Commission, March 16, 2015, No M.7480
EUROPEAN COMMISSION
Judgment
ACTAVIS/ ALLERGAN
Dear Sir/Madam,
Subject:M.7480 – Actavis/ Allergan
Commission decision pursuant to Article 6(1)(b) of Council Regulation No 139/20041 and Article 57 of the Agreement on the European Economic Area2
(1) On 17 February 2015, the European Commission received notification of a proposed concentration pursuant to Article 4 of the Merger Regulation by which which Actavis plc ("Actavis", Ireland) acquires within the meaning of Article 3(1)(b) of the Merger Regulation control of the whole of Allergan Inc. ("Allergan", US) by way of purchase of shares.3
1.THE PARTIES AND THE OPERATION
(2) Actavis is an integrated global specialty pharmaceutical company engaged in the development, manufacturing, marketing, sale and distribution of branded originator, generic, branded generic, biosimilar and over-the-counter pharmaceutical products. Actavis’s principal executive offices are located in Dublin, Ireland, and it is listed on the New York Stock Exchange.
(3) Allergan is a multi-specialty healthcare company with a portfolio of pharmaceuticals, biologics, medical devices and over-the-counter consumer products, with a focus on several medical specialties, including ophthalmology, neurology, medical aesthetics (including breast aesthetics), medical dermatology and urology. Allergan’s corporate headquarters are located in Irvine, California and it is listed on the New York Stock Exchange.
(4) The transaction consists in the acquisition of 100% of the share capital of Allergan by Actavis through cash and shares. A newly created, indirect wholly controlled subsidiary of Actavis will merge with Allergan, which will then become a wholly owned subsidiary of Actavis.
(5) Therefore, the proposed transaction constitutes a concentration within the meaning of Article 3(1)(b) of the Merger Regulation.
2.EU DIMENSION
(6) The undertakings concerned have a combined aggregate world-wide turnover of more than EUR 5 000 million4 (Actavis: EUR 8 128 million, Allergan: EUR 4 666 million). Each of them has an EU-wide turnover in excess of EUR 250 million (Actavis: […] million, Allergan: EUR […] million), but they do not achieve more than two-thirds of their aggregate EU-wide turnover within one and the same Member State.
(7) The notified operation therefore has an EU dimension within the meaning of Article 1(2) of the Merger regulation.
3.COMPETITIVE ASSESSMENT
3.1.Framework for assessment
(8) The Parties' activities overlap regarding ophthalmic pharmaceutical products (pharmaceuticals used in the treatment of diseases of the eye) and other eye care products (consumer vision products) that may be placed on the market as medical devices (CE-marked products) or as pharmaceuticals.
ATC classification for pharmaceuticals
(9) When assessing the relevant market definition in past decisions dealing with pharmaceutical products,5 the Commission noted that medicines may be subdivided into therapeutic classes by reference to the "Anatomical Therapeutic Classification" (ATC), devised by the European Pharmaceutical Marketing Research Association (EphMRA) and maintained by EphMRA and Intercontinental Medical Statistics (IMS).6
(10) The ATC system is a hierarchical and coded four-level system which classifies medicinal products according to their indication, therapeutic use, composition and mode of action. In the first and broadest level (ATC1), medicinal products are divided into the 16 anatomical main groups. The second level (ATC2) is either a pharmacological or therapeutic group. The third level (ATC3) further groups medicinal products by their specific therapeutic indications, i.e. their intended use (e.g. S1K - Artificial tears and ocular lubricants). Finally, the ATC4 level is the most detailed one (not available for all ATC3) and refers for instance to the mode of action (e.g. distinction of some ATC3 classes into topical and systemic depending on their way of action) or any other subdivision of the group.
(11) In its past merger decisions in the pharmaceutical sector, the Commission has referred to the third level (ATC3) as the starting point for defining the relevant product market. However, in a number of cases, the Commission found that the ATC3 level classification did not yield the appropriate market definition within the meaning of the Commission Notice on the Definition of the Relevant Market. As a result, where appropriate and based on the factual evidence collected during the market investigation, the Commission has defined the relevant product market at the ATC4 level or at a level of molecule or a group of molecules that are considered interchangeable so as to exercise competitive pressure on one another.7 The overlap in therapeutic uses does not necessarily imply any particular economic substitution patterns between products.
Consumer vision
(12) Consumer vision care products, such as ocular lubricants, are often not marketed as pharmaceutical products. In its past merger decisions,8 the Commission noted that for ocular lubricants the qualification as a pharmaceutical or a medical device may vary across Member States.9 The active ingredients in lens care preparations and ocular lubricants are generally common chemical compositions and not under patent protection. Notwithstanding that consumer vision products are often not pharmaceutical products, they are classified under the ATC2 (second level of the ATC classification) category S1. Each wider consumer vision product category is then grouped under a specific ATC3 class: for ease of reference, the relevant ATC3 level codes will therefore be used in the assessment below.
(13) Furthermore, consumer vision products can come in various galenic forms (e.g. drops, gels or ointments). As the Commission has acknowledged in its previous decisions,10 medicines are differentiated not only by their active ingredient(s), but also, in particular, as recognized by the European regulatory framework for medicines for human use, by their dosage, pharmaceutical form and route of administration and this may limit their substitutability.11
Geographic market definition
(14) The Commission considered in the past the national level as the relevant geographic level for analysing both pharmaceuticals and consumer vision products.12 The Notifying Party did not contest this, and the market investigation did not provide any indications to the contrary.
Pipeline products
(15) In its previous decisions, the Commission assessed the competitive constraint likely to be exerted by products in research & development on existing product markets as well on possible future markets.13 The Commission concluded that the potential for these products to enter into competition with other products which are either on the market or at the development stage should be assessed by reference to their characteristics, intended therapeutic use, and expected therapeutic and economic substitutability.14
(16) In particular generic companies are usually developing a number of pipeline generic drugs which are intended to compete with originators which come off- patent.15
(17) As regards the geographic dimension of pipeline pharmaceuticals, in line with previous decisions, the Commission considers that since pipeline products need to be assessed with reference to the R&D in a given area and if the extent that R&D for the relevant products is global, the geographic scope of the market should be global or at least be EEA-wide.16
(18) Both Actavis and Allergan invest in the development of new products. Contrary to some previous cases, none of the pipeline in this case concerns a situation whereby a Party would be the first generic entering the market after the loss of exclusivity of the other Party (the specific case of bimatoprost is discussed further below).
(19) The Parties have identified in the Form CO product markets where the other Party has an existing market share and the other party is planning to enter:
a. Actavis is planning to enter with a pipeline bimatoprost generic product, for which Allergan is the originator, and a pipeline travoprost product, which falls in the same ATC3 category (see section 3.4);
b. Allergan has three pipeline indications for Botox in therapeutic areas where Actavis has marketed treatments (see section 3.2);
c. Allergan is developing a nasal spray, SER-120 (AGN 235258), based on desmopressin, while Actavis currently sells a desmopressin nasal spray. However, as Actavis' market share for desmopressin on all countries where it is active is generally less than [0-5]% and in all cases under [5-10]%, this overlap will not be discussed further;
d. Allergan has a product, Restasis, based on cyclosporine, approved in the US but in phase II trials in the EU. Actavis filed an application to the US Food and Drug Administration for a generic version of Restasis in November 2011, but later withdrew its application. However, as Restasis is not an approved product in the EU and Actavis does not have any plans to develop or file a generic version of Restasis in the EEA, this overlap will not be discussed further.
3.2.Botox
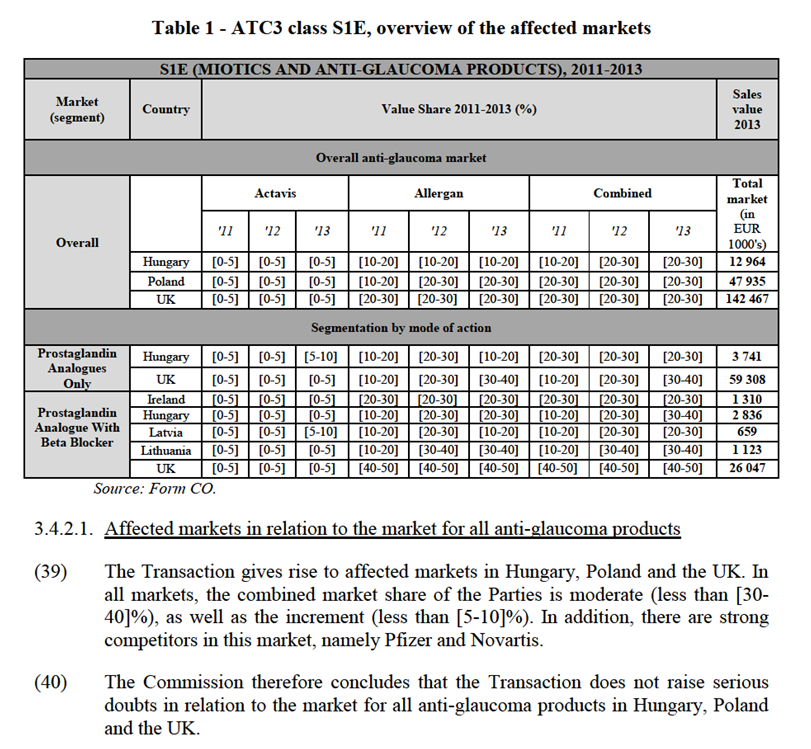
(20) One of Allergan’s main products is BOTOX (onabotulinum toxin A), a product that Allergan acquired from a physician and developed into a major product with multiple indications. BOTOX can be used for medical applications17, as well as non-medical (aesthetics) applications for which it is sold under the brand name Vistabel.18
(21) BOTOX (and Vistabel) are classified under the ATC class M3A – Peripherally Acting Muscle Relaxants. The Parties' activities overlap at ATC3 level due to Actavis marketing two M3A drugs19 in several countries where BOTOX (and Vistabel) are also marketed. The Parties also note that Actavis sells or is developing products that may overlap with two existing indications (chronic migraine and overactive bladder) and three pipeline indications (osteoarthritis pain, depression, and plaque psoriasis) for BOTOX. The great majority of Actavis products for these indications are in oral form20 and are genericized.
(22) Allergan further submits that it does not have BOTOX sales segmented by indication, as BOTOX is not sold in indication-specific packaging.21 While the quantity and mode of administration changes according to the indication, all vial sizes are distributed with a leaflet containing instructions for all indications in all languages. Since Allergan does not know what indication(s) any particular package of BOTOX has been used for, it cannot and does not apply different prices for different indications of BOTOX.
(23) Nonetheless, for the abovementioned therapeutic areas, the market investigation confirmed the Parties' view that BOTOX and Actavis products have distinct indications and uses, distinct mechanisms of action, and often distinct modes of administration (most of Actavis products are in tablet form while BOTOX is administed as an injectable solution). In addition, in most cases, for a given indication, a course of treatment with BOTOX has a significantly different price than a course of treatment with any of Actavis' products. While each of these elements taken separately is generally not sufficient to conclude on the absence of competitive concerns, in the specific case at hand, their combination allow to conclude that BOTOX is unlikely to closely compete with any of Actavis' products identified by the Parties, and that no serious doubts arise regarding these overlaps. As a result, these overlaps will not be assessed further in the decision.
3.3.Analysis of affected markets
(24) The Parties' activities give rise to affected markets in the following areas:
a.Miotics and anti-glaucoma preparations (ATC3 class S1E) in Hungary, Ireland, Latvia, Lithuania, Poland and the UK (section 3.4);
b. Artificial tears and ocular lubricants (ATC3 class S1K) in Denmark and the UK (section 3.5).
(25) Moreover, a vertical relationship occurs between the activities of Actavis' subsidiary Medis in the outlicensing and contract manufacturing of latanoprost, and Allergan's activities in the area of Miotics and anti-glaucoma preparations (for which latanoprost is an Active Pharmaceutical Ingredient), giving rise to affected markets in Italy and the UK (section 3.6).
3.4.Miotics and anti-glaucoma preparations (S1E)
3.4.1.Product market definition
(26) Glaucoma refers to a group of ocular disorders resulting in optic nerve damage or loss to the field of vision, typically caused by pressure build-up to the fluid of the eye. Anti-glaucoma products are aimed at reducing the intra-ocular pressure and are often in eye drop form. Other treatments include laser treatment and surgery.
3.4.1.1.Past Commission decisions
(27) The Commission previously considered that the ATC3 class S1E should be divided into (i) injectable miotics (used in ocular surgery to induce the constriction of the pupil), (ii) anti-glaucoma (used mainly as an eyedrop to relieve intraocular pressure, which frequently accompanies glaucoma), based on the different indications.22 It also considered that anti-glaucoma products can be further sub- divided based on:
a. their mode of action (namely into prostaglandin analogues, beta-blockers, alpha-adrenergic agonists, carbonic anhydrase inhibitors, non-injectable miotics);
b. the molecule that constitutes the active ingredient, depending on the specificities of each market.23
(28) The Commission has so far left the market definition for products in the S1E class open.
3.4.1.2.Notifying Party's views
(29) The Notifying Party submits that the appropriate market definition is the ATC3 class S1E, divided into (i) injectable miotics and (ii) anti-glaucoma products. The Notifying Party considers it inappropriate to sub-segment anti-glaucoma products based on mode of action, since all anti-glaucoma products are aimed at reducing intraocular pressure and should therefore be considered as substitutable.
3.4.1.3.Commission's assessment
(30) The market investigation provided indications that the S1E market should be subdivided into anti-glaucoma products and injectable miotics. Indeed, the majority of prescribers having responded to the Commission's market investigation indicated that they would not prescribe drops and injectable products interchangeably.24 Several prescribers further mentioned that injectables are not yet available for glaucoma and ocular hypertension.25 As there is no overlap in relation to the injectable miotics market, the decision will further consider only the anti-glaucoma market.
(31) Regarding anti-glaucoma products, the market investigation indicated that the different molecule groups (prostaglandin analogues, beta-blockers, alpha- adrenergic agonists, carbonic anhydrase inhibitors, non-injectable miotics) are typically used sequentially, switching from one category to the other depending on the observed effectiveness and tolerability. Most repondents to the market investigation indicated that prostaglandin analogues are usually prescribed first,26 in line with the European Glaucoma Society Guidelines.27
(32) If the reduction of intraocular pressure observed is unsufficient, depending on the reduction reached, physicians usually prescribe either another monotherapy (a prostaglandin analogue or a betablocker) or a combination of a betablocker with a prostaglandin analogue.28
(33) Other categories (alpha-adrenergic agonists and carbonic anhydrase inhibitors) are used if the abovementioned treatments do not show sufficient result or in case of side-effect or counterindication. Non-injectable miotics and in particular pilocarpine, are usally used in very specific cases, such as closed angle glaucoma.29
(34) Within the ATC3 class S1E, the Parties' activities overlap at molecule-level regarding prostaglandin analogues, for which four molecules exist:
a.latanoprost: the originator is Pfizer; latanoprost exists in generic version for several years;
b. bimatoprost: the originator is Allergan with its Lumigan products. The patent for some formulations of Lumigan already expired in the EEA30 (Lumigan 0.03 and 0.03 unit dose, not Lumigan 0.01), but they are protected by a supplementary protection certificate in some countries;
c. travoprost: the originator is Alcon (a subsidiary of Novartis); travoprost exists in generic version for several years; and
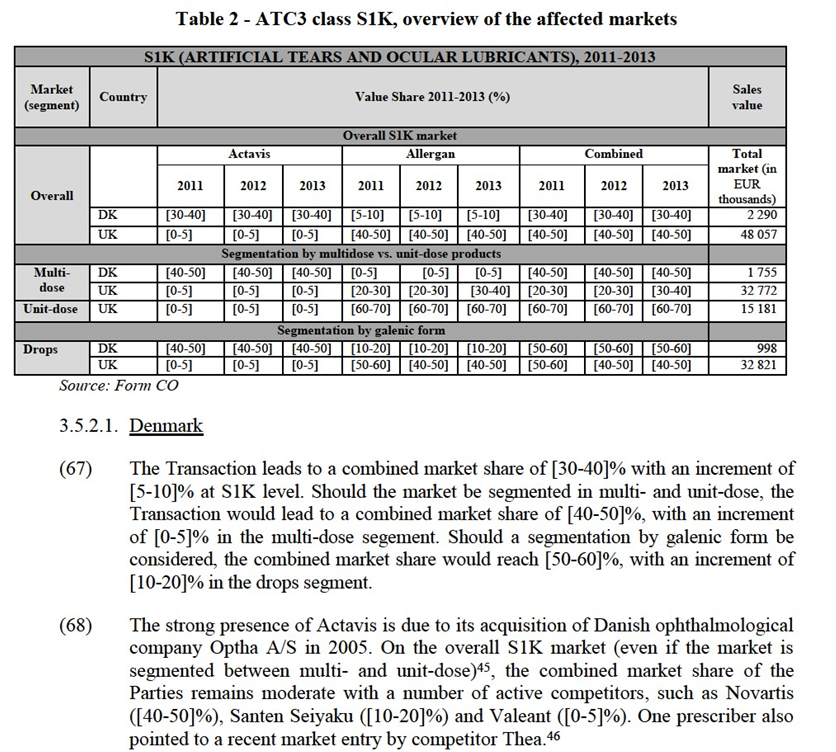
d. tafluprost: the originator is Santen; tafluprost is still under patent protection.
(35) Latanoprost is often the first choice of physicians within the group of prostaglandin analogues. This preference appears to be due to its good price/effectiveness ratio as it was the first genericised prostaglandin analogue (and is still the only genericised prostaglandin analogue in most European countries), than to a significant therapeutic difference compared to other prostaglandin analogues. Although bimatoprost is sometimes considered as more efficient but with more side effects than other prostaglandin analogues, and tafluprost as less efficient but with less side-effects,31 most respondents to the market investigation consider that the different prostaglandin analogues do not significantly differ from a therapeutic and side effect point of view.32 The absence of preservative also plays a role in the prescription of prostaglandin analogues. Tafluprost is the most cited as delivered without preservative, but other molecules also exist in this form.33
(36) In light of the above, the Commission considers that the S1E market could be subsegmented into injectable miotics and anti-glaucoma products. The latter could also be segmented by mode of action, and possibly further at molecule level depending on the category. In any event, the market definition can be left open for the purpose of this decision, as no serious doubts arise under any plausible market definition.
3.4.2.Competitive assessment
(37) Regarding anti-glaucoma products, the Parties are active in the following categories:
a.prostaglandin analogues: latanoprost (Actavis), bimatoprost (Allergan, Actavis pipeline) and travoprost (Actavis pipeline);
b.beta-blockers: timolol (Actavis and Allergan) and levobunolol (Allergan);
c.combinations of prostaglandin analogue and beta-blockers:
latanoprost/timolol (Actavis), bimatoprost/timolol (Allergan);
d.alpha-2 adrenergic receptor agonists (also known as alpha agonists or adrenoceptors): brimonidine and dipivefrine (both Allergan), and combination with a beta-blocker: brimonidine/timolol (Allergan);
e.carbonic anhydrase inhibitors: dorzolamide and brinzolamide (both Actavis, the first one is marketed, the second is a pipeline product), and combination with a beta-blocker: dorzolamide/timolol (Actavis);
f.non-injectable miotics: pilocarpine (Actavis and Allergan's pipeline).
(38) The Parties' activities give rise to affected markets:
a.at the level of anti-glaucoma products overall;
b.in relation to the following molecule groups: prostaglandin analogues, and prostaglandin analogues in combination with beta-blockers;
c.and at the following molecule levels: bimatoprost and pilocarpine.
(44) In the UK market for prostaglandin analogues combined with beta blockers, the Parties have a combined market share of [40-50]%, with an increment of [0-5]%. There are at least five other competitors, in particular Pfizer with a share of [20- 30]% and Novartis with a share of [10-20]%, as well as other suppliers such as Mylan, Teva and Beacon Pharmaceuticals. The Parties are not each other's closest competitors as their products are based on different prostaglandin analogue molecules (latanoprost for Actavis and bimatoprost for Allergan). Actavis' latanoprost/timolol combination products are likely competing more closely with other latanoprost/timolol combination products, such as Pfizer's or Novartis', than with Allergan's bimatoprost/timolol combination products.
(45) The Commission therefore concludes that the Transaction does not give rise to competition concerns in relation to the market for prostaglandin analogues in Hungary, Ireland, Latvia, Lithuania and the UK.
(46) Finally, Actavis also has a travoprost generic pipeline product, and it intends to sell travoprost in a number of countries in the EEA. In light of the combined market shares of the Parties on the market for prostaglandin analogues only (below [30- 40]%, even when only Allergan is present on the market) and the fact that a number of other companies already have marketing authorisations for travoprost in the EEA countries where Actavis intends to enter, this future overlap does not raise serious doubts.
(47) Overlap at molecule level
Bimatoprost
(48) The Transaction also gives rise to a pipeline overlap at bimatoprost molecule level, as Actavis intends to sell a generic version of bimatoprost in the EEA and Allergan is the originator company of this molecule (sold under the brand Lumigan).
(49) Actavis has purchased dossier rights from […], a third party out-licensor, on a non- exclusive basis. It has rights to register the dossier and obtain a marketing authorisation in the […] following countries: […]. In addition, there are other outlicensors, such as Alfred E. Tiefenbacher who own the dossier and could outlicense it.
(50) In all these countries except in […], at least one other competitor of the Parties such as global generic suppliers Novartis/Sandoz and Valeant/Pharmaswiss, as well as Polpharma and Alfred E. Tiefenbacher has marketing authorisations, even in the countries where a patent or supplementary protection certificate is still in place (namely in […]). Therefore, in these countries, competitors already began to sell generic bimatoprost or could easily begin to do so, while Actavis does not have yet obtained any marketing authorisation in the EEA so far.
(51) In […], only Lumigan 0.01 is under protection (via patent protection until 2026). Therefore, large generic companies such as Novartis/Sandoz which already have a generic version of Lumigan 0.03 for sale in other countries, could easily enter the market. In addition, should Actavis be the first entrant in […], there are no exclusivity rights or other protections associated with being the first generic supplier of a product that could prevent other competitors to enter the […] market. Competitors could obtain the dossier rights in […] from an outlicensor which sells these rights on a non-exclusive basis, such as Indoco.
(52) The Commission therefore concludes that the Transaction does not raise serious doubts in relation to the marketed / pipeline overlap in bimatoprost.
Pilocarpine
(53) Allergan has a pipeline product based on pilocarpine which is currently in Phase II and is expected to be launched in 2019, while Actavis markets three generic S1E products based on pilocarpine: Pilocarpine, Pilokarpin, and Pilokarpin Optha. However, Actavis sales of these products were minimal in 2013 (respectively EUR […] in Denmark, EUR […] in Norway, EUR […] in the UK) and have been decreasing since 2011. In addition, other competitors such as Merck are active on this market.
(54) The Commission therefore concludes that the Transaction does not raise serious doubts in relation to the marketed / pipeline overlap in pilocarpine.
3.4.2.4.Conclusion
(55) In light of the above, the Commission concludes that the Transaction does not raise serious doubts as to its compatibility with the internal market in anti-glaucoma markets, irrespective of the precise market definition.
3.5.Artificial tears and ocular lubricants (S1K)
3.5.1.Product market definition
(56) Ocular lubricants and artificial tears are ophthalmic preparations (solutions, gels or ointments) used for symptomatic relief of eye dryness, which can be the result of an insufficient quantity or quality of natural tears. They are classified under the ATC3 class S1K, which contains no ATC4 sub-category. Ocular lubricants do not contain active pharmaceutical ingredients, and are almost all available OTC.
3.5.1.1.Past Commission decisions
(57) In previous merger decisions,34 the Commission considered that the relevant market for S1K class products should be defined at ATC3 class level. While in Novartis/Alcon, the Commission contemplated that a division into some sub- segments would theoretically be possible, for example by galenic form (drops vs. gel/ointments), the presence of preservatives, or the lubricating ingredients, it found that these product differences would not justify a subdivision into separate markets.
(58) The Commission also observed that ocular lubricants may be placed on the market as medical devices (CE marketed products) or as pharmaceuticals, but concluded that this distinction does not have an impact on the market definition. Lastly, the Commission left open whether separate markets should be defined for prescription ("Rx") and over-the-counter ("OTC") ocular lubrictants.
3.5.1.2.The Notifying Party's views
(59) The Notifying Party submits that the relevant product market is the ATC3 class S1K. Regarding the OTC / Rx segmentation, the Notifying Party indicates that sub- dividing between OTC and Rx products would not be instructive, as all or virtually all S1K products are sold OTC. For completeness, the Notifying Party also provided market shares based on the different galenic forms and format (single-dose and multi- dose products).35
3.5.1.3.Commission's assessment
(60) The market investigation confirmed that most ocular lubricants are, at least to some degree, substitutable. Indeed, prescribers indicated that patients with dry eye symptoms are treated with different ocular lubricant products, with the exact choice depending on the severity of the symptoms, and the individual needs of the patients. In some situations a particular product might be better suited than the other, for example when a patient is allergic to a preservative contained in a given product.36
(61) The market investigation revealed that one distinguishing criterion could be the viscosity of the product. In general, it would appear that the higher the viscosity, the longer the effect of the product, but at the same time the more disturbing the effect on the vision. One prescriber indeed stated the following: "the frequency of use is an important criterion for the customers, as this impacts how often they need to apply the products every day […]. It therefore also impacts the recommendations of doctors depending on the gravity of the dry eye symptoms. A severe dryness requires more frequent usage and a thick product is then more appropriate."37 This difference in viscosity translates to some degree in different galenic forms. In general, drops have lower viscovity than gels.38 Ointments have the highest viscovity, and are more often used over night given the impact on a patient's vision.39 Nevertheless, this criterion would not seem suited to clearly delineate seperate markets as ocular lubricant products are located across a spectrum of viscosity levels. For example, some drops might be closer in viscosity (and thereby in effect) to gels than to other drops.40
(62) Competitors and prescribers also confirmed that a segmentation based on the molecule would not be meaningful for ocular lubricants. As indicated above, there are no active ingredients contained in these products, and the molecules used seem easy to obtain and largely similar in effect.41
(63) The market investigation also pointed towards a possible distinction between ocular lubricants which contain or do not contain preservatives42. Some patients do not tolerate (certain) preservatives, and in some instances, preservatives can be harmful to the eye, in particular if they are administered as frequently as 4 - 6 times a day. Therefore, it cannot be excluded that the relevant market would have to be delineated along this distinguishing criterion, and the Parties have, as means to approximate market shares in the preservative/preservative-free segments submitted data on unit-dose and multi-dose market shares43. Nonetheless, the market investigation did not put in question, aside from very specific instances, the overall therapeutic substitubility of these products.44
(64) In light of the above, the Commission considers that the relevant product market for ocular lubricants is likely to comprise the entire ATC3 class S1K, irrespective of the galenic form, whether it contains preservatives or not, or whether the drug is prescribed or OTC. In any event, the market definition in relation to S1K produts can be left open for the purpose of this decision, as no serious doubts arise regarding the compatibility with the internal market under any possible market definition.
3.5.2.Competitive assessment
(65) Actavis is active in this market with the following products: Viskose Øjendrab, Øjensalve Neutral Optha, Aquify and Focus Clerz. Allergan is active with Celluvisc, Lacril, Lacrilube, Liquifilm Tears, Optive, Optive Fusion, Optive Plus and Refresh. With the exception of the eye ointments Øjensalve Neutral Optha and Lacrilube, all the products are administered as drops.
(66) The Parties sell several of these products in the different countries concerned. Their activities give rise to affected markets at S1K level, at unit-dose/multi-dose level, and at drops level, in the UK and in Denmark.
 (70) In light of the above and of all available evidence, the Commission concludes that the Transaction does not raise serious doubts as regards artificial tears and ocular lubricants in Denmark.
(70) In light of the above and of all available evidence, the Commission concludes that the Transaction does not raise serious doubts as regards artificial tears and ocular lubricants in Denmark.
3.5.2.2. The UK
(71) At the S1K level the transaction leads to a combined market share of [40-50]% with an increment of [0-5]%. If the market were segmented in multi- and unit-dose, the transaction would lead to a combined market share of [60-70]%, with an increment of [0-5]% in the unit-dose segment48. Similarly, if a segmentation by galenic form were performed, the combined market share would be [40-50]%, with an increment of [0-5]% in the drops segement.
(72) In all the potential product markets, Actavis only contributes an insignificant increment. Moreover, there are other strong players active on the market such as Novartis ([10-20]% share), Valeant ([5-10]% share )and Scope Ophtalmics ([10- 20]% share), and more than 20 other competitors.
(73) In light of the above and of all available evidence, the Commission concludes that the Transaction does not raise serious doubts as regards artificial tears and ocular lubricants in the UK.
3.6. Contract manufacturing and out-licensing of latanoprost / Miotics and anti- glaucoma preparations (S1E)
(74) Actavis (through its subsidiary Medis) out-licenses generic pharmaceutical products to third parties. Where Medis out-licenses products to third parties, Actavis usually also manufactures these products for the third parties.49 In 2013, Medis generated total worldwide sales of approximately EUR [200-250] million from outlicensing and product manufacturing, of which approximately EUR [200- 250] million was derived from sales to customers situated in the EEA.
(75) In previous decisions, the Commission considered out-licensing as a separate activity upstream of the markets for finished pharmaceutical products. The Commission looked at the out-licensing of the relevant intellectual property rights for each individual API as potentially constituting a relevant market.50 The Commission has also considered contract manufacturing according to the pharmaceutical form manufactured, and also, in some cases, to the conditions of manufacture (e.g., the types of APIs used, toxicity levels, whether sterile environment is required).51
(76) Medis supplies latanoprost in the EEA, and Allergan is active in the downstream market of miotics and anti-glaucoma preparations (S1E). As discussed in section 3.4 above, the S1E market can be further sub-segmented by molecule group (latanoprost belonging to prostaglandin analogues) and at molecule-level (latanoprost). Affected downstream markets only arise with respect to prostaglandin analogues in Italy (where Allergan's share is at most [30-40]%) and the UK (where Allergan's share is [30-40]%). However, for these two markets, latanoprost supplied by Medis represent an immaterial share of sales of the downstream market ([0-5]% in Italy and [0-5]% in the UK). Therefore, other competitors are able to compete on the downstream market, either via contracts with other out-licensors and contract manufacturers, or by producing latanoprost internally.
(77) In light of the above and of all available evidence, the Commission concludes that the Transaction does not raise serious doubts as regards the vertical relationship between the contract manufacturing and out-licensing of latanoprost and the sale of miotics and anti-glaucoma products.
4.CONCLUSION
(78) For the above reasons, the European Commission has decided not to oppose the notified operation and to declare it compatible with the internal market and with the EEA Agreement. This decision is adopted in application of Article 6(1)(b) of the Merger Regulation and Article 57 of the EEA Agreement.
1 OJ L 24, 29.1.2004, p. 1 ('the Merger Regulation'). With effect from 1 December 2009, the Treaty on the Functioning of the European Union ('TFEU') has introduced certain changes, such as the replacement of 'Community' by 'Union' and 'common market' by 'internal market'. The terminology of the TFEU will be used throughout this decision.
2 OJ L 1, 3.1.1994, p.3 ("the EEA Agreement").
3 Publication in the Official Journal of the European Union No C 66, 24.02.2015 p. 10.
4 Turnover calculated in accordance with Article 5 of the Merger Regulation.
5 See for example cases M.6969 – Valeant Pharmaceuticals International/Bausch & Lomb Holdings of 5 August 2013; M.5778 – Novartis/Alcon of 9 August 2010, and M.5865 – Teva/Ratiopharm of 3 August 2010.
6 See for example cases M.6969 – Valeant Pharmaceuticals International/Bausch & Lomb Holdings of 5 August 2013; M.5865 – Teva/Ratiopharm of 3 August 2010; and M.5295 – Teva/Barr of 19 December 2008.
7 See cases M.6969 – Valeant Pharmaceuticals International/Bausch & Lomb Holdings of 5 August 2013; M.6705 – Procter & Gamble/Teva Pharmaceuticals OTC II of 9 November 2012; M.6613 – Watson/Actavis of 5 October 2012; and M.5865 – Teva/Ratiopharm of 3 August 2010.
8 See cases M.6969 – Valeant Pharmaceuticals International / Bausch & Lomb Holdings, Commission decision of 5 August 2013 and M.5778 – Novartis/Alcon, Commission decision of 9 August 2010.
9 Medical devices are subject to the EC Medical Device Directive 93/42/EEC.
10 See M.5778 – Novartis/Alcon of 9 August 2010; M.5865 – Teva/ Ratiopharm of 3 August 2010; and M.5253 – Sanofi-Aventis/Zentiva 4 February 2009.
11 See Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use (OJ L311, 28.11.2001, p.67), as amended by various subsequent acts.
12 See M.6969 – Valeant Pharmaceuticals International / Bausch & Lomb Holdings, Commission decision of 5 August 2013; and M.5778 – Novartis/Alcon, Commission decision of 9 August 2010.
13 See M.6969 – Valeant Pharmaceuticals International / Bausch & Lomb Holdings, Commission decision of 5 August 2013; M.6278 – Takeda/Nycomed, Commission decision of 29 July 2011; M.5778 – Novartis/Alcon, Commission decision of 9 August 2010; M.1878 – Pfizer/Warner Lambert, Commission decision of 22 May 2000; M.1846 – Glaxo Wellcome/Smithkline Beecham, Commission decision of 8 May 2000; and M.737 – Ciba-Geigy/Sandoz, Commission decision of 04 February 1998.
14 See M.7275 – Novartis/GlaxoSmithKline Oncology Business, Commission decision of 28 January 2015; M.5476 – Pfizer/Wyeth, Commission decision of 17 July 2009; M.1846 – Glaxo Wellcome/Smithkline Beecham, Commission decision of 8 May 2000.
15 See M.7275 – Novartis/GlaxoSmithKline Oncology Business, Commission decision of 28 January 2015; M.6258 – Teva/Cephalon, Commission decision of 13 October 2011; and M.6613 – Watson/Actavis, Commission decision of 5 October 2012.
16 See M.7275 – Novartis/GlaxoSmithKline Oncology Business, Commission decision of 28 January 2015; and M.737 – Ciba-Geigy/Sandoz, Commission decision of 17 July 1996.
17 BOTOX is indicated to treat eight medical conditions: (i) certain types of eye muscle problems (strabismus); (ii) abnormal spasm of the eyelids (blepharospasm); (iii) abnormal head position and neck pain that happens with cervical dystonia (CD); (iv) severe underarm sweating (severe primary axillary hyperhidrosis); (v) increased muscle stiffness in elbow, wrist, and finger muscles in people with upper limb spasticity; (vi) the prevention of headaches in adults with chronic migraine; (vii) leakage of urine (incontinence) in adults with overactive bladder due to neurologic disease who still have leakage; and (viii) overactive bladder symptoms such as a strong need to urinate with leaking or wetting accidents (urge urinary incontinence), a strong need to urinate right away (urgency), and urinating often (frequency) in adults.
18 Vistabel is a different brand name for BOTOX, with a dosage formulation and indication specific to the temporary improvement of glabellar lines and canthal lines.
19 Cisatracurium Actavis 2mg/ml and Atracurium Besylat.
20 Cisatracurium Actavis 2mg/ml and Atracurium Besylat are administered by intravenous injection, the oxybutynin-based Kentera is a transdermal patch, some of Actavis' non-steroidal anti-inflammatory drugs are under gel form, the coal tar solution Exorex is a skin lotion.
21 BOTOX is sold in three sizes (50, 100 and 200 Units per glass vial), which may be used for any indication.
22 See case M.5778 – Novartis/Alcon, Commission decision of 9 August 2010.
23 In particular, the Commission analysed competition at pilocarpine level (a miotic), while timolol (a beta-blocker) was considered as potentially substitutable with other beta-blockers.
24 Responses to questionnaire Q2 – S1E – Prescribers, question 5.
25 Responses to questionnaire Q2 – S1E – Prescribers, questions 4.
26 Responses to questionnaire Q2 – S1E – Prescribers, question 9.2.
27 Responses to questionnaire Q2 – S1E – Prescribers, questions 7 and 9.1.
28 Responses to questionnaire Q2 – S1E – Prescribers, questions 9.2, 9.3, 10.1; minutes of a conference call with a prescriber on 5 February 2015.
29 Responses to questionnaire Q2 – S1E – Prescribers, question 7; minutes of conference call with a prescriber on 5 February 2015.
30 A supplementary protection certificate is still valid in parts of the EEA until March 2017.
31 Responses to questionnaire Q2 – S1E – Prescribers, question 11; minutes of a conference call with a prescriber on 5 February 2015.
32 Responses to questionnaire Q2 – S1E – Prescribers, questions 11, 12 and 13.
33 Responses to questionnaire Q2 – S1E – Prescribers, question 11; minutes of a conference call with a prescriber on 5 February 2015.
34 See cases M.6969 – Valeant Pharmaceuticals International / Bausch & Lomb Holdings, Commission decision of 5 August 2013 and M.5778 – Novartis/Alcon, Commission decision of 9 August 2010.
35 According to the Notifying Party, unit-dose drops come in single use packages and thus generally do not contain preservatives, while most multi-dose products contain preservatives to avoid microbial contamination. The Notifying Party therefore provide market shares for unit-dose vs. multi-dose products, as a proxy for the presence vs. absence of preservatives.
36 Minutes of a conference call with a prescriber, 17 February 2015; minutes of a conference call with a presciber, 12 February 2015.
37 Minutes of a conference call with a prescriber, 4 February 2015.
38 Minutes of a conference call with a prescriber, 4 February 2015.
39 Minutes of a conference call with a prescriber, 12 February 2015.
40 Minutes of a conference call with a prescriber, 4 February 2015.
41 Minutes of a conference call with a competitor, 10 February 2015.
42 According to estimates by the Parties based on IMS data for Austria, Belgium, Croatia, Czech Republic, Denmark, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Netherlands, Norway, Poland, Portugal, Romania, Spain, Sweden and the UK, approximately 42% of all purchased ocular lubricants contain preservatives, whilst the rest do not.
43 There are currently two types of conservatives free products: those that comprise single-unit doses, and bottles with a filtering system preventing the entry of bacteria in the bottle (see minutes of a conference call with a prescriber, 4 February 2015; minutes of a conference call with a competitor, 9 February 2015. As data for sales of ocular lubricants with or without preservatives split by product are not readily available, the Parties provided the figures for the unit-dose / multi-dose levels. Whilst these numbers assign the sales of preservative free multi-dose products to the proxy for the products with preservative – multi-dose – they suffice in the case at hand. The preservative-free market segment would be slightly larger than the proxy suggests (11% in Denmark, 26% percent in the UK), whilst the market for ocular lubricants with preservatives would shrink accordingly. As demonstrated below, this eases rather than aggravates possible concerns in the affected markets
44 Minutes of a conference call with a prescriber, 12 February 2015, and minutes of a conference call with a prescriber, 4 February 2015.
45 As mentioned above, the distinction multi-dose/unit-dose mainly serves as a proxy for the plausible preservative/preservative free ocular lubricants market segments (multi-dose with preservatives 66.6% of the total S1K market, multi-dose without preservatives 11.4%, unit-dose 22%). Given that Actavis sells a preservative-free multi-dose product in Danemark, namely Ojensalve Neutral Optha, an eye ointment mainly used for the treatment of blepharitis symptoms, which can normally only be used overnight due to its heavy impact on a patient’s vision, the combined market shares of the parties in the ocular lubricants segment with preservatives would be even lower than what these proxy data suggest. Indeed, Ojensalve Neutral Optha is by far the post sold, if not only preservative free multi-dose product available in Danemark.
46 Minutes of a conference call with a prescriber, 4 february 2015.
47 Minutes of a conference call with a prescriber, 17 february 2015.
48 As mentioned above, the distinction multi-dose / unit-dose mainly serves as a proxy for the plausible preservative / preservative free ocular lubricants market segments (multidose with preservatives 44.8% of the total S1K market, multidose without preservatives 26%, unit-dose 29.2%). Given that neither Allergan nor Actavis sells any preservative-free multi-dose product in the UK, the combined market shares of the parties in the preservative free ocular lubricants segement would be slightly lower than what these proxy data suggest (and slightly higher in the ocular lubricants with preservatives segment, where the Parties have a much lower market share however).
49 In most cases these products are manufactured by Actavis manufacturing sites, rther than by Medis itself.
50 See Teva/Ratiopharm, Case COMP/M.5865, Commission decision of August 3, 2010, para. 396 and 408; and Teva/Cephalon, Case COMP/M.6258, Commission decision of October 13, 2011, para. 146 and 190; Actavis/Watson, Case COMP/ M.6613, Commission decision of October 5, 2012. para. 133; Takeda/Nycomed, Case COMP/M.6278, Commission decision of July 29, 2011, para. 19.
51 Sanofi-Aventis/Zentiva, Case COMP/M.5253, Commission decision of February 4, 2009, para. 189.