Commission, June 22, 2017, No M.8348
EUROPEAN COMMISSION
Decision
RAG STIFTUNG / EVONIK INDUSTRIES / HUBER SILICA
Subject: Case M.8348 – RAG STIFTUNG / EVONIK INDUSTRIES / HUBER SILICA
Commission decision pursuant to Article 6(1)(b) in conjunction with Article 6(2) of Council Regulation No 139/2004 (1) and Article 57 of the Agreement on the European Economic Area (2)
Dear Sir or Madam,
(1) On 27 April 2017, the European Commission received notification of a proposed concentration pursuant to Article 4 of the Merger Regulation and following a referral pursuant to Article 4(5) of the Merger Regulation by which Evonik Industries AG ("Evonik", Germany), controlled by RAG Stiftung (Germany), intends to acquire within the meaning of Article 3(1)(b) of the Merger Regulation sole control of the precipitated silica business ("Huber Silica") of J.M. Huber Corporation ("Huber", US) by way of a purchase of shares and purchase of assets ("the proposed Transaction"). Evonik and Huber are collectively designated hereinafter as "the Parties" and the combination of Evonik and Huber Silica as "the Merged Entity".
1. THE PARTIES
(2) Evonik is headquartered in Germany and listed on the Frankfurt Stock Exchange. In 2016, its world-wide turnover was EUR 12.9 billion (EUR 5.8 billion in the EEA). It is active in the production and marketing of speciality chemicals. Evonik has divided its activities into three business segments: Nutrition and Care, Resource Efficiency and Performanc Materials. Evonik's precipitated silica business is part of Evonik's Business Line Silica within the Resource Efficiency segment.
(3) Huber Silica is part of Huber, a privately owned business headquartered in the US, active in speciality chemicals and minerals, hydrocolloids and engineered woods as well as timber management. In 2016, Huber Silica's world-wide turnover was EUR […] (EUR […] in the EEA). Huber Silica produces precipitated silica with a particular focus on the dental end-user application which accounts for a majority of its sales. In addition, Huber Silica produces sodium silicate which is an input product to precipitated silica.
2. THE OPERATION
(4) On 9 December 2016, the Parties signed a Share and Asset Purchase Agreement for Evonik to acquire four legal entities as well as various assets of Huber, together forming Huber Silica. As a result of the proposed Transaction, Evonik will acquire sole control over Huber Silica.
(5) The notified operation therefore constitutes a concentration within the meaning of Article 3(1)(b) of the Merger Regulation.
3. REFERRAL REQUEST AND EU DIMENSION
(6) On 23 December 2016, the Commission received a referral request under Article 4(5) of the Merger Regulation with respect to the proposed Transaction, which was capable of being reviewed under the national competition laws of Austria, Germany and the United Kingdom. None of these Member States disagreed with the Parties' referral request.
(7) The notified operation therefore has an EU dimension pursuant to Article 4(5) of the Merger Regulation.
4. RELEVANT MARKETS
4.1. Introduction
(8) In the EEA, both Evonik and Huber Silica are active in the manufacture and supply of precipitated silica (see section 4.2.2), while only Huber Silica is active in the upstream market for sodium silicate (see section 4.2.1). In addition, Evonik is active in the neighbouring markets for organofunctional silanes, fumed silica, and betain (see sections 4.2.3, 4.2.4 and 4.2.5 respectively).
4.2. Relevant product markets
4.2.1. Sodium Silicate
4.2.1.1. Product market definition
(9) Sodium silicate, also known as "liquid glass" or "waterglass", is an inorganic chemical product, combining sodium (Na), silicon (Si) and oxygen (O), that can be supplied in a wide range of grades depending for example on purity, alkalinity (3) or water content and in various forms (aqueous solution or solid as powders, granules or lump blocks).
(10) Sodium silicate is mainly used in the manufacture of detergents, pulp/paper and other industrial applications such as the manufacture of precipitated silica. For the production of precipitated silica, sodium silicate can be either used under (i) liquid form (hydrothermal sodium silicate) or (ii) solid form (obtained from a melting process) which needs to be subsequently dissolved in preparation of the production process.
(11) In previous decisions (4), the Commission considered that the market for sodium silicate constitutes a separate relevant product market without any further subdivision and relied on market findings that "the various product presentations are functional and economic substitutes" (5) although switching between the different forms may incur additional costs.
Notifying Party’s views
(12) While noting that transportation costs for liquid sodium silicate are higher than for solid sodium silicate, the Parties agree with the Commission's previous approach and consider in particular that a segmentation of the market by type of industry supplied is not appropriate. The Parties also consider that switching between the different grades and product forms of sodium silicate is easy.
Commission’s assessment
(13) The Parties' competitors confirm that liquid and solid sodium silicate can be used for the manufacture of precipitated silica, namely "liquid as direct input or solid with preliminary dissolution step". (6) No distinction should be made between powders, granules and/or lump blocks (solid forms) since all of these forms need to be dissolved before use and require particular dissolution equipment. However, results of the market investigation show that a distinction could be made between solid and liquid forms of sodium silicate because of the difference in their transport cost and their respective necessary equipment. Similarly, there are indications that the alkalinity of sodium silicate is a key criterion for the production of precipitated silica.
(14) For the purpose of the present decision, the precise market definition can be left open i.e. whether there is an overall product market for sodium silicate or whether further distinctions need to be made according to solid or liquid forms or according to the alkalinity level since the Transaction does not raise serious doubts as to its compatibility with the internal market under any of the above market segmentations.
4.2.1.2. Geographic market definition
(15) The Commission has previously considered (7) the market for sodium silicate to be EEA-wide in geographic scope.
Notifying Party’s views
(16) The Parties consider the geographic market for sodium silicate to be at least EEA- wide.
Commission’s assessment
(17) The results of the market investigation indicated that the relevant geographic scope of the sodium silicate market is EEA-wide, most notably because most market respondents supply or source (as the case may be) their sodium silicate within 1500km away from the plant where it is produced. (8) Also, most market respondents consider that the sodium silicate market is EEA-wide in geographic scope. (9)
(18) In view of the fact that the Transaction does not raise serious doubts as to its compatibility with the internal market in relation to the sodium silicate market under any plausible market definition, the exact scope of the geographic market can be left open for the purposes of the competitive assessment of the Transaction.
4.2.2. Precipitated silica
4.2.2.1. Product market definition
(19) Silica are specialty chemical products manufactured through the acidification of a sodium silicate solution by a mineral acid (such as sulphuric acid) or by carbon dioxide. Silica can typically be classified according to their production process into: (i) precipitated silica, (ii) silica gels and (iii) colloidal silica. Each of these three types of silica is used for several and separate applications. In a previous decision (10), the Commission therefore considered that they are not substitutable from either the supply or the demand-side perspective and that they therefore belong to separate relevant product markets. For the purpose of the present case however, only precipitated silica is concerned by the proposed Transaction and no further reference will be made to silica gels and colloidal silica.
(20) Precipitated silica are manufactured through a precipitation (11) process during which several parameters (12) can be set up to achieve a large spectrum of different properties, related to the precipitated silica particles' pH value (13), absorption capacity, hardness or abrasiveness. During the manufacturing phases which follow precipitation, namely filtration, drying and potentially milling, granulating and/or coating, other physical properties of the final product can be adjusted to produce various average sizes, average specific surfaces, output shapes (such as powders, micro-pearls or granules), bulk densities, moisture contents, hydrophobic properties, etc.
(21) The various grades of precipitated silica give it specific functionalities that are adapted to the needs of various applications, mainly serving as free-flow agents, anti-caking agents, defoamers, carriers, thickening agents, absorbants, fillers, softeners or abrasive agents.
(22) In its previous decisions (14), the Commission has not assessed whether the overall market for precipitated silica could be further segmented into separate relevant product markets according to the end-use applications.
(23) Within the various potential applications of precipitated silica, a first distinction could be made between rubber applications (majority of EEA or worldwide volume), in which precipitated silica act as re-inforcing fillers, and non-rubber applications, in which precipitated silica can have several different functions. Within rubber applications, a distinction could possibly be made between tyre applications (such as conventional and/or fuel-efficient tyres), silicone rubber, footwear (shoe soles) and other rubber goods. Within non-rubber applications, a distinction may be envisaged between (i) dental, (ii) defoamer, (iii) paints and coatings, (iv) paper, (v) feed additive, (vi) food additive, (vii) battery separators, (viii) matting agents, (ix) agriculture applications and (x) other applications.
(24) Besides the above proposed market segmentation by end-use application, an alternative market segmentation could consist in the distinction of precipitated silica according to their chemical composition into (i) "standard" precipitated silica, (ii) aluminium silicate (involving the addition of aluminium sulfate as input raw material at the precipitation step) and (iii) calcium silicate (involving the addition of calcium chloride as input raw material at precipitation step) as well as according to the precipitated silica's hydrophilic/hydrophobic character into (i) hydrophilic precipitated silica (non-surface treated) and (ii) hydrophobic precipitated silica (surface treated).
Notifying Party’s views
(25) While recognising that precipitated silica are used in a multitude of different end- use applications, the Notifying Party does not consider the different areas of usage to constitute plausible relevant product markets because the precipitated silica for different end-uses is fully substitutable from a supply-side perspective.
(26) In this regard the Notifying Party explains that the precipitated silica supplied is chemically essentially the same across all usage areas and that there are no meaningful barriers to entry into any of the different end-uses. Manufacturers of precipitated silica can therefore switch from producing precipitated silica for one end-use application to another relatively quickly, with low sunk costs. (15) In addition the Notifying Party explains that there are various precipitated silica grades that are used interchangeably between a variety of different end-uses. The Notifying Party also claims that average prices depend primarily on customer specificities and not on the targeted end-use application.
(27) Similarly, while recognizing that aluminium silicate, calcium silicate and "standard" precipitated silica slightly vary from a chemical point of view, the Notifying Party is of the opinion that these products do not constitute separate relevant product markets. Apart from the addition of aluminium sulfate and calcium chloride at the precipitation stage for the production of aluminium and calcium silicate respectively, the Notifying Party explains that the overall production process is identical to the production of "standard" precipitated silica grades, that the same production lines can be used, that there are no materially different costs associated to switching between different "standard" precipitated silica grades, that aluminium and calcium silicate generally fulfil similar functionalities as "standard" precipitated silica and that these products are all supplied to several industries. At the Commission's request, the Notifying Party also provided information on the hypothetical markets for aluminium and calcium silicate, further segmented by end-use applications.
(28) At the Commission's request, the Notifying Party also put forward specific arguments concerning hydrophobic (water-repellent) precipitated silica, which is a surface treated as an additional production step. It consists of standard precipitated silica (hydrophilic) which has been surface-treated with silicone oil. The Notifying Party submits that hydrophobic precipitated silica does not constitute a distinct relevant product market and that hydrophilic and hydrophobic precipitated silica can be used interchangeably as free-flow agents. At the same time the Notifying Party recognises that hydrophobic precipitated silica may show superior effects for instance in defoamer and certain food and feed applications.
Commission's assessment according to the chemical nature of precipitated silica
(29) With regard to the hypothetical segmentation according to the chemical nature of precipitated silica into aluminium silicate, calcium silicate and "standard" precipitated silica, results of the market investigation show that aluminium silicate and calcium silicate are not perceived by customers as constituting substitute products to "standard" precipitated silica (16) and are used for their specific properties.
(30) Contrary to "standard" precipitated silica, and as recognized by the Notifying Party, aluminium and calcium silicate are characterized by higher pH values which make them more suitable for certain applications, such as certain plant protection and coating applications for which acid sensitivity is important (calcium silicate).
(31) Similarly, a majority of competitors agree on the fact that aluminium silicate constitute a separate product market from "standard" precipitated silica because they are "suitable only for certain end-use applications" (17), and because, "from a manufacturing perspective, the production process is different […], the products are not substitutable". (18) More specifically, one competitor is of the opinion that aluminium silicate forms a separate product market for certain applications in paper and paints and coatings, but not for remaining end-use applications within rubber, food, feed "where the aluminium silicate behaves as just another grade of precipitated silica". (19)
(32) Concerning calcium silicate, most competitors did not express any opinion. Only one competitor commented on the matter and considered, as for aluminium silicate, that calcium silicate forms a separate product market since calcium silicate "is suitable only for certain end-use applications". (20)
(33) In any event, were separate relevant product markets to be defined for aluminium silicate and/or calcium silicate, the Transaction would only give rise to one affected market in relation to aluminium silicate for paints and coating applications.
(34) In view of the fact that the Transaction does not raise serious doubts as to its compatibility with the internal market in relation to the market for aluminium silicate and calcium silicate, including at the narrower plausible market segmentations per end-use applications, the exact scope of the product market definition for aluminium silicate and calcium silicate can be left open for the purpose of the present case.
Commission's assessment according to the hydrophilic/hydrophobic property
(35) Contrary to standard (hydrophilic) precipitated silica, hydrophobic precipitated silica does "not disperse or mix with water" (21) and "can be made from precipitated silica or fumed silica" (22).
(36) The hydrophobic property of precipitated silica is achieved during the manufacturing process with a specific and final production step through which silicone oil is added at the particles' surface. According to one competitor, this hydrophobisation treatment "create[s] a wholly new manufacturing area with many special requirements, such as VOC (Volatile Organic Compounds), dangerous raw materials, and closed loop or scrubbing emission schemes that complicate production" (23).
(37) As hydrophobisation is performed as a final production step related to the surface treatment, hydrophobisation of particular (hydrophilic) precipitated silica grades can be realised either internally by the precipitated silica manufacturer (ready-to- use hydrophobic precipitated silica) or in situ by the end-user.
(38) Therefore hydrophobic precipitated silica does not constitute a separate end-use application as such but is used for its specific hydrophobic functionality in several end-use applications such as defoamer, paints and coatings and certain food and feed applications.
(39) Concerning the demand-side substitutability of hydrophobic precipitated silica, one competitor explains that, "[i]n most applications the polarity of the precipitated silica is critical. It is not possible to replace one grade for another with a different polarity. The hydrophilic or phobic character of the silica is one of the main contributors to that polarity" (24). Also in light of the results of the market investigation, such observation is confirmed by most customers of hydrophobic precipitated silica who explain that standard (hydrophilic) and hydrophobic precipitated silica grades cannot be used interchangeably because of their different performances (25).
(40) In light of the above, and in particular of the differentiating step in the manufacturing process as well as the absence of demand-side substitutability, the Commission will consider a separate relevant product market for hydrophobic precipitated silica (2% of EEA total market size for precipitated silica in value, 2016). The market for hydrophilic precipitated silica (98% of the total market) will be analysed in more details according to the different end-use applications for precipitated silica.
Commission’s assessment according to different end-use applications for precipitated silica
(41) With regard to the hypothetical segmentation according to end-use applications, the results of the market investigation show that the overall market for precipitated silica must be segmented according to different end-use applications.
(42) First, from a demand-side perspective, a majority of customers consider that there are specific markets for precipitated silica grades, at least for the industry they are active in. (26) For instance a customer indicated that; "[w]e view the precipitated silica market as having several markets due to the fact that the chemistry for each end use is drastically different and not interchangeable. For example, the technology and process needed to produce abrasive oral grades is very different than the process to make for rubber/tire outlets. This is also demonstrated by the fact that suppliers are unable to use tire lines to make the quality for oral care grades." (27)
(43) Another customer explained that "[t]he quality demands required for various markets can be very different. For example, the silica's applied in the Tire industry do not require specifications for heavy metals and dioxin while for Feed Applications, this is a requirement." (28) Accordingly, products target specific end- use applications.
(44) Also, a majority of customers confirmed that there are very different requirements for the performance of precipitated silica depending on their industry. (29) Therefore in the instances in which the base product is the same, or very similar, across end- use applications, customers are typically not aware of it and would not risk disrupting their own production processes by testing grades that are not designed or marketed for them.
(45) Second, from a supply-side perspective, a majority of competitors consider that there are specific markets for precipitated silica grades, which correspond to the following end-use applications; (i) dental, (ii) defoamer, (iii) paints and coatings (iv) paper, (v) rubber, (vi) food additive, (vi) feed additive, (vii) battery separators, (viii) matting agents and (ix) agriculture. (30) Competitors indicate there are different business dynamics in each of these end-use applications. (31)
(46) A majority of competitors consider that there are important price differences between the different end-use applications of precipitated silica products. (32) A customer also explains that "[t]he price mechanisms used can be very different per market." (33)
(47) Most competitors tend to specialize in a limited number of applications. Entering into a different end-use application does not appear feasible in a timely way and without incurring significant costs or risks.
(48) For instance a competitor explains that some markets "[…] have a highly technical know-how or require very specific modified silica grades, with a corresponding high investment cost before production (matting agents, defoamers, battery separators, and all those [where] [competitor name] is present). Though Intellectual Property rights used to be a big hurdle to entry in certain markets the situation has decreased the last ten years, but as indicated above, each market segment requires a specialized know how that cannot be acquired quickly through conventional research. Regulatory approvals and certifications are critical for food, feed, pharma and cosmetics, as well as usually requiring extra investment in the facilities and even some special raw materials." (34)
(49) Competitors also indicated that specific manufacturing equipment is essential for the production of precipitated silica for some end-use applications such as dental, defoamer and paints and / or coatings applications. (35) The Notifying Party also explained that if adaptations to the production facilities are necessary to start producing precipitated silica for a specific end-use application, the installation of new equipment can take up to [period]. (36)
(50) Most customers indicated that they have a qualification process for their suppliers' products which includes steps such as data verification and laboratory testing and can take from one month up to three years. (37) This qualification process can also apply to the production plants where the products are manufactured. (38) Competitors stated that other entities such as local authorities can also carry out qualification processes. (39) The Parties have also provided some information on the regulatory requirements and supplier certification processes per applications. (40) These requirements can vary significantly between applications.
(51) In view of the above the Commission considers that there are separate precipitated silica markets which correspond to the following end-use applications; (i) dental, (ii) defoamer, (iii) paints and coatings, (iv) paper, (v) rubber, (vi) food additive, (vi) feed additive, (vii) battery separators, (viii) matting agents and (ix) agriculture.
(52) Under the market segmentation as analysed above, the Transaction gives rise to the horizontally affected markets in relation to defoamer, dental, paints and coatings, paper, rubber and feed applications. Each of these affected markets is described in more details in the sections below.
The market for precipitated silica for defoamer end-use applications
(53) Certain precipitated silica grades can be used to increase performances of foam control and defoamer agents. Such agents enable to control the formation of foam by reducing the stability of foam films and consequently decompose bubbles.
(54) According to Evonik's technical brochure for "SIPERNAT ® speciality silica and AEROSIL ® fumed silica for defoamer" (41), "foam occurs in many natural and industrial processes as well as everyday life. […] [T]he formation of stable foams causes problems in most industrial processes. Examples are found in the paint and coating, textile, paper, detergent and the chemical industries. Here foam will either affect the quality of the final product directly or impede the manufacturing process, for example, by reducing the carrying capacity of containers or by causing pumping problems".
(55) The Parties do not consider precipitated silica used as defoamer agent to constitute a distinct product market. Furthermore, the Parties do not consider any further segmentation of the defoamer segment into grades with and without hydrophobic treatment to be meaningful because both types of products can be used for the same defoamer applications.
(56) From a supply-side perspective however, results of the market investigation indicated that the market for defoamer applications requires "a highly technical know-how or […] very specific modified silica grades, with a corresponding high investment cost before production" (42). Furthermore, one competitor considers that the defoamer market is "a minor segment in volume, though not in value, with too many obstacles for entry." (43) This observation is confirmed by the fact that average prices for precipitated silica marketed for defoamer applications show significantly higher average prices than in other end-use applications. Based on information provided by the Parties (in particular total market size and market value) (44), precipitated silica for defoamer applications are commercialized at [1.5- 2]€/kg, which is approximately [30-70]% higher than the average price on the hypothetical overall market for precipitated silica ([1.1-1.4]€/kg) (45).
(57) Moreover, as explained in the previous section, hydrophobic precipitated silica can be used in several different end-use applications and does constitute a separate relevant product market under the alternative approach of segmenting the market according to the hydrophilic and hydrophobic character. With respect to a market segmentation by end-use applications, it appears that the hydrophobic property seems to be of particular importance for defoamer applications.
(58) According to Huber's webpage dedicated to hydrophobic precipitated silica (46), "hydrophobic silica is an ideal solution for defoamers and can be used in food products as anti-caking and free-flow agents and in paints and coatings applications." Similarly, Evonik's technical brochure "SIPERNAT ® speciality silica and AEROSIL ® fumed silica for defoamer" (47) indicates that "it is particularly important for the defoaming effectiveness, that the silica has a hydrophobic surface character".
(59) Conversely, Huber's webpage dedicated to precipitated silica for defoamer applications (48), exclusively lists ready-to-use hydrophobic precipitated silica products or hydrophilic precipitated silica for in situ hydrophobisation. (49) And Evonik's technical brochure "SIPERNAT ® speciality silica and AEROSIL ® fumed silica for defoamer" (50) explains that "the use of in situ hydrophobisation in the manufacture of defoamers requires empirical knowledge and comes at high cost in terms of time and equipment. It is often easier to use silica that have already been optimized and hydrophobized for defoaming, like those sold by Evonik."
(60) In light of the above and in particular of the significant price difference of grades for defoamer applications and also of the entry barrier specific to defoamer applications, the Commission will consider the precipitated silica market for defoamer end-use applications to constitute a separate relevant product market.
The market for precipitated silica for dental end-use applications
(61) Precipitated silica has two main applications for dental end-use applications, both as an input for the production of toothpastes. It can be used to control rheology (51) in the manufacturing process and to provide the appropriate aesthetic qualities and stability to the finished toothpaste product (so-called “thickening” characteristics). Precipitated silica can also be used to enhance the cleaning/whitening performance of toothpaste (so-called “abrasive” characteristics).
(62) Some competitors consider that there are significant differences in the production process for each of these functionalities. (52) However most competitors consider that it would be relatively easy for a supplier only active in one of these functionalities to develop another. (53)
(63) The market for precipitated silica for dental end-use applications is described in Evonik's internal analysis as [an important market for silica]" (54)
(64) On the demand-side the market for precipitated silica for dental applications is characterised by the presence of some large customers; Procter and Gamble, GSK, Unilever and Colgate. These customers make up a large part of the overall demand. This is confirmed by Evonik's internal analysis […]. (55)
(65) This can have an impact on entry. As explained by a competitor: "[s]ome of these markets are dominated by a few global customers, where introduction of a new supplier is difficult, such as Dental." (56)
(66) As explained above, there are specific regulatory requirements, including GMP (57) and ISO 9001 requirements which are quite stringent. (58) This is confirmed by Evonik's internal analysis of this market [by referencing various aspects of regulatory requirements]. (59)
(67) Also, R&D and innovation play a significant role in this market. In this regard Evonik's internal analysis refers to innovation [as an important element in the dental market]. (60)
(68) Based on the above evidence, the Commission will consider the overall market for precipitated silica for dental end-use applications for the purposes of the competitive assessment in this case.
The market for precipitated silica for paints and coatings end-use applications
(69) Precipitated silica can be used as additives in emulsion paints (in which they can act as partial substitutes for white pigments) and as rheology modifiers for paint and coating products (in which they enable to fine-tune the proper flowability of the end-product).
(70) At the Commission's request, the Notifying Party identified the following hypothetical sub-applications within the overall market for precipitated silica for paints and coatings applications: (i) decorative paints and coatings (including for wood), (ii) automotive and transportation paints and coatings, (iii) printing inks, and (iv) industrial coatings.
(71) In view of the fact that the Transaction does not raise serious doubts as to its compatibility with the internal market in relation to the markets for paints and coatings applications, including at the narrower possible market definition as considered in the previous paragraph, the exact scope of the product market definition for precipitated silica for paints and coatings applications can be left open for the purpose of the present case.
The market for precipitated silica for paper end-use applications
(72) Within the paper industry, precipitated silica can serve either to increase the paper strength, toughness and resistance to folding (segment of paper pulp preparation / paper mass) or to enhance a paper's ability to absorb ink (61) (segment for surface coating). The intended effect of the latter usage is to achieve an instantly dried surface in order to improve the printed image's quality.
(73) The Transaction gives rise to horizontal overlaps only in the overall segment for paper applications: within the paper industry, Evonik's customers exclusively use Evonik's precipitated silica products for [application 1], while Huber Silica's customers exclusively use Huber Silica's precipitated silica products for [application 2].
(74) In view of the fact that the Transaction does not raise serious doubts as to its compatibility with the internal market in relation to the market for paper applications, the exact scope of the product market definition for precipitated silica for paper applications can be left open for the purpose of the present case.
The market for precipitated silica for rubber end-use applications
(75) Within rubber applications, a distinction can be made between tyre applications, silicone rubber, footwear (typically shoe soles) and other rubber goods.
(76) For tyre applications, precipitated silica are used to improve the performance of the rubber material such as its resistance to abrasion or to heat. Within the particular segment of fuel-efficient tyres, precipitated silica are essential to reduce the rolling resistance and abrasion and to improve the wet traction. In silicone rubber, precipitated silica act as fillers and are designated to mildly support reinforcing effects and provide some tear resistance. In footwear and other rubber goods, precipitated silica are used as reinforcing fillers to improve durability and resilience of the rubber products.
(77) In view of Huber's de minimis presence and the fact that the Transaction does not raise serious doubts as to its compatibility with the internal market, including at the narrower possible market definition as considered in the previous paragraphs, the exact scope of the product market definition for precipitated silica for rubber applications can be left open for the purpose of the present case.
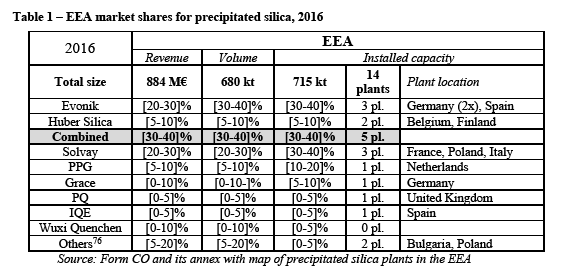
The market for precipitated silica for feed end-use applications
(78) As for food applications (62), precipitated silica are used as additives to improve the flow behaviour for animal feed and nutrition products. Associated effects are the reduction of clumping, of inter-particle interaction and of dust generation. Usage of precipitated silica also enables to improve the final product's storage stability and ability to absorb liquid components.
(79) In view of the fact that the Transaction does not raise serious doubts as to its compatibility with the internal market in relation to the market for feed applications, the exact scope of the product market definition for precipitated silica for feed applications can be left open for the purpose of the present case.
4.2.2.2. Geographic market definition
(80) In a previous case (63), the Commission considered the geographic market for precipitated silica to be at least EEA-wide, but left the precise geographic market definition open.
Notifying Party’s views
(81) The Parties consider the relevant geographic market for precipitated silica to be at least EEA-wide in scope, because it considers that there are no barriers for the trading of precipitated silica within the EEA and that transportation costs within Europe are low. The Parties also submit that the relevant geographic market may be worldwide, given that prices are interrelated on a global level and that some producers are active in Europe (such as Madhu, India) are operating in the EEA based on their intercontinental import capacities without having any production facility in Europe.
(82) The Notifying Party also explains that customers typically purchase internationally, and that large customers sometimes even purchase globally. The Notifying Party adds that due to large overcapacities in China and an erosion of prices in Asia, imports into Europe have been growing over the last years which affected prices in Europe.
Commission’s assessment
(83) The market investigation results indicate that the markets for precipitated silica are EEA-wide in scope.
(84) Although there are some imports from outside the EEA, a majority of customers indicate that they do not import precipitated silica from the US, Asia or from other regions outside Europe to their European facilities. (64) A customer explained that "[w]e do actually not import from outside Europe because of the lower lead times. We would import from outside Europe: - if the price would be lower, providing the required quality - if the manufacturer has a warehouse in Europe to keep a certain quantity in stock." (65)
(85) In parallel, competitors agree that the geographic proximity of suppliers to the customers plays an important role for the precipitated silica business in the EEA. (66) For instance a competitor explains that "[a] plant close to the specific customers could reduce lead-time, save logistics cost, serve customer better and more promptly." (67) Another competitor indicates that "[t]ransport cost contributes to the final price of precipitated silica. Therefore it is easier to be competitive in price with customers close-by." (68)
(86) A majority of competitors indicated that, for 80% of their supplies, the average distance from their production plants to their customers in the EEA is between 1000km and 1500km. (69) Competitors added that this distance does not vary significantly depending on end-use applications. (70)
4.2.3. Organofunctional silanes
(87) Organofunctional silanes are used as binders between inorganic materials such as glass, minerals and metals and organic polymers such as thermoplastics, as surfactants for inorganic and organic materials, and as cross-linking agents for polymers.
(88) In a previous decision (71), the Commission considered that three separate product markets for organofunctional silanes have to be distinguished, namely (i) organofunctional silanes for rubber applications, (ii) organofunctional silanes for non-rubber applications and (iii) alkyl silanes. In a previous decision (72), the Commission considered that the three products markets are at least EEA-wide in scope.
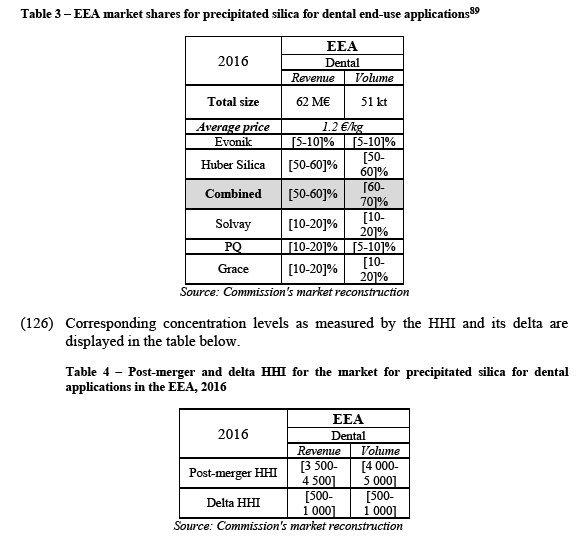
(89) The Notifying Party agrees with such product and geographic market definitions.
(90) For the purpose of the present case, the product and geographic market definitions for organofunctional silanes can be considered as per previous Commission practice. In particular, the question whether the geographic market for organofunctional silanes is EEA-wide or larger can be left open since the Transaction does not raise serious doubts as to its compatibility with the internal market.
4.2.4. Fumed silica
(91) Fumed silica is produced from silicon tetrachloride together with oxygen and hydrogen. The product is used as an additive in a variety of different products. The main areas of use are elastomers (improvement of the mechanical properties of silicone rubber, for example in sealants), thermosetting materials (improving the properties of unsaturated polyesters, epoxy resins and acrylates), and paints and varnishes.
(92) According to the Commission’s past practice (73), fumed silica forms a distinct product market with an EEA-wide geographic scope.
(93) The Notifying Party agrees with this product and geographic market definitions.
(94) For the purpose of the present case, the product and geographic market definitions for fumed silica can be considered as per previous Commission practice.
4.2.5. Betain
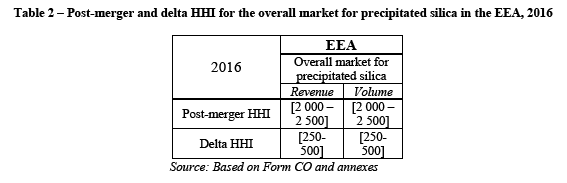
(95) Betain is a chemical compound which has application in dish washing liquids, hand disinfectants, oral care, professional car wash, shampoo/bodywash.
(96) There is no previous Commission practice in relation to betain.
(97) The Notifying Party considered a distinct product market for betain with an EEA- wide geographic scope.
(98) For the purpose of this case, the product and geographic market definitions for betain can be left open as the Transaction does not raise serious doubts as to its compatibility with the internal market.
5. COMPETITIVE ASSESSMENT
(99) Under Article 2(2) and (3) of the Merger Regulation, the Commission must assess whether a proposed concentration would significantly impede effective competition in the internal market or in a substantial part of it, in particular through the creation or strengthening of a dominant position.
(100) In this respect, a merger may entail horizontal, vertical and/or conglomerate effects.
(101) Within the EEA, the proposed Transaction gives rise to horizontally affected markets (see sections 5.1.2 to 5.1.4) in the markets for precipitated silica delineated (i) according to the precipitated silica's various end-use applications, namely the markets for dental, defoamer, paints and coatings, paper, rubber and feed end-use applications; (ii) according to the precipitated silica's hydrophilic/hydrophobic property, namely the market for hydrophobic precipitated silica; and (iii) according to the precipitated silica's chemical nature, namely the market for aluminium silicate.
(102) The proposed Transaction also gives rise to vertical links (see section 5.2) between the upstream market for sodium silicate (where only Huber Silica is active) and the downstream market for precipitated silica (where both Parties are active).
(103) In addition, potential conglomerate effects will also be examined between the closely related neighbouring markets for: precipitated silica for tyre applications and organofunctional silanes (see section 5.3.3); precipitated silica for paints and coatings and fumed silica (see section 5.3.4); and precipitated silica for dental applications and betain (see section (5.3.5).
5.1. Horizontal assessment
5.1.1 Analytical framework
(104) Horizontal effects are those deriving from a concentration where the undertakings concerned are actual or potential competitors of each other in one or more of the relevant markets concerned. The Commission appraises horizontal effects in accordance with the guidance set out in the relevant notice, that is to say the Horizontal Merger Guidelines.- (74)
(105) The Horizontal Merger Guidelines distinguish between two main ways in which mergers between actual or potential competitors on the same relevant market may significantly impede effective competition, namely non-coordinated and coordinated effects. Non-coordinated effects may significantly impede competition by eliminating important competitive constraints on one or more firms, which consequently would have increased market power, without resorting to coordinated behaviour. In that regard, the Horizontal Merger Guidelines consider not only the direct loss of competition between the merging firms, but also the reduction in competitive pressure on non-merging firms in the same market that could be brought about by the merger.
(106) The Horizontal Merger Guidelines also lists a number of factors which may influence whether or not significant non-coordinated effects are likely to result from a merger, such as the large market shares of the merging firms, the fact that the merging firms are close competitors, the limited possibilities for customers to switch suppliers, or the fact that a merger would eliminate an important competitive force. That list of factors applies equally if a merger would create or strengthen a dominant position, or would otherwise significantly impede effective competition.
(107) This decision will analyse whether the proposed Transaction is likely to raise doubts as to its compatibility with the internal market by the creation of non- coordinated effects in those markets on which the Parties' activities lead to horizontal overlaps and to affected markets, distinguishing between (i) the market segmentation for precipitated silica according to the products end-use applications (section 5.1.2), (ii) the market segmentation for hydrophobic precipitated silica (section 5.1.3) and (iii) the market segmentation for aluminium silicate (section 5.1.4).
5.1.2. Market segmentation of precipitated silica according to the products' end-use applications
5.1.2.1. Description of the hypothetical overall market for precipitated silica
(108) The table below shows the Parties' and their competitors' market shares, as well as the installed capacity in the EEA on the hypothetical market for precipitated silica in 2016. (75)
The Notifying Party's views
(109) The Notifying Party argues that the Transaction will not raise competition concerns on the overall precipitated silica market. In this regard the Notifying Party claims that the Merged Entity will remain subject to strong competition from suppliers active in the EEA as well as from additional suppliers from Asia. Moreover there is increasing overcapacity in the precipitated silica market and the capacity exceeds demand by around 48%. The Notifying Party also explains that competitive pressure increases due to imports from Asia. The Notifying Party indicates that Huber and Evonik have lost market shares in recent years to competitors because of the strong competitive pressure from low-cost producers with spare capacities.
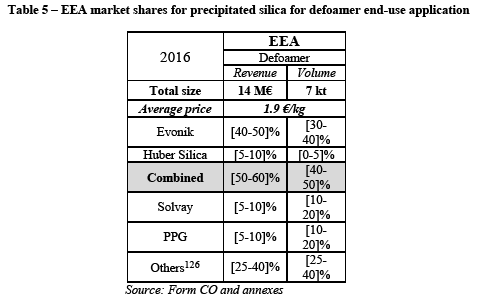
(110) The Notifying Party also argues that the Herfindahl-Hirschman-Index ("HHI") levels (see table above) do not indicate any serious competition concerns and that Evonik and Huber are not close competitors but their products are complementary in terms of product portfolio and geographic footprint. The Notifying Party further argues that customers have strong buyer power, strong leverage in price negotiations and are able to switch suppliers easily. Finally, the Notifying Party claims that there is strong competition in all end-use applications related to the high degree of supply-side substitutability.
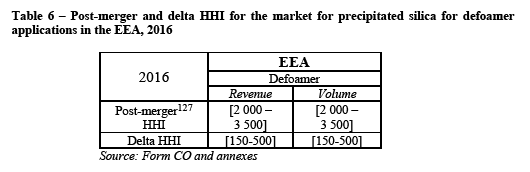
(111) For the purposes of general market intelligence, the Notifying Party relied, to some extent, on the industry report realized by Notch Consulting Inc. (the "Notch report"). (77)
(112) Concerning this report, the Parties made the following observations. First, the Notch report does not take into account the sales of Asian suppliers to EEA customers (78). Second, the report inaccurately lists Huber Silica's production facility for precipitated silica in Sweden among active capacities in Europe. (79) Third, it overestimates Huber Silica's total European sales in precipitated silica in 2016 (90M€ (80) instead of [30-60]€).
(113) The Notifying Party adds that the report underestimates the total European market size for 2016 in its last edition of March 2017 at 747M€ (830M$). Indeed, the Notifying Party notes that, in March 2016, the Notch report estimated the EU market size to be 774M€ (860M$) with an expected annual increase of 4-5%, which is in contradiction with its last estimation.
(114) To conclude, the Parties consider the data provided in the Notch report to be broadly reliable in some respects but to be incorrect in others. In particular, the Parties estimate that their own market intelligence and market estimates are in many instances more accurate than the one provided in the Notch report and correctly reflect the actual increase of precipitated silica production and the actual sales of Asian suppliers to EEA customers.
(115) At a narrower level, the Notifying Party is of the opinion that the Notch report [significantly misjudged Evonik's and Huber Silica's sales in this regard].
The Commission's preliminary observations
(116) Table 1 shows that post-Transaction the Merged Entity would be the market leader in the EEA with a market share of [30-40]% in terms of volume and [30- 40]% in terms of revenue with an increment of [5-10]% brought by Huber Silica. Solvay would be the second largest player in the market, with a market share of around [30-40]%. The next competitors would be smaller players, like PPG and Grace.
(117) Regarding the installed production capacity for precipitated silica, the Merged Entity would own more than one third of all 14 plants located within the EEA, representing [30-40]% of the total installed capacity.
(118) The Merged Entity would however still face strong competition, first from Solvay, which has comparable market shares in terms of revenue, volume and installed capacity. Also, most customers consider that Solvay is Evonik's closest or second closest competitor. (81) The Merged Entity would also face other significant players, such as PPG, which has higher market shares than Huber Silica.
(119) In the results of the market investigation, although most customers indicate that suppliers located outside the EEA do not exert significant competitive pressure on EEA-based suppliers (82), some customers nevertheless argue that they do exert some influence. For instance a customer explains that "quality from China and India is on par with European produced material and competitive with EEA produced material." (83)
(120) Almost all competitors with EEA-based capacities indicate in the market investigation to be aware of the Notch report (84) and none of them considers that it provides inaccurate data about the precipitated silica market, market players and their respective market shares. (85) Furthermore none of them identifies significant inaccuracies in the data as reported in the Notch report and raises concerns about any hypothetical lack of transparency in any market. (86)
(121) The Commission notes that the Parties adapted the total amount of EEA sales of precipitated silica provided by the Notch report from 747M€ to 884M€ to reflect its own market intelligence. Similarly, in terms of volumes, the Commission notes that the Parties estimated the total EEA volume of precipitated silica sold in 2016 to be 680kt, which corresponds to the volume sold in Europe as forecasted for 2020 in the Notch report. For 2016, the Notch report estimates the overall demand for precipitated silica to be 557kt in Europe. Such an estimate of the total volume sold in 2016 would lead to a combined market share for the Merged Entity of [40- 50]%.
(122) At a narrower level (i.e. in some markets for precipitated silica for particular end- use applications), the Commission notes that the Parties' best estimates for total market sizes correspond either to market data as provided in the Notch report for 2016 (e.g. markets for precipitated silica for rubber, paper or food applications), or to 2020 forecasts of the Notch report (87) (e.g. market for precipitated silica for dental applications), or to the worldwide demand in 2016 as provided in the Notch report (e.g. market for precipitated silica for defoamer applications). A consequence of the adjustments made by the Notifying Party and compared to the Notch report is an increase in the 'Other' category (88), which the Parties estimate to correspond to 19% of the total estimated volumes sold in the EEA in 2016 (126kt).
(123) In light of the above and as will be detailed below, some market reconstructions have been necessary to have access to an independent estimation of the total market sizes in volume and value for certain of the precipitated silica markets in which the Transaction gives rise to horizontal overlaps.
5.1.2.2. Precipitated silica for dental end-use application
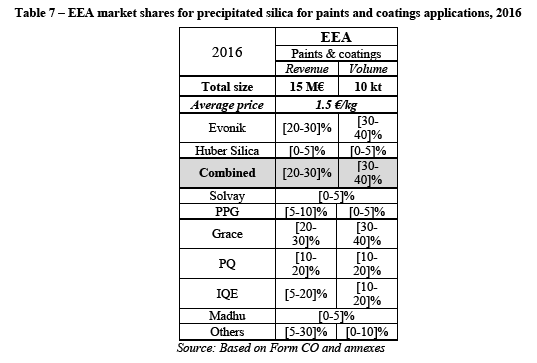
(124) As explained in the previous section, a brief market reconstruction has been necessary to estimate the total market size for precipitated silica for dental applications. In terms of value, the total market size of 60M€ as provided by the Parties has been roughly confirmed and slightly increased while the total market size in volume appears to be in line with the data provided in the Notch report for 2016 (51kt instead of 56kt as forecasted for 2020 and reported by the Parties).
(125) The table below shows the reconstructed market shares in the EEA for Evonik and Huber Silica as well as for their main competitors on the market for precipitated silica for dental end-use applications by value and volume.
The Notifying Party's view
(127) The Notifying Party argues that the Transaction will not raise competition concerns on the market for precipitated silica for dental end-use applications. First, the Notifying Party explains that the Merged Entity will remain subject to strong competition from suppliers such as Solvay and PQ.
(128) Second, the Notifying Party argues that Evonik's sales to dental customers are very limited and have decreased over the last years. Also, according to the Notifying Party Evonik ['s activities in this market are limited].
(129) Third, the Notifying Party submits that there are significant overcapacities in the market due among other things to growing competitive pressure from Asian suppliers.
(130) Fourth, the Notifying Party claims that customers have significant buyer power that will remain unchanged post-merger. The Notifying Party also argues that customers are able to request price decreases and shift orders to have better prices. Moreover, according to the Notifying Party customers can approach any precipitated silica manufacturer and request to start producing grades specifically suited for their use.
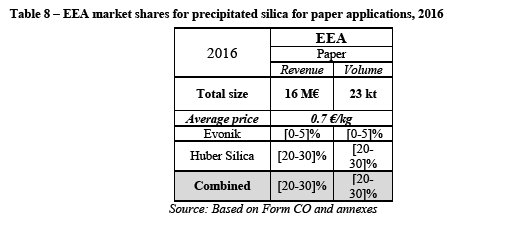
The Commission's assessment
(131) As displayed in Table 3, Huber Silica is the market leader pre-transaction, with a [50-60]% market share (based on revenue and volume data) based on the reconstruction resulting from the market investigation. Such market share levels are indicative of a potential pre-merger dominant position of Huber Silica in the market for precipitated silica for dental applications. With a significant combined market share of [55-70]%, the Merged Entity would enjoy a significantly stronger market position than the three other remaining competitors, with market shares between [5-20]%.
(132) The highly concentrated nature of this market is also reflected in the HHI levels as displayed in Table 4. The Transaction would also involve a significant increment in HHI of [500 – 1 000].
(133) According to Evonik's own internal analysis the "Huber's strong position on the market". (90) This observation has also been confirmed by the market investigation.
(134) First, a majority of customers consider that Huber is an unavoidable supplier for precipitated silica in the EEA. (91) In this regard a customer explains that "the silica qualities of the alternative suppliers works different in our end-use application (toothpaste) or packaging isn't suitable for us". (92) Another customer states that "[w]e cannot substitute the types/grades of one supplier 1:1 to the quality of another supplier, so we have to create "alternative" formulations which a very big effort to handle." (93) A third customer explains that Huber is an unavoidable supplier "[...] because the product are developed with this [Huber's] silicas". (94)
(135) In this regard, similarly to the situation in the overall precipitated silica market, all customers responding to the market investigation indicate that they qualify their suppliers (95). Half of the customers only approve the product, the other half also approve the plant (96). The procedure can take from one month to over a year and sometimes even longer.
(136) A majority of the customers say that it is not easy to switch suppliers (97). For instance a customer explains that switching suppliers "[...] takes a long time - many testing and it may not be in accordance with other raw materials." (98) Another customer indicates that "[s]witching is time consuming and there are significant costs involved. If tests are not made thoroughly there might be problems with our product quality." (99)
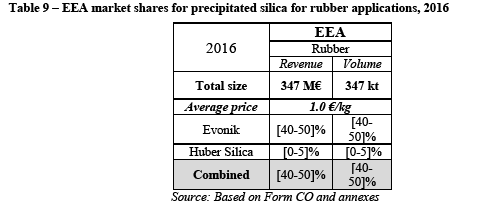
(137) Second, some respondents explain that entry is particularly challenging in this market, for instance a competitor explained that "[w]e did some developments in this area, but we consider this market segment suffers from oversupply so the cost of entry was not supported by good market expectatives [sic]. Also the strong links between each major customer and a precipitated silica supplier is a strong barrier, being limited in the short term to small companies or secondary brands." (100)
(138) Another competitor explains that "[s]ome of these markets are dominated by a few global customers, where introduction of a new supplier is difficult, such as Dental." (101) Indeed, as explained above, the demand on this market is mainly concentrated among four major customers (Colgate, Procter and Gamble, GSK and Unilever). There are indications that smaller suppliers would find it very challenging to supply these customers.
(139) Accordingly, in its internal analysis, Evonik [analyses the challenges to be a successful supplier in this field]. (102)
(140) In this regard, a large customer explains that "[…] its sourcing strategy now emphasizes the need for synergies in its qualification process. In this respect, global suppliers enable [customer name] to simply re-apply identical qualification procedures in several regions throughout the world. Also, [customer name] prefers to be supplied by companies of a certain scale with a global footprint and consistent manufacturing practices. [...] Evonik has the advantage of having a global footprint and could therefore supply [customer name] globally. Solvay, Huber, and Evonik are the only three manufacturers of precipitated silica for oral care to have such global footprint." (103)
(141) Post-merger the market position of Huber's dental business would be further enhanced; with [50-60]% market shares, the Merged Entity would become three times as large as the second largest competitor, Solvay. Three other competitors with shares of [5-20]% would remain active on the market (PQ, Grace and Madhu). Also, most customers confirmed that they do no import precipitated silica for dental end-use applications from outside of the EEA to their EEA facilities.
(142) Despite Evonik's limited activities in the dental segment it is still the fifth largest player in the EEA and has a global presence (104), which is important for the largest customers. Evonik lists [its areas of strengths in an internal document]. (105) Furthermore, Evonik explains in its internal analysis [its areas of strengths]. (106) Reportedly, some of Evonik's products [perform well against those of competitors]. (107)
(143) Evonik's internal analysis also refers to [success with dental customers] (108).(109)
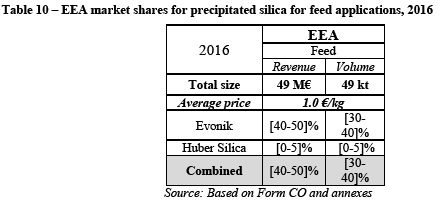
(144) In addition, Evonik explains that [levels of average sales prices to dental customers]. (110)
(145) In view of the above, in its internal analysis Evonik considers that [the advantage to expand in the dental market could outweigh the risks] (111) (112) (113) (114) (115),.
(146) By way of illustration of the opportunities which would be open to Evonik in this market [there is evidence of Evonik being in negotiations with a large toothpaste manufacture] (116) (117)
(147) This would lead to a two to [significant increase in the volumes sold by Evonik while no such decision has yet been reached] (118)
(148) In addition, the market investigation results show that Evonik and Huber are particularly close competitors. Indeed a majority of customers consider that Huber is Evonik's closest or second closest competitor. (119)
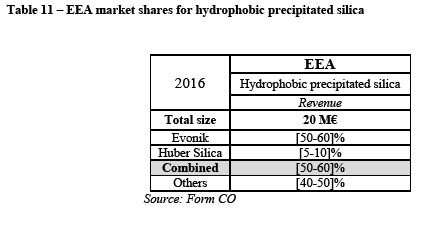
(149) In view of the above the Commission considers that the Transaction could lead to the removal to an important competitive force (Evonik). (120)
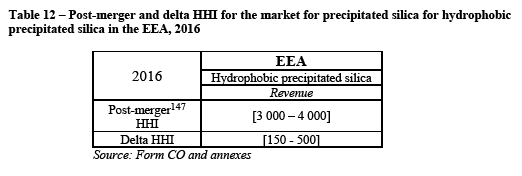
(150) As for the impact of the Transaction on customers' choice of suppliers, a customer explains that "[t]here are not sufficient suppliers on EU market as it is now, and there will be even less with this merger." (121) Another customer explains that "[t]oday, there are three global competitors with the capability to offer localized Oral Care capacity. With the merger, this number would be reduced to two. Two key points are 1) very few suppliers deliver good performance silica with a global footprint and 2) longer supply chains across continents often aren't competitive vs. local solutions." (122)
(151) Several customers, of different sizes, are also concerned about a potential price increase post-merger. (123) For instance a customer explains that "[t]here will be less competitors and the ones left will be free to operate the market as they please." (124). Another customer states that "[w]e expect price increase because there will be no direct competition between two suppliers that we know." (125)
(152) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the market for precipitated silica for dental end-use applications.
5.1.2.3. Precipitated silica for defoamer applications
(153) The market of precipitated silica for defoamer applications is relatively small in size but constitutes a niche market with one of the highest average price per ton (almost twice as high as in dental).
(154) The table below shows the EEA market shares for Evonik and Huber Silica as well as for the main competitors on the market for precipitated silica for defoamer applications by revenue and volume.
(155) Corresponding concentration levels as measured by the HHI and its delta are displayed in the table below.
The Notifying Party's view
(156) The Notifying Party considers that the Transaction does not give rise to competition concerns in relation to the market for precipitated silica for defoamer applications.
(157) First, The Notifying Party first submits that the Merged Entity will remain subject to strong competition in relation to the defoamer segment. The Notifying Party claims that all products sold to defoamer customers are grades of precipitated silica which can, in identical form, be used in different end-use applications and the vast majority of precipitated silica manufacturers can sell their products to defoamer customers without any need of investment or sunk costs.
(158) Second, the Notifying Party explains that the relatively limited demand for precipitated silica for defoamer applications, only enables opportunistic sales in this particular segment.
(159) Third, the Notifying Party indicates that there is significant overcapacity in the market for precipitated silica because of precipitated silica grades being in identical form.
(160) Fourth, the Notifying Party explains that defoamer customers have significant buyer power and an ability to negotiate favourable terms.
The Commission's assessment
(161) According to the Parties' best estimates for market shares, the Merged Entity would, post-transaction, become leader on the EEA market for precipitated silica for defoamer applications with a combined market share of [40-50]% in volume and [50-60]% in value. Already pre-transaction, Evonik enjoys a strong position in this market with [30-40]% in volume and [40-50]% in value. The increment brought by Huber Silica ranges between [0-5]% in volume and [5-10]% in value. According to the Parties, Solvay and PPG are the only precipitated silica manufacturers to be also active on this market with market shares between [5- 20]%. HHI considerations also highlight a relatively high degree of concentration and increment, in particular for market shares based on revenue considerations.
(162) As already explained, the total EEA market size in volume estimated by the Parties corresponds to the worldwide demand for precipitated silica for defoamer applications, which may lead to a dilution of the Parties' market shares and an increase of the market share attributed to the 'Others' category ([25-40]% according to the Parties). The market investigation sought to reconstruct the market.
(163) For confidentiality reasons, it is not possible to disclose the exact results of the market reconstruction. However, it should be noted that the total size of market in the EEA is significantly lower than the EUR 14 million estimated by the Parties. Consequently, the Parties' combined market share in value is significantly higher than [50-60]% (above [70-80]%).
(164) At the Commission's request, the Parties provided the names of precipitated silica grades of their competitors in the market for precipitated silica for defoamer applications. However, none of these grades are marketed as potential defoamer products on their respective webpages. Furthermore, contrary to the Parties' internet pages (128), none of Solvay's (129) or PPG's (130) webpages provide commercial or technical information with respect to their precipitated silica grades in the context of potential defoamer applications. The Commission observes that the Parties are the only precipitated silica manufacturers to have a targeted marketing strategy towards customers for the defoamer end-use applications. This means that, notwithstanding the relatively small size of the market, the Parties' presence goes well beyond 'opportunistic sales', while the same is not true for their competitors.
(165) As for the Parties' claim regarding overcapacity in the production of precipitated silica, the market investigation provided conflicting evidence of high capacity utilisation by several competitors. For those competitors which have free capacity available, the switch from other grades to defoamer grades is perceived as technically challenging. One competitor explained in the context of the market investigation that the market for defoamer end-use applications requires "a highly technical know-how or […] very specific modified silica grades, with corresponding high investment cost before production". (131)
(166) From a demand-side perspective, concerns are raised in particular by one customer of precipitated silica for defoamer applications who explains that the proposed Transaction would lead to a "monopoly situation". (132) Concerning the alleged buyer power of customers, results of the market investigation show that half of the respondents consider Evonik to be un unavoidable supplier of precipitated silica in the EEA. (133) Furthermore, it should be noted that these respondents include one major customer. It can be concluded that customers have limited ability to switch to alternative suppliers in the EEA.
(167) Some market participants also expressed concerns about potential reduction of competition and potential price increases in the market for precipitated silica for defoamer applications following the proposed Transaction.
(168) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the market for precipitated silica for defoamer end-use applications.
5.1.2.4. Precipitated silica for paints and coatings applications
(169) As illustrated in the table below, the market for precipitated silica for paints and coatings applications is affected in 2016, irrespective of the approach for market share calculation based either on value (combined market share of [20-30]%) or on volume (combined market share of [30-40]%).
The Notifying Party's views
(170) The Notifying Party does not put forward any specific arguments for this market. The Commission's assessment
(171) Based on the Parties' market share estimates in the market for precipitated silica for paints and coatings, the combined market shares remains fairly limited when considered per value while becoming [30-40]% when considered in volume. Under both possible approaches, the increment remains modest (below [0-5]%). Furthermore four major competitors would remain active in this segment post- transaction and the Merged Entity would have a similar size to Grace, forming the top two companies active in precipitated silica for paints and coatings applications.
(172) According the Guidelines on the assessment of horizontal mergers ("the Horizontal Merger Guidelines") (134), the Commission is unlikely to identify horizontal competition concerns in a merger with a post-merger HHI between 1 000 and 2 000 and a delta below 250, except where special circumstances are present. (135) At the overall level of precipitated silica for paints and coatings applications, it can also be noted that, under the approach of market share based on value, the post-merger HHI is between 1 000 and 2 000 ([1 500 – 2 000]) and the associated delta is below 250 ([50-150]).
(173) When segmenting the market for precipitated silica for paints and coatings applications into (i) decorative paints and coatings (including for wood), (ii) automotive and transportation paints and coatings, (iii) printing inks, and (iv) industrial coatings, it appears that the Parties' activities only overlap within the narrower segment of decorative paints and coatings (including for wood), which constitutes 73-90% of the total segment for paints and coatings. The Parties' combined market share remains in similar ranges ([20-30]% based on value and [25-40]% based on volume) as for the overall market for paints and coatings.
(174) Market participants did not raise substantiated concerns about the market for precipitated silica for paints and coatings and certain competitors also observed that "[w]ith the possibility of imports and the remaining suppliers there is enough product to avoid a dominance of the market" (136) or that "[t]here does not appear to be much overlap between the end use applications in which Evonik and Huber sell". (137)
(175) In light of the above, the Transaction does not raise serious doubts as to its compatibility with the internal market and concerning the Parties' activities in the market for precipitated silica for paints and coatings applications in the EEA, including at the narrower possible level of decorative paints and coatings (including for wood) applications.
5.1.2.5. Precipitated silica for paper applications
(176) As illustrated in the table below, the market for precipitated silica for paper applications is affected in 2016, irrespective of the approach for market share calculation based either on value (combined market share of [20-30 %]) or on volume (combined market share of [20-30%]).
The Notifying Party's views
(177) The Notifying Party does not put forward any specific arguments for this market, except on the [negligible magnitude of its sales].
The Commission's assessment
(178) Based on the Parties' market share calculations, the combined market share remains fairly limited (up to [20-30]%) and the increment brought by Evonik is de minimis (well below [0-5]%) under both possible approaches of market share calculations based on revenues or volume. Furthermore, several well-established competitors remain active on this market, in particular Grace ([20-30]%), PQ ([10-20]%), PPG ([5-10]%) and OSC ([5-10]%). (138)
(179) Results of the market investigation did not raise particular concerns with respect to the market for precipitated silica for paper applications. One single competitor identified a potential risk of decrease in competition in this market but without substantiating its view on top of the fact that the Merged Entity would enjoy a higher combined market share.
(180) According to the Horizontal Merger Guidelines (139), the Commission is unlikely to identify horizontal competition concerns in a merger with a post-merger HHI between 1 000 and 2 000 and a delta below 250 or in a merger with a post-merger HHI above 2 000 and a delta below 150, except where special circumstances are present. (140) With respect to the market for precipitated silica for paper applications, none of the special circumstances are met and, based on value, the post-merger HHI is between 1 000 and 2 000 ([1 500 – 2 000]) and the associated delta is below 250 ([0-100]), while, based on volume, the post-merger HHI is above 2 000 (2 000 – 3 000) and the associated delta is below 150 ( [0-100]).
(181) As already explained, the Transaction does not lead to any overlap at the narrower possible levels of precipitated silica for paper mass applications and for paper surface coatings applications since Evonik's customers exclusively use Evonik's precipitated silica products for […], while Huber Silica's customers exclusively use […].
(182) In light of the above, the Transaction does not raise serious doubts as to its compatibility with the internal market with regard to the Parties' activities in the market for precipitated silica for paper applications in the EEA, including at the narrower possible level of paper mass applications and paper surface coating applications.
5.1.2.6. Precipitated silica for rubber applications
(183) Among the different precipitated silica markets per end-use applications, the market for rubber applications is by far, the largest in size. According to the Parties' own estimations for 2016, this market alone accounts for 39% of all sales of precipitated silica in the EEA and for 51% of all volume traded in the EEA.
(184) As illustrated in the table below, the market for precipitated silica for rubber applications is affected in 2016, irrespective of the approach for market share calculation based either on value (combined market share of [40-50]%) or on volume (combined market share of [40-50]%).
The Notifying Party's views
(185) The Notifying Party does not put forward any specific arguments for this market. The Commission's assessment
(186) Based on the Parties' market share calculations, the Transaction is characterized by a de minimis increment (below [0-5]%) brought by Huber Silica and under both possible approaches of market share calculations based on revenues or volume. Huber Silica has no focus on this market for precipitated silica and generates almost no sales on it. Several other well-established competitors, including from Asia, are active in this market, in particular Solvay ([30-40]%), PPG ([5-20]%), Wuxi Quenchen (China) ([0-10]%), Grace ([0-5]%), Madhu (India) ([0-5]%) and several other competitors (accounting together for [5- 10]%). (141)
(187) The fact that Huber Silica is almost absent of this market holds true including at narrower possible levels for tyre, silicone rubber, footwear and other rubber applications.
(188) On the overall level of precipitated silica for rubber applications, it can also be noted that the post-merger HHI is above 2 000 ([2 000 – 3 000] in value and [2 000 – 3 000] in volume) and the associated delta is below [0-100] (<1 in value and volume). According to the Horizontal Merger Guidelines (142), the Commission is unlikely to identify horizontal competition concerns in such a situation, except where special circumstances are present. (143) None of these exceptional circumstances are present, in particular the fact that none of the Parties has a pre- merger market share of 50% or more.
(189) In light of the above, the Transaction does not raise serious doubts as to its compatibility with the internal market with regard to the Parties' activities in the market for precipitated silica for rubber applications in the EEA, including at the narrower possible level of tyre, silicone rubber, footwear and other rubber applications.
5.1.2.7. Precipitated silica for feed applications
(190) As illustrated in the table below, the market for precipitated silica for feed applications is affected in 2016, irrespective of the approach for market share calculation based either on value (combined market share of [40-50]%) or volume (combined market share of [30-40]%).
The Notifying Party's views
(191) The Notifying Party does not put forward any specific arguments for this market
The Commission's assessment
(192) Based on the Parties' market share calculations, the Transaction is characterized by a de minimis increment (below [0-5]%) brought by Huber Silica and under both possible approaches of market share calculations based on revenues or volume. Huber Silica has no focus on this market for precipitated silica and generates almost no sales on it. Several other well-established competitors, including from Asia are active in this market, in particular Solvay ([40-50]%), IQE ([5-20]%), Wuxi Quenchen (China) ([5-10]%), PQ ([0-5]%) and several other competitors (accounting together for [10-20]%). (144)
(193) It can also be noted that the post-merger HHI is above 2 000 ([2 000 – 3 000] in value and [2 000 – 3 000] in volume) and the associated delta is below 150 ([0- 50] in value and [0-50] in volume). According to the Horizontal Merger Guidelines (145), the Commission is unlikely to identify horizontal competition concerns in such a situation, except where special circumstances are present. (146) None of these exceptional circumstances are present, in particular the fact that none of the Parties has a pre-merger market share of 50% or more.
(194) In light of the above, the Transaction does not raise serious doubts as to its compatibility with the internal market with regard to the Parties' activities in the market for precipitated silica for feed applications in the EEA.
5.1.3. Market segmentation for hydrophobic precipitated silica
(195) The table below shows the EEA market shares for Evonik and Huber Silica on the market for hydrophobic precipitated silica based on revenue.
(196) Corresponding concentration levels as measured by the HHI and its delta are displayed in the table below.
The Notifying Party's view
(197) The Notifying Party argues that the Transaction will not raise competition concerns in relation to hydrophobic precipitated silica.
(198) First, it argues that Evonik and Huber Silica supply hydrophobic precipitated silica to different customer segments.
(199) Second, the Notifying Party explains that the Transaction will not increase Evonik's capacity as Huber does not produce hydrophobic precipitated silica but has a toll-manufacturing contract with Applied Material Solutions ("AMS") in the US.
(200) Third, sufficient supply alternatives remain to customers post-merger as the Notifying Party claims that several competitors active in the EEA also sell hydrophobic precipitated silica. To the best of the Parties' knowledge, Solvay, Madhu, Torrensil (China), Fuji Silysia (Japan/US), Tulco (US), Elementis (US), AMSI (US) and Hoffman Mineral (Germany) are also active on this market.
(201) Fourth, the Notifying Party adds that the possibility for toll-manufacturing may be more cost efficient for precipitated silica manufacturers than purchasing and operating its own equipment.
The Commission's assessment
(202) As displayed in the table above, out of the total market size for hydrophobic precipitated silica of EUR 20 million, Evonik captures a pre-merger significant market share of [50-60]% while the increment brought by Huber amounts for [5- 10]% when allocating its sales of toll-manufacturered products. Post-transaction, the Merged Entity would consequently have a [50-60]% combined market share in this market. The post-merger HHI of [3 000 – 4 000] also indicates the relatively important concentration level in the market for hydrophobic precipitated silica.
(203) As regards the Notifying Party's claim that Evonik and Huber Silica supply hydrophobic precipitated silica to different customer segments, as explained above, the Commission has identified one relevant product market for hydrophobic precipitated silica and has not been able to identify relevant sub-segments within this market. Therefore, the Commission considers that the Parties' activities overlap within this market.
(204) While it is true that the Transaction will not increase Evonik's hydrophobisation capacity in the EEA, the Notifying Party would nevertheless acquire Huber Silica's hydrophobic precipitated silica products with all associated customer base and market position. This would further reduce the competitive pressure in an already relatively concentrated market, where the Notifying Party's has a leading market position.
(205) Based on the information provided by the Parties, Solvay and Madhu are the only manufacturers of precipitated silica who also serve the market for hydrophobic precipitated silica in the EEA while Tulco, Elementis, AMSI and Hoffman Mineral are not precipitated silica manufacturers but serve the market as toll- manufacturers (as AMS for Huber Silica in the US).
(206) At the request of the Commission, the Parties provided the following grades of hydrophobic precipitated silica: SIPERNAT ® D10, D13* and D17 for Evonik, Zeoflo ® TL and 5169MD for Huber Silica, MFIL ® TS, TS100 and TS100D for Madhu, Perform-o-sil ® 35, 66, 70, 80 for AMSI and Dumancil ® 100, 300 and 402 for Elementis. No hydrophobic precipitated silica grades could be identified for Solvay.
(207) Although contacted by the Commission for pre-notification calls, market investigation and market test, Madhu did not provide any feedback to the Commission's request for information. In general terms and according to the Parties own estimate, Madhu has a limited presence in the EEA (up to 5% in the EEA market for precipitated silica for rubber applications).
(208) In any event, Evonik's pre-merger already very strong position in the EEA market for hydrophobic precipitated silica ([50-60]% pre-merger market share) reflects the fact that Evonik is the only precipitated silica manufacturer in the EEA to have integrated production capacities which indicates that these capacities constitute a competitive advantage compared to an outsourced solution. Huber Silica and Madhu (whose presence in the EEA market for hydrophobic precipitated silica could not be verified) are the only two manufacturers of precipitated silica which also offer own-branded hydrophobic precipitated silica grades. The Transaction would combine the only two precipitated silica manufacturers with EEA-based capacities that provide ready-to-use hydrophobic precipitated silica grades. This would lead to re-inforcing of Evonik's already dominant position in this market.
(209) With reference to the previous section, Evonik's strong market position in hydrophobic precipitated silica may be correlated to its similarly strong market position in the market of precipitated silica for defoamer applications. Indeed, [a significant part] of Evonik's sales for defoamer applications are ready-to-use hydrophobic precipitated silica grades and the Parties recognise that hydrophobicity may show superior effects in the defoaming end-usage. Similarly, [a large proportion] of Huber Silica's sales of hydrophobic silica are related to defoamer applications. Conversely, out of the three precipitated silica grades commercialized by Huber Silica for defoamer applications in the EEA, one grade (Zeofoam ® 166, which can be used in defoamers, paints and coatings or paper applications) is hydrophilic (non surface-treated) but described on Huber Silica's webpage as being "the first product to choose for in-situ surface treatment to make it hydrophobic" (148), while the two remaining grades (Zeoflo ® TL and 5169MD) are ready-to-use hydrophobic precipitated silica grades.
(210) It follows that competition concerns already identified above in the market for precipitated silica for defoamer applications are largely connected to potential competition concerns in the market for hydrophobic precipitated silica. This fact is due to the existing alternative relevant market segmentations either per end-use applications or per hydrophilic/hydrophobic nature of the precipitated silica grade.
(211) The respondents in the market investigation confirmed that none of Evonik's competitors currently supplies hydrophobic precipitated silica in the EEA (149) and there are indications that the ability to propose hydrophobic silica constitutes a strategic advantage (150).
(212) Concerning the availability of alternative suppliers of hydrophobic precipitated silica in the EEA, one customer indicates that "Evonik dominate[s]" (151) and several others consider that there is not a sufficient choice available. (152) Similarly, from a supply-side perspective, one competitor explains that "[…] only a few producers have strong market positions. The main provider of hydrophobic silica is Evonik. In principle, the ease and the cost of entering the market depends on the base equipment. The necessary capital investment could be quite significant and could be a hindering factor. Similarly, as it involves bringing organic components, it would be a further obstacle for producers who do not already have the necessary certifications for this." (153) It can be concluded from the above that there is a limited choice of suppliers of hydrophobic precipitated silica currently available in the EEA. Post-merger, this supply would be even further limited.
(213) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the market for hydrophobic precipitated silica.
5.1.4. Market segmentation for aluminium silicate
(214) Within aluminium silicate, the Parties' activities only overlap in the specific segment for paints and coatings applications.
(215) According to the Parties' best estimates provided in the table below, the market for aluminium silicate for paints and coatings is affected ([20-30]%) in 2016, when considering EEA market shares based on volume.
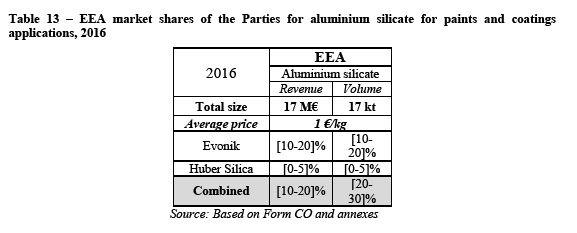
The Notifying Party's views
(216) The Notifying Party argues that the Parties have minimal market shares in this market and adds that there are many alternative suppliers of aluminium silicate.
The Commission's assessment
(217) On top of the fact that the combined market share remains fairly limited ([20- 30]%) and that the increment brought by Huber Silica is de minimis ([0-5]%), no market participant raised concerns with respect to the market for aluminium silicate for paints and coatings applications during the market investigation. Several other well-established competitors remain active in this market, in particular Grace ([30-40]%), IQE ([20-30]%) and Madhu ([0-5]%) as well as Solvay ([0-5]%), PQ ([0-5]%), Glassven ([0-5]%) and several other smaller competitors (accounting together for [10-20]% of the market). (154)
(218) Furthermore, according to the Horizontal Merger Guidelines (155), the Commission is unlikely to identify horizontal competition concerns in a merger with a post- merger HHI above 2 000 and a delta below 150, except where special circumstances are present. (156) With respect to the market for aluminium silicate for paints and coatings applications, none of the special circumstances are met, the post-merger HHI is above 2 000 ([2 000 – 3 000]) and the associated delta is below 150 ([0 - 100]).
(219) In light of the above, the Transaction does not raise serious doubts as to its compatibility with the internal market with regard to the Parties' activities in the market for aluminium silicate for paints and coatings applications in the EEA.
5.2. Vertical assessment
5.2.1. Analytical framework
(220) Vertical effects may arise from mergers of companies operating at different levels of the supply chain (157).
(221) A vertical merger may result in anti-competitive effects due to foreclosure. Foreclosure concerns a situation where actual or potential rivals' access to supplies or markets is hampered or eliminated as a result of the merger, thereby reducing these companies' ability and/or incentive to compete. (158)
(222) Two forms of foreclosure can be distinguished in a vertical relationship: input and customer foreclosure. The first is where the merger is likely to raise the costs of downstream rivals by restricting their access to an important input (input foreclosure). The second is where the merger is likely to foreclose upstream rivals by restricting their access to a sufficient customer base (customer foreclosure). (159)
(223) Input foreclosure arises where, post-merger, the new entity would be likely to restrict access to the products or services that it would have otherwise supplied absent the merger, thereby raising its downstream rivals' costs by making it harder for them to obtain supplies of the input under similar prices and conditions as absent the merger. (160)
(224) Customer foreclosure may occur when a supplier integrates with an important customer in the downstream market. Because of this downstream presence, the merged entity may foreclose access to a sufficient customer base to its actual or potential rivals in the upstream market (the input market) and reduce their ability or incentive to compete. In turn, this may raise downstream rivals' costs by making it harder for them to obtain supplies of the input under similar prices and conditions as absent the merger. (161)
(225) For an input or customer foreclosure scenario to raise competition concerns, three cumulative factors need to be taken into account: (i) the ability of the merged entity to engage in foreclosure; (ii) the incentives of the merged entity to do so; and (iii) whether a foreclosure strategy would have a significant detrimental effect on competition in the downstream market. (162)
5.2.2. Vertical link between sodium silicate and precipitated silica
(226) Huber Silica primarily produces sodium silicate as an input for its own precipitated silica production but also has, to a minor extent, external sales. Evonik does not produce any sodium silicate and purchases it from third party manufacturers as an input material for its precipitated silica production.
The Notifying Party's views
(227) According to the Notifying Party the vertical link between sodium silicate and precipitated silica will not give rise to competition concerns. First, the Notifying Party argues that there is no risk of input foreclosure. The notifying Party submits that Huber is a niche player on the silicate market in the EEA and worldwide as its market share on the merchant market is [0-5]% based on an estimated EEA- market size of about EUR 670 million. Thus, the Notifying Party claims that the Merged Entity will have no ability to foreclose silicate as an input product to competitors.
(228) Also, the Notifying Party alleges that there is no risk of customer foreclosure because Huber currently produces approximately […] kt per year of sodium silicate at its Taavetti plant and could expand the production to a maximum of […] kt per year. The Notifying Party notes that Evonik sources […].
(229) Furthermore, the Notifying Party describes the sodium silicate supply patterns of Huber and Evonik as follows.
(230) On the one hand, Huber Silica [produces sodium silicate and purchases only minor quantities from external sources. This will not change post-transaction].
(231) On the other hand, the Notifying Party explains that [Evonik’s sources of external sodium silicate supply will not change post-transaction].
The Commission's assessment
(232) The total EEA market size for sodium silicate accounts for 670M€ in 2016 and, according to the Parties' estimates, Huber Silica's market share on the merchant market is de minimis ([0-5]%). If the production of sodium silicate for captive use were to be allocated to Huber Silica' supply on the merchant market, Huber Silica's market share would remain fairly limited ([5-10]%). The largest player on the market for sodium silicate is PQ with [10-20]% market shares, and the Parties confirm that the EEA market for sodium silicate is quite fragmented with numerous active manufacturers, including from the glass industry.
(233) Although the vertical link between sodium silicate (upstream input product) and precipitated silica (downstream product) is affected, it should be noted that Huber Silica's limited market presence and market power in the upstream market for sodium silicate does not substantiate competitive concerns related to input foreclosure issues. This is also confirmed by one of the Commission's previous findings about the EEA market for sodium silicate which explain that sodium silicate is a commodity product for which customers "should have no difficulty in switching to alternative suppliers". (163)
(234) Similarly, Huber Silica's limited production of sodium silicate roughly accounting for its own pre-merger production does not substantiate any plausible customer foreclosure issue either. Moreover, due to the sodium silicate plant's location in Taavetti (Finland) and the fact that sodium silicate is a largely available input product, the Merged Entity is expected to have no ability to restrict it supplies from alternative manufacturers of sodium silicate ([…]).
(235) The market investigation indicated that neither input nor customer foreclosure could arise post-transaction with respect to the vertical link between precipitated silica and sodium silicate. Most respondents consider it would be easy for them to switch supplier of sodium silicate for the manufacture of precipitated silica (164). In particular, it is considered that "product specifications are met by large amount of suppliers in the market" (165) as well as that "the grade used for precipitated silica production is one of the main commercial grades worldwide and can be easily replaced with minimal work" (166). In addition, most competitors consider that there is sufficient supply of sodium silicate available in the EEA for the manufacture of precipitated silica (167).
(236) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction does not raise serious doubts as to its compatibility with the internal market with respect to the vertical link between sodium silicate and precipitated silica.
5.3. Conglomerate effects
5.3.1. Analytical framework
(237) Conglomerate mergers are mergers between firms that are in a relationship which is neither purely horizontal (as competitors in the same relevant market) nor vertical (as supplier and customer) but are active in closely related markets (e.g. mergers involving suppliers of complementary products or of products which belong to a range of products that is generally purchased by the same set of customers for the same end use) (168).
(238) According to the Non-Horizontal Merger Guidelines, in most circumstances, conglomerate mergers do not lead to any competition problems. (169)
(239) However, foreclosure effects may arise when the combination of products in related markets may confer on the merged entity the ability and incentive to leverage a strong market position from one market to another closely related market by means of tying or bundling or other exclusionary practices. The Non- Horizontal Merger Guidelines distinguish between bundling, which usually refers to the way products are offered and priced by the merged entity and tying, usually referring to situations where customers that purchase one good (the tying good) are required to also purchase another good from the producer (the tied good). Tying can take place on a technical or contractual basis. For instance, technical tying occurs when the tying product is designed in such a way that it only works with the tied product (and not with the alternatives offered by competitors). While tying and bundling have often no anticompetitive consequences, in certain circumstances such practices may lead to a reduction in actual or potential competitors' ability or incentive to compete. This may reduce the competitive pressure on the merged entity allowing it to increase prices. (170)
(240) In assessing the likelihood of such a scenario, the Commission examines, first, whether the merged firm would have the ability to foreclose its rivals (171), second, whether it would have the economic incentive to do so (172), and, third, whether a foreclosure strategy would have a significant detrimental effect on competition, thus causing harm to consumers (173). In practice, these factors are often examined together as they are closely intertwined.
5.3.2. Preliminary observations and Notifying Party’s general views
(241) In the EEA, Evonik manufactures and sells organofunctional silanes, fumed silica and betain which can be combined with precipitated silica for certain end-use applications. As long as they address the needs of the same customer base, they can be considered as closely related neighbouring markets where, post- transaction, the merged entity will be able to provide combined offers.
(242) Apart from the product-specific claims, described below, the Notifying Party alleges that the Transaction will not give rise to conglomerate effects for several reasons.
(243) First, it states that Evonik's portfolio will not change as a result of the Transaction.
(244) Second, according to the Notifying Party, it is common practice in the chemical industry that companies purchase and sell a multitude of products from and to each other, the terms and conditions being typically negotiated separately for different products.
(245) Third, the Notifying Party argues that customers choose to purchase products other than precipitated silica from the same precipitated silica supplier for reasons of convenience and efficiency only. Moreover, customers purchase products from the same supplier if this supplier offers the most advantageous price for both products. If customers can get a better price for one product at a different supplier, they would source the products from two suppliers. In addition, the Notifying Party, submits, that [its market behaviour will remain unchanged]..
(246) This decision analyses whether the proposed Transaction is likely to raise doubts as to its compatibility with the internal market by the creation on anticompetitive conglomerate effects
5.3.3.Organofunctional silanes and precipitated silica for tyre applications
The Notifying Party's views
(247) The Notifying Party provides Evonik's market shares in 2016 on the markets for organofunctional silanes, respectively for organofunctional silanes for rubber applications ([40-50]% in the EEA and [20-40]% worldwide), for organofunctional silanes for non-rubber applications ([35-45]% in the EEA and [20-30]% worldwide) and for akryl silanes (less than [10-20]% in the EEA and less than [10-20]% worldwide).
(248) The Notifying Party submits that only tyre manufacturers require both organofunctional silanes and precipitated silica for their production. The Notifying Party estimates that, in 2016, [60-70]% of Evonik's precipitated silica sales to tyre customers were achieved with customers purchasing also organofunctional silanes. Conversely, i.e. organofunctional silanes sales ratio to precipitated silica, Evonik's sales amount to [90-100]%. In this regard, the Notifying Party notes that Evonik is the only remaining producer of organofunctional silanes in Europe and that the rest of the market supply comes from Chinese producers. The Notifying Party explains that Evonik is supported by [Party's observation on market share].
(249) At the same time, the Notifying Party submits that [the transaction has no effect to Huber Silica’s customer relations]. Therefore, the Notifying Party argues that Evonik's position towards tyre customers in relation to organofunctional silanes and precipitated silica will not change post-transaction.
The Commission's assessment
(250) Huber Silica's sales of precipitated silica to customers in the tyre segment are negligible. Huber Silica has an EEA market share of [0-5]% in the market for precipitated silica for rubber applications. This market share is similarly low in the market for precipitated silica for tyre applications. Therefore, the Transaction does not lead to any merger-specific effects on the conglomerate relationship between the markets for organofunctional silanes and precipitated silica for rubber (and tyre) applications in which Evonik is active, but hardly Huber Silica.
(251) The market investigation confirmed the absence of any potential conglomerate effects, in particular that customers will not be forced to buy bundled products and that the Merged Entity is not likely to foreclose competitors. A competitor considers that bundling sales organofunctional silanes and precipitated silica is "not a necessary condition to expand on the market and clients can buy it from different suppliers" (174). None of the customers consider that post-merger the merged entity will be in a position to force its customers to buy both precipitated silica and organofunctional silanes from it (175).
(252) In the light of the above considerations, the proposed Transaction thus does not raise serious doubts as to its compatibility with the internal market with regard to the Parties' activities in the closely related neighbouring markets for precipitated silica for rubber (and tyre) applications and organofunctional silanes.
5.3.4. Fumed silica and precipitated silica for paints and coatings applications
The Notifying Party's views
(253) Evonik estimates its EEA market share in the market for fumed silica to be between [35-45]% in 2016. Huber Silica is not active in the market for fumed silica.
(254) Evonik notes that [the transaction will have only very limited or no effects due to Evonik’s and Huber Silica’s complimentary customer base].
The Commission's assessment
(255) Results of the market investigation showed that, despite concerns expressed by one competitor (176), none of the customers consider that post-merger the merged entity will be in a position to force them to buy both precipitated silica and fumed silica from it (177). Furthermore, the results of the market investigation also indicate that sufficient choice of suppliers of fumed silica will remain active in the EEA post-transaction (178).
(256) In the light of the absence of concerns from the market investigation and Huber Silica's limited presence on the market for precipitated silica for paints and coatings applications, the Transaction does not raise serious doubts as to its compatibility with the internal market with regard to the Parties' activities in the closely related neighbouring markets for precipitated silica for paints and coatings and fumed silica.
5.3.5. Betain and precipitated silica for dental applications
The Notifying Party's views
(257) Evonik estimates its EEA market share on the market for betain to be between [30-40]% in 2016. Huber Silica is not active in the market for betain.
(258) Evonik submits that less than [5-10]% of its customers purchasing betain also purchase precipitated silica. Furthermore, the revenues generated by these customers amount to less than EUR 3.5 million. The Notifying Party explains that [the transaction will unlikely have an appreciable effect in this regard, in particular due to multiple competitors offering betain].
The Commission's assessment
(259) According to the replies in the market investigation, customers confirmed that post-transaction, the Merged Entity would not be in the position to force its customers to buy both precipitated silica and betain from it. (179) In addition, customers consider that there is a sufficient choice of suppliers of betain in the EEA (180). The Merged Entity would therefore not have the ability nor the incentive to leverage its strong position in the market for precipitated silica for dental applications to the market for betain by means of bundling or tying and lead to foreclosure.
(260) In light of the above, in particular the absence of concerns expressed by customers and the fact that alternative sources of supply for betain remain available post-transaction, the proposed Transaction does not raise serious doubts as to its compatibility with the internal market with regard to the Parties' activities in the closely related neighbouring markets for precipitated silica for dental applications and betain.
6. PROPOSED REMEDIES
(261) In order to remove the serious doubts resulting from the Transaction and render the concentration compatible with the internal market, the Notifying Party has modified the notified concentration by formally submitting Commitments to the Commission on 20 June 2017 ("Final Commitments"). The Commitments are annexed to this decision and form an integral part thereof.
6.1. Framework for the assessment of the Commitments
(262) Where a concentration raises serious doubts as regards its compatibility with the internal market, the Parties may undertake to modify the concentration so as to remove the grounds for the serious doubts identified by the Commission.
(263) As set out in the Commission's Remedies Notice (181), the commitments have to eliminate the competition concerns entirely, and have to be comprehensive and effective from all points of view (182). Furthermore, commitments must be capable of being implemented effectively within a short period of time (183).
(264) From the point of view of the Merger Regulation's objective, divestiture commitments are normally the best way to eliminate competition concerns resulting from horizontal overlaps. However, the possibility cannot automatically be ruled out that other types of commitments may also be capable of preventing the significant impediment of effective competition (184). Divestiture commitments may be a divestiture of a business to a suitable purchaser, but also a removal of links with competitors, such as minority shareholding or the specific rights linked to such shareholding such as representations on the board, veto rights and also information rights (185). Other structural commitments may be suitable to resolve all types of concerns if those remedies are equivalent to divestitures in their effects (186).
(265) In assessing whether commitments will maintain effective competition, the Commission stresses that this question has to be examined on a case-by-case basis (187).
(266) It is against this background that the Commission analysed the proposed commitments in the present case.
6.2. Procedure
(267) To remedy the serious doubts identified following the phase I market investigation, on 31 May 2017 the Notifying Party proposed a set of Commitments ("Initial Commitments"). The Commitments were market tested by the Commission on 1 June 2017.
(268) Overall, the results of the market test were positive in that most respondent market participants agreed that the Commitments would remedy the Commission's serious doubts. At the same time many respondents suggested some amendments to the Commitments, to further ensure their effectiveness.
(269) The Commission informed the Party of the outcome of the market test during a conference call on 13 June 2017, with further interactions taking place in the following days.
(270) Following this feedback, the text of the commitments was amended and the Final Commitments were filed on 20 June 2017.
6.3. Description of the Initial Commitments
(271) The Initial Commitments consist of the divestment to a suitable purchaser of (i) Evonik's entire business relating to precipitated silica for dental applications in Europe, Middle East and Africa ("EMEA") region but excluding any production assets (hereinafter referred to as the "Sident Divestment Business") and (ii) Huber Silica's entire business relating to precipitated silica for defoamer applications in the EEA and hydrophobic precipitated silica for sale to any applications in the EEA, but excluding any production assets (hereinafter referred to as the "Zeoflo/Zeofoam Divestment Business"). Both divestment businesses will altogether be referred to as the "Divestment Businesses".
(272) A suitable purchaser for either the Sident Divestment Business or the Zeoflo/Zeofoam Divestment Business (the 'Purchaser') needs to be an established producer of precipitated silica with an existing market presence in the EEA and existing production facilities, preferably located in the EEA. Moreover, it must have the expertise and incentive to develop the Divestment Businesses by transferring and integrating the production of the divested products to its own production facilities.
(273) More in detail, the Sident Divestment Business, which the Purchaser has to transfer to its own production facility within a maximum of [period], with the possible extension of [period] if required, consists of:
a) all assets (with the exception of production assets) that are required for the current operation or are necessary to ensure the viability and competitiveness of the Sident Business, in particular:
· SIDENT ® 8, SIDENT ® 9, SIDENT ® 10, SIDENT ® 22 S trademarks in the EMEA;
· related intellectual property rights, know-how, product specifications, manufacturing recipe and technical documentation related to SIDENT ® 8, SIDENT ® 9, SIDENT ® 10 and SIDENT ® 22 S products to be exclusively used in a EEA plant and is not exported to Asia and the Americas. The intellectual property rights part of the Sident Divestment Business are transferred from the Merged Entity to the Purchaser, while the intellectual property rights that are also needed by the Merged Entity for their retained products. Therefore, the Purchaser grants an irrevocable, worldwide, non-exclusive and royalty-free license back to the Merged Entity for production of retained products and for dental grades sold in Asia and/or the Americas;
· relevant records and marketing materials;
· related licences, permits and authorisations issued by any governmental organisation;
· the entirety of Evonik’s portfolio of customers sourcing SIDENT ® 8, SIDENT ® 9, SIDENT ® 10, SIDENT ® 22 S for their EMEA requirements from Evonik's production facilities located in the EEA, including all related customer contracts and orders.
b) a transitional toll-manufacturing arrangement for the period necessary for the Purchaser to transfer production of SIDENT ® 8, SIDENT ® 9, SIDENT ® 10, SIDENT ® 22 S to its own production facility (i.e. a maximum of [period], with the possible extension of [period]if required), on the basis of which Evonik will supply the Purchaser with these products from its production site in Wesseling (Germany) in sufficient volumes (but not exceeding a […] increase in volume of the previous year and not exceeding […] per year and at a cost-oriented transfer price. The transfer price and the associated indexation formula, agreed by the Merged Entity and the Purchaser, are subject to review by the Monitoring Trustee who may be supported by an independent industry expert.
c) all necessary support (free of charge) to ensure the production transfer, including technical support for the implementation of SIDENT ® 8, SIDENT ® 9, SIDENT ® 10, SIDENT ® 22 S grades in one of the Purchaser’s own production sites and support for the customer qualification process related to those grades.
(274) Similarly, the Zeoflo/Zeofoam Divestment Business consists of:
a) all assets (with the exception of production assets) that are required for the current operation or are necessary to ensure the viability and competitiveness of the Zeoflo/Zeofoam Business, in particular:
· Zeoflo ® TL, Zeoflo ® MD and Zeofoam ® 166 trademarks for use in the EEA;
· related intellectual property rights, know-how, product specifications, manufacturing recipe and technical documentation to be licensed by Huber to the Purchaser under a royalty-free and non-exclusive licence for production in the EEA, which shall be limited to defoamer applications for Zeofoam ® 166;
· relevant records and marketing materials;
· related licences, permits and authorisations issued by any governmental organisation;
· the entirety of Huber Silica's portfolio of customers sourcing Zeoflo ® TL, Zeoflo ® MD and Zeofoam ® 166 for their EEA requirements, including all related customer contracts and orders.
b) a transitional toll-manufacturing arrangement for the period necessary for the Purchaser to transfer production of Zeoflo ® TL, Zeoflo ® MD and Zeofoam
® 166 to its own production facility (within a maximum of [period], on the basis of which Evonik will supply the Purchaser with these products in sufficient volumes (but not exceeding a […] increase in volume of the previous year and not exceeding […] of the sales of the Zeoflo/Zeofoam Business in 2016) and at a cost-oriented transfer price. The transfer price and the associated indexation formula, agreed by the Merged Entity and the Purchaser, are subject to review by the Monitoring Trustee who may be supported by an independent industry expert.
c) the existing toll-manufacturing arrangement with AMS for the hydrophobisation of hydrophilic precipitated silica marketed by Huber Silica under the Zeoflo ® TL and Zeoflo ® MD trademarks.
d) all necessary support (free of charge and for a maximum duration of [period]) to ensure the production transfer, including technical support for the implementation of Zeoflo ® TL, Zeoflo ® MD and Zeofoam ® 166 grades in one of the Purchaser’s own production sites in the EEA and support for the customer qualification process related to those grades.
6.4. Results of the market test of the Initial Commitments
(275) Overall, the results of the market test indicate that the divestment of the Sident and Zeoflo/Zeofoam Businesses to a suitable Purchaser with certain modifications is sufficient to remove the competition concerns raised by the Transaction. Customers' replies to the market test indicate that, in this case, the Commitments are suitable to effectively remove the competitive concerns with regard to each market for precipitated silica for dental end-use applications and for defoamer applications as well as for hydrophobic precipitated silica. In particular, with respect to the Sident Divestment Business, certain customers consider that the divestiture will "lessen the market power of Evonik in this sector" (188) and "would help maintain the current level of competition in the market for precipitated silica". (189) Similarly, with regard to the Zeoflo/Zeofoam Divestment Business, a customer notes that "generally, another player will contribute to competitiveness". (190) Even when highlighting the necessity of certain modifications in the Initial Commitments, a majority of competitors who responded to the market test indicate their potential interest in acquiring the Sident Divestment Business and the Zeoflo/Zeofoam Divestment Business. (191)
6.4.1. Sident Divestment Business
(276) According to the market test results, the geographic restriction to production in the EEA and sales in the EMEA is "a limitation to the ability to compete" (192) and "affects directly the production costs and the visibility of the products and therefore the competitiveness of the products" (193). The limitation "will make the divested unit less attractive to any multinational silica producer" (194), thus creating an obstacle to the viability of the Sident Divestment Business. Similarly, a competitor states that "a global scope for production and sales would allow purchaser to reach economies of scale and secure competitiveness as well as convince customers with global needs to keep sourcing from the divested business" (195). Customers confirmed potential viability problems too. They state that "with such limitations, there would be a limited number of customers for this purchaser and this may impact the viability of such a business" (196) as well as that "without an ability to resell globally by initial purchasers this may be a limited market and viability may be impacted" (197).
(277) In addition to the concern that they "could not export without transforming the silica" (198), the market participants indicated concerns related to the intellectual property ("IP") rights. A customer is in doubt whether there will be IP rights issues in case they "transformed the product within the EMEA and then exported that product that integrated silica" (199), which is not clear from the Initial Commitments.
(278) As to the production transfer period, the market test raised concerns that the production transfer period of a maximum duration of [period] with no possibility of extension would not be sufficient mainly due to the necessity and the duration of formulation adjustments, laboratory testing and customer acceptance testing (200). Similarly, the market test indicated that the duration of the tolling agreement of [period], as described in recital (273) above, is not sufficient and needs to be extended. (201)
(279) Also, market test results indicated that the Purchaser must have access to key personnel of Evonik, especially if the Purchaser does not have experience in the market for precipitated silica for dental end-use application (202). In particular, "it may be necessary for a purchaser without experience in the Cosmetics/toothpaste market to keep some of the technical marketing support, customer application support and toothpaste application researchers to join the purchaser to guarantee a good service to the customers and a continuity in any application developments and new product developments" (203). In addition to customer support, researchers and regulatory experts are considered necessary to guarantee the long-term viability of the production line (204). Furthermore, customers in the market test indicated that technical and product support is very important for them to continue purchasing precipitated silica for dental end-use application from the Purchaser (205).
(280) With regard to the production capacities, the market test provided indications about some customers' preference that the Purchaser has available production capacities (206). However, the market test replies from competitors suggest that manufacturers may not have sufficient spare capacity (207). In addition, most customers expressed a preference for the purchaser to have production facilities in the EEA in order to "reduce logistics / delivery costs" (208). The requirement is also related to securing the transition timing and the market knowledge (209).
(281) Furthermore, some competitors indicated the need for a non-compete agreement to be put in place. (210) In this regard, a competitor stated that "A non-compete for a period of time would be required to build customer loyalty and confidence in the abilities of the purchaser … would be a significant risk to the purchaser if customers would be in a position to source like products from Evonik/Huber shortly after the transaction" (211).
6.4.2. Zeoflo/Zeofoam Divestment Business
(282) Several competitors mentioned the lack of attractiveness of the defoamer package due to the very low production volumes it would involve (212). The concern is for example whether "a purchaser could maintain competitiveness" (213). A suggested way to mitigate these concerns was to enable the purchaser to sell the defoamer grades worldwide (214). A competitor suggested for pipeline projects to be included in the scope of the Zeoflo/Zeofoam Divestment Business to ensure viability and competitiveness (215).
(283) As to the production facilities, customers indicated that the purchaser should have production facilities in the EEA (216). It is considered that it would "reduce logistics and delivery cost and import tariffs. Also, management of production, stocks and storage will be more efficient if within EEA" (217).
(284) A competitor indicated that IP rights and know-know for products under development and R&D product development equipment should be included (218). The non-exclusive basis of the IP transfer could create concerns (219) because "the resultant production volume of each potential licensee would be so small that none of the EEA silica producer would have the economic incentive to start production of the grade of Silica within the scope of divestment, which negate completely the benefit of the divestment to restore competitive situation in the marketplace" (220). In addition, a competitor considered that in such a market segment multiple entrants could lead to revenue and profitability being unsustainable (221).
6.5. Description of the Final Commitments
(285) Following the results of the Initial market test, the Final Commitments include improved obligations More specifically, the Final Commitments include the following modifications.
6.5.1. Sident Divestment Business
(286) According to the Final Commitments:
· The Purchaser can globally supply the grades included in the Sident Divestment Business and is not restricted from supplying new customers and developing its own portfolio of customers in addition to those being transferred.
· The production transfer further includes precipitated silica products under development for dental end-use applications
· The production transfer is extended and must be completed within [period] after the acquisition by the Purchaser of the Sident Business, with a possible extension of [period] subject to approval of the Monitoring Trustee.
· The toll-manufacturing agreement is similarly extended and lasts until the Purchaser has transferred the Sident Divestment Business to its own production facility and for a maximum duration of [period] after the acquisition by the Purchaser of the Sident Business, with a possible extension of […] [period] subject to approval of the Monitoring Trustee.
· Provisions on personnel foresee, at the option of the Purchaser, the transfer of [number of Evonik employees] […] and the (part or full-time) secondment of […]. In addition, the Purchaser can identify key functions necessary to maintain the viability and competitiveness of the Sident Divestment Business and, if the Monitoring Trustee deems that these functions are necessary to maintain the viability and competitiveness of the Sident Divestment Business, it could make employment offers to [Evonik employee(s)].
· Provision on the IP rights explicitly provides that there is no limitation to the customers to sell transformed products containing precipitated silica grades of the Sident Divestment Business worldwide. IP rights and know-know for products under development are also licensed to the Purchaser. In relation to the non-exclusive basis of the IP transfer, the Final Commitments include an explicit provision that Evonik commits not to grant licenses as included in the Sident Divestment Business to any third party (excluding Evonik subsidiaries).
· The requirement for the Purchaser to have production facilities in the EEA.
6.5.2. Zeoflo/Zeofoam Divestment Business
(287) According to the Final Commitments:
· The Purchaser can globally supply the grades included in the Zeoflo/Zeofoam Divestment Business and is not restricted from supplying new customers and developing its own portfolio of customers in addition to those being transferred.
· The production transfer further includes precipitated silica products under development for defoamer end-use applications as well as hydrophobic precipitated silica under development.
· Provision on the IP rights explicitely provides that there is no limitation to the customers to sell transformed product containing precipitated silica grades of the Zeoflo/Zeofoam Divestment Business worldwide. IP rights and know- know for products under development are also licensed to the Purchaser. In relation to the non-exclusive basis of the IP transfer, the Final Commitments include an explicit provision providing that Evonik commits not to grant licenses as included in the Zeoflo/Zeofoam Divestment Business to any third party (excluding Evonik subsidiaries).
· Provision on personnel foresee that the Purchaser can identify key functions necessary to maintain the viability and competitiveness of the Zeoflo/Zeofoam Divestment Business and, if the Monitoring Trustee deems these functions are necessary to maintain the viability and competitiveness of the Zeoflo/Zeofoam Divestment Business, it could make employment offers to [Evonik employee(s)].
· The requirement for the Purchaser to have production facilities in the EEA.
6.6. Assessment of the Final Commitments
(288) As explained in this Decision, the serious doubts as to the compatibility of the proposed Transaction with the internal market reside in the combination of Evonik's and Huber Silica's activities in relation to the manufacture and supply of precipitated silica for dental end-use applications and for defoamer end-use applications, as well as in relation to the manufacture and supply of hydrophobic precipitated silica.
(289) The Final Commitments consist in the divestment of both Evonik's activities in precipitated silica grades for dental end-use applications and Huber Silica's activities in hydrophobic precipitated silica and grades for defoamer end-use applications, representing the full horizontal overlap between the Parties as regards precipitated silica for dental end-use applications and for defoamer end- use applications as well as for hydrophobic precipitated silica in the EEA.
(290) More specifically, the Divestment Businesses include all assets (with the exception of any production assets) necessary for their on-going activities. In particular, all necessary trademarks and other IP rights, know-how, licenses, authorizations, portfolio of customers with EMEA (Sident Divestment Business) / EEA (Zeoflo/Zeofoam Divestment Business and transitional toll-manufacturing agreements.
(291) The Commission considers that the Final Commitments address all the concerns raised by market participants in the context of the market test with regard to both the Sident Divestment Business and the Zeoflo/Zeofoam Divestment Business.
(292) The removal of geographic restrictions for sales limited to the EMEA region (Sident Divestment Business) or the EEA (Zeoflo/Zeofoam Divestment Business) increases the attractiveness of the Divestment Businesses and solves viability concerns by enabling the Purchaser to compete on a worldwide basis and have the opportunity to capture market growth in neighbouring regions and potentially increase its production by serving a larger customer base. The extended scope of the Divestment Businesses to also cover products under development, if any, further increases the attractiveness of the Divestment Businesses. As to the IP rights, the Commission notes that the Purchaser will have all IP rights allowing the Purchaser to produce the precipitated silica grades in the EEA. In addition, to address the comments of market participants, there will be no limitation for customers to sell their products, containing precipitated silica, on a worldwide basis. Also, the explicit provision that Evonik commits not to grant the Licenses to any third party (excluding Evonik subsidiaries) other than the Purchaser alleviates the concerns related to the non-exclusive nature of the IP transfer.
(293) Specifically for the Sident Divestment Business, the extension of the production transfer and toll-manufacturing periods allow sufficient time for the accomplishment of all necessary steps for the production transfer, including the customer certification.
(294) For both the Sident Divestment Business and the Zeoflo/Zeofoam Divestment Business, the condition for the Purchaser to have production capacities in the EEA addresses the customers' preferences as expressed in the results of the market test for ensuring reduced logistics and transport costs.
(295) The Commission considers that the Final Commitments are capable of being implemented effectively within a short period of time. The commitments contain an up-front buyer clause, meaning the Parties cannot close the proposed transaction before having entered into a final binding sale and purchase agreement for the sale of the Divestment Businesses and the Commission has approved the Purchaser and the terms of sale. This will create a strong incentive for the Parties to find a suitable purchaser quickly. The assets that are required for the operation of the Divestment Businesses will be transferred to the Purchaser shortly after the approval of the Purchaser and the terms of sale by the Commission. The elements of the commitments that have a long-term duration such as Evonik's commitment to provide support to the Purchaser to ensure the production transfer and the transitional agreements, can be effectively monitored by the Monitoring Trustee and the Commission.
6.7. Conclusion on the Commitments
(296) For the reasons outlined above, and in view of the results of the market test and the ensuing improvements to the Commitments, the Commission considers the Final Commitments are sufficient to eliminate the serious doubts as to the compatibility of the Transaction with the internal market, in relation to the manufacture and supply of precipitated silica for dental end-use applications, defoamer end-use application and hydrophobic precipitated silica.
6.8. Conditions and obligations
(297) The commitments in paragraphs 6(a), 6(b), 7(a) and 7(b) of section B of the Annex constitute conditions attached to this decision, as only through full compliance therewith can the structural changes in the relevant markets be achieved. The other commitments set out in the Annex constitute obligations, as they concern the implementing steps which are necessary to achieve the modifications sought in a manner compatible with the internal market.
7. CONCLUSION
(298) For the above reasons, the Commission has decided not to oppose the notified operation as modified by the commitments and to declare it compatible with the internal market and with the functioning of the EEA Agreement, subject to full compliance with the conditions in paragraphs 6(a), 6(b), 7(a) and 7(b) of section B of the commitments annexed to the present decision and with the obligations contained in the other sections of the said commitments. This decision is adopted in application of Article 6(1)(b) in conjunction with Article 6(2) of the Merger Regulation and Article 57 of the EEA Agreement.
Non-Confidential version
20 June 2017
Case M.8348 – Evonik Industries AG/Huber Precipitated Silica Business
COMMITMENTS TO THE EUROPEAN COMMISSION
Pursuant to Article 6(2) of Council Regulation (EC) No 139/2004 (the Merger Regulation), Evonik Industries AG (Evonik) hereby enters into the following Commitments (the Commitments) vis-à-vis the European Commission (the Commission) with a view to rendering the acquisition of sole control of the Huber Precipitated Silica Business (Huber Silica) (the Concentration) compatible with the internal market and the functioning of the EEA Agreement.
This text shall be interpreted in light of the Commission’s decision pursuant to Article 6(1)(b) of the Merger Regulation to declare the Concentration compatible with the internal market and the functioning of the EEA Agreement (the Decision), in the general framework of European Union law, in particular in light of the Merger Regulation, and by reference to the Commission Notice on remedies acceptable under Council Regulation (EC) No 139/2004 and under Commission Regulation (EC) No 802/2004 (the Remedies Notice).
Section A. Definitions
1. For the purpose of the Commitments, the following terms shall have the following meaning:
Affiliated Undertakings: undertakings controlled by Evonik and/or by the ultimate parents of Evonik, whereby the notion of control shall be interpreted pursuant to Article 3 of the Merger Regulation and in light of the Commission Consolidated Jurisdictional Notice under Council Regulation (EC) No 139/2004 on the control of concentrations between undertakings (the Consolidated Jurisdictional Notice).
Assets: the assets that contribute to the current operation or are necessary to ensure the viability and competitiveness of the Divestment Business as indicated in Section B, and described more in detail in the Schedule.
Closing: the transfer of the legal title to the Divestment Business to the Purchaser.
Closing Period: the period of 3 months from the approval of the Purchaser and the terms of sale by the Commission.
Confidential Information: any business secrets, know-how, commercial information, or any other information of a proprietary nature that is not in the public domain.
Conflict of Interest: any conflict of interest that impairs the Trustee's objectivity and independence in discharging its duties under the Commitments.
Divestiture Trustee: one or more natural or legal person(s) who is/are approved by the Commission and appointed by Evonik and who has/have received from Evonik the exclusive Trustee Mandate to sell the Divestment Business to the relevant Purchaser at no minimum price.
Divestment Business: the Sident Business and the Zeoflo/Zeofoam Business as defined in Section B and in the Schedule which Evonik commits to divest.
Effective Date: the date of adoption of the Decision.
EMEA: all countries of Europe, Africa and the Middle East.
Evonik Industries AG: a company incorporated under the laws of Germany and its affiliates, with its registered office at Rellinghauser Strasse 1-11, 45128 Essen and registered with the Commercial Register at Essen District Court under HRB number 19474.
First Divestiture Period: the period of 6 months from the Effective Date.
Hold Separate Manager: the person appointed by Evonik for the Divestment Business to manage the day-to-day business under the supervision of the Monitoring Trustee.
Key Personnel: all personnel necessary to maintain the viability and competitiveness of the Divestment Business, as listed in the Schedule, including the Hold Separate Manager(s).
Monitoring Trustee: one or more natural or legal person(s) who is/are approved by the Commission and appointed by Evonik, and who has/have the duty to monitor Evonik’s compliance with the conditions and obligations attached to the Decision.
Parties: Evonik and the undertaking that is the target of the concentration.
Purchaser: the entity or respective entities approved by the Commission as acquirer of the Divestment Business in accordance with the criteria set out in Section D. Insofar as the Divestment Business is not divested to the same entity, the term ‘Purchaser’ shall refer to the Purchaser of the Sident Business and/or the Zeoflo/Zeofoam Business respectively.
Purchaser Criteria: the criteria laid down in paragraph 18 of these Commitments that the Purchaser must fulfil in order to be approved by the Commission.
Schedule: the schedule to these Commitments describing in more detail the Divestment Business.
Sident Business: the business as defined in paragraphs 6 et seq. of Section B and in the Schedule and which Evonik commits to divest.
Sident Transfer Support Commitment: Evonik’s commitment to support the transfer of the Sident Business in accordance with Section B.
Sident Transitional Toll Manufacturing Agreement: Evonik’s commitment to supply on a temporary basis SIDENT ® 8, SIDENT ® 9, SIDENT ® 10, SIDENT ® 22 S to the Purchaser as defined in paragraph 6 of Section B and in the Schedule.
Technical Expert: one or more natural or legal person(s), appointed by and reporting to the Monitoring Trustee, who has/have industry expertise relevant to the Divestment Businesses and will assist and advise the Monitoring Trustee with regard to all technical aspects related to the Divestment Businesses, as described in more detail in paragraphs 6 and 7.
Transformed Product: any final or intermediary product which contains precipitated silica produced by the Sident Business and the Zeoflo/Zeofoam Business
Trustee(s): the Monitoring Trustee and/or the Divestiture Trustee as the case may be.
Trustee Divestiture Period: the period of six months from the end of the First Divestiture Period.
Zeoflo/Zeofoam Business: the business as defined in paragraphs 7 et seq. of Section B and in the Schedule and which Evonik commits to divest.
Zeofoam Transfer Support Commitment: Evonik’s commitment to support the transfer of the Zeoflo/Zeofoam Business in accordance with Section B.
Zeofoam Transitional Toll Manufacturing Agreement: Evonik's commitment to supply on a temporary basis Zeofoam to the Purchaser as defined in paragraph 7 of Section B and in the Schedule.
Section B. The commitment to divest and the Divestment Business
Commitment to divest
2. In order to maintain effective competition, Evonik commits to divest, or procure the divestiture of, the Divestment Business by the end of the Trustee Divestiture Period as a going concern to a purchaser and on terms of sale approved by the Commission in accordance with the procedure described in paragraph 19 of these Commitments. To carry out the divestiture, Evonik commits to find a purchaser and to enter into a final binding sale and purchase agreement for the sale of the Divestment Business within the First Divestiture Period. If Evonik has not entered into such an agreement at the end of the First Divestiture Period, Evonik shall grant the Divestiture Trustee an exclusive mandate to sell the Divestment Business in accordance with the procedure described in paragraph 31 in the Trustee Divestiture Period.
3. The proposed concentration shall not be implemented before Evonik or the Divestiture Trustee has entered into a final binding sale and purchase agreement for the sale of the Divestment Business and the Commission has approved the purchaser and the terms of sale in accordance with paragraph 19.
4. Evonik shall be deemed to have complied with this commitment if:
(a) by the end of the Trustee Divestiture Period, Evonik or the Divestiture Trustee has entered into a final binding sale and purchase agreement for the divestment of the Divestment Business and the Commission approves the proposed purchaser and the terms of sale as being consistent with the Commitments in accordance with the procedure described in paragraph 19;
(b) the Closing of the sale of the Divestment Business to the Purchaser takes place within the Closing Period and all related agreements have been concluded and;
(c) all support obligations and transitional services and supply agreements have been complied with.
5. In order to maintain the structural effect of the Commitments, Evonik shall, for a period of 10 years after Closing, not acquire, whether directly or indirectly, the possibility of exercising influence (as defined in paragraph 43 of the Remedies Notice, footnote 3) over the whole or part of the Divestment Business, unless, following the submission of a reasoned request from Evonik showing good cause and accompanied by a report from the Monitoring Trustee (as provided in paragraph 45 of these Commitments), the Commission finds that the structure of the market has changed to such an extent that the absence of influence over the Divestment Business is no longer necessary to render the proposed concentration compatible with the internal market.
Structure and definition of the Divestment Business
A. Sident Business
6. The Sident Business means Evonik’s EMEA business with the products marketed under the SIDENT ® 8, SIDENT ® 9, SIDENT ® 10, SIDENT ® 22 S trademarks. The Sident Business includes all precipitated silica produced in the EEA by Evonik for dental end-use applications in EMEA at Closing as well as any precipitated silica products for dental end- use applications under development within Evonik at Closing. It consists of:
(a) all assets (with the exception of any production assets) that are required for the current operation or are necessary to ensure the viability and competitiveness of the Sident Business, in particular:
(i) the SIDENT ® 8, SIDENT ® 9, SIDENT ® 10, SIDENT ® 22 S trademarks for use in EMEA;
(ii) all other intellectual property rights, know-how, product specifications, manufacturing recipes and technical documentation required for the continued viability and competitiveness of the Sident Business. This includes formulation know-how, current and historic customer files of customers purchasing for their EMEA requirements and current and historic product information relating to SIDENT ® 8, SIDENT ® 9, SIDENT ® 10, SIDENT ® 22 S, as follows.
- Intellectual property rights, know-how, product specifications, manufacturing recipes and technical documentation that are exclusively used for the production of SIDENT ® 8, SIDENT ® 9, SIDENT ® 10, SIDENT ® 22 S at a production site in the EEA will be fully transferred to the Purchaser.
- Intellectual property rights, know-how, product specifications, manufacturing recipes and technical documentation that are primarily used for the production of SIDENT ® 8, SIDENT ® 9, SIDENT ® 10, SIDENT ® 22 S but are also used for the production of other Evonik products will be fully transferred to the Purchaser and the Purchaser will grant an irrevocable, worldwide, non-exclusive and royalty-free licence back to Evonik for the production of other Evonik products and for SIDENT ® 8, SIDENT ® 9, SIDENT ® 10, SIDENT ® 22 S to the extent that these are sold in Asia and/or the Americas.
- Intellectual property rights, know-how, product specifications, manufacturing recipes and technical documentation that are primarily used for the production of other Evonik products but also for the production of SIDENT ® 8, SIDENT ® 9, SIDENT ® 10, SIDENT ® 22 S will be licenced by Evonik to the Purchaser under an irrevocable, royalty-free and non- exclusive licence for the production of precipitated silica dental grades in the EEA (the SIDENT Licence). Evonik commits not to grant the SIDENT Licence to any third party (excluding any Affiliated Undertakings) other than the Purchaser.
(iii) all relevant data, books, records, marketing and advertising/promotional materials, trade-dress, i.e. total image or overall design of appearance of products or its packaging and other documents to the extent exclusively or primarily related to or necessary for the operations of the Divestment Businesses;
(iv) all licences, permits and authorisations issued by any governmental organisation for the benefit of the Sident Business;
(v) the entirety of Evonik’s portfolio of customers sourcing SIDENT ® 8, SIDENT ® 9, SIDENT ® 10, SIDENT ® 22 S for their EMEA requirements from Evonik's production facilities located in the EEA (as listed in the Schedule), including all related customer contracts, leases commitments and customer orders of the Divestment Business and all customer, credit and other records of the Divestment Business. For the avoidance of doubt, the Purchaser is not restricted from supplying new customers with SIDENT ® 8, SIDENT ® 9, SIDENT ® 10, SIDENT ® 22 S and has the right to sell these products worldwide. In addition, there will be no limitation to the right of the customers to sell the Transformed Products worldwide.
(vi) at the option of the Purchaser,
A. the transfer of [number of Evonik employees], named in the Annex to these Commitments, who […] currently active in […]. During a period of [period] from Closing, Evonik will allow the Purchaser to have access to and make an employment offer to [number of Evonik employees]. Evonik will take all reasonable steps, or procure that all reasonable steps are being taken, including appropriate incentive schemes (based on industry practice), to encourage [ Evonik employee(s)], if […] received an employment offer from the Purchaser to transfer to the Purchaser, subject to applicable employment legislation.
B. the (part or full-time) secondment to the Purchaser of [Evonik employee(s)] currently responsible for […] and being [(a) member(s)] of the Production Transfer Team (see para. (d) below) for a period of up to [period].
C. the Purchaser can identify key functions for the Sident Business. If, following input from Evonik, the Monitoring Trustee deems that these functions meet the definition of Key Personnel (as set out in in para.1 above), Evonik will identify a pool of candidates for each of these functions, in consultation with the Monitoring Trustee. Evonik will allow the purchaser to have access to and make an employment offer to these candidates. The Parties will take all reasonable steps, or procure that all reasonable steps are being taken, including appropriate incentive schemes (based on industry practice), to encourage the candidates who have been identified and who have received an employment offer from the purchaser to transfer to the purchaser, subject to applicable employment legislation.
(b) the transfer of precipitated silica products of the Sident Business which are under development for dental end-use applications for use in the EMEA at the time of Closing including all relevant intellectual property rights, know-how, product specifications, manufacturing recipes and technical documentation required for the continued research and development of these products.
(c) a transitional toll-manufacturing arrangement (the Sident Transitional Toll Manufacturing Agreement)
(i) for the period until the Purchaser has transferred the Sident Business to its own production facility and for a maximum duration of [period] starting from Closing, extendable by [period] subject to approval of the Monitoring Trustee (together with the Technical Expert), if such extension is required in order to complete the transfer,
(ii) with the Purchaser having to transfer the Sident Business to its own production facility within [period] from Closing, extendable by [period] subject to approval of the Monitoring Trustee and the Purchaser having an option to effect such transfer at any earlier point in time with reasonable prior announcement to Evonik,
(iii) on the basis of which Evonik will produce SIDENT ® 8, SIDENT ® 9, SIDENT ® 10 and SIDENT ® 22 S at Evonik’s Wesseling production site (the Wesseling site) for supply to the Purchaser in sufficient volumes which allow the Purchaser to supply the transferred customers and new customers with SIDENT ® 8, SIDENT ® 9, SIDENT ® 10 and SIDENT ® 22 S,
(iv) at a cost-oriented transfer price, with the level of the presumed production costs to be agreed between Evonik and the Purchaser and the transfer price being cost-indexed;
i. The transfer price must be fixed and agreed in the sale and purchase agreement for the Divestment Business which Evonik shall provide to the Commission for approval pursuant to para. 19 of the present Commitments.
ii. Indexation formula of the transfer price shall be equally fixed and agreed in the sale and purchase agreement for the Divestment Business and based on standard cost related industry benchmarks which are outside the control of Evonik.
iii. The transfer price as well as the associated indexation formula agreed between Evonik and the Purchaser shall be subject to the review of the Monitoring Trustee who shall establish whether (and confirm to the Commission that) the agreed transfer price and its indexation are at the level of typical production costs in the precipitated silica industry. To this effect, Evonik and the Purchaser shall communicate the production costs in their various sites to the Monitoring Trustee. In order to carry out this review, the Monitoring Trustee may appoint an independent industry expert who would support the Monitoring Trustee in its review.
(v) at the option of the Purchaser and depending on the advancement level of the production transfer, with the annual product volumes that have to be made available being equal to, in a given year (Y), […] of the previous year's (Y-1) sales in metric tons of SIDENT ® 8, SIDENT ® 9, SIDENT ® 10 and SIDENT ® 22 S sold within the scope of the Divestment Business. For the volumes to be made available in 2017, the sales already made by Evonik to the divested customers in that year will be deducted from the product volume made available to the Purchaser; for determining the 2017 sales as a basis for the volumes to be made available in 2018, Evonik’s sales made in that year to the divested customers will be taken into account. The total capacity to be made available to the Purchaser in any given year shall not exceed[…]. The Purchaser shall inform Evonik about any future increased use of Evonik’s capacity (beyond the 2016 sales up to the limit indicated above) with sufficient time in advance so that Evonik is able to free up sufficient spare capacity for the Purchaser;
(d) a commitment to provide all support (free of charge) which is necessary to ensure the transfer of the Sident Business (the Sident Transfer Support Commitment), including the following:
(i) technical support and training necessary for the implementation of the Sident Business in one of the Purchaser’s own production sites by the end of the Sident Transitional Toll Manufacturing Agreement; and
(ii) support in the context of customer qualification processes for SIDENT ® 8, SIDENT ® 9, SIDENT ® 10, SIDENT ® 22 S which the Purchaser may need to undergo following the transfer of the Sident Business, on the terms set out in the Schedule. This obligation to provide support shall be carried out by a Production Transfer Team and shall apply until the point in time when the transfer of the production has been successfully achieved and at least the majority of the relevant customers (representing a substantial portion of the Divestment Business' demand) have qualified the Purchaser’s production.
(e) In the exercise of their various work tasks, the Production Transfer Team shall prioritise the support to be given to the Purchaser over their work for the retained businesses and make themselves available according to the requirements for a timely and effective implementation of the Production Transfers. Evonik will establish an appropriate incentive scheme for the [Evonik employee(s)] concerned in order to have the transfer of the production completed in a timely and an effective manner. The Production Transfer Team will be bound by appropriate confidentiality obligations which will be agreed in accordance with paragraphs 13 and 14 of the Commitments. Should any member of the Production Transfer Team involved in the support to the Purchaser leave the company, Evonik will ensure that other competent employees will step in and complete the Production Transfer Team, after consulting with Monitoring Trustee and the Technical Expert.
(f) If there is any asset (with the exception of manufacturing assets) which is not covered by points a) to d) but which is both used (exclusively or not) in the Sident Business and necessary for the continued viability and competitiveness of the Sident Business, that asset or adequate substitute will be offered to potential purchasers.
B.Zeoflo/Zeofoam Business
7. The Zeoflo/Zeofoam Business means Huber Silica’s EEA business with the products marketed under the Zeoflo ® TL, Zeoflo ® MD and Zeofoam ® 166 trademarks. The Zeoflo/Zeofoam Business includes any other hydrophobic precipitated silica products for defoamer and non-defoamer end-use applications and any other hydrophilic precipitated silica products for defoamer end-use applications sold by Huber Silica at Closing in the EEA as well as hydrophobic precipitated silica products under development, within Huber, for defoamer and non-defoamer end-use applications, hydrophilic precipitated silica products under development for defoamer end-use applications at Closing. It consists of:
(a) all assets (with the exception of any production assets) that are required for the current operation or are necessary to ensure the viability and competitiveness of the Zeoflo/Zeofoam Business, in particular:
(i) the Zeoflo ® TL, Zeoflo ® MD and Zeofoam ® 166 trademarks for use in the EEA;
(ii) all other intellectual property rights, know-how, product specifications, manufacturing recipes and technical documentation required for the continued viability and competitiveness of the Zeoflo/Zeofoam Business owned by Huber, including in particular, formulation know- how, current and historic files of customers purchasing for their EEA requirements and current and historic product information relating to Zeoflo ® TL, Zeoflo ® MD and Zeofoam ® 166, will be licenced by Huber to the Purchaser under an irrevocable, royalty-free and non- exclusive licence for production in the EEA, which shall in relation to Zeofoam ® 166 (but not with respect to Zeoflo ® TL and Zeoflo ® MD) be limited to defoamer applications (the Zeoflo/Zeofoam Licence). Evonik commits not to grant the Zeoflo/Zeofoam Licence to any third party (excluding Evonik subsidiaries) other than the Purchaser. For the avoidance of doubt, this latter commitment shall not prevent Evonik from entering into toll-manufacturing agreements with third parties for the purpose of hydrophobisation of precipitated silica.
(iii) all relevant data, books, records, marketing and advertising/promotional materials, trade-dress, i.e. total image or overall design of appearance of product or its packaging and other documents to the extent exclusively or primarily related to or necessary for the operations of the Divestment Businesses;
(iv) all licences, permits and authorisations issued by any governmental organisation for the benefit of the Zeoflo/Zeofoam Business;
(v) the entirety of Huber Silica’s portfolio of customers sourcing Zeoflo ® TL, Zeoflo ® MD and Zeofoam ® 166 for their EEA requirements (as listed in the Schedule), including all related customer contracts, leases, commitments and customer orders of the Divestment Business and all customer, credit and other records of the Divestment Business. The Purchaser is not restricted from supplying new customers with Zeoflo ® TL, Zeoflo ® MD and Zeofoam ® 166. For the avoidance of doubt, there will be no limitation to the right of the customers to sell the Transformed Products worldwide.
(vi) at the option of the Purchaser the Purchaser can identify key functions for the Zeoflo/Zeofoam Business. If, following input from Evonik, the Monitoring Trustee deems that these functions correspond to Key Personnel, Evonik will identify a pool of candidates for each of these functions, in consultation with the Monitoring Trustee. Evonik will allow the purchaser to have access to and make an employment offer to these candidates. The Parties will take all reasonable steps, or procure that all reasonable steps are being taken, including appropriate incentive schemes (based on industry practice), to encourage the candidates who have been identified and who have received an employment offer from the Purchaser to transfer to the Purchaser, subject to applicable employment legislation.
(b) the transfer of hydrophobic precipitated silica products of the Zeoflo/Zeofoam Business which are under development at the time of Closing for defoamer and non-defoamer end-use applications for use in the EEA and hydrophilic precipitated silica products under development for defoamer end-use applications for use in the EEA, including all relevant intellectual property rights, know-how, product specifications, manufacturing recipes and technical documentation required for the continued research and development of these products.
(c) a transitional toll-manufacturing arrangement (the Zeofoam Transitional Toll Manufacturing Agreement)
(i) for the period until either the Purchaser has transferred the production of Zeofoam 166 (which is a hydrophilic end-customer product and at the same time the upstream input product for the hydrophobic Zeoflo ® TL and Zeoflo ® MD) to its own production facility or for a maximum duration of [period],
(ii) with the Purchaser having an option to start the production of Zeofoam ® 166 at any of its own production facilities at any point in time during these [period] with reasonable prior notice to Evonik,
(iii) on the basis of which and subject to (iv) below, Evonik will produce the Zeofoam ® 166 at one of its US production sites and will procure the supply of Zeoflo ® TL, Zeoflo ® MD and Zeofoam ® 166 in sufficient volumes to the Purchaser in order to allow the Purchaser to supply the transferred customers with Zeoflo ® TL, Zeoflo ® MD and Zeofoam ® 166. Evonik will ship these volumes to an EEA facility of the Purchaser,
(iv) at the option of the Purchaser and depending on the advancement level of the production transfer, with the annual product volumes of Zeofoam that have to be made available (for either direct end customer sales as a hydrophilic product or as an upstream product for the conversion into Zeoflo ® TL and Zeoflo ® MD) being equal to, in a given year (Y), […] of the previous year's (Y-1) sales in metric tons of Zeoflo ® TL, Zeoflo ® MD and Zeofoam ® 166 to the divested customers, provided that the total capacity made available at any given year shall not exceed[…] of the sales of the Zeoflo/Zeofoam Business in 2016. For the volumes to be made available in 2017, the sales already made by Evonik to the divested customers in that year will be deducted from the product volume made available to the Purchaser; for determining the 2017 sales as a basis for the volumes to be made available in 2018, Evonik’s sales made in that year to the divested customers will be taken into account. The Purchaser shall inform Evonik about any future increased use of Evonik’s capacity (beyond the 2016 sales up to the limit indicated above) with sufficient time in advance so that Evonik is able to free up sufficient spare capacity for the Purchaser;
(v) at a cost-oriented transfer price (including any transport cost), with the level of the presumed production costs to be agreed between Evonik and the Purchaser and the transfer price being cost-indexed.
i. The transfer price must be fixed and agreed in the sale and purchase agreement for the Divestment Business which Evonik shall provide to the Commission for approval pursuant to para. 19 of the present Commitments.
ii. Indexation formula of the transfer price shall be equally fixed and agreed in the sale and purchase agreement for the Divestment Business and based on standard cost related industry benchmarks which are outside the control of Evonik.
iii. The transfer price as well as the associated indexation formula agreed between Evonik and the Purchaser shall be subject to the review of the Monitoring Trustee who shall establish whether (and confirm to the Commission that) the agreed transfer price and its indexation are at the level of typical production costs in the precipitated silica industry. To this effect, Evonik and the Purchaser shall communicate the production costs in their various sites to the Monitoring Trustee. In order to carry out this review, the Monitoring Trustee may appoint an independent industry expert who would support the Monitoring Trustee in its review.
(d) the existing toll-manufacturing arrangement with Applied Material Solutions (AMS) for the hydrophobisation of hydrophilic precipitated silica marketed by Huber Silica under the Zeoflo ® TL and Zeoflo ® MD trademarks; if the transfer of the tolling relationship with AMS should not be feasible despite Evonik's best efforts or if the Purchaser should not want the transfer, Evonik will itself supply the Purchaser with the relevant hydrophobic silica.
(e) for a period of [period], commencing on Closing, a commitment to provide all support (free of charge) which is necessary for the transfer of the production of Zeofoam ® 166 (the Zeofoam Transfer Support Commitment), including the following:
(i) technical support and training necessary for the Purchaser to start producing Zeofoam ® 166 in one of the Purchaser’s own production sites; and
(ii) support in the context of customer qualification proceedings for Zeoflo ® TL, Zeoflo ® MD and Zeofoam ® 166 which the Purchaser may need to undergo following the transfer of the production of Zeofoam, on the terms set out in the Schedule.
This obligation to provide support shall be carried out by a Production Transfer Team and shall apply until the point in time when the transfer of the production has been successfully achieved and at least the majority of the relevant customers (representing a substantial portion of the Divestment Business' demand) have qualified the Purchaser’s production.
(f) In the exercise of their various work tasks, the Production Transfer Team shall prioritise the support to be given to the Purchaser over their work for the retained businesses and make themselves available according to the requirements for a timely and effective implementation of the production pransfers. Evonik will establish an appropriate incentive scheme for the [employee(s)] concerned in order to have the transfer of the production completed in a timely and an effective manner. The Production Transfer Team will be bound by appropriate confidentiality obligations which will be agreed in accordance with paragraphs 13 and 14 of the Commitments. Should any member of the Production Transfer Team involved in the support to the Purchaser leave the company, Evonik will ensure that other competent employees will step in and complete the Production Transfer Team, after consulting with Monitoring Trustee and the Technical Expert.
(g) If there is any asset (with the exception of manufacturing assets) which is not covered by points a) to e) but which is both used (exclusively or not) in the Zeoflo/Zeofoam Business and necessary for the continued viability and competitiveness of the Zeoflo/Zeofoam Business, that asset or adequate substitute will be offered to potential purchasers.
C.General provisions in relation to the Divestment Business
8. Strict firewall procedures will be adopted so as to ensure that any competitively sensitive information relating to the Divestment Business and arising from the operation of the Sident Transitional Toll Manufacturing Agreement and the Zeofoam Transitional Toll Manufacturing Agreement will not be disclosed (whether within or outside Evonik’s operations) beyond what is reasonably required for the compliance with the obligations arising from this Sident Transitional Toll Manufacturing Agreement and the Zeofoam Transitional Toll Manufacturing Agreement.
9. The Divestment Business is further described in the Schedule, which forms an integral part of the Commitments.
Section C. Related commitments
Preservation of viability, marketability and competitiveness
10. From the Effective Date until Closing, Evonik shall preserve or procure the preservation of the economic viability, marketability and competitiveness of the Divestment Business, in accordance with good business practice, and shall minimise as far as possible any risk of loss of competitive potential of the Divestment Business. In particular Evonik undertakes:
(a) not to carry out any action that might have a significant adverse impact on the value, management or competitiveness of the Divestment Business or that might alter the nature and scope of activity, or the industrial or commercial strategy or the investment policy of the Divestment Business;
(b) to make available, or procure to make available, sufficient resources for the development of the Divestment Business, on the basis and continuation of the existing business plans;
(c) to take all reasonable steps, or procure that all reasonable steps are being taken, including appropriate incentive schemes (based on industry practice), to encourage all Key Personnel to remain in their current position. Where, nevertheless, individual members of the Key Personnel exceptionally leave their current position, Evonik shall provide a reasoned proposal to replace the person or persons concerned to the Commission and the Monitoring Trustee. Evonik must be able to demonstrate to the Commission that the replacement is well suited to carry out the functions exercised by those individual members of the Key Personnel. The replacement shall take place under the supervision of the Monitoring Trustee, who shall report to the Commission.
Hold-separate obligations
11. Evonik commits, from the Effective Date until Closing, and to the extent practically feasible, to keep the Divestment Business separate from the businesses it is retaining and to ensure that unless explicitly permitted under these Commitments: (i) management and staff of the businesses retained by Evonik have no involvement in the Divestment Business; (ii) the Key Personnel and Personnel of the Divestment Business have no involvement in any business retained by Evonik and do not report to any individual outside the Divestment Business.
12. Until Closing, Evonik shall assist the Monitoring Trustee in ensuring that the Divestment Business is managed as a distinct and saleable entity separate from the business(es) which Evonik is retaining. Immediately after the adoption of the Decision, Evonik shall appoint a Hold Separate Manager. The Hold Separate Manager, who shall be part of the Key Personnel, shall manage the Divestment Business independently and in the best interest of the business with a view to ensuring its continued economic viability, marketability and competitiveness and its independence from the businesses retained by Evonik. The Hold Separate Manager shall closely cooperate with and report to the Monitoring Trustee and, if applicable, the Divestiture Trustee. Any replacement of the Hold Separate Manager shall be subject to the procedure of these Commitments. The Commission may, after having heard Evonik, require Evonik to replace the Hold Separate Manager.
Ring-fencing
13. Evonik shall implement, or procure to implement, all necessary measures to ensure that it does not, after the Effective Date, obtain any Confidential Information relating to the Divestment Business and that any such Confidential Information obtained by Evonik before the Effective Date will be eliminated and not be used by Evonik. In particular, the participation of the Divestment Business in any central information technology network shall be severed to the extent possible, without compromising the viability of the Divestment Business. Evonik may obtain or keep information relating to the Divestment Business which is reasonably necessary for the divestiture of the Divestment Business or the disclosure of which to Evonik is required by law.
Non-solicitation clause
14. To the extent applicable, Evonik undertakes, subject to customary limitations, not to solicit, and to procure that Affiliated Undertakings do not solicit, the Key Personnel that may be transferred with the Divestment Business for a period of 2 years after Closing.
Due diligence
15. In order to enable potential purchasers to carry out a reasonable due diligence of the Divestment Business, Evonik shall, subject to customary confidentiality assurances and dependent on the stage of the divestiture process:
(a) provide to potential purchasers sufficient information as regards the Divestment Business;
(b) provide to potential purchasers sufficient information relating to the Personnel and allow them reasonable access to the Personnel.
Reporting
16. Evonik shall submit written reports in English on potential purchasers of the Divestment Business and developments in the negotiations with such potential purchasers to the Commission and the Monitoring Trustee no later than 10 days after the end of every month following the Effective Date (or otherwise at the Commission’s request). After one year, the frequency of the reports can be reduced to one report per year or per half year with the approval of the Commission. Evonik shall submit a list of all potential purchasers having expressed interest in acquiring the Divestment Business to the Commission at each and every stage of the divestiture process, as well as a copy of all the offers made by potential purchasers within five days of their receipt.
17. Evonik shall inform the Commission and the Monitoring Trustee on the preparation of the data room documentation and the due diligence procedure and shall submit a copy of any information memorandum to the Commission and the Monitoring Trustee before sending the memorandum out to potential purchasers.
Section D. The Purchaser
18. In order to be approved by the Commission, the Purchaser must fulfil the following criteria:
(a) The Purchaser shall be independent of and unconnected to Evonik and its Affiliated Undertakings (this being assessed having regard to the situation following the divestiture).
(b) The Purchaser shall have the financial resources, proven expertise and incentive to maintain and develop the Divestment Business as a viable and active competitive force in competition with the Parties and other competitors; to this effect it shall be an established producer of precipitated silica with an existing market presence in the EEA and production facilities located in the EEA.
(c) The Purchaser must at the latest by the point in time when the maximum duration of the Sident Transitional Toll Manufacturing Agreement expires have sufficient capacity, to transfer the Sident Business from Evonik’s Wesseling plant to one or more of its own production plants. In view of the maximum time period of [period] available to the Purchaser to transfer the production, the Purchaser must present a credible, feasible and committed business plan which sets out in which way the relevant capacity will be made available.
(d) In view of the maximum time period of [period] available to the Purchaser to transfer the Zeofoam/Zeoflo Business the Purchaser must present a credible, feasible and committed business plan which sets out in which way the Zeofoam/Zeoflo Business can be effectively operated and potentially transferred.
(e) The acquisition of the Divestment Business by the Purchaser must neither be likely to create, in light of the information available to the Commission, prima facie competition concerns nor give rise to a risk that the implementation of the Commitments will be delayed. In particular, the Purchaser must reasonably be expected to obtain all necessary approvals from the relevant regulatory authorities for the acquisition of the Divestment Business.
19. The final binding sale and purchase agreement (as well as ancillary agreements) relating to the divestment of the Divestment Business shall be conditional on the Commission’s approval. When Evonik has reached an agreement with a purchaser, it shall submit a fully documented and reasoned proposal, including a copy of the final agreement(s), within one week to the Commission and the Monitoring Trustee. Evonik must be able to demonstrate to the Commission that the purchaser fulfils the Purchaser Criteria and that the Divestment Business is being sold in a manner consistent with the Commission's Decision and the Commitments. For the approval, the Commission shall verify that the purchaser fulfils the Purchaser Criteria and that the Divestment Business is being sold in a manner consistent with the Commitments including their objective to bring about a lasting structural change in the market. The Commission may approve the sale of the Divestment Business without one or more Assets, or by substituting one or more Assets with one or more different assets, if this does not affect the viability and competitiveness of the Divestment Business after the sale, taking account of the proposed purchaser.
Section E. Trustee
I. Appointment procedure
20. Evonik shall appoint a Monitoring Trustee to carry out the functions specified in these Commitments for a Monitoring Trustee. Evonik commits not to close the Concentration before the appointment of a Monitoring Trustee.
21. If Evonik has not entered into a binding sale and purchase agreement regarding the Divestment Business one month before the end of the First Divestiture Period or if the Commission has rejected a purchaser proposed by Evonik at that time or thereafter, Evonik shall appoint a Divestiture Trustee. The appointment of the Divestiture Trustee shall take effect upon the commencement of the Trustee Divestiture Period.
22. The Trustee shall:
(i) at the time of appointment, be independent of Evonik and its Affiliated Undertakings;
(ii) possess the necessary qualifications to carry out its mandate, for example have sufficient relevant experience as an investment banker or consultant or auditor; and
(iii) neither have nor become exposed to a Conflict of Interest.
23. The Trustee shall be remunerated by Evonik in a way that does not impede the independent and effective fulfilment of its mandate. In particular, where the remuneration package of a Divestiture Trustee includes a success premium linked to the final sale value of the Divestment Business, such success premium may only be earned if the divestiture takes place within the Trustee Divestiture Period.
Proposal by Evonik
24. No later than two weeks after the Effective Date, Evonik shall submit the name or names of one or more natural or legal persons whom Evonik proposes to appoint as the Monitoring Trustee to the Commission for approval. No later than one month before the end of the First Divestiture Period or on request by the Commission, Evonik shall submit a list of one or more persons whom Evonik proposes to appoint as Divestiture Trustee to the Commission for approval. The proposal shall contain sufficient information for the Commission to verify that the person or persons proposed as Trustee fulfil the requirements set out in paragraph 22 and shall include:
(a) the full terms of the proposed mandate, which shall include all provisions necessary to enable the Trustee to fulfil its duties under these Commitments;
(b) the outline of a work plan which describes how the Trustee intends to carry out its assigned tasks;
(c) an indication whether the proposed Trustee is to act as both Monitoring Trustee and Divestiture Trustee or whether different trustees are proposed for the two functions.
Approval or rejection by the Commission
25. The Commission shall have the discretion to approve or reject the proposed Trustee(s) and to approve the proposed mandate subject to any modifications it deems necessary for the Trustee to fulfil its obligations. If only one name is approved, Evonik shall appoint or cause to be appointed the person or persons concerned as Trustee, in accordance with the mandate approved by the Commission. If more than one name is approved, Evonik shall be free to choose the Trustee to be appointed from among the names approved. The Trustee shall be appointed within one week of the Commission’s approval, in accordance with the mandate approved by the Commission.
New proposal by Evonik
26. If all the proposed Trustees are rejected, Evonik shall submit the names of at least two more natural or legal persons within one week of being informed of the rejection, in accordance with paragraphs 20 and 25 of these Commitments.
Trustee nominated by the Commission
27. If all further proposed Trustees are rejected by the Commission, the Commission shall nominate a Trustee, whom Evonik shall appoint, or cause to be appointed, in accordance with a trustee mandate approved by the Commission.
II. Functions of the Trustee
28. The Trustee shall assume its specified duties and obligations in order to ensure compliance with the Commitments. The Commission may, on its own initiative or at the request of the Trustee or Evonik, give any orders or instructions to the Trustee in order to ensure compliance with the conditions and obligations attached to the Decision.
Duties and obligations of the Monitoring Trustee
29. The Monitoring Trustee shall:
(i) propose in its first report to the Commission a detailed work plan describing how it intends to monitor compliance with the obligations and conditions attached to the Decision.
(ii) oversee, in close co-operation with the Hold Separate Manager, the on-going management of the Divestment Business with a view to ensuring its continued economic viability, marketability and competitiveness and monitor compliance by Evonik with the conditions and obligations attached to the Decision. To that end the Monitoring Trustee shall:
(a) monitor the preservation of the economic viability, marketability and competitiveness of the Divestment Business, and the keeping separate of the Divestment Business from the business retained by the Parties, in accordance with paragraphs 10 and 11 of these Commitments;
(b) supervise the management of the Divestment Business as a distinct and saleable entity, in accordance with paragraph 12 of these Commitments;
(c) with respect to Confidential Information:
- determine all necessary measures to ensure that Evonik does not after the Effective Date obtain any Confidential Information relating to the Divestment Business,
- in particular strive for the severing of the Divestment Business’ participation in a central information technology network to the extent possible, without compromising the viability of the Divestment Business,
- make sure that any Confidential Information relating to the Divestment Business obtained by Evonik before the Effective Date is eliminated and will not be used by Evonik and
- decide whether such information may be disclosed to or kept by Evonik as the disclosure is reasonably necessary to allow Evonik to carry out the divestiture or as the disclosure is required by law;
(d) monitor the splitting of assets and the allocation of Personnel between the Divestment Business and Evonik or Affiliated Undertakings;
(iii) propose to Evonik such measures as the Monitoring Trustee considers necessary to ensure Evonik’s compliance with the conditions and obligations attached to the Decision, in particular the maintenance of the full economic viability, marketability or competitiveness of the Divestment Business, the holding separate of the Divestment Business and the non-disclosure of competitively sensitive information;
(iv) review and assess potential purchasers as well as the progress of the divestiture process and verify that, dependent on the stage of the divestiture process:
(a) potential purchasers receive sufficient and correct information relating to the Divestment Business and the Personnel in particular by reviewing, if available, the data room documentation, the information memorandum and the due diligence process, and
(b) potential purchasers are granted reasonable access to the Personnel;
(v) act as a contact point for any requests by third parties, in particular potential purchasers, in relation to the Commitments;
(vi) provide to the Commission, sending Evonik a non-confidential copy at the same time, a written report within 15 days after the end of every month that shall cover the operation and management of the Divestment Business as well as the splitting of assets and the allocation of Personnel so that the Commission can assess whether the business is held in a manner consistent with the Commitments and the progress of the divestiture process as well as potential purchasers;
(vii) promptly report in writing to the Commission, sending Evonik a non-confidential copy at the same time, if it concludes on reasonable grounds that Evonik is failing to comply with these Commitments;
(viii) within one week after receipt of the documented proposal referred to in paragraph 19 of these Commitments, submit to the Commission, sending Evonik a non- confidential copy at the same time, a reasoned opinion as to the suitability and independence of the proposed purchaser and the viability of the Divestment Business after the Sale and as to whether the Divestment Business is sold in a manner consistent with the conditions and obligations attached to the Decision, in particular, if relevant, whether the Sale of the Divestment Business without one or more Assets or not all of the Personnel affects the viability of the Divestment Business after the sale, taking account of the proposed purchaser;
(ix) assume the other functions assigned to the Monitoring Trustee under the conditions and obligations attached to the Decision.
30. If the Monitoring and Divestiture Trustee are not the same [legal or natural] persons, the Monitoring Trustee and the Divestiture Trustee shall cooperate closely with each other during and for the purpose of the preparation of the Trustee Divestiture Period in order to facilitate each other's tasks.
Duties and obligations of the Divestiture Trustee
31. Within the Trustee Divestiture Period, the Divestiture Trustee shall sell at no minimum price the Divestment Business to a purchaser, provided that the Commission has approved both the purchaser and the final binding sale and purchase agreement (and ancillary agreements) as in line with the Commission's Decision and the Commitments in accordance with paragraphs 17 and 19 of these Commitments. The Divestiture Trustee shall include in the sale and purchase agreement (as well as in any ancillary agreements) such terms and conditions as it considers appropriate for an expedient sale in the Trustee Divestiture Period. In particular, the Divestiture Trustee may include in the sale and purchase agreement such customary representations and warranties and indemnities as are reasonably required to effect the sale. The Divestiture Trustee shall protect the legitimate financial interests of Evonik, subject to Evonik unconditional obligation to divest at no minimum price in the Trustee Divestiture Period.
32. In the Trustee Divestiture Period (or otherwise at the Commission’s request), the Divestiture Trustee shall provide the Commission with a comprehensive monthly report written in English on the progress of the divestiture process. Such reports shall be submitted within 15 days after the end of every month with a simultaneous copy to the Monitoring Trustee and a non-confidential copy to Evonik.
III. Duties and obligations of Evonik
33. Evonik shall provide and shall cause its advisors to provide the Trustee with all such co- operation, assistance and information as the Trustee may reasonably require to perform its tasks. The Trustee shall have full and complete access to any of Evonik’s or the Divestment Business’ books, records, documents, management or other personnel, facilities, sites and technical information necessary for fulfilling its duties under the Commitments and Evonik and the Divestment Business shall provide the Trustee upon request with copies of any document. Evonik and the Divestment Business shall make available to the Trustee one or more offices on their premises and shall be available for meetings in order to provide the Trustee with all information necessary for the performance of its tasks.
34. Evonik shall provide the Monitoring Trustee with all managerial and administrative support that it may reasonably request on behalf of the management of the Divestment Business. This shall include all administrative support functions relating to the Divestment Business which are currently carried out at headquarters level. Evonik shall provide and shall cause its advisors to provide the Monitoring Trustee, on request, with the information submitted to potential purchasers, in particular give the Monitoring Trustee access to the data room documentation and all other information granted to potential purchasers in the due diligence procedure. Evonik shall inform the Monitoring Trustee on possible purchasers, submit lists of potential purchasers at each stage of the selection process, including the offers made by potential purchasers at those stages, and keep the Monitoring Trustee informed of all developments in the divestiture process.
35. Evonik shall grant or procure Affiliated Undertakings to grant comprehensive powers of attorney, duly executed, to the Divestiture Trustee to effect the sale (including ancillary agreements), the Closing and all actions and declarations which the Divestiture Trustee considers necessary or appropriate to achieve the sale and the Closing, including the appointment of advisors to assist with the sale process. Upon request of the Divestiture Trustee, Evonik shall cause the documents required for effecting the sale and the Closing to be duly executed.
36. Evonik shall indemnify the Trustee and its employees and agents (each an “Indemnified Party”) and hold each Indemnified Party harmless against, and hereby agrees that an Indemnified Party shall have no liability to Evonik for, any liabilities arising out of the performance of the Trustee’s duties under the Commitments, except to the extent that such liabilities result from the wilful default, recklessness, gross negligence or bad faith of the Trustee, its employees, agents or advisors.
37. At the expense of Evonik, the Trustee may appoint advisors (in particular for corporate finance or legal advice), subject to Evonik’s approval (this approval not to be unreasonably withheld or delayed) if the Trustee considers the appointment of such advisors necessary or appropriate for the performance of its duties and obligations under the Mandate, provided that any fees and other expenses incurred by the Trustee are reasonable. Should Evonik refuse to approve the advisors proposed by the Trustee the Commission may approve the appointment of such advisors instead, after having heard Evonik. Only the Trustee shall be entitled to issue instructions to the advisors. Paragraph 36 of these Commitments shall apply mutatis mutandis. In the Trustee Divestiture Period, the Divestiture Trustee may use advisors who served Evonik during the Divestiture Period if the Divestiture Trustee considers this in the best interest of an expedient sale.
38. Evonik agrees that the Commission may share Confidential Information proprietary to Evonik with the Trustee. The Trustee shall not disclose such information and the principles contained in Article 17 (1) and (2) of the Merger Regulation apply mutatis mutandis.
39. Evonik agrees that the contact details of the Monitoring Trustee are published on the website of the Commission's Directorate-General for Competition and they shall inform interested third parties, in particular any potential purchasers, of the identity and the tasks of the Monitoring Trustee.
40. For a period of 10 years from the Effective Date the Commission may request all information from the Parties that is reasonably necessary to monitor the effective implementation of these Commitments.
IV. Replacement, discharge and reappointment of the Trustee
41. If the Trustee ceases to perform its functions under the Commitments or for any other good cause, including the exposure of the Trustee to a Conflict of Interest:
(a) the Commission may, after hearing the Trustee and Evonik, require Evonik to replace the Trustee; or
(b) Evonik may, with the prior approval of the Commission, replace the Trustee.
42. If the Trustee is removed according to paragraph 41 of these Commitments, the Trustee may be required to continue in its function until a new Trustee is in place to whom the Trustee has effected a full hand over of all relevant information. The new Trustee shall be appointed in accordance with the procedure referred to in paragraphs 20-27 of these Commitments.
43. Unless removed according to paragraph 41 of these Commitments, the Trustee shall cease to act as Trustee only after the Commission has discharged it from its duties after all the Commitments with which the Trustee has been entrusted have been implemented. However, the Commission may at any time require the reappointment of the Monitoring Trustee if it subsequently appears that the relevant remedies might not have been fully and properly implemented.
Section F. The review clause
44. The Commission may extend the time periods foreseen in the Commitments in response to a request from Evonik or, in appropriate cases, on its own initiative. Where Evonik requests an extension of a time period, it shall submit a reasoned request to the Commission no later than one month before the expiry of that period, showing good cause. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time send a non-confidential copy of the report to Evonik. Only in exceptional circumstances shall Evonik be entitled to request an extension within the last month of any period.
45. The Commission may further, in response to a reasoned request from Evonik showing good cause waive, modify or substitute, in exceptional circumstances, one or more of the undertakings in these Commitments. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time send a non-confidential copy of the report to Evonik. The request shall not have the effect of suspending the application of the undertaking and, in particular, of suspending the expiry of any time period in which the undertaking has to be complied with.
Section G. Entry into force
46. The Commitments shall take effect upon the date of adoption of the Decision.
………………………………… …………………………
Name: […] Name: […]
Title: Head of M&A Title: Legal Counsel M&A
duly authorised for and on behalf of duly authorised for and on behalf of
Evonik Industries AG Evonik Industries AG
SCHEDULE
A. Divestment commitment
1. The Divestment Business consists of :
a) Evonik’s entire business relating to precipitated silica for dental applications in EMEA and including SIDENT ® 8, SIDENT ® 9, SIDENT ® 10 and SIDENT ® 22 S grades, but excluding any production assets (the Sident Business);
b) Huber Silica’s entire business relating to precipitated silica for defoamer applications and hydrophobic precipitated silica for sale to defoamer and non- defoamer customers in the EEA and including Zeoflo ® TL, Zeoflo ® MD and Zeofoam ® 166 grades, but excluding any production assets (the Zeoflo/Zeofoam Business).
The Sident Business
Customers
2. Table 1 below lists all customers of Evonik’s Sident ® precipitated silica grades to be divested as part of the Commitments given by Evonik in relation to the Concentration.
Toll Manufacturing Agreement
3. The Sident Business will be divested together with a short-term toll manufacturing agreement (the Sident Transitional Toll Manufacturing Agreement). Under the Sident Toll Manufacturing Agreement Evonik will supply to the Purchaser SIDENT ® 8, SIDENT ® 9, SIDENT ® 10 and SIDENT ® 22 S in sufficient volumes, for a maximum duration of [period] from Closing, extendable by [period] subject to approval of the Monitoring Trustee (together with a technical expert which the Monitoring Trustee may appoint) to allow the Purchaser to supply the transferred customers with these precipitated silica grades.
4. In accordance with the Sident Toll Manufacturing Agreement Evonik will in any given year make available product volumes equal to (Y), […] of the previous year's (Y-1) sales in metric tons of SIDENT ® 8, SIDENT ® 9, SIDENT ® 10 and SIDENT ® 22 S sold within the scope of the Divestment Business to the divested customers in metric tons. The total capacity to be made available to the Purchaser in any given year shall not exceed […].
Transitional Support
5. Evonik commits to provide all necessary support to the Purchaser to ensure the transfer of the Sident Business. This includes technical support and training necessary for the transfer of the Sident Business to one of the Purchaser’s own production site as well as support regarding the customer qualification process in relation to the Sident Business.
Licences and Government permits
6. Evonik will transfer to the Purchaser all licences, permits and authorisations issued by any governmental organisation for the benefit of the Sident Business.
Intellectual Property Rights
7. Subject to the more detailed principles set out in para. 6 of the Commitments, Evonik will transfer to the Purchaser intellectual property rights, know-how, product specifications, manufacturing recipes and technical documentation that is exclusively, primarily or also used for the production of SIDENT ® 8, SIDENT ® 9, SIDENT ® 10 and SIDENT ® 22 S at a production site in the EEA.
8. Subject to the more detailed principles set out in para. 6 of the Commitments, Evonik also commits to transfer to the Purchaser all intellectual property rights required for the continued viability of the Sident Business.
Personnel
9. Evonik will provide at the option of the Purchaser,
· the transfer of the [number of Evonik employees ] (as listed in the Annex) who […] currently active in […]. During a period of [period] from Closing, Evonik will allow the Purchaser to have access to and make an employment offer to [Evonik employee(s)]. Evonik will take all reasonable steps, or procure that all reasonable steps are being taken, including appropriate incentive schemes (based on industry practice), to encourage [Evonik employee(s)], if […] received an employment offer from the Purchaser to transfer to the Purchaser, subject to applicable employment legislation.
· the (part or full-time) secondment to the Purchaser of [Evonik employee(s)] currently responsible for […] and being [(a) member(s)] of the Production Transfer Team, namely for a period of up to [period].
· the Purchaser can identify key functions for the Sident Business. If, following input from Evonik, the Monitoring Trustee deems that these functions correspond to Key Personnel, Evonik will identify a pool of candidates for each of these functions, in consultation with the Monitoring Trustee. Evonik will allow the purchaser to have access to and make an employment offer to these candidates. The Parties will take all reasonable steps, or procure that all reasonable steps are being taken, including appropriate incentive schemes (based on industry practice), to encourage the candidates who have been identified and who have received an employment offer from the purchaser to transfer to the purchaser, subject to applicable employment legislation.
10. Evonik will nominate a Production Transfer Team and will ensure that this Production Transfer Team will prioritise the support to be given to the Purchaser in relation to transitioning the Sident Business to the Purchaser’s own production site and will create appropriate incentive schemes to ensure that the transfer of the production is completed in an effective manner.
11. At the option of the Purchaser, Evonik will offer the transfer of the [number of Evonik employees] who […]currently active in […]. During a period of [period] from Closing, Evonik will allow the Purchaser to have access to and make an employment offer to [Evonik employee(s)] . Evonik will take all reasonable steps, or procure that all reasonable steps are being taken, including appropriate incentive schemes (based on industry practice), to encourage [Evonik employee(s)] , if […] received an employment offer from the Purchaser to transfer to the Purchaser, subject to applicable employment legislation. Evonik will also offer a (part or full-time) secondment to the Purchaser of [Evonik employee(s)] currently responsible for […] and being [(a) member(s)] of the Production Transfer Team for a period of up to [period].
Tangible Assets
12. The Divestment Business does not include any tangible assets relating to the Sident Business.
Any other assets
13. If there is any asset (with the exception of manufacturing assets) which is not covered by points a) to e) but which is both used (exclusively or not) in the Sident Business and necessary for the continued viability and competitiveness of the Sident Business, that asset or adequate substitute will be offered to potential purchasers.
The Zeoflo/Zeofoam Business
Customers
14. Table 2 below lists all customers of Huber Silica’s Zeoflo ® and Zeofoam ® precipitated silica grades to be divested as part of the Commitments given by Evonik in relation to the Concentration.
Toll Manufacturing Agreement
15. The Zeoflo/Zeofoam Business will be divested together with a toll manufacturing agreement (the Zeofoam Transitional Toll Manufacturing Agreement). Under the Zeofoam Toll Manufacturing Agreement Evonik will supply to the Purchaser Zeofoam ® 166 in sufficient volumes, for a duration of [period], to allow the Purchaser to supply the transferred customers with the precipitated silica grade.
16. In accordance with the Zeofoam Toll Manufacturing Agreement in any given year Evonik will make available product volumes being equal to (Y), […] of the previous year's (Y-1) sales in metric tons of Zeoflo ® TL, Zeoflo ® MD and Zeofoam ® 166 to the divested customers, provided that the total capacity made available at any given year shall not exceed […] of the sales of the Zeoflo/Zeofoam Business in 2016.
Transfer of AMS toll manufacturing agreement
17. Evonik will transfer to the Purchaser the existing toll-manufacturing arrangements with Applied Material Solutions for the hydrophobisation of hydrophilic precipitated silica marketed by Huber Silica under the Zeoflo ® TL and the Zeoflo ® MD trademarks.
Transitional Support
18. For a period of [period] Evonik commits to provide all necessary support to the Purchaser to ensure the transfer of the Zeoflo/Zeofoam Business. This includes technical support and training necessary for the transfer of the Zeoflo/Zeofoam Business to one of the Purchaser’s own production sites as well as support regarding the customer qualification process in relation to the Zeoflo/Zeofoam Business.
Licences and Government permits
19. Evonik will transfer to the Purchaser all licences, permits and authorisations issued by any governmental organisation for the benefit of the Zeoflo/Zeofoam Business.
Intellectual Property Rights
20. Evonik will transfer to the Purchaser the Zeoflo ® TL, Zeoflo ® MD and Zeofoam ® 166 trademarks for use in the EEA. For the avoidance of doubt, there will be no limitation to the right of the customers to sell the Transformed Products worldwide.
21. All other intellectual property rights, know-how, product specifications, manufacturing recipes and technical documentation required for the continued viability and competitiveness of the Zeoflo/Zeofoam Business owned by Huber, including in particular, formulation know-how, current and historic customer files of customers purchasing for their EEA requirements and current and historic product information relating to Zeoflo ® TL, Zeoflo ® MD and Zeofoam ® 166, will be licenced by Huber to the Purchaser under an irrevocable, royalty-free and non-exclusive licence for production in the EEA, which shall in relation to Zeofoam ® 166 (but not with respect to Zeoflo ® TL and Zeoflo ® MD) be limited to defoamer applications. Evonik commits not to grant the Zeoflo/Zeofoam Licence to any third party (excluding Evonik subsidiaries) other than the Purchaser. For the avoidance of doubt, this latter commitment shall not prevent Evonik from entering into a toll-manufacturing agreement with third parties for the purpose of hydrophobisation of precipitated silica.
Personnel
22. Evonik will nominate a Production Transfer Team and ensure that this Production Transfer Team will prioritise the support to be given to the Purchaser in relation to transitioning the Zeoflo/Zeofoam Business to the Purchaser’s own production site. In addition Evonik will create appropriate incentive schemes to ensure that the transfer of the production is completed in an effective manner.
Tangible Assets
23. The Divestment Business does not include any tangible assets relating to the Zeoflo/Zeofoam Business.
Any other Assets
24. If there is any asset (with the exception of manufacturing assets) which is not covered by points 7 a) to e) but which is both used (exclusively or not) in the Zeoflo/Zeofoam Business and necessary for the continued viability and competitiveness of the Zeoflo/Zeofoam Business, that asset or adequate substitute will be offered to potential purchasers.
1 OJ L 24, 29.1.2004, p. 1 (the 'Merger Regulation'). With effect from 1 December 2009, the Treaty on the Functioning of the European Union ('TFEU') has introduced certain changes, such as the replacement of 'Community' by 'Union' and 'common market' by 'internal market'. The terminology of the TFEU will be used throughout this decision.
2 OJ L 1, 3.1.1994, p. 3 (the 'EEA Agreement').
3 Capacity of neutralizing an acid.
4 Case M.4927 CARLYLE/INEOS/JV, paragraph 15 and Case M.6230 Solvay/Rhodia, paragraph 69.
5 Case CARYLE/INEOS/JV, paragraph 15.
6 Non-confidential reply to question 104 of the questionnaire Q1 to competitors.
7 Case M.4927 - CARYLE/INEOS/JV, paragraphs 35 to 37 and Case M.6230 - Solvay/Rhodia, paragraph 70.
8 Replies to question 99 of the questionnaire Q1 to competitors.
9 Replies to question 103 of the questionnaire Q1 to competitors.
10 Case M.4927 - CARYLE/INEOS/JV, paragraph 21.
11 Formation of a solid product (the precipitate) inside of a liquid solution in which a chemical reaction occurs. Precipitated silica are produced by the reaction of sulphuric acid with sodium silicate.
12 The main parameters are such as temperature, time, solid content, addition of calcium chloride or aluminium sulfate.
13 A measure of acidity or alkalinity (the capability to neutralize acidity) of water soluble substances (pH stands for 'potential of Hydrogen').
14 Cases M.4927 - CARYLE/INEOS/JV, paragraphs 21 and 22 and M.6230 - Solvay/Rhodia, paragraph 73.
15 With this respect, it should be noted that the Notifying Party however also recognises that the combination of Huber Silica and Evonik will enable the Merged Entity to [achieve efficiency gains by re-allocating production of certain grades].
16 Replies to question 8.1 of the questionnaire Q2 to customers.
17 Non-confidential reply to questions 31.1 of questionnaire Q1 to competitors.
18 Non-confidential reply to questions 31.1 of questionnaire Q1 to competitors.
19 Non-confidential reply to questions 31.1 of questionnaire Q1 to competitors.
20 Non-confidential reply to questions 32.1 of questionnaire Q1 to competitors.
21 Huber's webpage dedicated to hydrophobic precipitated silica (https://www.hubermaterials.com/hydrophobic-silica.aspx)
22 Huber's webpage dedicated to hydrophobic precipitated silica (https://www.hubermaterials.com/hydrophobic-silica.aspx)
23 Non-confidential reply to question 68.1 of the questionnaire Q1 to competitors.
24 Non-confidential reply to question 72.1 of the questionnaire Q1 to competitors.
25 Replies to question 10 of the questionnaire Q2 to customers.
26 Replies to question 13 of the questionnaire Q2 to customers.
27 A customer's reply to question 13 of the questionnaire Q2 to customers.
28 A customer's reply to question 13 of the questionnaire Q2 to customers.
29 Replies to question 17 of the questionnaire Q2 to customers.
30 Replies to question 16 of the questionnaire Q1 to competitors.
31 Replies to question 17 of the questionnaire Q1 to competitors.
32 Replies to question 30 of the questionnaire Q1 to competitors.
33 A customer's reply to question 13 of the questionnaire Q2 to customers.
34 A competitor's reply to question 22.1 of the questionnaire Q1 to competitors.
35 Replies to question 28 of the questionnaire Q1 to competitors.
36 The Notifying Party's reply to question 3.2 of the RFI of 20 February 2017.
37 Replies to question 25 of the questionnaire Q2 to customers.
38 Replies to question 26 of the questionnaire Q2 to customers.
39 Replies to question 29 of the questionnaire Q1 to competitors.
40 Annex 1 to the Form CO.
41 Technical Information 1313: "SIPERNAT ® speciality silica and AEROSIL ® fumed silica for defoamer", Evonik company publication
42 Non-confidential reply to question 22.1 of questionnaire Q1 to competitors.
43 Non-confidential reply to question 66 of questionnaire Q1 to competitors.
44 Annex 17 of the Form CO.
45 For comparison, average prices of precipitated silica per end-use application in 2016 are: [2.5-4]€/kg for matting agents, [1.4-1.9]€/kg for food applications, [1.3-1.8]€/kg for paints & coatings and agriculture applications, [1-1.5]€/kg for dental applications, [0.7-1.3]€/kg for rubber, feed and battery separators and [0.6-0.9]€/kg for paper applications.
46 https://www hubermaterials.com/hydrophobic-silica.aspx.
47 Technical Information 1313: "SIPERNAT ® speciality silica and AEROSIL ® fumed silica for defoamer", Evonik company publication.
48 https://www hubermaterials.com/products/silica-and-silicates/defoamers.aspx
49 On its webpage Huber's Zeofoam ® 166 is described as being "the first product to choose for in-situ surface treatment to make it hydrophobic" and the Zeofree ® 80 (not commercialized in the EEA) can "easily [be] made hydrophobic" (https://www hubermaterials.com/products/silica-and- silicates/defoamers.aspx).
50 Technical Information 1313: "SIPERNAT ® speciality silica and AEROSIL ® fumed silica for defoamer", Evonik company publication.
51 The flow properties of the product.
52 Replies to question 52 of the questionnaire Q1 to competitors.
53 Replies to question 53 of the questionnaire Q1 to competitors.
54 Evonik's internal presentation titled "Oral Care Strategy for Silica", [date].
55 Evonik's internal presentation titled "Oral Care Strategy for Silica", [date].
56 A competitor's reply to question 22.1 of the questionnaire Q1 to competitors.
57 Good Manufacturing Practices.
58 Replies to question 50 of the questionnaire Q1 to competitors.
59 Evonik's internal presentation titled "Oral Care Strategy for Silica", [date], p. 12.
60 Evonik's internal presentation titled "Oral Care Strategy for Silica", [date], p. 12.
61 Such as water-based inkjet inks (digital printing technology).
62 The hypothetical market for hydrophobic precipitated silica for food applications is not affected in the context of the present case.
63 M.6230 - Solvay/Rhodia, paragraph 75.
64 Replies to question 21 of the questionnaire Q2 to customers.
65 A customer's reply to question 21 of the questionnaire Q2 to customers.
66 Replies to question 11 of the questionnaire Q1 to competitors.
67 A competitor's reply to question 11 of the questionnaire Q1 to competitors.
68 A competitor's reply to question 11 of the questionnaire Q1 to competitors.
69 Replies to question 10 of the questionnaire Q1 to competitors.
70 Replies to question 10 of the questionnaire Q1 to competitors.
71 M.942 Veba/Degussa, European Commission decision of 3 December 1997 (paragraphs 25-27).
72 M.942 Veba/Degussa, European Commission decision of 3 December 1997 (paragraphs 36).
73 M.942 Veba/Degussa, European Commission decision of 3 December 1997 (paragraphs 30 and 36).
74 Guidelines on the assessment of horizontal mergers under the Council Regulation on the control of concentrations between undertakings ("Horizontal Merger Guidelines"), OJ C 31, 05.02.2004.
75 The competitive assessment would not change if, in addition to standard precipitated silica, aluminium and calcium silicate were to be taken into account, in view of the fact that they only account for a very small portion of a potential overall market including the three types of precipitated silica.
76 A significant number of smaller producers (less than [0-5]% market shares) have been agglomerated within the category 'Others'. These include Asian competitors such as Taiwan-based manufacturer OSC, Indian-based producer Madhu, as well as several other Chinese manufacturers (such as Zhuzhou Xinglon, Fujian Zhengchang, Tong Sheng, Wuxi Hengcheng).
77 Provided as Annexes 10 and 11 to the Form CO.
78 Market share figures have been adapted by the Parties' own and best market estimates to reflect their competitors' (including Asian competitors') market shares in the EEA.
79 Huber Silica closed this plant in Uddevalla in 2009.
80 Parties used an average exchange rate of 1.11$/€ for 2016 in the form CO.
81 Replies to question 23 of questionnaire Q2 for customers.
82 Replies to question 34 of questionnaire Q2 for customers.
83 A customer's reply to question 34 of questionnaire Q2 for customers.
84 Replies to question 41 of the questionnaire Q1 for competitors.
85 Replies to question 41.1 of the questionnaire Q1 for competitors.
86 Replies to question 41.2 of the questionnaire Q1 for competitors.
87 Exactly as for the total estimated volume sold in 2016.
88 As provided by the Parties in Annex 17 "Market Shares and Sales Shares per Application" of the Form CO.
89 For confidentiality reasons, market shares of competitors have been redacted as per the Guidance on the preparation of public versions of Commission Decisions adopted under the Merger Regulation available at http://ec.europa.eu/competition/mergers/legislation/guidance on preparation of public versions mergers 26052015.pdf.
90 Evonik's internal presentation titled "Oral Care Strategy for Silica", [date], p. 6.
91 Replies to question 31 of questionnaire Q2 for customers.
92 A customer's reply to question 31 of the questionnaire Q2 for customers.
93 A customer's reply to question 31 of the questionnaire Q2 for customers.
94 A customer's reply to question 31 of the questionnaire Q2 for customers.
95 Replies to Question 25 of Questionnaire Q2 – Customers.
96 Replies to Question 26 of Questionnaire Q2 – Customers.
97 Replies to Question 29 of Questionnaire Q2 – Customers.
98 A customer's reply to Question 29 of Questionnaire Q2 – Customers.
99 A customer's reply to Question 29 of Questionnaire Q2 – Customers.
100 A competitor's reply to question 48 of the questionnaire Q1 to competitors.
101 A competitor's reply to question 48 of the questionnaire Q1 to competitors.
102 Evonik's internal presentation titled "Oral Care Strategy for Silica", 6 March 2015, p. 12.
103 Minutes of a call with a customer in May 2017.
104 Evonik's internal presentation titled "Oral Care Strategy for Silica", [date], p. 12 and p. 14.
105 Evonik's internal presentation titled "Oral Care Strategy for Silica", [date], p. 14.
106 Evonik's internal presentation titled "Oral Care Strategy for Silica", [date], p. 8.
107 Evonik's internal presentation titled "Oral Care Strategy for Silica", [date], p. 8.
108 Evonik's internal presentation titled "Oral Care Strategy for Silica", [date], p. 6.
109 Evonik's internal presentation titled "Oral Care Strategy for Silica", [date], p. 14.
110 Form CO, paragraph 174.
111 Evonik's internal presentation titled "Oral Care Strategy for Silica", [date], p. 29.
112 Evonik's internal presentation titled "Oral Care Strategy for Silica", [date], p. 29.
113 Evonik's internal presentation titled "Oral Care Strategy for Silica", [date], p. 30.
114 Evonik's internal presentation titled "Oral Care Strategy for Silica", [date], p. 14.
115 Evonik's internal presentation titled "Oral Care Strategy for Silica", [date], p. 16.
116 Evonik's reply to question 12 of the RFI of 22 March 2017.
117 Minutes of a call with [company] of 12 May 2017.
118 Minutes of a call with [company] of 12 May 2017.
119 Replies to Question 23 of Questionnaire Q2 – Customers.
120 See paragraph 37 of the Horizontal Merger Guidelines.
121 Replies to Question 52 of Questionnaire Q2 – Customers.
122 Replies to Question 52 of Questionnaire Q2 – Customers.
123 Replies to Question 55 of Questionnaire Q2 – Customers.
124 A customer's reply to Question 55 of Questionnaire Q2 - Customers
125 A customer's reply to Question 55 of Questionnaire Q2 - Customers
126 The Parties lists several other competitors (PQ, Grace, IQE, Madhu, Wuxi Quenchen, OSC, Zhouzhou Xinglon, Fujian Zhengchang, Wuxi Hengchen and Tong Sheng) but attribute a market share of less than [0-5]% to each.
127 Given the ranges provided for Solvay and PPG and the large size of the Other category, a range is provided for the post-merger HHI. The lower limit is obtained for lower market shares levels of 5% per competitor (revenue) and 10% (volume); while the upper limit is obtained for upper market share levels of 10% (revenue) and 15% (volume).
128 Huber Silica's webpage (https://www.hubrmaterials.com/products/silica-and- silicates/defoamers.aspx) has a dedicated section for defoamers. Similarly, Evonik's wepage dedicated to the SIPERNAT ® product grades (http://www.sipernat.com/product/sipernat/en/services/downloads/defoamers/Pages/default.aspx) also mention applications for defoamers.
129 Solvay's webpage (http://www rhodia.com/en/markets and products/product finder/index.tcm) does not return any results in its search engine for the association of keywords "defoamer" (or "foam") and "precipitated silica" (or "silica").
130 PPG's webpage dedicated to precipitated silica (http://www.ppgsilica.com/Home.aspx) does not return any results in its search engine for the keyword "defoamer" (or "foam").
131 Non-confidential reply to question 22.1 of questionnaire Q1 to competitors.
132 Non-confidential reply to question 51.1 of questionnaire Q2 to customers.
133 Replies to question 30 of questionnaire Q2 to customers.
134 "Guidelines on the assessment of horizontal mergers under the Council Regulation on the control of concentrations between undertakings", OJ C 31, 5.2.2004 at p. 5.
135 Paragraph 20 of the Horizontal Merger Guidelines.
136 Non-confidential reply to question 63.1 of questionnaire Q1 to competitors.
137 Non-confidential reply to question 63.1 of questionnaire Q1 to competitors.
138 Estimated market share ranges for competitors reflect the competitor's actual market share both in value and volume.
139 "Guidelines on the assessment of horizontal mergers under the Council Regulation on the control of concentrations between undertakings", OJ C 31, 5.2.2004 at p. 5.
140 Paragraph 20 of the Horizontal Merger Guidelines.
141 Estimated market share ranges for competitors reflect the competitor's actual market shares both in value and volume.
142 "Guidelines on the assessment of horizontal mergers under the Council Regulation on the control of concentrations between undertakings", OJ C 31, 5.2.2004 at p. 5.
143 Paragraph 20 of the Horizontal Merger Guidelines.
144 Estimated market share ranges for competitors reflect the competitor's actual market shares both in value and volume.
145 "Guidelines on the assessment of horizontal mergers under the Council Regulation on the control of concentrations between undertakings", OJ C 31, 5.2.2004 at p. 5.
146 Paragraph 20 of the Horizontal Merger Guidelines.
147 Post-merger HHI has been estimated based on the assumption that the 'Others' category is split in competitors of identical size to Huber Silica ([0-5]%).
148 https://www hubermaterials.com/products/silica-and-silicates/defoamers.aspx
149 Replies to Question 75 of Questionnaire Q1 – Competitors
150 Replies to Question 79 of Questionnaire Q1 - Competitors
151 A customer's reply to Question 37 of Questionnaire Q2 – Customers.
152 Replies to Question 37 of Questionnaire Q2 – Customers.
153 Minutes of a call with a competitor of 3 April 2017.
154 Estimated market shares for competitors are based on volume.
155 "Guidelines on the assessment of horizontal mergers under the Council Regulation on the control of concentrations between undertakings", OJ C 31, 5.2.2004 at p. 5.
156 Paragraph 20 of the Horizontal Merger Guidelines.
157 “Guidelines on the assessment of non-horizontal mergers under the Council Regulation on the control of concentrations between undertakings”, OJ C 265, 18.10.2008, at p. 6 (‘Non-Horizontal Guidelines’), paragraph 4.
158 Non-Horizontal Guidelines, paragraphs 29-30.
159 Non-Horizontal Guidelines, paragraphs 29–30.
160 Non-Horizontal Guidelines, paragraph 31.
161 Non-Horizontal Guidelines, paragraph 58.
162 Non-Horizontal Guidelines, paragraphs 32 and 59.
163 Case M.4927 CARLYLE/INEOS/JV, paragraph 43.
164 A reply to Question 111 of questionnaire Q1 to competitors
165 A reply to Question 111.1 of questionnaire Q1 to competitors
166 A reply to Question 111.1 of questionnaire Q1 to competitors
167 A reply to Question 114 of questionnaire Q1 to competitors
168 Non-Horizontal Guidelines, paragraph 5.
169 Non-Horizontal Guidelines, paragraph 92.
170 Non-Horizontal Guidelines, paragraphs 91 and 93.
171 Non-Horizontal Guidelines, paragraphs 95 to 104.
172 Non-Horizontal Guidelines, paragraphs 105 to 110.
173 Non-Horizontal Guidelines, paragraphs 111 to 118.
174 A reply to Question 86.1 of questionnaire Q1 to competitors.
175 A reply to Question 42 of questionnaire Q2 to customers.
176 A reply to Question 94.1 of questionnaire Q1 to competitors
177 A reply to Question 50 of questionnaire Q2 to customers
178 A reply to Question 48 of questionnaire Q2 to customers
179 A reply to Question 86.1 of questionnaire Q1 to competitors
180 A reply to Question 44 of questionnaire Q1 to customers
181 Commission Notice on remedies acceptable under Council Regulation (EC) No 139/2004 and under Commission Regulation (EC) No 802/2004 (OJ C 267, 22.10.2008, p. 1-27.
182 Remedies Notice, paragraphs 9 and 61.
183 Remedies Notice, paragraph 9.
184 Remedies Notice, paragraph 15.
185 Remedies Notice, paragraphs 58-59.
186 Remedies Notice, paragraph 17.
187 Remedies Notice, paragraph 16.
188 A reply to question 2.1 of Questionnaire R2 – Customers
189 A reply to question 2.1 of Questionnaire R2 – Customers
190 A reply to question 32.1 of Questionnaire R2 – Customers
191 Replies to questions 30 and 61 of Questionnaire R1 - Competitors
192 A reply to Question 8.1 of Questionnaire R1 – Competitors
193 A reply to Question 22.1 of Questionnaire R1 – Competitors
194 A reply to Question 8.1 of Questionnaire R1 – Competitors
195 A reply to Question 22.1 of Questionnaire R1 – Competitors
196 Replies to Question 5.1 of Questionnaire R2 – Customers
197 Replies to Question 5.1 of Questionnaire R2 - Customers
198 Replies to Question 1.1 of Questionnaire R2 - Customers
199 Replies to Question 1.1 of Questionnaire R2 - Customers
200 Replies to Question 6.1 of Questionnaire R1 – Competitors
201 Replies to Questions 12.1 and 29.1 of Questionnaire R1 – Competitors
202 Replies to Question 9.1 of Questionnaire R1 – Competitors
203 A reply to Question 9.1 of Questionnaire R1 – Competitors
204 Replies to Question 9.1 and 10 of Questionnaire R1 – Competitors
205 Replies to Question 12 of Questionnaire R2 – Customers
206 206 Replies to Question 1.1 of Questionnaire R2 - Customers 207 Replies to Question 2.1 of Questionnaire R1 – Competitors 208 A reply to Question 19.1 of Questionnaire R2 - Customers
209 A reply to Question 19.1 of Questionnaire R2 - Customers
210 Replies to Question 6.1 and 7.1 of Questionnaire R1 – Competitors
211 A reply to Question 17.1 of Questionnaire R1 – Competitors
212 Replies to Question 38.1 and 39.1 of Questionnaire R1 – Competitors
213 A reply to Question 38.1 of Questionnaire R1 – Competitors
214 A reply to Question 38.1 of Questionnaire R1 – Competitors
215 A reply to Question 38.1 of Questionnaire R1 – Competitors
216 Replies to Question 50 of Questionnaire R2 - Customers
217 A Reply to Question 50.1 of Questionnaire R2 - Customers
218 A reply to Question 38.1 of Questionnaire R1 – Competitors
219 Replies to Question 38.1 and 40.1 of Questionnaire R1 – Competitors
220 A reply to Question 38.1 of Questionnaire R1 – Competitors
221 A reply to Question 40.1 of Questionnaire R1 – Competitors
222 Please note, that this list contains additional customers compared to the list of dental customers provided in response to the Commission’s request for information of 27 February 2017. This is because this list not only includes direct customers but also distributors sourcing a variety of precipitated silica grades including SIPERNAT ®.
223 Please note that this list contains additional customers compared to the list of defoamer customers provided in response to the Commission’s request for information of 27 February 2017. This is because this list not only includes direct customers but also distributors sourcing a variety of precipitated silica grades including Zeoflo ® and Zeofoam ®. In addition, please note that […] have been omitted from this list as they did not generate turnover in relation to precipitated silica for defoamer applications in 2016. Information previously provided to the Commission in this regard was historic.