Commission, February 11, 2021, No M.9686
EUROPEAN COMMISSION
Decision
MITSUI / BELCHIM CROP PROTECTION
Subject: Case M.9686 – Mitsui/Belchim Crop Protection
Commission decision pursuant to Article 6(1)(b) in conjunction with Article 6(2) of Council Regulation No 139/20041 2 and Article 57 of the Agreement on the European Economic Area3
Dear Sir or Madam,
(1) On 15.12.2020, the European Commission received notification of a proposed concentration pursuant to Article 4 of the Merger Regulation by which Mitsui & Co Ltd (‘Mitsui’, Japan) intends to acquire sole control within the meaning of Article 3(1)(b) of the Merger Regulation over Belchim Crop Protection NV (‘Belchim’ or the ‘Target’, Belgium) by way of purchase of shares (the ‘Transaction’).4 Mitsui is referred to hereinafter as the ‘Notifying Party’ and together with Belchim as the ‘Parties’. The undertaking that would result from the Transaction is referred to as the ‘Merged Entity’.
1. THE PARTIES
(2) Mitsui is a Japanese general trading company engaged in business activities covering several industries, such as steel, non-ferrous metals, machinery, electronics and chemicals, including chemical products for crop protection. In the EEA, Mitsui is active in crop protection through its subsidiary Mitsui AgriScience International SA NV (‘MASI’), which holds controlling interests in Dutch-based Certis Europe BV (‘Certis’) and German-based Spiess-Urania Chemical GmbH (‘Spiess-Urania’).
(3) Certis is active in the distribution of third-party and own formulated products for crop protection. Spiess-Urania mainly develops and manufactures copper-based active ingredients (‘AI’) and formulated products and is not active at the distribution level.
(4) Belchim is a Belgian-based company which, similarly to Certis, is active both in the distribution of third-party crop protection products and in the supply of own formulated products under proprietary brands, based on generic (off-patent) AIs, licensed AIs or own proprietary AIs. In the EEA, Belchim operates under its own name and those of its subsidiaries Orchem and Nordisk Alkali.
(5) Both Certis and Belchim specialise in crop protection products for smaller, high- value crops such as vines and potatoes, for which they have portfolios of products targeting some of the main pests and diseases. Neither have integrated R&D capabilities, but they are both known for introducing innovative products from their Japanese R&D partners to the European market and for formulating products based on Japanese-developed AIs. Both Certis and Belchim have Japanese (minority) shareholders active in crop protection development: Nisso and Kumiai for Certis and ISK for Belchim. Neither Certis nor Belchim has production capabilities for their own formulated products, which are produced by third parties under toll- manufacturing agreements. Certis’ sister company Spiess-Urania manufactures copper-based AIs and formulated products, in particular biocides for wood protection and fungicides for vines and potatoes.
2. THE OPERATION
(6) The proposed Transaction will be implemented pursuant to a sale and purchase agreement under which Mitsui, through its wholly owned subsidiary MASI, will acquire a controlling interest5 of 30% of the shares in Belchim followed by a second acquisition of a further 32% of the shares in Belchim.6 The Transaction value will not exceed EUR […].
3. EU DIMENSION
(7) The undertakings concerned have a combined aggregate world-wide turnover of more than EUR 5 000 million7 [Mitsui: […], Belchim: […]. Each of them has an EU-wide turnover in excess of EUR 250 million [Mitsui: […], Belchim: […] and they do not achieve more than two-thirds of their aggregate EU-wide turnover within one and the same Member State. The notified operation therefore has a Union dimension pursuant to Article 1(2) of the Merger Regulation.
4. INTRODUCTION TO CROP PROTECTION PRODUCTS
(8) This case concerns primarily the supply of formulated crop protection products. Crop protection products are designed to protect crops against different forms of damage caused by insects, weeds, fungi or other pests, such as nematodes.8
(9) The lifecycle of crop protection products involves several stages: (i) the discovery and development by companies active in R&D of new AIs, where these companies will apply for patents to protect the AIs they have discovered; (ii) the development of formulated products composed of one or more AI with inactive ingredients such as solvents, fillers or adjuvants, which includes field testing, and the manufacture thereof; (iii) the regulatory approvals and authorisations, where the AI is approved at the EU level by the Commission and the formulated product is approved at the Member state level by the relevant authority; and finally, (iv) the commercialisation of these formulated products to farmers and other customers.9
(10) Neither party has a research organisation engaged in the discovery and development of new AIs but they both formulate products based on AIs developed by third parties, in particular Japanese R&D partners. The Parties also supply third-party formulated products on the market, acting as wholesale distributors for these products. For the affected markets that arise in this Transaction, this distribution tends to be on an exclusive basis, either contractually or de facto.10
(11) Formulated crop protection products can be divided broadly into the main categories of herbicides (targeting weeds), insecticides (targeting insects) and fungicides (targeting diseases). Further, there are other crop protection products such as molluscicides (targeting slugs, snails and other types of molluscs), desiccants and plant growth regulators (‘PGRs’).
5. PRODUCT MARKET DEFINITION
5.1. Commission’s precedents
(12) In past cases, the Commission found that the relevant product markets for formulated crop protection products can be segmented on the basis of the pest(s) targeted and then by crop where each such combination constitutes a separate relevant product market.11 This applies to fungicides,12 herbicides,13 insecticides14 (including acaricides15), PGRs16 and seed treatment.17
(13) For fungicides, the Commission has previously taken the view that each crop/disease combination constitutes a separate relevant market.18 For herbicides, further distinctions have been made depending on the time of application of the relevant crop protection products. On this basis, the Commission found that the relevant product markets for herbicides can be segmented by crop, weeds targeted and timing of their application. For insecticides, it found a separate product market for each type of crop and per pest.19 It also considered relevant a distinction per method of application (i.e. foliar or soil), which is applicable for both insecticides and fungicides.20 For seed treatment, the Commission further segmented the market for each crop by indication.21
(14) As for PGRs, the Commission has previously taken the view that the relevant product markets can be segmented by individual crop.22 In UPL / Arysta Lifescience, the Commission in particular assessed markets for potato PGRs23, where it did not consider any further segmentations, such as those based on time of application.24
(15) Further, the Commission considered that molluscicides25 constitute a distinct product market.
(16) The Commission also found in an earlier decision that soil fumigants constitute a distinct product market but did not envisage any further segmentation.26 However, in Dow/Dupont, the Commission considered soil fumigants as part of the nematicide market.27
(17) As regard desiccants and paraffinic oils for virus control, the Commission has not addressed these markets in previous cases in the context of the agrochemical products. However, it did address paraffinic oils in the context of electrical oils in Castrol/Carless/JV.28 On that occasion, the Commission came to the conclusion that there might be a separate product market for paraffinic oils, instead of those products belonging to a market including all types of electrical oils.
(18) Paraffinic oils create a physical barrier on the crop which prevents certain insects from transmitting the virus they carry by sucking the leaves. Paraffinic oils do not kill the virus and are ineffective once the virus is transmitted and goes on to infect the crop. Paraffinic oils are usually used on crops which have requirements of being ‘virus-free’, such as seed potatoes and flower bulbs.
5.2. Notifying Party’s views
(19) The Notifying Party generally agrees with the approach followed by the Commission in past cases. The Notifying Party has however submitted arguments relating to the market definition for paraffinic oils for virus control and for potato PGRs.
5.2.1. Paraffinic oils for virus control
(20) As regards paraffinic oils for virus control, the Notifying Party submits that this should be a separate market from insecticides.29 The Notifying Party considers that paraffinic oils and insecticides operate in different ways and are used for different purposes. According to the Notifying Party, paraffinic oils have a different mode of action to insecticides, as the former do not kill the pests, but are used to avoid that they transmit the virus they may carry.30
(21) Further, the Notifying Party does not consider vegetable oils as competing with mineral oils for virus control (such as paraffinic oils).31 While the Notifying Party agrees that vegetable oils can be used for virus control to a certain extent, it argues that vegetable oils have a much lower effectiveness and higher cost than paraffinic oils.32 Moreover, the Notifying Party submits that, to the best of its knowledge, vegetable oils have never been registered as crop protection products and, therefore, their use would require market operators to undergo the full registration procedure for each specific crop, which would take a minimum of five to seven years.33
5.2.2. Plant growth regulators
(22) Both Parties are active in the supply of PGRs for potatoes. As regards potatoes, the use of PGRs is crucial to inhibit or control sprouting during storage in order to prevent damage and the loss of product quality.
(23) The Notifying Party submits that, as a result of the disruption in the market for PGRs for potatoes following the non-renewal of chlorpropham (‘CIPC’) in 2020 (the main active ingredient used to prevent potato sprouting), the traditional market definition of PGRs for potatoes needs to be revised.
(24) The Notifying Party considers the following sub-segmentations to be appropriate:
(a) First, based on time of application, between pre- and post-harvest,
(b) Second, within post-harvest, between products that prevent sprouting and those that act on the sprouts once they have appeared (referred to as preventative and curative products, respectively). Further, within post-harvest, the Notifying Party argues that cold storage is a credible alternative to chemical products.34
(25) The Notifying Party submits that Belchim’s product 1,4 Sight is a purely preventive product, while Certis’ spearmint oil35 product Biox-M is mainly a curative product.
(26) The Notifying Party notes that Certis may initially have suggested both preventive and curative use of Biox-M, when the product was first launched. It acknowledges that Biox-M could be used preventively [Mitsui’s business secrets]. However, the Notifying Party claims that [Mitsui’s business secrets]growers are unlikely to turn to Biox-M for preventive use. The Notifying Party claims that treatment with Biox-M allows growers to “wait and see” until the potatoes actually require a treatment against sprouting and that Biox-M is only purchased and applied once sprouting starts. It refers to a Dutch Biox-M product publication from Certis, the German Biox-M registration report and a French farming trade publication to support its claim that Biox-M is mainly used curatively.
5.3. Commission’s assessment
(27) The outcome of the market investigation has confirmed the Commission’s precedents defining the relevant product market for crop protection products on the basis of crop/target pest combinations (e.g. for fungicides, herbicides36, and insecticides), while molluscicides constitute a single product market. Moreover, the outcome of the market investigation has also confirmed that as regards fungicides and insecticides, the markets should be further segmented according to the mode of application, distinguishing between foliar and soil applications. In addition, the outcome of the market investigation has confirmed that, as regards herbicides, the market should be further segmented according to the time of application and whether they are used for agricultural or non-agricultural applications.37
5.3.1. Paraffinic oils for virus control
(28) The results of the market investigation support the Notifying Party’s view that paraffinic oils for virus control constitute a separate product market from insecticides. The majority of competitors expressing an opinion consider that paraffinic oils for virus control should constitute a separate market.38 Customers point at these products’ particularities and the lack of alternative products.39 The results of the market investigation did not contradict the Notifying Party’s claim that vegetable oils are not close substitutes to paraffinic oils.
(29) The results of the market investigation are mixed as regards the appropriateness to further subsegment the market of paraffinic oils for virus control per product, that is, to consider as separate markets paraffinic oils for virus control in (i) seed potatoes and (ii) flower bulbs. While the majority of competitors support a further segmentation,40 customers consider that it is not appropriate.41
(30) The Commission considers this distinction relevant and considers the markets for paraffinic oils for virus control in (i) seed potatoes and (ii) flower bulbs as separate markets. This is because not all paraffinic oils for virus control are registered for use on both flower bulbs and seed potatoes.42 Competitive conditions appear to be different, as customers are faced with different alternative suppliers depending on whether they want to treat flower bulbs or seed potatoes, as can be seen in Table 3 and Table 4 below.
5.3.2. Plant growth regulators
(31) The results of the market investigation support a sub-segmentation of potato PGRs based on crop and on time of application between pre- and post-harvest potato PGRs, as suggested by the Notifying Party.
(32) A large majority of both customers43 and competitors44 expressing an opinion indicate that potato PGRs should be segmented between products used before harvesting and those used after harvesting.
(33) Respondents do not view pre-harvest and post-harvest products as mutually substitutable, but more as complementary products that will be part of a typical treatment programme following the ban on CIPC. A competitor commented: “Both solutions complement but cannot replace each other (without suggesting farmers have to apply both options; they could choose at the expense of efficacy to go with only one). Storage strategy for sprout inhibition starts in the field. Depending on the season, with the use of a field treatment, it is possible to start with lower dose rate of the post-harvest treatment.”45
(34) Several respondents explain that pre-harvest products are not a reliable stand-alone solution for long-term sprout suppression, in particular because the effective application of pre-harvest products depends on the right weather conditions and produces only a short-term effect during storage. A customer commented in relation to pre-harvest potato PGRs: “Insufficient effect for the usual storage time for potatoes. Also the relevant general conditions in the field (e.g. weather) are not known beforehand.”46
(35) As regards a sub-segmentation between post-harvest products that prevent sprouting and products that burn off sprouts (preventive versus curative products) suggested by the Notifying Party, this is not supported by the market investigation.
(36) A majority of both customers47 and competitors48 expressing an opinion do not consider that post-harvest products should be segmented between products used before sprouting and products used after sprouting.
(37) Several respondents comment that, in any case, all post-harvest products should be applied before sprouting in order to obtain good results and to avoid damage. The feedback received suggests that it is not a viable option to wait for sprouts to appear and then burn them off. A customer commented: “Sprout suppressants have to applied before appearance.”49 A competitor remarked: “It is strongly recommended to use the potato sprout suppressants before the sprouts appear, to avoid any damage”50, while another competitor stated: “Any later application would impact the quality of the potato.”51
(38) Several respondents comment that the market is divided between pre- and post- harvest products, with all post-harvest products competing against each other.52 In the same vein, a customer stated: “Basically, it is sufficient to distinguish between treatment in the warehouse and treatment in the field”.53
(39) The Notifying Party’s claim that products based on spearmint oil are used mainly curatively has not been confirmed by the Commission’s investigation.
(40) In the market investigation, customers indicated that spearmint can be used both preventively and curatively, while competitors considered that it can only be used preventively. A majority of customers54 expressing an opinion consider that spearmint oil can be applied both before and after sprouts appear, while among the other customers, the same number consider that it can only be used before sprouting compared to only after sprouting. A majority of competitors55 expressing an opinion consider that spearmint oil can only be used before sprouting.
(41) As regards Certis’ Biox-M product in particular, most publicly available documents from both Certis and third parties suggest that the product is mainly used preventively. Sources describing the product as curative only, such as the French trade journal referred to by the Notifying Party, seem to be the exception to the rule.
(42) The Biox-M product label for instance provides the following instructions for use: “Biox-M is a sprouts inhibitor extracted from spearmint. It is used to inhibit the germination of potatoes during storage. The treatment of potato tubers must be done before they begin to germinate, but after healing”. 56
(43) Contrary to what the Notifying Party claims, Biox-M product brochures are still promoting it as a mainly preventive product with additional curative action. The Biox-M brochure currently available on the Belgian Certis website57 describes the mode of action of spearmint oil as follows: “At low concentration, mint oil acts on the hormonal system of the potato which has the effect of slowing the growth of sprouts. At higher concentration, the product causes damage to the sprouting cell membranes eventually leading to the general necrosis of the sprout.” […] Keeping a low concentration of Biox-M® in the storage building allows good sprout control to be maintained. At low concentration, Biox-M® acts as a growth regulator”.
(44) The brochure recommends the preventive use of Biox-M, while describing the curative use as an added benefit: “It is recommended to use Biox-M® preventively but the product is also completely able to act curatively on the sprouting by applying a dose of 60-90 ml / tonne of stored potatoes. The necrosed sprouts will fall off during the processing and marketing stages of the potatoes.”
(45) The same product brochure includes a treatment timeline which shows that without the use of pre-harvest products, under both ambient and cold storage conditions, Biox-M is recommended to be used from October onwards (i.e. similarly to 1,4 Sight, soon after potatoes are put into storage).
(46) Similarly, the Dutch Biox-M product publication from Certis referred to by the Notifying Party repeatedly describes the curative application of the product at higher doses as a useful option for growers rather than as its main type of use.58
(47) In addition, various third-party sources indicate that Biox-M is mainly used preventively: Belgian agronomic consultancy Inagro for instance states in its latest document on potato sprout suppression: “The mode of action of mint oil is aimed at inhibiting the hormonal sprouting system (preventive) but it can also destroy sprouts at higher doses (curative)”. 59
(48) Finally, the Parties have provided no evidence to substantiate their claim that side- by-side trials have revealed that Belchim’s 1,4 Sight is a much better compound for preventive purposes and that growers are unlikely to turn to Biox-M for preventive use. By contrast, agronomic studies seem to indicate similar efficacy between both products when used preventively. For instance, according to a presentation by agronomic consultancy Inagro, comparative product trials have indicated that the preventive use of both products yields similar results: after long-term treatment (with repeat applications at a preventive low dose of 30 ml/tonne for Biox-M), sprout length measured was not very different between the Parties’ products, at around 3 mm on average for both 1,4 Sight and Biox-M in the month of June, depending on the potato variety.60
(49) The Commission therefore considers that even if the market for post-harvest potato PGRs were segmented between preventive and curative products, which is not supported by the Commission’s investigation, the Parties’ products would compete directly in the preventive segment.
(50) As regards the Notifying Party’s claim that cold storage solutions should be included in the post-harvest market, this is not supported by the results of the market investigation.
(51) A majority of competitors61 expressing an opinion indicate that cold storage is not a credible alternative, while the responses from customers62 were less conclusive: the highest number of respondents replied that cold storage is not an alternative, with fewer respondents replying that it can always be an alternative or that it can be an alternative in certain circumstances. An example of where cold storage can be an alternative was the short-term storage of freshly harvested potatoes.
(52) Several respondents mention that cold storage using an effective refrigeration system can slow down or reduce sprouting in potatoes, depending on the variety and size.63 However, several respondents also point out that cold storage is not an option for potatoes to be processed as fries or crisps, due to health hazards for consumers.64 They explain that such potatoes cannot be stored at low temperatures because this creates reducing sugars65 that produce discoloration and acrylamide66, a substance officially labelled as a carcinogen, when the potatoes are subsequently processed or prepared at high temperatures. A customer commented: “Cold storage is not suitable for storage of potatoes for processing (frying chips or crisps). Sprout suppressants must be used to prevent low temperature sweetening and darkening of the product with increased acrylamide content.”67
(53) A supplier commented: “Low temperatures reduce sprouts but increase Acrylamide precursors (reducing sugars). Use of low T would engender toxical dangers to consumers”68 and pointed out that the formation of acrylamide in cold-stored potatoes processed at high temperatures is currently being investigated by the European Food Safety Authority (EFSA).69 The information leaflet ‘Acrylamide in Food’ available on the EFSA website warns the general public against cold-storing potatoes: “Do not store potatoes in the refrigerator as this increases sugar levels (potentially increasing acrylamide production during cooking)”.70
(54) In addition, the Parties’ internal documents suggest that [confidential internal documents].
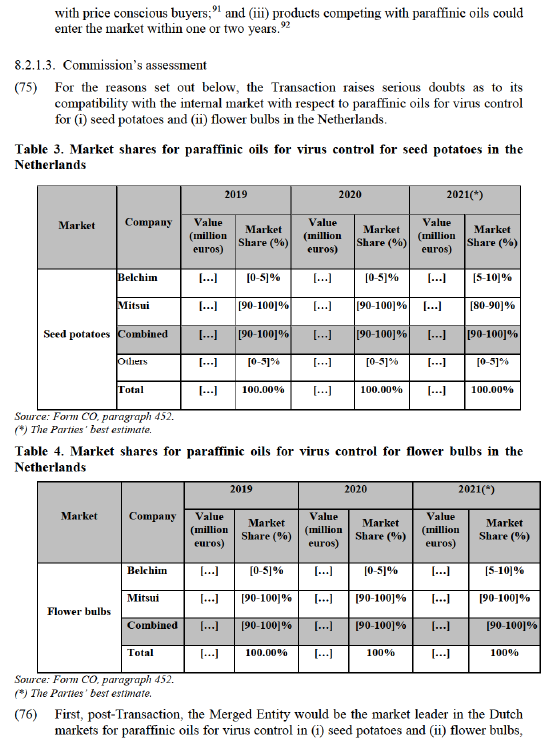
(55) In light of the results of its investigation, the Commission concludes that, for the purposes of assessing the Transaction, the relevant product market in relation to PGRs should be segmented between pre-harvest potato PGRs and post-harvest potato PGRs without the need for further sub-segmentations.
6. GEOGRAPHIC MARKET DEFINITION
(56) In previous decisions, the Commission has considered that the geographic market definition for crop protection products is national.71 This is because their authorisation is still regulated at the national level,72 each Member State remains responsible for maintaining specific national data requirements,73 distributors of crop protection products usually are adapted to varying demands of their customers in different Member States74 and pricing data indicated national differences in price levels and evolution for the same products.75
(57) The Notifying Party agrees with the Commission that the geographic market definition for crop protection products is national in scope.
(58) The vast majority of competitors and customers expressing an opinion agree with the Commission that the geographic market definition for crop protection products is national in scope.76 This includes the markets for fungicides, herbicides, insecticides, molluscicides, potato desiccants,77 paraffinic oils for virus control78 and PGRs for potatoes.
7. OVERVIEW OF THE OVERLAPS AND AFFECTED MARKETS
(59) Mitsui’s and Belchim’s activities in Europe mainly overlap in the supply of certain crop protection products for smaller crops such as potato, vines, fruits and vegetables as well as in flowers and ornamentals. For those crops, the Transaction gives rise to a number of nationally affected markets for the supply of fungicides, herbicides, Regulation (the ‘Horizontal Merger Guidelines’)80 distinguish between two main ways in which mergers may significantly impede effective competition, namely non- coordinated and coordinated effects.
(64) Non-coordinated effects may significantly impede effective competition by eliminating important competitive constraints on one or more firms, which consequently would have increased market power. The Horizontal Merger Guidelines consider not only the direct loss of competition between the merging firms, but also the reduction in competitive pressure on non-merging firms in the same market that could be brought about by the merger.81
(65) The Horizontal Merger Guidelines list a number of factors which may influence whether or not significant non-coordinated effects are likely to result from a merger, such as the large market shares of the merging firms, the fact that the merging firms are close competitors, the limited possibilities for customers to switch suppliers, or the fact that the merger would eliminate an important competitive force. That list of factors applies equally if a merger would create or strengthen a dominant position, or would otherwise significantly impede effective competition due to non-coordinated effects. Furthermore, not all of those factors need to be present to make significant non-coordinated effects likely and this is not an exhaustive list.82
(66) Furthermore, in accordance with the Horizontal Merger Guidelines, a merger with a potential competitor can have horizontal anti-competitive effects in two situations: (i) where the potential competitor constrains the behaviour of firms active in the market, notably when the potential competitor possesses assets that could easily be used to enter the market without incurring significant sunk costs or (ii) where the merging partner is very likely to incur the necessary sunk costs to enter the market in a relatively short period of time after which it would constrain the behaviour of firms currently active in the market.83
(67) For the merger to have significant anti-competitive effects, two basic conditions must be fulfilled. First, the potential competitor must already exert a significant constraining influence or there must be a significant likelihood that it would grow to become an effective competitive force. Evidence that a potential competitor has plans to enter a market in a significant way could help the Commission reach such a conclusion. Second, there must not be a sufficient number of other potential competitors, which could maintain sufficient competitive pressure after the merger.84
(68) In addition, when the Parties operate in closely related markets, a concentration may also give rise to conglomerate effects. The main concern regarding conglomerate effects is foreclosure. A concentration may lead to the foreclosure of rivals, by allowing the Merged Entity to leverage a strong market position from one market to another by means of tying, bundling or other exclusionary practices.85 The Commission appraises conglomerate effects in accordance with the guidance set out in the Non-Horizontal Merger Guidelines. In its assessment, the Commission examines whether the Merged Entity would have the (i) ability and (ii) incentive to foreclose rivals and (iii) whether such strategy would have a significant detrimental effect on competition.86
(69) In accordance with the legal framework set out above, the Commission has carried out an extensive competitive assessment. In the following sub-sections, the Commission (i) analyses the horizontally affected markets where the Commission considers that the Transaction would raise serious doubts in the absence of the remedies presented by the Notifying Party; and (ii) the markets where the Transaction does not raise serious doubts including horizontally affected markets and markets where conglomerate effects could rise.
8.2. Markets in which serious doubts arise
8.2.1. Paraffinic oils for virus control in (i) seed potatoes and (ii) flower bulbs in the Netherlands
(70) The Transaction would lead to the loss of potential competition in two markets in the Netherlands, those for paraffinic oils for virus control in (i) seed potatoes and (ii) flower bulbs.
8.2.1.1. Activities of the Parties
(71) Certis distributes paraffinic oils for virus control in the Netherlands, where its products are registered for seed potatoes and flower bulbs. Certis’ paraffinic oil for virus control products are sold under the brands Olie H and Kompaan.87
(72) Belchim intends to distribute Fibro in The Netherlands, a paraffinic oil product for virus control in seed potatoes and flower bulbs developed by Comptoir Commercial des Lubrifiants (‘CCL’), [Belchim’s confidential future product strategy]..88
(73) Consequently, there is a potential overlap between the Parties’ paraffinic oil for virus control in the Netherlands in seed potatoes and flower bulbs.89
8.2.1.2. Notifying Party’s views
(74) While the Notifying Party acknowledges that Certis is currently the largest, if not the only, distributor of paraffinic oil products in the Netherlands in the concerned markets, it argues that (i) it is uncertain whether Belchim will become a competitor as the registration process of its paraffinic oil product (Fibro) is ongoing;90 (ii) Certis has limited control over paraffinic oil pricing, as it is a relatively low value product
 being by far the largest player in the market for paraffinic oils for virus control in seed potatoes, and the only player in the market for paraffinic oils for virus control in flower bulbs.
being by far the largest player in the market for paraffinic oils for virus control in seed potatoes, and the only player in the market for paraffinic oils for virus control in flower bulbs.
(77) The results of the market investigation point at the importance of the products of the Parties for virus control in seed potatoes and flower bulbs in the Netherlands.93 A competitor indicated that Certis’ products are ‘an unmissable product in both crops [seed potatoes and flower bulbs] needing a high frequency of applications’.94 Another supplier of crop protection products argued that the Merged Entity will be a specialty crop company, and that it will be able to leverage this position.95 Several customers consider that Belchim will be Certis’ only potential competitor, with a customer stressing that ‘if the companies have both Olie H / Kompaan and Fibro in their portfolio they will monopolize the paraffinic oil market for virus control without competition’.96
(78) Second, the market is extremely concentrated, the only other player that would have a likely and timely entry in the market with a product similar to those of Certis would be Belchim.97
(79) Respondents to the market investigation have not confirmed the Notifying Party’s claim that there is uncertainty over Belchim’s entry in the Dutch markets for paraffinic oils for virus control in seed potatoes and flower bulbs.98
(80) Indeed, Belchim’s entry in the market appears likely. The Notifying Party has not argued nor provided evidence that the approval process is encountering difficulties or that the approval would be rejected. Belchim’s product Fibro is based on the same AI as Certis’ products (paraffinic oil). Given that Certis’ products are registered and are not under regulatory pressure, this would indicate that other products based on the same AI and in advanced stages of the registration process are unlikely to face difficulties for registration from the competent authorities. Moreover, several customers expect Belchim’s entry in the market.99
(81) Belchim’s entry in the market appears timely. As mentioned above, Belchim indicated that [Belchim’s confidential information on product registration].100
(82) Further, the results of the market investigation have not pointed at other products that would be credible alternatives to those of the Parties, nor at other competitors that would have a likely and timely entry in the markets and that would exert sufficient pressure on the Parties post-Transaction.101 Customers pointed at Certis’ products being the only ones registered for virus control in potatoes and flower bulbs in the Netherlands.102 In this line, a customer expressed that ‘Only Certis Olie H and Kompaan are used for virus control!’103
(83) In fact, the majority of respondents to the market investigation consider that there are no alternative products for treating virus control in seed potatoes and flower bulbs.104 While certain respondents cited alternatives such as insecticides for aphid control, including pyrethroids,105 other respondents point at the increasing resistance of pyrethroids and the cheaper price of paraffinic oils.106 Moreover, certain respondents consider that the two families of products have different technical characteristics and use. Paraffinic oils for virus control are specific to certain types of crops (such as seed potatoes and flower bulbs, which have requirements of being virus-free107).108 This is in line with the Notifying Party’s arguments, who explains that, while insecticides have a lethal effect on the insects, paraffinic oils are used as a physical barrier for insects, applied on the crops, and do not kill the virus nor insects.109
(84) Both the Notifying Party and some respondents to the market investigation consider that other alternatives to paraffinic oils such as vegetable oils are not credible alternatives. Vegetable oils seem to have lower efficacy than paraffinic oil110 and higher prices111 and paraffinic oils have characteristics that set them apart from vegetable oils, such as a specific chain length (a long carbon chain).112 Paraffinic oils can be used with high dose rate use specifically against non-persistent virus in seed potatoes and flower bulbs.113
(85) Third, the results of the investigation indicate that the Parties are fully autonomous in setting their own sales price.114 Further, and as has been mentioned in paragraph
(83) above, several respondents to the market investigation point at the lack of alternative products and suppliers to turn to.115
8.2.1.4. Conclusion
(86) In light of the above, particularly in view of the results of the market investigation, the Commission concludes that the Transaction raises serious doubts as to its compatibility with the internal market in relation to the supply of paraffinic oils for virus control in (i) seed potatoes and (ii) flower bulbs in the Netherlands.
8.2.2. Post-harvest PGRs for potatoes
(87) The Parties’ activities in the EEA overlap in the area of PGRs for potatoes. Based on the Parties’ activities in 2020, the Transaction gives rise to four affected markets for post-harvest potato PGRs: in Germany, Poland, Denmark and Sweden. In addition, the Transaction would lead to a loss of potential competition in the markets for post- harvest potato PGRs in Finland and Norway.116
(88) The potato PGRs market was dominated by the AI CIPC until its EU-wide ban took effect in early 2020. CIPC was traditionally used as an efficient preventive treatment with long-lasting residual action that required very few applications per storage season. Because the residual action of alternative products to CIPC is more limited, potato sprout suppression will now require several treatments, either with the same product or by combining different products.
8.2.2.1. Activities of the Parties
(89) When the EU-wide ban on CIPC took effect in early 2020, both Belchim and Certis were the first suppliers to have started selling, or to be about to sell, alternative products in the markets under review. In those markets Belchim is selling 1,4 Sight, a product based on dimethylnaphtalene (‘DMN’) sourced from Dormfresh, while Certis is selling, or is about to sell, Biox-M,117 a product based on spearmint oil sourced from Xeda. Both Certis and Belchim also sold CIPC-based products in various EEA markets before the ban came into force.
(90) The Parties offer both pre-harvest and post-harvest potato PGRs in various EEA countries. In Germany and Poland, an overlap in post-harvest potato PGRs has existed since 2019 and in Denmark and Sweden since 2020. In Finland and Norway, Belchim started selling 1,4 Sight in 2020, while Certis’ launch of Biox-M appears to be forthcoming. At the time of notification, both Belchim and Certis expected to be able to sell their post-harvest potato PGRs in Finland and Norway [Parties’ confidential business strategy]..
8.2.2.2. Notifying Party’s views on aspects common to the assessment of all post-harvest potato PGRs markets under review
(91) The Notifying Party raised a number of arguments that apply to all post-harvest potato PGRs markets.
(92) First, the Notifying Party submits that the Parties’ strong market position is a temporary situation following the ban on CIPC, as alternative products will be imminently registered and commercialised. It contends that several products are expected to replace CIPC, notably pre-harvest products based on maleic hydrazide and post-harvest products based on ethylene, DMN (which Belchim distributes), mint oil (which Certis distributes) and orange oil.
(93) Second, the Notifying Party claims that ethylene is an alternative product that may become the most popular potato sprout suppressant, at a treatment cost of EUR […] of potatoes. It contends that it is a biological product on which there are no regulatory restrictions as regards the residue level in the crop, which makes it suitable for use just before the potatoes are removed from the storage, unlike the other CIPC alternatives, and thus likely to win shares from the Parties’ products.
(94) Third, the Notifying Party submits that products based on mint oil are distant competitors to products based on DMN because they are applied post-harvest for the curative burning-off of sprouts, which must be renewed at each new sprouting, rather than to prevent sprouting as is the case for DMN-based products. It also points out that DMN increases the dormancy of the potato by a few weeks and has basically no curative activity and, therefore, needs to be applied in a stringent regime to avoid breaking the dormancy stage. Moreover, it claims that mint oil is expensive at a treatment cost of EUR […] of potatoes, which would make it suitable only for use in a limited set of circumstances. DMN has instead a treatment cost of EUR […] of potatoes.
(95) Fourth, the Notifying Party argues that Certis’ Biox-M distribution agreement [confidential third party agreement]. It contends that post-Transaction, it is likely that, if 1,4 Sight and Biox-M are considered as competing products, the distribution of both products would be reviewed and [confidential third party agreement]..
(96) Fifth, the Notifying Party contends that high market shares do not necessarily indicate strong market power, as the Parties cannot act independently from buyers or suppliers at the risk of being set aside by suppliers (as the Parties are mostly distributors of third-party products). It submits that distribution agreements are in general for the short term and the supplier may withdraw crop protection products from the Parties’ portfolio.
(97) In that respect, the Notifying Party claims that its products’ end-users’ choice is dictated by the buyers of potatoes, some of whom issue (binding) recommendations on the potato sprout suppressants to use or not to use.
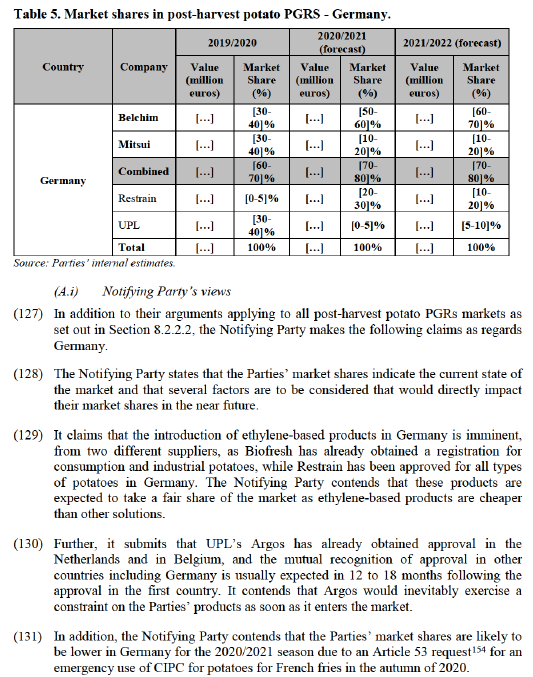
8.2.2.3. Commission’s assessment of the aspects common to the assessment of all post- harvest potato PGRs markets under review
(98) First, the concentration would lead to very high market shares of the Merged Entity in the affected and potentially affected markets for post-harvest potato PGRs. In each of those markets, the Notifying Party forecasts combined market shares of at least [70-80]% for the 2021/2022 season: Denmark ([80-90]%), Finland ([70-80]%), Germany ([70-80]%, see paragraph (126) below), Norway ([70-80]%), Poland ([80- 90]%, see paragraph (141) below) and Sweden ([90-100]%). In each of the post- harvest potato PGR markets the Parties seem to compete, or to be about to compete, with not more than three alternative suppliers.
(99) In addition to the products offered or about to be offered by the Parties based on DMN and spearmint oil respectively, there are only two other types of post-harvest PGRs that are in the process of being rolled out in the EEA: products based on ethylene and orange oil. However, majorities of customers118 and competitors119 responding to the market investigation are not aware of these, or any other competing products being sold or about to be sold as potato sprout suppressants in the next three years in Europe.
(100) Only two customers cited AIs other than spearmint oil and DMN: one cited orange oil and ethylene,120 while another mentioned that it was aware of SmartBlock,121 a product going through the regulatory process, but did not know whether it would be sold in the EU in the next three years.122 Only one competitor stated that it planned to enter in the affected markets in the next three years with one of the alternative AIs cited by the Notifying Party.123
(101) The Commission’s investigation did not reveal any potential entry by competing post-harvest potato PGRs based on AIs other than spearmint oil and DMN which could seem timely, likely and sufficient to counteract any harmful effects of the merger in any of the affected markets.
(102) Second, the Notifying Party’s claim that ethylene may become the most popular potato sprout suppressant is not supported by the Commission’s investigation.
(103) Several customers responding to the market investigation point out that ethylene is not recommended for certain applications such as processing into crisps or fries, due to discoloration issues,124 which is a disadvantage acknowledged by the Notifying Party.125 A competitor commented: “Ethylene is also a potato sprouts suppressant used in storage but is rarely used”.126
(104) Further, as recognised by the Notifying Party,127 ethylene’s effectiveness depends on a precise timing of application at the start of the storage and varies according to the potato variety. This is confirmed by comparative tests carried out by agronomic consultancy Inagro, which show that the long-term efficacy of ethylene shows much greater variance depending on the potato variety compared to competing products.128 The same tests indicated that ethylene had lower average efficacy than competing products, with an average sprout length of around 4 mm in the month of June compared to around 3 mm for Biox-M and 1,4 Sight.
(105) In addition, the use of ethylene affects product quality, as it has an aging effect on potatoes, which makes it less suitable for long-term storage. A supplier explained: “[…] ethylene is a poor product. It stops the growth of the sprouting but accelerates the ripening metabolism in potatoes. The result is an aged potato and a loss of starch (10%) – transformed into CO2 in storage.”.129
(106) Ethylene’s limited commercial prospects seem to be confirmed by market feedback. One market participant commented that he does not expect ethylene and products other than spearmint oil, DMN and orange oil to achieve market shares of more than 10-20% in Germany.130
(107) As regards orange oil, several respondents mention that the market authorisation of UPL’s product Argos is currently suspended in Germany.131 Market feedback indicates that Argos had its registration revoked in early December after a fire took place in a potato warehouse, which was in part attributed to the flammable nature of orange oil. A customer commented: “Argos has a very low flash point and is therefore highly flammable when used”.132 A supplier explained that the volatile nature of orange oil is also a disadvantage in terms of application: “Limonene/Orange oil: works on the same principle as mint oil. The problem is that it is much more volatile. It does not stay on the potato long enough, so it needs frequent reapplying: much more often than mint oil”.133 1,4 Sight, which is based on DMN, also had its authorisation revoked after a fire that took place at around the same time, but this revocation was lifted after two weeks subject to the requirement not to use fogging machines powered by combustion engines. A competitor comments that orange oil products may not be authorised in all regions134.135 It is at this stage unclear whether Argos could get its authorisation back in Germany and what the impact will be on the registration applications pending in other countries.
(108) In addition, market feedback suggests that despite the off-patent status of the Parties’ AIs, barriers to entry are high for potential upstream producers of products based on the same AIs, in particular because the proper and safe application of such products requires ownership and knowledge of the use of dedicated machines which are IP- protected.136
(109) Third, with regard to closeness of competition, the Parties and their products seem to be close competitors. A clear majority of customers137 and several competitors138 view Mitsui and Belchim as close to very close competitors in the markets for potato sprout suppressants in Germany, Poland, Denmark, Sweden, Norway and Finland.
(110) As regards closeness between the Parties’ respective active ingredients, the highest number of customers139 expressing an opinion view DMN, the AI used in Belchim’s product, as the best alternative for spearmint oil, the AI used in Mitsui’s product, and a majority of customers140 consider orange oil as the best alternative for DMN, followed by spearmint oil. Only one customer considers ethylene to be a close competitor to either of the Parties’ products, namely DMN. A customer commented: “1,4 sight - Argos - Biox M are similar products”.141
(111) A majority of competitors142 expressing an opinion consider orange oil as the best alternative for both spearmint oil and DMN. The other competitors chose DMN and spearmint oil respectively, with no competitor selecting ethylene as a close competitor to either of the Parties’ products.
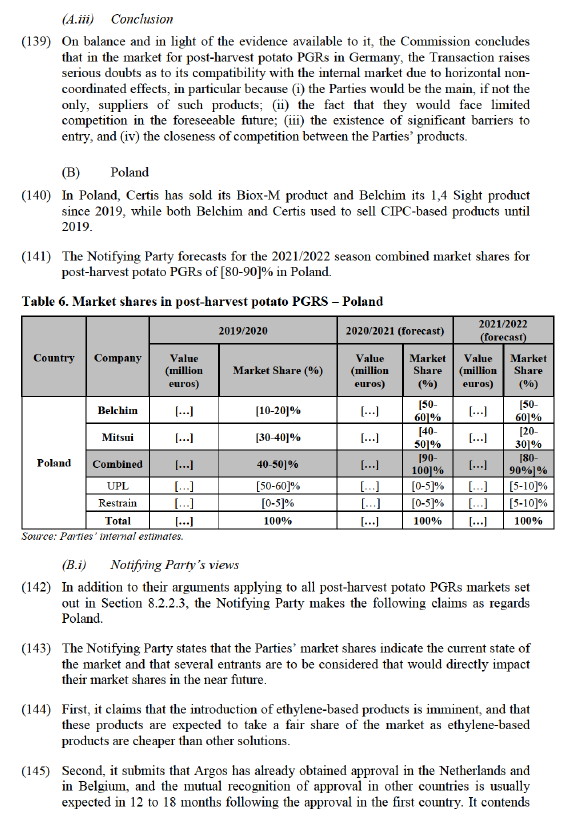
(112) The Commission therefore considers it likely that in countries where Argos, UPL’s orange oil product would not obtain, or would not recover, its market authorisation, the Parties’ products would be viewed as the only closely competing products.
(113) Beyond the fact that both Biox-M and 1,4 Sight seem to be used mainly preventively, before sprouts develop, as discussed in paragraphs (39) and (49), the Commission’s investigation has indicated that there are other elements that suggest closeness between the Parties’ products.
(114) In the first place, the products seem to have treatment costs which at least appear to be similar. Agronomic consultancy Inagro for instance estimates the treatment cost of 1,4 Sight, including application, at EUR 18.5 to EUR 23.5 per tonne, compared to at EUR 19 to EUR 27 per tonne for Biox-M.143
(115) Further, the effect of both products is not permanent, but is residual to a certain extent, which means that they both require limited repeat treatments, which are carried out at similar intervals of several weeks. A 2020 newsletter from the German NRW Chamber of Agriculture for instance recommends application of 1,4 Sight every four to six weeks and application of Biox-M every four weeks.144
(116) In addition, the Parties’ products have similar requirements in terms of application method and storage facility, which needs to be air-tight and equipped with an internal ventilation system. Agronomic consultancy Inagro for instance recommends similar fogging methods, warehouse closing times and internal ventilation intervals for both 1,4 Sight and Biox-M.145
(117) Finally, both have minimum recommended withholding times, i.e. periods during which the product should not be applied before the potatoes are removed from storage: the product labels recommend 12 days for Biox-M146 and 30 days for 1,4 Sight147.
(118) By contrast, the Commission’s investigation has indicated that several elements point to a lack of closeness between the Parties’ products and ethylene in addition to the limitations on its use due to its aging effect, discoloration issues, and lower and varying efficacy depending on the potato variety, as mentioned in paragraphs (102) to (106).
(119) As acknowledged by the Notifying Party148, ethylene requires an insulated storage facility, has a much lower price point, is not subject to any withholding time and has hardly any residual action, which means that it has to be applied constantly as sprouts can develop as soon as treatment is stopped. This makes ethylene unsuitable for fresh consumption potatoes sold to food retail, as explained by a supplier: “[…] products based on ethylene (such as Restrain) have only short-term activity. […] Potatoes need to be sold quickly after ethylene products are removed, which makes ethylene less suitable for fresh potatoes sold to food retail for instance”.149 This is also why ethylene is used mainly for seed potatoes150, as referenced by the leading Belgian farming trade journal Landbouwleven151, or even exclusively for seed potatoes in certain countries: an internal document from Belchim subsidiary Nordisk Alkali document notes that in Norway the ethylene product Restrain is sold for use only on seed potatoes152.
(120) Fourth, as regards the Notifying Party’s claim that the Merged Entity may have to give up one overlapping product under non-compete clauses in the distribution contracts, the Commission has not received evidence from the Notifying Party or feedback from the market to suggest that the Parties would indeed have to drop an overlapping product..
(121) Fifth, as regards the Notifying Party’s claim that the Parties cannot act independently from suppliers and customers, market feedback has indicated that the Parties are fully autonomous in setting their own sales prices and that agreements are typically concluded for the medium term. 153
(122) As regards the Notifying Party’s claim that potato PGR markets are driven by prescriptions issued by the buyers of potatoes, suggesting that the Transaction therefore could not reduce competition in markets that are not contestable, while it has provided some evidence of such PGR prescriptions by potato customers, it has not provided the Commission with evidence to indicate that potato PGR markets are predominantly prescription-driven.
(123) Indeed, the end market for potatoes is only partly, and not entirely, made up of industrial buyers who issue recommendations to potato suppliers on the potato PGRs to use or not to use. Moreover, these PGR recommendations by end customers also depend on product characteristics over which the distributors of PGRs have a degree of control, such as price.
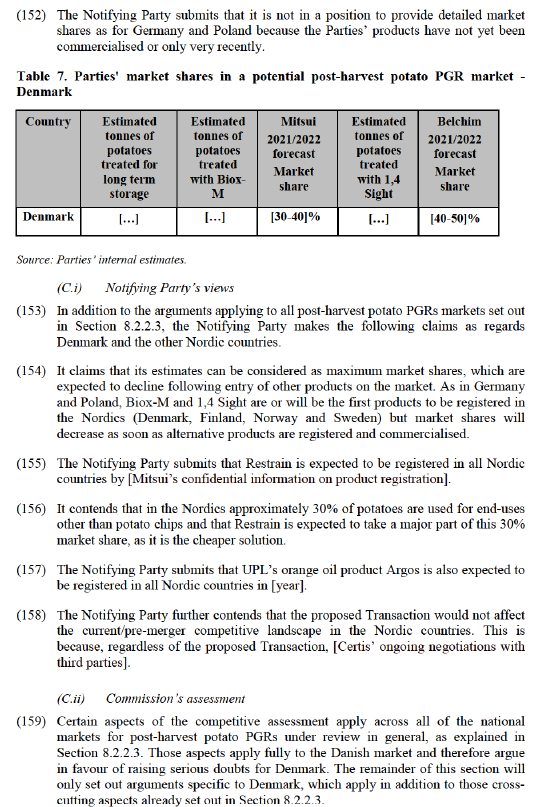
(124) The Commission therefore considers that the markets for the supply of potato PGRs are contestable and that competition on those markets can be reduced by the Transaction.
8.2.2.4. Competitive assessment of post-harvest potato PGRs by national market
(A) Germany
(125) In Germany, Certis has sold its Biox-M product since 2018 and Belchim its 1,4 Sight product since 2019, while both Belchim and Certis used to sell CIPC-based products until 2019.
(126) The Notifying Party’s forecast for their 2021/2022 season combined market shares for post-harvest potato PGRs in Germany is [70-80]%.
(A.ii) Commission’s assessment
(132) Certain aspects of the competitive assessment apply across all of the national markets for post-harvest potato PGRs under review in general, as explained in Section 8.2.2.3. Those aspects apply fully to the German market and therefore argue in favour of raising serious doubts for Germany. The remainder of this section will only set out arguments specific to Germany, which apply in addition to those cross- cutting aspects already set out in Section 8.2.2.3.
(133) Several customers replying to the market investigation commented that the Merged Entity will have a very strong or even dominant position in post-harvest potato PGRs in Germany.155 A German customer remarked: “From our point of view, the companies already have a high market strength in the field of post-harvest sprout inhibitors, which could be strengthened by the transaction.”156
(134) A German customer estimated that “in 2020, 1.4 Sight would have a market share of around 80% and Biox-M much of the remaining 20%”.157 A supplier estimated that the Parties’ post-CIPC market share in Germany would reach at least 60-70%, and 80-90% if Argos does not get its registration back.158
(135) With regard to the Notifying Party’s claim that the introduction of ethylene-based products is imminent in Germany and that these products are expected to take a fair share of the market, as discussed in paragraphs (102) to (106), the Commission’s investigation has indicated that the commercial prospects of ethylene are limited as it is unsuitable for certain applications such as processing into crisps, and has an aging effect on potatoes.
(136) As regards orange oil, as mentioned in paragraph (107), UPL’s product Argos recently had its registration withdrawn in Germany and it is uncertain whether Argos could get its authorisation back.
(137) As regards the emergency authorisation of CIPC requested for French fries in Germany in the autumn of 2020, the Commission notes that the Article 53 procedure provides only for a temporary exemption of four months.
(138) When asked about the impact of the Transaction on the post-harvest potato PGR markets, a majority of customers159 expressing an opinion have a neutral view, whereas a majority of competitors160 expressing an opinion expects price increases in the German market.
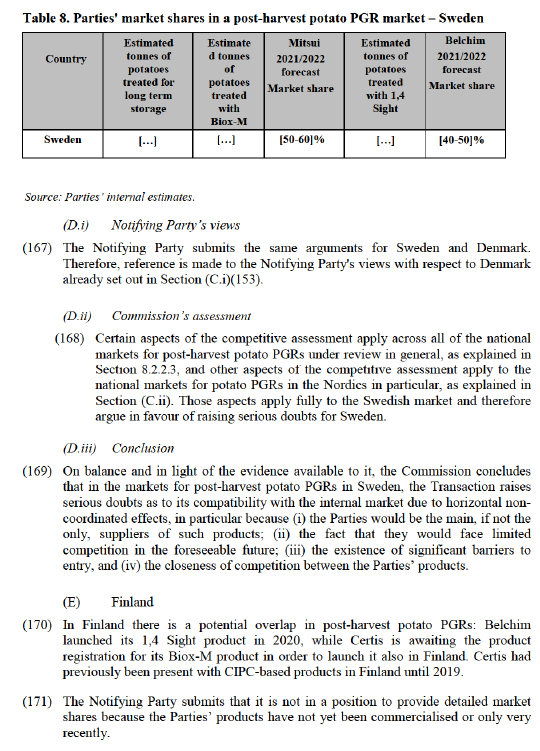
that it would inevitably exercise a constraint on the Parties’ products as soon as it enters the market.
(B.ii) Commission’s assessment
(146) Certain aspects of the competitive assessment apply across all of the national markets for post-harvest potato PGRs under review in general, as explained in Section8.2.2.3. Those aspects apply fully to the Polish market and therefore argue in favour of raising serious doubts for Poland. The arguments specific to Poland set out below applies in addition to those cross-cutting aspects already set out in Section8.2.2.3.
(147) With regard to the Notifying Party’s claim that the introduction of ethylene-based products is imminent in Poland and that these products are expected to take a fair share of the market, as discussed in paragraphs (102) to (106), the Commission’s investigation has indicated that the commercial prospects of ethylene are limited as it is unsuitable for certain applications such as processing into crisps and has an aging effect on potatoes.
(148) As regards orange oil, as mentioned in paragraph (107), UPL’s product Argos recently had its registration withdrawn in Germany and it is uncertain what the impact will be on the registration application pending in Poland.
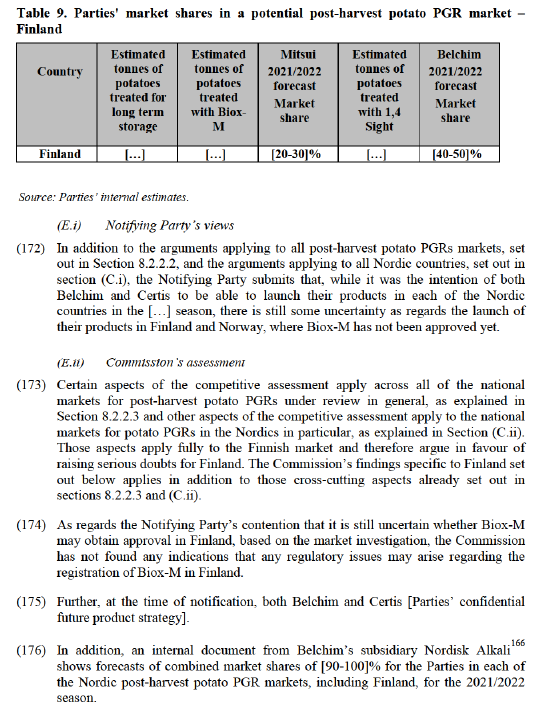
(149) In the Commission’s market investigation, when asked about the impact of the Transaction on the post-harvest potato PGR markets, a majority of customers161 expressing an opinion have a neutral view, whereas several competitors162 expressing an opinion expect price increases in the Polish market. A Polish customer stated with regard to the Merged Entity: “In Poland, it will be practically the only entity offering anti-sprouting agents, as a monopolist operating in the market”.163
(B.iii) Conclusion
(150) On balance and in light of the evidence available to it, the Commission concludes that in the market for post-harvest potato PGRs in Poland, the Transaction raises serious doubts as to its compatibility with the internal market due to horizontal non- coordinated effects in particular because (i) the Parties would be the main, if not the only, suppliers of such products; (ii) the fact that they would face limited competition in the foreseeable future; (iii) the existence of significant barriers to entry, and (iv) the closeness of competition between the Parties’ products.
(C) Denmark
(151) In Denmark, Certis launched its spearmint oil product under the brand name Xedamint in 2019, while Belchim launched its 1,4 Sight product in 2020. Certis had previously been present with CIPC-based products in Denmark until 2019.
(160) The Notifying Party’s claim that their estimates can be considered as maximum market shares, which are expected to decline following entry of other products on the market, seems to be contradicted by an internal document from Belchim’s164 subsidiary Nordisk Alkali. This document shows forecasts of combined market shares of [90-100]% for the Parties in each of the Nordic post-harvest markets for the 2021/2022 season, including in Denmark.
(161) In relation to the Notifying Party’s claim that ethylene-based product Restrain could capture a major part of the market for end uses other than potato crisps in the Nordics, the Commission’s investigation has indicated that ethylene is also less suitable for fresh potatoes sold to the retail channel, as explained in paragraph (119).
(162) As regards the Notifying Party’s claim that the Transaction would not affect the current/pre-merger competitive landscape in the Nordics, including Denmark, as Nordisk Alkali would in any event be the distributor of these products, the Commission considers that the Transaction does affect the current competitive landscape, as it brings the Parties together upstream and brings Nordisk Alkali under the same Merged Entity.
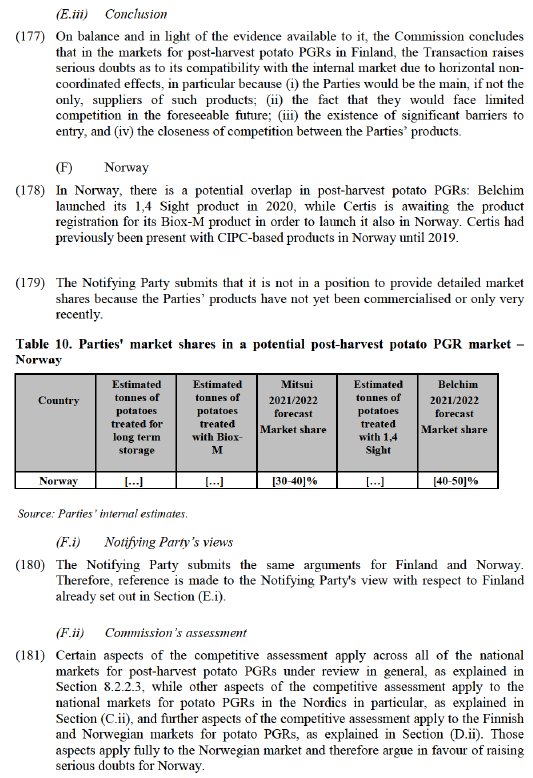
(163) As for the impact of the Transaction on the post-harvest potato PGR markets in the Nordic countries, including Denmark, the results of the market investigation were inconclusive.165 Respondents do not yet seem to be aware of the Parties’ recent overlapping offer of potato PGRs in Denmark.
(C.iii) Conclusion
(164) On balance and in light of the evidence available to it, the Commission concludes that in the markets for post-harvest potato PGRs in Denmark, the Transaction raises serious doubts as to its compatibility with the internal market due to horizontal non- coordinated effects, in particular because (i) the Parties would be the main, if not the only, suppliers of such products; (ii) the fact that they would face limited competition in the foreseeable future; (iii) the existence of significant barriers to entry, and (iv) the closeness of competition between the Parties’ products.
(D) Sweden
(165) In Sweden, Certis has been selling its Biox-M product and Belchim its 1,4 Sight product since 2020. Certis had previously been present with CIPC-based products in Sweden until 2019.
(166) The Notifying Party submits that it is not in a position to provide detailed market shares as for Germany and Poland because the Parties’ products have not yet been commercialised or only very recently.
(F.iii) Conclusion
(182) On balance and in light of the evidence available to it, the Commission concludes that in the market for post-harvest potato PGRs in Norway, the Transaction raises serious doubts as to its compatibility with the functioning of the EEA Agreement due to horizontal non-coordinated effects, in particular because (i) the Parties would be the main, if not the only, suppliers of such products; (ii) the fact that they would face limited competition in the foreseeable future; (iii) the existence of significant barriers to entry, and (iv) the closeness of competition between the Parties’ products.
8.3. Markets in which serious doubts do not arise
8.3.1. Fungicides
(183) The Transaction gives rise to six affected markets in fungicides, notably: (i) vine fungicides for downy mildew in Austria and in Germany and (ii) potato fungicides for late blight in Belgium, Germany, the Netherlands and the United Kingdom.
8.3.1.1. Vine Fungicides for downy mildew in Austria
(184) In Austria, Certis distributes Cuprozin and Funguran (both copper hydroxide based), which are developed and manufactured by Spiess-Urania, one of Mitsui’s subsidiaries. Belchim, on its end, distributes three third-party products, Mildicut (cyazofamid and disodium-phosphonate based), Videryo F (cyazofamid and folpet based) and Alleato-Duo (fosetyl and folpet based). 167
(185) In 2019, the Parties held an estimated combined market share of [30-40]% (Certis [20-30]% and Belchim [10-20]%). Post-Transaction, the Merged Entity would face competition from strong market players such as BASF (which holds a position similar to Certis with [20-30]%), Kwizda Agro (which holds a [10-20]% share), as well as other well-established suppliers also present in this market such as Bayer, ADAMA, Nufarm and Syngenta.168
(186) In the market investigation, a majority of respondents that expressed a view indicated that they do not consider the Parties to be close competitors nor do they expect the proposed Transaction to have an effect on prices. Moreover, no stakeholder has raised substantiated concerns.169
(187) In light of the above, particularly in view of the results of the market investigation, the Commission concludes that the proposed Transaction does not raise serious doubts as to its compatibility with the internal market in relation to the supply of vine fungicides for downy mildew in Austria.
8.3.1.2. Vine Fungicides for downy mildew in Germany
(188) Similarly to Austria, in Germany, Certis distributes Cuprozin and Funguran (both copper hydroxide based), whereas Belchim distributes three third-party products, Mildicut (cyazofamid and disodium-phosphonate based), Videryo F (cyazofamid and folpet based) and Airone (copper hydroxide and oxychloride based).170
(189) In 2019, the Parties held an estimated combined market share of [20-30]% (Certis [10-20]% and Belchim [10-20]%). Post-Transaction, the Parties would face strong competition from Adama (with a market share of [30-40]%), the market leader, as well as from other well-established suppliers such as BASF (with a [20-30]% market share), Bayer (with a [10-20]% market share) and Syngenta.171
(190) In the market investigation, a majority of respondents that expressed a view indicated that they do not consider the Parties to be close competitors nor do they expect the proposed Transaction to have an effect on prices. Moreover, no stakeholder has raised substantiated concerns.172
(191) In light of the above, particularly in view of the results of the market investigation, the Commission concludes that the proposed Transaction does not raise serious doubts as to its compatibility with the internal market in relation to the supply of vine fungicides for downy mildew in Germany.
8.3.1.3. Potato fungicides for late blight in Belgium
(192) In Belgium, Certis distributes two third-party products, Valbon (benthiavalicarb and mancozeb based) and Versilius (benthiavalicarb based), whereas Belchim distributes a mix of own and third-party products. Belchim sells their own formulated products: Cymbal, Cymopur and Danso (all cymoxanil based); Profilux, Ebrimax, Moximate and Cymozeb (all mancozeb and cymoxanil based); and Valis M and Emendo M (both valifenalate and mancozeb based). In addition, Belchim also sells the following third-party products: Kunshi (fluazinam and cymoxanil); Proxanil and Axidor (both propamocarb and cymoxanil based); Ranman Top (cyazofamid based); Unikat Pro (mancozeb and zoxamide based); Shirlan Gold (fluazinam based); Grifon (copper hydroxide oxychloride based); and Dithane (mancozeb based).173
(193) In 2018, the most recent year for which market shares are available, the Parties held an estimated combined market share of [20-30]% (Certis [5-10]% and Belchim [20- 30]%). In 2018, Corteva was the market leader (with a [20-30]% market share) and the Parties faced further competition from other strong players such as BASF, Bayer and Syngenta.174
(194) Due to the ban on mancozeb175 and in light of the regulatory pressure [Certis’ confidential future product strategy]176 cyazofamid177, the Parties expect their share to decrease going forward. In particular, Certis’ most sold product, Valbon, will soon exit the market ([Certis’ confidential future product strategy]])178 and [Certis’ confidential future product strategy]179. Belchim, on its end, faces the incertitude related to the renewal of the marketing authorisation for Ranman Top’s AI, cyazofamid.180 [Belchim’s confidential information relating to third parties’ agreements].181
(195) Although the Notifying Party is looking for alternatives to compensate for the loss of Valbon and the regulatory pressure on other products, [Certis’ confidential future product strategy].182 In addition, the Notifying Party also expects that Corteva will continue aggressively competing on this market with its new product Zorvec (oxathiapiprolin based).183
(196) In the market investigation, a majority of respondents that expressed a view indicated that they do not consider the Parties to be close competitors. The majority of competitors that expressed a view also do not expect the proposed Transaction to have an effect on prices. The Parties’ customers did not express a view on the impact of the Transaction on prices. Moreover no stakeholder has raised substantiated concerns.184
(197) In light of the above, particularly in view of the results of the market investigation, the Commission concludes that the proposed Transaction does not raise serious doubts as to its compatibility with the internal market in relation to the supply of potato fungicides for late blight in Belgium.
8.3.1.4. Potato fungicides for late blight in Germany
(198) In Germany, Certis distributes several third-party products: Valbon (benthiavalicarb and mancozeb based), Cuprozin, Funguran (both copper hydroxide based), and Dithane (mancozeb based); while Belchim distributes a mix of own and third-party products. Belchim sells its own formulated product Cymbal Flow (cymoxanil based). It also sells the following third-party products: Proxanil Extra (propamocarb and cymoxanil based), Ranman Top (cyazofamid based), Shirlan Gold (fluazinam based), Winby (fluazinam), Ranman Top + Proxanil Pack, Valis M (valifenalate and mancozeb based), Epok (fluazinam and metalaxyl-M based).185
(199) In 2019, the Parties held an estimated combined market share of [20-30]% (Certis [5-10]% and Belchim [10-20]%). They face competition from strong market players such as Syngenta (the market leader with a [30-40]% share), Bayer ([10-20]%), and Corteva ([10-20]%) in addition to other well-established players such as UPL, ADAMA and BASF.186
(200) Similarly to Belgium, [Certis’ confidential future product strategy]. To compensate for the loss of Valbon, and despite the incertitude related to its marketing authorization renewal, [Certis’ confidential future product strategy]. Certis will however face strong competition from Corteva that sells Versilus in a pack with its new product Zorvec.187 Similarly to Belgium, Ranman Top distributed by Belchim is facing the incertitude of not having the respective marketing authorisation renewed. [Certis’ confidential future product strategy]188 Going forward the Parties’ overlap is therefore likely to diminish.
(201) In the market investigation, the majority of respondents that expressed a view indicated that they do not consider the Parties to be close competitors. Whereas the few competitors who expressed a view were split as to impact of the Transaction on prices, the vast majority of the other respondents do not consider that the prices will change due to the Transaction. Moreover, no stakeholder has raised substantiated concerns.189
(202) In light of the above, particularly in view of the results of the market investigation, the Commission concludes that the proposed Transaction does not raise serious doubts as to its compatibility with the internal market in relation to the supply of potato fungicides for late blight in Germany.
8.3.1.5. Potato fungicides for late blight in the Netherlands
(203) In the Netherlands, Certis distributes two third-party products, Valbon (benthiavalicarb and mancozeb based) and Versilius (benthiavalicarb based); while Belchim distributes a mix of own and third-party products. Belchim sells its own formulated products Cymbal and Cymbal Flow (both cymoxanil based). In addition, Belchim sells the following third-party products: Kunshi (fluazinam and cymoxanil based); Proxanil (propamocarb and cymoxanil based); Ranman Top (cyazofamid based); Dithane, Phytane, Milcozeb (all mancozeb based); Profilux (mancozeb and cymoxanil based), Unikat Pro (mancozeb and zoxamide based).190
(204) In 2018, the most recent year for which shares are available, the Parties held an estimated combined market share of [30-40]% (Certis [10-20]% and Belchim [20-30]%). In 2018, the second position in this market was disputed between Bayer and Syngenta (each with a market share just above [10-20]%); Corteva followed very closely (with a market share of almost [10-20]%). For 2019, based on a smaller data set, the Parties have calculated an increase of both their market shares (Certis [20- 30]% and Belchim [20-30]%), totalling a combined market share of [40-50]%. The Parties were not able to estimate their competitors’ market shares.191
(205) Going forward, the Parties however expect a decrease in sales due to the ban on mancozeb and the incertitude surrounding the renewal of the marketing authorization for [Certis’ confidential information on product registration] cyazofamid. [Certis’ confidential information on product registration] . [Certis’ confidential information relating to third parties’ agreements].192 On its end, [Belchim’s confidential information relating to third parties’ agreements] [Belchim’s confidential information on product registration].
(206) In the market investigation, the majority of respondents, that expressed a view, indicated that they do not consider the Parties to be close competitors. The only two competitors that expressed a view considered that the prices may increase due to the proposed Transaction, whereas the vast majority of the other respondents indicated that they do not consider that the prices will change due to the Transaction. Moreover, no stakeholder has raised substantiated concerns.193
(207) In light of the above, particularly in view of the results of the market investigation, the Commission concludes that the proposed Transaction does not raise serious doubts as to its compatibility with the internal market in relation to the supply of potato fungicides for late blight in the Netherlands.
8.3.1.6. Potato fungicides for late blight in the United Kingdom
(208) In the United Kingdom, Certis distributes Valbon (benthiavalicarb and mancozeb based), Versilius (benthiavalicarb based) and Cuprokylt (copper hydroxide based); while Belchim distributes a mix of its own and third-party products. Belchim sells its own formulated products Cymbal and Drum (both cymoxanil based). In addition, Belchim sells the following third-party products: Kunshi (fluazinam and cymoxanil based); Ranman Top (cyazofamid based); Profilux and Cymax, (both mancozeb and cymoxanil based); Cymozeb (mancozeb and cymoxanil based); and Shirlan Gold (fluazinam based).194
(209) In 2019, the Parties held an estimated combined market share of [20-30]% (Certis [0-5]% and Belchim [20-30]%). In 2019, the Parties faced strong competition from Corteva (which was the market leader with a [20-30]% market share), Syngenta ([10-20]%), Bayer ([10-20]%), as well as from other well-established players such as BASF and UPL (both bigger shares than Certis).195 Also in the UK, Certis’ main product Valbon will exit the market.
(210) In the market investigation, the majority of respondents, that expressed a view, indicated that they do not consider the Parties to be close competitors, nor do they expect that the proposed Transaction will have an effect on prices. Moreover, no stakeholder has raised substantiated concerns.196
(211) In light of the above, particularly in view of the results of the market investigation, the Commission concludes that the proposed Transaction does not raise serious doubts as to its compatibility with the internal market in relation to the supply of potato fungicides for late blight in the United Kingdom.
8.3.2. Cross spectrum – pre emergence - herbicides for vegetable crops in the Netherlands
(212) The Parties are both active in the supply of cross spectrum – pre emergence - herbicides for vegetable crops in the Netherlands. Certis distributes two products from third parties, namely Bonanlan and Certis Chloor. Belchim sells its own formulated product, Lentagran, and a third-party product named Kerb Flo.197
(213) In 2018, the most recent year for which data are available, the Parties had a combined estimated market share of [40-50]% (Certis [10-20]% and Belchim [20- 30]%). The Notifying Party however argues that Certis’ share is likely to decrease due to the ban on CIPC-based products, which directly affects the sale of Certis Chloor. Indeed, this product, which was Certis’ stronger product has in the meantime exited the market.198 Despite their combined significant position, the Parties face competition from a strong vertically integrated player, Bayer, which led the market in 2018 (with a [30-40]% market share), and from other important players, such as BASF ([5-10]%) and Kwizda Agro (5-10]%).199
(214) In the market investigation, the majority of respondents that expressed a view consider that the prices are not likely to change due to the Transaction.200 Moreover, no stakeholder has raised substantiated concerns.
(215) In light of above, particularly in view of the results of the market investigation, the Commission concludes that the proposed Transaction does not raise serious doubts as to its compatibility with the internal market in relation to the supply of cross spectrum – pre emergence - herbicides for vegetable crops in the Netherlands.
8.3.3. Potato desiccants in the Netherlands
(216) In the Dutch potato dessicants market, Certis supplies a third-party product Quickdown (based on pyraflufen-ethyl), whereas Belchim supplies its own product Beloukha.
(217) According to the Notifying Party, since the ban on diquat, Belchim no longer distributes potato desiccants. In the Notifying Party’s view, the product Beloukha owned and distributed by Belchim is not a potato desiccant. The Notifying Party therefore claims that the proposed Transaction does not give raise to an overlap in any potato desiccant market. Alternatively, the Notifying Party submits that if Beloukha were to be considered a potato desiccant, it would not be a close competitor to the synthetic products, in particular to Certis’ Quickdown (based on pyraflufen-ethyl) and the increment brought by the Transaction would be negligible.201
(218) The Notifying Party submitted that Beloukha is a bio-based herbicide made up predominantly of pelargonic acid. Beloukha can be applied in a wide variety of plants and is registered and promoted as a broad-spectrum herbicide.202 In particular, as the Notifying Party acknowledged, Beloukha has been registered and, at least initially, promoted, as a potato desiccant in the Netherlands.
(219) The Notifying Party however argues that due to its lesser efficacy and higher price, Beloukha cannot be considered an alternative to the synthetic desiccants (based on pyraflufen-ethyl and carfentrazone-ethyl) currently available on the market. Beloukha would insufficiently attack the vast potato haulm and requires combination with mechanical solutions to be effective (it is better suited for smaller weeds). Moreover, to be effective, a higher dosage of the product is recommended per hectare (ha) and the price of the litter per ha is higher than the synthetic products.203
(220) The Commission tested the Notifying Party’s arguments in the market investigation. All competitors, that expressed a view, considered that Beloukha is used as a potato desiccant in particular in the so-called “potato countries”. Some of them have however confirmed the claims of lesser efficiency and higher costs.204 These two factors have also been raised by customers (and other respondents), which are split as regards the use of Beloukha as a potato desiccant.205 Overall, a majority of all respondents that expressed a view consider that Beloukha is used as a potato desiccant.
(221) Based on these results and on the fact that Beloukha has been registered, marketed and sold as a potato desiccant, the Commission considers that this product belongs to the potato desiccant market and has therefore assessed the overlap that the proposed Transaction raises in the potato desiccant market in the Netherlands.
(222) Until the summer of 2019, Belchim also supplied Roquat (based on diquatdibromide), which has exited the market due to the ban on diquat.206 Belchim also used to supply FMC’s Spotlight Plus (based on carfentrazone-ethyl) until the end of 2018.207 For these reasons, Belchim’s market shares in 2018 ([30-40]%) and in 2019 ([10-20]%) do not reflect Belchim’s position today. In fact, for 2020, the Notifying Party has estimated that with only one product (Beloukha), Belchim would have a market share below [0-5]%.208
(223) On the other hand, Certis seems to have benefited and continues to benefit from the ban on diquat. It increased its market share from 2018 ([10-20]%) to 2019 ([20- 30]%) and the Notifying Party forecasts a [30-40]% - [50-60]% market share in 2020.209 Notwithstanding the significant position of Certis, the increment brought by Belchim with its product Beloukha is not material.
(224) In addition, in this market, the Merged Entity would still face competition from FMC which owns and currently distributes Spotlight Plus, the other most known and used potato desiccant. The Parties estimate that FMC had a market share in 2020 between [40-50]% – [60-70]%.210
(225) A majority of respondents to the market investigation that expressed a view indicated that Certis and Belchim do not compete closely in the potato desiccants market in the Netherlands. Moreover, some competitors consider that Belchim’s Beloukha has a more limited use and performance than pyraflufen-ethyl based products, such as Certis’ Quickdown.211 Several customers pointed out that Beloukha is more expensive and less effective than the available alternatives and is used more in organic farming than in conventional agriculture.
(226) In addition a majority of respondents that expressed a view consider that the prices will not change due to the Transaction. In addition, no stakeholder has raised substantiated concerns.
(227) In light of the above, particularly in view of the results of the market investigation, the Commission concludes that the proposed Transaction does not raise serious doubts as to its compatibility with the internal market in relation to the supply of potato desiccants in the Netherlands.
8.3.4. Insecticides
(228) The Parties are both active in the supply of insecticides in the following markets: (i) sucking pests insecticides for stone fruit in Spain; and (ii) sucking pests insecticides for potato in the Netherlands.
8.3.4.1. Insecticides – sucking pest - stone fruit- Spain
(229) Both Parties supply third-party sucking pest insecticides for stone fruit in Spain. Certis supplies Mospilan (acetamiprid based), Breaker and Mansa (both natural pyrethrin based); whereas Belchim supplies Teppeki (flonicamid based).
(230) In 2018, the most recent year for which shares are available, the Parties held a combined market share of [30-40]% (Certis [20-30]% and Belchim [5-10]%). Post- Transaction, the Parties would still face competition from the market leader Bayer (which had [40-50]% market share in 2018), as well as from other established competitors, such as Syngenta.212
(231) In the market investigation, a majority of respondents that expressed a view indicated that they do not consider the Parties to be close competitors nor do they expect the proposed Transaction to have an effect on prices a majority of respondents. In addition, no stakeholder has raised substantiated concerns.213
(232) In light of above, particularly in view of the results of the market investigation, the Commission concludes that the notified Transaction does not raise serious doubts as to its compatibility with the internal market in relation to the supply of sucking pest insecticides for stone fruit in Spain.
8.3.4.2. Insecticides – sucking pest – potato – the Netherlands
(233) Both Parties supply third-party sucking pest insecticides for potato in the Netherlands. Certis supplies Gazelle (acetamiprid based); whereas Belchim supplies Teppeki (flonicamid based).214
(234) In 2019, the Parties held an estimated combined market share of [20-30]% (Certis [20-30]% and Belchim [5-10]%). In 2019, Belchim has however advised its clients to stop using Teppeki on potato crop for consumption [confidential],215 hence its share is likely to decrease going forward. In this market, the Parties face competition from well-established competitors such as Bayer (the market leader with [40-50]%), BASF (with [20-30]%), as well as FMC and Syngenta.216
(235) In the market investigation, the majority of respondents that expressed a view indicated that that they do not consider the Parties to be close competitors. Few respondents expressed a view as regards the impact of the Transaction on prices. Among those who did, a majority mentions that the Transaction may negatively affect prices, however when they explain their reasons, the respondents often refer to the Parties position in paraffinic oils (which are assessed below). No stakeholder has raised substantiated concerns.217
(236) In light of the above, particularly in view of the results of the market investigation, the Commission concludes that the proposed Transaction does not raise serious doubts as to its compatibility with the internal market in relation to the supply of sucking pest insecticides for potato in the Netherlands.
8.3.5. Molluscicides in Germany
(237) In 2019, the Parties’ activities overlapped in the supply of slug control molluscicides in Germany. Both Parties distributed third-party products based on metaldehyde. Certis distributed Frunol Delicia’s Patrol Metapads and SchneckenKorn products, Belchim has taken over the distribution of De Sangosse’s Metarex Inov.218
(238) However, Certis is no longer active in this market as its distribution agreement with Frunol Delicia has been terminated in 2020.219 In addition, [Certis’ confidential future product strategy].220
(239) In light of the above, the Commission concludes that the proposed Transaction does not raise serious doubts as to its compatibility with the internal market in relation to the supply of slug control molluscicides in Germany.
8.3.6. Pre-harvest PGRs for potatoes in Germany
(240) The Transaction gives rise to a potential overlap in pre-harvest potato PGRs in Germany, where an overlap existed until 2019. Certis had to withdraw its Crown product from the market in 2020 as its registration was revoked, while Belchim continues to sell its Himalaya product. However, both Certis and Belchim are in talks with the same supplier for the distribution of […], another pre-harvest potato PGR product, in Germany […].
(241) Based on 2019 data, the Parties had a combined market share of [50-60]% (Belchim [30-40]% and Certis [10-20]%) in pre-harvest sprout suppression products in Germany.
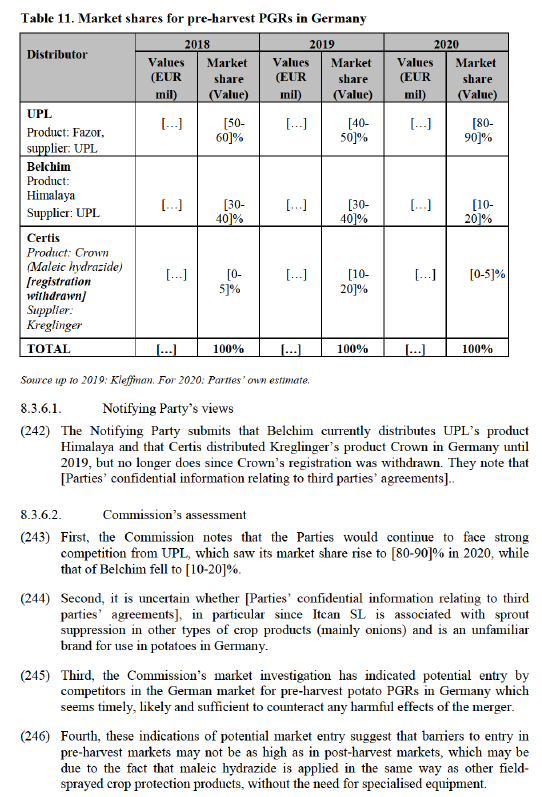
(247) Fifth, as regards the impact on prices, the market investigation was inconclusive: the majority of customers that expressed an opinion considered that the prices in the market for pre-harvest potato PGRs will not change as a result of the Transaction221, while a majority of competitors expressing an opinion considered that prices will increase.222
8.3.6.3. Conclusion
(248) In light of the above, in particular in view of potential entry by competitors, the Commission concludes that the proposed Transaction does not raise serious doubts as to its compatibility with the internal market in relation to the supply of pre-harvest potato PGRs in Germany.
8.3.7. Conglomerate Effects
(249) In the market investigation, some respondents mentioned that the Transaction would allow the Merged Entity to leverage its portfolio of products to engage in bundling practices across the different crop protection markets where it would be active. Some respondents also mentioned specifically the possibility of the Merged Entity to engage in this practice in relation to the different crop protection products for potato.223
(250) To assess the ability and incentive of the Merged Entity to adopt such strategy, the Commission has examined what would be the Merged Entity’s share of the overall sales of crop protection products in each of the countries where the Merged Entity would have a market share of at least [30-40]% in any crop protection market. In each of these countries (namely Austria, Denmark, Finland, Germany, Netherlands, Norway, Sweden, Poland, Spain), the Merged Entity’s share of the overall supply of crop protection products varies between [0-5]% and [5-10]% across these nine countries.224
(251) The Commission also assessed the Merged Entity’s ability and incentive to leverage its position in the markets for potato PGR’s (where it would have at least [30-40]% market share) to other crop protection products for potato. In these countries (namely, Denmark, Finland, Germany, Norway, Poland and Sweden), the Merged Entity’s share of the overall sales of crop protection products for potato varies between [5-10]% and [20-30]%. The Merged Entity would have its largest share ([20-30]%) in Norway where only Belchim is present.225 In none of these countries, the Merged Entity would be the leader in the sales of potato crop protection products and in all of these countries it would face competition from well-established players such as Bayer and Syngenta.226
(252) It is therefore unlikely that the Merged Entity would have the ability and the incentive to successfully engage in any exclusionary practice that could harm competition.
9. PROPOSED REMEDIES
9.1. Analytical Framework
(253) The following principles from the Remedies Notice227 apply where parties to a merger choose to offer commitments in order to restore effective competition.
(254) Where, as in this case, a notified concentration raises serious doubts as to its compatibility with the internal market, the Parties may modify the notified concentration so as to remove the grounds for the serious doubts identified by the Commission with a view to having it declared compatible with the internal market pursuant to Article 6(1)(b) in conjunction with Article 6(2) of the Merger Regulation.
(255) The Commission only has power to accept commitments that are capable of rendering the concentration compatible with the internal market in that they will prevent a significant impediment to effective competition in all relevant markets where competition concerns were identified.228 To that end, the commitments have to eliminate the competition concerns entirely229 and have to be comprehensive and effective from all points of view.230
(256) In assessing whether proposed commitments are likely to eliminate its competition concerns, the Commission considers all relevant factors, including inter alia the type, scale and scope of the commitments, judged by reference to the structure and particular characteristics of the market in which those concerns arise, including the position of the parties and other participants on the market.231 Moreover, commitments must be capable of being implemented effectively within a short period of time.232
(257) Where a proposed concentration threatens to significantly impede effective competition, the most effective way to maintain effective competition, apart from prohibition, is to create the conditions for the emergence of a new competitive entity or for the strengthening of existing competitors via divestiture by the merging parties.233
(258) While divestiture commitments are generally the best way to eliminate competition concerns resulting from horizontal overlaps, other structural commitments, such as access remedies, change of long-term exclusive contract, or other non-divestiture remedies may be suitable to resolve concerns if they are equivalent to divestitures in their effects.234
9.2. Description of the Proposed Remedies
(259) In order to address the serious doubts raised by the Transaction with a view to rendering the concentration compatible with the internal market, the Notifying Party has modified the notified Transaction by submitting to the Commission certain commitments.
(260) On 21 January 2021, the Notifying Party submitted the following commitments pursuant to Article 6(2) of the Merger Regulation (the ‘Commitments’).
(261) The Notifying Party proposed to divest or procure to divest its distribution business regarding the PGR product Biox-M, in Germany, Poland, Denmark, Sweden, Norway and Finland (the ‘Nordics’), keeping the distribution of this product in other EEA countries. In case it is not successful, it alternatively proposed to procure Belchim to divest the distribution business of the PGR product 1,4 Sight in the same national markets. Similarly to Certis under the first scenario, in this scenario Belchim also keeps the distribution of 1,4 Sight in other EEA countries (the ‘PGR Commitments’). Moreover, the Notifying Party proposed to procure to divest Belchim’s distribution agreement and regulatory package for paraffinic oils for virus control in flower bulbs and in seed potatoes in the Netherlands (the ‘Paraffinic Oils Commitments’). In particular, the Notifying Party proposed as a remedy:
(a) The divestment of the Biox-M distribution business in Germany and Poland (one divestment package) and in the Nordics (another divestment package), to a crop protection industry player(s) (the ‘Biox-M Divestment Business’). If one or both of these divestment packages cannot be transferred to a suitable purchaser(s) within a specific period of time, the Notifying Party commits to procure Belchim to transfer its 1,4 sight distribution business in Germany and Poland (one divestment package) and in the Nordics (another divestment package) to a crop protection industry player(s) (the ‘1,4 Sight Divestment Business’). The Biox-M Divestment Business and the 1,4 Sight Divestment business are together referred to as “PGR Divestment Business”.
(b) For paraffinic oil for virus control for seed potatoes and flower bulbs, to procure to transfer Belchim’s distribution agreement (and other relevant agreements) and regulatory package for Fibro, Belchim’s pipeline paraffinic oil product, in the Netherlands to an industry player (the ‘Paraffinic Oil Divestment Business‘).
(c) The PGR and Paraffinic Oil Divestment Businesses include all rights and assets, in particular distribution rights, customer contracts, commitments and orders, marketing and technical materials, access to brand IP and application machinery. At the request of the purchaser(s), the Notifying Party also commits to provide for a transitional period education and training, as well as support to obtain the registration of Fibro.
(d) Furthermore, the Notifying Party offered not to close the acquisition of Belchim until the Commission approves the sale of both the PGR and Paraffinic Oil Divestment Businesses to one or more suitable purchasers.
(262) In addition, the Notifying Party has entered into related commitments, inter alia regarding the preservation of the viability, marketability and competitiveness of both the PGR and Paraffinic Oil Divestment Businesses, including the appointment of a monitoring trustee and, if necessary, a divestiture trustee.
(263) The Notifying Party considers that the proposed divestments will eliminate the overlaps between the Parties in the national potato PGRs markets identified in section 8.2.2 above, and thus remove any competition concerns. Further, according to the Notifying Party, the transfer of the rights and assets relating to Fibro’s introduction in the Netherlands is likely to result in the emergence of a new competitive force, and thus it would address concerns relating to the removal of Belchim as a potential new entrant in the Dutch markets for paraffinic oils for virus control in seed potatoes and flower bulbs.
(264) On 2 February 2021, the Notifying Party submitted a revised version of the Commitments with the sole purpose of clarifying the language, no material changes were made.
10. ASSESSMENT OF THE PROPOSED REMEDIES
(265) The Commission's market test which was launched on 25 January 2020 and focused on (i) whether the Commitments were sufficient to entirely remove the competition concerns caused by the proposed Transaction; (ii) whether both the PGR and Paraffinic Oil Divestment Businesses constituted a viable business able to compete effectively with the Merged Entity on a lasting basis; (iii) whether there were specific conditions that a potential purchaser should fulfil and (iv) whether the Divestment Businesses were sufficiently attractive to find a suitable purchaser.
10.1. Removal of competition concerns
(266) In order to entirely remove the competition concerns in the potato PGR markets in Germany, Poland and the Nordics, the Notifying Party proposed to divest in the respective countries either the distribution business of the product it currently sells, Biox-M, or in case this is not successful, the distribution business of the product currently sold by Belchim, 1,4 Sight. The divestiture of one of these two distribution businesses would remove the overlaps brought by the proposed Transaction, and the negative effects thereof, in the potato PGR markets in Germany, Poland, Norway, Sweden, Denmark and Finland. The vast majority of respondents to the market test that expressed a view consider that the Commitment offered for the PGR markets is suitable to remove effectively the competition concerns raised in these markets.235
(267) In order to entirely remove the competition concerns in the paraffinic oils markets in the Netherlands, the Notifying Party proposed to transfer the regulatory package and the distribution agreement of Belchim’s pipeline paraffinic oil product, Fibro, as regards The Netherlands. In this way, the Notifying Party tries to ensure the emergence of a new competitive force, which otherwise would be lost with the Transaction, as Belchim was planning to enter these markets. By assigning or transferring Belchim’s position, potential competition can be protected and therefore the concerns raised with its loss can be removed. The vast majority of respondents to the market test that expressed a view consider that the Commitment offered for the Dutch paraffinic oil markets is suitable to remove effectively the competition concerns raised in these markets.236
(268) In addition, to maintain the structural effect of the remedies offered, the Notifying Party also proposed not to reacquire neither the PGR Divestment Business nor the Paraffinic oil Divestment business for a period of 10 years.
(269) The Commission therefore concludes that the Commitments are sufficient to remove entirely the concerns identified in the potato PGRs markets in Germany, Poland, Denmark, Sweden, Finland and Sweden, as well as, in the Dutch markets of paraffinic oils for virus control in seed potatoes and flower bulbs. Moreover, the Commission considers that the Commitments are able to create a lasting situation ensuring that the identified concerns will not materialise.
10.2. Viability and competitiveness of the Divestment businesses
(270) The Notifying Party proposed to divest all rights and assets that currently constitute the distribution business of Biox-M in six affected geographic markets (Germany, Poland, Norway, Sweden, Denmark and Finland), as well as all rights and assets that currently constitute the distribution business of 1,4 Sight in the respective countries (in case the assignment or transfer of the Biox-M is not successful). This means that the commitment to divest Biox-M or alternatively 1,4 Sight is a carve-out of the current distribution business that Certis, or Belchim, has for Biox-M, or 1,4 Sight (respectively). The scope of the divestment business packages for PGRs however includes all assets which contribute to their current operation and are necessary to ensure their viability and effective competitiveness in the respective countries and constitute on their own a stand-alone business in each of these six countries.
(271) The vast majority of the respondents to the market test indicated that they consider the scope of the divestment business is sufficient to ensure its immediate viability and competitiveness.237 As a competitor stated: “This Divested Business is a standalone business which can be easily handled by another agrochemical company being commercially active in these countries and having the ability to provide the technical support required by distributors and end-users. (…). The technical and commercial data and information, agreements and licenses provided with the transaction constitute a complete package that will allow the immediate viability, competitiveness and continuity of the sales and services to the customers and end- users”.238
(272) In addition, the majority of customers indicated that they would continue to purchase the products to be divested after the implementation of the remedy, when they are no longer distributed by the Parties.239 The suppliers also indicated that the remedy proposed would allow them to continue to have access to the different national markets, except for one supplier in relation to the Nordic countries. The reason behind its view was however not related to the scope or design of the PGR Commitments.
(273) The Notifying Party also proposed to transfer all rights and assets related to registration and distribution of Belchim’s pipeline for paraffinic oil for virus control in seed potatoes and flower bulbs. Similarly to the PGR packages, the paraffinic oil package also includes all assets that are necessary to bring the product onto the market and to operate the distribution business onwards.
(274) The vast majority of the respondents to the market test that expressed a view consider that the scope of the Paraffinic Oils divestment business is sufficient to ensure its immediate viability and competitiveness.240 The suppliers also indicated that the Paraffinic Oil Commitment would allow them to access the Dutch markets.241
(275) The majority of respondents to the market test that expressed a view also welcomed the Notifying’s Party commitment to offer education and training to the remedy- taker in relation to both PGRs and Paraffinic oils.242 The respondents’ views on the duration of such education and training varied but several respondents mentioned that it should cover at the very least one sales season.243 The majority of respondents that expressed a view also considered that a secondment of personnel would not be necessary.244
(276) The Commission therefore concludes that the scope of both the PGR and the Paraffinic Oil Divestment Businesses is sufficient to ensure their immediate viability and competitiveness.
10.3. Purchaser criteria
(277) The Notifying Party specifically proposed to assign or transfer the Divestment Businesses to a company that is already active in the crop protection industry. In fact, the majority of the respondents to the market test that expressed a view stressed the importance that the purchaser of the Divestment Businesses be already active in this industry. Although a few respondents mentioned that it is also necessary to have experience with the relevant crops and/or with the relevant national markets,245 the Commission considers that experience as a crop protection company would be sufficient to ensure the necessary know-how to conduct both the PGR and the Paraffinic Oil Divestment Businesses. In addition, the education and training offered by the Notifying Party gives additional support to the purchaser as regards the specific products and their current and potential customers.
(278) A majority of competitors and customers that expressed a view mentioned that having a single purchaser for both of the PGR packages would improve the viability, the effectiveness and the competitiveness of these businesses.246 Although this solution would indeed replicate the situation as it is today pre-Transaction, it could also limit the number of potential interested purchasers for each package. In addition, the proposed Commitments also envisage, and do not preclude this possibility: to have a single buyer for both PGR packages.
(279) More importantly, to limit the uncertainties of finding a suitable purchaser for the Biox-M divestment business in Germany and Poland on one hand, and in the Nordics on the other (and in case it does not find such suitable purchaser), the Notifying Party has committed to transfer the 1,4 sight business instead.
(280) In addition, the Notifying Party also proposed not to implement the proposed Transaction before entering into a binding agreement with a purchaser, which has to be approved by the Commission. This commitment limits the risks of not finding a suitable purchaser, in particular the risk of not being able to obtain the consent of the respective suppliers to proceed with the transfer or the assignment of the Divestment Businesses. Moreover, it also ensures that the identified concerns cannot materialise because the Notifying Party would not be able to find a suitable purchaser.
(281) In light of the above, in particular of (i) the alternative PGR divestment package, and (ii) the commitment not to close the proposed Transaction before the transfer of the Divestment businesses to suitable purchasers, the Commission considers that the purchaser criteria proposed by the Notifying Party are sufficient to ensure an effective implementation of the Commitments.
10.4. Attractiveness of the Commitments
(282) The majority of competitors and distributors that expressed a view in the market test, indicated that they consider the PGR Divestment Businesses and the Paraffinic Oils Divestment Business sufficiently interesting to attract suitable purchasers. The suppliers had a positive reaction as regards Paraffinic Oils but were less positive as regards the PGRs: the only supplier that expressed a view did not consider the PGR package to be sufficiently interesting to attract suitable purchasers.247 However, this supplier does not call into question the package itself. In any event, in the market test, several competitors showed interest in acquiring the different Divestment Businesses, including the PGR packages.248
(283) In light of the above, in particular of the interest shown in acquiring all Divestment Businesses, including both PGR packages, the Commission concludes that the Commitments are capable of being implemented effectively in a short period of time.
10.5. Conclusion of the Commitments Assessment
(284) For the reasons outlined above, the Commitments entered into by the undertakings concerned are sufficient to entirely eliminate the serious doubts as to the compatibility of the Transaction with the internal market.
(285) Under the first sentence of the second subparagraph of Article 6(2) of the Merger Regulation, the Commission may attach to its decision conditions and obligations intended to ensure that the undertakings concerned comply with the commitments they have entered vis-à-vis the Commission with a view to rendering the concentration compatible with the internal market.
(286) The fulfilment of the measures that give rise to the structural change of the market is a condition, whereas the implementing steps that are necessary to achieve this result are generally obligations on the parties. Where a condition is not fulfilled, the Commission's decision declaring the concentration compatible with the internal market is no longer applicable. Where the undertakings concerned commit a breach of an obligation, the Commission may revoke the clearance decision in accordance with Article 6(3) of the Merger Regulation. The undertakings concerned may also be subject to fines and periodic penalty payments under Articles 14(2) and 15(1) of the Merger Regulation.
(287) In accordance with the basic distinction between conditions and obligations described in the preceding paragraph, the Commitments in section B of the Annex constitute conditions attached to this decision, as only through full compliance therewith can the structural changes in the relevant markets be achieved. The other commitments set out in the Annex constitute obligations, as they concern the implementing steps which are necessary to achieve the modifications sought in a manner compatible with the internal market.
11. CONCLUSION
(288) For the above reasons, the Commission has decided not to oppose the notified operation as modified by the Commitments and to declare it compatible with the internal market and with the functioning of the EEA Agreement, subject to full compliance with the conditions in section B of the Commitments annexed to the present decision and with the obligations contained in the other sections of the said commitments. This decision is adopted in application of Article 6(1)(b) in conjunction with Article 6(2) of the Merger Regulation and Article 57 of the EEA Agreement.
Case M. 9686 – Mitsui / Belchim Crop Protection COMMITMENTS TO THE EUROPEAN COMMISSION
Pursuant to Article 6(2) of Council Regulation (EC) No 139/2004 (the “Merger Regulation”), Mitsui & CO Ltd. through its wholly owned subsidiary Mitsui AgriScience International SA NV (together “Mitsui” or the “Notifying Party”) hereby enter into the following Commitments (the “Commitments”) vis-à-vis the European Commission (the “Commission”) with a view to rendering the acquisition of sole control of Belchim Crop Protection NV (“Belchim”) (the “Concentration”) compatible with the internal market and the functioning of the EEA Agreement.
This text shall be interpreted in light of the Commission’s decision pursuant to Article 6(1)(b) of the Merger Regulation of the Merger Regulation to declare the Concentration compatible with the internal market and the functioning of the EEA Agreement (the “Decision”), in the general framework of European Union law, in particular in light of the Merger Regulation, and by reference to the Commission Notice on remedies acceptable under Council Regulation (EC) No 139/2004 and under Commission Regulation (EC) No 802/2004 (the “Remedies Notice”).
To the extent that any of the conditions or obligations contained in these Commitments need to be performed by Belchim, the Notifying Party procures to implement and comply with all such conditions and obligations on Belchim’s behalf, even where not expressly stated below.
Section A. Definitions
1. For the purpose of the Commitments, the following terms shall have the following meaning:
1,4 Sight PGR Germany and Poland Divestment Business: the business as described in the Schedule, paragraph 12.
1,4 Sight PGR Nordics Divestment Business: the business as described in the Schedule, paragraph 13.
Affiliated Undertakings: undertakings controlled by the Parties and/or by the ultimate parents of the Parties, whereby the notion of control shall be interpreted pursuant to Article 3 of the Merger Regulation and in light of the Commission Consolidated Jurisdictional Notice under Council Regulation (EC) No 139/2004 on the control of concentrations between undertakings (the "Consolidated Jurisdictional Notice").
Alternative PGR Divestment Package: the 1,4 Sight PGR Germany and Poland Divestment Business, and the 1,4 Sight PGR Nordics Divestment Business.
Assets: the tangible and intangible assets and rights that contribute to the current operation, or are necessary to ensure the viability and competitiveness of the Divestment Business as indicated in Section B, paragraph 2 and 3, and described more in detail in the Schedule.
Belchim: Belchim Crop Protection NV, incorporated under the laws of Belgium, with its registered office at Technologielaan 7, 1840 Londerzeel, Belgium and registered with the Commercial/Company Register at the Crossroads Bank of Enterprises under number 0458.909.077.
Biox-M PGR Germany and Poland Divestment Business: the business as described in the Schedule, paragraph 6.
Biox-M PGR Nordics Divestment Business: the business as described in the Schedule, paragraph 7.
Certis: Certis Europe BV, incorporated under the laws of the Netherlands, with its registered office at Stadsplateau 16, 3521az Utrecht, the Netherlands and registered with the Commercial/Company Register at Crossroads Bank of Enterprises under number 0450.470.176.
Closing: the Transfer of the Divestment Business to the Remedy-Taker(s).
Closing Period: the period of […] from the approval of the Remedy-Taker(s) and the terms of Transfer by the Commission.
Confidential Information: any business secrets, know-how, commercial information, or any other information of a proprietary nature that is not in the public domain.
Conflict of Interest: any conflict of interest that impairs the Trustee's objectivity and independence in discharging its duties under the Commitments.
Divestment Business: either (i) the Primary PGR Divestment Package; or in the event of the Notifying Party’s failure to transfer the Primary PGR Divestment Package within the First PGR Divestment Period, (ii) the Alternative PGR Divestment Package; and (iii) the Paraffinic Oil Netherlands Divestment Business – each as more fully described in the Schedule.
Divestiture Trustee: one or more natural or legal person(s) who is/are approved by the Commission and appointed by the Notifying Party and who has/have received from the Notifying Party the exclusive mandate to transfer the Divestment Business to one or more Remedy-Takers at no minimum price.
Effective Date: the date of adoption of the Decision.
First PGR Divestiture Period: the period of […] from the Effective Date, provided that this period may be shortened by the Monitoring Trustee, after consultation with the Commission, in response to a motivated request of the Notifying Party.
Hold Separate Manager: the person or persons appointed by the Notifying Party to undertake the functions set out in paragraph 10 of section B of these Commitments under the supervision of the Monitoring Trustee.
Monitoring Trustee: one or more natural or legal person(s) who is/are approved by the Commission and appointed by the Notifying Party, and who has/have the duty to monitor the Notifying Party’s compliance with the conditions and obligations attached to the Decision.
Nordics: comprise, for the purposes of these Commitments, Denmark, Finland, Norway and Sweden.
Paraffinic Oil Divestiture Period: the period of […] from the Effective Date.
Paraffinic Oil Netherlands Divestment Business: the business as described in the Schedule, paragraphs 8-10.
Paraffinic Oil Transitional Period: the period until […] after Closing or […] following the grant by the Dutch crop protection registration agency of the product registration for Fibro in the Netherlands (with a maximum of […] after Closing), whichever period is longer.
Parties: the Notifying Party and the undertaking that is the target of the concentration.
PGR: Plant Growth Regulators.
PGR Transitional Period: the period of […] starting from Closing, which can be extended at the discretion of the Monitoring Trustee for up to […], upon receipt of a motivated request by the Remedy-Taker(s).
Primary PGR Divestment Package: the Biox-M PGR Germany and Poland Divestment Business, and the Biox-M PGR Nordics Divestment Business.
Purchaser Criteria: the criteria laid down in paragraph 14 of these Commitments that the Remedy-Taker must fulfil in order to be approved by the Commission.
Remedy-Taker: the entity or entities approved by the Commission as transferee of the Divestment Business in accordance with the criteria set out in Section D, which for the avoidance of doubt may include a current Supplier of the products that are the subject matter of the Divestment Business provided the criteria in Section D are satisfied.
Remedy Markets: the PGR post-harvest market for potatoes in Sweden, Denmark, Finland, Norway, Germany and Poland; the paraffinic oil for application on seed potatoes market in the Netherlands and the paraffinic oil for application on flower bulbs market in the Netherlands.
Schedule: the schedule to these Commitments describing more in detail the Divestment Business.
Second PGR Divestiture Period: the period of […] from the end of the First Divestiture Period.
Supplier: Xeda, in respect of the supply of Biox-M to Certis, which is the subject matter of the Primary PGR Divestment Package; Dormfresh, in respect of the supply of 1,4 Sight to Belchim, which is the subject matter of the Alternative PGR Divestment Package; and CCL, with whom Belchim has a relationship in respect of Fibro, which is the subject matter of the Paraffinic Oil Netherlands Divestment Business.
Transfer: the divestment, whether by assignment or transfer, to the Remedy- Taker(s) of the Primary PGR Divestment Package or the Alternative PGR Divestment Package and the Paraffinic Oil Netherlands Divestment Business, it being understood that the Notifying Party will use its best efforts to make the Assets available to the Remedy Taker(s) on substantially the same terms as those enabling the distribution by the Parties of the products that are the subject matter of Divestment Business.
Trustee(s): the Monitoring Trustee.
Trustee Divestiture Period: the period of […] from the end of the Second PGR Divestiture Period and / or the Paraffinic Oil Divestiture Period.
Section B. The commitment to Transfer the Divestment Business
Commitment to Transfer
2. In order to maintain effective competition, the Notifying Party commits to Transfer, or procure the Transfer of the Divestment Business by the end of the Trustee Divestiture Period to one or more Remedy-Takers and on terms of Transfer approved by the Commission in accordance with the procedure described in paragraph 15 of these Commitments. To carry out the Transfer, the Notifying Party commits to find one or more Remedy-Takers and to enter into, or procure to enter into, final binding agreements for the Transfer of (i) the Primary PGR Divestment Package within the First PGR Divestiture Period or the Alternative PGR Divestment Package within the Second PGR Divestiture Period, and (ii) the Paraffinic Oil Netherlands Divestment Business within the Paraffinic Oil Divestiture Period, as described in paragraph 3 below.
3. More specifically, the Notifying Party commits to the following:
(a) To Transfer within the First PGR Divestiture Period:
(i) the Biox-M PGR Germany and Poland Divestment Business to one Remedy-Taker; and
(ii) the Biox-M PGR Nordics Divestment Business to one Remedy-Taker, which may be the same Remedy-Taker of the PGR Germany and Poland Divestment Business;
(b) If the Parties have not entered into such relevant agreement(s) relating to the Biox-M PGR Germany and Poland and the Biox-M PGR Nordics Divestment Business by the end of the First PGR Divestiture Period, the Notifying Party commits to Transfer within the Second PGR Divestiture Period:
(i) The 1,4 Sight PGR Germany and Poland Divestment Business to one Remedy-Taker; and
(ii) the 1,4 Sight PGR Nordics Divestment Business to one Remedy-Taker, which may be the same Remedy-Taker of the 1,4 Sight PGR Germany and Poland Divestment Business.
(c) To Transfer within the Paraffinic Oil Divestiture Period the Paraffinic Oil Netherlands Divestment Business to one Remedy-Taker, which may be the same Remedy-Taker of the PGR Germany and Poland Divestment Business and/or the PGR Nordics Divestment Business.
4. If the Parties have not entered into such relevant agreement(s) with respect to the Alternative PGR Divestment Package by the end of the Second PGR Divestiture Period and/or the Paraffinic Oil Netherlands Divestment Business by the end of the Paraffinic Oil Divestiture Period, the Notifying Party shall grant or procure Belchim to grant the Divestiture Trustee an exclusive mandate to Transfer such relevant Divestment Business in accordance with the procedure described in paragraph 27 in the Trustee Divestiture Period.
5. The Concentration shall not be implemented before the Parties or the Divestiture Trustee have entered into a final binding agreement with the Remedy-Taker(s) and, if different from the Remedy-Taker(s), the relevant Supplier for the Transfer of each Divestment Business, and the Commission has approved the Remedy-Taker(s) and the terms of each Transfer in accordance with paragraph 15.
6. The Notifying Party shall be deemed to have complied with these Commitments if by the end of the Trustee Divestiture Period, the Notifying Party, Belchim or the Divestiture Trustee, as the case may be, has entered into a final binding agreement for the Transfer of each Divestment Business, and the Commission approves the proposed Remedy-Taker(s) and the terms of each Transfer as being consistent with the Commitments in accordance with the procedure described paragraph 15, and the Transfer of the Divestment Business to the Remedy-Taker(s) has taken place within the Closing Period.
7. In order to maintain the structural effect of the Commitments, the Notifying Party shall, for a period of 10 years after Closing, not acquire, whether directly or indirectly, the possibility of exercising influence (as defined in paragraph 43 of the Remedies Notice, footnote 3) over the whole or part of the Divestment Business, unless, following the submission of a reasoned request from the Notifying Party showing good cause and accompanied by a report from the Monitoring Trustee (as provided in paragraph 41 of these Commitments), the Commission finds that the structure of the market has changed to such an extent that the absence of influence over the Divestment Business is no longer necessary to render the proposed concentration compatible with the internal market.
Structure and definition of the Divestment Business
8. The Divestment Business consists of the crop protection products from Certis or Belchim, as the case may be, from each of the Remedy Markets. The legal and functional structure of the Divestment Business as operated to date is described in the Schedules. The Divestment Business, described in more detail in the Schedules, includes all Assets that contribute to the current operation or are necessary to ensure the viability and competitiveness of the Divestment Business, in particular and to the extent relevant in the context of the Divestment Business:
(a) all tangible and intangible Assets (including intellectual property rights);
(b) all licences, permits and authorisations issued by any governmental organisation for the benefit of the Divestment Business; and
(c) all contracts, commitments and customer orders of the Divestment Business; all customer, credit and other records of the Divestment Business, including any lists of prospective customers for products in the scope of the Divestment Business which have not yet been brought to the market by the time of Transfer.
Section C. Related commitments
Preservation of viability, marketability and competitiveness
9. From the Effective Date until Closing, the Notifying Party shall preserve or procure the preservation of the economic viability, marketability and competitiveness of the Divestment Business, in accordance with good business practice, and shall minimise as far as possible any risk of loss of competitive potential of the Divestment Business. In particular the Parties undertake:
(a) not to carry out any action that might have a significant adverse impact on the value, management or competitiveness of the Divestment Business or that might alter the nature and scope of activity, or the industrial or commercial strategy or the investment policy of the Divestment Business;
(b) to make available, or procure to make available, sufficient resources for the development of the Divestment Business, on the basis and continuation of the existing business plans;
Ongoing viability obligations
10. Until Closing, the Notifying Party shall assist the Monitoring Trustee in ensuring that the Divestment Business is managed in the best interest of the business with a view to ensuring its continued economic viability, marketability and competitiveness and shall appoint a Hold Separate Manager for this purpose. The Hold Separate Manager shall closely cooperate with and report to the Monitoring Trustee and, if applicable, the Divestiture Trustee. The Commission may, after having heard the Parties, require the Parties to replace the Hold Separate Manager.
Due diligence
11. In order to enable potential Remedy-Takers to carry out a reasonable due diligence of the Divestment Business, the Parties shall, subject to customary confidentiality assurances and dependent on the stage of the divestiture process, provide to potential Remedy-Takers sufficient information as regards the Divestment Business.
Reporting
12. To the extent necessary, the Parties shall submit written reports in English on potential Remedy-Takers of the Divestment Business and developments in the negotiations with such potential Remedy-Takers to the Commission and the Monitoring Trustee no later than 10 days after the end of every month following the Effective Date (or otherwise at the Commission’s request). The Parties shall submit a list of all potential Remedy-Takers having expressed interest in acquiring the Divestment Business to the Commission at each and every stage of the divestiture process, as well as a copy of all the letters of intent made by potential Remedy- Takers within five days of their receipt.
13. The Parties shall inform the Commission and the Monitoring Trustee on the preparation of any data room documentation and any due diligence procedure and shall submit a copy of any information memorandum to the Commission and the Monitoring Trustee before sending any such memorandum out to potential Remedy- Takers.
Section D. The Remedy-Taker(s)
14. In order to be approved by the Commission, any Remedy-Taker must fulfil the following criteria:
(a) The Remedy-Taker shall be independent of and unconnected to the Parties and their Affiliated Undertakings (this being assessed having regard to the situation following the Transfer).
(b) The Remedy-Taker shall have the financial resources, proven expertise and incentive to maintain and develop the Divestment Business as a viable and active competitive force in competition with the Parties and other competitors;
(c) The Remedy-Taker(s) shall be companies already active in the crop protection industry;
(d) The acquisition of the Divestment Business by the Remedy-Taker must neither be likely to create, in light of the information available to the Commission, prima facie competition concerns nor give rise to a risk that the implementation of the Commitments will be delayed. In particular, the Remedy-Taker must reasonably be expected to obtain all necessary approvals from the relevant regulatory authorities for the acquisition of the Divestment Business.
15. The final binding documentation (as well as ancillary agreements) relating to the Transfer of the Divestment Business shall be conditional on the Commission’s approval. When the Parties have reached an agreement with a Remedy-Taker, the Notifying Party shall submit a fully documented and reasoned proposal, including a copy of any relevant final agreement(s), within one week to the Commission and the Monitoring Trustee. The Parties must be able to demonstrate to the Commission that the Remedy-Taker fulfils the Purchaser Criteria and that the Divestment Business is being transferred in a manner consistent with the Commission’s Decision and the Commitments. For the approval, the Commission shall verify that the Remedy-Taker fulfils the Purchaser Criteria and that the Divestment Business is being transferred in a manner consistent with the Commitments including their objective to bring about a lasting structural change in the market. The Commission may approve the Transfer of the Divestment Business without one or more Assets, or by substituting one or more Assets with one or more different assets, if this does not affect the viability and competitiveness of the Divestment Business after the Transfer, taking account of the proposed Remedy-Taker.
Section E. Trustee
I. Appointment procedure
16. The Notifying Party shall appoint a Monitoring Trustee to carry out the functions specified in these Commitments for a Monitoring Trustee.
17. If the Parties have not entered into any relevant binding agreement for the Transfer of the Divestment Business one month before the end of the Second Divestiture Period or the Paraffinic Oil Divestiture Period, or if the Commission has rejected a Remedy-Taker proposed by the Parties at that time or thereafter, the Notifying Party shall appoint a Divestiture Trustee. The appointment of the Divestiture Trustee shall take effect upon the commencement of the Trustee Divestiture Period.
18. The Trustee shall:
(i) at the time of appointment, be independent of the Notifying Party and its Affiliated Undertakings;
(ii) possess the necessary qualifications to carry out its mandate, for example have sufficient relevant experience as an investment banker or consultant or auditor;
(iii) neither have nor become exposed to a Conflict of Interest; and
(iv) during the term of his mandate and for a period of two years following termination of his mandate, not provide services to the Notifying Party or its Affiliated Undertakings without first obtaining the Commission’s prior approval.
19. The Trustee shall be remunerated by the Notifying Party in a way that does not impede the independent and effective fulfilment of its mandate. In particular, where the remuneration package of a Divestiture Trustee includes a success premium linked to the final transfer value of the Divestment Business, such success premium may only be earned if the Transfer takes place within the Trustee Divestiture Period.
Proposal by the Notifying Party
20. No later than two weeks after the Effective Date, the Notifying Party shall submit the name or names of one or more natural or legal persons whom the Notifying Party proposes to appoint as the Monitoring Trustee to the Commission for approval. No later than one month before the end of the First Divestiture Period or on request by the Commission, the Notifying Party shall submit a list of one or more persons whom the Notifying Party proposes to appoint as Divestiture Trustee to the Commission for approval. The proposal shall contain sufficient information for the Commission to verify that the person or persons proposed as Trustee fulfil the requirements set out in paragraph 18 and shall include:
(a) the full terms of the proposed mandate, which shall include all provisions necessary to enable the Trustee to fulfil its duties under these Commitments;
(b) the outline of a work plan which describes how the Trustee intends to carry out its assigned tasks;
(c) an indication whether the proposed Trustee is to act as both Monitoring Trustee and Divestiture Trustee or whether different trustees are proposed for the two functions.
Approval or rejection by the Commission
21. The Commission shall have the discretion to approve or reject the proposed Trustee(s) and to approve the proposed mandate subject to any modifications it deems necessary for the Trustee to fulfil its obligations. If only one name is approved, the Notifying Party shall appoint or cause to be appointed the person or persons concerned as Trustee, in accordance with the mandate approved by the Commission. If more than one name is approved, the Notifying Party shall be free to choose the Trustee to be appointed from among the names approved. The Trustee shall be appointed within one week of the Commission’s approval, in accordance with the mandate approved by the Commission.
New proposal by the Notifying Party
22. If all the proposed Trustees are rejected, the Notifying Party shall submit the names of at least two more natural or legal persons within one week of being informed of the rejection, in accordance with paragraphs 16 and 21 of these Commitments.
Trustee nominated by the Commission
23. If all further proposed Trustees are rejected by the Commission, the Commission shall nominate a Trustee, whom the Notifying Party shall appoint, or cause to be appointed, in accordance with a trustee mandate approved by the Commission.
II. Functions of the Trustee
24. The Trustee shall assume its specified duties and obligations in order to ensure compliance with the Commitments. The Commission may, on its own initiative or at the request of the Trustee or the Notifying Party, give any orders or instructions to the Trustee in order to ensure compliance with the conditions and obligations attached to the Decision.
Duties and obligations of the Monitoring Trustee
25. The Monitoring Trustee shall:
(i) propose in its first report to the Commission a detailed work plan describing how it intends to monitor compliance with the obligations and conditions attached to the Decision.
(ii) oversee, in close co-operation with the Hold Separate Manager, the ongoing management of the Divestment Business with a view to ensuring its continued economic viability, marketability and competitiveness and monitor compliance by the Parties with the conditions and obligations attached to the Decision. To that end the Monitoring Trustee shall:
(a) monitor the preservation of the economic viability, marketability and competitiveness of the Divestment Business, in accordance with paragraphs 9 and 10 of these Commitments;
(b) supervise the management of the Divestment Business as a saleable entity;
(c) with respect to Confidential Information:
- determine all necessary measures to ensure that the Parties do not after the Effective Date obtain any Confidential Information relating to the other Party’s Divestment Business, and
- decide whether such information may be disclosed to or kept by the Parties as the disclosure is reasonably necessary to allow the Parties to carry out the Transfer or as the disclosure is required by law;
(iii) propose to the Notifying Party such measures as the Monitoring Trustee considers necessary to ensure the Parties’ compliance with the conditions and obligations attached to the Decision, in particular the maintenance of the full economic viability, marketability or competitiveness of the Divestment Business, and the non-disclosure of competitively sensitive information;
(iv) review and assess potential Remedy-Takers as well as the progress of the Transfer process and verify that, dependent on the stage of the Transfer process, potential Remedy-Takers receive sufficient and correct information relating to the Divestment Business in particular by reviewing, if available, the data room documentation, the information memorandum and the due diligence process, and
(v) act as a contact point for any requests by third parties, in particular potential Remedy-Takers, in relation to the Commitments;
(vi) provide to the Commission, sending the Parties a non-confidential copy at the same time, a written report within 15 days after the end of every month that shall cover the operation and management of the Divestment Business so that the Commission can assess whether the business is held in a manner consistent with the Commitments and the progress of the Transfer process as well as potential Remedy-Takers;
(vii) promptly report in writing to the Commission, sending the Parties a non- confidential copy at the same time, if it concludes on reasonable grounds that the Parties are failing to comply with these Commitments;
(viii) within one week after receipt of the documented proposal referred to in paragraph 15 of these Commitments, submit to the Commission, sending the Parties a non-confidential copy at the same time, a reasoned opinion as to the suitability and independence of the proposed Remedy-Taker and the viability of the Divestment Business after the Transfer and as to whether the Divestment Business is discontinued in a manner consistent with the conditions and obligations attached to the Decision, Divestment Business taking account of the proposed Remedy-Taker;
(ix) assume the other functions assigned to the Monitoring Trustee under the conditions and obligations attached to the Decision.
26. If the Monitoring and Divestiture Trustee are not the same legal persons, the Monitoring Trustee and the Divestiture Trustee shall cooperate closely with each other during and for the purpose of the preparation of the Trustee Divestiture Period in order to facilitate each other's tasks.
Duties and obligations of the Divestiture Trustee
27. Within the Trustee Divestiture Period, the Divestiture Trustee shall assign at no minimum price the Divestment Business to a Remedy-Taker, provided that the Commission has approved both the Remedy-Taker and the final binding agreement for the Transfer of the Divestment Business (and ancillary agreements) as in line with the Commission's Decision and the Commitments in accordance with paragraphs 14 and 15 of these Commitments. The Divestiture Trustee shall include in the agreement for the Transfer of the Divestment Business (as well as in any ancillary agreements) such terms and conditions as it considers appropriate for an expedient Transfer in the Trustee Divestiture Period. In particular, the Divestiture Trustee may include in the agreement for the Transfer of the Divestment Business such customary representations and warranties and indemnities as are reasonably required to effect the Transfer. The Divestiture Trustee shall protect the legitimate financial interests of the Parties, subject to the Notifying Party’s unconditional obligation to Transfer at no minimum price in the Trustee Divestiture Period.
28. In the Trustee Divestiture Period (or otherwise at the Commission’s request), the Divestiture Trustee shall provide the Commission with a comprehensive monthly report written in English on the progress of the Transfer process. Such reports shall be submitted within 15 days after the end of every month with a simultaneous copy to the Monitoring Trustee and a non-confidential copy to the Parties.
III. Duties and obligations of the Parties
29. The Parties shall provide and shall cause its advisors to provide the Trustee with all such co-operation, assistance and information as the Trustee may reasonably require, to perform its tasks. The Trustee shall have full and complete access to any of the Parties or the Divestment Business’ books, records, documents, management or other personnel, facilities, sites and technical information necessary for fulfilling its duties under the Commitments and the Parties shall provide the Trustee upon request with copies of any document. The Parties shall make available to the Trustee one or more offices on their premises and shall be available for meetings in order to provide the Trustee with all information necessary for the performance of its tasks.
30. The Parties shall provide the Monitoring Trustee with all managerial and administrative support that it may reasonably request on behalf of the management of the Divestment Business. This shall include all administrative support functions relating to the Divestment Business which are currently carried out at headquarters level. The Parties shall provide and shall cause its advisors to provide the Monitoring Trustee, on request, with the information submitted to potential Remedy- Takers, in particular give the Monitoring Trustee access to the data room documentation and all other information granted to potential Remedy-Takers in the due diligence procedure. The Parties shall inform the Monitoring Trustee on possible Remedy-Takers, submit lists of potential Remedy-Takers at each stage of the selection process, including the offers made by potential Remedy-Takers at those stages, and keep the Monitoring Trustee informed of all developments in the Transfer process.
31. The Parties shall grant or procure Affiliated Undertakings to grant comprehensive powers of attorney, duly executed, to the Divestiture Trustee to effect the Transfer (including ancillary agreements), the Closing and all actions and declarations which the Divestiture Trustee considers necessary or appropriate to achieve the Transfer and the Closing, including the appointment of advisors to assist with the Transfer process. Upon request of the Divestiture Trustee, the Parties shall cause the documents required for effecting the Transfer and the Closing to be duly executed.
32. The Notifying Party shall indemnify the Trustee and its employees and agents (each an “Indemnified Party”) and hold each Indemnified Party harmless against, and hereby agrees that an Indemnified Party shall have no liability to the Notifying Party for, any liabilities arising out of the performance of the Trustee’s duties under the Commitments, except to the extent that such liabilities result from the wilful default, recklessness, gross negligence or bad faith of the Trustee, its employees, agents or advisors.
33. At the expense of the Notifying Party, the Trustee may appoint advisors (in particular for corporate finance or legal advice), subject to the Notifying Party’s approval (this approval not to be unreasonably withheld or delayed) if the Trustee considers the appointment of such advisors necessary or appropriate for the performance of its duties and obligations under the Mandate, provided that any fees and other expenses incurred by the Trustee are reasonable. Should the Notifying Party refuse to approve the advisors proposed by the Trustee the Commission may approve the appointment of such advisors instead, after having heard the Notifying Party. Only the Trustee shall be entitled to issue instructions to the advisors. Paragraph 32 of these Commitments shall apply mutatis mutandis. In the Trustee Divestiture Period, the Divestiture Trustee may use advisors who served the Notifying Party during the Divestiture Period if the Divestiture Trustee considers this in the best interest of an expedient transfer.
34. The Parties agree that the Commission may share Confidential Information proprietary to the Parties with the Trustee. The Trustee shall not disclose such information and the principles contained in Article 17 (1) and (2) of the Merger Regulation apply mutatis mutandis.
35. The Notifying Party agrees that the contact details of the Monitoring Trustee are published on the website of the Commission's Directorate-General for Competition and they shall inform interested third parties, in particular any potential Remedy- Takers, of the identity and the tasks of the Monitoring Trustee.
36. For a period of 10 years from the Effective Date the Commission may request all information from the Parties that is reasonably necessary to monitor the effective implementation of these Commitments.
IV. Replacement, discharge and reappointment of the Trustee
37. If the Trustee ceases to perform its functions under the Commitments or for any other good cause, including the exposure of the Trustee to a Conflict of Interest:
(a) the Commission may, after hearing the Trustee and the Notifying Party, require the Notifying Party to replace the Trustee; or
(b) the Notifying Party may, with the prior approval of the Commission, replace the Trustee.
38. If the Trustee is removed according to paragraph 37 of these Commitments, the Trustee may be required to continue in its function until a new Trustee is in place to whom the Trustee has effected a full hand over of all relevant information. The new Trustee shall be appointed in accordance with the procedure referred to in paragraphs 16-23 of these Commitments.
39. Unless removed according to paragraph 37 of these Commitments, the Trustee shall cease to act as Trustee only after the Commission has discharged it from its duties after all the Commitments with which the Trustee has been entrusted have been implemented. However, the Commission may at any time require the reappointment of the Monitoring Trustee if it subsequently appears that the relevant remedies might not have been fully and properly implemented.
Section F. The review clause
40. The Commission may extend the time periods foreseen in the Commitments in response to a request from the Notifying Party or, in appropriate cases, on its own initiative. Where the Notifying Party requests an extension of a time period, it shall submit a reasoned request to the Commission no later than one month before the expiry of that period, showing good cause. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time send a non- confidential copy of the report to the Notifying Party. Only in exceptional circumstances shall the Notifying Party be entitled to request an extension within the last month of any period.
41. The Commission may further, in response to a reasoned request from the Notifying Party showing good cause waive, modify or substitute, in exceptional circumstances, one or more of the undertakings in these Commitments. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time send a non-confidential copy of the report to the Notifying Party. The request shall not have the effect of suspending the application of the undertaking and, in particular, of suspending the expiry of any time period in which the undertaking has to be complied with.
Section G. Entry into force
42. The Commitments shall take effect upon the date of adoption of the Decision.
Duly authorised for and on behalf of Mitsui:
SCHEDULE
1. The Divestment Business is part of the Parties’ overall crop protection business offering and does not currently operate as a standalone business or entity. As a result, the Divestment Business will be carved out of Certis’ or Belchim’s (as the case may be) crop protection businesses.
2. In accordance with section B of these Commitments, the Notifying Party commits to the following:
(a) Transfer within the First PGR Divestiture Period
(i) the Biox-M PGR Germany and Poland Divestment Business the scope of which is defined in paragraph 6 of this Schedule; and
(ii) the Biox-M PGR Nordics Divestment Business the scope of which is defined in paragraph 7 of this Schedule.
(b) If the Parties have not entered into the relevant agreement(s) relating to the Biox-M PGR Germany and Poland Divestment Business and the Biox-M PGR Nordics Divestment Business at the end of the First Divestiture PGR Period, the Notifying Party commits to Transfer within the Second PGR Divestiture Period:
(i) the 1,4 Sight PGR Germany and Poland Divestment Business, the scope of which is defined in paragraph 12 of this Schedule; and
(ii) the 1,4 Sight PGR Nordics Divestment Business, the scope of which is defined in paragraph 13 of this Schedule.
(c) Transfer within the Paraffinic Oil Divestiture Period the Paraffinic Oil Netherlands Divestment Business, the scope of which is defined in paragraphs 8-10 of this Schedule.
3. Each Divestment Business is described in further detail below. For the avoidance of doubt, any Assets that are also used outside of the scope of the Divestment Business will be licensed to the Remedy-Taker on a royalty-free basis exclusively for use by the Remedy-Taker in relation to the Divestment Business as required pursuant to these Commitments, but will not be transferred to the Remedy-Taker. Where any such Assets are not owned by the Parties, the Parties shall use their best efforts to procure that they are made available to the Remedy-Taker by any third-party owner on reasonable commercial terms.
4. If there is any Asset which is not covered explicitly by these Commitments but which is used (exclusively or not) in relation to any Divestment Business and is necessary for the continued viability and competitiveness of any Divestment Business, as confirmed by the Monitoring Trustee, Asset (or adequate substitute) will be made available to the Remedy-Taker(s) to the extent that Asset is owned by the relevant party, and if it is not, the relevant party will use its best efforts to procure that the Asset (or adequate substitute) is made available to the Remedy- Taker on reasonable commercial terms.
Primary PGR Divestment Package
5. The Primary PGR Divestment Package comprises (i) the Biox-M PGR Germany and Poland Divestment Business; and (ii) the Biox-M PGR Nordics Divestment Business.
6. The Biox-M PGR Germany and Poland Divestment Business includes the following Assets, insofar as they are directly related to Biox-M in Germany and Poland:
(a) the distribution rights pursuant to Certis’ agreement with Xeda;
(b) all relevant marketing and technical materials;
(c) access, whether by means of an irrevocable, non-exclusive, royalty-free licence or otherwise, to the Biox-M logo developed by Certis;
(d) at the request of the Remedy-Taker, any relevant machinery for application of Biox-M;
(e) all relevant customer contracts, commitments and customer orders;
(f) all relevant customer, credit and other lists and records, including any lists of prospective customers and any non-binding season forecasts prepared by the customers, historical sales record by customer and future orders where available, and estimates of stock levels at customer level; and any of Certis’ own forecasts for the 2021-2022 season with the assumptions relied on;
(g) at the request of the Remedy-Taker, the benefit of education and training during the PGR Transitional Period;
(h) any remaining stock of Biox-M, at the price purchased from Xeda, with any applicable packaging and labels, pursuant to which the Remedy-Taker can sell out such remaining stock using Certis’ labels, or re-label existing packaging for sale by the Remedy-taker at the Remedy-Taker’s cost.
7. The Biox-M PGR Nordics Divestment Business includes the following Assets, insofar as they are directly related to Biox-M in Denmark, Finland, Norway and Sweden (the Nordics):
(a) the distribution rights pursuant to Certis’ agreement with Xeda;
(b) all relevant marketing and technical materials;
(c) access, whether by means of an irrevocable, non-exclusive, royalty-free licence or otherwise, to the Biox-M logo developed by Certis;
(d) at the request of the Remedy-Taker, any relevant machinery for the application of Biox-M;
(e) all relevant customer contracts, commitments and customer orders;
(f) all relevant customer, credit and other lists and records, including any lists of prospective customers and any non-binding season forecasts prepared by the customers, historical sales record by customer and future orders where available, and estimates of stock levels at customer level; and any of Certis’ own forecasts for the 2021-2022 season with the assumptions relied on;
(g) at the request of the Remedy-Taker, the benefit of any education and training during the PGR Transitional Period;
(h) any remaining stock of Biox-M, at the price purchased from Xeda, with any applicable packaging and labels pursuant to which the Remedy-Taker can sell out such remaining stock using Certis’ labels, or re-label existing packaging for sale by the Remedy-taker at the Remedy-Taker’s cost.
Paraffinic Oil Netherlands Divestment Business
8. The Paraffinic Oil Netherlands Divestment Business, includes the following Assets, insofar as they are directly related to Fibro in the Netherlands, it being understood that the objective of the Transfer of these Assets is to enable the Remedy-Taker to bring Fibro to the market in the Netherlands:
(a) the data development, distribution and custom formulation agreement in respect of Belchim’s Fibro based on paraffinic oil CAS 8042-47-5 in respect of the Netherlands for application on seed potatoes and flower bulbs;
(b) access, whether by means of an irrevocable, non-exclusive, royalty-free licence or otherwise, to the Fibro trademark, which is registered to Belchim, for use in connection with the Paraffinic Oil Netherlands Divestment Business;
(c) all relevant customer lists and records, including any lists of prospective customers and to the extent available any non-binding season forecasts prepared by the customers, their future orders; and any of Belchim’s own forecasts for the 2021-2022 season with the assumptions relied on;
(d) the product registration application (dossier number 20171129 ZTG) for Fibro in the Netherlands submitted to the Dutch crop protection registration agency, or, in the event that Fibro has been registered in the Netherlands by the time of Closing, the product registration of Fibro in the Netherlands;
(e) all relevant documentation in the possession of Belchim relating to the aforementioned product registration of Fibro in the Netherlands;
(f) access, whether by means of an irrevocable, non-exclusive, royalty-free licence or otherwise, to (i) all necessary data in relation to the active ingredient registration of paraffinic oil CAS 8042-47-5 to obtain or maintain the product registration of Fibro for application on seed potatoes and flower bulbs in the Netherlands; (ii) the studies (including internal studies) and field trial results started or completed at Closing in relation to the Paraffinic Oil Netherlands Divestment Business for the purpose of introducing and selling Fibro for application on seed potatoes and flower bulbs in the Netherlands. Where the consent of third parties is required, the Parties will use their best efforts to obtain those third-party consents. For the avoidance of doubt, nothing in the Paraffinic Oil Netherlands Divestment Business prevents the Parties from using the aforementioned studies, field trials or any other data to develop products with paraffinic oil in the EEA or elsewhere for any type of use, with the exception of products with paraffinic oil CAS 8042-47-5 for application on seed potatoes and flower bulbs in the Netherlands.
9. In addition, the Paraffinic Oil Netherlands Divestment Business may, at the request of the Remedy-Taker, include the following during the Paraffinic Oil Transitional Period:
(a) the benefit of education and training; and
(b) the benefit of reasonable support to obtain the registration of Fibro.
10. At the request of the Remedy-Taker, the Parties will use their best efforts to enable the Remedy-Taker to join any (i) existing task forces in relation to the active ingredient of paraffinic oil CAS 8042-47-5 to which the Parties are a member (subject to third party consents) and; (ii) any future task forces to which the Parties may become a member, provided that, in all cases, the Remedy-Taker shall agree to abide by the relevant terms and conditions of the applicable task force agreement and the standards that are applied by the Parties (as the case may be) in the ordinary course and pay its pro rata share of costs.
Alternative PGR Divestment Package
11. The Alternative PGR Divestment Package comprises (i) the 1,4 Sight PGR Germany and Poland Divestment Business; and (ii) the 1,4 Sight PGR Nordics Divestment Business. In accordance with section B of these Commitments, the Notifying Party commits to Transfer the Alternative PGR Divestment Package as a “crown jewel” remedy if the Parties have not entered into relevant Transfer agreement(s) relating to the Biox-M Germany and Poland Divestment Business, and/or the Biox-M PGR Nordics Divestment Business by the end of the First PGR Divestiture Period.
12. The 1,4 Sight PGR Germany and Poland Divestment Business includes the following Assets, insofar as they are directly related to 1,4 Sight in Germany and Poland:
(a) the distribution rights pursuant to Belchim’s agreement with DormFresh;
(b) all relevant marketing and technical materials;
(c) at the request of the Remedy-Taker, any relevant machinery for application of 1,4 Sight;
(d) all relevant customer contracts, commitments and customer orders;
(e) all relevant customer, credit and other lists and records, including any lists of prospective customers and any non-binding season forecasts prepared by the customers, historical sales record by customer and future orders where available, and estimates of stock levels at customer level; and any of Belchim’s own forecasts for the 2021-2022 season with the assumptions relied on;
(f) at the request of the Remedy-Taker, the benefit of any education and training during the PGR Transitional Period;
(g) any remaining stock of 1,4 Sight, at the price purchased from DormFresh, with any applicable packaging and labels pursuant to which the Remedy- Taker can sell out such remaining stock using Belchim’s labels, or re-label existing packaging for sale by the Remedy-taker at the Remedy-Taker’s cost.
13. The 1,4 Sight PGR Nordics Divestment Business includes the following Assets, insofar as they are directly related to 1,4 Sight in the Nordics:
(a) the distribution rights pursuant to Belchim’s agreement with DormFresh;
(b) all relevant marketing and technical materials;
(c) at the request of the Remedy-Taker, any relevant machinery for application of 1,4 Sight;
(d) all relevant customer contracts, commitments and customer orders;
(e) all relevant customer, credit and other lists and records, including any lists of prospective customers and any non-binding season forecasts prepared by the customers, historical sales record by customer and future orders where available, and estimates of stock levels at customer level; and any of Belchim’s own forecasts for the 2021-2022 season with the assumptions relied on;
(f) at the request of the Remedy-Taker, the benefit of any education and training during the PGR Transitional Period;
(g) any remaining stock of 1,4 Sight, at the price purchased from DormFresh, with any applicable packaging and labels pursuant to which the Remedy- Taker can sell out such remaining stock using Belchim’s labels, or re-label existing packaging for sale by the Remedy-taker at the Remedy-Taker’s cost.
1 OJ L 24, 29.1.2004, p. 1 (the ‘Merger Regulation’). With effect from 1 December 2009, the Treaty on the Functioning of the European Union (‘TFEU’) has introduced certain changes, such as the replacement of ‘Community’ by 'Union' and 'common market' by 'internal market'. The terminology of the TFEU will be used throughout this decision.
2 For the purposes of this Decision, although the United Kingdom withdrew from the European Union as of 1 February 2020, according to Article 92 of the Agreement on the withdrawal of the United Kingdom of Great Britain and Northern Ireland from the European Union and the European Atomic Energy Community (OJ L 29, 31.1.2020, p. 7), the Commission continues to be competent to apply Union law as regards the United Kingdom for administrative procedures which were initiated before the end of the transition period.
3 OJ L 1, 3.1.1994, p. 3 (the 'EEA Agreement').
4 Publication in the Official Journal of the European Union No C 447, 23.12.2020, p. 20.
5 Once it acquires the initial 30% of Belchim’s share capital, Mitsui [control rights](see Form CO, paragraph 6).
6 The remaining 38% will be controlled by ISK Biosciences (28%), wholly owned by Ishihara Sangyo Kaisha Ltd. (‘ISK’), and Mitsui Chemicals (10%).
7 Turnover calculated in accordance with Article 5 of the Merger Regulation and the Commission Consolidated Jurisdictional Notice (OJ C 95, 16.4.2008, p. 1).
8 See Form CO, paragraph 78.
9 See Form CO, paragraph 79.
10 Annex 210 to the Form CO.
11 Please note that in its precedents, for its competitive analysis, the Commission also considered crop groups for the sake of convenience. See, for example, M.7932 – Dow / DuPont (2017), paragraph 319.
12 Fungicides are products aimed at controlling fungal diseases in crops.
13 Herbicides are crop protection products used to kill unwanted weeds (i.e. weeds that compete with crops for water, light, nutrients and space).
14 Insecticides are crop protection products that target insects that damage cultivated crops. Insecticides have several modes of application, such as foliar application or soil application.
15 Acaricides are crop protection products that target acari, which includes ticks and mites.
16 PGRs are agrochemical products that influence the growth of crops or crop products, as they inhibit, stimulate, or modify plant growth and development.
17 Seed treatments are crop protection products that target seeds to protect them at an early stage of their development. They consist of either fungicides or insecticides or a combination of both with other chemical substances. Given that the Parties’ activities do not give rise to affected markets with regard to seed treatment, these products will not be further assessed in this decision.
18 M.9095 – UPL / Arysta Lifescience (2019), paragraph 17.
19 M.7932 – Dow / DuPont (2017), paragraph 1224. Due to the multitude of markets that result as the consequence of this approach, in its competitive analysis the Commission examined groupings of crops and groupings of types of pest. There are a number of different insects and insect groups which, for the sake of convenience for the competitive assessment, could broadly be classified as ‘sucking type’ insects or ‘chewing type’ insects. See M.7932 – Dow / DuPont (2017), paragraph 1162, M.7962 – ChemChina/Syngenta (2017), paragraphs 121 and 124.
20 M.7932 – Dow / DuPont (2017), paragraph 1224; M.7962 – ChemChina / Syngenta (2017), paragraph 124.
21 Case M.2547, Bayer/Aventis Crop Science (2002), paragraphs 810-823; Case M.1806, AstraZeneca/Novartis (2000), paragraph 77; Case M.3465, Syngenta CP/Advanta (2004), paragraph 28; Case M.5675, Syngenta/Monsanto’s Sunflower Seed Business (2010), paragraphs 102-109; Case M.6141, CNAC/Koor Industries/Makhteshim Agan Industries (2011), paragraphs 25 and 31; Case M.7962, ChemChina/Syngenta (2017), paragraph 143.
22 Case M.6141 – China National Agrochemical Corporation/Koor Industries/Makhteshim Agan Industries, OJ C 309/01, 21.10.2011, paragraph 27; Case M.1932 – BASF/American Cynamid (AHP), OJ C354/08, 09.12.2000, paragraph 28; Case M.1806 – AstraZeneca/Novartis, OJ L 110/1, 26.07.2000, paragraph 78; Case M.737 – Ciba-Geigey/Sandoz, OJ L201,/01, 17.07.1996, paragraph 123, M.7962 – ChemChina / Syngenta (2017), paragraph 138, M.9095 – UPL / Arysta Lifescience (2019), paragraph 17.
23 Potato PGRs are used for their effect on the crop’s products rather than the crop itself. Regardless of whether they are applied on the potato plants in the field (pre-harvest) or on the potato tubers in storage (post-harvest), they are all used to suppress sprouting in stored potatoes.
24 Case M.9095 – UPL / Arysta Lifescience (2019), paragraph 17.
25 Molluscicides are crop protection products designed to combat snails and other types of molluscs. They generally consist of chemically treated baits which are spread onto the soil. In case M.2547 – Bayer / Aventis Crop Science (2002), paragraph 527, the Commission considered that molluscicides form a separate product market as they cannot be substituted by other insecticides and that a breakdown by type of crop would not be appropriate since the formulation of molluscicides does not vary according to the plants affected.
26 Soil fumigants are used to prepare and clean up soil that has been used for intensive crop production by sterilising the soil and removing any remaining pests and diseases. They are applied by specialists before planting, since they also kill crops and they are specialised chemicals. They are usually used for high- value crops, because they are more effective but also more expensive than other chemicals. In case M.6141 – CNCA / Koor Industries /Makhteshim Agan Industries (2011), paragraph 24, the Commission found that soil fumigants constitute a distinct product market, but did not envisage any further segmentation.
27 Case M.7932 – Dow/Dupont (2017), paragraph 1642.
28 Case No. IV/M1591- Castrol/Carless/JV (1999), paragraph 14.
29 See Form CO, paragraph 109.
30 See Form CO, paragraph 106.
31 See Response to RFI 17, of 12 January, paragraph 18.
32 See Response to RFI 17, of 12 January, paragraphs 14 and 15.
33 See Response to RFI 17, of 12 January, paragraphs 15 and 16.
34 See Form CO, paragraph 140.
35 Spearmint oil is sometimes referred to as mint oil, which is the same substance.
36 Following its precedent regarding the possible segmentation of herbicides, the Commission also identified a potato desiccant market. Desiccants are a type of herbicides.
37 Responses to Q1 - Questionnaire to competitors, question 8; Responses to Q2 - Questionnaire to distributors, distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, question 9.
38 Responses to Q1 - Questionnaire to competitors, question 9.
39 Responses to Q2- Questionnaire to distributors, distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, question 24(1).
40 Responses to Q1 - Questionnaire to competitors, question 9.
41 Responses to Q2 - Questionnaire to distributors, distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, questions 10 and 10(1).
42 Paraffinic oils must be registered for a certain application before use. Products must obtain an authorisation before they can be used for a specific application.
43 Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, question 11.
44 Responses to Q1- Questionnaire to competitors, question 10.
45 Responses to Q1 - Questionnaire to competitors, question 10(1).
46 Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, question 11(1) (courtesy translation from German).
47 Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, question 12.
48 Responses to Q1 - Questionnaire to competitors, question 11.
49 Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, question 12(1).
50 Responses to Q1 - Questionnaire to competitors, question 11(1).
51 Responses to Q1 - Questionnaire to competitors, question 11(1).
52 Responses to Q1 - Questionnaire to competitors, question 11(1).
53 Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, question 12(1) (courtesy translation from German).
54 Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, question 20.
55 Responses to Q1 - Questionnaire to competitors, question 19.
56 Annex 288, Biox-M label Denmark – English version.
57 Biox-M product brochure available on the Belgian Certis website, last accessed on 11 January 2021 (https://www.certiseurope.be/fileadmin/BE/Downloads/Solutions/Factsheets/Biox-M/Certis_BIOX- M_folder_6p_FR-min.pdf).
58 Annex 109 to the Form CO, pages 1, 2 and 5.
59 Brochure Kiemremming 2020 (Brochure Sprout Suppression 2020), p. 12 (courtesy translation from Dutch), available at https://www.inagro.be/DNN_DropZone/Nieuws/7059/Brochure_kiemremming_2020.pdf (last accessed on 11 January 2021)
60 See Inagro presentation “Hoe aardappelen bewaren in 2020 zonder CIPC?” (How to store potatoes without CIPC in 2020?), 18 February 2020, slide 16, available at https://www.pibo-campus.be/wp- content/uploads/1-Kiemremming-Tongeren-200218-PIBO_Kurt.pdf (last accessed on 1 February 2021).
61 Responses to Q1 - Questionnaire to competitors, question 22.
62 Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, question 23.
63 Responses to Q1 - Questionnaire to competitors, questions 22(1) and 22(2).
64 Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, question 23(2).
65 A reducing sugar is any sugar that is capable of acting as a reducing agent because it has a free aldehyde group or a free ketone group.
66 Acrylamide is formed in a non-enzymatic reaction known as the Maillard reaction at processing temperatures above 120° C where reducing sugars combine with the free amino acid asparagine.
67 Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, question 23(2).
68 Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, question 23(2).
69 See minutes with a supplier, of 5 January 2021; paragraph 10.
70 Available at https://www.efsa.europa.eu/sites/default/files/acrylamide_en.png (last accessed on 20 January 2021).
71 M.8084 – Bayer / Monsanto (2018), paragraph 1366; M.7962 – ChemChina / Syngenta (2017), paragraph 174; M.7932 - Dow / DuPont (2017), paragraph 332; M.2547 – Bayer / Aventis Crop Science (2002), paragraph 27.
72 M.7932 - Dow / DuPont (2017), paragraph 330; M.2547 – Bayer/Aventis Crop Science (2002), paras. 23- 27; M.1806 – AstraZeneca/Novartis (2000), paras. 82-83; and M.1932 – BASF/American Cyanamid (AHP) (2000), paras. 29-31.
73 M.7932 - Dow / DuPont (2017), paragraphs 320 and 327.
74 M.7932 - Dow / DuPont (2017), paragraph 328.
75 M.7932 - Dow / DuPont (2017), paragraph 329.
76 Responses to Q1 - Questionnaire to competitors, question 12; Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, question 13.
77 Responses to Q1 – Questionnaire to competitors, question 13; Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations, and consultants, question 14.
78 Responses to Q1 - Questionnaire to competitors, questions 13 and 13(1); Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations, and consultants, questions 14 and 14(1).
79 As a result of EU-wide bans which took effect in the course of 2020, in particular in the markets for potato PGRs, the data presented relies exclusively on the Parties' forecasts, since no consultant has available data on these markets.
80 OJ C 31, 5.2.2004, p. 5.
81 Horizontal Merger Guidelines, paragraph 24.
82 Horizontal Merger Guidelines, paragraph 26.
83 Horizontal Merger Guidelines, paragraph 59.
84 Horizontal Merger Guidelines, paragraph 60.
85 Guidelines on the assessment of non-horizontal mergers under the Council Regulation on the control of concentrations between undertakings (“Non-Horizontal Merger Guidelines”) (2008/C 265/07), para 93.
86 Non-Horizontal Merger Guidelines paragraph 94.
87 See Form CO, paragraph 453.
88 See Form CO, paragraph 462.
89 While Certis also distributes paraffinic oil for virus control in Germany, where its products were only temporarily registered for potatoes, Belchim does not distribute paraffinic oil in Germany, [Belchim’s confidential business strategy]. Therefore, there is no current or potential overlap in Germany and this market will not be analysed further.
90 See Form CO, paragraph 457.
91 See Form Co, paragraph 468.
92 See Response to RFI19, of 18 January 2021, paragraphs 1-2. The Notifying Party argues that Certis confidential market intelligence).
93 Responses to Q1 - Questionnaire to competitors, question 23; Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations, and consultants, question 24.
94 Responses to Q1 - Questionnaire to competitors, question 23(1).
95 Responses to Q1 - Questionnaire to competitors, question 43(1).
96 Responses to Q2 - Questionaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations, and consultants, question 26.
97 See Form CO, paragraphs 451, 462, and 465; Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations, and consultants, question 26.
98 Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, question 26(1).
99 Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, question 26(1).
100 See Form CO, paragraph 462.
101 Responses to Q1 - Questionnaire to competitors, question 9(1); Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations, and consultants, questions 24(1) and 25; See Response to RFI to a supplier, of 11 January 2021. See minutes of a call with a customer, of 2 February 2021; paragraphs 4 and 6.
102 Responses to Q2 - Questionnaire to distributors, distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, questions 8(1) and 25.
103 Responses to Q2 - Questionnaire to distributors, distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, question 24(1).
104 Responses to Q1 - Questionnaire to competitors, question 9(1); Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations, and consultants, questions 24(1) and 25.
105 Responses to Q1 - Questionnaire to competitors, questions 9(1) and 24; Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations, and consultants, question 25.
106 Responses to Q1 - Questionnaire to competitors, question 9(1).
107 See Form CO, paragraph 106.
108 Questionnaire to competitors, question 9(1); Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations, and consultants, question 10(1).
109 See Form CO, paragraphs 104, 106 and 471.
110 See Annex 306 to the Response to RFI17, Brice Dupuis, 2017, “Development of a crop management method to control the spread of Potato Virus Y (PVY)”, p. 127.
111 See Response to RFI17, of 8 January 2021, para. 15.
112 See Response to RFI to a supplier, of 11 January 2021, and Questionnaire to competitors, question 9(1).
113 See Response to RFI to a supplier, of 11 January 2021, and Questionnaire to competitors, question 9(1).
114 See minutes of a call with a supplier, of 5 January 2021; paragraph 10; See minutes of a call with another supplier, of 5 January 2021, paragraph 7.
115 Responses to Q1 - Questionnaire to competitors, question 9(1); Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations, and consultants, questions 24(1) and 25.
116 Before the ban on CIPC took effect, there was an affected market under a market definition covering both pre- and post-harvest products in Belgium, where the Parties had a combined share of [60-70]% in 2018. These estimates include sales of CIPC-based products and are therefore not representative of the current or future market situation.
117 In Denmark Certis sells the same spearmint oil product under the brand name Xedamint.
118 Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations, and consultants, question 16.
119 Responses to Q1 - Questionnaire to competitors, question 15.
120 Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations, and consultants, question 19(1).
121 For completeness, the Parties submit that [Certis’ confidential future product strategy].
122 Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations, and consultants, question 16(1).
123 Responses to Q1 - Questionnaire to competitors, question 18.
124 Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations, and consultants, question 17(1).
125 Form CO, para 129.
126 Responses to Q1 - Questionnaire to competitors, question 16(1).
127 Form CO, para 129.
128 See Inagro presentation “Hoe aardappelen bewaren in 2020 zonder CIPC?” (How to store potatoes without CIPC in 2020?), 18 February 2020, slide 15, available at https://www.pibo-campus.be/wp- content/uploads/1-Kiemremming-Tongeren-200218-PIBO_Kurt.pdf (last accessed on 1 February 2021).
129 See minutes of a call with a supplier, of 5 January 2021; paragraph 21.
130 See minutes of a call with a supplier, of 5 January 2021; paragraph 17.
131 Responses to Q1 - Questionnaire to competitors, question 21.1; Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations, and consultants, questions 15.1, 22.1.
132 Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations, and consultants, question 17.1 (courtesy translation from German).
133 See minutes of a call with a supplier, of 5 January 2021; paragraph 22.
134 Responses to Q1 - Questionnaire to competitors, question 18.1.
135 See minutes with a supplier, of 5 January 2021; paragraph 10.
136 See minutes of a call with a supplier, of 5 January 2021; paragraphs 28 and 30.
137 Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations, and consultants, question 21.
138 Responses to Q1 - Questionnaire to competitors, question 20.
139 Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations, and consultants, question 17.
140 Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations, and consultants, question 18.
141 Responses to Q2 - Questionnaire to customers, distributors’ organisations, farmers’ organisations, EU Industry Associations, and consultants, question 20(1).
142 Responses to Q1 - Questionnaire to competitors, questions 16 and 17.
143 See Inagro presentation “Hoe aardappelen bewaren in 2020 zonder CIPC?” (How to store potatoes without CIPC in 2020?), 18 February 2020, slide 44, available at https://www.pibo-campus.be/wp- content/uploads/1-Kiemremming-Tongeren-200218-PIBO_Kurt.pdf (last accessed on 1 February 2021)
144 See NRW Chamber of Agriculture newsletter on plant protection Nr. 31 of 18 September 2020, available at https://www.isip.de/isip/servlet/resource/blob/317742/69051414655595055b522afb52bcc3c6/warndienst- nrw-2020---31--data.pdf (last accessed on 1 February 2020).
145 See Inagro presentation “Hoe aardappelen bewaren in 2020 zonder CIPC?” (How to store potatoes without CIPC in 2020?), 18 February 2020, slides 22-23, available at https://www.pibo-campus.be/wp- content/uploads/1-Kiemremming-Tongeren-200218-PIBO_Kurt.pdf (last accessed on 1 February 2021)
146 Annex 288 to the Form CO, Biox-M label Denmark – English version.
147 Annex 293 to the Form CO, 1,4Sight label Germany.
148 Form CO, para 129.
149 See minutes of a call with a supplier, of 5 January 2021; paragraph 4.
150 In 2018 seed potatoes accounted for 25.2% of the total value of intra-EU trade in potatoes. (Source : The EU potato sector: statistics on production & trade, available at https://europatat.eu/activities/the-eu- potato-sector/ (last accessed on 8 February 2021).
151 See Landbouwleven article “Tijd om alternatieven CIPC correct in te zetten” (Time to apply CIPC alternatives correctly), available at https://www.landbouwleven.be/art/d-20200212-3YUT1C (last accessed on 1 February 2021).
152 Annexes 287 and 302 to the Form CO.
153 See minutes of a call with a supplier, of 5 January 2021; paragraph 18; See minutes of a call with a supplier, of 5 January 2021; paragraph 11.
154 Article 53 of Regulation (EC) No 1107/2009 allows Member States to authorise the placing on the market of plant protection products, in special circumstances and derogating from the regular authorisation process for a period not exceeding 120 days, for a limited and controlled use where such a measure is necessary because of a danger which cannot be contained by measures that are available in the market.
155 Responses to Q2 – Questionnaire to distributors, distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants questions 45(1), 46(1).
156 Responses to Q2 – Questionnaire to distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, question 46(1).
157 See minutes of a call with a distributor, of 25 February 2021; paragraph 14.
158 See minutes of a call with a supplier, of 5 January 2021; paragraph 17.
159 Responses to Q2 – Questionnaire to distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, question 46.
160 Responses to Q1 – Questionnaire to competitors, question 45.
161 Responses to Q2 – Questionnaire to distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, question 46.
162 Responses to Q1 – Questionnaire to competitors, question 45.
163 Responses to Q2 – Questionnaire to distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, question 46(1).
164 Annex 255 to the Form CO.
165 Responses to Q2 – Questionnaire to distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, question 46.
166 Annex 255 to the Form CO.
167 Form CO, paragraphs 297, 298 and 299 and Annex 13.
168 Form CO, Table 1 and Annex 112.
169 Responses to Q1- Questionnaire to competitors, questions 36, 36.1, 37 and 37(1); Q2 – Questionnaire to distributors, distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, questions 37, 37(1), 38 and 38(1).
170 Form CO, paragraphs 297, 298 and 299 and Annex 13.
171 Form CO, Table 1 and Annex 112.
172 Responses to Q1- Questionnaire to competitors, questions 36, 36(1), 37 and 37(1); Q2 – Questionnaire to distributors, distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, questions 37, 37(1), 38 and 38(1).
173 Form CO, paras. 302 and 303 and Annex 13.
174 Form CO, Table 3 and Annex 113.
175 Commission Implementing Regulation (EU) 2020/2087 of 14 December 2020 concerning the non-renewal of the approval of the active substance mancozeb. A grace period of six months for the sale and distribution and then six months for the use has been granted. Distributors will no longer be authorized to sell mancozeb-based products from 4 July 2021 onwards – Notifying Party’s response to RFI 14, question 1.
176 The approval at the EU level of benthiavalicarb is expiring on 30 July 2021.
177 The EU approval period for cyazofamid has been extended by the Commission until 31 July 2021. However in 2019 the Commission circulated a draft proposal for non-renewal of cyazofamid, due to unacceptable risk to non-target arthropods. This draft proposal was followed by a EFSA review that indicates that the risk assessment for non-target arthropods is no longer a critical area of concern, but an open point (data gap) for outdoor uses remains: Form CO, paragraphs 331 and 332.
178 Form CO, paragraph 316 and Annexes 65, 66 and 67.
179 Form CO, paragraph 317.
180 Form CO, paragraph 328.
181 Form CO, paragraph 334.
182 Form CO paragraph 327 and Notifying Party’s response to RFI 14, question 2.
183 Form CO, paragraphs 225 and 314.
184 Responses to Q1- Questionnaire to competitors, questions 36, 36.1, 37 and 37.1; Q2 – Questionnaire to distributors, distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, questions 37, 37(1), 38 and 38(1).
185 Form CO, paragraphs 302 and 303 and Annex 13.
186 Form CO, Table 3 and Annex 113.
187 Form CO, paragraphs 321 and 325.
188 Form CO, paragraph 327.
189 Responses to Q1- Questionnaire to competitors, questions 36, 36(1), 37 and 37(1); Q2 – Questionnaire to distributors, distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, questions 37, 37(1), 38 and 38(1).
190 Form CO, paras. 302 and 303 and Annex 13.
191 Form CO, Table 3 and Annex 113.
192 Notifying Party’s response to RFI 14, question 2.
193 Responses to Q1 - Questionnaire to competitors, questions 36, 36(1), 37 and 37(1); Q2 – Questionnaire to distributors, distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, questions 37, 37(1), 38 and 38(1).
194 Form CO, paras. 302 and 303 and Annex 13.
195 Form CO, Table 3 and Annex 113.
196 Responses to Q1- Questionnaire to competitors, questions 36, 36(1), 37 and 37(1); Q2 – Questionnaire to distributors, distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, questions 37, 37(1), 38 and 38(1).
197 Form CO paragraphs 348 and 349; and Annex 13.
198 For all crops, the sales of Chloor were roughly the double of the sales of Bonanlan. Would this hold for the vegetable crops, Certis share would drop two thirds, resulting in a combined market share of [30-40]% instead of [40-50]%.
199 Form CO Table 4 and Annex 116.
200 Responses to Q1 – Questionnaire to competitors, question 27; and Q2 – Questionnaire to distributors, distributors and farmer organisations, and consultants, question 28.
201 Form CO paragraphs 184 and 401.
202 Form CO, paragraph 172.
203 Form CO, paragraphs 173, 174 and 180.
204 Responses to Q1 – Questionnaire to competitors, questions 28 and 28(1)
205 Responses to Q2 – Questionnaire to distributors, distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, questions 29 and 29(1).
206 Form CO, paragraph 171.
207 Form CO, paragraph 403.
208 Form CO, Table 12 and Annex 166.
209 Idem.
210 Ibidem.
211 Response to Q1 - Questionnaire to competitors, question 29(1) and 30(1).
212 Form CO Table 5 and Annex 117
213 Responses to Q1 - Questionnaire to competitors, questions 31, 31(1), 33 and 33(1); and Q2 – Questionnaire to distributors, distributors’ organisations, farmer organisations, EU Industry Associations and consultants, questions 32, 32(1), 34 and 34(1).
214 Form CO paragraphs 363 and 364; and Annex 13.
215 Form CO paragraph 365 and Annex 222.
216 Form CO Table 6B and Annex 220.
217 Responses to Q1 - Questionnaire to competitors, questions 32, 32(1), 33 and 33(1); Responses to Q2 - Questionnaire to distributors, distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, questions 33, 33(1), 34 and 34(1).
218 Form CO paragraphs 392 and 393; and Annex 13.
219 Form CO paragraph 395.
220 Notifying Party’s response to RFI 15, question 11.
221 Responses to Q2 – Questionnaire to distributors, distributors’ organisations, farmers’ organisations, EU Industry Associations and consultants, question 46.
222 Responses to Q1 – Questionnaire to competitors, question 45.
223 Responses to Q1 – Questionnaire to competitors, questions 43 and 43(1).
224 Notifying Party’s response to RFI 17, question 5, table 2.
225 Notifying Party’s response to RFI 17, question 6, table 3.
226 Idem.
227 Commission's Notice on Remedies acceptable under Council Regulation (EC) No 139/2004 and under Commission Regulation (EC) No 802/2004 (‘Remedies Notice’), OJ C 267, 22.10.2008, p. 1.
228 Remedies Notice, paragraph 9.
229 Case C-202/06 P Cementbouw Handel & Industrie v Commission [2007] ECR 2007 I-12129, paragraph 54.
230 Remedies Notice, paragraphs 9 and 61.
231 Remedies Notice, paragraph 12.
232 Remedies Notice, paragraph 9.
233 Remedies Notice, paragraph 22.
234 Remedies Notice, paragraph 61.
235 Responses to Q3 - Market test - Questionnaire on proposed remedies- Competitors, questions 1 and 1(1); Q4 - Market test - Questionnaire on proposed remedies- Distributors, questions 1 and 1(1); Q5 - Market test - Questionnaire on proposed remedies- Suppliers, questions 1 and 1(1).
236 Idem.
237 Responses to Q3 - Market test - Questionnaire on proposed remedies- Competitors, questions 2 and 2(1); Q4 - Market test - Questionnaire on proposed remedies- Distributors, questions 2 and 2(1); Q5 - Market test - Questionnaire on proposed remedies- Suppliers, questions 3 and 3(1).
238 Response to Q3 - Market test - Questionnaire on proposed remedies- Competitors, question 2(1).
239 Responses to Q4 - Market test - Questionnaire on proposed remedies- Distributors, questions 6 and 6(1).
240 Responses to Q3 - Market test - Questionnaire on proposed remedies- Competitors, questions 3 and 3(1); Q4 - Market test - Questionnaire on proposed remedies- Distributors, questions 3 and 3(1); Q5 - Market test - Questionnaire on proposed remedies- Suppliers, questions 4 and 4(1).
241 Q5 - Market test - Questionnaire on proposed remedies- Suppliers, questions 2 and 2(1).
242 Responses to Q3 - Market test - Questionnaire on proposed remedies- Competitors, question 4; Q4 - Market test - Questionnaire on proposed remedies- Distributors, question 4; Q5 - Market test - Questionnaire on proposed remedies- Suppliers, question 5; and Minutes of a call with a supplier on 28.01.2021 and Minutes of call with another supplier on 28.01.2021.
243 Responses to Q3 - Market test - Questionnaire on proposed remedies- Competitors, question 4(1); Q4 - Market test - Questionnaire on proposed remedies- Distributors, question 4(1); Q5 - Market test - Questionnaire on proposed remedies- Suppliers, question 5(1).
244 Responses to Q3 - Market test - Questionnaire on proposed remedies- Competitors, question 4.2; Q4 - Market test - Questionnaire on proposed remedies- Distributors, question 4(2); Q5 - Market test - Questionnaire on proposed remedies- Suppliers, question 5(2).
245 Responses to Q3 - Market test - Questionnaire on proposed remedies- Competitors, questions 8 and 8(1); Q4 - Market test - Questionnaire on proposed remedies- Distributors, questions 9 and 9(1); Q5 - Market test - Questionnaire on proposed remedies- Suppliers, questions 9 and 9(1).
246 Responses to Q3 - Market test - Questionnaire on proposed remedies- Competitors, question 12; Q4 - Market test - Questionnaire on proposed remedies- Distributors, questions 11 and 11(1);
247 Responses to Q3 - Market test - Questionnaire on proposed remedies- Competitors, questions 6 and 7; Q4 - Market test - Questionnaire on proposed remedies- Distributors, questions 7 and 8. Q5 - Market test -
248 Questionnaire on proposed remedies-Suppliers, questions 7 and 8. See also Minutes of call with a Supplier on 28.01.2021.
249 248 Responses to Q3 - Market test - Questionnaire on proposed remedies- Competitors, questions 9 and 9(1).