Commission, November 9, 2016, No M.7917
EUROPEAN COMMISSION
Decision
BOEHRINGER INGELHEIM / SANOFI ANIMAL HEALTH BUSINESS
Subject: Case No COMP M.7917 – BOEHRINGER INGELHEIM/ SANOFI ANIMAL HEALTH BUSINESS
Commission decision pursuant to Article 6(1)(b) in conjunction with Article 6(2) of Council Regulation No 139/20041 and Article 57 of the Agreement on the European Economic Area2
Dear Sir/Madam,
(1) On 19 September 2016, the European Commission received notification of a proposed concentration pursuant to Article 4 of the Merger Regulation by which Boehringer Ingelheim group (BI, Germany) acquires within the meaning of Article 3(1)(b) of the Merger Regulation control over Sanofi's animal health business (Merial, France), by way of purchase of shares and assets (the Transaction).3 BI and Merial are designated hereinafter as the 'Parties' and BI the 'Notifying Party'. The same concentration was initially notified to the Commission on 8 June 2016, however the notification was subsequently withdrawn on 22 July 2016.
I. THE PARTIES
(2) BI is a pharmaceutical company active in the development, production, distribution, and marketing of pharmaceuticals, in four business segments: prescription products, consumer healthcare products, biopharmaceuticals and animal health products.
(3) Merial is Sanofi's subsidiary specialised in animal health. Merial produces a wide range of pharmaceutical products and vaccines for companion and production animals.
II.THE OPERATION AND CONCENTRATION
(4) Pursuant to the agreement for the sale and purchase of Sanofi's animal health business (SAPA), BI intends to acquire control over Merial, by way of acquisition of shares (including 100% of Merial SAS shares) and assets.
(5) The operation is part of an asset swap whereby Merial would be transferred to BI in exchange for BI's consumer healthcare business (BI CHC). The proposed acquisition by Sanofi of BI CHC constitutes a separate concentration for the purposes of the EC Merger Regulation.4 An additional cash payment from BI to Sanofi will take place in order to bridge the value gap between the two swapped businesses.
(6) As a result of the Transaction, BI will have sole control over and ownership of Merial.
(7) The Transaction therefore constitutes a concentration within the meaning of Article 3(1)(b) of the Merger Regulation.
III.EU DIMENSION
(8) The undertakings concerned have a combined aggregate world-wide turnover of more than EUR 5 000 million5. Each of them has an EU-wide turnover in excess of EUR 250 million, but each does not achieve more than two-thirds of its aggregate EU-wide turnover within one and the same Member State.
(9) The notified operation therefore has an EU dimension pursuant to Article 1(2) of the Merger Regulation.
IV. ASSESSMENT
(10) In line with previous Commission's decisions,6 animal health products can generally be divided into three main areas:(i) Biologicals: products which trigger an immune response against viral and bacterial diseases as well as occasionally parasitic or fungal infections in animals. Biologicals include in particular animal vaccines.(ii) Pharmaceuticals: wide group of products that contain a variety of active substances to prevent or treat a large range of animal diseases and disorders.(iii) Feed supplements (medicinal and nutritional): pharmaceutical or nutritional substances which are not natural feedstuffs and are added to made-up and stored feeds for various purposes but chiefly to control infectious disease or to promote growth.
(11) The Parties' activities overlap in all three areas: animal health biologicals (vaccines) (IV.2), pharmaceuticals (IV.3) and feed supplements (IV.3.4).7
IV.1. Introduction - General features of animal health industry
IV.1.1. Animal health sector globally and in the EEA
(12) BI and Merial are among the largest companies active in animal health globally. Post- Transaction, the merged entity will rank number 2 in terms of net sales with a share of the global animal health business of approximately [10-20]%, after Zoetis.
[Graph on Global Animal Health Landscape in 2014, from BI internal document]
(13) The global animal health sector is concentrated with 70% of the business controlled by six global pharmaceutical companies, including the Parties as well as Zoetis (until recently the animal health division of Pfizer), Merck, Elanco (animal health division of Eli Lilly) and Bayer (focusing on animal health pharmaceuticals).
(14) In the EEA, the largest global players, including the Parties, Zoetis, Bayer, Elanco and Merck (known as MSD in Europe), are all active, together with smaller international players, such as Ceva Santé Animale (Ceva), Hipra, Vetoquinol and Virbac.
(15) Animal health companies expand their portfolio through organic growth, with the development of new products or improvements of existing products (also known as life cycle management), or inorganic growth. Recently, Elanco bought Novartis' animal health division,8 after having acquired certain animal health assets from Pfizer9 and Janssen Animal health10 in 2011. In 2013, Ceva acquired Sogeval and more recently, in 2015, Zoetis acquired the animal health division of Abbott.
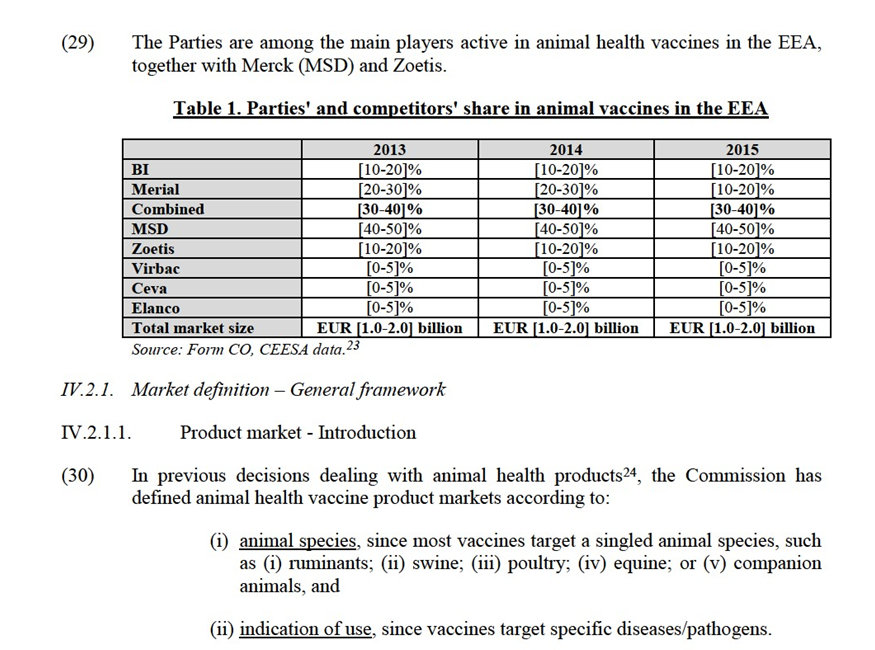
(16) The main barriers to entry in animal health markets are development costs and intellectual property rights associated with new products. In the area of swine and ruminant vaccines, BI, MSD and Zoetis are perceived as the strongest innovators.11 BI's R&D budget in vaccines has been growing over the last three years from EUR […] million in 2013 to EUR […] million in 2015, while Merial's vaccines R&D budget ranged between EUR […] million between 2013 and 2015.
(17) As to the expansion of existing products supplied in a limited number of EEA countries in new geographies, animal health suppliers need to obtain a marketing authorization (as described below), set-up a distribution and sales network and engage marketing costs. Once the distribution and sales networks are in place, the main investment in time and costs to commercialise additional products in this country generally consists in obtaining the regulatory approval.12
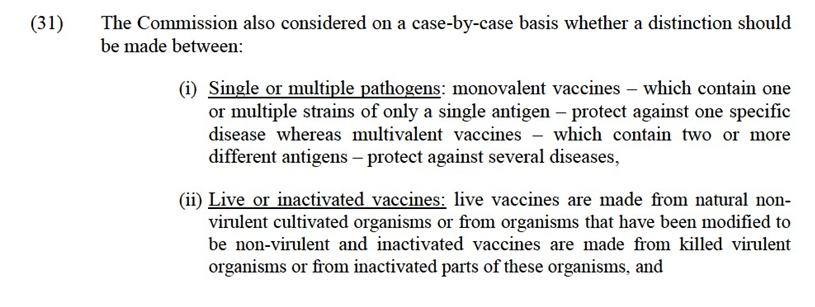
IV.1.2. Regulation of veterinary medicines in the EEA
(18) Like the human health sector, the animal health industry is regulated by both Member States and at the European Union level. More specifically, the manufacture and commercialisation of veterinary medicinal products (VMP) is subject to marketing authorizations.13
(19) However, contrary to the human health sector, VMPs are generally not reimbursed by public authorities except for in specific situations, such as (i) in the context of eradication schemes: by way of example, the German region of Hessen currently subsidies bovine viral diarrhoea (BVD) vaccines, or (ii) in the context of specific subsidies to farmers for some pharmaceuticals which vary by Member States. In some countries, for instance in Scandinavia and the United Kingdom, there is a possibility of private insurance in particular for pets whereby insured pet owners may claim reimbursement from their insurance companies subject to individual policies.
(20) As a consequence, prices of animal health products are generally not regulated and are freely set by manufacturers. The price of animal health products is thus function of competition in the market.
IV.1.3. Generics and brand importance
(21) In the animal health sector, competition essentially takes place between brands of various producers, to which customers attribute specific degree of efficacy, safety and price level based on the experience with the product and the manufacturer.14
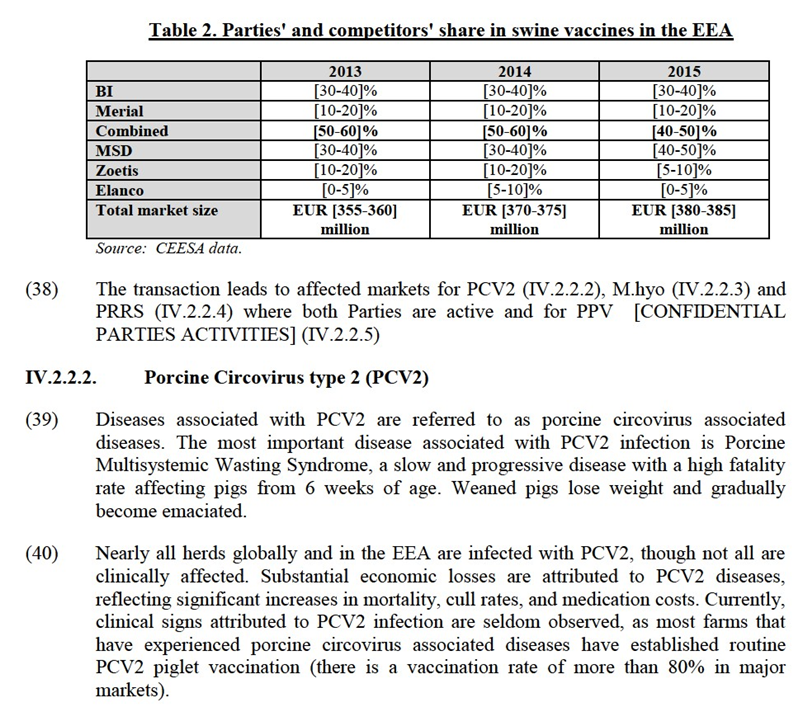
(22) As to the penetration of generic medicines, there are no generics of animal vaccines, as vaccines are biological products which do not exhibit bioequivalence. On the other hand, while animal pharmaceuticals do know generics, generic penetration is still rather limited as generally there is no regulatory incitation to introduce generics as is observed for human pharma.15 In addition, generic companies must demonstrate that(i) the product is a generic version of the reference VMP with respect to its composition (that it has qualitative and quantitative bioequivalence by demonstrating the equivalence of the rate and extent of drug absorption) and pharmaceutical formulation and that (ii) the generic drug is bioequivalent to the originator product (generic companies are only exempted to provide safety and efficacy documentation).The market investigation in this case confirmed the reluctance from some customers to use generics instead of originator products which are generally perceived as more efficacious. In this context, some customers mentioned that generics have different formulation and in some cases are only around 80% equivalent to originators and therefore not a perfect copy.16
(23) The low penetration of generics is also evidenced by high margins in animal vaccines and pharmaceuticals often reaching 70-80%.
IV.1.4.Customer base and purchasing patterns
(24) The animal health products customer base is split between two main categories, namely veterinarians (independent or attached to a farm or group of farms) and directly the farmers, in particular for production animals.
(25) The negotiation on prices as well as the choice of brands are generally made by veterinarians,17 which are the target audience of manufacturers' marketing.18 Farmers can also influence the decision, in particular the large farms and cooperatives.19 Price of specific products depends in particular on volume and the range of products purchased.20
(26) The market investigation indicated that customers typically multi-source in particular for vaccines where they generally have 2 to 4 vaccines suppliers for each specific disease.21 Veterinaries explain that multi-sourcing is necessary to negotiate prices and for security of supply. The choice of the vaccine will ultimately depend on its suitability for each farm.22
(27) The features of animal health industry described above will be reflected in the competitive assessment of the Transaction in the specific markets.
IV.2.Animal health vaccines
(28) Vaccines protect animals against future diseases or illnesses caused by exposure to bacterial, viral, parasitical or fungal agents (pathogens). Vaccines achieve this protection by introducing one or several antigens (harmless substances that stimulate an immune system response) into the animal’s body, in order to stimulate the production of antibodies (natural substances used by the animal’s immune system to protect against the relevant pathogen) or another protective immune response.
(iii)Marker or non-marker vaccines: marker vaccines allow distinguishing between animals that are immunised as a result of vaccination or as a result of exposure to a naturally occurring pathogenic strain of the virus.25
(32) The Commission further identified additional differentiating factors between vaccines, such as (i) animal target group within species (e.g. for swine, vaccines may be targeted at sows and/or piglets), (ii) the route of administration such as intramuscular or subcutaneous and (iii) the frequency of administration or number of doses.26
IV.2.1.2. Geographic market
(33) In previous decisions,27 the Commission found that despite the existence of some pan- European trends and the fact that the main players are active throughout the EEA, the relevant geographic market for animal health products was national in scope. This is mainly due to national legislation determining the selling conditions of the products, different prevalence of certain diseases in certain areas, and different competition landscape in different EEA countries in terms of market penetration, shares, price, distribution systems and local veterinarian preferences.
(34) The Notifying Party submits that the relevant geographic market definition in animal health products is indeed national. The Notifying Party points out the fact that most products on these markets remain subject to national and mutual recognition registration systems, causing products to be sold according to indications and uses prescribed by national registration and approval requirements.
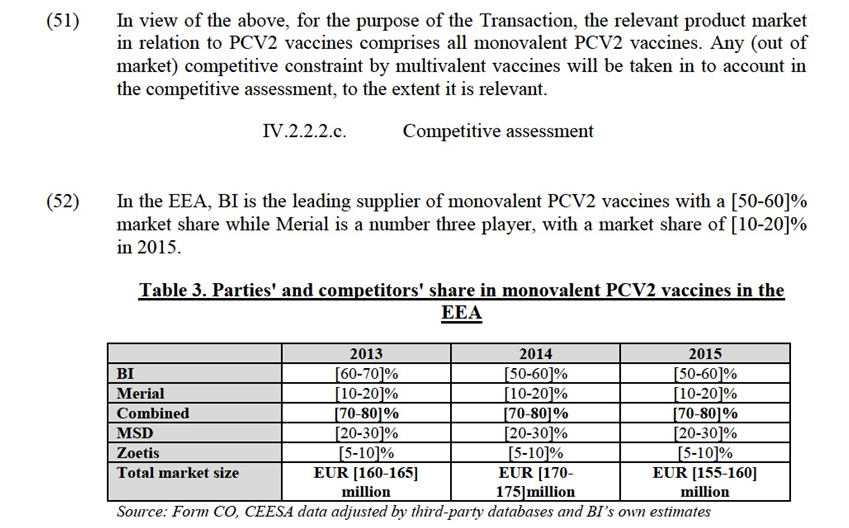
(35) In this case, the market investigation broadly confirmed that markets in the animal health sector are still national, as marketing authorizations are still subject to national regulations, the competitive landscapes varies from one Member State to another while pricing strategies of pharmaceutical companies also seem to be national.
(36) For the purpose of assessing the impact of the Transaction, the Commission therefore concludes that the relevant geographic markets in relation to animal health vaccines are national in scope.
IV.2.2. Swine vaccines
IV.2.2.1. Introduction
(37) At EEA level, the Parties are among the largest players in swine vaccines, together controlling around half of the market. The market has experienced strong growth over the last few years due to, among other things, the growing prevalence of some swine diseases and continuous innovation in the sector creating new demand.
IV.2.2.2.a. Parties' products
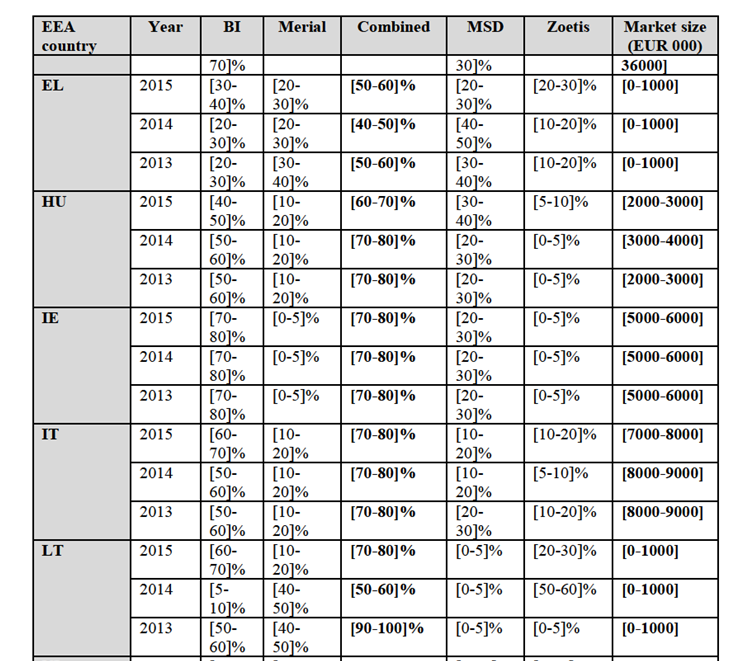
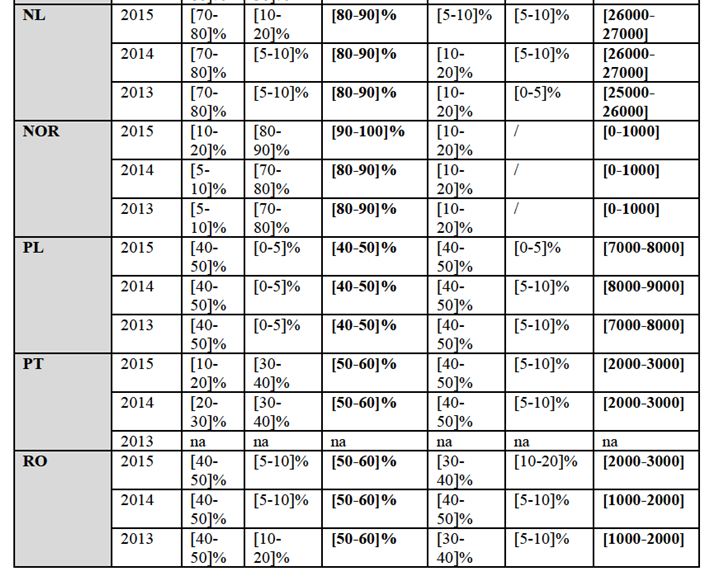
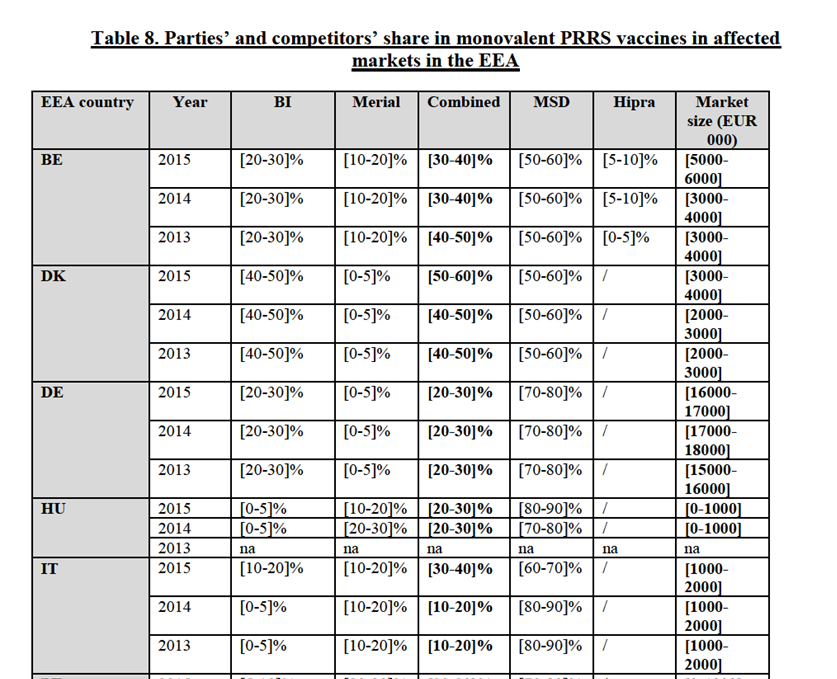
(41) The Parties offer only monovalent PCV2 vaccines.
(42) Merial markets a monovalent vaccine under the brand name Circovac, which was the first vaccine against circovirus to be approved in the EEA in 2007. Circovac is an inactivated vaccine. Circovac was initially authorized to be used for gilts and sows but obtained a marketing authorization for a use in piglets in 2010. The average profit margin at EEA level of Circovac is […]%.
(43) BI markets a monovalent vaccine under the brand Ingelvac CircoFLEX (CircoFLEX). CircoFLEX is a subunit28 vaccine. CircoFLEX was initially authorized to be used in piglets from 2 weeks of age but subsequently gained an authorization for sows and for all piglets. Since 2015, CircoFLEX can also be used during pregnancy and during lactation in sows. The average profit margin at EEA level of Circoflex is […]%.
(44) A combination of BI's PCV2 vaccine CircoFLEX and BI's M.Hyo vaccine MycoFLEX, for a mixing on site, is also authorized under the name FLEXCombo.
IV.2.2.2.b. Product market definition
Notifying Party's view
(45) The Notifying Party submits that the narrowest relevant product market is the market for monovalent PCV2 vaccines for swine.
(46) The Notifying Party however submits that multivalent swine vaccines which include PCV2, in particular the combo vaccines including PCV2 and MHyo, exert a competitive constraint on monovalent products since the vast majority of swine farmers would vaccinate against both diseases in the EEA.
(47) In addition, the Notifying Party submits that it is not necessary to distinguish between inactivated vaccines and subunit vaccines. Subunit vaccines include only the antigens that best stimulate the immune system; in a subunit vaccine only the most immunogenic protein of PCV2 (the capsid protein) is produced and used. A subunit- based vaccine is per definition a killed vaccine, but as a result of the production method it does not require additional inactivation. The Notifying Party submits that from a customer perspective, these concepts do not yield any meaningful differentiation.
The Commission's assessment
(48) As to the segmentation between monovalent and multivalent vaccines, the market investigation provided indications that, if monovalent PCV2 vaccines (used in combination with monovalent vaccines against other disease(s)) may, in some circumstances, be substitutable to multivalent vaccines including PCV2, the reverse is not true.
(49) The market investigation indicated that multivalent vaccines including PCV2 (and in particular PCV2/M.Hyo) can in some cases be preferred to administration of two





(57) In the area of PCV2 vaccines, BI is a clear market leader in the EEA and across the majority of EEA countries, with a value based market share of up to [90-100]% in Slovenia. While Merial's Circovac is generally a smaller player (in most EEA countries behind BI’s CircoFLEX and MSD’s Porcilis PCV), it still holds a substantial market share in many EEA countries, reaching up to [80-90]% in Norway.
(58) The market investigation generally confirmed BI's clear leading position. Many customers and competitors indicated that BI is dominating the market.34 BI's own internal documents qualify CircoFLEX as the leading and "gold standard"35 brand. In one internal document, BI states that "CircoFLEX is by far the global market leader ([60-70]% of market share). This is primarily based on the strong brand image [CONFIDENTIAL INFORMATION ON BI PRICES]".36
(59) As concerns Merial’s position, the market investigation indicated that Merial’s product would be less efficacious which is reflected in its generally lower market shares37 and more targeted at sows than piglets38. However, the market investigation also revealed a specific positioning of Circovac being priced at the lower end thus providing an interesting “value for money” proposition especially for large farms,.39 By way of example, one veterinarian indicated that "Merial's product is a good price product which is important, approximately […]% cheaper than the others, and used by big farms to reduce their costs"40 while another mentions that the "lowest price per dose for pig is Merial's vaccine."41 This is also confirmed by internal documents of BI [BI INTERNAL ANALYSIS OF MERIAL'S COMMERCIAL STRATEGY].42 [BI INTERNAL ANALYSIS OF MERIAL'S COMMERCIAL STRATEGY]43 [BI INTERNAL ANALYSIS OF MERIAL'S COMMERCIAL STRATEGY].
(60) As to other competitors active in the market, Zoetis' Suvaxyn CV product is generally perceived as less safe and efficacious.44 Customers did not comment on Zoetis' multivalent offering Suvaxyn Circo+MH RTU since it is not launched yet in the EEA.
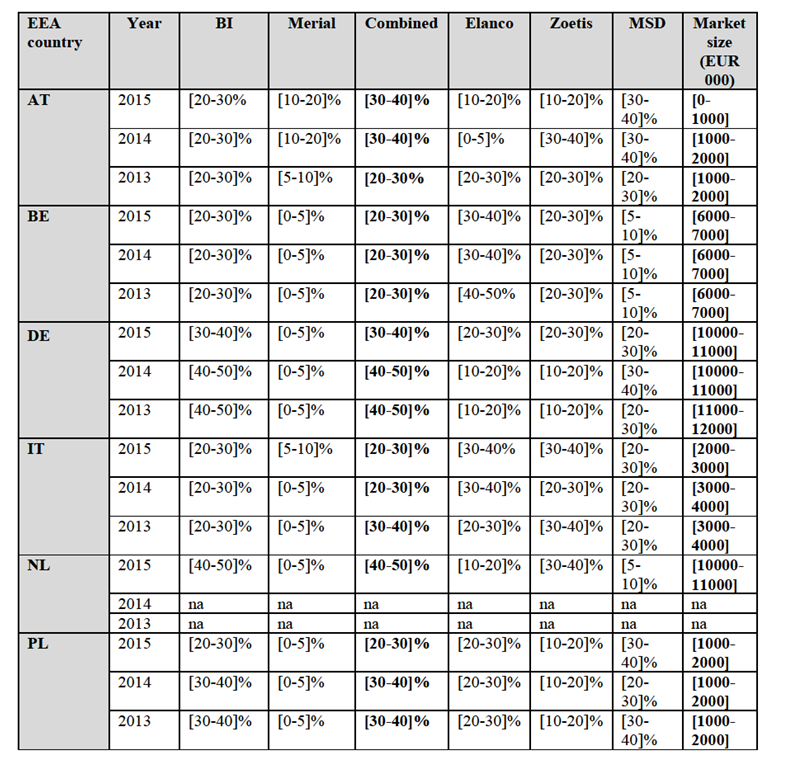
(61) As to the Parties' argument that MSD multivalent product Porcilis PCVM exert a competitive constraint on the Parties' monovalent PCV2 vaccines, in addition to the fact that Porcilis' PCVM is generally not substitutable to the Parties' monovalent PCV2 vaccines as explained above, even if its sales were all to be included in the market, the market shares of the Parties and of MSD would not substantially differ, the combined entity still leading by far the market in the 23 EEA affected countries in 2015 with more than [50-60]% of market shares in 16 EEA countries.45 This is because in general sales of multivalent vaccines are significantly less than sales of monovalent vaccines.
(62) Finally, some market participants identified a risk of price increase and reduced choice of products post-Transaction for PCV2 vaccines across EEA countries.46 One customer indicated that "the price [will] climb; [since] circovac [is] on cheap [side]" while others indicated that "the risk is that BI will suppress the products of Merial, and deprive the market of an alternative"47 and another one that the operation will have an impact on availability and choice as there is a "possibility that Circoflex will be withdrawn" and on price because "market share close to 90% for BI and Merial could have impact on prices".48
(63) As a result, the Transaction will eliminate actual competition for PCV2 vaccines in all 23 EEA countries where both Parties are active, which represent almost [90-100]% of each Party's EEA turnover, but also potential competition in other EEA countries where the two Parties are natural entrants.
(64) In view of the above, the Transaction raises serious doubts as to its compatibility with internal market in relation to monovalent PCV2 vaccines in the EEA in general and in Austria, Belgium, Bulgaria, Cyprus, Czech Republic, Denmark, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Lithuania, Netherlands, Norway, Poland, Portugal, Romania, Slovenia, Spain, Sweden and the UK in particular.
IV.2.2.3.Mycoplasma hyopneumoniae (M.hyo)
(65) M.Hyo is the primary etiological agent of enzootic pneumonia and a leading cause of respiratory disease throughout the swine industry. The typical clinical sign is a non- productive dry cough. Though mortality associated with the disease is typically low, significant losses are caused by reduced weight gain, increase feed conversion ratio and increased medication costs.
IV.2.2.3.a.Parties' products
(66) The Parties both only offer M.Hyo monovalent vaccines.
(67) BI sells its monovalent M.Hyo vaccines for swine under the brands IngelvacMycoFLEX (MycoFLEX) and Ingelvac M.Hyo. The main difference between the two products is that MycoFLEX enables mixing with BI’s PCV2 vaccine CircoFLEX. BI is currently phasing out Ingelvac M.Hyo. MycoFLEX is indicated for active immunization of pigs from three weeks of age or older to reduce lung lesions following the M.Hyo infection.
(68) Merial's monovalent vaccine is marketed under the brand Hyoresp. It is used for active immunization of suckling piglets from five weeks of age to reduce injection and lung lesions caused by M.Hyo.
IV.2.2.3.b. Market definition
(69) In its previous decisions,49 the Commission defined a market for monovalent mycoplasma (M.Hyo) vaccines for swine. The Commission further indicated that the distinction between live and inactivated is not relevant, given the fact that the products exist in an inactivated form only.
Notifying Party's views
(70) The Notifying Party submits that the narrowest relevant product market is the market for monovalent M.Hyo vaccines for swine, however multivalent swine vaccines which include M.Hyo, in particular the combo vaccines PCV2 and MHyo, exert a competitive constraint on monovalent products since the vast majority of swine farmers would vaccinate against both diseases in the EEA.
Commission's assessment
(71) As to the segmentation between monovalent M.Hyo vaccines and multivalent PCV2 and M.Hyo vaccines, in line with the developments in the section on PCV2, the market investigation indicated that multivalent vaccines address a specific customer demand and are thus likely to be part of a different product market.
(72) In view of the above, for the purpose of assessing this Transaction, the relevant product market in relation to MHyo vaccines comprises all monovalent MHyo vaccines. Any (out of market) competitive constraint by multivalent vaccines will be taken in to account in the competitive assessment, to the extent it is relevant.
IV.2.2.3.c. Competitive assessment
(73) In the EEA, BI is among the top 3 companies active in M.Hyo vaccines while Merial’s presence is negligible at the EEA level and several strong competitors are active.

Source: Form CO, CEESA data, CEESA data adjusted by third-party databases, BI's own estimates and Merial's actual sales, GfK data, Vetindex data50
Notifying Party's views
(75) The Notifying Party submits that the proposed acquisition of Merial's Hyoresp product will not lead to any notable reinforcement of BI's existing market position in relation to monovalent M.hyo vaccines since Merial's increment is practically non-existent (below [0-5]%) and the combined market share of the Parties, in particular at the EEA level, is not particularly high (around [20-30]%).
(76) Moreover, the Notifying Party submits that the market for monovalent M.Hyo vaccines will remain very competitive post-Transaction since:(i) At least three significant suppliers, namely Elanco, MSD and Zoetis, will remain on the market, and they represent strong competitors gaining market shares over the last years. Two additional smaller suppliers, Fatro and Hipra, are also present in some EEA countries markets and should quickly expand their geographic footprint.(ii) Ceva entered the EEA market in the third quarter of 2015. The Notifying Party expects Ceva to exert significant competitive constraint in the future.
Commission's assessment
(77) For M.Hyo vaccines, BI holds significant market shares in the EEA and across EEA countries, with up to [40-50]% in Netherlands. Merial's position is however limited, with a market share up to a maximum of [10-20]% in Austria and generally below [5- 10]%.
(78) The market investigation confirmed Merial's limited presence in monovalent M.Hyo vaccines across EEA countries.51 By way of example, one competitor indicated that "Hyoresp is a small and not significant Mhyo vaccine in the EEA market place", while another stressed that "after 20 years on the market, its product is at the end of its lifecycle and barely competitive."52
(79) The market investigation also indicated that the merged entity will continue to face strong competition from the remaining players, such as Elanco, Zoetis and MSD in all overlapping EEA countries. One market participant mentioned for instance that, for M.Hyo, "[there is] no defined leader. Similar sales [are generated by] Boehringer (Ingelvac Mycoflex), Elanco (Stellamune), Merck (Porcilis Mhyo), Zoetis (Suvaxyn Mhyo/Respisure)".53
(80) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction does not raise serious doubts as to its compatibility with the internal market with respect to the market for monovalent MHyo vaccines.
IV.2.2.4.Porcine reproductive and respiratory syndrome (PRRS)
(81) PRRS is a highly variable ribonucleic acid (RNA) virus causing both respiratory and reproductive patterns. Infected animals run a high temperature, develop severe respiratory disease and succumb to other illness, grow poorly and some may even die. Infected sows produce significantly fewer piglets and more stillborn pigs, mummified foetuses and weak piglets. The disease is grouped under Type 1 and Type 2, which were originally respectively restricted to Europe (Type 1) and North America (Type 2). Currently, both types are spread globally, although Type 1 is still highly predominantly present in Europe while Type 2 is prevalent in North America.
IV.2.2.4.a.Parties' products
(82) Both Parties supply only monovalent PRRS vaccines.
(83) BI's original PRRS vaccine is Ingelvac PRRS MVL, a modified-live vaccine based on Type 2 virus. BI subsequently obtained marketing authorizations for the commercialisation of two new products, Ingelvac PRRS FLEXEU (PRRS FLEXEU) and ReproCyc PRRS EU which are both modified-live vaccines targeting Type 1 virus. While the marketing authorization covers 24 EEA countries the products were launched since October 2015 in 10 EEA countries. PRRS FLEXEU is used for pigs, while ReproCyc PRRSEU is used for breeding gilts and sows and can be used at all stages of the reproductive cycle. In 2015, BI discontinued the sale of its killed PRRS vaccine, Inglevac PRRS KL, which was the same as Merial's product (see below) and was manufactured by Merial under a contract manufacturing agreement.
(84) Merial is currently active in the PRRS market only with its killed vaccine Progressis, which is a Type 1 vaccine specifically designed for sows and gilts to reduce reproductive disorders caused by PRRS. [CONFIDENTIAL INFORMATION ON THE PARTIES' ACTIVITIES]54 [CONFIDENTIAL INFORMATION ON THE PARTIES' ACTIVITIES].
IV.2.2.4.b. Market definition
Notifying Party's views
(85) The Notifying Party submits that monovalent PRRS vaccines for swine constitute a distinct product market.
(86) The Notifying Party however submits that the product characteristics and usage of PRRS vaccines can be differentiated between inactivated/killed (KV) and modified live (MLV) vaccines as well as by Type 1 and Type 2 vaccines. Killed PRRS vaccines are mainly used in sows and offer a high safety profile but arguably lower efficacy than MLVs. The Notifying Party submits that these factors should be taken into consideration in the competitive assessment of the Transaction.
Commission's assessment
(87) The market investigation broadly confirmed that the relevant product market should be defined as monovalent PRRS vaccines. Neither the Notifying Party nor market participants identified any competing multivalent vaccines in the EEA.
(88) The market investigation also confirmed that the type of vaccines (Type 1 or Type 2) and whether the vaccine is modified-live or killed are differentiating factors to be taken into consideration in the competitive assessment when assessing closeness of competition of available products. In this context, the market investigation indicated that:
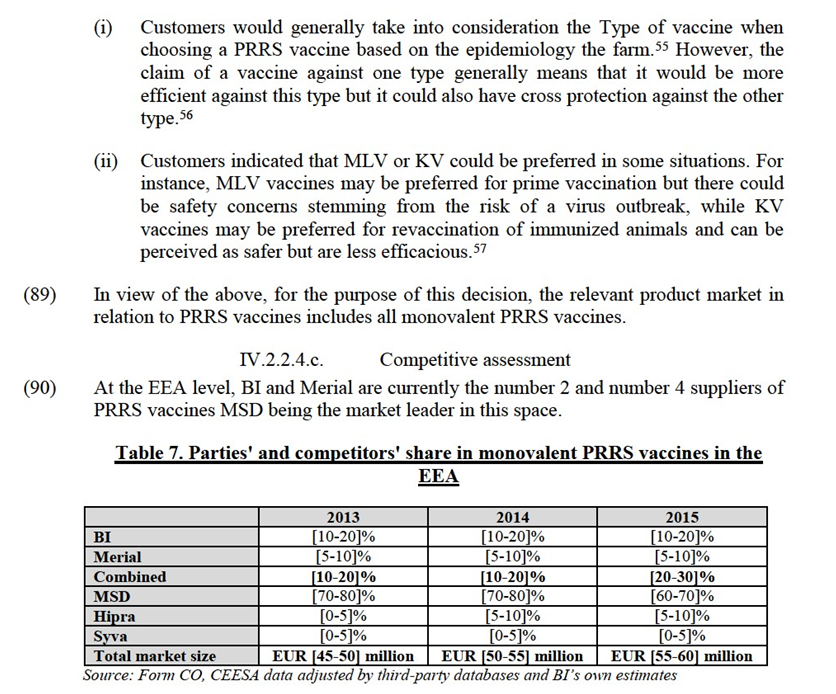


actually (all vaccines, type 1 and 2 plus KV) are around [20-30]%."60 Moreover, this is in line with BI’s own ambition set out in an internal document, whereby its objective is to [CONFIDENTIAL INFORMATION ON BI'S COMMERCIAL STRATEGY].61
(94) More importantly, the current market structure is generally contestable since the PRRS disease is not well controlled yet and there is a strong competition to innovate in this market which is set to grow. Many competitors indicated during the market investigation that PRRS vaccine is a major area of innovation: "PRRS is probably the biggest disease concern for the pig industry in the EEA. Vaccines have significant limitations in relation with efficacy and safety".62 The PRRS market has been growing over the last three years from EUR 45 million in 2013 to EUR 58 million in 2015. According to BI's own estimates, the market size would increase up to EUR [70-80] million in 2024.63
(95) In this context, while BI just launched two innovative products at the end of last year, [CONFIDENTIAL INFORMATION ON THE PARTIES' ACTIVITIES]64 [CONFIDENTIAL INFORMATION ON THE PARTIES' ACTIVITIES]65 [CONFIDENTIAL INFORMATION ON THE PARTIES' ACTIVITIES]66.
(96) As to competitors' products, MSD's product Porcilis which is the oldest modified live type 1 on the market and the current market leader is losing market share for the benefit of BI and possibly Hipra. This could be due to efficacy and safety issues, one veterinarian indicating that "the good attenuation [of MSD Porcilis] means that the vaccine is very sensitive to vaccination errors and vaccine storage conditions. I have studied several cases where the vaccine failed to induce significant immunity."67 As to Hipra's product Unistrain, which was introduced in the market in 2013, it seems its penetration remains limited in comparison to other modified-live vaccines. This is also confirmed in BI's internal documents indicating that Hipra's product has [BI INTERNAL ANALYSIS OF HIPRA'S MARKET POSITION].68 This could be explained by Hipra not having a large portfolio of swine vaccines and thus having a marketing disadvantage and more limited access to customers. Indeed, BI INTERNAL ANALYSIS OF HIPRA'S MARKET POSITION].69 In this context the market investigation confirmed the importance of having a portfolio of swine vaccines to be successful, swine veterinarians mentioned that discounts are often based on the range of swine vaccines purchased.70
(97) In view of the above, and in particular of BI's growing position and the importance of innovation in the PRRS area, [CONFIDENTIAL INFORMATION ON R&D].71
(98) As a result, the Transaction will eliminate actual competition for PRRS vaccines in all EEA countries where both Parties are active, which represent more than [90-100]% of BI's EEA turnover in relation to PRRS vaccines, as well as potential competition in other EEA countries, [CONFIDENTIAL INFORMATION ON R&D] and BI could expand the geographic coverage of its recently launched products.
(99) In view of the above, the Transaction raises serious doubts as to its compatibility with the internal market in relation to monovalent PRRS vaccines in the EEA in general and in Belgium, Denmark, Germany, Hungary, Italy, Portugal and Slovakia in particular.
IV.2.2.5.Porcine parvovirus (PPV)
(100) Porcine parvovirus (PPV) causes reproductive losses during pregnancy by infecting the fetus of naïve dams. PPV is the most common cause of infectious infertility in pigs.
IV.2.2.5.a. Parties' products
(101) Merial supplies both a monovalent PPV vaccine, Parvovax, and a multivalent PPV vaccine combined with erysipelas, Parvovurax.
(102) [INFORMATION ON BI ACTIVITIES].
IV.2.2.5.b. Market definition
(103) In a previous decision dated 199972 the Commission defined distinct product markets for, on the one hand, monovalent vaccines against PPV and, on the other hand, multivalent vaccines against both PPV and erysipelas.
Notifying Party's views
(104) The Notifying Party submits that monovalent PPV vaccines for swine form a distinct relevant product market. The Notifying Party however considers that there is a degree of competition between monovalent PPV vaccines and multivalent vaccines including PPV, although some farmers may choose to use a multivalent vaccine as a first shot and a monovalent vaccine as a booster.
(105) The Notifying Party further submits that a distinction between modified-live and killed vaccines is not relevant, since modified live and killed vaccines are sufficiently similar in terms of price, efficacy and safety to be viewed as equivalent from a veterinary and customer's perspective.

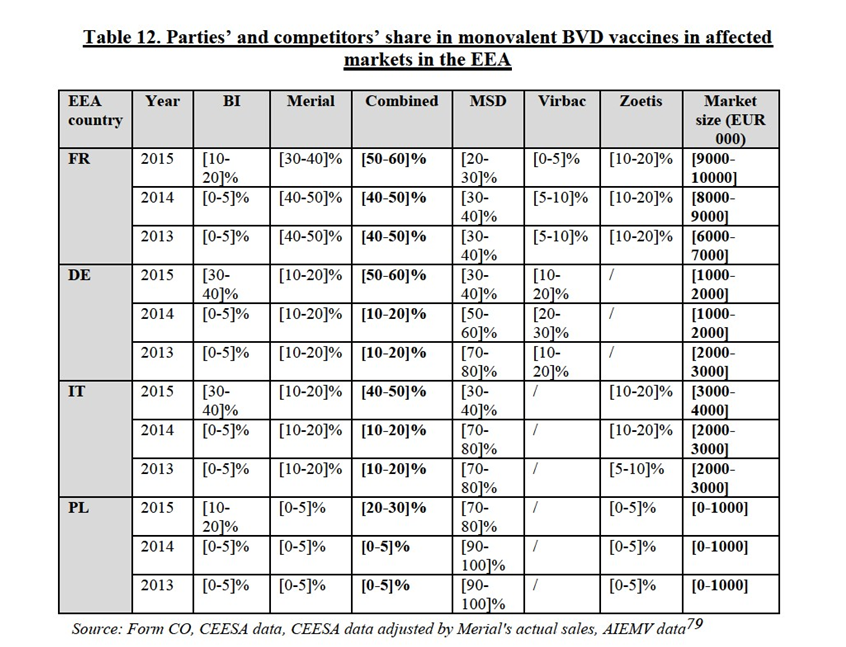
IV.2.3.1.a. Parties' products
(117) Both Parties produce and sell monovalent bovine viral diarrhoea (BVD) vaccines for ruminants. Merial also supplies a multivalent vaccine for respiratory cattle diseases including protection against BVD.
(118) BI entered the BVD market with its product Bovela in March 2015. Bovela is a modified-live vaccine that can be used for the control of both BVDV-1 and BVDV-2, it is the only vaccine in the EU licenced for the prevention of both types. Bovela is also currently the only BVD vaccine available on the market which offers foetal protection for both Type 1 and Type 2 BVDV as all other products are Type 1 vaccines which only offer cross-protection (not foetal protection) against BVDV-2.
(119) Merial’s monovalent BVD vaccine is marketed under the brand Mucosiffa. Mucosiffa is a modified-live vaccine. It is used for the active immunization of ruminants against BVDV-1 and mucosal disease. As regards multivalent vaccines Merial recently launched Bovalto Respi 4 which includes protection against BRSV, PI3, M.Haemolytica and BVD. [CONFIDENTIAL INFORMATION ON R&D]
IV.2.3.1.b. Market definition
The Notifying Party's view
(120) The Notifying Party submits that the relevant product market as regards vaccines against BVD should be a broader market for multivalent cattle respiratory vaccines including BVD. According to the Notifying Party, there is a non-negligible degree of competition between monovalent BVD vaccines and multivalent cattle vaccines which include protection against BVD. The Notifying Party however submits that while monovalent BVD vaccines are designated to eradicate BVD from the cattle population and therefore offer foetal protection (targeting breeding animals), multivalent vaccines including BVD protection do not offer foetal protection but are mainly focused at tackling the respiratory effects of BVD (and other pathogens included in the vaccine).
(121) The Notifying Party further submits that the distinction between live and inactivated vaccines would not be relevant in the case of BVD vaccines, since the Parties produce only modified-live BVD vaccines.
Commission's assessment
(122) As to the segmentation between monovalent and multivalent vaccines, the Commission has previously found that multivalent cattle respiratory vaccines, possibly including protection against BVD, constitute a distinct market from monovalent vaccines targeting only one pathogen.76 The market investigation in this case has not revealed any elements which would confirm the Notifying Party’s arguments. Indeed, customers did not identify any multivalent product as competing closely with the Parties' monovalent products and only one identified multivalent vaccines as a BVD offering.77 The market structure also seems to reflect this distinction, the two most important players in the area of BVD vaccines, namely MSD and BI, have only monovalent vaccines. Similarly, BI’s internal documents focus on the monovalent



The Commission's assessment
(131) BI and Merial are two significant suppliers of monovalent BVD vaccines across the EEA, with a combined market share up to more than [50-60]% in France and Germany in 2015.
(132) In addition, BI's market shares are not fully representative of its real market position as BI entered the market only in March 2015. Since its entry, BI already gained [20-30]% of the market in 2015 at EEA level and up to [30-40]% in Germany. Respondents to the market investigation expect BI's market share to continue to grow and eventually take over MSD as the market leader.80 One market participant indicated for instance that "Bovela has performed well since launch and has already reached #1 position in Germany and is already in a #2 position in most markets where it has launched. It looks set to take #1 position in Italy this year and also has the potential to do so in a number of key European markets over the next 9-18 months."81
(133) BI's internal documents confirm its growing position and show that BI expects to become the market leader in the near term. By way of example, one document mentions that "Bovela will MAKE HISTORY […] The secret of Bovela success will be [CONFIDENTIAL INFORMATION REGARDING BI COMMERCIAL STRATEGY] […] Mid-term 1-3 years (2016-2018): achieve at least 55% MS [market share] in Europe" / Long term > 3 years: extend market share in EU to 70%" and ultimately "achieve 80% of market share in the monovalent BVD market in Europe".82
(134) The market investigation indicated that the success of Bovela is due to a combination of factors including the fact that it is a single dose, modified live product and has cross protection against type 2 as opposed to the currently leading MSD Bovilis which is a two doses, killed vaccine with no cross protection against type 2. Indeed, market participants insisted on the importance of these criteria when choosing a BVD vaccine. For instance, one market participant indicated that "These factors are of important consideration only where the live vaccine is indicated as a single shot regime without the need for a 2 dose primary course",83 while others insisted on the "preference if it is a single dose by reducing labour cost it will increase BVD vaccination uptake (convenience)"84 and the fact that "although BVD type II is very rarely isolated in EU, farmers and vets like having a broader protection".85
(135) Moreover, the market investigation indicated that Merial’s Mucossifa is the closest competitor to BI’s Bovela.86 By way of example, one market participant indicated that "the closest competitor to Bovela is Mucosiffa where it is sold as it offers broadly the same convenience/usage attributes".87 Indeed, they are the only two modified live vaccines and one dose products.88 In addition, Mucossifa recently gained new claims which make the product even closer to Bovela.89 In particular, Merial recently obtained foetal protection in France and cross protection against type 2 in Italy. In addition, some market participants also noted that Mucossifa and Bovela both have twelve months duration of immunity.90 The strong competitive constraint exerted by Mucossifa on Bovilis can also be illustrated by BI's internal documents [CONFIDENTIAL INFORMATION REGARDING BI ANALYSIS OF COMPETITOR PRODUCT].91
(136) The market investigation did not confirm the competition exerted from multivalent offerings. Customers never identified any multivalent product as competing closely with the Parties' monovalent products.92 BI's internal documents also rarely mention multivalent vaccines within the BVD competitive landscape.93
(137) As a result, the Transaction will eliminate actual competition for BVD in all EEA countries where the Parties are both active, which represent almost [90-100]% of Merial's EEA turnover in the EEA for monovalent BVD vaccines, as well as potential competition in other countries and in particular in the UK and Spain in view of BI's presence and Merial's expansion plan in these countries.94
(138) In view of the above and in particular the strong market position and closeness of competition between the Parties' products, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the market for monovalent BVD vaccines in the EEA in general and in France, Germany, Italy and Poland in particular.
IV.3.Animal health pharmaceuticals
(139) Animal pharmaceuticals are a wide group of medicines containing a large variety of active pharmaceutical ingredients (APIs) that prevent or treat a range of animal diseases and disorders. Pharmaceuticals include (i) anti-inflammatories, (ii) antimicrobials (also known as antibiotics) and (iii) specialty products such as cardiopulmonary therapy for companion animals.
(140) In the present case, the Parties' activities overlap in all those three areas.
(141) The parties are among the main players active in animal health pharmaceuticals in the EEA, together with Zoetis, MSD, Elanco and Bayer.

IV.3.1.2.Geographic market
(145) In previous decisions,97 the Commission found that despite the existence of some pan- European trends and the fact that the main players are active throughout the EEA, the relevant geographic market for animal health pharmaceuticals was national in scope. This is mainly due to the fact that most products on these markets remain subject to national and mutual recognition registration systems. In addition, national legislation determines the selling conditions of the products, while competitive landscapes in EEA countries differ in terms of market penetration, shares, price, distribution systems and local veterinarian preferences.
(146) The Notifying Party agrees the geographic scope of the markets is national.
(147) In this case, the market investigation broadly confirmed that markets for pharmaceuticals in the animal health sector are still national, as marketing authorizations are still subject to national regulations, the competitive landscapes varies from one Member State to another while pricing strategies of pharmaceutical companies also seem to be national.
(148) For the purpose of assessing the impact of the Transaction, the Commission therefore concludes that the relevant geographic markets in relation to animal health pharmaceuticals are national in scope.
IV.3.2. Anti-inflammatories
IV.3.2.1. Market definition
(149) Anti-inflammatories are used to treat inflammation and to reduce the pain and fever associated with inflammation. In previous decisions,98 the Commission found that anti-inflammatories may be sub-divided into two categories: (i) non-steroidal anti- inflammatory drugs (NSAIDs) and (ii) corticosteroids. Although NSAIDs and corticosteroids both have anti-inflammatory properties, only NSAIDs have analgesic (anti-pain) and anti-pyretic (anti-fever) properties. Furthermore, NSAIDs can relieve pain and inflammation without the immunosuppressive and metabolic side-effects associated with corticosteroids. NSAIDs also tend to be more expensive than corticosteroids. NSAIDs are used in animal health primarily for pain relief and for treating inflammation. NSAIDs act by inhibiting the formation of prostaglandins synthesized via the cyclooxygenase pathway or the formation of leukotrienes via the lipoxygenase pathway to mediate the body’s inflammatory response to injury. Adverse effects of treating pain with NSAIDs are most commonly gastrointestinal ulceration and renal impairment.
(150) In previous decisions, the Commission considered distinction between NSAIDs based on :i. the mode of administration, distinguishing between (i) injectable and (ii) oral; andii. the animal species or groups of species, distinguishing between (i) companions animals, (ii) horses and (iii) ruminants, swine, horses and companion animals ("multi-species").99
(151) First, the market investigation generally confirmed the distinction between injectable and oral NSAIDs, injectable solutions being used for treating acute pain post-surgery for instance while oral solutions are typically administered by the animal owners for chronic pain.100
(152) Second, the market investigation confirmed the distinction by animal species or group of species, pharmaceuticals being generally authorized per animal species or group of species.
(153) The Notifying Party adds that other distinguishing factors should be accounted for when analysing the NSAID markets. While they may not impede substitutability between NSAID products to the extent that they form separate relevant product markets, they may still be relevant for the competitive assessment. These factors include (i) animal size for injectable NSAIDs, (ii) non selective Cyclooxygenase (COX-1)/cyclooxygenase 2 (COX-1) (COX) and selective COX-2 inhibitors (COXIB) treatments, (iii) treatment of acute or chronic inflammation and (iv) active substance of the pharmaceutical.
(154) The market investigation indeed indicated that in the area of animal pharmaceuticals the market should not be segmented by active pharmaceutical ingredients (API), since all NSAIDs compete together from a demand perspective.101
Injectable multiple species NSAIDs
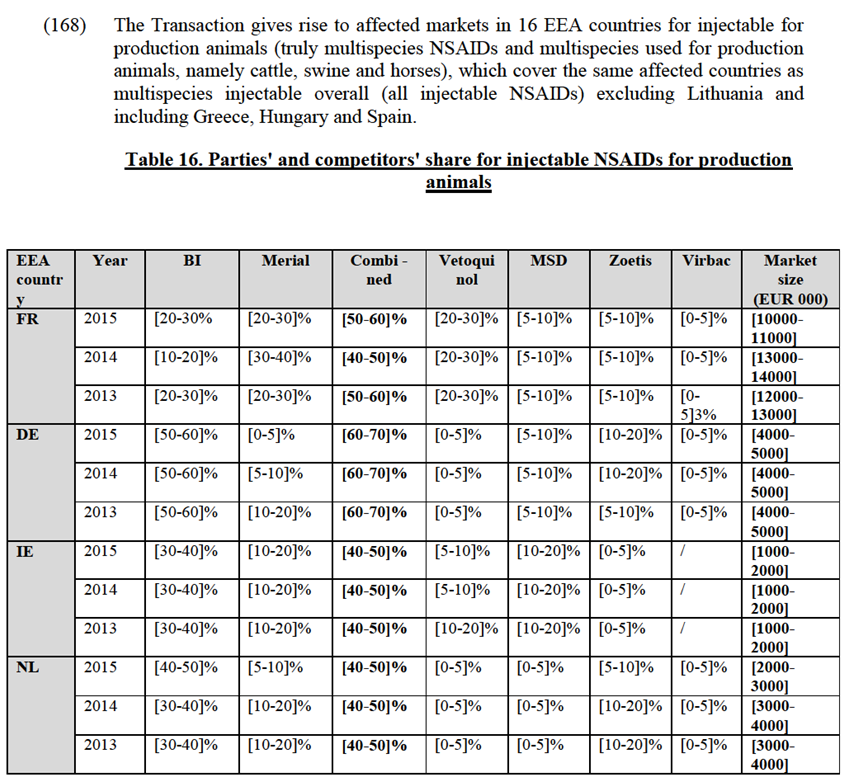
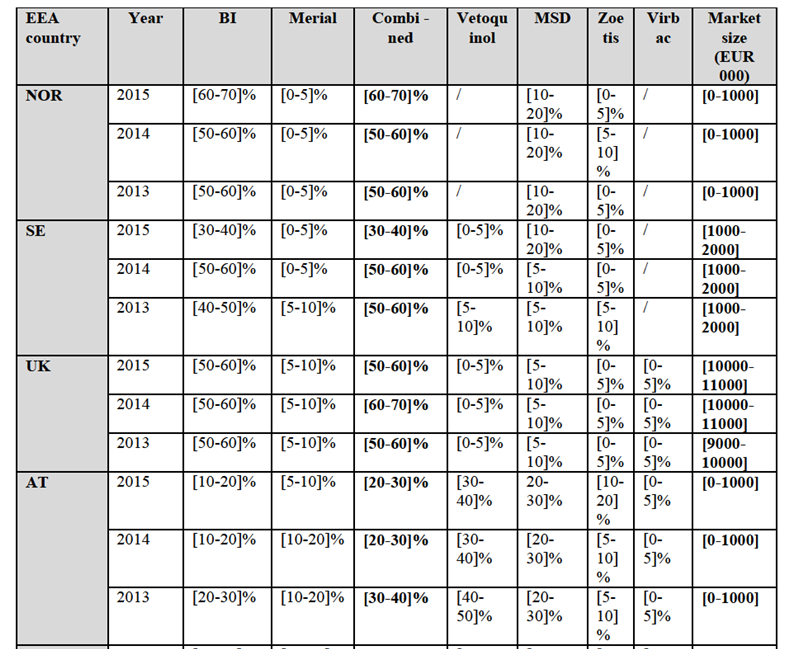
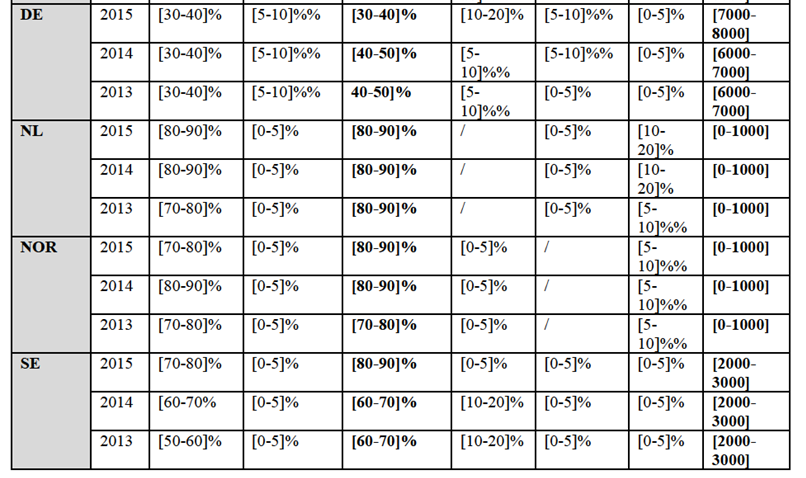
Notifying Party's views
(155) The Notifying Party submits that multi-species injectable NSAIDs constitute a distinct product market. The Notifying Party points out that most injectable NSAIDs are truly multi-species and that further segmentation according to species could lead to unrealistically small markets. However, the Notifying Party adds that certain injectables are specifically targeted for specific species.
Commission's assessment
(156) The Commission has previously found that although there are some injectable NSAIDs that are specifically targeted for horses, dogs and cats respectively, there are also injectable NSAIDs that are truly multi-species, which makes the task of estimating their use for each species very difficult.102
(157) The market investigation in this case broadly confirmed that the market for injectable NSAIDs would be multispecies. However, a distinction might be drawn between large animals (production animals such as cattle, horses and pigs) and small animals (companion animals such as dogs and cats) since products tend to have different concentrations and dosages depending on the animal's size and some of the Parties' products are used only for production animals (and one of Merial's product is even used for horses only). As a consequence, for specific specie (e.g. cattle), competition takes place between truly multi-species products and products authorized for use for this specie in particular (e.g. injectable NSAIDs for production animals such as cattle and swine).
(158) For the purpose of assessing the Transaction, the exact relevant product market in relation to injectable NSAIDs can be left open, since the Transaction raises serious doubts as to its compatibility with the internal market in relation to injectable multispecies NSAIDs, irrespective of the exact segmentation of that market.
Oral NSAIDs
Notifying Party's views
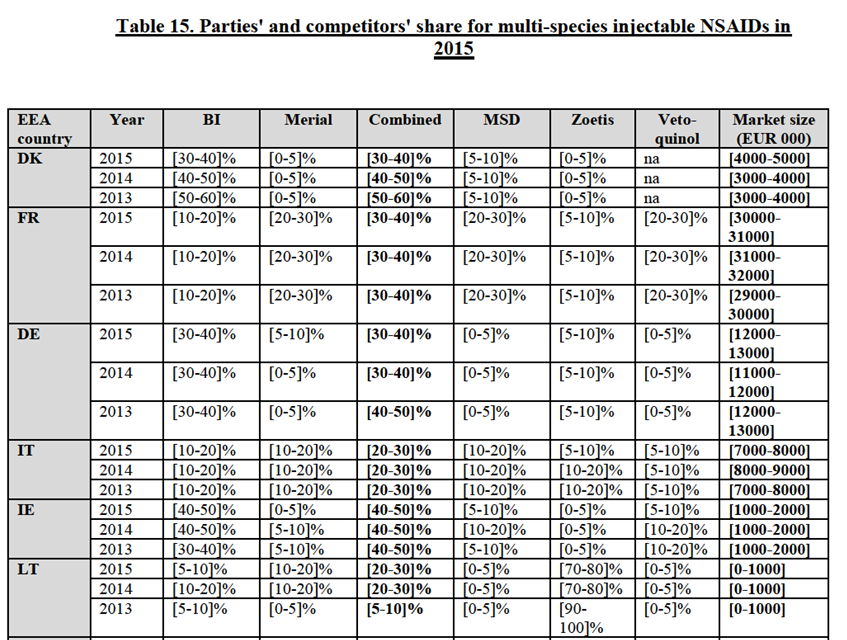
(159) In line with previous Commission decisions, the Notifying Party submits that orally administrated NSAIDs constitute a distinct product market.103 The Notifying Party also refers to the Commission's previous practice of further segmenting oral NSAIDs by the animal species for which they are intended. Thus, the Notifying Party submits that oral NSAIDs for horses and oral NSAIDs for companion animals constitute distinct product markets.
Commission's assessment
(160) In line with the principles identified above the Commission has previously defined product markets for oral NSAIDs for horses and oral NSAIDs for dogs and cats.104 The market investigation in this case confirmed this approach.
(161) In addition, the market investigation indicated that COX and COXIB also compete from a customer's perspective. Although COXIBs would be marketed as safer, many customers expressed doubts as to this better safety profile.105 It might however be a differentiating factor in particular for companion animals and horses (owners being more receptive to the safety argument).106
(162) For the purpose of assessing this Transaction, the relevant product markets are therefore the market for oral NSAIDs for horses and oral NSAIDs for pets.
(163) Based on the above, the Transaction leads to overlaps between the Parties' activities in:i. injectable multiple species NSAIDs,ii. oral NSAIDs for horses, and iii. oral NSAIDs for pets.
IV.3.2.2. Competitive assessment IV.3.2.2.a. Injectable multi-species NSAIDs IV.3.2.2.a.i. Parties' products
(164) BI sells its injectable NSAIDs under the brands Metacam and Novem. Meloxicam is the API of both brands of injectable NSAIDs. Metacam is licensed for use in several species and has different concentration and dosage depending on the animal's size: 40mg/ml for cattle and horses, 20mg/ml for cattle, pigs and horses, 5 mg/ml and 2 mg/ml for dogs and cats. Novem is licensed for use in cattle and swine only.






EEA countries.108 Metacam benefits from very strong brand recognition. This is confirmed by BI's internal document where it is stated that "Metacam is the world leading NSAID for the control of inflammation and pain in farm animals […] Metacam is and will remain the major global NSAID brand on the market."109 The market investigation confirmed also Merial’s strong position, number 2 at EEA level and among the top suppliers across EEA countries, with its products Ketofen and Equioxx.110
(172) The market investigation generally indicated the importance of branded products, as compared to generics.111 Indeed, Customers appear reluctant to consider generic as fully substitutable to originators as they experience issues with generics such as imprecise dosages. By way of example, one veterinarian indicated that "original products guarantee content and efficacy, based on long term experience. Copies often are experienced to have varying effects."112
(173) As to competitors active in the market, the market investigation indicated that they have weaker brands; MSD's Finadyna/Banamine (flunixin) product would be less efficacious113 and Vetoquinol, Zoetis and Ceva's products generally have market shares of less than 10% in affected markets.
(174) Furthermore, BI's Metacam and Merial's Ketofen would be the only two products with label claims for pain management. One market participant explained that "although all products have similar mechanisms of action, some have more complete set of label claims. For instance: Metacam and Ketofen are the only products with specific label claims for the management of pain".114
(175) The market investigation also provided indications that Merial tends to be cheaper than BI's strong brand and thus post-merger price increases are expected. In this context a customer explained that "merial is very aggressive with price. I don't think this will be the BI politics".115 Similarly, other market participants expressed concerns about a price increase post-Transaction116 one of which for instance stated there is a "risk of price increase due to significant market share of BI/Merial combined products".117
(176) As a result, the Transaction will eliminate actual competition in injectable NSAIDs in all 17 EEA countries where both Parties are active, where the Parties generated almost [90-100]% of their EEA sales in multispecies injectable NSAIDs, as well as potential competition in other EEA countries where the two Parties are natural entrants.
(177) In view of the above, the Transaction raises serious doubts as to its compatibility with the internal market in relation to injectable NSAIDs in the EEA in general and in Austria, Belgium, Denmark, Italy, Lithuania, France, Germany, Greece, Hungary, Ireland, the Netherlands, Norway, Poland, Spain, the UK, Slovakia and Sweden in particular.
IV.3.2.2.b. Oral NSAIDs for horses
IV.3.2.2.b..IV Parties' products
(178) BI sells its orally administered NSAIDs for horses under the brand Metacam Horse. Metacam Horse is based on meloxicam. Metacam is a COX product.
(179) Merial’s product is marketed under the brand Equioxx Paste. Equioxx Paste is an orally administered NSAID based on firocoxib. Equioxx Paste is a COXIB.

Source: CEESA data adjusted by BI's estimates, CEESA data adjusted by Merial's actual sales, GfK data118
The Notifying Party's view
(181) The Notifying Party submits that the merged entity will face significant competitive pressure across all of its product lines from global, regional and national originator manufacturers and generic suppliers.
(182) The Notifying party also claims that BI's and Merial’s products are not closest competitors, for the following reasons. The Notifying Party points out that:(i) Metacam is for short term use, whereas Equioxx is used for long term use,(ii) Metacam is a non-COXIB NSAID, whereas Equioxx is a COXIB, which is an important distinction for veterinarians,(iii) Metacam has a very short detection time whereas Equioxx has a longer detection time,(iv) Metacam is predominantly used for treatment of acute and chronic pain as well as during colic, whereas Equioxx focuses on (long-term) treatment, a chronic condition.
The Commission's assessment
(183) The market investigation confirmed the leading position of BI's Metacam Horse at EEA level and across EEA countries. Metacam Horse benefits from very strong brand recognition. For instance, a veterinary specialized in horses stated that it is "Top product for anti-inflammatory joint treatment, safety and brand recognition".119 This is also confirmed in BI's internal documents. As an example, a BI internal document states that "Metacam is the original top of mind brand in the main countries. Horse owners also know Metacam very well and ask for it".120
(184) The market investigation as well as BI’s internal documents also showed that Merial’s Equioxx although having a more limited market share across the EEA is a strong competitor to BI. For instance a BI internal document states that "Equioxx (Merial), a firocoxib, is a very strong competitor for us as well [as generics] […] Equioxx [Merial] has increasing market share".121 Merial tends in particular to use the COXIB nature of Equioxx (firocoxib) to gain market shares. BI noted in internal documents that "Merial is branding Equioxx strongly (the new “modern” NSAID has a better efficacy and is safer)."122 In addition, in some EEA countries, such as Sweden, Norway and the Netherlands, Merial's Equioxx is among very few products to compete with BI's leading branded product, which would lead to a combined market share post-Transaction of [80-90]% or more.
(185) Finally, some market participants expressed concerned about a price increase post- Transaction,123 one of them mentioning that "the newly combined entity would control the top brand name premium priced equine NSAIDS."124
(186) As a result, the Transaction will eliminate actual competition in all countries where the Parties are currently active, where the Parties generated almost [90-100]% of their EEA turnover, as well as potential competition in other EEA countries where the two Parties are natural entrants.
(187) In view of the above, the Transaction raises serious doubts as to its compatibility with the internal market in relation to oral NSAIDs for horses in the EEA in general and in Austria, Belgium, Denmark, Finland, France, Germany, the Netherlands, Norway and Sweden in particular.
IV.3.2.2.c. Oral NSAIDs for pets
IV.3.2.2.c..IV Parties' products
(188) BI sells its orally administered NSAIDs for pets under the brands Metacam Oral Suspension for Dogs, Metacam Chewable Tablets for Dogs and Metacam Oral Suspension for Cats. BI’s Metacam products are all based on meloxicam.
(189) Merial’s products are marketed under the brands Previcox CPR and Ketofen CPR. Previcox is used for dogs, it contains the API firocoxib, and is a COXIB. Ketofen CPR is used for dogs and cats, and is based on ketoprofen.
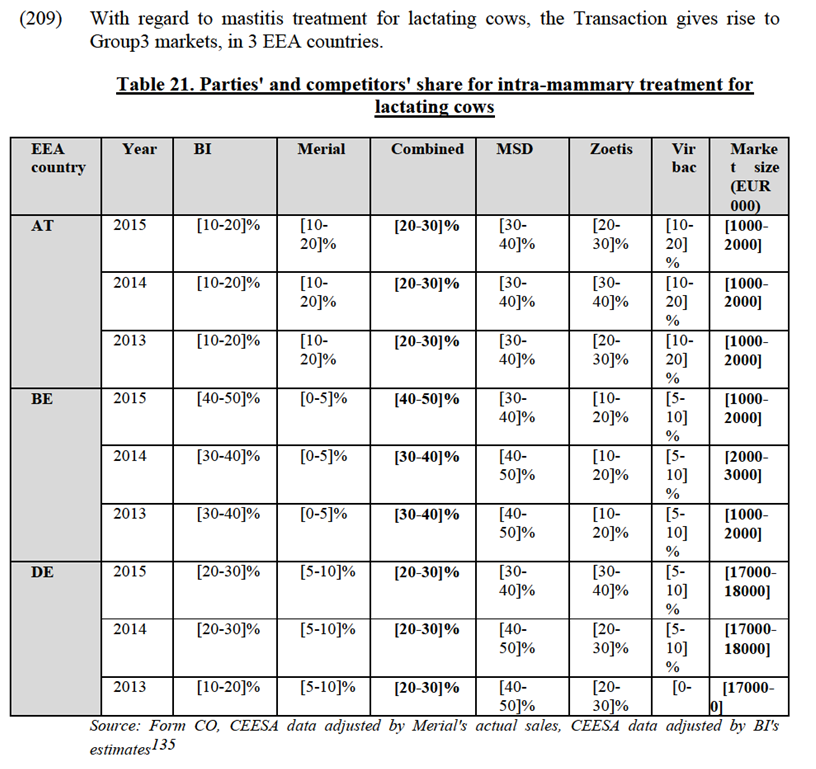
IV.3.2.2.d. Assessment
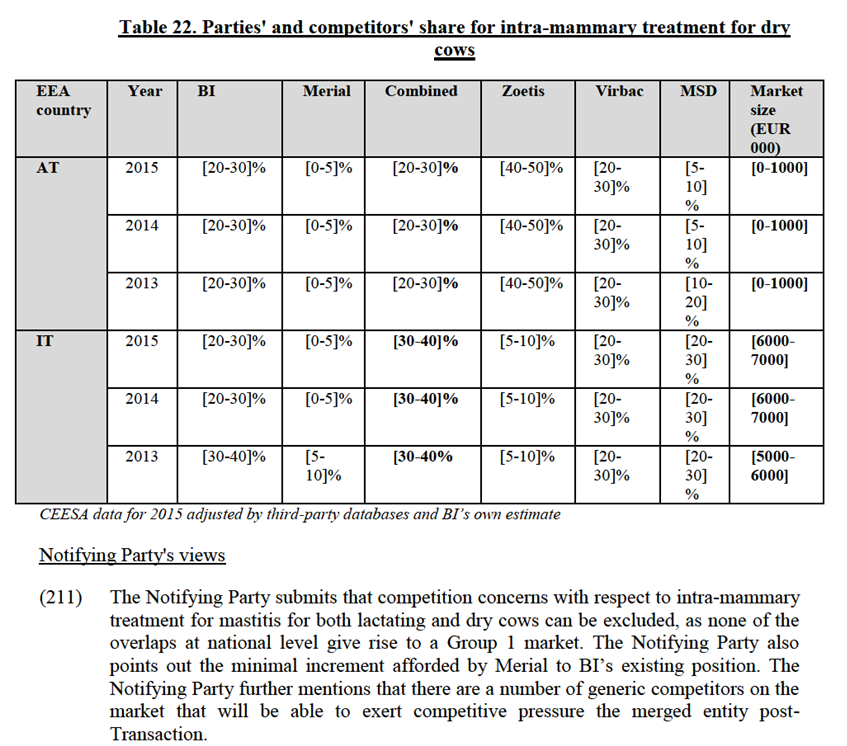
(190) The Transaction gives rise to affected markets for oral NSAIDs for pets in 12 EEA countries.108




identified Zoetis Rimadyl as the leading player in the oral NSAIDs for companion animals market.128 Several customers indicated that its products were "excellent" with regard to their safety and efficacy. Internal documents of BI also seem to confirm that Zoetis "is still by far the market leader with 40% Market Share"129, has an "efficacious product + added value services (Vet support…)" and is together with Metacam a "first choice" product.130 Ceva's products are also considered by many customers as having a good efficacy and safety profile, and would be close substitutes to BI's Metacam131.
(196) In view of the above and all the evidence available to the Commission, the Transaction does not raise serious doubts as to its compatibility with the internal market with respect to oral NSAIDs for companion animals.
IV.3.3. Anti-microbials
IV.3.3.1.Market definition
(197) Antimicrobials are pharmaceutical products that belong to the general group of anti- infectives for systemic, local or topical use. They are used to destroy and prevent the growth of microbes such as bacteria, mycoplasma (pathogens that lack cell walls) and treat associated diseases.
(198) In previous decisions,132 the Commission considered that the following factors could be relevant in defining product markets or influence the closeness of competition between antimicrobials:(i) active substance (sulphanomides, penicillins, cephalosporins, tetracyclines, etc.)(ii) route of administration (injectable products, products for oral administration and products for topical administration such as intra- mammary mastitis treatments); and(iii) animal's size (large animals such as horses, ruminants and swines and companion animals such as dogs and cats)
(199) The Notifying party agrees with this general approach with regard to antimicrobials.
(200) The overlap areas between the Parties in the antimicrobial segment concern mastitis treatment in dry and lactating cows. Mastitis treatments differ from other antimicrobials because of their singular mode of administration (generally intra- mammary) and the formulation of the drug that makes these products particularly effective against the relevant bacteria.
(201) In previous decisions,133 the Commission found that there are two different types of mastitis infections, which belong to separate product markets.(i) Acute mastitis which most commonly occurs during the lactation period (i.e., when the cow is producing milk). Treatment requires daily and repeated administration of therapeutic formulations (lactating cow products’). The drugs must produce results quickly and have a carefully controlled time of effectiveness as the milk must be discarded during the period in which the drug is active;(ii) Chronic infections (or sub-clinical mastitis) cause an increased number of white blood cells in the milk (somatic cells), but do not have any obvious clinical symptoms. Sub-clinical mastitis is typically treated during the days of the year when the cow is not milked (the so-called dry period).
(202) The Notifying party agrees with this approach. The distinction between treatment for dry and lactating cows was also confirmed by the market investigation.134
(203) Therefore, the Commission considers that for the purposes of this Transaction, the relevant product markets are (i) mastitis treatment for lactating cows and (ii) mastitis treatment for dry cows.
IV.3.3.2.Parties' products
IV.3.3.2.a. Parties' products for mastitis treatment for lactating cows
(204) BI sells its products for the treatment of mastitis in lactating cows under the brand Ubrolexin. The product is used for treatment of bacteria susceptible to the combination of cefalexin and kanamycin such as Staphylococcus aureus, Streptococcus dysgalactiae, Streptococcus uberis and Escherichia coli.
(205) Merial’s products are marketed under the brands Cefovet and Mastipent. Cefovet’s API is cefazolin, a first-generation cephalosporin antibiotic with a broad spectrum antibiotic indicated against both gram-negative and grampositive bacteria such as streptococci bacteria. Mastipent’s APIs are ampicillin and cloxacillin. This product is indicated for the treatment of mastitis caused by a wide range of gram-positive and gramnegative bacteria, such as Aerobacter aerogenes, Klebsiella species, Pseudomonas aeruginosa, and Escherichia coli. 128 



lactating cows136. Virbac was also mentioned as the market leader for dry cows by some respondents137.
(215) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction does not raise serious doubts as to its compatibility with the internal market with respect to the market for mastitis treatment for lactating cows and the market for mastitis treatment for dry cows.
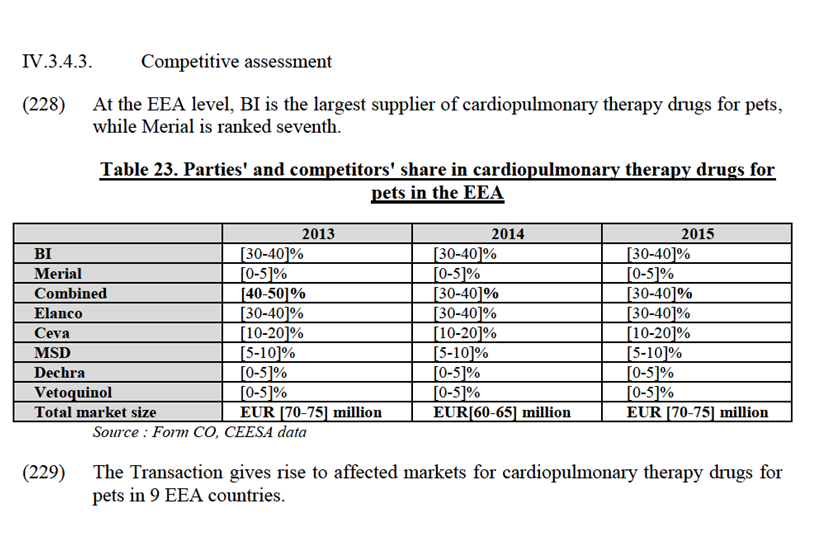
IV.3.4. Specialty products: cardiopulmonary therapy for pets
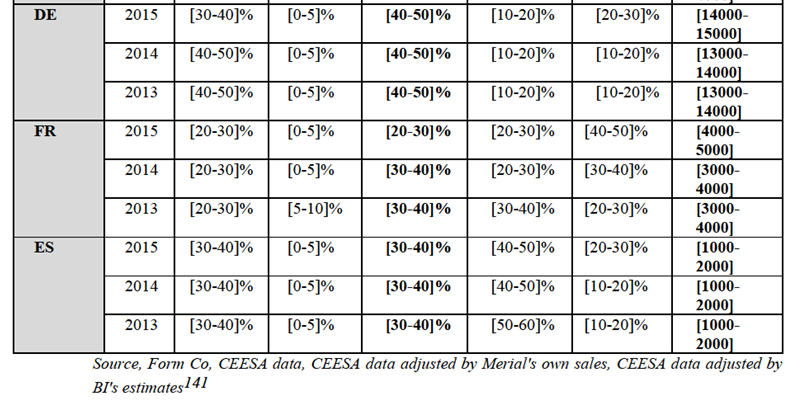
(216) Speciality products target very specific conditions and do not easily fit into any other pharmaceutical category, mainly because they lack the significance that they enjoy in the human health sector. These products include certain niche products such as insulin or diuretics which relieve oedemas.
(217) In the area of specialty products, the Parties' activities overlap in relation to cardiopulmonary for pets. The Commission has not previously assessed these types of pharmaceuticals.
IV.3.4.1. Market definition
(218) Cardiopulmonary drugs for pets are used to address congestive heart failure disease. These drugs significantly improve clinical signs and extend the life expectancy of dogs and cats.
The Notifying Party's view
(219) The Notifying Party submits that a distinction can be made between different modes of administration, such as oral and injectable formats, although cardiopulmonary drugs for pets are generally sold in oral format.
(220) The Notifying Party further submits that cardiopulmonary treatment for pets generally consists in a combination of different classes of drugs which target different aspects of the disease, including in particular:(i) Pimobendan which increases the strength of the contraction of the heart and also acts to dilate blood vessels. Pimobendan also relaxes vascular smooth muscle and elicits modest arterial vasodilation;(ii) ACE inhibitors which help block the activation of the reninangiotensin- aldosterone system (RAAS), which promotes fluid retention, vasoconstriction and myocardial and vascular remodelling;(iii) Diuretics which help to remove the fluid build-up in or around the lungs once signs of congestive heart failure develop;(iv) Beta blockers which slow down the heart rate and reduce the oxygen demand on the heart.
(221) The Notifying Party submits that the large majority of pets are treated with a therapy called "triple therapy" that is composed of an ACE inhibitor, a positive inotrope (like Pimobendan) and a diuretic for dogs, and beta-blockers, an ACE inhibitor and a diuretic for cats.
(222) In the view of the Notifying Party, this distinction does not impede substitutability between products to the extent that they would form separate relevant product markets, although the closest competitors tend to be other products from the same group. The Notifying Party thus submits that the relevant product market is the market for oral cardiopulmonary therapy drug for pets.
Commission's assessment
(223) The market investigation confirmed the existence of different classes of medication within the cardiopulmonary therapy products. Moreover, several respondents to the market investigation also confirmed that ACE inhibitors and Pimobendans are not substitutable but complementary since they have different modes of action and different therapeutic effects138.
(224) The market investigation also provided indications that cardiopulmonary drugs are used together mainly within a triple therapy, or sometimes within a quadruple therapy which the addition of a spironolactone139. According to one competitor, there is even a new trend on the market of cardiopulmonary for pets to produce a pill combining two or more active ingredients of the therapy140.
(225) In view of the above, ACE inhibitors for companion animals may constitute a separate relevant market.However, the precise product market definition with respect to cardiopulmonary therapy drugs for pets can be left open for the purpose of this decision as the Transaction does not raise serious doubts as to its compatibility with the internal market in relation to cardiopulmonary therapy drugs for pets irrespective of the precise product market definition.
IV.3.4.2. Parties' products
(226) BI sells its products under the brands Benefortin, Vetmedin, Vetmedin Chewables and Vetmedin Injection. Benefortin is an angiotensin-converting-enzyme inhibitor (ACE inhibitor) licensed for the treatment of congestive heart failure in dogs and chronic renal insufficiency in cats. Benefortin contains benazepril hydrochloride as its API. Vetmedin is therapy medicine for canine congestive heart failure. Vetmedin belongs to the new class of heart treatments termed inodilators and is indicated for the management of the signs of mild, moderate, or severe congestive heart failure in dogs.Vetmedin’s API is pimobendan and it is sold in injectable and chewable tablet form.
(227) Merial’s product is marketed under the brand Enacard which is an ACE inhibitor indicated for the treatment of mild, moderate and severe congestive heart failure in dogs. Enacard’s API is enalapril maleate.

Notifying Party's view
(230) The Notifying Party submits that the Parties’ combined market shares for cardiopulmonary therapy for pets would not reflect the dynamics of competition in these markets for the following reasons.
(231) First, the Notifying Party claims that the Parties' products are not closest competitors since Pimobendan and ACE inhibitors are complementary and not substitutable products, even though they belong to the same CEESA category. As a result, the Parties' activities solely overlap as regards ACE inhibitors.
(232) Second, the Notifying Party submits that the market for cardiopulmonary for pets will remain competitive post-Transaction since at least three strong competitors – namely Ceva, Elanco, MSD – will exert competitive constraints on the merged entity's products in all EEA markets. Moreover, a number of generic manufacturers such as Vetoquinol and Dechra have gained significant market shares in a short period of time and will continue to exert competitive constraint on the merged entity post- Transaction.
Commission's assessment.
(233) As regards the market for cardiopulmonary for pets, BI's product Vetmedin is one of the premium EEA brands, which benefits from very strong brand recognition and is leading the market together with Elanco's Fortekor.142 Reversely, Merial's product Enacard has small market shares, thus its increment to BI's position is relatively low, at no more than [5-10]% in all of the affected markets. The market investigation also indicated that BI and Merial are not particularly close competitors143.
(234) The transaction gives rise to Group 1 markets in 9 EEA countries. Nevertheless, in all of those countries, there are at least two strong competitors, namely Elanco and Ceva, which exert significant competitive constraint on BI's product. In addition MSD exercises some competitive constraints in the Netherlands, as does Vetoquinol (a generic manufacturer) in the Czech Republic.
(235) Moreover, the market investigation confirmed that BI's Vetmedin and Merial's Enacard are not close competitors, since one is a Pimobendan and the other an ACE inhibitor.
(236) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction does not raise serious doubts as to its compatibility with the internal market with respect to cardiopulmonary therapy drugs for pets.
IV.4. Animal feed supplements
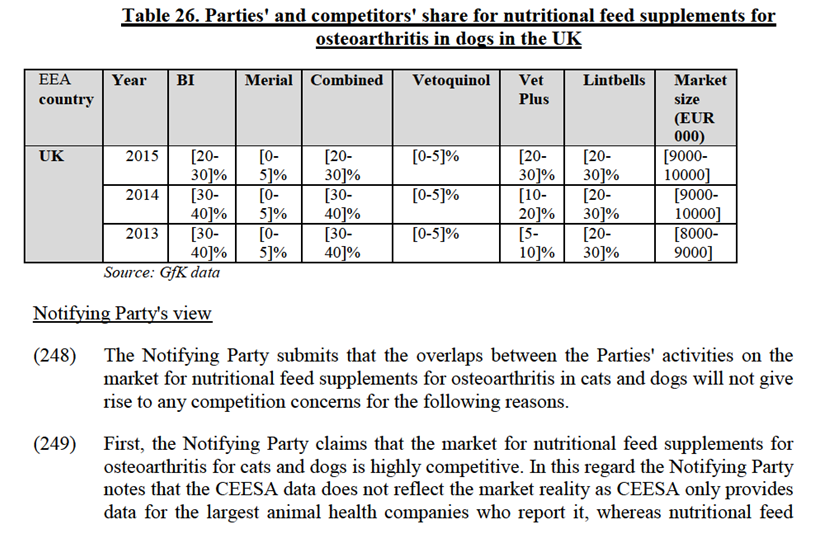
(237) Feed supplements are pharmaceutical or nutritional substances that are not natural feedstuffs and are added to made-up and stored feeds for various purposes but chiefly to control infectious disease or to promote growth. The Commission has previously considered markets for feed additives144.
(238) In the area of Animal feed Supplements, the Parties' activities overlap in relation nutritional feed supplements for osteoarthritis in cats and dogs. This area has not been previously analysed by the Commission.
IV.4.1. Market definition
Product market definition
(239) Osteoarthritis or degenerative joint disease is a slowly progressive, low-grade inflammatory syndrome causing deterioration of articular cartilage (the “shock absorber”) osteophytosis (new bone formation) and sclerosis of the subchondral bone. Nutraceuticals promote joint health and do not treat osteoarthritis as such because they are mainly intended to slow the progression of primary osteoarthrosis.
(240) The Notifying party makes a distinction by of species, target disease and method of application. The Notifying Party submits that the nutritional feed supplement for osteoarthritis in cats and dogs constitutes the narrowest possible product market.
(241) In view of the fact that the Transaction does not raise serious doubts as to its compatibility with the internal market in relation to nutritional feed supplements for osteoarthritis in cats and dogs under any plausible market definition, the exact scope of the product market can be left open for the purposes of the competitive assessment of the Transaction.
Geographic market definition
(242) In line with the principles mentioned at paragraph (145), the Commission has previously found that the relevant geographic market for animal health products, including feed additives, was national in scope.
(243) The Notifying Party agrees with this approach, which was also confirmed by the market investigation.
(244) In view of the above, animal feed supplements may constitute a separate relevant market. However, the precise product market definition with respect to animal feed supplements can be left open for the purpose of this decision as the Transaction does not raise serious doubts as to its compatibility with the internal market in relation to animal feed supplements irrespective of the precise product market definition.
IV.4.2. Parties' products
(245) BI sells its joint nutritional feed supplement for pets under the brand Seraquin that is intended for support of normal joint function in cats and dogs. Seraquin’s active ingredients are glucosamine hydrochloride, chondroitin sulphate and turmeric extract (curcumin). The product is sold in a chewable tablet format.
(246) Merial’s product is marketed under the brand Supleneo Flex that is used for the support of joint health in dogs. Supleneo Flex contains a combination of compounds

supplements are generally commercialised by pharmaceuticals nutraceutical and pet food manufacturers.
(250) Consequently, the Notifying Party estimates that the data provided by CEESA represents less than a third of the overall feed supplement market. To the Notifying Party's knowledge, at least six significant manufacturers of nutritional feed supplements are unaccounted for in the CEESA data.
(251) Second, the market for nutritional feed supplements for osteoarthritis for cats and dogs is a fast developing market with quick entry since regulatory requirements are less burdensome in terms of time and expense than those of vaccines or pharmaceuticals, with no marketing authorisation needed.
(252) Third, BI and Merial's products are not each other's closest substitutes since there are significant differences between their products with regard to composition and price.
Commission's assessment.
(253) While BI is an important player on these markets, Merial is a very small player, with an increment of no more than [0-5]% in all of the affected markets.
(254) The market investigation indicated that even though BI's Seraquin is one of the main premium brands, the market for nutritional feed supplements for osteoarthritis for cats and dogs is very competitive because products can be sold by veterinarians, pet shops, OTC or even supermarkets146.
(255) According to CEESA's limited data, at least three main competitors will exert competitive constraints on the merged entity post-Transaction (namely Vetoquinol, Virbac and Elanco, as well as Vet Plus and Lintbells in the UK). The Commission also notes that some of the market players have not been taken into account in CEESA's data and could also exert a constraint on BI's product.
(256) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction does not raise serious doubts as to its compatibility with the internal market with respect to nutritional feed supplements for osteoarthritis for cats and dogs.
IV.5. Conclusion of the Competitive assessment
(257) In light of the above assessment, the Commission concludes that the Transaction raises serious doubts as to its compatibility with the internal market in relation to (i) monovalent PCV2 vaccines in the EEA (§(64)); (ii) monovalent PPRS vaccines in the EEA (§(99)); (iii) monovalent PPV vaccines in the EEA (§(115)); (iv) monovalent BVD vaccines in the EEA (§(138)); (v) injectable NSAIDs in the EEA (§(177)); (vi) oral NSAIDs for horses in the EEA (§(186)).
V.COMMITMENTS
(258) In order to render the Transaction compatible with the internal market, the Parties have modified the Notified Transaction by entering into commitments.
V.1. Framework of assessment
(259) As background, the following principles, as referred to in Commission Regulation (EC) No 802/2004, and in the Commission Notice on remedies acceptable under the Merger Regulation ("the Remedies Notice")147, notably apply where the parties to a merger choose to offer commitments with a view to rendering the concentration compatible with the internal market.
(260) Where the Commission finds that a concentration raises competition concerns in that it could significantly impede effective competition, in particular as a result of the creation or strengthening of a dominant position, the parties may seek to modify the concentration in order to resolve the competition concerns and thereby gain clearance of their merger.148
(261) Under the Merger Regulation, it is the responsibility of the Commission to demonstrate that a concentration would significantly impede effective competition. The Commission then communicates its competition concerns to the parties to allow them to formulate appropriate and corresponding remedies proposals. It is then for the parties to the concentration to put forward commitments.149 The Commission only has power to accept commitments that are deemed capable of rendering the concentration compatible with the internal market so that they will prevent a significant impediment of effective competition in all relevant markets where competition concerns were identified.150 To this end, the commitments have to eliminate the competition concerns entirely and have to be comprehensive and effective from all points of view.151
(262) In assessing whether the proposed commitments will likely eliminate the competition concerns identified, the Commission considers all relevant factors including inter alia the type, scale and scope of the proposed commitments, judged by reference to the structure and particular characteristics of the market in which the competition concerns arise, including the position of the Notifying Party and other participants on the market.152
(263) A divested business has to include all the assets which contribute to its current operation or which are necessary to ensure its viability and competitiveness and all personnel which is currently employed or which is necessary to ensure the business' viability and competitiveness.153
(264) Commitments in Phase I can only be accepted where the competition concerns are readily identifiable and can be easily remedied. The remedies need to be so clear-cut that it is not necessary to enter into an in-depth investigation as to whether they are sufficient to rule out 'serious doubts' within the meaning of Article 6(1)(c) of the Merger Regulation.154
(265) As concerns the form of acceptable commitments, the Merger Regulation leaves discretion to the Commission as long as the commitments meet the requisite standard.155 In general, structural commitments are the best way to eliminate competition concerns resulting from horizontal overlaps. Structural commitments will meet the conditions set out above only in so far as the Commission is able to conclude with the requisite degree of certainty that it will be possible to implement them and that it will be likely that the new commercial structures resulting from them will be sufficiently workable and lasting to ensure that effective competition will be maintained.156
(266) In this regard divested activities must consist of a viable business that, if operated by a suitable purchaser, can compete effectively with the merged entity on a lasting basis and that is divested as a going concern.157 Normally, a viable business is a business that can operate on a stand-alone-basis, which means independently of the merging parties as regards the supply of input materials or other forms of cooperation other than during a transitory period.158 The Commission has a clear preference for an existing stand-alone business. A divestiture consisting of a combination of certain assets which did not form a uniform and viable business in the past creates risks as to the viability and competitiveness of the resulting business. In such circumstances, the package must be sufficient to allow the Commission to conclude that the resulting business will be immediately viable in the hands of a suitable purchaser.159
(267) In addition, in order for the commitments to be effective, commitments must be capable of being implemented effectively within a short period of time as the conditions of competition will not be maintained until the commitments have been fulfilled.160 The requisite degree of certainty concerning the implementation of the proposed commitments may in particular be affected by risks in relation to the transfer of a business to be divested.161These risks are generally higher in cases when commitments concern the transfer of production processes and technologies.
(268) It is against this background that the Commission assessed the viability, the workability, the effectiveness and the ability of the proposed commitments to entirely eliminate the competition concerns identified.
V.2. Procedure
(269) To remedy the serious doubts identified following the phase 1 market investigation, on 6 July 2016 the Notifying Party proposed a first set of commitments ("Initial Commitments"). The Initial commitments were market tested by the Commission on 8 July 2016 (“Initial market test”).
(270) The results of the initial market test were negative in that they provided indications that the commitments may not be comprehensive and effective in practice, as the implementation of the Initial commitments was deemed to be highly complex, long in duration and raised a number of risks which were not properly mitigated in the Initial commitments.
(271) The Commission informed the Parties of the outcome of the market test during a state of play meeting on 20 July 2016. On 22 July 2016, the Notifying Party withdrew the notification.
(272) The transaction was renotified on 19 September 2016 and a new set of commitments addressing issues identified during the Initial market test were submitted on 17 October 2016. Market test (“Second market test”) was launched on 18 October 2016. Following the results of the Second market test the text of the commitments was subsequently amended and finally filed on 7 November 2016 (the "Final Commitments").
V.3. Description of the Initial Commitments
(273) The Initial Commitments consisted in a divestiture of a number of Merial's animal health vaccines on a global basis (Vaccines Divestment Business)162 and some of Merial' NSAIDs on an EEA basis (NSAIDs Divestment Business).
V.3.1. Vaccines Divestment Business
(274) The Initial Commitments consisted in a divestiture of a number of Merial's animal health vaccines on a global basis. More specifically, it comprised the following swine vaccines: Circovac,163 Progressis, [OTHER PRODUCTS] and one ruminant vaccine: Mucossifa (the Divested Vaccines).
(275) The divestiture consisted in an upstream and downstream production technology transfer for all these products.
V.3.1.1. Introduction - Vaccine manufacturing processes
(276) The vaccine manufacturing process is composed of two steps: upstream manufacturing and downstream manufacturing.
V.3.1.1.a. Upstream manufacturing
(277) Upstream manufacturing process consists of the production of antigens, on the basis of a so called master seed. All antigen production technologies follow a similar process. The basic principle is to multiply the relevant – inactivated or live – virus or (inactivated) bacteria in growth media using specific equipment. The main difference between growing viruses and bacteria is that viruses require living cells for growth, whereas bacteria can grow by themselves. Media are required to provide the required nutrients for the growth of the bacteria, virus or cell. The required media vary depending on the organism in question.
(278) There are four major antigen production technologies: fermentors (for bacterial antigen production), bioreactors (for viral production), monolayer technology, and ovoculture. Bioreactors, monolayer technology and ovoculture represent different stages in the evolution of viral antigen manufacturing technology, with bioreactors being the most recent one.
(279) In terms of equipment, fermentors and bioreactors are similar stainless steel tanks that differ primarily in the stirring mechanism and how air is supplied to the culture.
(280) The antigen storage process varies depending on whether it is an inactivated or live vaccine. Live antigens are harvested and stored frozen to retain viability. Viruses or bacteria used for inactivated vaccines, on the other hand, are typically treated with chemicals that prevent further growth but retain the structure of the organism. After inactivation, these antigens can be stored at refrigerator temperatures. Further processing may remove water or further purify the antigens from the culture. The equipment used for downstream processing of viruses and bacteria (conventional or recombinant) is the same in most cases.
V.3.1.1.b. Downstream manufacturing
(281) The mixing process (formulation) for live and inactivated vaccines differs only in the additives used. Live vaccines require specific chemical additives that help keep the organism alive. Inactivated vaccines are mixed with diluents (e.g. water) and adjuvants to formulate the final vaccine. The mixing process for a monovalent versus multivalent vaccine only differs in the number of antigens that are put into the tank.
(282) Once formulation is completed, filling and finishing takes place; the liquids are filled into bottles or vials. Most live vaccines are freeze-dried (whereby the water content is removed under vacuum in a freezer).
(283) The unlabelled bottles or vials (intermediate finished products) are then labelled and final packaging takes place.
(284) By way of example the picture below provides an overview of the manufacturing process for Merial's vaccine Circovac.[PICTURE SHOWING CIRCOVAC PRODUCTION PROCESS FROM MERIAL INTERNAL DOCUMENT].
V.3.1.2. Merial's production capabilities for the Divested Vaccines
(285) Merial currently manufactures its PCV2, PRRS, BVD, and PPV vaccines at its Lyon Porte-des-Alpes ("LPA") facility, in France.
(286) LPA is Merial's […] biological production site globally, producing […] different live or killed vaccines. Merial's PCV2, PRRS, BVD, and PPV vaccines account for less than [CONFIDENTIAL INFORMATION ON LIMITED PROPORTION OF LPA’S FINISHED PRODUCT PRODUCTION CAPACITY REPRESENTED BY THE DIVESTED VACCINES] of finished products [CONFIDENTIAL INFORMATION ON LIMITED PROPORTION OF LPA’S FINISHED PRODUCT PRODUCTION CAPACITY REPRESENTED BY THE DIVESTED VACCINES] and less than [CONFIDENTIAL INFORMATION ON LIMITED PROPORTION OF LPA’S ANTIGEN PRODUCTION CAPACITY REPRESENTED BY THE DIVESTED VACCINES] of antigen [CONFIDENTIAL INFORMATION ON LIMITED PROPORTION OF LPA’S ANTIGEN PRODUCTION CAPACITY REPRESENTED BY THE DIVESTED VACCINES] manufactured at LPA.164
(287) In 2015, the revenues generated by all the products manufactured at LPA represented […]% of Merial's total EEA revenue and […]% of Merial's EEA vaccines revenue.165
V.3.1.3. Description of the Vaccines Divestment Business
(288) The Initial Commitments provided that the production technology transfers were to be effected either (i) to the Purchaser's own facility or (ii) to BI's manufacturing plant in […] which the Purchaser could acquire. As part of the latter option the Notifying Party committed to build the necessary manufacturing equipment (bioreactors) to produce the vaccines which […] currently does not have (except for monolayer technology used to produce Mucossifa and Parvovax).
(289) In addition, the Notifying Party committed to divest among other assets all IP rights, know-how, brands and customer information in relation to these vaccines. The divestment package also comprised transitional supply agreements under the supervision of a monitoring trustee and an industry expert.
(290) Pursuant to these commitments, the Vaccine Divestment Business should be sold to a single purchaser.
V.3.2. NSAIDs Divestment Business
(291) The Initial Commitments consisted in a divestiture of a number of Merial's animal health pharmaceuticals on an EEA basis. More specifically, it is composed of the following Merial's NSAIDs for multi-species; Ketofen, Wellicox, Allevinix, Genixine, Equioxx Injectable and the following oral NSAIDs for horses; Equioxx Paste, [OTHER PRODUCT] (NSAIDs Divested Products).
(292) The Initial Commitments consisted in a production transfer of the manufacturing process to the Purchaser's own plant or to a third party contract manufacturer. The divestment package also comprised transitional supply agreements under the supervision of a monitoring trustee and an industry expert.
(293) The NSAIDs divestment business should also be sold to a single purchaser, which could potentially be the same as the purchaser of the vaccine divestment business.
V.4.Results of the Initial market test
V.4.1. Vaccines Divestment Business
(294) The results of the Initial market test provided indications that transferring vaccine production technology is generally extremely complex and may fail in some cases, unless all risk mitigating factors are put in place, including the assurance of a suitable Purchaser being able to effectuate the transfer. In addition, the Initial market test provided indications the proposed technology transfer may not be effective in practice in that it would generate in a short period of time a viable competitor in the markets concerned as the transfer was deemed to be long in duration and raised a number of risks.
(295) Specifically, the initial market test raised issues that the antigen production transfer of the divested vaccines may not be feasible as the manufacturing processes may not be reproducible in a new manufacturing environment which highly depends on their consistency, stability and robustness.166 While one market participant indicated that to determine the feasibility of the transfer, information should be included on "stability test data as well as continued testing of stability batches for the product […] lab to lab consistency for confirmation testing of the quality attributes […] validation data for processes and analytical methods"167, another stated that "the consistency and robustness of the production technology available is crucial […] The lower such consistency/robustness, the higher the technical risks along the transfer process".16 The upstream production transfer will depend in particular on the "compatibility with other antigens and processes currently in place"169 at the receiving manufacturing site and more importantly on the equipment specifications, low yields impacting the costs of goods and products stability.170
(296) Second, the Initial market test revealed that upstream technology lengthy processes and most take up to 7-8 years (assuming it is successful). In this context a past example was referred to.
(297) Generally, the Initial market test provided indications that the success of such transfer highly depends on depends on the equipment of the receiving site meeting all specifications, all components, raw materials and packaging being already available and experienced team on both ends being fully dedicated to the project.171
(298) In this case, however, the market test indicated that it is unlikely that manufacturers would have the available bioreactors manufacturing capacity for the antigen production of the divested vaccines.172 In addition, the […] plant which the Notifying Party committed to sell, at the option of the purchaser, does not have the required bioreactor capacity. The market test also indicated that the time to build such manufacturing capability would be 2 to 3 years, which could be implemented in parallel to the downstream manufacturing process but would prevent the purchaser to start upstream transfer steps (qualification, stability batches, training of personnel etc.).173
(299) Therefore, the duration of the upstream production transfer, as of equipment being in place, would depend on multiple factors, including the complexity of the antigens and processes transferred, the culture periods needed and the time required to obtain the regulatory approvals on variations.174 In this regard market participants also identified risks of equipment validation failures, contamination and low yields, all having implications (amongst others) for the duration of the transfer.175
(300) As a result, some market participants raised that the transfer would have been lengthy: "based on current experience a minimum 5 years is realistic"; " Whole process (manufacturing / STA studies / regulatory) transfer minimum would take a minimum of 5 years and then with more vaccines it will take significantly more than 5 years.176
(301) In this context the Initial market test indicated that it is of crucial importance for the very success of the transfer that a suitable Purchaser be found. Specifically, such suitable purchaser would need to have expertise in bioreactor technology on site, successful track record in vaccines technology transfers, access to all relevant raw materials, R&D capabilities, a distribution network as well as experience with regulatory authorities in order to prevent delayed authorizations, product recalls, and supply interruptions.177
(302) In addition, respondents indicated that for successful commercialisation of Merial's Circovac it is important to already have a portfolio of swine vaccines,178 in particular a MHyo vaccine.179 This is in line with the market investigation which indicated that in zones where M.hyo is current, customers tend to purchase monovalent PCV2 and M.hyo vaccines from the same supplier, for convenience and pricing reasons.180 For PPV, the purchaser would need to also have a vaccine against Erysipelas, otherwise it would be a handicap.181 This is in line with the market investigation which showed that the vast majority of the revenue for vaccines against PPV is in multivalent offerings, the suppliers (Merial, Zoetis and MSD) have both monovalent and multivalent vaccines [CONFIDENTIAL INFORMATION ON R&D]..
V.4.2. NSAIDs Divestment Business
(303) No substantiated concerns were expressed as to the effectiveness of the production transfer as regards the NSAIDs Divestment Business.
(304) As to the assets included in the Commitments, some market respondents indicated that the know-how, data and regulatory documentations that will be transferred, should include manufacturing documentation, historical data trends for key process variables, equipment specifications, process change control documentation, analytical method documentation and raw data, as well as samples of product, reagents, key raw materials and excipients, packaging components and artwork.182 Many market respondents also indicated that Merial's patent rights for firocoxib should be included.183
(305) As to the Purchaser criteria, several customers indicated that a suitable purchaser would be any well-resourced and reliable pharma company with a local presence and an active commercial team.184 Customers also emphasised the need for the purchaser to already have a portfolio of animal NSAIDs or to have experience with them.185
V.4.3. Conclusion on the results of the Initial market test
(306) In light of the above, the Commission concluded that the Initial Commitments were not sufficiently clear-cut to eliminate the Commission's serious doubts with respect to PCV2, PRRS, PPV and BVD vaccines markets.
V.5. Additional fact finding
(307) Following the market test, the Commission conducted additional investigation to ascertain whether the technology production transfer is in principle feasible in relation to the vaccines included in the Commitments and what risk mitigating measures can be put in place to ensure that the Commitments will be effective in practice
(308) Specifically, the Commission gathered data on the manufacturing processes of the Divested Vaccines in their existing manufacturing site, and more specifically status and stability reports, Cpk (measure of process capability) reports, control charts and statistics on batches rejection.
(309) The analysis of such data (aided by independent experts) showed no remarkable out of scope results and indicated a compliant and consistent manufacturing process with minimal rejection of batches for all Divested Vaccines. While, no conclusion could be made on the specific difficulties that each of the products may pose during transfer, the analysis of the data did not reveal any specific risk factors which would in principle exclude the successful transfer. Indeed, while bioreactor technology (or emulsion for injection) is one of most challenging for vaccine production, all products concerned are of conventional technology that exists for many years in the field.
(310) The additional investigation also emphasised that experience and expertise of the purchaser and the receiving site in bioreactor technology is crucial for the success of the production transfer. 186 One company explained in particular that in the early stages of the transfer, in the receiving site for Merial's PCV2 vaccine Circovac, there should be skilled technical bioreactor operators and bioengineers to develop the vaccine as well as project leaders.187
(311) Furthermore, market respondents indicated that while location of receiving site outside the EEA was not prohibitive in principle it would involve some complexities in relation to regulatory approvals and would generally not put the Purchaser in the same position as Merial is prior to the transfer.188 Specifically, testing of the products bound for the EEA would be carried out in the US and there would be re-testing once the products arrive in the EEA. If discrepancies are found between these two series of tests additional information will be required by the authorities. Addressing this situation would require a good working relationship and support between the sending site and receiving site in the EEA, in order to avoid a loss of efficiency as compared to the original process.189
(312) Finally, market respondents also indicated that an effective transfer of the emulsion and reagents are critical steps to ensure the source of identical starting materials for the transferred vaccines. The success of the transfer would depend on the availability of reagents and other biological material190.
V.6. Description of the Final Commitments
(313) Following the results of the Initial market test and the additional investigation, the Final Commitments include improved obligations (in terms of personnel, support and access to reagents) and most importantlyidentify Ceva Santé Animale (Ceva) as the Purchaser fulfilling the criteria stemming from the Initial market test.
(314) More specifically, the Final Commitments include the following modifications.1.Vaccines i. AssetsAs for the marketed vaccines:· Merial's Parvoruvax (for PPV and erysipelas), in order to ensure the viability and competitiveness of the PPV part of the commitments,· Related recipes for the testing media and reagents,· The manufacturing know how now includes, but is not limited to the manufacturing of any reagent and adjuvant191 of the Vaccine Divestment Products,· The know-how required for or associated with obtaining and/or maintaining the related manufacturing and marketing approvals now includes stability/reproducibility data and periodic safety reports),· the obligation to provide any support to ensure an effective Production Transfer has been specified to last until six months after Ceva has successfully produced three validation batches of the relevant product in its production unit.As for the [OTHER PRODUCT]:· A best efforts obligation to transfer any contract or relationships with third parties concerning services related to […] Divestment Business· The recipes for the testing media and reagents that used are for the […] Divestment Business and relevant documentation required to carry out the relevant quality control tests ii. Transitional Agreements and Support· Supply of the reagents necessary for the manufacture and/or testing of any Vaccine Divestment Product for the duration of the TSA agreements. If Ceva is not able to source such reagents: back-to-back supply agreements with reagent suppliers for such period as required by Ceva to establish the Vaccine Divestment Businesses as viable and independent businesses, but not exceeding the duration of the TSA.· An obligation to provide any support to ensure an effective Production Transfer until six months after Ceva has successfully produced three validation batches of the relevant product in its production unit. The production transfer support will be provided by a team of expert employees of Merial.2. NSAID· The manufacturing know how, know-how required for or associated with obtaining and/or maintaining the related manufacturing and marketing approvals now includes stability/reproducibility data and periodic safety reports.
V.7. Assessment of the Final Commitments
(315) On 18 October 2016, the Commission launched the Second market test on the new set of commitments addressing the issues identified in the Initial market test, and specifying Ceva as the Purchaser.192
(316) Overall, the results of the market test were positive both as concerns the scope of the commitments and identity of the Purchaser.
(317) The commitments cover all product markets identified in paragraph (257) for which the Commission raised serious doubts as to the compatibility of the Transaction with the internal market.
(318) Concerning the Vaccines Divestment Business, a large majority of respondents to the market test stated that the assets which have been added under the Final Commitments are sufficient for a successful production transfer.193 There were no substantiated statements that other assets should be added.
(319) A large majority of respondents indicated that the commitments provide sufficient safeguards to ensure that all necessary steps will be undertaken to ensure a successful transitional supply of the final and intermediate products.194
(320) As regards quality control testing materials, a large majority of respondents indicated that the transferred media and reagents now included are comprehensive and will ensure that Ceva will be in a position to manufacture Divested Vaccines products on a sustainable basis in the same manner as Merial did before the transaction.195 There were no substantiated statements that additional safeguards should be included.
(321) As for the duration of the support obligation, in general respondents confirmed it should last until six months after Ceva has successfully produced three validation batches of the relevant product in its production unit. However one respondent indicated it should end following "satisfactory results after 3 consecutive manufacturing batches".196
(322) As regards Ceva's suitability as the purchaser of the Vaccine Divestment businesses, a large majority of respondents indicated they believe that with Ceva this business will continue to be viable and that Ceva will preserve the business' position in the market post-divestment.197
(323) Ceva's main strengths were identified as having an existing vaccine business with a strong reputation including a marketing and sales organization, commercial aggressiveness and R&D capabilities.198 One respondent stated that "Ceva is fully established in the European Animal Health sector, present in most of the key markets where this transaction is more relevant to the industry and with a product portfolio in the segments where these divestments will be complementary."199
(324) Respondents also stated that Ceva's acquisition of the Vaccines Divestment Business would be an opportunity for its development. In this regard one respondent indicated that Ceva was a "well established company in the Food Producing Animal Segment. […] the acquisition of innovative vaccine products will enhance company's reputation. Ceva will become an important player in finishing pig vaccines with the introduction of Circovac, together with its M. hyopneumoniae vaccine."200 Another respondent indicated that, with the Vaccine Divestment Business, "Ceva will have the chance to be a significant player in swine bio segment."201
(325) Moreover, a few respondents indicated it was possible Ceva could develop a stronger presence on the market than Merial.202 For instance, on respondent stated that Ceva "[...] will be stronger than Merial because they can complete their portfolio with several products not present in the hands of Merial."203
(326) With regard to the NSAIDs Divestment business, a large majority of respondents to the market test stated that the assets which have been added under the Final Commitments are sufficient for a successful production transfer.204 There were no substantiated statements that other assets should be added.
(327) As regards Ceva's suitability as the purchaser of the NSAIDs Divestment businesses, a large majority of respondents indicated they believe that with Ceva this business will continue to be viable and that Ceva will preserve the business' position in the market post-divestment.205 In this regard a respondent described Ceva as having an "established commercial presence and footprint in key European markets (sales force, marketing, technical services). Proven track record in integrating and growing acquired businesses/assets."206 Another respondent stated that Ceva's strenghts include"aggressive Marketing strategies consisting of providing any product at the lowest possible purchase Price to customers."207
V.8.Assessment of Ceva as a suitable purchaser
(328) The Notifying Party and Ceva concluded a binding put option on 16 September 2016, which was amended on 5 November 2016 to reflect the Final Commitments, pursuant to which Ceva undertakes to purchase the Divestment Businesses pursuant to an Asset Purchase Agreement and its exhibits (the Proposed Agreement).208
(329) Ceva is a global veterinary health company headquartered in Libourne, France which focuses on pharmaceuticals and vaccines for companion animals, livestock, swine and poultry.
V.8.1. Ceva is independent of an unconnected to BI
(330) Ceva is not structurally connected to BI in terms of direct or indirect ownership interests or board presence. Neither Ceva nor its affiliates or subsidiaries have any shares or direct or indirect interest in BI.
(331) As is customary in the pharmaceutical industry, BI and Ceva are part to a number of license and other types of customary commercial agreements. These agreements concern an insignificant number of products as compared to Ceva's overall portfolio of products and [INFORMATION ON BI AND CEVA'S COMMERCIAL ARRANGEMENTS]. As a result, Ceva is not economically dependent on BI.
(332) Based on the information provided, the Commission considers Ceva to be independent of and unconnected to BI, both from a legal and economic perspective.
V.8.2. Ceva has financial resources, proven relevant expertise and the incentive to be a viable and active competitor
V.8.2.1.Ceva has the financial resources to acquire the Divestment Businesses
(333) Ceva has shown a strong and consistent financial performance over the last decade. Ceva reported revenues of EUR 856.4 million in 2015. This represents an increase of 11.9% compared to 2014. In terms of profitability, the EBITDA margin of Ceva decreased in the last three years, from 18.6% in 2013, to 17.5% in 2015.209
(334) In terms of Ceva's capacity to finance the transaction, Ceva has secured the debt and cash requirements to finance the deal of EUR […] million.
(335) Ceva has a strong acquisition track record, with 30 acquisitions since 2000, such as the acquisition of Sogeval in 2013. Although the acquisition of the Divestment Businesses is one of the larger ones realised by Ceva, it only represents approximately […]% of its existing business which represents a health ratio.
(336) In addition, based on comparison of Ceva's key financials over the last three years with the relevant metrics of other pharmaceutical companies focused on animal health, Ceva’s indebtedness in terms of the Net Debt/EBITDA ratio appears below industry median and average.
(337) In view of the above, the Commission considers that Ceva has the financial capability to acquire the Divestment Businesses.
V.8.2.2. Ceva is a recognized animal health supplier with a complementary swine vaccine business
(338) Ceva is an independent company active in the animal health sector since 1999. Ceva focuses on research, development, production and marketing of pharmaceuticals and vaccines for poultry, swine, ruminants, horses and companion animals and has expertise centers in both pharmacology and biology.
(339) As to the swine vaccines, at global level, Ceva markets a number of vaccines. Ceva's portfolio is composed of Hyogen (against enzootic pneumonia, MHyo), Coglapix (against porcine pleuro pneumonia), Coglapest (against classical swine fever), Auphyl Plus (against aujesky disease) and Coglamune (against clostridial enteric disease).210 In the EEA, Ceva is supplying Hyogen, Coglapix and Coglamune. Amongst these vaccines, Hyogen is particularly important as M.Hyo vaccines and PCV2 vaccines are often administered and thus sold together.211 As a result, Ceva has in its portfolio a key complementary product which will allow it to market both products together.
(340) In addition, besides vaccines, Ceva is currently active in sow reproduction management with a portfolio of products, consisting of Altresyn, Fertipig, Enzaprost and in certain EEA countries also Alphabedyl.212
(341) Finally, Ceva is also active in R&D in this space [CONFIDENTIAL INFORMATION ON CEVA'S R&D].
(342) Based on the above, Ceva has an existing customer base and customer recognition in the swine industry which will allow it to access the market with divested products without the hurdle of establishing itself in the market.
V.8.2.3. Ceva has adequate manufacturing and regulatory capability to successfully implement the technology transfer in relation to Divestment Businesses
(343) As part of the Transaction, Ceva will not acquire any manufacturing assets from BI. Ceva will carry out the production technology transfer for the Vaccines Divestment Business to its Ceva-Phylaxia Campus in Hungary. The production of the NSAID Divestment Business’ products will be transferred to Libourne (France) for injectables and to Laval (France) or Loudeac (France) for tablets.
(344) In the past, Ceva has successfully transferred the production of multiple products internally, both animal vaccines and pharmaceuticals. By way of examples, [CONFIDENTIAL INFORMATION ON CEVA'S TECHNOLOGY TRANSFERS].
(345) In addition, Ceva has bioreactor expertise and know-how, including in the facility where the Vaccine Divestment Businesses would be transferred. [CONFIDENTIAL INFORMATION ON CEVA'S DEVELOPMENT PLANS].213
(346) Ceva has more than […] experienced staff fully dedicated to the industrial transfers and the process improvement on site. Ceva has gradually built up its bioreactor expertise during the last […] years, in particular by external hiring of experienced personnel and internal training in bioreactor technology. Therefore, Ceva already has trained and experienced personnel familiar with the bioreactor technology, and will have additional dedicated personnel […].214
(347) In addition, Ceva has strong experience in dealing with regulatory authorities for the commercialisation of animal health products and vaccines across the EEA and expertise in the required GMP certifications, quality assurance and pharmacovigilance.
(348) As regards the timeline for the production transfer, the fact that the project is already ongoing would allow Ceva to complete the transfer, i.e. obtain the approval for both antigen and finished dose product, within a shorter period of time than in a scenario where the equipment would yet have to be ordered.215
(349) Based on the above, Ceva has adequate manufacturing capability, in particular as to bioreactor technology in the receiving site, as well as regulatory expertise to successfully implement the technology transfers in a reasonable timeframe.
V.8.2.4. Ceva has adequate R&D capabilities to successfully develop the Divested Products
(350) Ceva has invested heavily in R&D and has growing R&D spends from EUR 59.6 million in 2013 to EUR 77.8 million in 2015.216 Ceva has 13 R&D sites around the world, of which 6 R&D sites are located in Europe, some of which are specialised in biologics R&D.
(351) Even absent these Commitments Ceva has been developing [CONFIDENTIAL INFORMATION ON CEVA'S R&D].
(352) Based on the above, Ceva has adequate R&D capabilities to develop the Divested Products.
V.8.2.5. Ceva has adequate distribution capabilities to supply the products in all EEA countries where Merial is currently present
(353) For all countries in which the Divestment Businesses generate revenues, Ceva either has an own distribution network or works with an external distributor. More specifically in relation to swine vaccines, Ceva is already present in all the swine production countries worldwide with dedicated swine teams.
(354) In addition, Ceva's current personnel will be reinforced in the EEA with 23 additional sales personnel from the Divestment Business located in five EEA countries.
(355) Based on the above, Ceva will be able to replicate Merial's current distribution and sales network and supply the Divested Products at least in all countries where Merial is currently active.
V.8.2.6. Ceva has strong incentives to develop the business
(356) The Commission has reviewed an overview of revenue and gross margin actuals and projections for all products in the Divestment Businesses covering the period FY 2013-2022. The Commission compared Ceva's projections to BI's revenue and gross margins projections for the period FY 2016-2018. Ceva's projections show healthy gross margins, which are generally in line with BI's own estimates217 and industry practise.
(357) The Commission also reviewed a complete Business Plan of Ceva for the Divestment Businesses starting in 2017. The Commission analysed in particular Ceva's projections in terms of operating expenses (selling expenses, distribution expenses, general and administrative expenses and R&D expenses), which take into account the one-off advertising and marketing expenses in 2017 following the acquisition, and capital expenditures, in particular in 2017 and 2018 to complete ramping up the bioreactor capacity for the Vaccines Divestment Business. Four alternative business cases were also analysed. Even in the more pessimistic scenarios, assuming a 20% volume and price decrease compared to Ceva's base case, the EBITDA margin of the Divestment Businesses is expected to remain at approximately [0-10]%.
(358) Therefore, Ceva's business plan for the Divestment Businesses is realistic and ensures continuous viability of the Divestment Businesses in the long run.
(359) In addition, Ceva demonstrated its commitment to develop the Divested Businesses which fully fit into its overall business development strategy in Europe existing prior to this acquisition.
(360) Based on the above analysis, the Commission concludes that Ceva has the incentives to develop the Divestment Business and run it in a viable and competitive manner in the long term.
(361) Based on the above and the evidence available, the Commission considers that Ceva possesses the financial resources, proven relevant expertise and has the incentive and ability to be a viable and active competitive force in the market in competition with the Parties and other competitors in all markets where the Commission identified serious doubts as to the compatibility of the Transaction with the internal market.
V.8.3.Ceva is unlikely to create prima facie competitive concerns
(362) As to the Vaccines Divestment Business, the only potential overlap relates to [CONFIDENTIAL INFORMATION ON CEVA'S ACTIVITIES].
(363) However, the acquisition by Ceva of Circovac is not likely to create prima facie competition concerns since:(i) [CONFIDENTIAL INFORMATION ON CEVA'S ACTIVITIES], there are already two competitors, namely [COMPETITOR NAMES], active on the market with larger market share than Merial.(ii) More importantly, [CONFIDENTIAL INFORMATION ON CEVA'S ACTIVITIES].218 [CONFIDENTIAL INFORMATION ON CEVA'S ACTIVITIES].219
(364) As to the NSAIDs Divestment Businesses, the only potential overlap relates to multispecies injectable NSAIDs. However, the acquisition by Ceva of Merial's injectable NSAIDs is not likely to create any prima facie competition concerns since:(i) Ceva's market position in multispecies injectable NSAIDs is rather limited. At EEA level, Ceva is ranked number 6 supplier, with a market share below [5- 10]%.(ii) Ceva supplies only generic products, while, as explained above, generic penetration in NSAIDs markets is rather low. As indicated above, the market investigation indicated that Ceva's products are perceived as having weaker brand recognition.(iii) BI, which currently leads the market, will remain Ceva's strongest competitor.
(365) Furthermore, the Proposed Agreement includes the sale of Merial's oral NSAID Ketofen. Although the Commission has not identified competition concerns on the market for oral NSAIDs for pets, Merial’s Ketofen oral pet NSAIDs were included in the business which is being divested to Ceva, along with the other Ketofen branded products (multi-species injectable NSAIDs), to avoid that complications arise as a result of a split of the Ketofen brand.
(366) The acquisition by Ceva of Merial's Ketofen oral pet NSAIDs gives rise to a limited overlap:(i) Ceva's market position in oral NSAIDs for pets is rather limited. At EEA level, Ceva is ranked number 6, with a market share below [5-10]%. Ceva supplies only generic products, while, as explained above, generic penetration in NSAIDs markets is generally low.(ii) BI and Zoetis, which currently lead the market, will remain Ceva's strongest competitors. As indicated above, the market investigation indicated that Ceva's products are perceived as being close to BI's. In addition, post-Transaction BI will supply the other oral NSAIDs for pets of Merial and in particular the COXIB Previcox, which currently generates more sales than Ketofen in the EEA.
(367) Finally, as to regulatory approvals, the only condition upon closing is the clearance from the Brazilian competition authority (CADE).
(368) In view of the above, the Commission considers the acquisition by Ceva of the Divestment Businesses pursuant to the Proposed Agreement is not likely to create any prima facie competition concerns nor give rise to a risk that the implementation of the Commitments will be delayed.
V.8.4. Conclusion
(369) In view of the above, the Commission considers that Ceva is a suitable Purchaser of the Divestment Business as specified in the Final Commitments.
V.9.Overall Conclusion
(370) In light of the above and in the very specific circumstances of this case, the Commission considers the Final Commitments capable of rendering the Transaction compatible with the internal market as it will prevent a significant impediment to effective competition in all relevant markets in which competition concerns were identified.
(371) Moreover, the Commission considers that the Proposed Agreement signed between BI and Ceva is in line with the Final Commitments and that Ceva is a suitable purchaser pursuant to the Final Commitments.220
V.10.Conditions and Obligations
(372) Pursuant to the second subparagraph of Articles 8(2) and 10(2) of the Merger Regulation, the Commission may attach to its decision conditions and obligations intended to ensure that the undertakings concerned comply with the commitments they have entered into vis-à-vis the Commission with a view to rendering the concentration compatible with the internal market.
(373) The fulfilment of the measure that gives rise to the structural change of the market is a condition, whereas the implementing steps which are necessary to achieve this result are generally obligations on the parties. Where a condition is not fulfilled, the Commission’s decision declaring the concentration compatible with the internal market is no longer applicable. Where the undertakings concerned commit a breach of an obligation, the Commission may revoke the clearance decision in accordance with Article 8(6) of the Merger Regulation. The undertakings concerned may also be subject to fines and periodic penalty payments under Articles 14(2) and 15(1) of the Merger Regulation.
(374) In accordance with the basic distinction as regards conditions and obligations, this Decision should be made conditional on full compliance by the Parties with of the Final Commitments and its Schedules and Sections B to C should be obligations within the meaning of Article 8(2) of the Merger Regulation. The other commitments set out in the Annex constitute obligations, as they concern the implementing steps which are necessary to achieve the modifications sought in a manner compatible with the internal market. The full text of the Final Commitments and its Schedules is attached as Annex to this Decision and forms an integral part thereof.
VI.CONCLUSION
(375) For the above reasons, the Commission has decided not to oppose the notified operation as modified by the commitments and to declare it compatible with the internal market and with the functioning of the EEA Agreement, subject to full compliance with the conditions in sections Sections B and C (including Schedules 1 and 2) of the commitments annexed to the present decision and with the obligations contained in the other sections of the said commitments. This decision is adopted in application of Article 6(1)(b) in conjunction with Article 6(2) of the Merger Regulation and Article 57 of the EEA Agreement.
(376) On the basis of the above assessment, the Commission approves Ceva Santé Animale (Ceva) as a suitable purchaser. On the basis of the Proposed Agreement, the Commission further concludes that the Divestment Business is being sold in a manner consistent with the Commitments.
(377) This decision only constitutes approval of the proposed purchaser identified herein and of the Proposed Agreement. This decision does not constitute a confirmation that BI has complied with its Commitments. 7 November 2016
1 OJ L 24, 29.1.2004, p. 1 (the 'Merger Regulation'). With effect from 1 December 2009, the Treaty on the Functioning of the European Union ('TFEU') has introduced certain changes, such as the replacement of 'Community' by 'Union' and 'common market' by 'internal market'. The terminology of the TFEU will be used throughout this decision.
2 OJ L 1, 3.1.1994, p. 3 (the 'EEA Agreement').
3 Publication in the Official Journal of the European Union No C349, 24.09.2016, p. 6.
4 See case M.7919 – Sanofi / Boehringer Ingelheim Consumer Healthcare Business.
5 Turnover calculated in accordance with Article 5(1) of the Merger Regulation and the Commission Consolidated Jurisdictional Notice (OJ C95, 16.4.2008, p. 1).
6 For example M.1681 - Akzo Nobel/Hoechst Roussel Vet, 22.11.1999, para 11; M.2922. Pfizer/Pharmacia, 27/02/2003, para 114; M.4691 – Schering-Plough / Organon biosciences, 11.10.2007, para 22; M.5476 – Pfizer/Wyeth, 17.07.2009, para 111.
7 The Transaction gave rise to vertical relationships derived from Merial's contract manufacturing activities in animal vaccines and pharmaceuticals. However, the Transaction does not lead to any vertically affected markets under all plausible market definitions.
8 M.7277 – Eli Lilly/Novartis Animal Health.
9 M.5843 – Eli Lilly/Certain Animal Health Assets of Pfizer.
10 M.5843 – Eli Lilly/Janssen Pharmaceutical Animal Health Business Assets.
11 Responses to Questionnaire Q1 to Competitors of 7 June 2016, questions 50 and 68.
12 Responses to Questionnaire Q1 to Competitors of 7 June 2016, questions 25-26 and 96-97.
13 Marketing authorizations can be obtained through three different procedures: (i) centralized procedure whereby the European Commission grants Community wide marketing authorization following the positive opinion of the European Medicines Agency (EMA) pursuant to Regulation (EC) No 726/2004 of 31 March 2004, (ii) decentralized procedure whereby manufacturers submit a single identical product dossier and applications simultaneously to multiple EEA Member States regulatory agencies and each agency issue its own approval and (iii) national procedure whereby manufacturers apply separately for marketing approval by individual Member State regulatory agencies, these approvals can be broaden to other Member States by subsequent mutual recognition requests.
14 Responses to Questionnaire Q2 to Swine customers of 7 June 2016, question 14.
15 Agreed minutes of a conference call held with a competitor dated 17 May 2016.
16 Responses to Questionnaires Q4 to Companion Animals customers and to Q5 to Horses customers of 8 June 2016, question 8.
17 Responses to Questionnaire Q2 to Swine customers of 7 June 2016, question 13.
18 Responses to Questionnaire Q1 to competitors of 8 June 2016, questions 11 and 77.
19 Agreed minutes of a conference call held with a customer dated 13 May 2016 and Responses to Questionnaire Q2 to Swine customers of 7 June 2016, question 13.
20 Responses to Questionnaire Q2 to Swine customers of 7 June 2016, question 9 and the minutes of a conference call held with a customer dated 11 May 2016.
21 Responses to Questionnaire Q2 to Swine customers of 7 June 2016, question 5.
22 Responses to Questionnaire Q2 to Swine customers of 7 June 2016, question 5.
23 The Notifying Party used data from Centre Européen d’Etude pour la santé animale (CEESA) a non-profit international association which collects sales data on the animal health market in 21EEA countries. It must be noted that manufacturers reports sales at différents levels, and Merial is the only manufacturer which report at level 3 which means that Merials sales in CEESA are not netted-of end of year customer specific rebates (level 4) or cash discount (level 5). In order to provide the most accurate market shares possible, Merial’s sales data into CEESA has been replaced by Merial’s actual net sales equivalent to level 5. Additionally , the CEESA data do not cover sales in all EEA countries and captures only the suppliers that report to the organisation. More local suppliers or suppliers of generics do not report into CEESA, as for example Hipra. The Notifying Party :therefore provided itsbest estimate as to Hipra’s market position in the different EEA countries where relevant.
24 M.5476-Pfizer/Wyeth 17.07.2009 and M.4691-Schering – Plough / Organon biosciences, 11.10.2007
25 The differentiation between marker and non-marker vaccines is not relevant with respect to the overlapping vaccines in this case.
26 M.1681 - Akzo Nobel/Hoechst Roussel Vet, 22.11.1999, para 46.
27 M.7277 - Eli Lilly/Novartis Animal Health, paras. 56-58, M.6205 - Eli Lilly/Janssen, para. 15 and M.4691- Schering-Plough/Organon Biosciences, paras. 42-45.
28 The term “subunit” describes the production of a single antigen using a recombinant expression technology.
29 Response to questionnaire Q2 to swine customers of 7 june 2016, question 16.
30 BI internal document, (…)
31 Response of a competitor to questionnaire Q1 to swine customers of 8 june 2016, question 30.
32 Response of a customer to questionnaire Q2 to swine customers of 7 june 2016, question 15.
33 Market share tables for Group 1 markets, Form CO, p.42-50.
34 Responses to Questionnaire Q2 to Swine customers of 7 June 2016, question 20 and responses to Questionnaire Q1 to competitors of 8 June 2016, question 33.
35 BI internal document, […].
36 BI internal document, […].
37 Agreed minutes of a conference call held with a customer dated 11 May 2016 and of a conference call held with a competitor dated 11 May 2016. See also responses to Questionnaire Q2 to Swine customers of 7 June 2016, question 18.
38 Responses to Questionnaire Q2 to Swine customers of 7 June 2016, questions 17 and 18.
39 Responses to Questionnaire Q1 to competitors of 8 June 2016, question 31 and responses to Questionnaire Q2 to Swine customers of 7 June 2016, question 18.
40 Agreed minutes of a conference call held with a customer dated 13 May 2016.
41 Response of a customer to Questionnaire Q2 to Swine customers of 7 June 2016, question 19.
42 BI internal document, […].
43 BI internal document, […].
44 Responses to Questionnaire Q2 to Swine customers of 7 June 2016, question 18.
45 Form CO, Chapter B, Table 14.
46 Responses to Questionnaire Q2 to Swine customers of 7 June 2016, question 38 and Responses to Questionnaire Q1 to competitors of 8 June 2016, question 69.
47 Agreed minutes of a conference call held with a customer dated 13 May 2016.
48 Response of a customer to Questionnaire Q2 to Swine customers of 7 June 2016, question 38.
49 M.5476 – Pfizer/Wyeth, 17.07.2009 and M.4691 – Schering-Plough / Organon biosciences, 11.10.2007.
50 Market share tables for Group 1 and Group 2 markets, Form CO, p. 90-91 and Annex A.12 to the Form CO.
51 Agreed minutes of conference calls with competitors dated 11 May 2016 and of a conference call with a competitor dated 18 May 2016.
52 Agreed minutes of conference calls with competitors dated 11 May 2016 and of a conference call with a competitor dated 18 May 2016.
53 Response of a competitor to to Questionnaire Q1 to competitors of 8 June 2016, question 37.
54 Animal health research programmes include three main phases: (i) the discovery phase, (ii) the exploratory development phase (or pre-development phase) and (iii) the full development phase. The discovery phase begins with a molecule or antigen identified as having potential therapeutic or prophylactic utility and being tested for approx.18 months. The exploratory development phase is aimed at showing proof of efficacy and safety as well as determining key elements of the end product (e.g formulation, target species, dosage etc.). This phase takes on average approx. 18 months. The full development phase can take four to five years, including regulatory review and approval. Testing is largely determined by the regulators involved and aims at proving shelf-life stability of the product, rationale and efficacy of the selected dose, and safe withdrawal periods. An environmental assessment is also mandatory.
55 Response to questionnaire Q2 to swine customers of 7 june 2016, question 24.
56 Response to questionnaire Q2 to swine customers of 7 june 2016, question 26.
57 Response to questionnaire Q2 to swine customers of 7 june 2016, question 23 and minutes of a conference call held with a competitor dated 11 may 2016 « As a killed vaccine Merial’s Progressis is perceived to be safer , in particular in sows, while the MLV vaccines have better efficacy. » See also minutes of a conference call held with a customers dated 11 may 2016 (KV vaccines) is essentially used for niche markets, in particular in PRRS free herds to boots the immunity without the risk of spreading the virus. »
58 Market share tables for Group 1 and Group 2 markets, Form CO, p.74 and Annex A.11 to the Form CO.
59 Form CO, Chapter B-Vaccines, paragraph 255.
60 Response of a competitor to Questionnaire Q1 to competitors of 8 June 2016, question 44.
61 BI internal document, […].
62 Response of a competitor to Questionnaire Q1 to competitors of 8 June 2016, question 40.
63 Form CO, Chapter B – Vaccines, paragraph 370.
64 Merial internal document, […].
65 Merial internal document, […].
66 Agreed minutes of a conference call held with a customer dated 13 May 2016.
67 Response of a customer to Questionnaire Q2 to Swine customers of 7 June 2016, question 26.
68 BI internal document, […].
69 BI internal document, […].
70 Responses to Questionnaire Q2 to Swine customers of 7 June 2016, question 9 and responses to Questionnaire Q1 to competitors of 8 June 2016, question 18.
71 Agreed minutes of a conference call held with a customer dated 13 May 2016.
72 M.1681 Akzo Nobel / Hoechst Roussel Vet, 22 November 1999. Para. 40 and following.
73 Agreed minutes conference call of 28 july with a competitor.
74 Market share tables, Form CO, p.96.
75 (Confidential information on bi activities)
76 Case M.5476 – Pfizer/Wyeth, paras 179-181.
77 Responses to Questionnaire Q3 to Ruminants customers of 8 June 2016, questions 10 and 16.
78 BI internal document (…)
79 Market share table, Form CO, p.124 and p.131 – 132.
80 Responses to Questionnaire Q3 to Ruminants customers of 8 June 2016, question 14 and responses to Questionnaire Q1 to competitors of 8 June 2016, question 64.
81 Response of a competitor to Questionnaire Q1 to competitors of 8 June 2016, question 64.
82 BI internal document, […]
83 Response of a customer to Questionnaire Q3 to Ruminants customers of 8 June 2016, question 8.
84 Response of a competitor to Questionnaire Q1 to competitors of 8 June 2016, question 54.
85 Response of a competitor to Questionnaire Q1 to competitors of 8 June 2016, question 57.
86 Responses to Questionnaire Q3 to Ruminants customers of 8 June 2016, question 16 and responses to Questionnaire Q1 to competitors of 8 June 2016, question 65.
87 Response of a competitor to Questionnaire Q1 to competitors of 8 June 2016, question 65.
88 Merial's Mucossifa is a single dose vaccine for animals aged over six months.
89 Responses to Questionnaire Q3 to Ruminants customers of 8 June 2016, question 5 and responses to Questionnaire Q1 to competitors of 8 June 2016, question 54.
90 Responses to Questionnaire Q1 to competitors of 8 June 2016, question 65.
91 BI internal document, […].
92 Responses to Questionnaire Q3 to Ruminants customers of 8 June 2016, questions 10 and 16.
93 BI's internal document, […].
94 Form CO, Chapter B – Vaccines, paragraph 313 and footnote 137.
95 See also Annex C.2 to the Form CO.
96 E.g. M4691 – Schering -Plough/Organon Biosciences perra.40 Case M.5476 – Pfizer/Wyeth, paras.121 and 122.
97 Case M.4691 - Schering-Plough/Organon Biosciences, paras. 42-45. M.7277 - Eli Lilly/Novartis Animal Health, paras. 56-58, Case M.6205 - Eli Lilly/Janssen, para. 15.
98 Case M.5476 – Pfizer/Wyeth, Paragraph 122.
99 Case M.4691 – Schering-Plough / Organon biosciences, paras. 305 and 306.
100 Responses to Questionnaire Q1 to competitors of 8 June 2016, question 79.
101 Responses to Questionnaire Q3 to Ruminants customers of 8 June 2016, question 55 and responses to Questionnaire Q1 to competitors of 8 June 2016, question 101.
102 Case M.4691, Schering-Plough/Organon Biosciences, decision of 11 October 2007, paragraph 305.
103 Case M.4691 – Schering-Plough / Organon biosciences, paragraphs 303 to 306.
104 Case M.4691 – Schering-Plough / Organon biosciences, para. 306.
105 Response of a competitor to Questionnaire Q1 to competitors of 8 June 2016, question 82.: "COXIB would be safer". See reply of a competitor to Questionnaire Q1 to competitors of 8 June 2016, question 82: "originally marketed as safer alternative but Any advantages have not borne out in the market place." See reply of a competitor to Questionnaire Q1 to competitors of 8 June 2016, question 82: "According to our opinion, the products are interchangeable. Left for individual preferences of a vet."
106 Responses to Questionnaire Q3 to Ruminants customers of 8 June 2016, question 39, responses to Questionnaire Q4 to Companion Animals customers of 8 June 2016, question 7. and responses to Questionnaire Q2 to Swine customers of 7 June 2016, question 50.
107 Market share table, Form CO, p.98 and Annexes A.11 and A.12 to the Form CO.
108 Responses to Questionnaire Q1 to competitors of 8 June 2016, question 88.
109 BI internal document, […].
110 Responses to Questionnaire Q5 to Horses customers of 8 June 2016, question 16.
111 Responses to Questionnaire Q3 to Ruminants customers of 8 June 2016, questions 36 and 37, responses to Questionnaire Q2 to Swine customers of 7 June 2016, questions 47 and 48 and responses to Questionnaire Q1 to competitors of 8 June 2016, questions 84 and 85.
112 See reply of a customer to Questionnaire Q5 to Horses customers of 8 June 2016, question 6.
113 Responses to Questionnaire Q2 to Swine customers of 7 June 2016, question 52.
114 Responses to Questionnaire Q1 to competitors, question 78.
115 Responses to Questionnaire Q2 to Swine customers, question Q66.
116 Responses to Questionnaire Q2 to Swine customers, question Q66.
117 Responses to Questionnaire Q2 to Swine customers, question Q66.
118 Market share table, Form CO, p. 66-67 and Annexes A.11 and A.12 to the Form CO.
119 Response of a customer to Questionnaire Q5 to Horses customers of 8 June 2016, question 17.
120 BI internal document, […].
121 BI internal document, […].
122 BI internal document, […].
123 Responses to Questionnaire Q1 to competitors of 8 June 2016, question 119.
124 Response of a competitor to Questionnaire Q1 to competitors of 8 June 2016, question 119.
125 Market share table, Form CO, p.74-78 and Annexes A.11 and A.12 to the Form CO.
126 Response to questionnaire Q4 to companion animals customers of 8 june 2016, question 21.
127 BI’s internal presentation (…)
128 Responses to Questionnaire Q4 to Companion Animals customers of 8 June 2016, question 21.
129 BI's internal presentation […].
130 BI's internal presentation […].
131 Responses to Questionnaire Q4 to Companion Animals customers of 8 June 2016, question 22.
132 Case M.5476 – Pfizer/Wyeth, paras. 324. Case COMP M. 4691-Schering-Plough/Organon Biosciences, paragraphs 325-346; Case COMP/M.2922-Pfizer/Pharmacia, paragraphs 122-123; Case COMP M. 1681- Akzo Nobel/Hoechst, paragraph 19.
133 M.4691 – Schering-Plough / Organon biosciences, 11.10.2007, para 341. M.2922 – Pfizer/Pharmacia, paragraphs 126-131 and 346. Case M.5476 – Pfizer/Wyeth, paras. 324 and following.
134 Responses to Questionnaire Q3 to Ruminants customers of 8 June 2016, question 55.
135 Market share tables for Group 3 markets Form CO, p.113 and Annex A.12 to the Form CO.
136 Responses to Questionnaire Q3 to Ruminants customers of 8 June 2016, questions 59 and 60.
137 Responses to Questionnaire Q3 to Ruminants customers of 8 June 2016, question 60.
138 Responses to Questionnaire Q4 to Companion Animals customers of 8 June 2016, question 34.
139 Responses to Questionnaire Q4 to Companion Animals customers of 8 June 2016, question 32.
140 Agreed minutes of a conference call held with a competitor dated 19 May 2016.
141 Market share tables, Form CO, p.119-120 and Annex c.12 to the Form CO.
142 Agreed minutes of a conference call held with a competitor dated 19 May 2016.
143 Responses to Questionnaire Q4 to Companion Animals customers of 8 June 2016, question 39.
144 M.5476 – Pfizer/Wyeth, 17.07.2009, para 123.
145 Annex c.12 to the Form CO.
146 Agreed minutes of a conference call held with a competitor dated 19 May 2016.
147 Commission notice on remedies acceptable under Council Regulation (EC) No 139/2004 and under Commission Regulation (EC) No 802/2004 (the "Remedies Notice"), OJ 2008/C 267/01.
148 See Commission notice on remedies acceptable under Council Regulation (EC) No 139/2004 and under Commission Regulation (EC) No 802/2004 (the "Remedies Notice"), OJ 2008/C 267/01, paragraph 5.
149 Remedies Notice, paragraph 6.
150 Remedies Notice, paragraph 9.
151 Remedies Notice, paragraph 9 and 61.
152 Remedies Notice, paragraph 12.
153 Remedies Notice, paragraph 25.
154 Remedies Notice, paragraph 81.
155 Case T-177/04 easyJet v Commission [2006] ECR II-1913, paragraph 197: "Article 6(2) of Regulation No 4064/89 provides that the Commission may authorise a merger if the commitments proposed by the parties dispel the serious doubts as to the compatibility of the merger with the common market. Regulation No 4064/89 thus lays down the objective to be achieved by the Commission, but leaves it a wide discretion as to the form which the commitments in question may take."
156 Remedies Notice, paragrah 10.
157 Remedies Notice, paragraph 23.
158 Remedies Notice, paragraph 32.
159 Remedies Notice, paragraph 37.
160 Remedies Notice, paragraph 9
161 Remedies Notice, paragraph 11.
162 To the exception of Circovac in the US.
163 Excluding the US.
164 Form RM, paragraph 92.
165 […]
166 Responses to Questionnaire R1 Market Test to competitors of 8 July 2016, question 1. 2.1, 14.2, 18 and 20.
167 Response of a competitor to Questionnaire R1 Market Test to competitors of 8 July 2016, question 1.
168 Response of a competitor to Questionnaire R1 Market Test to competitors of 8 July 2016, questions 20 and 28.
169 Response of a competitor to Questionnaire R1 Market Test to competitors of 8 July 2016, question 28.
170 Responses to Questionnaire R1 Market Test to competitors of 8 July 2016, question 28.
171 Responses to Questionnaire R1 Market Test to competitors of 8 July 2016, questions 1, 8 and 13.
172 Responses to Questionnaire R1 Market Test to competitors of 8 July 2016, questions 1, 2 and 3.
173 Responses to Questionnaire R1 Market Test to competitors of 8 July 2016, question 30.
174 Responses to Questionnaire R1 Market Test to competitors of 8 July 2016, question 19.
175 Responses to Questionnaire R1 Market Test to competitors of 8 July 2016, question 28.
176 Response of a competitor to Questionnaire R1 Market Test to competitors of 8 July 2016, question 19.
177 Responses to Questionnaire R1 Market Test to competitors of 8 July 2016, questions 1, 8, 15 and 32.
178 Responses to Questionnaire R1 Market Test to competitors of 8 July 2016, question 32.
179 Responses to Questionnaire R1 Market Test to competitors of 8 July 2016, questions 8 and 36.
180 Responses to Questionnaire Q2 to Swine customers of 7 June 2016, question 10.
181 Agreed minutes of the conference call with a competitor of 30 August2016. See responses to Questionnaire R1 Market Test to competitors of 8 July 2016, question 8
182 Responses to Questionnaire R1 Market Test to competitors of 8 July 2016, question 38.
183 Responses to Questionnaire R1 Market Test to competitors of 8 July 2016, question 40.
184 Responses to Questionnaire R2 Market Test to customers of 8 July 2016, question 8.
185 Response of a customer to Questionnaire R2 Market Test to customers of 8 July 2016, question 8: "[...] this only can assure , that the product will not disappear and will stay alive and available on the market." Responses to Questionnaire R2 Market Test to customers of 8 July 2016, question 8.
186 Agreed minutes of the conference call with a competitor of 30 August 2016. See also minutes of the conference call with a competitor of 29 August 2016.
187 Agreed minutes of the conference call with a competitor of 30 August 2016.
188 Agreed minutes of the conference calls with a competitor and another competitor of 29 and 30 August 2016.
189 Agreed minutes of the conference call with a competitor of 29 August 2016.
190 A reagent is a compound or mixture used to confirm the presence or absence of another substance. In this case reagents recognise the amount of antigen in the final product.
191 Inactivated vaccines are often formulated with compounds called adjuvants which enhance the immune response to the inactivated antigen.
192 On 16 September 2016 the Notifying Party and Ceva signed a binding put option, to which an asset purchase agreement (APA) and its exhibits are attached, with respect to the purchase of the Divestment Businesses.
193 Responses to Questionnaire R3 Market Test to competitors of 18 October 2016, question 1.
194 Responses to Questionnaire R3 Market Test to competitors of 18 October 2016, question 4.
195 Responses to Questionnaire R3 Market Test to competitors of 18 October 2016, question 3.
196 See reply of a competitor to Questionnaire R3 Market Test to competitors of 18 October 2016, question 5.
197 Responses to Questionnaire R4 Market Test to customers of 18 October 2016, question 9 and Responses to Questionnaire R3 Market Test to competitors of 18 October 2016, question 10.
198 Responses to Questionnaire R3 Market Test to competitors of 18 October 2016, question 11.
199 Response of a competitor to Questionnaire R3 Market Test to competitors of 18 October 2016, question 15.
200 Response of a customer to Questionnaire R4 Market Test to customers of 18 October 2016, question 4.
201 Response of a customer to Questionnaire R4 Market Test to customers of 18 October 2016, question 4.
202 Responses to Questionnaire R4 Market Test to customers of 18 October 2016, question 9.
203 Response of a customer to Questionnaire R4 Market Test to customers of 18 October 2016, question 9.
204 Responses to Questionnaire R3 Market Test to competitors of 18 October 2016, question 13.
205 Responses to Questionnaire R3 Market Test to competitors of 18 October 2016, question 10.
206 Response of a competitor to Questionnaire R3 Market Test to competitors of 18 October 2016, question 15.
207 Response of a competitor to Questionnaire R3 Market Test to competitors of 18 October 2016, question 15.
208 The binding put option entered into with an identified buyer during the Commission's procedure has similar effects as a "fix-it-fist remedy" under paragraph 50 of the Remedies Notice since Ceva (promesse d'achat) does not have any opt-out and will enter into the Asset Purchase Agreement and its exhibits attached to the put option once the option is exercised. The signature of a put option, instead of the Asset Purchase Agreement and its exhibits, is justified by the necessity to consult the Comite d'entreprise of the seller which is a mandatory requirement under French law. BI has an explicit obligation under the Commitments to sell the Divestments Businesses to Ceva, and therefore exercise the put option to the benefit of Ceva.
209 Ceva Annual Report, 2015, page 75.
210 http://www.ceva.com/Products/Swine/Vaccines
211 Minutes of the meeting with Ceva of 28 July 2016.
212 http://www.ceva.com/Products/Cattle/Reproduction-Management.
213 Agreed minutes of the meeting with Ceva of 28 July 2016.
214 Ceva's submission of 23 September 2016.
215 Agreed minutes of the meeting with Ceva of 28 July 2016.
216 Ceva Annual Report, 2015, page 75.
217 [INFORMATION ON CEVA'S PROJECTIONS]
218 Ceva's submission of 23 September 2016.
219 See paragraphs (59) above.
220 This is without prejudice of the fact that the transaction agreements ought to be interpreted in line with the commitments and in case of discrepancy the commitments take precedence.
Case M. 7917 – Boehringer Ingelheim/Merial COMMITMENTS TO THE EUROPEAN COMMISSION
Pursuant to Article 6(2) of Council Regulation (EC) No 139/2004 (the Merger Regulation), Boehringer Ingelheim (the Notifying Party or BI) and, to the extent applicable, Merial SAS (Merial) (together "the Parties") hereby enter into the following Commitments (the Commitments) vis-à-vis the European Commission (the Commission) with a view to rendering the acquisition of sole control over the animal health business of Sanofi (Merial) (the Concentration) compatible with the internal market and the functioning of the EEA Agreement.This text shall be interpreted in light of the Commission’s decision pursuant to Article 6(1)(b) of the Merger Regulation to declare the Concentration compatible with the internal market and the functioning of the EEA Agreement (the Decision), in the general framework of European Union law, in particular in light of the Merger Regulation, and by reference to the Commission Notice on remedies acceptable under Council Regulation (EC) No 139/2004 and under Commission Regulation (EC) No 802/2004 (the Remedies Notice).For the avoidance of doubt, the Schedules form an integral part of the Commitments.
Section A.Definitions
1. For the purpose of the Commitments, the following terms shall have the following meaning:
Affiliated Undertakings: undertakings controlled by the Parties and/or by the ultimate parents of the Parties, whereby the notion of control shall be interpreted pursuant to Article 3 of the Merger Regulation and in light of the Commission Consolidated Jurisdictional Notice under Council Regulation (EC) No 139/2004 on the control of concentrations between undertakings (the "Consolidated Jurisdictional Notice").
Assets: the assets that contribute to the current operation or are necessary to ensure the viability and competitiveness of the Divestment Businesses as indicated in Section B, paragraph 7 and described more in detail in the Schedules.
Best Efforts: Best effort obligations shall be interpreted in light of the Commission's decision pursuant to Article 6(1)(b) of the Merger Regulation to declare the Concentration compatible with the internal market and the functioning of the EEA Agreement, the Merger Regulation and the general principles of EU law. Any interpretation that may be given to this term under the law of other jurisdictions is not relevant solely for the purpose of interpreting and/or implementing the Commitments.
Binding Put Option: the binding put option agreement attached as Annex A entered into on 16 September 2016 whereby the Purchaser undertakes to acquire the Divestment Businesses in accordance with the terms of the Product Asset Purchase Agreement.
Boehringer Ingelheim (BI): Boehringer Ingelheim International GmbH, incorporated under the laws of Germany with its registered office at Ingelheim am Rhein, Germany and registered with the Commercial Register at the Local Court of Mainz under number HRB21063.
Closing: the transfer of the legal title to the Divestment Businesses to the Purchaser.
Closing Period: the period of [Conf] from the Effective Date, or, if the closing of the Concentration takes place after that period, the period of [Conf] from the closing of the Concentration.
Confidential Information: any business secrets, know-how, commercial information, or any other information of a proprietary nature that is not in the public domain.
Conflict of Interest: any conflict of interest that impairs the Trustee's objectivity and independence in discharging its duties under the Commitments.
Divestment Businesses: the businesses as defined in Section B and in Schedules 1 and 2 which the Notifying Party commits to divest.
Effective Date: the date of adoption of the Decision.
Gerland Antigen Supply: as defined in Schedule 1, Part D.
Hold Separate Manager: the person(s) appointed by the Notifying Party for the Divestment Businesses to manage the day-to-day business under the supervision of the Monitoring Trustee.
Key Personnel: all personnel necessary to maintain the viability and competitiveness of the Divestment Business, as listed in the Schedule, including the Hold Separate Manager(s).
Master Seed: master virus seed and master cell seed.
Merial: the animal health business of Sanofi to be acquired by BI.
Monitoring Trustee: one or more natural or legal person(s) who is/are approved by the Commission and appointed by the Notifying Party, and who has/have the duty to monitor the Notifying Party’s compliance with the conditions and obligations attached to the Decision.
Notifying Party: Boehringer Ingelheim. Where Boehringer Ingelheim cannot directly commit to the commitments described in the text below, Boehringer Ingelheim will use its Best Efforts to cause the relevant party to comply with the obligations hereby described below.
NSAIDs: non-steroidal anti-inflammatory drugs.
NSAID Divestment Business: the business as defined in Section B and Schedule 2.
NSAID Divestment Products: the NSAID products as defined in Section B and Schedule 2.
[Conf] Pipeline Product: [Conf] as described in Section B and Schedule 2.
NSAID TSA: as defined in Schedule 2.
Parties: the Notifying Party and the undertaking that is the target of the concentration.
Personnel: all staff currently employed by the Divestment Businesses, including staff seconded to the Divestment Businesses, shared personnel as well as the additional personnel listed in the Schedules.
Products Asset Purchase Agreement: the agreement for the sale and purchase of the Divestment Businesses to be executed between BI and the Purchaser in accordance with the terms of the Binding Put Option.
Production Transfer: as defined in Schedules 1 and 2 for the Vaccine Divestment Business and the NSAID Divestment Business respectively.
Production Transfer Personnel: all personnel necessary to ensure an effective production transfer of the Vaccine Divestment Businesses to a production location of the Purchaser’s choice, as described in Part D of Schedules 1 and 2.
Purchaser: Ceva Santé Animale.
Schedule(s): the schedules to these Commitments describing more in detail the Divestment Businesses.
Swine Vaccine Commercial Personnel: the Merial commercial employees in the EEA whom the Parties will allow the Purchaser to make an employment offer to under the terms and conditions described in the Products Asset Purchase Agreement.
Technical Expert: one or more natural or legal person(s), appointed by and reporting to the Monitoring Trustee, who has/have industry expertise relevant to the Divestment Businesses and will assist and advise the Monitoring Trustee with regard to all technical aspects related to the Divestment Businesses, as described in paragraph 27 below.
Trustee: the Monitoring Trustee.
TSA: Transitional Supply Agreement.
Vaccine Divestment Businesses: the vaccine businesses as defined in Section B and Schedule 1.
Vaccine Divestment Products: the vaccine products as defined in Section B and Schedule 1.
Vaccine TSA: as defined in Schedule 1.
Section B.The commitment to divest and the Divestment Businesses
Commitment to divest
2. In order to maintain effective competition in the EEA, the Notifying Party commits to divest, or procure the divestiture of the Divestment Businesses to the Purchaser.
3. The Notifying Party shall be deemed to have complied with this commitment if:(a) Pursuant to the Binding Put Option, BI sells at Closing the Divestment Businesses to the Purchaser and the Closing takes place within the Closing Period; and(b) the Production Transfers set forth in the Schedules have been completed.
4. In order to maintain the structural effect of the Commitments, the Notifying Party shall, for a period of 10 years after Closing, not acquire, whether directly or indirectly, the possibility of exercising influence (as defined in paragraph 43 of the Remedies Notice, footnote 3) over the whole or part of the Divestment Businesses, unless, following the submission of a reasoned request from the Notifying Party showing good cause and accompanied by a report from the Monitoring Trustee (as provided in paragraph 40 of these Commitments), the Commission finds that the structure of the market has changed to such an extent that the absence of influence over the Divestment Businesses is no longer necessary to render the proposed concentration compatible with the internal market.
Structure and definition of the Divestment Businesses Vaccine Divestment Businesses
5. The Vaccine Divestment Businesses consist of the rights, title and interests in the following products, including the right to develop, improve, manufacture and commercialise:(a) the worldwide (excluding U.S.) Circovac branded monovalent porcine circovirus type 2 (PCV2) swine vaccine business as described in more detail in Schedule 1 (the PCV2 Divestment Business);(b) the worldwide Progressis branded monovalent porcine reproductive and respiratory syndrome (PRRS) vaccine business (including [Conf]) as described in more detail in Schedule 1 (the PRRS Divestment Business);(c) the worldwide Parvovax branded monovalent inactivated porcine parvovirus (PPV) vaccine business and the worldwide Parvoruvax branded inactivated multivalent erysipelas and PPV vaccine business as described in more detail in Schedule 1 (the PPV Divestment Business); and(d) the worldwide Mucosiffa branded monovalent BVD ruminant vaccine business as described in more detail in Schedule 1 (the BVD Divestment Business).
NSAID Divestment Business
6. The NSAID Divestment Business1 consists of the rights, title and interests in the following products, including the right to develop, improve, manufacture and commercialise:(a) Merial’s injectable NSAIDs for multi-species on a EEA-wide basis, including the brands Ketofen, Romefen, Wellicox, Allevinix, Genixine and Equioxx Injectable, as described in more detail in Schedule 2; and(b) Merial’s Equioxx Paste branded oral NSAIDs for horses (including [Conf]) on an EEA-wide basis, as described in more detail in Schedule 2.
7. The legal and functional structure of the Divestment Businesses as operated to date is described in the Schedules. The Divestment Businesses, described in more detail in the Schedules, include all assets and staff that contribute to the current operation or are necessary to ensure the viability and competitiveness of the Divestment Businesses, in particular:(a) all tangible and intangible assets (including intellectual property rights);(b) all licences, permits and authorisations issued by any governmental organisation for the benefit of the Divestment Businesses;(c) all contracts, leases, commitments and customer orders of the Divestment Businesses; all customer credit and other records of the Divestment Businesses; and(d) the Personnel to the extent described in Schedules 1 and 2.
8. For the sake of clarity, the Divestment Businesses shall not include any physical production assets or manufacturing units owned or operated by the Parties.
9. The transfer of the Divestment Businesses will include for the Vaccine Divestment Businesses a production transfer to the Purchaser’s Phylaxia plant in Hungary and, for the NSAID Divestment Businesses a production transfer to one or several of the Purchaser’s existing facilities or a third-party toll manufacturer (CMO) (the Production Transfer), combined with transitional supply agreements (TSA) with the Purchaser, on the basis of which the Notifying Party will supply to the Purchaser the finished (and/or intermediate) products, and antigens when relevant, pending the completion of the production transfer process, as overseen by the Monitoring Trustee (together with the Technical Expert).
10. To support the transfer of the Divestment Businesses’ production process, the Notifying Party commits to provide the support necessary to ensure an effective Production Transfer of the Divestment Businesses to a production location of the Purchaser’s choice (Transfer Support Commitment).
11. Strict firewall procedures will be adopted so as to ensure that any competitively sensitive information relating to, or arising from such abovementioned arrangements (for example, product roadmaps) will not be shared with, or passed on to, anyone outside of the Divestment Businesses’ operations, beyond what is reasonably required for the compliance with the obligations relating to the Production Transfers and TSAs.
Section C.Related Commitments
Preservation of viability, marketability and competitiveness
12. From the Effective Date until Closing, the Parties shall preserve or procure the preservation of the economic viability, marketability and competitiveness of the Divestment Businesses, in accordance with good business practice, and shall minimise as far as possible any risk of loss of competitive potential of the Divestment Businesses. In particular the Parties undertake:(a) not to carry out any action that might have a significant adverse impact on the value, management or competitiveness of the Divestment Businesses or that might alter the nature and scope of activity, or the industrial or commercial strategy or the investment policy of the Divestment Businesses;(b) to make available, or procure to make available, sufficient resources for the development of the Divestment Businesses, on the basis and continuation of the existing business plans;(c) to continue to participate in tender processes in a manner consistent with past practice and ordinary course of business to ensure that the day-to-day operations of the Divestment Businesses are conducted on a “business as usual” basis; and(d) to take all reasonable steps, or procure that all reasonable steps are being taken, including appropriate incentive schemes (based on industry practice), to encourage all Key Personnel to remain with the Divestment Businesses, and not to solicit or move any Key Personnel to the Notifying Party's remaining business. Where, nevertheless, individual members of the Key Personnel exceptionally leave their current position, the Notifying Party shall provide a reasoned proposal to replace the person or persons concerned to the Commission and the Monitoring Trustee. The Notifying Party must be able to demonstrate to the Commission that the replacement is well suited to carry out the functions exercised by those individual members of the Key Personnel. The replacement shall take place under the supervision of the Monitoring Trustee, who shall report to the Commission.
Hold-separate obligations
13. The Notifying Party commits, from the completion of the Concentration until Closing, to keep the Divestment Businesses separate from the businesses it is retaining and to ensure that unless explicitly permitted under these Commitments: (i) management and staff of the businesses retained by the Notifying Party have no involvement in the Divestment Business; (ii) the Key Personnel and Personnel of the Divestment Business have no involvement in any business retained by the Notifying Party and do not report to any individual outside the Divestment Business to the extent reasonably practicable and in any case do not report to any individual having involvement in competing retained businesses. In addition, the Notifying Party commits to take all necessary steps to ensure that the Parties’ personnel involved in the transfer of the Divestment Businesses do not use any Confidential Information from the Purchaser other than information strictly required to assist in the transfer of the Divestment Business concerned, and that they only disclose such information to other of the Notifying Party’s personnel to the extent strictly required to assist in the transfer of the Divestment Businesses concerned.
14. Until Closing, the Notifying Party shall assist the Monitoring Trustee in ensuring that the Divestment Business is managed as a distinct and saleable entity separate from the business(es) which the Notifying Party is retaining. Immediately after the adoption of the Decision, the Parties, upon consultation with the Commission and the Monitoring Trustee, shall appoint one or more Hold Separate Managers who shall be responsible for the management of the Divestment Businesses, under the supervision of the Monitoring Trustee. The Hold Separate Manager(s), who shall be part of the Key Personnel, shall manage the Divestment Businesses in the best interest of the businesses with a view to ensuring their continued economic viability, marketability and competitiveness and their independence from the businesses retained by the Notifying Party.
15. The Parties will agree with the Monitoring Trustee and the Hold Separate Manager(s) on the scope of the ring-fencing and hold-separate measures and confidentiality obligations that will apply in the period between Closing and completion of the Production Transfers.
16. The Hold Separate Manager(s) shall closely cooperate with and report to the Monitoring Trustee who will be assisted by the Technical Expert. Any replacement of the Hold Separate Manager(s) shall be subject to the procedure laid down in paragraph 12(d) of these Commitments. The Commission may, after having heard the Notifying Party, require the Notifying Party to replace the Hold Separate Manager(s).
Ring-fencing
17. The Notifying Party, shall, to the extent possible, implement, or procure to implement, all necessary measures to ensure that it does not, from completion of the Concentration, obtain any Confidential Information relating to the Divestment Businesses and that any such Confidential Information obtained by the Notifying Party before the Effective Date will be eliminated and not be used by the Notifying Party. This includes measures vis-à-vis the Notifying Party's appointees on the supervisory board and/or board of directors of the Divestment Businesses. In particular, the participation of the Divestment Businesses in any central information technology network shall be severed to the extent possible, without compromising the viability of the Divestment Businesses. The Parties may obtain or keep information relating to the Divestment Businesses which is reasonably necessary for the divestiture of the Divestment Businesses or the disclosure of which to the Notifying Party is required by law.
Non-solicitation clause
18. To the extent applicable, the Notifying Party undertakes, subject to customary limitations2, not to solicit, and to procure that Affiliated Undertakings do not solicit, the Key Personnel that may be transferred with the Divestment Businesses for a period of 2 years after Closing.
Section D.Monitoring Trustee
I. Appointment procedure
19. The Notifying Party shall appoint a Monitoring Trustee to carry out the functions specified in these Commitments for a Monitoring Trustee. The Notifying Party commits not to close the Concentration before the appointment of a Monitoring Trustee.
20. The Monitoring Trustee shall be assisted by a Technical Expert with regard to all technical questions related to the Divestment Businesses, including technical aspects of the operation of the TSAs. The Technical Expert shall be appointed by and report to the Monitoring Trustee, with the Notifying Party having the right to be heard as to the suitability of the technical expert candidates. The Technical Expert will be independent of the Notifying Party and will not have or be exposed to any conflict of interest. The Notifying Party shall have the right to he heard with any reasoned objections against technical expert candidates, e.g., lack of competence or conflict of interest. In case of controversy between the Notifying Party and the Monitoring Trustee as to the suitability of the technical expert candidate, the Commission will decide on the matter.
21. The Trustee shall:(a) at the time of appointment, be independent of the Notifying Party and its Affiliated Undertakings;(b) possess the necessary qualifications to carry out its mandate, for example have sufficient relevant experience as an investment banker or consultant or auditor; and(c) neither have nor become exposed to a Conflict of Interest.
22. The Trustee and the Technical Expert shall be remunerated by the Notifying Party in a way that does not impede the independent and effective fulfilment of their mandate.
Proposal by the Notifying Party
23. Immediately after the Effective Date, the Notifying Party shall submit the name or names of one or more natural or legal persons whom the Notifying Party proposes to appoint as the Monitoring Trustee to the Commission for approval. The proposal shall contain sufficient information for the Commission to verify that the person or persons proposed as Trustee fulfil the requirements set out in paragraph 21 and shall include:(a) the full terms of the proposed mandate, which shall include all provisions necessary to enable the Trustee to fulfil its duties under these Commitments; and(b) the outline of a work plan which describes how the Trustee intends to carry out its assigned tasks.
Approval or rejection by the Commission
24. The Commission shall have the discretion to approve or reject the proposed Trustee and to approve the proposed mandate subject to any modifications it deems necessary for the Trustee to fulfil its obligations. If only one name is approved, the Notifying Party shall appoint or cause to be appointed the person or persons concerned as Trustee, in accordance with the mandate approved by the Commission. If more than one name is approved, the Notifying Party shall be free to choose the Trustee to be appointed from among the names approved. The Trustee shall be appointed within one week of the Commission’s approval, in accordance with the mandate approved by the Commission.
New proposal by the Notifying Party
25. If all the proposed Trustees are rejected, the Notifying Party shall submit the names of at least two more natural or legal persons within one week of being informed of the rejection, in accordance with paragraphs 19 and 24 of these Commitments.
Trustee nominated by the Commission
26. If all further proposed Trustees are rejected by the Commission, the Commission shall nominate a Trustee, whom the Notifying Party shall appoint, or cause to be appointed, in accordance with a trustee mandate approved by the Commission.
II.Functions of the Trustee
27. The Trustee shall assume its specified duties and obligations in order to ensure compliance with the Commitments. The Commission may, on its own initiative or at the request of the Trustee or the Notifying Party, give any orders or instructions to the Trustee in order to ensure compliance with the conditions and obligations attached to the Decision.
Duties and obligations of the Monitoring Trustee
28. The Monitoring Trustee shall:(a) propose in its first report to the Commission a detailed work plan describing how it intends to monitor compliance with the obligations and conditions attached to the Decision;(b) oversee, in close co-operation with the Hold Separate Manager(s), the on- going management of the Divestment Businesses with a view to ensuring its continued economic viability, marketability and competitiveness and monitor compliance by the Notifying Party with the conditions and obligations attached to the Decision. To that end the Monitoring Trustee shall:(i) monitor the preservation of the economic viability, marketability and competitiveness of the Divestment Businesses, and the keeping separate of the Divestment Businesses from the business retained by the Parties, in accordance with paragraphs 12 and 13 of these Commitments;(ii) supervise the management of the Divestment Businesses as a distinct and saleable entity, in accordance with paragraph 14 of these Commitments;(iii) with respect to Confidential Information:(A) determine all necessary measures to ensure that the Notifying Party does not after the Effective Date obtain any Confidential Information relating to the Divestment Businesses,(B) in particular strive for the severing of the Divestment Businesses’ participation in a central information technology network to the extent possible, without compromising the viability of the Divestment Businesses,(C) make sure that any Confidential Information relating to the Divestment Businesses obtained by the Notifying Party before the Effective Date is eliminated and will not be used by the Notifying Party, and(D) decide whether such information may be disclosed to or kept by the Notifying Party as the disclosure is reasonably necessary to allow the Notifying Party to carry out the divestiture or as the disclosure is required by law;(iv) monitor the splitting of assets and the allocation of Personnel between the Divestment Businesses and the Notifying Party or Affiliated Undertakings;(c) propose to the Notifying Party such measures as the Monitoring Trustee considers necessary to ensure the Notifying Party’s compliance with the conditions and obligations attached to the Decision, in particular the maintenance of the full economic viability, marketability or competitiveness of the Divestment Businesses, the holding separate of the Divestment Businesses and the non-disclosure of competitively sensitive information;(d) provide to the Commission, sending the Notifying Party a non-confidential copy at the same time, a written report within 15 days after the end of every month until Closing that shall cover the operation and management of the Divestment Businesses as well as the splitting of assets and the allocation of Personnel so that the Commission can assess whether the business is held in a manner consistent with the Commitments;(e) provide the Commission, sending the Notifying Party a non-confidential copy at the same time, a written report within 15 days after the end of every quarter during the first year after Closing, and every six months for the next three years, that shall cover the production transfer and the transfer supply agreement of the Divestment Products so that the Commission can assess whether these aspects are executed in a manner consistent with the Commitments;(f) promptly report in writing to the Commission, sending the Notifying Party a non-confidential copy at the same time, if it concludes on reasonable grounds that the Notifying Party is failing to comply with these Commitments; and(g) assume the other functions assigned to the Monitoring Trustee under the conditions and obligations attached to the Decision.
Duties and obligations of the Notifying Party
29. The Notifying Party shall provide and shall cause its advisors to provide the Trustee and Technical Expert with all such co-operation, assistance and information as the Trustee and Technical Expert may reasonably require to perform its tasks. The Trustee shall have full and complete access to any of the Notifying Party’s or the Divestment Businesses’ books, records, documents, management or other personnel, facilities, sites and technical information necessary for fulfilling its duties under the Commitments and the Notifying Party and the Divestment Businesses shall provide the Trustee upon request with copies of any document. The Notifying Party and the Divestment Businesses shall make available to the Trustee and Technical Expert one or more offices on their premises and shall be available for meetings in order to provide the Trustee with all information necessary for the performance of its tasks.
30. The Notifying Party shall provide the Monitoring Trustee with all managerial and administrative support that it may reasonably request on behalf of the management of the Divestment Businesses. This shall include all administrative support functions relating to the Divestment Businesses which are currently carried out at headquarters level.
31. The Notifying Party shall indemnify the Trustee and its employees and agents (each an “Indemnified Party”) and hold each Indemnified Party harmless against, and hereby agrees that an Indemnified Party shall have no liability to the Notifying Party for, any liabilities arising out of the performance of the Trustee’s and Technical Expert’s duties under the Commitments, except to the extent that such liabilities result from the wilful default, recklessness, gross negligence or bad faith of the Trustee, Technical Expert, or its employees, agents or advisors.
32. At the expense of the Notifying Party, the Trustee may appoint advisors (in particular for corporate finance or legal advice), subject to the Notifying Party’s approval (this approval not to be unreasonably withheld or delayed) if the Trustee considers the appointment of such advisors necessary or appropriate for the performance of its duties and obligations under the Mandate, provided that any fees and other expenses incurred by the Trustee are reasonable. Should the Notifying Party refuse to approve the advisors proposed by the Trustee the Commission may approve the appointment of such advisors instead, after having heard the Notifying Party. Only the Trustee shall be entitled to issue instructions to the advisors. Paragraph 31 of these Commitments shall apply mutatis mutandis.
33. The Notifying Party agrees that the Commission may share Confidential Information proprietary to the Notifying Party with the Trustee. The Trustee shall not disclose such information and the principles contained in Article 17 (1) and (2) of the Merger Regulation apply mutatis mutandis.
34. The Notifying Party agrees that the contact details of the Monitoring Trustee are published on the website of the Commission's Directorate-General for Competition and they shall inform interested third parties, in particular any potential purchasers, of the identity and the tasks of the Monitoring Trustee.
35. For a period of 10 years from the Effective Date the Commission may request all information from the Parties that is reasonably necessary to monitor the effective implementation of these Commitments.
III. Replacement, discharge and reappointment of the Trustee
36. If the Trustee ceases to perform its functions under the Commitments or for any other good cause, including the exposure of the Trustee to a Conflict of Interest:(a) the Commission may, after hearing the Trustee and the Notifying Party, require the Notifying Party to replace the Trustee; or(b) the Notifying Party may, with the prior approval of the Commission, replace the Trustee.
37. If the Trustee is removed according to paragraph 36 of these Commitments, the Trustee may be required to continue in its function until a new Trustee is in place to whom the Trustee has effected a full hand over of all relevant information. The new Trustee shall be appointed in accordance with the procedure referred to in paragraphs 19-26 of these Commitments.
38. Unless removed according to paragraph 36 of these Commitments, the Trustee shall cease to act as Trustee only after the Commission has discharged it from its duties after all the Commitments with which the Trustee has been entrusted have been implemented. However, the Commission may at any time require the reappointment of the Monitoring Trustee if it subsequently appears that the relevant remedies might not have been fully and properly implemented.
Section E.The review clause
39. The Commission may extend the time periods foreseen in the Commitments in response to a request from the Notifying Party or, in appropriate cases, on its own initiative. Where the Notifying Party requests an extension of a time period, it shall submit a reasoned request to the Commission no later than one month before the expiry of that period, showing good cause. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time send a non- confidential copy of the report to the Notifying Party. Only in exceptional circumstances shall the Notifying Party be entitled to request an extension within the last month of any period.
40. The Commission may further, in response to a reasoned request from the Notifying Parties showing good cause waive, modify or substitute, in exceptional circumstances, one or more of the undertakings in these Commitments. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time send a non-confidential copy of the report to the Notifying Party. The request shall not have the effect of suspending the application of the undertaking and, in particular, of suspending the expiry of any time period in which the undertaking has to be complied with.
Section F.Entry into force
41. The Commitments shall take effect upon the date of adoption of the Decision.
SCHEDULE 1
Part A – Vaccine Divestment Businesses
1. The Vaccine Divestment Businesses consist of the rights, title and interests in the following products, including the right to develop, improve, manufacture and commercialise:(a) the worldwide (excluding U.S.) Circovac branded monovalent porcine circovirus type 2 (PCV2) swine vaccine business as described in more detail in Part B of Schedule 1 (the PCV2 Divestment Business);(b) the worldwide Progressis branded monovalent porcine reproductive and respiratory syndrome (PRRS Marketed Divestment Business) vaccine business (including [Conf]) as described in more detail in Part B and C of Schedule 1 (the PRRS Divestment Business);(c) the worldwide Parvovax branded monovalent inactivated porcine parvovirus (PPV) vaccine business and the worldwide Parvoruvax branded inactivated multivalent erysipelas and PPV vaccine business as described in more detail in Part B of Schedule 1 (the PPV Divestment Business); and(d) the worldwide Mucosiffa branded monovalent BVD ruminant vaccine business as described in more detail in Part B of Schedule 1 (the BVD Divestment Business).
2. All reference to “exclusively or primarily” in the Commitments text, Schedules and Annexes should be interpreted as relating to the extent to which the relevant assets to be divested are used for the relevant Divestment Products as opposed to retained products. For the avoidance of doubt, even if a Vaccine Divestment Product generates the majority of its turnover outside the divested territory, the assets which relate exclusively or primarily to that product will be transferred to the Purchaser.
3. The tangible or intangible assets and rights that relate exclusively or primarily to the Divestment Businesses will be offered to the Purchaser by means of assignment. The Purchaser will subsequently grant the Notifying Party a licence, sub-licence or otherwise access to those tangible or intangible assets and rights that relate primarily to the Divestment Business but are shared between the Divestment Business and the retained business in view of the commercialisation of products not included in the Vaccine Divestment Businesses, which include Vaccine Divestment Products commercialised in the retained territory (US for the PCV2 Divestment Product) and other products. For the avoidance of doubt, the Notifying Party shall not have the right to sub-license or grant otherwise access in a manner which derogates from the rights granted to the Purchaser to any of the tangible or intangible assets and rights that are made available to the Notifying Party by means of the present provision.
4. Concerning the tangible and intangible assets and rights that are shared between the Divestment Businesses and the retained business but relate primarily to the retained business, the Notifying Party shall grant the Purchaser a licence, sub-licence, or access to such asset or right on a non-exclusive basis.
Part B – Scope of the Vaccine Divestment Businesses
1. The PCV2 Divestment Business, PRRS Marketed Divestment Business, [Conf] PPV Divestment Business and BVD Divestment Businesses as operated to date are not currently stand-alone businesses as they are integrated into a wider operational and commercial organisation; they will therefore be separated from current operations as described below. The PCV2 Divestment Business, PRRS Marketed Divestment Business, PPV Divestment Business and BVD Divestment Business are referred to as "Marketed Vaccine Divestment Businesses".
2. The Marketed Vaccine Divestment Businesses include, but are not limited to the transfer of:(a) all biological materials including the master virus/cells seeds (the Master Seed) and working seeds except for material necessary to sustain the retained US Circovac business and the monovalent Ruvax business. The Master Seed will transfer partially to the Purchaser promptly upon Closing and will be partially retained by the Notifying Party during the TSA. The remainder of the Master Seed and working seed will be transferred to the Purchaser at the end of the TSA;(b) finished goods inventory, existing lifecycle management projects, pipeline products and product improvements relating to the Vaccine Divestment Businesses, held at the date of Closing;(c) all recipes for the testing media and reagents that are used for the Vaccine Divestment Products and all relevant documentation required to carry out the relevant quality control tests;(d) all available inventory of Vaccine Divestment Products in an intermediate (nude bottled) form, to be replenished on an on-going basis until the Purchaser has complete downstream independence;(e) a [Conf] month antigen inventory stock for Circovac and Progressis to be delivered to the Purchaser as of completion by the Purchaser of its downstream independence,3 to be replenished on a continuous basis and, if required4 sufficient antigen inventory stock ([Conf] months) for Parvovax, Parvoruvax and Mucosiffa, to be replenished on an on-going basis until the Purchaser has complete upstream independence;(f) all relevant data, books, records, marketing and advertising/promotional materials, trade-dress, i.e. total image or overall design of appearance of product or its packaging and other documents to the extent exclusively or primarily related to or necessary for the operations of the Vaccine Divestment Businesses;(g) all know-how for the manufacturing of the Vaccine Divestment Products (including but not limited to the manufacturing of any active ingredient, antigen, reagent, adjuvant or other components of the Vaccine Divestment Products) as well as all know-how required for or associated with obtaining and/or maintaining manufacturing and marketing approvals for the Vaccine Divestment Products in the EEA, including but not limited to stability/reproducibility data (including process capability (CpK) data), periodic safety reports, any clinical reports, status reports, yearly product quality review reports;(h) with respect to all patent rights exclusively or primarily related to the Vaccine Divestment Businesses, the Notifying Party shall:(i) assign all patent rights that are exclusively owned by Merial; and(ii) use its best efforts, subject to third party rights, to assign the Merial rights under the patents that are jointly owned by Merial with a third party or currently in-licensed by Merial from a third party. Alternatively, the Notifying Party will provide the Purchaser with a licence or a sub-licence for the production and commercialisation of the Vaccine Divestment Products in the EEA territory.(i) all trademarks and the registered domain names that are exclusively or primarily used for the commercialisation of Vaccine Divestment Businesses (including the ones listed in Annex B);(j) all other IP rights (including, for the avoidance of doubt, in relation to the reagents), product formulations, know how, packaging specifications to the extent exclusively or primarily related to the manufacture and/or sale of Vaccine Divestment Businesses;(k) all licences, permits and marketing authorisations issued by any governmental organization and held by the Parties or their Affiliated Undertakings, as well as applications for variations in the context of the Production Transfer, that are related to the manufacture and/or sale of the Vaccine Divestment Businesses, including any dossiers relating to current or pending authorisations, to the extent transferrable (including the ones set out in Annex C). The transfer and possible updates of the abovementioned permits and authorisations in the EEA will be at the cost of the Notifying Party;(l) the Notifying Party will use its Best Efforts to transfer or assign, as appropriate, all customer contracts or relationships (including distribution agreements), and will transfer all available customer lists, customer credit and other records, and any other relevant customer information related to the Vaccine Divestment Businesses;(m) if requested by the Purchaser, the Notifying Party will use its Best Efforts to transfer, or assign, as appropriate, all contracts, agreements or relationships (including raw material and reagents supply agreements), leases, commitments and understandings with third-party suppliers of products or services related to the Vaccine Divestment Businesses (except to the extent required to be retained in order to manufacture for the Purchaser under the TSA);
3. For the avoidance of doubt, in addition to the abovementioned assets, the Marketed Vaccine Divestment Businesses will include all other assets and rights which are used and are necessary for the continued viability and competitiveness of the Marketed Vaccine Divestment Businesses. These assets will be offered to the Purchaser on the following basis:(a) an assignment of all tangible and intangible assets and rights that relate exclusively or primarily to the Marketed Vaccines Divestment Businesses. The Purchaser will subsequently grant the Notifying Party a licence, sub- licence or otherwise access to those tangible or intangible assets and rights that relate primarily to the Marketed Vaccine Divestment Business but are shared between the Marketed Vaccine Divestment Business and the retained business; and(b) a licence, sub-licence, or otherwise access to, on a non-exclusive basis, the shared tangible and intangible assets and rights that are shared between the Marketed Vaccine Divestment Businesses and the retained business but relate primarily to the retained business.The Monitoring Trustee shall supervise the Notifying Party’s performance in this regard.
4. At the option of the Purchaser and subject to applicable employment legislation, the Notifying Party will use its Best Efforts, including appropriate incentive schemes, to transfer to the Purchaser any of the operational/production, industrial/technical, R&D/Regulatory and/or commercial/marketing personnel in the EEA that are necessary to the Vaccine Divestment Businesses, on the following basis:(a) Key Personnel:(i) The Notifying Party has identified the following key functions for the Vaccine Divestment Business:· [Conf];· [Conf];· [Conf];· [Conf]; and· [Conf].(ii) The Key Personnel for each of the abovementioned key functions will be identified by the Parties in consultation with the Hold Separate Manager and the Monitoring Trustee as soon as possible following the Effective Date. During a period of [Conf] months from the Effective Date, the Parties will allow the Purchaser to have access to and make an employment offer to the Key Personnel in the abovementioned key functions. The Parties will take all reasonable steps, or procure that all reasonable steps are being taken, including appropriate incentive schemes (based on industry practice), to encourage the members of Key Personnel who have received an employment offer from the Purchaser to transfer to the Purchaser, subject to applicable employment legislation.(iii) Other personnel: At the request of the Purchaser, depending on the Purchaser’s needs and subject to applicable employment legislation, the Notifying Party will use its Best Efforts, including appropriate incentive schemes, to transfer any other personnel which the Purchaser may reasonably require for the operation of the Vaccine Divestment Business in the EEA. In particular, the Parties will use their Best Efforts (subject to applicable employment legislation), including appropriate incentive schemes, to transfer to the Purchaser the Swine Vaccine Commercial Personnel identified in the Products Asset Purchase Agreement. In the period between the Effective Date and Closing, the Monitoring Trustee will verify that the Purchaser will have the commercial personnel to replicate Merial’s existing commercial presence in the EEA, taking into account the Swine Vaccine Commercial Personnel to be transferred to, and the commercial personnel already available to the Purchaser.
5. For the avoidance of doubt, the Notifying Party shall retain:(a) Merial’s Circovac business in the US. This shall be effected by means of exclusion of any US-specific assets, and/or a reverse carve-out of US-specific rights, from the items listed in paragraph 2 above.(b) Merial’s monovalent Ruvax vaccine business worldwide, including sufficient erysipelas Master Seeds and working seeds. The Notifying Party shall retain the Ruvax business by means of an exclusion of any Ruvax specific assets, and/or reverse carve-out of assets or rights which relate primarily to Ruvax but are shared with the Parvoruvax Divestment Business, from the items listed in paragraph 2 above, whereby the Notifying Party shall grant the Purchaser a licence, sub-licence, or access to such retained assets or rights on a non- exclusive basis in accordance with the general principle set forth in Part A paragraph 4 above.
6. The Parties commit not to use or enable third parties to use any assets that are related to the Vaccine Divestment Products but are retained by the Parties for use in connection with their retained businesses for purposes of development, improvement and manufacture in view to commercialise the Vaccine Divestment Products or biologically identical products in the EEA territory.
7. Following the Production Transfer and the expiry of the TSA, the Purchaser will use its own manufacturing facilities and equipment at one or several sites for manufacturing and packaging of Vaccine Divestment Businesses.
8. For the avoidance of doubt, the Vaccine Divestment Businesses shall not include any right, title and/or interest in:(a) any production assets, manufacturing units, or R&D facilities;(b) the patent royalties which Merial is entitled to under the licence agreements it has concluded with BI and/or third parties;(c) the Parties’ company name, mark, or logo in any form;(d) all books and records required to be retained pursuant to any statue, rule, regulation or ordinance, provided that the Notifying Party will provide copies of such documents necessary for the Vaccine Divestment business to the Purchaser, upon request;(e) general books of account and books of original entry that comprise the Notifying Party’s or any of its Affiliated Undertakings’ permanent accounting or tax records provided that the Notifying Party will provide copies of such documents necessary for the Vaccine Divestment Business to the Purchaser, upon request; and(f) all books and records subject to the attorney-client or other legally recognised privilege, provided that the Notifying Party will provide copies of such documents necessary for the Vaccine Divestment Business to the Purchaser if the Purchaser and the Notifying Party enter into an arrangement that preserves any such privilege.
Part C – [Conf] Pipeline Divestment Business
1. [Conf] Pipeline Divestment Business consists of Merial’s rights to develop and commercialise [Conf] subject to the usual regulatory and technical risks inherent in a vaccine development project.
2. In accordance with paragraph 7 of these Commitments, the [Conf] Pipeline Divestment Business includes but is not limited to the transfer of:(a) all biological material already developed by Merial, including available Master Seeds;(b) all relevant data generated during the development project, including all material technical, preclinical, clinical and marketing files, reports, plans, know-how and records in the possession of or under control of Merial existing prior to Closing, which is exclusively or primarily related to or otherwise necessary for the development of commercialisation of the new [Conf] Pipeline Product;(c) all clinical data and studies exclusively or primarily relating to or otherwise necessary to the development of the [Conf] Pipeline Product existing prior to Closing;(d) all correspondence pertaining to regulatory filings and approvals (if any) relating to the commercialisation of the [Conf] Pipeline Product;(e) all recipes for the testing media and reagents that used are for the Vaccine Divestment Products and all relevant documentation required to carry out the relevant quality control tests;(f) any intellectual property rights where available which are primarily or exclusively related to the [Conf] Pipeline Product. These intellectual property rights include product formulations, manufacturing, know-how and other secret know-how, packaging specifications, rights to the trade dress, and all related copyright;(g) relevant data, books, records, and other documents exclusively or primarily related to or necessary for the development and commercialisation of the [Conf] Pipeline Product provided that the Parties redact from such copies any information that does not relate to the [Conf] Pipeline Product; and(h) to the extent applicable,1 the Notifying Party will use its Best Efforts to transfer to the purchaser any contract or relationships with third party contract development organisations concerning services related to the [Conf] Pipeline Divestment Business (except to the extent required to be retained in order to continue the development of the [Conf] Pipeline Divestment Business according to the plans and projections at the date of these Commitments).
3. For the avoidance of doubt, in addition to the abovementioned assets, the [Conf] Pipeline Divestment Businesses will include all other assets and rights which are used and are necessary for the continued viability and competitiveness of the [Conf] Pipeline Divestment Businesses. These assets will be offered to the Purchaser on the following basis:(a) an assignment of all tangible and intangible assets and rights that relate exclusively or primarily to the [Conf] Pipeline Divestment Businesses. The Purchaser will subsequently grant the Notifying Party a licence, sub-licence or otherwise access to those tangible or intangible assets and rights that relate primarily to the [Conf] Pipeline Divestment Business but are shared between the [Conf] Pipeline Divestment Business and the retained business; and(b) a licence, sub-licence, or otherwise access to, on a non-exclusive basis, the shared tangible and intangible assets and rights that are shared between the [Conf] Pipeline Divestment Businesses and the retained business but relate primarily to the retained business.The Monitoring Trustee shall supervise the Notifying Party’s performance in this regard.
4. The Parties commit not to use or enable third parties to use any assets that are related to the [Conf] Pipeline Divestment Products but are retained by the Parties for use in connection with their retained businesses for purposes of development, improvement and manufacture in view to commercialise the [Conf] Pipeline Divestment Products or biologically identical products in the EEA territory.
5. The [Conf] Pipeline Project will be transferred to the Purchaser at completion of the clinical development with the finalised, written reports. The Notifying Party commits (subject to circumstances entirely outside of its control) to continue the development of the [Conf] Pipeline Divestment Business according to the plans and projections at the date of these Commitments. The Notifying Party commits to update the Hold Separate Manager, the Purchaser, Monitoring Trustee and/or Technical Expert on the progress in the development of the [Conf] Pipeline Product and to grant them access to any relevant information and data regarding the development. Furthermore, the Notifying Party commits to provide assistance in obtaining the relevant marketing authorisation applications.
6. The [Conf] Pipeline Divestment Business will be transferred upon completion of the clinical development phase, according to the plans and projections at the date of these Commitments. Upon completion of the clinical development phase, the Notifying Party commits to separate and transfer the [Conf] Pipeline Divestment Businesses’ production process in accordance with the Production Transfer process described in Schedule 1, Part D, paragraphs 1-3.
7. For the avoidance of doubt, the [Conf] Pipeline Divestment Business shall not include any right, title and/or interest in:(a) raw materials, other than any raw materials used to develop the [Conf] Pipeline Product;(b) any production assets, manufacturing units, or R&D facilities;(c) the Parties’ company name, mark, or logo in any form;(d) all books and records required to be retained pursuant to any statue, rule, regulation or ordinance, provided that the Notifying Party will provide copies of such documents necessary for the [Conf] Pipeline Divestment Business to the Purchaser, upon request;(e) general books of account and books of original entry that comprise the Notifying Party’s or any of its Affiliated Undertakings’ permanent accounting or tax records provided that the Notifying Party will provide copies of such documents necessary for the [Conf] Pipeline Divestment Business to the Purchaser, upon request; and(f) all books and records subject to the attorney-client or other legally recognised privilege, provided that the Notifying Party will provide copies of such documents necessary for the [Conf] Pipeline Divestment Business to the Purchaser if the Purchaser and the Notifying Party enter into an arrangement that preserves any such privilege.
Part D – Vaccine Production Transfer
1. The Notifying Party commits to separate and transfer the Vaccine Divestment Businesses’ production process (the Production Transfer) to the Purchaser’s own production facility at one or several sites.
2. The Production Transfer will involve the following:(a) Downstream production process: transfer of the downstream processing, filing and packaging production process for the production of the relevant Vaccine Divestment Product in final form; and(b) Upstream production process: transfer of the relevant upstream Vaccine Divestment Product production process including, but not necessary limited to production of antigen.
3. To ensure the transfer of the production of the Vaccine Divestment Businesses to the location of the Purchaser’s choice, the Notifying Party commits to provide the Purchaser with all information and materials to allow the Purchaser to replicate Merial's existing manufacturing equipment and processes in its own manufacturing capabilities, including but not limited to:(a)detailed user requirement specifications for the design (equipment) and construction of a new upstream, and to the extent necessary, downstream facility;(b) detailed specifications of all relevant materials required for the production process;(c) relevant input materials, including reference and/or cell materials and reagents; and(d) detailed standard operating procedures for the execution of all in process controls and final product testing including training employees.
Gerland Antigen Supply
4. At the option of the Purchaser of the Vaccine Divestment Business, as of January 2019, the Notifying Party will dedicate its Gerland (France) production capacity exclusively or primarily to Circovac and Progressis antigen production (Gerland Antigen Supply) giving priority to the production of the Circovac and Progressis antigen production over the products of the retained business until the Purchaser has completed its upstream production capability at the Phylaxia plant. The relevant antigen shall be made available to the Purchaser at full manufacturing cost reflecting the current capacity utilization levels at the time of signing the relevant supply agreement, subject to the approval of the Monitoring Trustee and the Technical Expert. The relevant agreement between the Parties and the Purchaser will allow for a yearly revision of the costs of supply, subject to the approval of the Monitoring Trustee and the Technical Expert, in the event of an increase or decrease in the manufacturing cost of [Conf]% or more, it being understood that any increases in the costs of supply can be based on external factors only (e.g. a change requested by the Purchaser resulting in an increase in the manufacturing cost, an increase in the cost of raw materials or any other justified circumstances outside the control of the Parties resulting in an increase in the manufacturing costs).
5. Should the Purchaser opt for the Gerland Antigen Supply, the Notifying Party will make available the full nominal capacity of the Gerland manufacturing capacity for the production of the relevant Vaccine Divestment Product antigens and the Purchaser shall be granted permanent access and monitoring rights to ensure that the antigen production is undertaken according to product specification. The Purchaser’s technicians and operators will be granted access to observe the production process to facilitate the Production Transfer and training process. Additionally, if required, the Notifying Party will guarantee supplies of the Circovac, Progressis and Parvovirus antigen from the Lyon Portes-des-Alpes site, as a back-up supply source under the Gerland Antigen Supply option.
Transfer support commitment
6. To support the transfer of the Vaccine Divestment Businesses’ production process, the Parties commit to provide to the Purchaser, at no cost and until six months after the Purchaser has successfully produced three full scale manufacturing batches of the relevant product in its production unit, any support to ensure an effective Production Transfer of the Vaccine Divestment Businesses to the Purchaser’s Phylaxia plant.
7. In addition, until the Purchaser has obtained the required variations to the marketing authorizations of the Vaccine Divestment Products, the Parties commit to provide under supervision of the Monitoring Trustee and the Technical Expert, any support which the Purchaser may require to address manufacturing process issues in the production of the Vaccine Divestment Products and to achieve an acceptable robustness level of the relevant production processes of the Vaccine Divestment Products, as reflected in the relevant control charts for the products concerned.
8. The transfer support will at the Purchaser’s request be provided either at the Parties’ production site(s) or at the Purchaser’s site and will include:(a) support for the design, including providing general specifications and supporting the Purchaser in acquiring specific equipment, and the commissioning of a new production facility for the production of the Vaccine Divestment Products or the adjustment of an existing production facility at the Purchaser’s premises, on the basis of the know-how and technical documentation included in the Divestment Business;(b) technical training and transfer know-how to the Purchaser’s employees in relation to the production of the Vaccine Divestment Products, and any other aspects regarding the operation and maintenance of the relevant production assets, by training at the Purchaser’s facility after completion of the Production Transfer at the Notifying Party’s own expense;(c) R&D/clinical support by (a) advising on technical issues relating to research;(b) finishing on-going clinical studies; (c) transferring clinical studies, assays and technology; (d) providing assistance for pharmacovigilance and regulatory submissions, (d) support the Purchaser in quality control testing; and (e) train Purchaser’s designated personnel; and(d) advice on technical knowledge documentation; assistance to the Purchaser to make any necessary regulatory filings and obtain any necessary authorisations; and assist, where necessary in the transfer to the Purchaser of such licences, permits and authorisations concerning the Vaccine Divestment Businesses.
9. The production transfer support will be provided by a team of expert employees of the Parties (Production Transfer Personnel), listed at Annex D, who will prioritise the effective Production Transfer of the Vaccine Divestment Businesses over their work for the retained businesses and make themselves available according to the requirements for a timely and effective implementation of the Production Transfers. The Parties will implement an appropriate incentive scheme (based on industry practice) to incentivize the Production Transfer Personnel to complete the Production Transfers in a timely and effective manner. The Production Transfer Personnel will be bound by appropriate confidentiality obligations which will be agreed in accordance with paragraph 15 of the Commitments. Where individual members of the Production Transfer Personnel leave their position, the Parties shall replace the person or persons concerned and inform the Monitoring Trustee and the Technical Expert of the replacement.
10. The Production Transfer Personnel will be assisted by a steering committee, identified at Annex D, which will be composed of Merial employees with prior production transfer experience and will oversee/manage and make all necessary strategic decisions in relation to the execution of the Production Transfer of the Vaccine Divestment Products to the Purchaser’s Phylaxia plant.
11. Finally, at the request of the Purchaser, the Parties commit to provide to the Purchaser any support it may require to take over at, or as soon as possible after Closing the distribution of the Divestment Products.
Part E – Vaccine TSA
1. The Notifying Party shall enter into a Transitional Supply Agreement (TSA) and supply the products within the scope of Vaccine Divestment Businesses (including the [Conf] Pipeline Divestment Business, if it is completed successfully) until the Production Transfer has been completed. The TSA will be monitored by the Monitoring Trustee (together with the Technical Expert).
Supply of the intermediate or final product:
2. Subject to the requirements of the Purchaser, the Notifying Party shall supply the Vaccine Divestment Products in a finished or intermediate (nude bottled) form at full manufacturing costs on a cost pass-through basis (i.e. no mark-up) to the Purchaser, until the Purchaser has completed the downstream Production Transfer process for the relevant Vaccine Divestment Product or, in any case, for a maximum term of 30 months, extendable with approval of the Monitoring Trustee (together with the Technical Expert), if such extension is required in order to complete the transfer of the downstream production of the relevant Vaccine Divestment Product to the Purchaser’ own facilities. Costs will be fixed at the time of signing of the TSA for the duration of the agreement. The TSA will allow for a yearly revision of the costs of supply, subject to the approval of the Monitoring Trustee and the Technical Expert, in the event of an increase or decrease in the manufacturing cost of [Conf]% or more, it being understood that any increases in the costs of supply can be based on external factors only (e.g. a change requested by the Purchaser resulting in an increase in the manufacturing cost, an increase in the cost of raw materials or any other justified circumstances outside the control of the Parties resulting in an increase in the manufacturing costs). .
3. The available inventory of Vaccine Divestment Products in a finished or intermediate (nude bottled) form transferred to the Purchaser upon Closing will be replenished on an on-going basis at least at pre-Transaction announcement level until the Purchaser has complete downstream independence.
Antigen supply:
4. Once the Purchaser has completed the downstream Production Transfer process, the Notifying Party, shall supply the relevant Vaccine Divestment Products antigen at full manufacturing costs on a cost pass-through basis (i.e. no mark-up) to the Purchaser, until the Purchaser has completed the upstream antigen transfer process or, in any case, for a maximum term of 3 years extendable subject to approval of the Monitoring Trustee (together with the Technical Expert), if such extension is required in order to complete the transfer of the antigen production of the Vaccine Divestment Product to the Purchaser’s own facilities. Costs will be fixed at the time of signing of the TSA for the duration of the agreement. The TSA will allow for a yearly revision of the costs of supply, subject to the approval of the Monitoring Trustee and the Technical Expert, in the event of an increase or decrease in the manufacturing cost of [Conf]% or more, it being understood that any increases in the costs of supply can be based on external factors only (e.g. a change requested by the Purchaser resulting in an increase in the manufacturing cost, an increase in the cost of raw materials or any other justified circumstances outside the control of the Parties resulting in an increase in the manufacturing costs).
5. The [Conf] month antigen inventory stock for Circovac and Progressis and any antigen inventory stock for Parvovax, Parvoruvax and Mucosiffa to be transferred to the Purchaser upon completion by the Purchaser of downstream independence, will be replenished on an on-going basis at least at pre-Transaction announcement level until the Purchaser has complete upstream independence.
Reagents supply:
6. Subject to the requirements of the Purchaser, the Notifying Party, shall supply any reagents manufactured in-house by Merial necessary for the manufacturing and/or testing of the Vaccine Divestment Products at full manufacturing costs on a cost pass- through basis (i.e. no mark-up) to the Purchaser for the duration of the TSA. Costs will be fixed at the time of signing the TSA for the duration of the agreement. The TSA will allow for a yearly revision of the costs of supply, subject to the approval of the Monitoring Trustee and the Technical Expert, in the event of an increase or decrease in the manufacturing cost of [Conf]% or more, it being understood that any increases in the costs of supply can be based on external factors only (e.g. a change requested by the Purchaser resulting in an increase in the manufacturing cost, an increase in the cost of raw materials or any other justified circumstances outside the control of the Parties resulting in an increase in the manufacturing costs).
7. At the option of the Purchaser, the Notifying Party shall use its Best Efforts to assist the Purchaser to procure the reagents manufactured by third parties necessary for the manufacture and/or testing of any Vaccine Divestment Product for the duration of the TSA . If the Purchaser is not able to source such reagents, the Parties commit to enter, at the option of the Purchaser, into back-to-back supply agreements with reagent suppliers and to make such reagents available to the Purchaser at cost, for such period as required by the Purchaser to establish the Vaccine Divestment Businesses as viable and independent businesses, but not exceeding the duration of the TSA.
8. The TSA will have the following characteristics:(a) Sufficient Master Seed and working seed will be retained by the Notifying Party during the TSA to continue the production of the relevant Vaccine Divestment Products antigen for the Purchaser until the Purchaser has full upstream independency from the Notifying Party;(b) The Purchaser will grant the Notifying Party a temporary licence for the use of the relevant Master Seed, working seed, intellectual property, know-how and technical documentation required for the production of the Vaccine Divestment Products and relevant antigens;(c) The Notifying Party shall manufacture the Vaccine Divestment Products and/or antigen in accordance with specified existing product specifications and it shall continue to manufacture the Vaccine Divestment Products and the relevant antigen at the manufacturing facilities which are currently owned and used by Merial for the production of the relevant vaccines (with the exception of a shift of Circovac/Progressis antigen production to the Gerland site, at the Purchaser’s request) to ensure the continued supply of the Vaccine Divestment Products, giving priority to the production of the Vaccine Divestment Products over the products of the retained business should there be technical difficulties or shortage of supply.(d) The Vaccine Divestment Product or antigen shall be produced under the same cost structure and of the same quality and consistent with past practice as Merial produced the Vaccine Divestment Product or antigen prior to Closing.(e) The Notifying Party shall supply sufficient volumes of the finished Vaccine Divestment Product and antigen allowing the Purchaser to maintain and expand the existing market position until the Purchaser has established an alternative production capacity, with no limitation to the volume of production subject to Merial’s relevant existing manufacturing facilities’ capacity.(f) The Notifying Party will provide the Purchaser with assistance in order to implement any changes required to the packaging of the relevant Vaccine Divestment Products.
9. Under the terms of the TSA, the Purchaser will have the right to request on a transitional basis the Parties to assist in the distribution (for example via logistics and supply chain support) of the Vaccine Divestment Products on the Purchaser’s behalf in the EEA on a cost basis, until the Purchaser has established commercial independence and in any event for not longer than the duration of the TSA.
10. In the event of a dispute between the Notifying Party and the Purchaser regarding the Production Transfer or the TSA, the matter shall be referred to the Monitoring Trustee (together with the Technical Expert) for resolution.
SCHEDULE 2
Part A – NSAID Divestment Businesses
1. The NSAID Divestment Business consists of the rights, title and interests in the following products, including the right to develop, improve, manufacture and commercialise:(a) Merial’s injectable non-steroidal anti-inflammatory drugs (NSAIDs) for multi- species on an EEA-wide basis,2 including the brands Ketofen,Wellicox/Allevinix, Genixine and Equioxx Injectable; and(b) Merial’s Equioxx Paste branded oral NSAIDs for horses on an EEA-wide basis (including [Conf]) ((a) and (b) the NSAID Divestment Products).
2. All reference to “exclusively or primarily” in the Commitments text, Schedules and Annexes should be interpreted as relating to the extent to which the relevant assets to be divested are used for the relevant Divestment Products as opposed to retained products. For the avoidance of doubt, even if a NSAID divested product generates the majority of its turnover outside the divested territory, the assets which relate exclusively or primarily to that product will be transferred to the Purchaser.
3. The tangible or intangible assets and rights that relate exclusively or primarily to the Divestment Businesses will be offered to the Purchaser by means of assignment. The Purchaser will subsequently grant the Notifying Party a licence, sub-licence or otherwise access to those tangible or intangible assets and rights that relate primarily to the Divestment Business but are shared between the Divestment Business and the retained business in view of the commercialisation of products not included in the NSAID Divestment Businesses, which include NSAID Divestment Products commercialised in the retained territory (outside the EEA for the NSAID Divestment Products) and other products. For the avoidance of doubt, the Notifying Party shall not have the right to sub-license or grant otherwise access in a manner which derogates from the rights granted to the Purchaser to any of the tangible or intangible assets and rights that are made available to the Notifying Party by means of the present provision.
4. Concerning the tangible and intangible assets and rights that are shared between the Divestment Businesses and the retained business but relate primarily to the retained business, the Notifying Party shall grant the Purchaser a licence, sub-licence, or access to such asset or right on a non-exclusive basis.
Part B – Scope of the NSAID Divestment Businesses
1. The NSAID Divestment Business as operated to date is not currently a stand-alone business activity as it is integrated into a wider operational and commercial organisation; it will therefore be separated from current operations as described below.
2. The NSAID Divestment Business includes but is not limited to the transfer of:(a) the brands Ketofen, Wellicox/Allevinix, Genixine, Equioxx Injectable and Equioxx Paste in the EEA. For EEA countries where the brands are currently not registered, the Parties commit not to register any of them or oppose to such registration by the Purchaser;(b) finished goods inventory, work in progress, pipelines, product improvements relating to the NSAIDs Divestment Business held at the date of Closing;(c) all relevant clinical reports relating to the NSAID Divestment Business existing prior to Closing;(d) all know-how for the manufacturing of the NSAID Divestment Products as well as all know-how required for or associated with obtaining and/or maintaining manufacturing and marketing approvals for the NSAID Divestment Products in the EEA, including stability/reproducibility data (including process capability (CpK) data), periodic safety reports, any clinical reports, status reports, yearly product quality review reports;(e) all relevant data, books, records, marketing and advertising/promotional materials, trade-dress, i.e. total image or overall design of appearance of product or its packaging and other documents to the extent exclusively or primarily related to or necessary for the operation of the NSAID Divestment Business;(f) all trademarks and the registered domain names that are exclusively or primarily used for the commercialisation of the NSAID Divestment Products (including the ones set out in Annex E);(g) a licence or sub-licence to Merial’s [Conf] patent rights on a non-exclusive basis;(h) all other IP rights, product formulations, know-how, packaging specifications to the extent exclusively or primarily related to the manufacture and/or sale of the NSAID Divestment Products;(i) all licences, permits, and marketing authorisations issued by any governmental organization and held by the Parties or their Affiliated Undertakings, as well as to support applications for variations in the context of the Production Transfers, that are related to the NSAID Divestment Products including any dossiers relating to current or pending authorisations, to the extent transferrable (as set out in Annex F). The transfer and updates of the abovementioned permits and authorisations will be at the cost of the Notifying Party;(j) the Notifying Party will use its Best Efforts to transfer or assign, as appropriate, all customer contracts or relationships (including distribution agreements) and will transfer all available customer lists, customer credit and other records related to the NSAID Divestment Business;(k) if requested by the Purchaser, the Notifying Party will use its Best Efforts to transfer, or assign, as appropriate, all contracts, agreements or relationships (including raw material supply agreements), leases, commitments and understandings with third-party suppliers of products or services related to the NSAID Divestment Business (except to the extent required to be retained in order to manufacture for the Purchaser under the TSA);(l) at the option of the Purchaser, the Parties will use their Best Efforts to transfer (or otherwise provide) to the Purchaser sufficient rights under the agreement currently in place between Merial and [Conf] for Purchaser to obtain supply of [Conf];(m) at the option of the Purchaser, the Parties will use their Best Efforts to reach an arrangement with [Conf] pursuant to which Purchaser could purchase [Conf] directly from [Conf] under the agreement currently in place between Merial and [Conf], through the end of the current term thereof ([Conf]), without the Parties or Purchaser knowing the quantity of [Conf] purchased or forecasted for purchase by the other party.(n) access to any other tangible or intangible assets, with the exception of any physical production assets, which the Purchaser may require to successfully complete and transfer of the NSAID Divestment Business to an alternative production location; and
3. For the avoidance of doubt, in addition to the abovementioned assets, the NSAID Divestment Businesses will include all other assets and rights which are used and are necessary for the continued viability and competitiveness of the NSAID Divestment Businesses. These assets will be offered to the Purchaser on the following basis:(a) an assignment of all tangible and intangible assets and rights that relate exclusively or primarily to the NSAID Divestment Businesses. The Purchaser will subsequently grant the Notifying Party a licence, sub-licence or otherwise access to those tangible or intangible assets and rights that relate primarily to the NSAID Divestment Business but are shared between the NSAID Divestment Business and the retained business; and(b) a licence, sub-licence, or otherwise access to, on a non-exclusive basis, the shared tangible and intangible assets and rights that are shared between the NSAID Divestment Businesses and the retained business but relate primarily to the retained business.The Monitoring Trustee shall supervise the Notifying Party’s performance in this regard.
4. At the option of the Purchaser and subject to applicable employment legislation, the Notifying Party will use its Best Efforts, including appropriate incentive schemes to transfer to the Purchaser an employee for each of the key functions identified below:3(i) The Notifying Party has identified the following key functions for the NSAID Divestment Businesses:· [Conf];· [Conf];· [Conf]; and· [Conf].(ii) The Key Personnel for each of the abovementioned key functions will be identified by the Parties in consultation with the Hold Separate Manager and the Monitoring Trustee as soon as possible following the Effective Date. During a period of 12 months from the Effective Date, the Parties will allow the Purchaser to have access to and make an employment offer to the Key Personnel in the abovementioned key functions. The Parties will take all reasonable steps, or procure that all reasonable steps are being taken, including appropriate incentive schemes (based on industry practice), to encourage the members of Key Personnel who have received an employment offer from the Purchaser to transfer to the Purchaser, subject to applicable employment legislation.
5. The Parties commit not to use or enable third parties to use any assets that are related to the NSAID Divestment Products but are retained by the Parties for use in connection with their retained businesses for purposes of development, improvement and manufacture in view to commercialise the NSAID Divestment Products or biologically identical products in the EEA territory.
6. Following the Production Transfer and the expiry of the TSA, the Purchaser will either use its own manufacturing facilities and equipment at one or several sites and/or a CMO for manufacturing and packaging of the NSAID Divestment Products.
7. For the avoidance of doubt, the NSAID Divestment Business shall not include any right, title and/or interest in:(a) any production assets, manufacturing units, or R&D facilities;(b) the Parties’ company name, mark, or logo in any form;(c) all books and records required to be retained pursuant to any statue, rule, regulation or ordinance, provided that the Notifying Party will provide copies of such documents necessary for the NSAID Divestment Business to the Purchaser, upon request;(d) general books of account and books of original entry that comprise the Notifying Party’s or any of its Affiliated Undertakings’ permanent accounting or tax records provided that the Notifying Party will provide copies of such documents necessary for the NSAID Divestment Business to the Purchaser, upon request; and(e) all books and records subject to the attorney-client or other legally recognized privilege, provided that the Notifying Party will provide copies of such documents necessary for the NSAID Divestment Business to the Purchaser if the Purchaser and the Notifying Party enter into an arrangement that preserves any such privilege.
Part C – [Conf] Pipeline Product
The [Conf] pipeline product ([Conf] Pipeline Product)
1. The [Conf] Pipeline Product consists of the rights to develop and commercialise [Conf].
2. In accordance with paragraph 7 of these Commitments, the NSAID Pipeline Product includes but is not limited to the transfer of:(a) pharmaceutical material already developed by Merial in relation to the [Conf] Pipeline Product;(b) all relevant data generated during the development project, including all material technical, preclinical, clinical and marketing files, reports, plans, know-how and records in the possession of or under control of Merial existing prior to Closing in relation to the [Conf] Pipeline Product;(c) all clinical data and studies relating to the development of the [Conf] Pipeline Product, existing prior to Closing;(d) all correspondence pertaining to regulatory filings and approvals (if any) relating to the commercialisation of the [Conf] Pipeline Product;(e) any intellectual property rights which are primarily or exclusively related to the [Conf] Pipeline Product. These intellectual property rights include product formulations, manufacturing, know-how and other secret know-how, packaging specifications, rights to the trade dress, and all related copyright; and(f) the relevant data, books, records, and other documents exclusively or primarily related to or necessary for the development and commercialisation of the [Conf] Pipeline Product provided that the Parties redact from such copies any information that does not relate to the [Conf] Pipeline Product.
3. For the avoidance of doubt, in addition to the abovementioned assets, the [Conf] Pipeline Product will include all other assets and rights which are used and are necessary for the continued viability and competitiveness of the [Conf] Pipeline Product. These assets will be offered to the Purchaser on the following basis:(a) an assignment of all tangible and intangible assets and rights that relate exclusively or primarily to the [Conf] Pipeline Product. The Purchaser will subsequently grant the Notifying Party a licence, sub-licence or otherwise access to those tangible or intangible assets and rights that relate primarily to the [Conf] Pipeline Product but are shared between the [Conf] Pipeline Product and the retained business; and(b) a licence, sub-licence, or otherwise access to, on a non-exclusive basis, the shared tangible and intangible assets and rights that are shared between the [Conf] Pipeline Product and the retained business but relate primarily to the retained business.The Monitoring Trustee shall supervise the Notifying Party’s performance in this regard.
4. The Parties commit not to use or enable third parties to use any assets that are related to the [Conf] Pipeline Product but are retained by the Parties for use in connection with their retained businesses for purposes of development, improvement and manufacture in view to commercialise the [Conf] Pipeline Product or biologically identical products in the EEA territory.
5. The Notifying Party commits (subject to circumstances entirely outside of its control) to continue the development of the [Conf] Pipeline Product project, in the manner in which it is being developed at the date of these Commitments. Furthermore, the Notifying Party commits to provide assistance in obtaining the relevant marketing authorisation applications.
6. The [Conf] Pipeline Divestment Business will be transferred upon completion of the clinical development phase, according to the plans and projections at the date of these Commitments. Upon competition of the clinical development phase, the Notifying Party commits to separate and transfer the [Conf] Pipeline Divestment Businesses’ production process in accordance with the Production Transfer process described in Schedule 2, Part D, paragraph 1.
7. For the avoidance of doubt, the [Conf] Pipeline Product shall not include any right, title and/or interest in:(a) any personnel of the Parties;(b) raw materials, other than any raw materials used to develop the [Conf] Pipeline Product;(c) any production assets, manufacturing units, or R&D facilities;(d) the Parties’ company name, mark, or logo in any form;(e) all books and records required to be retained pursuant to any statue, rule, regulation or ordinance, provided that the Notifying Party will provide copies of such documents necessary for the [Conf] Divestment Business to the Purchaser, upon request;(f) general books of account and books of original entry that comprise the Notifying Party’s or any of its Affiliated Undertakings’ permanent accounting or tax records provided that the Notifying Party will provide copies of such documents necessary for the [Conf] Divestment Business to the Purchaser, upon request; and(g) all books and records subject to the attorney-client or other legally recognized privilege, provided that the Notifying Party will provide copies of such documents necessary for the development and commercialization of the [Conf] Pipeline Product to the Purchaser if the Purchaser and the Notifying Party enter into an arrangement that preserves any such privilege.
Part D – NSAID Transfer options
1. The Notifying Party commits to use its Best Efforts to facilitate the transfer to one or several of the Purchaser’s existing facilities or to a third party manufacturer of all manufacturing technology, IP and know-how necessary to enable the Purchaser or a third party manufacturer, to manufacture the NSAID Divestment Products.
2. As regards the production of [Conf], the Notifying Party will use its Best Efforts to ensure that the relationship currently in place with [Conf] for the supply of [Conf] is transferred to the Purchaser or to enable the Purchaser to conclude a new agreement. In any event that such arrangements cannot be made, the Notifying Party is prepared to conclude back-to-back supply agreements with the Purchaser.
Transfer support commitments
3. To support the transfer of the NSAID Divestment Business’s production process, the Notifying Party commits to provide any support to ensure an effective Production Transfer of the NSAID Divestment Business to the production location of the Purchaser’s choice at its own expenses. The Notifying Party envisages that technical assistance could include one or more of the following elements: advising on technical knowledge documentation, supporting the Purchaser in acquiring specific equipment, providing staff with suitable experience and skills to assist and/or advising on technical issues relating to research, assisting in trainings for the Purchaser’s staff, providing guidance on regulatory and legal aspects related to the transfer of any licence.
4. At the option of the Purchaser, the Notifying Party commits to support the transfer of the NSAID Divestment Businesses’ production process by providing, as required by the Purchaser:(a) for manufacturing, support for the preparation and equipping of the Purchaser’s chosen manufacturing site(s) and/or CMO(s); and(b) for R&D/clinical, a transitional service team in order to: (a) finish on-going clinical studies; (b) transfer clinical studies, assays and technology; (c) provide assistance for pharmacovigilance and regulatory submissions, and (d) train the Purchaser’s designated personnel.
5. The production transfer support will be provided by a team of expert employees of the Parties (Production Transfer Personnel), listed at Annex D, who will prioritise the effective Production Transfer of the NSAID Divestment Businesses over their work for the retained businesses and make themselves available according to the requirements for a timely and effective implementation of the Production Transfers. The Parties will implement an appropriate incentive scheme (based on industry practice) to incentivise the Production Transfer Personnel to complete the Production Transfers in a timely and effective manner. The Production Transfer Personnel will be bound by appropriate confidentiality obligations which will be agreed in accordance with paragraph 15 of the Commitments. Where individual members of the Production Transfer Personnel leave their position, the Parties shall replace the person or persons concerned and inform the Monitoring Trustee and the Technical Expert of the replacement.
6. The Production Transfer Personnel will be assisted by a steering committee, identified at Annex D, which will be composed of Merial employees with prior production transfer experience and will oversee/manage and make all necessary strategic decisions in relation to the execution of the Production Transfer of the NSAID Divestment Products to the Purchaser’s existing facilities or a CMO.
7. At the option of the Purchaser, the Notifying Party shall provide technical assistance to the Purchaser to facilitate the procurement of raw materials necessary for the manufacture of any NSAID Divestment Products. If the Purchaser is not able to source such raw materials, the Notifying Party commits to enter, at the option of the Purchaser, into back-to-back supply agreements with certain raw material suppliers and to make such raw materials available to the Purchaser at cost, for such period as required by the Purchaser to establish the NSAID Divestment Business as a viable and independent business, but not exceeding 2 years from the date of termination of the NSAIDs TSA. Under circumstances outside the control of the Notifying Party, this period can be extended by the Monitoring Trustee until the Purchaser has established the NSAID Divestment Business.
Part E – NSAID TSA
1. The Notifying Party, shall enter into a TSA and supply the NSAID Divestment Products (including [Conf]) (NSAID TSA), to the Purchaser, in sufficient volumes allowing the Purchaser to maintain and expand the existing market position of each of the abovementioned NSAID Divestment Products until the Purchaser has established an alternative production capability. The term of the NSAID TSA will be a maximum of 3 years, with the option for the Purchaser to extend the term subject to prior approval of the Monitoring Trustee (together with the Technical Expert). The TSA will be monitored by the Monitoring Trustee (together with the Technical Expert).
2. The NSAID TSA will have the following characteristics:(a) The Purchaser will grant the Notifying Party a temporary licence for the use of the relevant intellectual property, know-how and technical documentation required for the production of the NSAID Divestment Products;(b) The Notifying Party shall manufacture the NSAID Divestment Products (excluding [Conf])4 in accordance with specified existing product specifications and it shall continue to manufacture the NSAID Divestment Products (excluding [Conf]) at the manufacturing facilities which are currently owned and used by Merial for the production of the relevant NSAIDs to ensure the continued supply of the NSAID Divestment Products, giving priority to the production of the NSAID Divestment Products over the products of the retained business should there be technical difficulties or shortage of supply;(c) The NSAID Divestment Product (excluding [Conf]) shall be produced under the same cost structure and of the same quality and consistent with past practice as Merial produced the NSAID Divestment Product prior to Closing;(d) In the event that the relationship currently in place with [Conf]: (i) cannot be transferred; or (ii) cannot be renegotiated with the Purchaser to ensure immediate supply of [Conf], the Notifying Party will use its Best Efforts to enter into a back-to-back agreement with [Conf] for the supply of [Conf];(e) The Notifying Party commits to supply the NSAID Divestment Products to the Purchaser at full manufacturing cost on a cost pass-through basis (i.e. no mark-up), that will be fixed at the time of signing the TSA. The TSA will allow for a yearly revision of the costs of supply, subject to the approval of the Monitoring Trustee and the Technical Expert, in the event of an increase or decrease in the manufacturing cost of [Conf]% or more, it being understood that any increases in the costs of supply can be based on external factors only (e.g. a change requested by the Purchaser resulting in an increase in the manufacturing cost, an increase in the cost of raw materials or any other justified circumstances outside the control of the Parties resulting in an increase in the manufacturing costs).(f) The Notifying Party shall make available sufficient volumes of the finished NSAID Divestment Product allowing the Purchaser to maintain and expand the existing market position until the Purchaser has established an alternative production capacity, with no limitation to the volume of production subject to Merial’s relevant manufacturing facilities’ capacity. The Notifying Party shall replenish on an on-going basis Merial’s current stock levels in the hands of the Purchaser;(g) The Notifying Party will provide the Purchaser with assistance in order to implement any changes required to the packaging of the relevant NSAID Divestment Products.
3. Under the terms of the TSA, the Purchaser will have the right to request on a transitional basis the Parties to assist in the distribution (for example via logistics and supply chain support) of the NSAID Divestment Products on the Purchaser’s behalf in the EEA on a cost basis, until the Purchaser has established commercial independence and in any event for not longer than the duration of the TSA.
4. In the event of a dispute between the Notifying Party and the Purchaser regarding the manufacturing costs or the quantities, the matter shall be referred to the Monitoring Trustee (together with the Technical Expert) for resolution.
--------------------------------
1 The Notifying Party commits to divest all NSAID Divestment Products on an EEA-wide basis to solve the Commission’s potential competition concerns. However, the Notifying Party intends to divest the NSAID Divestment Products on a worldwide basis, excluding Anafen (Merial’s ketofen based multi- species injectable NSAID) in Canada and Merial’s Equioxx branded products (injectable and oral, including the [Conf]) in the U.S.
2 Customary limitations including, but not limited to, general advertising and approach of Personnel out of their own initiative, etc.
3 Should the Purchaser achieve downstream independence before January 2019, the Notifying Party shall transfer the maximum available inventory stock available to the Purchaser, supervised by the Hold Separate Manager and the Monitoring Trustee. Subsequently, the Notifying Party shall increase and replenish the antigen inventory so as to achieve a [Conf] month antigen inventory stock for the production of Circovac and Progressis by January 2019.
4 In the event that the downstream and upstream production transfer for Mucosiffa, Parvovax and Parvoruvax cannot be completed simultaneously.
5 It is currently expected that any studies in relation to the [Conf] Pipeline Product which are being undertaken by third party contract development organisations will have been completed by the time of Closing.
6 The Notifying Party commits to divest all NSAID Divestment Products on an EEA-wide basis to solve the Commission’s potential competition concerns. However, the Notifying Party intends to divest the NSAID Divestment Products on a worldwide basis, excluding Anafen (Merial’s ketofen based multi- species injectable NSAID) in Canada and Merial’s Equioxx branded products (including the [Conf]) in the U.S.
7 For the avoidance of doubt, the Key Personnel identified for the key functions described Schedule 1 Part B in relation to vaccines may qualify to fulfil the key functions for the NSAID Divestment Business.
8 Produced for Merial by a third-party toll manufacturer.
