Commission, June 15, 2015, No M.7435
EUROPEAN COMMISSION
Decision
MERCK/ SIGMA-ALDRICH
Dear Madam(s) and/or Sir(s),
Subject:Case M.7435 – Merck/ Sigma-Aldrich
Commission decision pursuant to Article 6(1)(b) in conjunction with Article 6(2) of Council Regulation No 139/20041 and Article 57 of the Agreement on the European Economic Area2
(1) On 21 April 2015, the European Commission received notification of a proposed concentration pursuant to Article 4 of the Merger Regulation by which the undertaking Merck KGaA ("Merck", Germany) acquires within the meaning of Article 3(1)(b) of the Merger Regulation control of the whole of Sigma-Aldrich Corporation ("Sigma", the United States) by way of purchase of securities (the "Transaction").3
(2) Merck is hereinafter referred to as "the Notifying Party". Merck and Sigma are collectively referred to as the "Parties".
I.THE PARTIES
(3) Merck is a German pharmaceutical and chemical company. Merck's operating activities are organized into four divisions, namely Merck Serono, Consumer Health Care, Performance Materials and Merck Millipore, each with a distinct business focus. The Merck's division concerned by the Transaction is Merck Millipore which focuses on developing, producing and selling tools and products for the life science industry. Merck Millipore is organized in three business units:(i) Bioscience, (ii) Lab Solutions, and (ii) Process Solutions.
(4) Sigma is a US company engaged in the development, production, and sale of life science tools and services as well as chemicals, analytical reagents and lab-ware. Sigma operates through three business units: (i) Research, (ii) Applied, and (iii) SAFC Commercial (custom manufacturing and services).
II.THE OPERATION AND THE CONCENTRATION
(5) On 22 September 2014, Merck and Sigma signed a share purchase agreement whereby, upon closing, Merck will acquire all issued and outstanding voting securities of Sigma. Sigma will be merged with and into an ad-hoc Merck subsidiary, thus becoming a wholly owned subsidiary of Merck. Outstanding share capital of Sigma will be cancelled at closing in exchange for consideration.
(6) Therefore, the proposed Transaction constitutes a concentration within the meaning of Article 3(1)(b) of the Merger Regulation.
(7) The total value of the Transaction is approximately USD 17 billion (EUR 13 billion).
III.UNION DIMENSION
(8) The undertakings concerned have a combined aggregate world-wide turnover of more than EUR 5 000 million (Merck: EUR 10 700 million, Sigma: EUR 2 036 million).4 Each of them has an EU-wide turnover in excess of EUR 250 million (Merck: EUR […]million, Sigma: EUR […]million), but each does not achieve more than two-thirds of its aggregate EU-wide turnover within one and the same Member State. The notified operation therefore has a Union dimension.
IV.ASSESSMENT
IV.1. INTRODUCTION
(9) The Transaction concerns the life science sector where both Parties are active. However, with the exception of laboratory chemicals representing […] of Merck's and […] of Sigma's life science businesses, the Parties' activities are mostly complementary. Indeed, the overall positioning of the Parties in the life science business is focused on different areas: while Merck is stronger in raw materials for pharmaceutical production (c.a. EUR […]for Merck versus EUR […]for Sigma in 2013) Sigma is focused on bioscience research (c.a. EUR […]for Sigma versus EUR […] for Merck in 2013).
(10) The main overlaps between the Parties' activities occur in relation to the supply of laboratory chemicals (section IV.5 of this Decision). Laboratory chemicals are chemicals used for research, analytical testing and quality control purposes by a wide range of customers, including academia, laboratories and pharmaceutical companies. The main role of laboratory chemicals is to allow for a repeated standardized testing with high precision and accuracy according to a predetermined testing protocol. As a result, laboratory chemicals have to meet high quality standards to avoid the presence of any contaminant. Given the nature of their use, laboratory chemicals are generally sold in catalogue quantities, i.e. less than 10 kilograms or litres per unit.
(11) In terms of the regulatory framework, quality standards for laboratory chemicals are promulgated by the International Organisation for Standardization (ISO), the reagent chapter of the European Pharmacopoeia (Reag. Pharm. Eur.) and the American Chemical Society (ACS).
(12) Merck is active in the laboratory chemicals field via its Lab Solutions division while Sigma competes in this space via its Research and Applied divisions, dedicated respectively to research and industrial customers. As detailed further below, Merck and Sigma are both strong suppliers of laboratory chemicals in the European Economic Area (EEA).
(13) The Parties' activities also overlap in the supply of raw materials for pharmaceutical production (section IV.4 of this Decision). These chemicals are essentially used by pharmaceutical and biopharmaceutical companies for the scale-up and production of therapeutic molecules, and include in particular ingredients to manufacture final drug dosage forms such as Active Pharmaceutical Ingredients (APIs), excipients and process chemicals ("biopharm ingredients"). Contrary to laboratory chemicals, raw materials used for manufacturing purposes are generally sold in larger volume.
(14) Importantly, because these products are used in the manufacturing of bio- pharmaceuticals, they generally meet higher regulatory standards compared to laboratory chemicals, and are normally produced at validated sites according to Good Manufacturing Practices (GMP) so as to ensure their traceability. In Europe, these standards are laid out in particular by the European Pharmacopoeia (Pharm. Eur.).
(15) Merck is active in the raw materials for pharmaceutical production field via its Process Solutions division and Sigma via its SAFC division. As detailed further below, while Merck is an important supplier of raw materials used for pharmaceutical production, Sigma's presence is limited.
(16) Both Parties are also active in the supply of chemicals and reagents used for bioscience research applications (section IV.2 of this Decision), such as genomics, proteomics, and molecular biology. These biochemicals are used by researchers to understand complex biological systems. Thus, biochemicals are tested to avoid any interference with biological systems (so called RNA/DNA free tests), but are not regulated by the quality standards described above for laboratory chemicals and raw materials for bio-pharmaceutical production. As opposed to laboratory chemicals, biochemicals are used in workflow protocols to develop new drugs, diagnostics and therapies, from the initial sample step to the detection step.
(17) Merck is active in the supply of bioscience products via its Bioscience division and Sigma via its Research division. As detailed below, while Sigma is one of the main suppliers in the EEA in this field, Merck's presence is limited.
(18) Finally, the Parties are both active in the supply of cell culture products (section
IV.3 of this Decision)5 where Parties' activities are largely complementary. Cell culture is a process by which cells are grown under controlled conditions, generally outside of their natural environment. Cell culture products can be used for research purposes, since it is one of the major tools used in cellular and molecular biology to study the physiology and biochemistry of cells and the effects of drugs and toxic compounds on cells. Cell culture products can also be used as raw materials for the production of biopharmaceuticals.
(19) For cell culture products for bio-production, Merck is active via its Process Solutions division and Sigma via its SAFC division. As concerns cell culture for research, Merck is active via its Bioscience division and Sigma via its Research division.
(20) It is also noted that the Parties are active as distributors of laboratory and life science products in the EEA, giving rise to a vertical relationship with their activities as suppliers of these products (section IV.6 of this Decision).
IV.2.BIOSCIENCE PRODUCTS
IV.2.1.Introduction
(21) Bioscience products consist of life science reagents and various kits used by researchers to understand complex biological systems and processes in their search for new drugs and therapies. Therefore, these products are used in a non- regulated discovery-driven environment, with the only requirement to avoid the presence of any substance that could interfere with the biological process being studied. The main customers of bioscience products are (i) academia and government bodies and (ii) pharma and biotech.
(22) Within bioscience, the Transaction leads to horizontally affected markets in the supply of cell culture, which will be discussed in section IV.3, and in the supply of a number of biochemicals.6
IV.2.2. Market definition
IV.2.2.1.Product market
(23) Within bioscience products, the Parties' activities give rise to affected markets for some general biochemicals, namely dyes and stains and carbohydrates, and bioactive small molecules.
Notifying Party's views
(24) According to the Notifying Party, general biochemicals are common, standardised chemicals that bioscience customers need for a wide variety of research applications and in significant quantities. These general biochemicals include among other products dyes and stains and carbohydrates7.
a. Dyes and stains are products used in different techniques which allow clinical researchers to study various constituent parts of tissue (e.g. nucleus, connecting tissue, etc.) by colouring it with a particular colour and making it more visible under microscope magnification. Dyes and stains are not only used for research applications, but can also be used for IVD (In-Vitro Diagnostics) applications. IVD tests are performed by clinical laboratory personnel in sampling and observing potentially diseased human tissue which has a direct implication on selecting a treatment for a patient. If used for IVD applications, dyes and stains must undergo the CE marking procedure as they are considered to be medical devices under the relevant EU legislation. In any event, research and IVD applications correspond to different chemicals that are not substitutable with each other.
b. Carbohydrates are basic components of cellular metabolism, as well as certain proteins receiving post-translational modification (such as glycosylation).
(25) Bioactive small molecules allow for the control of protein targets and cellular functions, and exist in many types.
(26) The Notifying Party submits that both general biochemicals (including dyes and stains and carbohydrates) and bioactive small molecules constitute separate relevant product markets which should not be further subdivided according to the type of chemical or molecule, due to the high level of supply-side substitutability within each category of products. Each supplier offers a wide range of these products, and is not restricted by material cost or time considerations from switching among them in response to customer demand.
Commission's assessment
(27) While in some previous decisions reference has been made to some of these products (such as dyes and stains), the Commission did not reach conclusions in relation to the scope of the product market concerning these products.8
(28) During the market investigation, most of the suppliers indicated that, within each product category (dyes and stains, carbohydrates and bioactive small molecules), they are able and do supply a wide range of products9, given the absence of regulatory constraints and the ordinary nature of these chemicals.
(29) In any event, for the purpose of this Decision, the Commission considers that the precise product market definitions in relation to general biochemicals and bioactive small molecules can be left open as this would not change the outcome of the competitive assessment.
IV.2.2.2.Geographic market
Notifying Party's views
(30) In line with the Commission's precedents in the field of life science industry,10 the Notifying Party considers that the market for various bioscience products, irrespective of any further product segmentation, is at least EEA-wide in scope. This is mainly due to low transport costs, the absence of regulatory barriers and global presence of suppliers.
Commission's assessment
(31) The market investigation did not provide any indications that the market for biochemicals would have a different geographic scope than other life science products markets dealt with in previous Commissions decisions11.
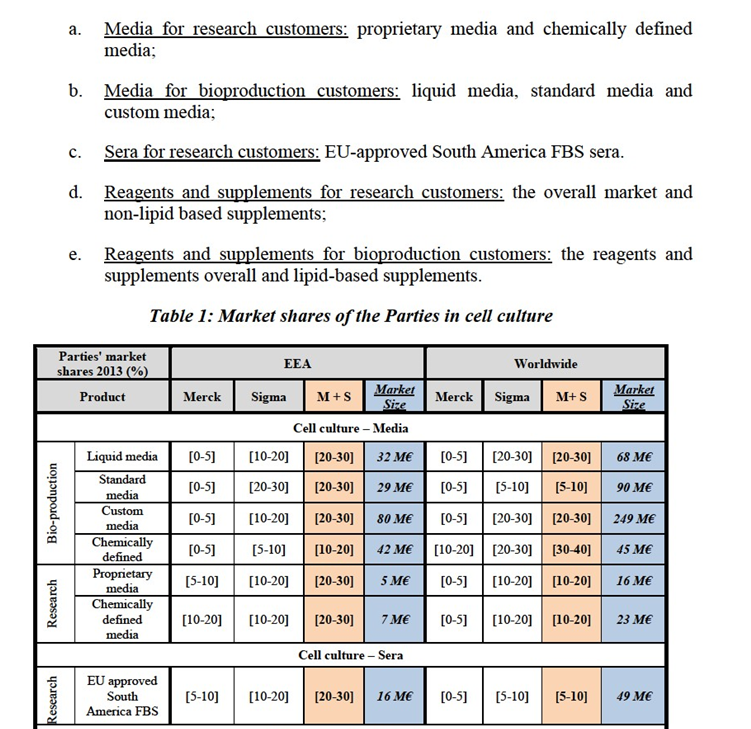
(32) In particular, replies obtained during the market investigation confirmed that suppliers are active on an EEA-wide and even global basis and products are sold under the same brand names regardless of the different geographic areas. Customers also indicated that their sourcing contracts have EEA or global dimension.12
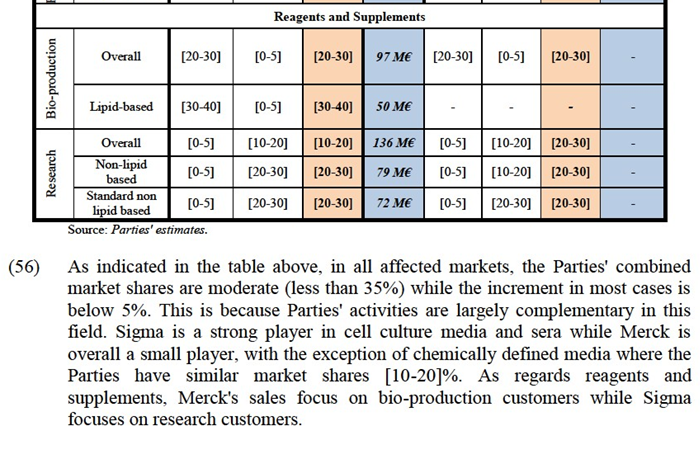
(33) In any event, for the purpose of this Decision, the Commission considers that the precise geographic market definition can be left open as this would not change the outcome of the competitive assessment.
IV.2.3.Competitive assessment
(34) The Transaction gives rise to horizontally affected markets in relation to dyes and stains and carbohydrates, as well as to bioactive small molecules. However, as further described below, the increment brought about by the Transaction is generally limited, large competitors will remain active on these markets, and no significant barriers to switching for customers have been identified.
(35) Specifically, as regards dyes and stains for research, the Parties' combined market share was [20-30]% with an increment of [0-5]% at EEA level in 2013. At worldwide level, the Parties' combined market share was [20-30]% with an increment of [0-5]% in 2013. Post-Transaction, there will still remain a large number of competitors active in this market, such as Thermo Fisher and Bio Techne.
(36) During the market investigation, some concerns were raised as to the Parties' position in dyes and stains. 13 However, further investigation confirmed that the Parties' activities in this field are mostly complementary. Indeed, Sigma is mainly active in dyes and stains for research applications, while Merck is mainly active in dyes and stains for IVD applications.14 Also, some of these complaints seem to stem from the current preference of some customers for the Parties' products and their specific coloring, rather than from the inability of customers to find alternative sources of supply.
(37) As regards carbohydrates, the Parties' combined market share was [20-30]% with an increment of [0-5]% at EEA level in 2013. At worldwide level, the Parties' combined market share was [20-30]% with an increment of [0-5]% in 2013. Moreover, there a large number of large competitors active in this market, such as Thermo Fisher, Bio Techne and Enzo, to whom customers can fairly easily turn to post-Transaction.
(38) As regards bioactive small molecules, the Parties' combined market shares would be below [30-40] % with a limited increment (at most [5-10]% in 2013) both at EEA and worldwide level, and a large number of suppliers in this market, such as Tocris/Techne and Abcam, to whom customers can fairly easily turn to post- Transaction.
(39) With the exception of dyes and stains which have been discussed above, most respondents to the market investigation indicated that they do not expect the Transaction to have any material impact as regards any bioscience products.15
(40) In light of the above, the Commission concludes that the Transaction is unlikely to significantly impede effective competition in relation to biochemicals.
IV.3.CELL CULTURE
IV.3.1. Introduction
(41) Cell culture is the process by which cells are grown in controlled conditions, generally outside of their natural environment. Cell culture is one of the main tools used in cellular and molecular biology, since it provides excellent model systems for studying the physiology and biochemistry of cells and the effects of drugs and toxic compounds on the cells. It is also used in the development of biological compounds (e.g. vaccines and therapeutic proteins).
(42) There are two categories of cell culture products, namely cell culture media (water-based) and cell culture sera (blood-based). They are aimed at supplying nutrients to human, animal, insect and plant to stimulate and support the cell growth in vitro (i.e. outside the living organism). For a complete cell culture, additional reagents and supplements are also required.
IV.3.2.Market definition
IV.3.2.1.Product market
Notifying Party's views
(43) In line with a Commission's precedent,16 the Notifying Party submits that cell culture products can be segmented based on the customer groups at which they are aimed, namely research customers and bio-production customers. This is due to the differences in terms of purchasing patterns, pricing and expected quality. Research customers do not require the same level of testing and validation that bio-production customers must undertake in order to comply with various regulations. As a result of these additional certification requirements bio- production products also tend to be priced at premium compared to research products.
(44) Apart from the distinction by end-customer group, the Notifying Party distinguishes between cell culture media, cell culture sera and reagents and supplements, as well as identifies several sub-categories within each above mentioned products group.
(45) Within media, distinctions can be made based on the form of the media (liquid or dry), the type of media (standard, custom or proprietary) and the use of animal components (chemically defined or non-chemically defined).
(46) Within sera, distinctions can be made based on animal type (Fetal Bovine Serum (FBS), calf serum, adult bovine serum and other species) and geographic origin (from Australia, New Zealand; the US; Canada and South American countries (EU approved)).
(47) Regarding reagents and supplements, the Notifying Party submits that cell culture supplements include lipid-based supplements and non-lipid based supplements.
Commission's assessment
(48) The market investigation confirmed the Commission's findings in one previous case17.
(49) As to the distinction by customer group, respondents to the market investigation indicated that there are differences mainly in terms of documentation requested and certification. Indeed, some suppliers indicated that bio-production customers require higher quality and additional documentation since they are more concerned about the risk of contamination and consistency.18
(50) As to the subcategories of products, some customers confirmed that they are specific and fulfil different needs. By way of example, one customer indicated that "cell culture products are specific for the different types of cells that are cultivated.For example they may need bovine serum or calf serum, activated inactivated etc".19
(51) In any event, for the purpose of this Decision, the Commission considers that the precise product market definition can be left open as this would not change the outcome of the competitive assessment.
IV.3.2.2. Geographic market
Notifying Party's views
(52) In line with the previous Commission's decisions pertaining to the life science industry, 20 the Notifying Party considers geographic market for cell culture products to be at least EEA-wide in scope. This is mainly due low transport costs, the absence of regulatory barriers and global presence of suppliers.
Commission's assessment
(53) The market investigation in this case tends to confirm the cell culture markets to be at least EEA wide, similarly to the markets for bioscience products and for raw materials for pharmaceutical production. This is mainly due to suppliers being active on an EEA wide or even global basis, products being sold under the same brand names regardless of the different geographic areas and customers concluding contracts at EEA or global level.21
(54) In any event, for the purpose of this Decision, the Commission considers that the precise geographic market definition can be left open as this would not change the outcome of the competitive assessment.
IV.3.3. Competitive assessment
(55) The Parties' activities overlap in a number of cell culture products in both customer segments namely research and bioproduction. Specifically, the following affected markets arise as a result of the proposed Transaction in this field:22
(57) The fact that the Parties are not close competitors in cell culture and that Merck is rather small while there are other strong suppliers active was also confirmed during the market investigation23. In this context, one customer explained that "Sigma is quite well known for supply of [cell culture] products, Merck is not. We believe Life Technology [Thermo Fisher] is still number one"24.
(58) Indeed, there are a number of large competitors active in cell culture such as Thermo Fisher who became a clear market leader following its recent acquisition of Life Technologies […]. Moreover, other strong competitors include Lonza and GE, who will continue to exert competitive constraint on the Parties post- Transaction with market shares similar to the one of the merged entity. Finally, cell culture seems to be a segment of life science where a number of specialized niche players are active, which was confirmed during the market investigation. In this context, one customer explained that "there are so many specialised suppliers in this area"25 that any merger-specific impact in these markets is unlikely.
(59) Therefore, while generally the respondents to the market investigation did not indicate any significant impediments to effective competition in relation to cell culture, some customers even expect a positive impact of the Transaction in this field as it may allow creating a stronger competitor vis-à-vis the market leader. For instance, one customer explained that "The current strong position of Thermo Fisher will be weakened, which is desirable from a competition point of view", while another stated that "On the other side a new real competitor could arise for Fisher".26
(60) In light of the above, the Commission concludes that the Transaction is unlikely to result in a significant impediment to effective competition in relation to cell culture.
IV.4.RAW MATERIALS FOR (BIO)PHARMACEUTICAL PRODUCTION
IV.4.1.Introduction
(61) Raw materials for (bio)pharmaceutical production include three broad categories of products, namely active pharmaceutical ingredients (API) and their intermediates; excipients (non-active ingredients used in a final drug dosage form); and biopharm ingredients (process chemicals used in biopharmaceutical processes).
(62) In addition, the Parties both provide various custom API synthesis services consisting of manufacturing API for which the manufacturing process is not well established, as well as process solutions services consisting of a broad and complementary set of support and guidance services to bio-manufacturers and developers.27 However, their activities in these fields are minimal and do no lead to any affected market.
IV.4.2. Market definition
IV.4.2.1. Product market
(63) Raw materials for (bio)pharmaceutical production cover a wide range of products used during the manufacturing process of pharmaceuticals, namely API and intermediates, excipients (non-active ingredients of a final dosage form, such as binders, fillers, diluents, lubricants, flavours, solvents sweeteners or preservatives, with inter alia lactose, starch, cellulose, magnesium, stearic acid, gelatine, sucrose, talc or sodium), and biopharm ingredients, which cover buffers and stabilizers (including inter alia amino acids, carbohydrates and polymers).
(64) Raw materials can be used at different stages of (bio)pharmaceutical manufacturing, namely the upstream phase, the downstream phase and the drug formulation phase. The upstream phase consists in the first manufacturing steps including biosynthesis and working cell bank preparation, up to the first purification steps to isolate the pure protein or peptide. The downstream phase includes processes from the chromatography steps to the final formulation of drugs, including e.g. virus inactivation and nanofiltration. Finally, during the drug formulation phase, excipients and/or adjuvants are added and the drug is filled and finished.
(65) When used in the upstream phase, raw materials do not typically end up in the final drug formulation and therefore generally do not need to meet the strict regulations for final drug formulations. On the other hand, when used in the downstream or drug formulation phases, raw materials have to adhere to Ph. Eur. (chemicals that adhere to Pharm. Eur. are also called compendial grade products) because they are likely to end up in the final drug formulation and thus create safety hazard. Therefore, even if chemically same or similar, the products used in the upstream and downstream phases are different due to different certification and quality control processes.
(66) Pharm. Eur. is legally enforced by directive 2001/83/EC (as amended).28 In order to market a product as being legally compliant with Ph. Eur., the product must comply with each of the specifications set forth in the monograph of the relevant article, and the manufacturer must also have in place a quality system capable of ensuring that the products consistently meet the requirement of the pharmacopoeia (such as a GMP-certified plant).
(67) In this space, the Parties' activities give rise to a horizontally affected market only with regards to GMP/compendial buffers29. Buffers, which may be organic or inorganic, are added to a solution to prevent a rapid change in acidity when acids or bases are added creating a product that has little reaction when it meets other liquids.
Notifying Party's views
(68) In light of the regulatory differences between raw materials used at the earlier stages and at the later stages of the manufacturing process described above, the Notifying Party submits that a distinction shall be made between non-compendial buffers, generally used in the earlier stages (upstream) of the biopharmaceutical process, and compendial buffers, generally used in the later stages (downstream) of the biopharmaceutical process. Indeed, as indicated above, pharmaceutical companies are legally obliged to use compendial grade buffers when they end up in the final drug formulation.
(69) To support their arguments, the Notifying Party explained that there are significant price differences between these two grades of buffers which reflect the substantially different regulatory burden of the two products.
Commission's assessment
(70) The market investigation broadly confirmed the Notifying Party's view that regulatory requirements play a role and vary according to the use of products in the pharmaceutical process, but was not conclusive as to which steps specifically require compendial products and whether there is, at least, one-way demand-side substitutability.
(71) Indeed, some respondents indicated that, for biopharm ingredients such as buffers (as opposed to excipients and APIs), a GMP/compendial grade would not always be required. For instance, one supplier stated: "For other raw materials [which include buffers], to our knowledge, GMP is not required", 30 and another considering that "The other raw materials for biopharma production (e.g. stabilisers and buffers) do not all require GMP certification"31. Customers also explained that the situation of regulatory requirements is unclear in relation to buffers. One customer indicated regarding biopharm ingredients such as buffers that, regarding regulatory requirements, they "file as little as possible", while for their internal requirements "no general rule applies. It could be Ph Eur, other compendia or just relevant parts of these, internal requirements or a combination of these, depending on type of material and use."32
(72) The market investigation however confirmed the trend of an increasing demand from pharmaceutical companies to purchase compendial raw materials, even for early stages (upstream) of the manufacturing process33. This illustrates a mutation in the pharmaceutical industry whereby responsibility on the quality and certification of raw materials for production is being shifted to the supplier. This evolution of customers' requirements is also in line with the last guidelines published by the European Commission in relation to excipients, which clarify the risk management procedure to be carried out by the manufacturing authorization holder regarding whether the appropriate GMP have been applied.34
(73) As to the supply-side substitutability, the market investigation confirmed that the supply of compendial buffers requires specific equipment, and in particular GMP plant certification which are not available for everyone and require substantial investment. In this respect one competitor currently not active in this area explained that "the investment would be significant to produce these chemicals on our own and would include the building of new manufacturing facilities".35
(74) In any event, for the purpose of this Decision, the Commission considers that the precise product market definition with respect to buffers, and in particular whether a distinction should be made between compendial and non-compendial buffers, can be left open as this would not change the outcome of the competitive assessment.
IV.4.2.2. Geographic market
Notifying Party's views
(75) The Notifying Party submits that, similarly to other life science products, the geographic market for the supply of raw materials for (bio)pharmaceutical production is at least EEA-wide. The Notifying Party indicates that the main suppliers sell their raw materials for pharmaceutical production on an EEA-wide basis and sometimes global basis, and use the same brand names throughout the EEA. Furthermore, the Notifying Party submits that there are no regulatory barriers that would prevent the sale of a product throughout the EEA, and that transport costs of such products are very low.
Commission's assessment
(76) The market investigation confirmed that suppliers are generally selling their raw materials for pharmaceutical production (which include buffers), under the same brands at European or even global level.36
(77) Contrary to laboratory chemicals (see section IV.5), the customers' demands are far less country-specific as the customer base is much more concentrated,37 with key customers being global or at least EEA-wide pharmaceutical manufacturers.
(78) Also, the market investigation did not reveal any other element, such as national pricing or local competitive landscape, which would tend to indicate a narrower geographic dimension than the EEA. Indeed, as detailed below, contrary to laboratory chemicals, there is no regional/local players active in these markets, but rather major chemicals manufacturers' active worldwide.
(79) Finally, as to compendial raw materials, the main regulations (GMP manufacturing and adherence to Pharm. Eur.) are defined at EEA-level.38
(80) In light of the above, and in line with the past decisions concerning raw materials for pharmaceutical production such as APIs,39 the Commission considers, for the purpose of the present Decision, that the markets for raw materials for bio- pharmaceutical production are at least EEA-wide in scope, while the specific market for compendial buffers is EEA-wide in scope.
IV.4.3. Competition assessment
(81) As regards biopharm ingredients, and in particular buffers for pharmaceutical production, the Parties have complementary activities. Merck only supplies compendial buffers (mainly inorganic) whereas Sigma mostly supplies organic buffers, the great majority of which are non-compendial. The Transaction thus only gives rise to a horizontally affected market in relation to compendial buffers.40
(82) The Parties' combined market share in the market for compendial buffers in the EEA in 2013 is moderate (below [20-30]%), with Sigma bringing a de minimis increment ([0-5]%). Also, a number of sizeable competitors such as Jungbunzlauer, Angus and Chemische Fabrik Budenheim, each of them with markets share above 10% (and up to [20-30]%), will continue to be active post- Transaction and exert competitive pressure on the merged entity.
(83) During the market investigation, some respondents indicated that the Parties are direct competitors in buffers.41 Nonetheless, the additional market investigation confirmed the small position of Sigma in the area of buffers for biopharmaceutical production, with one important supplier mentioning that "to [its] knowledge, Sigma is only selling small quantities of organic buffers for bio- pharmaceutical production. [It] does not consider Sigma as an important competitor in this field." 42 Similarly, only a limited number of customers indicated purchasing biopharm ingredients (which are already a larger category of production chemicals than buffers) from Sigma.43
(84) In light of the above, the Commission concludes that the Transaction is unlikely to significantly impede competition in relation to the markets for raw materials for (bio)pharmaceutical production and buffers in particular.
IV.5.LABORATORY CHEMICALS
IV.5.1.Introduction
(85) Merck and Sigma are both leading suppliers of laboratory chemicals in the EEA. They offer a wide portfolio of products to all customer segments, including universities, governments, pharmaceuticals, chemicals, cosmetics and food companies, as well as medical, clinical and diagnostic companies.
(86) The value chain for laboratory chemicals comprises the following main steps: production (including the down-filling or any other processing), marketing and rising of brand awareness and distribution to the fragmented customer base.
(87) Laboratory chemicals sold by Merck and Sigma in the EEA are either produced in-house or sourced from third party manufacturers. Even if the product is sourced from a third party bulk manufacturer, added value resides in the additional processing which is carried out by Merck and Sigma. This additional processing, depending on the chemical, may consist of quality control, packaging, down-filling, and/or labelling of the product under the Parties' own brands.
(88) As detailed further below, quality is an important factor in these markets. To differentiate themselves suppliers develop brands which are recognised by customers and represent, quality, certain set of specifications and consistency of the product. Both Merck and Sigma have strong brands, which are among the most recognized in the market.
(89) As to the distribution of laboratory chemicals, there are two main models, namely direct distribution, typically comprising a sales force and an e-commerce platform, or indirect distribution through exclusive or non-exclusive distributors who may be in charge of the marketing and promotion of the products.
(90) The competitive assessment of the Transaction contained in the following sections of this Decision focuses on the horizontal overlaps between the Parties' activities as suppliers of laboratory chemicals, and more specifically of solvents and inorganics in the EEA.
IV.5.2.Market definition
IV.5.2.1. Product market
(91) Laboratory chemicals are chemicals used for research, testing and quality control purposes and comprise thousands of different chemicals belonging to various chemical groups.
(92) The Commission has not so far analysed the market for laboratory chemicals.
(93) The Notifying Party submits that, within laboratory chemicals, several segmentations can be envisaged. Specifically, laboratory chemicals can be first segmented based on the product category, and within each category the intended use of the chemicals, that is chemicals used for multi-purpose analysis (classical analysis) or together with certain instruments and techniques (instrumental analysis). Second, laboratory chemicals can also be segmented based on the volume per unit sold into small-size catalogue format and bulk format.
(94) In order to determine the scope of the relevant product market in relation to laboratory chemicals, the Commission will assess different possible segmentations.
IV.5.2.1.a.Segmentation based on the product category and intended use
(95) The Parties' activities overlap in six categories of laboratory chemicals namely (i) solvents, (ii) inorganics, (iii) organics, (iv) standards and reference materials, (v) analytical chromatography and (vi) industrial microbiology.
Solvents
(96) In life sciences, analysis or synthesis of any given material may require the use of a solvent. Solvents are used to dissolve the target substance (a chemically different liquid, solid or gas). The resulting solution can then be used (i) for classic laboratory analysis or (ii) for instrumental analysis through techniques such as gas chromatography, liquid chromatography, or Nuclear Magnetic Resonance spectroscopy. Examples of solvents include acetonitrile, n-hexane, methanol, acetone, chloroform, ethanol, and even water.
(97) Solvents for classical laboratory analysis are mostly differentiated by their level of purity. Technical grade solvents are cost-efficient products used by laboratories, with lower purity levels (approximately 95% to ≥99%), while solvents for regulated industries generally attain a higher degree of purity and are used by pharmaceutical manufacturers. Finally, dried and anhydrous solvents are high-purity solvents with very low water content, used for moisture sensitive organic and biotech applications, such as chemical synthesis, high throughput screening, and organometallic synthesis and for moisture analysis.
(98) Solvents for instrumental analysis can be distinguished by the instrumental technique used. The most typical analytical techniques include various categories of spectroscopy, electrochemistry, chromatography polymer analysis, capillary gel electrophoresis, gas chromatography, liquid chromatography and mass spectroscopy.
Notifying Party's views
(99) The Notifying Party submits that, while from a demand-side perspective a distinction may be made between solvents used for classical laboratory analysis and solvents for instrumental analysis, there is a high degree of supply-side substitutability indicating that all solvents can be considered as part of a single relevant product market. Indeed, the Notifying Party submits that different solvent categories are produced using broadly similar equipment. Suppliers purchase raw materials in bulk, and then apply purification, distillation, special drying and quality control procedures to achieve desired level of purity and meet the requisite standard for each category of solvents. The Notifying Party also argues that solvents are not expensive or complicated to manufacture and their chemical formulae are not protected by patents or any other intellectual property which could limit the supply-side substitutability.
Commission's assessment
(100) The market investigation broadly confirmed the Notifying Party's views. From a demand-side perspective, product specifications, and in particular the level of purity and their suitability to certain specific techniques, make that various solvents are generally not substitutable in their specific use by customers44.
(101) The replies received during the market investigation also indicated a high degree of supply-side substitutability between all types of solvents. Most of the suppliers of solvents confirmed that they possess the adequate equipment and supply the whole range of solvents for classical analysis (at various level of purity and quality standards) and for instrumental analysis (such as HPLC, GC and spectroscopy).45
(102) These findings are consistent with the fact that most of the raw chemicals are actually produced by third-party bulk manufacturers, while laboratory chemicals suppliers apply standard purification, distillation and other processes to transform the product or make it adapted for the use in a laboratory environment. This in many cases implies quality control and certification of product specifications, which guarantee the precision and accuracy in standardized testing processes.
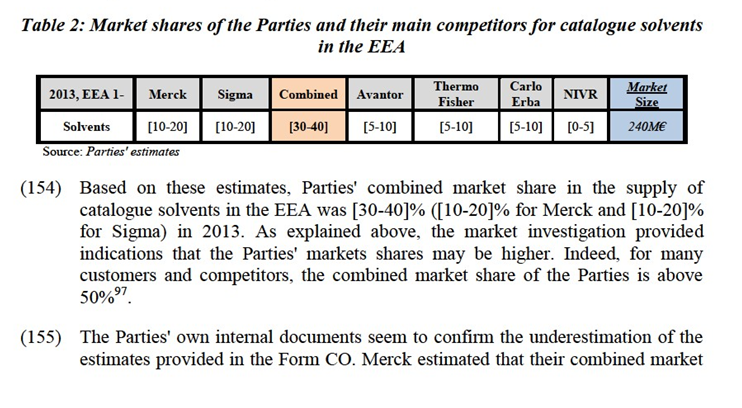
(103) In any event, for the purpose of the present Decision, the precise product market definition with respect to solvents, and in particular the potential distinction between solvents for classical laboratory analysis and solvents for instrumental analysis, as well as the sub-segmentations thereof, can be left open as this would not change the outcome of the competitive assessment.
Inorganics
(104) Inorganics are reagents meaning substances or compounds added to a system in order to bring about a chemical reaction or to see if a reaction occurs. The primary difference between organic and inorganic compounds is that organic compounds always contain carbon, while most inorganic compounds do not. Similarly to solvents, inorganics can be used for classical laboratory analysis and for instrumental analysis. Inorganics also includes auxiliaries, which are ancillary products such as absorbents for spilled liquids or drying agents used in association with inorganics.46
(105) Inorganics for classical laboratory analysis include a variety of compounds used in analytical processes, primarily for research, quality control, in-process quality control with qualitative and quantitative analytical chemical analysis, and biopharma and chemical manufacturing applications. They include the following categories:
a. acids: chemical substances whose aqueous solutions are characterized by the ability to react with bases to form salts and with most metals (like iron) to form salts and hydrogen;
b.bases: chemicals that in aqueous solution, react with acids to form salts, and promote certain chemical reactions (base catalysis);
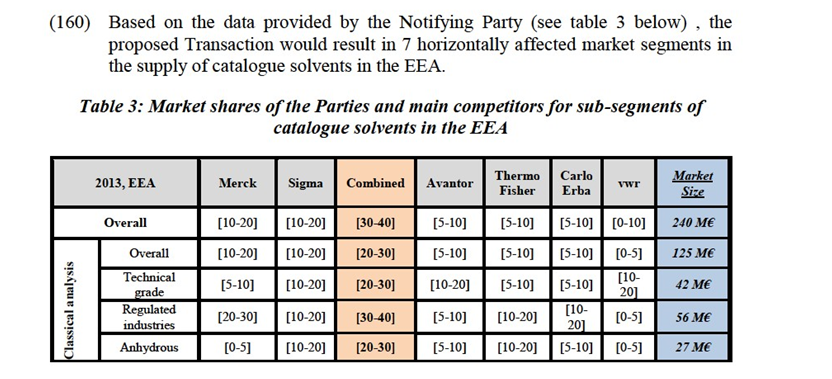
c. buffers: aqueous solutions used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications;
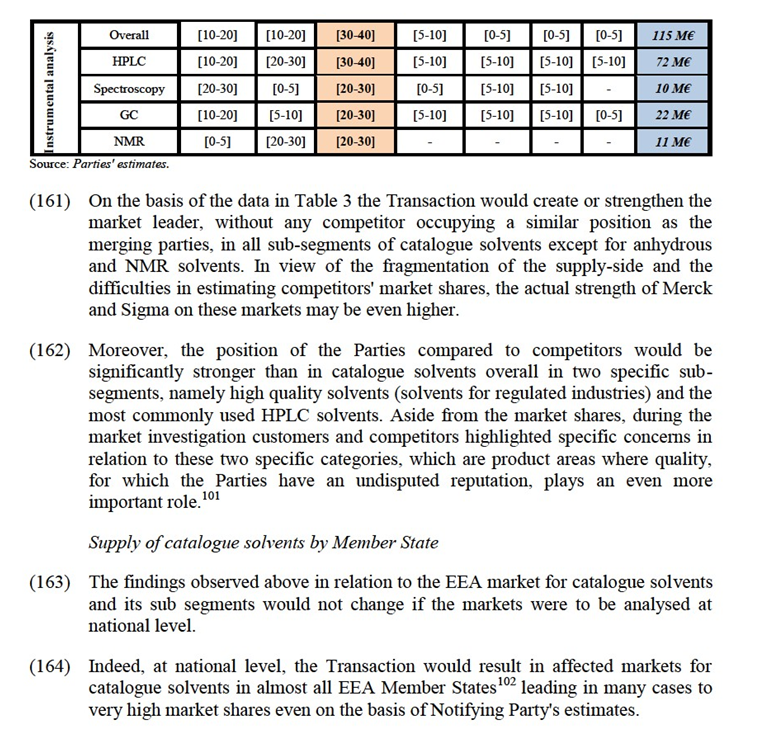
d. salts: ionic compounds that result from the neutralization reaction of an acid and a base, used in both qualitative and quantitative analysis of substances and substance mixtures;
e. metals/elements: materials (whether compounds, alloys or elements) that have high electrical conductivity, high thermal conductivity, and high density, used in a multitude of applications in R&D laboratories, production departments and in quality control for the chemical industry, the manufacture of ceramics or electronic components, or in food analytics.
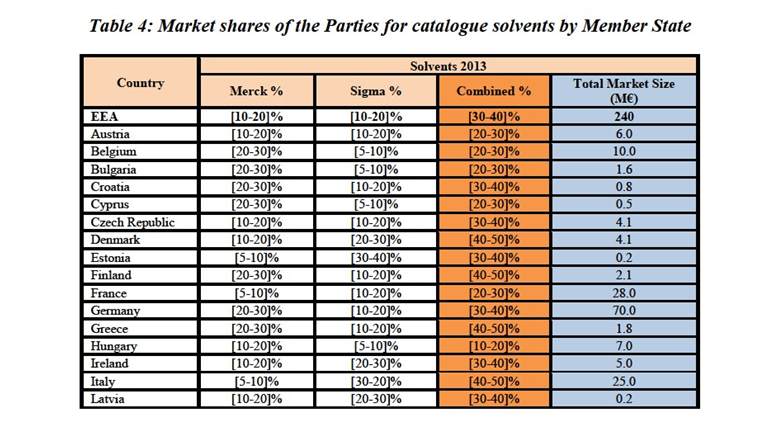
(106) Inorganics for instrumental analysis are sold as ready-to-use (pre mixed) materials for specific applications where customers require a high degree of precision in the results. In this case, the formulations and combinations of the different inorganic compounds are pre-set to minimize the risk of error in the analysis. Inorganics for instrumental analysis can be further distinguished on the basis of the applications for which they are designed, such as volumetric and titration solutions (used to determine the unknown concentration of any substance), inorganics used for water determination, sample preparation, calibration and qualification of analytical instruments, or X-ray fluorescence analysis.
(107) Auxiliaries are ancillary products used in association with inorganics. Key product groups under this category are cleaning reagents, silica gels, charcoals, absorbents for spilled liquids, filter aids and molecular sieves.
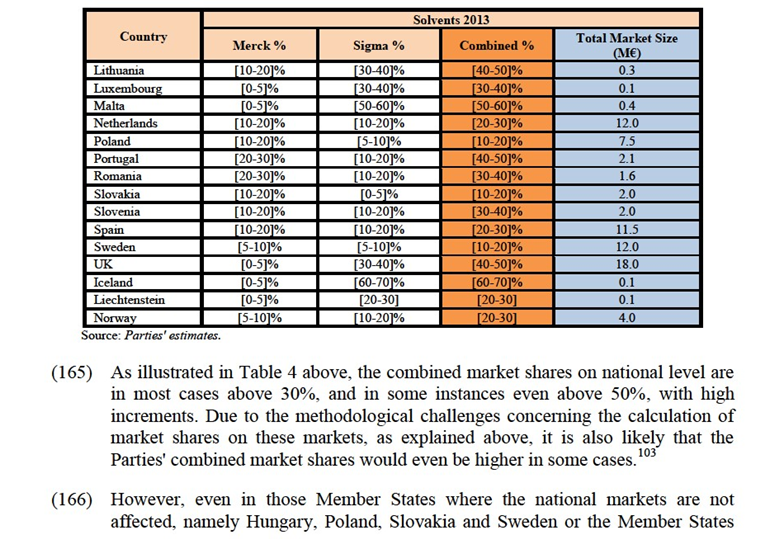
Notifying Party's views
(108) The Notifying Party considers that from demand-side a distinction can be drawn between inorganics used for classical analysis, inorganics used for instrumental analysis and auxiliaries. The products belonging to these categories are used in different applications, have a different chemical composition, and, thus, are not substitutable from the perspective of customers. Regarding auxiliaries in particular, the Notifying Party submits they are complementary, rather than substitutable, to other inorganic reagents, even if they are normally purchased together. However, from a supply-side perspective, the Notifying Party indicates that all inorganics are generally produced using similar manufacturing equipment, as the equipment used in the production plants is multi-purpose and can be used for different categories of inorganics. Also, the Notifying Party considers that inorganics are generally manufactured based on well-known processes in the industry.
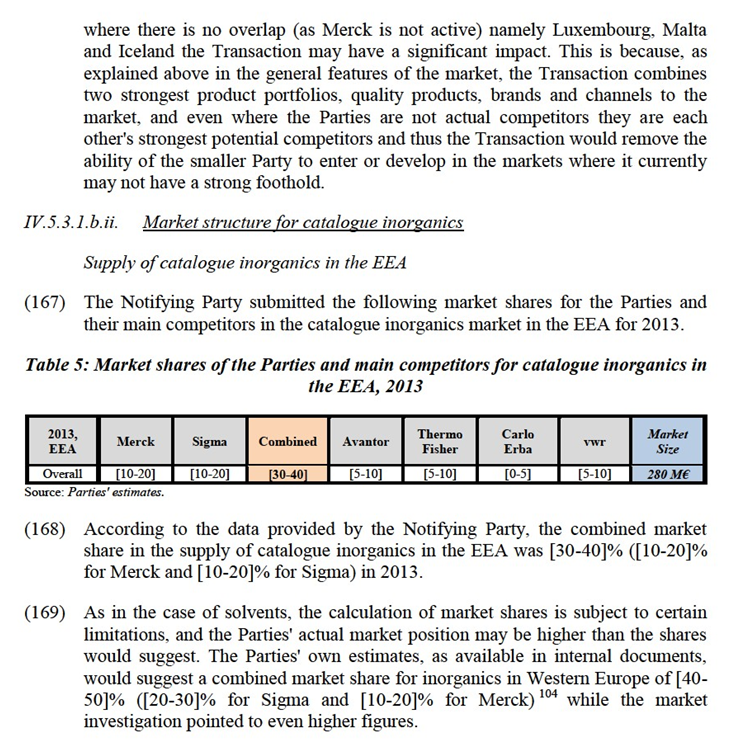
Commission's assessment
(109) The replies obtained in the market investigation partly confirmed the Notifying Party's view. From a demand-side perspective, the market investigation confirmed that inorganics can normally not be substituted by other chemicals for most of their applications, and that each specific category of inorganics (e.g. buffers) serve a specific purpose and cannot be substituted with other categories of inorganics. From a supply-side perspective, many suppliers of inorganics indicated that they supply a wide range of inorganic chemicals.47 However, the market investigation also indicated that for certain categories of inorganics the supply-side substitutability may be hindered by the existence of know-how and/or IP. For instance, high purity inorganics 48 or Karl Fischer titration solutions49 require specific equipment and know-how to be produced. Regarding Karl Fischer titration solutions, while the original technology is no longer patent- protected, Sigma owns IP rights and related know-how to a so called "second- generation" Karl Fischer titration technology.
(110) In any event, for the purpose of the present Decision, the precise product market definition with respect to inorganics, and in particular the potential distinction between inorganics for classical laboratory analysis and inorganics for instrumental analysis, as well as the sub-segmentations thereof, can be left open as this would not change the outcome of the competitive assessment.
Organics and other laboratory chemicals
(111) As opposed to inorganics, organics are reagents containing carbon-hydrogen (CH) bonds. Almost all molecules associated with living organisms are organic. Within organics, the Notifying Party identifies three categories of products where the Parties' activities overlap namely organic building blocks, synthesis reagents and catalysts.
(112) Organic building blocks are used in organic synthesis to construct new organic compounds by means of organic reactions. Synthesis reagents are substances to alter other chemicals, such as building blocks or larger molecules derived from combinations of building blocks, or for conditioning reaction mixtures. Catalysts are substances which are used in sub-stoichiometric amounts relative to the other reactants that accelerate their reaction or even cause reactions to occur when they otherwise would not occur without the catalyst and are generally compositionally unaltered throughout the reaction.
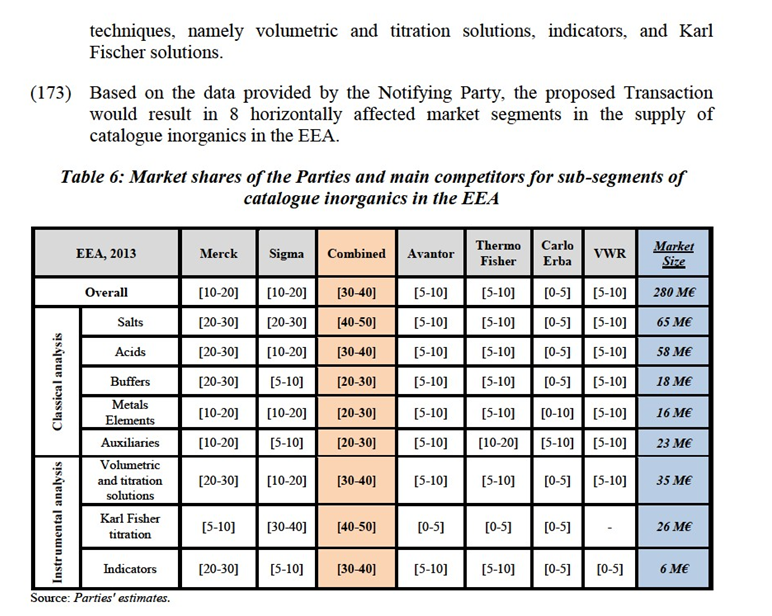
(113) In addition, the Notifying Party identifies three other laboratory chemicals categories where their activities overlap, namely standards and reference materials, analytical chromatography and industrial microbiology50.
(114) Reference materials are substances to support measurements concerned with chemical composition, biological, clinical, physical, engineering properties and other areas such as taste and odour. Suppliers of reference materials must follow ISO Guide 34 when they are accredited. In this area, the Parties' activities overlap in the following categories: HPLC standards, GC standards, UV Vis Standards and elemental standards.
(115) Analytical chromatography is an analytical process used to separate out one or more target substances from a sample in order to confirm the target's presence, or its concentration. In this area, the Parties' activities overlap with respect to HPLC columns, TLC plates and analytical sample preparation.
(116) Industrial microbiology includes products and services to detect the presence of specific microorganisms in a laboratory and workspace. Within industrial microbiology, the Notifying Party distinguishes media and instruments. Within the media category, the Notifying Party further distinguishes between dehydrated culture media, ready-to-use media (with a sub-segmentation between solid and liquid) and raw materials and culture media supplements .
(117) In any event, for the purpose of this Decision, the precise product market definition in relation to organics and other laboratory chemicals can be left open as this would not change the outcome of the competitive assessment.
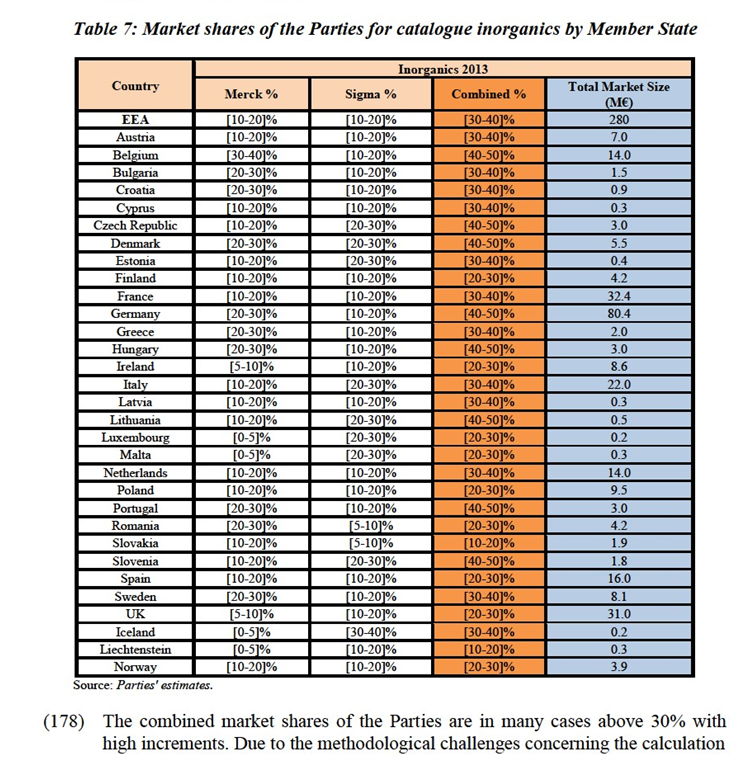
IV.5.2.1.b.Segmentation based on the size: catalogue and bulk formats
(118) The Notifying Party considers that the product market should be segmented according to the size of the unit sold into catalogue and bulk laboratory chemicals, whereby catalogue sales should include volumes up to 10 kilograms or litres per unit sold. The Notifying Party argues that, despite the fact that the products have the same characteristics from a chemical perspective, the purchasing patterns, customer categories and pricing can be quite different between those two categories of products. Bulk chemicals are used by end customers in the manufacturing industries as materials for analytics, quality control, small scale synthesis or even cleaning. Conversely, due to the inflammable, explosive, toxic and/or corrosive nature of many laboratory chemicals, and the fact that many testing research activities require only small amounts of chemicals, laboratories typically purchase laboratory chemicals through catalogue in small volumes.
(119) The market investigation broadly confirmed the Notifying Party's view, providing indications that laboratory chemicals sold in catalogue or bulk format evolve in different supplier landscapes, have different customer bases and pricing51.
(120) Concerning the customer base, the market investigation confirmed that bulk laboratory chemicals are dedicated to industrial customers having important needs. On the other hand, laboratories, having limited needs and using the products in small quantities, are not purchasing bulk volumes because of storage issues, safety requirements for refilling of hazardous materials, risks of obsolete materials and contamination during the refill.52 In this context one laboratory customer explained that "bulk material typically cannot be handled in laboratories or small scale manufacturing units [...] would not have the required equipment for repackaging. Furthermore even if repacked it would not be possible to use repacked volumes long term as chemicals have a maximum durability".53
(121) Concerning the supply-side substitutability, the market investigation confirmed that, while the majority of companies active at the catalogue level also supply some bulk volumes54, main chemicals manufacturers, such as BASF, Ineos and Dow, are only active with respect to bulk sales. This is because the business model of bulk manufacturers is not adapted to supplying small volumes to a fragmented customer base, irrespective of the price.55
(122) As to the definition of the threshold above which a volume of laboratory chemicals shall be considered as bulk, the market investigation indicated that it would generally be volume up to 10 kilograms or litres.56
(123) In light of the above, the Commission considers that for the purposes of assessing the competitive effects of the Transaction, a distinction should be made between catalogue sales (smaller-size volumes up to 10 kilograms or litres) and bulk sales.
IV.5.2.2. Geographic market
(124) The Notifying Party considers that the geographic dimension of the markets for the supply of laboratory chemicals is at least EEA-wide, following a similar reasoning than for bioscience products and raw materials for pharmaceutical production. First, the main players, such as the Parties and Thermo Fisher, are active on an EEA wide and even global basis. They sell their products under the same brand names throughout the EEA and ship their products from a limited number of warehouses located in the EEA. Second, an increasing number of international customers negotiate supply contracts covering their EEA or even global operations. Third, there is no intellectual property right or regulatory barriers that would limit the trade flows and transportation costs for laboratory chemicals would be low.
(125) The market investigation confirmed that suppliers are generally selling their laboratory chemicals under the same brands at European or even global level57. However, some elements pointed at a narrower geographic market definition than the EEA. Indeed, if some important customers negotiate contracts at European or even global level,58 the market investigation indicated that many customers negotiate their purchasing contracts at regional or national levels59 and suppliers organise their sales force symmetrically at regional or national level.60 This is because the customer base for laboratory chemicals is very fragmented, with numerous local customers, in particular research centres which require a local sales force/technical support.
(126) Also, even if some suppliers and customers indicated that prices are homogeneous at the EEA or Western Europe level, others mentioned price discrepancies (excluding transportation costs) between different EEA countries61. These price differences would not only be due to different distributors' margins, but also to specific customer demand and competitive and regulatory landscape. One competitor indicated that "buying methods and criteria differ [from] country to country",62 while another competitor stressed the "regional variations" within Europe, such as the language and "drivers of competition".63 Indeed, as to the competitive landscape, there are a number of regional and local suppliers of laboratory chemicals, such as PanReac Applichem (Germany), Carl Roth (Germany), Romil (UK), Rathburn (UK), and Armar (Switzerland).
(127) As to the applicable regulations, the Union legislation provides for certain packaging, labelling, transport and storage rules for these products64. There are also national requirements and regulations, such as rules on target individual container size, "ethanol taxes, ethanol denaturation methods, animal origin rules, storage and transportation requirements".65
(128) In any event, the exact geographic definition for the laboratory chemicals market and its sub-segments can however be left open because serious doubts arise from the Transaction irrespective of whether the markets for solvents and inorganics are considered at national or EEA level.
IV.5.3. Competition assessment
(129) Merck and Sigma are the two main suppliers of laboratory chemicals in the EEA. While they are present and widely recognised players across the whole spectrum of laboratory chemicals, such presence is more significant and has distinctive features in the markets for catalogue solvents and inorganics in the EEA. These markets are assessed in Section IV.5.3.1 of the present Decision66.
(130) The affected markets in the supply of organics and other laboratory chemicals, where the increment of market share is less significant, are also assessed in the present Decision in section IV.5.3.2.
IV.5.3.1. Solvents and inorganics
(131) This section will first present a general description of the main competitive characteristics which are common to solvents and inorganics markets (IV.5.3.1.a). Then, the markets structure for catalogue solvents and catalogue inorganics will be analysed (IV.5.3.1.b) and finally the main barriers to entry on these markets will be assessed ((179)).
IV.5.3.1.a. General competitive features of catalogue solvents and inorganics markets
IV.5.3.1.a..IV Reliability of market share data
(132) From the outset it should be noted that the market share data provided by the Notifying Party on these markets cannot be fully reliable in light of methodological challenges and the absence of public sources. Specifically, difficulties in providing an accurate overview of the market are mainly related to the presence, across all solvents and inorganics segments, of a substantial fringe of local smaller scale competitors and to the need for accounting for a distributor's margin in the cases where suppliers do not sell their products directly.
(133) During the market investigation, the Commission obtained turnover data for laboratory chemicals overall 67 and market share estimates for solvents and inorganics68 from the Parties' competitors. This exercise (with all its limitations) did not enable the Commission to fully reconstruct the market, but suggested, together with data from the Parties' own internal documents, that the Notifying Party may have underestimated the Parties' respective market shares.
(134) In any event, and irrespective of the exact market shares, the Commission's assessment in this case is complemented by qualitative elements collected during the market investigation which, taken together, reflect the competitive features of the markets for catalogue solvents and inorganics and the Parties' real position on the markets.
IV.5.3.1.a..IV Quality and brand recognition
(135) According to the Notifying party, solvents and inorganics are "basic commoditized" and "non-differentiated" products which can be supplied by a large number of companies with relative ease.
(136) However, the market investigation indicated a completely different reality. While the manufacturing of the basic chemical may be a commoditized process, selling laboratory chemicals requires a highly specialized business model. Because these products are used in standardized testing protocols, where high precision and accuracy are required, it is of utmost importance that customers are sure that the chemicals they purchase are compliant with the exact specifications prescribed by the protocol. Indeed, any minimal difference in the chemical composition or purity of a solvent or an inorganic may affect the behaviour of chemical substances handled in a laboratory and thus testing results.
(137) As a result, the market participants consistently stressed that quality, batch to batch consistency and reliability are key drivers of competition in the laboratory chemicals market, and that price considerations are to a large extent secondary69. This enables top quality producers, such as Merck and Sigma, to command price premiums.70
(138) It should also be stressed that quality of the product in this context refers not only to the chemical composition or the purity of the product but also to the level of confidence in the documentation and quality of the labelling which are particularly important due to the strong safety hazard in these markets. This quality is generally perceived in the market place through brands.
(139) The market investigation consistently indicated that customers acknowledge the importance of brands and associate quality with brand names.71 In this context one customer explained that "the reputation of a brand is a guarantee of quality, expertise and confidence".72 This is all the more important for certain segments of customers, such as pharma customers, which want to mitigate all risks and are thus generally less price sensitive. For instance, one customer indicated that brand recognition in these markets plays "a big role", since "a customer does link the brand with a good quality product, specifically in the pharma sector a good reputation plays an important role"73.
(140) Against this background, the market investigation consistently indicated that the Parties are the two players offering the catalogue solvents and inorganics of distinctively highest quality, and own the strongest brands in the market commanding a price premium.74 These are two of the key factors explaining their strength and position as market leaders across all segments, which are not matched by any other competitor.
(141) By way of example, one customer indicated that "Sigma and Merck offer laboratory chemicals of top quality. Their competitors' products, such as the products sold under VWR Prolabo brand, would be of lower quality [...] The products of Sigma and Merck are more expensive than their competitors due to the quality [...] [Company name]'s internal policy is quality and safety driven and it is willing to pay the 30% or 40% premium for Merck and Sigma-Aldrich's higher quality products [...] Merck and Sigma have a dominant role in the Lab chemicals market in Europe, in particular in the quality segment where they are de facto the only players"75. Similarly, other customers indicated in this context that the "first aspect to be considered is the quality. Second can be the economic reason"76 and that "brand is important because of the reliability of purchased products: we don't need to control product every time [and we are ready to pay a premium price] if it ensures quality"77.
(142) This perception is also shared by Parties' competitors78. One of them indicated that "Merck and Sigma are recognized as very high quality products and to quality sensitive customers they can certainly be defined as each other closest competitor and in many instances de facto the only options [...] Merck and Sigma are recognized as high quality brands without equivalent"79. Similarly, another competitor indicated that "brand recognition plays a very important role in the market of laboratory chemicals. Even if our company is capable of supplying some laboratory chemicals at a less expensive price than Merck and/or Sigma, customers often prefer their products to ours"80.
(143) The Parties' own internal documents also indicate that Merck and Sigma supply high quality catalogue solvents and inorganics under well-known brands. For instance, Merck when analysing the competitive landscape of the market considers one of the Parties' main competitors, [competitor name], as "not en par with SAF [Sigma] and MM [Merck] in terms of expertise & quality"81. It is also expressly stated that "[competitor name] plays in low tier chemicals" and that the "[…] competitors [have] low tier offerings" 82 . As to inorganics specifically, Merck and Sigma are considered as both having strong brands and good reputation 83 , whereas [competitor names] are generally considered as having "lower quality" products84.
IV.5.3.1.a..IV Breadth of the product portfolio
(144) Aside from product quality and brands, another important parameter of competition is the breadth of the product portfolio. This is explained by the fact that there is a wide variety of solvents and inorganics purchased by the customers and used in testing and quality control protocols. On the other hand, suppliers typically strive to supply the whole spectrum, often under the same brand.
(145) In this respect, the market investigation clearly indicated that the product portfolio is one of the main competitive parameters in these markets and represents key strength for both Merck and Sigma in this space. 85 Market investigation clearly indicated that Merck and Sigma are each other's closest competitors in relation to the breadth of the portfolio which is also evidenced by their respective market positions as overall laboratory chemicals number one and two with other competitors lagging behind. Some respondents expressly indicated that "no one can offer a comparable portfolio as Merck or Sigma"86 and that, in comparison, "Thermo Fisher has a small chemical portfolio"87.
(146) This is also confirmed in Merck's internal documents. Merck indicated that Sigma "[quote of internal assessment]"88. In another document Sigma is considered as Merck's "[quote of internal assessment]"89 for classical solvents and inorganics. As to inorganics specifically, Merck and Sigma are considered to have the widest product ranges 90 , whereas the other players would have "[quote of internal assessment]"91.
IV.5.3.1.a..IV Channels to the market
(147) Customers of solvents and inorganics are typically companies and other institutions, such as academic institutions, which have a research facility or a laboratory where they perform testing or quality control of their products. Sales are therefore scattered across a wide number of customers, which often need small quantities of several products from the portfolio. In order to be able to compete on this market, it is thus essential to own or have access to an efficient distribution system, also enabling a quick time of delivery of often hazardous or temperature sensitive material.
(148) The market investigation confirmed the importance of an efficient distribution system92 and suggested that Merck and Sigma have access to two of the most efficient channels to the market in the supply of catalogue solvents and inorganics in the EEA, with few other competitors being able to match their efficiency. These findings are in line with the market analysis of the Parties as outlined in their internal documents.
(149) Sigma's business model is focused on direct sales to customers and a very quick delivery, within 24 hours. Merck's internal documents indicate that Sigma is "[quote of internal assessment]"93 and that its "[quote of internal assessment]"94.
(150) In this context Sigma's e-commerce platform is widely considered as being the most sophisticated of the market, including by Merck referring to Sigma's platform is identified as "[quote of internal assessment]"95 . This is clearly a



of market shares on these markets, as explained above, it is also likely that the Parties' combined market shares would even be higher in some cases109.
(179) However, even in those Member States where the national markets are not affected, namely Slovakia, or the Member States where there is no overlap (as Merck is not active) namely Malta, Lichtenstein and Iceland, the Transaction may have a significant impact. This is because, as explained above in the general features of the market, the Transaction combines two strongest product portfolios, quality products, brands and channels to the market, and even where the Parties are not actual competitors they are each other's strongest potential competitors and thus the Transaction would remove the ability of the smaller Party to enter or develop in the markets where it currently may not have a strong foothold.
IV.5.3.1.c.Barriers to entry to the catalogue solvents and inorganics markets
(180) Despite the leading position and closeness of competition of Merck and Sigma in the whole EEA, the Notifying Party argues that, in any event, anticompetitive effects could be excluded since barriers to entry in the markets for the supply of catalogue solvents and inorganics are low. In this context the Notifying Party reiterates that suppliers of catalogue solvents and inorganics (which are often purchasing raw chemicals in bulk from the large chemical manufacturers) provide limited added value services, such as quality assurance and control, or purification, to the product originally produced by bulk manufacturers. Contracts with third party manufacturers would be widely available and raw materials producers, such as Ineos, Akzo Nobel and BASF, which are already present in respect to bulk sales, would be capable of entering the catalogue market quickly if a hypothetical price increase made such entry attractive. Finally, customers would easily switch among brands given the identity of the products purchased.
(181) The market investigation clearly contradicted the Notifying Party's views. In line with the general features of the market exposed above, the barriers to entry to these markets are considerable.
(182) First, the market investigation indicated that, because of brand loyalty, customers do not often switch suppliers and brand recognition requires years of work for a new supplier to establish a brand and more generally presence on these markets. As explained by one competitor, "once a relationship of trust is established, customers of solvents and inorganics become rather "sticky", i.e. they tend not to change suppliers unless so prompted by a significant increase in price, noticeable decrease in quality or chronic problems with ordering or delivery occur. The brand name epitomizes this relation of trust between supplier and customer"110. This analysis is also shared by many customers. One of them considered that "brand recognitions is the result of years of work of delivering high quality fine chemicals paired with strong customer relationships. In the fine chemical market, it is important to have the reliability in the products delivered because they impact the quality of testing results directly and therefore the decision made on the results indirectly"111
(183) Second, the market investigation identified the economies of scale and scope as another main barrier to entry in the catalogue solvents and inorganics markets. The utilization rate of a plant would typically be low and sales of solvents and inorganics in small quantities appear to be a viable activity only if a supplier can propose a wide portfolio of chemicals to an important customer base. In this respect, it is crucial for any party willing to enter this market to be able to offer a sufficiently broad product portfolio across the spectrum of solvents and inorganics. This analysis was confirmed by market participants112. For instance, one competitor considered that "this business needs to supply at least thousands of items of small scale chemicals. Nobody knows which item sells well in advance, so many items must be stored in each area to be delivered quickly. So sales will be relatively small compared to cost of inventory, test, repack and delivery"113. Another competitor stressed that "to be a credible supplier in laboratory chemicals requires the stocking of a very wide range of products. Additionally there is a significant degree of know-how required to be able to handle and distribute a wide range of laboratory chemicals both safely and in line with the required regulations".114
(184) Third, know-how and IP rights are also important barriers to entry in relation to some, typically most profitable, products. In particular, as to inorganics, Sigma had a long patent protection on first generation Karl Fisher titration solutions, from 1980 to 2000. On 2001, Sigma was granted a new patent protection for a second generation Karl Fisher titration solution, which will expire in 2021.
(185) To summarize, one competitor indicated that it is very difficult to enter the market for supply of catalogue solvents and inorganics because "the barriers of the recognized brand, the investment to perform the quality checks, the know-how, the sales force, and the regulatory aspects are too high"115.
(186) Thus, unsurprisingly, main large chemical manufacturers such as Ineos, Akzo Nobel, BASF and Dow, confirmed during the market investigation that they are unlikely to enter the markets for catalogue solvents and inorganics since it is a "different business model" from their current activities and because of the "lack of customer relationship" and the "investments needed to fill/repack".116
(187) Indeed, main chemicals manufacturers commonly supply solvents and inorganics in bulk format (e.g. rail car or tanker truck volumes) where they find it economically attractive, but do not intend participating in the sale of smaller laboratory catalogue volumes, which would require individual bottling/packaging facilities and stringent quality control to ensure these chemicals can be reliably used by the laboratories in their testing protocols.
(188) This was overwhelmingly confirmed in the market investigation. Bulk manufacturers explained that they would not be willing or able to start being active in the laboratory chemicals market post-merger. In this context one large chemical manufacturer mentioned that "[company] is not interested in the delivery of small volumes, this is not in line with [company] business model for the sale of respective products. [Company] does not have the facilities to pack in smallest volumes and to deliver such volumes to customers",117 while another explained that since it is "specialising in large volume sales to large volumes customers [it does not have] the infrastructure nor the manning to enter into the laboratory chemicals market".118
(189) The lack of interest in a merger induced entry by bulk manufacturers is further reinforced by the fact that the market where bulk manufacturers are active in has a totally different scale and economics when compared to laboratory chemicals markets. Indeed, as it was explained by a bulk manufacturer, "the size of the market and its business model would make it unattractive despite the better prices".119 Finally, another bulk manufacturer emphasised the difference in terms of organisation and business strategy between bulk and catalogue sales: "we don't want to sell small scale products to the customer directly due to our infrastructure and our cost structure".120 The fact that the two markets serve very different needs is further supported by the fact that many large chemical manufacturers purchase laboratory chemicals, including those originally produced by them, from companies like Merck and Sigma.121
(190) Finally, bulk manufacturers highlighted the long term process that would be needed to enter these markets. For example, one of them considered that "brand loyalty, brand recognition, scale of sales & distribution coverage, product range and e presence would make any ability to effectively compete [for third party manufacturers] subject to a multi-million dollar investment and a development period of approximately five years",122 while another also confirmed that the "main problem for them would be Brand image. Customers would not trust the brand. Process would take many years".123
(191) It follows that, in line with the general features of these markets, the barriers to entry are high, associated with a specific business model of small scale production and the need for brand recognition, customer acceptance and channel to the market. As a result, it is unlikely that bulk manufacturers would be able and/or willing to enter the market and exert competitive pressure on the merged entity post-Transaction.
IV.5.3.1.d.Conclusion on catalogue Solvents and Inorganics
(192) In view of the above, the Commission considers that the Parties are the two leading suppliers of catalogue solvents and inorganics in the EEA and each other's closest competitors in terms of the product portfolio, product quality and brands and channels to the market.
(193) The Transaction leads to the combination of the most extensive product portfolios, the strongest brands and the most effective channels to the market in the area of catalogue solvents and inorganics in the EEA. Besides these significant barriers to switching, barriers to entry are also high and would make it unlikely for other players, including large bulk chemical companies, to enter or expand on this market.
(194) Therefore, based on all available evidence, the Commission concludes that the proposed Transaction is likely to significantly impede effective competition and thus raises serious doubts as to its compatibility with the internal market in relation to the supply of catalogue solvents and inorganics in the EEA, irrespective of the precise product or geographic market definition.
IV.5.3.2.Organics and other laboratory chemicals
(195) According to data provided by the Notifying Party, the Transaction results in 9 horizontally affected markets or market segments124 in the supply of organics and other laboratory chemicals (reference materials, analytical chromatography, industrial microbiology) in the EEA.
(196) The Notifying party submits that the Transaction is unlikely to significantly impede competition, as the markets for organics and other laboratory chemicals are commoditised markets, where competitors are not affected by capacity constraints, and there are no significant barriers to entry and expansion.
(197) As regards organics in particular, the Notifying Party observes that third party manufacturers are available, and so are organic synthesis houses which would exert competitive constraints on the parties. The Parties rely to a very limited extent on IP rights (on products or on production steps), which cover less than [0- 10]% of Merck's and around [0-10]% of Sigma's sales of organics.
(198) As regards other laboratory chemicals, the Notifying party submits that the Parties have limited sales and a small product portfolio than other companies active on these markets, and this is a fragmented market with hundreds of competitors offering other chemicals such as reference materials.
(199) For the reasons set out below, the Commission finds that the Transaction is unlikely to significantly impede competition in relation to organics and other laboratory chemicals.
(200) First, the activities of the Parties in relation to these product markets are to a large extent complementary. Sigma has a strong market presence in organics where Merck is a less significant competitor, as in all affected markets and market segments in the EEA the market share increment (corresponding to Merck's market share) is below [5-10]%125 . Merck is a strong player for some other laboratory chemicals, such as TLC plates, with Sigma acting as a less significant competitor in all affected markets and market segments in the EEA, where the market share increment (corresponding to Sigma's market share) is below [5- 10]%126.
(201) Even though these market shares have been estimated by the Notifying Party and, thus, as for solvents and inorganics, might be underestimated, internal documents confirmed the more limited overlap between the Parties' activities in organics and other laboratory chemicals, such as chromatography. Merck's market shares were estimated in organics and chromatography as being below [10-20]%, and more specifically [5-10]%, in 2012 in Western Europe. It is explicitly mentioned that "Merck Millipore [is] strongest in Inorganics and Solvents" as opposed to other laboratory chemicals, such as organics and chromatography127.
(202) Second, the market investigation broadly confirmed that the merger between Sigma and Merck is unlikely to significantly impede competition on these product markets as opposed to solvents and inorganics. In particular, most customers reported other suppliers (such as Fisher / Acros, Alfa Aesar, GE, Invitrogen) as their main suppliers of organics, with few indicating Merck.128 This is consistent with the feedback of competitors.129
(203) As regards other laboratory chemicals, almost all customers130 and competitors131 indicated that Merck and Sigma are not among their three biggest suppliers.
(204) Third, concerning barriers to entry consisting of IP rights, no significant barriers exist. In those instances where the products concerned are materially subject to IP rights, as it is the case for instance for HPLC Columns, the parties do not have a significant share of the market.132
(205) Fourth, only as regards organics, certain competitors indicate that it may be costly and ultimately difficult to expand their presence on sub-segments of the organics market beyond their current activities. 133 However, this does not result in a significant impediment to effective competition in view of the limited overlaps between the parties in these markets, and of the availability of a sufficient number of competitors post-Transaction.
(206) In conclusion, in view of the above, the Commission concludes that the proposed Transaction is unlikely to significantly impede competition in relation to the market for organics and other laboratory chemicals in the EEA.
IV.6.DISTRIBUTION OF LABORATORY AND LIFE SCIENCE PRODUCTS
IV.6.1. Introduction
(207) Sigma operates a business whereby it distributes various (its own and other companies') life science industry products globally.134 To this end Sigma operates a traditional and an on-line (e-commerce) distribution system. Approximately […]% of Sigma's global sales are made through e-commerce. Sigma's sales of third party laboratory and life science products generated revenues of €[…] in 2013 in the EEA.
(208) The Transaction gives rise to vertically affected markets stemming from the link between the distribution of laboratory and life science products and the various life science markets analysed above
IV.6.2. Market definition
IV.6.2.1 Product market
(209) The Notifying Party submits that the relevant product market for distribution comprises the distribution of all laboratory and life science products. Distributors are able and do offer a range of products including laboratory chemicals as well as other laboratory consumables and life science products. Moreover, the same product market would comprise both traditional distributors and on-line distributors, as despite the different features of these channels, on-line distributors offer a range of products that is equivalent to that offered by traditional distributors.
(210) In its past decisional practice, the Commission found that distributors normally offer a range of products encompassing laboratory chemicals as well as other laboratory consumables and life science products.135 The Commission did not consider any subdivision of this market into different product markets depending on whether sales are made through traditional or online channels.
(211) Similarly, in its past decisional practice related to the distribution of commodity and speciality chemicals, 136 the Commission considered broad categories of chemicals as the relevant scope for distribution markets (e.g. the distribution of commodity chemicals).
(212) The market investigation in the case at hand did not provide any indications to depart from the Commission practice.
(213) The Commission therefore considers that the relevant product market is likely to be the distribution of laboratory and life science products.
IV.6.2.2.Geographic market
(214) The Notifying Party submits that there is a trend towards cross-border distribution of laboratory and life science products, although it acknowledges that the distribution market could be divided across national lines.
(215) In its past decisional practice in relation to life science products, the Commission found in particular that most distributors operate in a single Member State, and that most distributors have commercial negotiations with customers at national level.137 This is consistent with the need for local sales force and support in these markets.
(216) In any event, for the purposes of this Decision, the precise geographic market definition for distribution of laboratory chemicals can be left open as the competitive assessment would not differ under any of the plausible geographic market definitions.
IV.6.3. Competition assessment
IV.6.3.1. Customer foreclosure
(217) The Parties' combined market shares for the distribution of laboratory and life science products are below 30% in every Member State. As the Parties do not have a significant degree of market power on the downstream market, customer foreclosure is unlikely to be a concern in the case at hand.
(218) In this context, the Notifying Party also submits that Sigma is a relatively small distributor of third party products in every Member State in which it operates in the EEA, and that it faces significant competition from other distributors such as VWR (throughout the EEA), Euroclone (Italy, Spain, Greece, Germany), Omnilab (Germany and the Netherlands), Analis (France and Belgium), Dominique Dutscher (France and the UK), as well as local distributors active in one Member State only.
(219) The market investigation confirmed the existence of a number of global, EEA- wide and local distributors,138 and did not provide any indications to depart from the Parties' view as to Sigma's limited presence.
IV.6.3.2.Input foreclosure
(220) As evidenced in sections IV.2, IV.3, IV.4 and IV.5 above, the Parties' combined market share for a number of laboratory chemicals and life science products is higher than 30%.139 Therefore, all these upstream markets are vertically affected markets in relation to the downstream market for the distribution of laboratory and life science products. Should the merged entity be deemed to have a significant degree of market power regarding the upstream markets, it could potentially have an ability and incentive to leverage influence on the conditions of competition in these markets on prices and supply conditions in the downstream market for the distribution of laboratory and life science products.
(221) The Notifying Party submits in this context that the combined entity will not have a significant degree of market power for the supply of any laboratory and life science products in the EEA, and that customers do not perceive their products being particular "must have" brands for distributors of laboratory and life science products and thus any such foreclosure effects are unlikely.
(222) However, as evidenced in section IV.5 above, the Commission takes the view that the combined entity will have a significant degree of market power for a number of laboratory chemicals markets (e.g. solvents and inorganics). Nonetheless, these markets will only represent a fraction of the entire laboratory and life science portfolio of any given distributor, thereby mitigating the impact of any possible input foreclosure from the combined entity. Furthermore, the full overlap in relation to solvents and inorganics sold in the EEA will be removed by the Commitments offered by the Parties, and thus any vertical concerns will also be mitigated.
(223) More importantly, the combined entity is unlikely to have any incentive to undertake such input foreclosure strategy. Indeed, sales through distributors are a complementary sales channel to direct sales, and allow manufacturers of laboratory chemicals to expand their geographic and customer footprint. This is illustrated by Sigma itself, which still realizes […]% of its solvents sales and […]% of its inorganics sales through third party distributors, in spite of being vertically integrated with respect to distribution. Given the highly fragmented customer base in relation to laboratory chemicals, suppliers always have an incentive to rely on a number of distributors to achieve as wide sales of their products as possible. This is also illustrated by the strategy of Thermo-Fisher, a key vertically integrated competitor of the Parties, which besides its own distribution channels also sells a number of its products through a global distributor VWR.
(224) In light of the above, any input foreclosure is unlikely to be a concern, in particular because the combined entity would not have a strong incentive to pursue such foreclosure strategy.
IV.6.3.3. Conclusion
(225) In view of the information provided by the Parties, and of the results of the market investigation, the Commission therefore concludes that the proposed Transaction is unlikely to significantly impede competition in relation to the market for the distribution of laboratory and life science products.
V.COMMITMENTS
V.1. Framework for the assessment of the Commitments
(226) Where a concentration raises serious doubts as regards its compatibility with the internal market, the Parties may undertake to modify the concentration so as to remove the grounds for the serious doubts identified by the Commission.
(227) As set out in the Commission's Remedies Notice,140 the commitments have to eliminate the competition concerns entirely, and have to be comprehensive and effective from all points of view.141
(228) In assessing whether commitments will maintain effective competition, the Commission considers all relevant factors, including the type, scale and scope of the proposed commitments, with reference to the structure and particular characteristics of the market in which the Transaction is likely to significantly impede effective competition, including the position of the Parties and other participants on the market.142
(229) In order for the commitments to comply with those principles, they must be capable of being implemented effectively within a short period of time. Concerning the form of acceptable commitments, the Merger Regulation gives discretion to the Commission as long as the commitments meet the requisite standard. Structural commitments will meet the conditions set out above only in so far as the Commission is able to conclude with the requisite degree of certainty, at the time of its Decision, that it will be possible to implement them and that it will be likely that the new commercial structures resulting from them will be sufficiently workable and lasting to ensure that effective competition will be maintained.143 Divestiture commitments are normally the best way to eliminate competition concerns resulting from horizontal overlaps.
V.2.Commitments submitted by the Parties
(230) In order to ensure that effective competition will be maintained, the Parties submitted a set of commitments under Article 6(2) of the Merger Regulation on 22 May 2015 ("Initial Commitments"). The Commission market tested the Initial Commitments in order to assess whether they are sufficient and suitable to remedy serious doubts identified in the catalogue solvents and inorganics markets. Following the feedback received during the market test, the Initial Commitments were refined and improved, and amended commitments were submitted on 11 June 2015 ("Final Commitments"). These Final Commitments are annexed to this Decision and form an integral part thereof.
V.2.1. Initial Commitments
(231) The Initial Commitments consisted of the divestiture of the majority of Sigma's solvents and inorganics business in the EEA (the "Divestment Business").144
(232) The solvents and inorganics under the Divestment Business include:
a. for solvents: (i) HPLC solvents; (ii) regulated solvents; (iii) technical grade solvents; (iv) spectroscopy solvents, and (v) gas chromatography solvents;
b. for inorganics: (i) volumetric/titration solutions; (ii) inorganic salts; (iii) acids; (iv) bases; (v) buffers; (vi) auxiliaries; (vii) indicators; and (viii) Karl Fischer titration solutions.
(233) The Divestment Business generated EUR […]of sales in 2014 and included the following main elements:
a. the solvents and inorganics sold by Sigma worldwide under the Fluka brand (the "Fluka business"), as well as the full transfer of the Fluka brand and all associated brands and trademarks;
b. the solvents and inorganics sold by Sigma in the EEA under the Sigma- Aldrich brand, in so far as such products are produced in Sigma's plants in Seelze, (Germany), Buchs (Switzerland), and Steinheim (Germany) (the "Sigma-Aldrich business");
c. a royalty-free EEA-wide […]license to use the Sigma-Aldrich brand and associated trademarks for solvents and inorganics and an obligation on the Purchaser to rebrand those products in this period. The license is followed by an additional […]black-out period during which neither Merck nor the Purchaser are allowed to sell solvents and inorganics under the Sigma- Aldrich brand in the EEA;
d. all the assets currently owned and controlled by Sigma at its manufacturing facility located in Seelze (Germany) shared between Sigma and Honeywell Inc. ("Honeywell"),145 including all related production assets to the extent owned by Sigma (the "Seelze plant");
e. [Personnel];
f. [Personnel]; and
g. [Personnel] […].
(234) Moreover, the Initial Commitments included the following additional elements:
a. Sigma's know-how and associated IP rights used primarily or exclusively for the Divestment Business, as well as a royalty-free, irrevocable, non- exclusive, global licence under Sigma's know-how and associated IP rights used otherwise not primarily or exclusively for the Divestment Business;
b. at the option of the Purchaser, all assets and equipment solely or predominantly used at sites other than the Seelze plant for the production of products in the Divestment Business;
c. the trade names used exclusively or predominantly in connection with the Divestment Business specifically including the following names: Aerosol, Hydranal, Perdrogen, Riedel-De-Haen, TraceSelect, Tiron and Trolox;
d. all product descriptions and product specific information displayed on Sigma's e-commerce platform;
e. all assignable contracts and Sigma's best efforts to facilitate the transfer of all other contracts related to sourcing, customer supply, distribution and logistics; and
f. all customer lists of the Divestment Business and related information (regardless of the sales channel used) for the period from […]until the date of closing of the Transaction.
(235) Finally, the Initial Commitments foresee a number of transitional agreements between the Parties and the Purchaser for a period of […], including in particular:
a. technological support agreement providing support and training for the implementation of the licensed know-how in the Seelze plant;
b. technical assistance in relation with the manufacture of solvents and inorganics;
c. a transitional support agreement for sourcing the equipment and raw materials, and procuring logistics and distribution services, necessary for the Divestment Business; and
d. a supply agreement at manufacturing cost plus for products of the Divestment Business produced in sites other than the Seelze plant.
(236) In terms of Purchaser requirements, besides the standard requirements, the Initial Commitments provided that the Purchaser shall […]
(237) Finally, the Parties committed not to implement the Transaction before having entered into a final binding sale and purchase agreement for the sale of the Divestment Business and having received the Commission's approval of the Purchaser and the terms of sale.
V.2.2. Results of the market test and assessment of the Initial Commitments
(238) The market test was launched on 22 May 2015 and sought to assess mainly the scope and effectiveness of the Initial Commitments, their viability, the attractiveness of the Divestment Business as well as the suitability of the Purchaser criteria.
(239) While generally the market test yielded positive results, respondents identified a number of shortcomings. Specifically, the following key issues were raised:
a. lack of certain important trademarks used for solvents and inorganics, with particular reference to the Chromasolv, Fixanal and Trace Select Ultra trademarks;146
b. the possibility to circumvent the black-out of Sigma-Aldrich brand by selling the same products under the brands ´Sigma´ or ´Aldrich´;
c. as regards the re-branding aspect of the Commitments, the short duration of the re-branding and black-out periods for Sigma-Aldrich branded products in the perimeter of the Divestment Business as these periods were deemed insufficient for a Purchaser to successfully rebrand these products in particular in light of the industry characteristics and previous rebranding experiences in the market147;
d. lack of access to Sigma´s e-commerce platform, through which a vast number of orders are made by customers, and which would be necessary for the Purchaser to be able to continue to run the Divestment Business during a transitional period until it develops its sales channels;148
e. insufficient number of sales and marketing employees which was not deemed adequate to enable the Purchaser to be in a position to effectively market the products contained in the Divestment Business149; and
f. a number of other technicalities such as the duration of transitional agreements etc.
(240) As to the suitable Purchaser, respondents to the market test indicated that it should already be active in the laboratory chemicals business, to ensure its credibility vis-à-vis customers, and have a products portfolio in life science which includes but goes beyond solvents and inorganics to further strengthen the sustainability of its activities on a long term basis.150
V.2.3. Final Commitments
(241) The Parties were informed of the shortcomings identified during the market test within a framework of a State of play meeting held on 2 June 2015 and submitted a final text of Commitments addressing the issues on 11 June 2015.
(242) Specifically, the Final Commitments submitted by the Parties provide for the following additional improvements compared to the Initial Commitments:
a. worldwide rights to Chromasolv, Fixanal, and Trace Select Ultra trademarks;
b. extension of the scope of the Divestment Business to all EEA sales of Sigma-Aldrich branded solvents and inorganics (i.e. including those currently produced at other plants than Seelze, Buchs and Steinheim);
c. extension of the re-branding and black-out periods for the Sigma-Aldrich branded products included in the Divestment Business to reach the following structure:
· initial re-branding period of […], with possible extension for multiple […] periods up to a maximum of […] in aggregate if certain conditions are met; and
· […]black-out period;
d. inclusion of a commitment not to sell solvents and inorganics under the "Sigma" or "Aldrich" brands in the EEA during the periods foreseen in item
c. above;
e. [Personnel];
f.inclusion of an access obligation to Sigma's e-commerce platform for a period of […]with redirection to the Purchaser's platform when available, and a disclaimer that the sales are made on behalf of the Purchaser;
g. inclusion of a transitional support agreement to assist the purchaser in developing its e-commerce platform; and
h. possibility to extend the duration of transitional agreements by […].
(243) The Final Commitments also require, [Purchaser criteria].
(244) The full description of the assets and obligations of the Final Commitments is contained in the Schedule thereof.
V.2.4.Overall assessment of the Final Commitments
(245) The Commission analysed the suitability of the Final Commitments to remedy the serious doubts identified in relation to catalogue solvents and inorganics markets in the EEA. To this end, the Commission assessed whether the scope of the Commitments is sufficient and suitable to address the competition concerns identified and the nature of the industry, whether the Divestment Business is viable and the Commitments are likely to be effective in practice and whether the Commitments can be easily implemented and, finally, whether the Divestment Business is attractive for purchasers.
V.2.4.1. Scope of the Final Commitments and their suitability to remove the identified concerns
(246) As explained in this Decision, the significant impediments to effective competition stemming from this case reside in the combination of the most extensive product portfolios, the strongest brands and the most effective channels to the market in the area of solvents and inorganics in the EEA, in markets characterised by significant barriers to switching and significant barriers to entry.
(247) The Final Commitments seek to address these concerns taking account of the specific features of the market explained above in section (131) of this Decision, and in particular the need for scale.
(248) The Final Commitments consist of the divestment of a wide product portfolio representing the full overlap between the Parties as regards solvents and inorganics sold in the EEA. More specifically, the Divestment Business represents […]% of the overlap in solvents and inorganics in the EEA. Indeed, the Final Commitments comprise products in the area of solvents and inorganics encompassing all the product segments where Parties' position was strong, and in particular HPLC solvents, regulated industry solvents, salts, acids, buffers, volumetric and titration solutions, Karl Fisher titration and indicators.
(249) The Divestment Business includes all necessary assets from its pre-Transaction operation. Specifically, it contains manufacturing assets Sigma used in Seelze pursuant to the agreements with Honeywell, all important brands under which solvents and inorganics are sold in the EEA, and solutions for bringing the product to the market irrespective of the nature of the Purchaser. Indeed, even if the Purchaser were not to have any pre-existing sales capability, the Final Commitments would allow it to be immediately present in the market […].
(250) In terms of the geographic scope of the Final Commitments, the Divestment Business includes worldwide rights and worldwide customer base of the Fluka and associated brands in relation to solvents and inorganics. This, on the one hand, mitigates any risk of brand confusion and enhances chances for a long-term viability of the Divestment Business and, on the other hand, enlarges the scope of the Divestment Business beyond the EEA in relation to the main brand, and in particular the one under which the signature Karl Fisher titration solutions and many other premium quality solvents and inorganics are successfully sold worldwide.
(251) In addition, the divested assets at Seelze plant have sufficient spare capacity to increase the production as the case may be and thus readily compete with the merged entity and supply customers which currently purchase Sigma's products manufactured at other locations. In this respect, the option to acquire further equipment from Steinheim, Buchs and other locations where solvents and inorganics sold in the EEA are currently manufactured by Sigma further enhances the production capabilities of the Divestment Business.
(252) On this basis the Commission considers the Final Commitments are sufficient in scope and suitable to remove the competition concerns identified.
V.2.4.2. Viability and likelihood of effectiveness of the Final Commitments in practice
(253) The Divestment Business as specified in the Final Commitments generated EUR […]of sales in 2014, with a […]% gross margin. It does not include only assets but also critical elements to make a player successful in the solvents and inorganics markets in the EEA, which are a well-known brand, a wide portfolio of products, including high margin inorganics such as Karl Fisher titration solutions, various key customers information and the channels to the market.
(254) The divestiture of a wide portfolio of solvents and inorganics is crucial to the viability of the Divestment Business, in line with the findings of the market investigation and the market test, according to which it is indispensable for a player to establish itself as a competitor that it is capable to offer a broad range of products across the entire spectrum of solvents and inorganics. The product portfolio of solvents and inorganics under the Divestment Business is sufficiently broad to ensure viability as divested solvents and inorganics cover a wide spectrum of laboratory and inorganics, including best-in-class Sigma products such as Karl Fisher titration solutions.
(255) The only carve-out aspect of the divestiture concerns NMR and Anhydrous solvents, which are manufactured at different facilities than Seelze and using different production equipment which may be problematic to transfer. Given the small size of the sales associated with these products, the viability of the Divestment Business is unlikely to be affected.
(256) As explained above in relation to the scope of the Commitments, the assets contained in the Divestment Business cover the entire value chain of solvents and inorganics; from the production assets through the channel to the market to customer information. This further enhances the viability of the Divestment Business if operated by a suitable Purchaser.
(257) The transfer of the Fluka brand for an unlimited use worldwide further strengthens the viability of the Divestment Business, by enabling the Purchaser to build a complete set of offering at a global level based on one high quality brand. In addition, the transfer of the Fluka related know-how will foster customer retention as customers will have to turn to the Purchaser to obtain these products.
(258) As regards the rebranding of the products sold under the Sigma-Aldrich brand and the duration of the rebranding and blackout periods, these are in line with the criteria laid out in the Remedies Notice for the design of re-branding commitments, insofar as the licensed brands and trademarks are strong, license is exclusive in the EEA and the IP and other assets included in the Divestment Business increase the chances of the re-branding success. Indeed, the rebranding and black-out periods in relation to the Sigma Aldrich branded products will allow a suitable Purchaser for sufficient time to establish itself in the market with a new brand and develop customer relationships. In addition, the […] black-out period covering both the "Sigma" and "Aldrich" brands will ensure that the re- branding efforts in relation to the Sigma Aldrich brand are not undermined by a possible circumvention.
(259) The total duration of re-branding and black-out periods is satisfactory given the nature of the business (business-to-business as opposed to business-to-consumer) and that purchaser will have every incentive to swiftly rebrand, with the […] additional extension providing for further flexibility in case the circumstances justify it. In light of this additional extension, the Commission takes the view that co-branding is not necessary. Furthermore, as solvents and inorganics are very different products from other laboratory chemicals, and often sold under different trades names, the final scope of the Divestment Business would not affect the success of the re-branding.
(260) Finally, the transitional access to Sigma's best-in-class e-commerce platform for a period of […]will enable an effective and immediate transfer of the […]% of online sales represented in the Divested Business to the benefit of the Purchaser.
(261) The transfer of assignable distribution and logistics contracts will enable the Purchaser to develop a wide distribution network, after an interim period where the Parties will have granted access to their own distribution centres and warehouses. The transfer of […] further enhance the capacity of a suitable Purchaser to reach a fragmented customer base all over the EEA.
(262) On this basis, the Commission considers that the Divestment Business as set out in the Final Commitments provides a good portfolio of products, with only minor carve-outs from the original business of Sigma, and covers the full value chain. The retention of customers is safeguarded by the inclusion of a worldwide brand and sufficient rebranding periods for the Sigma-Aldrich brand, as well as by the access to Sigma's e-commerce platform which will allow immediate sales. Overall, the Divestment Business is therefore viable and can be run profitably by a suitable Purchaser. It follows the Commitments are likely to be effective in practice.
V.2.4.3. Ability of the Final Commitments to be implemented in practice
(263) Given that the core of the manufacturing assets are located in Seelze which is exploited by Sigma and Honeywell pursuant to a set of agreements […].
(264) First, the Final Commitments provide for an upfront buyer so that the Transaction cannot be implemented until the Commission has given its approval to the Purchaser.
(265) The upfront buyer clause is complemented by a Purchaser requirement whereby the Purchaser, […]. Thus, the Commission will approve the Purchaser only if it has assurances that the Purchaser […].
(266) [Third party information].
(267) On this basis, the Commission considers that the upfront buyer clause and the Purchaser requirements set out in the Commitments mitigate the implementation risk […].
V.2.4.4. Attractiveness of the package for Purchasers
(268) The attractiveness of the Divestment Business was evidenced by the number of potentially interested purchasers, including in particular large competitors of the Parties in the life science area already supplying laboratory chemicals.
(269) On this basis, and in particular in view of a number of interested Purchasers, the Commission considers that the Divestment Business is attractive and likely to be acquired by a suitable Purchaser.
V.2.4.5.Conclusion on Final Commitments
(270) For the reasons outlined above, and in view of the results of the market test and the ensuing improvements to the Commitments, the Commission considers the Final Commitments to be sufficient in scope and suitable to eliminate the serious doubts as to the compatibility of the Transaction with the internal market in relation to catalogue solvents and inorganics markets in the EEA.
V.3. Conditions and obligations
(271) Under the first sentence of the second subparagraph of Article 6(2) of the Merger Regulation, the Commission may attach to its Decision conditions and obligations intended to ensure that the undertakings concerned comply with the commitments they have entered into vis-à-vis the Commission with a view to rendering a notified concentration compatible with the internal market.
(272) The achievement of the measure that gives rise to the structural change of the market is a condition, whereas the implementing steps which are necessary to achieve this result are generally obligations on the Parties. Where a condition is not fulfilled, the Commission's decision declaring the concentration compatible with the internal market no longer stands. Where the undertakings concerned commit a breach of an obligation, the Commission may revoke the clearance decision in accordance with Article 8(6) of the Merger Regulation. The undertakings concerned may also be subject to fines and periodic penalty payments under Articles 14(2) and 15(1) of the Merger Regulation.
(273) In accordance with the distinction described above, the Decision in this case is conditioned on the full compliance with the requirements set out in section B of the Final Commitments (conditions), whereas the other sections of the Final Commitments constitute obligations on Merck.
(274) The detailed text of the Final Commitments is annexed to the present Decision. The full text of the final Commitments forms an integral part to this Decision.
VI.CONCLUSION
(275) For the above reasons, the Commission has decided not to oppose the notified operation as modified by the commitments and to declare it compatible with the internal market and with the functioning of the EEA Agreement, subject to full compliance with the conditions in section B of the commitments annexed to the present Decision and with the obligations contained in the other sections of the said commitments. This Decision is adopted in application of Article 6(1)(b) in conjunction with Article 6(2) of the Merger Regulation and Article 57 of the EEA Agreement.
Case No. COMP/M.7435 – MERCK/SIGMA-ALDRICH
COMMITMENTS TO THE EUROPEAN COMMISSION 11 June 2015
Pursuant to Article 6(2) of Council Regulation (EC) No 139/2004 (the “Merger Regulation”), Merck KGaA (“Merck” or the “Notifying Party”) and Sigma-Aldrich Corporation (“Sigma”; Merck and Sigma jointly referred to as the “Parties”) hereby provide the following Commitments (the “Commitments”) vis-à-vis the European Commission (the “Commission”) with a view to rendering the acquisition of sole control of Sigma (the “Transaction”) compatible with the common market and the functioning of the EEA Agreement. This text shall be interpreted in light of the Commission’s decision pursuant to Article 6(1)(b) of the Merger Regulation of the Merger Regulation to declare the Concentration compatible with the internal market and the functioning of the EEA Agreement (the “Decision”), in the general framework of European Union law, in particular in light of the Merger Regulation, and by reference to the Commission Notice on remedies acceptable under Council Regulation (EC) No 139/2004 and under Commission Regulation (EC) No 802/2004 (the “Remedies Notice”).
Section A. Definitions
1. For the purpose of the Commitments, the following terms shall have the following meaning:
Affiliated Undertakings: undertakings controlled by the Parties and/or by the ultimate parents of the Parties, whereby the notion of control shall be interpreted pursuant to Article 3 of the Merger Regulation and in light of the Commission Consolidated Jurisdictional Notice under Council Regulation (EC) No 139/2004 on the control of concentrations between undertakings (the "Consolidated Jurisdictional Notice").
Assets: the assets that contribute to the current operation or are necessary to ensure the viability and competitiveness of the Divestment Business as indicated in Section B, paragraph 2 and described more in detail in the Schedule.
Closing: the transfer of the legal title to the Divestment Business to the Purchaser.
Closing Period: the period of [CONFIDENTIAL] months from the approval of the Purchaser and the terms of sale by the Commission.
Confidential Information: any business secrets, know-how, commercial information, or any other information of a proprietary nature that is not in the public domain.
Conflict of Interest: any conflict of interest that impairs the Trustee's objectivity and independence in discharging its duties under the Commitments.
Divestment Business: the business or businesses as defined in Section B and in the Schedule which the Parties commit to divest.
Divestiture Trustee: one or more natural or legal person(s) who is/are approved by the Commission and appointed by Merck or a Merck Affiliated Undertaking and who has/have received from Merck or a Merck Affiliated Undertaking the exclusive Trustee Mandate to sell the Divestment Business to a Purchaser at no minimum price.
Effective Date: the date of adoption of the Decision.
First Divestiture Period: the period of [CONFIDENTIAL] months from the Effective Date.
Hold Separate Manager: the person appointed by Merck for the Divestment Business to manage the day-to-day business under the supervision of the Monitoring Trustee.
Key Personnel: all personnel necessary to maintain the viability and competitiveness of the Divestment Business, as listed in the Schedule, including the Hold Separate Manager.
Monitoring Trustee: one or more natural or legal person(s) who is/are approved by the Commission and appointed by Merck, and who has/have the duty to monitor Merck’s compliance with the conditions and obligations attached to the Decision.
Personnel: all staff currently employed by the Divestment Business, including staff seconded to the Divestment Business as well as additional personnel listed in the Schedule.
Purchaser: the entity approved by the Commission as acquirer of the Divestment Business in accordance with the criteria set out in Section D.
Purchaser Criteria: the criteria laid down in paragraph 17 of these Commitments that the Purchaser must fulfil in order to be approved by the Commission.
Schedule: the schedule to these Commitments describing more in detail the Divestment Business.
Trustee(s): the Monitoring Trustee and/or the Divestiture Trustee as the case may be.
Trustee Divestiture Period: the period of [CONFIDENTIAL] months from the end of the First Divestiture Period.
Section B. The commitment to divest and the Divestment Business
Commitment to divest
2. In order to maintain effective competition, the Parties commit to divest, or procure the divestiture of the Divestment Business by the end of the Trustee Divestiture Period as a going concern to a purchaser and on terms of sale approved by the Commission in accordance with the procedure described in paragraph 18 of these Commitments. To carry out the divestiture, the Parties commit to find a purchaser and to enter into a final binding sale and purchase agreement for the sale of the Divestment Business within the First Divestiture Period. If the Parties have not entered into such an agreement at the end of the First Divestiture Period, the Parties shall grant the Divestiture Trustee an exclusive mandate to sell the Divestment Business in accordance with the procedure described in paragraph 30 in the Trustee Divestiture Period.
3. The proposed concentration shall not be implemented before the Parties or the Divestiture Trustee has entered into a final binding sale and purchase agreement for the sale of the Divestment Business and the Commission has approved the purchaser and the terms of sale in accordance with paragraph 17.
4. The Parties shall be deemed to have complied with this commitment if: (a) by the end of the Trustee Divestiture Period, the Parties or the Divestiture Trustee have entered into a final binding sale and purchase agreement and the Commission approves the proposed purchaser and the terms of sale as being consistent with the Commitments in accordance with the procedure described in paragraph 18;(b) the Closing of the sale of the Divestment Business to the Purchaser takes place within the Closing Period; and (c) the transfer of assets and personnel specified in paragraphs 3-27 of the Schedule has been effected, the access obligations as specified in paragraph 33 of the Schedule have been complied with and customer information as specified in paragraph 32 has been transferred.
5. In order to maintain the structural effect of the Commitments, the Notifying Party shall, for a period of 10 years after Closing, not acquire, whether directly or indirectly, the possibility of exercising influence (as defined in paragraph 43 of the Remedies Notice, footnote 3) over the whole or part of the Divestment Business, unless, following the submission of a reasoned request from the Notifying Party showing good cause and accompanied by a report from the Monitoring Trustee (as provided in paragraph of these Commitments), the Commission finds that the structure of the market has changed to such an extent that the absence of influence over the Divestment Business is no longer necessary to render the proposed concentration compatible with the internal market.
Structure and definition of the Divestment Business
6. The Divestment Business consists of the business detailed in the Schedule which forms an integral part of the Commitments.
7. The Divestment Business, described in more detail in the Schedule, includes all assets and staff that contribute to the current operation or are necessary to ensure the viability and competitiveness of the Divestment Business, in particular: (a) all tangible and intangible assets (including rights in intellectual property);(b) all licences, permits and authorisations issued by any governmental organisation for the benefit of the Divestment Business;(c) all contracts, leases, commitments and customer orders of the Divestment Business; all customer, credit and other records of the Divestment Business; and(d) the Personnel.
8. Strict firewall procedures will be adopted so as to ensure that any competitively sensitive information related to, or arising from the transitional arrangements described in the Schedule will not be shared with, or passed on to, anyone, other than for the purpose of the implementation of the Commitments.
Section C.Related commitments
Preservation of viability, marketability and competitiveness
9. From the Effective Date until Closing, the Parties shall preserve or procure the preservation of the economic viability, marketability and competitiveness of the Divestment Business, in accordance with good business practice, and shall minimise as far as possible any risk of loss of competitive potential of the Divestment Business. In particular the Parties undertake:(a) not to carry out any action that might have a significant adverse impact on the value, management or competitiveness of the Divestment Business or that might alter the nature and scope of activity, or the industrial or commercial strategy or the investment policy of the Divestment Business;(b) to make available, or procure to make available, sufficient resources for the development of the Divestment Business, on the basis and continuation of the existing business plans;(c) to take all reasonable steps, or procure that all reasonable steps are being taken, including appropriate incentive schemes (based on industry practice), to encourage all Key Personnel to remain with the Divestment Business, and not to solicit or move any Personnel to the retained business or Merck. Where, nevertheless, individual members of the Key Personnel exceptionally leave the Divestment Business, the Parties shall provide a reasoned proposal to replace the person or persons concerned to the Commission and the Monitoring Trustee. The Parties must be able to demonstrate to the Commission that the replacement is well suited to carry out the functions exercised by those individual members of the Key Personnel. The replacement shall take place under the supervision of the Monitoring Trustee, who shall report to the Commission.
Hold-separate obligations
10. The Parties commit, from the Effective Date until Closing, to procure that the Divestment Business is kept separate from the retained businesses and, after closing of the notified transaction to keep the Divestment Business separate from the Notifying Party's other businesses and to ensure that unless explicitly permitted under these Commitments: (i) management and staff of the businesses retained have no involvement in the Divestment Business; (ii) the Key Personnel and Personnel of the Divestment Business have no involvement in any retained or Merck's business and do not report to any individual outside the Divestment Business.
11. Until Closing, the Parties shall assist the Monitoring Trustee in ensuring that the Divestment Business is managed as a distinct and saleable entity separate from the retained businesses and Merck. Immediately after the adoption of the Decision, the Parties, upon consultation with the Commission and the Monitoring Trustee, shall appoint a Hold Separate Manager. The Hold Separate Manager, who shall be part of the Key Personnel, shall manage the Divestment Business independently and in the best interest of the business with a view to ensuring its continued economic viability, marketability and competitiveness and its independence from the retained businesses and Merck. The Hold Separate Manager shall closely cooperate with and report to the Monitoring Trustee and, if applicable, the Divestiture Trustee. Any replacement of the Hold Separate Manager shall be subject to the procedure laid down in paragraph 8(c) of these Commitments. The Commission may, after having heard the Parties, require the Parties to replace the Hold Separate Manager. To the extent this may be required, to ensure that the Divestment Business is held and managed as a separate entity the Monitoring Trustee shall exercise Parties' rights as shareholder in the legal entity that constitutes the Divestment Business (except for its rights in respect of dividends that are due before Closing), with the aim of acting in the best interest of the business, which shall be determined on a stand-alone basis, as an independent financial investor, and with a view to fulfilling Parties' obligations under the Commitments. Furthermore, the Monitoring Trustee shall have the power to replace members of the supervisory board or non-executive directors of the board of directors, who have been appointed on behalf of any of the Parties. Upon request of the Monitoring Trustee, any of the Parties' nominees shall resign as a member of the boards or shall cause such members of the boards to resign.
Ring-fencing
12. The Parties shall implement, or procure to implement, all necessary measures to ensure that they do not, after the Effective Date, obtain any Confidential Information relating to the Divestment Business. Any such Confidential Information obtained by Merck before the Effective Date will be eliminated and not be used by Merck. This includes measures vis-à-vis Parties' appointees on the supervisory board and/or board of directors of the Divestment Business. In particular, the participation of the Divestment Business in any central information technology network shall be severed to the extent possible, without compromising the viability of the Divestment Business. The Parties may obtain or keep information relating to the Divestment Business which is reasonably necessary for the divestiture of the Divestment Business or the disclosure of which to the Parties are required by law.
Non-solicitation clause
13. Merck undertakes, subject to customary limitations, not to solicit, and to procure that Affiliated Undertakings do not solicit, the Key Personnel transferred with the Divestment Business for a period of three (3) years after Closing.
Due diligence
14. In order to enable potential purchasers to carry out a reasonable due diligence of the Divestment Business, the Parties shall, subject to customary confidentiality assurances and dependent on the stage of the divestiture process:(a) provide to potential purchasers sufficient information as regards the Divestment Business;(b) provide to potential purchasers sufficient information relating to the Personnel and Key Personnel and allow them reasonable access to the Personnel and Key Personnel.
Reporting
15. Merck shall submit written reports in English on potential purchasers of the Divestment Business and developments in the negotiations with such potential purchasers to the Commission and the Monitoring Trustee no later than ten (10) days after the end of every month following the Effective Date (or otherwise at the Commission’s request). Merck shall submit a list of all potential purchasers having expressed interest in acquiring the Divestment Business to the Commission at each and every stage of the divestiture process, as well as a copy of all the offers made by potential purchasers within five days of their receipt.
16. Merck shall inform the Commission and the Monitoring Trustee on the preparation of the data room documentation and the due diligence procedure and shall submit a copy of any information memorandum to the Commission and the Monitoring Trustee before sending the memorandum out to potential purchasers.
Section D. The Purchaser
17. In order to be approved by the Commission, the Purchaser must fulfil the following criteria:(a) The Purchaser shall be independent of and unconnected to the Notifying Party and its Affiliated Undertakings (this being assessed having regard to the situation following the divestiture);(b) The Purchaser shall have the financial resources, proven expertise and incentive to maintain and develop the Divestment Business as a viable and active competitive force in competition with the Parties and other competitors;(c) [CONFIDENTIAL];(d) [CONFIDENTIAL]; and(e) The acquisition of the Divestment Business by the Purchaser must neither be likely to create, in light of the information available to the Commission, prima facie competition concerns nor give rise to a risk that the implementation of the Commitments will be delayed. In particular, the Purchaser must reasonably be expected to obtain all necessary approvals from the relevant regulatory authorities for the acquisition of the Divestment Business.
18. The final binding sale and purchase agreement (as well as ancillary agreements) relating to the divestment of the Divestment Business shall be conditional on the Commission’s approval. When the Parties have reached an agreement with a purchaser, Merck shall submit a fully documented and reasoned proposal, including a copy of the final agreement(s), within one week to the Commission and the Monitoring Trustee. Merck must be able to demonstrate to the Commission that the purchaser fulfils the Purchaser Criteria and that the Divestment Business is being sold in a manner consistent with the Commission's Decision and the Commitments. For the approval, the Commission shall verify that the purchaser fulfils the Purchaser Criteria and that the Divestment Business is being sold in a manner consistent with the Commitments including their objective to bring about a lasting structural change in the market. The Commission may approve the sale of the Divestment Business without one or more Assets or parts of the Personnel, or by substituting one or more Assets or parts of the Personnel with one or more different assets or different personnel, if this does not affect the viability and competitiveness of the Divestment Business after the sale, taking account of the proposed purchaser.
Section E.Trustee
I. Appointment procedure
19. The Parties shall appoint a Monitoring Trustee to carry out the functions specified in these Commitments for a Monitoring Trustee.
20. If the Parties have not entered into a binding sale and purchase agreement regarding the Divestment Business one (1) month before the end of the First Divestiture Period or if the Commission has rejected a purchaser proposed by Merck at that time or thereafter, the Parties shall appoint a Divestiture Trustee. The appointment of the Divestiture Trustee shall take effect upon the commencement of the Trustee Divestiture Period.
21. The Trustee shall:(i) at the time of appointment, be independent of the Parties and its/their Affiliated Undertakings;(ii) possess the necessary qualifications to carry out its mandate, for example have sufficient relevant experience as an investment banker or consultant or auditor; and (iii) neither have nor become exposed to a Conflict of Interest.
22. The Trustee shall be remunerated by the Notifying Party in a way that does not impede the independent and effective fulfilment of its mandate. In particular, where the remuneration package of a Divestiture Trustee includes a success premium linked to the final sale value of the Divestment Business, such success premium may only be earned if the divestiture takes place within the Trustee Divestiture Period.
Proposal by Merck
23. No later than two (2) weeks after the Effective Date, the Parties shall submit the names of three natural or legal persons whom they propose to appoint as the Monitoring Trustee to the Commission for approval. No later than one month before the end of the First Divestiture Period or on request by the Commission, Merck shall submit a list of one or more persons whom Merck proposes to appoint as Divestiture Trustee to the Commission for approval. The proposal shall contain sufficient information for the Commission to verify that the person or persons proposed as Trustee fulfil the requirements set out in paragraph 21 and shall include:(a) the full terms of the proposed mandate, which shall include all provisions necessary to enable the Trustee to fulfil its duties under these Commitments;(b) the outline of a work plan which describes how the Trustee intends to carry out its assigned tasks;(c) an indication whether the proposed Trustee is to act as both Monitoring Trustee and Divestiture Trustee or whether different trustees are proposed for the two functions.
Approval or rejection by the Commission
24. The Commission shall have the discretion to approve or reject the proposed Trustee(s) and to approve the proposed mandate subject to any modifications it deems necessary for the Trustee to fulfil its obligations. If only one name is approved, Merck shall appoint or cause to be appointed the person or persons concerned as Trustee, in accordance with the mandate approved by the Commission. If more than one name is approved, Merck shall be free to choose the Trustee to be appointed from among the names approved. The Trustee shall be appointed within one week of the Commission’s approval, in accordance with the mandate approved by the Commission.
New proposal by the Merck
25. If all the proposed Trustees are rejected, Merck shall submit the names of at least two more natural or legal persons within one week of being informed of the rejection, in accordance with paragraphs 19 and 24 of these Commitments.
Trustee nominated by the Commission
26. If all further proposed Trustees are rejected by the Commission, the Commission shall nominate a Trustee, whom Merck shall appoint, or cause to be appointed, in accordance with a trustee mandate approved by the Commission.
II. Functions of the Trustee
27. The Trustee shall assume its specified duties and obligations in order to ensure compliance with the Commitments. The Commission may, on its own initiative or at the request of the Trustee or Merck, give any orders or instructions to the Trustee in order to ensure compliance with the conditions and obligations attached to the Decision.
Duties and obligations of the Monitoring Trustee
28. The Monitoring Trustee shall:(i) propose in its first report to the Commission a detailed work plan describing how it intends to monitor compliance with the obligations and conditions attached to the Decision.(ii) oversee, in close co-operation with the Hold Separate Manager, the on-going management of the Divestment Business with a view to ensuring its continued economic viability, marketability and competitiveness and monitor compliance by the Parties with the conditions and obligations attached to the Decision. To that end the Monitoring Trustee shall:(a) monitor the preservation of the economic viability, marketability and competitiveness of the Divestment Business, and the keeping separate of the Divestment Business from the business retained by the Parties, in accordance with paragraphs 9 and 10 of these Commitments;(b) supervise the management of the Divestment Business as a distinct and saleable entity, in accordance with paragraph 9 of these Commitments;(c) with respect to Confidential Information:- determine all necessary measures to ensure that the Parties do not after the Effective Date obtain any Confidential Information relating to the Divestment Business,- in particular strive for the severing of the Divestment Business’ participation in a central information technology network to the extent possible, without compromising the viability of the Divestment Business,- make sure that any Confidential Information relating to the Divestment Business obtained by Merck before the Effective Date is eliminated and will not be used by Merck, and- decide whether such information may be disclosed to or kept by Merck as the disclosure is reasonably necessary to allow Merck to carry out the divestiture or as the disclosure is required by law;(d) monitor the splitting of assets and the allocation of Personnel between the Divestment Business and retained business or Affiliated Undertakings;(iii) fulfil all functions assigned to him in paragraphs 14(b), 15, 24, 25, 33 and 34 of the Schedule;(iv) propose to the Parties such measures as the Monitoring Trustee considers necessary to ensure the Parties’ compliance with the conditions and obligations attached to the Decision, in particular the maintenance of the full economic viability, marketability or competitiveness of the Divestment Business, the holding separate of the Divestment Business and the non-disclosure of competitively sensitive information; (v) review and assess potential purchasers as well as the progress of the divestiture process and verify that, dependent on the stage of the divestiture process:(a) potential purchasers receive sufficient and correct information relating to the Divestment Business and the Personnel in particular by reviewing, if available, the data room documentation, the information memorandum and the due diligence process, and(b) potential purchasers are granted reasonable access to the Personnel;(vi) act as a contact point for any requests by third parties, in particular potential purchasers, in relation to the Commitments; (vii) provide to the Commission, sending the Parties a non-confidential copy at the same time, a written report within fifteen (15) days after the end of every month that shall cover the operation and management of the Divestment Business as well as the splitting of assets and the allocation of Personnel so that the Commission can assess whether the business is held in a manner consistent with the Commitments and the progress of the divestiture process as well as potential purchasers; (viii) promptly report in writing to the Commission, sending the Parties a non-confidential copy at the same time, if it concludes on reasonable grounds that one or both of the Parties is failing to comply with these Commitments; (ix) within one week after receipt of the documented proposal referred to in paragraph 18 of these Commitments, submit to the Commission, sending the Parties a non-confidential copy at the same time, a reasoned opinion as to the suitability and independence of the proposed purchaser and the viability of the Divestment Business after the Sale and as to whether the Divestment Business is sold in a manner consistent with the conditions and obligations attached to the Decision, in particular, if relevant, whether the Sale of the Divestment Business without one or more Assets or not all of the Personnel affects the viability of the Divestment Business after the sale, taking account of the proposed purchaser;(x) assume the other functions assigned to the Monitoring Trustee under the conditions and obligations attached to the Decision.
29. If the Monitoring and Divestiture Trustee are not the same legal or natural persons, the Monitoring Trustee and the Divestiture Trustee shall cooperate closely with each other during and for the purpose of the preparation of the Trustee Divestiture Period in order to facilitate each other's tasks.
Duties and obligations of the Divestiture Trustee
30. Within the Trustee Divestiture Period, the Divestiture Trustee shall sell at no minimum price the Divestment Business to a purchaser, provided that the Commission has approved both the purchaser and the final binding sale and purchase agreement (and ancillary agreements) as in line with the Commission's Decision and the Commitments in accordance with paragraphs 17 and 18 of these Commitments. The Divestiture Trustee shall include in the sale and purchase agreement (as well as in any ancillary agreements) such terms and conditions as it considers appropriate for an expedient sale in the Trustee Divestiture Period. In particular, the Divestiture Trustee may include in the sale and purchase agreement such customary representations and warranties and indemnities as are reasonably required to effect the sale. The Divestiture Trustee shall protect the legitimate financial interests of the Parties, subject to the Parties’ unconditional obligation to divest at no minimum price in the Trustee Divestiture Period.
31. In the Trustee Divestiture Period (or otherwise at the Commission’s request), the Divestiture Trustee shall provide the Commission with a comprehensive monthly report written in English on the progress of the divestiture process. Such reports shall be submitted within 15 days after the end of every month with a simultaneous copy to the Monitoring Trustee and a non-confidential copy to the Notifying Party.
III.Duties and obligations of the Parties
32. The Parties shall provide and shall cause their respective advisors to provide the Trustee with all such co-operation, assistance and information as the Trustee may reasonably require to perform its tasks. The Trustee shall have full and complete access to any of the Parties’ or the Divestment Business’ books, records, documents, management or other personnel, facilities, sites and technical information necessary for fulfilling its duties under the Commitments and the Parties and the Divestment Business shall provide the Trustee upon request with copies of any document. The Parties and the Divestment Business shall make available to the Trustee one or more offices on their premises and shall be available for meetings in order to provide the Trustee with all information necessary for the performance of its tasks.
33. The Parties shall provide the Monitoring Trustee with all managerial and administrative support that it may reasonably request on behalf of the management of the Divestment Business. This shall include all administrative support functions relating to the Divestment Business which are currently carried out at headquarters level. The Parties shall provide and shall cause its advisors to provide the Monitoring Trustee, on request, with the information submitted to potential purchasers, in particular give the Monitoring Trustee access to the data room documentation and all other information granted to potential purchasers in the due diligence procedure. The Parties shall inform the Monitoring Trustee on possible purchasers, submit lists of potential purchasers at each stage of the selection process, including the offers made by potential purchasers at those stages, and keep the Monitoring Trustee informed of all developments in the divestiture process.
34. The Parties shall grant or procure Affiliated Undertakings to grant comprehensive powers of attorney, duly executed, to the Divestiture Trustee to effect the sale (including ancillary agreements), the Closing and all actions and declarations which the Divestiture Trustee considers necessary or appropriate to achieve the sale and the Closing, including the appointment of advisors to assist with the sale process. Upon request of the Divestiture Trustee, Merck shall cause the documents required for effecting the sale and the Closing to be duly executed.
35. Merck shall indemnify the Trustee and its employees and agents (each an “Indemnified Party”) and hold each Indemnified Party harmless against, and hereby agrees that an Indemnified Party shall have no liability to Merck for, any liabilities arising out of the performance of the Trustee’s duties under the Commitments, except to the extent that such liabilities result from the wilful default, recklessness, gross negligence or bad faith of the Trustee, its employees, agents or advisors.
36. At the expense of Merck, the Trustee may appoint advisors (in particular for corporate finance or legal advice), subject to Merck’s approval (this approval not to be unreasonably withheld or delayed) if the Trustee considers the appointment of such advisors necessary or appropriate for the performance of its duties and obligations under the Mandate, provided that any fees and other expenses incurred by the Trustee are reasonable. Should Merck refuse to approve the advisors proposed by the Trustee the Commission may approve the appointment of such advisors instead, after having heard Merck. Only the Trustee shall be entitled to issue instructions to the advisors. Paragraph 28 of these Commitments shall apply mutatis mutandis. In the Trustee Divestiture Period, the Divestiture Trustee may use advisors who served Merck during the Divestiture Period if the Divestiture Trustee considers this in the best interest of an expedient sale.
37. The Parties agree that the Commission may share Confidential Information proprietary to the Parties with the Trustee. The Trustee shall not disclose such information and the principles contained in Article 17 (1) and (2) of the Merger Regulation apply mutatis mutandis.
38. The Parties agree that the contact details of the Monitoring Trustee are published on the website of the Commission's Directorate-General for Competition and they shall inform interested third parties, in particular any potential purchasers, of the identity and the tasks of the Monitoring Trustee.
39. For a period of ten (10) years from the Effective Date the Commission may request all information from the Parties that is reasonably necessary to monitor the effective implementation of these Commitments.
IV. Replacement, discharge and reappointment of the Trustee
40. If the Trustee ceases to perform its functions under the Commitments or for any other good cause, including the exposure of the Trustee to a Conflict of Interest:(a) the Commission may, after hearing the Trustee and Merck, require Merck to replace the Trustee; or(b) Merck may, with the prior approval of the Commission, replace the Trustee.
41. If the Trustee is removed according to paragraph 40 of these Commitments, the Trustee may be required to continue in its function until a new Trustee is in place to whom the Trustee has effected a full hand over of all relevant information. The new Trustee shall be appointed in accordance with the procedure referred to in paragraphs 19-26 of these Commitments.
42. Unless removed according to paragraph 40 of these Commitments, the Trustee shall cease to act as Trustee only after the Commission has discharged it from its duties after all the Commitments with which the Trustee has been entrusted have been implemented. However, the Commission may at any time require the reappointment of the Monitoring Trustee if it subsequently appears that the relevant remedies might not have been fully and properly implemented.
Section F. The review clause
43. The Commission may extend the time periods foreseen in the Commitments in response to a request from Merck or, in appropriate cases, on its own initiative. Where Merck requests an extension of a time period, it shall submit a reasoned request to the Commission no later than one month before the expiry of that period, showing good cause. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time send a non-confidential copy of the report to the Notifying Party. Only in exceptional circumstances shall Merck be entitled to request an extension within the last month of any period. Sigma agrees to be bound by any and all such extensions.
44. The Commission may further, in response to a reasoned request from the Parties showing good cause waive, modify or substitute, in exceptional circumstances, one or more of the undertakings in these Commitments. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time send a non-confidential copy of the report to the Parties. The request shall not have the effect of suspending the application of the undertaking and, in particular, of suspending the expiry of any time period in which the undertaking has to be complied with.
Section G. Entry into force
45. The Commitments shall take effect upon the date of adoption of the Decision.
[Signed]
……………………………………
duly authorised for and on behalf of Merck
[Signed]
duly authorised for and on behalf of Sigma
SCHEDULE
1. For purposes of the Commitments and this Schedule, the following terms shall have the following meaning:(a) Solvents: (i) high performance liquid chromatography solvents, (ii) regulated solvents, (iii) technical grade solvents, (iv) spectroscopy solvents, and (v) gas chromatography solvents.(b) Inorganics: (i) volumetric/titration solutions, (ii) inorganic salts, (iii) acids, (iv) bases, (v) buffers, (vi) auxiliaries, (vii) indicators, and (viii) Karl Fischer titration solutions.(c) For the avoidance of doubt, the above include all solvents and inorganics produced at Seelze.
2. The Divestment Business comprises the following elements:(a) Seelze, as described in paragraphs 3-6 below.(b) The Fluka Business, as described in paragraphs 7-11 below.(c) The Sigma-Aldrich Business, as described in and limited to paragraphs 12-14 below, and(d) The contracts, rights, and other assets described in paragraphs 15-end below.
Seelze
3. Seelze consists of the issued and outstanding shares of Sigma-Aldrich Laborchemikalien GmbH (“SAGL”), including the assets it currently owns and controls at its Seelze production site and all related assets, sourcing and distribution contracts relating to the Divestment Business.
4. Enclosed in Appendix 1 is an overview of the assets and equipment owned by Sigma at Seelze. For the avoidance of doubt these assets include all assets in Seelze currently used in relation to the Divestment Business and thus will be included in the Divestment Business. To the extent assets in the Divestment Business are currently packaged in returnable drums, returnable drums will be included.
5. Seelze shall also include:(a) the transfer of Sigma’s Seelze's inventory of Solvents and Inorganics at the time of closing of the divestiture sale to the Purchaser;(b) the transfer of Sigma’s authorizations, licenses, files and documentation relating to the Solvents and Inorganics manufactured by SAGL at Seelze [CONFIDENTIAL];(c) the transfer of existing sales and promotional material designed for and used in connection with the Solvents and Inorganics manufactured in Seelze;
6. [CONFIDENTIAL].
Fluka Business
7. The Fluka Business consists of Solvents and Inorganics sold by Sigma (both in catalogue format and in bulk format) worldwide under the Fluka brand.
8. The Parties will transfer to the Purchaser the Fluka brand and all associated trademarks and product names worldwide for unlimited use, and the Purchaser will have the perpetual and exclusive right to sell Fluka-branded products at a worldwide level. In any countries where there are registered Fluka trademarks or pending applications for registration at the time of divestment, these will be assigned to the Purchaser. In any countries where there are no registered trademarks at the time of divestment, Merck will cease to use the Fluka trademark.(a) For those products currently sold under the Fluka brand that are not included in the Divestment Business, Merck commits to re-brand these products to other Merck or Sigma brands within [CONFIDENTIAL] from closing of the sale of the Divestment Business.
9. The Parties will transfer to the Purchaser full rights to Sigma’s patents concerning the Fluka Business. The list of such patents is enclosed in Appendix 2.
10. The Parties will transfer to the Purchaser all of Sigma’s know-how that is used by Sigma exclusively or primarily to develop, manufacture, sell and use Fluka Solvents and Inorganics included in the Divestment Business and any intellectual property rights in such know-how (the “Exclusively or Primarily Used Know-how”). To the extent required by the Purchaser, the Parties will provide a royalty-free, irrevocable, non-exclusive, global license under Sigma’s intellectual property rights to use all know-how currently used by Sigma to manufacture, sell and use the Fluka Solvents and the Fluka Inorganics but which is also currently used by Sigma for the manufacturing of other products (or the same products under different brands) or is otherwise not exclusively or primarily used for the manufacturing of the Fluka Solvents and Fluka Inorganics (the “Licensed Know-How”).
11. To the extent there is any know-how of third-party manufacturers for the Fluka Solvents and Fluka Inorganics, the Parties will use their best efforts to provide the Purchaser with continued access to this know-how through agreements with the applicable manufacturers.
Sigma-Aldrich Business
12. The Sigma-Aldrich Business consists of Solvents and Inorganics sold by Sigma (both in catalogue form and in bulk format) under the Sigma-Aldrich brand in the EEA.
13. For the avoidance of doubt:(a) products sold by Sigma under the following brands shall not be included in the business to be divested: "Sigma," "Aldrich," "Supelco," "SAFC," "SAFC Hitech," "Proligo," "Cerilliant," "Vetec," "BioReliance," and "Cell Marque".(b) NMR, Dried Anhydrous solvents, derivatization reagents and ionophores sold by Sigma shall not be included in the business to be divested, and(c) It is intended that any reference to the Sigma-Aldrich Business, Sigma-Aldrich brand or Sigma-Aldrich in these Commitments shall be limited to Sigma-Aldrich Solvents and the Sigma-Aldrich Inorganics as defined in paragraph 1.
14. The Purchaser will receive a royalty-free license limited to the EEA to use the Sigma- Aldrich brand and any associated trademarks and product names for a period of [CONFIDENTIAL] from closing of the sale of the Divestment Business for the purposes set forth in subparagraph (a).(a) During this [CONFIDENTIAL] period, Purchaser will be allowed to sell existing inventory of finished products of Sigma-Aldrich Solvents and Sigma-Aldrich Inorganics brand in the EEA. During the same [CONFIDENTIAL] period, the Purchaser will be allowed to continue producing and selling Sigma-Aldrich Solvents and Sigma-Aldrich Inorganics in the EEA, but shall not be allowed to produce, launch into the market or otherwise sell any Sigma-Aldrich branded products other than the Sigma-Aldrich Solvents and Sigma-Aldrich Inorganics.(b) During this [CONFIDENTIAL] period the Purchaser shall re-brand the Sigma- Aldrich Solvents and Inorganics to one of its other brands, and Merck shall not be allowed to sell any Solvents or Inorganics under the Sigma-Aldrich brand in the EEA. The initial one-year period can be extended for multiple [CONFIDENTIAL] of extensions in the aggregate, if at the end of the initial [CONFIDENTIAL] period, notwithstanding its best efforts, the Purchaser will require additional time for the re- branding of the products. In such case, the Purchaser will submit a reasoned request for extension to the Monitoring Trustee no later than one month before the expiry of that period. The request shall be based on objective criteria to be determined under the supervision of the Monitoring Trustee who will in its judgment determine whether the criteria for the extension are met. The Monitoring Trustee will specifically consider in making its decision as to whether to grant an extension whether Purchaser’s actions prior to the date of the request represent Purchaser’s best efforts to complete the re-branding of the products during the initial one-year period (or during any extension period previously granted by the Monitoring Trustee).(c) At the expiry of this initial [CONFIDENTIAL] period or, as the case may be, the extended period as specified in b) above, Merck will negotiate an agreement with the Purchaser either (i) for Merck to purchase [CONFIDENTIAL]; or (ii) for the Purchaser to re-label [CONFIDENTIAL].(d) The initial [CONFIDENTIAL] period and any additional periods which may be determined in accordance to subparagraph b) above will be followed by an additional [CONFIDENTIAL] “black out” period during which neither Merck nor the Purchaser will be allowed to sell solvents or inorganics under the Sigma-Aldrich brand in the EEA. Notwithstanding anything herein to the contrary, in no event will the aggregate time periods described in the preceding subparagraphs a)-e) exceed [CONFIDENTIAL].(e) The Parties shall not sell solvents and inorganics in the EEA under the "Sigma" or the "Aldrich" brands during the periods referenced in sub-paragraphs 14 a) to e).
Ancillary Equipment
15. At the option of the Purchaser, the Parties shall make available to the Purchaser any additional assets and equipment for use in Seelze which may be necessary to produce the volumes of Solvents and Inorganics included in the Divestment Business which are currently manufactured in other sites. The Parties and the Purchaser will jointly and in good faith make this determination under the supervision of the Monitoring Trustee.
IP and Know-How
16. The Parties will transfer to the Purchaser the Exclusively or Primarily Used Technology.
17. To the extent required by the Purchaser, the Parties will provide the Purchaser with the Licensed Know-How.
18. Except for those rights that will be transferred, the Parties shall grant Purchaser a license to Sigma’s rights in the patents, other IP, and know-how owned by or licensed to Sigma that are used in the Divestment Business, including those related to the relevant labels and packaging, as well as product certification and documentation.
19. The Parties shall transfer all Sigma-held marketing and technical materials and know-how (or license them when these are shared) that are used in the Divestment Business, including any relevant videos, cds, database with applications, documented methodology techniques and instruments related to the Divestment Business.
20. The Parties shall transfer, to the extent legally possible, (i) all Sigma-held and relevant REACH registration/authorization and existing HACCP cGMP standard approvals/registrations/documentation (if any) for all products divested, including those that relate to pipeline products and (ii) all shared documents for quality certificates and approval. This documentation is product specific and will no longer be used by the Parties after closing of the sale of the Divestment Business.
21. The Parties shall transfer all Sigma-held data related to the quality management system of the divested products, including historic records on non-compliance and corrective/preventive actions.
22. The Parties shall transfer to the Purchaser full rights to the following trade names and trademarks: Aerosol, Hydranal, Perdrogen, Riedel-De-Haen, TraceSelect, Tiron Trolox Chromasolv, Fixanal, and Trace Select Ultra. In any countries where any of these trade names or trademarks are registered or pending applications for registration exist at the time of Closing, these will be assigned to the Purchaser. In any countries where there are no registered trademarks at the time of Closing, Merck will cease to use these trademarks. For those products currently sold under these trademarks that are not included in the Divestment Business, Merck will commit to cease using these trademarks and to re- brand these products to other Merck or Sigma brands/trademarks within [CONFIDENTIAL] from closing of the sale of the Divestment Business.
23. The Parties shall transfer to Purchaser all product descriptions and related product information displayed on Sigma’s e-commerce platform, to the extent that they relate solely or predominantly to the Divestment Business; provided, however, that Sigma’s e-commerce platform itself shall not be included in the business to be divested.
R&D
24. To the extent it concerns solely or predominantly new products or products under development within the scope of the Divestment Business, the Parties shall transfer all R&D and pipeline projects and related information existing at the Effective Date to the Purchaser or will facilitate such transfer, under the supervision of the Monitoring Trustee. To the extent it concerns new products or products under development which do not relate solely or predominantly to the Divestment Business, the Parties will provide a royalty-free, irrevocable, non-exclusive, global license to these R&D and pipeline projects.
25. To the extent any such agreements exist and concern solely or predominantly new products or products under development within the scope of the Divestment Business, the Parties will transfer to the Purchaser all assignable R&D agreements with third parties, and will facilitate the transfer of any such agreements which are non-assignable, under the supervision of the Monitoring Trustee.
Personnel
26. [CONFIDENTIAL]:
(a) [CONFIDENTIAL].
(b) [CONFIDENTIAL].
(c) [CONFIDENTIAL].
(d) [CONFIDENTIAL].
27. [CONFIDENTIAL].
Sourcing / supply contracts
28. The Parties will transfer all assignable contracts and will use their best efforts to facilitate the transfer to the Purchaser of all other contracts entered into by Sigma for the sourcing of the products included in the Divestment Business.
Distribution
29. Sigma will transfer to the Purchaser all assignable distribution contracts or will use its best efforts to facilitate the transfer of the other distribution contracts relating to the Divestment Business – Appendix 6 contains a list of the relevant distribution contracts.
Logistics
30. Sigma will transfer to the Purchaser all assignable distribution contracts or will use its best efforts to facilitate the transfer of the other distribution contracts relating to the Divestment Business. It will do the same in relation to the contracts with logistics companies that it currently uses for the delivery and shipping of the products included in the Divestment Business.
Customer supply agreements
31. The Parties will transfer all assignable customer supply agreements and will use their best efforts to facilitate the transfer of all other existing agreements related to the Divestment Business.
Customer lists
32. The Parties will transfer the list of all customers (including for bulk and catalogue products) of the Divestment Business, regardless of the sales channel used, for the period from January 2012 to the date of closing of the Merck/Sigma transaction, with contact details as well as all other available customer specific information, including but not limited to, customer records, customer reports, transactional data and customer accreditations.
Access obligations
33. For a transitional period of up to a maximum of [CONFIDENTIAL] after Closing, the Parties commit to provide the Purchaser with the following services [CONFIDENTIAL]:
(a) a sales channel via Sigma's e-commerce site for the purpose of selling the products included in the Divestment Business until the Purchaser has its own platform where these sales can be re-directed or until the Purchaser has effected the re-redirection to its own website. For these purchases Sigma's e-commerce site will provide a disclaimer, as agreed between the Parties and the Purchaser under the supervision of the Monitoring Trustee, indicating that the purchased products are those of the Purchaser, and re-direct/link to the Purchaser's own sales channel once this is operational.
(b) For a transitional period until the Purchaser has adequate facilities in place (but in any case up to a maximum of [CONFIDENTIAL] after Closing), a distribution service through the Parties' distribution centres and warehouses for the purpose of distribution of the products included in the Divestment Business to its customers, to be provided at a cost basis.
(c) For a transitional period until the Purchaser has adequate facilities in place (but in any case up to a maximum of [CONFIDENTIAL] after Closing), order entry services permitting orders from Purchasers’ customers to be entered, logged and processed by Sigma personnel and through Sigma’s systems on behalf of the Purchaser, to be provided at a cost basis.
Transitional Agreements
34. At the option of the Purchaser, the Parties will enter into the following transitional agreements under the supervision of the Monitoring Trustee, [CONFIDENTIAL], for a term of [CONFIDENTIAL] after the Closing, extendable for [CONFIDENTIAL]:
(a) A technological support agreement to provide support and training for the implementation of the Sigma licensed know-how in Seelze.
(b) A technical assistance agreement in relation with the manufacture of Solvents and Inorganics.
(c) A support agreement to provide the support in sourcing the necessary equipment and raw materials for the production of the products under the Divestment Business, and procuring logistics and distribution services for the distribution of products under the Divestment Business.
(d) A supply agreement with the Purchaser for the supply of the Solvents and Inorganics included in the Divestment Business which are currently manufactured in sites other than Seelze.
(e) A support agreement to use Parties' best efforts to advise, to the extent available within Sigma, the Purchaser in relation to building its own e-commerce platform or other sales channels.
[CONFIDENTIAL - Annexes 1 to 6 follow]
1 OJ L 24, 29.1.2004, p. 1 ('the Merger Regulation'). With effect from 1 December 2009, the Treaty on the Functioning of the European Union ('TFEU') has introduced certain changes, such as the replacement of 'Community' by 'Union' and 'common market' by 'internal market'. The terminology of the TFEU will be used throughout this Decision.
2 OJ L 1, 3.1.1994, p.3 ("the EEA Agreement").
3 Publication in the Official Journal of the European Union No C 139, 28.04.2015, p. 4.
4 Turnover calculated in accordance with Article 5(1) of the Merger Regulation and the Commission Consolidated Jurisdictional Notice (OJ C95, 16.04.2008, p.1).
5 Since cell culture products can be used both as bioscience research products and as raw materials for bio-pharmaceuticals production, for the sake of clarity, they are examined as a separate group of products in the present Decision.
6 Within bioscience for research, the Parties' activities also overlap in other areas (i.e. molecular and proteomics). However, the Transaction will not lead to affected market in these areas and therefore they would not be further discussed in this Decision.
7 General biochemicals also include products such as substrates, buffers, antibiotics, detergents, amino acids and peptides, nucleic acids, lipids and derivatives, protein separation, hormones and steroids and FLAG and other epitope tagging purification. There is no affected market in relation to these products and they will thus not be discussed further in this Decision.
8 See, e.g. case M.6944 – Thermo Fisher Scientific/Life Technologies; see also M.5264 –
Invitrogen/Applied Biosystems.
9 See replies to question 37 – Phase I questionnaire to competitors.
10 See, e.g., cases M.5264 - Invitrogen/Applied Biosystems; M.5863 - Merck/Millipore; and M.6944 –
Thermo Fisher Scientific/Life Technologies.
11 See, e.g., cases M.5264 - Invitrogen/Applied Biosystems; M.5611 – Agilent/Variant; M.6126 – Thermo Fisher/Dionex Corporation.
12 See replies to questions 38 and 39 – Phase I questionnaire to competitors. See also replies to questions 27 and 29 – Phase I questionnaire to customers.
13 See replies to questions 44, 79 and 88.3 – Phase I questionnaire to competitors.
14 Sigma withdrew from the IVD market in 2004 with the exception of a few legacy products. The Transaction does not give rise to an affected market regarding a hypothetical market for IVD dyes and stains.
15 See replies to questions 72 – Phase I questionnaire to customers.
16 See e.g. case M.6944 – Thermo Fisher Scientific / Life Technologies.
17 See case M.6944 – Thermo Fisher Scientific / Life Technologies.
18 See replies to question 35 – Phase I questionnaire to competitors.
19 See replies to question 23 – Phase I questionnaire to customers.
20 See, e.g., cases M.5264 - Invitrogen/Applied Biosystems; M.5863 - Merck/Millipore; and M.6944 –
Thermo Fisher Scientific/Life Technologies.
21 See replies to questions 38 and 39 – Phase I questionnaire to competitors, and replies to questions 27 and 29 - Phase I questionnaire to customers.
22 During the market investigation, several market participants also suggested that the Transaction may give rise to a vertical relationship between the upstream markets for the supply of raw materials for cell culture media and the downstream markets for the production of cell culture media for bioproduction (see, e.g. replies to questions 74 and 88.3 – Phase I questionnaire to competitors and comments submitted by one customer on 4.05.2015). However, as evidenced further below, the Parties have moderate market shares in the downstream markets for the supply of cell culture media for bioproduction. More importantly, regarding hypothetical markets for the supply of raw materials for cell culture media, the Parties supply a variety of raw materials used in different applications (including, but not limited to, the production of cell culture media), and they are not able to distinguish, let alone price discriminate by, the end application of these raw materials. The hypothetical vertical link between the Parties' activities in cell culture is therefore unlikely to result in a significant impediment to effective competition, and will not be discussed further.
23 See replies to question 61 – Phase I questionnaire to customers.
24 See reply of a customer to questions 61 – Phase I questionnaire to customers.
25 See reply of a customer to question 60 – Phase I questionnaire to customers.
26 See replies to question 72 – Phase I questionnaire to customers.
27 The Parties both provide good manufacturing practice services and contract manufacturing, as well as consulting services on validation and compliance.
28 Directive 2001/83/EC of 6 November 2001 on the Community code relating to medicinal products for human use.
29 The Parties' activities also overlap with regards to other biophram ingredients, such as amino acids and other processing chemicals. However, it does not lead to any affected market and, thus, these products will not be further discussed in the Decision.
30 See reply of a competitor to question 30 – Phase I questionnaire to competitors.
31 See reply of a competitor question 32 – Phase I questionnaire to competitors.
32 See reply of a customer to question 20 – Phase I questionnaire to customers.
33 See replies to question 20 – Phase I questionnaire to customers.
34 Guidelines of 19 March 2015 (2015/C95/02) on the formalized risk assessment for asserting the appropriate good manufacturing practice for excipients of medicinal products for human use.
35 See replies to question 29.3.3 – Phase I questionnaire to competitors.
36 See replies to question 39 – Phase I questionnaire to competitors. See also replies to question 29 – Phase I questionnaire to customers.
37 For instance, Merck's top ten cell culture media customers represent […]% of its sales and its top ten cell culture reagents and supplements customers represent more than […]% of its sales. Sigma's customer base is broader but the top 10 customers also cover an estimated […]% of the sales.
38 Directive 2011/62/EU (the "Falsified Medicines Directive") regarding GMP manufacturing, directive 2001/83/EC regarding the European Pharmacopeia.
39 See case M.3354 – Sanofi-Synthélabo / Aventis.
40 In the overall market for buffers for pharmaceutical production, the Parties' combined market shares is [10-20]% with and addition of [5-10]%.
41 See replies to question 47 – Phase I questionnaire to customers.
42 Agreed minutes of the call with a competitor, 12.05.2015.
43 See replies to question 34 – Phase I questionnaire to customers.
44 See agreed minutes of calls with customers dated 18.02.2015, 06.03.2015, 25.03.2015 and 26.03.2015 and agreed minutes of calls with competitors dated 18.02.2015, 19.02.2015, 23.02.2015 and 23.03.2015.
45 See replies to questions 19-20 – Phase I questionnaire to competitors.
46 The Parties also indicated being active in the supply of derivitization reagents, which are compounds used to modify a substrate or an inorganics surface into a derivate. However, as Merck's sales in this segment are de minimis (below EUR […] in 2014), they will not be discussed further
47 See replies to questions 21-22 - Phase I questionnaire to competitors.
48 See replies to questions 21-24 – Phase I questionnaire to competitors.
49 See replies to questions 21-24 – Phase I questionnaire to competitors.
50 The Parties also indicated very marginal overlaps in food and water testing and bottled lab water. Given the very limited market shares of the Parties on these markets, they will not be discussed further.
51 See replies to questions 14-18 – Phase I questionnaire to competitors and replies to questions 15-18 – Phase I questionnaire to customers.
52 See replies to question 18 – Phase I questionnaire to customers.
53 See reply of a customer to question 18 – Phase 1 questionnaire to customers.
54 See replies to question 15 – Phase 1 questionnaire to competitors.
55 See replies to question 10 – Phase I questionnaire to bulk manufacturers.
56 See replies to question 16 – Phase 1 questionnaire to customers and replies to question 14 – Phase 1 questionnaire to competitors.
57 See replies to question 39 – Phase 1 questionnaire to competitors. See also replies to question 29 – Phase 1 questionnaire to customers.
58 See replies to question 26 – Phase 1 questionnaire to customers.
59 See replies to question 26 – Phase 1 questionnaire to customers.
60 See replies to question 38 – Phase 1 questionnaire to competitors.
61 See replies to question 28 – Phase 1 questionnaire to customers and replies to question 40 – Phase 1 questionnaire to competitors.
62 See reply of a competitor to question 30.1 – Market test of the commitments questionnaire to competitors.
63 Reply of a competitor to question 30.1 – Market test of the commitments questionnaire to competitors.
64 See regulations as to the Atmosphères Explosibles (ATEX) Explosion prevention guidelines 94/9/EG (ATEX 95) and 1999/62/EG (ATEX 137) regulating use and handling of solvents, and the European Regulation on classification, labelling and packaging (GLP) adopting the globally harmonized system of chemical classification and labelling (GHS) created by the United Nations to inform users about such hazards.
65 Reply of a competitor to question 30.1 – Market test of the commitments questionnaire to competitors.
66 There is no affected market with respect to bulk sales of solvents and inorganics, where the Parties are competing with the main chemical manufacturers, such as Ineos, BASF, Akzo Nobel and Dow. Therefore, these markets will not be discussed further in the Decision.
67 See replies to question 2 – Phase I questionnaire to competitors.
68 See replies to question 43 – Phase I questionnaire to competitors.
69 See, in particular, replies to question 39.1 – Phase I questionnaire to customers and replies to questions 51 – Phase I questionnaire to competitors.
70 See replies to questions 39.1 and 40 – Phase 1 questionnaire to customers.
71 See replies to question 41 – Phase I questionnaire to customers.
72 See reply of a customer to question 41 – Phase I questionnaire to customers.
73 See reply of a competitor to question 53 – Phase I questionnaire to competitors.
74 See replies to questions 40 and 43 – Phase 1 questionnaire to customers and replies to question 53 – Phase 1 questionnaire to competitors.
75 Agreed minutes of the call with [Company Name], 06.02.2015.
76 See reply of a customer to question 9 – Market test of the commitments questionnaire to customers.
77 See reply of a customer to questions 41-42 – Phase I questionnaire to customers.
78 See replies to question 52 – Phase 1 questionnaire to competitors.
79 Agreed minutes of the call with [Company Name], 10.02.2015.
80 See reply of one competitor to question 53 – Phase 1 questionnaire to competitors.
81 "Advanced Analytics Lab Productivity – Franchise Strategy ", Merck Millipore, October 2014, Slide 11.
82 "Lab solutions – Lab essentials, Globally acknowledged premium brand for Labs all around the world", Merck Millipore, October 23, 2012, Slide 12.
83 See, in particular, for Sigma, "Inorganic reagents – Deep dive", Merck Millipore, 2013, Slide 14 and for Merck "High quality reagents for every lab", Merck Millipore, March 2015, Slides 64-65.
84 "High quality reagents for every lab", Merck Millipore, March 2015, Slides 64-65.
85 See replies to question 37.1 – Phase 1 questionnaire to customers.
86 Agreed minutes of the call with [Company Name], 19.02.2015.
87 Agreed minutes of the call with [Company Name], 10.02.2015.
88 "Proposed Barolo acquisition, taking our life science tools to the next level", Merck, September 19 2014, slide 13.
89 "Advanced Analytics Lab Productivity – Franchise Strategy", Merck Millipore, October 2014, Slide 11. Sigma is also considered as [quote of internal assessment] ("Lab Productivity – Comprehensive Class Room Training – oct 27th 2014", Merck Millipore, Slide 12).
90 "High quality reagents for every lab", Merck Millipore, March 2015, Slides 64-65.
91 "Inorganic reagents – Deep dive", Merck Millipore, 2013, Slide 14.
92 See replies to question 39.1 – Phase I questionnaire to customers and replies to question 51 – Phase I questionnaire to competitors.
93 "Proposed Barolo acquisition, taking our life science tools to the next level", Merck, September 19 2014, Slide 40.
94 "Proposed Barolo acquisition, taking our life science tools to the next level", Merck, September 19 2014, Slide 13.
95 "Proposed Barolo acquisition, taking our life science tools to the next level", Merck, September 19 2014, Slide 13; see also "MerckMillipore I Sigma Aldrich, PMI Proposal for support - Appendix", [author], Slide 34.
96 « Advanced Analytics Lab Productivity – Franchise Strategy » Merck Millipore , October 2014, Slide 11.
97 Agreed Minutes of the call with (company Name) 23.02.2015 ; Agreed minutes of the call with (company name) 25.03.2015 and replies to question 43 – Phase 1 questionnaire to competitors.
98 Merck Millipore I Sigma Aldrich PMI Proposal for support – Appendix (author) 9 october 2014 slide 4.6 ; see also « Western European market – Assessment of Lab Essentials « Merck Millipore 30 April 2013 slides 6 and 47.
99 See replies to question 30-Phase I questionnaire to customers and replies to question 43 – Phase I questionnaire to competitors.
100 Agreed minutes of the call with (company name) 18.02.15.
101 See replies to question 33-Phase I questionnaire to customers and replies to question 45 – Phase I questionnaire to competitors.
102 Based on the data provided by the Notifying Party for 2013 the only EEA menber states where the market for catalogue solvents would not be affected were Hungary, Poland, Slovakia and Sweden, where the combined market share of the Parties was slightly below 20% (respectively (10-20%), (10-20%) and (10-20%)and Iceland, Lichtenstein, Luxembourg and Malta, where Merck would hot be active but Sigma would have a significant presence, with a market share respectively of (60-70%), (20-30%), 30-40%), and (50-60%).
103 For instance as regards Germany, the Parties combined market shares could be above 50%von the markets concerned. This was suggested in the market investigation, see e.g. Agreed minutes of the call with (competitor name) 23.02.2015, Agreed minutes of the call with (competitor name) 02.02.15 and it is in line with the Partie’s estimates expressed in eternal documents, according to which for 2012 the Partie’s market shares in Germany were (50-60%) for solvents (30-40%) for Sigma and (20-30%) for Merck ) Germany representing (20-30%) of the western European market for solvents see « Western European market- Assessment of Lab Essentials » Merck Millipore 30 april 2013, slide 8 and 13 ; see also « Merck Millipore I Sigma Aldrich, PMI Proposal for support – Appendix (author) slide 13. This is also line with the findings of the German competition authority in 2011 in its decision B3-64/05 Merck/VWR.
104 Merck Millipore I sigma Aldrich, PMI Proposal for support -Appendix (author) 9 october 2014, slide 4.6 ; see also « Western European market – Assessment of Lab Essentials », Merck Millipore, 30 April 2013, slides 6 and 47.
105 See replies to question 33 – Phase 1 questionnaire to customers and replies to question 45-phase 1 questionnaire to competitors.
106 See reply of a competitor to question 69- Phase I questionnaire to competitors.
107 See replies to question 33 – Phase 1 questionnaire to customers and replies to question 45-phase 1 questionnaire to competitors.
108 Based on the data provided by the Notifying Party, the only EEA member states where the market for catalogue inorganics would not be affected were Slovakia, where the combined market share of the Parties was Slightly below 20% (10-20%) and Iceland, Malta and Lichtenstein, where Merck would not be active and Sigma would have a respective market share of (30-40%) (20-30%) and (10-15%).
109 For instance, as regards Germany, the Parties' combined market shares could be above [50-60]% on the markets concerned. According to an internal document, the Parties' market shares in Germany were [50-60]% for inorganics ([20-30]% for Sigma and [20-30]% for Merck) in 2012, Germany representing [20-30]% of the Western European market for inorganics, see "Western European market – Assessment of Lab Essentials", Merck Millipore, 30 April 2013, Slides 8 and 13; see also "Merck Millipore I Sigma Aldrich, PMI Proposal for support - Appendix", [author], Slide 13. This is also line with the findings of the German competition authority in 2011 in its decision B3-64/05 Merck/ VWR.
110 Reply of one competitor to question 4 – Market test of the commitments questionnaire to competitors.
111 See reply of one customer to question 41 – Phase I questionnaire to customers.
112 See replies to questions 61-63 – Phase I questionnaire to competitors.
113 See reply of one competitor to question 63 – Phase I questionnaire to competitors.
114 See reply of one competitor to question 61 – Phase I questionnaire to competitors.
115 See reply of a competitor to question 61.1 – Phase I questionnaire to competitors.
116 See replies to question 8.2 – Phase I questionnaire to bulk manufacturers.
117 See replies to question 9 – Phase I questionnaire to bulk manufacturers.
118 See replies to question 9 – Phase I questionnaire to bulk manufacturers.
119 See replies to question 10.1 – Phase I questionnaire to bulk manufacturers.
120 See reply of a bulk manufacturer to question 9 – Phase I questionnaire to manufacturers.
121 See replies to question 18 – Phase I questionnaire to manufacturers.
122 See reply of a competitor to question 63 – Phase I questionnaire to competitors.
123 See reply of a competitor to question 63 – Phase I questionnaire to competitors.
124 Based on value market shares calculated on 2013 sales, aside from catalogue organics, the market segments concerned are building blocks, catalysts, reagents, and within other chemicals, UV Vis Standards (reference materials), TLC plates (analytical chromatography), Ready to Use Liquid Media, Ready to Use Solid Media and Raw Materials/Supplements (industrial microbiology).
125 This is also confirmed at national level, where Merck's market share in organics was [5-10]% or below in all EEA member states except Denmark ([5-10]%), Germany ([5-10]%) and Slovenia ([10-20]%), based on the data provided by the Notifying Party for 2013. According to the Parties' own internal documents, Merck's market share in organics was limited in all the main Western European countries, up to a maximum of [10-20]% in France and Germany in 2012 (see "Western European market – Assessment of Lab Essentials", Merck Millipore, 30 April 2013, Slides 13, 61, 65, 69, 73, 77 and 81).
126 The absence of significant overlap is confirmed at national level, where the market share increment in reference materials, analytical chromatography and industrial microbiology is always [5-10]% or below in all EEA member states, based on the data provided by the Notifying Party for 2013. According to the Parties' own internal documents, Sigma's market share in chromatography was limited in all the main Western European countries, up to a maximum of [10-20]% in Italy in 2012 (see "Western European market – Assessment of Lab Essentials", Merck Millipore, 30 April 2013, Slides 13, 61, 65, 69, 73, 77 and 81).
127 See "Western European market – Assessment of Lab Essentials", Merck Millipore, 30 April 2013, Slide 58.
128 See replies to question 30 – Phase I questionnaire to customers.
129 See replies to question 43 – Phase I questionnaire to competitors.
130 See replies to question 32 – Phase I questionnaire to customers. There were scattered reports, with one customer reporting Merck's strength in TLC plates, an affected market on which Sigma has a market share below [0-5]%. One distributor also generically indicated reference standards as an area of strength.
131 See replies to question 44 – Phase I questionnaire to competitors.
132 In the case of HPLC Columns, the Parties have a combined market share of [5-10]%.
133 Replies to question 28 – Phase I questionnaire to competitors.
134 Merck also has a distribution business with total worldwide sales in 2013 of EUR […], but […]de minimis activities in the EEA, […].
135 See cases M.4242 – Thermo Electron/Fisher Scientific and M.6944 – Thermo Fisher Scientific/Life Technologies.
136 See e.g. cases M.7249 – CVC / ParexGroup and M.2244 – Royal Vopak / Ellis & Everard.
137 See cases M.4242 – Thermo Electron/Fisher Scientific and M.6944 – Thermo Fisher Scientific/Life Technologies.
138 Replies to question 6 – Phase 1 questionnaire to competitors.
139 Cell culture: lipid-based reagents and supplements for bio-production customers ([30-40]%).Laboratory chemicals: catalogue solvents in the EEA ([30-40]%) and catalogue inorganics in the EEA ([30-40]%), as well as a number of sub-segments and national markets.
140 Commission Notice on remedies acceptable under Council Regulation (EC) No 139/2004 and under Commission Regulation (EC) No 802/2004 (OJ C 267, 22.10.2008, p. 1-27.
141 Remedies Notice, paragraphs 9 and 61.
142 Remedies Notice, paragraph 12.
143 Remedies Notice, paragraph 10.
144 As well as some activities outside of the EEA relating to the Fluka brand (see below).
145 […]
146 See replies to question 2 – Questionnaire R1 Market test of the Commitments Competitors, and replies to question 1 – Questionnaire R2 Market test of the Commitments Customers.
147 See replies to questions 7 and 8 – Market test of the commitments questionnaire to competitors, and replies to questions 11 and 14 – Market test of the commitments questionnaire to customers.
148 See replies to questions 24, 27 and 32.2 – Market test of the commitments questionnaire to competitors, and replies to questions 24 and 29.2 – Market test of the commitments questionnaire to customers.
149 See replies to question 16 – Market test of the commitments questionnaire to competitors, and replies to question 27 – Market test of the commitments questionnaire to customers.
150 See replies to question 29 – Market test of the commitments questionnaire to competitors, and replies to question 31 – Market test of the commitments questionnaire to customers.