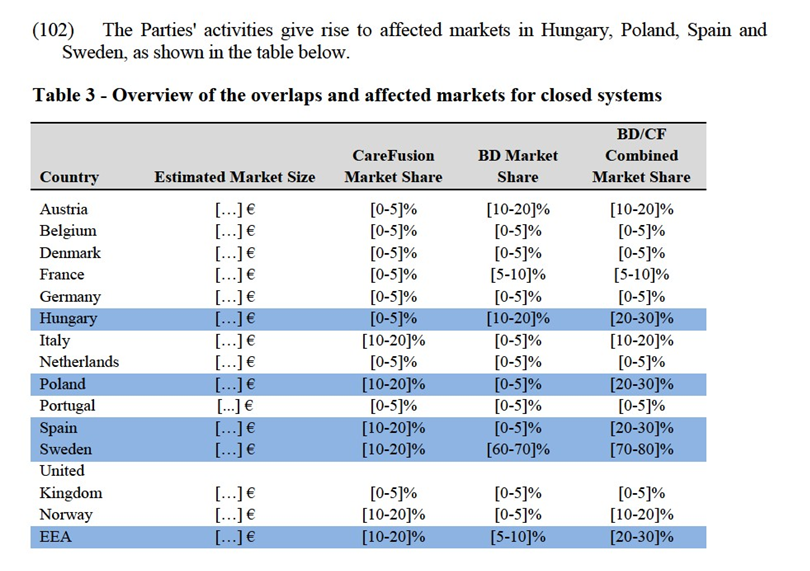
(77) In Spain, the Parties' combined market share amount to [30-40]% with an increment of [10-20]%. There are other competitors present on this market such as B Braun and Vygon, with market shares of [10-20]% and Cair with a market share of [5-10]%. In addition to these competitors, there are several distributors whom the Parties meet in tenders: Cair (French company), and Helianthus, Farmaban, IHT Medical, Nacatur, Palex, Prohosa, Amevisa, Krape and Oiarso (all are local distributors).
(78) Taking into account (i) the general characteristics of this market presented above, (ii) the moderate market shares of the Parties and small increments, (iii) the existence of other competitors that will continue to exert competitive pressure on the merged entity and (iv) the absence of any particular concern expressed during the market investigation, the Commission concludes that no serious doubts are likely to arise for stopcocks in Belgium, France, Spain and the UK as to the compatibility of the proposed Transaction with the internal market.
3.1.1.3.5.Caps
(79) At EEA level the market shares would reach [20-30]% (with an increment of [5- 10]%). Taking into account the relatively low market share of the Parties, the fact that there are other competitors like B Braun, Vygon, ICU, Codan and several other distributors that will continue to exert competitive pressure on the merged entity, the Commission concludes that no serious doubts are likely to arise for caps in the EEA as to the compatibility of the proposed Transaction with the internal market.
(80) At national level, the Parties' activities give rise to three affected national markets, in Belgium, France and the UK.
(81) In Belgium, the combined market shares reach [30-40]%, with an increment of [5- 10]% brought by CareFusion. The total estimated size of the Belgium market of caps is of only EUR […]. Taking this into account and the existence of other main players on this market, with considerable market shares like B Braun ([30-40]%), Fresenius and Baxter (each [10-20]%), the Commission concludes that no serious doubts are likely to arise for caps in Belgium as to the compatibility of the proposed Transaction with the internal market.
(82) In France, the combined market share reaches [30-40]% (with an increment of [10- 20]% of CareFusion), following the acquisition of Groupo Sendal in 2013. The other main competitors in this market include Didactic and B Braun, each with a market share of [10- 20]% and Prompela ([20-30]%market share). The market investigation replies have indicated the existence of several alternative suppliers in caps in the France.40
(83) The Parties submit that Becton Dickinson's sales of caps in France ([20-30]% market share) do not all correspond to the healthcare industry. They indicate that [70- 80]% (or EUR [<200 000]) of its sales of caps in France are made to an aeronautics company based in France. Only the remaining [20-30]% of BD’s sales (EUR [<100 000]) correspond to the healthcare industry. Therefore, the Parties submit that Becton Dickinson's share in caps is overstated and should be approximately [5-10]%.
(84) In the UK, the Parties' combined market share is approximately [40-50]% (with an increment of [5-10]% of Becton Dickinson). A number of other competitors including Vygon, B Braun, Fresenius, Codan, Universal, BMS and Sarstedt, with a comparable market share of [5-10]% each, will continue to exert competitive pressure on the merged entity. The market investigation replies have indicated the existence of several of these alternative suppliers in caps in the UK.41
(85) Taking into account (i) the general characteristics of this market presented above, (ii) the existence of other competitors that will continue to exert competitive pressure on the merged entity, (iii) the fact that a part of Parties' sales of caps in France are not all dedicated to health industry and (iv) the absence of any particular concern expressed during the market investigation, the Commission takes the view that no serious doubts are likely to arise for caps in Belgium, France and the UK as to the compatibility of the proposed Transaction with the internal market.
3.1.2.Closed systems for preparing and administering drugs
3.1.2.1.Relevant product market
(86) Closed system drug-transfer devices are medical devices used to prepare and administer drugs in hospital wards by nurses and in hospital pharmacies by dedicated healthcare personnel which allow for some protection against potential air and liquid leakage. There is a broad range of products used for this purpose from "semi closed" systems to "fully closed" systems depending on the degree of potential leakage.
3.1.2.1.1.Notifying Party's view
(87) The Notifying Party submits that various types of devices are used to prepare and administer drugs, and the choice of system used largely depends on the type of drugs that are handled and the safety level required. Such drug handling can be done by using "open" systems, which do not have any particular valves or device to avoid leakages and represent [40-50]% of the vials prepared, using "semi closed" systems that do not exchange unfiltered air or contaminants with the environment, but are not fully air tight and leak-free and represent [50-60]% of the market, or using "fully" closed systems (i.e. fully air tight and leak-free) that represent [5-10]% of the vials prepared (most of that volume occurring in Sweden).
(88) According to the Notifying Party, hospital pharmacies usually prepare hazardous drugs (such as chemotherapy cytotoxic drugs), while hospital wards usually prepare non-hazardous drugs (such as antibiotics). The former therefore place high importance on avoiding exposure to drugs, and often use fully closed systems or closed handling cabinets. According to the Notifying Party, Sweden is a notable exception, as fully closed systems are also used for the preparation and administration of antibiotics. This can be partly explained by more stringent regulation for handling hazardous drugs (AFS 2005:5, "Chemotherapy and other drugs with lasting toxic effects").
(89) The main components of closed systems are vial access devices (to hook in the vial containing the drug), closed male luers and valves (to transfer the drug into the syringe), and bag access devices with valves (to ensure that no air leaks). Closed vial access devices, closed male luer and bag access devices with valves can be sold and used separately in a non-closed system context and usually do not need to be used with connectors because they are ISO standard (and therefore compatible with components from multiple manufacturers). Nevertheless, this is not the case for all products on the market: some of BD’s PhaSeal components, as well as some of Teva’s Tevadaptor components have proprietary connections, and may therefore not be used with other manufacturers’ components. In addition, according to the Notifying Party, all competitors active in the closed system market sell entire systems, and the various components are intended by the manufacturer to be used together.
(90) The Notifying Party considers that customers perceive all closed systems (from semi closed to fully closed) as broadly substitutable despite the degree of differentiation within them. It submits that the Parties’ tender data reflect this statement, as semi closed and fully closed systems very often compete head to head in the same tenders.
(91) From the supply perspective, the Notifying Party considers that switching production from a semi closed system to a fully closed system is costly and cumbersome as it requires significant investments in the creation of new product lines and as well as patent registrations for the new products and its components. The costs associated with switching production would amount approximately to EUR […] million. The Notifying Party nevertheless underlines that CareFusion was recently able to move closer to the fully closed systems space by adding an airtight vial access device sourced from Yukon that it markets under its SmartSite Vial Shield brand. This SmartSite Vial Shield is then used in conjunction with CareFusion’s Texium to compete with fully closed systems.
(92) The Notifying Party therefore considers that the relevant market is the market for closed systems used to prepare and administer drugs, encompassing semi closed to fully closed systems.
3.1.2.1.2.Commission's assessment
(93) Most customers indicated that usually pharmacists prepare hazardous drugs and nurses antibiotics, including in Sweden.42 The market investigation indicated that there are various ways to prepare drugs, namely using mixing sets, partially and fully closed systems, as well as clean chambers with laminar air flows. Clean chambers and closed systems (either fully or semi-closed) appear to be often used for hazardous drugs such as cytotoxic drugs. Nevertheless, some customers indicate using other devices than closed systems and clean chambers, even to prepare hazardous drugs.43
(94) Likewise regarding the devices that medical staff uses to administer drugs, responses were also overall mixed, as some use closed systems and others use infusion/injection devices also for some hazardous drugs.44 As indicated by a Swedish customer, "Some departments in a hospital make 1 preparation of antibiotics or hazardous drugs per day; some might need to do 50 such preparations. Therefore the risk of exposure is also totally different. An oncology department, which prepares a lot of chemotherapeutic drugs might prefer to only use the fully closed systems, however an orthopaedic department might need only one such a preparation per day". Although some customers and closed systems suppliers indicated that health care personnel in Sweden is more inclined to use fully closed systems even for antibiotics, there was no homogeneous picture in Sweden either.45
(95) Market participants indicated that fully closed systems are more expensive than semi closed systems. Nevertheless, it appears that semi closed systems and fully closed systems can compete in the same tenders.46 The scope of the tenders very much varies from hospital to hospital even in a given country. The market investigation indicated that the scope of tenders depends on their habits and needs: some customers define separate lots for systems for hazardous and systems for antibiotics, while others have one single tender for closed systems without further subcategories.47
(96) From a supply-side perspective, competitors confirmed that fully closed systems are difficult to enter due to very strong IP protections and high investment costs. A competitor indicates that 3 to 5 years and several millions of euros are necessary to develop fully closed systems. Another competitor explains that 1-2 years are required to enter the market and approximately half a million euros to establish evidence data, promotion material and sales representative training.48
(97) In light of the above, the Commission considers that the question whether all closed systems together constitute a product market or whether fully closed and semi closed systems should be considered as separate product markets can be left open, as no competition issues arise under either market definition.
3.1.2.2.Geographic market
(98) The Parties consider that there is significant evidence suggesting that the relevant geographic market for closed systems is EEA-wide. For example, European markets for medical devices such as closed systems are characterised by low regulatory barriers (CE Mark) and low transportation costs.
(99) A large majority of customers indicated in the market investigation that suppliers need to have local representatives based in their country, as presentations of the products, on-site training and assistance in the national language are required.49 Although competitors consider that these support services are typically required by customers, they do not necessarily consider that such a local presence represents a significant barrier to be selected as a supplier. In addition, most competitors consider that there is no certification or any other specific obstacle for suppliers to be able to sell in a given country.50
(100) In the present case, the geographic market for closed systems can be left open, as the proposed concentration does not raise serious doubts as to its compatibility with the internal market with respect to any plausible geographic market definition.
3.1.2.3.Competitive assessment
(101) Becton Dickinson sells a fully closed system called "PhaSeal", while CareFusion markets "Chemo Safety System", which utilises its needle-free connector SmartSite and its luer lock connector Texium.

product is an alternative to CareFusion, these same customers also mention BBraun, Teva or ICU, as close competitors to CareFusion. Some respondents even clearly consider on one hand, CareFusion, ICU (with its Spiros system), BBraun and Codan, as competing against each other, and on the other hand, Becton Dickinson, Teva and the recent entrant Equashield as each other's best alternatives.51
3.1.2.3.2.Hungary, Poland, Spain
(107) In Hungary, Poland and Spain, the combined market shares are between [20-30]%, with increments of [0-5]%. In each of these countries, the Parties face other international competitors such as Teva, BBraun and / or ICU.
(108) In Hungary, CareFusion has a limited increment (between […]EUR over the last 3 years). The market size being small ([<500] kEUR), it is unlikely to sustain a high number of competitors. In any event, other competitors such as Teva and BBraun are present in this market (with [70-80]% and [5-10]% market shares, according to the Parties).
(109) In Spain, ICU (via its distributor Hospira) and BBraun are present (respectively with [40-50]% market share and [10-20]%, according to the Parties).
(110) In Poland, ICU, BBraun and Teva are present (respectively with [40-50]% market share, [20-30]% and [10-20]% according to the Parties).
(111) The Transaction therefore does not give rise to competition concerns in Hungary, Poland, and Spain as regards closed systems for preparing and administering drugs.
3.1.2.3.3.Sweden
(112) On the Swedish market, the combined market shares of the Parties reach [70-80]% with an increment of [10-20]% from CareFusion.
(113) According to the Parties, Becton Dickinson's high market penetration can be explained by the fact that Sweden differs from the other Member States as regards the level of protection imposed by the users of these systems. The Parties consider that this is due to the fact that nurses in wards usually also use fully closed systems even for the preparation of non-hazardous drugs. One competitor confirmed this specificity of Sweden, although another considers that this concerns more broadly Nordic countries.52
(114) Swedish hospitals do not necessarily consider CareFusion's device as suitable for the preparation and administration of hazardous drugs. Conversely, a majority of them mentioned Becton Dickinson and Teva as competing closely against each other in this area. One customer for example explains that Becton Dickinson is the market leader with "a very complete product", and mentions EquaShield and Teva as its closest competitors. Likewise, a competitor indicates that Becton Dickinson has a "leading position", "followed by Teva (with its Tevadaptor which has proven being a fully closed system)"..53
(115) The analysis of the Parties' tender data in Sweden shows that Becton Dickinson and Teva appears to be the strongest market players, as they won a significantly larger number of tenders than the other players. CareFusion, BBraun and/or ICU (distributed by Mediplast) were the other usual participants. When no distinction was drawn between systems for cytotoxic drugs and systems for antibiotics, there were usually slightly more participants in the tender. When hospitals made a distinction between systems for cytotoxic vs antibiotics drugs in their tenders, the contract related to systems for cytotoxic drugs was mostly attributed to Becton Dickinson or Teva.54
(116) In addition, according to the Parties and market participants, Equashield entered the Swedish market in 2015, with Codan distributing its system. Equashield being a fully closed system, it is likely to be a closer alternative to Becton Dickinson than CareFusion currently is and therefore exert competitive pressure on Becton Dickinson. According to one Swedish hospital, "Equashield's [closed system] could then possibly become a competitor to BD or Teva".55
(117) The Transaction therefore does not give rise to competition concerns in Sweden as regards closed systems.
3.1.3.Antimicrobial scrubs
3.1.3.1.Product market definition
(118) Antimicrobial scrubs are single-use, disposable brushes typically used for pre- surgical sterilization and cleaning of the hands and lower arms of medical personnel. These brushes are sold either dry or impregnated with an antimicrobial solution, such as chlorhexidine gluconate (CHG), chloroxylenol (PCMX), or povidone iodine (PVP-I). Thus, if a customer purchases dry brushes, a bulk antimicrobial solution will be required as well.
(119) The Notifying Party submits that the market should be defined as antimicrobial scrubs, comprising both dry and impregnated brushes, as from the customer point of view they are fully substitutable.
(120) From the supply side, the Notifying Party argues that competitors offer either both types of scrubs or only dry scrubs. In addition, the Notifying Party submits that while it would be easy for a manufacturer of impregnated scrubs to produce dry scrubs, impregnated antimicrobial scrubs are more difficult to produce in terms of manufacturing (handling of antiseptic) and regulatory framework. The applicable regulation depends on the intended use of the impregnated scrub and could either be classified as a biocide product, falling within the ambit of the biocide directive, or as a drug.56 In both cases, this would trigger a regulatory-heavy registration process. Finally, impregnated antimicrobial scrubs also have to undergo more quality testing (concentration, stability, shelf life) than dry antimicrobial scrubs.
(121) Although the Commission has not previously analysed the market for antimicrobial scrubs as such, in past decisions concerning surgical products it has divided the market into a number of sub-markets for each different end-use like drapes, gowns, caps, masks, swabs, and scrub suits.57 In addition, the Commission has previously analysed the market for antiseptics used for skin cleansers and concluded that it represents a distinct market from other surgical products.58
(122) In the present case, the product market definition for antimicrobial scrubs can be left open, as the proposed concentration does not raise serious doubts as to its compatibility with the internal market with respect to any plausible market definition.
3.1.3.2.Geographic market definition
(123) The Notifying Party submits that there is significant evidence suggesting that the relevant geographic market for antimicrobial scrubs is EEA-wide such as low regulatory barriers (single marketing requirement being to obtain the CE mark) or low transport costs.
(124) In previous cases dealing with antiseptic solutions and disinfectants, the Commission has considered the markets to be national.59 In a previous case60 dealing with sterile single use medical devices, the Commission has indicated that it may be either EEA-wide or national.
(125) In the present case, the geographic market definition for antimicrobial scrubs can be left open, as the proposed concentration does not raise serious doubts as to its compatibility with the internal market, irrespective of whether the market is defined as national or EEA-wide.
3.1.3.3.Competitive assessment
(126) Both Parties are active in the market for antimicrobial scrubs. Should the market be further divided into dry and impregnated antimicrobial scrubs, the Parties would overlap only on the dry antimicrobial scrubs segment, as none of them are producing impregnated scrubs.61
(127) At EEA level, neither the overall market for all antimicrobial scrubs, nor the dry brushes segment would be an affected market. The combined market shares of the Parties reach [10-20]% on the wider market for all antimicrobials and [10-20]% on the dry segment.
(128) On a narrow national level, the Transaction would result in only one affected market for antimicrobial scrubs in France, with a combined market share of [40-50]% (and a [5- 10]% increment brought by Becton Dickinson). On the dry brushes segment, there are three affected markets: France (combined market share of [60-70]%, increment of [5-10]%), Spain (combined market share of [20-30]%, increment of [5-10]%) and UK (combined market share of [20-30]%, increment of [10-20]%).
(129) The Notifying Party submits that these market share figures vastly overstate its current market position in all these markets, as CareFusion used a third party distributor, [details on distribution strategy]. In addition, the Parties' sales were already declining since 2012, in particular in France.
(130) CareFusion internally projects that its revenues for antimicrobial scrub brushes in France for 2015 are likely to give it a market share of less than [5-10]% and below [10- 20]% in the dry brushes segment. In addition, there are also other competitors present in France like Asept, Apotechnia, Medline, which offer the same products in the market. Equally, CareFusion expects to make no sales in Spain in 2015 and to achieve minimum sales in UK (of only EUR [0-50 000]), reducing its share in the dry brushes segment from [10-20]% in 2014 to only [0-5]% in 2015.
(131) Therefore, in view of the present situation and the declining market shares of CareFusion up to a level of non-affected markets and the existence of other competitors, it follows that no serious doubts are likely to arise for either antimicrobial scrubs or the dry brushes segment to the compatibility of the proposed Transaction with the internal market, no matter the geographic market definition.
3.1.4.Biopsy needles
3.1.4.1.Product market definition
(132) A biopsy is a procedure involving removal of tissue in order to examine it for disease. This can be performed with two types of needles: (i) fine needle aspiration ("FNA") and (ii) core needle biopsy needles ("CNB").
(133) The Notifying Party submits that the relevant product market should comprise the two types of biopsy needles. The Notifying Party argues that further segmentation into "soft tissue needles" and "bone marrow biopsy needles" would be relevant from the demand side, since physicians use different needles in different types of biopsies (depending on the tissue type). However, the Notifying Party considers that a further distinction based on automatisation (into manual, semi-manual or automatic) or reusability (single use and multiple use needles) would not be appropriate as all these types are fully interchangeable from a functional point of view. Equally, on the supply side, the Notifying Party argues that from a manufacturing and know-how standpoint, there is no real barrier to switching from manufacturing one needle type to the other.
(134) The Commission has never analysed the market for biopsy needles. However in a previous decision concerning blood micro sampling devices the Commission confirmed that sterile single use medical devices could constitute one product group or could be split in a number of submarkets.62
(135) In the present case, the product market definition for biopsy needles can be left open, as the proposed concentration does not raise serious doubts as to its compatibility with the internal market with respect to any plausible market definition.
3.1.4.2.Geographic market definition
(136) The Notifying Party submits that there is significant evidence suggesting that the relevant geographic market for biopsy needles is EEA-wide. For example, European markets for medical devices such as biopsy needles are characterised by low regulatory barriers (CE mark) or low transport costs.
(137) In a previous case63 dealing with sterile single use medical devices, the Commission has indicated that it may be either EEA-wide or national. In another case64, the Commission asserted that the guiding catheters and Endoscopic Vessel Harvesting Systems should be analysed at a national level.
(138) In the present case, the geographic market definition for biopsy needles can be left open, as the proposed concentration does not raise serious doubts as to its compatibility with the internal market no matter if the market would be defined as national or EEA- wide level.
3.1.4.3.Competitive assessment
(139) The Parties' activities overlap on the overall market for all biopsy needles and, if further segmented, only in the soft tissue segment. Both are active in the provision of biopsy needles in Austria, Belgium, Czech Republic, France, Germany, Ireland, Italy, Poland, Spain, Sweden, United Kingdom, and Norway.
(140) Regardless of the market segmentation, the Transaction results in only two affected markets: Ireland (combined market share of [20-30]% in the overall biopsy needles market and [20-30]% in soft tissue segment) and the UK ([30-40]% and [30-40]% respectively). The increments are minimal (in Ireland [0-5]% in the overall biopsy needles market and [0- 5]% in soft tissue segment and less than [0-5]% in both markets in the UK). At EEA-wide level, the Parties' combined market share in all biopsy needles is of [5-10]% and in the soft tissue biopsy needles of [5-10]%.
(141) The Notifying Party argues that Becton Dickinson and CareFusion are not close competitors, as Becton Dickinson only sells needles for FNA biopsy, which is a somehow outdated and declining procedure. CareFusion on the contrary sells mainly CNB biopsy needles, which is seen as the dominant soft tissue biopsy method today. There are also a number of important competitors in biopsy needles on both markets.
(142) The Parties submit that UK market is a mature, competitive market with several major players. The main customer is the NHS, which represents over [90-100]% of the market. As described above, the NHS requires that competitive tenders with qualifying criteria be organized for the purchase of biopsy needles. The vast majority of these tenders appear in the Official Journal of the EU. The UK is therefore under significant pressure to make cost savings within the NHS and it renders the landscape very competitive.
(143) In addition, a number of significant competitors including Argon, Bard, and Cook in the UK and Somatex, Bard and Cook in Ireland will continue to exert competitive pressure on the merged entity
(144) Therefore, in view of the low or moderate market shares and the small increments, and the fact that the merged entity will continue to face competitive constraints from other players in this market, it follows that no serious doubts are likely to arise for biopsy needles as to the compatibility of the proposed Transaction with the internal market.
3.2.Vertical effects
(145) The Transaction gives rise to one vertical link between the sales of syringes by Becton Dickinson (upstream market) to CareFusion which resells them with its syringe based pumps or use them for testing with its syringe based pumps (downstream market).
3.2.1.Upstream market: syringes
3.2.1.1.Product market definition
(146) Syringes are devices used to inject/infuse or withdraw fluids or gas. Syringes are typically constructed of glass or plastic and consist of a barrel with measurement lines and a plunger. Syringes are most commonly used for the administration of medications or for blood sampling. They come in various sizes and for various applications, but all syringes share the same general construction and are therefore typically considered by users as part of the same product category.
(147) The Parties estimate that between [10-20]% of the total volume of syringes are currently acquired with the potential intention to be used with syringe pumps. Syringes are not dedicated to pumps and can be used either with pumps or as a standalone product. The same type of syringe is used manually as standalone device and for a lesser extent with pumps that have been set up to work with syringes from different manufacturers. Therefore, according to the Parties, there is no basis for defining a sub- segment for syringes to be used in pumps, and the relevant market is the supply of syringes on an original equipment manufacturer (OEM) basis.
(148) The Commission has not considered the market for syringes in previous decisions.
(149) In the present case, the product market definition for syringes on an OEM basis can be left open, as the proposed concentration does not raise serious doubts as to its compatibility with the internal market with respect to any plausible market definition.
3.2.1.2.Geographic market definition
(150) The Parties submit that the market for supply of syringes between major OEMs is at least EEA-wide, if not worldwide: contracts are concluded for global purchases. According to the Parties, the competitors on this market are global companies and products are manufactured across the globe and delivered to any worldwide location regardless of the final destination for these products.
(151) In the present case, the geographic market definition for syringes can be left open, as the proposed concentration does not raise serious doubts as to its compatibility with the internal market with respect to any plausible market definition
3.2.2.Downstream market: syringe based-pumps
(152) Syringe pumps are used to gradually administer small amounts of fluid (with or without medication) to a patient or for use in chemical and biomedical research. Syringe pumps are useful for delivering IV medications over several minutes, as they save staff time and reduce errors. Syringe pumps use a regular syringe as a reservoir. Syringes and extension sets used with the syringe pumps are generally “non-dedicated” (i.e. any company can offer them).
(153) Syringe pump producers source syringes for two purposes: (i) testing the syringe in order to ensure that the pump is compatible with a syringe of a given producer and (ii) reselling the syringes together with the pump. However, in the present case, the product market definition for syringe pumps can be left open, as the proposed concentration does not raise serious doubts as to its compatibility with the internal market with respect to any plausible market definition.
(154) The Parties submit that the market for syringe pumps on an OEM basis is EEA wide, since the syringe pump producers are global players and the OEM agreements are also usually concluded on a global scale.
(155) In any event, the geographic market definition for syringe based pumps can be left open, as the proposed concentration does not raise serious doubts as to its compatibility with the internal market under any geographical market definition.
3.2.3.Competitive assessment
(156) Becton Dickinson sells conventional syringes to CareFusion under an OEM agreement. CareFusion then uses these syringes for two purposes. One is for testing in order to ensure that the Alaris pumps are compatible with the syringes manufactured by Becton Dickinson.65 Sometimes, these are offered for free. The second purpose is for selling syringes after sterilisation with its Alaris range of syringe pumps. They sell the pumps together with a syringe as a kit mainly in Spain, due to market practice in Spain, according to the Parties.66 CareFusion purchases about [10-20]% of the syringes it sells in Spain from Becton Dickinson and the remaining [80-90]% from [supplier].
(157) According to the Parties, Becton Dickinson's market share in the syringe market is [40-50]% EEA-wide. The value of Becton Dickinson's sales to CareFusion in the EEA was only EUR [<200 000], representing [0-5]% of total Becton Dickinson's sales of syringes.67 CareFusion's share of syringe pumps in the EEA is approximately [20-30]%.
(158) The Commission investigated whether CareFusion could start procuring all of its syringes from Becton Dickinson and whether this would deny access to the market to Becton Dickinson's competitors.
(159) Even if CareFusion was to source all of its requirements exclusively from Becton Dickinson, the other supplier of CareFusion, [supplier], could find other customers of syringe pumps producers, as the CareFusion's market share on the syringe pumps in the EEA is moderate (only [20-30]%). B Braun is leading the market with around [30-40]% market share, followed by Fresenius Kabi (approximately [20-30]%), Smiths Medical (approximately [10-20]%), McKinley Medical and KD Scientific. Therefore there are other strong alternative producers of syringe pumps.
(160) With the view of a moderate market share of CareFusion in the syringe pumps market and the presence of strong competitors active in the downstream market, the Transaction will not give the merged entity the ability and incentive to enter into a customers' foreclosure strategy.
(161) Secondly, Becton Dickinson could stop supplying syringes to competitors in the syringe pumps market and thus cut off their access to syringes for resale with their pumps an input (input foreclosure).
(162) The Parties consider that the combined entity will not have the ability to foreclose rivals in the supply of syringes, since syringes are mature technologies, very commoditized and there are other strong alternative suppliers of syringes. Those include B.Braun, Terumo, Nipro, Covidien, Weigao and Hindustan Medical Devices (the last two are low cost manufacturers). In addition, CareFusion's purchases represent only EUR [<1 000 000] in the EEA, i.e. a small part of the market (as a comparison, in the EEA, Becton Dickinson's sales of syringes to other producers of syringe-based pumps represent EUR [<2 500 000] and its syringe sales to any type of customer represent EUR [80 000 000 - 90 000 000]). The Parties note that all the main syringe pump producers are already vertically integrated and also sell syringes (e.g. BBraun, Fresenius): they would therefore be able to compete with the merged entity.
(163) The Parties submit that the combined entity would have no incentive to engage in the input foreclosure strategy. Firstly, the supply of syringes to other syringe pump manufacturers is a source of additional revenue. Becton Dickinson further asserts that it is a trusted supplier of syringes to the medical world and if it disrupted the supply of syringes by raising prices, its reputation would be severely affected. Furthermore, the sales of syringes to other producers of syringe-based pumps represent a small part ([0- 5]%) of Becton Dickinson's syringe business in the EEA.
(164) In view of the presence of other strong alternative suppliers in both syringes and syringe pumps and a modest market share of the Parties in both segments, it follows that no serious doubts are likely to arise for with regard to the vertical link between syringes and syringe based pumps as to the compatibility of the proposed Transaction with the internal market
4.CONCLUSION
(165) For the above reasons, the European Commission has decided not to oppose the notified operation and to declare it compatible with the internal market and with the EEA Agreement. This decision is adopted in application of Article 6(1)(b) of the Merger Regulation and Article 57 of the EEA Agreement.
1 OJ L 24, 29.1.2004, p. 1 ('the Merger Regulation'). With effect from 1 December 2009, the Treaty on the Functioning of the European Union ('TFEU') has introduced certain changes, such as the replacement of 'Community' by 'Union' and 'common market' by 'internal market'. The terminology of the TFEU will be used throughout this decision.
2 OJ L 1, 3.1.1994, p.3 ("the EEA Agreement").
3 Publication in the Official Journal of the European Union No C55, 14.02.2015, p. 14.
4 The Transaction also gives rise to three other vertical links. First one is the manufacturing of different components (connectors and secondary IV set) for closed systems. Becton Dickinson is currently buying these components only from CareFusion, and CareFusion has no other customer for such products. The second link refers to the supply of non-dedicated IV components (by CareFusion) under OEM agreement for re-selling. These products are (i) luer-lock adapters with caps, (ii) Tutodrop IV flow controllers and (iii) female luer-lock adapters, which Becton Dickinson currently buys only from CareFusion. In view of this and the low market shares of CareFusion on these potential markets, the Commission will not further analyse these two vertical links. Finally, BD is selling peripheral intravenous catheters, which CareFusion is buying in the EEA from other suppliers for the sale in UK, France and Spain. Taking into account the minor sales made by BD in the EEA of only EUR [<200 000] and also the sales of these products by CareFusion of EUR [100 000-300 000] and that each of the Parties' market shares in the respective markets are relatively low, this link will also not be further analysed.
5 Some infusion pump producers manufacture devices which can be referred to as ‘open systems’ that can operate with or without a ‘dedicated’ IV set. The generic term "open systems" refers to pumps that can accept compatible IV sets produced by competing manufacturers such as, for example, B.Braun or Codan. However, these open-systems used in combination with compatible IV sets do not offer the same guarantees in terms of infusion accuracy and safety than dedicated IV sets used with pumps. Such systems are not as sophisticated or as precise as infusion pumps with dedicated IV sets.
6 Case M. 6851 – Baxter International / Gambro.
7 Case C-0523/13 CareFusion/Grupo Sendal.
8 Replies to questionnaire to customers "Q1- Questionnaire IV products", question 4 and to questionnaire "Q3 – Questionnaire to competitors", question 31.
9 Replies to questionnaire to customers "Q1- Questionnaire IV products", question 5.
10 Replies to questionnaire to customers "Q1- Questionnaire IV products", question 8.
11 Replies to questionnaire "Q3 – Questionnaire to competitors", question 37.
12 Replies to questionnaire to customers "Q1- Questionnaire IV products", question 7.
13 Replies to questionnaire to customers "Q1- Questionnaire IV products", question 8; Minutes of the conference call with a customer on 22 January 2015; Minutes of the conference call with a customer from 19 January 2015.
14 Replies to questionnaire "Q3 – Questionnaire to competitors", question 37.
15 Replies to questionnaire to customers "Q1 - Questionnaire IV products", question 10.
16 Replies to questionnaire "Q3 - Questionnaire to competitors", question 34.
17 Replies to questionnaire to customers "Q1 - Questionnaire IV products", question 9.
18 Minutes of the conference call with a customer on 19 January 2015.
19 Replies to questionnaire to customers "Q1 - Questionnaire IV products", question 11.
20 Replies to questionnaire "Q3 – Questionnaire to competitors", question 39.
21 To estimate market sizes for IV sets and accessories, BD used European IV Therapy and Enteral Nutrition Devices Market (Publisher: Frost & Sullivan – Published: September 2009, submitted as Confidential Annex 101) and IMS Data for Germany (GPI Dataview® medical devices - Publisher: IMS Data – Published: November 2014) and derived country market sizes based on population.
22 Replies to questionnaire to customers "Q1- Questionnaire IV products", question 17, and minutes of the conference call with a customer on 19 January 2015.
23 Minutes of the conference call with a customer on 22 January 2015, minutes of the conference call with a customer of 19 January 2015.
24 Replies to questionnaire to customers "Q1- Questionnaire IV products", question 13.
25 Replies to questionnaire "Q3 – Questionnaire to competitors",question 40.
26 Replies to questionnaire to customers "Q1- Questionnaire IV products", question 14.
27 Minutes of the conference call with a customer of 19 January 2015.
28 Replies to questionnaire to customers "Q1- Questionnaire IV products", question 18.
29 Replies to questionnaire to customers "Q1- Questionnaire IV products", question 19.
30 Replies to questionnaire to customers "Q1- Questionnaire IV products", question 18.
31 In 2013 the Danish market represented [0.8-1] million EUR and [1-1.2] the Finnish one.
32 Replies to questionnaire to customers "Q1- Questionnaire IV products", question 14.
33 Replies to questionnaire "Q3 – Questionnaire to competitors", question 44.
34 Replies to questionnaire to customers "Q1- Questionnaire IV products", question 20 and replies to questionnaire "Q3 – Questionnaire to competitors", question 40.
35 Minutes of the conference call with a customer from 22 January 2015.
36 Replies to questionnaire "Q3 – Questionnaire to competitors", question 44.
37 Minutes of the conference call with a customer from 19 January 2015.
38 Replies to questionnaire to customers « Q1-Questionnaire IV products », question 18.
39 Replies to questionnaire to customers « Q3-Questionnaire to competitors » question 44.
40 Replies to questionnaire to customers "Q1- Questionnaire IV products", question 18.
41 Replies to questionnaire to customers "Q1- Questionnaire IV products", question 18.
42 Replies to questionnaire "Q2 - Questionnaire to customers – closed systems drug-transfer devices", question 6.
43 Replies to questionnaire "Q2 - Questionnaire to customers – closed systems drug-transfer devices", questions 6.2 and 13, and to questionnaire "Q3 – Questionnaire to competitors", question 8.
44 Replies to questionnaire "Q2 - Questionnaire to customers – closed systems drug-transfer devices", question 7 and to questionnaire "Q3 – Questionnaire to competitors", question 9.
45 Minutes of the conference call with a customer on 26 January 2015, paragraphs 7 and 8; Minutes of the conference call with a competitor on 23 January 2015, paragraph 17.
46 Minutes of the conference call with a competitor on 23 January 2015, paragraph 16; Minutes of the conference call with a customer on 26 January 2015, paragraph 8; Parties' reply to Request For Information #6, Annex 125 (Parties' tender data in Sweden).
47 Replies to questionnaire "Q2 - Questionnaire to customers – closed systems drug-transfer devices", question 10 and replies to questionnaire "Q3 – Questionnaire to competitors", question 14; Parties' reply to Request For Information #6, Annex 125 (Parties' tender data in Sweden).
48 Replies to questionnaire "Q3 – Questionnaire to competitors", question 11; Minutes of the conference call with a competitor on 23 January 2015, paragraph 18.
49 Replies to questionnaire "Q2 - Questionnaire to customers – closed systems drug-transfer devices", question 11.
50 Replies to questionnaire "Q3 – Questionnaire to competitors", questions 16 and 22.
51 Replies to questionnaire "Q2 - Questionnaire to customers – closed systems drug-transfer devices", questions 14, 17 and 18 and replies to questionnaire "Q3 – Questionnaire to competitors", question 20 and 21; Minutes of the conference call with a competitor on 23 January 2015, paragraphs 13 and 15; Minutes of the conference call with a competitor on 26 January 2015, paragraph 8; Minutes of the conference call with a customer on 26 January 2015, paragraph 9.
52 Replies to questionnaire "Q3 – Questionnaire to competitors", question 12; Minutes of the conference call with a competitor on 23 January 2015, paragraph 17.
53 Replies to questionnaire "Q2 - Questionnaire to customers – closed systems drug-transfer devices", questions 13, 14, 17 and 18; Minutes of the conference call with a customer on 26 January 2015, paragraph 3; Minutes of the conference call with a competitor on 28 January 2015, paragraph 8.
54 Replies to questionnaire "Q3 – Questionnaire to competitors", question 12; Parties' reply to Request For Information #6, Annex 125 (Parties' tender data in Sweden).
55 Minutes of the conference call with a customer on 26 January 2015, paragraph 9.
56 According to the Notifying Party, in a vast majority of cases, antiseptic liquids for scrubs are classified as biocides under the EU directive 98/8/EC (amended by directive 2013/7/EU) and by the EU Biocide Product regulation 528/2012. The classification depends on the substance, and on the claims. Scrub brushes used for pre-operative skin preparation of the patient would have to be registered as drugs in most EU countries.
57 Case M.4229 - APHL/Netcare/General Healthcare, Case M.3816 - Apax/Mölnlycke, Case M.4367 - APW / APSA / NORDIC CAPITAL / CAPIO, Case M.3816 - Apax/Mölnlycke.
58 Case M.4229 - APHL/Netcare/General Healthcare
59 Case M.4007 - Reckitt Benckiser/Boots Healthcare International.
60 Case M.4367 - APW/Apsa/Nordic Capital/Capio.
61 In February 2010, in consideration of the evolution of these regulations and local implementation, BD decided to discontinue the sale of antimicrobial scrubs impregnated with CHG or PCMX in the EU and the EEA. Iodine Povidone impregnated antimicrobial scrubs were then finally discontinued in December 2010. Since then, BD does not sell impregnated antimicrobial scrubs in the EU and the EEA.
62 Case M.7058 - EQT VI / Terveystalo Healthcare, paragraph 19; Case M.4367 - APW/Apsa/Nordic Capital/Capio, para 17 and 28.
63 Case M.4367 - APW/Apsa/Nordic Capital/Capio.
64 Case M.3687 - JOHNSON&JOHNSON/GUIDANT.
65 Syringe pumps need a syringe in order to operate and syringe pumps producers must ensure that these syringes are compatible with their syringe pumps. A lack of compatibility would jeopardise the accuracy and safety provided by syringe pumps. CareFusion makes sure that the pump’s software work together to deliver the medication optimally. The pump’s software must recognise the specifications of the different syringes used with the pump. Therefore, CareFusion tests various brands of syringes internally and adapt the software before listing other manufacturers’ syringes as compatible with their pump.
66 According to the Parties, CareFusion sold syringes only marginally as standalone products. In 2014, this amounted to a one-off sale worth approximately EUR [<50 000].
67 Becton Dickinson's sales to other producers of syringe-based pumps represented only [0-5]% of total Becton Dickinson's sales of syringes.