Commission, February 19, 2021, No M.9945
EUROPEAN COMMISSION
Decision
SIEMENS HEALTHINEERS / VARIAN MEDICAL SYSTEMS
Subject: Case M.9945 – Siemens Healthineers / Varian Medical Systems Commission decision pursuant to Article 6(1)(b) in conjunction with Article 6(2) of Council Regulation No 139/20041 2 and Article 57 of the Agreement on the European Economic Area3
Dear Sir or Madam,
(1) On 23 December 2020, the Commission received notification of a proposed concentration pursuant to Article 4 of the Merger Regulation, by which Siemens AG (“Siemens”, Germany), through its subsidiary Siemens Healthineers AG (“Siemens Healthineers”, […]* Germany), intends to acquire within the meaning of Article 3(1)(b) of the Merger Regulation control of the whole of Varian Medical Systems, Inc. (“Varian”, USA).4 Siemens is referred […]** with Varian as the “Parties”.
1. THE PARTIES AND THE OPERATION
(2) Siemens is a technology group headquartered in Munich (Germany), which is active worldwide and focuses on various areas including medical technology and digital healthcare services. Its subsidiary, Siemens Healthineers provides healthcare solutions and services worldwide under three business segments: (i) Imaging; (ii) Laboratory Diagnostics; and (iii) Advanced Therapies.
(3) Varian is a public corporation headquartered in Palo Alto (USA) and listed on the New York Stock Exchange. Varian is a global provider of medical devices and software solutions for treating cancer and other medical conditions with radiotherapy and other advanced treatments.
(4) On 2 August 2020, the Parties entered into an Agreement and Plan of Merger pursuant to which Siemens will acquire 100% of the shares in Varian (the “Transaction”). Siemens will thus acquire sole control of the whole of Varian. The Transaction is therefore a concentration within the meaning of Article 3(1)(b) of the Merger Regulation.
2. EU DIMENSION
(5) The undertakings concerned have a combined aggregate world-wide turnover of more than EUR 5 000 million5 (Siemens: EUR […]; Varian: EUR […]). Each of them has an EU-wide turnover in excess of EUR 250 million (Siemens: EUR […]; Varian: EUR […]), but none of the Parties achieved more than two-thirds of its aggregate EU-wide turnover within one and the same Member State. The notified operation therefore has an EU dimension.
3. GENERAL INTRODUCTION
(6) Siemens and Varian are both active in the provision of medical solutions to healthcare providers. Although the Transaction does not give rise to horizontal overlaps, the following activities of the Parties are closely related:
· Siemens provides imaging solutions, which are primarily used to diagnose a wide variety of medical conditions but can also be used to support the planning and delivery of oncology treatments, such as radiotherapy planning. The relevant imaging solutions are the following: computed tomography (“CT”) scanners, magnetic resonance (“MRI”) scanners, positron emission tomography CT (“PET/CT”) scanners.
· Varian supplies radiotherapy solutions used to plan and deliver radiotherapy treatments, including linear accelerators (“Linacs”), proton therapy equipment, brachytherapy equipment, motion management devices, as well as oncology software (such as oncology information system (“OIS”) and treatment planning software (“TPS”)).6
(7) Radiotherapy is an oncology treatment that uses radiation to extinguish cancer cells, shrink tumours and provide palliative treatment for cancer symptoms such as pain. The treatment algorithm for cancer patients receiving radiotherapy treatment involves a scanner to diagnose whether a tumour is cancerous. If so, the patient and the oncologist will discuss and decide the best treatment options, which may include radiotherapy. In such a case, a radiation oncologist will perform a radiotherapy simulation with a scanner (most often a CT scanner specifically equipped for radiotherapy, “CT simulator”). The simulation images are used to plan the radiotherapy, in particular (i) to contour the tumour and organs at risk (in order to ensure that the radiation beam hits the tumour without harming the surrounding healthy tissues); and (ii) to calculate the relevant radiation dose. On this basis, the TPS constructs a treatment plan to be executed by the radiotherapy equipment (most often Linacs). The above images/data are transferred to the OIS, which ensures the workflow between the various equipment and solutions needed for radiotherapy simulation, planning and delivery.
(8) It follows that Siemens’ imaging solutions (used for radiotherapy simulation) and Varian’s radiotherapy solutions are part of an integrated ecosystem and, thus, closely related, giving rise to conglomerate links.7
4. MARKET DEFINITION
4.1. Product Market Definitions
4.1.1. Imaging Solutions
(9) Imaging equipment is employed to create visual representations of the interior of the human body, which can be used for a variety of diagnostic and treatment purposes across multiple medical disciplines (including notably radiotherapy).8 Siemens supplies imaging equipment based on different technologies, including in particular:
- CT scanners. CT is a non-invasive procedure that uses X-rays to create detailed pictures, or scans, of areas inside the body;
- MRI scanners. MRI is a non-invasive procedure that uses a magnetic field and computer-generated radio waves to create images of areas inside the body. MRI does not use X-rays and therefore does not produce ionizing radiation;
- PET/CT scanners. PET imaging uses radioactive substances to visualize and measure metabolic processes in the body. During a PET exam, a radioactive tracer is injected into the patient’s blood. PET/CT scanners combine PET and CT technology in one single unit equipment and acquire sequential images from both in the same session, which are combined into a single superimposed image.
(A) The Commission’s precedents
(10) In past decisions, the Commission concluded that a segmentation by imaging modality – i.e. CT, MRI and PET/CT in the present case – was appropriate, while it left open whether further segmentations within imaging modalities (e.g. by product range or by end-use) were necessary.9
(11) More specifically, in relation to CT-scanners, the Commission considered possible segmentations between (i) single-slice or multi-slice, and/or (ii) low, mid, and high- end, but left the market definition open in the absence of competition concerns.10
(12) Likewise, for MRI scanners, the Commission considered segmenting the market between (i) open MRI scanners, using non-cylindrical magnets and are open vertically or horizontally, and closed MRI scanners, using cylindrical magnets that surround the patient who is placed in a gantry,11 and/or (ii) low, mid, and high-end MRI scanners12 but ultimately left the market definition open.
(13) Furthermore, the Commission assessed but ultimately left open whether nuclear imaging equipment should be further segmented according to type, in Gamma Cameras and PET scanners.13
(B) The Notifying Party’s view Segmentation by imaging modality
(14) In line with the Commission’s decisional practice, the Notifying Party submits that CT scanners, MRI scanners and PET/CT scanners (together referred as “Scanners”) constitute distinct product markets. As detailed below, it also argues that these markets should not be further segmented.
Segmentation by end-use
(15) As regards a potential segmentation by end-use, the Notifying Party argues that it is not relevant to define markets limited to CT scanners, MRI scanner and PET/CT scanners used for radiotherapy simulation (i.e. so-called “CT simulators”, “MRI simulators” and “PET/CT simulators”, together referred as “Simulators”). In particular, the Notifying Party claims that, from a demand-side perspective, Scanners and Simulators are fully substitutable and equally capable of performing radiotherapy simulation. According to the Notifying Party, Simulators are standard Scanners with certain characteristics and additional features, which for the most part are not intrinsic to the Scanner itself and which customers can procure separately from the Scanner equipment (including from third-party suppliers). Moreover, the Notifying Party argues that [commercial strategy]. From the supply-side perspective, the Notifying Party submits that (i) all suppliers of imaging equipment offer a broad portfolio of Scanners for all clinical uses, including radiotherapy; and (ii) Simulators are [information on Simulators’ manufacturing process] and do not require significant additional manufacturing steps or specific know-how.14
Other possible segmentations
(16) The Notifying Party also contests the relevance of the other potential segmentations envisaged by the Commission in the past, arguing that (i) all Scanners can be used for all or most clinical applications regardless of whether there are low-, mid-, or high-end; (ii) in the case of CT scanners, the distinction between single- or multi- slice is no longer appropriate, since the former are no longer considered “state of the art” and that all CT scanners sold today are multi-slice; and (iii) in the case of MRI scanners, the distinction between open and closed is not appropriate as it does not impact the end-use application of the Scanner per se.15
(C) The Commission’s assessment Segmentation by imaging modality
(17) The results of the market investigation confirmed that CT scanners, MRI scanners and PET/CT scanners constitute distinct product markets. Customers and competitors generally consider that different Scanners cannot be used interchangeably for the same medical procedure,16 revealing limited demand-side substitutability. For instance, a competitor stressed the fact that CT scanners, MRI scanners and PET/CT scanners are “used for different medical purposes”, rely on a “different technology” and have “different price”,17 while a customer indicated that “the different scanners have different intended use and technical feature.”18 The above conclusion is also corroborated by the market data provided by the Notifying Party, which show that market conditions for CT, MRI and PET/CT scanners are not homogeneous, each type of Scanners being characterised by (i) different prices19 and (ii) different competitive landscape.20
Segmentation by end-use
(18) As regards the existence of a potential segment for imaging equipment used for radiotherapy simulation, the market investigation provided mixed results.
(19) First, contrary to the Notifying Party’s claims, the market investigation provided indications that a distinction between Scanners and Simulators could be appropriate due to limited demand-side substitutability. Indeed, the vast majority of respondents consider that, for radiotherapy simulation, the use of Simulators is more efficient and convenient than the use of standard Scanners, explaining that Simulators are “optimized for radiotherapy planning”.21 In particular, respondents stressed that the size of the bore, which is an intrinsic feature of the Simulator itself that cannot be procured separately is critical in radiotherapy simulation: “in extremis the CT scanners can be used for radiotherapy planning, however the difference in bore size restricts the procedure considerably”.22 That said, several market participants also indicated that Scanners could be used for radiotherapy simulation, provided that additional features or “extra options” are installed on them.23
(20) Second, most respondents to the market investigation indicated that the supply conditions for Scanners and Simulators are not the same, stressing in particular a difference in terms of pricing.24 This is also corroborated by the EEA/UK market share estimates provided by the Notifying Party, which vary significantly for the supply of CT scanners and CT simulators.25 In particular, Siemens’ market share in CT simulators in the EEA/UK, over the 2017-2019 period, is more than [20-30] percentage points higher than its market share for the supply of CT scanners ([60- 70]% vs. [40-50]%). Moreover, the feedback received from the market investigation revealed that the number of credible and competitive suppliers is more limited for CT simulators than for CT scanners. Indeed, market participants generally consider that Canon is not competitive for the supply of CT simulators, whereas it is perceived as a competitive player for the supply of CT scanners.26 That being said, the vast majority of competitors also indicated that all companies manufacturing standard CT scanners have the capabilities to manufacture CT simulators.27
(21) Third, the Commission notes that Siemens’ internal documents support the existence of distinct market segments for Simulators.28 In particular, these documents illustrate that [commercial strategy])29.
(22) In any event, for the purpose of this Decision, the question of whether CT simulators, MRI simulators and PET/CT simulators constitute distinct market segments can be left open, as it has no impact on the Commission’s competitive assessment of the Transaction.
Other possible segmentations
(23) As regards other possible segmentations, the Commission notes that the elements in the file show that the markets for CT scanners, MRI scanners and PET/CT scanners are differentiated markets, where low- and high-end Scanners may not be fully substitutable. However, the results of the market investigation did not provide indications that a segmentation between low-, mid-, and high-end Scanners, as well as the other alternative segmentations envisaged in the past decisional practice (see Section 4.1.1(A) above) would be relevant in the present case.
(D) Conclusion
(24) Based on the results of the market investigation, for the purpose of this Decision, the Commission concludes that (i) CT scanners, MRI scanners and PET/CT scanners constitute distinct product markets and that (ii) it can be left open whether the above products markets should further segmented according to their end-use, as these alternative market delineations do not affect the Commission’s conclusion regarding the compatibility of the Transaction with the internal market.
4.1.2. Radiotherapy solutions
4.1.2.1. Radiotherapy equipment
(25) Radiotherapy uses radiation to kill cancer cells, shrink tumours and provide palliative treatment for cancer symptoms such as pain. Radiotherapy is one of the main therapies for treating cancer. It is used alone or in combination with other cancer therapies such as surgery, chemotherapy, immunotherapy or interventional oncology.
(A) The Commission’s precedents
(26) The Commission has not previously examined the markets for radiotherapy equipment.
(B) The Notifying Party’s view
(27) In the absence of Commission precedents, the Notifying Party submits that there are four types of equipment used to deliver radiotherapy treatment, based on the position and radiation source, which each constitute separate product markets:30
(i) External beam radiotherapy (“EBRT”), which involves radiation being directed at the tumour from outside the body. EBRT can be delivered with:
· Linear accelerators (“Linacs”), which use high energy X-rays (photons) to destroy the cancerous cells. Linacs are the most common form of radiotherapy and are used to treat most types of cancer; and
· Proton therapy, which uses protons instead of X-rays to destroy cancerous cells and it is used when there are great risks associated with damage to healthy tissue (e.g. in paediatric cancers) as it allows the delivery of radiation in a more targeted way with fewer side-effects. Proton therapy involves significant capital investments and its operation requires dedicated facilities (there are only around 20 proton therapy centres in Europe);
(ii) Brachytherapy, which uses a radiation source located inside the body and is, thus, unlike ERBT, an invasive treatment procedure. Brachytherapy is mainly used to treat certain types of cancer (e.g. cervical, breast, skin); and
(iii) Systemic radioisotope therapy, which involves radioactive materials administered by infusion or orally. Since Varian is not active in systemic radiation therapy, this Decision will not discuss this radiotherapy equipment further.
(28) The Notifying Party claims that a further segmentation is not warranted as within each type of radiotherapy equipment, the various models are merely the result of technological and scientific advancements.31
(C) The Commission’s assessment
(29) The results of the market investigation and the evidence available in the Commission’s file support the Notifying Party’s arguments.
(30) First, respondents to the market investigation confirmed that (i) Linacs, (ii) proton therapy equipment and (iii) brachytherapy equipment cannot be used interchangeably for the same medical procedures, revealing limited demand-side substitutability.32 In that respect, a customer explained that, “each type of treatment (external [EBRT], proton [therapy], brachy[therapy]) requires suitable equipment which cannot be interchangeable”,33 whilst another indicated that the use of the various equipment differs “because of their special inherent physics in dose application and their natural behaviour in human tissue (dose distribution)”.34 Some respondents however indicated that Linacs and proton therapy share some applications (as both equipment are considered EBRT techniques) whereas proton therapy uses newer technology and is significantly more expensive.35
(31) Second, the market data provided by the Parties show that market conditions for Linacs, proton therapy and brachytherapy are not homogeneous, each type of radiotherapy equipment being characterised by (i) different prices36 and (ii) different competitive landscape.37
(32) Third, the Commission notes that Siemens’ internal documents,38 as well as independent industry reports39 support the existence of distinct markets for each type of radiotherapy equipment.
(33) Finally, the results of the market investigation did not provide any indication that a further segmentation of the Linacs, proton therapy and brachytherapy was warranted.
(D) Conclusion
(34) Based on the above, the Commission considers that, for the purpose of this Decision, Linacs, brachytherapy and proton therapy constitute separate product markets, without the need for a further segmentation.
4.1.2.2. Oncology Software
(35) In the field of radiotherapy, Varian offers two main types of oncology software,40 including:
- Treatment planning software (“TPS”), which allows physicians to plan how the radiotherapy equipment will be used to deliver the treatment;
- Oncology information software (“OIS”), which is a software solution providing a single portal to facilitate management of the profile and treatment of cancer patients. It integrates information about a patient’s diagnosis and therapy from the range of healthcare professionals and equipment involved in the patient’s oncology treatment.
(A) The Commission’s precedents
(36) There is no Commission precedent analysing the markets for oncology software. However, in previous cases assessing software solutions, the Commission considered that software can be segmented based on (i) their functionality, (ii) the sector concerned, and (iii) their end-use.41 With respect to healthcare software more specifically, the Commission also considered a potential segmentation by module (i.e Hospital Information System or “HIS”, Electronic Medical Record or “EMR”, etc.), leaving the exact product market definition open.42
(B) The Notifying Party’s view TPS
(37) The Notifying Party, referring to precedents at national level,43 submits that TPS for EBRT constitute a distinct product market which should not be further segmented between Linacs and proton therapy as EBRT TPS is designed for external radiation delivery, irrespective of whether that is proton based (i.e. proton therapy) or X-ray based (i.e. Linacs). In that respect, the Notifying Party notes that Varian offers the same software platform for both Linacs and proton therapy.44 The Notifying Party also claims that brachytherapy equipment and TPS for brachytherapy are part of the same market on the basis that Varian does not sell TPS for brachytherapy as a stand- alone product.45 Ultimately, the Notifying Party considers that the product market definition can be left open.
OIS
(38) The Notifying Party submits that OIS has specific features that are not necessarily the same as the ones in other healthcare software. However, it also considers that OIS present complementary features to EMR or HIS and that suppliers of EMR and HIS could easily enter the OIS market with limited investment in capital and time. Due to this supply-side substitutability, the Notifying Party concludes that HIS, EMR and OIS should be viewed as being part of the same market.46
(C) The Commission’s assessment TPS
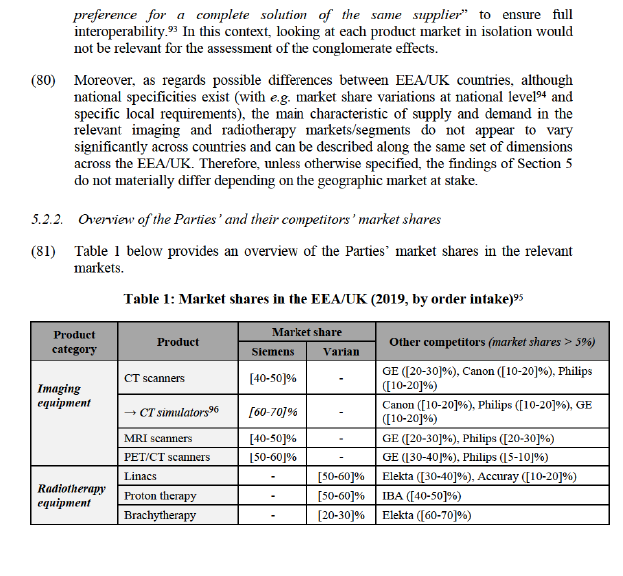
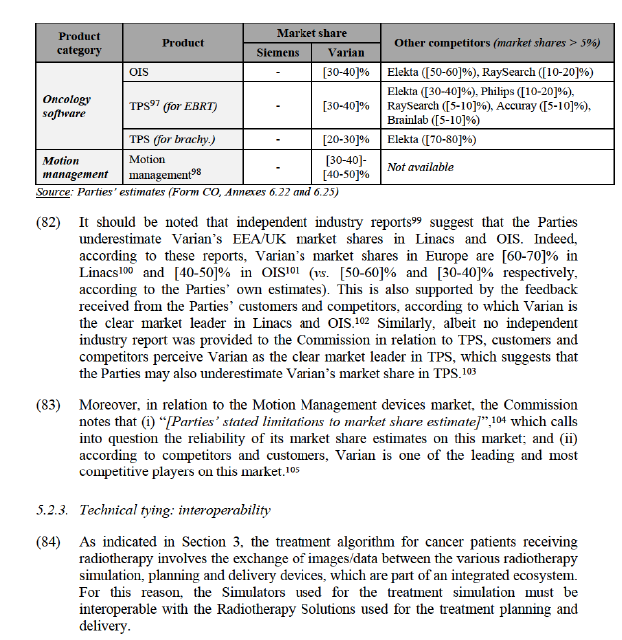
(39) The results of the market investigation confirmed the Notifying Party’s claim that TPS constitute a distinct product market from other healthcare software.47 Indeed, a majority of respondents indicated that, while TPS share some functionalities with other software solutions (e.g. contouring), TPS is not substitutable with other software solutions as it presents a specific set of functionalities that remain its sole remit, such as dose calculation for the planning of radiotherapy.48
(40) The market investigation also provided indications that TPS for EBRT and TPS for brachytherapy constitute separate product markets, as the supply conditions for these two software are different.49 In that respect, one customer indicated that “some suppliers are more focused on brachytherapy and others on EBRT” while another one added that “complexity, pricing [and] suppliers are different”.50 This is also corroborated by the market share data provided by the Parties, which reveal that in the EEA/UK the competitive landscape in TPS for brachytherapy is characterised by a quasi-duopoly between Elekta and Varian, which is not the case in TPS for EBRT (see Table 1 below). However, the results of the market investigation do not allow the Commission to determine whether TPS for brachytherapy should be part of the overall product market for brachytherapy equipment.
(41) Based on the above considerations, the Commission concludes that, for the purpose of this Decision (i) TPS constitute a separate product market, and that (ii) it can be left open whether the market for TPS should be further segmented between TPS for brachytherapy and TPS for EBRT, as these alternative market delineations do not affect the Commission’s conclusion regarding the compatibility of the Transaction with the internal market.
OIS
(42) The results of the market investigation did not support the Notifying Party’s view and provided clear indications that OIS constitute a distinct market from other healthcare software.
(43) The vast majority of customers responding to the market investigation indicated that OIS cannot be replaced by other software solutions,51 “because of the complex radiotherapy workflow and specific functionality, integration with TPS and delivery systems, […] transferring the functionality of the OIS to the HIS is extremely difficult, certainly in a large hospital facility”.52 While customers who responded to the market investigation acknowledged that OIS share some features with other healthcare software such as HIS and EMT, they also confirmed that “OIS has crucial features that the HIS/EMR/PACS does NOT have”.53 Suppliers also confirmed the differing functionalities and purposes of these products and did not raise any suggestion that there is strong supply side substitutability between the products.54
(44) In addition, whilst the majority of customers who responded to the market investigation explained that they typically procure OIS together with other radiotherapy solutions,55 the results of the market investigation and evidence in the Commission’s file also show that the competitive dynamics for OIS differ from other radiotherapy solutions’. This is notably illustrated by the fact that the competitive landscape for the supply of OIS in the EEA/UK differ from the supply of other radiotherapy solutions, in terms notably of the identity of the suppliers56 and market share estimates.57
(45) Based on the above, the Commission considers that, for the purpose of this Decision, OIS constitute a distinct product market, with no need for further segmentation.
(D) Conclusion
(46) Based on the results of the market investigation, for the purpose of this Decision, the Commission concludes that (i) OIS and TPS constitute separate product markets and that (ii) it can be left open whether the market for TPS should be further segmented between TPS for brachytherapy and TPS for EBRT, as these alternative market delineations do not affect the Commission’s conclusion regarding the compatibility of the Transaction with the internal market.
4.1.2.3. Motion Management Devices
(47) Motion management devices track and manage a patient’s motion (e.g. respiration, movements) during the radiotherapy simulation and treatment (“Motion Management devices”). Motion Management devices are used either (i) with imaging equipment (such as CT simulators) while the image is acquired during radiotherapy simulation (“Imaging Motion Management devices”) or (ii) with radiotherapy equipment (such as Linacs) while the radiotherapy treatment is delivered (“Treatment Motion Management devices”). Motion Management devices have an interface, composed of a hardware and a software, whose intended purpose is to connect the former with the relevant imaging or radiotherapy equipment.
(48) During the radiotherapy simulation, the Imaging Motion Management device tracks the patient’s motion and breathing patterns. The obtained data are then used by the Treatment Motion Management device (combined with the radiotherapy equipment), so that those same motions and patterns can be accounted for during the delivery of the radiotherapy treatment to avoid distortions of the target volume and incorrect positional and volumetric information.58
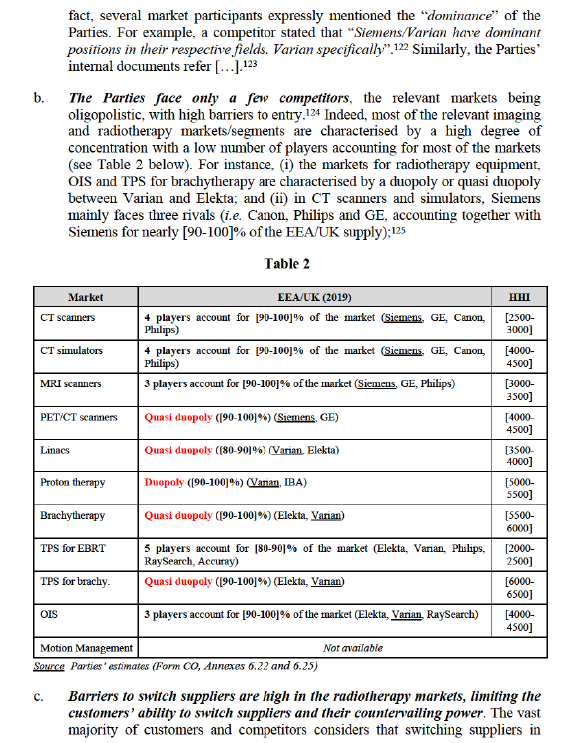
(A) The Commission’s precedents
(49) The Commission has not previously examined the market for Motion Management devices.
(B) The Notifying Party’s view
(50) The Notifying Party did not expressly provide its view on the scope of the relevant product market for the production and supply of Motion Management devices. However, in the Form CO, Siemens (i) makes a distinction between Imaging and Treatment Motion Management devices59 and (ii) stresses that Imaging and Treatment Motion Management devices do not need to be purchased from the same supplier.60
(C) The Commission’s assessment
(51) The results of the Commission’s market investigation provide indications that Motion Management devices constitute a distinct product market. In particular, the investigation revealed that the main suppliers for Motion Management devices are not identical to the suppliers of other radiotherapy and imaging solutions.61 For instance, respondents to the market investigation identified that the main suppliers for motion management devices (apart from Varian and Elekta) are VisionRT, Brainlab, Anzai, Dyn’R and C-Rad (among others),62 none of which is a radiotherapy or medical imaging solutions supplier.
(52) However, the elements in the file do not allow the Commission to conclude whether the market should be further segmented between Imaging and Treatment Motion Management devices.
(D) Conclusion
(53) Based on the above considerations, the Commission concludes that, for the purpose of this Decision: (i) Motion Management devices constitute a separate product market, and that (ii) it can be left open whether the market should be further segmented between Imaging and Treatment Motion Management devices, as it does not affect the Commission’s conclusion regarding the compatibility of the Transaction with the internal market.
4.2. Geographic Market Definition
4.2.1. Imaging solutions, radiotherapy equipment and Motion Management devices
(A) The Commission’s precedents
(54) In previous cases, the Commission has assessed the relevant geographic markets for all types of imaging solutions both at an EEA/UK-wide and national level, while leaving the precise geographic scope open in the absence of competition concerns.63
(55) As explained above, the Commission has not examined in the past the geographic market definition in relation to either radiotherapy equipment or Motion Management devices. That said, in past decisions related to other types of medical devices, the Commission has generally considered that the markets for medical devices are national in scope, in particular in view of the national regulatory and reimbursement schemes.64
(B) The Notifying Party’s view
(56) The Notifying Party submits that the scope of the relevant geographic markets for imaging solutions and radiotherapy equipment should be at least EEA/UK-wide for the following reasons. First, suppliers are active globally, delivering their solutions worldwide and across the EEA/UK, bearing relatively low transportation costs compared to the value of the equipment.65 Second, to be marketed in the EEA/UK, imaging solutions, radiotherapy equipment and Motion Management devices must be certified to conform to the essential requirements of the EU Medical Devices Directive66 and must obtain a CE-mark.67 Third, Siemens argues that procurement rules are, to some extent, harmonized at EEA/UK level,68 which render the supply relatively homogenous across the EEA/UK.69 However, it also acknowledges the existence of national specificities, such as procurement procedures and reimbursement policies. The Notifying Party concludes that the precise geographic scope of the above product markets can be left open.70
(C) The Commission’s assessment
(57) The Commission’s market investigation provided mixed results as regards the geographic scope of the relevant markets for (i) imaging solutions (namely CT, MRI and PET/CT scanners), (ii) radiotherapy equipment (namely Linacs, brachytherapy equipment and proton therapy equipment) and (iii) Motion Management devices, and their potential sub-segments.
(58) On the demand-side, the investigation yielded mixed results. Customers and competitors confirmed that the procurement of the above products is to some extent harmonised at EEA/UK level with the application of the EU rules on public procurement,71 but also stressed the existence of local specificities at national level, in terms of public procurement procedures and practices, as well as reimbursement policies.72
(59) On the supply-side, the market investigation confirmed that the main suppliers are active across the EEA/UK and more generally at global level.73 This is illustrated by the fact that, regardless of their location, customers identified suppliers active worldwide as the largest credible players in each of the relevant markets.74 While it was noted that customers may require maintenance and repair services for medical devices at the local level, in practice major suppliers’ (or their distributors’) engineers are present throughout the EEA/UK so as to meet this demand.75
(60) Finally, the Commission also notes that the Parties’ internal documents assess market conditions both at national and EEA/UK level (if not worldwide level).76
(61) The above provides indications that, despite the existence of national specificities, from a supply-side perspective, the Parties compete to some extent with their rivals at a broader geographic level (at least at EEA/UK level).
(D) Conclusion
(62) Based on the results of the market investigation, for the purpose of this Decision, the Commission concludes that the geographic scope of the imaging solutions, radiotherapy equipment and motion management devices markets can be left open (between national or at least EEA/UK-wide) as the exact geographic scope of the markets does not affect the Commission’s conclusions regarding the compatibility of the Transaction with the internal market.
4.2.2. Oncology Software
(A) The Commission’s precedents scope of the market for healthcare software solutions to be either national or EEA/UK wide, leaving the
(63) In past decisions, the Commission considered the geographic exact scope open.77 However, there is no Commission precedent analysing the markets for oncology software, including TPS and OIS.
(B) The Notifying Party’s view
(64) In relation to TPS, the Notifying Party considers that the same arguments as the ones raised for radiotherapy equipment apply (see recitals (56)-(56) above).78 Thus, the Notifying Party considers that the market should be defined as being EEA/UK-wide in scope but ultimately concludes that it can be left open.
(65) Similarly, with respect to OIS, the Notifying Party considers the geographic scope of the market to be at least EEA/UK-wide, if not worldwide in scope. This is because the main suppliers are active globally, and the same software products are homogenous and available at worldwide level. However, the Notifying Party submits that in some instances, interfaces might have to be translated into the local language.79
(C) The Commission’s assessment
(66) The results of the market investigation and the evidence in the Commission’s file did not provide any indications that the Commission should depart from its decisional practice regarding healthcare software in the present case. In particular, the results of the market investigation were the same for OIS and TPS as for imaging solutions, radiotherapy equipment and Motion Management devices, confirming that: (i) EU procurement rules apply, (ii) there are some variations at national level in procurement and reimbursement rules and practices, (iii) the main and most credible suppliers are the same across the EEA/UK.80
(D) Conclusion
(67) Based on the results of the market investigation, for the purpose of this Decision, the Commission concludes that the geographic scope of the OIS and TPS markets can be left open (between national or at least EEA/UK-wide) as the exact geographic scope of the markets do not affect the Commission’s conclusions regarding the compatibility of the Transaction with the internal market.
5. COMPETITIVE ASSESSMENT
5.1. Framework of analysis - Assessment of conglomerate non-coordinated effects
(68) Article 2 of the Merger Regulation provides that the Commission has to appraise concentrations with a view to establishing whether or not they are compatible with the internal market. For that purpose, the Commission must assess, pursuant to Article 2(2) and (3), whether or not a concentration would significantly impede effective competition, in particular as a result of the creation or strengthening of a dominant position in the common market or a substantial part of it.
(69) In this Decision, the Commission’s assessment focuses on conglomerate non- coordinated effects due to the combination of the Parties’ complementary equipment in the imaging and radiotherapy space.
(70) Conglomerate mergers consist of mergers between companies that are active in closely related markets, for instance suppliers of complementary products or of products which belong to a range of products that is generally purchased by the same set of customers for the same end use.81
(71) Pursuant to the Non-Horizontal Merger Guidelines, in most circumstances, conglomerate mergers do not lead to any competition problems.82 However, foreclosure effects may arise when the combination of products in related markets may confer on the merged entity the ability and incentive to leverage a strong market position from one market to another closely related market by means of tying or bundling or other exclusionary practices.83
(72) The Non-Horizontal Merger Guidelines distinguish between bundling, which usually refers to the way products are offered and priced by the merged entity and tying, which usually refers to situations where customers that purchase one good (the tying good) are required to also purchase another good from the producer (the tied good).84
(73) Within bundling practices, a distinction is also made between pure bundling and mixed bundling. In the case of pure bundling the products are only sold jointly in fixed proportions. With mixed bundling the products are also available separately, but the sum of the stand-alone prices is higher than the bundled price.85
(74) Tying can take place on a technical or contractual basis. For instance, technical tying occurs when the tying product is designed in such a way that it only works with the tied product (and not with the alternatives offered by competitors).86
(75) The main concern in the context of conglomerate mergers is that of foreclosure. The combination of products in related markets may confer on the merged entity the ability and incentive to leverage a strong market position from one market to another by means of tying or bundling or other exclusionary practices. While tying and bundling have often no anticompetitive consequences, in certain circumstances such practices may lead to a reduction in actual or potential competitors' ability or incentive to compete. This may reduce the competitive pressure on the merged entity allowing it to increase prices or deteriorate supply conditions in other ways. 87
(76) In assessing the likelihood of such a scenario, the Commission examines, first, whether the merged firm would have the ability to foreclose its rivals,88 second, whether it would have the economic incentive to do so89 and, third, whether a foreclosure strategy would have a significant detrimental effect on competition, thus causing harm to consumers.90 In practice, these factors are often examined together as they are closely intertwined.
5.2. Assessment of the conglomerate non-coordinated effect of the Transaction
5.2.1. Introduction
(77) The elements in the Commission’s file show that the combination of the Parties’ activities could potentially result in technical tying (through the degradation of interoperability with third-party products) and commercial bundling91 between the following products:
(i) Siemens’ imaging solutions used for radiotherapy simulation, i.e. CT simulators, MRI simulators and PET/CT simulators (together referred as Simulators), on the one side; and
(ii) Varian’s radiotherapy solutions, i.e. radiotherapy equipment (namely Linacs, brachytherapy equipment and proton therapy equipment), OIS, TPS and Imaging Motion Management (together referred as “Radiotherapy Solutions”), on the other side.
(78) The remainder of this Decision will assess whether the two above-mentioned practices could result in the foreclosure of the Parties’ rivals in the relevant markets.
(79) As a preliminary remark, it should be noted that, unless otherwise specified, the findings set out in Section 5.2.3 (on technical tying) and Section 5.2.4 (on commercial bundling) of this Decision and, in particular, the results of the market investigation92 do not materially differ depending on the type of Simulators and the type of Radiotherapy Solutions. This is mainly due to the fact that all the various equipment and solutions used for radiotherapy simulation, planning and treatment are part of an integrated ecosystem and, therefore, highly intertwined. This is particularly true for Radiotherapy Solutions, for which customers may “have ax
(85) In this context, during the market investigation, several competitors of both Parties proactively approached the Commission expressing strong concerns about the Transaction in relation to a potential risk of technical tying between Siemens’ Simulators and Varian’s Radiotherapy Solutions. More specifically, they argue that, post-Transaction, the Parties will have the ability and incentive to foreclose their rivals by degrading the interoperability (i) between Siemens’ Simulators and third parties’ Radiotherapy Solutions; and (ii) between Varian’s Radiotherapy Solutions and third parties’ Simulators.
(A) The Notifying Party’s view
(86) The Notifying Party contests the above, arguing that, post-Transaction, the combined entity would have no ability and no incentive to implement a technical tying strategy. The Notifying Party’s main arguments are detailed below.106
(87) First, the Notifying Party claims that Siemens and Varian do not have a sufficient degree of market power to foreclose competitors since, in each of the relevant imaging and radiotherapy markets, the Parties face (i) vigorous competition from several strong players and (ii) sophisticated customers, with countervailing power, relying on open tender processes.
(88) Second, the Notifying Party argues that the existence of a de facto industry-wide and non-proprietary standard, named Digital Imaging and Communications in Medicine (“DICOM”), including its radiotherapy extension (“DICOM RT”), prevents the degradation of interoperability. More specifically, the Notifying Party states that (i) DICOM is sufficient to ensure full interoperability between imaging and radiotherapy solutions,107 with no need for specific data formats/interfaces108 or cooperation between vendors; and (ii) compliance with DICOM is a commercial obligation as it is requested by customers who favour open ecosystems allowing them to source products from different vendors to ensure the best possible treatment for patients.
(89) Third, Siemens submits that moving away from DICOM would not be profitable as it would entail significant losses and costs outweighing any potential gains, including notably (i) substantial sales losses (customers, which have a strong preference for DICOM-compliant solutions, would likely switch to alternative suppliers); in particular, the Notifying Party notes that removing interoperability at the prospect of gaining market share on the Imaging Motion Management market would put at risk the sales of CT simulators, [pricing information]; (ii) reputational damage for Siemens beyond the radiotherapy segment; and (iii) material investments to develop and roll out an alternative non-DICOM standard.
(90) Fourth, the Notifying Party claims that abandoning DICOM would have no adverse impact on competition and would not result in the foreclosure of competitors. On the contrary, according to it, such a strategy would make the Parties’ products commercially unsaleable, which would only alienate customers and benefit competitors.
(91) Moreover, the Notifying Party argues that (i) with respect to Simulators, the degradation of interoperability would not materially reduce the sales prospects of Siemens’ competitors as it would only affect their sales of Simulators, which account for a minor share of the overall sales of Scanners; and (ii) with respect to Motion Management devices, only a small portion of the EEA/UK demand for the latter is used with Siemens’ Simulators, which means that the degradation of interoperability with Siemens’ Simulators would only affect the sales of Varian’s Motion Management rivals to a limited extent.
(B) The Commission’s assessment
(92) In the remainder of this Section, the Commission assesses the risk of technical tying between Siemens’ Simulators and Varian’s Radiotherapy Solutions, through the degradation of interoperability with third-party products.
Ability to foreclose
(93) For the reasons set out below, the Commission finds that, post-Transaction, the Parties would have the ability to foreclose their rivals by degrading the interoperability between the above-mentioned products.
(94) First, contrary to the Notifying Party’s claim, the market structure and the evidence in the Commission’s file show that Siemens and Varian have a significant degree of market power:109
a. The Parties are the clear market leaders on their respective markets, with very substantial market shares, close to or above [50-60]% in most of the relevant imaging and radiotherapy markets/market segments at EEA/UK level (in 2019).110 In particular:
− Imaging markets: Siemens has a market share of [60-70]% in CT simulators, which are by far the most prevalent imaging equipment used for radiotherapy simulation and, thus, the most relevant imaging equipment for the assessment of the conglomerate effects of the Transaction. On the broader CT scanner market, Siemens’ market share is lower but remain very significant ([40-50]%). With respect to other types of Scanners, Siemens’ market shares are close to or above [50-60]%, i.e. [40-50]% in MRI scanners and [50-60]% in PET/CT scanners;111 In line with the above, customers and competitors generally perceive Siemens as the leading player in imaging equipment (overall) and CT simulators.112 The Commission also notes that internal documents of Siemens emphasise […];113
− Radiotherapy markets: As regards radiotherapy equipment, Varian has a market share of up to [60-70]% in Linacs114 (which are by far the most common form of radiotherapy), as well as a market share of [50-60]% in Proton therapy. In the brachytherapy market, despite a more moderate market share ([20-30]%), Varian is a very important supplier as it is one of the only two players active in this market (which is characterised by a duopoly). In line with the above, both customers and competitors perceive Varian as the “undisputed” leading player in radiotherapy equipment (overall), as well as in Linacs.115
As regards oncology software, Varian’s market share is close to [40-50]% ([40-50]% according to an independent industry report)116 in OIS (which is central to ensure the workflow between the various solutions used for radiotherapy simulation, planning and treatment and which directly interfaces with Simulators).117 In TPS, Varian’s market share estimates are more moderate (between [20-30]% and [30-40]% depending on the segment), however the Commission notes that (i) Varian is one of the only two players in TPS for brachytherapy and the second largest player in TPS for EBRT (with a market share very close to the market leader) and (ii) the feedback received from customers and competitors, according to which Varian is the clear market leader in TPS (as well as in OIS) suggests that the Parties underestimate Varian’s market share in TPS.118
As regards Motion Management devices, according to the Parties, Varian has a market share of [30-40]-[40-50]%. However, the Commission notes (i) according to the Form CO, “[Parties’ stated limitations to market share estimates]”,119 which calls into question the reliability of the Parties’ market share estimate on this market; and (ii) according to competitors and customers, Varian is one of the leading and most competitive players on this market.120
According to well-established case law, very large market shares above 50% may in themselves be evidence of the existence of a dominant position.121 In
these markets is difficult, a long and expensive process. The main difficulties in switching from one radiotherapy supplier to another are (i) the need to train staff in order to be able to use the new equipment, (ii) the high costs involved, (iii) potential loss of patient data and (iv) lack of interoperability/compatibility between the solutions of different suppliers.126 Consequently, only a minority of customers switched Linac, OIS and/or TPS suppliers in the past 10 years.127
(95) Second, the Parties have a large pool of common customers128 since all radiotherapy customers must procure both Simulators and Radiotherapy Solutions, which are closely interconnected. This is illustrated by the fact that, in 2012, the Parties entered into a strategic global partnership (called “EnVision”) including sales and marketing cooperation. In this respect, the Parties’ internal documents [strategy information relating to the EnVision partnership].129 Similarly, another internal document reads: [strategy information relating to the EnVision partnership].130 In line with the above, internal presentations of Siemens assessing the impact of the Transaction reveal that Siemens expects the Transaction to [strategy information].131 Moreover, the Commission notes that technical tying would allow the combined entity to tie imaging and radiotherapy products purchased separately and, thus, to “overcome the fact that only a small share of customers procures their Linacs and CT scanners at the same time” (as opposed to commercial tying and bundling – see Section 5.2.4 below).132
(96) Third, customers confirmed that interoperability between Simulators and Radiotherapy Solutions, in particular between Simulators and OIS/TPS, is very important, considering it “crucial”/“vital”/“essential” for the entire radiotherapy process to ensure “efficient and safe operations” with a “good workflow”.133 The Parties’ internal documents also stress that […].134
(97) Fourth, the results of the market investigation support the Notifying Party’s claims that the DICOM standard (i) facilitates the exchange of images/data between Simulators and Radiotherapy Solutions; and (ii) is widely adopted (despite the absence of regulatory obligation to adhere to it), which is explained by the fact that customers request it.135 However, the Commission also found that:
a. DICOM does not in itself guarantee full interoperability. A large majority of competitors and a material number of customers confirmed it, explaining that DICOM suffers limitations.136 In particular, several respondent stressed that DICOM covers only the least common denominator and is slow to adapt to new features and functionalities, which are often subject to specific formats (such as “private image tags”) and proprietary extensions to DICOM. The above is corroborated by (i) the DICOM Standards Committee, which states that the DICOM standard “facilitates interoperability of systems claiming conformance in a multi-vendor environment, but does not, by itself, guarantee interoperability” (emphasis added),137 and (ii) the Parties’ internal documents illustrating Siemens […].138
b. DICOM’s implementation requires active and voluntary collaboration between OEMs, which was confirmed by the vast majority of competitors139 and corroborated by the Parties’ internal documents.140 According to competitors, the collaboration required to ensure interoperability between Simulators and Radiotherapy Solutions is material and consists mainly in exchange of technical information (e.g. specific file format details) and joint testing.141 This bilateral collaboration takes place primarily during the development process but also, to a more limited extent, on the ground at the customers’ sites.142
c. Interoperability between DICOM-compliant solutions can be compromised. In particular, most competitors confirmed that interoperability could be hindered
(i) when the relevant solutions do not properly implement the DICOM standard or implement different versions of it (e.g. by failing to quickly incorporate new elements of the standard, such as new DICOM objects/tags, that are created as part of new technological advancements) or (ii) when a vendor does not disclose to other vendors (or delays the disclosure of) relevant information (e.g. private image tags used by the solution).143 For example, a competitor stated that “minor differences in the interpretation and implementation of [DICOM] are common and can make data exchanges challenging”.144 Another respondent explained that “vendors must be aware of any changes to DICOM tags with enough advanced notice to make any required changes to their own product” and that it could “be quite difficult to get a sample data sets of DICOM RT files including all tags from the vendors” since “these are not made publicly available”.145
(98) The above DICOM limitations and the need for active cooperation between vendors are also supported by the fact that [confidential information about the EnVision partnership].146 In fact, internal documents reveal that Varian’s main goal [confidential information about the EnVision partnership].147
(99) Fifth, Imaging Motion Management devices require a direct interface with Simulators, whose development, implementation and validation involve cooperation between the manufacturers. This is notably illustrated by the fact that, under the EnVision partnership, [confidential information about the EnVision partnership].148[…].
(100) Sixth, the market investigation also revealed that a significant number of market participants consider that, post-Transaction, the Parties would be able to degrade the interoperability between Simulators and Radiotherapy Solutions.149
(101) Finally, the Commission considers that the Parties’ rivals would be unable to deploy effective counter-strategies,150 given (i) Siemens’ and Varian’s leadership and potential dominance in the relevant markets, (ii) the absence of other integrated players providing both Simulators and Radiotherapy Solutions and (iii) the fact that respondents to the market investigation were unable to identify any effective workaround mechanisms or solutions whereby they could circumvent an attempt by the Parties to degrade interoperability.151
Incentive to foreclose
(102) Based on the evidence available in the file and the results of the market investigation, the Commission considers that, post-Transaction, the Parties would have incentives to foreclose their rivals by degrading the interoperability between the above-mentioned products for the following reasons.
(103) First, the argumentation raised by the Notifying Party to show that technical tying would not be profitable rely on the key assumption that degrading interoperability between Simulators and Radiotherapy Solutions would require the combined entity to “move away from DICOM”, which would entail significant losses of sales and costs.152 However, as explained in the previous Section, this underlying assumption is contradicted by the results of the market investigation, which revealed that the combined entity would have the ability to hinder the interoperability between their products and third-party products while remaining DICOM-compliant. For example, the Parties could, at no or limited cost, reserve certain new functionalities of Siemens’ Simulators for cases where Siemens’ Simulators are used in combination with Varian’s Radiotherapy Solutions simply by e.g. refusing to disclose technical information (such as private image tags used by Siemens’ Simulators) to Varian’s competitors. More broadly, the Parties could refuse to cooperate in providing technical information and support necessary to enable suppliers to understand and account for its particular implementation of the standard (which respondents suggest is to a degree open to interpretation).153
(104) Second, as explained above, the relevant radiotherapy markets are characterised by high barriers to switch suppliers.154 The customers’ limited ability to switch suppliers of Radiotherapy Solutions (such as OIS and Linacs) would de facto limit the risk of sales losses for Parties. Indeed, the degradation of the interoperability between the Parties’ and their competitors’ Simulators and Radiotherapy Solutions would not put at risk the Parties’ sales of Radiotherapy Solutions. This is all the more true considering that Radiotherapy Solutions, and in particular Linacs, drive the customers’ procurement decisions notably because they are much more expensive and may raise additional non-merger specific interoperability issues.155
(105) Similarly, the Notifying Party’s claim according to which degrading interoperability between Siemens’ Simulators and third-party Imaging Motion Management devices at the prospect of gaining market share on the Imaging Motion Management market would put at risk sales of a significantly more profitable product (CT simulator) is not consistent with the fact that […].156
(106) Lastly, the market investigation revealed that a significant number of competitors believe that, post-Transaction, the Parties would find it profitable to degrade the interoperability between Simulators and Radiotherapy Solutions (including Imaging Motion Management devices).157 According to them, such technical tying would allow the combined entity to leverage Varian’s leadership in the radiotherapy space to further gain market shares in Siemens’ imaging markets (and vice versa). In support of their claim, many of them indicated that, already pre-Transaction, Varian adopts similar practices to limit or delay the interoperability between its radiotherapy solutions and other competing products (e.g. between Varian’s Linac and Elekta’s OIS).158 This is also corroborated by some customers, who explained that “Varian has a history where OIS/TPS is not "speaking" as good as possible with third-party devices” and that “already today the Varian environment is considered to be relatively closed and not very well suited for a true multi-vendor approach”.159 In accordance with the Non-Horizontal Merger Guidelines,160 the Commission considers that the above suggests that a strategy consisting in degrading interoperability in the field of radiotherapy may be profitable, which would incentivise the new entity is implement such strategy post-Transaction.
Impact on competition
(107) Based on the evidence available in the file and the results of the market investigation, the Commission considers that the degradation of interoperability between the above-mentioned products would have a significant detrimental effect on competition, thus causing harm to patients.
(108) First, the market investigation stressed the importance of interoperability for the entire radiotherapy process and revealed that if a vendor of imaging or radiotherapy solutions ceases to collaborate with other vendors, interoperability limitations would arise with “severe consequences” for customers and patients. In particular, (i) customers could lose workflow efficiencies and functionalities (including “critical” ones); (ii) new features could become “useless”. Competitors also emphasized that workaround solutions would be difficult (“technically close to impossible”) and would lead to “additional costs”, “complexity” and “safety issues”.161
(109) Second, given (i) Siemens’ and Varian’s very high market shares – close to or above [50-60]% in most of the relevant imaging and radiotherapy markets/segments (up to [60-70]% in CT simulators and Linacs) at EEA/UK level (in 2019)162 – and (ii) the absence of other integrated players, the degradation of the interoperability between the Parties’ products and their competitors’ would likely significantly reduce their rivals’ sales prospects in the relevant markets/segments and, thus, lead to a reduction in their ability or incentive to compete and innovate with the new entity on these markets/segments.
(110) As regards the Notifying Party’s claim that the degradation of interoperability would only affect the sales of Simulators, which account for a minor share of the overall sales of Scanners, the elements in the Commission’s file show that (i) a technical tying strategy would have the ability to significantly affect the rivals’ sales of CT simulators and that (ii) CT simulators and scanners are differentiated products, which may constitute distinct market segments. A significant reduction of the rivals’ sales prospects for CT simulators may reduce their incentive to compete and innovate in this potential market segment, which would be detrimental for both customers and patients.
(111) As regards the Notifying Party’s claim that only a small portion of the EEA/UK demand for Motion Management devices would be affected by foreclosure resulting from the merger, the Commission notes that:
− Knowing that [Parties’ stated limitations to market share estimates], the Parties’ computation of the total addressable demand for Imaging Motion Management devices that could be foreclosed appears highly uncertain and unreliable;163
− Most of the CT simulators sold by Siemens with an interface for Imaging Motion Management (i.e. […]% worldwide and […]% in the EEA/UK) are used in combination with Imaging Motion Management devices marketed by Varian’s rivals164 which, given Siemens’ market share in the EEA/UK CT simulator segment ([60-70]%), suggest that a sufficiently large fraction of the market output would be affected by foreclosure resulting from the Transaction; and
− Assuming that the Parties’ computation of the addressable demand for Imaging Motion Management devices is reliable (which as previously explained is doubtful), the new entity would still be in a position to foreclose more than [40- 50]% of the addressable demand in the EEA/UK,165 which is substantial enough to raise concerns.
(112) Third, many competitors actively complained and confirmed that the degradation of the interoperability between the Parties’ and their competitors’ Simulators and Radiotherapy Solutions would significantly affect competition on the relevant markets/segment.166
(113) In contrast, only a (material) minority of customers raised concerns about a risk of degradation of interoperability167 and that most of them consider that DICOM is sufficient to ensure full interoperability.168 However, it should be noted that customers may not be not fully aware of the collaboration required between imaging and radiotherapy vendors to ensure full interoperability. Indeed, as already indicated, the market investigation revealed that this cooperation takes place primarily during the development process, i.e. upstream of the onsite installation of the equipment, without involving customers.169 Therefore, customers experience the result collaboration to ensure interoperability, rather than the collaboration effort itself.
(114) Fourth, the Commission observes that, pre-Transaction, the countervailing buyer power of the customers does not prevent Varian from limiting or delaying the interoperability between its Radiotherapy Solutions and other competing products,170 which suggests that customers do not have sufficient countervailing buyer power to defeat similar practices implemented by the new entity post-Transaction.
(115) Lastly, the Commission notes that the degradation of interoperability between the Parties’ and their competitors’ Simulators and Radiotherapy Solutions would not bring any efficiencies.171
(C) Conclusion
(116) In view of the above considerations, the Commission concludes that the Transaction raises serious doubts as to its compatibility with the internal market by providing the new proposed entity with the ability and incentive to foreclose its rivals by degrading the interoperability between (i) Siemens’ Simulators and third parties’ Radiotherapy Solutions; and (ii) Varian’s Radiotherapy Solutions and third parties’ Simulators.
5.2.4. Commercial bundling172
(117) The Transaction leads to conglomerate links between Siemens’ Simulators and Varian’s Radiotherapy Solutions (including Imaging Motion Management Devices), which could result in the implementation of commercial bundling between the above-mentioned products.
(A) The Notifying Party’s view
(118) The Notifying Party argues that, post-Transaction, the combined entity would have no ability and no incentive to foreclose competitors through the implementation of commercial bundling strategy. The Notifying Party’s main arguments are detailed below.
(119) First, the Notifying Party submits that the combined entity will not have the ability to (a) leverage its position in Simulators to foreclose Varian’s Radiotherapy Solution rivals (including Imaging Motion Management rivals), or (b) leverage its position in Radiotherapy Solutions (including Imaging Motion Management Devices) to foreclose Siemens’ Simulators rivals. The Notifying Party claims that, despite their relatively high market shares (see Table 1 above), Siemens and Varian face sizeable and credible alternatives in the relevant markets for Simulators (e.g. GE, Philips and Canon) and Radiotherapy Solutions (e.g. Elekta, IBA, Accuray, VisionRT, C-Rad, Brainlab). In addition, the Notifying Party submits that Simulators and Radiotherapy Solutions are rarely procured together in a single tender so that the combined entity will have limited opportunities to bundle these products, notably due to the different lifespans of the equipment concerned, the public tender procedure rules applicable across the EEA/UK, and customers’ preferences for certain equipment.173
(120) Second, the Notifying Party argues that the combined entity will lack the incentive to engage in a bundling strategy. It points out that, to persuade customers to purchase a bundled offer, the combined entity would have to offer steep bundle discounts (e.g. to compensate for the premature renewal of equipment or to overcome customers’ preference) with no real prospects of recuperating the lost margins. The Notifying Party claims that the lack of incentive of the combined entity to engage in any bundling strategy in the future is highlighted by the fact that, pre-Transaction, (i) Varian does not [commercial strategy], (ii) Siemens does not [commercial strategy], and (iii) even when Siemens was active in the supply of Radiotherapy Solutions (i.e. Linacs)174 it [commercial strategy].175
(121) Third, the Notifying Party is of the view that, in any event, any bundling strategy would not have any detrimental effect on the market since, without changing customers’ procurement behaviour, a bundling strategy would affect only a minor part of the demand for Simulators and Radiotherapy Solutions176 and this is unlikely to change significantly post-Transaction given that Simulators and Radiotherapy Solutions have different average lifespans. Moreover, competitors can implement timely and effective counter-strategies to defeat any attempted foreclosure by granting discounts on their products or by partnering up and offering competing bundles.177
(122) Finally, the Notifying Party submits that the Transaction will result in efficiencies for customers resulting in lower prices and availability of innovative new products in a faster and more effective way.178
(B) The Commission’s assessment
(123) In the remainder of this Section, the Commission assesses whether the commercial bundling between Siemens’ Simulators and Varian’s Radiotherapy Solutions (including Imaging Motion Management Devices) could result in the foreclosure of the Parties’ rivals.
Ability to foreclose
(124) As explained below, the Commission finds that, despite their significant degree of market power in the relevant markets/segments,179 the Parties would not have the ability to foreclose their rivals by bundling commercially Siemens’ Simulators and Varian’s Radiotherapy Solutions (including Imaging Motion Management Devices).
(125) First, the market investigation generally supported the Notifying Party’s claim that, for the reasons set out below, radiotherapy customers typically procure Simulators and Radiotherapy Solutions separately, which considerably limits the Parties’ opportunities to bundle the above products:
a. The pool of common customers procuring Simulators and Radiotherapy Solutions at the same time is limited.180 As rightly pointed out by the Notifying Party, commercial bundling can only apply to products purchased at the same time (contrary to technical tying).181 It follows that any potential ability of the combined entity to offer a bundle would be limited to the share of customers that purchase the relevant Simulators at the same time as the Radiotherapy Solution. Yet, the results of the market investigation confirmed that radiotherapy equipment is rarely procured at the same time as imaging equipment, and vice versa due to the products’ different life cycles. In that respect, one customer noted that “typically CT and linac replacements are planned years ahead and have their own lifespans, so if it would go to the same year, procurement bundle would be an option” while another one explained that since hospitals “require business continuity and redundancy, [they] tend to treat radiotherapy imaging equipment separate from linac”.182
b. The market investigation confirmed that most Simulators and Radiotherapy Solutions are procured as part of public procurement processes.183 Thus, the vast majority of Simulators and Radiotherapy Solutions sold in the EEA/UK is subject to the EU rules on public procurement ([…]% in the case of Siemens’ imaging solutions),184 whereby it is the customer that determines its requirements and the scope of the tenders. Although respondents to the market investigation recognised that public procurement rules do not prevent customers from purchasing Simulators and Radiotherapy Solutions in a bundle (with the exception of a few EEA/UK countries, such as France),185 in practice, the guiding principle under public procurement rules is to tender by individual product lots to ensure an even competitive field for all suppliers.186 In that respect, one customer indicated that “for competitive reasons and respect of the equality between providers it is impossible to bundle because actually only SIEMENS and Varian could do so [post-Transaction]”187 and another one explained that “supplier[s] of imaging equipment and radiotherapy equipment are not [the] same, it’s more easy to dissociated both tenders”.188 In other words, customers would “not risk not to get the best (price and quality) imaging equipment and best radiotherapy equipment”.189
c. The Parties’ sales data corroborate the claim that opportunities to commercially bundle Simulators and Radiotherapy Solutions are limited. In particular, Siemens’ sales data show that less than […]% of Siemens’ CT and MRI scanners are procured with Radiotherapy Solutions in a single tender.190 Moreover, in 2019 in the EEA/UK, only […] Siemens’ CT simulators were sold to radiation oncology department that also purchased a Varian’s Linac that year (representing only around […]% of Siemens’ total sales of CT simulators in the EEA/UK).191 Taking into consideration the Parties’ high EEA/UK market shares in CT simulators ([60-70]%) and Linac ([60-70]%),192 which are by far the most prominent equipment used for radiotherapy simulation and treatment delivery, the above objective […]% figure support the Notifying Party’s claim that Simulators and Radiotherapy Solutions are typically not procured together.193
Regarding specifically Imaging Motion Management Devices, the results of the market investigation suggest that these products tend to be purchased together with Simulators more often than other Radiotherapy Solutions.194 However, the sale data provided by the Parties reveal that only […]% of Varian’s Imaging Motion Management Devices are sold to imaging solutions suppliers (namely […]) – who may sell these products together with Simulators195 – the remaining […]% being sold by Varian directly to customers. The above means that, already today, while customers have the opportunity to procure Varian’s Imaging Motion Management Devices from imaging solutions suppliers ([…]), the vast majority of customers choose to procure Varian’s Imaging Motion Management Devices on a standalone basis (at least around […]% of Varian’s EEA/UK sales of Imaging Motion Management Devices).196 According to some market participants, this is due to the fact that “customers have their own preferences regarding e.g. motion management devices”.197
(126) Second, the evidence in the Commission’s file show that the combined entity’s rivals would have the ability and incentive to deploy counterstrategies to react to a bundling offer of the combined entity,198 by partnering to offer competing bundles or lowering their price for standalone products. In that respect, there are already established relationships between suppliers of radiotherapy equipment and imaging equipment199 and new ones could be implemented post-Transaction, for instance between Elekta and Siemens’ competitors.Such a possibility of increased collaboration with imaging players was already made public by Elekta.200 Alternatively, the level of margins generated by manufacturers of Simulators and Radiotherapy Solutions in the EEA/UK should enable them to grant substantial discounts on their equipment, while remaining profitable.201 This is notably illustrated by the Parties’ margin data in the relevant markets in the EEA/UK in 2019: for instance, Siemens achieves a gross margin of around […]% in imaging equipment (between […]% and […]% depending on the type of Scanners), whereas Varian’s gross margin reaches […]% in Linacs, […]% in TPS, […]% in OIS and up to […]% in Brachytherapy.202 In this respect, one customer explained that “if a 3rd party can offer a comparable product at a lower cost, or additional features which are not yet available from Siemens or Varian, then we would look very closely at them and potentially choose them if it is felt that this is the direction we need to go”.203
Incentive to foreclose
(127) Based on the evidence available in the file and the results of the market investigation, the Commission cannot exclude that the Parties would have incentives to commercially bundle their Simulators and Radiotherapy Solutions (to the extent possible given the limited opportunities).204 However, even if the Parties had incentives to engage in a commercial bundling strategy, for the reasons set out in the previous section, the Commission considers that such strategy would unlikely result in the foreclosure of competitors.
Impact on competition
(128) Based on the evidence available in the file and the results of the market investigation, the Commission considers that the adoption of a bundling strategy by the new entity is unlikely to have a significant detrimental effect on competition in the relevant imaging and radiotherapy markets for the following reasons.
(129) First, although some competitors complained about the risk of commercial bundling resulting from the Transaction,205 the Commission recalls that the reduction in sales by competitors is not it itself a problem and raises concerns only if this reduction is significant enough.206 In this respect, as explained above,207 the evidence in the Commission’s file suggest that the Parties’ opportunities to bundle their Simulators and Radiotherapy Solutions will be rather limited since these are typically procured separately. It follows that, despite the high market shares of Siemens and Varian in the relevant markets/segments, the implementation of a bundling strategy by the combined entity is unlikely to reduce significantly the sales prospects of the Parties’ rivals in the relevant imaging and radiotherapy markets/segments and a fortiori to reduce their ability to compete on these markets/segments. The above elements suggest that the Parties would not have the ability to successfully leverage any potential strength in any of the relevant imaging solutions and/or radiotherapy solutions markets. Any hypothetical bundling strategy of the combined entity is therefore unlikely to result in foreclosure of Siemens’ or Varian’s rivals.
(130) Second, the Commission notes that customers responding to the market investigation did not raise concerns as to the risk of a bundling strategy or its potential impact on their business or on the market.208 On the contrary, many of them stress that such commercial bundling could bring efficiencies (lower prices)209 and more generally expect the Transaction to result in innovation benefits for customers. For example, one customer pointed out that the Transaction could lead to “increased product development”210 while another customer expect the production of “new features in the Siemens/Varian portfolio that will benefit patients”.211 Overall, the Transaction could be expected “to have its main impact on pushing the technological innovation”,212 which will ultimately benefit “oncology patients within the EU”.213
(C) Conclusion
(131) In light of the above and the evidence available to the Commission, and in view of the outcome of the market investigation, the Commission considers that the Transaction is unlikely to raise serious doubts as to its compatibility with the internal market on the relevant imaging and radiotherapy markets as a result of commercial bundling practices.214
6. PROPOSED REMEDIES
(132) In order to render the Transaction compatible with the internal market, the Notifying Party submitted a set of commitments under Article 6(2) of the Merger Regulation on 29 January 2021 (the “First Commitments”). The Commission market tested the First Commitments on 1 February 2021 in order to assess whether they were sufficient and suitable to remedy the serious doubts identified at Section in Section 5.2.3 above. Following the feedback received from market test, amended commitments were submitted on 12 February 2021 (the “Final Commitments”). The Final Commitments are annexed to this Decision and form an integral part thereof.
6.1. Framework for the assessment of the commitments
(133) Where a concentration raises serious doubts as regards its compatibility with the internal market, the merging parties may undertake to modify the concentration to remove the grounds for the serious doubts identified by the Commission. Pursuant to Article 6(2) of the Merger Regulation, where the Commission finds that, following modification by the undertakings concerned, a notified concentration no longer raises serious doubts, it shall declare the concentration compatible with the internal market pursuant to Article 6(1)(b) of the Merger Regulation.
(134) As set out in the Commission Notice on remedies acceptable under Council Regulation (EC) No 139/2004 and under Commission Regulation (EC) No 802/2004 (the “Remedies Notice”)215, the commitments have to eliminate the competition concerns entirely, and have to be comprehensive and effective from all points of view.216
(135) In assessing whether commitments will maintain effective competition, the Commission considers all relevant factors, including the type, scale and scope of the proposed commitments, with reference to the structure and particular characteristics of the market in which the transaction is likely to significantly impede effective competition, including the position of the parties and other participants on the market.217
(136) In order for the commitments to comply with those principles, they must be capable of being implemented effectively within a short period of time. Concerning the form of acceptable commitments, the Merger Regulation gives discretion to the Commission as long as the commitments meet the requisite standard.
(137) When assessing the remedies proposed by the parties, the Commission has the duty to ensure that the remedies would be effective in practice. In order for the commitments to remove the competition concerns entirely and be comprehensive and effective, there has to be an effective implementation and ability to monitor the commitments.218 Whereas divestitures once implemented do not normally require any further monitoring measures, other types of commitments require effective monitoring mechanisms in order to ensure that their effect is not reduced or even eliminated by the parties. Otherwise such commitments would have to be considered as mere declarations of intentions by the parties and would not amount to binding conditions and obligations, as, due to the lack of effective monitoring mechanisms, it is unlikely that the Commission would be able to detect any breach and, if necessary, to revoke the decision according to Article 8(6)(b) of the Merger Regulation or to impose fines as per Article 14(2)(d) of the Merger Regulation.
(138) Where the parties submit remedies proposals that are so extensive and complex that it is not possible for the Commission to determine with the requisite degree of certainty, at the time of its decision, that they will be fully implemented and that they are likely to maintain effective competition in the market, an authorisation decision cannot be granted. The Commission may reject such remedies in particular on the grounds that the implementation of the remedies cannot be effectively monitored and that the lack of effective monitoring diminishes, or even eliminates, the effect of the commitments proposed.219
(139) It is against those principles that the Commission assessed the viability, the effectiveness and the ability of the proposed commitments to entirely eliminate the serious doubts identified in Section 5.2.3 of this Decision.
6.2. The First Commitments
6.2.1. Description of the First Commitments
(140) The First Commitments consist of a behavioural undertaking to ensure the interoperability between the combined entity’s and its rivals’ imaging/radiotherapy solutions, in particular between the following products:
· Simulators and OIS/TPS (the “Simulator and OIS/TPS Commitment”): The Notifying Party commits to ensure interoperability (i) between the Parties’ Simulators220 and third-party OIS/TPS and (ii) between the Parties’ OIS/TPS and third-party Simulators, and in particular:
− to provide rival suppliers with the information necessary to ensure the interoperability and the testing of interoperability (upon written request, without charge and on a non-discriminatory basis);
− to commits to continue to support the DICOM standard (and its radiotherapy extension) in its Simulators, OIS and TPS.
· Simulators and Imaging Motion Management devices (the “Simulator and Imaging Motion Management Commitment”): The Notifying Party commits to ensure interoperability between Siemens’ Simulators and third-party Imaging Motion Management devices, and in particular:
− to maintain, upgrade and update the existing interfaces between the above products (on a non-discriminatory basis, free of charge for maintenance and at cost including a reasonable margin for updates and upgrades);
− to provide rival suppliers with (i) technical documentation and information required to achieve interoperability; (i) reasonable feedback and technical guidance to ensure interoperability; and (iii) access to Siemens’ Simulators to enable Imaging Motion Management suppliers to perform the necessary tests of interoperability. These services shall be provided on a non- discriminatory basis, free of charge or at cost including a reasonable margin (depending on the service).
(141) More generally, the combined entity will refrain from implementing any features or functions (to its Simulators and/or OIS/TPS) that is designed to negatively affect the performance and functionality of third-party Simulators, OIS/TPS and/or Imaging Motion Management devices by degrading interoperability. Customers will also be provided with support to ensure the interoperability of the above-mentioned products on a non-discriminatory basis and on terms that are no less favourable than those provided to customers seeking to ensure interoperability between the combined entity’s products.
(142) The First Commitments are proposed to last 5 years in duration and apply to products that have obtained or are in the process of obtaining a CE mark in the EEA or the UK equivalent. The First Commitments would be supervised by a Monitoring Trustee and supported by a fast-track dispute resolution mechanism to promptly resolve disagreements that arise between the combined entity and suppliers of the products covered by the Simulator and OIS/TPS Commitment and Simulator and Imaging Motion Management Commitment.
6.2.2. The Notifying Party’s views
(143) The Notifying Party argues that the First Commitments would eliminate the Commission’s serious doubts in relation to the Transaction. In particular, the Notifying Party is of the view that the Simulator and OIS/TPS Commitment ensures and facilitates the continued interoperability between Siemens’ Simulators and the OIS/TPS of third party suppliers, and vice versa. In this respect, Siemens also submits that interoperability between Simulators and OIS/TPS is sufficient to ensure interoperability between Simulators and radiotherapy equipment (such as Linacs), which thus do not need to be included in the scope of the First Commitments.221 Similarly, the Notifying Party considers that the Simulator and Imaging Motion Management Commitment ensures and facilitates the continued interoperability between Siemens’ Simulators and third-party Imaging Motion Management devices.
6.2.3. Assessment of the First Commitments
(144) The Commission initiated a market test of the First Commitments on 1 February 2021 (the “Market Test”) to investigate the adequacy of the First Commitments in nature and in scope to resolve the competition concerns identified in Section 5.2.3 above, as well as their viability and effectiveness. The Commission’s assessment of each of these elements in light of the results of the Market Test is as follows:
(A) Scope and adequacy to address concerns
(145) According to the Remedies Notice, “the commitments have to eliminate the competition concerns entirely and have to be comprehensive and effective from all points of view”.222
(146) The results of the Market Test were largely positive regarding the suitability of the First Commitments to resolve the concerns arising from the Transaction:
· Simulator and OIS/TPS Commitment: The majority of suppliers that expressed a view considered that the First Commitments would maintain interoperability between Siemens’ and rivals’ Simulators and OIS/TPS.223 Moreover, the majority of respondents confirmed that ensuring the interoperability of Simulators with OIS TPS was sufficient to ensure that Simulators would be interoperable with radiotherapy equipment (such as Linacs).224
· Simulator and Imaging Motion Management Commitment: Similarly, the majority of suppliers that expressed a view confirmed that the First Commitments are suitable and sufficient to resolve the potential interoperability concerns between Siemens’ Simulators and rivals’ Imaging Motion Management devices.225
(147) The Market Test confirmed that the mechanisms envisaged in the First Commitments to ensure interoperability (i.e. the disclosure of information, provision of technical assistance, provision of non-discriminatory assistance to customers, maintenance/update/upgrade of existing interfaces and adherence to the DICOM standard) are sufficiently clear and exhaustive to ensure an efficient, safe and reliable interoperability, and that the concept of interoperability is clearly defined.226
(148) However, the results of the Market Test strongly indicated that a duration of 5 years for the First Commitments would not be sufficient to protect competition in the relevant imaging and radiotherapy markets.227 The large majority of respondents that expressed a view confirmed that this period would be insufficient, noting that: (i) market conditions are unlikely to have changed sufficiently in 5 years as to remove the need for commitments to ensure interoperability with the Parties’ products, (ii) the average lifetime of the relevant imaging and radiotherapy products can be 10 years or more, and (iii) the typical lead time for the development of new products is such that by the time the combined entity brings new imaging or radiotherapy solutions to market the First Commitments may be close to expiring (or may have expired). The vast majority of respondents indicated that in order to effectively address the interoperability concerns, the First Commitments should last at least 15 years, and some players even indicated that they should have an unlimited duration.228
(149) In light of the above, the Commission considers that the First Commitments would be suitable in nature to remove the serious doubts arising from the Transaction, but the limited duration proposed means that the First Commitments are not sufficiently comprehensive to remove these doubts.
(B) Viability and effectiveness of the First Commitments
(150) As noted above, the Market Test generally confirmed the suitability of the First Commitments to resolve the competition concerns. However, the Market Test also pointed to certain shortcomings of the First Commitments that could impact the viability and effectiveness of the proposed remedy.
(151) First, respondents to the Market Test identified that it would be important not only for information necessary to ensure interoperability to be provided to suppliers who request it, but also that the same suppliers be automatically provided with updates to that information without undue delay.229 This would preserve the ongoing ability for rival suppliers to ensure interoperability during the lifetime of the commitments.
(152) Second, the Market Test indicated that the requirement contained in the Simulator and Imaging Motion Management Commitment to provide reasonable feedback and technical guidance to facilitate testing activities should also be included as part of the Simulator and OIS/TPS Commitment, to grant the same level of protection.230
(153) Third, the results of the Market Test pointed to a concern that the effectiveness of the First Commitments might be circumvented through communications made by the combined entity regarding the (possible lack of) interoperability between its Simulators, OIS/TPS and/or Imaging Motion Management devices and those of rivals.231 Similarly, respondents considered that, having completed the necessary testing and validation, “the third party vendor must be allowed to communicate openly, in marketing and with customers, that their products are interoperable with the Varian/Siemens products in question. Varian/Siemens must not impose any restrictions on the third party to make such statements”. 232
(154) Fourth, respondents to the Market Test identified that the combined entity may seek to develop a product that combines imaging processing software with OIS and/or TPS, and that in this situation the interoperability obligations in the First Commitments would not necessarily be effective in ensuring that Simulators are interoperable with Radiotherapy Solutions (as independent OIS/TPS software as such may not be required).233
(155) Fifth, the Market Test indicated that it would be appropriate for the First Commitments to apply not only to (third-party) products which have obtained or have commenced the formal regulatory process to obtain a CE mark in the EEA (or the UK equivalent), but to products for which there is a clear and demonstrated intention to obtain such a mark.234 This is as otherwise the information or assistance necessary to ensure interoperability would be received too late in the product development process to be effectively actionable.
(156) Sixth, on the whole the Market Test confirmed that the disclosure mechanisms and the monitoring and enforcement mechanisms in the First Commitments are adequate. However, respondents noted that to ensure the effectiveness and timely implementation of the remedy the time granted to the combined entity to respond to requests for interoperability information should be shortened.235
(157) Other than the above, the Market Test did not give rise to any reasons to doubt the viability and effectiveness of the First Commitments.
6.3. The Final Commitments
6.3.1. Description of the Final Commitments
(158) In response to the Commission's feedback regarding the outcome of the Market Test and its preliminary assessment, the Notifying Party submitted the Final Commitments on 12 February 2021. The Final Commitments represent an amended version of the First Commitments, with the following main changes seeking to address the shortcomings identified above.236
· The duration of the Final Commitments has been increased to an initial term of 10 years, which, can be extended for a further 5 year period by the Commission (if it is considered necessary to address the serious doubts identified in this Decision), having afforded the combined entity the opportunity to make submissions in this regard.
· Suppliers who request interoperability information from the combined entity will also automatically receive updates to that information without undue delay.
· The requirement contained in the Simulator and Imaging Motion Management Commitment to provide reasonable feedback and technical guidance is also be included as part of the Simulator and OIS/TPS Commitment.
· The combined entity shall be prevented from imposing any restrictions on third- party suppliers’ ability to communicate in accordance with applicable laws on the interoperability between their Simulators, OIS/TPS and/or Imaging Motion Management devices products and those of the combined entity, and the combined entity shall refrain from proactively communicating on the (potential) lack of such interoperability unless the (potential) lack of interoperability can be demonstrated by means of a reasoned submission to the Monitoring Trustee.
· The Simulator and OIS/TPS Commitment shall also apply between Simulators and radiotherapy equipment (namely Linacs, Brachytherapy and proton therapy equipment) to the extent they directly interact to ensure the ongoing interoperability of the imaging solution functionality.
· The scope of the Final Commitments shall cover products that have obtained a CE mark in the EEA (or the UK equivalent) or taken steps to do so (including steps undertaken during the design and development phases in preparation for the regulatory approval, even though such steps may take place before the regulatory process is initiated).
· The period of time in which the combined entity must respond to a request for interoperability information has been reduced.
(159) All of these changes have been incorporated into and form an integral part of the Final Commitments annexed to this Decision.
6.3.2. Assessment and conclusion on the Final Commitments
(160) The amendments introduced by the Notifying Party into the Final Commitments resolve all of the concerns that the Commission had regarding the First Commitments. Accordingly, based on the information available to it, the Commission is satisfied that the Final Commitments are feasible, viable, effective and adequate to remove the identified competition concerns.
(161) The Final Commitments ensure the ongoing, long-term interoperability of rivals’ Simulators with Radiotherapy Solutions of the combined entity. Likewise, the interoperability of third parties’ Radiotherapy Solutions with the combined entity’s Simulators is assured on a long-term basis. The Final Commitments are effective and have sufficient safeguards to ensure that the combined entity would be prevented from engaging in the foreclosure strategies that gave rise to the Commission’s serious doubts. As a result, different suppliers’ products will continue to work together efficiently, reliably and safely. Hospitals in the EEA/UK will continue to enjoy the ability to mix and match imaging and radiotherapy devices and components from those suppliers they prefer on the merits of the supplier’s product.
(162) Moreover, the Final Commitments have effective and timely compliance and monitoring mechanisms. The Commission will be able to monitor compliance through the appointment of an independent trustee. In addition, third parties would have recourse to a fast track dispute resolution procedure, which would enable third parties to receive speedy and effective adjudication through independent arbitrators. Importantly, the Commission would retain control of the procedure as the arbitrators would have to seek and be bound by the Commission's interpretation of the Commitments where necessary.
(163) Accordingly, the Commission concludes that the Final Commitments entered into by the undertakings concerned and as submitted to the Commission on 12 February 2021 are sufficient to eliminate the serious doubts identified by the Commission in relation to interoperability issues identified in Section 5.2.3.
6.4. Conclusion on remedies
(164) Under the first sentence of the second subparagraph of Article 6(2) of the Merger Regulation, the Commission may attach to its decision conditions and obligations intended to ensure that the undertakings concerned comply with the commitments they have entered into vis-à-vis the Commission with a view to rendering a notified concentration compatible with the internal market.
(165) The achievement of the measure that gives rise to the structural change of the market is a condition, whereas the implementing steps which are necessary to achieve this result are generally obligations on the Parties. Where a condition is not fulfilled, the Commission’s decision declaring the concentration compatible with the internal market no longer stands.237 Where the undertakings concerned commit a breach of an obligation, the Commission may revoke the clearance decision in accordance with Article 8(6) of the Merger Regulation. The undertakings concerned may also be subject to fines and periodic penalty payments under Articles 14(2) and 15(1) of the Merger Regulation.
(166) In accordance with the distinction described in the previous paragraph, the Decision in this case is conditioned on the full compliance with the requirements set out in Sections B and C of the Final Commitments, which constitute conditions. The remaining requirements set out in the other Sections of the Final Commitments constitute obligations, as they concern the implementing steps that are necessary to achieve the modifications sought in a manner compatible with the internal market.
(167) The full text of the Final Commitments is annexed to this Decision and forms an integral part thereof.
7. CONCLUSION
(168) For the above reasons, the Commission has decided not to oppose the notified operation as modified by the commitments and to declare it compatible with the internal market and with the functioning of the EEA Agreement, subject to full compliance with the conditions in Sections B and C of the Final Commitments annexed to the present Decision and with the obligations contained in the other sections of the said Final Commitments. This Decision is adopted in application of Article 6(1)(b) in conjunction with Article 6(2) of the Merger Regulation and Article 57 of the EEA Agreement.
COMMITMENTS TO THE EUROPEAN COMMISSION
1. Pursuant to Article 6(2) of Council Regulation (EC) No 139/2004 (the “Merger Regulation”), Siemens Healthineers AG hereby enters into the following Commitments (the “Commitments”) vis-à-vis the European Commission (the “Commission”) with a view to render the acquisition of sole control over Varian Medical Systems, Inc. (“Varian”) by Siemens Healthineers AG (the “Concentration”) compatible with the internal market and the functioning of the EEA Agreement.
2. This text shall be interpreted in light of the Commission’s decision pursuant to Article 6(2) of the Merger Regulation to declare the Concentration compatible with the internal market and the functioning of the EEA Agreement (the “Decision”), in the general framework of European Union law, in particular in light of the Merger Regulation, and by reference to the Commission Notice on remedies acceptable under Council Regulation (EC) No 139/2004 and under Commission Regulation (EC) No 802/2004 (the “Remedies Notice”).
3. The Commitments shall take effect upon the Effective Date, provided that if the completion of the Concentration does not subsequently take place, Siemens Healthineers AG shall not be bound by these Commitments.
SECTION A - DEFINITIONS
4. For the purpose of the Commitments, the following terms shall have the following meaning:
Affiliated Undertakings: undertakings controlled by the Siemens Healthineers and/or by the ultimate parents of Siemens Healthineers, whereby the notion of control shall be interpreted pursuant to Article 3 of the Merger Regulation and in light of the Commission Consolidated Jurisdictional Notice under Council Regulation (EC) No 139/2004 on the control of concentrations between undertakings (the “Consolidated Jurisdictional Notice”).
Anzai: Anzai Medical Co., Ltd.
Anzai Interface: existing interface between Siemens Healthineers’ Medical Imaging Solutions and Anzai’s MM Imaging Devices composed of a hardware and a software interface.
Certification: a declaration under Article 12 of the Medical Device Directive and/or any certification customarily requested by Customers in relation to Interoperability.
Closing Date: closing date of the Concentration.
Conflict of Interest: any conflict of interest that impairs the Monitoring Trustee’ objectivity and independence in discharging its duties under the Commitments.
CT Scanner: computed tomography scanners.
Customer: an entity that owns and/or operates a Siemens Healthineers’ Medical Imaging Solution, OIS, TPS and/or MM Imaging Device.
DICOM Data: images and associated objects (including RT objects), each as defined in the Standard, produced and/or processed by Siemens Healthineers’ Medical Imaging Solutions, OIS, and TPS that are currently commercially available or that may be developed by Siemens Healthineers in the future, and that are required to fulfil the product’s respective intended purpose in the field of radiation therapy.
DICOM Standard or DICOM: Digital Imaging and Communications in Medicine standard to transmit, store, retrieve, print, process, and display medical imaging information, including its radiotherapy extension (DICOM RT).
Disclosed by Appropriate Means: communicate the fact that a new Siemens Healthineers’ Medical Imaging Solution, OIS or TPS has been developed (including, but not limited to the development of a new product, an updated/upgraded version of an existing product, a new image, a new functionality and/or any other type of new features), either in industry publications, industry events (e.g., industry conferences), relevant DICOM Committee meetings, or directly to third party Vendors of Medical Imaging Solutions, OIS, TPS and MM Imaging Devices and, in any event, no later than completion of product development (i.e. the time of submission of the CE- marking application (if applicable) or UK-equivalent (if applicable)).
End of Support Status: the point in time when the manufacturer ceases to guarantee the availability of parts, maintenance, service or updates, e.g., due to product age, lack of personnel, obsolescence of parts or software components.
Imaging Processing Software: software used to process medical images.
Interoperability: for the purpose of the Commitments, Interoperability means the ability of two or more devices, including hardware and/or software, from the same manufacturer or from different manufacturers to interact reliably and safely in a way that enables each device to fully achieve its intended purpose in the field of radiation therapy, including, but not limited to, by (a) exchanging information and mutually using the information that has been exchanged for the correct execution of a specified function without changing the content and/or format of the data, (b) communicating with each other, and (c) working together as intended.
Medical Imaging Solutions: CT Scanners, PET CT Scanners, MRI Scanners, and Imaging Processing Software, as well as add-on options or features to these products, currently or in the future commercially available whose intended purpose includes to assist in the planning of radiation therapy.
MM Imaging Device: motion management devices used with Medical Imaging Solutions to track and manage a patient’s motion while the image is acquired during the radiation therapy simulation.
MM Imaging Device Interface: interface whose intended purpose is to connect a Medical Imaging Solution with an MM Imaging Device composed of a hardware and a software interface.
Monitoring Trustee: one or more natural or legal person(s) who is/are approved by the Commission and appointed by Siemens Healthineers, and who has/have the duty to monitor compliance with the obligations attached to the Decision.
MRI Scanner: magnetic resonance imaging scanners.
OIS: software whose primary intended purpose is to manage and process an oncology department’s administrative and clinical patient data for radiation therapy purposes.
Open Interface: existing interface between Siemens Healthineers’ Medical Imaging Solutions and MM Imaging Devices of Vendors composed of hardware and a software interface.
Open Interface Documentation: documentation (in electronic and/or written form) with information about the technical specifications of the Open Interface.
PET CT Scanners: positron emission tomography CT scanners.
Required Imaging Information: information in the possession of or otherwise known to Siemens Healthineers required to ensure the Interoperability between Siemens Healthineers’ Medical Imaging Solutions and any Vendor’s OIS, TPS and/or MM Imaging Devices, including, but not limited to, information on DICOM Data (including necessary information or explanations about such data) as well as information on how Siemens Healthineers interprets or implements the DICOM Standard, each provided in electronic and/or written form.
Required MM Information: information in the possession of or otherwise known to Siemens Healthineers required to ensure Interoperability between Siemens Healthineers’ Medical Imaging Solutions and Vendor’s MM Imaging Devices in electronic and/or written form.
Required Treatment Information: information in the possession of or otherwise known to Siemens Healthineers required to ensure the Interoperability between Siemens Healthineers’ OIS and/or TPS and any Vendor’s Medical Imaging Solutions, including, but not limited to, information and explanation on how Siemens Healthineers’ OIS and/or TPS consume DICOM Data as well as information on how Siemens Healthineers interprets or implements the DICOM Standard, each provided in electronic and/or written form.
RT: radiation therapy.
RT Equipment: Linear accelerators, Brachytherapy equipment and Proton therapy equipment.
Siemens Healthineers: Siemens Healthineers AG and its affiliated undertakings, including Varian Medical, Inc. and its affiliated undertakings post-transaction.
Timely Manner: without undue delay and, at the latest, within 20 Working Days from receiving a Written Request.
TPS: software whose primary intended purpose is to generate a plan for the delivery of radiation therapy to oncology patients.
Working Days: days as defined in Commission Regulation (EC) No 802/2004 of 21 April 2004 implementing Council Regulation (EC) No 139/2004 on the control of concentrations between undertakings.
Written Request: request made by electronic means to the following email addresses of Siemens Healthineers: DICOM-RT_interop.func@siemens- healthineers.com (for requests under Section B.1 and requests under Section B.3 related to Section B.1) and (for requests under Section B.2 and requests under Section B.3 related to Section B.2). The Written Request must contain sufficient information on the Interoperability issue for Siemens Healthineers to discharge its obligations under these Commitments; Siemens Healthineers shall inform Vendor without undue delay, but no later than 10 Working Days, if this is not the case.
Vendor: third party vendor of the relevant devices (including hardware and software).
SECTION B – COMMITMENTS
B.1 – Medical Imaging Solutions and OIS/TPS
5. Siemens Healthineers shall, in a Timely Manner, upon a Written Request, without charge or other form of consideration, on a non-discriminatory basis, and in English language:
a. provide to a Vendor of OIS and/or TPS the Required Imaging Information necessary to ensure Interoperability, the testing and Certification of Interoperability, between the requesting Vendor’s OIS and/or TPS and Siemens Healthineers’ Medical Imaging Solutions; provided, however, with respect to a new product development nothing therein shall require Siemens Healthineers to provide the Required Imaging Information before it has been Disclosed by Appropriate Means; and
b. provide to a Vendor of Medical Imaging Solutions the Required Treatment Information necessary to ensure Interoperability, the testing and Certification of Interoperability, between the requesting Vendor’s Medical Imaging Solutions and Siemens Healthineers’ OIS and/or TPS; provided, however, with respect to a new product development nothing therein shall require Siemens Healthineers to provide the Required Treatment Information before it has been Disclosed by Appropriate Means.
6. In the event of any relevant modifications to the Required Imaging Information and the Required Treatment Information that could have an impact on Interoperability, Siemens Healthineers shall provide updates to the Required Imaging Information and the Required Treatment Information automatically to the Vendors previously provided with any such information without undue delay.
7. Siemens Healthineers shall, upon Written Request, at cost and including a reasonable margin, in a timely fashion and on a non-discriminatory basis, provide reasonable feedback and technical guidance, including for example to facilitate testing and Certification activities, to Vendors of Medical Imaging Solutions and/or OIS and/or TPS that is necessary to ensure Interoperability (i) between Siemens Healthineers’ Medical Imaging Solutions and Vendors’ OIS and/or TPS and (ii) between Siemens Healthineers’ OIS and/or TPS and Vendors’ Medical Imaging Solutions.
8. Siemens Healthineers shall refrain from implementing any features or functions to its Medical Imaging Solutions or to the way they interact with Vendors’ OIS and/or TPS that are designed to negatively affect the performance and functionalities of Vendors’ OIS and/or TPS, or Siemens Healthineers’ Medical Imaging Solutions used in combination with such Vendors’ OIS and/or TPS, by degrading Interoperability. Siemens Healthineers shall refrain from implementing any features or functions to its OIS and TPS or to the way they interact with Vendors’ Medical Imaging Solutions that are designed to negatively affect the performance and functionalities of Vendors’ Medical Imaging Solutions, or Siemens Healthineers’ OIS and/or TPS used in combination with such Vendors’ Medical Imaging Solutions, by degrading Interoperability.
9. Siemens Healthineers (i) shall not impose any restrictions on the Vendors’ ability to communicate in accordance with applicable laws on the Interoperability between their OIS and/or TPS and Siemens Healthineers’ Medical Imaging Solutions; and (ii) shall refrain from actively communicating on the lack of Interoperability (or potential lack of Interoperability) between Vendors’ OIS and/or TPS and Siemens Healthineers’ Medical Imaging Solutions unless the lack of Interoperability (or potential lack of Interoperability) can be demonstrated by Siemens Healthineers by means of a reasoned and documented submission to the Monitoring Trustee. For the avoidance of doubt, the above shall not prevent Siemens Healthineers from passively communicating on Interoperability in response to a Customer request in accordance with applicable laws. Nothing therein shall limit Siemens Healthineers’ ability to comply with applicable laws, including its regulatory duties as a Medical Device manufacturer.
10. Siemens Healthineers (i) shall not impose any restrictions on the Vendors’ ability to communicate in accordance with applicable laws on the Interoperability between their Medical Imaging Solutions and Siemens Healthineers’ OIS and/or TPS; and (ii) shall refrain from actively communicating on the lack of Interoperability (or potential lack of Interoperability) between Vendors’ Medical Imaging Solutions and Siemens Healthineers’ OIS and/or TPS unless the lack of Interoperability (or potential lack of Interoperability) can be demonstrated by Siemens Healthineers by means of a reasoned and documented submission to the Monitoring Trustee. For the avoidance of doubt, the above shall not prevent Siemens Healthineers from passively communicating on Interoperability in response to a Customer request in accordance with applicable laws. Nothing therein shall limit Siemens Healthineers’ ability to comply with applicable laws, including its regulatory duties as a Medical Device manufacturer.
11. Siemens Healthineers shall provide, on a non-discriminatory basis, Customers with support to ensure Interoperability between (i) Vendor’s OIS and/or TPS and Siemens Healthineers’ Medical Imaging Solutions, or (ii) Vendor’s Medical Imaging Solutions and Siemens Healthineers’ OIS and/or TPS, at a level, timeline, and on terms that are no less favourable than those provided to Customers that use Siemens Healthineers’ Medical Imaging Solutions with Siemens Healthineers’ OIS and/or TPS.
12. Siemens Healthineers shall continue to support the DICOM Standard (and its RT extension) in its Medical Imaging Solutions, OIS, and TPS and continue to contribute as a member of the corresponding DICOM and IHE organs, and in particular to participate in the yearly IHE-RO connectathons.
13. Interoperability between (i) Siemens Healthineers’ Medical Imaging Solutions and Vendor’s OIS and/or TPS and (ii) Siemens Healthineers’ OIS and/or TPS and Vendor’s Medical Imaging Solutions shall be ensured by support of the DICOM Standard (and its RT extension) to the greatest extent possible. In particular, Siemens Healthineers shall not be required to implement the Commitments under Section B.1. in a way that could prevent conformance of its Medical Imaging Solutions, OIS and/TPS with the DICOM Standard, or with respect to Vendor’s Medical Imaging Solutions, TPS, and/or OIS that do not conform to the DICOM Standard.
14. If Siemens Healthineers combines its Medical Imaging Solutions with other products (e.g. OIS or TPS), the Section B.1 provisions and the other relevant provisions of the Commitments would remain applicable to the Medical Imaging Solution functionality of such a combined product.
15. The Section B.1 provisions and the other relevant provisions of the Commitments shall also remain applicable with respect to the Medical Imaging Solution functionality between Siemens Healthineers’ Medical Imaging Solutions and Vendors’ RT Equipment to the extent that they directly interact.
B.2 – Medical Imaging Solutions and MM Imaging Device
16. Siemens Healthineers shall, on a non-discriminatory basis, maintain, without charge or other form of consideration, and update and upgrade, at cost and including a reasonable margin:
a. The existing Anzai Interface between Siemens Healthineers’ existing and future Medical Imaging Solutions and Anzai’s existing and future MM Imaging Devices to achieve Interoperability between them; and
b. The existing Open Interface between Siemens Healthineers’ existing and future Medical Imaging Solutions and Vendor’s existing and future MM Imaging Devices to achieve Interoperability between them.
17. The Commitments in Section B.2 shall apply to Siemens Healthineers’ Medical Imaging Solutions currently commercially available incorporating the Anzai Interface or Open Interface and to Medical Imaging Solutions interfacing with MM Imaging Device that Siemens Healthineers may market in the future.
18. Siemens Healthineers shall, upon Written Request, free of charge or other consideration, on a non-discriminatory basis, and in English language, provide Open Interface Documentation and/or Required MM Information to Vendors of MM Imaging Devices to enable them to achieve Interoperability between Siemens Healthineers’ Medical Imaging Solutions and their MM Imaging Devices. In the event of any relevant modifications to the Open Interface Documentation and/or Required MM Information that could have an impact on Interoperability, Siemens Healthineers shall provide updates to the Open Interface Documentation and/or Required MM Information automatically to the Vendors previously provided with any such information without undue delay.
19. Siemens Healthineers shall, upon Written Request, at cost and including a reasonable margin, without undue delay and on a non-discriminatory basis, cooperate with Vendors of MM Imaging Devices to provide reasonable feedback and technical guidance, including facilitate testing and validation activities, to ensure Interoperability between Siemens Healthineers’ Medical Imaging Solutions and their MM Imaging Devices through the Open Interface or the Anzai Interface. This may include reasonable feedback and technical guidance for the purpose of issuing a declaration under Article 12 of the Medical Device Directive.
20. Siemens Healthineers shall refrain from implementing any features or functions to its Medical Imaging Solutions or to the way they interact with Vendors’ MM Imaging Devices that is designed to negatively affect the performance and functionalities of Vendors’ MM Imaging Devices, or Siemens Healthineers’ Medical Imaging Solutions used in combination with such Vendor MM Imaging Devices, by degrading Interoperability.
21. Siemens Healthineers (i) shall not impose any restrictions on the Vendors’ ability to communicate in accordance with applicable laws on the Interoperability between their MM Imaging Devices and Siemens Healthineers’ Medical Imaging Solutions; and (ii) shall refrain from actively communicating on the lack of Interoperability (or potential lack of Interoperability) between Vendors’ MM Imaging Devices and Siemens Healthineers’ Medical Imaging Solutions unless the lack of Interoperability (or potential lack of Interoperability) can be demonstrated by Siemens Healthineers by means of a reasoned and documented submission to the Monitoring Trustee. For the avoidance of doubt, the above shall not prevent Siemens Healthineers from passively communicating on Interoperability in response to a Customer request in accordance with applicable laws. Nothing therein shall limit Siemens Healthineers’ ability to comply with applicable laws, including its regulatory duties as a Medical Device manufacturer.
22. Siemens Healthineers shall, upon Written Request, for a reasonable period, and at cost, provide or facilitate access to a Siemens Healthineers’ Medical Imaging Solutions or a reasonable facsimile thereof, to enable Vendors of MM Imaging Devices to perform the necessary tests of Interoperability between their MM Imaging Devices and Siemens Healthineers’ Medical Imaging Solutions.
23. Siemens Healthineers shall provide, on a non-discriminatory basis, Customers with support to ensure Interoperability between Vendor’s MM Imaging Device and Siemens Healthineers’ Medical Imaging Solutions at a level, timeline, and on terms that are no less favourable than those provided to Customers that use Siemens Healthineers’ Medical Imaging Solutions with Siemens Healthineers’ MM Imaging Devices.
B.3 – Common Provisions
24. Nothing in these Commitments shall be interpreted to require Siemens Healthineers to provide confidential information that is not necessary to ensure Interoperability between (i) Vendor’s OIS and/or TPS and Siemens Healthineers’ Medical Imaging Solutions, or (ii) Vendor’s Medical Imaging Solutions and Siemens Healthineers’ OIS and/or TPS.
25. These Commitments do not apply to Medical Imaging Solutions, OIS, TPS, and MM Imaging Devices that have reached End of Support Status.
26. Siemens Healthineers shall, upon Written Request, keep all information received from the requesting Vendor in the context of the Commitments in Sections B.1 and B.2 confidential and shall use this information only to discharge its obligations under these Commitments and for no other purpose.
27. Siemens Healthineers may request that a third party receiving information according to the Commitments in Sections B.1 and B.2 be bound by a confidentiality agreement obliging that party to use the information for purposes directly related to the Commitments and for no other purpose. In case of disagreement concerning the terms of the confidentiality agreement, the Commission shall have the power to decide its terms and Siemens Healthineers undertakes that it will enter into such agreement as required by the Commission.
28. Siemens Healthineers shall advertise the email address for Written Requests on its website in an easily visible position and publish a version of the Commitments on its website (any redactions must be approved by the Commission).
29. Nothing in these Commitments shall be interpreted in a way that prevents Siemens Healthineers from developing new products and/or integrated systems, provided that these new products and/or integrated systems retain Interoperability with Vendor’s devices and software, in accordance with the provisions of the Commitments in Section B above.
SECTION C – DURATION AND SCOPE
30. The Commitments shall be in force for a period of (10) years from the Closing Date. The Commission may, during the final year of the initial (10) year period, decide to extend the duration of the Commitments for an additional period of up to (5) years if such extension is necessary to address the competition concerns identified in the merger clearance decision to which the Commitments are attached. Before extending the duration of the Commitments, the Commission must afford Siemens Healthineers the possibility to submit its views in writing and orally at least eight weeks prior to taking a position on the necessity of an extension.
31. The Commitments shall apply to Medical Imaging Solutions, TPS, OIS and MM Imaging Devices that have obtained or are in the process of obtaining or have taken steps to obtain a CE mark in the EEA or the UK equivalent or to functions/features of Medical Imaging Solutions, TPS, OIS and MM Imaging Devices products that have obtained or are in the process of obtaining or have taken steps to obtain a CE mark in the EEA or the UK equivalent or that do not require a CE mark to be commercialized in the EEA.
32. If the Concentration is abandoned, unwound or otherwise terminated, the Commitments shall automatically cease to apply.
SECTION D – MONITORING TRUSTEE
D.1 – Appointment
33. Siemens Healthineers shall appoint a Monitoring Trustee to carry out the functions specified in these Commitments for a Monitoring Trustee. Siemens Healthineers commits not to close the Concentration before the appointment of a Monitoring Trustee.
34. The Monitoring Trustee shall:
a. at the time of appointment, be independent of Siemens Healthineers;
b. possess the necessary qualifications to carry out its mandate; and
c. neither have nor become exposed to a Conflict of Interest.
35. The Monitoring Trustee shall be remunerated by Siemens Healthineers in a way that does not impede the independent and effective fulfilment of its mandate.
Proposal by Siemens Healthineers
36. No later than (2) weeks after the Effective Date, Siemens Healthineers shall submit the name or names of one or more natural or legal persons whom Siemens Healthineers proposes to appoint as the Monitoring Trustee to the Commission for approval. The proposal shall contain sufficient information for the Commission to verify that the person or persons proposed as Monitoring Trustee fulfil the requirements set out in paragraph 34 and shall include:
a. the full terms of the proposed mandate, which shall include all provisions necessary to enable the Monitoring Trustee to fulfil its duties under the Commitments; and
b. the outline of a work plan which describes how the Monitoring Trustee intends to carry out its assigned tasks.
Approval or rejection by the Commission
37. The Commission shall have the discretion to approve or reject the proposed Monitoring Trustee(s) and to approve the proposed mandate subject to any modifications it deems necessary for the Trustee to fulfil its obligations. If only one name is approved, Siemens Healthineers shall appoint or cause to be appointed the person or persons concerned as Monitoring Trustee, in accordance with the mandate approved by the Commission. If more than one name is approved, Siemens Healthineers shall be free to choose the Monitoring Trustee to be appointed from among the names approved. The Monitoring Trustee shall be appointed within one week of the Commission’s approval, in accordance with the mandate approved by the Commission.
New proposal by Siemens Healthineers
38. If all the proposed Monitoring Trustees are rejected, Siemens Healthineers shall submit the names of at least two more natural or legal persons within one week of being informed of the rejection, in accordance with paragraphs 33 and 37 of the Commitments.
Monitoring Trustee nominated by the Commission
39. If all further proposed Monitoring Trustees are rejected by the Commission, the Commission shall nominate a Monitoring Trustee, whom Siemens Healthineers shall appoint, or cause to be appointed, in accordance with a trustee mandate approved by the Commission.
D.2 – Functions of the Monitoring Trustee
40. The Monitoring Trustee shall assume its specified duties and obligations in order to ensure compliance with the Commitments. The Commission may, on its own initiative or at the request of the Monitoring Trustee or Siemens Healthineers, give any orders or instructions to the Monitoring Trustee in order to ensure compliance with the conditions and obligations attached to the Decision.
Duties and obligations of the Monitoring Trustee
41. The Monitoring Trustee shall:
a. Propose in its first report to the Commission a detailed work plan describing how it intends to monitor compliance with the obligations and conditions attached to the Decision;
b. Monitor compliance by Siemens Healthineers with the conditions and obligations attached to the Decision;
c. Propose to Siemens Healthineers such measures as the Monitoring Trustee considers necessary to ensure Siemens Healthineers’ compliance with the conditions and obligations attached to the Decision;
d. Act as a contact point for any requests by third parties, in relation to the Commitments;
e. Subject to the fast track dispute resolution mechanism provided for in Section E below, broker a resolution of any dispute that would arise between Siemens Healthineers and a Vendor of a Medical Imaging Solution, OIS, TPS, or MM Imaging Device regarding compliance with the conditions and obligations set out in Section B if Siemens Healthineers and the Vendor are unable to resolve the dispute within a period of thirty (30) working days from the date Siemens Healthineers is contacted in writing regarding the dispute;
f. Provide to the Commission, sending Siemens Healthineers a non-confidential copy at the same time, a written report within (15) Working Days after the end of every quarter from the Effective Date during the term of the Commitments, so that the Commission can assess whether the Commitments are being correctly implemented. The Commission can amend the frequency of these reports after consulting with the Monitoring Trustee;
g. Promptly report in writing to the Commission, sending Siemens Healthineers a non-confidential copy at the same time, if it concludes on reasonable grounds that Siemens Healthineers is failing to comply with the Commitments; and
h. Assume the other functions assigned to the Monitoring Trustee under the conditions and obligations attached to the Decision.
D.3 – Duties and Obligations of Siemens Healthineers
42. Siemens Healthineers shall provide and shall cause its advisors to provide the Monitoring Trustee with all such cooperation, assistance and information as the Monitoring Trustee may reasonably require to perform its tasks. The Monitoring Trustee shall have full and complete access to Siemens Healthineers’ books, records, documents, management or other personnel, facilities, sites and technical information necessary for fulfilling its duties under the Commitments and Siemens Healthineers shall provide the Monitoring Trustee, upon request and without undue delay, with copies of any document. Siemens Healthineers shall be promptly available for meetings in order to provide the Monitoring Trustee with all information necessary for the performance of its tasks.
43. In carrying out its mandate, the Monitoring Trustee shall have due regard for Siemens Healthineers’ legitimate interests in avoiding unjustified burden and interference in Siemens Healthineers’ business operations, subject to Siemens Healthineers’ unconditional obligation to comply with the Commitments.
44. Siemens Healthineers shall indemnify the Monitoring Trustee and its employees and agents (each an “Indemnified Party”) and hold each Indemnified Party harmless against, and hereby agrees that an Indemnified Party shall have no liability to Siemens Healthineers for, any liabilities arising out of the performance of the Monitoring Trustee’s duties under the Commitments, except to the extent that such liabilities result from the willful default, recklessness, gross negligence or bad faith of the Monitoring Trustee, its employees, agents or advisors.
45. At the expense of Siemens Healthineers, the Trustee may appoint advisors (in particular a legal advisor or an expert in medical imaging and RT), subject to Siemens Healthineers’ approval (this approval not to be unreasonably withheld or delayed) if the appointment of such advisors is necessary or appropriate for the performance of the Monitoring Trustee’s duties and obligations under the mandate, provided that any fees and other expenses incurred by the Monitoring Trustee are reasonable. Should Siemens Healthineers refuse to approve the advisors proposed by the Monitoring Trustee, the Commission may approve the appointment of such advisors instead if the requirements above are met, after having heard Siemens Healthineers. Only the Monitoring Trustee shall be entitled to issue instructions to the advisors. Paragraph 44 of these Commitments shall apply mutatis mutandis.
46. Siemens Healthineers agrees that the Commission may share confidential information proprietary to Siemens Healthineers with the Monitoring Trustee. The Trustee shall not disclose such information and the principles contained in Articles 17(1) and (2) of the Merger Regulation apply mutatis mutandis.
47. Siemens Healthineers agrees that the contact details of the Monitoring Trustee are published on the website of the Commission’s Directorate-General for Competition and shall inform interested third parties of the identity and the tasks of the Monitoring Trustee.
48. For the duration of the Commitments, the Commission may request all information from Siemens Healthineers that is reasonably necessary to monitor the effective implementation of these Commitments.
D.4 – Replacement, discharge and reappointment of the Monitoring Trustee
49. If the Monitoring Trustee ceases to perform its functions under the Commitment or for any other good cause, including the exposure of the Monitoring Trustee to a Conflict of Interest:
a. the Commission may, after hearing the Monitoring Trustee and Siemens Healthineers, require Siemens Healthineers to replace the Monitoring Trustee; or
b. Siemens Healthineers may, with the prior approval of the Commission, replace the Monitoring Trustee.
50. If the Trustee is removed according to paragraph 49 of the Commitments, the Monitoring Trustee may be required to continue in its function until a new Monitoring Trustee is in place to whom the Monitoring Trustee has effected a full hand over of all relevant information. The new Monitoring Trustee shall be appointed in accordance with the procedure referred to in paragraphs 33-39 of the Commitments.
51. Unless removed according to paragraph 49 of the Commitments, the Monitoring Trustee shall cease to act as Monitoring Trustee only after the Commission has discharged it from its duties after all the Commitments with which the Monitoring Trustee has been entrusted have been implemented. However, the Commission may at any time require the reappointment of the Monitoring Trustee if it subsequently appears that the relevant remedies might not have been fully and properly implemented.
SECTION E – FAST TRACK DISPUTE RESOLUTION
52. In the event that a Vendor of a Medical Imaging Solution, OIS, TPS, or MM Imaging Device, showing a sufficient legitimate interest (the “Requesting Party”), claims that Siemens Healthineers is failing to comply with its obligations arising from these Commitments, the fast track dispute resolution procedure as described herein shall apply.
53. The Requesting Party shall notify Siemens Healthineers and the Monitoring Trustee of its request and specify the reasons why it believes that Siemens Healthineers is failing to comply with the Commitments. The Requesting Party and Siemens Healthineers shall use their best efforts to resolve all differences of opinion and to settle all disputes that may arise through co-operation and consultation within a reasonable period of time not to exceed (15) Working Days after receipt of the request (such period being extendable by mutual consent of Siemens Healthineers and the Requesting Party).
54. The Monitoring Trustee shall present its own proposal for resolving the dispute within eight working days to Siemens Healthineers, the Requesting Party and the Commission, specifying in writing the action, if any, to be taken by Siemens Healthineers in order to ensure compliance with the Commitments vis-à-vis the Requesting Party, and be prepared, if requested, to facilitate the settlement of the dispute.
55. Should Siemens Healthineers and the Requesting Party fail to resolve their differences of opinion through cooperation and consultation, the Requesting Party may initiate the arbitration process described below. The arbitration process shall be used only to resolve disputes regarding compliance with the Commitments.
56. To initiate the arbitration process, the Requesting Party shall serve a notice (the “Notice”), in the sense of a request for arbitration, to the International Chamber of Commerce (“ICC”, hereinafter the “Arbitral Institution”), with a copy of such Notice and request for arbitration to Siemens Healthineers.
57. The Notice shall set out in detail the dispute, difference or claim (the “Dispute”) and shall contain, inter alia, all issues of both fact and law, including any suggestions as to the procedure, and all documents relied upon shall be attached, e.g. documents, agreements, expert reports, and witness statements. The Notice shall also contain a detailed description of what is required of Siemens Healthineers and the Monitoring Trustee Proposal, including a comment as to its appropriateness.
58. Siemens Healthineers shall, within (20) Working Days from receipt of the Notice, submit its response (the “Response”). The Response shall provide detailed reasons for its conduct and set out, inter alia, all issues of both fact and law, including any suggestions as to the procedure, and all documents relied upon, e.g. documents, agreements, expert reports, and witness statements. The Response shall, if appropriate, contain a detailed description of the action that Siemens Healthineers proposes to undertake vis-à-vis the Requesting Party.
Appointment of the Arbitrators
59. The Arbitral Tribunal shall consist of three persons. The Requesting Party shall nominate its arbitrator in the Notice; Siemens Healthineers shall nominate its arbitrator in the Response. The arbitrator nominated by the Requesting Party and by Siemens Healthineers shall, within (5) Working Days of the nomination of the latter, nominate the chairman, making such nomination known to the Requesting Party and Siemens Healthineers and the Arbitral Institution, which shall confirm the appointment of all three arbitrators.
60. Should the Requesting Party wish to have the Dispute decided by a sole arbitrator it shall indicate this in the Notice. In this case, the Requesting Party and Siemens Healthineers shall agree on the nomination of a sole arbitrator within (5) Working Days from the communication of the Response, communicating this to the Arbitral Institution.
61. Should Siemens Healthineers fail to nominate an arbitrator, or if the two arbitrators fail to agree on the chairman, or should the Requesting Party and/or Siemens Healthineers fail to agree on a sole arbitrator, the default appointment(s) shall be made by the Arbitral Institution.
62. The three-person arbitral tribunal or, as the case may be, the sole arbitrator, are herein referred to as the “Arbitral Tribunal”.
Arbitration Procedure
63. The Dispute shall be finally resolved by arbitration under the ICC Rules of Arbitration, with such modifications or adaptations as foreseen herein or necessary under the circumstances (the “Rules”). The arbitration shall be conducted in Zurich, Switzerland, in the English language. For good cause, any Party may apply to the Arbitral Institution (or Arbitral Tribunal as may be appropriate) for an extension of the timelines provided below.
64. The procedure shall be a fast-track procedure. For this purpose, the Arbitral Tribunal shall shorten all applicable procedural time-limits under the Rules as far as appropriate in the circumstances. The Requesting Party and Siemens Healthineers (hereinafter each a “Party” and together the “Parties”) shall consent to the use of e- mail for the exchange of documents.
65. The Arbitral Tribunal shall, as soon as practical after the confirmation of the Arbitral Tribunal, hold an organisational conference to discuss any procedural issues with the Parties to the arbitration. Terms of reference shall be drawn up and signed by the Parties to the arbitration and the Arbitral Tribunal at the organisational meeting or thereafter and a procedural timetable shall be established by the Arbitral Tribunal. An oral hearing shall, as a rule, be established within (2) months of the confirmation of the Arbitral Tribunal.
66. In order to enable the Arbitral Tribunal to reach a decision, it shall be entitled to request any relevant information from the Parties to the Arbitration, to appoint experts and to examine them at the hearing, and to establish the facts by all appropriate means. Any order for the production or disclosure of documents shall be limited to the documents on which each Party specifically relies in its submission(s). The Arbitral Tribunal is also entitled to ask for assistance by the Monitoring Trustee in all stages of the procedure if the Requesting Party and/or Siemens Healthineers agree.
67. The arbitrators shall not disclose confidential information and shall apply the legal standards covering the treatment of confidential information under the Merger Regulation and the Treaty of the Functioning of the European Union. The Arbitral Tribunal may take the measures necessary for protecting confidential information in particular by restricting access to confidential information to the Arbitral Tribunal, Monitoring Trustee and outside counsel and experts of the opposing party.
68. The burden of proof in any dispute governed under the Rules shall be borne as follows: (i) the Requesting Party must produce evidence of a prima facie case; (ii) if the Requesting Party does so, the Arbitral Tribunal must find in favour of the Requesting Party unless Siemens Healthineers can produce sufficient evidence to the contrary.
Involvement of the Commission
69. The Commission shall be allowed and enabled to participate in all stages of the procedure by:
a. receiving all written submissions (including documents and reports, etc.) made by the Parties to the arbitration;
b. receiving all orders, interim and final awards and other documents exchanged by the Arbitral Tribunal with the Parties to the arbitration (including the terms of reference and procedural timetable);
c. filing any Commission amicus curiae briefs; and
d. being present at the hearing(s) and being allowed to ask questions to Parties, witnesses and experts.
70. The Arbitral Tribunal shall forward, or shall order the Parties to the arbitration to forward, the documents mentioned to the Commission without delay.
71. In the event of disagreement between the Parties to the arbitration regarding the interpretation of the Commitments, the Arbitral Tribunal shall inform the Commission and may seek the Commission’s interpretation of the Commitments before finding in favour of any Party to the arbitration and shall be bound by the interpretation.
Decisions of the Arbitral Tribunal
72. The Arbitral Tribunal shall decide the dispute on the basis of the Commitments and the Decision. The Commitments shall be construed in accordance with the Merger Regulation, EU law and general principles of law common to the legal orders of the Member States without a requirement to apply a particular national system. The Arbitral Tribunal shall take all decisions by majority vote.
73. Upon the request of the Requesting Party, the Arbitral Tribunal may make a preliminary ruling on the Dispute. The preliminary ruling shall be rendered within one month after the confirmation of the Arbitral Tribunal, shall be applicable immediately and, as a rule, remain in force until a final decision is rendered.
74. The Arbitral Tribunal shall, in the preliminary ruling as well as in the final award, specify the action, if any, to be taken by Siemens Healthineers in order to comply with the Commitments vis-à-vis the Requesting Party.
75. The final award shall be final and binding on the Parties to the arbitration and shall resolve the dispute and determine any and all claims, motions or requests submitted to the Arbitral Tribunal. The arbitral award shall also determine the reimbursement of the costs of the successful party and the allocation of the arbitration costs. In case of granting a preliminary ruling or if otherwise appropriate, the Arbitral Tribunal shall specify that terms and conditions determined in the final award apply retroactively.
76. The final award shall, as a rule, be rendered within (6) months after the confirmation of the Arbitral Tribunal. The timeframe shall, in any case, be extended by the time the Commission takes to submit an interpretation of the Commitments if asked by the Arbitral Tribunal.
77. The Parties to the arbitration shall prepare a non-confidential version of the final award, without business secrets. The Commission may publish the non-confidential version of the award.
78. Nothing in the above-described arbitration procedure shall affect the powers of the Commission to take decisions in relation to the Commitments in accordance with its powers under the Merger Regulation and the Treaty on the Functioning of the European Union.
SECTION F – REVIEW CLAUSE
79. The Commission may, where appropriate, in response to a reasoned request from Siemens Healthineers showing good cause, waive, modify or substitute, in exceptional circumstances, one or more of the undertakings in the Commitments. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time, send a non-confidential copy of the report to Siemens Healthineers. The request shall not have the effect of suspending the application of the undertaking and, in particular, of suspending the expiry of any time period in which the undertaking has to be complied with.
1 OJ L 24, 29.1.2004, p. 1 (the “Merger Regulation”). With effect from 1 December 2009, the Treaty on the Functioning of the European Union (“TFEU”) has introduced certain changes, such as the replacement of “Community” by “Union” and “common market” by “internal market”. The terminology of the TFEU will be used throughout this decision.
2 For the purposes of this Decision, although the United Kingdom withdrew from the European Union as of 1 February 2020, according to Article 92 of the Agreement on the withdrawal of the United Kingdom of Great Britain and Northern Ireland from the European Union and the European Atomic Energy Community (OJ L 29, 31.1.2020, p. 7), the Commission continues to be competent to apply Union law as regards the United Kingdom for administrative procedures which were initiated before the end of the transition period.
3 OJ L 1, 3.1.1994, p. 3 (the “EEA Agreement”).
* Should read “or the Notifying Party,”
** Should read “together”
4 Publication in the Official Journal of the European Union No C 8, 11.1.2021, p. 14.
5 Turnover calculated in accordance with Article 5(1) of the Merger Regulation and the Commission Consolidated Jurisdictional Notice (OJ C95, 16.4.2008, p. 1).w
6 Siemens exited the radiotherapy business, where they used to supply Linacs, in 2011.
7 The Transaction also gives rise to minor potential conglomerate links between (i) Siemens’ fixed C-arms and Varian’s embolization devices, and (ii) Siemens’ CT scanners and Varian’s ablation devices, which are unlikely to raise serious doubts as to the compatibility of the Transaction with the internal market for several reasons. First, the potential links between the above products are quite remote since Siemens’ fixed C-arms and CT scanners are predominantly used for applications that do not involve embolization and ablation. In fact, the Parties’ above products are never sold together. Second, Varian’s EEA/UK market shares are moderate (between [0-5]% and [20-30]%), with several competitors, including players with higher market shares. Siemens’ EEA/UK market shares remain below [40-50]% (between [30-40]% and [40-50]%), with a number of significant competitors with market shares above [10-20]%. Finally, none of the competitors and customers that participated in the market investigation expressed concerns about these potential links. For completeness, the Transaction also gives rise to limited vertical links since Siemens supplies certain components (i.e. MRI components, dose chambers, multi-leaf collimators) principally to [customer information]. According to the Parties’ estimates, Siemens’ market shares are relatively modest for these products (no more than [30-40]% for MRI components worldwide, [10-20]% for dose chambers worldwide, [0-5]% for multi-leaf collimators worldwide). These links are unlikely to give rise to input foreclosure concerns since [customer information]’s supply from Siemens is contractually protected for at least the next […] years and several rivals are capable of meeting its needs. Moreover, some of the concerned inputs are commodity products that can be purchased off the shelf from several suppliers (e.g. dose chambers). Finally, none of the respondents to the market investigation (including [customer information]) expressed concerns in relation to the above products.
8 Form CO, paras. 74-76 and 107-109. Imaging equipment is usually sold together with an image processing software, which is used to record, display and manipulate the images acquired through the imaging equipment in order to help the physician visualizing, reading and interpreting these images. Siemens’ image processing software - syngo.via - is usually incorporated as an add-on to its scanners. Syngo.via has an extension called syngo.via RT Image Suite (“RTIS”) which incorporates a number of functionalities specific to radiotherapy simulation.
9 See cases M.2256 – Philips/Agilent Health Care Solutions, decision dated 2 March 2001; M.2537 – Philips/Marconi Medical Systems, decision dated 17 January 2001; M.3083 – GE/Instrumentarium, decision dated 2 September 2003; and M.3304 – GE/Amersham, decision dated 21 January 2004.
10 M.2537 – Philips / Marconi Medical Systems, decision dated 17 January 2001 paras. 8-10, 18 and 20.
11 M.2537 – Philips / Marconi Medical Systems, decision dated 17 January 2001 paras. 11-14 and 20.
12 M.2537 – Philips / Marconi Medical Systems, decision dated 17 January 2001 paras. 19-20.
13 M.2537 – Philips / Marconi Medical Systems, decision dated 17 January 2001 paras. 15-17.
14 Form CO, paras. 179 and ff.
15 Form CO, paras. 195, 210 and 222.
16 Question 4 of questionnaire Q1 to competitors and question 4 of questionnaire Q2 to customers.
17 Question 4 of questionnaire Q1 to competitors (emphasis added). Similarly, another a competitor indicated that “each of the three imaging modalities serve specific needs, which are dependent on the type of diagnosis to be performed, the tissue (soft tissue, bone) and diagnostic location” (emphasis added).
18 Question 4 of questionnaire Q2 to customers.
19 In the EEA/UK, in 2019, Siemens’ average sales prices are EUR […] for CT scanners, EUR […] for MRI scanners and EUR […] for PET/CT scanners (see Form CO, Annex 8.3(a)).
20 For example, based on the Parties’ estimates (in the EEA/UK, over the 2017-2019 period), the number and identity of the main players (with market shares above 10%) differ depending on the type of Scanners: (i) there are four main players for the supply of CT scanners (namely Siemens ([40-50]%), GE ([20- 30]%), Canon ([10-20]%) and Philips ([10-20]%)), whereas (ii) the supply of PET/CT scanners is characterised by a quasi-duopoly between Siemens ([40-50]%) and GE ([40-50]%) and (iii) the supply of MRI scanners is characterised by three main players (namely Siemens ([50-60]%), GE ([20-30]%) and Philips ([20-30]%)).
21 Questions 5-6 of questionnaire Q1 to competitors and questions 5-6 of questionnaire Q2 to customers.
22 Question 5.1 of questionnaire Q2 to customers.
23 For example, a customer stated: “the difference between a standard CT scanner and CT simulator is largely down to the additional peripherals purchased. The basic equipment is now the same (although you would normally choose a large bore scanner for the CT simulator)” (Question 6.1 of questionnaire Q2 to customers).
24 Question 6.1 of questionnaire Q2 to customers.
25 The Parties were unable to provide market share estimates for MRI simulators and PET/CT simulators.
26 Questions 14 and 15.1-15.2 of questionnaire Q1 to competitors and questions 13 and 14.1-14.2 of questionnaire Q2 to customers.
27 Questions 6 and 6.1 of questionnaire Q1 to competitors and questions 6 and 6.1 of questionnaire Q2 to customers.
28 Response to RFI 5, Annex 1.1 and response to RFI 3, Annex 10.
29 Response to RFI 5, Annex 1.1, p.7.
30 Form CO, paras. 252 and ff.
31 Form CO, paras. 252 and ff.
32 Question 9 of questionnaire Q1 to competitors and question 8 of questionnaire Q2 to customers.
33 Question 8.1 of questionnaire Q2 to customers.
34 Question 8.1 of questionnaire Q2 to customers.
35 Question 9.1 of questionnaire Q1 to competitors and question 8.1 of questionnaire Q2 to customers. In that respect, one customer indicated that “[p]roton therapy is much more expensive (both price and cost of utilisation), but more comparable with Linacs in medical procedures”. Similarly, competitors noted that “[l]inacs and Proton have similar applications, but there is a big difference in price point” and that “[p]roton is significantly more expensive (30 to 50x higher) compared to LINAC, but is highly precise, highly conformal, has low entrance dose and zero exit dose and low normal tissue dose compared to LINAC”.
36 In the EEA/UK, in 2019, Varian’s average sales prices are EUR […] for Linacs, EUR […] for proton therapy and EUR […] for brachytherapy (see Form CO, Annex 8.3(b)).
37 For example, based on the Parties’ estimates (in the EEA/UK, over the 2017-2019 period), the number and identity of the main players (with market shares above [10-20]%) differ depending on the type of radiotherapy equipment: (i) there are three main players for the supply of Linacs (namely Varian ([40- 50]%), Elekta ([30-40]%), and Accuray ([10-20]%)), whereas (ii) the main players in the supply of proton therapy are Varian ([40-50]%), IBA ([40-50]%) and Hitachi ([10-20]%), and (iii) the supply of brachytherapy is characterised by a quasi-duopoly between Eleka ([70-80]%) and Varian ([10-20]%).
38 See e.g. response to RFI 1, Annex 5.4.-2, dated 13 March 2020, pp. 8 and 33; and Annex 5.4-30, dated June 2020, p.18. See also response to RFI 3, Annex 11, pp. 9-12.
39 See e.g., Grand View Research, Radiation Oncology: Market estimates & trend analysis from 2020 to 2027”, 2020; and MEDraysintel, “Proton Therapy, world market report & Directory”, 2019.
40 For completeness, the Notifying Party submits that Varian’s oncology information and software solutions offering includes also software solutions with three main applications: (i) care management (these are new products that are still in a nascent phase), (ii) quality assurance, and (iii) analytics. Varian’s offering also includes Velocity, an intelligence platform that creates a map of imaging and treatment information, integrating it into a comprehensive dashboard. Siemens Healthineers does not offer care management, quality assurance, or analytics software that could be considered to compete with Varian’s oncology software. However, Varian’s sales of these solutions in the EEA/UK are negligible (amounting to less than EUR […] in 2019) and, in any case, these software solutions are not relevant to the Commission’s analysis of the conglomerate effects arising as a result of this Transaction.
41 Specifically concerning healthcare software, see case M.6237, Computer Sciences Corporation/iSoft Group, decision dated 20 June 2011.
42 Case M.6237, Computer Sciences Corporation/iSoft Group, decision dated 20 June 2011, para. 26.
43 With respect to TPS, the Notifying Party notes that several national competition authorities have examined concentrations in the radiotherapy solutions industry in the EEA/UK, including the market for TPS. In particular, the Notifying Party submits that (i) the UK Office of Fair Trading and the Portuguese Competition Authority considered that TPS for EBRT and TPS for brachytherapy constitute separate product markets due to the fact that TPS are designed to be specific to one type of radiotherapy (decisions of the UK Office of Fair Trading, acquisition by Elekta AB of Nucletron BV ME/5118/11; and of the Portuguese Competition Authority, Elekta / Nucletron - Ccent. 24/2011); and that (ii) the Polish Competition Authority concluded that brachytherapy equipment and TPS for brachytherapy were part of the same market on the basis that competitors’ brachytherapy software offerings were not compatible with equipment from other manufacturers (decision of the Polish Competition Authority, Elekta AB/RTA VC - DKK2-421/42/14/DL, Varian Medical Systems International AG/VRT Polska - DKK-113/2016).
44 Form CO, para. 262.
45 Form CO, paras. 265-266.
46 Form CO, para. 272-275.
47 Questions 12 and 12.1 of questionnaire Q1 to competitors and questions 11-11.2 of questionnaire Q2 to customers.
48 Questions 12 and 12.1 of questionnaire Q1 to competitors and questions 11-11.3 of questionnaire Q2 to customers.
49 Question 11 of questionnaire Q1 to competitors and question 10 of questionnaire Q2 to customers.
50 Question 10.1 of questionnaire Q2 to customers.
51 Question 12 of questionnaire Q2 to customers.
52 Question 12.2 of questionnaire Q2 to customers.
53 Question 12.2 of questionnaire Q2 to customers.
54 Questions 13, 13.2 and 13.2.1 of questionnaire Q1 to competitors
55 Question 9.1 of questionnaire Q2 to customers.
56 Question 15.5 of questionnaire Q1 to competitors and Question 14.6 of questionnaire Q2 to customers.
57 Form CO, paras. 358, 368, 378 and 399 and tables 19-24, 29 and 30.
58 Form CO, para. 462.
59 Form CO, paras. 463-464. In fact, Varian’s Imaging Motion Management devices (namely RGSC and Identify CT) and Treatment Motion Management devices (namely RPM, Identify and RMM) are distinct products.
60 Form CO, para. 466.
61 Questions 15.7 and 15.7.1 of questionnaire Q1 to competitors and questions 14.7 and 14.7.1 of questionnaire Q2 to customers.
62 Questions 15.7 and 15.7.1 of questionnaire Q1 to competitors and questions 14.7 and 14.7.1 of questionnaire Q2 to customers.
63 Cases M.2537 – Philips/Marconi Medical Systems, decision dated 17 October 2001, para. 24; and M.3304 – GE/Amersham, decision dated 21 January 2004, para. 17.
64 See e.g. cases M.8941 – EQT/Widex/JV, decision dated 13 February 2019, para. 62; and M.3687 - Johnson & Johnson/Guidant, decision dated 25 August 2005, para. 69.
65 Form CO, paras. 229 and 257.
66 Council Directive 93/42/EEC of 14 June 1993 concerning medical devices, OJ L 169 of 12 July 1993 as amended.
67 Form CO, paras. 230 and 257.
68 Directive 2014/24/EU of the European Parliament and of the Council of 26 February 2014 on public procurement and repealing Directive 2004/18/EC.
69 Form CO, para. 230.
70 Form CO, para. 230.
71 See e.g. Non-confidential minutes of the calls (i) with customers dated 10 November 2020, 12 November 2020 and 1 December 2020; and (ii) with competitors dated 3 November 2020, 17 November 2020 and 18 November 2020.
72 Questions 29-31 of questionnaire Q1 to competitors and questions 25-26 of questionnaire Q2 to customers
73 See e.g. Non-confidential minutes of the calls (i) with customers dated 10 November 2020, 12 November 2020, 1 December 2020 and (ii) with competitors dated 3 November 2020, 17 November 2020 and 18 November 2020.
74 Questions 14.1-14.1.1 and 14.7-14.7.1 of questionnaire Q2 to customers.
75 Form CO, paras. 634-637.
76 Response to RFI 5, Annex 1.1; response to RFI 3, Annex 10; and Form CO, Annexes 5.4(a)(43), 5.4(b)(7), 8.3(a), 8.3(b) and 8.4.
77 See e.g. COMP/M.6237 – Computer Sciences Corporation/iSOFT Group, decision of dated 20 June 2011, para. 32.
78 Form CO, para. 269.
79 Form CO, para. 280.
80 See e.g. Question 15 of questionnaire Q1 to competitors and Question 14 of questionnaire Q2 to customers; Form CO, paras. 634-637; Non-confidential minutes of the calls (i) with customers dated 10 November 2020, 12 November 2020 and 1 December 2020; and (ii) with competitors dated 3 November 2020, 17 November 2020 and 18 November 2020.
81 Commission Guidelines on the assessment of non-horizontal mergers under the Merger Regulation, OJ C 265, 18.10.2008, p. 6 (“Non-Horizontal Merger Guidelines”), para. 91.
82 Non-Horizontal Merger Guidelines, para. 92
83 Non-Horizontal Merger Guidelines, para. 93.
84 Non-Horizontal Merger Guidelines, paras. 95 -97.
85 Non-Horizontal Merger Guidelines, para. 96.
86 Non-Horizontal Merger Guidelines, para. 97.
87 Non-Horizontal Merger Guidelines, para. 93.
88 Non-Horizontal Merger Guidelines, paras. 95104.
89 Non-Horizontal Merger Guidelines, paras. 105-110.
90 Non-Horizontal Merger Guidelines, paras. 111-118.
91 More specifically mixed bundling (see para. (73) above).
92 This is notably illustrated by the fact that, when answering to the questions of the Commission related to the assessment of the conglomerate effect resulting from the Transaction (see e.g. Sections E, F and G of questionnaires Q1 to competitors and Q2 to customers), market participants generally refer to “imaging" and “radiotherapy” products, rather than a specific type of equipment/solution.
93 Response to RFI 2, question 7(a). This is notably corroborated by Varian's bidding database, which shows that [Varian's bidding information) (see Response to RFI 1, Annex 25.2).
94 See footnote 95 below.
95 Market share estimates are only presented at EEA/UK level in this Decision. National-level market shares are not presented in this Decision for the following reasons. At national level, market shares for each product are volatile, due to the bidding nature of the markets and the lumpy demand, and, therefore, unlikely to give reliable indications of the Parties' market power. Moreover, as explained above in para. (80), the main characteristics of the supply and demand in the relevant imaging and radiotherapy markets/segments do not appear to vary significantly across countries. However, for the sake of completeness, the Commission notes that, in 2019 (by order intake), (i) there were a large number of affected markets at national level with significant market shares above 30% (for instance, there were 23 EEA/UK countries in which Siemens' market share for CT scanners exceeded 30% and 23 EEA/UK countries in which Varian's market share for Linacs exceeded 30%) and (ii) the Parties even had a monopoly in several national markets, namely the markets for CT simulators in Belgium, Bulgaria, Hungary and Luxembourg (Siemens), the markets for CT scanners, MRI scanners and PET/CT scanners in Cyprus (Siemens) and the market for proton therapy equipment in Norway (Varian) (Form Co, Annexes 6.22 and 6.25).
96 The Parties were unable to provide similar market share estimates for MRI and PET/CT simulators.
97 The Parties did not provide market share estimates for a broader TPS market encompassing both TPS for EBRT and TPS for brachytherapy.
98 The Parties were unable to provide market share estimates for Imaging Motion Management devices.
99 Grand View Research, "Radiation Oncology- Market Estimates & Trend Analysis from 2020 to 2027" (2020) and Frost & Sullivan, "Siemens Healthineers' acquisition of Varian- Implications for the industry at large" (2020).
100 Grand View Research 2020. This is also corroborated by an internal presentation of Siemens [...].
101 Frost & Sullivan 2020.
102 Question 15 of questionnaire Q1 to competitors and question 14 of questionnaire Q2 to customers.
103 Question 15 of questionnaire Q1 to competitors and question 14 of questionnaire Q2 to customers.
104 Form CO, footnote 290.
105 Question 15 of questionnaire Q1 to competitors and question 14 of questionnaire Q2 to customers.
106 Form CO, paras. 557-600; RBB Economics’ report on conglomerate effects dated 23.11.2020 (the “RBB Report”), Section 5; the Supplementary Submission dated 24 January 2021; and the reply to RFI 7 dated 29 January 2021.
107 The Notifying Party also explained that imaging equipment used for radiotherapy simulation directly interact only with OIS, TPS and Imaging Motion Management devices. There is no direct data flow between Siemens’ Simulators and Varian’s radiotherapy equipment (such as Linacs), the relevant images and data being transferred from the Simulators to the radiotherapy equipment, through the OIS.
108 To the exception of Imaging Motion Management devices, which require a specific interface to interact with Simulators.
109 Non-Horizontal Merger Guidelines, para. 99.
110 See Table 1 above.
111 The Parties were unable to provide market share estimates for MRI and PET/CT simulators.
112 Question 15 of questionnaire Q1 to competitors and question 14 of questionnaire Q2 to customers.
113 See response to RFI 5, Annex 1.1, slides1 and 7. See also response to RFI 3, Annex 10.
114 Based on Frost & Sullivan, 2020. [50-60]% based on the Parties’ estimates. See also an internal presentation of Siemens assessing the Transaction stating: […].
115 Question 15 of questionnaire Q1 to competitors and question 14 of questionnaire Q2 to customers.
116 Based on Grand View Research, 2019. [30-40]% based on the Parties’ estimates.
117 See an internal presentation of the Parties stating that “[commercial strategy information]” (response to RFI 1, Annex 5.4.4, p.7).
118 Question 15 of questionnaire Q1 to competitors and question 14 of questionnaire Q2 to customers. Moreover, as explained above in para. (82), independent industry reports reveal that the Parties underestimate Varian’s EEA/UK market shares in other (closely related) radiotherapy markets (i.e. Linacs and OIS) (no independent industry report was provided to the Commission in relation to TPS).
119 Form CO, footnote 290.
120 Question 15 of questionnaire Q1 to competitors and question 14 of questionnaire Q2 to customers.
121 Case T-221/95, Endemol, para. 234 and Case T-102/96, Gencor, para. 205. See also Horizontal Merger Guidelines, para. 17.
122 Questions 34 and 35 of questionnaire Q1 to competitors. See also questions 14 and 15 of questionnaire Q2 to customers.
123 Form CO, Annex 5.4(b)-8, p. 6.
124 Question 17.3 of questionnaire Q1 to competitors.
125 The feedback received from the market suggests that the number of competitive suppliers is more limited in the CT simulator segment where Canon is not perceived as competitive (question 15 of questionnaire Q1 to competitors and question 14 of questionnaire Q2 to customers).
126 Question 18.2 of questionnaire Q1 to competitors and question 16.2 of questionnaire Q2 to customers. Conversely, most respondents consider that switching suppliers of imaging equipment is easy or not difficult (Questions 18.2 of questionnaire Q1 to competitors and 16.2 of questionnaire Q2 to customers).
127 Questions 17.2-17.4 of questionnaire Q2 to customers.
128 Non-Horizontal Merger Guidelines, para. 100.
129 Response to RFI 5, Annex 1.1 (Siemens), pp. 1 and 4.
130 Response to RFI 5, Annex 1.2 (Siemens), pp. 5 and 33. See also response to RFI 3, Annex 11 (Varian), p.3.
131 See response to RFI 1, Annex 5.4.-2 (dated 13 March 2020), slide 33 […].
132 See RBB Report, p.12.
133 Question 21 of questionnaire Q2 to customers (e.g. “great importance to avoid additional risk of mistakes and better manage patients and equipment”; “good interoperability is key to the provision of a safe, efficient, and innovative clinical service”).
134 […]
135 Question 21 of questionnaire Q1 to competitors. See also question 19 of questionnaire Q2 to customers where virtually all customers confirmed that they would not consider procuring Simulators and Radiotherapy Solutions that are not DICOM-compliant.
136 Question 20 of questionnaire Q1 to competitors and question 18 of questionnaire Q2 to customers.
137 DICOM PS3.1 2020e, Sect. 1.2: http://dicom nema.org/medical/dicom/current/output/html/part01 html#sect 1.2
138 See response to RFI 3, question 1(e) and Annex 5.
139 Question 22 of questionnaire Q1 to competitors (e.g. “The implementation and testing & validation of the standards between vendors […] require the willingness of both parties to collaborate”).
140 See e.g. response to RFI 3, Annexes 3 and 4 (exchange of technical information between Siemens and RaySearch to allow RaySearch’s TPS to fully display a new type of images produced by Siemens’ CT simulators).
141 Questions 23-25 of questionnaire Q1 to competitors.
142 Question 24.3 of questionnaire Q1 to competitors. Customer also confirmed that they need assistance from suppliers (on the ground) to achieve interoperability between Simulators and Radiotherapy Solutions (question 22 of questionnaire Q2 to customers).
143 Question 26 of questionnaire Q1 to competitors.
144 Question 26 of questionnaire Q1 to competitors.
145 Question 22 of questionnaire Q1 to competitors.
146 […]
147 Response to RFI 3, Annex 11.
148 Response to RFI 1, Table 32.
149 Question 28 of questionnaire Q1 to competitors and question 24 of questionnaire Q2 to customers.
150 Non-Horizontal Merger Guidelines, para. 103.
151 Question 24 of questionnaire Q1 to competitors.
152 See e.g. RBB Report, p.51: “a reduction of interoperability with competitor equipment would require the Parties to make their equipment incompatible with DICOM”.
153 Questions 24.1, 24.3 and 26.1 of questionnaire Q1 to competitors.
154 See para. (94) above.
155 See para. (79) above and footnote 158 below
156 Form CO, para. 473. See also, para. 471 of the Form CO: [pricing information].
157 Question 28 of question Q1 to competitors and question 24 of questionnaire Q2 to customers.
158 Many market participants also expressed concerns in relation to a potential of degradation of the interoperability between Varian’s and its competitors’ Radiotherapy Solutions (e.g. Linacs, OIS, TPS). However, pre-Transaction, Siemens is not active on these markets. Therefore, the Commission considers that the above a claim is not merger specific. For the sake of clarity, the outcome of the present assessment under the Merger Regulation is without prejudice to the application of other competition rules, including in particular Article 102 TFEU.
159 Question 24 of questionnaire Q2 to customers.
160 Non-Horizontal Merger Guidelines, para.109.
161 Question 24.4 of questionnaire Q1 to competitors and question 21 of questionnaire Q2 to customers.
162 See Section 5.5.2 above.
163 Form CO, footnote 290 (emphasis added).
164 […]. See response to RFI 7, question 2.
165 Taking only into consideration Imaging Motion Management devices (i.e. excluding Treatment Motion Management devices, which are not relevant to assess the foreclosure resulting from the merger). See response to RFI 7, Table 2.
166 Questions 28, 35 and 37 of questionnaire Q1 to competitors.
167 Questions 24, 27 and 29 of questionnaire Q2 to competitors.
168 Question 18 of question Q2 to customers.
169 See para. (97) above.
170 See para. (106) above.
171 Non-Horizontal Merger Guidelines, para.115.
172 In this section, the risk of commercial tying and commercial bundling (pure and mixed bundling) are discussed together and referred to as “bundling” for convenience, as the same considerations largely apply in all instances.
173 Form CO, paras. 500-515 and 525-533.
174 […]
175 Form CO, paras. 516 and 534-535.
176 This argument also applies to other imaging and radiotherapy equipment.
177 RBB Report.
178 RBB Report.
179 See Section 5.5.2 above.
180 Non-Horizontal Merger Guidelines, para.100.
181 See para. (95) above.
182 Question 26.2.1 of questionnaire Q2 to customers.
183 Question 26.2.1 of questionnaire Q2 to customers.
184 Form CO, para. 154
185 Question 32 of questionnaire Q1 to competitors and Question 26 of questionnaire Q2 to customers.
186 Question 30.1 of questionnaire Q1 to competitors.
187 Question 26.2.1 of questionnaire Q2 to customers. In that respect, it should be noted that the EU rules on public procurement provide that “award criteria […] should remain stable throughout the entire procedure, and should not be subject to negotiations, in order to guarantee equal treatment of all economic operators”.
188 Question 26.2.1 of questionnaire Q2 to customers.
189 Question 26.2.1 of questionnaire Q2 to customers.
190 Form CO, para. 506.
191 Form CO, para. 512 and Response to question 1 of RFI 7.
192 See above para. (82) and footnote 114.
193 According to the Parties’ estimates, over the period 2015-2019, in […] (i.e. the Parties’ main national markets in the EEA/UK), (i) only […]% of the customers procuring a Varian’s Linac also procured a CT scanner (of any supplier) in the same year and (ii) […]% of the customers procuring a Siemens’ CT scanner also procured a Linac (of any supplier) in the same year (see Form CO, Tables 34 and 37 and RBB Report).
194 Question 30.2 of questionnaire Q1 to competitors.
195 Form CO, para. 468.
196 Form CO, para. 468.
197 Question 21.4.2 of questionnaire Q2 to customers. Emphasis added. This is reinforced by other respondents, who explain the benefits of procuring Motion Management Devices from third-party suppliers: “While 10 years ago the linac vendors provided the better motion management systems, today mainly 3rd party products are mo[re] competitive” and another one added “companies concentrating to develop motion management systems [e.g. VisionRT, C-Rad, Brainlab] seem to develop [the] best products” (Question 14.7.2 of questionnaire Q2 to customers).
198 Non-Horizontal Merger Guidelines, para.103.
199 In that respect, it should be noted that Siemens and Varian already pre-Transaction are entered into a strategic partnership for imaging and radiotherapy solutions, including sale cooperation (see para. (95) above).
200 Form CO, para. 516. See also “Elekta AB CEO Gustaf Salford on Q1 2021 Results – Earnings Call Transcript” available at https://seekingalpha.com/amp/article/4370931-elekta-ab-publ-ektaf-ceo-gustaf- salford-on-q1-2021-results-earnings-call-transcript.
201 RBB Report. In the report, it is explained that the average absolute margin realised by Varian on a Linac is EUR […] in the EEA/UK (EUR […] in globally), which [pricing information]. Based on the public information available, competitors’ margins should be of the same level.
202 Response to RFI 1, Tables 5 and 6.
203 Question 29.1 of questionnaire Q2 to customers.
204 These limited opportunities may explain why, pre-Transaction, (i) Varian does not bundle its various radiotherapy solutions and (ii) Siemens does not bundle its various Simulators.
205 Question 34 of questionnaire Q1 to competitors.
206 Non-Horizontal Merger Guidelines, paras.93 and 111.
207 See para. (125) above.
208 Question 29 of questionnaire Q2 to customers.
209 Question 27.7.1 of questionnaire Q2 to customers.
210 Question 29.1 of questionnaire Q2 to customers.
211 Question 27 of questionnaire Q2 to customers.
212 Question 30 of questionnaire Q2 to customers.
213 Question 30 of questionnaire Q2 to customers.
214 For the sake of clarity, the outcome of the present assessment under the Merger Regulation is without prejudice to the application of other competition rules, including in particular Article 102 TFEU.
215 OJ C 267, 22.10.2008, p. 1.
216 Paragraph 9 of the Remedies Notice.
217 Paragraph 12 of the Remedies Notice.
218 Paragraph 13 of the Remedies Notice.
219 Paragraph 14 of the Remedies Notice.
220 Including CT simulators, MRI simulators and PET/Simulators, as well as the image processing software and other add-on options of features to these products.
221 See footnote 107 above.
222 Paragraph 9 of the Remedies Notice.
223 Questions 10-12 of questionnaire R1 to competitors.
224 Questions 4 of questionnaire R1 to competitors.
225 Questions 11-12 of questionnaire R1 to competitors. In addition, some respondents noted that the First Commitments would not address the risk that, post-Transaction, the combined entity may seek to degrade interoperability between its radiotherapy equipment (such as Linacs) and third parties’ OIS/TPS, or vice versa. A similar concern was raised regarding the risk that the combined entity may seek to degrade interoperability between its Radiotherapy Solutions and third parties’ Treatment Motion Management devices. However, as previously explained, these concerns do not appear to be merger-specific (see
footnote 158 above). Accordingly, these concerns do not affect the efficacy of the First Commitments to eliminate the serious doubts identified as arising from the Transaction.
226 Questions 2 and 5 of questionnaire R1 to competitors.
227 Question 9 of questionnaire R1 to competitors.
228 Question 9.1 of questionnaire R1 to competitors.
229 Question 5 of questionnaire R1 to competitors.
230 Questions 5-6 of questionnaire R1 to competitors.
231 Question 6 of questionnaire R1 to competitors.
232 Question 6 of questionnaire R1 to competitors.
233 Question 4 of questionnaire R1 to competitors. As one respondent explained: “Further interoperability must also be ensured between Medical Imaging Solutions and radiotherapy hardware, as OIS/TPS software is not necessarily needed to link these two stages of the oncology treatment process. For example, the already available Siemens Healthineers syngo Via RT Image Suite provides for functionalities such as contouring, dose accumulation analysis and [radiotherapy] workflow integration management, which traditionally has been included in TPS/OIS. [COMPANY] expects that this will further be developed towards an integrated software solution combining Imaging software with TPS and potentially OIS”.
234 Question 8 of questionnaire R1 to competitors.
235 Questions 3 and 12 of questionnaire R1 to competitors. For completeness, two respondents suggested that the time period for the fast track dispute resolution could be shortened to expedite the process. However, this concern was not raised by other respondents, who did not identify any need for additional protections for third party suppliers as part of the Commitments; accordingly, no amendment is considered necessary.
236 The Final Commitments also included certain clarificatory modifications that were not of major significance and so are not discussed in this decision.
237 See recital 31 of the Merger Regulation and para. 20 of the Remedies Notice