Commission, September 26, 2019, No M.9547
EUROPEAN COMMISSION
Decision
JOHNSON & JOHNSON / TACHOSIL
Subject: Case M.9547 - Johnson & Johnson/Tachosil
Request for referral of 6 September 2019 by France to the Commission pursuant to Article 22(2) of Council Regulation (EC) No. 139/2004 (1) and Article 57 of the Agreement on the European Economic Area (2)
Ref.: Letter of 6 September 2019 by Ms. Isabelle de Silva, President of the Autorité de la Concurrence, the competent Competition Authority of France, to the European Commission.
Dear Madam,
1. INTRODUCTION
(1) With the above-mentioned request of 6 September 2019 the Autorité de la Concurrence (the “French Competition Authority”) formally requested the Commission to examine, in application of Article 22(3) of the Merger Regulation, the concentration whereby Johnson & Johnson (“J&J”, USA) acquires, from Takeda Pharmaceuticals International AG (“Takeda”, Switzerland), sole control over Tachosil, a haemostatic patch product (“Tachosil” or the “Target”), through the acquisition of Topaz Investment AS (“Topaz”, Norway) and additional assets related to Tachosil (the “Transaction”). J&J and the Target are hereafter referred to as the “Parties”.
(2) Pursuant to Article 22(1) of the Merger Regulation, one or more Member States may request the Commission to examine any concentration, as defined in Article 3 of the Merger Regulation, that does not have a Union dimension within the meaning of Article 1 of the Merger Regulation but affects trade between Member States and threatens to significantly affect competition within the territory of the Member State or States making the request. Such a request must be made within 15 working days of the date of the notification of the concentration. Pursuant to Article 22(2) of the Merger Regulation, any other Member State may join the initial request within a period of 15 working days of being informed by the Commission of the initial request. Pursuant to Article 6(3) of Protocol 24 to the EEA Agreement, any EFTA State may join the request within a period of 15 working days from the day on which the Commission informed the EFTA Surveillance Authority of the initial request.
(3) In the present case, J&J notified the Transaction to the German Competition Authority on 1 August 2019. (3) The Commission received from Germany a referral request pursuant to Article 22(1) of the Merger Regulation on 21 August 2019, i.e. within 15 working days of the date of the notification as foreseen in Article 22(1) of the Merger Regulation. On 22 August 2019, in accordance with Article 22(2) of the Merger Regulation, the Commission informed the competent authorities of the other Member States and the EFTA Surveillance Authority of the above request. On 3 September 2019, the Commission also shared with them additional information received from the German Competition Authority relating to the Parties’ market shares in the EEA.
(4) On 6 September 2019, the French Competition Authority requested to join the initial referral request made by Germany. (4)
2. THE PARTIES AND THE OPERATION
(5) J&J is the ultimate parent company of a global group of companies active in three business sectors: (i) consumer, (ii) pharmaceuticals, and (iii) medical devices.
(6) The Target consists of (i) Topaz, which holds the majority of the rights assets and obligations connected to Tachosil (and its predecessor products), as well as (ii) additional assets related to these products.
(7) The Transaction notified to the German Competition Authority consists in the acquisition of sole control by J&J over the Target. Therefore, the Transaction constitutes a concentration within the meaning of Article 3(1)(b) of the Merger Regulation.
(8) The Transactions would not constitute a concentration with a Union dimension within the meaning of Article 1 of the Merger Regulation, according to the information provided by the competent authorities.
3. ASSESSMENT OF THE REFERRAL REQUEST
(9) Pursuant to Article 22 of the Merger Regulation, in order for a Member State to join the initial referral request made by another Member State, one procedural and two substantive conditions must be fulfilled. As to the procedural precondition, the initial referral shall be made within 15 working days of the date on which the concentration was notified (or if no notification is required, otherwise made known) to the Member State concerned and other Member States mayjoin the initial request within a period of 15 working days of being informed by the Commission of the initial request. As to the substantial conditions, the concentration must (i) affect trade between Member States and (ii) threaten to significantly affect competition within the territory of the Member State or States making the request. (5)
(10) If the above legal requirements are met, the Commission may exercise discretion with regard to whether or not it is appropriate that the concentration is examined by the Commission. The Commission has, in the Referral Notice, set out in a general manner its understanding regarding the appropriateness of particular cases or categories of cases for referral.
3.1. Procedural Criterion
(11) J&J formally notified the Transaction to the German Competition Authority on 1 August 2019. The Commission received the referral request made by the Federal Republic of Germany on 21 August 2019, i.e. within the time limit foreseen in Article 22(1) second indent of the Merger Regulation
(12) The Commission informed the competent authorities of the other Member States and the EFTA Surveillance Authority of the referral request made by Germany on 22 August 2019. On 6 September 2019, the Commission received France’s request to join the initial referral request made by Germany.
(13) Therefore, France joined the initial referral request within 15 working days following the date on which it was informed of the referral request by the Commission, that is to say within the time limit foreseen in Article 22(2) second indent of the Merger Regulation.
3.2. Substantive criteria
(14) The Parties’ activities overlap in the field of haemostatic and tissue sealing products. These products are developed to stop bleeding during surgery and are typically used when traditional techniques (such as suture, ligation or cauterisation) are either ineffective or impractical. Haemostatic and tissue sealing products are available in different forms and with various product properties.
(15) Tachosil is a wound patch made of human fibrinogen and human thrombin, which has both a haemostatic and tissue sealing effect. J&J offers several haemostatic and tissue sealing products in the EEA, including Evicel (fibrin sealant liquid), Surgiflo (flowable gelatine-based foam), Tabotamp/Surgicel (cellulose-based absorbable haemostat) and Spongostan (haemostatic gelatine sponge). J&J’s product portfolio also includes Evarrest, a surgical patch with dual haemostatic […]*, similar to Tachosil, which is currently marketed outside of the EEA. Evarrest was sold in the EEA between 2013 and late 2017 until J&J filed an application to have its marketing authorisation revoked for commercial reasons.
(16) The Transaction is not notifiable in France. Therefore, the French Competition reaches the conclusion that the two substantive legal requirements of Article 22 of the Merger Regulation are met on the basis of the information provided by the German Competition Authority in the initial referral request.
Effect on trade between Member States
(17) Regarding the first substantive criterion, paragraph 43 of the Referral Notice provides that a concentration fulfils this requirement to the extent that it is liable to have some discernible influence on the pattern of trade between Member States. (6)
(18) In the present case, as explained in the preliminary analysis of the German Competition Authority, the Transaction affects trade between Member States because the Parties have sales of haemostatic and tissue sealing products in almost all Member States. Moreover, Tachosil is manufactured in one of Takeda’s production sites in Austria, from which it is distributed in the EEA and globally. Similarly, J&J’s haemostatic and tissue sealing products are each manufactured in [locations] (Evicel and Evarrest are manufactured in [the Middle East], Surgiflo and Spongostan in [Europe] and Tabotamp in [Europe] and [Latin America]), from which they are distributed in the EEA and globally.
(19) Based on the above, the Commission considers that the Transaction is capable of having an appreciable impact on cross-border economic activity involving several Member States. Indeed, the Transaction gives rise to overlaps involving products sold in several Member States and, therefore, is by its very nature capable of affecting trade between Member States. (7) In view of the foregoing, the Commission concludes that the first substantive legal requirement for an Article 22 referral is met.
Concentration threatens to significantly affect competition
(20) Regarding the second criterion, paragraph 44 of the Referral Notice provides that a referring Member State should demonstrate that, based on a preliminary analysis, there is a real risk that the transaction may have a significant adverse effect on competition within the territory of the Member State making the request and thus deserves close scrutiny, without prejudice to the outcome of a full investigation.
(21) On the basis of the elements provided by the German Competition Authority, the French Competition Authority argues that the concentration threatens to significantly affect competition, at least within France, in the field of haemostatic and tissue sealing products.
(22) In the absence of precedents in this sector, the German Competition Authority has considered, in its preliminary assessment of the Transaction, three plausible product market definitions: (i) the overall market for haemostatic and tissue sealing products, as well as two potential narrower market segments, i.e. (ii) a market based on the European Pharmaceutical Marketing Research Association’s (EphMRA) ATC 3 classification (Group B2F “Tissue Sealing Preparation”) and (iii) a market limited to haemostatic patches with dual effect. As regards the geographic market, the German Competition Authority has considered that the market is likely to be national in scope but that it can be left open as competition concerns would arise under all plausible geographic market definitions (national and EEA).
(23) The file of the German Competition Authority includes estimates of the Parties’ market share in France only with respect to the market based on the EphMRA ATC 3 classification (Group B2F “Tissue Sealing Preparation”). Based on the IMS Health’s data for the first quarter of 2018, on this market, the Parties have high combined market shares above [50-60]% in France ([60-70]% in volume and [50-60]% in value) and the increment brought by the Transaction is significant ([20-30]% in volume and [10-20]% in value). The third largest player on this market is Baxter, with a market share of approximately [30-40]%. The other players active on this market have a marginal position.
(24) On the narrower market limited to haemostatic patches with dual effect, J&J has currently no marketed products in the EEA, while Tachosil is the market leader in the EEA with a market share of approximately 80-90% according to the German referral request. On this market, Tachosil and Evarrest are the only two haemostatic patches with dual effect based on human fibrinogen and thrombin and are classified as pharmaceuticals. Competing haemostatic patches with dual effect, sold by Baxter and Medtronic in the EEA, support the haemostatic process through synthetic ingredients and are classified as medical devices. The German Competition Authority submits in its initial referral request, to which the French Competition Authority refers, that it can be assumed that J&J could launch Evarrest in the EEA without any substantial effort since this product had already been authorised and distributed in the EEA, between 2013 and 2017, until the withdrawal of the European marketing authorisation at J&J’s request (reportedly for economic reasons). In view of the fact that Evarrest has already been authorised and distributed in the EEA and that such authorisation was only removed pursuant to J&J’s own request, the acquisition of Tachosil and the likely negative impact that this could have on J&J’s incentives to recommence distribution of Evarrest in the EEA could result in the elimination of Tachosil’s closest potential competitor.
(25) On the basis of the above and without prejudice to the outcome of the investigation by the Commission, the request to join the referral indicates that the concentration in question threatens to significantly affect competition at least within the territory of France. In view of the foregoing, the Commission concludes that the second substantive legal requirement for an Article 22 referral is met.
3.3. Appropriateness of a referral
(26) Pursuant to paragraph 45 of the Referral Notice, referrals of concentrations already notified should normally be limited to those cases which appear to present a real risk of negative effects on competition and trade between Member States and where it appears that these would be best addressed at the Community level.
(27) One of the categories of cases normally most appropriate for referral under Article 22 of the Merger Regulation are cases giving rise to serious competition concerns in a series of national or narrower than national markets located in a number of EEA countries, in circumstances where coherent treatment of the case (regarding possible remedies, but also, in appropriate cases, the investigative efforts as such) is considered desirable, and where the main economic impact of the concentration is connected to such markets. (8)
(28) In the present case, at this stage of the procedure, it appears that the Transaction could threaten to significantly affect competition in a series of national markets in EEA countries which have requested a referral to the European Commission, namely in Germany, Austria, Spain, France, Finland and Norway. In this respect, it should be noted that the main economic impact of the Transaction is connected to these EEA countries, which account for more than […]% of the Target’s turnover in the EEA.
(29) Without prejudice to the outcome of a full investigation, and based on the information available at this stage, the Parties’ combined market shares in the above- mentioned EEA countries appears to be very high (under one possible market definition for which market shares are available at this stage):
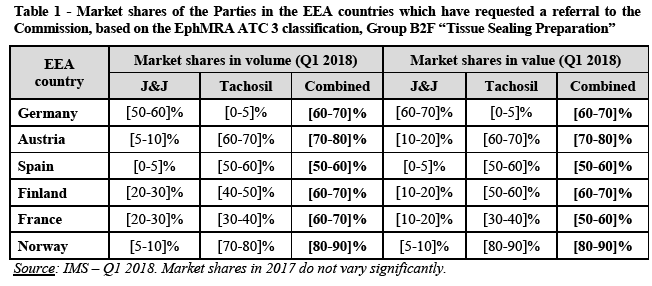
(30) A coherent treatment of the case in terms of investigative efforts is desirable for the several reasons.
(31) First, the Transaction is notifiable in three Member States (namely Germany, Austria, and Spain). These multiple notifications of the same Transaction increase legal uncertainty and may lead to conflicting assessments. In particular, in the absence of precedents from the Commission or national competition authorities in this sector, it is important to adopt a coherent product market definition across the EEA.
(32) Second, the examination of the Transaction will have to address the competitive significance of J&J’s branded dual effect haemostatic patch Evarrest and thus to clarify why J&J decided to exit the whole of the EEA in 2017 and whether (and under what conditions) a (re)authorisation by the European Medicines Agency would be possible.
(33) Third, the Parties’ competitors are active throughout the EEA (directly or through distributors) and it would be more efficient for the Commission to centralise contacts with competitors in one merger review procedure.
(34) Finally, a coherent treatment of the case in terms of potential remedies is desirable, taking into account that the Parties’ manufacturing facilities are located in few locations worldwide, serving several EEA countries.
(35) Therefore, the Commission has concluded that it is, in the present circumstances, be the best placed authority to assess this concentration.
4. CONCLUSION
(36) In view of the foregoing, the Commission has concluded that the Transaction is a concentration within the meaning of Article 3 of the Merger Regulation. The Commission considers that the request by the French Competition Authority to join the initial referral request made by Germany for the application of Article 22(3) is admissible as the concentration meets the requirements laid down in Article 22(2) and 22(3) of the Merger Regulation and paragraphs 42-45 of Referral Notice. The Commission therefore has decided to examine the proposed concentration under the Merger Regulation.
1 OJ L 24, 29.1.2004, p. 1 (the “Merger Regulation”). With effect from 1 December 2009, the Treaty on the Functioning of the European Union (“TFEU”) has introduced certain changes, such as the replacement of “Community” by “Union” and “common market” by “internal market”. The terminology of the TFEU will be used throughout this decision.
2 OJ L 1, 3.1.1994, p.3 (the “EEA Agreement”).
3 On the same day, J&J notified the Transaction to the Austrian Competition Authority. Besides Germany and Austria, in the EEA, the Transaction is also subject to merger control in Spain but has not been notified in this country yet.
4 Austria (23 August 2019), Spain (3 September 2019), Finland (9 September 2019), and Norway (12 September 2019) also joined the initial referral request made by Germany within a period of 15 working days of being informed by the Commission of the referral request (on 22 August 2019), thus within the time limit of Article 22(2), second indent, of the Merger Regulation.
5 See also Commission Notice on Case Referral in respect of concentrations (the “Referral Notice”), paragraphs 42-44 (OJ C 56, 05.03.2005, p. 2).
* Should read: “effect”.
6 The Referral Notice also refers by analogy to the Commission Guidelines on the effect on trade concept contained in Articles 81 and 82 of the Treaty OJ C 101, 27.4.2004, p. 81.
7 See by analogy Commission Guidelines on the effect on trade concept contained in Articles 81 and 82 of the Treaty, paragraph 61.
8 Referral Notice, paragraph 45.