Commission, January 18, 2019, No M.8674
EUROPEAN COMMISSION
Decision
BASF/Solvay's Polyamide Business
COMMISSION DECISION of 18.1.2019
declaring a concentration to be compatible with the internal market and the EEA Agreement
(Case M.8674 BASF/Solvay's Polyamide Business)
(Only the English text is authentic)
THE EUROPEAN COMMISSION,
Having regard to the Treaty on the Functioning of the European Union,
Having regard to the Agreement on the European Economic Area, and in particular Article 57 thereof,
Having regard to Council Regulation (EC) No 139/2004 of 20 January 2004 on the control of concentrations between undertakings1, and in particular Article 8(2) thereof,
Having regard to the Commission's Decision of 26 June 2018 to initiate proceedings in this case, Having regard to the opinion of the Advisory Committee on Concentrations2,
Having regard to the final report of the Hearing Officer in this case3, Whereas:
1. INTRODUCTION
(1) On 22 May 2018, the European Commission ("Commission") received notification of a proposed concentration pursuant to Article 4 of Council Regulation (EC) No 139/2004 (the “Merger Regulation”) by which BASF SE (“BASF”, Germany) intends to acquire sole control of Solvay S.A. (“Solvay”, Belgium)’s worldwide polyamide business (“the Business”) within the meaning of Article 3(1)(b) of the Merger Regulation (“the Transaction”).4 BASF is referred to in this Decision as “the Notifying Party” while BASF and the Business are collectively referred to as “the Parties" and the undertaking that would result from the Transaction is referred to as “the Merged Entity".
2. THE PARTIES AND THE CONCENTRATION
(2) BASF is a diversified company headquartered in Ludwigshafen, Germany, active in chemicals, performance products, functional materials & solutions, agricultural solutions and oil & gas. BASF is active in the polyamide value chain in the production of hexamethylenediamine (“HMD”), adipic acid,5 Hexamethylenediamine adipate Salt (“AH Salt”), PA 6.6 polyamide base polymer (“PA 6.6 BP”), co-polyamide 6/6.6 and polyamide engineering plastic (“PA 6.6 EP”).
(3) The Business consists of Solvay’s worldwide polyamide activities (with the exception of certain assets located in Paulinia and Santo André, Brazil), with production facilities located in the EEA (France, Poland, Spain and Germany), the Americas (Brazil, Mexico), and Asia (South Korea, China and India), as well as sales and research organisations, e.g., in Italy, the United States of America and Japan. As a whole, the Business is active in the production of adiponitrile (“ADN”), HMD, adipic acid, AH Salt, PA 6.6 BP, PA 6.6 EP and PA 6.6 Performance Fibres. Thus, the Business does include Solvay’s 50% stake in Butachimie, Société en Nom Collectif (“Butachimie”, France), a 50/50 joint venture with INVISTA Equities, LLC (“Invista”, United States of America) active in the production of ADN and HMD.
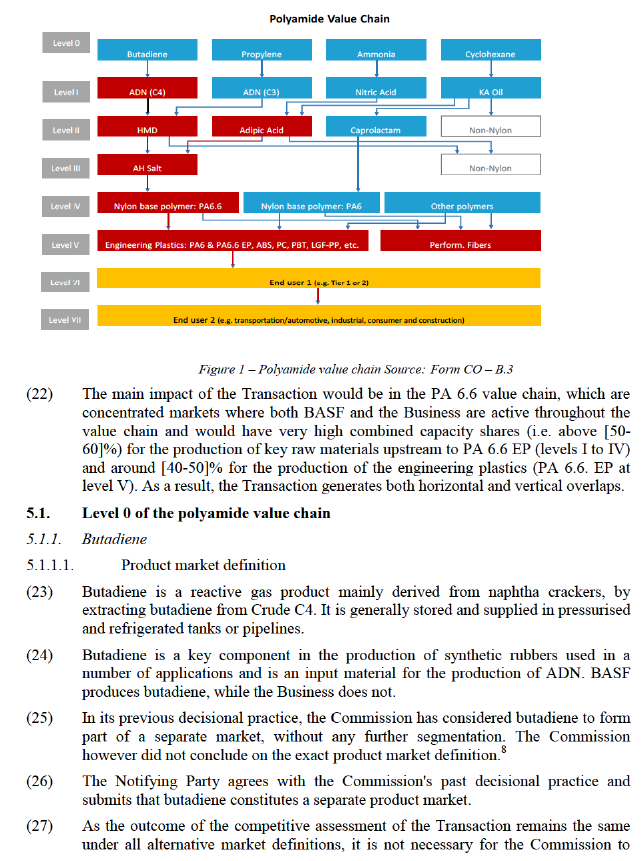
(4) As a result of the Transaction, BASF would acquire sole control of the Business, primarily by means of share transfers of existing Solvay affiliates. The Transaction would therefore result in a concentration within the meaning of Article 3(1) of the Merger Regulation.
3. UNION DIMENSION
(5) The undertakings concerned have a combined aggregate world-wide turnover of more than EUR 5 000 million (EUR 58 886 million in 2016). Each of them has Union-wide turnover in excess of EUR 250 million (BASF: EUR […] million in 2016; Business: EUR […] million in 2016). They do not achieve more than two- thirds of their respective aggregate Union-wide turnovers within one and the same Member State. The notified operation therefore has a Union dimension within the meaning of Article 1(2) of the Merger Regulation.
4. THE PROCEDURE
(6) After a preliminary examination of the notification and based on the Phase I market investigation, on 26 June 2018, the Commission decided to initiate proceedings under Article 6(1)(c) of the Merger Regulation (the "Article 6(1)(c) Decision"). In the Article 6(1)(c) Decision, the Commission concluded that the Transaction raised serious doubts as to its compatibility with the internal market and with the functioning of the EEA Agreement in relation to (i) EEA-wide markets for: ADN, HMD, adipic acid, AH salt, PA 6.6 BP, and PA 6.6 EP based on horizontal non- coordinated effects; and (ii) EEA-wide markets for ADN, HMD, adipic acid, AH aalt and PA 6.6 BP due to likely vertical input foreclosure effects.
(7) On 9 July 2018, the Notifying Party submitted its response to the Article 6(1)(c) Decision (the "Response to the Article 6(1)(c) Decision" or "Response"), in which it challenged aspects of the Commission's assessment as set out in the Article 6(1)(c) Decision.
(8) On 12 July 2018, a formal State of Play meeting took place between the Commission and the Parties.
(9) On 7 August 2018, the Commission adopted a decision pursuant to Article 11(3) of the Merger Regulation, following Solvay's failure to provide complete information in response to an information request ("RFI 8") from the Commission ("the Article 11(3) Decision of 7 August 2018"). That Decision suspended the time limits referred to in the first subparagraph of Article 10(3) of Regulation (EC) 139/2004. Solvay responded to RFI 8 on 28 August 2018.
(10) On 19 July 2018, the Commission adopted a decision pursuant to Article 11(3) of the Merger Regulation, following BASF's failure to provide complete information in response to an information request ("RFI 7") from the Commission ("the Article 11(3) Decision of 19 July 2018"). That Decision suspended the time limits referred to in the first subparagraph of Article 10(3) of Regulation (EC) 139/2004. BASF responded to RFI 7 on until 30 August 2018 and the suspension expired at the end of that day.
(11) Based on a Phase II investigation which supplemented the findings of the Phase I investigation, a State of Play meeting between the Commission and the Parties took place on 20 September 2018.
(12) During that meeting, the Commission informed the Parties of its preliminary conclusion that the Transaction would likely lead to a significant impediment of effective competition as a result of horizontal non-coordinated effects in the EEA markets ADN, HMD, adipic acid, AH aalt, PA 6.6 BP and PA 6.6 EP, as well as of vertical input foreclosure effects in the EEA markets for ADN, HMD, adipic acid, AH Salt and PA 6.6 BP and vertically related markets.
(13) On 25 September 2018 and 10 October 2018, two separate extensions of the time limit for adopting a final decision (of ten working days each) were granted under Article 10(3) second subparagraph, third sentence of the Merger Regulation in order to allow the Parties to present commitments to the Commission.
(14) In order to address the competition concerns identified by the Commission, of which the Parties were informed in the course of the procedure, the Parties formally submitted a set of commitments to the Commission (the "Initial Commitments") on 15 October 2018. The Initial Commitments were market tested by the Commission on 16 October 2018.
(15) The results of the market test identified risks on the viability and competitiveness of the business which the Notifying Party committed to divest (the “Divestment Business”) as foreseen in the Initial Commitments. The Commission informed the Parties of the outcome of the market test on 24 October 2018.
(16) Taking into account the market test results, the Parties submitted revised commitments to the Commission on 31 October 2018, which include several improvements to the Initial Commitments. Eventually, the Parties submitted a final set of commitments to the Commission on 11 December 2018 ("the Final Commitments").
(17) The Advisory Committee discussed a draft of this Decision on 17 December 2018 and issued a favourable opinion.6
5. RELEVANT MARKETS
(18) The Transaction leads to horizontal overlaps at all levels of the polyamide value chain namely for ADN, HMD, AH Salt, adipic acid, PA 6.6 BP, PA6 3D printing powders and PA 6.6. EP. The Transaction also generates vertical relationship along all the polyamide value chain and, outside the polyamide value, between HMD and hexamethlyene diisocynate ("HDI") derivatives. This Decision therefore focuses on the horizontal overlaps and the vertical links at all levels of the polyamide value chain and, outside the polyamide value chain, on the vertical links between HMD and HDI derivatives.
(19) The main nexus of the Transaction are nylon products ("PA 6" and "PA 6.6", where PA stands for polyamide).7 PA 6 and PA 6.6 are produced from oil derivatives through a number of chemical and physical reactions, illustrated in Figure 1. The production process results in PA 6 BP and PA 6.6 BP, which are then compounded into PA 6 EP and PA 6.6 EP or transformed into performance fibres.
(20) PA 6 EP and PA 6.6 EP are used in a wide number of end use applications in the automotive, electronics, construction, clothing and food industries. They are found for example in automobiles, electrical and electronic appliances, windows frames, film for food packaging. PA 6 Performance Fibres and PA 6.6 Performance Fibres are used among other for carpets and clothing applications.
(21) As illustrated in Figure 1, the polyamide value chain is composed of eight levels (0 to VII), and the Transaction affects Level I to V. In red are indicated the steps of the value chain for PA 6.6 EP where the Business is active.
conclude on the exact product market definition. For the purposes of this Decision, butadiene will be regarded as a separate product market.
5.1.1.2. Geographic market definition
(28) The Commission has previously considered the geographic scope for butadiene to be at least "Western Europe +", which is composed of Western Europe, Poland and the Czech Republic. The precise geographic market definition was however ultimately left open.9
(29) The Notifying Party argues that there are strong arguments for a global market definition. In particular, it notes that Western Europe is a net exporter of butadiene and that North America is a net importer of butadiene, which advocates for a global market.
(30) As the outcome of the competitive assessment of the Transaction remains the same under all alternative market definitions, it is not necessary for the Commission to conclude on the exact product market definition.
5.1.2. Ammonia
5.1.2.1. Product market definition
(31) Ammonia is manufactured by reacting nitrogen from the air with hydrogen in the presence of a catalyst at high temperature and high pressure. At room temperature and ambient pressure ammonia is gaseous. It can be refrigerated or compressed and thus stored as a liquid. Ammonia can be diluted in demineralised water, typically at approximately 25% of ammonia and approximately 75% of water. In distinction to “anhydrous ammonia” (not diluted in water) the product diluted in water is named “aqueous ammonia”. Ammonia is used as an input material for the production of nitrogen-based fertilisers and for other industrial applications.
(32) BASF produces anhydrous ammonia and aqueous ammonia, while the Business does not produce ammonia, but rather sources it from market players including BASF.
(33) In past decisions, the Commission considered that anhydrous ammonia is part of product market, distinct from all other chemicals.10
(34) The Notifying Party concurs with the Commission’s decisional practice as regards anhydrous ammonia, however does not take a position as regards aqueous ammonia.
(35) The market investigation indicated that anhydrous ammonia likely forms part of a separate product market. As regards a plausible sub-segmentation of that market by end application (i.e. industrial applications and fertiliser production), the market investigation was largely inconclusive. It however gave some indications that both from a supply and demand side perspective there might be a certain degree of substitutability between the various applications.11
(36) As the outcome of the competitive assessment of the Transaction remains the same under all alternative market definitions, it is not necessary for the Commission to conclude on the exact product market definition.
5.1.2.2. Geographic market definition
(37) For anhydrous ammonia, the Commission has in the past found that, whilst the possible market for the supply of large quantities (to the fertiliser industry for example) is global in scope as the product is shipped worldwide in large vessels, the geographic scope of the possible market for anhydrous ammonia for smaller industrial customers covered a territory smaller than the EEA. In a previous case, the Commission considered North Western Europe (consisting of France, Germany, Denmark, Belgium, the Netherlands and Luxembourg) as the relevant geographical market due to the intense transportation infrastructure in that area.
(38) The Notifying Party does not take a position regarding the geographic scope of the market, but claims at the precise definition can be left open.
(39) The market investigation broadly supported the conclusions of the Commission' previous decisional practice. On the demand side, the majority of customers responding to the market investigation indicated that they source anhydrous ammonia within the EEA, and a significant proportion thereof indicated that they source only from nearby regions, and don’t consider the entirety of the EEA.
(40) On the supply side, none of the responding competitors indicated that there is any import of anhydrous ammonia from outside the EEA.
(41) Based on the assessment in recitals 31 to 41, the Commission concludes that the geographic scope of the market for ammonia is at most EEA wide and most likely narrower. However, as the outcome of the competitive assessment of the Transaction remains the same under all alternative market definitions, it is not necessary for the Commission to conclude on the exact product market definition.
5.2. Level I of the polyamide value chain
5.2.1. ADN
5.2.1.1. Introduction
(42) Adiponitrile ("ADN") is an aliphatic diamine consisting of 6 Carbon atoms. It can be manufactured following two commercially established routes: the C4 route (with primary feedstock butadiene) and the C3 route (with primary feedstock propylene). The only producer of ADN in the EEA is Butachimie, the joint venture between Solvay and Invista which uses the C4 route.
(43) The only commercial use of ADN is for the production of Hexamethylene Diamine ("HMD") within the polyamide value chain.
5.2.1.2. Product market definition
5.2.1.3. The Commission precedents
(44) The Commission has not previously analysed the market for ADN.
5.2.1.4. The Notifying Party's view
(45) The Notifying Party submits that ADN constitutes a distinct product market (without any further segmentation) due to its sole use for the production of HMD, the absence of substitutes for that purpose, and given that there are no individual product specifications within ADN.
(46) In the Response to the 6(1)(c) Decision, the Notifying Party provides further arguments and claims that ADN C3 and ADN C4 are perfect substitutes from the demand side. First, the Notifying Party claims that while C3 ADN may be less pure, having higher water content than ADN C4, HMD production would ultimately be unaffected from a product quality point of view by using ADN C3. According to the Notifying Party, this applies in particular to […], […].
(47) Second, the Notifying Party further explains that in its understanding the HMD produced by […] is used […].
(48) Third, the Notifying Party claims that HMD producers' choice of input (i.e. ADN C3 or ADN C4) mainly depends on raw material costs. To illustrate this point, the Notifying Party provides evidence that, while ADN C4 usually has a cost advantage over ADN C3, the price difference may be and is subject to changes.
(49) Fourth, the Notifying Party claims that switching from using ADN C3 and ADN C4 is easy and only requires that the HMD process parameters in the synthesis and purification steps need to be adapted. […].
5.2.1.5. The Commission's assessment
(50) The market investigation carried out by the Commission during in Phase I provided strong indications that ADN C3 and ADN C4 belong to separate product markets.
(51) From the demand-side, respondents to the market investigation indicated that they can only use one type of ADN in their production process (in the EEA, that is ADN C4) because the production assets are designed to use ADN derived from a specific feedstock (propylene or butadiene). Any switch in feedstock would require significant investments. Respondents also indicated that ADN C4 is preferred over ADN C3 because has a higher purity and is available in the EEA, while ADN C3 is not.12
(52) The investigation carried by the Commission in Phase II, contrary to the Notifying Party's claim in the Response to the 6(1)(c) Decision, further supported these findings.
(53) First, with regards to the Notifying Party's claim that ADN C3 and ADN C4 are technically substitutable, the information provided by BASF13 does not support this finding. The Notifying Party in fact indicates that switching between ADN C3 and ADN C4 is possible, but such a switch cannot be made seamlessly and in a short period of time without causing at least some disruption to the HMD production process. […]. The Commission understands that these costs are additional to […] indicated in the Response to the 6(1)(c) Decisions necessary to then perform the switch from ADN C3 to ADN C4 itself. The Commission also understand that these costs, in direct investment, time and production disruption, would occur each time that a company producing HMD were to switch between ADN C3 and ADN C4, or vice versa.
(54) In its Response to the 6(1)(c) Decision, the Notifying Party further explains that HMD producers choice of raw material depends upon the cost, and that the alleged perfect substitutability of the two type of ADN would allow for an almost immediate switch. The Notifying Party however did not provide any evidence supporting this statement, but rather provided information pointing towards the opposite conclusion:
(a) First, notwithstanding the change in price differential between ADN C3 and ADN C4, the Notifying Party […];
(b) Second, the Notifying Party explains that switching from ADN C4 to ADN C3 without needing to renegotiate the feedstock supply contracts is possible only for a marginal proportion of the HMD production. […].
(55) In its reply to the request for information number 10, the Notifying Party further claims that it considers multiple switching between using ADN C3 and ADN C4 as a viable business model to react to price fluctuation of either type of ADN. […] Switching from using ADN C4 to ADN C3 requires additional time, estimated by the Notifying Party to be 3 to 4 months […].
(56) The Commission takes the view that – contrary to the Notifying Party's claim – it would not be sustainable to switch between ADN C4 and ADN C3 in response to a 5-10% price increase as such a switch would cause disruption to the production process which would make the cost savings less attractive.
(57) The Notifying Party explains that "switching from ADN C4 to ADN C3 possibly leads to an increase of necessary financial expenses as the catalyst consumption in the hydration process […] compared to the use of ADN C4 in the HMD production. The use of ADN C3 leads to additional by-products in the HMD production" […].
(58) The Commission therefore concludes that the additional cost and time required to switch a meaningful proportion of supply from ADN C4 to ADN C3 (and vice-versa) further advocates against substitutability of ADN C3 and ADN C4.
(59) This conclusion is further supported by the information provided by […], which indicated that "(t)he change in the system that would be needed to start using C3 ADN would be huge in terms of costs and time", the magnitude of the investment would be so significant that "together with the logistic difficulties and the higher price of the inputs (ADN from Ascend is more expensive) would force […] out of the market". These significant investment and long time frame are caused by technical barriers to perform such a switch, and more in detail "ADN needs to be hydrogenized and distilled to produce HMD. The condition reactions with the catalysts to hydrogenize the ADN are different depending on the technology used to produce the ADN (C3 vs C4)".14
(60) […] indicated that switching from using ADN C4 to using ADN C3 requires "customer qualification and to modify the facility’s environmental permit".15 Such regulatory requirements pose an administrative burden on HMD producers, further negating the substitutability of ADN C3 and ADN C4.
(61) This is partially contradicted by […], a manufacturer of ADN C3, which stated – though without providing any level of detail – that it can use both ADN C3 and ADN C4 in its production process. The Commission notes, however, that […] utilises ADN C3 and not ADN C4 and therefore […]’s reply may not be material for the purposes of the Commission’s assessment. The production process of ADN C3 generates more by-products than the production process of ADN C4 and, as acknowledged by the Notifying Party in its Response to the 6(1)(c) Decision, ADN C3 is less pure and has a higher content of water than ADN C4. The Commission therefore contends that producers of ADN C3 can switch more easily to ADN C4 than ADN C4 producers can switch to ADN C3.
(62) With reference to the Notifying Party's argument that the water content of HMD is irrelevant for the production of AH Salt, the Commission observes that this is not relevant in the assessment of substitutability of ADN C3 and ADN C4. According to paragraph 17 of the Commission Notice on the definition of the relevant market for the purposes of Community competition law,16 the relevant test for assessing whether two products belong to the same product market is "whether the parties' customers would switch to readily available substitutes (…)". In order to assess whether two products are substitutable, the Commission must therefore assess the behaviour of the customers of ADN (the producers of HMD) and not of those of HMD (e.g. the producers of AH Salt). Whether the water content of HMD is important for AH Salt manufacturer is therefore not of relevance for this assessment as AH Salt manufacturers are not ADN customers, but rather they purchase HMD.
(63) From the supply-side, the market investigation carried out in the first phase indicated that switching from producing ADN C4 to producing ADN C3 and vice versa is not feasible for both economic and technical reasons. Respondents to the market investigation indicated that both the investment and the time required to perform such a switch would be significant. The switch from ADN C3 to ADN C4 is further
complicated by the fact that access to proprietary intellectual property (“IP”) belonging to Invista is necessary, and Invista is not licensing that IP on the market.17 This finding was not contested by the Notifying Party.
5.2.1.6. Conclusion on product market definition
(64) Based on the assessment in recitals 50 to 63, the Commission concludes that, for the purpose of assessing the Transaction, ADN C4 constitutes a distinct product market.
5.2.1.7. Geographic market definition
5.2.1.8. The Commission precedents
(65) The Commission has not analysed the geographic scope of the market for ADN in the past.
5.2.1.9. The Notifying Party's view
(66) In the Form CO, the Notifying Party argues that the geographic market is global in scope for the following reasons:
(a) ADN is predominantly manufactured in the USA and the EEA and demand in Asia is mainly supplied by imports due to insufficient local capacities;
(b) most global sales are attributed to Invista and the Notifying Party estimates that the ADN market price in 2016 is at most [0-10]% lower in China than in the EEA;
(c) the EEA exports approximately [0-10]% ([…] kts) of its ADN production to either Brazil or Asia. The USA exports approximately [10-20]% ([…] kts) of its ADN production to either Brazil or Asia. China and Brazil import [90- 100]% of their ADN requirements from either the EEA or the USA;
(d) transport costs between the USA and the EEA and between the EEA and Brazil amount to approximately [10-20]% of the overall product value;
(e) in case of production shortfalls in the EEA, additional capacities are shipped from the USA to the EEA within a short timeframe.
(67) In its Response to the 6(1)(c) Decision, the Notifying Party further submits that the existence of significant interregional trade of ADN in Asia in particular demonstrates the economical transportability of ADN. The Notifying Party claims that the responses to the Phase I market investigation claiming that a business model based on importing ADN is unsustainable are implausible. The fact that HMD / PA 6.6 BP demand in Asia is constantly growing on the basis of ADN imported from the EEA and the USA shows that any technical challenge that may exist can be dealt with easily and in an economical way.
(68) As regards the price of ADN, and absent any available public information, the Notifying Party restates in the Response to the 6(1)(c) Decision that it assumes that the ADN market price in 2016 is at most [10-20]% lower in China than that in the EEA. In the Response to the 6(1)(c) Decision, the Notifying Party further claims that the lower ADN price in China makes transportation of ADN economically viable.
(69) Finally, the Notifying Party claims that the increased value chain complexity in sourcing ADN from outside the EEA is not an impediment. According to the Notifying Party, effective demand planning is manageable for ADN customers and the very fact that significant amounts of ADN are traded interregionally shows that such demand planning is possible.
5.2.1.10. The Commission's assessment
(70) The market investigation carried out during the first phase strongly contradicted the Notifying Party's views, and gave indications pointing towards an EEA-wide market. It emerged that there are both technical and economic consideration that limit the transportability of ADN.
(71) The investigation in Phase II further strengthened the initial finding of an EEA-wide market.
(72) As regards the Notifying Party's claim that the existence of interregional trade is evidence of a worldwide market, the Commission disagrees In the Commission’s view, the Notifying Party focuses almost exclusively on trade flows to China as evidence of a broader geographic market yet there is no ADN production in China, thereby forcing Chinese suppliers to resort to import. As Chinese customers do not have any alternative to importing ADN, it makes no sense to consider it as a proof that the market is worldwide. The situation for Brazil is the same as for China.
(73) The Notifying Party also stated that it […], referencing an EEA-wide market. Information obtained by the Commission in the course of the market investigation further confirms the absence of any material trade flow of ADN into the EEA. To the contrary, the result of the market investigation indicates that ADN is only exported from the EEA to Asia and South America.18
(74) With regard to the Notifying Party claim that ADN can be technically transported, the Commission observes that it never argued that transporting ADN is impossible, but rather that doing so is not a sustainable business model and therefore customers would not resort to imports in response to a 5-10% price increase. In the words of […], an indirect customer, imports “may be acceptable short term and for small volumes to ensure the continued supply in response to a force majeure event, but they are unsustainable over a mid- to long-term, i.e. as a business model.”19 Contrary to the Notifying Party's claim, this finding strongly supports the determination of an EEA-wide market: in a shortage situation, the alternative to importing from outside the EEA is to shut down production, and it is only under these extreme conditions that EEA customers would source from outside the EEA. Conversely, no respondent to the market investigation indicated that it has or would import ADN from outside the EEA in response to a price increase. 20
(75) This is further supported by the information provided by […] in the course of the Phase II market investigation, which explained that "on cost point of view, obviously, the transport from overseas country compared with the one from Chalampé (F) is completely different and uncompetitive", this is because "the more realistic way to transport ADN from US to […] plant is in ISO Container, notoriously more expensive compared with transport via Train, as […] normally does from Chalampé. The cost composition is made by, the fulfilment of every ISO, the inland movement from Decatur (Alabama) to the nearest port (typically, Savannah port in Georgia), the trans-oceanic shipment to Genoa port (Italy), the inland transport to […] Plant in Novara (Italy)". […] further specifies that "In a normal market situation (historically speaking) the magnitude of the transport cost from North America, even considering an ideal price parity of the material on FCA basis, lead to compromise any marginality or, even more, to create losses".21
(76) The Phase I market investigation also indicated that import tariffs are significant, at 6.5% for ADN, and further point towards an EEA-wide market. This was confirmed by the Phase II market investigation.
(77) The Phase I market investigation also indicated that long delivery times for imported ADN also create additional costs and complexity in the supply chain. This conclusion was supported by the Phase II market investigation. For example, […] explained that, in addition to the increased costs and complexity, "The main constrain in […] supply chain, in case of regular ADN import from North America would consist in a pure additional action, which would consist in the control and in the management of the blend, at the right rate, of the two kinds of material. That it means new storage equipment, mixers, checks and analysis, plus another kind of transport (ISO’S vs Train) to be managed". 22
(78) Finally, with reference to the alleged [10-20]% difference between the price of ADN in the EEA and the price of ADN in China mentioned in recital (67(b)), the Commission firstly observes that – as acknowledged by the Notifying Party itself – there is no publicly available data to support this statement. Secondly, to the extent it is relevant, this information rather advocates in favour of the fact that the conditions of competition, at least in terms of price, differ in different regions of the world, thus excluding a worldwide market for ADN.
5.2.1.11. Conclusion on geographic market definition
(79) Based on the assessment in recitals (70) to (78), the Commission concludes that the market for the supply of ADN C4 is EEA-wide in scope.
5.2.1.12. Conclusion on the market definition for ADN
(80) In conclusion, the Commission will carry out its competitive assessment on a market for ADN C4 defined as EEA-wide in scope.
5.2.2. Nitric acid
5.2.2.1. Introduction
(81) Nitric acid is an aqueous solution of hydrogen nitrate. Nitric acid is used predominantly in the fertiliser industry (approximately 80% of the total nitric acid consumption). In the area of polyamide (approximately 3% of the total nitric acid consumption), it serves as raw material for the production of adipic acid (Level II) when processed with KA Oil.
(82) Nitric acid has numerous other uses in the chemical industry, such as metal treatment, cleaning, adhesives, as intermediate, in the production of polyurethanes, of dyestuff and explosives and also of adipic acid.
(83) Nitric acid is supplied in several concentrations: weak nitric acid (concentration between 54% and 65%), azeotropic nitric acid (68% concentration) and concentrated nitric acid (concentration of 98% to 99%).
(84) Both BASF and the Business produce nitric acid mainly for their captive demand, however part of the production is also sold on the merchant market.
5.2.2.2. Product market definition
(85) In a previous decision, the Commission considered that weak nitric acid, azeotropic nitric acid and concentrated nitric acid form part of separate product markets. These findings were due to lack of demand side substitutability, as each type of nitric acid has different applications and prices, and the very limited supply side substitutability.23 The exact product market definition was, however, left open.
(86) The Notifying Party agrees with the Commission's previous decisional practice.24
(87) The results of the market investigation largely confirm the Commission's decisional practice.
(88) On the demand side, customers responding to the market investigation indicated that they purchase only one grade of nitric acid and that switching – albeit technically possible – would require a significant investment. A customer also indicated that normally market participants would not switch between grades.25
(89) On the supply side, producers of nitric acid responding to the market investigation indicated that they generally produce only one grade, mostly for internal consumption. […] explained that the technology required to produce the different grades varies and the investment required to perform the switch between the different technologies is significant.26
(90) As the outcome of the competitive assessment of the Transaction remains the same under all alternative market definitions, it is not necessary for the Commission to conclude on the exact product market definition.
5.2.2.3. Geographic market definition
(91) As regards weak nitric acid the Commission has considered in a previous decision that the geographic market is mainly national or regional, e.g., in the area of North Western Europe (France, Germany, Denmark, Belgium, The Netherlands and Luxembourg). In a more recent decision, the Commission also took into account a possible wider regional geographic market within a radius of 1,000 km around each production plant.27
(92) The Notifying Party, in line with the Commission’s decisional practice, submits that the geographic market is at least 1,000 km around each production plant, and can ultimately be left open.
(93) The results of the market investigation broadly corroborated the Commission’ recent decisional practice. The overwhelming majority of customers responding to the market investigation in fact indicated that they source nitric acid within 1,000 km from their plants. Respondents indicated that this is due both to the high transport cost compared to the overall price of the product and to the logistic complication inherent in the corrosive nature of nitric acid.
(94) As the outcome of the competitive assessment of the Transaction remains the same under all alternative geographic market definitions, it is not necessary for the Commission to conclude on the exact geographic market definition.
5.2.3. KA Oil
5.2.3.1. Introduction
(95) KA Oil is the industry term for the unrefined mixture of cyclohexanone and cyclohexanol (ketone and alcohol) which is produced by the oxidation of cyclohexane. KA Oil can be used directly for the production of adipic acid without further processing.28 For the production of caprolactam, pure cyclohexanone is needed. Such purecyclohexanone can be derived by separating KA Oil into cyclohexanone and cyclohexanol.
(96) The most significant volumes of KA Oil are used for the production of caprolactam (approximately 65%); the remaining volume is mainly used for the production of adipic acid (30%) and solvents (<5%).
5.2.3.2. Product market definition
(97) The Notifying Party submits that KA Oil constitutes a distinct product market, as there are no real substitutes for KA Oil to produce AA, and because individual product specifications are absent. KA Oil in its pure form can be used without further distillation in the production process for AA.
(98) The Commission has not previously analysed the product market for KA Oil.
(99) The Notifying Party is of the opinion that both distilled and undistilled KA Oil belong to the same product market, as the production process of adipic acid in any case involves producing undistilled KA Oil. In some cases, KA Oil will then be distilled into cyclohexanol and cyclohexanone. All KA Oil producers are able to do this, without much added difficulty or cost.
(100) The evidence in the Commission's file has not provided any indication which would suggest that further sub-segmenting the KA Oil market would be appropriate.
(101) As the outcome of the competitive assessment of the Transaction remains the same under all alternative market definitions, it is not necessary for the Commission to conclude on the exact product market definition. However, for the purpose of this Decision, KA Oil will be regarded as a separate product market.
5.2.3.3. Geographic market definition
(102) The Commission has not previously analysed the geographic market for KA Oil.
(103) The Notifying Party submits that the relevant geographic market for KA Oil is worldwide or at least EEA-wide, in particular because transportation costs are low, there are no barriers to trade and imports taxes are not relevant.
(104) The Commission takes the view that it is not necessary to conclude on the exact geographic market definition as the Transaction does not raise competition concerns irrespective of the precise geographic market definition.
5.3. Level II of the polyamide value chain: HMD
5.3.1. Introduction
(105) HMD (level II in the polyamide value chain) is, together with adipic acid (also level II), a precursor of AH Salt (level III) which is then polymerised into PA 6.6 BP (level IV). Outside the polyamide value chain, it is also used to produce Hexamethlyene diisocynate ("HDI"), a chemical product that is used for the production of paints and coatings.
(106) HMD is produced through the hydrogenation of ADN (Level I). The hydrogenation of HMD is a two-step process through which ADN is first hydrogenated into a crude product (“crude HMD”) and then purified (“pure HMD”).
(107) HMD is a monomer used, in particular, for the production of different polyamides. The major part of the HMD that is produced globally is consumed in the PA 6.6 BP production (approximately 85%). The remainder is used in a number of different applications including as an intermediate for other polyamides such as PPA, PARA, and PA 6.10, and for coatings and biocides. The most significant non-polyamide application of HMD is the coatings precursor HDI. Further, HMD is used for the production of plastic additives, adhesives, inks, water treatment and construction.
5.3.2. Product market definition
5.3.2.1. The Commission’s precedents
(108) The Commission has previously considered that HMD may form a distinct product market. In Solvay/Rhodia, the Commission carried out a market investigation, which implied no need for a further segmentation of the market for HMD, but ultimately left open the exact product market definition. 29
5.3.2.2. The Notifying Party's view
(109) The Notifying Party agrees with the Commission's past decisional practice and submits that HMD constitutes a separate product market and that no further segmentation is appropriate.
(110) According to the Notifying Party, there are only two types of HMD, i.e. anhydrous HMD (HMD content >99,5%) and less concentrated HMD (typically 98% HMD content or less).
(111) According to the Notifying Party, on the supply-side, all producers in the EEA manufacture anhydrous HMD, and if necessary add water to the product to obtain less concentrated HMD.
(112) In the Response to the 6(1)(c) Decision, the Notifying Party adds that Anhydrous HMD can be easily de-filtered to less concentrated HMD. Such de-filtering technology is easily available and inexpensive. De-filtering is a standard process that does not require special technology and costs for this process are negligible. In practice, water is pumped into a tank with anhydrous HMD, diluting it to the desired degree. In the past, BASF produced [90-100]% HMD from anhydrous HMD using this process.
(113) On the demand-side, HDI producers need anhydrous HMD. AH Salt producers can use both anhydrous and less concentrated HMD (minimum 90% HMD content) but typically source anhydrous HMD given the supply conditions.
(114) BASF produces, sells and uses […]. The Commission understands, based on the information provided in the Form CO that the Business […].
5.3.2.3. The Commission's assessment
(115) The market investigation results indicate that HMD should be considered a separate product market. AH Salt and HDI can only be produced through HMD and there are no alternatives.
(116) With regard to the differentiation between anhydrous HMD (HMD content >99,5%) and less concentrated HMD (typically 98% HMD content) the investigation was inconclusive as to whether they belonged to the same or different product markets.
(117) On the demand-side, most of the customers only source anhydrous HMD, however it is possible to use less concentrated HMD for the production of AH Salt. In contrast, HDI producers can only use anhydrous HMD because “water content specification is a key requirement for the phosgenation process (used for the production of HDI) as water will lead to the formation of highly insoluble amine hydrochloride salts. These compounds lead to many production issues (drop of yields, clogging of filters and equipment ...) as well as safety issues (leakages due to corrosion)”.30
(118) On the supply-side, the market investigation showed that in the EEA both anhydrous HMD and less concentrated HMD are being produced. However, contrary to the Notifying Party's claims, one producer indicated that it is only producing anhydrous HMD and that “to produce different grades it would be necessary to invest in a loading station with tanks”.31
5.3.2.4. Conclusion on product market definition
(119) In the light of the considerations in recitals (105) to (118) , as the outcome of the competitive assessment of the Transaction remains the same and the Final Commitments entered into by the Notifying Party address the Commission’s concerns under all alternative market definitions, irrespective of whether the relevant product market is defined as comprising anhydrous HMD and less concentrated HMD or should be further segmented into these two categories, it is not necessary for the Commission to conclude on the exact product market definition.
5.3.3. Geographic market definition
5.3.3.1. The Commission’s precedents
(120) As regards the geographic market definition the Commission has previously considered the geographic scope of the market for HMD to be at least EEA-wide but ultimately left open the exact market definition.32
5.3.3.2. The Notifying Party's view
(121) The Notifying Party submits that there is significant evidence suggesting that the relevant geographic market for HMD is global in scope. For example, some market players source HMD on a worldwide basis, there […] and there are imports from the USA to the EEA as well as from the USA and the EEA to Turkey, Israel and Asia. The Notifying Party also submits that international trade flows represent [20-30]% of the worldwide sales and transportation costs for HMD are rather low (approximately [10-20]% of the total sales price) supporting a global reach.
(122) In the Response to the 6(1)(c) Decision, the Notifying Party continues to sustain that the HMD market is global in scope and provide further arguments.
(123) First, the Notifying Party submits that transportation costs for HMD are rather low (approx. [10-20]% of the total sales price)33 and that transportation costs for sourcing HMD on a global level amounts to an additional [0-10]% of the overall sales value at most. According to the Notifying Party, the main reason would be that important HMD customers like […] would benefit from convenient locations for the sourcing of imported HMD. The Notifying Party also submits that […] transportation costs would increase only slightly if it stopped sourcing HMD from the Business' production plant in Belle-Etoile (France), irrespective of whether the alternative HMD supply would come from a production site within or outside the EEA.
(124) Second, according to the Notifying Party, there are significant exports from the EEA to other regions of the world, mainly to Asia, thus supporting a global scope of the HMD market.
(125) Third, the Notifying Party points out that the technical requirements for the transportation of HMD into and out of the EEA do not differ from the requirements for the transportation within the EEA.
(126) Fourth, the Notifying Party submits that while the existing swap agreements between HMD producers help to reduce overall costs, the 6(1)(c) Decision errs when denying the economic transportability of HMD by referring to [REFERENCE TO THE BUSINESS’ COMMERCIAL STRATEGY].
(127) Fifth, the Notifying Party claims that long delivery time are easily manageable through demand planning and a consistent procurement organisation. Further, statements of market participants arguing that imports may only be acceptable in the short term in response to a force majeure, show that the delivery time cannot be of relevance for the supply chain because a force majeure event by definition require a fast reaction.
(128) Finally, according to the Notifying Party, regulatory provisions constitute only a negligible barrier to trade.
5.3.3.3. The Commission's assessment
(129) The results of the market investigation in Phase I provided strong indications that the market for HMD is EEA wide in scope. The investigation in Phase II has not provided any element speaking in favour of a global market and has confirmed that the geographic market is EEA in scope.
(130) First, transportation costs and custom tariffs increase significantly the price of HMD imported into the EEA. The Notifying Party submits that HMD custom tariffs are usually 6.5% and the extra costs for freight from outside the EEA would amount to approximately [0-10]% of the total sales price and that these costs are […].34 However, the market investigation shows that transport costs from outside the EEA, in addition to the tariffs, have a very significant impact on the overall cost and are significantly higher than what the Notifying Party claims. Indeed, transport costs are identified as a barrier to trade across regions of the world by some producers and virtually all customers.35 Along this line, […] explains that “sourcing from non-EEA countries is possible but currently uneconomical and has a negative impact on the competitive positioning on the downstream market(s). In particular, the price for transcontinental imports of HMD is significantly higher than for HMD produced locally”.36 Another important customer of HMD, […], adds that “sourcing ADN or HMD from outside Europe is not competitive. Technically it is possible to bring HMD from USA, but it is much more expensive”.37 […] continue explaining that, “the price for HMD increases significantly if it is imported from outside Europe: in addition to high transportation costs relating to hazardous goods, the product needs to be shipped with specific conditions to maintain its integrity (insulated & heated)”38. It adds that “non-European producers do not constitute viable alternatives to Solvay due to significantly increased prices for potential import of HMD from overseas”.39 […] also submits that "transport of HMD is expensive. Transport by sea, e.g., requires ships with specially equipped tanks. Shipping HMD from the US to Europe, e.g., increases the variable costs significantly, e.g. by 15- 20%".40
(131) Second, according to the Notifying Party, imports to the EEA were as follows: […] kts in 2014; […] kts in 2015 and […] kts in 2016. These figures represent a very small part of the total HMD production in the EEA and of the merchant market. For example, in 2016 imports represented less than [0-10]% of the total HMD production in the EEA and less than [0-10]% of the merchant market. This is confirmed by the market investigation in which all respondents confirmed that they purchase HMD to be used in their plants located in the EEA either exclusively or for the vast majority of their needs within the EEA.41 […] explains that imports are done in response to shortages or force majeure events, but not as business model: “imports from outside Europe may be acceptable short term and for small volumes to ensure the continued supply in response to a force majeure event, but they are unsustainable over a mid- to long-term, i.e. as a business model”.42 Another customer, […], adds that “the HMD can be sourced from the USA, but it is much more expensive and supply chain is very complex as product has to be heated”.43
(132) The Notifying Party also provide a number of examples of trade across regions and in particular of exports from the EEA towards Asia. However, the existence of exports from the EEA to other regions does not prove that the geographic market is broader in scope. The rationale of the exports from the EEA to Asia can find their explanation in other factors, like for example the lack of ADN production in Asia or structural shortages of the HMD market. For example, […] explains that "in China, the market for HMD is short, but they have a big capacity in the downstream markets. Therefore, demand of HMD in China is strong. In […] opinion, this can make exports to China attractive for HMD producers".44 The relevant factor in this case is that imports into Europe do not represent a competitive constraint because they represent negligible volumes and because the overwhelming majority of customers consider than importing from outside the EEA is uneconomical as explained in this section.
(133) Third, HMD is difficult to transport over long distances due to certain technical characteristics, as it needs to be heated and insulated from moisture. All customers responding to the market investigation considers that there are technical characteristics of HMD that limit its transportability.45 “HMD's transportation requires careful handling its exposure to moisture and to be kept at temperatures above 40°C, so that it remains liquid, which makes it significantly more complicated and expensive to source from outside Europe”.46 In general, customers consider that the main requirement is to keep temperature constant and avoid exposure to air or light, which is very costly.47 The vast majority of competitors also confirmed in the market investigation that there exist technical characteristics of HMD that limit its transportability.48 For example, […] explains that temperature should be kept constant, that HMD should travel in a nitrogen atmosphere to avoid exposure to oxygen and that special safety handling is required.49 […] adds that "another barrier is the availability of specialized logistics and handling. Special ships and tanks and storage at both loading and receiving ends are required"50.
(134) Moreover, while the Notifying Party claims that the technical requirements for the transportation of HMD into and out of the EEA do not differ from the requirements for the transportation within the EEA, importing from outside the EEA means that the distance is higher and delivery time is longer, making logistics more complicated. Storage time influence HMD final characteristics. […] explains that "during transportation, storage and re-heating process of HMD, side reactions with air or oxygen together with temperature exposure could occur if HMD is improperly stored or exposed for too long. These reactions will damage product characteristics and performance such as color, reactivity…".51 […] continues explaining that "obviously, transportation costs, long delivery periods, as well as risks from transhipments shall be taken into consideration in case of sourcing non negligible volumes on lasting basis from outside the EEA. The technical requirements of HMD transportation has also significant impact on the cost of imported HMD".52 […] submits that for imports from outside the EEA "delivery time [is] almost 6 weeks".53 In the same vein, […] explains that "HMD is costly to transport. It is toxic and corrosive and must be shielded from oxygen and kept under a nitrogen blanket the entire time. It must also be kept above a temperature of 40°C as it otherwise solidifies. For these reasons, the transport of HMD is expensive. Transport by sea, e.g., requires ships with specially equipped tanks. Normally HMD being transported by sea in bulk is diluted with water to depress the freezing point, which adds to the costs as the water is transported as well. Shipping HMD from the US to Europe, increases the variable costs significantly, e.g. by 15-20%."54
(135) Fourth, the existence of swap agreements between HMD producers with facilities in different regions indicates that transport between regions is often not economical. For example, [REFERENCE TO THE BUSINESS’ COMMERCIAL STRATEGY]. Contrary to the Notifying Party view, this argument holds true even if [REFERENCE TO THE BUSINESS’ COMMERCIAL STRATEGY]. As the Notifying Party recognises, the rationale of the existing swap agreements between HMD producers is to "help to reduce overall costs".55 The distance that the HMD needs to travel between the […] and […] is less than the distance that the HMD would have to travel between the […] and […]. Moreover, […].
(136) Finally, while half of the competitors consider that there are no barriers to trade at global level, the other half believe that high transportation costs and long delivery periods are barriers to trade at global level.56 On the customer side, contrary to the Notifying Party's claim that long delivery times are easily manageable, all customers unanimously consider that long delivery periods are important barriers to trading HMD across regions of the world.57 Furthermore, the statements of market participants arguing that imports may only be acceptable for short term in response to a force majeure event do not show that delivery times are irrelevant. These responses from customer show under what conditions EEA customers would source HMD from outside the EEA. That is, only when there is no availability of HMD in the EEA. In a shortage situation, there is no consideration of price differences between regions of the world or logistic difficulties, because the alternative to importing from outside the EEA is to shut down production. It is only under these extreme conditions that EEA customers would source from outside the EEA. It is also important to note that force majeure situations are relatively frequent in this industry and may last up to several months. Under these circumstances, customers accept the significant additional costs and logistical difficulties involved with imports, including longer lead times.
(137) Moreover, all customers responding to the market investigation indicate that transportation costs are barriers to trading HMD across the world. The majority of those customers also consider that tariffs are a barrier to trade, while other customers also mention regulatory barriers. […], a major customer, explains “obviously, transportation costs, long delivery periods, as well as risks from transhipments shall be taken into consideration in case of sourcing non negligible volumes on lasting basis from outside the EEA. The technical requirements of HMD transportation have also significant impact on the cost of imported HMD”.58 Another customer, […], explains that regulatory barriers exist, as HMD must be REACH-registered.59 Although the importance of regulatory barriers should not be overstated, it adds to the numerous and important barriers described in this section.
5.3.3.4. Conclusion on geographic market definition
(138) Based on the assessment in recitals (120) to (137), the Commission concludes that, for the purpose of assessing the Transaction, the market for the supply of HMD is EEA-wide in scope
5.3.4. Conclusion on the market definition for HMD
(139) As the outcome of the competitive assessment of the Transaction remains the same and the Final Commitments entered into by the Notifying Party address the Commission’s concerns under all alternative market definitions, it is not necessary for the Commission to conclude on the exact product market definition for HMD, which is EEA-wide in scope for the purpose of assessing the Transaction.
(140) In conclusion, the Commission will carry out its competitive assessment on a market for HMD defined as EEA-wide in scope.
5.4. Level II of the polyamide value chain: adipic acid
5.4.1. Introduction
(141) Adipic acid is an aliphatic diacid consisting of 6 carbon atoms. The most established commercial production process entails a mixture of cyclohexanol and cyclohexanone (commonly known as KA Oil, and represented at level I in Figure 1). The KA Oil is oxidised with nitric acid (also level I) to generate Adipic acid, via a multistep pathway (i.e., through several production steps), as follows:
(a) Liquid cyclohexane is air-oxidised through a cyclohexyl hydroperoxide intermediate to a cyclohexanone-cyclohexanol mixture;
(b) KA Oil is oxidised to adipic acid by nitric acid in the presence of a catalyst; and
(c) Adipic acid is cleaned from impurities.
(142) Within the polyamide value chain, adipic acid is mixed with HMD to produce AH Salt (level III), the precursor to PA 6.6 BP (level IV). Outside the polyamide value chain, adipic acid has other equally important applications including polyurethane, flexible foam, plasticisers, polyester TPU thermoplastic and synthetic leather.
5.4.2. Product market definition
5.4.2.1. The Commission’s precedents
(143) In Solvay/Rhodia, the Commission considered that adipic acid may form a distinct product market.60 In that case, the Commission carried out a market investigation in which the respondents did not point to any further segmentation of the product in question. Ultimately, the Commission left the product market definition open.
5.4.2.2. The Notifying Party's view
(144) The Notifying Party agrees with the Commission's past decisional practice and submits that adipic acid constitutes a separate product market and that no further segmentation is warranted.61
5.4.2.3. The Commission's assessment
(145) The outcome of the market investigation indicates that adipic acid should be considered a separate product market, as there is no alternative to produce AH Salt and other products like polyurethane, flexible foam, or plasticisers. Conversely, the market investigation did not reveal a need to segment this market further.
(146) Certain respondents – and the Notifying Party – have occasionally referred to different adipic acid grades. Others have objected that adipic acid is a single commoditised product, though quality levels may vary across producers, notably in relation to imports from China. Thus […] explained that: "Adipic Acid is a commodity product that can vary by quality and colours".62 Likewise, […] indicated that adipic acid "is the same everywhere" but that there can be "quality differences between different market players", pointing in particular to supplies from China.63
(147) Overall, the market investigation has not revealed that adipic acid produced in the EEA could be of such different nature, composition or presentation so as to be suitable for certain applications and not others. Conversely, EEA suppliers can typically accommodate varying customer requirements. Thus, respondents have indicated that "Adipic Acid quality is consistent in Europe",64 and that “European producers of Adipic Acid produce roughly equivalent products. Customers specify the characteristics of Adipic Acid they want and [the producer] provides the product. Once a quality is approved, it does not matter who the actual manufacturer is”.65
5.4.2.4. Conclusion on product market definition
(148) The Commission therefore concludes that, for the purpose of assessing the Transaction, adipic acid constitutes a distinct product market.
5.4.3. Geographic market definition
5.4.3.1. The Commission’s precedents
(149) In terms of geographic market definition, the Commission considered in Solvay/Rhodia that the geographic scope of the market for adipic acid was at least EEA-wide in scope. Respondents to the market investigation in that case indicated that, in principle, they source adipic acid on an EEA or worldwide basis. Ultimately, the Commission left open the precise market definition in that case.66
5.4.3.2. The Notifying Party's view
(150) The Notifying Party originally submitted that the geographic market for adipic acid should be defined as global in scope because, in essence, transportation costs and import duties do not hamper the possibility to trade adipic acid over long distances and thus to procure from suppliers located outside of the EEA, notably in view of the existing price gap between the EEA and China.
(151) In response to the Article 6(1)(c) Decision, the Notifying Party elaborated as follows:67
(a) Adipic acid quality requirements do not constitute an obstacle to imports for the vast majority of EEA customers. Whereas different adipic acid grades (standard and TPU) can be distinguished in relation to Chinese imports, the Notifying Party considers that the majority of EEA customers do not require adipic acid of a specific "better" quality, that the Chinese standard adipic acid quality would be sufficient to cover most of the EEA demand and that the largest Chinese adipic acid producers produce the higher adipic acid grade of a quality comparable to that of EEA producers;
(b) Long distance transportation does not affect the quality of adipic acid to make it an obstacle to imports. In particular, the Notifying Party considers that the solidification of adipic acid resulting from its storage over a long period and its exposure to humidity – known as "caking" – does not constitute a significant impediment to importing adipic acid from outside the EEA due to the possibility of sourcing adipic acid in big bags and to the availability of low-cost "crushing machines" to break up "clumped" adipic acid;
(c) Logistical arrangements required by the importation of adipic acid to the EEA do not constitute a significant limitation to import from China either. Specifically, the Notifying Party contends that the supply of imported adipic acid in big bags rather than bulk is not such as to make imports significantly more difficult or uncompetitive because a majority of European customers take delivery of adipic acid in big bags while the additional costs of processing adipic acid delivered in bags are limited. Moreover, a number of EEA distributors are active in the import of adipic acid and may alleviate the logistical constraints faced by smaller customers;
(d) Transportation costs, tariffs and longer delivery periods do not constitute an obstacle to imports of adipic acid either. In the Notifying Party's view, transportation costs and import tariffs are insignificant in the context of importing adipic acid into the EEA and the REACH registration process does not constitute a real barrier to entry;
(e) Longer delivery time and associated financial risks do not further constitute a significant barrier to imports. In effect, longer lead time between each delivery can be catered for by planning additional storage or compensated by procuring on the European spot market, whereas the financial risks due to price variations are low given the significant price gap between the European and Chinese adipic acid market;
(f) Chinese adipic acid capacity is sufficient to supply a large part of the entire EEA market and Chinese suppliers have a significant cost advantage over EEA producers. According to the Notifying Party, significant adipic acid spare capacity is available in China and capacity will continue to rise, whereas Chinese suppliers will adjust to the enforcement of environmental legislations in order to address temporary shutdowns. Moreover, the price gap between EEA and Chinese adipicacid remains material due to the significant cost advantage of Chinese over European suppliers;
(g) EEA customers can – and already do – rely on non-EEA suppliers for a very significant proportion of their adipic acid needs. The fact that a large number of EEA customers are procuring adipic acid from Chinese producers and that imports have increased significantly in recent years show that Chinese supply is clearly an alternative to domestic EEA production, thereby putting pressure on EEA prices. In effect, adipic acid imports from China increased because the price of imports became relatively cheaper than the price of adipic acid produced in the EEA. Moreover, the price of the adipic acid produced in the EEA and the price of Chinese imports of adipic acid are positively correlated and the correlation increases if the EEA prices are lagged, thus suggesting that EEA prices are influenced by Chinese import prices; and
(h) The Parties' customers often use Chinese producers of adipic acid as a credible threat in negotiations. In that regard, the Notifying Party provides anecdotal evidence of correspondences with customers over the 2012-2018 period allegedly […].
5.4.3.3. The Commission's assessment
(152) The outcome of the market investigation in Phase II does not concur with the Notifying Party's claims but rather supports the view that the market for adipic acid is EEA-wide in scope. In the Article 6(1)(c) Decision, the Commission acknowledged that the evidence available back then was inconclusive and that it would continue investigating the appropriate scope of the adipic acid market for the purpose of assessing the impact of the Transaction. While market participants continue to share different views, an overall assessment of the evidence obtained over the course of the in-depth investigation points more clearly in favour of an EEA-wide market.
(153) At the outset, in line with the Notice on the definition of relevant market,68 the Commission notes that, according to the Notifying Party, the overall demand (merchant + captive) for sdipic scid in the EEA amounted to approx. […] kts in 2016, of which imports represented approx. [0-10]% ([…] kts, the lowest import level since 2011).69 Imports have increased significantly in 2017, reaching approx. [10-20]% of the total demand of […] kts ([…] kts, the highest import level since 2011).70
(154) While imports from the United States have dropped significantly in 2016 (to approx. […] kts from […] kts in 2015 and up to […] kts in 2013), imports from China have increased to approx. […] kts in 2017, compared to approx. […] kts in 2016 and […] kts in 2015. Overall, it appears to be a commonly held view among market participants,71 including the Notifying Party,72 that Chinese imports to the EEA have replaced imports from the USA over the past couple of years due to certain plant closures in North America.73
(155) If, as claimed by the Notifying Party,74 imports of adipic acid for the purposes of the merchant market are primarily derived from China, these represented approx. [0- 10]% of EEA merchant sales in 2016 and [10-20]% in 2017. In contrast, the Parties accounted for more than [50-60]% of EEA merchant sales and [60-70]% of total EEA demand (merchant + captive), based on 2016 data.75 Altogether, EEA producers including the Parties, Radici and Lanxess Deutschland GmbH ("Lanxess", Germany), account for more than [90-100]% of total EEA demand.
(156) Imports from outside the EEA therefore represent a relatively moderate proportion of total EEA demand or merchant sales, even though they increased in 2017, thus questioning the reality of a global market. The Commission notes that the median adipic acid import volume between 2011 and 2017 was around […] kts/year, representing approx. [0-10]% of the 2017 EEA demand.
(157) In line with the Notice on the definition of relevant market, the reasons behind the market shares figures need to be explored based on an analysis of demand characteristics, to establish whether companies in different areas (primarily China) do indeed constitute a real alternative source of supply for consumers, and possibly an analysis of supply factors, to assess the impediments in developing sales throughout the whole alleged geographic market.76 While doing so, the Commission also addresses the Notifying Party's arguments.
5.4.3.3(A) Demand-side considerations
(158) As noted in the Article 6(1)(c) Decision, a significant number of customers are sourcing adipic acid from China, but these typically represent only a small percentage of the required amount.77 The Phase II investigation confirmed that those EEA customers sourcing adipic acid from China to feed their downstream manufacturing processes tend to import limited volumes, in line with the overall share of Chinese imports into the EEA, though variations apply depending on the applications for which adipicacid is used and the design of the customer's manufacturing processes. 78 Importantly, various limitations to a wider reliance on Chinese imports appear to remain in the opinion of EEA customers.
(a) Quality issues remain a source of concern for EEA customers
(159) The market investigation in Phase II confirmed the conclusions of the Article 6(1)(c) Decision in that a number of EEA customers, including very large ones,79 continue to view the quality of adipic acid supplies from China as being either inferior to that available from EEA producers, or as being of inconsistent quality across suppliers.80
(160) Admittedly, the quality concern may not be of equal magnitude across all industries. Thus, using adipic acid from China appears particularly problematic (and even "not an option"81) for food contact goods due to hygiene specifications but also for polymer (bioplastic) production, due to purity issues.82 Generally, quality development has been mentioned as an important requirement for a greater acceptance of Chinese imports,83 for "Chinese quality hasn't been improved enough yet".84
(161) Traders tend to be more positive about the quality of the material that they procure from China on behalf of certain EEA customers.85 However, they also concede that "[s]ome Chinese producers are not in line with the European producers' requirements".86 Likewise, the quality required by EEA customers is sometimes available only from a particular production line (e.g., Huafon's third production line).87 Moreover, the Chinese material appears to frequently require repacking and sometimes reprocessing to improve the physical appearance of the product.88
(162) Thus, notwithstanding possible improvements over the recent past,89 the quality of Chinese supplies appears to be an issue for a number of EEA customers and, combined with other considerations spelled out in this section continues to affect demand dynamics differently than vis-à-vis EEA producers.
(b) The "caking" issue and the unavailability of imports in bulk remain an important constraint for a number of EEA customers
(163) The fact that adipic acid is hygroscopic in nature and solidifies in block when transported over long distances by cargo, appears to be well known within the industry. Likewise, respondents to the in-depth investigation (including traders) concur to the effect that "clumped" adipic acid cannot be used as such in production lines, i.e., is "inoperable".90 Since that phenomenon derives from the physical characteristics of adipic acid, it is not clear how it could be alleviated in the short to medium term.91
(164) The Notifying Party argues that "it is in fact easy to break up the clumped AA with a simple crusher, to revert to a powder form AA".92 Admittedly, the impact of the caking issue may be more or less significant depending on the industrial equipment of the customer. Still, additional cost to crush caked adipic acid has been estimated between [5-10]% of the purchase price,93 which is not negligible and does affect the competitiveness of Chinese imports.
(165) Likewise, respondents to the market investigation are unanimous that Chinese material is not available in bulk.94 This constraint may also affect certain customers more than others. For example, […] explains that it can purchase both packaged and bulk quantities, which ensure greater flexibility, including in procuring from overseas.95 However, this is a significant limitation for other customers operating silo storage tanks from which the material goes directly into production. For these customers, the procurement of adipic acid in bags rather than bulk would entail efficiency losses and labour cost increases, while also raising safety concerns.96
(166) The procurement of adipic acid from China therefore faces significant physical constraints limiting the availability or attractiveness of Chinese supplies, at least for a category of customers, including large customers. As a corollary, these limitations, combined with other factors, also affect the constraints that Chinese imports can exercise on domestic EEA producers.
(c) Longer delivery periods and associated financial risks also limit the competitiveness of Chinese imports
(167) The investigation in Phase II has confirmed the findings of the Article 6(1)(c) Decision to the effect that importing from China entails longer and inconsistent delivery times, translating in greater financial risks and working capital requirements (due to the necessary adjustment of stocks and storage needs).97 In turn, the outcome of the market investigation in Phase II does not support the Notifying Party's views to the effect that price differentials between European and Chinese adipic acid would naturally mitigate financial risks, and that so would the possibility of outsourcing risks to traders and/or turning to the EEA spot market in case of shortage.
(168) In response to the market investigation, both large customers and traders have insisted on the volatility of Chinese adipic acid prices. Thus, according to […], that volatility of Chinese prices is significant, and significantly greater than that of EEA prices, whereas the delivery time differs between two months for Chinese supplies and one week from EEA deliveries.98 While generally more positive about the availability of Chinese supplies, […] also highlighted the risks arising from the longer supply chain when resorting to imports from outside Europe.99 This is consistent with […] earlier explanation that “Adipic Acid is traded on monthly basis. From one month to another the price difference can be very high (for example in 2017 we have seen changes of 25% and more within a month) depending on raw material price developments and product availability. Imports e.g. from Asia take around 6-8 weeks by sea transport. The European market could completely different at the time of product arrival”.100
(169) Interestingly, trader […] confirmed that the long transit time for adipic acid from China is a main disadvantage and has a material impact on the price risk for imported product. To become comparable to EEA supplies, according to […], Chinese suppliers would need to offer pricing based on delivery date in the EEA not shipment date from China, thus illustrating the significance of the risks in question and resulting lack of homogeneity in supply conditions.101 Likewise, […] explained that hedging is not a viable option as the derivative market for adipic acid is not well developed.102
(170) Overall, the significance of the financial risks associated with the long delivery time should also be considered against the narrowing down and possible reversal of the price gap between EEA and Chinese supplies in recent years, as discussed in the present section. For the sake of completeness, the Commission also notes that emergency supplies from China (Huafon) have occasionally taken place by train in recent times, thus shortening the delivery time to 18 days, but that these remain exceptional and are thus not representative of normal supply conditions.103
(d) Security of supply considerations significantly limit the attractiveness of Chinese imports
(171) In addition to confirming considerations already spelled out in the Article 6(1)(c) Decision, the in-depth market investigation has elicited additional concerns, especially in relation to the security of Chinese supplies as a result of recent disruptions resulting from regulatory enforcement.
(172) Based on the results of the market investigation, the Commission considers that the so-called "Blue Sky" policy has led to the tightening of the enforcement of environmental standards in China over the past couple of years, leading to the closure of certain production facilities, even if on a temporary basis (winter). These developments appear to have profoundly affected the perceived reliability of Chinese supplies in the eyes of EEA customers.
(173) Thus, according to […], "the Chinese supply is unreliable, since not available year round. Importing AA from China is therefore not a reliable option for the long- term".104 Likewise, […] highlights the "low supply reliability [of Chinese producers] due to unpredictable plant shutdowns in China because of environmental reasons or natural gas shortage".105 […] equally underlines that "with the recent local environment constraints in China, regular and consistent deliveries are not even guaranteed any longer which makes sourcing from China even less viable due to the ensuing security of supply issues" or, put otherwise, "due to safety, environmental or other local regulations, the supply reliability from China is very poor in the light of a number of unplanned shutdowns or significant reduction on plant output and their corresponding impact on the security of the supply".106 […] is also "concerned that the reliability of Adipic Acid supply from Chinese producers is significantly lower than from European producers (mainly driven by regulatory decisions)"; in turn, according to […], "supply reliability could generate a very big financial impact".107
(174) Traders equally acknowledge the seasonality of adipic acid supplies from China "as the offer is tighter in winter and larger in summer".108 Moreover, delivery delays have been experienced due to the enforcement of Chinese environmental regulations.109 For chemical distributor […], "[the] point is that the material [i.e., adipic acid from China] is not regular[ly] available, and that makes sustainable distribution very difficult".110
(175) There are therefore significant doubts among EEA customers about the reliability of Chinese imports, which mitigates their suitability as an alternative for a significant portion of demand and, as a result, limits the ability of such imports to materially constrain the Merged Entity in the future. Notably, regulatory enforcement appears to have significantly impacted the pricing of Chinese supplies, contributing to narrowing down the price differential with material produced in the EEA.
(e) Chinese imports do not seem to benefit from a significant price advantage (any longer)
(176) The Notifying Party's argument about the global scope of the adipic acid market relies heavily on an alleged price gap benefiting Chinese supplies and ensuring their competitiveness in spite of the costs associated with the various logistical constraints to overcome (transportation, customs, reprocessing, storage, etc.). The outcome of the market investigation does not support the existence of such significant price differential to the advantage of material imported from China. To the contrary, respondents consider that the gap has narrowed down significantly in recent years, or even reversed, thus cancelling out any price advantage.
(177) The outcome of the market investigation reveals indeed that the prices of adipic acid delivered by Chinese producers are now similar to – or even less attractive than – prices that can be obtained from EEA producers. The primary cause of that turn of event appears to lie with the need to improve production processes in order to address the stricter enforcement of Chinese environmental standards and achieve higher quality requirements, thus providing additional value to customers.111
(178) Thus, according to distributor […], adipic acid originating from China benefited from lower prices until some years ago but this is not the case anymore: "[t]here is currently no price advantage with regards to Chinese supplier. …Haili and Shenma and other State-owned companies, are still under restrictions and their prices are still higher than the ones offered by European suppliers".112 This coincides with the experience of a customer like […] facing higher purchase prices for Chinese imports since a couple of years: "[a]lthough the price per unit in China is similar to the one offered by European producers, there are additional costs due to logistic" which, in the end, affect the competitiveness of material originating from China.113 Likewise, […] explains that Chinese prices are similar to the European ones at the moment but are expected to increase in the coming months "leading Chinese materials to be more expensive than European materials".114 Similarly, for a large customer like […], "supply from China is seldom attractive from a price point of view" and Chinese imports are overall not a viable option in view of total delivered costs combined with security of supply issues.115
(179) Aside from the apparent inability of Chinese suppliers to offer preferential prices, contrary to the Notifying Parties' argument, the market investigation has also revealed limited incentives in doing so. Distributor […], for example, reported having entered into a strategy with a Chinese supplier aimed at "not damaging the European market and also respecting the market price level when using Adipic Acid imported from China", including by designing pricing formulas similar to those used by EEA producers.116 In the Commission's view, this illustrates a willingness to opportunistically serve the EEA market for residual demand requirements instead of exercising effective constraints on domestic suppliers.
(180) At the end, it appears that the constraints in terms of quality and delivery translate into a competitiveness issue for Chinese producers, who appears unable to leverage – at least to the same extent as before – comparative advantages in terms of labour costs and costs of capital. Combined with security of supply concerns, this seriously questions the constraints that Chinese imports exercise today on EEA producers and could effectively impose on the Merged Entity in the future. In that regard, the next section discusses the scope and relevance of the alleged correlation between Chinese and EEA prices.
(f) The reality of the constraint exercise by Chinese imports over EEA prices is questionable and the Notifying Party's price correlation data are not determinative
(181) The Notifying Party submitted that the geographic market for adipic acid is global in scope, and that, in case of a hypothetical price increase across the EEA, the sales lost by EEA producers to Chinese or other non-EEA producers would be sufficient to make this hypothetical price increase unprofitable.117 The Notifying Party also claimed that this would likely be achieved through EEA customers marginally increasing their purchases from Chinese suppliers, as it is not needed that all EEA customers switch all of their demand towards Chinese suppliers to defeat a hypothetical price increase.
(182) In support of its arguments, the Notifying Party submitted an empirical analysis with the objective of demonstrating that imports of adipic acid into the EEA exercise "a significant competitive constraint" on the adipic acid produced in the EEA.118 This analysis looks at the relation between the prices of adipic acid in the EEA and in China, according to two different methods.
(183) The first method is a price correlation analysis between the price of adipic acid produced in the EEA and the price of imported adipic acid from China. The price of adipic acid produced in the EEA is based on the lower bound of the monthly EEA price of adipic acid, as provided by PCI Wood Mackenzie. The price of imported adipic acid from China is constructed from the lower bound of the monthly spot price of standard-grade CFR adipic acid, as provided by PCI Wood Mackenzie, to which various cost components representing the cost of importing the product from China are added (these include transport costs, duty, etc.). The price series used by the Parties cover the period January 2015 to August 2017.119 The Notifying Party found that "the correlation coefficient between the price of AA produced in the EEA and the price of imports at the same date is equal to […], it increases to […] when the price of imports is lagged by one month, and it further increases to […] when the price of imports is lagged by […]. This suggests that the price of AA produced in the EEA reacts to the price of imports, with a lag of […] On the contrary, the correlation coefficient decreases when the price of AA produced in the EEA is lagged by […] (coefficient equal to […]), and it further decreases (coefficient equal to […]) when the price of AA produced in the EEA is lagged by […]. This confirms that it is the price of AA produced in the EEA that reacts to the price of imports and not vice versa".120 The Parties also claimed that this price correlation pattern cannot be explained by common shocks on both the price of adipic acid in China and that in the EEA, because, if it were to be the case, the correlation would be stronger for contemporaneously observed prices, and not for lagged values.
(184) The second method is a time series analysis of the price of adipic acid in one of the relevant geographic areas (EEA or China) of past variations in prices in this area and of past variations in prices in the other area (China or EEA), over the period January 2011 to June 2017.121 The Notifying Party claimed that past monthly variations in the (change in) prices of imports from China "Granger-cause" variations in the (change in) price of EEA-produced adipic acid with a one-month lag with a statistical significance at the 1% level, meaning that price-changes of adipic acid produced in the EEA are better explained by past price-changes of adipic acid produced in China and past price-changes of the EEA production together, rather than by the past price- changes of the EEA production alone. The Notifying Party submitted that "[t]he conclusion reached by this analysis is identical to the one reached with the price correlation analysis. The price of imports Granger-causes the price of AA produced in the EEA but the reverse is not true. In other words, past prices of imports help to predict the price of AA produced in the EEA above and beyond the own past prices but the reverse is not true".122
(185) Finally, the Notifying Party also observed that the difference between price in the EEA and the price of imports in China is strongly correlated with the volume of imports with a correlation coefficient of […] consistent with a competitive constraint from imports.123
(186) The Commission disagrees with the Parties' conclusions for the following reasons.
(187) First, the Commission acknowledges that Chinese imports of adipic acid have increased in recent years. However, as shown in Section 5.4, such imports have remained moderate. The Commission also observes that, in 2017, import volumes appeared correlated with the difference between EEA prices and the price of Chinese imports reported by the Notifying Party. However, respondents to the in-depth market investigation have contested the existence of a meaningful price gap to the advantage of Chinese imports, in 2018 and over the previous few years, which seriously questions the assumptions underlying the Notifying Party's calculations. Moreover, respondents to the market investigation have contested the existence of a possible correlation between prices in China and prices in Europe, finding it "rather weak" and concluding instead that "the two markets are very much influenced by sudden changes to the local supply/demand balance and by the different approach of players on the market".124 Furthermore, as discussed in the previous section, not only the ability but also the incentive of Chinese producers to constraint EEA prices is questionable.
(188) Second, with respect to the Parties' empirical correlation analysis, the Commission, in line with past decisions,125 considers that the use of price correlation analysis to quantify the degree of co-movement of prices over time, and indicating whether the underlying products belong to the same relevant market or not, is best suited as a "separation" test rather than an "inclusion" test. More specifically, while an absence of correlation between two price series indicates that two products do not belong to the same relevant product market, the existence of even high positive correlations does not indicate that the products are in the same market. A positive correlation is also consistent with that correlation being the result of other influences such as common movements in raw material costs. The correlation coefficients reported by the Notifying Party, which are within the range of […], are not particularly high and may also be spurious or the result of trends or non-stationarity of the underlying series as acknowledged by the Notifying Party in its Granger causality analysis.
(189) Third, claims by the Notifying Party that the correlation coefficients increase when EEA prices are correlated with lags of the price of Chinese imports are based on results which are not robust, as such increase in correlation appears to depend on the time series under consideration. Indeed, the updated analysis provided by the Notifying Party in its reply to RFI 10 indicates that, when considering the period from January 2015 to May 2018, the correlation coefficients remain relatively stable in lags of the price of Chinese imports (only moving from […] to […]). In addition, the Commission considers that, even if a robust relation was found and that the correlation coefficients were to increase when EEA prices are correlated with lags of the price of Chinese imports, and not vice versa, this would not directly imply that Chinese prices explain EEA prices. Indeed, correlations may still be spurious, the result of trends, or due to common underlying influences that manifest themselves at different lags. Moreover, an observed increase in correlation with lags need not imply a causal relationship or a working arbitrage that would lead to a constraint on price levels.
(190) Fourth, the empirical time series analysis provided by the Notifying Party to determine Granger causality does not allow drawing conclusions as to whether or not the market should be wider than the EEA. The analysis is performed on changes of prices and its results, if taken at face value, only imply that past changes in the Chinese prices have some statistically explanatory value in relation to current changes in the EEA price. This does not allow inferences as to whether this empirical relationship is such that it implies a market that is wider than the EEA.
(191) Moreover, the main result reported by the Notifying Party does not seem to be significantly impacted when narrowing down the time period considered in order to exclude the most recent years, when imports from China have increased. This implies that the relationship between past price-changes in China and current price- changes in the EEA remains statistically significant even when considering time windows for which, according to the Notifying Party, imports from China stood at very low levels (e.g., over the period January 2011 to December 2014)126 and were, therefore, unlikely to constitute a competitive threat to EEA producers. This casts doubts on the validity of the results put forward by the Notifying Party, as this raises the likelihood that the effect put forward by the Parties is spurious and not related to any real-world business or economic rationale that would lie behind the statistical results.
(192) Fifth, an analysis of the difference between EEA prices and the price of Chinese imports in the EEA as measured by the Notifying Party, indicates that the difference is non-stationary.127 In other words, in contrast to what one would expect if there was strong arbitrage between regions that would ensure that prices cannot diverge over time, there is no statistical tendency that shocks leading to changes in the price gap are reversed over time. This indicates that the geographic market should not include China.
(193) Overall, the Commission does not consider the results of the empirical analysis put forward by the Notifying Party to support the Notifying Party's claims that the market for adipic acid should be defined as global or that imports of adipic acid into the EEA exercise a "significant competitive constraint" on the adipic acid produced in the EEA. Instead, the Notifying Party's empirical analysis is inconclusive with respect to the geographical market definition or with respect to the strength of the potential competitive constraints exerted by producers located in China. Also, and in addition to the results of the market investigation, the non-stationarity of the price gap indicates that the constraint from imports is not strong enough to consider the market to be wider than the EEA.
(g) Threats to switch supply to Chinese producers do not appear systematic or show that Chinese imports represent a viable alternative to EEA supplies
(194) In its Reply to the Article 6(1)(c) Decision, the Notifying Party contends that customers often use Chinese producers of adipic acid as a credible threat in negotiations. To support its argument, the Notifying Party provides anecdotal and often indirect evidence of (sometimes dated) interactions with customers, primarily […].
(195) However, in response to the market investigation, […] has denied the existence of any global benchmark for adipic acid, allegedly influenced by Chinese prices, used to put pressure on EEA suppliers.128 Moreover, […] explained that "[n]egotiations take into account the latest market knowledge, such as expected supply/demand balance, access to raw materials, strength of the various downstream markets, gathered by each negotiating party" and added that "due to price volatility, cost and reliability of supply issue, China offers for AA cannot be used as leverage during negotiations with EU producers". In addition, […] notes: "most of the time, Chinese prices are higher on a delivered basis as European prices".129
(196) This is not to say that Chinese prices are not sometimes used as a bargaining chip on top of other considerations such as quantities, feedstock levels or indexation.130 Still, in view of all the elements considered in section 5.4, the Commission seriously questions the credibility and weight that can be ascribed to the alleged threats of switching to Chinese imports for a significant proportion of customers' requirements.
(197) This inference is reinforced by the outcome of the in-depth market investigation and the opinion expressed by a majority of respondents to the effect that conditions for the procurement of adipic acid from China are not comparable to those of European suppliers and that the supply of significant volumes of adipic acid originating from China is unlikely to become comparable to adipic acid produced in the EEA over the next 3 to 5 years.131
(198) The Commission concludes that the various demand-side considerations discussed in section 5.4 seriously undermine the Notifying Party's claim that the relevant geographic market for adipic acid should be global in scope. To the contrary, in spite of a recent increase, the competitiveness of Chinese imports appears to be subject to serious limitations, thereby questioning the ability of Chinese supplies to constraint effectively the Merged Entity in the future. Put otherwise, the conditions of competition appear to remain appreciably different in the EEA, which supports an EEA-wide definition of the relevant market.
5.4.3.3(B) Supply-side considerations
(199) For the surplus, the Commission also investigated a number of supply-side considerations, along the arguments put forward by the Notifying Party, to assess the degree of market interpenetration at global level. Overall, these considerations tend to support the existence of different conditions of competition in the EEA, compared to China, and thus to confirm the EEA-wide nature of the market for the supply of adipic acid.
(200) First of all, as claimed by the Notifying Party, the outcome of the in-depth market investigation has clearly dismissed the materiality of REACH registration as a barrier to trade with EEA customers.132 The main Chinese suppliers do have a REACH registration in place and EEA distributors can assist in processing such registration. Generally, according to […], there is no regulatory requirement limiting the transportability of adipic acid across world regions.133
(201) Secondly, however, the in-depth market investigation has revealed the inappropriateness of considering relatively low transportation costs separately from an array of other costs affecting the competitiveness of Chinese imports, including custom duties but also reprocessing, repacking and crushing costs or financial costs and working capital costs associated with long delivery periods. The Article 6(1)(c) Decision mentioned that transportation costs are not significantly higher for procuring adipic acid from inside or outside the EEA ([0-10]%). However, as discussed in relation to demand-side factors, transportation costs are just one among various other cost factors affecting the final delivered price of Chinese supplies, in particular. Logistic costs altogether have been considered by respondents to the in- depth market investigation to range between 15 and 20% of the final delivered price of material originating in China, thereby significantly hampering the competitiveness thereof.134
(202) Third, respondents to the in-depth market investigation indicated that adipic acid from China is "not an option" for food contact goods and that many Chinese producers do not provide the certification necessary to enable such applications.135 In response to a request for information, however, the Notifying Party submitted food contact certificates originating from suppliers […] and […].136 On balance, the Commission appreciates that certification may be available from some Chinese suppliers but not others, and notes that the availability of certificates might not be sufficient as such to counter the perception or experience of certain EEA customers in relation to adipic acid supplies from China. Otherwise, certain respondents have explained that Chinese suppliers are reluctant to assume liability for product defects, in compliance with the industry standards applicable in the EEA,137 which the Notifying Party also denies.
(203) Generally, distributors have acknowledged that the business culture of Chinese suppliers was not aligned with the European market, even though practices are evolving and some of them are adopting a certification model designed to accommodate European industry requirements.138 As leading chemical trader […] also pointed out, "[t] o become comparable [with EEA supplies], Chinese need to offer pricing based on delivery date in Europe not shipment date from China". Moreover, "big contracts in Europe are based on raw-material formulas, using European raw material prices [and] Chinese producers need to offer such formulas as well – currently they only base formulas on Asian feedstocks or product prices".139
(204) Fourth, various EEA customers still view Chinese suppliers as opportunistic players that are unreliable and uninterested in establishing long-term industrial partnerships but rather attracted by gains deriving from occasional price differentials between the EEA and their domestic market.140 Conversely, when supply gets short, Chinese producers would tend to favour domestic supplies.141 The fact that Chinese suppliers do not have an established physical presence in the EEA or a domestic sales organisation, and do not offer logistic services on their own but only through distributors, is also viewed negatively by certain EEA customers.142
(205) In closing, the fact that large production capacities are available in China, as argued by the Notifying Party and confirmed by certain market participants,143 does not as such alleviate the impediments revealed by the in-depth market investigation as to the ability (and incentives) of Chinese suppliers to develop sales within the EEA or to the existence of different conditions of competition prevailing within the EEA. The in-depth market investigation has also revealed that overcapacities have been reduced recently due to the tighter enforcement of environmental standards,144 though new investments are possible at the plants of certain suppliers.145
5.4.3.4. Conclusion on geographic market definition
(206) Based on the assessment in recitals (149) to (205), and in light of the evidence collected during its investigation in Phase II, the Commission concludes that, for the purpose of assessing the Transaction, the adipic acid market is EEA-wide in scope.
5.4.4. Conclusion on the market definition for adipic acid
(207) In conclusion, the Commission will carry out its competitive assessment on a market for adipic acid defined as EEA-wide in scope.
5.5. Level II of the polyamide value chain: Caprolactam
5.5.1. Introduction
(208) Caprolactam is a monomer intermediate produced primarily from cyclohexane and used almost entirely for production of PA 6 BP and – to a lesser extent – in conjunction with AH Salt for the production of co-polyamide 6/6.6 base polymer ("Co-Polyamide 6/6.6 BP") or co-polyamide 6.6/6 base polymer ("Co-Polyamide 6.6/6 BP).
(209) Caprolactam can be produced by different methods (including HSO, HPO, HSNO, moximation and other methods). Caprolactam is produced in liquid form and can be transformed into solid form to transport it over longer distances (and is then subsequently melted by the customers).
5.5.2. Product market definition
5.5.2.1. The Commission’s precedents
(210) In CVC / Royal DSM, the Commission analysed the market for caprolactam and left open the exact market segmentation for Caprolactam.146
(211) The Commission ultimately looked at market shares both for caprolactam overall, and based on individual production methods.147
5.5.2.2. The Notifying Party's view
(212) The Notifying Party submits that caprolactam constitutes a distinct product market, as there is no substitute for caprolactam in the context of PA 6 BP or co-polyamide production.
(213) The Notifying Party submits that there is no need to further sub-segment the market for caprolactam since there is no meaningful difference between caprolactam sold for different applications. The Notifying Party also states that there is one uniform caprolactam specification regardless of the application in which it is used.
5.5.2.3. Conclusion on product market definition
(214) As the outcome of the competitive assessment of the Transaction remains the same regardless of the exact product market definition for caprolactam, it is not necessary for the Commission to conclude on the exact product market definition.
5.5.3. Geographic market definition
5.5.3.1. The Commission’s precedents
(215) In CVC / Royal DSM, the Parties claimed that the market for caprolactam was worldwide in scope. The Commission noted that third party industry reports typically track prices on a regional basis (e.g. Asia, Europe, United States), but left open the exact market segmentation for Caprolactam.148
(216) The Commission ultimately looked at market shares both at worldwide and at the EEA level in its competitive assessment.149
5.5.3.2. The Notifying Party's view
(217) The Notifying Party submits that the caprolactam market is global in scope in particular because customers would generally source from a range of caprolactam producers worldwide, transport costs would represent less than […]% of the total caprolactam sales prices, and caprolactam would be priced globally.
5.5.3.3. Conclusion on geographic market definition
(218) As the outcome of the competitive assessment of the Transaction remains the same regardless of the exact geographic market definition for caprolactam , it is not necessary for the Commission to conclude on the exact geographic market definition
5.5.4. Conclusion on the market definition for Caprolactam
(219) The exact market segmentation for caprolactam can be left open as the Transaction does not give rise to serious doubts as to its compatibility with the internal market under any plausible market definition.
5.6. Level III of the polyamide value chain: AH Salt
5.6.1. Introduction
(220) AH Salt, also known as Nylon Salt, is an intermediate used in the production process of PA 6.6. AH Salt is produced by mixing HMD and adipic acid in an aqueous reaction. AH Salt is then used predominantly to produce of PA 6.6 BP products (Level IV) and – to a lesser extent – in conjunction with caprolactam for the production of Co-Polyamide 6/6.6 BP or Co-Polyamide 6.6/6 BP. It can be used also to produce other PA engineering plastics grades (such as PA 6.12 EP).
(221) AH Salt can be found in both liquid and dry format. Liquid AH Salt has a high content of water. Dry AH Salt is obtained by reducing the content of water of liquid AH Salt. For this purpose, an AH Salt dryer is needed.
(222) The production process consists of pure mixing of HMD with adipic acid. As a consequence, according to the Notifying Party almost all PA 6.6 producers also produce AH Salt themselves, as they possess the required simple production assets.
5.6.2. Product market definition
5.6.2.1. The Commission’s precedents
(223) The Commission assessed AH Salt as part of the PA 6.6 BP production process in two previous decisions. In the context of those decisions it was unnecessary for the Commission to define a specific product market with regard to AH Salt.150
5.6.2.2. The Notifying Party's view
(224) The Notifying Party submits that AH Salt forms a distinct product market that does not require to be further segmented because there is no substitutes for AH Salt in the context of the production of PA 6.6 BP, and given the absence of product differentiation within AH Salt, as all suppliers produce the same quality of AH Salt.
5.6.2.3. The Commission's assessment
(225) The market investigation results indicate that AH Salt should be considered a separate product market, as there is no alternative to produce PA 6.6 BP or co- polyamide.
(226) As regards the differentiation between dry and liquid AH Salt, the market investigation has indicated that they may belong to different product markets.
(227) On the supply side, in the EEA only BASF produces dry AH Salt. Indeed, the market investigation confirmed that in the EEA all producers, other than BASF, produce only liquid AH Salt. Moreover, in order to produce dry AH Salt, it is needed to invest in AH Salt dryer capacity. In this vein, […] explains that it uses (liquid) AH Salt mainly for captive use, so it has no interest to invest in other kind of AH Salt.151
(228) On the demand side, all customers in the EEA source exclusively liquid AH Salt. Customers of AH Salt consider that sourcing AH Salt in powder would entail significant additional costs. For example, […] explains that it buys AH Salt in bulk and dry AH Salt is only available in small packages (bags) which requires high manual effort. Moreover, dry AH Salt needs to be dissolved later on which implies additional costs. Switching to dry AH Salt would require to change the current plant set up.152 In the same vein, […] indicates that “the powder needs to be dissolved before use. That process represents extra costs and would need an extra investment”.153 […] adds that "the switch to Solid AH Salt would require investments in dissolution and filtering equipment in addition to the additional costs for handling and processing”.154
5.6.2.4. Conclusion on product market definition
(229) Based on the assessment in recitals (220) to (228), the Commission concludes that, for the purpose of assessing the Transaction, AH Salt constitutes a separate product market. Since the outcome of the competitive assessment remains the same irrespective of whether the market is defined as comprising dry AH Salt in addition to liquid AH Salt, the market definition can be left open in that respect.
5.6.3. Geographic market definition
5.6.3.1. The Commission’s precedents
(230) The Commission mentioned AH Salt as part of the PA 6.6 BP production process in two previous decisions. In the context of those decisions, it was unnecessary for the Commission to define a specific product or geographic market with regard to AH Salt.155
5.6.3.2. The Notifying Party's view
(231) The Notifying Party submits that the geographic market for AH Salt would be global in scope. In particular, the Notifying Party submits that there are no legislative barriers to the cross border trade of AH Salt, there are no import duties or other barriers which would prevent the import of AH Salt into the EEA; transportation costs amount at most to [10-20]% of the sales price and exports to Asia exist in dry format.
(232) In the Response to the Article 6(1)(c) Decision, the Notifying Party provided further arguments to support that the AH Salt market is global in scope.
(233) First, the Notifying Party submitted that the transport of dry AH Salt is much cheaper than the transport of AH Salt in liquid form and the cheaper transport costs compensate for the longer travel distance, allowing transport across regions. Moreover, according to the Notifying Party, the dissolving process of dry AH Salt into liquid AH Salt is a standard process which can be done relatively quickly at very limited extra costs.
(234) Second, the Notifying Party submitted that several market participants in Asia (including […]) source AH Salt delivered in bags.
5.6.3.3.The Commission's assessment
(235) The results of the Phase I market investigation provided strong indications that the market for AH Salt is EEA-wide in scope. The investigation in Phase II has not provided any element speaking in favour of a global market and has therefore confirmed that the geographic market for AH Salt is EEA-wide in scope.
(236) First, contrary to the Notifying Party's views, customers consider that several barriers to trade across regions exist. Transportation costs, long delivery periods and need to purchase dry AH Salt when sourcing from outside the EEA and need for further processing (dissolution of dry AH Salt) were identified by customers as the most important barriers to trading AH Salt across regions of the world. Other barriers mentioned were tariffs and regulatory barriers.156 […], a very important customer, explains “longer transportation distances increase logistical costs significantly. It also requires additional storage capacity which is currently not needed due to short distance to the suppliers”.157 Moreover, it is clear that AH Salt does not travel long distances in liquid format. […], an important customer, explains that “AH Salt is in a great part liquid, the transportation would be expensive, it would make no sense to transport water over long distances”.158 Indeed, the Notifying Party recognises that “the majority of AH Salt is in liquid format. Due to a rather high water content, AH Salt is typically transported within a radius of less than 1.000 km around of the production site”. Nevertheless, the Notifying Party also points out that “transport in dry format is possible and practiced globally. For example, AH Salt is occasionally shipped to Asia in dry format. […]”.159 However, customers of AH Salt consider that sourcing AH Salt in powder would entail significant additional costs. For example, […], a customer of AH Salt, indicates that “AH Salt is bought as a solution by […], therefore AH Salt is not easily transportable. Indeed, for long distance transportation (importation) purposes, it needs to be transformed into a powder. The powder needs to be dissolved before use. That process represents extra costs and would need an extra investment”.160 In the same vein, […] explains that "the switch to Solid AH Salt would require investments in dissolution and filtering equipment in addition to the additional costs for handling and processing”.161
(237) Indeed, the market investigation confirmed that in the EEA all producers other than the Parties produce only liquid AH Salt. In this vein, […] explains that it uses (liquid) AH Salt mainly for captive use, so it has no interest to invest in other kind of AH Salt.162 All customers in the EEA source exclusively liquid AH Salt. For example, […] explains that it buys AH Salt in bulk and dry AH Salt is only available in small packages (bags) which requires high manual effort. Moreover, dry AH Salt needs to be dissolved later on which implies additional costs. Switching to dry AH Salt would require to change the current plant set up.163
(238) Second, contrary to the Notifying Party's claim, long distance transportation increases significantly the price of AH Salt and is difficult logistically. For example, […] points out that it “cannot seek sourcing AH Salt overseas since the material does not transport easily, for heating purposes”.164 In the same line, […] explains that “for liquid salt, exposure to oxygen during transload or shipping operations can cause deterioration of the salt (e.g., colour, impurities)”. It continues explaining also that “proper handling of AH Salt is key. Heated vessels and proper use of nitrogen is critical”, although "distance is not critical if the AH Salt is handled properly". 165 Customers' views do not differ on this point. For example, […] explains that “AH Salt delivered to […] must stay above a temperature of 85°C. Otherwise it solidifies". It continues explaining that "the above condition limits the distance for the transportation of liquid AH Salt”.166 […] adds that AH Salt needs “constant temperature and nitrogen blanketing” and that “it cannot buy the product from long geographical distances, due to the cost of transporting water solution”.167
(239) Finally, imports into the EEA are negligible, as the Notifying Party recognises, while “some AH Salt is exported from the EEA to Asia. There are currently no imports into the EEA […]. While AH Salt is typically transported over shorter distances, the transport costs would still allow for imports into the EEA from third countries in a wider geographic perimeter”.168 The market investigation confirmed that producers do not import AH Salt into the EEA,169 that all customers source AH Salt exclusively at EEA level,170 and that in the last 7 years customers of AH Salt based in the EEA have sourced exclusively from the EEA.171
(240) The Notifying Party also provided a number of examples of exports from the EEA towards Asia. However, exports from the EEA to other regions do not prove that the geographic market is broader that EEA in scope. The rationale of the exports from the EEA to Asia can find their explanation in other factors, such as structural shortages of AH Salt in Asia. The relevant factor is that imports into the EEA do not represent a competitive constraint because customers are not purchasing outside the EEA and do not consider imports a viable alternative.
5.6.3.4. Conclusion on geographic market definition
(241) Based on the assessment in recitals (230) to (240), the Commission concludes that, for the purpose of assessing the Transaction, the market for AH Salt is EEA-wide in scope.
5.6.4. Conclusion on the market definition for AH Salt
(242) As the outcome of the competitive assessment of the Transaction remains the same and the Final Commitments entered into by the Notifying Party address the Commission’s concerns under all alternative market definitions, it is not necessary for the Commission to conclude on the exact product market definition for AH Salt, which is EEA-wide in scope for the purpose of assessing the Transaction.
(243) In conclusion, the Commission will carry out its competitive assessment on a market for AH Salt defined as EEA-wide in scope.
5.7. Level IV of the polyamide value chain: PA 6 and PA 6.6 BP
5.7.1. Introduction
(244) PA 6 BP and PA 6.6 BP are medium performance polyamides and belong to the family of aliphatic polyamides. They are each manufactured through different processes using different production equipment and inputs.
(245) PA 6 BP is made from the ring opening polymerisation of caprolactam (Level II). PA 6.6 BP is made from the polycondensation of the aqueous salt (AH Salt, Level III) made of HMD and adipic acid (Level II).
(246) Both PA 6 BP and 6.6 BP are predominantly used for the production of fibres (including textile and carpet) and EP. These two uses together represent approximately 80% of the use of PA 6 BP and PA 6.6 BP. The remainder is used for films for food packaging and mainly involves PA 6 BP.
5.7.2. Product market definition
5.7.2.1. The Commission’s precedents
(247) In Solvay/Rhodia, the Commission left the exact product market definition open, in particular on possible further segmentations according to the different materials involved (e.g. between PA 6 BP and PA 6.6 BP). The Commission however specifically analysed the impact of the Transaction on the PA 6.6 BP market.172
5.7.2.2. The Notifying Party's view
(248) The Notifying Party notes that in the past the Commission has indicated that PA 6 BP and PA 6.6 BP may belong to the same product market without expressly analysing this market.173
(249) The Notifying Party argues that PA 6 BP and PA 6.6 BP belong to the same product market.174
(250) The Notifying Party argues in particular that PA 6 BP and PA 6.6 BP have similar technical performance characteristics (including glass transition temperature, melting point, deflection temperature, water absorption, stiffness and strength) and are used for the same application base (namely fibres, films and EP). The Notifying Party also points out the price correlation across PA 6 BP and PA 6.6 BP to suggest the two products belong to the same market.
(251) The Notifying Party also suggests that there are no distinct product markets within PA 6 BP or PA 6.6 BP on the basis of its potential applications (i.e. for EP, fibres or films), since typically there is no meaningful price difference between the grades used for the production of fibres and EP. According to the Notifying Party, every PA 6 BP producer in the EEA produces PA 6 BP for films, and the production of PA 6.6 BP for film for food packaging amounts to just [0-10]% of the total PA 6.6 BP market and neither BASF nor the Business are active in this segment.
5.7.2.3. The Commission's assessment
(252) As a preliminary remark, the Commission notes that the product market definition of PA 6.6 BP is intrinsically linked to that of PA 6.6 EP, which is further discussed in Section 6.8.2. PA 6 BP cannot be used to produce PA 6.6 EP, and conversely, PA 6.6 BP cannot be used to produce PA 6 EP. As a result, analysing the substitutability of PA 6.6 and PA 6 at base polymer level is largely redundant.
(253) The market investigation did not validate the Notifying Party's arguments claiming that PA 6 BP and PA 6.6 BP belong to the same product market.
(254) On the demand-side, a majority of responding customers for PA 6.6 BP contradicted the argument of the Notifying Party, that PA 6 BP and PA 6.6 BP have similar technical performance.175 A large majority of customers indicated that it not possible to switch any of their PA 6.6 BP purchases to PA 6 BP.176 Customers noted in particular the better thermal resistance and lower water absorption properties of PA 6.6 BP, as opposed to PA 6 BP, which impacts end applications and the customers' ability to switch between the two products.177
(255) Moreover, regarding pricing, the Commission notes that PA 6.6 BP is more expensive than PA 6 BP on a per ton basis.178 Nevertheless, results of the market investigation overwhelmingly indicate that PA 6.6 BP customers would not be able to switch at all their supply requirements from PA 6.6 BP to PA 6 BP, in the event of an increase in the already existing price differential between PA 6.6 BP and PA 6 BP. 179
(256) Several responses to the market investigation and minutes of calls made with market participants pointed out that, in particular since PA 6.6 BP is already more expensive, all applications that could technically and practically switch to PA 6 BP have already done so.180
(257) Regarding price correlation between PA 6.6 BP and PA 6 BP, customers indicated that this correlation is not constant and stressed that the price differential has increased in the recent past, when PA 6.6 BP became 50% more expensive than PA 6 BP.181
(258) On the supply-side, all of the PA 6 BP producers having responded to the market investigation indicated that they cannot start producing PA 6.6 BP in the short term.182 Indeed the market investigation confirmed that production processes of PA 6 BP and PA B6 BP differ completely and involve different equipment and inputs.183 Starting to produce PA 6.6 BP would entail constructing new production facilities and establishing a supply chain for important inputs such as ADN, HMD, or AH Salt which are in tight supply.184 Conversely, producers of PA 6.6 BP also consider that producing PA 6 BP is not possible.185
(259) The Commission also investigated whether the market for PA 6.6 BP could be further sub-segmented according to the various types/grades/final uses of PA 6.6 BP (for EP, fibres, food packaging, etc.), or not. Both at producers' and at customers' levels, the market investigation indicated that there is some degree of supply and demand side substitutability.186
5.7.2.4. Conclusion on product market definition
(260) Based on the assessment in recitals (247) to (259), the Commission concludes that, for the purpose of assessing the Transaction, PA 6 BP and PA 6.6 BP constitute distinct product markets.
5.7.3. Geographic market definition
5.7.3.1. The Commission’s precedents
(261) In Solvay/Rhodia,187 the Commission found that relevant geographic market for different polyamides was at least EEA wide in scope.
5.7.3.2. The Notifying Party's view
(262) The Notifying Party submits that the markets for PA 6 BP and PA 6.6 BP are global in scope, because of the existence of significant interregional trade flows,low transport costs, and the fact that customers can and do source on a global scale.188 According to the Notifying Party, imports into the EEA will become more attractive in the upcoming years […]. The Notifying Party expects Asian producers to utilise their capacities and lower prices, and states that imports from the USA (particularly from […]) into the EEA have increased during the last four years.
(263) In its Response to the 6(1)(c) Decision, the Notifying Party further insists that the market for PA 6.6 BP in particular should be considered as global in scope. First, the Notifying Party reaffirms that interregional trade is possible, as evidenced by the fact that a majority of market respondents stated there was no technical or regulatory characteristics of PA 6.6 BP that would limit its transportability across regions, apart from ensuring that the products are adequately protected against humidity.189 Second, the Notifying Party mentions that interregional trade of PA 6.6 BP exists, in particular due to substantial imports from […].190 Third, the Notifying Party also considers that transportation costs, tariffs, and the REACH regulatory framework are not significant barriers to trade between the EEA and third countries.191
5.7.3.3. The Commission's assessment
(264) The market investigation has not revealed any elements indicating that, contrary to what the Notifying Party claims that the markets for PA 6 BP and PA 6.6 BP are global in scope.
(265) Firstly, the fact that respondents to the market investigation state there is no technical or regulatory feature of PA 6.6 BP that would limit its transportability192 does not imply that the conditions of competition for the sale of the product are sufficiently homogeneous worldwide. Indeed, importing may not be a commercially viable option, in particular with regards to PA 6.6 BP, for which transportation costs and duties, based on the Notifying Party's own estimates de facto raise the price of imported products by around [10-20]%.
(266) The majority of customers procures PA 6.6 BP from inside the EEA only.193 Customers flag long delivery periods, import/export duties, higher costs, as well as considerations related to the REACH regulation and customers' sourcing requirements.194 Those customers which indicated procuring PA 6.6 BP from outside the EEA also procured limited volumes typically representing less than 30% of their requirements from imports. Out of this minority of customers who indicated procuring PA 6.6 BP from outside the EEA:
· […] is a US-based company, which procured 90% of its PA 6.6 BP requirements from outside the EEA195
· […] specified that its "Primary EEA demand is served by producers within EEA"196
· […] only sourced PA 6.6 BP from the USA for industrial trials, amounting to less than 1% of the company's requirements.197
(267) Respondents to the market investigation also flag the preference or requirement for PA 6.6 BP to be of European origin. […], which imports limited PA 6.6 BP in the EEA, acknowledges that, with regards to PA 6.6 BP "Most EEA clients prefer an EEA origin product as most clients want a local supply if possible".198 […] mentions that there is a "market requirement for polymer of European origin".199
(268) Secondly, with regards to existing trade flows, the share of imports remains limited, and imports only serve the merchant market to a marginal extent.
(269) Imports of PA 6.6 BP from Asia were virtually non-existent in the EEA in 2017, based on the Notifying Party's own market shares estimates.200 Imports from North America exist and have become more viable following the various force majeure situations in Europe, which led to the historical price gap between the USA and Europe to narrow in 2017 (and to European prices exceeding American prices in 2018). Based on IHS estimates, such price difference was historically around USD [0.1-0.6] / kg (see Figure 2), making imports from the USA less attractive. Similarly, based on the Tecnon index, price for PA 6.6 BP in the USA was on average higher by USD [0.1-0.6] / kg in 2015 and 2016.201 Force majeure situations in the US, in particular the recent fire at Ascend's Pensacola site, will likely impact the supply of PA 6.6 BP, including to EEA customers, and may further revert the current pricing trend, relativising the attractiveness of imports from the US.202
[GRAPH SHOWING HISTORICAL PA6.6 PRICING BY REGION]
Figure 2 PA6.6 Historical Pricing by Region Source: IHS203
(270) Separately, the volume of Invista's exports to the EEA has been steadily decreasing between 2014 and 2017. Invista has local capacity in the EEA for PA 6.6 BP, from its Rozenburg (Netherlands) facility, and […] acknowledges that, with regards to PA 6.6 BP, EEA customers have a preference for local supply.204
(271) As per Ascend, which accounts for most of imports to the EEA merchant market, the company supplies PA 6.6 BP globally from its facilities in the USA.205 However while the company has announced capacity increases, including with regards to PA 6.6 BP, only a fraction of such increase is scheduled to reach the European market, in spite of the tightness of the market in the EEA.206 […],207 […].208 As noted in recital 269, a recent force majeure situation at Ascend's largest PA 6.6 BP plant in the USA may further impede the company's ability to deliver volumes to EEA customers.
(272) Internal documents also highlight the cost disadvantages presented by imports from the US. The vendor due diligence report prepared for Solvay reads that "[QUOTE FROM CONFIDENTIAL INTERNAL DOCUMENT]" (emphasis added). The difference in profitability between assets in the Union and assets in the USA is even projected to increase by 2020.209
(273) Similarly, price differences exist between PA 6 BP across different regions. In particular, over 2017, PA 6 BP was around USD 0.3-0.4 / kg more expensive in North America than in Europe, according to both PCI and Tecnon indexes.210
(274) Thirdly, in addition to customers identifying transportation costs and duties as barriers to trade PA 6.6 BP across continents (see recital 266), the submissions of the Notifying Party also indicate that it considers such barriers as relevant. The Commission notes that swap agreements are commonplace with regards to PA 6.6 BP. Producers of PA 6.6 BP, including Ascend, DowDuPont, Solvay and BASF regularly enter into swap agreements, typically to receive products in regions where they do not have any production facility, which indicates that regional PA 6.6 BP markets are clustered. In particular, the Notifying Party itself recognises that swap agreements are concluded by producers to circumvent such barriers. The Notifying Party states that "logistic swap agreements may exist between producers in the EEA and the USA, in order to save transportation costs and tariffs".211 […]212 Internal documents of the Parties also seemingly consider that swap agreements (rather than exports) are required to ensure a profitable business in regions where a company has no production facility. […].213 […].214
5.7.3.4. Conclusion on geographic market definition
(275) Based on the assessment in recitals 261 to274, the Commission concludes that, for the purpose of assessing the Transaction, the market for the supply of PA 6.6 BP is EEA-wide in scope.
(276) As the outcome of the competitive assessment of the Transaction remains the same under all alternative market definitions, it is not necessary for the Commission to conclude on the exact geographic market definition for PA 6 BP.
5.7.4. Conclusion on the market definition for PA 6 BP and PA 6.6 BP
(277) The Commission therefore concludes that, for the purpose of assessing the Transaction, the markets for the supply of PA 6 BP and for PA 6.6 BP constitute distinct product markets and that the market for PA 6.6 BP is EEA-wide in scope in scope.
(278) As the outcome of the competitive assessment of the Transaction remains the same under all alternative market definitions, it is not necessary for the Commission to conclude on the exact geographic market definition for PA 6 BP.
5.8. Level IV of the polyamide value chain: Co-Polyamide 6/6.6 BP and Co- Polyamide 6.6/6 BP
5.8.1. Introduction
(279) Co-Polyamide 6/6.6 BP consists of 80-85% caprolactam and 15-20% AH Salt, whereas Co-Polyamide 6.6/6 BP typically consists of 80%-90% AH Salt and 10-- 20% caprolactam.
5.8.2. Product market definition
5.8.2.1. The Commission’s precedents
(280) The Commission has not previously analysed the market for Co-Polyamide 6/6.6 BP.
5.8.2.2. The Notifying Party's view
(281) The Notifying Party suggests that both co-polyamides (as well as PA 6 BP and PA 6.6 BP) should be considered as belonging to the same product market, because they are used in the same end use applications and have similar production processes.215 Indeed, the Notifying Party stresses that from a supply point of view, the only differentiation between Co-Polyamide 6/6.6 BP is the dosage between caprolactam and AH Salt. From a demand point of view, it submits that indeed, there are no end-applications for which there is a specific customer demand for Co-Polyamide 6/6.6 BP.
5.8.2.3. The Commission's assessment
(282) On the demand-side none of the responding customers for Co-Polyamide 6/6.6 BP or Co-Polyamide 6.6/6 BP indicated that it was possible to switch to any of Co-PA 6 BP and/or PA 6.6 BP, or Co-Polyamide 6.6/6 BP and Co-Polyamide 6/6.6 BP respectively.216 Moreover, the market investigation indicated that on the demand- side, Co-Polyamide PA 6/6.6 BP and Co-Polyamide 6.6/6 BP have some special characteristics. Additionally, according to the data submitted by the Notifying Party in the Form CO,217 Co-Polyamide PA 6.6/6 BP is more expensive than Co- Polyamide PA 6/6.6 BP, which is itself much more expensive than either PA 6 BP, or PA 6.6 BP.
(283) On the supply-side, the Commission's market investigation did not confirm the affirmation of the Notifying Party that a producer of any of Co-Polyamide 6.6/6 BP, PA 6 BP and/or PA 6.6 BP can start producing Co-Polyamide 6/6.6 BP.218 The reasons put forward are the different production processes & technologies, access to inputs and the necessary investments.219
5.8.2.4. Conclusion on product market definition
(284) Based on the assessment in recitals 280 to283, the Commission concludes that, for the purpose of assessing the Transaction, Co-Polyamide 6.6/6 BP and Co-Polyamide 6/6.6 BP constitute distinct product markets.
5.8.3. Geographic market definition
5.8.3.1. The Commission precedents
(285) In Solvay/Rhodia,220 the Commission found that relevant geographic market for different polyamides is at least EEA wide in scope, but left open whether it could also be defined on a worldwide basis.
5.8.3.2. The Notifying Party's view
(286) In line with its position for PA 6.6 BP, the Notifying Party submits that the markets for co-polyamide BP is global in scope, in particular because significant interregional trade can be observed; transport costs are low (less than [0-10]% of the total sales prices) and customers source on a global scale.221
5.8.3.3. The Commission's assessment
(287) The Notifying Party’s argument was not validated by the Commission's market investigation. No customer of the Merged Entity indicated sourcing Co-Polyamide 6/6.6 BP or Co-Polyamide 6.6/6 BP from outside the EEA,222 while a majority of competitors indicated that their EEA customers source co-polyamide BP exclusively from the EEA.223
5.8.3.4. Conclusion on geographic market definition
(288) Based on the assessment in recitals 285 to287, the Commission concludes that the market for the supply of Co-Polyamide 6/6.6 BP and Co-Polyamide 6.6/6 BP are EEA-wide in scope.
5.8.4. Conclusion on the market definition for Co-Polyamide 6/6.6 BP and Co-Polyamide 6.6/6 BP
(289) Based on the assessment in recitals 280 to287, the Commission concludes that Co- Polyamide 6/6.6 BP and Co-Polyamide 6.6/6 BP constitute distinct product markets, which are EEA-wide in scope.
5.9. Level V of the polyamide value chain: PA 6 and PA 6.6 Engineering Plastics
5.9.1. Introduction
(290) PA 6 EP and 6.6 EP belongs to the group of engineering plastics. These have been developed in the 1960s and 1970s to replace materials like metal, alloys, wood and glass. Compared to those materials, engineering plastics are often lighter and easier to process into complex shapes. Their qualities make them suitable for diverse industry segments, i.e., transportation/automotive, industrial and consumer products and construction.
(291) Engineering plastics are produced in a polymerisation process by mixing molten base polymer (PA 6 BP for PA 6 EP and PA 6.6 BP for PA 6.6 EP) with additional ingredients such as additives (e.g. flame retardants), stabilisers (e.g. to improve resistance against heat or UV light) and various fillers from minerals to fibres. These additional ingredients are, depending on the intended field of application, added for stiffness, strength, impact or other performance features such as thermal or electrical conductivity. After this compounding step, they are sold to customers in form of granules. Customers process the granules into the final product form by using processes like injection moulding, blow moulding or extrusion.
5.9.2. Product market definition
5.9.2.1. The Commission’s precedents
(292) The Commission has so far not specifically analysed the market for PA 6 EP or PA 6.6 EP. Regarding engineering plastics more broadly, in Sabic/GE Plastics,224 the decision left open whether Polycarbonate (or “PC”, a type of engineering plastic) formed part of a wide market for engineering plastics or whether a separate PC market, or narrower markets should be defined.225
(293) In Rhône Poulenc / Caffaro,226 the Commission analysed the market for PA engineering plastics (“plastiques techniques en polyamide” or PTP). That decision acknowledged “fringes” of substitutability between different categories of EP, but concludes that the technical characteristics of polyamide EP (including quality and prices) and demand inelasticity distinguish them from other EP.227 The Commission ultimately left open the question of whether PA 6 EP and PA 6.6 EP belonged to separate markets, as the distinction had no impact on the competitive assessment.228
5.9.2.2. The Notifying Party’s view
(294) The Notifying Party submits its view that at least PA 6 EP and PA 6.6 EP belong to one and the same relevant product market as they are sufficiently substitutable both from a demand- and a supply-side perspective. In addition, the Notifying Party argues that in instances where substitutability between PA 6 EP and PA 6.6 EP may be low, each of them remains sufficiently substitutable by other materials, including other engineering plastics.229
(295) The Notifying Party considers the approach is justified by demand-side and supply- side substitutability considerations.
(296) On the demand-side, the Notifying Party argues that PA 6 EP, PA 6.6 EP, other EP, and other non-EP materials (like metals or alloys) are all substitutes and/or exert competitive pressure on each other.
(297) The Notifying Party argues that PA 6 EP and PA 6.6 EP are substitutable with non- EP materials. The Notifying Party states in particular that EPs have been developed to replace other materials like metal, alloys, wood and glass, and share similar characteristics. As a result, PA 6 EP and PA 6.6 EP can be functionally and economically substituted with these other materials.230
(298) The Notifying Party also submits that PA 6 EP and PA 6.6 EP can be substitutable with other EP.231 From a customer's point of view depending on the specific application there can exist extensive substitutability between PA 6 EP, PA 6.6 EP and other EP such as polyamide 4.6 engineering plastics ("PA 4.6 EP"), polyphenylene sulfide ("PPS"), polybutylene terephthalate ("PBT"), polyphthalamide ("PPA") and polysulfone ("PSU").
(299) Similarly as with regards to base polymers (see Section 6.6.2), the Notifying Party also argues that there is even closer substitutability between PA 6 EP and PA 6.6 EP. In particular, according to the Notifying Party, both share similar chemistry, properties (mechanical strength, rigidity and thermal stability) and processing and are sold at equivalent prices, although the average price for PA 6.6 EP is higher than that of PA 6 EP, for use in the same applications (with the exception of a few niche applications). In total the Notifying Party estimates that over [50-60]% of PA 6.6 volumes could be substituted by PA 6 and vice versa.232
(300) On the supply-side, the Notifying Party focuses on arguing the substitutability between PA 6 EP and PA 6.6 EP. In particular, the Notifying Party submits that most of the suppliers are able to (and do) offer PA 6 and PA 6.6 EP immediately, and that increasing the supply of PA 6 or PA 6.6 EP would not entail significant costs. Compounding processes are similar for both products which are produced in the same plants, using the same compounding equipment and personnel, and (to a large extent) the same ingredients. PA 6 EP and PA 6.6 EP thus possess similar technical characteristics which make that a producer of PA 6 EP can easily start producing PA 6.6 EP and vice versa.233
(301) The Notifying Party further argues that there is no reason to segment the PA EP market on the basis of grades or applications, as each PA 6 or PA 6.6 EP could accommodate different performance characteristics and serve various applications, and all PA EP producers are able to produce EP for all industries with the same equipment and can switch production between different grades of PA 6 and PA 6.6 EP without a significant cost increase.234
5.9.2.3. The Commission’s assessment
(302) On the demand-side, a majority of customers of PA 6.6 EP who responded to the market investigation state it is not possible to replace PA 6.6 EP by PA 6 EP for the applications they use PA 6.6 EP for.235 An even larger majority of such customers reject the claim that PA 6.6 EP can be replaced by any other material.236
(303) Indeed, respondents indicate that PA 6 EP is not a suitable preplacement for EP 6.6 EP, since PA 6.6 EP presents different technical characteristics.237 Moreover, according to respondents, switching to PA 6 EP, where possible, would be costly and time consuming (1 to 2 years).238
(304) The state of the PA 6.6 value chain, characterised by important price differences between PA 6 and PA 6.6, further evidences the lack of substitutability between PA 6.6 EP and PA 6.EP. Less than 10% of the respondents managed to replace some of their PA 6.6 EP volumes by PA 6 EP.239
(305) Furthermore, the market investigation clearly demonstrates that customers cannot replace the PA 6.6 EP volumes they use in their own production processes by PA 6 EP, or any other material. Most of PA 6.6 EP customers undergo a supplier qualification process in place by their own customers (e.g. OEMs). In particular, some end-customers require approval of the specific material used (such as PA 6.6 EP). As a result, substitutability of PA 6.6 EP by other non-PA 6.6 EP materials is virtually impossible, at least in the short term.240
(306) The Commission also notes that PA 6.6 EP is considerably more expensive than PA 6 EP on a per ton basis, i.e. between [10-20]% and [20-30]% according to the Parties,241 or exceeding 25% according to respondents to the market investigation.242 However, demand for both materials remains stable and customers for PA 6.6 EP do not switch to PA 6 EP even if the price differential increases.243 As one market participant puts it "The price per ton of PA 6.6 [EP] has been 400 EUR higher than for PA 6[EP] historically. Currently the price is 700-800 EUR higher for PA 6.6 [EP] than for PA 6.[EP] This price increase is not sufficient to substitute PA 6.6 and PA 6."244
(307) On the supply-side, responses of PA 6 EP producers to the market investigation confirm that technically compounding equipment can produce both PA 6 EP and PA 6.6 EP.245 However, respondents also clearly indicated that to produce PA 6.6 EP, access to PA 6.6 BP and its upstream components (ADN, HMD and/or AH Salt) is also necessary, which limits significantly their ability to switch production to PA 6.6 EP. 246
(308) The Commission also investigated whether the market for PA 6.6 EP could be further sub-segmented according to the various grades or applications of PA 6.6 EP. The market investigation indicated that there is some degree of supply- and demand- side substitutability.247 In particular, a majority of customers indicated in particular that the same grades or types of PA 6.6 EP can be used in different industry sectors (i.e. transportation, industrial, consumer, construction, etc.) and that there is no grade or type of PA 6.6 EP that cannot be used in certain industry sectors.248
5.9.2.4. Conclusion on product market definition
(309) Based on the assessment in recitals 292 to308, the Commission concludes that, for the purpose of assessing the Transaction, PA 6 and PA 6.6 EP constitute distinct product markets.
5.9.3. Geographic market definition
5.9.3.1. The Commission’s precedents
(310) In Sabic/GE Plastics, cited by the Notifying Party, the Commission has found that the affected markets (including EP) were at least EEA-wide in scope, but left the exact market definition open.249
(311) In Rhône Poulenc/Caffaro, the Commission considered the reference geographic market for PA EP to be EEA-wide in scope.250
5.9.3.2. The Notifying Party’s view
(312) The Notifying Party considers that the relevant geographic market for PA 6 EP and PA 6.6 EP is global in scope.251 The Notifying Party argues in particular that (i) EP suppliers sell their products to customers globally since there are no significant barriers to the flow of PA 6 EP and PA 6.6 EP between regions; (ii) PA 6 EP and PA
6.6 EP are non-perishable, non-hazardous products which can be easily stored for a long period and transported across great distances at low costs (less than [0-10]% of the sales price); (iii) the Parties already export PA 6 EP and PA 6.6 EP […].
(313) In its Response to the 6(1)(c) Decision, the Notifying Party further insists that the market for PA 6.6 EP should be considered as global in scope. Firstly, the Notifying Party points out to the fact that a majority of market respondents stated there were no technical or regulatory characteristics of PA 6.6 EP that would limit its transportability across regions, apart from ensuring that the products are adequately protected against humidity.252 Secondly, the Notifying Party considers that the market investigation focuses on barriers to trade and that its results are not in line with those in the Solvay/Rhodia case,253 in which the Commissions concluded that the market for the different polymers was at least EEA-wide in scope.254
5.9.3.3. The Commission’s assessment
(314) The market investigation indicates that the PA 6.6 EP market is EEA-wide in scope.
(315) Firstly, as mentioned in recital 265 the fact that there is no technical or regulatory barrier to interregional trade, does not imply that the conditions of competition are sufficiently homogeneous worldwide. In particular cross-region trade may not be a commercially viable option, in particular for cost-related considerations.
(316) On the demand-side, the majority of customers that responded to the market investigation only procure PA 6.6 EP volumes from inside the EEA to supply their EEA plants.255 If customers do source from outside the EEA, imported volumes typically remain a small part (i.e. less than 20% and typically less than 10%) of their overall needs in PA 6.6 EP.256 Customers' purchasing of PA 6.6 EP from outside the EEA. In fact, only one responding customer ([…]) reported procuring a significant share of its requirement from sources outside the EEA.257 Barriers limiting trading of PA 6.6 EP across regions, include transport costs, the ensuing long delivery periods and import/export duties.258 Transport costs and duties, by the Notifying Party's own estimates, suffice to increase the price of PA 6.6 EP by around [10-20]%.259
(317) The Notifying Party does not contest the fact that additional lead-time makes imports a less viable solutions for customers, especially those which do not typically store products and/or requiring timely or even just-in-time delivery, as is commonplace in the automotive industry. Most customers of PA 6.6 EP do not keep stocks for more than 2-4 weeks of production, making delays in deliveries a potential issue.260
(318) In addition, end customers may prefer or require PA 6.6 EP to be manufactured in Europe. […] for instance mentions that there are "Customers requirements for material with European origin".261 Similarly, […] states that commercial limitations include "preferential origin regulation (i.e. EEA)".262
(319) On the supply-side, producers of PA 6.6 EP, similarly to customers, do not believe that there are any technical or regulatory characteristics of PA 6.6 EP that would limit its transportability.263 However, this does not imply a broader than EEA geographic market dimension. Indeed, a majority of responding PA 6.6 EP producers indicated that they do not import PA 6.6 EP into the EEA.264 Among those who do import PA 6.6, only Ascend, imports volumes which are significant and has a limited production footprint in the EEA. Out of the other players who import PA 6.6 EP, […], which recycles and distributes PA 6.6 EP imports PA 6.6 EP from Ascend from the US.265 […] only started importing in 2018 due to the recent shortages in PA 6.6 BP.266 […] however acknowledges the need to have a local production, and recently purchased a compounder to this effect.267 Barriers cited as limiting trading of PA 6.6 EP across regions also include import tariffs,268 and long delivery periods.269
(320) Secondly, the Solvay/Rhodia case does not call into question the Commission's assessment in the presence case. First, the reference to Solvay/Rhodia, seems out of place with regards to PA 6.6 EP, as that previous decision, which refers to "polyamides", focused on BP (and not EP), as evidenced by the competitive assessment, which identifies the main players in the PA 6.6 BP space, namely Rhodia (now the Business), Invista, Ascend, Radici and DuPont (now DowDuPont).270 The geographic market definition for PA 6.6 BP is further discussed in Section 6.6.3. Second, the Notifying Party misrepresents the conclusions of the Commission in Solvay/Rhodia. Contrary to the Notifying Party's claim, the decision does not exclude that the relevant market would be worldwide in scope. In fact the decision reads that "[w]hile the majority of respondents source polyamides on a worldwide basis, some respondents indicated a narrower sourcing area, limited to the EEA. The market investigation also indicated that transport costs represent a small fraction of the overall price for the above polyamides, that customers tend to source on a global or EEA-wide scale and that producers of polyamides usually supply a number of diverse geographic areas" and concludes that "the relevant geographic market for the different polyamides of the parties, in particular […] PA6.6, is at least EEA-wide in scope" (emphasis added).271 Third, the Commission is not bound by the results of the market investigation in a separate case, in particular a previous case in which market investigation took place over 7 years ago. The Commission conducted in the present case a market investigation that evidences recent and current commercial practices of the relevant market participants.
5.9.3.4. Conclusion on geographic market definition
(321) The Commission therefore concludes that, for the purpose of assessing the Transaction, the market for the supply of PA 6.6 EP is EEA-wide in scope.
(322) As the outcome of the competitive assessment of the Transaction remains the same under all alternative market definitions, it is not necessary for the Commission to conclude on the exact geographic market definition for PA 6 EP.
5.9.4. Conclusion on the market definition for PA 6 and PA 6.6 EP
(323) Based on the assessment in recitals 292 to322, the Commission concludes that, for the purpose of assessing the Transaction, the market for the supply of PA 6 EP and for PA 6.6 EP constitute distinct product markets and that the market for PA 6.6 EP is EEA-wide in scope in scope.
(324) As the outcome of the competitive assessment of the Transaction remains the same under all alternative market definitions, it is not necessary for the Commission to conclude on the exact geographic market definition for PA 6 EP.
5.10. Level V of the polyamide value chain: PA 6.6 Performance Fibres
5.10.1. Introduction
(325) In addition to engineering plastics, base polymers can be processed into performance fibres.
(326) Performance fibres can be obtained from PA 6.6 BP or from PA 6 BP. The Business only produces PA 6.6 BP-based performance fibres (whereas BASF does not produce performance fibres at all).
5.10.2. Product market definition
5.10.2.1. The Commission’s precedents
(327) In previous decisions, the Commission considered that the market for polyamide yarns and fibers could be subdivided into several segments according to the intended application of the product: i) fibres for carpets; ii) fibres for spinning to use in textile reinforcement (mainly for garments); fibres for “non-woven” (technical) applications and tow for flock (is used mainly in the automotive sector, in upholstery and in packaging).272
(328) The Commission considered that the fibres for each of the applications have different characteristics in terms of their quality, weight, thickness, softness and resistance and the customers for each of these applications are different. However, in the absence of competition concerns, the Commission left the product market definition open and, in particular, whether each of the application segments constituted a distinct relevant market.273
5.10.2.2. The Notifying Party’s view
(329) On the basis of its submission that PA 6 BP and PA 6.6 BP belong to the same product market, the Notifying Party argues there is no need to define distinct markets for fibres produced from PA 6 BP and fibres produced from PA 6.6 BP.274
(330) The Notifying Party also submits that there may be distinct product markets for carpet, textile, flock and non-woven applications.275
(331) Such considerations notwithstanding, the Notifying Party provides market shares for fibres made of PA 6 BP and of PA 6.6 BP, for an overall market as well as for the distinct product markets mentioned in recitals 329 and 330.
5.10.2.3. The Commission’s assessment
(332) The Commission's market investigation confirmed that there are distinct markets for fibres produced from PA 6 BP and fibres produced from PA 6.6 BP.276 The main reasons are differences in product characteristics, investment and different manufacturing processes.277
(333) The responses from manufacturers of one type of performance fibres also stated that it is not possible for them to start producing other types of performance fibres278 due in particular to the associated high investment costs.279 While Invista produces all categories of performance fibres, other companies only produce specific categories of performance fibres. Radici produces all types of fibres with the exception of non- woven applications. PHP fibers only produces fibres for spinning to use in textile reinforcement.280
(334) Customers of performance fibres on the other hand generally indicated during the initial market investigation that there are no types of PA 6.6 performance fibres that can only be used in a specific industry sector.281 A large share of respondents indicated having a limited knowledge as to whether the performance fibres they purchased could be used for other applications.282 Those who responded were split as to whether this was the case or not, regardless of the type of fibre concerned.283
(335) Variations in the average price of performance fibres reportedly paid by customers of performance fibres were also inconclusive and thus not sufficient to infer whether the products belonged to separate markets or whether they should form part of the same one.
5.10.2.4. Conclusion on product market definition
(336) As the outcome of the competitive assessment of the Transaction remains the same and the Final Commitments entered into by the Notifying Party address the Commission’s concerns under all alternative market definitions, it is not necessary for the Commission to conclude on the exact product market definition for PA 6.6 Performance Fibres and in particular on the relevance of possible sub-segmentation of the market between carpet, textile, flock and non-woven applications.
5.10.3. Geographic market definition
5.10.3.1. The Commission’s precedents
(337) The Commission decisions cited in Section 5.10.2.1, which date back to the 1990s, held that the relevant markets were EEA-wide, mainly in light of the limited imports into the EEA, the relatively limited trade flows with other regions like the USA and the Far East, the need for proximity to one’s supplier (for technical support and customisation reasons) and the rather high custom duties on imports of yarns and fibres from outside the EEA (approximately 9%).284
5.10.3.2. The Notifying Party's view
(338) The Notifying Party submits that the performance fibres markets have evolved towards a global dimension,285 since trade flows of performance fibres between the EEA and other regions of the world have become significant (it claims that imports of PA 6 and PA 6.6-based performance fibres from […] represented […] kts, i.e., [60-70]% of the market) and transport costs have gone down (representing less than [0-10]% of the overall selling price).
5.10.3.3. The Commission's assessment
(339) No technical or regulatory characteristics of PA 6.6 Performance Fibres are seen as limiting their transportability across different regions, by either suppliers or customers.286
(340) On the supply-side, the market investigation indicated that among the manufacturers of performance fibres active in the EEA, only […] reported importing volumes from other regions.287
(341) On the demand-side, while in the context of Phase I investigation a majority of customers for performance fibres indicated that they sourced globally for performance fibres used in their EEA plants,288 respondents in Phase II overwhelmingly mentioned they procured PA 6.6 Performance Fibres within the EEA.289 Reasons put forward for not procuring performance fibres from outside the EEA include price, risk management and quality.290
5.10.3.4. Conclusion on geographic market definition
(342) Based on the assessment in recitals 337 to341, the Commission concludes that, for the purpose of assessing the Transaction, the market for the supply of PA 6.6 Performance Fibres is EEA-wide in scope.
5.10.4. Conclusion on the market definition for PA 6.6 Performance Fibres
(343) As the outcome of the competitive assessment of the Transaction remains the same and the Final Commitments entered into by the Notifying Party address the Commission’s concerns under all alternative market definitions, it is not necessary for the Commission to conclude on the exact product market definition for PA 6.6 Performance Fibres, which is EEA-wide in scope.
5.11. Level V of the polyamide value chain: PA6 3D printing powders
5.11.1. Introduction
(344) PA6 powders are 3D printing powders designed for selective laser sintering (SLS), which is an additive manufacturing (AM) technique. These polymer powders are well-suited for many applications in automotive, appliances, sporting goods, plumbing, transportation, construction and electrical markets.
(345) PA6 powder is produced through cryogenic grinding of PA6 base polymer, then packed and sold to the customer.
(346) Other 3D Polymer Printing Powders are mostly based on PA12 technology, but there is also a part based on PA11 technology or PA13.
(347) According to the Notifying Party, the most widely used 3D printing powder is […], followed by […]. According to the Notifying Party, the […].
(348) According to the Notifying Party, PA6 is only slowly introduced to the market. This material offers higher heat resistance which makes it suitable for under the hood applications in the automotive industry. The disadvantage of this material is its smaller processing window and the need to control humidity and oxygen at low levels during 3D printing.
5.11.2. Product market definition
5.11.2.1. The Commission’s precedents
(349) The Commission has not previously analysed the product market for PA6 3D printing powders.
5.11.2.2. The Notifying Party's view
(350) The Notifying Party submits that market reports generally refer to the 3D printing powder market as segmented on the basis of powder type (i.e., metal, plastic, ceramic, and others). While PA6 powders are an improvement with regard to resistance, they are in principle interchangeable with the more basic varieties. There are, moreover, other 3D printing methods such as fused filament fabrication (FFF), also known as fused deposition modeling (FDM), based on extruding thermoplastic materials (e.g., Polylactic acid and Acrylonitril) layer by layer. Other methods include resin photopolymerisation, laminated object manufacturing and electro beam freeform fabrication
(351) The Notifying Party therefore contends that PA6 3D printing powders for laser sintering belong to a larger market for materials to be used in various 3D printing technologies.
5.11.2.3. The Commission's assessment.
(352) Even though some respondent to the market investigation, such as Ricoh, gave indications that could point toward a certain degree of substutability with other types of printing powder, the Commission considers that demand side considerations univocally indicate that PA6 3D printing powder form part of a separate product market from other 3D printing powders.
(353) More precisely, […] explained that the "that the machine they use with PA6 3D printing powder is also compatible with other kind of plastic powders such as PA12, PA12 glass beads, PA11, PA6 glass beads and polypropylene offered by […]", and that "in order to switch between the different types of printing powders, the machine has to been cleaned. The cleaning process is short and, according to […], lasts around two hours"291.
(354) On the demand side, although some respondents to the market investigation indicate that other types of 3D printing powder have technical characteristics similar to those of PA6 3D printing powder, the majority of respondents indicated that PA6 3D printing powder have different technical characteristic compared to other 3D printing powders and that other types of 3D printing powders have some weaknesses compared to the PA6 3D printing powder.292
(355) Further, customers responding to the market investigation gave indication that from the demand side PA6 3D printing powders are not substitutable with other types of 3D printing powders. In this regard, […] – a customer of 3D printing powder – explained that "[…] uses PA6 3D printing powder in order to get functional prototypes that aim at being as close as possible to the series production components that will be made out of PA6, and that will be produced by injection moulding" and it further explained that "there is currently no substitute to PA6 3D printing powder on the market". […] nonetheless asserted that "one of the alternatives to PA6 3D printing powder would be the injection moulding or to use another prototyping technology. However alternatives would be much more costly, but also would take much more time, as building the relevant tools for injection moulding is much lengthier than printing".293
(356) Based on the assessment in recitals 352 to 355, the Commission concludes that PA6 3D printing powder constitutes a distinct product market for the purpose of assessing the Transaction.
5.11.3. Geographic market definition.
5.11.3.1. The Commission precedents
(357) The Commission has not previously analysed the product market for PA6 3D printing powders.
5.11.3.2. The Notifying Party's view
(358) The Notifying Party submits that the market for production and sale of PA6 3D printing powder is global. In the Notifying Party's view, this is because suppliers of PA6 3D printing powder offer the product globally.
5.11.3.3. The Commission's assessment.
(359) Some of the respondents to the market investigation indicated that they source PA6 3D printing powder for their EEA needs within the EEA. However, this may also be due to the very limited size of the market, as explained for example by "[…] has not looked at other alternative suppliers outside of Europe because their PA6 3D printing powder need is not large enough and is not […]'s priority".294
(360) Some respondents gave however indications advocating for a broader geographic scope of the market, for example […], a 3D printing powder customer, indicated that it "is sourcing 100% of its materials from outside the EU". 295.
(361) However, as the outcome of the competitive assessment of the Transaction remains the same under all alternative market definitions, it is not necessary for the Commission to conclude on the exact geographic market definition.
5.12. Products outside the polyamide value chain
5.12.1. HDI derivatives
5.12.1.1. Introduction
(362) HMD can be processed into hexamethlyene diisocyanate ("HDI") which is an organic compound in the class known as isocyanates. More specifically, it is an aliphatic diisocyanate. HDI can be used for the production of HDI oligomer (HDI derivatives) or for biopolymer applications.
(363) HDI oligomer (or "HDI derivatives") also referred to as aliphatic polyisocyanate is used as cross linker for two component polyurethane coatings, i.e., as a hardener in the coatings industry and to an insignificant extent in adhesives.
5.12.1.2. Product market definition
5.12.1.2(A) The Commission’s precedents
(364) The Commission has not previously analysed the product market for HDI derivatives.
5.12.1.2(B) The Notifying Party's view
(365) The Notifying Party submits that HDI oligomer constitutes a distinct product market which does not need to be further sub-segmented by application as the vast majority of the product goes into the coatings industry (more than [90-100]%) and to a far lesser extent in adhesives. Within the coatings industry a distinction between industrial, automotive, refinish and other coatings applications is not relevant as the same HDI oligomer can be used in any coating application without any quality or functional differences.
5.12.1.2(C) The Commission's assessment
(366) The evidence in the Commission's file has not provided any indication which would suggest that further sub-segment the HDI derivatives market would be appropriate.
5.12.1.2(D) Conclusion on product market definition
(367) Based on the assessment in recitals 362 to 366, the Commission concludes that, for the purpose of this Decision, HDI derivatives constitute a separate product market and that no further segmentation is appropriate.
5.12.1.3. Geographic market definition
5.12.1.3(A) The Commission’s precedents
(368) The Commission has not previously analysed the geographic market for HDI derivatives.
5.12.1.3(B) The Notifying Party's views
(369) The Notifying Party submits that the geographic market for this product is at least EEA-wide or global due in particular to low transportation costs which amount to approximately [0-10]% of the total sales value and the existence of significant imports into the EEA from Asia and exports from the EEA to the United States and South America. The Notifying Party also submits that the Chinese competitor Wanhua supplies the product globally from its production sites in China and BASF supplies the product to […].
5.12.1.3(C) The Commission's assessment
(370) The investigation in Phase II has provided indications that, on balance, the market for HDI derivatives is EEA-wide in scope.
(371) First, the majority of customers (67%) indicate that they source HDI derivatives at EEA level. Moreover, while the volumes sourced from outside the EEA represent a significant amount of the total needs of the customers importing from outside the EEA296 and other customers consider that they could start importing the product, the overall volumes imported to the EEA accounts for a limited share of the total needs of the EEA customers (i.e. less than [10-20]% according to the Notifying Party data). In this vein, […] explains that "we import some HDI products from China, as these have traditionally been more competitive than European sources. However, the current market situation has resulted in significant shortages particularly in Asia, which will result in minimal import 2H2018 onwards".297
(372) Customers sourcing at EEA level indicate that lead time and availability are among the consideration not to source globally. For example, […] explains that "[they] need a very short leadtime". However, other customers mention historic reasons ([…]) or that there are not particular reasons for not importing HDI derivatives […].298 Further, […] recognises that technically and commercially could source from outside the EEA299 and […] that they "do not have any constraint" to source outside the EEA.300 […] also recognises that "historically [they] prefer to use only EEA products. Technically [they] could [import] after a positive test in Laboratory".301
(373) Second, there are some product and market characteristics that limit the transportability of HDI derivatives, even if they do not appear to be of such magnitude that impedes completely trade across continents per se. In this regard, 67% of customers responding to the market investigation consider that there are technical or regulatory characteristics of HDI Derivatives that limit its transportability while 33% are of the opinion that HDI derivatives travel without limitations.302 In particular customers explain that humidity and temperature need to be controlled. For example, […]) explains that "the product must for example be heated to be transported, which can take a day or two, so short term supply can sometimes be difficult". 303 However, the most important characteristics mentioned by customers is the relatively short shelf life of the HDI (6 months) which implies that transportation has to be done within a short time. For example, […] considers that "delivery time is very important and on that case the short shelf life time of the raw material" is a factor to be considered.304 […] points to regulatory issues "HDI product need proper labeling and handling as HDI products contain hazardous ingredients according to Directive 1999/45/EC".305 Nevertheless, while […] is one of the respondents that considers that some characteristics limits the transportability of HDI derivatives, it also recognises that "the products can be transported as long as the temperature is controlled. It is technically feasible to transport product from one continent to the other".306 […] also considers that "the product is transportable over large distances".307
(374) Finally, while 30% of the customers believe that there are no barriers to trade at global level, the vast majority consider that long delivery periods are a barrier to trade. For example, […] explains that "long delivery time will increase inventory costs".308 In the same vein, […] also explains that "although products are sourced on a worldwide basis, if a supplier is further away there is longer lead time, a bigger supply chain risk and more risk of failure".309 […] explains that "if a producer from outside the EU has a warehouse in the EU and if he can deliver the quantities in bulk [[…]] need in a very short time", […] would buy material from outside the EEA.310 […] adds that "long lead time/ delivery time impacts inventory management and production planning. Long lead times decrease flexibility and increase risk, but it is not considered an absolute barrier to trading HDI products across regions globally".311 Transportation costs and tariffs are also mentioned as barriers by some of respondents.312 On the other hand […] explains that "in general the transport cost is not a major component of the HDI product cost, relatively speaking, as the cost of these products is already quite high".313
(375) On balance, while a number of customers source an important part of their needs from outside the EEA, showing therefore that it is technically feasible, there are some characteristics that limit the transportability of HDI derivatives and there appear to be some logistics difficulties to import HDI derivatives. Actually, most customers only source at EEA level and the total volumes imported to the EEA in recent years represent a small fraction of the total requirements of EEA customers.
5.12.1.3(D) Conclusion on geographic market definition
(376) Based on the assessment in recitals (368) to (375), the Commission concludes that, for the purpose of this Decision, the market for HDI Derivatives is EEA-wide in scope.
5.12.1.4. Conclusion on the market definition for HDI Derivatives
(377) The Commission concludes that HDI Derivatives constitute a separate product market and that no further segmentation is appropriate.
(378) The Commission further concludes that the relevant geographic market is EEA-wide in scope.
5.12.2. Microcellular Polyurethanes
5.12.2.1. Introduction
(379) Microcellular polyurethanes are anti-vibration products that are produced by the condensation of an isocyanate or polyisocyanate with a low-molecular-weight polymer containing hydroxyl functional groups — either a polyester polyol or a polyether polyol (e.g., polypropylene glycol). Adipic acid is an indirect input into microcellular polyurethanes.
5.12.2.2. Product market definition
5.12.2.2(A) The Commission precedent
(380) So far, the Commission has not considered a market for microcellular polyurethanes as such. It has assessed a market for anti-vibration systems with possible further sub- segmentation into various types, such as microcellular polyurethane bumpers.314
5.12.2.2(B) The Notifying Party's views
(381) The Notifying Party considers that the market for microcellular polyurethane bumpers should include rubber bumpers. From a demand-side perspective, the degree of substitutability between microcellular polyurethane and rubber for bumpers is high as customers decide between either rubber bumpers or microcellular polyurethane bumpers. While rubber bumpers may be cheaper with respect to their raw materials input, microcellular polyurethane competes directly with rubber as the total cost of a wheel suspension is lower when using microcellular polyurethane.
5.12.2.2(C) The Commission's assessment
(382) The evidence in the Commission's file has not provided any indication that would suggest that the product market should not include rubber bumpers.
5.12.2.2(D) Conclusion on product market definition
(383) The Commission therefore concludes that microcellular polyurethane, including rubber bumpers, constitutes a separate product market and that no further segmentation is appropriate.
5.12.2.3. Geographic market definition
5.12.2.3(A) The Commission precedent
(384) The Commission considers the geographic market for anti-vibration systems for automobiles and possible sub-markets for various types, such as micro cellular polyurethane bumpers, to be at least EEA-wide.
5.12.2.3(B) The Notifying Party's views
(385) The Notifying Party submits that the geographic scope of microcellular polyurethanes is at least EEA-wide. Transportation costs amount to 3% of the total sales value.
5.12.2.3(C) The Commission's assessment
(386) The Commission considers the market for microcellular polyurethane, including rubber bumpers, as EEA-wide in scope.
5.12.2.3(D) Conclusion on geographic market definition
(387) The Commission concludes thatthe market for microcellular polyurethane, including rubber bumpers, is EEA-wide in scope.
5.12.2.4. Conclusion on the market definition for microcellular polyurethanes
(388) The Commission concludes that microcellular polyurethane including rubber bumpers constitutes a separate product market and that no further segmentation is appropriate.
(389) The Commission further concludes that the relevant geographic market is EEA-wide in scope
5.12.3. Aliphatic Polyester TPU
5.12.3.1. Introduction
(390) TPU is produced by the condensation of an isocyanate or polyisocyanate with a low- molecular-weight polymer containing hydroxyl functional groups — either a polyester polyol or a polyether polyol (e.g., polypropylene glycol). Only the polyester based TPU contains adipic acid. Adipic acid is an indirect input for the production of polyester TPU trough processing polyester polyol. TPU has many applications including automotive, films, wire and cable jacketing, hose and tube, in adhesive and textile, sporting goods, footwear and inflatable rafts.
5.12.3.2. Product market definition
5.12.3.2(A) The Commission’s precedents
(391) The Commission has not previously analysed the market for Aliphatic Polyester TPU.
5.12.3.2(B) The Notifying Party's View
(392) The Notifying Party submits that polyester TPU constitutes the relevant product market with possible further sub-segmentations into (i) aliphatic polyester TPU and (ii) aromatic polyester TPU.
(393) From a demand-side perspective, the Notifying Party argues that there is a limited substitutability between aliphatic polyester TPU and aromatic polyester TPU as they have different product characteristics. Aliphatic polyester based TPU is used for specialty applications while aromatic polyester TPU is used as a standard polyester TPU.
5.12.3.2(C) The Commission's assessment
(394) The evidence in the Commission's file has not provided any indication which would suggest that the product market should not be segmented into aliphatic polyester TPU and aromatic polyester TPU, taking into account the limited substitutability argued by the Notifying Party. Only aliphatic polyester TPU constitutes an affected market.
5.12.3.2(D) Conclusion on product market definition
(395) The Commission concludes that aliphatic polyester TPU constitutes a separate product market and that no further segmentation is appropriate
5.12.3.3. Geographic market definition
5.12.3.3(A) The Commission’s precedents
(396) The Commission has not previously analysed the market for Aliphatic Polyester TPU.
5.12.3.3(B) The Notifying Party's View
(397) The Notifying Party submits that the geographic market for polyester TPU is at least EEA-wide. For aliphatic polyester TPU the geographic market may well be global in scope as the transportation costs amount to less than 5% of the total sales value. In the Response to the 6(1)(c) Decision, the Parties did not bring new evidence that would support a wider geographic scope of the market.
5.12.3.3(C) The Commission's assessment
(398) The evidence in the Commission's file has not provided any indication that the geographic scope of the market should be wider than EEA-wide.
5.12.3.3(D) Conclusion on geographic market definition
(399) The Commission concludes that the market for aliphatic polyester TPU is EEA-wide in scope.
5.12.3.4. Conclusion on the market definition for aliphatic polyester TPU
(400) The Commission concludes that aliphatic polyester TPU constitutes a separate product market and that no further segmentation is appropriate.
(401) The Commission further concludes in light of the results of the market investigation that the relevant geographic market is EEA-wide in scope.
5.13. Additives
5.13.1. Melamine cyanurates and melamine polyphosphates
5.13.1.1. Product market definition
5.13.1.1(A) The Commission’s precedents
(402) Melamine cyanurates and melamine polyphosphates are flame retardants. Flame retardants are chemicals which are incorporated into a variety of manufactured materials in order to increase the resistance of these materials to ignition or slow down combustion.315
(403) Melamine cyanurates are largely commoditised flame retardants employed by polyamide, PBT, and PU substrates.
(404) Melamine polyphosphates are specialty flame retardants, which are typically used by resin producers and compounders as ingredients for synergistic blends with DEPAL (Diethyl Phophinate Aluminum). Melamine polyphosphates find applications in glass-fiber, reinforced polyamides, PBT and epoxy resins, especially when superior thermal stability is required.
5.13.1.1.(B) The Notifying Party's view
(405) The Notifying Party submits that the relevant product market is the market is the market for melamine cyanurate and polyphosphate without further sub- segmentation.316
(406) The Notifying Party argues that from a demand-side perspective, there is a high degree of substitutability between melamine cyanurate and polyphosphate as both products are used as flame retardants, although the temperature range for both products may differ to some extent.317
5.13.1.1.(C) The Commission's assessment
(407) The precise definition of the relevant product market can be left open as the Transaction will leave effective competition unaffected under any alternative definition of the relevant market.
5.13.1.1.(D) Conclusion
(408) The precise definition of the relevant product market can be left open as the Transaction will leave effective competition unaffected under any alternative definition of the relevant market.
5.13.1.2. Geographic market definition
5.13.1.2.(A) The Commission's precedent
(409) Regarding the relevant geographic market, and when considering the market for flame retardants generally, the Commission has left open the geographic scope of the market. 318
5.13.1.2.(B) The Notifying Party's view
(410) The Notifying Party submits that the geographic scope of the melamine cyanurate and polyphosphate markets is at least EEA-wide. 319
5.13.1.2.(C) The Commission's assessment
(411) The precise geographic market definition can be left open as the Transaction will leave effective competition unaffected under any alternative definition of the geographic market.
5.13.1.2.(D) Conclusion
(412) The precise geographic market definition can be left open as the Transaction will leave effective competition unaffected under any alternative definition of the geographic market.
5.13.2. HALS
5.13.2.1. Product market definition
5.13.2.1.(A) The Commission's precedent
(413) Hindered Amine Light Stabilisers ("HALS") are light stabilisers that are radical scavengers, i.e. additives that inhibit the propagation of free radicals within the substrate caused by light. HALS are used in plastics including PA 6 EP and PA 6.6 EP and coatings.320 The Commission has left the precise product market definition for HALS open.321
5.13.2.1.(B) The Notifying Party's view
(414) The Notifying Party submits that there is a separate product market for HALS in line with the Commission's precedent.322
5.13.2.1.(C) The Commission's assessment
(415) The precise definition of the relevant product market can be left open as the Transaction will leave effective competition unaffected under any alternative definition of the relevant market.
5.13.2.2. Geographic market definition
5.13.2.2.(A) The Commission's precedent
(416) The Commission has previously defined the relevant geographic market for light stabilisers to be at least EEA-wide,323 but later also considered it to be wider than the EEA.324
5.13.2.2.(B) The Notifying Party's view
(417) The Notifying Party submits that the relevant geographic market for HALS should be global, and at least EEA-wide, as there is substantial intercontinental trade of HALS products and many customers source HALS from Chinese suppliers.325
5.13.2.2.(C) The Commission's assessment
(418) The precise geographic market definition can be left open as the Transaction will leave effective competition unaffected under any alternative definition of the geographic market.
5.13.3. Antioxidants
5.13.3.1. Product market definition
(419) Antioxidants are additives which are used for a variety of applications including food, rubber, lubricants and fuel. They are used primarily in plastic resins and protect polymers during the production process as well as after the polymers have been incorporated in finished goods. In particular they stabilise most polymers against radical driven oxidation happening both in processing and in use).
(420) They are sold mainly to producers of polyolefins (polypropylene and polyethylene), PVC, engineered thermoplastics, urethanes and rubber materials.326
(421) Antioxidants are generally added to these materials irrespectively of application. Polyolefins are driving the largest demand due to their largest volumes and highest sensitivity to oxidation. Polyamides also require certain antioxidant stabilisation.
(422) The Commission has considered different types of additives, including antioxidants to constitute separate product markets. The Commission has also considered a potential further segmentation into primary and secondary antioxidants. Primary antioxidants (such as amines or phenolics) are used to protect polymers from degradation during the production and processing stages. Secondary antioxidants (such as organophosphites (OPH) or thioesters) are used to protect polymers from
degradation once they are manufactured.327
5.13.3.1.(A) The Notifying Party's view
(423) The Notifying Party submits that the exact market definition can be left open in the present case noting that only primary phenolic as well as secondary antioxidants are used as additives for PA EP applications.328
5.13.3.1.(B) The Commission's assessment
(424) The precise definition of the relevant product market can be left open as the Transaction will leave effective competition unaffected under any alternative definition of the relevant market.
5.13.3.2. Geographic market definition
5.13.3.2.(A) The Commission's precedent
(425) The Commission previously defined the geographic markets for all antioxidants to be at least EEA-wide.329
5.13.3.2.(B) The Notifying Party's view
(426) The Notifying Party submits, in line with the Commission's precedent that the possible markets for antioxidants are at least EEA-wide in scope.
5.13.3.2.(C) The Commission's assessment
(427) The precise geographic market definition can be left open as the Transaction will leave effective competition unaffected under any alternative definition of the geographic market.
5.13.4. Kaolin
5.13.4.1. Product market definition 5.13.5.1.(A) The Commission's precedents
(428) Kaolin is a mineral filler used in the production of EP, like other white minerals, such as wollastonite. Kaolin is not regarded as a plastic additive. 330
(429) The Commission has previously assessed the product market for Kaolin and identified Kaolin as a separate product market with further distinction between (i) paper filling, (ii) paper coating as well as (iii) specialty applications such as plastics.331
5.13.5.1.(B) The Notifying Party's view.
(430) The Notifying Party agrees with this approach 5.13.5.1.(C) The Commission's assessment
(431) The precise definition of the relevant product market can be left open as the Transaction will leave effective competition unaffected under any alternative definition of the relevant market.
5.13.4.2. Geographic market definition 5.13.4.2.(A) The Commission's precedents
(432) In previous cases the Commission held that (i) the market for Kaolin used in coating applications was global in scope332, while (ii) the market for Kaolin used in filling applications was held to be EEA-wide in scope 333.The Commission left open the geographic definition for the market for Kaolin processed in plastics. 334
5.13.4.2.(B) The Notifying Party's view
(433) The Notifying Party agrees with this approach 5.13.4.2.(C) The Commission's assessment
(434) The precise geographic market definition can be left open as the Transaction will leave effective competition unaffected under any alternative definition of the geographic market.
5.13.5. Optical brighteners (stilbene/thiophene-benzoxazol-based OBA)
5.13.5.1. Product market definition 5.13.5.1.(A) The Commission's precedents
(435) Optical brighteners, also known as optical brightening agents (OBA), are additives that enhance the white appearance of fabrics and paper. They are dissolvable organic compounds with a high affinity for cellulosic material.
(436) The main types of optical brighteners are (i) stilbene/thiophene-benzoxazol-based OBA and (ii) Dinatrium-4,4-bis-(2-sulfostyryl)-biphenyl (DSBP)-based OBA. Stilbene/thiophene-benzoxazol-based OBA are used for detergent, textile, paper and plastics applications. DSBP-based OBA are mainly used for detergent and textile applications.335
(437) In a previous case, the Commission considered stilbene/thiophene-benzoxazol- based OBA to constitute a separate product market.336.
5.13.5.1.(B) The Notifying Party's view
(438) The Notifying Party agrees with the approach outlined in the Commission's previous decisional practice.
5.13.5.1.(C) The Commission's assessment
(439) The precise definition of the relevant product market can be left open as the Transaction will leave effective competition unaffected under any alternative definition of the relevant market.
5.13.5.2. Geographic market definition 5.13.5.2.(A) The Commission's precedents
(440) In previous cases, the Commission has left open the precise definition of the geographic scope but considered that the market for OBA was global in scope.337
5.13.5.2.(B) The Notifying Party's view
(441) The Notifying Party submits that the geographic market for stilbene/thiophene- benzoxazol-based and DSBP-based OBA is global as customers are supplied globally and transport costs do not exceed [0-10]% of the total sales price.338
(442) Nonetheless, the Notifying Party argues that if the Commission would consider an EEA-wide market, the Transaction will leave effective competition unaffected.
5.13.5.2.(C) The Commission's assessment
(443) The precise geographic market definition can be left open as the Transaction will leave effective competition unaffected under any alternative definition of the geographic market.
6. COMPETITIVE ASSESSMENT
6.1. Framework of assessment
(444) Article 2 of the Merger Regulation requires the Commission to examine whether notified concentrations are to be declared compatible with the internal market, by assessing whether they would significantly impede effective competition in the internal market or in a substantial part of it.
(445) The Commission Guidelines on the assessment of horizontal mergers under the Council Regulation on the control of concentrations between undertakings339 (the "Horizontal Merger Guidelines") distinguish between two main ways in which mergers between actual or potential competitors on the same relevant market may significantly impede effective competition, namely non-coordinated effects and coordinated effects.
(446) Non-coordinated effects may significantly impede effective competition by eliminating the competitive constraint imposed by each merging party on the other, as a result of which the Merged Entity would have increased market power without resorting to coordinated behaviour. In this regard, the Horizontal Merger Guidelines consider not only the direct loss of competition between the merging firms, but also the reduction in competitive pressure on non-merging firms in the same market that could be brought about by the merger. According to recital (25) of the preamble of the Merger Regulation, a significant impediment to effective competition can result from the anticompetitive effects of a concentration even if the Merged Entity would not have a dominant position on the market concerned.
(447) The Horizontal Merger Guidelines list a number of factors which may influence whether or not significant non-coordinated effects are likely to result from a merger, such as the large market shares of the merging firms, the fact that the merging firms are close competitors, the limited possibilities for customers to switch suppliers or the fact that the merger would eliminate an important competitive force. Not all of these factors need to be present for significant non-coordinated effects to be likely. The list of factors, each of which is not necessarily decisive in its own right, is also not an exhaustive list.
(448) In addition, the Commission Guidelines on the assessment of non-horizontal mergers under the Council Regulation on the control of concentrations between undertakings340 (the "Non-Horizontal Merger Guidelines") distinguish between two main ways in which vertical mergers may significantly impede effective competition, namely input foreclosure and customer foreclosure.
(449) As explained in recital (805), the assessment of potential vertical effects would be limited to input foreclosure, in particular whether the Transaction may give rise to the to the possibility of the Merged Entity to restrict competing firms' access, in downstream markets, to a key input, or to provide access at less favourable terms.
(450) For a Transaction to raise input foreclosure competition concerns, the Merged Entity must have a significant amount of market power upstream.341 In assessing the likelihood of an anticompetitive input foreclosure strategy, the Commission has to examine whether (i) the Merged Entity would have the ability to substantially foreclose access to inputs, (ii) whether it would have the incentive to do so and (iii) whether a foreclosure strategy would have a significant detrimental on competition downstream. Each one of these points will be analysed separately although the Commission recognises that they are closely intertwined.342
(451) In addition, the Non-Horizontal Merger Guidelines also state that a concentration may entail conglomerate effects. Conglomerate effects may arise in a concentration where the undertakings involved are active on closely related markets (for example, as suppliers of complementary products). In most circumstances, conglomerate mergers do not lead to any competition concerns. However, foreclosure effects may arise when the combination of products in related markets may confer on the Merged Entity the ability and incentive to leverage a strong market position from one market to another closely related market by means of tying or bundling or other exclusionary practices.343
(452) In accordance with the legal framework set out in recitals (444) to (452), the Commission has carried out an extensive competitive assessment of the Transaction in Phase I and Phase II in order to assess whether the Transaction significantly impedes effective competition within the internal market on account of horizontal non-coordinated effects in the relevant markets for ADN, HMD, adipic acid, AH Salt, PA 6.6 BP PA6 3D Printing Powder and PA 6.6 EP. The Commission has also assessed whether the Transaction significantly impedes effective competition within the internal market on account of input foreclosure in the supply of butadiene, ammonia, ADN, HMD, adipic acid, caprolactam, AH Salt, PA 6.6 BP, and additives to the respective downstream markets. Lastly, the Commission has also assessed whether the Transaction significantly impedes effective competition within the internal market as a result of conglomerate effects.
6.2. Preliminary methodological remarks regarding market shares
(453) As a preliminary introduction to the assessment of both the horizontal overlaps and the vertical links generated by the Transaction, , the Commission notes, for the reasons set out in the present section, that it is relevant to consider various measures of market shares in this case.
(454) Market shares provide useful first indications of the market structure and of the competitive importance of both the merging parties and their competitors. Typically, the Commission uses “merchant” market shares, that is market shares based on sales to third parties. Nevertheless, the choice of a particular market share measure depends on the circumstances of the specific industry in question. Different market share measures may have different advantages and shortcomings in indicating market power. It may therefore be useful to analyse a combination of different market share measures as complementary first indicators for market power. In particular, in order to better reflect the market power of integrated producers at each level of the value chain, and in line with previous decisions,344 the Commission may decide to rely on other shares than merchant sales shares.
(455) In the present case, the Commission considers three complementary market share measures to be informative as first indicators of market power:
(a) Sales shares (or merchant sales shares), which reflect sales to third parties and include imports into the geographical market considered.
(b) Capacity shares, which reflect the production capabilities within the geographical market that is considered. Capacity shares do not encompass imports into the geographical market considered, but, instead, encompass exports.
(c) The sum of merchant sales and captive production, which reflects both the sales to third parties and internal consumption or captive use by integrated players, in the geographical market that is considered. This metric provides an indicator of the role of each producer in supplying internal and external consumption within a given geographical area. These shares also take into consideration the role of imports into the geographical market that is considered.
(456) The Commission considers that these complementary market share measures may be necessary in industries with integrated firms, in order to evaluate the market power stemming from captive production and use. This is particularly true because relying on these complementary metrics is useful to take into consideration the complementary effects set out in recitals (458)-(462).
(457) First, a merger between firms that sell in the merchant market would relax the direct constraints they exert on prices in such merchant market. The positions of the various competitors are usually well captured by sales shares, which also reflect constraints from outside the relevant market (imports).
(458) Second, whether or not firms participate in the merchant market, they may exert some constraints on merchant market prices whenever they have (or are perceived as having) the ability to participate or to expand their sales in such market. This ability could materialise in actual (or further) merchant sales when they have the incentives to do so. For example, the competitive constraint of a firm active only at one level of a vertical chain, and in particular the likelihood that it can defeat a possible price increase by the merging parties, depends in particular on its ability and incentives to expand or to redirect output between markets. Therefore, market shares based on production capabilities can reflect such market power. A vertically-integrated firm will face the same type of trade-offs but, in addition, its ability and incentives to redirect captive sales into the merchant market will also play a role. This, again, will not be reflected by merchant sales shares only.
(459) Therefore, measures including captive volumes are more likely to reflect the constraints imposed on prices in the merchant markets as referred to in recital (803), and measures related to capacity shares are also likely to be relevant to understand the constraints that firms can exert in the merchant market.
(460) More generally, the Commission regards capacity shares as an important driver of competitive dynamics, as they constitute a more structural metric of market power than sales shares. Capacity shares, and particularly changes thereof, may better reflect lasting changes in the structure of a market as well as the magnitude of such structural change. This measure captures the structure of the market at a level of production where barriers to entry are the highest, and is an indicator of the capabilities of an integrated firm also at further levels of the supply chain, and as such a potential indication of its market power.
(461) Third, whether or not vertically-integrated firms sell in the merchant market, they exert indirect constraints on the price arising in such market if they compete with merchant market customers (or these customers’ customers, etc.) at some levels further down the supply chain. The economic literature has suggested that the magnitude of these indirect effects can be large, as the indirect constraints exerted by vertically-integrated firms can even be stronger than the hypothetical direct constraints which would be exerted by the independent upstream arms of the vertically-integrated firms.345 This effect also confirms that market share measures which take into account capacity or production volumes for captive use are relevant indicators of market power.
6.2.1. The Notifying Party’s view
(462) The Notifying Party claimed that, in assessing the effects of the Transaction, only sales on the merchant market are relevant.346 In particular, the Notifying Party developed their arguments for the HMD product level, in relation to assessing the horizontal effects of the Transaction. These arguments were then used also for other levels of the supply chain, both in relation to horizontal and vertical effects.347More precisely, the Notifying Party claimed that “the level of captive use does not and cannot exert any competitive constraint on the merchant market. Only the prices and quantities traded on the merchant market do affect the costs of competitors sourcing HMD from third parties but not the volumes captively retained as these are simply not traded in the market.”348
(463) The Notifying Party also claimed that merchant market sales constitute the relevant measure of market power when assessing the vertical aspects of the Transaction that relate to foreclosure concerns.349 More precisely, the Notifying Party claimed that “the volumes that matter for the purposes of the foreclosure analysis are the volumes that the merged entity “would have otherwise supplied”. This can only correspond to its merchant sales. The Parties’ shares in terms of total production and capacity are thus irrelevant since, even absent the Proposed Transaction, the volumes used captively are by definition not available to the merchant market.” 350
6.2.2. The Commission’s assessment
(464) The Commission disagrees with the various claims made by the Notifying Party that only merchant sales should be relevant in assessing the horizontal and vertical effects of the transaction.
(465) With respect to horizontal effects, captive use can exert a competitive constraint on the merchant market. The production which is used captively exerts several types of constraint on the merchant market.
(466) First, there is no technological barrier to diverting production used captively towards various merchant market, as the merging parties both made sales in the merchant markets for HMD, adipic acid, AH salt, and PA 6.6 BP. Thanks to possible diversion of captive production into merchant sales, the merging parties thus possess the ability to easily increase their merchant sales when they face the incentives to do so. This ability to enter or to expand in the merchant market represents a competitive constraint for other firms active in the merchant market, as the price in such market should remain low enough not to give the merging parties the incentives to expand their merchant sales. Similarly, customers in the merchant market rely on the Notifying Party’s ability to serve them at a certain price, in order to negotiate with other firms active in the merchant market. In this vein, […] believes that “it will be very difficult to negotiate prices because there will be only one supplier and […] will not be able to shift volumes to other competitor in case of price increases”.351
(467) Second, and because (part of) the merging parties’ production which is used captively is ultimately transformed down the supply chain into products which are typically used to compete against the customers in the merchant market, the production which is used captively exerts an indirect constraint on the merchant market. Indeed, the competitive constraint originating from captive use and occurring at the downstream level of the supply chain prevents the merchant market price to be raised too high, in order for customers in the merchant market to face input costs allowing them to make positive sales downstream, as positive sales downstream translate into positive sales of input for the firms active in the merchant market.
(468) Moreover, with respect to vertical effects, the Commission considers again that merchant sales do not constitute the only relevant measure to be taken into account. While merchant sales provide an indication of the positions of the suppliers in the merchant market, the merging parties’ incentives to foreclose competitors, fully or partially, in the merchant market depend, at least in part, on the merging parties' overall presence and market power which can be reflected by market share measures that account for capacity and/or captive use. For example, the incentives to foreclose depend to a great extent on the presence at levels further down the supply chain. Hence, a consolidation of the market downstream would raise the incentives to foreclose at a level upstream.
6.3. Horizontal non-coordinated effects
6.3.1. Introduction
(469) The transaction leads to a number of horizontally affected markets in relation to the production of ADN, nitric ccid, KA Oil, HMD, adipic acid, AH Salt, PA 6.6 BP, PA
6.6 EP and PA6 3D Printing Powder. The horizontally affected markets are discussed in the present section.
6.3.2. Level I of the polyamide value chain: ADN
(470) As mentioned in Section 5.2.1.1., there is only one producer of ADN in the EEA: Butachimie. Butachimie is a 50/50 joint venture between Solvay and Invista, and produces ADN using the C4 route. Butachimie has a nameplate capacity (i.e. a theoretical capacity) of […] kts of ADN per year, […]. Pursuant to the [JV AGREEMENT] concluded between Butachimie and its current two shareholders (“Butachimie ADN Contract”), each shareholder has the right to purchase [50-60]% of the actual capacity of the ADN plant. Butachimie has no market facing activities other than the sales to its parents, which then use the ADN captively or sell it independently on the merchant market.
(471) In the Form CO, as well as in the Response to the 6(1)(c) Decision, the Notifying Party submitted that, since BASF is not active in the production of ADN, the Transaction does not generate any horizontal overlap on that market.
(472) The Commission acknowledges that BASF does not have any ADN production capacity pre-transaction, however, for the reasons set out in recitals (473)-(474), the Commission took the preliminary view in the 6(1)(c) Decision that the Transaction directly generates a significant change in the market structure.
(473) […].
(474) As a consequence, the Commission concluded that BASF would not only control the [50-60]% production share in Butachimie by replacing Solvay as a shareholder, but would control an additional [10-20]% on a long term and structural basis as a result of its contract with Invista.352 Hence, the Commission took the view in the 6(1)(c) Decision, and maintains in this Decision, that the Transaction would result in a significant change to the market structure and would not amount to a mere substitution of Solvay by BASF, as submitted by BASF. In fact, two suppliers are present on the market pre-transaction, each with 50% of the capacity shares on the market, whereas post-transaction BASF would control [60-70]% of the production capacity of ADN and Invista would control the remaining [30-40]%. This would be akin to BASF acquiring two separate positions in the market for the supply of ADN, which puts it into a different position compared to Solvay's prior to the Transaction. As a result of this shift in the post-transaction market structure, the competitive dynamics between the two suppliers of ADN would change and the competitive impact of such a change has to be assessed.
6.3.2.1. Market structure
(475) On the EEA market for ADN C4, the combined market shares of the Parties is illustrated in Table 1:353
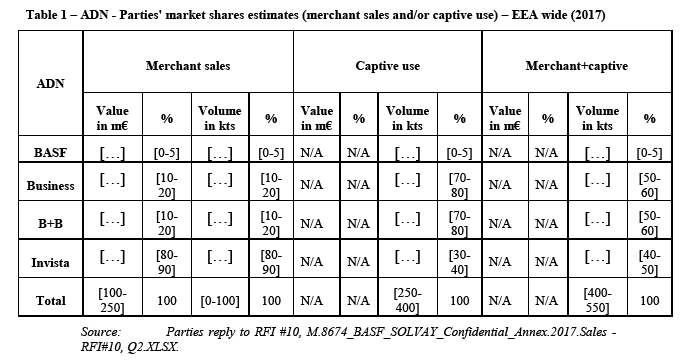
(476) As explained in recital (474), the production capacity in the EEA would be split equally between the Business and Invista absent the Transaction, however the production capacity shares would be [60-70]% BASF and [30-40]% Invista post- transaction.
6.3.2.2. Competitive assessment: non-coordinated effects 6.3.2.2.(A) The Notifying Party's arguments
(477) In the Form CO the Notifying Party claimed that, lacking any horizontal overlap, the market for ADN, irrespective of the exact market definition, is not affected by the Transaction.
(478) In its Response to the 6(1)(c) Decision, the Notifying Party claims that any concern in relation to ADN falls outside the Commission's jurisdiction under the the Merger Regulation as (i) the Transaction does not lead to a significant change in the structure of the ADN market, and (ii) any concern is not merger specific.
(479) More specifically, the Notifying Party submits that the Transaction only entails the replacement of a player (Solvay) with another (BASF) as BASF is not active in the ADN market, but is only a customer. On that basis, the Notifying Party claims that the Transaction does not generate any horizontal overlap.
(480) In the Response to the 6(1)(c) Decision, the Notifying Party also claims that the Commission approach in that Decision is flawed as the assessment of horizontal non- coordinated effects therein is based on the increase in production capacity share brought about by a long term ADN supply agreement entered into by and between BASF and Invista. In this respect, the Notifying Party claims that the production capacity shares corresponding to the contracted volumes cannot be allocated to BASF as it will not have control over those volumes as defined by the Merger Regulation. According to the Notifying Party, the long term contract entered into with Invista is purely a commercial relationship and does not grant BASF any de facto or de jure decisive influence over an undertaking. As such, any change in the structure of the ADN market is not merger specific.
(481) The Notifying Party further claims that the concerns raised by customers in the Phase I market investigation that post-transaction the Merged Entity will no longer supply ADN to the merchant market are not merger specific as this would have happened even absent the Transaction. According to the Notifying Party this is because:
(a) BASF […]. Absent the Transaction, this situation would have remained unchanged; and,
(b) The Business […].
(482) According to the Notifying Party, the correct counterfactual is therefore not the Business selling ADN on the merchant market in competition with Invista, but rather a situation where neither BASF nor the Business will pose a competitive constraint on Invista.
(483) The Notifying Party also claims that the concern raised by the Commission in the Decision that Invista would hold and exploit a dominant position on the ADN market following the proposed Transaction is not a direct and immediate effect of it. According to the Notifying Party, Invista is already the dominant player on the ADN market and this dominance will not be in any way affected by the Proposed Transaction.
6.3.2.2.(B) The Commission' Assessment
(484) As a preliminary remark, the Commission observes that there is only one competitor on the ADN C4 market, Invista, as well as only one customer, […]. However, as ADN is an essential input for the value chain as a whole, a number of downstream market participants active at different levels of the value chain commented also on the ADN level.
(485) In the 6(1)(c) Decision, the Commission concluded that the Transaction raises serious doubts as to its compatibility with the internal market for the reasons set out in the present section.
(486) Respondents to the market investigation indicated that they expect the Merged Entity to internalise all, or almost all, the ADN production available to it. Respondents further explain that if BASF was to fully internalise Butachimie's production, only Invista would be left as supplier of ADN on the market. Therefore, the pre- transaction competitive constraint on Invista's ability to unilaterally increase prices for ADN would be removed.
(487) […] indicated that "With the acquisition of Solvay’s portion of the Butachimie JV, the amount of “non-captive” ADN available in Europe will be reduced by 27% from 366kt per year to 267kt per year (221KT from Invista and 46KT from Solvay/BASF)", explaining that "Of greater concern, this will then result in Invista having a near monopoly control (≈85%) over the remaining freely available ADN in the market in Europe".354
(488) This concern is further raised by […] which indicates that "post-transaction, the combined firm would have the ability and incentive to divert Solvay's current merchant sales of ADN to BASF to meet BASF's captive demand internally. Given the anticipated growth of the downstream PA66 compounded engineering resins market, where both BASF and Solvay are major suppliers, the combined firm would need to increase the portion of ADN (and HMD) production that it uses captively for the production of PA66 polymer and PA66 compounded engineering resins. As a matter of fact, […] estimates that Solvay's ADN capacity in the EU available to the merchant market (either directly as ADN or indirectly as HMD) would likely be fully consumed by BASF's ADN demand".355 And another market participant further explained that "BASF has a very strong internal demand of HMD for its engineering plastic business and they will prioritize internal requirements. It is […] assessment that unless the combined BASF/Solvay entity can maintain the existing level of merchant ADN and HMD quantities, these markets will be dominated by Invista".356
(489) The analysis of the internal documents carried out by the Commission indicated that the market concerns are well-founded. In one internal document assessing the opportunity to pursue the Transaction, it is explained that “[QUOTE FROM CONFIDENTIAL INTERNAL DOCUMENT]”357 and in an internal presentation concerning the Transaction it was clearly indicated that [QUOTE FROM CONFIDENTIAL INTERNAL DOCUMENT]”.358
(490) The further analysis of BASF' internal documents carried out during the Phase II investigation, confirmed the market concerns. In Figure 3, for example, […]:359
[GRAPH SHOWING BASF PROJECTIONS ON AND USE]
Figure 3. BASF projections on ADN use (1)
(491) From Figure 3, […].
(492) Another internal document (Figure 4) […]:360
[GRAPH SHOWING BASF PROJECTIONS ON AND USE]
Figure 4. BASF projections on ADN use (2)
(493) A further internal document (Figure 5) clarifies […]:361
[PRESENTATION SLIDE RE. BASF PROJECTIONS ON AND USE]
Figure 5. BASF projections on ADN use (3)
(494) Additional internal documents of BASF indicate that […]362-[…].363 […]364[…]365 […].366
(495) In the 6(1)(c) Decision, the Commission preliminarily concluded that the most likely outcome of the Transaction on the market is that BASF would use a significant proportion of ADN internally, therefore reducing the quantities available on the merchant market. The findings of the Phase II market investigation presented in recitals (484)-(494) further support this conclusion.
(496) Under the current market conditions, this would result in BASF withholding from the merchant market a significant proportion of the quantities that Solvay was making available on the market. As a result, the competitive constraint faced by Invista would be significantly reduced, if not entirely removed, giving Invista the ability and all the incentives to increase the price of ADN for all the downstream players.
(497) The Transaction would therefore directly result in customers losing the only alternative supplier to Invista and therefore a significant degree of buyer power, to the extent that they would no longer be able to defeat, totally or partially, price increases imposed by Invista. In this sense, a respondent to the market investigation indicated that "it is vital to have at least two suppliers in order to be able to negotiate acceptable prices, which already today are very high".367
(498) The Commission concludes that, with the only competitor pulling out of the merchant market and focusing on internal sales - […] – Invista would in fact enjoy a quasi-monopoly situation, and would be able to extract monopoly rent on the market.
(499) The Commission also assessed the impact that the planned increase in production capacity of Butachimie would have on the availability of ADN on the merchant market.
(500) On 1 December 2015, Solvay and Invista agreed to carry out the so-called “Retrofit project” by means of which Butachimie's production process will be updated to the latest C4 technology available to Invista, which is the exclusive owner of the relevant IP rights. The Notifying Party expects as a result of the Retrofit project an increase in production capacity of circa […] kts […].368
(501) The Commission notes that […].369 Contrary to this forecast, […] has confirmed that the Retrofit project is scheduled for 2019.370 In light of the available information, the Commission therefore considers likely that the retrofit project will be carried out in 2019.
(502) The Commission considers, however, that the expected capacity increase would not be sufficient to sustainably guarantee the delivery of additional quantities of ADN to the merchant market for the following reasons:
(a) First, the EEA market for PA 6.6. EP is expected to grow vigorously in the next few years, mainly driven by demand from the automotive industry. Public sources indicate that an increase in demand between 2% and 5% per year can be expected.371 This finding was also supported by respondents to the market investigation. As ADN is the key input in the PA 6.6 value chain and even assuming a constant share market for BASF, its internal demand of ADN will most likely increase, absorbing an increasing proportion of the ADN production capacity added with the Retrofit project;
(b) Second, BASF's publicly stated that it has the strategic objective to strengthen its position on the EP markets, […];
(c) Third, increases in nameplate capacity will not necessarily fully translate in increased production. The Notifying Party has indicated in the Form CO that capacity utilisation rates in the ADN industry have been historically between [80-90]% in recent years. Therefore, absent any indication to the contrary, utilisation rates are likely to remain within that range in the future.
(d) Fourth, ADN is exported from the EEA into Asia and South America. Specifically, according to the information provided by the Notifying Party, as well as from the data reported in industry reports, there is no ADN production capacity in China, nor is it expected to be set up in the near future. Hence, China will continue to rely on import for its ADN demand and to accommodate any further increase in demand.
(503) In the 6(1)(c) Decision, the Commission also observed that Solvay indicated that […]. The Commission notes in this regard that capacity utilisation rates have been in the area of [80-90]% in recent years. At the same time, Solvay indicated that the profitability loss connected to running the plant at lower capacity utilisation rates is not excessive. The Notifying Party has indicated that a reduction in capacity of utilisation of […]% would have an impact on production cost of approximately [5- 10]%, while a reduction of [10-20]% would generate an increase of production cost of circa [10-20]%.372
(504) The Commission therefore considered that – should the Merged Entity decide to continue supplying the merchant market, by controlling the vast majority of Butachimie's production capacity could profitably increase prices for ADN. In this market, the Commission preliminary concluded that Invista would have no incentive to defeat such a price increase to maximise its profit on that market. The increase in prices could also be a direct result of a decision by Butachime to reduce its output, thus exacerbating the supply/demand imbalances on the market. In this context, the lost volumes could easily be recovered by extracting higher prices. The Commission is of the preliminary opinion that Invista's likelihood to not align with such a strategy is further diminished by the fact that it has secured sales […], which would make the trade-off even more profitable.
(505) In the 6(1)(c) Decision, the Commission observed that the result of the Phase I market investigation, as well as public sources,373 indicated that entry in this market in not expected or likely, due to the existence of very high entry barriers. Respondents to the market investigation indicated that the very high investment required, as well as the need to access IP rights make entry into the market very unlikely. As explained by […] "(…) the market for the manufacture of ADN is further characterized by high entry barriers: the manufacture of ADN is complex, requires know-how, IP and considerable investment. Therefore, the likelihood of a new entrant into the ADN market in Europe is highly unlikely. This is underlined by the 2015 case in China where a greenfield ADN plant was permanently shut down after a fatal explosion".374 Another respondent further explained that "the production of ADN is difficult due to the required technical know-how which only a few suppliers have. There are only two technologies available, owned by Invista and by Ascend. Entry barriers are high. An investment of USD 500-700 million and an expected lead time of 5 years are required to build a new plant. For a plant extension, which would not be sufficient to satisfy future ADN demand, the plant would need to be taken offline for several months thereby increasing the scarcity of ADN in the market".375 […] also gave information supporting this finding as it stated that "Barriers to entry for the production of ADN are very high. The investment required for an ADN production plant is between USD 500 and 700 million and the time needed to build a new plant is around five years. Moreover, there are technical barriers and special know-how/IP required that makes entry by new suppliers very difficult"376
(506) This finding is not contested by the Notifying Party, which explained in the Form CO that "The main reason for the absence of entry is that the cost of entering production of 4-based ADN is relatively high. […]"377 and did not contest this finding in its Response to the 6(1)(c) Decision.
(507) The Commission therefore concludes that there is no likelihood of timely and sufficient entry, able to counter the anticompetitive effects of the Transaction.
(508) The Commission therefore concludes that the Merged Entity may either unilaterally increase prices on the ADN merchant market or withhold entirely volumes to the merchant market, in this latter case leaving Invista in a quasi-monopoly situation. This also would result in an increase in price for ADN as well as in a structural shortage of supply.
(509) As explained in Section 6.3.2.2. (A), the Notifying Party, in its Response to the 6(1)(c) Decision, argued that any concerns in relation to ADN fall outside the Commission’s jurisdiction under the Merger Regulation as they cannot be merger- specific as the effects on the market of the long term ADN supply agreement entered into by and between BASF and Invista fall outside of the Commission's scope of assessment.
(510) First, the Commission considers that the Transaction brings about a structural change on the ADN market. This is because, prior to the Transaction, there were two suppliers each with 50% of the capacity shares on the market, whereas post- transaction, one supplier (BASF) would control [60-70]% of the production capacity of ADN and the other supplier (Invista) would control the remaining [30-40]%. This would be akin to BASF acquiring two separate positions in the market for the supply of ADN, which puts it in a different position to Solvay prior to the Transaction. As a result of this change in the post-transaction market structure, the competitive dynamics between the two suppliers of ADN would change.
(511) The Commission observes that BASF procured the volumes from a producer on its own account and will be, for a […] period, the only undertaking that has the possibility of selling, or otherwise utilising, those volumes. More precisely, the Commission takes the view that it is appropriate to allocate the production capacities contracted with Invista to the Notifying Party because the Notifying Party will be the only undertaking to have the power to determine the economic destination of the contracted volumes on a structural basis, by deciding whether to sell on the merchant market or use it for its internal consumption. Conversely, Invista will be entirely deprived of the decision making power in this regard.
(512) Second, the Commission considers that the structural change is merger specific. […].378 […].
(513) It is clear that the structural change in the market brought about by the ADN supply agreement is caused by the Transaction. This is because, absent the Transaction, the supply agreement would not have been entered into by and between Invista and BASF. Hence, that change of the structure of the ADN market is caused directly by the Transaction.
(514) With respect to customers’ concerns that the Merged Entity will no longer supply ADN to the merchant market post-transaction, the Notifying Party contends that these are not merger specific as this would have happened even absent the Transaction. In that regard, the Commission notes that BASF's argument openly contradicts the statement made in the Form CO. In particular, in paragraphs C.122 to C.124 of the Form CO, the Notifying Party claimed the following:
– "C.122. For the merged entity, the PA6.6 base polymer capacity (incl. Fiber) is not sufficient to absorb all of the ADN that it will have available. In particular, based on the ADN input factors for PA6.6 of […], the merged entity can convert a maximum of […] kts of ADN in the EEA into PA6.6 base polymer or Fiber. The remaining […] kts have to leave the internal PA value chain in the EEA at Levels I to III (ADN, HMD and AH Salt), either as sales in the EEA or exports globally.
– C.123. For the remaining […] kts of ADN, the merged entity has in fact some limited other captive uses in the amount of max […].
– C.124. This means that the merged entity must either sell the remaining available ADN volume of […] as ADN, HMD or AH Salt in the market, or leave some ADN capacity idle."
(515) The Commission therefore considers that the correct counterfactual is not the one presented by the Notifying Party in the Response to the 6(1)(c) Decision (i.e. neither BASF nor the Business imposing a competitive constraint on Invista), but rather Solvay selling some volumes of ADN on the merchant market in the next 3 to 5 years. Although it is undisputed that […]. This is also acknowledged by Solvay in its reply to RFI 10: "[CONFIDENTIAL RESPONSE TO REQUEST FOR INFORMATION]"
(516) The Commission also considers that, contrary to the Notifying Party's claim, absent the Transaction the Retrofit project would result in Solvay having sufficient quantities of ADN exceeding its internal demand to serve the merchant market in the next […] to […] years. This can be deduced from the information provided by the Notifying Party in the economic annex to the Response to the 6(1)(c) Decision (Figure 6):
[CONFIDENTIAL FIGURE ILLUSTRATING THE BUSINESS’ ADN NEEDS BETWEEN 2016 - 2025]
Figure 6. BASF projections for ADN capacities
(517) This conclusion is further corroborated by an internal document of Solvay (Figure 7) where it is mentioned that "[QUOTE FROM CONFIDENTIAL INTERNAL DOCUMENT]":379
[CONFIDENTIAL FIGURE ILLUSTRATING THE BUSINESS’ ADN NEEDS BETWEEN 2016 - 2025]
Figure 7 Solvay’s projections on ADN use
(518) According to the PCI yellowbook 2018, the global "PA 6.6 demand will increase by 254kt (+2.1% CAGR)" which translate in a 2018 to 2028 increase in ADN requirement of 2%. According to this public information, the Commission conservatively assumes that the Business' increase in demand will be in line with the market increase, and therefore will be at most the 2.4% estimated by the Notifying Party in Table 1, if not lower. The Commission concludes that, absent the Transaction, Solvay would have ADN volumes to sell on the merchant market until 2025, at least. The Commission therefore concludes that the appropriate counterfactual is Solvay competing with Invista on the ADN merchant market.
(519) The Commission considers the argument put forward by the Notifying Party thatthe Transaction would not effect Invista’s existing position of dominance and that the Commission's concerns as therefore ungrounded, as flawed.
(520) The Commission does not deny that, on the merchant market, Invista may well hold a dominant position pre-transaction as well as post-transaction. However, the Commission contends that the Transaction, by eliminating the only competitive force on the merchant market, will remove the most important competitive constraint to this dominant position, and therefore create a significant impediment of effective competition. As explained in paragraph 24 of the Horizontal Merger Guidelines "Non-merging firms in the same market can also benefit from the reduction of competitive pressure that results from the merger" and, at paragraph 25, "mergers in oligopolistic markets involving the elimination of important competitive constraints that the merging parties previously exerted upon each other together with a reduction of competitive pressure on the remaining competitors may, even where there is little likelihood of coordination between the members of the oligopoly, also result in a significant impediment to competition".
(521) In the present case, the most likely outcome of the Transaction is, as already explained in the 6(1)(c) Decision, that the Merged Entity will internalise most or all of the ADN production and this will result in a significant lessening of the competitive constraint which was imposed pre-transaction by Solvay. More precisely, the Commission considers that a direct effect of the Transaction is that the Merged Entity will not have the incentive to sell ADN on the merchant market beyond small, opportunistic volumes. On the one hand, and contrary to the allegations of the Notifying, Party BASF will most likely need ADN volumes in excess of those contracted with Invista and on the other hand the impact of any loss of upstream sales will be mitigated by the larger operations of the Merged Entity at all the downstream levels. With respect to the conclusion that the Merged Entity will need more ADN than the volumes […].
(522) This means that the post-transaction behaviour of the Merged Entity (internalising all the ADN) will directly impact the characteristics and the structure of the market, making Invista's increase of market power merger specific.
(523) Further to that, the increase of Invista's dominance is material, as the presence of Solvay as a supplier of ADN did constitute a negotiation leverage for ADN customers which disciplined Invista's behaviour. Absent that alternative, no other supplier will discipline Invista's behaviour which will be able to extract monopoly rent on the supply of ADN.
(524) The Transaction will therefore precisely result in that "reduction of competitive pressure on the remaining competitors" listed by the Horizontal Merger Guidelines as one of the instances giving rise to a significant impediment of effective competition.
(525) This preliminary finding is also supported by the findings of the Phase II market investigation. […] in fact explained that "It’s clear that ADN price depends on PA 66 market for instance, but it also clear that making a negotiation with only one supplier lead to a very difficult situation for the buyer (in the attached contracts you have a crystal clear example of what does it mean deal with a monopolistic entity such as Invista). Invista got the ability and the incentives to increase prices of ADN and […] was forced to accept a steep increase of the Pc,380 because of lack of alternatives".381
6.3.2.2.(C) Conclusion on non-coordinated effects: ADN
(526) Based on the assessment in recitals (484) to (525) and in light of the result of the market investigation and of all the evidence available to it, the Commission concludes that the Transaction would significantly impede effective competition as a result of horizontal non-coordinated effects arising in the supply of ADN C4 in the EEA because of the high capacity shares of the Merged Entity and the lack of alternatives for customers in a very concentrated market with high barriers to entry.
6.3.3. Level I of the polyamide value chain: nitric acid
(527) The Transaction leads to a horizontally affected market in relation to the production of azeotropic nitric acid only. On the market for weak nitric acid the combined market share of the Parties will be below [5-10]% irrespective of the market definition retained. Finally, the Business does not produce concentrated nitric acid therefore there is no overlap with regards to that market.
(528) For the reasons set out in the present section, the Commission takes the view that the Transaction will not significantly affect competition in the azeotropic nitric acid market.
(529) First, on the markets for azeotropic nitric acid in 2017, the combined market share of the Parties based on merchant sales plus captive use is [30-40]% on the geographic market encompassing 1 000 km around the Ludwigshafen Plant, [30-40]% on the geographic market encompassing 1 000 km around the Antwerp Plant, [30-40]% on the geographic market encompassing 1 000 km around the Roussilion Plant, and [30- 40]% on the geographic market encompassing 1 000 km around the Chalampé Plant. However, on each of these markets, the increment brought about the Business is of [0-5]% only.
(530) Second, the Merged Entity will continue to face competition from a number of credible players having smaller, but sizeable, market shares: taking into account merchant sales plus captive use Covestro ([20-30]%, depending on the geographic market), Yara ([10-20]%, depending on the geographic market), FC Fertilizers ([10- 20]%, depending on the geographic market) and Borealis ([5-10]%, depending on the geographic market).
(531) Third, respondents to the market investigation do not perceive BASF as a particularly important player on the market, they rather consider Yara, OstChem and to a certain extent Borealis as important players.382
(532) Finally, the vast majority of customers responding to the market investigation indicated that post-transaction they will continue to have a sufficient number of suppliers of nitric acid.383
(533) Based on the assessment in recitals 527 to 532, the Commission concludes that the Transaction will not significantly impede effective competition as a result of horizontal non-coordinated effects arising in the supply of azeotropic nitric acid, under any plausible geographic market definition.
6.3.4. Level I of the polyamide value chain: KA Oil
(534) The Transaction leads to a horizontally affected market in relation to the production of KA Oil only if the market is considered EEA-wide and if capacity or both merchant and captive market shares are taken into account. The Transaction does not give rise to horizontally affected markets for cyclohexanone or cyclohexanol (distilled KA Oil). The Transaction does not lead to horizontally affected markets at worldwide level.
(535) The Business is not active in the production of KA Oil distilled into pure cyclohexanol or of KA Oil distilled into pure cyclohexanone.
(536) The combined production capacity of the Parties for KA Oil is [40-50]% ([20-30]% BASF and [10-20]% Solvay) in 2017 in the EEA.
(537) The combined market shares in 2017 of the Parties for KA Oil used for adipic acid production taking into account merchant sales plus captive use is [60-70]% in 2017, followed by Lanxess ([20-30]%), Versalis ([5-10]%), Radici ([5-10]%) and Aztoy ([5-10]%).
(538) The Transaction does not give rise to horizontally affected markets, based on the merchant sales of the Parties for KA Oil, regardless of the product and geographic market definition.384
(539) The Commission concludes that the horizontal relationship leaves all effective competition unaffected.385
(540) The combined market share of the Parties based on merchant sales at EEA level in 2017 for KA Oil is below [0-5]%. The clear market leader is Versalis ([70-80]%), followed by Fibrant ([10-20]%) and Domo ([5-10]%). If a narrower market for only pure KA Oil for adipic acid production is considered, the combined position of the parties on the merchant market would also remain below [0-5]%, while Versalis would account for [90-100]% of such EEA market.
(541) In fact, the combined market shares of the Parties based on merchant sales of KA Oil have steadily been below [0-5]% in the EEA during the last 4 years (2014 -[0-5]%-, 2015 -[0-5]%- and 2016 -[0-5]%-). The market share would not be significantly different taking into consideration a narrower product market.
(542) The merchant market accounts for less than [5-10]% of the total production in the EEA and customers of KA Oil responding to the market investigation unanimously consider that the Transaction will not have any impact on the KA Oil market in the EEA.386
(543) Based on the assessment in recitals (534) to (542), the Commission therefore concludes that the Transaction will not significantly affect competition in the KA Oil market in the EEA, under any plausible product market definition and sub-segments thereof.
6.3.5. Level II of the polyamide value chain: HMD
(544) The Transaction leads to a horizontally affected market in relation to the production of HMD at EEA level. The vast majority of HMD sold in Europe is anhydrous HMD and the HMD market is EEA in scope. For the purpose of this Decision, the Commission will carry out the competitive assessment for an overall HMD market. Taking into consideration that the Parties are not active in less concentrated HMD and the very limited size of a potential market for less concentrated HMD, the Commission takes the view that the competitive assessment set out in Section 6.3.5.1 would apply to a potential market for anhydrous HMD, as the only difference would be a slight increment of the market share of the Parties.
6.3.5.1. Market structure
(545) The Parties’ combined market shares in the market for HMD in 2017 are:
(a) [30-40]% in value for merchant sales ([20-30]% Business and [0-5]% BASF). The only competitors are Invista with [60-70]% and Ascend with [5-10]%. However, it is worth to note that the Business sells HMD mainly outside the polyamide value chain;
(b) [50-60]% in volume for merchant sales plus captive use ([40-50]% Business and [10-20]% BASF); and,
(c) [60-70]% for production capacity ([50-60]% Business and [10-20]% BASF).
(546) Table 2 provides the Parties’ sales and captive use for 2017 (along with the corresponding market shares) in an EEA market for HMD. The shares of the Business387 and BASF388 in terms of merchant sales have remained stable during the last years.
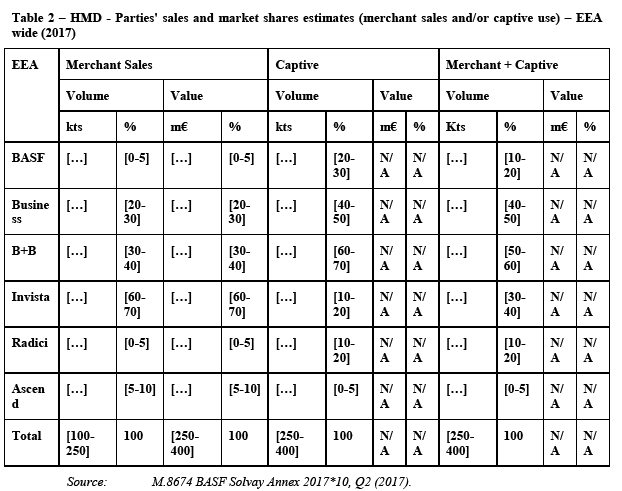
(547) Tables 3 and 4 differentiate between HMD sold to polyamide manufacturers and HMD sold to HDI producers.
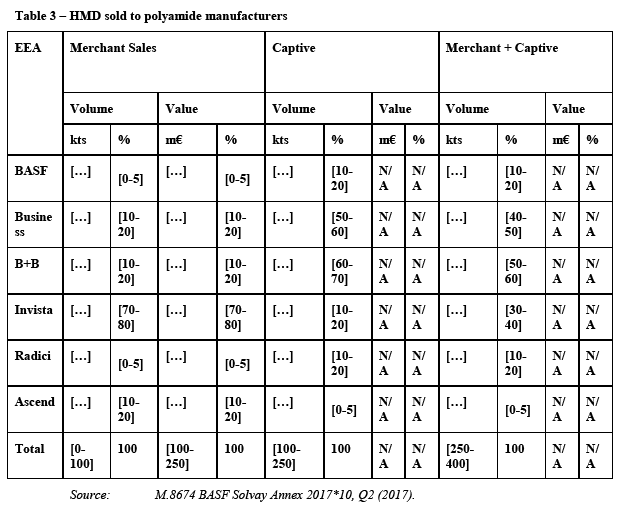
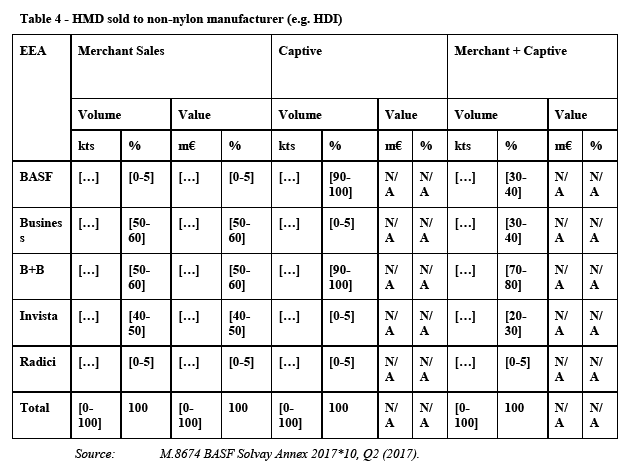
(548) Table 5 provides the Parties’ sales and captive use for 2017 at worldwide level for HMD.
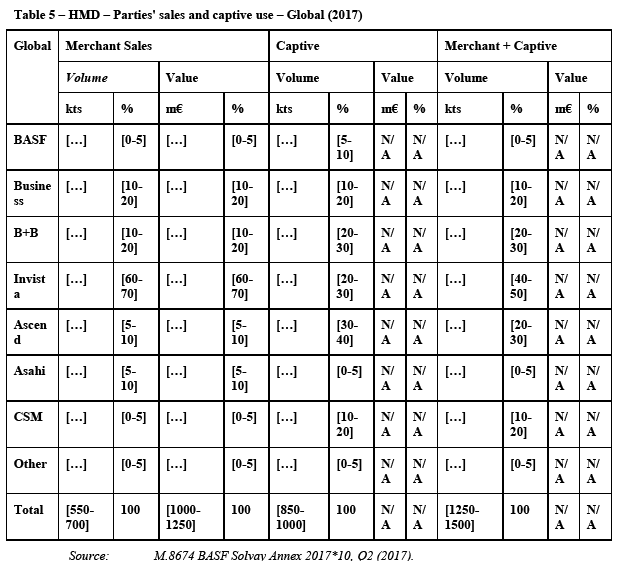
(549) An overview of the HMD capacity estimates and utilisation rate is provided in Table 6:
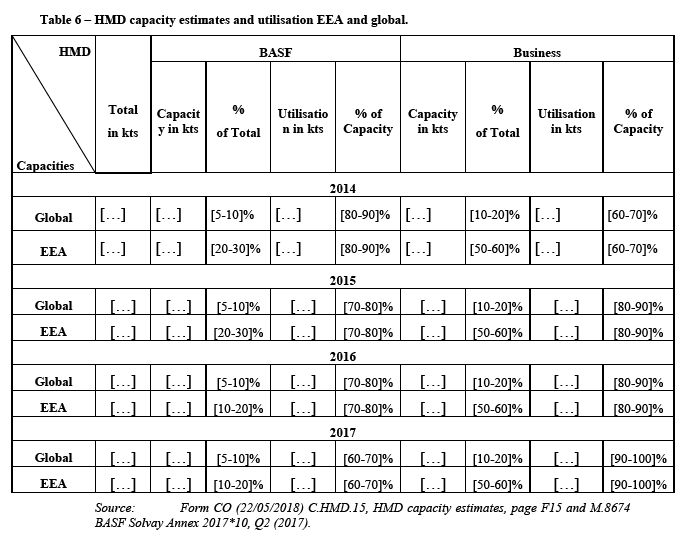
6.3.5.2. Competitive assessment: non-coordinated effects 6.3.5.2.(A) The Notifying Party's arguments
(550) The Notifying Party's position is that the Transaction would not bring any competitive harm, based on the arguments set out in the present section.
(551) First, the Notifying Party submits, as explained in Section 5.2.3, that the market for HMD is worldwide in scope, leading to low combined market shares. In a worldwide market, the combined market shares of the Parties would remain below [20-30]% (merchant market [10-20]% by value; captive sales plus merchant market [20-30]% by volume; and production capacity [10-20]%). Therefore, according to the Parties, the Merged Entity’s market share would remain moderate and it would not enjoy any market power at worldwide level.
(552) The Notifying Party also submits that substantial overcapacities of HMD exist globally, with a utilisation rate of approximately [70-80]%. Installed capacities of approximately […] kts in the USA face a demand of only approximately […] kts. In Asia a capacity of approximately […] kts faces a demand of only approximately […] kts. Such overcapacity represents a considerable constraint in terms of pricing. According to the Notifying Party, there are no technical or cost barriers to selling HMD worldwide and transporting it over long distances.
(553) Second, in the Response to the 6(1)(c) Decision, the Notifying Party submits that only sales on the merchant market for HMD are relevant for the assessment of the Proposed Transaction's horizontal effects in the HMD market and that the Commission’s approach to take into account capacity shares and captive use shares for its assessment in addition to merchant sales is incorrect as: (i) it is not in line with established case law; and (ii) it is not appropriate for the purposes of the assessment of the horizontal concerns at hand.
(554) According to the Notifying Party, captive production should be excluded from the assessment because it cannot be used to further withdraw volumes from the merchant market, since these volumes would not be diverted to the merchant market in response to a price increase or an output reduction for the product in question. Captive production volumes were unavailable to the merchant market pre- transaction, and will remain unavailable to the merchant market post-transaction. Therefore, as these volumes are not "in the market", they cannot form a competitive constraint on the market. To the contrary, the Notifying Party submits that captive volumes can be an element to dismiss doubts as to the compatibility of a transaction with the internal market where merchant market shares are high but the merchant market is small, as they were used in Outokumpu/Inoxum389.
(555) Third, the Notifying Party points out that market shares in the merchant market in the upstream products do not indicate concerns, either at EEA or worldwide level. Further, the Proposed Transaction will only give rise to an insignificant and de minimis increment of [0-5]%. The Notifying Party claims in the Response to the 6(1)(c) Decision that BASF’s sales on the merchant market have historically been minimal and opportunistic and such small volumes cannot pose a competitive constraint on competitors present in the market. Instead, the market structure post- transaction is virtually unchanged, with Invista continuing to be the dominant player in the EEA and BASF merely replacing the Business on the merchant market as the second competitor. Further, BASF could not materially expand their HMD production capacity or merchant market sales absent the Transaction because, similar to Radici, BASF lacks access to its own ADN and is dependent on ADN supplied by Invista.
(556) Fourth, in the Response to the 6(1)(c) Decision, the Notifying Party claims that Invista’s very high market shares on the ADN and the HMD merchant markets, combined with the limited spare capacity of both BASF and the Business, allows it to set prices and impose supply conditions without reacting to the actions of the Parties. The combination of a small sales base and a lack of ability to expand this sales base means that BASF cannot compete with Invista for more than a limited fraction of sales. It would be more profitable for Invista to ignore competition from BASF altogether in this scenario than to cut profitability of its entire sales base in order to chase the Parties’ modest sales.
(557) Fifth, the Notifying Party considers that the necessary investment […] would bring about substantial efficiencies.
(558) Finally, the Notifying Party submits that to overcome any HMD supply issues polyamide-producing customers that purchase HMD can also purchase at a different level of the supply chain, and in particular they can also purchase PA 6.6 BP.
6.3.5.2.(B) The Commission's assessment
(559) As explained in Section 5.2.3, the market investigation shows that the market should be considered EEA-wide and that importing from outside the EEA is not an alternative for most competitors due to higher prices derived from import tariffs, transportation costs, as well as financial risks or logistic and technical difficulties. Customers of HMD see imports as an option to overcome supply issues like force majeure events, but consider that imports as business model is not sustainable. For this reason, the Commission's assessment will be carried out on the basis of an EEA wide geographic market, where the role of imports is limited.
6.3.5.2.(B.i) Market is short and concentrated
(560) The vast majority of competitors consider that demand exceeds supply, i.e. the market is short.390 This view is also supported by 80% of customers responding to the market investigation.391 This problem will be accentuated in the near future, as competitors believe the market will continue growing in the coming years.392 Indeed, the vast majority of customers consider that HMD demand will grow vigorously (>2%) in the next 5 years.393
(561) […] explains that “the HMD market is already very concentrated and tight and is very difficult to get enough quantities. The HMD market is completely dependent upon the ADN market because there is no other route to produce HMD except through ADN. The situation is already getting worse and worse”.394 […] adds “HMD and ADN are key intermediates for PA66 production, and the availability of HMD and ADN is currently very tight”.395 In the same vein, […] explains that "availability of HMD is tight globally due to ADN constraints. The supply in EMEA is even tighter".396
(562) The strong combined position of the Merged Entity, that would control almost two thirds of the EEA production capacity, combined with the lack of alternatives and the tightness in the market, would give the Merged Entity the power to increase prices in the merchant market, as explained in Section 6.3.5.2.(B.ii).
6.3.5.2.(B.ii) Competitive landscape
(563) The number of competitors active in the HMD production is very limited. In addition to the Parties, only 2 companies are present in this market in the EEA in 2017: Invista and Ascend. Ascend did not sell any volumes in the merchant market in 2016.
(564) The Business' share in the merchant market is [20-30]% while it is [40-50]% for merchant sales plus captive use. While the Business has the largest captive use in the EEA compared to its competitors ([…] kts against […] kts for BASF, […] kts for Invista, […] kts for Radici and […] for Ascend), it also sells HMD in the market, mainly to non-polyamide manufacturers (i.e. HDI producers). The Business accounts for [10-20]% of the HMD sold to polyamide manufacturers in 2017 ([5-10]% in 2016).
(565) Table 7 provides the split in volume for Business’ HMD production used for AH Salt, HDI and other uses in 2016:
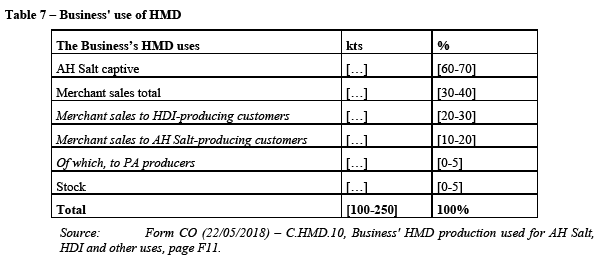
(566) The nameplate HMD production capacities of the Business are provided in Table 8:
(567) BASF accounts for [0-5]% of merchant sales and [10-20]% for merchant sales plus captive use in the EEA. BASF uses the […] of its HMD production entirely for the production of […]. BASF also uses part of its HMD to produce […]. BASF only makes […].
(568) Table 9 provides the split in volume for BASF’s HMD production used for AH Salt, HDI and other uses in 2016:
(569) The nameplate HMD production capacities of BASF are provided in Table 10:

(570) The Notifying Party submits that BASF has […].
(571) The Notifying Party also submits that BASF is not an active player on the merchant market. BASF uses the majority of the HMD for the production of PA 6.6. The Notifying Party submits that as a consequence of the Transaction, BASF would be replacing the Business as a supplier on the merchant market.
(572) According to the Notifying Party, Invista is the clear leader in the merchant market with [60-70]% market share while it accounts for [30-40]% for merchant sales plus captive use in the EEA. Importantly, Invista's sales in 2017 account for [70-80]% ([90-100]% in 2016) of the HMD sold to polyamide manufacturers. This is explained by the fact that Invista is not active in the PA 6.6 EP downstream market and its captive consumption is smaller than this of their competitors. Invista's strategy is to sell ADN, HMD and PA 6.6 BP in the market, but it is not vertically integrated into PA 6.6 EP.
(573) Radici's share in the merchant market in 2016 was [0-5]% while it has not sold HMD into the market in 2017. It accounts for [10-20]% of the total production in the EEA. Radici uses almost its entire production captively to produce AH Salt and further transform it into PA 6.6 BP and PA 6.6 EP.
(574) Ascend's share in the merchant market is [10-20]% in 2017 while according to the data submitted by the Notifying Party it did not sell any volumes on 2016 in the EEA. These volumes were imported, as Ascend does not produce in the EEA.
6.3.5.2.(B.iii) The Parties’ position in the market.
(575) Combined market shares of the Parties range from significant to very high depending on the measure chosen ([30-40]% in value for merchant sales; [50-60]% volume for merchant sales plus captive use; and [60-70]% for production capacity). The Merged Entity would control approximately two thirds of the HMD production capacity in the EEA. These measures of market shares are a first clear signal of market power of the Merged Entity. This is supported by market participants that claim that the Merged Entity would have a dominant position in HMD and that the Transaction would reduce choice. Indeed, all customers responding to this question consider that BASF and Solvay are among the top 5 producers of HMD in the world and the majority consider that they are the top 2 producers of HMD in the EEA.397
(576) The Notifying Party points out that market shares in the merchant market do not indicate concerns, either at EEA or worldwide level, that the increment brought by the Transaction is small and that capacity or total production shares should not be considered for the competitive assessment.
(577) First, as explained in Section 6.3.2.2, the market is very tight and concentrated even pre-transaction, with a market leader in the merchant market (Invista) that accounts for [60-70]% ([70-80]% in 2016) of the sales in the market. Under these circumstances, other competitors necessarily have moderate market shares in the merchant market and even a competitor accounting for a small share of the merchant sales represents a relevant economic constraint in a market that is already very uncompetitive.
(578) Second, as explained in Section 6.2, the Commission considers that captive use and capacity shares are also relevant metrics to assess the market power of the Merged Entity. The high shares in terms of merchant sales plus captive use and capacity shares clearly indicate market power of the Merged Entity which is in fact supported by the views of the large majority of the respondents to the market investigation.
(579) Indeed, capacity shares and merchant sales plus captive use are important for the horizontal assessment. Controlling the majority of the production of HMD would allow the Merged Entity to exert a substantial degree of control on the market in this particular case, given the absence of alternatives. Were the Merged Entity to increase the prices, reduce supply or supply in less favourable terms, customers would not have any other option than to accept these conditions given the structure of the market, as the only alternative to the Merged Entity would be Invista. Further, the market investigation shows that there are no companies in the EEA which could be active in the merchant market on a lasting basis.398 In particular, […] explains that "[…] is short HMD in the EEA and has to supplement its EEA production capacity using swap arrangements with other HMD manufacturers and by importing extra HMD from its HMD plants in Texas, United States. To expand capacity, a new HMD facility would need to be constructed. This would take around 3 years and cost approximately [[…] Internal Cost Estimation]. Butachimie’s HMD plant (50% […]- owned) could not be significantly expanded because it has previously been expanded".399
(580) […] explains that it “does not sell HMD into the merchant market because Invista is the main supplier of HMD. Should […] start competing in the HMD market, Invista could increase prices or stop selling ADN to […]”.400
(581) On the other hand, […] submits that it could serve some additional volumes in the market by importing HMD from the USA.401 However, as explained in Section 5.2.3, customers do not consider imports from outside the EEA as a viable alternative.
(582) Furthermore, contrary to the Notifying Party claim that the Parties do not constrain Invista, customers fear that removing the Business from the market would significantly deteriorate their negotiating position vis-à-vis Invista.
(583) For example, […], an important customer claims that “BASF has a very strong internal demand of HMD for its engineering plastic business and they will prioritize internal requirements. It is […] assessment that unless the combined BASF/Solvay entity can maintain the existing level of merchant ADN and HMD quantities, these markets will be dominated by Invista”.402
(584) […] states that “HMD is a very concentrated market even before the transaction. There are currently three suppliers of HMD in Europe: BASF, Solvay and Invista. […] If, post-transaction, BASF decides not to supply HMD into the merchant market, […] prices will increase and […] ability to compete in the market will be significantly reduced. The ability to buy inputs at competitive prices depends on the existence of competition upstream […]. Invista has no available capacity to increase its supply of HMD in the merchant market. […] Choice will be reduced and the market very stretched”.403
(585) […] “anticipates a price increase for HMD since the two currently main competing suppliers would merge. There will be less choice and prices will increase […] If HMD was facing a price increase, between 10 and +20%, […] would try to pass on the increase to downstream market but this will not work for all markets/accounts as there are contracts in place and buyers are expected to resist”.404 […] believes that “Invista has no available capacity to increase its supply of HMD in the merchant market”.405 Yet […] adds “BASF will have an absolutely dominant position in Europe in the production of HMD […]”.406 […] is also of the opinion that "the post- merger entity will have a dominant position on the ADN, HMD and AH Salt markets".407
(586) It transpires from the market investigation, that the vast majority of customers consider that the Business and BASF constrains the prices of Invista. Post- transaction, therefore one option would disappear and the vast majority of customers fear that the Merged Entity could unilaterally increase prices for HMD in the merchant market, given that the only alternative for customers would be capacity constrained and that the market is tight (see Section 6.3.2). Indeed, given the strong market power of the Merged Entity that would control [50-60]% of the merchant sales plus captive use and [60-70]% of the capacity in the EEA, it could decide to increase prices for HMD in the merchant market.
(587) Internal documents of BASF […]408-[…]409 […]410 […]411 […]412. […]413 […]414
(588) In this vein the vast majority of the respondents to the market investigation consider that if the Transaction goes ahead they would not have a sufficient choice of suppliers of HMD in the area they operate in.415 Actually, the vast majority of customers responding to the market investigation consider that the acquisition by BASF of the Business has a negative impact on the market for HMD in the EEA.416 For example, […] considers that “the new entity will have a dominant position in EEA”417 and […] adds that the “majority of HMD sources in Europe will be under a control of one company”.418
(589) Finally, it should be noted that, contrary to the Notifying Party's claim that BASF will replace the Business as supplier to the market and that therefore the Transaction would not bring any change to the market structure, the fact that the Merged Entity will have a very strong position also in downstream markets (AH Salt, PA 6.6 BP and PA 6.6 EP) with very significant increments in terms of shares, changes completely the incentives of the Merged Entity to sell HMD into the market at a certain price. Indeed, the Merged Entity may decide to increase prices for two reasons: first, because it will have a stronger combined position as a result of the Transaction in a market that is short and without competitors with spare capacity to expand their sales and second, because even if it lost some sales, it may expect to recover the profits downstream where the Merged Entity will be the clear market leader in all markets (AH Salt, PA 6.6 BP and PA 6.6 EP).
6.3.5.2.(B.iv) Barriers to entry
(590) The market investigation has shown that barriers to entry are very high and entry is very unlikely in this market and has also clearly indicated that access to input (ADN) is the key in order to produce HMD. One of the factors making entry into this market very difficult is that ADN is controlled by Butachimie - the joint venture between Invista (50%) and the Business (50%) - and therefore any potential competitors wishing to produce HMD post-transaction would have to rely on BASF or Invista, which would be at the same time competitors in the HMD or downstream markets. Competitors also mention that the high level of investment required to set up a production facility, intellectual property rights and the required know-how together with lack of expertise in the production of HMD are important barriers to entry in
this market.419 Customers also indicate that the main barriers to entry are access to raw material (ADN), lack of expertise, the level of investment required to set up a production plan and intellectual property right and know-how.420 For example, […] explains that "HMD production requires specific know-how in terms of hydrogenation (catalyst), purification and separation specific to HMD production and depending also on ADN routes”.421 No new entrants have been observed in the EEA in the last 5 years.422
6.3.5.2.(B.v) Multi-sourcing
(591) Multi-sourcing appears important for customers for security of supply reasons, but difficult in practice due to the lack of options in the market. “Multi sourcing is key to secure supply but sourcing options are limited, in EEA, to 2 suppliers sharing same ADN production site. Given that transportation is a source of a non-negligent increase of sourcing costs and potential risk to the product integrity, the possibilities for multi-sourcing outside of EEA remain by definition limited in practice”.423
6.3.5.2.(B.vi) […]
(592) […]
(593) […]
(594) […].424 […].425 […] However, it is clear that the Merged Entity will enjoy a high degree of flexibility post-transaction […] and therefore it could decide capacity of the market and use this strategically.
(595) […]
6.3.5.2.(B.vii) Switching to PA 6.6 BP or PA.6 BP if prices for HMD increase
(596) The Notifying Party submits that to overcome any HMD supply issues polyamide- producing customers that purchase HMD can also purchase at a different level of the supply chain, and in particular they can also purchase PA 6.6 BP.
(597) According to the Notifying Party, for companies using PA 6.6 BP as an input, the difference in cost between (a) sourcing HMD and adipic acid, and processing HMD and adipic acid into PA 6.6 BP; and (b) sourcing an equivalent volume of PA 6.6 BP is minor. The Notifying Party claims that the total cost of option (a) is less than 5% lower than the cost of option (b), or the sourcing an equivalent volume of PA 6.6 BP.
(598) Contrary to the Notifying Party’s claim, the Commission considers that this option is not a viable alternative. First, as explained in Section 6.3.5, the Merged Entity would enjoy market power at the level of the PA 6.6 BP (level IV) and therefore could also increase prices at this level of the polyamide value chain. Second, if HMD prices were to rise, PA 6.6 BP competitors sourcing HMD in the merchant market would be less competitive, accentuating the market power of the Merged Entity at the PA 6.6 BP level. Third, AH Salt producers (level III) sourcing HMD in order to produce co- polyamide do not have an alternative to source the base polymer and the market share of the Merged Entity in the merchant market for AH Salt is above [90-100]%. Finally, HDI producers cannot source products other than HMD for their production of HDI derivatives for coatings and, therefore, this argument would not apply to them.
(599) Additionally, the Notifying Party claims that the Merged Entity would also face the indirect competitive constraint posed by PA 6 BP. Should its customers manufacturing PA 6.6 BP be exposed to a price increase, they would switch to a large extent to manufacturing PA 6 BP (for which HMD is not an input), substantially reducing their demand of HMD.
(600) However, as described in Section 5.5.2, the market investigation has shown that PA 6.6 BP is different and standalone product market.
6.3.5.2.(C) Conclusion on non-coordinated effects: HMD
(601) Based on the assessment in recitals (544) to (600), as a result of the high combined market shares of the Merged Entity, the reduced number of competitors, the reduction of choice and the lack of alternatives for customers in a very concentrated market with high barriers to entry and in which demand exceeds supply, the Commission concludes that the Transaction would significantly impede effective competition as a result of horizontal non-coordinated effects arising in the supply of HMD in the EEA.
(602) The horizontal non-coordinated effects would be reinforced by the strong position of the Merged Entity in the downstream markets because, even if the Merged Entity were to lose some sales in the merchant market, its stronger combined position in all levels downstream to the polyamide value chain would make it more likely that it would increase substantially its benefits downstream as further detailed in Section 6.7.4.
6.3.6. Level II of the polyamide value chain: adipic acid
(603) The Transaction leads to a horizontally affected market in relation to the production of adipic acid at EEA level.
6.3.6.1. Market structure
(604) In the market for adipic acid, the combined 2016 market shares of the Parties are as follow:
(a) [50-60]% in volume for merchant sales ([30-40]% Business and [10-20]% BASF). The other EEA competitors are Radici ([20-30]%) and Lanxess ([10- 20]%);
(b) [60-70]% in volume for total demand (merchant sales + captive use: [40-50]% Business and [20-30]% BASF); and,
(c) [60-70]% for production capacity ([40-50]% Business and [20-30]% BASF). (605) Table 11 provides the Parties’ market share estimates for 2016 (merchant sales
and/or captive use) on an EEA market.
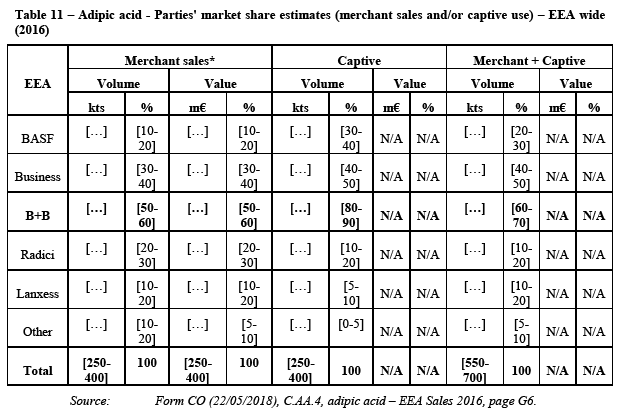
(606) Over the course of the in-depth investigation, the Parties submitted 2017 market share estimates, which differ materially from the 2016 ones, as apparent from Table 12. The difference lies primarily with The Business' merchant sales, which would have dropped by ten percentage point in 2017 due to an overall reduction in production. However, the combined market shares of the Parties still represented [40- 50]% of merchant sales, [60-70]% of total EEA demand in 2017 (merchant + captive) and [60-70]% in terms of production capacity.
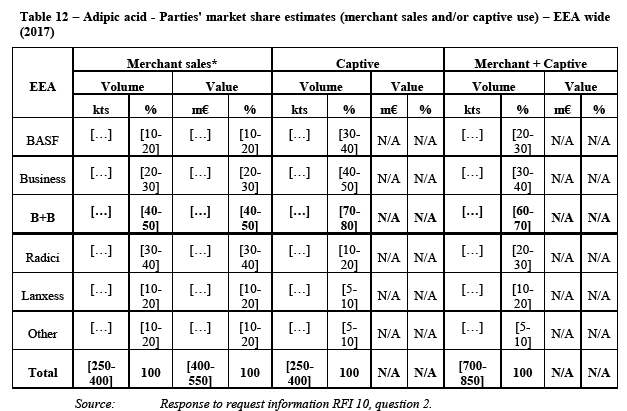
(607) An overview of the adipic acid capacity estimates and utilisation rate is provided in Table 13:
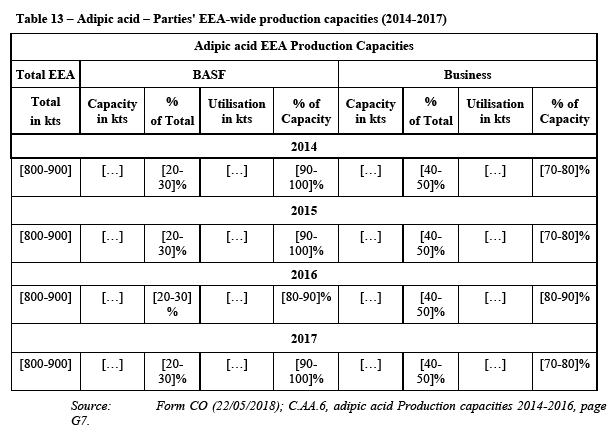
(608) The Parties’ EEA production sites have the following individual capacities (2014- 2016):
(a) BASF: Ludwigshafen (Germany): […] kts.
(b) Business: Chalampé (France): […] kts.426
6.3.6.2. Competitive assessment: non-coordinated effects 6.3.6.2.(A) The Notifying Party's arguments
(609) The Notifying Party considers that the Transaction would not lead to any significant impediment to effective competition as a result of horizontal non-coordinated effects in relation to the supply of adipic acid.
(610) First, according to the Notifying Party, the market for adipic acid is worldwide in scope, leading to combined market shares below [20-30]%.
(611) Second, adipic acid is a commodity product and price competition is strong. As a result, the Merged Entity would not have market power and would be unable to increase its prices to remain competitive.
(612) Third, imports from China exercise a significant constraint on market price levels in the EEA, notably given the significant cost advantage of Chinese suppliers. Hence, any attempt to increase prices would be at the expense of the Merged Entity.
(613) Fourth, competitors, in particular Chinese suppliers, would be able to absorb the switching demand resulting from any price increase due to significant available capacities. Moreover, EEA producers that use most of their production captively could increase their supply substantially if prices were to increase, and both Radici and Lanxess have been successful in winning volumes from the Parties in recent years.
(614) Fifth, financial and technical barriers to entry are low and know-how is available. Only standard government authorisations regarding the production of chemical products are required. Given the overcapacity and low barriers to entry, irrespective of its current market share, the Merged Entity would not enjoy market power even at EEA level.
(615) Finally, large customers represent a significant share of demand and exercise countervailing buyer power, notably by threatening to switch to Chinese suppliers.
(616) As a result, the Merged Entity would have no ability or incentive to reduce its outputs and/or increase its prices post-transaction.
6.3.6.2.(B) The Commission' assessment
(617) In Section 5.3, the Commission concludes that the market for adipic acid is EEA- wide in scope. In support of that conclusion, the Commission discusses the limitations to the ability and incentives of Chinese supplies to exercise effective constraints on the Merged Entity and, as a corollary, the different conditions of competition prevailing in the EEA. Hence, the following assessment builds on and cross-refers to the market definition analysis, as appropriate.
6.3.6.2.(B.i) The market is concentrated and imports are moderate
(618) According to the Notifying Party, adipic acid is used for PA and non-PA applications with a […] split between those two segments. Around [40-60]% of the adipic acid production is used by manufacturers of high-end polyurethane producers, special long chain PA producers and plasticisers, while around [40-60]% is used by manufacturers that will combine adipic acid with HMD to produce AH Salt with a view to its polymerisation into PA 6.6 BP. The Parties sell adipic acid to both polyurethane producers and PA 6.6 producers.
(619) The number of domestic suppliers active in the production of adipic acid in the EEA is very limited. Thus, in addition to the Parties, only 2 companies are present in this market in the EEA: Lanxess and Radici.
(620) The Business' 2017 share of the merchant market is [20-30]%, down from [30-40]% in 2016, while it amounts to [30-40]% of total demand, down from [40-50]% in 2016. While the Business has the largest captive use in the EEA if compared to its competitors ([…] kts against […] kts for BASF, […] kts for Radici and […] kts for Lanxess), it is also the market leader in the merchant market for adipic acid. In effect, the Business produces adipic acid for captive use (for approximately [60- 70]%) and for the merchant market (approximately [40-50]%).
(621) The Business produces adipic acid out of Chalampé, France. The nameplate production capacities for adipic acid for the Business are provided in Table 14:

(622) BASF' accounts for [10-20]% of the merchant market and [20-30]% of the total demand in the EEA. BASF is using the largest part […] of its adipic acid production captively for the production of AH Salt and other products outside the polyamide value chain in which BASF is active. BASF therefore gives priority to its internal consumption over sales to the market.
(623) BASF produces adipic acid out of Ludwigshafen, Germanyand its nameplate capacities for the production of adipic acid are provided in Table 15:

(624) Radici is the third supplier in the EEA with a share of [20-30]% of total demand and [30-40]% of the merchant market, up from [20-30]% in 2016 according to the Notifying Party.
(625) Lanxess is also active in the merchant market with a share of [10-20]%, while it accounts for [10-20]% of the total production in the EEA. Lanxess has a lower internal demand of adipic acid and therefore is more market oriented.
(626) As noted in Section 5.3.3, imports of adipic acid into the EEA have historically been moderate with an average volume of […] kts/year over the 2011-2017 period (or [0- 10]% of total 2017 demand). Imports were historically low in 2016, with only […] kts representing [0-10]% of total demand, and historically high in 2017, reaching […] kts representing [10-20]% of total demand.
6.3.6.2.(B.ii) The Parties’ have a strong market position and the market investigation has elicited concerns
(627) Combined market shares of the Parties are very high at EEA level ([40-50]% of merchant sales, down from [50-60]% in 2016; [60-70]% of total demand, down from [60-70]% in 2017; and [60-70]% for production capacity with [40-50]% for the Business and [20-30]% for BASF). Therefore, the Merged Entity would control approximately two thirds of the adipic acid production in the EEA. This market share may in itself be evidence of the existence of a dominant market position.427 Moreover, the market investigation has revealed that BASF and the Business are consistently considered the top 2 suppliers at EEA level.428
(628) A very important number of customers consider that the Transaction would lead to a significant lessening of competition on the EEA adipic acid market.429 For example, […] believes that there would be “limited choice of European suppliers”430 and […] fears that the Merged Entity “might impact prices and choice options, as only three manufacturers will remain in Europe”.431 In the same vein, […] believes that “in the future […] will have less choice to negotiate its contracts [...]. In Europe, amongst four suppliers, two are merging. The merged entity will have 50% of the market share, and that will have an impact on the price”.432
(629) Overall, large customers seem more concerned about the Transaction than smaller customers because large customers find fewer options in the market that can serve the volumes they require. For example, […] explains that “the concentration will merge the two main suppliers in Europe. There are only other 2 suppliers because the rest are very small and unable to serve big customers, and prices […] may rise further, making production of downstream products in Europe less competitive”.433
Another large customer, […], states that “post-transaction, BASF will also dominate the AA market, with a market share post-transaction of approx. 65%. Solvay and BASF are the only two companies with capacity to meet […] needs of AA”.434
6.3.6.2.(B.iii) Market is tight
(630) While producers consider, on average, that adipic acid production is balanced in the EEA,435 an important number of customers indicate the market has become short due to recent plant closures and force majeure declarations.436
(631) For example, an important customer, […], explains that “supply and demand is unbalanced in Europe. Invista closed its plant in the UK (Wilton) and then in the US (Orange/TX, 220KT in 2015). To be able to produce their PA6.6 in Rosenberg, NL, Invista did a contract with Solvay to get AA. There is no new capacity in Europe. New capacity is set in China but with big limitations due to quality and safety. Producing AA is not delivering profit. AA is mainly consumed in PA6.6 productions. Therefore producers keep AA for their own captive use and prefer to sell Polyamide than intermediates like AA. BASF has stopped selling AA on the market (mostly captive use). On top the AA from BASF is yellow AA (standard is white). Consequently they are regularly buying big volumes from co-producers like Solvay and Radici. BASF has 4 production lines. Only 3 lines are running, one was stopped 4 years ago and never re-started. Solvay has no extra volume to offer. […] could not get more volume. Radici is more and more approached by the market but sold out. Lanxess is the smallest producer and they have no volume to offer. Market price for AA has increased substantially since 2 yrs. European producers are in regular shutdowns for maintenance causing problems of supply”.437 […] adds that “all European producers are already sold out and demand for AA is increasing drastically. They have preference for small and medium customers rather than big ones”.438
(632) Along the same line, the 2017 PCI Yellowbook, the most important reference in the industry, reads as follow: “the outlook for adipic acid in Europe has changed considerably in recent years. After years of over-capacity and asset closures, the market is now moving quickly back towards balance and as anticipated this balance condition will endure […].The YB17 scenario for adipic acid in Europe therefore, has Solvay/Rhodia re-opening its mothballed capacity in 2015 and Lanxess expanding over 2017-2018 […]. If these assets are not returned to service, Europe shall quickly move into deficit”.439
(633) The availability of adipic acid in the EEA may become even more problematic in the short term, as customers unanimously believe that the adipic acid market will grow in the next 5 years, either moderately or significantly (>2%).440 Moreover, an internal document of BASF shows that […], thus further stretching the availability of adipic acid in the merchant market: “[…]”.441
6.3.6.2.(B.iv) Spare capacities are limited and customers have to multi-source
(634) The Notifying Party submits that adipic acid is a commodity product, that price is the main competition driver and that competitors could increase supply to the merchant market significantly if prices were to increase. As a result, the Merged Entity would not have market power and would be unable to remain competitive if it were to increase prices.
(635) However, while the market is tight or at least balanced, spare capacities are scarce in the EEA, whereas customers have to multi-source for security of supply reasons. Therefore, if the Merged Entity were to increase prices, customers would be unlikely to be able to find sufficient alternative sources of supply for adipic acid in the EEA.
(636) Indeed, only two competitors have indicated that they have spare capacity to increase their production and serve additional volumes into the merchant market in the EEA.442 […] submits that it could serve an additional 10 kts per year,443 while […] mentioned around 50 kts per year.444 However, […] is not based in the EEA and, therefore, all the disadvantages that customers face when importing adipic acid into the EEA would apply to those extra volumes. Moreover, the size of the merchant market in the EEA in 2017 was 359 kts; […]'s 10 kts of additional capacity would represent less than 3% thereof and is insufficient to absorb any significant diversion of supply post-concentration.
(637) In addition, customers fear that the Merged Entity might shut down capacity further, thus worsening sourcing conditions for adipic acid in the EEA. For example, […] explains “if BASF shuts down capacity as a result of the Transaction, it will impact very negatively the market… Radici and Lanxess will not be able to cover the market needs”.445 That concern is supported […].446
(638) Moreover, customers consider that multi-sourcing is important, mainly for security of supply reasons.447 For example, […] explains that “there are four suppliers of Adipic Acid (AA) in the European market… For security reasons, they source from all the four of the suppliers”.448 Other customers have explained that they never source more than 30% of their total requirements from one single source due to the risk of supply failures impacting their own production. […] also reports that "[c]ustomers usually have two to four supply sources in general in addition to some volumes that they get on spot base".449 Hence, it is unlikely that customers would be able to circumvent the Merged Entity in case of price increase.
6.3.6.2.(B.v) High barriers to entry and low incentives to invest
(639) The Notifying Party claims that barriers to entry in the adipic acid market are low. In contrast, competitors responding to the market investigation have pointed to the existence of significant barriers to entry, starting with the high level of investment required to set a production facility.450 One of them also explains that, to be economical, a high volume of output is required and this implies absorbing a relevant portion of the production captively.451 Know-how and lack of expertise are also mentioned as barriers to entry.452
(640) The vast majority of customers also consider that the level of investment required to set up a production facility is a barrier to enter the adipic acid market. For example, […] thinks that “Adipic acid is a low margin product which probably doesn't justify new investments in North-Western Europe”.453 Other barriers mentioned by customers include the lack of expertise or regulatory requirements.454
(641) As a result, respondents to the market investigation have indicated that no domestic entry has occurred over the last 5 years in the EEA adipic acid market.455 Conversely, competitors have consistently mentioned that Chinese producers have entered the EEA market over the past years.456 However, these entries are limited to imports and Chinese suppliers' commercial activity in the EEA is undertaken via local independent distributors.457 Moreover, the ability (and incentive) of Chinese suppliers to exercise competitive constraints on the Merged Entity are limited, as explained in Section 5.3.3.
6.3.6.2.(B.ii) Limited constraints arising from imports
(642) The market investigation in Phase II revealed the significant and lasting limitations to the exercise of competitive constraints over EEA adipic acid production by imports, and Chinese imports in particular, as purported by the Notifying Party. These limitations are physical and technical but also of a financial and commercial nature, as discussed in Section 5.3.3. Moreover, the level of imports fluctuates and remains limited overall, ranging between [0-10]% (in 2016) and approx. [10-20]% (in 2017) of total EEA adipic acid demand.
(643) As such, the outcome of the market investigation is consistent with the assessment of the 2017 PCI Yellowbook, the most important reference in the industry, according to which: “Although Europe already imports some adipic acid from China, these volumes are limited and only used as incremental supply/pricing leverage. Transitioning large amounts of European adipic demand to Chinese materials will present logistical, quality, legal and financial hurdles which are not trivial”.458
(644) In terms of physical limitations, the market investigation has pointed to remaining quality issues with Chinese imports. In short, quality is inconsistent, insufficient for certain applications and/or requires repacking and sometimes reprocessing to satisfy the requirements of EEA customers. A particular concern relates to the solidification of adipic acid when shipped over long distances ("caking"), which make it inoperable without further processing. The lack of bulk supplies is also a significant limitation for those large customers operating silo storage tanks from which the material goes directly into production.
(645) In turn, longer delivery period generate financial risks and working capital requirements due to the volatility of Chinese adipic acid prices and the need to adjust stocks and storage facilities. Moreover, contrary to the Notifying Party's contentions, the existence of a significant price differential between China and the EEA, allegedly capable of mitigating these risks, is not supported by the outcome of the market investigation. To the contrary, the market investigation has revealed that the prices of adipic acid delivered by Chinese producers are now similar to – or even less attractive than – prices available from EEA producers. The reason lies in the necessary improvement of production processes to address the stricter enforcement of Chinese environmental standards and achiever higher quality requirements.
(646) Tighter enforcement of Chinese environmental standards under the so-called "Blue Sky" policy has also resulted in supply disruptions, including due to plant closures, which appear to have profoundly affected the reliability of Chinese supplies in the eyes of EEA customers, thus limiting the attractiveness thereof due to security of supply requirements. Likewise, the seasonality of adipic acid supplies from China due to environmental issues appears to have become a known fact to EEA distributors, which mandates specific adjustments due to the irregular availability of the material.
(647) Finally, the market investigation has indicated that Chinese imports remain opportunistic in nature, in addition to being still limited. Thus, Chinese suppliers do not have an EEA-based organisation and do not offer logistical services on their own but rather channel volumes mainly through independent distributors. Hence, they are regarded by some respondents as uninterested in long-term industrial partnerships but as mainly attracted by short-term gains when faced with excess production. Moreover, sophisticated suppliers engaged in regular deliveries with the assistance of domestic distributors, like Huafon with […], appear to favour a price-follower strategy in serving residual demand requirements. Combined with a business culture that is not yet fully aligned with the needs of the EEA market, this positioning testifies of limited incentives to constraint EEA production.
(648) On balance, based on the results of the market investigation, the Commission therefore finds that the competitive constraint exercised by Chinese imports over EEA adipic acid production is limited and insufficient to outweigh the risk of a significant impediment to effective competition arising from the Transaction. In closing, the Notifying Party has not explained how the existence of overcapacities in China would concretely alleviate the limitations revealed by the market investigation in terms of ability and incentives of Chinese suppliers to constraint the Merged Entity in the future.
6.3.6.2.(C) Conclusion on non-coordinated effects: adipic acid
(649) Based on the assessment in recitals (603) to (648), the Commission concludes that the Transaction would significantly impede effective competition as a result of horizontal non-coordinated effects arising in the supply of adipic acid in the EEA.
6.3.7. Level III of the polyamide value chain: AH Salt
(650) The Transaction leads to a horizontally affected market in relation to the production of AH Salt at EEA level. As noted, only liquid AH Salt is sold in Europe459 and the AH Salt market is EEA in scope. The Commission has therefore carried out its competitive assessment on an overall AH Salt market at EEA level.
6.3.7.1. Market structure
(651) On this market, the combined market shares of the parties are:
(a) [90-100]% in volume for merchant sales ([80-90]% Business and [5-10]% BASF);
(b) [50-60]% in volume in volume for merchant sales plus captive use ([40-50]% Business and [10-20]% BASF); and,
(c) [60-70]% for production capacity ([40-50]% Business and [10-20]% BASF). (652) Table 16 provides the Parties’ 2017 sales (along with the corresponding market
shares) of AH Salt at EEA level.
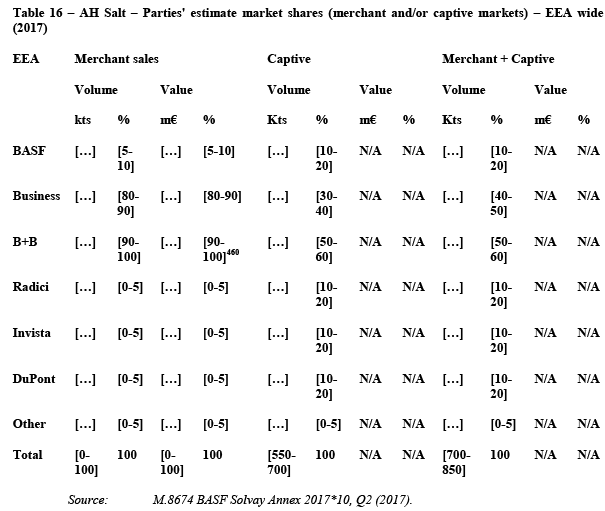
(653) The merchant sales of the Parties at worldwide level would be lower, although still very high. Table 17 provides the Parties’ sales for 2016 at worldwide level for AH Salt.
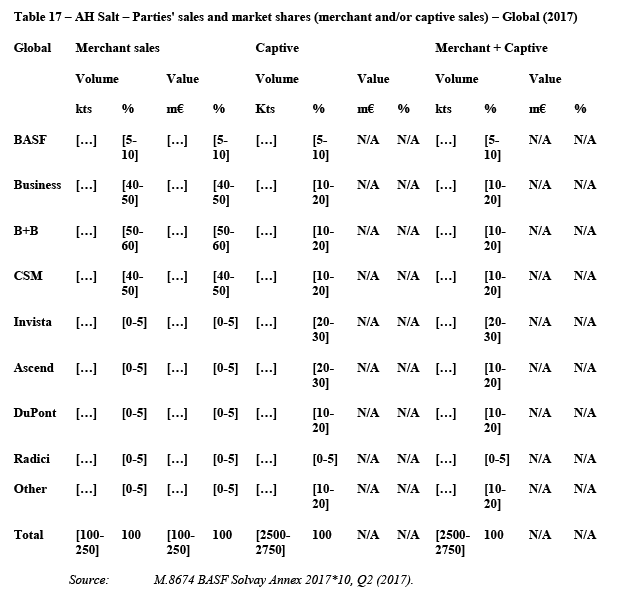
(654) An overview of the AH Salt capacity estimates and utilisation rate is provided in Table 18:
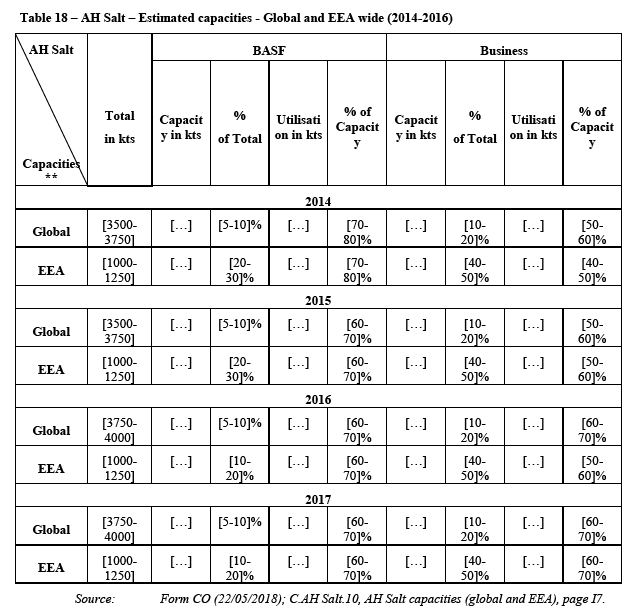
(655) The Parties’ production sites have the following individual capacities
(a) BASF:
(1) Ludwigshafen (Germany): […] kts
(b) Business:
(1) Chalampé (France): […] kts,
(2) Belle Etoile (France): […] kts,
(3) Blanes (Spain): […] kts,
(4) Onsan (South Korea): […] kts.
6.3.7.2. Competitive assessment: non-coordinated effects 6.3.7.2.(A) The Notifying Party's arguments
(656) The Notifying Party's position is that the Transaction would not bring any competitive harm.
(657) First, the Notifying Party claims that the market for AH Salt is worldwide in scope and on the global market the Merged Entity’s market share are moderate. Therefore, the Merged Entity would not enjoy any market power. Moreover, the Notifying Party claims that irrespective of the geographic market definition, the Merged Entity would face significant competition from non-EEA producers through imports in the EEA.
(658) Further, in the Response to the 6(1)(c) Decision the Notifying Party submits that Ascend and CSM as reliable global producers of AH Salt. Although Ascend and CSM do not have AH Salt production capacity in the EEA, they both have significant excess AH Salt capacity on a worldwide basis. According to the Notifying Party, Ascend and CSM each would have the means and resources to enter the EEA market and supply the needs of third-party AH Salt customers in the EEA.
(659) Second, the Notifying Party is of the view that the Merged Entity would face significant competition from EEA producers that keep their production for captive use, and that could trade in the merchant market if prices increased. In the Response to the 6(1)(c) Decision, the Notifying Party further claims that Radici is active in the merchant market and can easily become a credible alternative supplier to those customers that are concerned about the elimination of a ‘second supply’ source or multi-sourcing.
(660) Further, the Notifying Party claims that overcapacities exist in the merchant market and considers that any base polymer producer could easily start supplying AH Salt to third parties and could do so in a timely manner. Likewise, current producers could easily expand their respective capacities in a timely manner. According to the Notifying Party, the existing unused capacity in the EEA exceeds the total level of merchant sales ([…] kts in the EEA in 2016).
(661) In this vein, the Notifying Party submits that the combined total production capacity available to Invista, DowDuPont and Radici is […] kts of which they used only [70- 80]% or […] kts in 2016. Hence, the excess capacity available to third parties on the EEA market for AH Salt was […] kts in 2016, while the size of the merchant market in 2016 was […] kts. Therefore, even if the Merged Entity were to withdraw from the merchant market, the remaining AH Salt producers would be able to supply the entire merchant market on the basis of their excess capacity alone without having to divert volumes used for captive sales. Although these suppliers may not be active in the merchant market simply because of its small- sized nature and the fact that the market is well-served, nothing prevents them from entering at any point in time. In case of an attempted price increase, each of Invista, DowDuPont and Radici would have the ability and incentive to step in.
(662) Third, according to the Notifying Party, barriers to entry are low and most base polymer producers (including PA 6.6 BP and Co-Polyamide 6/6.6 and 6.6/6 BP) produce AH Salt internally or would be able to do so in case of price increase. According to the Notifying Party, AH Salt is easily obtained by mixing HMD with adipic acid in an aqueous reaction, no specific knowhow is required and only relatively simple assets are required. The total costs required to install an AH Salt mixing facility are moderate and it also takes a moderate time to build a salinification unit. Finally, the Notifying Party claims that the raw materials (adipic acid and HMD) are readily available and only need to be mixed to produce AH Salt. In the Response to the 6(1)(c) Decision the Notifying Party submits that the Merged Entity’s activities will also be constrained by the likelihood of new entry in case of attempted price increases because barriers to entry for the production of AH Salt are significantly lower than for other levels of the polyamide value chain as the cost and time investments to build an AH Salt mixing asset are moderate (less than EUR […] million / approx. […] months) and AH Salt production does not require specific know how, sophisticated production facilities, IP rights or a particular expertise.
(663) The Notifying Party further submits that as regards the access to the key inputs of adipic acid and HMD, which was identified by the market respondents as the highest barrier to entry, such barriers do not exist (or not to the same extent) for Radici and Invista, two alternative AH Salt producers. Radici and Invista have privileged access to HMD in the EEA and both produce adipic acid on the global market. They moreover have the required know how and experience and can readily increase AH Salt production capacity to effectively compete with the volumes supplied by the Merged Entity.
(664) Finally, the Notifying Party claims that customer could, in case of a price increase, purchase PA 6.6 BP or Co-Polyamide 6/6.6 BP or 6.6/6 BP instead of AH Salt or sourcing adipic acid and HMD and mixing such materials themselves subject to a limited investment.
6.3.7.2.(B) The Commission' assessment
6.3.7.2.(B.i) EEA market and imports
(665) The Notifying Party claims that there are no technical or cost barriers to selling AH Salt worldwide and transporting it over long distances and therefore market players present in other regions of the world could import AH Salt into the EEA thus representing a considerable constraint in terms of pricing. In particular, the Notifying Party claims that Ascend and CSM would have significant spare capacity at worldwide level and that they could enter the EEA market.
(666) However, the market investigation has shown that the market should be considered EEA-wide and that importing from outside the EEA is not an alternative for customers due to higher prices derived from import tariffs, transportation costs, logistic and technical difficulties, special conditions for transportation of liquid AH Salt, technical difficulties and increased costs to import solid AH Salt and long delivery times (see Section 5.4.3).
(667) Additionally, the existence of swap agreements between competitors in order to avoid imports and AH Salt transport over long distances provides another indication that imports may not impose an effective competitive constraint to local production. For example, BASF […]. [REFERENCE TO THE BUSINESS’ COMMERCIAL STRATEGY].461
(668) Finally, it is important to note that while the majority of customers consider that the market is short (see. Section 6.3.4.2 (B.ii)) and therefore it is difficult to secure enough volumes of AH Salt in the EEA merchant market, the market investigation has shown that customers have not imported any volumes of AH Salt into the EEA in the last 7 years462. This further demonstrates that customers do not see imports as a
viable alternative to defeat a shortage of supply or a price increase even when it is difficult to source enough volumes in the EEA market.
6.3.7.2.(B.ii) Market is short and concentrated
(669) The vast majority of customers consider that the market is short, i.e. demand exceeds supply.463 This view is also supported by producers responding to the market investigation.464 This problem will be accentuated in the near future, as competitors believe that the market will continue growing in the next 5 years either moderately (1-2% per year) or vigorously (over 2% per year).465 Indeed, the majority of customers also consider that AH Salt demand will grow vigorously (>2%) in the next 5 years.466
6.3.7.2.(B.iii) Competitive landscape
(670) The number of potential competitors active in the AH Salt production is very limited in the EEA. In addition to the Parties, only 3 companies produce AH Salt in the EEA: Radici, Invista and DowDuPont. […] are not active in the merchant market. In the case of […], the Notifying Party submits that it is active in the merchant market and that it accounts for [0-5]% of the merchant sales. However, the market investigation has indicated that […] is not active in the merchant market and therefore, the Parties are the only competitors active in the merchant market in the EEA. Indeed, customers responding to the market investigation have not sourced any volumes467 from […] in the last 7 years468 and […] has indicated that it uses 100% of its production for internal consumption469. Therefore, the Parties likely underestimated their market share for merchant sales.
(671) According to the data provided by the Notifying Party, the Business' share in the merchant market is [80-90]% while it is [40-50]% for merchant sales plus captive use. While the Business has the largest captive use in the EEA if compared to its competitors ([…] kts against […] kts of BASF, […] kts of […], […] kts of […] and […] kts of […]), it is also the market leader in the AH Salt merchant market.
(672) The Business’ global capacity in 2017 amounted to […] kts with a production of […] kts of AH Salt. The three EEA production sites had a combined production capacity of […] kts and produced […] kts of AH Salt. The Notifying Party claims that 80% of the AH Salt sales of the Business (constituting at the same time approximately 80% of the entire EEA-wide merchant market), are generated […].
(673) BASF' accounts for [5-10]% of the merchant market according to the data submitted by the Notifying Party, although as discussed in recital (670) the market investigation contains indications that it may be higher, and [10-20]% of the merchant sales plus captive use in the EEA. BASF is using the largest part of its AH Salt production captively for the production of PA 6.6 BP and co-polyamides. BASF’s production site for AH Salt is located in Ludwigshafen. In 2017, the production site in Ludwigshafen had a capacity of […] kts and produced […] kts of AH Salt. […].
(674) Radici accounts for [10-20]% of the total production in the EEA. While according to the data submitted by the Notifying Party, Radici accounts for [0-5]% of the merchant sales, the market investigation has indicated that Radici is not active470 in the merchant market471.
(675) Invista and DowDuPont are not active in the merchant market while they account respectively for [10-20]% and [10-20]% of the merchant sales plus captive use in the EEA. All their production is used captively for the production of PA 6.6 BP.
(676) Moreover, as explained in Section 6.3.4.2(B.vii), […] are capacity constrained or do not have access to enough raw materials to produce AH Salt at competitive terms, and they do not have plans to enter the merchant market.
6.3.7.2.(B.iv) The Parties' position in the market and closeness of competition
(677) Combined shares of the Parties are very high ([90-100]% in 2017 in value for merchant sales according to the Notifying Party and closer to [90-100]% according to the Commission; [50-60]% volume for merchant sales plus captive use; and [60- 70]% for production capacity). Therefore, the Merged Entity would control approximately two thirds of the AH Salt production in the EEA and virtually all the merchant market. Moreover, it should be noted that capacity shares are measured in terms of nameplate (or plant processing) capacity without considering access to key inputs. Taking into consideration that other producers like […] do not have access to enough raw materials to use their nameplate capacity at full, if availability of inputs is considered, the share of the Merged Entity in terms of real production capacity would be even higher. These market shares indicate a presumption of dominance which is supported by market participants that claim that the Merged Entity would have a dominant position in AH Salt and that the Transaction would reduce choice. In fact, virtually all customers and producers consider that BASF and Solvay are the top 2 producers of AH Salt in the EEA.472
(678) Customers unanimously are of the opinion that if the Transaction goes ahead they would not have a sufficient choice of suppliers of AH Salt in the area they operate in.473 Moreover, the market investigation shows that customers consider that the only two options in the market are BASF and the Business. For example, […] explains that “currently, there are only two merchant suppliers of AH Salt in Europe, Solvay and BASF”.474 Another very important customer, […] explains “Solvay and BASF are the only 2 suppliers of liquid AH Salt with 62% concentration level. Other AH Salt producers either are not capable of loading of AH Salt or do not produce AH Salt with 62% concentration. After the merger […] will have only 1 supplier for its main raw material”.475 Another customer, […] claims that there is “no free capacity outside of BASF-Solvay”476 and therefore the Merged Entity would enjoy a monopoly.477 […] adds that “BASF and Solvay are the only suppliers of AH Salt active in merchant market and therefore 100% of their final production depends on AH Salt procured by either Solvay, or BASF... The merger might have a detrimental negative effect, since its two suppliers of AH Salt (which are the only ones who make AH Salt commercially available) will become one company. This is a merger to monopoly situation”.478
(679) The situation described in recitals (677)-(678) explains why the majority of the respondents to the market investigation consider that the Transaction would have a negative impact on the market for AH Salt.479 […] fears that the Transaction would “eliminate our current second supply situation and will lead to single sourcing. Price pressure will continue as it has during the last months”.480 In the same vein, several customers fear that the Transaction would lead to an increment of the price of AH Salt. For example, […] claims that “there are other AH Salt producers, like Radici, but they do not sell their product to the merchant market […]. Indeed, the post- merger entity will be in a monopolistic situation for AH Salt […].That monopolistic situation also raises concerns about the price of the product”.481 Similarly, […] believes that “it will be very difficult to negotiate prices because there will be only one supplier and […] will not be able to shift volumes to other competitor in case of price increases”.482 In the same vein, […] adds that it "has been trying to source AH Salt from Radici but the latter uses its production in-house as the market is expected to be tight. […] has not found other alternatives in the EEA market for AH Salt. Consequently, […] relies on BASF or Solvay to purchase AH Salt. […] […] cannot import AH Salt. […] likely not to get any AH Salt next year and could not therefore produce certain grades and will have ultimately to reduce […] output for some PA products, including PA 6.6".483
(680) The strong position of the Merged Entity that would control almost two thirds of the EEA production and virtually all the merchant sales, combined with the lack of alternatives and the tightness in the market, would give the Merged Entity the power to increase prices in the merchant market.
6.3.7.2.(B.v) Barriers to entry
(681) Contrary to the Notifying Party’s views, the respondents to the market investigation consider that barriers to entry in the AH Salt market are high. Producers of AH Salt identify as barriers to entry the level of investment required to set up a production facility, know-how, lack of expertise and especially access to raw materials (i.e. HMD and adipic acid).484
(682) In the same vein, customers consider that the most important barrier to enter the AH Salt market is access to HMD and adipic acid, followed by the level of investment required, the lack of expertise, the know-how and regulatory requirements.485 For example, […] explains that “an AH Salt strike unit would require a capital investment in a mixing station of HMD and Adipic Acid as well as filtration and silo capacity. There are only about 6 AH salt strike units existing in EEA which restricts expertise and availability of know how. The handling of the materials (especially HMD) is strictly regulated due to its hazardous properties. The availability of HMD which is produced from ADN is similarly restricted as AH Salt”.486
(683) Indeed, as explained in Sections 6.3.2 and 6.3.3, the merged entity would enjoy market power in the HMD and adipic acid markets which are very tight and concentrated, thus making access to these raw materials very difficult for a company that wishes to compete with the merged entity in the AH Salt market. Moreover, no new entrants have been observed in the EEA in the last 5 years.487
(684) Finally, in the Response to the 6(1)(c) Decision, the Notifying Party claims that as regards to access to HMD and adipic acid, which has been identified by the market respondents as the highest barrier to entry, such barriers do not exist (or not to the same extent) for […]. However, the market investigation has indicated that […] do not have spare capacity to sell AH Salt into the merchant market and that they are not interested in entering in this market nor they have any plans to increase capacity or enter the market488.
6.3.7.2.(B.vi) Multi-sourcing
(685) Multi-sourcing appears important for customers for security of supply reasons and they fear that following the Transaction this possibility would disappear. For example, […] explains that it “sources actively from BASF and Solvay because in the interest of supply security it is important to have 2 suppliers”489 and […] adds that its “actual strategy is to source their total requirements from two suppliers. After the merger, that possibility will disappear. Indeed […] it will be difficult to find other suppliers (apart from the merged entity) for AH Salt in Europe”.490
6.3.7.2.(B.vii) Increase of merchant sales by other producers
(686) Contrary to the Notifying Party's claim that market players have unused assets, such as Invista, Radici and DowDuPont, allowing each customer to choose among several potential suppliers now and post-transaction, the market investigation has not indicated that there are companies with additional capacities to serve additional volumes in the market.491 Indeed, […] has indicated that it does not have spare capacity to sell AH Salt into the merchant market492 and that it uses 100% of its AH Salt production captively.493 This is the reason why "[…] doesn't intend to enter on the EEA merchant market of AH Salt".494 […] adds that "enter the AH Salt merchant market is not contemplated as a […] market strategy. The intention, from the very beginning, was to produce AH salt to serve PA 66 production".495 Indeed, […] considers that "BASF and Solvay are the only two companies that sells AH Salt in the merchant market. Post- transaction there will be a monopoly for AH Salt in Europe".496 Meanwhile, […] has also confirm that it "does not have spare production capacity of AH salt that could be sold on the merchant market"497 and this "is the reason why […] does not intend to proactively enter the AH Salt merchant market in the EEA".498
(687) Finally, […] cannot serve the market because it does not have access to enough HMD. […] recognises that it "does have excess AH salt capacity […]. But this capacity cannot be utilized because there is no HMD available necessary to produce the AH salt".499 Therefore, […] does not intend to enter on the EEA merchant market because it "does not have access to the required HMD to produce AH salt for the merchant market. […] consumes all the HMD quantities it can secure in Europe to supply its PA66 EP customers".500 Indeed, "[…] has been approached by third parties seeking AH salt supply in the EEA. But […] is not in a position to supply AH salt into the market as this would require to have access to the intermediates (HMD and AA) at competitive terms".501
6.3.7.2.(B.viii) Switching to PA 6.6 BP or to HMD and adipic acid if prices for AH Salt increase
(688) The Notifying Party claims that to overcome any AH Salt supply issues polyamide- producing customers that purchase AH Salt can also purchase at a different level of the supply chain, and in particular they can also purchase HMD and adipic acid in order to produce themselves AH Salt or, in the alternative, purchase PA 6.6 BP.
(689) However, contrary to the Notifying Party’s views, regarding the option to purchase HMD and adipic acid, first, as explained in Sections 6.3.2 and 6.3.3, customers and producers consider that barriers to entry are high and point to high investment costs, access to raw materials and know-how. Second, as explained in Sections 6.3.2 and 6.3.3, the Merged Entity would enjoy market power also at the level of HMD and adipic acid and therefore it can also increase prices at this level of the polyamide value chain. Indeed, access to raw materials is seen by producers and customers the most important barrier to start producing AH Salt. Third, contrary to what the Notifying Party claims, market players are of the opinion that starting to produce AH salt would take a considerable amount of time. For example, an important customer, […], explains that if it “had to purchase the raw materials (HMD and adipic acid) in order to produce its own AH Salt, the set process would take between two and four years (to obtain the approval from the government or the State to build the plant and to go through the investment and building process) and would cost approximately 10 million euros”.502 These constraints are reflected in the opinion of another important customer of AH Salt, […], which is of the view that to develop its own new AH Salt production site in the EEA, “[t]he key problem would […] lie on finding the necessary HMD and AA volumes, their transportation and storage. […] would need some time to achieve the quality needed. The preparation of the plant cost millions of euros. It is worth noting that the merged entity would also be a dominant player in the commercial supply of HMD and AA. Therefore, it is not clear that […] would have access to HMD and AA even if it makes the investment in a new plant for AH Salt”.503
(690) Moreover, contrary to the Notifying Party’s view, the market investigation shows that customers would not be able to source PA 6.6 BP. As explained in Section 6.3.5, the Merged Entity would enjoy market power at the level of the PA 6.6 BP and therefore could increase prices at this level of the polyamide value chain. Further, producers sourcing AH Salt in order to produce co-polyamide are unable to source PA 6.6 BP. Finally, yarn producers would face significant technical difficulties and an increase in costs if they were to switch to sourcing PA 6.6 BP. For example, […] explains “[a]lternatively, […] could be pushed to buy the Base Polymer and concentrating on producing the yarns. But that mean to close down the polymerisation lines and long approval procedures (by their own downstream customers) for the use of these third party polymers. This could take between 18 to 24 months. Due to very strict safety requirements for airbags and tyres, this process would be very burdensome for customers. Moreover, closing the polymerisation and purchasing the base polymer would entail an increase of costs”.504
6.3.7.2.(C) Conclusion on non-coordinated effects: AH Salt
(691) Based on the assessment in recitals (650) to (690), the Commission concludes that the Transaction would significantly impede effective competition as a result of horizontal non-coordinated effects arising in the supply of AH Salt due to the high market shares of the Merged Entity, the reduced number of competitors and the lack of alternatives for customers in a very concentrated market with high barriers to entry and in which demand exceeds supply.
6.3.8. Level IV of the polyamide value chain: PA 6.6 BP
6.3.8.1. Market structure
(692) Based on the Notifying Party's own estimates, the Parties’ combined market shares in the EEA market for PA 6.6 BP in 2017 were as follow:
(a) [30-40]% or [30-40]% (respectively in value and volume) for merchant sales ([30-40]% Business and [0-5]% BASF). Next competitors, both in value and volume, are Invista ([20-30]%), Radici ([20-30]%) and Ascend ([10-20]%);
(b) [40-50]% in volume for merchant sales and captive use (including volumes used internally, [30-40]% Business and [10-20]% BASF). Next competitors are Invista ([10-20]%), Radici ([10-20]%) and DowDuPont ([10-20]%); and
(c) [40-50]% for production capacity ([30-40]% Business and [10-20]% BASF). (693) Table 19 provides the Merged Entity's market share estimates for 2017 (merchant sales and/or captive use) for the EEA market.
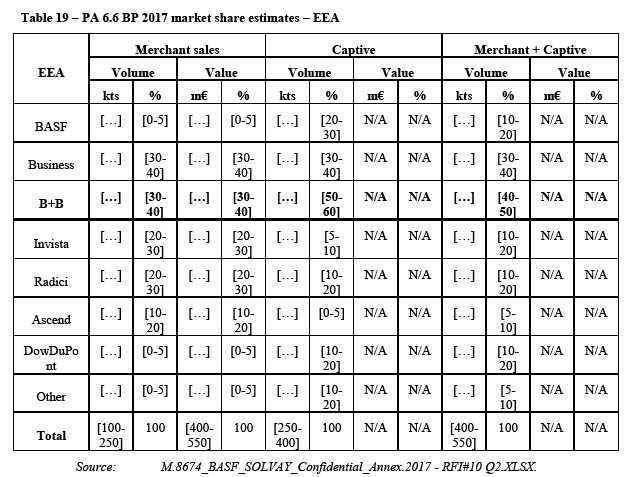
(694) Table 20 provides the Parties' capacity share estimates for 2017.
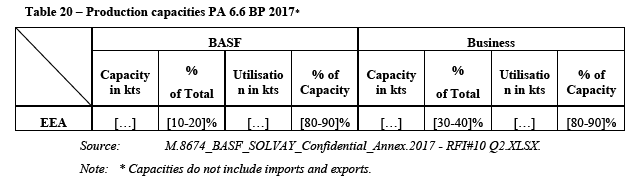
(695) The market investigation indicates that sales of competitors of the Merged Entity for 2017 (merchant sales and/or captive use) for the EEA market differ from the Notifying Party's own estimates. Market shares based on the results of the market investigation are provided in Table 21:
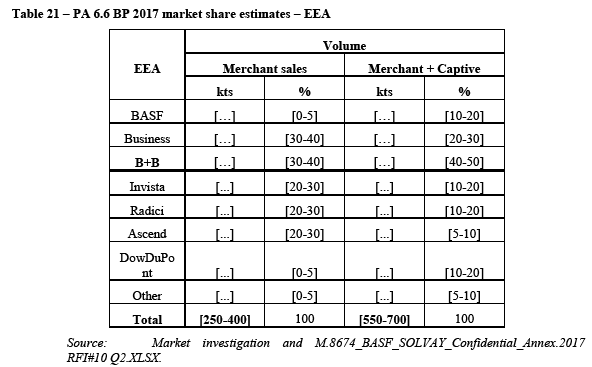
6.3.8.2. Competitive assessment: horizontal non-coordinated effects 6.3.8.2.(A) The Notifying Party's view
(696) The Notifying Party argues that the Transaction would not lead to horizontal non- coordinated effects, as the Merged Entity would not enjoy market power, for the reasons laid out in the present section.
(697) The Notifying Party considers that merchant sales are the only appropriate metric to look at for the purposes of the competitive assessment. The Notifying Party's underlying arguments are further detailed in Section 6.2.1. Based on merchant sales, the Notifying Party argues that the Merged Entity's market share does not indicate market power, and that the Transaction only gives rise to a marginal increment in such share.505
(698) Irrespective of the market definition, the Notifying Party considers that the Merged Entity would still face competition from significant market participants including in particular Invista, Ascend, and Radici. Customers would thus have the possibility to switch supplier, as switching costs are irrelevant, determined by the qualification of the relevant base polymer, when required.506
(699) The Notifying Party further argues that there is substantial over-capacity in the market, as well as unused capacity including from the Parties, and competitors can increase supplies if prices increase. The Merged Entity would thus have no incentive to reduce output or increase prices.507
(700) The Notifying Party also argues that the Parties are not close competitors and that the Transaction thus does not eliminate an important competitive constraint. While BASF is mainly active on the PA 6 BP segment, the Business’ activities focus on the PA 6.6 BP segment.508
(701) According to the Notifying Party, the Merged Entity would be constrained by EP producers as customers exercising countervailing buyer power, since compounders switch suppliers if the price offered by the Merged Entity is not satisfactory.509
(702) The Notifying Party also argues that, regardless of market definition, PA 6 BP exerts a competitive constraint on PA 6.6 BP,510 and that imports exert a constraint on EEA sales of PA 6.6 BP.511
6.3.8.2.(B) The Commission's assessment
(703) The results of the market investigation contradict the Notifying Party's claim that there would be sufficient viable alternative suppliers of PA 6.6 BP post-transaction.
(704) As detailed in Section 6.2.2, market shares based on merchant sales plus captive use, as well as market shares based on capacity are complementary to the use of market shares based on merchant sales, to assess the impact of the Transaction throughout the polyamide value chain. This is also relevant in the context of the PA 6.6 BP market, in particular as the Notifying Party argues that unused/excess capacity exists, generating a highly competitive environment, and as market participants downstream raised input foreclosure concerns. The Merged Entity's [40-50]% market share in 2017, based on production capacity, or [40-50]% based on merchant sales plus captive volumes, is itself evidence of market power over the EEA-wide market for PA 6.6 BP, particularly considering Invista is only a distant second with a [10-20]% market share based on its captive use and merchant sales in the EEA.
(705) Furthermore, regardless of the increment in market share, Solvay’s internal documents show that the Business takes into account BASF's supply situation when planning prices and output. In particular, following BASF's force majeure declaration on HMD, AH Salt and PA 6.6 BP and compounds […], [MEMBER OF THE SENIOR GBU MANAGEMENT] tells its team "[QUOTE FROM CONFIDENTIAL INTERNAL DOCUMENT]".512
(706) A majority of customers of PA 6.6 BP also believes, contrary to the Notifying Party's view, that post-transaction, they would no longer have a sufficient choice of suppliers.513 Reasons put forward include the already small number of suppliers of PA 6.6 BP on the market.514 By the Notifying Party's own account, in addition to BASF and the Business, only Invista, Radici, Ascend and DowDuPont supplied PA 6.6 BP to customers in the EEA in 2017.515 Also, […] confirmed that it is typically not supplying PA 6.6 BP to the merchant market, but only sporadically making one- off sales in specific circumstances, such as following an incident in Ludwigshafen.516
(707) Market participants stress the importance of backwards and forwards integration into the entire polyamide chain from ADN and HMD to PA 6.6 EP.517 Out of the PA 6.6 BP suppliers listed in recitals (703) to (706) , both Radici and DowDuPont are not fully backwards integrated in the value chain, and may thus be foreclosed from essential inputs, as further described in Sections 6.3.2, 6.3.3 and 6.3.4.
(708) There has been no market entry in PA 6.6 BP in the past five years,518 and a large majority of market participants does not expect any new entry in the short term (next two to three years) in the market.519 The main barriers to entry cited include access to raw materials and the levels of investment to be made.520 Market participants estimate that a polymerisation unit to produce 20 to 30 kts of PA 6.6 BP would cost around EUR 50-100 million.521
(709) Regarding available capacity, responding customers and competitors also overwhelmingly indicate that the supply/demand situation for PA 6.6 BP in the EEA is short, i.e. that demand exceeds supply.522 Market participants also believe that in the future the demand for PA 6.6 BP will grow,523 while capacity is expected to remain identical in the foreseeable future.524
(710) While the Notifying Party states that there is overcapacity of PA 6.6 BP in the market, it arguably overestimates such excess capacity. The Notifying Party indicates that the difference between the nameplate capacity and the actual production implies available spare capacity.525 However, nameplate capacity cannot equal production, as it would imply a 100% utilisation rate of all production facilities which, in practice, is hardly achievable. In particular, with regards to PA 6.6 BP specifically, when making its own projections, the Notifying Party uses a [90-100]% utilisation rate "to estimate the volumes that each supplier could reliably deliver in practice" (emphasis added).526 In 2017, according to PCI estimates, the total EEA capacity for PA 6.6 BP was [600-700] kts, while the production volumes amounted to [500-600] kts.527 These figures imply an average utilisation rate of capacity of approx. [70-80]%, which is lower than [90-100]%, but in line with that of BASF over the last two years.528 Assuming a [90-100]% utilisation rate, the extra volumes that could be brought to the market would amount to around 73 kts. Such additional volumes would not only include additional volumes produced by the Merged Entity, but they would also be lower than those supplied by the Business to the merchant market.
(711) Also contrary to the Notifying Party's claim, excess capacity of PA 6.6 BP cannot accommodate the demand of non-integrated PA 6.6 BP customers in the EEA. Firstly, the Notifying Party's analysis which mentions that Ascend, Invista and DowDuPont have around "[…]" of available PA 6.6 BP surplus, focuses on global production capabilities. Regardless of the accuracy of this specific figure, for reasons laid out in Section 6.6.3, the market for PA 6.6 BP cannot be viewed as worldwide, and the analysis of available capacity should focus on EEA-based capacity. Secondly, the Notifying Party seemingly underestimates the demand of PA 6.6 BP from non-integrated players. The Notifying Party itself estimates merchant sales (largely to non-integrated players) amount to […] kts in 2017, far exceeding the "[…]" estimated as sufficient to cover the needs of all downstream users of PA 6.6 BP which are not vertically integrated in the Notifying Party's economic analysis.529
(712) The assessment presented by the Notifying Party, based on 2016 data, also does not factor the estimated demand growth over the next years. Based on PCI estimates, PA 6.6 BP demand in the EEA will increase by over 7% between 2016 and 2020.530 In fact BASF itself expects its internal consumption […].531
(713) Competitors of the Merged Entity in the PA 6.6 BP market state that they have indeed some spare capacity to serve additional volumes into the merchant market in the EEA - in case of a permanent increase of demand, but such volumes are dwarfed by the Parties' current merchant sales.532 […] says that they could bring into the market “at least 20Kt per year”,533 however, such volumes would be imports to the EEA, since […] has no EEA-based polymerisation capacity (and such statement predates the company's force majeure situation in Pensacola). As a result, while these volumes are relevant for the competitive assessment, they are not evidence of excess capacity in the EEA. […] states they could bring about 10 kts.534 […] could also increase the volumes of its sales to the EEA market.535 These volumes, added together, do not nearly equate the Parties' merchant sales which reached […] kts in 2017.
(714) The Commission notes that PA 6.6 BP is not a particularly differentiated product - with a possible exception for viscosity which some market participants mentioned as potentially relevant-536 which mitigates the importance of the Parties' being each other's closest competitor (or not) for the competitive assessment. Also, there are few suppliers of PA 6.6 BP. Regardless of whether the two companies are each other's closest competitors, BASF is able to exert a competitive constraint on the Business, in particular due to its ability to increase merchant sales, should it so choose, or as a threat waived by customers in supply negotiations. Respondents to the market investigation can and regularly do approach BASF for a supply of PA 6.6 BP. For instance, […] recently approached BASF for supply of PA 6.6 BP for 2019.537
(715) Customers overwhelmingly respond that the Transaction would have an impact on the PA 6.6 BP market.538 In particular, customers consider that the Transaction would lead to higher prices539 and a reduced choice of suppliers.540
(716) The downstream EP market, which consumes most of the PA 6.6 BP sold in the EEA involves an important number of compounders with relatively minor market presence (with a market share typically not exceeding [5-10]%), besides the leading players which are BASF, the Business and DowDuPont. Compounders are typically only active at this last level of the PA 6.6 value chain. As a result they do not have a bargaining power that would compare to that of BASF or Solvay, which are active throughout most of the value chain and have a significant presence at every relevant level. In addition, for the reasons outlined in the present section, compounders could not effectively threaten to switch immediately to another source of supply, due to the limited number of viable suppliers, nor vertically integrate, in particular due to the significant costs linked to the installation of a polymerisation unit.
(717) With regards to the Parties' argument that PA 6 BP exerts a competitive constraint on PA 6.6 BP, the Commission refers to recital (252). As PA 6 BP is used to produce PA 6 EP and PA 6.6 BP is used to produce PA 6.6 EP, the competitive constraint can only be relevant through the interactions at the downstream level. The competitive pressure that PA 6 EP exerts on PA 6.6 EP is further discussed in Section 6.3.9.
(718) On the other hand, the impact of imports of PA 6.6 BP from outside the EEA, as a competitive constraint on local players, is particularly relevant.
(719) Such impact is reflected in the market share estimates, mainly in Ascend's share of supply ([…]). […] has local capacity in the EEA for PA 6.6 BP, from its Rozenburg (Netherlands) facility, which capacity is estimated to be 90 kts per year by PCI. […] European plant typically serves the company's EEA customers.541
(720) Imports from China appear inconsequential with regards to PA 6.6 BP. According to PCI's 2018 YellowBook, in 2018, the Chinese market's requirements dwarfed the local production, thus making China a net importer of PA 6.6 BP, due to a lack of local production assets.542 Imports from China are thus unlikely to constrain players in the EEA market, at least in the short term. This is further evidenced by the Notifying Party's own market share estimates, which mention no sales of PA 6.6 BP by Chinese suppliers in 2017. Market participants also flagged that, not only are Chinese suppliers dependent on ADN from outside China,543 but the quality of PA
6.6 BP offered by Chinese suppliers is not considered up to the level of its European and American counterparts by some market participants,544 and while they can be technically similar, end customers prefer not to use Chinese materials.545 In terms of reliability, […] mentions for instance that even if the company could potentially procure one truck of PA 6.6 BP from Huafon, it would be impossible to rely on Chinese suppliers for the totality of its BP requirements, in particular for consistency and reliability reasons.546
6.3.8.2.(C) Conclusion on non-coordinated effects: PA 6.6 BP
(721) Based on, the Commission concludes that the Transaction would significantly impede effective competition as a result of horizontal non-coordinated effects arising in the supply of PA 6.6 BP in the EEA.
6.3.9. Level V of the polyamide value chain: PA 6.6 Engineering Plastic
6.3.9.1. Market structure
(722) Based on the Notifying Party's estimates, the Merged Entity would have a market share of [30-40]% (increment of [10-20]% from the Business) in value. The nearest competitor is DowDuPont with [10-20]%. Other competitors include Radici, Celanese and Ascend ([5-10]% each) followed by Ravago and Lanxess ([5-10]% each).
(723) In volume, the Merged Entity's market share is [30-40]% (increment of [10-20]% from the Business). The picture of the competitive landscape for competitors remains the same as for value.
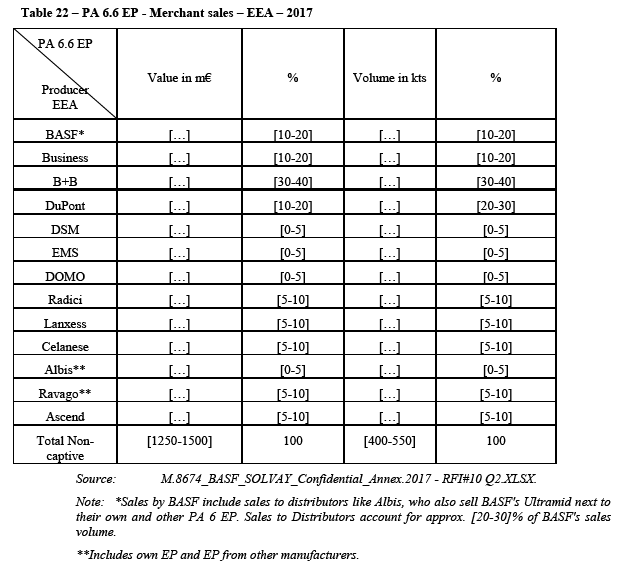
(724) According to the Notifying Party, it is not appropriate to differentiate between capacities for PA EP, PA 6 EP and PA 6.6 EP because compounding facilities are generally multi-purpose facilities that are able to produce, inter alia, both PA 6 EP and PA 6.6 EP. In terms of production capacity, the share of the Merged Entity of the overall PA EP, PA 6 EP and PA 6.6 EP production capacity is [20-30]% (increment of [5-10]% from the Business).
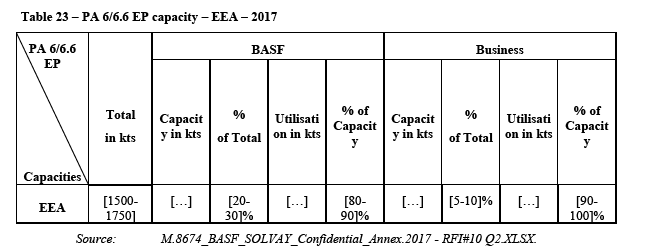
(725) The market investigation did not reveal any significant discrepancy between the participant's actual sales and those estimated by the Notifying Party. As such, the estimates provided by the Notifying Party can be used as a reliable proxy for the PA
6.6 EP suppliers' market shares in the EEA.
(726) […], by reference to a FIDES / Actreu report547 estimates the combined market shares of the Parties to be much higher, i.e. over 60%. However such estimates rely on data obtained via self-reporting by a limited number of companies (namely BASF, Solvay, Lanxess, EMS, DSM and DowDuPont), which in turn may overestimate the share of the participants, including the Parties.548 As a result, the competitive assessment primarily relies on the estimates provided by the Notifying Party.
6.3.9.2. Competitive assessment: horizontal non-coordinated effects 6.3.9.2.(A) The Notifying Party's view
(727) The Notifying Party argues that the Transaction would not have horizontal effects.
(728) The Notifying Party argues that the Transaction would not lead to non-coordinated effects, as it does not eliminate the Business as an important competitive constraint, thus preventing the Transaction from increasing the Merged Entity's market power, for the reasons laid out in Section 6.3.9.2.(A.i).
6.3.9.2.(A.i) Market share levels
(729) The Notifying Party considers that the market shares in PA 6.6 EP, are not high and are not in themselves evidence of a potential dominant position of the Merged Entity post-transaction. The Notifying Party argues that market characteristics (including the fact that the PA 6.6 EP market is tender-based) further relativise the Parties' combined shares.549
6.3.9.2.(A.ii) Closeness of competition
(730) The Notifying Party also argues that the Parties are not close competitors. Arguments raised in that context include the lack of meaningful customer overlaps between the Parties, the different segment focus of BASF (mainly focused on […]) and the Business (mainly focused on […]). This lack of closeness of competition is evidenced in particular by recent customer surveys conducted by BASF.550
(731) In paragraphs K.286-K.288 of the Form CO, the Notifying Party argues that “[d]iversion ratios based on recent customer survey indicate that the Parties are indeed distant competitors”. In support of their claim, the Notifying Party uses a “customer survey with PA EP and PBT EP customers” conducted by BASF. On the basis of responses to a question asking customers “[w]ho is currently your main / second main supplier for engineering plastics?”,551 the Notifying Party produces table C.EP.51 which displays “diversion ratios” between BASF and some of its competitors. In the economic literature, the diversion ratio between BASF and a given competitor is defined as the share of BASF sales that would be lost to that competitor in the event of a price increase by BASF. In the Response to the 6(1)(c) Decision, the Notifying Party also claims that this customer survey shows that […].552
(732) In the Response to the 6(1)(c) Decision, the Notifying Party further argues that BASF and Solvay are not each other's closest competitor. Firstly, the Notifying Party considers that the 6(1)(c) Decision does not provide substantive evidence of the alleged closeness of competition between the Parties. Secondly, the Notifying Party submits the underlying data relating to the customer surveys analysis conducted by BASF. Thirdly, the Notifying Party argues that there is no meaningful customer overlap between BASF and the Business.553
6.3.9.2.(A.iii) Alternative suppliers
(733) Furthermore, according to the Notifying Party, customers have numerous possibilities to switch supplier. The Notifying Party considers that the Merged Entity would still face competition from alternative global suppliers such as DowDuPont, Lanxess, DSM, Radici/Invista, Toray, Ascend, EMS and KingFa, as well as smaller EP producers. Customers would thus have the possibility to switch supplier easily, as supply contracts are normally short term (3 to 6 months).554 The Notifying Party further states that the market investigation also indicates there is a high number of competitors active in the EEA.555
(734) The Notifying Party further argues that competitors are likely to increase supply if prices increase, as they have the capacity to do so. In fact, there are large over- capacities in the PA 6.6 EP market and suppliers have the capability to produce different grades.556
6.3.9.2.(A.iv) Barriers to entry and expansion
(735) The Notifying Party also considers that the Merged Entity would not have the ability to hinder expansion by competitors post-transaction, due to the market being dynamic, and the fact that a large number of players operating in the PA 6.6 EP market are not backwards integrated.557
6.3.9.2.(A.v) Customer countervailing buyer power
(736) The Notifying Party also considers that customers of the Merged Entity hold significant countervailing buyer power and would have the ability to defeat any price increase post-transaction.558
6.3.9.2.(A.vi) Competitive constraint of PA 6 EP (and other materials) as well as PA 6.6 EP from outside the EEA on PA 6.6 EP in the EEA
(737) The Notifying Party also argues that, regardless of product market definition, PA 6 EP and other materials exert a competitive constraint on PA 6.6 EP, which should be taken into account for the purposes of the Commission's competitive assessment.559
6.3.9.2.(B) The Commission's assessment
6.3.9.2.(B.i) Market share levels
(738) Contrary to the Notifying Party's claim, concentrations giving rise to market shares below 40% can significantly impede effective competition, regardless of "exceptional" circumstances. The Commission's Horizontal Merger Guidelines mention indeed that "[a] merger involving a firm whose market share will remain below 50 % after the merger may also raise competition concerns in view of other factors such as the strength and number of competitors, the presence of capacity constraints or the extent to which the products of the merging parties are close substitutes. The Commission has thus in several cases considered mergers resulting in firms holding market shares between 40 % and 50 %, and in some cases below 40 %, to lead to the creation or the strengthening of a dominant position" (emphasis added). In the present case, these conditions apply, as further discussed in the present section.
(739) On a structural level, based on the Notifying Party's own estimates, the pre- transaction HHI is […], while the post-transaction HHI is […], leading to a delta of […]. These HHI and HHI delta levels exceed the thresholds mentioned in paragraphs 19 and 20 of the Horizontal Merger Guidelines, and as such are evidence of a significant change in the PA 6.6 EP market structure that require an extensive analysis.
(740) Contrary to the Notifying Party's claim that the market is largely tender-based, which would relativise the importance of market shares as evidence of market power, most responding customers indicated engaging in bilateral commercial negotiations for the supply of PA 6.6 EP.560 As such, existing market shares are evidence of PA 6.6 EP suppliers' market power (or lack thereof).
(741) Furthermore, the Commission notes that the Parties' (and their competitors') market shares for PA 6.6 EP in the EEA have only changed to a very limited extent (i.e. less than 5 percentage points, and usually less than 2) over the last four years (2014 to 2017), further evidencing that market shares are a relevant proxy for market power.561
(742) Current market share levels may on the contrary underestimate the market power of the Merged Entity post-transaction. Respondents to the market investigation regularly identify vertical integration as granting the Parties market power on the PA 6.6 EP market.562 The vertical effects of the Transaction are further discussed in Section 6.4.5.
(743) The Merged Entity's market shares, in light of the structural features of the PA 6.6 EP market in the EEA, characterised by the presence of only three large players including both Parties (as well as DowDuPont) and numerous smaller players with market shares below [5-10]%, as well as the Merged Entity's enhanced vertical integration throughout the PA 6.6 EP value chain, are evidence that the Transaction may raise competition concerns.
6.3.9.2.(B.ii) Closeness of competition
(744) The Phase I market investigation contradicts the Notifying Party's argument that the Parties are only "distant competitors". Over half of the responding customers of PA 6.6 EP considered that the Business and BASF are each other's closest competitor.563 Others customers place them as each other's second or third closest competitor, implying they are at least relatively close competitors, contrarily to the Notifying Party's claims.
(745) In the course of the Phase II investigation, customers of PA 6.6 EP further confirmed that they considered the Parties as close competitors. In fact, around three-fifths of responding customers considered BASF and the Business to be each other's closest competitor.564 Respondents mainly identified the Parties' vertical integration and product characteristics, as reasons justifying such closeness of competition. Other reasons mentioned (by order of decreasing importance) include the Parties' portfolio breadth, pricing strategy and R&D capabilities.565 […], […] and […] also indicated that the fact that the Parties have the largest production capacities of PA 6.6 EP in the EEA makes them each other's closest competitors.566 On the other hand, no market respondent claimed that the Parties had unique technical capabilities that no other market player would have.567
(746) With regard to vertical integration, while, in the EEA, only Solvay is a PA 6.6 EP supplier that is backwards integrated into ADN, both BASF and Solvay are integrated from level II (HMD) downwards. Out of the remaining PA 6.6 EP suppliers, only DowDuPont and Radici are also vertically integrated into AH Salt and into HMD respectively in the EEA, while Ascend is vertically integrated from Level I (ADN), but outside of the EEA.
(747) In terms of product characteristics, the market investigation indicates that BASF and the Business are recognised as particularly close competitors with regard to heat- resistant PA 6.6 EP grades.568 For instance, […] considers that the Solvay's Technyl A 218 V35 Black 34NG and BASF's Ultramid A3WG7 BK20560 are each other's closest substitute.569 Similarly, […] considers that "BASF Ultramid A3WG7 and Solvay Technyl A218v35 are very similar interchangeable grades".570 Separately, both […] and […] consider that Solvay's Technyl A 218 V30 Black 34NG and BASF's Ultramid A3HG6 HR BK23591 are each other's closest substitutes.571 This closeness is competitively significant, in particular due to the fact that heat stabilised grades are the Parties' primary bestselling grades. Heat stabilised grades represent over [60-70]% of BASF's572 and approximately [40-50]% of the Business'573 PA 6.6 EP sales.
(748) The market investigation however also indicates that there are competitors offering PA 6.6 EP presenting similar characteristics as those cited in recital (747), including DowDuPont, Lanxess, EMS and A.Schulman.574 Also, in line with the Notifying Party's claim, the market investigation confirms that there is virtual supply-side substitutability across all grades and that PA 6.6 EP suppliers, even those smaller than the Parties or DowDuPont, are able to offer most grades, in particular if given the opportunity by a customer.575 Furthermore, as mentioned in recital (747), no market respondent claimed that the Parties had unique technical capabilities that no other market player would have. As a result, while the Parties may be close competitors, or even each other's closest competitors, these factors need to be taken into account, to relativise the importance of closeness of competition for the purposes of the competitive assessment.
(749) Significant customer overlaps, while potentially evidence of closeness of competition, are not required to establish such closeness. Regardless of whether both BASF and the Business are suppliers to specific customers, the fact that PA 6.6 EP customers regard them as each other's closest competitors, and that many of them have qualified both BASF and the Business as suppliers of PA 6.6 EP for the EEA, is evidence that the Parties are close competitors. An important number of PA 6.6 EP customers in the EEA have qualified both BASF and the Business and/or regularly source from both, evidencing the closeness of competition between the Parties. Such customers include […].576 For instance, while […] is listed as a top customer of the Business for PA 6.6 EP, the company identifies products of BASF as the closest competitors of products from the Business, and lists both Solvay and BASF as qualified suppliers. As such, BASF would likely be a particularly viable alternative to the Business, should […] decide to switch supplier for these specific grades.
(750) Regarding the customer survey submitted by the Notifying Party to evidence that BASF and the Business are not close competitors, and the supporting documentation, the Commission does not consider that the analysis of the Notifying Party's customer survey provides any evidence that BASF and Solvay are distant competitors for PA 6.6 EP in the EEA. In particular, the methodology used by the Notifying Party does not seem to measure the "diversion ratios" between BASF and its competitors. The question used by the Notifying Party in its customer survey577 relates to the identification of the customers' two main suppliers, apparently in terms of business size. It is usually difficult to extrapolate, from the replies to such question,
information on the diversion ratios between suppliers. Indeed, the fact that two given suppliers are rarely together the main and second main suppliers of some customers does not imply, in isolation, that these two suppliers are distant competitors. For example, it could indicate that, amongst rare but important contracts, these suppliers have been competing and only one has been selected.
(751) Moreover, the analysis submitted by the Notifying Party does not support the claim that […].578 Indeed, in contrast to what the Notifying Party claim, customers may not divert sales to the supplier identified in the customer survey as their second main supplier, after a price increase by BASF, for the reasons developed in recital (750).
(752) Furthermore, the survey covers ex-EEA customers of BASF for PA EP and PBT EP, and as such is not limited to either customers in the EEA or to customers purchasing PA 6.6 EP.579
(753) Therefore, the evidence put forward by the Notifying Party does not demonstrate that the parties are distant competitors for PA 6.6 EP in the EEA and does not allow the Commission to infer any conclusions as to the intensity of competition between suppliers.
(754) BASF and the Business are therefore close competitors on the market for PA 6.6 BP in the EEA, even if the importance of such closeness of competition should be relativised for the reasons laid out in in recital (748).
6.3.9.2.(B.iii) Alternative suppliers
(755) A majority of the responding customers believes that they would not have a sufficient choice of alternative suppliers of PA 6.6 EP if the Transaction took place.580 The reasons put forward by such customers include mainly the loss of one main supplier in the market, as well as the concentration in the upstream markets for inputs.581 On top of that, a majority of respondents indicate that they expect a price increase if the Transaction were to go ahead.582
(756) The fact that other players, identified in Response to the 6(1)(c) Decision,583 are active on the PA 6.6 EP market in the EEA, as pointed out by the Notifying Party, is not sufficient in itself to constrain the Merged Entity post-transaction.
(757) DowDuPont is overwhelmingly considered by respondents to the Phase II market investigation as a viable competitor on the PA 6.6 EP market for the EEA, with regard to its technical ability, existing production capacity, commercial ability, and R&D capabilities, which has a reputation that is acceptable for direct customers as well as their own customers (e.g. OEMs).584 As such, the company could represent a competitive force that would constrain the Merged Entity post-transaction. The competitive constraint exerted by DowDuPont is further evidenced by the fact that numerous grades offered by the company typically compete against BASF's and/or the Business' portfolio.585
(758) However, half of the respondents who expressed an opinion consider that DowDuPont does not offer customers the necessary security of supply for them to manage their supply chain, due to the company's limited vertical integration.586 While the potential input foreclosure that may affect DowDuPont is further discussed, in particular in Sections 6.3.3 and 6.3.4, the fact that customers value vertical integration (which DowDuPont is considered as lacking) is also relevant for the purposes of assessing the Transaction's horizontal non-coordinated effects.
(759) Respondents also overwhelmingly consider that DowDuPont's pricing strategy is not competitive (which some respondents link to its limited vertical integration).587 Considering the Transaction would only leave two large players on the PA 6.6 EP EEA market, including the Merged Entity and DowDuPont, the fact that DowDuPont is more expensive than its main competitors is an indication that prices may further increase post-transaction. This is in particular the case, considering BASF's high pricing reputation on the market, and the fact that customers expect as a result prices for the Merged Entity's products to increase post-transaction.588
(760) Radici is also largely considered by respondents to the Phase II market investigation as a viable competitor, with regard to its technical ability, existing production capacity, commercial ability, and R&D capabilities, which has an acceptable reputation to PA 6.6 EP customers.589 However, similarly as for DowDuPont, half of the respondents who expressed an opinion consider that Radici does not offer customers the necessary security of supply for them to manage their supply chain, due to its limited vertical integration, which implies a reliance on third parties for ADN, which would be even more problematic post-transaction.590 The potential input foreclosure that may affect Radici with regard to ADN is further discussed, in particular in Section 6.4.1.
(761) Ascend is also generally viewed as a viable supplier of PA 6.6 EP, based on its technical and R&D ability, production capacity, and reputation.591 However, the fact that it has limited compounding facilities in the EEA, and needs to import products from the US, affects the company's viability as a supplier of PA 6.6 EP to EEA customers. In particular, respondents consider the security of supply offered by Ascend is limited,592 either due to potential delivery delays risks,593 or the fact that they may prefer to supply other regions depending on foreign exchange movements,594 as well as the fact the company is prone to force majeure events.595 While force majeure is normally not a structural feature defining the competitive constraint exerted by a company, the fact that Ascend's main production site is located in Florida, i.e. in area subject to hurricane risks, increases the risk of supply disruptions.596
(762) Lanxess, EMS, and DSM are perceived by a majority of respondents as technically viable players in the PA 6.6 EP space in the EEA.597 A large majority of customers also consider the companies to have strong R&D capabilities, as well as an acceptable reputation to be suppliers of PA 6.6 EP.598 However, with regard to EMS, customers point out that the company does not supply standard PA 6.6 grades in large volumes that e.g. automotive customers may require.599 DSM is primarily viewed as a supplier of PA 6 EP, with a more recent/limited PA 6.6 EP presence by some market respondents.600
(763) Celanese is perceived by a smaller majority of respondents as a technically viable competitor, in particular due do its recent acquisitions of Softer and Nilit's compounding business.601 Again, a majority of customers also consider the company to have sufficient R&D capabilities, as well as an acceptable reputation to be a supplier of PA 6.6 EP.602
(764) Ravago is dismissed as a competitive supplier of PA 6.6 EP by most market respondents which provided information relating to the company.603 Ravago is a supplier for recycled PA 6.6 EP,604 and is also a distributor of PA 6.6 EP from other suppliers,605 but does not offer its own products. […] confirmed that, with regard to PA 6.6 EP "[they] currently don’t have the capacity on prime but [they] do recycle PA 6.6". As such, the company cannot be considered as a competitive alternative to BASF or the Business, of which […] is an important customer.606
(765) DOMO is also dismissed by a large share of market respondents as a valid alternative for various reasons. The company is primarily perceived as a supplier of PA 6 EP, and not as a valid competitor in PA 6.6 EP.607 The lack of competitive constraint that DOMO would exert over the Merged Entity is further evidenced by the fact that the Parties only list […] competing against BASF's or the Business' portfolio.608
(766) Importantly, a large majority of respondents consider that (non-integrated) suppliers of PA 6.6 EP do not offer sufficient guarantees in terms of security of supply, mainly because they rely on input provided by third parties including the Parties, which are also their competitors. Such dependence also can have an impact on the pricing, production capacity, and commercial ability of such suppliers to offer PA 6.6 EP to their customers according to the respondents. These statements apply to each of Celanese,609 Lanxess,610 611 Ravago,612 EMS,613 DSM,614 and Domo.615 The potential input foreclosure that may affect non-integrated PA 6.6 EP suppliers with regard to PA 6.6 BP is further discussed in Section 6.4.5.
(767) Furthermore, a vast majority of the customers of EP indicated that they would not be able to switch easily to other suppliers.616 Only a minority of customers indicated that they would be able to find alternative sources of PA 6.6 EP.617 This is further evidenced by the limited number of instances PA 6.6 EP customers indicated having replaced an existing supplier.618 The vendor due diligence report prepared for Solvay for the divestment of the Business also indicates that switching costs in EP can be significant; the report notes that, in EP, "[QUOTE FROM CONFIDENTIAL INTERNAL DOCUMENT]".619
(768) Customers typically qualify their suppliers of PA 6.6 EP.620 The qualification process is overall similar, involving first testing in the laboratory, then testing of the product itself and validation. Sometimes certificates issued by independent organisations are necessary as well.621 The whole certification process lasts on average one year per individual product. The approval criteria vary, but the technical competence of the supplier and his ability to deliver the product are crucial.622 Moreover, most customers approve the specific production site of their PA 6.6 EP supplier.623 Most customers qualify more than one supplier for the same PA 6.6 EP grade/type, mostly in order to maintain a steady and secure flow of supply and to mitigate risks related and delivery problems and better negotiate prices.624 Customers of PA 6.6 EP who responded to the Phase II market investigation have overwhelmingly qualified and/or purchased from both BASF and the Business in the EEA.625
(769) Direct customers of PA 6.6 EP (e.g. Tier 1 suppliers to OEMs) require approval from their own customers (e.g. OEMs) of their PA 6.6 EP supplier(s), which may render switching more complicated.626 Finally, almost none of the responding customers ever switched to a supplier which was not qualified by that customer.627 This implies that smaller compounders not qualified by a customer of the Parties would not be a viable alternative to replace BASF and/or the Business. Also, regardless of supply- side substitutability, PA 6.6 EP customers would typically not retain a manufacturer that did not offer the specific grade at the time of the award/tender as a supplier of PA 6.6 EP, even if it could develop such capability afterwards.628
(770) Therefore, on the basis of the market investigation, the Commission considers that there is a risk that PA 6.6 EP customers would not be able to find alternative sources of supply if the Merged Entity were to raise prices post-transaction.
6.3.9.2.(B.iv) Barriers to entry and expansion
(771) The market investigation confirms the Notifying Party's position that expanding production capacity in PA 6.6 EP is not excessively costly. For instance, […] estimates the cost of adding up approximately 20 kts of capacity to be around EUR 10-15 million.629 Entry into the market is different, as creating a full compounding unit is much more costly, and could reach around EUR 50-100 million.630
(772) However, both producers and customers of PA 6.6 EP confirm that the PA 6.6 EP market in the EEA is already short.631 Therefore, additional volumes of PA 6.6 EP are already required to meet the growing demand, notwithstanding any attempt by the Merged Entity to exert market power.
(773) When asked whether they would be able to increase their PA 6.6 EP production if there was an increase in demand, producers replied positively conditionally to the availability of upstream inputs.632
(774) Both producers and customers, while they expect demand to grow in the next 5 years, unanimously believe that no new producer of PA 6.6 EP will enter the market in the short term.633 Access to inputs is by far the main barrier to entry put forward.634
(775) Additionally, when asked whether they can supply sufficient volumes of PA 6.6. EP to meet the demands of large customers, producers of PA 6.6 EP replied negatively to a large extent.635 The underlying reasons include once again the unavailability of upstream inputs.636
(776) Barriers to entry and expansion are therefore important in the PA 6.6 EP market, particularly considering that access to input, which are in short supply and of which the Merged Entity would control a large share, is a key requirement to enter the market or expand an existing production base.
6.3.9.2.(B.v) Customer countervailing buyer power
(777) The Commission notes that, contrary to the Notifying Party's claim, the customer base of the Parties is not heavily concentrated. Indeed, in the EEA BASF's top 10 and top 30 PA 6.6 EP represent respectively around [30-40]% and [50-60]% of its total PA 6.6 EP sales in the EEA. BASF has over […] customers […], and over […] customers […].637 Similarly, the Business' top 30 PA 6.6 EP customers in the EEA represent around [70-80]% of its total PA 6.6 EP sales in the EEA. The Business has over 15 customers who purchased more than 1 kt of PA 6.6 EP in 2017 in the EEA, and also has over 50 customers which purchases exceeded EUR 1 million in 2017 in the EEA.638 These figures are not evidence of a strong concentration of customers, each of which would be indispensable to run the Merged Entity profitably.
(778) The Commission acknowledges however that end customers have a certain degree of bargaining power. Internal documents of the parties reveal for instance that when […].639
(779) However, while the Notifying Party indicates that […].640 This evidences that the extent to which PA 6.6 EP customers may exert countervailing buyer power is limited.
(780) Furthermore, an important number of PA 6.6 EP customers in the EEA have qualified both BASF and the Business and/or regularly source from the Parties, and would thus likely be particularly dependent on the Merged Entity post-transaction, in particular in case of price increases. Such customers include […].641
(781) The vendor due diligence report prepared for Solvay also indicates that the EP market is characterised by strong customer loyalty, reading for instance that "[QUOTE FROM CONFIDENTIAL INTERNAL DOCUMENT]", in particular in the automotive sector as "[QUOTE FROM CONFIDENTIAL INTERNAL DOCUMENT]".642
(782) While customers, and in particular OEMs, may indeed exert some degree of countervailing buyer power, it does not appear sufficient to counterbalance any attempt by the Merged Entity to increase prices or otherwise worsen supply conditions.
6.3.9.2.(B.vi) Competitive constraint of PA 6 EP (and other materials) as well as PA 6.6 EP from outside the EEA on PA 6.6 EP in the EEA
(783) The evidence presented by the Notifying Party643 further establishes that PA 6 and PA 6.6 can both be used in a number of the same applications. However, this does not imply that both are equally suitable for specific applications. As mentioned in Sections 6.6.2 and 6.8.2, customers that use PA 6.6 consider they are unable to switch to PA 6 in the short term and/or with limited financial investments. For applications requiring high temperature resistance or water absorption, only PA 6.6 is viewed as a viable option by customers, which are important features for the automotive and E&E sectors.
(784) As a result, the competitive constraint exerted by PA 6 EP over PA 6.6 EP for the applications for which PA 6.6 EP is typically used is limited.
(785) Furthermore, significant price differentials between PA 6 and PA 6.6 are evidence of the weak constraint exerted by PA 6 on the PA 6.6 market. PA 6.6 has consistently been more expensive than PA 6 in the EEA. PCI estimates the price differential between EP Grade PA 6 and PA 6.6 to amount to [30-40]% for 2016 and [20-30]% for 2017,644 while consumption of PA 6.6 EP increased between 2015 and 2017 in the EEA,645 which is evidence of limited impact of PA 6 which, even with a lower price fails to capture PA 6.6 EP demand (Figure 8).
[GRAPH SHOWING PRICE DIFFERENCE PA6.6 POLYMER VS. PA6 POLYMER]
Figure 8. Price difference PA66 polymer vs PA6 polymer Source: PCI
(786) The Phase II market investigation confirms the limited impact that PA 6 plays on PA 6.6 demand. Customers of PA 6.6 EP are mixed when asked whether the price and performance of other materials (besides PA 6.6 EP) impacts their commercial negotiations with PA 6.6 EP suppliers,646 which evidences the limited constraint exerted by other materials on PA 6.6 EP.
(787) Furthermore, the competitive constraint exerted by PA 6 EP or other materials on PA 6.6 EP is also typically limited primarily to the design / development stage of a product, and not to later stages of the project, such as production or commercialisation.647 The situation is different with regards to PA 6.6 EP, as customers may replace one supplier of PA 6.6 EP with another one throughout the lifecycle of the end product, even if such replacement generates additional costs, and have done so in the past. For instance, customers including […], […], […], or […] have displaced PA 6.6 EP suppliers in the last five years, replacing them by a competing provider of PA 6.6 EP.648
(788) Regarding the competitive constraint exerted by imports of PA 6.6 EP into the EEA, while such constraint exists, and is already reflected in the market share estimates, the Notifying Party overestimates the importance of such constraint exerted by imports of PA 6.6 EP.
(789) Ascend is the only player in the PA 6.6 EP market with significant imports of PA 6.6 EP into the EEA and a limited local production capacity. While customers overall consider Ascend as a viable competitor in the supply of PA 6.6 EP,649 its limited European footprint is perceived as a disadvantage on the market. Ascend's limited compounding capacity in the EEA has indeed been identified as a commercial disadvantage by various customers.650 The company itself seemingly acknowledges the need to have a local production, and recently purchased a compounder with capacity in the EEA, Britannia Techno Polymer to this effect. Ascend's CEO stated at the occasion that "[t]his acquisition provides [Ascend] with a sixth manufacturing location and a dedicated footprint for serving our European customers with world- class nylon 6,6 compounds and regional manufacturing expertise"651 (emphasis added).
(790) As per producers from Asia, they are seemingly largely absent from the European market to date.
(791) Responding customers to the Phase II market investigation have not qualified Asian suppliers of PA 6.6 EP for the EEA, with only very limited exceptions. Only one respondent to the market investigation, […], considers one Chinese supplier, namely Kingfa, as a potentially viable supplier of PA 6.6 EP in the EEA.652 LG Chem, Polyplastics, and Kolon Plastic, cited by the Notifying Party653 as suppliers that could supply EEA customers have not been cited by respondents.654
(792) The Notifying Party notes that some countries like Korea are exempted from import duties into the EEA, which potentially makes Korea-based companies a stronger constraint on European players.655 Based on PCI estimates, BASF and the Business are the largest producers of PA EP in Korea. Other producers mainly include local Korean players (Kolon, Kopla, Korea Engineering Plastics or Mando Advanced Materials) as well as DowDuPont. The Parties hold a combined share of [40-50]% of the total PA EP capacity in Korea,656 which is in line with the Merged Entity's EEA capacity share ([40-50]%), implying that the impact of imports from Korea into the EEA, should they increase, would likely benefit the Parties.
6.3.9.2.(C) Conclusion on non-coordinated effects: PA 6.6 EP
(793) Based on the Parties' market shares, the closeness of competition between BASF and the Business, the lack of viable alternative suppliers, and the barriers to entry and expansion mainly due to the lack of available input, the Commission concludes that the Transaction would significantly impede effective competition as a result of horizontal non-coordinated effects arising in the supply of PA 6.6 EP in the EEA.
6.3.10. Level V of the polyamide value chain: PA6 3D printing powders
6.3.10.1. Market structure
(794) The Notifying Party is unable to provide reliable market share estimates, it however estimate that the Parties' combined market share on the global market for printing powders does not exceed [0-5]%. The Notifying Party could not provide market share estimates for the narrower market encompassing PA 6 3D printing powders only.
(795) The market investigation carried out by the Commission univocally indicated that the Parties are the only suppliers of PA6 3D printing powder worldwide.
(796) Respondents to the market investigation listed only BASF and Solvay as suppliers of PA6 3D printing powder apart from Daimler who also indicated Ricoh and Prowdays. These companies are however using printing powders originating from Solvay and BASF respectively.
(797) The Notifying Party identified in the Form CO Toray, a Japanese company, as a producer of PA6 3D printing powders. This statement could not however be corroborated by the market investigation and is contradicted by an internal document of Solvay where it is stated that "A ma connaissance Toray ne commercialise pas de poudre de PA6 pour le SLS....mais du PPS que ASpect utilise by the way".657
(798) The Commission market investigation has sought to identify other possible suppliers of PA 6 3D printing powders. However, none of the responding customers of PA 6 3D printing powders named alternative suppliers other than the Parties. Additionally, no other responding potential supplier indicated they sell this product. Therefore, the Commission concludes that the Transaction results in a merger to monopoly on the market for PA6 3D printing powders.
(799) The Commission reconstructed the market shares on the PA 6 3D printing powder based on the sales of the Parties and on the conclusion that only the Parties are active on that market. On that basis, in 2017 in the EEA BASF had a market share of [40- 50]% while Solvay of [50-60]%. At a global level, BASF' share was of [10-20]% and Solvay's of [80-90]%.
6.3.10.2. Conclusion on non-coordinated effects: PA6 3D printing powders
(800) The Commission therefore concludes that the Transaction would significantly impede effective competition in a substantial part of the internal market in the global market for PA6 3D printing powders, in particular because the Merged Entity will have a monopoly on the market.
6.3.11. Conclusion on horizontal effects
(801) Based on the assessment in Section 6.3 and in view of the results of the market investigation and of all the evidence available to it, the Commission concludes that the Transaction would result in a significant impediment to effective competition as a result of horizontal non-coordinated effects arising in the supply of ADN, HMD, adipic acid, AH Salt, PA 6.6 BP, and PA 6.6 EP in the EEA and of PA6 3D printing powders globally.
6.4. Vertical non-coordinated effects
6.4.1. Introduction
(802) The transaction leads to a number of vertically affected markets in relation to the production of Ammonia, ADN, nitric acid, KA Oil, HMD, adipic acid, Caprolactam, AH Salt and PA 6.6 BP. The vertically affected markets are discussed in the present section.
(803) The Commission will not analyse customer foreclosure effects of this Transaction for HMD, HDI, AH Salt, PA 6.6 BP or PA 6.6 EP for the following reasons: first, there are no indications of customer foreclosure with reference to the relevant evidence; second, customers have difficulties to secure enough volumes due to the tightness of supply of all these markets and therefore the problem is precisely the lack of enough volumes to purchase in the merchant market and no the absence of customer to sell the products; third, BASF and the Business did not buy important quantities of these products in the merchant market (except BASF purchasing ADN in order to produce HMD); fourth, although […]; finally, none of the respondents to the extensive market investigation has raised customer foreclosure as a potential negative effect of the Transaction.
6.4.2. Level 0 of the polyamide value chain: Ammonia
6.4.2.1. Ammonia: input foreclosure to nitric acid producers.
(804) Ammonia is a feedstock to nitric acid. On the upstream market for Ammonia, only BASF is active. BASF's market share is limited, irrespective of the market definition retained. Such share exceeds [30-40]% only under the narrower plausible product and geographic market definition, that is to say on the market for anhydrous ammonia for industrial uses in North Western Europe in which BASF’s market share is estimated at [30-40]%. If only merchant sales are considered, BASF share is estimated at [10-20]%.
(805) In light of the circumstances set out in recitals (806) to (808), the Commission concludes that the Merged Entity will not have the ability to foreclose its downstream competitors from accessing ammonia.
(806) First, the upstream market is rather competitive. The Notifying Party has identified a number of competitors with sizeable market shares, such as Yara, INEOS, OCI, SKW. Respondents to the market investigation also identified additional competitors, such as Borealis, Fertiberia and Eurochem, further supporting the finding of a competitive market.658
(807) […], to which downstream competitors of the Parties could have access.
(808) Third, if only the merchant market is considered (i.e. once discounted ammonia manufactured exclusively for internal use), the position of BASF is rather limited, at most [10-20]%, and other major players, such as Yara, INEOS and OCI, have comparable or stronger presence on the merchant market. These companies, in fact, seem to be more focused on the merchant market than BASF.
(809) The Commission therefore concludes that the Transaction would not significantly impede effective competition as a result of vertical non-coordinated effects arising from vertical links between the upstream EEA market for ammonia and the downstream EEA market for nitric acid, irrespective of the market definition ultimately retained.
6.4.3. Level I of the polyamide value chain: ADN
6.4.3.1. ADN: customer foreclosure to Butadiene producers659
(810) In the EEA only ADN C4 is produced, and only by Butachimie. Butadiene is a feedstock to ADN C4, and BASF manufactures it.
(811) The Commission concludes that the vertical relationship between the upstream EEA market for Butadiene and the downstream market for ADN C4 leaves effective competition unaffected.
(812) First, BASF has a […] market share in the upstream market for butadiene. In 2017, BASF had a market share amounting to [5-10]% in volume and [0-5]% in value of merchant sales at global level, and [10-20]% in volume and [5-10]% in value of merchant sales at EEA level. If a narrow geographic market definition encompassing only Western Europe is retained, BASF's estimated market share would be of [10- 20]% of merchant sales both in volume and in value.
(813) Second, the Business is a minor purchaser of butadiene through Butachimie. Its requirements for 2016 represented less than [10-20]% of total demand on the merchant market in the EEA and less than [5-10]% globally.
(814) Third, the Commission observes that butadiene is used in a wide variety of synthetic rubbers and polymer resins as well as a few chemical intermediates. According to public sources, the largest single use for butadiene is in the production of styrene butadiene rubber ("SBR"), which is principally used in the manufacture of automobile tyres. SBR is also used in adhesives, sealants, coatings and rubber articles such as shoe soles. Butadiene is one of the components used in the manufacture of acrylonitrile-butadiene-styrene ("ABS"), which is the largest-volume engineering thermoplastic resin. Other polymers made from butadiene include styrene-butadiene ("SB") copolymer latex, which is used in paper coatings, carpet back coatings, foam mattresses and adhesives. SB block copolymers have many applications ranging from asphalt modifiers in road and roofing construction to adhesives, footwear and toys.660
(815) The polyamide value chain absorbed only [0-10]% of the 2016 global production of butadiene. In Western Europe, that proportion increases but remains low, at [10- 20]%, whereas there is no recorded sales of butadiene to the polyamide value chain in Central and Eastern Europe.661
(816) Based on the assessment in recitals 810 to 815 the Commission considers that, even if Butachimie was to source its butadiene need exclusively from BASF post- transaction, upstream competitors would still have access to a significant number of alternative customers, albeit active in markets other than the ADN market. The Commission therefore concludes that the Transaction would not significantly impede effective competition as a result of vertical non-coordinated effects arising from vertical links between the upstream market for Butadiene in the EEA and the downstream market for ADN C4 in the EEA.
6.4.3.2. ADN: customer foreclosure to producers of Ammonia.662
(817) Ammonia is, as butadiene, a feedstock for the production of ADN C4. As BASF manufactures Ammonia, the Transaction gives rise to a vertically affected relation.
(818) The Commission concludes that the vertical relationship between the upstream EEA market for anhydrous ammonia and the downstream EEA market for ADN C4 leaves all effective competition unaffected.
(819) First, (anhydrous) ammonia is an input for a wide range of other products for many other customers. Anhydrous ammonia for industrial applications is used for the manufacturing of industrial urea or nitric acid, or as an input material for isocyanates and polyamides, amines and others. More precisely, according to the Notifying Party, ADN comes up for approximately [5-10]% of the consumption of anhydrous ammonia in the EEA.
(820) Second, The Business’ requirements in 2016 represented a minor proportion of the total anhydrous ammonia merchant demand, estimated at less than [0-5]%.
(821) Based on the assessment in recitals 817 to 820, the Commission therefore considers that, even if Butachimie was to source its anhydrous ammonia needs exclusively from BASF post-transaction, upstream competitors would still have access to a significant number of alternative customers, albeit active in markets other than the ADN market. Therefore, the Commission concludes that the Transaction would not significantly impede effective competition as a result of vertical non-coordinated effects arising from vertical links between the upstream market for anhydrous ammonia in the EEA and the downstream market for ADN C4 in the EEA.
6.4.3.3. ADN: input foreclosure to HMD producers
(822) ADN is used essentially for the production of HMD at level II of the polyamide value chain. The Transaction therefore generates a vertically affected relation between ADN and HMD.
(823) In assessing the likelihood of an anticompetitive input foreclosure strategy, the Commission has to examine whether (i) the Merged Entity would have the ability to substantially foreclose access to inputs, (ii) whether it would have the incentive to do so and (iii) whether a foreclosure strategy would have a significant detrimental on competition downstream. Each one of these points will be analysed separately although the Commission recognises that they are closely intertwined.663
6.4.3.4. Ability to foreclose
6.4.4.1.(A) The Notifying Party's view
(824) In the Form CO, the Notifying Party submitted that the Merged Entity, lacking any degree of market power on the market for ADN, would lack the ability to foreclose access to ADN.
(825) In the Form CO, The Notifying Party further claimed that the Transaction will not in any way affect the Merged Entity ability to foreclose downstream competitors as it will not result in a change of the structure of the upstream ADN market nor in any addition of capacity shares. This was further restated in the Response to the 6(1)(c) Decision.
(826) In the Form CO, the Notifying Party claimed that the Merged Entity would continue to sell ADN on the merchant market and the volumes of ADN available on the merchant market would remain unchanged. As already explained in Section 6.2.1.2(a), the Notifying Party contends that the Transaction merely results in the substitution of Solvay with BASF as a shareholder of Butachimie. The Notifying Party further submits that even […], the Transaction would not have an impact on the Parties' availability or requirements of ADN. In the Response to the 6(1)(c) Decision the Notifying Party claims that the Merged Entity will not have the need to divert any ADN because of the combination of BASF and the Business, […] rather than by the Business' share in Butachimie.
(827) In the Form CO, the Notifying Party also claimed that should the Merged Entity withdraw capacities from the merchant market, Invista would have sufficient capacity to supply to the Business' customers. Also, as Invista does not have enough HMD production capacity to process all the ADN it manufactures, it would have the incentive to continue selling on the merchant market without increasing prices.
(828) Finally, the Notifying Party claimed that […] Invista has sufficient spare capacity to cater for all of […] ADN needs.
(829) In its Response to the 6(1)(c) Decision, the Notifying Party claims that the Transaction will have no effect on the upstream market for ADN, and therefore that there is no risk of foreclosure, neither inadvertent nor strategic. The Notifying Party further specifies that the ADN volumes required by BASF will be met by the ADN supply agreement with Invista while the Business' ADN requirements will continue to be met by its share in Butachimie, while any spare capacity made available by Solvay on the merchant market will be rapidly absorbed by the growth of the Business' captive ADN demand.
(830) The Notifying Party therefore claims that the concerns raised by the Commission in the 6(1)(c) Decision are not substantiated.
(831) The Notifying Party further claims that the Business is already vertically-integrated, selling only limited volumes of ADN on the merchant market. The Notifying Party contends that the concerns raised by the Commission in the 6(1)(c) Decision stems from the fact that BASF will stop supplying volumes to the merchant market, which is a legitimate business decision. According to the Notifying Party, as the structure of the ADN market will not be affected, the Commission cannot challenge such a business decision.
6.4.4.1.(B) The Commission's assessment
(832) In the 6(1)(c) Decision, the Commission took the preliminary view that the Merged Entity would have the ability to foreclose access to ADN to its downstream competitors, as explained in recitals (833) to (841).
(833) First, the Merged Entity would have a significant degree of market power in the upstream market. The high shares in terms of total production and capacity clearly indicate market power, and the indications of the market investigation corroborate this conclusion.
(834) The Commission took the view that Transaction does not result in the mere change of ownership over 50% of Butachimie, leaving the structure of the market unchanged. The Commission maintains this view and, as explained in Section 6.2.1.2.(b), considers that as a direct result of the Transaction BASF would gain control on additional ADN volumes from Invista, and therefore the percentage of capacity share it would control would be of [60-70]%.664 Hence, the Merged Entity would be the largest player on the upstream market.
(835) Second, the Commission took the preliminarily view that Merged Entity would have sufficient HMD production capacity to fully internalise its ADN production, as shown by Table 24 submitted by the Notifying Party. That table shows that, if regarded in isolation, BASF has a capacity of […] kts of HMD at level II. In order to produce those volumes, BASF needs […] kts ADN, which is more than what the control of [60-70]% grants to it.
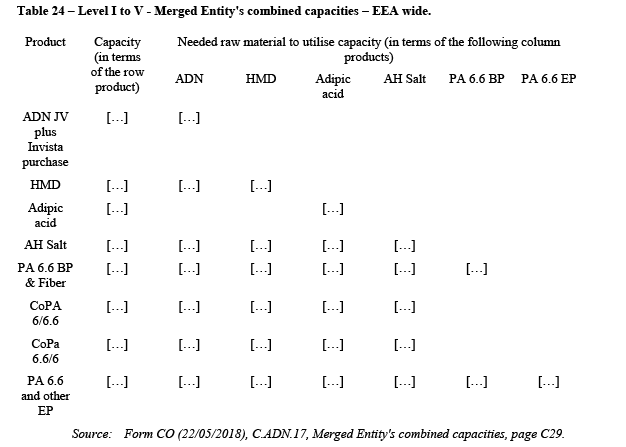
(836) Also, in the 6(1)(c) Decision the Commission preliminarily concluded that ADN is the essential input in the polyamide value chain, both technically and in term of costs and availability. As the Phase I market investigation clearly indicated that switching to ADN C3 as input for the production of HMD is not a credible and viable solution, in particular in the EEA, the Commission preliminarily concluded that the Merged Entity will have the ability to foreclose downstream competitors access to a key input.
(837) Moreover, the Commission preliminarily concluded that the Transaction would directly result in customers losing the only alternative supplier to Invista and therefore a significant degree of buyer power. Customers would therefore no longer be able to defeat, totally or partially, price increases imposed by Invista. This was confirmed by the Phase I market investigation. For instance, a respondent to the market investigation indicated that "it is vital to have at least two suppliers in order to be able to negotiate acceptable prices, which already today are very high".665
(838) As regards the Notifying Party's argument in the Form CO that Invista would have sufficient ADN capacity to supply the market post-transaction, the Commission took the view in the 6(1)(c) Decision that this may not be entirely sufficient and that this issue would be further investigated in Phase II. The investigation in Phase II supported that Notifying Party's argument. […] indicated that "ADN will be used in EU to fully satisfy the HMD needs in the region as the first priority",666 and that all the excess volumes will be devoted to the export market.
(839) The Commission argued in the 6(1)(c) Decision that, even if Invista had sufficient volumes of ADN C4 to serve the market, a refusal by the Merged Entity to supply ADN C4 to Radici would directly result in a worsening of the conditions at which Radici would be able to source ADN in the future.
(840) The Commission notes that for input foreclosure to materialise, "it is sufficient that the rivals are disadvantaged and consequently led to compete less effectively".667 Similarly to the assessment of the horizontal effects in Section 6.3, the fact that the plausible price increase for downstream competitors will be carried out by a third party, Invista, has no impact on a possible finding of a significant impediment of effective competition, as explained by the Guidelines on the assessment of non- horizontal mergers: "such foreclosure is regarded as anti-competitive where the merging companies – and, possibly, some of its competitors as well – are as a result able to profitably increase the price charged to consumers".668
(841) All of the conclusions set out in recitals (832) to (840) remain relevant and valid after the market investigation in Phase II. Moreover, with regard to the additional arguments put forward by the Notifying Party in its Response to the 6(1)(c) Decision, the Commission explains in Section 6.2.1.2(B) that it considers that the Transaction brings about a significant change in the structure of the upstream ADN market.
(842) Further to that , the Commission observes that the Notifying Party's claim that BASF demand will be met by […] is questionable as between […] and […]BASF consistently purchased volumes […], and BASF needs are expected to increase further in the future in line with the market's growth. This statement contradicts the Notifying Party's statements in the form CO as well: at paragraph C.10, referring to the […], in fact it is stated that: "[…]". This means that, as already explained in Section 6.3.1(B) concerning the assessment of the horizontal non-coordinated effects, the Merged Entity will likely divert ADN volumes sold to the merchant market to internal use, thus lessening the competitive constraint faced by Invista. In the Commission’s assessment, this will inevitably result in an increase in ADN prices, which will lead Radici to compete less effectively.
(843) The Commission noted, in Section 6.3.2.2.(B.vi), that BASF is evaluating […].
(844) The Commission considers that at this point it is not possible to understand whether capacity of HMD controlled by BASF will remain the same or increase […]. However, irrespective of the outcome, BASF's ADN requirement will remain the same or increase. The Commission therefore considers that […], irrespective of the outcome, would further strengthen BASF's ability to foreclose.
(845) The Commission has also already explained in Section 6.2.1.2(B) why it considers that, contrary to the Notifying Party's allegation in the Response to the 6(1)(c) Decision, the additional ADN volume available to the Business, including those brought about by the Retrofit Project, will be absorbed by the Business own demand in the near future.
(846) The Commission further observes that the approach of the Notifying Party is methodologically flawed as, while the Notifying Party claims that BASF's growth is capped by the BP production capacity,669 it does not do so with respect to the Business' growth expectations despite the fact that it ran at [80-90]% capacity utilisation in 2017.670 In this respect, the Commission understands that in order for the Business to absorb the additional ADN volumes brought about by the Retrofit project, […].
6.4.4.1.(C) Conclusion on ability to foreclose
(847) The Commission therefore concludes that the Merged Entity would have the ability to foreclose access to ADN to downstream HMD competitors.
6.4.3.5. Incentive to foreclose
6.4.4.2.(A) The Notifying Party's view
(848) In the Form CO, The Notifying Party submitted that, even if the Merged Entity had the ability to foreclose customers (i.e. […]), access to ADN, it would not have the incentive to do so. According to the Notifying Party this is because:
(a) First, even if the Merged Entity refuses to sell ADN to […], […] would still be able to either source ADN from Invista and from non-EEA market players. The Merged Entity would therefore lose sales without gaining shares downstream;
(b) Second, there are overcapacities at various levels of the value chain, and the Merged Entity’s downstream competitors would always be able to source other midstream products instead of ADN (such as HMD, AH Salt or PA 6.6 BP) from other market players. Ultimately, the Merged Entity would thus not be able to force […] (or […] customers) out of the BP/EP markets, or to make them lose BP/EP market shares;
(c) Third, the Merged Entity would have no incentive to foreclose ADN to HMD- producing customers also because the Business’ sales share in the HMD market is moderate (less than [20-30]% in 2016 in the EEA) and there is effectively no increment in this share brought about by the Transaction, since BASF’s sales in this market are only small and opportunistic;
(d) Fourth the difference in margins between ADN and HMD is small. This implies that the “critical diversion ratio” in HMD is large, and the Merged Entity would need to recapture four fifth of each unit of HMD diverted from its potentially foreclosed rivals for it to have an incentive to foreclose input.
(849) In the Response to the 6(1)(c) Decision, the Notifying Party claims that – as the Transaction does not bring about any change in the structure of the ADN market – the ability and, relevant for this section, incentive to foreclose will remain unchanged and any concern raised in the 6(1)(c) Decision to that effect is not merger specific.
6.4.4.2.(B) The Commission's assessment
(850) In the 6(1)(c) Decision, the Commission preliminarily concluded that, contrary to the Notifying Party' claims, the Merged Entity would have the incentive to foreclose access to ADN.
(851) First, as indicated in table 24, the Merged Entity would need […]. The Merged Entity's incentives to make volumes of ADN available on the merchant market are therefore significantly hindered.
(852) Second, the effects and incentives of a foreclosure strategy are to be analysed globally in the polyamide value chain and not merely focused in the current customers of the Parties. Were the Merged Entity to reduce the volumes of ADN in the merchant market, prices would increase for all customers and availability would be reduced, making it difficult for customers to secure the volumes needed to compete in downstream markets. Therefore, the Merged Entity would benefit from a less competitive landscape not only for HMD but also for all products downstream of HMD. Hence, the Merged Entity would be able to compensate for any lost sales not only on the HMD market, but also on all markets along the value chain, therefore strengthening its incentives to foreclose.
(853) Third, even if the difference in margins between ADN and HMD is small according to the Notifying Party, the margins between ADN and the products sold in the other markets further downstream, preliminary identified as AH Salt, PA 6.6 BP, co- polyamide, PA 6.6 EP in the polyamide value chain and as HDI derivatives in the HDI value chain, can be large. Therefore, the corresponding “critical diversion ratios” are lower than for HMD, likely providing the Merged Entity with the incentives to foreclose competitors. Moreover, the Notifying Party's analysis of the "critical diversion ratio" in HMD underestimates the incentives to foreclose, because it does not take into account the price increases in HMD which would result from the strong position of the Merged Entity.
(854) Finally, the Commission takes the view that the planned increase in capacity discussed in recitals (843) to (844) and in section 6.3.2.2(B.vi) will further increase BASF's incentive to foreclose downstream competitors access to ADN. If the HMD capacity increase will materialise, BASF will have the incentive to devote all the necessary ADN to run that capacity efficiently, further depriving the market of ADN volumes.
(855) Internal documents of BASF also support the preliminary findings of the Commission […]671. In an internal presentation […].672
(856) The conclusions set out in recitals (850) to (855) remain valid after the Phase II market investigation. With regards to the further arguments put forward by the Notifying Party in its Response to the 6(1)(c) Decision, the Commission explains in section 6.2.1.2(B) that it considers that the Transaction brings about a significant change in the structure of the upstream ADN market.. As regards the alleged lack of increase of incentive to foreclose, the Commission concludes, that the increased position of the Merged Entity on the various downstream markets further increases the incentive to foreclose access to ADN.
6.4.4.2.(C) Conclusion on incentives to foreclose
(857) The Commission therefore concludes that the Merged Entity would have the incentive to foreclose access to ADN to downstream HMD competitors.
6.4.3.6. Effects of a potential foreclosure strategy with respect to the supply of ADN 6.4.4.3.(A) The Notifying Party's view
(858) In the Form CO, the Notifying Party claimed that the Merged Entity does not have the ability or the incentive to foreclose. However, should a foreclosure strategy be pursued it would have no effects on competition for the following reasons:
(a) The Business [REFERENCE TO THE BUSINESS’ COMMERCIAL STRATEGY]. Hence, a hypothetical foreclosure strategy would not entail any change of the current supply conditions;
(b) The Business [REFERENCE TO THE BUSINESS’ COMMERCIAL STRATEGY]. Therefore, irrespective of the Merged Entity’s strategy, […] would be guaranteed to receive ADN for another year;
(c) […] could also switch to C3 ADN. The HMD produced would be identical and […] would be able to continue to effectively compete on the downstream market; and,
(d) Radici only has a merchant market share of [0-5]% on the EEA market for HMD. Therefore, it does not exert any competitive role. Its foreclosure would not lead to a lessening of competition on the downstream HMD market. In particular, the Merged Entity would still face competition from the largest player: Invista.
(859) The Notifying Party has not presented any additional arguments in its Response to the 6(1)(c) Decision.
6.4.4.3.(B) The Commission's assessment
(860) In the 6(1)(c) Decision, the Commission preliminarily concluded that, should a foreclosure strategy be pursued, it is likely to have a significant effect on competition.
(861) First, Solvay states that it does not supply […] with ADN "because [REFERENCE TO THE BUSINESS’ COMMERCIAL STRATEGY]".673 While it may not supply ADN to Radici on a structural basis, Solvay may have supplied ADN to Radici in the past and could do so in the future. Should the Merged Entity engage in a foreclosure strategy, the reduction of suppliers for Radici would be structural rather than
occasional. […]. Thus the impact on competition would only be deferred, not excluded.
(862) Second, as already explained in section 5.2.2.3, a switch to ADN C3 does not, at this stage of the proceedings, appear possible. Therefore, if the Merged Entity would foreclose access to its ADN C4, there would be no alternative input for HMD producers active in the EEA. However, even if this was possible, the additional costs linked to importing ADN C3 in the EEA would be material and would therefore significantly deteriorating the competitiveness of the downstream players.
(863) Finally, Radici is the only HMD producer in the EEA non-integrated into ADN. Would the Merged Entity foreclose access to ADN, it would create a duopoly on the HMD market, and possibly a monopoly should it elect to internalise HMD production as well. As indicated by […] "Invista will have the power to control […] production because post-transaction they will be the only available supplier in the market and because of that […] would have two potential problems: 1) not be able to increase production if Invista denies an increase of the ADN volumes supplied, and 2) not able to negotiate acceptable price condition, because of lack of alternative suppliers. As a consequence […] risks that it won't be able to grow and it won’t be able to compete at same conditions of integrated players".674
(864) Fourth, albeit representing only [10-20]% of the merchant market, the ADN sold by the Business is the only alternative to the ADN sold by Invista. Hence, the competitive relevance of those volumes is higher than what suggested by the market share. In fact, in the Commission's view, they represent the only credible threat to counter a price increase imposed by Invista. In that sense, withdrawing them from the merchant market would significantly increase the costs of Merged Entity's downstream rivals.
(865) All of the above remains valid after the market investigation in Phase II.
6.4.4.3.(C) Conclusion on effect on competition
(866) The Commission therefore concludes that, were the Merged Entity to pursue an input foreclosure strategy of ADN C4, there would be significant negative effects on competition in the polyamide value chain.
6.4.3.7. Conclusion
(867) Based on the assessment in recitals 824 to 866 and in light of the results of the market investigation and of all the evidence available to it, the Commission concludes that the Transaction would significantly impede effective competition as a result of vertical non- coordinated effects arising from vertical links between the upstream EEA marker for ADN C4 and the downstream EEA market for HMD.
6.4.4. Level I of the polyamide value chain: Nitric acid
6.4.4.1. Nitric acid: customer foreclosure to Ammonia producers.
(868) The Commission takes the view that the Merged Entity will not have the ability to engage in customer foreclosure.
(869) First, ammonia is not used exclusively in the polyamide value chain, rather it used in a number of industrial applications, such as for the manufacturing of industrial urea or nitric acid, or as an input material for isocyanates and polyamides, amines and others, and as an input for the fertilisers industry. Even if the Merged Entity would internalise the sourcing of ammonia for its adipic acid-related production of nitric acid, upstream suppliers of ammonia will still have a sufficient number of alternative customers in the fertiliser industry.
(870) Second, ammonia is used as an input material in a number of industrial application and not only for the production of nitric acid. Hence, if the Merged Entity would internalise the sourcing of ammonia for its adipic acid-related production of nitric acid, upstream suppliers of ammonia will still have a sufficient number of alternative customers in other industrial applications.
(871) Finally, none of the respondents to the market investigation indicated that they expect an adverse impact on competition arising from the Transaction675.
(872) Hence, the Commission concludes that the Merged Entity will not have the ability to foreclose its upstream competitors from accessing a sufficient customer base for Ammonia.
(873) Based on the assessment in recitals (868) to (872), the Commission concludes that the Transaction would not significantly impede effective competition as a result of vertical non-coordinated effects arising from vertical links between the upstream EEA market for ammonia and the downstream EEA market for nitric acid, irrespective of the market definition ultimately retained.
6.4.4.2. Nitric acid: input foreclosure to adipic acid producers
(874) The Commission concludes that the vertical relationship leaves all effective competition unaffected.
(875) First, under the narrower product and geographic market definition, the combined market share of the Parties on the upstream market is just above [30-40]%, and the Transaction leaves the structure of the upstream market is largely unaffected, with an increment of [0-5]% brought about by the Business and on the upstream market, irrespective of the geography, a number of credible players will continue to be active.676
(876) Second, the vast majority of nitric acid customers responding to the market investigation indicated that they will continue to have a sufficient number of alternative suppliers of nitric acid post-transaction.677
(877) The Commission therefore concludes that the Merged Entity will not have the ability to foreclose adipic acid producers from accessing nitric acid.
(878) Based on the assessment in recitals (874) to (877), the Commission concludes that the Transaction would not significantly impede effective competition as a result of vertical non-coordinated effects arising from vertical links between the upstream market for nitric acid, irrespective of the exact market definition, and the downstream EEA market for adipic acid.
6.4.5. Level II of the polyamide value chain: KA Oil
6.4.5.1. KA Oil: input foreclosure to adipic acid producers
(879) The Commission concludes that the vertical relationship arising from the activities of BASF and the Business in the production of KA Oil and Adicpid Acid leaves all effective competition unaffected.
(880) First, as explained in Section 6.3.2, the combined market shares of the Parties in 2017 in the EEA for KA Oil for adipic acid production is below [0-5]%. In 2016, […].
(881) Second, the merchant market accounts for less than [5-10]% of the total production in the EEA. All adipic acid producers in the EEA (BASF, Solvay, Lanxess and Radici) are integrated into KA Oil production. Only one of the adipic acid producers need to source additional volumes on the merchant market to complement its internal production and does not source from the Parties. In fact, none of the adipic acid producers or KA Oil customers raised input foreclosure concerns.678
(882) The Commission therefore concludes that the Transaction will not grant the Merged Entity any ability to engage in input foreclosure.
6.4.5.2. KA Oil: input foreclosure to cyclohexanone and caprolactam producers
(883) KA Oil can be distilled into Cyclohexanone which is used in the production of Caprolactam. BASF is active on all these markets.
(884) The Business does not produce or sell Cyclohexanone or Caprolactam.
(885) As explained in section 6.3.2, the combined market shares of the Parties in 2017 for merchant sales in the EEA for KA Oil is below [0-5]%. Neither Party has sales of KA Oil to competing producers of Cyclohexanone. Therefore, the Merged Entity will have no ability to foreclose access to KA Oil.
(886) The EEA market share of BASF in 2017 based on merchant sales for cyclohexanone is below [0-5]%, with Versalis being the market leader ([60-70]%), followed by Fibrant ([10-20]%), CHP ([10-20]%) and Domo ([5-10]%).679 Therefore, the Merged Entity will have no incentive to foreclose access to KA Oil to Cyclohexanone producers and will not have the ability to foreclose access to Cyclohexanone to Caprolactam producers.680
(887) The Commission therefore concludes that the Transaction will not grant the Merged Entity any ability to engage in input foreclosure behaviours.
6.4.6. Level II of the polyamide value chain: HMD input foreclosure
(888) A very large number of customers have expressed concerns that the Merged Entity could choose not to sell HMD in the merchant market or do it on less favourable terms and thus deny them access to an important input for their business. Some of these customers further claim that HMD sourced from the Parties account for a significant proportion of their needs and that they are therefore dependent on being able to buy from the Parties because there are no alternatives to find the extra
volumes required.681
6.4.6.1. Ability to foreclose access to HMD in the polyamide value chain and HDI producers
6.4.7.1.(A) The Notifying Party's view
(889) The Notifying Party is of the opinion that the Merged Entity would have no ability to foreclose HMD (level II) to customers producing AH Salt (level III) or HDI for the following reasons:
(890) First, the Notifying Party claims that the Business’ sales share in the HMD market is moderate and there is effectively no increment in this share brought about by the Transaction, because BASF’s sales in this market are […]. Further, in the Response to the 6(1)(c) Decision, the Notifying Party submits that looking at production or capacity shares to analyse vertical relationships is inappropriate. According to the Notifying Party, the Parties’ shares in terms of total production and capacity are thus irrelevant since, even absent the Transaction, the volumes used captively are by definition not available to the merchant market.
(891) Second, none of the Business’ HMD customers is active in the AH Salt market and only one of them is active in further downstream markets where an overlap with the Merged Entity may arise, in particular in the market for PA 6.6 EP. This is […], who in 2016 bought about […] kts of HMD from the Business, corresponding to about a half of its total requirements. The Transaction would thus not affect the overall availability of HMD for AH Salt producers: as the Parties already sell only limited volumes of HMD, if they stop selling these volumes, the potential reduction in the HMD volumes available on the market would be very limited.
(892) Furthermore, in the Response to the 6(1)(c) Decision, the Notifying Party submits that only DowDuPont and Nilit purchase HMD on the merchant market for use in PA 6.6-based products that are sold in the EEA. The Parties pre-transaction and the Merged Entity post-transaction are not, and would not be able to have a material impact on the competitive conditions including most importantly the prices that the said sole EEA polyamide customers (i.e. DowDuPont and Nilit) achieve for HMD – irrespective of whether they decide to withdraw from the merchant market. They thus have no ability to foreclose downstream polyamide players by raising rivals’ costs by withdrawing from supplying these polyamide manufacturers, and thereby weaken them as competitors on the downstream markets. To support this argument, the Notifying Party submits that while the Parties’ combined HMD merchant market sales to polyamide manufacturers in the EEA was just […] kts in 2016, Invista supplied over ten times those volumes ([…]kts) to polyamide manufacturers during the same year in the EEA. Even absent the Proposed Transaction, neither BASF nor the Business would be able to meet the HMD requirement of DowDuPont and Nilit, which amount to about […] kts per year and mainly purchase from other suppliers.
(893) Third, the Notifying Party considers that AH Salt producers could always purchase HMD from other suppliers like Radici and especially Invista who is the main HMD supplier with a global market share of [60-70]% and a [60-70]% share in the EEA. Equally, HMD could be imported from the USA, where Invista has additional capacities which it could use to supply the EEA market. Furthermore, in the Response to the 6(1)(c) Decision, the Notifying Party submits that even if the Merged Entity decided to stop supplying Nilit or other customers, Invista would have sufficient capacity to meet the entirety of Nilit's HMD requirements. Invista would also have enough capacity (globally) to serve the other HDI-producing HMD customers of the Business. Moreover, Invista will be incentivised to supply Nilit because their activities do not overlap in the downstream markets and since customers would not be able to pass price increases on, Invista will supply them at competitive terms. The Notifying Party also submits that the Merged Entity only supplies marginal quantities to DowDuPont, which purchases most of its required HMD from Invista. In addition, the Notifying Party claims that DowDuPont […] could continue sourcing its HMD needs on competitive terms.
(894) Fourth, the Notifying Party also submits that if, in turn, Invista attempted to exploit the Merged Entity’s reduction in supplies by demanding a higher price, customers could turn in particular to Ascend. According to the Notifying Party, the HMD exported from […] by […] to […] has increased by […] since 2015 and price of HMD exported to Israel is similar to the price of HMD in the EEA. However, it also recognises that the volumes of HMD exported by Ascend in the EEA are still marginal.
(895) Fifth, the Notifying Party considers that the HMD market is characterised by over- capacity. There is thus enough capacity to replace the supplies of the Merged Entity. Any foreclosure strategy would be unsuccessful as other producers would be able to increase their production rate. The global utilisation rate is approximately [70-80]% with important overcapacities in the USA and China.
(896) Sixth, the Notifying Party argues that the possibility for customers to source PA 6.6 BP instead of or sourcing HMD and adipic acid also prevents any input foreclosure strategy from being successful.
(897) Seventh, regarding the HDI value chain, the Notifying Party submits that there are two HDI-producing customers in the HMD market in the EEA, and these are […] and […]. In the Business’s estimation, […] is already supplying about four fifths of […]’s HMD requirements and about one quarter of that of […]. […] has a long-term contract with the Business for the supply of HMD that runs until the end of […] and includes a tacit renewal period of […] years, which would extend the contract until the end of […]. This contractual commitment would prevent the Merged Entity from attempting to foreclose […]. In the Response to the 6(1)(c) Decision, the Notifying Party submits that the Business also has a contractual obligation to supply HMD to […] in force until […].
(898) Finally, with regard also to the HDI value chain, the Notifying Party submits in the Response to the 6(1)(c) Decision that imports exert a strong competitive constraint in the EEA. According to the Notifying Party, this would be true for HMD but also for HDI Derivatives.
6.4.7.1.(B) The Commission's assessment
(899) First, as explained in section 6.3.2 the combined market shares of the Parties are very high682 and the Merged Entity would control approximately two thirds of the HMD production in the EEA. Those market shares indicate a presumption of dominance which is supported by market participants that claim that the Merged Entity would have a dominant position in HMD. The vast majority of customers consider that BASF and Solvay are the top 2 producers of HMD in the EEA.683
(900) The high shares in terms of total production and capacity clearly indicate market power of the Merged Entity which is in fact supported by the views of the large majority of the respondents to the market investigation.
(901) The Notifying Party claims in its Response to the 6(1)(c) Decision that taking into consideration merchant sales plus captive use and capacity shares is inadequate for the vertical assessment. However, as explained in section 6.2, the Commission consider that captive use and capacity shares are also relevant metrics to assess the market power of the Merged Entity. Indeed, capacity shares and total production are important for the vertical assessment. Controlling the majority of the production of HMD would allow the Merged Entity to control the market in this particular case, given the absence of alternatives for customers. Were the Merged Entity to increase the prices, reduce supply or supply in less favourable terms, customers would not have any other option than to accept these conditions given the structure of the market, as the only alternative to the Merged Entity would be Invista which is capacity constraint. Further, the market investigation shows that there are no companies based in the EEA that can serve additional volumes in the merchant market or can do it only occasionally.684 In fact, the large capacity share of the Parties means that they can successfully determine the conditions of supply in the merchant market by selling or deciding not to sell part of their captive production. Therefore, the Merged Entity would have the ability to discipline the market.
(902) Moreover, the Merged Entity would control [30-40]% of the merchant sales. Taking into account that the market is short and that EEA competitors cannot increase their volumes sold to the market, if the Merged Entity decided not to sell HMD in the EEA or do it at less competitive conditions, prices would rise and customers would not be able to secure enough volumes of HMD to compete in downstream markets, as there is no competitor in the EEA with spare capacity to substitute the volumes sold by the Parties on the market.
(903) Indeed, the vast majority of customers responding to the market investigation consider that the acquisition by BASF of Solvay's polyamide business would have a negative impact on the market for HMD in the EEA.685
(904) In particular, […] fears that "BASF decision on HMD usage (Nylon vs. HDI) may limit the availability of HMD to the merchant market".686 Another respondent to the market investigation, […], considers that "the new entity will have a dominant position in EEA" and another customer adds that the "majority of HMD sources in Europe will be under a control of one company".687
(905) Second, the Notifying Party claims that important competitors downstream cannot be foreclosed, since they purchase only marginal volumes of HMD from the Parties and do not source inputs from the Parties on other levels of the value chain. However, if the Merged Entity increased prices of HMD or reduced supply in the merchant market, this strategy would negatively impact the availability and the price level of HMD for the whole market and not only for the current customers of the Parties. As explained in section 6.3.2, the market is short and reducing further the volumes available for the merchant market would likely not only increase prices but also affect the ability of customers to secure the volumes required and therefore their ability to compete downstream. If the Merged Entity decided to reduce HMD supply to the merchant market, including to both polyamide and HDI producers, all the customers of the business (including customers active outside the polyamide value chain) would have to turn to Invista, the only EEA competitor of the Parties, which is capacity constraint. Taking into consideration that Invista cannot increase its HMD production in the EEA, as explained in section 6.3.2, a bigger demand from customer to Invista will result in higher prices and difficulties to secure enough volumes. Therefore, […] and […] would also be affected by a foreclosure strategy of the Merged Entity. Moreover, the Commission's file does not contain any element to ascertain that […] would have a long term contractual protection as claimed by the Notifying Party.
(906) For example, one important market player, […], explains that "the HMD market is already very concentrated and tight and is very difficult to get enough quantities. The HMD market is completely dependent upon the ADN market because there is no other route to produce HMD except through ADN. The situation is already getting worse and worse […]. The liquidity of HMD in the market is really bad and if BASF stops supplying the market, the market will be under more stress".688 […] adds "HMD and ADN are key intermediates for PA66 production, and the availability of HMD and ADN is currently very tight".689
(907) Similarly, an important customer of HMD, […] "is concerned that it [will] be foreclosed from the supply of HMD and ADIPIC ACID because BASF will control these key upstream ingredient markets and it will be the largest competitor of […] in the downstream market (the production of PA 6.6 EP)[…] If, post-transaction, BASF decides not to supply HMD into the merchant market, […] prices will increase and […]'s ability to compete in the market will be significantly reduced. The ability to buy inputs at competitive prices depends on the existence of competition upstream".690
(908) Another customer, […], fears that "post-merger the much increased market position in PA 6.6 of BASF might result in an increase of BASF's captive use of ADN, HMD and Adipic acid and therefore reduce considerably the volumes currently available in the respective merchant markets".691
(909) Moreover, the volumes of HMD sold by the Parties to polyamide manufacturers increased to […] kts in 2017, while the volumes sold by Invista decreased to […] kts. Therefore, contrary to the Notifying Party claim, the importance of the Parties as suppliers to polyamide producers is far from insignificant. By denying supplies to HMD customers, the Merged Entity would take away the only competitive constraint to Invista, which will be able to act as pure monopolist and increase prices. Following this strategy, the Merged Entity would raise the cost of the competitors, to the advantage of the Merged Entity downstream products.
(910) Third, concerning the argument of the Notifying Party that AH Salt producers could also purchase HMD from other suppliers, as explained in section 6.3.2, the market investigation shows that there are no suppliers in the EEA with capacity to serve additional volumes in the market. Indeed, […] and […] confirmed that they cannot serve additional volumes in the merchant market or can do it only occasionally but not on a permanent basis.692 In particular, […] explains that "[…] is short HMD in the EEA and has to supplement its EEA production capacity using swap arrangements with other HMD manufacturers and by importing extra HMD from its HMD plants in Texas, United States. To expand capacity, a new HMD facility would need to be constructed. This would take around 3 years and cost approximately [[…] Internal Cost Estimation]. Butachimie’s HMD plant (50% […]-owned) could not be significantly expanded because it has previously been expanded".693
(911) […] explains that it “does not sell HMD into the merchant market because Invista is the main supplier of HMD. Should […] start competing in the HMD market, Invista could increase prices or stop selling ADN to […]”.694
(912) On the other hand, […] submits that it could serve some additional volumes in the market by importing HMD from the USA.695 However, as explained in section 5.2.3, customers do not consider imports from outside the EEA as a viable alternative.
(913) Moreover, customers also consider that replacing the volumes sold by the Parties on the merchant market would be very difficult. For example, […], an important customer, explains that "it would be extremely difficult and even impossible to replace them with another supplier. […] fears that they will not find alternatives to source equivalent volumes because the market is very stretched already".696
(914) Fourth, regarding the argument that global overcapacities exist and that customers could turn to imports from outside the EEA, as explained in section 5.2.3, the geographic scope of the market is EEA-wide and customers do not consider imports as a viable alternative due to higher prices derived from import tariffs, transportation costs, financial risks or logistic and technical difficulties. Customers of HMD see imports as a temporary option to overcome supply issues like force majeure events, but consider that imports are not sustainable as a business model.697
(915) Fifth, the Commission rejects the argument that customers could source PA 6.6 BP instead of HMD and adipic acid, as explained in section 6.3.2. The Merged Entity would enjoy market power at the level of the PA 6.6 BP (level IV) and therefore could increase prices at this level of the polyamide value chain. Moreover, if HMD prices were to rise, competitors in the PA 6.6 BP market which source HMD in the merchant market would be less competitive, accentuating the market power of the Merged Entity at the PA 6.6 BP level. Furthermore, AH Salt producers (level III) sourcing HMD in order to produce co-polyamide do not have the alternative option of sourcing the base polymer and the market share of the Merged Entity in the merchant market for AH Salt is above [90-100]%. In addition, HDI producers cannot source other products than HMD for their production of HDI derivatives for coatings and therefore this argument would not apply to them. Finally, companies make strategic decisions and investments as to the most efficient level of vertical integration. Changing the level at which they buy the inputs would bring inefficiencies through write offs and double marginalisation.
(916) Sixth, the Notifying Party submits that HDI producers source significant volumes of HMD from other suppliers and that the Merged Entity would therefore not have the ability to foreclose them. However, as explained in the present section a strategy to reduce HMD supply in the market would negatively impact not only the Parties’ current customers, but also the price level and availability of HMD for the whole market. Moreover, the Business accounts for [50-60]% of the total volumes of HMD purchased in the EEA by HDI producers.
(917) Seventh, regarding the long-term contract in place with […] or […], the Merged Entity would have the possibility to renegotiate the conditions as soon as in […] and it cannot therefore be considered as a long-term constraint for the Merged Entity.
(918) Finally, regarding the argument of the Notifying Party that imports of HDI derivatives exert a strong competitive constraint in the EEA, it should be noted in the first place that competitors like Asahi or Wanhua importing HDI into the EEA account for less than [10-20]% of the merchant sales in the EEA.698 While imports are technically feasible and a number of customers import some volumes into the EEA as described in section 5.9.1.3, overall, the volumes imported represent only a relatively small fraction of the merchant market in the EEA. Therefore, contrary to the Notifying Party views, imports represent only a limited constraint in terms of prices in the EEA. Second, as discussed in this section the ability to foreclosure HMD to competitors should be assessed on an overall market for HMD, including customers active in both the polyamide and the HDI value chain. The HMD sold in the EEA to both types of customers is not different and therefore there is only one market for HMD. By artificially trying to separate customers active in the polyamide and HDI value chain, the assessment as to the ability of the Merged Entity to foreclose HMD to EEA customers would be biased. If the Merged Entity decided to stop supplying HMD to EEA customers or doing it at a higher price, customers active in both value chains would not have other option than turning to Invista which is capacity constrained. Therefore, prices would increase and customers would likely not be able to secure enough volumes, given that the market is already short.
6.4.7.1.(C) Conclusion on ability to foreclose
(919) The Commission therefore concludes that the Merged Entity would have the ability to foreclose access to HMD to PA and HDI producers.
6.4.6.2. Incentive to foreclose access to HMD in the polyamide value chain 6.4.7.2.(A) The Notifying Party's view
(920) The Notifying Party considers that the Merged Entity would have no incentive to foreclose HMD to AH Salt-producing customers, since none of them is present in the AH Salt merchant market.
(921) The Notifying Party is of the view that the incentive of the Merged Entity to foreclose HMD to AH Salt producers is also not present if further downstream markets are considered because the customers of the Business or BASF do not compete (or do it to a very limited extent) in the downstream markets where the Merged Entity would be active, as they use HMD to produce grades of PA where the Merged Entity is not active or products outside the polyamide value chain where the Merged Entity is not active.
(922) In addition, the Notifying Party claims that the difference in margins between HMD and AH Salt is relatively small and therefore does not give incentives to stop selling HMD into the merchant market. This is because the “critical diversion ratio” in AH Salt would be, according to the calculation of the Notifying Party, about [50-60]%.
6.4.7.2.(B) The Commission's assessment
(923) First, the Notifying Party submits that the Merged Entity would have no incentive to foreclose HMD to the Parties' customers because they do not compete or do it to a very limited extent in the downstream markets. As explained in section 6.3.2, taking into consideration that the market is short and that there are no alternatives to source additional volumes of HMD, the effects and incentives of a foreclosure strategy are to be analysed globally in the HMD market and not merely focused on the Parties’ current customers. If the Merged Entity reduced the volumes of HMD in the merchant market, the prices would increase for all customers and the availability of HMD would be reduced, making it difficult for customers to secure the volumes needed to compete in downstream markets. Therefore, the Merged Entity would benefit from a less competitive landscape in all products downstream to HMD where it would have in addition a strong position and where the margins are higher, given that its main competitors would have access to a critical input in less favourable terms.
(924) The Merged Entity could decide to increase prices for HMD or reduce volumes in the HMD merchant market. Even if the Merged Entity were to lose some sales in the HMD merchant market following this price increase (which is doubtful given that the market is short and there are no alternatives in the HMD merchant market for customers to source their needs of HMD) or following a decision to reduce supply, it could recuperate the profits in the downstream markets (i.e. AH Salt, PA 6.6 BP or PA 6.6 EP) where it would also be by far the market leader.
(925) Were the Merged Entity to increase prices for HMD, given that the market is short and that there are no suppliers with spare capacity to increase their supply into the HMD merchant market, customers would likely continue sourcing from it to a large extent.The Merged Entity could decide to increase prices also downstream (i.e. AH Salt, PA 6.6 BP and PA 6.6 EP) and competitors in these markets that source HMD in the merchant market would have to follow the price increase because their input costs (i.e. HMD) would be higher.
(926) For example, […] explains that "the availability of ADN in the market is very tight. Invista has more ADN capacity than HMD capacity and therefore, it sells into the merchant market. Solvay has a more balanced capacity between HMD and ADN, but it also sells into the market. BASF has a big HMD production capacity while it has no ADN capacity at all. Moreover, BASF has a very strong production capacity downstream (base polymer and engineering plastics) that will be further reinforced with the acquisition of Solvay's business. Solvay, on the other hand, does not have enough polymerization capacity in Europe to transform all its HMD into PA 6.6 polymer and therefore sells HMD in the merchant market. Post-transaction, BASF will have a balance between upstream and downstream capacities. Therefore, the transaction is not a mere change in the ownership of the JV Butachimie because the Transaction changes completely the incentives to sell ADN and HMD in the merchant market".699
(927) Indeed, […] fears that "BASF will have the ability and the incentives to reduce supply of HMD in the market and in particular to […]. BASF currently purchases ADN in the merchant market. Post-transaction, BASF will have the incentives to use ADN and HMD internally to satisfy its internal demand. BASF will have enough capacity to absorb all its ADN and HMD and is unlikely to sell large and long term volumes into the merchant market".700
(928) Another customer, […], "is of the opinion that post-merger the much increased market position in PA6.6 of BASF might result in an increase of BASF's captive use of ADN, HMD and Adipic acid and therefore reduce considerably the volumes currently available in the respective merchant markets - whether these were to be ultimately used by either direct competitors of the merged entity downstream, or by other, downstream non-competing users".701
(929) In the same vein, an important customer, […], "is worried that the amount of HMD in the market will drop down and Invista will become the dominant HMD supplier by restricting ADN access to other HMD producers. […]'s concern is that BASF will abandon the ADN and HMD sales into the merchant market and make up the forgone profits in those products by increased margins in downstream products […] BASF has a very strong internal demand of HMD for its engineering plastic business and they will prioritize internal requirements".702
(930) Internal documents of BASF also show […]703 […]704 […]705 […].706 […]707:
[BASF INTERNAL PRESENTATION SLIDE]
Figure 9. BASF projections on ADN use (4)
[BASF INTERNAL PRESENTATION SLIDE]
Figure 10. BASF projections on ADN use (5)
(931) Regarding the argument that the difference in margins between HMD and AH Salt is relatively small and therefore does not give incentives to stop selling HMD into the merchant market, first it should be noted that the Merged Entity is not only active in the AH Salt market, but also in other further downstream markets. The Merged Entity would also have a very strong position in the PA 6.6 BP, co-polyamide and PA 6.6 EP. HMD price increases or reductions of HMD volumes sold by the Merged Entity into the merchant market would impact the ability of competitors that source HMD in the merchant market to secure the volumes required and to compete in downstream markets (i.e. AH Salt, PA 6.6 BP and PA 6.6 EP) with the Merged Entity. Therefore, the Merged Entity would benefit from the cost increase of its rivals' input not only in the AH Salt market, but also in the PA 6.6 BP, co-polyamide and PA 6.6 EP markets. As a result, the Merged Entity could increase prices downstream and/or gain market shares from their competitors that would have a competitive disadvantage due to higher input costs.
(932) Moreover, regarding the argument that the difference in margins between HMD and AH Salt is relatively small, leading to a quite large “critical diversion ratio”, it should be noted that the Merged Entity would have an extremely strong position in the merchant market for AH Salt. This implies that the diversion ratio in AH Salt would be very high, likely giving the Merged Entity the incentives to foreclose HMD to AH Salt-producing customers. Moreover, the Parties are active in other markets further downstream (the PA 6.6 BP, co-polyamide and PA 6.6 EP markets) where they compete with HMD customers. Given that the margins in these markets can be larger than the AH Salt margin, the corresponding “critical diversion ratios” would be significantly lower than for AH Salt, likely providing the Merged Entity with the incentives to foreclose competitors. Finally, such analysis of “critical diversion ratios” is highly conservative because it does not take into account the price increases in AH Salt and other markets further downstream (e.g. PA 6.6 BP, co-polyamide and PA 6.6 EP) which would result from the strong position of the Merged Entity in these markets. This analysis thus understates the incentives to foreclose of the Merged Entity.
(933) Finally, the Merged Entity could export some volumes of HMD to China, as there is no ADN production in Asia and there exists demand for HMD. In this vein, […] explains that "in China, the market for HMD is short, but they have a big capacity in the downstream markets. Therefore, demand of HMD in China is strong. In […] opinion, this can make exports to China attractive for HMD producers. Exports of HMD from Europe to China are small currently. If HMD were imported to China, the European market would be short".708 Exporting some volumes to China would reduce potential losses in the upstream sales, at the same time that the Merged Entity increase its sales base downstream or increases prices.
6.4.7.2.(C) Conclusion on incentive to foreclose
(934) The Commission therefore concludes that the Merged Entity would have the incentive to foreclose access to HMD to PA producers.
6.4.6.3. Effects of a potential input foreclosure strategy with respect to HMD in the polyamide value chain
6.4.7.3.(A) The Notifying Party's view
(935) The Notifying Party claims that the Merged Entity would have neither the ability nor the incentive to foreclose HMD to AH Salt-producing customers and therefore no effect on effective competition would emerge.
6.4.7.3.(B) The Commission's assessment
(936) HMD is a key input for the production of AH Salt which is in turn the precursor of PA 6.6 BP and co-polyamide which are further processed into PA 6.6 EP and co- polyamide EP respectively. If the prices of HMD increases or the availability is reduced, the competitors in these markets downstream that source HMD in the merchant market would thus be in a competitive disadvantage steaming from the higher costs of a key input.
(937) If the Merged Entity engaged in a strategy of input foreclosure, the availability of HMD in the market would be reduced and/or the prices would increase. Therefore other competitors in downstream markets (AH Salt, PA 6.6 BP, PA 6.6 EP and co- polyamide) would no longer have access to HMD with the same conditions and thus could not offer customers the same prices or volumes. One important competitor in the downstream markets, […], considers that "permitting the acquisition will limit supply of critical raw materials, damage our ability to supply existing customers and
severely restrict our ability to grow".709 Another market player, […] adds that in general the “the liquidity of the market will be limited. The number of participants on the supply side is very reduced and this consolidation will further reduce it. There will be business discontinuity in some cases”.710
6.4.7.3.(C) Conclusion on effects of a foreclosure strategy
(938) The Commission therefore concludes that, were the Merged Entity to pursue an HMD input foreclosure strategy, it would have significant negative effects on competition in the polyamide value chain.
6.4.6.4. Incentive to foreclose access to HMD to HDI producers 6.4.7.4.(A) The Notifying Party's view
(939) The Notifying Party claims that the Merged Entity would have no incentive to foreclose HMD to HDI-producing customers because neither party is present in the HDI merchant market.
(940) According to the Notifying Party, this remains the case even considering further downstream markets in which BASF’s sales share in 2017 in the EEA was less than [20-30]%, the Business’ was not active and the other suppliers were Vencorex ([20- 30]%) and Covestro ([40-50]%). The Notifying Party considers that this is the case for the reasons set out in recitals (941) to (946):
(941) First, […], if the Merged Entity attempted to foreclose HMD to […], […] could retaliate […]. This would defy any hypothetical attempt to increase the profits on its sales of HDI derivatives by increasing its rivals’ costs. In the Response to the 6(1)(c) Decision the Notifying Party further submits that without […], the Merged Entity would not be able to continue to serve the HDI Oligomer market at all. […] therefore has the ability to inflict costs on the Merged Entity that would by far exceed any hypothetical gains that the Merged Entity may make from a foreclosure strategy.
(942) Second, the Notifying Party states that considering the market share of BASF in the EEA, it is unlikely that the Merged Entity would have an incentive to foreclose HMD to […] since, even if any sales were to be diverted from […] most of them would be expected to go to […] because it is the largest supplier in the market. Moreover, in the Response to the 6(1)(c) Decision, the Notifying Party submits that the Merged Entity will not be fully backward integrated into HMD because it won't control HDI production assets. Therefore, the Merged Entity will not have full control over the entire production along the value chain. This excludes upfront the ability of the Merged Entity to expand its HDI Oligomer production without recurring to HDI suppliers.
(943) Third, given the relatively low transportation costs of HDI derivatives, there are significant imports into the EEA from Asia, and on a global level, there are further suppliers (e.g. Asahi and Wanhua), which reduces any potential benefits for the Merged Entity in the merchant market for HDI derivatives if it tried to foreclose access to HMD to […].
(944) Fourth, the Notifying Party is of the view that in the hypothetical scenario that the Merged Entity would try to foreclose […], it could also source HDI to produce HDI derivatives rather than producing it itself. Even if this would likely be more expensive than producing itself, […] could still maintain its HDI derivatives production.
(945) Fifth, the Notifying Party submits that Vencorex is also selling HDI on the merchant market. If Vencorex faced a reduced supply of HMD, it would first likely reduce its HDI sales to the merchant market to preserve its downstream sales, which are likely to be more profitable.
(946) Finally, in the Response to the 6(1)(c) Decision, the Notifying Party submits that a 30% increase in HDI Oligomer prices would be required to offset the upstream profits that the Merged Entity would forego by withholding merchant HMD sales to HDI producers and that such increase is unachievable because it is unlikely that HDI Oligomer players could pass on such a high price increase to their customers and because it would be cheaper for downstream customers to import their HMD, HDI and/or HDI Oligomer needs from outside the EEA.
6.4.7.4.(B) The Commission's assessment
(947) BASF is not active in the production of HDI but sources […], with the balance sourced from other suppliers. BASF processes the HDI it receives from […] into HDI derivatives which it sells in the merchant market under the trade name Basonat and also uses it captively in its coatings production.
(948) BASF uses HDI predominantly (more than [90-100]%) for the production of HDI derivatives. BASF uses the remaining volumes of HDI in other non-coating applications. The Business is not active in the production of HDI or HDI derivatives and does not source any HDI.
(949) On the market for HDI, […] the combined market shares of the parties are:
(a) [0-5]% for merchant sales.
(b) [30-40]% in volume for captive use plus merchant sales (including volumes used internally for production of HDI Derivatives and in biopolymer applications -[0-5]% Business and [30-40]% BASF); and,
(c) [0-5]% for production capacity.
(950) Table 25 provides the Parties’ market share estimates for 2017 (merchant sales and/or captive use). These market share estimates consider the volumes sourced by BASF from […] as external sales by […].
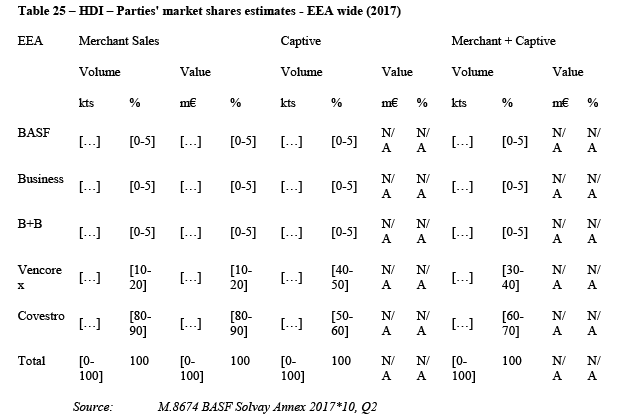
(951) Table 26 provides the Parties’ market share estimates for 2017 (merchant sales and/or captive use) considering the HDI volumes tolled by […] as captive use of BASF.
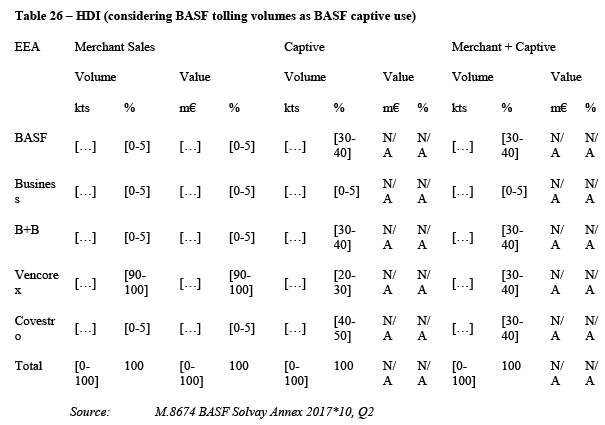
(952) The Commission considers that the tolling agreement with […] gives BASF the control over this part of the HDI production and therefore, it should be considered as captive use of BASF.
(953) The Parties’ combined market shares in the market for HDI derivatives in 2017 in the EEA are:
(a) [10-20]% for merchant sales;
(b) [10-20]% in volume for merchant sales plus captive use (including volumes used internally for production of coatings).
(954) Table 27 provides the Parties’ market share estimates for 2017 (merchant sales and/or captive use).
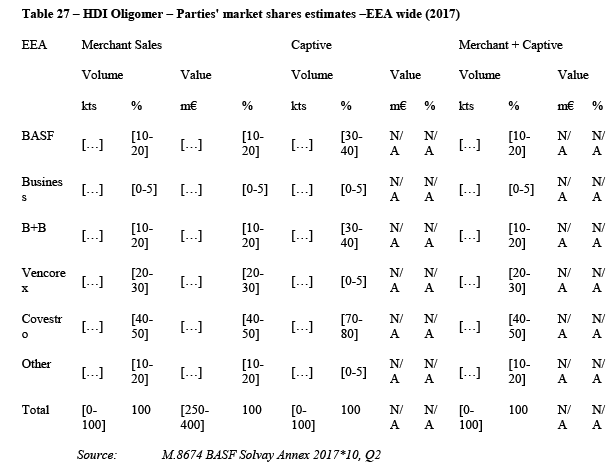
(955) The Parties’ combined market shares in the market for HDI derivatives in 2017 at global level are:
(a) [10-20]% for merchant sales;
(b) [10-20]% in volume for merchant sales plus captive use (including volumes used internally for production of coatings).
(956) Table 28 provides the Parties’ market share estimates for 2017 (merchant sales and/or captive use).
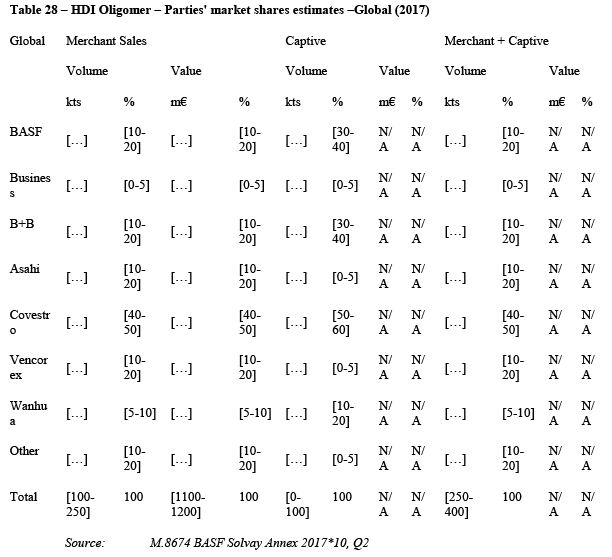
(957) The Commission considers that the relevant link to assess a potential input foreclose strategy in the HDI value chain in the present case is between HMD and HDI derivatives and not between HMD and HDI. This is because HDI is being used mainly captively by the only three producers in the EEA and the merchant market represents only [0-10]% of the total production. Therefore, the relevant vertical relation to consider in order to analyse a possible input foreclose strategy is between HMD and HDI derivatives and not only between HMD and HDI.
(958) First, the Notifying Party submits that it would have no incentive to foreclose HMD to […] because it could retaliate […]. However, as explained in section 6.3.2, taking into consideration that the market is short and that there are no suppliers with spare capacity to supply additional volumes of HMD in the merchant market, the effects and incentives to pursue a foreclosure strategy are to be analysed globally in the HMD market and not merely focused in one particular customer or competitor. In any event, the capacity of […] to retaliate cannot be assumed, given that […] sources a significant part of its HMD requirements from the Business, and therefore, post- transaction, it would depend on the Merged Entity for those volumes.
(959) If the Merged Entity reduced the volumes of HMD available for the merchant market, the prices would increase for all customers and the availability of HMD would be reduced. In this scenario, customers of HMD would face more difficulties to secure the volumes of HMD needed to compete in downstream markets. Therefore, the Merged Entity does not need to target a specific customer/competitor, because if it pursued a foreclosure strategy it would affect the whole market and therefore it would affect also to non-customers of the Parties. Moreover, even if the Merged Entity continued selling to […] at the same conditions than pre-transaction, it could decide to stop supplying HMD or doing it at a higher price to all other customers active in the HDI and polyamide value chain. If the Merged Entity decided to follow such strategy, given than the volumes sold to […] are limited compared to the total sales of HMD of the Parties, the effects on the HMD market would be the almost the same than if it also targeted […]. Furthermore, in this scenario, […] would also face difficulties to source HMD even if the Merged Entity continued selling to it the same volumes of HMD than pre-transaction, because […] sources a very important part of its requirements from Invista which would have a higher demand from other customers. Therefore, the competitive assessment remains the same even if […] is not specifically foreclosed by the Merged Entity.
(960) Second, the Notifying Party claims that the Merged Entity would not have incentives to foreclose […] because if any sales were to be diverted from […] most of them would be expected to go to […] as it is the largest supplier in the market and because the Merged Entity does not control HDI production assets and therefore it would depend on HDI producers to expands its HDI derivatives production. However, the Commission considers that the effects and incentives to pursue a foreclosure strategy are to be analysed globally in the HMD market and not individually. As discussed in this section, following a foreclosure strategy the Merged Entity would benefit from the increased costs of its rivals not only in the HDI value chain but also in the polyamide value chain. Moreover, […] could also face difficulties in sourcing enough quantities of HMD at competitive prices, while the Merged Entity would be the only HDI derivatives producer controlling HMD and ADN production assets. Moreover, the HDI yearly maximum volume that […].711 […].
(961) […] fears that "after the merger, […]'s competitor, since BASF is active in the HDI derivative market, will then also become their supplier of essential raw material. Solvay is active neither in HDI nor derivatives production".712 This competitor continues explaining that its "competitor on the downstream HDI & derivatives market (BASF) will become its supplier for its upstream essential raw material, HMD. BASF could leverage on its position on the upstream HMD market to reinforce its market position on the downstream market using HDI as an input. […] is therefore afraid that it will be foreclosed by the merged entity if their strategy is to only use their HMD captively and decide not to sell to the market or at some conditions that will jeopardize […]'s sustainability. BASF may give priority to its internal consumption and may limit sales of HMD into the market to HDI producers".713
(962) BASF could also increase prices given the reduced choice of suppliers for customers. Taking into consideration that the market for HDI derivatives is already concentrated with three suppliers and that the two competitors of the Merged Entity would be depend on it for significant volumes of HMD, the Merged Entity could increase prices. Given that […] and […] would likely not be able to increase their production of HDI derivatives because they would not have access to enough HMD (the market for HMD would be controlled by the Merged Entity as explained in section 6.3.2), customers would not find alternatives to the Merged Entity to secure the volumes they source from them. Moreover, […] and […], would likely have the incentives to follow the price increase. This is aggravated by the HDI derivatives market, which according to the majority of customers is short714 and demand will continue to grow in the next 5 years.715 Moreover, multi-sourcing from at least two suppliers is common in the industry716 and barriers to entry are high. The most important barriers identified by customers are the level of investment required to set up a production facility and access to raw materials (i.e. HMD). Regulatory requirements, lack of expertise and intellectual property rights or know-how are also mentioned by some customers.717 For example, […] explains that "setting a production facility is generally expensive and setting a manufacturing facility able to handle isocyanates is highly regulated and involves management of dangerous chemicals. Know-how of process, optimization of the plant, integrated transformation of by-products and appropriate waste handling are significant barrier to entry. Access to raw material, unless manufacturer is a manufacturer of HMD or HDI as well, can impact the cost to manufacture HDI Derivatives".718 In the same vein, […] explains that "high investment costs due to chemical processes and key is to control the phosgene, which is a restricted raw material (stringent security measures are needed to work with this material, may have high investment costs)".719 […] adds "the manufacture of HDI derivatives is technically demanding, and also the number of raw material suppliers in the market is limited. You would need access to both the expertise and the raw materials to be able to produce".720
(963) Third, regarding the argument that HDI derivatives transportation costs are low and imports from Asia exist, according to the data provided by the Notifying Party competitors like Asahi or Wanhua importing HDI into the EEA account for less than [10-20]% of the merchant sales in the EEA.721 Therefore, while imports are technically feasible and a number of customers import some volumes into the EEA as described in section 5.9.1.3, overall, the volumes imported represent only a relatively small fraction of the merchant market in the EEA. Therefore, it does not seem that companies importing HDI derivatives can successfully constrain prices and serve the needs of the market if the Merged Entity decided to foreclose its competitors. Moreover, […] also sources some HMD volumes from the Business ([…] kts in 2016).722
(964) Fourth, as the Notifying Party recognises, sourcing HDI to produce HDI derivatives rather than producing it internally, would likely be more expensive than producing the HDI in-house. Therefore, this strategy would affect the ability to compete in the HDI derivatives market of a company trying to implement this approach.
(965) Fifth, the Notifying Party claims that, if […] faced a reduced supply of HMD, it would first likely reduce its HDI sales into the HDI merchant market to preserve its HDI derivatives downstream sales. However, these sales represent only 10% of […]'s HDI production while 90% is being used captively in order to produce HDI derivatives. Therefore, such a strategy of […] would have very limited effects.
(966) Finally, the argument of the Notifying Party that a 30% increase in HDI Oligomer prices would be required to offset the upstream profits that the Merged Entity would forego by withholding merchant HMD sales to HDI producers, does not hold for several reasons. First, this calculation is done under the assumptions that Invista would guarantee the same volumes as the Business at the same pricing conditions and HDI competitors would not lose market shares. As demonstrated in section 6.4.2.1, these assumptions are not credible and therefore, the entire calculation is not valid. Second, the market is short and […] is capacity constrained, therefore if the Merged Entity reduced its HMD supply to the market, competitors would face difficulties to secure enough volumes of HMD for their production downstream and prices of HMD would likely increase. Under this scenario, competitors in the HDI derivatives market would likely lose market shares in favour of the Merged Entity.
(967) Moreover, the Merged Entity could export some volumes of HMD to China, thus reducing potential losses in the upstream sales and contributing to offset the upstream profits. In this vein, […] explains that "in China, the market for HMD is short, but they have a big capacity in the downstream markets. Therefore, demand of HMD in China is strong. In […] opinion, this can make exports to China attractive for HMD producers".723
6.4.7.4.(C) Conclusion on incentive to foreclose
(968) The Commission therefore concludes that the Merged Entity would have the incentive to foreclose access to HMD to HDI producers.
6.4.6.5. Effects of a potential input foreclosure strategy with respect to the supply of HMD to HDI producers.
6.4.7.5.(A) The Notifying Party's view
(969) The Notifying Party claims that the Merged Entity would have neither the ability nor the incentive to foreclose HMD to HDI-producing customers and therefore no effect on effective competition would emerge. It also claims that theoretical price effects with respect to HDI-Oligomers resulting from an assumed input foreclosure strategy would in any case need to be balanced against the efficiencies resulting from the Transaction due to lower input costs for BASF’s production of HDI-Oligomers.
6.4.7.5.(B) The Commission's assessment
(970) HMD is a key input for the production of HDI and HDI derivatives. If the prices of HMD increases or the availability is reduced, the competitors in these markets downstream that source HMD in the merchant market would thus be in a competitive disadvantage steaming from the higher costs of a key input. For example, one important competitor, […], claims that "one of the impacts of the transaction is regarding the HDI value chain. With their new position on HMD, BASF will have a significant market power on the HDI value chain. HDI being also used by BASF for downstream businesses (HDI derivatives and coatings divisions), this new position may potentially impact the global isocyanate/HDI industry for the benefit of BASF".724
(971) As explained in section 6.4.2.1, if the Merged Entity engaged in an input foreclosure strategy, the availability of HMD in the HMD merchant market would be reduced and/or the prices of HMD would increase. Therefore, the costs of a key input would increase for other competitors in downstream market (HDI and HDI derivatives) or they would face difficulties to secure the volumes of HMD that they need for their production of HDI and HDI derivatives. As a result, they could not offer to their customers the same prices or volumes.
6.4.7.5.(C) Conclusion on effects of a foreclosure strategy
(972) The Commission therefore concludes that, were the Merged Entity to pursue an input foreclosure strategy, there would be significant negative effects in competition in the HDI derivatives market.
6.4.6.6. Conclusion
(973) Based on the assessment in recitals (888) to (972) and in light of the results of the market investigation and of all the evidence available to it, the Commission concludes that the Transaction would significantly impede effective competition as a result of vertical non-coordinated effects arising from vertical links between the upstream EEA market for HMD and the downstream PA (AH Salt, PA 6.6 BP and PA 6.6 EP) and the HDI (HDI and HDI derivatives) value chains in the EEA.
6.4.7. Level II of the polyamide value chain: adipic acid, input foreclosure in the polyamide value chain
(974) Vertically affected markets arise when adipic acid is used as a key input into other materials such as AH Salt within the polyamide value chain, as well as Microcellular Polyurethanes, Aliphatic Polyester TPU, Plasticisers (polymeric and adipate plasticisers), and HDO. Indeed, the Parties' combined market share exceeds 30% on a market for adipic acid defined as EEA-wide in scope for the purpose of assessing the Transaction.
(975) At the outset, the Commission recalls its findings as to the significant impediment to effective competition arising from the Transaction as a result of horizontal non- coordinated effects in the supply of adipic acid in the EEA, i.e., at the level of the upstream product, and in the supply of AH Salt in the EEA, which is the direct downstream product in the polyamide value chain.725 Likewise, the Commission has found that the intermediate levels in the polyamide value chain involved a limited number of suppliers and purchasers, whereas the products in question are largely commoditised.
(976) Moreover, participants in the market investigation have excluded foreclosure risks in the EEA in relation to polymeric and adipate plasticisers, as well as HDO. These vertical relations are therefore not discussed further as part of the present competitive assessment.
6.4.7.1. Ability to foreclose access to adipic acid to the polyamide value chain 6.4.8.1.(A) The Notifying Party's view
(977) Adipic acid is mixed with HMD (level II) to produce AH Salt (level III), within the polyamide value chain. In the EEA, about [50-60]% of the adipic acid production and about [40-50]% of adipic acid merchant market sales are used for the production of AH Salt. In 2016, […] kts of adipic acid were sold at EEA-level. About […] kts ([40-50]%) were sold to AH Salt producers.
(978) From its plant based in Ludwigshafen, BASF produced approximately […] kts of adipic acid in 2016. BASF utilised […] captively and sold only […] kts in the EEA and […] kts in Asia. Also in 2016, the Business kept approximately [50-60]% of its global production of […] kts ([…] kts in the EEA and […] kts in Korea) for captive use and sold adipic acid both in the EEA ([…] kts) and elsewhere ([…] kts).
(979) According to the Notifying Party, the Transaction would not affect the overall availability of adipic acid on the market. Post-transaction, adipic acid customers would still be able to source adipic acid from alternative EEA producers, but also to import adipic acid, in particular from China. As far as EEA producers are concerned, the Notifying Party referred to […] plan to increase it adipic acid production capacity by 20%. Moreover, the Notifying Party argued that there are global overcapacities, especially in […] (around […] kts), whereas the utilisation rate of adipic acid production plants would be only [70-80]% globally, and below [50-60]% in […]. As a result, according to the Notifying Party, adipic acid producers that sell limited quantities on the merchant market could supply the Parties’ customers in case the Merged Entity was to internalise its production further and/or limit merchant market supplies. In this context, the Merged Entity would therefore not have the ability to foreclose access to adipic acid, as competing suppliers could expand output in response to a hypothetical supply restriction.
(980) The Notifying Party also claims that it is easy for adipic acid customers to switch supplier because: (i) adipic acid is a commodity product for which only one standard specification exists; (ii) supply agreements are usually concluded for relatively short periods of time; and (iii) switching supplier is an easy task for customers who only have to spend around one hour to clean their facilities.
(981) The Notifying Party therefore concludes that given the global nature of the adipic acid market, the Merged Entity's adipic acid customers would face no constraint in replacing it as an adipic acid supplier. The Merged Entity would therefore not have the ability to foreclose access to adipic acid.
6.4.8.1.(B) The Commission's assessment
(982) For the purpose of assessing the Transaction, the Commission finds that the adipic acid market is EEA-wide (and not global) in scope. Hence, the Merged Entity would account for [40-50]% of the EEA merchant market of adipic acid and would control more than [60-70]% of EEA production capacities.726 Moreover, the market share of the Merged Entity in the EEA would be significantly higher that the market share of its remaining competitors (Radici [20-30]% and Lanxess approx. [10-20]%).
(983) The Commission further notes that the debottlenecking of […]' adipic acid production plant, even if it were to materialise with the effects claimed by the Notifying Party, is not such as to alleviate the Merged Entity's ability to foreclose downstream competitors as it would amount to a mere [5-10]% of 2017 EEA merchant sales, whereas overall demand for adipic acid is expected to increase in the coming years.727 Moreover, it was explained in details in section 5.4.3. that imports from China into the EEA have been historically low, irrespective of recent increases, and that various elements (quality, logistics, security of supply, etc.) constrain the ability of EEA customers to consider Chinese suppliers on par with EEA ones.
6.4.8.1.(C) Conclusion on ability to foreclose
(984) The Commission therefore concludes that the Merged Entity is likely to have the ability to foreclose access to adipic acid to the polyamide value chain.
6.4.7.2. Incentive to foreclose access to adipic acid in the polyamide value chain 6.4.8.2.(A) The Notifying Party's view
(985) According to the Notifying Party, the Merged Entity would have no incentive to foreclose adipic acid, neither in general, nor specifically, to suppliers present in each of the downstream PA markets. According to the Notifying Party, any restriction in the Merged Entity’s supply of adipic acid to its customers would only lead to a loss of adipic acid sales and margins without an offsetting gain of downstream sales and margins. This is because adipic acid customers can obtain the adipic acid they require from other suppliers, in particular through imports, without affecting their downstream operations. Lower sales and margins of adipic acid by the Merged Entity would therefore not be compensated by higher sales and margins in downstream markets.
(986) In particular, any input foreclosure strategy would be costly for the Merged Entity, according to the Notifying Party because: (a) its customers sell in multiple downstream markets; (b) in some of these markets they are not in competition with the Merged Entity; and (c) the Merged Entity cannot discriminate between input sales that are destined to competing applications and input sales that are not. For this reason the Merged Entity would need to forego a large amount of upstream sales to ensure that at least some of them are destined to competing downstream applications. This makes input foreclosure costly, not profitable and thus unlikely.
(987) In relation to AH Salt (level III), The Notifying Party argues that the only other supplier active in the merchant market in the EEA is Radici. As Radici is backwards integrated in the production of adipic acid and does not purchase AH Salt, according to the Notifying Party, the Merged Entity cannot foreclose adipic acid to any AH Salt supplier on the adipic acid merchant market in the EEA.
(988) In relation to PA 6.6 BP (level IV), the Notifying Party contends that their only two customers active on that market are Invista and DowDuPont, which have both access to alternative sources of supply. Moreover, Invista's position in the ADN supply chain, notably as the Merged Entity's partner in Butachimie, renders any foreclosure strategy highly hypothetical. Likewise, DowDuPont is considered by the Notifying Party to be a minor EEA supplier of PA 6.6 BP to the merchant market, thus not an attractive target for a foreclosure strategy.
(989) Finally, in relation to PA 6.6 EP, the Notifying Party contends that DowDuPont, in particular, given its significant resources, would have various ways to procure adipic acid, AH Salt and/or PA 6.6 BP from alternative sources in case the Merged Entity was to reduce merchant supplies. Otherwise, the Notifying Party views Celanese as a small EP player in the EEA, thus not an attractive target for a foreclosure strategy.
6.4.8.2.(B) The Commission's assessment
(990) During the market investigation market participants stated that post-transaction the Merged Entity would dominate the adipic acid market, and that for some of them Solvay and BASF are the only with capacity to meet their requirements. According to these market participants, the Merged Entity would have the ability and incentive to implement price increases for adipic acid, due to their nature as a critical input for the production of AH Salt, PA 6.6 BP and eventually PA 6.6 EP.728 Therefore, according to these market participants, the Merged Entity would control, including in relation to adipic acid, the PA 6.6 value chain by dominating all key inputs for the production of PA 6.6.
(991) Taking into account the significant market power that the Merged Entity would have post-transaction in the adipic acid market, and the dependence of certain key customers, the incentives to foreclose adipic acid as an input cannot be excluded, especially towards BP producers, with a knock-on effect on EP producers. In effect, the arguments put forward by the Notifying Party in its Reply to the Article 6(1)(c) Decision with respect to […] and […] respective ability to source adipic acid from third-parties are unconvincing. The duration of the […] contract is limited in time and imports from the USA are unlikely to be as attractive as domestic EEA supply. In addition, the leverage that Invista could gain as the Merged Entity's partner in Butachimie is unspecified. Moreover, the market investigation has revealed that […] is a very large adipic acid purchaser in the EEA with limited sourcing options, and that investing in own production is not a credible solution, even in the medium term.729
(992) In relation to EP producers, the argument that […] has a small position in PA 6.6 EP in the EEA is of no comfort. To the contrary, it may also put the Merged Entity in a position to weaken downstream competition at limited costs.
6.4.8.2.(C) Conclusion on incentive to foreclose
(993) The Commission therefore concludes that the Merged Entity is likely to have an incentive to foreclose access to adipic acid to the polyamide value chain.
6.4.7.3. Effects of a potential input foreclosure strategy with respect to the supply of adipic acid to the polyamide value chain
6.4.8.3.(A) The Notifying Party's view
(994) The Notifying Party claims that the Merged Entity would have neither the ability nor the incentive to foreclose access to adipic acid to gain additional sales on the relevant downstream markets. As a result, according to the Notifying Party, no impact on effective competition is expected to arise from the existence of these vertical relationships.
6.4.8.3.(B) The Commission's assessment
(995) Adipic acid is a key input in order to produce AH Salt, and then subsequently PA 6.6 BP and PA 6.6 EP. If the price of adipic acid increases or the availability is reduced, the competitors in the downstream markets that source adipic acid in the merchant market would be at a competitive disadvantage stemming from the higher costs of a key input.
(996) If the Merged Entity engages in an input foreclosure strategy, the availability of adipic acid in the merchant market would be reduced and/or the prices of adipic acid would increase. Therefore, the costs of a key input would increase for other competitors in the AH-Salt and other downstream markets and/or they would face difficulties to secure the volumes of adipic acid they need for their own production at competitive terms.
6.4.8.3.(C) Conclusion on effects of a foreclosure strategy
(997) The Commission therefore concludes that an input foreclosure strategy applied by the Merged Entity is likely to have significant negative effects on competition in the polyamide value chain.
6.4.7.4. Conclusion
(998) Based on the assessment in recitals (974) to (997) and in light of the results of the market investigation and of all the evidence available to it, the Commission concludes, that the Transaction would significantly impede effective competition as a result of vertical non-coordinated effects arising from vertical links between adipic acid, AH Salt and the downstream polyamide value chain in the EEA.
6.4.7.5. Adipic acid: input foreclosure in relation to Microcellular Polyurethanes 6.4.8.5.(A) The Notifying Party's View
(999) Tables 29 and 30 provide 2017 sales and market share estimates on a global and EEA-wide market for microcellular polyurethanes including rubber bumpers.
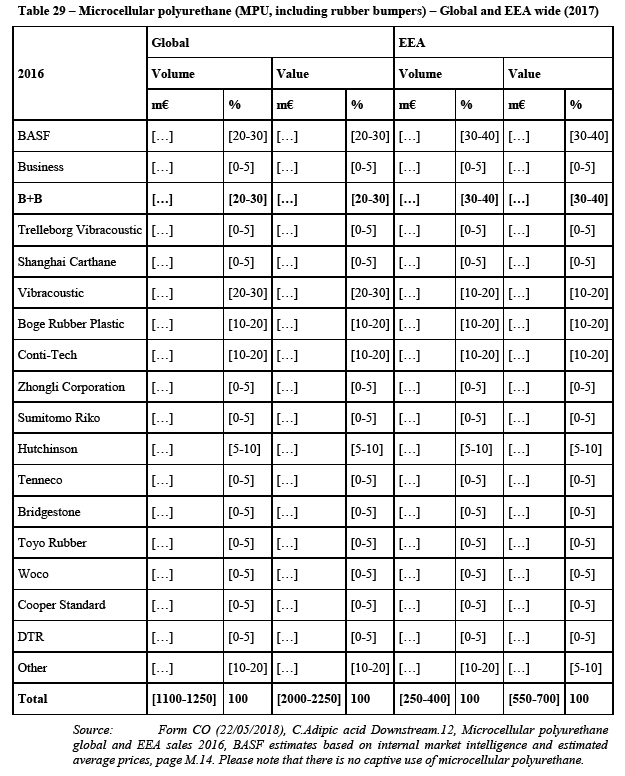
(1000) The Notifying Party also provided market share estimates for microcellular polyurethanes excluding rubber bumpers at the EEA level, as follows for 2016:
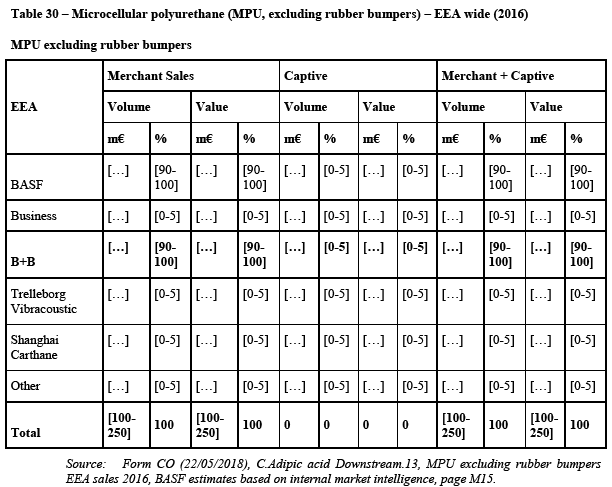
(1001) BASF produces microcellular polyurethanes in Lemförde (Germany), Shanghai & Nansha (China), Wyandotte (USA), Shinshiro (Japan), Guaratinguetá (Brazil), Dahej (India) and markets the product under the trade name Cellasto. BASF sells the vast amount of its microcellular polyurethane production (more than [90-100]%) to the automotive industry as top-mounts, coil spring isolators or jounce bumpers for wheel suspensions.
(1002) According to the Notifying Party, there is no vertical relationship between the business’ adipic acid activities and BASF’s microcellular polyurethane production, as microcellular polyurethane is a downstream product of aliphatic polyester polyol, of which adipic acid is the main input.
(1003) The Notifying Party therefore argues that, in spite of BASF's market share of (at least) [30-40]% (by volume) and [30-40]% (by value) on the downstream market for microcellular polyurethanes, the Transaction would leave effective competition unaffected due to the absence of input and customer foreclosure risks.730 In particular, the Notifying Party claims that there are many large suppliers of aliphatic polyester polyol in the EEA, such as Coim and Covestro.
(1004) Moreover, the Notifying Party contends that microcellular polyurethane can also be produced on the basis of polycaprolactone polyols, which are not produced on the basis of adipic acid. Likewise, low performance polyurethanes do not require polyester polyols, which may be substituted for by cheaper polyether polyols.
(1005) Furthermore, BASF is already vertically integrated with regards to adipic acid, polyester polyol and microcellular polyurethane so that the Transaction would not materially change the market structure. In addition, given the much smaller size of EEA competitors, the possible gain in market shares that could result from a hypothetical foreclosure strategy would not be large enough to cover profit loss upstream in the supply of adipic acid.
(1006) Finally, the Notifying Party indicates that many non-polyamide customers of adipic acid are also active in the production of other non-polyamide products, including plasticizers, polyurethane foam and HDO, with limited ability for the Merged Entity to determine for which product the adipic acid will eventually be used. In turn, the impossibility to target specific downstream markets reduces the likelihood that the Notifying Party could profitably foreclose access to adipic acid due to the costs associated with a foreclosure strategy that would apply to non-PA customers irrespective of the final application.
6.4.8.5.(B) The Commission's assessment
(1007) For the purpose of this Decision, the Commission considers that the adipic acid market is EEA-wide in scope. In turn, given the Parties' combined market shares both in terms of merchant sales and capacity, the Merged Entity is likely to have the ability to foreclose adipic acid as an input into the production of microcellular polyurethanes. Notably, the market share of the Merged Entity in the EEA is significantly higher than the market share of its remaining competitors, which indicates that alternative sources of supply are limited, as discussed in section 6.3.3.
(1008) The market investigation has not clearly confirmed that adipic acid is only an indirect input into microcellular polyurethane. Conversely, the Notifying Party acknowledged in the Reply to the Article 6(1)(c) Decision that […] and […], which are large suppliers of aliphatic polyester polyol in the EEA, are customers of the Business for adipic acid. In turn, […] repeatedly indicated over the course of the market investigation that adipic acid supplies from China were not comparable to adipic acid produced in Europe, and raised serious concerns about the post-transaction availability of adipic acid on the EEA merchant market.731 Similarly, […] expressed concerns that the Transaction could lead to an increase in the market price of adipic acid, and pointed to a loss in competitiveness of Chinese material that renders unlikely any material increase of imports in the foreseeable future.732
(1009) As a result, the Commission raised serious doubts in Phase I in relation to the risks of input foreclosure arising from the Transaction and its effects in the supply of microcellular polyurethane. These concerns have not been clearly dispelled over the course of the investigation in Phase II, as apparent from […] and […] contributions referred to in recital (1008). Conversely, the Commission's Phase II investigation did confirm the existence of a significant impediment to effective competition resulting from horizontal non-coordinated effects arising in the supply of adipic acid in the EEA. On balance, since the Final Commitments entered into by the Notifying Party will address these effects and associated vertical concerns, it is not necessary to discuss in details the likelihood that any foreclosure strategy in relation to microcellular polyurethane could be profitable and/or materialise.
6.4.8.5.(C) Conclusion
(1010) Based on the assessment in recitals (999) to (1008) and in light of the results of the market investigation and of all the evidence available to it, the Commission concludesthat, in view of the Final Commitments entered into by the Notifying Party, it is not necessary to conclude as to whether the Transaction would lead to a significant impediment to effective competition in the internal market as a result of vertical non-coordinated effects arising from vertical links between adipic acid and microcellular polyurethane in the EEA, as the commitments would in any event address such potential concerns.
6.4.7.6. Adipic acid: input foreclosure in relation to Aliphatic Polyester TPU
(1011) Table 31 provides the Parties' 2017 sales and estimated market shares on a global and EEA-wide market for aliphatic polyester TPU.
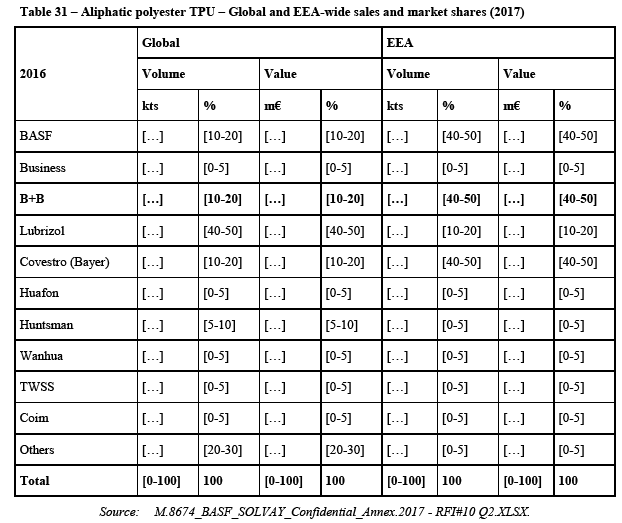
6.4.8.6.(A) The Notifying Party's views
(1012) BASF produces polyester TPU and sells it under the trade name Ellastolan. The Business is not active in the production of and has not sourced TPU in 2016. For this reason, and according to the Parties, the competitive structure of this market would not be altered by the Transaction. In particular, BASF has […].733
(1013) The Notifying Party also claims that there is no direct vertical relationship between adipicacid and TPU, since TPU is also a downstream product of aliphatic polyester polyol, of which adipic acid is the main input, and various other suppliers of aliphatic polyester polyol are active in the EEA.
(1014) The Notifying Party argues that the Merged Entity would be unable and without incentive to foreclose downstream TPU producers from access to adipic acid due to the presence of sufficient alternative adipic acid suppliers and overcapacity on the market.
(1015) Moreover, according to the Notifying Party, the size of the EEA aliphatic polyester TPU market is small, with […] and […] being the only other two suppliers, and competition is constrained by foreign imports. Conversely, the Notifying Party considers that […] and […] are important customers of adipic acid, thus leaving sufficient demand for third-party suppliers, […].
(1016) Eventually, the Notifying Party contends that the Parties' adipic acid customers use it as an input in other downstream applications for which no incentive to foreclose arises, which would increase the costs associated with any foreclosure strategy as it would need to be implemented irrespective of the final application.
6.4.8.6.(B) The Commission's assessment
(1017) For the purpose of this Decision, the Commission considers that the adipic acid market is EEA-wide in scope. In turn, given the Parties' combined market shares both in terms of merchant sales and capacity, the Merged Entity is likely to have the ability to foreclose adipic acid as an input into the production of aliphatic polyester TPU, taking into account the Parties combined market share. Notably, the market share of the Merged Entity in the EEA is significantly higher than the market share of its remaining competitors, which indicates that alternative sources of supply are limited, as discussed in section 6.3.3. Moreover, market participants generally doubt the suitability of Chinese adipic acid in terms of quality in order to produce Aliphatic Polyester TPU.734
(1018) The market investigation has not clearly confirmed that adipic acid is only an indirect input into aliphatic polyester TPU. EEA production for aliphatic polyester TPU is highly concentrated, with […] being one of only three domestic suppliers. In turn, […] has voiced significant concerns throughout the market investigation as to the post-transaction availability and pricing of adipic acid in the EEA, thereby impacting the competitiveness of downstream production, including aliphatic polyester.735
(1019) As a result, the Commission raised serious doubts in Phase I in relation to the risks of input foreclosure arising from the Transaction and its effects in the supply of aliphatic polyester TPU. These concerns have not been clearly dispelled over the course of the investigation in Phase II, as apparent from […] contribution referred to in recital (1018). Conversely, the Commission's Phase II investigation did confirm the existence of a significant impediment to effective competition resulting from horizontal non-coordinated effects arising in the supply of adipic acid in the EEA. On balance, since the commitments entered into by the Notifying Party will address both these effects and associated vertical concerns, it is not necessary to discuss in details the likelihood that any foreclosure strategy in relation to aliphatic polyester TPU could be profitable and/or materialise.
6.4.8.6.(C) Conclusion
(1020) Based on the assessment in recitals (1011) to (1019) and in light of the results of the market investigation and of all the evidence available to it, the Commission concludes that, in view of the Final Commitments entered into by the Notifying Party, it is not necessary to conclude as to whether the Transaction would lead to a significant impediment to effective competition in the internal market as a result of vertical non-coordinated effects arising from vertical links between adipic acid and aliphatic polyester TPU in the EEA, as the commitments would in any event address such potential concerns.
6.4.8. Level II of the polyamide value chain: Caprolactam
(1021) Caprolactam is used almost entirely for production of PA 6 BP (as well as Co- Polyamide 6/6.6 BP and 6.6/6 BP).
(1022) Irrespective of market definition, the Notifying Party argues that the Transaction would leave effective competition unaffected, in particular due to the absence of input foreclosure risks.
6.4.8.1. Market structure
(1023) The Business does not produce caprolactam and does not have the equipment to do so. BASF's market shares for caprolactam are provided in Table 32, which shows the Parties' and competitors' EEA and global market share estimates for 2017.
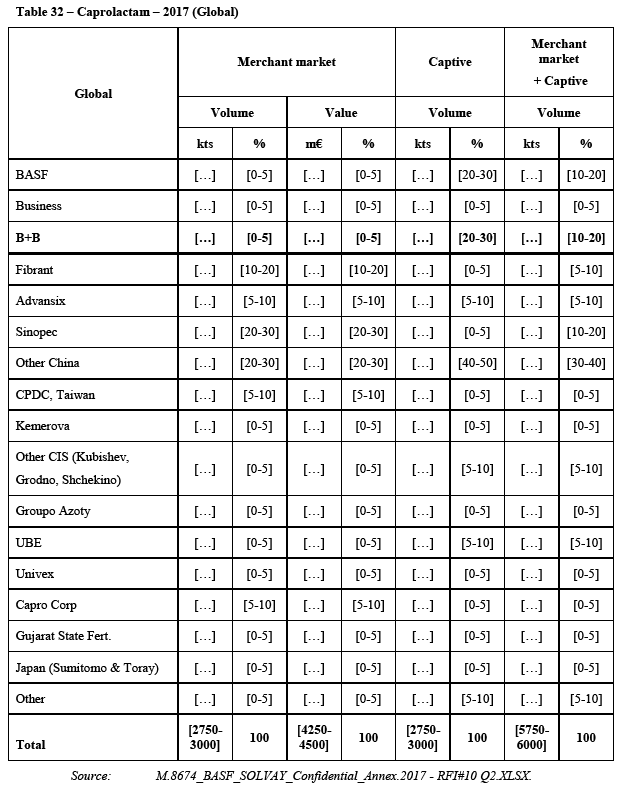
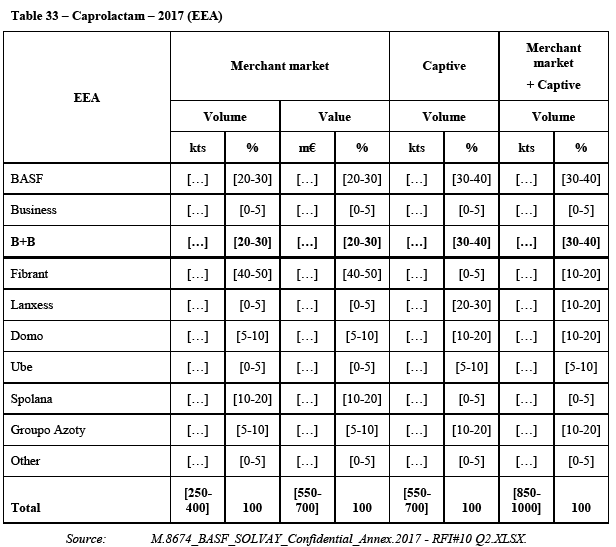
(1024) Both BASF and (to a much lesser extent) the Business produces PA 6 BP. The Parties' market shares for PA 6 BP are provided in Table 34, which shows the Parties'
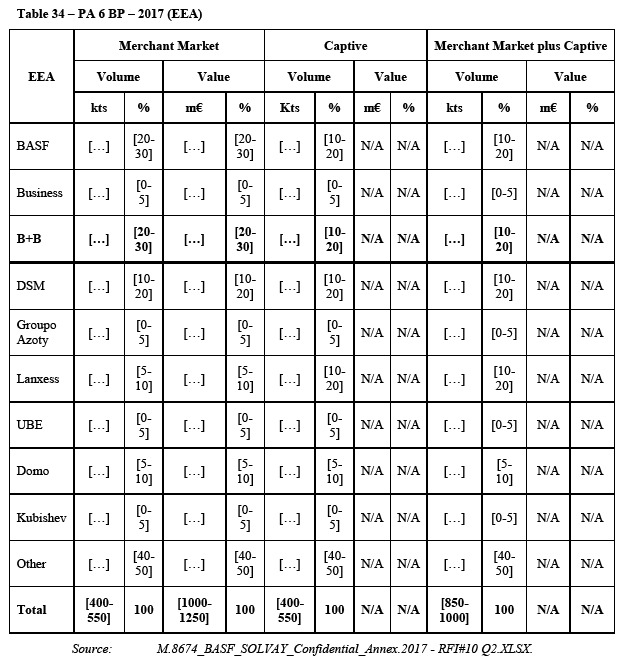
(1025) The Parties' market shares for Co-Polyamide 6/6.6 BP, are provided in Table 35, which shows the Parties' and competitors' EEA market share estimates for 2017.
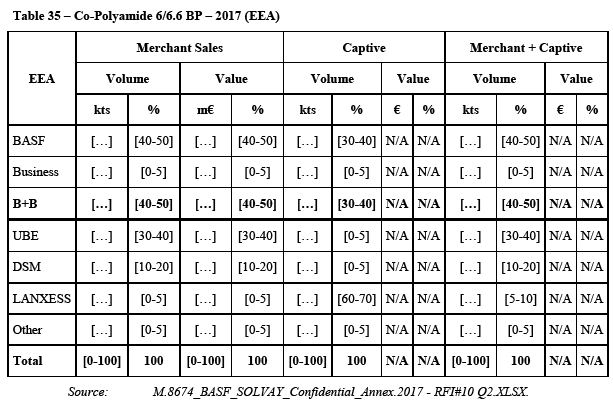
(1026) The Parties are unable to provide market shares estimates for Co-Polyamide 6.6/6 base polymer due to their very limited activity in the field and the fact that Co- Polyamide 6.6/6 BP is not distinguished from PA 6.6 BP in sources such as the PCI Yellowbook or in imports / exports statistics. As a result, market shares for PA 6.6 BP (see Section 6.3.8) may be used as a proxy. The Parties' merchant sales and captive use of Co-Polyamide 6.6/6 BP in the EEA are provided in Table 36.
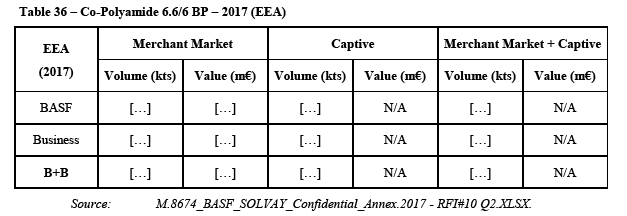
6.4.8.2. The Notifying Party's view
(1027) The Notifying Party submits that the Merged Entity will have no market power in the market for caprolactam and will thus be unable and without incentive to foreclose producers of PA 6 BP, Co-Polyamide 6/6.6 BP and/or Co-Polyamide 6.6/6 BP.
6.4.8.3. The Commission's assessment
(1028) As stated in recital (450), for a Transaction to raise input foreclosure competition concerns according to the Non-Horizontal Merger Guidelines, the Merged Entity must have a significant amount of market power upstream. In the present case however, the Merged Entity's market share in the upstream market, caprolactam is limited to [20-30]%, in volume and value, based on merchant sales, and [30-40]% in volume based on merchant sales plus captive use. Such shares are not prima facie evidence of market power.
(1029) Only BASF produces caprolactam. As a result the Transaction does not enhance the Merged Entity's (in)ability to engage in input foreclosure strategy.
(1030) In addition, the Parties face various strong competitors in the supply of caprolactam. Such competitors include pure merchant players, such as Fibrant and Spolana, which market shares' in the EEA amount to approximately [40-50]% and [10-20]%, in volume and value, based on merchant sales. Competitors also include integrated players such as Lanxess, Ube, Domo and Groupo Azoty.
(1031) PA 6 BP producers are the main customers of caprolactam. A number of such producers are backward integrated into caprolactam (including Lanxess, Ube, Domo and Groupo Azoty). As a result, the Merged Entity only has limited incentives to engage in a foreclosure strategy. Integrated producers could potentially increase their caprolactam production, thus defeating the foreclosure strategy of the Merged Entity that would face difficulties to compensate a drop in its caprolactam sales by increasing its PA 6 BP sales.
(1032) Furthermore, the Business only has little to no production of PA 6 BP, Co-Polyamide 6/6.6, and Co-Polyamide 6.6/6 BP, and only generates merchant sales for Co- Polyamide 6.6/6 BP, which are limited in volume ([…] kts). As a result it is unlikely that the Transaction would enhance the Merged Entity's (lack of) incentive to engage in input foreclosure strategy.
(1033) Finally, no respondent to the market investigation raised concern regarding the supply of caprolactam.
6.4.8.4. Conclusion on input foreclosure
(1034) The Commission concludes that the Transaction is unlikely to significantly impede effective competition as a result of vertical non-coordinated effects arising from vertical links between caprolactam (upstream) and PA 6 BP, Co-Polyamide 6/6.6 BP and Co-Polyamide 6.6/6 BP (downstream) in the EEA.
6.4.9. Level III of the polyamide value chain: AH Salt, input foreclosure to co-polyamide producers
(1035) A large number of customers producing co-polyamide (level IV) have expressed concerns that the Merged Entity could choose not to sell AH Salt (level III) in the merchant market or do it in less favourable terms and thus deny them access to an important input for their business. Some of these customers further claim that AH Salt sourced from the Parties account for a significant proportion of their needs and that they are therefore dependent on being able to buy from the Parties because there are no alternatives to find the extra volumes required.
(1036) BASF’s is the clear market leader for PA 6/PA 6.6 Co-Polyamide BP in the EEA with a market share of [40-50]% in the merchant market and [40-50]% for captive use plus merchant sales, while the Business is not present at all. The other main suppliers in the merchant market are UBE ([30-40]%), DSM ([10-20]%) and Lanxess ([0-5]%). Therefore, the Merged Entity would hold a strong market position in both the upstream market for AH Salt ([90-100]% market share in the merchant market) and the downstream market for Co-Polyamide 6/6.6 BP ([40-50]% market share in the merchant market).
6.4.9.1. Ability to foreclose access to AH Salt to Co-Polyamide PA 6/6.6 Producers 6.4.10.1.(A) The Notifying Party's view
(1037) The Notifying Party submits that the Merged Entity would have no ability to foreclose AH Salt to customers producing co-polyamide for the reasons set out in recitals (1038) to (1040).
(1038) First, the Notifying Party claims that the combined market share of the Parties overestimate their competitive importance since AH Salt producers who do not use their entire capacity could enter the merchant market or expand their merchant sales. The Merged Entity would continue to face the competitive constraint posed by the capacity available to third parties in the EEA. Such third parties include DowDuPont, Invista and Radici. In the Response to the 6(1)(c) Decision the Notifying Party further submits that third party demand for AH Salt on the merchant market for the production of Co-Polyamide 6/6.6 BP in the EEA only amounted to approximately 3- 4 kts in 2016. The Notifying Party estimates that, even after taking account of their respective captive needs of AH Salt (including supplies needed for the PA 6.6 BP that it sells on the merchant market), Invista, DuPont and Radici would each individually have sufficient free AH Salt capacity in the EEA to supply this 3-4 kts requirement in full. According to the Notifying Party, if the Merged Entity were to withdraw supply or attempt to raise prices, there is no reason to doubt that Invista or DowDuPont or Radici would be able to supply the Merged Entity’s Co-Polyamide 6/6.6 BP customers.
(1039) Moreover, the Notifying Party submits that none of Invista, DowDuPont and Radici and Ascend or CSM are active in the production of Co-Polyamide 6/6.6 BP. They would thus gain nothing from refusing to supply these customers with their excess capacities or supplying these customers at uncompetitive terms. According to the Notifying Party, given the large number of third-party suppliers with surplus capacity, customers would be expected to be supplied on competitive terms .Second, the Notifying Party considers that customers could always start producing AH Salt themselves and enter the upstream market.
(1040) Finally, the Notifying Party submits that Ascend in particular has significant production capacities of AH Salt in the USA and customers could import from it into the EEA.
6.4.10.1.(B) The Commission's assessment
(1041) First, as explained in section 6.3.4 the combined market shares of the Parties are very high. The Merged Entity would control approximately [70-80%] of the AH Salt production in the EEA and virtually all the merchant market. These market shares indicate a presumption of dominance which is supported by market participants that claim that the Merged Entity would have a dominant position in AH Salt. In fact, virtually all customers and producers indicate that BASF and Solvay are the top 2 producers of AH Salt in the EEA.736
(1042) The situation described in recital (1041) explains why the majority of the respondents to the market investigation consider that the acquisition by BASF of Solvay's polyamide business would have a negative impact on the market for AH Salt in the EEA.737 For example, […] explains that it "produces Co-polyamide PA 6- PA6.6, for which it procures AH Salt from both Solvay (5%) and BASF (95%). BASF and Solvay are the only suppliers of AH Salt active in merchant market and therefore 100% of their final production depends on AH Salt procured by either Solvay, or BASF. About 15% of […] PA 6 compounds are based in Co-polyamide".738 Other customer of AH Salt, […], adds "BASF will dominate the AH Salt market, being the only alternative in the market".739
(1043) Second, the Notifying Party submits that the Merged Entity would continue to face the competitive constraint posed by producers who do not use their entire capacity and that could enter the merchant market. However, as explained in section 6.3.4, the market investigation does not show that there are companies with additional capacities to serve additional volumes in the market.740 Moreover, […]741, […]742 and […]743 explained that they are not active in the merchant market, they do not have spare capacity to sell AH Salt into the merchant market, they do not have access to enough raw materials and that they are not interested in entering that market nor they have any plans to increase capacity or enter the market.744
(1044) Third, regarding the argument that customers could always start producing AH Salt themselves, as explained in section 6.3.4, customers and producers consider the barriers to start producing AH Salt as high in relation to investment costs, access to raw materials and required know-how. Moreover, the Merged Entity would also enjoy market power at the level of HMD and adipic acid (the inputs needed to produce AH Salt) and therefore could also increase prices at this level of the polyamide value chain. This is why customers do not consider producing AH Salt themselves as a viable alternative. Indeed, access to raw materials is the most important barrier to the production of AH Salt according to producers and customers. […] explains that "[t]he option to produce own AH salt by […] is likewise limited by the supply of ADN (1 plant in EU, as JV of Invista and Solvay-BASF) and HMD. Moreover it requires heavy investment and a technology which we do not have. An investment in own AH salt, or separate purchase of HMD and AA would likewise increase our cost and reduce our competitivity with BASF on the final PA6/66 film resin. An investment in PA6 film resin development without AH salt is not considered as feasible on medium term and rather unrealistic as to chances of success".745
(1045) Finally, regarding the notion that customers could import AH Salt from outside the EEA, the market is EEA-wide and, as explained in section 5.4.3, customers do not consider imports as a viable alternative due to higher prices derived from import tariffs, transportation costs, logistic and technical difficulties, long delivery times and technical difficulties and related costs related to importing solid AH Salt.
6.4.10.1.(C) Conclusion on ability to foreclose
(1046) The Commission therefore concludes that the Merged Entity would have the ability to foreclose access to AH Salt to Co-Polyamide PA 6/6.6 producers.
6.4.9.2. Incentive to foreclose access to AH Salt to Co-Polyamide 6/6.6 Producers 6.4.10.2.(A) The Notifying Party's view
(1047) The Notifying Party considers that the Merged Entity would have no incentives to foreclose AH Salt (level III) to customers producing co-polyamide 6/6.6 (level IV) for the reasons set out in recitals (1048) to (1049).
(1048) First, the Notifying Party claims that there are no applications for which there is a specific customer demand for co-polyamide 6/6.6. This implies that if the Merged Entity tried to foreclose access to AH Salt to some co-polyamide 6/6.6 producers, in the worst case these customers could replace it with PA 6 BP or PA 6.6 BP. Thus, as Co-Polyamide 6/6.6 BP can be easily substituted by PA 6 BP and PA 6.6 BP, the Merged Entity cannot expect that sales of Co-Polyamide 6/6.6 BP lost by foreclosed competitors would be diverted to the Merged Entity, and in any event not in the same proportion to its share of sales of Co-Polyamide 6/6.6 BP.
(1049) Second, given the small volumes of AH Salt required to produce Co-Polyamide 6/6.6 BP and also given the small volumes purchased by the AH Salt customers of the merging parties, these customers should easily find another AH Salt supplier.
6.4.10.2.(B) The Commission's assessment
(1050) As explained in Section 5.6, Co-Polyamide 6/6.6 BP belongs to a different product market than PA 6.6 BP or PA 6 BP.
(1051) BASF’s is the clear market leader for Co-Polyamide 6/6.6 BP, while the Business is not present at all. The strong market position of the Merged Entity in both the upstream market for AH Salt ([90-100]% or more market share in the merchant market) and the downstream market for co-polyamide 6/6.6 ([40-50]% market share in the merchant market) makes a foreclosure strategy a very plausible scenario. Indeed, customers of AH Salt which are competitors in the Co-Polyamide PA 6/6.6 BP market fear that the Merged Entity would deny them access to AH Salt or would supply them at less favourable terms, thus increasing their input costs and reducing their ability to compete.
(1052) This is confirmed by an important customer of AH Salt, […], that explains "Solvay is the only integrated ADN-HMD/AA-AH-PA66 producer still having enough free capacity in the upstream part of the PA 6.6 value chain (ADN-HMD/AA-AH salt) to supply the AH salt quantities we require. Solvay was not a competitor, as they are not in the downstream PA6/66 high viscosity film resin business. BASF will be able to integrate and reinforce its PA66 chain, and utilize the former free Solvay capacity to assure the growth of its PA66 engineering plastics business and its PA6/6,6 high viscosity film resin business. BASF will have good reason to reserve the supply of AH salt to its captive consumption and thereby enjoy a competitive advantage over its PA 6/66 film resin competitors that will have to import lower quality and higher price AH salt solution from China or USA".746
(1053) […] fears that the "merger might have a detrimental negative effect, since its two suppliers of AH Salt (which are the only ones who make AH Salt commercially available) will become one company. This is a merger to monopoly situation, in a market where […] would strongly prefer to have two suppliers. Moreover, the Merged Entity will be a competitor downstream, for Co-polyamide PA 6/PA 66, which clearly creates worries of a possible input foreclosure for […].747 The same customer adds "BASF's increased captive requirements and the disappearance of the other merchant seller will have a detrimental and negative impact on the merchant market. It would be in BASF's best interest to foreclose the market".748
(1054) Similarly, […] fears that "[i]n the long term, the Merged Entity will potentially increase its market share in the downstream market and therefore will have a bigger captive consumption of AH Salt to produce more PA 6.6. In that case, AH Salt will become short in the market. That monopolistic situation also raises concerns about the price of the product. If AH Salt is not available in the merchant market, […] would have to look for alternatives (potentially causing investments) or even exit the Engineering Plastics PA 6.6 market for food packing. BASF will be dominant in the AH-Salt market".749
(1055) As explained in Section 6.3.4, contrary to the Notifying Party’s claim, the market investigation has not revealed the presence of other EEA market participants with spare capacities to serve additional volumes in the EEA market.750 Therefore, other producers of AH Salt cannot be considered as a constraint to the Merged Entity.
6.4.10.2.(C) Conclusion on incentives to foreclose
(1056) The Commission therefore concludes that the Merged Entity would have the incentive to foreclose access to AH Salt to Co-Polyamide PA 6/6.6 producers.
6.4.9.3. Effects of a potential input foreclosure strategy with respect to AH Salt in the Co- Polyamide PA 6/6.6 value chain
6.4.10.3.(A) The Notifying Party's view
(1057) The Notifying Party claims that the Merged Entity would have neither the ability nor the incentive to foreclose AH Salt to co-polyamide producing customers and therefore no effect on effective competition would emerge.
(1058) Furthermore, the Notifying Party claims that even if the price for AH Salt increases, it would not have effects on the Co-Polyamide PA 6/6.6 market. This is because only low quantities of AH Salt go into the production of PA 6/6.6 Co-Polyamide BP and, thus, only accounts for a small share of production costs. If a foreclosed input represents a small share of the rivals’ production costs, an increase in the cost of the input results in a much smaller increase in the overall production costs.
(1059) Finally, in the Response to the 6(1)(c) Decision, the Notifying Party submits that given the marginal relevance of AH Salt for the production of Co-Polyamide 6/6.6 BP, the concern that the downstream competitors would suffer a competitive disadvantage because the Merged Entity would know “their commercial strategy and production figures” as well as “the minimum price it may offer” does not hold. The production cost of AH salt only represents a minor cost (approx. 20%) element in total production cost of Co-Polyamide 6/6.6 BP. Hence, sourcing AH Salt from the Merged Entity would not allow BASF to easily reverse engineer competitors’ commercial strategies or to calculate the minimum price that they can offer, and this would thus not put downstream competitors at a competitive disadvantage.
6.4.10.3.(B) The Commission's assessment
(1060) AH Salt is a key input for the production of Co-Polyamide PA 6/6.6 BP which is in turn the precursor of Co-Polyamide PA 6/6.6 EP. If the prices of AH Salt were to increase or if availability were to be reduced, purchasers of AH Salt would be at a competitive disadvantage.
(1061) The market share of the Merged Entity in the AH Salt merchant market would be above [90-100]% or more as a result of the concentration, and over [50-60]% if captives sales were included. The market investigation has not revealed the existence of other market participants with spare capacities to serve additional volumes of AH Salt in the EEA. Accordingly, were the Merged Entity to engage in a strategy of input foreclosure the availability of AH Salt in the market would be drastically reduced and/or prices would increase.Therefore, the costs of a key input would increase for other competitors in the downstream market (Co-Polyamide PA 6/6.6 BP) or they would face difficulties securing the volumes of AH Salt needed for their production of Co-Polyamide PA 6/6.6 BP. As a result, they could not continue offering the same prices or volumes to their customers.
(1062) […] confirms this position by stating that "BASF will dominate the AH Salt market, being the only alternative in the market. At the same time, BASF is a competitor of […] in the co-polyamide. Therefore, BASF may increase the prices of AH Salt to […] or foreclose them completely. This will lead to higher prices for final consumers".751
(1063) Moreover, competitors in the Co-Polyamide PA 6/6.6 are concerned that if they are forced to source AH Salt exclusively from the Merged Entity, which would be their competitor in the downstream market, they would be at a competitive disadvantage because the Merged Entity would know have access to commercially sensitive information (such as commercial strategy and production figures) in advance. "[…] will have a clear disadvantage if they are forced to purchase AH Salt from the Merged Entity, because BASF will know their commercial strategy and production figures. Indeed, […] claims that by knowing their AH Salt volumes, BASF can calculate the material […] will put in the market and the minimum price it may offer. If […] purchases 1000 metric tons of AH Salt, BASF will know that […] will put in the market 6000 metric tons of Co-Polyamide. […] would have to go through a qualification process if it was to change supplier. Indeed, […] provides materials for the automotive and food industry, where a high level of security and health is required. In any case, post-transaction there will be only one supplier in Europe, which is their competitor".752
(1064) Each metric ton of AH Salt produces 6 metric tons of Co-Polyamide PA 6/6.6 base polymer.753 Therefore, contrary to the Notifying Party’s claim that it will not be able to know the volumes that downstream competitors will sell into the merchant market, the Merged Entity will be able to determine the volumes of AH Salt that each competitor is sourcing (if the Merged Entity does not foreclose them completely) as the Merged Entity will be the only supplier of AH Salt in the merchant market in the EEA and will therefore be able to calculate the maximum volumes of Co-Polyamide PA 6/6.6 base polymer that competitors will be able to produce.
6.4.10.3.(C) Conclusion on effects of a foreclosure strategy
(1065) The Commission therefore concludes that, were the Merged Entity to pursue an input foreclosure strategy, there would likely be a negative impact on competition in the Co-Polyamide PA 6/6.6 market.
6.4.10.3.(D) Conclusion
(1066) Based on the assessment in recitals (1035) to (1065) and in light of the results of the market investigation and of all the evidence available to it, the Commission concludes that the Transaction would significantly impede effective competition as a result of vertical non-coordinated effects arising from vertical links between the upstream EEA marker for AH Salt and the downstream EEA market for Co- Polyamide PA 6/6.6.
6.4.10. Level IV of the polyamide value chain: PA 6.6 BP, Input foreclosure of PA 6.6 EP producers
(1067) Engineering plastics are typically produced in a polymerisation process by mixing molten polymers with additional ingredients including additives, stabilisers and fillers. As such, producers of PA 6.6 BP sell the base polymer to producers of PA 6.6 EP.
(1068) Irrespective of market definition, the Notifying Party argues that the Transaction would leave effective competition unaffected, in particular due to the absence of input foreclosure risks.
6.4.10.1. Ability to foreclose access to PA 6.6 BP to PA 6.6 EP producers 6.4.11.1.(A) The Notifying Party's view
(1069) According to the Notifying Party, PA 6.6 EP producers would still have access to alternative suppliers post-transaction (see Section 7.2.5(A)) with sufficient capacity, which demonstrate the lack of ability of the Merged Entity to foreclose access to PA 6.6 BP.
(1070) The Notifying Party also argues that the significance of imports would defeat any attempt at price increase of PA 6.6 BP. In particular, there are significant PA 6.6 BP imports from the USA (mainly from Invista and Ascend) which would represent an actual competitive constraint on the Merged Entity. In addition to the US, the Notifying Party also highlights trade flows with other regions indicating that imports are a credible alternative for PA 6.6 BP customers in the EEA.754 The Notifying Party further argues that prices of imports do not significantly differ from the selling price of the Parties in the EEA, evidencing that any attempt of a price increase of PA 6.6 BP could be defeated through imports.755
(1071) In the Response to the 6(1)(c) Decision, the Notifying Party reiterates these arguments, insisting on the strength and excess capacity of Ascend and Invista,756 and submitted an economic analysis discussing how trade flows and overcapacity globally render a foreclosure strategy unlikely.757 The Notifying Party also renews the claim that only market shares based on merchant sales are relevant for the purposes of the competitive assessment.758
6.4.11.1.(B) The Commission's assessment
(1072) The Parties' market shares in the relevant segments as detailed in Section 6.3.8.1 (for PA 6.6 BP) and Section 6.3.9.1 (for PA 6.6 EP) are significant. In particular, based on the market reconstitution carried out by the Commission for 2017, the Merged Entity would have a market share for PA 6.6 BP in the EEA reaching [30-40]% for merchant sales (in volume respectively), [40-50]% based on captive use plus merchant sales (in volume), and [40-50]% based on capacity (based on the Notifying Party's own estimates). Market shares based on captive use plus merchant sales, as well as production capacity are particularly relevant for the competitive assessment of the polyamide value chain, in complement with those based on merchant sales.
(1073) As mentioned in Section 6.3.8, customers of PA 6.6 BP who responded to the market investigation largely disavow the Notifying Party's claim that sufficient alternative competitors would remain available in the PA 6.6 BP market post-transaction. The conclusion remains identical when looking at responses of producers of PA 6.6 EP, which are active on the downstream market. A large majority of producers of PA 6.6 EP mentioned they would not have a sufficient choice of suppliers post- transaction.759 In particular, some customers fear BASF would stop selling the Business' products on the merchant markets, instead using PA 6.6 BP volumes internally, for its own PA 6.6 EP production.760
(1074) These concerns appear legitimate when looking at BASF's plans for Solvay's PA 6.6 BP production. Internal documents of the Parties also show that […]761 […].762 […].763
(1075) Furthermore, most responding PA 6.6 EP producers confirmed sourcing PA 6.6 BP locally, thus challenging the Notifying Party's argument that imports would defeat any input foreclosure strategy.764 The competitive constraint exerted by imports of PA 6.6 BP from outside the EEA is further discussed in Section 6.3.8.
6.4.11.1.(C) Conclusion on ability to foreclose
(1076) The Commission therefore concludes that the Merged Entity would have the ability to foreclose access to PA 6.6 BP to PA 6.6 EP producers.
6.4.10.2. Incentive to foreclose access to PA 6.6 BP to PA 6.6 EP producers 6.4.11.2.(A) The Notifying Party's view
(1077) Based on the difference in margins between PA 6.6 BP and PA 6.6 EP, the Notifying Party also argues that the Merged Entity would have no incentive to foreclose PA 6.6 EP producers. In particular, the critical diversion ratio, i.e. the ratio of the PA 6.6 BP and the PA 6.6 EP margin, above which an incentive to foreclose PA 6.6 BP arises, is quite large. The Merged Entity would thus need to recapture a large share (at least half) of each unit of PA 6.6 EP diverted from its potentially foreclosed rivals, to have an incentive to foreclose input.765
6.4.11.2.(B) The Commission's assessment
(1078) Regarding the analysis of the “critical diversion ratio” between PA 6.6 BP and PA6.6 EP, the Commission notes that such analysis is largely conservative because it does not take into account price increases in PA 6.6 EP which would result from the strong position of the Merged Entity in this market, as discussed in Section 6.3.9.
(1079) The market investigation also indicates that producers of PA 6.6 EP expect the Merged Entity to have an incentive to engage in an input foreclosure strategy.766 Some market participants even expect at least some producers of PA 6.6 EP to be driven out of the market.767
(1080) The analysis of BASF and the Business' internal documents […],768 […] 769
6.4.11.2.(C) Conclusion on incentive to foreclose
(1081) The Commission therefore concludes that the Merged Entity would have the incentive to foreclose access to PA 6.6 BP to PA 6.6. EP producers.
6.4.10.3. Effects of a potential input foreclosure strategy with respect to PA 6.6 BP to PA 6.6 EP producers
6.4.11.3.(A) The Notifying Party's view
(1082) The Notifying Party claims that the Merged Entity would have neither the ability nor the incentive to foreclose access to PA 6.6 BP to PA 6.6 EP producers and therefore no effect on effective competition would emerge.770
6.4.11.3.(B) The Commission's assessment
(1083) PA 6.6 BP is a key input for the production of PA 6.6 EP. If the price for PA 6.6 BP increases or the quantity supplied decreases, the competing producers of PA 6.6 EP that source PA 6.6 BP in the merchant market would thus be at a competitive disadvantage compared to the Merged Entity.
(1084) A large majority of producers of PA 6.6 EP who responded to the market investigation mentions that the Transaction would have a negative impact on their business.771 Vertical effects, particularly input foreclosure, are one of the concerns expressed by PA 6.6 EP producers.772 One of the largest non-integrated PA 6.6 EP producer in particular, […], spontaneously submitted an analysis of such potential input foreclosure scenario in the BP market,773 and even argued that since the announcement of the Transaction, the Business already started to restrict sales of PA 6.6 BP to third parties.774
6.4.11.3.(C) Conclusion on effects on competition
(1085) The Commission therefore concludes that, were the Merged Entity to pursue an input foreclosure strategy of PA 6.6 BP, there would be significant negative effects on competition in the PA 6.6 EP market.
6.4.11.3.(D) Conclusion
(1086) Based on the Parties' increased market power in both PA 6.6 BP and of PA 6.6 EP and in light of their internal documents, the Commission concludes that the Transaction would significantly impede effective competition as a result of vertical non-coordinated effects arising from vertical links between PA 6.6 BP and PA 6.6 EP in the EEA.
6.4.11. Level IV of the polyamide value chain: PA 6.6 BP, Input foreclosure of performance fibres producers
(1087) Performance fibres are typically produced in a process during which base polymer (e.g. PA 6.6 BP) is spun directly into filaments, extruded through spinnerets. These filaments are then gathered together, in parallel, to form a tow. This tow is subsequently stretched and can either be left flat or be crimped, heat set, dried and/or cut, depending on the application. As such, producers of PA 6.6 BP sell the base polymer to producers of performance fibres.
(1088) Irrespective of the market definition, the Notifying Party argues that the Transaction would leave effective competition unaffected, in particular due to the absence of input foreclosure risks.
6.4.11.1. Ability to foreclose access to PA 6.6 BP to performance fibres producers 6.4.12.1.(A) The Notifying Party's view
(1089) According to the Notifying Party, performance fibres producers would still have access to alternative suppliers of PA 6.6 BP post-transaction with sufficient capacity, which demonstrate the lack of ability of the Merged Entity to foreclose access to PA 6.6 BP.775
6.4.12.1 (B) The Commission's assessment
(1090) The Parties' market shares for PA 6.6 BP are detailed in Section 6.3.8. In particular, based on 2017 data, the Merged Entity would have a market share for PA 6.6 BP in the EEA reaching [30-40]% for merchant sales (in value and volume respectively) and [40-50]% based on captive use plus merchant sales volumes.
(1091) BASF divested its performance fibres activities in 2002. The Business' market shares for PA 6.6 Performance Fibres are provided in Table 37, which shows the Parties' and competitors' EEA market share estimates for 2017.776
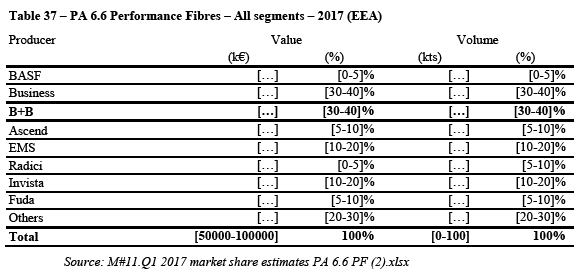
(1092) The Merged Entity's market shares vary depending on potential sub-segments. In particular, the Merged Entity's market shares in the EEA (see Tables 38-41) reach or exceed [40-50]% in the textile and carpet segments, respectively.
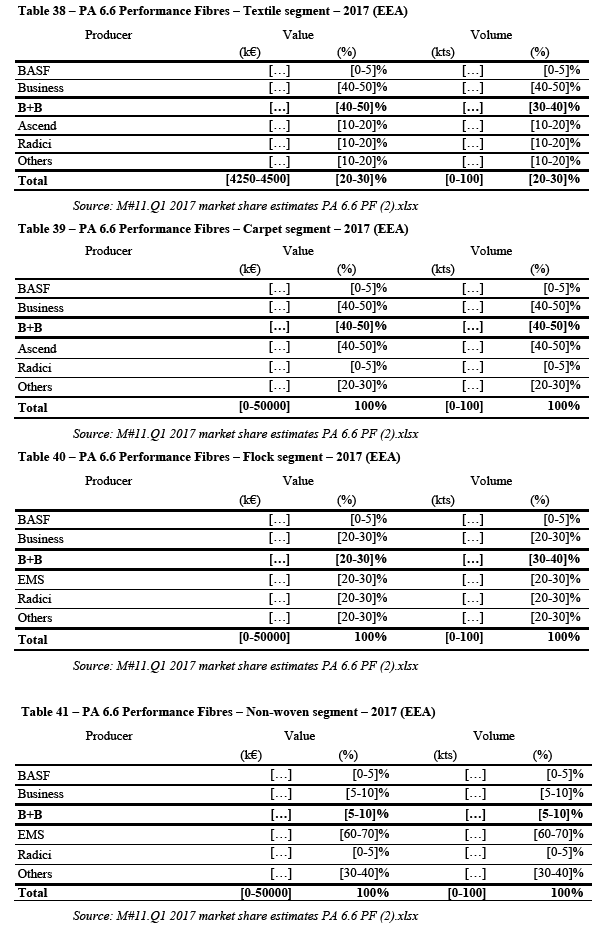
(1093) As mentioned in Section 6.3.8, customers of PA 6.6 BP who responded to the market investigation largely disavow the Parties' claim that sufficient alternative competitors would remain available in the PA 6.6 BP market post-transaction. In particular, the Merged Entity would hold almost [50-60]% of total EEA capacity of PA 6.6 BP, evidencing its market power in the segment.
(1094) The Merged Entity's PA 6.6 EP capacity post-transaction would be larger than the Business' one pre-transaction. As such, the Merged Entity could thus more effectively redirect PA 6.6 BP sales to the EP activity, or threaten to do so, in order to impose higher prices or deliver lower quantities to non-integrated PA 6.6 Performance Fibres manufacturers, such as EMS.
6.4.12.1 (C) Conclusion on ability to foreclose
(1095) The Commission therefore concludes that the Merged Entity would have the ability to foreclose access to PA 6.6 BP to performance fibres producers.
6.4.11.2. Incentive to foreclose access to PA 6.6 BP to performance fibres producers
6.4.12.2 (A) The Notifying Party's view
(1096) The Notifying Party considers that the incentives to foreclose access to PA 6.6 BP suppliers remain unaffected post-transaction, in particular since BASF only has minor merchant market sales of PA 6.6 BP and has no sales of performance fibres, nor does it sell PA 6.6 BP for fibres.
6.4.12.2(B) The Commission's assessment
(1097) Some market participants indicate concerns that the Merged Entity would reduce or interrupt the supply of PA 6.6 BP to performance fibres manufacturers. […] notes for instance that in the past BASF decided not to supply PA 6.6 BP to the performance fibres market, and fear that the Merged Entity would likewise post-transaction cut the supplies offered by Solvay.777 […] customer of performance fibres also worries that a concentration in the PA 6.6 value chain may lead to significant price increases which would impact the PA 6.6 Performance Fibres market.778
(1098) The Merged Entity's sizeable share in the downstream markets for PA 6.6 Performance Fibres, where it is a market leader (with the exception of the non-woven segment in which its market share would be lower), means the company would be well-placed to replace non-integrated players as a supplier of PA 6.6 Performance Fibres.
6.4.12.2 (C) Conclusion on incentive to foreclose
(1099) The Commission considers that, in view of the Final Commitments entered into by the Notifying Party, it is not necessary to conclude as to whether the Merged Entity would have the incentive to foreclose access to PA 6.6 BP to performance fibres producers, as the commitments would in any event address input foreclosure concerns.
6.4.11.3. Effects of a potential input foreclosure strategy with respect to PA 6.6 BP to PA 6.6 Performance Fibres producers
6.4.12.3 (A) The Notifying Party's view
(1100) The Notifying Party claims that the Merged Entity would have neither the ability nor the incentive to foreclose access to PA 6.6 BP to PA 6.6 Performance Fibres producers and therefore no effect on effective competition would emerge. However, even if the Merged Entity were to engage in such a strategy, this would not materially affect the competitive conditions according to the Notifying Parties. Reasons put forward include the presence of other suppliers of inputs (including PA 6.6 BP) to PA 6.6 Performance Fibres manufacturers, as well as imports to the EEA.779
6.4.12.3.(B) The Commission's assessment
(1101) PA 6.6 BP is an important input for the production of PA 6.6 Performance Fibres. If the price for PA 6.6 BP increases or the quantity supplied decreases, the competing producers of PA 6.6 Performance Fibres that source PA 6.6 BP in the merchant market would thus be at a competitive disadvantage compared to the Merged Entity.
(1102) As mentioned in Section 6.3.8, customers of PA 6.6 BP who responded to the market investigation largely disavow the Parties' claim that sufficient alternative competitors would remain available in the PA 6.6 BP market post-transaction, even including imports.
(1103) Customers purchasing PA 6.6 Performance Fibres are also concerned about the vertical integration of the parties and how it may impact the fibres market, with half of the respondents considering the Transaction would ultimately reduce the choice of PA 6.6 Performance Fibres suppliers.780
6.4.12.3.(C) Conclusion on effects on competition
(1104) The Commission therefore concludes that, were the Merged Entity to pursue an input foreclosure strategy of PA 6.6 BP, there would be significant negative effects on competition in the PA 6.6 Performance Fibres market or any of its sub-segments (for carpet, textile, flock and non-woven applications).
6.4.12.3 (D) Conclusion
(1105) The Commission concludes that, in view of the Final Commitments entered into by the Notifying Party, it is not necessary to conclude as to whether the Transaction would lead to significant impediment to effective competition in the internal market as a result of vertical non-coordinated effects arising from vertical links between PA 6.6 BP and PA 6.6 Performance Fibres in the EEA, as the commitments would in any event address such potential concerns.
6.4.12. Products outside the polyamide value chain
6.4.13. Additives
6.4.13.1. Stilbene/thiophene-benzoxazol-based OBA for plastics
(1106) Table 42 provides the Parties' sales for 2017 and the Parties' estimates on market shares on EEA-wide market for stilbene/thiophene-benzoxazol-based OBA for plastics.
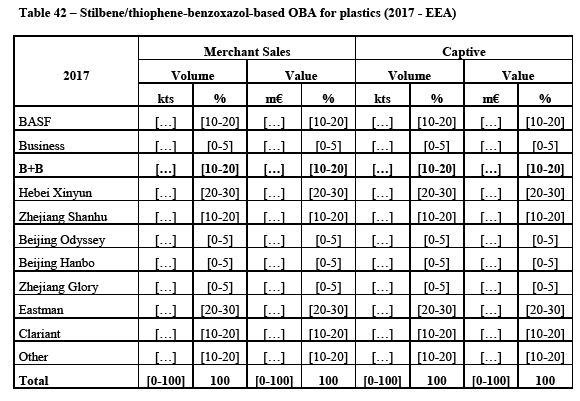
6.4.13.2. Melamine cyanurates and melamine polyphosphates
(1107) Tables 43-48 provide the Parties' sales for 2017 and the Parties' estimates on market shares on a global and EEA-wide market for melamine cyanurate and melanime polyphosphate.
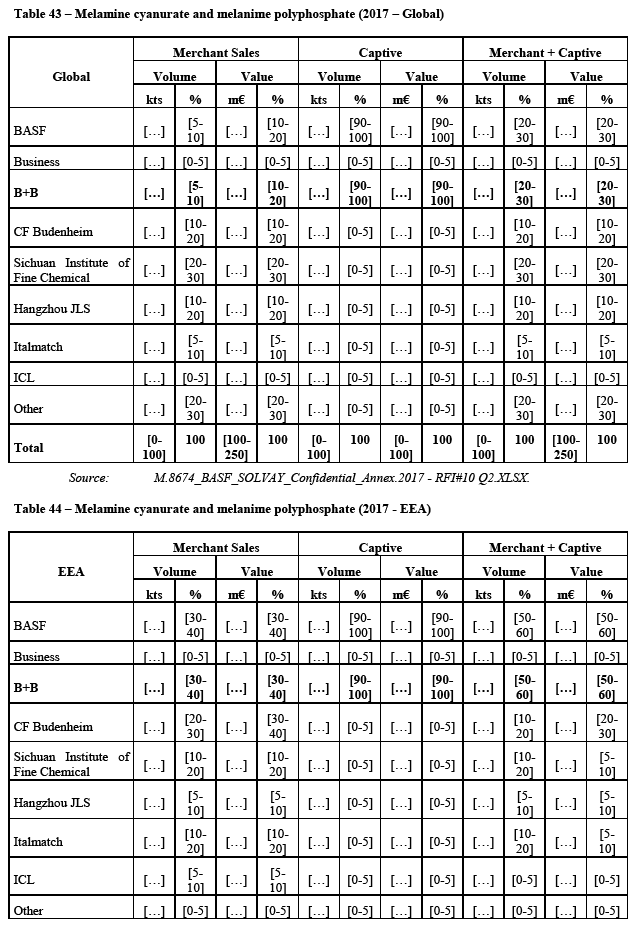
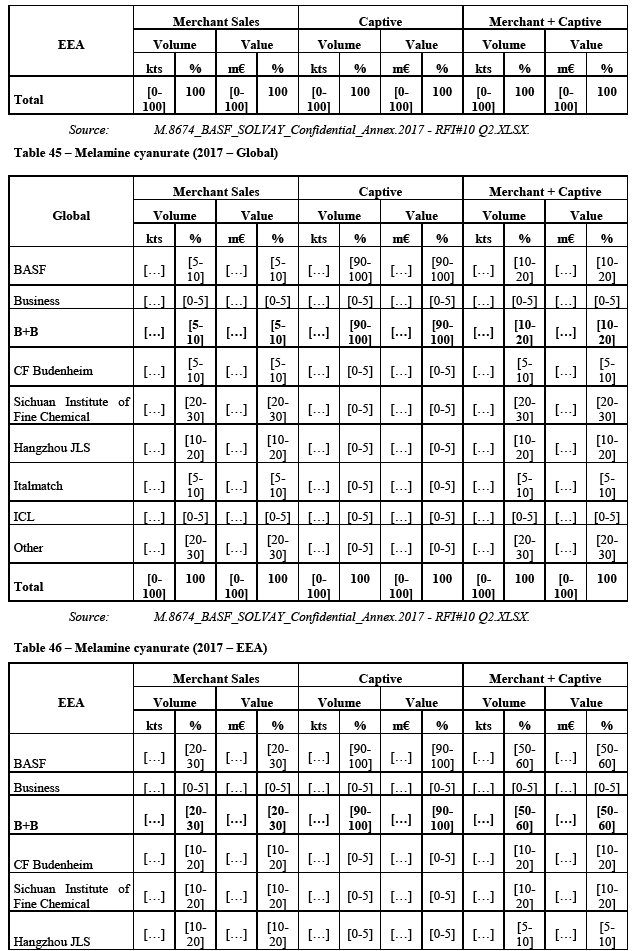
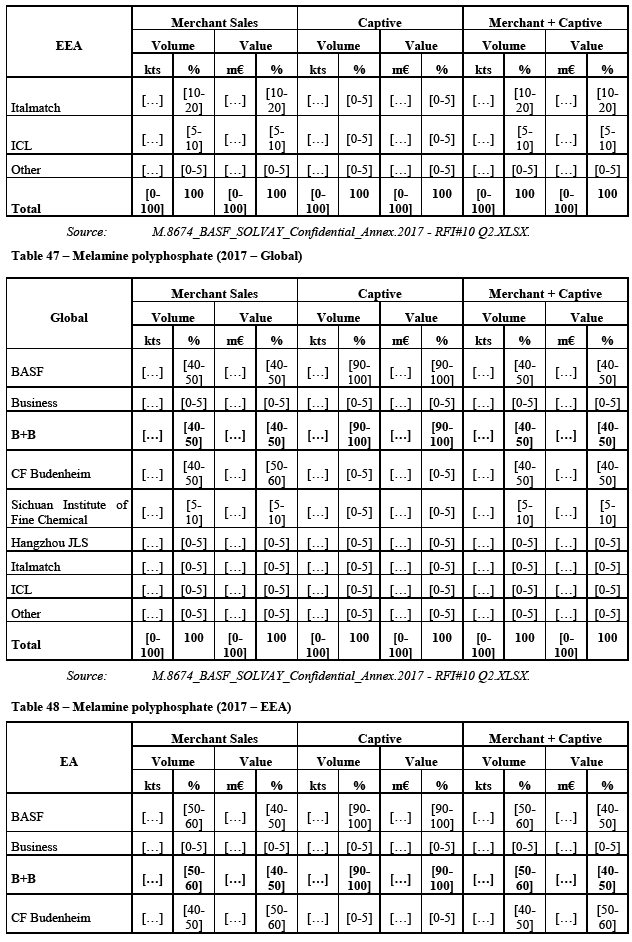
6.4.13.3. HALS
(1108) Tables 49 and 50 provide the Parties’ sales for 2017 and the Parties’ estimates on market shares on a global and EEA-wide market for HALS for plastics.
(1109) Tables 51 and 52 provide the Parties’ sales for 2017 and the Parties’ estimates on market shares on an EEA-wide market for LMW standard HALS and HMW standard HALS781.
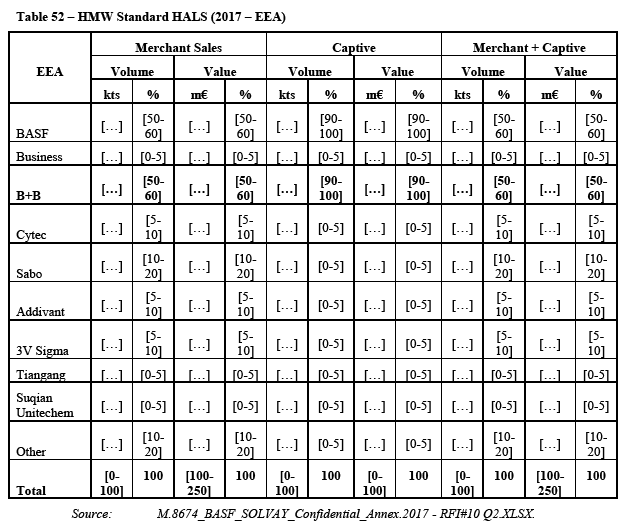
6.4.13.4. Antioxydants
(1110) Tables 53-56 provide the Parties’ sales for 2017 and the Parties’ estimates on market shares on a global and EEA-wide market for primary phenolic antioxidants as well as secondary OPH which are the only antioxidants used in polyamide applications.
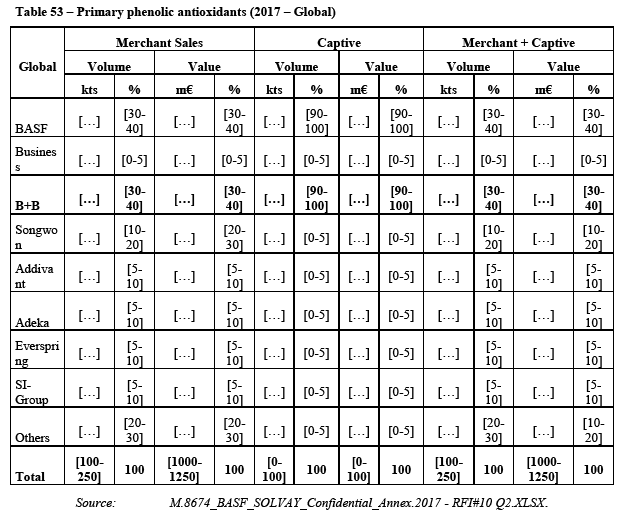
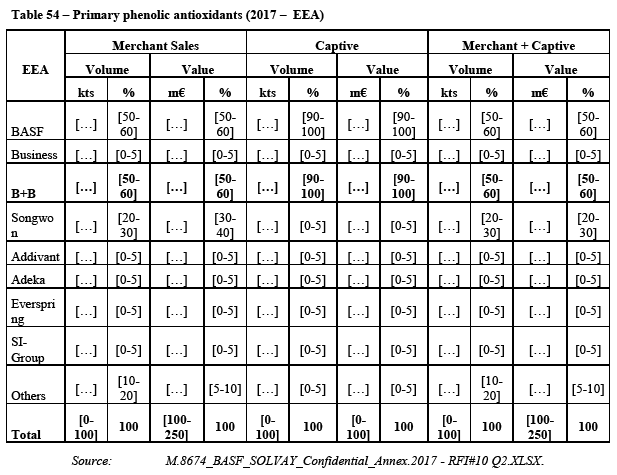
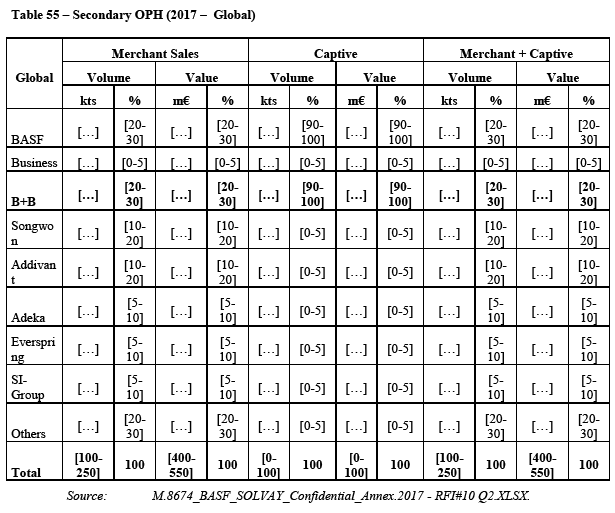
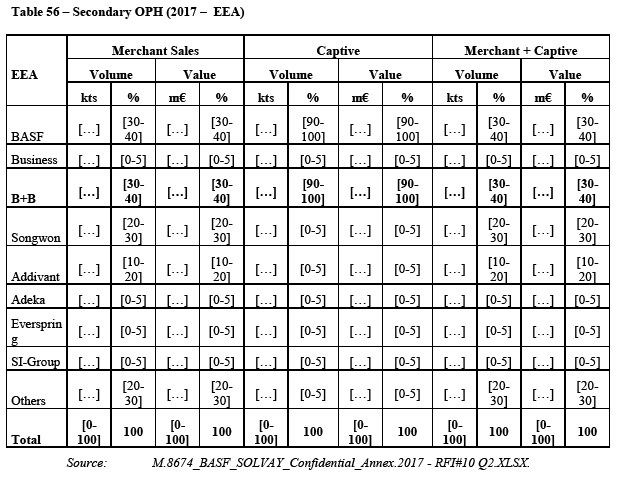
6.4.13.5. Kaolin
(1111) Tables 57-58 provide the Parties’ sales for 2017 and the Parties’ estimates on market shares on a global and EEA-wide market for kaolin used in plastic applications.
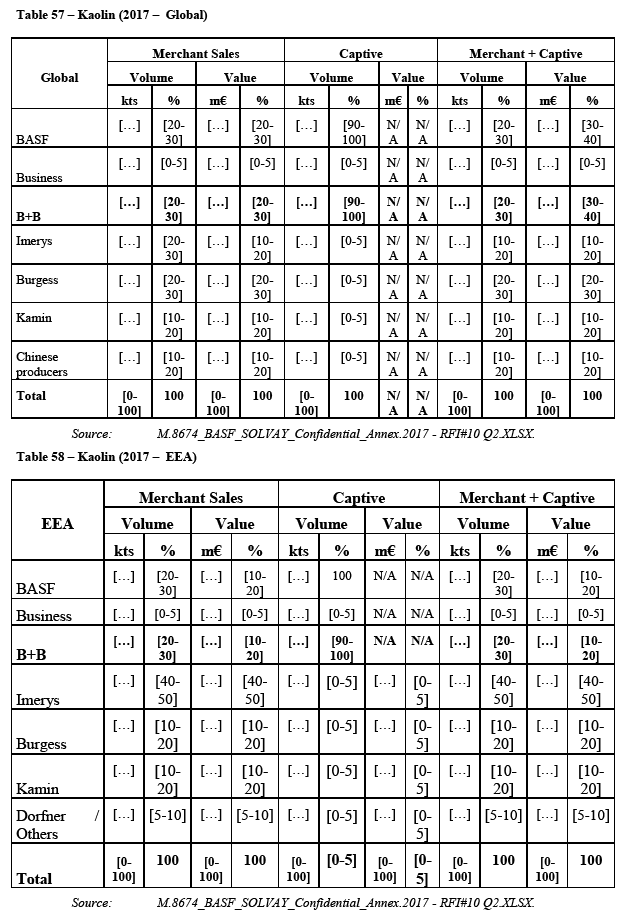
6.4.13.6. Overall assessment on Additives
(1112) The Business is not active in any of the different additives markets mentioned in Section 6.4.13.
(1113) Competitors have indicated the presence of other top additives suppliers besides BASF, such as Budenheim, Italmatch, Adeka Group, Songwon & SABO, 3V Sigma,
Omnova, or other Chinese suppliers.782
(1114) All of the competitors who responded to the market investigation indicated that their customers source additives from anywhere in the world.783 The majority of competitors have also confirmed that they can serve additional volumes if an increased demand of additives in the merchant market in the EEA would arise.784 Competitors that responded to the market investigation also indicated that there are new entrants in the additives market in the last 5 years.785
(1115) The majority of customers who responded to the market investigation confirm that they purchase additives to be used in their plants located in the EEA on a worldwide basis.786 Customers generally multi-source when buying additives.787 Even so, virtually all of the customer-respondents confirmed having sufficient choice of additives suppliers if the Transaction were to go ahead.788 While BASF is mentioned as only one of the potential additives suppliers amongst many competitors, Solvay is not considered as a supplier in this area.
(1116) All the customers that responded confirm also that the proposed Transaction will have no impact on the additives markets.789
(1117) Therefore, the Merged Entity would be unlikely to have any kind of ability to foreclose customers of additives post-transaction, due to the presence of viable competitors in the additives space.
6.4.13.7. Commission's conclusion
(1118) The Commission therefore concludes that the Transaction would not impede effective competition in a substantial part of the internal market in terms of vertical input foreclosure effects in the EEA markets for additives.
6.4.14. Conclusion on vertical effects
(1119) The Commission therefore concludes that the Transaction would significantly impede effective competition in a substantial part of the internal market in terms of vertical non-coordinated effects due to input foreclosure at least in the following EEA markets :
– the upstream market for ADN and the downstream market for HMD.
– the upstream market for HMD and the downstream market for HMD and the downstream PA (AH Salt, PA 6.6 BP and PA 6.6 EP) and HDI (HDI and HDI derivatives) value chains.
– the upstream market for adipic acid and the downstream market for AH Salt.
– the upstream market for AH Salt and the downstream market for Co-Polyamide PA 6/6.6HMD.
– the upstream market for PA 6.6 BP and the downstream market for PA 6.6 EP.
6.5. Conglomerate effects
(1120) The Transaction gives rise to conglomerate relationships between the Parties’ activities in different polymers and plastics product categories.
(1121) As a preliminary remark, the Commission notes that its assessment is restricted to potential exclusionary tying and bundling practices that could result from or be strengthened by the Transaction. As such, the Commission cannot take a view on potential bundling and tying practices within the existing product categories offered by BASF or the Business respectively.
(1122) In line with the Non-Horizontal Merger Guidelines, the Commission also notes that the mere fact that the Merged Entity would be able to offer a broader range of products is insufficient to conclude that the Transaction would significantly increase the risk of abusive tying and bundling and be incompatible with the internal market.790
(1123) Similarly, tying and bundling as such are common practices that often have no anticompetitive consequences.
6.5.1. Level IV of the polyamide value chain: PA 6 BP and PA 6.6 BP
6.5.1.1. The Notifying Party's view
(1124) The Notifying Party submits that the Merged Entity will neither have the ability nor the incentive to foreclose rivals on the market for PA 6 BP and PA 6.6 BP as it would not have the required market power on either of these markets, as there are many alternative suppliers, and as in practice a tying or bundling strategy is unlikely to be workable and profitable.
6.5.1.2. Commission's assessment
(1125) In the context of the market investigation some market participants highlighted BASF's strong position in the PA 6 markets and the Business' corresponding strength in the PA 6.6 markets, which would lead to some form of market power of the Merged Entity over the whole (PA 6 / PA 6.6) nylon industry.791
(1126) However, the Commission notes that these respondents did not substantiate whether the Transaction could increase the ability and incentive of the Merged Entity to tie and/or bundle its PA 6 BP and PA 6.6 BP products, nor did they elaborate on any mechanics and the potential success of a bundling or tying strategy.
(1127) For the reasons laid out in Section 6.3.7, the Commission considers that the Merged Entity would likely enjoy a significant degree of market power in the market for PA
6.6 BP in the EEA as a result of the Transaction, which could in turn translate into the ability to engage in tying and/or bundling strategies with regards to PA 6.6 BP.
(1128) The Commission notes however that tying and bundling between PA 6.6 BP and PA 6 BP does not appear to be a common practice in the industry.
(1129) As mentioned in Section 6.4.5, the Business has no merchant sales of PA 6 BP, and only little production of PA 6 BP ([…] kts in 2017), which it fully uses captively. In addition, prior to the Transaction, despite its market shares of [30-40]% on the EEA PA 6.6 BP market (regardless of the relevant metric), [THE BUSINESS COMMERCIAL STRATEGY].792
(1130) Similarly, BASF does not sell PA 6 BP bundled/tied with PA 6.6 BP or vice versa. RFQs, tenders or contracts for PA 6 BP are typically independent from those for PA 6.6 BP. BASF sets prices for each product individually and does not engage in contractual tying or bundling of PA 6 BP and PA 6.6 BP.
(1131) Besides the Parties, out of the leading producers of PA 6.6 BP supplying the EEA market, i.e. Radici, Invista, Ascend or DowDuPont, only Radici also produces PA 6 BP (and is only a minor player in the area).
(1132) There is a large number of established suppliers for PA 6 BP in the EEA including DSM, Lanxess, Grupa Azoty, Domo, UBE, and Kubishev with whom suppliers of PA 6.6 BP could potentially partner, to counter the Merged Entity, in case a bundling/tying strategy would be profitable.
(1133) In addition, customers of PA 6 BP and PA 6.6 BP may largely differ. This is in particular the case for the Parties' own customers. […].793 As a result, any bundling or tying strategy would likely entail losses of both PA 6 BP and PA 6.6 BP sales, irrespective of which product is tied or leveraged.
6.5.1.3. Conclusion
(1134) The Transaction is therefore unlikely to lead to a significant impediment of effective competition as a result of conglomerate effects between PA 6 BP and PA 6.6 BP in the EEA.
6.5.2. Level V of the polyamide value chain: PA 6 EP and PA 6.6 EP
6.5.2.1. The Notifying Party's view
(1135) The Notifying Party submits that Merged Entity will neither have the ability nor the incentive to foreclose rivals on the market for PA 6 EP and PA 6.6 EP as it would not have the required market power on either of these markets, as there are many alternative suppliers, and as in practice a tying or bundling strategy is unlikely to be workable and profitable.
6.5.2.2. The Commission's assessment
(1136) As mentioned in Section 6.5.1, some market participants highlighted BASF's strong position in the PA 6 markets and the Business' corresponding strength in the PA 6.6 markets, which would lead to some form of market power of the Merged Entity over the whole (PA 6 / PA 6.6) nylon industry. These respondents did not specifically explain whether their concerns related to the BP or the EP level.
(1137) Again, the Commission notes that the same respondents did not substantiate whether the Transaction could increase the ability and incentive of the Merged Entity to tie and/or bundle its PA 6 EP and PA 6.6 EP products, nor did they elaborate on any mechanics and the potential success of a bundling or tying strategy.
(1138) For the reasons laid out in Section 6.3.8, the Commission considers that the Merged Entity would like enjoy a significant degree of market power in the market for PA 6.6 EP in the EEA as a result of the Transaction, which could in turn translate into the ability to engage in tying and/or bundling strategies with regards to PA 6.6 EP.
(1139) With regard to PA 6 EP, based on the Notifying Party's estimates (Table 59), the Merged Entity would have a market share of [10-20]% in the EEA (increment of [0- 5]% from the Business) in both value and volume. The nearest competitor is Lanxess with [10-20]%, followed by DSM ([10-20]% in value, [10-20]% in volume). Other competitors include EMS, Domo and Albis ([5-10]% each) followed by Radici and Celanese ([0-5]% each).
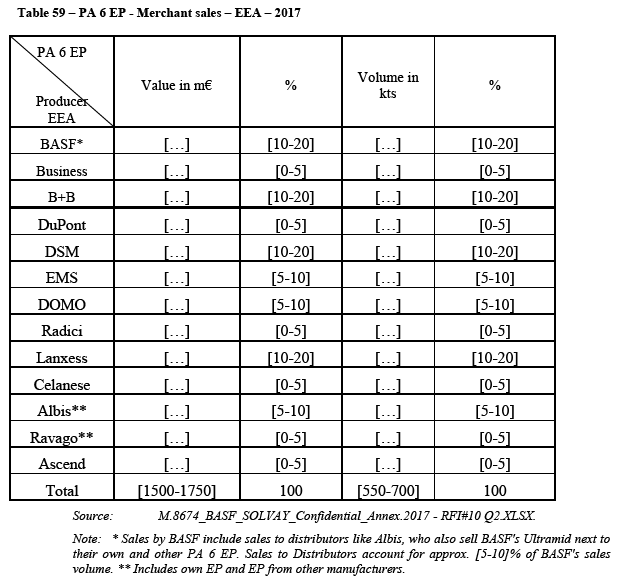
(1140) The Parties' market shares are below [20-30]% regardless of the metric used and as such are prima facie evidence of a lack of significant market power in PA 6 EP in the EEA. Furthermore the increment brought about by the Transaction in PA 6 EP in the EEA ([0-5]%) is minimal.
(1141) An overwhelming majority of EP customers (all but one) which responded to the market investigation indicated that they purchased both PA 6.6 EP and PA 6 EP.794 However, customers' procurement patterns may be incompatible with a bundling strategy in particular as customers purchase different products at different point in time. Some EP customers operate under a just in time delivery regime for PA 6.6 EP, or with limited stocks, which makes bundling PA 6.6 EP with other products, including PA 6 EP, unworkable.795 Furthermore, some customers based their procurement strategy on lifecycle tenders per part (i.e. of a specific plastic type) or on long-term contracts (with periodical price negotiations), which also make bunding strategies difficult to enforce.796
(1142) The Commission notes that tying and bundling between PA 6.6 EP and PA 6 EP do not appear to be a common practice in the industry.
(1143) BASF, which is the market leader in the market for PA 6 EP in the EEA, and the second most important supplier in the market for PA 6.6 EP in the EEA does not in practice bundle or tie PA 6 and PA 6.6 EP. All tenders and sales of PA 6 EP and PA 6.6 EP are independent from each other as customers order them separately for the relevant projects.
(1144) Furthermore, a number of the Parties' competitors at EP level could themselves replicate to a bundling/strategy of the Merged Entity post-transaction. Indeed, suppliers of both PA 6.6 EP and PA 6 EP include DowDuPont, Celanese, Lanxess, DSM, Radici, Domo, as well as other smaller compounders.797
(1145) As a result of the commitments, the Merged Entity's market share in PA 6.6 EP will be equal to that of BASF pre-transaction. As a result, the Merged Entity will not have any additional ability or incentive to bundle or tie PA 6.6 EP with PA 6 EP than BASF did pre-transaction.
6.5.2.3. Conclusion
(1146) The Transaction is therefore unlikely to lead to a significant impediment of effective competition as a result of conglomerate effects between PA 6 EP and PA 6.6 EP in the EEA.
6.5.3. Level V of the polyamide value chain: other conglomerate effects
(1147) Internal documents of the Parties refer to possible cross-selling opportunities as a result of the Transaction.798
(1148) The following products of BASF are potentially eligible for cross-selling together with the Business’ PA EP799 following the Transaction: (i) PBT; (ii) POM; (iii) PAES; (iv) PU foam systems and (v) Thermoplastic Polyurethane (TPU) (jointly referred to as "Cross-Selling Products").
6.5.3.1. The Notifying Party's view
(1149) The Notifying Party submits that the Merged Entity will neither have the ability nor the incentive to foreclose rivals on any of these markets as it would not have the required market power on the market for PA EP, or on any of the markets of the Cross-Selling Products
6.5.3.2. Commission's assessment
(1150) The Non-Horizontal Merger Guidelines state clearly that tying and bundling as such are common practices that often have no anticompetitive consequences, except in certain circumstances where these practices may lead to a reduction in rivals' ability or incentive to compete.800
(1151) The Business does not offer any of the cross-selling products.801
(1152) BASF estimates its market share for PBT to be around [10-20]% globally and [20- 30]% in the EEA. BASF estimates its market share for POM to be around [5-10]% globally and [10-20]% in the EEA. BASF estimates its market share for PAES to be around [20-30]% globally and [40-50]% in the EEA. BASF estimates its market share for PU foam systems to be around [10-30]% in the EEA. BASF's market shares for TPU, as detailed in Section 6.4.8.4 is estimated to be around [10-20]% globally and [40-50]% in the EEA. BASF, which is the second most important supplier in the market for PA 6.6 EP in the EEA, and a sizeable player for certain Cross-Selling Products (in particular PAES and TPU) does not in practice bundle or tie PA 6.6 EP with any of the Cross-Selling Products. All tenders and sales of PA EP products are independent from those of the Cross-Selling Products. BASF sets prices for these products individually and does not engage in contractual tying or bundling as regards the Cross-Selling Products.
(1154) Finally, none of the respondents to the market investigation raised specific concerns relating to the anti-competitive bundling or tying of PA 6.6 EP with any of the cross- selling products.
(1155) As a result of the commitments, the Merged Entity's market share in PA 6.6 EP will be equal to that of BASF pre-transaction. As a result, the Merged Entity will not have any additional ability or incentive to bundle or tie PA 6.6 EP with the Cross-Selling Products than BASF did pre-transaction.
6.5.3.3. Conclusion
(1156) The Transaction is therefore unlikely to lead to a significant impediment of effective competition as a result of conglomerate effects between PA EP and any of the cross- selling products in the EEA.
6.5.4. Conclusion on conglomerate effects
(1157) The Commission concludes that the Transaction is unlikely to lead to significant impediment to effective competition in the internal market as a result of conglomerate effects.
6.6. Conclusion
(1158) In conclusion, the Commission finds that the Transaction would significantly impede effective competition in the internal market or a substantial part of it, within the meaning of Article 2(3) of the Merger Regulation, and in the territory covered by the EEA Agreement or a substantial part of it, within the meaning of Article 57(1) of that Agreement:
(1159) as a result of horizontal non-coordinated effects arising in the supply of ADN, HMD, adipic acid, AH Salt, PA 6.6 BP, and PA 6.6 EP in the EEA and of. PA6 3D printing powders globally; and
(1160) in terms of vertical non-coordinated effects due to input foreclosure for the upstream market for ADN and the downstream market for HMD; the upstream market for HMD and the downstream market for HMD and the downstream PA (AH Salt, PA 6.6 BP and PA 6.6 EP) and HDI (HDI and HDI derivatives) value chains; the upstream market for adipic acid and the downstream market for AH Salt; the upstream market for AH Salt and the downstream market for Co-Polyamide PA 6/6.6 HMD and the upstream market for PA 6.6 BP and the downstream market for PA 6.6 EP in the EEA.
7. COMMITMENTS
7.1. Analytical framework for the assessment of the Commitments
(1161) When a concentration raises competition concerns because it would significantly impede effective competition, the parties may seek to modify the concentration so as to remove the significant impediment to effective competition identified by the Commission, with a view to having the concentration declared compatible with the internal market pursuant to Article 8(2) of the Merger Regulation.
(1162) In assessing whether or not commitments are likely to remove its competition concerns, the Commission must consider all relevant factors including, inter alia, the type, scale and scope of the commitments with reference to the structure and particular characteristics of the markets in which the Commission has identified a significant impediment to effective competition.802
(1163) The commitments must eliminate the competition concerns entirely and must be comprehensive and effective in all respects.803 The commitments should also be proportionate to the competition concerns identified.804 Furthermore, the commitments must be capable of being implemented effectively within a short period of time as the conditions of competition on the market will not be maintained until the commitments have been fulfilled.805
(1164) Under the Merger Regulation, the Commission must show that a concentration would significantly impede effective competition in the internal market or in a substantial part of it. By contrast, it is for the parties to the concentration to propose appropriate commitments. The Commission only has the power to accept commitments that are deemed capable of rendering the concentration compatible with the internal market so that they will prevent a significant impediment to effective competition in all relevant markets in which competition concerns were identified.
(1165) Pursuant to Article 10(2) of the Merger Regulation, the Commission has to take a clearance decision as soon as the serious doubts referred to in the decision initiating proceedings are removed as a result of the commitments submitted by the parties. This rule applies to commitments proposed in second phase proceedings before the Commission has issued a statement of objections.806
(1166) It is against this background and the standard set out in the Commission Notice on Remedies that the Commission has assessed the viability, workability, effectiveness and ability of the proposed commitments to entirely eliminate the competition concerns identified.
7.2. Procedure
(1167) In order to render the notified concentration with the internal market and the EEA Agreement in relation to the EEA markets for ADN, HMD, adipic acid, AH Salt, PA 6.6 BP, PA6 3D Printing Powder and PA 6.6 EP, the Parties has modified the notified concentration pursuant to Article 8(2) of the Merger Regulation by submitting commitments. The Parties submitted a first set of commitments on 15 October 2018 (the "Initial Commitments"). The Initial Commitments were market tested by the Commission on 16 October 2018. Subsequently, in order to address the result of the market test, the Parties submitted an amended set commitments on 31 October 2018. Finally, the Parties submitted a new set of commitments on 11
December 2018 ("the Final Commitments").807
7.3. Description of the Initial Commitments
(1168) The Initial Commitments consist in the divestiture of a business (the "Divestment Business"), which is composed of the following main assets:
(a) the Business’ facility (including its relevant production units of HMD, AH Salt, PA 6.6 BP, PA6 3D printing powder and PA 6.6 EP) located at Belle- Etoile, France;
(b) the Business’ facility (including its relevant production units of PA 6 BP, PA T4E BP and PA 6.6 EP) located at Gorzow, Poland;
(c) the Business’ facility (including its relevant production units of HMD distillation, AH Salt and PA 6.6 BP) located at Blanes, Spain;
(d) the Business’ facility (including its relevant production units for PA 6.6 BP and PA 6.6 Performance Fibres) located at Valence, France; and
(e) a 49% interest in a production joint venture (the "Chalampé JV") to which the entirety of the assets owned by the Business on the Chalampé site will be transferred including the production facilities of KA Oil, nitric acid, adipic acid and AH Salt excluding the Business’ shares in Butachimie which will be transferred to BASF. The purchaser of the Divestment Business will hold a 49% interest and BASF will hold a 51% interest. The purchaser of the Divestment Business will have the right to offtake 49% of the products manufactured by the Chalampé JV. BASF has the right to offtake 51%.
(1169) The Divestment Business includes tangible and intangible assets (including intellectual property rights) and personnel that contribute to its current operation or are necessary to ensure its viability and competitiveness.
(1170) It also includes licences, permits and authorisations issued by any governmental organisation for the benefit of the Divestment Business, as well as all contracts, leases, commitments and customer orders of the Divestment Business; all customer credit and other records of the Divestment Business.
(1171) The Initial Commitments also include three supply agreements for ADN:
(a) The assignment by BASF in favour of the Divestment Business of a long term supply agreement with an ADN supplier (Invista) for the supply of […] kts of ADN for […] years which shall enter into effect on the later of 1 January 2020 or closing (“ADN Agreement 1”). The price formula would be based on a provisional price benchmarked on a raw material based formula which will be subject to a retroactive price adjustment mechanism in order to determine the final ADN price. The price adjustment mechanism is based on the aggregate average HMD sales price of Invista over a period of one year.
(b) A medium term supply agreement with BASF for the supply of […] kts of ADN for […] years which shall enter into effect on the later of 1 January 2020 or closing (“ADN Agreement 2”). The price formula will be based on a provisional price benchmarked on a raw material based formula which will be subject to a retroactive price adjustment mechanism based on the average HMD sales price of Invista over a period of one year as defined for ADN Supply Agreement 1.
(c) Provided that closing occurs in 2019, BASF will supply as of closing on an interim basis (i.e. until ADN Supply Agreements 1 and 2 enter into effect) the relevant ADN volumes in 2019 (pro rata on a daily basis of […] kts per year) to the Divestment Business at market price.
(1172) In addition, the Divestment Business includes the benefit, for a transitional period after closing and on terms and conditions equivalent to those at present afforded to the Divestment Business, of all current arrangements under which Solvay or its affiliated undertakings supply products or services to the Divestment Business, as detailed in the Schedule, unless otherwise agreed with the purchaser of the Divestment Business.
(1173) The Divestment Business will also include:
(a) a transitional supply agreement pursuant to which the Divestment Business will offer master batches808 to BASF or one or more of its affiliated undertakings for a period of […] years;
(b) a transitional supply agreement pursuant to which the Divestment Business will offer specialty base polymers to BASF or one or more of its affiliated undertakings for a period of […] years and
(c) either a Technyl Grade Supply Agreement, or a Technyl Grade (EP) Service Agreement (at the option of the purchaser of the the Divestment Business).
(1174) The Initial Commitments provide for the Divestment Business to be sold to a purchaser with an established presence in the production and sale of chemical products.
(1175) The Initial Commitments also provide that the proposed concentration shall not be implemented before BASF or the Divestiture Trustee (in case of its appointment by BASF) has caused Solvay to enter into a put option agreement for the sale of the Divestment Business and the Commission has approved the purchaser of the Divestment Business and the terms of sale; a so-called upfront buyer remedy.
7.4. Results of the market test
(1176) The responses to the market test were numerous, and covered all levels of the polyamide value chain. However, a significant proportion of the observations made by the respondents did not address whether the Initial Commitments would solve the competition concerns identified by the Commission or whether the Divestment Business would be viable and competitive. Rather, several observations reflected the widespread dissatisfaction with the pre-transaction (un)competitive situation on the upstream markets of the value chain (mainly ADN and HMD). The Commission will therefore address separately observations that are merger-specific and those that are not.
7.4.1. Merger specific observations
7.4.1.1. ADN
(1177) None of the respondents to the market test raised observations as to the fact that the Initial Commitments would not solve the competition concern identified. In fact, the proposed commitments will entirely remove the overlap generated by the Transaction and will grant the purchaser of the Divestment Business additional ADN volumes.
(1178) Some respondents observe that the viability of the Divestment Business is significantly impaired by the fact that it is not vertically integrated into ADN, like Solvay was pre-transaction. […] explained that "The Divestment Business will lack of a certain level of upstream integration, as far as ADN is concerned",809 and […] further explained that "ADN is so strictly controlled, that the Divestment Business will not be independent, and probably not will not be viable, at least for the pa 66 part of it" and that "The Divestment Business will depend too heavily from the ADN supplier".810
(1179) A number of respondents to the market test indicated that the Initial Commitments are unable to guarantee the viability of the Divestment Business. According to the respondents, the Divestment Business would need a steady, long term access to ADN and the length of the ADN Agreement 2 is not sufficient to guarantee that the Divestment Business will be a viable and competitive player on the PA 6.6 value chain. […] explained that "Agreement 1 runs for 10-20 years, but agreement 2 runs for 4-7 years. Viability of Divestment business is questionable after year 7".811
(1180) A number of respondents also indicated that the total volume of ADN that the Divestment Business will have access to via the ADN Agreement 1 and ADN Agreement 2 is too low, especially once the ADN Agreement 2 will come to an end, to allow the Divestment Business to be a competitive force on the different merchant markets along the polyamide value chain. […] explained that "The mid- and long- term amount of ADN required for HMDA unit in Belle Etoile is not covered, especially with respect to the merchant HMDA market",812 and […]" (…) it is very likely that there will be a lack of ADN capacity which will result in Divestment Business’ HMD plants not being fully utilized".813 Similarly, […] observed that "the proposed Commitments also do not remedy the effective removal of Solvay as a merchant market supplier of ADN and/or HMD in Europe… the Divestment Business will not be able to supply ADN (and, consequently, HMD) to the merchant market in sufficient volumes".814
(1181) Several respondents to the market test also observed that the pricing formula provided for by in the Initial Commitments could put the Divestment Business at a competitive disadvantage vis-à-vis the integrated players. Particularly, some respondents observed that the pricing formula proposed does not reflect the ADN market dynamics and that a price formula based on cost, rather than on HMD prices, would be more appropriate.815
(1182) Also, a respondent to the market test observed that the proposed remedy could be improved by providing the price adjustment mechanism to be on a monthly basis.816 On a partially opposite note, some respondents observed that the ADN price formula – being based on the price of the downstream HMD may increase transparency on the market and ultimately reduce competition.817
7.4.1.2. HMD
(1183) Half of the upstream competitors/customers and the majority of downstream competitors consider that the Initial Commitments would be sufficient to remove the competition concerns raised by the Transaction.818 For example, a large downstream customer, […], is of the opinion that "the commitments provided will create sufficient space for a competitor to rival BASF for supplies of intermediates".819 […] adds that "with the Commitment to install a joint venture for the Solvay production in Chalampe and that the production from Belle-Etoile will be part of the divestment business, access to essential inputs for PA 6.6 producers is given".820
(1184) Important players upstream consider that, taking into consideration the Initial Commitments, the competitive situation would not change with the Transaction. For example, […] believes that "the market of HMD will be very similar of today in term of number of players and in term of reciprocal capacities".821 In the same vein, […] considers that the Transaction as modified by the Initial Commitments would have a "neutral impact to HMD".822
(1185) On the other hand, half of the upstream competitors/customers and a number of downstream competitors expressed doubts about the ability of the Initial Commitments to remove the competition concerns. The most important concern raised in the market test is that the competitiveness of the Divestment Business would depend on the conditions of the ADN supply. For example, […]submits that "decisive are the conditions (price, contract duration, payment terms) of the ADN (raw material for HMD) supply".823 In the same vein, […] is of the opinion that the extent to which the Commitments remove the competition concerns in the HMD market "depends on the competitiveness of the ADN supply contract between BASF and/or Invista to the purchaser. The proposed contract defines a minimum price level for HMD".824
(1186) The market test also shows that it is important that ADN Supply Agreements cover the ADN needs of the Divestment Business. For example, […] points out that the "volumes laid down in the long-term ADN Supply Agreement(s) must be sufficient to fully utilize the Divestment Business’ HMD plants".825 […] further adds that the Divestment Business "cannot be viable, competitive, and an independent merchant market supplier of ADN and HMD if it does not have unrestricted long-term access to sufficient ADN to meet both its own and the merchant market needs. Therefore, Solvay’s current share in the Butachimie JV would need to be fully and perpetually transferred to the Divestment Business. Alternatively, the Divestment Business would, at least, need to be granted a perpetual right from BASF to access Solvay’s current ADN production capacity in the Butachimie JV, at cost".826 […] further adds that the second ADN supply agreement, "that is assumed by DowDuPont to be of five years will only cover a small part of the merchant market but does not work for the long term and does not provide the security of supply and certainty that is required by the divestment business as well as the divestment business's EP customers".827 Another competitor828 is of the same opinion and submits that "BASF is proposing divesting the 142KT HMD unit at St. Fons / Belle Etoile and the 27.2KT HMD unit at Blanes (which together would require approximately 160KT of ADN to fully operate HMD) but as referenced above regarding the ADN supply would only be 60-80KT under a long-term agreement. By provided a reduced amount of ADN, BASF is ensuring that the prospective buyer would only be able to operate the HMD facility at less than 50% capacity, which reduces the viability of the Divestment Business and makes the economics highly unattractive and uncompetitive. Effectively, BASF is forcing the divestment purchaser to operate the HMD unit at low rates which will necessarily result in higher costs and therefore higher pricing for merchant HMD".829
(1187) Another issue raised in the market test is that nickel based catalyst technology is key to transform ADN into HMD.830 Access of the Divestment Business to nickel catalyst should thus be guaranteed.
(1188) Finally, […] submits that the Initial Commitments would not completely remove the full overlap for HMD because "the Divestment Business does not include BASF’s HMD capacity in the Seal Sands (UK) plant or Solvay’s HMD capacity in Butachimie. This non-divestiture combined HMD capacity of the parties still accounts for over 40% of the total HMD capacity in Europe. Therefore, the horizontal competition concern remains"831 and further adds that "unlike Solvay, the divestment business would be unable to serve the merchant markets for HMD".832 In the same vein, another competitor considers that "under the current proposal, BASF would possess around 215KT or 45% of this capacity (e.g. 125KT at Seal Sands and 50% of the capacity at Butachimie)".833
7.4.1.3. Adipic acid
(1189) Discounting for the non merger-specific considerations elicited by the market test, a majority of respondents consider that the Initial Commitments would remove the competition concerns raised by the Transaction in relation to the supply of adipic acid in the EEA.834 Moreover, the overwhelming majority of respondents to the market test who expressed an opinion consider that the Chalampé JV arrangement
proposed as part of the Initial Commitments would constitute a viable solution enabling the purchaser of the Divestment Business to compete effectively on the merchant market, including with BASF.835 Likewise, the governance principles of the Chalampé JV are considered appropriate to protect the purchaser's entitlement on a lasting basis by a clear majority of respondents to the market test.836
(1190) Most of the reservations expressed by market participants relate to the identity and market position of the potential purchaser of the Divestment Business, chiefly in case it would be an existing EEA producer of adipic acid but also whether it would have the expertise for running such an operation.837 In addition, two market participants voiced concerns at the sufficiency of the Initial Commitments, though in opposite ways.
(1191) One market participant who requested anonymity considered that the Initial Commitments would remove the concerns raised in relation to adipic acid by ensuring that sufficient capacity is made available to the purchaser of the Divestment Business to support the downstream production assets.838 However, that same market participant expressed the view that the available capacity was in fact too large relative to the captive needs of the Divestment Business, both in relation to the adipic acid and KA Oil produced by the Chalampé JV, thereby burdening the purchaser of the Divestment Business with fixed costs that are disproportionate to its actual needs.839 According to that market participant, the Divestment Business would have more than 100 kts of capacity in excess of its internal requirements.
(1192) In contrast, the other market participant – […] – submitted that the Divestment Business would not be able to supply adipic acid to the merchant market in sufficient volumes.840 Thus the Divestment Business would have less than 40 kts available for merchant market sale.841 Moreover, the purchaser of the Divestment Business would not become BASF's independent competitor, according to that market participant, because of the important control rights that BASF would retain over the Chalampé JV.842
(1193) Moreover, during a subsequent meeting, […] submitted that the Chalampé JV would create structural links and dependencies between BASF and the Divestment Business affecting the Divestment Business’ viability and autonomy and raising additional concerns in an already concentrated industry.843 No other respondent to the market test raised similar issues.
7.4.1.4. AH Salt
(1194) None of the respondents to the market test made observations as to the fact that the Initial Commitments would not include sufficient capacity or assets at the AH Salt level. For instance, […] explained that "the Divested Business, the Purchaser will have access to 3 AH Salt plants (Belle Etoile, Blanes & Chalampe)".844 Similarly, […] is of the opinion that the "market of AH Salt will be very similar of today. Eventually there will be more players with smaller capacities".845
(1195) On the other hand, some important players in the AH Salt market made a number of observations. The most important issue raised is that AH Salt supply will ultimately depend on access to ADN on the long term and at competitive prices. For example, […] mentions that the extent to which the proposed remedy would restore competition on the market "depends on the competitiveness of the ADN supply contract between BASF and/or Invista to the purchaser".846 Similarly, […] explains that "ADN->HMD is the critical component in AH salt. Even if adipic acid has a more liquid market and more suppliers, the AH availability and price will be directed by the HMD supply ability and implicitly the ADN availability and conditions".847
(1196) Finally, an important competitor, also submits that the Divestment Business would have an excess of capacity at the AH Salt level compared to its access to inputs (i.e. ADN and HMD) and that the inclusion of the 49% of the Chalampé JV burdens the Divestment Business with high cost obligations for unneeded assets.848
(1197) An important competitor who requested anonymity also calls into question whether the AH Salt Blanes' facility is actually cost-competitive compared to larger facilities retained by BASF given that its size is relatively small (60 kts).849
7.4.1.5. HDI
(1198) The majority of the respondents to the market test consider that the scope of the Initial Commitments would be sufficient to allow sufficient competition in the HDI market.850 […] submits that the "proposed commitments release the concerns previously raised regarding the competitive situation on HDI and HDI derivatives if BASF would have acquired the whole polyamide business of Solvay, as BASF is active on the downstream value chain of HDI".851 However, […] considers that "the availability of HDI derivatives strongly depends on the merchant HMDA market".852
7.4.1.6. PA 6.6 BP
(1199) Around half of the responding upstream competitors/customers of the Parties consider that the Initial Commitments would be sufficient to remove the competition concerns raised by the Transaction with regards to PA 6.6 BP.853 Out of the others, only few presented arguments explaining potential shortcomings of the Initial Commitments specifically relevant at PA 6.6 BP level.854
(1200) Some market respondents expressed concerns that the supply and tolling agreements included in the Initial Commitments would harm the Divestment Business' to compete, as the Divestment Business would need to dedicate a significant share of its PA 6.6 BP capacity to honour agreements with the Merged Entity.855
(1201) Some respondents also complained that the Divestment Business did not include the Freiburg facility, which produces PA 6.6 BP, in particular for the production of performance fibres.856
(1202) At least one market participants indicated that the Divestment Business would not have sufficient polymerisation capacity to meet its own needs, and would thus not be able to offer PA 6.6 BP on the merchant market.857
7.4.1.7. Co-Polyamide PA 6/6.6 Base Polymer
(1203) The large majority of the upstream customers and competitors responding to the market test consider that the remedies proposed would be sufficient to allow effective competition in the Co-Polyamide PA 6/6.6 base polymer market.858 For example, […] submits that "the remedies proposed are enough to remove the competition concerns. The number of players and their reciprocal capacities into the market place will be the same of today"859 and […] considers that "the divested capacity is big enough for a new independent supplier".860
7.4.1.8. PA 6.6 EP
(1204) Around half of the responding upstream competitors/customers of the Parties consider that the Initial Commitments would be sufficient to remove the competition concerns raised by the Transaction with regards to PA 6.6 EP.861 Over 60% of responding PA 6.6 EP customers considered that the Commitments were suitable to effectively remove the Commission's concerns with regard to the market for PA 6.6 EP in the EEA.862 Out of the others, only few presented arguments explaining potential shortcomings of the Initial Commitments specifically relevant at PA 6.6 EP level.
(1205) Some PA 6.6 EP customers,863 as well one competing PA 6.6 EP producer,864 indicated that the purchaser of the Divestment Business should have experience in working with the relevant downstream industries, and in particular the automotive industry.
(1206) Some respondents, mostly downstream customers,865 as well as one competing PA 6.6 EP producer866 expressed concerns that, following the Transaction, the Divestment Business will go from a global player, to a local (European) player, which would affect its ability to supply customers which purchase some products globally.867
7.4.1.9. PA6 3D printing powders
(1207) Over 80% of responding upstream competitors/customers of the Parties consider that the Initial Commitments would be sufficient to remove the competition concerns raised by the Transaction with regards to PA 6.3D Printing Powders. Furthermore, none of the respondents presented arguments explaining potential shortcomings of the Initial Commitments.868
7.4.1.10. Performance fibres
(1208) Over two thirds of responding upstream competitors/customers of the Parties consider that the Initial Commitments would be sufficient to remove the competition concerns raised by the Transaction with regards to PA 6.6 Performance Fibres.869 Furthermore, no customer of PA 6.6 Performance Fibres presented arguments explaining potential shortcomings of the Initial Commitments.870 Concerns raised by market participants are largely non merger-specific (see Section 6.10.2.8).
7.4.2. Non-merger specific observations
7.4.2.1. ADN
(1209) A number of respondents to the market test raised several observations that are related to the pre-transaction (un)competitive situation on the upstream markets of the value chain.
(1210) The main observation, which clearly reflects the discontent of the market about the pre-transaction situation, is that as the Notifying Party is not proposing a structural remedy for the ADN, the production assets will remain in the hands of only two suppliers. As a few examples of a broader pool of critical observations, […] observed that "Adiponitrile (“ADN”) remains the essential feedstock for the whole Nylon/HDI downstream chain. The access to 50% of this essential feedstock manufactured in EU will be held by BASF, […] competitor. If there is an ADN shortage in the market, which has consequences on both the HMD and Nylon 6.6 markets, BASF may dedicate its internal capacity to captive use. This would create a shortage for HDI. BASF may not be a pure merchant player while both prior members of the Butachimie’s JV were".871 On a similar note, […] observed that "BASF would remain in control of the ADN plant at Chalampee as 50% JV partner of INVISTA (…)"872 and […] "No change compared to the original proposal. The owner of ADN capacity dominates the supply chain".873 […] also appeared dissatisfied by the lack of a structural remedy in the ADN market: "The Divestment Business does not include ADN production capacity and thus does not even get close to replicating Solvay as it will not have access to its own ADN, the most critical input product for the Divestment Business’s downstream production and its future merchant market participation",874 an observation also mirrored by […] "The Proposed Commitments do not include divesting any ADN producing assets. Rather, BASF would obtain Solvay’s 50% interest in the Butachimie JV, which is effectively the sole supplier of ADN in Europe".875 […] more clearly observes that "the structure of the ADN market after the proposed transaction will not be competitive and a small player will have no possibility to be competitive and independent".876 On a similar note, […] observes that "The Divestment Business does not include ADN production capacity and thus does not even get close to replicating Solvay", and later restates "the proposed Commitments fall far short of replicating Solvay or creating an adequate and viable downstream competitor".877 "
(1211) With respect to this comment, the Commission observes that the acquisition of the 50% stake in Butachimie does not per se represent a structural change in the market structure; rather this is represented by the addition of the [50-60]% capacity share deriving from that ownership and the additional volumes deriving from the long term contract with Invista. With respect to the comment that the ADN production assets will be owned by only two market participants, the Commission observes that this does not change as a result of the Transaction: with regard to the ownership of the assets, in fact, the Transaction only entails the change in the identity of one of the Butachimie partners (BASF instead of Solvay) and not the reduction of number of players owning such assets.
(1212) Also, the Commission observes that the aim of the Final Commitments is not to replicate Solvay, rather is to remove the competition concerns identified and restore the competitive situation as it was pre-transaction. Therefore, it is not necessary for the Commitments to be acceptable that they replicate the position of the Seller pre- transaction.
(1213) Furthermore, some respondents indicated that the Divestment Business profitability may be impacted by a shortage of ADN expected in 2019 and 2020 (due to the Retrofit Project). For example, […] observed that "(…) ADN is currently in tight availability worldwide. This will even last for the next few years. Further market concentration to a potential key user of ADN could still keep the markets under pressure because of the maybe preferred captive use".878
(1214) The Commission observes that the expected shortage of ADN is unrelated to the Transaction as the Retrofit Project was agreed before and independently from the Transaction. This means that the shortage of ADN would have been observed even if the Transaction would have not been carried out. Hence, facing that shortage is part of the normal course of business for all players, including the Divestment Business. In any event, the Final Commitments, by ensuring that the Divestment Business has for at least 5 years sufficient ADN to satisfy its historical needs, addresses this comment.
7.4.2.2. HMD
(1215) A number of respondents to the market test raised several observations that are related to the pre-transaction (un)competitive situation on the upstream markets of the value chain.
(1216) The tightness and shortages of the ADN market are viewed as a risk to the ability of the Divestment Business to effectively compete in the HMD market. For example, […] is of the opinion that "ADN is very tight and if BASF receives Solvay’s ADN capacities and capacities from Butachimie (which BASF already has), there will be less ADN for the merchant market, i.e. for HMD producers".879 Similarly, […] considers that the Divestment Business "will depend on ADN, which is and which will remain in very short supply, and which as soon as the ADN assets will be transferred from Solvay to BASF will be in a very short supply position, as BASF will switch a significant amount of its ADN capacity to its own HMD - Base Polymer - Engineering Plastic captive business".880 […] adds that "the divested HMD capacity can hardly compete freely, as the ADN capacity will remain tight".881
(1217) However, the shortages of ADN and the tightness of the market are not related to the Transaction. On the contrary, they are characteristics of this market as described is Section 6.3.2. These characteristics were taken into consideration in the competitive assessment of the Transaction because they would increase the effects of the high combined market shares of the Merged Entity (absent commitments), together with the lack of alternatives for customers in a very concentrated market with high barriers to entry. Moreover, as described in Section 7.6, the Commitments include two ADN supply agreements that ensure long-term access to competitive ADN for the Divestment Business which will have sufficient quantities of ADN to run the Belle-Etoile plant at virtually full capacity. These quantities will cover the captive needs of the Divestment Business and will also allow significant merchant sales of HMD and other downstream products. Finally, it should be noted that BASF which was identified during the market investigation as one of the strongest competitors in the PA 6.6 value chain (see Section 6.3.2) was not vertically integrated into ADN and was fully dependent on the merchant market to secure its ADN needs.
(1218) The possibility that the purchaser of the Divestment Business decides to sell HMD to plastics producers rather than to companies active in the fibres industry was also raised during the market test. For example, […] submits that "the purchaser of La Belle Etoile could direct its HMD production to plastic application (or reduce the supply of HMD to factories selling to textile sector)".882
(1219) Given that companies active in the fibres sector source PA 6.6 base polymer and not HMD, this argument is discussed in Section 7.2.4.8.
(1220) Finally, […] submits that the purchaser of the Divestment Business should not be active in the production of HMD because "the Concentration may lead to a less competitive landscape than today if Belle Etoile HMD unit is acquired by an existing HMD producer".883
(1221) The Commission agrees that it is of paramount importance that the acquisition of the Divestment Business by the purchaser of the Divestment Business does not create, in light of the information available to the Commission, prima facie competition concerns, as stated in the Final Commitments under Section D ("The Purchaser").
7.4.2.3. Adipic acid
(1222) The market test elicited a number of non-merger-specific considerations in relation to the Initial Commitments' ability to remove the competitive concerns raised by the Transaction. These considerations revolved essentially around the availability of nitric acid, cyclohexane and ammonia as input into the production of adipic acid.884 In that regard, market participants shared somewhat conflicting views as to the position of BASF in the supply of ammonia with […] pointing to BASF as a "major ammonia producer" whereas […] underlined that the supply of ammonia (and cyclohexane) is "not dominated by BASF".885
(1223) The market investigation has not identified specific concerns in relation to the supply of nitric acid, cyclohexane and ammonia, and BASF's or the Merged Entity's position as a supplier thereof. Moreover, the market test confirmed that there are sufficient sources of ammonia, nitric acid and cyclohexane available in the EEA to support the production of adipic acid according to a large majority of respondents who expressed an opinion.886
7.4.2.4. AH Salt
(1224) […] submits that there is a risk that the purchaser of the Divestment Business directs AH Salt to the factories producing PA 6.6 and to the production of plastic application to the detriment of factories selling to textile sector.887
(1225) Given that companies active in the textile sector source mainly PA 6.6 BP and not AH Salt, the notion of redirection is discussed in Section 7.2.4.8.
7.4.2.5. HDI
(1226) While […] is of the opinion that the Commitments would be sufficient to render the Transaction compatible with the internal market, it submits that the "Commitments shall ensure that Purchaser shall dedicate enough HMD competitive volumes to support the HDI growing market needs and to ensure fair and equitable competition on the HDI market…" and that "the Commission needs to ensure that the new Purchaser shall ensure the access to HMD for HDI manufacturing, at market conditions, inter alia via competitive ADN".888
(1227) The Commitments cannot ensure that the Divestment Business would sell HMD to certain customers or fix the conditions at which HMD should be sold to certain customers. Rather, the commitments should be able to replicate the competitive situation that existed pre-transaction. As described in Section 7.6, the Commitments allow for the creation of a new market player that will have enough volumes of HMD to sell to the merchant market. In fact, contracts to supply HMD to HDI customers, including […], will be transferred to the Divestment Business.
7.4.2.6. PA 6.6 BP
(1228) Some producers of performance fibres expressed concerns that the Divestment Business would not serve the performance fibres industry, but would instead focus on serving the plastics industry (including the Divestment Business' own needs).889 However such commercial strategy would be unrelated to a foreclosure strategy as it would not aim at favouring the Divestment Business' own performance fibres sales,
but sales of PA 6.6 EP to other customers. Such concerns are not merger-specific. Indeed, the Business could already decide pre-transaction to favour sales to EP producers rather than to PF suppliers, and market participants already acknowledge it is the case to some extent pre-transaction, and could increasingly happen in the future, regardless of the Transaction.890
7.4.2.7. PA 6.6 EP
(1229) […] noted that "The PA6.6 EP assets to form part of the divestment commitments are estimated to be old".891 This point, which is not per se merger specific, as the age of the PA 6.6 EP assets remains unaffected by the Transaction, is nonetheless addressed in Section 7.6.3 on the viability of the Initial Commitments.
7.4.2.8. Performance fibres
(1230) As set out in Section 7.4.2.6, some performance fibres manufacturers and customers raised a concern that the purchaser of the Divestment Business may decide to supply the EP industry instead of the performance fibre industry.
(1231) In addition, some customers of performance fibres mention that prices in the PA 6.6 value chain are high and expected to increase, which would impede the ability of the Divestment Business to be competitive, in particular in order to find new customers.892 However, the same considerations would apply to the Business (and any other market participant) regardless of the Transaction.
7.5. Description of the Final Commitments
(1232) Following the result of the market test of the Initial Commitments, the Commission communicated to the Parties a summary of the observations made by respondents. The Commission also informed the Notifying Party that it considers some of the observations justified and amendments to the Initial Commitments necessary in order to remedy the concerns raised.
(1233) In order to address the observations made by the Commission as a result of the market test, the Parties submitted an improved version of the Initial Commitments containing:
– a possible extension of the ADN Supply Agreement 2, at the discretion of the purchaser of the Divestment Business for an additional period of up to […] years, with a maximum duration of […] years. In addition, BASF commits to provide an additional […] kts ADN volumes per year to the Divestment Business, which are sufficient to cover the Divestment Business’ historic ADN needs.
– changes to the governance of the Chalampé JV […];
– the provision by BASF of up to [90-100]% of the purchaser’s annual requirement of Nickel Raney for the production of HMD in Belle-Etoile for […] years;
– a modification of the Transitional Agreement to take into account the feedback to the market test and the reduction of the duration of the Masterbatch Transitional Supply Agreement to […] years, which also includes a ramping-down of volumes supplied. The final terms of the Specialty Base Polymer Transitional Supply Agreement and of the Tolling Agreement and in particular the fee, duration and volumes will be negotiated in good faith between BASF and the purchaser of the Divestment Business.
– a modification of the purchaser criteria to take into account the feedback to the market test. According to the wording of the Final Commitments and regardless of the geographic dimension of the relevant product markets, the purchaser of the Divestment Business needs to be able to compete for global customers (and not only regional customers). The purchaser criteria also explicitly require that the purchaser of the Divestment Business be able to service one key category of customers, namely automotive customers.
– a clear statement that BASF will transfer all patents, licenses and know-how that have been used solely or predominantly by the Divestment Business to the purchaser of the Divestment Business. Additionally, the background patents licenses and the licenses for background know-how necessary to implement an innovation generated by the Divestment Business for the upstream products will be perpetual, irrevocable and non-exclusive.
7.6. Assessment of the Final Commitments
7.6.1. Removal of competition concerns
7.6.1.1. ADN
7.6.1.1.(A) Horizontal non-coordinated effects
(1234) As already explained throughout this Decision, the only ADN producer in the EEA is Butachimie. Prior to the Transaction the shareholders in Butachimie, Invista and Solvay, shared on equal terms the output of Butachimie thus each having a share equal to [50-60]% of the EEA ADN market in terms of capacity.
(1235) As explained in Section 6.3.1., […]. In order to obtain Invista's consent, BASF agreed to enter into a long term agreement with Invista, whereby Invista will supply to BASF up to […] kts of ADN per year for the a duration of […] years. The Commission is therefore of the opinion that the structural change in the market derives by the addition of BASF acquiring control over the 50% of Butachimie and the long term contract with Invista.
(1236) The Commission therefore takes the view that BASF would not only control the [50- 60]% production share in Butachimie from replacing Solvay as a shareholder, but also an additional [10-20]% on a long term and structural basis deriving from its contract with Invista. This additional [10-20]% in capacity is therefore the overlap brought about by the Transaction on the ADN market.
(1237) Post-transaction, BASF's capacity share on the ADN market will at most be [50- 60]%, with Invista having a capacity share of [30-40]% and the Divestment Business of [10-20]% deriving from the ADN Agreement 1. If also the ADN Agreement 2 is taken into account, BASF will have a share of [40-50]%, Invista of [30-40]% and the Divestment Business of [20-30]%.
(1238) If the Retroift Project is taken into account, BASF will still have a market share of [50-60]% post-transaction while Invista will have a capacity share of [30-40]% and the Divestment Business of [10-20]%. If the ADN agreement 2 and the retrofit project are taken into account BASF will have a capacity share of [40-50]%, Invista of 37% and the Divestment Business of [20-30]%.893
(1239) The Commission therefore considers that the Final Commitments entirely remove the horizontal relationship on the ADN market as the long term contract by and between BASF and Invista will be attributed at the same terms and conditions to the Divestment Business. The Final Commitments therefore entirely remove the overlap brought about by the Transaction. The Commission also considers that the Final Commitments go beyond the mere removal of the overlap as it grants to the Divestment Business additional […] kts for a minimum of […] years, but potentially up to […] years, by means of the ADN Agreement 2.
(1240) The structural change in the market brought about by the Transaction consisted in fact in the long term supply agreement of ADN entered into by and between BASF and Invista. That contract will be transferred to the purchaser of the Divestment Business thus entirely removing the horizontal relationship.
(1241) The Commission therefore concludes that the Final Commitments are sufficient to remove the competition concerns identified with regards to the market for ADN.
7.6.1.1 (B) Vertical effects
(1242) The Commission notes that the Final Remedies entirely eliminate the overlap on the ADN market and therefore restore the competitive situation existing pre-transaction in the upstream market.
(1243) With regards to the effects of the Transaction concerning the vertical relationship existing between the upstream market for ADN and the downstream market for HMD, the Commission concluded that – absent the Final Commitments – BASF would have the ability and the incentive to foreclose access to ADN to its downstream competitors. As a result, downstream competitors would be faced with a significant increase in their downstream costs as, albeit representing only [10-20]% of the merchant market, the ADN sold by the Business is the only alternative to the ADN sold by Invista. The Transaction would have therefore removed the only price constraint on Invista.
(1244) The Commission considers that, by eliminating BASF's incentive to engage in any such foreclosure behaviour, the Final Commitments entirely remove such a competition concern.
(1245) The Final Commitments ensure that the Divestment Business have sufficient access to ADN to cater for virtually all the historical needs of Belle-Etoile's HMD production capacity, as further detailed in Section 7.6.1.2. The Divestment Business will therefore have the ability, and most likely the incentive, to captively use all of its ADN for the production of HMD. Hence, the Divestment Business will likely not represent an alternative to Invista.
(1246) However, the Final Commitments will result in BASF having more ADN capacity than it will be able to process into HMD. Post-transaction, BASF's production capacity of HMD will be […] kts, while the Business had a capacity of […] kts pre- transaction. Even discounting […]kts (and possibly […] kts) of ADN which BASF will sell to the Divestment Business under the ADN Agreement 2, BASF will have volumes of ADN which it will not be able to process into HMD.
(1247) Even if adopting a very conservative calculation method (i.e. considering an 85% efficiency rate of the ADN production assets and a 1:1 conversion ratio from ADN to HMD to discount for possible fluctuation of the efficiency rate), BASF will have between […] kts (without the Retrofit) and at least […] kts (with the Retrofit) of ADN in excess of what is required to feed its HMD production capacity. It would therefore make economic sense for BASF to sell the additional volumes on the ADN merchant market, therefore eliminating BASF’s incentive to engage in foreclosure behaviour.
(1248) Further, as the Business sold […] kts of ADN on the merchant market in 2017, the Final Commitments replicate – and possibly increase – the competitive constraint on Invista compared to the pre-transaction situation and are therefore will replicate, and possibly improve, the competitive situation existing pre-transaction
(1249) It should be noted […]. The most likely scenario is that – due to the retrofit project – BASF will have excess ADN volumes to sell on the merchant market.
(1250) The Final Commitments will therefore remove the significant impediment of effective competition as a result of vertical effects identified by the Commission with regards to the vertical relation between the upstream market for ADN and the downstream market for HMD.
7.6.1.2. HMD
7.6.1.2.(A) Horizontal non-coordinated effects
(1251) None of the respondents to the market test raised observations as to the fact that the remedy proposed by the Notifying Party would not solve the competition concerns identified in terms of capacity or assets included in the remedy package, except […] and (to a lesser extent) another competitor. In fact, the proposed commitments will entirely remove the overlap generated by the Transaction.
(1252) The Parties’ combined market shares in the market for HMD in 2017 are:
(a) [30-40]% in value for merchant sales ([20-30]% Business and [0-5]% BASF);
(b) [50-60]% in volume for merchant sales plus captive use ([40-50]% Business and [10-20]% BASF); and,
(c) [60-70]% for nameplate production capacity ([50-60]% -[…] kts- Business, and [10-20]% -[…] kts- BASF).
(1253) The Final Commitments include the Belle Etoile and Blanes plants with […] kts and […] kts of pure HMD nameplate production capacity respectively. Therefore, the horizontal overlap -[…] kts- is more than entirely removed.894
(1254) Table 60 shows the production capacity pre-transaction and post-transaction (including Commitments) in the EEA:
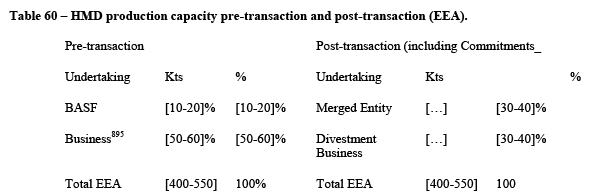
(1255) Therefore, contrary to […] view that the commitments do not completely remove the full overlap for HMD and that the non-divested HMD assets of the parties still accounts for over [40-50]% of the total HMD capacity in Europe, the Final Commitments go beyond the […] kts overlap created by the Transaction; the Merged Entity will account for [30-40]% of the nameplate capacity in the EEA while the Divestment Business will control [30-40]% of the HMD nameplate capacity in the EEA. The Final Commitments will create a competitor at the HMD level with a capacity significantly exceeding BASF’s capacity.
(1256) The Initial Commitments included two ADN supply agreements to the Divestment Business of […] kts and […] kts per year respectively. Moreover, under the Final Commitments, BASF committed to supply an additional […] kts of ADN per year, increasing the volumes of either supply agreement. Therefore, the Divestment Business will have guaranteed access to […] kts of ADN per year in total. These quantities of ADN are sufficient to run Belle-Etoile plant virtually at full capacity taking into account the historical production rate.896 In fact, the Business' non- divested assets supplied […] kts of ADN from Butachimie to Belle-Etoile in 2017. The Final Commitments increased the quantities of ADN to be supplied to the Divestment Business by […] kts with regards to the First Commitments in order to cater for virtually the historical needs of Belle Etoile. […]. Therefore, the Final Commitments ensure access to sufficient ADN to meet both the Divestment Business captive needs and the merchant market needs, as several marker players demanded inthe market test. […]. These volumes are enough to cover the captive needs of the Divestment Business ([…] kts approximately)897 and serve the merchant market ([…] kts).898
(1257) Moreover, a significant number of upstream competitors/customers indicated that the ADN supply conditions and in particular the price, would be crucial to determine the ability of the Divestment Business to compete in the market. As explained in Section 7.6.3, the ADN price formula guarantees access to ADN at competitive prices, based on historical data.
(1258) The Final Commitments also include a Tolling Agreement whereby the Merged Entity will provide crude HMD to the Divestment Business, in exchange for PA 6.6 BP.899 While the First Commitments stipulated that the Merged Entity would provide […] kts of crude HMD per year, the Final Commitments ensure that the purchaser of the Divestment Business will be able to negotiate at arm's length the main terms of
such agreement, including the quantities and relevant fee.
(1259) In addition, the Business’s existing HMD merchant sales contracts for HDI customers will be transferred to the Divestment Business, meaning that the Divestment Business will inherit a material sales base on which it can build a merchant presence from the outset.
(1260) Table 61 shows the merchant sales pre-transaction and post-transaction (including Commitments) in the EEA:
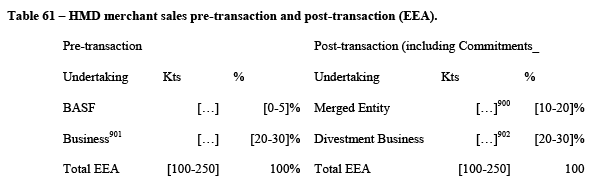
(1261) The Final Commitments therefore eliminate the increments in market shares brought about by the Transaction, regardless of the metric (merchant market sales, merchant sales and captive production, or capacity). In fact, because the Divestment Business will be larger than BASF was in terms of capacity and merchant sales, levels of market concentration by these metrics will be lower following the modified Transaction than pre-transaction.
(1262) Moreover, in addition to the Divestment Business, the Merged Entity will also have sufficient HMD capacity and access to ADN to sell into the merchant market. While the Merged Entity will control […] kts of HMD nameplate capacity and […] kts of HMD actual production capacity,903 it will account for […] kts of AH Salt real production capacity and […] kts of real production capacity at the level of PA 6.6 BP in the EEA. Given that the quantity of HMD needed to manufacture 1 kt of AH Salt is 0.445 kts904, the Merged Entity would be able to produce […] kts of AH Salt. The real capacity of the Merged Entity at the level of the PA 6.6 BP is […] kts. Given that 1,17 kts of AH Salt are needed to produce 1 kt of PA 6.6 BP, the Merged Entity would require […] kts of AH Salt to run its PA 6.6 BP plants at full operational capacity. The total size of the AH Salt merchant market in the EEA in 2017 was […] kts. Therefore, even if the Merged Entity accounted for 100% of the AH Salt merchant market, it would require a maximum of […] kts of AH Salt. The Merged Entity would require approximately […] kts of HMD to produce […] kts of AH Salt. Therefore, the Merged Entity would have up to […] kts of HMD available for the merchant market. Even taking into consideration the Merged Entity global capacity (i.e. including capacity in Onsan, South Korea and San Bernardo, Brazil) at PA 6.6. BP level, the Merged Entity would have approximately […] kts of HMD available for the market.905 It is also important to note that pre-transaction, in 2017, the Business sold […] kts of HMD into the merchant market, mostly to HDI producers and the supply contracts to HDI producers will be transferred to the Divestment Business.
(1263) Finally, the Final Commitments also ensure that the Merged Entity will provide up to [90-100]% of the purchaser’s annual requirement of Nickel Raney for the production of HMD for […] years. […].906
(1264) The Commission therefore concludes that the Final Commitments are sufficient to remove the competition horizontal concerns identified with regards to the market for HMD.
7.6.1.2.(B) Vertical effects
(1265) The vast majority of respondents to the market test have not raised any concern as to the vertical relation of HMD with regards to either the polyamide value chain or the HDI value chain. […] submits that the "proposed commitments release the concerns previously raised regarding the competitive situation on HDI and HDI derivatives if BASF would have acquired the whole polyamide business of Solvay, as BASF is active on the downstream value chain of HDI".907
(1266) However, […] considers that BASF will have the incentives to foreclose its rivals and that the Divestment Business will not be able to serve the merchant market because it will not be backward integrated into ADN. According to […]"BASF will likely divert the ADN available from the Chalampé plant to its own internal global downstream needs. BASF will thus probably choose to convert it into HMD in China, through tolling agreements, or would build an HMD plant in China. ADN would thus not go into the European merchant market".908
(1267) As explained in Section 7.6.2.1, the Divestment Business will inherit the Business’s existing HMD merchant sales contracts for HDI customers and therefore will sell into the EEA market […] kts of HMD909, while the Business sold […] kts of HMD into the merchant market in 2017 and BASF sold […] kts. Moreover, after expiration of these contracts,910 the Divestment Business will have the ability and incentives to continue selling HMD to third parties. In fact, the Divestment Business will have access to a minimum of […] kts of ADN per year which are sufficient to producing […] kts of HMD. This quantity of HMD is in turn sufficient to produce […] kts of AH Salt.911 The real capacity of the Divestment Business at the PA 6.6 BP level (including volumes of polymer that are produced on the Technyl line as part of the continuous EP production process) is […] kts approximately.912 Therefore, the Divestment Business would require approximately […] kts of AH Salt to run its plants of PA 6.6. BP at full capacity.913 The total size of the AH Salt merchant market in the EEA in 2017 was […] kts and the Merged Entity will […]. It follows that the Divestment Business will use captively a maximum of […] kts of AH Salt while it could produce up to […] kts of AH Salt. Therefore, the Divestment Business will need […] kts of HMD approximately for captive use and will have approximately […] kts of HMD available for merchant sales.914 It is also important to note that while […] claims that the Divestment Business will not be able to serve the merchant market because it will not be backward integrated into ADN, it also recognises that "two to three years ago, Solvay and BASF both indicated being interested in participating in discussions with […] once its contract with Invista expired".915 At this time, BASF was not backward integrated into ADN either.
(1268) Moreover, in addition to the Divestment Business, the Merged Entity will also have approximately […] kts of HMD available for the merchant market or […] kts of HMD after taking into consideration all of its global captive needs.916 Therefore, both, the Merged Entity and the Divestment Business will have volumes available for the merchant market. Importantly, the Merged Entity will be smaller than Solvay was in the downstream market for PA 6.6 BP. As the Notifying Party rightfully submits "the Proposed Transaction will also not give rise to an increase in the incentive to foreclose rivals at downstream levels within the polyamide supply chain as the scale of the downstream activities of the Merged Entity and Divestment Business will each be smaller than that of the Business absent the Proposed Transaction. In other words, to the extent that the Business would gain anything from withholding its sales of HMD absent the Proposed Transaction, the Merged Entity and Divestment Business would each gain even less".917 It follows that the incentive of the Merged Entity to sell HMD to third parties will not be lesser than that of Solvay pre- transaction. In fact, as […] explains "the main reason for Solvay to sell on the merchant market is that it has more capacity on the upstream level, than it does at the downstream level. […] argues that upstream excess capacity is necessary to have a viable committed merchant player on the market. For instance, if a market player has 100 kts capacity upstream, and only 25 kts are sufficient for its own requirements, then there will likely be an upstream merchant market. Such excess capacity upstream establishes a commonality of interest between suppliers and buyers to have a functioning merchant market, all along the value chain".918 The Final Commitments by decreasing the Merged Entity's downstream presence lead to a lower internal demand for HMD which may translate into merchant sales by the Merged Entity.
(1269) Finally, […] claims that BASF could build a new HMD plant in China or choose to transform ADN into HMD in China through tolling agreements. In its extensive review of internal documents of BASF, the Commission did not find any indication that BASF would follow such a strategy.919 It is also important to note that Solvay also had, and would have absent the Transaction, the possibility to build a new HMD plant or to enter into tolling agreements with Chinese companies.
(1270) The Commission therefore concludes that the Final Remedies are sufficient to remove the competition vertical concerns identified with regards to the market for HMD.
7.6.1.3. Adipic acid
7.6.1.3.(A) Horizontal effects
(1271) As noted, a limited number of respondents to the market test put forward conflicting considerations in terms of the volumes of adipic acid (and associated materials) included as part of the remedy package and the related ability of the commitments to solve the competition concerns identified in the EEA market for adipic acid. […] considers that the Divestment Business will not be able to supply adipic acid to the merchant market in sufficient volumes,920 whereas another anonymous market participant indicates that the Divestment Business will be endowed by excessive adipic acid capacity – double its own internal requirements – translating into competitive disadvantages due to a disproportionate cost burden.921 In turn, […] considers that the purchaser's share of the Chalampé JV should be increased, whereas the other market participant takes the view that the purchaser's share should be reduced.922
(1272) […], the Final Commitments enable the Divestment Business to serve a greater share of the merchant market than BASF did pre-transaction, while remaining proportionate and not creating an undue burden for the purchaser of the Divestment Business. This is because the purchaser's 49% interest in the Chalampé JV will enable it to offtake 49% of the adipic acid (and other products) manufactured by the Chalampé JV amounting to […] kts of nameplate adipic acid capacity.923
(1273) Thus, based on data submitted by the Parties, the real production capacity available to the purchaser of the Divestment Business can be estimated at […] kts, i.e., 49% of [90-100]% of Chalampé's adipic acid nameplate capacity of […] kts. This figure is consistent with the actual adipic acid production of Chalampé, for example in 2016. In turn, the captive needs of the Divestment Business can be estimated at a total of approximately […] kts, including for the purpose of serving the downstream merchant markets and of complying with the terms of the temporary supply agreement to BASF that is part of the Commitments. This would leave the purchaser of the Divestment Business with approximately […] kts of adipic acid to serve the merchant market, which is significantly more than BASF's merchant sales of adipic acid in 2016 and 2017 (of […] kts).
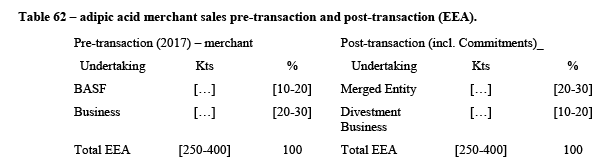
(1274) The Divestment Business includes significantly more capacity available to third parties than estimated by […], to a level that would enable the purchaser of the Divestment Business to, e.g., continue supplying DowDuPont with the volumes that it sources from the Chalampé plant.924 Conversely, the Divestment Business' captive needs are significantly more important than estimated by the other market participant, by more than [30-40]%, whereas the real offtake capacity is materially lower than estimated by that same market participant, by more than [10-20]%. The result is that the risk of disproportionate cost burden appears inexistent. In fact, the resulting difference matches the excess volumes identified by the market participant in question on the basis of inaccurate assumptions and its proposal to reduce the purchaser's interest in the Chalampé JV accordingly. In conclusion, the concerns expressed as part of the market test appear misplaced.
(1275) The Final Commitments go beyond the overlap created by the Transaction in relation to the supply of adipic acid based on merchant sales while ensuring that the Divestment Business would be able to cover its own captive requirements, including for merchant sales of downstream products. In turn, volumes freed by the termination of the temporary supply agreement with BASF that is part of the Commitments (PA 6.6 Base Polymer Tolling Agreement) will enable the purchaser of the Divestment Business to expand its available production capacity in the future. Furthermore, the Commitments provide that the purchaser of the Divestment Business will have the right to offtake at cost any production capacity not utilised by BASF as shareholder in the Chalampé JV, and vice-versa, thus providing for additional flexibility as may be required in the future, to the benefit of customers of adipic acid.
(1276) In addition, as noted, the market test largely endorsed the governance principles of the Chalampé JV and their appropriateness to protect the purchaser's entitlement, with the notable exception of […]. However, contrary to […] claims,925 these principles include the purchaser's involvement […].
(1277) Finally, the Chalampé JV arrangement is not such as to put in doubt the viability of the Divestment Business as a stand-alone business, or to create structural links or dependencies likely to raise competition issues.926 In that regard, each shareholder in the Chalampé JV will procure feedstock materials separately and determine its production volumes and sales prices independently from the other. In other words, the Chalampé JV will be a pure production joint venture and each Shareholder will be able to manage and dispose of its offtake share autonomously, without being constrained by the other. Moreover, the General Manager will also act solely in the best interest of the Chalampé JV and be incentivised accordingly. The Chalampé JV- arrangement is therefore a proportionate structural remedy that will create a viable adipic acid competitor in the short-term, whereas alternatives would have been inferior in terms of independence and/or certainty.927 Furthermore, links between competitors have not been identified as a source of competition concerns as part of the assessment of the Transaction, whereas […] is the only respondent to the market test that raised such a potential issue, without much substantiation. In view of the Commission, the Commitments will not materially increase the likelihood that firms will be able to coordinate their behaviour on the market in view, in particular, of the governance principles of the Chalampé JV and the associated independence of the Chalampé JV Shareholders in setting production levels and prices, as well as of the post-transaction asymmetry of their respective market position and related incentives.
(1278) The Commission concludes that the Final Commitments are suitable to remove the competition horizontal concerns identified with regards to the supply of adipic acid in the EEA.
7.6.1.3 (B) Vertical effects
(1279) The market test has not elicited particular concerns as to the risk of lasting foreclosure concerns arising from the Commitments in relation to the supply of adipic acid to the polyamide value chain or to producers of microcellular polyurethanes. The only (conflicting) concerns related to the scope of the Divestment Business' ability to supply the merchant market, thus including downstream competitors in the polyamide value chain.
(1280) As noted, data submitted by the Notifying Party indicates that the Divestment Business will have sufficient and proportionate volumes available to supply the merchant market in addition to satisfying its own captive needs, in excess of […] amounting to approximately a third of its real production capacity. As a result, the Commitments remove the overlap and restore the status quo in terms of number of market participants with material volumes of surplus capacity to supply the merchant market. In addition, the Divestment Business' proportion of merchant sales compared to total capacity is in line with that of the Business, thereby ensuring similar incentives to serve the merchant market.928
(1281) Moreover, as a result of the Final Commitments, the Merged Entity's sales base will be no larger than those of the Business at the downstream levels of the polyamide value chain that directly or indirectly use adipic acid. As a result, the Merged Entity will have no stronger ability or incentive to foreclose third-party producers than BASF or the Business did prior to the Transaction.
(1282) Furthermore, the Divestment Business does not include assets for the production of microcellular polyurethanes, with the result that it cannot as such give rise to foreclosure concerns in that respect and, generally, outside of the polyamide value chain
(1283) The Commission therefore concludes that the Final Remedies are sufficient to remove the vertical concerns identified in relation to the supply of adipic acid in the EEA.
7.6.1.4. AH Salt
7.6.1.4(A) Horizontal non-coordinated effects
(1284) None of the respondents to the market test raised observations as to the fact that the remedy proposed by the Notifying Party would not solve the competition concerns identified in terms of capacity or assets included in the remedy package. In fact, the proposed commitments will entirely remove the overlap generated by the Transaction.
(1285) On this market, the combined market shares of the parties are:
(a) [90-100]% in volume for merchant sales ([80-90]% Business and [5-10]% BASF);
(b) [50-60]% in volume in volume for merchant sales plus captive use ([40-50]% Business and [10-20]% BASF); and,
(c) [60-70]% for production capacity ([40-50]% Business -[…] kts- and [10-20]% BASF -[…] kts).
(1286) The Final Commitments offered by the Notifying Party include Belle Etoile and Blanes plants with […] and […] kts of AH Salt nameplate production capacity respectively, plus a 49% stake in the Chalampé JV which entails […] kts of additional capacity. Therefore, the horizontal overlap -[…] kts- is entirely removed.
(1287) Table 63 shows the production capacity pre-transaction and post-transaction (including Commitments) in the EEA:
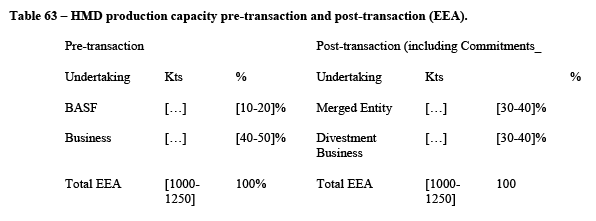
(1288) Therefore, the Commitments offered by the Notifying Party go beyond the overlap created by the Transaction which was of […] kts.
(1289) Although a competitor points out that the Divestment Business will have an excess of capacity at this level of the value chain taking into consideration the quantities of upstream inputs that will control and that this may increase fixed costs and therefore the 49% stake in the Chalampé JV would not be needed for the Divestment Business, it should be noted that fixed costs of the AH Salt stake in Chalampé are negligible compared to the total fixed costs of the Divestment Business (i.e. less than 2%).929 On the contrary, the 49% stake in the Chalampé JV will give a high degree of flexibility to the Divestment Business as to where to produce the AH Salt. It may also allow the Divestment Business to enter into tolling agreements.
(1290) Moreover, the competitor claims that the Divestment Business would only require […] kts of capacity of AH Salt. However, as discussed in Section 7.6.1.2, the Divestment Business would require producing up to […] kts of AH Salt to run its PA 6.6 BP plants at full capacity and sell some volumes of AH Salt to third parties. The nameplate capacity of the Divestment Business without including the 49% stake in the Chalampé JV would be […] kts with a real production capacity of […] kts of AH Salt. Furthermore, the Divestment Business will control through the two ADN supply agreements […] kts of ADN which are sufficient to produce […] kts of AH Salt. Therefore, without the 49% stake in the Chalampé JV, the Divestment Business would lack flexibility to decide whether to increase production at the level of AH Salt and try to gain market shares at this level, let alone to compete with the Merged Entity to supply […], the main customer of AH Salt which requires volumes about […] kts per year. Therefore, reducing the capacity of the Divestment Business at this level would reduce potential competition in the AH Salt merchant market.
(1291) With regards to the argument that the Blanes facility may not be competitive due to its small size, it should be noted that the Business has continued utilising this plant to date even if it had an excess of capacity at the AH Salt level (real production capacity of […] kts vs a production of […] kts in 2017)930. Should this plant not be competitive, the Business would have had the incentives to stop utilising it. Moreover, the Divestment Business will have flexibility to decide in which plant it will produce AH Salt. Additional information on the margin levels of the Blanes plant are provided in Section 7.6.2.2.
(1292) Table 64 shows merchant sales pre-transaction and post-transaction (including Commitments) in the EEA:
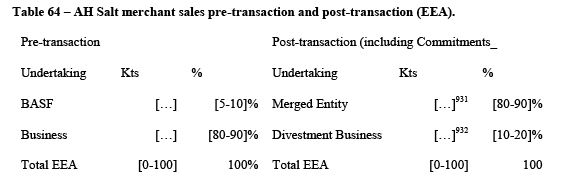
(1293) The Commitments will therefore fully eliminate the increments in market shares brought about by the Proposed Transaction based on both merchant sales and capacity. In fact, because the Divestment Business will be larger than BASF was in terms of capacity, the level of market concentration based on this metric will be lower post-transaction than pre-transaction.
(1294) Finally, with regards to the point raised by several market players that the extent to which the Divestment Business will be an effective competitor in the AH Salt market would ultimately depend on the conditions of supply of ADN and in particular on the competitiveness of the ADN price, the Commission notes that the Divestment Business will have access to ADN at market prices as discussed in Section 7.6.2.1, thus ensuring its competitiveness.
(1295) The Commission therefore concludes that the Final Commitments are sufficient to remove the competition horizontal concerns identified with regards to the market for AH Salt in the EEA.
7.6.1.4.(B) Vertical effects
(1296) None of respondents to the market test have raised any concern as to the vertical relation of AH Salt with regards to Co-Polyamide PA 6/6.6 base polymer and the large majority of the upstream customers and competitors responding to the market test consider that the remedies proposed are sufficient to allow effective competition in the Co-Polyamide PA 6/6.6 base polymer market.933
(1297) According to the data provided by the Notifying Party, third-party Co-Polyamide 6/6.6 BP producers in the EEA produced only […] kts in 2016.934 The amount of AH Salt required to produce 18 kts of Co-Polyamide PA 6/6.6 BP amounts to only […] kts. As explained in Section 7.6.1.4(A), the Divestment Business will have sufficient surplus capacity to supply more than […] kts of AH Salt to third parties.
(1298) The Divestment Business will also have the same incentive to supply AH Salt as the Business does pre-transaction, since (like the Business pre-transaction) the Divestment Business will not have any Co-Polyamide 6/6.6 BP production or sales. This means that neither the Business nor the Divestment Business would gain anything from foreclosing Co-Polyamide 6/6.6 BP producers. It is also foreseen that the Business’ Co-Polyamide PA 6/6.6 BP customer ([…]) will be transferred as part of the Divestment Business.
(1299) The Commission therefore concludes that the Final Commitments are sufficient to remove the competition vertical concerns identified with regards to the market for AH Salt in the EEA.
7.6.1.5. PA 6.6 BP
7.6.1.5.(A) Horizontal non-coordinated effects
(1300) The Divestment Business produces PA 6.6 BP at Belle-Etoile, Blanes and Valence. The Business also produces PA 6.6 BP at its Freiburg facility, which is not part of the Divestment Business and will thus remain part of the Merged Entity post- transaction.
(1301) The Final Commitments involve the divestment of substantial PA 6.6 BP production capacity in the form of the facilities at Belle-Etoile (installed capacities of […] kts from its Polaris unit and […] kts from its Technyl unit),935 Blanes ([…] kts) and Valence ([…] kts), for a total amounting to […] kts.
(1302) The Divestment Business will have access to sufficient AH Salt capacity through in- house production at Belle Etoile and Blanes as well as at the Chalampé JV to operate these PA 6.6 BP production facilities at full capacity (see Section 7.6.1.4 for discussions on AH Salt).
(1303) Table 65 shows the capacity for PA 6.6 BP pre-transaction and post-transaction (including the Final Commitments) in the EEA:
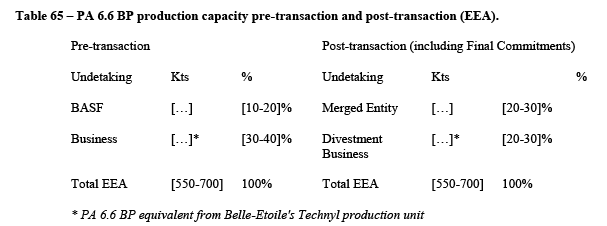
(1304) The Final Commitments offered by the Notifying Party go beyond the overlap created by the Transaction which was of […] kts, as the capacity of the Divestment Business in terms of PA 6.6 BP in the EEA in 2017 exceeds […] kts (or […] kts when disregarding the Technyl production unit in Belle-Etoile).
(1305) At the EEA level, the Divestment Business will hold [20-30]% of the PA 6.6 BP capacity, compared to [20-30]% for the Merged Entity. The Merged Entity's market shares, following the Final Commitments, thus do not indicate market power.
(1306) The same applies when looking at actual production volumes. Table 66 shows the production of PA 6.6 BP pre-transaction and post-transaction (including the Final Commitments) in the EEA, based on 2017 figures:
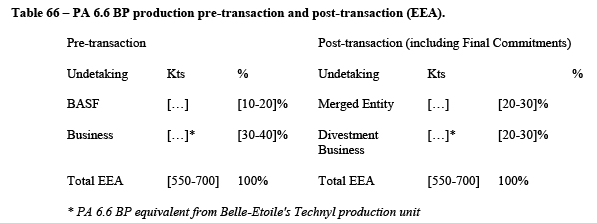
(1307) The Final Commitments offered by the Notifying Party go beyond the overlap created by the Transaction which was of […] kts, as the volumes produced of the Divestment Business in the EEA in 2017 exceed […] kts (or […] kts when disregarding the Technyl production unit in Belle-Etoile).
(1308) At the EEA level, the Divestment Business will hold [20-30]% of the PA 6.6 BP production, compared to [20-30]% for the Merged Entity. The Merged Entity's market shares, following the Final Commitments, thus do not indicate market power.
(1309) Furthermore, the Divestment Business will also have sufficient PA 6.6 BP capacity and access to AH Salt to sell PA 6.6 BP into the merchant market.
(1310) Pre-transaction, in 2017, facilities of the Divestment Business sold around […] kts of PA 6.6 BP into the merchant market. Table 67 shows the merchant sales of PA 6.6 BP pre-transaction and post-transaction (including the Final Commitments) in the EEA:
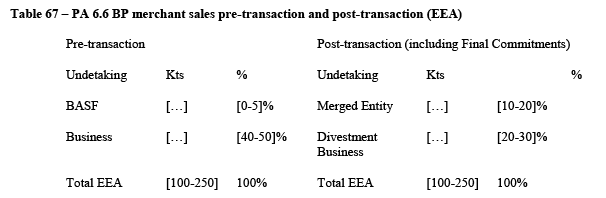
(1311) At the EEA level, the Divestment Business would hold [20-30]% of the PA 6.6 BP market share based on merchant sales, compared to [10-20]% for the Merged Entity. The Merged Entity's market shares, following the Final Commitments, do not indicate market power, and in fact are even much lower (over 50% lower) than the Business' pre-transaction.
(1312) As of 2017, the Divestment Business has capacity to produce around […] kts of PA 6.6 EP ([…] kts from Gorzow and […] kts from Belle Etoile's Technyl unit - this capacity is planned to increase to […] kts in 2019 as a result of investments that have already been made)936 and […] kts of PA 6.6 Performance Fibres. To function at full capacity, these facilities require around […] kts of PA 6.6 BP (approx. […] kts for Gorzow and approx. […] kts for Valence). PA 6.6 EP from Belle-Etoile’s Technyl unit is polymerised and compounded continuously from AH Salt. Continuous production implies that the PA 6.6 EP is manufactured directly from AH Salt, and thus does not use PA 6.6 BP as raw material input.
(1313) Considering that, even disregarding the Technyl production unit in Belle-Etoile, the Divestment Business has capacity of […] kts for the production of PA 6.6 BP and produced […] kts in 2017, it has sufficient capacity to meet its downstream requirements which amount to […] kts in terms of capacity, and […] kts in terms of production volumes, in 2017. The Divestment Business would thus have at least between […] kts (based on capacity) and […] kts (based on actual production volumes) of PA 6.6 BP available to third parties, which represents between [10-20]% and [20-30]% of total merchant sales on the market in 2017.
(1314) Projections made by respondents claiming the Divestment Business would not have sufficient PA 6.6 BP capacity are inaccurate, mainly because they ignore the fact that a share of the Divestment Business' EP is produced directly from AH Salt, and thus do not require the intermediate state of polymerisation to obtain PA 6.6 BP.937 Also, these calculations also typically deduct volumes that would have been provided to the Merged Entity under the transitional supply and tolling agreements, which were fixed under the Initial Commitments. Under the Final Commitments, the transitional supply and tolling agreement that the Divestment Business should negotiate with the Merged Entity do not include any fixed volumes and will be negotiated in good faith between the purchaser of the Divestment Business and the Merged Entity. Such freely negotiated sales share more characteristics with merchant sales than with captive use.
(1315) Also, the fact that the Divestment Business is not vertically integrated into ADN does not disqualify it as a competitive supplier of PA 6.6 BP. Pre-transaction, in the EEA, companies including DowDuPont, BASF, and Radici already produce PA 6.6 BP without being integrated into ADN. Some of which, in particular Radici, also offer substantial amounts of polymer to the merchant market. Furthermore, the Divestment Business will have access to a long term supply of ADN on market conditions that will allow it to fully utilise its downstream capacity for the supply of PA 6.6 BP.
(1316) The Commission concludes that the Final Commitments are sufficient to remove the horizontal competition concerns identified with regards to the market for PA 6.6 BP in the EEA.
7.6.1.5.(B) Vertical effects
(1317) As a result of the Final Commitments, which eliminate entirely the overlap (and more) at PA 6.6 BP level, the Merged Entity will have a much smaller presence in the upstream market for PA 6.6 BP in the EEA. Consequently, the Merged Entity will not have the ability to engage in input foreclosure strategies with regards to PA 6.6 BP. Its market share in particular evidences a lack of market power. Based on either capacity ([20-30]%), production ([20-30]%) or merchant sales ([10-20]%), the Merged Entity's market share in the EEA is much smaller than the Divestment Business', and is lower than either BASF's or the Business' pre-transaction.
(1318) With regards to the supply of PA 6.6 BP to producers of performance fibres, the Merged Entity will not be active in the production of PA 6.6 Performance Fibres, whereas the Business was. As such, the Merged Entity would have no incentive whatsoever to foreclose producers of PA 6.6 Performance Fibres, as it will not be competing with them downstream. In fact, the Final Commitments increase the number of players able to supply the performance fibres industry. While only the Business (and not BASF) had the ability to supply PA 6.6 BP for use in performance fibres, both the Merged Entity (in Freiburg) as well as the Divestment Business (in Valence, as well as in Belle-Etoile and Blanes to a lesser extent) will have the ability to produce PA 6.6 BP for use in performance fibres.
(1319) With regards to the supply of PA 6.6 BP to producers of PA 6.6 EP, the Merged Entity will also have a more limited market presence downstream as a result of the Commitments, which de facto remove the entire overlap on the PA 6.6 EP market in the EEA, decreasing any incentive to foreclose competitors (see Section 7.6.5 for additional information on the impact of the Final Commitments on the PA 6.6 EP market).
(1320) In addition, an important share of the Business' merchant sales of PA 6.6 BP (approximately [40-50]% of total merchant sales in 2017) was carried through its Freiburg facility, which will remain part of the Merger Entity post-transaction. The Parties expect the facility to continue to supply customers on the merchant market post-transaction.
(1321) Furthermore, the Divestment Business will be able to supply downstream customers of PA 6.6 BP, due to the fact that its own requirements are not sufficient to absorb the totality of its PA 6.6 BP production.
(1322) The Commission concludes that the Final Commitments are sufficient to remove the vertical competition concerns identified with regards to the market for PA 6.6 BP (upstream), and PA 6.6 EP and PA 6.6 Performance Fibres (downstream) in the EEA.
7.6.1.6. PA6 3D printing powder
(1323) The Final Commitments involve the divestment of all of the Business’s PA 6 3D printing powder activities, therefore entirely removing the horizontal overlap brought about by the Transaction.
(1324) The Commission therefore concludes that the Final Commitments are sufficient to remove the horizontal competition concerns identified with regards to the market for PA 6 3D printing powder.
7.6.1.7. PA 6.6 EP
(1325) The Divestment Business’ produces PA 6.6 EP in Belle-Etoile and Gorzow. Belle- Etoile’s Technyl line produces large volumes of standard grade PA 6.6 EP which have been homologated or accepted for use by large automobile manufacturers. Gorzow produces customised and specialty grades of PA 6.6 EP on demand.
(1326) The Final Commitments involve the divestment of all of the Business’s PA 6/PA 6.6 EP production units, with combined operational capacities amounting to around 107 kts.
(1327) The Divestment Business will have access to sufficient PA 6.6 BP capacity through in-house production to operate these PA 6.6 EP production facilities at full capacity (see Section 7.6.1.5).
(1328) Table 68 shows the merchant sales of PA 6.6 EP pre-transaction and post-transaction (including the Final Commitments) in the EEA based on 2017 sales data
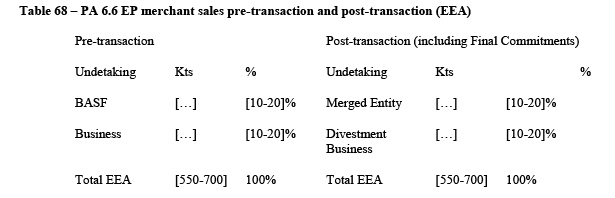
(1329) The Final Commitments offered by the Notifying Party totally remove the overlap created by the Transaction which was of […] kts, as the Divestment Business includes all of the Business' PA 6.6 EP production units (in Belle-Etoile and Gorzow).
(1330) […] notes that the Divestment Business cannot exert the same competitive pressure on the PA 6.6 EP market as the Business pre-transaction, in particular due to the limited access of the Divestment Business to upstream raw materials, and specifically ADN, on a sustainable basis. Customers, including in particular the automotive industry need to have visibility in terms of security of supply over a long period of time (covering e.g. the lifetime of specific model series), and would thus typically look for suppliers able to guarantee such security of supply. Again, the Commission notes that the fact that the Divestment Business is not vertically integrated into ADN does not disqualify it as a competitive supplier of PA 6.6 EP. Pre-transaction, in the EEA, companies including DowDuPont, BASF, Radici, Celanese or Lanxess already produce PA 6.6 EP without being integrated into ADN. Some of them, including Celanese, Lanxess and many compounders, are not integrated at all upstream and only offer PA 6.6 EP but not any of its precursors. Furthermore, the Divestment Business will have access to a long term supply of ADN on market conditions that will allow it to fully utilise its downstream capacity for the supply of PA 6.6 EP.
(1331) […] also notes that the Initial Commitments may hinder innovation due to the lack of upstream supply security. In addition to the points raised regarding ADN supply, the Commission notes that Divestment Business includes all of the relevant R&D assets necessary to continuously innovate and compete on the PA 6.6 EP markets. The Business has two R&D centers: (a) The Research and Innovation Centre of Lyon ("RICL") focused on intermediate products, located in Saint Fons (close to the Belle- Etoile site), and (b) the Technyl Innovation Center, focused on EP, located at Belle- Etoile. Both the RICL and the Technyl Innovation Center including all the relevant installed equipment (including testing, laboratory equipment and computers) will be part of the Divestment Business. In addition over 90% of the Business' research staff, including key inventors, will be transferred to the Divestment Business.938 As a result, the Divestment Business will have the same ability (and incentives) to innovate as the Business did pre-transaction.
(1332) As mentioned in Section 7.4, some market respondents raised a concern that the Divestment Business will be a regional player and may thus be unable to effectively supply customers with global platforms, in particular in the automotive space, which would impact on its ability to compete in the PA 6.6 EP space. The Commission notes that the market investigation indicated that the market for PA 6.6 EP was EEA- wide in scope rather than global. Regardless, the wording of Final Commitments ensures that the Divestment Business can participate in global platforms, which may require some presence or sales outside of the EEA. Indeed, the Final Commitments include as a purchaser criterion, the fact that "The Purchaser shall have an established presence in the production and global sale of chemical products. The Purchaser shall be in a position to effectively continue the Divestment Business as a viable and active competitor with BASF and other competitors. For example, the Purchaser shall be in a position to service global automotive customers" (emphasis added). By imposing that the purchaser of the Divestment Business is active globally in the sales of chemicals, the Final Commitments ensure that the Divestment Business continues to be a strong competitor, including for global projects.
(1333) The Commission concludes that the Final Commitments are sufficient to remove the horizontal competition concerns identified with regards to the market for PA 6.6 EP in the EEA.
7.6.2. Viability of the Final Commitments
(1334) The Commission takes the view that the Divestment Business as set up under the Final Commitments is viable.
7.6.2.1. Access to ADN
(1335) First, the Commission takes the view that the Divestment Business will have access to sufficient volumes of ADN, under competitive conditions and on a long term basis.
(1336) Following the results of the market test, the Parties improved the Initial Commitments by (i) extending, at the option of the purchaser of the Divestment Business, the duration of the ADN Agreement 2 by up to […] years and (ii) increasing the total volume of ADN to which the purchaser of the Divestment Business will have access by […] kts, through either ADN Agreement 1 or 2. Under the terms of the Final Commitments, the Divestment Business will have access to a volume of ADN equivalent to its historical needs, for a period of up to […] years. The Divestment Business will be therefore have access on a long-term basis to the same samount of AND as it did pre-transaction. Also, if the purchaser of the Divestment Business intends to utilise the Divestment Business’ HMD plants at full capacity, it would only need to purchase an additional […] kts of ADN from the market.
(1337) The Commission also observes that the […] year extension of ADN Agreement 2 is at the option of the purchaser of the Divestment Business, which provides the purchaser of the Divestment Business with a sufficient degree of flexibility in its sourcing strategy to adapt to any change in market conditions or pricing structures on the ADN market.
(1338) The Commission also considers that the contractual links created by the Final Commitments will not create any dependency between BASF and the Divestment Business. […].
(1339) Furthermore, links between competitors have not been identified as a source of competition concerns as part of the assessment of the Transaction.
(1340) The Commission takes the view that Invista will not have the ability to strategically manipulate its HMD prices in order to increase the ADN prices paid by the Divestment Business. This is because Invista will be constrained in its pricing strategy by BASF, which as explained in Section 7.6.1.2, will remain a competitive force on the HMD market. […].
(1341) The Commission also considers that the price formula provided for in ADN Agreement 2 will not increase transparency in the market to such an extent that it would raise competition concerns. […].
(1342) The Commission further takes the view that the price formula proposed by the Parties for ADN Agreement 1 and ADN Agreement 2 reflect the market prices of ADN and therefore will not place an excessive and undue burden on the Divestment Business' cost structure and overall viability.
(1343) To assess the suitability of the price formula, the Commission asked the Parties to apply the price formulas provided to their past purchases of ADN and compare the resulting prices with the prices actually paid. By performing this exercise, it emerged that the price actually paid is comparable to the prices resulting from the application of the formulas.
(1344) […].
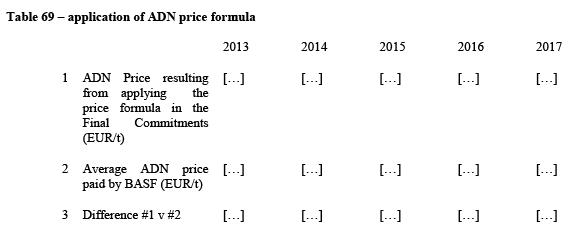
(1345) Table 69 shows that the price formula included in the Final Commitments closely reflect the prices paid by BASF on the merchant market and therefore do not impair the Divestment Business' viability on the market.
(1346) The Commission also compared the price that would have resulted in applying the price formula of ADN Agreement 1 since 2014 and the price actually charged by Invista in the same period. While the Commission cannot divulge the precise figures resulting from that exercise as they are confidential, it not that the ADN prices resulting from the application of the formula would have not been dissimilar from the average prices actually charged by Invista.
(1347) The Commission notes that the Divestment Business will incur ADN procurement costs which closely replicate those of BASF pre-transaction, which allowed BASF to be a viable and competitive force in the polyamide value chain in the past. The Commission therefore concludes that, just as with BASF, the price formulas provided for by in the Final Commitments will allow the Divestment Business to be a viable and competitive force.
(1348) Some respondents to the market test have indicated that the fact that the Divestment Business is not vertically integrated into ADN – like Solvay had been pre-transaction – significantly impacts its viability.
(1349) The Commission notes that the observations of the respondents to the market test contradicts the result of the market investigation as BASF has been consistently identified as a strong and credible competitive force along the polyamide value chain, despite its lack of vertical integration into ADN. However, despite replicating the same model as BASF pre-transaction and having access to larger volumes of ADN and larger production capacity than other intermediates along the value chain, the Divestment Business is considered as not being viable by the same respondents.
(1350) Secondly, the Commission notes that the Commitments are not aimed at replicating Solvay on the market, but rather at solving the competition concerns raised. With regards to the ADN market, the Final Commitments replicate the market structure while increasing ADN availability, thereby improving the pre-transaction market situation.
(1351) Pre-transaction, two competitors were vertically integrated into ADN in the EEA – Invista and Solvay – while BASF and Radici were sourcing ADN and integrated downstream. Post-transaction, the market structure will be similar, with BASF replacing Solvay as a fully integrated supplier and the Divestment Business replacing BASF as purchaser that is integrated further down the PA 6.6 value chain, but with access to ADN via long term contracts and for significantly higher volumes than those available to BASF pre-transaction.
(1352) Thirdly, the Commission considers that the Final Commitments will allow and provide the incentives to the Divestment Business to act as a competitive force on the market. As mentioned in section 7.6.1, the Divestment Business will have access to volumes of ADN on a long term basis ([…] years), which will allow it to make intermediates available to the merchant market. In particular, the Divestment Business will be able to produce HMD at almost full capacity939 with the […] kts of ADN to which it will have access. It will also be able to produce an additional […] kts of HMD to sell on the merchant market in the EEA, while the Business sold […] kts of HMD in the EEA in 2017. The Divestment Business, therefore, has enough available volume of ADN to replicate the merchant position of Solvay.
(1353) If the analysis is then carried out for the rest of the value chain, the Divestment Business will have sufficient spare capacity of intermediates to be an active player on the relative merchant markets.
(1354) Fourth, and contrary to the claims of some respondents to the market test, the Commission notes that Radici is an active, viable and credible competitor throughout the polyamide value chain, albeit not vertically integrated into ADN.
(1355) Finally, and as already explained, the Commission considers that the Divestment Business will have access to ADN on market terms. The Commission therefore takes the view that the costs of sourcing ADN will not prevent the Divestment Business from making profitable sales on the merchant market.
7.6.2.2. General considerations on the viability of the Divestment Business
(1356) Respondents to the markets investigation raised concerns about the viability of the Divestment Business.
7.6.2.2.(A) Age and location of the Divestment Business' facilities
(1357) Some respondents flagged the geographic dispersion of the Divestment Business’ facilities, which may generate high logistic costs, and affect the Divestment Business' integration,940 and others stated that the Divestment Business' assets are old, and that BASF may have wanted to get rid of them either way.941
(1358) The Commission notes that the Divestment Business already functions as an integrated cluster, regardless of proximity of the different facilities, as evidenced by the trade flows between the different facilities and their specialisation. For instance:
– the Divestment Business facilities largely procure their key raw material inputs (except ADN) from each other. Belle-Etoile’s HMD is used as an input by Gorzow, Blanes and Valence. Valence sources it’s AH Salt from Belle- Etoile. Belle-Etoile and Blanes source adipic acid from Chalampé.
– Belle-Etoile and Gorzow are complementary in respect of the types of Engineering Plastics customers they supply. Belle-Etoile produces large volumes of PA 6.6 EP for large automotive customers, while Gorzow produces smaller volumes of specific grades of customised EP.
– Belle-Etoile and Valence are relatively close geographically and share similar continuous polymerisation technology and a team of engineers.
(1359) Furthermore, regardless of the age of the facilities of the Divestment Business, it is important to note that all facilities have shown constant profitability over the last five years, with the exception of Blanes in 2013, as evidenced by Figure 11 […]”]
[…]
(1360) Based on internal documents, […], other assets of the Divestment Business, such as Solvay's EP Business, were considered particularly profitable.
(1361) Furthermore, geographic proximity between facilities is not necessarily required to effectively run a vertically integrated business in the polyamide value chain. Similarly, competitive businesses may include old facilities. For instance, BASF's own Seal Sands HMD facility is relatively old and geographically isolated from the rest of BASF's PA 6.6 production assets, but BASF remains a leading player in the
PA 6.6 value chain.942
7.6.2.2.(B) Intellectual property rights
(1362) […] also submits that the Divestment Business would include proprietary IP rights, i.e. all IP rights, including trade secrets and know-how, which are required in order to innovate and compete. According to […], these rights would need to be exclusively transferred to the Divestment Business. 943 Co-ownership of these rights with BASF would restrict the Divestment Business’ ability to innovate.
(1363) However, contrary to what […] appears to assume, the Final Commitments ensure that BASF will effectively transfer all the patents and licenses, including the rights thereto, which are owned by the Business and are (or have been) used solely or predominantly by the Divestment Business. The Final Commitments also provide for: (i) perpetual, irrevocable, non-exclusive, fully paid-up EEA-wide licences for the patents that the Divestment Business uses in connection with the manufacture of the upstream products, if these patents are not used solely or predominantly by the Divestment Business; and (ii) perpetual, irrevocable, non-exclusive, fully paid-up worldwide licences for the patents that the Divestment Business uses in connection with the manufacture of the downstream products.
(1364) The Commitments also provide that BASF will transfer to the Divestment Business: (i) all the know-how, trade secrets, other confidential information, and technical documentation for the production of the upstream products owned by the Business that are used or have been used solely or predominantly by the Divestment Business; and (ii) perpetual, irrevocable, non-exclusive, fully paid-up EEA-wide licences for the know-how that the Divestment Business uses in connection with the manufacture of the upstream products, if this know-how is not used solely or predominantly by the Divestment Business.
(1365) In addition, the Final Commitments grant the purchaser of the Divestment Business the option to share with BASF co-ownership of all know-how, trade secrets and other confidential information and technical documentation for the worldwide production and sale of the downstream products. This possibility is at the sole discretion of the purchaser of the Divestment Business. Therefore, the purchaser of the Divestment Business will have flexibility to choose between: (i) ownership over all the know- how, trade secrets, other confidential information, and technical documentation for the production of the downstream products owned by the Business that are used or have been used solely or predominantly by the Divestment Business, plus a license for the know-how that the Divestment Business uses in connection with the manufacture of the Dowstream Products, if this know-how is not used solely or predominantly by the Divestment Business; or, alternatively, (ii) shared co- ownership with BASF for both, the know-how and other IP rights used exclusively or predominantly by the Divestment Business and over the know-how that is not used solely or predominantly by the Divestment Business. This flexibility will allow the purchaser of the Divestment Business to choose the option that will allow it to innovate and compete more effectively in the downstream products.
(1366) […] also submits that BASF will have access to the purchaser´s background patents. […] states that "Hence Purchaser/Divested Business has less flexibility to exploit such Purchaser´s background patents in the future (e.g. with regard to exclusive licensing)".944
(1367) The Commission notes that BASF will transfer all the patents and licenses, including the rights thereto, owned by the Business that are (or have been) used solely or predominantly by the Divestment Business. The purchaser of the Divestment Business will grant perpetual, irrevocable, non-exclusive, fully paid-up licences to BASF for the use of these patents, in so far they are needed to continue running the non-divested business. In addition, the purchaser of the Divestment Business will grant to BASF a worldwide license for any background patents that are required to implement an innovation generated by the non-divested business.
(1368) The Divestment Business will have full ownership of these patents. Granting a license for background patents to BASF will not have any impact on the flexibility that the Divestment Business will have on its own use of these patents. The purpose of these background licenses is to allow the non-divested assets to continue investing in the development of new products and innovation. In fact, the Divestment Business also includes a worldwide license for any background patents that are required in connection with the manufacture of upstream products to implement an innovation generated by the Divestment Business where BASF is the predominant user of the patents that are therefore not transferred to the Divestment Business, The licensing arrangements under the Final Commitments thus offer both the Divestment Business and BASF an enhanced ability to innovate in the PA 6.6 value chain.
(1369) Separately, according to […], the Divestment business might require to increase its production footprint outside EEA to ensure its viability and competitiveness. In such case it might be necessary to have a perpetual, irrevocable, sub-licensable, fully paid- up worldwide licence of the patents and know-how for the manufacture of upstream products.945 In this vein, […] submits that "the territory “EEA” of the license will most probably not be sufficient for the Divestment Business to ensure its business activities and establish customer relationships outside the “EEA” territory, as products/compounds manufactured in the EEA sites covered by the transaction might also be intended for offering and sale outside the EEA territory where, however, the patents and know-how (not licensed for such territory) might prohibit such offering and sale".946
(1370) First, BASF is only active in the production of upstream products in the PA 6.6 value chain in the EEA (in Seal Sands in the UK for HMD, and in Ludwigshafen in Germany for AH Salt, adipic acid and PA 6.6 BP). BASF does not produce any of the upstream products in the PA 6.6 value chain outside the EEA. Regardless, BASF is one of the strongest competitors in the PA 6.6 value chain in the EEA. Therefore, having production capabilities for the upstream products outside the EEA do not appear necessary to be a competitive or viable competitor.
(1371) Second, BASF will transfer all the patents and licenses, including the rights thereto, owned by the Business that are (or have been) used solely or predominantly by the Divestment Business, as well as perpetual, irrevocable, non-exclusive, fully paid-up EEA-wide licences for the patents that the Divestment Business uses in connection with the manufacture of the upstream products, if these patents are not used solely or predominantly by the Divestment Business. Moreover, the EEA scope of the licenses used in connection with the manufacture of the upstream products does not limit the possibility of the Divestment Business to sell these products outside the EEA; it only limits the possibility to use these licenses to engage in the production of any of the upstream products outside the EEA. Therefore, the Divestment Business will be able to sell globally. In fact, the Final Commitments include a supply agreement with […] whereby the Divestment Business will supply […] kts of HMD to […] in […].947
(1372) Finally, a competitor who requested anonymity submits that licenses should also include improvements on background technology to ensure long-term viability. It also adds that "the Licensee should be able to practice any improvement BASF makes if a licensed patents still reads on that improvement".948
(1373) The Divestment Business includes licenses for background patents and know-how that will allow it to implement an innovation generated by the Divestment Business. Such background licenses will be perpetual, irrevocable, non-exclusive, and fully paid-up. For the avoidance of doubt, the Divestment Business cannot benefit from innovations developed by the Merged Entity post-closing. Similarly, the Merged Entity cannot benefit from innovations developed by the Divestment Business. Allowing the Divestment Business to benefit from future innovations developed by the Merged Entity (or conversely) would go beyond what is necessary to ensure the viability of the Final Commitments and would reduce the incentives of the Merged Entity (and/or the Divestment Business) to invest in improvements and innovation because the Merged Entity (and/or the Divestment Business) would not be in a position to extract exclusively all the benefits that its own innovation entails.
7.7. Conclusion
(1374) Based on the assessment in recitals (1168) to (1373), the Commission concludes that the Final Commitments are sufficient in scope and suitable to remove entirely the significant impediments to effective competition to which the Transaction would otherwise have given rise and that, therefore, the Final Commitments render the concentration brought about by the Transaction compatible with the internal market and the EEA Agreement. In conclusion, the Commission finds that, following modification in accordance with the Final Commitments, the concentration brought about by the Transaction would not significantly impede effective competition in the internal market or within the territory covered by the EEA Agreement, or in a substantial part of either of them.
8. CONDITIONS AND OBLIGATIONS
(1375) Pursuant to the first sentence of the second subparagraph of Article 8(2) of the Merger Regulation, the Commission may attach to its Decision conditions and obligations intended to ensure that the undertakings concerned comply with the commitments they have entered into with the Commission with a view to rendering the concentration compatible with the internal market.
(1376) The fulfilment of a measure that gives rise to a structural change of the market is a condition, whereas the implementing steps which are necessary to achieve that result are generally obligations on the parties. Where a condition is not fulfilled, the Commission’s decision declaring the concentration compatible with the internal market and the EEA Agreement will not be, or no longer be, applicable. Where the undertakings concerned commit a breach of an obligation, the Commission may revoke the clearance decision in accordance with Article 8(6) (b) of the Merger Regulation. The undertakings concerned may also be subject to fines and periodic penalty payments under Articles 14(2) and 15(1) of the Merger Regulation.
(1377) In accordance with the distinction between conditions and obligations described in recital (1376), this Decision should be conditional on the full compliance by the Parties with the requirements set out in Sections B, C and D of the Final Commitments, including the Schedule and the Annexes to the Schedule, which constitute conditions. The requirements set out in Sections E and F of the Final Commitments constitute obligations on the Parties within the meaning of Article 8(2) of the Merger Regulation. The full text of the Final Commitments, together with the Schedule and the Annexes to the Schedule, is attached as Annex I to this Decision and forms an integral part thereof.
HAS ADOPTED THIS DECISION:
Article 1
The notified concentration whereby BASF SE acquires sole control of Solvay S.A.’s worldwide polyamide activities within the meaning of Article 3(1)(b) of Regulation (EC) No 139/2004 is declared compatible with the internal market and the Agreement on the European Economic Area.
Article 2
Article 1 is subject to compliance with the conditions set out in Sections B, C and D of Annex I, the Schedule of Annex I and the Annexes to the Schedule of Annex I.
Article 3
BASF and Solvay SA shall comply with the obligations set out in Section E and F of Annex I.
Article 4
This Decision is addressed to:
BASF SE
Carl-Bosch Straße 38
67056 Ludwigshafen
Germany
Done at Brussels, 18.1.2019
Case M. 8674 – BASF/SOLVAY'S POLYAMIDE BUSINESS
COMMITMENTS TO THE EUROPEAN COMMISSION
Pursuant to Articles 8(2) and 10(2) of Council Regulation (EC) No 139/2004 (the “Merger Regulation”), BASF SE (“BASF” or the “Notifying Party”) hereby enters into the following Commitments (the Commitments) vis-à-vis the European Commission (the “Commission”) with a view to rendering the proposed acquisition of sole control of Solvay S.A.’s (“Solvay”) Polyamide Business (“the Business”) (the “Concentration”) compatible with the internal market and the functioning of the EEA Agreement.
This text shall be interpreted in light of the Commission’s decision pursuant to Article 8(2) of the Merger Regulation to declare the Concentration compatible with the internal market and the functioning of the EEA Agreement (the “Decision”), in the general framework of European Union law, in particular in light of the Merger Regulation, and by reference to the Commission Notice on remedies acceptable under Council Regulation (EC) No 139/2004 and under Commission Regulation (EC) No 802/2004 (the “Remedies Notice”).
Section A. Definitions
1. For the purpose of the Commitments, the following terms shall have the following meaning:
Affiliated Undertakings: undertakings controlled by the Parties and/or by the ultimate parents of the Parties whereby the notion of control shall be interpreted pursuant to Article 3 of the Merger Regulation and in light of the Commission Consolidated Jurisdictional Notice under Council Regulation (EC) No 139/2004 on the control of concentrations between undertakings (the "Consolidated Jurisdictional Notice").
Assets: the assets that contribute to the current operation or are necessary to ensure the viability and competitiveness of the Divestment Business as indicated in Section B, paragraph 6 and described more in detail in the Schedule.
BASF: BASF SE, incorporated under the laws of Germany, with its registered office at Carl- Bosch-Straße 38, 67056 Ludwigshafen, Germany including its Affiliated Undertakings.
Chalampé JV: a production joint venture to which the entirety of the assets owned by the Business on the Chalampé site will be transferred including the production plants of KA Oil, Nitric Acid, AA and AH Salt (Chalampé) excluding the Business’ shares in the Butachimie JV which will be transferred to BASF. The Purchaser will hold a 49% interest (the Chalampé JV Interest) and BASF will hold a 51% interest. The Chalampé JV Interest will give the Purchaser the right to offtake 49% of the Upstream Products manufactured by the Chalampé JV. BASF has the right to offtake 51% of the Upstream Products manufactured by the Chalampé JV. For the avoidance of doubt, the Purchaser will have the right to use 49% of the actual available capacity of the Chalampé JV at its own discretion, and to offtake at cost any additional production capacity not utilised by BASF. BASF will have the right to use 51% of the actual available capacity of the Chalampé JV at its own discretion, and to offtake at cost any additional production capacity not utilized by the Divestment Business.
Closing: the transfer of the legal title to the Divestment Business to the Purchaser.
Closing Period: the period of up to […] from the approval of the Purchaser and the terms of the initial binding agreement (Put Option Agreement) by the Commission.
Confidential Information: any business secrets, know-how, commercial information, or any other information of a proprietary nature that is not in the public domain.
Conflict of Interest: any conflict of interest that impairs the Trustee’s objectivity and independence in discharging its duties under the Commitments.
Divestment Business: the business or businesses as defined in Section B and in the Schedule 1 which the Notifying Party commits to divest.
Divestiture Trustee: one or more natural or legal person(s) who is/are approved by the Commission and who may be appointed by BASF and who – in case of the appointment by BASF – has/have received from BASF the exclusive Trustee Mandate to sell the Divestment Business to a Purchaser at no minimum price.
Downstream Products: all products of the polyamide value chain other than the Upstream Products produced, marketed and commercialised by the Divestment Business, including but not limited to in particular master batches, PA6.6 EP, PA6 EP, PA6.10 EP, PA T4E EP, PA HT EP, PA 6.6 PF and PA6 3D printing powder.
Effective Date: the date of adoption of the Decision.
First Divestiture Period: the period of […] from the Effective Date including any potential extensions.
Hold Separate Manager: the person appointed by BASF in consultation with Solvay for the Divestment Business to manage the day-to-day business under the supervision of the Monitoring Trustee.
Key Personnel: all personnel necessary to maintain the viability and competitiveness of the Divestment Business, as referred to in section 2 of the Schedule, including the Hold Separate Manager.
Monitoring Trustee: one or more natural or legal person(s) who is/are approved by the Commission and appointed by BASF in consultation with Solvay, and who has/have the duty to monitor BASF’s compliance with the conditions and obligations attached to the Decision.
Non-Divested Assets: any production asset or manufacturing units located at the Business’ sites at Freiburg, Roussillon or located outside the EEA, Solvay’s 50% equity stake in the ADN and HMD joint venture with INVISTA named “Butachimie” (located in Chalampé, France), as well as BASF’s 51% interest in the Chalampé JV.
Non-Divested Business: the business for products manufactured by the Non-Divested Assets including the licences for all patents and know-how, trade secrets, confidential information, technical documentation granted by the Business or one of its Affiliated Undertakings to (i) the Butachimie JV, (ii) Solvay or one of its Affiliated Undertakings (excluding the Divestment Business), or (iii) to the Chalampé JV.
Parties: the Notifying Party and the undertaking that is the target of the concentration.
Personnel: all staff currently employed by the Divestment Business, including staff seconded to the Divestment Business, shared personnel as well as the additional personnel.
Products: Upstream and Downstream Products.
Purchaser: the entity approved by the Commission as acquirer of the Divestment Business in accordance with the criteria set out in Section D. The Divestment Business shall be sold to a single purchaser which does not exclude that such purchaser has more than one shareholder.
Purchaser Criteria: the criteria laid down in paragraph 17 of these Commitments that the Purchaser must fulfil in order to be approved by the Commission.
Put Option Agreement: initial binding agreement between the Purchaser and Solvay regarding the transfer of the Divestment Business to the Purchaser. The exercise of the put option is conditional upon the French works council consultation only.
Schedule: the schedule to these Commitments describing more in detail the Divestment Business.
Trustee(s): the Monitoring Trustee and/or the Divestiture Trustee as the case may be.
Trustee Divestiture Period: in case of the appointment of a Divestiture Trustee by BASF the period of […] from the end of the First Divestiture Period.
Transaction: the Concentration.
Upstream Products: all polyamide intermediate and base polymer products of the polyamide value chain produced, marketed and commercialised by the Divestment Business, including but not limited to in particular AA, Nitric Acid, KA Oil, AGS, DBA, HMD, AH Salt, PA6.6 BP, PA6 BP, PA6.10 BP, PA T4E BP, and PA HT BP.
Section B. The commitment to divest and the Divestment Business
Commitment to divest
2. In order to maintain effective competition, BASF commits to divest, or procure the divestiture of the Divestment Business by the end of the First Divestiture Period as a going concern to a purchaser and on terms of sale approved by the Commission in accordance with the procedure described in paragraph 18 of these Commitments. To carry out the divestiture, BASF commits to find a purchaser and to cause Solvay to enter into a Put Option Agreement for the sale of the Divestment Business within the First Divestiture Period. If Solvay has not entered into such an agreement at the end of the First Divestiture Period, BASF may appoint, at its sole discretion a Divestiture Trustee and grant the Divestiture Trustee an exclusive mandate to sell the Divestment Business in accordance with the procedure described in paragraph 30 in the Trustee Divestiture Period. If BASF has not found a purchaser within the First Divestiture Period and decides not to appoint a Divestiture Trustee, the Transaction lapses without prejudice to the contractual obligations between the Parties.
3. The proposed Concentration shall not be implemented before BASF or the Divestiture Trustee (in case of its appointment by BASF) has caused Solvay to enter into a Put Option Agreement for the sale of the Divestment Business and the Commission has approved the Purchaser and the terms of sale in accordance with paragraph 18.
4. BASF shall be deemed to have complied with this commitment if:
(a) by the end of the Trustee Divestiture Period, BASF or the Divestiture Trustee (in case of its appointment by BASF) has caused Solvay to enter into a Put Option Agreement and the Commission approves the proposed purchaser and the terms of sale as being consistent with the Commitments in accordance with the procedure described in paragraph 18; and
(b) the Closing of the sale of the Divestment Business to the Purchaser takes place within the Closing Period.
5. In order to maintain the structural effect of the Commitments, the Notifying Party shall, for a period of 10 years after Closing, not acquire, whether directly or indirectly, the possibility of exercising influence (as defined in paragraph 43 of the Remedies Notice, footnote 3) over the whole or part of the Divestment Business, unless, following the submission of a reasoned request from the Notifying Party showing good cause and accompanied by a report from the Monitoring Trustee (as provided in paragraph 44 of these Commitments), the Commission finds that the structure of the market has changed to such an extent that the absence of influence over the Divestment Business is no longer necessary to render the proposed Concentration compatible with the internal market.
Structure and definition of the Divestment Business
6. The Divestment Business consists of the EEA polyamide business of the Business, as detailed in the Schedule including:
(i) the Business’ facility (including its relevant production units of HMD, AH Salt, PA6.6 BP, PA6 3D printing powder and PA6.6 EP) located at Belle-Etoile, France (Belle-Etoile). All of Belle-Etoile will be included in the Divestment Business;
(ii) the Business’ facility (including its relevant production units of PA6 BP, PA T4E BP and PA6.6 EP) located at Gorzow, Poland (Gorzow). All of Gorzow will be included in the Divestment Business;
(iii) the Business’ facility (including its relevant production units of HMD distillation, AH Salt and PA6.6 BP) located at Blanes, Spain (Blanes). All of Blanes will be included in the Divestment Business;
(iv) the Business’ facility (including its relevant production units for PA6.6 BP and PA6.6 Performance Fibres) located at Valence, France (Valence) All of Valence will be included in the Divestment Business; and
(v) a 49% interest in the Chalampé JV (Chalampé JV Interest).
7. The legal and functional structure of the Divestment Business as operated to date is described in the Schedule. The Divestment Business includes all assets and staff that contribute to the current operation or are necessary to ensure the viability and competitiveness of the Divestment Business as detailed in the Schedule, in particular:
(a) all tangible and intangible assets (including intellectual property rights);
(b) all licences, permits and authorisations issued by any governmental organisation for the benefit of the Divestment Business;
(c) all contracts, leases, commitments and customer orders of the Divestment Business; all customer, credit and other records of the Divestment Business;
(d) the Personnel; and
(e) three supply agreements (the ADN Supply Agreements) that will be entered into between BASF and/or [ADN Supplier] and the Purchaser pursuant to which BASF and/or [ADN Supplier] will supply ADN to the Divestment Business on the terms described in the Schedule.
8. In addition, the Divestment Business includes the benefit, for a transitional period after Closing and on terms and conditions equivalent to those at present afforded to the Divestment Business, of all current arrangements under which Solvay or its Affiliated Undertakings supply products or services to the Divestment Business, as detailed in the Schedule, unless otherwise agreed with the Purchaser. Strict firewall procedures will be adopted so as to ensure that any competitively sensitive information related to, or arising from such supply arrangements (for example, product roadmaps) will not be shared with, or passed on to, anyone outside the relevant Solvay business unit providing the relevant products/services.
Section C. Related commitments
Preservation of viability, marketability and competitiveness
9. From the Effective Date until Closing, the Notifying Party shall preserve or procure the preservation of the economic viability, marketability and competitiveness of the Divestment Business, in accordance with good business practice, and shall minimise as far as possible any risk of loss of competitive potential of the Divestment Business. In particular BASF undertakes:
(a) not to carry out any action that might have a significant adverse impact on the value, management or competitiveness of the Divestment Business or that might alter the nature and scope of activity, or the industrial or commercial strategy or the investment policy of the Divestment Business;
(b) to make available, or procure to make available, sufficient resources for the development of the Divestment Business, on the basis and continuation of the existing business plans;
(c) to take all reasonable steps, or procure that all reasonable steps are being taken, including appropriate incentive schemes (based on industry practice) to be agreed between BASF and Solvay, to encourage all Key Personnel to remain with the Divestment Business, and not to solicit or move any Personnel to BASF's remaining business. Where, nevertheless, individual members of the Key Personnel exceptionally leave the Divestment Business, BASF in consultation with Solvay shall provide a reasoned proposal to replace the person or persons concerned to the Commission and the Monitoring Trustee. BASF in consultation with Solvay must be able to demonstrate to the Commission that the replacement is well suited to carry out the functions exercised by those individual members of the Key Personnel. The replacement shall take place under the supervision of the Monitoring Trustee, who shall report to the Commission.
Hold-separate obligations
10. The Notifying Party commits, from the Effective Date until Closing, to procure that the Divestment Business is kept separate from the businesses that the Notifying Party will be retaining and, after closing of the notified Transaction to keep the Divestment Business separate from the business that the Notifying Party is retaining and to ensure that unless explicitly permitted under these Commitments: (i) management and staff of the business(es) retained by BASF have no involvement in the Divestment Business; (ii) the Key Personnel and Personnel of the Divestment Business have no involvement in any business retained by BASF and do not report to any individual outside the Divestment Business.
11. Until Closing, BASF shall assist the Monitoring Trustee in ensuring that the Divestment Business is managed as a distinct and saleable entity separate from the business(es) which BASF is acquiring. Immediately after the adoption of the Decision, BASF shall appoint a Hold Separate Manager in consultation with Solvay. The Hold Separate Manager, who shall be part of the Key Personnel, shall manage the Divestment Business independently and in the best interest of the business with a view to ensuring its continued economic viability, marketability and competitiveness and its independence from the businesses acquired by BASF. The Hold Separate Manager shall closely cooperate with and report to the Monitoring Trustee and, if applicable, the Divestiture Trustee. Any replacement of the Hold Separate Manager shall be subject to the procedure laid down in paragraph 8(c) of these Commitments. The Commission may, after having heard BASF, require BASF to replace the Hold Separate Manager.
Ring-fencing
12. BASF shall implement, or procure to implement, all necessary measures to ensure that it does not, after the Effective Date, obtain any Confidential Information relating to the Divestment Business and that any such Confidential Information obtained by BASF before the Effective Date will be eliminated and not be used by BASF. This includes measures vis-à-vis BASF’s appointees on the supervisory board and/or board of directors of the Divestment Business. In particular, the participation of the Divestment Business in any central information technology network shall be severed to the extent possible, without compromising the viability of the Divestment Business. BASF may obtain or keep information relating to the Divestment Business which is reasonably necessary for the divestiture of the Divestment Business or the disclosure of which to BASF is required by law.
Non-solicitation clause
13. The Parties undertake, subject to customary limitations, not to solicit, and to procure that Affiliated Undertakings do not solicit, the Key Personnel transferred with the Divestment Business for a period of 2 years after Closing.
Due diligence
14. In order to enable potential purchasers to carry out a reasonable due diligence of the Divestment Business, BASF shall, subject to customary confidentiality assurances and dependent on the stage of the divestiture process:
(a) provide or procure to Solvay to provide to potential purchasers sufficient information as regards the Divestment Business; and
(b) provide or procure to Solvay to provide to potential purchasers sufficient information relating to the Personnel and allow them reasonable access to the Personnel.
Reporting
15. BASF shall submit written reports in English on potential purchasers of the Divestment Business and developments in the negotiations with such potential purchasers to the Commission and the Monitoring Trustee no later than 10 days after the end of every month following the Effective Date (or otherwise at the Commission’s request). BASF shall submit a list of all potential purchasers having expressed interest in acquiring the Divestment Business to the Commission at each and every stage of the divestiture process, as well as a copy of all the offers made by potential purchasers within five days of their receipt.
16. BASF shall inform the Commission and the Monitoring Trustee on the preparation of the data room documentation and the due diligence procedure and shall submit a copy of any information memorandum to the Commission and the Monitoring Trustee before sending the memorandum out to potential purchasers.
Section D. The Purchaser
17. In order to be approved by the Commission, the Purchaser must fulfil the following criteria:
(a) The Purchaser shall be independent of and unconnected to the Notifying Party and its/their Affiliated Undertakings (this being assessed having regard to the situation following the divestiture);
(b) The Purchaser shall have the financial resources, proven expertise and incentive to maintain and develop the Divestment Business as a viable and active competitive force in competition with the Parties and other competitors;
(c) The Purchaser shall have an established presence in the production and global sale of chemical products. The Purchaser shall be in a position to effectively continue the Divestment Business as a viable and active competitor with BASF and other competitors. For example, the Purchaser shall be in a position to service global automotive customers.
(d) The acquisition of the Divestment Business by the Purchaser must neither be likely to create, in light of the information available to the Commission, prima facie competition concerns nor give rise to a risk that the implementation of the Commitments will be delayed. In particular, the Purchaser must reasonably be expected to obtain all necessary approvals from the relevant regulatory authorities for the acquisition of the Divestment Business.
18. The (final) binding agreements relating to the divestment of the Divestment Business (Put Option Agreement, as well as ancillary agreements including the agreement procuring the transfer of the Divestment Business upon exercise of the put option) shall be conditional on the Commission’s approval. When BASF has reached an agreement with a purchaser, it shall submit a fully documented and reasoned proposal, including a copy of the final agreement(s), within one week the Commission and the Monitoring Trustee. BASF must be able to demonstrate to the Commission that the purchaser fulfils the Purchaser Criteria and that the Divestment Business is being sold in a manner consistent with the Commission's Decision and the Commitments. For the approval, the Commission shall verify that the purchaser fulfils the Purchaser Criteria and that the Divestment Business is being sold in a manner consistent with the Commitments including their objective to bring about a lasting structural change in the market. The Commission may approve the sale of the Divestment Business without one or more Assets or parts of the Personnel, or by substituting one or more Assets or parts of the Personnel with one or more different assets or different personnel, if this does not affect the viability and competitiveness of the Divestment Business after the sale, taking account of the proposed purchaser.
Section E. Trustee
I. Appointment procedure
19. BASF shall appoint in consultation with Solvay a Monitoring Trustee to carry out the functions specified in these Commitments for a Monitoring Trustee. The Notifying Party commits not to close the Concentration before the appointment of a Monitoring Trustee.
20. The Monitoring Trustee shall:
(i) at the time of appointment, be independent of the Notifying Party and its Affiliated Undertakings;
(ii) possess the necessary qualifications to carry out its mandate, for example have sufficient relevant experience as an investment banker or consultant or auditor; and
(iii) neither have nor become exposed to a Conflict of Interest.
21. If BASF has not caused Solvay to enter into a binding put option agreement regarding the Divestment Business one month before the end of the First Divestiture Period or if the Commission has rejected a Purchaser proposed by BASF at that time or thereafter, BASF may appoint, at its sole discretion a Divestiture Trustee. The appointment of the Divestiture Trustee shall take effect upon the commencement of the Trustee Divestiture Period.
22. The Trustee shall be remunerated by the Notifying Party in a way that does not impede the independent and effective fulfilment of its mandate. In particular, where the remuneration package of a Divestiture Trustee includes a success premium linked to the final sale value of the Divestment Business, such success premium may only be earned if the divestiture takes place within the Trustee Divestiture Period.
Proposal by BASF
23. No later than two weeks after the Effective Date, BASF shall submit the name or names of one or more natural or legal persons whom BASF proposes to appoint as the Monitoring Trustee to the Commission for approval. The proposal shall contain sufficient information for the Commission to verify that the person or persons proposed as Trustee fulfil the requirements set out in paragraph 20 and shall include:
(a) the full terms of the proposed mandate, which shall include all provisions necessary to enable the Monitoring Trustee to fulfil its duties under these Commitments;
(b) the outline of a work plan which describes how the Monitoring Trustee intends to carry out its assigned tasks;
In case BASF decides to appoint a Divestiture Trustee, no later than one month before the end of the Divestiture Period or on request by or after a respective consultation with the Commission, BASF will submit a list of one or more persons whom BASF proposes to appoint as Divestiture Trustee to the Commission for approval. The appointment procedure described herein for the Monitoring Trustee shall be applicable mutatis mutandis to the appointment of the Divestiture Trustee, if applicable.
Approval or rejection by the Commission
24. The Commission shall have the discretion to approve or reject the proposed Monitoring Trustee and to approve the proposed mandate subject to any modifications it deems necessary for the Monitoring Trustee to fulfil its obligations. If only one name is approved, BASF shall appoint or cause to be appointed the person or persons concerned as Monitoring Trustee, in accordance with the mandate approved by the Commission. If more than one name is approved, BASF shall be free to choose the Trustee to be appointed from among the names approved. The Monitoring Trustee shall be appointed within one week of the Commission’s approval, in accordance with the mandate approved by the Commission.
New proposal by BASF
25. If all the proposed Monitoring Trustees are rejected, BASF shall submit the names of at least two more natural or legal persons within one week of being informed of the rejection, in accordance with paragraphs 19 and 24 of these Commitments.
Trustee nominated by the Commission
26. If all further proposed Trustees are rejected by the Commission, the Commission shall nominate a Monitoring Trustee, whom BASF shall or, in case of the nomination of a Divestiture Trustee may appoint, or cause to be appointed, in accordance with a trustee mandate approved by the Commission.
II. Functions of the Trustee
27. The Trustee shall assume its specified duties and obligations in order to ensure compliance with the Commitments. The Commission may, on its own initiative or at the request of the Trustee or BASF, give any orders or instructions to the Trustee in order to ensure compliance with the conditions and obligations attached to the Decision.
Duties and obligations of the Monitoring Trustee
28. The Monitoring Trustee shall:
(i) propose in its first report to the Commission a detailed work plan describing how it intends to monitor compliance with the obligations and conditions attached to the Decision.
(ii) oversee, in close co-operation with the Hold Separate Manager, the on-going management of the Divestment Business with a view to ensuring its continued economic viability, marketability and competitiveness and monitor compliance by BASF with the conditions and obligations attached to the Decision. To that end the Monitoring Trustee shall:
(a) monitor the preservation of the economic viability, marketability and competitiveness of the Divestment Business, and the keeping separate of the Divestment Business from the business retained by the Parties, in accordance with paragraphs 9 and 10 of these Commitments;
(b) supervise the management of the Divestment Business as a distinct and saleable entity, in accordance with paragraph 12 of these Commitments;
(c) with respect to Confidential Information:
- determine all necessary measures to ensure that BASF does not after the Effective Date obtain any Confidential Information relating to the Divestment Business,
- in particular strive for the severing of the Divestment Business’ participation in a central information technology network to the extent possible, without compromising the viability of the Divestment Business,
- make sure that any Confidential Information relating to the Divestment Business obtained by BASF before the Effective Date is eliminated and will not be used by BASF, and
- decide whether such information may be disclosed to or kept by BASF as the disclosure is reasonably necessary to allow BASF to carry out the divestiture or as the disclosure is required by law;
(d) monitor the splitting of assets and the allocation of Personnel between the Divestment Business and BASF or Affiliated Undertakings;
(iii) propose to BASF such measures as the Monitoring Trustee considers necessary to ensure BASF’s compliance with the conditions and obligations attached to the Decision, in particular the maintenance of the full economic viability, marketability or competitiveness of the Divestment Business, the holding separate of the Divestment Business and the non- disclosure of competitively sensitive information;
(iv) review and assess potential purchasers as well as the progress of the divestiture process and verify that, dependent on the stage of the divestiture process:
(a) potential purchasers receive sufficient and correct information relating to the Divestment Business and the Personnel in particular by reviewing, if available, the data room documentation, the information memorandum and the due diligence process, and
(b) potential purchasers are granted reasonable access to the Personnel;
(v) act as a contact point for any requests by third parties, in particular potential purchasers, in relation to the Commitments;
(vi) provide to the Commission, sending BASF a non-confidential copy at the same time, a written report within 15 days after the end of every month that shall cover the operation and management of the Divestment Business as well as the splitting of assets and the allocation of Personnel so that the Commission can assess whether the business is held in a manner consistent with the Commitments and the progress of the divestiture process as well as potential purchasers;
(vii) promptly report in writing to the Commission, sending BASF a non-confidential copy at the same time, if it concludes on reasonable grounds that BASF is failing to comply with these Commitments;
(viii) within one week after receipt of the documented proposal referred to in paragraph 18 of these Commitments, submit to the Commission, sending BASF a non-confidential copy at the same time, a reasoned opinion as to the suitability and independence of the proposed purchaser and the viability of the Divestment Business after the Sale and as to whether the Divestment Business is sold in a manner consistent with the conditions and obligations attached to the Decision, in particular, if relevant, whether the Sale of the Divestment Business without one or more Assets or not all of the Personnel affects the viability of the Divestment Business after the sale, taking account of the proposed purchaser;
(ix) assume the other functions assigned to the Monitoring Trustee under the conditions and obligations attached to the Decision.
29. In case of the appointment of a Divestiture Trustee and if the Monitoring and Divestiture Trustee are not the same [legal or natural] persons, the Monitoring Trustee and the Divestiture Trustee shall cooperate closely with each other during and for the purpose of the preparation of the Trustee Divestiture Period in order to facilitate each other's tasks
Duties and obligations of the Divestiture Trustee
30. Within the Trustee Divestiture Period, if appointed, the Divestiture Trustee shall sell at no minimum price the Divestment Business to a purchaser, provided that the Commission has approved both the purchaser and the final binding sale and purchase agreement (and ancillary agreements) as in line with the Commission's Decision and the Commitments in accordance with paragraphs 17 and 18 of these Commitments. The Divestiture Trustee shall include in the sale and purchase agreement (as well as in any ancillary agreements) such terms and conditions as it considers appropriate for an expedient sale in the Trustee Divestiture Period. In particular, the Divestiture Trustee may include in the sale and purchase agreement such customary representations and warranties and indemnities as are reasonably required to effect the sale. The Divestiture Trustee shall protect the legitimate financial interests of BASF, subject to the Notifying Party’s unconditional obligation to divest at no minimum price in the Trustee Divestiture Period.
31. In the Trustee Divestiture Period (or otherwise at the Commission’s request), the Divestiture Trustee shall provide the Commission with a comprehensive monthly report written in English on the progress of the divestiture process. Such reports shall be submitted within 15 days after the end of every month with a simultaneous copy to the Monitoring Trustee and a non-confidential copy to the Notifying Party.
III. Duties and obligations of the Parties
32. BASF shall provide and shall cause its advisors to provide the Trustee with all such co-operation, assistance and information as the Trustee may reasonably require to perform its tasks. The Trustee shall have full and complete access to any of BASF or the Divestment Business’ books, records, documents, management or other personnel, facilities, sites and technical information necessary for fulfilling its duties under the Commitments and BASF and the Divestment Business shall provide the Trustee upon request with copies of any document. BASF and the Divestment Business shall make available to the Trustee one or more offices on their premises and shall be available for meetings in order to provide the Trustee with all information necessary for the performance of its tasks.
33. BASF shall provide the Monitoring Trustee with all managerial and administrative support that it may reasonably request on behalf of the management of the Divestment Business. This shall include all administrative support functions relating to the Divestment Business which are currently carried out at headquarters level. BASF shall provide and shall cause its advisors to provide the Monitoring Trustee, on request, with the information submitted to potential purchasers, in particular give the Monitoring Trustee access to the data room documentation and all other information granted to potential purchasers in the due diligence procedure. BASF shall inform the Monitoring Trustee on possible purchasers, submit lists of potential purchasers at each stage of the selection process, including the offers made by potential purchasers at those stages, and keep the Monitoring Trustee informed of all developments in the divestiture process.
34. BASF shall grant or procure Affiliated Undertakings to grant comprehensive powers of attorney, duly executed, to the Divestiture Trustee to effect the sale (including ancillary agreements), the Closing and all actions and declarations which the Divestiture Trustee considers necessary or appropriate to achieve the sale and the Closing, including the appointment of advisors to assist with the sale process. Upon request of the Divestiture Trustee, BASF shall cause the documents required for effecting the sale and the Closing to be duly executed.
35. BASF shall indemnify the Trustee and its employees and agents (each an “Indemnified Party”) and hold each Indemnified Party harmless against, and hereby agrees that an Indemnified Party shall have no liability to BASF for, any liabilities arising out of the performance of the Trustee’s duties under the Commitments, except to the extent that such liabilities result from the wilful default, recklessness, gross negligence or bad faith of the Trustee, its employees, agents or advisors.
36. At the expense of BASF, the Trustee may appoint advisors (in particular for corporate finance or legal advice), subject to BASF’s approval (this approval not to be unreasonably withheld or delayed) if the Trustee considers the appointment of such advisors necessary or appropriate for the performance of its duties and obligations under the Mandate, provided that any fees and other expenses incurred by the Trustee are reasonable. Should BASF refuse to approve the advisors proposed by the Trustee the Commission may approve the appointment of such advisors instead, after having heard BASF. Only the Trustee shall be entitled to issue instructions to the advisors. Paragraph 30 of these Commitments shall apply mutatis mutandis. In the Trustee Divestiture Period, the Divestiture Trustee may use advisors who served BASF during the Divestiture Period if the Divestiture Trustee considers this in the best interest of an expedient sale.
37. BASF agrees that the Commission may share Confidential Information proprietary to BASF with the Monitoring Trustee. The Monitoring Trustee shall not disclose such information and the principles contained in Article 17 (1) and (2) of the Merger Regulation apply mutatis mutandis.
38. The Notifying Party agrees that the contact details of the Monitoring Trustee are published on the website of the Commission's Directorate-General for Competition and they shall inform interested third parties, in particular any potential purchasers, of the identity and the tasks of the Monitoring Trustee.
39. For a period of 10 years from the Effective Date the Commission may request all information from the Parties that is reasonably necessary to monitor the effective implementation of these Commitments.
IV. Replacement, discharge and reappointment of the Trustee
40. If the Trustee ceases to perform its functions under the Commitments or for any other good cause, including the exposure of the Trustee to a Conflict of Interest:
(a) the Commission may, after hearing the Trustee and BASF, require BASF to replace the Trustee; or
(b) BASF may, with the prior approval of the Commission, replace the Trustee.
41. If the Trustee is removed according to paragraph 40 of these Commitments, the Trustee may be required to continue in its function until a new Trustee is in place to whom the Trustee has effected a full hand over of all relevant information. The new Trustee shall be appointed in accordance with the procedure referred to in paragraphs 19-26 of these Commitments.
42. Unless removed according to paragraph 40 of these Commitments, the Trustee shall cease to act as Trustee only after the Commission has discharged it from its duties after all the Commitments with which the Trustee has been entrusted have been implemented. However, the Commission may at any time require the reappointment of the Monitoring Trustee if it subsequently appears that the relevant remedies might not have been fully and properly implemented.
Section F. The review clause
43. The Commission may extend the time periods foreseen in the Commitments in response to a request from BASF or, in appropriate cases, on its own initiative. Where BASF requests an extension of a time period, it shall submit a reasoned request to the Commission no later than one month before the expiry of that period, showing good cause. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time send a non-confidential copy of the report to the Notifying Party. Only in exceptional circumstances shall BASF be entitled to request an extension within the last month of any period.
44. The Commission may further, in response to a reasoned request from BASF showing good cause waive, modify or substitute, in exceptional circumstances, one or more of the undertakings in these Commitments. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time send a non-confidential copy of the report to the Notifying Party. The request shall not have the effect of suspending the application of the undertaking and, in particular, of suspending the expiry of any time period in which the undertaking has to be complied with.
Section G. Entry into force
45. The Commitments shall take effect upon the date of adoption of the Decision.
SCHEDULE
1. The Divestment Business as operated to date has the following legal and functional structure:
a) the Divestment Business is currently owned and operated by a number of Solvay entities and it is not incorporated separately. It belongs to and entails a large part of Solvay’s Performance Polyamides (PePol) Global Business Unit (GBU).
b) However, within Solvay’s PePol GBU, the Divestment Business is part of the same organizational cluster whose core centre lies in Belle-Etoile with complementary activities in terms of operations, common technology and intra-group supply and sourcing arrangements. For this reason, although the Divestment Business is not currently a stand-alone business activity, it already encompasses a large part of Solvay’s PePol GBU and will therefore be separated from current operations as described below.
c) The Divestment Business also contains the Chalampé JV Interest in the Chalampé JV. BASF will hold the 51% interest in the Chalampé JV (BASF and the Purchaser are referred to hereinafter as the Chalampé JV Shareholders).
aa. The Chalampé JV Interest will give the Purchaser the right to offtake 49% of the Upstream Products manufactured by the Chalampé JV. The fixed costs of the Chalampé JV will be split between the Chalampé JV Shareholders whereby […].
bb. In relation to all Upstream Products manufactured by the Chalampé JV, in case one JV Shareholder does not utilize its share of production capacity in a given month […], the other JV Shareholder has the right to utilize such capacity at full cost, […].
cc. [CONFIDENTIAL INFORMATION ON JV MANAGEMENT/GOVERNANCE].
dd. [CONFIDENTIAL INFORMATION ON JV INTERNAL SHAREHOLDER AGREEMENTS].
ee. [CONFIDENTIAL INFORMATION ON JV INTERNAL SHAREHOLDER AGREEMENTS].
ff. [CONFIDENTIAL INFORMATION ON JV MANAGEMENT/GOVERNANCE].
gg. [CONFIDENTIAL INFORMATION ON JV INTERNAL SHAREHOLDER AGREEMENTS].
hh. [CONFIDENTIAL INFORMATION ON JV INTERNAL SHAREHOLDER AGREEMENTS].
An organisational chart of the Divestment Business is attached as Annex 1.1-1.7.
2. In accordance with paragraph 6 of these Commitments, the Divestment Business includes, but is not limited to:
a) the following main tangible assets:
aa. (i) the Business’ facility located at Avenue Albert Ramboz, 69190 Saint-Fons, France owned by Rhodia Opérations (Belle-Etoile). All of Belle-Etoile will be included in the Divestment Business. However, for the sake of clarity, BASF may propose that a limited number of specific employees not necessary for the operation of the Divestment Business, having regards to its viability and competitiveness, may be transferred to BASF, unless the Purchaser objects with good cause. In this case, the Monitor Trustee, in consultation with the European Commission, shall provide a reasoned opinion as to whether the specific employees are needed for the operation of the Divestment Business. The reasoned opinion will be binding on BASF and the Purchaser.
(ii) the Business’ facility located at 25 Walczaka street, 66-407 Gorzow Wielkopoliski, Poland, owned by Rhodianyl SAS (Gorzow). All of Gorzow will be included in the Divestment Business;
(iii) the Business’ facility located at 74 Avenida de la Estación, 17300 Blanes, Girona, Spain owned by Solvay France S.A. (Blanes). All of Blanes will be included in the Divestment Business;
(iv) the Business’ facility located at 220 Avenue des Auréats, 26000 Valence, France owned by Rhodia Opérations (Valence). All of Valence will be included in the Divestment Business; and
(v) the Chalampé JV Interest.
The nameplate capacities of the relevant production sites are included in Annex 2.
bb. Belle-Etoile, Gorzow, Blanes and Valence are owned and operated by the Business and include the land on which these plants are located as well as all of the manufacturing equipment, buildings and non-manufacturing facilities installed at the above-mentioned sites which contribute to the current operation or are necessary to ensure the viability of the Divestment Business including:
(i) all non-manufacturing facilities and buildings owned by the said production units including office buildings, warehouse and storage units, (un)loading facilities, packaging assets, control rooms and equipment, as well as quality insurance, laboratory and testing equipment;
(ii) all tangible assets located at the R&D centres of Saint-Fons (RICL; focused on upstream P&I activities) and the Technyl Innovation Centre (focused on downstream EP activities), including testing and laboratory equipment, and computers, except in the event of duplicate equipment used equally by the Divestment Business and the Non-Divested Business, which can be adequately split if the Monitoring Trustee agrees that specific aforementioned duplicate equipment is not necessary to ensure the viability and competitiveness of the Divestment Business ; and
(iii) all raw materials, stock, semi-finished and finished goods including by- products held at the mentioned production sites at the time of the transfer to the Purchaser of the Divestment Business.
cc. The Chalampé JV Interest consists of 49% of the shares and the right for the Purchaser to offtake 49% of the products manufactured by the Chalampé JV. For the avoidance of doubt, the Purchaser will have the right to use 49% of the actual available capacity of the Chalampé JV at its own discretion, and to offtake at cost any additional production capacity not reserved by BASF. BASF will have the right to use 51% of the actual available capacity of the Chalampé JV at its own discretion, and to offtake at cost any additional production capacity not utilized by the Divestment Business. The Chalampé JV will consist of the entirety of the assets currently owned by the Business on the Chalampé site and includes the land on which these assets are located as well as all of the manufacturing equipment, buildings and non-manufacturing facilities installed at the above-mentioned sites which contribute to the current operation or are necessary to ensure the viability of the Divestment Business including;
(i) the Business’ production facilities for the production of AA, AH Salt, Nitric Acid and KA Oil;
(ii) all non-manufacturing facilities and buildings owned by the Business on the Chalampé site including office buildings, warehouse and storage units, (un)loading facilities, packaging assets, control rooms and equipment, as well as quality insurance, laboratory and testing equipment; and
(iii) all raw materials, stock, semi-finished and finished goods including by products relating to Upstream Products to be manufactured by the Chalampé JV owned by the Business on the Chalampé site at the time of the transfer to the Purchaser of the Divestment Business. The Chalampé JV shall use these raw materials, stock, semi-finished and finished goods including by products in accordance with the equity split of the JV Shareholders
b) the following main intangible assets which contribute to the current operation or which are necessary to ensure the viability and competitiveness of the Divestment Business, including the intangible assets with the exception of those intangible assets necessary for the viability of the Chalampé JV as such:
Patents
(i) BASF will transfer all the patents and licenses, including the rights thereto, owned by the Business that are or have been previously used solely or predominantly by the Divestment Business. The Purchaser will grant perpetual, irrevocable, non-exclusive, fully paid-up licences to BASF for the use of these patents, in so far they are needed to continue running the Non-Divested Business. The Purchaser will grant to BASF additionally a worldwide license for any background patents of the Business, that are required to implement an innovation generated by the Non-Divested Business. Such background patents license will be perpetual, irrevocable, non-exclusive and fully paid-up. For the avoidance of doubt, patents and licenses which are predominantly used by the Non-Divested Business will remain in the ownership of BASF and will be subject to the licensing arrangements as described under 2.(b)(ii) and (iii) below. A non-exhaustive list of the patents to be transferred is included as Annex 2.1 to the Commitments. The respective patent owner (BASF or Divestment Business) will have the right to decide on the maintenance of its patents at its own discretion. For the avoidance of doubt, if a patent is not maintained it will end; in which case any party will be able and allowed to make use of the corresponding (foreground or background) technology, without any limitations.
(ii) perpetual, irrevocable, non-exclusive, fully paid-up EEA-wide licences for the patents that the Divestment Business uses in connection with the manufacture of the Upstream Products, if these patents are not used solely or predominantly by the Divestment Business (and therefore covered by (i)) and in so far they are needed to continue running the Divestment Business.
· The Divestment Business will additionally include a worldwide license for any background patents of the Business that are required in connection with the manufacture of Upstream Products to implement an innovation generated by the Divestment Business. Such background patents license will be perpetual, irrevocable, non-exclusive, and fully paid-up.
· BASF will provide to the Chalampé JV an additional perpetual, irrevocable, non-exclusive, fully paid-up licence for the Upstream Products manufactured at the Chalampé JV; BASF will additionally provide to the Chalampé JV a license for any use of the background patents of the Business, that are required in connection with the manufacture of the Upstream Products manufactured at the Chalampé JV to implement an innovation generated by the Chalampé JV. Such background patents license will be perpetual, irrevocable, non-exclusive, and fully paid-up.
(iii) perpetual, irrevocable, non-exclusive, fully paid-up worldwide licences for the patents that the Divestment Business uses in connection with the manufacture of the Downstream Products, to the extent that they are not exclusively or predominantly used by the Divestment Business (and therefore covered by (i)). These licences will cover all patents and licences owned by the Business for the Downstream Products. BASF will grant to the Divestment Business additionally worldwide license for any background patents of the Business, that are required in connection with the manufacture of Downstream Products to implement an innovation generated by the Divestment Business. Such background patents license will be perpetual, irrevocable, non-exclusive, and fully paid-up.
Know-how
(iv) With respect to Upstream Products BASF will transfer to the Divestment Business all the know-how, trade secrets, other confidential information, and technical documentation for the production of the Upstream Products owned by the Business that are used or have been used previously solely or predominantly by the Divestment Business. The Purchaser will grant perpetual, irrevocable, non-exclusive, fully paid- up licences to BASF for the use of this know-how in so far it is needed to continue running the Non-Divested Business. The Purchaser will grant to BASF additionally a worldwide license for any background know-how of the Business that is required in connection with the manufacture of Upstream Products to implement an innovation generated by the Non-Divested Business. Such background know-how of the Business license will be perpetual, irrevocable, non-exclusive, and fully paid-up. For the avoidance of doubt, know-how, trade secrets, other confidential information, and technical documentation which are predominantly used by the Non-Divested Business will remain at BASF and will subject to the licensing arrangements as described under 2.(b)(v) below.
(v) perpetual, irrevocable, non-exclusive, fully paid-up EEA-wide licences for the know-how that the Divestment Business uses in connection with the manufacture of the Upstream Products, if this know-how is not used solely or predominantly by the Divestment Business (and therefore covered by (iv)) and in so far they are needed to continue running the Divestment Business.
· The Divestment Business will additionally include a worldwide license for any background know-how of the Business that is required in connection with the manufacture of Upstream Products to implement an innovation generated by the Divestment Business. Such background know-how of the Business license will be perpetual, irrevocable, non- exclusive and fully paid-up.
· BASF will provide to the Chalampé JV an additional perpetual, irrevocable, non-exclusive, fully paid-up licence for the Upstream Products manufactured at the Chalampé JV; BASF will additionally provide to the Chalampé JV a license for any use of the background know-how of the Business, that is required in connection with the manufacture of the Upstream Products manufactured at the Chalampé JV to implement an innovation generated by the Chalampé JV. Such background know-how license will be perpetual, irrevocable, non- exclusive, and fully paid-up.
(vi) With respect to the Downstream Products, at the option of the Purchaser, the Divestment Business and BASF will share co-ownership of all know-how, trade secrets, other confidential information and technical documentation for the worldwide production and sale of the Downstream Products. More particularly, this includes all recipe formulations of the Downstream Products and related know-how derived from practical experience and master batches formulations. In this case, points 2.(b)(vii) and 2.(b)(viii) below shall not apply.
(vii) Alternatively, with respect to the Downstream Products, the Divestment Business will include all the know-how, trade secrets, other confidential information, and technical documentation for the production of the Downstream Products owned by the Business that are used solely or predominantly by the Divestment Business. More particularly, this includes all recipe formulations of the Downstream Products and related know-how derived from practical experience and master batches formulations. The Purchaser will grant perpetual, irrevocable, non-exclusive, fully paid-up licences to BASF for the use of this know-how of the Business in so far they are needed to ensure that the Non-Divested Business can continue (i) offering the products that have been produced up to the Effective Date and (ii) R&D projects which are ongoing as of the Effective Date; and offering products resulting from such ongoing R&D projects to the relevant customers. The Purchaser will grant to BASF additionally a worldwide license for any background know-how of the Business that is required in connection with the manufacture of Downstream Products to implement an innovation generated by the Non-Divested Business. Such background know-how of the Business licences will be perpetual, irrevocable, non-exclusive, and fully paid-up. For the avoidance of doubt, know-how, trade secrets, other confidential information, and technical documentation which are predominantly used by the Non-Divested Business will remain at BASF and will subject to the licensing arrangements as described under 2.(b)(viii) below.
(viii) perpetual, irrevocable, non-exclusive, fully paid-up world-wide licences for the know-how that the Divestment Business uses in connection with the manufacture of the Downstream Products, if this know-how is not used solely or predominantly by the Divestment Business (and therefore covered by (vii)) and in so far they are needed to continue running the Divestment Business, as well as (i) offering the products that have been produced up to the Effective Date and (ii) R&D projects which are ongoing as of the Effective Date; and offering products resulting from such ongoing R&D projects to the relevant customers. BASF will grant to the Divestment Business additionally a worldwide license for any background know-how of the Business that is required in connection with the manufacture of Downstream Products to implement an innovation generated by the Divestment Business. Such licenses of background know- how of the Business will be perpetual, irrevocable, non-exclusive, and fully paid-up.
Predominant use of patents and know-how
(ix) For the avoidance of doubt, the determination of the predominant use of patents and know-how also considers current licensing activities by the Business or one of its Affiliated Undertakings to (i) the Butachimie JV, (ii) Solvay or one of its Affiliated Undertakings (excluding the Divestment Business), or (iii) to the Chalampé JV as use by the Non-Divested Business to be transferred to BASF to the same extent as self- used production capacities.
Trademarks
(x) the trademarks owned and used by the Business for the sale of relevant products at the date of the completion of the divestment including, in particular: (a) the Technyl brand, currently used by the Business for the marketing of Engineering Plastics; (b) the Stabamid brand, currently used by the Business for the marketing of Base Polymers and (c). the Sinterline brand, currently used by the Business for the marketing of PA Printing Powders. The Divestment will license the Technyl and the Stabamid trademarks for a transitional period of two years in order to allow BASF the rebranding of the Non-Divested Business the rebranding of the relevant products. Irrespective of the transfer of the trademarks BASF will still maintain the right to use the pre- and suffixes to the trademarks (commonly used as a general description to identify the product/grade).
c) all the permits and authorisations needed to operate the Divestment Business including local authority, environmental and health and safety permits. Permits and authorisations necessary for the operation of the Chalampé JV as such will not be transferred to the Purchaser, but to the Chalampé JV itself, which is not part of the Divestment Business;
d) all the contracts, agreements, leases, commitments and understandings, in particular and all key supply contracts held by Solvay in relation to the Divestment Business. The Divestment Business will include also an additional HMD supply agreement contract for minimum […] kt/y currently supplied by the Business in Chalampé to a […] until the end […] as described in Annex 3. The contracts, agreements, leases, commitments and understandings necessary for the operation of the Chalampé JV will be transferred to the Chalampé JV itself. A summary of the main customer contracts per plant is attached as Annex 3. A summary of the main supply contracts per plant is attached as Annex 4.
e) all current customer orders and records, as well as historic customer records relating to the Divestment Business (including certain customer contracts and records relevant to the viable operation of the Chalampé JV Interest) dating back to 2016;
f) all the personnel as specified in Annex All Personnel Divestment Business of the Business who directly supports the Divestment Business’ activities in the polyamide supply chain in the EEA including […]. All personnel based at Chalampé that is directly involved in the day-to-day operation of the Chalampé JV as specified in Annex All Personnel Chalampé JV will be transferred to the Chalampé JV.
g) the Key Personnel as specified in Annex 5.
h) The Divestment Business will include the following supply arrangements with respect to the supply of products or services by BASF or one of its Affiliated Undertakings to the Divestment Business:
(i) ADN Supply Agreements
The Divestment Business will include three ADN Supply Agreements.
ADN Supply Agreement 1
BASF commits to assign to the Purchaser of the Divestment Business the currently negotiated new [ADN SUPPLIER]-BASF contract for the supply of […] of ADN for […] which shall enter into effect on the later of […]. Annex 6 contains the proposed term sheet, setting out the main terms of ADN Supply Agreement 1. The price formula would be based on a provisional price ruled by a raw material based formula reflecting the respective ADN market price which will be subject to an […] price adjustment mechanism in order to determine the final ADN price. The price adjustment mechanism is based on the weighted (volume) average [ADN SUPPLIER] sales price over a period of one year as defined in Annex 6. After each price adjustment, the adjusted price will be taken as the basis for the new provisional ADN price for the following […].
ADN Supply Agreement 2
BASF commits to supply up to […] of ADN per year to the Purchaser which shall enter into effect on the later of […] for at least […], and, at the option of the Purchaser prior to signing of the Put Option Agreement, for up to an additional […] (leading to a maximum duration of […]). The price formula would be based on a provisional price ruled by a raw material based formula reflecting the ADN market price which will be subject to an […] price adjustment mechanism based on the weighted (volume) average [ADN SUPPLIER] HMD sales price over a period of […] as defined for ADN Supply Agreement 1. After each price adjustment, the adjusted price will be taken as the basis for the new provisional ADN price for the following […].
In addition, BASF commits to provide additional ADN volumes of […] per year to the Divestment Business, either by increasing the volumes to be supplied under ADN Supply Agreement 1 or ADN Supply Agreement 2. The Divestment Business (as BASF today) would have the opportunity to source any additional ADN from the market (including from the Merged Entity). Annex 6 contains the proposed term sheet, setting out the main terms of ADN Supply Agreement 2.
ADN Supply Agreement 3 (ADN Supply 2019)
Provided that Closing occurs in […], BASF will supply as of Closing on an interim basis (i.e. until ADN Supply Agreements 1 and 2 enter into effect) the relevant ADN volumes in […] (pro rata on a daily basis of […] per year) to the Divestment Business at […]. Annex 6 contains the proposed term sheet, setting out the main terms of ADN Supply Agreement 3.
(ii) Nickel Raney transitional supply agreement
The Divestment Business will include, at the option of the Purchaser, a transitional supply agreement pursuant to which BASF or one or more of its Affiliated Undertakings will offer for sale [90-100]% of the Purchaser’s requirement of Nickel Raney for the production of HMD by the Divestment Business during each calendar year for a period of […]. The requirement of the Purchaser is estimated to be up to […] per year of Nickel Raney. Annex 7 contains a proposed term sheet, setting out the main terms of the Nickel Raney transitional supply agreement.
(iii) PA6.6/6 co-polyamide BP transitional supply agreement
The Divestment Business will include, at the option of the Purchaser, a transitional supply agreement pursuant to which BASF or one or more of its Affiliated Undertakings will offer for sale up to […] per year of PA6.6/6 co-polyamide BP to the Purchaser for a period of […]. Annex 8 contains a proposed term sheet, setting out the main terms of the PA6.6/6 co-polyamide BP transitional supply agreement.
(iv) Transitional agreement for the provision of IT services
The Divestment Business will include, at the option of the Purchaser, a transitional agreement pursuant to which Solvay or one or more of its Affiliated Undertakings will provide IT services to the Purchaser, for a period of up to […](renewable for an additional period of maximum […], following a reasoned request from the Purchaser and a positive opinion of the Monitoring Trustee).
(v) Transitional agreement for the provision of finance services
The Divestment Business will include, at the option of the Purchaser, a transitional agreement pursuant to which Solvay or one or more of its Affiliated Undertakings will provide finance services to the Purchaser, for a period of up to […] (renewable for an additional period of maximum […], following a reasoned request from the Purchaser and a positive opinion of the Monitoring Trustee).
(vi) Other transitional service agreement between Solvay and the Purchaser
The Divestment Business will include, at the option of the Purchaser, further transitional agreements pursuant to which Solvay or one or more of its Affiliated Undertakings will provide other services to the Purchaser required for operation of the Divestment Business, to the Purchaser, for a period of up to […]((renewable for an additional period of maximum […], following a reasoned request from the Purchaser and a positive opinion of the Monitoring Trustee).
i) The Divestment Business will include the following supply arrangements with respect to the supply of products or services by the Divestment Business to BASF or one of its Affiliated Undertakings:
(i) Masterbatch Transitional Supply Agreement
The Divestment Business will include a transitional supply agreement pursuant to which the Divestment Business will offer for sale up to […] per year master batches to BASF or one or more of its Affiliated Undertakings for a period of […]. The Masterbatch Transitional Supply Agreement will only cover the master batches that are required to ensure that the Non-Divested Assets can continue manufacturing the products they produced before the Effective Date. It will contain the following ramp down schedule (i) years 1-2: [90-100]% of BASF´s need; (ii) year 3: [50-60]% of BASF´s need in year 2; and (iii) year 4: [20-30]% of BASF´s need in year 2. Annex 9 contains a proposed term sheet, setting out the main terms of the proposed Masterbatch Supply Agreement.
(ii) Specialty Base Polymer Transitional Supply Agreement
The Divestment Business will include a transitional supply agreement pursuant to which the Divestment Business will offer for sale specialty base polymers to BASF or one or more of its Affiliated Undertakings. The final terms of the Specialty Base Polymer Transitional Supply Agreement and in particular the fee, duration and volumes will be negotiated in good faith between BASF and the Purchaser prior to the signing of the Put Option Agreement. The Specialty Base Polymer Transitional Supply Agreement will only cover the specialty base polymers that are required to ensure that the Non-Divested Assets can continue manufacturing the products they produced before the Effective Date. BASF considers a volume of up to […] per year of speciality base polymers per year for a period of […] to be required to ensure that the Non-Divested Assets can continue manufacturing the products they produced before the Effective Date. Annex 10 contains a term sheet setting out the main terms to be initially proposed by BASF.
(iii) Technyl Grade (EP) Transitional Supply or Service Agreement
At the Option of the Purchaser the Divestment Business will either include a Technyl Grade Supply Agreement, or a Technyl Grade (EP) Service Agreement.
Under the Technyl Grade (EP) Supply Agreement, the Divestment Business would supply limited volumes of EP Products to BASF or one or more of its Affiliated Undertakings which are currently indirectly supplied by the Divestment Business through Non-Divested Assets to customers located outside the EEA. Annex 11 contains a proposed term sheet, setting out the main terms of the proposed Technyl Grade (EP) Supply Agreement.
Alternatively, under the Technyl Grade (EP) Transitional Service Agreement, for a period of up to […], BASF would provide the Purchaser with services relating to logistic and customer support for existing non-EEA customers of the Divestment Business which are currently indirectly supplied by the Divestment Business through Non-Divested Assets. In particular, BASF would offer to the Purchaser all suppor needed to supply customers located outside the EEA that are currently indirectly supplied by the Divestment Business through Non-Divested Assets.
(iv) PA6.6 Base Polymer Tolling Agreement
The Divestment Business will include a PA6.6 base polymer agreement pursuant to which the Divestment Business will supply a specific quantity of PA6.6 BP to BASF who will provide the relevant amounts of raw materials HMD and AA. The final terms of the Tolling Agreement and in particular the fee, duration and volume will be negotiated in good faith between BASF and the Purchaser prior to the signing of the Put Option Agreement. BASF requires these quantities of PA6.6 base polymer to ensure the continued supply of the Non-Divested Assets. BASF considers a volume of […] of PA6.6 base polymer per year for a period of […] to be required to ensure the continued supply of the Non-Divested Assets. Annex 12 contains a proposed term sheet setting out the main terms to be initially proposed by BASF.
(v) PA6.6 Base Polymer Supply Agreement
The Divestment Business will include a PA6.6 base polymer supply agreement pursuant to which the Divestment Business will offer for sale […] per year of PA6.6 base polymer to BASF or one or more of its Affiliated Undertakings for a period of […].
3. The Divestment Business shall not include the Non-Divested Assets and the Non- Divested Business
4. If there is any asset or personnel which is not be covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset (or a substitute thereto) will be offered to potential purchasers.
Annex 1.1
[CONFIDENTIAL]
Annex 1.2
[CONFIDENTIAL]
Annex 1.3
[CONFIDENTIAL]
Annex 1.4
[CONFIDENTIAL]
Annex 1.5
[CONFIDENTIAL]
Annex 1.6
[CONFIDENTIAL]
Annex 1.7
[CONFIDENTIAL]
Annex 2
[CONFIDENTIAL]
Annex 2.1
[CONFIDENTIAL]
Annex 3
[CONFIDENTIAL]
Annex 4
[CONFIDENTIAL]
Annex 5
[CONFIDENTIAL]
Annex 6
[CONFIDENTIAL]
Annex 7
[CONFIDENTIAL]
Annex 8
[CONFIDENTIAL]
Annex 9
[CONFIDENTIAL]
Annex 10
[CONFIDENTIAL]
Annex 11
[CONFIDENTIAL]
Annex 12
[CONFIDENTIAL]
1 OJ L 24, 29.1.2004, p. 1 ("the Merger Regulation"). With effect from 1 December 2009, the Treaty on the Functioning of the European Union ("TFEU") introduced certain changes, such as the replacement of "Community" by "Union" and "common market" by "internal market". The terminology of the TFEU is used throughout this Decision.
2 OJ C …,… 200., p....
3 OJ C …,… 200., p....
4 OJ C 185, 30.05.2018, p. 4.
5 Also referred to in the industry as “AA”.
6 At the Advisory Committee all present Member States agreed that that the Transaction must be declared compatible with the internal market and the EEA Agreement in accordance with Article 2(2) and 8(2) of the Merger Regulation and Article 57 of the EEA Agreement.
7 Nylon is a generic designation for a family of synthetic polymers, based on aliphatic or semi-aromatic polyamides. Within this Decision, nylon will be used interchangeably with polyamides, or PA.
8 Commission Decision in case M.8605, INEOS/Solvay/JV.
9 Commission Decision in case M.6905, INEOS /Solvay / JV.
10 Commission Decision in case M.4730, Yara/Kemira Growhow.
11 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to questions 1 and 11
12 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 29
13 Replies to RFI #10, doc. ID 1865.
14 Non confidential reply to the request for information of 13 August 2018 addressed to […], doc. ID 2122.
15 Doc. ID 1884.
16 OJ 97/C 372/03.
17 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 15.
18 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 30
19 Non-confidential minutes of the call with […], 9 February 2018, 14:30h, doc. ID 399.
20 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to questions 30,31and 32
21 Non-confidential reply to the request for information of 13 August 2018 addressed to […], doc. ID
2122.
22 Non-confidential reply to the request for information of 13 August 2018 addressed to […], doc. ID 2122.
23 Commission Decision in case. M.7784 – CF Industries Holldings/OCI Business.
24 Form CO, Paragraph D.13, p. D4.
25 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 57
26 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 45.
27 Commission Decision in case M.6900 – Boeralis/Rosier/GPN.
28 The only exception among EEA adipic acid producers active in the EEA is BASF, as it needs a pure cyclohexanol for its adipic production process.
29 Commission Decision in Case M.6230 – Solvay/Rhodia, paras. 52 et seq.
30 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 110.
31 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 94.
32 Commission Decision in Case M.6230 – Solvay/Rhodia, paras. 57 et seq.
33 Form CO, paragraph F.49.
34 Form CO, paragraphs F.49 and F.153.
35 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to questions 100 and 113.
36 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to questions 100 and 111.
37 Non-confidential minutes of the call with […], 09 February 2018, 14.30h, doc. ID 399.
38 Non-confidential minutes of the call with […], 12 February 2018, 14.00h, doc. ID 1416.
39 Ibid
40 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 99, doc. ID 1189.
41 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 111.
42 Non-confidential minutes of the call with […], 09 February 2018, 14.30h, doc. ID 399.
43 Non-confidential minutes of the call with […], 12 February 2018 16.30h, doc. ID 327.
44 Non-confidential minutes of the call with […], 05 July 2018, 10.30h, doc. ID 274.
45 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 112.
46 Non-confidential minutes of the call […], 09 February 2018, 14.30h, doc. ID 399.
47 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 112.
48 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to questions 99 and 100.
49 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 99, doc. ID 915.
50 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 100, doc. ID 1189.
51 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 112, doc. ID 1014.
52 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 113, doc. ID 1014.
53 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 100, doc. ID 915.
54 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to
question 99, doc. ID 1189.
55 Response to 6(1)(c) Decision, paragraph 114.
56 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 100.
57 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 113.
58 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 113, doc. ID 1014.
59 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 113, doc. ID 657.
60 Commission Decision in Case M.6230 – Solvay/Rhodia, paras. 52 et seq.
61 Response to the 6(1)(c) Decision, para. 157.
62 Non-confidential minutes of the call with […], 5 July 2018, 14.00h, doc. ID 1599.
63 Non-confidential minutes of the call with […], 5 July 2018, 11.30h, doc. ID 1970.
64 Non-confidential minutes of the call with […], 5 July 2018, 10.30h, doc. ID 1602; non-confidential minutes of the call with […], 4 July 2018, 14.00h, doc. ID 1590.
65 Non-confidential minutes of the call with […], 19 February 2018 12.00h, doc. ID 330.
66 Commission Decision in Case M.6230 – Solvay/Rhodia, paras. 57 et seq.
67 Response to the Article 6(1)(c) Decision, section C.I.2.b.
68 Commission Notice on the definition of relevant market for the purposes of Community competition law, [1997] O.J. C 372/03, para. 28.
69 Form CO (22/05/2018), C.AA.4, AA – EEA Sales 2016, page G6. Import data have been retrieved from the EU Commission Market Access Database available at http://madb.europa.eu/madb/statistical form.htm (code 291712 -- adipic acid, its salts and esters). The Commission notes that import volumes recorded in the Market Access Database differ markedly from the 2017 data provided by the Notifying Party in response to the Commission's request for information RFI 10, question 28. However, the Notifying Party's import data are also difficult to reconcile with the 2017 market share data supplied by the Notifying Party in response to the same request for information RFI 10, question 2. In any event, even the 2017 import data submitted by the Notifying Party in reply to question 28 of RFI 10 would be limited to less than [10-20]% of total EEA demand.
70 The Notifying Party estimates total captive and merchant sales of adipic acid in 2017 to amount to […] kts (see BASF's response to the Commission's request for information RFI 10, question 29).
71 See, e.g., […]'s response to the Commission request for information on adipic acid, question 3, b), doc. ID 2173; non-confidential minutes of the call with […], 26 July 2018, 15.00h, doc. ID 2135.
72 See BASF internal document, doc. ID 1938-33514.
73 Plant closures in Ukraine have also been reported sporadically during the in-depth investigation. See, e.g., non-confidential minutes of the call with […], 26 July 2018, 14.00h, doc. ID 1967.
74 This was confirmed by respondents to the in-depth market investigation. See, e.g., non-confidential minutes of the call with […], 4 July 2018, 14.00h, doc. ID 1590.
75 According to 2017 data submitted by the Notifying Party in response to the Commission request for information Q.10, question 2, the combined market share of the Parties dropped to [60-70]% of total demand (merchant + captive) and [40-50]% of total merchant sales.
76 Commission Notice on the definition of relevant market for the purposes of Community competition law, [1997] O.J. C 372/03, paras. 29-30.
77 Article 6(1)(c) Decision, para. 66.
78 In that regard, the 2017 PCI Yellowbook, which is the most important reference in the industry, indicates that: “Although Europe already imports some adipic acid from China, these volumes are limited and only used as incremental supply/pricing leverage. Transitioning large amounts of European adipic demand to Chinese materials will present logistical, quality, legal and financial hurdles which are not trivial” (2017 PCI Yellowbook, pp. 17-18).
79 This is notably the case for […] (see […] response to the Commission request for information of 8 August 2018, doc. ID 2010).
80 Non-confidential minutes of the call with […], 07 February 2018, 15.30h, doc. ID 236; […] response to the Commission request for information of 8 August 2018, doc. ID 2010; […] response to the
Commission request for information on adipic acid, doc. ID 2036.
81 […] response to the Commission request for information on adipic acid, doc. ID 2031. See also […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), response to question 145, doc. ID 1189.
82 […] response to the Commission request for information on adipic acid, doc. ID 2036.
83 […] response to the Commission request for information on adipic acid, doc. ID 2042.
84 […] response to the Commission request for information on adipic acid, doc. ID 2258.
85 See, e.g., non-confidential minutes of the call with […], 26 July 2018, doc. ID 1967.
86 Non-confidential minutes of the call with […], 26 July 2018, doc. ID 2135.
87 Idem.
88 […] response to the Commission request for information on adipic acid, doc. ID 2173.
89 See, e.g., non-confidential minutes of the call with […], 19 February 2018 12.00h, doc. ID 330; […] response to the Commission request for information on adipic acid, doc. ID 2173.
90 Non-confidential minutes of the call with […], 07 February 2018, 15.30h, doc. ID 236; Non- confidential minutes of the call with […], 26 July 2018, doc. ID 2135; Non-confidential minutes of the call with […], 26 July 2018, doc. ID 1967; […] response to the Commission request for information of 8 August 2018, doc. ID 2010. See also […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), response to question 144, doc. ID 962; […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), response to question 131, doc. ID 915; […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), response to question 144, doc. ID 653; […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 144, doc. ID 1183.
91 […] mentioned that some improvements could be done by adding "anti-caking" agents to the material but that these solutions would need to be tested and qualified, which would in any event take time ([…] response to the Commission request for information on adipic acid, doc. ID 2166).
92 Reponse to the Article 6(1)(c) Decision, para. 170.
93 […] estimates the costs of its "de-caking" service to 5-7% of the purchase price, whereas […] refers to an "around 10%" estimate. See Non-confidential minutes of the call with […], 26 July 2018, doc. ID
1967; […] response to the Commission request for information of 8 August 2018, doc. ID 2010.
94 See responses to the Commission request for information on adipic acid.
95 […] response to the Commission request for information on adipic acid, doc. ID 2013.
96 See, e.g., […] response to the Commission request for information on adipic acid, doc. ID 2042; See also […] reply to Questionnaire Q3 to competitors/customers of the nylon business - adipic acid, responses to question 6, doc. ID 996; […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 144, doc. ID 1189.
97 See responses to the Commission request for information on adipic acid. See also non-confidential minutes of the call with […], 21 February 2018, 14.00h, doc. ID 335.
98 […] response to the Commission request for information of 8 August 2018, doc. ID 2010. See also […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 143, doc. ID 1189.
99 […] response to the Commission request for information on adipic acid, doc. ID 2013.
100 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 145, doc. ID 653. See also […] response to the Commission request for information on adipic acid, doc. ID 2272.
101 […] response to the Commission’s request for information on adipic acid, doc. ID 2173 (pointing also to the fact that large contracts in Europe are based on raw-material formulas, using European raw material prices, whereas Chinese producers use only formulas based on Asian feedstocks or product prices). See also Non-confidential minutes of the call with […], 19 February 2018, 12.00h, doc. ID 330. See also […] response to the Commission request for information on adipic acid, doc. ID 2272.
102 Non-confidential minutes of the call with […], 19 February 2018, 12.00h, doc. ID 330.
103 Non-confidential minutes of the call with […], 26 July 2018, doc. ID 2135.
104 Non-confidential minutes of the call with […], 05 July 2018, doc. ID 1602.
105 […] response to the Commission request for information on adipic acid, doc. ID 2031.
106 […] response to the Commission request for information on adipic acid, doc. ID 2111.
107 […] response to the Commission request for information on adipic acid, doc. ID 2168.
108 Non-confidential minutes of the call with […], 26 July 2018, doc. ID 1967.
109 Idem.
110 […] response to the Commission request for information on adipic acid, doc. ID 2154.
111 See e.g., non-confidential minutes of the call with […], 26 July 2018, doc. ID 1967; non-confidential minutes of the call with […], 26 July 2018, doc. ID 2135; non-confidential minutes of the call with […], 4 July 2018, 14.00h, doc. ID 1590; Non-confidential minutes of the call with […], 07 February 2018, 15.30h, doc. ID 236.
112 Non-confidential minutes of the call with […], 26 July 2018, doc. ID 2135.
113 Non-confidential minutes of the call with […], 5 July 2018, 14.00h, doc. ID 1599.
114 Non-confidential minutes of the call with […], 5 July 2018, 10.30h, doc. ID 1602.
115 […] response to the Commission request for information of 8 August 2018, doc. ID 2010. While being generally more positive about the availability of Chinese imports, […] notes soberly that "China Adipic acid price delivered into Europe market can, at times, be competitive with local supply" ([…] response to the Commission request for information on adipic acid, doc. ID 2013).
116 Non-confidential minutes of the call with […], 26 July 2018, doc. ID 2135.
117 Response to the Article 6(1)(c) Decision, para. 158-159.
118 Response to the Article 6(1)(c) Decision, Technical Annex on AA, para. 1.
119 In its reply to RFI 10, the Notifying Party presented an updated version of this analysis over the period January 2015 – May 2018, which did not change the overall conclusion.
120 Notifying Party's Reply to Q42 of RFI 10, para. 55.
121 In its reply to RFI 10, the Notifying Party presented an updated version of this analysis over the period January 2011 – May 2018, which did not change the overall conclusion.
122 Notifying Party's Reply to Q42 of RFI 10, para. 55.
123 Response to the Article 6(1)(c) Decision, Technical Annex on AA, para. 13.
124 […] response to the Commission request for information of 8 August 2018, doc. ID 2010.
125 Case M. 7061 – Huntsman Corporation / Equity interests held by Rockwood Holdings.
126 Notifying Party's Reply to RFI 10, Q34 and Q40. In particular, as explained by the Notifying Party, in the reply to Q40, it is "reasonable to focus on the period starting in 2015 to obtain economically meaningful results".
127 The Commission has run statistical stationarity tests on (i) the difference in levels of the EEA price and the price of Chinese imports in the EEA and (ii) the log of the ratio between these prices. The Augmented Dickey Fuller (ADF) test fails to reject the null hypothesis of non-stationarity once a modest number of lags is included while the Kwiatkowski–Phillips–Schmidt–Shin (KPSS) test rejects the null hypothesis of stationarity in most specifications, and regardless of whether the full data period or the period after 2015 is considered.
128 […] response to the Commission request for information of 8 August 2018, doc. ID 2010
129 Idem.
130 For a reference, see, e.g., non-confidential minutes of the call with […], 4 July 2018, 14.00h, doc. ID 1590.
131 See responses to the Commission request for information on adipic acid.
132 Idem.
133 […] response to the Commission request for information of 19 July 2018, question 12, doc ID 1648
134 Non-confidential minutes of the call with […], 26 July 2018, doc. ID 1967; non-confidential minutes of the call with […], 5 July 2018, doc. ID 1599.
135 Non-confidential minutes of the call with […], 5 July 2018, doc. ID 1602; Non-confidential minutes of the call with […], 5 July 2018, doc. ID 1599.
136 Reponse to request for information RFI 10, question 31.
137 Non-confidential minutes of the call with […], 5 July 2018, doc. ID 1602.
138 Non-confidential minutes of the call with […], 26 July 2018, doc. ID 2135.
139 […] response to the Commission request for information on adipic acid, doc. ID 2173.
140 Non-confidential minutes of the call with […], 4 July 2018, doc. ID 1590.
141 […] response to the Commission request for information on adipic acid, doc. ID 2042.
142 Non-confidential minutes of the call with […], 4 July 2018, doc. ID 1590.
143 Non-confidential minutes of the call with […], 26 July 2018, doc. ID 2135; non-confidential minutes of the call with […], 26 July 2018, doc. ID 1967; […] response to the Commission request for information of 19 July 2018, question 20, doc ID 1648.
144 Non-confidential minutes of the call with […], 07 February 2018 15.30h, doc. ID 236; non-confidential minutes of the call with […], 12 February 2018 16.30h, doc. ID 327.
145 Non-confidential minutes of the call with […], 26 July 2018, doc. ID 2135; non-confidential minutes of the call with […], 26 July 2018, doc. ID 1967.
146 Commission Decision in M.7614 CVC Capital Partners / Royal DSM (Fibre Intermediates And Composite Resins), paras. 19-22.
147 Commission Decision in M.7614 CVC Capital Partners / Royal DSM (Fibre Intermediates And Composite Resins), para. 71.
148 Commission Decision in M.7614 CVC Capital Partners / Royal DSM (Fibre Intermediates And Composite Resins), paras. 23-25.
149 Commission Decision in M.7614 CVC Capital Partners / Royal DSM (Fibre Intermediates And Composite Resins), para. 71.
150 Commission Decision in Case M.6230 – Solvay/Rhodia, para. 54 and Commission Decision in Case – M.214 Du Pont/ICI, para. 7.
151 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 165, doc. ID 915.
152 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 181, doc. ID 987.
153 Non-confidential minutes of the call with […], 22 February 2018, 14.00h, doc. ID 274.
154 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 184, doc. ID 860.
155 Ibid
156 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 184.
157 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 184, doc. ID 860.
158 Non-confidential minutes of the call with […], 12 February 2018, 15.30h, doc. ID 285.
159 Form CO, paragraphs I.62 and I.63.
160 Non-confidential minutes of the call with […], 22 February 2018, 14.00h, doc. ID 274.
161 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 184, doc. ID 860.
162 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 165, doc. ID 915.
163 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 181, doc. ID 987.
164 Non-confidential minutes of the call with […], 13 February 2018, 17.00h, doc. ID 228.
165 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 170, doc. ID 1183.
166 […] reply to Questionnaire 1 to competitors/customers nylon business (PA), responses to question 183, doc. ID 860.
167 […] reply to Questionnaire 1 to competitors/customers nylon business (PA), responses to question 183, doc. ID 984.
168 Form CO, paragraphs I.65 and I.66.
169 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 169.
170 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 182.
171 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 185 and 186.
172 Commission Decision in Case M.6230 – Solvay/Rhodia, para. 99 et seq.
173 Form CO, paragraph J.20.
174 Form CO, paragraph J.30.
175 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 221.
176 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 224.
177 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 221. […] notes for instance that “PA6 is complementary to PA66. But the products are not interchangeable. The performance characteristics of PA66 (temperature resistance, mechanical strength, moisture absorption, dimensional stability, etc.) can’t be achieved with PA6. Components made from the two products will have different functional performance” ([…], doc. ID 1189); […] states that “The key performance characteristic of PA6.6 over PA6 is heat resistance, PA6.6 can withstand much higher temperatures which give it specific applications where high temperatures are prevalent. In addition, PA6.6 has water absorption qualities which make it particularly effective in electrical applications” (doc. ID 1170); […], another customer of PA 6.6 BP points out the “Well-Known performance difference btw PA6.6 base Polymer and PA 6 Base Polymer, especially in Automotive Industry” (doc. ID 1119); another ([…]) confirms that “PA66 has higher thermo resistence and this is why impossible to use PA6 for airbag applications as well as some tyres and MRG applications. With PA6 is impossible to achieve as high tenacities as with PA66 polymer” (doc. ID 937); […] states that “Our Company purchase both PA6 and PA6.6, there polyamides are totally different so the 6.6 cannot replace the 6 and vice versa” (doc. ID 606). Note that all quotes of respondents to the market investigation are quoted in this Decision as textually given by them.
178 See Notification, _Confidential_Annex.MS#7, Q3.xlsx.
179 Questionnaire 1 to competitors/customers of the nylon business (PA), responses to question 224.
180 Non-confidential minutes of the call with […], 06 February 2018, 10.15h, doc. ID 215: "PA66 has always been more expensive than PA6 (And way more expensive than Polyester). Where PA66 has not been substituted already by import, or by other fibers, there are always very good technical reasons which prevented and which will prevent that from happening. […] PA6, as said above, where possible, has taken away market from PA 66, because is in general cheaper than PA66. However, most of the possible substitution took place already in the industry. It will be very hard to substitute the current remaining use of PA 66". Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 224.1. […] confirms that “All possible substitute has happened, the Incumbent demand for PA6.6 is must to have, rare chance to be further substituted by PA6 base polymers. Linked with the performance difference” (doc. ID 1119).
181 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to questions 224.1, 225 and 226.
182 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 200.
183 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 201. One
PA 6 BP producer, […], mentions for instance that “A new PA66 plant would have to be engineered and built, which would take several years” (doc. ID 984).
184 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 200. One PA 6 BP producer ([…]) mentions that “We could produce PA66 if we have access to sufficient quantity of AH salt” (doc. ID 984); another PA 6 BP producer ([…]) states that “By investing in captive AH Salt and PA66 Polymerization a company would just move its dependency from 3 or 4 supplier of PA66 polymer to an even lesser number of suppliers of ADN/HMDA.”(doc. ID 883); another PA 6 BP producer ([…]) states that “Producing PA66 base polymer would require investment and access to ADN, which will not be available after the acquisition, and HMD” (doc. ID 987).
185 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 200.1. […], which is a PA 6.6 BP producer stated that “[…] only produces Nylon 6,6 base polymer. Nylon 6 is a different product with different capabilities that is not an option given [the company]'s focus based on Nylon 6,6” (doc. ID 1183).
186 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to questions 201 and 219.
187 Commission Decision in Case M.6230 – Solvay/Rhodia, para. 51.
188 Form CO, paragraph J.40.
189 Response to the Article 6(1)(c) Decision, para. 332.
190 Response to the 6(1)(c) Decision, para. 333.
191 Response to the 6(1)(c) Decision, paras. 334-335.
192 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 228.
193 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 227.
194 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 229 and
229.1. One customer of PA 6.6 BP, […], mentions “The REACH regulations in Europe create a barrier to source PA6.6 from outside the EU. There can also be tariff barriers depending on the producing country and transportation costs can impact relative competitiveness.” (doc. ID 1170); another one, […], confirms that “REACH regulations limit the number of suppliers active within the EU” (doc. ID 1134); […] also states that “Transportation cost and Import Tariffs, makes the product more expensive. The long delivery periods, makes it difficult to adapt to the fast changing market.” (doc. ID 834); […] mentions “[…] customer requirements for PA66 of European origin […]” and in its reply to Questionnaire Q1, response to question 210.1, it also states: “Others: higher market prices in Asia and US compared to EU. Regulatory barriers: market requirement for polymer of European origin.” (doc. ID 987). Also see: "[…] has thought about procuring PA 6.6 BP from the United States but it is difficult because of the REACH legislation. This certification need especially applies to the automotive sector where this qualification may, in many cases, be a barrier of entry in the market" (doc. ID 2280).
195 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 227.1, doc. ID 972.
196 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 227.1, doc. ID 1119.
197 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 227.1, doc. ID 937.
198 […] response to the Commission’s request for information of 1 August 2018, question 25, doc. ID 1884.
199 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 210.1, doc. ID 987.
200 M.8674_BASF_SOLVAY_Confidential_Annex.2017 - RFI#10 Q2.XLSX.
201 Doc. ID 1964-9.
202 See for instance http://www rubbernews.com/article/20180717/NEWS/180719958/nylon-66-shortage- looking-worse-ascend-declares-force-majeure-following-plant-fire
203 As cited […] in their spontaneous submission dated 12 June 2018, doc. ID 2116, p. 5.
204 […] response to the Commission’s request for information of RFI 2, question 25 and 26, doc. ID 1884.
205 Non-confidential minutes of the call with […], 27 June 2018, doc. ID 2108, para. 12.
206 […] response to the Commission’s request for information of 19 July 2018, question 27, doc. ID 1648.
207 BASF’s response to the Commission’s request for information RFI 10, question 55.
208 […] response to the Commission’s request for information of 6 August 2018, doc. ID 2117, question 7.
209 Doc. ID 1902-35781, slide 183.
210 Doc. ID 1902-136.
211 Form CO, paragraph J.128.
212 […]
213 […]
214 […]
215 Form CO, paragraph J.66.
216 Questionnaire Q1 to competitors/customers of the nylon business (PA) responses to questions 261 to 266 and 296 to 301.
217 Form CO, paragraphs J.44, J.58 and J.68.
218 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 245.1 and 280.1.
219 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 245. […], a producer of co-polyamide 6/6.6 states for instance that to switch production towards other products "that would need a different and new plant", and confirms that "Investment cost, access to secure and competitive source of AH salt, and technology are the barriers to build a PA 66 or COPA 66/6 plant" and adds that "AH salt availability is limited, and therefore switch is limited". ([…] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to questions 245.1,
245.2 and 245.4, doc. ID 984). […], another producer flags the "Investment costs and time needed"
(doc. ID 987).
220 Commission Decision in Case M.6230 – Solvay/Rhodia, para. 51.
221 Form CO, paragraph J.67.
222 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 268 and 284.
223 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 246 and 281.
224 Commission Decision in Case M.4737 – Sabic/GE Plastics.
225 Commission Decision in Case M.4737 – Sabic/GE Plastics, para. 11 to 17.
226 Commission Decision in Case IV.427 – Rhône-Poulenc-Caffaro.
227 Commission Decision in Case IV.427 – Rhône-Poulenc-Caffaro para. 21.
228 Commission Decision in Case IV.427 – Rhône-Poulenc-Caffaro para. 22.
229 Form CO, paragraph K.12.
230 Form CO, paragraphs K.16 et seq.
231 Form CO, paragraphs K.37 et seq.
232 Form CO, paragraphs. K.41 et seq.
233 Form CO, paragraphs K.63 et seq.
234 Form CO, paragraphs K.100 et seq.
235 Questionnaire Q2 to customers of PA Engineering Plastics, responses to question 48.
236 Questionnaire Q2 to customers of PA Engineering Plastics, responses to questions 49 and 50.
237 Questionnaire Q2 to customers of PA Engineering Plastics, responses to questions 48.1 and 48.2. […], a customer of PA 6.6 EP active in the automotive industry notes that P6 EP and PA 6.6 EP “[…] have different physical properties (mechanical, electrical, thermal,..), so there are applications that must be used just PA6 or PA6.6 depending on the part design and its application” (doc. ID 1008); […], another customer active in the same sector, notes that “the higher temperatures coming from downsizing trends in automotive sector requires temperature resisting materials like PA 66” (doc. ID 877); […] notes that PA 6 EP is not a valid alternative to the company as “Material cannot be used […] because material must be resistant to hydrolysis. Furthermore, water absorption of PA6 is too high.” (doc. ID 1259); while […] states that; “PA 6.6 EP could be replaced by PA 6 only for limited [company] components and would be not convenient in terms of investments and timing” (doc. ID 909).
238 Questionnaire Q2 to customers of PA Engineering Plastics, responses to question 48.2. […] notes that “In some cases PA 6.6 EP can be replaced by PA 6 EP, but significant R&D efforts are needed to test and qualify PA 6 EP where PA 6.6 EP is currently used for the production of the relevant product. Also, where a high melting point is required, PA 6 EP is not suitable as engineering plastic” (doc. ID 1058). Customers estimates for the costs and timing involved in switching from PA 6.6 to PA 6 vary: “Minimum: 18 months including Laboratory approval, Product and Process validation, and Customer approval Cost between 200 K€ and 500 K€” ([…] reply to Questionnaire Q2 to customers of PA Engineering Plastics, responses to question 48.2, doc. ID 990); “New materials lead to the fact that all the testing on all parts need to be done again. This is an automotive basic standard. This could lead to high costs depending on the product. On average project testing costs are approx. EUR 100.000 – lead time for tests are in average 10 to 30 weeks. Moreover, the change will lead to tool modifications (costs).”; “12 month @ 100.000 € for each application” ([…] reply to Questionnaire Q2 to customers of PA Engineering Plastics, responses to question 48.2, doc. ID 877); “From a minimum of 6 months to more than 1 or 2 years, depending on the complexity of components, not including timing and costs for requalification, testing and final customers validations that aren’t predictable by […]” ([…] reply to Questionnaire Q2 to customers of PA Engineering Plastics, responses to question 48.2, doc. ID 909).
239 Questionnaire Q2 to customers of PA Engineering Plastics, responses to question 51.
240 Questionnaire Q2 to customers of PA Engineering Plastics, responses to questions 64 and 64.1. […], a purchaser of PA 6.6 EP, mentions “Our customers are car makers, they want that our suppliers are
selected from their lists of approved suppliers” (doc. ID 854); […] states that “Some Customers have a specific material list per application and even specific needs requiring additional tests” (doc. ID 990); while […] mentions that “Some of our OEM Customer require that the Material (PA6.6) is listed and approved in their database In order to achieve the listing it requires time and resources” (doc. ID 947); and finally […] states the practice is standard in the automotive sector: “Compound name is shown in PPAP documents and therefore visible to OEMs. Hence, change of material must be indicated and approved by customer – automotive standard procedure”. (doc. ID 1259).
241 Form CO, Annex Notification, _Confidential_Annex.MS#7, Q3.xlsx.
242 Questionnaire Q2 to customers of PA Engineering Plastics, responses to questions 54 and 54.1
243 Questionnaire Q2 to customers of PA Engineering Plastics, responses to questions 53 and 54.
244 Non-confidential minutes of the call with […], 06 February 2018, 16.00h, doc. ID 296.
245 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 355.3. […] notesfor instance that “The equipment (Compounding Extruders) required to make Engineering Polymer compounds based on PA66, PA6 or PA66/6 are very similar or can be adapted” (doc. ID 1189).
246 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 355.4. […] notes for instance that a “Switch is only possible in case of available polymer for compounding, which requires availability of ADN/AH salt (for PA66 and copolymers).” (doc. ID 987); […] notes that “[…] Of course there could be problems with the raw materials to produce PA 66 (mainly ADN and therefore HMD) […]”(doc. ID 915); […] confirms that “Switching to a higher usage of PA6.6 would be an issue due to the tightness in that market. This tightness is driven by availability of ADN” (doc. ID 1170).
247 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to 357 to 359 and Questionnaire Q2 to customers of PA Engineering Plastics, responses to questions 12-15.
248 Questionnaire Q2 to customers of PA Engineering Plastics, responses to questions 12- to 15.
249 See Case No COMP/M.4737 Sabic/GE Plastics, para. 22.
250 See Case No COMP/IV.427 Rhône-Poulenc/Caffaro para. 24.
251 Form CO, para. K.119.
252 Response to the 6(1)(c) Decision, para. 372.
253 See Case No COMP/M.6230 Solvay/Rhodia.
254 Response to the 6(1)(c) Decision, paras. 373 to 375.
255 Questionnaire Q2 to customers of PA Engineering Plastics, responses to questions 26 and 26.1. […] notes that “For our plants located in the EEA we only purchase PA 6.6. EP form suppliers located within the EEA” (doc. ID 1125); […] confirms that “[they] do sourcing only in the region for the region” (doc. ID 947). Note that some respondents who replied positively to Question 26 indicated in the following question that they in fact did not purchase any PA 6.6 EP from outside the EEA for their EEA plant, including […] who stated "For our plants located in the EEA we only purchase PA 6.6. EP form suppliers located within the EEA” (doc. ID 1125), […], who purchases "[…] PA66 compounds from Ascend, where the base resin is solely produced in the US, but the compounding process is done in the EU" (doc. ID 848).
256 Questionnaire Q2 to customers of PA Engineering Plastics, responses to question 26.1.
257 Questionnaire Q2 to customers of PA Engineering Plastics, responses to question 26.1. ([…], doc. ID 844).
258 Questionnaire Q2 to customers of PA Engineering Plastics, responses to 29, 29.1, and 30. Most respondents identify transport costs as the main barrier. […] states that “Strong OEM demand variation (every week) doesn’t allow long distances due to long lead-time in sea transportation (between 4 to 8 weeks) and cost of Air ship in case of too high demand from OEMs (versus supplies lead-time)” (doc. ID 990); […] confirms that “[d]istance could cause additional cost and supply risk. This has to be taken under consideration for a “total cost” comparison and risk management” (doc. ID 1067); […] emphasizes that costs increase with distance, stating that “[t]o move material from A to B there is a cost. This cost increases the longer the distance. At the same time the co-orditation in supply chain increases with distance and quantities. Shipping goods from China to Europe take longer then sourcing in Europe so your lead-time increases. Tariffs and duties only increase the cost effect of supply chain” (doc. ID 947); […] notes the existence of “[u]ncertainty of potential import duties due to increasing protectionism, higher inventory and lead time because of sea freight. Increasing transport costs” (doc. ID 1259); […] states that “[l]ocalization is a preferred condition due to supply efficiencies (transportation costs, supply security, delivery timing, etc.)” and that “[t]ransportation costs and Import/Exports duties will highly impact the costs of the materials” (doc. ID 909); […] identifies as barriers to trade globally “long delivery time, exchange rate risk” (doc. ID 844).
259 Response to the 6(1)(c) Decision, para. 375.
260 Questionnaire Q2 to customers of PA Engineering Plastics, responses to question 46.
261 […] reply to Q1 to competitors/customers of the nylon business (PA), doc. ID 987, response to question 223.
262 […] reply to Q1 to competitors/customers of the nylon business (PA), doc. ID 915, response to question 356.4.
263 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 364. Some customers however mention that regulatory issues exist which limit the transportability of PA 6.6 EP. See Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question
365.1 […] indicates as a barrier “REACH for importing PA66 EP into EEA” (doc. ID 912); […] notes that “There are local regulatory requirements which can act as a barrier to move product from region to region i.e. REACH” (doc. ID 1170); […] considers that “Many producers outside the EU do not register their products under REACH Legislation, ruling themselves out of the European market" (doc. ID 1134).
264 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 363.
265 […] reply to Q1 to competitors/customers of the nylon business (PA), doc. ID 1020, response to question 363.1: "All PA6.6 from Ascend (US) that we bought and distributed".
266 […] reply to Q1 to competitors/customers of the nylon business (PA), response to question 363.1. Imports by […] "Only started 2018 due to unavailability of PA66 polymer in Europe", doc. ID 987.
267 See https://www.ascendmaterials.com/news/ascend-performance-materials-acquires-britannia-techno- polymer: "This acquisition provides us with a sixth manufacturing location and a dedicated footprint for serving our European customers with world-class nylon 6,6 compounds and regional manufacturing expertise".
268 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 365.1 […] notes that “There are duties in China” (doc. ID 1020); […] also notes that “as this is cost competitive market, taxes and import duties can make the product less economically attractive and seen as a kind of 'barrier'”(doc. ID 1119); […] mentions that “Some countries, such as China, impose punitive anti-dumping duties" (doc. ID 1134); […] notes that "Tariffs can make importing or, exporting uncompetitive" (doc. ID 1170).
269 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 365.1 […] states that, by importing “Lead times can be longer and therefore from a security of supply
perspective sourcing outside of the region may not be advisable"” (doc. ID 1170).
270 See Case No COMP/M.6230 Solvay/Rhodia para. 100.
271 See Case No COMP/M.6230 Solvay/Rhodia paras. 49 to 51.
272 COMP/IV/M.1083 – Rhône-Poulenc/Novalis/Nyltech, 15 April 1998; COMP/IV/M.206 – Rhône- Poulenc/SNIA, 10 August 1992.
273 COMP/IV/M.206 – Rhône-Poulenc/SNIA, 10 August 1992.
274 Form CO, para. L.24.
275 Form CO, para. L.25.
276 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 376.1.
277 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to questions 376.1 and 376.2. […] notes for instance that "PA 6.6 and PA 6 industrial yarn have limited interchangeability due to different characteristics of the fibers” and that “Yarn types are specific to applications due to
their intrinsic properties. I.e. PA 6.6 yarns are used for airbags due to the higher heat resistance compared to PA 6 yarns” (doc. ID 860); […] mentions that “[…] assets are designed for Nylon 6,6 and it is not feasible to convert them to Nylon 6 production assets” (doc. ID 1183).
278 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 377.1.
279 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to questions 377.2 to
377.4. […] notes that switching “would require completely different equipment with significant investments. It is like starting a new business” (doc. ID 860); […] confirms it is “impossible to produce other type of performance fibres because of different type of machines” and that switching would require “high investment and specific know-how” (doc. ID 937); […] also states that “Any Change could mean a high investment of time and money” (doc. ID 834).
280 […], […] and […] replies to Questionnaire Q1 to competitors/customers of the nylon business (PA), doc. ID 1183, 915 and 860 respectively, responses to question 377.1.
281 Questionnaire Q5 to customers of PA 6.6 Performance Fibres, responses to question 11.
282 […] (doc. ID 2335) and […] (doc. ID 2224), responses to PF 6.6 questions, response to question 3.
283 […] (doc. ID 2245), […] (doc. ID 3051), […] (doc. ID 2221), […] (doc. ID 2236) and […] (doc. ID 3056) responses to PF 6.6 questions, response to question 3. For instance, […] notes that "the PA 66 fiber that we buy can be used in several sectors, for example Fiber PA 66 3.3 50 can be used for spinning and also for non-woven" (doc. ID 3051). On the other hand, […] states that "The fibres that we use, are specific for the carpet industry because of the dtex and the texturation of the fibres" (ID 2221), and […] that "Polyamide tow is mainly used for producing flock, flock is like a textile powder used in some fields: automotive, packaging, textile, etc. There are very few Polyamide tow suppliers" (doc. ID 3056).
284 COMP/IV/M.1083 – Rhône-Poulenc/Novalis/Nyltech, 15 April 1998; COMP/IV/M.206 – Rhône- Poulenc/SNIA, 10 August 1992.
285 Form CO, para. L.29.
286 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 3. Questionnaire Q5 to customers of PA 6.6 Performance Fibres, responses to 20.
287 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 389.
288 Questionnaire Q5 to customers of PA 6.6 Performance Fibres, responses to question 20.
289 […] (doc. ID 2250), […] (doc. ID 2245), […] (doc. ID 3051), […] (doc. ID 2221), […] (doc. ID 2224) […] (doc. ID 2196) and […] (doc. ID 2236) responses to PF 6.6 questions, response to question 4.
290 Questionnaire Q5 to customers of PA 6.6 Performance Fibres, responses to question 26. Also see […] (doc. ID 2250), […] (doc. ID 2245), […] (doc. ID 3051), […] (doc. ID 2221), […] (doc. ID 2224), […] (doc. ID 2196) and […] (doc. ID 2236) responses to PF 6.6 questions, response to questions 4 and 5. For instance, […] mentioned needing a local supplier because of the "Logistics in terms of full containers from the Far East & the lead times involved. Our business is very irregular & impossible to forecast so require a “local” supplier" (doc. ID 2250); […] states that "In the textile world of today it is difficult to import from very far because the customers with the fashions need everything for tomorrow, of quality and cheap and according to my opinion if I have to bring PA 66 from outside the European Union the textile business is complicated" (doc. ID 3051); […] notes "[they] only use Material with European Origin, due to tax reasons" (doc. ID 2224).
291 Minutes of a conference call with […], doc. ID 3112
292 For example, […] mentioned that other types of 3D print powders offer inferior thermal stability
293 Minutes of a conference call with […], doc. ID 2337.
294 Ibid
295 Doc. ID 1113.
296 Questionnaire Q9 HDI Derivatives, responses to question 5.
297 Jotun Paints reply to Questionnaire Q9 HDI Derivatives, responses to question 10, doc. ID 2063.
298 Questionnaire Q9 HDI Derivatives, responses to question 6.
299 […] reply to Questionnaire Q9 HDI Derivatives, responses to question 6, doc. ID 1551.
300 […] reply to Questionnaire Q9 HDI Derivatives, responses to question 6, doc. ID 1614.
301 […] reply to Questionnaire Q9 HDI Derivatives, responses to question 6, doc. ID 1939.
302 Questionnaire Q9 HDI Derivatives, responses to question 8.
303 […] reply to Questionnaire Q9 HDI Derivatives, responses to question 8, doc. ID 1848.
304 […] reply to Questionnaire Q9 HDI Derivatives, responses to question 7, doc. ID 2023.
305 […] Paints reply to Questionnaire Q9 HDI Derivatives, responses to question 8, doc. ID 2063
306 […] reply to Questionnaire Q9 HDI Derivatives, responses to question 8, doc. ID 1851.
307 […] reply to Questionnaire Q9 HDI Derivatives, responses to question 6, doc. ID 1848.
308 […] reply to Questionnaire Q9 HDI Derivatives, responses to question 9, doc. ID 1857.
309 […] reply to Questionnaire Q9 HDI Derivatives, responses to question 7, doc. ID 1848.
310 […] reply to Questionnaire Q9 HDI Derivatives, responses to question 11, doc. ID 1551.
311 […] reply to Questionnaire Q9 HDI Derivatives, responses to question 9, doc. ID 2063.
312 Questionnaire Q9 HDI Derivatives, responses to question 9.
313 […] reply to Questionnaire Q9 HDI Derivatives, responses to question 7, doc. ID 2063.
314 Commission, Decision of 04/05/2016, Case No. M.7864, Trelleborg/CGS Holding; para. 52; Commission, Decision of 14/05/2012, Case No. COMP/M.6339, Freudenberg & Co/Trelleborg/JV, para. 21.
315 See COMP/ M. 8261, Lanxess/ Chemtura, para 26.
316 Reply to rfi#2, question 36, para 155.
317 Reply to rfi#2, question 26, para 156.
318 See COMP/ M. 8261, Lanxess/ Chemtura, para 32; Case COMP/ M. 7393, Albemarle/ Rockwood, para 73.
319 Reply to rfi#2, question 36, para 159.
320 See COMP/ M.5355 BASF/ CIBA, para 237.
321 COMP/M.5355, BASF/CIBA, para 241.
322 See Reply to RFI#2, Q36, para. 165.
323 Commission, Decision of 15/06/2005, Case No. COMP/M.3805, Crompton/Great Lakes, para. 36.
324 Commission, Decision of 12/03/2009, Case No. COMP/M.5355, BASF/CIBA, para. 243; See Reply to RFI#2, Q36, para. 166.
325 See Reply to RFI#2, Q36, para. 167.
326 See COMP/M.6093, BASF/INEOS/Styrene/JV, para. 9 and reply to RFI#2, Q36, para. 169.
327 Commission, Decision of 15/06/2005, Case No. COMP/M.3805, Crompton/Great Lakes, para. 10.
328 See Reply to RFI#2, Q36, para. 173.
329 Commission, Decision of 15/06/2005, Case No. COMP/M.3805, Crompton/Great Lakes, paras. 31-35.
330 See Reply to RFI#2, Q36, para. 177.
331 Commission, decision of 10/02/2003, Case No. COMP/M.3064, Ahlström Capital/Capman/Nordkalk, para. 18.
332 Commission, Decision of 26/04/1999, Case No. IV/M.1381, IMETAL/English China Clays, para. 48.
333 Commission, Decision of 26/04/1999, Case No. IV/M.1381, IMETAL/English China Clays, para. 49.
334 Commission, Decision of 10/02/2003, Case No. COMP/M.3064, Ahlström Capital/Capman/Nordkalk, para. 29; See Reply to RFI#2, Q36, para. 181.
335 Commission, Decision of 12/03/2009, Case No. COMP/M.5355, BASF/CIBA, para. 395
336 Commission, Decision of 12/03/2009, Case No. COMP/M.5355, BASF/CIBA, para. 397; See Reply to RFI#2, Q36, para. 141.
337 Commission, Decision of 12/03/2009, Case No. COMP/M.5355, BASF/CIBA, para. 399.
338 See Reply to RFI#2, Q36, para. 145.
339 OJ C3See Reply to RFI#2, Q36, para. 145.1, 5.2.2004, p.5.
340 OJ L24, 29.1.2004, p.1
341 Non-horizontal Merger Guidelines, paragraph 35.
342 Non-horizontal Merger Guidelines, paragraph 32.
343 Non-horizontal Merger Guidelines, paragraphs 92-93.
344 M.6471 – Outokumpu / Inoxum, recitals 282 and following. In that case, which assessed a merger in the flat stainless steel industry, the Commission considered merchant market sales as well as capacity and production shares at the EEA level at each level of the value chain.
345 See Inderst, R., and Valletti, T., “Indirect versus Direct Constraints in Markets with Vertical Integration,” The Scandinavian Journal of Economics, 2009, 111:527-546.
346 Response to the 6(1)(c) Decision, paragraphs 126-134.
347 See, for instance, the Response to the 6(1)(c) Decision, paragraphs 60, 146, 248, 343.
348 Response to 6(1)(c) Decision, paragraph 133. Similarly, the Notifying Party claimed, in the Response to the 6(1)(c) Decision, paragraph 248, in relation to adipic acid, that “volumes captively used by the Parties are not available to the merchant market and should therefore not be taken into account for the purpose of assessing the Merged Entity to have an impact on such merchant market.” They also stated, in the Response to the 6(1)(c) Decision, paragraph 343, in relation to PA 6.6 BP, that “it is appropriate to only look at and carry out the competitive assessment on the basis of the merchant sales market shares only.”
349 See, for instance, the Response to the 6(1)(c) Decision, paragraphs 60, 146, 248.
350 Response to the 6(1)(c) Decision, paragraph 146. Similarly, the Notifying Party submitted, at paragraph 248, in relation to foreclosure concerns for adipic acid, that other market share measures than those relying on merchant sales “are not a relevant tool to measure the Merged Entity market power and ability to foreclose customers’ access to AA on the merchant market.”.
351 Non-confidential minutes of the call with […], 13 February 2018, 17.00h, doc. ID 228.
352 The additional share of calculated on the total nameplate production capacity of Butachimie would actually amount to [10-20]%. However, the Notifying Party indicated in the Form CO that the overall equipment efficiency in the industry varies between [80-90]% and [90-100]%. Assuming an efficiency rate of [80-90]%, the increase in the production share generated by the contract with Invista will be of […]%, as indicated in the text.
353 Figures refer to year 2016. Volumes sold by BASF are just resell on the market of volumes it purchased by Invista. Hence, in the competitive assessment Invista is considered as having performed those sales.
354 Briefing Document submitted by […] in response to: COMP/M.8674 - BASF / SOLVAY'S EP AND P&I, doc. ID 2269.
BUSINESS - Q1 - Competitors/Customers Nylon Business (PA), pages 8 to 10. It has to be noted that this customer is not taking into account the long term contract entered into by and between BASF and Invista for the supply of […] kts of ADN a year for the next […] years.
355 Annex I to Questionnaire Q1 to competitors/customers of the nylon business (PA), submitted by […] doc. ID 1395.
356 Non-confidential minutes of the call with […], 21 February 2018, h 14:00, doc. ID 335.
357 […].
358 […].
359 Doc. ID 001938-044517.
360 Doc. ID 001938-045780.
361 Doc. ID 001938-047930.
362 […]
363 […]
364 […]
365 […]
366 […]
367 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 34.1.
368 Form CO, C.26.
369 […]
370 Non-confidential minutes of the call with […], 11 June 2018, h 17:30, doc. ID 1442.
371 PCI Yellow book 2017, page 17.
372 Form CO, C59.
373 Ibid
374 […] in response to: COMP/M.8674 - BASF / SOLVAY'S EP AND P&I BUSINESS - Q1 - Competitors/Customers Nylon Business (PA), pages 8 to 10, doc ID 2269.
375 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 34.1.
376 Non-confidential minutes of the call with […], 9 February 2018, h 14:30, doc. ID 399
377 Form CO, para C.92
378 […]
379 […].
380 Pc, or unit price, is the part of the ADN price formula which is determined by the commercial negotiation between the parties to the contract, and is not linked to any kind of index.
381 Non confidential reply to the request for information of 13 August 2018 addressed to […], doc. ID 2122.
382 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 56.2.
383 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 62.
384 As explained in the methodological introduction, the Commission has considered in its assessment ofthe case 3 metrics: merchant sales, capacity, and merchant sales plus captive use. The Transaction leads to affected markets taking into consideration capacity or merchant sales plus captive use in the EEA. On the contrary, the Transaction does not lead to affected markets if merchant sales are considered.
385 Nevertheless, the Commitments include the divestment of […] kts of KA Oil Capacity equivalent to [40-50]% of the capacity of the Business or [5-10]% of the capacity in the EEA,
386 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 93.
387 [20-30]% in value for merchant sales in 2014, [20-30]% in value for merchant sales in 2015 and [20- 30]% in value for merchant sales in 2016, according to the data provided by the Notifying Party.
388 [0-5]% in value for merchant sales in 2014, [0-5]% in value for merchant sales in 2015 and [0-5]% in value for merchant sales in 2016 according to the data provided by the Notifying Party.
389 M.6471 – Outokumpu/Inoxum, para 282ff. In that case, which assessed a merger in the flat stainless steel industry, the Commission considered merchant market sales as well as capacity and production shares at the EEA level at each level of the value chain.
390 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 103.
391 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 117.
392 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 107.
393 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 121.
394 Non-confidential minutes of the call with […], 21 February 2018, 14.00h, doc. ID 335.
395 Non-confidential minutes of the call with […], 29 November 2017, 09.30h, doc. ID 64.
396 Non-confidential response of […] to RFI to […] dated 20 July 2018, doc. ID 2050.
397 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 122.
398 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 102.
399 Non-confidential response of […] to RFI 2 to […] dated 1 August 2018, doc. ID 1884.
400 Non-confidential minutes of the call with […], 23 January 2018, 15.00h, doc. ID 245.
401 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 102, doc. ID 1219.
402 Non-confidential minutes of the call with […], 21 February 2018, 14.00h, doc. ID 335.
403 Non-confidential minutes of the call with […], 9 February 2018, 14.30h, doc. ID 399.
404 Non-confidential minutes of the call with […] 12 February 2018, 16.30h, doc. ID 327.
405 Non-confidential minutes of the call with […], 09 February 2018, 14.30h, doc. ID 399.
406 Non-confidential minutes of the call with […] 29 November 2017, 09.30h, doc. ID 64.
407 Non-confidential minutes of the call with […], 05 July 2018, 10.30h, doc. ID 1602.
408 […]
409 […]
410 […]
411 […]
412 […]
413 […]
414 […]
415 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 116.
416 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 126.
417 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 126, doc. ID 915.
418 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 126, doc. ID 937.
419 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 104.
420 Q1 to competitors/customers of the nylon business (PA), responses to question 118.
421 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), response to question 118, doc. ID 1014.
422 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to questions 105 and 119.
423 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 125, doc. ID 1014.
424 […]
425 […]
426 The Business also operates a plant with […] kts in adipic acid generation capacity based in Onsan, South Korea.
427 Horizontal Merger Guidelines, para. 17.
428 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 159.
429 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to questions 153 and 163; non-confidential version of the call with […], 5 July 2018, 14.00h, doc. ID 1599; […] response to the Commission adipic acid request for information, doc. ID 2042.
430 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 163, doc. ID 1020.
431 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to
question 163, doc. ID 1176.
432 Non-confidential minutes of the call with […], 12 February 2018 16.30h, doc. ID 327.
433 Non-confidential minutes of the call with […], 21 February 2018 14.00h, doc. ID 335.
434 Non-confidential minutes of the call with […], 9 February 2018 14.30h, doc. ID 399.
435 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 135 and Questionnaire Q3 to competitors/customers of the nylon business (PA) - adipic acid, response to question 16.
436 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 154.
437 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 157, doc. ID 1189.
438 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 153, doc. ID 1189.
439 2017 PCI Yellowbook, pp. 17-18.
440 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 158.
441 M.8674_BASF_SOLVAY_13 - 6.02.2017 - Expertise II.PDF, pp 17.
442 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 134.
443 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 134, doc. ID 915.
444 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to
question 134, doc. ID 1219.
445 Non-confidential minutes of the call with […], 7 February 2018 15.30h, doc. ID 236.
446 M.8674_BASF_SOLVAY_13 - 6.02.2017 - Expertise II.PDF, pp 17.
447 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 162.
448 Non-confidential minutes of the call with […], 21 February 2018 14.00h, doc. ID 335.
449 Non-confidential version of minutes of a call with […], 26 July 2018, 15.00h, doc. ID 2135.
450 Questionnaire Q3 to competitors/customers of the nylon business (PA) - adipic acid, responses to question 17 and Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 136.
451 […] reply to Questionnaire Q3 to competitors/customers of the nylon business (PA) - adipic acid, response to question 17, doc. ID 1002.
452 Questionnaire Q3 to competitors/customers of the nylon business (PA) - adipic acid, responses to question 17 and Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 136.
453 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 155, doc. ID 921.
454 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 155.
455 Questionnaire Q3 to competitors/customers of the nylon business (PA) - adipic acid, responses to question 18; and Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 137.
456 Questionnaire Q3 to competitors/customers of the nylon business (PA) - adipic acid, responses to question 18; and Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 137.
457 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 156; non- confidential version of minutes of a call with […], 19 February 2018 12.00h, doc. ID 330; non- confidential version of minutes of a call with […], 26 July 2018, 15.00h, doc. ID 2135; non-confidential version of minutes of a call with […], 26 July 2018, 10.00h, doc. ID 1967; […] response to the Commission request for information on adipic acid, doc. ID 2111; […] response to the Commission request for information on adipic acid, doc. ID 2272; […] response to the Commission request for information on adipic acid, doc. ID 2036; […] response to the Commission request for information on adipic acid, doc. ID 2166.
458 2017 PCI Yellowbook, pp. 17-18.
459 Taking into consideration that in the EEA only BASF produces dry AH Salt and with the sole purpose of exporting some volumes to Asia and that customers in the EEA do not purchase dry AH Salt, the Commission takes the preliminary view that the competitive assessment in Section 6.3.7.2 would apply to a potential market for liquid AH Salt, as market shares and all other considerations would remain the same.
460 See reply to RFI#3, Q89, para. 215.
461 See reply to RFI#3, Q89, para. 215.
462 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to questions 185 and 186.
463 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 188.
464 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 174.
465 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 178.
466 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 192.
467 The only exception is […] that has indicated that it sources 2% of its needs from […]. The volumes sourced by […] from […] represents a tiny fraction of the European market (i.e. between 0.1% and 0.5%), doc. ID 984.
468 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to questions 185 and 186.
469 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 167, doc. ID 915.
470 With the exception of a de minimis sale to […].
471 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to questions 185 and 186 and […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 167, doc. ID 915.
472 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to questions 179 and 193.
473 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 187.
474 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 187, doc. ID 987.
475 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 187, doc. ID 860.
476 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 187, doc. ID 984.
477 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 187.
478 Non-confidential minutes of the call with […], 08 February 2018, 09.30h, doc. ID 190.
479 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 197.
480 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 197, doc. ID 860.
481 Non-confidential minutes of the call with […], 22 February 2018, 14.00h, doc. ID 274.
482 Non-confidential minutes of the call with […], 13 February 2018, 17.00h, doc. ID 228.
483 Non-confidential minutes of the call with […], 5 July 2018, 10.30h, doc. ID 1602.
484 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 175.
485 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 189.
486 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 189, doc. ID 860.
487 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to questions 176 and 190.
488 See Section 6.3.4.2(B.vii).
489 Non-confidential minutes of the call with […], 8 February 2018, 9.30h, doc. ID 190.
490 Non-confidential minutes of the call with […], 13 February 2018, 17.00h, doc. ID 228.
491 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 173.
492 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to
question 173 and Non-confidential response of […] to RFI to […] dated 13 August 2018, q11, doc. ID 915.
493 Non-confidential response of […] to RFI to […] dated 13 August 2018, q12, doc. ID 915.
494 Non-confidential response of […] to RFI to […] dated 13 August 2018, q13, doc. ID 915.
495 Non-confidential response of […] to RFI to […] dated 13 August 2018, q14, doc. ID 915.
496 Non-confidential minutes of the call with […], 23 January 2018, 15.00h, doc. ID 245.
497 Non-confidential response of […] to RFI2 to […] dated 1 August 2018, q19, doc. ID 1884.
498 Non-confidential response of […] to RFI2 to […] dated 1 August 2018, q20, doc. ID 1884.
499 Non-confidential response of […] to RFI to […] dated 20 July 2018, q12, doc. ID 2010.
500 Non-confidential response of […] to RFI to […] dated 20 July 2018, q13, doc. ID 2010.
501 Non-confidential response of […] to RFI to […] dated 20 July 2018, q13, doc. ID 2010.
502 Non-confidential minutes of the call with […], 13 February 2018, 17.00h, doc. ID 228.
503 Non-confidential minutes of the call with […], 12 February 2018, 15.30h, doc. ID 285.
504 Non-confidential minutes of the call with […], 13 February 2018, 17.00h, doc. ID 228.
505 Response to the 6(1)(c) Decision, paras. 342 to 345.
506 Form CO, para. J.144 et seq. Also see Response to the 6(1)(c) Decision, paras. 346 to 349.
507 Form CO, para. J.149 et seq. Response to the 6(1)(c) Decision, paras. 350 to 354.
508 Form CO, para. J.152 et seq. Response to the 6(1)(c) Decision, paras. 355 and 356.
509 Form CO, para. J. 155.
510 Response to the 6(1)(c) Decision, paras. 359 and 360.
511 Response to the 6(1)(c) Decision, paras. 357 and 358.
512 […].
513 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 234.
514 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to questions 234.1 and 448. […] mentions that “already to day the choice is quite limited” (doc. ID 1020); […] further states that “The number of players is already limited. An additional consolidation would reduce further the available suppliers” (doc. ID 1037); other statements by customers of PA 6.6 BP include the following: “Our access to the product will be severely impacted” ([…], doc. ID 987) and “Not really a sufficient choice. There will be just few” ([…], doc. ID 576).
515 M.8674_BASF_SOLVAY_Confidential_Annex.2017 - RFI#10 Q2.XLSX.
516 Non-confidential response of […] to RFI dated 20 July 2018, question 14, doc. ID 2066.
517 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 214,
214.1 and 238.1. […] notes in particular that “A producer of PA 6,6 Base Polymer would need to secure access to both HMD and adipic acid to produce salt to then produce polymer” (doc. ID 1183).
518 Form CO, para. J. 108.
519 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to questions 216, 237 and 238.
520 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 214, 214.1 and 238.1. […] states that “As mentioned above, the supply of raw material (ADN-HMDA) is controlled by the same companies controlling the Polymer market.” (doc. ID 883); […] notes that “Due to lack of raw materials the investment in new polymerization capacity is difficult to justify since there is high risk that the plant cannot be operated at expected levels.” (doc. ID 860).
521 Non-confidential minutes of the call with […], 5 July 2018, 10.30h, doc. ID 1602.
522 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to questions 234.1 and 235. […] notes in particular that “We believe the combined company will be tight on PA66 Polymerization capacity” (doc. ID 1189). […] is concerned that the combined company will likely use all available polymer capacity for their captive needs.” ([…], doc. ID 1179). Also see responses to question 213.1. […] notes that “As everybody knows, ADN availability is limited, and PA66 Polymer demand in Engineering “Plastic, automotive in particular, has been rising significantly” (doc. ID 883).
523 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to questions 217 and 239.
524 Annex.RFI#10.Q01.PCIYellowbook2018.pdf, p. 137.
525 Response to the 6(1)(c) Decision, para. 350.
526 M.8674 - Parties R6(1)(c) - Economic Annex Vertical forecloure.pdf, para. 60.
527 Annex.RFI#10.Q01.PCIYellowbook2018.pdf, p. 137.
528 Annex#10.Q04.BASF.AnnualProductionVolume.xlsx.
529 M.8674 - Parties R6(1)(c) - Economic Annex Vertical forecloure.pdf, para. 61.
530 Annex.RFI#10.Q01.PCIYellowbook2018.pdf, p. 137.
531 […]
532 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 212.
533 […] reply to Questionnaire Q1 to competitors/customers of the nylon business, question 212.1, doc. ID 1219.
534 […] reply to Questionnaire Q1 to competitors/customers of the nylon business, doc. ID 915, question
212.1.
535 […] reply to Questionnaire Q1 to competitors/customers of the nylon business, doc. ID 1183, question 212.1.
536 Non-confidential minutes of the meeting with […], 12 July 2018, doc. ID 1596.
537 Non-confidential response of […] to RFI dated 6 August 2018, doc. ID 2117, question 5.
538 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 244.
539 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 239, Numerous customers of PA 6.6 BP raised the impact on prices that the Transaction would bring about: […] stated that “All PA66 compounders, i.e competitor to BASF in the compound market, will be confronted with reduction and /or interruption of supplies and higher prices.” (doc. ID 987); […] mentions that “less competition will lead to higher prices”(doc. ID 889); […] also states that “Pa6.6 will become probably more expensive than less in a mid-term horizon”(doc. ID 981); […] notes that “Merchant volume for market could be significantly reduced; Consequently, supply will be tight, and price will be very high” (doc. ID 1119); […] also notes that “Less availability of base polymer means increase of prices for us”(doc. ID 831).
540 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 244.1. One PA 6.6 BP customer, […], states that “BASF will be fully integrated […].We mainly see this as an impact on the choice” (doc. ID 1020); another one […] mentions that “Die Vielfalt und die Angebotsmenge wird sich reduzieren” (doc. ID 785).
541 […] response to Phase II RFI (Question 25), doc. ID 1884 "[…] attempts to serve EEA customers from […] EEA plant […]."
542 Annex.RFI#10.Q01.PCIYellowbook2018.pdf, pp. 23 and 101.
543 At least until Invista's project to install an ADN facility in China, which would not occur before 2019 at least Non-confidential minutes of the call with […], 5 July 2018, doc. ID 1602, para. 16; Non- confidential minutes of the meeting with […], 12 July 2018, doc. ID 1596, para. 21.
544 Non-confidential minutes of the meeting with […] 12 July 2018, doc. ID 1596, para. 21: "The quality of PA6.6 BP from local Chinese suppliers is not up to the level of European or American suppliers. The quality gap explains why China remains a significant importer of PA6.6 BP". Similarly, […] also stated that "[…], importing from Asia in to Europe is not possible primarily due to poor quality and consistency, as evidenced by the continued high level of imports in to China"; Non-confidential minutes of the call with […], 12 July 2018, doc. ID 1593, para. 11: "In the unlikely event that Huafeng (The only Company in China producing PA66 Polymer) will offer and will deliver one truck of PA 6.6 to […] but it would be impossible to rely on Huafeng for […] production needs."
545 Non-confidential response of […] to RFI dated 6 August 2018, doc. ID 2117, question 12: "While on a purely technical basis, the materials produced by local Chinese Nylon 6,6 BP producers and others outside China are similar, there remains a preference by our end customers in Europe and Americas not to use these materials."
546 Non-confidential minutes of the call with […], 12 July 2018, doc. ID 1593, para. 11.
547 FIDES is a trade organization of which the Parties are a member, and which is references in para. K.328 of the Form CO.
548 Non-confidential response of […] to RFI dated 20 July 2018, doc. ID 2066, question 17.
549 Response to the 6(1)(c) Decision, paras. 380 to 383.
550 Form CO, paras. K346 et seq.
551 Form CO, Annex 77, slide 45.
552 Response to the 6(1)(c) Decision, para. 398.
553 Response to the 6(1)(c) Decision, paras. 388 to 401.
554 Form CO, paras. K.346 et seq.
555 Response to the 6(1)(c) Decision, paras. 384 to 387.
556 Form CO, para. K.348.
557 Form CO, para. K.349.
558 Form CO, para. K.352. Response to the 6(1)(c) Decision, paras. 402 to 413.
559 Response to the 6(1)(c) Decision, paras. 414 to 416.
560 Questionnaire Q10 to customers of PA 6.6 EP, responses to question 20.
561 See 51 M.8674 BASF_SOLVAY_Confidential_Annex.MS.Estimates#7, Q3.xlsx and M.8674_BASF_SOLVAY_Confidential_Annex.2017 - RFI#10 Q2.XLSX.
562 Questionnaire Q2 to customers of PA Engineering Plastics, responses to question 66.1. For instance, […] notes "The problem is in the supply chain for the monomers to produce a PA6.6. Solvay is one of the few key producers of specific PA6.6 monomers. There is a risk that after the merger the supply to smaller players will be limited by BASF/Solvay If the few producer of the monomers does not increase their production capacity the supply is limited and therefore the quantity of PA6.6 which has an direct effect on the prices of PA6.6” (doc. ID 947); […] also states that the “Number of suppliers who are fully or almost fully backwards integrated will be reduced by 1. After the merger, 3 players dominate the PA66 intermediate market/ PA66 value chain” (doc. ID 1259).
563 Questionnaire Q2 to customers of PA Engineering Plastics, responses to questions 21.1 and 22.1.
564 Questionnaire Q10 to customers of PA 6.6 EP, responses to question 18.
565 Questionnaire Q10 to customers of PA 6.6 EP, responses to question 18.1.
566 […], […], and […]replies to Questionnaire Q10 to customers of PA 6.6 EP, responses to question 18.2, doc. IDs 1818, 1777 and 1617 respectively.
567 Questionnaire Q10 to customers of PA 6.6 EP, responses to question 18.1.
568 Questionnaire Q10 to customers of PA 6.6 EP, responses to question 19. […] states for instance that "BASF and Solvay are the major suppliers of heat-resistant PA 6.6 EP" (doc. ID 1777).
569 […] reply to Questionnaire Q10 to customers of PA 6.6 EP, response to question 19 (doc. ID 1663).
570 […] reply to Questionnaire Q10 to customers of PA 6.6 EP, response to question 19 (doc. ID 1691).
571 […] reply to Questionnaire Q10 to customers of PA 6.6 EP, response to question 19 (doc. ID 2005). […] reply to Questionnaire Q10 to customers of PA 6.6 EP, response to question 19 (doc. ID 1840).
572 Annex#10.Q64.BASF.CompetitorGrades.xlsx
573 Form CO, para. K.132, C.EP.29.
574 Form CO, Annex#10.Q64.BASF.CompetitorGrades.xlsx and Annex#10.Q64.Business.CompetitorGrades.xlsx.
575 […] minutes paras. 10, 14-15, doc. ID 2280; Non-confidential minutes of the call with […], 5 July 2018, doc. ID 1602, paras. 9 and 13-14.
576 […]
577 Which are respectively "Who is currently your main supplier for (product category)?" and "In addition, who is currently your second main supplier? Other". See PM Europe questions 42 and 43.xlsx.
578 […]
579 Which are respectively "Who is currently your main supplier for (product category)?" and "In addition, who is currently your second main supplier? Other". See PM Europe questions 42 and 43.xlsx.
580 Questionnaire Q2 to customers of PA Engineering Plastics, responses to question 66.
581 Questionnaire Q2 to customers of PA Engineering Plastics, responses to question 66.1. As a result of the Transaction […] states that “RHODIA and BASF together represent more than 95% of our purchases of PA66 EP in Europe It would take us years until we build alternative sources” (doc. ID 854); […] confirms that “The available supplier portfolio for […] is already limited and the merger of BASF and Solvay will enforce this situation” (doc. ID 877); others note that the two companies are the largest players for products to the automotive industry – […] notes for instance that “BASF and Solvay are the 2 biggest manufacturers of PA6.6 for Automotive compounds” (doc. ID 1197); […] that “In Europe BASF and Solvay are the dominant Producers of PA6.6 for the automotive industry. The alternative suppliers in Europe do not have enough capacity to take a significant share of the demands to keep a sufficient […]” (doc. ID 956); […] further states that "The problem is in the supply chain for the monomers to produce a PA6.6. Solvay is one of the few key producers of specific PA6.6 monomers. There is a risk that after the merger the supply to smaller players will be limited by BASF/Solvay If the few producer of the monomers does not increase their production capacity the supply is limited and therefore the quantity of PA6.6 which has an direct effect on the prices of PA6.6” (doc. ID 947). […] states that the “Number of suppliers who are fully or almost fully backwards integrated will be reduced by 1. After the merger, 3 players dominate the PA66 intermediate market/ PA66 value chain” (doc. ID 1259).
582 Questionnaire Q2 to customers of PA Engineering Plastics, responses to question 67.
583 Response to the 6(1)(c) Decision, para. 385.
584 Questionnaire Q10 to customers of PA 6.6 EP, responses to questions 4.1 to 4.4, and 4.7. […] notes for instance that "In contrast to Solvay (in Europe) and Ascend (in USA), DowDupont is not fully integrated in PA66 value chain (mainly to produce one of the key precursors: Adiponitrile). It can be a constraint and weakness to secure the supply" (doc. ID 1818).
585 See Annex#10.Q64.BASF.CompetitorGrades.xlsx and Annex#10.Q64.Business.CompetitorGrades.xlsx. Exceptions include high flow grades and impact modified grades, for which the Notifying Party does not list any grade offered by DowDuPont.
586 Questionnaire Q10 to customers of PA 6.6 EP, responses to question 4.5. See in particular […], […], […], […], […] and […] replies to Questionnaire Q10 to customers of PA 6.6 EP, responses to question 4.5, doc. IDs 1818, 1777, 1514 , 1663, 1697 and 1617 respectively. […] notes for instance that "In contrast to Solvay (in Europe) and Ascend (in USA), DowDupont is not fully integrated in PA66 value chain (mainly to produce one of the key precursors: Adiponitrile). It can be a constraint and weakness to secure the supply" (doc. ID 1818). […] notes that "there is a shortage of ADN in the market, and there is only one ADN factory in Europe. Following the BASF/Solvay transaction, we expect that it will become more difficult for suppliers of PA 6.6 EP to get access to sufficient ADN. This is an observation that is true not only for DowDupont but for all compounding companies that are not vertically integrated" (doc. ID 1777). […] also notes that DowDuPont has "limited sources of necessary raw materials. BASF/Solvay would control the market, because of vertical integration" (doc. ID 1514). […] confirms that "DuPont no longer controls the assets producing their intermediates - they can no longer fully control the supply chain" (doc. ID 1663). […] also states that "As Dupont is buying intermediates, the supply chain is not 100% secure" (doc. ID 1697). Similarly, […] considers that "DowDupont is fully dependent by PA66 producers (= intermediates producers)" (doc. ID 1617).
587 Questionnaire Q10 to customers of PA 6.6 EP, responses to question 4.6. See in particular […], […], […], […], […], […], […], […], […], […], […], […], […], […], and […] replies to Questionnaire Q10 to customers of PA 6.6 EP, responses to question 4.5, doc. IDs 2005, 1511, 1681, 1818, 1777, 1691, 1981, 1560, 1514, 1663, 1840, 1821, 1617, 2002 and 1765 respectively. […] notes for instance that "Dupont is more expensive than their direct competitors of PA66 most probably because they are not fully integrated" (doc. ID 1818). […] states that "generally speaking large plastic producers and compounders are less flexible and customer-oriented than smaller companies." (doc. ID 1765).
588 Questionnaire Q2 to customers of PA Engineering Plastics, responses to question 67. […] notes for instance that they “expect price increase for Solvay material because BASF price politicy is agresiv. BASF has more expencive materials then the other producers” (doc. ID 851); […] states that “Our expectation with this merger is that will see price increases due to the shortage (limited number of monomer supplier). BASF will have an interest that Solvay sell the monomer primarily to BASF and not to other” (doc. ID 947); […] states that “Clearing the proposed merger would, to the estimation of […], result in higher Prices for PA 6.6 EP at least within the EEA” (doc. ID 950).
589 Questionnaire Q10 to customers of PA 6.6 EP, responses to questions 5.1 to 5.4, and 5.7.
590 Questionnaire Q10 to customers of PA 6.6 EP, responses to question 5.5. See in particular […], […], […], […], […], […] and […] replies to Questionnaire Q10 to customers of PA 6.6 EP, responses to question 4.5, doc. IDs 1818, 1511, 1514, 1663, 1840, 1697 and 1617 respectively. […] notes for instance that Radici would be viable from a security of supply standpoint "if they have supply on ADN" (doc. ID 1511). […] states that "In contrast to Solvay (in Europe) and Ascend (in USA), Radici is not fully integrated in PA66 value chain (mainly to produce one of the key precursors: Adiponitrile). It can be a constraint and weakness to secure the supply" (doc. ID 1818). […] again states Radici would have "limited sources of necessary raw materials. BASF/Solvay would control the market, because of vertical integration" (doc. ID 1514). […] confirms that "Radici is dependent upon the Solvay supply chain for ADN - […] are not aware of the sources they are using - so their ability to secure supplies in not clear". […] states that "Radici has not lifted their Force Majeure declaration and within the PA66 value chain, Radici relies on ADN produced by Butachimie" (doc. ID 1663). […] states that "[a]s Radici is buying intermediates, the supply chain is not 100% secure" (doc. ID 1697). […], while acknowledging that Radici's vertical integration is an advantage compared to non-integrated suppliers, notes that "the fully dependence on ADN by external sources (fully owned by Solvay, Invista, Ascend + negligible Asahi) is a structural risk" (doc. ID 1617).
591 Questionnaire Q10 to customers of PA 6.6 EP, responses to questions 6.1 to 6.4, and 6.7.
592 Questionnaire Q10 to customers of PA 6.6 EP, responses to question 6.5. See in particular […], […], […], […], […], […], […], and […] replies to Questionnaire Q10 to customers of PA 6.6 EP, responses to question 6.5, doc. IDs 2020, 2005, 1511, 1681, 1818, 1809, 1821 and 1697 respectively. […] notes for instance that Ascend does not offer security of supply because "Ascend have not plant in Europe" (doc. ID 1511). Similarly, […] states that "For EEA, Ascend will have to increase its compound capacity to improve its market share with our Company, and secure the supply chain." (doc. ID 1818).
593 […] reply to Questionnaire Q10 to customers of PA 6.6 EP, response to question 6.5, doc. ID 2020. […] states: "As they have to import PA 6.6 there are sometimes delays in the supply".
594 […] reply to Questionnaire Q10 to customers of PA 6.6 EP, response to question 6.5, doc. ID 1681. […] states that Ascend "may prefer other régions and / or other customers, depending on regional pricing and / or exchange".
595 […] and […] Replies to Questionnaire Q10 to customers of PA 6.6 EP, responses to question 6.5, doc. IDs 2005 and 1809 respectively. […] states that "these days they have Force MAjeure therefore I can not say that they there is security in supply of the material". […] mentions that "the PA66 marked is already very critical (Force Majeure) were declared in Q2/2018 and supplies are already critical".
596 […] reply to Questionnaire Q10 to customers of PA 6.6 EP, response to question 6.5, doc. ID 1821. […] states that Ascend's "base resin factory is in the US in an hurricane area", which impacts security of supply.
597 Questionnaire Q10 to customers of PA 6.6 EP, responses to questions 8.1, 9.1, 10.1 and 11.1.
598 Questionnaire Q10 to customers of PA 6.6 EP, responses to question 8.4, 8.7, 9.4, 9.7, 10.4, 10.7, 11.4, and 11.7.
599 Questionnaire Q10 to customers of PA 6.6 EP, responses to question 10.2. See in particular […], […], and […] replies to Questionnaire Q10 to customers of PA 6.6 EP, doc. IDs 1560, 1809 and 1840 respectively. […] states that "EMS is a supplier for special PA based raw materials. They do not supply standard PA66 grades in the volumes […] needs". […] also states that "As far as [they] know EMS doesn't produce any "normal" PA6.6 types, that we use. Only Special types like Grivory, which is only used for Special requirements, because it is way too expensive". […] also states that "PA66 is a niche product - our volumes are to big for them".
600 Questionnaire Q10 to customers of PA 6.6 EP, responses to questions 11.1 and 11.2. See in particular […] and […] replies to Questionnaire Q10 to customers of PA 6.6 EP, doc. IDs 1818 and 1663 respectively. […] notes that DSM is "used by […] as a main source of PA6 - they are venturing now into PA66 with polymer from Invista". Similarly, […] states that "DSM is only producing PA6 and not PA66. PA66 is only a complement of range".
601 Questionnaire Q10 to customers of PA 6.6 EP, responses to question 7.1.
602 Questionnaire Q10 to customers of PA 6.6 EP, responses to question 7.4 and 7.7.
603 It is meaningful to note that an important share of respondents (over half) did not provide information regarding Ravago, in particular due to the lack of experience or contact with the company, which is evidence in itself that the company is not viewed as a credible alternative to the Parties.
604 […] reply to Questionnaire Q10 to customers of PA 6.6 EP, response to question 9.1, doc. ID 1681. […] states that Ravago is "Not [an alternative] for Virgin material, only for recycled material". […] also states that "Ravago are a well know recycler - not known as a prime supplier - based upon this it is unlikely they have the right Capacities".
605 See Form CO, fn. K.80.
606 See 44 M.8674 BASF_SOLVAY_Confidential_Annex.Contact Details.b) (former Annex 8.15 b) - Business_Customers.xlsx
607 Questionnaire Q10 to customers of PA 6.6 EP, responses to questions 12.1 and 12.2. See in particular […], […], […], […], and […] replies to Questionnaire Q10 to customers of PA 6.6 EP, doc. IDs 2020, 1511, 1818, 1821 and 1694 respectively. […] notes that DOMO "have the facilities for PA6 EP" and that the company is "focused on PA6 EP". […] states that DOMO "are lacking the neat resin to produce and they focus on PA 6". […] states that DOMO does not have the necessary production capacity to supply customers of PA 6.6 EP "because tey are on PA6". […] also notes that "DOMO is only producing PA6 and not PA66. PA66 is only a complement of range". […] states that the company "[is] strong only on PA6".
608 […]
609 Questionnaire Q10 to customers of PA 6.6 EP, responses to questions 7.2, 7.3, 7.5 and 7.6. See in particular […], […], […], […], […], and […] replies to Questionnaire Q10 to customers of PA 6.6 EP, doc. IDs 1777, 1981, 1812, 2020, 1818 and 1617 respectively. […] states that "[d]ue to their non integration in PA66 value chain, their production capacity will depend on their ability to source and contractualize volumes with PA66 producers", and similarly that "[Celanese's] commercial ability to supply […] with competitive conditions will depend on their compound production capacity and their purchasing conditions to get PA66 base resin". With regards to pricing, […] states that "Celanese has less chance to be competitive vs pure producers (Solvay, Ascend), mainly due to their non industrial integration in PA66". […] also notes more generally that "Celanese is a pure compouder (with no industrial integration in PA66 value chain). They are highly dependent on PA66 producers (like Solvay and Ascend). The security of supply for such a company is at stake for PA66 high consumers like our Company". […] states that: "[d]ue to they are not backward integrated in PA6.6 they have to buy material from the market as others. This is a strategical disadvantage, which we as customers experience (lead time, price) too". […] notes that "as Celanese is already facing restraints in their supply chain, […] are concerned that Celanese will not be able to secure the supplies for […]". […] notes that the ability of Celanese to provide sufficient guarantees relating to the security of supply "[a]s they are compounder […] depends on the availability of the neat resin. We think this situation will improve as from 2020.". […] notes that Celanese "are performing only compounding and they are fully dependent by other sources (PA66 producers)", which has for instance an impact on pricing: "Being exposed to the impact coming from their few PA66 sources, also their pricing policy is fully dependent on spot availability and spot prices". Respondents reiterate some of these statements in relation to other non-integrated suppliers.
610 While Lanxess is a producer of adipic acid, the company is not vertically integrated with regards to PA 6.6 BP or other levels of the polyamide value chain.
611 Questionnaire Q10 to customers of PA 6.6 EP, responses to questions 8.2, 8.3, 8.5 and 8.6. See in particular […], […], […], […], […], […], […], […], […], […], […], and […] replies to Questionnaire Q10 to customers of PA 6.6 EP, doc. IDs 1777, 1981, 1812, 2020, 1818, 1617,, 1511, 1809, 1796, 1514, 1815 and 1812 respectively. […] notes that Lanxess' "capacity for PA66 is limited due to the fact that Lanxess is dependent on external base material supply form BASF and / or Solay" and that "Lanxess is I depending on external base (BASF and / or Solay) material supply, this has a negative effect on the competitiveness of Lanxess". […] states that "[Lanxess] have no polymerization, and only ADIPIC ACID as intermediate (that they cannot use for PA66 since they do not perform polymerization). Intermediates estimated capacity ktons/year: ADN=0 / HMD=0 / ADIPIC ACID= 105 They are fully dependent by other sources". […] also states that Lanxess offers security of supply "[o]nly to some extent. As they are a Compounder and have to buy the PA66-Base-Polymer from the market". […] states that there are "limited sources of necessary raw materials. BASF/Solvay would control the market, because of vertical integration". […] also responded with regards to Lanxess' ability to guarantee sufficient security of supply "definitely not for PA66 (not backwards integrated)". […] states with regards to pricing that "Lanxess is considered a […] preferred supplier in specific plastics (PA6, PBT) and in general their pricing policy is in line with […] expectations. However, for PA6.6 grades we experience in the last months a mismatch to our expectations as Lanxess are dependent on the pricing policy of the PA6.6 polymerization suppliers".
612 As a recycler and distributor of PA 6.6 EP, Ravago does not offer customer security of supply for their PA 6.6 EP requirements, as it itself relies on supplies from PA 6.6 EP suppliers, including the Business. Also see Questionnaire Q10 to customers of PA 6.6 EP, responses to questions 9.2, 9.3, 9.5 and 9.6. See in particular […] and […] replies to Questionnaire Q10 to customers of PA 6.6 EP, doc. IDs 1617 and 1809 respectively.
613 Questionnaire Q10 to customers of PA 6.6 EP, responses to questions 10.2, 10.3, 10.5 and 10.6. See in particular […], […], […], […], […], […], […], […], and […] replies to Questionnaire Q10 to customers of PA 6.6 EP, doc. IDs 1981, 1812, 1818, 1617, 1809, 1796, 1514, 1812 and 1863 respectively. For instance, […] states that "EMS is not fully backward integrated. EMS is a PA6.6 compounder and sources PA6.6 EP polymers from the same limited sources available (Invista, Ascend, Solvay, BASF etc). Therefore we are concerned if EMS can provide security of supply to our supply chain. This is because EMS is depending on limited PA6.6 polymerization sources." […] states that "EMS is not an integrated provider to enable them to give this [security of supply] guarantee".
614 Questionnaire Q10 to customers of PA 6.6 EP, responses to questions 11.2, 11.3, 11.5 and 11.6. See in particular […], […], […], […], […], […], […], […], […], […], […] and […] replies to Questionnaire Q10 to customers of PA 6.6 EP, doc. IDs 1777, 1981, 1812, 1818, 1617, 1511, 1809, 1514, 1815, 1812, 1663 and 1821 respectively. […] notes for instance that "DSM is purchasing the base PA66 from Ascent, in case of supply issues (Force Majeure), DSM will have delivery issues as well. Due to the very difficult supply chain situation, there is a high risk of material shortage at least until 2022". […] states that "[DSM] miss base resin" to offer sufficient security of supply guarantees. […] schematizes "no backwards integration => no securisation of supply".
615 Questionnaire Q10 to customers of PA 6.6 EP, responses to questions 12.2, 12.3, 12.5 and 12.6. See in particular […], […], […], […], […], […], and […] replies to Questionnaire Q10 to customers of PA 6.6 EP, doc. IDs 1981, 2020, 1818, 1617, 1511, 1514 and 1681 respectively. […] states that "[a]s [Domo] are not integrated, their production capacity will depend on the fluidity of the market of ADN / HMD/ PA66 resin"
616 Questionnaire Q2 to customers of PA Engineering Plastics, responses to question 61.
617 Questionnaire Q2 to customers of PA Engineering Plastics, responses to questions 51 and 51.1.
618 Questionnaire Q10 to customers of PA 6.6 EP, responses to questions 26.
619 […].
620 Questionnaire Q2 to customers of PA Engineering Plastics, responses to question 55.
621 Questionnaire Q2 to customers of PA Engineering Plastics, responses to question 55.
622 Questionnaire Q2 to customers of PA Engineering Plastics, responses to question 57. 623 Questionnaire Q2 to customers of PA Engineering Plastics, responses to question 57.
624 Questionnaire Q2 to customers of PA Engineering Plastics, responses to question 57.
625 Questionnaire Q10 to customers of PA 6.6 EP, responses to question 1.
626 Questionnaire Q2 to customers of PA Engineering Plastics, responses to questions 64 and 64.1. […] notes that “Our customers are car makers, they want that our suppliers are selected from their lists of approved suppliers” (doc. ID 854); […] further states that “Some Customers have a specific material list per application and even specific needs requiring additional tests” (doc. ID 990); […] confirms that “Some of our OEM Customer requiere that the Material (PA6.6) is listed and approved in their database In order to achieve the listing it requieres time and resources” (doc. ID 947); […] also states that “For some applications, customers request to directly approve and validate PA 6.6 EP suppliers” (doc. ID 909).
627 Questionnaire Q2 to customers of PA Engineering Plastics, responses to question 63.
628 Questionnaire Q10 to customers of PA 6.6 EP, responses to questions 17.
629 Non-confidential minutes of the call with […], 05 July 2018, 10.30h, doc. ID 1602, para. 15
630 Ibid
631 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 368. Questionnaire Q2 to customers of PA Engineering Plastics, responses to question 33
632 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to questions 367 and
367.1. […] notes for instance that they could increase supply “but not at the moment, because there was a big shortage of PA 66 virgin polymer in Q1 due to FM” (doc. ID 915).
633 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 372. Questionnaire Q2 to customers of PA Engineering Plastics, responses to question 35.
634 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to questions questions 371, 371.1 and 371.2. […] notes for instance “The main limiting factor for any new entrant would be the availability of raw materials on a consistent basis”; and […] notes that “Producers of base polymers have no volume to give new entrants” (doc. ID 1170). Questionnaire Q2 to customers of PA Engineering Plastics, responses to question 35.2. […] notes that “Entering the production of PA 6.6 EP is rendered difficult by the following factors: • Complexity of the supply chain of PA6.6 Polymerization • Limited availability of ADN (which is an input product to the polymerization process) as only a limited number of suppliers have the requisite know-how limited advantages for a company to enter into the production of PA 6.6 EP in EEA” (doc. ID 1058); […] states that “On PA6.6 the entry barrier is the cost to establish the value chain. There is no company that invest that money in this product area” (doc. ID 947); […] also states that “Costs in order to secure raw materials availability (vertical integration) are very high and extremely penalizing. Indeed, without vertical integration, it’s not possible to compete with the 4 integrated major players on this concentrated market” (doc. ID 909); […] further states that “ADN bottleneck as we reached 97% of the capacity at this point of time. Only new players that could enter are compounders that could buy resins from the big players but according to major force majeure not possible. it is one on the main reason why we think this acquisition is going to bring to lack of competition meaning price competitiveness and supplies reliabilities” (doc. ID 897).
635 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 369.
636 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 369.1. […] notes for instance that “We are cutting orders to date, mainly due to the recent FM's. Historically, it was always tight but, in the last 12 months it has been severe. We believe that the avaialbility of PA6.6 will remain tight for the remainder of 2018 and all of 2019. As no new capacity of ADN is announced, we further believe that the structural tightness will remain moving forward" (doc. ID 1170); […] states that “current supplu PA is tight. We are experiencing supply interruptions to our customers” (doc. ID 1119).
637 Annex#10.Q67.BASF.SalesEPAndCoPa.xlsx
638 31 M.8674_BASF_SOLVAY_Confidential_Annex#5, Q43.xlsx
639 See doc. ID 1938-5715, an internal BASF presentation dated January 2018, focusing on PA 6.6 to the industrial and transportation segments, which reads "[QUOTE FROM CONFIDENTIAL INTERNAL DOCUMENT]".
640 […].
641 […]
642 […]
643 Response to the 6(1)(c) Decision, paras. 359 and 360.
644 Doc. ID 1902-20065, slide 36.
645 Annex.RFI#10.Q01.PCIYellowbook2018.pdf, p. 137.
646 Questionnaire Q10 to customers of PA 6.6 EP, responses to question 28.2.
647 Questionnaire Q10 to customers of PA 6.6 EP, responses to question 28.1.
648 Questionnaire Q10 to customers of PA 6.6 EP, responses to question 26.
649 Questionnaire Q10 to customers of PA 6.6 EP, responses to questions 6.1 to 6.8.
650 Questionnaire Q10 to customers of PA 6.6 EP, responses to question 6.2. This is for instance pointed out by […] which states that "Ascend is a vertically integrated producer of PA66. That's a real industrial advantage. Their only weakness is their poor compound capacity in Europe to produce PA66 EP." (doc. ID 1818). […] also notes that "They miss some compounding capacity in Europe. They recently bought a compounder (BTP) but still seem to be looking for additional capacity." (doc. ID 2020). […] also notes that Ascend has "not enough compounding capacity installed" (doc. ID 1815). Also see Questionnaire Q10 to customers of PA 6.6 EP, response to questions 6.5 and 6.6. […] point out that Ascend's competitiveness is dependent on import duties, and that the fact that they import the relevant products sometimes lead to delays in the supply of PA 6.6 EP. […] states that "As they have to import PA 6.6 there are sometimes delays in the supply" and that Ascend "are competitive on pricing, but in the future this can depend on import duties…" (doc. ID 2020).
651 See https://www.ascendmaterials.com/news/ascend-performance-materials-acquires-britannia-techno- polymer.
652 […] reply to Questionnaire Q10 to customers of PA 6.6 EPEP, responses to questions 13.1, 13.2, 13.3, 13.4, 13.5, doc. ID 1812. […] states that "KingFa is an alternative supplier for PA6.6 EP grades.". However […] highlights the lack of backward integration of Kingfa: "KingFa is not fully backward integrated. KingFa is a PA6.6 compounder and sources PA6.6 EP polymers from the same limited sources available (Invista, Ascend, Solvay, BASF etc). Therefore we are concerned if KingFa can provide the security of supply to our supply chain. KingFa is dependend on limited PA6.6 polymerization sources".
653 Form CO. Paras. K296 and K.325.
654 Ravago reply to Q1 to competitors/customers of the nylon business (PA), response to question 372.1, doc. ID 1020. […], which distributes and recycles EP listed LG and Kingfa as potential entrants into the EEA for PA 6/6.6 EP.
655 Response to the 6(1)(c) Decision, para. 375.
656 Annex.RFI#10.Q01.PCIYellowbook2018.pdf, p. 371.
657 Internal email exchange of Solvay, doc. ID 1902-22724.
658 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 13.
659 In light of the fact that Butachimie is the only one producer of ADN in Europe, there is no scope for input foreclosure in this case.
660 https://www.icis.com/explore/resources/news/2007/11/01/9075172/butadiene-uses-and-market-data/
661 Doc. ID: 1938-41922.
662 As for butadiene, in light of the fact that Butachimie is the only one producer of ADN in the EEA, there is no scope for input foreclosure in this case.
663 Non-horizontal Merger Guidelines, paragraph 32.
664 As explained in footnote 352, the additional share of calculated on the total nameplate production capacity of Butachimie would actually amount to [10-20]%. However, the Notifying Party indicated in the Form CO that the overall equipment efficiency in the industry varies between [80-90]% and [90- 100]%. Assuming an efficiency rate of [80-90]%, the increase in the production share generated by the contract with Invista will be of [10-20]%, as indicated in the text.
665 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 34.1.
666 […] responses to RFI#2 of 1 August 2018, doc. ID 1884.
667 Guidelines on the assessment of non-horizontal mergers under the Council Regulation on the control of concentrations between undertakings, OJ 200/C 265/07, paragraph 29
668 Ibid
669 M.8674 - Parties R6(1)(c) - Economic Annex Vertical forecloure.pdf, para 9
670 M.8674_BASF_SOLVAY_Confidential_Annex.2017 - RFI#10 Q2.XLSX.
671 Annex 5.4.14 to the form CO.
672 Doc. ID 000870-001121.
673 Form CO, paragraph C.149.
674 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 44, Doc ID. 915.
675 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 14.
676 Section 6.3.2.
677 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 62.
678 Nevertheless, the Commitments include the divestment of […] kts of KA Oil Capacity equivalent to […]% of the capacity of the Business or […]% of the capacity in the EEA,
679 The market shares of BASF for merchant sales plus captive production remain also below [30-40]%.
680 The ability or incentives to foreclose access to Cyclohexanone to Caprolactam producers is not changed by the Transaction because the Business does not produce or sell Cyclohexanone or Caprolactam.
681 See Section 6.4.2.1(A.ii)
682 [30-40]% in value for merchant sales; [50-60]% volume for merchant sales plus captive use; and [60- 70]% for production capacity -[50-60]% Business and [10-20]% BASF.
683 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 122.
684 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 102.
685 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 126.
686 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 126, doc. ID 1014.
687 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 126, doc. ID 915.
688 Non-confidential minutes of the call with […], 21 February 2018, 14.00h, doc. ID 335.
689 Non-confidential minutes of the call with […], 29 November 2017, 09.30h, doc. ID 64.
690 Non-confidential minutes of the call with […], 9 February 2017, 14.30h, doc. ID 399.
691 Non-confidential minutes of the call with […], 12 February 2018, 16.30h, doc. ID 327.
692 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 102.
693 Non-confidential response to RFI to […] dated 1 August 2018, doc. ID 1884.
694 Non-confidential minutes of the call with […], 23 January 2018, 15.00h, doc. ID 245.
695 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 102, doc. ID 1219.
696 Non-confidential minutes of the call with […], 12 February 2017, 14.00h, doc. ID 1416.
697 See Section 5.2.3
698 M.8674 BASF – Solvay Confidential Annex 2017*10, Q2.
699 Non-confidential minutes of the call with […], 9 February 2018, 14.30h, doc. ID 399. 700 Non-confidential minutes of the call with […], 9 February 2017, 14.30h, doc. ID 399. 701 Non-confidential minutes of the call with […], 12 February 2017, 16.30h, doc. ID 327.
702 Non-confidential minutes of the call with […], 21 February 2018, 14.00h, doc. ID 335.
703 […]
704 […]
705 […]
706 […]
707 […]
708 Non-confidential minutes of the call with […], 05 July 2018, 10.30h, doc. ID 1602.
709 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 447, doc. ID 1170.
710 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 447, doc. ID 1037.
711 […]
712 Non-confidential minutes of the call with […], 12 February 2017, 14.00h, doc. ID 1416.
713 Non-confidential minutes of the call with […], 12 February 2017, 14.00h, doc. ID 1416.
714 Questionnaire Q9 HDI Derivatives, responses to question 13.
715 Questionnaire Q9 HDI Derivatives, responses to question 17.
716 Questionnaire Q9 HDI Derivatives, responses to question 21.
717 Questionnaire Q9 HDI Derivatives, responses to question 14.
718 […] reply to Questionnaire Q9 HDI Derivatives, responses to question 14, doc. ID 1851.
719 […] reply to Questionnaire Q9 HDI Derivatives, responses to question 14, doc. ID 1848.
720 […] reply to Questionnaire Q9 HDI Derivatives, responses to question 14, doc. ID 1854.
721 M.8674 BASF – Solvay Confidential Annex 2017*10, Q2.
722 Response to the 6(1)(c) Decision, para. 151.
723 Non-confidential minutes of the call with […], 05 July 2018, 10.30h, doc. ID 1602.
724 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 447, doc. ID 1014.
725 See Sections 6.3.3. and 6.3.4.
726 The Notifying Party contests the relevance of capacity shares (see Reply to the Article 6(1)(c) Decision, para. 248). The counter-arguments put forward in Section 6.2 are equally valid in the present context.
727 As noted, […] is the only EEA supplier that indicated in response to the market investigation that it could serve additional volumes into the merchant market, and for volumes limited to 10 kts per year, i.e., less than 3% of 2017 EEA merchant sales ([…] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 134, doc. ID 915).
728 Non-confidential version of minutes of a call with […], 9 February 2018, doc, ID 399; non-confidential version of minutes of a meeting with […], 9 October 2018, (doc. ID 3059). See also non-confidential version of minutes of meeting with […], 12 July 2018, doc. ID 1596; […] reply to a request for information, 6 August 2018, doc. ID 2117.
729 Non-confidential version of minutes of a meeting with […], 9 October 2018, doc. ID 3059.
730 Reply to RFI#2, Q16, paras. 63-66; Reply to the Article 6(1)(c) Decision, paras. 267-279.
731 Non-confidential version of minutes of a call with […], 21 February 2018, 14:00, doc. ID 335; […] reply to eQuestionnaire Q3 – Competitors/Customers Nylon Business (PA) – adipic acid, question 36, doc. ID 996; […] reply to the Commission request for information on adipic acid, 24 August 2018, doc. ID 2164.
732 […] reply to eQuestionnaire Q3 – Competitors/Customers Nylon Business (PA) – adipic acid, questions 30 and 36, doc. ID 1002; non-confidential version of minutes of a call with […], 5 July 2018, 14:00,
doc. ID 1599.
733 […]
734 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 143.2.
735 Non-confidential version of minutes of a call with […], 21 February 2018, 14:00, doc. ID 335; […]
reply to eQuestionnaire Q3 – Competitors/Customers Nylon Business (PA) – adipic acid, questions 31 and 36, doc. ID 996; […] reply to the Commission request for information on adipic acid, 24 August 2018, doc. ID 2164. Likewise, […] raised concerns about the reduction of availability of adipic acid in the free market, also in relation to the production of aliphatic polyester ([…] reply to eQuestionnaire Q3 – Competitors/Customers Nylon Business (PA) – adipic acid, question 31, doc. ID. 1002).
736 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to questions 179 and 193.
737 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 197.
738 Non-confidential minutes of the call with […], 8 February 2018, 9.30h, doc. ID 190.
739 Non-confidential minutes of the call with […], 12 February 2018, 15.30h, doc. ID 285.
740 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 173.
741 Non-confidential response of […] to RFI to […] dated 13 August 2018, doc. ID 2122.
742 Non-confidential response of […] to RFI to […] dated 20 July 2018, doc. ID 2010.
743 Non-confidential response of […] to RFI2 to […] dated 1 August 2018, q19, doc. ID 1884.
744 See Section 6.3.4 for more details.
745 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 197, doc. ID 984.
746 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 197, doc. ID 984.
747 Non-confidential minutes of the call with […], 8 February 2018, 9.30h, doc. ID 190.
748 […] reply to Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 187, doc. ID 987.
749 Non-confidential minutes of the call with […], 22 February 2018, 14.00h, doc. ID 274.
750 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 173.
751 Non-confidential minutes of the call with […], 12 February 2018, 15.30h, doc. ID 285.
752 Non-confidential minutes of the call with […], 12 February 2018, 15.30h, doc. ID 285.
753 Non-confidential minutes of the call with […], 12 February 2018, 15.30h, doc. ID 285.
754 Form CO, para. J.170 et seq.
755 Form CO, para. J.174 et seq.
756 Response to the 6(1)(c) Decision, paras. 363 and 365.
757 M.8674 - Parties R6(1)(c) - Economic Annex Vertical forecloure.pdf.
758 Response to the 6(1)(c) Decision, para. 364.
759 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 234.1. For instance, one producer of PA 6.6 EP, […], states that "In PA 66, […] is acting as a compounder. Our access to the product will be severely impacted" (doc. ID 987). Another one, […], states that in terms of BP 6.6 suppliers "already to day the choice is quite limited" (doc. ID 1020).
760 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to questions 234.1 and 244.1. […] states that “[…] BASF might decide to withdraw the PA66 Polymer sales of Solvay from PA66 Polymer sales […]. This would not come as a suprise as BASF already did this with its own PA66 Polymer capacity in the past […]” (doc. ID 883); […] raises a similar concern, stating that “Integrated Producer(enhanced position) might consume majority of the PA6.6 base Polymer (Legacy for market choice) for promoting their own downstream.” and also states that “There will be a reduction on the availabiltiy of material for “merchant” buyers” and that “Merchant volume for market could be significantly reduced” as a result of the Transaction(doc. ID 1119). […] notes that “Availability for other PA66 producers and suppliers to the base resin market will be strongly decreased” (doc. ID 987); […] confirms, stating that the Merged Entity's BP capacity would also be tight: “We believe the combined company will be tight on PA66 Polymerization capacity. […] is concerned that the combined company will likely use all available polymer capacity for their captive needs” ([…], doc. ID 1179); […] further states “Less suppliers possible, Basf will use the polymer for their own” (doc. ID 831[…] points to the fact that not supplying PA 6.6 BP would be in line with BASF's existing strategy “It is not BASF strategy to sell PA66 base resin.” (doc. ID 987).
761 […]
762 […]
763 […][QUOTE FROM CONFIDENTIAL INTERNAL DOCUMENT]
764 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to questions 227 and
227.1. […] mentions for instance that "Primary EEA demand is served by producers within EEA" (doc. ID 1119). In a separate spontaneous submission to the Commission, […] mentioned that "[i]mporting of Polyamide 6.6 in to Europe from North America has not been commercially possible over the last five years due to the relatively high price of US material […]. Additionally, importing from Asia in to Europe is not possible primarily due to poor quality and consistency, as evidenced by the continued high level of imports in to China." (doc. ID 2269).
765 Form CO, para. J.177 et seq.
766 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 244. […] notes that "BASF is a major consumer of ADN and downstreams, used to be a merchant buyer of ADN and will be strongly incentivised to use the material captively and dry up the merchant market and continue in their value chain to PA 66 compounds downstream. It is not BASF strategy to sell PA66 base resin" (doc. ID 987).
767 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 244.1. […] notes for instance that "less competition will lead to higher prices. Or they will reduce prices that the competitors cannot keep up an economic production process and will leave the PA66 market" (doc. ID 889).
768 […] [QUOTE FROM CONFIDENTIAL INTERNAL DOCUMENT]
769 […] [QUOTE FROM CONFIDENTIAL INTERNAL DOCUMENT]
770 Form CO, para. J.180.
771 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 375.
772 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 234.1. […] mentions for instance that "Integrated Producer (enhanced position) might consume majority of the PA6.6 base Polymer (Legacy for market choice) for promoting their own downstream" (ID 1119). Also see replies to question 239, […] states that "All PA66 compounders, i.e competitor to BASF in the compound markt, will be confronted with reduction and /or interruption of supplies and higher prices." (doc. ID 987).
773 Non-confidential presentation of […] dated 12 July 2018, doc. ID 2116, slides 7 to 10.
774 Non-confidential response of […] to RFI dated 6 August 2018, doc. ID 2117, question 8.
775 Form CO, para. L.44. Also see recital (1071).
776 As there are no captive sales of performance fibres based on the market share estimates provided by the Notifying Party, sales in the merchant market equal total sales for all segments.
777 Minutes of a call held on 6 February 2018 with […]read: "BASF used to supply limited amounts of PA66 polymers to the European textile fiber producers until the time when some of its Ludwigshafen PA66 Polymerization Assets where shut down, (Approx 10 year ago). […] opinion is that BASF main focus is and will be after Solvay assets acquisition on the Engineering Plastic Business" (doc. ID 215, para. 7) as well as "BASF's acquisition of Solvay will have a very significant impact on the fiber industry. BASF will control 50% of the JV Butachimie and therefore the production of ADN. BASF will also acquire Solvay's capacity to produce PA 66 base polymer. While SOLVAY remained a reliable supplier for the fiber industry, BASF decided to withdrew from the PA 66 base polymer for fibers market. In post-merger situation, the Polymer that SOLVAY makes available will possibly fade out because BASF might decide not to supply any more to the fiber industry, or to impose significantly higher prices in order to maintain the supply. The demand of PA 66 for the automotive industry is very high, and is increasing. BASF will probably confirm its focus on this sector. Post-merger, the main European PA66 polymer supplier for the fiber industry will disappear" (doc. ID 215, para. 14).
778 […] (doc. ID 2236): "PA 66 raw material is highly restricted in supply on a Worldwide level. Especially Europe is price competitive in PA 66, even on Worldwide scale. This is not true for PA 6. A further consolidation of the market would add a significant additional thread to the supply-chain as only a few large players will dominate the whole market. Already we have “strategic” indications that - driven by the plastic industry - the price level of PA 66 is supposed to significantly increase over the coming years. Price increases of 200% were mentioned. This trend would be further accelerated with additional consolidation in the market".
779 Form CO, paras. L.47-49.
780 Questionnaire Q5 to customers of PA 6.6 Performance Fibres, responses to question 54.
781 See Reply to RFI#5, Q 16.
782 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 329.
783 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 316.
784 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 324.
785 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 327. One respondent mentions that "Chinese additives suppliers are competitive", and another points out that "Many Chinese or Asian additives producers established business either directly or through agent/ distributor in the last five years". A third adds that they are "seeing a strong activity and capacity build in Asia", doc IDs 1071, 903 and 1096 respectively.
786 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 333.
787 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 353.
788 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 341.
789 Questionnaire Q1 to competitors/customers of the nylon business (PA), responses to question 354.
790 Non-horizontal Merger Guidelines, para. 104.
791 […]reply to Questionnaire Q10 to customers of PA 6.6 EP, response to question 18.2, doc. ID 2002, […]states that "The main problem is that this acquisition will drive BASF to be dominant into the PA (nylon) market. PA market is made by PA66 and PA6. Today BASF is vertical on PA6 and SOLVAY is vertical on PA66. After the acquisition BASF will lead the nylon market". Non-Confidential Minutes of meeting with […] 25 October 2018, 11:00 Brussels time (doc. ID 3065). […] stated that "BASF is also by far the leader in the broader global nylon business due to its presence in the nylon 6 market and in some raw materials including caprolactam." Also see Non-Confidential Minutes of meeting with […] 9 October 2018, 14:00 Brussels time (doc. ID 3059) "PA 6 is a competing value chain within the nylon industry. PA 6 and PA 6.6 are different products in particular due to the relevant price delta; if customers do not use PA 6, it is because they specifically need nylon 6.6. BASF is the strongest player in PA 6 in Europe. It is also one of the two biggest producers of caprolactam, an essential input into the PA 6 value chain. The transaction would grant the merged entity dominance over the nylon industry in general."
792 […]
793 […].
794 Questionnaire Q2 to customers of PA Engineering Plastics, responses to question 4.
795 Questionnaire Q2 to customers of PA Engineering Plastics, responses to question 43.
796 Questionnaire Q10 to customers of PA 6.6 EP, responses to question 20.
797 The input foreclosure strategy that may affect these companies is assessed separately in Section 6.4.11.
798 See […] [QUOTE FROM CONFIDENTIAL INTERNAL DOCUMENT]
799 Including PA 6 EP and PA 6.6 EP, as well as smaller niche products such as PA 6.10 EP, which are produced in small volumes (less than 1 kt in 2017) by the Parties in the EEA).
800 Non-horizontal Merger Guidelines, para. 93.
801 The Business sells PBT to a limited extent, but outside of the EEA.
802 Commission Notice on remedies acceptable under Council Regulation (EC) No 139/2004 and under Commission Regulation (EC) No 802/2004, 2008/C 267/01 (“ Commission Notice on Remedies”), paragraph 9
803 Merger Regulation, recital 30; see also paragraph 9 of the Commission notice on remedies acceptable
under Council Regulation (EC) No 139/2004 No 802/2004 (OJ C 267, 22.10.2008, p.1) (the ‘Remedies Notice’).
804 Merger Regulation, recitals 30.
805 Commission Notice on Remedies, paragraph 9.
806 Commission Notice on Remedies, paragraph 18.
807 The purpose of the Final Commitments as submitted on 11 December 2018 was to clarify the wording of the sections relating to intellectual property rights and Know-How.
808 In order to produce EP, additives need to be added in a relatively low concentration. A production unit at Belle-Etoile mixes a low quantity of polymers with highly concentrated additives. The resulting products are referred to as "master batches". These master batches are then mixed with BP, in order to produce EP. Producing master batches predominantly involves IP rights and know-how (i.e. the recipes), more than a particular mixing asset.
809 […] response to questionnaire R1 to upstream customers/competitors responses to question 2.1, doc. ID 2867.
810 […] response to questionnaire R1 to upstream customers/competitors responses to question 2.1, doc. ID 2799.
811 […] response to questionnaire R1 to upstream customers/competitors responses to question 2.1, doc. ID 2802.
812 […] response to questionnaire R1 to upstream customers/competitors responses to question 2.1, doc. ID 2682.
813 […] response to questionnaire R1 to upstream customers/competitors responses to question 2.1, doc. ID 2795.
814 […] response to questionnaire R1 to upstream customers/competitors responses to question 1.2.1, doc. ID 2924
815 Responses of […] to questionnaire R1 to upstream customers/competitors question 14.1. doc. IDs 2772, 2673, 2802, 2920 and 2682 respectively.
816 Responses to questionnaire R1 to upstream customers/competitors
817 Responses to questionnaire R1 to upstream customers/competitors
818 Questionnaire R1 to upstream customers/competitors responses to question 1.2 and Questionnaire R2 to EP customers responses to question 3.
819 […] response to Questionnaire R2 to EP customers responses to question 3, doc. ID 2806.
820 […] response to Questionnaire R2 to EP customers responses to question 3, doc. ID 2276.
821 […] response to questionnaire R1 to upstream customers/competitors responses to question 1.2, doc. ID. 2867.
822 […] response to questionnaire R1 to upstream customers/competitors responses to question 1.2, doc. ID 2917.
823 […] response to questionnaire R1 to upstream customers/competitors responses to question 1.2, doc. ID 2657.
824 […] response to questionnaire R1 to upstream customers/competitors responses to question 1.2, doc. ID 2792. Also see […] spontaneous submission dated 14 December 2018, doc. ID 3136, p. 4.
825 […] response to questionnaire R1 to upstream customers/competitors responses to question 1.11, doc. ID 2795.
826 […] response to questionnaire R1 to upstream customers/competitors responses to question 1.11, doc.
ID 2924.
827 Non-Confidential Minutes of meeting with […] 25 October 2018, 11:00 Brussels time, doc. ID 3065.
828 This competitor requested confidentiality.
829 Anonymous competitor to questionnaire R1 to upstream customers/competitors responses to question 1.2. ID 3061.
830 […] response to questionnaire R1 to upstream customers/competitors responses to question 6.1, doc. ID 2673. […] spontaneous submission dated 14 December 2018, doc. ID 3136, p. 2.
831 […] response to questionnaire R1 to upstream customers/competitors responses to question 1.2, doc. ID 2924
832 Non-Confidential Minutes of Meeting with […] on 25 October, 11.00h, doc. ID 3065.
833 Anonymous competitor response to questionnaire R1 to upstream customers/competitors responses to question 1.2. ID 3061.
834 Questionnaire R1 to upstream customers/competitors, responses to question 1.4
835 Questionnaire R1 to upstream customers/competitors, responses to question 17
836 Questionnaire R1 to upstream customers/competitors, responses to question 15
837 […] responses to questionnaire R1 to upstream customers/competitors, responses to question 1.4., doc. IDs 2684, 2938, 2682.
838 Anonymous competitor's response to questionnaire R1 to upstream customers/competitors, question 1.4, doc. ID 3061.
839 Anonymous competitor's response to questionnaire R1 to upstream customers/competitors, question 15, doc. ID 3061.
840 […] response to questionnaire R1 to upstream customers/competitors, responses to question 1.4., doc. ID 2924.
841 Annex 1 to […] response to questionnaire R1 to upstream customers/competitors, para. 17. (doc.ID 2924) See also non-confidential minutes of a meeting with […], 25 October 2018, doc. ID 3065.
842 Idem. See also Annex 1 to […] response to questionnaire R1 to upstream customers/competitors, paras. 6 and 22 (doc. ID 2924).
843 Non-confidential minutes of a meeting with […], 25 October 2018, doc. ID. 3065.
844 […] response to questionnaire R1 to upstream customers/competitors responses to question 1.2, doc. ID 2886.
845 […] response to questionnaire R1 to upstream customers/competitors responses to question 1.2, doc. ID 2867.
846 […] response to questionnaire R1 to upstream customers/competitors responses to question 1.3, doc. ID 2792.
847 […] response to questionnaire R1 to upstream customers/competitors responses to question 1.3, doc. ID 2772.
848 Anonymous competitor presentation to the EC for the meeting of 8 November 2018, doc. ID 3119.
849 Anonymous competitor response to questionnaire R1 to upstream customers/competitors responses to question 1.3.1., doc. ID 3061.
850 Questionnaire R1 to upstream customers/competitors responses to question 1.9
851 […] response to questionnaire R1 to upstream customers/competitors responses to question 1.9, doc. ID 2920.
852 […] response to questionnaire R1 to upstream customers/competitors responses to question 1.9, doc. ID 2682.
853 Questionnaire R1 to upstream customers/competitors responses to question 1.5
854 Many respondents renewed concerns expressed in relation to upstream products, and in particular ADN and HMD. See Sections 7.4.1.1, 7.4.1.2, 7.4.2.1, and 7.4.2.2.
855 […] responses to questionnaire R1 to upstream customers/competitors responses to question 1.5.1. An anonymous purchaser of PA 6.6 BP notes that "if BASF takes its full capacity under the tolling and supply agreements, the Divestment Business will actually be short on base polymer and will need to buy from the market, thereby actually worsening the competitive landscape for base polymer. Since the divestment business will have to pay market price for PA6,6 base polymer, it will be unable to compete on price with BASF which will be acquiring the base polymer under advantaged supply and tolling arrangements" (doc. IDs 3061, 2895, 2795). […] notes for instance that "Divestment Business will use the available ADN for its HMD production instead of supplying PA6.6 BP manufacturers and it will rather use the limited raw material availability for its own PA6.6. EP production and for the PA6.6 BP tolling and supply agreement" (doc. ID 2795). […] notes that "The primary problem with the Proposed Commitments is that the divestment of the PA 6.6 production would be in name only. By entering into the tolling agreement with the Divestment Business, BASF would be retaining priority and control over the PA 6.6 BP manufacturing capacity for itself, limiting the Divestment Business’s capacity to manufacture PA 6.6 BP for third party compounders. […] Further, the toll arrangement would mean that BASF is still able to leverage the Divestment Business’s manufacturing capacity to manufacture PA 6.6 BP at slightly above cost. Meanwhile, the ADN supply agreement to the Divestment Business is a market-based contract, ensuring that BASF would be able to obtain PA 6.6 BP from the Divestment Business at significantly less cost than the PA 6.6 BP will be available to any of BASF’s compounding competitors" (doc. ID 2895).
856 […] response to questionnaire R1 to upstream customers/competitors responses to question 1.5.1, doc. ID 2673. Anonymous competitor presentation to the EC for the meeting of 8 November 2018, doc. ID 3119. Also see […] spontaneous submission dated 14 December 2018, doc. ID 3136, p. 2.
857 Anonymous response to Questionnaire R2 to PA 6.6 EP customers, responses to question 1.5.1. The PA 6.6 EP supplier makes calculations based on available data and concludes that "the divested business may have little to no merchant PA6.6 base polymer to sell after fulfilling its own downstream compounding needs" (doc. ID 3061). Also see […] response to questionnaire R1 to upstream customers/competitors, Annex 1 (doc. ID 2924).
858 Questionnaire R1 to upstream customers/competitors responses to question 1.8.
859 […] response to questionnaire R1 to upstream customers/competitors responses to question 1.8, doc. ID 2867.
860 […] response to questionnaire R1 to upstream customers/competitors responses to question 1.8, doc. ID 2727.
861 Questionnaire R1 to upstream customers/competitors responses to question 1.6.
862 Questionnaire R2 to PA 6.6 EP customers, responses to question 2.
863 Questionnaire R2 to PA 6.6 EP customers, responses to question 15.1 and 17.1.For instance, […]notes that the purchaser should "be a global player to cover worldwide industrial platforms" (doc. ID 2935). […] notes that "The production of EP products requires the knowledge of the complexity of operating in the automotive sector where approvals and high and sustained quality levels are key - failure to maintain either of these could result in deselection of the divested business on grounds of unsustainability" (doc. ID 2806).
864 Anonymous response to Questionnaire R2 to PA 6.6 EP customers, responses to question 21. The PA 6.6 EP supplier mentions that "For the downstream EP business the buyer would need to have experience in the following sectors: • Automotive • Electrical and Electronics • Industrial • Consumer" (doc. ID 3061).
865 Questionnaire R2 to PA 6.6 EP customers, responses to questions 2.1 and 6.1. […] states for instance that "customers are expecting company with a global footprint to avoid requalification costs for global programs. They are almost all acting in different regions of the world. Divested company would be excluded from RFQ (Request For Quotation) requesting production in ADN outside of EEA. For this reason, Solvay's current customers active in our industry might shift away from the new entity after the transaction" (Doc ID 2935). […] notes that "SOLVAY that used to be a GLobal Player could become a local one as all the assetts outside of Europe would be sold to BASF" (Doc ID 2742). […] also notes that "Customers will also lose "backup solutions" in case supplier's plant in Europe has technical failures, if the Purchaser does not purchase the Solvay PA6.6 EP facilities outside Europe" (Doc ID 2751).
866 Non-confidential minutes of the meeting with […], 25 October 2018, (doc. ID 3065): "[…] also stressed that the lack of global presence of the divestment business was critical. While a global presence does not imply there are PA 6.6 material flows worldwide, customers make specification and purchasing decisions on a global basis. For instance, in the automotive industry, OEMs typically moved from a regional approach to a global platform-based approach even if vehicles differ e.g. in terms of characteristics or place of assembly, depending on the regions."
867 Also see […] spontaneous submission dated 14 December 2018, doc. ID 3136, p. 4.
868 Questionnaire R1 to upstream customers/competitors responses to question 1.10
869 Questionnaire R1 to upstream customers/competitors responses to question 1.7.
870 Questionnaire R3 to PA 6.6 PF customers, responses to question 2.
871 Response of […] to questionnaire R1 of […] to upstream customers/competitors, doc. ID 2920.
872 Response of […] to questionnaire R1 to upstream customers/competitor, doc. ID 2717.
873 Response of […] to questionnaire R1 to upstream customers/competitors, doc. ID 2792.
874 Response of […] to questionnaire R1 to upstream customers/competitors, doc. ID 2924.
875 Response of […] to questionnaire R1 to upstream customers/competitors, doc. ID 2895.
876 Response […] questionnaire R1 to upstream customers/competitors, doc. ID 2799.
877 Response of […] to questionnaire R1 to upstream customers/competitors responses to question 1.2, doc. ID 2924.
878 Response of […] to questionnaire R1 to upstream customers/competitors, doc. ID 2906.
879 […] response to questionnaire R1 to upstream customers/competitors responses to question 1.2, doc. ID 2795.
880 […] response to questionnaire R1 to upstream customers/competitors responses to question 1.2, doc. ID 2673.
881 […] response to questionnaire R1 to upstream customers/competitors responses to question 1.2, doc. ID 2772.
882 […] response to questionnaire R1 to upstream customers/competitors responses to question 1.2, doc. ID 2913.
883 […] response to questionnaire R1 to upstream customers/competitors responses to question 1.2, doc. ID 2684.
884 Responses of […] to questionnaire R1 to upstream customers/competitors, question 1.4, doc. IDs 2880, 2795 and 2772.
885 Responses of […] to questionnaire R1 to upstream customers/competitors, question 1.4, doc. IDs 2880 and 2772.
886 Responses to questionnaire R1 to upstream customers/competitors, question 16.
887 […] response to questionnaire R1 to upstream customers/competitors responses to question 1.3, doc. ID 2913.
888 […] response to questionnaire R1 to upstream customers/competitors responses to question 1.2 and 1.9, doc. ID 2920.
889 […] responses to questionnaire R1 to upstream customers/competitors responses to question 1.3, doc. IDs 2913 and 2673.
890 Non-confidential minutes of a call with […], 12 July 2018, doc. ID 1593 read that "[…] is of the opinion that if the transaction did not go through, Solvay would perhaps move out some capacity from their NTF clients towards engineering plastics clients regardless […]however notes that until now Solvay has supplied NTF fibres clients, which could mean that Solvay still values the NTF fibres sector. However, while Solvay has a strong commitment to engineering plastics, BASF's is much stronger". Also see […] spontaneous submission dated 14 December 2018, doc. ID 3136, p. 5.
891 […] response to questionnaire R2 to PA 6.6 EP customers, response to question 19.1.
892 […], Questionnaire R3 to PA 6.6 PF customers, responses to questions 2.1, 7.1 and 11 (doc. ID 2968 and 2611).
893 All these calculations are made assuming an 85% efficiency rate of the ADN assets.
894 Even without considering the HMD purification capacity at Blanes ([…] kts), the full overlap would be more than removed.
895 Figures in the table are based on pure HMD. This means that the Business’s capacity at Blanes – which can produce pure, but not crude – HMD has been included in the capacity figures for both the Business and the Divestment Business.
896 Blanes HMD plant is a purification which does not process ADN into HMD. Instead, it processes crude HMD into pure HMD.
897 See Section 7.6.1.2.B.
898 These volumes include the contract with […] to supply […] kts per year which will be transferred to the Divestment Business.
899 Blanes plant has a purification nameplate capacity of […] kts. This plant can purify crude HMD into pure HMD which is in turn used to produce AH Salt and subsequently PA 6.6 BP.
900 Estimate provided for the Notifying Party.
901 Figures in the table are based on pure HMD. This means that the Business’s capacity at Blanes – which can produce pure – but not crude – HMD has been included in the capacity figures for both the Business and the Divestment Business.
902 These figures reflect the minimum and maximum quantities provided for in the contract transferred to the Divestment Business for year 2018.
903 Form RM I.B
904 The methodology to compute the quantity of a given upstream product needed to produce 1 kt of the downstream product consists in applying a fix conversion rate between both. The conversion rates used in this Decision are the actual average conversion rates of the Business, which are at the same time very similar to the standard conversion rates of the industry as published in the PCI Yellowbook.
905 The nameplate capacity of the Merged Entity in South Korea is […] kts and in Brazil […] kts. The total real capacity at an effective utilisation rate of […]% would be […] kts approximately. Therefore, […] kts of AH Salt and […] kts of HMD would be required to run these plants at full real capacity.
906 […]
907 […] response to questionnaire R1 to upstream customers/competitors responses to question 1.9, doc. ID 2920.
908 Non-Confidential Minutes of meeting with […] 25 October 2018, 11:00, doc. ID 3065.
909 […].
910 Contract with […] expires on […] (renewal at the sole request of […] until […]. Contract with […] expires on […].
911 The real capacity of the Divestment Business for AH Salt is […] kts approximately.
912 Taking as reference […]% utilisation rate of the nameplate capacity. However, the Notifying Party submits that the real capacity of the Divestment Business is […] kts and the production […] kts.
913 Without taking into consideration the PA 6.6. BP tolling agreement with BASF for which the later would provide raw materials needed.
914 In 2021 the Divestment Business will have […] kts of HMD contracted for the EEA merchant market plus […] kts contracted with […]. While the Divestment Business would have at least circa […] kts of HMD available for the market if it runs the PA 6.6 BP plants at full capacity, given the ADN input provided as part of the Remedies. Moreover, it should be noted that BASF will provide additional volumes of crude HMD to the Divestment Business in the framework of the PA 6.6 BP tolling agreement. The exact duration and volumes will be subject to negotiation between the purchaser of the Divestment Business and BASF.
915 Non-Confidential Minutes of meeting with […] 9 October 2018, 14:00, doc. ID 3059.
916 See Section 7.6.2.1
917 Form RM paragraph 77.
918 Non-Confidential Minutes of meeting with […] 25 October 2018, 11:00, doc. ID 3065.
919 The Commission found internal documents showing BASF's plans […].
920 […] response to questionnaire R1 to upstream customers/competitors, doc. ID 2924; non-confidential minutes of a meeting with […], 25 October 2018, doc. ID. 3065.
921 Anonymous competitor's response to questionnaire R1 to upstream customers/competitors, question 1.4, doc. ID 3061; Anonymous competitor presentation to the EC for the meeting of 8 November 2018, doc. ID 3123.
922 […] response to questionnaire R1 to upstream customers/competitors, doc. ID 2924; Anonymous competitor presentation to the EC for the meeting of 8 November 2018, doc. ID 3123.
923 The capacity included as part of the Divestment Business therefore amounts to more than [70-80]% of BASF's current adipic acid nameplate production capacity, whereas the Divestment Business' captive needs amounts to approx. [60-70]% of BASF's current captive sales.
924 The Notifying Party has confirmed that the contract with […] will effectively be transferred to the purchaser of the Divestment Business (see Form RM, para. 220).
925 See non-confidential version of minutes of a meeting with […], 25 October 2018, doc. ID 3065.
926 Non-confidential minutes of a meeting with […], 25 October 2018, doc. ID 3065.
927 In particular, the Commission understands that BASF is subject to an obligation stemming from its agreement with Invista to take-over Solvay's interest in Butachimie to […]. As a result, BASF is not contractually able to relinquish control over the Chalampé JV without Invista's consent, and forcing such an outcome appears unwarranted to the Commission as a matter of sufficiency and viability – including certainty – of the Commitments.
928 In other words, the Divestment Business will be in a similar situation of interdependency towards third- party customers for the disposal of its adipic acid production.
929 Paragraph 77 of the AA Addendum to the Form RM submitted on 19 October 2018.
930 Form RM submitted on 19 October 2018, table 3.
931 Estimate provided for the Notifying Party.
932 These figures reflect the minimum and maximum quantities provided for in the contract transferred to the Divestment Business.
933 Questionnaire R1 to upstream customers/competitors responses to question 1.8. For example, […]submits that "the remedies proposed are enough to remove the competition concerns. The number of players and their reciprocal capacities into the market place will be the same of today"and […] considers that "the divested capacity is big enough for a new independent supplier", doc. ID 2867
934 Form RM paragraph 83.
935 PA6.6 EP from Belle-Etoile’s Technyl unit is polymerized and compounded continuously from AH Salt. Continuous production implies that PA 6.6 EP is manufactured directly from AH Salt, without any separation step.
936 See Parties Reply to RFI#13, Q 23.
937 Anonymous response to Questionnaire R1 to upstream customers/competitors, responses to question1.5.1 (doc. ID 3061). Also see […] response to questionnaire R1 to upstream customers/competitors, Annex 1 (doc. ID 2924).
938 Only eight research employees of the Business (out of 91) will remain with the Merged Entity. These are employees focusing on polymer business related research which partially remains with the Merged Entity, as well as employees located in Europe with global roles pertaining to business outside of Europe.
939 In this regards, the Commission carries out the assessment based on real capacity of the plants and not on nameplate capacity, which is theoretical.
940 […] responses to questionnaire R1 to upstream customers/competitors, questions 1.11, 2.1 and 3.1. […] states that "Blanes site is very far away and it is not anymore downstream integrated" (doc. ID 2867). […] states for instance that "Scattered assets, little internal synergies (prima facie)" negatively impact the ability of the Divestment Business to compete effectively (doc. ID 2772). […] also states that "The final scenario will have in fact BASF free of the need to run multiple location, and happy to achieve (with limited investments in AH Salt and in Polymerization Capacity) in its own manufacturing location a more efficient manufacturing platform. Perhaps, the shutdown of the Blanes, Gorzow (And maybe even Belle Etoile) sites was in BASF Business Plan since the very beginning and what was exactly their plan, is now presented as a ....."remedy"."
941 See […] response to questionnaire R2 to PA 6.6 EP customers, response to question 19.1 (doc. ID 2751). […] states that "The PA6.6 EP assets to form part of the divestment commitments are estimated to be old". Also see […] response to questionnaire R1 to upstream customers/competitors, questions 2.1 and 4.1 (doc. ID 2772). […] states for instance that due to "Scattered plants - lack of integration, small scale, old sites with ageing premises, negative economy of scale higher logistic costs, Dismembered management and IT" "Restructuring will be complicated and costly" and further states that "Old and scattered plants, with potential liabilities in labour, soil contamination, little logistic sustainability" raise uncertainties as to the ability of the Divestment Business to be competitive.
942 […].
943 […] reply to Questionnaire R1 to upstream customers/competitors responses to question 4, doc. ID 2924.
944 […] reply to Questionnaire R1 to upstream customers/competitors responses to question 4, doc. ID 2795.
945 […] reply to Questionnaire R1 to upstream customers/competitors responses to question 10.1, doc. ID 2920.
946 […] reply to Questionnaire R1 to upstream customers/competitors responses to question 10.1, doc. ID 2795.
947 Annex 3 to the Commitments.
948 Anonymous competitor replies to Questionnaire R1 to upstream customers/competitors responses to question 11.1, doc. ID 3061.