Commission, November 17, 2017, No M.8522
EUROPEAN COMMISSION
Decision
AVANTOR / VWR
Subject: Case M.8522 - Avantor / VWR
Commission decision pursuant to Article 6(1)(b) of Council Regulation No 139/2004 (1) and Article 57 of the Agreement on the European Economic Area (2)
Dear Sir or Madam,
(1) On 11 October 2017 (3), the Commission received notification of a proposed concentration pursuant to Article 4 of Council Regulation (EC) No 139/2004 by which Avantor, Inc. ("Avantor", USA), controlled by New Mountain Capital, LLC ("New Mountain", USA), acquires within the meaning of Article 3(1)(b) of the Merger Regulation control of the whole of VWR Corporation ("VWR", USA), by way of a purchase of shares. Avantor is designated hereinafter as the Notifying Party and Avantor and VWR together as the Parties.
1. THE PARTIES
(2) Avantor, a corporation organised under the laws of the State of Delaware (USA), is a global supplier of ultra-high purity materials for the life sciences and advanced technology sectors, including laboratory chemicals. It supplies products for use in production and research, to customers in a range of sectors including biotechnology, pharmaceuticals, medical devices, diagnostics, aerospace and defence, and semiconductors. Avantor is controlled by New Mountain (4), an investment firm that manages private equity, public equity and credit funds. Its portfolio companies are active in a range of sectors including healthcare and healthcare services, pharmaceuticals, technology services, clothing, logistics and shipping.
(3) VWR, a publicly traded company listed on the Nasdaq Stock Market, is a global distributor of laboratory products and services. It distributes chemicals, reagents, consumables, durable products and scientific equipment and instruments, and offers both branded and private label products. VWR is also active in the manufacture of bioscience products and laboratory chemicals, both for its own private label and for other suppliers.
2. THE OPERATION
(4) On 4 May 2017 Avantor and VWR entered into an agreement under which Avantor will acquire the whole of VWR for USD 33.25 in cash per share of VWR common stock, reflecting an enterprise value of approximately $6.4 billion (the "Transaction"). Because Avantor is indirectly solely controlled by New Mountain, following the Transaction, New Mountain will indirectly control VWR.
(5) As a result of the Transaction, VWR will become a wholly-owned subsidiary of Avantor. The Board of VWR will consist of a Chief Executive Officer and ten other board members. New Mountain will be entitled to appoint the Chief Executive Officer and seven (or at least five) (5) of the other board members, thus allowing it to exercise sole control of VWR.
(6) In view of the above, New Mountain will acquire within the meaning of Article 3(1)(b) of the Merger Regulation sole control over VWR as a result of the Transaction.
3. EU DIMENSION
(7) The undertakings concerned have a combined aggregate world-wide turnover of more than EUR 5 000 million (6) (EUR […]). Each of them has EU-wide turnover in excess of EUR 250 million (New Mountain: EUR […] million; VWR: EUR […] million), but neither achieves more than two-thirds of its aggregate EU-wide turnover within one and the same Member State. The notified operation therefore has an EU dimension pursuant to Article 1(2) of the Merger Regulation.
4. MARKET DEFINITIONS
4.1. Introduction
(8) Avantor is a supplier of ultra-high purity materials for the life sciences and advanced technology sectors. It is active globally and across the EEA and has a particularly strong presence in the US, where it is based.
(9) VWR is both a manufacturer and a distributor of laboratory and bioscience products and also provides various laboratory services. Similarly to Avantor, VWR is active globally and across the EEA. It is a particularly strong player in the western European markets.
(10) The Parties' activities overlap in relation to the production of three main types of life science product: bioscience products, raw materials for (bio)pharmaceutical production, and laboratory chemicals. The Transaction, however, only creates horizontally affected markets in one of these areas: laboratory chemicals.
(11) The Transaction also creates a vertical relationship, due to the link between VWR's activities as a distributor of laboratory chemicals and both Parties' presence on the upstream (production) market(s) for laboratory chemicals. VWR is currently a distributor for Avantor, meaning that the Transaction internalises an existing vertical relationship.
(12) For the purposes of this decision, any references made to life sciences products, laboratory chemicals and laboratory products are to be understood as follows: i) "life sciences products" denotes products used in bioscience (such as genomics, proteomics, and molecular biology) as well as cell culture products and other raw materials for (bio)pharmaceutical production (both biopharma and small molecule), and including laboratory chemicals; ii) "laboratory chemicals" denotes chemicals that are used for research, analytical testing and quality control purposes in a laboratory setting; iii) "laboratory products" denotes laboratory instruments, consumables and equipment in addition to laboratory chemicals.
4.2. Production of laboratory chemicals
(13) The Commission most recently assessed the market for laboratory chemicals in case M.7435 Merck/Sigma-Aldrich. Laboratory chemicals were defined as chemicals used for research, testing and quality control purposes. In that case, the Commission considered six main categories of laboratory chemicals ((i) solvents, (ii) inorganics, (iii) organics, (iv) standards and reference materials, (v) analytical chromatography, and (vi) industrial microbiology), and considered the various possible market definitions within each of these categories.
(14) In the present case, the two categories of laboratory chemicals where horizontally affected markets arise are solvents and inorganics. These will each be discussed in more detail below.
4.2.1. Product market definition
(15) The relevant product market for the production of laboratory chemicals is to be defined based on product characteristics that determine demand- and supply side substitutability as well as potential competition. (7) A complainant suggested that a separate market exists that comprises only sales of specific laboratory chemicals to distributors. (8) However, such a limitation would artificially reduce the actual market size because producers of laboratory chemicals can and do sell at the same time directly to final customers as well as via distributors. (9) For the purpose of this case, the Commission does therefore not consider a segmentation of the relevant market by type of customer on the production level. The relevant downstream distribution market is considered separately below. (10)
4.2.1.1. Solvents
(16) In case M.7435 Merck/Sigma-Aldrich, the Commission defined solvents as laboratory chemicals used to dissolve a target substance (a chemically different liquid, solid, or gas). The Commission also observed that solvents can be used either for classical laboratory analysis or for instrumental analysis, and found that separate product markets could therefore be considered for each of these areas, and for sub-segments within these areas.
(17) Within solvents for classical laboratory analysis, narrower classes of products can be identified on the basis of purity: technical grade solvents have lower purity levels than regulated industry grade solvents, which are certified according to published standards. Dried and anhydrous solvents have even higher purity levels and are used in specific processes where very low water content is needed.
(18) Within solvents for instrumental analysis, a distinction can be made according to the technique used. The most common techniques include spectroscopy, electrochemistry, chromatography polymer analysis, capillary gel electrophoresis, gas chromatography, liquid chromatography, and mass spectroscopy.
(19) In case M.7435 Merck/Sigma-Aldrich, the Commission found that the different types of solvents mentioned above are generally not substitutable from a demand-side perspective, due to individual product's specification, level of purity and suitability for use in certain techniques. From a supply-side perspective, however, the Commission found that there was generally a high level of substitutability, as suppliers are generally able to produce a comprehensive range of solvents for both classical and instrumental analysis. The Commission ultimately left the precise product market definition open with respect to solvents and all possible sub-segments thereof.
(20) The Notifying Party agrees with the Commission's findings as to supply-side substitutability in the Commission decision in case M.7435 but submits no further views on the appropriate product market definition for solvents in this case.
Results of the market investigation: demand-side substitutability
(21) The results of the market investigation in the present case generally confirmed the Commission's findings in M.7435 Merck/Sigma-Aldrich, with the vast majority of customers stating that there is little if any substitutability between the different grades and specific types of solvent. (11) The market investigation explored, in particular, the distinctions between: i) solvents for classical analysis and solvents for instrumental analysis; ii) within solvents for classical analysis: the difference between technical grade, regulated industry grade, and dried and anhydrous solvents; and iii) within solvents for instrumental analysis: the difference between solvents for use in each specific technique (in particular spectroscopy, gas chromatography and high-performance liquid chromatography (HPLC) analysis).
Solvents for classical analysis v solvents for instrumental analysis
(22) Solvents can be distinguished by the purpose for which they are used. Solvents that can be used for multi-purpose analyses are typically referred to as "classical analysis" solvents as opposed to specialised solvents for "instrumental analysis" that are designed to be used with certain instruments and techniques such as gas chromatography, liquid chromatography or Nuclear Magnetic Resonance spectroscopy.
(23) Almost all customers confirmed that solvents for classical analysis and solvents for instrumental analysis are used for entirely distinct purposes. One respondent stated, for example: "they are bought for different purposes, we need better quality for instrumental analysis" (12). Other respondents also confirmed that the main difference between the two grades is their purity. A number of customers also mentioned the price difference between solvents for instrumental analysis and solvents for classical analysis, explaining that it would therefore be illogical to use a solvent that is designed for instrumental analysis in classical analysis (even if this would be technically possible). (13)
(24) Customers emphasised that using a solvent of a lower grade in a procedure where the purity is important could jeopardise the results. One stated, for example: "we do not choose to [change between solvents for instrumental analysis and solvents for classical analysis] as the quality of solvents influence the results we provide to customers, mixing grades means for us less reliability". (14)
(25) Furthermore, almost all customers responding to the market investigation stated that they would not switch from solvents for instrumental analysis to solvents for classical analysis if the price of solvents for instrumental analysis were to increase by 5-10%. (15) A small minority would consider switching if there was a very significant increase in price of certain types of solvent, but often mentioned that this would be dependent on the particular product. One customer stated, for example, that the company would only switch "if it is necessary for the company, if it is beneficial and technically compatible". (16) The responses generally confirmed, however, that customers could envisage very few circumstances in which such a switch would be possible.
Solvents for classical analysis: technical grade, regulated industry grade, dried and anhydrous grade solvents
(26) Within solvents for classical analysis, customers also perceived there to be an important distinction between the different grades, namely technical grade (a lower purity product), regulated industry grade (a higher purity product) and dried and anhydrous solvents (a high purity grade for specific uses). (17) The vast majority of responding customers confirmed that technical grade and regulated industry grade solvents are bought for different purposes, according to their specifications. A number of respondents mentioned, for example, that they use technical grade solvents mainly for cleaning, as the purity is not high enough for them to be suitable for use in other areas. One customer explained: "We buy technical grade acetone for washing while analysis grade is used for reactions […] the stabiliser in dichloromethane is different in technical and analysis grade and that can matter in addition to impurities". (18)
(27) Only a very small proportion of customers would consider switching between regulated industry grade and technical grade solvents as a result of a change in price of 5-10%, and of those who would switch, most would only be able to change a limited proportion of their purchases. (19) One respondent explained that "analysis procedures are designed for defined levels of variability, a change in the purity of the solvent could negate this work". (20) Another customer also stressed the impossibility of switching to lower grade solvents: "if our work requires an anhydrous solvent then it would not be possible to swap to a technical grade because the work would not be successful". (21)
Solvents for instrumental analysis: spectroscopy, gas chromatography and HPLC analysis
(28) Considering the different types of solvents for instrumental analysis, responding customers were, again, mainly of the opinion that there was very limited substitutability between their uses. (22) One customer stated, for example "each product is qualified for its intended use. We cannot change without extra qualification". (23) A number of customers did, however, identify examples of circumstances where a solvent sold as being for use in one technique could be used in another. This type of 'crossover' in usage included, for example, using solvents designed for spectroscopy in HPLC and using solvents designed for HPLC in gas chromatography. Views on this varied, however, with some respondents also stating that they would not use spectroscopy or HPLC grades for gas chromatography. (24) Moreover, respondents emphasised that such cases were rather the exception than the rule. One stated, for example "[it would] need to be checked for each individual analysis [...] some types of analysis have specific requirements that are not present in all instrumental grade solvents". (25)
(29) In addition, as was mentioned in relation to the distinction between solvents for classical analysis and solvents for instrumental analysis, price also appears to play a role. It would often be theoretically possible to use a higher quality solvent in a process where a lower grade solvent would be adequate, but customers are unlikely to have any interest in doing this. One customer explained: "spectroscopic grade solvents could often be used for other uses (e.g. HPLC) but this would not be cost effective because it would cost many times more". (26)
Results of the market investigation: supply-side substitutability
(30) From a supply-side perspective, the results of the market investigation were also generally in line with the findings in case M.7435 Merck/Sigma-Aldrich. The majority of competitors offer a wide range of solvents and the major international manufacturers typically produce solvents of all of the types discussed above (i.e. solvents for both classical and instrumental analysis, and all of the subtypes within each of these two categories). Smaller producers sometimes do not have such an extensive range, but confirm that they could easily start producing those types of solvents which they do not currently offer. (27) Competitors are not aware of any particular type of solvents which would require particular expertise or equipment beyond that necessary for the production of solvents in general. (28) The results of the market investigation therefore appear to confirm that there is a high level of supply-side substitutability between different types of solvent.
High Purity Acetonitrile
(31) The Commission has not previously concluded on possible markets for any specific, individual types of solvent. It considered that HPLC solvents may form a separate product market but left the precise delineation of the market open. (29) During the market investigation for this case, a small number of market participants suggested and expressed concern that the merged entity could have a significant market share in a market for one specific type of HPLC solvent, high purity acetonitrile. (30) VWR (through its subsidiary PTI) is active in the production of bulk size high purity acetonitrile by purifying crude acetonitrile and selling it to competitors of the Parties in the downstream market which down-pack the high purity acetonitrile and resell it under their own respective brands. Avantor is also active in the upstream market by producing catalogue sized high purity acetonitrile on an OEM basis. (31) It produces, packs and labels high purity acetonitrile for competitors downstream. In addition, both VWR and Avantor are also active on the downstream market, selling catalogue sized high purity acetonitrile to distributors and end customers (laboratory customers, (bio)pharmaceutical producers and agricultural chemical manufacturers) under their own respective brands. In order to assess the Parties' activities in this area, the Commission therefore discusses the possible scope of a hypothetical product market for high purity acetonitrile.
(32) The Notifying Party estimates that sales of high purity acetonitrile account for around 50% of all sales of HPLC solvents in the EEA. The Notifying Party submits high purity acetonitrile should be distinguished from standard-grade acetonitrile. The Notifying Party explains that standard-grade acetonitrile is sold in much larger quantities, for use in various types of industrial application, whereas high purity acetonitrile is used by (bio)pharmaceutical and laboratory customers. The acetonitrile captured within the wider product market for HPLC solvents would therefore concern only high purity acetonitrile and not the standard-grade product. Furthermore, the distinction between bulk and catalogue sizes (discussed below in Section 4.2.1.3 for all solvents and inorganics) would apply equally to high purity acetonitrile.
(33) The Notifying Party submits that the three main customer groups purchasing high purity acetonitrile are: i) users of laboratory chemicals, ii) (bio)pharmaceutical manufacturers, and iii) agricultural chemical manufacturers. It further explains that, as a laboratory chemical, high purity acetonitrile is used in various types of instrumental analysis, such as HPLC and liquid chromatography–mass spectrometry.
(34) The Notifying Party explains that there are two different processes for purifying acetonitrile: distillation and purification. It submits that purification may provide certain efficiencies as compared to distillation, but that the end product is the same irrespective of which method is used. The Notifying Party further submits that the equipment required and the process followed for distilling acetonitrile is the same as for any other solvent. The Notifying Party claims that any company currently distilling solvents can distil acetonitrile without any special equipment, technology or expertise beyond that required for any other type of HPLC solvent.
(35) The Notifying Party submits that high purity acetonitrile should not be considered to constitute a separate market of its own, but that it should be considered as part of the market for HPLC solvents (or as part of a wider market for solvents, including HPLC solvents).
(36) In their responses to the market investigation, competitors active in the production of crude acetonitrile as a raw material explained that acetonitrile is produced as a co-product of acrylonitrile. (32) The volumes of acetonitrile produced are therefore dependent on decisions regarding acrylonitrile production. (33) Some manufacturers have spare capacity at their current acrylonitrile production plants, (34) but would not necessarily make use of this as a result of any changes on the acetonitrile market, and constructing a new acrylonitrile plant would represent a very significant investment (at least EUR 500 million). (35) The Parties are not active in the production of crude acrylonitrile but both are active purifying crude acetonitrile to higher purity grades.
(37) Competitors’ responses also generally confirmed the distinction made by the Notifying Party between the high purity acetonitrile produced for use in laboratories, and a lower, standard-grade acetonitrile. One manufacturer also explained that it sells crude acetonitrile, which then has to be further purified, and is thus not typically bought by laboratory chemicals suppliers, for whom it is more cost efficient to buy acetonitrile that is already of a sufficiently high purity level to sell to their customers. (36)
(38) Suppliers of high purity acetonitrile to end customers (i.e. competitors to VWR and Avantor on the downstream market) typically source high purity acetonitrile in one of two ways: either they purchase high purity acetonitrile in bulk and repackage it into catalogue sizes, or they purchase the product already in catalogue sizes (and usually already packaged with their brand name), i.e. they contract out the production entirely. (37) Some suppliers use both these options simultaneously, and all respondents confirmed that no specific technology is needed for repackaging acetonitrile different to that used for other laboratory chemicals. (38) Nevertheless, the largest players in the market such as Merck, Honeywell or Thermo Fischer also buy crude acetonitrile and further purify it. (39)
(39) Competitors on the downstream market also confirm that it would not be feasible for them to start producing acetonitrile as a raw material. Most have never considered the idea and do not regard it as realistic for their type of business. The level of investment needed and the time taken to start production is also seen as prohibitive. One supplier mentions that it would be difficult to be competitive in the production of acetonitrile, given that other suppliers produce it as a by-product. (40)
(40) In view of the above, there would seem to be very little supply-side substitutability for the production of crude acetonitrile, as a production plant producing acrylonitrile, of which acetonitrile happens to be a by-product, could not easily switch to producing other solvents, and a producer of other solvents would appear to have to make a significant investment to set up an acrylonitrile production plant.
(41) Nonetheless, there would appear to be supply-side substitutability at the level of production of high purity acetonitrile level. Suppliers that manufacture high purity acetonitrile by further purifying crude acetonitrile confirmed that the process of purification of the crude acetonitrile is not materially different from other solvents.
(42) Moreover, suppliers that do not distil or purify but simply purchase high purity acetonitrile in bulk size to downpack it also confirmed that no specific technology is needed for repackaging high purity acetonitrile as opposed to any other laboratory chemical.
(43) From a demand-side perspective, the same arguments as presented above in the sections on the various types of solvents would strongly suggest that there would only be very limited, if any, substitutability between high purity acetonitrile and other solvents, even other HPLC solvents. At least where a customer has already established a protocol or started a research study using high purity acetonitrile, it would be very difficult to switch to another HPLC (or other) solvent.
(44) For the purposes of this case, the question as to whether high purity acetonitrile constitute a separate product market can be left open, as the Transaction does not lead to competition concerns under any plausible market definition.
Conclusion on market definition for solvents
(45) For the purposes of this case, the exact product market definition for solvents and any sub-segments thereof can, in any case, be left open, as the Transaction does not give rise to competition concerns on these markets under any possible market definition.
4.2.1.2. Inorganics
(46) In case M.7435 Merck/Sigma-Aldrich, the Commission defined inorganics as substances or compounds added to a system in order to bring about a chemical reaction or to see if a reaction occurs. The Commission noted that the primary difference between organic and inorganic compounds is that organic compounds always contain carbon, while most inorganic compounds do not. The Commission also observed that, similarly to solvents, inorganics can be used for classical laboratory analysis and for instrumental analysis. In addition, the category of inorganics also includes a third group of products, auxiliaries, which are ancillary products such as absorbents and drying agents that are used in conjunction with inorganics.
(47) In case M.7435, the Commission found inorganics for classical laboratory analysis to include a number of different types of products, namely acids, bases, buffers, salts, and metals/elements. Each of these types of inorganic is defined by its particular composition and serves a different purpose in laboratory applications, as follows:
a. acids: chemicals that, in aqueous solutions, react with bases to form salts and with most metals (such as iron) to form salts and hydrogen;
b. bases: chemicals that, in aqueous solutions, react with acids to form salts, and promote certain chemical reactions (base catalysis);
c. buffers: aqueous solutions used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications;
d. salts: ionic compounds that are produced as a result of the neutralisation reaction of an acid and a base, used in both qualitative and quantitative analysis of substances and substance mixtures;
e. metals/elements: materials (whether compounds, alloys, or elements) that have high electrical conductivity, high thermal conductivity and high density, and that are used for various purposes in research and production.
(48) Inorganics for instrumental analysis can be further categorised on the basis of the purpose for which they are used, e.g. for determining the concentration (volumetric titration solutions), for determining the presence of water (Karl Fischer titration), or for calibration and qualification of analytical instruments.
(49) The Commission found in its market investigation in case M.7435 that inorganics cannot normally be substituted for one another from a demand-side point of view. Each type of organic serves a specific purpose, for which other types would not be suited. There is, however, a large degree of supply-side substitutability, and many suppliers are able to offer a wide range of inorganics. Nonetheless, supply-side substitutability may also be limited in some cases by the presence of IP rights or the needs for specific knowledge to produce certain types of inorganics, most notably high purity organics and Karl Fischer titration solutions. In case M.7435 the Commission left the product market definition open for inorganics and all possible sub-segments thereof.
(50) The Notifying Party submits no views on the appropriate product market definition for the market for inorganics or any part thereof.
Results of the market investigation: demand-side substitutability
(51) The results of the market investigation in the present case also confirmed that there is very little demand-side substitutability between different types of inorganic, (41) as had been found in the Commission's previous case M.7435 Merck/Sigma-Aldrich. The market investigation considered in particular the distinctions between: i) inorganics for classical analysis and inorganics for instrumental analysis; ii) within inorganics for classical analysis: the difference between specific types of inorganics (acids, bases, salts, buffers and metals); and iii) within inorganics for instrumental analysis: the difference between inorganics for use in each specific technique (in particular volumetric titration solutions, Karl Fischer products, inorganics for calibration and qualification of analytical instruments, inorganics for sample preparation, inorganics for X-ray fluorescence analysis and auxiliary products).
Inorganics for classical analysis v inorganics for instrumental analysis
(52) The findings of the market investigation in relation to this distinction were very similar to those discussed above for the distinction between solvents for classical analysis and solvents for instrumental analysis. Customers generally confirmed that inorganics for classical analysis and inorganics for instrumental analysis are considered to have different uses, as a result of their different levels of purity, and would not be bought interchangeably. (42) One stated, for example: "we would not expect to see an overlap in their usage, inorganics for instrumental analysis tend to be of a different grade to any purchased for classical analysis". (43) Whilst a number of customers did acknowledge that inorganics for instrumental analysis could theoretically be used for classical analysis, it would be too expensive to do this in practice. One respondent explained: "it is unlikely that an end user would utilise a higher grade more expensive chemical where a lower grade is fit for purpose and likewise would not use a lower grade where clearly for technical reasons a higher grade is required". (44)
(53) Only a very small proportion of customers would consider switching from inorganics for instrumental analysis to inorganics for classical analysis, and this is usually only for a relative small proportion of their total purchases of inorganics for instrumental analysis. (45) The reasons for customers reluctance to do this are very similar to those discussed above for solvents, namely that using a product with lower purity levels could jeopardise the results of the procedure. One respondent referred, for example, to the "risk of inferior results and interferences". (46) A small number of respondents also mentioned that there may be theoretical overlaps in usage, but that they would not typically investigate these. One mentioned, for example, "We purchase inorganics against a specification for the work we need to conduct. In theory inorganics could be used for multiple types of work and as a result there might be an overlap […] but specification is the important factor and not cost". (47) It would seem, therefore, that there very little overlap in usage in practice.
Inorganics for classical analysis: acids, bases, salts, buffers and metals
(54) The results of the market investigation also confirmed that there is very little demand-side substitutability within the category of inorganics for classical analysis. Respondents explained that each of the main types of inorganics for classical analysis (acids, bases, salts, buffers and metals) has a unique composition and function, and can thus not be substituted for with another inorganic. (48) One customer explained, for example: "Every substance is unique, especially in analytical matters. Bases cannot be replaced by acids. Also, sulphuric acid cannot be replaced by nitric acid and so on. Every substance has unique properties, they rarely can be swapped." (49)
(55) As explained above, customers almost without exception follow the specifications of the product, and would not set about testing whether it could be used for a different purpose. One stated, for example: "we basically follow the ISO/AOAC/AFNOR/... classifications. They clearly state which products can be used. We are not testing if we can substitute a type of product with another." (50)
(56) A very small proportion of respondents did see there being some scope for substitution between different types of inorganics for classical analysis, (51) but this was often restricted to particular procedures (such as, for example, that "a buffer can be made from acid/salt or base/salt" (52)) and was not generally stated to be the case. Furthermore, some respondents that mentioned a theoretical overlap in usage also acknowledged that this occurs only very rarely in practice. (53)
Inorganics for instrumental analysis: volumetric titration solutions, Karl Fischer products, inorganics for calibration and qualification of analytical instruments, inorganics for sample preparation, inorganics for X-ray fluorescence analysis, and auxiliary products
(57) Similarly to inorganics for classical analysis, customers also see there being little scope for substitution between the different inorganics for instrumental analysis. The vast majority of respondents confirmed that inorganics designed for a specific type of instrumental analysis are used exclusively for this designated purpose. (54) One respondent explained that they "all have a specific use within the organisation, no double use possible". (55)
(58) As is the case for inorganics for classical analysis, a small number of respondents did identify potential limited overlaps (such as using titration grade inorganics as sample preparation reagents). (56) Nonetheless, it is clear that even these limited theoretical overlaps are almost never exploited in practice. One respondent stated for example, that overlaps in usage are "theoretically present but not in practice, too time consuming". (57) Another respondent explained that the way in which the chemicals are sold would also make it even less likely that customers would use chemicals for any purpose other than that stated in the specification. "Companies […] sell a range of reagents as a calibration kit for example. Some of the kit contents could well be chemicals that would have other uses but an end user is not likely to split a kit for another use." (58)
Results of the market investigation: supply-side substitutability
(59) Responses from competitors indicated that most manufacturers are able to offer a wide range of inorganics. Competitors generally produce both inorganics for classical analysis and inorganics for instrumental analysis. Within inorganics for classical analysis, almost all manufacturers offer a full range including all (or most) of the different types of product (acids, bases, salts, buffers and metals). Those who do not offer a particular category could easily start producing it should they choose to. Within inorganics for instrumental analysis, meanwhile, there is slightly more differentiation between manufacturers. The major global players tend to offer the full range (volumetric titration solutions, Karl Fischer products, inorganics for calibration and qualification of analytical instruments, inorganics for sample preparation, inorganics for X-ray fluorescence analysis and auxiliary products). A number of smaller manufacturers, however, do not currently produce and would not be able to start producing some of these types of inorganics, in particular inorganics for x-ray fluorescence analysis, auxiliary products (such as absorbents and drying agents), some Karl Fischer products and (to a lesser extent) inorganics for sample preparation. This is due to the specific technology and expertise required to produce these products. (59)
(60) Overall, therefore, it can be concluded that there is generally a high degree of supply-side substitutability between most inorganics, but that the expertise required to produce certain types of inorganics for instrumental analysis limits this. The set of manufacturers able to offer these products is therefore more restricted and it would be difficult for other competitors to enter these areas.
High purity acids
(61) As mentioned in reference to acetonitrile (above), the Commission has not previously assessed potential product markets for individual solvents and inorganics, but has been led to do so in this case on the basis of information provided by market participants. A small number of market participants indicated that VWR's presence in the upstream market for the production of high purity acids on an OEM basis (i.e. the form in which this type of acid is then bought by Avantor and other suppliers of laboratory chemicals) could create potential for input foreclosure. VWR (through Seastar) purifies, packs in catalogue size and labels high purity acids for downstream competitors reselling the product under their own brands. As Avantor is active in such dowstream market and a customer of Seastar, the merged entity could hypothetically stop selling on an OEM basis to other downstream competitors.
(62) As VWR is itself present on the downstream market for retail sales, the Transaction also creates a horizontal overlap. In order to assess both the vertical and horizontal relationships between the Parties' activities in this area, the Commission has therefore considered the existence of a possible market for high purity acids. (60)
(63) High purity acids are generally considered to be acids of a level of purity where the contaminants are measured in parts per billion (known as 'PPB acids') or parts per trillion (known as 'PPT acids'). Possible markets could be considered for all high purity acids or for PPT acids only, as the production of PPT acids requires additional steps and authorisation compared to the production of PPB acids. (61)
(64) The Commission noted in case M.7435 Merck/Sigma-Aldrich that high purity acids were one of the categories of inorganics for which supply-side substitutability may be limited due to the need for particular expertise and IP rights related to their production.
(65) The main differences in the production of PPB and PPT acids compared to other acids for laboratory use are: i) the distillation equipment and piping used for the production of PPB and PPT acids must be made from high purity plastic or quartz; ii) the equipment used for distilling and packaging PPB and PPT acids must be enclosed in a clean-room environment to prevent potential environmental contamination; and iii) PPB and PPT acids are usually packaged in high purity and acid-resistant plastic bottles to prevent contamination and potential leaching from packaging material that could introduce contaminants into the product.
(66) In addition, the production of PPT acids has some additional requirements relative to the production of PPB acids. A second distillation may be required for some PPT acids and producers may have to meet higher requirements in their clean rooms in relation to air exchanges for the production of PPT acids in comparison to PPB production.
(67) The Notifying Party submits that the process of upgrading an existing lower purity acids manufacturing line to manufacture PPB and PPT acids would take approximately […] and would require investment of approximately EUR […] to EUR […], (for a manufacturer that already has the necessary expertise).
(68) The Notifying Party submits that it is not appropriate to define a separate market for high purity acids, and that they should instead be considered as part of the product market for acids that belongs to the overall market for inorganics. It argues that acid producers can and do adapt their manufacturing processes to make acids of differing purities, and that there are no barriers (such as technology or intellectual property) preventing producers of other types of acid from starting to manufacture high purity acids. The additional investment required to be able to produce PPB and even PPT grades is described as 'relatively modest'. The Notifying Party explains that suppliers may choose to purchase high purity acids from other manufacturers on an OEM basis (i.e. under contract manufacturing, to sell under their own brand name) rather than producing these acids themselves simply because this is the most economical option, but not because they would be incapable of producing high purity acids.
(69) Contrary to the statements made by the Notifying Party, the results of the market investigation confirmed that specific equipment and expertise is required to produce high purity acids that would not be needed for producing other acids (or other inorganics more generally). Competitors active in the production of high purity acids mentioned in particular, that the equipment used when manufacturing PPT and PPB acids needs to be made of specific types of material. In general, the higher purity the acid, the more tightly controlled the production process has to be, and additional technology and expertise is therefore required for producing PPT acids relative to PPB, for example, sophisticated analysis techniques to measure the impurities in PPT acids. (62)
(70) The replies received from competitors active in production also suggest that the markets for high purity acids for industrial use (mainly in the semiconductor industry) and for laboratory use are quite separate. A number of other manufacturers only produce high purity acids for industrial customers, mainly because they do not have the production lines for packaging the acids in catalogue sizes, or the general set-up for selling to other types of customer. (63) There may also be some small differences in the specifications for high purity acids for laboratory and industrial use respectively but the main difference is the pack sizes; one respondent explained that "there is no difference in high purity acids for industrial use compared to high purity acids for classical laboratory analysis". (64) Additional responses from suppliers to end customers (i.e. competitors to VWR and Avantor in the laboratory chemicals market) also confirmed this. (65)
(71) For a company that currently sells to industrial customers only, it would therefore require some investments to start selling to laboratory customers (either directly or to laboratory suppliers), and would entail a change in business strategy, given that the market for high purity acids for industrial use is much larger than that for laboratory use because customers request significantly larger amounts. (66) One competitor that currently produces high purity acids for industrial customers only explained that, starting to produce for laboratory use would involve constructing dedicated production facilities including a cleanroom for down-packing, and that it would be at least a year before it were able to commercialise high purity acids for laboratory use. (67)
(72) Given the above, it would appear that there is limited supply-side substitutability between high purity acids and other types of acid (or of inorganics more generally). Producers of other types of inorganic could not easily start producing high purity acids, and a significant investment would be required in new production lines, in addition to which not all suppliers of laboratory chemicals would have the necessary expertise. Furthermore, there are some differences between the processes for producing PPT and PPB acids, meaning that a competitor active in the production of PPB acids could not necessarily start producing PPT acids although it is likely to be easy for a producer of PPT acids to also produce PPB quality products. This is because required purity levels required for PPT acids are 1 000 times higher than for PPB acids. Distillation or purification has to be done using highly specialised equipment and piping (distillation columns, tubing, filling machines etc.) that are made of special materials like Teflon or quartz that do not contaminate the acid. Stainless steel equipment for example is not suitable for these types of materials. Filling of small sized containers is typically done manually in clean room environment with particularly high requirements.
(73) Similarly, there is very little if any demand-side substitutability between high purity acids and other acids. High purity acids are chosen for procedures where a certain level of purity is necessary, thus also determining whether a PPT or a PPB acid should be used. Whilst it would theoretically be possible to use acids of higher purity than needed (e.g. PPT when PPB would be adequate, or PPB where a normal acid could be used), this would clearly not be cost efficient and does not occur in practice.
(74) Based on the above, there would seem to be strong indications that it may be appropriate to define a separate market for high purity acids, distinct from other acids. There are also some grounds to suggest that PPT acids may constitute a market of their own. Should a market for high purity acids (or any sub-segment thereof) be considered appropriate, this may be limited to only high purity acids for laboratory use, to reflect the lack of supply-side substitutability between high purity acids for industrial and laboratory use respectively. (68)
(75) For the purposes of this case, the question as to whether high purity acids (or any sub-segment thereof) constitute a separate product market can be left open, as the Transaction does not lead to competition concerns under any plausible market definition.
Conclusion on market definition for inorganics
(76) For the purposes of this case, the exact product market definition for inorganics and any sub-segments thereof can be left open, as the Transaction does not give rise to competition concerns on these markets under any plausible market definition.
4.2.1.3. Package sizes and brands
Bulk Size v catalogue size
(77) In case M.7435 Merck/Sigma-Aldrich, the Commission also considered the importance of the sizes of the units in which laboratory chemicals are sold. It was concluded that it would be relevant to make a distinction between catalogue size chemicals (defined as being up to 10 kg or 10 litres per unit) and larger, ‘bulk size’ purchases. The main reasons for defining separate markets for bulk and catalogue size chemicals were that the types of customer and the pricing of the chemicals can be quite different, with laboratory customers more likely to purchase catalogue sizes and customers in the manufacturing industries tending to buy bulk sizes. Furthermore, it was found that customers purchasing catalogue sizes could not easily switch to bulk sizes, due to a lack of storage facilities and for safety reasons. From a supply-side perspective, the Commission noted that, whilst most suppliers active in catalogue size chemical also supply bulk volumes to some customers, there are also a significant number of suppliers only active in the bulk size market, as selling in catalogue sizes would require an entirely different business model. The Commission concluded that 10kg or 10 litres was the unit size that reflected a natural divide in the market between catalogue size and bulk size.
(78) The Notifying Party agrees with the Commission’s findings in M.7435 and thus with the definition of separate markets for bulk and catalogue sizes of laboratory chemicals.
(79) The results of the market investigation in the present case also suggested that a distinction between bulk and catalogue sizes of laboratory chemicals would be appropriate. (69) The customers contacted were the Parties' main customers for catalogue size chemicals and the majority of respondents stated in their responses that they did not buy bulk chemicals. Considering the different main categories of customer for laboratory chemicals (pharmaceutical and biopharmaceutical manufacturers, professional laboratories, and research institutions and universities), it can be noted that a slightly higher proportion of pharmaceutical and biopharmaceutical manufacturers buy in bulk compared to the other two types of customer, but even within this group, the majority only buy catalogue sizes. (70) Bulk size customers are typically industrial customers in different sectors (including electronics and semi-conductors but also a wide range of other industrial manufacturers) that need large quantities of specific chemicals for their production processes. The needs of those bulk size customers are very different from the needs of laboratory customers that require a range of laboratory chemicals for testing and research purposes in small quantities.
(80) Moreover, almost all of those customers who do not currently buy bulk chemicals stated that they would not be able to buy in bulk. The main reasons given for this were safety considerations, related to the possible risks involved in transferring chemicals between containers, and practical considerations, such as a lack of appropriate equipment and/or storage space for larger volumes and insufficient usage (meaning that the chemicals would go out of date). (71) One customer stated, for example, that "transferring [laboratory chemicals] is not desirable because of safety concerns". (72) Another mentioned that the company was "not equipped to do so properly. It would just not be efficient." (73)
(81) Some customers were of the opinion that bulk chemicals were probably cheaper, but most had little knowledge of the potential price difference having never investigated the possibility of buying in bulk. (74) Furthermore, the price difference does not appear to play any role in customers' decision as to whether to buy chemicals in bulk or catalogue sizes, as for those who only need small volumes and do not have the facilities for repacking, buying in bulk is simply not considered to be a realistic option.
(82) A large proportion of customers reported that they typically buy quantities of laboratory chemicals of 2.5kg/l or 5kg/l. Some even buy in smaller quantities of 1kg or less. In terms of defining an upper limit as to what can be considered 'catalogue sizes', most respondents were of the opinion that either 10kg/l or lower would be an appropriate ceiling. (75)
(83) In light of the above, the Commission considers that, as regards relevant markets for laboratory chemicals, a distinction could be made between catalogue sizes and bulk size. However, for the purposes of this case, the question as to whether catalogue size and bulk size laboratory chemicals can be left open as the Transaction does not give rise to competition concerns under any possible market definition. In any event, the Transaction only results in affected markets for certain solvents and inorganics in catalogue size and this will be the focus of the analysis below. (76) The Transaction does not lead to affected markets for any laboratory chemicals in bulk size considered separately, nor when catalogue sizes and bulk size are considered together.
Importance of branding
(84) Branding is generally important in the industry because specific brands are associated with high and stable quality levels. At the same time, more commoditised chemicals are also available from distributors under their own private labels. The Commission found in case M.7435 Merck/Sigma-Aldrich that in order to differentiate themselves suppliers develop brands which are recognised by customers and represent quality, certain set of specifications and consistency of the product. (77) It also found that quality of the product in this context refers not only to the chemical composition or the purity of the product but also to the level of confidence in the documentation and quality of the labelling which are particularly important due to the strong safety hazard in these markets. This quality is generally perceived in the market place through brands. (78)
(85) The Notifying Party explains that some customers, such as pharmaceutical companies and other customers that place a high importance on quality, or are risk-averse generally, favour suppliers with branded products, rather than private (or "house") labelled products. On the other hand, more cost-conscious customers like academic institutions are typically more likely to purchase from suppliers with less expensive private label products. The Parties do not believe that branded products and other chemicals belong to separate markets.
(86) The Commission considers that the specification of chemicals is a scientific definition and that customers in the relevant sectors have a good understanding of the chemical composition of specific products, including purity levels. Therefore, customers can substitute branded and other products as long as they comply with the required specifications. Typically, private labelled products are cheaper alternatives to high priced branded products from the original manufacturers sold under distributor's names. From a supply side perspective, there is also no reason to believe that branded and private label products differ to a significant degree. To the contrary, companies like the Parties that produce branded products can and often do produce private label products that are sold on an OEM basis to other market participants that market them as their own private label products. In this regard, OEM manufacturing refers to a situation where a manufacturer produces chemicals and also packs and labels it for final sales using private brand names of third parties. Such OEM manufacturing also happens on behalf of producers that have strong brands themselves. An example of the latter would be Merck that purchases some of its global sales of high- purity acids from Seastar on an OEM basis without any further processing, repackaging, or labelling done by Merck itself.
(87) In light of the above and for the purpose of this decision, the Commission concludes that private label and branded products belong to one and the same product market. However, it has to be considered that differences particularly in price exist that play a role when analysing the closeness of competition between different products.
4.2.2. Geographic market definition
(88) In its most recent case in this area, M.7435 Merck/Sigma-Aldrich, the Commission noted that the production market for laboratory chemicals had some features of an EEA-wide market but that there were also indications of national markets. From a supply-side perspective, the Commission found that the majority of major manufacturers sell their laboratory chemicals under the same brands across the EEA and sometimes even globally. Alongside these major suppliers, there are, however, also a number of regional and local suppliers of laboratory chemicals, such as PanReac Applichem (Germany), Carl Roth (Germany), Romil (UK), and Rathburn (UK).
(89) From a demand-side perspective, meanwhile, it was noted that many customers organise purchasing of laboratory chemicals at national or regional level, even if some very large customers have global supply contracts. Furthermore, it was found that suppliers often have sales teams operating at national or regional level. This reflects the highly fragmented nature of the customer base, with suppliers needing local sales forces and technical support to meet the needs of large numbers of small customers. It was also noted that customers in different countries often have different preferences in terms of how they purchase laboratory chemicals and that the variation in customers’ habits and in language requirements was also an important factor that suppliers had to take into account.
(90) In addition, the market investigation carried out in case M.7435 gave some indications that there could at times be significant price differences between countries, which could not be attributed to variations in transport costs or distribution margins alone. Furthermore, the Commission noted that there are both EU and national regulations on the packaging, labelling, transport and storage of laboratory chemicals.
(91) The Notifying Party submits that the geographic market for laboratory chemicals may be at least EEA-wide. It highlights that there are a number of players active on an EEA-wide and even global basis, such as the Parties, Merck, Carlo Erba, Honeywell and Thermo Fisher, and that these suppliers sell their products under the same brand names throughout the EEA and ship their products from a limited number of warehouses located in the EEA. The Notifying Party also claims that an increasing number of international customers negotiate supply contracts that cover their entire EEA or even global operations. Lastly, the Notifying Party submits that there are no intellectual property rights or regulatory barriers that would limit trade flows and that transportation costs for laboratory chemicals are low.
(92) The results of the market investigation in the present case contain a number of indications that the markets for different laboratory chemicals are likely to be national, although there are also a number of characteristics which would suggest a wider geographic scope.
(93) Firstly, a large proportion of customers purchasing laboratory chemicals are active in only one country. This is particularly true of professional laboratories and of research institutions and universities, which, by their very nature, often only have one site. Even universities and other research institutions that coordinate their purchases or organise combined tenders typically do so only on national or even smaller scale. The third group of customers, pharmaceutical and biopharmaceutical manufacturers, does include more organisations with a wide geographic presence, but there are nonetheless a large proportion only present in one EEA Member State. Amongst those customers that are present in more than one country, purchasing can be organised at either national or EEA level (or wider). Where customers conclude contracts at global level (usually in order to benefit from the greater leverage they can exercise through having larger volumes), there is often also some purchasing carried out at local level, on a more ad hoc basis. (79)
(94) The majority of customers reported that transport costs do not have any influence on their purchases. Transport costs are usually included in the prices quoted by distributors, and because smaller customers are typically buying from distributors that have a local presence, transport costs are not a major consideration when choosing suppliers or distributors, at least within the EEA. (80) One customer stated, for example: "transport cost is not a limitation irrespective of whether we buy from a manufacturer or from a distributor". (81) A small minority of respondents do, however, state that transport costs may influence their purchasing decisions. Some customers mentioned that they try to source only from within the EU, with transport costs being one of the reasons why they would not source from further afield. One explained, for example, that "transport costs always influence our purchasing decisions independently of whether we buy directly from the manufacturer or from the distributor". (82)
(95) Customers' opinions varied as to whether there are significant differences in prices between EEA Member States. A fairly large proportion had no insight into this, due to the fact that they are active in only one country. Of those that did express an opinion, some noted that prices vary between countries, even within the same region (e.g. between western European countries). (83)
(96) The responses to the market investigation from manufacturers and distributors of laboratory chemicals also suggested that national markets may be relevant.
(97) Whilst the majority of the major manufacturers of laboratory chemicals are active at least across the EEA (and often globally), there are also a number of smaller local and regional manufacturers present, which often operate from only one production site and serve a much more limited geographic area. (84)
(98) The majority of competitors organise their sales forces at national level, (85) and some also tend to sell a larger proportion of their laboratory chemicals direct in the countries where they are based, whereas they prefer to use distributors for other markets, where they do not have a local presence. (86) One competitor explained, for example, that "the huge number of customers is better covered by local actors and having local players in the country allows a better level of service", (87) thus highlighting the importance of local distribution networks and personnel. A number of the smaller European producers explained that, although they do sell to customers in other EEA countries, they are generally strongest in the countries where they are based, as they have their own sales forces and can offer shorter delivery times. (88)
(99) A significant majority of customers also stated that their prices vary either between individual countries or between regions in Europe. (89) Some competitors (including the largest players) also offer separate catalogues for each country, mainly due to language requirements. (90)
(100) The barriers to trading between countries and regions most often mentioned by competitors included regulatory barriers (at regional or national level) and transport costs. One competitor explained, for example, that local regulations can change very quickly, making it difficult for manufacturers to adapt. Another also emphasised that without a commercial structure already in place in a different country, it would be difficult to extend operations due to the need for staff familiar with national regulations. Transport costs can also play a role for some products, especially if the product is of lower value (and the transport costs therefore proportionally higher) or if certain packaging or other requirements apply which make transport more expensive. One competitor also explained that the lack of brand recognition for their products outside their home market would make it very difficult to compete effectively. (91)
(101) The results of the market investigation did, however, also confirm one of the arguments put forward by the Notifying Party in favour of a wider geographic market. The majority of competitors sell their products under the same brand names at least across the EEA and often globally. (92) Manufacturers that are active across several countries or even globally (like the Parties) tend to concentrate manufacturing for the whole of the EEA in only few production sites, sell their products under the same brand names throughout the EEA and ship their products from a limited number of warehouses located in the EEA.
High purity acids and acetonitrile
(102) Notwithstanding the above, it should also be noted that the geographic market definition may vary depending on the exact point in the supply chain that is being assessed. It is often the case that suppliers of laboratory chemicals buy the chemical in bulk from a manufacturer, and then purify it further (often via distillation) before packaging it into quantities suitable for laboratory use. Suppliers do not, therefore, always manufacture the actual raw material themselves.
(103) In the case of the market for high purity acids, VWR is active via Seastar in Canada which sells on an OEM basis to third parties globally. The geographic market for this upstream level of production may be different from the geographic market for the downstream sale of the finished product.
(104) The Notifying Party submits that the appropriate geographic market for the production of high purity acids is at least EEA wide, as manufacturers ship these products throughout the EEA.
(105) The location of manufacturers of high purity acids (as a raw material) and their customers (i.e. suppliers of high purity acids to end customers) would indeed seem to suggest that the market for the production of high purity acids is at least EEA wide, if not global, while the downstream market is likely to be national. Upstream competitors also confirmed in their responses to the market investigation that they sell high purity acids to customers across the EEA and often more widely, (93) while competitors on the downstream market (i.e. suppliers of laboratory chemicals to end customers) often sell high purity acids within only one or a small group of countries, as is the case for other laboratory chemicals. (94)
(106) The considerations discussed above in relation to high purity acids largely also hold for acetonitrile. Competitors supplying acetonitrile to end-users very often achieve all or the vast majority of their sales in one country, (95) (although the major laboratory chemicals suppliers that are active across the EEA also market acetonitrile in all the areas where they are present) while raw materials manufacturers serve customers at least across a large region, if not globally. (96)
(107) In light of this, the geographic market for the production of high purity acids and acetonitrile is likely to be at least EEA wide in scope, given the trade flows that can be observed and the standard nature of the product, i.e. the lack of differentiation according to regional preferences.
(108) When considering the downstream market for the supply of laboratory chemicals to end customers, the geographic market for high purity acids and acetonitrile is likely to be national, notwithstanding some indications of a wider geographic scope.
Conclusion on the geographic market definition for solvents and inorganics
(109) For the purposes of this case, the exact geographic market definition for laboratory chemicals and any sub-segments thereof can, in any case, be left open, as the Transaction does not give rise to competition concerns on these markets irrespective of whether they are considered to be national or EEA-wide in scope.
4.3. Distribution of laboratory products
(110) The Commission most recently assessed the market for the distribution of laboratory products, including laboratory chemicals in cases M.7435 Merck/Sigma-Aldrich and M.6944 Thermo Fisher Scientific/Life Technologies. While laboratory chemicals were defined as chemicals used for research, testing, and quality control, including thousands of chemicals belonging to different chemical groups, (97) a larger set of products was also considered as a market for laboratory products, including laboratory chemicals but also consumables like glassware and plastic products typically used by laboratory customers (such as pipettes and vials, safety-related products such as gloves, masks, etc.). (98)
(111) The market investigation in case M.7435 largely confirmed the Commission’s findings in the earlier case (M. 6944) and the same approach was taken in both those cases.
4.3.1. Product market definition
(112) In its past decisional practice (as referred to above), the Commission's market investigations indicated that distributors normally offer a wide range of products including a large number of laboratory chemicals from different manufacturers, laboratory consumables (i.e. glassware and single-use equipment such as disposable pipettes and gloves), equipment and instruments, as well life science products (99). Furthermore, one of the reasons why customers often prefer to source laboratory products from distributors was found to be the convenience of being able to buy a wide range of products from one source. In light of this, in the past, the Commission concluded that the relevant product market was likely to be the market for distribution of laboratory and life science products. (100) The distribution of clinical diagnostics equipment for use in hospitals and by pathologists was considered to constitute a separate market (101) (and thus excluded from the market for distribution of laboratory and life sciences products) due to the presence of specific regulatory requirements and differences in the way these products are purchased and in the services required by customers.
(113) The Commission has not previously considered any segmentation of the market for the distribution of laboratory and life sciences products on the basis of the channel through which the products are sold, e.g. online sales as opposed to purchases made from sales reps.
(114) The Notifying Party agrees with the Commission’s previous findings and considers the relevant product market to be the distribution of all laboratory and life science products.
(115) The results of the market investigation in the present case broadly confirm the Commission's findings in its previous cases relating to the distribution of laboratory and life science products. Many customers consider the range of products offered to be an important criterion when choosing a distributor (102) and almost all state that it is preferable if not essential for a distributor to offer a comprehensive range of products. (103) One customer explained, for example, that "[offering a wide range of products] is important / preferable as [the distributor] acts like a one stop shop model for the users". (104) One of the main advantages of buying from a distributor (rather than from the manufacturer) is also considered to be the convenience of having fewer suppliers to deal with, (105) which also stems from the range of products offered by the distributor.
(116) The responses from distributors also suggest that the market for distribution is likely to cover at least all laboratory products and likely also life sciences products, rather than to be limited to laboratory chemicals alone. The vast majority of distributors supply laboratory chemicals, equipment and consumables, and sell all these products to the same customers. (106) Most distributors are of the opinion that other distributors also offer a full range (107) and consider this to be an important part of their business model. (108) One distributor stated, for example: "the customers expect a broad range of products as they do not want to have too many different suppliers". (109) Furthermore, most distributors state that there are no laboratory or life sciences products that they could not start distributing. Only a small minority of respondents felt that there were areas where specific expertise would be needed which they did not have. (110)
(117) The responses provided by some distributors did, however, indicate that the requirements for distributing laboratory chemicals can be slightly different to those for distributing other laboratory products. Distributing chemicals is subject to stricter regulations (such as the ADR regulation) and can also be more time sensitive, as compared to distribution of consumables or equipment. (111)
(118) As regards laboratory chemicals specifically, most distributors confirmed that they are able to distribute certified products. Although some distributors mentioned specific requirements they have to adhere to for the distribution of certified products (e.g. temperature control, separate storage areas and safety measures), only a minority stated that they needed any particular accreditation. (112) In addition, the majority of distributors believe that they would be able to distribute any type of laboratory chemical. (113)
(119) There are, however, also a small number of distributors on national level that specialise in particular types of laboratory chemicals, such as solvents for spectroscopy and/or chromatography. These distributors offer a more specialised range of products and typically do not sell other laboratory chemicals or other laboratory products such as equipment and consumables. Their presence on the market overall appears, however, to be rather limited, with the majority of distributors aiming to offer a wider range so as to be able to better meet customers' needs. (114)
(120) Most distributors serve a wide range of different customer groups and a large majority state that there are no specific groups of customer they would not be able to serve. For those that do mention customers they would not be able to serve, this is mainly large customers, such as pharma companies (for which the distributor would not be able to provide the volumes required and/or which typically enter into global supply agreements and therefore do not use local distributors). (115)
(121) Given that Avantor is not active as a distributor of third party products, no overlap arises in relation to the distribution of third party products. However, the present Decision also assesses potential harm to competition which may arise from the vertical integration of Avantor's activities on the upstream laboratory chemicals markets and VWR's activities as a distributor downstream. The Commission's guidelines on non-horizontal merges specify that when intermediate customers (i.e. distributors) are actual or potential competitors of the parties to the merger, the Commission focusses on the effects of the mergers on the consumers to which the merged entity and those competitors are selling. (116) Accordingly, whilst the Commission in this case has carefully analysed potential input foreclosure of access by competing distributors to laboratory chemicals, the assessment focuses on the impact on final customers. For the purpose of this decision, the Commission therefore has considered in its assessment of the downstream market all sales to final customers, including direct sales and sales via distributors (i.e., internal and external distribution). In addition, the Commission has also assessed the potential customer foreclosure of access by competing manufacturers of laboratory chemicals to distributors by considering external distribution separately.
(122) All the foregoing elements, taken together indicate that the relevant downstream distribution market for the purposes of the assessment of the present Transaction covers at least all laboratory chemicals. The market investigation in the present case did not indicate that a narrower segmentation of that potential downstream market (for example, on the basis of specific laboratory chemicals or particular types of laboratory chemicals) would be pertinent. The elements presented above, rather, tend to indicate that the relevant downstream market is potentially broader and also includes also other laboratory products in addition to laboratory chemicals, and may, in line with the Commission's findings in past cases, also comprise life sciences products (in addition to laboratory products). The elements presented above also indicate that it is not necessary to further segment the market according to the type of customer.
Conclusion on the product market definition for the distribution of laboratory products
(123) For the purposes of this case, the exact product market definition at the distribution level can be left open as to whether it encompasses the distribution of all laboratory and life science products, all laboratory products, or only laboratory chemicals. The Transaction does not give rise to competition concerns under any potential alternative market definition, even considering the narrower potential markets for the distribution of laboratory products alone or for the distribution of laboratory chemicals alone. On that basis, in the present Decision, the Commission will carry out its assessment of the vertical link between the upstream laboratory chemical product markets and the narrower of the potential downstream distribution markets, that is, those comprising (i) only laboratory chemicals and (ii) all laboratory products.
4.3.2. Geographic market definition
(124) In case M.6944 Thermo Fisher Scientific/Life Technologies, the Commission concluded that the market for the distribution of laboratory and life sciences products was national in scope and assessed the market on this basis. This finding was based on the market investigation carried out in that case which confirmed that most distributors operate in a single Member State. The results in that case further suggested that most distributors that are active in several countries organise their sales forces at national level and offer different catalogues in different Member States, as customers typically make purchases at national level. In addition, the Commission noted that prices and purchasing habits (e.g. the use of tenders) vary significantly between different EEA countries.
(125) In its more recent case in this area, M.7435 Merck/Sigma-Aldrich, the Commission confirmed these findings, noting that most distributors operate in a single Member State and that even larger distributors tend to carry out commercial negotiations with customers at national level. This is consistent with the need for a local sales force and local technical support in these markets. However, the Commission left the geographic market definition open in this case.
(126) The Notifying Party submits that the precise geographic market definition for the distribution of laboratory chemicals and life sciences products may be at least EEA-wide.
(127) The results of the market investigation in the present case are consistent with the Commission's findings in its earlier cases. Responses from distributors indicated that the majority are active only in one country (or occasionally in a small group of neighbouring countries) and that they operate from a single warehouse or storage facility. Even distributors that are active across a wider geographic area tend to have national sales forces and different catalogues for each country. As explained in the geographic market definition for the market for the production of laboratory chemicals, the majority of customers conclude contracts at national level, and even multinational companies with global purchasing agreements often also purchase at local level.
(128) A small number of distributors also reported that transport costs play an important role in competition on the market, at least for some types of product. (Although a larger proportion did not consider transport costs to be an important factor, this is in many cases a reflection of the fact that they are only active in one country.) Transport costs are most likely to be significant when the products are subject to the ADR regulation on the transport of dangerous goods by road, as this creates extra costs. A small number of distributors do therefore see transport costs as a factor limiting their ability to distribute beyond the country in which they have their storage facilities. (117)
(129) Furthermore, very few distributors would envisage any expansion of the geographic scope of their business. If prices were to rise in neighbouring markets, only a small minority would consider starting to distribute in these countries. (118) Most consider that they do not have the expertise or the capacity to expand their activities in this way, and that it would be extremely risky to do so. One distributor explained that "[starting] direct sales with our own salesmen [in a neighbouring market] would have very high costs and enormous commercial risks" and is thus of the opinion that the best way to move into a new market is by acquiring a distributor already present there, as they would have the necessary local knowledge. (119) This is confirmed by another distributor that stated: "we are not competitive enough to distribute outside our area of influence, which is regional, and where we can compete effectively for business". (120) Other respondents also mentioned that differences in regulations and the contracts they have with manufacturers would prevent them from expanding their activities to a wider geographic area, and that distributors present in a particular country can offer shorter delivery times. (121)
(130) There are, however, also a number of indications that the market for distribution of laboratory products may have some characteristics of an EEA market. First, large distributors like Thermo Fisher or VWR operate distribution centres that serve a number of countries. Second, a number of smaller distributors mentioned that they struggle to compete for business with larger customers that prefer to conclude agreements for purchasing covering their entire operation or at least a
wider geographic region. (122) A small number of customers also cited a distributor's ability to supply all EEA countries as an important factor in their choice. (123)
(131) Furthermore, the results of the market investigation suggest that online sales platforms are preferred by a majority of customers as they consider them an easy and fast method to buy, making the concept of national catalogues less important. Nevertheless, distributors would still need to provide the platform in different languages, this nonetheless demands less organisation at national level than producing separate physical catalogues adapted to each country. (124). At the same time, however, the results of the market investigation confirmed the continuing importance of local sales reps as a main channel to market, thus strongly suggesting that local presence and knowledge of the local market is critical to being able to compete effectively, especially for non-international customers. (125) The majority of manufacturers and distributors consider that online platforms, traditional catalogues, and local sales forces are necessary and complementary. For example, an important manufacturer explains "Ideally we cover all channels. E-commerce is a must today and the backbone for transactions. A sales force who is capable to hook up e-systems between customer and supplier is a key differentiator. The classical sales rep who “sells” products moves more and more from a product sales approach to a contract specialist and process enabler to automate buying processes". (126)
Conclusion on the geographic market definition for the distribution of laboratory and life science products
(132) For the purposes of this case, the exact geographic market definition for the distribution of laboratory and life science products can, in any case, be left open, as the Transaction does not give rise to competition concerns on these markets irrespective of whether they are considered to be national or EEA-wide in scope.
5. COMPETITIVE ASSESSMENT
5.1. Introduction
(133) The transaction leads to a number of horizontally affected markets in relation to the production of laboratory chemicals. It also creates vertically affected markets due to both Parties’ presence on the upstream production market and VWR’s activities as a distributor on the downstream market. The horizontally and vertically affected markets are discussed in turn below.
5.2. Laboratory chemicals: horizontally affected market
(134) Within laboratory chemicals, the Notifying Party has identified affected markets on the basis of the possible market definitions discussed above, namely for the overall markets for each of solvents and inorganics, and for the various sub- segments within each of these categories. Similarly, both the EEA and national markets are considered within the competitive assessment.
(135) The markets for solvents and inorganics (and the various sub-segments thereof) share many similarities in terms of both the competitive landscape and customers’ requirements and buying habits. An overall competitive assessment is therefore presented first, which applies equally to all the various horizontally affected markets within solvents and inorganics. Market shares and further assessment (where relevant) are then provided for specific sub-segments and national markets.
5.2.1. Overall assessment: solvents and inorganics
5.2.1.1. Limited availability of reliable market share data
(136) As was noted by the Commission in case M.7435 Merck/Sigma-Aldrich, the availability of market share data appears to be very limited in the markets in question here. In that case, the Commission commented on a number of sources of information that seemed to suggest that the Parties’ market shares could be higher than was stated by the Notifying Party (including both internal documents provided by the Parties and other market participants' perceptions of the Parties' strength). Furthermore, the market shares provided by the Notifying Party left a large proportion of the market in that case not attributed to any particular competitor.
(137) The Notifying Party in the present case submits that it is very difficult to produce reliable market shares, in particular for the narrower potential sub- segmentations, as there is very little publicly available information on total market sizes or competitors' sales. The Notifying Party has used the estimates disclosed in M.7435 Merck/Sigma-Aldrich, which, as stated in the Commission's decision in that case cannot be considered fully reliable in light of methodological challenges and absence of public sources.
(138) In view of the above, the Commission has taken a comprehensive approach to its assessment of the case, whereby the analysis has covered potentially relevant markets, not only when identified as being affected on the basis of market shares, but also when both Parties both have a meaningful presence. On the one hand, all respondents in the market investigation were asked to provide their views on other potentially affected markets, enabling comments on the overall markets for solvents and inorganics as well as potentially smaller markets. On the other hand, the customers whose contact details were provided for a particular narrow (potential) sub-segment were not only asked about these sub- segments but were also asked to provide to provide information in relation to their purchases and uses of solvents and inorganics more generally. Given that customers purchase a range of solvents and inorganics (as was confirmed by the market investigation itself), this approach enabled to validate the quantitative evidence provided via the market share data with qualitative information from market participants. Market shares provided by the Parties therefore constitute only one of several sources on which the analysis in this decision is based. The qualitative evidence gathered did not reveal any indications that other markets might be affected or that any other segments exist that warrant further assessment in addition to those discussed below. (127)
5.2.1.2. Competitive landscape
(139) In its most recent case relating to the markets for solvents and inorganics, M.7435 Merck/Sigma-Aldrich, the Commission noted the presence on the market of both a small number of major global manufacturers like Honeywell and Thermo Fisher and a larger set of smaller, local or regional players including PanReach Applichem, Carl Roth, Romil, and Amar. (128) The results of the market investigation for this case largely confirmed these findings.
Overview of main competitors and their respective brands
(140) Most of the major manufacturers of laboratory chemicals offer solvents and inorganics of all types, within both classical and instrumental analysis. The results of the market investigation also show that the range of products offered by manufacturers and the perceived quality/purity levels of their products appear to vary. (129)
(141) Both the market shares and market participants' views of the market indicate that Merck remains the clear market leader, and enjoys an unrivalled reputation for the range and quality of its products. A large proportion of customers named it as the top manufacturer (and an even larger proportion as one of the top two). (130) The small group of other global manufacturers further includes Honeywell, Thermo Fisher, Avantor, and VWR. Panreac AppliChem and Carlo Erba also appear to be amongst the main suppliers at EEA level (although their strength is very variable across different national markets in the EEA). At EEA level, Merck has estimated market shares of [20-30]% in solvents and [10-20]% in inorganics, Honeywell [10-20]% in solvents and [10-20]% in inorganics, Thermo Fisher [5-10]% in solvents and [5-10]% in inorganics and Carlo Erba and Panreac both [5-10]% in solvents and inorganics.
(142) In addition to this group of leading competitors, there are also a very large number of smaller manufacturers often only active in one or a small number of Member States. These include, for example, Scharlau, Carl Roth, Chempur, Romil, and Rathburn.
(143) While general chemicals producers such as BASF, Dow, Solvay, and Ineos exist, these focus on bulk production of laboratory chemicals and should not be considered as competitors to the Parties in the area of laboratory chemicals in catalogue size. This was the indication resulting from the market investigation, where the vast majority of responding customers stated that they had not bought either catalogue or bulk size laboratory chemicals from such general chemicals producers, and it was clear from responses that for most customers this would not be a viable alternative. (131) A large number of customers explained that the volumes they purchase are too small to source from general chemicals producers and that there would be "no rational reasons to buy bulk laboratory chemicals". (132) General chemicals producers are not viewed as meeting the needs of the types of customers that buy laboratory chemicals, and given the other options available, most have never seen any reason to explore this route. (133)
(144) The range of alternative suppliers indicates that viable alternatives remain on the market post-merger. There are also no indications for any specific substance for which customers require at least two suppliers and where post-merger not at least three suppliers would be readily available.
The Parties' products and brands
(145) Avantor sells its products in the EEA under the brand names J.T. Baker, Macron Fine Chemicals and POCH. (134) The Notifying Party explains that Avantor positions the J.T. Baker range as its premium brand, with its other brands priced at lower price points to attract more price sensitive customers. Laboratory chemicals sold under the Macron brand are typically priced around […]% lower than J.T. Baker, and those sold under the POCH brand around […]% lower. The Notifying Party therefore submits that the Macron and POCH brands are positioned closer to distributor brands rather than to the other main manufacturers. POCH is only available in Poland and is discussed in more detail in the section on national markets.
(146) VWR's main brand in the EEA is the VWR Chemicals brand. VWR does, however, also continue to sell under the brand names of regional and national companies it has acquired in the past, such as Klinipath in the Netherlands and Qpath in France. Although these brands keep their own name, they are still sold under the VWR Chemicals umbrella, e.g. as 'VWR Chemicals Klinipath'. Customers also seemed to be familiar with VWR's BDH Prolabo label.
Importance of brand recognition
(147) The results of the market investigation presented a mixed picture in terms of the importance of brand names. The majority of customers were of the opinion that brand is not especially important, explaining that they look at the product specification and the formula and choose on this basis. At the same time, however, a number of customers did acknowledge that brand was sometimes used as an indication that a product is likely to meet their requirements, i.e. they know that products from manufacturers with strong brand names are likely to have the right documentation and reliable purity levels. (135)
(148) In addition, a significant proportion of respondents confirmed that they would consider buying private label products and that these are often assessed alongside branded products when choosing a supplier. One respondent stated, for example: "all suppliers are considered, if the quality (both of the chemicals and the delivery) meet our requirements." (136) Another customer expressed a similar view, stating that "the decision would be made based on price and product equivalence. If the composition and expected quality are the same we would consider purchasing own brand products." (137) Customers with these types of opinions were therefore rarely prepared to pay a premium for branded products. (138)
(149) A smaller proportion of respondents did, however, state that brand plays a role in their decisions, and that they would not be prepared to buy private label products for some uses. One explained, for example: "we buy private labels for generic chemicals and solvents where purity is not of prime importance for testing purpose", (139) suggesting that brand is perceived as a guarantee of a certain quality. Similarly, another respondent explained that brand is important for solvents and inorganics for instrumental analysis but less so for solvents and inorganics for classical analysis, as the quality is not as critical for this second group. At least a certain proportion of respondents do, therefore, associate brand with the quality and purity of the product. One customer explained the link between brand and quality as follows: "usually the quality of a solvent goes hand in hand with its brand, the better known the brand the higher the quality, in general". (140) Another customer stated: "branded products guarantee reliable test results." (141)
(150) The customers for whom brand plays a role are often prepared to pay a premium for a branded product, although responses suggest that this only applies to chemicals being bought for procedures where quality is of paramount importance. One customer explained "in some cases [where we are performing] critical analysis, we would pay a premium for a branded product that guarantees the quality we need". (142)
(151) Views on the importance of brand names were generally similar across all types of customers. In all three categories (pharma and biopharma producers, professional laboratories, and research institutes and universities), customers mainly said that brand does not play a major role for them, but, there was also a strong perception that brand could serve as in indication of quality. Whether or not customers would be prepared to use private label products appears to depend more on the specific purpose for which each laboratory chemical is being bought rather than the type of customer. Customers would often be prepared to buy private label products for more 'standard' usage but would prefer known brand names where the quality is a critical factor. (143)
(152) Distributors' views on the importance of brand names provide a similar picture. One distributor stated, for example, "we think that customers prefer to use branded products for particular uses because they associate them with higher quality", whilst another was of the opposite view: "unless the internal protocol obliges the customer to buy a particular brand, the product specification and the price is what matters". (144) This seems to indicate that brands are more important for some than for others. In as far as brands play a role, they seems to be indirectly important as indicators for higher quality and reliable consistency in purity levels.
(153) This is further supported by some distributors that shared the views expressed by customers that brands are important in some circumstances but not others, depending on the sensitivity of the procedure. One explained, for example: "in unproblematic applications they [branded and private label products] are in competition; in more sensitive uses generally not". (145) A small number of distributors also made a distinction between different types of customers, observing that pharma companies would be more likely to prefer branded products, whilst smaller laboratories and potentially universities and research institutes may be more willing to consider private labels. (146)
(154) A major competitor who the Commission spoke to also observed similar tendencies in customers' choices with respect to brands, explaining that "Brands are very important to at least some customers […] in the area of technical grade laboratory chemicals the products are more commoditised and customers are less inclined to stick to a specific brand". This competitor further emphasised that the importance of brand can vary significantly between customers: "some customers will look for a particular brand, often because this brand is specified in their procedures whereas others will look for a particular type of chemical and then choose the lowest priced product that meets their needs. (147)
(155) In conclusion, it would appear that brand names can be important in at least some applications, as a significant proportion of customers associate them with a certain level of quality. As a result, customers are more likely to consider only branded products for sensitive uses, where the exact purity of the product can influence results, even if for more routine usage many would be prepared to substitute between branded and private label products on the basis of other factors, such as price. Therefore, high priced branded products do not seem to be in close competition with cheaper "commoditized" products of average quality.
Closeness of competition
(156) The findings on the importance of brands in the industry above are in line with the perception of the majority of customers that do not consider Avantor and VWR to be particularly close competitors. (148) In general, for sensitive uses, customers differentiate between branded products which are regarded as high quality products and sell at a premium price and lower quality products that are typically cheaper. Merck is typically named at the upper end of the price/quality range where Avantor positions its flagship brand JT Baker. On the other hand, VWR that is primarily a distributor sells products under its own label typically to more price sensitive customers with a lower affinity for specific brands.
(157) The few customers that consider Avantor and VWR as being similar are generally customers in markets where Avantor has a stronger presence than it does in the EEA overall, in particular in Poland. One Polish customer, for example, stated: "Avantor and VWR are close competitors to each other's own brands. They have similar customer targets and prices for solvents for classical analysis and inorganics for classical analysis." (149) These views seem to be limited to a small minority of customers and here particularly in Poland where Avantor is active via POCH that is a traditionally strong brand in Poland but not representative for Avantor's overall portfolio. These situations will be discussed in more detail in the respective sections on national markets below, as relevant. In general, even where customers were of the opinion that Avantor and VWR offer similar products, these products were also considered to be equally similar to those of various other suppliers so that VWR and Avantor are not seen as particularly close competitors by market participants. This is reflected in the view of one customer who stated: "we consider that VWR and Avantor offer similar chemical products to other manufacturers, such as Merck Sigma, Panreac, Honeywell and so on". (150)
(158) Only a small proportion of respondents expressed the opinion that Avantor and VWR were each other's closest competitors. (151) Customers mainly named Merck as the closest competitor to Avantor in both solvents and inorganics, with Thermo Fisher also mentioned quite frequently. Merck and Thermo Fisher were also considered by most customers to be the closest competitors to VWR. Honeywell was named as the closest competitor to Avantor and VWR by a smaller number of customers.
(159) Around one fifth of customers reported having switched from Avantor to VWR or vice versa for laboratory chemicals. Where customers had made such a change, it was more often from Avantor to VWR, and the reasons given were generally fairly generic reasons, such as price and availability of products. A number of customers also explained that the switch had occurred within the context of their regular tenders, i.e. other suppliers were also competing for the business, and on this occasion VWR had the best offer. (152) It is important to note that while switching from Avantor to VWR products is rare, switching between products of different laboratory manufacturers occurs more frequently. (153) This again suggests that there is no particular closeness of competition between Avantor and VWR and that customers of either would consider a wider range of other suppliers as equally viable alternatives.
(160) Distributors also named Merck most often as the leading manufacturer, with VWR, Honeywell and Thermo Fisher fairly evenly rated behind Merck. Avantor was only very occasionally named amongst the top five, and then generally after Merck, Honeywell, Thermo Fisher and Panreac. (154) Competitors and distributors also mentioned Merck and Thermo Fisher most often as being the closest competitors to VWR. A smaller proportion also named Honeywell, Avantor, and a range of other competitors. (155) Competitors and distributors views on the closest competitors to Avantor were more mixed, with all the major suppliers (including Merck, VWR, and Honeywell) being mentioned with similar frequency. (156)
(161) Most customers did not consider there to be any particular closeness between Avantor and VWR's brands. The characteristics that customers typically associate with Avantor and VWR's respective brands are also quite different. On the one hand, J.T. Baker has a very positive image for most customers, and is seen as a well-established, high quality brand. It is associated above all with high prices and high quality. There is generally much less awareness amongst customers of Avantor's (lower priced) Macron brand. (157) On the other hand, VWR's own brand (VWR Chemicals) is generally very well regarded by customers because it offers a good price to quality ratio. The quality is generally viewed as being acceptable or good, and pricing is average or low. Customer's views on the VWR BDH Prolabo brand were mainly fairly similar to those for other VWR Chemicals. It is perceived as a brand that offers good quality at a low price. (158) Whilst both Avantor and VWR's main brands enjoy a good reputation amongst customers, it appears therefore that customers associate them with different characteristics.
(162) Avantor and VWR are generally also not considered to have any particular common strengths in specific types of solvents and inorganics. They also do not seem to have any particular characteristics that set them apart from other manufacturers. While a very small minority of customers mentioned HPLC solvents as an area where both have a good product offering, (159) Honeywell and Merck are also strong players in this area as will be discussed in the respective sections below. Therefore, Avantor and VWR do not appear as neither unique players in any particular way nor as particularly close competitors to each other. Given that the Parties' products are typically not the closest alternatives, the merger does also not raise concerns with regards to customers that require dual sourcing. There are no indications that the Transaction would reduce choice to a critically small number of alternatives in any potential market.
Conclusion
(163) Based on the considerations above, a clear picture of the competitive landscape can be deducted: Merck is very consistently seen by market participants as being the leading supplier of laboratory chemicals and as having the highest quality products. (160) Customers and competitors generally consider Merck's main strengths to be the quality and range of its products, its fast delivery times and other aspects of its delivery service and logistics.
(164) Avantor is generally regarded by customers and competitors as also offering quality products, but at quite high prices (in particular under its main brand name, JT Baker). (161) Avantor's main strength in the eyes of customers is the quality of its product, this being mentioned by a large proportion of customers from all categories. Its main weaknesses were considered to be the availability of products, delivery times and prices. (162) Avantor is also generally not considered to have as wide a portfolio as some competitors. Combined with the perceived weaknesses in its own distribution network, which is reflected in rather long delivery times, (163) Avantor seems to be a close competitor to Merck but also more dependent on distributors to reach its end customers. However, it is also clear from the general level of awareness that Avantor has a more limited presence on the market than many of its main competitors, in particular Merck, Thermo Fisher, and Honeywell. (164)
(165) In terms of characteristics, a large proportion of customers rate VWR highly for its prices, quality, fast delivery times and its range of products. Others, however, mention that it cannot offer the full range of laboratory chemicals, in particular products for very specific uses. (165) One distributor emphasised this point, comparing VWR's range of laboratory chemicals to that of Merck: "VWR has been offering a wide range of laboratory chemicals but still their selection is negligible compared to Merck-Sigma". (166) While this is true for VWR's own product range, it still has an undisputed strength as a distributor with a wide range of products from other manufacturers which further differentiates it from Avantor that lacks a strong distribution network in Europe.
(166) Of the other main competitors: Thermo Fisher's main strengths are seen as being its wide portfolio, the quality of its products and also, similarly to VWR, its prices, while its customer service and delivery times are often mentioned as a weakness. Honeywell is considered to offer good quality products, but price, reliability and availability are considered as weaknesses for several customers. Views on the other competitors are generally more variable, although customers often mentioned price and quality as amongst the strengths for both Panreac and Carlo Erba. Waters (active across the EEA) was generally regarded as having high quality products, whilst its product range and price were seen as weaknesses. Chempur (the third largest player in Poland behind Merck and Avantor) was consistently rated favourably in terms of its very competitive prices although the quality of its products was rated relatively less well. Other brands which customers that are aware of regarded as good quality and price are Romil, Rathburn, Reagecon or Biosolve. (167)
(167) Overall, it can therefore be concluded that Avantor and VWR are not particularly close competitors on the market for solvents and inorganics, and that the other competitors, including competitors which are well-rated for their prices and for their quality, would continue to offer customers a wide range of choice following the merger. This finding is reflected in the Parties' brand perception: The image of J.T. Baker, Avantor's main brand in Europe, is quite different to that of VWR's own label lines and it is therefore unlikely that either is the main force exerting competitive pressure on the other prior to the merger. Furthermore, awareness of the J.T. Baker brand remains fairly limited in at least some EEA countries, and where it is present it is more likely to be competing for business with the other branded ranges, such as those of Merck, Honeywell, and Thermo Fisher than with VWR's brands. Moreover, Avantor's (lower priced) Macron brand has generally much less awareness amongst customers and was not generally indicated as a close competitor to VWR's brands. Panreac, Carlo Erba, and private label brands of Thermo Fisher, and several national competitors such as Boom, Roth, Romil, Scharlau, Rathburn, Chempur, Chemlab, and others represent alternatives for more price sensitive customers that are at least as close competitors to VWR's brands and typically more widely known than Macron. Merck has also recently introduced in the market a new lower price brand in bottles with red caps (referred to by the Parties as "Merck Red") targeting more price sensitive customers. These products are not distributed via VWR. The Notifying Party submits that Merck Red is in direct competition with VWR products.
(168) In light of the above, it can be concluded that the Parties are not particularly close competitors to each other.
5.2.1.3. Barriers to entry
(169) The Notifying Party claims that barriers to entry are low in the markets for laboratory chemicals, including in the markets for solvents and inorganics.
(170) It submits that the main regulatory requirement that a new competitor would have to fulfil to start competing on the market for laboratory chemicals is compliance with the REACH regulation. It also explains that there are additional EU rules applying specifically to solvents and that individual Member States set their own requirements and regulations, for example on storage and transport, in addition to those set at EU level. The Notifying Party further submits that barriers to entry are lowered by the fact that customers tend to use multiple suppliers. It also adds that there are no material intellectual property rights which would prevent a new competitor from entering the market.
(171) The Commission found in case M.7435 Merck/Sigma-Aldrich that barriers to entry were very high for the laboratory chemicals market, due in particular to brand loyalty, the need for scale and IP rights. In addition, it was found that general chemicals manufacturers whose business model is focused on supplying bulk size chemicals would have no interest in entering the market for catalogue solvents and inorganics, as this would require a totally different business model and significant investment in facilities for repackaging into small volumes and in the development of distribution channels.
(172) The results of the market investigation in this case also confirmed that barriers to entry are rather high in the markets for laboratory chemicals. Firstly, as discussed in the geographic market definition, differences in national regulation make it very difficult for existing competitors to extend their geographic scope. Secondly, the results of the market investigation confirmed that specific expertise and technology is required, in particular for some types of inorganics in particular (as discussed in the product market definition).
(173) The results of the market investigation confirmed that entry to the market is rare. Respondents were not aware of any new entrants in the last three years (168) or of any companies planning to enter the market. (169)
(174) In view of the above, the competitive assessment is based on the assumption that no new competitors are expected to enter the market for laboratory chemicals in the near future.
5.2.2. Market shares and market structure: EEA level
5.2.2.1. Solvents
(175) The table below shows the Parties' and their competitors market shares on the overall EEA markets for solvents and for the various possible sub-segments thereof to the extent that there are horizontal overlaps between the Parties' activities.
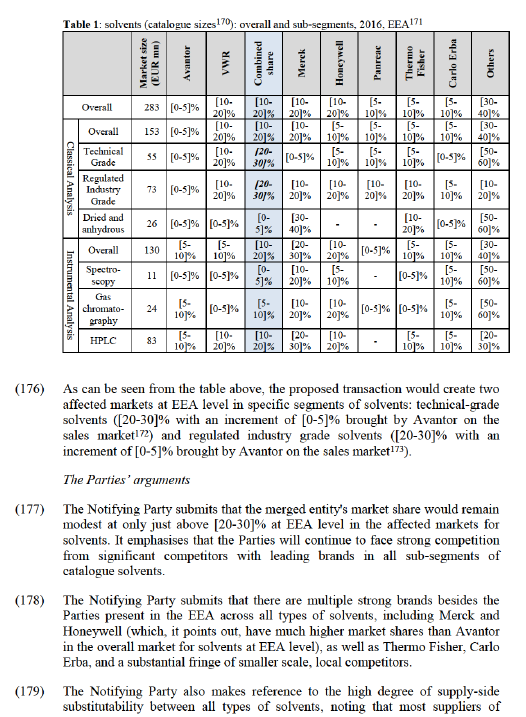
solvents possess adequate equipment to manufacture and supply the whole range of solvents for classical analysis and for instrumental analysis. The Notifying Party further submits that third-party bulk manufacturers produce most of the raw chemicals used to make solvents, that solvents are not expensive or complicated to manufacture, and that their chemical formulae are not protected by patents or other intellectual property.
The Commission’s assessment
(180) According to the market share data provided by the Notifying Party, the merged entity would become the largest competitor in both affected markets within solvents (technical grade solvents and regulated industry grade solvents). In the market for technical grade solvents, the merged entity would hold the highest market share ([20-30]%). The transaction does not, however, significantly change the market structure as VWR was already the market leader with [10- 20]% while Avantor brings an increment of less than [0-5]%. Furthermore, Honeywell and Panreac (each having shares of [5-10]%) and other competitors, including those named in the table above, will remain active on the market and will continue to exert competitive pressure on the merged entity.
(181) The merged entity will become the market leader on the second affected market, regulated industry grade solvents. However, on this market, Merck also appears to have a comparable position (only slightly behind the merged entity with [10- 20]% market share) and a further three main competitors all having market shares above [10-20]% (Panreac, Honeywell and Thermo Fisher) will also remain active on the market and will continue to exert competitive pressure on the merged entity. In addition, a number of smaller competitors will also remain active on this market.
(182) In relation to both the affected markets, it can also be noted that these types of solvents are at the lower range of the quality spectrum (both being solvents for classical analysis rather than instrumental analysis). Technical grade solvents are the lowest grade type of solvents and, as explained in the product market definition, typically used for tasks such as cleaning where the quality is not of great importance. As discussed in the competitive assessment, customers can generally switch brands very easily for this type of low grade solvent, thus limiting the ability the merged entity would have to increase prices. It was also found, as discussed above, that brand names generally carry value only in relation to solvents for use in more sensitive applications, where purity is critical (generally solvents for use in instrumental analysis). For lower grade solvents, meanwhile, customers often see branded and private label products as being largely equivalent. As a result, customers would have a relatively wide choice of possible suppliers in the two types of solvents that have been identified by the Notifying Party as affected markets.
(183) It can also be noted that the merged entity would not become the leading player in the overall market for solvents at EEA level (which has not been identified by the Notifying Party as an affected market). Merck, with a market share of [10- 20]%, would remain larger than the merged entity ([10-20]%) and Honeywell would also have a significant market share ([10-20]%). Thermo Fisher and Carlo Erba (which each have a presence on all potential sub-segments of the overall market) as well as other market players remain present on this market
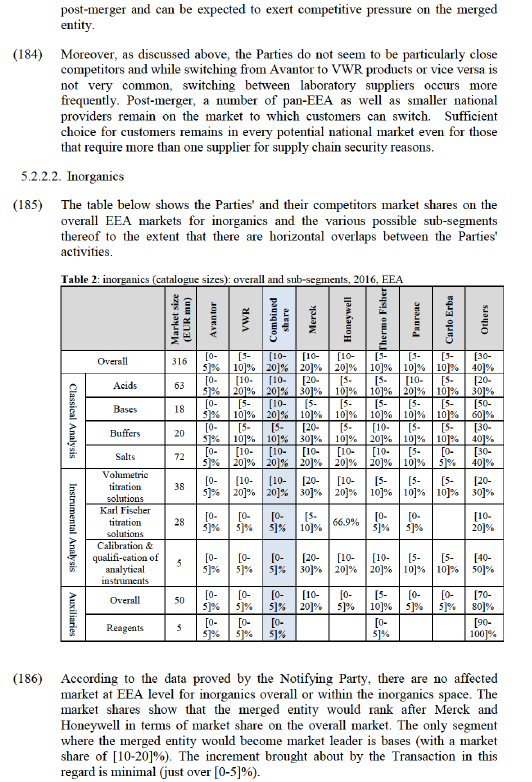
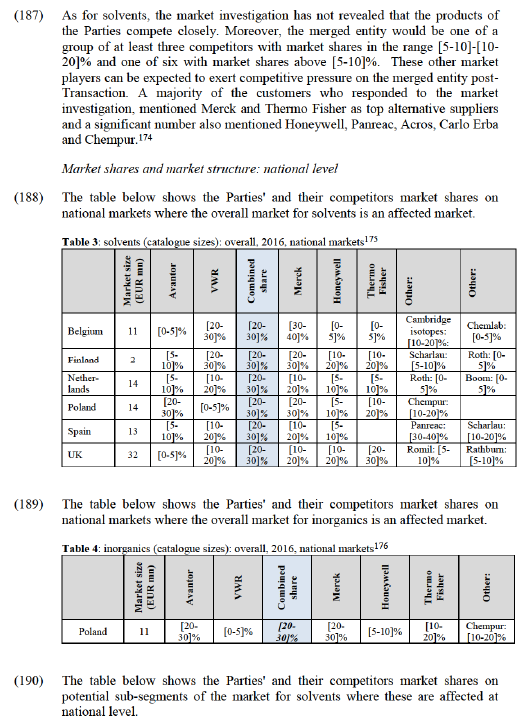
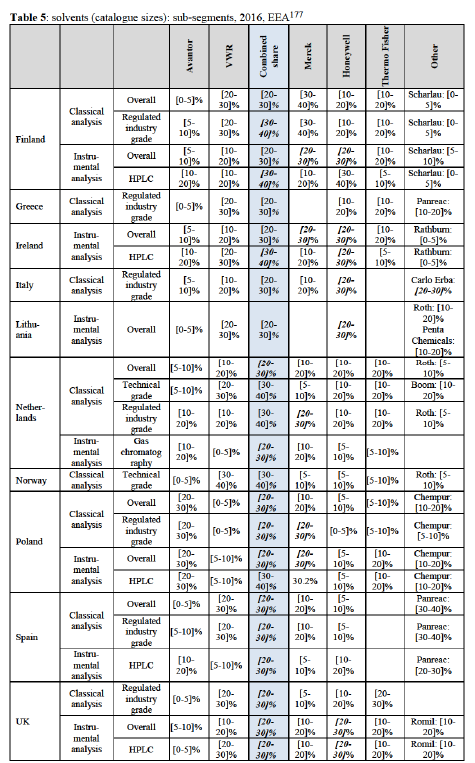
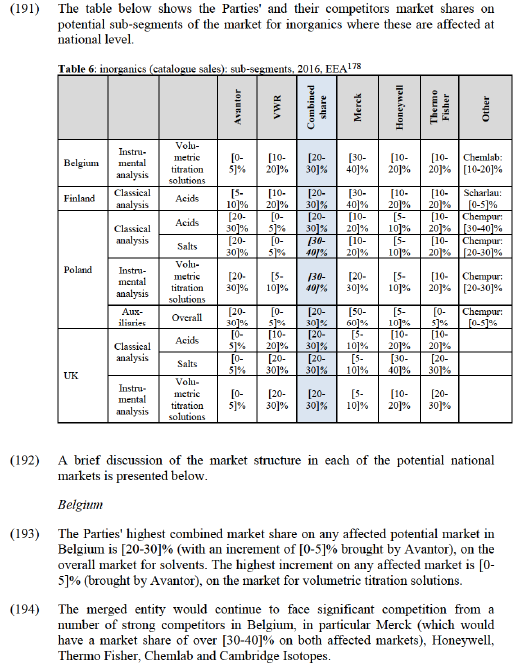
Finland
(195) The Parties' highest combined market share on any affected potential market in Finland is [30-40]% (with an increment of [5-10]% brought by Avantor), on the market for regulated industry grade solvents. The highest increment on any affected potential market is [10-20]% (brought by Avantor), on the potential market for HPLC solvents.
(196) The merged entity would continue to face significant competition from a number of strong competitors in Finland, in particular Merck (which would have market shares of around or over [30-40]% in all affected markets except HPLC solvents, where, however, Honeywell would have a market share of over [30- 40]%), Honeywell, Thermo Fisher, Scharlau, and Roth.
Greece
(197) The Parties' combined market share on the one affected potential market in Greece (regulated industry grade solvents) is [20-30]% (with an increment of [0- 5]% brought by Avantor). The change to the market structure brought by the transaction is therefore very modest.
(198) The merged entity would continue to face significant competition from a number of strong competitors in Greece, in particular Thermo Fisher (which would have a market share of [10-20]%), Honeywell, and Panreac.
Ireland
(199) The Parties' highest combined market share on any affected market in Ireland is [30-40]% (with an increment of [10-20]% brought by Avantor), on the potential market for HPLC solvents. This is also the highest increment on any affected potential market.
(200) The merged entity would continue to face significant competition from a number of strong competitors in Ireland, in particular Merck (which would have a market share of close to [30-40]% on one of the two affected potential markets), Honeywell (which would have a market share of close to [30-40]% on the other affected potential market), Thermo Fisher, and Rathburn.
Italy
(201) The Parties' combined market share on the one affected potential market in Italy (regulated industry grade solvents) is [20-30]% (with an increment of [5-10]% brought by Avantor).
(202) The merged entity would continue to face significant competition from a number of strong competitors in Italy, in particular Honeywell (which would have a market share of just over [20-30]% on the affected potential market), Merck, and Carlo Erba.
Lithuania
(203) The Parties' combined market share on the one affected potential market in Lithuania (solvents for instrumental analysis) is [20-30]% (with an increment of [0-5]% brought by Avantor). The change to the market structure brought by the transaction is therefore very modest.
(204) The merged entity would continue to face significant competition from a number of strong competitors in Lithuania, in particular Honeywell (which would have a market share of [20-30]% on the affected potential market), Roth, and Penta Chemicals.
Netherlands
(205) The Parties' highest combined market share on any affected potential market in the Netherlands is [30-40]% (with an increment of [5-10]% brought by Avantor), on the potential market for technical grade solvents. The highest increment on any affected market is [10-20]% (brought by Avantor), on the potential market for regulated industry grade solvents.
(206) The merged entity would continue to face significant competition from a number of strong competitors in the Netherlands, in particular Merck (which would have a market share of [10-20]% on the overall market for solvents and significant market shares of up to [20-30]% on affected segments), Honeywell, Thermo Fisher, Roth, and Boom.
(207) Avantor has historically had a strong presence in the Netherlands as this was the location of its main production site in the EEA. Having acquired POCH in 2011, Avantor subsequently decided to transfer all its production to the POCH site in Poland. The Netherlands production facility will therefore be closing this year.
(208) The Notifying Party submits that the presence of Avantor's production sites in the Netherlands has allowed it to develop direct relationships with customers in this country, as a result of which it has a higher proportion of direct sales than in the EEA on average. This also seems to have helped it to establish a stronger market position overall. Nonetheless, there is a particularly wide range of strong competitors active in the Netherlands, which would continue to act as a constraint on the merged entity.
(209) Furthermore, all of the affected markets in the Netherlands (with the exception of solvents for gas chromatography, where the Parties have a combined market share of only just above [20-30]%) are in solvents for classical analysis, where (as mentioned in the assessment of the market shares and market structure at EEA level), customers are most easily able to switch and typically choose from a wide range of branded and private label alternatives.
Norway
(210) The Parties' combined market share on the one affected potential market in Norway (technical grade solvents) is [30-40]% (with an increment of [0-5]% brought by Avantor). The change to the market structure brought by the transaction is therefore very modest.
(211) The merged entity would continue to face significant competition from a number of competitors in Norway, in particular Merck, Honeywell, Thermo Fisher, and Roth.
Spain
(212) The Parties' highest combined market share on any affected potential market in Spain is [20-30]% (with an increment of [5-10]% brought by Avantor), on the potential market for regulated industry grade solvents. The highest increment on any affected potential market is [5-10]% (brought by VWR), on the market for HLPC solvents, leading to combined shares of [20-30]%.
(213) The merged entity would continue to face significant competition from a number of strong competitors in Spain, in particular Panreac (which would have a market share of almost [30-40]% on the overall market for solvents and above [20-30]% on all affected sub-segments), Scharlau, Merck, Honeywell, and Carlo Erba.
UK
(214) The Parties' highest combined market share on any affected market in the UK is [20-30]% (with an increment of [0-5]% brought by Avantor), on the potential market for regulated industry grade solvents. The highest increment on any affected potential market is [5-10]% (brought by Avantor), on the market for solvents for instrumental analysis.
(215) The merged entity would continue to face significant competition from a number of strong competitors in the UK, in particular Thermo Fisher (which would have a market share of over [20-30]% on the overall market for solvents and up to [20-30]% on affected sub-segments within solvents and inorganics), Honeywell, Merck, Romil, and Rathburn.
Poland
(216) The Parties' highest combined market shares in affected potential markets in Poland are [30-40]% (with an increment of [5-10]% brought by VWR) in volumetric titration solutions, [30-40]% (with an increment of [5-10]% brought by VWR) in solvents for HPLC, and [30-40]% (with an increment of [0-5]% brought by VWR) in salts.
(217) The situation created by the merger is slightly different in Poland than in the other EEA markets due to Avantor’s more significant presence and particular profile on the market. Avantor bought a Polish laboratory chemicals manufacturer, POCH, in 2011 and continues to sell under the POCH brand in Poland. As a result, Avantor has stronger position on the market in Poland than in most other EEA countries (with a market share of around [20-30]% in solvents and [20-30]% in inorganics overall). Avantor also achieves a higher proportion of its sales via direct distribution in Poland than elsewhere in the EEA as it acquired POCH's established distribution network.
(218) Avantor sells both its main EEA-wide brand, J.T. Baker, and the POCH brand in Poland. The Notifying Party has explained that it positions POCH at a lower price point than J.T. Baker (approximately […]% lower), thus targeting more price sensitive customers and avoiding competition between its own brands. The Notifying Party states that POCH is "generally positioned close to distributor brands", meaning that POCH and VWR's own label products could compete more closely than do J.T. Baker and VWR. Both J.T. Baker and POCH appear to enjoy strong brand recognition amongst customers in Poland.
(219) As can be seen from the tables above, the market shares suggest that Merck would be slightly smaller than the merged entity, with a share of around [20- 30]% in the overall solvents market (merged entity: [20-30]%) and [20-30]% (merged entity: [30-40]%) in the overall inorganics market. The other main competitors present are Chempur (solvents [10-20]%, inorganics [10-20]%), Thermo Fisher (solvents [10-20]%, inorganics [10-20]%) and Honeywell (solvents [5-10]%, inorganics [10-20]%). The Notifying Party also names a number of other national competitors understood to be present on the Polish market (e.g. Alchem and Argenta) but no estimates of sales figures are available for these.
(220) The results of the market investigation confirm that there is greater awareness of the Avantor brands in Poland, and that Avantor’s image in this market is strongly influenced by the POCH brand. Customers in Poland more often name Avantor as being among the top manufacturers than do customers in other EEA Members States and there are also a small number of respondents which consider Avantor to be the leading manufacturer, a view not generally held by respondents in other markets. (179)
(221) Customers in Poland name VWR and Avantor as each other’s closest competitors slightly more often than in other countries, but Merck and Chempur are, nonetheless, both mentioned more often than the Parties as closest competitor to each of Avantor and VWR. (180) In addition, a higher proportion of customers in Poland reported having switched between Avantor and VWR as suppliers of laboratory chemicals in the last three years, (181) which would appear to confirm that they may be closer competitors than in other markets.
(222) The influence of the POCH brand is most evident in the strengths and weaknesses that customer associated with the different manufacturers. Whereas customers in other countries generally considered Avantor to be a high quality premium manufacturer, customers in Poland more often described it as a lower price, good value supplier. (182) This is consistent with the positioning of POCH compared to J.T. Baker, and suggests that Polish customers are more aware of the POCH brand than of the EEA wide J.T. Baker name. Furthermore, the POCH brand is generally very highly regarded by Polish customers, with most describing it as offering good value, competitive pricing and good quality.
(223) In spite of the stronger position of Avantor in Poland, the majority of customers in Poland do not expect the transaction to have any impact on competition on the market for laboratory chemicals or on their companies in particular. A small minority, however, are concerned about possible price increases and a potential reduction of choice due to products being withdrawn. (183) As mentioned above, the market investigation indicates that there will remain a significant number of other strong competitors on the market. A significant number of respondent to the market investigation mentioned Merck, Chempur, Acros, or Honeywell as one of the top suppliers. (184) This further supports the view of the majority of market participants in Poland, who are not concerned about the effects of the transaction.
(224) In conclusion and in light of all the above, it appears on balance that even though Avantor has a stronger presence on the Polish market (with higher market shares than in other EEA countries) and that Avantor and VWR’s brand names are closer competitors than in other countries, Merck and Chempur are perceived as close competitors to the Parties and other competitors remain which represent valid alternatives to the Parties' products, such as, Acros or Honeywell. (185) Overall, therefore it is unlikely that the Transaction would lead to unilateral effects. Merck has a relatively strong presence too in the overall markets for solvents and inorganics in Poland. In addition to Merck, Chempur, Honeywell and Thermo Fisher would also remain present with significant market shares, alongside a number of smaller competitors, all of which would continue to exert competitive pressure on the Parties.
5.2.3. Conclusion
(225) The analysis above has to be complemented by the finding that the Parties are not particularly close competitors as discussed in the competitive assessment under the heading "closeness of competition", which is also confirmed by the views expressed by the majority of customers on the possible impact of the Transaction, with many referring to the fact that there would be a large number of other competitors still active. One customer states, for example, "there are a sufficient number of other competitors supplying alternative products. These competitors include global/regional players like Thermo Fisher and Sigma Chemicals as well as numerous local players." (186) Another customer explained that the Transaction would not limit their choice because "Merck, Thermo group, Honeywell or Carlo Erba have reliable alternatives". (187)
5.2.4. Potential narrower markets within solvents and inorganics
(226) As mentioned above in the product market definition, the Commission is, for the purposes of the present case, also considering two hypothetical markets (188) for individual solvents and inorganics, due to responses from some market participants which indicated that there could be reasons to consider these specific solvents and inorganics as constituting separate product markets. These potential markets are on the one hand a possible market for high purity acetonitrile, a type of solvent, and on the other hand a possible market for high purity acids, a specific set of inorganic laboratory chemicals.
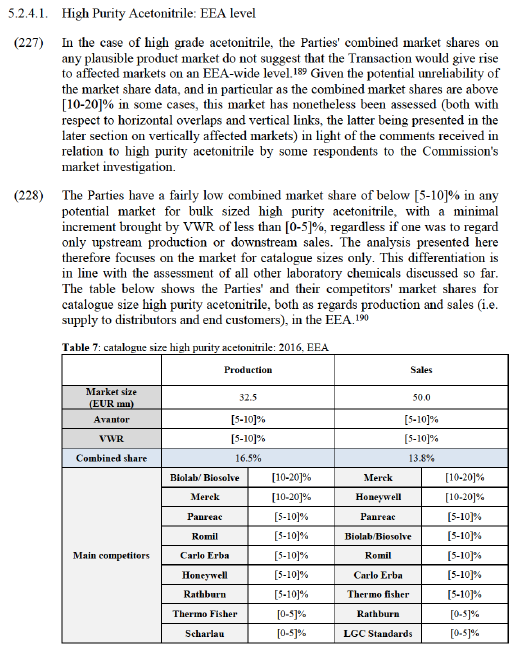
(229) According to the data provided by the Notifying Party, as set out in the table above, the merged entity would not become the market leader in either the production or the retail market. In both cases, the merged entity would be one of the three top players, all of which would have market shares between [10-20]% and [20-30]%. The rest of the market is fairly fragmented, with (in both cases) five further competitors having between [5-10]% and [10-20]% market share, followed by various smaller competitors with market shares of below [5-10]%. There will thus be a large range of other competitors present on the market, including strong international players such as Merck, Thermo Fisher and Honeywell that will continue to exert competitive pressure on the merged entity.
(230) Some replies to the market investigation from competitors to VWR and Avantor in the supply of high purity acetonitrile on the retail market indicated that the Parties are seen as two of the top suppliers. VWR, Avantor, and Merck were mentioned most often as amongst the leading players in this market, suggesting that the Parties may have a stronger position than would be assumed on the basis of their declared market shares alone. Nonetheless, the other main competitors in laboratory chemicals (including Thermo Fisher, Carlo Erba, and Honeywell) were also considered as major suppliers. (191)
(231) The market investigation supported the general picture that can be drawn from the market shares above. In fact, a major competitor confirmed that the "market will continue to be fragmented".192 This clearly supports the finding from the market shares above that the Parties are not particularly strong players and that alternative sources continue to exist post-merger.
(232) The finding that other suppliers remain active can also be understood from other replies received. Even though one other competitor who currently procures some high purity acetonitrile from PTI was critical about the Transaction, it could be verified that this already procures high purity acetonitrile on an OEM basis form an alternative third party supplier. (193)
(233) Based on the analysis above, it would appear unlikely that the merged entity would be able to exercise market power, such as would allow it to raise prices or restrict supply. A small number of competitors mention generic concerns that the market would become more concentrated, but without substantiating these views further. Yet, most competitors active as suppliers of high purity acetonitrile to end customers do not believe that the proposed Transaction would have any negative effect on this market. (194)
(234) On the basis of the above reasoning, it appears unlikely that the Transaction would lead to unilateral effects on a potential EEA-wide market for high purity acetonitrile.
5.2.4.2. High purity acetonitrile: national level
(235) If the market for high purity acetonitrile would be considered to be national in scope rather than EEA wide, (195) the Transaction would, on the basis of the information submitted by the Notifying Party, result in one affected, that is, the market in Poland, where the Parties have a combined market share of [30-40]% with an increment of [5-10]% brought by VWR. (196) Avantor is already the leading supplier of high purity acetonitrile in Poland, with a market share of [20- 30]%. The other main competitors present are Merck (approximately [20-30]% market share), Chempur ([10-20]%), Thermo Fisher ([10-20]%) and Honeywell ([5-10]%). The Transaction is therefore unlikely to significantly change the competitive dynamics on the market as customers will continue to have a variety of choice of other main suppliers available to choose from.
(236) In view of the above, and given the general absence of concerns on the part of market participants in Poland, it appears unlikely that the post-merger situation in Poland will be other than that described for the EEA as a whole or the specific post-merger situation for solvents in Poland already discussed above. Even if the Parties' have a higher combined market share in this country, unilateral effects appear unlikely to result from the proposed Transaction.
5.2.4.3. High purity acids: global level
(237) The second specific hypothetical market(s) which the Commission has assessed in light of some comments received from market participants responding to the market investigation is that related to high purity acids. On the basis of the information submitted by the Notifying Party, the proposed Transaction leads to affected markets in the production of catalogue size high purity acids at global level while at EEA level there would be no overlap, as VWR (Seastar) produces these products only in Canada. The Parties' and their competitors' market shares at global level are shown in the table below. The Parties' combined market share remains modest in all of the other hypothetical sub-segments/markets for the hypothetical high purity acids market discussed (197).
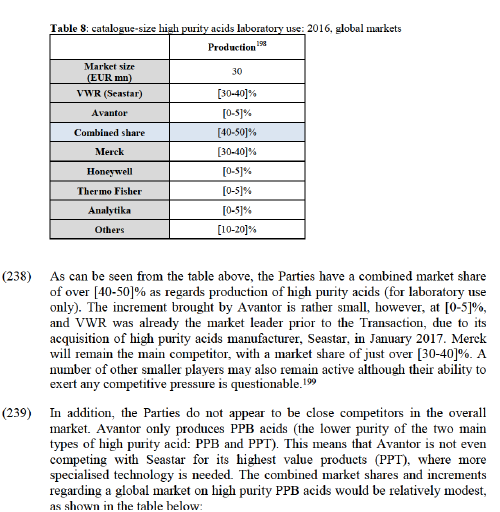
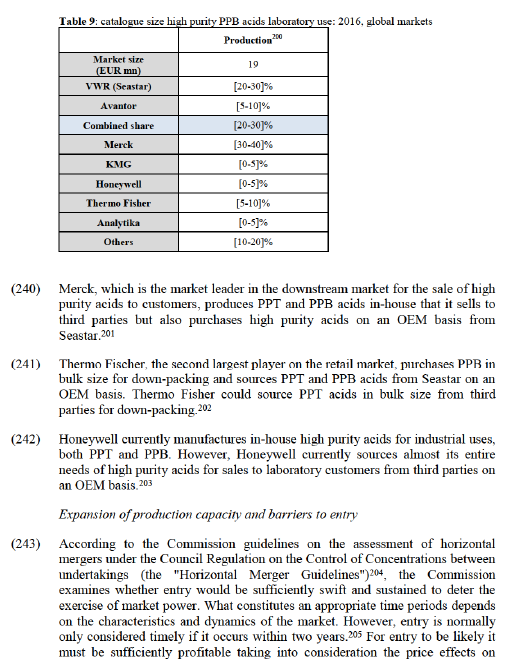
injecting additional output into the market. (206) Entry is particularly likely if suppliers in other markets already possess production facilities that could be used to enter the market in question. (207)
(244) As analysed in more detail in section 5.3.5.2, the largest players on the market, Merck, Thermo Fisher, and Honeywell, could start or expand their in-house production of both PPB and PPT acids undertaking moderate investments in a market with high margins and within relative short periods of time of around one year, making it likely to be a profitable investment. They already have production facilities that can be used to start or expand their production and Merck is currently expanding its production capacity. (208) Moreover, GFS, a US producer of high purity acids has confirmed that it has recently doubled its capacity and has spare capacity available for both PPT and PPB acids. (209) The market investigation did not reveal others barriers that could prevent the main competitors from expanding their production, such as regulatory barriers, IP rights, technical advantages or access to raw materials. Therefore, potential expansion of capacity of the largest players that are already present in the market is likely to constrain the behaviour of the merged entity.
Conclusion
(245) In light of all the above, it can therefore be concluded that the Transaction will not raise competition concerns in relation to a hypothetical global production market for high purity acids, given the relatively modest increment brought by Avantor, the presence of other competitors and the relative ease by some market players to expand production or to enter the market.
5.2.4.4. High purity acids: national level
(246) While VWR is active on the production level via its subsidiary Seastar in Canada, producing both PPB and PPT acids, Avantor produces only relatively small amounts of PPB acids in a plant in the US.
(247) As for high purity acetonitrile, the downstream market for high purity acids could also be considered to be national in scope rather than EEA wide or global (whilst the production market is likely to be an EEA wide or global market). The Transaction results in three affected markets at national level – two in high purity acids overall and one in the narrower market for PPT acids. The market shares are shown in the table below:
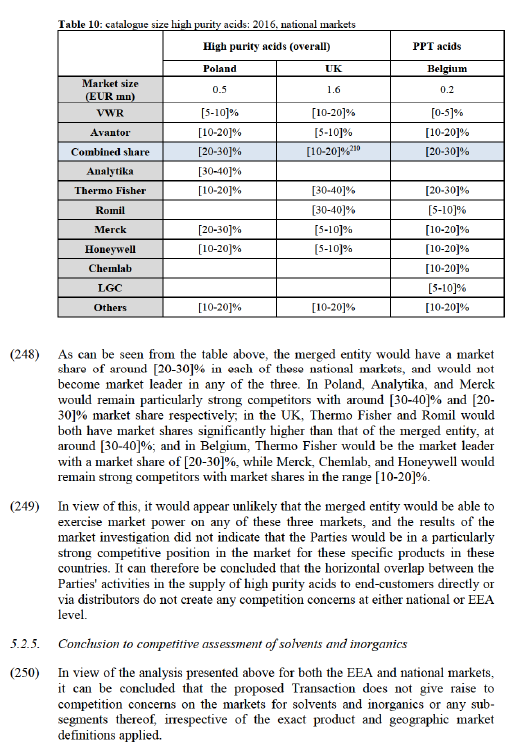
5.3. Vertical link between the production and the downstream distribution market
(251) Besides being active in the production of laboratory products, VWR is also active in the distribution of its own as well as third party laboratory and life sciences products. VWR is therefore already partly vertically integrated. Avantor's sales of laboratory chemicals are done partly via VWR, with the rest of sales being effected either by Avantor directly or via other (non-VWR) third party distributors. Avantor does not distribute third party laboratory and life science products and therefore its activities in the distribution of laboratory chemicals are limited to sales of its own products. Given that VWR already distributes Avantor laboratory chemicals, a vertical link between the Parties already exists pre-merger. The proposed Transaction will internalise this vertical link.
(252) The Notifying Party submits that the (further) vertical integration transpiring from the proposed Transaction, is a main part of the rationale for the Transaction and that it expects to generate significant synergies that will allow customers to benefit from a supplier with a wider product portfolio and improved business model.
5.3.1. Importance of distribution channels
(253) Laboratory chemicals are sold both directly by manufacturers through their own sales networks and via distributors, which typically sell the products of a range of manufacturers (including, potentially, their own products if they are vertically integrated). Distributors do not only sell chemicals but typically a wider range of laboratory products, including equipment, glassware, and other consumables.
(254) According to the data provided by the Notifying Party, approximately […]% of total EEA sales of laboratory products and approximately […]% of total EEA sales of laboratory chemicals are made via distributors. The proportion sold through each route varies significantly between manufacturers, and often also between countries for any given manufacturer. In general, however, most manufacturers make use of both types of sales channel, to varying degrees.
(255) In 2016, Avantor realised […]% of its EEA sales of solvents and […]% of its EEA sales of inorganics via its own sales network and […]% of sales of solvents and […]% of sales of inorganics via distributors. These proportions vary significantly between EEA Member States as there are some countries where Avantor has no direct distribution network and therefore relies entirely on distributors and others, such as Poland, where it has a very strong direct distribution operation and achieves the vast majority (over […]% in solvents and over […]% in organics) of its sales directly.
(256) Responses to the market investigation showed that the majority of customers purchase laboratory chemicals both directly from the manufacturer and through distributors. A small proportion purchase only from distributors, whilst only very few source laboratory chemicals uniquely directly from manufacturers.
(257) As will be described in some more detail below, the results of the market investigation indicate that smaller customers tend to source more often from distributors whereas larger customers have easier access to direct distribution. The larger the amount of one specific chemical needed by a customer, the higher is the chance that direct supplies are economically advantageous. However, distributors also enable to simplify purchasing by offering a wide range of products from various manufacturers in one single purchasing order, providing the advantage of a one-stop solution which can appeal to smaller as well as larger customers. Relative to the other types of customer, slightly more universities and research institutes purchase laboratory chemicals only from distributors, and only a few purchase only from the manufacturer. (211) Where customers buy from both distributors and manufacturers, the relative proportions they purchase from each vary considerably. (212)
(258) Customers' responses overall however suggested that a significant proportion do not have a strong preference for buying either from a distributor or direct from the manufacturer, and that this is more a question of availability. One customer stated, for example: "it all depends on the presence of the manufacturers. If the manufacturers have presence in the same geography/country, we buy it from them directly or via their local distribution network." (213) Other respondents also confirmed that it would simply depend where they could source the product most easily and for the best price.- (214) Some customers did, however, express a preference for buying standard products from distributors and more specialised products from the manufacturer. Similarly, products where only small quantities are needed are more likely to be purchased from a distributor that offers the possibility to source a range of smaller orders rather than handling various purchasing orders for small amounts each with a number of manufacturers. (215)
(259) Customers generally mentioned price and convenience as the main reasons for choosing to buy from a distributor. Smaller customers in particular often prefer to reduce the administrative work associated with managing orders by buying as much as possible from one source, which is more often a distributor due to the range of products they offer. A number of customers also explained that they can often negotiate better prices from distributors as they are buying a larger volume from one source, and also that the distributor may be able to negotiate better prices from the manufacturer than could small customers. (216)
(260) Some competitors (manufacturers) also shared the view that smaller customers are more likely to prefer buying from distributors, whilst larger customers may feel it is to their advantage to deal directly with the supplier. One competitor observed that "wholesale customers choose suppliers because of favourable pricing and after-sales service. Small customers prefer distributors because of the availability of chemical reagents and quicker delivery of smaller orders." (217) A regional distributor expressed a similar view, explaining that “distributors […] are more flexible, in particular due to their ability to stock products. The products needed are therefore often available immediately.” This respondent also pointed out that distributors’ proximity can make it easier for them to ensure the correct storage of certain types of chemicals that have to be kept under special conditions. (218)
(261) The main advantages of buying directly from a manufacturer, meanwhile, were price (especially when the customer is buying larger volumes) and the possibility of negotiating better terms, for example for delivery. (219) One customer explained, for example, that purchasing direct "helps build a strong relationship directly with the manufacturer which results in a strategic partnership, if the spend with the manufacturer is high and has global presence" and also referred to the ability to "negotiate on standard discounts with the manufacturer". (220) In general, purchasing directly from the manufacturer was seen to be an attractive option when a customer is wishing to buy larger volumes, whereas customers sourcing smaller volumes do not see it as a realistic alternative. One customer commented, for example, "I'm afraid that many manufacturers still would not be interested in selling their products directly to us given the scale of our consumption". (221)
(262) Overall, responses from both customers themselves and from manufacturers suggested that, whether a customer chooses to buy from a distributor or a manufacturer is often, to a certain extent at least, a question of the individual customer's strategy, as there can be advantages of each route. One competitor explained that "some purchasers will prefer to consolidate their supply with global distributors and buy everything from one or two suppliers mainly for service reasons ( […] less admin costs, […] more power to negotiate...). Some others prefer to buy from suppliers for the more adapted answer to their needs and specificity." (222)
(263) From a manufacturer's perspective there are also advantages and disadvantages of direct sales and sales via distributors respectively. Competitors mention that direct distribution gives them direct contact with customers and thus allows them to gain a better understanding of customer needs and to ensure certain service levels. Another major advantage is of course the higher margins. (223) Direct distribution is, however, seen as being more labour intensive, and requires suppliers to hold more inventory and to manage a large number of small accounts. The main motivation for using third party distributors is to reach customers beyond the geographic scope of their own distribution network or who are otherwise difficult to reach. Relative to direct sales, using distributors is also simpler for the manufacturer from an organisational perspective, as it avoids the need to hold large volumes of stock, to manage invoices and to handle local marketing. Using distributors does, however, mean that the supplier accepts lower margins, and some competitors also mentioned the risk of the distributor changing the brand. The supplier has less power to try to prevent customers switching to other brands as it has no direct contact with them, and distributors could even encourage this if it is in their interest to do so. More generally, the supplier is put in direct competition with other brands that the distributor may be offering. (224)
(264) One major manufacturer also explained that distributors play a critical role as they can offer a wider range of products than can any single manufacturer, thus allowing customers to benefit from a "one-stop shop". This competitor also explained that some distributors offer bundles of products at attractive discounts and that customers would tend to only buy from the manufacturer for larger volumes or if they need the technical expertise of the manufacturer, for example for specialist products. An additional reason for ordering from the distributor is that manufacturers often have minimum order sizes which some customers would not meet, whereas distributors' minimums are typically much lower. (225)
(265) It follows from the above that both direct distribution and sales via distributors can be important for suppliers, depending on the type and preferences of its customers and its own strength in distribution. Distributors thus appear to be an important albeit not the only channel for suppliers to obtain access to customers.
5.3.2. Overview of the competitive landscape and the Parties' position
(266) In general, two types of distributors can be distinguished: global/pan-EEA players on the one hand and more regional or national players on the other hand that have a clear advantage of closeness to its customers and knowledge of the local markets but do not have the same geographical coverage. VWR clearly belongs to the first group of distributors with a global (and pan-EEA) reach.
(267) VWR is active on the distribution market by distributing third party products alongside its own products ("external and internal distribution"), while Avantor is active only in direct sales of its own products ("internal distribution") but relies to a large extent on distribution of its products through a wide range of local and global third party distributors. Given that the Parties do not overlap in external distribution, no horizontally affected markets arise in that regard.
(268) VWR's share of the distribution market is moderate as can be seen from the table below that provide the market share estimates submitted by the Notifying Party on an EEA-wide and national level in relation to the distribution (internal and external) of laboratory products (where, in the EEA overall, VWR's market share is below 10% and, at national level, most often VWR's market shares are generally below 10% and are above 20% only in one instance). (226)
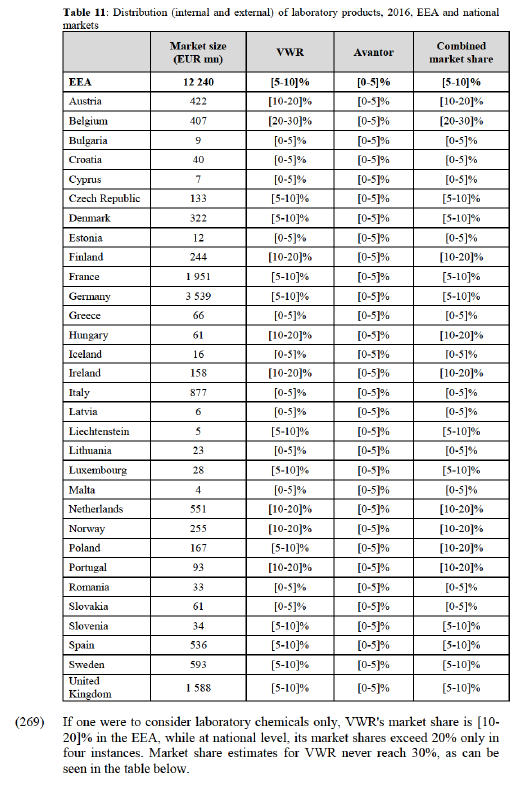
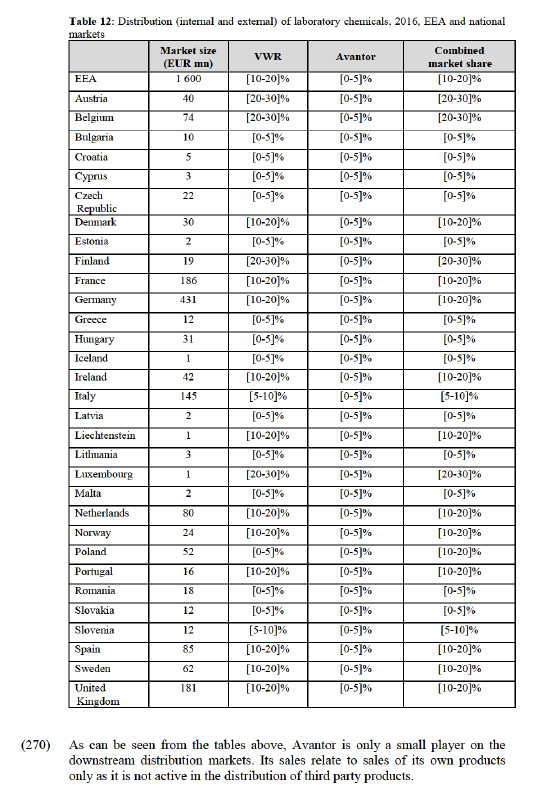
(271) When analysing the potential market for distribution of laboratory chemicals only, the market shares of the Parties are slightly higher when compared to a broader laboratory products market, as can be seen from table 12 above. However, the Parties' market shares (both individual and combined) never reach 30%. Indeed, the Transaction would not lead to an affected market on an EEA- wide level and on a potential national level; market shares exceed 20% only in Austria, Belgium, Finland, and Luxembourg but remain below 30%. In all these cases, the increment brought by Avantor is significantly below [0-5]%.
(272) The general perception on the market, however, is that VWR has a stronger position than would be suggested by these market share estimates. The vast majority of VWR's competitors in distribution rated it as the leading player on the market. Thermo Fisher is often ranked as the second most significant competitor, with a wide range of other names then mentioned as the next strongest (dependent on the particular national market). These included Dominque Dutscher and Grosseron in France, Th. Geyer, Carl Roth and Omnilab in Germany, Mikro+Polo, Kefo, and Sanolabor in Slovenia, Scharlab and Serviquimia in Spain, Ocon and Lennox in Ireland, and many others. (227) These are typical examples of more national or at best regionally focussed distributors which together appear, from the data submitted by the Notifying Party, to account for a significant part of the total market.
(273) Manufacturers of laboratory chemicals also consistently view VWR as the market leader in the distribution of laboratory chemicals in the EEA. (228) One major manufacturer described VWR as "by far the biggest laboratory chemical distributor in the EEA". (229) Its main strengths are seen as being its large portfolio of brands and products, its extensive network of warehouses and logistics across the EEA and its strong sales team. (230)
(274) An additional strength of VWR in view of market participants is its special relationship with Merck. VWR used to be part of the Merck group until it was spun off in 2004. Even after that, it used to be an exclusive supplier for Merck products for some countries and still has preferential agreements with Merck in several European countries and for a range of product groups. […].The existing agreements are described by the Notifying Party as commercial agreements at arm’s length and will expire at the end of [YEAR].
(275) Thermo Fisher is described by market participants as another strong player on the distribution market. It is described as having many of the same strengths as VWR, including a large variety of products, an international structure and sales force, and significant negotiating power. Its weaknesses, as seen by other distributors, were also largely the same as those mentioned for VWR: poor customer service, a lack of contact with customers, and a lack of flexibility due to its size and global set-up. (231)
(276) In light of this feedback and the general concerns about potential unreliability of market share data, it seems that VWR might have a stronger actual position than seems to be suggested by the market share data, even though there are other strong distributors present. Therefore, the vertical aspects of the Transaction are assessed in more detail even if the market shares are below the thresholds as of which the Commission typically considers vertical theories of harm.
(277) According to the Commission guidelines on the assessment of non-horizontal mergers under the Council Regulation on the Control of Concentrations between undertakings (the "Non-horizontal Merger Guidelines") (232), non-coordinated effects may significantly impede effective competition as a result of a non- horizontal merger if such merger gives rise to foreclosure. Foreclosure occurs where actual or potential rivals' access to supplies or markets is hampered or eliminated as a result of the merger, thereby reducing these companies' ability and/or incentive to compete. Such foreclosure may discourage entry or expansion of rivals or encourage their exit. (233)
(278) The non-horizontal Merger Guidelines distinguish between two forms of foreclosure. Input foreclosure occurs where the merger is likely to raise the costs of downstream rivals by restricting their access to an important input. Customer foreclosure occurs where the merger is likely to foreclose upstream rivals by restricting their access to a sufficient customer base. (234) In this case, both input and customer foreclosure theories will be discussed in the following sections.
5.3.3. Input foreclosure
(279) In the context of the current decision, input foreclosure relates to the possibility of the merged entity to restrict competing distributors' access to input, i.e. products manufactured by Avantor that may be exclusively or preferentially distributed via VWR post-merger to the detriment of other distributors.
(280) A number of distributors have expressed concerns that the merged entity could choose to distribute Avantor products exclusively via VWR and thus deny them access to an important input for their business. Some of these distributors further claim that Avantor products account for a significant proportion of their sales and that they are therefore dependent on being able to buy from Avantor.
(281) One distributor based in Eastern Europe, for example, submits that the transaction will have a significant impact on the market for the distribution of laboratory chemicals in the countries in which it is present. It explains that Avantor represents a large proportion of its sales of laboratory chemicals and that, without Avantor products, it would no longer be able to offer an attractive range. It would lose business as it would not be able to persuade customers to buy other manufacturers' products instead of Avantor's and customers would instead go to VWR to source Avantor products. (235)
(282) This complainant further voiced concerns that VWR holds a dominant position in the respective national market and engages already in behaviours which can be labelled as unfair competition practices, e.g. hiring away its competitors’ top local sales staff along with the clients allocated to them. According to this distributor, VWR's activities may thus ultimately force smaller distributors out of the market. (236)
(283) A distributor in Western Europe similarly fears that Avantor may give preferential treatment to VWR post-Transaction and that it may thus lose access to Avantor products completely. It would be forced to promote other brands, which it would try to do, but predicts that it would lose a significant proportion of its customers who would move to VWR to continue purchasing Avantor products. (237) Equally, if it was able to continue supplying Avantor products but on worse terms than VWR, it believes that the majority of customers would move to VWR in view of the price difference. (238)
(284) An additional complainant further accentuated the concerns in light of the special relationship between VWR and Merck which is perceived as giving other distributors already pre-merger worse access to Merck products than VWR. (239) The combination of the contractual links between VWR and Merck arising from their current arrangement and the structural link between VWR and Avantor arising from the Transaction would result, in the view of the complainant, in a compounded or worsened potential foreclosure of third party distributors' access to Merck and Avantor products. This complaint could be considered as implying a coordinated input foreclosure of other distributors by the merged entity and Merck or as assuming that post-merger VWR would be a vehicle for coordination between Avantor/the merged entity and Merck. The present section of the Decision will assess potential input foreclosure effects. The Decision also assesses potential coordinated effects of the Transaction in light of the arrangement currently in place between VWR and Merck.
5.3.3.1. The Parties' arguments
(285) The Notifying Party argues that an input foreclosure scenario is implausible because:
a. It is important for Avantor, like other suppliers, to maximise its customer reach by distributing its products via as a range of channels as wide as possible.
b. No single distributor in the EEA is dependent on Avantor's products and could reasonably claim to be materially harmed by not having access to Avantor products. Avantor estimates that its products represent less than []% of the downstream sales of any one EEA distributor (with a certain number of exceptions).
c. If the merged entity were to stop selling Avantor products via other distributors, at least some customers who currently buy Avantor products from those distributors would stay with the same distributor and switch to a different brand (rather than starting buying Avantor products from VWR). Avantor is described by the Notifying Party as a "small player" in the EEA, which does not have the type of must-have products that would allow it to keep customers if it employed such a strategy.
d. Currently, only around […]% of Avantor's sales of life sciences and laboratory products via distributors (and […]% of its sales of laboratory chemicals via distributors) are achieved through VWR. It would therefore be a highly risky strategy for Avantor to stop selling via distributors that represent the major proportion of its indirect sales. Selling through a large number of different distributors is a fundamental part of Avantor's strategy and choosing to sell only through VWR would therefore represent a major departure from its current business model.
(286) In view of the above arguments, the Notifying Party maintains that the merged entity would have neither the ability nor the incentive to engage in an input foreclosure strategy.
(287) As regards a potential coordinated foreclosure effect the Notifying Party submits that such a strategy would be equally impossible to pursue. First of all, such a strategy could not be implemented by the merged entity but rather depends on Merck over which it has no control.
(288) In addition, Merck and VWR already terminated their agreement with respect to approximately […]% of Merck’s laboratory chemicals portfolio including the most commonly used solvents and inorganics and particularly regarding the German market because as a result of a 2009 decision by the German antitrust authority. Merck's products are therefore available to other distributors on the same terms as for VWR.
(289) […].
(290) Furthermore, the Notifying Party claims that Merck is not interested in pushing VWR as a preferential route to market. To the contrary, it has actively converted VWR Merck business to direct sales following its acquisition of Sigma-Aldrich, and is expected to continue to do so. [Confidential argument by the Parties on recent development of Merck sales via VWR].
(291) To the extent the Transaction affects VWR’s incentives at all, the Notifying Party claims that it will push VWR to favour sales of Avantor products over sales of Merck products, which would make any coordinated effort between Avantor/VWR and Merck unsustainable.
(292) In light of the expansion of direct sales of Merck, any attempted foreclosure of other distributors would be without effect because Merck's products are available via direct sales.
5.3.3.2. The Commission's assessment
(293) The Transaction would only raise serious doubts in relation to input foreclosure if the merged entity would have the ability and incentive to foreclose rival distributors and if such a foreclosure would have a negative effect on downstream customers. As regards the latter, the Commission Guidelines on non-horizontal mergers explain that "When intermediate customers are actual or potential competitors of the parties to the merger, the Commission focuses on the effects of the merger on the customers to which the merged entity and those competitors are selling. Consequently, the fact that a merger affects competitors is not in itself a problem. It is the impact on effective competition that matters, not the mere impact on competitors at some level of the supply chain". (240) In the context of this case, a potential effect on distributors would only be problematic if it would also harm final customers further downstream.
Ability to foreclose
(294) In order to raise competition concerns, the merged entity must have a significant amount of market power upstream. (241) In this case, this would imply the power of Avantor on the production market for laboratory chemicals. This would mean that the merged entity could operate successfully without selling Avantor via any other channels (i.e. depending only on Avantor's direct sales network and sales of Avantor's products via VWR). Such a scenario would imply that customers would rather switch distributors than switching from Avantor products to comparable products from competitors.
(295) First, Avantor's market shares are modest overall as can be seen from the horizontal assessment above, giving a first indication that it is unlikely to possess market power. In fact, Avantor's market shares are below [5-10]% in solvents overall and below [0-5]% in inorganics overall assuming an EEA-wide market. The situation is not materially different in most national markets with the exception of Poland. However, even in relation to the Polish market, as indicated in paragraph 213 above, Avantor would appear to lack market power. (242)
(296) Second, it has to be noted that the results of the market investigation did not reveal any facts that would contradict the Parties' claim that Avantor has no "must-have" product. Even if specifications of laboratory chemicals sometimes deviate slightly between manufacturers, there is no one product that is only available from Avantor and not also produced by other manufactures on the market.
(297) Third, whilst the results of the market investigation in relation to the ability of customers to switch between suppliers are mixed, they do not support a claim that switching would be impossible or prohibitively expensive for a significant part of customers.
(298) Some customers' responses appear to suggest that they are reluctant to switch between manufacturers (as can also be seen from responses to the market investigation suggesting overall that the choice of manufacturer tends to be more important for customers than the choice of distributor). One customer explains, for example: "[There is a] choice between distributors only if they offer exactly the same product. Then price, availability, delivery time... become the main selection criteria." (243) Another customer confirmed similarly: "we rather focus on the product (the producer is more important than the distributor)". (244)
(299) Although a limited number of customers therefore seem to have difficulties in switching, others clearly do not. This can be seen from the results of the market investigation, which show that switching takes place regularly. Around half of customers (across all categories: biopharma and pharma producers, professional laboratories, and research institutes and universities) reported that they had switched supplier (manufacturer) at some point during the last three years. (245) In addition, a quarter of respondents had switched from VWR's (own) products to products of another manufacturer or vice versa and a significant number of respondents confirmed having switched to or away from Avantor products. (246)
(300) The main reasons given for switching were price and the availability of products (e.g. if there regular supplier experienced as shortage). A smaller number of respondents also mentioned poor performance (e.g. problems with deliveries or service) and new products becoming available (i.e. customers having more choice than previously and thus choosing to move to a different supplier). (247) Switching of manufacturers therefore occurs regularly in the relevant markets.
(301) Overall, the picture is mixed and customers' choices can be influenced by a range of factors. While customers consider the quality of the product to be of prime importance, which is dependent on the manufacturer, they also take into account delivery times, where the distributor plays the major role. (248)
(302) Presented with a scenario where a particular distributor stops selling a certain brand (that they currently buy), some customers would stay with this distributor and switch to another brand. However, the majority of customers would consider moving to another distributor or direct sales for this one product. Only a minority would consider switching the whole range of its purchases to another distributer in such a scenario. (249) One explains, for example: "our experience with a specific brand in combination with our instruments pushes us to stick to the same brand." (250) However, customers’ responses also indicate that this is very much dependent on the particular product, and where it is a standard purpose chemical, they may be more inclined to stay with the distributor for convenience. Where, however, the chemical is critical, then it seems there would be no obstacle to finding another source, as explained by one customer "the answer would depend on the details of the switch, and may vary as some end users might be happy to change while others might need/prefer to continue with that brand, in which case we would need to find an alternative source." (251) Similarly, some customers say that they might test an alternative proposed by the distributor, but if the alternative does not meet their needs in terms of quality, then again, they would switch: "If the alternative [offered by the distributor] is unacceptable we would source from the manufacturer" (252).
(303) One competitor also stated: "if the supplier’s brand is 'locked in' in SOPs, recipes or other documentation, they may change the distributors but try to avoid supplier change". (253) Another competitor also expressed a similar view, that if a particular supplier's products were no longer available from a certain distributor, then "probably customers would change to another distributor, because supplier is the main thing". (254)
(304) The results quoted above seem to indicate that manufacturers of a specific product could enjoy some degree of market power because customers would rather change distributors than manufacturers. If this was the case, the merged entity might have an ability to force customers to switch their distributor in order to stay loyal to Avantor's products. However, the Commission considers that is unlikely to be the case for Avantor's products in view of the fact that Avantor does not seem to have "must have" products.
(305) In addition, the results of the market investigation also show some indications that distributors in general may be able to influence customers' choice of brands. One competitor claimed, for example, that "as a result of their customer relation, distributors have a better chance to proactively substitute brands to their advantage". (255) Other respondents were of the view that this may depend on the type of customer. Whilst, this could on the one hand be seen to support a possibility by VWR to seek to substitute competing brands by Avantor's brands, on the other hand, the same ability to sway customers to competing brands could be argued in relation to other distributors in the event that they would have no or only limited access to Avantor's brands. In a scenario where the distributor is no longer offering a certain supplier's products, one competitor observed that "for researcher and university customers, the name of the distributor prevails", whereas other customers may be more tied to suppliers. (256) This is likely to be due to the fact that universities tend to procure a very broad range of chemicals in relatively small quantities, which makes the services of a distributor as a "one-stop-shop" particularly attractive. Other customers, such as professional laboratories and manufacturing companies may purchase for more specific needs, and may therefore have a stronger preference for staying with a product they know. Customers from the environmental sector did, however, also clearly state: "We would prefer to stay with the distributor and would switch brand after testing the offered alternative. If the alternative is unacceptable we would source from the manufacturer". (257)
(306) The majority of customers also reported that there are no particular barriers to switching suppliers (manufacturers) for laboratory chemicals. Some mentioned that they may have to individually qualify a new chemical, but explained that this depends on the specific chemical and its usage. The documents and validation procedures vary between different types of chemicals and the extra work created by switching suppliers is therefore very dependent on the particular situation. One customer stated, for example, that "general laboratory chemicals can be changed without any obstacles. Chemicals for instrumental analyses have to be checked internally." (258) Another customer expressed a similar view, explaining that "for critical chemicals we would need to test from a new supplier, for standard chemicals not". (259) It should be noted in this regard that laboratory chemicals are different from other products like raw materials for (bio)pharma production where switching costs might be different. However, even for laboratory chemicals, individual situations of specific customers might vary. Some customers explained in this regard that there are circumstances in which it would be more difficult to switch from one manufacturer's product to another. (260)
(307) Customers with specific needs are typically aware of the risks associated with potential problems of availability and therefore have other suppliers already qualified as potential back-ups. (261) One explains, for example: "our company tries to rationalise purchases to a limited number of suppliers/distributors but our primary goal is to seek better price conditions and we would not like to enter into an exclusivity agreement and would ensure alternative sources of supply." (262) In total, more than 60% of customers that gave meaningful replies to the market investigation confirmed that they are actively multi-sourcing or at least try to have a second qualified supplier available for critical inputs. Customers that need particular products therefore do not typically depend on only one supplier. Such multi sourcing is equally prevalent amongst all types of customers (professional laboratories, pharma producers, and universities and research institutions).- (263)
(308) Competitors also confirmed that customers typically source from two or more suppliers of laboratory chemicals simultaneously (264) although there was some disparity of views concerning the percentage of their purchases that customers would allocate to one supplier within a multi-sourcing strategy. One competitor explained that it could be "very different for each customer depending on its purchasing strategy, ranging from a balanced position with each of its suppliers to a big majority to one and a small piece of cake to the other, just to keep it referenced 'in case'." (265)
(309) The main reasons mentioned by competitors for multi-sourcing were the availability of products, the ability to negotiate better prices by dealing with different suppliers at once, security of supply and avoiding dependency on any one supplier. (266) One competitor explained, for example, that customers multi- source in order to "get the cheapest prices by being able to ask different competitors for an offer and to have another alternative source if a supplier is not able to deliver within a short time from their stock" while another mentioned that "customers do not want to rely on just one supplier". (267)
(310) In conclusion, it therefore seems that customers in general do have the ability to switch between suppliers (manufacturers). This is particularly the case for general laboratory chemicals that are already more commoditised and available from a broad range of manufacturers. Pharmaceutical producers and other customers that may have higher switching costs are typically aware of critical dependencies and already have more than one suitable supplier available. On this basis, the Commission concludes that switching is possible, has been done frequently in the past and would most likely occur, depending on the individual situation of a specific customer if Avantor's products would no longer be available at competitive prices. (268) The time that customers stated it would take them to switch between suppliers varied considerably, but was most often around 1-3 months.
(311) Therefore, overall the results of the market investigation indicate that alternative products are available and switching between suppliers (manufacturers) is typically possible.
(312) In light of all the above, it appears unlikely that the merged entity would possess the required ability to engage in a successful input foreclosure strategy.
Incentive to foreclose
(313) In order to engage in a successful input foreclosure strategy, the merged entity would also need to find it profitable to foreclose its downstream competitors. The table below gives an overview of the current relationship between Avantor and VWR. The first table shows the proportion of Avantor's sales of laboratory chemicals that it achieves via distributors, and, of these sales via distributors, the proportion that are sold through VWR.
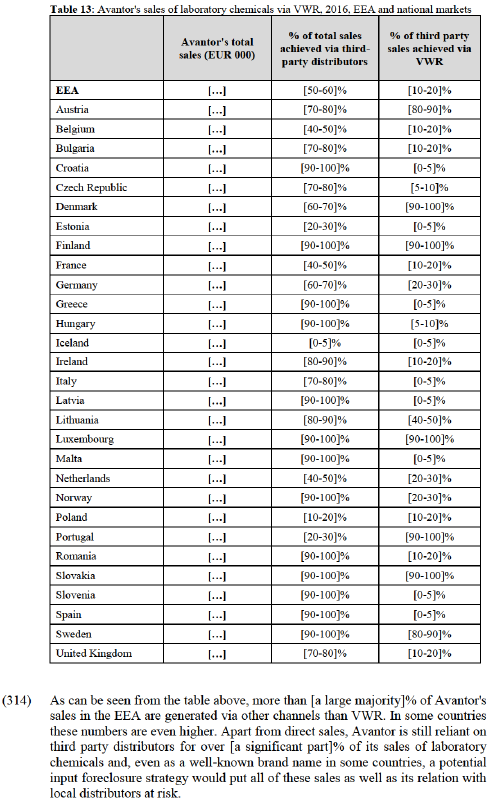
(315) As discussed in the section on ability to foreclose above, the results of the market investigation indicated that most customers could easily switch between products from different customers. Consistent with this, customers also confirmed that, if the price of a particular brand sold by a distributor were to increase, they would look for alternatives, including by potentially switching to other brands. The merged entity would therefore face a realistic risk of losing a significant amount of Avantor sales to other third party producers by foreclosing distributors' access to these products.
(316) Even given VWR's strength as a distributor, it would be a highly risky strategy for the merged entity to stop supplying the extensive range of distributors that Avantor sells through and thus to cut its current link with a significant proportion of customers currently served via those distributors. Some smaller (often local) distributors have close connections to their customers and a deep understanding of their needs as well as local market developments. This knowledge is most likely not easily replicable by a global player like VWR.
(317) Moreover, even though VWR is generally active across the EEA, its strength does vary significantly between different countries and, given the importance for some customers of a genuine local presence (including contact with sales reps, for example), it could not be assumed that VWR would always be capable of 'replacing' Avantor's local distributors.
(318) Overall, it therefore seems unlikely that the merged entity would have the incentive to risk losing sales by restricting other distributors' access to Avantor products.
Impact on effective competition
(319) Were the merged entity to engage in a strategy of input foreclosure as described above, other distributors would no longer have access to Avantor products (or would only have access on worse terms than VWR) and thus could not offer customers the same choice of brands as they do currently.
(320) It is to be noted, that consumer harm appears doubtful in such a situation given that customers would still have access to Avantor brands via the merged entity and to competing suppliers' products, whether directly or via distributors. As explained above, there is strong competition between the products of these different suppliers making it unlikely that any decrease in intra-brand competition may have negative effects on competition overall.
(321) In addition, distributors are not completely defenceless. Given that the business model of distributors always involves advice to customers, they possess at least some possibilities to leverage their close relationship with customers in order to convert them to third party products.
(322) Given Avantor's limited overall market share and its position within the portfolio of most competing distributors, it also seems unlikely that a significant number of distributors would be forced out of the market in the unlikely event that the merged entity decided to foreclose access to Avantor products. The Commission's investigation showed that the vast majority of distributors do not have a critical dependency on Avantor products. (269) As mentioned above, the Notifying Party claims that Avantor products represent less than […]% of the downstream sales of any one EEA distributor (with a certain number of exceptions). In the market investigation, most distributors confirmed that Avantor products represent at most 15% of their total sales of laboratory chemicals and often much less. (270)
(323) The exceptional case where Avantor constitutes a slightly larger share of third party distributors' revenues, relates to countries in Eastern Europe. (271) However, the merged entity is unlikely to have an incentive to foreclose considering the market share estimates, which indicate that VWR has no strong presence in these countries. Accordingly, any attempted foreclosure would be specifically risky given the narrower base on which potential foreclosure benefits could be reaped. In any event, also in these countries, a number of alternative products exist to which final customers (with their support of local distributors) could switch. (272) This is particularly the case given that exclusivity agreement between manufacturers and distributors typically do not exist. (273)
(324) Moreover, the majority of distributors are not concerned by the transaction and, even those that indicate that they might lose access to Avantor products seem confident that they could replace Avantor in their product portfolio with other manufacturers or have already alternatives in their portfolio. (274)
(325) Also, a number of responding distributors have not had access or only very limited access to some Merck products in some countries based on the preferential agreement between VWR and Merck (Merck being, most often, the market leader in relation to laboratory chemicals and enjoying a strong brand recognition) in recent years and have continued to compete effectively on the market, also suggesting that the loss of access to Avantor, a much smaller competitor, is unlikely to have a significant effect on their business model.
(326) If one were to consider a hypothesis where the merged entity could increase prices for customers who previously bought Avantor brands via other distributors and who want to continue buying these products from the merged entity (via VWR), the Commission considers that, on the basis of the following elements, such a hypothesis is unlikely to transpire.
(327) Responses to the market investigation strongly suggested that the merged entity, even as the sole source of Avantor products, would be unable to increase prices significantly taking into consideration the relative wide choice of laboratory chemicals supplied by different manufacturers. As discussed in the section on ability to foreclose above, the results of the market investigation indicated that most customers could easily switch between products (of different manufacturers). Consistent with this, customers also confirmed that, if the price of a particular brand sold by a distributor were to increase, they would look for alternatives, including by potentially switching to other brands. Even if there are specific chemicals for which customers would 'tolerate' a certain price increase, because this particular chemical is critical for a certain procedure or is named in protocols, it is unlikely that these would be the same chemicals for all customers, or that, for any particular customer, all of the chemicals it purchases from Avantor would be critical inputs. It would therefore be very difficult for the merged entity to introduce any type of general price increase without risking losing significant sales. In addition, given the number of customers for laboratory customers and the wide range of laboratory products bought by each, it is highly unlikely that the merged entity could know which specific product is critical for a particular customer, and thus be able to increase the price of this product for this customer.
(328) In light of the above, a significant number of customers are likely move to other brands if the merged entity would offer the same products via its own distribution network at increased prices. Therefore, even assuming the merged entity would have the ability and incentive to foreclose competing distributors, it would most likely not be in a position to increase prices for its products.
(329) In light of the above it can be concluded that even in the unlikely event of an attempted input foreclosure, such a strategy would not lead to a significant detrimental effect for downstream customers. Therefore, and considered alongside the limited ability and incentive that the merged entity would have to pursue a foreclosure strategy, the Commission considers that the Transaction is unlikely to raise serious doubts as a result of potential input foreclosure.
Impact of VWR's preferential agreement with Merck
(330) The special relationship between VWR and Merck does not change the above assessment of a potential foreclosure. First, the merged entity has no ability to unilaterally foreclose customers from access to Merck's products and in any case, such a scenario is not merger specific. Whether Merck had and used its ability to restrict its sales to VWR at the expense of other distributors is not subject to this decision.
(331) In as far as the Transaction impacts the Merck-VWR relationship, it rather seems to lead to increased competition between these two players. (275) [Discussion of potential VWR strategy based on internal documents]. Therefore, it would be a reasonable reaction of Merck to react to the merger by also increasing its competitive efforts. This might likely entail the broadening of its scope of distributors rather than keeping a dependency on the merged entity. In any case, the current agreements between VWR and Merck expire in [YEAR] and the probability that VWR and Merck will replace the existing agreements at the end of their contractual term in [YEAR] with a similarly restrictive agreement is highly unlikely in light of the Transaction. In addition, it should also be noted that exclusivity agreements have been significantly restricted in Germany following an intervention of the national competition authority. (276)
(332) The results of the market investigation also support a claim that competition between the merged entity and Merck may actually increase rather than be lessened by the Transaction. One French competitor of the Parties stated for example: "VWR distributes Merck chemicals in an exclusive manner (although the agreement is being broken). Merck is market leader in quality and reputation and very important for French market. Any factor weakening the relationship between Merck and VWR is beneficial. So in my opinion the purchase of VWR by Avantor is good". (277)
(333) The Parties' claim that Merck actively tries to convert Merck sales via VWR to direct sales is another example that competition appears to be actually working in spite of the agreements in place. Moreover, in the national market to which the complaint referred to in paragraph (284) relates, Merck already sells the majority of its products direct, and also uses other distributors than VWR. The addition of Avantor to VWR's portfolio will not change its incentive to reduce any of these sales.
(334) Moreover, it is pertinent to note that the existence of a preferential agreement between VWR and Merck does not in any case increase the risk of a potential input foreclosure of other distributors from Avanto
(335) Finally, the arguments above as regards effects of an attempted foreclosure are also not affected by the existing contractual agreements between Merck and VWR. Even if the transaction would in some way lead to a restriction of availability of Avantor and/or Merck products via distributors, final customers still have the possibility to switch to other suppliers like Thermo Fisher, Honeywell, Carl Roth, Carlo Erba and many others in addition to the possibility of sourcing directly from Merck.
5.3.3.3. Conclusion
(336) In light of the above, the Commission considers that the Transaction does not raise serious doubts as to the compatibility with the internal market based on input foreclosure concerns.
5.3.4. Customer foreclosure
(337) Customer foreclosure may occur when an upstream supplier integrates with an important customer in the downstream market.
(338) A small number of competitors in the upstream production market have raised concerns that the merged entity could foreclose other manufacturers of laboratory chemicals, either by no longer selling their products or by 'demoting' their products in terms of their ranking in catalogues, the level of prominence given to them in marketing material and the resources devoted to their marketing (e.g. via sales reps). This could be considered to give Avantor a competitive advantage relative to other suppliers and would potentially deny these suppliers a route to market. [Discussion of confidential commercial agreements between VWR and Merck] (278). This is acknowledged by VWR itself and the special relationship between VWR and Merck appears to be general knowledge on the market.
(339) A major competitor explained that VWR's search engine is programmed to favour certain products over others. Customers that go to the VWR website to look for a particular product will therefore see results with certain products presented more prominently than others. At present, VWR gives the top placing to its own brand, followed by Merck products, and then other competitors. (279) It would be reasonable to assume that a similar level of prominence could be expected for Avantor products once Avantor becomes a VWR in-house brand.
(340) Concerns have been expressed that manufacturers might find it difficult post- merger to achieve the same treatment as today in VWR's search engine. (280) And that this could have a detrimental effect on their sales, as VWR represents an important channel to market for them and customers' choices are likely to be swayed by VWR's efforts to promote Avantor products above those of other manufacturers. (281)
(341) A small number of customers also considered it possible that the merged entity may give favourable treatment to Avantor at the expense of all other manufacturers' products. One customer suggested, for example: "It can be speculated that VWR might be forced to increase its efforts in selling brands like J.T. Baker and neglect its service level for other brands." (282)
(342) Another scenario that could be hypothesised is that the merged entity could maintain VWR's current preferred relationship with Merck, and promote (only or more strongly) Merck, Avantor, and VWR's own-label products, at the expense of all other manufacturers. In such a scenario, the merged entity would engage in (full or partial) foreclosure of other (non-Merck) upstream competitors. A complaint received from a distributor was suggestive of this latter scenario. (283) Other respondents to the market investigation also voiced concerns, in more general terms, about the existing relationship between VWR and Merck together with the merged entity's ability to promote its own brands. (284)
5.3.4.1. The Parties' arguments
(343) The Notifying Party explains that VWR's business model involves carrying a broad range of third-party brands as well as its own-brand products and that there is no intention to change this model.
(344) While the Parties anticipate that this Transaction will provide an opportunity to strengthen the Avantor brand, they submit that they do not have any plans to exclude other suppliers from VWR’s distribution network. Given Avantor’s very small size in the EEA relative to VWR’s existing sales of third party brands, the Transaction would not create either the ability or the incentive to engage in customer foreclosure. On the contrary, the Parties submit that VWR distributing more Avantor products will benefit the market by making Avantor a stronger brand and competitor to Merck, Thermo Fisher, and others, thus increasing choice for end customers.
5.3.4.2. The Commission's assessment
(345) Potential full or partial customer foreclosure, whether through a reduction of purchases or by purchasing on less favourable terms, could only arise if the merged entity would have the ability and incentive to engage in such a strategy and if such a foreclosure would result in significant detrimental effects on consumers.
(346) Partial foreclosure could be considered in situations where VWR would (i) buy fewer volumes from an upstream competitor or at less favourable terms, and/or (ii) where VWR would decide to promote its own and Avantor's brands over those of competitors in its catalogues/demote competing brands in its portfolio. The latter is relevant for customers that search for a certain chemical but do not have a specific brand loyalty. In these cases, electronic catalogues have search engines that provide suggestions ranked according to different criteria as was already discussed above. (285) The operator of a catalogue has the ability to change these criteria and influence the resulting ranking.
Ability to foreclose
(347) When considering whether an entity would have the ability to foreclose access to downstream markets, the Commission examines whether there are sufficient alternatives in the downstream market to which upstream rivals could sell their output. The Commission also considers whether there are possible counter- strategies, that would be effective, possible to implement in the short time, and sustainable over time, and that rival firms would be likely to employ.
(348) There is no indication that VWR would be the only route to market for manufacturers. On the contrary, alternative routes to market do exist for competing suppliers.
(349) Firstly, direct sales are not uncommon in the laboratory chemicals industry, especially with respect to larger customers. Should the merged entity try to foreclose other manufacturers, it is highly possible that a significant part of the customer base would increase their direct purchases.
(350) A number of smaller competitors on the upstream production market (i.e., suppliers) reported in the market investigation that they are planning to increase their percentage of direct sales (independently of the merger and of any change to their relationship with VWR). Some are planning to achieve this expansion within the next two of years. (286)
(351) As regards larger suppliers, as indicated above, […]. Thus, at least until that date, the ability of the merged entity to foreclose Merck's access to distribution via VWR is rather questionable. Thermo Fisher has taken steps to strengthen its European distribution network by opening a new distribution centre in Sweden this year. Thermo Fisher is active as a distributor also of third party products and confirmed that it is prefers to carry a wide range of products. (287) In addition, a wide range of other distributors exist on a pan-EEA but also on smaller scale as already discussed above. Honeywell currently utilises a diverse distributorship channel as a route to market.
(352) One competitor (288), which currently relies significantly on VWR for the distribution of its products, indicated that for certain reasons (289) (including, amongst these constraints, an inability to establish required customer relationships), it would be unable, in the short term, to increase its direct sales to counteract a possible foreclosure strategy. The evidence on file indicates, however, that this competitor has already been engaging in increased direct sales (notwithstanding some of these constrains which it cites) and that the majority of its sales are already made directly in most EEA member states. (290) Besides this, and with respect to this competitors' stated concern that it would not be able establish required customer relationships in the short term, it should be noted that this competitor currently uses other distributors (291) as well as VWR and could therefore still make use of the customer relationships already in place between third party distributors and customers going forward. In any event, the information submitted by this competing supplier indicates that plans to employ a multi-channel strategy and to capitalise on its existing own distribution network by gradually increasing its direct sales team on a medium- to long-term basis. In this regard, it is also worth noting that this competitor could be expected to benefit from the brand recognition which it enjoys.
(353) Another competitor indicated that it relies on VWR for a non-insignificant portion of its sales and that this channel represents a de facto important route to market for it. (292) However, evidence on file indicates that this competitor already envisages a potential viable alternative (to VWR) route to market and would consider distributing its products via an alternative main distributor. (293)
(354) Besides increasing direct sales (even in markets where they also sell via distributors), an alternative route to market for other competing manufacturers generally would be the range of other distributors (294), which have an incentive to seek to carry to a broad portfolio of products available for their customers. Thus, even considering the distribution channel consisting of external distributors (i.e. distributors that sell third party products) alone, the Commission has found that there are sufficient alternative distributors present for manufacturers to distribute their products, including global players like Thermo Fisher as well as important national or regional players such as Th. Geyer, Boom, Chemlab, Bartelt, Omnilab, Chempur, Metlab, and others.
(355) Should the merged entity decide to foreclose fully or partially access to its catalogue for other manufacturers, these competing suppliers would therefore still appear to have alternative routes to market. A partial foreclosure by demoting a specific producer in the search engine of VWR's catalogue would also not affect sales to those customers that search actively for a specific brand. However, those customers that have no brand loyalty might find alternatives more easily in VWR's catalogue. In this sense, the merged entity in its function as a distributor has a limited ability to influence sales of specific products. However, that ability is limited not only by the fact that some customers approach distributors with requests for specific brands but also by the overall moderate market power of VWR. Other competitors with strong e-commerce platforms exist, including not only distributors like Thermo Fisher but also Sigma, which was recently acquired by Merck. (295)
(356) The Notifying Party estimates that on an EEA-wide or national markets for the distribution of laboratory products, VWR's market shares are below 30% ([5- 10]% at EEA level and below [10-20]% in all national markets except Belgium with [20-30]%). As indicated above however, the market investigation results appear to suggest that VWR's market position is stronger than what these market share estimates might suggest, in particular for the distribution of laboratory chemicals considered in isolation. However, for customer foreclosure to be a concern it must be the case that the vertical merger involves a company which is an important customer with a significant degree of market power in the downstream market. The foregoing elements call into question whether VWR does in fact have significant market power and it appears unlikely, on balance, that the merged entity would have the ability to effectively foreclose other manufacturers' access to customers other than VWR given the alternative routes to market available to competing manufacturers.
(357) In any event however, even if one were to assume that the merged entity would have the ability to foreclose its competitors, foreclosure of competing manufacturers (whether partial or otherwise) would not appear to be a likely outcome of the Transaction for lack of incentives (at least in the short term) and would in any event have limited effects on effective competition, for the reasons which will be explained below.
Incentive to foreclose
(358) Even assuming that the merged entity would have the ability to foreclose rival manufacturers, it would not appear to have the incentive to do so as engaging in customer foreclosure would expose it to significant economic risk.
(359) The choice and breadth of the portfolio of products (and also of brands and of different quality grades of each product) that a distributor offers is seen as an important criterion by most customers when choosing a supplier of laboratory chemicals. This characteristic appears to be one of the main reasons why distributors like VWR and Thermo Fisher constitute attractive suppliers for customers and consequently, an attractive route to market for competitors. (296) It would therefore seem, all other things being equal, economically unsound for VWR to take an active decision to reduce its product portfolio by offering only Avantor products in addition to its own label. A customer foreclosure strategy would be likely to have a significant negative impact on VWR's current market position, as a distributor that has a wide range of products available for its customers to choose from.
(360) Many customers state that purchasing from VWR would not have the same appeal if they could no longer source the same range of products, and that they might therefore move to other distributors. One customer stated, for example: "this would affect our relation with VWR as the product mix and the ability to supply this product range makes them interesting as a supplier." (297) Other customers, however, would continue purchasing from VWR even if its product range was reduced, explaining, for example "the range of products offered by VWR does not influence our decision to purchase from them". (298) Even if by no means all customers would stop purchasing from VWR were it to limit its range of products, it is clear that some would. This would represent a loss of sales that would potentially be even more difficult to compensate for with fewer brands in its portfolio with which to attract new customers. This is especially so if alternative foreclosed brands enjoy, at least in the short term, significant brand recognition which supersedes that of Avantor products.
(361) Choosing not to stock products from other major suppliers such as Honeywell and Thermo Fisher could have an impact on VWR's appeal as a sourcing channel for customers. The possibility that Merck might not be affected (at least for the duration of the contract and beyond that date, should the arrangement be extended) by a customer foreclosure strategy does not materially change this assessment. The three product portfolios of Merck, Avantor and VWR cannot replicate the currently existing breadth of VWR portfolio. The merged entity would most likely risk losing its attractiveness as a one-stop-shop distributor, if it were to engage in customer foreclosure, regardless of its current / potential future agreement with Merck.
(362) Opinions amongst competitors as to whether VWR may choose to stop selling or to 'demote' their products were varied. While a small number of competitors raised specific concerns with respect to customer foreclosure, these concerns were not shared by all manufacturers. For example, one small supplier that currently achieves a small proportion of its sales via VWR explained that it was not concerned about the transaction, and did not believe that VWR would stop selling its products as there is customer demand for them. (299)
(363) As mentioned above, sales of Avantor products currently account for only a small part of VWR's sales of third parties' laboratory chemicals ([…]%) (300). VWR revenue depends to a significant extent on the sales of some competing suppliers' products. Notably, Merck represents approximately […]% of its sales of third-party laboratory chemicals. (301)
(364) More generally, Avantor's position on the overall markets for solvents and inorganics appears to be modest, and the market investigation confirmed that its brands do not have the same consistent reputation across EEA markets as Merck's. On some markets VWR has a stronger market position than Avantor and still it has continued to rely significantly on sales of third party products, especially those which enjoy a certain degree of brand recognition. It is at best questionable therefore that the addition of Avantor to VWR's own products would materially change VWR/the merged entity's incentives to such an extent to elect to fully foreclose competitors.
(365) As explained in paragraph (352), one of the customers which have raised foreclosure concerns is planning to work with a multi-channel strategy (that is relying on both direct sales and sales via distributors) and to gradually increase its direct sales team. This would decrease possible dependency on the capabilities of VWR on a medium to long term perspective. Also as noted above, such a strategy on the part of this competitor could benefit from both its significant direct distribution network and brand recognition. Moreover, also as noted above, this competitor does and could sell via other distributors (and again in this respect brand recognition could aid it in this strategy).
(366) As indicated in paragraph (353), another competitor which has raised customer foreclosure concerns has indicated that it is considering another main player as a viable alternative distributor of its products as it has the capacity and business model to promote its brand successfully in Europe and in the US. (302) It does not appear to be concerned by the fact that this alternative route to market carries its own competing products and appears to be able to seek to utilise this alternative route to market in the short term.
(367) VWR's longstanding promotion of Merck's brand appears to have contributed to its strong reputation. The relationship between VWR and Merck is based on a long common history - it originates from the time when both belonged to the same group and VWR continued to invest in the promotion of the Merck's products/brand during the period within which it was Merck's exclusive distributor and subsequently as its preferred distributor. Similarly, even assuming that the merged entity does start in the short term to promote Avantor as strongly as it currently does for Merck products, it is reasonable to argue that developing Avantor's brand recognition and its reputation and market position in the EEA will, similarly, take some time. It therefore seems unlikely that VWR on its own could, in the short term, develop Avantor's brand such that it would become possible or economically interesting to demote other brands. At the same time, it is also not plausible that Merck would lose its current level of brand recognition and reputation simply because other manufacturers are also promoted (whether as significantly or more significantly than Merck products).
(368) In any case, the merged entity would lack incentives to fully foreclose competing suppliers at least in the short term. Indeed, if the merged entity were to promote Avantor products at the expense of all others, it would appear to be exposing itself to significant economic risk. Firstly, it would risk not being able to 'convert' all of its sales of other manufacturers' chemicals to sales of Avantor products, and, secondly, its whole business model as a global distributor may become less attractive due to its more restricted product range, meaning that its sales of the remaining products could also suffer because the attractiveness of VWR as a distributor is that it can place its products within a broader portfolio, alongside those of other suppliers. This same logic also applies to a strategy based on selective foreclosure of smaller suppliers: a distributor's strength in these markets is dependent on offering the broadest possible portfolio. The risk of potential losses that could be incurred as a result of losing this advantage, i.e. reducing the range of products offered, is likely to outweigh any theoretical gains that could be made from promoting Avantor and VWR brands only.
(369) A partial foreclosure would be equally unlikely because a potential reduction of purchases or potential purchasing on less favourable terms would (i) negatively impact the merged entity's portfolio breadth and (ii) competing suppliers could sell (directly or indirectly) via alternative channels; both of which would impact on the merged entity's incentives to foreclose. The merged entity would risk not being able to 'convert' all of its sales of other manufacturers' chemicals to sales of Avantor products. While the results of the market investigation show that some customers have difficulties to switch easily, others were generally confident that, should a particular laboratory chemical that they currently purchase from VWR become unavailable, they would be able to find another source for it. (303)
(370) A partial foreclosure by demoting a specific producer in the search engine of VWR's catalogue would not affect sales to those customers that search actively for a specific brand. Making it more difficult to find specifically branded products on VWR's catalogue will not necessarily lead to a conversion of third party sales to Avantor products but rather carries the risk of the merged entity losing sales altogether to different distributors or direct sales.
(371) The above mentioned elements and risks are, taken together, likely to outweigh any theoretical gains by the merged entity to fully or partially foreclose competing brands.
Impact on effective competition
(372) In their responses to the market investigation, customers were generally confident that, should a particular laboratory chemical that they currently purchase from VWR become unavailable, they would be able to find another source and would do so if the chemical was a critical input. One customer stated, for example: "if the chemical is needed, a new purchase source will be tracked down". (304) For many customers, both sourcing directly from the manufacture and from other distributors would be possible solutions. One stated, "we would try to source the product first directly from the manufacturer and if this wasn't possible from other distributors." (305)
(373) In any event, it seems that most customers would not feel in any way 'trapped' with VWR if the products they want to buy were suddenly to become unavailable. Instead they would compare prices from other sources and make a decision as to the best option in the particular circumstances. Therefore, given that manufacturers have alternative ways to market their products via alternative distributors or via direct sales, a potential foreclosure of other manufacturers from VWR's catalogue is unlikely to have any negative effect on customers downstream as is also confirmed in customers' responses in the replies to the market investigation.
(374) As explained in paragraph (274), VWR has preferential agreements in place with Merck under which [Discussion of confidential commercial agreements between VWR and Merck].
(375) Some competitors also consider the proposed Transaction to have potential advantages. One competitor predicts that the merger may lead to improved availability and lower prices due to the improved channels to market for Avantor products. (306) The Transaction could also have positive effects if the merged entity decided to compete more aggressively with Merck, pushing Merck to look for alternative distributors or to further increase its direct sales.
(376) Moreover, as indicated above, there is no indication that VWR would be the only route to market for manufacturers. Quite the contrary – alternative routes to market do exist for competing suppliers.
(377) Direct sales are not uncommon in the industry, especially in relation to larger customers. Should the merged entity try to engage in foreclosing other manufacturers, an increased supply via direct sales is a realistic scenario for a significant part of the customer base.
(378) In this regard, it can be noted that Thermo Fisher has taken steps to strengthen its European distribution network by opening a new distribution centre in Sweden this year. Thermo Fisher is active as a distributor of third party products, as well as its own products, and confirmed that it prefers to carry a wide range of products. In addition, a wide range of other distributors are present in specific countries or regions, as discussed above. Were the merged entity to stop selling other brands, these distributors would see the opportunity to promote them and gain sales. For example, one competitor that is also a distributor sees the merger as offering a good opportunity to improve its sales of brands that compete with Avantor and that VWR may no longer want to sell. (307)
(379) A number of smaller competitors on the upstream production market (i.e., suppliers) also reported in the market investigation that they are planning to increase their percentage of direct sales (independently of the merger and of any change to their relationship with VWR). Some are planning to achieve this expansion within the next two of years. This suggests that even fairly small manufacturers are able to extend their own sales network and are potentially becoming less dependent on distributors.
(380) As indicated above, one of the upstream competitors which have raised customer foreclosure concerns submits that it faces some constraints in the short term in relation to direct sales and sales via other distributors. However, already today this competitor has a significant direct sales presence. It is present directly in all EEA States, and in fact a significant amount of its sales are made directly in most EEA States. (308) It would also seem reasonably possible for this competitor to further increase its own direct sales. Besides this, it is to be noted that this competitor currently utilises distributors (309) in addition to VWR and could therefore seek to rely on the customer relationships already in place between third party distributors and customers going forward. Moreover, the evidence on file indicates that this competitor is already planning to work with a multi-channel strategy (that is relying on both direct sales and sales via distributors) and to gradually increase its direct sales going forward. This would decrease potential dependency on the capabilities of VWR on a medium to long term perspective. Also as noted above, in pursuing such a strategy, this competitor would be in a position to benefit from both its own significant direct distribution network and brand recognition.
(381) As indicated above, another competitor which has raised customer foreclosure concerns, whilst suggesting that smaller distributors may not be a viable option for it to achieve its desired plan/level of distribution, has indicated that it is considering another main player as a viable alternative distributor (to VWR) for the distribution of its products going forward. Besides this, other competing manufacturers overall do not appear to be concerned by customer foreclosure. (310)
(382) Besides the potential of other manufacturers to expand direct sales, a number of other (smaller in comparison to VWR and Thermo Fisher) local distributors appear to be present in most Member States and these could be a potential route to market for such other suppliers should these face full foreclosure of access to the merged entity's distribution network or access at less favourable terms. (311)
(383) In light of all the above, therefore, the overall impact of any customer foreclosure on both competitors' ability to compete (given alternative routes to market) as well as on consumers is not likely to be significant.
Conclusion
(384) In light of all the above, the Transaction is not likely to lead to customer foreclosure in relation to the vertical links between the Parties upstream activities and the downstream market for distribution of laboratory chemicals or a broader market for laboratory products at EEA or national level.
5.3.5. Vertical links between the production of high purity acids and acetonitrile respectively and sales on the retail market
(385) While the above relates to the vertical link between the potential markets for the production of laboratory chemicals upstream and their distribution downstream, specific vertical relations between the Parties exist in the potential markets for high purity acetonitrile and high purity acids that are discussed separately below.
5.3.5.1. Acetonitrile
(386) The activities of the parties overlap in the production of high purity acetonitrile, which is obtained by purifying crude acetonitrile. The parties are not active in manufacturing crude acetonitrile as a raw material. A small number of competitors voiced concern about the possibility that the merged entity could pursue an input foreclosure strategy in light of the fact that VWR (through its subsidiary PTI) sells bulk size high purity acetonitrile to Whyte (a third party distributor) that then sells it on to competitors of the Parties that down-pack and sell the high purity acetonitrile under their own respective brands. In addition, Avantor is also active in the production of catalogue sized high purity acetonitrile on an OEM basis. It produces and already packs and labels high purity acetonitrile for competitors such as […]. Both, VWR and Avantor are active downstream on the downstream market, selling catalogue-sized high purity acetonitrile to laboratory customers.
(387) As explained above, input foreclosure occurs where the merger is likely to raise the costs of downstream rivals by restricting their access to an important input. Therefore input foreclosure in the current context relates to the possibility of the merged entity to restrict competing distributors' access to high purity acetonitrile (either by restricting OEM sales from Avantor or bulk sales from PTI). However, such a scenario is unlikely and would in any case not harm downstream customers as can be seen from the analysis below.
Ability to foreclose
(388) In order to raise competition concerns, the merged entity must have a significant amount of market power upstream. In this case, this would imply the power of the merged entity on the production market for high purity acetonitrile.
(389) As described in section 5.2.5.1, the merged entity would not become the market leader in a hypothetical high purity acetonitrile production market, with a modest combined market share of [10-20]%. There will remain strong international players such as Biosolve ([10-20]%) or Merck ([10-20]%) with market shares above or similar to those of the merged entity, plus five further competitors having between [5-10]% and [10-20]% market share, followed by a long tail of smaller competitors with market shares of below [5-10]%.
(390) Given Avantor and VWR’s relatively modest combined market share in the production of high purity acetonitrile, and the number of other strong players active in this market, it would appear unlikely that the merged entity would be able to restrict supply. A majority of competitors active as suppliers of high purity acetonitrile to end customers do not believe that the proposed Transaction would have any effect on this market. (312)
(391) Suppliers of high purity acetonitrile use three different approaches to manufacture it: (i) buy crude acetonitrile and purify it by means of distillation, (ii) buy high purity acetonitrile in bulk size and down-pack it and (iii) and buy catalogue size packs on an OEM basis.
(392) VWR, through PTI, is a supplier of high purity acetonitrile in bulk sizes and could therefore potentially only foreclose manufacturers that buy high grade acetonitrile to down-pack it, notably by not supplying them or doing so on less favourable terms. On the other hand, following its integration with an upstream supplier, Avantor as an OEM provider could potentially foreclose competitors by stopping its production on an OEM basis or worsening the terms.
(393) Nevertheless, most of the largest suppliers of high purity acetonitrile to end customers (i.e. competitors to VWR and Avantor on the retail market) use more than one of these option simultaneously (i.e. purifying crude acetonitrile, buying on an OEM basis or down-packing high purity bulk size acetonitrile).
(394) As regards VWR's ability to engage in input foreclose, it has to be noted that PTI is not active as a manufacturer on an OEM basis for high purity acetonitrile, neither is VWR. All of PTI's high purity acetonitrile is sold to Whyte that then further distributes it. Therefore the only competitors of the Parties that might be affected from an attempted foreclosure would be Whyte's customers in the EEA that purchase high purity acetonitrile for further down-packaging. Besides Avantor, these include […] have confirmed their capability to buy crude acetonitrile (from third parties which are not affiliated to VWR and/or PTI) and further purify it. (313)
(395) In addition, most of the largest players currently buy the majority of their input from other sources than PTI (314) and most of the respondents to the market investigation confirmed not to be depending on the Parties' supply. (315) VWR would therefore not have an ability to foreclose its current customers from access to high purity acetonitrile.
(396) Avantor is active in OEM sales but only on a very limited scale. The only customer that generated sales with Avantor in OEM high purity acetonitrile above EUR […] in 2015 and 2016 was […] Given that […] has its own capacities to produce acetonitrile (i.e. purify or in other ways process it for sale in catalogue format), the ability to engage in an effective foreclosure can be ruled out. This is particularly the case given the respective quantities: While Avantor delivered less than […] liters of high purity acetonitrile as an OEM manufacturer to […] in 2016, the Parties estimated Merck's quantity of high purity acetonitrile sold in the laboratory chemicals market in Europe to be above […] million litres. Data provided by Merck confirm that Avantor is not a relevant supplier for them and that they do not procure any high purity acetonitrile from VWR. (316) However, Merck confirmed that it did purchase some high purity acetonitrile from PTI, but these purchases are not significant in comparison to total purchases. (317)
(397) In light of the moderate market shares of the Parties upstream, the fact that most of the largest competitors on the downstream market for the supply of acetonitrile to distributors and end-customers procure only a small part of their respective supplies from the Parties, and the fact that alternative sources are readily available, the Commission considers that the merged entity will not have the ability to effectively foreclose competitors' access to high purity acetonitrile.
Incentives to foreclose
(398) Avantor has a limited presence downstream in a catalogue sales retail market for high purity acetonitrile ([5-10]%). Given the presence of competitors downstream, with Merck ([10-20]%) and Honeywell ([10-20]%) having a higher market share than the merged entity ([10-20]%) and other competitors also having a significant presence, such as Panreac ([5-10]%), Biosolve ([5-10]%) and Romil ([5-10]%), it would be difficult for PTI to recover the losses from a potential foreclosure strategy of these larger players. It therefore seems unlikely that the merged entity (through PTI) would have any incentive to pursue a foreclosure strategy.
Impact on effective competition
(399) In light notably of the fact that most of the largest competitors on the downstream market for the sale of high purity acetonitrile procure only a small part of their respective supplies from the Parties, and the fact that alternative sources are readily available, the Commission considers that any potential impact on effective competition of a hypothetical input foreclosure is not likely to be significant.
Conclusion
(400) Based on the analysis above, it seems that the merged entity would neither have the ability nor the incentives to pursue an input foreclosure strategy. In any event, an attempted foreclosure would also not have a detrimental effect downstream given that alternative sources for high purity acetonitrile exist in the downstream market that are not dependent on Avantor's OEM manufacturing or VWR and its subsidiary PTI. (318) Therefore, the Transaction does not raise vertical concerns in relation to a potential market for acetonitrile.
5.3.5.2. High purity acids
(401) High purity acids are used in industrial production processes for example in the semi-conductor industry as well as for laboratory purposes. While these products are typically sold to industrial customers in larger quantities, smaller catalogue sizes are typically sold for laboratory usages. A number of large chemical producers like BASF, Ineos, Solvay, and KMG are active in the production for industrial customers, but these customers are not served by VWR. Therefore, only customers that require high purity acids for laboratory purposes are relevant for the assessment.
(402) On the overall potential downstream market for the supply of high purity acids in catalogue size to distributors and end-customers, the Parties' combined shares remain below 10%, with Avantor bringing an increment of under [0-5]%, as can be seen from the table below. Therefore, horizontal effects are unlikely to arise in this regard. (319) Should the market be segmented between PPB and PPT, the combined market shares remain below 10%. For PPB the combined market share is [5-10]% with an increment brought by Avantor of [0-5]%. For PPT the combined market share is [5-10]% with an increment brought by Avantor of [0- 5]%. However, the production process for these types of acids requires a more detailed analysis.
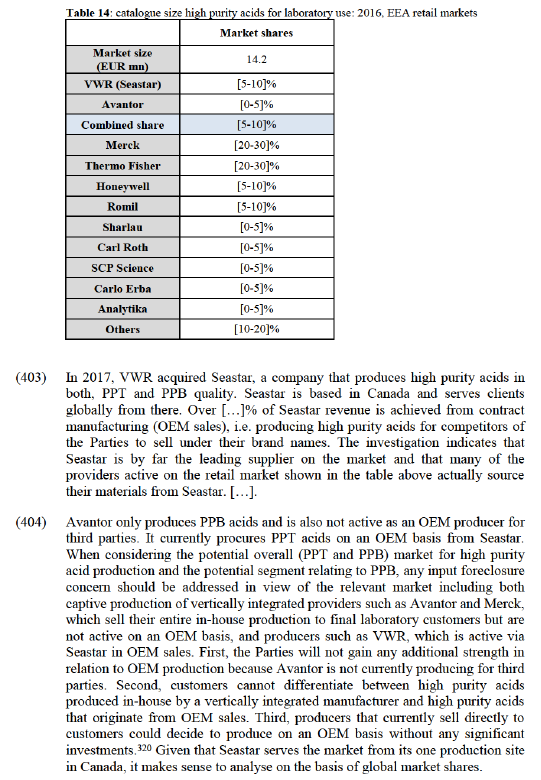
(405) On an overall production market (PPT and PPB), the merged entity would have market shares of slightly over [40-50]% with a small increment of c.[0-5]% brought by Avantor. For PPB acids, the combined market share overall would be lower at [20-30]% with a slightly higher increment brought by Avantor of [5- 10]%. Merck is the second largest producer with over [30-40]% market share and the rest being attributable to a number of smaller producers.
(406) Several competitors in the downstream market have voiced concerns that they might be foreclosed post-merger, claiming that Seastar is the only available source of supply. Hypothetically, the merged entity might seek to either: fully foreclose competitors' access to high purity acids; or alternatively raise prices/costs of its rivals. Both strategies could entail price increases for downstream customers in the event that there are no alternative high purity manufactures to which lab chemical competitors could turn.
Ability to foreclose
(407) The main competitors upstream (i.e. production level) do not currently sell to downstream competitors on an OEM basis. They are either active as vertically integrated providers (like Merck) selling to end laboratory customers or focussed on industrial customers and not active in catalogue sized sales to laboratory customers. Only Analytika, a small supplier in Poland, appears to be active as an OEM manufacturer, albeit at a significantly smaller scale than Seastar. Given that Avantor does also not sell high purity acids to downstream competitors on an OEM basis, the merged entity would not gain any additional ability to foreclose (whether fully or partially) downstream competitors that depend on OEM producers for high purity acids.
(408) Even if VWR acquired Seastar already in January 2017, it has not engaged in any input foreclosure (there has been no significant changes in prices or volumes since the acquisition). On the contrary, OEM production is Seastar's core business model as the Notifying Party submits and current OEM customers have confirmed that Seastar rather tries to extend rather than reduce its OEM services. (321).
(409) The ability to effectively foreclose downstream rivals also depends on the capabilities of the downstream customers to react to such a foreclosure attempt. On the one hand, existing producers of high purity acids that are currently not strong as OEM producers in Europe could offer replacing Seastar and increase sales to its current customers. GFS has confirmed that it has the ability to start contract manufacturing and that it has recently increased its capabilities to produce high purity acids; while it does not currently produce on an OEM basis for sales in the EEA, it would consider doing so if commercially attractive. Given the recent expansion of capacity, GFS would be able to start offering such services in the short term. (322)
(410) On the other hand, large customers of Seastar that are currently using their OEM services have stated their ability to purchase high purity acids from other suppliers like Tama, Canto, GFS, Merck, or KMG. If required they could increase their capabilities to down-pack these products from bulk to catalogue sizes. While not all customers of Seastar might have the ability to start down- packaging, it is at least an alternative option that limits VWR's ability to effectively foreclose downstream customers from high purity acids in general.
(411) In light of the above, it cannot be excluded that VWR has the ability to foreclose some downstream rivals but that that potential ability might be more limited in relation to some of its downstream competitors. However, this ability is not linked to the current Transaction but related to VWR's prior acquisition of Seastar. Indeed, the increment brought about through the transaction (Avantor's in-house production of PPB acids, which it does not even sell to OEMs) is insignificant at less than [0-5]% of an overall hypothetical high purity acid production market and c.[5-10]% of a hypothetical PPB segment and therefore does not significantly alter any potential pre-existing market power upstream on the part of VWR.
Incentive to foreclose
(412) Seastar achieved (pre-acquisition by VWR) and still achieves (post-acquisition by VWR) the vast majority ([…]%) of its revenue from OEM sales. Out of these, Avantor represents […]% and VWR purchases an additional […]% from Seastar's OEM production while […] represents almost half of Seastar's OEM sales. In sum, Seastar would risk losing almost three quarters of its OEM revenues if it would engage in a foreclosure strategy.
(413) Given the limited share the Parties currently have of the downstream market, it seems unlikely that all lost sales could be converted back to the merged entity. The market share increment on the downstream retail market brought by Avantor is minimal ([0-5]%) and in any event lower than the market share of VWR ([5-10]%). This small increment would not appear to significantly change the incentives to supply competing downstream players, also as the combined market share of the merged entity also remains relatively moderate below 10%, as shown in the table above.
(414) Given the presence of competitors downstream, with Merck ([20-30]%) and Thermo Fisher ([20-30]%) having significantly higher market shares than the merged entity ([5-10]%) and other competitors also having a significant presence, such as Honeywell ([5-10]%), Romil ([5-10]%) or Sharlau ([5-10]%), it would be difficult for Seastar to recover the losses from a potential foreclosure strategy. This is even more the case given that these competitors might find alternatives to the current dependency on OEM production.
(415) Merck, which is the market leader in the downstream market, produces high purity acids in-house but also purchases high purity acids on an OEM basis from Seastar. Currently, Merck can serve much of the European market for PPB and PPT acids with its own capacity while Seastar is mainly used as an OEM producer for the American market. (323) Merck is currently expanding its production capacity and will continue making investments in order to expand its capacity and reduce its dependency on Seastar, but expects to keep them as a supplier going forwards. Given that Seastar's business model depends on OEM manufacturing and that they have tried to expand rather than reduce their OEM sales since the acquisition by VWR, it is not obvious that the Transaction would change the merged entities' incentive and restrict sales to Merck which itself could threaten to further its own production and further decrease its dependency on Seastar.
(416) Thermo Fischer, the second largest player on the retail market, already has down-packing facilities and is not concerned about the Transaction even if it relies largely on Seastar for high purity acids. Thermo Fischer already purchases PPB in bulk size for down-packing. In the event of foreclosure by Seastar, it would increase OEM purchases from other sources (324) and bulk purchases for down-packing. As regards PPT, it would turn to the bulk suppliers and would down-pack these acids. (325) Bulk suppliers (eg: BASF, INEOS) are not capacity constrained as regards PPB and PPT.
(417) Honeywell currently manufactures in-house high purity acids for industrial uses, both PPT and PPB. However, Honeywell currently sources almost its entire needs of high purity acids for sales to laboratory customers from third parties on an OEM basis. High purity acids destined to laboratory use must be packaged into smaller containers. This difference of packaging size would require the packaging operation to be done in a different cleanroom. Honeywell does not own the required facility, even if already disposes of a cleanroom dedicated to the production of high purity acids for industrial use (bulk packages). Honeywell would need to invest approximately EUR1 million in order to build a purification plant and a cleanroom readily available for packaging smaller units of high purity acids destined to laboratory use. The investment necessary to build such a facility would be of approximately EUR 1 million and it would take approximately a year to obtain such a facility. (326)
(418) Moreover, customers that depend on OEM manufacturing could also arguably turn to other smaller competitors of Seastar like Analytika or GFS that is likely to be able to quickly start producing high purity acids for laboratory usages for European customers on an OEM basis.
(419) It therefore seems also unlikely that the merged entity (through Seastar) would have any incentive to pursue a foreclosure strategy.
(420) The incentive to engage in any such attempt is further reduced because wholesale margins seem to be higher than retail margins downstream. The risk of losing significant parts of these upstream margins in exchange for an uncertain chance of gaining some of the lower downstream margins does not seem to be an attractive strategy.
(421) Finally, a decision by VWR to stop selling Seastar's high purity acids to third party competitors could jeopardise its relationship with them. The downstream high purity acids market (at retail level) represents only a very small part of the laboratory chemicals market (EUR 14 million of EUR 1 600 million), meaning that it would arguably be illogical for VWR to engage in any strategy in relation to high purity acids that could be damaging to its business elsewhere when total gains would be small in absolute numbers anyway.
(422) Therefore, based of the above elements, it appears doubtful whether the merger significantly changes or strengthens incentives to foreclose competitors.
Impact on effective competition
(423) Significant effects on consumers are unlikely given that alternative sources for high purity acids would remain in the market even if the merged entity would pursue a foreclosure strategy.
(424) Alternative sources of supply for end customers with incentives to compete for share beyond the merged entity would exist, such as Thermo, Merck, or Honeywell. Even if these currently use OEM manufacturing services from Seastar for some of their sales, alternatives are available within and outside of OEM manufacturing that these manufacturers could pursue. It seems highly unlikely that any of these competitors would be forced to completely stop selling high purity acids in case of input foreclosure. Even a hypothetical foreclosure would therefore not reduce choice on the market.
(425) Should the merged entity nevertheless try to foreclose downstream competitors, it seems highly unlikely that it could at the same time increase downstream prices. To the contrary, the required conversion of customers would only be possible with additional incentives, effectively reducing prices for end customers.
(426) In light of the above, even a hypothetical foreclosure would not lead to significant detrimental effects of downstream customers.
Conslusion
(427) Based on the analysis above, the Transaction is not likely to lead to a foreclosure of downstream competitors from access to high purity acids.
5.4. Coordinated effects
(428) In addition to unilateral effects discussed above, the Commission assessed the possibility of the Transaction to raise coordinated effects. A merger in a concentrated market may significantly impede effective competition, through the creation or the strengthening of a collective dominant position, because it increases the likelihood that firms are able to coordinate their behaviour in this way and raise prices, even without entering into an agreement or resorting to a concerted practice. A merger may also make coordination easier, more stable or more effective for firms that were already coordinating before the merger, either by making the coordination more robust or by permitting firms to coordinate on higher prices.
(429) A distributor has issued concerns during the Commission's investigation suggesting that the combination of the existing the vertical commercial relationship between Merck and VWR and the structural link created between VWR and another upstream competitor, Avantor as a result of the proposed Transaction, could lead to the merged entity to foreclosing other manufacturers or distributors. These concerns are discussed in the vertical sections above. (327) The following section focusses on potential coordination on the two horizontal levels of manufacturing and distribution respectively.
(430) As regards a potential horizontal coordination on the manufacturing level, in light of suggestions by the distributor referred to in the preceding paragraph that the combination of the Transaction and the pre-existing commercial link between VWR and Merck might incentivise the merged entity to compete less strongly with Merck, the Commission examined whether/the extent to which the Transaction lead to coordination between the merged entity and Merck or otherwise increase the likelihood that Merck and Avantor coordinate to compete less strongly. The Commission considered in this context whether fact that VWR generates a significant part of its sales with Merck's products would mean that it would not be in the merged entity's interest for Avantor to compete too strongly with Merck. In such a scenario, Avantor and Merck could use VWR as a vehicle for coordination.
(431) The Commission also assessed potential coordination on the distribution market in light of statements by a distributor, claiming that in the country in which it is present, VWR, Merck, and Thermo Fisher already coordinate to dictate the conditions on the distribution market. In its view, as the three biggest competitors, these three players accept that they have to "endure one another’s presence" anyway, making some sort of cooperation between them inevitable. This distributor further claims that VWR, Merck, and Thermo Fisher have concluded formal and informal agreements at international level, and that they exchange intermediary and final products and sell these products to one another. (328)
The Parties' arguments
(432) The Notifying Party submits that the Transaction does not materially changes their position on the production market. Existing horizontal overlaps do not lead to a significant concentration because Avantor has only a moderate presence in Europe and the transaction does therefore not materially affect the market structure.
(433) With regards to VWR's existing agreements with Merck the Notifying Party submits that Merck will remain a third-party supplier with an arm’s-length agreement with VWR. However, even an assessment of "combined" market shares of Merck and VWR/Avantor would not lead to any type of market dominance. To the extent the Transaction affects VWR’s incentives at all, it would push VWR to favour sales of Avantor products over sales of Merck products, which would make any coordinated effort between Avantor/VWR and Merck unsustainable.
The Commission's assessment
(434) As regards potential coordination on the distribution market, the market for distribution of laboratory chemicals and potentially other laboratory products at EEA and national level is not particularly concentrated because of the strong presence of several national or regional players on the market that account for a significant share of the market in addition to global players like VWR and Thermo Fisher. In light of the fact that the Transaction does not increase the Parties' presence on a potential market for third party distribution, no signs arise that the Transaction would increase a risk of coordinated behaviour on this downstream market. The portfolios of downstream players vary and the multiple products carried by distributors may be considered as rendering the achievement of potential coordination more difficult. Even if one were to hypothesise that the merged entity and Merck, were to compete less strongly post-merger, there are a number of very heterogeneous national or regional distributors with whom any attempt to coordinate would be highly unlikely to be stable. There are also no signs that the distributors active across several national markets could engage in any type of market sharing. To the contrary, Thermo Fisher has recently invested into the expansion of its network in the EEA and no stable geographic pattern can be seen among the large players.
(435) Coordinated effects are equally unlikely on the upstream market for production of laboratory chemicals and the potential sub-markets thereof. (329) The limited availability of reliable market share data does not support a claim that monitoring market behaviour is easily possible. In addition, end-user prices vary between direct distribution and third party distribution and depend significantly on the volumes purchased. There are no indications for particularly high transparency on effective prices paid by end customers.
(436) As regards the special agreement between VWR and Merck and the hypothetical post-Transaction scenario where only Merck and the merged entity would seek to coordinate their behaviour in relation to laboratory chemicals, [Discussion of potential VWR strategy based on internal documents]. These documents also support the claim that VWR sees Merck's recent acquisition of Sigma's distribution network as a threat and it would be reasonable to consider that Merck has incentives, going forward, to build on its own distribution network in light of that acquisition.
(437) In addition, the incentives of the merged entity and Merck do not appear to be aligned, especially given the disparity between Avantor/the merged entity's and Merck's market shares. The vertical integration resulting from the transaction would seem more likely to incentivise the merged entity to compete with Merck for market share rather than to coordinate so as to maintain the status quo. Incentives for coordination would therefore seem to be, if anything, reduced rather than increased by the merger. First signs for such an increased competition can already be seen by Merck introducing "Merck Red" products that is perceived by the Notifying Party as a "fighter brand" in direct competition to VWR's product line and not distributed via VWR. Even if these first signs should be misleading, attempts to coordinate between Merck and the merged entity to increase prices of their lower priced brands would likely be frustrated by other competitors such as Carl Roth, Panreac, Carlo Erba, and the range of national manufacturers such as Boom, Roth, Romil, Scharlau, Rathburn, Chempur, Chemlab, and others, whose brands seek to compete directly within this segment of products targeting more price sensitive customers as analysed in section 5.2.1.2. The number of players and their diversity do not support a claim that coordination among them would be a likely scenario.
(438) Coordination between Merck and Avantor/ the merged entity to increase prices of their premium brands seems equally unlikely due to their market shares disparities. While Merck is the market leader, the market share of Avantor is modest. One of the main rationales of the transaction for Avantor seems to be to improve its channels to the market in order to compete and increase its market share.
(439) Moreover, Thermo Fisher (which is seeking to increase its direct European presence) and Honeywell (which recently acquired the business divested in the context of the Merck/Sigma merger) may be considered as having an incentive to compete for market share against the market leader, Merck (whether or not the current/similar preferred supplier/distributor relationship between Merck and VWR is extended) and the merged entity, rather than to coordinate. Indeed, even if one were to hypothesise about a scenario where only Merck and the merged entity would seek to coordinate their behaviour, Honeywell and Thermo Fisher could jeopardise the outcome of any expected coordination in seeking to gain market share.
(440) Moreover and in any event, the internal documents of the Parties do not contain evidence of potential current or post-Transaction coordination but rather provide a picture of a sector with increased competition between vertically integrating players.
(441) In light of the above, and taking into account the presence of other major providers and distributors of laboratory chemicals, as well as a range of smaller local players active on both markets which would seek to frustrate attempts to coordination, it seems unlikely that the Transaction increases the risk of coordinated effects in the markets where the Parties are active.
5.5. Conglomerate effects
(442) The Commission has to assess if a transaction could lead to conglomerate effects. These may arise if the merged entity had the ability and incentive to leverage a strong market position from one market to another by for example tying or bundling products, leading to a foreclosure of competitors. (330) In order to raise foreclosure risks, a significant large number of customers would need to demand a specific set of products whereby the merged entity would have a strong market position for at least one of these products.
(443) In the markets assessed, only a small proportion of customers reported that they buy laboratory chemicals in bundles. This often related to combinations of laboratory chemicals and either consumables or equipment. (331) Some customers felt that there could be a price advantage to buying in bundles (such as a discount on chemicals if bought in combination with laboratory equipment), but most had little knowledge of this, having never bought in this way. (332) A small number of respondents also mentioned other advantages of buying in bundles, such as efficiency and correct use of the equipment. (333) Respondents confirmed that there were, in any case, no products that they could not obtain outside bundles. (334)
(444) Relative to the other types of customer, proportionally more universities and research institutes choose to buy in bundles. Respondents mentioned both sets of laboratory chemicals and combinations of equipment and chemicals being bought in this way. (335) One university explained, for example: "suppliers may do promo periods where for example a PCR machine may be offered along with PCR reagents as a promo offer […] These offers generally are not exclusive, the individual components can normally be purchased individually under separate SKU’s." (336) It would appear that the buying habits of this type of customer possibly lend themselves more to buying in bundles (whereas some other types of customer mentioned being aware of bundles but that the products offered did not meet their needs), but that even for these customers it remains possible to buy all products separately as well.
(445) A number of competitors referred specifically to distributors' ability to sell in bundles, claiming this to be an advantage that distributors may have over manufacturers selling direct to customers. Given that VWR already stocks Avantor's (and many other manufacturer's products), the proposed transaction could not be reasonably seen as increasing VWR's ability to bundle products.
(446) One distributor voiced some concern that the proposed transaction could allow the merged entity to bundle products in a way that VWR does not already. (337) This distributor explained that VWR is very strong in consumables but weaker in laboratory chemicals (as a manufacturer), whilst Avantor is very strong in laboratory chemicals. Given that it is often much cheaper to buy chemicals and consumable as a package, this respondent claims that the merged entity could attract customers with this type of bundle. Another distributor claimed that VWR will become more dominant post-Transaction due to a considerable extension of its already significantly large portfolio. (338)
(447) Firstly, the market investigation did not indicate that the Parties (whether individually or combined) hold a strong market position in relation to any laboratory chemical product market which it could leverage from one market to another, nor did it indicate that they hold any 'must have' products. Thus, at the outset, it appears unlikely that the merged entity would have the ability to engage in the leveraging of a strong market position from one market to another.
(448) Secondly, responses to the market investigation do not generally suggest that customers are very inclined to buy in bundles, as their needs are very specific and price is not always the most important consideration. It is therefore unlikely that the merged entity would have a great deal to gain from a strategy of bundling.
(449) Thirdly, if the merged entity did offer some products at better prices in bundles, providing these products are also available individually, there would not appear to be any harm to customers. The markets investigation did not give any indication that suppliers 'force' customers to buy in bundles and there is no indication that the merged entity could effectively tie specific products in such a way. The fact that suppliers have never attempted this in the past strongly suggests that they know the strategy would be doomed to failure, because customers could easily obtain equivalent products separately elsewhere. On this basis, it is also highly unlikely that he merged entity would try to restrict customers' choice by offering certain products only in bundles as a way of preventing customers from buying some products from it and some from other suppliers. In any event, the market shares of each of the parties remain rather modest (339) and in any event, the market investigation has not indicated that Avantor or VWR carry a must have product within their portfolio. (340) It is also important to note that VWR already distributes Avantor's products and therefore, its product portfolio will not vary.
(450) In conclusion, therefore, there seems to be very little indication that the proposed transaction could lead to any increased ability to bundle specific products and to thereby reduce competition or restrict customers' choice and in any event, the ability to bundle remains questionable as do potential effects given that alternative suppliers remain available for customers to turn to should the merged entity seek to engage in anti-competitive bundling. Thus, overall, the Transaction is unlikely to result in significant negative conglomerate effects.
6. CONCLUSION
(451) For the above reasons, the European Commission has decided not to oppose the notified operation and to declare it compatible with the internal market and with the EEA Agreement. This decision is adopted in application of Article 6(1)(b) of the Merger Regulation and Article 57 of the EEA Agreement.
1 OJ L 24, 29.1.2004, p. 1 (the 'Merger Regulation'). With effect from 1 December 2009, the Treaty on the Functioning of the European Union ('TFEU') has introduced certain changes, such as the replacement of 'Community' by 'Union' and 'common market' by 'internal market'. The terminology of the TFEU will be used throughout this decision.
2 OJ L 1, 3.1.1994, p. 3 (the 'EEA Agreement').
3 The Transaction was originally notified to the Commission on 15 September 2017. On 9 October 2017, the notifying party informed the Commission that it withdrew its notification. On 11 October 2017, the Transaction was re-notified.
4 Approximately [80-90]% of Avantor's outstanding voting common stock is owned by investment funds and holding vehicles controlled by affiliates of New Mountain
5 If other Avantor shareholders syndicate convertible preferred stock to additional investors, these investors, depending on the size of their investment, may gain the right to appoint one or two of the board members which New Mountain currently has the right to appoint. Even if this further syndication occurs, none of these investors will obtain any control rights over VWR, and New Mountain will retain sole control on the basis of its right to appoint at least six of the eleven members of the Board, including the CEO.
6 Turnover calculated in accordance with Article 5 of the Merger Regulation and the Commission Consolidated Jurisdictional Notice (OJ C 95, 16.4.2008, p. 1).
7 See Commission Notice on the Definition of Relevant Markets (OJ 372, 9 December 1997. p. 5).
8 Complaint received on 2 August 2017.
9 Over two thirds of responding customers responding to the market investigation explained that they source from both, manufacturers and distributors (Questionnaire 3, 4, 5, and 01 to customers, q 30). The competitive pressure from customers' ability to switch from distributors to direct sales is further supported by internal documents of the Parties.
10 See section 4.3.
11 Questionnaires 3, 4 and 5 to customers, Q8-14.
12 Questionnaire 4 to customers, Q8.
13 Questionnaires 3, 4 and 5 to customers, Q8.
14 Questionnaire 4 to customers, Q9.
15 Questionnaires 3, 4 and 5 to customers, Q10.
16 Questionnaire 4 to customers, Q10.
17 Questionnaires 3, 4 and 5 to customers, Q11-13.
18 Questionnaire 3 to customers, Q8.
19 Questionnaires 3, 4 and 5 to customers, Q13.
20 Questionnaire 5 to customers, Q13.
21 Questionnaire 4 to customers, Q13.
22 Questionnaires 3, 4 and 5 to customers, Q14.
23 Questionnaire 4 to customers, Q14.
24 Questionnaires 3, 4 and 5 to customers, Q14.
25 Questionnaire 5 to customers, Q14.
26 Questionnaires 4 to customers, Q14.
27 Questionnaire 1 to competitors, Q8.
28 Questionnaire 1 to competitors, Q10.
29 M.7435 Merck/Sigma-Aldrich, paragraph 101.
30 No additional potential submarkets of solvents were identified in the market investigation that would warrant further assessment.
31 As discussed further in paragrapgh (86), OEM manufacturing refers to a situation where a third party manufacturer produces, packs, and labels a specific product using the brand name (or private label) of another company. The latter actively sells the product under its own name but is thus not directly involved in the actual manufacturing process.
32 Questionnaire 1 to upstream acetonitrile competitors, Q5.
33 Questionnaire 1 to upstream acetonitrile competitors, Q8, Q9.
34 Questionnaire 1 to upstream acetonitrile competitors, Q9.
35 Questionnaire 1 to upstream acetonitrile competitors, Q11.
36 Questionnaire 1 to upstream acetonitrile competitors, Q3, Q4, Q9.
37 Questionnaire 2 to downstream acetonitrile competitors, Q2.
38 Questionnaire 2 to downstream acetonitrile competitors, Q7.
39 Questionnaire 2, downstream acetonitrile competitors, Q2, Q6 and Q7. Panreac can also purify for certain uses (Questionnaire 2 to downstream acetonitrile competitors, Q8).
40 Questionnaire 2 to downstream acetonitrile competitors, Q8.
41 Questionnaires 3, 4 and 5 to customers, Q16-20.
42 Questionnaires 3, 4 and 5 to customers, Q16.
43 Questionnaire 3 to customers, Q16.
44 Questionnaire 3 to customers, Q17.
45 Questionnaires 3, 4 and 5 to customers, Q18.
46 Questionnaire 5 to customers, Q17.
47 Questionnaire 4 to customers, Q17.
48 Questionnaires 3, 4 and 5 to customers, Q19.
49 Questionnaire 5 to customers, Q19.
50 Questionnaire 5 to customers, Q19.
51 Questionnaires 3, 4 and 5 to customers, Q19.
52 Questionnaire 3 to customers, Q19.
53 Questionnaire 3 to customers, Q19.
54 Questionnaires 3, 4 and 5 to customers, Q20.
55 Questionnaire 5 to customers, Q20
56 Questionnaire 4 to customers, Q20.
57 Questionnaire 3 to customers, Q20.
58 Questionnaire 3 to customers, Q20.
59 Questionnaire 1 to competitors, Q9.
60 No additional potential submarkets of inorganics were identified in the market investigation that would warrant further assessment.
61 It would also be technically possible to consider a separate market for PPB acids only, but given that the requirements for producing these acids are less stringent than those for producing PPT acids, and that a PPT acid could generally be used in place of a PPB acid, but not vice-versa (given that PPT acids are higher purity), such a market would seem far less plausible than a market for PPT acids only, from both a supply- and a demand-side perspective.
62 Questionnaire 1 to upstream high purity acids competitors, Q7.
63 Questionnaire 1 to upstream high purity acids competitors, Q6.
64 Questionnaire 1 to upstream high purity acids competitors, Q5.
65 Questionnaire 2 to downstream high purity acids competitors, Q4.
66 Questionnaire 1 to upstream high purity acids competitors, Q5, Q7.
67 Questionnaire 1 to upstream high purity acids competitors, Q5.
68 This is also consistent with the general approach taken for other, broader, classes of laboratory chemicals.
69 Questionnaires 3, 4 and 5 to customers, Q21-24.
70 Questionnaires 3, 4 and 5 to customers, Q21.
71 Questionnaires 3, 4 and 5 to customers, Q24.
72 Questionnaire 5 to customers, Q24.
73 Questionnaire 4 to customers, Q24.
74 Questionnaires 3, 4 and 5 to customers, Q23.
75 Questionnaires 3, 4 and 5 to customers, Q22.
76 Nonetheless, the Commission analyses in section 5.2.4.1. the potential market for high purity acetonitrile which is produced and resold by the Parties in bulk size.
77 M.7435 Merck/Sigma-Aldrich paragraph 88.
78 M.7435 Merck/Sigma-Aldrich paragraph 138.
79 Questionnaires 3, 4 and 5 to customers, Q25
80 Questionnaires 3, 4 and 5 to customers, Q27.
81 Questionnaire 5 to customers, Q27.
82 Questionnaire 5 to customers, Q27.
83 Questionnaires 3, 4 and 5 to customers, Q29.
84 Questionnaire 1 to competitors, Q11.
85 Questionnaire 1 to competitors, Q12.
86 Questionnaire 1 to competitors, Q14-15.
87 Questionnaire 1 to competitors, Q15.
88 Questionnaire 1 to competitors, Q17.
89 Questionnaire 1 to competitors, Q18.
90 Questionnaire 1 to competitors, Q19.
91 Questionnaire 1 to competitors, Q20.
92 Questionnaire 1 to competitors, Q16.
93 Questionnaire 1 to upstream high purity acids competitors, Q9.
94 Questionnaire 2 to downstream high purity acids competitors, Q14.
95 Questionnaire 2 to downstream acetonitrile competitors, Q9.
96 Questionnaire 1 to upstream acetonitrile competitors, Q6.
97 M.7435 – Merck/Sigma-Aldrich, paragraph 91.
98 M.6944 – Thermo Fisher Scientific/Life Technologies, paragraph 339.
99 Life science products include laboratory chemicals as well as products used in bioscience research (such as genomics, proteomics, and molecular biology) as well as cell culture products (M.7435 – Merck/Sigma-Aldrich).
100 M.7435 – Merck/Sigma-Aldrich, paragraph 213.
101 M.6944 – Thermo Fisher Scientific/Life Technologies, paragraph 27 and 42.
102 Questionnaires 3, 4 and 5 to customers, Q45.
103 Questionnaires 3, 4 and 5 to customers, Q74.
104 Questionnaire 4 to customers, Q74.
105 Questionnaires 3, 4 and 5 to customers, Q60.
106 Questionnaire 2 to distributors, Q6-7.
107 Questionnaire 2 to distributors, Q10.
108 It is pertinent to note that whilst the portfolios of distributors are a relevant factor for the definition of the downstream distribution market for the purpose of the assessment of this merger, the breadth of the portfolio of (upstream) suppliers is not relevant in this regard. In fact, an important value added by distributors is the consolidation of a broad range of products that individual manufacturers might not be able to offer. In this context, even if an upstream market to a distribution market may be considered to have a potentially narrow scope (for example, in this case, inorganics or solvents), a downstream distribution market may well have a wider scope. The market investigation results in the present case indeed do indicate that the relevant market for distribution encompasses more products than the potential narrower product markets upstream.
109 Questionnaire 2 to distributors, Q10.
110 Questionnaire 2 to distributors, Q8.
111 Questionnaire 2 to distributors, Q9.
112 Questionnaire 2 to distributors, Q13.
113 Questionnaire 2 to distributors, Q12.
114 Questionnaire 2 to distributors, Q10.
115 Questionnaire 2 to distributors, Q14-15.
116 See non-horizontal Merger Guidelines (Guidelines on the assessment of non-horizontal mergers; OJ C 265, 18.10.2008, p. 6), paragraph 16.
117 Questionnaire 2 to distributors, Q18.
118 Questionnaire 2 to distributors, Q21.
119 Questionnaire 2 to distributors, Q21.
120 Questionnaire 2 to distributors, Q21.
121 Questionnaire 2 to distributors, Q21.
122 Questionnaire 2 to distributors, Q15; one distributor also gave an example of such a situation: "We are currently in discussions with one customer with whom we have a long standing and successful relationship. […] This customer has recently been acquired by a global organisation that has a “global” supply agreement […] and we now understand we are to lose this business […] we have not been allowed to compete at the local/ regional level because of agreements forged by a global competitor in areas of the world in which we do not have a presence."
123 Questionnaires 3,4 and 5 to customers, Q45.
124 Questionnaires 3, 4 and 5 to customers, Q68.
125 Questionnaires 3, 4 and 5 to customers, Q68.
126 Questionnaire 2 to distributors, Q36 and Questionnaire 1 to competitors, Q76 and.
127 The potential markets for acetonitrile and high purity acids were the only ones where the Commission has received indications of potential issues wherefore they have been included in separate sections in the competitive assessment.
128 M.7435 Merck/Sigma-Aldrich, paragraph 156.
129 The range of products offered by a manufacturer also appears to have a bearing on the channels to market it can use. One major competitor explained that distributors are important for it because customers prefer to limit the number of suppliers they work with, and it does not have a very wide portfolio of laboratory chemicals itself. Whilst a very large supplier can sometimes meet customers' needs alone and thus keep business in direct sales, suppliers that do not offer the full portfolio of products would risk losing customers if they did not build relationships with distributors (Non- confidential minutes of call with a manufacturer, 28 June 2017).
130 Questionnaires 3, 4 and 5 to customers, Q34
131 Questionnaires 3, 4 and 5 to customers, Q44.
132 Questionnaire 3 to customers, Q44.
133 See also above section 4.2.1.3.
134 Avantor's brand Puritan Products is also available in the EEA but only upon request. This brand is mainly targeted at the US market and is not actively marketed in the EEA.
135 Questionnaires 3, 4 and 5 to customers, Q48
136 Questionnaire 5 to customers, Q50.
137 Questionnaire 5 to customers, Q50.
138 Questionnaire 5 to customers, Q48.
139 Questionnaire 4 to customers, Q47.
140 Questionnaire 4 to customers, Q47.
141 Questionnaire 3 to customers, Q49.
142 Questionnaire 4 to customers, Q48.
143 Questionnaires 3, 4 and 5 to customers, Q47-50.
144 Questionnaire 2 to distributors, Q61.
145 Questionnaire 2 to distributors, Q62.
146 Questionnaire 2 to distributors, Q62 and Q65.
147 Non-confidential minutes of call with a manufacturer, 28 June 2017
148 Questionnaires 3, 4 and 5 to customers, Q35.
149 Questionnaire 4 to customers, Q35.
150 Questionnaire 5 to customers, Q52.
151 Questionnaires 3, 4 and 5 to customers, Q56.
152 Questionnaires 3, 4 and 5 to customers, Q37.
153 See paragraph (299).
154 Questionnaire 2 to distributors, Q53.
155 Questionnaire 1 to competitors, Q46. Questionnaire 2 to distributors, Q68.
156 Questionnaire 1 to competitors, Q46. Questionnaire 2 to distributors, Q68.
157 The POCH brand is discussed in the section on Poland, as it is not available in other EEA countries.
158 Questionnaires 3, 4 and 5 to customers, Q53.
159 Questionnaires 3, 4 and 5 to customers, Q43.
160 Questionnaires 3, 4 and 5 to customers, Q36; questionnaire 2 to distributors, Q56.
161 Questionnaires 3, 4 and 5 to customers, Q36; questionnaire 1 to competitors, Q32.
162 Questionnaires 3, 4 and 5 to customers, Q40. Very few respondents also mentioned price as an advantage of Avantor. The apparent contradiction between Avantor being viewed as a high-price supplier by some customers and price being named as a weakness, while others rate price as a strength (suggesting that prices are very competitive) is most likely due to the relative positioning of Avantor's different brands. The JT Baker brand is known to be a higher price, higher quality brand whereas POCH (only available in Poland) and Macron Fine Chemicals are aimed at more price sensitive customers.
163 Questionnaire 1 to competitors, Q32; questionnaire 2 to distributors, Q58; questionnaires 3, 4 and 5 to customers, Q40.
164 Questionnaire 2 to distributors, Q57; questionnaire 1 to competitors, Q26.
165 Questionnaires 3, 4 and 5 to customers, Q40; questionnaire 1 to competitors, Q32; questionnaire 2 to distributors, Q67. A minority of respondents name customer service and delivery times amongst its weaknesses and mention that VWR cannot offer the full range of laboratory chemicals, in particular more specialist products. While the latter appears to be linked to VWR's own portfolio range, it does notr apply to VWR as a distributor.
166 Questionnaire 2 to distributors, Q10.
167 Questionnaires 3, 4 and 5 to customers, Q40.
168 Honeywell was mentioned by some as having entered the market. Whilst this is technically true, the acquisition of the Fluka business involved the transfer of a pre-existing business rather than the creation of a new brand or set of products.
169 Questionnaires 3, 4 and 5 to customers, Q80-81.
170 No affected markets arise in relation to bulk size products (see section 4.2.1.3 above).
171 All market share data presented in this decision are estimates submitted by the Notifying Party.
172 The combined market share on a production would be (10-20)% with an increment of (0-5)% brought by Avantor.
173 The combined market share on a production market would be (20-30)% with an increment of (5-10)% brought by Avantor.
174 Question 3, 4 and 5 to cutomers, Q34.
175 The market solvents (catalogue size) is also technically an affected market, but with only a very small increment (<(0-5)%) in Slovenia.
176 The market for inorganics (catalogue size) is also technically an affected market, but with only a very small increment (<(0-5)%) in France.
177 There are also technically affected markets within solvents in Croatia, Denmark, France, Malta and Slovenia, but the increment always remains below (0-5)%.
178 There are also technically affected markets within solvents in Denmark, France and Hungary, but the increment always remains below (0-5)%.
179 Questionnaires 3, 4 and 5 to customers, Q34.
180 Questionnaires 3, 4 and 5 to customers, Q56.
181 Questionnaires 3, 4 and 5 to customers, Q37.
182 Questionnaires 3, 4 and 5 to customers, Q53.
183 Questionnaires 3, 4 and 5 to customers, Q96-97.
184 Questionnaires 3, 4 and 5 to customers, Q34.
185 Questionnaires 3, 4 and 5 to customers, Q56 and Q34.
186 Questionnaire 5 to customers, Q96.
187 Questionnaire 4 to customers, Q96.
188 The potential markets for acetonitrile and high purity acids were the only ones where the Commission has received indications of potential issues wherefore they are discussed in separate sections in the competitive assessment.
189 Market shares remain below [20-30]% considering markets within acetonitrile defined on the basis of the purity level (as only higher-grade acetonitrile is used in laboratories), catalogue v bulk sizes and laboratory v industrial use; for the EEA.
190 Should the production market for acetonitrile be considered to be global, market shares for the combined entity would still remain below [20-30]% with Avantor having approximately (5-10]% and VWR [10-20]%. Merck would in such a scenario have higher market shares, amounting to [20-30]%. Thermo Fisher would follow the merged entity with [10-20]% and Biolab/Biosolve and Honeywell have each slightly below [10-20]%. The Notifying Party named additional 16 suppliers with each less than [5-10]% of global market share that make up the remaining - [30-40]% of such a market.
191 Questionnaire 2 to downstream acetonitrile competitors, Q10.
192 Reply to RFI on acetonitrile received on 23 October 2017.
193 Reply to RFI on acetonitrile received on 30 October 2017.
194 Questionnaire 2 to downstream acetonitrile competitors, Q11.
195 The production market is unlikely to be smaller than the EEA in any case, given the centralised structure of production.
196 Slovenia is also technically an affected market as the parties' have a combined market share of [50- 60]%, but the increment brought by VWR is less than [0-5]%. The Transaction can thus be assumed to have a minimal impact.
197 The highest combined market share on the retail market is [10-20]% (with an increment of [0-5]% brought by Avantor) in the market for catalogue size PPT acids at EEA level (for laboratory and other uses).
198 Market data includes only production of catalogue-sized high purity acids used by laboratory customers. The market investigation revealed that general chemicals manufacturers also produce limited amounts of high purity acids in catalogue sizes for selling to industrial customers. These general manufacturers do not sell to laboratory customers and therefore are not relevant for the analysis of the present case. In any event, the Parties' market share would be diluted if such additional suppliers were taken into account. Therefore, if the Cominission can exclude competition concerns on the basis of the narrower scope of the market, concerns can also be excluded considering a wider market scope.
199 The next largest competitor would have a market share of less than [0-5]%. Furthermore, responses to the market investigation suggest that some of the smaller competitors named by the Notifying Party are not or are no longer active on this market Questionnaires 1 and 2 to upstream and downstream competitors).
200 market data includes only production of catalogue-sized high purity acids by laboratory customers, See footnote 199 above.
201 Non-confidential minutes of call with Merk, 27 October 2017.
202 Question 2 to downstream high purity acid competitors, Q5, Q8, Q9 and Q16.
203 Non-confidential minutes of call with Honeywell, 24 October 2017. Questionnaire 2 to downstream high purity acids competitors, Q5.
204 OJ C031, 05.02.2004, p 5.
205 See Horizontal Merger Guidelines, paragraph 74.
206 See Horizontal Merger Guidelines, paragraph 69.
207 See Horizontal Merger Guidelines, paragraph 73.
208 Non-confidential minutes of call with Merck 27 October 2017.
209 Non-confidential minutes of call with GFS, 27 October 2017.
210 Given that the estimate submitted by the Parties is very close to (20-30)% in light of the specificities of this case, this market is being considered as an affected market for the purpose of this Decision.
211 Questionnaires 3, 4 and 5 to customers, Q30.
212 Questionnaires 3, 4 and 5 to customers, Q31.
213 Questionnaires 4 to customers, Q30.
214 Questionnaires 3, 4 and 5 to customers, Q30.
215 Questionnaire 5 to customers, Q30.
216 Questionnaires 3, 4 and 5 to customers, Q30 and Q60.
217 Questionnaire 1 to competitors, Q77.
218 Non-confidential minutes of call with a distributor.
219 Questionnaires 3, 4 and 5 to customers, Q61.
220 Questionnaire 4 to customers, Q61.
221 Questionnaire 3 to customers, Q62.
222 Questionnaire 1 to competitors, Q77.
223 Avantor's average margins on direct sales of solvents and inorganics in 2016 were […]% and […]% respectively, whilst its margins on sales via third party distributors were somewhat lower at […]% and […]% respectively.
224 Questionnaire 1 to competitors, Q71.
225 Non-confidential minutes of call with a manufacturer, 23 June 2017.
226 In case of a combined market for laboratory and life sciences products VWR's EEA-wide share would still remain below [10-20]% and its national market shares would be in a comparable range to those in the potential market for laboratory products shown in the tables below.
227 Questionnaire 2 to distributors, Q28.
228 Questionnaire 1 to competitors, Q67.
229 Questionnaire 1 to competitors, Q75.
230 Questionnaire 1 to competitors, Q75.
231 Questionnaire 2 to distributors, Q29.
232 OJ C265, 18.10.2008, p. 6.
233 See non-horizontal Merger Guidelines, paragraph 18.
234 See non-horizontal Merger Guidelines, paragraph 29.
235 Non-confidential minutes of call held with a distributor, 20 July 2017.
236 Non-confidential minutes of call held with a distributor, 20 July 2017. It is at the outset worth noting that these arguments are not merger specific. Whilst it could be argued that a significant market position downstream could imply that the merged entity would be incentivised to engage in foreclosure of competitors downstream as it would have a broader base on which to reap input potential foreclosure benefits, as indicated in this Decision, Avantor and the merged entity would not appear to hold market power upstream in the market in which the complainant is located. Besides this, whilst this particular complainant purchases between 10-20% of its supplies from Avantor, other manufacturers to whom this complainant could turn are active in this national market. Moreover, and in any event, end customers in this market would continue to have access to several manufacturers (via direct sales) and distributors in the sourcing laboratory chemicals.
237 Non-confidential minutes of call held with a distributor, 3 July 2017.
238 Non-confidential minutes of call held with a distributor, 3 July 2017.
239 Complaint received by the Commission on 2 August 2018.
240 Non-horizontal Merger Guidelines, paragraph 16.
241 Non-horizontal Merger Guidelines, paragraph 35.
242 For the Commission's assessment and findings of the likely lack of market power by Avantor and the merged entity in Poland, please see paragraph (216).
243 Questionnaire 5 to customers, Q72.
244 Questionnaire 4 to customers, Q72.
245 Questionnaires 3, 4 and 5 to customers, Q89.
246 Questionnaire 3 to customers, Q38 and 39.
247 Questionnaires 3, 4 and 5 to customers, Q89.
248 Questionnaires 3, 4 and 5 to customers, Q72.
249 Questionnaires 3, 4 and 5 to customers, Q72.
250 Questionnaire 5 to customers, Q72.
251 Questionnaire 3 to customers, Q72.
252 Questionnaire 3 to customers, Q72.
253 Questionnaire 1 to competitors, Q77.
254 Questionnaire 1 to competitors, Q77.
255 Questionnaire 1 to competitors, Q77.
256 Questionnaire 1 to competitors, Q77.
257 Questionnaire 3 to customers, Q72.2.1.
258 Questionnaire 3 to customers, Q91.
259 Questionnaire 3 to customers, Q91.
260 Questionnaire 5 to customers, Q90; questionnaire 4 to customers, Q91.
261 Questionnaires 3, 4 and 5 to customers, Q73.
262 Questionnaire 5 to customers, Q73.
263 Questionnaires 3, 4, and 5 to customers, question 94.
264 Questionnaire 1 to competitors, Q23 and Q63. (All competitors that responded to the market investigation answered positively to the question "Do your customers typically source laboratory chemicals from two or more suppliers simultaneously?").
265 Questionnaire 1 to competitors, Q63.
266 Questionnaire 1 to competitors, Q63.
267 Questionnaire 1 to competitors, Q63.
268 Questionnaires 3, 4 and 5 to customers, Q90; Questionnaire 4 to customers, Q62.
269 Questionnaire 2 to distributors, question 42.
270 Questionnaire 2 to distributors, Q49.
271 Even a complainant that claimed a specific dependency on Avantor products realises more than 80% of its sales on group level with other products.
272 Customers would also have the possibility of seeking to acquire directly from manufacturers.
273 The preferential relationship between VWR and Merck is an exception in this regard.
274 Minutes of call with distributor, 14 July 2017. and Minutes of call with another distributor, 21 June 2017.
275 One competitor at the distribution level claimed that the Parties together with Merck would have significant upstream market power in at least one EEA country and that VWR's market position downstream in the distribution of particular products would also be significant. The market shares presented for VWR by that competitor deviate from the Commission's findings set out in section 4.3. above. In the Commission's view, the market share data provided by the competitor are not relevant for the purpose of this decision because they refer to the distribution of subsegments of laboratory chemicals, whereas, on the basis of its market investigation, the Commission found that the distribution market encompasses at least the distribution of laboratory chemicals as a whole. However, even if the market was defined more narrowly, the merged entity would still face competitive pressure both at production and at distribution level. At the upstream production level, competing producers like Honeywell, Carl Roth, and many others remain present. At the downstream distribution level, large global players like Thermo Fisher as well as important national players in each EEA country will also remain on the market. The assessment of a potential foreclosure would equally
not change because the methodology of market share calculation does not influence the assessment of ability, incentive, and impact on competition as presented in this decision.
276 See Oberlandesgericht Düsseldorf, decision dating 13 November 2013 in case VI – Kart 5/09 (V).
277 Questionnaire 2 to distributors, reply to question 81.
278 […].
279 Non-confidential minutes of call with manufacturer, 28 June 2017.
280 Non-confidential minutes of call with a manufacturer, 9 August 2017.
281 Non-confidential minutes of call with a manufacturer, 28 June 2017.
282 Questionnaire 5 to customers, Q96.
283 Complaint received by the Commission on 2 August 2018.
284 Questionnaire 4 to customers, Q96.
285 See paragraph (339) above.
286 Questionnaire 1 to competitors, Q82.
287 Non-confidential minutes of a call with a manufacturer 23 June 2017,
288 Non-confidential minutes of call with a competitor, 23 June 2017.
289 Non-confidential minutes of call with a competitor, 23 June 2017.
290 Competitors response to RFI dated 29 September 2017.
291 Reply to request for Information received on 5 October 2017.
292 Non-confidential minutes of call with a competitor, 28 June 2017.
293 Non-confidential minutes of call with a competitor, 28 September 2017.
294 Thermo Fisher and a wide range of national or regional distributors' such as Serviquimia, Boom, Atlantic Labo, Witko, Th Geyer and many others.
295 See M.7435 Merck/Sigma-Aldrich.
296 Non-confidential minutes of call with a competitor, 28 September 2017.
297 Questionnaire 5 to customers, Q78.
298 Questionnaire 5 to customers, Q78.
299 Questionnaire 1 to competitors, Q81.
300 Form CO, paragraph 635.
301 Form CO, paragraph 635.
302 Non-confidential minutes of call with a competitor, 28 September 2017.
303 See section 5.3.3.2 for a detailed analysis.
304 Questionnaire 5 to customers, Q64.
305 Questionnaire 5 to customers, Q64.
306 Questionnaire 1 to competitors, Q86.
307 Questionnaire 1 to competitors, Q86.
308 Competitors response to RFI dated 29 September 2017 and non/confidential minutes of call, 23 June 2017.
309 Reply to request for Information received on 5 October 2017.
310 Questionnaire 1 to competitors, Q85 and Q86.
311 Form CO paragraphs 512 and 640, confirmed by a competitor in reply to a Request for Information received on 5 October 2017.
312 Questionnaire 2 to downstream acetonitrile competitors, Q11.
313 There appears to be a wide number of suppliers of crude Acetonitrile. Among others, Ineos, Alkyl Amines, Tedia, Anhui Fultime Specialized Solvents, Cornerstone, Sinopec, Petro, Imperial chemicals.
Ineos has spare capacity to increase production up to 50% Questionnaire 2 to downstream acetonitrile competitors, Q3 and Q4. Questionnaire 1 to upstream acetonitrile competitors, Q1, Q9 and Q10.
314 Questionnaire 2 to downstream acetonitrile competitors, Q3.
315 Questionnaire 2 to downstream acetonitrile competitors, Q4.
316 Questionnaire 2 to upstream acetonitrile competitors, Q1 and Q3.
317 Questionnaire 2 to upstream acetonitrile competitors Q3.
318 There are a number of suppliers downstream, offering high purity acetonitrile in catalogue sizes, including Merck, Panreac, Biosolve, Romil, Carlo Erba, Thermo Fisher, Rathburn, Mikrochem, Bernd Kraft, and Chempur.
319 The Transaction results in two affected market at national level – one in high purity acids overall and one in the narrower potential market for PPT acids only. However, the combined market shares remain moderate: [20-30]% in Poland and [20-30]% in Belgium for PPT acids.
320 For more detailed assessment of existing production capacities and planned capacity expansions see above section 5.2.4.3.
321 Non-confidential minutes of call with Seastar customer, 27 October 2017.
322 Non-confidential minutes of call with GFS, 27 October, 2017.
323 Non-confidential minutes of call with Merck on 27 October 2017.
324 Thermo Fisher mentioned Tama, Canto, GFS, Merck, KMG and High Purity Products. Questionnaire 2 to downstream high purity acids competitors,Q9.
325 Questionnaire 2 to downstream high purity acids competitors, Q5, Q8, Q9 and Q16.
326 Non-confidential minutes of call with Honeywell, 24 October 2017..Questionnaire 2 to downstream high purity acids competitors, Q5.
327 See section 5.3.3. on input foreclosure and 5.3.4. on customer foreclosure.
328 Non-confidential minutes of teleconference call with a distributor, 20 July 2017.
329 Including the potential sub-markets for high purity acids and acetonitrile discussed above.
330 Non-horizontal guidelines, paragraph 93.
331 Questionnaires 3, 4 and 5 to customers, Q82.
332 Questionnaires 3, 4 and 5 to customers, Q83.
333 Questionnaires 3, 4 and 5 to customers, Q84.
334 Questionnaires 3, 4 and 5 to customers, Q85.
335 Questionnaire 3 to customers, Q82.
336 Questionnaire 3 to customers, Q82.
337 Questionnaire 2 to distributors, Q81.
338 Non-confidential minutes of call held with a distributor, 20 July 2017.
339 See above, section 5.2.2.
340 See above, section 5.3.3.2.