Commission, April 22, 2020, No M.9517
EUROPEAN COMMISSION
Decision
MYLAN / UPJOHN
Subject: Case M.9517 – Mylan/Upjohn
Commission decision pursuant to Article 6(1)(b) in conjunction with Article 6(2) of Council Regulation No 139/20041 and Article 57 of the Agreement on the European Economic Area2
Dear Sir or Madam,
(1) On 28 February 2020, the European Commission (the “Commission”) received notification of a proposed concentration pursuant to Article 4 of the Merger Regulation by which Mylan N.V. (“Mylan”, the Netherlands) and Upjohn, a business division of Pfizer Inc. (“Pfizer”, the United States of America) intend to merge (the “Transaction”).3 Mylan and Upjohn are designated hereinafter as the “Notifying Parties” or “Parties” to the Transaction.
1. THE PARTIES
(2) Mylan is a publicly listed global pharmaceutical company, which develops, licenses, manufactures, markets, and distributes (i) generic, (ii) branded generic and (iii) specialty pharmaceuticals. Mylan offers a broad product portfolio of medicines, including more than 1 500 products (generics, branded generics, prescription, and non-prescription). Mylan has a vertically integrated global supply chain that includes over 40 manufacturing facilities.
(3) Upjohn is a global business division of Pfizer. It operates a portfolio of 20 off-patent branded and generic molecules under 21 brands in five therapeutic areas: (i) cardiovascular, (ii) central nervous system/psychiatry, (iii) pain/neurology, (iv) urology, and (v) ophthalmology. In addition, Upjohn includes the generic business of Greenstone LLC, a generic business exclusively active in the United States of America.
2. THE OPERATION
(4) On 29 July 2019, the Parties and Pfizer entered into a business combination agreement pursuant to which the businesses of the Parties will be combined. The Transaction will take place in three steps.(a) First, Pfizer will contribute and transfer Upjohn’s assets and liabilities to Spinco, a special-purpose vehicle wholly owned by Pfizer.(b) Second, Pfizer will distribute Spinco’s common stock to its stockholders.4(c) Third, Spinco and Mylan will combine, by a merger or an asset sale, resulting in the transfer of Mylan’s assets and liabilities to Spinco.
(5) Upon completion of the Transaction, the merged entity (comprising Upjohn and Mylan) will be wholly owned by Spinco, which will be renamed “Viatris”. Former Mylan shareholders will hold 43% and Pfizer’s shareholders will hold 57% of Viatris. None of the individual shareholders of Mylan or Pfizer will exercise control over Viatris, which will be an independent undertaking.
(6) The Transaction therefore constitutes a merger between Mylan and Upjohn within the meaning of Article 3(1)(a) of the Merger Regulation.
3. UNION DIMENSION
(7) The undertakings concerned have a combined aggregate worldwide turnover of more than EUR 5 000 million (Mylan: EUR 9 551 million; Upjohn: EUR 10 582 million).5 Each of them has a Union-wide turnover in excess of EUR 250 million (Mylan: EUR […] million; Upjohn: EUR […] million), but each does not achieve more than two- thirds of its aggregate Union-wide turnover within one and the same Member State. The notified operation therefore has a Union dimension.
4. OVERVIEW OF THE OVERLAPS AND VERTICAL RELATIONSHIPS
(8) The Transaction will combine one of the top five generic suppliers in the EEA6 (Mylan) with an originator,7 whose products were the first launched on the market for a specific molecule but that have lost exclusivity following patent expiries (Upjohn).
(9) The Transaction gives rise to horizontally affected markets in the supply of finished dose pharmaceuticals (“FDPs”), which are assessed in Section 5 of this Decision.8
(10) In addition, the Transaction leads to vertically affected markets for (i) the supply of active pharmaceutical ingredients (“APIs”), upstream, and the supply of FDPs, downstream, and (ii) the outlicensing of rights to FDPs, upstream and the supply of FDPs, downstream. These vertical links are respectively assessed in Sections 6 (regarding the supply of APIs) and 7 (regarding the outlicensing of rights to FDPs) of this Decision.9
5. FINISHED DOSE PHARMACEUTICALS
(11) FDPs are pharmaceutical products that have undergone all stages of production (including packaging in the final container and labelling). They are the final pharmaceutical products received by pharmacies and other healthcare professionals, and ready to use by patients.
(12) Both Parties supply genericized FDPs in the EEA, but have a slightly different business focus. While Mylan offers mostly unbranded generics (among its offering of more than 1 500 FDPs), Upjohn is an originator supplier, whose portfolio comprises 21 brands based on 20 molecules that were the first launched on the market for a specific molecule but most of which lost exclusivity following patent expiry more than five years ago.
5.1. Market definition
5.1.1. Product market definition
5.1.1.1. Product market definition in the pharmaceutical sector
(13) FDPs may be subdivided into therapeutic classes by reference to the Anatomical Therapeutic Classification (“ATC”), devised by the European Pharmaceutical Marketing Research Association (“EphMRA”) and maintained by EphMRA and IQVIA, formerly known as Intercontinental Medical Statistics (“IMS”).
(14) The ATC system is a hierarchical and coded four-level system, which classifies medicinal products according to their indication, therapeutic use, composition, and mode of action. In the first and broadest level (ATC1), medicinal products are divided into the 16 anatomical main groups. The second level (ATC2) is either a pharmacological or a therapeutic group. The third level (ATC3) further groups medicinal products by their specific therapeutic indications. Finally, the ATC4 level is generally the most detailed one (not available for all ATC3 classes) and refers for instance to the mode of action or any other subdivision of the relevant products.
(15) When defining relevant markets in past decisions dealing with FDPs, the Commission often referred to the third level (ATC3) as the starting point for defining the relevant product market.10 However, in a number of cases, the Commission found that the ATC3 level classification did not yield the appropriate market definition within the meaning of the Commission Notice on the Definition of the Relevant Market.11
(16) In decisions involving genericized FDP markets, the Commission generally defined the relevant product market at the level of the relevant molecule (i.e. based on the same active pharmaceutical ingredient) or group of molecules (for instance all benzodiazepines or all anticholinergics)12 that are considered interchangeable.13
(17) In previous decisions, the Commission found that, at molecule level, the originator (i.e. the first product that was launched on the market for a specific molecule) and generics (i.e. products that were launched after the originator’s loss of exclusivity)14 generally form part of the same market. This is because generics are versions of originator medicines, which are specifically designed to compete with those medicines and normally represent the closest substitute to them.15
(18) The Commission has acknowledged in previous decisions that additional segmentations may also apply.16 FDPs may be differentiated not only by their active ingredient(s), but also by galenic form and route of administration, which may limit their substitutability. The Commission also considered separate markets for FDPs, which can be dispensed only against a prescription and those which can be sold over the counter (or "OTC").17
5.1.1.2. The Notifying Parties' views
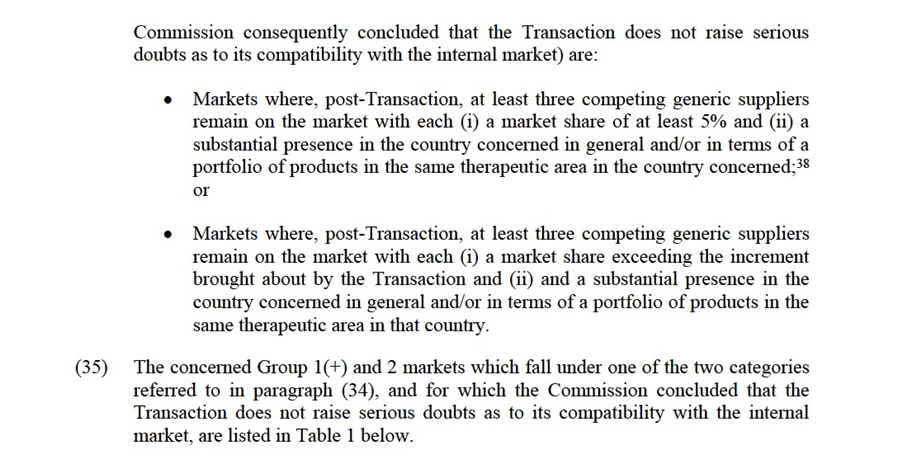
(19) The Notifying Parties did not provide any indications that the Commission should depart from the more recent approach to define the product market for genericized FDPs at the level of molecules within the same ATC3 class, and considered all alternative market definitions (at ATC3, ATC4, and multi-molecule levels, based on galenic form, as well as between prescription and OTC products) in line with applicable precedents.18
5.1.1.3. The Commission's assessment
(20) For the purpose of this Decision, the Commission considers that the relevant product markets for FDPs should be defined at molecule level.
(21) In the present case, the responses to the market investigation suggest that, for the molecules involved in this case, different genericized molecules, including from the same ATC3 class, do not form part of the same product market, in particular since they are not interchangeable for patients and pharmacies,19 and their price-setting modalities differ.20 Relatedly, respondents to the market investigation also indicated that certain genericized molecules belonging to wider groups of molecules with the same or similar mode of actions, such benzodiazepines, are generally not interchangeable.21 However, with regard to anticholinergics specifically, while the majority of respondents also consider that different molecules generally do not form part of the same product market, 22 the Commission notes that the answers received in the market investigation to this question were mixed regarding the Lithuanian market.23 These elements indicate that, with the possible exception of anticholinergics,24 the molecules offered by the Parties and analysed in the present case each form a separate product market.
(22) Respondents to the market investigation also took the view that, for the same genericized molecule, originator and generics form part of the same market, as they are generally perceived as substitutes to each other and interchangeable.25 This indicates that, concerning the products offered by the Parties and therefore analysed in the present case, products based on the same molecule(s) that fall within the same therapeutic indication (namely within a same ATC3 class) belong to the same product market.
(23) The market investigation was inconclusive regarding whether products with different galenic forms are substitutable.26 A large majority of responding pharmacies indicated that different form factors might not be interchangeable to treat the same symptoms or illness, especially for nervous system treatments,27 while an overwhelming majority of competitors consider that different galenic forms do compete with each other.28 The Commission notes that the Commission’s assessment does not significantly differ in the present case should the relevant product markets be sub-segmented at the galenic form level or comprise all galenic forms. Moreover, no additional Group 1(+) or 2 markets arise from the Transaction if the relevant product markets were defined based on different galenic forms, except where this is explicitly mentioned in Section 5, and in none of those cases the competitive assessment changes. Therefore, the question of whether the relevant molecule markets should be further segmented based on the galenic form of FDPs can be left open, as it has no impact on the competitive assessment of the Transaction.
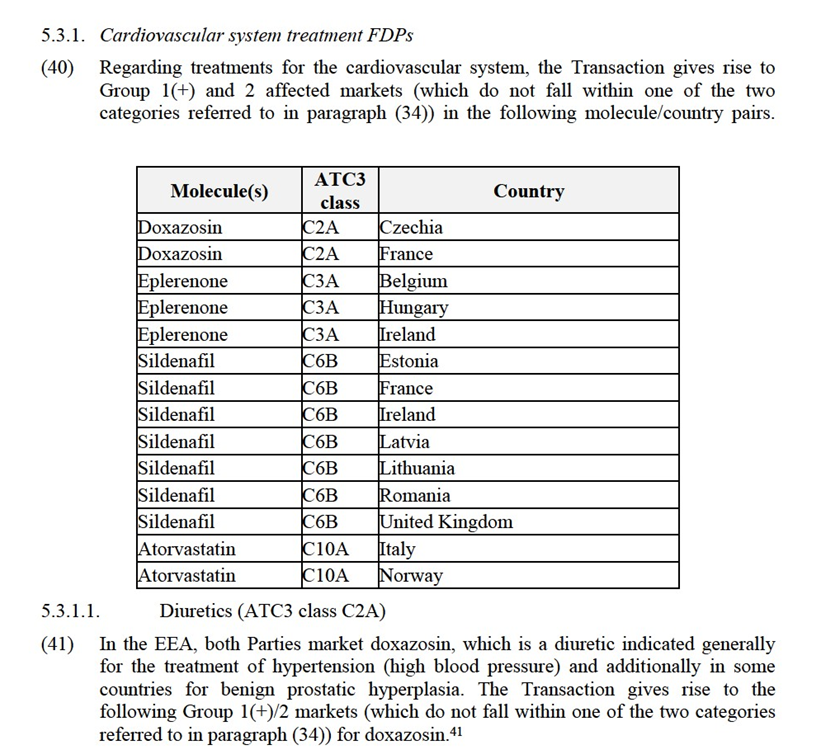
(24) In summary, based on the results of the market investigation and any other evidence available to it, the Commission has no reason to depart from its decisional precedents in the area of genericized FDPs (see paras. 15 to 18 above), and concludes that the relevant product markets should be defined at molecule level, while the question of the sub-segmentation based on the galenic form can be left open.
5.1.2. Geographic market definition
(25) The Commission has consistently defined the geographic markets for FDPs as being national in scope.29
(26) The Notifying Parties, in line with the Commission’s decisional practice, provided market share data at national level for FDP overlaps.
(27) The market investigation in this case confirmed the national dimension of the markets for FDPs, in particular in view of the differing national regulatory and reimbursement schemes, and the fact that competition between pharmaceutical suppliers still predominantly takes place at a national level.30
(28) Therefore, for the purpose of this Decision, the Commission considers that the geographic scope of all relevant FDP product markets is national.
5.2. Methodology for the identification and the assessment of affected markets
(29) In line with Commission precedents,31 the Notifying Parties primarily used sales data of pharmaceutical products compiled by IQVIA to identify the affected markets that the Transaction gives rise to.32
(30) In addition, given a large number of affected markets in pharmaceutical mergers (involving numerous product and geographic markets), the Commission has applied a system of filters aimed at determining the group of markets where concerns are most likely and on which it focuses its analysis. In line with Commission precedents in the pharmaceutical sector,33 affected markets can be classified in four categories- Group 1, where the Parties' combined market share exceeds 35% and the increment exceeds 1%;- Group 1+, where either (i) the combined market share is below 35% (but above 20%), and only one other competitor remains on the market, or where(ii) the combined market share exceeds 35% and the increment is below 1%, but the party with the small increment is a recent entrant.34- Group 2, where the Parties' combined market share exceeds 35% but the increment is below 1%; and- Group 3, where the Parties' combined market share is between 20% and 35%.
(31) The Commission has analysed all markets affected by the Transaction. Regarding Group 1(+) markets (comprising Group 1 and Group 1+ markets), the Commission assessed the markets under the narrowest plausible market definition, namely at the molecule level (with potential sub-segmentation by galenic form where relevant). Depending on the results of the market investigation on the scope of the relevant market in relation to these molecules, the Commission also assessed these markets at "multi-molecule" level (namely a combination of potentially interchangeable molecules within the same ATC4 or ATC3 class).
(32) The Commission's assessment focused primarily on volume-based market shares. As generics are in general less expensive than the originator drugs, the competitive interactions between Mylan (and other generic players) and Upjohn, are more accurately reflected in volume-based market shares. In addition, the products concerned by the Transaction have typically been genericised over five years ago, thus mitigating any first-mover advantage that a specific generic player may have in particular on pricing. Under these circumstances, the competitive pressure that generics exert on the originator largely depends on the volumes that they can divert from the originator, and the competitive position of each company is therefore better captured with volume market shares. However, the Commission also reviewed the value-based market shares of the Parties and their competitors for the affected markets. In the present case, the combined value-based market share of the Parties is generally higher than their volume-based combined market share, as the former originator product of Upjohn is generally sold at a higher price point than its generic competitors. In any event, the Commission notes that the competitive assessment does not differ substantially when considering value market shares for any affected market, in particular because the number of competitors and their ability to exert a credible competitive pressure on the merged entity does not change.
5.3. Competitive assessment
(33) In line with precedents,35 Group 3 markets are not discussed individually in this Decision.36 The Commission assessed the competitive situation in these markets by considering the combined market shares of the Parties and their competitors over the last three years, other factors including the presence of competitors with a significant presence in the generics markets, the date of patent expiry, the recent evolution of prices, the level of complexity of the Parties’ products,37 the Parties’ pipeline products, as well as the results of the market investigation. The Commission reached the conclusion that the Transaction does not raise serious doubts as to its compatibility with the internal market in relation to the Group 3 markets arising from the Transaction, due to the limited market shares of the Parties and the presence of significant competitors remaining on the market post-Transaction that will likely sufficiently constrain the merged entity.
(34) The Commission has assessed all Group 1(+) and 2 markets individually, but does not discuss individually in this Decision the Group 1(+) and 2 markets which fall within one of the two following sets of criteria. These markets (for which the

(36) In these markets, the Parties' combined market share generally remains below [50- 60]%,39 under any plausible market definition.40 In this case, which involves a merger between an originator and a generic company, high market shares alone do not equate to market power. Genericised FDPs are typically heavily regulated, which limit the opportunity to engage in price increases and favours the entry of new and lower cost generic players. In addition, Mylan, as a generic player, competes more closely with other generic players. If a sufficient number of credible competitors remain in the market, combining the market shares of Mylan and Upjohn thus does not automatically translate into increased market power.
(37) Moreover, the overwhelming majority of respondents to the market investigation considered that the Transaction would have a neutral or positive impact on these markets.
(38) In light of the foregoing, the Commission finds that the Transaction does not raise serious doubts as to its compatibility with the internal market in relation to the markets listed in Table 1.
(39) In the following, the Commission individually assesses all Group 1(+) and Group 2 markets, which do not fall under one of the two categories referred to in paragraph (34). For the reasons detailed in paragraph (32) above, the Commission relied as a starting point on volume-based market shares to assess the competitive dynamics of the affected markets. However, as noted above, the Commission considered the value market shares for the markets affected by the Transaction and notes that the competitive assessment does not differ substantially when considering value market shares for any affected market.


Commission’s assessment
(47) The combined market share of the Parties at molecule level amounts to nearly [40- 50]% in 2018. In addition, only one significant competitor would remain post- Transaction, namely Zentiva with a volume-based market share of [40-50]% in 2018 and hence, the combined entity would face limited competitive constraints post- Transaction.
(48) Moreover, if the market was further segmented based on galenic form, the Parties are the only two manufacturers supplying extended release tablets of doxazosin in Czechia. Therefore, the Transaction would lead to a monopoly if a market segmented by galenic form at molecule level were considered.
Conclusion
(49) In view of the above considerations, taking into account the market investigation and all the evidence available to it, the Commission concludes that the Transaction raises serious doubts as to its compatibility with the internal market due to its likely horizontal non-coordinated effects (i) in the market for doxazosin in Czechia given the high combined market share post-Transaction and the reduced number of significant competitors in the market, as well as (ii) in a possible market for extended release tablets of doxazosin in Czechia given that no competitor would remain on this possible market.
(b) Doxazosin in France
(50) Both Parties supply doxazosin (ATC3 class C2A) in France. This molecule is genericized in France since 2009. While Upjohn markets doxazosin under the brand name Zoxan, Mylan supplies an unbranded version of doxazosin.
Market shares
(51) A Group 1 market arises at the molecule level for doxazosin in France.44
(52) The volume market shares of the Parties and their competitors for the supply of doxazosin in France are provided below in Table 3.




Notifying Parties’ views
(63) The Parties did not submit any views in relation to this market. To expedite the clearance of the Transaction, the Parties offered to divest Mylan’s eplerenone product in Belgium to a suitable purchaser.47
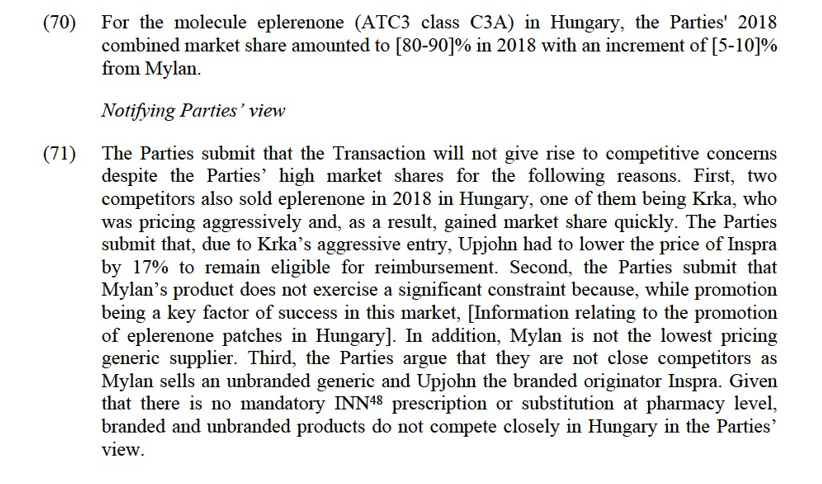
Commission’s assessment
(64) The combined market share of the Parties is [90-100]% at molecule level, with a significant increment from Upjohn (of [20-30]% based on 2018 figures). As a result, no competitor would remain post-Transaction and the Transaction would therefore lead to a monopoly for the supply of eplerenone in Belgium.
(65) The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the merged entity would have a monopoly on the market post-Transaction and there would be no competitor present.
Conclusion
(66) In view of the above considerations, taking into account the market investigation and all the evidence available to it, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market as regards the supply of eplerenone in Belgium due to its likely horizontal non-coordinated effects in the market for eplerenone in Belgium given the high combined market share and the fact that no competitors will remain on the market post-Transaction.
(b) Eplerenone in Hungary
(67) Both Parties supply eplerenone (ATC3 class C3A) in Hungary. This molecule is genericized in Hungary since 2010. In this country, while Upjohn markets eplerenone under the brand name Inspra, Mylan sells an unbranded version of eplerenone.
Market shares
(68) The Transaction gives rise to a Group 1 market at the molecule level for eplerenone in Hungary.

Commission’s assessment
(72) Structurally, the Transaction leads to an important change in the market. Post- Transaction, the merged entity’s market share would be very high (reaching [80- 90]% based on 2018 figures) with a material increment from Mylan (of [5-10]% based on 2018 figures). Such market shares may in themselves be indicative of a dominant position of the merged entity post-Transaction.49
(73) In addition, the market investigation conducted by the Commission does not fully support the Notifying Parties’ arguments.50
(74) First, the Transaction will result in a reduction in the number of players from four to three. Between the two other players active in the supply of eplerenone in Hungary, only one competitor has a significant presence in Hungary overall and in diuretics (ATC2 class C3) more specifically, namely Krka, which is a recent entrant with a market share remaining below [0-5]% in 2018. Alvogen, the only other supplier of eplerenone in Hungary, has consistently had a higher market share than Mylan in the last 3 years. However, this player does not have a strong presence in Hungary in general, nor in the therapeutic area (ATC2 level). As a result, the constraints exercised by competitors on the merged entity for the supply of eplerenone in Hungary are likely to remain limited post-Transaction.
(75) Second, the Parties’ claim that generics/unbranded products do not compete closely with originators/branded products was not supported by the market investigation. Replies to the market investigation were inconclusive as to whether originator and generic products are considered interchangeable.51 In addition, the Hungarian national authority states that pharmacies have incentives to substitute originator products with generics.52
(76) In addition, wholesalers in Hungary indicated that they carry both the originator and generic versions of genericized molecule, and try to offer as many generics as possible,53 evidencing as well the importance of a sufficient number of generic suppliers to maintain effective competition.

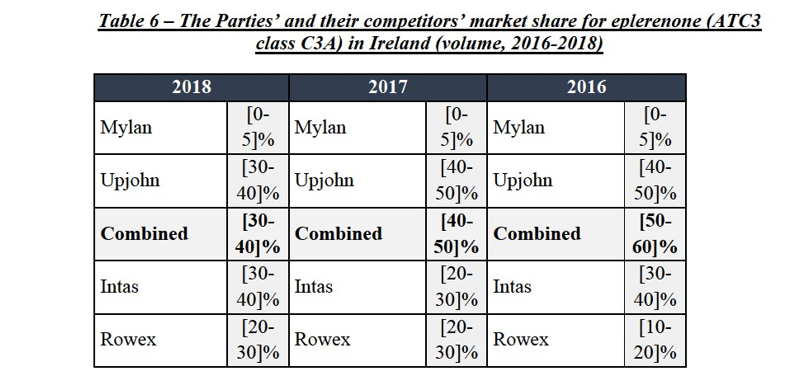

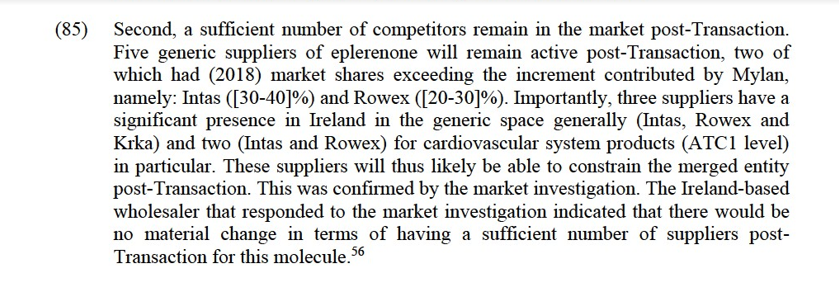
(86) Mylan’s limited increment and the constraints exercised by competitors suggest that the Transaction will not alter significantly the structure of the market for the supply of eplerenone in Ireland. This was confirmed by the market investigation. The majority of respondents consider that the Transaction will not have a negative impact on the market for eplerenone in Ireland, either on prices or on product availability.57
(87) Finally, the results of the market investigation did not reveal any substantiated concerns as regards the impact of the Transaction in the eplerenone market in Ireland.
Conclusion
(88) In view of the above considerations, taking into account the market investigation and all the evidence available to it, the Commission considers that the Transaction does not raise serious doubts as to its compatibility with the internal market with respect to the supply of eplerenone in Ireland.
5.3.1.3. Pulmonary arterial hypertension (ATC3 class C6B)
(89) In the EEA, both Parties market sildenafil, which is a so-called PDE-5 inhibitor. This molecule is used to treat pulmonary arterial hypertension (“PAH”) and is indicated for the prevention of cardiovascular disease in adults and for the treatment of hyperlipidemia. The Transaction gives rise to the following Group 1(+)/2 markets (which do not fall within one of the two categories referred to in paragraph (34)) for sildenafil (PAH).
(a) Sildenafil in Estonia
(90) Both Parties supply sildenafil for the treatment of PAH (ATC3 class C6B) in Estonia.58
(91) This molecule is genericized in Estonia since 2015 for the treatment of PAH. Both Parties market a branded version of sildenafil for the treatment of PAH in Estonia. While Upjohn markets sildenafil (C6B) under the brand name Revatio, Mylan markets this product under the name Mysildecard.
Market shares
(92) The Transaction gives rise to a Group 1+ market at the molecule level for the supply of sildenafil (C6B) in Estonia.




Notifying Parties’ views
(103) The Parties did not submit any views in relation to this market. To expedite the clearance of the Transaction, the Parties offered to divest Mylan’s sildenafil product (C6B) in France to a suitable purchaser.60
Commission’s assessment
(104) Structurally, for the supply of sildenafil (C6B) in France, the combined market share of the Parties is extremely high (namely [90-100]%). Besides the Parties, no other competitor is active for the supply of sildenafil (C6B) in France and thus no competitor would remain post-Transaction on the market. Therefore, the Transaction would lead to a monopoly for the supply of sildenafil (C6B) in France.
(105) The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the merged entity would have a monopoly on the market post-Transaction and there would be no competitor present.
Conclusion
(106) In view of the above considerations, taking into account the market investigation and all the evidence available to it, the Commission concludes that the Transaction raises serious doubts as to its compatibility with the internal market as regards the supply of sildenafil for the treatment of PAH (ATC3 class C6B) in France, due to its horizontal non-coordinated effects in the market for sildenafil in France given the high combined market share and the fact that no competitors will remain in the market post-Transaction.
(c) Sildenafil in Ireland
(107) Both Parties supply sildenafil (ATC3 class C6B) in Ireland for the treatment of PAH. This molecule is genericized in Ireland since 2015 for the treatment of PAH. In this country, both Parties market a branded version of sildenafil (C6B) under the brand names Revatio concerning Upjohn and Mysildecard concerning Mylan.
Market shares
(108) The Transaction gives rise to a Group 1+ market at the molecule level for sildenafil (C6B) in Ireland.


[5-10]% and [5-10]% respectively) and have a strong presence in the therapeutic area as well (namely a market share of [5-10]% and [5-10]% respectively at ATC2 level in Ireland).
(114) In addition, the results of the market investigation indicate that there will remain a sufficient number of suppliers of sildenafil (C6B) in Ireland. All responding Irish wholesalers indicated that a sufficient number of suppliers of sildenafil (C6B) would remain in the market post-Transaction.61
(115) Finally, the results of the market investigation did not reveal any substantiated concerns as regards the impact of the Transaction regarding the supply of sildenafil (C6B) in Ireland.
Conclusion
(116) In view of the above considerations, taking into account the market investigation and all the evidence available to it, the Commission considers that the Transaction does not raise serious doubts as to its compatibility with the internal market with respect to the supply of sildenafil for the treatment of PAH (C6B) in Ireland.
(d) Sildenafil in Latvia
(117) Both Parties supply sildenafil (ATC3 class C6B) in Latvia for the treatment of PAH. This molecule is genericized in Latvia since 2015 for this therapeutic indication. Both Parties market a branded version of sildenafil for the treatment of PAH in Latvia. While Upjohn markets sildenafil (C6B) under the brand name Revatio, Mylan markets this product under the name Mysildecard.
Market shares
(118) The Transaction gives rise to a Group 1 market at the molecule level for the supply of sildenafil (C6B) in Latvia.
(119) The volume market shares of the Parties and their competitors for the supply of sildenafil in Latvia are provided below in Table 10




Commission’s assessment
(130) Structurally, the combined market share of the Parties is very high at molecule level ([90-100]% in 2018), with a significant market share from Upjohn ([90-100 ]% in 2018), and Mylan’s increment is small (below [0-5]% in 2018). Mylan is, however, a recent entrant, which started supplying sildenafil for PAH in Lithuania in 2017. In addition, only two competitors are active in the supply of this product in Lithuania, namely Teva and Intas, one of which (Intas) does not rank among the top 10 suppliers of generics in Lithuania. As a result, the Transaction would reduce the number of players from four to three. These elements are indicative of a likely dominant position of Upjohn pre-Transaction, which would be further strengthened by the Transaction.
(131) The market investigation did not provide any elements to dispel the serious doubts arising from the fact that Upjohn would likely have a dominant position pre- Transaction, which would be further strengthened by the Transaction and that the merged entity would only face limited competition post-Transaction.
Conclusion
(132) In view of the above considerations, taking into account the market investigation and all the evidence available to it, the Commission concludes that the Transaction raises serious doubts as to its compatibility with the internal market as regards the supply of sildenafil in Lithuania for the treatment of PAH (ATC3 class C6B), due to its likely horizontal non-coordinated effects, in particular given the high combined market share and the limited number of significant suppliers.
(f) Sildenafil in Romania
(133) Both Parties supply sildenafil (ATC3 class C6B) in Romania for the treatment of PAH. This molecule is genericized in Romania since 2015 for this therapeutic indication. Both Parties market a branded version of sildenafil for the treatment of PAH in Romania. While Upjohn markets sildenafil (C6B) under the brand name Revatio, Mylan markets this product under the name Mysildecard.
Market shares
(134) The Transaction gives rise to a Group 1 market at the molecule level for the supply of sildenafil in Romania.
(135) The volume market shares of the Parties and their competitors for the supply of sildenafil in Romania are provided below in Table 12.




Notifying Parties’ views
(145) The Parties did not submit any views in relation to this market. To expedite the clearance of the Transaction, the Parties offered to divest Mylan’s sildenafil product (C6B) in the United Kingdom to a suitable purchaser.66
Commission’s assessment
(146) Structurally, the combined market share of the Parties is very high at molecule level ([80-90]% in 2018), with a significant market share from Upjohn ([50-60]% in 2018), and Mylan’s increment is also high (namely [30-40]% in 2018). These elements are indicative of a likely dominant position of Upjohn pre-Transaction, which would be further strengthened by the Transaction.
(147) The market investigation did not provide any elements to dispel the serious doubts arising from the fact Upjohn would likely have a dominant position pre-Transaction, which would be further strengthened by the Transaction and that the merged entity would only face limited competition post-Transaction. To the contrary, three wholesalers based in the United Kingdom believe that, for the supply of sildenafil, the Transaction will have a negative impact on prices or product availability in the United Kingdom.67
Conclusion
(148) In view of the above considerations, taking into account the market investigation and all the evidence available to it, the Commission concludes that the Transaction raises serious doubts as to its compatibility with the internal market as regards the supply of sildenafil in the United Kingdom for the treatment of PAH (ATC3 class C6B), due to horizontal non-coordinated effects, in particular given the high combined market share and the limited number of significant suppliers.
5.3.1.4. Cholesterol and Triglyceride preparations (ATC3 class C10A)
(149) In the EEA, both Parties market atorvastatin, which is a molecule indicated for the prevention of cardiovascular disease in adults and for the treatment of hyperlipidemia. The Transaction gives rise to the following Group 1(+)/2 markets (which do not fall within one of the two categories referred to in paragraph (34)) for atorvastatin.
(a) Atorvastatin in Italy
(150) Both Parties supply atorvastatin (ATC3 class C10A) in Italy. This molecule is genericized in Italy since 2012. In this country, Upjohn markets a branded version of

Commission’s assessment
(155) Structurally, the Parties’ combined market share is high, but remained below [50- 60]% in the past three years. Mylan’s increment amounted to [5-10]% in 2018, and remained below [10-20]% in 2016 and 2017.
(156) In addition, a high number of players, namely nineteen, will remain active in the supply of atorvastatin in Italy post-Transaction. Importantly, two of the Parties’ competitors, Menarini and Teva, have a significant presence in this market. Menarini and Teva’s market share exceeded [5-10]% in the past three years ([20-30]% and [5- 10]% respectively in 2018). In addition, both Menarini and Teva have a significant presence at the level of the ATC1 class to which atorvastatin belongs.68 An additional player, Doc Generici, which ranks among the top 10 suppliers of generics in Italy, is also active in the supply of atorvastatin in Italy, although with a market share slightly below [5-10]% ([0-5]% in 2018, but with a small increase in the past three years). These competing suppliers will thus likely be able to constrain the merged entity post-Transaction. This was confirmed by the market investigation. The Italy-based customers that responded to the market investigation indicated that there would be no material change in terms of having a sufficient number of suppliers post-Transaction for this molecule.69
(157) Finally, the results of the market investigation did not reveal any substantiated concerns as regards the impact of the Transaction in the atorvastatin market in Italy. None of the respondents to the market investigation considers that the Transaction will have a negative impact on the market, either on prices or on product availability.70
Conclusion
(158) In view of the above considerations, taking into account the market investigation and all the evidence available to it, the Commission considers that the Transaction does not raise serious doubts as to its compatibility with the internal market with respect to the supply of atorvastatin in Italy.
(b) Atorvastatin in Norway
(159) Both Parties supply atorvastatin (ATC3 class C10A) in Norway. This molecule is genericized in Norway since 2002. In this country, while Upjohn markets a branded






Commission’s assessment
(175) Post-Transaction, the Parties will face competition from a number of established generic manufacturers, with a significant market presence in Austria (Sanofi is among the top 10 competitors in generics in Austria), with a significant presence in urinary incontinence products (Montavit has a market share of [5-10]% at ATC2 level), as well as Astellas Pharma (market share of [10-20]% in anticholinergics). Furthermore, Upjohn faces competition from two additional competitors (namely Intas with a market share of [5-10]% and Easypharm Generika with a market share of [5-10]%) at the molecule level (tolterodine).
(176) Furthermore, the market investigation confirmed the argument of the Parties that the Parties’ products are not close substitutes, as the Parties do not market the same genericized molecule.72.
(177) In addition, the results of the market investigation indicate that there will remain a sufficient number of suppliers of anticholinergics in Austria. All responding Austrian wholesalers indicated that a sufficient number of suppliers of anticholinergics would remain on the market post-Transaction.73
(178) Finally, the results of the market investigation did not reveal any substantiated concerns as regards the impact of the Transaction in the anticholinergics market in Austria.
Conclusion
(179) In view of the above considerations, taking into account the market investigation and all the evidence available to it, the Commission considers that the Transaction does not raise serious doubts as to its compatibility with the internal market with respect to the supply of anticholinergics in Austria.
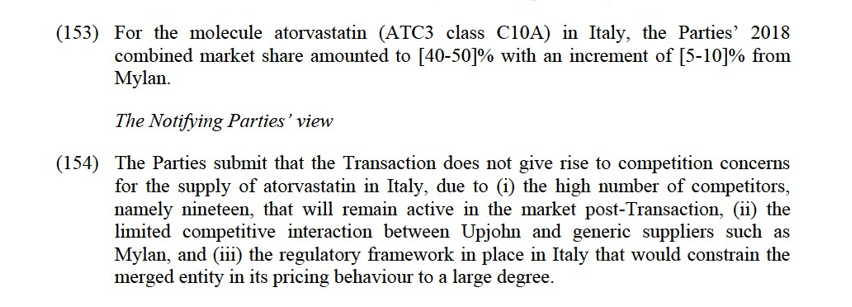
(b) Anticholinergic agents in Lithuania
(180) The Transaction does not give rise to Group 1(+) or 2 market at molecule level for the treatment of urinary incontinence (ATC3 G4D) in Lithuania. However, the Transaction gives rise to a Group 1 market if the relevant market were defined at the multi-molecule level for the supply of anticholinergics in Lithuania.
(181) With regard to anticholinergics in Lithuania, Upjohn sells the molecule tolterodine, under the brand name Detrusitol, while Mylan markets a branded version of oxybutynin, under the brand name Driptane.


respectively [30-40]% and [10-20]%). Respondents to the market investigation based in Lithuania indicated that these two companies are among the ten leading suppliers of generics in Lithuania.74 This indicates that Zentiva and Teva could exert constraints on the behaviour of the merged entity, including in terms of pricing.
(187) Second, the market investigation confirmed the argument of the Parties that the Parties’ products are not close substitutes, as the Parties do not market the same genericized molecule.75
(188) In addition, the results of the market investigation indicate that there will remain a sufficient number of suppliers of anticholinergics in Lithuania. All responding Lithuanian wholesalers indicated that a sufficient number of suppliers of anticholinergics would remain on the market post-Transaction.76
(189) Finally, the results of the market investigation did not reveal any substantiated concerns as regards the impact of the Transaction in the anticholinergics market in Lithuania.
Conclusion
(190) In view of the above considerations, taking into account the market investigation and all the evidence available to it, the Commission considers that the Transaction does not raise serious doubts as to its compatibility with the internal market with respect to the supply of anticholinergics in Lithuania.
5.3.3. Musculoskeletal system treatment FDPs
(191) Regarding treatments for the musculoskeletal system, the Transaction also gives rise to a Group 1 affected market (which does not fall within one of the two categories referred to in paragraph (34)) in the market for the supply of celecoxib in Italy.
(192) In the EEA, both Parties market celecoxib, which is a molecule indicated for the treatment of musculoskeletal inflammation. A Group 1 affected market arises in Italy.
(193) Both Parties supply celecoxib (ATC3 class M1A) in Italy. This molecule is genericized in Italy since 2014. In this country, Upjohn sells both an unbranded and a branded version (Celebrex) of celecoxib, and Mylan supplies an unbranded version of celecoxib.

(199) Second, a sufficient number of competitors remain in the market post-Transaction. 11 generic suppliers of celecoxib will remain active post-Transaction, five of which had market shares exceeding the increment contributed by Mylan, namely: S.F. Group ([5-10]%), Teva ([5-10]%), Doc Generici ([0-5]%), Novartis ([0-5]%) and Stada ([0-5]%). Importantly, these five suppliers have a significant presence in Italy in the generic space. These five suppliers will thus likely be able to constrain the merged entity post-Transaction. Another six competitors offer the same molecule. Therefore, a sufficient number of competitors will remain in the market post- Transaction. This was confirmed by the market investigation. Indeed, Italian-based wholesalers indicated unanimously that they would have a sufficient number of suppliers post-Transaction for this molecule.77
(200) In addition, the presence of five suppliers with market shares larger than Mylan suggests that the Transaction will not alter significantly the structure of supply and will not lead to a negative impact in terms of product availability or frequency of price reductions. This was confirmed by the market investigation. Respondents to the market investigation unanimously confirmed that the Transaction would not have a negative impact on the market for celecoxib in Italy, either on prices or on product availability.78
(201) Third, the market investigation did not indicate that the Transaction could meaningfully alter the competitive dynamics in the market. S.F. Group, the second largest supplier of celecoxib in Italy following Upjohn (in terms of market shares), is the only competing supplier besides Upjohn offering branded celecoxib in Italy. All other suppliers, including Mylan, offer undifferentiated generic products.79
(202) Finally, the results of the market investigation did not reveal any substantiated concerns as regards the impact of the Transaction in the celecoxib market in Italy.
Conclusion
(203) In view of the above considerations, taking into account the market investigation and all the evidence available to it, the Commission considers that the Transaction does not raise serious doubts as to its compatibility with the internal market with respect to the supply of celecoxib in Italy.



competitive constraint, as they can enter or re-enter the market very easily from neighbouring countries.
Commission’s assessment
(211) The market investigation conducted by the Commission does not fully support the Notifying Parties' arguments.
(212) First, the combined market share of the Parties is very high ([70-80]%) with a very significant increment brought by Mylan ([20-30]%). The Parties were the two largest suppliers of Eletriptan in Denmark in 2018. The combined market share of [70-80]% may in itself be evidence of a dominant position of the merged entity post-Transaction.80
(213) Moreover, only one competitor with a significant presence would remain in the market post-Transaction, namely Orifarm, a parallel importer81 of Upjohn’s eletriptan product with a [20-30]% market share. Although market shares fluctuate significantly in Denmark, only three suppliers have gained market shares above 10% in the last three years, showing clearly that the Parties are among the top three suppliers of eletriptan in Denmark.
(214) While the market investigation confirms the Notifying Parties' claims that barriers to entry are not particularly high in Denmark, the Commission was not able to confirm entry plans for this market in Denmark before 2023.82 Moreover, the market investigation was inconclusive as to whether parallel importers are effective competitors. While the views of competitors are mixed, the retailer responding to the market investigation does not consider parallel importers as effective competitors.83
(215) Second, the Commission observes that while the tenders system in Denmark fosters competition, it is important to have a sufficient number of credible suppliers participating in such tenders in order to keep downward pressure on prices and to ensure product availability. The Transaction would reduce the number of suppliers who have achieved market shares above [10-20]% in the last years from three to two. Moreover, over the period between 2016 and 2018, only one additional supplier achieved market shares above [5-10]% and only in 2016.
(216) Moreover, wholesalers in Denmark indicated that they carry both the originator and generic versions, and try to offer as many generics as possible,84 evidencing as well


Orion, one of the largest pharmaceutical suppliers in Finland, obtained a marketing authorization for eletriptan in October 2018. Second, the Parties submit that the main reason for the limited generic uptake in this segment is Upjohn’s decision to price Relpax (eletriptan) within the price corridor applied under the Finnish generic substitution policy. Upjohn’s strategy to price within it generally limits the incentive for patients to switch to a generic. In Finland, pharmacies may dispense not just the lowest priced generic alternative for a prescribed medicinal product, but all products priced within the acceptable price range (referred to as the "price corridor"). In many instances where the originator prices within the price corridor, which is what Upjohn does for Relpax, patients tend to prefer the originator product and pharmacies tend to dispense it.
Commission’s assessment
(223) The market investigation conducted by the Commission does not validate the Notifying Parties' arguments.
(224) First, the combined market shares of the Parties are very high ([90-100]%). Although the increment is very limited, only one additional supplier (Orifarm) achieved sales of eletriptan in Finland in 2018.
(225) Orifarm is a parallel importer of Upjohn’s own eletriptan, Relpax, and does not offer a generic version of eletriptan. Therefore, the Transaction would remove from the market the only generic supplier of eletriptan in 2018. Even if Orion were to enter the market, as suggested by the Parties, the number of generic competitors would remain very limited.
(226) Second, despite the decision of some suppliers to price their originator drugs within the price corridor, it is important to have generic suppliers to maintain price competition. The vast majority of competitors who responded to the market investigation indicated that generics exert a competitive constraint on originators in Finland.85 Removing the only generic eletriptan player would limit the competitive constraint exerted on Upjohn's Relpax, the only other molecule sold in Finland.
Conclusion
(227) In view of the above considerations and taking account of the results of the market investigation and all of the evidence available to it, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market, due to its likely horizontal non-coordinated effects in the market for eletriptan in Finland, in particular given the high combined market shares of the Parties and the limited number of suppliers.














market post-Transaction. Among those, both Novartis ([0-5]%) and Stada ([0-5]%) achieve market shares higher than Mylan.
(270) However, the Parties submitted remedies for Luxembourg for pregabalin and explained that, for the purpose of marketing pregabalin, [Information relating to the promotion and distribution of pregabalin in Luxembourg]. Therefore, to expedite the clearance of the Transaction, the Parties offered to divest Mylan’s pregabalin product in Belgium and Luxembourg to a suitable purchaser. 93
Commission’s assessment
(271) The combined market shares of the Parties are very high ([90-100]% in 2018), with an increment brought by Mylan of [0-5]% on top of Upjohn’s significant market share ([80-90]% in 2018). Moreover, only two competitors with market shares above the increment would remain in the market. No competitor achieves market shares above [5-10]%. These elements are indicative of a likely dominant position of Upjohn pre-Transaction that would be further strengthened by the Transaction.
(272) The market investigation did not provide enough elements to dispel the serious doubts arising from the fact that Upjohn likely has a dominant position pre- Transaction, which would be strengthened by the Transaction, and given that the merged entity would only face limited competition.
Conclusion
(273) In view of the above considerations and taking account of the results of the market investigation and all of the evidence available to it, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market due to its likely horizontal non-coordinated effects in the market for pregabalin in Belgium, in particular given the high combined market share and the limited number of significant suppliers.
(b) Pregabalin in Czechia
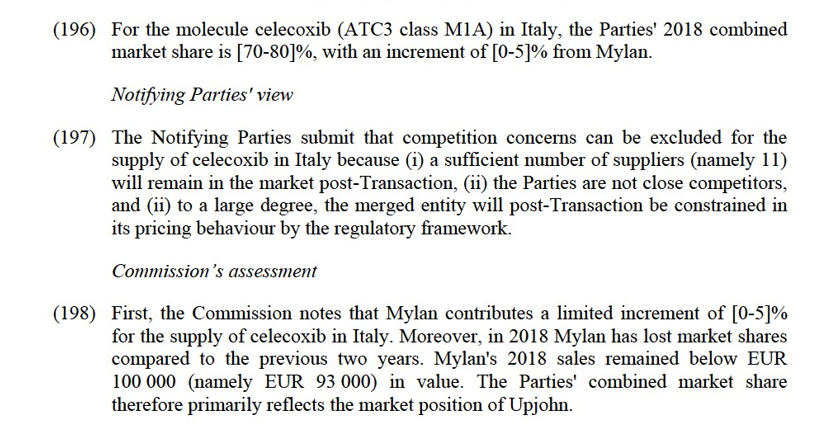
(274) Both Parties supply pregabalin (ATC3 class N3A) in Czechia. This molecule is genericized in Czechia since 2014. In this country, while Upjohn sells a branded version (Lyrica) of pregabalin, Mylan supplies an unbranded version of pregabalin.


Commission’s assessment
(279) The market investigation conducted by the Commission does not fully support the Notifying Parties' arguments.
(280) First, the combined market share of the Parties is high ([50-60]%) with a significant increment brought by Mylan ([5-10]%). Although, Upjohn has lost market shares over the last three years, it remains the clear market leader. The increment brought by Mylan would lead to market shares above [50-60]%.
(281) Second, while a large number of competitors are active in the market, only two competitors with a significant presence would remain in the market post- Transaction, namely Krka with a [10-20]% market share and Novartis with a [5- 10]% market share. All the other competitors would have markets shares below [5- 10]% and below the increment created by the Transaction.
(282) Regardless of the fact that the regulatory framework would constrain potential price increases, it cannot be excluded that the removal of one of the few significant competitors to Upjohn would lead to less frequent price decreases or less product availability.
(283) The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the merged entity would have a strong position on the market post Transaction and only face limited competition.
Conclusion
(284) In view of the above, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market, due to its likely horizontal non-coordinated effects in the market for pregabalin in Czechia, in particular given the high combined market shares and the limited number of significant suppliers.
(c) Pregabalin in Greece
(285) Both Parties supply pregabalin (ATC3 class N3A) in Greece. This molecule is genericized in Greece since 2014. In this country, while Upjohn sells a branded version (Lyrica) of pregabalin, Mylan supplies an unbranded version of pregabalin.

Commission’s assessment
(290) First, the Commission notes that Mylan contributes a limited increment of [0-5]% for the supply of pregabalin in Greece. The Parties' combined market share therefore primarily reflects the market position of Upjohn.
(291) Second, a sufficient number of competitors would remain in the market post- Transaction. Six generic suppliers of pregabalin will remain active post-Transaction in addition to the Parties, four of which with market shares exceeding the increment contributed by Mylan, namely: Novartis ([0-5]%), Teva ([0-5]%), Medochemie ([0- 5]%) and Elpen ([0-5]%). Another two competitors offer the same molecule and two additional suppliers have entered in 2019. Therefore, a sufficient number of competitors remain in the market post-Transaction. This was confirmed by the market investigation. Indeed, wholesalers active in Greece unanimously indicated that they would have a sufficient number of suppliers for this molecule post-Transaction.96
(292) In addition, the presence of four suppliers with market shares larger than Mylan suggests that the Transaction will not alter significantly the structure of supply and will not lead to a negative impact in terms of product availability or frequency of price reductions. This was confirmed by the market investigation.97
(293) Respondents to the market investigation generally consider that the Transaction will not have a negative impact on the market for pregabalin in Greece.98
Conclusion
(294) In view of the above considerations, taking into account the market investigation and all the evidence available to it, the Commission considers that the Transaction does not raise serious doubts as to its compatibility with the internal market with respect to the supply of pregabalin in Greece.
(d) Gabapentin in Ireland
(295) Both Parties supply gabapentin (ATC3 class N3A) in Ireland. This molecule is genericized in Ireland since 1999. In this country, while Upjohn sells a branded version (Neurontin) of gabapentin, Mylan supplies an unbranded version of gabapentin.

recently become substitutable at pharmacy level and that consequently Upjohn would face more competition from generic players, and that given the number of remaining suppliers the Transaction cannot have a negative impact on product availability. Finally, the Notifying Parties submit that the merged entity will be constrained in its pricing behaviour by the regulatory framework in Ireland.
Commission’s assessment
(301) The market investigation conducted by the Commission does not fully support the Notifying Parties' arguments.
(302) First, the combined market share of the Parties is high ([50-60]%) with a material increment brought by Mylan ([5-10]%). The increment brought by Mylan would lead to market shares above [50-60]%. Market shares for the first two months of 2020 are still not sufficiently meaningful, as the period of two months is too short to be indicative of the market position of the Parties. In any event, market shares do not change significantly ([40-50]% combined for the first two months of 2020) and there is only one competitor with a market share above [5-10]% or the increment, namely Teva.
(303) Second, while four competitors are active in the market, only one competitor with a significant presence would remain in the market post-Transaction, namely Teva with a [30-40]% market share. All the other competitors would have markets shares below [5-10]% and below the increment created by the Transaction.
(304) Third, as recognized by the Notifying Parties and confirmed by the market investigation,100 gabapentin is substitutable at pharmacy level. This increases competition between the originator and generics. Mylan is the second largest generic supplier of gabapentin in Ireland and thus it is well positioned to compete closely with Upjohn to gain market shares.
(305) Fourth, respondents to the market investigation indicate that the Transaction would likely have a negative impact, in particular on product availability.101
Conclusion
(306) In view of the above, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market, due to its likely horizontal non-coordinated effects in the market for gabapentin in Ireland, in particular given the high combined market shares of the Parties and the limited number of significant suppliers.


Notifying Parties' view
(311) The Parties submit that the Transaction does not give rise to competition concerns regarding the supply of pregabalin in Italy because (i) a sufficient number of suppliers (namely 16) will remain in the market post-Transaction, (ii) Upjohn lost significant market share since generic entry in 2015 (-[30-40]% in volume) and (iii) the merged entity will post-Transaction be constrained in its pricing behaviour by the regulatory framework.
Commission’s assessment
(312) First, a sufficient number of competitors remain in the market post-Transaction. 16 generic suppliers of pregabalin will remain active post-Transaction, three of which have market shares exceeding the increment contributed by Mylan and above [5- 10]%, namely: Teva ([10-20]%), Doc Generici ([5-10]%) and Novartis ([5-10]%). Importantly, these three suppliers also have a significant presence in in Italy in the generic space. Another 13 competitors offer the same molecule. Therefore, a sufficient number of competitors remain in the market post-Transaction which will be able to exert a meaningful competitive constraint for the supply of pregabalin in Italy. This was confirmed by the market investigation. Indeed, Italian-based wholesalers indicated unanimously that they would have a sufficient number of suppliers post-Transaction for this molecule.102
(313) In addition, the presence of three suppliers with market shares larger than Mylan suggests that the Transaction will not alter significantly the structure of supply and will not lead to a negative impact in terms of product availability or frequency of price reductions. This was confirmed by the market investigation. Respondents to the market investigation unanimously confirmed that the Transaction would not have a negative impact on the market for pregabalin in Italy, either on prices or on product availability.103
(314) Second, Upjohn has lost market shares very rapidly in the last years ([20-30] percentage points between 2016 and 2018), since the loss of exclusivity for pregabalin in Italy in 2014, while the market shares of Mylan have grown modestly ([0-5] percentage points between 2016 and 2018). Other suppliers such as Teva or Novartis have gained market shares faster than Mylan. Suppliers such as Stada or Ecupharma have also gained market shares in the last three years. This suggests that other suppliers compete more closely with Upjohn than Mylan and exert a competitive pressure on Upjohn.






(333) The market investigation indicated that parallel importers sell branded products and do not price at generic level.106 Wholesalers also indicated that parallel importers are less reliable suppliers.107 Therefore, they do not exert a strong competition constraint on originator products.
(334) Third, the Commission was not able to verify the entry plans of other suppliers before 2023 in the pregabalin market in Norway.108 The Transaction would eliminate Mylan as the only potential competitor for a generic product in Norway.
(335) Finally, the Commission does not find that it is appropriate to consider as the most likely counterfactual scenario a lack of entry by Mylan in the market. [information about Mylan’s commercialization of pregabalin in Norway].
Conclusion
(336) In view of the above, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market, due to its likely horizontal non-coordinated effects in the market for pregabalin in Norway given the removal of the only potential generic competitor.
5.3.4.3. Antipsychotics (ATC3 class N5A)
(337) In the EEA, both Parties market ziprasidone, a molecule indicated for the treatment of mental disorders including schizophrenia and mania. The Transaction gives rise to the following Group 1(+)/2 affected markets (which do not fall within one of the two categories referred to in paragraph (33)) for ziprasidone.
(a) Ziprasidone in Czechia
(338) Both Parties supply ziprasidone (ATC3 class N5A) in Czechia. This molecule is genericized in Czechia since 2013. In this country, while Upjohn markets ziprasidone under the brand name Zeldox, Mylan supplies an unbranded version of ziprasidone.
Market shares
(339) The Transaction gives rise to a Group 1 market at the molecule level for ziprasidone in Czechia.109




(350) For the molecule ziprasidone (ATC3 class N5A) in Portugal, the Parties' combined market share amounted to [40-50]% in 2018, with an increment of [10-20]% from Upjohn.
Notifying Parties' view
(351) The Parties submit that competition concerns can be excluded for the supply of ziprasidone (ATC3 class N5A) in Portugal, in particular due to the actual and potential competitors on the market (including holders of dormant market authorisations), the lack of closeness of competition between the Parties, and constraints due to the applicable regulatory framework which would limit the possibility to increase prices.
Commission’s assessment
(352) The combined market shares of the Parties would remain below [40-50]% post- Transaction. The increment is brought about by Upjohn, evidencing the largely genericized nature of the ziprasidone market in Portugal.
(353) Post-Transaction, at least two other significant generic players will remain on the market, namely Stada and Aurobindo, with market shares of [20-30]% and [20-30]% respectively. In addition, the responding Portuguese national health authorities indicate that entry in the market is easy, and in particular, that generic companies holding dormant marketing authorisations are credible entrants which could enter within a short period.112
(354) The market investigation also indicates that, in Portugal, generics compete with originators primarily on prices. However, the market investigation also indicates that Mylan is perceived as not being a particularly price-aggressive player by some pharmacies and wholesalers. Respondents note in particular [Information relating to the commercialization of ziprasidone in Portugal].113
(355) In addition, virtually all responding wholesalers indicated that a sufficient number of suppliers of ziprasidone would remain on the market post Transaction.114
(356) Finally, the results of the market investigation did not reveal any substantiated concerns as regards the impact of the Transaction in the ziprasidone market in Portugal.


(361) For the molecule ziprasidone (ATC3 class N5A) in Spain, the Parties' combined market share amounted to [80-90]% in 2018, with an increment of [0-5]% from Mylan.
Notifying Parties’ view
(362) The Parties submit that competition concerns can be excluded for the supply of ziprasidone (ATC3 class N5A) in Spain, in particular due to the limited increment brought about by Mylan, the number of competitors remaining on the market, the lack of closeness of competition between the Parties, and constraints due to the applicable regulatory framework, which would limit the possibility to increase prices.
Commission’s assessment
(363) The Commission notes that the increment brought about by Mylan (less than [0-5]%) is particularly limited. The merged entity’s significant market share thus mainly reflects Upjohn’s position and is largely not merger-specific.
(364) Importantly, substitutability between originators and generics for ziprasidone may be limited in Spain. In the area of anti-psychotics, such as ziprasidone, doctors would normally not switch a patient’s prescription when one specific product is found to be effective, as switching can be counterproductive or even lead to side effects. Responding Spanish pharmacies confirmed that for antipsychotics, pharmacies would not switch a patient’s prescription from a branded product to a generic, while they generally have the ability to do convert prescription towards generics under the applicable rules.116 This situation may explain the relative stability of Upjohn’s market share over the years.
(365) In addition, responding pharmacies and wholesalers do not consider Mylan’s ziprasidone as a must-have product, i.e. a product that they need to have in stock to meet patients' demand, in Spain.117
(366) Competitors to the Parties, with a more significant footprint than Mylan will remain on the market post-Transaction. At least three players, including large generic companies such as Krka (with a [5-10]% market share) and Stada ([0-5]%), as well as Infarco ([0-5]%), which have a market share exceeding the increment ([0-5]%), will remain active on the market.
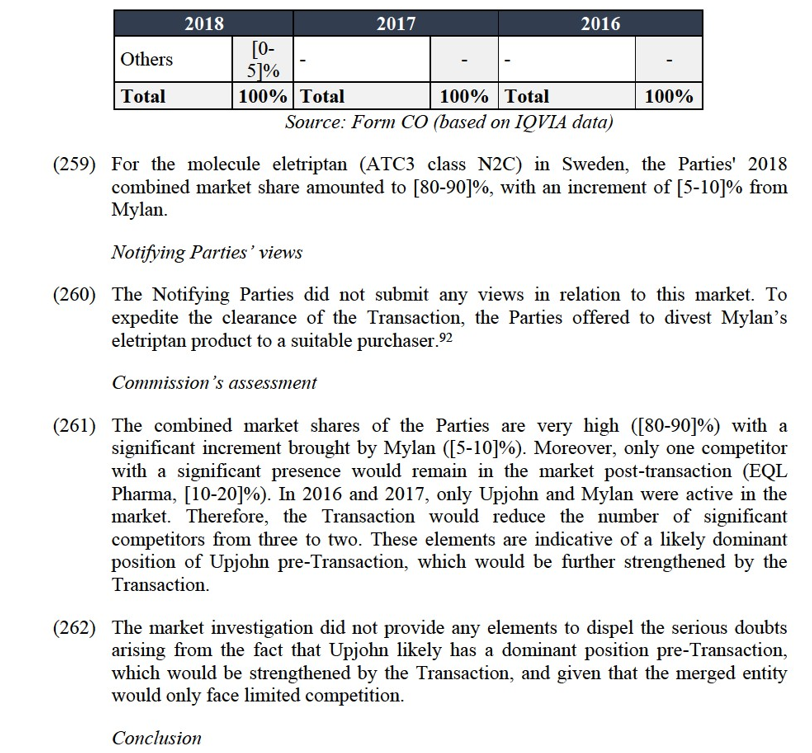
(367) Furthermore, virtually all responding wholesalers also indicated that a sufficient number of suppliers of ziprasidone would remain on the market post Transaction.118
(368) Finally, the results of the market investigation did not reveal any substantiated concerns as regards the impact of the Transaction in the ziprasidone market in Spain.
Conclusion
(369) In view of the above considerations, taking into account the market investigation and all the evidence available to it, the Commission considers that the Transaction does not raise serious doubts as to its compatibility with the internal market with respect to the supply of ziprasidone in Spain.
5.3.4.4.Tranquilisers (ATC3 class N5C)
(370) In the EEA, both Parties market alprazolam, a molecule indicated for the treatment of anxiety, panic disorders, alcohol withdrawal syndrome, depression, and anxiety.
(371) Alprazolam belongs to the category of benzodiazepines, which are molecules affecting a key neurotransmitter, effectively slowing nerve impulses throughout the body. While Mylan markets other benzodiazepines (including for instance oxazepam, lorazepam, bromazepam, hydroxyzine and buspirone), Upjohn only markets alprazolam.
(372) The Transaction gives rise to the following Group 1(+)/2 markets (which do not fall within one of the two categories referred to in paragraph (34)) for alprazolam.119
(a) Alprazolam in Greece
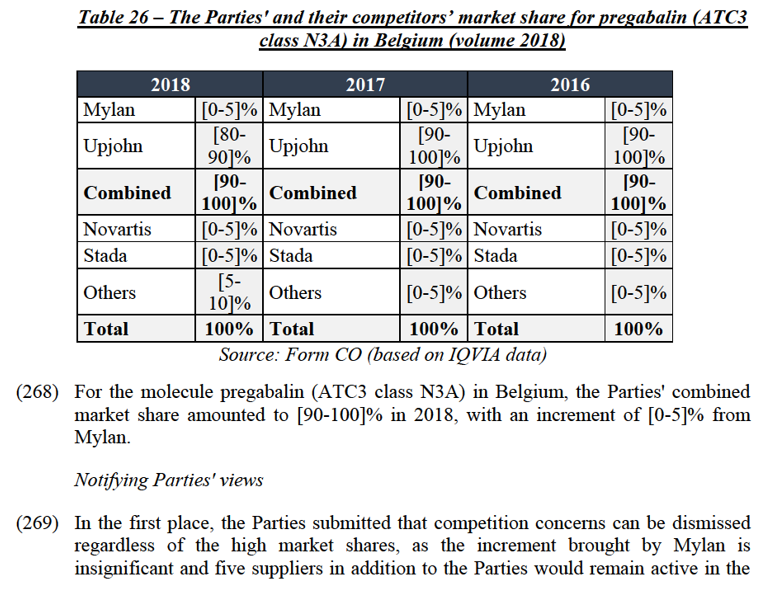
(373) Both Parties supply alprazolam (ATC3 class N5C) in Greece. This molecule is genericized in Greece for at least 20 years. In this country, while Upjohn markets alprazolam under the brand name Xanax, Mylan supplies an unbranded version of alprazolam.
Market shares
(374) The Transaction gives rise to a Group 2 market at the molecule level for alprazolam in Greece.


(381) First, Upjohn’s Xanax is by far the primary alprazolam product sold in Greece, and only faces limited competition to date. Most respondents, including virtually all responding pharmacies/hospitals and a majority of wholesalers consider Upjohn’s products including Xanax as a must-have.120 In addition, […].
(382) Competition from all third parties, including Mylan, is important to guarantee supply of alprazolam to Greek patients and hospitals. Pfizer suffered out of stock situations for alprazolam in Greece over the last three years.121 In addition, supply is further limited due to parallel exports from Greece.122 As a result, product availability from smaller players, including Mylan, is likely to be particularly critical for patients and health systems.
(383) Only one player, namely Adelco, would remain active in the market post- Transaction with a market share of [0-5]%. Adelco and Mylan hold a similar market share for the supply of alprazolam in Greece. Contrary to Mylan, Adelco is not among the top 10 generic players in Greece. Adelco thus presumably exerts a similar or lesser level of competitive constraint on Upjohn pre-Transaction than Mylan.
(384) Regardless of any price increase, the removal of one of only two competitors to Upjohn’s Xanax could potentially lead to less frequent price decreases or less product availability.
(385) A majority of responding customers expect a negative impact of the Transaction on prices and/or product availability, in relation to the supply of alprazolam in Greece.123 One customer notes for instance that “"Xanax of Pfizer & alprazolame (sic) of Mylan cover almost [90-100]% of patient needs”.124
(386) Lastly, documents provided by the Parties [[Information relating to Mylan’s commercial activities in Greece]. As a result, it is not clear that the correct counterfactual scenario, absent the Transaction, would be [[Information relating to Mylan’s commercial activities in Greece].
Conclusion
(387) In view of the above considerations and taking account of the results of the market investigation and all of the evidence available to it, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market, due to its likely horizontal non-coordinated effects in the market for alprazolam in


Commission’s assessment
(393) The Parties’ combined market shares are very high, exceeding [80-90]%, with a significant increment, approaching [20-30]%, from Upjohn, on top of Mylan’s significant market share ([50-60]% in 2018) These elements are indicative of a likely dominant position of Mylan pre-Transaction, which would be further strengthened by the Transaction.
(394) On such market, the Parties only face one competitor, namely Krka. As a result, the Transaction essentially amounts to a reduction in the number of players active on the market from three to two.
(395) Furthermore, when looking only at oral solid immediate release alprazolam products (NFC1 class A) in Iceland, the situation is even more problematic, as Mylan's market share would be [80-90]% and Upjohn's [10-20]%. On this market, the merger would essentially amount to a merger to monopoly.
(396) The market investigation did not provide any elements to dispel the serious doubts arising from the fact that Mylan likely has a dominant position pre-Transaction, which would be further strengthened by the Transaction, and given that the merged entity would only face limited competition post-Transaction.
Conclusion
(397) In view of the above considerations and taking account of the results of the market investigation and all of the evidence available to it, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market, due to its likely horizontal non-coordinated effects in the market for alprazolam in Iceland, in particular given the high combined market shares and the limited number of suppliers.
(c) Alprazolam in Ireland
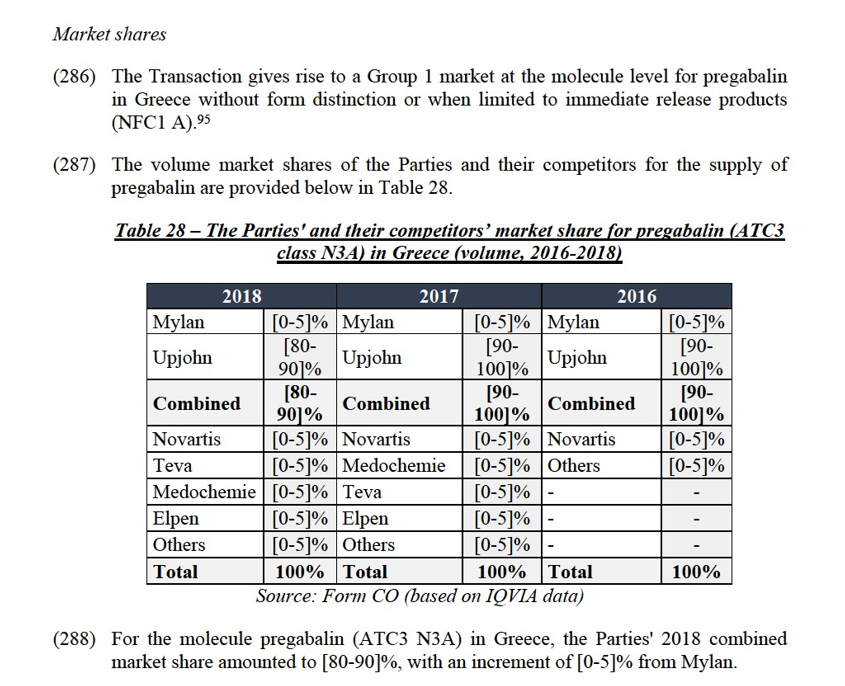
(398) Both Parties supply alprazolam (ATC3 class N5C) in Ireland. This molecule is genericized in Ireland since at least 20 years. In this country, Upjohn markets alprazolam under the brand name Xanax, Mylan markets alprazolam under the brand name Gerax.
Market shares
(399) The Transaction gives rise to a Group 1 market at the molecule level for alprazolam in Ireland.

(404) The market investigation did not provide any elements to dispel the serious doubts arising from the fact that Upjohn likely has a dominant position pre-Transaction, which would be further strengthened by the Transaction, and given that the merged entity would only face limited competition post-Transaction. In fact, half of responding customers (pharmacies and wholesalers) considered that the Transaction would have a negative impact on prices or product availability with regards to alprazolam in Ireland.128
Conclusion
(405) In view of the above considerations and taking account of the results of the market investigation and all of the evidence available to it, the Commission concludes considers that the Transaction raises serious doubts as to its compatibility with the internal market, due to its likely horizontal non-coordinated effects in the market for alprazolam in Ireland, in particular given the high combined market shares and the limited number of significant suppliers.
(d) Alprazolam in Italy
(406) Both Parties supply alprazolam (ATC3 class N5C) in Italy. This molecule is genericized in Italy since at least 20 years. In this country, while Upjohn markets alprazolam under the brand name Xanax, Mylan supplies both an unbranded version of alprazolam and a branded version, under the brand name Frontal.
Market shares
(407) The Transaction gives rise to a Group 1 market at the molecule level for alprazolam in Italy.129
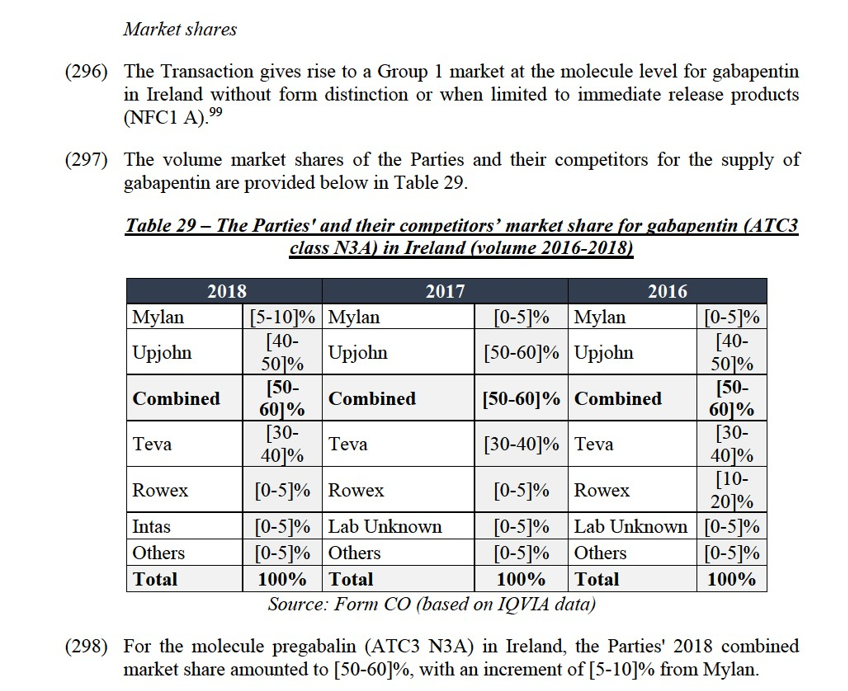
(408) The volume market shares of the Parties and their competitors for the supply of alprazolam in Italy are provided below in Table 39.


share ([60-70]%). These elements are indicative of a likely dominant position of Upjohn pre-Transaction, which would be further strengthened by the Transaction. In addition, the market investigation conducted by the Commission does not fully support the Notifying Parties’ arguments.
(413) First, the market investigation indicates that generic penetration remains quite low for alprazolam in Italy (below one third of the total market).130 This is confirmed by Upjohn’s consistently high market shares over the last three years, in spite of Xanax’s loss of exclusivity occurring over 20 years ago.
(414) A majority of responding Italian customers (pharmacies and wholesalers) consider that the price of generic products in Italy generally affects the price level of originators. For instance, prices of originators would likely decrease if all generic players reduced their prices by 5-10%.131 Respondents to the market investigation also took specifically the view that the price of generic alprazolam constrains the pricing of Upjohn’s Xanax.132
(415) Second, Mylan exerts a competitive constraint on Upjohn. The market investigation confirms that brand loyalty is very high in Italy and is a key parameter of competition in the supply of pharmaceutical products in Italy.133 However, as mentioned in paragraph [414], generic alprazolam exerts a competitive constraint on Xanax, in particular in terms of pricing. As a result, removing the largest provider of generic alprazolam is likely to strengthen Xanax’s already strong market position.
(416) Third, independently from any kind of product differentiation, Mylan can be set apart from other generic players on the alprazolam market in Italy. Mylan is the largest generic player in the alprazolam market, with a market share nearly three times as large as the second generic player, Stada, whose market shares does not exceed [5-10]%. One responding competitor even considers the Parties to be close competitors in Italy because they both have high market shares.134 The respondents to the market investigation indicate that companies with low market shares (that is to say below 5%) do not exert a strong competitive constraint on an originator.135 Combined, all other generic suppliers besides Stada have a market share lower than Mylan’s. As a result, Mylan is among one of the only two generic players able to exert an effective competitive constraint on Upjohn’s Xanax.
(417) In addition, part of Mylan’s sales (accounting for around [0-5]% of the sales in the Italian alprazolam market) result from the sale of its branded product Frontal, [Information concerning alprazolam in Italy].136 This product is priced at a premium, closer to Upjohn’s than Mylan’s unbranded generic alprazolam.
(418) Lastly, alprazolam is not a reimbursed product in Italy. It belongs to the so-called “class C” of pharmaceutical products. The pricing of class C products, such as alprazolam is set freely by the manufacturers, and can be increased every odd year, in line with anticipated inflation, under the monitoring of the Italian public health authorities. Information provided by the Notifying Parties shows that both Upjohn, Mylan, as well as other generic suppliers have used this possibility in 2017 and 2019.
(419) In addition to potential price increases, the Transaction could potentially lead to less frequent price reductions, as a result of the more limited competitive interactions between Xanax and generic alprazolam.
Conclusion
(420) In view of the above considerations and taking account of the results of the market investigation and all of the evidence available to it, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market, due to its likely horizontal non-coordinated effects in the market for alprazolam in Italy, in particular given the high combined market shares and the limited number of significant suppliers.
(e) Alprazolam in Poland
(421) Both Parties supply alprazolam (ATC3 class N5C) in Poland. This molecule is genericized in Poland since at least 20 years. In this country, while Upjohn markets alprazolam under the brand name Xanax, Mylan supplies an unbranded version of alprazolam.
Market shares
(422) The Transaction gives rise to a Group 1 market at the molecule level for alprazolam in Poland.137


(427) Post-Transaction, the Parties will face competition from a number of established generic purchasers, with a significant market presence, including Orion (with a [10- 20]% market share), Servier ([10-20]%), Krka ([10-20]%), and Delfarma ([5-10]%), all with market shares exceeding [5-10]% and even exceeding [10-20]% for the former three.
(428) In addition, virtually all responding Polish wholesalers indicated that a sufficient number of suppliers of alprazolam would remain on the market post-Transaction.138
Conclusion
(429) In view of the above considerations, taking into account the market investigation and all the evidence available to it, the Commission considers that the Transaction does not raise serious doubts as to its compatibility with the internal market with respect to the supply of alprazolam in Poland.(f) Alprazolam in Portugal
(430) Both Parties supply alprazolam (ATC3 class N5C) in Portugal. This molecule is genericized in Portugal since at least 20 years. In this country, while Upjohn markets alprazolam under the brand name Xanax, Mylan supplies an unbranded version of alprazolam.
Market shares
(431) The Transaction gives rise to a Group 1 market at the molecule level for alprazolam in Portugal.139
(432) The volume market shares of the Parties and their competitors for the supply of alprazolam in Portugal are provided below in Table 41.


Commission’s assessment
(436) Structurally, the Transaction leads to an important change in market dynamics. Based on 2018 figures, the merged entity’s market share would reach nearly [50- 60]%, with a significant increment from Upjohn ([20-30]%).
(437) In addition, the market investigation conducted by the Commission does not fully support the Notifying Parties’ arguments.
(438) First, while there is a significant number of companies offering alprazolam in Portugal, only few have a significant market presence. Indeed, only four, including Upjohn and Mylan (who is the market leader), have a market share exceeding [5- 10]%, including Teva (with an [10-20]% market share) and Atral (around [10-20]%). Out of these, only Teva is perceived by responding pharmacies as having a salesforce matching Mylan’s in the country.140
(439) In addition, Portugal is pharmacy-driven market, where discounts and broad portfolio offering regarding or regardless of the therapeutic are key advantages. A large majority of responding pharmacies indicated receiving multi-product discounts from pharmaceutical companies, including from those offering both generics and originators.141 One Portuguese wholesaler states for instance that “[…] when there are rebates, these are applied to all portfolio, and not to a specific range of products. We don't have rebates that favor generics against branded products”.142 The market investigation indicates that Upjohn’s Xanax is considered as must-have by a majority of responding customers. The results of the market investigation are more mixed as to whether Mylan’s alprazolam is also a must-have product. While a large majority of wholesalers consider it to be the case (but also list other suppliers of must-have alprazolam including for instance Teva), pharmacies are more split.143 As a result, the market position of the merged entity post Transaction may be even stronger than the combination of Mylan and Upjohn’s position pre Transaction.
(440) Second, regardless of any consideration relating to closeness of competition, the Parties can be considered as exerting a competitive constraint on each other. A majority of respondents to the market investigation consider generics exert competitive constraints on originators (and vice versa) in Portugal, proportionally more than on average in the EEA.144 The pricing of generics also move in parallel to the pricing of the originator based on information provided by the Notifying Parties. As the leading generic supplier for alprazolam in Portugal, Mylan is thus a primary competitor to Upjohn.
(441) Finally, the market investigation confirms price increases of generics in Portugal are infrequent.145 However, the transaction may lead to less frequent price decreases in relation to alprazolam in Portugal, as prices of generics tend to decrease over time. While information provided by the Notifying Parties indicates that no voluntary price reduction occurred over the last six years, there are no indications that future price reductions would not take place absent the Transaction.
Conclusion
(442) In view of the above considerations and taking account of the results of the market investigation and all of the evidence available to it, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market, due to its likely horizontal non-coordinated effects in the market for alprazolam in Portugal, in particular given the high combined market shares and the limited number of significant suppliers.
5.3.4.5. Anti-depressants and mood stabilisers (ATC3 class N6A)
(443) In the EEA, both Parties market venlafaxine and sertraline, which are molecules used to treat depression and anxiety disorders. In the EEA, their only common indication is for the treatment and prevention of the recurrence of major depressive episodes. In addition, sertraline is also indicated for the treatment of panic, obsessive-compulsive, social anxiety and post-traumatic stress disorders. The Transaction gives rise to the following Group 1(+)/2 markets (which do not fall within one of the two categories referred to in paragraph (34)) for venlafaxine and sertraline.
(a) Venlafaxine in Belgium
(444) Both Parties supply venlafaxine (ATC3 class N6A) in Belgium. This molecule is genericized in Belgium since 2008. In this country, while Upjohn markets venlafaxine under the brand name Effexor-Exel, Mylan supplies an unbranded version of venlafaxine.
Market shares
(445) The Transaction gives rise to a Group 1 market at the molecule level for venlafaxine in Belgium.146


(451) First, there are only two significant suppliers of venlafaxine in Belgium besides the Parties, namely Novartis and Stada, with market shares around half or less compared to the merged entity’s. The Transaction would thus largely result in a reduction in the number of significant players from four to three.
(452) In addition, the market investigation provided some indication that, among generic players, Mylan was perceived as having a more aggressive price policy, or as initiating price decreases, compared to other generic players. Half of the responding pharmacies flagged this.147
(453) Second, regarding out of stock situations in relation to venlafaxine, the market investigation results are more mixed. A majority of responding customers as well as the national authority indicate that these situations are not frequent in relation to the molecule in Belgium.148 However, the market investigation did not indicate that the recent supply issues experienced by the Parties’ competitors have been resolved. One customer also expressed concerns that the Transaction could lead to further issues in terms of product availability, including for the provision of venlafaxine.149
(454) Finally, the market investigation confirms that prices are fixed by law including for venlafaxine. However, the market investigation, and in particular the national health authority, confirms that significant price reductions are also common several years after first generic entry.150 As a result, the Transaction, which involves the merger between the originator manufacturer, and an established generic player, potentially aggressive in terms of pricing, could lead to less frequent price reductions for venlafaxine in Belgium.
Conclusion
(455) In view of the above considerations and taking account of the results of the market investigation and all of the evidence available to it, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market, due to its likely horizontal non-coordinated effects in the market for venlafaxine in Belgium, in particular given the high combined market shares and the limited number of significant suppliers.


increment brought about by Mylan, [information concerning the commercialization of venlafaxine in Greece].
Commission’s assessment
(461) The Commission notes that the increment brought about by Mylan is minimal (less than [0-5]%). Mylan’s limited market share has been consistent over the last three years. The merged entity’s strong market share thus mainly reflects Upjohn’s and is largely not merger-specific.
(462) The Parties will face competition from a number of established generic purchasers, with a significant and stable market presence, including Innovis (with a [10-20]% market share), Gap ([5-10]%), Medochemie ([5-10]%), all with market shares exceeding [5-10]%, and other players including Qualia or SJA, whose share are much higher (around or nearly tenfold) than the increment brought about by Mylan. In total, the Parties will continue facing competition from 14 generic suppliers active in the market.
(463) In addition, virtually all responding Greek wholesalers indicated that a sufficient number of suppliers of venlafaxine would remain on the market post Transaction.151
(464) Finally, the results of the market investigation did not reveal any substantiated concerns as regards the impact of the Transaction in the venlafaxine market in Greece.
Conclusion
(465) In view of the above considerations, taking into account the market investigation and all the evidence available to it, the Commission considers that the Transaction does not raise serious doubts as to its compatibility with the internal market with respect to the supply of venlafaxine in Greece.
(c) Sertraline in Greece
(466) Both Parties supply sertraline (ATC3 class N6A) in Greece. This molecule is genericized in Greece since 1996. In this country, while Upjohn markets sertraline under the brand name Zoloft, Mylan supplies an unbranded version of sertraline.


over the last three years. The merged entity’s market share thus mainly reflects Upjohn’s and is largely not merger-specific.
(472) The Parties will face competition from a number of established generic purchasers, with a significant and stable market presence, including in particular Teva, with a market share nearing [20-30]% (at [20-30]%), followed by Novartis ([5-10]%), and other players including Vianex ([0-5]%), whose shares are higher than the increment brought about by Mylan. In total, the Parties will continue facing competition from nine generic suppliers active in the market.
(473) In addition, virtually all responding Greek wholesalers indicated that a sufficient number of suppliers of venlafaxine would remain on the market post Transaction.152
(474) Finally, the results of the market investigation did not reveal any substantiated concerns as regards the impact of the Transaction in the sertraline in Greece.
Conclusion
(475) In view of the above considerations, taking into account the market investigation and all the evidence available to it, the Commission considers that the Transaction does not raise serious doubts as to its compatibility with the internal market with respect to the supply of sertraline in Greece.
(d) Venlafaxine in Italy
(476) Both Parties supply venlafaxine (ATC3 class N6A) in Italy. This molecule is genericized in Italy since 2008. In this country, Upjohn markets both an unbranded version of venlafaxine as well as branded venlafaxine under the name Effexor, while Mylan only supplies an unbranded version of venlafaxine.
Market shares
(477) The Transaction gives rise to a Group 1 market at the molecule level for venlafaxine in Italy.153
(478) The volume market shares of the Parties and their competitors for the supply of venlafaxine in Italy are provided below in Table 45.


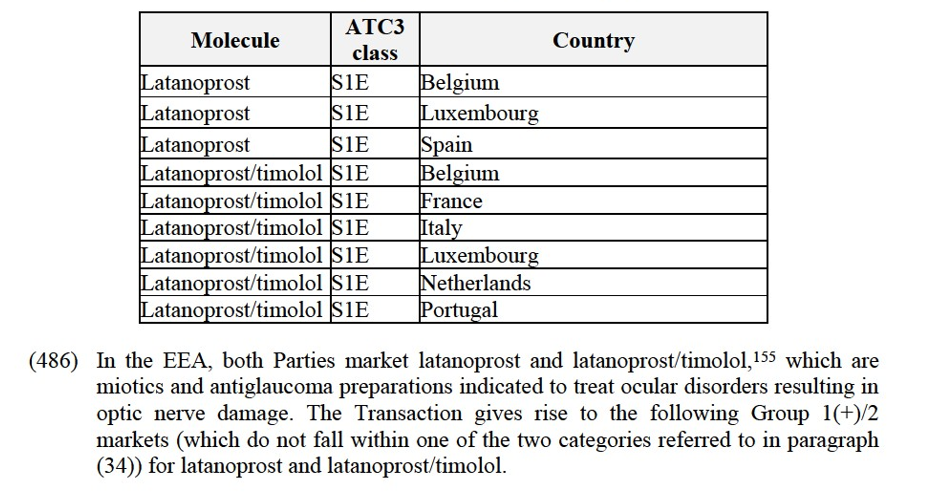

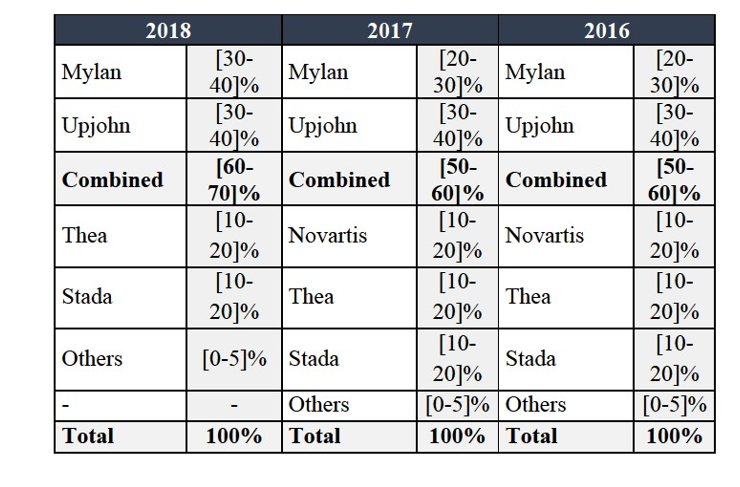
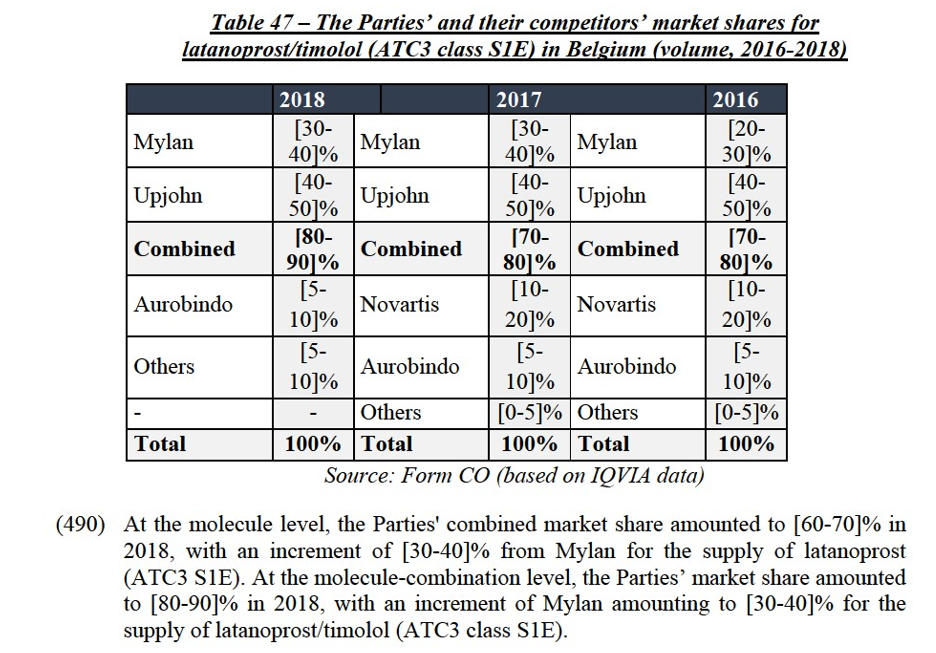







with generic players such as Stada, Teva, and Zentiva, and less so with branded products.158 Third, the Parties claim that the applicable regulatory framework constrains the pricing of latanoprost/timolol in Italy, including the merged entity.159
(508) In a separate submission following the State of Play meeting, the Notifying Parties additionally submit pricing analyses evidencing [information concerning Mylan’s pricing strategy of latanoprost/timolol in Italy].
Commission’s assessment
(509) Structurally, the Transaction leads to an important change in market dynamics. The combined market share of the Parties is very high at molecule combination level ([60-70]% in 2018), and Mylan’s increment is material ([5-10]% in 2018), on top of Upjohn’s significant market share ([60-70]% in 2018).
(510) In addition, the market investigation conducted by the Commission does not fully confirm the Notifying Parties’ arguments.
(511) First, while eight additional competitors are also active in the supply of latanoprost/timolol in Italy, only two of them have market shares exceeding [5-10]% and/or the increment brought by Mylan (namely Visufarma and Novartis). While Novartis is a well-established generic player in Italy, Visufarma does not rank among the top ten generic suppliers in Italy. The results of the market investigation further indicate that Visufarma is not a leading generic player in Italy160 and is not perceived by responding pharmacies as having a salesforce matching Mylan’s in the country.161
(512) Second, the results of the market investigation did not confirm the Parties’ claim that Mylan does not exert a material competitive constraint on Upjohn. The market investigation confirms that in Italy, while brand loyalty is very high and constitutes a key parameter of competition in the supply of pharmaceuticals,162 generics do exert a competitive constraint on originators, and in particular that the price of generic products in Italy impacts the price level of originators. These elements are indicative of a likely dominant position of Upjohn pre-Transaction, which would be further strengthened by the Transaction.
(513) In addition, the market investigation revealed that the latanoprost/timolol molecule combination faced out of stock incidents in Italy in the past years.163
(514) Finally, neither the market investigation nor the additional submission of the Parties provided sufficient elements to dispel the serious doubts arising from the fact that the merged entity would have a dominant position on the market post-Transaction and would only face limited competition. To the contrary, for example, a leading generic supplier specifically mentioned that the Transaction would have a negative impact on prices for the supply of latanoprost/timolol in Italy.164
Conclusion
(515) In view of the above considerations, taking into account the market investigation and all the evidence available to it, the Commission concludes that the Transaction raises serious doubts as to its compatibility with the internal market due to horizontal non- coordinated effects as regards the supply of latanoprost/timolol in Italy, as the Transaction would likely lead to the creation or strengthening of dominance in this market.
(d) Latanoprost and latanoprost/timolol in Luxembourg
(516) Both Parties supply latanoprost and latanoprost/timolol (both ATC3 class S1E) in Luxembourg. This molecule and molecule-combination are genericized in Luxembourg since 2012. Both Parties market latanoprost as branded products. Upjohn’s latanoprost and latanoprost/timolol products are branded under the respective names Xalatan and Xalacom while Mylan’s products are branded under the names Latanotears for latanoprost and Timolatears for latanoprost/timolol. In addition, […].
Market shares
(517) The Transaction gives rise to a Group 1 market at the molecule level for latanoprost, as well as at the molecule combination level for latanoprost/timolol in Luxembourg.
(518) The volume market shares of the Parties and their competitors for the supply of latanoprost and of latanoprost/timolol in Luxembourg are provided below in Tables 50 and 51.


Commission’s assessment
(521) Structurally, for the supply of latanoprost, the combined market share of the Parties is very high (reaching [70-80]% in 2018), with a significant increment from Mylan (of [10-20]% in 2018). Moreover, only one competitor, Thea, would remain active post-Transaction with a market share higher than [0-5]%.
(522) Regarding the supply of latanoprost/timolol, the combined market share of the Parties is even higher (namely [90-100]%), with an increment from Mylan of [0- 5]%. As a result, no competitor would remain active post-Transaction for the supply of latanoprost/timolol. Therefore, the Transaction would reduce the number of significant competitors from three to two for latanoprost and from two to one for latanoprost/timolol. As a result, the Transaction will lead to a significant change in the structure of the market, both at molecule level for latanoprost, and even more so at molecule-combination level for latanoprost/timolol. These elements are indicative of a likely dominant position of Upjohn pre-Transaction for the markets of latanoprost and latanoprost/timolol, which would be further strengthened by the Transaction.
(523) The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the merged entity would have a dominant position on the markets for latanoprost and latanoprost/timolol in Luxembourg post-Transaction and would only face limited effective competition (if any for latanoprost/timolol) as a result of the Transaction.
Conclusion
(524) In view of the above considerations, taking into account the market investigation and all the evidence available to it, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market as regards the supply of latanoprost and of latanoprost/timolol in Luxembourg, due to horizontal non- coordinated effects, in particular given the high combined market share and the limited number of competitors remaining post-Transaction.
(e) Latanoprost/timolol in Netherlands
(525) Both Parties supply latanoprost/timolol (ATC3 class S1E) in the Netherlands. This molecule combination is genericized in the Netherlands since 2012. In this country, while Upjohn markets a branded version of latanoprost/timolol, under the name Xalacom, Mylan markets an unbranded version of latanoprost/timolol.
Market shares
(526) The Transaction gives rise to a Group 1 market at the molecule combination level for the supply of latanoprost/timolol in the Netherlands.



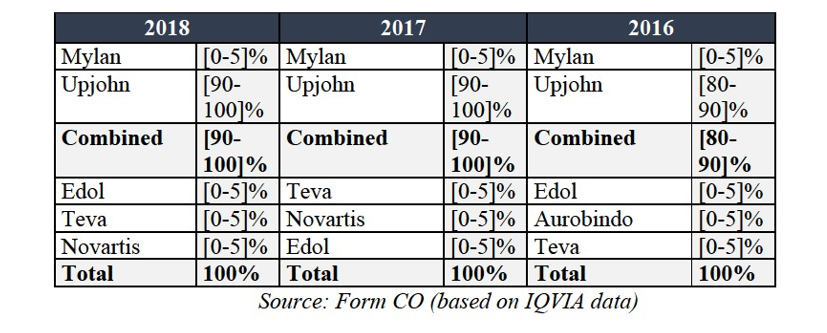
(536) For the molecule combination latanoprost/timolol in Portugal, the Parties' 2018 combined market share amounted to [90-100]%, with an increment of [0-5]% from Mylan.
Notifying Parties’ views
(537) The Notifying Parties submit that the Transaction will not raise competitive concerns regarding the supply of latanoprost/timolol (ATC3 class S1E) in Portugal for the following reasons. First, the Parties consider that, because Mylan’s increment is small, the Transaction will neither (meaningfully) strengthen the pre-merger position of Upjohn, nor alter the structure of supply in the market. Second, the Parties argue that post-Transaction the merged entity will face competition constraints from four generic players active on this market, namely Edol, Teva, Aurobindo and Novartis, and three additional competitors (Stada, Bruschettini and Thea, which have a registered latanoprost/timolol product in Portugal and could easily enter the market. Third, the Parties claim that the applicable regulatory framework constrains the pricing of latanoprost/timolol in Portugal.168
Commission’s assessment
(538) Structurally, the combined market share of the Parties is very high at molecule combination level ([90-100]% in 2018), and Mylan’s increment remains small ([0- 5]% in 2018).
(539) In addition, the market investigation conducted by the Commission does not fully support the Notifying Parties’ arguments.
(540) Besides the Parties, four players are active in Portugal for the supply of latanoprost/timolol but none of the Parties’ competitors have a market share exceeding [5-10]% and only two have a market share slightly exceeding Mylan’s increment, namely Edol and Teva (with respective 2018 market shares of [0-5]% and [0-5]%). While Teva is a well-established generic player in Portugal, Edol does not rank among the top ten generic suppliers in Portugal. The results of the market investigation further indicate that Edol is not a leading generic player in Portugal169 and is not perceived by responding pharmacies as having a salesforce matching Mylan’s in the country.170
(541) The results of the market investigation also indicate that post-Transaction there will not remain a sufficient number of suppliers for latanoprost/timolol in Portugal. A Portuguese-based competitor indicated that the Parties are among the few suppliers for this eye drop product in Portugal171 and a Portuguese-based wholesaler indicated that there would not be a sufficient number of suppliers for this combination of molecules.172 In addition, the market investigation did not reveal that the companies that have a registered latanoprost/timolol product in Portugal have plans to enter the market in the next three years.173
(542) The market investigation further indicates that Upjohn’s Xalacom is considered as must-have by some responding customers, which is indicative of market power. In addition, Portugal is a “pharmacy-driven” market, where discounts and broad portfolio offering regarding or regardless of the therapeutic are key advantages. A large majority of responding pharmacies indicated receiving multi-product discounts from pharmaceutical companies, including from those offering both generics and originators.174 One Portuguese wholesaler states for instance that “[…] when there are rebates, these are applied to all portfolio, and not to a specific range of products. We don't have rebates that favor generics against branded products”.175 Therefore, despite Mylan’s small increment, the Transaction will likely strengthen the merged entity’s dominance in this market.
(543) In addition, the market investigation revealed that suppliers of latanoprost/timolol faced out of stock incidents in Portugal in the past years.176
(544) Finally, the market investigation confirms that price increases of generics in Portugal are infrequent.177 However, the Transaction may lead to less frequent price decreases in relation to latanoprost/timolol in Portugal. While information provided by the Notifying Parties indicates that no voluntary price reduction occurred over the last six years, there are no indications that future price reductions would not take place absent the Transaction.
Conclusion
(545) In view of the above considerations, taking into account the market investigation and all the evidence available to it, the Commission concludes that the Transaction raises serious doubts as to its compatibility with the internal market due to horizontal non- coordinated effects as regards the supply of latanoprost/timolol in Portugal, in particular given the high combined market share and the limited number of competitors remaining post-Transaction.

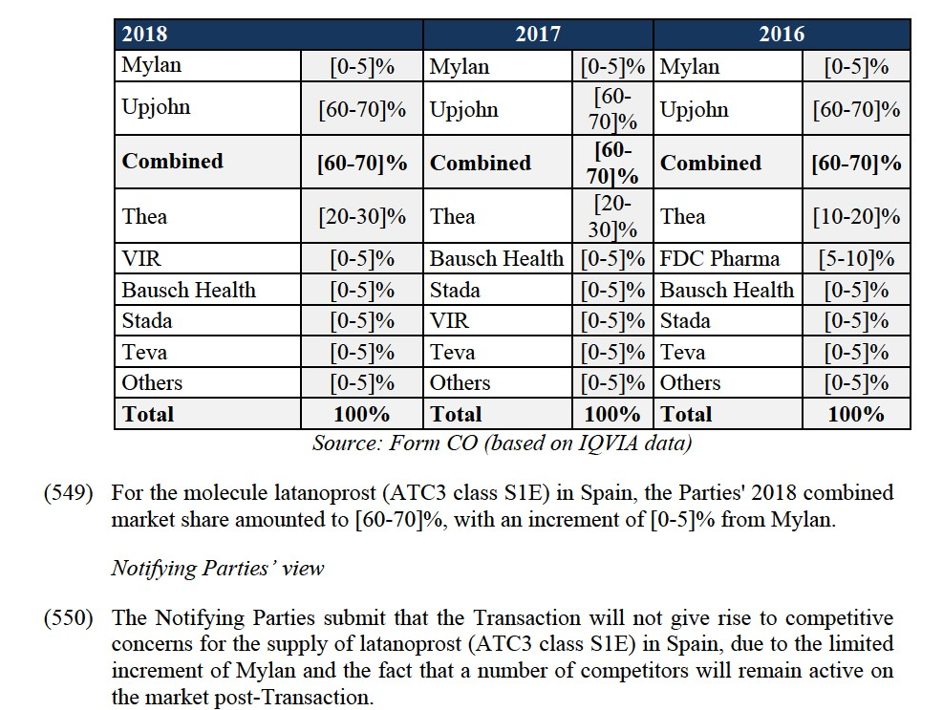
Commission’s assessment
(551) The Commission notes that Mylan’s increment is small (consistently below [0-5]% during the last three years) and therefore, the market structure is unlikely to significantly change as a result of the Transaction.
(552) The Parties will face competition from a number of established generic manufacturers including Thea ([20-30]%), Bausch Health ([0-5]%), Vir ([0-5]%) and Stada ([0-5]%), all with market shares exceeding Mylan’s as regards latanoprost sales.
(553) Competitors did not raise any concerns regarding the impact of the transaction in relation to latanoprost in Spain.178 In addition, a responding pharmacy confirmed that it expected a neutral impact of the transaction on prices for latanoprost in Spain.179 In addition, all responding Spanish wholesalers indicated that a sufficient number of suppliers of latanoprost would remain on the market post Transaction.180
(554) In summary, the results of the market investigation did not reveal any substantiated concerns as regards the impact of the Transaction in the latanoprost market in Spain.
Conclusion
(555) In view of the above considerations, taking into account the market investigation and all the evidence available to it, the Commission considers that the Transaction does not raise serious doubts as to its compatibility with the internal market with respect to the supply of latanoprost in Spain.
5.4. Conclusion
(556) It derives from the assessment conducted in Section 5.3 that the Transaction raises serious doubts as to its compatibility with the internal market, due to its likely horizontal non-coordinated effects in the following molecule/country pair markets:(a) Atorvastatin in Norway(b) Doxazosin in Czechia(c) Doxazosin in France(d) Eplerenone in Belgium(e) Eplerenone in Hungary(f) Sildenafil (PAH) in Estonia(g) Sildenafil (PAH) in France(h) Sildenafil (PAH) in Latvia(i) Sildenafil (PAH) in Lithuania(j) Sildenafil (PAH) in Romania(k) Sildenafil (PAH) in the United Kingdom(l) Eletriptan in Denmark(m) Eletriptan in Finland(n) Eletriptan in France(o) Eletriptan in Norway(p) Eletriptan in Sweden(q) Pregabalin in Czechia(r) Pregabalin in Belgium(s) Pregabalin in Luxembourg(t) Pregabalin in Norway(u) Gabapentin in Ireland(v) Ziprasidone in Czechia(w) Alprazolam in Greece(x) Alprazolam in Iceland(y) Alprazolam in Ireland(z) Alprazolam in Italy (aa) Alprazolam in Portugal (bb) Venlafaxine in Belgium (cc) Latanoprost in Belgium(dd) Latanoprost/timolol in Belgium (ee) Latanoprost/timolol in France (ff) Latanoprost/timolol in Italy (gg) Latanoprost in Luxembourg(hh) Latanoprost/timolol in Luxembourg(ii) Latanoprost/timolol in the Netherlands (jj) Latanoprost/timolol in Portugal
6. ACTIVE PHARMACEUTICAL INGREDIENTS
(557) An active pharmaceutical ingredient ("API") is the substance in a FDP that is pharmaceutically active and is suspended in excipients (that is, inert substances taking the form, for instance, of a tablet or a solution), for the purposes of administration.
(558) Both Parties are active in the production of APIs for pharmaceutical products.
6.1. Relevant market
6.1.1. Product market
(559) In past decisions, the Commission considered that the supply of APIs for each individual molecule might constitute a separate product market that is upstream to the markets for the supply of FDPs, whilst noting that it was not excluded that certain APIs may be substitutable with each other for all, or for a range of, applications.181
(560) The Notifying Parties agree with the previous practice of the Commission and submit that the competitive analysis of the supply of APIs does not have to be conducted at the ATC3 or ATC4 level if competition concerns can be excluded at the level of individual APIs.
(561) The Commission's file does not contain any indication that would suggest departing from the previous practice and the views of the Notifying Parties.
(562) Therefore, for the purpose of this Decision, the product market definition proposed by the Notifying Parties is retained. For the purpose of this case, and for the molecules involved in this case, the Commission therefore considers that the supply of the APIs for each molecule concerned by the Transaction constitutes a separate relevant product market.
6.1.2. Geographic market
(563) In past decisions, the Commission considered that API markets are, from a geographic perspective, at least EEA-wide and possibly global in scope.182 The Notifying Parties submit that in this case the upstream API markets should be defined as global in scope.
(564) The Notifying Parties submit that API suppliers are active globally. The Parties indicate that only [30-40]% of the valid certificates awarded to control the quality of pharmaceutical substances183 were held by European manufacturers, while Chinese and Indian manufacturers held together [50-60]% of these certificates in 2017.184 The Parties further submit that the share of Indian and Chinese manufacturers has steadily increased over recent years. In the Parties’ view, the fact that the majority of CEP holders are from outside of Europe shows that suppliers are able to compete in Europe regardless of their location.
(565) In view of the fact that the Transaction does not raise serious doubts as to its compatibility with the internal market in relation to the API markets under any plausible geographic market definition, the exact scope of the geographic market (i.e. whether it is at least EEA wide or worldwide) can be left open for the purpose of the competitive assessment of the Transaction.185
(566) For the purpose of this case, the Commission considers that for the supply of APIs the geographic market is at least EEA-wide and possibly global in scope.
6.2. Competitive assessment
(567) Mylan generated total worldwide sales of approximately EUR […] million from the sale of APIs to third parties in 2018, of which only [20-30]% (i.e. EUR […] million) are sales achieved in the EEA. Upjohn does not generally supply APIs to third parties, with only very limited exceptions which do not give rise to any affected market in the EEA.
(568) Based on the Commission’s practice, the Commission has applied a system of filters aimed at determining the groups of markets where concerns are most likely and on which it focuses its analysis regarding the supply of APIs, namely:· Horizontally affected markets, where the parties have a combined market share exceeding 35% at the level of the supply of an individual API;186· Downstream vertically affected markets where either party has a market share of more than 30% in the upstream API market and the other party has a market share that exceeds 5% in the downstream FDP market; and· Upstream vertically affected markets where either party has a market share of more than 5% in the upstream API market and the other party has a market share that exceeds 30% in the downstream FDP market.187
(569) In the present case, the Transaction does not result in any horizontal overlaps or downstream vertically relationships falling under these criteria.
(570) The Transaction, however, gives rise to five upstream vertically affected markets falling under these criteria, namely for the supply of alprazolam, celecoxib, eletriptan, sertraline and sildenafil, where Mylan has an estimated worldwide market share of [5-10]% or more on the upstream API market and Upjohn has a market share of [30-40]% or more at the downstream FDP market at the molecule level in various EEA countries.
6.2.1. Alprazolam
(571) While only Mylan is active for the supply of the alprazolam API (Upjohn is not active in this market), both Parties supply alprazolam FDPs in the EEA.
(572) The Parties’ upstream market shares (stemming exclusively from Mylan) for the supply of alprazolam API are provided in Table 55 below:






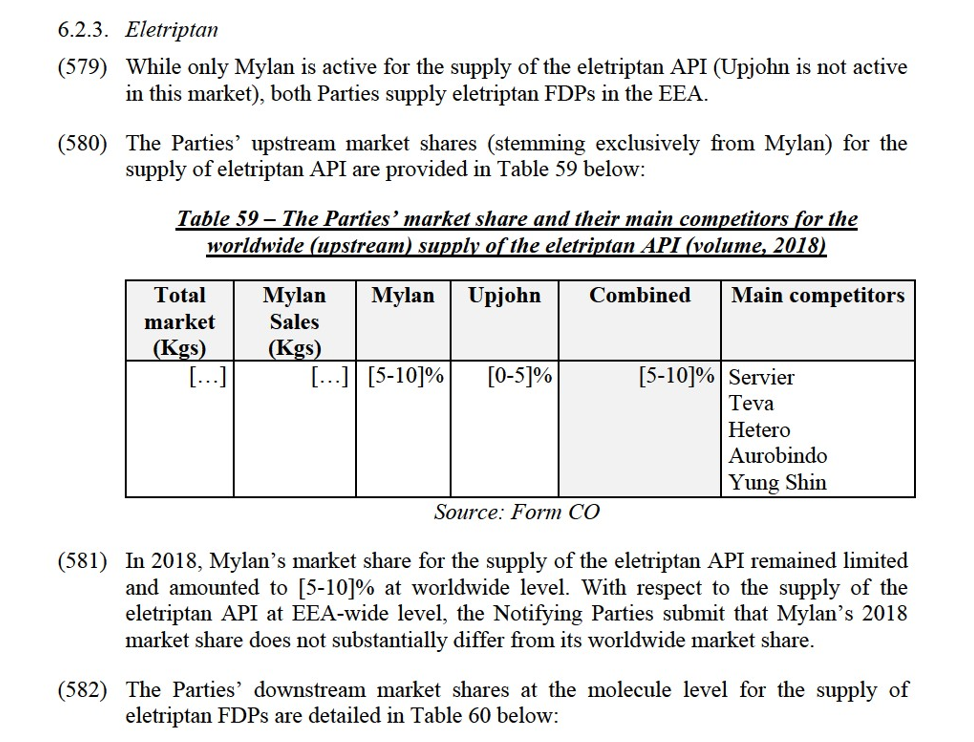








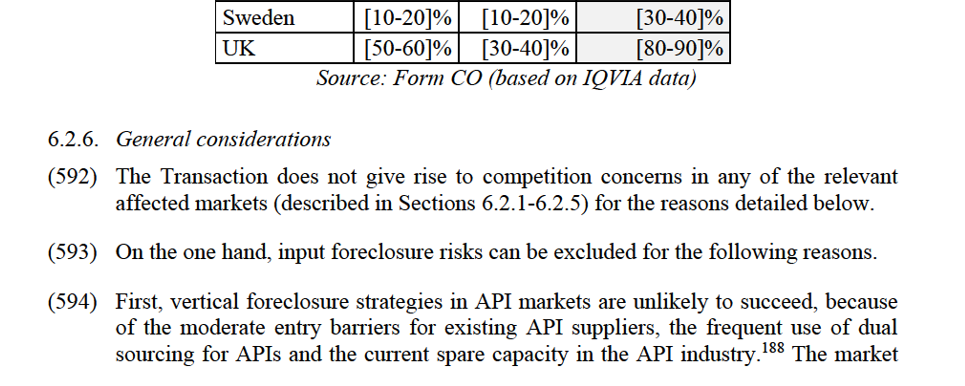
investigation in the present case confirmed that companies rely on multi-sourcing strategies and do not have difficulties to purchase APIs.189
(595) Second, the market shares of the merged entity for the supply of the relevant APIs are not high. In fact, as detailed in Tables 55, 57, 59, and 61, the market shares of the merged entity remain below [5-10]% (volume, 2018) for alprazolam, celecoxib, eletriptan and sertraline. For the supply of sildenafil, the market share of the merged entity is [20-30]% (volume, 2018). In all the relevant API markets (namely alprazolam, celecoxib, eletriptan, sildenafil, and sertraline), only Mylan is active and Upjohn does not contribute any increment. Therefore, the merged entity does not have market power upstream, and consequently does not have the ability to engage in input foreclosure.
(596) Third, the merged entity is unlikely to restrict access to APIs for alprazolam, celecoxib, eletriptan, sertraline or sildenafil to downstream FDP competitors because the merged entity will face competition from multiple companies active in the upstream markets with valid CEPs. The market investigation confirmed that there are a sufficient number of suppliers of APIs for alprazolam, celecoxib, eletriptan, sertraline and sildenafil.190 In particular for sildenafil, which is the only upstream market where the merged entity’s share will exceed [5-10]%, the majority of API suppliers who responded to the market investigation confirmed that they do not face any constraint to increase the volumes of production and that they would be interested in supplying more volumes to existing or new customers.191
(597) Finally, competitors indicated almost unanimously that the Transaction would not have any impact on their ability to purchase APIs, including for alprazolam, celecoxib, eletriptan, sertraline and sildenafil.192
(598) On the other hand, customer foreclosure risks can also be excluded for the following reasons.
(599) [Information on the Parties’ API sourcing]. Therefore, the Transaction does not remove an important customer from the market, and the merged entity will not have the ability or incentive to engage in customer foreclosure.
(600) In addition, competitors active upstream indicated almost unanimously that the Transaction would not have any impact on their ability to sell alprazolam, the APIs for celecoxib, eletriptan, sertraline or sildenafil.193
6.3. Conclusion
(601) Based on the above considerations, taking into account the market investigation and all the evidence available to it, the Commission concludes that the Transaction does not raise serious doubts as to its compatibility with the internal market in relation to any vertical links between the Parties’ activities for APIs and FDPs.
7. OUTLICENSING
(602) Outlicensing in the pharmaceutical industry refers to a company (the licensor) licensing to another company (the licensee) rights to use a dossier to obtain a marketing authorization for a product in one or more countries and commercialize the licensed product in those countries.
7.1. Relevant market
7.1.1. Product market definition
(603) In past decisions, the Commission considered outlicensing for each individual molecule to potentially constitute a separate product market which is upstream of the market for the supply of FDPs.194
(604) Neither the Notifying Parties nor the market investigation provided any indications that the Commission should depart from this approach regarding product market definition.
(605) For the purpose of this case, the Commission therefore considers that individual molecules constitute the relevant product market for outlicensing.
7.1.2.Geographic market definition
(606) In past decisions, the Commission considered the upstream outlicensing market(s) to be at least EEA-wide.195
(607) Neither the Notifying Parties nor the market investigation provided any indications that the Commission should depart from this approach regarding geographic market definition.
(608) For the purpose of this case, the Commission considers that the market(s) of FDP outlicensing to be EEA-wide in geographic scope.




of harm seems however unlikely to materialize in practice due to the below considerations.
(614) Firstly, the merged entity lacks the ability to foreclose FDP competitors, as neither Party is a particularly important competitor in the EEA outlicensing markets upstream for the relevant molecules. In addition, competitors in the market investigation agreed that the merged entity has no competitive advantage in relation to the outlicensing of generics post-Transaction.
(615) Numerous competitors remain on the upstream outlicensing markets for the relevant molecules, including (i) Esteve and Aurobindo for doxazosin; (ii) Teva, Esteve, Adamed, Grupo Tecnimede, Accord, Aurobindo for amlodipine; (iii) Teva, Esteve, and Aurobindo for atorvastatin; (iv) Adamed and Grupo Tecnimede for eplerenone and (v) Esteve and Aurobindo for venlafaxine.202
(616) Secondly, the merged entity would lack the incentive to foreclose FDP competitors. Its existing licensees only account for a minor share of FDP sales downstream and thus the licensees’ sales that could be diverted to the Parties as a result of a potential foreclosure would remain limited. Hence, it would likely not be profitable for the merged entity to forego license fees from those downstream competitors if it were to terminate their license agreements. In most instances, the market share of the Parties' licensee accounts for less than [5-10]% in the supply of the relevant FDP market at molecule level. For instance, [licensee] presence in the Italian doxazosin and amlodipine markets is limited (with respective shares of [0-5]% and [0-5]% in 2018). Similarly, Mylan's [licensee], has only a [0-5]% market share for venlafaxine in Belgium. Regarding atorvastatin in Italy, while Upjohn’s licensee, [licensee], has an important market share (of [5-10]% in 2018), [licensee] share from the sale of the out-licensed product amounts to less than 1%.203 Regarding eplerenone in Spain, Upjohn’s licensee, [licensee], has a high share (of [40-50]% in 2018). However, the Commission notes in this respect that the merged entity would not necessarily recuperate [licensee] market share if it were to terminate [licensee] licensing contract. First, [licensee] could switch to an alternative outlicensor (e.g. Adamed and Grupo Tecnimede). Second, even if [licensee] would not switch to an alternative licensor, other competitors at the FDP level would compete with the merged entity to recuperate [licensee] market share. In particular, for the supply of eplerenone in Spain (downstream), the Parties’ market share is limited (it amounted to [5-10]%) while the merged entity will continue to face strong competition from a number of other competitors than [licensee] with shares greater than [5-10]%, including Vir ([10-20]%), Infarco ([5-10]%), Teva ([5-10]%), Stada ([5-10]%), Normon ([5-10]%)and Novartis ([5-10]0%).
(617) In addition, the market investigation did not reveal any substantiated concerns in relation to outlicensing activities. Competitors also generally confirm that in their view, the merger has no impact on the outlicensing markets in the EEA.204
7.3. Conclusion
(618) Based on the above considerations, taking into account the market investigation and all the evidence available to it, the Commission concludes that the Transaction does not raise serious doubts as to its compatibility with the internal market in relation to the Parties' outlicensing activities in the EEA.
8. COMMITMENTS
8.1. Framework for the assessment of the Commitments
(619) Where a notified concentration raises serious doubts as to its compatibility with the internal market, the parties may undertake to modify the concentration to remove the grounds for the serious doubts identified by the Commission. Pursuant to Article 6(2) of the Merger Regulation, where the Commission finds that, following modification by the undertakings concerned, a notified concentration no longer raises serious doubts, it shall declare the concentration compatible with the internal market pursuant to Article 6(1)(b) of the Merger Regulation.
(620) As set out in the Commission's Remedies Notice,205 the commitments have to eliminate the competition concerns entirely, and have to be comprehensive and effective from all points of view.206
(621) In assessing whether commitments will maintain effective competition, the Commission considers all relevant factors, including the type, scale and scope of the proposed commitments with reference to the structure and the particular characteristics of the market in which the Transaction is likely to significantly impede effective competition, including the position of the Parties and other participants on the market.207
(622) In order for the commitments to comply with those principles, they must be capable of being implemented effectively within a short period. Concerning the form of acceptable commitments, the Merger Regulation gives discretion to the Commission as long as the commitments meet the required standards. Structural commitments will meet the conditions set out above only in so far as the Commission is able to conclude with the requisite degree of certainty, at the time of its Decision, that it will be possible to implement them and that it will be likely that the new commercial structures resulting from them will be sufficiently workable and lasting to ensure that effective competition will be maintained.208 Divestiture commitments are normally the best way to eliminate competition concerns resulting from horizontal overlaps.
(623) The divested activities must consist of a viable business that, if operated by a suitable purchaser, can compete effectively with the merged entity on a lasting basis and that is divested as a going concern. The business must include all the assets which contribute to its current operation or which are necessary to ensure its viability and competitiveness and all personnel which are currently employed or which are necessary to ensure the business' viability and competitiveness.209
(624) The intended effect of the divestiture will only be achieved if and once the business is transferred to a suitable purchaser in whose hands it will become an active competitive force in the market. The potential of a business to attract a suitable purchaser is an important element of the Commission's assessment of the appropriateness of the proposed commitment.210
(625) Even though normally the divestiture of an existing viable stand-alone business is required, the Commission, taking into account the principle of proportionality, may also consider the divestiture of businesses which have existing strong links or are partially integrated with businesses retained by the parties and therefore need to be ‘carved out’ in those respects. Commitments including a carve-out of a business can only be accepted by the Commission if it can be certain that, at least at the time when the business is transferred to the purchaser, a viable business on a stand-alone basis will be divested and the risks for the viability and competitiveness caused by the carve-out will thereby be reduced to a minimum.
(626) It is against this background that the Commission analysed the proposed commitments in this case.
8.2. Procedure
(627) In order to render the concentration compatible with the internal market, the Parties have submitted a set of commitments under Article 6(2) of the Merger Regulation on 27 March 2020 (the "Initial Commitments"). The Commission market tested the Initial Commitments on 30 March 2020 in order to assess whether they were sufficient and suitable to remedy the serious doubts identified in Section 5.4 above. Following the feedback received during the market test, the Initial Commitments were refined and improved, and amended commitments were submitted on 17 April 2020 (the “Final Commitments”). The Final Commitments are annexed to this Decision and form an integral part thereof.
8.3. The Initial Commitments
(628) Under the Initial Commitments, the Parties offered to divest Mylan’s products, in markets where serious doubts were identified following the Commission's market investigation, to one or more suitable third party purchasers (“the Purchaser(s)”).
(629) The products that the Parties offered to divest under the Initial Commitments (hereafter referred together as the “Divestment Businesses”, and each individually as a “Divestment Business”) are listed below:
(i) Mylan's atorvastatin in Norway;
(ii) Mylan's doxazosin in Czechia;
(iii) Mylan's doxazosin in France;
(iv) Mylan's eplerenone in Belgium;
(v) Mylan's eplerenone in Hungary;
(vi) Mylan's sildenafil (PAH) in Estonia;
(vii) Mylan's sildenafil (PAH) in France;
(viii) Mylan's tadalafil in France;
(ix) Mylan's sildenafil (PAH) in Latvia;
(x) Mylan's sildenafil (PAH) in Lithuania;
(xi) Mylan's sildenafil (PAH) in Romania;
(xii) Mylan's sildenafil (PAH) in the United Kingdom;
(xiii) Mylan's eletriptan in Denmark;
(xiv) Mylan's eletriptan in Finland;
(xv) Mylan's eletriptan in France;
(xvi) Mylan's eletriptan in Norway;
(xvii) Mylan's eletriptan in Sweden;
(xviii) Mylan's pregabalin in Belgium;
(xix) Mylan's pregabalin in Czechia;
(xx) Mylan's pregabalin in Luxembourg;
(xxi) Mylan's pregabalin in Norway;
(xxii) Mylan's gabapentin in Ireland;
(xxiii) Mylan's ziprasidone in Czechia;
(xxiv) Mylan's alprazolam in Greece;
(xxv) Mylan's alprazolam in Iceland;
(xxvi) Mylan's alprazolam in Ireland;
(xxvii) Mylan's alprazolam in Italy;
(xxviii) Mylan's alprazolam in Portugal;
(xxix) Mylan's venlafaxine in Belgium;
(xxx) Mylan's latanoprost in Belgium;
(xxxi) Mylan's latanoprost in Luxembourg;
(xxxii) Mylan's latanoprost/timolol in Belgium;
(xxxiii) Mylan's latanoprost/timolol in France;
(xxxiv) Mylan's latanoprost/timolol in Italy;
(xxxv) Mylan's latanoprost/timolol in Luxembourg;
(xxxvi) Mylan's latanoprost/timolol in the Netherlands; and
(xxxvii) Mylan's latanoprost/timolol in Portugal.
(630) The Divestment Businesses are structured as an asset carve-out; no legal entity of Mylan is to be divested. Specifically, under the Initial Commitments, the Parties committed that the Divestment Business(es) include the following assets and rights:i. all tangible and intangible assets (including intellectual property rights, which contribute to the current operation and are necessary to ensure the viability, marketability and competitiveness of the Divestment Business);ii. all licences, permits and authorizations issued by any governmental organisation for the benefit of the Divestment Business;iii. all contracts, commitments and customer orders of the Divestment Business, insofar they relate to the Divestment Business; all customer, credit and other records of the Divestment Business;iv. all advertising, marketing, sales, publicity and presentational materials related to the Divestment Business, as applicable;v. a best efforts obligation for the Parties to obtain the assignment (at the Purchaser’s option) of existing contract manufacturing contracts and/or API supply contracts, where applicable, or, in the event that consent for the assignment cannot be obtained, the benefit of a back-to-back arrangement for the supply of the product to the Purchaser for the duration of the relevant third party contract and, in any case, for no longer than three years;vi. a best efforts obligation for the Parties to obtain the assignment (at the Purchaser’s option) of existing licensing agreements and/or any other relevant contract currently in place including any awarded tender contracts (to the extent that national legislation allows for assignment), where applicable;vii. the benefit, for a period of up to 3 years after the closing of the divestment (the "Divestment Closing"), of (i) an at-cost transitory manufacturing or supply arrangement relating to the existing forms of the product in the Member State of the Divestment Business to be agreed with the Purchaser and overseen by the person(s) approved by the Commission, and entrusted with the duty to monitor Mylan's compliance to this Decision (the “Monitoring Trustee”) (such transitory arrangement(s) shall include the appropriate provisions to ensure the continued supply of the product to the Purchaser, including prioritization of the supply to the Purchaser in case of shortages); and/or (ii) reasonable technical assistance to the Purchaser or a third party manufacturer indicated by the Purchaser, provided on a no cost basis, to assume responsibility for the manufacture, sale and marketing of the relevant Divestiture Business, to be agreed with the Purchaser and overseen by the Monitoring Trustee; andviii. an option for the Purchaser to hire one or more Mylan personnel, who work for the relevant Divestment Business and would be considered necessary to maintain the viability, marketability and competitiveness of that Divestment Business to be supervised by the Monitoring Trustee. This option is to be exercised within a period of one year after Divestment Closing.
(631) In addition, the Parties have offered related commitments, inter alia regarding the separation of the Divestment Businesses from their retained businesses, the preservation of the economic viability, marketability and competitiveness of the Divestment Businesses, including the appointment of a Monitoring Trustee and, if necessary, the person(s) approved by the Commission with a mandate to sell the Divestment Business to a Purchaser at no minimum price (the “Divestiture Trustee”).
(632) The Initial Commitments also include specific purchaser requirements in particular the need for the Purchaser(s) to be either (i) an established generic supplier with presence in the EEA that can market the Divestment Businesses through its own commercial infrastructure or through distributors in the relevant countries where each relevant Divestment Business is currently active, or (ii) an established generic supplier that is not yet present in the EEA but has an advanced plan to enter the EEA which existed prior and unrelated to the divestment transaction and will persuade the Monitoring Trustee and the Commission that it can effectively start competing in the relevant markets within the required timeframe.
8.4. Assessment of the Initial Commitments
(633) The Commission analysed the suitability of the Commitments to remedy the serious doubts raised by the Transaction, in particular under the principles set out in the Commission Remedies Notice. In its assessment, the Commission relied inter alia on the results of the market test launched on 30 March 2020.
(634) Respondents to the market test were generally positive about the Initial Commitments and confirmed that they are suitable to eliminate the competition concerns identified by the Commission.211 In particular, the majority of respondents considered that, provided they are divested to suitable purchasers, the Divestment Businesses include all the necessary assets to successfully market the specific molecules in the markets where the Commission identified competition concerns and to subsequently compete effectively with the merged entity on these markets.212 In addition, respondents highlighted that the transfer/assignment of existing contracts (licensing, manufacturing, tender contracts etc.) proposed in the Initial Commitments is crucial for the Divestment Businesses to remain a viable and competitive force after the transfer to suitable purchasers.213 Moreover, some respondents highlightedthat some individual molecule markets offered for divestment may not be viable when transferred on a standalone basis and hence, a certain grouping of Divestment Businesses could be required.214 For this reason, and the reasons described in paragraph (636)ii, the Commission welcomes the requirement set out in the Initial Commitments according to which, in the event that multiple Purchasers were proposed to acquire the Divestment Businesses, the Commission will approve the different Purchasers and correlated sale and purchase agreements at the same time. Such approach ensures that the transfer of each individual molecule to a suitable purchaser is carried out, regardless of the grouping ultimately adopted.
(635) However, the results of the market test also identified issues with the Initial Commitments requiring further improvements.
(636) First, while the Initial Commitments allowed for the closing of the Transaction before completing the transfer of the Divestment Businesses, the Commission considers for the following reasons, based on the results of the market test and the evidence available to it, that an upfront buyer is necessary to ensure the viability and competitiveness of the Divestment Businesses:i. Only a limited number of companies with an existing presence in the EEA have expressed an interest in purchasing all Divestment Businesses together. A more significant number of respondents expressed interest in purchasing some Divestment Businesses (as opposed to all). However, even adding up these respondents would still leave some Divestment Businesses with no suitable Purchaser.215ii. While the responses to the market test were mixed as to whether the viability and competitiveness of the Divestment Businesses will be negatively affected if its transfer occurs after the closing of the Transaction,216 a significant number of respondents potentially interested in purchasing the Divestment Business(es) considered it necessary to sell the Divestment Business(es) before the closing of the Transaction in this case. The reasons expressed by the respondents included the following: (i) the merged entity has a strategic advantage vis-à-vis the Purchaser(s), being active with branded originator products that are considered “must-haves” and not having to deal with contract assignments, transfers and the uncertainty of robust supply, which is important for customers of generic manufacturers; and (ii) the “dispersed” composition of the Divestment Business in terms of molecules and countries led to limited interest in the market test of potential buyers to purchase the whole Divestment Business.iii. In addition, the Commission notes that a number of divested molecules have declining sales and are not among the most sought after molecules, given that loss of exclusivity happened between five to over twenty years ago, and may therefore face difficulties to attract a suitable Purchaser.
(637) Second, with regard to the Purchaser requirements, the results of the market test indicated, that a Purchaser with no existing generic presence in the EEA is unlikely to be suitable. However, respondents to the market test expressed the view that the presence could be direct or indirect via third party distributor/s, as the importance of a direct salesforce seems to vary from country to country.217 As a consequence, the Commission considers that the Purchaser criteria as set out in the Initial Commitments required further improvements so as to require the Purchaser(s) to be an established generic supplier with presence in the EEA that can market the products of the Divestment Businesses through its own commercial infrastructure or through distributors in the relevant countries where each relevant Divestment Business is currently active.
(638) Third, with regard to the transitional supply agreements, the results of the market test indicated that the proposed duration of three years might be insufficient to ensure the viability of the Divestment Businesses and allow the Purchaser to be fully operational.218 Therefore, the Commission considers that the duration of the transitional supply agreements should be extendable beyond three years (for at least an additional year) at the option of the Purchaser and under the supervision of the Monitoring Trustee.
8.5. The Final Commitments
(639) On 17 April 2020, the Parties submitted a revised set of commitments (the "Final Commitments") taking into account the results of the market test of the Initial Commitments.
(640) First, the Parties included an upfront buyer clause in the Final Commitments. As a result, the Transaction shall not be implemented before Mylan (or the Divestiture Trustee) has entered into a final binding sale and purchase agreement for the sale of the Divestment Businesses and the Commission has approved the Purchaser or Purchasers and the terms of sale. Upon the Parties’ reasoned request accompanied by a reasoned opinion by the Monitoring Trustee, the Commission may (i) approve the Purchaser(s) on the basis of (a) legally binding agreement(s) for the sale of the Divestment Business(es) setting out all material terms and conditions of such sale, but prior to the execution of final sale and purchase agreement(s) and (ii) approve the implementation of the Transaction. In that case, the final sale and purchase agreement(s) (including any ancillary agreement relevant for the purpose of the Final Commitments) shall be approved separately by the Commission.
(641) Second, the Parties amended the Purchaser requirements to reflect the market test feedback. Under the Final Commitments, a Purchaser with no existing generic presence in the EEA would no longer be suitable. However, an established generic supplier with presence in the EEA that can market products of the Divestment Businesses through its own commercial infrastructure or through distributors in the relevant countries where each relevant Divestment Business is currently active, would be a suitable Purchaser.
(642) Third, the Parties amended the duration of transitional supply agreements. Under the Final Commitments, these agreements would have an initial term of up to three years, and may be extended for an additional year, up to two times (for a total duration of five years) at the request of the Purchaser/s and subject to the approval of the Monitoring Trustee.
8.6. Assessment of the Final Commitments
(643) The Commission considers that these amendments adequately address the concerns raised by the market test respondents and the Commission itself in relation to the Initial Commitments. The Commission notes in particular that the introduction of an upfront purchaser requirement, in light of the limited interest from market participants and the specificities of the Divestment Businesses, contributes to the viability and competitiveness of the Divestment Businesses, by guaranteeing timely divestment to (a) suitable Purchaser(s).
(644) In addition, the Commission may, if it considers it appropriate in the circumstances,(i) approve the Purchaser(s) on the basis of (a) legally binding agreement(s) setting out all material terms and conditions of the sale of the Divestment Businesses but prior to Mylan (or the Divestiture Trustee) having entered into (a) final sale and purchase agreement(s) for the sale of the Divestment Businesses and (ii) approve the implementation of the Transaction, based on the Parties’ reasoned request accompanied by a reasoned opinion by the Monitoring or Divestiture Trustee. Indeed, the Final Commitments imply the negotiation of numerous ancillary agreements, including transitional supply agreements for the 37 Divestment Businesses across 20 different EEA countries. However, the exceptional circumstances brought about by the coronavirus outbreak, and the disruptions caused by it to the usual course of business, notably in the pharmaceutical industry, may delay the finalisation of these ancillary agreements. Moreover, even if the Parties request that the Commission uses this possibility and the Commission accepts it, the final sale and purchase agreement(s), including all ancillary agreement(s), will remain subject to the Commission’s approval.
(645) Based on the above, the Commission concludes that the Divestment Businesses are viable businesses and the modalities foreseen for their transfer under the Final Commitments will allow suitable Purchaser(s) to operate them in a competitive and viable manner.
(646) The Final Commitments fully address the competition concerns identified in this Decision as they remove the overlap between Mylan and Upjohn in all markets where serious doubt arise, replacing Mylan with an alternative generic player.
(647) Moreover, the Commitments are comprehensive, effective, and are capable of being implemented effectively within a short period.
(648) The Commission therefore considers that the Final Commitments are sufficient to eliminate all serious doubts as to the compatibility of the Transaction with the internal market and the EEA Agreement.
8.7. Conditions and obligations
(649) Under the first sentence of the second subparagraph of Article 6(2) of the Merger Regulation, the Commission may attach to its decision conditions and obligations intended to ensure that the undertakings concerned comply with the commitments they have entered into vis-à-vis the Commission with a view to rendering a notified concentration compatible with the internal market.
(650) The achievement of the measure that gives rise to the structural change of the market is a condition, whereas the implementing steps which are necessary to achieve this result are generally obligations on the Parties. Where a condition is not fulfilled, the Commission’s decision declaring the concentration compatible with the internal market no longer stands. Where the undertakings concerned commit a breach of an obligation, the Commission may revoke the clearance decision in accordance with Article 8(6) of the Merger Regulation. The undertakings concerned may also be subject to fines and periodic penalty payments under Articles 14(2) and 15(1) of the Merger Regulation.
(651) In accordance with the distinction described above, this Decision in this case is conditioned on the full compliance with the requirements set out in Section B of the Final Commitments (including the Schedules), which constitute conditions. The remaining requirements set out in the other sections of the Final Commitments constitute obligations on the Parties.
(652) The detailed text of the Final Commitments is annexed to this Decision. The full text of the Final Commitments forms an integral part of this Decision.
9. CONCLUSION
(653) For the above reasons, the Commission has decided not to oppose the notified operation as modified by the Commitments and to declare it compatible with the internal market and with the functioning of the EEA Agreement, subject to full compliance with the conditions in Section B (including the Schedules) of the Commitments annexed to this Decision and with the obligations contained in the other sections of the said Commitments. This Decision is adopted in application of Article 6(1)(b) in conjunction with Article 6(2) of the Merger Regulation and Article 57 of the EEA Agreement.
Case M.9517 | Commitments
COMMITMENTS TO THE EUROPEAN COMMISSION
Pursuant to Article 6(2) of Council Regulation (EC) No 139/2004 (the "Merger Regulation"), Mylan N.V. ("Mylan") and Upjohn (“Upjohn”) hereby enters into the following Commitments (the "Commitments") vis-à-vis the European Commission (the "Commission") with a view to rendering their merger (the "Transaction") compatible with the internal market and the functioning of the EEA Agreement.
This text shall be interpreted in light of the Commission’s decision pursuant to Article 6(1)(b) of the Merger Regulation to declare the Transaction compatible with the internal market and the functioning of the EEA Agreement (the "Decision"), in the general framework of European Union law, in particular in light of the Merger Regulation, and by reference to the Commission Notice on remedies acceptable under Council Regulation (EC) No 139/2004 and under Commission Regulation (EC) No 802/2004 (the "Remedies Notice").
SECTION A – DEFINITIONS
(1) For the purpose of the Commitments, the following terms shall have the following meaning:
Affiliated Undertakings: undertakings controlled by the Parties and/or by the ultimate parents of the Parties, whereby the notion of control shall be interpreted pursuant to Article 3 of the Merger Regulation and in light of the Commission Consolidated Jurisdictional Notice under Council Regulation (EC) No 139/2004 on the control of concentrations between undertakings (the "Consolidated Jurisdictional Notice").
Assets: the assets that contribute to the current operation or are necessary to ensure the viability and competitiveness of the Divestment Business as indicated in Section B, paragraph 6 (a), (b) and (c) and described more in detail in the Schedule.
Divestment Closing: the transfer of the legal title to the Divestment Business to the Purchaser.
Divestment Closing Period: the period of […] months from the approval of the Purchaser and the terms of sale by the Commission.
Confidential Information: any business secrets, know-how, commercial information, or any other information of a proprietary nature that is not in the public domain.
Conflict of Interest: any conflict of interest that impairs the Trustee's objectivity and independence in discharging its duties under the Commitments.
Divestment Business: each of the businesses that Mylan commits to divest as defined in Section B and in the Schedules.
Divestiture Trustee: one or more natural or legal person(s) who is/are approved by the Commission and appointed by Mylan and who has/have received from Mylan the exclusive Trustee mandate to sell the Divestment Business to a Purchaser at no minimum price.
Effective Date: the date of adoption of the Decision.
First Divestiture Period: the period of […] months from the Effective Date.
Hold Separate Manager: the person appointed by Mylan to manage the day-to-day operations of the Divestment Business under the supervision of the Monitoring Trustee.
Monitoring Trustee: one or more natural or legal person(s) approved by the Commission, appointed by Mylan, and entrusted with the duty to monitor Mylan's compliance with the conditions and obligations attached to the Decision.
Mylan: Mylan N.V. a private limited liability company organized and existing under the laws of the Netherlands, with its corporate seat in Amsterdam, the Netherlands.
Parties: Mylan and Upjohn or following the closing of the Transaction, Viatris.
Personnel: the staff that could be considered necessary to maintain the viability, marketability and competitiveness of the Divestment Business.
Pfizer: Pfizer Inc. a pharmaceutical company active worldwide in the research, development, manufacturing and marketing of innovative medicines with its corporate headquarters in 235 East 42nd Street, New York, NY 10017, USA.
Purchaser: the entity approved by the Commission as acquirer of the Divestment Business in accordance with the criteria set out in Section D.
Purchaser Criteria: the criteria laid down in paragraph 17 of these Commitments that the Purchaser must fulfil to be approved by the Commission.
Retained Business(es): Upjohn’s overlapping products in the relevant markets, which give rise to the need for a divestment and which are not part of the Commitments.
Schedule: the schedule to these Commitments describing the Divestment Business in more detail.
Trustee(s): the Monitoring Trustee and/or the Divestiture Trustee as the case may be.
Trustee Divestiture Period: the period of […] months from the end of the First Divestiture Period.
Upjohn: the Upjohn business being spun-off and combined with Mylan. Upjohn is made up of (a) a portfolio of 21 established brands organised across the following key therapeutic areas: (i) Cardiovascular, (ii) Central Nervous System/Psychiatry, (iii) Pain/Neurology, (iv) Urology and (v) Ophthalmology; and (b) Greenstone LLC (Greenstone), a US-focused generics business. Greenstone sells non-branded authorized generic versions of Pfizer branded products (and a very small number of authorized generics from Allergan) exclusively in the US.
SECTION B – THE COMMITMENT TO DIVEST AND THE DIVESTMENT BUSINESS
Commitment to divest
(2) To maintain effective competition, Mylan commits to divest, or procure the divestiture of, the Divestment Business by the end of the Trustee Divestiture Period to one or more purchasers and on terms of sale approved by the Commission in accordance with the procedure described in paragraph 18 of these Commitments. To carry out the divestiture, Mylan commits to finding a purchaser/purchasers and entering into one or more final binding sale and purchase agreements for the sale of the Divestment Business within the First Divestiture Period. In the event that Mylan proposes to divest the Divestment Business to multiple purchasers, Mylan commits to submitting the reasoned proposals and the relevant sale and purchase agreements to the Commission for approval at the same time. If Mylan has not entered into such an agreement (or agreements) at the end of the First Divestiture Period, Mylan shall grant the Divestiture Trustee an exclusive mandate to sell the Divestment Business in the Trustee Divestiture Period in accordance with the procedure described in paragraph 30.
(3) The Transaction shall not be implemented before Mylan or the Divestiture Trustee has entered into (a) final binding sale and purchase agreement(s) for the sale of the Divestment Businesses and the Commission has approved the purchaser or purchasers and the terms of sale in accordance with paragraph 19. Upon the Parties’ reasoned request accompanied by a reasoned opinion by the Trustee and the previous sentence notwithstanding, the Commission may (i) approve the purchaser or purchasers on the basis of (a) legally binding agreement(s) between Mylan or the Divestiture Trustee and the purchaser(s) for the sale of the Divestment Business(es) setting out all material terms and conditions of such sale but prior to Mylan or the Divestiture Trustee having entered into (a) final sale and purchase agreement(s) for the sale of the Divestment Businesses and (ii) approve the implementation of the Transaction. The final sale and purchase agreement(s) (including any ancillary agreement relevant for the purpose of these Commitments) shall be subject to approval in line with paragraph 19.


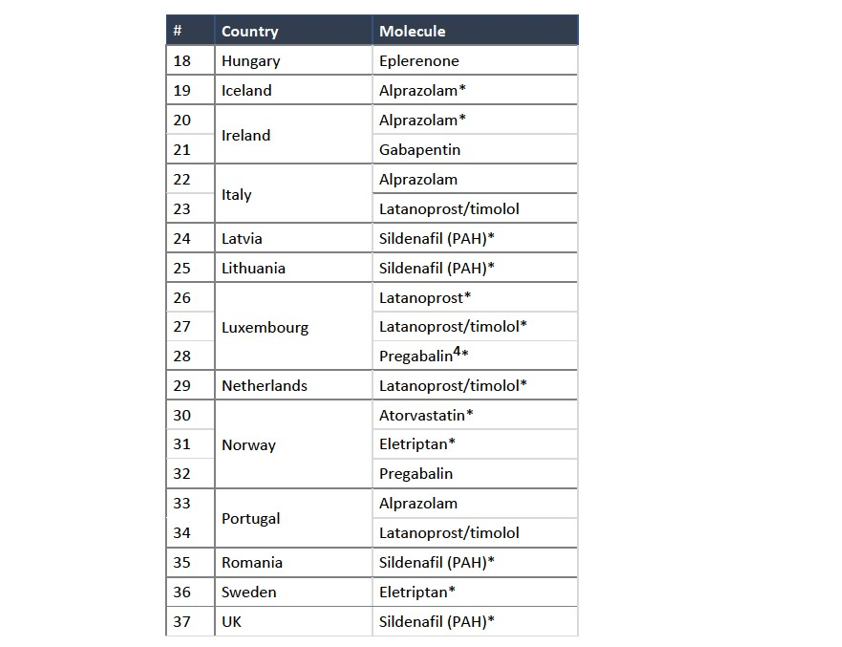

no longer than three years;(f) if such contract exists, a best efforts obligation to obtain the assignment (at the purchaser’s option) of existing licensing agreements and/or any other relevant contract currently in place including any awarded tender contracts (to the extent that national legislation allows for assignment);(g) the benefit, for a period of up to 3 years after Divestment Closing, which period may be extended by one year, and an additional year thereafter, upon request by the Purchaser and subject to the approval of the Monitoring Trustee on the basis that such extension is necessary for the Purchaser to continue commercializing the Divestment Business, of (i) an at-cost transitory manufacturing or supply arrangement relating to the existing forms of the product in the Member State of the Divestment Business to be agreed with the Purchaser and overseen by the Monitoring Trustee in accordance with paragraph (29)(i)(iii) (such transitory arrangement(s) shall include the appropriate provisions to ensure the continued supply of the product to the Purchaser, including prioritization of the supply to the Purchaser in case of shortages); and/or (ii) reasonable technical assistance to the Purchaser or a third party manufacturer indicated by the Purchaser, provided on a no cost basis, to assume responsibility for the manufacture, sale and marketing of the relevant Divestiture Business, as detailed in the Schedules to be agreed with the Purchaser and overseen by the Monitoring Trustee in accordance with paragraph (29)(i)(iii); and(h) in relation to the Divestment Businesses set out in the Schedules, subject to applicable local employment legislation, an option for the Purchaser to hire one or more Mylan Personnel, who work for the relevant Divestment Business and who would be considered necessary to maintain the viability, marketability and competitiveness of that Divestment Business to be supervised by the Monitoring Trustee. This option is to be exercised within a period of one year after Divestment Closing.6
(8) The Divestment Business is structured as an asset carve-out; no legal entity of Mylan is to be divested.
SECTION C – RELATED COMMITMENTS
Preservation of viability, marketability and competitiveness
(9) From the Effective Date until Divestment Closing, the Parties shall preserve or procure the preservation of the economic viability, marketability and competitiveness of the Divestment Businesses, in accordance with good business practice, and shall minimize as far as possible any risk of loss of competitive potential of the Divestment Businesses. In particular, Mylan undertakes:(a) not to carry out any action that might have a significant adverse impact on the value, management or competitiveness of the Divestment Businesses or that might alter the nature and scope of activity, or the industrial or commercial strategy or the investment policy of the Divestment Businesses;(b) to make available, or procure to make available, sufficient resources for the development of the Divestment Businesses, on the basis and continuation of the existing business plans.
Hold-separate obligations
(10) The Parties commit from the Effective Date until Divestment Closing, to the extent reasonably practicable, to keep the Divestment Businesses separate from the Retained Business. The Parties also commit to ensuring that the Personnel of the Divestment Businesses – including the Hold Separate Manager – will not be involved in the Retained Business. The Parties likewise commit to ensuring that the personnel of the Retained Business will not be involved in the Divestment Businesses.
(11) Until Divestment Closing, Mylan shall assist the Monitoring Trustee in ensuring that the Divestment Businesses are managed separately and as saleable businesses from the Retained Business. Immediately after the adoption of the Decision, Mylan shall appoint a Hold Separate Manager. The Hold Separate Manager shall manage the Divestment Businesses independently of the Retained Business and in the best interest of each Divestment Business with a view to ensuring its continued economic viability, marketability and competitiveness. The Hold Separate Manager shall closely cooperate with and report to the Monitoring Trustee and, if applicable, the Divestiture Trustee. In case of any replacement of the Hold Separate Manager, Mylan shall provide a reasoned proposal to replace the person(s) concerned to the Commission and the Monitoring Trustee. Mylan must be able to demonstrate to the Commission that the replacement is well-suited to carry out the functions exercised by the Hold Separate Manager. The replacement shall take place under the supervision of the Monitoring Trustee, who shall report to the Commission. The Commission may require Mylan to replace the Hold Separate Manager, after having heard Mylan.
Ring-fencing
(12) Mylan shall implement, or procure to implement, all necessary measures to ensure that its personnel that manages the Divestment Businesses shall not obtain commercially sensitive and/or product specific Confidential Information relating to the Retained Business.
(13) Mylan shall implement, or procure to implement, all necessary measures to ensure that its personnel that manages the Retained Business shall not obtain commercially sensitive and/or product specific Confidential Information relating to the Divestment Businesses.
Non-Solicitation clause
(14) In the instance that the Purchaser exercises the option as described in paragraph 6(g), the Parties undertake, subject to customary limitations, not to solicit, and to procure that Affiliated Undertakings do not solicit Personnel hired by (as opposed to seconded to) the Purchaser according to paragraph 6(g) for a period of 24 months after Divestment Closing.
Due diligence
(15) In order to enable potential purchasers to carry out a reasonable due diligence of the Divestment Businesses, Mylan shall, subject to customary confidentiality assurances and subject to confidentiality obligations vis-à-vis third parties and dependent on the stage of the divestiture process:(a) provide to potential purchasers sufficient information as regards the Divestment Businesses;(b) provide to potential purchasers sufficient information relating to the Personnel and allow them reasonable access to the Personnel.
Reporting
(16) Mylan shall submit written reports in English on potential purchasers of the Divestment Businesses and developments in the negotiations with such potential purchasers to the Commission and the Monitoring Trustee no later than 10 days after the end of every month following the Effective Date (or otherwise at the Commission’s request). Mylan shall submit a list of all potential purchasers having expressed interest in acquiring the Divestment Businesses to the Commission at each and every stage of the divestiture process, as well as a copy of all the offers made by potential purchasers within five days of their receipt.
(17) Mylan shall inform the Commission and the Monitoring Trustee on the preparation of the data room documentation and the due diligence procedure and shall submit a copy of any information memorandum to the Commission and the Monitoring Trustee before sending the memorandum out to potential purchasers.
SECTION D – THE PURCHASER
(18) In order to be approved by the Commission, the Purchaser must fulfil the following criteria:(a) The Purchaser shall be independent of and unconnected to the Parties and their Affiliated Undertakings (this being assessed having regard to the situation following the divestiture);(b) The Purchaser shall have the financial resources, proven expertise and incentive to maintain and develop the relevant Divestment Business (or Divestment Businesses) as a viable and active competitive force in competition with the Parties and other competitors;(c) The Purchaser shall be an established generic supplier with presence in the EEA that can market the relevant Divestment Business (or Divestment Businesses) through its own commercial infrastructure or through distributors in the relevant countries where each relevant Divestment Business is currently active; and(d) The acquisition of the relevant Divestment Business (or Divestment Businesses) by the Purchaser must neither be likely to create, in light of the information available to the Commission, prima facie competition concerns nor give rise to a risk that the implementation of the Commitments will be delayed. In particular, the Purchaser must reasonably be expected to obtain all necessary approvals from the relevant regulatory authorities for the acquisition of the Divestment Business(es).
(19) Subject to paragraph 3, the final binding sale and purchase agreement(s) (as well as ancillary agreements) relating to the divestment of the Divestment Businesses shall be conditional on the Commission’s approval. When Mylan has reached an agreement with a purchaser, it shall submit a fully documented and reasoned proposal, including a copy of the final agreement(s), within one week to the Commission and the Monitoring Trustee. Mylan must be able to demonstrate to the Commission that the purchaser fulfils the Purchaser Criteria and that the relevant Divestment Business is being sold in a manner consistent with the Commission's Decision and the Commitments. For the approval, the Commission shall verify that the purchaser fulfils the Purchaser Criteria and that the Divestment Business is being sold in a manner consistent with the Commitments including their objective to bring about a lasting structural change in the market. The Commission may approve the sale of the Divestment Businesses without one or more Assets or parts of the Personnel, or by substituting one or more Assets or parts of the Personnel with one or more different assets or different personnel, if this does not affect the viability and competitiveness of the Divestment Businesses after the sale, taking account of the proposed purchaser(s).
SECTION E – TRUSTEE
I. Appointment procedure
(20) Mylan shall appoint a Monitoring Trustee to carry out the functions specified in these Commitments for a Monitoring Trustee. Mylan commits not to close the Transaction before the appointment of a Monitoring Trustee.
(21) If Mylan has not entered into binding sale and purchase agreements regarding the Divestment Businesses one month before the end of the First Divestiture Period or if the Commission has rejected a purchaser proposed by Mylan at that time or thereafter, Mylan shall appoint a Divestiture Trustee. The appointment of the Divestiture Trustee shall take effect upon the commencement of the Trustee Divestiture Period.
(22) The Trustee shall:(i) at the time of appointment, be independent of the Parties and their Affiliated Undertakings;(ii) possess the necessary qualifications to carry out its mandate, for example have sufficient relevant experience as an investment banker or consultant or auditor; and(iii) neither have nor become exposed to a Conflict of Interest.
(23) The Trustee shall be remunerated by Mylan in a way that does not impede the independent and effective fulfilment of its mandate. In particular, where the remuneration package of a Divestiture Trustee includes a success premium linked to the final sale value of the Divestment Business, such success premium may only be earned if the divestiture takes place within the Trustee Divestiture Period.
Proposal by Mylan
(24) No later than two weeks after the Effective Date, Mylan shall submit the name or names of one or more natural or legal persons whom Mylan proposes to appoint as the Monitoring Trustee to the Commission for approval. No later than one month before the end of the First Divestiture Period or on request by the Commission, Mylan shall submit a list of one or more persons whom Mylan proposes to appoint as Divestiture Trustee to the Commission for approval. The proposal shall contain sufficient information for the Commission to verify that the person or persons proposed as Trustee fulfil the requirements set out above and shall include:(a) the full terms of the proposed mandate, which shall include all provisions necessary to enable the Trustee to fulfil its duties under these Commitments;(b) the outline of a work plan which describes how the Trustee intends to carry out its assigned tasks;(c) an indication whether the proposed Trustee is to act as both Monitoring Trustee and Divestiture Trustee or whether different trustees are proposed for the two functions.
Approval or rejection by the Commission
(25) The Commission shall have the discretion to approve or reject the proposed Trustee(s) and to approve the proposed mandate subject to any modifications it deems necessary for the Trustee to fulfil its obligations. If only one name is approved, Mylan shall appoint or cause to be appointed the person or persons concerned as Trustee, in accordance with the mandate approved by the Commission. If more than one name is approved, Mylan shall be free to choose the Trustee to be appointed from among the names approved. The Trustee shall be appointed within one week of the Commission’s approval, in accordance with the mandate approved by the Commission.
New proposal by Mylan
(26) If all the proposed Trustees are rejected, Mylan shall submit the names of at least two more natural or legal persons within one week of being informed of the rejection, in accordance with paragraphs (20) and (25) of these Commitments.
Trustee nominated by the Commission
(27) If all further proposed Trustees are rejected by the Commission, the Commission shall nominate a Trustee, whom Mylan shall appoint, or cause to be appointed, in accordance with a trustee mandate approved by the Commission.
II. Functions of the Trustee
(28) The Trustee shall assume its specified duties and obligations in order to ensure compliance with these Commitments. The Commission may, on its own initiative or at the request of the Trustee or Mylan, give any orders or instructions to the Trustee in order to ensure compliance with the conditions and obligations attached to the Decision.
Duties and obligations of the Monitoring Trustee
(29) The Monitoring Trustee shall:(i) propose in its first report to the Commission a detailed work plan describing how it intends to monitor compliance with the obligations and conditions attached to the Decision.(ii) oversee, in close co-operation with the Hold Separate Manager, the on-going management of the Divestment Businesses with a view to ensuring their continued economic viability, marketability and competitiveness and monitor compliance by Mylan with the conditions and obligations attached to the Decision. To that end the Monitoring Trustee shall:(a) monitor the preservation of the economic viability, marketability and competitiveness of the Divestment Businesses, and the keeping separate of the Divestment Businesses from the Retained Business, in accordance with paragraphs 8 and 9 of these Commitments;(b) supervise the management of the Divestment Businesses as distinct and saleable entities, in accordance with paragraph 10 of these Commitments;(c) with respect to Confidential Information:- determine all necessary measures to ensure that Mylan does not after the Effective Date obtain any Confidential Information relating to the Divestment Businesses,- in particular strive for the severing of the Divestment Businesses’ participation in a central information technology network to the extent possible, without compromising the viability of the Divestment Businesses,- make sure that any Confidential Information relating to the Divestment Businesses obtained by Mylan before the Effective Date is eliminated and will not be used by Mylan and- decide whether such information may be disclosed to or kept by Mylan as the disclosure is reasonably necessary to allow Mylan to carry out the divestiture or as the disclosure is required by law;(d) monitor the splitting of assets and the allocation of Personnel between the Divestment Businesses and Mylan or Affiliated Undertakings (should the Purchaser make use of the option in paragraph 6(g));(iii) oversee the determination of the reasonable cost basis for the transitory manufacturing or supply arrangements and/or for the provision of technical assistance to the Purchaser (see paragraph 00 above), which will be provided by Mylan to the Purchaser at no cost;(iv) propose to Mylan such measures as the Monitoring Trustee considers necessary to ensure Mylan's compliance with the conditions and obligations attached to the Decision, in particular the maintenance of the full economic viability, marketability or competitiveness of the Divestment Businesses, the holding separate of the Divestment Businesses and the nondisclosure of competitively sensitive information;(v) review and assess potential purchasers as well as the progress of the divestiture process and verify that, dependent on the stage of the divestiture process:(a) potential purchasers receive sufficient and correct information relating to the Divestment Business and the Personnel in particular by reviewing, if available, the data room documentation, the information memorandum and the due diligence process, and(b) potential purchasers are granted reasonable access to the Personnel;(vi) act as a contact point for any requests by third parties, in particular potential purchasers, in relation to these Commitments;(vii) provide to the Commission, sending Mylan a non-confidential copy at the same time, a written report within 15 days after the end of every month that shall cover the operation and management of the Divestment Businesses as well as the splitting of assets and the allocation of Personnel so that the Commission canassess whether the business is held in a manner consistent with these Commitments and the progress of the divestiture process as well as potential purchasers;(viii) promptly report in writing to the Commission, sending Mylan a non-confidential copy at the same time, if it concludes on reasonable grounds that Mylan is failing to comply with these Commitments;(ix) within one week after receipt of the documented proposal referred to in paragraph18 of these Commitments, submit to the Commission, sending Mylan a non- confidential copy at the same time, a reasoned opinion as to the suitability and independence of the proposed purchaser and the viability of the Divestment Businesses after the Sale and as to whether the Divestment Businesses are sold in a manner consistent with the conditions and obligations attached to the Decision, in particular, if relevant, whether the sale of the Divestment Businesses without one or more Assets or not all of the Personnel affects the viability of the Divestment Businesses after the sale, taking account of the proposed purchaser;(x) assume the other functions assigned to the Monitoring Trustee under the conditions and obligations attached to the Decision.
(30) If the Monitoring and Divestiture Trustee are not the same legal or natural persons, the Monitoring Trustee and the Divestiture Trustee shall cooperate closely with each other during and for the purpose of the preparation of the Trustee Divestiture Period in order to facilitate each other's tasks.
Duties and obligations of the Divestiture Trustee
(31) Within the Trustee Divestiture Period, the Divestiture Trustee shall sell at no minimum price the Divestment Businesses to a purchaser, provided that the Commission has approved both the purchaser(s) and the final binding sale and purchase agreement(s) (and ancillary agreements) as in line with the Decision and paragraphs (16) and (17) of these Commitments. The Divestiture Trustee shall include in the sale and purchase agreement (as well as in any ancillary agreements) such terms and conditions as it considers appropriate for an expedient sale in the Trustee Divestiture Period. In particular, the Divestiture Trustee may include in the sale and purchase agreement such customary representations and warranties and indemnities as are reasonably required to effect the sale. The Divestiture Trustee shall protect the legitimate financial interests of Mylan, subject to the Parties' unconditional obligation to divest at no minimum price in the Trustee Divestiture Period.
(32) In the Trustee Divestiture Period (or otherwise at the Commission’s request), the Divestiture Trustee shall provide the Commission with a comprehensive monthly report written in English on the progress of the divestiture process. Such reports shall be submitted within 15 days after the end of every month with a simultaneous copy to the Monitoring Trustee and a non-confidential copy to the Parties.
III. Duties and obligations of the Parties
(33) Mylan shall provide and shall cause its advisors to provide the Trustee with all such co- operation, assistance and information as the Trustee may reasonably require to perform its tasks. The Trustee shall have full and complete access to any of Mylan's or the Divestment Business’ books, records, documents, management or other personnel, facilities, sites and technical information necessary for fulfilling its duties under the Commitments and Mylan and the Divestment Business shall provide the Trustee upon request with copies of any document. Mylan and the Divestment Business shall make available to the Trustee one ormore offices on their premises and shall be available for meetings in order to provide the Trustee with all information necessary for the performance of its tasks.
(34) Mylan shall provide the Monitoring Trustee with all managerial and administrative support that it may reasonably request on behalf of the management of the Divestment Businesses. This shall include all administrative support functions relating to the Divestment Businesses which are currently carried out at headquarters level. Mylan shall provide and shall cause its advisors to provide the Monitoring Trustee, on request, with the information submitted to potential purchasers, in particular give the Monitoring Trustee access to the data room documentation and all other information granted to potential purchasers in the due diligence procedure. Mylan shall inform the Monitoring Trustee on possible purchasers, submit lists of potential purchasers at each stage of the selection process, including the offers made by potential purchasers at those stages, and keep the Monitoring Trustee informed of all developments in the divestiture process.
(35) Mylan shall grant or procure Affiliated Undertakings to grant comprehensive powers of attorney, duly executed, to the Divestiture Trustee to effect the sale (including ancillary agreements), the Divestment Closing and all actions and declarations which the Divestiture Trustee considers necessary or appropriate to achieve the sale and the Divestment Closing, including the appointment of advisors to assist with the sale process. Upon request of the Divestiture Trustee, Mylan shall cause the documents required for effecting the sale and the Divestment Closing to be duly executed.
(36) Mylan shall indemnify the Trustee and its employees and agents (each an "Indemnified Party") and hold each Indemnified Party harmless against, and hereby agrees that an Indemnified Party shall have no liability to Mylan for, any liabilities arising out of the performance of the Trustee’s duties under these Commitments, except to the extent that such liabilities result from the wilful default, recklessness, gross negligence or bad faith of the Trustee, its employees, agents or advisors.
(37) At the expense of Mylan, the Trustee may appoint advisors (in particular for corporate finance or legal advice), subject to Mylan's approval (this approval not to be unreasonably withheld or delayed) if the Trustee considers the appointment of such advisors necessary or appropriate for the performance of its duties and obligations under the Trustee mandate, provided that any fees and other expenses incurred by the Trustee are reasonable. Should Mylan refuse to approve the advisors proposed by the Trustee the Commission may approve the appointment of such advisors instead, after having heard Mylan. Only the Trustee shall be entitled to issue instructions to the advisors. Paragraph 35 of these Commitments shall apply mutatis mutandis. In the Trustee Divestiture Period, the Divestiture Trustee may use advisors who served Mylan during the Divestiture Period if the Divestiture Trustee considers this in the best interest of an expedient sale.
(38) Mylan agrees that the Commission may share Confidential Information proprietary to Mylan with the Trustee. The Trustee shall not disclose such information and the principles contained in Article 17(1) and (2) of the Merger Regulation apply mutatis mutandis.
(39) Mylan agrees that the contact details of the Monitoring Trustee are published on the website of the Commission's Directorate-General for Competition and they shall inform interested third parties, in particular any potential purchasers, of the identity and the tasks of the Monitoring Trustee.
(40) For a period of 10 years from the Effective Date the Commission may request all information from the Parties that is reasonably necessary to monitor the effective implementation of these Commitments.
IV. Replacement, discharge and reappointment of the Trustee
(41) If the Trustee ceases to perform its functions under the Commitments or for any other good cause, including the exposure of the Trustee to a Conflict of Interest:(a) the Commission may, after hearing the Trustee and Mylan, require Mylan to replace the Trustee; or(b) Mylan may, with the prior approval of the Commission, replace the Trustee.
(42) If the Trustee is removed according to paragraph 40 of these Commitments, the Trustee may be required to continue in its function until a new Trustee is in place to whom the Trustee has effected a full hand over of all relevant information. The new Trustee shall be appointed in accordance with the procedure referred to in paragraphs 19-26 of these Commitments.
(43) Unless removed according to paragraph 40 of these Commitments, the Trustee shall cease to act as Trustee only after the Commission has discharged it from its duties after all the Commitments with which the Trustee has been entrusted have been implemented. However, the Commission may at any time require the reappointment of the Monitoring Trustee if it subsequently appears that the relevant remedies might not have been fully and properly implemented
SECTION F – THE REVIEW CLAUSE
(44) The Commission may extend the time periods foreseen in these Commitments in response to a request from Mylan or, in appropriate cases, on its own initiative. Where Mylan requests an extension of a time period, it shall submit a reasoned request to the Commission no later than one month before the expiry of that period, showing good cause. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time send a non-confidential copy of the report to Mylan. Only in exceptional circumstances shall Mylan be entitled to request an extension within the last month of any period.
(45) The Commission may further, in response to a reasoned request from the Parties showing good cause waive, modify or substitute, in exceptional circumstances, one or more of the undertakings in these Commitments. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time send a non-confidential copy of the report to Mylan. The request shall not have the effect of suspending the application of the undertaking and, in particular, of suspending the expiry of any time period in which the undertaking has to be complied with.
SECTION G – ENTRY INTO FORCE
(46) The Commitments shall take effect upon the date of adoption of the Decision.
…………………………………….
duly authorized for and on behalf of Mylan
…………………………………….
duly authorized for and on behalf of Upjohn
PRODUCT: MYLAN'S EPLERENONE PRODUCTS
Territory: Belgium
(1) The Divestment Business consists of Mylan's rights, title and interests in eplerenone in Belgium (currently marketed under the name Eplerenone Mylan) including the right to develop, manufacture and use eplerenone with a view to its sale and marketing in any form in Belgium. Eplerenone is no longer under exclusivity and is used to treat hypertension and specific types of heart failure.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing eplerenone in Belgium. It includes in particular:(a) the sale of existing eplerenone finished product inventory, sales and promotional material in Belgium to the extent available;(b) all eplerenone-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to eplerenone in Belgium specifically will be provided; 7(c) the transfer of the marketing authorization for eplerenone in Belgium including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorizations available to Mylan; and(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in Belgium.The items referred to under (a) - (d) are hereinafter collectively referred to as "Assets of the Divestment Business".8
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of eplerenone in Belgium, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of eplerenone in Belgium.
(4) Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding eplerenone in Belgium with contract manufacturer […] to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the supply agreement it has in place concerning the Divestment Business, subject to the consent of the third party ([…]).
(5) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(6) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(7) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to eplerenone after Divestment Closing;(d) all marketing authorizations currently held by the Parties outside of Belgium for eplerenone;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;9 and(g) monies owed to the Parties by customers for the purchase of eplerenone, and monies owed by the Parties to suppliers for materials used in the production of eplerenone.
(8) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
* * * * *
PRODUCT: MYLAN'S LATANOPROST PRODUCTS
Territory: Belgium
(1) The Divestment Business consists of Mylan's rights, title and interests in latanoprost inBelgium (currently marketed under the name Latanotears) including the right to develop, manufacture and use latanoprost with a view to its sale and marketing in any form in Belgium. Latanoprost is no longer under exclusivity and is used to reduce inter ocular pressure in patients with various types of glaucoma and ocular hypertension.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing latanoprost in Belgium. It includes in particular:(a) the sale of existing latanoprost finished product inventory, sales and promotional material in Belgium to the extent available;(b) all latanoprost-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to latanoprost in Belgium specifically will be provided;10(c) the transfer of the marketing authorization for latanoprost in Belgium including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorizations available to Mylan;(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in; and(e) Full transfer of the national trademark related to latanoprost in Belgium or, in case of a wider than national specific latanoprost trademark, an irrevocable, assignable, sub-licensable, perpetual and royalty free license to use the trademark for the Divestment Business.The items referred to under (a) - (e) are hereinafter collectively referred to as "Assets of the Divestment Business".11
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of latanoprost in Belgium, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of latanoprost in Belgium.
(4) Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding latanoprost in Belgium with contract manufacturer […] to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the supply agreement it has in place concerning the Divestment Business, subject to the consent of the third party. In the event that consent for the assignment cannot be obtained, at the option of the Purchaser Mylan commits to offer a back-to-back arrangement for the supply of the product to the Purchaser for the duration of the relevant third party contract and, in any case, for no longer than three years.
(5) Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding latanoprost in Belgium with licensor […] to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the license agreements it has in place concerning the Divestment Business, subject to the consent of the third party.
(6) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(7) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(8) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to latanoprost after Divestment Closing;(d) all marketing authorizations currently held by the Parties outside of Belgium for latanoprost;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;12 and(g) monies owed to the Parties by customers for the purchase of latanoprost, and monies owed by the Parties to suppliers for materials used in the production of latanoprost.
(9) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
* * * * *
PRODUCT: MYLAN'S LATANOPROST/TIMOLOL PRODUCTS
Territory: Belgium
(1) The Divestment Business consists of Mylan's rights, title and interests in latanoprost/timolol in Belgium (currently marketed under the name Timolatears) including the right to develop, manufacture and use latanoprost/timolol with a view to its sale and marketing in any form in Belgium. Latanoprost/timolol is no longer under exclusivity and is used to reduce inter ocular pressure in patients with various types of glaucoma and ocular hypertension.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing latanoprost/timolol in Belgium. It includes in particular:(a) the sale of existing latanoprost/timolol finished product inventory, sales and promotional material in Belgium to the extent available;(b) all latanoprost/timolol-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to latanoprost/timolol in Belgium specifically will be provided;13(c) the transfer of the marketing authorization for latanoprost/timolol in Belgium including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorizations available to Mylan;(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in Belgium; and(e) Full transfer of the national trademark related to latanoprost/timolol in Belgium or, in case of a wider than national specific latanoprost/timolol trademark, an irrevocable, assignable, sub-licensable, perpetual and royalty free license to use the trademark for the Divestment Business.The items referred to under (a) - (e) are hereinafter collectively referred to as "Assets of the Divestment Business".14
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of latanoprost/timolol in Belgium, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of latanoprost/timolol in Belgium.
(4) Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding latanoprost/timolol in Belgium with contract manufacturers […] to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the supply agreements it has in place concerning the Divestment Business, subject to the consent of the third party. In the event that consent for the assignment cannot be obtained, at the option of the Purchaser Mylan commits to offer a back-to-back arrangement for the supply of the product to the Purchaser for the duration of the relevant third party contract and, in any case, for no longer than three years.
(5) Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding latanoprost/timolol in Belgium with licensor […] to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the license and supply agreements it has in place concerning the Divestment Business, subject to the consent of the third party.
(6) Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding latanoprost/timolol in Belgium with licensor […] to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the supply agreements it has in place concerning the Divestment Business, subject to the consent of the third party.
(7) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(8) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(9) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to latanoprost/timolol after Divestment Closing;(d) all marketing authorizations currently held by the Parties outside of Belgium for latanoprost/timolol;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;15 and(g) monies owed to the Parties by customers for the purchase of latanoprost/timolol, and monies owed by the Parties to suppliers for materials used in the production of latanoprost/timolol.
(10) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
* * * * *
PRODUCT: MYLAN'S PREGABALIN PRODUCTS
Territory: Belgium
(1) The Divestment Business consists of Mylan's rights, title and interests in pregabalin in Belgium (currently marketed under the name Pregabalin Mylan) including the right to develop, manufacture and use pregabalin with a view to its sale and marketing in any form in Belgium. Pregabalin is no longer under exclusivity and is indicated and primarily used for peripheral and central neuropathic pain, but is also used as an ‘add-on' to existing treatment in patients who have partial seizures that cannot be controlled with their current treatment.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing pregabalin in Belgium. It includes in particular:(a) the sale of existing pregabalin finished product inventory, sales and promotional material in Belgium to the extent available;(b) all pregabalin-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to pregabalin in Belgium specifically will be provided;16(c) access to Mylan’s dossier for pregabalin in order for the Purchaser to obtain its own marketing authorization for pregabalin;17(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in Belgium.The items referred to under (a) - (d) are hereinafter collectively referred to as "Assets of the Divestment Business".18
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of pregabalin in Belgium, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of pregabalin in Belgium.
(4) Pending the Purchaser applying for its own marketing authorizations for the Divestment Business, Mylan will appoint the Purchaser as the exclusive distributor of the Divestment Business for a period of up to two years and will, during this period, support the Purchaser in applying for its own marketing authorizations. During this two-year period, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in Belgium.
(5) At the option of the Purchaser, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in Belgium for an additional year after the two-year period noted above (i.e., up to three years in total). Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Mylan to the Purchaser. It shall not contain provisions requiring the delivery of minimum supply volumes or batches.
(6) Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding pregabalin in Belgium with […] for the packaging of the product to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the agreement it has in place concerning the Divestment Business, subject to the consent of the third party. In the event that consent for the assignment cannot be obtained, at the option of the Purchaser Mylan commits to offer a back-to-back arrangement for the supply of the product to the Purchaser for the duration of the relevant third party contract and, in any case, for no longer than three years.
(7) At the option of the Purchaser, Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(8) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(9) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to pregabalin after Divestment Closing;(d) all marketing authorizations currently held by the Parties for pregabalin;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;19 and(g) monies owed to the Parties by customers for the purchase of pregabalin, and monies owed by the Parties to suppliers for materials used in the production of pregabalin.
(10) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
* * * * *
PRODUCT: MYLAN'S VENLAFAXINE PRODUCTS
Territory: Belgium
(1) The Divestment Business consists of Mylan's rights, title and interests in venlafaxine in Belgium (currently marketed under the name Venlafaxine Mylan) including the right to develop, manufacture and use venlafaxine with a view to its sale and marketing in any form in Belgium. Venlafaxine is no longer under exclusivity and is used to treat depression and anxiety disorders.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing venlafaxine in Belgium. It includes in particular:(a) the sale of existing venlafaxine finished product inventory, sales and promotional material in Belgium (to the extent available);(b) all venlafaxine-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to venlafaxine in Belgium specifically will be provided;20(c) the transfer of the marketing authorizations for venlafaxine in Belgium including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorizations available to Mylan; and(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in Belgium;The items referred to under (a) - (d) are hereinafter collectively referred to as "Assets of the Divestment Business".21
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of venlafaxine in Belgium, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of venlafaxine in Belgium.
(4) Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding venlafaxine in Belgium with contract manufacturer […] to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the supply agreement it has in place concerning the Divestment Business, subject to the consent of the third party. In the event that consent for the assignment cannot be obtained, at the option of the Purchaser Mylan commits to offer a back-to-back arrangement for the supply of the product to the Purchaser for the duration of the relevant third party contract and, in any case, for no longer than three years.
(5) At the option of the Purchaser, Mylan shall enter into a transitory non-exclusive packaging agreement relating to the existing forms of the product in Belgium for up to three years, which period may be extended by one year, and an additional year thereafter, upon request by the Purchaser and subject to the approval of the Monitoring Trustee on the basis that such extension is necessary for the Purchaser to continue commercializing the Divestment Business. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Mylan to the Purchaser. It shall not contain provisions requiring the delivery of minimum supply volumes or batches.
(6) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(7) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(8) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to venlafaxine after Divestment Closing;(d) all marketing authorizations currently held by the Parties outside of Belgium for venlafaxine;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;22 and(g) monies owed to the Parties by customers for the purchase of venlafaxine, monies owed by the Parties to suppliers for materials used in the production of venlafaxine.
(9) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
* * * * *
PRODUCT: MYLAN'S DOXAZOSIN PRODUCTS
Territory: the Czech Republic
(1) The Divestment Business consists of Mylan's rights, title and interests in doxazosin in the Czech Republic (currently marketed under the name Doxazosin Mylan) including the right to develop, manufacture and use doxazosin with a view to its sale and marketing in any form in the Czech Republic. Doxazosin is no longer under exclusivity and is used as an anti- hypertensive and as a treatment for benign prostatic hyperplasia (BPH).
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing doxazosin in the Czech Republic. It includes in particular:(a) the sale of existing doxazosin finished product inventory, sales and promotional material in the Czech Republic to the extent available;(b) all doxazosin-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to doxazosin in the Czech Republic specifically will be provided;23(c) the transfer of the marketing authorization for doxazosin in the Czech Republic including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorizations available to Mylan; and(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development,manufacture, use of the Divestment Business with a view to its sale in the Czech Republic.The items referred to under (a) - (d) are hereinafter collectively referred to as "Assets of the Divestment Business".24
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of doxazosin in the Czech Republic, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of doxazosin in the Czech Republic.
(4) Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding doxazosin in the Czech Republic with licensor and contract manufacturer […] to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the license and supply agreements it has in place concerning the Divestment Business, subject to the consent of the third party. In the event that consent for the assignment cannot be obtained, at the option of the Purchaser Mylan commits to offer a back-to-back arrangement for the supply of the product to the Purchaser for the duration of the relevant third party contract and, in any case, for no longer than three years.
(5) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(6) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(7) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials(c) any research and development, clinical data and studies or intellectual property relating to doxazosin after Divestment Closing;(d) all marketing authorizations currently held by the Parties outside of the Czech Republic for doxazosin;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;25 and(g) monies owed to the Parties by customers for the purchase of doxazosin, and monies owed by the Parties to suppliers for materials used in the production of doxazosin.
(8) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
****
PRODUCT: MYLAN'S PREGABALIN PRODUCTS
Territory: Czech Republic
(1) The Divestment Business consists of Mylan's rights, title and interests in pregabalin in the Czech Republic (currently marketed under the name Pregabalin Mylan) including the right to develop, manufacture and use pregabalin with a view to its sale and marketing in any form in the Czech Republic. Pregabalin is no longer under exclusivity and is indicated and primarily used for peripheral and central neuropathic pain, but is also used as an ‘add-on' to existing treatment in patients who have partial seizures that cannot be controlled with their current treatment.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing pregabalin in the Czech Republic. It includes in particular:(a) the sale of existing pregabalin finished product inventory, sales and promotional material in the Czech Republic to the extent available;(b) all pregabalin-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to pregabalin in the Czech Republic specifically will be provided;26(c) access to Mylan’s dossier for pregabalin in order for the Purchaser to obtain its own marketing authorization for pregabalin;27 and(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in the Czech Republic.The items referred to under (a) - (d) are hereinafter collectively referred to as "Assets of the Divestment Business".28
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of pregabalin in the Czech Republic, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of pregabalin in the Czech Republic.
(4) Pending the Purchaser applying for its own marketing authorizations for the Divestment Business, Mylan will appoint the Purchaser as the exclusive distributor of the Divestment Business for a period of up to two years and will, during this period, support the Purchaser in applying for its own marketing authorizations. During this two-year period, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in the Czech Republic.
(5) At the option of the Purchaser, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in the Czech Republic for an additional year after the two-year period noted above (i.e., up to three years in total). This period may be further extended by one year, and an additional year thereafter, upon request by the Purchaser and subject to the approval of the Monitoring Trustee on the basis that such extension is necessary for the Purchaser to continue commercializing the Divestment Business. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Mylan to the Purchaser. It shall not contain provisions requiring the delivery of minimum supply volumes or batches.
(6) Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding pregabalin in the Czech Republic with […] for the packaging of the product to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the agreement it has in place concerning the Divestment Business, subject to the consent of the third party. In the event that consent for the assignment cannot be obtained, at the option of the Purchaser Mylan commits to offer a back-to-back arrangement for the supply of the product to the Purchaser for the duration of the relevant third party contract and, in any case, for no longer than three years.
(7) At the option of the Purchaser, Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business.
(8) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(9) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to pregabalin after Divestment Closing;(d) all marketing authorizations currently held by the Parties for pregabalin;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;29 and(g) monies owed to the Parties by customers for the purchase of pregabalin, and monies owed by the Parties to suppliers for materials used in the production of pregabalin.
(10) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
* * * * *
PRODUCT: MYLAN'S ZIPRASIDONE PRODUCTS
Territory: the Czech Republic
(1) The Divestment Business consists of Mylan's rights, title and interests in ziprasidone in the Czech Republic (currently marketed under the name Ziprasidone Mylan) including the right to develop, manufacture and use ziprasidone with a view to its sale and marketing in any form in the Czech Republic. Ziprasidone is no longer under exclusivity and is used to treat schizophrenia and (paediatric) mania.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing ziprasidone in the Czech Republic. It includes in particular:(a) the sale of existing ziprasidone finished product inventory, sales and promotional material in the Czech Republic to the extent available;(b) all ziprasidone-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to ziprasidone in the Czech Republic specifically will be provided;30(c) the transfer of the marketing authorization for ziprasidone in the Czech Republic including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorizations available to Mylan; and(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in the Czech Republic.The items referred to under (a) - (d) are hereinafter collectively referred to as "Assets of the Divestment Business".31
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of ziprasidone in the Czech Republic, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of ziprasidone in the Czech Republic.
(4) At the option of the Purchaser, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in the Czech Republic for up to three years, which period may be extended by one year, and an additional year thereafter, upon request by the Purchaser and subject to the approval of the Monitoring Trustee on the basis that such extension is necessary for the Purchaser to continue commercializing the Divestment Business. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Mylan to the Purchaser. It shall not contain provisions requiring the delivery of minimum supply volumes or batches.
(5) Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding ziprasidone in the Czech Republic with […] for the packaging of the products to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the packaging agreement it has in place concerning the Divestment Business, subject to the consent of the respective parties. In the event that consent for the assignment cannot be obtained, at the option of the Purchaser Mylan commits to offer a back-to-back arrangement for the supply of the product to the Purchaser for the duration of the relevant third party contract and, in any case, for no longer than three years.
(6) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(7) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(8) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to ziprasidone after Divestment Closing;(d) all marketing authorizations currently held by the Parties outside of the Czech Republic for ziprasidone;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;32 and(g) monies owed to the Parties by customers for the purchase of ziprasidone, and monies owed by the Parties to suppliers for materials used in the production of ziprasidone.
(9) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
* * * *
PRODUCT: MYLAN'S ELETRIPTAN PRODUCTS
Territory: Denmark
(1) The Divestment Business consists of Mylan's rights, title and interests in eletriptan in Denmark (currently marketed under the name Eletriptan Mylan) including the right to develop, manufacture and use eletriptan with a view to its sale and marketing in any form in Denmark. Eletriptan is no longer under exclusivity and is used for the acute treatment of the headache phase of migraine attacks, with or without aura.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing eletriptan in Denmark. It includes in particular:(a) the sale of existing eletriptan finished product inventory, sales and promotional material in Denmark to the extent available;(b) all eletriptan-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to eletriptan in Denmark specifically will be provided;33(c) the transfer of the marketing authorization for eletriptan in Denmark including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorizations available to Mylan; and(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in Denmark.The items referred to under (a) - (d) are hereinafter collectively referred to as "Assets of the Divestment Business".34
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of eletriptan in Denmark, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of eletriptan in Denmark.
(4) At the option of the Purchaser, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in Denmark for up to three years, which period may be extended by one year, and an additional year thereafter, upon request by the Purchaser and subject to the approval of the Monitoring Trustee on the basis that such extension is necessary for the Purchaser to continue commercializing the Divestment Business. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Mylan to the Purchaser. It shall not contain provisions requiring the delivery of minimum supply volumes or batches.
(5) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(6) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(7) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to eletriptan after Divestment Closing;(d) all marketing authorizations currently held by the Parties outside of Denmark for eletriptan;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;35 and(g) monies owed to the Parties by customers for the purchase of eletriptan, and monies owed by the Parties to suppliers for materials used in the production of eletriptan.
(8) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
* * * * *
PRODUCT: MYLAN'S SILDENAFIL (PAH) PRODUCTS
Territory: Estonia
(1) The Divestment Business consists of Mylan's rights, title and interests in sildenafil in Estonia (currently marketed under the name Mysildecard) including the right to develop, manufacture and use sildenafil (PAH) with a view to its sale and marketing in any form in Estonia, only for the pulmonary arterial hypertension (PAH) indication. Sildenafil (PAH) is no longer under exclusivity.(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing sildenafil (PAH) in Estonia. It includes in particular:(a) the sale of existing sildenafil (PAH) finished product inventory, sales and promotional material in Estonia to the extent available;(b) all sildenafil (PAH)-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to sildenafil (PAH) in Estonia specifically will be provided;36(c) access to Mylan’s dossier for sildenafil (PAH) in order for the Purchaser to obtain its own marketing authorization for sildenafil (PAH);37(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in Estonia; and(e) Full transfer of the national trademark related to sildenafil (PAH) in Estonia or, in case of a wider than national specific sildenafil (PAH) trademark, an irrevocable, assignable, sub-licensable, perpetual and royalty free license to use the trademark for the Divestment Business.(f) The items referred to under (a) - (e) are hereinafter collectively referred to as "Assets of the Divestment Business".38
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of sildenafil (PAH) in Estonia, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of sildenafil (PAH) in Estonia.
(4) Pending the Purchaser applying for its own marketing authorizations for the Divestment Business, Mylan will appoint the Purchaser as the exclusive distributor of the Divestment Business for a period of up to two years and will, during this period, support the Purchaser in applying for its own marketing authorizations. During this two-year period, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in Estonia.
(5) At the option of the Purchaser, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in Estonia for an additional year after the two-year period noted above (i.e., up to three years in total). This period may be further extended by one year, and an additional year thereafter, upon request by the Purchaser and subject to the approval of the Monitoring Trustee on the basis that such extension is necessary for the Purchaser to continue commercializing the Divestment Business. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Mylan to the Purchaser. It shall not contain provisions requiring the delivery of minimum supply volumes or batches.
(6) Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, with the Estonian Sick Fund to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the price agreement it has in place concerning the Divestment Business, subject to the consent of the respective parties.
(7) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate thetransfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(8) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(9) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to sildenafil (PAH) after Divestment Closing;(d) all marketing authorizations currently held by the Parties for sildenafil (PAH);(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;39 and(g) monies owed to the Parties by customers for the purchase of sildenafil (PAH), and monies owed by the Parties to suppliers for materials used in the production of sildenafil (PAH).
(10) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
* * * * *
PRODUCT: MYLAN'S ELETRIPTAN PRODUCTS
Territory: Finland
(1) The Divestment Business consists of Mylan's rights, title and interests in eletriptan in Finland (currently marketed under the name Eletriptan Mylan) including the right to develop, manufacture and use eletriptan with a view to its sale and marketing in any form in Finland. Eletriptan is no longer under exclusivity and is used for the acute treatment of the headache phase of migraine attacks, with or without aura.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing eletriptan in Finland. It includes in particular:(a) the sale of existing eletriptan finished product inventory, sales and promotional material in Finland to the extent available;(b all eletriptan-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to eletriptan in Finland specifically will be provided;40(c) the transfer of the marketing authorization for eletriptan in Finland including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorizations available to Mylan; and(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in Finland.The items referred to under (a) - (d) are hereinafter collectively referred to as "Assets of the Divestment Business".41
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of eletriptan in Finland, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of eletriptan in Finland.
(4) At the option of the Purchaser, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in Finland for up to three years, which period may be extended by one year, and an additional year thereafter, upon request by the Purchaser and subject to the approval of the Monitoring Trustee on the basis that such extension is necessary for the Purchaser to continue commercializing the Divestment Business. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Mylan to the Purchaser. It shall not contain provisions requiring the delivery of minimum supply volumes or batches.
(5) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(6) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation,who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(7) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to eletriptan after Divestment Closing;(d) all marketing authorizations currently held by the Parties outside of Finland for eletriptan;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;42 and(g) monies owed to the Parties by customers for the purchase of eletriptan, and monies owed by the Parties to suppliers for materials used in the production of eletriptan.
(8) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
* * * * *
PRODUCT: MYLAN'S DOXAZOSIN PRODUCTS
Territory: France
(1) The Divestment Business consists of Mylan's rights, title and interests in doxazosin in France (currently marketed under the name Doxazosin Mylan) including the right to develop, manufacture and use doxazosin with a view to its sale and marketing in any form in France. Doxazosin is no longer under exclusivity and is used as an anti-hypertensive and as a treatment for benign prostatic hyperplasia (BPH).
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing doxazosin in France. It includes in particular:(a) the sale of existing doxazosin finished product inventory, sales and promotional material in France to the extent available;(b) all doxazosin-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details,whilst only the information related to doxazosin in France specifically will be provided;43(c) the transfer of the marketing authorization for doxazosin in France including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorizations available to Mylan; and(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in France.The items referred to under (a) - (d) are hereinafter collectively referred to as "Assets of the Divestment Business".44
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of doxazosin in France, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of doxazosin in France.
(4) Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding doxazosin in France with licensor and contract manufacturer […] to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the license and supply agreements it has in place concerning the Divestment Business, subject to the consent of the respective parties. In the event that consent for the assignment cannot be obtained, at the option of the Purchaser Mylan commits to offer a back-to-back arrangement for the supply of the product to the Purchaser for the duration of the relevant third party contract and, in any case, for no longer than three years.
(5) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(6) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(7) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to doxazosin after Divestment Closing;(d) all marketing authorizations currently held by the Parties outside of France for doxazosin;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;45 and(g) monies owed to the Parties by customers for the purchase of doxazosin, and monies owed by the Parties to suppliers for materials used in the production of doxazosin.
(8) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
*****
PRODUCT: MYLAN'S ELETRIPTAN PRODUCTS
Territory: France
(1) The Divestment Business consists of Mylan's rights, title and interests in eletriptan in France (currently marketed under the name Eletriptan Mylan) including the right to develop, manufacture and use eletriptan with a view to its sale and marketing in any form in France. Eletriptan is no longer under exclusivity and is used for the acute treatment of the headache phase of migraine attacks, with or without aura.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing eletriptan in France. It includes in particular:(a) the sale of existing eletriptan finished product inventory, sales and promotional material in France to the extent available;(b) all eletriptan-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to eletriptan in France specifically will be provided;46(c) the transfer of the marketing authorization for eletriptan in France including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorizations available to Mylan; and(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in France.The items referred to under (a) - (d) are hereinafter collectively referred to as "Assets of the Divestment Business".47
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of eletriptan in France, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of eletriptan in France.
(4) At the option of the Purchaser, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in France for up to three years, which period may be extended by one year, and an additional year thereafter, upon request by the Purchaser and subject to the approval of the Monitoring Trustee on the basis that such extension is necessary for the Purchaser to continue commercializing the Divestment Business. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Mylan to the Purchaser. It shall not contain provisions requiring the delivery of minimum supply volumes or batches.
(5) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(6) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(7) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to eletriptan after Divestment Closing;(d) all marketing authorizations currently held by the Parties outside of France for eletriptan;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any 48of its subsidiaries; and(g) monies owed to the Parties by customers for the purchase of eletriptan, and monies owed by the Parties to suppliers for materials used in the production of eletriptan.
(8) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
*****
PRODUCT: MYLAN'S LATANOPROST/TIMOLOL PRODUCTS
Territory: France
(1) The Divestment Business consists of Mylan's rights, title and interests in latanoprost/timolol in France (currently marketed under the name Latanoprost/timolol Mylan) including the right to develop, manufacture and use latanoprost/timolol with a view to its sale and marketing in any form in France. Latanoprost/timolol is no longer under exclusivity and is used to reduce inter ocular pressure in patients with various types of glaucoma and ocular hypertension.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing latanoprost/timolol in France. It includes in particular:(a) the sale of existing latanoprost/timolol finished product inventory, sales and promotional material in France to the extent available;(b) all latanoprost/timolol-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to latanoprost/timolol in France specifically will be provided;49(c) the transfer of the marketing authorization for latanoprost/timolol in France including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorizations available to Mylan; and(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in France.The items referred to under (a) - (d) are hereinafter collectively referred to as "Assets of the Divestment Business".50
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of latanoprost/timolol in France, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of latanoprost/timolol in France.
(4) Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding latanoprost/timolol in France with contract manufacturer […] to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the supply agreement it has in place concerning the Divestment Business, subject to the consent of the respective parties. In the event that consent for the assignment cannot be obtained, at the option of the Purchaser Mylan commits to offer a back-to-back arrangement for the supply of the product to the Purchaser for the duration of the relevant third party contract and, in any case, for no longer than three years.
(5) Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding latanoprost/timolol in France with licensor and supplier […] to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the license and supply agreement it has in place concerning the Divestment Business, subject to the consent of the third party.
(6) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(7) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(8) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to latanoprost/timolol after Divestment Closing;(d) all marketing authorizations currently held by the Parties outside of France for latanoprost/timolol;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;51 and(g) monies owed to the Parties by customers for the purchase of latanoprost/timolol, and monies owed by the Parties to suppliers for materials used in the production of latanoprost/timolol.
(9) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
*****
PRODUCT: MYLAN'S SILDENAFIL (PAH) PRODUCTS
Territory: France
(1) The Divestment Business consists of Mylan's rights, title and interests in sildenafil in France (currently marketed under the name Mysildecard) including the right to develop, manufacture and use sildenafil (PAH) with a view to its sale and marketing in any form in France, only for the pulmonary arterial hypertension (PAH) indication. Sildenafil (PAH) is no longer under exclusivity.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing sildenafil (PAH) in France. It includes in particular:(a) the sale of existing sildenafil (PAH) finished product inventory, sales and promotional material in France to the extent available;(b) all sildenafil (PAH)-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to sildenafil (PAH) in France specifically will be provided;52(c) access to Mylan’s dossier for sildenafil (PAH) in order for the Purchaser to obtain its own marketing authorization for sildenafil (PAH)53(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in France; and(e) Full transfer of the national trademark related to sildenafil (PAH) in France or, in case of a wider than national specific sildenafil (PAH) trademark, an irrevocable, assignable, sub-licensable, perpetual and royalty free license to use the trademark for the Divestment Business.The items referred to under (a) - (e) are hereinafter collectively referred to as "Assets of the Divestment Business".54
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of sildenafil (PAH) in France, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of sildenafil (PAH) in France.
(4) Pending the Purchaser applying for its own marketing authorizations for the Divestment Business, Mylan will appoint the Purchaser as the exclusive distributor of the Divestment Business for a period of up to two years and will, during this period, support the Purchaser in applying for its own marketing authorizations. During this two-year period, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in France.
(5) At the option of the Purchaser, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in France for an additional year after the two-year period noted above (i.e., up to three years in total). This period may be further extended by one year, and an additional year thereafter, upon request by the Purchaser and subject to the approval of the Monitoring Trustee on the basis that such extension is necessary for the Purchaser to continue commercializing the Divestment Business. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Mylan to the Purchaser. It shall not contain provisions requiring the delivery of minimum supply volumes or batches.
(6) In relation to the existing tender contracts, Mylan will transfer all historical information (orders; price; etc.) concerning its relationship with the […] to which Mylan is selling its sildenafil (PAH) products through tenders. Mylan commits to make its best efforts to support the Purchaser to obtain the […] consent for the transfer the tender contracts.
(7) Mylan commits to continue its participation in tenders for the Divestment Business up until Divestment Closing. If Mylan were to win any tenders pertaining to sildenafil (PAH) before Divestment Closing, Mylan commits to make its best efforts to facilitate the assignment of the relationship or the contract in line with the provisions contained in this Schedule concerning existing tender contracts.
(8) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(9) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(10) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to sildenafil (PAH) after Divestment Closing;(d) all marketing authorizations currently held by the Parties for sildenafil (PAH);(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;55 and(g) monies owed to the Parties by customers for the purchase of sildenafil (PAH), and monies owed by the Parties to suppliers for materials used in the production of sildenafil (PAH).
(11) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
*****
PRODUCT: MYLAN'S TADALAFIL (PAH) PRODUCTS
Territory: France
(1) The Divestment Business consists of Mylan's rights, title and interests in tadalafil (PAH) in France (currently marketed under the name Talmanco) including the right to develop, manufacture and use tadalafil (PAH) with a view to its sale and marketing in any form in France. Tadalafil (PAH) is no longer under exclusivity and is used to treat pulmonary arterial hypertension (PAH).
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing tadalafil (PAH) in France. It includes in particular:(a) the sale of existing tadalafil (PAH) finished product inventory, sales and promotional material in France to the extent available;(b) all tadalafil (PAH)-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details,whilst only the information related to tadalafil (PAH) in France specifically will be provided;56(c) access to Mylan’s dossier for tadalafil (PAH) in order for the Purchaser to obtain its own marketing authorization for tadalafil (PAH);57(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in France; and(e) Full transfer of the national trademark related to tadalafil (PAH) in France or, in case of a wider than national specific tadalafil (PAH) trademark, an irrevocable, assignable, sub-licensable, perpetual and royalty free license to use the trademark for the Divestment Business.The items referred to under (a) - (e) are hereinafter collectively referred to as "Assets of the Divestment Business".58
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of tadalafil (PAH) in France, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of tadalafil (PAH) in France.
(4) Pending the Purchaser applying for its own marketing authorizations for the Divestment Business, Mylan will appoint the Purchaser as the exclusive distributor of the Divestment Business for a period of up to two years and will, during this period, support the Purchaser in applying for its own marketing authorizations. During this two-year period, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in France.
(5) At the option of the Purchaser, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in France for an additional year after the two-year period noted above (i.e., up to three years in total). This period may be further extended by one year, and an additional year thereafter, upon request by the Purchaser and subject to the approval of the Monitoring Trustee on the basis that such extension is necessary for the Purchaser to continue commercializing the Divestment Business. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Mylan to the Purchaser. It shall not contain provisions requiring the delivery of minimum supply volumes or batches.
(6) In relation to the existing tender contracts, Mylan will transfer all historical information (orders; price; etc.) concerning its relationship with the […] to which Mylan is selling its tadalafil (PAH) products through tenders. Mylan commits to make its best efforts to support the Purchaser to obtain the […] consent for the transfer the tender contracts.
(7) Mylan commits to continue its participation in tenders for the Divestment Business up until Divestment Closing. If Mylan were to win any tenders pertaining to tadalafil (PAH) before Divestment Closing, Mylan commits to make its best efforts to facilitate the assignment of the relationship or the contract in line with the provisions contained in this Schedule concerning existing tender contracts.
(8) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(9) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(10) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to tadalafil (PAH) after Divestment Closing;(d) all marketing authorizations currently held by the Parties for tadalafil (PAH);(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;59 and(g) monies owed to the Parties by customers for the purchase of tadalafil (PAH), and monies owed by the Parties to suppliers for materials used in the production of tadalafil (PAH).
(11) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
*****
PRODUCT: MYLAN'S ALPRAZOLAM PRODUCTS
Territory: Greece
(1) The Divestment Business consists of Mylan's rights, title and interests in alprazolam in Greece (currently marketed under the name Alprazolam Mylan) including the right to develop, manufacture and use alprazolam with a view to its sale and marketing in any form in Greece. Alprazolam is no longer under exclusivity and is used to treat anxiety or panic disorders.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing alprazolam in Greece. It includes in particular:(a) the sale of existing alprazolam finished product inventory, sales and promotional material in Greece to the extent available;(b) all alprazolam-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to alprazolam in Greece specifically will be provided;60(c) the transfer of the marketing authorization for alprazolam in Greece including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorizations available to Mylan; and(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in Greece.The items referred to under (a) - (d) are hereinafter collectively referred to as "Assets of the Divestment Business".61
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of alprazolam in Greece, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of alprazolam in Greece.
(4) At the option of the Purchaser, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in Greece for up to three years, which period may be extended by one year, and an additional year thereafter, upon request by the Purchaser and subject to the approval of the Monitoring Trustee on the basis that such extension is necessary for the Purchaser to continue commercializing the Divestment Business. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Mylan to the Purchaser. It shall not contain provisions requiring the delivery of minimum supply volumes or batches.
(5) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(6) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(7) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to alprazolam after Divestment Closing;(d) all marketing authorizations currently held by the Parties outside of Greece for alprazolam;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;62 and(g) monies owed to the Parties by customers for the purchase of alprazolam, and monies owed by the Parties to suppliers for materials used in the production of alprazolam.
(8) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
*****
PRODUCT: MYLAN'S EPLERENONE PRODUCTS
Territory: Hungary
(1) The Divestment Business consists of Mylan's rights, title and interests in eplerenone in Hungary (currently marketed under the name Eplerenone Mylan) including the right to develop, manufacture and use eplerenone with a view to its sale and marketing in any form in Hungary. Eplerenone is no longer under exclusivity and is used to treat hypertension and specific types of heart failure.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing eplerenone in Hungary. It includes in particular:(a) the sale of existing eplerenone finished product inventory, sales and promotional material in Hungary to the extent available;(b) all eplerenone-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to eplerenone in Hungary specifically will be provided; 63(c) the transfer of the marketing authorization for eplerenone in Hungary including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorizations available to Mylan; and(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in Hungary.The items referred to under (a) - (d) are hereinafter collectively referred to as "Assets of the Divestment Business".64
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of eplerenone in Hungary, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of eplerenone in Hungary.
(4) Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding eplerenone in Hungary with contract manufacturer […] to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the supply agreement it has in place concerning the Divestment Business, subject to the consent of the third party ([…]). In the event that consent for the assignment cannot be obtained, at the option of the Purchaser Mylan commits to offer a back-to-back arrangement for the supply of the product to the Purchaser for the duration of the relevant third party contract and, in any case, for no longer than three years.
(5) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(6) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(7) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to eplerenone after Divestment Closing;(d) all marketing authorizations currently held by the Parties outside of Hungary for eplerenone;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;65 and(g) monies owed to the Parties by customers for the purchase of eplerenone, and monies owed by the Parties to suppliers for materials used in the production of eplerenone.
(8) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
* * * * *
PRODUCT: MYLAN'S ALPRAZOLAM PRODUCTS
Territory: Iceland
(1) The Divestment Business consists of Mylan's rights, title and interests in alprazolam in Iceland (currently marketed under the name Alprazolam Mylan) including the right to develop, manufacture and use alprazolam with a view to its sale and marketing in any form in Iceland. Alprazolam is no longer under exclusivity and is used to treat anxiety or panic disorders.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing alprazolam in Iceland. It includes in particular:(a) the sale of existing alprazolam finished product inventory, sales and promotional material in Iceland to the extent available;(b) all alprazolam-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details,whilst only the information related to alprazolam in Iceland specifically will be provided;66(c) the transfer of the marketing authorization for alprazolam in Iceland including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorizations available to Mylan; and(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in Iceland.The items referred to under (a) - (d) are hereinafter collectively referred to as "Assets of the Divestment Business".67
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of alprazolam in Iceland, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of alprazolam in Iceland.
(4) At the option of the Purchaser, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in Iceland for up to three years, which period may be extended by one year, and an additional year thereafter, upon request by the Purchaser and subject to the approval of the Monitoring Trustee on the basis that such extension is necessary for the Purchaser to continue commercializing the Divestment Business. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Mylan to the Purchaser. It shall not contain provisions requiring the delivery of minimum supply volumes or batches.
(5) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(6) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(7) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to alprazolam after Divestment Closing;(d) all marketing authorizations currently held by the Parties outside of Iceland for alprazolam;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;68 and(g) monies owed to the Parties by customers for the purchase of alprazolam, and monies owed by the Parties to suppliers for materials used in the production of alprazolam.
(8) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
*****
PRODUCT: MYLAN'S ALPRAZOLAM PRODUCTS
Territory: Ireland
(1) The Divestment Business consists of Mylan's rights, title and interests in alprazolam in Ireland (currently marketed under the name Gerax69 Mylan) including the right to develop, manufacture and use alprazolam with a view to its sale and marketing in any form in Ireland. Alprazolam is no longer under exclusivity and is used to treat anxiety or panic disorders.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing alprazolam in Ireland. It includes in particular:(a) the sale of existing alprazolam finished product inventory, sales and promotional material in Ireland to the extent available;(b) all alprazolam-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to alprazolam in Ireland specifically will be provided;70(c) the transfer of the marketing authorization for alprazolam in Ireland including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorizations available to Mylan; and(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in Ireland.The items referred to under (a) - (d) are hereinafter collectively referred to as "Assets of the Divestment Business".71
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of alprazolam in Ireland, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of alprazolam in Ireland.
(4) Mylan commits neither to register the Gerax brand in Ireland nor to oppose the future registration of the Gerax brand name by the Purchaser in Ireland. The Purchaser will have the right to use the Gerax brand in Ireland.
(5) At the option of the Purchaser, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in Ireland for up to three years, which period may be extended by one year, and an additional year thereafter, upon request by the Purchaser and subject to the approval of the Monitoring Trustee on the basis that such extension is necessary for the Purchaser to continue commercializing the Divestment Business. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Mylan to the Purchaser. It shall not contain provisions requiring the delivery of minimum supply volumes or batches.
(6) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(7) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(8) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to alprazolam after Divestment Closing;(d) all marketing authorizations currently held by the Parties outside of Ireland for alprazolam;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;72 and(g) monies owed to the Parties by customers for the purchase of alprazolam, and monies owed by the Parties to suppliers for materials used in the production of alprazolam.
(9) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
*****
PRODUCT: MYLAN'S GABAPENTIN PRODUCTS
Territory: Ireland
(1) The Divestment Business consists of Mylan's rights, title and interests in gabapentin in Ireland (currently marketed under the name Gabapentin Ireland) including the right to develop, manufacture and use gabapentin with a view to its sale and marketing in any form in Ireland. Gabapentin is no longer under exclusivity and is used both as a monotherapy to prevent partial epileptic seizures (epileptic fits starting in one specific part of the brain) and also as an ‘add-on' to existing treatments in patients who have partial seizures.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing gabapentin in Ireland. It includes in particular:(a) the sale of existing gabapentin finished product inventory, sales and promotional material in Ireland to the extent available;(b) all gabapentin-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to gabapentin in Ireland specifically will be provided;73(c) the transfer of the marketing authorization for gabapentin in Ireland including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorizations available to Mylan; and(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in Ireland;The items referred to under (a) - (d) are hereinafter collectively referred to as "Assets of the Divestment Business".74
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of gabapentin in Ireland, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of gabapentin in Ireland.
(4) Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding gabapentin in Ireland with licensor […] to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the license agreement it has in place concerning the Divestment Business, subject to the consent of the third party.
(5) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(6) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(7) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to gabapentin after Divestment Closing;(d) all marketing authorizations currently held by the Parties outside of Ireland for gabapentin;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;75 and(g) monies owed to the Parties by customers for the purchase of gabapentin, and monies owed by the Parties to suppliers for materials used in the production of gabapentin.
(8) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
* * * *
PRODUCT: MYLAN'S ALPRAZOLAM PRODUCTS
Territory: Italy
(1) The Divestment Business consists of Mylan's rights, title and interests in alprazolam in Italy (currently marketed under the names Frontal and Alprazolam Mylan) including the right to develop, manufacture and use alprazolam with a view to its sale and marketing in any form in Italy. Alprazolam is no longer under exclusivity and is used to treat anxiety or panic disorders.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing alprazolam in Italy. It includes in particular:(a) the sale of existing alprazolam finished product inventory, sales and promotional material in Italy to the extent available;(b) all alprazolam-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to alprazolam in Italy specifically will be provided;76(c) the transfer of the marketing authorization for alprazolam in Italy including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorizations available to Mylan;(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in Italy; and
(e) if applicable, full transfer of the national trademark related to alprazolam in Italy or, in case of a wider than national specific alprazolam trademark, an irrevocable, assignable, sub-licensable, perpetual and royalty free license to use the trademark for the Divestment Business.The items referred to under (a) - (e) are hereinafter collectively referred to as "Assets of the Divestment Business".77
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of alprazolam in Italy, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of alprazolam in Italy.
(4) With respect to Alprazolam Mylan oral drops, Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding alprazolam in Italy with contract manufacturer […] to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the supply agreement it has in place concerning the Divestment Business, subject to the consent of the third party. In the event that consent for the assignment cannot be obtained, at the option of the Purchaser Mylan commits to offer a back-to-back arrangement for the supply of the product to the Purchaser for the duration of the relevant third party contract and, in any case, for no longer than three years.
(5) Mylan will also transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding Alprazolam Mylan oral drops with […] for the supply of the product to the Purchaser in accordance with applicable law.
(6) With respect to Frontal, Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding alprazolam in Italy with licensor […] to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the license agreement it has in place concerning the Divestment Business, subject to the consent of the third party.
(7) Also with respect to Frontal, in the (unlikely) event that the transfer to the Purchaser occurs prior to the closing of the Transaction, Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding the Frontal trademark license in Italy with licensor […] to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the license agreement it has in place concerning the Divestment Business, subject to the consent of the third party. If the transfer to the Purchaser occurs following the closing of the Transaction, […]
(8) At the option of the Purchaser, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of product in Italy for up to three years, which period may be extended by one year, and an additional year thereafter, upon request by the Purchaser and subject to the approval of the Monitoring Trustee on the basis that such extension is necessary for the Purchaser to continue commercializing the Divestment Business. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Mylan to the Purchaser. It shall not contain provisions requiring the delivery of minimum supply volumes or batches.
(9) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(10) In relation to the existing tender contracts, Mylan commits to make its best efforts to suggest the Purchaser of the Divestment Business to the relevant tender authorities as the new supplier of the product for the remainder of the tender duration.
(11) Mylan commits to continue its participation in tenders for the Divestment Business up until Divestiture Closing. If Mylan were to win any tenders pertaining to alprazolam in Italy before Divestiture Closing, Mylan commits to make its best efforts to suggest the Purchaser of the Divestment Business to the relevant tender authorities as the new supplier of the product for the remainder of the tender duration.
(12) At the option of the Purchaser, and in case any of the tender contracting entities would decide not to accept the Purchaser as the new supplier with respect to the existing tender contracts, Mylan will enter into a transitional dual distributorship arrangement related to the Divestment Business lasting until the relevant marketing authorization is transferred to the name of the Purchaser on an at-cost basis which determination will be overseen by the Monitoring Trustee. Mylan commits to make its best efforts to ensure that no supply disruption will occur or any other supply issue that might lead to the termination of the contract.
(13) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(14) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to alprazolam after Divestment Closing;(d) all marketing authorizations currently held by the Parties outside of Italy for alprazolam;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;78 and(g) monies owed to the Parties by customers for the purchase of alprazolam, and monies owed by the Parties to suppliers for materials used in the production of alprazolam.
(15) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
*****
PRODUCT: MYLAN'S LATANOPROST/TIMOLOL PRODUCTS
Territory: Italy
(1) The Divestment Business consists of Mylan's rights, title and interests in latanoprost/timolol in Italy (currently marketed under the name Latanoprost/timolol Mylan) including the right to develop, manufacture and use latanoprost/timolol with a view to its sale and marketing in any form in Italy. Latanoprost/timolol is no longer under exclusivity and is used to reduce inter ocular pressure in patients with various types of glaucoma and ocular hypertension.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing latanoprost/timolol in Italy. It includes in particular:(a) the sale of existing latanoprost/timolol finished product inventory, sales and promotional material in Italy to the extent available;(b) all latanoprost/timolol-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to latanoprost/timolol in Italy specifically will be provided;79(c) the transfer of the marketing authorization for latanoprost/timolol in Italy including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorizations available to Mylan; and(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in Italy.The items referred to under (a) - (d) are hereinafter collectively referred to as "Assets of the Divestment Business".80
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of latanoprost/timolol in Italy, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of latanoprost/timolol in Italy.
(4) Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding latanoprost/timolol in Italy with contract manufacturer […] to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the supply agreement it has in place concerning the Divestment Business, subject to the consent of the respective parties. In the event that consent for the assignment cannot be obtained, at the option of the Purchaser Mylan commits to offer a back-to-back arrangement for the supply of the product to the Purchaser for the duration of the relevant third party contract and, in any case, for no longer than three years.
(5) Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding latanoprost/timolol in Italy with licensor and supplier […] to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the license and supply agreement it has in place concerning the Divestment Business, subject to the consent of the third party.
(6) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(7) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(8) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to latanoprost/timolol after Divestment Closing;(d) all marketing authorizations currently held by the Parties outside of Italy for latanoprost/timolol;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;81 and(g) monies owed to the Parties by customers for the purchase of latanoprost/timolol, and monies owed by the Parties to suppliers for materials used in the production of latanoprost/timolol.
(9) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
* * * * *
PRODUCT: MYLAN'S SILDENAFIL (PAH) PRODUCTS
Territory: Latvia
(1) The Divestment Business consists of Mylan's rights, title and interests in sildenafil in Latvia (currently marketed under the name Mysildecard) including the right to develop, manufacture and use sildenafil (PAH) with a view to its sale and marketing in any form in Latvia, only for the pulmonary arterial hypertension (PAH) indication. Sildenafil (PAH) is no longer under exclusivity.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing sildenafil (PAH) in Latvia. It includes in particular:(a) the sale of existing sildenafil (PAH) finished product inventory, sales and promotional material in Latvia to the extent available;(b) all sildenafil (PAH)-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to sildenafil (PAH) in Latvia specifically will be provided;82(c) access to Mylan’s dossier for sildenafil (PAH) in order for the Purchaser to obtain its own marketing authorization for sildenafil (PAH);83(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in Latvia; and(e) Full transfer of the national trademark related to sildenafil (PAH) in Latvia or, in case of a wider than national specific sildenafil (PAH) trademark, an irrevocable, assignable, sub-licensable, perpetual and royalty free license to use the trademark for the Divestment Business.The items referred to under (a) - (e) are hereinafter collectively referred to as "Assets of the Divestment Business".84
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of sildenafil (PAH) in Latvia, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of sildenafil (PAH) in Latvia.
(4) Pending the Purchaser applying for its own marketing authorizations for the Divestment Business, Mylan will appoint the Purchaser as the exclusive distributor of the Divestment Business for a period of up to two years and will, during this period, support the Purchaser in applying for its own marketing authorizations. During this two-year period, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in Latvia.
(5) At the option of the Purchaser, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in Latvia for an additional year after the two-year period noted above (i.e., up to three years in total), which period may be extended by one year, and an additional year thereafter, upon request by the Purchaser and subject to the approval of the Monitoring Trustee on the basis that such extension is necessary for the Purchaser to continue commercializing the Divestment Business. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Mylan to the Purchaser. It shall not contain provisions requiring the delivery of minimum supply volumes or batches.
(6) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(7) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(8) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to sildenafil (PAH) after Divestment Closing;(d) all marketing authorizations currently held by the Parties for sildenafil (PAH);(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;85 and(g) monies owed to the Parties by customers for the purchase of sildenafil (PAH), and monies owed by the Parties to suppliers for materials used in the production of sildenafil (PAH).
(9) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
* * * * *
PRODUCT: MYLAN'S SILDENAFIL (PAH) PRODUCTS
Territory: Lithuania
(1) The Divestment Business consists of Mylan's rights, title and interests in sildenafil in Lithuania (currently marketed under the name Mysildecard) including the right to develop, manufacture and use sildenafil (PAH) with a view to its sale and marketing in any form in Lithuania, only for the pulmonary arterial hypertension (PAH) indication. Sildenafil (PAH) is no longer under exclusivity.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing sildenafil (PAH) in Lithuania. It includes in particular:(a) the sale of existing sildenafil (PAH) finished product inventory, sales and promotional material in Lithuania to the extent available;(b) all sildenafil (PAH)-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to sildenafil (PAH) in Lithuania specifically will be provided;86(c) access to Mylan’s dossier for sildenafil (PAH) in order for the Purchaser to obtain its own marketing authorization for sildenafil (PAH);87(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in Lithuania; and(e) Full transfer of the national trademark related to sildenafil (PAH) in Lithuania or, in case of a wider than national specific sildenafil (PAH) trademark, an irrevocable, assignable, sub-licensable, perpetual and royalty free license to use the trademark for the Divestment Business.The items referred to under (a) - (e) are hereinafter collectively referred to as "Assets of the Divestment Business".88
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of sildenafil (PAH) in Lithuania, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of sildenafil (PAH) in Lithuania.
(4) Pending the Purchaser applying for its own marketing authorizations for the Divestment Business, Mylan will appoint the Purchaser as the exclusive distributor of the Divestment Business for a period of up to two years and will, during this period, support the Purchaser in applying for its own marketing authorizations. During this two-year period, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in Lithuania.
(5) At the option of the Purchaser, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in Lithuania for an additional year after the two-year period noted above (i.e., up to three years in total). This period may be extended by one year, and an additional year thereafter, upon request by the Purchaser and subject to the approval of the Monitoring Trustee on the basis that such extension is necessary for the Purchaser to continue commercializing the Divestment Business. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Mylan to the Purchaser. It shall not contain provisions requiring the delivery of minimum supply volumes or batches.
(6) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(7) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(8) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to sildenafil (PAH) after Divestment Closing;(d) all marketing authorizations currently held by the Parties for sildenafil (PAH);(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;89 and(g) monies owed to the Parties by customers for the purchase of sildenafil (PAH), and monies owed by the Parties to suppliers for materials used in the production of sildenafil (PAH).
(9) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
* * * *
PRODUCT: MYLAN'S LATANOPROST PRODUCTS
Territory: Luxembourg
(1) The Divestment Business consists of Mylan's rights, title and interests in latanoprost in Luxembourg (currently marketed under the name Latanotears) including the right to develop, manufacture and use latanoprost with a view to its sale and marketing in any form in Luxembourg. Latanoprost is no longer under exclusivity and is used to reduce inter ocular pressure in patients with various types of glaucoma and ocular hypertension.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing latanoprost in Luxembourg. It includes in particular:(a) the sale of existing latanoprost finished product inventory, sales and promotional material in Luxembourg to the extent available;(b) all latanoprost-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to latanoprost in Luxembourg specifically will be provided;90(c) the transfer of the marketing authorization for latanoprost in Luxembourg including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorizations available to Mylan;(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in Luxembourg; and (e) Full transfer of the national trademark related to latanoprost in Luxembourg or, in case of a wider than national specific latanoprost trademark, an irrevocable, assignable, sub-licensable, perpetual and royalty free license to use the trademark for the Divestment Business.The items referred to under (a) - (e) are hereinafter collectively referred to as "Assets of the Divestment Business".91
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of latanoprost in Luxembourg, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of latanoprost in Luxembourg.
(4) Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding latanoprost in Luxembourg with contract manufacturer […] to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the supply agreement it has in place concerning the Divestment Business, subject to the consent of the third party. In the event that consent for the assignment cannot be obtained, at the option of the Purchaser Mylan commits to offer a back-to-back arrangement for the supply of the product to the Purchaser for the duration of the relevant third party contract and, in any case, for no longer than three years.
(5) Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding latanoprost in Luxembourg with licensor […] to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the license agreements it has in place concerning the Divestment Business, subject to the consent of the third party.
(6) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(7) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(8) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to latanoprost after Divestment Closing;(d) all marketing authorizations currently held by the Parties outside of Luxembourg for latanoprost;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;92 and(g) monies owed to the Parties by customers for the purchase of latanoprost, and monies owed by the Parties to suppliers for materials used in the production of latanoprost.
(9) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
* * * * *
PRODUCT: MYLAN'S LATANOPROST/TIMOLOL PRODUCTS
Territory: Luxembourg
(1) The Divestment Business consists of Mylan's rights, title and interests in latanoprost/timolol in Luxembourg (currently marketed under the name Timolatears) including the right to develop, manufacture and use latanoprost/timolol with a view to its sale and marketing in any form in Luxembourg. Latanoprost/timolol is no longer under exclusivity and is used to reduce inter ocular pressure in patients with various types of glaucoma and ocular hypertension.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing latanoprost/timolol in Luxembourg. It includes in particular:(a) the sale of existing latanoprost/timolol finished product inventory, sales and promotional material in Luxembourg to the extent available;(b) all latanoprost/timolol-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to latanoprost/timolol in Luxembourg specifically will be provided;93(c) the transfer of the marketing authorization for latanoprost/timolol in Luxembourg including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorizations available to Mylan;(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in Luxembourg; and(e) Full transfer of the national trademark related to latanoprost/timolol in Luxembourg or, in case of a wider than national specific latanoprost/timolol trademark, an irrevocable, assignable, sub-licensable, perpetual and royalty free license to use the trademark for the Divestment Business.The items referred to under (a) - (e) are hereinafter collectively referred to as "Assets of the Divestment Business".94
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of latanoprost/timolol in Luxembourg, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of latanoprost/timolol in Luxembourg.
(4) Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding latanoprost/timolol in Luxembourg with contract manufacturer […] to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the supply agreement it has in place concerning the Divestment Business, subject to the consent of the third party. In the event that consent for the assignment cannot be obtained, at the option of the Purchaser Mylan commits to offer a back-to-back arrangement for the supply of the product to the Purchaser for the duration of the relevant third party contract and, in any case, for no longer than three years.
(5) Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding latanoprost/timolol in Luxembourg with licensor […] to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the license and supply agreement it has in place concerning the Divestment Business, subject to the consent of the third party.
(6) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(7) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(8) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to latanoprost/timolol after Divestment Closing;(d) all marketing authorizations currently held by the Parties outside of Luxembourg for latanoprost/timolol;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;95 and(g) monies owed to the Parties by customers for the purchase of latanoprost/timolol, and monies owed by the Parties to suppliers for materials used in the production of latanoprost/timolol.
(9) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
* * * * *
PRODUCT: MYLAN'S PREGABALIN PRODUCTS
Territory: Luxembourg
(1) The Divestment Business consists of Mylan's rights, title and interests in pregabalin in Luxembourg (currently marketed under the name Pregabalin Mylan) including the right to develop, manufacture and use pregabalin with a view to its sale and marketing in any form in Luxembourg. Pregabalin is no longer under exclusivity and is indicated and primarily used for peripheral and central neuropathic pain, but is also used as an ‘add-on' to existing treatment in patients who have partial seizures that cannot be controlled with their current treatment.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing pregabalin in Luxembourg. It includes in particular:(a) the sale of existing pregabalin finished product inventory, sales and promotional material in Luxembourg to the extent available;(b) all pregabalin-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to pregabalin in Luxembourg specifically will be provided;96(c) access to Mylan’s dossier for pregabalin in order for the Purchaser to obtain its own marketing authorization for pregabalin;97(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in Luxembourg; and The items referred to under (a) - (d) are hereinafter collectively referred to as "Assets of the Divestment Business".98
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of pregabalin in Luxembourg, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of pregabalin in Luxembourg.
(4) Pending the Purchaser applying for its own marketing authorizations for the Divestment Business, Mylan will appoint the Purchaser as the exclusive distributor of the Divestment Business for a period of up to two years and will, during this period, support the Purchaser in applying for its own marketing authorizations. During this two-year period, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in Luxembourg.
(5) At the option of the Purchaser, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in Luxembourg for an additional year after the two-year period noted above (i.e., up to three years in total). This period may be extended by one year, and an additional year thereafter, upon request by the Purchaser and subject to the approval of the Monitoring Trustee on the basis that such extension is necessary for the Purchaser to continue commercializing the Divestment Business. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Mylan to the Purchaser. It shall not contain provisions requiring the delivery of minimum supply volumes or batches.
(6) Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding pregabalin in Luxembourg with […] for the packaging of the product to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the agreement it has in place concerning the Divestment Business, subject to the consent of the third party. In the event that consent for the assignment cannot be obtained, at the option of the Purchaser Mylan commits to offer a back-to-back arrangement for the supply of the product to the Purchaser for the duration of the relevant third party contract and, in any case, for no longer than three years.
(7) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(8) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(9) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to pregabalin after Divestment Closing;(d) all marketing authorizations currently held by the Parties for pregabalin;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;99 and(g) monies owed to the Parties by customers for the purchase of pregabalin, and monies owed by the Parties to suppliers for materials used in the production of pregabalin.
(10) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
* * * * *
PRODUCT: MYLAN'S LATANOPROST/TIMOLOL PRODUCTS
Territory: the Netherlands
(1) The Divestment Business consists of Mylan's rights, title and interests in latanoprost/timolol in the Netherlands (currently marketed under the name Latanoprost/timolol Mylan) including the right to develop, manufacture and use latanoprost/timolol with a view to itssale and marketing in any form in the Netherlands. Latanoprost/timolol is no longer under exclusivity and is used to reduce inter ocular pressure in patients with various types of glaucoma and ocular hypertension.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing latanoprost/timolol in the Netherlands. It includes in particular:(a) the sale of existing latanoprost/timolol finished product inventory, sales and promotional material in the Netherlands to the extent available;(b) all latanoprost/timolol-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to latanoprost/timolol in the Netherlands specifically will be provided;100(c) the transfer of the marketing authorization for latanoprost/timolol in the Netherlands including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorizations available to Mylan; andd) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in the Netherlands.The items referred to under (a) - (d) are hereinafter collectively referred to as "Assets of the Divestment Business".101
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of latanoprost/timolol in the Netherlands, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of latanoprost/timolol in the Netherlands.
(4) Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding latanoprost/timolol in the Netherlands with contract manufacturer […] to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the supply agreement it has in place concerning the Divestment Business, subject to the consent of the respective parties. In the event that consent for the assignment cannot be obtained, at the option of the Purchaser Mylan commits to offer a back-to-back arrangement for the supply of the product to the Purchaser for the duration of the relevant third party contract and, in any case, for no longer than three years.
(5) Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding latanoprost/timolol in the Netherlands with licensor […] to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the license agreement it has in place concerning the Divestment Business, subject to the consent of the third party.
(6) In relation to the existing tender contracts, Mylan will transfer all historical information (orders; price; etc.) concerning its relationship with […]. Mylan commits to make its best efforts to support the Purchaser to obtain the […] consent for the transfer the tender contracts.
(7) Mylan commits to continue its participation in tenders for the Divestment Business up until Divestment Closing. If Mylan were to win any tenders pertaining to latanoprost/timolol before Divestment Closing, Mylan commits to make its best efforts to facilitate the assignment of the relationship or the contract in line with the provisions contained in this Schedule concerning existing tender contracts.
(8) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(9) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(10) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to latanoprost/timolol after Divestment Closing;(d) all marketing authorizations currently held by the Parties outside of the Netherlands for latanoprost/timolol;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;102 and(g) monies owed to the Parties by customers for the purchase of latanoprost/timolol, and monies owed by the Parties to suppliers for materials used in the production of latanoprost/timolol.
(11) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
* * * * *
PRODUCT: MYLAN'S ATORVASTATIN PRODUCTS
Territory: Norway
(1) The Divestment Business consists of Mylan's rights, title and interests in atorvastatin in Norway (currently marketed under the name atorvastatin Mylan) including the right to develop, manufacture and use atorvastatin with a view to its sale and marketing in any form in Norway. Atorvastatin is no longer under exclusivity and is used to prevent cardiovascular disease in adults and to treat hyperlipidemia.103
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing atorvastatin in Norway. It includes in particular:(a) the sale of existing atorvastatin finished product inventory, sales and promotional material in Norway to the extent available;(b) all atorvastatin-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to atorvastatin in Norway specifically will be provided;104(c) the transfer of the marketing authorization for atorvastatin in Norway including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorizations available to Mylan; and(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in Norway.The items referred to under (a) - (d) are hereinafter collectively referred to as "Assets of the Divestment Business".105
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of atorvastatin in Norway, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of atorvastatin in Norway.
(4) At the option of the Purchaser, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in Norway for up to three years, which period may be extended by one year, and an additional year thereafter, upon request by the Purchaser and subject to the approval of the Monitoring Trustee on the basis that such extension is necessary for the Purchaser to continue commercializing the Divestment Business. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Mylan to the Purchaser. It shall not contain provisions requiring the delivery of minimum supply volumes or batches.
(5) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(6) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(7) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to atorvastatin after Divestment Closing;(d) all marketing authorizations currently held by the Parties outside of Norway for atorvastatin;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;106 and(g) monies owed to the Parties by customers for the purchase of atorvastatin, and monies owed by the Parties to suppliers for materials used in the production of atorvastatin.
(8) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
*****
PRODUCT: MYLAN'S ELETRIPTAN PRODUCTS
Territory: Norway
(1) The Divestment Business consists of Mylan's rights, title and interests in eletriptan in Norway (currently marketed under the name Eletriptan Mylan) including the right to develop, manufacture and use eletriptan with a view to its sale and marketing in any form in Norway. Eletriptan is no longer under exclusivity and is used for the acute treatment of the headache phase of migraine attacks, with or without aura.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing eletriptan in Norway. It includes in particular:(a) the sale of existing eletriptan finished product inventory, sales and promotional material in Norway to the extent available;(b) all eletriptan-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to eletriptan in Norway specifically will be provided;107(c) the transfer of the marketing authorization for eletriptan in Norway including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorizations available to Mylan; and(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in Norway.The items referred to under (a) - (d) are hereinafter collectively referred to as "Assets of the Divestment Business".108
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of eletriptan in Norway, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of eletriptan in Norway.
(4) At the option of the Purchaser, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in Norway for up to three years, which period may be extended by one year, and an additional year thereafter, upon request by the Purchaser and subject to the approval of the Monitoring Trustee on the basis that such extension is necessary for the Purchaser to continue commercializing the Divestment Business. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Mylan to the Purchaser. It shall not contain provisions requiring the delivery of minimum supply volumes or batches.
(5) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(6) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(7) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to eletriptan after Divestment Closing;(d) all marketing authorizations currently held by the Parties outside of Norway for eletriptan;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;109 and(g) monies owed to the Parties by customers for the purchase of eletriptan, and monies owed by the Parties to suppliers for materials used in the production of eletriptan.
(8) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
* * * * *
PRODUCT: MYLAN'S PREGABALIN PRODUCTS
Territory: Norway
(1) The Divestment Business consists of Mylan's right, title and interest in pregabalin in Norway including the right to develop, manufacture and use pregabalin to sell in any form. Pregabalin is no longer under exclusivity and is indicated and primarily used for peripheral and central neuropathic pain, but is also used as an ‘add-on' to existing treatment in patients who have partial seizures that cannot be controlled with their current treatment.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing pregabalin in Norway. It includes in particular:(a) access to Mylan’s dossier for pregabalin in order for the Purchaser to obtain its own marketing authorization for pregabalin;110 and(b) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in Norway, including in particular the information in the registration dossier.
(3) The items referred to under (a) - (b) are hereinafter jointly referred to as "Assets of the Divestment Business".111
(4) If and to the extent that the know-how listed in paragraph 2(e) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of pregabalin in Norway, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of pregabalin in Norway.
(5) Pending the Purchaser applying for its own marketing authorizations for the Divestment Business, Mylan will appoint the Purchaser as the exclusive distributor of the Divestment Business for a period of up to two years and will, during this period, support the Purchaser in applying for its own marketing authorizations. During this two-year period, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in Norway.
(6) At the option of the Purchaser, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in Norway for an additional year after the two-year period noted above (i.e., up to three years in total). This period may be extended by one year, and an additional year thereafter, upon request by the Purchaser and subject to the approval of the Monitoring Trustee on the basis that such extension is necessary for the Purchaser to continue commercializing the Divestment Business. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Mylan to the Purchaser. It shall not contain provisions requiring the delivery of minimum supply volumes or batches.
(7) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(8) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(9) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to pregabalin after the sale of the Divestment Product to the Purchaser;(d) all marketing authorizations relating to pregabalin held by the Parties outside of Norway;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business; and(f) the "Mylan" name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries.
(10) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
PRODUCT: MYLAN'S ALPRAZOLAM PRODUCTS
Territory: Portugal
(1) The Divestment Business consists of Mylan's rights, title and interests in alprazolam in Portugal (currently marketed under the name Alprazolam Mylan) including the right to develop, manufacture and use alprazolam with a view to its sale and marketing in any form in Portugal. Alprazolam is no longer under exclusivity and is used to treat anxiety or panic disorders.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing alprazolam in Portugal. It includes in particular:(a) the sale of existing alprazolam finished product inventory, sales and promotional material in Portugal to the extent available;(b) all alprazolam-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to alprazolam in Portugal specifically will be provided;112(c) the transfer of the marketing authorization for alprazolam in Portugal including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorizations available to Mylan; and(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in Portugal.The items referred to under (a) - (d) are hereinafter collectively referred to as "Assets of the Divestment Business".113
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of alprazolam in Portugal, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of alprazolam in Portugal.
(4) Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding alprazolam modified release tablets in Portugal with licensor and contract manufacturer […] to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the license agreement it has in place concerning the Divestment Business, subject to the consent of the third party. In the event that consent for the assignment cannot be obtained, at the option of the Purchaser Mylan commits to offer a back-to-back arrangement for the supply of the product to the Purchaser for the duration of the relevant third party contract and, in any case, for no longer than three years.
(5) At the option of the Purchaser, and to the extent national legislation allows for it, Mylan will license the name “Alprazolam Mylan” to the Purchaser for a period of two years (or more, should the Purchaser express an interest to that effect). To the extent that this is not allowed by national legislation and at the option of the Purchaser, Mylan will appoint the Purchaser as the exclusive distributor of the Divestment Business for a period of up to two years (or more, should the Purchaser express an interest to that effect).
(6) Mylan commits to make its best efforts to support the Purchaser in communicating the upcoming name change to physicians and pharmacists. Mylan will also support the Purchaser’s representations vis-a-vis physicians and pharmacies that the Purchaser’s product is identical to the product carrying the Mylan name for a period of up to 2 years (or more, should the Purchaser express an interest to that effect).
(7) In relation to the existing tender contracts, Mylan will transfer all historical information (orders; price; etc.) concerning its relationship with the different […] Mylan has tender agreements in Portugal for alprazolam. Mylan commits to make its best efforts to support the Purchaser to obtain the […] consent for the transfer the tender contracts.
(8) Mylan commits to continue its participation in tenders for the Divestment Business up until Divestment Closing. If Mylan were to win any tenders pertaining to alprazolam beforeDivestment Closing, Mylan commits to make its best efforts to facilitate the assignment of the relationship or the contract in line with the provisions contained in this Schedule concerning existing tender contracts.
(9) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(10) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(11) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to alprazolam after Divestment Closing;(d) all marketing authorizations currently held by the Parties outside of Portugal for alprazolam;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;114 and(g) monies owed to the Parties by customers for the purchase of alprazolam, and monies owed by the Parties to suppliers for materials used in the production of alprazolam.
(12) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
* * * * *
PRODUCT: MYLAN'S LATANOPROST/TIMOLOL PRODUCTS
Territory: Portugal
(1) The Divestment Business consists of Mylan's rights, title and interests in latanoprost/timolol in Portugal (currently marketed under the name Latanoprost/timolol Mylan) including the right to develop, manufacture and use latanoprost/timolol with a view to its sale and marketing in any form in Portugal. Latanoprost/timolol is no longer under exclusivity and is used to reduce inter ocular pressure in patients with various types of glaucoma and ocular hypertension.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing latanoprost/timolol in Portugal. It includes in particular:(a) the sale of existing latanoprost/timolol finished product inventory, sales and promotional material in Portugal to the extent available;(b) all latanoprost/timolol-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to latanoprost/timolol in Portugal specifically will be provided;115(c) the transfer of the marketing authorization for latanoprost/timolol in Portugal including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorizations available to Mylan; and(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in Portugal.The items referred to under (a) - (d) are hereinafter collectively referred to as "Assets of the Divestment Business".116
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of latanoprost/timolol in Portugal, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of latanoprost/timolol in Portugal.
(4) Mylan will transfer all historical information (orders, price, etc.) concerning its relationship, if applicable, regarding latanoprost/timolol in Portugal with licensor and supplier […] to the Purchaser in accordance with applicable law. Mylan commits to make its best efforts to facilitate the assignment to the Purchaser of the license and supply agreement it has in place concerning the Divestment Business, subject to the consent of the third party. In the event that consent for the assignment cannot be obtained, at the option of the Purchaser Mylan commits to offer a back-to-back arrangement for the supply of the product to the Purchaser for the duration of the relevant third party contract and, in any case, for no longer than three years..
(5) In relation to the existing tender contracts, Mylan will transfer all historical information (orders; price; etc.) concerning its relationship with the […]. Mylan commits to make its best efforts to support the Purchaser to obtain the […] consent for the transfer the tender contracts.
(6) Mylan commits to continue its participation in tenders for the Divestment Business up until Divestment Closing. If Mylan were to win any tenders pertaining to latanoprost/timolol before Divestment Closing, Mylan commits to make its best efforts to facilitate the assignment of the relationship or the contract in line with the provisions contained in this Schedule concerning existing tender contracts.
(7) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(8) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(9) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to latanoprost/timolol after Divestment Closing;(d) all marketing authorizations currently held by the Parties outside of Portugal for latanoprost/timolol;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;117 and(g) monies owed to the Parties by customers for the purchase of latanoprost/timolol, and monies owed by the Parties to suppliers for materials used in the production of latanoprost/timolol.
(10) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
* * * * *
PRODUCT: MYLAN'S SILDENAFIL (PAH) PRODUCTS
Territory: Romania
(1) The Divestment Business consists of Mylan's rights, title and interests in sildenafil in Romania (currently marketed under the name Mysildecard) including the right to develop, manufacture and use sildenafil (PAH) with a view to its sale and marketing in any form in Romania, only for the pulmonary arterial hypertension (PAH) indication. Sildenafil (PAH) is no longer under exclusivity.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing sildenafil (PAH) in Romania. It includes in particular:(a) the sale of existing sildenafil (PAH) finished product inventory, sales and promotional material in Romania to the extent available;(b) all sildenafil (PAH)-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to sildenafil (PAH) in Romania specifically will be provided;118(c) access to Mylan’s dossier for sildenafil (PAH) in order for the Purchaser to obtain its own marketing authorization for sildenafil (PAH);119(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in Romania; and(e) Full transfer of the national trademark related to sildenafil (PAH) in Romania or, in case of a wider than national specific sildenafil (PAH) trademark, an irrevocable, assignable, sub-licensable, perpetual and royalty free license to use the trademark for the Divestment Business.(f) The items referred to under (a) - (e) are hereinafter collectively referred to as "Assets of the Divestment Business".120
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of sildenafil (PAH) in Romania, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of sildenafil (PAH) in Romania.
(4) Pending the Purchaser applying for its own marketing authorizations for the Divestment Business, Mylan will appoint the Purchaser as the exclusive distributor of the Divestment Business for a period of up to two years and will, during this period, support the Purchaser in applying for its own marketing authorizations. During this two-year period, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in Romania.
(5) At the option of the Purchaser, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in Romania for an additional year after the two-year period noted above (i.e., up to three years in total). This period may be extended by one year, and an additional year thereafter, upon request by the Purchaser and subject to the approval of the Monitoring Trustee on the basis that such extension is necessary for the Purchaser to continue commercializing the Divestment Business. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Mylan to the Purchaser. It shall not contain provisions requiring the delivery of minimum supply volumes or batches.
(6) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(7) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(8) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to sildenafil (PAH) after Divestment Closing;(d) all marketing authorizations currently held by the Parties for sildenafil (PAH);(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;121 and(g) monies owed to the Parties by customers for the purchase of sildenafil (PAH), and monies owed by the Parties to suppliers for materials used in the production of sildenafil (PAH).
(9) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
* * * * *
PRODUCT: MYLAN'S ELETRIPTAN PRODUCTS
Territory: Sweden
(1) The Divestment Business consists of Mylan's rights, title and interests in eletriptan in Sweden (currently marketed under the name Eletriptan Mylan) including the right to develop, manufacture and use eletriptan with a view to its sale and marketing in any form in Sweden. Eletriptan is no longer under exclusivity and is used for the acute treatment of the headache phase of migraine attacks, with or without aura.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing eletriptan in Sweden. It includes in particular:(a) the sale of existing eletriptan finished product inventory, sales and promotional material in Sweden to the extent available;(b) all eletriptan-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to eletriptan in Sweden specifically will be provided;122(c) the transfer of the marketing authorization for eletriptan in Sweden including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorizations available to Mylan; and(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in Sweden.The items referred to under (a) - (d) are hereinafter collectively referred to as "Assets of the Divestment Business".123
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of eletriptan in Sweden, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of eletriptan in Sweden.
(4) At the option of the Purchaser, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in Sweden for up to three years, which period may be extended by one year, and an additional year thereafter, upon request by the Purchaser and subject to the approval of the Monitoring Trustee on the basis that such extension is necessary for the Purchaser to continue commercializing the Divestment Business. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Mylan to the Purchaser. It shall not contain provisions requiring the delivery of minimum supply volumes or batches.
(5) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(6) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(7) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to eletriptan after Divestment Closing;(d) all marketing authorizations currently held by the Parties outside of Sweden for eletriptan;(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;124 and(g) monies owed to the Parties by customers for the purchase of eletriptan, and monies owed by the Parties to suppliers for materials used in the production of eletriptan.
(8) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
* * * * *
PRODUCT: MYLAN'S SILDENAFIL (PAH) PRODUCTS
Territory: United Kingdom
(1) The Divestment Business consists of Mylan's rights, title and interests in sildenafil in the UK (currently marketed under the name Mysildecard) including the right to develop, manufacture and use sildenafil (PAH) with a view to its sale and marketing in any form in the UK, only for the pulmonary arterial hypertension (PAH) indication. Sildenafil (PAH) is no longer under exclusivity.
(2) The Divestment Business includes the transfer of all assets predominantly or exclusively used for the purposes of marketing sildenafil (PAH) in the UK. It includes in particular:(a) the sale of existing sildenafil (PAH) finished product inventory, sales and promotional material in UK to the extent available;(b) all sildenafil (PAH)-related contracts, commitments, and customer records, meaning customer credit records, customer invoices, purchase orders and contact details, whilst only the information related to sildenafil (PAH) in the UK specifically will be provided;125(c) access to Mylan’s dossier for sildenafil (PAH) in order for the Purchaser to obtain its own marketing authorization for sildenafil (PAH);126(d) an irrevocable, assignable, sub-licensable and royalty-free license for all relevant intellectual property rights, data books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Business with a view to its sale in UK; and(e) Full transfer of the national trademark related to sildenafil (PAH) in the UK or, in case of a wider than national specific sildenafil (PAH) trademark, an irrevocable, assignable, sub-licensable, perpetual and royalty free license to use the trademark for the Divestment Business.(f) The items referred to under (a) - (e) are hereinafter collectively referred to as "Assets of the Divestment Business".127
(3) If and to the extent that the know-how listed in paragraph 2(d) above of this Schedule is not exclusively or predominantly related to, and not exclusively used in respect of, the manufacture, use and sale of sildenafil (PAH) in UK, Mylan shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of sildenafil (PAH) in UK.
(4) Pending the Purchaser applying for its own marketing authorizations for the Divestment Business, Mylan will appoint the Purchaser as the exclusive distributor of the Divestment Business for a period of up to two years and will, during this period, support the Purchaser in applying for its own marketing authorizations. During this two-year period, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in the UK.
(5) At the option of the Purchaser, Mylan shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of the product in the UK for an additional year after the two-year period noted above (i.e., up to three years in total). This period may be extended by one year, and an additional year thereafter, upon request by the Purchaser and subject to the approval of the Monitoring Trustee on the basis that such extension is necessary for the Purchaser to continue commercializing the Divestment Business. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Mylan to the Purchaser. It shall not contain provisions requiring the delivery of minimum supply volumes or batches.
(6) The Parties also undertake to ensure that the applicable supply conditions described in the preceding two paragraphs will enable the Divestment Business to remain competitive irrespective of any adverse conditions resulting from the consequences of the exit of the United Kingdom from the European Union.
(7) Mylan will transfer all historical information (orders; price; etc.) concerning its relationship with […] regarding the regional tender contracts for sildenafil (PAH) in the UK. Mylan commits to make its best efforts to support the Purchaser to obtain […] consent for the transfer the tender contract.
(8) Mylan commits to continue its participation in tenders for the Divestment Business up until Divestment Closing. If Mylan were to win any tenders pertaining to sildenafil (PAH) before Divestment Closing, Mylan commits to make its best efforts to facilitate the assignment of the relationship or the contract in line with the provisions contained in this Schedule concerning existing tender contracts.
(9) Mylan commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer. Until transfer of the Divestment Business, Mylan will bear the costs for updates to maintain the current registration of the dossier of the Divestment Business. In addition, Mylan will bear the dossier preparation and regulatory filing costs for all the updates of the dossier triggered by the transfer of the Divestment Business to the Purchaser.
(10) The Purchaser will be given an option (to be exercised within one year after the Divestment Closing) to hire one or more Personnel, subject to applicable local employment legislation, who would be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
(11) The Divestment Business shall not include:(a) any manufacturing facility;(b) raw materials;(c) any research and development, clinical data and studies or intellectual property relating to sildenafil (PAH) after Divestment Closing;(d) all marketing authorizations currently held by the Parties for sildenafil (PAH);(e) any other asset not part of the Divestment Business or which is used in relation to a business of the Parties other than the Divestment Business;(f) the “Mylan” name, Mylan trademark and Mylan trade dress or trade dress of any of its subsidiaries;128 and(g) monies owed to the Parties by customers for the purchase of sildenafil (PAH), and monies owed by the Parties to suppliers for materials used in the production of sildenafil (PAH).
(12) If there is any asset or personnel which is not covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or personnel or adequate substitute will be offered to the Purchaser.
* * * * *
1 OJ L 24, 29.1.2004, p. 1 (the "Merger Regulation"). With effect from 1 December 2009, the Treaty on the Functioning of the European Union ("TFEU") has introduced certain changes, such as the replacement of "Community" by "Union" and "common market" by "internal market". The terminology of the TFEU will be used throughout this Decision.
2 OJ L 1, 3.1.1994, p. 3 (the "EEA Agreement").
3 Publication in the Official Journal of the European Union No C 72, 5.3.2020, p. 13.
4 Pursuant to the Transaction’s agreements, Pfizer will distribute Spinco common stock to its stockholders, either through a pro rata distribution as a stock dividend or an offer of Spinco common stock to Pfizer’s stockholders as a non-pro rata exchange offer
5 Turnover calculated in accordance with Article 5(1) of the Merger Regulation and the Commission Consolidated Jurisdictional Notice (OJ C95, 16.4.2008, p. 1).
6 For the purpose of this Decision, although the United Kingdom withdrew from the European Union as of 1 February 2020, according to the Withdrawal Agreement, Union law continues to apply to the United Kingdom during the transition period. Accordingly, any references made to the EEA in this Decision are meant to also include the United Kingdom.
7 In the pharmaceutical space, while an “originator” refers to the first product that was launched on the market for a specific molecule, “generics” refer to products that were launched after the loss of exclusivity of the originator.
8 The Commission notes that the Parties’ activities also overlap for (i) the supply of active pharmaceutical ingredients, (ii) the outlicensing of rights to FDPs, and (iii) the contract manufacturing of FDPs. However, the Transaction does not give rise to any affected market for these horizontal overlaps, regardless of market definition.
9 The Commission notes the Transaction also gives rise to a vertical relationship between the Parties’ activities in the contract manufacturing of FDPs (upstream) and the supply of FDPs (downstream). However, the Transaction does not give rise to any affected market for this vertical relationship, regardless of market definition.
10 See, for example M.9274 – GlaxoSmithKline/Pfizer Consumer Healthcare Business; M.8889 – Teva/PGT OTC.
11 OJ C 372, 9.12.1997, pages 5 to 13.
12 In past decisions, the Commission considered that anticholinergics could form a separate relevant product market. See M.7379 – Mylan/Abbott EPD-DM, para. 444 and M.7975 – Mylan/Meda, paras. 404-409.
13 See for example M.7975 – Mylan/Meda, para. 13 and M.7746 – Teva/Allergan, para. 13.
14 Generics are in general less expensive, bioequivalent versions of originator drugs. In regulatory approval procedures, a generic drug manufacturer has to demonstrate that the generic version of the originator drug has the same qualitative and quantitative composition in terms of active substance and the same pharmaceutical form and is bioequivalent to the originator drug.
15 See M.7975 – Mylan/Meda, M.5253 - Sanofi-Aventis/Zentiva.
16 See M.5778 - Novartis/Alcon, M.5865 - Teva/Ratiopharm, and M.5253 - Sanofi-Aventis/Zentiva.
17 See M.9274 - GlaxoSmithKline / Pfizer Consumer Healthcare Business. In the present case, drugs giving rise to (Group 1) affected markets are only prescription drugs and this distinction is thus not relevant.
18 Form CO, paras. 86-89.
19 Questionnaire Q2 to retailers, non-confidential replies to question 14.
20 Questionnaire Q1 to competitors, non-confidential replies to question 13.
21 Questionnaire Q2 to retailers, non-confidential replies to questions 14.2 and 14.3 and questionnaire Q1 to competitors, non-confidential replies to question 43.
22 Questionnaire Q2 to retailers, non-confidential replies to questions 14.2 and 14.3 and questionnaire Q1 to competitors, non-confidential replies to question 43.
23 Questionnaire Q1 to competitors, non-confidential replies to question 43. Significant competitors active in the supply of anticholinergics in Lithuania indeed consider that these molecules are interchangeable, especially because they are reimbursed for the same diagnosis (i.e. overactive bladder).
24 With regard to anticholinergics, the Commission considers that the precise relevant product market, whether it includes (i) all anticholinergic agents combined or (i) each separate anticholinergic agent separately, can be left open. Indeed, the competitive assessment of the Transaction does not raise serious doubts regardless of whether the relevant product market comprises (i) all anticholinergic agents combined or (i) each separate anticholinergic agent separately.
25 Questionnaire Q2 to retailers, non-confidential replies to question 13. Questionnaire Q3 to wholesalers, non-confidential replies to question 8.
26 For the purpose of this Decision, the Commission has looked at “galenic form” with reference to the first letter of the typology of form codes (the so-called “New Form Code” or NFC) used by IMS/EphMRA. In general, the first letter ("NFC-1") differentiates between forms for systemic and topical effect, site of application (e.g. oral, nasal, parenteral or rectal), and long-acting and ordinary forms.
27 Questionnaire Q2 to retailers, non-confidential replies to question 15.
28 Questionnaire Q1 to competitors, non-confidential replies to question 5.
29 See M.9274 – GlaxoSmithKline / Pfizer Consumer Healthcare Business, paras. 23-26. See also, e.g., M.7975 – Mylan/Meda, para. 24, and M.7746 – Teva/Allergan, para. 19.
30 See Questionnaire Q4 to national authorities, non-confidential replies, as well as Questionnaire Q1 to competitors, non-confidential replies, Questionnaire Q2 to retailers, non-confidential replies, and Questionnaire Q3 to wholesalers, non-confidential replies.
31 See M.7746 – Teva/Allergan, e.g. paras 47, 126, 168; M.7975 – Mylan/Meda, para 67. In those decisions, IQVIA’s former name, “IMS”, is mentioned as source of market share data.
32 The Notifying Parties relied on IQVIA data for most EEA countries, except those that are not fully covered by IQVIA, namely Cyprus, Iceland, Malta, and the United Kingdom (as well as the Netherlands for which the Notifying Parties relied on, the Farminform database). With regard to the years covered, the Notifying Parties did not provide full year 2019 market share because such data was not available at the time of notification from the relevant databases. As year-to-date market share data was however available from the relevant databases, at the request of the Commission, the Notifying Parties identified the additional affected markets arising from the Transaction based on 2019 year-to-date (January-November 2019) market share data. The Notifying Parties provided 2019 year-to-date (January-November 2019) market share data for these additional affected markets and for those where Mylan is a recent entrant (i.e. where it entered in the last three years). This Decision therefore contains 2019 market share data for the period January-November 2019 in the individual assessment of these markets where relevant.
33 See M.8889 – Teva / PGT OTC Assets, para. 35.
34 See M.5778 – Novartis/Alcon, para. 25.
35 See M.8889 – Teva / PGT OTC Assets, para. 36.
36 Based on 2018 market shares provided by the Parties, Group 3 markets arise in the following molecules (or combination of molecules)/country pairs: Nitroglycerin (C1E) in Spain; Doxazosin (C2A) in Poland and Spain; Doxazosin/Prazosin (C2A) in Spain; Sildenafil (C6B) in Germany and Sweden; Amlodipine (C8A) in Ireland and Portugal; atorvastatin (C10A) in Bulgaria, Croatia, Czechia, Greece, the Netherlands, Portugal, Slovakia, and the United Kingdom; anticholinergics (G4D) in Finland and Italy; sildenafil (G4E) in Czechia, Denmark, Hungary, Iceland, Ireland, Portugal, Slovakia, and the United Kingdom; celecoxib (M1A) in Germany; gabapentin (N3A) in Belgium, Iceland, and Italy; pregabalin (N3A) in Bulgaria, Germany, Iceland, and the Netherlands; ziprasidone (N5A) in Slovakia; alprazolam (N5C) in Belgium; benzodiazepines (N5C) in France and Portugal; sertraline (N6A) in Belgium, Czechia, Ireland, and Sweden; venlafaxine (N6A) in Czechia, Ireland, the Netherlands, Portugal, and Spain; latanoprost (S1E) in France (see Form CO, Annexes 10a-b).
37 Complex generics commonly refer to products, which require comparably higher investment before they may be launched on the market, because they are more difficult to manufacture or formulate, or require a drug/device combination.
38 See M.5865- Teva/Ratiopharm, paras.386-390.
39 However, in some instances the Parties’ combined market share may reach [60-70] or [60-70]%. The molecule/country markets in which the Parties’ combined market shares are between [50-60]% and [60- 70]% (based on volume shares) in 2018 are the following: amlodipine in Italy (Upjohn: [40-50]%, Mylan: [5-10]%, combined: [50-60]%, competitors Teva: [5-10]%, Doc Generici: [5-10]%, Novartis: [5-10]%); alprazolam in the Netherlands (Upjohn: [40-50]%, Mylan: [10-20]%, combined: [50-60]%, competitors Novartis: [20-30]%, Stada:[10-20]%, Teva: [5-10]%); alprazolam in Spain (Upjohn: [5-10]%, Mylan: [40- 50]%, combined: [50-60]%, competitors Infarco: [10-20]%, Normon: [5-10]%, Stada: [5-10]%, Aristo Pharma [5-10]%, Sun Pharma: [5-10]%); doxazosin in Italy (Upjohn: [50-60]%, Mylan: [5-10]%, combined: [60-70]%, competitors Stada: [5-10]%, Teva; [5-10]%, Doc Generici; [5-10]%); eplerenone in the United Kingdom (Upjohn: [40-50]%, Mylan: [10-20]%, combined: [60-70]%, competitors: Accord: ca. [10-20]%, Zentiva: ca. [10-20]%, Aspire Pharma: ca. [5-10]% based on the Parties’ estimates); pregabalin in Ireland (Upjohn: [50-60]%, Mylan: [0-5]%, combined: [50-60]%, competitors Krka: [10-20]%, Intas: [10-20]%, Teva: [5-10]%); pregabalin in Slovakia (Upjohn :[20-30]%, Mylan: [30-40]%, combined: [50- 60]%, competitors Krka: [10-20]%, Novartis: [5-10]%, Stada: [5-10]%, Glenmark Pharma: [5-10]%); sertraline in Italy (Upjohn: [10-20]%, Mylan: [40-50]%, combined: [50-60]%, competitors Doc Generici: [10-20]%, Novartis: [10-20],% Teva: [5-10]%, Stada: [5-10]%).
40 See Form CO, Section 7A.
41 The commission notes that, for the supply of doxazusim (within the ATC3 C2A) in Poland, the Transaction gives rise to a Group 3 market at the molecule level and to a Group 3 market at the molecule level, and to a Group 1 market at the molecule level for the galenic form NFC1 B (which corresponds to extended release tablets). At the molecule level (including both immediate and extended release forms), the Partie’s combined market share remain below (20-30%) which is indicative of a limited market power. At the molecule level, limited to the NFC1 B, the Partie’s 2018 combined share (70-80%) with a small increment of (0-5%) from Mylan while two competitors remain on the market, namely KrKaand Tva with respective 2018 share of (10-20%) and (0-5%) These two competitors rank among the 10 generic suppliers in Poland, have an increment higher than Mylan and did not raise substantiated concerns with regard to the supply of doxazosin in Poland. For these reasons, and the evidence available to it the Commission concludes that the competitive assessment does not differ regard less of whether the market’s is defined at molecule level, or further subdivided by galenic form.
42 The Partie were not able to provide the exact year when loss of exclusivity occurred as Czechia acceded to the EU in 2004 and Cardura was approved in Czechia in 1992. The Parties suspect that by the time of the accession of Czechia to the E.U, regulator exclusivity of Cardura in the country is likely to have expired.
43 See Section 8 of this Decision.
44 A Group 1 market also arises at the multi-molecule level (doxazosin and prazosin) in France.
45 See Section 8 of this Decision.
46 Horizontal Merger Guidelines, paras 17.
47 See Section 8 of this Decision.
48 INN stands for international non-proprietary name and is an official generic and non-brand name given to a drug or an active ingredient.
49 Horizontal Merger Guidelines, para 17.
50 No Hungary-based retailers responded to the market investigation.
51 Questionnaire Q3 to wholesalers, non-confidential replies to question 8. As indicated in footnote 50, no Hungary-based retailers responded to the market investigation.
52 Questionnaire Q4 to national authorities, non-confidential replies to question 5.
53 Questionnaire Q3 to wholesalers, non-confidential replies to questions 6 and 7.
54 Questionnaire Q3 to wholesalers, non-confidential replies to question 36.
55 Questionnaire Q3 to retailers, non-confidential replies to questions 21 and 22.
56 Questionnaire Q3 to wholesalers, non-confidential replies to question 25.
57 Questionnaire Q2 to retailers, non-confidential replies, non-confidential replies to question 28.
58 Sildenafil is a molecule falling within both (i) the ATC3 class C6B, for the treatment of PAH and (ii) the ATC3 class G4E, for the treatment of erectile dysfunctions. In this Decision, the Commission refers to “sildenafil (C6B)” for sildenafil products marketed for the treatment of PAH, and to “sildenafil (G4E)” for sildenafil products marketed for the treatment of erectile dysfunction.
59 See Section 8 of this Decision
60 See Section 8 of this Decision
61 Questionnaire Q3 to wholesalers, non-confidential replies to question 25
62 See Section 8 of this Decision
63 See Section 8 of this Decision
64 See Section 8 of this Decision
65 Questionnaire Q3 to wholesalers, non-confidential replies to question 22.
66 See Section 8 of this Decision.
67 Questionnaire Q3 to wholesalers, non-confidential replies to question 22.
68 The Commission notes that Upjohn has an outlicensing arrangement with [Upjohn’s outlicensees] for the supply of atorvastatin in Italy. However, this outlicensing relationship is very limited. It only relates to [details of the outlicensing agreement] (with a market share at molecule level of [0-5]% in terms of volume and value). The licensee, [name of the licensee], has its own independent marketing authorization for [galenic form], which accounts for the majority of [the licensee’s] sales in that molecule market.
69 Questionnaire Q3 to wholesalers, non-confidential replies to question 25.
70 Questionnaire Q1 to competitors, non-confidential replies to question 22. Questionnaire Q2 to retailers, non-confidential replies to question 28.
71 See Section 8 of this Decision
72 Questionnaire Q2 to retailers, non-confidential replies to question 14.3 and questionnaire Q1 to competitors, non-confidential replies to question 43.
73 Questionnaire Q3 to wholesalers, non-confidential replies to question 25.
74 Questionnaire Q3 to wholesalers, non-confidential replies to question 15.
75 Questionnaire Q2 to retailers, non-confidential replies to question 14.3 and questionnaire Q1 to competitors, non-confidential replies to question 43.
76 Questionnaire Q3 to wholesalers, non-confidential replies to question 25.
77 Questionnaire Q3 to wholesalers, non-confidential replies to question 25.
78 Questionnaire Q3 to wholesalers, non-confidential replies to question 22. Questionnaire Q2 to retailers, non-confidential replies to question 28.
79 Questionnaire Q3 to wholesalers, non-confidential replies to question 19. Questionnaire Q2 to Retailers, non-confidential replies to question 22.
80 Horizontal Merger Guidelines, para. 17.
81 Based on IQVIA, Orifarm also supplied its own unbranded product (eletriptan Orifarm).
82 Questionnaire Q1 to competitors, non-confidential replies to question 40.
83 Questionnaire Q1 to competitors, non-confidential replies to question 47. Questionnaire Q2 to retailers, non-confidential replies to question 35.
84 Questionnaire Q3 to wholesalers, non-confidential replies to questions 6 and 7.
85 Questionnaire Q1 to competitors, non-confidential replies to question 56.
86 See Section 8 of this Decision
87 As explained in footnote 32, market shares for the full year 2019 were not available at the time of notification. The notifying Parties provided market share figures for the supply of electriptan in Italy for 2019 year to date (namely from January to November 2019). The commission notes that these 2019 year to date market share data for the supply of eletriptan in Italy are useful to observe the evolution of reunt entrants.
88 Questionnaire Q3 to wholesalers, non-confidential replies to question 39.
89 Questionnaire Q3 to wholesalers, non-confidential replies to question 25.
90 Questionnaire Q1 to competitors, non-confidential replies to question 22. Questionnaire Q3 to retailers, non-confidential replies to question 28.
91 See Section 8 of the Decision.
92 See Section 8 of the Decision.
93 See Section 8 of this Decision.
94 The Transaction also gives rise to a Group 1 affected market, when looking only at immediate release products (NFCI1 class A). However, the competitive dynamics do not differ compared to those at molecule level, and will thas not be assessed separately.
95 The Transaction also gives rise to a Group 1 affected market, when looking only at immediate release products (NFCI1 class A). However, the competitive dynamics do not differ compared to those at molecule level, and will thas not be assessed separately.
96 Questionnaire Q3 to wholesalers, non-confidential replies to question 25.
97 Questionnaire Q3 to wholesalers, non-confidential replies to question 22. Questionnaire Q2 to retailers, non-confidential replies to question 28.
98 Questionnaire Q3 to wholesalers, non-confidential replies to question 22. Questionnaire Q2 to retailers, non-confidential replies to question 28.
99 The Transaction also gives rise to a Group 1 affected market, when looking only at immediate release products (NFCI1 class A). However, the competitive dynamics do not differ compared to those at molecule level, and will thas not be assessed separately.
100 Questionnaire Q2 to retailers, non-confidential replies to question 50.
101 Questionnaire Q3 to wholesalers, non-confidential replies to question 22. Questionnaire Q2 to retailers, non-confidential replies to question 28.
102 Questionnaire Q3 to wholesalers, non-confidential replies to question 25.
103 Questionnaire Q3 to wholesalers, non-confidential replies to question 22. Questionnaire Q2 to retailers, non-confidential replies to question 28.
104 See Section 8 of this Decision
105 A secundary medical use patient for the neuropathic pain indication of Lyria expired in July 2019.
106 Questionnaire Q3 to wholesalers, non-confidential replies to question 45. Questionnaire Q2 to retailers, non-confidential replies to question 51.
107 Questionnaire Q3 to wholesalers, non-confidential replies to question 45.
108 Questionnaire Q1 to competitors, non-confidential replies to question 41
109 The Transaction also gives rise to a Group 1 affected market, when looking only at oral solid immediate release products (NFC1 class A). However, the competitive dynamics do not differ compared to those at molecule level, as the Parties and their competitor’s market shares are similar , and will thus not be assessed separately.
110 See Section 8 of this Decision
111 Questionnaire Q2 to retailers, non-confidential replies to question 28.
112 Questionnaire Q4 to national authorities, non-confidential replies to question 11.
113 Questionnaire Q2 to retailers, non-confidential replies to question 17. Questionnaire Q3 to wholesalers, non-confidential replies to question 16.
114 Questionnaire Q3 to wholesalers, non-confidential replies to question 25.
115 The Transaction also gives rise to a Group 1 affected market, when looking only at oral solid immediate release products (NFC1 class A). However the competitive dynamics do not differ compared to those at molecule level, as the Parties and their Competitor’s market shares are similar and will thus not be assessed separately.
116 Questionnaire Q2 to retailers, non-confidential replies to questions 4 and 6.
117 Questionnaire Q2 to retailers, non-confidential replies to question 22. Questionnaire Q3 to wholesalers, non-confidential replies to question 19.
118 Questionnaire Q3 to wholesalers, non-confidential replies to question 25.
119 No additional affected Group 1(+) or 2 market would arise, considering benzodiazepines as a whole, with the exception of Austria. In Austria, Upjohn sells alprazolam (under the brand Xanor) and Mylan sells a different molecule, namely oxazepam. Regardless of market definitions, competition concerns can be excluded as the Parties’ combined market share is [40-50]% and there remain at least three providers with each, a market share of at least [5-10]% and a substantial presence in the country concerned and/or in terms of a portfolio of products in the same therapeutic area in the country.
120 Questionnaire Q2 to retailers, non-confidential replies to question 21. Questionnaire Q3 to wholesalers, non-confidential replies to question 18.
121 Questionnaire Q2 to retailers, non-confidential replies to question 27.
122 Questionnaire Q4 to national authorities, non-confidential replies to question 11.
123 Questionnaire Q2 to retailers, non-confidential replies to questions 28 and 29. Questionnaire Q3 to wholesalers, non-confidential replies to questions 22 and 23.
124 Questionnaire Q3 to wholesalers, non-confidential replies to question 26.
125 The Transaction also gives rise to a Group 1 affected market, when looking only at oral solid immediate release products (NFC 1 Class A).
126 See Section 8 of this Decision.
127 See Section 8 of this Decision.
128 Questionnaire Q2 to retailers, non-confidential replies to question 28 Questionnaire Q3 to wholesalers, non-confidential replies to question 22.
129 The Transaction also gives rise to a Group 1 affected market, when looking only at oral solid immediate release products (NFC1 class A) or oral liquid ordinary products (NFC1 class D). However, the competitive dynamics do not differ significantly compared to those at molecule level, as the Parties' combined market share remains similar (between [70-80]% and [70-80]% at NFCA1 A and D level respectively, with similar increments of [10-20]% and [10-20]% respectively) and the same competitors are generally active in both format, and will thus not be assessed separately.
130 Questionnaire Q1 to competitors, non-confidential replies to question 51.2.
131 Questionnaire Q1 to competitors, non-confidential replies to question 51.3.
132 Questionnaire Q2 to retailers, non-confidential replies to question 43.2. Questionnaire Q3 to wholesalers, non-confidential replies to question 38.2.
133 Questionnaire Q1 to competitors, non-confidential replies to question 50. Questionnaire Q2 to retailers, non-confidential replies to question 43. Questionnaire Q3 to wholesalers, non-confidential replies to question 38.
134 Questionnaire Q1 to competitors, non-confidential replies to question 57.
135 Questionnaire Q1 to competitors, non-confidential replies to question 51.3.
136 [Information concerning alprazolam in Italy]
137 The Transaction also gives rise to a Group 1 affected market, when looking only at oral solid immediate release products (NFC1 class A). However, the competitive dynamics do not differ compared to those at molecule level, as the Parties and their competitors' market shares are similar, and will thus not be assessed separately.
138 Questionnaire Q3 to wholesalers, non-confidential replies to question 25.
139 The Transaction also gives rise to a Group 1 affected market, when looking only at oral solid immediate release products (NFC1 class A) or only oral solid extended release formulations (NFC class B). However, the competitive dynamics do not differ significantly compared to those at molecule level, and will thus not be assessed separately. As the Parties' combined market share remains similar (between [50- 60]% and [60-70]% at NFCA1 A and B level respectively, with similar increments of [20-30]% and [30- 40]% respectively) and the same competitors are generally active in both format.
140 Questionnaire Q2 to retailers, non-confidential replies to question 26.
141 Questionnaire Q2 to retailers, non-confidential replies to question 19.
142 Questionnaire Q3 to wholesalers, non-confidential replies to question 13.
143 Questionnaire Q2 to retailers, non-confidential replies to question 54. Questionnaire Q3 to wholesalers, non-confidential replies to questions 20.1 and 48.
144 Questionnaire Q1 to competitors, non-confidential replies to question 55.
145 Questionnaire Q4 to national authorities, non-confidential replies to question 5.
146 The Transaction also gives rise to a Group 1 affected market, when looking only at oral solid extended release formulations (NFC class B). However, the competitive dynamics do not differ compared to those at molecule level, as the Parties and their competitors' market shares are similar, and will thus not be assessed separately.
147 Questionnaire Q2 to retailers, non-confidential replies to question 17. Questionnaire Q3 to wholesalers, non-confidential replies to question 16.
148 Questionnaire Q2 to retailers, non-confidential replies to question 33.1. Questionnaire Q3 to wholesalers, non-confidential replies to question 28.
149 Questionnaire Q3 to wholesalers, non-confidential replies to question 22.
150 Questionnaire Q4 to national authorities, non-confidential replies to question 4.
151 Questionnaire Q3 to wholesalers, non-confidential replies to question 25.
152 Questionnaire Q3 to wholesalers, non-confidential replies to question 25.
153 The Transaction also gives rise to a Group 1 affected market, when looking only at oral solid extended release formulations (NFC class B). However, the competitive dynamics do not differ compared to those at molecule level, as the Parties and their competitors' market shares are similar, and will thus not be assessed separately.
154 Questionnaire Q3 to wholesalers, non-confidential replies to question 25.
155 Latanoprost is a prostaglandine analogue, while latanoprost/timolol is molecule combination consisting of a prostaglandine analogue and a beta blocker. Pfizer/Upjohn is not the originator of timolol. The molecule combination latanoprost / timolol was originally developed by Pharmacia, a Company acquired by Pfizer in 2003.
156 See Section 8 of this Decision
157 See Section 8 of this Decision
158 Besides Upjohn, in Italy, latanoprost/timolol is marketed as branded generic by Bausch (with Ocuprostim), Doc Generici (with Latafix), Visufarma (with Imolast) and Angelini (with Droplacomb).
159 The Parties state that the Italian authorities do not allow for price increases of reimbursed products (latanoprost/timolol is a reimbursed product).
160 Questionnaire Q3 to wholesalers, non-confidential replies to question 15.
161 Questionnaire Q2 to retailers, non-confidential replies to question 26.
162 Questionnaire Q1 to competitors, non-confidential replies to question 50. Questionnaire Q2 to retailers, non-confidential replies to question 43. Questionnaire Q3 to wholesalers, non-confidential replies to question 38.
163 Questionnaire Q2 to retailers, non-confidential replies to question 27.
164 Questionnaire Q1 to competitors, non-confidential replies to question 57.
165 See Section 8 of this Decision
166 See Section 8 of this Decision
167 Questionnaire Q1 to competitors, non-confidential replies to question 57.
168 The Parties explain that in Portugal, for prescription products, (i) public authorities establish a maximum ex-factory price and regulate the wholesale and pharmacy margins, which would cap the public price paid by patients in the pharmacy and (ii) price increases require a request which would occur rarely and be only exceptionally granted.
169 Questionnaire Q3 to wholesalers, non-confidential replies to question 15.
170 Questionnaire Q2 to retailers, non-confidential replies to question 26.
171 Questionnaire Q1 to competitors, non-confidential replies to question 61.
172 Questionnaire Q3 to wholesalers, non-confidential replies to question 25.
173 Questionnaire Q1 to competitors, non-confidential replies to question 23.
174 Questionnaire Q2 to retailers, non-confidential replies to question 19.
175 Questionnaire Q3 to wholesalers, non-confidential replies to question 13.
176 Questionnaire Q2 to retailers, non-confidential replies to question 27.
177 Questionnaire Q4 to national authorities, non-confidential replies to question 5.
178 Questionnaire 1 to competitors, non-confidential replies to question 57.
179 Questionnaire 2 to retailers, non-confidential replies to question 28.
180 Questionnaire Q3 to wholesalers, non-confidential replies to question 25
181 See M.7645 Mylan/Perrigo and M.5865 Teva/Ratiopharm,
182 See M.7645 - Mylan/Perrigo and M.5865 - Teva/Ratiopharm.
183 Certificate(s) of suitability to the monographs of the European Pharmacopoeia, or "CEPs".
184 See https://www.edqm.eu/sites/default/files/presentationpheur-training-ppr general presentation
on cep-may2018.pdf
185 See paragraph 605.
186 See M.5295 – Teva/Barr.
187 See M.7746 – Teva/Allergan
188 M.5865-Teva Ratiopharm para.406 and M.5295 Teva/Ban, para.202.
189 Questionnaire Q1 to competitors, non-confidential replies to questions 72, 73 and 76.
190 Questionnaire Q1 to competitors, non-confidential replies to questions 73 and 76.
191 Questionnaire Q1 to competitors, non-confidential replies to question 72.
192 Questionnaire Q1 to competitors, non-confidential replies to question 76.
193 Questionnaire Q1 to competitors, non-confidential replies to question 76
194 See e.g. M.7746 – Teva/Allergan, para. 596; M.7480 – Actavis/Allergan, para. 75 and M.6613 – Watson/Actavis, para. 120. In the past the Commission did not always distinguish between contract manufacturing and outlicensing (see e.g., M.5865 – Teva/Ratiopharm, paras. 408-420 and M.6258 – Teva/Cephalon, paras. 145-151).
195 M.7746 – Teva/Allergan, para. 599; and M.6613 – Watson/Actavis, para. 120
196 M.7746 – Teva/Allergan, para. 607-609; and M.7975-Mylan-Meda, para.605.
197 With respect to (iii) while in past decisions the Commission has relied on a market share thereshold of 25% regarding the combined share of the Parties ad their licensee(s) the Commission relies on a 30% threshold suice, as of 1 January 2014, the 25% threshold for vertically affected market was changed to 30% (see the Commission implementing regulator n°1269/2013, Annex 1, Section 6-3).
198 M.7746 – Teva/Allergan, para. 607-609
199 Combined market shares include the market share of Upjohn/Mylan’s licensee.
200 The commission notes that, based on value market shares the Transaction gives rise to an addictional Group 1 vertically affected market-where the combined market share of the Parties and their licensee(s) is in excess of (30-40%) and the increment is over 1% for the following markets-for the supply of doxazosin (ATC3 C2A) in Spain where Upjohn is active upstream and both Parties are active downstream (with a combined market share in 2018 of (30-40%) in volume and (30-40%) in value.
201 Non-Horizontal Merger Guidelines, paras.47.
202 Questionnaire Q1 to competitors, non-confidential replies to question 63.
203 In the case of atorvastatin in Italy, the outlicensing relationship is very limited. It only relates to [details of the outlicensing agreement] (with a market share at molecule level of [0-5]% in terms of volume and value). The licensee, [name], has its own independent marketing authorization for [product with a different galenic form], which accounts for the majority of [licensee]’s sales in that molecule market.
204 Questionnaire Q1 to competitors, non-confidential replies to question 65.
205 Commission Notice on remedies acceptable under Council Regulation (EC) No 139/2004 and under Commission Regulation (EC) No 802/2004 (OJ C 267, 22.10.2008, p. 1-27), the "Remedies Notice".
206 Remedies Notice, paras. 9 and 61.
207 Remedies Notice, para. 12
208 Remedies Notice, para. 10.
209 Remedies Notice, paras. 23-25.
210 Remedies Notice, para. 47
211 Market test of the commitments (R1, R2 and R3), non-confidential replies to question 2.
212 Market test of the commitments (R1, R2 and R3), non-confidential replies to question 3.
213 Market test of the commitments (R1, R2 and R3), non-confidential replies to question 3
214 The responses were, however, inconclusive as to the criterion (by molecule, by country, or by therapeutic area) by which a grouping would be most effective. See Market test of the commitments (R1, R2 and R3, non-confidential replies to question 4.
215 Market test of the commitments (R1, R2 and R3), non-confidential replies to question 18.
216 Market test of the commitments (R1, R2 and R3), non-confidential replies to question 5.
217 Market test of the commitments (R1, R2 and R3), non-confidential replies to questions 13-15.
218 Market test of the commitments (R1, R2 and R3), non-confidential replies to question 8.
1 As explained in the Form RM, in lieu of transfering its marketing authorization to the Purchaser in these countries Mylan will allow the Purchaser to access Mylan’s dossier in order for the Purchaser to obtain its own marketing authorization. See the relevant commitments below.
2 Please note that the products marked cuith * are the products initially offered up for divestment.
3 Throughout this Form RM, PAH refers to pulmonary arterial hypertension.
4 Please note that (information relating to the commercialization of pregabalin in Luxembourg).
5 Best efforts obligations in this context are in line with the Commission’s pratice in the context of pharmaceutical mergers. See, for example, the remedes accepted in case M.7746. Teva /Allergan (2016).
6 This entails that Purchaser(s) would have to adduce specific circumstances based on which it can demonstrate objectively that in order to be able to competitively commercialize the relevant products it needs certain Mylan Personnel. Mylan will cooperate with the Monitoring Trustee to provide information necessary for the Purchaser to conduct this assessment.
7 Mylan will include all customer lists and records since 2014 in the Divestment Business.
8 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
9 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
10 Mylan will include all customer lists and records since 2014 in the Divestment Business.
11 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
12 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
13 Mylan will include all customer lists and records since 2014 in the Divestment Business.
14 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
15 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
16 Mylan will include all customer lists and records since 2014 in the Divestment Business.
17 Mylan’s marketing authorization for pregabalin was obtained via the centralized procedure and therefore cannot be transferred only as it relates to one country to the Purchaser (see explanation in the Form RM).
18 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
19 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
20 Mylan will include all customer lists and records since 2014 in the Divestment Business.
21 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favour of third parties, including those as to development, manufacture, distribution, marketing and sale.
22 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
23 Mylan will include all customer lists and records since 2014 in the Divestment Business.
24 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
25 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
26 Mylan will include all customer lists and records since 2014 in the Divestment Business.
27 Mylan’s marketing authorization for pregabalin was obtained via the centralized procedure and therefore cannot be transferred only as it relates to one country to the Purchaser (see explanation in the Form RM).
28 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
29 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
30 Mylan will include all customer lists and records since 2014 in the Divestment Business.
31 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
32 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
33 Mylan will include all customer lists and records since 2014 in the Divestment Business.
34 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
35 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
36 Mylan will include all customer lists and records since 2014 in the Divestment Business
37 Mylan’s marketing authorization for sildenafil (PAH) was obtained via the centralized procedure and therefore cannot be transferred only as it relates to one country to the Purchaser (see explanation in the Form RM).
38 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
39 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
40 Mylan will include all customer lists and records since 2014 in the Divestment Business.
41 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
42 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
43 Mylan will include all customer lists and records since 2014 in the Divestment Business.
44 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
45 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
46 Mylan will include all customer lists and records since 2014 in the Divestment Business.
47 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
48 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
49 Mylan will include all customer lists and records since 2014 in the Divestment Business.
50 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
51 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
52 Mylan will include all customer lists and records since 2014 in the Divestment Business.
53 Mylan’s marketing authorization for sildenafil (PAH) was obtained via the centralized procedure and therefore cannot be transferred only as it relates to one country to the Purchaser (see explanation in the Form RM).
54 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
55 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
56 Mylan will include all customer lists and records since 2014 in the Divestment Business.
57 Mylan’s marketing authorization for tadalafil (PAH) was obtained via the centralized procedure and therefore cannot be transferred only as it relates to one country to the Purchaser (see explanation in the Form RM).
58 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
59 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
60 Mylan will include all customer lists and records since 2014 in the Divestment Business.
61 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
62 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
63 Mylan will include all customer lists and records since 2014 in the Divestment Business.
64 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
65 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
66 Mylan will include all customer lists and records since 2014 in the Divestment Business.
67 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
68 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
69 Gerax is not a registered trademark.
70 Mylan will include all customer lists and records since 2014 in the Divestment Business.
71 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
72 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
73 Mylan will include all customer lists and records since 2014 in the Divestment Business.
74 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
75 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
76 Mylan will include all customer lists and records since 2014 in the Divestment Business.
77 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
78 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
79 Mylan will include all customer lists and records since 2014 in the Divestment Business.
80 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
81 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
82 Mylan will include all customer lists and records since 2014 in the Divestment Business.
83 Mylan’s marketing authorization for sildenafil (PAH) was obtained via the centralized procedure and therefore cannot be transferred only as it relates to one country to the Purchaser (see explanation in the Form RM).
84 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
85 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
86 Mylan will include all customer lists and records since 2014 in the Divestment Business.
87 Mylan’s marketing authorization for sildenafil (PAH) was obtained via the centralized procedure and therefore cannot be transferred only as it relates to one country to the Purchaser (see explanation in the Form RM).
88 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
89 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
90 Mylan will include all customer lists and records since 2014 in the Divestment Business.
91 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
92 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
93 Mylan will include all customer lists and records since 2014 in the Divestment Business.
94 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
95 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
96 Mylan will include all customer lists and records since 2014 in the Divestment Business.
97 Mylan’s marketing authorization for pregabalin was obtained via the centralized procedure and therefore cannot be transferred only as it relates to one country to the Purchaser (see explanation in the Form RM).
98 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
99 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
100 Mylan will include all customer lists and records since 2014 in the Divestment Business.
101 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
102 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser
103 Abnormally elevated levels of lipids or lipoproteins in the blood.
104 Mylan will include all customer lists and records since 2014 in the Divestment Business.
105 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
106 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
107 Mylan will include all customer lists and records since 2014 in the Divestment Business.
108 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
109 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
110 Mylan’s marketing authorization for pregabalin was obtained via the centralized procedure and therefore cannot be transferred only as it relates to one country to the Purchaser (see explanation in Form RM).
111 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favour of third parties, including those relating to the development, manufacture, distribution, marketing and sale of pregabalin in Norway.
112 Mylan will include all customer lists and records since 2014 in the Divestment Business.
113 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
114 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
115 Mylan will include all customer lists and records since 2014 in the Divestment Business.
116 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
117 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
118 Mylan will include all customer lists and records since 2014 in the Divestment Business.
119 Mylan’s marketing authorization for sildenafil (PAH) was obtained via the centralized procedure and therefore cannot be transferred only as it relates to one country to the Purchaser (see explanation in the Form RM).
120 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
121 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
122 Mylan will include all customer lists and records since 2014 in the Divestment Business.
123 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
124 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.
125 Mylan will include all customer lists and records since 2014 in the Divestment Business.
126 Mylan’s marketing authorization for sildenafil (PAH) was obtained via the centralized procedure and therefore cannot be transferred only as it relates to one country to the Purchaser (see explanation in the Form RM).
127 For the avoidance of doubt, the Divestment Business is transferred subject to any contractual obligations (including any confidentiality obligations) or restrictions applicable thereto in favor of third parties, including those as to development, manufacture, distribution, marketing and sale.
128 With the exception of the sale of inventory existing at Divestment Closing and sold to the Purchaser.