Commission, December 18, 2019, No M.9331
EUROPEAN COMMISSION
Decision
DANAHER / GE HEALTHCARE LIFE SCIENCES BIOPHARMA
Subject: Case M.9331 – DANAHER/GE HEALTHCARE LIFE SCIENCES BIOPHARMA
Commission decision pursuant to Article 6(1)(b) in conjunction with Article 6(2) of Council Regulation No 139/2004 (1) and Article 57 of the Agreement on the European Economic Area (2)
Dear Sir or Madam,
(1) On 29 October 2019, the European Commission received notification of a proposed concentration pursuant to Article 4 of the Merger Regulation by which Danaher Corporation (“Danaher”, United States) acquires sole control of GE Healthcare Life Sciences’ Biopharma Business (“GE Biopharma”, United States), within the meaning of Article 3(1)(b) of the Merger Regulation. (3) Danaher is hereinafter referred to as “the Notifying Party”. Danaher and GE Biopharma are hereinafter collectively referred to as “the Parties”.
1. THE PARTIES, THE OPERATION AND THE CONCENTRATION
(2) Danaher is the ultimate holding company of a group that designs, manufactures and markets professional, medical, industrial and commercial products and services. In the life sciences area, Danaher is primarily active through its wholly-owned subsidiaries Pall Corporation (“Pall”), Molecular Devices, L.L.C (“MolDev”), Leica Microsystems GmbH (“Leica”), Beckmann Coulter, IDBS, Integrated DNA Technologies, Phenomenex, Inc. (“Phenomenex”) and SCIEX.
(3) GE Biopharma is part of General Electric’s (“GE”) Healthcare Life Sciences business unit. GE Biopharma supplies instruments, consumables and software for the research, discovery, process development and manufacturing workflows of biopharmaceutical drugs, such as monoclonal antibodies, vaccines, and cell and gene therapies. GE Biopharma is not organised as a separate stand-alone group of companies, but is held by different legal entities within GE. (4) GE Biopharma comprises all properties, assets, goodwill and rights that relate primarily to the manufacture and distribution of instruments, consumables, and software that support the research, discovery, process development and manufacturing workflows of biopharmaceutical drugs and that are currently owned by GE, either directly or indirectly though GE’s subsidiaries.
(4) On 25 February 2019, Danaher and GE entered into an Equity and Asset Purchase Agreement under which Danaher would acquire GE Biopharma by means of an acquisition of equity interests and assets for USD 21 400 million (EUR 18 100 million) (the “Transaction”). Upon completion of the Transaction, Danaher would, either directly or indirectly own all equity interests and all assets belonging to GE Biopharma. Therefore, the Transaction constitutes a concentration pursuant to Article 3(1)(b) of the Merger Regulation.
2. UNION DIMENSION
(5) The combined aggregate turnover of the Parties is more than EUR 5 000 million (Danaher: EUR […] million; GE Biopharma: EUR […] million) and the aggregate Union-wide turnover of each of the Parties is more than EUR 250 million (Danaher: EUR […] million; GE Biopharma: EUR […] million). Neither of the Parties achieves more than two-thirds of their Union-wide turnovers within one and the same Member State. The Transaction therefore has a Union dimension pursuant to Article 1(2) of the Merger Regulation.
3. COMMISSION’S APPROACH AND STRUCTURE OF THE COMMISSION’S ASSESSMENT
(6) The Parties’ activities horizontally overlap in the manufacture and sale of various products and services used in the bioprocessing industries. Moreover, there are also some vertical links to be assessed between the Parties respective activities, as well as possible conglomerate effects. In section 4 of this decision, the Commission will firstly introduce the area of biologics and bioprocessing, followed by an assessment of possible horizontal non-coordinated effects in the various relevant markets, which form part of the bioprocessing workflow (section 4.2 to 4.7). Where relevant, the Commission will assess possible non-coordinated vertical effects (sections 4.2.1.2(B), 4.2.2.2(B) and 4.7.2.2) and possible coordinated effects in section 4.8. In section 4.9, the Commission will then assess possible conglomerate effects.
(7) Moreover, the Parties’ activities horizontally overlap in the manufacture and supply of products and services which, while not directly forming part of the bioprocessing workflow, are closely related to this. In section 5, the Commission assesses possible horizontal non-coordinated effects in relation to the Parties’ activities in those other life sciences research segments, as well as possible coordinated effects.
(8) Sections 7 and 8 will be dedicated to the Commission’s assessment of the Commitments offered by the Notifying Party.
4. MARKET DEFINITION AND COMPETITIVE ASSESSMENT CONCERNING THE PARTIES’
ACTIVITIES IN BIOPROCESSING
4.1. Introduction
4.1.1. Biologics and bioprocessing
(9) Bioprocessing is a broad term encompassing the research, development and, mainly, manufacturing of products prepared from or used by biological systems – namely, cells. Those products are known as biopharmaceuticals or biologics. Although cell- derived pharmaceutical products – such as vaccines and blood products – have long been used for therapeutic purposes, in the last two decades a new class of therapeutics has emerged that is based on proteins produced by genetically engineered living cells. New types of biologics include most notably monoclonal antibodies (“mAbs”). mAbs therapies were developed through a series of scientific advances since the 1980s, and currently are the largest product group within biologics. Most recent classes of biologics therapies are cell and gene therapy. In cell therapy, cells (for instance, stem cells) are injected in patients to treat a certain disease. In the gene therapy, faulty genes causing a disease are modified or inactivated. The latter can be performed by harvesting, curing and re-injecting the patient’s own cells, or by administering modified viruses (“viral vectors”) containing the corrected gene(s).
(10) Although biologics are a relatively young class of therapies, their benefits have fuelled rapid growth. One report estimates that the biologics market was valued at approximately USD 255 000 million in 2017 and is expected to more than double by 2026. (5) The development of biologics and in particular mAbs since the 1980s has also led to the emergence and growth of an industrial bioprocessing sector that supplies equipment and consumables to develop and manufacture biologics, which likewise has seen significant expansion. Its five-year expected annual growth rate is almost 9%. (6)
(11) While the manufacturing process of biologics differs somewhat depending on the specific (type of) product, a typical and standardised process such as the one for mAbs begins with cell culture – growing cells within the laboratory – followed by a scale-up process in which the cell culture is grown to the levels required for manufacturing. After sufficient cell growth is achieved, the desired protein must be separated from the cells and the growth media purified. This involves various stages of filtering and clarification, as well as mixing the protein with sterile solutions at the desired level of dilution for administration to the patient. (7)
(12) Bioprocessing can be divided into two broad manufacturing steps: an “upstream” process, during which cells are grown and the biologic target harvested, and a “downstream” process during which the desired drug product is isolated from the cells, purified and ultimately formulated and packaged into a final drug product – typically a filled vial or syringe. (8)
4.1.2.Customer preferences and regulatory constraints
(13) Choices of bioprocessing consumables and hardware are often made at the early stages of the drug development process, based principally on technical considerations. A key feature of the industry is that biologics manufacturers must provide full characterisation of their products for regulatory approval. Such characterisation includes testing of the production process and its control.
(14) In the Union, the European Medicines Agency (“EMA”) is responsible for central evaluation and authorisation of biologics (in EMA terminology, advanced therapy medicinal products or “ATMPs”). In addition to physicochemical testing, biologics require biological testing for full characterisation. Such characterisation combines testing of the active substance and the final medicinal product together with the production process and its control. Thus, the characterised and tested production process, which is designed by the manufacturer of the ATMP, becomes part of the basis for regulatory approval of the ATMP or biologic.
(15) Thus, once regulatory approval to produce a new biologic using a defined manufacturing process has been obtained, and a manufacturer has selected a particular product for a step in this process, it often tries to avoid switching because doing so would require re-characterisation/recertification. It also appears that manufacturers typically do not qualify more than one product for the same use in a given production process, and that they are thus largely locked-in with their suppliers once the product decision has been made.
(16) The Parties explain that it is typical for customers to select hardware and consumables from many different suppliers for a single bioprocessing setup. In light of the replies provided in the course of the market investigation, the Commission finds that customers appear in general to be able to “mix-and-match” equipment from different suppliers. (9)
(17) It should be noted that a significant trend within bioprocessing – especially for commercial production – is the rapidly growing use of recently developed single-use technology (“SUT”) products made of disposable plastic instead of conventional glass or stainless-steel vessels and tubings. SUT provide benefits in terms of lower initial investment cost, ease of use and flexibility, although consumables are more expensive. In particular, it appears that traditional steel hardware requires manufacturers to file with regulators a cleaning protocol, which they need to demonstrate ensures that there is no cross-contamination between product batches produced with the same hardware. Non-SUT also require the actual cleaning of hardware between production campaigns.
(18) Another trend in bioprocessing appears to be that, while traditional biopharma manufacturing occurs through a “batch” process – with the raw materials used to create the biologic being processed as discrete quantities at each stage of manufacturing – there are emergent methods that involve continuous processing at least at certain stages.
4.1.3. Bioprocessing products
(19) Bioprocessing technologies in manufacturing processes include both hardware and various types of consumables. Bioprocessing hardware includes equipment such as mixers, bioreactors, columns for housing the chromatography process and “skids” or “systems” that manage the flow of fluids being processed. Bioprocessing consumables, which account for the vast majority of sales in the industry, include a large number of products, such as cell culture media, chromatography resins or filters, offered by a broad range of suppliers. (10)
(20) Pursuant to explanation of the Notifying Party, (11) bioprocessing at the upstream level generally involves three types of technologies and related products: bioreactors; filtration equipment; and various mixing and storage instruments.
- Bioreactors are the key element of the upstream manufacturing process. They are vessels in which cells are grown in a controlled environment. Most commonly the cells are suspended in a liquid solution that is stirred or rocked to encourage cell growth.
- Filtration solutions are used at various stages of the upstream process to purify and clarify the cell growth solution and ensure its sterility before it is introduced into the bioreactor. The most prevalent type of filtration is direct flow filtration where a mixture flows through the filter. Another type of filtration is tangential flow filtration where the mixture flows alongside the filter.
- Mixers and storage instruments are used to prepare and store cell growth solutions. Mixers can also be used to mix other inputs, such as powders.
(21) Throughout the downstream manufacturing process, various types of equipment are used to separate unwanted (for example cell mass) from wanted (for example proteins) products of the upstream cell production process. The technologies generally fall into two areas:
- Chromatography involves purification technologies that separate desired from undesired elements through chemical processes by running a liquid “mobile phase” through a “stationary phase” of chemically treated resins.
- Filtration typically removes unwanted from wanted elements of the cell growth process through physical separation by running liquids through porous membranes.
(22) The Commission’s assessment of the Parties’ activities in the bioprocessing industry will start with horizontal non-coordinated effects and vertical relationships concerning the upstream process followed by an assessment of the horizontal non- coordinated effects and vertical relationships between the Parties’ activities in the downstream process before moving to possible conglomerate and coordinated effects.
4.2. SUT products
(23) The Parties sell SUT bioreactors, mixers, connectors and bags for use in the upstream bioprocessing steps. The Parties consider each of these functions as different product markets. (12) SUT products have consumable elements made of flexible or semi-rigid plastic. They are intended for one-time use and subsequent disposal, which improves safety and reduce cleaning and sterilisation costs. These consumables are designed to work only with the SUT hardware purchased from a given supplier. SUT products are growing rapidly in bioprocessing.
(24) Bioreactors are vessels that create the appropriate environment through several conditions(13) that enable cell culture growth for the purposes of producing biologics. The hardware part consists of a tank with a motor into which the bag is inserted and an automation and process control system, as well as sensors to control critical operating parameters, such as oxygen, pH, temperature and pressure, and mass flow controllers to regulate the gas flow into the bioreactor. The mixing technology may consist of a rocking platform (rocking bioreactor), a tank (stirred tank bioreactors) or a docking station and equipment to control sensors, mixing or gassing (fixed-bed bioreactor). The consumable part is the disposable plastic assemblies designed for specific hardware systems that contain the cells and cell culture media during cell growth.
(25) SUT mixers are used to perform process steps such as preparing media and buffers, as well as virus inactivation. They are only used for mixing purposes. Most mixers consist of a sterile bag assembly containing a magnetically driven impeller supported by plastic or stainless-steel hardware.
(26) SUT connectors allow to manage fluid between separate fluid pathways in the bioprocessing industry. SUT connectors have largely replaced traditional tube welding (that is joining two pieces of thermoplastic tubing by using high heat), as they provide a faster and easier solution. While it is not possible to use one supplier’s connector on one end with another supplier’s connector on the other end, they can be connected to the SUT system of a different supplier.
(27) SUT bioprocess bags are used for the collection, storage, transport and feed-in of biopharmaceutical liquids in bioproduction processes.
(28) The vast majority of customers and competitors (14) who expressed an opinion in the market investigation on this point submitted that SUT bioreactors, SUT mixers and SUT connectors may be distinguished from each other based on their functions. As some market respondents stated: (15) “they all have different functions which have different performance requirements, thereby making their classifications distinct”, “each product has its own use and can be use independently from each other. None can be replaced by another one” or “these products play different roles in bioprocessing and should therefore be distinguished from each other”.
(29) In light of these considerations as well as all evidence available to it, the Commission will assess the different SUT product categories separately.
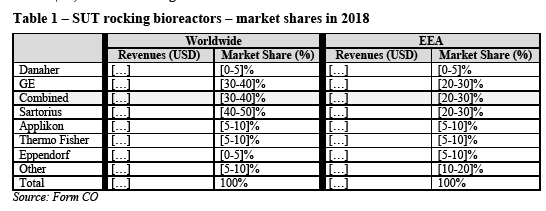
4.2.1. SUT bioreactors
4.2.1.1. Market definition
(A) Product market definition
(A.i) Notifying Party’s arguments
(30) The Notifying Party submitted that (i) SUT rocking bioreactors; (ii) SUT stirred tank bioreactors; and (iii) SUT fixed-bed bioreactors belong to separate product markets. (16) The Notifying Party provides a number of arguments in this regard:
(a) Rocking and stirred tank bioreactors would most commonly be used in suspension cell culture. Rocking and stirred tank bioreactors would be used at different stages of the bioprocess workflow (17): whereas rocking bioreactors may be used as a seed train bioreactor for the stirred tank, (18) stirred tank bioreactors are generally used for larger scale operations, such as development and production.
(b) Fixed-bed bioreactors would represent a niche that can only be used for adherent cell culture. (19) The Notifying Party submits that the cases where either fixed-bed bioreactors or microcarriers in stirred tank bioreactors can be used interchangeably to grow adherent cells are limited to small-scale R&D activities (20) and to instances where customers are interested in what the cell produces rather than in the cells themselves. (21) While fixed-bed bioreactors are not well suited for very large-scale cell culture, stirred tanks in combination with microcarriers may be used for large-scale production of vaccines. (22)
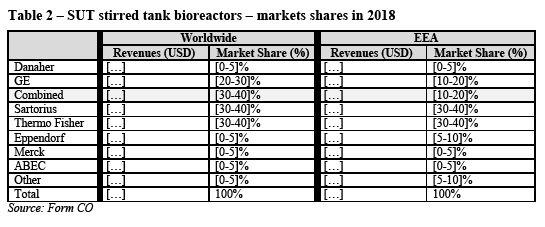
(31) The Notifying Party submits that there is generally no substitution between SUT fermenters (vessels that used for the growth of microorganisms in non-mammalian microbial fermentation applications) and SUT bioreactors and therefore excludes the former from its proposed assessment. (23)
(32) The Notifying Party considers that it is not necessary to distinguish between different sizes of SUT bioreactors because the size of the product is determined by the application it is used in and all suppliers generally offer a range of sizes and volumes to address these needs. (24)
(33) The Notifying Party submits that markets should not be further segmented by types of customers because the majority of the SUT products are sold to bioprocessing with only a small part being sold to academia customers. (25)
(34) The Notifying Party considers that it is not necessary to define a separate product market for the hardware (tanks and skids) used to hold and control the consumable part of SUT bioreactors. (26) In this regard, the Notifying Party submits that the hardware and consumable parts are initially sold together as an SUT bioreactors and that a given supplier’s SUT bioreactor generally cannot be used with consumable SUT bioreactor bags supplied by another supplier. (27)
(A.ii) Commission’s assessment
(35) In a previous decision (28), the Commission left open whether SUT bioreactors, SUT bags, SUT mixers and SUT transfer sets constitute separate product markets or whether they belong to a single SUT product market. The Commission did not differentiate between different types of bioreactors in that decision.
(36) A majority of customers who expressed an opinion in the market investigation on this point submitted that they are not generally able to use SUT rocking bioreactors, SUT stirred tank bioreactors and SUT fixed-bed bioreactors interchangeably or are only able to do so to a limited extent. (29) In this regard, a customer submitted that “[e]ach type of SUT bioreactor have its own specification and range of use. [The respondent] choose the one that best fits the cultivation constrains imposed by the research project objectives. On very seldom occasion (once in 6 years), [the respondent] has hesitated between stirred tank and rocking bioreactor”. (30) “[Stirred tank and rocking bioreactors] are used at different steps in the manufacturing process and are not interchangeable”.
(37) Some customers referred to the size of the batch and the individual steps of the bioprocessing workflow as factors limiting the interchangeability between different types of SUT bioreactors. One customer submitted that “[r]ocking bioreactors are used in early steps to grow cells and are limited to a volume of 200 litres. On the other hand, stirred-tank bioreactors are used in the production phase; they are more advanced in their parameters and technical settings, and may be used with a volume of 1 500 - 2 000 litres. While technically both types of bioreactors could be used for the same purpose of growing cells, stirred-tanks bioreactors are generally too complex for smaller scale where rocking bioreactors can be used”. (31) Another customer stated that “[d]ifferent bioreactors as listed above are best fit depending on the processes that you are running as well as the size of a batch that you need to do. For example, rocking bioreactors will be smaller and not able to handle cultures which are higher titer or require greater O2 control and/or consumption”. Another customer stated that “[these different types of bioreactors] have different functions at different scales and so in general are not interchangeable”. Another customer stated that these are “[u]sed for different volume and steps of the process”.
(38) Other customers referred to the differences between suspension and adherent cell culture. In this regard, one customer stated that “[e]specially fixed-bed and stirred tank are not interchang[e]able for suspension cell cultures”. (32) Another customer expressed that “[e]ach of the type of reactors quoted has a specific purpose in a production system, rocking systems are typically used in preculture applications. Stirred tank reactors are used in for production cultures, and fixed bed cultures are specific in their use with adherent cultures. None of these uses are interchangeable”.
(39) Moreover, none of the competitors who expressed an opinion on this point indicated that customers are generally able to use these three types of SUT bioreactors interchangeably. (33)
(40) In addition, a majority of customers who expressed an opinion in the market investigation on this point submitted that, for the specific purpose of growing adherent cell cultures, they are not generally able to use interchangeably fixed-bed bioreactors and rocking/stirred tank bioreactors in combination with microcarriers. (34) In this regard, a customer stated that “[f]or adherent cells, the Company uses (i) small culturing vessels or plastic pipes at an initial phase; (ii) Danaher’s fixed- bed bioreactors at an intermediate phase; and (iii) stirred-tank bioreactors in combination with microcarriers when a larger commercial-scale is required”. (35)
(41) The Commission notes, based on the information provided by the Notifying Party, that a variety of suppliers offer SUT rocking bioreactors with working volumes between 1-5l and 25-50l and SUT stirred tank bioreactors with working volumes between 1-5l and 2,000l. (36) This has been confirmed by a majority of customers who expressed an opinion in the market investigation on this point, which consider that suppliers of SUT bioreactors have a credible offering of differently sized SUT bioreactors (from 1l to 2,000l) to address different needs. (37) The response is however mixed within competitors. (38)
(42) As to SUT fermenters, over the course of the market investigation, no market participant put forward any arguments in relation to a potential substitutability between SUT fermenters and SUT bioreactors.
(43) For the purposes of this decision and in light of all information available to it, the Commission considers that (i) SUT rocking bioreactors; (ii) SUT stirred tank bioreactors; and (iii) SUT fixed-bed bioreactors constitute separate product markets, given that these three types of SUT bioreactors are mostly used at different stages of the bioprocess workflow and that they generally serve different applications. Moreover, the Commission does not consider necessary to further segment these product markets based on the size of the SUT bioreactor, since suppliers have a credible offering of differently sized bioreactors. The Commission does not consider it necessary to define a market for SUT fermenters as those are not relevant for the assessment of the Transaction.
(B) Geographic market definition
(B.i) Notifying Party’s arguments
(44) The Notifying Party submitted that SUT bioreactors are worldwide and in any event, not narrower than EEA-wide in scope, as these products are manufactured at centralised sites and shipped via regional distribution hubs to customers globally. (39)
(45) Moreover, the Notifying Party submitted that (i) transportation costs are low as a proportion of total costs, representing around […]% of the sales prices of SUT bioreactors; (ii) there are no regulatory differences within the EEA; (iii) custom duties do not affect transportation globally; and (iv) pricing is similar across the EEA. (40)
(B.ii) Commission’s assessment
(46) The market investigation confirmed the Notifying Party’s submission regarding geographic market definition.
(47) In relation to rocking bioreactors, stirred tank bioreactors and fixed-bed bioreactors, the large majority of customers and competitors who expressed an opinion on this point stated that (i) they procure SUT bioreactors at worldwide level; (ii) after-sale services are provided at worldwide level; (iii) prices are comparable at worldwide level; and (iv) the same suppliers are active at worldwide level. (41)
(48) For the purposes of this decision, it can be left open whether the market for SUT rocking bioreactor, SUT stirred tank bioreactor and SUT fixed-bed bioreactors is global or EEA-wide in scope. The Transaction does not give rise to serious doubts as to their compatibility with the internal market or the functioning of the EEA Agreement under any of these plausible geographic market definitions.
4.2.1.2. Competitive assessment
(A) Horizontal overlaps
(49) The Transaction leads to a horizontal overlap between the Parties’ offerings in SUT rocking bioreactors and SUT stirred tank bioreactors.
(50) Danaher also offers its iCELLis SUT fixed-bed bioreactors, where GE is not active. (42) Moreover, GE offers SUT fermenters, while Danaher does not. Given the absence of any horizontal overlap, SUT fixed-bed bioreactors and SUT fermenters will not be discussed further for the purpose of this decision.
(A.i) SUT rocking bioreactors
(51) According to the Parties’ estimates set out in Table 1 above, the combined entity would hold a market share of [30-40]% (GE: [30-40]%; Danaher: [0-5]%) in a worldwide market for SUT rocking bioreactors. Following the Transaction, Sartorius will continue being the market leader with a market share of [40-50]%. Danaher would […] add a modest [0-5]% market share, and it is currently a smaller player in SUT rocking bioreactors than Applikon ([5-10]%), Thermo Fisher ([5-10]%) or Eppendorf ([0-5]%). Danaher’s small market share is due to it being a recent entrant in this market with only a small rocking bioreactor limited to 25L.
(52) At the EEA-level, and according to the Parties’ estimates set out in Table 1 above, the combined entity would hold a market share of [20-30]% (GE: [20-30]%; Danaher: [0-5]%) for SUT rocking bioreactors. Following the Transaction, Sartorius will continue being the market leader with a market share of [20-30]%. Danaher would only add a modest [0-5]% market share, and it is currently a smaller player in SUT rocking bioreactors than Applikon ([5-10]%), Thermo Fisher ([5-10]%) or Eppendorf ([5-10]%).
(53) A large majority of customers who expressed an opinion in the market investigation on this point submitted that they would have credible alternative suppliers of SUT rocking bioreactors post-Transaction. (43) In this regard, a customer stated that it “considers there to be sufficient competitors and options in this space. Post- Transaction, [the respondent] is comfortable that it will be able to find sufficient credible alternative suppliers of SUT bioreactors to the merged entity”. This view was unanimously backed by all competitors who expressed an opinion in the market investigation on this point. (44)
(54) Moreover, the results of the market investigation indicated that GE and Danaher would not be close competitors in SUT rocking bioreactors. A clear majority of customers and competitors who expressed an opinion in the market investigation on this point considered that GE is the strongest player and that Sartorius is the second strongest player in this market. (45)
(55) In addition, the replies from the market investigation confirmed the Notifying Party’s argument that GE’s and Sartorius’ strong position is due to the fact that they respectively acquired Wave Biotech LLC in 2007 and Wave Biotech AG in 2008, which resulted from the split of Wave Biotech, the inventor of SUT rocking bioreactors, into two companies. (46) In this regard, a customer elaborated as to GE’s and Sartorius’ relative position in a market for SUT rocking bioreactors: “[t]he rocking bioreactor technology was developed by Wave Biotech over 20 years ago and subsequently acquired by GE. A European based copy of the same technology was purchased by Sartorius. These have been the dominant players in this market place”. (47) This is consistent with the replies from competitors, who stated that “[s]trongest players were first to market with their SUT bioreactors” and that “Thermo has had a long-standing leadership in classical single-use bioreactors”. (48)
(56) A majority of customers and competitors who expressed an opinion in the market investigation on this point does not expect an impact of the Transaction on price, quality, product range, innovation or security of supply. (49)
(57) In light of the considerations in paragraphs (51) to (56) above as well as all evidence available to it, the Commission concludes that, in the worldwide or EEA-wide market for SUT rocking bioreactors, the Transaction will not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to horizontal non-coordinated effects given in particular (i) the moderate combined share of the Parties; (ii) the small increment contributed by Danaher, which is a recent entrant to this market, to GE’s position; (iii) the fact that the parties are not close competitors to one another; and (iv) the presence of a strong competitor, Sartorius, matching the combined entity’s market shares.
(A.ii) SUT stirred tank bioreactors
(58) According to the Parties’ estimates set out in Table 2 above, the combined entity would hold a market share of [30-40]% (GE: [20-30]%; Danaher: [0-5]%) in a worldwide market for SUT stirred tank bioreactors. Following the Transaction, Sartorius and Thermo Fisher will remain very similar in size to the combined entity, both with market shares of [30-40]%.
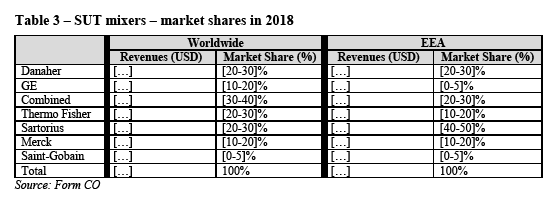
(59) At the EEA-level, and according to the Parties’ estimates set out in Table 2 above, the combined entity would hold a market share of [10-20]% (GE: [10-20]%; Danaher: [0-5]%) for SUT stirred tank bioreactors. Following the Transaction, Sartorius and Thermo Fisher will continue being the market leaders, both with market shares of [30-40]%.
(60) A large majority of customers who expressed an opinion in the market investigation on this point submitted that they would have credible alternative suppliers of SUT stirred tank bioreactors post-Transaction. (50) This view was unanimously backed by all competitors who expressed an opinion in the market investigation on this point. (51)
(61) Moreover, the results of the market investigation indicated that GE and Danaher would not be close competitors in SUT stirred tank bioreactors. Customers who expressed an opinion in the market investigation on this point were divided as to whether GE or Sartorius are the strongest player in SUT stirred tank bioreactors. (52) A majority of competitors and a significant number of customers selected Thermo Fisher as the strongest player in this market. (53) Very similar views on this point were expressed in relation to the second strongest player in SUT stirred tank bioreactors, with customers divided between GE and Sartorius.
(62) A majority of customers and competitors who expressed an opinion in the market investigation on this point does not expect an impact of the Transaction on price, quality, product range, innovation or security of supply. (54)
(63) In light of the considerations in paragraphs (58) to (62) above as well as all evidence available to it, the Commission concludes that, in the worldwide or EEA-wide market for SUT stirred tank bioreactors, the Transaction will not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to horizontal non-coordinated effects given in particular (i) the moderate combined share of the Parties; (ii) the small increment contributed by Danaher to GE’s position; (iii) the fact that the Parties are not close competitors to one another; and (iv) the presence of two strong competitors, Sartorius and Thermo Fisher, matching the combined entity’s market shares.
(B) Vertical relationships
(64) The Transaction leads to a vertical link between the upstream supply of direct flow filtration (“DFF”) consumables (described in detail in section 4.5) and the downstream supply of SUT rocking bioreactors and SUT stirred tank bioreactors. DFF consumables are generally used in SUT bioreactors, while tangential flow filtration (“TFF”) hollow fibre consumables are used in SUT bioreactors for perfusion applications. (55)
(65) An internal email of Danaher identified […]. (56) […]. (57)
(66) The Notifying Party explains that “DFF filters used in bioreactors are always chosen by the manufacturer of the bioreactor to ensure that the overall specifications of the bioreactor are met”. (58) While the Notifying Party recognises that, theoretically, DFF filters from other suppliers could be substituted, this would only be possible at the point of manufacture and would be expensive and time consuming, without adding any significant value to the customer. Because of this, the Notifying Party submits that “no bioreactor supplier offers customers the ability to customize the bioreactor bag with their DFF filter of choice because there is no customer demand for this”. (59)
(67) Against this background, the Commission investigated whether the Transaction would lead to non-coordinated vertical effects in the form of input or customer foreclosure.
(68) As regards a possible input foreclosure, the Commission assessed whether post- Transaction, the merged entity could restrict access to its DFF consumables or TFF hollow fibre consumables and thereby foreclose its competitors on the market for SUT bioreactors.
(69) In this regard, the Commission firstly notes that as described in section 4.5, Pall sells DFF consumables and holds a market share of [20-30]% in the EEA and [30-40]% at the worldwide level. In turn, GE is active only […], with a […] market share of [0-5]% in both the EEA and worldwide level. Moreover, there are a number of credible competitors active in DFF consumables (see section 4.5.3.1) so that any attempt by the merged entity to foreclose access to its DFF consumables is likely to be defeated by its competitors in DFF consumables, such as Merck Millipore or Sartorius, who both have a broad portfolio of DFF solutions. (60) Therefore, the Commission considers that the merged entity would not have a sufficiently strong market position in DFF consumables to engage in input foreclosure to the detriment of its competitors.
(70) As regards TFF hollow fibre consumables, the Commission notes that while GE holds a strong position (see paragraph (264) below), […]. Thus, there is no merger- specific change as regards the future market position of the merged entity compared to GE’s current market position in TFF hollow fibre consumables. Against this background, the Commission considers it unlikely that the merged entity would engage in input foreclosure with respect to TFF hollow fibre consumables as a result of the Transaction.
(71) As regards possible customer foreclosure, the Commission assessed whether the merged entity would have the ability to foreclose competing suppliers of DFF or TFF hollow fibre consumables from accessing customers.
(72) In this context, the Commission firstly notes that bioprocessing filtration consumables such as DFF and TFF hollow fibre consumables are not only sold as an input for SUT bioreactors as part of the upstream process, but also on a stand-alone basis or together with hardware used in the downstream process. Thus, even if the merged entity could successfully retro-fit its own consumables into SUT rocking or SUT stirred-tank bioreactors, this would not impact on competitors’ sales of DFF and TFF hollow fibre consumable sales that are unconnected to bioreactors.
(73) Secondly, the Commission notes that the merged entity’s market share in SUT rocking bioreactors would amount to [30-40]% at worldwide level and [20-30]% at EEA level. For SUT stirred tank bioreactors, the merged entity’s market share would amount to [30-40]% and [10-20]% respectively at worldwide and EEA level. This implies that a large part of SUT rocking and stirred tank bioreactors sales would not be affected by a possible retro-fitting strategy of the merged entity.
(74) Thirdly, the Commission considers that any possible customer foreclosure strategy by the merged entity would also be limited by customer preferences. Contrary to the submission by the Notifying Party, a majority of customers who expressed an opinion in the market investigation on this point considers that they typically decide which DFF consumable is included in the SUT bioreactor, with a minority considering that the supplier of SUT bioreactors selects the DFF consumable to be used. (61) In this regard, a customer stated that “[t]ypically, the relevant supplier of the bioreactor would recommend the DFF consumables that it normally offers (usually its own filters) but will always be willing to include a different filter if requested. While their own filter is likely the default, suppliers tend to be flexible following a request”. (62)
(75) Therefore, the Commission considers that the merged entity would also lack the ability to foreclose competing suppliers of DFF and TFF hollow fibre consumables to access respective customers.
(76) In light of the considerations in paragraphs (64) to (75) above as well as all evidence available to it, the Commission concludes that, in the worldwide or EEA-wide market for SUT bioreactors and the worldwide or EEA-wide markets for DFF and TFF hollow fibre consumables, the Transaction will not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to vertical non-coordinated effects.
4.2.2. SUT mixers and related IP
(77) SUT mixers are used to perform process steps such as preparing media and other process liquids, (63) as well as virus inactivation. (64) SUT mixers make solutions by mixing various ingredients and cannot be used for adherent or suspension cell culture. (65) Most mixers consist of a sterile bag assembly containing a magnetically driven impeller supported by plastic or stainless-steel hardware. (66)
4.2.2.1. Market definition
(A) Product market definition
(A.i) Notifying Party’s arguments
(78) The Notifying Party submitted that it is not necessary to segment SUT mixers into various categories given that all suppliers offer all types of mixers and can provide a solution based on the user requirement specification of the customer. (67)
(79) The Notifying Party considers that it is not necessary to define a separate product market for the hardware (tanks and skids) used to hold and control the consumable part of SUT mixers. (68) In this regard, the Notifying Party submits that the hardware and consumable parts are initially sold together as an SUT mixer and that a given supplier’s SUT mixer generally cannot be used with consumable SUT mixer bags supplied by another supplier. (69)
(80) The Notifying Party submitted that a further segmentation of the mixer portfolio could nevertheless be made based on the functionality that the mixer provides (that is pH or temperature control), and whether the mixers are locally controlled or connected to the customer’s automation systems. (70) On this basis, it may be possible to identify the following sub-segments: (i) mixers with sensors; (ii) mixers with jackets; (iii) mixers with load cells; and (iv) mixers that are controlled locally or remotely. (71)
(A.ii) Commission’s assessment
(81) In a previous decision, (72) the Commission left open whether SUT bioreactors, SUT bags, SUT mixers and SUT transfer sets constitute separate product markets or whether they belong to a single SUT product market. The Commission did not differentiate between different types of SUT mixers in that decision.
(82) The market investigation in this decision has largely confirmed the Parties’ submissions regarding product market definition.
(83) A majority of customers and competitors who expressed an opinion in the market investigation on this point considered that SUT mixers should not be further segmented according to separate categories based, inter alia, on the different functionality they provide (namely, SUT mixers with sensors, SUT mixers with jackets, SUT mixers with load cells and SUT mixers that are controlled locally or remotely). (73) In this regard, a customer expressed that “[t]he SU mixing is the main functionality and all the others are technical options that end users will request based on the specificities of their processes”. Another customer stated that it “often require[s] an SUT mixer to have sensors, load cells and jackets and be capable of being controlled locally and remotely. [The respondent] does not consider it makes sense to split SUT mixers by such categories given the requirement to mix and match functionality on a machine”. Moreover, a competitor expressed that “[a]ll these variants […] (as well as gas handling) exist and are used at times and not at other times and for various process steps”. This competitor moreover stated that “[i]n general, […] general mixers seems to be a reasonable split”.
(84) Over the course of the pre-notification market investigation, it was put forward that an additional distinction could be made between top-mounted and bottom-mounted impeller mixers
(85) A majority of those customers who expressed an opinion in the market investigation on this point considered that they were generally able to use top-mounted SUT impeller mixers for the same applications as for which bottom-mounted impeller mixers are used. (74) In this regard, a customer expressed that “[t]he only difference between both options is purely operational and [that the] decision would be based on the clean room infrastructure where the SU mixing systems would be installed”. Another customer expressed that, “[i]n practice, [it has] used both top-mounted SUT impellers mi[x]ers and bottom-mounted impeller mixers for the same applications in the past. Ultimately, both function as mixers but with differing entry points. [The respondent] does not consider there to be a substantive difference between the two”. Another customer said that, “when designing a manufacturing process [they] would be able to select either a top- or bottom-mounted impeller mixer regardless of the product”.
(86) Moreover, a majority of competitors who expressed an opinion in the market investigation on this point considered that customers are generally able to use top- mounted impellers for the same applications as for which bottom-mounted impeller mixers are used. (75) In this regard, a competitor expressed that both types of impeller mixers are targeted towards “the same customers and the same applications. Top- mounted impellers have certain advantages, bottom-mounted have other advantages. It depends on the priorities of the customer regarding flexibility, openness, cleaning and other factors”.
(87) For the purposes of this decision, in the light of all evidence available to it, the Commission considers that there is a single relevant market including all types of SUT mixers.
(B) Geographic market definition
(B.i) Notifying Party’s arguments
(88) The Notifying Party submitted that SUT mixers are worldwide and in any event, not narrower than EEA-wide in scope, as these products are manufactured at centralised sites and shipped via regional distribution hubs to customers globally. (76)
(89) Moreover, the Notifying Party submitted that (i) transportation costs are low as a proportion of total costs, representing around […]% of the sales prices of SUT mixers; (ii) there are no regulatory differences within the EEA; (iii) custom duties do not affect transportation globally; and (iv) pricing is similar across the EEA. (77)
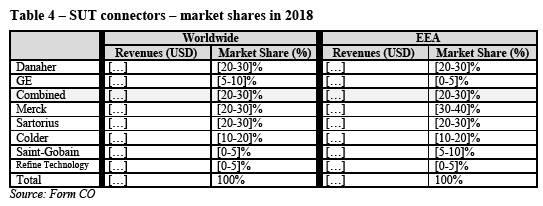
(B.ii) Commission’s assessment
(90) The market investigation confirmed the Notifying Party’s claims regarding geographic market definition.
(91) The large majority of customers and competitors who expressed an opinion on this point stated that (i) they procure SUT mixers at worldwide level; (ii) after-sale services are provided at worldwide level; (iii) prices are comparable at worldwide level; and (iv) the same suppliers are active at worldwide level. (78)
(92) For the purposes of this decision, it can be left open whether the market for SUT mixers is global or EEA-wide in scope. The Transaction does not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement under any of these plausible geographic market definitions.
4.2.2.2. Competitive assessment
(A) Horizontal overlaps
(93) In SUT mixers, the Transaction does not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement for the following reasons.
(94) The combined entity will have moderate market shares at both the worldwide and the EEA-levels. As Table 3 shows, the Transaction will combine the current […] and […] players in the worldwide market for SUT mixers and will position the combined entity as the […] player in the market with a market share of [30-40]%. The combined entity will be closely followed by Thermo Fisher and Sartorius, which are roughly similar in size ([20-30]% and [20-30]% respectively). At the EEA-level, Sartorius will continue being the largest player in the market, with a market share of [40-50]%.
(95) A majority of customers and all competitors who expressed an opinion in the market investigation on this point considered there will be credible alternative suppliers of SUT mixers post-Transaction. (79)
(96) A majority of customers and competitors who expressed an opinion in the market investigation on this point considered Sartorius as the closest competitor to Danaher in SUT mixers. (80)
(97) Moreover, a majority of customers and competitors who expressed an opinion on this point did not expect the Transaction to have a negative impact on price, quality, product range, innovation or security of supply. (81)
(98) In light of the considerations in paragraphs (94) to (97) above as well as all evidence available to it, the Commission concludes that, in the worldwide or EEA-wide market for SUT mixers, the Transaction will not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to horizontal non-coordinated effects.
(B) Vertical relationships
(99) There is a vertical link between Danaher’s upstream licensing of intellectual property rights […] and the downstream supply of SUT mixers by its competitors.
(100) Danaher [Danaher’s intellectual property rights]. (82)
(101) Currently, [Danaher’s intellectual property rights and license agreements]. (83)
(102) Moreover, [Danaher’s distribution agreements]. (84)
(103) Against this background, the Commission investigated whether the Transaction would lead to non-coordinated vertical effects in the form of input foreclosure. Specifically, the Commission assessed whether post-Transaction, Danaher could restrict access to its intellectual property rights […] and thereby foreclose its competitors on the market for SUT mixers.
(104) In assessing the likelihood of an anticompetitive input foreclosure scenario, the Commission examines, first, whether the merged entity would have, post-merger, the ability to substantially foreclose access to inputs, second, whether it would have the incentive to do so, and third, whether a foreclosure strategy would have a significant detrimental effect on competition downstream. In practice, these factors are often examined together since they are closely intertwined. (85)
(B.i) Ability to foreclose
(105) In light of the fact that Danaher’s intellectual property rights […] are used by many manufacturers of SUT mixers, the Commission has firstly assessed whether Danaher would have the ability to restrict access to those rights.
(106) In this context, the Commission notes that [Danaher’s intellectual property rights]. (86) According to the Notifying Party, [Danaher’s license agreements]. (87)
(107) During the market investigation, all the companies currently licensing these intellectual property rights from Danaher have confirmed that the term of their licence agreements matches the duration of Danaher’s patent protection. (88)
(108) Moreover, the Commission notes that none of the concerned producers of SUT mixers considered that the Transaction or [Danaher’s intellectual property rights] will have an implication on their production of mixers for which they licence IP rights […]. (89)
(109) As the existing licensing agreements cover a sufficient number of operators in the market, the Commission finds that Danaher would not have the ability to restrict access to its intellectual property rights […] post-Transaction.
(B.ii) Incentive to foreclose
(110) As regards a potential incentive for the merged entity to engage in input foreclosure, the Notifying Party submits that [Danaher’s intellectual property rights and license agreements]. (90)
(111) The Commission notes that no competitor currently sourcing components from Danaher to be used in SUT mixers considers it likely that Danaher will restrict access to them post-Transaction. (91) In this regard, a licensee stated that “Danaher is already supplying […] to competitors, the transaction will not change that competitive landscape, or their incentive to restrict access […]”. (92)
(112) In light of this and of the fact that Danaher is bound by the terms of existing licences for the life of the licenced patents, the Commission finds that Danaher would not have the incentive to restrict access to its intellectual property rights […] post- Transaction. In particular, the Transaction will not change the trade-off that Danaher already faces pre-Transaction between the profit that it could lose in the upstream market by engaging in an input foreclosure strategy and the potential profit gain from expanding its sales of SUT mixers downstream.
(B.iii) Impact
(113) Given the absence of the ability or the incentive to foreclose other manufacturers of SUT mixers, the Commission considers that the Transaction will not lead to increased prices in the downstream market thereby significantly impeding effective competition. In particular, the Commission has seen no evidence that a potential anticompetitive foreclosure would increase the cost of downstream competitors in the sale of SUT mixers nor raise the barriers to entry to potential competitors. Moreover, any potential input foreclosure would only affect part of a single market comprising all SUT mixers, as has been defined in paragraph (87) above, [Danaher’s intellectual property rights].
(114) Moreover, the Commission notes that, with the exception of one, all competitors in the downstream market also sell mixers that are not affected by Danaher’s intellectual property rights and would thus be in a position to offer SUT mixers even in the event of restricted access to Danaher’s intellectual property rights. (93)
(B.iv) Conclusion on vertical relationships
(115) In light of the considerations in paragraphs (99) to (114) above as well as all evidence available to it, the Commission concludes that, in the worldwide or EEA- wide market for SUT mixers, the Transaction will not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to vertical non-coordinated effects.
4.2.3. SUT connectors
(116) SUT connectors provide reliable sterile connections between separate fluid pathways. They are used throughout single-use bioprocessing. Sterile connectors have largely displaced traditional tube welding as they provide faster and easier solutions to ensure a permanent connection.
(117) In upstream single-use bioprocessing, sterile connectors may be incorporated in SUT bioreactors to enable fluid transfer in or out, pH probe insertion, or sampling. They can also be incorporated in mixer bags to enable fluid transfer in or out of the mixer bag, or for pH/temperature probe insertion during media preparation. Sterile connectors are also key components of transfer sets, which enable the sterile transfer of fluid between unit operations. They can also be a component of an SUT bag, or part of a single-use filtration manifold.
(118) In downstream single-use bioprocessing, sterile connectors are used extensively in transfer sets to enable the sterile transfer of fluid from one bioprocessing unit operation to another. They can also be a component of an SUT bag or incorporated in a mixer, or for pH/temperature probe insertion during buffer preparation. Sterile connectors can also be a component of single-use filtration manifolds or single-use chromatography systems to enable the connection and transfer of fluid from one source through the inline device to another component such as an SUT bag.
(119) It is not possible to use one supplier’s connector on one end with another supplier’s connector on the other end. However, it is possible and common to integrate one supplier’s connectors in another supplier’s single use system according to the specifications of the customer.
4.2.3.1. Market definition
(A) Product market definition
(A.i) Notifying Party’s arguments
(120) The Notifying Party submits that gendered and genderless connectors (94) compete on the same market, as they both offer the same applications and functionalities and are similar in price. (95)
(121) The Notifying Party submits that aseptic connectors may compete with sterile connectors (96) in applications where sterile connections are not essential, but they generally do not compete where sterile connections are important, such as in the last steps of the downstream bioprocessing workflow, such as in filtration. (97) The Notifying Party submits that sterile and aseptic connectors do not compete with quick connectors (98) as they are intended for different applications. Quick connectors do not allow customers to maintain the cleanliness offered by sterile and aseptic connectors. (99)
(122) The Notifying Party provided market shares on the basis of a relevant product market comprising sterile and aseptic connectors (that is to say excluding quick connectors). (100)
(A.ii) Commission’s assessment
(123) In a previous decision (101), the Commission left open whether SUT bioreactors, SUT bags, SUT mixers and SUT transfer sets constitute separate product markets or whether they belong to a single SUT product market. The Commission did differentiate between different types of SUT connectors in that decision.
(124) In this decision, a wide majority of customers who expressed an opinion in the market investigation on this point indicated that they can replace sterile genderless and sterile gendered SUT connectors as regards price, use applications, technical characteristics and efficiency. (102) In this regard, a customer stated that “[e]ven though users usually prefer genderless connectors (they allow easier designs and better materials management), these should always be interchangeable with gendered ones, provided they are also validated by the drug manufacturer”. Another customer submitted that in its experience, “it is possible to replace a sterile genderless connector with a sterile gendered connector. The two connectors simply differ in their approach to connecting (genderless connectors involves identical connectors and gendered connectors require a female and male component) but otherwise, either can be utilised. Genderless is where the consoles are both identical at the end”.
(125) Moreover, competitors who expressed an opinion in the market investigation on this point have largely supported this view in relation to price, technical characteristics and efficiency. (103) Responses where however mixed in relation to use applications. In this regard, a competitor pointed to certain limitations: “[the respondent] typically chooses between gendered connectors based on application. Genderless connectors are used for more generic, mass quantity production while gendered connectors are used for more customised production. Choice usually lies solely on what makes sense for the given design”.
(126) Competitors who expressed an opinion on this point in response to a request for information submitted that their customers may only replace a sterile connector by an aseptic connector in rare occasions. Some competitors stated that customers may move from connectors that are non-certified for sterile connections (that is aseptic) to those which are certified for this purpose, but not the other way around. (104)
(127) For the purposes of this decision, the Commission considers that the market for SUT connectors comprise both gendered and genderless connectors, as they generally serve the same applications in the bioprocess workflow. Given that the Transaction does not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement in the market for SUT connectors, it can be left open for the purposes of this decision whether SUT aseptic connectors and SUT sterile connectors belong to the same product market.
(B) Geographic market definition
(B.i) Notifying Party’s arguments
(128) The Notifying Party submitted that SUT connectors are worldwide and in any event, not narrower than EEA-wide in scope, as these products are manufactured at centralised sites and shipped via regional distribution hubs to customers globally. (105)
(129) Moreover, the Notifying Party submitted that (i) transportation costs are low as a proportion of total costs, representing around […]% of the sales prices of SUT connectors; (ii) there are no regulatory differences within the EEA; (iii) custom duties do not affect transportation globally; and (iv) pricing is similar across the EEA. (106)
(B.ii) Commission’s assessment
(130) The large majority of customers and competitors who expressed an opinion on this point stated that (i) they procure SUT connectors at worldwide level; (ii) after-sale services are provided at worldwide level; (iii) prices are comparable at worldwide level; and (iv) the same suppliers are active at worldwide level. (107)
(131) For the purposes of this decision, it can be left open whether the market for SUT connectors is global or EEA-wide in scope. The Transaction does not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement under any of these plausible geographic market definitions.
4.2.3.2. Competitive assessment
(132) The Transaction leads to a horizontal overlap between the Parties’ offerings in SUT connectors. Danaher offers sterile SUT connectors (both gendered and genderless), through different generations of its Kleenpak connectors. GE only offers genderless aseptic SUT connectors, namely its ReadyMate connectors. Should SUT aseptic connectors and SUT sterile connectors be considered to belong to separate product markets, the Transaction would not lead to a horizontal overlap. For this reason, the assessment carried out in this section will be based on a market for overall SUT connectors.
(133) According to the Parties’ estimates set out in Table 4 above, the combined entity would hold a market share of [20-30]% in a worldwide market for SUT connectors. At the EEA-level, the combined entity’s market shares would be of [20-30]%. According to these estimates, Merck would remain the market leader at both the worldwide and EEA levels (with market shares of [20-30]% and [30-40]% respectively), closely followed by Sartorius.
(134) During the market investigation, a number of elements suggested that the Parties’ would hold higher combined market shares in a market for SUT connectors:
(a) First, ordinary course of business documents of Danaher suggest that the combined entity would hold a combined market share of [50-60]% in a worldwide market for SUT connectors in 2015, with GE bringing an increment of [10-20]%. (108) The Notifying Party has explained that the estimates provided in the Form CO are more sophisticated than in this internal document, given that the Form CO is based on customer replies and relies on GE’s actual data. (109)
(b) Second, a number of competitors who expressed an opinion in the market investigation on this point provided estimates of the combined entity’s market shares in SUT connectors, which ranged between [40-50]% and [60-70]% at the worldwide level, with an increment from GE ranging between [10-20]% and [20-30]%. (110)
(135) In view of this discrepancy, the Commission carried out a market reconstruction based on actual sales figures of competitors in order to verify the position of the combined entity in a market encompassing all SUT connectors at the worldwide and EEA levels. (111) The market reconstruction showed that the competitors’ actual sales data was in fact much lower than what had been provided in the Form CO. According to the results of the market reconstruction, the combined entity would hold a market share of [30-40%].
(B) Limited potential entry of new suppliers
(136) A significant majority of customers who expressed an opinion in the market investigation on this point did not expect additional companies to start supplying SUT connectors in the next two to three years. (112) A number of these customers pointed towards the existence of high barriers to entry, as newcomers are required to meet the quality and business requirements of mature pharmaceutical companies. In particular, one customer indicated that “[c]osts and scale of suppliers must be such that new companies coming to the market may not be easy to come by”. (113) The view of competitors in this regard was mixed, with some competitors referring to this market as being “already occupied by clear market leaders, high technical and quality barriers to entry” and that, as such, “[a]ny new entrant would only be able to capture a very small market share”. (114)
(C) Credible alternatives suppliers
(137) A majority of customers and competitors who expressed an opinion in the market investigation on this point considered that, post-Transaction, there will be sufficient credible alternative suppliers of SUT connectors for new production processes prior to obtain regulatory approval of a biopharmaceutical . (115)
(D) Competitors’ expansion of capacity
(138) Several competitors that responded to a Commission’s request for information indicated that they currently have spare capacity for SUT connectors. (116) Moreover, some of these competitors submitted that they would be able to increase their output of SUT connectors without incurring into additional high costs (117) and that they have also plans for expanding their total capacity for SUT connectors in the coming year. (118)
(139) Moreover, one of these competitors indicated that a “significant increase in the price of the SUT competitor connectors” or “SUT competitor connector supply or quality issues” would trigger an expansion in capacity. (119) An additional number of competitors referred in general to an increase in the demand for SUT connectors as a triggering factor for an expansion in total capacity. (120)
(E) Closeness of competition
(140) The Notifying Party submitted that, while GE markets its SUT connectors as aseptic, Danaher offers only sterile certified SUT connectors. According to the Notifying Party, the difference in quality derived from the SUT connectors being aseptic or sterile is reflected in the sharp differences in the price of the Parties’ SUT connectors. (121)
(141) The views of competitors who expressed an opinion on this point in response to a Commission request for information support this argument. The respondents submitted that their customers are only able to replace a sterile connector by an aseptic connector in rare occasions: some customers may move from aseptic SUT connectors to certified sterile SUT connectors, but not the other way around. (122)
(142) Moreover, the Notifying Party submitted that […]. (123)
Figure 1 – Overview of the main suppliers’ offering of SUT connectors (2000-2025)
[…]
Source: Form CO, Annex DHR 580
(143) In this regard, the Commission notes (as illustrated in Figure 1 above) that GE’s SUT connectors (ReadyMate) have been present in the market since before 2010. In turn, […]. Figure 1 above shows that the main competitors to the combined entity have recently introduced new models.
(144) This appears consistent with the […]. (124)
(F) Conclusion
(145) While the Commission notes the discrepancy in market shares and also the responses of customers and competitors regarding the limited scope for potential entry of new suppliers in SUT connectors, further elements lead to the conclusion that the Transaction will not give rise to serious doubts, namely (i) the existence of credible alternative suppliers in the market; (ii) the fact that competitors currently have spare capacity and are able to increase output without incurring into additional high costs; and (iii) the fact that the Parties’ are not close competitors to one another.
(146) In light of the considerations in paragraphs (132) to (145) above as well as all evidence available to it, the Commission concludes that, in the worldwide or EEA- wide market for SUT connectors, the Transaction will not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to horizontal non-coordinated effects.
4.3. Cell culture media and sera
(147) Cell culture bioprocessing typically requires growing different types of cells. Nutrients are provided to cultivated cells in the form of media, sera and other reagents such as growth factors and hormones, which are consumables used in cell culture. Cell culture media are water-based liquids, while sera are liquid blood-based animal products.
(148) The Parties’ activities overlap in all three areas, namely cell culture media, cell culture sera and other process liquids but the Transaction gives rise to affected markets only in cell culture sera.
4.3.1. Product market definition
4.3.1.1. Commission’s precedents
(149) The Commission has examined cell culture sera in the past and, while the precise market definition was left opened, it considered that the market for cell culture sera could be segmented based on the customer groups, the type of animal the blood originates from, and the geographic origin of the blood provided. (125)
4.3.1.2. Notifying Party’s views
(150) In line with Commission’s precedents, (126) the Notifying Party considers cell culture sera as a separate market within the overall cell culture segment. The Notifying Party further submits that cell culture sera could likely be segmented based on customer type, type of animal the blood originates from, and geographic origin of the blood provided. However, the precise product market definition was left open, since there would not be any competitive concerns under any such market definition.
4.3.1.3. Commission’s assessment
(151) The majority of customers and competitors who expressed an opinion on this point considered that the Commission’s past definitions for the relevant market in cell culture sera are still pertinent. (127)
(152) Therefore, for the purpose of this case, it can be left open whether there are relevant markets based on the different segmentations like the customer type, the type of animal that the blood originates from, or the geographic origin of the blood provided, as the transaction does not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement under any of these plausible product market definitions (128). Considering the overlaps in the Parties’ activities, for the purpose of this decision, the Commission will carry out its competitive assessment only with regard to (i) overall cell culture sera; and
(ii) Ultroser G serum substitute product markets.
4.3.2. Geographic market definition
4.3.2.1. Commission’s precedents
(153) In previous decisions, the Commission considered the geographic market for cell culture sera to be worldwide or at least EEA-wide in scope. (129)
4.3.2.2. Notifying Party’s views
(154) In line with past Commission previous definitions, the Notifying Party considers the geographic market for cell culture sera to be worldwide or at least EEA-wide in scope.
4.3.2.3. Commission’s assessment
(155) The majority of customers and competitors who expressed an opinion on this point tend to confirm the cell culture sera market to be worldwide. This is mainly due to comparable prices at a worldwide level, the fact that the same suppliers of sera are active at a worldwide level and that customers procure sera at a worldwide level. (130)
(156) In any event, for the purpose of this decision, the Commission considers that the precise geographic market definition can be left open, as this would not change the outcome of the competitive assessment.
4.3.3. Competitive assessment
(157) Danaher sources various media from Fujifilm and Stemcell Technologies and resells it for research stages of cell culture, under the under the MolDev brand. Also, Danaher offers a serum substitute (Ultroser G serum substitute) that can replace fetal calf serum (FCS) for small-scale experiments and diagnostic applications and reagents, buffers and solutions that are not used in cell culture. The serum is not suited for bioproduction, human, or animal use.
(158) GE Biopharma, under the HyClone product line, offers various cell culture consumables, for example media, (131) sera, and other supplements, buffers and process liquids. GE Biopharma cell culture media and sera are predominantly used in the manufacturing stage of cell culture and, thus, supplied in high volumes.
(159) The Parties’ activities overlap in the overall sera market and in the possible market for Ultroser G serum substitute and fetal calf serum. The Transaction would lead to affected markets in the possible markets for overall cell culture sera and Ultroser G serum substitute at a worldwide level. However, in none of the possible affected markets, the Transaction raises serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement for the following reasons.
(160) First, according to the Parties’ estimates, in all affected markets, the combined market shares are […] ([20-30]%), with a […] increment from Danaher which is [0-5]%. The […] increment would not lead to a substantial change of the competitive structure of the market in any of the possible affected markets.
(161) Second, the combined entity will face several well-established suppliers post- Transaction, such as Thermo Fisher, Merck Millipore, Corning and other smaller ones.
(162) Third, the majority of customers and competitors who expressed an opinion in the market investigation on this point did not expect that the Transaction would have a negative impact in relation to cell culture sera regarding any of the following parameters: price, quality, innovation, security of supply and product range. (132)
(163) In light of the considerations in paragraphs (157) to (162) above as well as all evidence available to it, the Commission considers that, in the worldwide or EEA- wide market for cell culture sera and Ultroser G serum substitute, the Transaction will not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to horizontal non-coordinated effects under any plausible market definition.
4.4. Microcarriers
(164) Microcarriers are consumables used in cell culture bioprocessing. They provide a surface for the anchorage of adherent cells to attach and grow in cell culture vessels and bioreactors for adherent cell culture.
(165) Microcarriers consist of two parts: the core or matrix and the surface.
(166) The core or matrix of microcarriers can be of different porosity. Accordingly, microcarriers can be described as either microporous with pore sizes up to around 20 µm or as macroporous with pore diameters from around 20 µm up to around 400 µm. In a microporous structure, cells cannot enter the pores and thus grow in monolayers on the surface of the microcarrier. In a macroporous structure, cells can access the pores and grow inside the microcarrier in multilayers. As a general matter, microporous microcarriers may be used when the cell itself is the target product (for instance, in the manufacturing of stem cells), whereas macroporous microcarriers are mainly used when the cell secretes the target product (for instance, in the manufacturing of certain protein or viruses for vaccine manufacturing).
(167) The core material of microcarriers can be for example plastic (polystyrene), cotton (cellulose), protein (for example gelatin), or sugar (polysaccharide). The surface of microcarriers can be untreated or coated with proteins (collagen, recombinant proteins or synthetic peptides). The coating material is either animal-free or contains animal product (for example porcine collagen). Moreover, microcarriers can bear a positive charge (cationic) or different chemistries.
4.4.1. Market definition
(168) There are no precedents of the Commission concerning the product or geographic market definition in microcarriers.
4.4.1.1. Product market definition
(A) Notifying Party’s arguments
(169) The Notifying Party submits that microcarriers constitute a single overall product market. (133) Within this overall product market, the Notifying Party considers that microcarriers can be differentiated between microporous and macroporous structures. (134)
(170) Moreover, the Notifying Party considers that a distinction may be drawn between microcarriers using animal-free components and microcarriers with animal-based components. (135) Whereas some microcarrier customers are sensitive to the risk associated with animal component consumables, animal-free consumables do not raise the same concerns.
(171) Finally, the Notifying Party submits that industry reports may categorise microcarriers based on surface coating or surface modification, distinguishing untreated microcarriers, cationic microcarriers, collagen-coated microcarriers and protein-coated microcarriers (other than collagen). The Notifying Party submits that these attributes are not mutually exclusive and that they do not provide a meaningful basis for separate relevant product market definitions.
(B) Commission’s assessment
(172) A wide majority of customers who expressed an opinion in the market investigation on this point submitted that they cannot or they can only to a limited extent use microporous and macroporous microcarriers for the same applications. (136) Likewise, no competitor has indicated during the market investigation that its customers are able to use both types of microcarriers for the same applications. (137)
(173) A wide majority of customers who expressed an opinion in the market investigation on this point were not able, or only able to a limited extent, to use animal-free and animal-based microcarriers for the same applications. (138) In this regard, a customer indicated that “[a]nimal component-free microcarriers are generally regarded as preferable in the pharmaceutical manufacturing setting and we would never replace animal-free with animal-based microcarriers” (non-confidential). A large majority of competitors who expressed an opinion on this point took the same view. (139)
(174) A slight majority of customers who expressed an opinion on this point and a wide majority of competitors who expressed an opinion on this point considered that it is not possible to substitute between cationic microcarriers and collagen-coated microcarriers taking into consideration use applications, technical characteristics and efficiency. (140) In this regard, one competitor stated that “[w]hile for performance testing purposes Sartorius cannot substitute between cationic microcarriers and collagen-coated microcarriers this may well not be the case for other applications”. While a majority of competitors who expressed an opinion on this point considered that it is possible to substitute between cationic microcarriers and collagen-coated microcarriers with regard to their price, the Commission takes the view that the limited substitutability in relation to use applications, technical characteristics and efficiency would suggest that these constitute separate product segments.
(175) Given that the Transaction gives rise to serious doubts as to its compatibility with the internal market and the functioning of the EEA Agreement in the market for microcarriers under any plausible product market definitions, it can be left open for the purposes of this decision whether there is a single relevant market comprising all types of microcarriers or whether further segmentations could be made based on (i) their porosity (between microporous and macroporous microcarriers); (ii) whether they use animal-free or animal-based components; or (iii) their surface coating (between cationic microcarriers and collagen-coated microcarriers).
4.4.1.2.Geographic market definition
(A) Notifying Party’s arguments
(176) The Notifying Party submits that the market for microcarriers is worldwide in scope and, in any event, not smaller than EEA-wide, given the low costs of transport and that microcarriers are produced in a limited number of sites for worldwide shipping. (141)
(B) Commission’s assessment
(177) A significant majority of customers who expressed an opinion in the market investigation on this point confirmed that they procure microcarriers at worldwide level; after-sale services are provided at worldwide level; prices are comparable at worldwide level; and the same suppliers are active at worldwide level.142 Competitors have unanimously confirmed this view. (143)
(178) For the purpose of assessing this Transaction, the Commission considers that the market for microcarriers is worldwide in scope.
4.4.2. Competitive assessment
4.4.2.1. Notifying Party’s views
(179) The Notifying Party submits that the Transaction does not lead to a significant impediment of effective competition for microcarriers. (144)
(180) While the Notifying Party considers that GE is a supplier of microcarriers overall and in a potential sub-segment comprising microporous microcarriers, it submits that the Transaction will not change the competitive structure of the market. (145) In this regard, the Notifying Party argues that the Transaction will combine GE with one of several similarly sized small competitors. It moreover argues that the Parties are not close competitors, given that (i) GE manufactures microcarriers mainly for large scale bioprocess applications and Danaher produces smaller-scale batches (146); and (ii) while GE offers both microporous microcarriers and macroporous microcarriers with a polysaccharide core, Danaher offers only microporous microcarriers with a plastic core. (147)
(181) The Notifying Party argues that, given the absence of chance in the competitive structure of the market, the combined entity will continue to face significant competitive constraints by at least five credible and viable suppliers with higher or similar sales and market shares as Danaher, namely Corning, Fujifilm, Advanced Biomatrix, Percell and Global Cell Solutions. (148)
4.4.2.2. Commission’s assessment
(182) The Transaction leads to a horizontal overlap between the Parties’ offerings in microcarriers. Through Pall, Danaher offers its SoloHill microporous-only microcarriers, which have a plastic core and are supplied as animal-free or with animal components (collagen coating) that can also bear cationic charges. GE offers both microporous microcarriers (Cytodex) and macroporous microcarriers (Cytopore), with a sugar-based and a cotton-based core respectively. GE’s microcarriers are supplied with animal components as well as animal-free, that can also bear cationic charges. The Transaction does not lead to a horizontal overlap in relation to macroporous microcarriers.
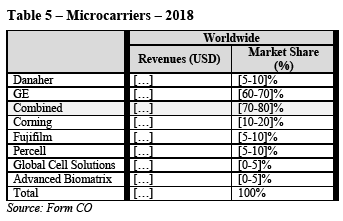
(183) According to the Parties’ estimates set out in Table 5 above, the combined entity would hold a market share of [70-80]% (GE: [60-70]%; Danaher: [5-10]%) in a worldwide market comprising all types of microcarriers. The Transaction would reinforce GE’s current […] position in this market and would place the combined entity well ahead of Corning ([10-20]%), Percell ([5-10]%), Global Cell Solutions ([0-5]%) and Advanced Biomatrix ([0-5]%).
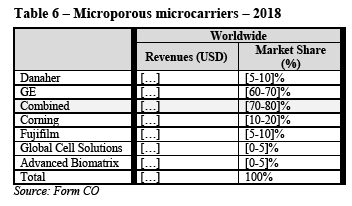
(184) In a worldwide market comprising only microporous microcarriers, the combined entity would hold a market share of [70-80]%, as set out in Table 6 above. As in a market comprising all types of microcarriers, the Transaction would reinforce GE’s current […] position ahead of Corning ([10-20]%), Fujifilm ([5-10]%), Global Cell Solutions ([0-5]%) and Avanced Biomatrix ([0-5]%).
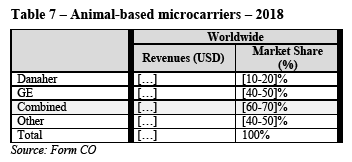
(185) In a worldwide market comprising only animal microcarriers, the combined entity would hold a market share of [60-70]%, as set out in Table 7 above. On this basis, the Transaction would reinforce GE’s current […] position with an increment of [10-20]%.
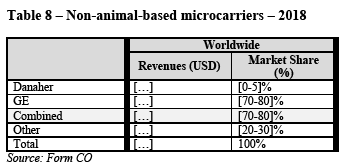
(186) In a worldwide market comprising only non-animal microcarriers, the combined entity would hold a market share of [70-80]%, as set out in Table 8 above. On this basis, the Transaction would reinforce GE’s current […] position with an increment of [0-5]%.
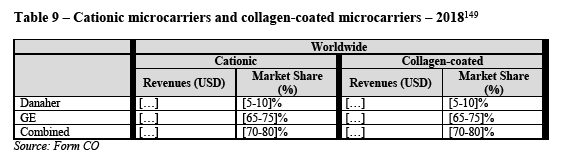
(187) In a worldwide market for cationic microcarriers as well as in such a market for collagen-coated microcarriers, the combined entity would hold a combined market share of [70-80]%, as set out in Table 9 above. On this basis, the Transaction would reinforce GE’s current […] position with an increment of [5-10]%.
(188) Contrary to the Notifying Parties’ arguments, the Parties appear to compete closely with each other in a market for overall microcarriers. A majority of customers and competitors who expressed an opinion in the market investigation on this point indicated that Danaher is the closest competitor to GE. (150) […] (151) […], as it shows that […]. In comparison, […].
(189) Moreover, customers appear to have limited possibilities of changing suppliers in this market. The views of customers who expressed an opinion in the market investigation on this point were mixed as to whether they will be able to find sufficient credible alternative suppliers of microcarriers post-Transaction. (152) A majority of customers and all competitors who expressed an opinion on this point found at least some limitations in the number of credible alternatives for some use cases. (153)
(190) Furthermore, the market investigation suggested that entry of new competitors in this market is not likely. A majority of customers and competitors who expressed an opinion on this point does not expect additional companies to start supplying microcarriers in the next two to three years. (154) Some competitors indicated that the market for microcarriers is characterised by high barriers to entry. In this regard, a competitor expressed the view that “[t]he extent of expertise, development, and testing required to gain meaningful adoption are high barriers to entry”. (155) The Commission considers that the lack of expected entry of additional competitors would not pose a sufficient competitive constraint on the combined entity post- Transaction.
(191) While customers did not express concerns in relation to the impact of the Transaction, the views of competitors who expressed an opinion in the market investigation on this point were mixed (50-50%) on whether the Transaction will have an impact on price, product range or security of supply. (156) While no competitor is concerned about a negative impact on the quality of microcarriers, a majority of competitors who expressed an opinion on this point expects a decrease in innovation. In this regard, a competitor stated that “[w]ith limited competition, price increases would be expected by the combined GE and Danaher offering, while security of supply would decrease given the combined entity’s control over the microcarrier market”. (157)
(192) In light of the considerations in paragraphs (182) to (191) above as well as all evidence available to it, the Commission concludes that, in the worldwide market for microcarriers (either overall, or in the potential market segments for microporous microcarriers, animal-based microcarriers, non-animal-based microcarriers, cationic microcarriers or collagen-coated microcarriers), the Transaction will give rise to serious doubts as to its compatibility with the internal market and the functioning of the EEA Agreement due to horizontal non-coordinated effects given in particular (i) the significant combined market share of the Parties; (ii) the meaningful increment contributed by Danaher to GE’s pre-existing leading position; (iii) the close competition between the Parties; and (iv) the lack of strong competitors or entry prospects.
4.5.Bioprocess filtration
4.5.1. Introduction
(193) Bioprocess filtration constitutes a method for separating components based on size. It takes place at several steps in the bioprocessing production chain of the production of for instance mAbs, biologics used in cell and gene therapy, and vaccines. Different types of filtration are used depending on the production step and specific application.
(194) Similar as for other areas in the bioprocessing industry, it is for customers very difficult to change suppliers once a production process is in place and regulatory approval has been obtained, in particular where products are in contact with the molecule. (158)
(195) The Commission has not previously dealt with cases in the area of bioprocess filtration, and has therefore not previously assessed relevant markets in this area.
(196) Bioprocess filtration setups consist of systems (sometimes also referred to as ‘skids’), consumables, and equipment. Systems are used to control the filtration process and are composed of pumps, sensors, tubings, manifolds among other components. The filtration process is performed by filters (consumables) inserted into equipment (housing or holders that hold the equipment) that are deployed in the filtration system.
4.5.1.1. Direct flow filtration and tangential flow filtration
(197) There are two filtration techniques, direct flow filtration (“DFF”) and tangential flow filtration (“TFF”). In DFF, process fluids are passed directly through a membrane. This is generally used at stages of the bioprocessing work flow where the target product has to be separated from other particles based on size. In TFF, the process fluid flows in parallel to the membrane surface. TFF is typically used when the final product needs to be concentrated, or when the buffer in which the product is contained need to be exchanged. Both Parties are active in DFF and TFF.
(198) Figure 2 and Figure 3 below portray DFF and TFF techniques respectively, illustrating the manner in which the feed is passed through the membrane, and how the filtration flux rate varies according to the volume filtered.
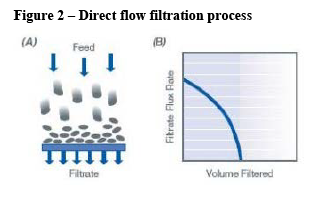
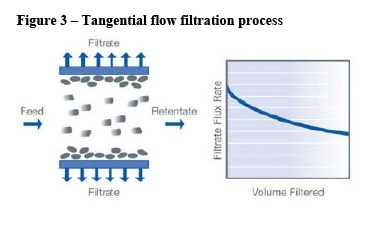
(199) Figure 4 depicts the usage of bioprocess filtration in a generic production process for mAbs, which is one of the most common types of biopharmaceutical products. The figure indicates at which steps of the production whether DFF or TFF is used. The Parties submit that DFF and TFF cannot be interchanged (or when it is, it is application specific). (162)
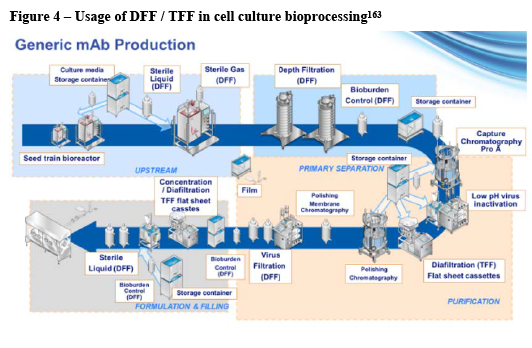
4.5.1.2. Different types of consumables used in bioprocessing filtration
(200) Different types of DFF filters include (i) pre-filters; (ii) sterile filters; (iii) depth filters; (iv) air/gas filters; and (v) virus filters.
(201) As regards TFF, there are two main technologies and corresponding consumables: TFF flat sheet and TFF hollow fibre. In flat sheets, the feed passes between sheets and the permeate is collected from the opposite side of the sheets, while in hollow fibre the feed passes through hollow fibres and the permeate is collected outside the fibres. These technologies result in different fluid dynamic characteristics. Figure 5 and Figure 6 below portray diagrams of hollow fibre and flat sheet consumables respectively.
4.5.2. Market definition
4.5.2.1. Product market definition
(A) Systems, equipment, and consumables
(202) The Notifying Party considers that systems, equipment and consumables constitute distinct product markets. First, these perform different functions in the filtration process. Consumables perform the actual filtering, while the equipment is used to support and host these consumables, and the system performs and steers the filtration process. Second, systems, equipment and consumables are generally sold separately and can often be mixed and matched between different suppliers. Systems of one supplier may be used with equipment and consumables from the same or other suppliers, because of standardisation in size, form and format (but not quality and performance) of consumables. Third, customers do not make a buying decision at the same moment but will at an earlier stage already decide the consumable needed for the production process before the system or equipment is chosen.
(203) The Commission notes that this claim is supported by the fact that there are suppliers that are active only in either systems, equipment or consumables. For instance, 3M is only active as a supplier of consumables, without offering systems or equipment; GE is active in systems and consumables, but only very limitedly in equipment; and YMC is only active in systems, but not in equipment or consumables.
(204) This is further confirmed by the market investigation, where customers predominantly indicate that they predominantly mix and match hardware and consumables from different suppliers. (166) (167)
(205) On this basis, the Commission considers that systems, equipment and consumables constitute distinct product markets.
(B) Consumables
(B.i) Notifying Party’s arguments
(206) The Notifying Party claims that customers’ choice between DFF or TFF is driven by technical requirements in the process, that there would be very little (if any) applications for which both DFF and TFF could be used. (168) From a supply side perspective, there would be large differences in the production of DFF and TFF consumables and equipment, but not for systems. It therefore argues there to be distinct product markets for DFF and TFF consumables and equipment, but not for systems.
(207) As regards specifically DFF consumables, the Notifying Party indicates that the different types, namely (i) pre-filters; (ii) sterile filters; (iii) depth filters; (iv) air/gas filters; and (v) virus filters, are used for different purposes and are used for different applications. These segmentations might therefore potentially each constitute a distinct product market.
(208) As regards specifically TFF consumables, the Notifying Party submits that product and process needs determine whether one or the other technique and corresponding consumable has to be used. TFF hollow fibre and flat sheet consumables are used mainly in different applications. While both are capable of performing similar applications to some extent, there are many of such applications where one technique would be more effective or suitable than the other. Because of this, customers would have clear preferences for one over the other depending on the customer’s particular process and application needs.
(209) The Notifying Party submitted an overview of the advantages and constraints of each TFF technique (hollow fibre vs flat sheet), depending on the application, indicating where one technique would be preferred over the other. (169) The Notifying Party indicates that each has a technical ‘sweet spot’ for a certain application, and that depending on the application, customer process requirements and a drug’s physical characteristics (that is to say the target molecule) gives one technique advantages over the other.
(210) Typically, TFF steps upstream in the production process use hollow fibre consumables, while those downstream use flat sheet consumables.
(211) Furthermore, the production of equipment and consumables of these technologies would be highly specific and different. Suppliers of hollow fibre consumables and equipment would not be able to easily switch to the production of flat sheet consumables and equipment in case of a relative price increase, and vice versa. Therefore, the Parties consider that TFF hollow fibre and flat sheet consumables constitute two separate product markets.
(B.ii) Commission’s assessment
(212) As regards consumables used for bioprocessing filtration, the market investigation showed that customers do not use DFF and TFF filtration techniques and respective consumables for the same purposes. Customers explain: “DFF and TFF are used for different purposes in a protein purification process. They are rarely interchangeable although each type of products from different vendors are often interchangeable for a specific application (i.e. a different DFF filter may replace the existing DFF filter in use, and same for TFF)”;”DFF (direct or normal flow) and TFF (tangential or “cross-flow”) tend to be used to for different purposes. They are different technology and they involve the performance of different process. As a result of the differing operational performance, [Company] considers that DFF and TFF are generally not able to be used for the same applications”; “[d]ifference in surface layer formation between the two process forms”. (170)
(213) As regards specifically consumables for DFF, the Commission notes that GE’s activities […]. It is active only as […] of specific categories of DFF consumables (pre-filters and sterile filters). If these were to constitute distinct relevant product markets, affected markets would arise, but with only a […] increment from GE’s activities (between [0-5]% and [0-5]% on a worldwide and EEA level). For these reasons, the Commission does not further assess these segments and leaves open whether these would constitute distinct relevant product markets.
(214) As regards specifically consumables for TFF, the market investigation showed that customers do not use flat sheet and hollow fibre filtration for the same applications, or only limitedly. Customers explain: “[w]e use both and while they can be interchanged to an extent, the availability of various membranes at different sizes for different scales means we are limited in how much cross over we can exploit. Also product specific characteristics cause certain filter types to be unsuitable”; “[i]n process applications, they are rarely in competition with each other. TFF hollowfibre is used in 2 important niche applications – cell culture perfusion and harvest and virus removal applications. The vast bulk of the remaining TFF applications use flat-sheet style membrane devices”; “[t]he TFF flat sheet and TFF hollow fibre filtration utilize different mechanics depending on the application; accordingly, it is not practical to use these for the same applications”; “[u]sed for different purposes (higher through put, etc)”. A smaller minority however does consider these interchangeable: “[… f]rom [customer name]’s perspective, TFF hollow fibre filtration is simply a more modern version of the TFF flat sheet but both ultimately produce similar results”. (171)
(215) As to supply-side substitutability between different types of bioprocessing filtration consumables, the results of the market investigation do not contradict this. One competitor for instance indicates: “[t]he barriers to introducing a new TFF cassette are mostly a long ROI, as swapping a cassette in an existing process would be very difficult, so you would need to specific at Clinical phase 1 and grow with the process. This could take 5+ years before you are in manufacturing and seeing any ROI. In the meantime you would incur all the costs of manufacturing supporting and validating the device”. (172)
(216) On this basis, the Commission considers it likely that there are separate product markets for each consumable (DFF, flat sheet and hollow fibre TFF). In any event, this can be left open as the Transaction will not raise serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement in the area of consumables in either an overall market for consumables or segmented by technology (DFF, flat sheet, hollow fibre TFF).
(C) Systems
(C.i) Notifying Party’s arguments
(217) The Notifying Party argues that there is an overall market for bioprocessing filtration systems encompassing DFF, as well as TFF hollow fibre and TFF flat sheet systems, although DFF and TFF systems might also belong to two different markets. (173)
(218) It explains that bioprocessing filtration systems are composed of the same components, including for example pumps and valves, but that the difference is mainly driven by the way the feed stream flows, leading to different system designs and set-ups. DFF systems for instance only need a pump and pressure sensors to monitor the filtration process, while TFF systems require more complex functionalities. Because of this, in the Notifying Party’s view DFF systems generally do not work with TFF consumables. TFF systems would also not work with DFF consumables, because of ‘differences in control functionalities and the geometry for the pipe arrangement’. (174)
(219) However, the production process of TFF systems and DFF systems would be the same in terms of engineering practices and capabilities. In that regard, the Notifying Party submits that it is possible for one supplier producing a particular system to build another type of system within a time frame of approximately […]. Switching costs would amount to around USD […]. Nonetheless, the Notifying Party also stresses the importance for a supplier to have experience and reputation in order to start producing a different type of filtration system.
(220) In addition, the Notifying Party submits that there are existing systems that are able to use both hollow fibre and flat sheet TFF consumables, or both TFF and DFF consumables. The Notifying Party in that regard submits an example of GE’s Äkta readyflux. At the same time, [use of Äkta readyflux by GE].
(221) As regards specifically TFF systems, the Notifying Party submits additional arguments that would show supply-side substitutability between TFF hollow fibre and TFF flat sheet systems.
(222) In that regard, it would be possible to modify a flat sheet TFF system to work with hollow fibre consumables. Such modification would take a [timeframe] and would cost under USD […]. (175) The opposite modification for a hollow fibre TFF system to work with flat sheet consumables would be possible in [timeframe] with an investment of about USD […].
(223) As an illustration for there being little engineering differences, the Notifying Party refers to the possibility to use both hollow fibre and flat sheet consumables with a number of GE’s TFF systems. For these systems, customers would only have to purchase appropriate accessories and set the appropriate flow rate on the system for it to work with either hollow fibre or flat sheet. Similarly, the Notifying Party claims that Repligen and LEWA/YMC would also market and sell TFF systems compatible with both types of consumables.
(224) The Notifying Party further indicates that TFF systems are typically heavily customised (instead of being “off the shelf”), and that a custom system typically take [timeframe] to design and deliver, or up to [timeframe] including installation at the customer’s site. This time for a supplier to deliver a custom system would be the same regardless of whether the system is for use with flat sheet or hollow fibre consumables, and irrespective of whether it is supplied by a system supplier active only in flat sheet, hollow fibre, or both. Therefore, the Notifying Party considers that the test for supply-side substitutability is met, as the condition for supply-side substitutability to occur ‘in the short term’ (176) has to be seen in light of these general industry characteristics (in particular, the prevalence of long lead times).
(225) Lead times for a standard (“off the shelf”) system would be significantly shorter, namely [timeframe]. To design, test, validate and commercialise a new standard system would however take [timeframe]. Nonetheless, the Notifying Party indicates that […]% of Danaher’s TFF system sales are for custom systems or customised ‘to at least some extent’’ (indicating that […] are purely custom). In hollow fibre systems, it only offers custom systems and has no standard systems in its portfolio. GE estimates that […]% of its TFF system sales in 2018 were custom.
(C.ii) Commission’s assessment
(C.ii.a) Demand-side substitutability
(226) The Commission notes that the Notifying Party does not contest that customers generally (177) cannot use systems for DFF, and TFF hollow fibre and TFF flat sheet interchangeably.
(227) In terms of demand-side substitutability between systems used for DFF and flat sheet and hollow fibre TFF, as indicated in paragraphs (212) and (214), the market investigation showed that customers do not use DFF, flat sheet and hollow fibre TFF filtration for the same applications, or only in a limited way. In that regard, the Commission understands that different systems are generally used for each of these filtration techniques. In light of the foregoing, the Commission considers that, from a demand-side perspective, customers are generally not able to substitute between DFF and TFF systems. As regards, hollow fibre and flat sheet TFF systems, the market investigation is inconclusive. However, concerning the Notifying Party’s arguments relating to existing supply-side substitutability, the Commission observes the following:
(C.ii.b) Supply-side substitutability
(228) With respect to supply-side substitutability, the Commission assessed (i) the competitive landscape for DFF, flat sheet and hollow fibre TFF consumables, (ii) the extent to which there are systems today that can process all (or some) of these consumables, (iii) to what extent suppliers that are not yet active in systems for a particular consumable can design and supply such a system from scratch.
a. Competitive landscape
(229) The Commission first notes that the competitive landscape for DFF and TFF systems differs significantly. While Danaher has a sizeable position in DFF, GE is hardly present. Competitors in DFF are not identical to those active in TFF and generally hold a stronger position than those active in TFF. In TFF, Danaher holds a sizeable position and GE has a stronger presence than in DFF. Moreover, one of the main competitors in TFF, namely Repligen, is not active in DFF. Similarly, the Parties’ largest competitors in flat sheet systems are not present in hollow fibre systems, while Repligen, the largest competitor in hollow fibre systems is not present in flat sheet systems. This can be seen as a first indication of the absence of supply-side substitutability.
b. Multi-functional systems
(230) Secondly, the Commission investigated to what extent systems, beyond those of the Parties, are today able to work with both DFF, flat sheet and hollow fibre TFF consumables.
(231) In the market investigation, a large minority of customers who expressed an opinion on this point (21 out of 46) indicated that skids can generally only work with one type of consumable, while others (14 out of 46) say that skids are generally able to work with flat sheet and hollow fibre TFF but not with DFF consumables and yet others (11 out of 46) say that skids are generally able to work with all types of consumables. (178)
(232) In that regard, the Commission notes that GE’s Äkta readyflux systems can be used for both consumables. However, it is unclear to what extent there are other systems that can also work with both. In this regard, the Notifying Party provided a link to Repligen and YMC’s websites where, according to it, it is indicated that they offer systems for both hollow fibre and flat sheet. The Commission however observes that the reference is unclear as to whether this would entail a single device that can work with both consumables, or whether these companies’ product portfolio would contain both systems for hollow fibre and systems for flat sheet consumables (that is, as individual devices). (179) Similarly, the other competitors’ websites referred to by the Notifying Party in the Form CO did not indicate that their systems would process both types of consumables. (180) Repligen’s website indicates that it provides certain TFF skids within its product portfolio that would be compatible with both flat sheet and hollow fibre consumables. (181) To request a quote on the respective devices however, customers are referred to a different link depending on the choice for flat sheet or hollow fibre filters. This would indicate that modifications or adaptations might be needed. Furthermore, these devices seem also limited to lab and pilot scale production scales.
(233) Further, while the Parties indicated that GE’s skid can also work with both DFF and TFF consumables, they also indicate that this system [use of GE’s systems]. The fact that TFF skids are more sophisticated is also reflected by comments made in the market investigation, for instance: “TFF is a more complex and time-consuming unit operation than with DFF. As such the required equipment is also necessarily more complex with more ability to control / modulate the process. […]”. The Commission in this regard questions the relevance of responses in the market investigation (referred to in paragraph (231) above) that indicated that skids can generally work with all types of consumables, regardless of whether these are flat sheet, hollow fibre TFF or DFF.
(234) Competitors are split in their views as to whether systems can typically accommodate different types of bioprocessing filtration consumables. Five indicate that skids can work only with one type of consumable, two that skids can work with both flat sheet and hollow fibre, and three that skids work with all consumables, including DFF. (182) On the basis of their explanations, the Commission understands that skids could potentially work with both flat sheet and hollow fibre consumables, but not readily so as this would require adaptations: “Skids are typically set up to cater either TFF or DFF and to specific consumable types which limits the interchangeability between flat sheet and hollow fiber consumables”; “[t]his is possible it is just a question of plumbing and assembly design”; “[t]he different types of filtration consumables have different requirements for the equipment. In most cases the systems are dedicated to the consumables. Still there are some overlaps that can be managed for TFF HF & TFF FS but need to be considered during the planning/construction of the equipment”; “[a] skid needs to be designed to meet the flow rate, pressure and physical connectivity requirements of the device that it is connected to. In general, the flow rate demands of a hollow fibre device are different from those of a flat sheet device, to do an equivalent separation job. That being said, customers who know up-front that they want the flexibility to have a skid to do both could have one designed. The key features of connecting the pump to the skid (inlet, permeate outlet, retentate outlet, valves, pressure sensors, etc.) are all the same”. (183)
(235) On the basis of the above considerations, the Commission concludes that devices able to process both DFF and TFF filters, or flat sheet and hollow fibre filters are not common in the industry.
c. Suppliers’ ability to switch
(236) Third, the Commission investigated to what extent suppliers can supply any type of system (regardless of whether they are an established supplier for that type of filtration) within the typical lead times in which customers source bioprocessing filtration skids.
(237) In this regard, it appears that the timeframe of […] indicated by the Parties as the timeframe in which a supplier can provide any type of skid (regardless of whether it has a presence in only flat sheet TFF, hollow fibre TFF or DFF) would in essence be in line with the typical lead times for customers to source bioprocessing filtration skids (overall). (184) The Commission notes that this might however be different for DFF systems in particular, as these are less customised than TFF systems and therefore would have shorter lead times. It is therefore not surprising that the Notifying Party only claims in this context that TFF systems are typically customised (while not claiming this for DFF systems), as well as with replies from the market test: “TFF skids are usually customized thus inducing a longer lead time. For DFF skids, standard skids are used with shorter lead time”; “[f]or our DFF filtration needs our requirements are very simple in that a pump and pressure monitoring are all we require, and these are mostly available ‘off the shelf’”. (185)
(238) A majority of competitors who expressed an opinion on this point indicate that they have the ability to produce all types of skids (DFF, flat sheet or hollow fibre TFF) within a short time period and without incurring significant sunk costs. (186) However, Repligen, the largest competitor in hollow fibre systems, indicates that it would not be able to produce these within a short time period and without incurring significant costs, and indicates in that regard that this would be a multi-year effort. (187)
(239) The market investigation also generally showed that lead times vary according to degree of customisation. For instance, a competitor indicated that “[i]t depends if the systems are custom made as they would also have longer lead times if the systems are large”. (188) While the Notifying Party claims that […]% of Danaher's TFF system sales are “custom and customized to at least some extent”, (189) customers indicated that they do not see it as mostly a supplier of customised systems. (190) Competitors also generally seem to not consider the Notifying Party as mostly a supplier of customised skids. (191)
(240) Therefore, the Commission considers that it is likely that the lead times for DFF systems as well as the Parties’ off-the-shelf TFF systems would be shorter than the time period needed by a supplier to start producing these systems when it is not already doing so, and that supply-side substitutability would therefore be limited in these areas.
(241) Moreover, it appears that there are other barriers to entry than engineering considerations. Competitors explain that reputation and experience as well as associated consumables are important: “[r]eputation and supply of core tech are they key barriers”; “[e]xperience, reputation and associated consumable products”; “[i]n filtration, reputation, brand, and customer validation are very strong differentiators”; “[e]xperience and reputation”; “[l]a non-fourniture des consommables (media filtrant) est un obstacle car le fournisseur du media filtrant peut influencer le client en conditionnant son support et ses garanties au choix de son skid et de ses “filter holders””; (192) “[l]a réputation est aussi très difficile à obtenir si on ne propose pas ces consommables”; (193) “[c]ustomers value experience/reputation as a top priority. No customer in this industry wants to be the first to try something new or bring something new before the regulators. Sophisticated Quality Systems is another barrier to entry. Customers expect that their suppliers will have extensive Quality Systems (essentially to the level of their own) and that they can be audited by customers at any time”; (194) “[s]uppliers design their skids for the TFF products that they offer. For example, Repligen offers both cassettes and hollow fibers, so their systems are capable of operating with both form factors. Pall and Millipore offer the cassette configuration for TFF, so their systems are designed to operate with cassettes”. (195) In light of this, the Commission considers that there are barriers to entry other than engineering considerations, such as reputation, experience and having an offering of associated consumables.
(242) The Commission considers the importance attached to reputation and experience particularly relevant given that it appears from the market shares that competitors seem much more present in certain segments and less in others. Similarly, it considers the importance attached to offering associated consumables particularly relevant given the differences of the suppliers present in each consumable type.
(243) In addition, relative price increases in systems of a particular type, such as those that could hypothetically be introduced as a result of the Transaction, do not seem likely to incentivise suppliers to switch (nor push suppliers to sell more of a particular system they do not have a focus on), as other factors such as the strengths and opportunities in associated consumables seem to be a main driver of such behavior. Competitors explain: “[d]riven by our consumable growth. If high, Repligen will enter into the market with differentiating technology”; “[t]he consumable part would likely include components that are specified by the end user. Besides, it generates the recurring revenue stream”; “[c]urrently our company does not provide any standard skids for any technology for which we do not have the consumable” (196); “[w]e have decided that our return on investment (ROI) to provide a standard hollow fibre skid is weak because customers are likely to go to a supplier who ‘understands’ how to design the skid based on the consumables […]”. (197)
(244) In light of the foregoing, the Commission considers that there are strong indications that there is no supply-side substitutability between different types of bioprocessing filtration systems. Therefore, the Commission will assess DFF, flat sheet and hollow fibre TFF systems as distinct markets, as well as systems overall and TFF systems overall. For the purpose of this decision, it can ultimately be left open. On the more narrowly defined plausible markets for TFF systems (SUT), flat sheet systems (SUT) and hollow fibre systems (conventional), the Transaction would give raise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to horizontal non-coordinated effects. The Notifying Parties have submitted Commitments that would address those. On the broader plausible markets, the Transaction would not raise serious doubts. In light of this, the market definition does not change the outcome of the competitive assessment.
(D) Conventional and single use technologies
(245) Bioprocess filtration processes can be either conventional (stainless steel, “SS”) or SUT. Different systems are used for either technology, whereby the SUT systems have a disposable flow kit and tubing. The equipment is also different. For both conventional and SUT processes, the filters (consumables) are disposable. For some applications however, the SUT equipment has an integrated consumable.
(D.i) Notifying Party’s arguments
(246) According to the Notifying Party, customers decide between the two technologies on a case by case basis. As SUT does not require cleaning between batches, it saves the customer time and would be particularly interesting for a customer with plants that make several products. On the other hand, conventional technology might be more interesting at large production scales due to cost-efficiencies.
(247) The Notifying Party considers that markets for systems and equipment should not be further segmented between conventional and SUT, due to supply-side substitutability. They claim that there are no material technical differences that would prevent a supplier of conventional products to produce SUT and vice versa. The system’s size and design for SUT would follow the same engineering principles and practices as for stainless-steel skids, with the difference that the flow path components and sensors used are made of a different material.
(D.ii) Commission’s assessment
(248) The market investigation indicated that there are large engineering differences between the two technologies (SUT and conventional). (198) Competitors explain: “[i]t is on a scale between a push bike and motorbike. SS skids are far more complex as you have to include all the support around cleaning and sterilisation, not something you need for SU”; “[f]or stainless steel, it’s engineering and at least a $5M R&D investment to start the program”; “[…] [i]l nous faudrait environ 1 à 2 ans pour proposer un skid “single use” en filtration”. (199) (200)
(249) In addition, the majority of competitors who expressed an opinion on this point that was not yet active in both technologies indicated that they would not be able to produce the other technology within a short period time (relative to customers’ average lead times) or without incurring significant costs. (201) Among those indicating that it could not switch is Repligen, the Parties’ largest competitor in hollow fibre, who specifies the need to “[b]uilding infrastructure for all the elements needed for stainless steel skids”. (202)
(250) In light of the foregoing, the Commission considers that there are strong indications that there is no supply-side substitutability between the SS and SUT technologies. Therefore, the Commission will assess the relevant markets both overall, and by segmentation into SS and SUT. For the purpose of this decision, it can ultimately be left open. On the more narrowly defined plausible markets for TFF systems (SUT), flat sheet systems (SUT) and hollow fibre systems (conventional), the Transaction would give raise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to horizontal non-coordinated effects. The Notifying Parties have submitted Commitments that would address those. On the broader plausible markets, the Transaction would not raise serious doubts. In light of this, the market definition does not change the outcome of the competitive assessment.
(E) Segmentation by size
(251) The Notifying Party submits that all main competitors sell small and large systems, and that engineering houses build all types of systems. Table 11 below provides an overview of systems supplied by the Parties and their competitors, ordered by size.
(252) On the basis of the above, the Commission does not further segment the relevant product markets by size, for the assessment of the current Transaction.
(F) Conclusion on plausible relevant product markets
(253) On the basis of the above, the Commission analyses the competitive impact of the proposed Transaction under the following plausible relevant product markets: overall bioprocessing filtration consumables, DFF consumables, flat sheet TFF consumables, hollow fibre TFF consumables, overall bioprocessing filtration systems, DFF systems (overall and segmented by SUT and conventional technology), TFF systems (idem), flat sheet TFF systems (idem) and hollow fibre TFF systems (idem).
4.5.2.2. Geographic market definition
(A) Notifying Party’s arguments
(254) Similarly as for the other product markets in the area of bioprocessing instruments and consumables, the Notifying Party considers the relevant product markets to be worldwide or at least EEA-wide in scope, for the following reasons: (i) all major players are active and sell globally; (ii) transportation costs and custom tariffs are low; and (iii) regulatory barriers have no or at most a very low impact on the geographic market definition. As regards regulatory differences within the EEA, the Notifying Party submits that these are technically the same, with the European Medicines Agency being centrally responsible for central evaluation and authorisation of biologics. While the process would slightly differ in the rest of the world, this is based on the same principles. In addition, the Notifying Party refers to the mutual recognition agreements with third-country authorities concerning the conformity assessment of biologics.
(B) Commission’s assessment
(255) The Commission considers that there are strong indications that the relevant geographic markets for bioprocessing filtration systems and consumables are world- wide in scope. (204)
(256) First, the Parties’ plants ship their bioprocess filtration products for all plausible markets from central locations to all regions in the world. (205)
(257) Second, customers who expressed an opinion in the market investigation on this point predominantly indicated that it is the worldwide level in which (i) they procure bioprocessing filtration systems; (ii) suppliers typically provide sales and after-sales services; (iii) prices are comparable; and (iv) the same suppliers are active. One customer however indicated that it is easier to use ‘European-active’ suppliers for customised systems, because of the importance of after-sales services. (206) This is also the case for consumables. (207) Customers did not indicate any differences between the different plausible product markets for consumables or systems in this regard.
(258) Third, competitors in their feedback agree with this, although a majority of those who expressed an opinion on this point indicate that prices for systems (not consumables) are comparable at the EEA-wide level (instead of at the worldwide level). (208)
(259) For the purpose of this decision, the Commission considers that the precise geographic market definition is most likely to be worldwide, although the exact market definition can be left open as the serious doubts raised by the Transaction are remedied by the Notifying Party’s Final Commitments.
4.5.3. Competitive assessment
4.5.3.1. Consumables
(260) There is a de minimis overlap in consumables overall, where the combined market share would be [20-30]% worldwide or in the EEA, with an increment of [0-5] and [0-5] percentage points respectively. Given (i) the negligible overlap; and (ii) considering that Danaher’s market shares are moderate; (iii) the lack of closeness given that the majority of this combined market share originates from Danaher’s activities in DFF consumables where GE is only active as […], while customers do not consider DFF and TFF to be substitutable; and (iv) due to an absence of substantiated concerns raised in the market investigation in this area, the Commission considers that the proposed Transaction does not lead to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement as regards a potential overall market for consumables.
(261) There is a de minimis overlap in DFF consumables, where GE is active only […]. GE’s market share in 2018 amounted to [0-5]% in both the EEA and worldwide (Danaher has a market share of about [20-30]% and [30-40]% respectively). (209) Given (i) the negligible overlap; and (ii) considering that Danaher’s market shares are moderate; and (iii) due to an absence of substantiated concerns raised in the market investigation in this area, the Commission considers that the proposed Transaction does not lead to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement as regards DFF consumables.
(262) There is an overlap in TFF consumables, where the merged entity would in 2018 obtain a combined market share of [20-30]% worldwide and [10-20]% in the EEA, with an increment of [10-20] and [5-10] percentage points respectively. Given (i) the moderate market shares; (ii) lack of closeness due to Danaher and GE’s focus on flat sheet and hollow fibre respectively; (iii) limitations of customers to switch between flat sheet and hollow fibre, as indicated in section 4.5.2.1(B); (iv) the presence of several other competitors, including a market leader that is almost twice as strong as the merged entity; and (v) the lack of substantiated concerns raised in the market investigation in this area, the Commission considers that the proposed Transaction does not lead to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement as regards a potential overall market for TFF consumables.
(263) Similarly, the overlap in flat sheet consumables would be de minimis […]. GE’s market share in 2018 amounted to [0-5]% on a worldwide level, and to [0-5]% in the EEA (Danaher’s market shares were [10-20]% and [10-20]% respectively). (210) Given (i) the negligible overlap; and (ii) considering that Danaher’s market shares are moderate, and (iii) due to an absence of substantiated concerns raised in the market investigation in this area, the Commission considers that the proposed Transaction does not lead to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement as regards flat sheet consumables.
(264) In hollow fibre consumables, the overlap would also be de minimis. Danaher, active […]. According to the market shares provided by the Parties, GE would in 2018 have had a market share of [40-50]% both worldwide and in the EEA (Danaher would have had a market share of [0-5]% and [0-5]% respectively).
(265) [Danaher’s R&D strategy and pipeline information].
(266) In this respect, the Notifying Party considers that no potential competition issues would arise from the Transaction in this area for the following reasons.
(267) First, [pipeline information and Danaher’s R&D strategy].
(268) Second, [pipeline information].
(269) Third, [pipeline information].
(270) Fourth, [GE’s R&D strategy].
(271) Fifth, [pipeline information].
Figure 7 – [Internal assessment of competitive relationships] (211)
[…]
(272) Sixth, the Notifying Party assumes that [pipeline information].
(273) Therefore, the Notifying Party considers that [internal assessment of competitive relationships]. In addition, [pipeline information; internal assessment of competitive relationships]. In addition, the Notifying Party considers that [pipeline information].
(274) The Parties indicated not to be in a position to provide detailed market shares for this application, [pipeline information].
(275) The Commission further investigated this matter, in order to verify to what extent [competitive assessment and pipeline information], and in that regard would potentially cause the Transaction to raise doubts given [pipeline information].
(276) The market investigation confirms that [pipeline information]. (212) [Pipeline information]. (213) The Commission understands that this is linked to the fact that [pipeline information]. (214)
(277) More importantly, [competitive assessment and pipeline information]. (215)
(278) In addition, the Commission understands that [assessment of the competitive landscape]. (216)
(279) On the basis of the above, the Commission considers that the Transaction would not raise serious doubts as to its compatibility with the internal market as regards [pipeline information].
(280) A large majority of customers who expressed an opinion in the market investigation on this point indicated that they did not expect that the Transaction would have an impact on price, quality, innovation, product range or security of supply as regards the market for flat sheet or hollow fibre TFF consumables. (217) This is largely in line with the replies from competitors, of which a majority of those who expressed an opinion on this point did not consider there to be an impact these markets. (218)
(281) In light of the considerations in paragraphs (260) to (280) above as well as all evidence available to it, the Commission concludes that, in the possible worldwide or EEA markets for DFF, TFF consumables, flat sheet TFF consumables and hollow fibre TFF consumables, the Transaction will not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to horizontal non-coordinated effects.
4.5.3.2. Equipment
(282) There is a minimal overlap in flat sheet equipment, where GE had […] sales of USD […] worldwide in 2018. This would not be an affected market, and the combined market share would in 2018 amount to [5-10]% (GE [0-5]% and Danaher [5-10]%) worldwide, and [5-10]% (GE [0-5]% and Danaher [5-10]%) in the EEA. The decision does not further address equipment. There is no overlap in DFF and TFF hollow fibre equipment. (219)
4.5.3.3. Systems
(283) According to the Notifying Party, an overall market for systems would not be an affected market. The Parties would have a combined market share of [10-20]% (GE [0-5]%, Danaher [5-10]%) worldwide and [5-10]% (GE [0-5]%, Danaher [5-10]%) in the EEA in 2018. (220)
(284) A market for DFF systems overall or markets segmented by SUT and stainless steel are not affected markets. The combined worldwide market share would in 2018 be highest in SUT DFF systems, namely at [10-20]% (GE [0-5]%, Danaher [5-10]%). GE […] in the EEA. (221)
(285) An overall market for TFF systems would be an affected market worldwide, but not in the EEA. The combined market share in 2018 was [20-30]% (GE [5-10]% and Danaher [10-20]%) worldwide and [10-20]% (GE [0-5]% and Danaher [10-20]%) in the EEA.
(286) The Commission recalls that segmentations of TFF systems which possibly constitute relevant product markets are the following: (i) TFF systems (SUT); (ii) TFF systems (conventional); (iii) flat sheet TFF systems (overall); (iv) flat sheet TFF systems (SUT); (v) flat sheet TFF systems (conventional); (vi) hollow fibre TFF systems (overall); (vii) hollow fibre TFF systems (SUT); and (viii) hollow fibre TFF systems (conventional).
(287) The possible markets for flat sheet TFF systems (conventional) and hollow fibre TFF systems (SUT) are not affected. In the potential market for flat sheet TFF systems (conventional), the merged entity’s combined market share would amount to [10-20]% worldwide and [10-20]% in the EEA with an increment of [0-5] and [0-5] percentage points respectively (2018 data). While the market share on a worldwide level is below the 20% threshold for affected markets, it is nonetheless […]. In light of the Parties’ declaration that it is difficult to estimate market shares, the Commission has performed spot-checks on their estimations on their competitors’ revenues. Based on the information at its availability, the Commission finds that the combined market share in 2018 was indeed [10-20]%. (222) As regards the possible market for hollow fibre TFF systems (SUT), the Commission notes that Danaher is […] in this possible market and thus there is […], neither worldwide or EEA-wide, in the activities of the Parties.
(288) Other plausible product markets are affected. According to the market share data submitted by the Notifying Party, the merged entity had a market share in TFF systems (conventional) of [20-30]% worldwide and not affected in the EEA; for TFF systems (SUT) of [20-30]% worldwide and not affected in the EEA; for flat sheet TFF systems (overall) of [20-30]% worldwide and not affected in the EEA; for flat sheet TFF systems (SUT) of [30-40]% worldwide and [20-30]% in the EEA; for hollow fibre TFF systems (overall) of [20-30]% worldwide and not affected in the EEA; and for hollow fibre TFF systems (conventional) of [30-40]% worldwide and not affected in the EEA. Each of these potential markets will be analysed below, as well as the overall market for TFF systems.
(A) Market shares
(289) The Notifying Party declared that it had difficulties to provide market share estimates for bioprocess filtration systems. While Danaher would not systematically estimate market size and market shares for bioprocess filtration systems, it more systematically tracks the opportunities that it is contacted for. It has based its estimates for systems on this information. It consider that it misses in this data a large number of system opportunities that are bid out to engineering companies, or other suppliers or system fabricators, that do not give Danaher to opportunity to quote for system business. In addition, not all opportunities are made public.
(290) The market investigation has not yielded any reliable third party data as regards market size estimations. The SDi Report Bioprocessing Technologies 2018 report (223) for instance attributes a higher amount of turnover to GE than Danaher, […]. (224)
(291) In its market investigation, the Commission has therefore collected turnover data of the Parties’ competitors, in order to gain comfort on the estimations used by the Notifying Party. As explained in sections (D), (G) and (H), it appears that the Parties’ market shares have been significantly understated for hollow fibre skids (overall and SUT) and flat sheet skids (SUT).
(292) The market shares provided by the Notifying Party are summarised in the tables below. (225)
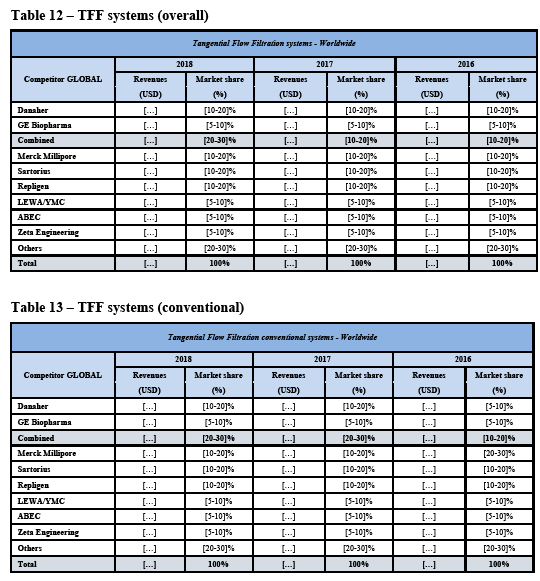
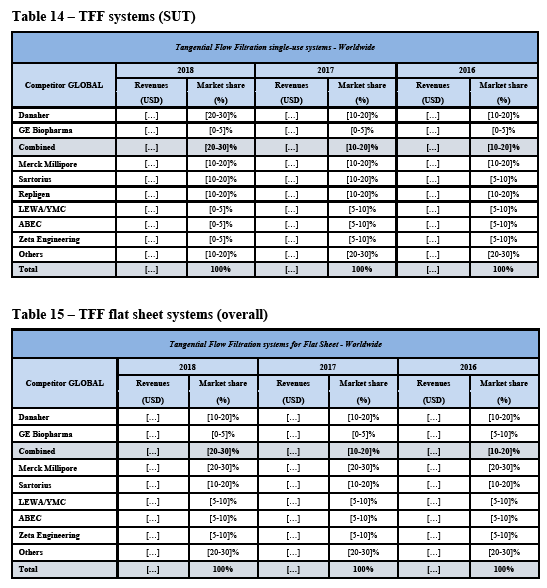
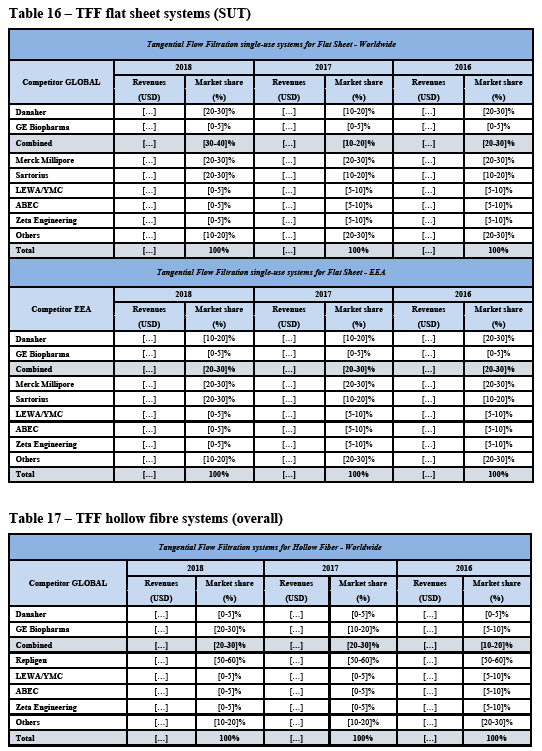
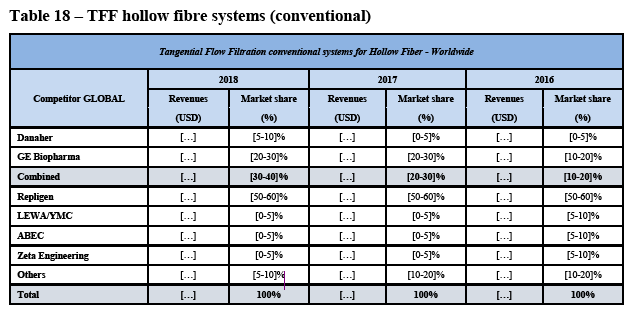
(293) In the above tables provided by the Notifying Party, the term ‘others’ refers to other engineering companies, as well as some activities of Merck Millipore and Sartorius.
(B) TFF systems (overall)
(B.i) Notifying Party’s arguments
(294) The Notifying Party submits that (i) the merged entity’s combined market shares and/or the increment brought about by the Transaction will not be high, (ii) there will be strong competition remaining from bioprocess filtration suppliers and engineering firms, (iii) the merged entity would face substantial countervailing buyer power, (iv) Danaher’s win-loss data shows […], and (v) that the assessment for TFF will not change when segmenting between conventional and SUT technologies or between flat sheet and hollow fibre filtration.
(B.ii) Market reconstruction
(295) According to the market shares submitted by the Notifying Party, the merged entity would obtain a combined market share of [20-30]% worldwide with an increment of [5-10] percentage points in 2018. An EEA market would not be affected. Apart from the Parties, there would be three other large integrated competitors, as well as a number of engineering firms.
(296) The Commission has requested competitors to submit 2018 turnover data and has performed a market reconstruction on the basis of the information obtained.(226) (227) The Commission is not able to disclose detailed market share information due to the confidential nature of such information. According to this reconstruction, the merged entity would obtain a worldwide market share of [20-30]% in an overall TFF systems market, with an increment of [5-10] percentage points. An EEA market would not be affected on the basis of the data at the Commission’s availability, although sales information of engineering firms was not obtained for verification. As the Commission can therefore not exclude that this would also be an affected market, the Commission also assesses the EEA market for TFF systems.
(B.iii) Closeness
(B.iii.a) Focus on flat sheet vs hollow fibre systems
(297) The Notifying Party claims that GE so far has not been a close competitor to Danaher. (228) As regards an overall TFF market, Danaher would primarily focus on flat sheet TFF systems and GE on hollow fibre TFF systems. The Commission however considers that, as set out in section 4.5.2.1(C.ii), flat sheet and hollow fibre systems are likely to constitute distinct product markets and are therefore assessed also individually in this section. To the extent that there would be a potential overall TFF market, the Commission considers that the Parties would not be close competitors as regards their focus on flat sheet and hollow fibre systems in such an overall market.
(B.iii.b) Scale
(298) The Notifying Party further indicates that the Parties are not close as regards the scale of systems sold, as over […]% of GE’s standard TFF systems revenues were from lab-scale systems in 2018, whereas this only amounted to […]% of Danaher’s standard TFF system revenues. More than […]% of Danaher’s standard TFF system revenues for 2018 were from pilot-and process-scale systems. (229) The Parties however underline that revenues from custom TFF system offerings are not included, and that such data segregated by scale for custom systems is not tracked in its reporting system. The Commission therefore does not consider this data representative, also in light of Danaher’s claim that it would sell predominantly custom TFF systems. (230)
(B.iii.c) Custom and off the shelf systems
(299) Filtration skids can be sold as a standard, off-the-shelf product, or designed and customised for individual customer needs. (231) In this regard, GE indicates that it estimates that […]% of its TFF systems in 2018 were custom, while the Notifying Party claims that […]% of its TFF system sales are ‘custom and customized to at least some extent’. (232) However, a small majority of customers who expressed an opinion on this point indicated that they do not see it as mostly a supplier of customised systems. (233) Competitors also generally seem to not consider the Notifying Party as mostly a supplier of customised skids. (234)
(B.iii.d) Conclusion on closeness
(300) A significant number of customers indicated that Danaher is the closest competitor of GE in TFF systems, and vice versa. The Commission notes that Merck, Sartorius and Repligen are also frequently mentioned. Out of a large number of respondents, none have indicated an engineering firm as being a close competitor. (235)
(301) On the basis of the above, the Commission considers that the Parties and their competitors Merck, Sartorius and Repligen are close competitors in an overall market of TFF systems, but engineering firms (236) (to whom the Notifying Party attributed significant shares) less so. On the other hand, the Commission considers that the Parties are likely close competitors within the segments for flat sheet and hollow fibre TFF skids.
(B.iv) Competition from engineering firms
(302) The Notifying Party claims that engineering firms compete closely with the Parties and other bioprocessing equipment suppliers. (237) These engineering firms would be addressing the same customers, and have deep technical knowledge, experience and established supply relationships with some of the major bioprocessing customers.
(303) It however appears that the customers that source from the Parties actually in only very few instances have sourced from suppliers other than the traditional suppliers in the bioprocessing industry (the Parties, Merck, Sartorius and Repligen). In this regard, the Commission found in the market investigation that out of 41 respondents (which were overall a sample of the Parties’ customers), only 1 indicated to have sourced TFF skids from the three larger engineering firms to whom the Notifying Party had attributed a significant amount of market share. (238)
(304) In addition, the competitive strength of such engineering firms, such as LEWA/YMC, ABEC, Cotter Brothers, Zeta and Bilfinger, appears to be perceived by most customers as much lower compared to the traditional suppliers in the bioprocessing industry (including the Parties). (239) Customers explain: “we do not consider such firms to compete equally with the Parties”; “[i]neffective engagement with the Bioprocessing industry from the engineering firms, especially smaller biotech companies and those engaging in pre-clinical or clinical work”; “[w]e view engineering companies compete on custom SS skids but not on SUT”; “[i]n our experience, an engineering company and the bioprocessing companies play complementary roles in the sense that an engineering company can help customize a design of a product that is made by the bioprocessing company. The engineering company does not ‘manufacture’ anything other than drawings”; “engineering firms end up going to the original suppliers for support. Our company prefers to work directly with the established bioprocessing players where we can count on them for troubleshooting and service in the long run”; “GE will charge an engineering company a higher price for equipment than they would charge us if we went directly to GE for the design work. they compete with the engineering companies for the design work so will discount the equipment if you use them for design as well as equipment”. (240)
(305) Competitor also explain that these firms might be dependent on the established suppliers to the bioprocessing industry: “[t]hey tend to use the established players as subcontractors. They will issue a URS on behalf of their clients and project manage the purchase of the skid”; “[a]s a supplier we receive User Requirement Specifications either from customers or from engineering companies. They define in detail what kind of functionality is required. The specifications sometimes even go down to Component / BOM level”. (241)
(306) The Commission points out that the Notifying Party indicated, as a caveat to the market shares it provided, to have limited visibility on the presence of engineering firms. It explains that these would namely participate in tenders where Danaher itself is generally not invited to. The Commission considers this also to confirm that the established bioprocessing suppliers such as the Parties might serve a different part of the market than these engineering firms.
(307) Furthermore, [Parties’ views of the engineering firms’ presence].
Figure 8 – […] (242)
[…]
(308) The Commission therefore finds that engineering firms potentially compete only in a neighbouring but different area in the market, and would in any event only exert a limited competitive constraint on the merged entity.
(B.v) Expansion
(309) As the Parties pointed out, Repligen announced in its 2018 Annual report that it will increase its production capacity for flat sheet TFF systems and hollow fibre TFF systems. (243) The Commission has assessed this in its market investigation but is unable to elaborate on its findings due to the inherent confidential nature of such strategic information. Nonetheless, the Commission takes note of this, but does not consider the expansion of a single competitor sufficient to alleviate serious doubts in the markets concerned, also in light of the differentiation in these markets where Repligen is currently focussed on hollow fibre TFF skids (SUT), whereas the Parties combined are stronger in flat sheet TFF skids (SUT) and hollow fibre TFF skids (SS). In addition, Repligen does not have a material presence in flat sheet skids today. A production capacity increase in this regard would not guarantee a successful entry, as it will enter the market as a new player that would still have to convince the market of its reputation and experience, as indicated in the market definition that these are important factors for a supplier to enter a particular segment. The Commission therefore considers that this cannot be taken into account to assess the extent to which the merged entity would be competitively constrained. In any event, the Commission does not find serious doubts in the overall market for TFF systems, regardless of the materialization of this claim.
(B.vi) Entrants
(310) The Notifying Party argues that there are no significant barriers that would prevent other firms already operating in the bioprocess filtration space to redirect or adapt their supply to other types of systems, and that market entry therefore is possible and not impeded by high market barriers.
(311) As indicated in section 4.5.2.1(C.ii), the Commission finds that there are barriers to entry, namely reputation, experience, and the need to offer associated consumables. In addition, entry would only be incentivised when a producer would have an associated consumable in its offering. In that regard, the Commission does not consider it likely that a price increase in systems would trigger entry and to that extent would sufficiently constrain the merged entity. In any event, the Commission does not find serious doubts in the overall market for TFF systems, regardless of the materialisation of this claim.
(B.vii) Buyer power
(312) The Notifying Party submits that […]% of its revenue comes from […]% of its customers, and that […] of its clients represent […]% of its revenue. (244) It claims that the merged entity would face substantial countervailing power from its customers, in particular biopharmaceutical companies, with large purchasing departments, using purchasing methods based on detailed request for proposals.
(313) The Commission however considers it unlikely that customers’ buyer power would be able to countervail competition concerns brought forth by the Transaction. In particular, the Commission notes that customers typically buy several products from suppliers in the bioprocessing industry, and are for any of these products that are specified in a commercial scale production line locked in to their supplier as switching would require revalidation and often re-filing with the relevant regulatory approval bodies. (245)
(B.viii) Impact
(314) While a majority of customers who expressed an opinion on this point does not expect the Transaction to have an impact on flat sheet TFF skids or hollow fibre TFF skids, some do expect a price increase, quality decrease, product range decrease, a decrease in innovation and/or a decrease in security of supply. (246) Competitors’ replies are in line with this. (247)
(B.ix) Complaint by a competitor
(315) There was one complaint by a competitor, who submitted on an anonymous basis that the Parties would be the largest skid providers and would obtain a dominant position post-Transaction. (248) In that regard, the Commission finds in its market reconstruction that the merged entity would indeed be the largest supplier, but however with moderate market shares. On that basis, the Commission does not accept this competitor’s claim.
(316) The complainant also submitted that GE would be dominant in small (lab scale) systems. (249) As regards GE’s alleged dominant position in lab scale systems, according to the Notifying Party, [Danaher’s sales of lab-scale systems]. (250) In any event, this competitor did not substantiate this claim of dominance, and its concern (as regards dominance or its theory more broadly) was not shared by any customer or other competitor. (251)
(B.x) Complaint by a customer
(317) A customer anonymously submitted that the Parties are each other’s closest competitors in TFF skids, and the only viable suppliers that are able to meet its specifications. In that sense, it expects the transaction to lead to the removal of a competitive constraint with higher prices as a consequence. (252)
(318) The Commission questions the allegations made by the complainant. As regards indicated in paragraph (300), the Commission found that Merck, Sartorius and Repligen are also frequently mentioned as the Parties’ closest competitors in the market investigation. In addition, the Parties are also focussed on different filtration techniques, while the complainant indicated in the questionnaire that it is not able to substitute between these techniques.
(319) To the extent that the complainant would refer to the Parties’ closeness in the segments where they are strongest (TFF SUT, TFF flat sheet SUT, TFF SS HF), the Commission notes that the commitments by the Notifying Party would remove the full overlap in these segments.
(B.xi) Conclusion
(320) In light of the considerations in paragraphs (295) to (319) above as well as all evidence available to it, the Commission concludes that, in the worldwide or EEA-wide market for TFF systems (overall), the Transaction will not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to horizontal non-coordinated effects given the Parties’ moderate market shares, and the presence of at least three other significant competitors.
(C) TFF systems (SUT)
(321) According to the market shares submitted by the Notifying Party, the merged entity would in 2018 obtain a combined market share of [20-30]% worldwide, with an increment of [0-5] percentage points, while an EEA market would not be affected. Apart from the Parties, there would be three other large integrated competitors, as well as a number of engineering firms.
(322) The Commission has requested competitors to submit 2018 turnover data and has performed a market reconstruction on the basis of the information obtained. (253) (254) The Commission is not able to disclose detailed market share information due to the confidential nature of such information. According to this reconstruction, the merged entity would be market leader with a market share of [30-40]% and an increment of [5-10] percentage points on a worldwide level with only one other competitor left as a significant player. The combined market share in the EEA would amount to [20-30]%, with an increment of [0-5] percentage points.
(323) In addition, a large part of the market has been attributed by the Parties to engineering firms and a category of ‘others’ that is not specified. The Commission’s market investigation did not obtain turnover data of these latter firms in order to verify their presence. Generally however, the market shares of such firms in other segments have been overestimated by the Parties. In this regard, the Commission cannot exclude that this would also be the case here and that therefore the Parties’ worldwide market share would be even higher than [30-40]%.
(324) As indicated in section (B.iv), these engineering firms to whom the Notifying Party attributed a large part of the market would only exert a limited competitive constraint on the merged entity.
(325) Similarly as indicated in section (B.v), Repligen’s announced capacity expansion cannot be taken into account to assess the extent to which the merged entity would be competitively constrained and the presence of buyer power (if any) would not suffice to guarantee that the merged entity would be competitively constrained.
(326) While the Parties argue that even if supply side substitutability would not lead to a single overall market for systems, producers active in one type of system are potential entrants into the other. However, in light of the large engineering differences, as well as importance for a supplier to have reputation and experience, as described in section 4.5.2.1(C.ii), the Commission does not consider that entry would be easy and would not constrain the merged entity.
(327) In addition, the Commission finds that the Parties are particularly close competitors, considering that both focus on flat sheet filtration within this segment, while the other significant competitor in this segment predominantly focuses on hollow fibre filtration.
(328) In light of the considerations in paragraphs (321) to (327) above as well as all evidence available to it, the Commission concludes that, in the worldwide or EEA- wide market for TFF systems (SUT), the Transaction will raise serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to horizontal non-coordinated effects given in particular (i) the uncertainty as to the market shares; (ii) the limited number of remaining traditional suppliers other than engineering firms; and (iii) the closeness of competition due to their common focus on flat sheet filtration. In any event, this therefore also replicates the findings in the segment of flat sheet TFF (SUT).
(D) Flat sheet TFF systems (SUT)
(329) According to the market shares submitted by the Notifying Party, the merged entity would in 2018 obtain a combined market share of [30-40]% worldwide or [20-30]% in the EEA, with an increment of [0-5] and [0-5] percentage points respectively. Apart from the Parties, there would be two other large integrated competitors, as well as a number of engineering firms.
(330) The Commission has requested competitors to submit 2018 turnover data and has performed a market reconstruction on the basis of the information obtained. (255) (256) The Commission is not able to disclose detailed market share information due to the confidential nature of such information. According to this reconstruction, the merged entity would be market leader with a market shares of [50-60]% on a worldwide level, which is [Parties’ presence], with an increment of [5-10] percentage points. In the EEA, the merged entity would have a market share of [40-50]%, with an increment of [5-10] percentage points.
(331) In addition, a large part of the market has been attributed by the Parties to engineering firms (for which the Party had identified market shares per individual supplier, as shown in the tables in section (A)) and a category of ‘others’ (which is not specified by individual supplier). The Commission’s market investigation found that these engineering firms are only to a limited extent present. The Commission cannot exclude that the Notifying Party also overestimated the presence of the unspecified category of ‘others’, as market shares obtained from the market investigation have generally also been overestimated. In this regard, the Commission cannot exclude that this would also be the case here and that therefore the Parties’ worldwide and EEA market shares would even be higher.
(332) As indicated in section (B.iv), these engineering firms to whom the Notifying Party attributed a large part of the market would only exert a limited competitive constraint on the merged entity.
(333) Similarly as indicated in section (B.v), Repligen’s announced capacity expansion and the presence of buyer power (if any) would not suffice to guarantee that the merged entity would be competitively constrained.
(334) While the Parties argue that even if supply side substitutability would not lead to a single overall market for systems, producers active in one type of system are potential entrants into the other. However, in light of the barriers set out in section 4.5.2.1(C.ii.b) (large engineering differences, as well as importance for a supplier to have reputation and experience), the Commission does not consider that entry would be easy and would not constrain the merged entity.
(335) As indicated in paragraph (B.viii), while a majority of customers who expressed an opinion on this point does not expect the Transaction to have an impact on flat sheet TFF skids, some do expect a price increase, quality decrease, product range decrease, a decrease in innovation and/or a decrease in security of supply. Due to the market investigation questionnaire being very lengthy, no particular questions were asked on the impact for flat sheet TFF skids (SUT) in specific.
(336) In light of the considerations in paragraphs (329) to (335) above as well as all evidence available to it, the Commission concludes that, in the worldwide or EEA- wide market for flat sheet TFF systems (SUT), the Transaction will give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to horizontal non-coordinated effects given in particular the high combined market share at a worldwide level.
(E) Flat sheet TFF systems (overall)
(337) According to the market shares submitted by the Notifying Party, the merged entity would obtain in 2018 a combined market share of [20-30]% worldwide with an increment of [0-5] percentage points, while an EEA market would not be affected. Apart from the Parties, there would be two other large integrated competitors, as well as a number of engineering firms.
(338) Based on the Commission’s market reconstruction, the combined worldwide market share was slightly higher, but [20-30]% and with an increment that would remain below [0-5] percentage points. In addition, the presence of other strong competitors, namely Merck and Sartorius, as well as some larger engineering firms, have been confirmed. The market share in the EEA would be [20-30]% with an increment of [0-5]%.
(339) The Commission did not receive any further evidence that would raise concerns in the markets for TFF systems (overall) that the Commission considers plausible.
(340) In light of the considerations in paragraphs (337) to (339) above as well as all evidence available to it, the Commission concludes that, in the worldwide or EEA- wide market for flat sheet TFF systems (overall), the Transaction will not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to horizontal non-coordinated effects.
(F) TFF systems (conventional)
(341) According to the market shares submitted by the Notifying Party, the merged entity would in 2018 obtain a combined market share of [20-30]% worldwide with an increment of [5-10] percentage points while an EEA market would not be affected. Apart from the Parties, there would be three other large integrated competitors, as well as a number of engineering firms.
(342) Based on the Commission’s market reconstruction, the combined worldwide market share was slightly higher, but [20-30]%. In addition, the presence of other strong competitors, namely Merck and Sartorius, have been confirmed. An EEA market would not be affected.
(343) The Commission did not receive any further evidence that would raise concerns in the markets for TFF systems (conventional) that the Commission considers plausible.
(344) In light of the considerations in paragraphs (341) to (342) above as well as all evidence available to it, the Commission concludes that, in the worldwide or EEA- wide market for TFF systems (conventional), the Transaction will not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to horizontal non-coordinated effects.
(G) Hollow fibre TFF systems (conventional)
(345) According to the market shares submitted by the Notifying Party, the merged entity would in 2018 obtain a combined market share of [30-40]% worldwide or [5-10]% in the EEA, with an increment of [5-10] and [0-5] percentage points respectively. Apart from the Parties, there would be one other large integrated competitor, as well as a number of engineering firms.
(346) As indicated in section (B.iv), the Commission finds that engineering firms would not competitively constrain the merged entity.
(347) Furthermore, the market investigation revealed that Repligen is not active within this segment, (257) leaving thus only the Parties and a number of fringe players. Based on the market reconstruction, the merged entity would obtain a market share of [70-80]% on a worldwide level, which is [Parties’ presence], with an increment of [10-20] percentage points. In the EEA, there is no overlap as Danaher is not active.
(348) As indicated in section (B.viii), while a majority of customers who expressed an opinion on this point does not expect the Transaction to have an impact on hollow fibre TFF skids, some do expect a price increase, quality decrease, product range decrease, a decrease in innovation and/or a decrease in security of supply. Due to the market investigation questionnaire being very lengthy, no particular questions were asked on the impact for hollow fibre TFF skids (conventional) in specific.
(349) In light of the considerations in paragraphs (345) to (348) above as well as all evidence available to it, the Commission concludes that, in the worldwide market for hollow fibre TFF systems (conventional), the Transaction will give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to horizontal non-coordinated effects.
(H) Hollow fibre TFF systems (overall)
(350) According to the market shares submitted by the Notifying Party, the merged entity would in 2018 obtain a combined market share of [20-30]% worldwide with an increment of [0-5] percentage points, while an EEA market would not be affected as [Danaher’s presence] in the EEA. Apart from the Parties, there would be one other large integrated competitor, as well as a number of engineering firms.
(351) As indicated in section (B.iv), the Commission finds that engineering firms would not competitively constrain the merged entity. In that regard, the transaction would result in a three to two, with only fringe players remaining.
(352) As indicated in section (B.viii), while a majority of customers who expressed an opinion on this point does not expect the Transaction to have an impact on hollow fibre TFF skids, some do expect a price increase, quality decrease, product range decrease, a decrease in innovation and/or a decrease in security of supply.
(353) Based on the market reconstruction, the merged entity would in 2018 obtain a combined worldwide market share of [30-40]% with an increment of [5-10] percentage points. An EEA market would not be affected.
(354) The Commission did not receive any further evidence that would raise concerns in the plausible markets for TFF systems (conventional).
(355) In light of the considerations in paragraphs (350) to (353) above as well as all evidence available to it, the Commission concludes that, in the worldwide or EEA- wide market for hollow fibre TFF systems (overall), the Transaction will not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to horizontal non-coordinated effects, given the moderate market shares and increment.
(I) Conclusion
(356) On the basis of the considerations in paragraphs (260) to (354) above, the Commission considers that the Transaction would raise serious doubts in regards to the plausible markets of (i) TFF systems (SUT); (ii) flat sheet TFF systems (SUT); and (iii) hollow fibre TFF systems (conventional).
4.6. Chromatography
(357) Chromatography is an important step in downstream bioprocessing because it is the core process for purifying the cell mass created in upstream bioprocessing. Chromatography refers to a method of separating and analysing the components or solutes within complex chemical mixtures. This separation is achieved by allowing a sample within a solvent to be carried by a mobile phase (in liquid or gaseous form) through an adsorbent serving as a stationary phase (typically in a column, gel or paper)
(358) The wider area of chromatography may generally be segmented according to the technique used in the chromatography process. One can generally distinguish between nine technology segments: (i) analytical high-pressure liquid chromatography (“HPLC”); (ii) preparative HPLC; (iii) clinical HPLC; (iv) gas chromatography (“GC”); (v) ion chromatography (“IC”); (vi) supercritical fluid chromatography (“SFC”); (vii) low-pressure liquid chromatography (“LPLC”); (viii) flash chromatography; and (ix) thin chromatography. (258) The Parties’ activities overlap only in the field of LPLC as this is the only area of chromatography in which GE BioPharma is active.
(359) LPLC chromatography is performed in a setup of three major components, which performs different tasks and are not substitutes:
– the chromatography skid, which is essentially the hardware component controlling liquid flow in the chromatography process.
– the chromatography column, which is the container holding the resins, that is the stationary phase, in which the chromatography process takes place.
– the chromatography resins are complex chemical products and in general are the most critical component of the chromatography process. Resins are placed in columns to bind & capture designed proteins.
(360) The chromatography market also includes new technologies which are sometimes used instead of traditional column chromatography for certain applications, such as membrane chromatography, a technology where the fluid is not run through a resin- bed to capture certain molecules, but through a membrane with specific adsorbent features and continuous chromatography, a setup for the chromatography process in bioprocessing that allows for the simultaneous processing of multiple columns.
(361) The Commission has not analysed in previous cases the various potential markets in the area of LPLC chromatography.
4.6.1. Product market definitions
4.6.1.1.Chromatography skids
(362) Chromatography skids are the hardware component controlling liquid flow in the chromatography process. They comprise of a metal frame housing a pumping system of pumps, valves, sensors and tubing operating the flow of the fluid that goes through the column and electronic components that control and steer the process. The electronic components are in turn controlled and monitored through a system operating software. Figure 9 portrays a chromatography skid.

(363) As described in paragraph (17) above, there is a growing trend in the bioprocessing industry to move from traditional clean-and-reuse stainless-steel products to single- use products. SUT chromatography skids are generally similar to conventional process chromatography skids with the main difference that the part of the skid that has contact to the product, that is the flow path through the chromatography control skid (which includes air traps, sensors, valves, fittings and pump tubing) is available as a single-use flow path. The SUT skid of one supplier is in general only compatible with the SU flow path of the same supplier (the skid and the flow path generally constitute a closed system). Figure 10 below portrays a SUT skid.
(364) The Notifying Party considers that there are notable differences between conventional LPLC skids and SUT LPLC skids that may warrant the definition of separate product markets for SUT LPLC skids. This is because, direct competition typically takes places not between SUT and non-SUT equipment, but within SUT and non-SUT equipment (similarly for other products). A number of customers are determined to use only SUT (or only non-SUT) products in their process facilities depending on their analysis of what is the best economically viable option for them and their setup. In addition, the range of suppliers for non-SUT and SUT skids is somewhat different some engineering companies that offer conventional LPLC skids have only a limited presence (or none presence at all) in the field of SUT LPLC skids. (259)
(365) A majority of customers who expressed an opinion on this point have confirmed in the market investigation that they would not consider stainless steel chromatography skids and SUT chromatography skids as suitable alternatives for the same applications. (260) As explained by one customer “not all applications of stainless steel chroma skids can be substituted by SUT chroma skids”. (261)
(366) For the purpose of this decision, and in the light of the fact that the market investigation confirmed the views of the Notifying Party that stainless steel chromatography skids and SUT chromatography skids do not constitute alternatives for the same application, the Commission will carry out its assessment with a distinction between stainless steel chromatography skids and SUT chromatography skids.
4.6.1.2. Continuous chromatography
(367) In continuous chromatography several (smaller) columns are operated in series over a larger number of cycles. By allowing simultaneous operation on many columns, continuous chromatography is generally considered to be more efficient. Moreover, continuous chromatography is associated with less resin consumption because more product can be applied to a given volume of resin as any unbound product from the first column is easily collected by the second column in the load phase. In terms of equipment and consumables, the difference between regular and continuous chromatography is limited to the skid. While continuous chromatography has for some time been an accepted practice by small-molecule manufacturers, it is not yet widely used in larger bio-manufacturing processes. (262) The Notifying Party considers that continuous chromatography skids constitutes a growing separate product market from other chromatography skids, which has been confirmed by a majority of respondents who expressed an opinion in the market investigation on this point. (263)
(368) For the purpose of this decision, and in the light of the finding that continuous chromatography skids are different products than stainless steel or SUT skids, the Commission will carry out its assessment distinguishing between continuous chromatography skids and stainless steel or SUT chromatography skids.
4.6.1.3. Chromatography columns
(369) Chromatography columns are the containers holding the resins, that is, the stationary phase, in which the chromatography process takes place. From a customer perspective, skids and columns serve different purposes in the chromatography process and are generally sourced separately. They therefore constitute a separate market from chromatography skids Pre-filled columns are a specific form of columns in that they constitute an integrated product comprising both the column and the resin.
(370) Danaher is essentially not active in pre-filled columns and therefore the overlap between the Parties 'activities lies only in non-prefilled chromatography columns. For the purpose of this decision, the Commission will carry out its assessment on traditional non-prefilled columns.
4.6.1.4. Resins
(371) Resins are complex chemical products and in general are the most critical component of the chromatography process. Resins are consumables that need to be more frequently exchanged over time. The choice of the particular resin defines the efficiency, flow resistance, selectivity and binding capacity of the process. Due to the differences in their chemical characteristics and features, different types of resin are used for specific purification and production steps and the processing of particular molecules. (264)
(372) The Notifying Party distinguishes between different types of resins based on their performance and capabilities and considers that they are not substitutable from a demand side perspective. (265) (266)
– Affinity resins utilise specific binding interactions between a ligand that is immobilised to a resin and its binding partner. The most commonly used affinity resin in biomanufacturing of monoclonal antibodies is Protein A resin because Protein A is a simple and effective means to purify antibodies. The Notifying Party distinguishes between Protein A and other affinity resins because the latter are selective for other biological targets and not antibodies.
– Ion exchange (“IEX”) resins separate molecules based on their total electric charge. For targets such as monoclonal antibodies, IEX is typically an effective second purification step, while affinity resins are typically the initial step in the purification workflow. For non-antibody molecules, IEX resins are often a good starting point for the first purification step.
– Mixed mode resins utilise matrices that have been functionalised with ligands capable of multiple interactions. This method is useful when purifying target proteins where other methods fail, because of its unique selectivity.
(373) A vast majority of customers who expressed an opinion on this point have confirmed in the market investigation that they would not consider different types of resins as suitable alternatives for the same applications. As explained by one customer, “[e]ach resin has specific capacity and selectivity whereas the mechanism of action is similar (e.g., affinity, cationic or anionic exchange, hydrophobicity)”. Another customer claimed that “[a]ny resin is specific to the process, ion exchange resins cannot be replaced with size exclusion resins and smaller beads can not be replaced with larger beads”. (267)
(374) For the purpose of this decision, and in particular the light of the findings of the market investigation that confirmed the views of the Notifying Party that different types of resins are not substitutable from a demand-side perspective, the Commission will carry out its assessment with a distinction between protein A resins, other affinity resins, ion-exchange resins and mixed mode resins, which are the resins in which the activities of Danaher band GE BioPharma overlap.
4.6.1.5. Membrane chromatography
(375) Membrane chromatography is a technology where the fluid is not run through a resin-bed to capture certain molecules, but through a membrane with specific adsorbent features. For certain applications, this makes them a superior solution because even if membrane chromatography is limited in dynamic binding capacity compared with column/resin technology, it allows for much faster flow-through rates.
(376) For that reason, while column chromatography (notably based on ion exchange resins) and membrane chromatography may be substitutable for certain applications, the Notifying Party considers that there are differences in the demand structure that may indicate that membrane chromatography constitutes a separate product market from column chromatography performed with resins. In terms of supply, there are also differences in the structure of supply between the two areas. This is, for example, illustrated by the fact that Sartorius is the largest supplier of membrane chromatography absorbers, while it has no presence in conventional chromatography columns. (268)
(377) Respondents to the market investigation have in general confirmed this lack of substitutability between resins-based chromatography and membrane chromatography. As explained by one customer, “[a]lthough they use similar ligands, their mass transfer properties are very different. As such they work better in different applications and there is not really that much overlap in reality. IEX resins dominate in protein application, while IEX membranes are better suited for larger macro-molecules like viruses”. (269)
(378) For the purpose of this decision, and in the light of the findings of the market investigation that confirmed the views of the Notifying Party resin-based chromatography and membrane chromatography are not substitutable from a demand-side perspective the Commission will carry out its assessment on membrane chromatography as a separate product market.
4.6.2. Geographic market definition
(379) The Notifying party submits that all relevant product markets for chromatography laid out above are at least EEA-wide in scope, for the following reasons: (270)
– Transportation costs are low in absolute terms and not significant compared to the value of the relevant products, generally accounting for a very low proportion of […]% of the price for skids, columns and resins.
– The relevant products are mostly manufactured at one single site worldwide and shipped from those sites to regional distribution hubs around the world. For example, GE BioPharma manufactures […] its skids in its factory in […], from where it ships its products to regional distribution hubs around the world.
– There are only limited regulatory differences with respect to the sale of these products between different regions of the world and product design and technical specifications for skids, columns and resins are also generally uniform globally.
– All major suppliers of skids, columns and resins operate globally and are present in every geography.
(380) Respondents to the market investigation have overwhelmingly confirmed that they source these products on a worldwide basis, with more sourcing within the EEA as regards complex chromatography skids, to ease the relationships with and services from the supplier. (271)
(381) On the basis of the above, the Commission can leave open the precise geographic definition for chromatography products as the competitive assessment remains the same regardless of whether a worldwide or an EEA-wide geographic definition is retained.
4.6.3. Competitive assessment
(382) The Danaher company operating in the areas of chromatography in which GE Biopharma is active is Pall. Pall is offering skids, columns, affinity, ion exchange and mixed-mode resins ([Danaher’s contractual relationship with its trading partner]), membrane chromatography products and continuous chromatography products. GE BioPharma offers standard and SUT skids, standard and pre-filled columns, all types of resins, continuous chromatography skids and […].
4.6.3.1. Chromatography skids
(A) Market shares and the Notifying party’s views
(383) As regards standard chromatography skids, the Notifying Party has estimated its combined market shares at worldwide level at [30-40]% (with an increment from Danaher of [0-5]%) and at EEA-level at [20-30]% (with an increment of [5-10]%). The Notifying Party has identified as alternative suppliers Merck Millipore ([5-10]% at world-wide and EEA level), Novasep ([5-10]% at both levels), Lewa ([0-5]% at both levels), as well as companies specializing in plant/process engineering services such as ABEC, Bilfinger, Cotter Boothers, Vogelsbuch and Zeta which together would account for [20-30]% of sales at worldwide and EEA level.
(384) As regards SUT chromatography skids, the Notifying Party has estimated its combined market shares at worldwide level at [80-90]-[90-100]% (with an increment of Danaher of [0-5]%) and at EEA-level at [70-80]-[80-90]% (with an increment of [0-5]%). The Notifying Party has identified as alternative suppliers Merck Millipore ([5-10]% at world-wide and EEA level), Verdot ([0-5]% at both levels), and Flexbiosis ([0-5]% at both levels).
(385) The Notifying Party therefore submits that the combined entity’s share of the conventional LPLC skids area will be moderate and not exceed [30-40]% worldwide or in the EEA. According to Danaher, customers will continue to have a choice between multiple suppliers, and the combined entity will continue to face effective competition from these suppliers. Danaher also submits there are also no substantial barriers that would prevent new suppliers from entering the skids segment.
(386) As regards SUT skids, the Transaction will result only in a small increase in segment shares given that Danaher’s revenues are small. The Notifying party also submits that Sartorius and Thermo-Fisher are potential entrants in this area.
(B) Commission’s assessment
(387) As regards standard chromatography skids, the Commission has found internal documents which indicate that the combined share is higher than what Danaher submits, in the area of [80-90]% for both skids and columns. (272) The columns on the left of the graph refer to pilot-scale chromatography skids and columns (small instruments) and shows that GE would hold a market share between [60-70]% for each of the skids and columns with an increment of [10-20]% from Danaher. The columns on the centre refer to process-scale chromatography skids and columns (larger instruments) and shows that GE would hold a market share [50-60]% for each of the skids and columns with an increment [20-30]% from Danaher.
[…]
(388) Customers having responded to the market investigation have indicated that in that regard Pall would be the closest substitute to GE for both stainless skids and that therefore the Transaction would eliminate a significant alternative to GE. (273) As explained by one customer, “[a]lternatives are fewer due to past mergers. However due to costs and regulatory timings, we currently would be exposed to a single supplier as we only use Pall & GE”. (274) Another customer put forward that “[t]his would only leave Millipore as a reasonable alternative but systems are not available to cover labscale (process development) and large scale making scale up more cumbersome and also software is less sophisticated”. (275)
(389) With market shares [70-80]% at worldwide levels and [80-90]% at EEA level, GE already holds as regards SUT chromatography skids a very significant market position which is at this stage […] and the elimination of one of the alternatives, albeit small, would risk strengthening [GE’s market position]. In particular in SUT skids, GE [GE’s market position] the process development and pilot scales with their Unicorn systems offering.
(390) Moreover, a majority of customers who expressed an opinion in the market investigation on this point do not considered engineering companies as suitable alternatives due to inability to custom build a skid and lack in experience in the chromatography field suited to meet customer’s requirements. This is particularly the case for SUT skids. One customer explained that they might compete in “[o]nly for stainless steel fabrications where we will supply the design. But they have very limited ability to compete in the pilot and smaller scales as a high level of automation is required and they cannot compete with the software packages from GE”. (276)
(391) Finally, respondents to the market investigation do not expect additional companies to start supplying chromatography skids (either stainless steel or SUT) in the next two to three years, because of costs, technologies and entry barriers such as regulatory timing. (277)
(392) Because of this elimination of suitable alternatives, a majority of respondents who expressed an opinion on this point expect negative impacts on competition a regards chromatography skids, both for stainless steel and SUT, notably price increases. As explained by a university customer, “[i]n the first few years, not many/any company will be able to provide the products (with the same quality). Some companies will then increase their prices”. Another customer put forward that “[c]ombining GE and Danaher will impact on the overall market for bioprocessing skids (chrom & TFF) as one of the major competitors will be removed from the bidding processes and not replaced”. (278)
(393) In light of the considerations in paragraphs (387) to (392) above as well as all evidence available to it, the Commission concludes that, in the worldwide or EEA- wide market for stainless steel chromatography skids, as well as in the worldwide or EEA-wide market for SUT chromatography skids, the Transaction will give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to horizontal non-coordinated effects.
4.6.3.2. Continuous chromatography skids
(A) Market shares and the Notifying Party’s views
(394) As regards continuous chromatography skids, the Notifying Party has estimated its combined market shares at worldwide level at [30-40]-[40-50]% (with an increment from GE of [10-20]%) and at EEA-level at [20-30]-[40-50]% (with an increment of less than [0-5]%). The Notifying Party has identified as alternative suppliers Novasep ([10-20]% at world-wide and EEA level), Chromacon ([10-20]% at both levels) and Semba ([0-5]% at both levels).
(395) The Notifying Party therefore submits that the combined entity’s share of the continuous chromatography skids area will be moderate and not exceed [40-50]% worldwide or in the EEA. According to Danaher, a combined Danaher/GE Biopharma will face competition from three current suppliers – […] – and customers will, thus, continue to have the choice between a number of suppliers of continuous chromatography skids with an established product offering on the market. Danaher also submits that given the rather low barriers to enter the continuous chromatography skids area, there are a number of suppliers who are on the verge of entering or at least likely to enter the market, like Merck-Millipore or Sartorius.
(B) Commission’s assessment
(396) The Commission takes the view that in light of the replies from the market investigation, the Transaction would eliminate the most important source of rivalry in the continuous chromatography skids market, creating a new market leader with a share twice as high at its nearest rival, with little prospects of new entry and potential negative impact on conditions of supply in the continuous chromatography skids market
(397) In particular, customers having responded to the market investigation have indicated that in that regard GE would be the closest substitute to Pall and that therefore the Transaction would eliminate a significant alternative to Danaher in continuous chromatography skids. As explained by one customer, “[t]echnology assessments show these suppliers (Pall and GE) as potential competitors for this technology”. (279) Another customer put forward that “SEMBA and CHROMACON are small players and wouldn’t be considered as credible partners in large biopharma companies”. (280) A third customer explained that there are “[l]imited number of major vendors in this space. Danaher purchased one of the major supplier Tarpen a few years ago”. (281)
(398) Moreover, respondents to the market investigation do not expect additional companies to start supplying continuous chromatography skids in the next two to three years – rather established suppliers in other fields could grow in this market through acquisition, as claimed by one customer. “The matter is complex, and barriers are high, we expect that the existing small players will be acquired by resin providers or by TFS, MM or Sartorius”. (282)
(399) Because of this elimination of suitable alternatives, some customers expect negative impacts on competition a regards continuous chromatography skids, notably price increases and elimination of one product range, although the future appears at this stage highly uncertain. As explained by one customer, “[t]he merger may lead to discontinuation of either Pall or GE’s continuous system offering, which may reduce market competition and potentially increase the pricing. But the two players may leverage each other’s IPs and further improve their product features and capabilities”. (283)
(400) In light of the considerations in paragraphs (396) to (399) above as well as all evidence available to it, the Commission concludes that, in the worldwide or EEA- wide market for continuous chromatography skids, the Transaction will give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to horizontal non-coordinated effects.
4.6.3.3. Chromatography columns
(A) Market shares and the Notifying Party’s views
(401) As regards chromatography columns, the Notifying Party has estimated its combined market shares at worldwide level at [40-50]% (with an increment from Danaher of [5-10]%) and at EEA-level at [30-40]-[40-50]% (with an increment of [0-5]%). The Notifying Party has identified as alternative suppliers Merck Millipore ([10-20]% at world-wide and [10-20]% at EEA level) and Novasep ([0-5]% at both levels). The overlap in pre-filled columns is minimal ([0-5]%).
(402) The Notifying Party therefore submits that the combined entity’s share of the chromatography columns will be moderate and not exceed [40-50]% worldwide or in the EEA. According to Danaher, customers will continue to have a choice between multiple suppliers, and the combined entity will continue to face effective competition from these suppliers. Danaher also submits there are also no substantial barriers that would prevent new suppliers from entering the columns segment.
(B) Commission’s assessment
(403) The Commission takes the view that in light of the replies from the market investigation, the Transaction would eliminate the most important source of competitive interaction in the chromatography columns market, creating a new market leader with a share at least twice as high at its nearest rival (and possibly much above), with little prospects of new entry and potential negative impact on conditions of supply in the continuous chromatography skids market
(404) Similarly to chromatography skids, the internal document showed in paragraph (387) chromatography skids, would tend to indicate that the combined share is higher than what Danaher submits, in the area of [80-90]% for columns.
(405) Customers having responded to the market investigation have indicated that in that regard Pall would be the closest alternative to GE and that therefore the Transaction would eliminate a significant alternative to GE in columns. As explained by one customer, “[f]or some applications, there will be no alternatives”. Another customer put forward that “[o]fferings available from alternative suppliers however Danaher and GE are 2 of the top 3 suppliers of industrial columns so potential to restrict choice”. (284)
(406) Moreover, respondents to the market investigation do not expect additional companies to start supplying chromatography columns in the next two to three years, because, as explained by one customer, “[i]t takes a long time to get into market and create supply channels etc. so unlikely to be new entrants in timeline above”. (285)
(407) Because of this elimination of suitable alternatives, a majority of respondents who expressed an opinion on this point expect negative impacts on competition a regards chromatography columns, notably price increases. As explained by one customer, “[i]f there are not many suppliers, the prices could increase and the quality could decrease. The security of supply could be decrease as well, if there are not many suppliers”. (286)
(408) In light of the considerations in paragraphs (403) to (407) above as well as all evidence available to it, the Commission concludes that, in the worldwide or EEA-wide market for chromatography columns, the Transaction will give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to horizontal non-coordinated effects.
4.6.3.4. Chromatography resins
(A) Market shares and the Notifying Party’s views
(409) As regards resins, the Notifying Party has provided market shares at worldwide and EEA level in the table below.
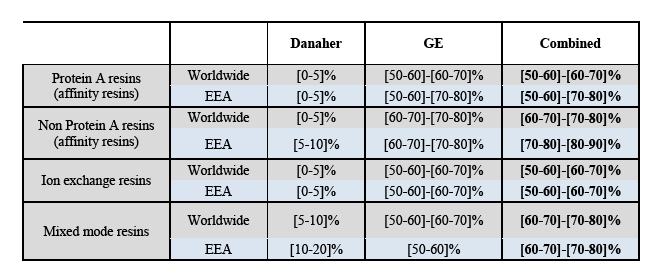
(410) As regards Protein A resins, the Notifying Party submits that Danaher does not manufacture Protein A, and its activities in this segment are limited to the resale of small amounts of Protein A [Danaher’s market shares and sales as well as contractual relationship].
(411) For other resins, Danaher submits that it is only a minor supplier and by no means a close competitor to GE Biopharma. In any event, the Notifying Party submits customers post-Transaction will continue to have a choice between several viable alternative suppliers that are all established players in the chromatography segment, including Merck Millipore, Bio-Rad, Tosoh and Thermo Fisher.
(B) Commission’s assessment
(412) With market shares systematically [50-60]% and in some cases, [60-70]%, GE already holds a [GE’s market position] both at worldwide level and EEA level which [GE’s market position].
(413) In particular as regards Protein A, internal documents of GE show that GE is able to [GE’s price structure for Protein A] but also to the market position that it enjoys in the resin space, as shown in the graph below. (287) Similar graphs have been produced by GE in relation to other types of resins.
[…]
(414) This […] in the resin field is also reflected in this internal document, which show the overall share of the resin business and demonstrates how GE is clearly ahead of all its rivals in resins. (288)
[…]
(415) Customers having responded to the market investigation have indicated that in that regard the Transaction would eliminate one of the few alternative to GE in resins. As explained by one customer, “[o]ur gmp production suites rely heavily on GE resins with minimal use of Thermo Fisher POROs AEX resin. Based on our R&D team’s experience, Thermo Fisher would be able to provide comparable performance of polishing resins (non-ProA), however ProA applications do not have a viable substitute apart from GE. Additionally, regarding Thermo Fisher, we are unsure of their ability to commercially supply large quantities of polishing resins because we only have experience with procuring smaller quantities”. (289)
(416) Consistent with such expectations of this elimination of one suitable alternative to GE resins, a majority of respondents who expressed an opinion on this point expect negative impacts on competition a regards resins, notably price increases.
(417) As regards affinity non-protein A resins, the Commission takes the view that the very high market share of GE ([60-70]%), combined with the elimination of the few alternatives, is likely to strengthen the [market position] of GE. This has been confirmed by a majority of respondents to the market investigation having expressed an opinion on this point.
(418) As regards ion exchange resins, the Commission takes the view that the very high market share of GE ([50-60]%), combined with the elimination of one of the few alternatives, albeit small, is likely to strengthen the [market position] of GE. This has been confirmed by a majority of respondents to the market investigation having expressed an opinion on this point.
(419) As regards mixed-mode resins, the Commission takes the view that the very high market share of GE ([50-60]%), combined with the elimination of the few alternatives, with a non-negligible market share, is likely to strengthen the [market position] of GE. This has been confirmed by a majority of respondents to the market investigation having expressed an opinion on this point.
(420) The Commission, however, also notes that Danaher’s position specifically regarding Protein A has been […] and that its […]. (290) Thus, while there was a […] in relation to Protein A […], this horizontal overlap no longer exists.
(421) In light of the considerations in paragraphs (412) to (420) above as well as all evidence available to it, the Commission concludes that, in the worldwide or EEA-wide markets for non-Protein A affinity resins, ion exchange resins and mixed-mode resins, the Transaction will give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to horizontal non-coordinated effects.
4.6.3.5. Membrane chromatography
(422) The Transaction does not lead to the combination of actual suppliers of membrane chromatography as GE Biopharma is essentially not active in the supply of membrane chromatography. While GE Biopharma is party to a distribution agreement […] under which GE Biopharma resells […] chromatography membranes, revenues derived from such resales were […], accounting for [0-5]% of worldwide and EEA-wide total sales of chromatography membranes. (291)
(423) Danaher on the other hand has a combined share of [20-30]% while the market leader is Sartorius with [50-60]% share at both worldwide and EEA level. Accordingly, the Transaction does not affect the structure of supply of membrane chromatography and customers have not expressed concerns as regards the impact of the transaction in membrane chromatography. (292)
(424) In light of the considerations in paragraphs (422) to (423) above as well as all evidence available to it, the Commission concludes that, in the worldwide or EEA-wide market for membrane chromatography, the Transaction will not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to horizontal non-coordinated effects.
4.7. Integrated solutions
(425) ‘Integrated solutions’ constitute a relatively nascent, dynamic and diverse offering for which there does not appear to be a generally agreed or common definition at this point in time. It encompasses the sale as a single product of a diverse range of combinations of multiple components, equipment and systems of the bioprocessing workflow together with related services, such as biomanufacturing process design, systems and process automation, and project management.
(426) Integrated solutions seem to be appealing in particular to smaller or less sophisticated customers for which time to market and a single point of contact provide greater value than the needed investment to optimise yield with a choice of the best technical fit for each bioprocessing product. (293) Their sales are forecast to increase significantly in the coming years.
(427) In that regard, key customer groups appear to be small biotechnology companies (notably those active in cell and gene therapy), firms with projects in emerging markets (notably China) or to produce biosimilars, as well as contract development and manufacturing organisations (‘CDMOs’).
(428) More generally, integrated solutions seem to contribute to a broader trend for greater standardisation and use of platforms, but also a parallel trend for more customised and flexible production in view of more individualised therapies.
4.7.1. Market definition
4.7.1.1. Product market definition
(429) The Commission has not previously dealt with cases in the area of integrated solutions.
(430) Depending on the supplier and specific sale opportunity, integrated solutions in particular involve multiple components, equipment or systems in (i) a single step of the manufacturing process; (ii) two or more steps in either of the upstream or downstream process; (iii) two or more steps across both upstream and downstream processes; (iv) a complete factory line module; or (v) a working biomanufacturing facility.
(431) Different integrated solutions may also be split between, among others, (i) manufacturing platforms (upstream, downstream or both); (ii) full prefabricated manufacturing facilities; and (iii) associated services such as process development services, plant design or construction, and contract manufacturing.
(432) Given their diversity of content, the Parties view integrated solutions as a marketing initiative set up internally by suppliers to enhance individual product sales – rather than responding to a discrete customer demand – that cannot properly be defined as a distinct relevant product market. (294)
(433) On the contrary, the large majority of customers and competitors who expressed an opinion on this point indicated in their responses to the market investigation that integrated solutions would constitute a product that is distinct from the sum of its components. (295)
(434) Similarly, at least the Parties have teams, brands, reporting channels, strategic presentations and marketing material dedicated to integrated solutions. Moreover, integrated solutions are offered [Parties’ price structure]. (296)
(435) In this light, and considering all available information in the file, for the purposes of this decision, the Commission considers that there is a differentiated market including all integrated solutions as opposed to several distinct relevant product markets to meet identified customer needs within a broader integrated solutions area, for instance upstream or downstream manufacturing platforms. The Transaction does not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement in thi
4.7.1.2. Geographic market definition
(436) Integrated solutions are sales of bioprocessing equipment and associated products and services to customers in several regions of the world, mainly by suppliers active globally.
(437) There do not appear to be significant differences in the conditions of competition across geographies compared to those for bioprocessing products generally, which are the components of integrated solutions, as described for instance in section 4.2.1.1(B).
(438) Accordingly, there are indications that the relevant geographic market for integrated solutions could be global or at least EEA-wide.
(439) For the purposes of this decision, it can be left open whether the market for integrated solutions is global or EEA-wide in scope. The Transaction does not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement under any of these plausible geographic market definitions.
4.7.2. Competitive assessment
4.7.2.1. Horizontal overlaps
(440) Danaher (through Pall) has since 2018 supplied ‘Integrated Solutions’ – [Danaher’s sales strategy and marketing plans]. Pall advertises two Integrated Solutions platforms equipped with Pall hardware systems and consumables for upstream and downstream bioprocessing: (i) one for 2 000 litre monoclonal antibody batch processing; and (ii) another for adeno-associated virus vector production (used in gene therapy). It also sales a wide variety of more tailor-made solutions. (297)
(441) Danaher’s sales in Integrated Solutions (excluding associated services) amounted to approximately […]. (298)
(442) GE supplies ‘Entreprise solutions’: (i) FlexFactory, an application-tailored manufacturing platform; and (ii) KUBio: a prefabricated configurable biomanufacturing facility. It also supplies (iii) Fast Trak Services such as process development. GE launched FlexFactory for gene therapy in October 2019. (299)
(443) GE’s sales in integrated solutions (only FlexFactory and KUBio) amounted to approximately USD […] in 2018 and USD […] to date in 2019. (300)
(444) Both Parties also offer some associated or other CDMO services such as process development. (301)
(445) Each of the Parties’ integrated solutions are largely built around their respective portfolio of proprietary products, although they are usually willing to incorporate components from competitors at the customer’s request and in any event do so to fill their own portfolio gaps.
(446) The Parties’ sales figures illustrate that, being a nascent market, integrated solution sales have been […]. It is not disputed, though, that they are forecast to […] in the coming years.
(447) The Parties argue that there are no competition concerns with regard to integrated solutions in spite of this horizontal overlap. In particular, the Parties submit that the merged entity would post-Transaction continue to be constrained by a number of competitors active in the supply of at least some elements of integrated solutions.
(448) In addition to the supply of individual bioprocessing products, suppliers such as Merck, Thermo Fisher and Sartorius would offer process development services and other services such as cell line development, facility design of equipment selection. (302)
(449) In particular, the Parties submit that Sartorius would offer bioprocess automation platforms, and Merck would offer end-to-end solutions under its BioReliance brand. Sartorius notably appears to have developed a 2 000 litre single-use mAb generic bioprocessing platform. (303)
(450) The Parties further submit that engineering firms would similarly be able to combine individual components sourced from bioprocess suppliers into custom-designed automated platforms to meet customer needs. The Parties notably list Jacobs, Cotter Brothers and ABEC as relevant firms. (304)
(451) Moreover, the Parties submit that, in a context where customers seek to procure the best product – from a technical perspective – for each step, a broad or even complete product portfolio is not a significant competitive advantage and suppliers of integrated solutions are constrained by mix-and-match procurement by customers from several suppliers.
(452) Finally, the Parties submit that the merged entity would continue to have significant portfolio gaps.
(453) The market investigation and the Parties’ internal documents confirmed that [GE’s market position and sales strategy]. (305)
(454) The Parties’ internal documents suggest that among the four main competitors to GE in integrated solutions [Danaher’s market position and performance based on GE’s insight and intelligence]. (306) As noted above, Pall has been offering integrated solutions only since 2018.
(455) Consequently, the competitive change brought about by the Transaction in the current market for integrated solutions can be considered to be limited.
(456) [Danaher’s market position and performance based on GE’s insight and intelligence]. (307)
(457) Moreover, [Danaher’s market position and performance based on GE’s insight and intelligence] in relation to GE needs to be considered in a context of a nascent and significantly growing market, in which no standard solution appears to have emerged yet, as submitted by the Parties.
(458) Indeed, Thermo Fisher (Brammer and Patheon) and Merck each have large CDMO divisions, which – the Parties submit – may be viewed by prospective customers of integrated solutions as competitive alternatives to setting-up in-house manufacturing capabilities. (308) Other CDMOs such as Lonza, Wuxi, Boehringer Ingelheim and FujiFilm could also be perceived as offering competitive alternative solutions.
(459) Regarding other players, the market investigation overall confirmed engineering firms to constitute only second-tier alternatives. (309) In particular, these firms would lack the crucial knowledge of bioprocessing products and processes to be able to provide sufficiently tailored and good recommendations to customers. For this, they would need to revert to the product suppliers (on which they also vertically depend).
(460) The fact that several competitive alternatives may remain post-Transaction is confirmed by the fact that a majority of customers who expressed an opinion on this point does not expect the Transaction to have a negative impact regarding the market for integrated solutions, in particular on price. (310) It should be noted, however, that a number of customers and a majority of competitors who expressed an opinion on this point are concerned with possible price increases. (311) Some respondents also mentioned a negative impact on quality, choice, security of supply and innovation.
(461) Furthermore, the majority of customers in the bioprocessing industry are sophisticated firms, which may be able to adjust their behaviour to resist potential price increases.
(462) On balance, it appears that a sufficient number of competitive alternatives to the merged entity in integrated solutions would remain in place post-Transaction, at least for the majority of customers.
(463) In light of the considerations in paragraphs (440) to (462) as well as all evidence available to it, the Commission concludes that, in the worldwide or EEA market for integrated solutions, the Transaction will not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement with regard to horizontal non-coordinated effects.
4.7.2.2. Non-horizontal overlaps
(464) The Transaction also gives rise to non-horizontal overlaps in the area of integrated solutions since bioprocess equipment is an input for the supply of integrated solutions to customers. Against this background, the Commission assessed whether the Transaction could lead to input or customer foreclosure in relation to integrated solutions.
(465) On the one hand, with respect to customer foreclosure, some competitors and customers who expressed an opinion on this point appear to be concerned with the possibility that the merged entity would no longer purchase products from its competitors to incorporate into its own integrated solutions – thereby foreclosing significant revenues from competitors). (312)
(466) With respect to possible customer foreclosure, the Commission firstly notes that while being a growing market, integrated solutions only constitute a small share of the overall bioprocessing market. Significant single product demand will remain post Transaction, which remains accessible for competing producers of bioprocessing hardware and consumables. In other words, even if competitors could no longer sell components to the merged entity for incorporation into integrated solutions to be sold to customers, they would continue to sell their products directly to customers. These direct sales account for the vast majority of their current sales, and will likely continue to do so in the coming years. It thus seems unlikely that the Parties’ competitors could significantly be foreclosed from bioprocessing markets overall.
(467) Moreover, it appears that the incorporation of components from competing bioprocess suppliers into a given player’s integrated solutions are limited. Such is certainly the case for the Parties, [Parties’ sales strategies].
(468) This indicates that the merged entity would not have the ability to engage in customer foreclosure by not incorporating its competitors components into its own offering of integrated solutions.
(469) On the other hand, with respect to input foreclosure, customers do not appear to identify it as a concrete concern. (313)
(470) Indeed, the fact that there is only limited use of components from competing bioprocess suppliers into a given operator’s integrated solutions, is consistent with a finding of lack of ability to foreclose inputs.
(471) In light of the considerations in paragraphs (464) to (468) as well as all evidence available to it, the Commission concludes that, in the worldwide or EEA market for integrated solutions, the Transaction will not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to non-horizontal non-coordinated effects.
4.7.2.3. Conclusion
(472) In light of the above considerations, the Commission concludes that the Transaction does not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement in the possible worldwide or EEA-wide market for integrated solutions due to either horizontal or non-horizontal non- coordinated effects.
4.8. Coordinated effects
(473) The Notifying Party submits that tacit coordination in any of the bioprocessing product markets would be unlikely because (i) pricing policy is not transparent; (ii) prices are less of a focal point as customers focus more on quality, expertise, and supply chain reliability; and (iii) major suppliers in bioprocess filtration have unbalanced and varying market positions, market shares and business models, limiting their ability to come to an understanding or to discipline others’ behaviour.
(474) The market investigation did not reveal concerns of customers or competitors regarding coordinated effects in any of the bioprocessing markets. (314) The Notifying Party’s arguments are supported by respondents to the market investigation that take a position. A majority of customers that take a position indicate that effective prices are generally not known among market participants, that products are rather differentiated, and that they have not observed coordination in the industry.
(475) The Commission has furthermore not received evidence that would support the finding of coordinated effects of the proposed Transaction.
(476) On this basis, the Commission concludes that the Transaction does not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to coordinated effects.
4.9. Conglomerate effects involving bioprocessing products
4.9.1. Introduction
(477) Conglomerate mergers are mergers between firms that are in a relationship which is neither purely horizontal (as competitors in the same relevant market) nor vertical (as supplier and customer). In practice, the focus is on mergers between companies that are active in closely related markets (for example mergers involving suppliers of complementary products or of products that belong to a range of products that is generally purchased by the same set of customers for the same end use). This Transaction exhibits such a feature as most customers purchase complementary products from the Parties for the same bioprocessing operation. For example, some customers purchase GE chromatography products and Danaher/Pall filters for their bioprocessing operations.
(478) The Commission Guidelines on the assessment of non-horizontal mergers (“Non- Horizontal Merger Guidelines”) (315) recognise that “conglomerate mergers in the majority of circumstances will not lead to any competition problems,” while noting that “in certain specific cases there may be harm to competition” (316) In particular, the Non-Horizontal Merger Guidelines indicate that the “main concern in the context of conglomerate mergers is that of foreclosure”. (317) They also stress that the combination of products in related markets is “a common practice that often ha[s] no anticompetitive consequences” because companies engage in such practices “in order to provide their customers with better products or offerings in cost-effective ways”. (318)
(479) Nevertheless, in certain circumstances, this practice “may lead to a reduction in actual or potential rivals’ ability or incentive to compete. This may reduce the competitive pressure on the merged entity, allowing it to increase prices”. (319) In particular, “[i]n order to be able to foreclose competitors, the new entity must have a significant degree of market power, which does not necessarily amount to dominance, in one of the markets concerned”. (320)
(480) The Non-Horizontal Merger Guidelines clarify the framework of assessment of such practice: “[i]n assessing the likelihood of such a scenario, the Commission examines, first, whether the merged firm would have the ability to foreclose its rivals, second, whether it would have the economic incentive to do so and, third, whether a foreclosure strategy would have a significant detrimental effect on competition, thus causing harm to consumers. In practice, these factors are often examined together”. (321)
(481) In doing so, the Commission typically distinguishes three different types of practices: mixed bundling, pure bundling and tying. “Mixed bundling” usually refers to a situation where a company offers and prices together, as a bundle, several products and, at the same time, offers and prices the same product individually. “Pure bundling” refers to a situation where a company offers and prices several products only together in a bundle, and does not offer them individually. Eventually, “tying” refers to a situation in which customers that purchase one good, the tying good; are also required to purchase another good, the tied good, from the same company, and not from alternative suppliers.
(482) These practices can be implemented through various means. Rebates, when made dependent on the purchase of other goods, may be considered a form of mixed bundling. Tying can take the form of technical tying, by which the tying product will only work with the tied one, or can result from contractual terms, which would specify that a customer purchasing the tying good undertakes only to purchase the tied good from the samizee company.
(483) In the course of its investigation, the Commission received two complaints partly or entirely focused on conglomerate effects. One complainant explained that “[i]n the therapeutic pipeline dimension, [… it] is concerned that because GE’s ÄKTA™ purification chromatography platform dominates the market as a process development tool, the potential combination of Danaher’s technologies with that platform could represent a limitation of customer choice at exactly the point where customers are locking in their product choices for future production. This potential bundling would prevent other suppliers from competing in the pipeline” (original emphasis). (322)
(484) Another complainant expressed concerns that “the merged entity would force it to purchase Danaher’s chromatography filters alongside GE’s Protein A resin. […] GE does not manufacture chromatography filters, and [the complainant] currently has a choice between Danaher and Sartorius as the two viable suppliers of these specific filters. Therefore, such a bundling and tying strategy would foreclose Sartorius and leave with no choice in chromatography filters. [The complainant] is also concerned about such bundling and tying and the consequent foreclosure effect more broadly, for example, for other filters and related consumables”. (323) These concerns arise “from the must-have nature of GE’s Protein A”. (324) This complaint was supported by an economic model commissioned to Oxera entitled “Analysis of the incentives of the merged entity to bundle” (“Oxera report”) that aimed at assessing “the merged entity’s incentives to adopt different bundling strategies, in light of their market shares and margins from different products”. (325)
(485) For the convenience of the description, this decision assesses separately possible anticompetitive effects resulting from bundling practices and from (technical) tying practices. In doing so, the Commission assesses the ability and the incentives of the merged entity to engage in such practices post-Transaction.
4.9.2. Bundling
(486) The Notifying Party argues that the Transaction will not give rise to anticompetitive conglomerate effects. The arguments put forward to support this conclusion are, in summary, the following: (326) (i) none of the Parties currently bundle products to any substantive extent; (ii) the merged entity will not have the ability to foreclose rival suppliers through bundling; (iii) the merged entity will not have the incentives to foreclose rivals through bundling; and (iv) a foreclosure strategy through bundling would not succeed in producing anticompetitive effect.
(487) In order to assess the likelihood of anticompetitive conglomerate effects and test the Notifying Party’s arguments, the Commission conducted an extensive investigation related to the ability and incentive by the merged entity to engage in such practices. It held numerous interviews with customers and competitors, including the two complainants, in particular, in order to verify the claims made by these market participants and the Parties. A market investigation has been carried out by means of written questionnaires pursuant to Article 11(2) of the EUMR. The Commission further obtained non-confidential versions of the above-mentioned complaints and provided the Notifying Party with an opportunity to comment on them.
(488) As a starting point, the Commission considers that with market shares systematically [50-60]% and in some cases, [60-70] or even [70-80]%, GE already holds both at worldwide level and EEA level a [market position] in the various types of chromatography resins, which is at this stage indicative of [GE’s market position] – see paragraph (412). Thus, it has to be assessed whether post-Transaction, the merged entity would have the ability and the incentives to leverage [GE’s market position]. The Commission understands that chromatography is used in downstream bioprocessing, which uses different forms of biofiltration processes. Thus, the Commission considers that these markets can be considered as closely related and are therefore the most likely candidates for bundling practices. Moreover, as mentioned above, one of the complainants voiced the fear that the merged entity would engage in bundling its chromatography resins, in particular protein A, with chromatography filters or other types of filter consumables used in the downstream process.
4.9.2.1. Ability to bundle
(489) In this respect, the Notifying Party firstly submits that none of the Parties currently bundle products to any substantive extent. While each of the Parties offer and sell multiple products to their customers, and therefore would be in a position to bundle some of these products, “the Parties do not engage in contractual bundling, nor do they offer as a matter of policy discounts conditional on the customer buying multiple products”, resulting [Parties’ sales strategy]. (327)
(490) Secondly, despite [GE’s market position], according to the Notifying Party, the merged entity will not have the ability to foreclose rival suppliers through bundling for the following reasons: (328)
(a) “There are no real ‘must have’ products in bioprocessing, making it difficult to leverage any strength from a market to another”;
(b) “Customers typically purchase products for each bioprocessing step separately, making it difficult to offer bundled sales”;
(c) “Bioprocessing procurement often involves independent decision makers across each step, which reduces the effectiveness of any bundling strategy”;
(d) “Customers often evaluate several competing solutions, resulting in intense competition in each bioprocessing step [and d]ecision-makers typically have strong preferences for sourcing the product best suited to their technical requirements, and are unlikely to change their decision in order to obtain a discount on another product”; and
(e) “Customers often make their selection decisions at different points in time, which [...] makes it difficult to bundle products”.
(491) As regards the lack of “must have”, the Notifying Party explains that, “[o]n an ex- ante basis, i.e. before the process for producing a biologic is specified, there are no ‘must have’ products, as – for each product – customers have multiple choices from credible vendors, typically including, in addition to the parties, ThermoFisher, Merck (MilliporeSigma), Sartorius and Repligen. Moreover, for specific areas there also exist more narrowly-focused venders […]”. (329) GE thus “considers that there are credible ex-ante alternatives for its products”, (330) even for the supply of Protein A resins where GE has high market shares and Danaher “considers that there is no product in Pass’s portfolio that is ex-ante a ‘must have’ product”. (331)
(492) The market feedback is inconclusive as regards the presence of must-have products, from either the Parties or their competitors. While a large number of customers who expressed an opinion on this point have listed several products as must-have, it appears that these have been interpreted as not interchangeable due to the lock-in effect that occurs for a large number of products once it has been selected for usage in the bioprocessing production process or once this process is set in place, irrespective of the market power of the supplier of that product and thereto applicable to any supplier of these products, as exemplified by statements such as: “[b]ecause the company is single source and it is difficult to switch supplier” or “[a]ll components have been qualified and are included in registered, commercial processes”. Other customers provide clearer statements on whether they consider the Parties’ products as must have: “there are no alternatives in term of performance”; or whether they do not: “there are other companies offering similar products”; “are considered must haves but not necessarily from the parties”; “[a]lthough it takes time, the products are interchangeable”; “[n]one are truly unique and irreplaceable”; “[t]here are no ‘must have’ products in our view”. (332)
(493) As regards customers’ purchase patterns and procurement processes, the Notifying Party submits that customers typically purchase products for each bioprocessing step separately. In this regard, the Notifying Party submits customers’ business cases collected from the Parties showing that in […]% of cases, customers purchased separately in each step. The Commission notes however that it appears that […] of medium-sized customers in fact did not purchase all steps separately, although it generally also recognises that, given that the sample size is small (for customers overall and then naturally even more when segmenting), it is difficult to infer general conclusions from these business cases. (333)
(494) As for bioprocessing filtration and chromatography, which are Danaher and GE’s respectively strongest areas, the Notifying Party submits that such products are not often purchased together. [GE’s sales]. In addition, both areas mostly are represented [GE’s sales] (accounting for […]% and […]% by count respectively). (334)
(495) A majority of customers who expressed an opinion on this point confirmed that most purchases (or requests for quotations) concerned single products or products for a specific step in the bioprocessing production chain (for instance for chromatography hardware and consumables). (335) This seems to validate the Notifying Party’s claim in this respect.
(496) The Notifying Party also argues that, typically, different actors are responsible for purchasing different products used in the bioprocessing production process. Large customers would have different technical teams for upstream and downstream processes, and may further differentiate across technologies.
(497) The market investigation, based on customer feedback, confirms that mostly different actors are responsible for the upstream and downstream processes, although mostly the same people are responsible within the upstream or downstream parts, and overall, in a majority of cases, there are persons/teams responsible for purchasing decisions for more than one step in the bioprocessing workflow. (336) For bioprocessing filtration and chromatography products in specific, a large majority of customers who expressed an opinion on this point indicate that purchasing decisions are made by the same people/teams. (337)
(498) As regards customers’ evaluation and preferences, the Notifying Party stipulates that decision makers typically have strong preferences for sourcing the product that is best suited to their technical requirements and that price is not the primary factor in purchasing decisions. Customers evaluate a number of options. The financial impact of even a limited loss in productivity from using sub-optimal solutions would substantially outweigh any discount offered by suppliers as this could result in significant delays in the product coming to market, or in productivity losses. [Danaher’s sales strategy and customer preferences] (338).
(499) The market investigation generally confirms this, as both customers and competitors who expressed an opinion on this point consider that product quality (including technical fit) and supply chain reliability are the most important purchasing parameters. The product price is clearly less important than those two parameters. (339)
(500) The importance of finding the best technical fit for a bioprocessing product is generally confirmed by customers and competitors who expressed an opinion on this point. Choosing a product with possibly lower productivity would unlikely be compensated by other gains (such as lower purchase price or discounts): “[q]uality and production yield typically outweigh any lower purchasing price offered”; “it is never compensated by other gains”; “[l]ower productivity has a much higher impact than reduced purchase price”; “[d]ifficult to assess. As standard, a loss in yield, even of less than 1% can impact costs more than a possible reduction in purchased material pricing. Time to market may not be a benefit if the product cost is higher due to lower yields”. This may however also depend on the particular case: “[t]rade offs are always evaluated. We judge each opportunity based on multiple factors than make the most business sense for a given situation”. (340)
(501) Aiming to purchase the “best in class” and maintaining flexibility are considered as the as drivers for the “mix-and-match” approach that today characterises the industry to some extent: “we are seeking the best solution for each element of the process”; “[t]his approach in based on the need of fitting the hardware in the manufacturing suite with the highest efficiency. Said efficiency depends on both economical and technical needs”. (341) Indeed, customers and competitors who expressed an opinion on this point confirm that it is generally possible to combine hardware components from different suppliers, as well as hardware and consumables from different suppliers. (342)
(502) As regards the timing of purchasing decisions, roughly half of customers who expressed an opinion on this point indicate that decisions with regards to chromatography and bioprocessing filtration are made at a similar point in time. (343) This does not support the picture put forth by the Notifying Party, who claims that these decisions are typically been made at different points in time. The majority of the competitors who expressed an opinion on this point however support the Notifying Party’s view that the respective purchasing decisions are made at different points in time. (344) The explanations provided vary significantly. That being said, from the market feedback, it appears that for chromatography, hardware is mostly chosen at the clinical or scale-up manufacturing stage, while consumables are mostly chosen at the drug discovery and pre-clinical stage. For DFF filtration, it seems that systems are typically chosen at the clinical or scale-up manufacturing stage, but customers and competitors do not agree on DFF consumables where customers say that these are also chosen at the clinical or scale-up manufacturing stage while competitors mostly say that these are chosen at the drug discovery and pre-clinical stage. (345)
(503) In light of the results of the market investigation, the Commission considers that the arguments of the Notifying Party appear pertinent as regards (i) the separate purchasing of different bioprocessing products; and (ii) the importance of quality and the “best technical fit” for purchasing decisions. The picture is however somewhat more mixed as regards the claims that (i) there are no real ‘must have’ products in bioprocessing; (ii) the identity of decision makers for purchasing decisions concerning the downstream processing comprising chromatography and filtration is different; and that (iii) those decisions are being taken at different points in time.
(504) In any event, a large majority of customers who expressed an opinion on this point indicates that the Parties or their competitors did not attempt to leverage must-have products to enhance sales of other products or to enforce less favourable contractual terms. (346) This clearly supports the Notifying Party’s claim in this respect.
(505) Moreover, the Commission notes that overall, a majority of customers who expressed an opinion on this point does not consider that it would be a successful and profitable strategy for the merged entity to leverage GE’s market position in chromatography into other areas by conditioning the sale of chromatography products on the sale of other products (pure bundling). For example, a customer clarified that “[p]roducts even though they may be similar may not be identical to what customers already use or need and so technically this strategy would cause major issues and negative feedback to GE so it’s unlikely they would pursue it” Moreover, another customer indicated that: “[o]ur experience to date would suggest that the market would not support this strategy”. Another customer also emphasised that: “[w]e do not like to be locked into products because of unforeseen technical issues and/or price destabilization. So if there was some kind of tie between the current supply of GE’s chrom products to PALL’s product, this would be far too risky”. (347)
(506) Moreover, a majority of customers who expressed an opinion on this point would not accept the merged entity forcing them to buy all of the merged entity’s products for a certain production steps or the entire bioprocessing workflow, and state that, if faced with such situation, they would switch supplier. This seems to be driven by customers’ needs to be able to pick individual components from different suppliers that work optimally for their production process, as exemplified by the following statements:
(a) “I would not be pushed into using products for new processes which are not the most suitable for the process. As now we would test different skids and materials and choose the best ones for our process and product regardless of the supplier. If a company tried to force us to use only their products then we would avoid working with them unless they agreed that we can combine products from mutiple suppliers”;
(b) “We only buy the products we are interested in because we want to use the most suitable product for each process. In new projects we would not accept requirements to force us to buy the full portfolio instead of just cherry picking the product we are interested in”;
(c) “We seek to source and implement technologies that offer best in class performance for our processes. We do not want to be tied to a single set of products from a single supplier that may not be the best performance for our process”;
(d) “For new processes there isn’t a typical constraint so we can choose alternative suppliers”;
(e) “We would resist any attempt to be influenced or directed to a single vendor option. However, the technical realities may be that in some cases we will have no option. In such instances we are likely to develop alternates over time to ensure we address any such vulnerabilities”;
(f) “We will rely on these suppliers and products for many years to come and we do not need to be forced into use of it’s products (or those of the merged entity). The supplier does not tell us what we can and cannot use nor do they have a way to enforce this. We would likely go to another supplier very quickly to avoid predatory behavior” (348) (emphasis added).
(507) As regards mixed bundling, the Commission notes that a majority of competitors who expressed an opinion on this point takes the view that the merged entity could successfully leverage its position in chromatography into other areas by granting specific rebates. While also a majority of customers who expressed an opinion on this point considers that the merged entity could leverage its strength in chromatography by offering discounts on combined purchases of chromatography and filtration products, customers nevertheless indicate that the acceptance of this practice would be conditional on the technical fit of the products. This feedback is in line with the high importance of quality and best technical fit for purchasing decisions in the bioprocessing sector. Two major customers underline though that the selection of products does not only depend on price. (349). (350)
(508) In light of the foregoing, and considering all elements in the file, the Commission considers that the merged entity will not have the ability to leverage its market position in chromatography resins by pure or mixed bundling practices.
4.9.2.2. Incentives to bundle
(509) According to the Notifying Party, the merged entity will not have the incentives to foreclose rivals through bundling. Indeed, according to the Notifying Party, “[t]he proportion of customers who would take the bundle [for example of Protein A resin and DFF consumables] is likely going to be limited […] given that many customers will have a strong preference for a ‘best of breed’ product which best suits their specific technical processing needs […]”. (351) For these reasons, [GE’s price structure and sales strategy] (352) and [Danaher’s price structure]. (353) The Notifying Party concludes that [GE’s price structure and sales strategy]. (354)
(510) This is generally confirmed in the market investigation. As indicated above in paragraph (500), customers and competitors who expressed an opinion on this point generally confirm the importance of finding the best technical fit for a bioprocessing product, with quality (including best technical fit) and supply chain reliability being considered by customers who expressed an opinion on this point to be more important than price considerations. Furthermore, competitors who expressed an opinion on this point also confirm the relatively high level of margins in chromatography resins and filtration consumables who indicated their margins for these products, as described in paragraph (529) below.
(511) However, a complainant submitted a report by Oxera assessing the incentives of the merged entity to bundle. The report “set[s] out an economic model of the merged entity’s incentives to adopt different bundling strategies, in light of their market shares and margins from different products. [It] focus[es] on the possibility of bundling of Danaher’s chromatography filters [referred to as ion exchange membrane in this decision] with GE’s Protein A (for new products)”. (355)
(512) The report compares the merged entity’s gains between the current situation, in which Protein A and ion exchange membranes are sold separately, and two different scenarii: a pure bundling scenario in which the merged entity would sell exclusively the two products in a bundle; and a specific mixed bundling scenario in which the merged entity would “increase the stand-alone price of Protein A in order to incentivise customers to buy the bundle with Danaher’s chromatography filters [that is, ion exchange membranes]”. (356) In doing so, the report assumes that the merged entity has the ability to implement such strategies.
(513) In the complainant’s specific mixed bundling scenario, by increasing its price of Protein A, the merged entity would face the following trade-off between: (357)
(a) an increase in profits on the supply of Protein A from customers that will maintain their purchase of Protein A from the merged entity and of ion exchange membranes from a competitor;
(b) an increase of profits on the supply of ion exchange membranes, as some customers that chose the merged entity’s Protein A and a competitor’s ion exchange membrane will now buy the ion exchange membrane proposed in the bundle; and
(c) a decrease in profits from customers deciding to select an alternative supplier.
(514) The report estimates the overall gain that could be derived from this specific mixed bundling scenario and concludes that, for level of gross margins of 70-80% for Protein A and 50-70% for ion exchange membranes, and for market shares around 90% for Protein A and 40-60% for ion exchange membranes, the merged entity would always have an incentive to engage into such practice.
(515) The Commission first notes that the specific mixed bundling scenario described by the complainant does not fully reflect the incentives of the merged entity for the pricing of these two products. This scenario assesses only the indirect unilateral effect resulting from the competition between the bundle and one of its component and it ignores the possible complementarity of the bundled products. For complementary goods, a reduction in price of one of the product will increase its demand as well as the one of its complement. As the merged entity would benefit from this increase in demand in the complementary good, it has an incentive to lower the price of such goods. When assessing mixed bundling practices for complementary goods, the economic literature supports the fact that the price of the bundle tends to decrease because of the internalization of their complementarity.
(516) Moreover, the Commission also notes that there are several assumptions and factors that drive these results, in particular: (i) the assumption that the commercial interactions between the merged entity and its customers are limited to these two products, and therefore gains and losses from such practices need to be assessed on these two products only; (ii) the assumption that the merged entity can price discriminate customers on the basis of the application they buy the products for; (iii) the assumption that the merged entity would price the bundle at the sum of pre- Transaction prices of the individual products, and therefore that the merged entity would not use the price of the bundle to optimise its strategy; (iv) the proportion of usage of Protein A and ion exchange membranes by customers, which is assumed to be one unit of Protein A for one unit of ion exchange membrane; (v) the level of diversion ratios for ion exchange membranes, which are assumed to be driven by the current level of market shares; and (vi) the level of profit margin earned on the ion exchange membranes.
(517) The Notifying Party commissioned Compass Lexecon to review both the pure bundling and mixed bundling scenario discussed in the Oxera report (“Compass Lexecon report”). (358) For both the pure bundling and the specific mixed bundling scenario, the Notifying Party challenges most of the assumptions listed above.
(518) In particular, the Notifying Party disputes that, as assumed by the complainant, the merged entity would be able to “sell Protein A as a standalone product to a customer for processes which do not use ion exchange membranes, but would refuse to supply standalone Protein A to that same customer for processes which do use ion exchange membranes (instead insisting upon a tie of Protein A and ion exchange membranes)”. (359) Indeed, this would require a drastic change in the contractual framework currently used by the Parties. It would also be “difficult for the merged entity to monitor the process in which specific purchases of Protein A were used, as– absent inspection rights which the customers would be unlikely to grant – the merged entity would need to rely on the customer’s representation”. (360)
(519) In the absence of the ability to price discriminate, a pure bundling strategy would have to be implemented on all the merged entity’s sales of Protein A. In such context, using the model developed by the complainant but correcting the proportion of usage (in value) between Protein A and ion exchange membranes from 1.17 to 1, used by the complainant, to […], based on the relative value of these markets, (361) the Notifying Party shows that there is no incentives to engage in pure bundling practices.
(520) The Commission indeed notes that the market for Protein A is significantly ([value of the market], worldwide) larger than that of ion exchange membranes.
(521) The Notifying Party also argues that “customers have significant buyer power and have the ability to retaliate against an attempt by the merged entity to tie or bundle. This is because customers purchase multiple products from GE and Pall, in each case with many viable alternatives”. (362) In support of this submission, the Notifying Party “estimate[s] the proportion of the Parties’ sales which are at risk of retaliation by the customers” and shows that, “on average, [GE’s customers purchases and quantities sold]. (363) The Notifying Party indicates that this percentage is significantly understated due to data limitations.
(522) On the argument related to buyer power, the Commission notes that a significant share of the Parties’ revenues are made by few “large, highly knowledgeable, and sophisticated companies, often with strong global presence [: …] [Danaher’s customers purchases and quantities sold]. (364) Nevertheless, while these customers enjoy, by their characteristics, some level of buyer power, other customers, for example smaller or less knowledgeable customers, might not, arguably, benefit from similar level of buyer power.
(523) The Commission notes that, pre-Transaction, GE [GE’s market position] in chromatography resins (see section 4.6.3.4) and that GE’s product portfolio is already substantial. In particular, GE is also active in neighbouring markets of chromatography resins. Amongst those, the Notifying Party identifies five markets in which GE’s market shares and margins are similar to those of Danaher in the most likely candidates for bundling: ion exchange membranes and bioprocess filtration. (365) For example, GE supplies ion exchange resins, which can also be used for mAb polishing like ion exchange membranes. According to the Notifying Party, GE has a share of [50-60]-[60-70]% in ion exchange resins generally, (366) with a worldwide market value of USD […] and a standard margin of […]%, (367) while GE controls a […] share in ion exchange resins used for mAb polishing. Against this background, GE (368) and GE’s customers who expressed an opinion on this point (369) state that GE does not currently engage in bundling practices. Therefore, pre-Transaction, GE does lack the ability and/or the incentives to bundle its products in the bioprocessing sector for all its customers.
(524) The Transaction will bring together GE’s product portfolio and Danaher’s product portfolio. As indicated in paragraph (488) above, the most likely candidates for bundling practices are chromatography filters or other types of filter consumables. In these markets, Danaher has similar market positions and margins than GE currently enjoys other closely related markets. As GE lacks the ability and/or incentives to engage into bundling practices on these closealy related markets, it is likely that the merged entity will also lack the ability and/or incentives to engage in bundling GE chromatography resins and chromatography filters and other types of filter consumables.
(525) In light of the foregoing and all information available to it, the Commission considers that the merged entity will not have the incentives to leverage its market position in chromatography resins by pure or mixed bundling practices.
4.9.2.3. Effect on competition
(526) The Notifying Party submits that a foreclosure strategy through bundling would not succeed in producing anticompetitive effects by reference to the following elements: (370)
(a) “existing rivals have counted-strategies available to them”;
(b) “customer’s buyer power would prevent any anticompetitive effect from materialising”; and
(c) “a tying or bundling strategy could not force rival suppliers out of the market”.
(527) In particular, as regards the rival’s counter-strategies, the Notifying Party notes that “Sartorius and Merck Millipore have more complete offerings than the Parties and could therefore offer competing bundles of products”. (371) According to the Notifying Party, Sartorius has announced to the public to provide “complete offerings in filtration, fluid management and fermentation, a 25% complete offering in purification and a 75% complete offering in cell culture media […and the Parties] would thus be in a similar or less complete position compared to Sartorius and Merck”. (372)
(528) The above submissions are consistent with the findings from the Commission’s investigation. In particular, several of the merged entity’s competitors offer a large portfolio of products in the bioprocessing sector. This suggests that they could provide competing bundles to the merged entity, should the merge entity engage into such practice.
(529) The Commission also notes that suppliers in this industry experience high levels of profit margins for chromatography resins and filtration consumables. (373) Such level of margin generally appears to be sufficiently high to leave margin for rebates, in particular should the merged entity engage in bundling practices.
(530) Moreover, the Commission notes that several respondents to the market investigation also indicated that competitors to the merged entity could resort to improving quality and reducing prices as a counterstrategy that would reduce the attractiveness of any bundles by the merged entity. As one customer put it, competitors could offer “products or services that are competitive technically and in price”. Another customer explained that “best ways will be to increase innovation and be less expensive”. (374) Similarly, several competitors considered the focus on higher quality product as a key element for a counter strategy. According to a competitor, competition was possible “[b]y developing and supplying superior performance products”. (375) Another competitor explained: “Small to medium sized companies can compete with better service, support lead times and last but not least better performing products with lower cost of ownership”. (376)
4.9.2.4. Conclusion on bundling
(531) In light of above considerations as well as all evidence available to it, the Commission concludes that the Transaction does not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement with regard to conglomerate effects from pure and mixed bundling.
4.9.3. Technical tying
(532) When a supplier engages in tying practices, customers who purchase a (tying) good are also required to purchase another good (the tied good). Typically, the tying good would be a product in which the seller has a strong market position which it tries to leverage to market of the tied good.
(533) For the Notifying Party, the merged entity will not have the incentive to foreclose rival suppliers through tying. (377) Its arguments are, overall, similar to those it makes in respect to bundling. The Notifying Party also notes that “the large majority of conventional bioprocessing products are interoperable, and the scope for any potential technical tying is very limited. In particular, the only conventional products that are not agnostic regarding the supplier of complements are, typically, consumables bags for bioreactors and mixers, and flow kits for single use chromatography skids and filtration systems”. (378)
(534) The Commission received a complaint related to tying practices in relation to Unicorn, GE’s proprietary operating software which is common to GE’s Äkta product offering. (379)
(535) The Commission notes that the areas where the Parties are active are characterised by a large degree of interoperability, both in between product areas as well as between hardware and consumables. This seems to be driven by the “mix-and- match” approach by customers who aim to purchase the “best in class” products that accommodate their needs for individual steps of the bioprocessing workflow (as described in paragraph (501)). On the basis of this and absence of other evidence that would suggest the contrary, the Commission does not find general concerns for technical tying. As regards merger specificity, the Commission notes that the proposed Transaction brings together GE’s strength in chromatography with Danaher’s portfolio of bioprocessing filtration. Given also the specific complaint in this regard, the Commission has further assessed this area. This section will focus on the assessment of the possible technical tie of lab-scale chromatography and filtration systems.
(536) The usage of chromatography resins and columns require customers to program a chromatography method. [GE’s products and know-how]. For process scales, while the consumables may be different, the technologies are identical, and in turn can also influence the choice of large scale systems.
(537) According to the complainant, GE has a very strong position in lab-scale chromatography systems and it has leveraged this strength in lab-scale systems. This resulted, according to the complainant, in GE obtaining very high market shares in chromatography consumables and hardware. Post-Transaction, the complainant argues that the merged entity would extend these practices to Danaher’s filtration offering in the lab-scale instrumentation. As a result, the merged entity’s products would become the default choices for customers in early stages of the development of new production processes. Moreover, as customers generally experience difficulties to switch products in a production process, GE’s strength in lab-scale systems would be transferred to process-scale production processes.
(538) The complainant argues that such a strategy could be implemented by designing Danaher’s filtration products to work with GEs Äkta platform, whereby the Äkta instrument would similarly have the pre-loaded recipes for these products. This would require the following modifications: (i) modifying Danaher’s filtration products to match the input connections to the Äkta device; (ii) modifying GE’s pumps and plumbing on the Äkta device to work with filtration; and (iii) creating pre-loaded Unicorn methods to operate filtration experiments. The complainant estimates that it would take roughly 3 years for this mechanism to materialise as a technical offering and 7-10 years for the financial effects to be realised.
(539) The Commission understands that the proposed theory of harm would require, inter alia, (i) a meaningful difference for customers to use GE’s or the merged party’s resins compared to competitor resins on the Unicorn/Äkta platform (pre- or post- Transaction); that (ii) this pre-loading would similarly be required for filtration; (iii) the ability and incentive for the merged entity to develop a single system for chromatography and filtration; that (iv) the parties would no longer sell a stand-alone device; and that (v) competitors would not have countervailing strategies.
(540) In relation to this complaint, the Notifying Party has brought to the Commission’s attention the following arguments:
(a) It would be technically challenging to develop a process development system that combines chromatography and filtration.
(b) This would in any event not be useful or efficient as technical challenges to combine functionalities would result in too many compromises for usage in chromatography.
(c) The Parties would not have an incentive to only offer such a combined system. There would not be substantial customer demand for such a system, downtime on chromatography systems are small, and as prices of chromatography systems are a multiple of those of filtration system, it would be costly to use such system only for filtration. The Parties would therefore lose a substantial proportion of their current sales (if not most).
(d) The current practice in terms of pre-loading of settings does not confer any significant advantage.
(e) The Parties would not be able or incentivised to close a combined system. Customers require lab-scale process development to try different types of consumables to optimise the production process, and would not purchase a system that does not allow testing consumables from different suppliers.
(f) Customers could purchase stand-alone filtration systems from the Parties’ competitors in order to test filtration consumables from other suppliers. Furthermore, there is a large installed base of open systems in use today, which customers could continue to use. These have service lifetimes often in the range of 10-15 years.
(541) Most customers who expressed an opinion on this point in the market investigation indicate that there are no meaningful obstacles in using competitor resins on GE’s Äkta chromatography skids with Unicorn software. They for instance indicate that “[n]on-GE and GE resins can be used equally well on GE systems using Unicorn software”, that “[t]he parameters can be easily loaded into the software for non-GE resins - this is not an issue”, and that, overall, “[t]his makes almost no difference. We routinely use the AKTA and rarely use it in association with GE resins, yet it works very well”. (380) Most competitors who expressed an opinion on this point agree. (381)
(542) This feedback from market participants is sufficiently concrete to justify doubts about the complainant’s assertion that GE’s alleged strength in lab-scale instruments has successfully translated into a corresponding strength in consumables and subsequently in process-scale products. Nonetheless, the market investigation results do not, in themselves, exclude that the merged entity could engage post-Transaction in limiting interoperability. Therefore, the Commission investigated whether the merged entity would have the ability and incentive to pursue the strategy set out by the complainant.
(543) The Commission finds that, while customers and competitors who expressed an opinion in the market investigation on this point indicate that parameters also have to be loaded for filtration, this seems to be simpler than for chromatography. (382) Furthermore, it appears that most of these parameters would not be specific to the particular filter in use, but rather to the requirements of the customer’s production process. As put by one customer, “some parameters for the use of bioprocess filtration consumables do have to be loaded into the software of a filtration skid. For example, a filter will often have a maximum differential pressure. If this maximum is exceeded, the filter will shut off. This maximum will be loaded into the software both as a safety feature and to protect the product. Otherwise, nothing further needs to be loaded”. Other customers point to the same view: “parameters tend to be process- specific for filtration operations (not consumable specific) and these can be readily used with any software packages”; and “[t]he system software is programmed for the specific customer’s process parameters and during the production process, these parameters (pressure, flow, temperature, weight, volume, conductivity, etc.) are controlled. No specific filters data are loaded in the software. So, if filters fit the installation and if these filters is suitable to the process parameters there is no difference from which producer filters are used”. (383)
(544) In light of this, the Commission considers that limiting interoperability could be more difficult for filtration consumables than for chromatography resins. Considering that GE does not appear to currently engage in limiting interoperability of systems for resins, in which it already has a strong position today, the Commission has doubts as to the extent to which the addition of Danaher’s position in filtration consumables (which is furthermore less pronounced than GE’s in resins) would alter the merged entity’s incentives to limit interoperability post-Transaction for filtration (or chromatography, for that matter).
(545) Nevertheless, the Commission notes that a majority of customers and competitors who expressed an opinion on this point consider that the merged entity would technically and commercial be able to create a closed hardware system in the next five years with chromatography and bioprocessing filtration capabilities that would be compatible only with own consumables. (384) (385) However, the commercial prospects of such a combined and closed system appear to be rather limited.
(546) First, the complainant acknowledges that larger customers typically employ different personnel responsible for chromatography and filtration, and that production steps are developed sequentially (first a bioreactor, then products for clarification, chromatography, etc.). The characteristics of the production process are therefore not consistent with the use of such a device by these customers.
(547) Second, while some customers indicate that they would be commercially interested and/or see added value in a system that combines chromatography and filtration in a single machine, most customers would not be interested, or only be interested for specific applications. One customer “[c]an’t imagine that this would be relevant or practical”. Other customers do not see the benefits for their current activities (without, however, completely excluding its utility for some applications): “[w]e do not have a specification at this time where this would be considered an advantage”, “[t]his kind of set-up is not relevant for our research purposes” and “[w]e would loose flexibility in the R&D processes” or “[w]e are not interested in combined chromatography and filtration systems in a single machine, at least not in a lab- scale level where optimisation processes take place and each of the mentioned systems need to be independent and versatile”. Another customer speculates about potential benefits: “[t]his may be interesting for continuous processing but in general we would prefer to use separate skids since this gives more flexibility with respect to the products and suppliers”. (386)
(548) The Commission therefore considers that to the extent that there would be commercial interest in such an integrated device, demand would not be sufficient for the merged entity to profitably limit its offering to such a device by discontinuing the offering of stand-alone devices at the same time. Furthermore, to the extent that the merged entity would only offer such a device, the Commission considers it likely that customers would use stand-alone devices of competitors.
(549) Customers and competitors who expressed an opinion on this point also confirm that chromatography skids are typically more expensive than bioprocessing filtration skids, with “much more higher [prices]” according to a customer. (387) This is in line with the Notifying Party’s submission and therefore also confirms that it would be unlikely for customers to use such system only for filtration.
(550) As regards the feasibility of a combined chromatography and filtration skid, customers generally consider that such a device is technically feasible, although some express reservations that are in line with the Notifying Party’s submission: “[t]here would need to be so many different options to allow the use of both chromatography and filtration at different scales and with multiple variables that it would likely be too cumbersome to use”. Another customer state that this is “[t]echnically feasible but expensive and lengthily across all modalities […]”. Several competitors also consider it feasible and indicate that the development phase could take between one and five years. Again, some express reservations. One competitor for instance submitted that “[i]t could work for filtration and membrane absorber based chromatography but I don’t see it working for the conventional chrome. Also the price and maintenance on such a machine could be prohibitive. Millipore have tried this with SMART Flexready”. (388)
(551) On the basis of all the available evidence and in light of the above, the Commission considers that essential conditions for the theory of harm proposed by the complainant are unlikely to materialise.
4.9.4. Conclusion on conglomerate effects
(552) On balance and in light of above considerations, the Commission concludes that the Transaction does not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to conglomerate effects.
5. MARKET DEFINITION AND COMPETITIVE ASSESSMENT CONCERNING THE PARTIES’
ACTIVITIES IN OTHER LIFE SCIENCES RESEARCH SEGMENTS
(553) This section includes the Commission’s market definition and competitive assessment concerning life science areas outside of bioprocessing where GE and a Danaher operating company are active. These life science areas include molecular characterisation; microscopy; high-content screening; and laboratory filtration. In light of the horizontally overlapping activities of the Parties in these areas, the Commission’s competitive assessment will focus on horizontal non-coordinated effects. Given that the products the Parties and their competitors offer are differentiated and the suppliers in the relevant markets have differentiated profiles, horizontal coordinated effects are only briefly assessed at the end of this section.
5.1. Molecular characterisation
(554) Molecular characterisation focuses on the study of characteristics of and interactions between molecules (most often, proteins). (389) Molecular characterisation is used in various industry sectors, including biotechnology (for the development of biological therapeutic drugs); pharmaceuticals (to measure the interaction between large and small molecules); and academia (for various biological research purposes). (390)
(555) Depending on whether the sample studied requires tagging, molecular characterisation techniques can be classified as follows:
(a) Label-based detection technologies, which use labels or tags (foreign substances such as dyes) attached to the molecule of interest, to detect molecular presence and measure the relevant molecular properties and
(b) Label-free (“LF”) detection techniques, which perform these measurements without the aid of labels, based on measurement of the optical, calorimetric, electrical, acoustic, or other physical reaction of the sample to various stimuli. (391) There are two broad categories of commercialised LF detection systems, (i) surface plasmon resonance (“SPR”) systems (392); and (ii) non-SPR systems which are based on various different technologies. The most widespread non-SPR LF technology is bio-layer interferometry (“BLI”). (393)
(556) LF detection systems are composed of a main instrument and integrated software. They also use consumables (for example, biosensors, various reagents and buffers, and microtiter plates).
5.1.1. Market definition
5.1.1.1. Product market definition
(A) Notifying Party’s arguments
(557) The Notifying Party submits that there is an overall product market encompassing all molecular characterisation systems, irrespective of their technology. The Notifying Party justifies this by stating that customers use various technologies for common applications and that customer preferences are not driven by technology but the needs of a specific application.
(558) Having said this, the Notifying Party also provides detailed information regarding a plausible product market including only LF detection systems. However, the Notifying Party suggests that a product market definition including all LF detection systems would be inconsistent with the fact that there are highly differentiated LF technologies, such as SPR and BLI. For this reason, the Notifying Party takes the view that SPR-based LF detection systems and BLI-based LF detection systems constitute two separate (but neighbouring) markets. (394)
(559) Finally, the Notifying Party takes the view that it is not necessary to define separate product markets for the LF detection initial system and the LF detection system aftermarkets (including components, consumables, services, and software). Third- party reports do not break down this segment into initial systems and aftermarket elements and customers do not typically source aftermarket elements separately, but rather usually buy them from the manufacturer/supplier of the initial system. (395)
(B) Commission’s precedents
(560) The Commission has not previously defined the product scope of the relevant markets concerning molecular characterisation.
(C) Commission’s assessment
(561) The Commission assessed product market definition in relation to molecular characterisation, taking into account the functionality of different devices; their use applications; and the technologies they deploy.
(562) The vast majority of customers and the majority of competitors who expressed an opinion in this respect took the view that LF detection systems constitute a distinct relevant market within the field of molecular characterisation. (396) Customers and competitors indicated that LF detection systems are typically used to measure the kinetics (that is the speed of interactions) between molecules. (397) The majority of customers and competitors who expressed an opinion on this point also noted that customers are able to use label-based techniques only for some – but not most -- of the applications for which they use LF techniques. (398) One customer explained: “[LF] detection is used to evaluate binding affinity and binding kinetics of antibodies and antibody drug conjugates. We do not recognize other label-based technologies that provide equivalent data”. (399) As competitors put it, “[i]t is not possible to measure kinetics with ELISA [a label-based technique]” (400) and “for kinetics you need label free”. (401)
(563) Within LF detection systems, neither customers nor competitors supported separate relevant product markets for SPR-based and BLI-based LF detection systems. For example, one customer noted that “BLI [detection systems of Danaher] can compete with [GE’s SPR system]” and another added: “[GE’s SPR detection system and Danaher’s BLI detection system] offer among the best throughput performance in the market”. (402) One competitor stated “SPR and BLI [...] devices are both strongly use[d] in the market of antibodies and thus have a strong overlap”. (403)
(564) In the light of the above and considering all information in the file, the Commission concludes that there is no single overall product market including all molecular characterisation techniques. Instead, the relevant product market comprises only LF detection systems. (404) This differentiated market comprises both SPR-based and BLI- based LF detection systems. The Commission will take into account the differences between these systems when to determining the closeness of the Parties’ offerings, in the framework of the competitive assessment.
(565) Finally, the relevant market also comprises after-market elements which are typically sourced by the instrument supplier. This is also consistent with the results from the market investigation, where the majority of customers and competitors who expressed an opinion on this point stated that “[t]he distinction between instruments and aftermarket does not seem a relevant consideration for the purposes of determining the relevant product market” in molecular characterisation and that “[a]ftermarket products and services are generally provided by suppliers only for their own instruments”3. (405) For this reason, the Commission does not distinguish separate markets for instruments and aftermarket elements in LF detection systems. (406)
5.1.1.2. Geographic market definition
(A) Notifying Party’s arguments
(566) The Parties consider that the relevant market for LF detection systems is worldwide and in any event, not narrower than EEA-wide.
(B) Commission’s precedents
(567) The Commission has not previously defined the geographic scope of the markets concerning molecular characterisation or LF detection systems.
(C) Commission’s assessment
(568) There are indications of the existence of a worldwide relevant market, as the majority of customers who expressed an opinion in the market investigation on this point stated that they procured LF detection systems at worldwide level. (407) Also, after-sale services are provided at worldwide level, prices are comparable and the same suppliers are active worldwide. (408) These findings are consistent with the responses from the vast majority of competitors who expressed an opinion on this point. (409) However, the views of competitors were split as to whether price levels of molecular characterisation products are homogeneous or not across different global regions (not narrower than the EEA). (410)
(569) For the purposes of this decision, the Commission concludes that it can be left open whether the market for LF detection systems is global or EEA-wide in scope. The Transaction gives rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement under both these plausible geographic market definitions for the reasons explained in paragraphs (570) to (595) below.
5.1.2. Competitive Assessment
(570) In LF detection systems, Danaher is active through Fortébio, a division of its subsidiary Molecular Devices (“MolDev”) and offers an SPR-based product line (Pioneer) (411) and BLI-based product lines, Octet (412) and BLItz. SPR-based products represented […]% of Danaher’s 2018 turnover from LF detection instrument sales worldwide. (413) GE offers its SPR-based product line, Biacore. (414) GE does not offer BLI-based LF detection systems.
(571) In the market for LF detection systems, the combined entity would be the far-and- away market leader both worldwide and in the EEA. As shown in Table 19 below, the combined market share of the Parties in 2018 was [60-70]% worldwide and [50-60]% in the EEA. Danaher contributed a […] under both geographic market delineations, namely [20-30]% in the worldwide market for LF detection systems and [10-20]% in the EEA.
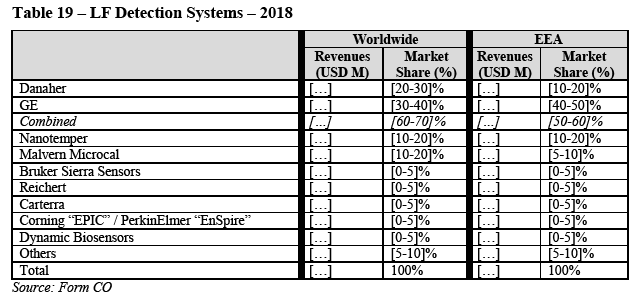
(572) As Table 19 shows, the Transaction will combine the top two players in the market for LF detection systems. As the Parties themselves acknowledge, “GE is one of the main vendors” in this area “due in large part to its first mover advantage and the large installed base for its systems”. (415) In 2018, Danaher was the strongest competitor to GE in this market both at worldwide and at EEA level. Post- Transaction, the remaining competitors will include only two players with shares [10-20]% (Nanotemper and Malvern Microcal) and a long tail of small players with shares that do not exceed [0-5]%.
5.1.2.1. Notifying Party’s Arguments
(573) The Parties argue that these combined shares overstate the extent to which they constrain one another’s competitive behaviour in LF detection systems.
(A) The Parties’ technologies are very different and they do not compete for the same use cases
(574) The Notifying Party claims that the Parties do not compete closely in LF detection systems. It takes the view that they are not close competitors because Danaher focuses on BLI-based LF detection systems while GE only offers SPR-based systems. According to the Notifying Party, SPR and BLI have different strengths and weaknesses. While BLI would deliver higher throughput and lower sensitivity, SPR would deliver higher sensitivity and lower throughput.
(575) The Notifying Party adds that because of the technical differences between SPR and BLI, the main applications for which GE and Danaher offer their products are distinct. The Parties distinguished between different types of customers that use LF detection systems, namely pharma companies; biotechnology companies; and academia. Within the biotechnology segment, the Parties also considered different use cases, including lead generation and optimisation (“LGO”); process development (“PD”); cell line development (“CLD”); and quality control (“QC”).
(576) According to the Notifying Party, Danaher’s BLI product line Octet has [market positioning]. On the other hand, GE’s SPR products appear to be [market positioning].
(B) The Parties will continue facing strong competition constraints post-Transaction
(577) The Notifying Party acknowledges that there are some use cases for which both Parties’ instruments compete, [market positioning]. For these use cases, the Notifying Party argues that post-Transaction, the combined entity will continue to face significant competitive constraints from rivals.
(578) First, post-Transaction, the Notifying Party submits that Danaher and GE will face strong competition from other LF detection system suppliers (including other suppliers of SPR- and BLI-based instruments), as well as from label-based and other suppliers, such as Bruker/Sierra, Reichert, Carterra, Nicoya, Malvern Microcal, Nanotemper, and Corning.
(579) Second, according to the Notifying Party, barriers to entry in the market for LF detection systems are low, as in the past 10 years companies such as Sierra Sensors, Reichert, Creoptix, ProbeLife, and Biametrics have introduced new LF detection systems.
5.1.2.2. Commission’s Assessment
(580) The market investigation did not confirm the Parties’ arguments regarding the competitive interaction between Danaher and GE in LF detection systems.
(A) The Parties compete closely in most use cases
(581) The Commission found that the Parties’ products compete closely in LF detection systems because they are used for the same types of measurements. The Parties products also compete closely in terms of throughput and sensitivity/precision and they are often purchased for the same use cases. Finally, the Parties’ recent product launches and […] appear to be moving their respective product lines closer towards the other’s.
(582) First, the results of the market investigation suggested that, contrary to the Parties’ claims, Danaher and GE are close competitors in (worldwide or EEA-wide) market for LF detection systems. During the market investigation, irrespective of the differences between SPR and BLI technologies, the vast majority of customers who expressed an opinion on this point identified Danaher as the closest competitor to GE in LF detection systems for most types of measurements, including binding affinity; binding specificity; and kinetics. (416) The vast majority of competitors who expressed an opinion on this point identified Danaher as the closest competitor to GE in LF detection systems for binding affinity; binding specificity; kinetics; and concentration. (417)
(583) Second, the majority of customers and competitors who expressed an opinion in the market investigation on this point took the view that Danaher’s (SPR- and BLI-based) and GE’s SPR-based instruments compete strongly or at least moderately as regards throughput. (418) As one customer put it, “Danaher and GE seem to be directly competing against each other in terms of throughput”. (419) Another customer added: “Biacore 8K+ (SPR) and Danaher Octet HTX (BLI) offer among the best throughput performance in the market”. (420)
(584) The majority of customers and competitors who expressed an opinion in the market investigation on this point also submitted that Danaher and GE compete strongly or at least moderately as regards sensitivity/precision. (421) While respondents acknowledged the technical differences between SPR- and BLI-based LF detection systems, they still found that the one can compete with the other. According to one competitor, “GE is generally consider[ed] having higher precision, but in many applications, it is not necessary and thus competition is strong”. (422)
(585) Third, in the market investigation, several customers and competitors also indicated that both SPR- and BLI-based LF detection systems are purchased for most uses cases, including use cases for which the Parties claimed that SPR- or BLI-based LF detection systems have difficulties to compete, such as drug discovery (for pharma customers); CLD (for biotech customers); and PD (for biotech customers). (423)
(586) Fourth, it appears that the Parties’ recent product launches and […] may be moving their respective product lines closer towards the other’s. In 2016Q4, GE launched Biacore 8K series, an SPR-based LF detection system which however has established itself as a high-throughput product. (424) High-throughput is a typical characteristic of BLI-based LF detection systems. […]. (425) On the other hand, in 2015, Danaher launched the Oktet K2 System, which is based on BLI technology but was meant [Danaher’s business strategy]. (426) In 2012, Danaher also purchased SensIQ which offers SPR LF detection instruments. […]. (427)
(587) In light of the results of the market investigation as well as all evidence available to it, the Commission concludes that, contrary to the Parties’ claims, the products of Danaher and GE in LF detection systems compete closely in most use cases.
(B) Competitive constraints to the combined entity post-Transaction will be limited
(588) The Commission found that post-Transaction, the combined entity will have significant market power in LF detection systems worldwide and in the EEA and that it will face only limited constraints.
(589) First, while several customers acknowledged that credible alternatives might remain in the market for LF detection systems, (428) the market investigation did not provide substantiated evidence that the rivals mentioned by the Notifying Party in this market could compete strongly with the combined entity in terms of price. (429) One customer stated: “[w]e think the Biacore / ForteBio combination is likely to continue to be dominant in market share” (430) and also “[w]e can expect [the combined entity] to rationalize its product range and perhaps leverage its market position to raise prices”. (431)
(590) In addition, the majority of informative customers who expressed an opinion on this point are concerned that post-Transaction, their company will not have credible alternatives for all or at least for some use cases. (432) As one customer put it, “[GE’s] Biacore [and Danaher’s] Fortebioare the industry gold standard [...] [it] is not a matter of existing alternatives or cost [...] [it] is a matter of producing results that are trusted in the community [and] thus have value”. (433)
(591) In the same vein, the majority of competitors who expressed an opinion on this point noted that in the market for LF detection systems, post-Transaction the combined entity will not be constrained by strong rivals. (434) As one competitor put it, “[...] label-free instruments SPR, BLI and similar are commonly purchased on instrument at a time [. ...] The leading SPR instrument (Biacore) has spent the last 25-30 years to build its installed base. [Danaher] has spen[t] many years as well to reach its #2 position in the market. No other competitor is close. Each will need 5+ years to build a profitable installed base while competing against entrenched competitors GE and Danaher. It is likely many of these companies will exit the market due to cost/time involved to profitably compete [...]”. (435) Another competitor summarised the situation as follows: “yes [there will be] alternatives but difficult to compete with the combined level of market [strength]” that the Parties will have post-Transaction. (436)
(592) Second, the market investigation results overall did not confirm the Parties’ claims that recent and new entrants can constrain the combined entity post-Transaction in LF detection systems (worldwide or in the EEA). While the majority of customers who expressed an opinion on this point expect additional companies to enter the market for LF detection systems in the next two to three years, (437) several customers expressed concerns about the success of such entry in the long run. According to one customer, “[t]here has been continuous turn-over of start-ups in this field with new players entering and many leaving it again”. (438) Another customer indicated: “entrance of further players is to be expected but it’s not clear when this will happen and whether they will be able to sustain”. (439) The answers of competitors during the market investigation were also inconclusive. While a small majority of competitors who expressed an opinion on this point expects that additional companies will enter the development and supply of LF detection systems in the next two to three years, one competitor stated “[LF] market is dominated by Fortebio and Biacore, besides these two there is nothing relevant” (440) and regarding recent entrants, one competitor added: “[these] companies are less established players may not have the extent of portfolio or the reach to ensure a full replacement opportunity for the customer, particularly from a single vendor”. (441)
(593) Further confirming that post-Transaction the combined entity will have significant market power in LF detection systems and it will not face important competitive constraints, the majority of customers who expressed an opinion on this point (442) and the vast majority of competitors who expressed an opinion on this point took the view that the Transaction could result in price increases in the market. (443)
(594) In light of the results of the market investigation as well as all evidence available to it, the Commission concludes that, contrary to the Parties’ claims, the competitive constraints to the combined entity post-Transaction will be limited and as a result, there is a risk of price increases in LF detection systems.
5.1.2.3. Conclusion
(595) In light of the above considerations as well as all evidence available to it, the Commission concludes that in the worldwide or EEA-wide market for LF detection systems, the proposed Transaction gives rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to horizontal non-coordinated effects given in particular (i) the significant combined share of the Parties and the meaningful increment contributed by Danaher; (ii) the close competition between the Parties; (iii) the lack of strong competitors or entry prospects; and (iv) the concerns for price increase expressed in the market investigation.
5.2. Microscopy
(596) Microscopes are instruments for making enlarged images of small objects and minute structures. There are several different microscopy techniques, including optical microscopy; electron microscopy; and scanning probe microscopy. (444)
(597) This section will focus on optical microscopy, where the activities of the Parties overlap horizontally. Optical microscopes are instruments that use the spectral range of light to image the object of interest through a system of magnifying lenses. The Parties have no product offerings using other microscopy techniques.
(598) Optical microscopes can be further distinguished based on the illumination technique followed and on the field of application:
(a) A microscope’s illumination technique determines which part of the sample is illuminated and how. Widefield microscopy (where the whole sample is permanently illuminated) can be distinguished from confocal microscopy (where only one single focal spot is illuminated at a time).
(b) A microscope’s field of application is based on the instrument’s achievable resolution. Microscopes that can surpass the so-called “diffraction limit” and achieve resolution below 200-250 nm are called super-resolution microscopes. Ultra-high resolution microscopes are microscopes which, albeit highly developed, do not surpass the diffraction limit of light. Super- resolution microscopes and ultra-high resolution microscopes can be further sub-segmented based on the illumination technique followed and other criteria.
5.2.1. Market definition
5.2.1.1. Product market definition
(A) Notifying Party’s arguments
(599) The Parties consider that not all optical microscopes are part of a single product market. In the Parties’ view, a general market definition at that level would be too wide.
(600) The Parties also do not consider appropriate to define product markets only based on the illumination technique. A possible product market for widefield microscopes would include instruments of very different quality and level of development, from the most advanced to the most basic. It would include, for example, simple microscopes used in schools and highly advanced microscopes used in biomedical research. The same would be the case for a broad possible product market encompassing all confocal microscopes.
(601) In the Parties’ view, super-resolution microscopes constitute one single product market, separate from ultra-high resolution microscopes. Within this market, the Parties reject any further sub-segmentations, for example by resolution level or by illumination technique (that is to say confocal and widefield). The Parties argue that the key characteristic of super-resolution microscopes is their ability to surpass the diffraction limit. According to the Parties, the resolution level and the illumination techniques are not decisive for the customer.
(602) As regards ultra-high resolution microscopes, the Parties distinguished advanced inverted research microscopes as a separate product market. These are highly developed (widefield) microscopes that make it possible to resolve detail in high resolutions from 1000 nm up to 250 nm. They are called “inverted” because the slide with the specimen is above and the objective below (while in “upright” microscopes the slide is below and the objective above). Inverted microscopes are typically used to observe living cells, which are commonly grown in liquid solutions. In contrast, upright microscopes are commonly used with fixed samples.
(B) Commission’s precedents
(603) The Commission has not previously defined the relevant product market for microscopy. In Danaher/Beckman Coulter, the Commission took the view that surface science techniques (that is microscopy techniques) could be segmented into different product groups by reference to the specific analytical technique, but ultimately, the exact product market definition was left open. (445)
(C) Commission’s assessment
(604) The Commission assessed the product market definition in relation to optical microscopy, taking into account the achievable resolution; the illumination technique used by the microscope; and also its structure.
(605) The majority of customers who expressed an opinion on this point took the view that optical microscopes with different levels of achievable resolution and thus different fields of do not compete with each other in terms of price; use applications; technical characteristics; or efficiency. Most customers indicated that an ultra-high resolution microscope and a super-resolution microscope are not substitutable, regardless of the illumination technique used. (446) Several competitors confirmed this. (447) As one competitor put it, “super-resolution microscopes [...] tend to be significantly more expensive, in general require more support infrastructure, [and] safety training requirements due to exposed laser illumination”. (448)
(606) The results of the market investigation are, however, inconclusive as to whether super-resolution microscopes should be further sub-segmented into two separate product markets based on the illumination technique: (i) confocal super-resolution microscopes; and (ii) widefield super-resolution microscopes. The majority of customers who expressed an opinion on this point stated that confocal super- resolution microscopes do not compete with widefield super-resolution microscopes in terms of use applications; technical characteristics; or efficiency. (449) But the views of customers were evenly split as regards substitutability in terms of price. (450) The opinions of competitors on the substitutability between confocal super-resolution microscopes and widefield super-resolution microscopes were equally split. (451) According to one competitor, in some cases “purchasers already know and describe in detail what type of super-resolution microscope they prefer (e.g., based on confocal technique [...] or on widefield technique)” but other customers “might include less details on the requirements of the microscope because they are after versatile tools and they are mainly seeking high uptime”. (452)
(607) In light of their product characteristics and taking into account the results of the market investigation, the Commission concluded that super-resolution microscopes do not belong in the same market as ultra-high resolution microscopes. The Commission also takes the view that for the purposes of this decision, it can be left open whether there is a relevant market including all super-resolution microscopes or there are separate markets for (i) confocal super-resolution microscopes; and (ii) widefield super-resolution microscopes. The Transaction does not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement under any of these plausible product market definitions.
(608) As for ultra-high resolution microscopes, the customers who expressed an opinion in the market investigation on this point confirmed that advanced inverted research microscopes cannot be replaced by an advanced upright microscope in terms of technical characteristics and use applications. According to one customer, “[t]here are applications (eg looking at plates with liquid cultures) that can only be performed in an inverted microscope. I would say an upright can be substituted by an inverted microscope, but an inverted microscope can not always be substituted by an upright microscope. This depends on the ability to turn your sample upside down [...]”. (453) The views of competitors on this question were more varied, but most competitors did confirm that inverted microscopes are not substitutable with upright microscopes in terms of technical characteristics. (454)
(609) In light of the results of the market investigation as well as all evidence available to it, for the purposes of this decision, the Commission considers that there is a distinct relevant product market within ultra-high resolution microscopes including only advanced inverted research microscopes. (455)
5.2.1.2. Geographic market definition
(A) The Notifying Party’s arguments
(610) The Notifying Party submits that the markets for super-resolution microscopes and for advanced inverted research microscopes are worldwide and in any event, not narrower than EEA-wide.
(B) Commission’s precedents
(611) The Commission has not previously defined the geographic scope of the market for advanced inverted research microscopes.
(C) Commission’s assessment
(612) The market investigation confirmed the Notifying Party’s claims regarding geographic market definition.
(613) The majority of customers who expressed an opinion on this point stated that they procure (super-resolution and advanced inverted) microscopes at worldwide level; after-sale services are provided at worldwide level; prices are comparable at worldwide level; and the same suppliers are active at worldwide level. Customers confirmed that this applies to super-resolution microscopes and advanced inverted research microscopes. (456) The vast majority of competitors who expressed an opinion on this point took this view as well. (457) However, several competitors also indicated that their market position differs significantly across different regions (for example in the EEA or outside the EEA).
(614) Therefore, the results of the market investigation were inconclusive as to the precise geographic scope of the plausible markets for super-resolution microscopes and for advanced inverted research microscopes. For the purposes of this decision, the Commission took the view that it can be left open whether the markets for super- resolution microscopes and for advanced inverted research microscopes are global or EEA-wide in scope. The Transaction does not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement under any of these plausible geographic market definitions (namely, worldwide or EEA-wide markets).
5.2.2. Competitive assessment
(615) In super-resolution microscopy, Danaher is active through its subsidiary Leica Microsystems (“Leica”). In this space, Danaher offers a confocal super-resolution microscope, namely Leica TCS SP8 STED. (458) GE Biopharma offers widefield super- resolution microscopes, namely, GE Deltavision OMX SR and OMX Flex, which are both based on the SIM technology. (459)
(616) As explained above, the Transaction does not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement in super-resolution microscopy, under any possible market delineation. If separate markets were defined for each of widefield super-resolution microscopes and confocal super-resolution microscopes, the Transaction would not give rise to a horizontal overlap between the activities of the Parties.
(617) If one single market including all super-resolution microscopes were defined, then the Transaction would give rise to an affected market. However, in that relevant market, no competition concerns arise for the following reasons.
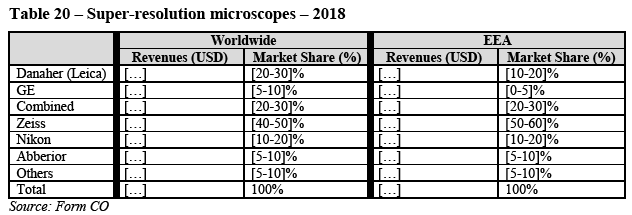
(618) First, based on the Parties’ estimates in Table 20 above, the combined share of the Parties in super-resolution microscopes in 2018 would be limited to [20-30]% worldwide. Post-Transaction, the combined entity would continue facing competition by several large competitors, including Zeiss (the number one player in the market worldwide) and also Nikon, Abberior, and others (including Olympus, Yokogawa, Andor, and Bruker/Vutara). (460)
(619) In the EEA, the combined share of the Parties in super-resolution microscopes in 2018 would be [20-30]% (see Table 20 above). (461) The increment contributed by GE Biopharma would remain […], namely [0-5]%. Post-Transaction, several large rivals will remain in the market including Zeiss (the number one player in the market also in the EEA), Nikon, Abberior, and others (including Olympus, Yokogawa, Andor, and Bruker/Vutara).
(620) Second, Danaher and GE Biopharma are not close competitors in super-resolution microscopes:
(a) The Parties use different illumination principles in their super-resolution microscopes. Danaher’s super-resolution microscopes use confocal STED technology, whereas GE Biopharma uses SIM technology.
(b) The lack of close competition between the Parties is also reflected in the [Danaher’s bidding data]. (462)
(c) [Parties’ internal documents]. (463)
(d) The results of the market investigation also support the conclusion that Danaher and GE Biopharma do not compete closely in the market for super- resolution microscopes. Most customers indicated that Zeiss, Olympus, and Nikon are the closest competitors to Danaher (in terms of price; use applications; technical characteristics; and overall assessment). (464) All the competitors who expressed an opinion in the market investigation on this point identified Zeiss as the closest competitor to Danaher in super-resolution microscopes and the majority of competitors who expressed an opinion on this point identified Nikon as the second closest competitor. (465)
(621) Third, the vast majority of customers and competitors who expressed an opinion in the market investigation on this point did not expect that the Transaction would have a negative impact on the supply of super-resolution microscopes, in terms of price, quality, product range, innovation, or security of supply.
(622) In light of the considerations in paragraphs (615) to (621) above as well as all evidence available to it, the Commission concludes that the Transaction does not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement in the market for super-resolution microscopes in the EEA or worldwide.
(623) In advanced inverted research microscopes, Danaher offers Leica DMi8 and GE offers DeltaVision Ultra.
(624) According to the Parties, the Transaction does not give rise to an affected market in advanced inverted research microscopes, as the combined share of the Parties would remain [10-20]%, worldwide and in the EEA. During the market investigation, the vast majority of customers and competitors who expressed an opinion on this point did not expect that the Transaction would have a negative impact on the supply of advanced inverted research microscopes in terms of price, quality, product range, innovation, or security of supply. (466)
(625) In light of the considerations in paragraphs (623) and (624) above as well as all evidence available to it, the Commission concludes that the Transaction does not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to horizontal non-coordinated effects in the (non-affected) market for advanced inverted research microscopes worldwide or in the EEA.
5.3. High content screening
(626) High content screening (“HCS”) is a screening method used to identify substances that alter the phenotype of a cell in a desired manner. An HCS system combines microscopy, advanced imaging processing, and analysis software. As a result, an HCS system can provide a description and quantitative information on complex inter- and intra-cellular events (for example changes in cellular appearance or in protein expression).
(627) There are three types of HCS systems:
(a) Widefield HCS systems. These devices are based on widefield microscopy. (467) Widefield HCS systems provide a two-dimensional (“2D”) image of the cell observed and they are generally considered easier to use than confocal HCS systems. Widefield HCS systems can be further distinguished into (i) personal imaging/automated cell imaging systems; and (ii) other widefield HCS systems. Personal imaging/automated cell imaging systems are based on the same technology as the other widefield HCS systems, but they offer lower assay sensitivity and resolution. As such, they are less expensive than other widefield HCS systems and they are typically used for basic research.
(b) Confocal HCS systems. These devices are based on confocal microscopy. (468) Confocal HCS systems are more sophisticated than widefield HCS systems. They are used to create three-dimensional (“3D”) images with increased contrast.
(c) Laser scanning cytometers. These devices provide a 2D image of the cells observed. This technology allows for very fast cell analysis but the quality of the 2D imaging provided is very low.
5.3.1. Market definition
5.3.1.1. Product market definition
(A) The Notifying Party’s arguments
(628) The Notifying Party considered a relevant market including both widefield and confocal HCS systems but not laser scanning cytometers. According to the Notifying Party, laser scanning cytometry is a technology distinct from widefield and confocal imaging. It can only enumerate cells and provides imaging of a very low quality. In any event, the Notifying Party submits that the precise market delineation can be left open for the purposes of this decision, as neither Danaher nor GE Biopharma are present in laser scanning cytometry.
(629) If the market for HCS systems were to be sub-segmented further, the Notifying Party suggests that confocal HCS systems constitute a relevant product market, separate from widefield HCS systems. The functionality and price of widefield and confocal HCS systems is very different. As a result, widefield and confocal HCS systems are often used in different applications and by different customers.
(630) A widefield HCS system can be upgraded to a confocal based system in two ways: (i) through a (cost-intensive) hardware overhaul; or (ii) through (less costly) software updates which allow for improved image quality. The Notifying Party suggests that the possibility for an upgrade does not change its view that confocal HCS systems constitute a separate market. Even benefiting from a hardware overhaul or a software update, a widefield HCS system would not be substitutable to a confocal HCS system. (469)
(631) Within widefield HCS systems, the Notifying Party note that a distinction can be made between (i) personal imaging/automated cell imaging systems; and (ii) all other widefield HCS devices. This is because of the lower resolution and price of personal imaging/automated cell imaging systems compared to other widefield HCS devices. Ultimately, in the Notifying Party’s view, the exact market definition for personal imaging/automated cell imaging systems and other widefield HCS devices can be left open, because the Transaction does not raise competition concerns under any plausible product market delineation.
(B) Commission’s precedents
(632) The Commission has not previously defined the relevant product market for HCS systems. In Danaher/Beckman Coulter, the Commission referred to life instrumentation, which encompasses a range of distinct technologies, including HCS technology. (470) The Commission added that the technologies in question can be further sub-segmented into different product groups. However, the exact market definition was left open. (471)
(C) Commission’s assessment
(633) The Commission assessed product market definition in HCS systems, taking into account their different techniques and use cases.
(634) The results of the market investigation suggested that separate product markets should be defined for widefield HCS systems and confocal HCS systems. The majority of customers who expressed an opinion on this point stated that even an upgraded widefield HCS system (472) cannot replace a confocal HCS system. (473) The majority of customers who expressed an opinion on this point also flagged that the two types of HCS systems are not substitutable specifically in terms of price. (474) This was confirmed by competitors. As one competitor put it, “confocal-based HCS systems are significantly more expensive than widefield-based HCS systems, and generally offer superior resolution”. (475) Customers and competitors indicated that a confocal HCS system could replace a widefield HCS system in terms of use applications, technical characteristics, and efficiency – but not in terms of price. (476)
(635) As regards specifically widefield HCS systems, most customers stated that overall, they would not replace any type of widefield HCS device with a personal imaging/automated cell imaging system. According to both customers and competitors, personal imaging/automated cell imaging systems often lack flexibility and efficiency (for example they are slower in handling samples and they offer lower technical performance). (477)
(636) In light of the results of the market investigation as well as all evidence available to it, the Commission concludes that confocal HCS systems and widefield HCS systems do not compete with each other, in particular in terms of price. In addition, the Commission finds that personal imaging/automated cell imaging systems do not compete with other widefield HCS systems because of differences in flexibility and efficiency. For the purposes of this case, the Commission will therefore assess a relevant product market for confocal HCS systems and another relevant product market for widefield HCS systems (excluding personal imaging/automated cell imaging systems). (478)
5.3.1.2. Geographic market definition
(A) The Notifying Party’s arguments
(637) The Notifying Party submits that the markets for confocal HCS systems and widefield HCS systems are worldwide and in any event, not narrower than EEA- wide.
(B) Commission’s precedents
(638) The Commission has not previously defined the geographic scope of the market for (confocal or widefield) HCS systems.
(C) Commission’s assessment
(639) The market investigation confirmed the Parties’ claims regarding geographic market definition.
(640) The majority of customers who expressed an opinion on this point stated that they procure HCS systems at worldwide level; after-sale services are provided at worldwide level; prices are comparable at worldwide level; and the same suppliers are active at worldwide level. Customers added that this applies to all types of HCS systems. (479) Competitors also confirmed this. (480) Most competitors added that their market position in HCS systems does not differ significantly across different regions. (481)
(641) For the purposes of this decision, it can be left open whether the markets for confocal HCS systems and for widefield HCS systems are global or EEA-wide in scope. The Transaction does not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement under any of these plausible geographic market definitions (namely, global or EEA-wide).
5.3.2. Competitive assessment
(642) The Transaction gives rise to horizontally affected markets in confocal HCS systems and widefield HCS systems. (482)
5.3.2.1. Confocal HCS systems
(643) In confocal HCS systems, Danaher is active through its subsidiary MolDev and offers one device, ImageXpress Micro Confocal. GE Biopharma offers IN Cell Analyzer 6500.
(644) Regarding confocal HCS systems, the Transaction does not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement for the following reasons.
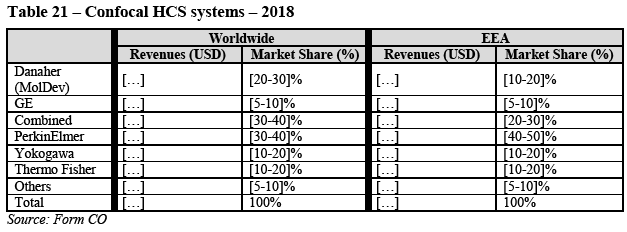
(645) First, the Parties held a combined share of [30-40]% in confocal HCS systems in 2018 worldwide, with GE Biopharma contributing a share increment of [5-10]% (see Table 21 above). Post-Transaction, the combined entity would continue facing competition by several large rivals including PerkinElmer (the number one player in the market), Yokogawa, and Thermo Fisher.
(646) In the EEA, the Parties held a combined share of [20-30]% in confocal HCS systems in 2018, with GE Biopharma contributing a share increment of [5-10]% (see Table 21 above). Post-Transaction, the combined entity would continue facing competition by the same rivals that exert competitive constraints also at the worldwide level.
(647) Second, Danaher and GE are not close competitors in confocal HCS systems:
(a) Each of the Parties offer confocal HCS systems at a different price point. Danaher’s Image Xpress Micro Confocal is available at a broad price range of USD […] (depending on the specifications) while GE’s InCell 6500 is priced at USD […]. As a result, Danaher competes with rival products across the spectrum of prices in confocal HCS systems (including Perkin Elmer’s Operetta CLS priced at USD […]), while GE is only present at the higher end of the segment.
(b) [Danaher’s internal documents], (483) […]. (484)
(c) The majority of the customers who expressed an opinion in the market investigation on this point confirmed that PerkinElmer is competing head-to- head with Danaher in confocal HCS systems. (485)
(648) Third, post-Transaction, in addition to competing with established players like PerkinElmer and Thermo Fisher, the combined entity will continue to face competition from newer confocal HCS competitors, such as Zeiss, which entered the market of confocal HCS systems in April 2019, integrating its confocal laser scanning technology to its Celldiscover 7 platform.
(649) Fourth, the majority of customers who expressed an opinion on this point expects that the proposed Transaction will not have a negative impact on the price, quality, product range, innovation, and security of supply of confocal HCS systems. (486)
(650) In light of the considerations in paragraphs (643) to (649) above as well as all evidence available to it, the Commission concludes that, in the plausible worldwide and EEA-wide market for confocal HCS systems, the Transaction will not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to horizontal non-coordinated effects.
5.3.2.2. Widefield HCS systems
(651) In widefield HCS systems, Danaher is active through MolDev and offers two devices, Image Xpress Micro 4 High-Content Imaging System and Image Xpress Nano Automated Imaging System. GE Biopharma offers IN Cell Analyzer 2500 HS HCA Imaging System.
(652) In widefield HCS systems, the Transaction does not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement for the following reasons.
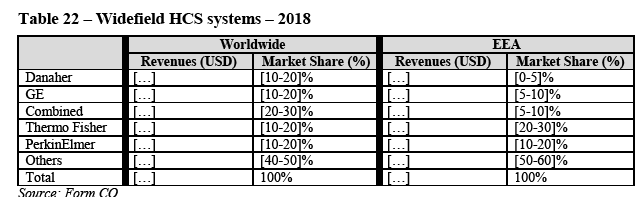
(653) First, the Parties held a combined share of [20-30]% in widefield HCS systems in 2018 worldwide (see Table 22 above). (487) The Transaction would not lead to an affected market at EEA level. Post-Transaction, the combined entity would continue facing competition by several large competitors, including PerkinElmer, Thermo Fisher and others (such as Nexelcom, Vala, Synentec, and Nikon). According to the majority of the customers and competitors who expressed an opinion in the market investigation on this point, PerkinElmer is competing head-to-head with Danaher in widefield HCS systems. (488)
(654) Second, the combined entity will also face strong competition from recent entrants, such as Zeiss, who is an established microscopy player expanding its activities in HCS systems. Zeiss launched its Celldiscoverer 7 platform in mid-2016. This product is marketed as combining the ease of use of an automated boxed microscope with auto adjustable optics and non-supervised multi-location imaging and the image quality and flexibility of a classic inverted research microscope. (489) The majority of the customers who expressed an opinion in the market investigation on this point confirmed that they consider widefield HCS systems as a dynamic space with new players entering and existing players launching new products. (490)
(655) Third, the majority of customers who expressed an opinion on this point expects that the proposed Transaction will not have a negative impact on the price, quality, product range, innovation, and security of supply of widefield HCS devices. (491)
(656) In light of the considerations in paragraphs (651) to (655) above as well as all evidence available to it, the Commission concludes that, in the plausible worldwide and EEA-wide market for widefield HCS systems, the Transaction will not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to horizontal non-coordinated effects.
5.4. Laboratory filtration
(657) Laboratory filtration (“lab filtration”) products are the instruments and accessories used for laboratory-scale physical and chemical processing to separate solid particles form fluids using a filtration medium.
(658) Lab filtration products have applications in the biopharmaceutical, food and beverage, semiconductor and electronics manufacturing, and environmental industries. The main difference between lab filtration and bioprocessing filtration (492) is that lab filtration products are for small-scale use in laboratories while bioprocessing filtration products are used in large-scale manufacturing. Also, lab filtration products do not need the regulatory approval that is required for bioprocessing filtration.
(659) Lab filtration products can be distinguished in three broad categories:
(a) Filter Media. This category includes different consumables that can be used as filters. There are two types of filter media: (i) fibrous filters (including cellulose filter papers and glass microfibre filters); and (ii) membrane filters.
(b) Disposable Devices/Media encapsulation. Filters are often integrated in a ready-to-use plastic device. Examples of such devices include syringe filters (where the filter is attached at the end of the syringe), plate filters, etc.
(c) Lab filtration accessories. This category includes hardware that is used for various lab filtration techniques (for example, filter holders, filter funnels, microbiology manifolds).
5.4.1.Market definition
5.4.1.1. Product market definition
(A) The Notifying Party’s arguments
(660) According to the Notifying Party, the widest possible product market definition could be an overall single product market for laboratory filtration.
(661) Alternatively, the Notifying Party considered narrower product market definitions. (662) In filter media, the Notifying Party distinguished between fibrous filters (which are inexpensive and have a high flow rate) and membrane filters (which are costlier and have a lower flow rate). Within fibrous media, the Parties distinguished between cellulose filter papers, which are used in basic research and glass microfiber filters, which have a higher chemical, temperature, and mechanical stability and are therefore used in more sophisticated applications.
(663) In disposable devices/media encapsulation, the Notifying Party submitted that there is significant supply-side substitutability between different devices. However, the Parties also suggested that demand-side substitutability is limited, because different disposable devices must be used for different applications (depending on the amount of sample to be filtered and the objective of the filtration). For example, syringe filters are used to filter small sample volume sizes in the following applications: sterile filtration, sample preparation, sterile venting, etc.
(664) Finally, the Notifying Party takes the view that there is an overall single market including all lab filtration accessories (493). While there are differences between various types of accessories, they all share similarities in terms of basic function and there is strong supply side substitutability.
(665) In any event, the Notifying Party has submitted that the precise product market definition can be left open as the Transaction would not raise competition concerns under any plausible product market delineation.
(B) Commission’s precedents
(666) In its decisional practice, the Commission has not examined in detail the relevant product market definition for the lab filtration segment. In Thermo Electron/Fisher Scientific (494), the Commission considered that all plastic microplates filters (a type of media encapsulation device) belonged to the same relevant product market without further distinction based on sizes or number of individual wells. Also, in Thermo Fisher/Phadia (495), the Commission discussed separate markets for microplates for general use and microplates for diagnostic applications, but ultimately, the exact product market definition was left open.
(C) Commission’s assessment
(667) The market investigation is inconclusive as to whether there is an overall lab filtration product market or whether narrower markets should be defined.
(668) The majority of customers who expressed an opinion on this point consider there would be demand side substitutability in terms of price, types of applications, quality, and efficiency for all lab filtration products. (496)
(669) However, the explanations provided by several customers and competitors suggested that narrower relevant product markets could be defined on the basis of the materials used or the application of each product. For example, one customer indicated that “the different applications need to be considered” while other customers added that “specific process parameters require specific filtration parameters, incl. filter material, technical parameters (pore size, adhesion, binding, etc.)” and “the physical characteristics of the filter and filtration performance drivers like device type and accessory are critical for product selection in lab applications”. (497) Competitors also indicated that there should be different product markets for the various sub-segments of lab filtration. (498)
(670) Considering the different product characteristics and applications of laboratory filtration products, it can be left open for the purposes of this decision whether there is a relevant market including (i) all lab filtration products or there are separate markets for (ii) lab filter media; (iii) all fibrous filter media; (iv) membrane filters; (v) non-microbiology membrane filters segment; (vi) microbiology membrane filters segment; (vii) cellulose filter papers; (viii) glass microfiber filter media; (ix) quartz filter papers (x) overall disposable devices/media encapsulation filters; (xi) syringe filters; (xii) non-sterile syringe filters; (xiii) sterile syringe filters; (xiv) 50/60 mm in- line filters; (xv) capsule filters segment; (xvi) vent filters segment; (xvii) plate filters segment; (xviii) spin devices; (xix) overall lab filtration accessories; (xx) filter holder segment; (xxi) filter funnels segment; (xxii) hardware segment; (xxiii) microbiology manifold segment; (xxiv) vacuum pumps segment; and (xxv) cut membrane discs. The Transaction does not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement under any of these plausible product market definitions.
5.4.1.2. Geographic market definition
(A) The Notifying Party’s arguments
(671) The Notifying Party submits that the markets in the lab filtration area are worldwide and in any event, not narrower than EEA-wide.
(B) Commission’s precedents
(672) The Commission has not examined in detail relevant geographic market for the lab filtration segment in its decisional practice.
(C) Commission’s assessment
(673) The majority of customers and competitors who expressed an opinion on this point views the lab filtration market or markets as global, but there are some market participants that put forward that there are different prices and lead times across regions (499).
(674) The investigation in this case has confirmed that these markets are at least EEA- wide, but the exact scope of the geographic market can be left open, as the Transaction does not raise competition concerns under any of these market delineations.
5.4.2. Competitive assessment
(675) Danaher (through Pall Laboratory and Phenomenex) supplies various products in all categories of lab filtration products.
(676) GE Biopharma (through Whatman Lab Filtration) supplies a wide range of products in all categories of lab filtration products.
(677) The Transaction results in affected markets only in the following categories which constitute possible product markets: (i) all fibrous filter media; (ii) glass microfiber filter media; and (iii) microbiology manifolds. (500)
5.4.2.1. Fibrous filter media overall and glass microfiber filter media segment
(678) In the possible market for all fibrous filter media, the combined entity would have, according to the Notifying Party’s estimates for 2018, a market share of [30-40]% worldwide (with an increment of [0-5]% from Danaher) and [20-30]% market share in the EEA (with a [0-5]% increment from Danaher). The Transaction does not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement at worldwide and EEA levels, in particular because the […] increment attributable to Danaher ([0-5]%) is confined to the mere re-sale of Ahlstrom-Munksjö’s products.
(679) When considering the different segments of fibrous filter media as separate product markets, the Parties’ activities would only overlap in the possible market for glass microfiber filter media. In the possible market for glass microfiber filter media, the Transaction does not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement for the following reasons.
(680) First, if a glass microfiber filter media market were defined, the combined entity would have, according to the Notifying Party’s estimates for 2018, a market share of [20-30]% worldwide (with an increment of [0-5]% from Danaher) and [20-30]% market share in the EEA (with a [0-5]% increment from Danaher). The […] increment from Danaher’s side is likely to have no impact on the competitive environment; also, Danaher’s small market share comes from reselling the products bought from Ahlstrom-Munksjö.
(681) Second, according to the Parties (501), the merged entity will continue to face effective competition from a number of suppliers like Ahlstrom-Munktell, Advantec, Macherey-Nagel and Hahnemuehle Fineart GmbH.
(682) Third, the vast majority of customers who expressed an opinion on this point expects (502) that the proposed Transaction will not have a negative impact on the price, quality, product range, innovation, and security of supply of glass microfiber filter media. The views of competitors regarding the negative impact on price were evenly split, but the majority of competitors who expressed an opinion on this point do not expect any negative impact on quality, product range, innovation, and security of supply.
(683) In light of the considerations in paragraphs (678) to (682) above as well as all evidence available to it, the Commission concludes that, in the potential worldwide or EEA-wide markets for all fibrous filter media and glass microfiber filter media, the Transaction will not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to horizontal non- coordinated effects.
5.4.2.2. Microbiology manifold segment
(684) In the microbiology manifold segment, the Transaction does not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement for the following reasons.
(685) First, if a relevant product market would be defined for the microbiology manifold filter, the combined entity would have, according to the Notifying Party’s estimates for 2018, a market share of [10-20]% worldwide (which would not be an affected market) and a [20-30]% market share in the EEA (with a [0-5]% increment from GE Biopharma). Post transaction, the merged entity market share would be [20-30]% (503) with a […] increment from GE’s side.
(686) Second, the vast majority of customer who expressed an opinion on this point expects that the proposed Transaction will have no impact on the price, quality, product range, innovation, and security of supply of microbiology manifold segment (504). Also, most competitors do not expect (505) any negative impact on quality, product range, innovation, and security of supply.
(687) Third, the majority of competitors who expressed an opinion on this point answered (506) that it would be easy to start producing in the microbiology manifold segment.
(688) Even though the majority of competitors who expressed an opinion on this point expressed concerns about a potential price increase post-transaction, in light of the Notifying Party's arguments (507) that are in line with the above findings and considerations, the Commission concludes that the Transaction does not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement in the possible worldwide or EEA-wide market for the microbiology manifolds.
5.4.2.3. Assessment of the non-affected markets of overall lab filtration products, syringe filters, cut membrane discs and lab filter media overall
(689) As some competitors raised competition concerns regarding the (non-affected) market for overall lab filtration products, the Commission also assessed a possible overall market for lab filtration products.
(690) However, in this market, based on the results of the market investigation, the Commission considers that the Transaction does not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement for the following reasons. The combined entity would have, according to the Parties estimates for 2018 (508), a combined market share below 20% both at a worldwide level and at EEA level; the merged entity would still face competitive pressure from a number of suppliers like Merck Millipore, Sartorius, Advantec, Ahlstrom Munktell, Thermo Fisher and Macherey Nagel.
(691) The market investigation shows (509) that customers consider that there would be enough suppliers on the market of overall lab filtration post-Transaction, including new entrants from China. In addition, the vast majority of customers who expressed an opinion on this point does not expect any negative impact of the proposed transaction on price, quality, product range, innovation, and security of supply (510). Also, the majority of competitors who expressed an opinion on this point does not expect any negative impact of the proposed transaction on quality, product range, innovation, and security of supply.
(692) Also, the market investigation shows that customers consider that there are no barriers to switch suppliers as lab filtration products are not subject to regulatory filing as the bioprocessing filters are (511).
(693) One competitor raised competition concerns regarding the (non-affected) markets for syringe filters and cut membrane discs (512) and another competitor raised concerns regarding the (non-affected) lab filter media. However, in these markets, based on the results of the market investigation, the Commission considers that the Transaction does not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement for the following reasons.
(a) Syringe filters: The combined entity would have, according to the Parties estimates for 2018, a combined market share below 20% (513) both at a worldwide level and at a EEA level; the merged entity would still face effective competition competition from a number of suppliers like Merck Millipore, Sartorius, Thermo Fisher and many small players, including new low-cost entrants from China, more particularly in the analytical segment as stated by the Parties (514).
(b) Cut membrane discs: The combined entity would have a combined market share below 20% (515) both at a worldwide level and at a EEA level; the merged entity would still face effective competition (516) from a number of suppliers like Merck Millipore, Sartorius, Thermo Fisher, Cobetter and many small players like Advantec, MIDI, Sterlitech, Donaldson and others.
(c) Lab filter media: The combined entity would have a combined market share below 20% (517) both at a worldwide level and at an EEA level; the merged entity would still face effective competition competition from a number of suppliers like Merck Millipore, Sartorius, Thermo Fisher, and Corning.
(694) In light of the considerations in paragraphs (684) to (693) above as well as all evidence available to it, the Commission considers that, in the potential worldwide or EEA-wide markets for overall lab filtration, syringe filters, cut membrane discs and lab filter media, the Transaction will not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to horizontal non-coordinated effects under any plausible market definition.
5.5. Coordinated effects
(695) The Notifying Party takes the view that the Transaction does not give rise to coordinated effects in the (worldwide or EEA-wide) markets for LF detection systems; super-resolution microscopes; confocal HCS systems; widefield HCS systems; fibrous filters; glass microfiber filter media; or microbiology manifolds. (518)
(696) The Commission assessed whether the Transaction gives rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement in terms of coordinated effects in the markets mentioned in paragraph (695) above, taking into account the following:
(a) LF detection systems: In this market, Danaher focuses on LF detection systems based on BLI technology while GE only offers systems based on SPR technology. While the Commission considered the Parties to compete closely, the market for LF detection systems has to be regarded as a differentiated market. Not only the product offerings, but also the suppliers of LF detection systems are also differentiated including large suppliers (such as the Parties, PerkinElmer, and Bruker); medium-sized suppliers (such as Malvern Microcal); and smaller suppliers (such as Carterra and Creoptix). The market investigation did not reveal concerns of customers or competitors regarding coordinated effects in LF detection systems. (519)
(b) Super-resolution microscopes: The products of the Parties are highly differentiated, as Danaher offers confocal-based super-resolution microscopes and GE supplies widefield-based super-resolution microscopes. The market investigation did not reveal concerns of customers or competitors regarding coordinated effects in super-resolution microscopes. (520)
(c) Confocal HCS systems and widefield HCS systems: In HCS systems, post- Transaction, several players will remain in the market, including established competitors, such as PerkinElmer, and recent entrants, such as Zeiss. Furthermore, in confocal HCS systems, the Parties’ products are offered at highly differentiated price points. The market investigation did not reveal concerns of customers or competitors regarding coordinated effects in confocal HCS systems and widefield HCS systems. (521)
(d) Fibrous filter media, glass microfiber filter media, and microbiology manifolds. The relevant markets are highly asymmetric as reflected in the significant market share differences between Danaher and GE. (522) The market investigation did not reveal concerns of customers or competitors regarding coordinated effects in these plausible markets. (523)
(697) In light of the considerations above as well as all evidence available to it, the Commission concludes that, in the worldwide or EEA markets for mentioned in paragraph (695) above, the Transaction will not give rise to serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement due to horizontal coordinated effects.
6. CONCLUSION ON COMPETITIVE ASSESSMENT
(698) In view of sections 4 and 5 above, the Commission concludes that the following markets will give rise to serious doubts as to their compatibility due to horizontal non-coordinated effects: (i) in the worldwide market for microcarriers (either overall, or in the potential markets for microporous microcarriers, animal-based microcarriers, non-animal-based microcarriers, cationic microcarriers or collagen- coated microcarriers); (ii) the worldwide or EEA-wide market for TFF systems (SUT); (iii) the worldwide or EEA-wide market for flat sheet TFF systems (SUT); (iv) the worldwide market for hollow fibre TFF systems (conventional); (v) the worldwide or EEA-wide market for stainless steel chromatography skids; (vi) the worldwide or EEA-wide market for SUT chromatography skids; (vii) the worldwide or EEA-wide market for continuous chromatography skids; (viii) the worldwide or EEA-wide market for non-Protein A affinity resins; (ix) the worldwide or EEA-wide market for ion exchange resins; (x) the worldwide or EEA-wide market for mixed- mode resins; (xi) the worldwide or EEA-wide market for chromatography columns; and (xii) the worldwide or EEA-wide market for LF detection systems.
7. MODIFICATION OF THE TRANSACTION
7.1. Framework for the assessment of the commitments
(699) The following principles, as referred to in the Commission Regulation (EC) No 802/2004 and in the Commission Notice on remedies acceptable under the Merger Regulation (the “Remedies Notice”) (524) apply where parties to a concentration choose to offer commitments in order to restore effective competition following serious doubts identified by the Commission with a view to having the transaction approved in phase 1.
(700) In phase 1, commitments offered by the parties can only be accepted where the competition problem is readily identifiable and can easily be remedied. The competition problem therefore needs to be so straightforward and the remedies so clear-cut that it is not necessary to enter into an in-depth investigation and that the commitments are sufficient to clearly rule out serious doubts within the meaning of Article 6(1)(c) of the Merger Regulation. Where the assessment confirms that the proposed commitments remove the grounds for serious doubts on this basis, the Commission clears the merger in phase 1.
(701) In assessing whether the proposed commitments will likely eliminate the competition concerns identified, the Commission considers all relevant factors including inter alia the type, scale and scope of the proposed commitments, judged by reference to the structure and particular characteristics of the market in which the competition concerns arise, including the position of the parties and other participants on the market. As set out in the Remedies Notice, the commitments have to eliminate the competition concerns entirely, and have to be comprehensive and effective from all points of view. The Commission only has power to accept commitments that are capable of rendering the concentration compatible with the internal market or the functioning of the EEA Agreement in that they will prevent the significant impediment to effective competition in all relevant markets where competition concerns were identified.
(702) In order for the commitments to comply with those principles, they must be capable of being implemented effectively within a short period of time. The Commission must determine with the requisite degree of certainty, at the time of its decision, that they will be fully implemented and that they are likely to maintain effective competition in the market.
(703) As concerns the form of acceptable commitments, the Merger Regulation leaves discretion to the Commission as long as the commitments meet the requisite standard.
(704) Divestiture commitments are often the most effective way to eliminate competition concerns resulting from horizontal overlaps. The intended effects of a divestiture will only be achieved if and once the business to divest is transferred to a suitable purchaser in whose hands it will become an active competitive force in the market.
(705) To that end, the divested activities must consist of a viable business that, if operated by a suitable purchaser, can compete effectively with the merged entity on a lasting basis and that is divested as a going concern. (525) The business has to include all the assets which contribute to its current operation or which are necessary to ensure its viability and competitiveness and all personnel which is currently employed or which is necessary to ensure the business’ viability and competitiveness. Normally, a viable business is a business that can operate on a stand-alone basis, which means independently of the merging parties as regards the supply of input materials or other forms of cooperation other than during a transitory period.
(706) Even though the divestiture of an existing viable stand-alone business is normally required, the Commission, taking into account the principle of proportionality, may also consider the divestiture of businesses which have existing strong links or are partially integrated with businesses retained by the parties and therefore need to be ‘carved out’. (526) The Commission will only be able to accept commitments which require the carve-out of a business if it can be certain that, at least at the time when the business is transferred to the Purchaser, a viable business on a stand-alone basis will be divested and the risks for the viability and competitiveness caused by the carve-out will thereby be reduced to a minimum. (527)
(707) In order to ensure that the business is divested to a suitable purchaser, the commitments have to include criteria to define its suitability which will allow the Commission to conclude that the divestiture of the business to such a purchaser will likely remove the competition concerns identified.
(708) An up-front buyer may be necessary in cases which cause considerable risks of preserving the competitiveness and saleability of the divestment business in the interim period until divestiture. This category comprises cases where the risks of a degradation of the divestment business appear to be high, in particular due to a risk of losing employees being key for the business, or where the interim risks are increased as the parties are not able to undertake the carve-out process in the interim period, but the carve-out process can only take place once a sales and purchase agreement with a purchaser is entered into. The up-front buyer provision may accelerate the transfer of the business to be divested — given the increased incentives for the parties to close the divestiture in order to be able to complete their own concentration — to such an extent that the commitments may allow the Commission to conclude with the requisite degree of certainty that those risks are limited and the divestiture will be effectively implemented. (528)
7.2. COMMITMENTS SUBMITTED BY THE NOTIFYING PARTY
(709) In order to render the concentration compatible with the internal market and the functioning of the EEA Agreement, on 27 November 2019 the Notifying Party submitted commitments pursuant to Article 6(2) of the Merger Regulation. The Notifying Party made some technical modifications on the text of the commitments and resubmitted them on 28 November 2019 (the “Initial Commitments”).
(710) The Commission launched a market test of the Initial Commitments on 29 November 2019, seeking responses from competitors and customers. The Commission informed the Notifying Party of the results of the market test on 6 December 2019. Following the Commission’s feedback on the market test and the assessment of the Initial Commitments, the Notifying Party submitted a revised set of commitments on 10 December 2019 (the “Final Commitments”).
(711) The Final Commitments are annexed to this decision and form an integral part of it.
7.3. Initial Commitments
7.3.1. Description of the Initial Commitments
(712) In substance, the Initial Commitments include the divestiture of five businesses controlled by the Notifying Party (jointly, the “Divestment Business”): (a) the FortéBio molecular characterisation business from Danaher’s Molecular Devices subsidiary (“the FortéBio molecular characterisation business”); (b) Danaher’s Pall Biotech SoloHill microcarriers and the particle validation standards (“PVS”) business (“Microcarriers Divestment Business”); (c) Danaher’s Pall Biotech chromatography resins business; (d) Danaher’s Pall Biotech chromatography hardware business comprising conventional chromatography columns, conventional and single-use technology chromatography skids and BioSMB continuous chromatography Process Development and Process Scale skids (“Danaher’s Pall Biotech chromatography hardware business”); and (e) Danaher’s Pall Biotech Single-Use Tangential Flow Filtration ("SUT TFF") systems and stainless-steel Hollow Fibre TFF (“SS HF TFF”) systems business (“the TFF Divestment Business”).
7.3.1.1. The FortéBio molecular characterization business
(713) The FortéBio molecular characterisation business operates out of legal entity ([…]) within Molecular Devices LLC.
(714) The FortéBio Divestment business consists of Danaher’s business in label-free detection (a field within molecular characterization) that offers instruments and consumables for BLI under its Octet and BLItz product lines, as well as for SPR which it offers under its Pioneer product line.
(715) It includes: (i) tangible and intangible assets; (ii) licences, permits and authorisations;(iii) contracts, leases, commitments and customer orders; (iv) customer, credit and other records; and (v) personnel, including salesforce. The Notifying Party furthermore undertake the required preparation work to ensure post- closing business continuity (notably, regarding the [certifications]). The Notifying Party further commits to allow the Purchaser to rely on Pall’s existing certification under a TSA.
7.3.1.2. It does not include the […] trademark and logo (although a license will be provided for a transitory period of […], extendable by […]). Microcarriers Divestment Business
(716) The “Microcarriers Divestment Business” operates out of [Danaher’s organisation structure] located in Ann Arbor, Michigan (USA).
(717) The business transferred includes: (i) tangible and intangible assets including equipment and intellectual property; (ii) contracts, leases and commitments; (iii) customer, credit and other records; (iv) personnel (excluding those attributed to the activities listed below); and (v) Danaher will assist the Purchaser with preparation work to obtain its own [certifications] to ensure business continuity after the divestiture and will allow the Purchaser to rely on Pall’s existing certification under a TSA until it obtains it.
(718) It does not include: (ii) the rights to use the Pall brand [Danaher’s intellectual property rights and timeframe].
(719) The business transferred does not include: (i) back office functions; (ii) salesforce (but the Notifying Party commits to train the Purchaser’s salesforce under a TSA for [timeframe]); and (iii) Process Development Services (“PDS”), which are currently located in the […] facility and which provides targeted development and consultation services for the development of bioprocessing systems and processes.
7.3.1.3. Danaher’s Pall Biotech chromatography resins business
(720) The “Chromatography resins business” consists of […] located in Cergy (France) which will be transferred to the Purchaser.
(721) The business transferred includes: (i) the Cergy facility and its equipment; (ii) tangible and intangible assets; (iii) personnel (excluding salesforce); and (iv) Danaher will assist the Purchaser with preparation work for the Purchaser to obtain an [certifications] for the business to be transferred.
(722) The business transferred does not include: (i) salesforce (but the Notifying Party commits to train the Purchaser’s salesforce under a TSA); (ii) the right to use the Pall trade mark or the Pall logo for chromatography resins for a period of […], extendable by […]; and (iii) employees and facilities that are within the Cergy facility but unrelated to the chromatography resins operations.
7.3.1.4. Danaher’s Pall Biotech chromatography hardware business
(723) The Initial Commitments (529) foresee to (i) consolidate Danaher’s activities in columns and skids in a segregated section of Pall’s […] facility on an interim basis; and to (ii) subsequently relocate these to a suitable new location in the […] area.
(724) Process development skids BioSMB continuous chromatography skids are currently assembled in [location], and will as per the commitments be relocated to a new location in […].
(725) The business transferred includes: (i) tangible and intangible assets (including intellectual property rights); (ii) licences, permits and authorisations issued by any governmental organisation for the benefit of the hardware chromatography business; (iii) contracts, leases, commitments and customer orders of the hardware chromatography business; all customer, credit and other records; (iv) the Personnel, and notably the engineers, but excluding those attributed to the activities listed below; and (v) Danaher will assist the Purchaser with preparation work for the Purchaser to obtain an [certifications] for the business to be transferred.
(726) The business transferred does not include: (i) back office functions; (ii) salesforce (but the Notifying Party commits to train the Purchaser’s salesforce under a TSA for [timeframe]); and (iii) the […] Trademark, which will be licenced under a TSA.
7.3.1.5. The TFF Divestment Business
(727) Danaher’s facilities for SUT flat sheet and SS hollow fibre TFF skids are located in […]. The Commitments foresee that the personnel and activities in […] for these products will move within the facility to a segregated section. The […] activities for these products will be relocated to an already leased new location in […]. The […] activities for these products will be relocated to available space in the transferring […] facility. Post-closing, the […] activities will be relocated to a new location in the […] area. Danaher will use its Best Efforts to help the Purchaser in this relocation exercise.
(728) The TFF Divestment Business includes Danaher’s businesses in TFF systems (SUT) and hollow fibre TFF systems (conventional).
(729) The TFF Divestment Business does not include: (i) the trademark for […], although Danaher will license these to the purchaser for a transitory period of […]; (ii) Danaher’s salesforce (but the Party commits to train the Purchaser’s salesforce under a TSA); (iii) no [location] personnel or functions will transfer (inventory will transfer); (iv) Danaher’s activities in the assembly of flow kit consumables and valves for SUT TFF skids (the Purchaser will receive a license to any trade secrets of the business relating to the design, manufacture and assembly of SUT flow kits); and (v) Danaher will assist the Purchaser with preparation work for the Purchaser to obtain an [certifications] for the locations to be transferred.
(730) The entire Divestment Business is complemented by a Transitional Trademark Licence Agreement for the retained Trademark and Logos, namely […], whereby the Purchaser obtains a […] license to use those Trademarks and brands for the Divestment Business for [timeframe]. However, no specific [timeframe]. (530)
(731) In addition, the Initial Commitments provide for special criteria that a potential purchaser needs to fulfil. With regard to the entire Divestment Business, the Purchaser must (i) have proven expertise in the biotechnology process equipment and/or consumables industry; (ii) have an established presence in at least one Union Member State other than the United Kingdom; and (iii) have at its disposal back- office support functions (531). With regard to the SoloHill microcarriers and PVS business, the chromatography resins business, the chromatography hardware business and the SUT TFF and SS HF TFF systems businesses, the potential purchaser must also have at its disposal a salesforce with experience of selling and promoting biotechnology process equipment and/or consumables in EMEA, the Americas and Asia, including China.
(732) Moreover, the Notifying Party commits not to implement the Transaction before it, or the Divestiture Trustee, has entered into a final binding sale and purchase agreement for the sale of the Divestment Business and the Commission has approved the Purchaser and the terms of sale in accordance with the procedure set out in the Final Commitments.
(733) Finally, the Notifying Party has entered into related commitments, inter alia regarding the separation of the divested businesses from their retained businesses, the preservation of the viability, marketability and competitiveness of the divested businesses, including the appointment of a monitoring trustee and, if necessary, a divestiture trustee.
7.3.2. Results of the market test
(734) The commission launched a market test of the Initial Commitments on 29 November 2019, which was addressed to competitors and customers. Overall, the feedback of the market test was positive as to the elimination of competition concerns by the proposed commitments. (532)
(735) With respect to all the areas of concern, the majority of the respondents who expressed an opinion on this point considered that after the transfer of the entire Divestment Business, the suitable Purchaser would be able to operate it as a viable competition constraint to the merged entity. (533)
(736) The majority of market respondents consider that the purchaser criteria are appropriate to identify a suitable purchaser the Divestment Business. (534) Two market respondents expressed the opinion on this point that the potential purchaser should not discontinue the Divestment Business: “it should be added to above considerations that the Divestment Business should not be discontinued by the purchaser”, “The criteria selected are acceptable to guarantee customers that the business will see continuity, as it is a very specialized market segment. As mentioned in point F.1.1 the issue will be having the purchaser guarantee they will not discontinue product lines, as a potential company which fits the selection criteria listed would inevitably be a company which already has similar products on the market”. (535)
(737) However, with respect to the different commitments packages, some additional areas for improvement were identified:
(a) With the exception of the FortéBio business, the commitment does not include a ‘go-to-market’ sales staff. While the purchaser criteria require a suitable purchaser to have its own sales organisation, several respondents highlighted that the lack of sales force in the commitments package may impede upon the competitiveness of the divested businesses. (536)
(b) While the Notifying Party proposes transitional license agreements for brands that are used by the divestment businesses (537), and as well by the retained business, several respondents considered that the length of the transitional licensing agreements would not be long enough.
(c) Moreover, the feedback was mixed as to whether a change in the brand name of products that are specified in the regulatory filings would require a modification of the regulatory approval and how cumbersome this would be. The majority of the market respondents who expressed an opinion on this point were not worried about the impact of the brand change in the regulatory filling for Danaher’s Pall Biotech chromatography resins business (538) and Danaher’s Pall Biotech chromatography hardware business. (539)
(d) Regarding the Danaher’s Pall Biotech chromatography hardware business, some market participants expressed their worries about the relocation of continuous chromatography in the […] (540) area and of the rest of the activities in the […] area (541). In particular, these moves would create uncertainties about the losses of know-how and technical capabilities; there would also be a need to limit the distance to the new facilities as much as possible.
(e) Regarding the Danaher’s Pall Biotech chromatography resins business, some respondents specified that the [supply source] agreement for Protein A and the membrane chromatography should be included in the commitments package. (542)
(f) Regarding the filtration skids remedy, some market participants indicated the importance for the Purchaser to have associated consumables (filters and flow kits, as discussed further below).
(g) The results of the market test were mixed as to whether the exclusion of the PDS will hinder the Purchaser’s ability to operate the Microcarriers Divestment Business as a viable and competitive force. (543)
7.3.3. The Commission’s assessment of the Initial Commitments
7.3.3.1. Scope of the Divestment Businesses
(738) The Commission firstly notes that the Initial Commitments remove the full overlap in those markets where the transaction would have raised serious doubts as to its compatibility with the internal market and the EEA-Agreement, namely (i) the worldwide market for microcarriers (either overall, or in the potential markets for microporous microcarriers, animal-based microcarriers, non-animal-based microcarriers, cationic microcarriers or collagen-coated microcarriers); (ii) the worldwide or EEA-wide market for TFF systems (SUT); (iii) the worldwide or EEA- wide market for flat sheet TFF systems (SUT); (iv) the worldwide market for hollow fibre TFF systems (conventional); (v) worldwide or EEA-wide market for stainless steel chromatography skids; (vi) the worldwide or EEA-wide market for SUT chromatography skids; (vii) the worldwide or EEA-wide market for continuous chromatography skids; (viii) the worldwide or EEA-wide market for non-Protein A affinity resins; (ix) the worldwide or EEA-wide market for ion exchange resins; (x) the worldwide or EEA-wide market for mixed-mode resins; (xi) the worldwide or EEA-wide market for chromatography columns; and (xii) the worldwide or EEA- wide market for LF detection systems. Thus, the Commission considers the Initial Commitments in principle to be sufficiently broad to address the identified competition concerns.
(739) Moreover, the Commitments only comprise assets from the Notifying Party and therefore do not include any mix-and-match elements, which allows the Purchaser to realise certain efficiencies (in particular as regards the Chromatography Hardware and TFF Divestment Businesses.
(740) The Commission, however, notes that the Chromatography Hardware and TFF Divestment Businesses do not constitute stand-alone businesses and form currently part of a larger business that also includes other product areas that are not being divested. The Commitments foresee that the activities for divestiture will be segregated within the facilities where they are currently located, and subsequently moved to new locations in the proximity. The Commission considers that this may entail complexities and risks to be carefully assessed. In this context, the Commission firstly considers the nature of the activities carried out by the Chromatography Hardware and TFF Divestment Businesses. After the design or customisation of the design of a specific chromatography or TFF hardware, which is carried out [production secrets], all of which will be transferred or made available to the Purchaser as part of the Chromatography Hardware and TFF Divestment Business). The Commission moreover notes that the Chromatography Hardware Business was successfully moved from a different location to its current location in […] when Danaher acquired it in 2005. Also, a majority of respondents who expressed an opinion in the market test on this point took the view that the envisaged concentration and relocation procedure would enable the Purchaser to run the Hardware Chromatography and TFF Divestment Businesses as a viable and competitive force. (544) Furthermore, the purchaser criteria require that the Purchaser has proven expertise in the biotechnology process equipment and/or consumables industry. On this basis, the Commission is able to accept the proposed carve-out as suitable for the Commitments in the current Transaction, in particular due to the nature in which the relevant activities are performed today, and in light of the purchaser criteria defined in the Commitments.
(741) The Commission considers that the lack of inclusion of sales personnel could entail risks for the Divestment Businesses to get access to the market in a timely manner. This concern has also been reflected in the market test where this was generally brought forward for each of the Divestment Businesses. (545) One competitor considered that Divestment Business would in that regard be dependent on the salesforce of [Danaher]. (546) Overall, while a majority of respondents who expressed an opinion in the market test on this point did not consider any specific difficulties or risks associated with the fact that sales personnel would not transfer, a large minority did. (547) Based on feedback from the market investigation, the Commission finds that it might take time to build up customer relationships for the products involved: “[s]ales personnel are the key to customers; this is an important asset, as it takes a long time to win a new customer, but takes only seconds to loose” (548); “[s]ales personnel in many cases build up a long-term relationship to the purchaser”; “loss of built long-term business relationships and knowledge of target market needs”; “[f]or this kind of equipment a long term relation with clients is rather relevant (experience, interaction, know how and reliability). This relation needs to be reinstalled”. (549)
(742) The Commission however also takes note that a significant majority of respondents who expressed an opinion on this point considered that the Commitment of the Notifying Party to train the Purchaser’s salesforce would enable the Purchaser to run the Divestment Business as a viable and competitive force.- (550)
(743) While the Commission therefore accepts that the entirety Danaher’s salesforce generally does not transfer with the Divestment Business, it considers that the Commitments should foresee flexibility in this regard, allowing some sales personnel to also transfer to the Purchaser.
(744) In addition, the Commission notes that the Notifying Party considered the number of [production secrets] that it committed to transfer for the Chromatography Hardware Divestment Business and TFF Divestment Business to be confidential, and that in that regard it was not able to obtain assurance from the market test whether this number would be sufficient. In particular, according to the Notifying Party, [production secrets]. (551) While there are currently a large number [production secrets] within Danaher’s organisation [production secrets], the Notifying Party estimated the number of transferring engineers for the Chromatography Hardware and TFF Divestment Businesses by a review of projects executed in the period 2016-2018 and an analysis of current pending projects. (552)
(745) In light of the above, the Commission considers that overall the scope of the Divestment Business appears to be sufficient as regards the number of engineers who are transferring, and that the lack of a general sales team appears to be compensated by the special purchaser criteria in combination with the respective training commitment. That being said, the Commission considers that it would be necessary to fine-tune the number of transferring engineers and sales personnel in light of the identity of the Purchaser.
(746) The Commission notes that one complainant considers that the scope of the divestment is not wide enough, in that a divestiture of Danaher’s entire SUT and stainless steel hardware should be included. In this regard, the Commission notes that the entire SUT TFF business of Danaher is included in the Commitments, as well as the stainless steel systems for hollow fibre. While the stainless steel flat sheet systems are not included, as set out in section 4.5.3.3 above, the Commission did not find serious doubts in that respective area. Moreover, the Commission takes note of the Notifying Party’s argument that an inclusion of its stainless steel flat sheet systems into the commitments would not be warranted in order to ensure the viability and competitiveness of the TFF Divestment Business, as (1) customers do not require suppliers to be active in all TFF systems segments, (2) transferring the Notifying Party’s stainless steel flat sheet systems activities, which are [production secrets] would only add low-added value services to the TFF Divestment Business, such as logistics and procurement and support, as well as space for factory acceptance testing, and (3) the Divestment Business as a whole does not make or procure [production secrets]. Thus, the Commission considers that the inclusion of the Notifying Party’s stainless steel flat sheet TFF activities is not be warranted for viability purposes.
(747) As regards the concern that the Chromatography resins business does not include the supply agreement with [supply source] for Protein A, the Commission notes that this has been terminated and hence cannot be transferred. Moreover, the Commission does not find that the Transaction raises serious doubts as to its compatibility with the internal market or the functioning of the EEA Agreement in relation to the supply of Protein A. Likewise, the Commission does not raise serious doubts in relation to membrane chromatography and therefore considers that the respective activities doesn’t need to be included in the commitments.
(748) Finally, a complainant who had in the market investigation been concerned on potential conglomerate effects brought forth by the proposed Transaction (as set out in sections 4.9.2 and 4.9.3) reiterated its concerns in the market test, and concluded that the proposed Commitments would not address these. However, as set out in section 4.9.4, the Commission does not raise serious doubts as regards the compatibility of the proposed Transaction in the internal market or the functioning of the EEA Agreement as regards conglomerate effects. This complainant also considered that the Commitments would not address its concerns in an overall market of TFF systems, for which the Commission points out that it did not find serious doubts either, as set out in section 4.5.3.3.
7.3.3.2. Viability and competitiveness of the Divested Businesses
(749) While a majority of respondents who expressed an opinion in the market test on this point indicated that they did not see any specific difficulties or risks associated with the transitory supply or service agreements to the Purchaser, (553) the Commission found that the commitment to not transfer certain of Danaher’s brands but instead to license these for a transitory period could pose difficulties to customers. The Commission recalls that once customers have specified a product in their production process, it might be difficult for them to replace such a product, given the requirements in terms of validation and regulatory filings. In this regard, the market test namely revealed that a change in brand name might also have an impact on the validity of a product concerned in a regulatory filing. (554) Also, several respondents questioned whether the transitional trademark licence agreements were sufficiently long to allow the Purchaser to successfully rebrand the respective products.
(750) The Commission furthermore considers that it would be important for the Purchaser to offer associated consumables to go with the TFF divestment business, as indicated also in paragraph (242), as well as flow kits, which are an integral part of SUT TFF skids.
(751) This was also reflected by comments of several respondents in the market investigation. (555) In this regard, the Commission will pay particular attention in the buyer approval for the Purchaser to be able and incentivised to compete in light of, inter alia, its offering of consumables.
(752) Similarly, several respondents indicated the importance for the Purchaser to be able to supply the flow kits that go with the SUT TFF skids. A minority of respondents to the market test that took a position considered that the exclusion thereof would hinder the Purchaser’s ability to operate the TFF Divestment Business as a viable and competitive force. (556) The Commission in this regard notes that the Commitments foresee to transfer Danaher’s manufacturing capabilities to the Purchaser, and that, according to the Notifying Party, its internal estimates suggest that “[Danaher’s] flow kits account for only about […]% of the expected flow kit consumption associated with [its] SUT TFF systems”. (557) Nonetheless, in order to safeguard what could potentially be a vital element, the Commission will pay particular attention in the buyer approval process for the Purchaser to be able to replicate Danaher’s production of these flow kits.
7.3.3.3. Potential purchaser
(753) In response to the market test, more than 10 companies active in the bioprocessing industry expressed a general interest in the acquisition of the Divestment Business or parts thereof. (558) The Commission also notes that the Notifying Party has already entered into a sales and purchase agreement for parts of the Divestment Business with Sartorius and is currently negotiating to extend the sales and purchase agreement to the entire Divestment Business.
(754) In this context, the Commission also notes that a large majority of respondents who expressed an opinion on this point considered Sartorius to be a suitable purchaser for the Divestment Business, if proposed by the Notifying Party. (559) Nevertheless, the assessment of the suitability of any proposed purchaser by the Commission would be a subject of a separate procedure, as envisaged under the Initial Commitments.
(755) The Commission also notes the Notifying Party's commitment not to implement the Transaction before the Commission approves the Purchaser, which further limits the risk related to not finding a suitable purchaser.
(756) In light of the foregoing, the Commission considers that there is sufficient credible interest for the acquisition of the Divestment Business by potential purchasers.
7.3.3.4. Conclusion on the Initial Commitments
(757) On the basis of the above, the Commission concludes that the Initial Commitments could generally be suitable to address the competition concerns identified by the Commission in sections 4.3-4.6. However, as explained in section 7.3.3, the Initial Commitments showed certain shortcomings which could negatively impact on the viability and competitiveness of the Divestment Business.
7.4. Final Commitments
7.4.1. Description of the Final Commitments
(758) The Final Commitments essentially follow the structure of the Initial Commitments. The Notifying Party made improvements to address the shortcomings identified during the market test. In particular, the Final Commitments include
(a) a commitment at the option of the Purchaser to transfer a specified number of sales personnel in North America, Europe and Asia should the Purchaser’s sales organisation be augmented by the transferring Microcarriers Divestment Business; the chromatography resins business; the chromatography hardware business, and the TFF Divestment Business.
(b) a commitment at the option of the Purchaser to transfer to the Purchaser a maximum of [production process] in [location], as the case may be.
(c) a commitment at the option of the Purchaser to transfer the PDS personnel, which are not included in the Microcarriers Divestment Business;
(d) as regards the transitional trade mark licence agreements for the non-transferring trademarks and logos ([…]), the Final Commitments clarify that the general term shall be […], extendable by a period of […] at the request of the Purchaser and followed by a […];
(e) a commitment at the request of the Purchaser to extend on a customer specific basis […];
(f) a commitment at the request of the Purchaser to extend on a customer specific basis […];
(g) as regards the transitional supply agreement for flow kit consumables, it has been clarified that this should be (i) at cost; or (ii) at current terms and conditions, whichever is lower.
7.4.2. Assessment of the Final Commitments
(759) The Commission considers that the Final Commitments effectively address the specific shortcomings of the Initial Commitments.
(760) The Commission notes the importance for the Purchaser to find a suitable location in […], in order to guarantee the viability and competitiveness of the Chromatography Hardware and TFF Divestment Business, and will pay particular attention in the buyer approval process to the respective planning and efforts made by the Purchaser in this regard.
(761) The Commission will also pay particular attention in the buyer approval process for the Purchaser to be able and incentivised to (i) replicate Danaher’s production of flow kits that are used with SUT TFF systems; and (ii) compete with the TFF Divestment Business in light of, inter alia, its offering of consumables.
7.4.3. Conclusion on the Final Commitments
(762) For the reasons outlined above, the Final Commitments made by the Notifying Party are sufficient to eliminate the Commission’s serious doubts, identified in sections 4.3-4.6, as to the compatibility of the Transaction with the internal market and the functioning of the EEA Agreement.
8. CONDITIONS AND OBLIGATIONS
(763) Under the first sentence of the second subparagraph of Article 6(2) of the Merger Regulation, the Commission may attach to its decision conditions and obligations intended to ensure that the undertakings concerned comply with the commitments they have entered vis-à-vis the Commission with a view to rendering the concentration compatible with the internal market
(764) The fulfilment of the measures that gives rise to the structural change of the market is a condition, whereas the implementing steps that are necessary to achieve this result are generally obligations on the parties. Where a condition is not fulfilled, the Commission's decision declaring the concentration compatible with the internal market is no longer applicable. Where the undertakings concerned commit a breach of an obligation, the Commission may revoke the clearance decision in accordance with Article 6(3) of the Merger Regulation. The undertakings concerned may also be subject to fines and periodic penalty payments under Articles 14(2) and 15(1) of the Merger Regulation.
(765) In accordance with the basic distinction between conditions and obligations described in the preceding paragraph, the commitments in section B, and the respective Schedules 1 to 5 and their annexes of the Annex constitute conditions attached to this decision, as only through full compliance therewith can the structural changes in the relevant markets be achieved. The other commitments set out in the Annex constitute obligations, as they concern the implementing steps which are necessary to achieve the modifications sought in a manner compatible with the internal market and the functioning of the EEA Agreement.
9. CONCLUSION
(766) For the above reasons, the Commission has decided not to oppose the notified operation as modified by the commitments and to declare it compatible with the internal market and with the functioning of the EEA Agreement, subject to full compliance with the conditions in section B, and the respective Schedules 1 to 5 and their annexes of the commitments annexed to this decision and with the obligations contained in the other sections of the said commitments. This decision is adopted in application of Article 6(1)(b) in conjunction with Article 6(2) of the Merger Regulation and Article 57 of the EEA Agreement.
10 December 2019
By hand, email and fax: 00 32 2 296 4301 European Commission
DG Competition Place Madou 1
1210 Saint-Josse-ten-Noode, Belgium
Case M. 9331 – Danaher / GE Biopharma
COMMITMENTS TO THE EUROPEAN COMMISSION
Pursuant to Article 6(2) of Council Regulation (EC) No 139/2004 as amended (the “Merger Regulation”), Danaher Corporation (“Danaher”) proposes the following Commitments (the “Commitments”) vis-à-vis the European Commission (the “Commission”) with a view to rendering the acquisition of sole control by Danaher over the General Electric Company’s (“GE’s”) Healthcare Life Sciences Biopharma Business (“GE Biopharma”) (GE, together with Danaher, referred to as the “Parties”) by means of an acquisition of equity and assets (the “Concentration”) compatible with the internal market and the functioning of the EEA Agreement.
This text shall be interpreted in light of the Commission’s decision pursuant to Article 6(1)(b) of the Merger Regulation to declare the Concentration compatible with the internal market and the functioning of the EEA Agreement (the “Decision”), in the general framework of European Union law, in particular in light of the Merger Regulation, and by reference to the Commission Notice on remedies acceptable under Council Regulation (EC) No 139/2004 and under Commission Regulation (EC) No 802/2004 (the “Remedies Notice”).
The Commitments shall take effect upon the date of adoption of the Decision (the “Effective Date”).
Section A. Definitions
1. For the purpose of the Commitments, the following terms shall have the followingmeaning:
Affiliated Undertakings: undertakings controlled by the Notifying Party, whereby the notion of control shall be interpreted pursuant to Article 3 of the Merger Regulation and in light of the Commission Consolidated Jurisdictional Notice under Council Regulation (EC) No 139/2004 on the control of concentrations between undertakings (the "Consolidated Jurisdictional Notice").
Assets: the assets that contribute to the current operation or are necessary to ensure the viability and competitiveness of the Divestment Businesses as indicated in Section B and described more in detail in the Schedules and Annexes.
Best Efforts: Best effort obligations shall be interpreted solely in light of the Commission’s decision pursuant to Article 6(1)(b) of the Merger Regulation to declare the Concentration compatible with the internal market and the functioning of the EEA Agreement, the Merger Regulation and the general principles of EU law.
Closing: the transfer of the legal title and/or assets of the Divestment Businesses to the Purchaser.
Closing Period: the period of […] from the approval of the Purchaser and the terms of sale by the Commission.
Confidential Information: any business secrets, know-how, commercial information, or any other information of a proprietary nature that is not in the public domain.
Conflict of Interest: any conflict of interest that impairs the Trustee’s objectivity and independence in discharging its duties under the Commitments.
Divestment Businesses: the businesses as defined in Section B and in the Schedules that the Notifying Party commit to divest.
Divestiture Trustee: one or more natural or legal person(s) who is/are approved by the Commission and appointed by the Notifying Party and who has/have received from the Notifying Party the exclusive Trustee Mandate to sell the Divestment Businesses to a purchaser at no minimum price.
Effective Date: the date of adoption of the Commission’s 6(1)(b) decision.
First Divestiture Period: the period of […] from the Effective Date.
Hold Separate Manager: the person(s) appointed by the Notifying Party for the Divestment Businesses to manage the day-to-day business under the supervision of the Monitoring Trustee.
Key Personnel: all personnel necessary to maintain the viability and competitiveness of the Divestment Businesses, as listed in the Schedules, including the Hold Separate Manager(s).
Monitoring Trustee: one or more natural or legal person(s) who is/are approved by the Commission and appointed by the Notifying Party, and who has/have the duty to monitor the Notifying Party’s compliance with the conditions and obligations attached to the Decision.
Notifying Party: Danaher Corporation with its registered office at 2200 Pennsylvania Ave. N.W., Suite 800W, Washington, D.C., USA.
PD: Process Development
Parties: the Notifying Party and General Electric Company, with its registered office at 3135 Easton Turnpike Fairfield, Connecticut 0682, USA.
Personnel: all staff currently employed by the Divestment Businesses, including staff seconded to the Divestment Businesses, shared personnel as well as the additional personnel listed in the Schedules.
Purchaser: Sartorius AG, a stock corporation (Aktiengesellschaft) incorporated under the laws of Germany, or any other entity approved by the Commission as acquirer of Divestment Businesses in accordance with the criteria set out in paragraph 37 of these Commitments.
Purchase Agreement: the purchase agreement entered into on 18 October 2019 whereby Sartorius undertakes to acquire the Divestment Businesses (1) or any equivalent purchase agreement where an alternate Purchaser undertakes to acquire the Divestment Businesses.
Purchaser Criteria: the criteria laid down in paragraph 37 of these Commitments that the Purchaser must fulfil in order to be approved by the Commission.
PVS: Particle Validation Standards.
Schedules: the schedules to these Commitments describing more in detail the Divestment Businesses.
SSA: Supply and Service Agreement.
SUT: Single Use Technology
Trustee: the Monitoring Trustee and/or the Divestiture Trustee as the case may be. ;
Trustee Divestiture Period: the period of […] from the end of the First Divestiture Period.
TSA: Transition Services Agreement.
Section B. The commitment to divest and the Divestment Businesses
Commitment to divest
2. In order to maintain effective competition, the Notifying Party commits to divest, or procure the divestiture of the Divestment Businesses to the Purchaser by the end of the Trustee Divestiture Period as a going concern and on terms of sale approved by the Commission in accordance with the procedure described in paragraph 38 of these Commitments. To carry out the divestiture, the Notifying Party commits to find a purchaser and to enter into a final binding sale and purchase agreement for the sale of the Divestment Businesses within the First Divestiture Period. If the Notifying Party has not entered into such an agreement at the end of the First Divestiture Period, the Notifying Party shall grant the Divestiture Trustee an exclusive mandate to sell the Divestment Businesses in accordance with the procedure described in paragraph 49 of these Commitments in the Trustee Divestiture Period. The divestiture of the Divestment Businesses shall not be implemented unless and until the Commission has approved the terms of divestiture in accordance with these Commitments.
3. The proposed concentration shall not be implemented before the Notifying Party or the Divestiture Trustee has entered into a final binding sale and purchase agreement for the sale of the Divestment Business and the Commission has approved the Purchaser and the terms of sale in accordance with paragraph 38 of these Commitments.
4. The Notifying Party shall be deemed to have complied with these Commitments if
(a) by the end of the Trustee Divestiture Period, the Notifying Party or the Divestiture Trustee has entered into a final binding sale and purchase agreement for the Divestment Businesses and the Commission approves the proposed purchaser and the terms of sale as being consistent with the Commitments in accordance with the procedure described in paragraph 38 of these Commitments and
(b) the Closing of the sale of the Divestment Businesses to the Purchaser takes place within the Closing Period.
5. In order to maintain the structural effect of the Commitments, the Notifying Party shall, for a period of […] after the Effective Date, not acquire, whether directly or indirectly, the possibility of exercising influence (as defined in paragraph 42 of the Remedies Notice, footnote 3) over the whole or part of the Divestment Businesses, unless, following the submission of a reasoned request from the Notifying Party showing good cause and accompanied by a report from the Monitoring Trustee (as provided in paragraph 63 of these Commitments), the Commission finds that the structure of the market has changed to such an extent that the absence of influence over the Divestment Business or Businesses is no longer necessary to render the proposed concentration compatible with the internal market.
Structure and definition of the Divestment Businesses
6. The Divestment Businesses consist of the divestiture of five businesses:
(a) the Notifying Party’s MolDev FortéBio molecular characterization business;
(b) the Notifying Party’s Pall Biotech SoloHill microcarriers and PVS business;
(c) the Notifying Party’s Pall Biotech chromatography resins business;
(d) the Notifying Party’s Pall Biotech chromatography hardware business comprising conventional chromatography columns, conventional and single-use technology chromatography skids and BioSMB continuous chromatography Process Development and Process Scale skids; and
(e) the Notifying Party’s Pall Biotech Single-Use Tangential Flow Filtration ("SUT TFF") systems and stainless-steel Hollow-Fibre TFF (“SS HF TFF”) systems business.
a) FortéBio molecular characterisation business
7. FortéBio is a provider of analytical systems designed to accelerate biotherapeutic drug discovery and development. The business manufactures molecular characterisation instruments and consumables and provides services for those instruments. FortéBio’s main analytical technology is bio-layer interferometry (“BLI”), which is used in its Octet and BLItz product lines. The company also offers instruments based on surface plasmon resonance (“SPR”) technology, which is used in its Pioneer product line. Molecular characterisation consumables include potentiometric sensors and reagents used with molecular characterisation instruments.
8. The legal and functional structure of this business as operated to date is described in Schedule 1. The FortéBio molecular characterisation business, described in more detail in Schedule 1 and Annexes, includes all assets and staff that contribute to the current operation or are necessary to ensure the viability and competitiveness of the business, in particular:
(a) the legal entity […]
(b) all tangible and intangible assets (including intellectual property rights);
(c) all licences, permits and authorisations issued by any governmental organisation for the benefit of the FortéBio molecular characterisation business;
(d) all contracts, leases, commitments and customer orders of the FortéBio molecular characterisation business; all customer, credit and other records; and
(e) the Personnel to the extent described in Schedule 1.
9. In addition, the FortéBio molecular characterisation business includes the benefit, for a transitional period of up to […] and on terms and conditions equivalent to those at present afforded to the business or at cost, of all current arrangements under which the Notifying Party or its Affiliated Undertakings supply products or services to the FortéBio molecular characterisation business, as detailed in Schedule 1 and relevant Annexes. The Commission may, in response to a reasoned request from the Purchaser showing good cause, extend the duration of the transitional period by up to […]. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time send a non-confidential copy of the report to the Notifying Party.
10. Strict firewall procedures (e.g. IT segregation) will be adopted so as to ensure that any competitively sensitive information relating to, or arising from such abovementioned arrangements (for example, product roadmaps) will not be shared with, or passed on to, anyone outside of the divestment business’ operations, beyond what is reasonably required for the compliance with the obligations relating to the SSAs and/or TSAs.
b) SoloHill microcarriers and PVS business
11. The Notifying Party manufactures and sells microcarriers. The business operates out of […] (SoloHill), located in Ann Arbor, Michigan.
12. Microcarriers are consumables used in cell culture bioprocessing. They provide a surface for the anchorage dependent cells to attach and grow in cell culture vessels and bioreactors for adherent cell culture. SoloHill manufactures and sells different types of microcarriers (e.g., plastic, collagen-coated, Fact III, Plastic Plus, Star-Plus and Hillex) used in various cell culture applications, vaccine, rProtein and stem cell production.
13. SoloHill also offers PVS, which includes kits and support for biopharmaceutical companies to identify contaminants during bioprocessing and compare the level of contamination against manufacturing standards
14. The legal and functional structure of this business as operated to date is described in Schedule 2. The SoloHill microcarriers and PVS business, described in more detail in Schedule 2 and Annexes, includes all assets and staff that contribute to the current operation or are necessary to ensure the viability and competitiveness of the business, in particular:
(a) all tangible and intangible assets (including intellectual property rights);
(b) all contracts, leases, commitments and customer orders of the SoloHill microcarriers and PVS business; all customer, credit and other records; and
(c) the Personnel as described in Schedule 2.
15. In addition, the SoloHill microcarriers and PVS business includes the benefit, for a transitional period of up to […] and on terms and conditions equivalent to those at present afforded to the business or at cost, of all current arrangements under which Danaher or its Affiliated Undertakings supply products or services to the SoloHill microcarriers and PVS business, as detailed in Schedule 2 and relevant Annexes. The Commission may, in response to a reasoned request from the Purchaser showing good cause, extend the duration of the transitional period by up to […]. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time send a non-confidential copy of the report to the Notifying Party.
16. Strict firewall procedures (e.g. IT segregation) will be adopted so as to ensure that any competitively sensitive information relating to, or arising from such abovementioned arrangements will not be shared with, or passed on to, anyone outside of the Divestment Business’ operations, beyond what is reasonably required for the compliance with the obligations relating to the SSAs and/or TSAs.
c) Chromatography resins business
17. The Notifying Party manufactures and sells different types of chromatography resins.
18. This includes ion exchange resins, mixed mode resins, and affinity resins excluding Protein A. The resins business operates out of a […] at Cergy, France.
19. The legal and functional structure of this business as operated to date is described in Schedule 3. The Cergy chromatography resins business, described in more detail in Schedule 3 and Annexes, includes all assets and staff that contribute to the current operation or are necessary to ensure the viability and competitiveness of the business, in particular:
(a) all tangible and intangible assets (including intellectual property rights and a dedicated site);
(b) all licences, permits and authorisations issued by any governmental organisation for the benefit of the chromatography resins business;
(c) all contracts, leases, commitments and customer orders of the chromatography resins business; all customer, credit and other records; and
(d) the Personnel as described in Schedule 3.
20. In addition, the chromatography resins business includes the benefit, for a transitional period of up to […] and on terms and conditions equivalent to those at present afforded to the business or at cost, of all current arrangements under which Danaher or its Affiliated Undertakings supply products or services to the chromatography resins business, as detailed in Schedule 3 and relevant Annexes. The Commission may, in response to a reasoned request from the Purchaser showing good cause, extend the duration of the transitional period by up to […]. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time send a non-confidential copy of the report to the Notifying Party.
21. Strict firewall procedures (e.g. IT segregation) will be adopted so as to ensure that any competitively sensitive information relating to, or arising from such abovementioned arrangements (for example, product roadmaps) will not be shared with, or passed on to, anyone outside of the divestment business’ operations, beyond what is reasonably required for the compliance with the obligations relating to the SSAs and/or TSAs.
d) Chromatography hardware business
22. The Notifying Party’s chromatography hardware business comprises conventional chromatography columns, conventional (stainless steel) and SUT chromatography skids, and BioSMB continuous chromatography skids (the BioSMB skids include a process development offering known as BioSMB PD and two process scale offerings known as BioSMB Process 80 and 350). [Business’ future locations and business relationships].
23. The functional structure of this business is described in Schedule 4. The chromatography hardware business, described in more detail in Schedule 4 and Annexes, includes all assets and staff that are necessary to ensure the viability and competitiveness of the business, in particular:
(a) all tangible and intangible assets (including intellectual property rights);
(b) all licences, permits and authorisations issued by any governmental organisation for the benefit of the hardware chromatography business;
(c) all contracts, leases, commitments and customer orders of the hardware chromatography business; all customer, credit and other records; and
(d) the Personnel, and notably the engineers, as described in Schedule 4 and Annex 21 on the Chromatography Hardware Divestment Business Personnel.
24. In addition, the chromatography hardware business includes the benefit, for a transitional period of up to […] and on terms and conditions equivalent to those at present afforded to the business or at cost, of all current arrangements under which Danaher or its Affiliated Undertakings supply products or services to the chromatography hardware business, as detailed in Schedule 4 and relevant Annexes. The Commission may, in response to a reasoned request from the Purchaser showing good cause, extend the duration of the transitional period by up to […]. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time send a non-confidential copy of the report to the Notifying Party.
25. Strict firewall procedures (e.g. IT segregation) will be adopted so as to ensure that any competitively sensitive information relating to, or arising from such abovementioned arrangements (for example, product roadmaps) will not be shared with, or passed on to, anyone outside of the divestment business’ operations, beyond what is reasonably required for the compliance with the obligations relating to the SSAs and/or TSAs.
e) SUT TFF and SS HF TFF systems businesses
26. The Notifying Party’s SUT TFF and SS HF TFF systems businesses comprise filtration systems used to separate and purify biomolecules in bioprocessing. Danaher designs, manufactures and sells SUT TFF systems and SS HF TFF systems. Each system is tailored to specific customer needs. Danaher conducts some design, assembly, and/or testing of the divested TFF systems in facilities located in […].
27. The legal and functional structure of this business as operated to date is described in Schedule 5.
28. The Notifying Party’s SUT TFF and SS HF TFF systems businesses, described in more detail in Schedule 5 and Annexes, include all assets and staff that contribute to the current operation or are necessary to ensure the viability and competitiveness of the business, in particular:
(a) all tangible and intangible assets (including intellectual property rights);
(b) all licences, permits and authorisations issued by any governmental organisation for the benefit of the Notifying Party’s SUT TFF and SS HF TFF systems businesses;
(c) all contracts, leases, commitments and customer orders of the Notifying Party’s SUT TFF and SS HF TFF systems businesses; all customer, credit and other records; and
(d) the Personnel to the extent described in Schedule 5.
29. In addition, the Notifying Party’s SUT TFF and SS HF TFF systems businesses includes the benefit, for a transitional period of up to […] and on terms and conditions equivalent to those at present afforded to the business or at cost, of all current arrangements under which Danaher or its Affiliated Undertakings supply products or services to the Notifying Party’s SUT TFF and SS HF TFF systems businesses, as detailed in Schedule 5 and relevant Annexes. The Commission may, in response to a reasoned request from the Purchaser showing good cause, extend the duration of the transitional period by up to […]. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time send a non-confidential copy of the report to the Notifying Party.
30. Strict firewall procedures (e.g. IT segregation) will be adopted so as to ensure that any competitively sensitive information relating to, or arising from such abovementioned arrangements will not be shared with, or passed on to, anyone outside of the Divestment Business’ operations, beyond what is reasonably required for the compliance with the obligations relating to the SSAs and/or TSAs.
Section C. Related commitments
Preservation of viability, marketability and competitiveness
31. From the Effective Date until Closing, the Notifying Party shall preserve or procure the preservation of the economic viability, marketability and competitiveness of the Divestment Businesses, in accordance with good business practice, and shall minimise as far as possible any risk of loss of competitive potential of the Divestment Businesses. In particular, the Notifying Party undertakes:
(a) not to carry out any action that might have a significant adverse impact on the value, management or competitiveness of the Divestment Businesses or that might alter the nature and scope of activity, or the industrial or commercial strategy or the investment policy of the Divestment Businesses;
(b) to make available, or procure to make available, sufficient resources for the development of the Divestment Businesses, on the basis and continuation of the existing business plans;
(c) to take all reasonable steps, or procure that all reasonable steps are being taken, including appropriate incentive schemes (based on industry practice), to encourage all Key Personnel to remain with the Divestment Businesses, and not to solicit or move any Personnel to their remaining business. Where, nevertheless, individual members of the Key Personnel exceptionally leave the Divestment Businesses, the Notifying Party shall provide a reasoned proposal to replace the person or persons concerned to the Commission and the Monitoring Trustee. The Notifying Party must be able to demonstrate to the Commission that the replacement is well suited to carry out the functions exercised by those individual members of the Key Personnel. The replacement shall take place under the supervision of the Monitoring Trustee, who shall report to the Commission.
Hold-separate obligations
32. The Notifying Party commits, from the Effective Date until Closing, to procure that the Divestment Businesses are kept separate from the businesses it will be retaining, and to ensure that unless explicitly permitted under these Commitments and the relevant agreements required to ensure the viable transfer of the businesses (i.e. SSAs and TSAs), such as for instance some of the Divested Businesses’ continued reliance on the Notifying Party’s support for sales and quotations: (i) management and staff of the businesses retained by the Notifying Party have no involvement in the Divestment Businesses; (ii) the Key Personnel and Personnel of the Divestment Businesses have no involvement in any business retained by the Notifying Party and do not report to any individual outside the Divestment Businesses to the extent reasonably practicable and in any case do not report to any individual having involvement in competing retained businesses. In addition, the Notifying Party commits to take all necessary steps to ensure that its personnel involved in the transfer of the Divestment Businesses do not use any Confidential Information from the Purchaser other than information strictly required to assist in the transfer of the Divestment Businesses concerned, and that they only disclose such information to other of their personnel to the extent strictly required to assist in the transfer of the Divestment Businesses concerned.
33. Until Closing, the Notifying Party shall assist the Monitoring Trustee in ensuring that the Divestment Businesses are managed as distinct and saleable entities separate from the businesses which the Notifying Party is retaining. Immediately after the adoption of the Decision, the Notifying Party, upon consultation with the Commission and the Monitoring Trustee, shall appoint one Hold Separate Manager per divested business. The Hold Separate Managers, who shall be part of the Key Personnel, shall manage the Divestment Businesses independently and in the best interest of each business with a view to ensuring continued economic viability, marketability and competitiveness as well as independence from the businesses retained by the Notifying Party. The Hold Separate Managers shall closely cooperate with and report to the Monitoring Trustee. Any replacement of Hold Separate Managers shall be subject to the procedure laid down in paragraph 31(c) of these Commitments. The Commission may, after having heard the Notifying Party, require the Notifying Party to replace a Hold Separate Manager.
Ring-fencing
34. The Notifying Party shall, to the extent possible and subject to transitional arrangements put in place to assist in the transfer of the Divested Businesses, implement, or procure to implement, all necessary measures to ensure that they do not, from the Effective Date, obtain any Confidential Information relating to the Divestment Businesses and that any such Confidential Information obtained before the Effective Date will be eliminated and not be used by them. In particular, the participation of the Divestment Businesses in any central information technology network shall be severed to the extent possible, without compromising the viability of the Divestment Businesses. The Notifying Party may obtain or keep information relating to the Divestment Businesses which is reasonably necessary for the divestiture of the Divestment Businesses or the disclosure of which to the Notifying Party is required by law.
Non-solicitation clause
35. The Notifying Party undertakes, subject to customary limitations, not to solicit, and to procure that Affiliated Undertakings do not solicit, the Key Personnel transferred with the Divestment Businesses for a period of […].
Due diligence
36. If Sartorius is not the Purchaser and in order to enable potential alternate purchasers to carry out a reasonable due diligence of the Divestment Businesses, the Notifying Party shall, subject to customary confidentiality assurances and dependent on the stage of the divestiture process:
(a) provide to potential purchasers sufficient information as regards the Divestment Businesses;
(b) provide to potential purchasers sufficient information relating to the Personnel and allow them reasonable access to the Personnel.
Section D. The Purchaser
37. In order to be approved by the Commission, the Purchaser must fulfil the following criteria:
(a) the Purchaser shall be independent of and unconnected to the Parties and their Affiliated Undertakings (this being assessed having regard to the situation following the divestiture).
(b) the Purchaser shall have the financial resources, proven expertise and incentive to maintain and develop the Divestment Businesses as a viable and active competitive force in competition with the Notifying Party and other competitors. In particular, the Purchaser
(i) with regard to all Divestment Businesses must have proven expertise in the biotechnology process equipment and/or consumables industry,
(ii) with regard to all Divestment Businesses must have an established presence in at least one EU Member State other than the United Kingdom,
(iii) with regard to all Divestment Businesses must have at its disposal back- office support functions, such as information technology (“IT”) staff, IT operations, general back-office software (e.g., an enterprise resource planning system and payroll system), HR functions, finance, accounting, tax, and legal and compliance support, and
(iv) with regard to the SoloHill microcarriers and PVS business pursuant to Schedule 2, the Chromatography resins business pursuant to Schedule 3, the Chromatography hardware business pursuant to Schedule 4 and the SUT TFF and SS HF TFF systems businesses pursuant to Schedule 5 must have at its disposal a salesforce with experience of selling and promoting biotechnology process equipment and/or consumables in EMEA, the Americas and Asia, including China.
(c) the acquisition of the Divestment Businesses by the Purchaser must neither be likely to create, in light of the information available to the Commission, prima facie competition concerns nor give rise to a risk that the implementation of the Commitments will be delayed. In particular, the Purchaser must reasonably be expected to obtain all necessary approvals from the relevant regulatory authorities for the acquisition of the Divestment Businesses.
38. The final binding sale and Purchase Agreement (as well as ancillary agreements) relating to the divestment of the Divestment Business shall be conditional on the Commission’s approval. When the Notifying Party has reached an agreement with Sartorius or an alternate Purchaser, it shall submit a fully documented and reasoned proposal, including a copy of the final agreement(s), within two weeks to the Commission and the Monitoring Trustee. The Notifying Party must be able to demonstrate that the Purchaser fulfils the Purchaser Criteria and that the Divestment Businesses are being sold in a manner consistent with the Commission’s Decision and the Commitments. For the approval, the Commission shall verify that the purchaser fulfils the Purchaser Criteria and that the Divestment Business is being sold in a manner consistent with the Commitments including their objective to bring about a lasting structural change in the market. The Commission may approve the sale of the Divestment Businesses without one or more Assets or parts of the Personnel, or by substituting one or more Assets or parts of the Personnel with one or more different assets or different personnel, if this does not affect the viability and competitiveness of the Divestment Businesses after the sale, taking account of the proposed Purchaser.
Section E. Trustee
I. Appointment procedure
39. The Notifying Party shall appoint a Monitoring Trustee to carry out the functions specified in these Commitments for a Monitoring Trustee. The Notifying Party commits not to close the Concentration before the appointment of a Monitoring Trustee.
40. If the Notifying Party has not entered into a binding sale and purchase agreement regarding the Divestment Business one month before the end of the First Divestiture Period or if the Commission has rejected a purchaser proposed by the Notifying Party at that time or thereafter, the Notifying Party shall appoint a Divestiture Trustee. The appointment of the Divestiture Trustee shall take effect upon the commencement of the Trustee Divestiture Period.
41. The Trustee shall:
(a) at the time of appointment, be independent of the Parties and their Affiliated Undertakings;
(b) possess the necessary qualifications to carry out its mandate, for example have sufficient relevant experience as an investment banker or consultant or auditor; and
(c) neither have nor become exposed to a Conflict of Interest.
42. The Trustee shall be remunerated by the Notifying Party in a way that does not impede the independent and effective fulfilment of its mandate. In particular, where the remuneration package of a Divestiture Trustee includes a success premium linked to the final sale value of the Divestment Business, such success premium may only be earned if the divestiture takes place within the Trustee Divestiture Period.
Proposal by the Notifying Party
43. No later than two days after the Effective Date, the Notifying Party shall submit the name or names of one or more natural or legal persons whom the Notifying Party proposes to appoint as the Monitoring Trustee to the Commission for approval. No later than one month before the end of the First Divestiture Period or on request by the Commission, the Notifying Party shall submit a list of one or more persons whom the Notifying Party proposes to appoint as Divestiture Trustee to the Commission for approval. The proposal shall contain sufficient information for the Commission to verify that the person or persons proposed as Trustee fulfil the requirements set out in paragraph 40 and shall include:
(a) the full terms of the proposed mandate, which shall include all provisions necessary to enable the Trustee to fulfil its duties under these Commitments;
(b) the outline of a work plan which describes how the Trustee intends to carry out its assigned tasks;
(c) an indication whether the proposed Trustee is to act as both Monitoring Trustee and Divestiture Trustee or whether different trustees are proposed for the two functions.
Approval or rejection by the Commission
44. The Commission shall have the discretion to approve or reject the proposed Trustee(s) and to approve the proposed mandate subject to any modifications it deems necessary for the Trustee to fulfil its obligations. If only one name is approved, the Notifying Party shall appoint or cause to be appointed the person or persons concerned as Trustee, in accordance with the mandate approved by the Commission. The Trustee shall be appointed within one week of the Commission’s approval, in accordance with the mandate approved by the Commission.
New proposal by the Notifying Party
45. If all the proposed Trustees are rejected, the Notifying Party shall submit the names of at least two more natural or legal persons within one week of being informed of the rejection, in accordance with paragraphs 39 and 43 of these Commitments.
Trustee nominated by the Commission
46. If all further proposed Trustees are rejected by the Commission, the Commission shall nominate a Trustee, whom the Notifying Party shall appoint, or cause to be appointed, in accordance with a trustee mandate approved by the Commission.
II. Functions of the Trustee
47. The Trustee shall assume its specified duties and obligations in order to ensure compliance with the Commitments. The Commission may, on its own initiative or at the request of the Trustee or Notifying Party, give any orders or instructions to the Trustee in order to ensure compliance with the conditions and obligations attached to the Decision.
Duties and obligations of the Monitoring Trustee
48. The Monitoring Trustee shall:
(i) propose in its first report to the Commission a detailed work plan describing how it intends to monitor compliance with the obligations and conditions attached to the Decision.
(ii) oversee, in close co-operation with the Hold Separate Manager(s), the on-going management of the Divestment Businesses with a view to ensuring its continued economic viability, marketability and competitiveness and monitor compliance by the Notifying Party with the conditions and obligations attached to the Decision. To that end the Monitoring Trustee shall:
(a) monitor the preservation of the economic viability, marketability and competitiveness of the Divestment Businesses, and the keeping separate of the Divestment Businesses from the business retained by the Notifying Party, in accordance with paragraphs 31 and 32 of these Commitments;
(b) supervise the management of the Divestment Businesses as a distinct and saleable entity, in accordance with paragraph 33 of these Commitments;
(c) with respect to Confidential Information:
- determine all necessary measures to ensure that the Notifying Party does not after the Effective Date obtain any Confidential Information relating to the Divestment Businesses,
- in particular strive for the severing of the Divestment Businesses’ participation in a central information technology network to the extent possible, without compromising the viability of the Divestment Businesses and taking into account what is reasonably necessary to implement the TSAs and SSAs concluded with the Purchaser,
- make sure that any Confidential Information relating to the Divestment Businesses obtained by the Notifying Party before the Effective Date is eliminated and will not be used by the Notifying Party, and
- decide whether such information may be disclosed to or kept by the Notifying Party as the disclosure is reasonably necessary to allow the Notifying Party to carry out the divestiture or as the disclosure is required by law;
(d) monitor the splitting of assets and the allocation of Personnel between the Divestment Businesses and the Notifying Party or Affiliated Undertakings;
(iii) propose to the Notifying Party such measures as the Monitoring Trustee considers necessary to ensure the Notifying Party’s compliance with the conditions and obligations attached to the Decision, in particular the maintenance of the full economic viability, marketability or competitiveness of the Divestment Businesses, the holding separate of the Divestment Businesses and the non- disclosure of competitively sensitive information;
(iv) review and assess potential purchasers as well as the progress of the divestiture process and verify that, if Sartorius is not the Purchaser and dependent on the stage of the divestiture process:
(a) potential purchasers receive sufficient and correct information relating to the Divestment Business and the Personnel in particular by reviewing, if available, the data room documentation, the information memorandum and the due diligence process, and
(b) potential purchasers are granted reasonable access to the Personnel;
(v) act as a contact point for any requests by third parties, in particular, if applicable, potential purchasers, in relation to the Commitments;
(vi) provide to the Commission, sending the Notifying Party a non-confidential copy at the same time, a written report within 15 days after the end of every month that shall cover the operation and management of the Divestment Businesses as well as the splitting of assets and the allocation of Personnel so that the Commission can assess whether the business is held in a manner consistent with the Commitments and the progress of the divestiture process as well as potential purchasers;
(vii) promptly report in writing to the Commission, sending the Notifying Party a non- confidential copy at the same time, if it concludes on reasonable grounds that the Notifying Party is failing to comply with these Commitments;
(viii) within one week after receipt of the documented proposal referred to in paragraph 38 of these Commitments, submit to the Commission, sending the Notifying Party a non- confidential copy at the same time, a reasoned opinion as to the suitability and independence of the Purchaser and the viability of the Divestment Businesses after the Sale and as to whether the Divestment Businesses are sold in a manner consistent with the conditions and obligations attached to the Decision, in particular, if relevant, whether the Sale of the Divestment Business without one or more Assets or not all of the Personnel affects the viability of the Divestment Business after the sale, taking account of the Purchaser;
(ix) assume the other functions assigned to the Monitoring Trustee under the conditions and obligations attached to the Decision.
49. If the Monitoring and Divestiture Trustee are not the same legal or natural persons, the Monitoring Trustee and the Divestiture Trustee shall cooperate closely with each other during and for the purpose of the preparation of the Trustee Divestiture Period in order to facilitate each other's tasks.
Duties and obligations of the Divestiture Trustee
50. Within the Trustee Divestiture Period, the Divestiture Trustee shall sell at no minimum price the Divestment Businesses to a Purchaser, provided that the Commission has approved both the Purchaser and the final binding sale and purchase agreement (and ancillary agreements) as in line with the Commission's Decision and the Commitments in accordance with paragraphs 37 and 39 of these Commitments. The Divestiture Trustee shall include in the sale and purchase agreement (as well as in any ancillary agreements) such terms and conditions as it considers appropriate for an expedient sale in the Trustee Divestiture Period. In particular, the Divestiture Trustee may include in the sale and purchase agreement such customary representations and warranties and indemnities as are reasonably required to effect the sale. The Divestiture Trustee shall protect the legitimate financial interests of the Notifying Party, subject to the Notifying Party’s unconditional obligation to divest at no minimum price in the Trustee Divestiture Period.
51. In the Trustee Divestiture Period (or otherwise at the Commission’s request), the Divestiture Trustee shall provide the Commission with a comprehensive monthly report written in the English language on the progress of the divestiture process. Such reports shall be submitted within 15 days after the end of every month with a simultaneous copy to the Monitoring Trustee and a non-confidential copy to the Notifying Party.
III. Duties and obligations of the Parties
52. The Notifying Party shall provide and shall cause their advisors to provide the Trustee with all such co-operation, assistance and information as the Trustee may reasonably require to perform its tasks. The Trustee shall have, to the extent possible, full and complete access to any of the Notifying Party’s or the Divestment Businesses’ books, records, documents, management or other personnel, facilities, sites and technical information necessary for fulfilling its duties under the Commitments and the Notifying Party and the Divestment Businesses shall provide the Trustee upon request with copies of any document. The Notifying Party and the Divestment Businesses shall make available to the Trustee one or more offices on their premises and shall be available for meetings in order to provide the Trustee with all information necessary for the performance of its tasks.
53. The Notifying Party shall provide the Monitoring Trustee with all managerial and administrative support that it may reasonably request on behalf of the management of the Divestment Businesses. This shall include all administrative support functions relating to the Divestment Businesses which are currently carried out at headquarters level.
54. The Notifying Party shall provide and shall cause its advisors to provide the Monitoring Trustee, on request, with the information submitted to potential purchasers, in particular give the Monitoring Trustee access to the data room documentation and all other information granted to potential purchasers in the due diligence procedure. The Notifying Party shall inform the Monitoring Trustee on possible purchasers, submit lists of potential purchasers at each stage of the selection process, including the offers made by potential purchasers at those stages, and keep the Monitoring Trustee informed of all developments in the divestiture process.
55. The Notifying Party shall grant or procure Affiliated Undertakings to grant comprehensive powers of attorney, duly executed, to the Divestiture Trustee to effect the sale (including ancillary agreements), the Closing and all actions and declarations which the Divestiture Trustee considers necessary or appropriate to achieve the sale and the Closing, including the appointment of advisors to assist with the sale process. Upon request of the Divestiture Trustee, the Notifying Party shall cause the documents required for effecting the sale and the Closing to be duly executed.
56. The Notifying Party shall indemnify the Trustee and its employees and agents (each an “Indemnified Party”) and hold each Indemnified Party harmless against, and hereby agrees that an Indemnified Party shall have no liability to the Notifying Party for, any liabilities arising out of the performance of the Trustee’s duties under the Commitments, except to the extent that such liabilities result from the wilful default, recklessness, gross negligence or bad faith of the Trustee, its employees, agents oradvisors.
57. At the expense of the Notifying Party, the Trustee may appoint advisors (in particular for corporate finance or legal advice), subject to the Notifying Party’s approval (this approval not to be unreasonably withheld or delayed) if the Trustee considers the appointment of such advisors necessary or appropriate for the performance of its duties and obligations under the Mandate, provided that any fees and other expenses incurred by the Trustee are reasonable. Should the Notifying Party refuse to approve the advisors proposed by the Trustee the Commission may approve the appointment of such advisors instead, after having heard the Notifying Party. Only the Trustee shall be entitled to issue instructions to the advisors. Paragraph 54 of these Commitments shall apply mutatis mutandis. In the Trustee Divestiture Period, the Divestiture Trustee may use advisors who served the Notifying Party during the Divestiture Period if the Divestiture Trustee considers this in the best interest of an expedient sale.
58. The Notifying Party agrees that the Commission may share Confidential Information proprietary to the Notifying Party with the Trustee. The Trustee shall not disclose such information and the principles contained in Article 17 (1) and (2) of the Merger Regulation apply mutatis mutandis.
59. The Notifying Party agrees that the contact details of the Monitoring Trustee are published on the website of the Commission's Directorate-General for Competition and they shall inform interested third parties, in particular the Purchaser, of the identity and the tasks of the Monitoring Trustee.
60. For a period of 10 years from the Effective Date the Commission may request all information from the Notifying Party that is reasonably necessary to monitor the effective implementation of these Commitments.
IV. Replacement, discharge and reappointment of the Trustee
61. If the Trustee ceases to perform its functions under the Commitments or for any other good cause, including the exposure of the Trustee to a Conflict of Interest:
(a) the Commission may, after hearing the Trustee and the Notifying Party, require the Notifying Party to replace the Trustee; or
(b) the Notifying Party may, with the prior approval of the Commission, replace the Trustee.
62. If the Trustee is removed according to paragraph 59 of these Commitments, the Trustee may be required to continue in its function until a new Trustee is in place to whom the Trustee has effected a full hand over of all relevant information. The new Trustee shall be appointed in accordance with the procedure referred to in paragraphs 39-45 of these Commitments.
63. Unless removed according to paragraph 59 of these Commitments, the Trustee shall cease to act as Trustee only after the Commission has discharged it from its duties after all the Commitments with which the Trustee has been entrusted have been implemented. However, the Commission may at any time require the reappointment of the Monitoring Trustee if it subsequently appears that the relevant remedies might not have been fully and properly implemented.
Section F. The review clause
64. The Commission may extend the time periods foreseen in the Commitments in response to a request from the Notifying Party or, in appropriate cases, on its own initiative. Where the Notifying Party requests an extension of a time period, it shall submit a reasoned request to the Commission no later than one month before the expiry of that period, showing good cause. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time send a non-confidential copy of the report to the Notifying Party. Only in exceptional circumstances shall the Notifying Party be entitled to request an extension within the last month of any period.
65. The Commission may further, in response to a reasoned request from the Notifying Party showing good cause waive, modify or substitute, in exceptional circumstances, one or more of the undertakings in these Commitments. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time send a non-confidential copy of the report to the Notifying Party. The request shall not have the effect of suspending the application of the undertaking and, in particular, of suspending the expiry of any time period in which the undertaking has to be complied with.
Section G. Entry into force
66. The Commitments shall take effect upon the date of adoption of the Decision. (signed)
duly authorised for and on behalf of Danaher Corporation
Schedule 1 - The FortéBio Divestment Business
1. The FortéBio Divestment Business as operated to date is a stand-alone molecular characterisation business unit, which comprises various assets [Danaher’s legal and organisational structure] within Molecular Devices LLC (“MolDev”). MolDev is one of the eight operating companies of Danaher’s Life Sciences platform. The relevant organisational chart for the FortéBio Divestment Business is attached as Annex 1.
2. In accordance with paragraph 7 of these Commitments, the FortéBio Divestment Business includes, but is not limited to:
(a) the legal entity […];
(b) the following main tangible assets:
- furniture and fixtures […];
- equipment […];
- lab equipment […]; and
- other instruments and consumables currently used by the business: […].
(c) the following main intangible assets:
- patent portfolio […];
- all trade secrets […];
- trademarks, […];
- [other intangibles assets]. […].
More details on the FortéBio patents, patent applications, registered trademarks, common law trademarks and other intellectual property rights are contained in Annex 2 on the FortéBio Divestment Business Intellectual Property.
(d) the following main licences, permits and authorisations:
- Danaher will undertake all required preparation work to ensure post-closing business continuity (notably, [certifications]). Danaher understands that the purchaser must merely change the name on [certifications], given that the entire processes, procedures and employees will transfer with the facilities in Fremont and Shanghai, thus the certification will remain intact. Danaher commits to allow the Purchaser to rely on Pall’s existing certification under a TSA until it obtains its own as stated in Annex 3 on Potential Services to be Provided to the Divestment Businesses.
(e) the following main contracts, agreements, leases, commitments and understandings:
- Danaher commits to use its Best Efforts to obtain, if and to the extent necessary, the consent of third parties to transfer all FortéBio customer contracts, supplier agreements, and distributor agreements in their entirety, [Danaher’s business strategy].
- The main contracts are:
- the leases for two primary facilities:
o […];
o […].
- [Danaher’s business relationships].
- [Danaher’s business relationships].
- [Danaher’s supply sources]. The records of such suppliers and purchase orders will transfer to the Purchaser.
For further details please see Annex 4 on the FortéBio Divestment Business Main Suppliers.
(f) the following customer, credit and other records:
All available records of the FortéBio Divestment Business customers, credits and other records will be transferred to the Purchaser. For further details on the main customers please see Annex 5 on the FortéBio Divestment Business Main Customers.
(g) the following Personnel: […]
(h) the following Key Personnel: […]
(i) the arrangements for the supply with the following products or services by Danaher or Affiliated Undertakings for a transitional period after Closing:
Please see Annex 3 on Potential Services to be Provided to the Divestment Businesses, Annex 6 on Supply and Service Agreement and Annex 7 on Transitional Trademark License Agreement.
3. [Danaher’s intellectual property rights]. In any case Danaher will provide a licence to the Purchaser to use [Danaher’s intellectual property rights] trademarks, alone and in such combinations with other words, phrases and logos during the transition period, the main terms of which are provided in Annex 7 on Transitional Trademark License Agreement.
4. If there is any asset or personnel which is not be covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the FortéBio Divestment Business and necessary for the continued viability and competitiveness of the FortéBio Divestment Business, that asset or an adequate substitute will be offered to the Purchaser.
Schedule 2 - The Microcarriers and PVS Divestment Business
1. The Microcarriers and PVS Divestment Business operated independently until its acquisition by Pall in November 2013, roughly two years before Pall’s acquisition by Danaher. The business is currently organised as a business unit within Pall. The Microcarriers and PVS Divestment Business is housed in SoloHill Engineering, located at 4370 Varsity Drive Suite B, Ann Arbor, Michigan 48108, USA. The relevant organisational chart for the Microcarriers and PVS Divestment Business is attached as Annex 8.
2. In accordance with paragraph 11 of these Commitments, the Microcarriers and PVS Divestment Business includes, but is not limited to:
(a) the following main tangible assets:
- office furniture;
- relevant microcarriers equipment […]
- relevant PVS equipment […].
(b) the following main intangible assets:
- patents […];
- trade secrets […];
- trademarks, […]
- [intellectual property rights and timeframe]
- [other intangible assets].
More details on the Microcarriers and PVS Divestment Business patents and trademarks are contained in Annex 9 on the Microcarriers and PVS Divestment Business Intellectual Property.
(c) the following main licences, permits and authorisations:
- […] certification will need to be re-established post-closing under the new purchaser. Danaher commits that all required preparation work will be done to ensure post-closing business continuity. [Certifications]. Danaher commits to allow the Purchaser to rely on Pall’s existing certification under a TSA until it obtains its own as stated in Annex 3 on Potential Services to be Provided to the Divestment Businesses.
There are no other licences, permits or authorisations.
(d) the following main contracts, agreements, leases, commitments and understandings;
Danaher commits to use its Best Efforts to obtain, if and to the extent necessary, the consent of third parties to transfer [Danaher’s business strategy]. For further details see Annex 10 on the Microcarriers and PVS Divestment Business Main Suppliers. [Danaher’s business strategy].
For further details on the main customers please see Annex 11 on the Microcarriers and PVS Divestment Business Main Customers.
(e) the following customer, credit and other records:
All available records of the Microcarriers and PVS Divestment Business customers, credits and other records will be transferred to the Purchaser.
(f) the following Personnel:
The table below lists the employees assigned to the Microcarriers and PVS Divestment Business and their respective functions:
[…]
Upon a reasoned request by the Purchaser, […], Danaher commits to use its Best Efforts to transfer […] in […] North America, Europe and Asia, [marketing plans and sales strategy].
(g) the following Key Personnel: [Danaher’s personnel]
(h) The arrangements for the supply with the following products or services by Danaher or Affiliated Undertakings for a transitional period of […]:
Please see Annex 3 on Potential Services to be Provided to the Divestment Businesses, Annex 6 on Supply and Service Agreement and Annex 7 on Transitional Trademark License Agreement.
3. The Microcarriers and PVS Divestment Business shall not include the following:
(a) [Danaher’s organisational structure]. The Purchaser shall provide these functions, subject to certain services being provided on a transitional basis pursuant to Annex 3 on Potential Services to be Provided to the Divestment Businesses).
(b) [Danaher’s marketing plans and sales strategy]. Danaher commits to provide any necessary training for the Purchaser’s sales force in conjunction with the transferring subject-matter experts and relevant training materials to help them identify and pursue sales opportunities under a TSA [timeframe].
(c) [Danaher’s intellectual property rights and quantities produced and sold].
For the sake of completeness, the SoloHill facility also houses Process Development Services (“PDS”). PDS provides targeted development and consultation services for development of bioprocessing systems and processes as part of [Danaher’s organisations structure and the Transaction]. Upon a reasoned request from the Purchaser, [timeframe and process], showing that the remaining PDS employees currently working on the SoloHill site have skills necessary to the microcarriers business, Danaher commits to use its Best Efforts to […]. [Parties sales strategy].
Other than the exclusion of PDS from the divestiture, there is no difference between the nature and scope of the business as currently operated and the one to be divested.
4. If there is any asset or personnel which is not be covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Microcarriers and PVS Divestment Business and necessary for the continued viability and competitiveness of the Microcarriers and PVS Divestment Business, that asset or an adequate substitute will be offered to the Purchasers.
Schedule 3 - The Chromatography Resins Divestment Business
1. The Chromatography Resins Divestment Business has been operating out of [Danaher’s organisational structure] owned and operated by Pall at 48 Avenue des Genottes, 95800, Cergy, France. The relevant organisational chart for the Chromatography Resins Divestment Business is attached as Annex 12.
2. In accordance with paragraph 17 of these Commitments, the Chromatography Resins Divestment Business includes, but is not limited to:
(a) the following main tangible assets:
- […] facility at Cergy […];
- all chromatography resins manufactured in Cergy as well as additional products and associated key inputs to manufacture those products; and
- machinery, office and laboratory equipment.
(b) the following main intangible assets:
- […] patents [business know-how];
- trade secrets […]
- trademarks, […]. [Intellectual property rights].
More details on the Chromatography Resins Divestment Business patents, patent applications, registered trademarks and trademark applications as well as common law trademarks are contained in Annex 13 on the Chromatography Resins Divestment Business Intellectual Property.
(c) the following main licences, permits and authorisations:
- Danaher will undertake all required preparation work to ensure post-closing business continuity (notably, [certifications]. Danaher commits to allow the Purchaser to rely on Pall’s existing certification under a TSA until it obtains its own as stated in Annex 3 on Potential Services to be Provided to the Divestment Businesses;
- [Certifications].
(d) the following main contracts, agreements, leases, commitments and understandings;
- Danaher commits to use its Best Efforts to obtain, if and to the extent necessary, the consent of third parties to transfer all customer and supplier contracts and/or purchase orders to the Purchaser and to use its Best Efforts to ensure such consents are obtained for the main contracts before the Commission’s purchaser approval. Danaher does not anticipate any difficulty in obtaining these consents. [Danaher’s commitments and business strategy].
Please see Annex 14 on the Chromatography Resins Divestment Business Main Suppliers for further details.
(e) the following customer, credit and other records:
All available records of the Chromatography Resins Divestment Business customers, credits and other records will be transferred to the Purchaser.
For further details on the main customers please see Annex 15 on the Chromatography Resins Divestment Business Main Customers.
(f) the following Personnel:
The table below lists the transferring employee census for the Chromatography Resins Divestment Business:
[…]
Upon a reasoned request by the Purchaser […], Danaher commits to use its Best Efforts to transfer […] in […] North America, Europe and Asia, [Parties’ marketing plans and sales strategy].
(g) the following Key Personnel: [Danaher’s personnel]
(h) the arrangements for the supply with the following products or services by Danaher or Affiliated Undertakings for a transitional period of […]:
Please see Annex 3 on Potential Services to be Provided to the Divestment Businesses, Annex 6 on Supply and Service Agreement, and Annex 7 on Transitional Trademark License Agreement.
3. The Chromatography Resins Divestment Businesses shall not include the following:
a) [Danaher’s sales strategy]. Danaher commits to provide any necessary training for the Purchaser’s sales force in conjunction with the transferring subject-matter experts and relevant training materials to help them identify and pursue sales opportunities under a TSA [timeframe].
b) [Supply source]. For this reason, Danaher will not transfer this supplier contract, [supply source], and make introductions to [supply source] for the potential negotiation of a new supply agreement with the purchaser, if the Purchaser so desires (see Annex 3 on Potential Services to be Provided to the Divestment Businesses).
[Danaher’s organisational structure and production secrets].
4. If there is any asset or personnel which is not be covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Chromatography Resins Divestment Business and necessary for the continued viability and competitiveness of the Chromatography Resins Divestment Business, that asset or adequate substitute will be offered to the Purchaser.
Schedule 4 - The Chromatography Hardware Divestment Business
1. The Chromatography Hardware Divestment Business comprises conventional chromatography columns, conventional and single-use technology chromatography skids and BioSMB continuous chromatography process development (“PD”) and process scale skids. The Chromatography Hardware Divestment Business is housed in Pall. The relevant organisational charts for the Chromatography Hardware Divestment Business is attached as Annex 16.
2. Danaher commits to carry out the divestiture of the Chromatography Hardware Divestment Business [Danaher’s commitments]:
- Danaher commits to provide the Purchaser with a fully operational, competitively capable chromatography hardware business: [Danaher’s commitments and location of its facilities].
- Post-closing, [location and strategy].
3. In accordance with paragraph 22 of these Commitments, the Chromatography Hardware Divestment Business includes, but is not limited to:
(a) the following main tangible assets:
- […].
(b) the following main intangible assets:
- a patent portfolio [intellectual property rights];
- a patent portfolio [intellectual property rights];
- all trade secrets [business know-how and organisation];
- know-how [business know-how and organisation];
- any trade secrets [business know-how and organisation];
- [business know-how and organisation];
- all relevant software;
- [intellectual property rights];
- trade secrets and other IP.
Danaher commits […]. The main terms of this agreement are outlined in Annex 17 on the Intellectual Property Licence Agreement.
More details on the Chromatography Hardware Divestment Business patents, patent applications, registered trademarks and trademark applications are contained in Annex 18 on the Chromatography Hardware Divestment Business Intellectual Property.
(c) the following main licences, permits and authorisations:
- [certifications]; and
- […] certification for the existing facilities, which will need to be re-established post- closing under the Purchaser. Danaher commits to use Best Efforts to support the Purchaser in obtaining such […] certification. Similar to other transferring facilities, all required preparation work will be done to ensure post-closing business continuity. Danaher commits to allow the Purchaser to rely on Pall’s existing certifications under a TSA until it obtains its own as stated in Annex 3 on Potential Services to be Provided to the Divestment Businesses.
- [Certifications]. Danaher understands that the extension process could take [timeframe] and may require an audit of the facility. Post-closing, the Purchaser would be able to use the Pall licence and operate under Pall’s quality system under a TSA, allowing it to produce and ship units from closing [timeframe and certification].
(d) the following main contracts, agreements, leases, commitments and understandings:
- Danaher commits to use its Best Efforts to obtain, if and to the extent necessary, the consent of third parties to transfer [supply sources and business strategy];
- [supply sources and business strategy].
- [supply sources and business strategy]. Further details are contained in Annex 3 on Potential Services to be Provided to the Divestment Businesses.
- [business strategy]; and
- Danaher commits to facilitate establishment of direct commercial relationships between the supplier and the Purchaser [business strategy].
Further details are contained in Annexes 19 and 20 on the Chromatography Hardware Divestment Business Main Suppliers and Main Customers.
(e) the following customer, credit and other records:
All available records of the Chromatography Hardware Divestment Business customers, credits and other records will be transferred to the Purchaser.
(f) the following Personnel:
The list of transferring employees can be found in Annex 21 on the Chromatography Hardware Divestment Business Personnel.
Upon a reasoned request by the Purchaser, […], Danaher commits to use its Best Efforts to transfer […] in […] North America, Europe and Asia, [information on the Transaction, the Parties’ marketing plans and sales strategy].
(g) the following Key Personnel: […]
(h) the arrangements for the supply with the following products or services by Danaher or Affiliated Undertakings for a transitional period after Closing:
See Annex 3 on Potential Services to be Provided to the Divestment Businesses, Annex 6 on Supply and Service Agreement, Annex 7 on Transitional Trademark License Agreement and Annex 22 on Intellectual Property License Agreement for Business Secrets.
4. The Chromatography Hardware Divestment Business does not exclude any areas where the commitments offered differ from the nature and scope of the business as currently operated. However, the Chromatography Hardware Divestment Business does not include certain functions and/or accreditations which will be fulfilled by the purchaser:
(a) For the entire business
- [marketing plans and sales strategy]. Danaher commits to provide any necessary training for the Purchaser’s sales force in conjunction with the transferring subject- matter experts and relevant training materials to help them identify and pursue sales opportunities under a TSA [timeframe];
- [organisational structure]; and
- titles to its facilities, [organisational structure].
(b) Specific to columns
- Danaher is aware of one accreditation that likely cannot be obtained pre-closing. It is [certifications]. Danaher will use its Best Efforts to support this process and will provide the purchaser with a data extract of its current QMS to ensure that the purchaser has the required documentation to schedule an audit. Danaher does not expect any issue in obtaining a new [certifications].
(c) Specific to chromatography skids except BioSMB PD
- [Danaher’s commitments];
- The […] trademark, [intellectual property rights]. This trademark will be licensed to the Purchaser under a transitional agreement as required for the chromatography skids business. [Intellectual property rights].
(d) Specific to BioSMB PD
- [Danaher’s commitments].
- The […] trademark, [intellectual property rights]. This trademark will be licensed to the Purchaser under a transitional agreement as required for the BioSMB PD business. [Intellectual property rights].
5. If there is any asset or personnel which is not be covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Chromatography Hardware Divestment Business and necessary for the continued viability and competitiveness of the Chromatography Hardware Divestment Business, that asset or adequate substitute will be offered to the Purchaser.
Schedule 5 - The SUT TFF and SS HF TFF Systems Divestment Business
1. The Pall Biotech SUT TFF and SS HF TFF systems business comprises Pall Biotech’s Single-Use Tangential Flow Filtration (“SUT TFF”) systems and stainless-steel Hollow-Fibre TFF (“SS HF TFF”) systems business (together the “SUT TFF and SS HF TFF Systems Divestment Business”). The SUT TFF and SS HF TFF systems Divestment Business is housed in Pall. The relevant organisational chart for the SUT TFF and SS HF TFF systems Divestment Business is attached as Annex 22.
2. Danaher commits to carry out the divestiture of the SUT TFF and SS HF TFF Systems Divestment Business [Danaher’s commitments]:
- Danaher commits to provide the Purchaser with a fully operational SUT TFF and SS HF TFF systems business, preserving as much as possible the current business from a manufacturing, geographic and personnel organization of the business: [location and employees].
- Post-Closing, [location and strategy].
3. In accordance with paragraph 26of these Commitments, the SUT TFF and SS HF TFF systems Divestment Business includes, but is not limited to:
(a) the following main tangible assets:
- Equipment used [tangible assets];
- […].
(b) the following main intangible assets:
- know-how and trade secrets […];
- all relevant software;
- [intellectual property rights];
- the transitional rights to use […] trademarks, [intellectual property rights]. This right does not extend to any new TFF systems launched by the Purchaser post-Closing, nor to any new customer won by the Purchaser post-Closing; and
- [intellectual property rights].
[Intellectual property rights]. The main terms of this agreement are outlined in Annex 17 on the Intellectual Property Licence Agreement.
(c) the following main licences, permits and authorisations:
- Danaher will allow the purchaser to rely upon existing certifications such as manufacturing site registrations, product registrations, listings, licensing and/or labelling to market under a TSA.
- [Certifications] for the existing facilities will need to be re-established post-closing under the new purchaser. [Certifications]. Danaher commits to allow the Purchaser to rely on Pall’s existing certification under a TSA until it obtains its own as stated in Annex 23 on Potential Services to be Provided to the SUT TFF and SS HF TFF Divestment Businesses.
- The purchaser will need to comply with safety and environmental standards, although there is no notified body. The purchaser must issue a declaration of conformity with the standards. Because these are common requirements, the purchaser should already meet these standards. [Standards].
- [Licenses].
(d) the following main contracts, agreements, leases, commitments and understandings
- Danaher commits to use its Best Efforts to obtain, if and to the extent necessary, the consent of third parties to transfer SUT TFF and SS HF TFF systems supplier contracts and third-party patent licence agreements to the Purchaser;
- Danaher will transfer and assign all relevant software [supply sources, business relationships and business strategy];
- [Business strategy]
- Danaher commits to facilitate establishment of direct commercial relationships between the supplier and the Purchaser [business strategy].
For further details please see Annexes 24 and 25 on SUT TFF and SS HF TFF systems Main Customers and Suppliers.
(e) the following customer, credit and other records:
All available records of the SUT TFF and SS HF TFF systems Divestment Business customers, credits and other records will be transferred to the Purchaser.
(f) the following Personnel:
The list of transferring employees can be found in Annex 26 on SUT TFF and SS HF TFF systems Divestment Business’ Personnel.
Upon a reasoned request from the Purchaser, […], Danaher commits to use its Best Efforts to transfer to the Purchaser […] in […], as the case may be.
Upon a reasoned request by the Purchaser, […], Danaher commits to use its Best Efforts to transfer […] in […] North America, Europe and Asia, [information on the Transaction, Danaher’s marketing plans and sales strategy].
(g) the following Key Personnel: […]
(h) the arrangements for the supply with the following products or services by Danaher or Affiliated Undertakings for a transitional period of […]:
Please see Annex 23 on Potential Services to be Provided to the SUT TFF and SS HF TFF Divestment Businesses, Annex 6 on Supply and Service Agreement, Annex 7 on Transitional Trademark License Agreement and Annex 17 on Intellectual Property License Agreement.
4. The SUT TFF and SS HF TFF systems Divestment Business does not exclude any areas where the commitments offered differs from the nature and scope of the business as currently operated. However, the SUT TFF and SS HF TFF systems Divestment Business does not include certain functions and/or accreditations which will be fulfilled by the purchaser:
[Information on the Transaction, Danaher’s marketing plans and sales strategy]. Danaher commits to provide any necessary training for the Purchaser’s sales force and after sales service in conjunction with the transferring subject-matter experts and relevant training materials to help them identify and pursue sales opportunities under a TSA for [timeframe].
[Danaher’s commitments].
For the sake of completeness, the custom-designed conventional flat sheet (“FS”) TFF systems are not included in the divestiture for several reasons. First, Danaher understands that the Commission does not have serious doubts as to the effects of the transaction on conventional FS TFF systems; accordingly, there is no substantive justification for including these activities in the scope of the proposed remedy. Second, [business structure].
5. If there is any asset or personnel which is not be covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the SUT TFF and SS HF TFF systems Divestment Business and necessary for the continued viability and competitiveness of the Chromatography Hardware Divestment Business, that asset or adequate substitute will be offered to the Purchaser.
Annex 1 on the FortéBio Divestment Business Organisational Chart
[…]
Annex 2 on the FortéBio Divestment Business Intellectual Property
[…]
Annex 3 on Potential Services to be Provided to the Divestment Businesses
For the avoidance of doubt, Danaher commits to transitional service agreements as specified above in the main body of the Commitments for a duration of […], extendable, upon a reasoned request from the Purchaser showing good cause and approval by the Commission, for an additional […], and on terms and conditions equivalent to those at present afforded to the business or at cost, of all current arrangements under which the Notifying Party or its Affiliated Undertakings supply products or services to the Divestment Businesses, as detailed in Schedules and relevant Annexes. The Duration column in the table below lists an indicative time period at the end of which Danaher would anticipate that the Purchaser would no longer require the corresponding services.
[…]
Annex 4 on the FortéBio Divestment Business Main Suppliers
[…]
Annex 5 on the FortéBio Divestment Business Main Customers
[…]
Annex 6 on Supply and Services Agreement
[…]
Annex 7 on Transitional Trademark License Agreement
[…]
Annex 8 on the Microcarriers and PVS Divestment Business Organisational Chart
[…]
Annex 9 on the Microcarriers and PVS Divestment Business Intellectual Property
[…]
Annex 10 on the Microcarriers and PVS Divestment Business Main Suppliers
[…]
Annex 11 on the Microcarriers and PVS Divestment Business Main Customers
[…]
Annex 12 on the Chromatography Resins Divestment Business Organisational Chart
[…]
Annex 13 on the Chromatography Resins Divestment Business Intellectual Property
[…]
Annex 14 on the Chromatography Resins Divestment Business Main Suppliers
[…]
Annex 15 on the Chromatography Resins Divestment Business Main Customers
[…]
Annex 16 on the Chromatography Hardware Divestment Business Organisational Chart
[…]
Annex 17 on the Intellectual Property Licence Agreement
[…]
Annex 18 on the Chromatography Hardware Divestment Business Intellectual Property
[…]
Annex 19 on the Chromatography Hardware Divestment Business Main Suppliers
[…]
Annex 20 on the Chromatography Hardware Divestment Business Main Customers
[…]
Annex 21 on the Chromatography Hardware Divestment Business Personnel
[…]
Annex 22 on the SUT TFF and SS HF TFF Systems Divestment Business Organisational Structure
[…]
Annex 23 on Potential Services to be Provided to the SUT TFF and SS HF TFF Systems Divestment Businesses
For the avoidance of doubt, Danaher commits to transitional service agreements as specified above in the main body of the Commitments for a duration of […], extendable upon a reasoned request from the Purchaser showing good cause and approval by the Commission for an additional […], and on terms and conditions equivalent to those at present afforded to the business or at cost, of all current arrangements under which the Notifying Party or its Affiliated Undertakings supply products or services to the Divestment Businesses, as detailed in Schedules and relevant Annexes. The Duration column in the table below lists an indicative time period at the end of which Danaher would anticipate that the Purchaser would no longer require the corresponding services.
[…]
Annex 24 on SUT TFF and SS HF TFF systems Main Suppliers
[…]
Annex 25 on SUT TFF and SS HF TFF systems Main Customers
[…]
Annex 26 on SUT TFF and SS HF TFF systems Divestment Business Personnel
[…]
1 OJ L 24, 29.1.2004, p. 1 (the ‘Merger Regulation’). With effect from 1 December 2009, the Treaty on the Functioning of the European Union (‘TFEU’) has introduced certain changes, such as the replacement of ‘Community’ by ‘Union’ and ‘common market’ by ‘internal market’. The terminology of the TFEU will be used throughout this decision.
2 OJ L 1, 3.1.1994, p. 3 (the ‘EEA Agreement’).
3 Publication in the Official Journal of the European Union No C 375, 6.11.2019, p. 28.
4 The entities within GE Biopharma that are active in the affected markets in the EEA are: [...].
5 See the market report “Global Biologics Market Size, Market Share, Application Analysis, Regional Outlook, Growth Trends, Key Players, Competitive Strategies and Forecasts, 2018 to 2026”, April 2018, Acute Market Reports.
6 See the market report “Bioprocessing Technologies 2018, Market Analysis & Perspectives”, page 9, September 2018, SDi.
7 Form CO, paragraph 84.
8 Form CO, paragraph 85.
9 Questionnaire Q1, questions B.8 and B.9.
10 Form CO, paragraph 91.
11 Form CO, paragraphs 87 and 88.
12 In Commission decision of 23 August 2017 in Case No COMP/M.8541 – Thermo Fisher Scientific/Patheon, the Commission considered a differentiation according to functions into bioreactors, bags, mixers, transfer assembles and other SUT products, but ultimately left open the exact product market definition.
13 Such as temperature, pH, oxygen, nutrient concentrations and removal of metabolic waste products.
14 Questionnaire Q1, question E.B.2 and questionnaire Q3, question E.B.2.
15 Questionnaire Q1, question E.B.2.1 and questionnaire Q3, question E.B.2.1.
16 Form CO, paragraphs 809-810 and 816.
17 Form CO, paragraph 823.
18 Form CO, paragraph 820.
19 Form CO, paragraph 829.
20 Form CO, paragraph 831.
21 Form CO, paragraph 830.
22 Form CO, paragraph 833.
23 Form CO, paragraph 863.
24 Form CO, paragraphs 811-814.
25 Form CO, paragraph 815.
26 Form CO, paragraph 876.
27 Form CO, paragraph 877.
28 Commission decision of 23 August 2017 in Case No COMP/M.8541 – Thermo Fisher Scientific/Patheon, recitals 21 and 23.
29 Questionnaire Q1, question E.B.A.1.
30 Questionnaire Q1, question E.B.A.1.
31 Minutes of a call with a customer of 17 May 2019.
32 Questionnaire Q1, question E.B.A.1.
33 Questionnaire Q3, question E.B.A.1.
34 Questionnaire Q1, question E.B.A.2.
35 Minutes of a call with a customer of 17 May 2019.
36 Form CO, tables 33 and 34.
37 Questionnaire Q1, question E.B.A.4.
38 Questionnaire Q3, question E.B.A.4.
39 Form CO, paragraph 881.
40 Form CO, paragraph 883.
41 Questionnaires Q1 and Q3, question E.C.A.1.
42 The Parties are only active in SUT (non-conventional) bioreactors.
43 Questionnaire Q1, question E.D.A.1.
44 Questionnaire Q3, question E.D.A.1.
45 Questionnaires Q1 and Q3, question E.D.A.2.
46 Form CO, paragraph 918.
47 Questionnaire Q1, question E.D.A.2.1.
48 Questionnaire Q3, question E.D.A.2.1.
49 Questionnaires Q1 and Q3, question E.D.A.4.
50 Questionnaire Q1, question E.D.A.1.
51 Questionnaire Q3, question E.D.A.1.
52 Questionnaire Q1, question E.D.A.2.
53 Questionnaire Q1 and Q3, question E.D.A.2.
54 Questionnaires Q1 and Q3, question E.D.A.5.
55 RFI 8, question 7, annex DHR 566.
56 DHR 138.
57 RFI 5, question 25.
58 RFI 7, question 25.
59 RFI 7, question 25.
60 Form CO, paragraph 1325 and table 77.
61 Questionnaire Q1, question I.9.
62 Questionnaire Q1, question I.9.
63 Commission decision of 23 August 2017 in Case No COMP/M.8541 – Thermo Fisher Scientific/Patheon, recital 21.
64 Form CO, paragraph 766.
65 Form CO, paragraph 767.
66 Form CO, paragraph 768.
67 Form CO, paragraphs 872 and 875.
68 Form CO, paragraph 876.
69 Form CO, paragraph 877.
70 Form CO, paragraph 873.
71 Form CO, paragraph 874.
72 Commission decision of 23 August 2017 in Case No COMP/M.8541 – Thermo Fisher Scientific/Patheon, recitals 21 and 23.
73 Questionnaires Q1 and Q3, question E.B.B.2.
74 Questionnaire Q1, question E.B.B.1.
75 Questionnaire Q3, question E.B.B.1.
76 Form CO, paragraph 881.
77 Form CO, paragraph 883.
78 Questionnaires Q1 and Q3, question E.C.B.1.
79 Questionnaires Q1 and Q3, question E.D.B.1.
80 Questionnaires Q1 and Q3, question E.D.B.2.
81 Questionnaires Q1 and Q3, question E.D.B.4.
82 Form CO, paragraphs 938 and 939.
83 Form CO, paragraph 939.
84 Form CO, paragraph 939.
85 Commission Guidelines on the assessment of non-horizontal mergers under the Council Regulation on the control of concentrations between undertakings, OJ C 265, 18.10.2008, p. 6, paragraph 32.
86 Form CO, paragraph 942.
87 Form CO, paragraphs 941 and 946.
88 Questionnaire Q3, question E.D.B.6.
89 Questionnaire Q3, questions E.D.B.7 and E.D.B.8.
90 Form CO, paragraph 946.
91 Questionnaire Q3, question E.D.B.9.1.
92 Questionnaire Q3, question E.D.B.9.1.
93 Form CO, paragraph 939.
94 SUT connectors may be gendered or genderless. Gendered connectors are provided as two different components (a male and a female component), which form a sterile connection when fitted together. In genderless connectors, a single component design fits together to form the sterile connection.
95 Form CO, paragraph 877.
96 Aseptic connectors provide aseptic connections for non-aseptic environments. Sterile connectors constitute a niche market within aseptic connectors, as they are certified for sterile connections and have undergone additional validation that aseptic connections do not have.
97 Form CO, paragraph 878.
98 Quick connectors allow for the connection and disconnection of fluid paths. As opposed to aseptic and sterile connectors, quick connectors do not allow customers to maintain the same cleanliness in their connections.
99 Form CO, paragraph 879.
100 Form CO, paragraph 880.
101 Commission decision of 23 August 2017 in Case No COMP/M.8541 – Thermo Fisher Scientific/Patheon, recitals 21 and 23.
102 Questionnaire Q1, question E.B.C.1.
103 Questionnaire Q3, question E.B.C.1.
104 RFI 16, question 2.
105 Form CO, paragraph 881.
106 Form CO, paragraph 883.
107 Questionnaires Q1 and Q3, question E.C.C.1.
108 Form CO, annex DHR 148 “Baseball ABC 20181007”, slide 13.
109 Form CO, paragraph 956.
110 Questionnaire Q3, question E.D.C.1.
111 The Commission requested the Notifying Party’s competitors in SUT connectors to provide (i) their total worldwide sales (in thousand USD) of overall SUT connectors in 2018; (ii) their total worldwide sales (in thousand USD) of sterile-only connectors in 2018; and (iii) an explanation as to whether the figures provided were representative of the competitor’s total worldwide sales of SUT connectors in 2016 and 2018. For the purpose of this request, the Commission followed a methodology consistent with the Parties’ approach. In particular, the Commission identified “overall SUT connectors” as comprising
(i) both sterile and aseptic SUT connectors; (ii) both genderless and gendered SUT connectors; and
(iii) exclusively stand-alone sales (that is excluding aseptic SUT connectors integrated into hardware, such as bioreactor or mixer systems).
112 Questionnaire Q1, question E.D.C.4.
113 Questionnaire Q1, question E.D.C.4.
114 Questionnaire Q3, question E.D.C.4.
115 Questionnaires Q1 and Q3, question E.D.C.2.
116 RFI 16, question 1.a.
117 RFI 16, question 1.b.
118 RFI 16, question 1.c.
119 RFI 16, question 1.e (non-confidential quotes).
120 RFI 16, question 1.e.
121 Form CO, paragraphs 962 and 963 and Notifying Party’s letter of 20 November 2019. According to the figures provided by the Notifying Party, […].
122 RFI 16, question 2.
123 Notifying Party’s letter of 20 November 2019.
124 Danaher’s bidding data (M.9331 – Danaher GE Biopharma – SUT Connectors Win Loss Analysis, Doc ID277).
125 Commission decision of 23 August 2017 in Case No COMP/M.8541 – Thermo Fisher Scientific/Patheon, recitals 12-16; Commission decision of 26 November 2013 in Case No COMP/M.6944 – Thermo Fisher Scientific/Life Technologies, recital 51; Commission decision of 15 June 2015 in Case No COMP/M.7435
– Merck/Sigma-Aldrich, recitals 48-51.
126 Commission decision of 23 August 2017 in Case No COMP/M.8541 – Thermo Fisher Scientific/Patheon, recitals 12-16; Commission decision of 26 November 2013 in Case No COMP/M.6944 – Thermo Fisher Scientific/Life Technologies, recital 51; Commission decision of 15 June 2015 in Case No COMP/M.7435
– Merck/Sigma-Aldrich, recitals 48-51.
127 Questionnaire Q1, question C.B.1 and questionnaire Q3, question C.B.1.
128 There are separate product markets for (i) overall cell culture sera; (ii) bioproduction customers and research customers; (iii) types of sera from different animals (FBS, calf, bovine, etc.); and (iv) geographic origins.
129 Commission decision of 26 November 2013 in Case No COMP/M.6944 – Thermo Fisher Scientific/Life Technologies, recitals 52-54.
130 Questionnaire Q1, question C.C.1 and questionnaire Q3, question C.C.1.
131 Cell culture media and other supplements, buffers and process liquids will not be further assessed in this chapter as the proposed transaction does not lead to affected markets, under any possible market definition.
132 Questionnaire Q1, question C.D.1 and questionnaire Q3, question C.D.1.
133 Form CO, paragraph 487.
134 Form CO, paragraph 489.
135 Form CO, paragraph 491.
136 Questionnaire Q1, question D.B.1.
137 Questionnaire Q3, question D.B.1.
138 Questionnaire Q1, question D.B.2.
139 Questionnaire Q3, question D.B.2.
140 Questionnaires Q1 and Q3, question D.B.3.
141 Form CO, paragraphs 505 to 507.
142 Questionnaire Q1, question D.C.1.
143 Questionnaire Q3, question D.C.1.
144 Form CO, paragraph 594.
145 Form CO, paragraph 596.
146 Form CO, paragraph 597.
147 Form CO, paragraph 615.
148 Form CO, paragraphs 596 and 608.
149 The Notifying Party could only provide a rough estimate of the Parties’ market shares estimates for these segments/markets.
150 Questionnaires Q1 and Q3, question D.D.1.
151 […].
152 Questionnaire Q1, question D.D.2.
153 Questionnaires Q1 and Q3, question D.D.2.
154 Questionnaires Q1 and Q3, question D.D.3.
155 Questionnaire Q3, question D.D.3.
156 Questionnaires Q1 and Q3, question D.D.4.
157 Questionnaire Q3, question D.D.4.
158 Form CO, paragraphs 1572-1583.
159 Form CO, figure 35.
160 Form CO, figure 46.
161 Form CO, figure 47.
162 Form CO, tables 67-69.
163 Form CO, figure 33.
164 Form CO, figure 40.
165 Questionnaire Q1, question B.9.
166 In addition, a majority of customers seem to decide upon their choice of TFF skid at the clinical stage or later, while a majority of customers seem to decide upon their choice of TFF consumables before the clinical stage. Questionnaire Q1, question B.7).
167 Form CO, annex 57.
168 Form CO, figure 41.
169 Form CO, tables 71, 72 and 73.
170 Questionnaire Q1, questions G.B.1 and G.B.1.1.
171 Questionnaire Q1, questions G.B.2 and G.B.2.1.
172 Questionnaire Q3, question G.B.13.
173 Form CO, paragraph 1292.
174 Form CO, paragraphs 1211-1213.
175 Though the particular example given by the Notifying Party appears to only refer to a SUT system (Form CO, paragraph 1245).
176 Commission Notice on the definition of relevant market for the purposes of Community competition law, OJ C 372, 9.12.1997, p. 5-13, paragraph 20.
177 Pursuant to the Notifying Party, GE’s Äkta readyflux skid constitutes a notable exception in as far as it can be used with both hollow fibre and flat sheet consumables, and to some extent with DFF consumables when used with supporting flow kits.
178 Questionnaire Q1, question G.B.6.
179 “Turnkey systems for tangential flow filtration (TFF), single-use hollow fiber filters and flat sheet cassettes enable process and cost efficiencies” (Repligen), https://www.repligen.com/technologies/tff and “[w]e manufacture TFF systems using either flat sheet cassettes or hollow fiber (HF) modules”, https://www.ymcpt.com/resource-library/tff-uf-mf-membrane-systems-overview; Doc ID3121 and ID3122.
180 https://www.abec.com/process-engineering-equipment/; https://www.abec.com/single-use/; https://www.zeta.com/en/downstream-systems_62 htm; https://www.zeta.com/en/tff-skid_20_d_184 htm; http://cotterbrothers.com/products/.
181 https://www repligen.com/technologies/krosflo-tff/lab/kr2i; https://www repligen.com/technologies/krosflo-tff-1/systems/kmpi.
182 Questionnaire Q3, question G.B.6.
183 Questionnaire Q3, question G.B.6.1.
184 Questionnaire Q1, question G.B.9.
185 Questionnaire Q1, question G.B.9.1.
186 Questionnaire Q3, question G.B.11.
187 Questionnaire Q3, question G.B.11.1.
188 Questionnaire Q3, question G.B.9.1.
189 Form CO, paragraph 1265.
190 Questionnaire Q1, question G.B.10.2.
191 Questionnaire Q3, question G.B.10.2.
192 Courtesy translation: “the absence of supply of consumables (filtering media) is an obstacle because the filtering media supplier can influence the client by conditioning its support and warranties to the choice of its own skid and of its filter holders”.
193 Courtesy translation: “achieving a level of reputation is also very difficult if you don't supply such consumables”.
194 Questionnaire Q3, question G.B.12.2.
195 Questionnaire Q3, question G.B.7.1.
196 Questionnaire Q3, question G.B.12.3.
197 Questionnaire Q3, question G.B.13
198 Questionnaire Q3, question G.B.15.
199 Courtesy translation: it would take us about one or two years to offer a SUT filtration skid.
200 Questionnaire Q3, question G.B.15.1. Courtesy translation of the last quote: “[w]e would need 1 to 2 years to supply a single use skid for filtration”.
201 Questionnaire Q3, question G.B.14.
202 Questionnaire Q3, question G.B.14.1.
203 Form CO, table 105.
204 The Commission did not further look into equipment due to GE’s minimal footprint in this area.
205 Form CO, paragraphs 1529-1532.
206 Questionnaire Q1, question G.C.1.
207 Questionnaire Q1, question G.C.2.
208 Questionnaire Q3, question G. C.1.
209 Form CO, paragraph 1153.
210 Form CO, paragraph 1153 and RFI 5, question Q1.
211 […].
212 Questionnaire Q1, question G.B.3.
213 Questionnaire Q1, question G.B.4.
214 Questionnaire Q3, question G.B.4.
215 Questionnaire Q1, question G.B.5.
216 Questionnaire Q3, question G.D.7.
217 Questionnaire Q1, questions G.D.9.2 and G.D.9.3.
218 Questionnaire Q3, questions G.D.12.2 and G.D.12.3.
219 Form CO, paragraph 1153.
220 RFI 5, question 1, update of 10 October 2019.
221 RFI 5, question 1, update of 10 October 2019.
222 Questionnaire Q3, question G.D.3 and RFI to a competitor dated 18 November 2019.
223 Doc ID17.
224 Form CO, paragraph 1314.
225 Parties’ updated response to RFI 5 question 1, annex 1.
226 Questionnaire Q3, question G.D.3; RFIs to competitors dated 14 November, 15 November, 18 November, 21 November, 25 November, 26 November and 2 December.
227 In the segment of flat sheet TFF systems (conventional), the Parties’ low market share was confirmed. In the segment for hollow fibre systems (SUT), there would in any event […] be an […] as [Danaher’s presence].
228 Form CO, paragraph 1383.
229 Form CO, paragraph 1398.
230 Form CO, paragraph 1265.
231 Form CO, paragraph 1172.
232 Form CO, paragraph 1265.
233 Questionnaire Q1, question G.B.10.2.
234 Questionnaire Q3, question G.B.10.2.
235 Questionnaire Q1, question G.D.3.
236 See further below in the next section.
237 Form CO, paragraph 1328.
238 Questionnaire Q1, question G.D.1.
239 Questionnaire Q1, question G.D.2, and follow-up RFI to competitors of 11 and 13 November 2019.
240 Questionnaire Q1, question G.D.4.
241 Questionnaire Q3, question G.D.5.
242 […].
243 http://www.snl.com/Cache/1500119405.PDF?O=PDF&T=&Y=&D=&FID=1500119405&iid=4325751; Doc ID3123.
244 Form CO, paragraph 1358.
245 Questionnaire Q1, question B2.
246 Questionnaire Q1, question G.D.8.2.
247 Questionnaire Q3, questions G.D.11.2 and G.D.11.3.
248 Doc ID859.
249 Doc ID859.
250 Letter from the Notifying Party to the FTC dated 9 October 2019.
251 Questionnaire Q1, question G.D.8.4 and questionnaire Q3, question G.D.11.4.
252 Doc ID2562.
253 Questionnaire Q3, question G.D.3; RFIs to competitors dated 14 November, 15 November, 18 November, 21 November, 25 November, 26 November and 2 December.
254 In the segment of flat sheet TFF systems (conventional), the Parties’ low market share was confirmed. In the segment for hollow fibre systems (SUT), there would in any event […] be an […] as [Danaher’s presence].
255 Questionnaire Q3, response G.D.3; RFIs to competitors dated 14 November, 15 November, 18 November, 21 November, 25 November, 26 November and 2 December.
256 In the segment of flat sheet TFF systems (conventional), the Parties’ low market share was confirmed. In the segment for hollow fibre systems (SUT), there would in any event […] be an […] as [Danaher’s presence].
257 RFI to a competitor dated 13 November 2019.
258 Commission decision of 13 May 2011 in Case No COMP/M.6126 – Thermo Fisher Scientific/Dionex Corporation.
259 Form CO, paragraph 1783 to 1786.
260 Questionnaire Q1, question F.B.1.
261 Questionnaire Q1, question F.B.1.
262 Form CO, paragraphs 17514-1754.
263 Questionnaire Q1, question F.B.3.
264 Form CO, paragraphs 1736 and 1737.
265 Form CO, paragraphs 1740-1745.
266 The Parties’ activities do not overlaps as regards two different types of resins, namely size-exclusion resins and hydrophobic interaction resins as Danaher does not supply these resins.
267 Questionnaire Q1, question F.B.2.
268 Form CO, paragraphs 1806-1808.
269 Questionnaire Q1, question F.B.5.1.
270 Form CO, paragraphs 1812-1815.
271 Questionnaire Q1, question F.C.1.
272 […].
273 Questionnaire Q1, question F.D.A.1.
274 Questionnaire Q1, question F.D.A.2.
275 Questionnaire Q1, question F.D.A.2.
276 Questionnaire Q1, question F.D.A.3.
277 Questionnaire Q1, question F.D.A.4.
278 Questionnaire Q1, question F.D.A.5.
279 Questionnaire Q1, question F.D.E.2.
280 Questionnaire Q1, question F.D.E.2.
281 Questionnaire Q1, question F.D.E.2.
282 Questionnaire Q1, question F.D.E.3.
283 Questionnaire Q1, question F.D.E.4.
284 Questionnaire Q1, question F.D.B.2.
285 Questionnaire Q1, question F.D.B.3.
286 Questionnaire Q1, question F.D.B.4.
287 DHR 66.
288 DHR 57.
289 Questionnaire Q1, question F.D.C.2.
290 Form CO, paragraph 1880.
291 […].
292 Questionnaire Q1, question F.D.D.4.
293 Form CO, paragraphs 3593, 3616 and 3705-3707.
294 Form CO, paragraphs 3472 and 3591.
295 Questionnaire Q1, question H.3 and questionnaire Q3, question H.1.
296 See for instance GE 285, GE 290, DHR 443 and DHR 555 to DHR 558.
297 Form CO, chapter 9, B.I.
298 RFI 13, question 1.
299 Form CO, chapter 9, B.I. and submission on integrated solutions of 22 November 2019.
300 RFI 13, question 1.
301 Form CO, chapter 9, B.III.
302 Form CO, chapter 9, A.II.
303 RFI of 22 November 2019, annex 1.
304 Form CO, chapter 9, A.II.4.
305 Questionnaire Q1, question H.5.1; questionnaire Q3, question H.3.1; GE 285; DHR 443.
306 GE 285, page 68.
307 GE 400.
308 Form CO, paragraphs 3603 and 3634.
309 Questionnaire Q1, question H.5.2 and questionnaire Q3, question H.3.2.
310 Questionnaire Q1, question H.10.
311 Questionnaire Q1, question H.10 and questionnaire Q3, question H.9.
312 Questionnaire Q1, question H.8 and questionnaire Q3, question H.7.
313 Questionnaire Q1, question H.9 and questionnaire Q3, question H.8.
314 Questionnaire Q1, questions J.1, J.2, and J.3 and questionnaire Q3, question J.2.
315 Commission Guidelines on the assessment of non-horizontal mergers under the Council Regulation on the control of concentrations between undertakings, OJ C 265, 18.10.2008, p. 6.
316 Non-Horizontal Merger Guidelines, paragraph 92.
317 Non-Horizontal Merger Guidelines, paragraph 93.
318 Non-Horizontal Merger Guidelines, paragraph 93.
319 Non-Horizontal Merger Guidelines, paragraphs 91 and 93.
320 Non-Horizontal Merger Guidelines, paragraph 99.
321 Non-Horizontal Merger Guidelines, paragraph 94.
322 Doc ID474.
323 Report entitled “Analysis of the incentives of the merged entity to bundle” (Doc ID2571), paragraph 1.2.
324 Report entitled “Analysis of the incentives of the merged entity to bundle” (Doc ID2571), paragraph 1.3.
325 Oxera report (Doc ID2571), paragraph 1.7 as well as sections 2 and 3.
326 Form CO, chapter 10.
327 Form CO, paragraphs 3739 and 3740.
328 Form CO, paragraph 3772.
329 Form CO, paragraph 3774.
330 Form CO, paragraph 3779.
331 Form CO, paragraph 3778.
332 Questionnaire Q1, questions B12 and B13.
333 Form CO, table 264.
334 Form CO, tables 260 and 261.
335 Questionnaire Q1, question B.4.
336 Questionnaire Q1, question B.5.
337 Questionnaire Q1, question B.6.
338 Form CO paragraphs 3824-3825 and table 267: [Danaher’s sales strategy and customer preferences].
339 Questionnaire Q1, question B.1 and questionnaire Q3, question B.1.
340 Questionnaire Q1, question B.3 and questionnaire Q3, question B.3.
341 Questionnaire Q1, question B.9.1
342 Questionnaire Q1, question B.9 and questionnaire Q3, question B.9.
343 Questionnaire Q1, question B.6.2.
344 Questionnaire Q3, question B.6.2.
345 Questionnaires Q1 and Q3, question B.7.
346 Questionnaire Q1, question I.2.
347 Questionnaire Q1, questions I.6.1, I.6.1.1, 1.6.2 and I.6.2.1.
348 Questionnaire Q1, questions I.7, I.7.1, I.8 and I.8.1.
349 Questionnaire Q1, questions I.6.2 and I.6.2.1.
350 Questionnaire Q1, question I.5.2.
351 Form CO, paragraph 3853.
352 Form CO, paragraph 3852.
353 Form CO, paragraph 3854.
354 Form CO, paragraph 3852.
355 Oxera report, paragraph 1.7.
356 Oxera report, paragraph 3.1.
357 In essence, the model analyses the marginal incentive for the merged entity to increase the stand-alone price of Protein A while keeping the price of the bundle at pre-merger levels. In particular, the model calculates an index that is similar to the Gross Upward Pricing Pressure Index (GUPPI), which is used to estimate the incentive of the merging parties to increase prices post-merger.
358 Compass Lexecon report “Comments on Oxera’s analysis of incentives to bundle”, dated 22 November 2019 (Doc ID2595).
359 Compass Lexecon report, paragraph 4.5.
360 Compass Lexecon report, paragraph 4.6.
361 Compass Lexecon report, paragraph 4.1: “The Oxera paper does not account for the fact that the Protein A market is significantly larger than the market for ion exchange membranes. As described in the Form CO [in tables 129 and 145], [value of the market]. The Oxera paper’s analysis instead assumes that the ratio of the value of Protein A to the value of ion exchange membranes is 1.17:1”.
362 Compass Lexecon report, paragraph 2.11.
363 [GE’s customers purchases and quantities sold].
364 Form CO, paragraph 3881.
365 Compass Lexecon report, paragraphs 3.7-3.8 and annex 2.
366 Form CO, paragraph 1895 and Compass Lexecon report, paragraph 3.2.
367 Form CO, table 133 and Compass Lexecon report, paragraph 3.2.
368 Form CO, paragraph 3765 and Compass Lexecon report, paragraphs 3.1-3.2.
369 Questionnaire Q1, question I.2.
370 Form CO, paragraph 3857.
371 Form CO, paragraph 3860.
372 Form CO, paragraphs 3862-3863.
373 Questionnaire Q3, question I.1.
374 Questionnaire Q1, question I.6.3.
375 Questionnaire Q3, question I.5.3.
376 Questionnaire Q3, question I.5.3.
377 Form CO, chapter 10, sections C, D, E and F.
378 Form CO, paragraph 3731.
379 Doc ID474, Doc ID859, Doc ID949.
380 Questionnaire Q1, question I.10.
381 Questionnaire Q3, question I.9.
382 Questionnaire Q1, question I.11 and questionnaire Q3, question I.10.
383 Questionnaire Q1, question I.11.
384 Questionnaire Q1, I.15, question I.16.
385 Questionnaire Q3, I.14, question I.15.
386 Questionnaire Q1, question I.13.
387 Questionnaire Q1, question I.14 and questionnaire Q3, question I.13.
388 Questionnaire Q1, question I.13.1 and questionnaire Q3, question I.12.1.
389 Form CO, paragraph 2232.
390 Form CO, paragraphs 2238-2245.
391 Form CO, paragraph 2246.
392 SPR systems typically use biosensors made from thin films of gold. Shining incident light on the gold film creates an energy field around the biosensor that enables detection of the molecules which bind to the biosensor.
393 BLI systems are based on an optical analytical technique. They detect biomolecules that are typically absorbed to the tips of optical fibers by analysing the interference pattern of white light reflected from two surfaces: a layer of immobilised protein to the biosensor tip and an internal reference layer.
394 Form CO, paragraph 2436.
395 Form CO, paragraphs 2402-2407.
396 Questionnaire Q2, question B.C.2 and questionnaire Q4, question B.C.2.
397 Questionnaire Q2, questions B.B.1, B.C.2.2 and questionnaire Q4, question B.C.3.1.
398 Questionnaire Q2, question B.C.3 and questionnaire Q4, question B.C.3.
399 Questionnaire Q2, question B.C.3.1.
400 Questionnaire Q4, question B.C.3.1.
401 Questionnaire Q4, question B.E.2.1.
402 Questionnaire Q2, question B.E.4.1.
403 Questionnaire Q4, question B.C.2.1.
404 Label-based detection systems for molecular characterisation are not discussed further as the Transaction does not give rise to horizontally or non-horizontally affected markets in this respect.
405 Questionnaire Q2, question B.C.1.
406 See also Commission decision of 16 June 2011 in Case No COMP/M.6175 – Danaher/Beckman Coulter, recital 20.
407 Questionnaire Q2, question B.D.1.
408 Questionnaire Q2, question B.D.1.
409 Questionnaire Q4, question B.D.1.
410 Questionnaire Q4, question B.D.2.
411 Including Pioneer and Pioneer FE systems.
412 Including Octet RED384, Octet QKe, Octet HTX, Octet K2, and Octet RED96e systems.
413 Form CO, table 180.
414 Including Biacore C, Biacore X100, Biacore T200, Biacore S200, Biacore 8K, and Biacore 8K+.
415 Form CO, paragraph 2492.
416 Questionnaire Q2, question B.E.3.
417 Questionnaire Q4, question B.E.3.
418 Questionnaire Q2, question B.E.4 and questionnaire Q4, question B.E.4.
419 Questionnaire Q2, question B.E.4.
420 Questionnaire Q2, question B.E.4.
421 Questionnaire Q2, question B.E.5 and questionnaire Q4, question B.E.5.
422 Questionnaire Q2, question B.E.5.
423 Questionnaire Q2, question B.E.1 and questionnaire Q4, question B.E.1.
424 [GE’s internal documents].
425 [GE’s internal documents].
426 [Danaher’s internal documents].
427 [Danaher’s internal documents].
428 Questionnaire Q2, question B.E.7.
429 Questionnaire Q2, question B.E.7.
430 Questionnaire Q2, question B.E.7.1.
431 Questionnaire Q2, question B.E.10.1.
432 Questionnaire Q2, question B.E.9.
433 Questionnaire Q2, question B.E.9.1.
434 Questionnaire Q4, questions B.E.8 and B.E.10.
435 Questionnaire Q4, question B.E.7.
436 Questionnaire Q4, question B.E.10.1.
437 Questionnaire Q2, question B.E.8.
438 Questionnaire Q2, question B.E.8.1.
439 Questionnaire Q2, question B.E.8.1.
440 Questionnaire Q2, question B.E.8.1.
441 Questionnaire Q4, question B.E.8.1.
442 Namely, customer respondents excluding respondents who do not purchase LF detection systems at all or they do not purchase such systems as end-customers (that is they are distributors).
443 Questionnaire Q2, question B.E.10 and questionnaire Q4, question B.E.10.
444 Commission decision of 16 June 2011 in Case No COMP/M.6175 – Danaher/Beckman Coulter, recital 27.
445 Commission decision of 16 June 2011 in Case No COMP/M.6175 – Danaher/Beckman Coulter, recitals 21, 27 and 31.
446 Questionnaire Q2, question C.C.1.
447 Questionnaire Q4, question C.C.1.
448 Questionnaire Q4, question C.C.1.
449 Questionnaire Q2, question C.C.2.
450 Questionnaire Q2, question C.C.2.
451 Questionnaire Q4, question C.C.2.
452 Minutes of call with competitor, 13 August 2019, Doc ID1075, paragraph 17.
453 Questionnaire Q2, question C.C.3.
454 Questionnaire Q4, question C.C.3.
455 Upright ultra-high resolution microscopes are not discussed further as the Transaction does not give rise to horizontal or non-horizontal overlaps in this respect.
456 Questionnaire Q2, question C.D.1.
457 Questionnaire Q4, question C.D.1.
458 STED stands for stimulated emission depletion and is a technique in confocal super-resolution microscopy.
459 SIM stands for structured illumination microscopy and is a technique in widefield super-resolution microscopy.
460 The Commission verified with one competitor the market share estimates provided by the Parties in the worldwide market for super-resolution microscopes and confirmed that the combined share estimate for the Parties properly reflects (and if anything, overstates) the Parties’ position in the market (Minutes of call with competitor, 13 August 2019, Doc ID1075, paragraph 15).
461 See also Horizontal Merger Guidelines, paragraph 18.
462 [Danaher’s bidding data].
463 [Danaher’s bidding data]. [GE internal documents].
464 Questionnaire Q2, question C.E.6.
465 Questionnaire Q4, question C.E.6.
466 Questionnaire Q2, question C.E.8 and questionnaire Q4, question C.E.8.
467 Described in paragraph (599)(a) above.
468 Described in paragraph (599)(b) above.
469 Form CO, paragraphs 2869 and 2872.
470 Commission decision of 16 June 2011 in Case No COMP/M.6175 – Danaher/Beckman Coulter, recital 23.
471 Commission decision of 16 June 2011 in Case No COMP/M.6175 – Danaher/Beckman Coulter, recital 31.
472 Upgraded through either a hardware overhaul or a software tool.
473 Questionnaire Q2, question D.C.3.
474 Questionnaire Q2, question D.C.2.
475 Questionnaire Q4, question D.C.2.
476 Questionnaire Q2 question D.C.2 and questionnaire Q4, question D.C.2.
477 Questionnaire Q2, question D.C.1.1 and questionnaire Q4, question D.C.1.1.
478 In the remainder of the decision, “widefield HCS systems” will be meant to exclude personal imaging/automated imaging systems.
479 Questionnaire Q2, question D.D.1.
480 Questionnaire Q4, question D.D.1.
481 Questionnaire Q4, question D.D.2.
482 A plausible (global or EEA-wide) market for personal imaging/automated cell imaging systems would not be affected by the Transaction.
483 [Danaher’s internal documents].
484 [Danaher’s internal documents].
485 Questionnaire Q2, question D.E.4.
486 Questionnaire Q2, question D.E.6.
487 In the EEA, the Parties held a combined share of [5-10]% in widefield HCS systems in 2018. Therefore, the Transaction does not give rise to an affected market and the competitive assessment in the remainder of this section will focus on the worldwide market for widefield HCS systems.
488 Questionnaire Q2, questions D.E.3 and questionnaire Q4, question D.E.3.
489 Minutes of call with competitor, 13 August 2019, Doc ID1075, paragraph 21.
490 Questionnaire Q2, question D.E.5.
491 Questionnaire Q2, question D.E.6.
492 See section 4.5 above.
493 Lab filtration accessories include various different type of products like: filter holders, filter funnels, filter flasks, hardware (that is membrane dispensers, peristaltic pumps, vacuum pumps, microbiology manifolds, microbiology manifold accessories, multi-well plate manifolds, shakers and pressures vessels) and other filtration accessories (that is tubes and seals).
494 Commission decision of 9 November 2006 in Case No COMP/M.4242 – Thermo Electron/Fisher Scientific, recital 23.
495 Commission decision of 18 August 2011 in Case No COMP/M.6293 – Thermo Fisher/Phadia, recital 67.
496 Questionnaire Q2, question E.C.1 and questionnaires Q4, question E.C.1.
497 Questionnaire Q2, question E.C.1.1.
498 Questionnaire Q4, questions E.C.1.1 and E.C.2.
499 Questionnaire Q2, question E.D.1.1 and questionnaire Q4, question E.D.1.
500 According to the data provided by the Parties, the Transaction does not give rise to affected markets in
• all lab filtration products or if there are separate markets for (ii) lab filter media; (iii) membrane filters;
(iv) non-microbiology membrane filters segment; (v) microbiology membrane filters segment;
(vi) cellulose filter papers; (vii) overall disposable devices/media encapsulation filters; (viii) syringe filters; (ix) non-sterile syringe filters; (x) sterile syringe filters; (xi) 50/60 mm in-line filters; (xii) capsule filters segment; (xiii) vent filters segment; (xiv) plate filters segment; (xv) spin devices; (xvi) overall lab filtration accessories; (xvii) filter holder segment; (xviii) filter funnels segment; (xix) hardware segment;
(xx) vacuum pumps segment; (xxi) cut membrane discs; and (xxii) quartz filter papers.
501 Form CO, paragraph 2180.
502 Questionnaire Q2, question E.E.4.
503 See also Horizontal Merger Guidelines, paragraph 18.
504 Questionnaire Q2, question E.E.5.
505 Questionnaire Q4, question E.E.6.
506 Questionnaire Q4, question E.E.3.
507 Form CO, paragraphs 2190-2194.
508 Form CO, paragraph 2168
509 Questionnaire Q2, question E.E.3.
510 Questionnaire Q2, question E.E.3.
511 Questionnaire Q2, question E.E.3.1.
512 RFI 15 of 19 November 2019, question 2: “Cut membrane discs are circular discs cut from larger rolls of membrane; cut membrane discs would be a sub-segment of the membrane filters segment”.
513 Form CO, paragraph 2049.
514 Pre-notification RFI 5, question 95.
515 RFI 15 of 19 November 2019, question 2.
516 RFI 15 of 19 November 2019, question 1.
517 Form CO, paragraph 2048.
518 Form CO, paragraphs 2197ff, 2527ff, 2948ff, and 3335ff.
519 Questionnaire Q2, questions F.1, F.3, and G.2 and questionnaire Q4, question F.2.
520 Questionnaire Q2, questions F.1, F.3, and G.2 and questionnaire Q4, question F.2.
521 Questionnaire Q2, questions F.1, F.3, and G.2 and questionnaire Q4, question F.2.
522 See paragraphs (679), (681), and (686) above.
523 Questionnaire Q2, questions F.1, F.3 and G.2 and questionnaire Q4, question F.2.
524 Commission's Notice on Remedies acceptable under Council Regulation (EC) No 139/2004 and under Commission Regulation (EC) No 802/2004, OJ C 267, 22.10.2008, p. 1.
525 Remedies Notice, paragraph 23.
526 Remedies Notice, paragraph 35.
527 Remedies Notice, paragraph 36.
528 Remedies Notice, paragraph 55.
529 The Initial Commitments package includes the entire skids business from Pall.
530 Annex 7 of the Initial Commitments.
531 Such as IT staff and operations, general back-office software, HR functions, finance, accounting, tax and legal and compliance support.
532 Market test questionnaire, question E.B.1 (FortéBio molecular characterisation business); questions D.B.1 and D.B.2 (TFF Divestment business); question A.B.1 (Microcarriers Divestment Business); question C.B.1 (Danaher’s Pall Biotech chromatography resins business); question B.B.1, B.B.2 and B.B.3 (Danaher’s Pall Biotech chromatography hardware business).
533 Market test questionnaire, question F.1 (Entire Divestment Business Package), question E.B.2 (FortéBio Divestment Business); question D.B.3 (TFF Divestment business); question A.B.2 (Microcarriers Divestment Business); question C.B.2 (Danaher’s Pall Biotech chromatography resins business); question B.B.2 (Danaher’s Pall Biotech chromatography hardware business).
534 Market test questionnaire, question F.A.1.
535 Market test questionnaire, question F.A.1.1.
536 Market test questionnaire, questions B.D.5, C.D.5 and D.D.5.
537 Danaher’s Pall Biotech chromatography resins business, Danaher’s Pall Biotech chromatography hardware business, the TFF Divestment Business.
538 Market test questionnaire, question C.C.4.
539 Market test questionnaire, question B.C.4.
540 Market test questionnaire, question B.B.6.
541 Market test questionnaire, question B.C.5.
542 Market test questionnaire, question C. B.1.1.
543 Market test questionnaire, question A.B.4.
544 Market test questionnaire, questions B.B.5, B.B.6 and D.B.4.
545 Except for the FortéBio Divestment business, which did include ‘go-to-market’ sales staff.
546 Market test questionnaire, question D.B.1.
547 Market test questionnaire, question B.D.5 and C.D.5.
548 Market test questionnaire, question A.D.5.1.
549 Market test questionnaire, question D.D.1, question F.1.1.
550 Market test questionnaire, questions A.D.6, B.D.6, C.D.6 and D.D.6.
551 Form RM, paragraph 283.
552 Form RM, footnotes 32 (chromatography) and 61 (TFF).
553 Market test questionnaire, questions A.D.4, B.D.4, C.D.4, D.D.4 and E.D.4.
554 Market test questionnaire, questions A.D.4, B.D.4, C.D.4, D.D.4 and E.D.4.
555 Market test questionnaire, questions D.B.1.1 and D.B.3.1.
556 Market test questionnaire, question D.B.3.1, D.B.6 and D.B.6.1.
557 RFI 19, question 13.
558 Market test questionnaire, question F.A.5.
559 Market test questionnaire, question F.A.6.
Annex
1 On 18 October 2019, the Notifying Party concluded a Purchase Agreement with Sartorius AG to acquire the Divested Businesses described in Section B.(a) to (d) above. A copy of this Purchase Agreement has been submitted to the European Commission on 22 October 2019. Pursuant to Article X (Conditions to Closing), section 10.1(a), the obligations of each party under the Purchase Agreement are subject to all required approvals having been obtained, including that of the European Commission. The Notifying Party is currently negotiating with Sartorius AG an amendment to the Purchase Agreement to include the Divested Business described in Section B.(e) above.
