Commission, June 8, 2020, No M.9554
EUROPEAN COMMISSION
Decision
ELANCO ANIMAL HEALTH / BAYER ANIMAL HEALTH DIVISION
Subject: Case M.9554 – Elanco Animal Health/Bayer Animal Health Division Commission decision pursuant to Article 6(1)(b) in conjunction with Article 6(2) of Council Regulation No 139/20041 and Article 57 of the Agreement on the European Economic Area2
Dear Sir or Madam,
(1) On 14 April 2020, the Commission received notification of a proposed concentration pursuant to Article 4 of Council Regulation (EC) No 139/2004 (the “Merger Regulation”) by which Elanco Animal Health Inc. (“Elanco”, USA) acquires sole control of Bayer AG’s (“Bayer”, Germany) animal health business (“BAH”) (the “Transaction”).3 Elanco and BAH are designated hereinafter as the “Parties” and Elanco as the “Notifying Party” to the Transaction.
1. THE PARTIES AND THE OPERATION
(2) Elanco is a US-based animal health company that develops, manufactures and markets veterinary products for companion animals (“CA”) and production animals (“PA”) worldwide. Formerly part of the US pharmaceutical group Eli Lilly, Elanco became a fully independent company in March 2019.
(3) BAH comprises Bayer’s animal health business, which is active in the development, production and marketing of veterinary products for CA and PA worldwide. BAH represented approximately 3.5% of Bayer’s turnover in 2019.
(4) On 20 August 2019, the Parties entered into a share and asset purchase agreement pursuant to which Elanco will acquire sole control over BAH. The Transaction would therefore give rise to a concentration within the meaning of Article 3(1)(b) of the Merger Regulation.
2. UNION DIMENSION
(5) Based on 2019 data, the combined aggregate world-wide turnover of the Parties was more than EUR 2 500 million (Elanco: EUR 2 744 million; BAH: EUR 1 571 million). Each of the Parties had an EU-wide turnover of more than EUR 100 million (Elanco: […]; BAH: […]). In at least three Member States, the Parties had combined turnover of more than EUR 100 million and individual turnover of more than EUR 25 million ([…]). Neither of the Parties achieved more than two-thirds of its aggregate EU-wide turnover within one and the same Member State.
(6) The concentration therefore has a Union dimension pursuant to Article 1(3) of the Merger Regulation.
3. MARKET DEFINITION
3.1. Product market definition
3.1.1. Introduction
(7) The Transaction will combine the number four (Elanco) and five (BAH) players in the animal health industry, creating the number two supplier in the EEA and worldwide (in terms of sales) after Zoetis.
(8) As regards product market definition, in previous cases in this sector,4 the Commission has divided animal health products into three main categories: (i) biologicals (such as vaccines);5 (ii) pharmaceuticals; and (iii) feed supplements.6
(9) In the present case, the Parties’ activities mainly overlap in animal pharmaceuticals.7 Animal pharmaceuticals encompass a wide group of products that contain a variety of active substances to prevent or treat a large range of animal diseases and disorders. Within pharmaceuticals, the Commission’s past decisional practice8 distinguishes: (i) parasiticides,9 (ii) otitis products, (iii) antimicrobials, (iv) endocrine treatments, (v) anti-inflammatories, and (vi) analgesics.
(10) Furthermore, when defining relevant product markets in the area of animal pharmaceuticals, the Commission considered the following factors as important:10 (i) species (e.g. cats and dogs within CA; poultry, swine and cattle within PA), (ii) active ingredient/chemical class, (iii) targeted pathology/indication, (iv) mode of administration (“MoA”) (e.g. oral, topical, injection), (v) duration of efficacy for PA; and (vi) duration of the withdrawal period for PA.11 Not each factor was considered for each type of pharmaceuticals.
(11) In the present case, the Transaction gives rise to horizontally affected markets in the supply of pharmaceuticals for PA and CA. The Parties’ activities mainly overlap in relation to the following products: (i) CA parasiticides, antimicrobials, and otitis products and (ii) PA anticoccidials, parasiticides and antimicrobials.12
3.1.2. Companion animals
3.1.2.1. Parasiticides
(a) Commission’s precedents
(12) In its previous decisional practice, the Commission considered that feline and canine parasiticides belong to a single market for CA parasiticides.13
(13) Within parasiticides, the Commission found that products targeting multi-celled parasites (e.g. fleas and worms) and products targeting single-celled parasites (“anticoccidials”) constitute distinct product markets.14 As regards multi-celled parasiticides, the Commission distinguished three categories.15 (i) ectoparasiticides (or ecto products), used to control external parasites (e.g. fleas, ticks), (ii) endoparasiticides (or endo products), used to control internal parasites (e.g. flukes, tapeworms, roundworms), and (iii) endectocides (or endecto products), used to control both external and internal parasites. Given the existence of a certain degree of substitutability, the Commission also envisaged alternative market segments comprising (i) ecto/endecto products together and (ii) endo/endecto products together, but ultimately left the market definition open.16
(14) The Commission also envisaged additional sub-segmentations of the CA parasiticides markets including (i) a segmentation by mode of administration (e.g. oral, injectable, topical), which was ultimately left open;17 and (ii) a segmentation based on the indication/targeted parasites, considering that heartworm products constitute a separate market.18 In contrast, previous decisions did not envisage a segmentation of parasiticides by active ingredient/chemical class (such as isoxazolines (“isox”)) or by duration of efficacy.19
(b) The Parties’ views
(15) The Parties do not contest the fact that products targeting multi-celled parasites and anticoccidials are part of distinct product markets. They also agree with the distinction between (i) ectoparasiticides, (ii) endoparasiticides, and (iii) endectocides. However, they consider that market segments comprising (i) ecto/endecto products and (ii) endo/endecto products are not plausible. The Parties also submit that CA parasiticides are usually authorised for use specifically in either cats or dogs, and cannot be used interchangeably. Conversely, they do not consider meaningful to further segment the markets for parasiticides by mode of administration, indication or chemical class. In particular, they contest the existence of a distinct market segment for isox products on the ground that (i) the chemical class of parasiticides does not drive customer choices and that (ii) products may combine various compounds in order to extract the benefit of multiple different chemical classes. The Parties claim that the differences in terms of mode of administration, indication and chemical class are relevant when assessing closeness of competition rather than identifying separate product markets. The Parties also argue that, within these hypothetical sub-segments, the overlaps between their activities are limited.20
(c) The Commission's assessment
Segmentation between ecto, endo and endecto products
(16) Nothing in the market investigation suggested that the Commission should depart in the present case from its previous practice concerning the distinction between endo, ecto and endecto products.
(17) As regards the alternative market segments, which were envisaged in previous decisions, comprising (i) CA ecto/endecto products and (ii) CA endo/endecto products, the market investigation yielded mixed results.
(18) On the one hand, several market participants consider that endo and ecto products are not interchangeable with endecto products as they are generally used for different indications or situations. For instance, a competitor indicated that, in situations where veterinarians have to “address a specific issue related either to gastroinstestinal worms (EndoP) or fleas and flies (EctoP) […], EndectoP are not relevant from both a medical and customer point of view.” Another player stated that “endecto products are typically used for prevention (e.g., for prevention of heartworm, lungworm, or flea and tick)”, while “Endo products […] are typically used for the treatment of [gastrointestinal] parasites”. Similarly, a customer submitted that “for serious infections, exclusively endo should be used”.21
(19) On the other hand, a number of respondents confirmed the existence of a certain degree of substitutability indicating that endecto products may be used interchangeably with ecto or endo products in some situations. For example, a customer stressed that endo and endecto products are interchangeable “for minor infections” and a competitor submitted that “ENDECTOS products can replace ENDOS products as they cover the endoparasiticide spectrum”. Market participants also explained that “in practice, this depends very much on the customer and prescribing vet’s preferences, as well as the individual products at issue”. Some competitors also emphasised the fact that endecto sales have a “direct impact” on the revenues and trends of endo and ecto products.22
(20) In view of the above, for the purpose of this Decision, the Commission concludes that (i) endo, ecto and endecto products constitute distinct product markets and that (ii) the question of whether there exist alternative product markets comprising endo / endecto products and ecto / endecto products can be left open as it does not affect the Commission’s conclusions regarding the compatibility of the Transaction with the internal market.
Segmentation by species
(21) The results of the market investigation largely confirmed the Parties’ claim that CA parasiticides are usually authorised for use specifically in either cats or dogs, and cannot be used interchangeably. In particular, market participants stressed the facts that (i) CA parasiticides indicated for both cats and dogs are “very rare”, that (ii) drugs are “developed, tested and approved for [a particular pet] and should not be used on a different animal (and vets would not do so)” and that (iii) “giving a dog product to a cat is dangerous and can kill the cat”. Some competitors also explained that, albeit parasiticides for cats and dogs can be marketed under the same brand and based on the same active ingredient, the dosage and the mode of administration is likely to vary from one species to another. For instance, “dogs get a much higher dose of active depending on their weight” and oral parasiticides are more commonly used on dogs than on cats (as it is difficult to administer pills to cats).23
(22) In view of the above, for the purpose of this Decision, the Commission concludes that parasiticides for cats and dogs are likely to constitute distinct product markets. Segmentation by mode of administration
(23) The market investigation was inconclusive on the question of whether the CA parasiticides markets should be further segmented by mode of administration. Although a majority of market participants indicate that CA parasiticides with different modes of administration can be used interchangeably, a significant number of customers disagree.24 Moreover, both customers and competitors consider that the mode of administration is an important parameter of competition in the markets for CA parasiticides.25 This is notably illustrated by the fact that, in some CA parasiticides markets (in particular canine parasiticides), the demand tends to switch to oral products due to their ease of administration.
(24) In any event, for the purpose of this Decision, the segmentation of the markets for CA parasiticides by mode of administration can be left open as it does not affect the Commission’s conclusions regarding the compatibility of the Transaction with the internal market.
Segmentation by indication (heartworm)
(25) The results of the market investigation revealed that market participants have a clear preference for products with a broad spectrum of claims as it is more convenient (i.e. customers favour products that can be used to treat or prevent several parasites rather than using several different products).26 […].27 That being said, the market investigation also confirmed that products for the treatment and prevention of heartworms are likely to constitute a distinct product market. Heartworm is an endoparasitic worm residing in the heart of the host animal, which can be fatal and difficult to treat once contracted. In the EEA, heartworm is endemic only in southern Europe. Given these specific features, market participants consider that heartworm “is a very specific claim and also a unique product”.28 The results of the market investigation did not suggest the existence of distinct market segments for other indications.29
(26) In view of the above, and in line with its previous practice, the Commission concludes that, for the purpose of this Decision, products for the treatment and prevention of heartworm constitute a distinct product market. Segmentation by chemical class
(27) Subject to isox (see below), the results of the market investigation generally suggest that a segmentation of the markets for CA parasiticides by chemical class is not relevant. All competitors and half of the customers consider that parasiticides for CA with different chemical classes can be used interchangeably.
(28) More specifically, a large number of respondents indicated that products based on isox,30 i.e. the newest chemical class of ectoparasiticides (which is also used as endecto products in combination with endo chemical classes), are interchangeable with other parasiticides.31
(29) However, the Commission also found that isox products are subject to different competitive dynamics, with different pricing (isox products are significantly more expensive) and competitive landscape (only a limited number of players have isoxbased products). In recent years, isox products have rapidly increased sales at the expense of older ecto/endecto products and market participants expect the demand for isox products to increase further in the coming years. Some respondents consider that isox products are “changing the dynamic” in CA parasiticides.32 The success of isox products is linked to the facts that (i) they are effective against a wide range of ectoparasites (including fleas and ticks), (ii) they can be administered orally, (iii) they have a long duration of efficacy (between one and three months) and (iv) they act systematically, protecting all parts of the animals’ body. By comparison, older ecto chemical classes are either topical or injectable products, only effective against a sub-set of ectoparasites (or have encountered resistance issues with fleas or ticks) or do not produce systemic effects for one month or longer. For these reasons and also given that isox products are prescription-only (whereas older ecto chemical classes are often also available in the other-than-vet (“OTV”) distribution channel (including over-the-counter (“OTC”) and online distribution channels), isox products are increasingly endorsed by vets.33
(30) In any event, for the purpose of this Decision, the exact delineation of the product market can be left open since the Transaction gives rise to serious doubts as to its compatibility with the internal market regardless of whether isox-based parasiticides for CA constitute a distinct market segment or are part of broader markets for CA ecto and endecto.
Segmentation by duration of efficacy
(31) As previously indicated, the past decisional practice did not consider a segmentation of the CA parasiticides markets by duration of efficacy. Nothing in the market investigation suggested that the Commission should depart in the present case from its previous practice.
(d) Conclusion
(32) Based on the results of the market investigation, for the purposes of this Decision, the Commission concludes that (i) endo, ecto and endecto products for CA; (ii) parasiticides for cats and for dogs; and (iii) products for the treatment and prevention of heartworm constitute distinct product markets/market segments.
(33) For the purposes of this Decision, the Commission also considers that it can be left open (i) whether there exist alternative product markets comprising endo / endecto products and ecto / endecto products for CA; (ii) whether the markets for CA parasiticides should be further segmented by mode of administration; and (iii) whether isox-based parasiticides for CA constitute a distinct market segment or are part of broader markets for CA ecto and endecto, as these alternative market delineations do not affect the Commission’s conclusions regarding the compatibility of the Transaction with the internal market.
3.1.2.2. Antimicrobials
(34) Antimicrobials (also known as antibiotics) are pharmaceutical products that destroy or prevent the growth of microbes such as bacteria, mycoplasma or fungi and treat diseases associated with them. Antimicrobials can be administered in various ways, notably by injection and orally (including as a tablet; a soluble powder, concentrate or solution that is added to drinking water; and as a pre-mix). Antimicrobial products can be used to treat multiple species. There are a number of chemical classes used in antimicrobials.
(a) Commission’s precedents
(35) In previous decisions, the Commission considered possible segmentations based on the following factors: (i) the species, (ii) the active ingredient/chemical class (including sulphonamides, penicillins, cephalosporins, phenicols, fluoroquinolones and tetracyclines), and (iii) the mode of administration (oral vs. injectable).34 The exact delineation of the market was ultimately left open.35
(b) The Parties’ views
(36) The Parties agree with the Commission’s previous position that there is a limited demand-side substitutability between antimicrobials based on different chemical classes. For instance, different chemical classes have received different categorisations from the European Medicines Agency (“EMA”) regarding their impact on disease resistance in humans,36 and those defined as higher risk are not recommended for use except as a last resort. Lower risk classes include macrolides (which form the base of the majority of Elanco’s antimicrobials), alongside pleuromutilins, various types of penicillins, cephalosporins, aminoglycosides, lincosamides and tetracyclines. Higher risk classes include, in particular, fluoroquinolones (which is the basis of BAH’s core Baytril product, which makes up the […] majority of BAH’s sales in Group 1 affected markets37). Moreover, different chemical classes have different properties, meaning that they may typically be used at different stages of a given treatment.
(37) As regards methods of administration, the Parties agree with the Commission’s past assessment that there is limited demand-side substitutability between different methods of administration.
(38) As regards a potential segmentation by species, the Parties argue that many antimicrobials can be administered to a range of species and that distinguishing between different species for the purposes of market definition is not appropriate from both a supply- and demand-side perspective.
(c) The Commission’s assessment
(39) As regards a segmentation by mode of administration (i.e. oral and injectable), the results of the market investigation were mixed. From the demand side, a large majority of customers do not consider antimicrobials with different modes of administration to be interchangeable and some respondents specified that the choice may depend on the circumstances of the individual animal.38 On the other side, the majority of suppliers consider them to be interchangeable, specifying that often a vet will administer the same antimicrobial by injection and the pet owner will follow the treatment at home with an oral version.39
(40) As regards a segmentation by species (i.e. cats and dogs), the results of the market investigation were mixed. From the demand side, the large majority of customers do not consider antimicrobials for different species to be interchangeable, but some respondents specified that most products are indicated for use in both cats and dogs.40 Most suppliers consider that customers use antimicrobials for different species interchangeably to treat the same disease, confirming that many antimicrobials are authorised for use in both cats and dogs.41
(41) Also as regards segmentation by chemical class (the relevant classes for the Parties’ CA antimicrobials being fluoroquinolones, penicillin and sulphonamides), the results of the market investigation were mixed. From the demand side, a large majority of customers do not consider antimicrobials with different chemical classes to be interchangeable, and some respondents further specified that it is linked to the target bacteria.42 However, most suppliers consider that antimicrobials with different chemical classes are interchangeable, while vets tend to have first and second line preferences to treat certain infections.43 One supplier mentioned that “The choice of active ingredient will usually vary depending on the pathogen/bacteria causing the disease. A disease can be caused by a pathogen and several antibiotics can be acceptable for use.”
(d) Conclusion
(42) In the present case, the question of the segmentation of the market by mode of administration, species or chemical class can be left open as the Transaction does not raise serious doubts as to its compatibility with the internal market under any plausible market definition.
3.1.2.3. Otitis products
(a) Commission’s precedents
(43) Otitis externa is an inflammation of the external ear canal. Sometimes, otitis may progress further into the ear (otitis media). Otitis is not a disease in itself but rather a symptom of other diseases, such as an infection. It is common in dogs but less frequent in cats.
(44) According to the Commission’s past decisional practice, otitis pharmaceuticals are considered as a separate market. Previously, the Commission has left open the exact delineation of the relevant market for otitis products as to whether further segmentation based on the mode of administration was warranted, indicating however that the otitis externa market is mainly a topical market and that oral and injectable products are rarely used.44
(b) The Parties’ views
(45) The Parties agree with the Commission’s precedents in that otitis products constitute a separate market and submits that there exist significant differences between (i) a new generation of long-acting otitis products that are applied only once or twice by the veterinarians (“long-acting otitis products”) and (ii) older daily-use otitis products that need to be applied daily for one to three weeks by pet owners (“dailyuse otitis products”). The Parties point to the important difference in price between both products. RBB Economics’ analysis of third party data sources indicates that, for example, in the UK, Osurnia (long-acting product) was approximately […] more expensive than Canaural (daily-use product) in 2019.
(c) The Commission’s assessment
(46) The market investigation confirmed that in line with precedents and the Parties’ views, otitis products constitute a separate product market.45 The market investigation also confirmed the views of the Parties that very significant differences exist based on the duration of efficacy of the products, between long-acting and daily-use otitis products.
(47) Long-acting otitis products currently include in the EEA only the Parties’ products: Elanco’s Osurnia and BAH’s recently launched Neptra. These products are administered by a veterinarian and only require a single (in the case of Neptra) or a dual (in the case of Osurnia) application. Both products are effective against Grampositive bacteria and fungal infections. Long-acting products are significantly more expensive than daily dose products.
(48) Daily-use otitis products cover most of the topical otitis products in the market, including Elanco’s Surolan, Dechra’s Canaural, Vetoquinol’s Aurizon and Oridermyl, Ecuphar’s Conofite Forte, Virbac’s Easotic and Merck’s Posatex. These products normally require daily ear drop administration by pet owners for one to three weeks. Most of these products are effective against both Gram-positive and Gram-negative bacteria, as well as fungal infections.
(49) As long-acting products, Osurnia and Neptra only need to be applied once or twice and are administered by a vet. They eliminate or greatly reduce the risk of noncompliance (i.e. non-adherence to the treatment regimen due to difficulties to apply the products to the animal by pet owners without any training). A significant share of customers are ready to pay a significant premium for a convenient solution which eliminates the need for them to apply the product and the risk of ineffective treatment. In contrast, daily dose products are seen as a cheap option, and will be used in cases where compliance is not a primary concern or for pet owners which prefer a more economical alternative.
(50) In this respect, half of the suppliers consider that long-acting and daily-use otitis product are not interchangeable, while the other half indicate they are interchangeable for customers.46 However, it seems that suppliers that answered that long-acting and daily-use product are interchangeable refer only to substitutability from a medical point of view. For example, one of the suppliers who deemed these products substitutable explains that “from a medical point of view they are interchangeable. The main benefit of long duration products is perceived by pet owners who no longer apply any product at home as it has been administered at the veterinary clinic”.47 Other suppliers considering the products interchangeable, explain that ease of administration and price are key factors for customers and vets to decide on a specific product, as well as the ability of the owner to administer the product.48 Some suppliers consider that even if they believe long-acting and daily-use products are interchangeable, “long acting treatments are more and more used as first line otitis treatment in dogs.”49
(51) Another supplier explains that “compliance with the treatment regime is critical to success. In cases where the vet is concerned about an owners ability to administer the treatment on a daily basis they will opt for a long term treatment option […] The choice would therefore be predominantly based upon […] the ability of the owner to apply the ear suspension for several consecutive days. Both Neptra (Bayer) and Osurnia (Elanco) are applied by the veterinary surgeon in the clinic rather than by the pet owner at home which is ensuring full compliance of treatment and therefore peace of mind for the vets as when a treatment does not work we can never be sure if the diagnostic was not good, if the treatment protocol was not respected by the petowner at home (not easy to put a liquid in the ear of a dog) or if the product failed. For this reason, vets tend to prescribe these long acting products at the place of the short acting ones”.50
(52) Moreover, the vast majority of customers considers that long-acting and daily-use otitis products cannot be used interchangeably.51 For example, one customer explains that “the advantage of long duration products is highly appreciated by customers and veterinarians”.52
(53) In fact, competitors indicated clearly that the most important parameters to choose an otitis product are duration of efficacy and frequency of administration while price, chemical class or reputation of suppliers would be less relevant.53 Customers also identify duration of efficacy and frequency of administration as the most important parameters to select an otitis product, followed by price, while chemical class or reputation of the supplier would be less relevant.54 Long-acting and daily-use products’ main differences are precisely duration of effect and frequency of administration.
(54) Based on the significant differences in terms of price, duration of effect, frequency of administration and customers’ preferences and taking into consideration the results of the market investigation, the Commission considers that long-acting and daily-use products belong to separate product markets.
(55) With regards to mode of administration, the Commission notes that most otitis products are topical and the market investigation did not provide any element suggesting that a segmentation by mode of administration would be meaningful. Moreover, this differentiation would be only relevant for daily-use products. Should the market be segmented by mode of administration, there would be no overlap between the daily-use products of the Parties. Therefore, this distinction will not be further considered in this Decision.
(56) With regards to a distinction by species, it would not be relevant for long-acting products, as long-acting products are only indicated for dogs. With regards to daily-use otitis products, the question as to whether a segmentation by species is needed can be left open as the Transaction does not raise concerns with regards to daily-use otitis products irrespective of the exact product market definition retained.
(d) Conclusion
(57) The Commission concludes, in light of the above, that, for the purposes of this case, long-acting and daily-use otitis products constitute distinct product markets. The precise market definition can be left open as to whether daily-use products for different species (cats and dogs) or via different modes of administration constitute a separate market or form part of a wider daily-use otitis products market, as the Transaction does not raise serious doubts as to its compatibility with the internal market for daily-use otitis products irrespective of whether the relevant product market is defined as comprising all species and all modes of administration or not.
3.1.3. Production animals
3.1.3.1. Parasiticides
(a) Commission’s precedents
(58) The Commission’s decisional practice referred to in Section 3.1.2.1(a) above concerning parasiticides for CA is to a large extent also relevant for PA. In particular, in Eli Lilly/Novartis Animal Health, the Commission adopted for PA the same approach as for CA, (i) distinguishing ecto, endo and endecto products and (ii) envisaging alternative market segments comprising ecto/endecto products and endo/endecto products. In that decision, the Commission also envisaged to subsegment the PA parasiticides markets (i) by species, (ii) by mode of administration, and (iii) by indication, but ultimately left open the exact delineation of the market.55 In contrast, previous decisions did not envisage a segmentation of PA parasiticides by chemical class, period of efficacy or period of withdrawal.
(b) The Parties’ views
(59) The Parties did not provide any specific views regarding the product market definition for PA parasiticides and referred to the approach taken for CA.56
(c) The Commission’s assessment
Segmentation between ecto, endo and endecto products
(60) Nothing in the market investigation suggested that the Commission should depart in the present case from its previous practice concerning the split between endo, ecto and endecto products. As regards the alternative market segments comprising (i) PA ecto/endecto products and (ii) PA endo/endecto products, the market investigation was inconclusive, with respondents providing mixed responses. For instance, a competitor submitted that these products cannot be used interchangeably as “it depends on the type of prevention program and breeding conditions of the target animal group”, while another player indicated that “most customers use endecto for comfort and consider specific products when necessary”, suggesting the existence of a certain degree of demand-side substitutability.57 In any event, for the purpose of this Decision, the Commission considers that the exact scope of the product market can be left open since the Transaction does not give rise to serious doubts as to its compatibility with the internal market under any plausible product market definition.
Segmentation by species
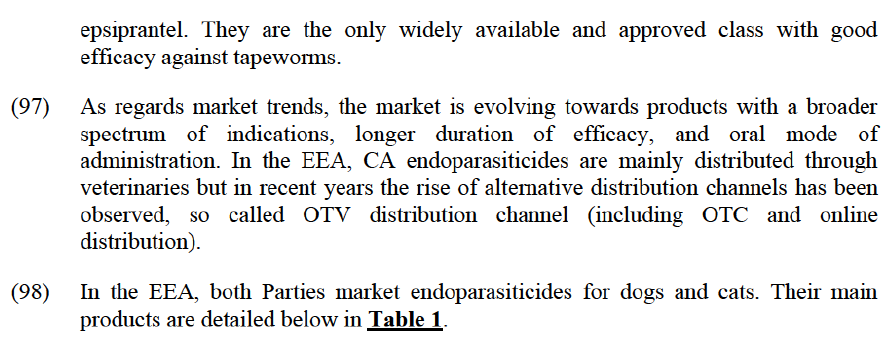
(61) The results of the market investigation largely confirmed that PA parasiticides for different species (e.g. swine, cattle, etc.) cannot be used interchangeably. For example, a competitor explained that “many products are only indicated for a particular species or group of species. This limitation of use is largely driven by: (i) the size of the animal which may justify a different strength/dosage; and (ii) the animal’s intended use (e.g. human consumption).”58 In view of the above, and similarly to CA, the Commission consider that, for the purpose of this Decision, the markets for PA parasiticides should be segmented by species.
Segmentation by mode of administration
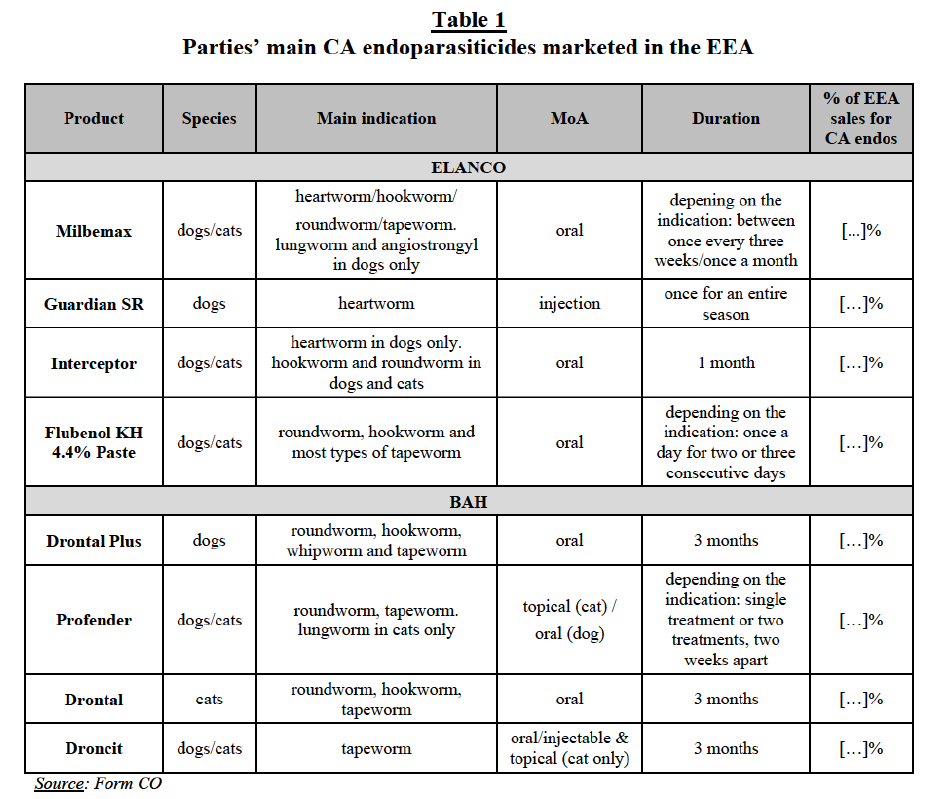
(62) The results of the market investigation suggest some demand-side substitutability between PA parasiticides with different modes of administration. However, some customers also stressed the fact that depending on the situations products with different modes of administration may not be used interchangeably (e.g. “it depends whether I have to do mass treatments, if I have to treat only a few animals, it depends on age and how the animals are housed”).59 In any event, for the purpose of this Decision, the segmentation of the markets for PA parasiticides by mode of administration can be left open since the Transaction does not give rise to serious doubts as to its compatibility with the internal market under any plausible product market definition.
Segmentation by indication
(63) The results of the market investigation were inconclusive as to whether the markets for PA parasiticides should be further segmented by indication. In any event, for the purpose of this Decision, the exact relevant product market definition can be left open since the Transaction does not give rise to serious doubts as to its compatibility with the internal market under any plausible product market definition.
Segmentation by chemical class
(64) The market investigation revealed that PA parasiticides with different chemical classes can be used interchangeably and that the chemical class is not a key parameter of competition. For instance, a competitor stated: “efficacy and price are the main drivers, the chemical class is not that relevant”.60 In any event, for the purpose of this Decision, the exact relevant product market definition can be left open since the Transaction does not give rise to serious doubts as to its compatibility with the internal market under any plausible product market definition.
Segmentation by duration of efficacy or withdrawal period
(65) The past decisional practice did not consider a segmentation of the PA parasiticides markets by period of efficacy or by period of withdrawal. Nothing in the market investigation suggested that the Commission should depart in the present case from its previous practice.
(d) Conclusion
(66) Based on the results of the market investigation, for the purposes of this Decision, the Commission concludes that endo, ecto and endecto products for PA constitute distinct product markets/market segments and that the markets for PA parasiticides should be segmented by species.
(67) For the purposes of this Decision, the Commission also considers that it can be left open (i) whether there exist alternative product markets comprising PA endo / endecto products and PA ecto / endecto products; (ii) whether the markets for PA parasiticides should be further segmented by mode of administration, indication or chemical class, since the Transaction does not give rise to serious doubts as to its compatibility with the internal market under any plausible product market definition.
3.1.3.2. Antimicrobials
(a) Commission’s precedents
(68) The Commission’s decisional practice and the approach outlined in Section 3.1.2.2 (a) above concerning antimicrobials for CA are also applicable to PA. As regards the mode of administration for PA antimicrobials, the Commission previously also considered a possible further sub-segmentation of oral PA antimicrobials into premixes and solubles, but ultimately left the question open.61 In previous cases, the Commission has also considered that mastitis treatments for cattle can be distinguished from other forms of antimicrobial treatment on the basis of their singular mode of administration, namely intra-mammary injections.62 As the Parties’ activities do not overlap in mastitis treatments, this segment will not be considered in the rest of this Decision.
(b) The Parties’ views
(69) The Parties submit the same views for PA antimicrobials as for CA antimicrobials described in Section 3.1.2.2(b). In respect of defining markets for antimicrobials by reference to chemical class or mode of administration, the Parties agree with the Commission’s previous paractice that there can be limited demand-side substitutability between antimicrobials that make use of different chemical classes or modes of administration. Moreover, within oral antimicrobials, the Parties consider that there is a meaningful difference from a customer perspective between pre-mixes and other products like water solubles. As regards a potential segmentation by reference to species, the Parties do not consider this appropriate from both supplyand demand-side substitutability.
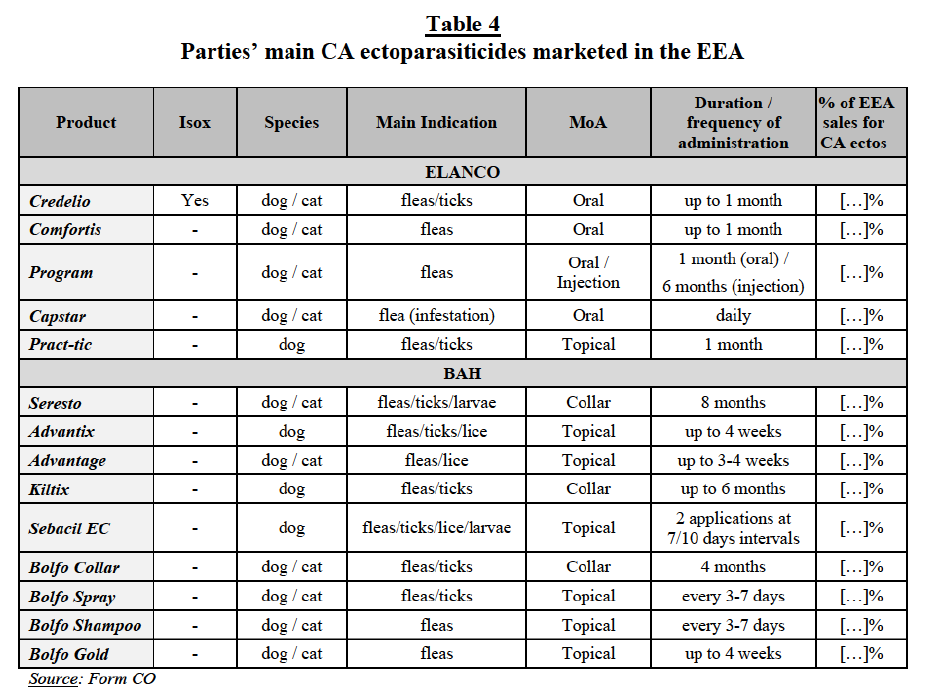
(c) The Commission’s assessment
(70) As regards a segmentation by mode of administration (i.e. oral vs. injectable), the results of the market investigation were not conclusive. From the demand side, most customers do not consider antimicrobials with different modes of administration to be interchangeable, with some respondents specifying “except in certain cases.”63 The majority of suppliers do consider them to be interchangeable, however specifying that it depends on the target indication.64
(71) As regards a segmentation by species, the results of the market investigation confirmed that this is not appropriate. The large majority of customers65 and suppliers responded that most antimicrobials are indicated for multiple species.66
(72) As regards a segmentation by chemical class, the results of the market investigation were mixed. From the demand side, the majority of customers do not consider antimicrobials with different chemical classes to be interchangeable,67 while the large majority of suppliers responded that antimicrobials with different chemical classes are considered interchangeable, specifying that it depends on the target bacteria.68
(d) Conclusion
(73) Based on the results of the market investigation, for the purposes of this Decision the Commission does not consider further segmentation of the PA antimicrobials market by species. The question of segmentation of the market by mode of administration or chemical class can be left open in the present case as the Transaction does not raise serious doubts as to its compatibility with the internal market under any plausible market definition.
3.1.3.3. Anticoccidials
(a) Commission’s precedents
(74) Anticoccidials act against single-celled parasites known as coccidia. Various types of drug are used for coccidiosis prevention and control: (i) prophylactic anticoccidials approved on an EEA-wide scale as preventative feed-in anticoccidials to be administered routinely, in particular for poultry (including turkey); (ii) pharmaceutical coccidiostats authorised as veterinary medicinal products available throughout the EEA for prevention, treatment or aid in the control of coccidiosis (dependent on the pharmacokinetic characteristics of their active substances); and (iii) coccidiosis vaccines: used as a preventative preparation, to induce active immunity against coccidiosis, in particular for poultry.
(75) The Commission has considered PA anticoccidials in previous cases.69 In these cases, the Commission has defined a separate market for poultry anticoccidials compared to anticoccidials for other species. Within poultry anticoccidials, the Commission further defined the following categories: (i) prophylactic anticoccidials, (combining categories (i) and (iii) above in paragraph 74) with a possible market segmentation between vaccines and feed additives, and (ii) pharmaceutical coccidiostats, corresponding to category (ii) above in paragraph 74.70 The subsegmentation of prophylactic anticoccidials between vaccines and feed additives was ultimately left open.
(76) As regards other species (cattle, sheep and swine), the Commission has not previously assessed these products and therefore has not considered any potential segmentation.
(b) The Parties’ views
(77) As regards poultry, the Parties support the above segmentation distinguishing between pharmaceutical coccidiostats and prophylactic anticoccidials, potentially with separate sub-markets within the latter for vaccines and feed additives.
(78) As regards other species, the Parties submit that only pharmaceutical coccidiostats are available on the market and, that for each of cattle, sheep and swine, there is a single market for species-specific pharmaceutical coccidiostats.
(79) The Parties submit that there is no basis for further segmentation by mode of administration or indication, as the relevant anticoccidials are all administered orally, and all products indicated for each of cattle, sheep and swine target all of the coccidiosis-causing species relevant for the respective species.
(c) The Commission’s assessment
(80) As regards the separation of anticoccidials for poultry from other species, and the segmentation of poultry anticoccidials into prophylactic anticoccidials and pharmaceutical coccidiostats, the market investigation did not provide any element to depart from the previous decisional practice and the Parties’ views.71 On this basis, no overlap among the Parties’ activities would arise and therefore poultry products will not be considered further in this Decision.
(81) As regards segmentation by single species for non-poultry species, the market investigation did not confirm the Parties’ views that this is appropriate. The majority of responding competitors and customers confirm that PA pharmaceutical coccidiostats are indicated for multiple species and not single species. Respondents highlighted that there are products indicated for ruminants (i.e. both cattle and sheep) suggesting this possible grouping of species within the non-poultry species72.
(82) As only pharmaceutical coccidiostats are available for non-poultry species, further segmentation between prophylactic anticoccidials and pharmaceutical coccidiostats for these species was not considered meaningful and as such was not investigated further.
(83) The Commission does not consider any further segmentation by mode of administration or indication to be meaningful as the relevant anticoccidials are all administered orally, and all products indicated for each of cattle, sheep and swine target all of the coccidiosis-causing species relevant for the respective species. Furthermore, the market investigation results did not indicate that any segmentations by chemical class, period of efficacy or period of withdrawal are relevant and they are not considered for the purpose of this case.
(d) Conclusion
(84) In the present case, the question of segmentation of the non-poultry PA anticoccidial market by (groups of) species (i.e. considering ruminants and swine separately or together) can be left open as the Transaction raises serious doubts as to its compatibility with the internal market under any plausible market definition.
3.2. Geographic market definition
(a) Commission’s precedents and the Parties’ view
(85) The Commission has consistently defined the geographic markets for marketed animal pharmaceuticals as being national in scope.73 With respect to pipeline pharmaceuticals, the Commission has consistently held that the geographic scope of the market is either global or EEA-wide.74
(86) The Parties agree with the above approach but submits, with respect to marketed products, that there is a growing trend towards competition across borders in the EEA (due to the increasing harmonisation of the regulation at European level and the fact that many animal health players are active in multiple EEA countries), which should be taken into account in the competitive assessment.75
(b) Commission’s assessment and conclusion
(87) As regards marketed products, the results of the market investigation confirmed that the geographic market for animal pharmaceuticals should be defined at national level. However, the market investigation also supports, at least to some extent, the growing supranational dimension of competition put forward by the Parties. For example, although most competitors fix prices and organise their sales and marketing teams at national level, this is also done by some players at regional or EEA level.76 Similarly, although customers typically source animal pharmaceuticals at national level, some of them organise their procurement at regional or EEA level.77 Therefore, for the purpose of this Decision, the Commission considers that the geographic market for marketed animal pharmaceuticals is national in scope, and it will consider the growing supranational dimension of competition in its competitive assessment.
(88) As regards pipeline products, nothing in the market investigation suggests that the Commission should depart in the present case from its previous decisional practice concerning the geographic market definition. In fact, companies which invest significant resources in R&D to develop new products expect to sell those products in as many countries as possible. It is not possible to allocate pipeline products to specific countries. Therefore, pipeline products should be considered at EEA or global level.
4. COMPETITIVE ASSESSMENT
4.1. Methodology for the identification and the assessment of affected markets
(89) In line with Commission precedents in pharmaceutical mergers with a large number of affected markets (involving numerous product and geographic markets), the Commission has applied a system of filters aimed at determining the group of markets where concerns are most likely and on which it focuses its analysis. In line with Commission precedents in the pharmaceutical sector,78 affected markets can be classified in four categories: Group 1, where the Parties' combined market share exceeds 35% and the increment exceeds 1%; Group 1+, where either (i) the combined market share is below 35% (but above 20%), and only one other competitor remains on the market, or where (ii) the combined market share exceeds 35% and the increment is below 1%, but the party with the small increment is a recent entrant;79 Group 2, where the Parties' combined market share exceeds 35% but the increment is below 1%; and Group 3, where the Parties' combined market share is between 20% and 35%, and Group 1+ conditions are not met.
(90) The Commission has analysed all markets affected by the Transaction under all plausible market definitions.
(91) In line with precedents,80 Group 3 markets are not discussed individually in this Decision.81 The Commission assessed the competitive situation in these markets by considering the combined market shares of the Parties and their competitors over the last three years, other factors including the presence of competitors with a significant presence, the date of patent expiry and competition from generics, the Parties’ pipeline products, as well as the results of the market investigation. The Commission reached the conclusion that the Transaction does not raise serious doubts as to its compatibility with the internal market in relation to the possible Group 3 markets arising from the Transaction, due to the limited market shares of the Parties and the presence of significant competitors remaining on the market post-Transaction that will likely sufficiently constrain the merged entity.
(92) Similarly, in line with precedents,82 Group 2 markets are not discussed individually in this Decision. Generally, the Parties have combined market shares below 50%83 with a de minimis increment below 1%. In some markets, combined market shares are above 50%,84 with a de minimis increment below 1%. Post-Transaction, the Parties will face at least three competitors with market shares equal or above the increment (including meaningful players with market share above 5%) in all these markets. Moreover, the market investigation did not reveal any concerns in these markets. In light of the above, the Commission considers that the Transaction is unlikely to remove any significant competitive constraint on the Group 2 markets and, thus, will not lead to any competition concerns on these markets.
(93) In the following, the Commission individually assesses all Group 1 and 1+ markets.
4.2. Companion animals
4.2.1. Parasiticides
4.2.1.1. Endoparasiticides85
(a) Overview of the market and the Parties’ products
(94) As explained in Section 3 above, endoparasiticides are used to control internal parasites, such as flukes, tapeworms and roundworms. Each type of endo-product has a different mode of administration and works on endoparasiticides more or less effectively. The Parties estimate that, in 2018, the total size of the endoparasiticides segment in the EEA was […].86 The Parties explained that the demand for CA endoparasiticides is growing and expected to further grow in the next years due to increasing pet adoption and expenditure on veterinary healthcare.
(95) Endoparasiticides for CA can be administered (i) topically, with a typical frequency of application/duration of a month or once every three months; (ii) by injection, with a typical frequency of application/duration of one time per year; (iii) orally (including both preventive and treatment endoparasiticides; in the former case, they can be administered monthly or quarterly, while in the latter case, they should be administered daily, for a certain number of days).
(96) There are a number of chemical classes used in endo products. The core chemical classes used in currently marketed endo products are: avermectins, benzimidazoles and isoquinoles. Avermectins are a class of endos used also as endectos. This class is effective against intestinal worms, heartworms and lungworms, as well as ectoparasites. Benzimidazoles are a class of endos with a broad spectrum of action and a high safety profile; they are effective against roundworms (such as hookworms and whipworms) and some tapeworms. Isoquinolines include praziquantel and


players (i.e. BI, Merck and Zoetis), which have a significant presence in most EEA national markets, and (ii) several smaller competitors (such as Ceva and Virbac), with market shares comparable to Elanco’s in certain national markets. Second, the Parties claim that their products face fierce competition from generics (several of the Parties’ products being off-patent, including Elanco’s Milbemax, as well as most of BAH’s Drontal family of products). Third, the Parties argue that their products do not closely compete with each other as they are differentiated (i) in terms of spectrum of indications. Elanco’s Milbemax can be used against a wider range of worms, while BAH’s Drontal, Drontal Plus and Profender are not effective against either heartworm or lungworm. In addition, Droncit can only be used in cases of tapeworm and is not effective against other gastro-intestinal worms; and (ii) in terms of distribution channels, Elanco focusing on veterinaries, whereas BAH increasingly distributes its products through the OTV channel. Finally, the Parties argue that barriers to entry from neighbouring geographic markets in which certain CA endoparasiticides are already sold are relatively low.
(102) In any event, the Parties have agreed to divest BAH’s Drontal and Profender families of products in the EEA in order to remove the full overlap between their activities in the market for CA endoparasiticides. More details on this remedy are provided in Section 5 below.
(d) The Commission’s assessment
(103) As a preliminary remark, it should be noted that, unless otherwise specified, the findings set out in this Section 4.2.1.1, and in particular the results of the market investigation, do not materially differ depending on the species and MoAs.89 As regards possible differences depending on the analysed EEA country, although national specificities exist (with e.g. market share variations at national level), the main characteristics of supply and demand in the markets for feline and canine endoparasiticides (and sub-segments) do not appear to vary significantly across countries. Therefore, unless otherwise specified, the findings of Section 4.2.1.1 (regarding e.g. closeness of competition, generic competition and the feedback received from market participants) do not materially differ depending on the geographic market at stake.
(104) For the reasons set out below, the Commission considers that the Transaction raises serious doubts as to the compatibility of the Transaction with the internal market and the functioning of the EEA Agreement in some markets for CA endoparasiticides (and potential sub-segments).
(105) First, the Commission notes that the Transaction leads to large or very large combined market shares in a number of affected markets (see Table 3 above).90 As set out in the Horizontal Merger Guidelines, market shares provide a useful first indication of the market structure and of the competitive importance of both the Parties and their competitor.91 In the present case, the Commission considers that the high market shares would lead to a significant increase of market power and/or the strengthening or creation of a dominant position of the merged entity post- Transaction. More specifically:
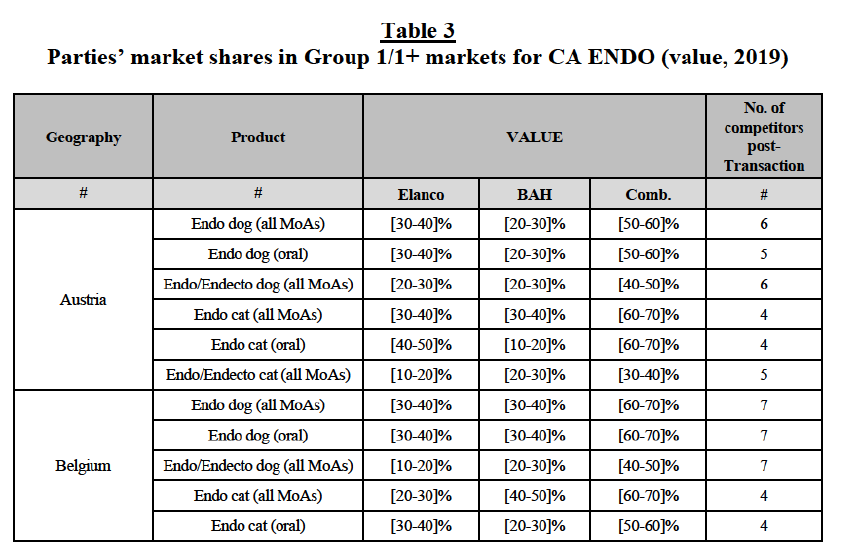
(a) In Austria: the Parties have high combined market shares with a significant increment in (i) the canine endo market, both encompassing all modes of administration and including oral products only ([50-60]% combined market share, with a [20-30]% increment brought by BAH); in (ii) the feline endo market ([60-70]% combined market share, with a [30-40]% increment brought by Elanco); and in (iii) the feline endo market including only products administered orally ([60-70]% combined market share, with a [10- 20]% increment brought by BAH). Post-Transaction, (a) in the canine endo market, both encompassing all modes of administration and including oral products only, the new entity would face five competitors, with Ceva and Virbac being the largest ([10-20]-[10-20]%), followed by Zoetis, Merck and Vetoquinol (with market shares between [5-10]% and below [0-5]%); and (b) in the feline endo market, both encompassing all modes of administration and considering oral products only, the new entity would face four competitors, the largest being Ceva and Virbac, with market shares between [10-20]-[10- 20]%, followed by Merck, Zoetis, Vetoquinol and BI, with market shares below [5-10]%.
(b) In Belgium: the Parties have high combined market shares in (i) the canine endo market, both encompassing all modes of administration and including oral products only ([60-70]% combined market share, with a [30-40]% increment brought by Elanco), in (ii) the feline endo market ([60-70]% combined market share, with a [20-30]% increment brought by Elanco), similarly in the (iii) the feline endo market including only products administered orally ([50-60]% combined market share, with a [20-30]% increment brought by BAH). Post-Transaction, (a) in the canine endo market, both encompassing all modes of administration and considering the products administered orally only, the new entity would face seven competitors, with Ceva and Virbac being the largest (with market shares of [5-10]% and [10- 20]%, respectively); and (b) in the feline endo market, both encompassing all modes of administration and considering oral products only, the new entity would face four competitors, the largest being Virbac and Zoetis, with market shares between [10-20]-[10-20]%, followed by Ceva and Krka, with market shares between [0-5]-[5-10]%.
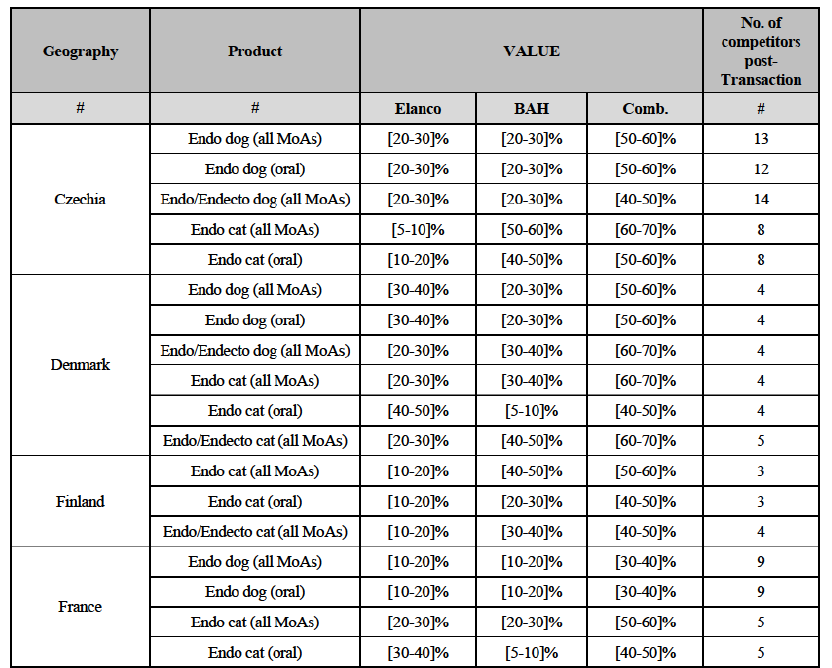
(c) In Czechia: the Parties have high combined market shares in (i) the canine endo market, both encompassing all modes of administration and including oral products only ([50-60]% combined market share, with a [20-30]% increment brought by BAH); and in (ii) the feline endo market, both encompassing all modes of administration and considering oral products only ([60-70]-[50-60]% combined market share, with a [5-10]-[5-10]% increment brought by Elanco). Post-Transaction, (a) in the canine endo market, both encompassing all modes of administration and considering only the products administered orally, the new entity would face 13-12 competitors, all with a very limited market presence (between [5-10]% and below [0-5]% market share), and only with Krka having a larger market share of [10-20]%; and (b) in the feline endo market, both encompassing all modes of administration and considering oral products only, the new entity would face eight competitors, all with a limited market presence (between [5-10]% and below [0-5]% market share) and only with Krka having a larger market share between [10- 20]-[20-30]%.
(d) In Denmark: the Parties have high combined market shares in (i) the canine endo market, both encompassing all modes of administration and considering only oral products ([50-60]% combined market share, with a [20-30]% increment brought by BAH), in (ii) the feline endo market ([60-70]% combined market share, with a [20-30]% increment brought by Elanco). Similarily, the Parties have high combined market shares in (iii) the canine endo/endecto market ([60-70]% combined market share, with a [20-30]% increment brought by Elanco) and (iv) the feline endo/endecto market ([60- 70]% combined market share, with a [20-30]% increment brought by Elanco). Post-Transaction, (a) in the canine endo market, both encompassing all modes of administration and considering only the products administered orally, the new entity would face four competitors, two of which (Merck and Virbac) with a larger market presence between [20-30]-[10-20]% market share and the other two (Ceva and Zoetis) with a negligible market share between [0-5]% and below [0-5]%; similarily, (b) in the feline endo market, the new entity would face four competitors, all with a limited market presence (between [5-10]% and below [0-5]% market share), with the only exception represented by Virbac holding a larger market share of [20-30]%; (c) in the canine endo/endecto market, the new entity would face four competitors, the largest ones being Merck ([10-20]%) and Virbac ([10-20]%) and the other two with a market share between [5-10]% and [0-5]%; and (d) in the feline endo/endecto market, the new entity would face five competitors, the largest one being Virbac ([20-30]%) and the others with a negligible market share between [0-5]% and [0-5]%.
(e) In Finland: the Parties have high combined market shares in the feline endo market ([50-60]% combined market share, with a [10-20]% increment brought by Elanco). Post-Transaction, in the feline endo market, encompassing all modes of administration, the new entity would face only three competitors, the largest one being Merck with a [30-40]% market share and the two remaining ones (Virbac and Zoetis), with market shares between [10-20]% and below [0-5]%.
(f) In France: the Parties have high combined market shares in the feline endo market ([50-60]% combined market share, with a [20-30]% increment brought by BAH). Post-Transaction, in the feline endo market, encompassing all modes of administration the new entity would face five competitors, with the larger ones being Ceva ([10-20]%) and Virbac ([10-20]%), followed by Clément Thekan ([10-20]%), and Merck and Zoetis with a market share between [0-5]% and below [0-5]%.
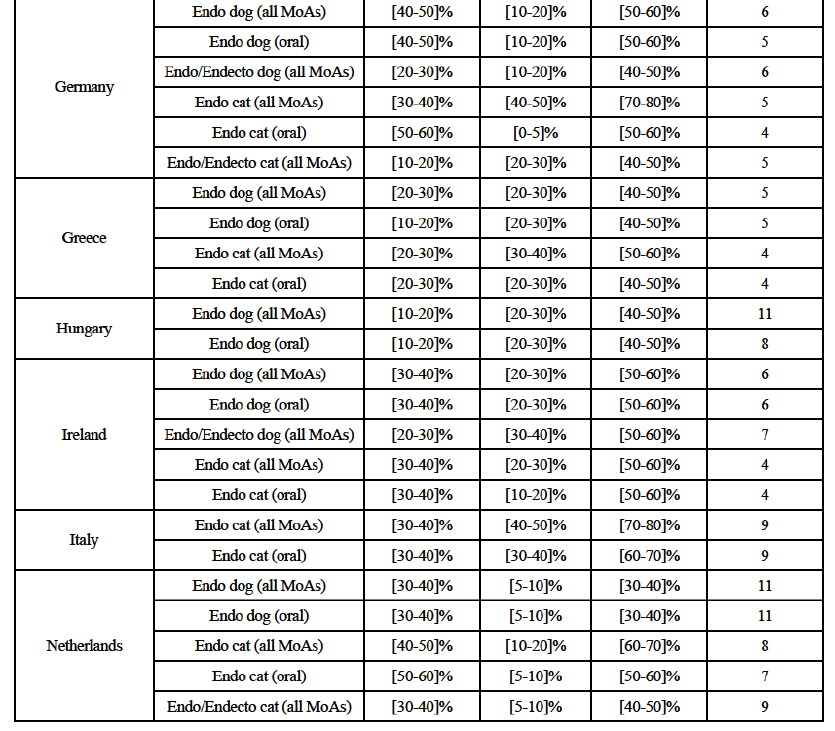
(g) In Germany: the Parties have high combined market shares in (i) the canine endo market, both encompassing all modes of administration and consideringonly oral products ([50-60]% combined market share, with a [10- 20]% increment brought by BAH), in (ii) the feline endo market ([70-80]% combined market share, with a [30-40]% increment brought by Elanco). When considering (iii) the feline endo market, including oral products only, the increment brought by BAH is less significant but still contributes to high combined market shares for the Parties ([50-60]% combined market share, with a [0-5]% increment brought by BAH). Post-Transaction, (a) in the canine endo market, both encompassing all modes of administration and considering only the products administered orally, the new entity will face 6- 5 competitors, the largest being Virbac, Merck and Ceva ([20-30]-[10-20]%) and the others with a more negligible market presence; and (b) in the feline endo market, both encompassing all modes of administration and considering oral products only, the new entity would face 5-4 competitors, the largest being Virbac ([10-20]-[20-30]%) and the others holding a more negligible market share.
(h) In Greece: the Parties have high combined market shares in the feline endo market, encompassing all modes of administration ([50-60]% combined market share, with a [20-30]% increment brought by Elanco). Post- Transaction, in the feline endo market, encompassing all modes of administration, the new entity would face four competitors, with Virbac and Zoetis being the largest ([10-20]-[20-30]%) and the others with a more limited market presence ([10-20]-[0-5]%).
(i) In Ireland: the Parties have high combined market shares in (i) the canine endo market, both encompassing all modes of administration and considering oral products only ([50-60]% combined market share, with a [20-30]% increment brought by BAH); (ii) the feline endo market, both encompassing all modes of administration and considering oral products only ([50-60]-[50- 60]% combined market share, with a [20-30]-[10-20]% increment brought by BAH) and (iii) the canine endo/endecto market ([50-60]% combined market share, with a [20-30]% increment brought by Elanco). Post-Transaction, (a) in the canine endo market, both encompassing all modes of administration and considering only the products administered orally, the new entity would face six competitors, the largest being Virbac ([30-40]%) and the others having a more negligible presence (between [5-10]% and below [0-5]%); (b) in the feline endo market, both encompassing all modes of administration and considering only the products administered orally, the new entity would face four competitors, the largest one being Virbac ([30-40]-[30-40]%) and the others having a more negligible market presence (between [5-10]% and below [0-5]%); and (c) in the canine endo/endecto market, the new entity would face seven competitors, the largest one being Virbac ([30-40]%) and the others having a more negligible market presence (all well below [5- 10]%).
(j) In Italy: the Parties have high combined market shares in the feline endo market, both encompassing all modes of administration and considering only oral products ([70-80]-[60-70]% combined market share, with a [30-40]-[30- 40]% increment brought by Elanco). Post-Transaction, in the feline endo market, both encompassing all modes of administration and considering only oral products, the new entity would face nine competitors, the largest one being Merck ([10-20]-[10-20]%) and all the others with a very negligible market presence equal to or well below [5-10]%.
(k) In the Netherlands: the Parties have high combined market shares in (i) the feline endo market, both encompassing all modes of administration and considering oral products only ([60-70]-[50-60]% combined market share, with a [10-20]-[5-10]% increment brought by BAH). Post-Transaction, in the feline endo market, both encompassing all modes of administration and considering only the products administered orally, the new entity would face 8-7 competitors, with Virbac and Beaphar being the largest ([10-20]-[10- 20]%) and the others with a more limited market presence (between [5-10]% and below [0-5]%).
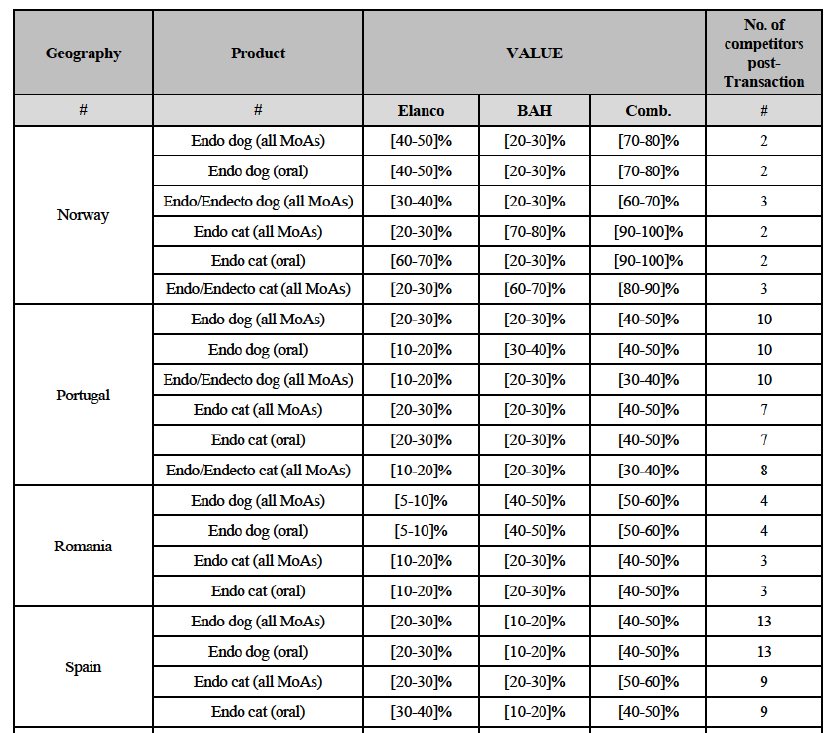
(l) In Norway: the Parties have high combined market shares (i) in the canine endo market, both encompassing all modes of administration and considering oral products only ([70-80]% combined market share, with a [20-30]% increment brought by BAH); (ii) in the feline endo market, the increment brought by Elanco being equally significant, leading to a situation of quasimonopoly ([90-100]% combined market share, with a [20-30]% increment brought by Elanco) and (iii) in the feline endo market, including oral products only ([90-100]% combined market share, with a [20-30]% increment brought by BAH). Still very high is the increment brought by BAH and Elanco, respectively, (iv) in the canine endo/endecto market ([60- 70]% combined market share, with a [20-30]% increment brought by BAH) and (v) in the feline endo/endecto market ([80-90]% combined market share, with a [20-30]% increment brought by Elanco). Post-Transaction, across the various segments, the new entity would face only 2-3 competitors, the largest being Merck and Zoetis.
(m) In Romania: the Parties have high combined market shares in the canine endo market, both encompassing all modes of administration and considering only oral products ([50-60]-[50-60]% combined market share, with a [5-10]- [5-10]% increment brought by Elanco). Post-Transaction, in the canine endo market, both encompassing all modes of administration and considering only oral products, the new entity would face only four competitors, the largest ones being Ceva ([30-40]%) and Krka ([10-20]%), the two others with a negligible market presence equal to or well below [0-5]%.
(n) In Spain: the Parties have high combined market shares in the feline endo market, encompassing all modes of administration ([50-60]% combined market share, with a [20-30]% increment brought by BAH). Post- Transaction, in this segment, the new entity would face 9 competitors, the largest being Virbac ([10-20]%) and the others with a moderate to negligible market presence (between [10-20]% and below [0-5]%).
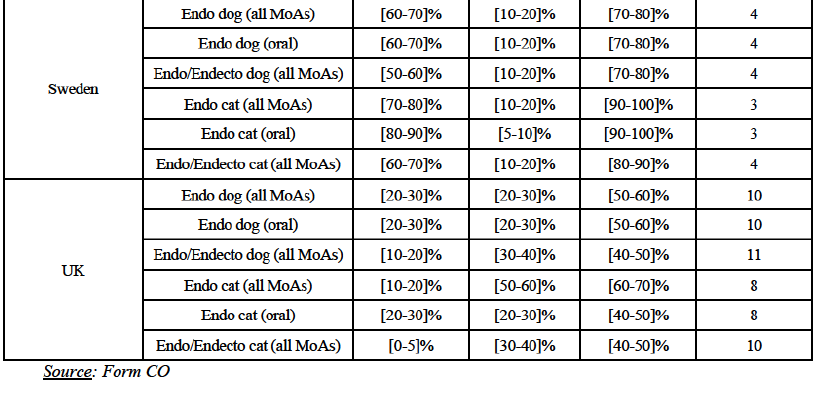
(o) In Sweden: the Parties have high combined market shares (i) in the canine endo market, both encompassing all modes of administration and considering oral products only ([70-80]% combined market share, with a [10-20]% increment brought by BAH); (ii) in the feline endo market, both encompassing all modes of administration and considering oral products only ([90-100]% combined market share, with a [10-20]-[5-10]% increment brought by BAH), leading to a situation of quasi-monopoly, and (iii) in the canine and feline endo/endecto markets ([70-80]-[80-90]% combined market share, with a [10-20]% increment brought by BAH). Post-Transaction, in all segments, the new entity would face only 3-4 competitors, the largest being Merck ([0-5]-[10-20]%) and the others (Zoetis, Virbac and BI) with a much more limited market presence (equal to or well below [5-10]%, with the exception of Zoetis which holds a [10-20]% in the canine endo/endecto market).
(p) In the UK: the Parties have high combined market shares with a significant increment brought by Elanco in (i) the canine endo market, both encompassing all modes of administration and including oral products only, ([50-60]-[50-60]% combined market share, with a [20-30]% increment brought by Elanco); and (ii) the feline endo market, encompassing all modes of administration ([60-70]% combined market share, with a [10-20]% increment brought by Elanco). Post-Transaction, across the various segments, the new entity would face between 10 and 8 competitors, the largest being BI, Merck, Virbac and Zoetis (with market shares varying between [20-30]% and [0-5]% across the different segments) and the others with a more limited market presence (between [5-10]% and, more frequently, below [0-5]%).
(106) Second, the results of the market investigation confirm that Elanco and BAH are perceived as close competitors and as the two largest players in the EEA markets for CA endoparasiticides, followed by BI, Ceva and Virbac. Zoetis and Merck are also mentioned among the top five competitors, although to a lesser extent.92 Competitors also stressed that (i) Elanco is an innovative player and is particularly strong thanks to its well known brands and large portfolio of products and (ii) BAH also benefits from a large portfolio of strong brands and, although less innovative than Elanco, it has a significant presence in the OTV distribution channel, which represents one of its major strengths, against other competitors.93
(107) Third, a large majority of competitors indicated that the Parties are the only alternatives available or among the very few suppliers in the EEA with respect to certain CA endoparasiticides. One competitor indicated that the merged entity would be “Dominant on the tapewormer market with Milbemax, Drontal and Profender”.94 95
(108) Fourth, the market investigation results were mixed with regards to the competitive pressure exercised by generic products over branded products in the market for CA endoparasiticides.
(109) Fifth, respondents to the market investigation indicated that there has been no material originator entry in the EEA in the past three years nor is such entry expected to occur in the next three years in the market for CA endoparasiticides and that, generally, entry in this market is perceived as difficult.96
(110) Finally, both competitors and customers, who responded to the market investigation, confirmed the existence of serious competition concerns in relation to the combination of the Parties’ marketed feline and canine endoparasiticides.
(e) Conclusion
(111) In view of the above considerations, taking into account the market investigation and all the evidence available, the Commission concludes that the Transaction raises serious doubts as to its compatibility with the internal market and functioning of the EEA Agreement with respect to overlaps between the Parties’ marketed endoparasiticides for CA in the markets/market segments listed and assessed in paragraph 105. The Commission notes that, in relation to the markets mentioned in Table 3 but not listed and assessed in paragraph 105 (see foonote 90 above), the Parties’ combined market shares and the presence of a sufficient number of competitors post-Transaction do not raise serious doubts. In any event, the Final Commitments aimed at remedying the horizontal non-coordinated effects of the Transaction in other CA endoparasiticides markets also exclude the possibility that the Transaction would lead to horizontal non-coordinated effects in those markets. Indeed, the Final Commitments will fully remove the overlap between the Parties’ activities in CA endoparasiticides at EEA level.
4.2.1.2. Ectoparasiticides97
(a) Overview of the market and the Parties’ products
(112) As explained in Section 3 above, ectoparasiticides are used to control external parasites, such as fleas and ticks.
(113) The Parties estimate that the total size of the CA ectoparasiticide sector was […] in the EEA in 201898 and explain that demand is growing due to increasing pet adoption and expenditure on veterinary healthcare.
(114) A number of chemical classes are used in ecto products. Most of them were originally developed for crop protection applications and subsequently applied to animal health. The main ecto chemical classes currently available on the market (which can be used alone or in combination) are: pyrazoles, neonicotinoids, pyrethroids, insect growth regulators, spinosyns and isox products (the newest class

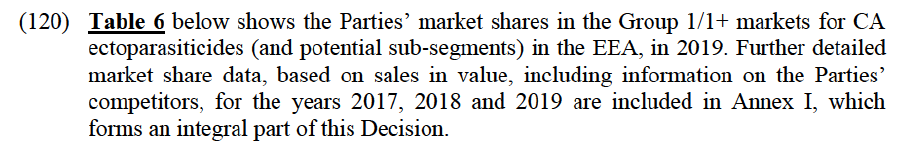
new entity would face significant competition across the EEA from a range of competitors, including (i) three significant players (i.e. BI, Merck and Zoetis), with at least two of them equalling or surpassing BAH in size, and (ii) several smaller rivals (such as Ceva and Virbac), with market shares comparable to Elanco’s. Third, the Parties claim that their products face strong competitive constraints from generics (several of their products being off-patent, including Elanco’s Comfortis and Capstar, as well as most of BAH’s Advantage family of products). Fourth, it is argued that competitors supplying CA ectoparasiticides in other EEA countries can easily and quickly expand to countries giving rise to Group 1 markets. Finally, Elanco and BAH argue that their products do not closely compete as they are differentiated (i) in terms of MoAs, Elanco’s key brands (i.e. Comfortis, Credelio, Capstar) being all administered orally, whereas BAH’s key brands (i.e. Advantix, Advantage and Seresto) are administered topically or by collar; (ii) in terms of spectrum of indications, BAH’s key brands being indicated for use against a broader range of ectoparasites compared to Elanco’s portfolio (Comfortis and Capstar, being only active against fleas) and (iii) in terms of distribution channels, Elanco focusing on veterinarians, whereas BAH increasingly distributes its products through the OTV channel.
(d) The Commission’s assessment
(122) As a preliminary remark, it should be noted that, unless otherwise specified, the findings set out in this Section 4.2.1.2, and in particular the results of the market investigation, do not materially differ depending on the species or the mode of administration.102 As regards possible differences depending on the analysed EEA country, although national specificities exist (with e.g. market share variations at national level), the main characteristics of supply and demand in the markets for feline and canine ectoparasiticides do not appear to vary significantly across countries. Therefore, unless otherwise specified, the findings of Section 4.2.1.2 (regarding e.g. closeness of competition, generic competition and the feedback received from market participants) do not materially differ depending on the geographic market at stake.
(123) The evidence in the Commission’s file generally confirms the Parties’ claims. It allows the Commission to exclude serious doubts as to the compatibility of the Transaction with the internal market and the functioning of the EEA Agreement resulting from the overlap of the Parties’ activities in feline and canine ectoparasiticites regardless of possible further segmentations.
(124) First, it appears from Table 6 above that the Transaction gives rise to Group 1 markets for CA ecto in only seven countries, with combined shares not exceeding 50% and a moderate increment brought by Elanco comprised between 1% and 10% (except in two cases where the increment is above 10%). More specifically:
(a) In Belgium: the Parties have medium combined market shares with a limited or moderate increment brought by Elanco in (i) the canine ecto market ([30- 40]%, with a [0-5]% increment), and (ii) the feline ecto market ([40-50]%, with a [5-10]% increment). Post-Transaction, in both markets, the new entity would face significant competitors, with market shares above [20-30]%, namely Merck and BI, as well as several other players (including Zoetis, Ceva and Virbac);
(b) In Czechia: the Parties have medium combined market shares with a modest increment brought by Elanco in (i) the canine ecto market ([30-40]%, with a [0-5]% increment), (ii) the feline ecto market ([40-50]%, with a [0-5]% increment), and (iii) the canine ecto/endecto market ([30-40]%, with a [0-5]% increment). Post-Transaction, in the above markets, the new entity would face at least three competitors with market shares well above the increment (between [5-10]% and [40-50]%), namely Merck, BI and Zoetis, as well as several other players, such as Ceva, Virbac, Vetoquinol and Krka (whose market shares are comparable to Elanco’s);
(c) In France: the Parties have a combined market share of [40-50]% in the feline ecto market ([10-20]% for Elanco and [20-30]% for BAH). Post- Transaction, the new entity would face (i) two significant competitors, with market shares above [10-20]%, namely BI ([20-30]%) and Merck ([10- 20]%), (ii) several other meaningful players, including Clement Thekan ([5- 10]%) and Virbac ([5-10]%), as well as (iii) generic players (such as Biocanina, Krka and Vetoquinol);
(d) In Italy: the Parties have medium combined market shares with a modest increment brought by Elanco in (i) the canine ecto market ([40-50]%, with a [0-5]% increment), and (ii) the canine ecto/endecto market ([30-40]%, with a [0-5]% increment). Post-Transaction, in both markets, the new entity would face two significant competitors, with market shares comprised between [10- 20]% and [30-40]%, namely BI and Merck, and several other players, including three rivals with market share above the increment (i.e. Zoetis, Ceva and Virbac);
(e) In the Netherlands: the Parties’ combined market shares do not exceed [50- 60]%, with a limited or moderate increment brought by Elanco in (i) the canine ecto market ([30-40]%, with a [0-5]% increment), (ii) the feline ecto market ([50-60]%, with a [5-10]% increment), and (iii) the feline ecto/endecto market ([30-40]%, with a [5-10]% increment). Post-Transaction, in the above markets, the new entity would face many competitors, including significant players with market shares well above [10-20]% (such as Merck and BI, as well as Zoetis in the feline ecto/endecto market) and several other players (such as Ceva, Virbac, and Fengo);
(f) In Portugal: the Parties have medium combined market shares with a moderate increment brought by Elanco in (i) the feline ecto market ([40- 50]%, with a [5-10]% increment), and (ii) the feline ecto/endecto market ([30-40]%, with a [5-10]% increment). Post-Transaction, in both markets, the new entity would face two significant competitors, with market shares comprised between [10-20]% and [20-30]%, namely BI and Merck, as well as several other players (such as Calier, Ceva, Virbac, Zoetis, and Vetoquinol); and
(g) In Spain: the Parties have a medium combined market share of [30-40]% in the feline ecto market ([10-20]% for Elanco and [20-30]% for BAH). Post- Transaction, the new entity would face many competitors, including (i) one rival with a comparable market share, namely BI ([30-40]%), (ii) two other meaningful players, i.e. Merck ([5-10]%) and Virbac ([5-10]%), and (iii) several other competitors (e.g. Ceva, Ecuphar, Beaphar, Karizoo, Vetoquinol).
(125) Second, the results of the market investigation confirmed that, post-Transaction, the new entity would continue to face strong competitive constraints from a number of rivals. Although BAH is generally perceived by competitors as the main player in the EEA markets for CA ectoparasiticides, Elanco is typically ranked number five in the EEA, after Merck, BI and Zoetis.103 In fact, the vast majority of customers and most competitors indicated that there would remain sufficient alternative suppliers post-Transaction.104
(126) Third, a large majority of customers confirmed the Parties’ claim that generics exert pressure on the prices of branded CA ectoparasiticides that are off-patent.105
(127) Fourth, the market investigation confirmed the Parties’ claim that their marketed products are differentiated. Indeed, most customers and a large number of competitors consider that Elanco’s and BAH’s marketed CA ectoparasiticides do not closely compete.106 The market investigation also confirmed that the MoAs and the spectrum of indications are key factors in the choice of CA ectoparasiticides for customers,107 two parameters that differentiate the Parties’ main products: (i) BAH’s key brands (i.e. Advantix, Advantage and Seresto, which together account for more than […] of BAH’s CA ecto sales in the EEA) are administered topically or by collar and are indicated for use against a broad range of ectoparasites, whereas (ii) Elanco’s key brands (i.e. Comfortis, Credelio, Capstar, which together account for more than […] of Elanco’s CA ecto sales in the EEA) are all administered orally, and two of these brands are only active against fleas.
(128) Finally, market participants did not express competition concerns in relation to the combination of the Parties’ marketed feline and canine ectoparasiticides.108
(e) Conclusion
(129) In view of the above considerations, taking into account the market investigation and all the evidence available to it, the Commission concludes that the Transaction does not raise serious doubts as to its compatibility with the internal market and the functioning of the EEA Agreement with respect to overlaps between the Parties’ marketed ectoparasiticides for CA.109
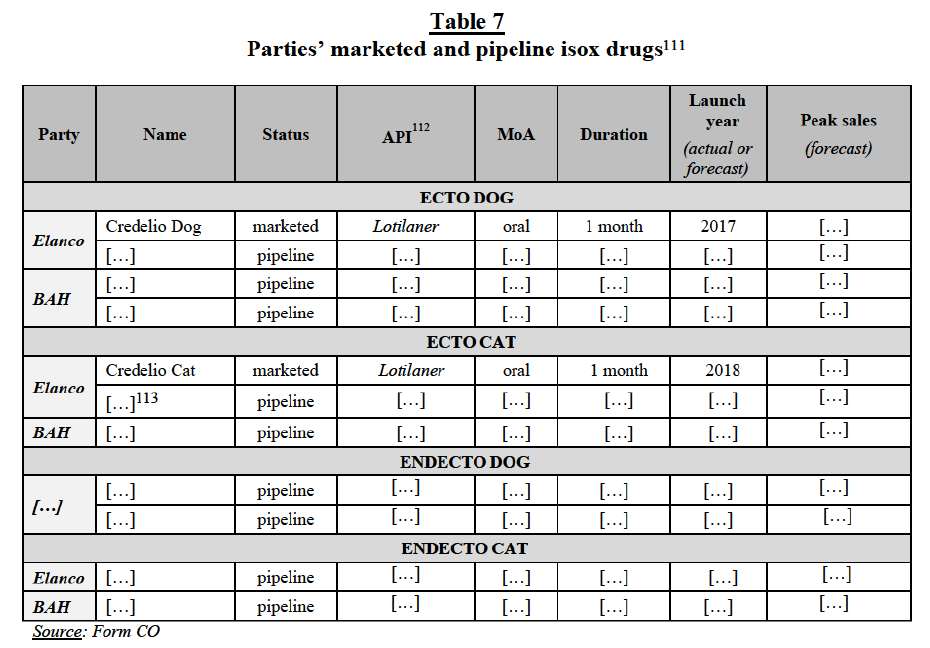
(133) As detailed above: Elanco has two marketed isox products in the EEA, which are marketed under the same brand and based on the same API (Lotilaner), namely Credelio Dog (canine ecto product) and Credelio Cat (feline ecto product). In addition to its marketed products, Elanco has […] isox pipelines (including both feline and canine ecto and endecto products), […]114 […]; BAH has no marketed isox products in the EEA (and worldwide) but […] pipeline isox products, […].115 […]116, […].
(b) The Parties’ views
(134) The Parties submit that the Transaction does not raise competition concerns in relation to isox for several reasons.117 First, as indicated in Section 3.1.2.1(b) above, they strongly contest the existence of a distinct market segment for isox products, arguing that isox products compete with other chemical classes. Second, they claim that, in the hypothetical isox segment, Elanco is “weak” on the ground that Credelio (i) is the fourth isox launched in the EEA, with modest sales and market shares across the EEA (well behind Merck, BI and Zoetis) and (ii) does not enjoy any beneficial differentiation compared to other isox products. Third, the Parties submit that, from a chemical point of view, BAH’s pipelines are not strictly speaking isox compounds ([…]). Fourth, they argue that many of the overlapping pipelines are at an early stage of development, with low probability of success and several years away from an hypothetical market launch. The Parties consider that such early-stage pipelines are too speculative to have an impact on the competitive assessment. Fifth, the Parties claim that, post-Transaction, the new entity would face many competitors (irrespective of the market definition), including (i) the established and well-known isox products marketed by BI (NexGard), Merck (Bravecto) and Zoetis (Simparica); (ii) several “isox-like” products currently on the market (i.e. products which are not based on isox but have similar features and closely compete with isox products) (such as BI’s Frontline and Broadline and Ceva’s Vectra 3D); (iii) generics, with the expiration of Bravecto (Merck)’s and NexGard (BI)’s patents in the coming years; and (iv) potential entrants, with the existence of considerable R&D opportunities regarding both isox and isox-like compounds. Finally, the Parties also claim that, post-Transaction, the new entity would have incentives to continue the development of BAH’s pipeline products, which are differentiated from and, thus, complementary with Elanco’s product portfolio.
(c) The Commission’s assessment
(135) As detailed in Table 7 above, the Parties’ isox activities give rise to several marketto- pipeline and pipeline-to-pipeline overlaps.
(136) In line with the four-layer competitive assessment framework118 used in the Commission’s past decisional practice,119 the Commission has assessed the overlaps involving the Parties’ isox pipeline products in terms of both (a) potential (product and price) competition, assessing the overlaps (i) between the Parties’ existing (marketed) and pipeline products at advanced stages of development and (ii) between the Parties’ pipeline products at advanced stages of development; and (b) innovation competition in relation to the Parties’ early stage pipeline products/projects, assessing the risk of significant loss of innovation competition resulting from the discontinuation, delay or redirection of one party’s early stage pipeline products/projects overlapping with the other Party’s existing products or advanced or early stage pipeline products/projects .
(137) For the reasons set out below, the results of the market investigation and the evidence in the Commission’s file strongly suggest that the combination of the Parties’ isox activities would raise serious doubts as to its compatibility with the internal market and the functioning of the EEA Agreement under any plausible market definitions.
Isox products as a distinct market segment
(138) Should isox products constitute a distinct market segment, the Transaction gives rise to (i) marketed-to-pipeline overlaps in the canine ecto segment between Elanco’s Credelio Dog (marketed), on the one hand, and BAH’s […] (pipelines) on the other hand; (ii) a marketed-to-pipeline overlap in the feline ecto segment between Elanco’s Credelio Cat (marketed) and BAH’s […] (pipeline);120 and (iii) a pipeline-to-pipeline overlap in the feline endecto segment between […].
(139) The Commission finds that the above overlaps would raise competition concerns for the following reasons.
(140) First, the number of players with marketed isox products is limited. As detailed in Table 8 below, in the EEA, only four companies are currently supplying isox products. In several sub-segments, the number of players is even more limited, with duopolies in the feline ecto and endecto segments, as well as in the canine endecto segment.
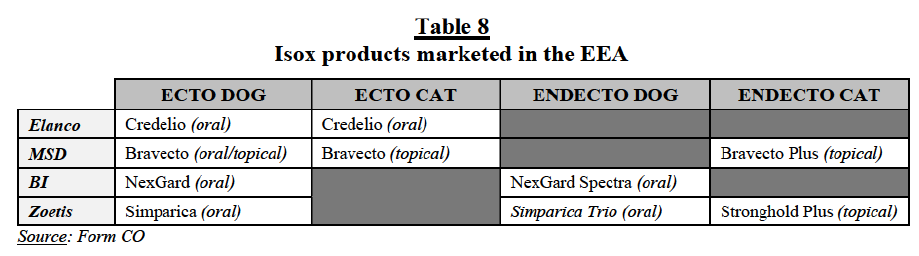

compounds of the family (those with better profile), too significant research and development costs for mid-size players (huge barriers of entry).”124
(146) Fourth, the market investigation revealed that very few players are currently developing either isox products or isox-like products (i.e. compounds, which are not isox from a chemical point of view but have similar features in terms notably of spectrum of claim, method and ease of administration, duration of efficacy and systemic effects).125
(147) In fact, the Phase I investigation did not allow the Commission to identify potential entrants other than BAH.
(148) The Parties contest the above on the ground that BAH’s […] pipelines are not strictly speaking isox compounds (from a chemical point of view). In this respect, the Commission notes that […],126 […] some industry reports127 and some market participants128, qualify these pipelines as isox. Similarly, the World Health Organisation also classifies […] as an “isoxazoline compound”, together with Afoxolaner, Fluralaner, Lotilaner and Sarolaner (i.e. the APIs of the isox products currently marketed in the EEA).129
(149) In any event, the Parties acknowleged that […]’s features are similar to isox in terms of spectrum of claim, method and ease of administration, duration of efficacy and systemic effects. In fact, the Parties expressly indicated that […]130
(150) As regards the Parties’ claim that BAH’s pipelines are at a too early stage to have an impact on the market and that forecasts about market entry are too speculative, the Commission notes that (i) in previous pharmaceutical cases, the Commission has regularly assessed overlaps involving pipelines which were several years away from the market and requested their divestment when necessary;131 (ii) BAH’s pipelines are expected to reach the market in the short/medium term: […] should be launched in less than […], while […] pipelines’ commercialisation is anticipated in […] (by comparison the total development process of animal pharmaceuticals can take up to 13 years according to the Parties and up to 15 years according to competitors);132 and (iii) the methodology used by BAH to compute […] probability of success is unclear.
(151) Fifth, BAH’s pipelines are expected to be very competitive due […]. In this respect, the Parties’ internal documents stress […].133 Internal documents also emphasise […]. For instance, an internal presentation of BAH indicates that […].134 Similarly, another internal presentation states that […].135 The competitivity of BAH’s pipelines is also supported by the fact that […].
(152) It stems from the above that the Parties’ isox products will likely be close competitors, with a significant risk of cross-cannibalisation. In this context, post- Transaction, the new entity will most likely have limited incentives to develop and market in parallel BAH’s and Elanco’s isox products. Conversely, it is likely that Elanco will have the incentive to maximise the profits it could generate by developing and marketing its isox products, by avoiding to the maximum extent possible competition between them. To this end, Elanco would likely make changes to the development programs that Elanco and BAH had planned absent the merger. Changes may include the termination of specific programs, a change in their timing (for example to introduce a new product on the market only when another product is losing patent protection), or a change in their scope (for example overlapping indications with other products). Therefore, the Commission considers that there is a significant risk that the new entity would discontinue, delay or reorient the isox products of either one of the Parties to eliminate or mitigate the risk of cannibalisation. As a result, the Transaction would lead to a reduction in the number of players that is already very low (especially in the feline isox segments), leading to potentially less choice and higher prices.
Isox products as part of broader markets for CA ecto and endecto
(153) Should isox products be part of the markets for CA ecto and endecto, the Transaction gives rise to several overlaps between the Parties’ marketed and pipeline ecto and endecto products for CA, the most relevant ones being the marketed-topipeline and pipeline-to-pipeline overlaps listed in paragraph 138 above.
(154) The risk of discontinuation, delay or reorientation of the isox products of either one of the Parties (see previous Section) would also have a detrimental impact on these broader markets for the following reasons.
(155) First, as explained in Section 3.1.2.1(c) above, the market investigation largely confirmed that isox have a singular profile resulting from the combination of various features (in terms of mode of administration, duration, efficacy, distribution channel, etc.). For instance, a competitor stated that isox products are very “strong” due to their “larger spectrum (including ticks), the oral administration which is seen by owners as a safe and environmental friendly option, and for some of them, the longer time of action, up to 3 months” and noted that “when topical and collar parasiticides became OTC, new prescription-only oral products [isox] were favoured by a growing number of vets”.136 It follows that, within the markets for CA ecto and endecto products, isox products are close competitors.
(156) Conversely, the market investigation revealed that non-isox based ecto and endecto products for CA currently marketed in the EEA are “distant competitors” to isoxbased ecto and endecto products for CA. In particular, the results of the market investigation contradicted the Parties’ claim that (i) BI’s Frontline/ Broadline; (ii) Virbac’s Effipro/Effitix, and (iii) Ceva’s Vectra/Vectra 3D have similar features and closely compete with isox products. For instance, a respondent indicated that “None of these products come close to Isox products. […] Isox products are mainly tablets that work 4 to 12 weeks, which is completely different in administration to a topical” and that isox are favoured by vets “giving the advise that Isox products are better than anything else on the market (mainly because it brings them more margin).” Another competitor indicated: “All the products above are based on fipronil and are topical. The products they are compared to have a new active instead of Fipronil (Isox) which is able to overcome any developing resistance and have a long duration action (monthly or longer) when given orally.”137
(157) Second, the Commission found that isox products are cannibalising the sales of other ecto/endecto products and are perceived by several market participants as ‘game changer’.138 In fact, the market investigation showed that isox products are very popular and have rapidly increased market share at the expense of other ecto/endecto products. According to the Parties’ estimates, oral isox products account now for […]% of total global canine flea treatments and […]% of total global feline flea treatments, and will continue to gain market shares.
(158) Competitors generally share the Parties’ view on the above. For instance, a rival stressed the fact that “the market dynamic is largely in favor of isox-based products”, explaining that in EEA, (i) between 2015 and 2019, all isox-based product (ecto and endecto) had compound annual growth rate of +34% vs. -3% for non isox-based products and that (i) since 2015, isox-based ecto and endecto products have grown respectively by 27% and +117% per year, while ecto and endecto products based on other chemical class have dropped respectively by -2% and -7% per year over the same period.139
(159) The fact that isox products are cannibalising other CA ecto and endecto products is also corroborated by the Parties’ internal documents. […].140
(160) Finally, several market participants raised concerns about the combination of the Parties’ isox products and its negative impact on the other ecto/endecto classes. For example, a competitor indicated that “the combined entity will have a very strong position in the ecto- and endecto markets due to leveraging isox mono and combination products”.141 Another player submitted that the combination of the Parties’ isox products may lead to the foreclosure of mid-size players: “the merged entity will probably hold three products from this isoxazoline class of compounds […] the unchallenged […] positions of the top 4 leaders on this segment makes the competition totally unfair on the total parasiticides segments by leveraging strong ectos positions […] to dominate the full market, also developing ENDECTOS to eat the ENDOS market of mid-size players like us. […] ELANCO/BAYER are already dominating the ENDOS and ECTOS segments. By combining their forces and launching the [isox] products they have in their pipeline (ENDECTOS) they will dominate the entire market”.142
(161) As a result of the above, some market participant expressly stressed the need for a remedy in relation to isox: “In our mind, the Parties should divest also in the flea and tick category, in particular an ISOX compound (CREDELIO)”.143
(d) Conclusion
(162) In view of the above considerations, taking into account the market investigation and all the evidence available to it, the Commission concludes that the Transaction raises serious doubts as to its compatibility with the internal market and the functioning of the EEA Agreement with respect to overlaps between the Parties’ isox products due to its likely horizontal non-coordinated effects in the EEA markets for feline ecto and endecto products and their potential sub-segments for isox-based feline ecto and endecto products. In the present case, the Commission does not need to take a view on whether the overlaps between the Parties’ isox products raise serious doubts in the EEA markets for canine ecto markets and their potential sub-segments for isoxbased canine ecto, as in any event the remedy offered by the Parties covers the full overlap in the EEA for viability reasons.
4.2.1.4. Heartworm products
(a) Overview of the market and the Parties’ products
(163) Heartworm is an endoparasitic worm that primarily resides in the heart of the host animal (mainly dogs and to a lesser extent cats) and that is spread through mosquito bites. Heartworms can be fatal and are difficult to treat once contracted, so preventative treatments are typically used. In the EEA, heartworm is endemic only in southern Europe, including in particular in Greece, Italy, Portugal, Spain and France.
(164) Treatments against heartworm for cats and dogs (i) include endoparasiticides that are exclusively indicated for heartworms, as well as (ii) endo and endecto products that have a broader spectrum of claims and can also treat other parasites.
(165) In the EEA, the Parties market several products indicated for the treatment/prevention of heartworm in CA (see Table 9 below). Only Elanco’s Guardian SR product is indicated for heartworm alone.
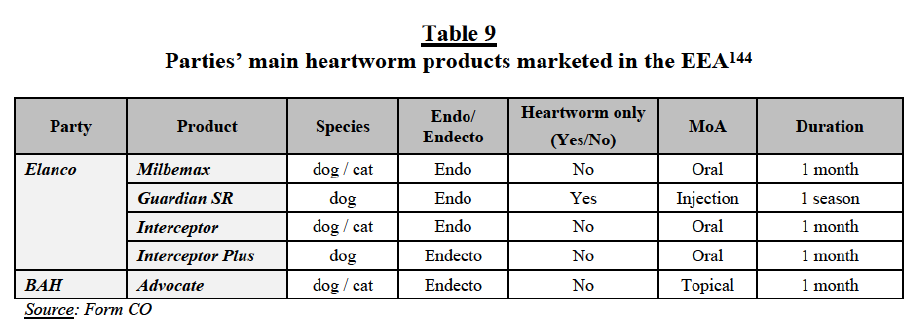

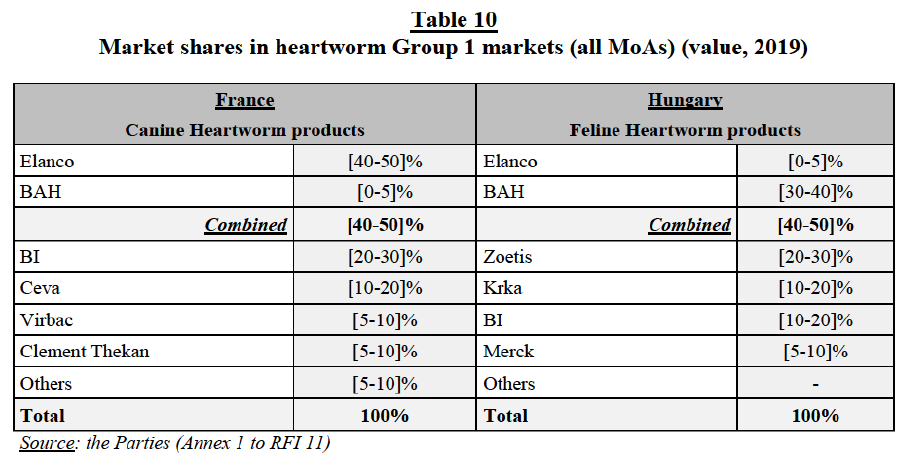
(c) The Parties’ views
(167) The Parties submit that the Transaction does not raise competition concerns in relation to the supply of heartworm products for CA in France and Hungary for several reasons.147 First, the Parties submit that the increment brought by the Transaction is modest, with no material impact on the market, and that their market shares are declining. Second, post-Transaction, the new entity would face a wide range of competing originator products (both endo and endecto) indicated for the treatment of heartworm, including BI’s Broadline and Heartgard, Zoetis’ Revolution/Stronghold, as well as numerous generic versions of their own products which are all off-patent. Finally, the Parties claim that their products are differentiated: while BAH’s Advocate product is a spot-on endecto, Elanco’s main heartworm products are endos that are administered orally or by injection.
(d) The Commission’s assessment
(168) As a preliminary remark, it should be noted that, unless otherwise specified, the findings set out in this Section 4.2.1.4, and in particular the results of the market investigation, do not materially differ depending on the species.148 As regards possible differences depending on the analysed EEA country, although national specificities exist, the overall structure and the main characteristics of supply and demand in the markets for heartworm products do not appear to vary significantly in France and Hungary. Therefore, unless otherwise specified, the findings of Section 4.2.1.4 (regarding e.g. closeness of competition, generic competition and the feedback received from market participants) do not materially differ depending on the geographic market at stake.
(169) The evidence in the Commission’s file generally confirms the Parties’ claims. It allows the Commission to exclude serious doubts as to the compatibility of the Transaction with the internal market and the functioning of the EEA Agreement resulting from the overlap of the Parties’ activities in heartworm products for CA.
(170) First, it appears from Table 10 above that the Transaction gives rise to only two Group 1 markets for the supply of heartworm product for CA in France and Hungary, where (i) the Parties have combined shares below 50% and declining sales, (ii) the increment brought by the Transaction is modest (below 5%) and (iii) several competitors will remain post-Transaction. More specifically:
(a) In France, in the canine heartworm market, the Parties have (i) a combined market shares of [40-50]%, with a [0-5]% increment brought by BAH, and (ii) declining sales (in the past four years, Elanco and BAH lost respectively [10-20] and [5-10] percentage points of market shares). Post-Transaction, the new entity would face many rivals, including four competitors with market shares above the increment, namely BI ([20-30]%), Ceva ([10-20]%), Virbac ([5-10]%) and Clement Thekan ([5-10]%); and
(b) In Hungary, in the feline heartworm market, the Parties (i) have a combined market share of [40-50]%, with a [0-5]% increment brough by Elanco, and (ii) declining market shares (in the past four years, BAH and Elanco lost respectively [10-20] and [0-5] percentage points of market shares). Post- Transaction, the new entity would face four competitors with market shares above the increment, namely Zoetis ([20-30]%), Krka ([10-20]%), BI ([10- 20]%) and Merck ([5-10]%).
(171) It stems from the above that the Transaction is unlikely to have a material impact on competition in the above markets. In fact, Elanco has de minimis sales in Hungary (below […]), whereas BAH has de minimis sales in France (below […]).
(172) Second, the results of the market investigation confirmed that, post-Transaction, the new entity would continue to face strong competitive constraints from a number of rivals.149 In particular, market participants emphasised that BI has a very efficacious heartworm product, with a well-known and established brand (i.e. Heartguard).150 Other significant competing (originator) products include Stronghold Plus (Zoetis) and Broadline (BI). The Parties also stressed the fact that Zoetis has recently launched in the EEA an isox-based endecto with a heartworm claim (Simparica Trio), which is expected to be very successful: Elanco expects Simparica Trio to gain a [20-30]% market share in the EEA countries where heartworm is endemic in the next six years. In fact, the vast majority of customers and competitors indicated that there would remain sufficient alternative suppliers post-Transaction.151
(173) Third, a large majority of customers confirmed that generics exert pressure on the prices of originator heartworm products.152 Several competitors offer generic endo or endecto product with heartworm claim, including generic versions of the Parties’ products. For instance, in France and Hungary, generic versions of Elanco’s key product (Milbemax) are offered by Ceva (Milbactor), Virbac (Milpro) and Krka (Milprazon), which explains the decline in Elanco’s sales in these two countries.
(174) Fourth, the market investigation confirmed the Parties’ claim that their marketed products are differentiated. Indeed, most customers and competitors consider that Elanco and BAH do not closely compete.153 The market investigation also confimed the MoAs, the spectrum of claim and the duration of efficacy are key factors in the choice of heartworm products for customers,154 three parameters that differentiate the Parties’ main products: (i) all of BAH’s sales derive from Advocate, which is an endecto product administered topically once a month, whereas (ii) Elanco’s two main products (i.e. Guardian SR and Milbemax) are pure endo products administered orally or by injection and one of them (Guardian SR) is only applied once for the entire season.
(175) Finally, the market investigation did not reveal any substantiated competition concerns in relation to the combination of the Parties’ heartworm products.155

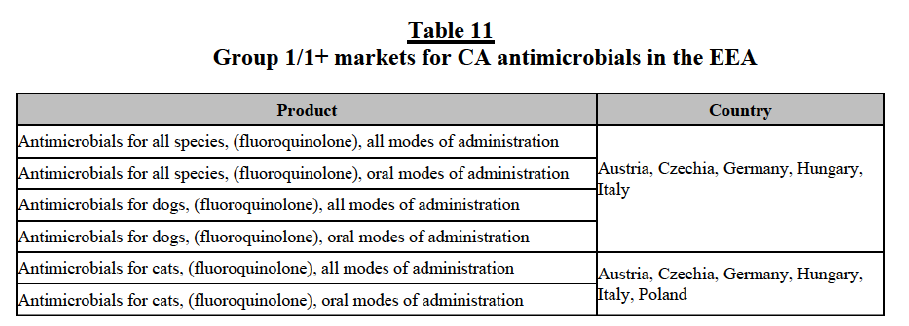
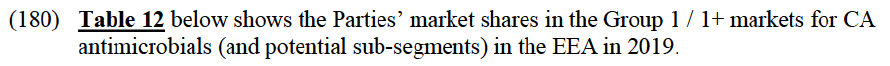
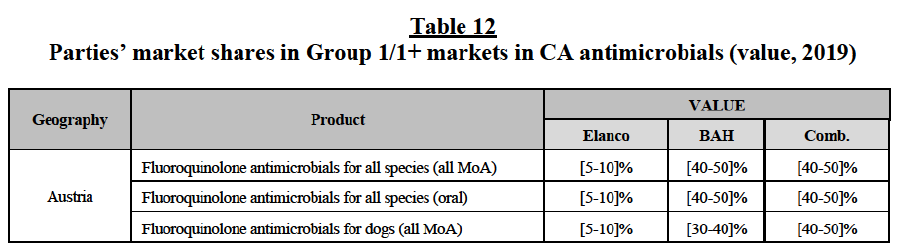
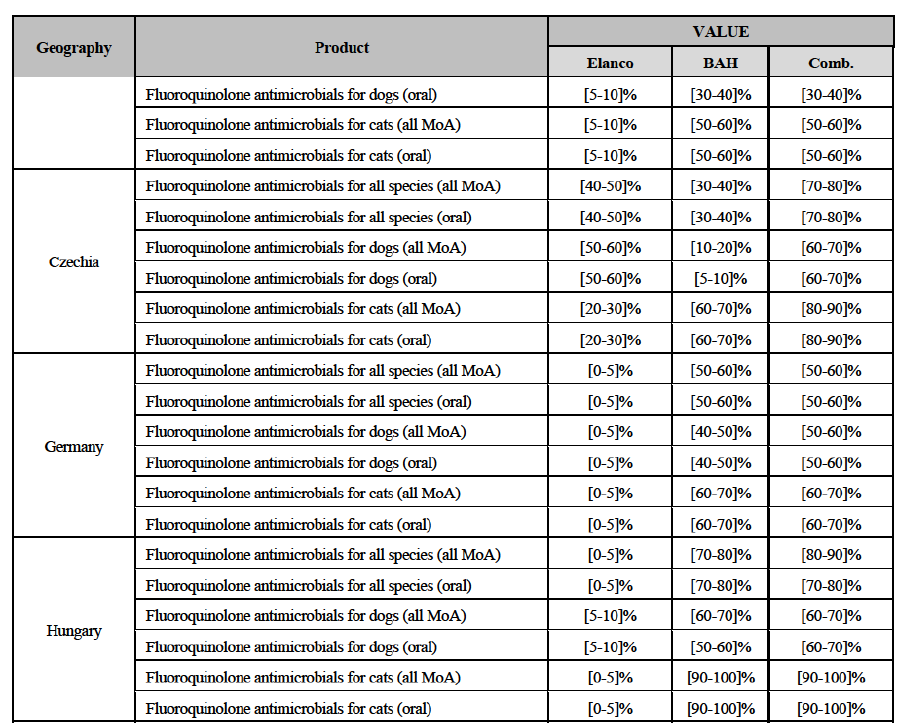
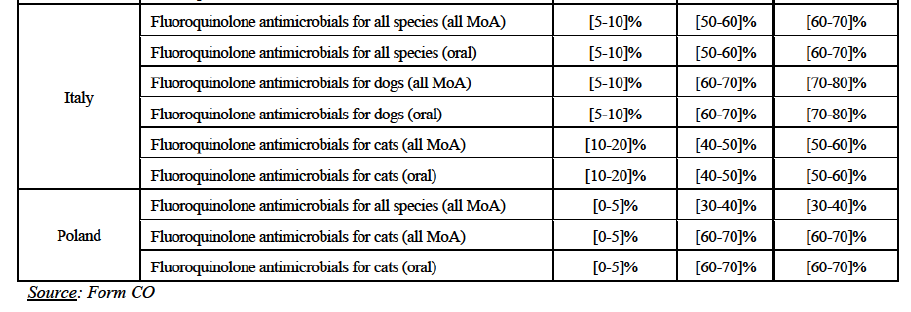

(c) The Parties’ views
(182) The Parties make the following observations on these Group 1 markets for fluoroquinolone antimicrobials for CA. The increment is caused by Elanco’s Zobuxa product in all markets except Czechia, where in four putative segments the increment is caused by BAH’s Baytril. Notably, in all the countries in which Group 1 affected markets for CA antimicrobials arise and in which Zobuxa represented at some point in the 2016-2018 period more than 5% of the total market, typically, more than 50% of all fluoroquinolones sold comprise Baytril generics, the majority of which are not attributable to Zobuxa. As such, the Parties argue that the Transaction would not lead to a material loss of competitive pressure on Baytril, since other generic versions of Baytril would continue to constrain it, and Baytril generics present in other geographies could also enter these Group 1 markets in the short-term. Moreover, the Parties note that the extent and strength of the presence of generic Baytril products may not be fully captured in the market share estimates, given the general lack of visibility into generic activity by reference to the CEESA156 dataset.
(d) The Commission’s assessment
(183) The evidence in the Commission’s file generally confirms the Parties’ claims. It allows the Commission to exclude serious doubts as to the compatibility of the Transaction with the internal market and the functioning of the EEA Agreement resulting from the overlap of the Parties’ activities in antimicrobials for CA.
(184) First, the market investigation confirmed the Parties’ claim that generics exert pressure on the prices of branded CA antimicrobials.157 In addition to Elanco’s Zobuxa, there are several enrofloxacin generics marketed in the EEA by Vetoquinol, Virbac and Ceva. In each of the countries listed in Table 12 except for Czechia, there is at least one other enrofloxacin generic besides Zobuxa. Further, in each of the countries listed in Table 12 including Czechia, there is at least one other fluoroquinolone antimicrobial (marbofloxacin being the active ingredient) marketed by competitors.
(185) Second, the results of the market investigation confirm that, generic entry into the antimicrobials market is straightforward. One supplier explained that “We believe this [entry] would be relatively easy for generics…”158 and another mentioned that “…these [development and registration] costs are significantly lower when the product is a generic copy…The level of cost and difficulty would also be lower depending on the supplier – a supplier that has an existing product, established in one country market would be able to enter another country with that product at a lower cost and with less difficulty than a supplier developing and establishing a product from scratch.” Provided they have established commercial presence in the country, other players could also enter these segments with generic products.
(186) Third, the use of fluoroquinolones is expected to decline due to the impact of EMA regulation159, according to which it is classified as higher risk, and pressure on prices may be expected in such shrinking markets, with customers seeking alternatives to antimicrobial use altogether. The overarching trend in respect of antimicrobials is the growing awareness of bacterial resistance to human antibiotics. As a result of this growing awareness, there continues to be significant regulatory focus (at both the European and national levels). This focus has already resulted in a significant decline in antimicrobial usage across Europe. This decline (at a rate of an estimated 5-10% per annum, based on annual declines in antimicrobial usage to date) will continue in the near and longer term in the EEA. While concerns have been more focused on the use of antimicrobials in PA, the use of high risk antibiotics is also discouraged by regulation for CA. Indeed, the majority of competitors and customers confirmed that regulation has had an impact on the CA antimicrobials market as well. 160 One CA customer explained that “Antimicrobials in the veterinary sector have been reduced, especially … and Fluorochinolons.” 161 As such, competitors and customers both stressed the fact that while BAH has a strong brand, a key weakness is that the molecule is a fluoroquinolone. 162
(187) Fourth, the market investigation did not reveal any substantiated competition concerns in relation to the combination of the Parties’ CA antimicrobials. Overall, the large majority of customers and competitors indicated that there would remain sufficient alternative suppliers post-Transaction and do not expect a price increase in CA antimicrobials.163
(188) Lastly, looking at the individual countries, the Transaction gives rise to Group 1 markets for CA antimicrobials in six countries. More specifically:
(a) In Austria: the Parties have combined market shares below [50-60]% with moderate increments in (i) fluoroquinolone antimicrobials for all CA ([5- 10]% for Elanco and [40-50]% for BAH), (ii) oral fluoroquinolone antimicrobials for all CA ([5-10]% for Elanco and [40-50]% for BAH), (iii) canine fluoroquinolone antimicrobials ([5-10]% for Elanco and [30-40]% for BAH), (iv) oral canine fluoroquinolone antimicrobials ([5-10]% for Elanco and [30-40]% for BAH), and above [50-60]% in (v) feline fluoroquinolone antimicrobials ([5-10]% for Elanco and [50-60]% for BAH) and (vi) oral feline fluoroquinolone antimicrobials ([5-10]% for Elanco and [50-60]% for BAH). Post-Transaction, in all segments, the new entity would continue to face three competitors, two of which have market shares significantly above the increment. Specifically, Ceva has market shares of [30-40]%, [30-40]%, [30-40]%, [40-50]%, [20-30]% and [20-30]% in markets (i)-(vi) respectively, while Vetoquinol has market shares of [10-20]%, [10-20]%, [10-20]%, [10- 20]%, [10-20]% and [10-20]% respectively. The new entity would also continue to face the possibility of further generic entry. Moreover, the market investigation did not reveal any substantiated concerns, and the majority of customers and competitors consider that there will remain sufficient suppliers and do not see a risk of price increases in this market.
(b) In Czechia: the Parties have combined market shares above [50-60]% in (i) fluoroquinolone antimicrobials for all CA ([40-50]% for Elanco and [30- 40]% for BAH), (ii) oral fluoroquinolone antimicrobials for all CA ([40- 50]% for Elanco and [30-40]% for BAH), (iii) canine fluoroquinolone antimicrobials ([50-60]% for Elanco and [10-20]% for BAH), (iv) oral canine fluoroquinolone antimicrobials ([50-60]% for Elanco and [5-10]% for BAH), (v) feline fluoroquinolone antimicrobials ([20-30]% for Elanco and [60-70]% for BAH) and (vi) oral feline fluoroquinolone antimicrobials ([20-30]% for Elanco and [60-70]% for BAH). The increment brought by Elanco in these segments ranges between [5-10]% and [30-40]% (corresponding to between EUR […] and […]). Post-Transaction, the new entity would continue to face one competitor, Vetoquinol, with market shares above [10-20]% in all segments, as well as the possibility of further generic entry. In addition to Vetoquinol, a number of suppliers (Ceva, Krka, Chanelle, Pharmagal and Le Vet Beheer) have marketing authorizations for generic CA fluoroquinolones in Czechia. In addition, BI was active in the market until 2018, so could reenter. Moreover, the market investigation did not reveal any concerns, and the majority of customers and competitors consider that there will remain sufficient suppliers and do not see a risk of price increases in this market.
(c) In Germany: the Parties have combined market shares above [50-60]% with a limited increment brought by Elanco in (i) fluoroquinolone antimicrobials for all CA ([0-5]% for Elanco and [50-60]% for BAH), (ii) oral fluoroquinolone antimicrobials for all CA ([0-5]% for Elanco and [50-60]% for BAH), (iii) canine fluoroquinolone antimicrobials ([0-5]% for Elanco and [40-50]% for BAH), (iv) oral canine fluoroquinolone antimicrobials ([0-5]% for Elanco and [40-50]% for BAH), (v) feline fluoroquinolone antimicrobials ([0-5]% for Elanco and [60-70]% for BAH) and (vi) oral feline fluoroquinolone antimicrobials ([0-5]% for Elanco and [60-70]% for BAH). Post-Transaction, the new entity would continue to face three competitors, Vetoquinol, Ceva and Virbac (at least one of which has significantly higher market shares than the increment, and above [10-20]% in any case), as well as the possibility of further generic entry. Moreover, the market investigation did not reveal any substantiated concerns, and the majority of customers and competitors consider that there will remain sufficient suppliers and do not see a risk of price increases in this market.
(d) In Hungary: the Parties have combined market shares above [50-60]% with a limited increment brought by Elanco in (i) fluoroquinolone antimicrobials for all CA ([0-5]% for Elanco and [70-80]% for BAH), (ii) oral fluoroquinolone antimicrobials for all CA ([0-5]% for Elanco and [70-80]% for BAH), (iii) canine fluoroquinolone antimicrobials ([5-10]% for Elanco and [60-70]% for BAH), (iv) oral canine fluoroquinolone antimicrobials ([5- 10]% for Elanco and [50-60]% for BAH), (v) feline fluoroquinolone antimicrobials ([0-5]% for Elanco and [90-100]% for BAH) and (vi) oral feline fluoroquinolone antimicrobials ([0-5]% for Elanco and [90-100]% for BAH). The increment brought by Elanco in these segments ranges between [0-5]% and [5-10]% (corresponding to between EUR […] and […]). Post- Transaction, the new entity would continue to face two competitors, Vetoquinol and Ceva (both of which have higher market shares than the increment in all segments), as well as the possibility of further generic entry. Indeed Ceva has increased its market shares significantly since entering in 2017, while BAH’s have dropped in the same period. In addition to Vetoquinol and Ceva (Krka, Le Vet, Animedica, Livisto, and CP-Pharma) have marketing authorizations for generic CA fluoroquinolones in Hungary. Moreover, the market investigation did not reveal any substantiated concerns, and the majority of customers and competitors consider that there will remain sufficient suppliers and do not see a risk of price increases in this market.
(e) In Italy: the Parties have combined market shares above [50-60]% with a moderate increment brought by Elanco in (i) fluoroquinolone antimicrobials for all CA ([5-10]% for Elanco and [50-60]% for BAH), (ii) oral fluoroquinolone antimicrobials for all CA ([5-10]% for Elanco and [50-60]% for BAH), (iii) canine fluoroquinolone antimicrobials ([5-10]% for Elanco and [60-70]% for BAH), (iv) oral canine fluoroquinolone antimicrobials ([5- 10]% for Elanco and [60-70]% for BAH), (v) feline fluoroquinolone antimicrobials ([10-20]% for Elanco and [40-50]% for BAH) and (vi) oral feline fluoroquinolone antimicrobials ([10-20]% for Elanco and [40-50]% for BAH). Post-Transaction, the new entity would continue to face four competitors, Vetoquinol, Virbac, Fatro and Ceva (at least one of which has a higher market share than the increment and above [10-20]% in all segments), as well as the possibility of further generic entry. Indeed Ceva has increased its market shares significantly since entering in 2017, while BAH’s have dropped in the same period. Moreover, the market investigation did not reveal any substantiated concerns, and the majority of customers and competitors consider that there will remain sufficient suppliers and do not see a risk of price increases in this market.
(f) In Poland: the Parties have combined market shares above [50-60]% with a limited increment brought by Elanco in (i) feline fluoroquinolone antimicrobials ([0-5]% for Elanco and [60-70]% for BAH) and (ii) oral feline fluoroquinolone antimicrobials ([0-5]% for Elanco and [60-70]% for BAH), and below [50-60]% in (iii) fluoroquinolone antimicrobials for all CA ([0- 5]% for Elanco and [30-40]% for BAH). Post-Transaction, the new entity would continue to face two competitors, Vetoquinol and Ceva (both of which have higher market shares than the increment, and at least one of which has a market share above [20-30]% in all segments), as well as the possibility of further generic entry. Moreover, the market investigation did not reveal any substantiated concerns, and the majority of customers and competitors consider that there will remain sufficient suppliers and do not see a risk of price increases in this market.
(e) Conclusion
(189) In view of the above considerations, taking into account the market investigation and all the evidence available to it, the Commission concludes that the Transaction does not raise serious doubts as to its compatibility with the internal market and the functioning of the EEA Agreement with respect to antimicrobials for CA in Austria, Czechia, Germany, Hungary, Italy, Poland under any potential market definition.
4.2.3. Otitis
4.2.3.1. Long-acting otitis products
(190) In the EEA, both Parties market long-acting otitis products. However, based on 2019 market shares, the Transaction does not give rise to any overlap, as BAH started commercializing its product Neptra in 2020. In 2019, Elanco was the only supplier commercializing a long-acting otitis product in the EEA. Therefore, currently Elanco’s Osurnia and BAH’s Neptra are the only long-acting otitis products currently available in the EEA.
(a) The Parties’ views
(191) To expedite the clearance of the Transaction, the Parties propose to divest Elanco’s Osurnia otitis product at global level to a suitable purchaser.
(b) The Commission’s assessment
(192) Osurnia was launched in 2015 in the EEA, and Neptra launched in 2020. Following the launch of BAH’s Neptra otitis product across the EEA at the beginning of 2020, horizontal overlaps arise in the long-acting otitis product market. Market shares are not available as Neptra was launched in 2020. Neptra and Osurnia being the only long-acting otitis products available in the EEA, the Transaction would suppose a merger to monopoly in this market and the merged entity would control 100% of the market without other alternatives available. Osurnia and Neptra being the only two long-acting products in the EEA will compete closely against each other.
(193) This is confirmed by the market investigation. Competitors indicated that the only alternatives available in the EEA for long-acting otitis products are the Parties’ products. For example, one competitor explains that “Osurnia from Elanco was the unique long lasting offer until Bayer launched Neptra in Europe”.164 In the same vein, several customers also explained that only the Parties offer long-acting otitis products. For example, one customers submitted that “Osurnia and Neptra are the only long duration otitis products”.165
(c) Conclusion
(194) In view of the above considerations, taking into account the market investigation and all the evidence available to it, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market and the functioning of the EEA Agreement due to its likely horizontal non-coordinated effects as regards long-acting otitis products in the EEA, in particular given the absence of alternative competitors.
4.2.3.2. Daily-use otitis products
(195) In the EEA, both Parties market daily-use otitis products. The Transaction does not give rise to any Group 1/1+ in 2019 considering a market for daily-use otitis
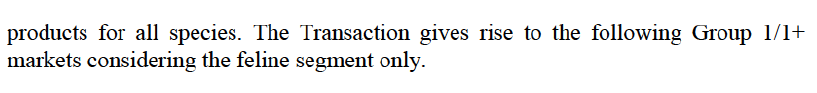

otitis externa. Moreover, even if a systemic antibiotic were used by a vet, because Baytril contains a fluoroquinolone, which is classified as Group B ‘Restrict’ in the EMA Antimicrobial Expert Group (“AMEG”) list (and has been classified as a restricted antibiotic since the first AMEG list was published in 2014), it would typically be used as a second-choice antibiotic. Indeed, the use of high risk antibiotics is discouraged by EMA regulation for CA166. In this vein, the majority of competitors and customers confirmed that regulation has had an impact on the CA antimicrobials market as well. One CA customer explained that “Antimicrobials in the veterinary sector have been reduced, especially … and Fluorochinolons.” Finally the mode of administration is also different: Baytril Injectable is administered by injection and Baytril Tablets are administered orally. In contrast, Surolan is administered topically to the ear, as is the case for most otitis externa treatments.
(202) Second, BAH is not a strong player in otitis daily-use products. Market shares across all EEA countries are very low, generally below [0-5]% and always below [0-5]%. This is confirmed by the market investigation. Competitors indicated that Elanco, Virbac, Vetoquinol, Merck and Dechra are the top suppliers. BAH was not identified among the top 5 suppliers by any of the competitors responding to the market investigation.167 Similarly, customers identified as top suppliers Elanco, Virbac, Vetoquinol, Merck and Dechra, but not BAH. Moreover, customers identified a significant number of additional suppliers of otitis daily-use products.168
(203) Third, the majority of suppliers and customers consider that post-Transaction there will remain sufficient alternative suppliers of otitis products for customers in the EEA to obtain competitive offers.169
(204) Finally, the majority of suppliers and customers consider that the Transaction will not lead to price increases with respect to otitis products in the EEA.170
Austria
(205) A group 1 market arises in Austria for the supply of daily-use otitis products for cats171.
(206) The value market shares of the Parties and their competitors for daily-use otitis products for cats are provided below in Table 14.
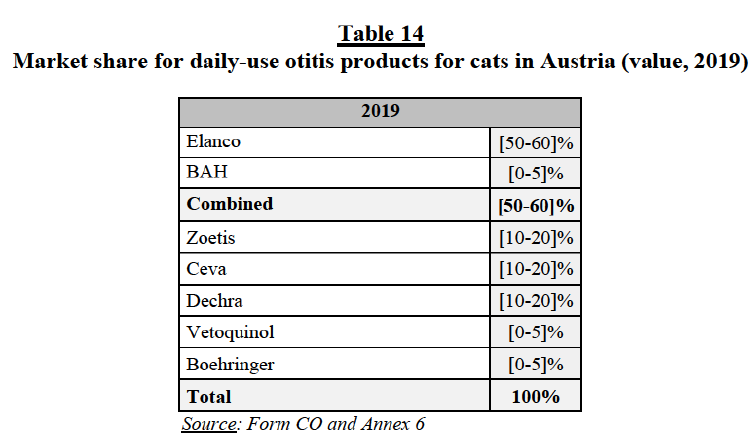
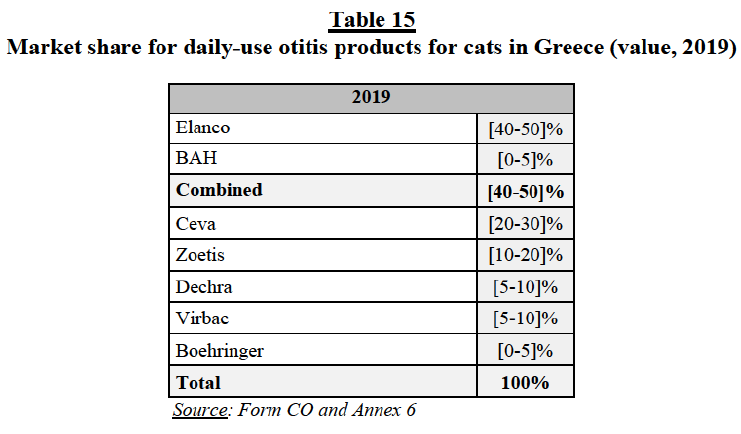
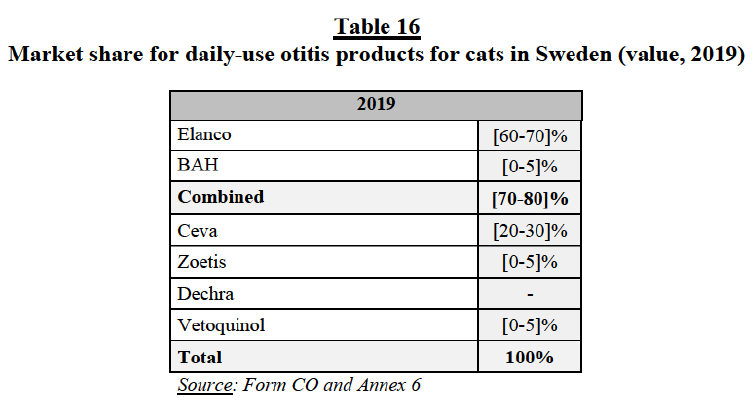


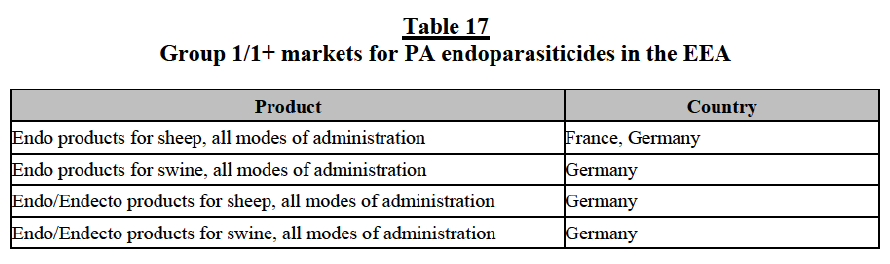

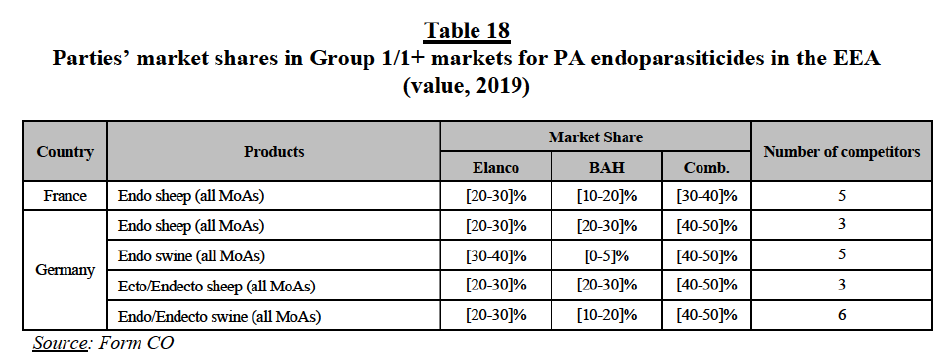
number of competitors active in Germany and in other EEA countries which exert significant competitive pressure on the Parties.
(d) The Commission’s assessment
(235) As a preliminary remark, it should be noted that, unless otherwise specified, the findings set out in this Section 4.3.1.1, and in particular the results of the market investigation, do not materially differ depending on the species. As regards possible differences depending on the analysed EEA country, although national specificities exist, the overall structure and the main characteristics of supply and demand in the markets for endoparasiticides and endo/endecto products for PA do not appear to vary significantly across countries. Therefore, unless otherwise specified, the findings of Section 4.31.1 (regarding e.g. closeness of competition, generic competition and the feedback received from market participants) do not materially differ depending on the geographic market at stake.
(236) The evidence in the Commission’s file generally confirms the Parties’ claims. It allows the Commission to exclude serious doubts as to the compatibility of the Transaction with the internal market and the functioning of the EEA Agreement resulting from the overlap of the Parties’ activities in PA endoparasiticides and endo/endecto products, under all plausible market definitions.
(237) First, it appears from Table 18 above that the Transaction gives rise to Group 1 markets for PA endoparasiticides and PA endo/endecto products in only two countries, with combined shares not exceeding 50%. More specifically:
(a) In France: the Parties have moderate combined market shares of [30-40]%, with a [10-20]% increment brought by BAH, in the PA endoparasiticides market for sheep. Post-Transaction, the new entity would face five competitors, the largest one being Zoetis with a market share higher than the Parties’ combined market share ([40-50]%).
(b) In Germany: the Parties have higher combined market shares in (i) the endoparasiticides market for sheep ([40-50]% combined market share, with a 20% increment brought by Elanco), (ii) endoparasiticides market for swine ([40-50]% combined market share, with a minor increment of [0-5]% brought by BAH), (iii) endo/endecto products market for sheep ([40-50]% combined market share, with a [20-30]% increment brought by Elanco) and (iv) endo/endecto products market for swine ([40-50]% combined market share, with a [10-20]% increment, brought by BAH). Albeit the higher combined market shares, post-Transaction, the new entity would face competition from a significant large player, Zoetis, with [40-50]-[40-50]% market share, depending on the segment, and other competitors with a more negligible market presence (Merck, BI, Ceva, Virbac).
(238) Second, the market investigation results indicated that Merck is perceived as the market leader, followed by Elanco and Zoetis in endoparasiticides and endo/endectoparasitices for PA. BAH is also mentioned among the top five competitors but to a lesser extent.



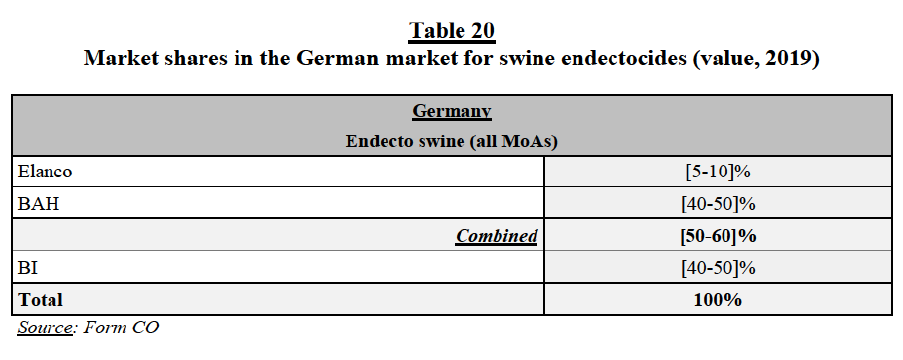
(251) Third, similarly to the market feedback received for PA endoparasiticides, according to most competitors, there are no specific PA parasiticides for which the Parties are either the only alternatives available or among the very few suppliers in the EEA.
(252) Fourth, similarly to the market feedback received for PA endoparasiticides, competitors indicated that generics exert a competitive constraint over branded PA endectocides.
(253) Fifth, the large majority of market respondents indicated that Elanco and BAH are not close competitors in the market for PA endectocides, since their products can be differentiated by indication and withdrawal period.
(254) Finally, customers and competitors did not indicate any substantiated competition concerns in relation to the combination of the Parties’ marketed endectocides for PA.
(e) Conclusion
(255) In view of the above considerations, taking into account the market investigation and all the evidence available to it, the Commission concludes that the Transaction does not raise serious doubts as to its compatibility with the internal market and the functioning of the EEA Agreement with respect to overlaps between the Parties’ marketed endectocides for swine in Germany, under all plausible market definitions.
4.3.1.3. Ectoparasiticides
(a) Overview of the market and the Parties’ products
(256) As explained in Section 3 above, ectoparasiticides are used to control external parasites and present similar characteristics to ectoparasiticides for CA. There are a number of classes of ecto products, with different mechanisms of action, that are used alone or in combination. The major chemical classes of ecto products are organophosphates, synthetic pyrethroids, formamidines and insect growth regulators. With specific regard to ecto products for sheep, they are generally administered topically.
(257) Similarily to endoparasiticides for PA (see paragraph 228 above), the Parties expect a reduction of the demand for PA ectoparasiticides, as a consequence of the decrease in the number of farm animals and farms in the EEA in the future. They also observe an increase in buyer power, through the concentration of veterinaries into large clinics and hospitals, buying groups and the expansion of large corporate customers in the PA sector. Another trend indicated by the Parties is the increase in the presence of generics, which would lead to downward pricing pressure on animal health companies in the future.
(258) In the EEA, both Parties market several PA ectoparasiticides and the Transaction gives rise to the following Group 1/1+ markets in 2019.

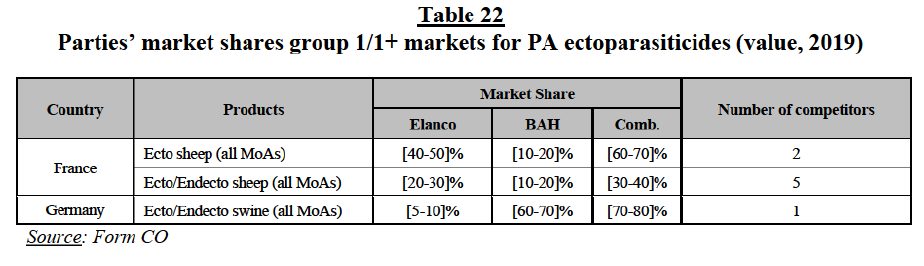

(d) The Commission’s assessment
(262) As a preliminary remark, it should be noted that, unless otherwise specified, the findings set out in this Section 4.3.1.3, and in particular the results of the market investigation, do not materially differ depending on the species. As regards possible differences depending on the analysed EEA country, although national specificities exist, the overall structure and the main characteristics of supply and demand in the markets for ectoparasiticides and ecto/endecto products for PA do not appear to vary significantly across countries. Therefore, unless otherwise specified, the findings of Section 4.3.1.3 (regarding e.g. closeness of competition, generic competition and the feedback received from market participants) do not materially differ depending on the geographic market at stake.
(263) The evidence in the Commission’s file generally confirms the Parties’ claims. It allows the Commission to exclude serious doubts as to the compatibility of the Transaction with the internal market and the functioning of the EEA Agreement resulting from the overlap of the Parties’ activities in PA ectoparasiticides, under all plausible market definitions.
(264) First, it appears from Table 22 above that the Transaction gives rise to Group 1 markets for PA ectoparasiticides in only two countries. More specifically:
(a) In France: the Parties have (i) high combined market shares of [60-70]%, with a [10-20]% increment brought by BAH, in the PA ectoparasiticides market for sheep and (ii) more moderate combined market shares of [30- 40]%, with a [10-20]% increment brought by BAH, in the PA ecto/endecto products market for sheep. Albeit the significant combined market shares in the PA ectoparasiticides market for sheep, post-Transaction, the new entity would face a strong competitor, Merck ([30-40]% market share)176. The new entity would face strong competitors also in the ecto/endecto products market for sheep, such as BI ([20-30]% market share), followed by Merck ([10- 20]%) and Zoetis ([10-20]%). In addition, the Parties indicated that the market share estimates provided to the Commission do not capture all the generic competitors active in the above market (the Parties’ estimates are based on the CEESA database, which does not systematically report the sales of all the generic players active in the EEA). Therefore, it is likely that the new entity will also face competition from generics, which is corroborated by the feedback received from market participants (see paragraphs 266 and 267 below).
(b) In Germany: the Parties have high combined market shares of [70-80]%, with an increment of [5-10]% brought by Elanco, in the ecto/endecto products market for swine. Albeit the higher combined market shares, the increment brought by the Transaction ([5-10]% brought by Elanco) is relatively small; in addition, post-Transaction, the new entity would still face competition from a strong player (BI, with [30-40]% market share). In addition, the Parties indicated that the market share estimates provided to the Commission do not capture all the generic competitors active in the above market (the Parties’ estimates are based on the CEESA database, which does not systematically report the sales of all the generic players active in the EEA). Therefore, it is likely that the new entity will also face competition from generics, which is corroborated by the feedback received from market participants (see paragraphs 266 and 267 below).
(265) Second, the market investigation results indicated that, with regard to, more broadly, the EEA market for ectoparasiticides for PA, Merck is perceived as the market leader, followed by Elanco, BAH and Zoetis in ectoparasiticides for PA. Other players, such as BI, Virbac and Bimeda, are also often mentioned among the top five competitors in this segment.
(266) Third, similarly to the considerations made above for PA endoparasiticides and PA endectocides, according to most competitors, there are no specific PA parasiticides for which the Parties are either the only alternatives available or among the very few suppliers in the EEA.
(267) Fourth, similarly to the considerations made above for PA endoparasiticides and PA endectocides, competitors indicated that generics exert a competitive constraint over branded PA ectoparasiticides.
(268) Finally, customers and competitors did not indicate any substantiated competition concerns in relation to the combination of the Parties’ marketed ectoparasiticides for PA.
(e) Conclusion
(269) In view of the above considerations, taking into account the market investigation and all the evidence available to it, the Commission concludes that the Transaction does not raise serious doubts as to its compatibility with the internal market and the functioning of the EEA Agreement with respect to overlaps between the Parties’ marketed ectoparasiticides for sheep in France and ecto/endecto products for sheep in France and for Swine in Germany, under all plausible market definitions.
4.3.2. Antimicrobials
(a) Overview of the market and the Parties’ products
(270) As explained in Section 3 above, antimicrobials (also known as antibiotics) are pharmaceutical products that destroy or prevent the growth of microbes such as bacteria, mycoplasma or fungi and treat diseases associated with them.
(271) Elanco supplies two products in countries giving rise to Group 1 / 1+ markets; Tylan and Denagard. Tylan is a macrolide antimicrobial indicated for swine, poultry and cattle; Denagard is a pleuromutilin antimicrobial indicated for swine and poultry. Both are chiefly administered orally.
(272) BAH supplies one product in the same markets; Baytril, a fluoroquinolone antimicrobial indicated for cattle, poultry, swine, goats and rabbits (as well as CA). Baytril is predominantly administered by way of injection, but also comes in oral pharmaceutical forms. Fluoroquinolones are categorised as higher risk antimicrobials than macrolides or pleuromutilins, mentioned above.

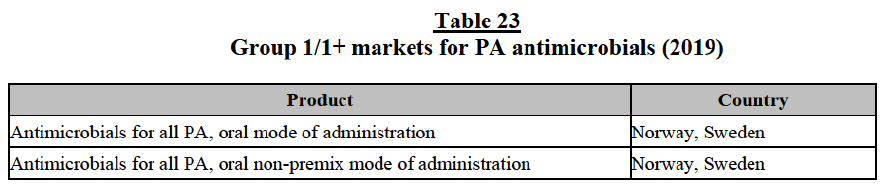

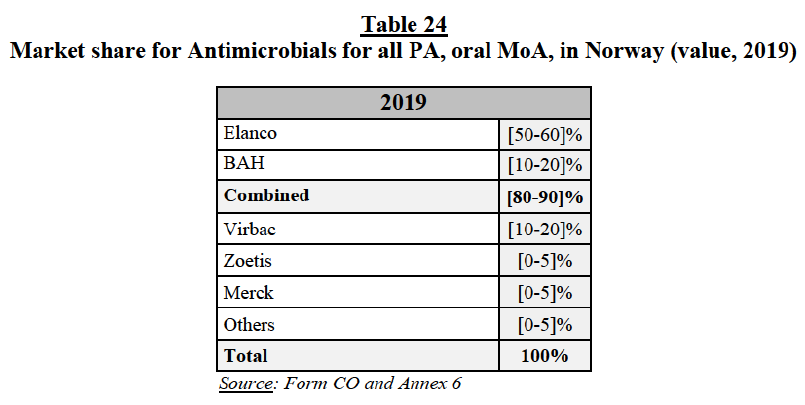

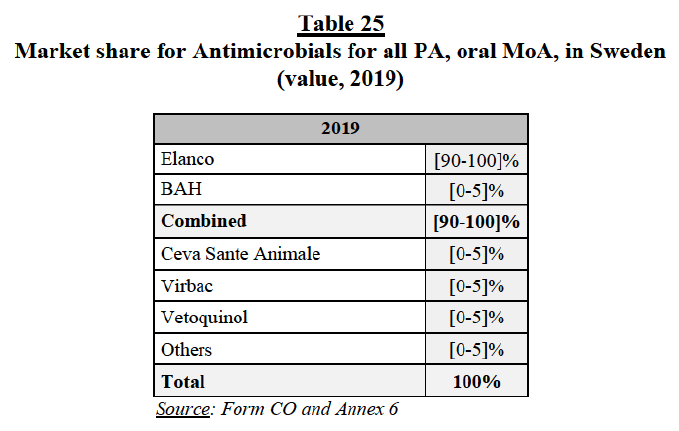

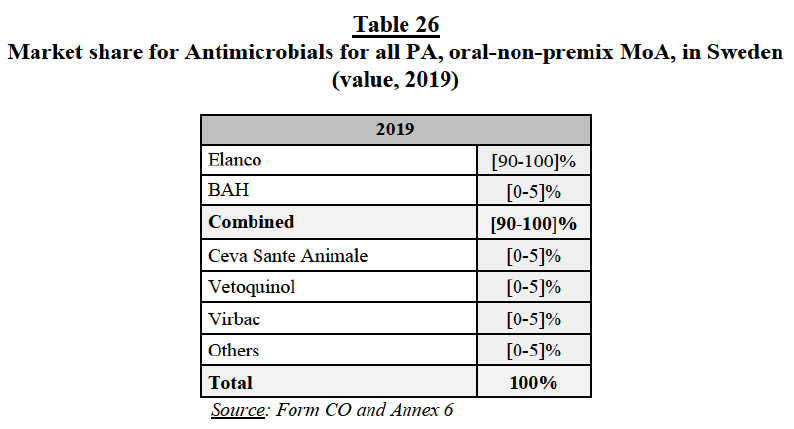
macrolides, pleuromutilins or other) have already been used. Importantly, Baytril (including the Baytril 10% oral solution product that determines BAH’s market position in all Group 1 affected markets) has a specific contraindication that states that it should not be used for prophylaxis i.e. preventative treatments. Thus they are typically used at different stages of the treatment.
(279) On the other hand, a number of competitor products are in the same chemical family as one or other of the Parties’ products. For example, Zoetis and Virbac both market macrolide antimicrobials, which compete more closely with Elanco’s products, and Hipra markets fluoroquinolone antimicrobials, which compete more closely with BAH’s product.
(280) Further, the Parties’ respective portfolios differ materially in the withdrawal periods following administration, before which animals cannot be slaughtered. These relative differences reflect the portfolios’ differing underlying chemical classes: Baytril’s oral products require withdrawal periods of a minimum of 5-13 days depending on the species, compared to 0-1 days for Elanco’s oral products. From a customer perspective, therefore, switching between the Parties’ products would require meaningful adjustment to planning and organisation in order to incorporate different withdrawal periods.
(281) Lastly, all of the Parties’ key antimicrobial products are off-patent and generics play a particularly significant role in the supply of antimicrobial products in Europe.
(d) The Commission’s assessment
(282) The evidence in the Commission’s file generally confirms the Parties’ claims. It allows the Commission to exclude serious doubts as to the compatibility of the Transaction with the internal market and the functioning of the EEA Agreement resulting from the overlap of the Parties’ activities in antimicrobials for PA.
(283) First, most customers confirm the Parties’ views that antimicrobials with different chemical classes are not interchangeable. Indeed, with the EMA categorisation of antibiotics by chemical class177, and consequent restriction of the use of antibiotics belonging to certain chemical classes, there are elements to indicate that antibiotics with chemical classes in different risk categorisations (as are Elanco’s and BAH’s products respectively) do not compete closely. Indeed, one customer elaborated further that “Avoiding antimicrobial resistance plays a big part in product choice…”178 Most customers also confirmed that the withdrawal period is the most important factor in the selection for a PA antimicrobial179. As such, the Parties’ products, being of different chemical classes, and different withdrawal periods, are not close competitors. In the EEA Virbac (Pharmasin), BI (Suanovil) and Zoetis (Zactran and Draxxin) all supply macrolides (the same class as Elanco’s Tylan); and Virbac (Stalimox), Calier (Caliermutin) and Ceva (Timavet) all supply pleuromutilins (the same class as Elanco’s Denagard); while as regards BAH’s Baytril product, Zoetis (Dicural and Advocin), Hipra (Hiprolana), Calier (Roxacin) and Ceva (Flumisol) all supply fluoroquinolones.
(284) Second, the results of the market investigation confirm that, post-Transaction, the new entity would continue to face competitive constraints from rivals. Competitors and customers consider Zoetis as the leader in the overall EEA PA Antimicrobials markets, followed by Merck, Ceva, BAH, BI and Virbac, whereas Elanco is not widely considered to be among the top players180. The results of the market investigation also confirm that the Parties are not close competitors.181
(285) Third, the market investigation confirmed the Parties’ claim that generics exert pressure on the prices of branded PA antimicrobials182. In the EEA there are several generics of Tylan, Denagard and Baytril marketed by larger players such as Ceva, Vetoquinol, Huvepharma and Krka as well as a number of smaller, regional players. Further, competitors noted that generic entry into the antimicrobials market is straightforward and that suppliers active in some EEA countries can also enter others. One supplier explained that “It [entry] will be easier / less intensive capital investment for generic entrants.” Another noted “Overall there is no significant different barriers of entry between … countries in the EEA zone.” 183 Provided they have established commercial presence in the country, other players could also enter these segments with generic products. The Parties estimate that it would be low cost ([…]) and quick ([…]) for suppliers to obtain authorisations in Norway and Sweden for generic versions of the Parties’ products.
(286) Fourth, the market investigation did not reveal any substantiated competition concerns in relation to the combination of the Parties’ PA antimicrobials.184 Overall, the large majority of customers and competitors indicated that there would remain sufficient alternative suppliers post-Transaction and do not expect a price increase in PA antimicrobials.
(287) Lastly, looking at the individual countries, the Transaction gives rise to Group 1 markets for PA antimicrobials in two countries. More specifically:
(a) In Norway: the Parties have a combined market share of [80-90]% with BAH’s increment of [10-20]% (or EUR […]) in the oral antimicrobials for all species and oral non-premix antimicrobials for all species segments, which are the same in Norway. However, in Norway Elanco’s only product in these segments is Denagard, which is exclusively used for swine in Norway (Denagard is not registered for species other than swine in Norway). BAH’s products (Baytril 2.5% and 10%), on the other hand, are used solely for poultry and cattle185. As such, the Parties are effectively not competing in these segments. The third supplier in this market, Virbac, targets niche species other than swine, poultry or cattle, and thus in the pre-transaction situation none of the three suppliers compete closely. Moreover, the so-called PA antimicrobials market leaders Zoetis and Merck were present in these segments in previous years, and would presumably have the commercial presence to be able to re-enter and compete with the new entity. Further, for generic fluoroquinolone products there is currently a marketing authorisation in Norway for Exoflox (Le Vet Beheer) and were previously a number of additional marketing authorizations which were then withdrawn by the holders (including Enrotron of antiMedica and Fenoflox of Chanelle). The Parties estimate that it would be low cost ([…]) and quick ([…]) for suppliers to obtain Norwegian authorisations for these products. Lastly, no competitors or customers have raised concerns for this market during the investigation.
(b) In Sweden: the Parties have high combined market shares with limited increments; (i) [90-100]% ([0-5]% or EUR […] increment from BAH) in the oral antimicrobials for all species segment and (ii) [90-100]% ([0-5]% or EUR […] increment from BAH) in the overall oral non-premix antimicrobials for all species segment. In Sweden, Elanco supplied Denagard and Tylan in 2018, over [70-80]% of which is supplied to swine, with the remainder going to poultry. BAH’s products, on the other hand (Baytril 2.5% and 10%), are used predominantly for poultry ([80-90]%) and cattle ([20- 30]%). As such the Parties are not considered to compete closely in these segments. The new entity will face competitors with higher market shares than BAH, namely Ceva ([0-5]% and [0-5]% respectively) and Vetoquinol ([0-5]% and [0-5]% respectively). Further, for generic fluoroquinolone products there is currently a marketing authorisation in Norway for Exoflox (Le Vet Beheer) and Fenoflox (Chanelle), and there were previously a number of additional marketing authorizations which were then withdrawn by the holders (including Enrotron of antiMedica). The Parties estimate that it would be low cost ([…]) and quick ([…]) for suppliers to obtain Swedish authorisations for these products. Lastly, no competitors or customers have raised concerns for this market during the investigation.
(e) Conclusion
(288) In view of the above considerations, taking into account the market investigation and all the evidence available to it, the Commission concludes that the Transaction does not raise serious doubts as to its compatibility with the internal market and the functioning of the EEA Agreement with respect to antimicrobials for PA under any potential market definition.
4.3.3. Anticoccidials
(a) Overview of the market and the Parties’ products
(289) As explained in Section 3 above, anticoccidials act against coccidiosis, a parasitic disease of the intestinal tract of animals caused by pathogenic coccidia.
(290) The Parties’ products giving rise to Group 1 / 1+ markets are Elanco’s Vecoxan indicated for cattle and sheep and BAH’s Baycox product indicated for cattle, sheep and swine. Both products are licensed for the prevention rather than treatment of coccidiosis.186 These products are principally used to prevent coccidiosis in an entire

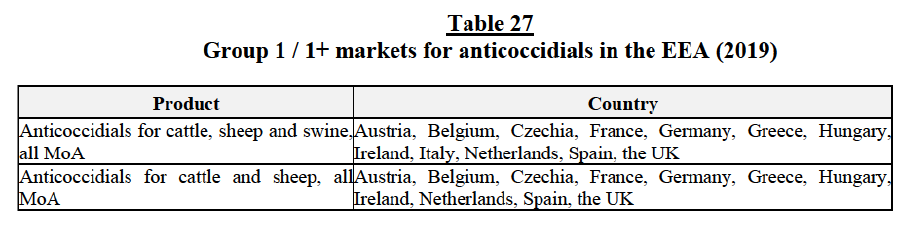
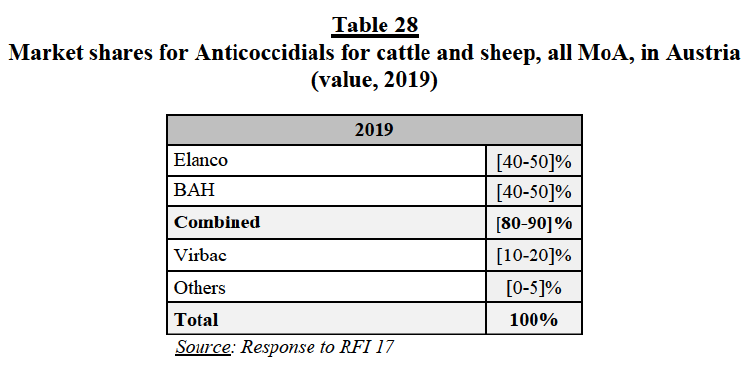

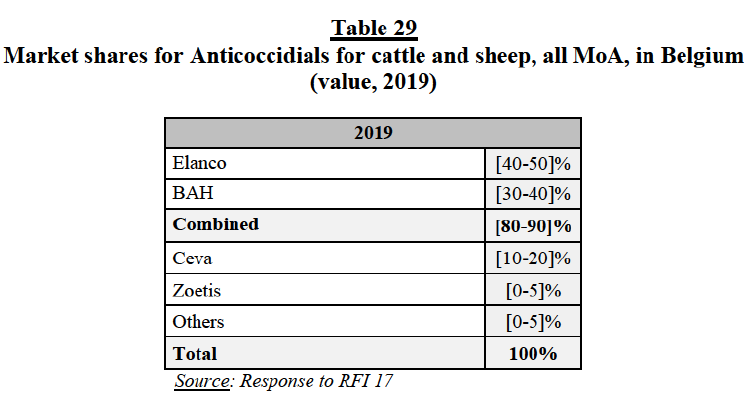

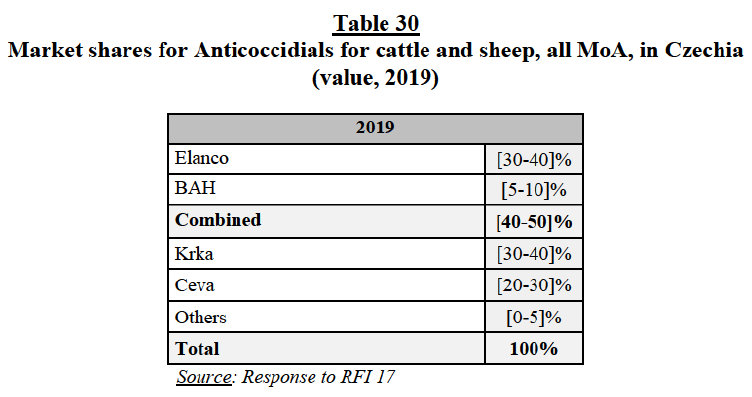

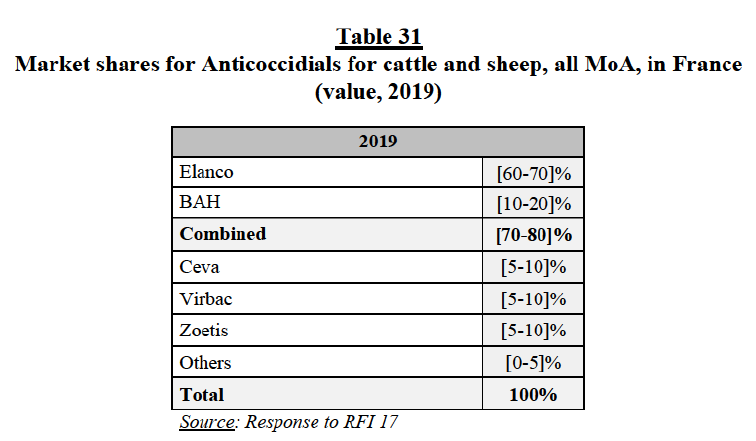

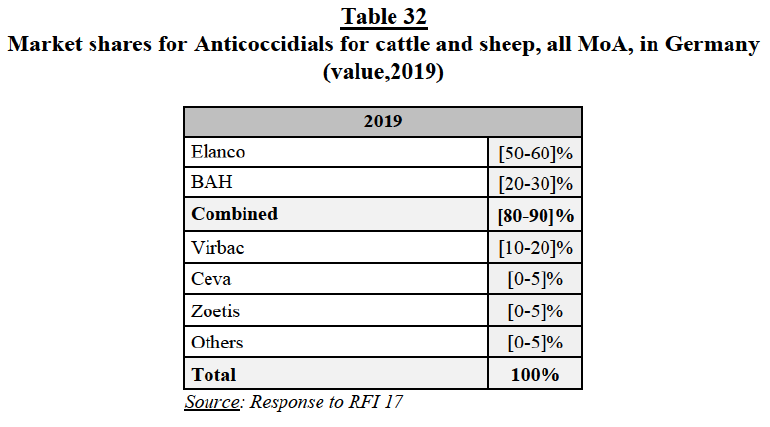

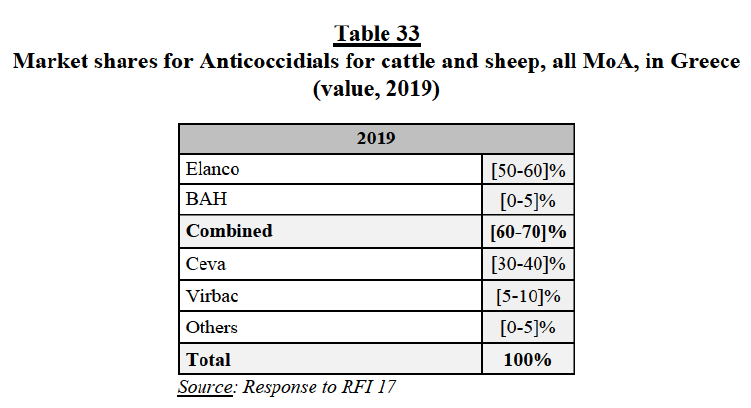

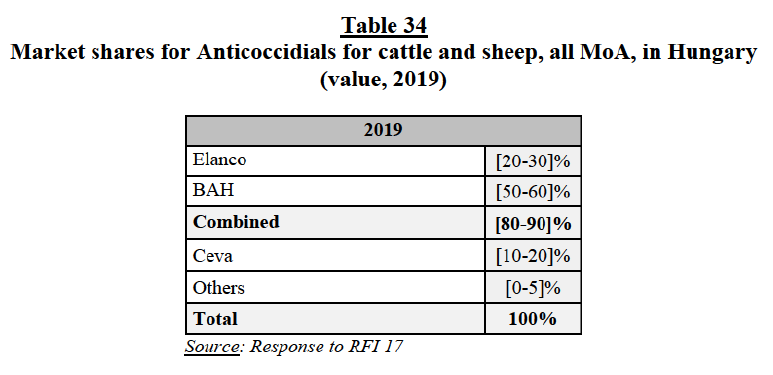

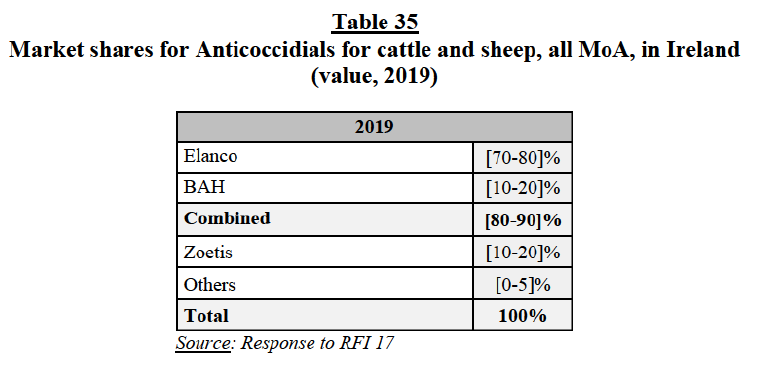

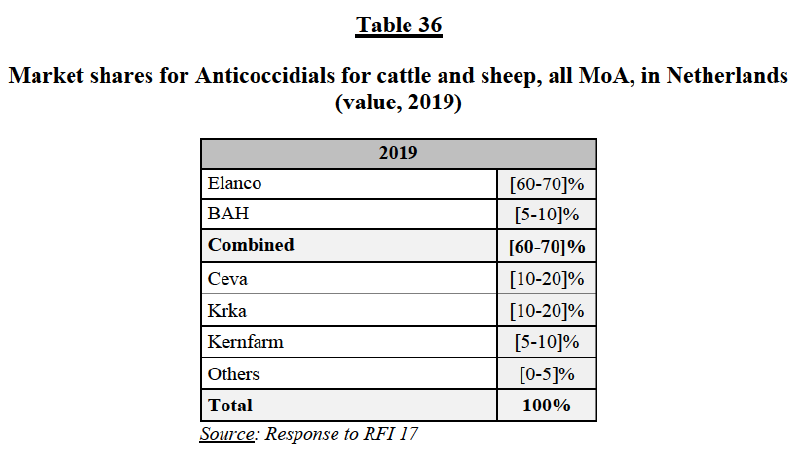

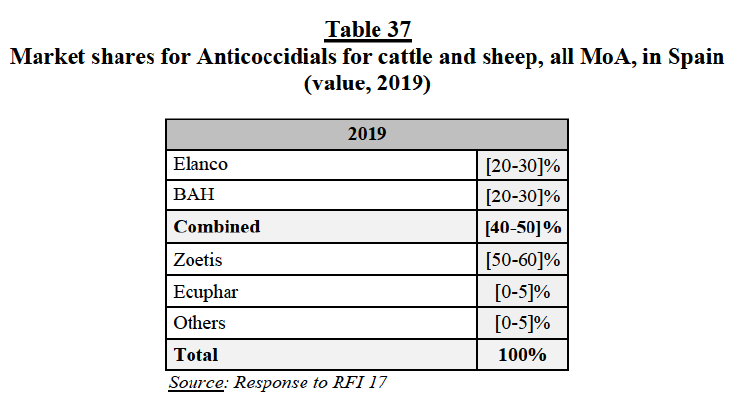

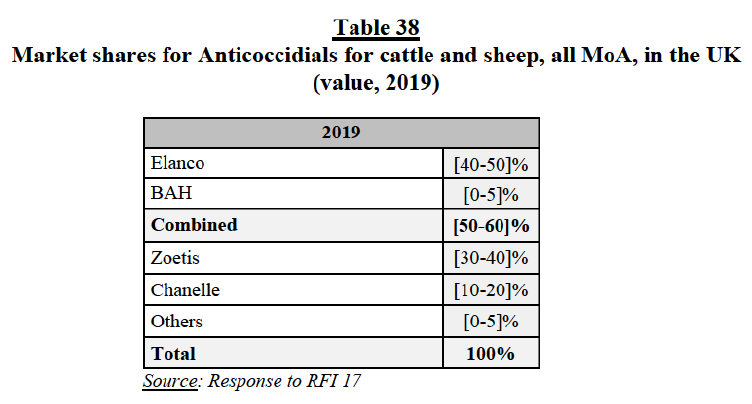
(309) First, most competitors consider that the Parties are close competitors in anticoccidials for cattle and sheep.188 A number of customers also highlighted that for ruminant anticoccidials the Parties’ products are the only alternatives or are among very few suppliers available189. Moreover, both customers and competitors consider BAH and Elanco to be in the top three players in ruminant anticoccidials190.
(310) Second, while the market investigation does confirm the Parties’ claim that generics exert pressure on the prices of branded products, generics accounted for less than 20% of most customers’ PA anticoccidial purchases191. One supplier points out that for PA anticoccidials “Generics create price competition but branded products benefit from brand loyalty.”
(311) Third, the large majority of competitors consider that entering the EEA markets for PA anticoccidials is difficult, with the main barriers to entry cited being R&D costs, access to APIs, and the need for large volume production facilities.192.
(312) Fourth, most competitors consider that there is a risk of price increase in ruminant anticoccidials as a result of the transaction.193 One supplier notes that “… Elanco & Bayer are major providers of … (toltrazuril, diclazuril). There is no alternative except generics that are not a lot.”
(313) Lastly, looking at the individual countries, the Commission notes that the Transaction leads to high or moderate combined market shares in a number of affected markets. More specifically:
(a) In Austria: the Parties have high combined market shares in Anticoccidials for cattle and sheep ([80-90]%, with a [40-50]% increment). There are two competitors, even the larger of which has significantly a lower market share ([10-20]%) than the increment.
(b) In Belgium: the Parties have high combined market shares in Anticoccidials for cattle and sheep ([80-90]%, with a [30-40]% increment). There is one competitor with a significantly lower market share ([10-20]%) than the increment.
(c) In Czechia: the Parties have moderate combined market shares in Anticoccidials for cattle and sheep ([40-50]%, with a [5-10]% increment). There are two competitors, both with higher market shares ([30-40]% and [20-30]%) than the increment.
(d) In France: the Parties have high combined market shares in Anticoccidials for cattle and sheep ([70-80]%, with a [10-20]% increment). There are three competitors, all with market shares ([5-10]%, [5-10]%, [5-10]% respectively) lower than the increment.
(e) In Germany: the Parties have high combined market shares Anticoccidials for cattle and sheep ([80-90]%, with a [20-30]% increment). There are two competitors, both with significantly lower market shares ([10-20]% and [0- 5]%) than the increment.
(f) In Greece: the Parties have high combined market shares in Anticoccidials for cattle and sheep ([60-70]%, with a [0-5]% increment). There are two competitors, one with a significantly higher market share ([30-40]%) than the increment.
(g) In Hungary: the Parties have high combined market shares in Anticoccidials for cattle and sheep ([80-90]%, with a [20-30]% increment). There is only one competitor, with a lower market share ([10-20]%) than the increment.
(h) In Ireland: the Parties have high combined market shares in Anticoccidials for cattle and sheep ([80-90]%, with a [10-20]% increment). There is one competitor with a lower market share ([10-20]%) than the increment.
(i) In the Netherlands: the Parties have high combined market shares in Anticoccidials for cattle and sheep ([60-70]%, with a [5-10]% increment). There are four competitors, two of which have higher market shares ([10- 20]% and [10-20]%) than the increment.
(j) In Spain: the Parties have moderate combined market shares in Anticoccidials for cattle and sheep ([40-50]%, with a [20-30]% increment). There are two competitors, one with a higher market share ([50-60]%) than the Parties’ combined market share.
(k) In the UK: the Parties have high combined market shares in Anticoccidials for cattle and sheep ([50-60]%, with a [0-5]% increment). There are two competitors, both with significantly higher market shares ([30-40]% and [10- 20]%) than the increment.
(e) Conclusion
(314) The Commission considers that the high market shares, combined with the closeness of competition between the Parties’ products, the lack of sufficient competitors and the other elements of the market investigation described above, point to a significant increase of market power of the merged entity post-Transaction in a number of countries. It derives from this assessment that the Transaction raises serious doubts as to its compatibility with the internal market, due to its likely horizontal noncoordinated effects in Anticoccidials for cattle and sheep in Austria, Belgium, France, Germany, Hungary, and Ireland. In the present case, the Commission does not need to take a view on the markets in Czechia, Greece, the Netherlands, Spain and the UK, as in any event the remedy offered by the Parties covers the full overlap on an EEA-wide basis for viability purposes.
4.4. Innovation
(a) The Commission’s assessment
(315) The Commission has assessed whether the Transaction could lead to a reduction in innovation competition in relation to the capability to innovate in certain innovation spaces, assessing the risk of a significant loss of innovation competition resulting from a structural reduction of the overall level of innovation, in line with the fourlayer competitive assessment framework used in previous decisions.194 This assessment has been conducted looking at the general innovation capabilities of the Parties and their competitors, over and beyond the competitive situation for specific marketed and pipeline products, which have been assessed in detail in the sections above.
(316) The market investigation showed that barriers to entry with regards to innovation in the animal health sector are high in particular for parasiticides due to substantial development costs and the synergies stemming from internal R&D capabilities to develop parasiticides for crops in the development of parasiticides for animal health.195
(317) This notwithstanding, the Commission found that the Transaction will not result in a significant loss of innovation for several reasons.
(318) First, the Parties are well below the top 3 undertakings in term of R&D (i.e. Zoetis, Merck, and BI). In particular, the results of the market investigation revealed that:
(a) According to the data provided by the Parties, […]. Moreover, the market investigation revealed that the Parties underestimated the R&D expenditure and number of full time employees of several of their competitors. It follows that, post-Transaction, several competitors will have significant resources in terms of budget and number of employees to compete in innovation with the merged entity;
(b) BAH has not been a particularly strong innovator in recent years, including in parasiticides. For instance, contrary to its competitors (including also medium size companies), BAH has launched no new parasiticides (based on new molecules) in recent years. In this vein, one of the top competitors explained that “BAH is not considered in the same league as Zoetis, BI, Merck and Elanco in terms of innovation” and explained that animal health was no longer a priority of Bayer.196 This is indeed confirmed by Bayer’s decision to exit the animal health business;
(c) Customers perceive Merck, Zoetis and Boehringer as the strongest innovators in animal health and in parasiticides in particular, while Elanco, BAH and Ceva would be number four, five and six respectively.197
(319) Second, the market investigation showed that medium-size companies have the capability to develop new products in all relevant product categories concerned by this Transaction, including parasiticides. In fact, thirteen companies indicated they would be capable of developing new originator products based on new molecules or active ingredients and fourteen companies have the capabilities to develop new products based on life cycle management.198 The market investigation revealed that medium-size companies (without internal crop R&D) have the possibility to conclude partnerships with third-parties. In fact, in recent years, several parasiticides originator products have been launched to the market by medium-size companies, such as Ceva, Fatro or Beaphar.199
(320) Finally, the overwhelming majority of customers and competitors indicated that the impact of the Transaction in R&D and innovation would be neutral or positive. In fact, some of the most important competitors believe that the Transaction may create synergies and lead to faster development of products.200
(b) Conclusion
(321) Based on the above considerations, taking into account the market investigation and all the evidence available to it, the Commission concludes that the Transaction does not raise serious doubts as to its compatibility with the internal market or the functioning of the EEA agreement in relation to effects on innovation.
5. COMMITMENTS
5.1. Framework for the assessment of the Commitments
(322) Where a notified concentration raises serious doubts as to its compatibility with the internal market, the parties may undertake to modify the concentration to remove the grounds for the serious doubts identified by the Commission. Pursuant to Article 6(2) of the Merger Regulation, where the Commission finds that, following modification by the undertakings concerned, a notified concentration no longer raises serious doubts, it shall declare the concentration compatible with the internal market pursuant to Article 6(1)(b) of the Merger Regulation.
(323) As set out in the Commission's Remedies Notice,201 the commitments have to eliminate the competition concerns entirely, and have to be comprehensive and effective from all points of view.202
(324) In assessing whether commitments will maintain effective competition, the Commission considers all relevant factors, including the type, scale and scope of the proposed commitments with reference to the structure and the particular characteristics of the market in which the Transaction is likely to significantly impede effective competition, including the position of the Parties and other participants on the market.203
(325) In order for the commitments to comply with those principles, they must be capable of being implemented effectively within a short period. Concerning the form of acceptable commitments, the Merger Regulation gives discretion to the Commission as long as the commitments meet the required standards. Structural commitments will meet the conditions set out above only in so far as the Commission is able to conclude with the requisite degree of certainty, at the time of its Decision, that it will be possible to implement them and that it will be likely that the new commercial structures resulting from them will be sufficiently workable and lasting to ensure that effective competition will be maintained.204 Divestiture commitments are normally the best way to eliminate competition concerns resulting from horizontal overlaps.
(326) The divested activities must consist of a viable business that, if operated by a suitable purchaser, can compete effectively with the merged entity on a lasting basis and that is divested as a going concern. The business must include all the assets which contribute to its current operation or which are necessary to ensure its viability and competitiveness and all personnel which are currently employed or which are necessary to ensure the business' viability and competitiveness.205
(327) The intended effect from the divestiture will only be achieved if and once the business is transferred to a suitable purchaser in whose hands it will become an active competitive force in the market. The potential of a business to attract a suitable purchaser is an important element of the Commission's assessment of the appropriateness of the proposed commitment.206
(328) Even though normally the divestiture of an existing viable stand-alone business is required, the Commission, by observing the principle of proportionality, may also advise the Parties to consider the divestiture of businesses which have existing strong links or are partially integrated with businesses retained by the parties and therefore need to be ‘carved out’ in those respects. Conversely, carving-out a business from the scope of the commitments can only be accepted by the Commission if it can be certain that, at least at the time when the business is transferred to the purchaser, a viable business on a stand-alone basis will be divested and the risks for the viability and competitiveness caused by the carve-out will thereby be reduced to a minimum.
5.2. Procedure
(329) In order to render the concentration compatible with the internal market, the Parties have submitted a set of commitments under Article 6(2) of the Merger Regulation on 13 May 2020 (the "Initial Commitments"). The Commission market tested the Initial Commitments on 14 May 2020 in order to assess whether they were sufficient and suitable to remedy the serious doubts identified at Section 4 above. Following the feedback received from market test, amended commitments were submitted on 3 June 2020 (the “Final Commitments”). The Final Commitments are annexed to this Decision207 and form an integral part thereof.
5.3. The Initial Commitments
5.3.1. Description of the Initial Commitments
(330) The Initial Commitments are set out in two submissions: the “Main Commitments” and the “Additional Commitments”.
(331) Under the Main Commitments, the Parties offered the divestiture of the following products (together referred as the “Main Divestment Businesses”):
(i) Elanco’s VECOXAN (anticoccidials for ruminants) at global level (the “Anticoccidials Divestment Business”);
(ii) Elanco’s OSURNIA (long lasting otitis treatment) at global level (the “Otitis Divestment Business”); and
(iii) BAH’s Drontal and Profender family of products208 (CA endoparasiticides) at EEA level (the “Parasiticides Divestment Business”).
(332) The Main Commitments provide that the divestiture of the Anticoccidial Divestment Business, the Otitis Divestment Busines and the Parasiticide Divestment Business would be implemented by way of asset sales and that each of them may be sold to different suitable purchasers (the “Purchaser(s)”). The Main Divestment Businesses would include all rights, title, and interest in the concerned products (including the rights to develop, improve, manufacture, commercialise and distribute these products), as well as all the assets that are necessary to ensure its viability, in particular: (i) all contracts related to these products (such as supply and distribution agreements); (ii) all licences, product registrations and authorisations; (iii) all intellectual property rights related to the development, manufacturing and exploitation of the concerned products; (iv) all customer orders and records and (v) all other tangible and intangible assets (e.g., inventory, regulatory files, books and records). At the option of the Purchaser(s), the Main Divestment Businesses would also include staff currently employed by the Main Divestment Businesses (the “Personnel”). Furthermore, with the aim of ensuring a smooth transfer of the Main Divestment Businesses, the Parties committed to offer a number of transitional supply arrangements to the Purchaser(s).
(333) In addition to the standard purchaser requirements contained in the Commission’s template for divestiture remedies, the Main Commitments provide that the Purchaser(s) must have: (i) established capabilities or a track record in the manufacture, marketing and distribution of animal health products in the EEA; (ii) adequate manufacturing and regulatory capabilities to successfully implement a production transfer in relation to the Main Divestment Businesses (where relevant); (iii) sufficient R&D ressources and experience to develop the relevant pipeline products included in the scope of the Main Divestment Businesses (where relevant); (iv) complementary products and expertise to the Main Divestment Businesses.
(334) Furthermore, the Main Commitments provide for an upfront buyer provision pursuant to which the Transaction cannot be implemented until the Commission has given its approval to the purchase of the Main Divestment Businesses by the Purchaser(s).
(335) Under the Additional Commitments, the Parties offered to divest globally to one or several Purchaser(s) BAH isox pipelines, comprising […] (together referred as the “ESP Divestment Business”) or, alternatively, Elanco’s marketed and pipeline isox products […] at EEA level (the “Alternative Divestment Business”).209 […],210 […]. The Parties would be obliged to implement the Alternative Divestment Business if they are not able to implement the divestiture of the BAH Divestment Business within the time frame of the first Divestiture Period.211
(336) The ESP Divestment Businesses, or the Alternative Divestment Business (as applicable), would be divested by way of asset sales. They would include all rights, title, and interests to develop, improve, manufacture and commercialise the products concerned, as well as all the assets necessary to ensure their viability (insofar as they relate to the ESP Divestment Business or the Alternative Divestment Business), in particular: (i) all contracts related to these products; (ii) all product licences, permits, registrations and authorisations; (iii) all intellectual property rights related to to the development, improvement, manufacturing and exploitation of the concerned products; and (iv) all other tangible and intangible assets (e.g., inventory, regulatory files, books and records). In addition, the ESP Divestment Business includes certain employees working on the development of BAH’s isox pipeline (the “Key Personnel”). Furthermore, with the aim of ensuring a smooth transfer of the ESP Divestment Business, or the Alternative Divestment Business (as applicable), the Parties committed to offer a number of transitional supply arrangements to the Purchaser.
(337) Moreover, at the option of the Purchaser(s), the ESP Divestment Businesses, or the Alternative Divestment Business (as applicable), would also include personnel.212
(338) […].
(339) In addition to the standard purchaser requirements contained in the Commission’s template for divestiture remedies, the Additional Commitments provide that the Purchaser(s) must have: (i) established capabilities or a track record in the clinical development of animal health products in the EEA (including with regard to having interactions with relevant EEA-wide or national bodies that decide on approval of animal health products); (ii) established capabilities or a track record in the manufacture, commercialisation and distribution of animal health products in the EEA; (iii) sufficient R&D ressources and experience to develop the relevant pipeline products included in the scope of the ESP Divestment Business or, if applicable, the Alternative Divestment Business; (iv) complementary products and expertise to the ESP Divestment Business or, if applicable, the Alternative Divestment Business.
5.3.2. Assessment of the Initial Commitments
(340) The Commission analysed the suitability of the Initial Commitments to remedy the serious doubts raised by the Transaction, in particular under the principles set out in the Commission Remedies Notice. In its assessment, the Commission relied inter alia on the results of the market test launched on 14 May 2020.
(a) The Initial Commitments appear generally capable to address the competition concerns identified in Section 4.
(341) For the reasons set out below, the Commission considers that the Initial Commitments appear generally capable to fully address the competition concerns identified in Section 4.
(342) First, respondents to the market test were generally positive about the Initial Commitments and confirmed that they are suitable to eliminate the competition concerns identified by the Commission.213
(343) Second, in terms of scope, the vast majority of market participants considered that the Divestment Businesses contain all the necessary assets to remain a viable and competitive force in the relevant markets.214 In fact, virtually all market participants generally consider that the divestiture of the Divestment Businesses will not result in a reduction of their sales.215 More specifically, competitors confirmed that no transfer of other assets or personnel is needed to ensure the viability and competitiveness of the Divestment Businesses, other than the Key Personnel of ESP Divestment Business. In this respect, competitors confirmed that the Key Personnel as identified in the Additional Commitments covered all the necessary roles.216
(344) Third, the results of the market test confirmed that all the Divestment Businesses are attractive businesses.217 In particular, many competitors expressed interest in the acquisition of either the ESP Divestment Business or the Alternative Divestment Business.218
(345) Fourth, the results of the market test confirmed the relevance of the purchaser requirements included in the Initial Commitments. Indeed, respondents consider almost unanimously that the Purchaser Criteria are sufficient to ensure the Purchaser(s) is/are able to compete actively and effectively in the relevant markets.219
(346) Fifth, virtually all competitors consider that the proposed transitional agreements provided for in the Initial Commitments were sufficient, both in terms of scope and duration, to ensure a smooth transfer of the Divestment Businesses.220
(347) Finally, respondents to the market test were generally positive about the Initial Commitments and confirmed, almost unanimously, that the sales of the Divestment Businesses would be sufficient to solve any competition concerns arising from the Transaction in the relevant markets.221
(b) Shortcomings of the Initial Commitments
(348) Nonetheless, the results of the market test also identified a few issues with the Initial Commitments requiring further improvements in order to make the Parasiticides Divestment Business and the Additional Divestment Business viable and competitive. The market test did not identify any shortcomings with regards to the Otitis Divestment Business or the Anticoccidials Divestment Business. On the basis of the results of the Market Test, the Commission considered the following amendments to the Initial Commitments to be necessary.
(349) As regards the Parasiticides Divestment Business, the market test revealed that the […] Pipeline is closely related to one of the […] products included in the Parasiticides Divestment Business, namely […]. Indeed, the […] pipeline product is […]. As a result, the […] pipeline product is expected to closely compete with […]. Taking these considerations into account, some respondents to the market test […] and indicated that this product should be divested together with […] to ensure the viability of the Parasiticides Divestment Business ([…]). In particular, one competitor indicated that “the only possibility to remove them [the competition concerns] completely is to couple the sale of the Endoparasiticides Divestment Business and of […]”.222 […].223
(350) Moreover, the market test also revealed that the […] Pipeline is very advanced, the drug having reached the final process of registration, with only a few ongoing studies, and that the marketing authorisation of the […] pipeline could be jeopardized without the support of BAH to finalise the ongoing studies and the final process with the EMA.
(351) As regards the Additional Commitments, and more specifically the Alternative Divestment Business, the Commission considered that (i) additional safeguards were needed to ensure that the development of the pipeline products included in the Alternative Divestment Business (which is conducted under the supervision of the Purchaser and the Monitoring Trustee) and that (ii) to ensure the viability of the Alternative Divestment Business, all the obligations for the interim period (e.g., preservation of viability, ring fencing mechanism, hold-separate manager) (the “Interim Obligations”) should apply to the Alternative Divestment Business from the date of the adoption of the present Decision (the “Effective Date”).
5.4. The Final Commitments
(352) In order to address the shortcomings discussed in Section 5.3, the Parties submitted the Final Commitments, on 3 June 2020.
5.4.1. Description of the Final Commitments
(353) The Final Commitments are set out in two submissions: the “Final Main Commitments” and the “Final Additional Commitments”.
(354) With regards to the Parasiticides Divestment Business, the Final Commitments include the following improvements:
(a) The […] Pipeline is now part of the Parasiticides Divestment Business and will be sold to the same Purchaser together with all assets included in the Initial Commitments with regards to the Parasiticides Divestment Business.
(b) The Final Commitments include an obligation for the Parties to support the Purchaser of the Parasiticides Divestment Business […].
(355) With regards to the Additional Divestment Business, the Final Commitments include the following improvements:
(a) Enhanced safeguards for the Alternative Divestment Business in relation to the development of the pipeline products included in the Alternative Divestment Business. In particular: (i) the clinical development will be conducted under the supervision of the Purchaser, as well as the Monitoring Trustee; (ii) Elanco should devote sufficient resources in terms of budget and personnel, specifically, to conclude the clinical development phase without delays; (iii) the Purchaser will be granted a final say on all decisions related to the clinical development of the pipeline, which could impact the development and entry into market of the products in the EEE and; (iv) the Final Commitments provide for a fast-track dispute resolution mechanism in case of disputes between Elanco and the Purchaser in relation to the development of the the above-mentioned pipeline products.
(b) Interim Obligations will be applicable to the Alternative Divestment Business as of the Effective Date. All the obligations for the interim period (e.g., preservation of viability, ring fencing mechanism, hold-separate manager) apply to the Alternative Divestment Business from the Effective Date.
5.4.2. Assessment of the Final Commitments
(356) The Commission considers that these amendments adequately address the concerns raised by the market test respondents and the Commission itself in relation to the Initial Commitments. The Commission notes in particular that the changes done to the Initial Commitments as described in Section 5.3, contributes to the viability and competitiveness of the Divestment Businesses.
(357) On the basis of the above, the Commission concludes that the Divestment Businesses are viable businesses and the modalities foreseen for their transfer under the Final Commitments will allow suitable Purchaser(s) to operate them in a competitive and viable manner.
(358) The Final Commitments fully address the competition concerns identified in the Decision as they remove the overlap between Elanco and BAH in all markets where serious doubt arise.
(359) Moreover, the Final Commitments are comprehensive, effective, and are capable of being implemented effectively within a short period of time.
5.4.3. Conclusion
(360) Based on the reasoning in Sections 5.3 and 5.4 and taking into consideration the results of the market test, the Commission therefore considers that the Final Commitments are sufficient to eliminate all serious doubts as to the compatibility of the Transaction with the internal market and the EEA Agreement.
5.5. Conditions and obligations
(361) Under the first sentence of the second subparagraph of Article 6(2) of the Merger Regulation, the Commission may attach to its decision conditions and obligations intended to ensure that the undertakings concerned comply with the commitments they have entered into vis-à-vis the Commission with a view to rendering a notified concentration compatible with the internal market.
(362) The achievement of the measure that gives rise to the structural change of the market is a condition, whereas the implementing steps which are necessary to achieve this result are generally obligations on the Parties. Where a condition is not fulfilled, the Commission’s decision declaring the concentration compatible with the internal market no longer stands. Where the undertakings concerned commit a breach of an obligation, the Commission may revoke the clearance decision in accordance with Article 8(6) of the Merger Regulation. The undertakings concerned may also be subject to fines and periodic penalty payments under Articles 14(2) and 15(1) of the Merger Regulation.
(363) In accordance with the distinction described above, this Decision in this case is conditioned on the full compliance with the requirements set out in Section B or Section C, as the case may be, of the Final Commitments (including the Schedules), which constitute conditions. The remaining requirements set out in the other sections of the Final Commitments constitute obligations on the Parties.
(364) The detailed text of the Final Commitments is annexed to this Decision. The full text of the Final Commitments forms an integral part of this Decision.
6. CONCLUSION
(365) For the above reasons, the Commission has decided not to oppose the notified operation as modified by the Final Commitments and to declare it compatible with the internal market and with the functioning of the EEA Agreement, subject to full compliance with the conditions in Section B (or Section C as applicable) (including the Schedules) of the Final Commitments annexed to this Decision and with the obligations contained in the other sections of the said Final Commitments. This Decision is adopted in application of Article 6(1)(b) in conjunction with Article 6(2) of the Merger Regulation and Article 57 of the EEA Agreement.
FOOTNOTES :
1 OJ L 24, 29.1.2004, p. 1 (the "Merger Regulation"). With effect from 1 December 2009, the Treaty on the Functioning of the European Union ("TFEU") has introduced certain changes, such as the replacement of "Community" by "Union" and "common market" by "internal market". The terminology of the TFEU will be used throughout this Decision. Although the United Kingdom (the “UK”) withdrew from the European Union as of 1 February 2020, under the Agreement on the withdrawal of the United Kingdom of Great Britain and Northern Ireland from the European Union and the European Atomic Energy Community (the “Withdrawal Agreement”), Union law continues to apply to the UK during the transition period. Accordingly, any reference to Member States shall be understood as including the UK. Similarly any references made to the EEA in this Decision are meant to also include the UK.
2 OJ L 1, 3.1.1994, p. 3 (the "EEA Agreement").
3 Publication in the Official Journal of the European Union No C 132, 23.4.2020, p. 12.
4 See notably cases M.7917 - Boehringer Ingelheim/Sanofi Animal Health Business, paragraph 10; M.7277 - Eli Lilly/Novartis Animal Health, paragraph 12; M.6205 - Eli Lilly/Janssen Pharmaceutical Animal Health Business Assets, paragraph 11; and M.2922, Pfizer / Pharmacia, paragraph 111.
5 Biologicals are products triggering an immune response against viral and bacterial diseases, as well as occasionally parasitic or fungal infections in animals. Biologicals include in particular animal vaccines. The Parties’ activities do not give raise to affected markets with regards to vaccines or biologicials more broadly.
6 Feed supplements are pharmaceutical or nutritional substances which are not natural feedstuffs and are added to made-up and stored feeds, chiefly to control infectious diseases or to promote growth. The Parties’ activities give rise to minor horizontal overlaps in relation to feed supplements, but without affected market.
7 Additionally, both Parties supply certain additional, miscellaneous products: (i) ketosis products for cattle, (ii) rehydration treatments, (iii) premise applications, (iv) anaesthetics, (v) dermatitis products, (vi) topical ear cleaners, (vii) hormones, (viii) probiotics, (ix) immuno-stimulants. No competition concern arises with respect to any of these additional products, regardless of the market definition (see Section 4 below).
8 See footnote 4 above.
9 As further explained in Section 3.1.2.1 below, within parasiticides, the Commission distinguished products targeting multi-celled parasites (e.g. fleas and worms) and products targeting single-celled parasites (“anticoccidials”) (see e.g. case M.7277, Eli Lilly/ Novartis Animal Health, paragraph 16). In this Decision, any references to “parasiticides” shall be understood as parasiticides targeting multi-celled parasites (i.e. excluding anticoccidials).
10 See cases M.7917 - Boehringer Ingelheim/Sanofi Animal Health Business, paragraph 142; M.7277 - Eli Lilly/Novartis Animal Health, paragraph 14; M.6205 - Eli Lilly/Janssen Pharmaceutica Animal Health Business Assets, paragraph 14; and M.5476 - Pfizer/Wyeth, paragraph 120.
11 The withdrawal period corresponds to the period after treatment during which an animal’s meat or milk is considered unsuitable for human consumption.
12 Within pharmaceuticals, minor overlaps also arise for anti-inflammatories and analgesics. No competition concern arises with respect to these products, regardless of the market definition (see Section 4 below).
13 See cases M.7277 - Eli Lilly/Novartis Animal Health, paragraph 14; M.5476 - Pfizer/Wyeth, paragraph 110; M.4691 - Schering-Plough/Organon Biosciences, paragraph 21; and M.885 - Merck/Rhone-Poulenc-Merial, paragraph 36.
14 See cases M.7277 - Eli Lilly/Novartis Animal Health, paragraph 16; M.5476 - Pfizer/Wyeth, paragraph 287; and M.4691 - Schering-Plough/Organon Biosciences, paragraph 422.
15 See cases M.5476 - Pfizer/Wyeth, paragraph 289; and M.4691 - Schering-Plough/Organon Biosciences, paragraph 422.
16 See case M.7277 - Eli Lilly/Novartis Animal Health, paragraph 24.
17 See cases M.7277 - Eli Lilly/Novartis Animal Health, paragraph 19; and M.4691 - Schering-Plough/Organon Biosciences, paragraph 438.
18 See case M.7277 - Eli Lilly/Novartis Animal Health, paragraph 26.
19 See case M.6205 - Eli Lilly/Janssen Pharmaceutica Animal Health Business Assets, paragraph 57.
20 Form CO, paras. 6.93 and ff.
21 Questionnaires Q1 to competitors (question 11) and Q2 to CA customers (questions 10 and 11).
22 Questionnaires Q1 to competitors (question 11) and Q2 to CA customers (questions 10 and 11).
23 Questionnaires Q1 to competitors (question 15) and Q2 to CA customers (question 9).
24 Questionnaires Q1 to competitors (question 14) and Q2 to CA customers (question 14).
25 Questionnaires Q1 to competitors (question 17) and Q2 to CA customers (question 15).
26 Questionnaires Q1 to competitors (question 12) and Q2 to CA customers (question 12). For instance, a competitor expressly stated that: “customers ─ prescribers, veterinarians and end customers ─ increasingly favor broad spectrum products”. See also non-confidential minutes of a conference call with a competitor dated 6 February 2020, p. 2: “veterinaries generally prescribe two different products to treat ectoparasites like fleas and ticks, and endoparasites like heartworm and lungworm but, since there are products which can treat both at the same time, these would be more appealing to vets and pet owners.”
27 […].
28 Questionnaires Q1 to competitors (questions 12 and 13) and Q2 to CA customers (question 12).
29 Questionnaires Q1 to competitors (question 12) and Q2 to CA customers (question 12).
30 Isox products are GABA (gamma-aminobuyric acid) receptor antagonists, causing the uncontrolled activity of the nervous system of the ectoparasites, which leads to its paralysis and death.
31 Questionnaires Q1 to competitors (question 16) and Q2 to CA customers (question 13).
32 Questionnaire Q1 to competitors (question 18). See Section 4.2.1.3 below.
33 See Annex 78 to the Form CO.
34 See cases M.7277 - Eli Lilly/Novartis Animal Health, paragraphs 36 and 39; M.5476 - Pfizer/Wyeth, paragraph 324; and M.4691 - Schering-Plough/Organon Biosciences, paragraph 329.
35 See case M.7277 - Eli Lilly/Novartis Animal Health, paragraph 43.
36 Growing awareness of antimicrobial resistance in bacteria has led to regulation surrounding the use of antibiotics in animals. Prudent and responsible use of antibiotics in both animals and humans can lower the risk of bacteria becoming resistant. This is particularly important for antibiotics that are used to treat both people and animals and for antibiotics that are the last line of treatment for critical infections in people. See https://www.ema.europa.eu/en/documents/report/categorisation-antibiotics-use-animals-prudent-responsibleuse en.pdf
37 See paragraph 89 below.
38 Questionnaire Q2 to to CA customers, question 26.
39 Questionnaire Q1 to competitors, question 31.
40 Questionnaire Q2 to CA customers, question 27.
41 Questionnaire Q1 to competitors, question 32.
42 Questionnaire Q2 to CA customers, question 28.
43 Questionnaire Q1 to competitors, question 33.
44 See cases M.7277 - Eli Lilly/Novartis Animal Health, paragraph 33; and M.4691 - Schering-Plough/Organon Biosciences, paragraph 327.
45 Considering that otitis is an inflammation of the external ear canal which sometimes may progress further into the ear, a further sub-segmentation by indication is not meaningful. Moreover, the market investigation did not provide any element suggesting that a further segmentation by chemical class would be adequate.
46 Questionnaire Q1 to competitors, non-confidential replies to question 47.
47 Questionnaire Q1 to competitors, non-confidential replies to question 47.
48 Questionnaire Q1 to competitors, non-confidential replies to question 47.
49 Questionnaire Q1 to competitors, non-confidential replies to question 47.
50 Questionnaire Q1 to competitors, non-confidential replies to question 47.
51 Questionnaire Q2 to customers, non-confidential replies to question 40.
52 Questionnaire Q2 to customers, non-confidential replies to question 40.
53 Questionnaire Q1 to competitors, non-confidential replies to question 48.
54 Questionnaire Q2 to customers, non-confidential replies to question 41.
55 See case M.7277 - Eli Lilly/ Novartis Animal Health, paragraphs 44 to 51.
56 Form CO, para. 6.146. See also Section 3.1.2.1(b) above.
57 Questionnaires Q1 to competitors (question 62) and Q3 to PA customers (questions 10 and 11).
58 Questionnaires Q1 to competitors (question 64) and Q3 to PA Customers (question 9).
59 Questionnaires Q1 to competitors (question 63) and Q3 to PA customers (question 13).
60 Questionnaires Q1 to competitors (questions 65 and 66) and Q3 to PA customers (questions 12 and 14).
61 See case M.7277 - Eli Lilly/Novartis Animal Health, paragraph 41.
62 See cases M.4691 - Schering-Plough/Organon Biosciences, paragraph 340; M.5476 - Pfizer/Wyeth, paragraph 358; and M.7917 - Boehringer Ingelheim/Sanofi Animal Health Business, 200.
63 Questionnaire Q3 to PA customers, question 41.
64 Questionnaire Q1 to competitors, question 99.
65 Questionnaire Q3 to PA customers, question 42.
66 Questionnaire Q1 to competitors, question 100.
67 Questionnaire Q3 to PA customers, question 44.
68 Questionnaire Q1 to competitors, question 101.
69 See cases M.6205 - Eli Lilly/Janssen Pharmaceutica Animal Health Business Assets; and M.885 - Merck/Rhone- Poulenc-Merial.
70 See case M.6205 - Eli Lilly/Janssen Pharmaceutica Animal Health Business Assets, paragraph 19.
71 Questionnaires Q1 to competitors (questions 81 and 82) and Q3 to PA customers (questions 27 and 28).
72 Questionnaires Q1 to competitors (question 80) and Q3 to PA customers (question 26).
73 See cases M.7917 - Boehringer Ingelheim/Sanofi Animal Health Business, paragraph 148; M.7277 - Eli Lilly/Novartis Animal Health, paragraph 58; M.6205 - Eli Lilly/Janssen Pharmaceutica Animal Health Business Assets, paragraph 15; and M.5476 - Pfizer/Wyeth, paragraph 126.
74 See notably case M.8955 - Takeda/Shire, paragraph 56.
75 In particular, Elanco submits that, although the geographic market for marketed animal pharmaceutical is still national in scope, the barriers to entry/expand to other geographies have been significantly reduced and that suppliers in a given EEA country are constrained not just by actual competitors but also by potential entrants already active in other EEA countries.
76 Questionnaire 1 to Competitors, non-confidential replies to questions 5 and 6.
77 Questionnaires Q2 to CA Customers (question 5), Q3 to PA Customers (question 5) and Q1 to Competitors (question 7).
78 See cases M.8889 - Teva/PGT OTC Assets, paragraph 35; M.7746 – Teva/Allergan Generics, paragraph 58; and M.9044 - CVC/Recordati, paragraph 25.
79 See case M.5778 - Novartis/Alcon, paragraph 25.
80 See cases M.8889 - Teva/PGT OTC Assets, paragraph 36; M.7746 – Teva/Allergan Generics, paragraph 58; M.7645 – Mylan/Perrigo, paragraph 30; and M.7379 – Mylan/Abbott EPD-DM, paragraph 36.
81 Based on 2019 market shares provided by the Parties, Group 3 markets arise in the following possible product markets/country pairs: Antimicrobials for CA, oral mode of administration, in Austria; Antimicrobials for PA, oral mode of administration, in Hungary, Ireland and Spain; Antimicrobials for PA, oral-non-premix mode of administration, in Greece and Netherlands; Antimicrobials for cats, oral mode of administration, in Austria; Antimicrobials for dogs, all modes of administration, fluoroquinolones in Spain; Antimicrobials for dogs, oral mode of administration, fluoroquinolones in Spain; Antimicrobials for cats, all modes of administration, fluoroquinolones in Spain; Antimicrobials for cats, oral mode of administration, fluoroquinolones in Spain; Antimicrobials for CA, all modes of administration, fluoroquinolones in Spain; Antimicrobials for CA, oral mode of administration, fluoroquinolones in Spain; Ecto products for cats, all modes of administration, in Austria, Bulgaria, Poland and the UK; Ecto and Endecto products for cats, all modes of administration, in Belgium, Czechia, France, Poland, Romania and the UK; Ecto products for dogs, all modes of administration, in Austria, Bulgaria, France, Portugal and Spain; Ecto and Endecto products for dogs, all modes of administration, in Austria, Belgium, Bulgaria, France, Hungary, Ireland, Netherlands, Portugal, Spain and the UK; Ecto and Endecto products for cattle, all modes of administration, in Germany; Ecto and Endecto products for sheep, all modes of administration, in Germany; Endecto products for cattle, all modes of administration, in Germany; Endecto products for dogs, all modes of administration, in Austria and Poland; Endo products for dogs, all modes of administration, in Finland, Italy and Poland; Endo products for dogs, oral mode of administration, in Finland, Italy and Poland; Endo products for dogs against heartworm, all modes of administration, in Greece, Portugal and Spain; Endo and Endecto products for dogs, all modes of administration, in Finland, France, Greece, Hungary, Italy, Netherlands, Poland, Romania and Spain; Endo products for cats, all modes of administration in Hungary and Poland; Endo products for cats against heartworm, all modes of administration, in France; Endo and Endecto products for cats, all modes of administration, in Belgium, Czechia, France, Greece, Hungary, Ireland and Poland; Endo products for poultry, all modes of administration, in Germany; Endo and Endecto products for poultry, all modes of administration, in Germany; Endo and Endecto products for sheep, all modes of administration in France; Endo products for swine, all modes of administration, in Austria; Otitis daily-use products for CA (all species), all modes of administration in Belgium, Czechia, Greece, Italy, Netherlands, Romania and Sweden; Otitis daily-use products for cats, all modes of administration in Italy and Netherlands; Otitis daily-use products for dogs, all modes of administration in Austria, Belgium, Czechia, Greece, Ireland, Italy, Netherlands and Romania; Ketosis products in Spain; Dermatitis products for dogs and for CA in Germany; Rehydration treatments for cattle in Germany; Sedatives and tranquilizers for CA in the UK; Injectable NSAIDS for cats in Germany; and Corticosteroids for all species in Italy.
82 See cases M.7645 – Mylan/Perrigo, paragraph 30; M.7379 – Mylan/Abbott EPD-DM, paragraph 36; and M.5865 - Teva/Ratiopharm, paragraphs 386-390.
83 Based on 2019 market shares provided by the Parties, Group 2 markets arise in the following possible product markets/country pairs: Otitis daily-use products for CA (all species), all modes of administration, in Austria, Finland, Germany, Ireland and the UK; Otitis daily-use products for dogs, all modes of administration, in Germany and the UK; Ecto/Endecto products for dogs, all modes of administration, in Poland; Ecto products for dogs, all modes of administration, in Poland; Endecto products for dogs, all modes of administration in Germany and the UK; Ecto products for cats, all modes of administration in Romania.
84 Based on 2019 market shares provided by the Parties, Group 2 markets with combined market shares above 50% arise in the following possible product markets/country pairs: Otitis daily-use products for dogs, all modes of administration, in Finland (Combined market shares: [50-60]%, number of competitors above or equal to increment: 6); Otitis daily-use products for cats, all modes of administration, in Belgium (Combined market shares: [50-60]%, number of competitors above increment: 4), Czechia (Combined market shares: [60-70]%, number of competitors above or equal to increment: 7), Denmark (Combined market shares: [50-60]%, number of competitors above or equal to increment: 6), Germany (Combined market shares: [70-80]%, number of competitors above or equal to increment: 6), Ireland (Combined market shares: [70-80]%, number of competitors above or equal to increment: 4), the UK (Combined market shares: [50-60]%, number of competitors above or equal to increment: 7).
85 Endoparasiticides include heartworm products which are assessed separately in Section 4.2.1.4.
86 See Form CO, paragraph 6.266.
![]()
![]()
89 Questionnaires Q1 to competitors (e.g. questions 18.1, 18.2, 21.2, 21.3, 22.6) and Q2 to CA customers (e.g. questions 16.1, 22.6, 23.6).
90 The Commission notes that, in relation to the markets mentioned in Table 3 but not assessed individually in paragraph 105, that is to say (i) Austria, endo/endecto canine and endo/endecto feline, (ii) Belgium, endo/endecto canine, (iii) Czechia, endo/endecto canine, (iv) Denmark, endo feline, (v) Finland, endo feline (oral) and endo/endecto feline, (vi) France, endo canine (all MoAs), endo canine (oral) and endo feline (oral), (vii) Germany, endo/endecto canine, (viii) Greece, endo canine (all MoAs), endo canine (oral) and endo feline (oral), (ix) Hungary, endo canine (all MoAs) and endo canine (oral), (x) the Netherlands, endo canine (all MoAs), endo canine (oral) and endo/endecto feline, (xi) Portugal, endo canine (both all MoAs and oral only), endo feline (both all MoAs and oral only), endo/endecto (both canine and feline), (xii) Romania, endo feline (both all MoAs and oral only), (xiii) Spain, endo canine (all MoAs and oral only) and endo feline (oral), and (xiv) the UK, endo/endecto (both canine and feline) and endo feline (oral), the Parties’ combined market shares are moderate, with the presence of a sufficient number of competitors post-Transaction, and thus do not raise serious doubts. In any event, the Final Commitments aimed at remedying the horizontal non-coordinated effects of the Transaction in other endoparasiticides markets also exclude the possibility that the Transaction would lead to horizontal non-coordinated effects in those markets. Indeed, the Final Commitments will fully remove the full overlap between the Parties’ activities in the markets for CA endoparasiticides at EEA level.
91 Guidelines on the assessment of horizontal mergers under the Council Regulation on the control of concentrations between undertakings (“Horizontal Merger Guidelines”), OJ C 31, 5.2.2004, p. 5, para. 14.
92 Questionnaires Q1 to competitors (question 18) and Q2 to CA customers (question 16).
93 Questionnaires Q1 to competitors (question 19) and Q2 to CA customers (question 17).
94 Some competitors stressed the existence of specific concerns with regards to some of the Parties’ CA endoparasiticides products targeting tapeworms. As indicated above in Section 3.1.2.1, the market investigation did not suggest the existence of a distinct market segment for tapeworms. In any event, any potential competition concern related to tapeworms would be addressed by the divestiture of the Drontal and Profender families of products.
95 Questionnaire Q1 to competitors (question 20.1).
96 Questionnaires Q1 to competitors (question 24) and Q2 to CA customers (question 21).
97 The present Section only assesses the marketed-to-marketed overlaps in CA ectoparasiticides. In CA ectoparasiticides, the Transaction also gives rise to overlaps involving pipeline products (isox) which are assessed in Section 4.2.1.3 below.
98 See Form CO, paragraph 6.213.
102 Questionnaires Q1 to competitors (e.g. questions 18.1, 18.2, 21.2, 21.3, 22.6) and Q2 to CA customers (e.g. questions 16.1, 22.6, 23.6).
103 Questionnaires Q1 to competitors (question 18) and Q2 to CA customers (question 16).
104 Questionnaires Q1 to competitors (question 27, see also question 20) and Q2 to CA customers (question 22, see also question 18).
105 Questionnaire Q2 to CA customers (question 20).
106 Questionnaires Q1 to competitors (question 22) and Q2 to CA customers (question 19).
107 Questionnaires Q1 to competitors (question 17) and Q2 to CA customers (question 15).
108 Questionnaires Q1 to competitors (questions 27, 28 and 131) and Q2 to CA customers (questions 22, 23 and 58).
109 In CA ectoparasiticides, the Transaction also gives rise to overlaps involving pipeline products (isox) which are assessed in Section 4.2.1.3 below.

114 […].
115 […].
116 […].
117 See Annex 74 to the Form CO, and the Parties’ submission on isox theory of harm dated 11 May 2020.
118 The four layers correspond to the overlaps between the parties’ activities in terms of: (1) Actual (product and price) competition (i.e. assessment of the overlaps between the parties' existing (marketed) products), (2) Potential (product and price) competition (i.e. assessment of the overlaps (i) between the parties’ existing (marketed) and pipeline products at advanced stages of development and (ii) between the parties’ pipeline products at advanced stages of development); (3) Innovation competition in relation to the parties’ early stage pipeline projects (i.e. assessment of the risk of significant loss of innovation competition resulting from the discontinuation, delay or redirection of one party’s early stage pipeline products/projects overlapping with the other party’s existing products or advanced or early stage pipeline products/projects; and (4) Innovation competition in relation to the capability and incentives to innovate (i.e. assessment of the risk of a significant loss of innovation competition resulting from a structural reduction of the overall level of innovation).
119 See cases M.7932 - Dow/Dupont, recitals 272-302; M.8084 - Bayer/Monsanto, recitals 48-54; M.9294 – BMS/Celgene, paragraph 22; and M.9461-– AbbVie/Allergan, paragraph 19.
120 In the canine and feline ecto segment, Elanco also has […] pipelines, […].
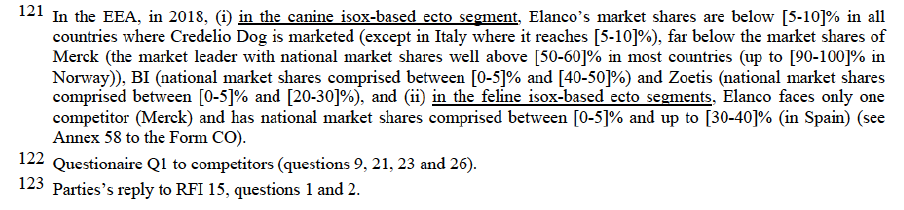
124 Questionaire Q1 to competitors (questions 9, 23 and 26). See also non-confidential minutes of a conference call with a competitor dated 21 January 2020, para. 18.
125 Questionaires Q1 to competitors (question 9) and R1 to competitors (question 36). See also the replies to the RFI sent to KOL on 18 May 2020.
126 […].
127 Parties’ Reply to RFI 1, Attachment A.26, Vetnosis report “Bayer PP July 2019”, (pp.78 and 82) and the Parties’ Reply to RFI 4, question 26.
128 Non-confidential minutes of a conference call with a competitor dated 17 January 2020, p.2.
129 See https://www.who.int/medicines/services/inn/StemBook_2018.pdf (p.116). Fluralaner is the API of Bravecto (Merck); Sarolaner is the API of Simparica (Zoetis); Afoxolaner is the API of NexGard (BI) and Lotilaner is the API of Credelio (Elanco). […].
130 Form CO, Annex 74, para. 1.16.
131 See case M.8401 – J&J/Actelion.
132 See Form CO, para. 8.77 and Questionnaire Q1 to competitors (question 128).
133 […].
134 Parties’ reply to RFI 11, Annex 2.2.5, p.18.
135 […].
136 Non-confidential minutes of a conference call with a competitor dated 21 January 2020, para. 17. Similarly, the Parties submitted that, contrary to isox, “other ecto chemical classes are either only effective against a sub-set of ectoparasites, do not produce systemic effects for one month or greater, or have encountered resistance issues with fleas or ticks […]” and that “for these reasons and also given that isox products are prescription-only (whereas ecto spot-on products are also broadly available in OTV channels where applicable), isox products are increasingly endorsed by vets” (Form CO, Annex 74, para. 1.8).
137 Questionnaires R1 to competitors (question 37) and R2 to CA customers (question 10). See also the replies to the RFI sent to KOL on 18 May 2020.
138 For instance a competitor indicated that isox products are “changing the dynamic” in CA parasiticides (see Questionnaire Q1 to competitors, question 18). See also Non-confidential minutes of a conference call with a competitor dated 17 January 2020, p.2: “The antiparasiticide segment has recently had new entrants leveraging a new innovative isoxazoline molecule. […] This is changing the antiparasiticide landscape […].”
139 Questionnaire R1 to competitor, question 37.
140 […].
141 Questionnaire Q1 to competitor, question 27.
142 Questionnaire Q1 to competitor, question 18.
143 Questionnaire Q1 to competitor, question 29.

147 See Form CO, paras. 6.347 and ff.
148 Questionnaires Q1 to competitors (e.g. questions 18.1, 18.2, 21.2, 21.3, 22.6) and Q2 to CA customers (e.g. questions 16.1, 22.6, 23.6).
149 Questionnaires Q1 to competitors (question 18) and Q2 to CA customers (question 16).
150 Questionnaire Q1 to competitors (question 19).
151 Questionnaires Q1 to competitors (question 27, see also question 20) and Q2 to CA customers (question 22, see also question 18).
152 Questionnaire Q2 to CA customers (question 20).
153 Questionnaires Q1 to competitors (question 22) and Q2 to CA customers (question 19).
154 Questionnaires Q1 to competitors (question 17) and Q2 to CA customers (question 15).
155 Questionnaires Q1 to competitors (questions 27, 28 and 131) and Q2 to CA customers (questions 22, 23 and 58).
156 In Europe, the main third-party data source for the animal health industry is the European Animal Health Study Centre or Centre Européen d’Etudes pour la Santé Animale (“CEESA”). CEESA is a non-profit international association, based in Belgium, which collects sales data on the animal health market worldwide, including data on 22 countries in the EEA. There are 31 reporting members within the EEA. However, most regional or local firms and most generic manufacturers do not report their sales to CEESA. To estimate market shares, the Parties relied on CEESA data in addition to their own sales data and business intelligence.
157 Questionnaire Q1 to competitors (questions 39) and Questionnaire Q2 to CA customers (question 35).
158 Questionnaire Q1 to competitors, question 42.
159 See paragraph 36.
160 Questionnaire Q1 to competitors (questions 35) and Questionnaire Q2 to CA customers (question 30).
161 Questionnaire Q2 to CA customers (question 30).
162 Questionnaire Q1 to competitors (questions 37) and Questionnaire Q2 to CA customers (question 32).
163 Questionnaire Q1 to competitors (questions 44, 45) and Questionnaire Q2 to CA customers (question 37, 38).
164 Questionnaire Q1 to competitors, non-confidential replies to question 48.
165 Questionnaire Q2 to customers, non-confidential replies to question 44.
166 See Section 4.2.2.
167 Questionnaire Q1 to competitors, non-confidential replies to question 49.
168 Questionnaire Q2 to customers, non-confidential replies to question 42.
169 Questionnaire Q1 to competitors, non-confidential replies to question 57 and Questionnaire Q2 to customers, nonconfidential replies to question 48.
170 Questionnaire Q1 to competitors, non-confidential replies to question 58 and Questionnaire Q2 to customers, nonconfidential replies to question 49.
171 Market shares for otitis daily-use products may include sales of general antibiotics with an otitis indication, similar to BAH products.
![]()
![]()
![]()
![]()
176 Also Vetoquinol is active in this segment with a negligible market share of below 1%.
177 See paragraph 36.
178 Questionnaire Q3 to PA customers (question 44).
179 Questionnaire Q3 to PA customers (questions 41, 45).
180 Questionnaire Q1 to competitors (question 104) and Questionnaire Q3 to PA customers (question 47).
181 Questionnaire Q1 to competitors (question 108) and Questionnaire Q3 to PA customers (question 50).
182 Questionnaire Q1 to competitors (question 107) and Questionnaire Q3 to PA customers (question 51).
183 Questionnaire Q1, Questionnaire to competitors (question 110).
184 Questionnaire Q1 to competitors (questions 112, 113) and Questionnaire Q3 to PA customers (questions 53, 54).
185 Indeed, the usage of fluoroquinolones in PA is increasingly rare in Nordic countries in particular for swine (given the detrimental effects on antibiotic resistance for human health purposes) – for instance, in Denmark the usage of fluoroquinolones is prohibited in swine.
186 Only in the UK, Vecoxan is licensed for both prevention and treatment of coccidiosis.

188 Questionnaire Q1 to competitors (question 91) and Questionnaire Q3 to PA customers (question 35).
189 Questionnaire Q3 to PA customers (question 34).
190 Questionnaire Q1 to competitors (question 87) and Questionnaire Q3 to PA customers (question 32).
191 Questionnaire Q1 to competitors (question 90) and Questionnaire Q3 to PA customers (questions 4, 36).
192 Questionnaire Q1 to competitors (question 93).
193 Questionnaire Q1 to competitors (question 96) and Questionnaire Q3 to PA customers (question 39).
194 See paragraph 138 and foonote 119 above.
195 Questionnaires Q1 to Competitors, non-confidential replies to questions 127 and 128.
196 Questionnaires Q1 to Competitors, non-confidential replies to question 124.
197 Questionnaires Q2 to Customers, non-confidential replies to question 55 and Questionnaire Q3 to Customers, non-confidential replies to question 60.
198 Questionnaires Q1 to Competitors, non-confidential replies to question 116.
199 Questionnaires Q1 to Competitors, non-confidential replies to question 117.
200 Questionnaires Q2 to Customers, non-confidential replies to question 55; Questionnaires Q3 to Customers, nonconfidential replies to question 61; and Questionnaires Q1 to Competitors, non-confidential replies to question 129.
201 Commission Notice on remedies acceptable under Council Regulation (EC) No 139/2004 and under Commission Regulation (EC) No 802/2004 (OJ C 267, 22.10.2008, p. 1-27), the "Remedies Notice".
202 Remedies Notice, paras. 9 and 61.
203 Remedies Notice, para. 12.
204 Remedies Notice, para. 10.
205 Remedies Notice, paras. 23-25.
206 Remedies Notice, para. 47.
207 See Annex II, which comprises two parts: part 1 includes the Final Main Commitments ("Annex II.1") and part 2 includes the Final Additional Commitments ("Annex II.2").
208 Including the following products: Drontal (Cat), Drontal Plus (Dog), Drontal Puppy/Welpan (Dog), Droncit Injectable (Cat and Dog) / Spot On (Cat) / Tablet (Cat and Dog); Profender Spot-on (Cat) / Tablet (Dog), Dronspot (Cat), and Procox (Dog).
209 […]. For more details, see Table 7 in Section 4.2.1.3(a) above.
210 […].
211 The Anticcocidials Divestment Business, the Otitis Divestment Business, the Parasiticides Divestment Business, as well as the ESP Divestment Business and the Alternative Isox Divestment Business are together referred as the “Divestment Businesses”. In addition, with regard to the scope of the Parties’ obligation to implement the Alternative Divestment Business, paragraph 2 of the Additional Commitments provides that, should Elanco not enter into a final binding sale and purchase agreement for the sale of the ESP Divestment Business by the end of the first Divestiture Period, it shall sell the Alternative Divestment Business.
212 Paragrapghs 9 and 18 of the Additional Commitments.
213 Market test of the Initial Commitments (R1, R2 and R3).
214 Questionnaires R1 to Competitors (questions 1, 11, 21 and 31); R2 to CA Customers (questions 1, 4 and 7); and R3 to PA Customers (question 1).
215 Questionnaires R1 to Competitors (questions 6, 16 and 26); and R2 to CA Customers (questions 13 and 15); and R3 to PA Customers (question 6).
216 Questionnaire R1 to Competitors (question 2, 12, 22, 32 and 33).
217 Questionnaire R1 to Competitors (questions 3, 13, 23 and 34).
218 Questionnaire R1 to Competitors (questions 35.1 and 35.2).
219 Questionnaires R1 to Competitors (questions 4, 14, 24 and 38); R2 to CA Customers (question 11); and R3 to PA Customers (question 4).
220 Questionnaire R1 to Competitors (questions 7, 17, 27 and 39).
221 Questionnaires R1 to Competitors (questions 10, 20 and 30); R2 to CA Customers (question 3, 6 and 8); and R3 to PA Customers (question 3).
222 Questionnaire R1 to Competitors (question 30).
223 […].
Annex 1
2016 - 2019 market share data base of affected potential segments (in 2016-18 and/or 2019) […]
Case M.9554 – Elanco / Bayer Animal Health
COMMITMENTS TO THE EUROPEAN COMMISSION
Pursuant to Article 6(2) of Council Regulation (EC) No 139/2004 (the “Merger Regulation”), Elanco Animal Health Inc. (“Elanco”) and Bayer AG (together with its affiliates referred to as “Bayer”) (the “Parties”, each a “Party”) hereby enter into the following Commitments (the “Commitments”) vis-à-vis the European Commission (the “Commission”) with a view to rendering Elanco’s acquisition of Bayer’s animal health business (“Bayer Animal Health” or “BAH”) (the “Concentration”) compatible with the internal market and the functioning of the EEA Agreement. The Commitments bind Bayer only in so far as necessary with regard to actions that must be taken by Bayer with regards to the Parasiticides Divestment Business (as defined below).
This text shall be interpreted in light of the Commission’s decision pursuant to Article 6(1)(b) of the Merger Regulation to declare the Concentration compatible with the internal market and the functioning of the EEA Agreement (the “Decision”), in the general framework of European Union law, in particular in light of the Merger Regulation, and by reference to the Commission Notice on remedies acceptable under Council Regulation (EC) No 139/2004 and under Commission Regulation (EC) No 802/2004 (the “Remedies Notice”).
SECTION A. DEFINITIONS
1. For the purpose of the Commitments, the following terms shall have the following meaning:
Affiliated Undertakings: undertakings controlled by the Parties and/or by the ultimate parents of the Parties, whereby the notion of control shall be interpreted pursuant to Article 3 of the Merger Regulation and in light of the Commission Consolidated Jurisdictional Notice under Council Regulation (EC) No 139/2004 on the control of concentrations between undertakings (the "Consolidated Jurisdictional Notice").
Anticoccidials Divestment Business: the Divestment Business described in more detail in Schedule 1.
API: active pharmaceutical ingredient(s).
Assets: the tangible and intangible assets that contribute to the current operation or are necessary to ensure the viability and competitiveness of the Divestment Businesses including but not limited to the assets in Section B, paragraphs 6.1, 6.2, and 6.3 and described in more detail in the Schedules.
Best Efforts: Best effort obligations shall be interpreted in light of the Commission's decision pursuant to Article 6(1)(b) of the Merger Regulation to declare the Concentration compatible with the internal market and the functioning of the EEA Agreement, the Merger Regulation and the general principles of EU law. Any interpretation that may be given to this term under the law of other jurisdictions is not relevant solely for the purpose of interpreting and/or implementing the Commitments.
Closing: the transfer of the legal title to the Divestment Businesses to the Purchaser(s).
Closing Period: For each Divestment Business the period of […] from the approval of the Purchaser and the terms of sale by the Commission for that Divestment Business.
Confidential Information: any business secrets, know-how, commercial information, or any other information of a proprietary nature that is not in the public domain.
Conflict of Interest: any conflict of interest that impairs the Trustee's objectivity and independence in discharging its duties under the Commitments.
Contract: any written contract, agreement, lease, sublease, licence, sub-licence or other legally binding commitment or arrangement.
Divestment Businesses: the businesses as defined in Section B and in the Schedules which Elanco commits to divest, and “Divestment Business” shall mean any one of the Divestment Businesses, as relevant.
Divestiture Trustee: one or more natural or legal person(s) who is/are approved by the Commission and appointed by Elanco and who has/have received from Elanco the exclusive trustee mandate to sell the Divestment Businesses to one or more Purchasers at no minimum price.
Effective Date: the date of adoption of the Decision.
Endectocide Assets: part of the Parasiticides Divestment Business, described in further detail in Part 2 of Schedule 3.
Endoparasiticides Assets: part of the Parasiticides Divestment Business, described in further detail in Part 1 of Schedule 3.
Field: means animal health.
First Divestiture Period: the period of […] from the Effective Date.
Hold Separate Manager(s): the person(s) appointed by Elanco and/or Bayer (as relevant) for the Divestment Businesses to manage the day-to-day business under the supervision of the Monitoring Trustee.
Key Personnel: all personnel necessary to maintain the viability and competitiveness of the Endectocide Assets, as listed in Schedule 3.
Monitoring Trustee: one or more natural or legal person(s) who is/are approved by the Commission and appointed by Elanco, and who has/have the duty to monitor the Parties’ compliance with the conditions and obligations attached to the Decision.
Otitis Divestment Business: the Divestment Business described in more detail in Schedule 2.
Overlapping Retained Business: (a) in the case of Personnel of the Anticoccidials Divestment Business, any business to be retained by Elanco that is related to anticoccidials for lambs and calves; (b) in the case of Personnel of the Otitis Divestment Business, any business to be retained by Elanco that is related to long-lasting canine otitis externa treatments; (c) in the case of Personnel of the Parasiticides Divestment Business, any business to be retained by the Parties that is related to canine and/or feline parasiticides (or in the case of Key Personnel, any business to be retained by the Parties that is related to canine and/or feline isoxazoline or isoxazoline-like parasiticides).
Parasiticides Divestment Business: the Divestment Business described in more detail in Schedule 3, including both the Endoparasiticides Assets and the Endectocide Assets.
Personnel: all staff currently employed by the Divestment Businesses, including staff seconded to the Divestment Business and shared personnel.
Purchaser(s): one or more entities approved by the Commission as the acquirer(s) of the Divestment Businesses in accordance with the criteria set out in Section D. For the avoidance of doubt, (i) the Anticoccidials Divestment Business cannot be split and shall be sold to one entity approved by the Commission; (ii) the Otitis Divestment Business cannot be split and shall be sold to one entity approved by the Commission; and (iii) the Parasiticides Divestment Business cannot be split and shall be sold to one entity approved by the Commission.
Purchaser Criteria: the criteria laid down in paragraph 21 of these Commitments that the Purchaser(s) must fulfil in order to be approved by the Commission.
Schedules: the schedules to these Commitments describing more in detail the Divestment Businesses.
Trustee(s): the Monitoring Trustee and/or the Divestiture Trustee as the case may be.
Trustee Divestiture Period: the period of […] from the end of the First Divestiture Period.
SECTION B. THE COMMITMENT TO DIVEST AND THE DIVESTMENT BUSINESSES
Commitment to divest
2. In order to maintain effective competition, Elanco commits to divest, or procure the divestiture of, each of the Divestment Businesses by the end of the Trustee Divestiture Period as a going concern to a Purchaser or Purchasers and on terms of sale approved by the Commission in accordance with the procedure described in paragraph 22 of these Commitments. To carry out the divestiture, Elanco commits to find a Purchaser or Purchasers and to enter into a final binding sale and purchase agreement or agreements for the sale of the Divestment Businesses by the end of the First Divestiture Period. If Elanco has not entered into such an agreement at the end of the First Divestiture Period in relation to any Divestment Business, Elanco shall grant the Divestiture Trustee an exclusive mandate to sell the relevant Divestment Business in accordance with the procedure described in paragraph 34 in the Trustee Divestiture Period.
3. The Concentration shall not be implemented before Elanco or the Divestiture Trustee has entered into a final binding sale and purchase agreement or agreements for the sale of the Divestment Businesses and the Commission has approved these Purchaser(s) and the terms of sale in accordance with paragraph 21.
4. Elanco shall be deemed to have complied with this commitment if:
(i) By the end of the Trustee Divestiture Period, Elanco or the Divestiture Trustee has entered into a final binding sale and purchase agreement or agreements and the Commission approves the proposed Purchaser(s) and the terms of sale as being consistent with the Commitments in accordance with the procedure described in paragraph 21; and
(ii) The Closing of the sale of the Divestment Businesses to the Purchaser(s) takes place within the Closing Period.
5. In order to maintain the structural effect of the Commitments, Elanco shall, for a period of 10 years after Closing, (i) not acquire, whether directly or indirectly, the possibility of exercising influence (as defined in paragraph 43 of the Remedies Notice, footnote 3) over the whole or part of the Divestment Businesses or (ii) not market generic versions of the divestment products, unless, following the submission of a reasoned request from Elanco showing good cause and accompanied by a report from the Monitoring Trustee (as provided in paragraph 48 of these Commitments), the Commission finds that the structure of the market has changed to such an extent that the absence of influence over the Divestment Businesses is no longer necessary to render the proposed Concentration compatible with the internal market.
Structure and definition of the Divestment Businesses
6. The Divestment Businesses comprise:
6.1 The Anticoccidials Divestment Business, as described in more detail in Schedule 1, which includes all assets that contribute to the current operation or are necessary to ensure the viability and competitiveness of the Anticoccidials Divestment Business globally, inter alia:
(i) All contracts related exclusively or primarily to the Anticoccidials Divestment Business and, according to the Purchaser’s needs, the rights and benefits of the relevant portion of contracts that are material and/or necessary to, but not exclusively or primarily related to, the Anticoccidials Divestment Business to the extent they are capable of being assigned. Elanco undertakes to use Best Efforts to obtain all necessary third-party consents where applicable;
(ii) All Assets;
(iii) All licences, product registrations and authorisations issued by governmental organisations or other bodies for the benefit of the Anticoccidials Divestment Business;
(iv) All intellectual property and intellectual property rights (including productspecific know-how), which are owned, maintained, used and/or controlled by Elanco (as applicable), and related to the development, manufacturing and exploitation of the divestment products in the Field or for the maintenance of the product registrations being transferred;
(v) All customer orders and customer records of the Anticoccidials Divestment Business; and
(vi) The benefit of transitional arrangements to ensure the viability and competitiveness of the Anticoccidials Divestment Business for a transitional period following Closing.
6.2 The Otitis Divestment Business, as described in more detail in Schedule 2, which includes all assets that contribute to the current operation or are necessary to ensure the viability and competitiveness of the Otitis Divestment Business globally, inter alia:
(i) All contracts related exclusively or primarily to the Otitis Divestment Business and, according to the Purchaser’s needs, the rights and benefits of the relevant portion of contracts that are material and/or necessary to, but not exclusively or primarily related to, the Otitis Divestment Business to the extent they are capable of being assigned Elanco undertakes to use Best Efforts to obtain all necessary third-party consents where applicable;
(ii) All Assets;
(iii) All licences, product registrations and authorisations issued by governmental organisations or other bodies for the benefit of the Otitis Divestment Business;
(iv) All intellectual property and intellectual property rights (including productspecific know-how), which are owned, maintained, used and/or controlled by Elanco (as applicable), and related to the development, manufacturing and exploitation of the divestment products in the Field or for the maintenance of the product registrations being transferred;
(v) All customer orders and customer records of the Otitis Divestment Business; and
(vi) The benefit of transitional arrangements to ensure the viability and competitiveness of the Otitis Divestment Business for a transitional period following Closing.
6.3 The Parasiticides Divestment Business, as described in more detail in Schedule 3, which includes all assets that contribute to the current operation or are necessary to ensure the viability and competitiveness of the Parasiticides Divestment Business including the Endoparasiticides Assets in the EEA/UK and the Endectocide Assets globally, for which purposes:
(i) The Endoparasiticides Assets include inter alia:
(a) All contracts related exclusively or primarily to the Endoparasiticides Assets and, according to the purchaser’s needs, the rights and benefits of the relevant portion of contracts that are material and/or necessary to, but not exclusively or primarily related to, the Endoparasiticides Assets to the extent they are capable of being assigned Elanco/BAH undertake to use Best Efforts to obtain all necessary third-party consents where applicable;
(b) All Assets;
(c) All licences, product registrations and authorisations issued by governmental organisations or other bodies for the benefit of the Endoparasiticides Assets;
(d) All intellectual property and intellectual property rights (including product-specific know-how), which are owned, maintained and/or control by the Parties (as applicable), and related to the development, manufacturing and exploitation of the divestment products in the Field in the EEA/UK or for the maintenance of the product registrations being transferred;
(e) All customer orders and customer records of the Endoparasiticides Assets; and
(f) The benefit of all transitional arrangements (including a transitional manufacture and supply agreement, pending completion of the technology transfer) that are necessary to ensure the viability and competitiveness of the Endoparasiticides Assets for a transitional period following Closing.
(ii) The Endectocide Assets include inter alia:
(a) All rights, title and interests to develop, improve, manufacture and commercialise (including the right to conduct the ongoing clinical trials for) the products concerned for use in the Field;
(b) All contracts related exclusively or primarily to the Endectocide Assets and, according to the Purchaser’s needs, the rights and benefits of the relevant portion of contracts that are material and/or necessary to, but not exclusively or primarily related to, the Parasiticide Divestment Business to the extent they are capable of being assigned. Elanco undertakes to use Best Efforts to obtain all necessary third party consents where applicable;
(c) All Assets;
(d) Key Personnel;
(e) All product licences, permits, authorisations and registrations (“regulatory approvals”) for the products concerned as, and all other product registrations or regulatory approvals to the extent exclusively or primarily relating to the products concerned for use in the Field;
(f) All intellectual property and intellectual property rights (including product-specific know-how) which are owned, maintained, used and /or controlled by Bayer (as applicable) and related to the development, improvement, manufacturing and commercialisation of the products concerned for use in the Field or for the maintenance of the regulatory approvals being transferred;
(g) All customer orders and customer records of the Endectocide Assets; and
(h) The benefit of transitional arrangements, including an R&D and regulatory transitional service agreement, transitional manufacturing and supply services, and a technology transfer (including know-how) to the Purchaser(s), to ensure the viability and competitiveness of the Endectocide Assets for a transitional period of […] following Closing […], to ensure the smooth transfer of the Endectocide Assets.
7. All the Assets, which are necessary to, but not exclusively or primarily related to, the viability and competitiveness of the Divestment Business(es) and also relate to products/businesses retained by the Parties, shall also be transferred to the Purchaser(s) but only to the extent they relate to the Divestment Business(es) (and at the Purchaser’s option). In the event that materials to be transferred to the Purchaser(s) pursuant to the Commitments contain information that is confidential to the Elanco/BAH’s retained businesses and not relevant to the Divestment Business(es), the information shall be redacted as appropriate.
8. For the sake of clarity, with the exception of the Otitis Divestment Business, the Divestment Businesses shall not include any physical production assets, equipment or manufacturing units owned or operated by Elanco or the Parties (as applicable). Further, the Divestment Businesses shall not include any Personnel (save for Key Personnel and as provided for at paragraph 9 below).
9. At the option of the Purchaser(s), the Divestment Business(es) will include the Personnel who would reasonably be considered necessary to maintain the viability, marketability and competitiveness of the Divestment Business(es). The exercise of such option shall be supervised by the Monitoring Trustee and subject to applicable local employment legislation and employee consent. Should Elanco not agree with the request of the Purchaser, the Monitoring Trustee shall prepare a reasoned opinion within 7 working days and the Commission shall ultimately decide on the merits of the Purchaser proposal.
10. The sale of each Divestment Business shall be structured as an asset sale with licences and rights of access to certain shared assets. The manufacturing transfer for each Divestment Business will be effected as follows.
(i) As regards the Anticoccidials Divestment Business and the Otitis Divestment Business, a transfer of the relevant third-party supply contracts (or transfer of the rights and benefits of the relevant portion thereof where the supply contract is not exclusively or primarily used for that Divestment Business) related to the manufacture of the relevant product(s).
(ii) As regards the Parasiticides Divestment Business:
(a) For the Endoparasiticides Assets, a technology transfer (including know-how) relating to the production of the Drontal/Profender Family, together with transitional arrangements for the manufacture and supply of finished products to the Purchaser for a period of […] following Closing ([…]), pending completion of the technology transfer process, as overseen by the Monitoring Trustee.
(b) For the Endectocide Assets, a technology transfer (including knowhow), transitional manufacturing and supply services and an R&D and regulatory transitional service agreement for a period of […] following Closing ([…]).
11. Strict firewall procedures will be adopted so as to ensure that any competitively sensitive information related to, or arising from, such supply arrangements (for example, product roadmaps) will not be shared with, or passed on to, anyone outside the Divestment Businesses’ operations, beyond what is reasonably required for the compliance with obligations relating to the transfer of the Divestment Businesses and supply of transitional services.
SECTION C. RELATED COMMITMENTS
Preservation of viability, marketability and competitiveness
12. From the Effective Date until Closing, the Parties shall preserve or procure the preservation of the economic viability, marketability and competitiveness of the Divestment Businesses, in accordance with good business practice, and shall minimise, as far as possible, any risk of loss of competitive potential of the Divestment Businesses. In particular, the Parties undertake:
(i) Not to carry out any action that might have a significant adverse impact on the value, management or competitiveness of the Divestment Businesses or that might alter the nature and scope of activity, the industrial or commercial strategy, and/or the investment policy of the Divestment Businesses;
(ii) To make available, or procure to make available, sufficient resources for the development of the Divestment Businesses, on the basis and continuation of the existing business plans; and
13. Furthermore, Elanco undertakes to take all reasonable steps, or procure that all reasonable steps are being taken, including appropriate incentive schemes (based on industry practice), to encourage all Key Personnel to remain with the Parasiticides Divestment Business, and not to solicit or move any Personnel to Elanco’s remaining business. Where, nevertheless, individual members of the Key Personnel exceptionally leave the Parasiticides Divestment Business, Elanco shall provide a reasoned proposal to replace the person or persons concerned to the Commission and the Monitoring Trustee. Elanco must be able to demonstrate to the Commission that the replacement is well suited to carry out the functions exercised by those individual members of the Key Personnel. The replacement shall take place under the supervision of the Monitoring Trustee, who shall report to the Commission.
Hold-separate obligations
14. The Parties commit, from the Effective Date until Closing, to procure that the Divestment Businesses are kept separate from the business(es) that the Parties will be retaining and, after closing of the Concentration to keep the Divestment Businesses separate from the businesses they are retaining and to ensure that unless explicitly permitted under these Commitments:
(i) Management and staff of the businesses retained by the Parties have no involvement in the Divestment Businesses; and
(ii) Key Personnel and Personnel can be involved in businesses retained by the Parties only to the extent that (a) they are bound by the terms of non-disclosure agreements or similar arrangements preventing the disclosure of any information related to the Divestment Businesses; (b) they are not involved in a relevant Overlapping Retained Business; and (c) their involvement in other businesses retained by the Parties is compatible with their required involvement in the Divestment Businesses.
15. Until Closing, the Parties shall assist the Monitoring Trustee in ensuring that the Divestment Businesses are managed as distinct and saleable entities separate from the businesses which they are retaining. Immediately after the adoption of the Decision, the Parties shall appoint a Hold Separate Manager or Managers for the Divestment Businesses. The Hold Separate Manager(s) shall manage the Divestment Businesses independently and in the best interests of the relevant Divestment Business with a view to ensuring its continued economic viability, marketability and competitiveness and its independence from the businesses retained by the Parties. The Hold Separate Manager(s) shall closely cooperate with and report to the Monitoring Trustee and, if applicable, the Divestiture Trustee. In case of replacement of a Hold Separate Manager, the Parties shall provide a reasoned proposal to replace the person or person concerned to the Commission and the Monitoring Trustee. The Parties must be able to demonstrate to the Commission that the replacement is well suited to carry out the functions exercised by that individual. The replacement shall take place under the supervision of the Monitoring Trustee, who shall report to the Commission. The Commission may, after having heard the Parties, require the Parties to replace a Hold Separate Manager.
Ring-fencing
16. The Parties shall implement, or procure to implement, all necessary measures to ensure that they do not, after the Effective Date, obtain any Confidential Information relating to the Divestment Businesses and that any such Confidential Information obtained by the Parties before the Effective Date will be eliminated and not be used by them. In particular, the participation of the Divestment Businesses in any central information technology network shall be severed to the extent possible, without compromising the viability of the Divestment Businesses. The Parties may obtain or keep information relating to the Divestment Businesses which is reasonably necessary for the divestiture of the Divestment Businesses or the disclosure of which to the Parties is required by law.
Non-solicitation clause
17. The Parties undertake, subject to customary limitations, not to solicit, and to procure that Affiliated Undertakings do not solicit, any Key Personnel or Personnel transferred with the Divestment Businesses for a period of two years after Closing. Due diligence
18. In order to enable potential purchasers to carry out reasonable due diligence of the Divestment Businesses, the Parties shall, subject to customary confidentiality assurances and dependent on the stage of the divestiture process:
(i) Provide to potential purchasers sufficient information as regards the Divestment Businesses;
(ii) Provide to potential purchasers sufficient information relating to the Personnel and allow them reasonable access to the Personnel to the extent required under paragraph 9 above.
Reporting
19. Elanco shall submit written reports in English on potential purchasers of the Divestment Businesses and developments in the negotiations with such potential purchasers to the Commission and the Monitoring Trustee no later than 10 days after the end of every month following the Effective Date (or otherwise at the Commission’s request). Elanco shall submit a list of all potential purchasers having expressed interest in acquiring the Divestment Businesses to the Commission at each and every stage of the divestiture process, as well as a copy of all the offers made by potential purchasers within five days of their receipt.
20. Elanco shall inform the Commission and the Monitoring Trustee on the preparation of the data room documentation and the due diligence procedure and shall submit a copy of any information memorandum to the Commission and the Monitoring Trustee before sending such memorandum out to potential purchasers.
SECTION D. THE PURCHASER
21. In order to be approved by the Commission, the Purchaser(s) must fulfil the following criteria:
(i) The Purchaser(s) shall be independent of and unconnected to Elanco and BAH and their Affiliated Undertakings (this being assessed having regard to the situation following the divestiture);
(ii) The Purchaser(s) shall have the financial resources, proven expertise and incentive to maintain and develop the Divestment Businesses as a viable and active competitive force in competition with the Parties and other competitors;
(iii) The acquisition of the Divestment Businesses by the Purchaser(s) must neither be likely to create, in light of the information available to the Commission, prima facie competition concerns nor give rise to a risk that the implementation of the Commitments will be delayed. In particular, the Purchaser(s) must reasonably be expected to obtain all necessary approvals from the relevant regulatory authorities for the acquisition of the Divestment Businesses; and
(iv) The Purchaser(s) shall have:
(a) Established capabilities or a track record in the manufacture, marketing and distribution of animal health products in the EEA/UK;
(b) Adequate manufacturing and regulatory capabilities to successfully implement a production transfer in relation to the Divestment Businesses (where relevant);
(c) Sufficient R&D resources and experience to develop the relevant pipeline products included in the scope of the Divestment Business(es); and
(d) Complementary products and expertise relevant to the Divestment Businesses.
22. The final binding sale and purchase agreement or agreements (as well as ancillary agreements) relating to the divestment of the Divestment Businesses shall be conditional on the Commission’s approval. When Elanco has reached an agreement with a purchaser, it shall submit a fully documented and reasoned proposal, including a copy of the final agreement(s), within one week to the Commission and the Monitoring Trustee. Elanco must be able to demonstrate to the Commission that the purchaser(s) fulfil the Purchaser Criteria and that the Divestment Businesses is being sold in a manner consistent with the Commission's Decision and these Commitments. For the approval, the Commission shall verify that the purchaser fulfils the Purchaser Criteria and that the Divestment Businesses are being sold in a manner consistent with the Commitments including their objective to bring about a lasting structural change in the market. The Commission may approve the sale of the Divestment Businesses without one or more Assets or any or part of any Personnel, or by substituting one or more Assets or parts of any Personnel with one or more different assets or different personnel or support, if this does not affect the viability and competitiveness of the Divestment Businesses after the sale, taking account of the proposed purchaser(s).
SECTION E. TRUSTEE
I. Appointment procedure
23. Elanco shall appoint a Monitoring Trustee to carry out the functions specified in these Commitments for a Monitoring Trustee. Elanco commits not to close the Concentration before the appointment of a Monitoring Trustee.
24. If Elanco has not entered into a binding sale and purchase agreement or agreements regarding the Divestment Businesses one month before the end of the First Divestiture Period, or if the Commission has rejected a purchaser proposed by Elanco at that time or thereafter, Elanco shall appoint a Divestiture Trustee. The appointment of the Divestiture Trustee shall take effect upon the commencement of the Trustee Divestiture Period.
25. The Trustee shall:
(i) At the time of appointment, be independent of Elanco and its Affiliated Undertakings;
(ii) Possess the necessary qualifications to carry out its mandate, for example having sufficient relevant experience as an investment banker or consultant or auditor; and
(iii) Neither have nor become exposed to a Conflict of Interest.
26. The Trustee shall be remunerated by Elanco in a way that does not impede the independent and effective fulfilment of its mandate. In particular, where the remuneration package of a Divestiture Trustee includes a success premium linked to the final sale value of the Divestment Businesses, such success premium may only be earned if the divestiture takes place within the Trustee Divestiture Period.
Proposal by Elanco
27. No later than two weeks after the Effective Date, Elanco shall submit the name or names of one or more natural or legal persons whom Elanco proposes to appoint as the Monitoring Trustee to the Commission for approval. No later than one month before the end of the First Divestiture Period or on request by the Commission, Elanco shall submit a list of one or more persons whom Elanco proposes to appoint as Divestiture Trustee to the Commission for approval. The proposal shall contain sufficient information for the Commission to verify that the person or persons proposed as Trustee fulfil the requirements set out in paragraph 25 and shall include:
(i) The full terms of the proposed mandate, which shall include, for the avoidance of doubt, all provisions necessary to enable the Trustee to fulfil its duties under these Commitments;
(ii) The outline of a work plan which describes how the Trustee intends to carry out its assigned tasks; and
(iii) An indication whether the proposed Trustee is to act as both Monitoring Trustee and Divestiture Trustee or whether different trustees are proposed for the two functions.
Approval or rejection by the Commission
28. The Commission shall have the discretion to approve or reject the proposed Trustee(s) and to approve the proposed mandate subject to any modifications it deems necessary for the Trustee to fulfil its obligations. If only one name is approved, Elanco shall appoint or cause to be appointed the person or persons concerned as Trustee, in accordance with the mandate approved by the Commission. If more than one name is approved, Elanco shall be free to choose the Trustee to be appointed from among the names approved. The Trustee shall be appointed within one week of the Commission’s approval, in accordance with the mandate approved by the Commission.
New proposal by Elanco
29. If all the proposed Trustees are rejected, Elanco shall submit the names of at least two more natural or legal persons within one week of being informed of the rejection, in accordance with paragraphs 23 and 28 of these Commitments.
Trustee nominated by the Commission
30. If all further proposed Trustees are rejected by the Commission, the Commission shall nominate a Trustee, whom Elanco shall appoint, or cause to be appointed, in accordance with a trustee mandate approved by the Commission.
II. Functions of the Trustee
31. The Trustee shall assume its specified duties and obligations in order to ensure compliance with the Commitments. The Commission may, on its own initiative or at the request of the Trustee or Elanco, give any orders or instructions to the Trustee in order to ensure compliance with the conditions and obligations attached to the Decision.
Duties and obligations of the Monitoring Trustee
32. The Monitoring Trustee shall:
(i) Propose in its first report to the Commission a detailed work plan describing how it intends to monitor compliance with the obligations and conditions attached to the Decision;
(ii) Oversee, in close co-operation with the Hold Separate Manager(s), the ongoing management of the Divestment Businesses with a view to ensuring their continued economic viability, marketability and competitiveness and monitor compliance by the Parties with the conditions and obligations attached to the Decision. To that end the Monitoring Trustee shall:
(a) Monitor the preservation of the economic viability, marketability and competitiveness of the Divestment Businesses, and the keeping separate of the Divestment Businesses from the business retained by the Parties, in accordance with paragraphs 12 and 14 of these Commitments;
(b) Supervise the management of the Divestment Businesses as distinct and saleable entities, in accordance with paragraph 15 of these Commitments;
(c) With respect to Confidential Information:
(I) Determine all necessary measures to ensure that the Parties do not after the Effective Date obtain any Confidential Information relating to the Divestment Businesses,
(II) In particular, strive for the severing of the Divestment Businesses’ participation in a central information technology network to the extent possible, without compromising the viability of the Divestment Businesses,
(III) Make sure that any Confidential Information relating to the Divestment Businesses obtained by the Parties before the Effective Date is eliminated and will not be used by the Parties, and
(IV) Decide whether such information may be disclosed to or kept by the Parties as the disclosure is reasonably necessary to allow the Parties to carry out the divestiture or as the disclosure is required by law;
(d) Monitor the splitting of assets and the allocation of Personnel (to the extent required under paragraph 9) between the Divestment Businesses and the Parties or Affiliated Undertakings;
(iii) Propose to the Parties such measures as the Monitoring Trustee considers necessary to ensure the Parties’ compliance with the conditions and obligations attached to the Decision, in particular the maintenance of the full economic viability, marketability or competitiveness of the Divestment Businesses, the holding separate of the Divestment Businesses and the non-disclosure of competitively sensitive information;
(iv) Review and assess potential purchasers as well as the progress of the divestiture process and verify that, dependent on the stage of the divestiture process:
(a) Potential purchasers receive sufficient and correct information relating to the Divestment Businesses and Personnel (to the extent required under paragraph 9) in particular by reviewing, if available, the data room documentation, the information memorandum and the due diligence process, and
(b) Potential purchasers are granted reasonable access to Personnel (to the extent required under paragraph 9);
(v) Act as a contact point for any requests by third parties, in particular potential purchasers, in relation to the Commitments;
(vi) Provide to the Commission, sending Elanco a non-confidential copy at the same time, a written report within 15 days after the end of every month that shall cover the operation and management of the Divestment Businesses as well as the splitting of assets and the allocation of Personnel (to the extent required under paragraph 9) so that the Commission can assess whether the business is held in a manner consistent with the Commitments and the progress of the divestiture process as well as potential purchasers;
(vii) Promptly report in writing to the Commission, sending Elanco a non-confidential copy at the same time, if it concludes on reasonable grounds that the Parties are failing to comply with these Commitments;
(viii) Within one week after receipt of the documented proposal referred to in paragraph 22 of these Commitments, submit to the Commission, sending Elanco a non-confidential copy at the same time, a reasoned opinion as to the suitability and independence of the proposed purchaser(s) and the viability of the Divestment Businesses after the sale and as to whether the Divestment Businesses are sold in a manner consistent with the conditions and obligations attached to the Decision, in particular, if relevant, whether the sale of the Divestment Businesses without one or more Assets or none, or not all, of the Personnel (to the extent required under paragraph 9) affects the viability of the Divestment Businesses after the sale, taking account of the proposed purchaser(s); and
(ix) Assume the other functions assigned to the Monitoring Trustee under the conditions and obligations attached to the Decision.
33. If the Monitoring and Divestiture Trustee are not the same legal or natural persons, the Monitoring Trustee and the Divestiture Trustee shall cooperate closely with each other during and for the purpose of the preparation of the Trustee Divestiture Period in order to facilitate each other's tasks.
Duties and obligations of the Divestiture Trustee
34. Within the Trustee Divestiture Period, the Divestiture Trustee shall sell at no minimum price the Divestment Businesses to a purchaser or purchasers, provided that the Commission has approved both the purchaser(s) and the final binding sale and purchase agreement or agreements (and ancillary agreements) as in line with the Commission's Decision and the Commitments in accordance with paragraphs 21 and 22 of these Commitments. The Divestiture Trustee shall include in the sale and purchase agreement (as well as in any ancillary agreements) such terms and conditions as it considers appropriate for an expedient sale in the Trustee Divestiture Period. In particular, the Divestiture Trustee may include in the sale and purchase agreement or agreements such customary representations and warranties and indemnities as are reasonably required to effect the sale. The Divestiture Trustee shall protect the legitimate financial interests of Elanco, subject to Elanco’s unconditional obligation to divest at no minimum price in the Trustee Divestiture Period.
35. In the Trustee Divestiture Period (or otherwise at the Commission’s request), the Divestiture Trustee shall provide the Commission with a comprehensive monthly report written in English on the progress of the divestiture process. Such reports shall be submitted within 15 days after the end of every month with a simultaneous copy to be sent to the Monitoring Trustee and a non-confidential copy to be sent to Elanco.
III. Duties and obligations of the Parties
36. The Parties shall provide and shall cause its advisors to provide the Trustee with all such co-operation, assistance and information as the Trustee may reasonably require to perform its tasks. The Trustee shall have full and complete access to any of the Parties’ or the Divestment Businesses’ books, records, documents, management or other personnel, facilities, sites and technical information necessary for fulfilling its duties under the Commitments and the Parties and the Divestment Businesses shall provide the Trustee upon request with copies of any document. The Parties and the Divestment Businesses shall make available to the Trustee one or more offices on their premises and shall be available for meetings in order to provide the Trustee with all information necessary for the performance of its tasks.
37. The Parties shall provide the Monitoring Trustee with all managerial and administrative support that it may reasonably request on behalf of the management of the Divestment Businesses. This shall include all administrative support functions relating to the Divestment Businesses which are currently carried out at headquarters level. Elanco shall provide and shall cause its advisors to provide the Monitoring Trustee, on request, with the information submitted to potential purchasers, and in particular give the Monitoring Trustee access to the data room documentation and all other information granted to potential purchasers in the due diligence procedure. Elanco shall inform the Monitoring Trustee on possible purchasers, submit lists of potential purchasers at each stage of the selection process, including the offers made by potential purchasers at those stages, and keep the Monitoring Trustee informed of all developments in the divestiture process.
38. The Parties shall grant or procure Affiliated Undertakings to grant comprehensive powers of attorney, duly executed, to the Divestiture Trustee to effect the sale (including ancillary agreements), the Closing and all actions and declarations which the Divestiture Trustee considers necessary or appropriate to achieve the sale and the Closing, including the appointment of advisors to assist with the sale process. Upon request of the Divestiture Trustee, the Parties shall cause the documents required for effecting the sale and the Closing to be duly executed.
39. Elanco shall indemnify the Trustee and its employees and agents (each an “Indemnified Party”) and hold each Indemnified Party harmless against, and hereby agrees that an Indemnified Party shall have no liability to Elanco for, any liabilities arising out of the performance of the Trustee’s duties under the Commitments, except to the extent that such liabilities result from the wilful default, recklessness, gross negligence or bad faith of an Indemnified Party.
40. At the expense of Elanco, the Trustee may appoint advisors (in particular for corporate finance or legal advice), subject to Elanco’s approval (such approval not to be unreasonably withheld or delayed) if the Trustee considers the appointment of such advisors necessary or appropriate for the performance of its duties and obligations under the mandate, provided that any fees and other expenses incurred by the Trustee are reasonable. Should Elanco refuse to approve the advisors proposed by the Trustee the Commission may approve the appointment of such advisors instead, after having heard Elanco. Only the Trustee shall be entitled to issue instructions to the advisors. Paragraph 39 of these Commitments shall apply mutatis mutandis. In the Trustee Divestiture Period, the Divestiture Trustee may use advisors who served Elanco during the Divestiture Period if the Divestiture Trustee considers this in the best interest of an expedient sale.
41. The Parties agree that the Commission may share Confidential Information proprietary to the Parties with the Trustee. The Trustee shall not disclose such information and the principles contained in Article 17 (1) and (2) of the Merger Regulation apply mutatis mutandis.
42. Elanco agrees that the contact details of the Monitoring Trustee are published on the website of the Commission's Directorate-General for Competition and they shall inform interested third parties, in particular any potential purchasers, of the identity and the tasks of the Monitoring Trustee.
43. For a period of 10 years from the Effective Date the Commission may request all information from the Parties that is reasonably necessary to monitor the effective implementation of these Commitments.
IV. Replacement, discharge and reappointment of the Trustee
44. If the Trustee ceases to perform its functions under the Commitments or for any other good cause, including the exposure of the Trustee to a Conflict of Interest:
(i) The Commission may, after hearing the Trustee and Elanco, require Elanco to replace the Trustee; or
(ii) Elanco may, with the prior approval of the Commission, replace the Trustee.
45. If the Trustee is removed according to paragraph 44 of these Commitments, the Trustee may be required to continue in its function until a new Trustee is in place to whom the Trustee has effected a full hand over of all relevant information. The new Trustee shall be appointed in accordance with the procedure referred to in paragraphs 23 to 30 of these Commitments.
46. Unless removed according to paragraph 44 of these Commitments, the Trustee shall cease to act as Trustee only after the Commission has discharged it from its duties after all the Commitments with which the Trustee has been entrusted have been implemented. However, the Commission may at any time require the reappointment of the Monitoring Trustee if it subsequently appears that the relevant remedies might not have been fully and properly implemented.
SECTION F. THE REVIEW CLAUSE
47. The Commission may extend the time periods foreseen in the Commitments in response to a request from Elanco or, in appropriate cases, on its own initiative. Where Elanco requests an extension of a time period, it shall submit a reasoned request to the Commission no later than one month before the expiry of that period, showing good cause. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time, send a non-confidential copy of the report to Elanco. Only in exceptional circumstances shall Elanco be entitled to request an extension within the last month of any period.
48. The Commission may further, in response to a reasoned request from Elanco showing good cause, waive, modify or substitute, in exceptional circumstances, one or more of the undertakings in these Commitments. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time, send a non-confidential copy of the report to Elanco. The request shall not have the effect of suspending the application of the undertaking and, in particular, of suspending the expiry of any time period in which the undertaking has to be complied with.
SECTION G. ENTRY INTO FORCE
49. The Commitments shall take effect upon the date of adoption of the Decision.
(Signed)
duly authorised for and on behalf of Elanco Animal Health Inc.
duly authorised for and on behalf of Bayer AG
SCHEDULE 1
1. The Anticoccidials Divestment Business consists of the worldwide rights, title and interests in (including the right to develop, improve, manufacture and commercialise worldwide) the products marketed under the brand Vecoxan (“Vecoxan”) and a private label version of Vecoxan currently sold in the UK, Cocci-Drench.1
2. The Anticoccidials Divestment Business as operated to date is not a stand-alone business, rather it is integrated into a wider operational and commercial organisation; it will therefore be separated from Elanco’s current operations as described below at paragraph 3 of this Schedule. As regards its legal and functional structure:
(i) The Anticoccidials Divestment Business forms part of Elanco’s production animal Ruminants & Swine business segment, which also encompasses vaccines, antibiotics, implants and other parasiticides targeting ruminants and swine species. The Anticoccidials Divestment Business covers the entirety of Elanco’s anticoccidials product line for cattle and sheep. Please refer to the structure chart provided as Annex 1 for further detail.
(ii) […]. Specifically, the EEA/UK supply chain is as follows:
(a) The API, diclazuril microfine, is manufactured by […]. The API is stored […]. The API is then shipped to […].
(b) […].
(iii) The sales, technical and marketing Personnel for the Anticoccidials Divestment Business are located in […]. (iv) In the EEA/UK, Vecoxan is distributed by […].
3. In accordance with Section B, paragraph 6.1 of these Commitments, subject to third party consent where relevant and to the extent in Elanco’s ownership, control or possession at Closing, the Anticoccidials Divestment Business includes, but is not limited to, the following on a worldwide basis:
(i) All Contracts exclusively or primarily related to Vecoxan, and the rights and benefits of the portion of Contracts which are material or necessary to, but not exclusively or primarily used in, the Anticoccidials Divestment Business or the manufacture of Vecoxan (being ‘shared contracts’), including, but not limited to, the contracts defined and listed in Annex 2. Elanco undertakes to use Best Efforts to obtain all necessary third-party consents where applicable. To the extent any such third-party consent could not be obtained, or any contract could not be otherwise transferred, the Elanco will, as appropriate, either: (i) assist the Purchaser and the relevant third party to put in place arrangements to transfer any work product and work in progress, and support the Purchaser to put in place alternative arrangements; or (ii) enter into back-to-back agreements with the Purchaser under the same terms and conditions as the relevant contract for a transitional period of […] and up to the duration of the existing relevant contract (if longer than […]).
(ii) All inventory related to Anticoccidials Divestment Business (to the extent that it has not been sold to a third party), as well as all rights to market and sell such inventory including but not limited to:
(a) Vecoxan in finished packaged form (together with any product packaging materials) labelled and held for sale for the treatment or prevention of coccidial infections in lambs, calves and other ruminants; and
(b) Bulk API used or held for use exclusively or primarily in the manufacture of Vecoxan for sale by the Anticoccidials Divestment Business.
(iii) Product registrations for Vecoxan listed at Annex 3, and all other product registrations to the extent exclusively or primarily relating to Vecoxan for use in the treatment or prevention of coccidial infections in lambs, calves and other ruminants.
(iv) All documentation comprising the product registrations in Annex 3 and, to the extent exclusively or primarily related to Vecoxan for use in the treatment or prevention of coccidial infections in lambs, calves and other ruminants:
(a) Correspondence and reports submitted to or received from governmental authorities;
(b) Other dossiers or compilations necessary to obtain or maintain any of the product registrations in Annex 3;
(c) Literature safety reports and documents relating to good manufacturing practices or issues, animal clinical trials, animal research, including laboratory and target animal research and all veterinary master files contained or referenced to in the product registrations in Annex 3;
(d) Environmental and safety documentation; and
(e) Data referenced in any documentation and materials referred to above or necessary to commercialise and develop Vecoxan or any pipeline transferred as part of the divestment business, but in all cases except where explicitly excluded, and excluding all intellectual property rights of any third party unless they are necessary for the functioning of the Anticoccidials Divestment Business (in which instance, Elanco shall use Best Efforts to work with the Purchaser to reach an agreement with the entity that is able to grant a licence).
(v) All books and records, including customer records, relating exclusively or primarily to the Anticoccidials Divestment Business in such form as maintained by Elanco (except where explicitly excluded).
(vi) All existing advertising, promotional and media materials, sales training materials, customer lists, other marketing data and materials and trade show materials and videos in such form as maintained by Elanco to the extent they are exclusively or primarily used or held for exclusive or primary use in the Anticoccidials Divestment Business (except where explicitly excluded).
(vii) All intellectual property rights exclusively or primarily related to the Anticoccidials Divestment Business, including, but not limited to, the intellectual property rights listed at Annex 4, which, broadly, comprise:
(a) Product-specific know-how and registered or unregistered intellectual property that is identified or identifiable in tangible form that is or has been used for or held for use by or on behalf of Elanco exclusively or primarily for or exclusively in connection with the manufacturing of Vecoxan for the treatment or prevention of coccidial infections in lambs, calves and other ruminants, or the maintenance of any product registration being transferred;
(b) The trademark Vecoxan, as registered in certain territories; for the avoidance of doubts, the Purchaser will have all rights to market and sell the inventory transferred as part of the Anticoccidials Divestment Business;
(c) Any copyrights or know-how owned by Elanco that comprise or are contained or embodied in any of the product promotional materials being transferred; and
(d) An exclusive, perpetual, irrevocable, royalty-free, worldwide sublicensable and transferable licence to with respect to necessary registered and unregistered intellectual property and know-how used or held for use by or on behalf of Elanco for or in connection with the conduct of the Anticoccidials Divestment Business or the manufacturing of Vecoxan (as applicable and further specified in Annex 4).
(viii) Details of customers of the Anticoccidials Divestment Business, including, but not limited to, the customer details listed at Annex 5.
(ix) Arrangements for the supply of services by Elanco to the Purchaser for a transitional period […] following Closing, […], to ensure the smooth transfer of the Anticoccidials Business (including, inter alia, quality release and quality control testing, regulatory activities support, pharmacovigilance activities support and assistance in connection with the sale of Vecoxan in the EEA/UK).
4. All the Assets, which are necessary to, but not exclusively or primarily related to, the viability and competitiveness of the Anticoccidials Divestment Business and also relate to products/businesses retained by Elanco, shall also be transferred to the Purchaser (at the option of the Purchaser) but only to the extent they relate to the Anticoccidials Divestment Business.
5. In the event that materials to be transferred contain information that is confidential to the Parties’ retained businesses and not relevant to the Anticoccidials Divestment Business, the information shall be redacted as appropriate.
6. The Anticoccidials Divestment Business shall not include any right, title or interest in or to any of the assets of the Parties or their Affiliated Undertakings other than those specified in paragraphs 6.1 and 7 of the Commitments and paragraphs 3 and 4 of this Schedule and, for the avoidance of doubt, will not include (inter alia):
(i) Any right to manufacture, market or sell any other product of the Parties other than Vecoxan for use in the treatment or prevention of coccidial infections in lambs and calves or any licence to use any asset of the Parties in connection with any product other than Vecoxan.
(ii) Any asset that is not an Asset, and any asset that does not relate to the manufacture, marketing and sale of Vecoxan.
(iii) The Parties’ names (specifically, Elanco, Bayer or Bayer Animal Health), or any trading name of the Parties, together with all variations thereof and all trademarks, service marks, domain names, trade names, corporate names, logos and other identifiers of source containing, incorporating or associated with any of the foregoing.
(iv) Personnel, except where (at the option of the Purchaser) reasonably considered necessary to maintain the viability, marketability and competitiveness of the Divestment Business(es) as further set out in paragraph 9 of these Commitments).
(v) Real property and tangible personal property of Elanco.
(vi) Any shared Contracts retained by Elanco.
(vii) Human resources and any other employee books and records (subject to paragraph 9 of the Commitments).
(viii) Items to the extent applicable law prohibits their transfer.
(ix) […].
7. If there is any asset or personnel which is not covered by paragraph 3 of this Schedule but which is both used (exclusively or not) in the Anticoccidials Divestment Business and necessary for the continued viability and competitiveness of the Anticoccidials Divestment Business, that asset or an adequate substitute will be offered to potential Purchaser.
SCHEDULE 2
1. The Otitis Divestment Business consists of the worldwide rights, title and interests in (including the right to develop, improve, manufacture and commercialise in at worldwide level) Osurnia ear gel (“Osurnia”), […] (as defined below).
2. The Otitis Divestment Business as operated to date is not a stand-alone business, rather it is integrated into a wider operational and commercial organisation; it will therefore be separated from Elanco’s current operations as described below at paragraph 3 of this Schedule. As regards its legal and functional structure:
(i) The Otitis Divestment Business forms part of Elanco’s companion animal therapeutics business segment, which also encompasses pain, osteoarthritis, cardiovascular and dermatological products targeting cats and dogs. The Otitis Divestment Business is part of Elanco’s wider otitis product line which also includes Surolan, a daily dose product applied by the pet owner for otitis externa in felines and canines. Please refer to the structure chart provided as Annex 6 for further detail.
(ii) The manufacturing process is as follows:
(a) The three APIs are manufactured by, and purchased from, […].
(b) […].
(c) […].
(iii) The EEA/UK (a) sales, technical and marketing and (b) manufacturing and quality Personnel for the Otitis Divestment Business are located in […].
(iv) […].
3. In accordance with Section B, paragraph 6.2 of these Commitments, subject to third party consent where relevant and to the extent in Elanco’s ownership, control or possession at Closing, the Otitis Divestment Business includes but is not limited to, the following on a worldwide basis:
(i) All Contracts exclusively or primarily related to Osurnia, […], and the rights and benefits of the portion of Contracts which are material and necessary to, but not exclusively used in, the Otitis Divestment Business or manufacture of Osurnia (being ‘shared contracts’), including, but not limited to, the contracts listed in Annex 7. Elanco undertakes to use Best Efforts to obtain all necessary thirdparty consents where applicable. To the extent any such third-party consent could not be obtained, or any contract could not be otherwise transferred, Elanco will, as appropriate, either: (i) assist the Purchaser and the relevant third party to put in place arrangements to transfer any work product and work in progress, and support the Purchaser to put in place alternative arrangements; or (ii) enter into back-to-back agreements with the Purchaser under the same terms and conditions as the relevant contract for a transitional period of […] and up to the duration of the existing relevant contract (if longer than […]).
(ii) According to the Purchaser’s needs, rebate, promotional and other similar programs related to the Otitis Divestment Business.
(iii) All inventory of (to the extent that it has not been sold to a third party) including all rights to market and sell such inventory
(a) Osurnia in finished packaged form (together with any product packaging materials thereon) labelled and held for sale for the treatment of otitis externa complicated by susceptible strains of yeast in dogs;
(b) Bulk API, work-in-progress, excipients, labelling materials and packaging materials held for use exclusively or primarily in the manufacture of Osurnia, […] for sale in the relevant fields;2 and
(c) Samples of Osurnia, […] in finished packaged form labelled and held for use in the relevant fields.
(iv) The manufacturing equipment listed in Annex 8.
(v) The product registrations for Osurnia as listed in Annex 9.
(vi) All documentation comprising the product registrations in Annex 9, and to the extent exclusively or primarily related to Osurnia, […] and the relevant fields for the products and necessary to, or otherwise limiting the ability to, exploit those products in the relevant fields at Closing:
(a) Correspondence and reports submitted to or received from governmental authorities;
(b) Other dossiers or compilations necessary to obtain or maintain any product registrations in Annex 9 with regard to any of Osurnia, […];
(c) Literature safety reports and documents relating to good manufacturing practices or issues, animal clinical trials, animal research, including laboratory and target animal research and all veterinary master files contained or referenced in the product registrations in Annex 9 with regard to each of Osurnia, […]; and
(d) Data (including clinical and pre-clinical data) referenced in any of the documentation and materials referred to above or necessary to develop and commercialise Osurnia, […], but in all cases except where explicitly excluded and excluding all intellectual property rights of any third party (in which instance, Elanco shall use Best Efforts to work with the Purchaser to reach an agreement with the entity that is able to grant a licence).
(vii) All books and records, including customer records, relating exclusively or primarily to the manufacture of Osurnia, […] for exploitation in the relevant fields or the product business in such form as maintained by Elanco to the extent necessary and used to manufacture or exploit Osurnia, […] in the relevant fields as manufactured or exploited by or on behalf of Elanco at Closing (but excluding any assets explicitly excluded and all intellectual property rights of any third party).
(viii) All existing advertising, promotional and media materials, sales training materials, customer lists, other marketing data and materials and trade show materials and videos, in such form as maintained by Elanco, to the extent exclusively or primarily used or held for exclusive and primary use in the product business, and necessary to exploit (but not manufacture) Osurnia, […] in the relevant fields as exploited by Elanco as of Closing (but excluding any assets explicitly excluded and all intellectual property rights of any third party).
(ix) All intellectual property rights exclusively or primarily relating to the Otitis Divestment Business including, but not limited to, the intellectual property rights listed at Annex 10 which broadly comprise:
(a) Product-specific know-how and registered or unregistered intellectual property that is identified or identifiable in tangible form that is owned by Elanco and is used by or on behalf of Elanco exclusively or primarily for or exclusively in connection with the manufacturing of Osurnia, […] for the exploitation in the relevant fields, or the maintenance of any product registration being transferred;
(b) The copyrights set out in Annex 10;
(c) Registered domain names for Osurnia, as registered in certain territories;
(d) The trademark Osurnia; for the avoidance of doubts, the Purchaser will have all rights to market and sell the inventory transferred as part of the Otitis Divestment Business; and
(e) A non-exclusive, perpetual, irrevocable, royalty-free and licence with respect to necessary registered and unregistered copyright and knowhow owned or controlled by Elanco as of Closing and used by or on behalf of Elanco in connection with the exploitation or manufacturing of Osurnia, […] for exploitation in the relevant fields or the maintain of any project registration being transferred (as applicable and further specified in Annex 10).
(x) All rights to claims, demands, causes of action or litigation set out in Annex 11.
(xi) Arrangements for the supply of services by Elanco to the Purchaser for a transitional period of […] following Closing, […], to ensure the smooth transfer of the Otitis Business (including, inter alia, qualified person release services, quality control of batch release testing and stability services, pharmacovigilance activities support in line with a separate pharmacovigilance agreement, regulatory affairs support for the ongoing product registration applications in certain territories, regulatory chemistry manufacturing and control activities to support certain ongoing technology transfers, preparation of renewals for marketing authorisations, and in the jurisdictions where Elanco is not the holder of record, provide the Purchaser with support to transfer product registrations to the Purchaser).
4. All the Assets, which are necessary to, but not exclusively or primarily related to, the viability and competitiveness of the Otitis Divestment Business and also relate to products/businesses retained by Elanco, shall also be transferred to the Purchaser (at the option of the Purchaser) but only to the extent they relate to the Otitis Divestment Business.
5. In the event that materials to be transferred contain information that is confidential to the Parties’ retained businesses and not relevant to the Otitis Divestment Business, the information shall be redacted as appropriate.
6. The Otitis Divestment Business shall not include any right, title or interest in or to any of the assets of the Parties or their Affiliated Undertakings other than those specified in paragraphs 6.2 and 7 of the Commitments and paragraphs 3 and 4 of this Schedule and, for the avoidance of doubt, will not include (inter alia):
(i) Any right to manufacture, market or sell any other product of the Parties other than Osurnia or any licence to use any asset of Elanco in connection with any product other than Osurnia, […].
(ii) Any asset that is not an Asset and any asset that does not relate to the development, manufacture, marketing and sale of Osurnia, […].
(iii) The Parties’ names (specifically, Elanco, Bayer or Bayer Animal Health), or any trading name of the Parties, together with all variations thereof and all trademarks, service marks, domain names, trade names, corporate names, logos and other identifiers of source containing, incorporating or associated with any of the foregoing.
(iv) Personnel, except where (at the option of the Purchaser) reasonably considered necessary to maintain the viability, marketability and competitiveness of the Divestment Business(es) as further set out in paragraph 9 of these Commitments).
(v) Real property and tangible personal property of Elanco.
(vi) All manufacturing-related assets of Elanco (other than as listed in Annex 8).
(vii) Any shared Contracts to be retained by Elanco.
(viii) Trade secrets unrelated to the Otitis Divestment Business.
(ix) Human resources and any other employee books and records (subject to paragraph 9 of the Commitments).
(x) Items to the extent applicable law prohibits their transfer.
7. If there is any asset or personnel which is not covered by paragraph 3 of this Schedule but which is both used (exclusively or not) in the Otitis Divestment Business and necessary for the continued viability and competitiveness of the Otitis Divestment Business, that asset or an adequate substitute will be offered to potential purchasers.
SCHEDULE 3
1. The Parasiticides Divestment Business comprises the Endoparasiticides Assets and the Endectocide Assets as described at Parts 1 and 2 of this Schedule 3.
PART 1:
2. The Endoparasiticides Assets consist of the EEA/UK-wide rights, title and interests in the BAH canine and feline endoparasiticide products marketed under the following brands, […] and all associated assets (including the right to develop, improve, manufacture and commercialise in the EEA/UK):
(i) Drontal (Cat), which is indicated for the treatment of gastrointestinal roundworms, hookworm and tapeworm in cats, and is administered orally as a tablet.3 Its active ingredients are praziquantel and pyrantel;
(ii) Drontal Plus (Dog), which is indicated for the treatment of roundworms, hookworms, whipworms and tapeworms in dogs, and is administered orally as chewable or non-chewable and flavoured or non-flavoured tablets in a boneshaped form.4 Its active ingredients are febantel, pyrantel and praziquantel;
(iii) Drontal Puppy/Welpan (Dog), which is indicated for the treatment of roundworm, hookworm and whipworm in puppies and young dogs up to one year of age. It is administered orally as a solution. Its active ingredients are pyrantel and febantel;
(iv) Droncit Injectable (Cat and Dog) / Spot On (Cat) / Tablet (Cat and Dog): Droncit Tablet, which are used for the treatment of tapeworm in cats and dogs, and is administered orally as a tablet. Droncit spot-on is used in cats only and Droncit injection is available for cats and dogs. All Droncit versions have praziquantel as their active ingredient;
(v) Profender Spot-on (Cat), which is indicated for treatment of mixed parasitic infections caused by roundworm, tapeworm, and lungworm in cats. It is administered as a spot-on treatment,5 Profender’s active ingredients are emodepside and praziquantel;
(vi) Profender Tablet (Dog), which is indicated for treatment of mixed parasitic infections caused by roundworm, and tapeworm in dogs. It is administered orally as a tablet6 and has emodepside and praziquantel as active ingredients;
(vii) Dronspot (Cat), which is indicated for treatment of mixed parasitic infections in cats caused by roundworms and tapeworms. It is administered as a spot-on treatment and has emodepside and praziquantel as active ingredients;
(viii) Procox (Dog), which is an oral suspension that indicated for the treatment of mixed parasitic infections caused by roundworms and coccidia in dogs, and is primarily used in puppies with coccidia. It is administered orally as a suspension for dogs only and has emodepside and toltrazuril as its active ingredients;
(ix) All second brands and private label versions of the above-mentioned products;7
(x) […]
(xi) […], as more fully outlined in Annex 12.
(together, the “Drontal/Profender Family”).
3. The Endoparasiticides Assets as operated to date are not currently run as a stand-alone business, rather are integrated into a wider operational and commercial organisation; they will therefore be separated from BAH’s current operations as described below at paragraph 4 of this Schedule. As regards its legal and functional structure:
(i) The Endoparasiticides Assets is the EEA/UK product line within BAH’s companion animal endoparasiticides business, which encompasses the Drontal/Profender Family products (for sale in EEA/UK). Please refer to the structure chart provided as Annex 13 for further detail.
(ii) The manufacture of finished products intended for sale in the EEA/UK is conducted […].
(a) Specifically, for the Drontal products8 (including private label versions):
(I) Praziquantel (for Drontal tablets, Drontal Plus and Droncit) was until recently supplied by […], but BAH has now terminated that contract and is in the process of switching supplier to […].
(II) Pyrantel (for Drontal, Drontal Plus, and Welpan) is supplied by […].
(III) Febantel is supplied by […].
(IV) The artificial flavouring used in Drontal tablets is supplied by […].
(b) For the Profender products:9
(I) BAH has licensed emodepside (for Profender and Procox) for use in the animal health field from Bayer AG. The pre-cursor “PF1022A” of emodepside (for Profender and Procox) is currently produced […]. Emodepside is neither patent protected by Bayer AG, nor […].
(II) Toltrazuril (for Procox) is produced by […].
(III) Praziquantel (for Profender) is sourced in the same way as for the Drontal products.
(iii) BAH conducts the production, fill and packaging of the finished products intended for sale in the EEA/UK […].
(iv) The Personnel for the Endoparasiticides Assets are based […].
(v) The Endoparasiticides Assets includes […]:
(a) […]; and
(b) […].
(vi) In the EEA/UK, the Drontal/Profender Family is distributed […].
4. In accordance with Section B, paragraph 6.3 of these Commitments, subject to third party consent where relevant and to the extent in Elanco/BAH’s ownership, control or possession at Closing, the Endoparasiticides Assets includes, but is not limited to, the following on an EEA/UK-wide basis (as Elanco/BAH will continue to market the Drontal/Profender Family products ex-EEA/UK):
(i) All Contracts exclusively or primarily related to the Drontal/Profender Family for exploitation in the EEA/UK, and the rights and benefits of the relevant portion of Contracts which are material and/or necessary to, but not exclusively or primarily related to, the Endoparasiticides Assets (being ‘shared contracts’), including, but not limited to, the contracts listed in Annex 14. The Parties undertake to use Best Efforts to obtain all necessary third-party consents where applicable. To the extent any such third-party consent could not be obtained, or any contract could not be otherwise transferred, the Parties will, as appropriate, either: (i) assist the Purchaser and the relevant third party to put in place arrangements to transfer any work product and work in progress, and support the Purchaser to put in place alternative arrangements; or (ii) enter into back-toback agreements with the Purchaser under the same terms and conditions as the relevant contract for a transitional period of at least […]) and up to the duration of the existing relevant contract (if longer than […]).
(ii) Elanco/BAH commit to use their commercially reasonable efforts to procure that Purchaser shall have the rights to acquire emodepside directly from […].
(iii) All rebates, promotional and other similar programs related to the Endoparasiticides Assets.
(iv) All inventory (to the extent that it has not been sold to a third party) of Drontal/Profender Family products including inventory in finished packaged form labelled and held for sale in the EEA/UK and all forms of non-saleable inventory intended for the sale of Drontal/Profender Family products in the EEA/UK (e.g. blister cards with tablets, bulk tablets, printed packaging materials, API). For the avoidance of doubts, the purchaser will have all rights to market and sell the inventory transferred as part of the divestment business;
(v) All relevant regulatory files and product registrations which Elanco/BAH owns or has the right to own (as applicable) that are used by or on behalf of Elanco/BAH for, or exclusively or primarily in connection with, the Endoparasiticides Assets including, but not limited to, the regulatory files and product registrations listed at Annex 15.
(vi) All documentation related exclusively or primarily to the Endoparasiticides Assets, including inter alia:
(a) Documentation comprising the product registrations as listed at Annex 15; and
(b) To the extent exclusively or primarily related to the Drontal/Profender Family products in the EEA/UK and/or necessary to (or limiting the ability to) exploit these products in the EEA/UK:
(I) Correspondence and reports submitted to or received from governmental authorities;
(II) Other dossiers or compilations necessary to obtain or maintain any product registrations to be transferred with regard to such products;
(III) Literature safety reports and documents relating to good manufacturing practices or issues, animal clinical trials, animal research, including laboratory and target animal research and all veterinary master files contained or referenced in the product registrations to be transferred with regard to such products; and
(IV) Data (including clinical and pre-clinical data) referenced in any of the documentation and materials referred to above, or necessary to commercialise or develop any product or pipeline included in the Endoparasiticides Assets. but in all cases except where explicitly excluded, and excluding all intellectual property rights of any third party (in which instance, Elanco shall use Best Efforts to work with the Purchaser to reach an agreement with the entity that is able to grant a licence).
(vii) All books and records relating exclusively or primarily to the Endoparasiticides Assets, in such form as maintained and to the extent necessary and used to develop, manufacture or exploit the Drontal/Profender Family products in the EEA/UK.
(viii) All customer lists, customer orders and customers records of the Endoparasiticides Assets.
(ix) All existing advertising, promotional and media materials, sales training materials, other marketing data and materials, trade show materials and videos to the extent exclusively or primarily used or held for exclusive or primary use in the Endoparasiticides Assets.
(x) All intellectual property rights exclusively or primarily relating to the Endoparasiticides Assets including but not limited to the intellectual property rights listed in Annex 16, which, broadly, comprise:
(a) Product-specific know-how used exclusively or primarily for or exclusively or primarily in connection with the development, manufacturing, exploitation or the maintenance of any purchased product registrations of the Drontal/Profender Family products in the EEA/UK (“Purchased Product Know-How”); as well as a nonexclusive, perpetual, irrevocable, and royalty-free licence (covering the exploitation or manufacture of the Drontal/Profender Family of products in the EEA/UK) for know-how and any proprietary information other than Purchaser Product Know-How that are used by Elanco/BAH for or in connection with the manufacturing of the Drontal/Profender Family products or the maintenance of these product registration (“Licensed Product Know-How”);
(b) All copyrights used exclusively or primarily for or exclusively or primarily in connection with (i) the manufacturing of the Drontal/Profender Family products for exploitation in the EEA/UK, or (ii) the Endoparasiticides Assets (“Purchased Copyrights”); as well as a non-exclusive, perpetual, irrevocable, and royalty-free licence (covering the exploitation or manufacture of the Drontal/Profender Family products in the EEA/UK) with respect to the copyrights other than Purchased Copyrights that are used for or in connection with the exploitation of the Drontal/Profender Family products in the EEA/UK (“Licensed Copyrights”);
(c) The domain names for the Drontal/Profender Family products in the EEA/UK (“Purchased Domain Names”);
(d) All trademarks registered in the EEA/UK and used exclusively or primarily for or exclusively or primarily in connection with the Endoparasiticides Assets (“Purchased Trademarks”); for the avoidance of doubts, the Purchaser will have all rights to market and sell the inventory transferred as part of the Endoparasiticides Assets;
(e) An exclusive, perpetual, irrevocable, and royalty-free licence, under all patents necessary for the exploitation of the Drontal/Profender Family products in the EEA/UK; and a non-exclusive, perpetual, irrevocable, and royalty-free licence, under all patents necessary for the manufacture of the Drontal/Profender Family products (and product improvements) in and outside the EEA/UK for exploitation in the EEA/UK (together the “Licensed Patents”).
(xi) All rights to claims, demands, causes of action or litigation set out in Annex 17.
(xii) All goodwill relating to the Drontal/Profender Family products in the EEA/UK.
(xiii) In order to ensure the smooth transfer of production technology and sales activities to the Purchaser, arrangements for:
(a) A transitional manufacturing and supply agreement – For a period of […], the Purchaser will be able to benefit from manufacturing and supply arrangement, […], pursuant to which Elanco/BAH (or a subcontractor) will manufacture the Drontal/Profender Family products, and will be responsible for procuring the product API and maintaining product API inventory sufficient to meet the Purchaser’s forecasts. Elanco/BAH will also assist the Purchaser with technology transfer including the CMC transfer of the manufacturing process for the Drontal/Profender Family products, however not including any technology transfer relating to the manufacture of the product API. In that regard, Elanco/BAH will provide, free of charge, training for the Purchaser’s personnel with regard to all product presentations (i.e. pipettes, tablets, non-sterile liquid). Concurrently, under the supervision of the Monitoring Trustee, Elanco/BAH and the Purchaser will enter into a quality agreement, […], that will detail the roles and responsibilities, expectations, timelines, deliverables and quality standards between the parties with regards to the manufacture, packaging, quality control, quality assurance, testing, release, storage and shipping of the Drontal/Profender Family products and Elanco/BAH’s obligations relating to the maintenance of records and the rights to inspect the premises;
(b) A transitional services agreement – For a period of […]), and under the supervision of the Monitoring Trustee, Elanco/BAH shall also provide (or cause to be provided), […], to the Purchaser a number of services (for specified periods which shall not exceed […] following Closing) including (inter alia) qualified person release services, quality control of batch release testing services, pharmacovigilance activities support in line with a separate pharmacovigilance agreement, assistance for the transfer of production, regulatory affairs support for the transfer and preparation of renewals of the marketing authorizations for the Drontal/Profender Family products, sales force training (provision of product technical training (including adverse events training), and product-positioning training to sales force, marketing, customer service, veterinary team) and procurement support in setting up point of contact with API suppliers and the other critical suppliers engaged in the manufacturing process for the Drontal/Profender Family products; and
(c) A (transitional) distribution compliance agreement – During the transition period, for so long as Elanco/BAH is the holder of any Purchased Product Registrations (on a country by country basis), which shall not exceed […] following Closing, the Purchaser is authorised to distribute the Drontal/ Profender Family products in the EEA/UK.
5. All the Assets, which are necessary to, but not exclusively or primarily related to, the viability or competitiveness of the Endoparasiticides Assets and also relate to products/businesses retained by the Parties, shall also be transferred to the Purchaser (at the option of the Purchaser) but only to the extent they relate to the Endoparasiticides Assets.
6. In the event that materials to be transferred to the Purchaser pursuant to the Commitments contain information that is confidential to the Elanco/BAH’s retained businesses and not relevant to the Endoparasiticides Assets, the information shall be redacted as appropriate.
7. BAH shall retain, and Elanco shall acquire at Closing of the Concentration, the Drontal/Profender Family outside of the EEA/UK. This shall be effected by means of exclusion of any ex-EEA/UK specific rights, title or interests in the Drontal/ Profender Family, or any ex-EEA/UK assets relating to the same, in the sale and asset purchase agreement, and/or a reverse carve-out of ex-EEA/UK specific rights, from the items listed in paragraph 4 of this Schedule.
8. Subject to the provisions in Part 2 of Schedule 3, the Endoparasiticides Assets shall not include any right, title or interest in or to any of the assets of the Parties or their Affiliated Undertakings other than those specified in paragraphs 6.3 and 7 of the Commitments and paragraphs 4 and 5 of this Schedule (including, for clarity, any right, title or interest in the Drontal/Profender Family outside of the EEA/UK) and, for the avoidance of doubt, will not include (inter alia):
(i) Any right to manufacture, market or sell any products of the Parties other than the Drontal/Profender Family in the EEA/UK or any licence to use any asset of the Parties in connection with any products other than the Drontal/Profender Family in the EEA/UK.
(ii) Any asset that is not an Asset and any asset that does not relate to the manufacture, marketing and sale of the Drontal/Profender Family in the EEA/UK.
(iii) The Parties’ names (specifically, Elanco, Bayer or Bayer Animal Health), or any trading name of the Parties, together with all variations thereof and all trademarks, service marks, domain names, trade names, corporate names, logos and other identifiers of source containing, incorporating or associated with any of the foregoing.
(iv) Personnel, except where (at the option of the Purchaser) reasonably considered necessary to maintain the viability, marketability and competitiveness of the Divestment Business(es) as further set out in paragraph 9 of these Commitments).
(v) Real property and tangible personal property of Elanco/BAH.
(vi) All manufacturing assets of the Parties.
(vii) Any rights or interests exclusively or primarily relating to any Drontal/Profender Family product outside of the EEA/UK.
(viii) Any shared Contract retained by the Parties.
(ix) Trade secrets unrelated to the Endoparasiticides Assets.
(x) Human resources and any other employee books and records (subject to paragraph 9 of these Commitments).
(xi) Items to the extent applicable law prohibits their transfer.
9. If there is any asset or personnel which is not covered by paragraph 4 of this Schedule but which is both used (exclusively or not) in the Endoparasiticides Assets and necessary for the continued viability and competitiveness of the Endoparasiticides Assets, that asset or an adequate substitute will be offered to potential purchasers.
PART 2:
10. The Endectocide Assets consist of:
(i) […] (the “Full Stage Pipeline Product”).10
11. The Endectocide Assets as operated to date is not a stand-alone business, rather it is integrated into a wider operational and commercial organisation; it will therefore be separated from BAH’s current operations as described below at paragraph 12. As regards its legal and functional structure:
(i) The Endectocide Assets forms part of BAH’s companion animal therapeutics business segment and part of BAH’s R&D function.
(ii) The Full Stage Pipeline Product […].
12. In accordance with Section B of these Commitments, subject to third party consent where relevant and to the extent in the Parties’ ownership, control or possession at Closing, the Endectocide Assets includes, but is not limited to, the following on a worldwide basis:
(i) All rights, title and interests in the Full Stage Pipeline Product (including the right to conduct the ongoing clinical trials (listed inter alia in Annex [18]) and to develop, improve, manufacture and commercialise) for use in the Field.
(ii) All Contracts exclusively or primarily related to the Full Stage Pipeline Products, and the benefit of the portion of Contracts which are material or necessary to, but not exclusively or primarily related to the Full Stage Pipeline Product, including but not limited to, the Contracts listed in Annex [19]. The Parties undertake to use Best Efforts to obtain all necessary third-party consents where applicable. To the extent any such third-party consent could not be obtained, or any contract could not be otherwise transferred, the Parties will, as appropriate, either: (i) assist the Purchaser and the relevant third party to put in place arrangements to transfer any work product and work in progress, and support the Purchaser to put in place alternative arrangements; or (ii) enter into back-toback agreements with the Purchaser under the same terms and conditions as the relevant contract for a transitional period of […] and up to the duration of the existing relevant contract (if longer than […]).
(iii) Elanco/BAH commit to use their commercially reasonable efforts to procure that the Purchaser shall have the rights to acquire emodepside directly from […].
(iv) All inventory of (to the extent that it has not been sold to a third party) including, but not limited to, the inventory listed at Annex 20:
(a) Full Stage Pipeline Product in finished form (together with any product packaging materials and any samples) labelled and held for use by the Endectocide Assets; and
(b) bulk API, work-in-progress, excipients, labelling materials and packaging materials used or held for use exclusively or primarily in the manufacture of Full Stage Pipeline Product for use by the Endectocide Assets.
(v) All licences, permits, authorisations and registrations (“Regulatory Approvals”) for the Full Stage Pipeline Product, and all other product registrations or regulatory approvals to the extent exclusively or primarily relating to the Full Stage Pipeline Product for use in the Field.
(vi) All documentation comprising any Regulatory Approvals, and to the extent exclusively or primarily related to the Full Stage Pipeline Product for use in the Field:
(a) correspondence and reports submitted to or received from relevant regulatory authorities relating to the research, development, manufacture, testing, storage, import, export, use, labelling, distribution, sale, offer for sale, commercialisation, licensing, advertising, marketing and promotion (“exploitation”) of the Full Stage Pipeline Product;
(b) other dossiers or compilations necessary to obtain or maintain any of the product registrations and Regulatory Approvals;
(c) literature safety reports and documents relating to good manufacturing practices or issues, animal clinical trials, animal research, including laboratory and target animal research and all veterinary master files contained or referenced to in the product registrations and Regulatory Approvals;
(d) environmental and safety documentation; and
(e) data that resulted from any of BAH’s research or development activities related to the Full Stage Pipeline Product, or data referenced in any documentation and materials referred to above, but in all cases except where explicitly excluded and excluding all intellectual property rights of any third party unless they are necessary for the functioning of the Endectocide Assets (in which instance, Elanco shall use Best Efforts to work with the Purchaser to reach an agreement with the entity that is able to grant a licence).
(vii) All relevant reports, databases and analysis (including all technical, clinical and marketing files, protocols, clinical data and studies, reports, plans, books and records) relating exclusively or primarily to the Endectocide Assets in such form as maintained by BAH (except where explicitly excluded).
(viii) All existing marketing materials, plans and forecasts which are specific to the Full Stage Pipeline Product in such form as maintained by BAH to the extent they are exclusively or primarily related to the Endectocide Assets (except where explicitly excluded).
(ix) All intellectual property rights, to the extent exclusively or primarily relating to the Full Stage Pipeline Product for use in the Field, including, but not limited to, the intellectual property rights listed at Annex 21 which, broadly, comprise:
(a) The patents exclusively or primarily relating to the Full Stage Pipeline Product for use in the Field, which include […]
(b) Worldwide licenses to the patents shared with the Drontal/Profender family and relating to the Full Stage Pipeline Product, for use in the development, manufacture or sale of the Full Stage Pipeline Product, which are: […];
(c) the proposed trademarks relating to the brands […];
(d) product-specific know-how that is identified or identifiable in tangible form that is or has been used for or held for use by or on behalf of BAH exclusively for or exclusively in connection with the Full Stage Pipeline Product for use in the Field;
(e) any copyrights or know-how owned by BAH that comprise or are contained or embodied in any of the marketing materials, plans and forecasts being transferred; and
(f) an exclusive, perpetual, irrevocable, royalty-free sub-licensable and transferable licence with respect to necessary unregistered intellectual property and know-how used or held for use by or on behalf of BAH for or in connection with the conduct of the Endectocide Assets or the development, manufacture or sale of the Full Stage Pipeline Product.
(x) The Key Personnel listed at Annex 22.
(xi) The obligation for Elanco/BAH to use Best Efforts to: (a) complete the Full Stage Pipeline Product related ongoing studies in a timely manner; (b) inform the Purchaser of any information or communication exchanged with the EMA in relation to the Full Stage Pipeline Product; and (c) provide the Purchaser with monthly reports on development process of the Full Stage Pipeline Product.
(xii) The obligation for Elanco/BAH to use Best Efforts (a) to file before Closing all then-available studies and claims consistent with the summary of product characteristics of the Full Stage Pipeline Product with the EMA and (b) to obtain the targeted product profile claims of the Full Stage Pipeline Product.
(xiii) The benefit of transitional arrangements to ensure the smooth transfer of the Endectocide Assets and to enable the continuity of the clinical trials, including an R&D and regulatory transitional service agreement, transitional manufacturing and supply services, and a technology transfer (including knowhow) to the Purchaser, to ensure the viability and competitiveness of the Endectocide Assets for a transitional period of […] following Closing […].
13. All the Assets, which are necessary to, but not exclusively or primarily related to, the viability and competitiveness of the Endectocide Assets and also relate to the products/businesses retained by the Parties, shall also be transferred or licensed to the Purchaser but only to the extent that they relate to the Endectocide Assets (at the option of the Purchaser). In the event that materials to be transferred contain information that is confidential to the Parties’ retained businesses and not relevant to the Endectocide Assets, the information shall be redacted as appropriate.
14. The Parties also undertake to respond promptly (to the best of their abilities) to any questions from the Purchaser of the Endectocide Assets.
15. Subject to the provisions of Part 1 of Schedule 3, the Endectocide Assets shall not include any right, title or interest in or to any of the assets of the Parties or their Affiliated Undertakings other than those specified in paragraph 12 of this Schedule and, for the avoidance of doubt, will not include, (inter alia):
(i) Any right to develop, manufacture, market or sell any product of the Parties other than the Full Stage Pipeline Product for use in the Field.
(ii) Any asset that is not an Asset, and any asset that does not relate to the development, manufacture, marketing and sale of the Full Stage Pipeline Product.
(iii) The Parties’ names (specifically, Elanco, Bayer or Bayer Animal Health), or any trading name of the Parties, together with all variations thereof and all trademarks, service marks, domain names, trade names, corporate names, logos and other identifiers of source containing, incorporating or associated with any of the foregoing.
(iv) Subject to the Commitments with regard to Personnel and Key Personnel in paragraphs 6 and 9, employees, real property and tangible personal property of the Parties.
(v) Any shared Contracts retained by the Parties.
(vi) Trade Secrets unrelated to the Endectocide Assets. : (vii) Human resources and any other employee books and records (subject to paragraphs 6 and 9 of these Commitments).
(viii) Items to the extent applicable law prohibit their transfer.
16. If there is any asset or personnel which is not covered by paragraph 12 of this Schedule but which is both used (exclusively or not) in the Endectocide Assets and necessary for the continued viability and competitiveness of the Endectocide Assets, that asset or an adequate substitute will be offered to the Purchaser.
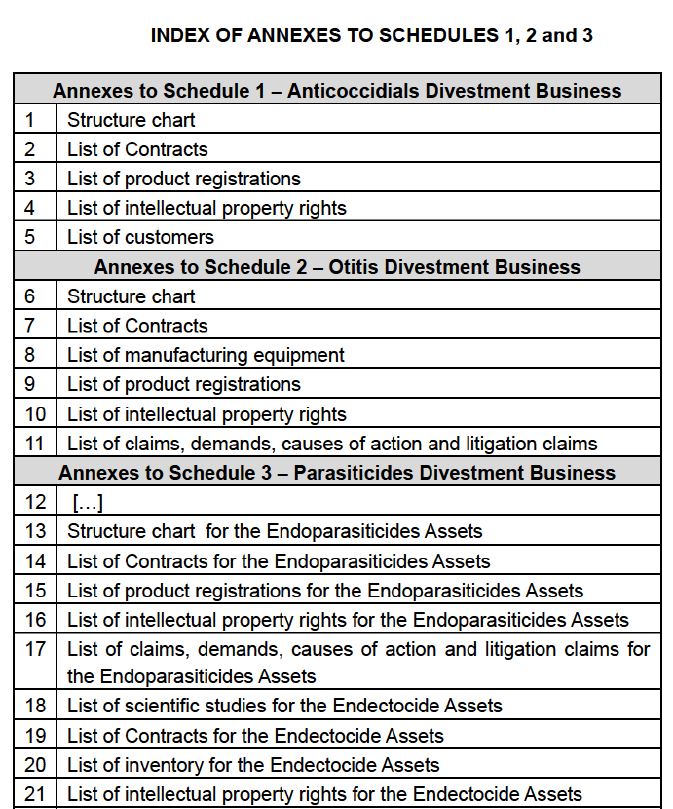

Case M.9554 – Elanco / Bayer Animal Health
ADDITIONAL COMMITMENTS TO THE EUROPEAN COMMISSION
Pursuant to Article 6(2) of Council Regulation (EC) No 139/2004 (the “Merger Regulation”), Elanco Animal Health Inc. (“Elanco”) and Bayer AG (together with its affiliates referred to as “Bayer”) (the “Parties”, each a “Party”) hereby enter into the following Additional Commitments (the “Additional Commitments”) vis-à-vis the European Commission (the “Commission”) with a view to rendering Elanco’s acquisition of Bayer’s animal health business (“Bayer Animal Health” or “BAH”) (the “Concentration”) compatible with the internal market and the functioning of the EEA Agreement. The Additional Commitments bind Bayer only in so far as necessary with regards to actions that must be taken by Bayer with regards to the ESP Divestment Business (as defined below).
This text shall be interpreted in light of the Commission’s decision pursuant to Article 6(1)(b) of the Merger Regulation to declare the Concentration compatible with the internal market and the functioning of the EEA Agreement (the “Decision”), in the general framework of European Union law, in particular in light of the Merger Regulation, and by reference to the Commission Notice on remedies acceptable under Council Regulation (EC) No 139/2004 and under Commission Regulation (EC) No 802/2004 (the “Remedies Notice”).
SECTION A. DEFINITIONS
1. For the purpose of the Additional Commitments, the following terms shall have the following meaning:
Affiliated Undertakings: undertakings controlled by the Parties and/or by the ultimate parents of the Parties, whereby the notion of control shall be interpreted pursuant to Article 3 of the Merger Regulation and in light of the Commission Consolidated Jurisdictional Notice under Council Regulation (EC) No 139/2004 on the control of concentrations between undertakings (the "Consolidated Jurisdictional Notice").
Alternative Divestment Business: the business as described in further detail in Section C of the Additional Commitments and Schedule 2.
API: active pharmaceutical ingredient(s).
Assets: the tangible and intangible assets that contribute to the current operation or are necessary to ensure the viability and competitiveness of the ESP Divestment Business or, if applicable, the Alternative Divestment Business as including but not limited to the assets in paragraphs 6 and 14 respectively and described in more detail in the Schedules 1 and 2.
Best Efforts: Best effort obligations shall be interpreted in light of the Commission's decision pursuant to Article 6(1)(b) of the Merger Regulation to declare the Concentration compatible with the internal market and the functioning of the EEA Agreement, the Merger Regulation and the general principles of EU law. Any interpretation that may be given to this term under the law of other jurisdictions is not relevant solely for the purpose of interpreting and/or implementing the Additional Commitments.
Closing: the transfer of the legal title to the ESP Divestment Business to the Purchaser or, if applicable, of the Alternative Divestment Business, to the Purchaser.
Closing Period: the period of […] from the approval of the Purchaser and the terms of sale by the Commission.
Confidential Information: any business secrets, know-how, commercial information, or any other information of a proprietary nature that is not in the public domain.
Conflict of Interest: any conflict of interest that impairs the Trustee's objectivity and independence in discharging its duties under the Additional Commitments.
Contract: any written contract, agreement, lease, sublease, licence, sub-licence or other legally binding commitment or arrangement.
Divestiture Trustee: one or more natural or legal person(s) who is/are approved by the Commission and appointed by Elanco and who has/have received from Elanco the exclusive trustee mandate to sell the Alternative Divestment Business to a Purchaser at no minimum price.
Elanco Pipeline Products: means the pipeline products as described in further detail in Schedule 2.
Effective Date: the date of adoption of the Decision.
ESP Divestment Business: the business as described in further detail in Section B of the Additional Commitments and Schedule 1.
Fast-Track Dispute Resolution Procedure: the procedure provided for in Section G below and in Annex 2.7.
Field: means animal health.
First Divestiture Period: the period of […] from the Effective Date.
Hold Separate Manager: the person appointed by Elanco or Bayer (as relevant) for the ESP Divestment Business or, if applicable, the Alternative Divestment Business, to manage the day-to-day business under the supervision of the Monitoring Trustee.
Key Personnel: all personnel necessary to maintain the viability and competitiveness of the ESP Divestment Business, as listed in Schedule 1.
Licensor: as described in further detail in Schedule 1.
Licensor Agreement: as described in further detail in Schedule 1.
Monitoring Trustee: one or more natural or legal person(s) who is/are approved by the Commission and appointed by Elanco, and who has/have the duty to monitor the Parties’ compliance with the conditions and obligations attached to the Decision.
Overlapping Retained Business: any business to be retained by Elanco or BAH that is related to canine and/or feline isoxazoline or isoxazoline-like parasiticides.
Personnel: all staff currently employed by the ESP Divestment Business or, if applicable, the Alternative Divestment Business, including staff seconded to the ESP Divestment Business or, if applicable, the Alternative Divestment Business and shared personnel.
Purchaser: the entity approved by the Commission as the acquirer of the ESP Divestment Business or, if applicable, the Alternative Divestment Business in accordance with the criteria set out in Section D.
Purchaser Criteria: the criteria laid down in paragraph 21 of these Additional Commitments that the Purchaser must fulfil in order to be approved by the Commission.
Trustee(s): the Monitoring Trustee and/or the Divestiture Trustee as the case may be.
Trustee Divestiture Period: the period of […] from the end of the First Divestiture Period.
SECTION B. THE COMMITMENT TO DIVEST AND THE DIVESTMENT BUSINESS
Commitment to divest
2. In order to maintain effective competition, Elanco commits to divest, or procure the divestiture of, the ESP Divestment Business by the end of the First Divestiture Period as a going concern to a Purchaser and on terms of sale approved by the Commission in accordance with the procedure described in paragraph 22 of these Additional Commitments. To carry out the divestiture, Elanco commits to find a Purchaser and to enter into a final binding sale and purchase agreement for the sale of the ESP Divestment Business by the end of the First Divestiture Period. If Elanco has not entered into such an agreement at the end of the First Divestiture Period, Elanco shall grant the Divestiture Trustee an exclusive mandate to sell the Alternative Divestment Business in accordance with the procedure described in paragraph 34 in the Trustee Divestiture Period.
3. Elanco shall be deemed to have complied with these Additional Commitments if:
(a) by (i) the end of the First Divestiture Period, Elanco has entered into a final binding sale and purchase agreement for the ESP Divestment Business or the Alternative Divestment Business (as applicable) or (ii) by the end of the Trustee Divestiture Period, the Divestiture Trustee has entered into a final binding sale and purchase agreement for the Alternative Divestment Business, provided that the Commission approves the proposed Purchaser and the terms of sale as being consistent with the Additional Commitments in accordance with the procedure described in paragraph 22; and
(b) the Closing of the sale of the ESP Divestment Business or the Alternative Divestment Business (as applicable) to the Purchaser takes place within the Closing Period.
4. In the event that the Licensor terminates the Licensor Agreement within a week following the acquisition of BAH by Elanco, Elanco shall instead be deemed to have complied with these Additional Commitments and, for the avoidance of doubt, shall not be required to sell the Alternative Divestment Business. Elanco undertakes to use Best Efforts to ensure that the Licensor does not terminate the Licensor Agreement upon the acquisition of BAH by Elanco.
5. In order to maintain the structural effect of the Additional Commitments, Elanco shall, for a period of 10 years after Closing, (i) not acquire, whether directly or indirectly, the possibility of exercising influence (as defined in paragraph 43 of the Remedies Notice, footnote 3) over the whole or part of the ESP Divestment Business and (ii) not market generic versions of the divestment products, unless, following the submission of a reasoned request from Elanco showing good cause and accompanied by a report from the Monitoring Trustee (as provided in paragraph 48 of these Additional Commitments), the Commission finds that the structure of the market has changed to such an extent that the absence of influence over the ESP Divestment Business is no longer necessary to render the proposed Concentration compatible with the internal market. For the avoidance of doubt, Elanco will still be bound by this obligation even if the Licensor terminates the Licensor Agreement.
Structure and definition of the ESP Divestment Business
6. The ESP Divestment Business, as described in more detail in Schedule 1, includes all assets that contribute to the current operation or are necessary to ensure the viability and competitiveness of the ESP Divestment Business at a worldwide level, in particular:
(i) All rights, title and interests to develop, improve, manufacture and commercialise (including the right to conduct the ongoing clinical trials for) the products concerned for use in the Field;
(ii) All contracts related exclusively or primarily to the ESP Divestment Business and, according to the Purchaser’s needs, the rights and benefits of the relevant portion of contracts that are material and/or necessary to, but not exclusively or primarily related to, the ESP Divestment Business to the extent they are capable of being assigned;
(iii) Elanco undertakes to use Best Efforts to obtain all necessary third party consents where applicable;
(iv) All Assets;
(v) Key Personnel;
(vi) All product licences, permits, authorisations and registrations (“regulatory approvals”) for the products concerned as, and all other product registrations or regulatory approvals to the extent exclusively or primarily relating to the products concerned for use in the Field;
(vii) All intellectual property and intellectual property rights (including productspecific know-how) which are owned, maintained, used and /or controlled by the Parties (as applicable) and related to the development, improvement, manufacturing and commercialisation of the products concerned for use in the Field or for the maintenance of the regulatory approvals being transferred;
(viii) All customer orders and customer records of the ESP Divestment Business; and
(ix) the benefit of transitional arrangements, including an R&D and regulatory transitional service agreement, transitional manufacturing and supply services, and a technology transfer (including know-how) to the Purchaser, to ensure the viability and competitiveness of the ESP Divestment Business for a transitional period of […] following Closing […], to ensure the smooth transfer of the ESP Divestment Business.
7. All the Assets, which are necessary to, but not exclusively or primarily related to, the viability and competitiveness of the ESP Divestment Business and also relate to the products/businesses retained by the Parties, shall also be transferred to the Purchaser but only to the extent they related to the ESP Divestment Business (and at the Purchaser’s option). In the event that materials to be transferred to the Purchaser pursuant to the Additional Commitments contain information that is confidential to Elanco/BAH’s retained businesses and not relevant to the ESP Divestment Business, the information shall be redacted as appropriate.
8. For the sake of clarity, the ESP Divestment Business shall not include any physical production assets, equipment or manufacturing units owned or operated by the Parties. Further, the ESP Divestment Business shall not include any Personnel (save for Key Personnel and as provided for at paragraph 9 below).
9. At the option of the Purchaser, the ESP Divestment Business will include the Personnel who would reasonably be considered necessary to maintain the viability, marketability and competitiveness of the ESP Divestment Business. The exercise of such option shall be supervised by the Monitoring Trustee and subject to applicable local employment legislation and employee consent. Should Elanco not agree with the request of the Purchaser, the Monitoring Trustee shall prepare a reasoned opinion within seven working days and the Commission shall ultimately decide on the merits of the Purchaser proposal.
10. The sale of the ESP Divestment Business shall be structured as an asset sale with licences and rights of access to certain shared assets. Strict firewall procedures will be adopted so as to ensure that any competitively sensitive information related to, or arising from, transitional arrangements (for example, product roadmaps) will not be shared with, or passed on to, anyone outside the ESP Divestment Business’s operations, beyond what is reasonably required for the compliance with obligations relating to the transfer of the ESP Divestment Business and supply of transitional services.
SECTION C. THE ALTERNATIVE DIVESTMENT BUSINESS
11. During the First Divestiture Period, Elanco can choose to divest the Alternative Divestment Business instead of the ESP Divestment Business to remove competition concerns where the European Commission has found serious doubts as to the compatibility of the Transaction with the internal market (the “Alternative Divestiture Commitment”). Elanco’s decision to divest the Alternative Divestment Business shall be notified to the Monitoring Trustee. To carry out the divestiture, Elanco commits to find a Purchaser and enter into a final binding sale and purchase agreement for the sale of the Alternative Divestment Business within the First Divestiture Period. To the extent that at the end of the First Divestiture Period Elanco has not entered into such an agreement, Elanco shall grant the Divestiture Trustee an exclusive mandate to sell the Alternative Divestment Business within the Trustee Divestiture Period in accordance with the procedure described in paragraph 34 below.
12. Elanco shall be deemed to have complied with the Additional Commitments if the conditions set out in paragraph 3 or 4 are met.
13. In order to maintain the structural effect of the Additional Commitments, Elanco shall, for a period of 10 years after Closing, (i) not acquire, whether directly or indirectly, the possibility of exercising influence (as defined in paragraph 43 of the Remedies Notice, footnote 3) over the whole or part of the Alternative Divestment Business and (ii) not market generic versions of the divestment products, unless, following the submission of a reasoned request from Elanco showing good cause and accompanied by a report from the Monitoring Trustee (as provided in paragraph 48 of these Additional Commitments), the Commission finds that the structure of the market has changed to such an extent that the absence of influence over the Alternative Divestment Business is no longer necessary to render the proposed Concentration compatible with the internal market.
Structure and definition of the Alternative Divestment Business
14. The Alternative Divestment Business, as described in more detail in Schedule 2, includes all assets that contribute to the current operation or are necessary to ensure the viability and competitiveness of the Alternative Divestment Business at EEA/UK level, in particular:
(i) All rights, title and interests to develop, improve, manufacture and commercialise (including the right to conduct the ongoing clinical trials for) the products concerned for use in the Field;
(ii) All contracts related exclusively or primarily to the Alternative Divestment Business and, according to the Purchaser’s needs, the rights and benefits of the relevant portion of contracts that are material and/or necessary to, but not exclusively or primarily related to, the Alternative Divestment Business to the extent they are capable of being assigned;
(iii) Elanco undertakes to use Best Efforts to obtain all necessary third party consents where applicable;
(iv) All Assets;
(v) All product licences, permits, authorisations and registrations (“regulatory approvals”) for the products concerned as, and all other product registrations or regulatory approvals to the extent exclusively or primarily relating to the products concerned for use in the Field;
(vi) All intellectual property and intellectual property rights (including productspecific know-how) which are owned, maintained, used and /or controlled by Elanco (as applicable) and related to the development, improvement, manufacturing and commercialisation of the products concerned for use in the Field or for the maintenance of the regulatory approvals being transferred;
(vii) All customer orders and customer records of the Alternative Divestment Business; and
(viii) the benefit of transitional arrangements, including an R&D and regulatory transitional service agreement, transitional manufacturing and supply services, and a technology transfer (including know-how), a transitional agreement for the manufacture and supply of finished products to the Purchaser, to ensure the viability and competitiveness of the Alternative Divestment Business for a transitional period of […] following Closing […], to ensure the smooth transfer of the Alternative Divestment Business.
15. The Alternative Divestment Business includes a perpetual, irrevocable, royalty-free exclusive licence for the Purchaser to develop, improve, manufacture and market the Elanco Pipeline Products (including the right to sub-license to third parties) in the EEA/UK for use in the Field upon completion of clinical development. The clinical development of the Elanco Pipeline Products shall be conducted and completed by Elanco under the supervision of the Purchaser (to the extent necessary for the exploitation of the Elanco Pipeline Products in the Field under the license in the EEA/UK) and in accordance with the provisions of Schedule 2. Any of the assets outlined in paragraph 14 relating to the Elanco Pipeline Products will be transferred upon completion of clinical development, to the extent necessary or useful for the development, improvement, manufacture and marketing of the Elanco Pipeline Products in the Field under the licence.
16. All the Assets, which are necessary to, but not exclusively or primarily related to, the viability and competitiveness of the Alternative Divestment Business and also relate to products/businesses retained by the Elanco, shall also be transferred to the Purchaser but only to the extent they relate to the Alternative Divestment Business (at the Purchaser’s option). In the event that materials to be transferred to the Purchaser pursuant to the Additional Commitments contain information that is confidential to the Elanco/BAH’s retained businesses and not relevant to the Alternative Divestment Business, the information shall be redacted as appropriate.
17. For the sake of clarity, the Alternative Divestment Business shall not include any physical production assets, equipment or manufacturing units owned or operated by the Parties. Further, the Alternative Divestment Business shall not include any Personnel (save as provided for at paragraph 18 below).
18. At the option of the Purchaser, the Alternative Divestment Business will include the Personnel who would reasonably be considered necessary to maintain the viability, marketability and competitiveness of the Alternative Divestment Business. The exercise of such option shall be supervised by the Monitoring Trustee and subject to applicable local employment legislation and employee consent. Should Elanco not agree with the request of the Purchaser, the Monitoring Trustee shall prepare a reasoned opinion within seven working days and the Commission shall ultimately decide on the merits of the Purchaser proposal.
19. The sale of the Alternative Divestment Business shall be structured as an asset sale with licences and rights of access to certain shared assets. Strict firewall procedures will be adopted so as to ensure that any competitively sensitive information related to, or arising from, transitional arrangements (for example, product roadmaps) will not be shared with, or passed on to, anyone outside the Alternative Divestment Business’s operations, beyond what is reasonably required for the compliance with obligations relating to the transfer of the Alternative Divestment Business and supply of transitional services.
SECTION D. RELATED COMMITMENTS
Preservation of viability, marketability and competitiveness
20. From the Effective Date until Closing, the Parties shall preserve or procure the preservation of the economic viability, marketability and competitiveness of the ESP Divestment Business, in accordance with good business practice, and shall minimise, as far as possible, any risk of loss of competitive potential of the ESP Divestment Business. In particular, the Parties undertake:
(i) not to carry out any action that might have a significant adverse impact on the value, management or competitiveness of the ESP Divestment Business or that might alter the nature and scope of activity, the industrial or commercial strategy, and/or the investment policy of the ESP Divestment Business;
(ii) to make available, or procure to make available, sufficient resources for the development of the ESP Divestment Business, on the basis and continuation of the existing business plans; and
21. Furthermore, Elanco undertakes to take all reasonable steps, or procure that all reasonable steps are being taken, including appropriate incentive schemes (based on industry practice), to encourage all Key Personnel to remain with the ESP Divestment Business, and not to solicit or move any Personnel to Elanco’s remaining business. Where, nevertheless, individual members of the Key Personnel exceptionally leave the ESP Divestment Business, Elanco shall provide a reasoned proposal to replace the person or persons concerned to the Commission and the Monitoring Trustee. Elanco must be able to demonstrate to the Commission that the replacement is well suited to carry out the functions exercised by those individual members of the Key Personnel. The replacement shall take place under the supervision of the Monitoring Trustee, who shall report to the Commission.
Hold-separate obligations
22. The Parties commit, from the Effective Date until Closing, to procure that the ESP Divestment Business is kept separate from the business(es) that the Parties will be retaining and, after closing of the Concentration to keep the ESP Divestment Business separate from the retained business and to ensure that unless explicitly permitted under these Additional Commitments:
(i) Management and staff of the retained business have no involvement in the ESP Divestment Business; and
(ii) Key Personnel and Personnel can be involved in businesses retained by the Parties only to the extent that (a) they are bound by the terms of non-disclosure agreements or similar arrangements preventing the disclosure of any information related to the ESP Divestment Business; (b) they are not involved in the Overlapping Retained Business; and (c) their involvement in other businesses retained by the Parties is compatible with their required involvement in the ESP Divestment Business.
23. Until Closing, the Parties shall assist the Monitoring Trustee in ensuring that the ESP Divestment Business is managed as a distinct and saleable entity separate from the businesses which they are retaining. Immediately after the adoption of the Decision, the Parties shall appoint a Hold Separate Manager(s) for the ESP Divestment Business. The Hold Separate Manager shall manage the ESP Divestment Business independently and in the best interests of the business with a view to ensuring its continued economic viability, marketability and competitiveness and its independence from the businesses retained by the Parties. The Hold Separate Manager shall closely cooperate with and report to the Monitoring Trustee and, if applicable, the Divestiture Trustee. In case of replacement of a Hold Separate Manager, the Parties shall provide a reasoned proposal to replace the person or person concerned to the Commission and the Monitoring Trustee. The Parties must be able to demonstrate to the Commission that the replacement is well suited to carry out the functions exercised by that individual. The replacement shall take place under the supervision of the Monitoring Trustee, who shall report to the Commission. The Commission may, after having heard the Parties, require the Parties to replace a Hold Separate Manager.
Ring-fencing
24. The Parties shall implement, or procure to implement, all necessary measures to ensure that they do not, after the Effective Date, obtain any Confidential Information relating to the ESP Divestment Business and that any such Confidential Information obtained by the Parties before the Effective Date will be eliminated and not be used by them. In particular, the participation of the ESP Divestment Business in any central information technology network shall be severed to the extent possible, without compromising the viability of the ESP Divestment Business. The Parties may obtain or keep information relating to the ESP Divestment Business which is reasonably necessary for the divestiture of the ESP Divestment Business or the disclosure of which to the Parties is required by law.
Non-solicitation clause
25. The Parties undertake, subject to customary limitations, not to solicit, and to procure that Affiliated Undertakings do not solicit, any Key Personnel or Personnel transferred with the ESP Divestment Business or, if applicable, the Alternative Divestment Business for a period of […] years after Closing.
Due diligence
26. In order to enable potential purchasers to carry out reasonable due diligence of the ESP Divestment Business and, if applicable, the Alternative Divestment Business, the Parties shall, subject to customary confidentiality assurances and dependent on the stage of the divestiture process:
(i) provide to potential purchasers sufficient information as regards the ESP Divestment Business and, if applicable, the Alternative Divestment Business;
(ii) provide to potential purchasers sufficient information relating to the Personnel and allow them reasonable access to the Personnel to the extent required under paragraph 9 above (and, if applicable, under paragraph 18 above).
Reporting
27. Elanco shall submit written reports in English on potential purchasers of the ESP Divestment Business and, if applicable, the Alternative Divestment Business and developments in the negotiations with such potential purchasers to the Commission and the Monitoring Trustee no later than 10 days after the end of every month following the Effective Date (or otherwise at the Commission’s request). Elanco shall submit a list of all potential purchasers having expressed interest in acquiring the ESP Divestment Business and, if applicable, the Alternative Divestment Business to the Commission at each and every stage of the divestiture process, as well as a copy of all the offers made by potential purchasers within five days of their receipt.
28. Elanco shall inform the Commission and the Monitoring Trustee on the preparation of the data room documentation and the due diligence procedure and shall submit a copy of any information memorandum to the Commission and the Monitoring Trustee before sending such memorandum out to potential purchasers.
Other
29. The obligations described in paragraphs 12 to 24 above shall also apply to the Alternative Divestment Business from the Effective Date until Closing (or some earlier time as agreed with the Commission).
30. As soon as Elanco informs the Monitoring Trustee of its decision to divest the Alternative Divestment Business pursuant to paragraph 11, the commitments provided for in Section D shall no longer apply to the ESP Divestment Business.
SECTION E. THE PURCHASER
31. In order to be approved by the Commission, the Purchaser must fulfil the following criteria:
(i) the Purchaser shall be independent of and unconnected to Elanco and BAH and their Affiliated Undertakings (this being assessed having regard to the situation following the divestiture);
(ii) the Purchaser shall have the financial resources, proven expertise and incentive to maintain and develop the ESP Divestment Business or, if applicable, the Alternative Divestment Business as a viable and active competitive force in competition with the Parties and other competitors;
(iii) the acquisition of the ESP Divestment Business, or if applicable, the Alternative Divestment Business by the Purchaser must neither be likely to create, in light of the information available to the Commission, prima facie competition concerns nor give rise to a risk that the implementation of the Additional Commitments will be delayed. In particular, the Purchaser must reasonably be expected to obtain all necessary approvals from the relevant regulatory authorities for the acquisition of the ESP Divestment Business or, if applicable, the Alternative Divestment Business; and
(iv) the Purchaser shall have:
(a) Established capabilities or a track record in the clinical development of animal health products in the EEA/UK, including with regard to having interactions with relevant EEA-wide and national bodies that decide on approval of animal health products;
(b) Established capabilities or a track record in the manufacture, commercialisation and distribution of animal health products in the EEA/UK;
(c) Sufficient R&D resources and experience to develop the relevant pipeline products included in the scope of the ESP Divestment Business or, if applicable, the Alternative Divestment Business; and
(d) Complementary products and expertise relevant to the ESP Divestment Business or, if applicable, the Alternative Divestment Business.
32. The final binding sale and purchase agreement (and, if applicable, with respect to the Alternative Divestment Business, a licence agreement) (as well as ancillary agreements) relating to the divestment of the ESP Divestment Business or, if applicable, the Alternative Divestment Business, shall be conditional on the Commission’s approval. When Elanco has reached an agreement with a purchaser, it shall submit a fully documented and reasoned proposal, including a copy of the final agreement(s), within one week to the Commission and the Monitoring Trustee. Elanco must be able to demonstrate to the Commission that the Purchaser fulfils the Purchaser Criteria and that the ESP Divestment Business or, if applicable, the Alternative Divestment Business is being sold in a manner consistent with the Commission's Decision and these Additional Commitments. For the approval, the Commission shall verify that the purchaser fulfils the Purchaser Criteria and that the ESP Divestment Business or, if applicable, the Alternative Divestment Business are being sold in a manner consistent with the Additional Commitments including their objective to bring about a lasting structural change in the market. The Commission may approve the sale of the ESP Divestment Business or, if applicable, the Alternative Divestment Business without one or more Assets or any or part of any Personnel, or by substituting one or more Assets or parts of any Personnel with one or more different assets or different personnel or support, if this does not affect the viability and competitiveness of the ESP Divestment Business or, if applicable, the Alternative Divestment Business after the sale, taking account of the proposed purchaser.
SECTION F. TRUSTEE
I. Appointment procedure
33. Elanco shall appoint a Monitoring Trustee to carry out the functions specified in these Additional Commitments for a Monitoring Trustee. Elanco commits not to close the Concentration before the appointment of a Monitoring Trustee.
34. If Elanco has not entered into a binding sale and purchase agreement regarding the ESP Divestment Business or, if applicable, the Alternative Divestment Business one month before the end of the First Divestiture Period, or if the Commission has rejected a purchaser proposed by Elanco at that time or thereafter, Elanco shall appoint a Divestiture Trustee. The appointment of the Divestiture Trustee shall take effect upon the commencement of the Trustee Divestiture Period.
35. The Trustee shall:
(i) at the time of appointment, be independent of Elanco and its Affiliated Undertakings;
(ii) possess the necessary qualifications to carry out its mandate, for example having sufficient relevant experience as an investment banker or consultant or auditor; and
(iii) neither have nor become exposed to a Conflict of Interest.
36. The Trustee shall be remunerated by Elanco in a way that does not impede the independent and effective fulfilment of its mandate. In particular, where the remuneration package of a Divestiture Trustee includes a success premium linked to the final sale value of the Alternative Divestment Business, such success premium may only be earned if the divestiture takes place within the Trustee Divestiture Period.
Proposal by Elanco
37. No later than two weeks after the Effective Date, Elanco shall submit the name or names of one or more natural or legal persons whom Elanco proposes to appoint as the Monitoring Trustee to the Commission for approval. No later than one month before the end of the First Divestiture Period or on request by the Commission, Elanco shall submit a list of one or more persons whom Elanco proposes to appoint as Divestiture Trustee to the Commission for approval. The proposal shall contain sufficient information for the Commission to verify that the person or persons proposed as Trustee fulfil the requirements set out in paragraph 25 and shall include:
(i) the full terms of the proposed mandate, which shall include, for the avoidance of doubt, all provisions necessary to enable the Trustee to fulfil its duties under these Additional Commitments;
(ii) the outline of a work plan which describes how the Trustee intends to carry out its assigned tasks; and
(iii) an indication whether the proposed Trustee is to act as both Monitoring Trustee and Divestiture Trustee or whether different trustees are proposed for the two functions.
Approval or rejection by the Commission
38. The Commission shall have the discretion to approve or reject the proposed Trustee(s) and to approve the proposed mandate subject to any modifications it deems necessary for the Trustee to fulfil its obligations. If only one name is approved, Elanco shall appoint or cause to be appointed the person or persons concerned as Trustee, in accordance with the mandate approved by the Commission. If more than one name is approved, Elanco shall be free to choose the Trustee to be appointed from among the names approved. The Trustee shall be appointed within one week of the Commission’s approval, in accordance with the mandate approved by the Commission.
New proposal by Elanco
39. If all the proposed Trustees are rejected, Elanco shall submit the names of at least two more natural or legal persons within one week of being informed of the rejection, in accordance with paragraphs 23 and 28 of these Additional Commitments.
Trustee nominated by the Commission
40. If all further proposed Trustees are rejected by the Commission, the Commission shall nominate a Trustee, whom Elanco shall appoint, or cause to be appointed, in accordance with a trustee mandate approved by the Commission.
II. Functions of the Trustee
41. The Trustee shall assume its specified duties and obligations in order to ensure compliance with the Additional Commitments. The Commission may, on its own initiative or at the request of the Trustee or Elanco, give any orders or instructions to the Trustee in order to ensure compliance with the conditions and obligations attached to the Decision.
Duties and obligations of the Monitoring Trustee
42. The Monitoring Trustee shall:
(i) propose in its first report to the Commission a detailed work plan describing how it intends to monitor compliance with the obligations and conditions attached to the Decision.
(ii) oversee, in close co-operation with the Hold Separate Manager, the on-going management of the ESP Divestment Business or, if applicable, the Alternative Divestment Business, with a view to ensuring its continued economic viability, marketability and competitiveness and monitor compliance by the Parties with the conditions and obligations attached to the Decision. To that end the Monitoring Trustee shall:
(a) monitor the preservation of the economic viability, marketability and competitiveness of the ESP Divestment Business or, if applicable, the Alternative Divestment Business, and the keeping separate of ESP Divestment Business or, if applicable, the Alternative Divestment Business from the business retained by the Parties, in accordance with paragraphs 21 and 24 of these Additional Commitments;
(b) supervise the management of the ESP Divestment Business or, if applicable, the Alternative Divestment Business as distinct and saleable entities, in accordance with paragraph 22 of these Additional Commitments;
(c) with respect to Confidential Information:
(I) determine all necessary measures to ensure that the Parties do not after the Effective Date obtain any Confidential Information relating to the ESP Divestment Business or, if applicable, the Alternative Divestment Business,
(II) in particular, strive for the severing of the ESP Divestment Business’s participation in a central information technology network to the extent possible, without compromising the viability of the ESP Divestment Business or, if applicable, the Alternative Divestment Business, as applicable,
(III) make sure that any Confidential Information relating to the ESP Divestment Business or, if applicable, the Alternative Divestment Business, obtained by the Parties before the Effective Date is eliminated and will not be used by the Parties; and
(IV) decide whether such information may be disclosed to or kept by the Parties as the disclosure is reasonably necessary to allow the Parties to carry out the divestiture or as the disclosure is required by law;
(d) monitor the splitting of assets and the allocation of Personnel (to the extent required under paragraph 9) between the ESP Divestment Business or, if applicable, the Alternative Divestment Business, and the Parties or Affiliated Undertakings;
(iii) propose to the Parties such measures as the Monitoring Trustee considers necessary to ensure the Parties’ compliance with the conditions and obligations attached to the Decision, in particular the maintenance of the full economic viability, marketability or competitiveness of the ESP Divestment Business or, if applicable, the Alternative Divestment Business, the holding separate of the ESP Divestment Business or, if applicable, the Alternative Divestment Business, and the non-disclosure of competitively sensitive information;
(iv) review and assess potential purchasers as well as the progress of the divestiture process and verify that, dependent on the stage of the divestiture process:
(a) potential purchasers receive sufficient and correct information relating to the ESP Divestment Business or, if applicable, the Alternative Divestment Business, and Personnel (to the extent required under paragraph 9) in particular by reviewing, if available, the data room documentation, the information memorandum and the due diligence process, and
(b) potential purchasers are granted reasonable access to Personnel (to the extent required under paragraph 9);
(v) act as a contact point for any requests by third parties, in particular potential purchasers, in relation to the Additional Commitments;
(vi) provide to the Commission, sending Elanco a non-confidential copy at the same time, a written report within 15 days after the end of every month that shall cover the operation and management of the ESP Divestment Business or, if applicable, the Alternative Divestment Business as well as the splitting of assets and the allocation of Personnel (to the extent required under paragraph 18) so that the Commission can assess whether the business is held in a manner consistent with the Additional Commitments and the progress of the divestiture process as well as potential purchasers;
(vii) promptly report in writing to the Commission, sending Elanco a non-confidential copy at the same time, if it concludes on reasonable grounds that the Parties are failing to comply with these Additional Commitments;
(viii) within one week after receipt of the documented proposal referred to in paragraph 22 of these Additional Commitments, submit to the Commission, sending Elanco a non-confidential copy at the same time, a reasoned opinion as to the suitability and independence of the proposed purchaser and the viability of the ESP Divestment Business or, if applicable, the Alternative Divestment Business after the sale and as to whether the ESP Divestment Business is sold in a manner consistent with the conditions and obligations attached to the Decision, in particular, if relevant, whether the sale of the ESP Divestment Business without one or more Assets or none, or not all, of the Personnel (to the extent required under paragraph 9) affects the viability of the ESP Divestment Business or, if applicable, the Alternative Divestment Business after the sale, taking account of the proposed purchaser; and
(ix) assume the other functions assigned to the Monitoring Trustee under the conditions and obligations attached to the Decision.
43. If the Monitoring and Divestiture Trustee are not the same legal or natural persons, the Monitoring Trustee and the Divestiture Trustee shall cooperate closely with each other during and for the purpose of the preparation of the Trustee Divestiture Period in order to facilitate each other's tasks.
Duties and obligations of the Divestiture Trustee
44. Within the Trustee Divestiture Period, the Divestiture Trustee shall sell at no minimum price the Alternative Divestment Business to a purchaser, provided that the Commission has approved both the purchaser and the final binding sale and purchase agreement (and ancillary agreements) as in line with the Commission's Decision and the Additional Commitments in accordance with paragraphs 21 and 22 of these Additional Commitments. The Divestiture Trustee shall include in the sale and purchase agreement (as well as in any ancillary agreements) such terms and conditions as it considers appropriate for an expedient sale in the Trustee Divestiture Period. In particular, the Divestiture Trustee may include in the sale and purchase agreement such customary representations and warranties and indemnities as are reasonably required to effect the sale. The Divestiture Trustee shall protect the legitimate financial interests of Elanco, subject to Elanco’s unconditional obligation to divest at no minimum price in the Trustee Divestiture Period.
45. In the Trustee Divestiture Period (or otherwise at the Commission’s request), the Divestiture Trustee shall provide the Commission with a comprehensive monthly report written in English on the progress of the divestiture process. Such reports shall be submitted within 15 days after the end of every month with a simultaneous copy to be sent to the Monitoring Trustee and a non-confidential copy to be sent to Elanco.
III. Duties and obligations of the Parties
46. The Parties shall provide and shall cause their advisors to provide the Trustee with all such co-operation, assistance and information as the Trustee may reasonably require to perform its tasks. The Trustee shall have full and complete access to any of the Parties’ or the ESP Divestment Business’ or, if applicable, the Alternative Divestment Business’ books, records, documents, management or other personnel, facilities, sites and technical information necessary for fulfilling its duties under the Additional Commitments and the Parties and the ESP Divestment Business’ or, if applicable, the Alternative Divestment Business’ shall provide the Trustee upon request with copies of any document. The Parties and the ESP Divestment Business or, if applicable, the Alternative Divestment Business’ shall make available to the Trustee one or more offices on their premises and shall be available for meetings in order to provide the Trustee with all information necessary for the performance of its tasks.
47. The Parties shall provide the Monitoring Trustee with all managerial and administrative support that it may reasonably request on behalf of the management of the ESP Divestment Business or, if applicable, the Alternative Divestment Business. This shall include all administrative support functions relating to the ESP Divestment Business or, if applicable, the Alternative Divestment Business which are currently carried out at headquarters level. Elanco shall provide and shall cause its advisors to provide the Monitoring Trustee, on request, with the information submitted to potential purchasers, and in particular give the Monitoring Trustee access to the data room documentation and all other information granted to potential purchasers in the due diligence procedure. Elanco shall inform the Monitoring Trustee on possible purchasers, submit lists of potential purchasers at each stage of the selection process, including the offers made by potential purchasers at those stages, and keep the Monitoring Trustee informed of all developments in the divestiture process.
48. The Parties shall grant or procure Affiliated Undertakings to grant comprehensive powers of attorney, duly executed, to the Divestiture Trustee to effect the sale (including ancillary agreements), the Closing and all actions and declarations which the Divestiture Trustee considers necessary or appropriate to achieve the sale and the Closing, including the appointment of advisors to assist with the sale process. Upon request of the Divestiture Trustee, the Parties shall cause the documents required for effecting the sale and the Closing to be duly executed.
49. Elanco shall indemnify the Trustee and its employees and agents (each an “Indemnified Party”) and hold each Indemnified Party harmless against, and hereby agrees that an Indemnified Party shall have no liability to Elanco for, any liabilities arising out of the performance of the Trustee’s duties under the Additional Commitments, except to the extent that such liabilities result from the wilful default, recklessness, gross negligence or bad faith of an Indemnified Party.
50. At the expense of Elanco, the Trustee may appoint advisors (in particular for corporate finance or legal advice), subject to Elanco’s approval (such approval not to be unreasonably withheld or delayed) if the Trustee considers the appointment of such advisors necessary or appropriate for the performance of its duties and obligations under the mandate, provided that any fees and other expenses incurred by the Trustee are reasonable. Should Elanco refuse to approve the advisors proposed by the Trustee the Commission may approve the appointment of such advisors instead, after having heard Elanco. Only the Trustee shall be entitled to issue instructions to the advisors. Paragraph 50 of these Additional Commitments shall apply mutatis mutandis. In the Trustee Divestiture Period, the Divestiture Trustee may use advisors who served Elanco during the Divestiture Period if the Divestiture Trustee considers this in the best interest of an expedient sale.
51. The Parties agree that the Commission may share Confidential Information proprietary to the Parties with the Trustee. The Trustee shall not disclose such information and the principles contained in Article 17 (1) and (2) of the Merger Regulation apply mutatis mutandis.
52. Elanco agrees that the contact details of the Monitoring Trustee are published on the website of the Commission's Directorate-General for Competition and they shall inform interested third parties, in particular any potential purchasers, of the identity and the tasks of the Monitoring Trustee.
53. For a period of 10 years from the Effective Date the Commission may request all information from the Parties that is reasonably necessary to monitor the effective implementation of these Additional Commitments.
IV. Replacement, discharge and reappointment of the Trustee
54. If the Trustee ceases to perform its functions under the Additional Commitments or for any other good cause, including the exposure of the Trustee to a Conflict of Interest:
(i) the Commission may, after hearing the Trustee and Elanco, require Elanco to replace the Trustee; or
(ii) Elanco may, with the prior approval of the Commission, replace the Trustee.
55. If the Trustee is removed according to paragraph 44 of these Additional Commitments, the Trustee may be required to continue in its function until a new Trustee is in place to whom the Trustee has effected a full hand over of all relevant information. The new Trustee shall be appointed in accordance with the procedure referred to in paragraphs 21 to 28 of these Additional Commitments.
56. Unless removed according to paragraph 44 of these Additional Commitments, the Trustee shall cease to act as Trustee only after the Commission has discharged it from its duties after all the Additional Commitments with which the Trustee has been entrusted have been implemented. However, the Commission may at any time require the reappointment of the Monitoring Trustee if it subsequently appears that the relevant remedies might not have been fully and properly implemented.
SECTION G. DISPUTE RESOLUTION
57. In the event that Elanco divests the Alternative Divestment Business, the Purchaser may avail itself of the Fast-Track Dispute Resolution Procedure described in Annex 2.7 if it believes that Elanco is failing to comply with these Additional Commitments in relation to the development of the Elanco Pipeline Products.
SECTION H. THE REVIEW CLAUSE
58. The Commission may extend the time periods foreseen in the Additional Commitments in response to a request from Elanco or, in appropriate cases, on its own initiative. Where Elanco requests an extension of a time period, it shall submit a reasoned request to the Commission no later than one month before the expiry of that period, showing good cause. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time, send a non-confidential copy of the report to Elanco. Only in exceptional circumstances shall Elanco be entitled to request an extension within the last month of any period.
59. The Commission may further, in response to a reasoned request from Elanco showing good cause, waive, modify or substitute, in exceptional circumstances, one or more of the undertakings in these Additional Commitments. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time, send a nonconfidential copy of the report to Elanco. The request shall not have the effect of suspending the application of the undertaking and, in particular, of suspending the expiry of any time period in which the undertaking has to be complied with.
SECTION I. ENTRY INTO FORCE
60. The Additional Commitments shall take effect upon the date of adoption of the Decision.
(Signed)
duly authorised for and on behalf of Elanco Animal Health Inc.
duly authorised for and on behalf of Bayer AG
SCHEDULE 1: The ESP Divestment Business
1. The ESP Divestment Business consists of:
(i) The worldwide rights, obligations, title and interest that Elanco will acquire from Bayer under the licence agreement dated […] between BAH and […]. (the “Licensor” and the “Licensor Agreement”), subject to the Licensor not exercising its termination rights under the Licensor Agreement, which relate to the following pipeline products based on the […] compound, being developed by BAH pursuant to the Licensor Agreement (the “Early Stage Pipeline Products”):
(a) […];
(b) […]
(c) […].
[…].
2. The ESP Divestment Business as operated to date is not a stand-alone business, rather it is integrated into a wider operational and commercial organisation; it will therefore be separated from BAH’s current operations. As regards its legal and functional structure:
(i) The ESP Divestment Business forms part of BAH’s companion animal therapeutics business segment and part of BAH’s R&D function.
(ii) The Early Stage Pipeline Products’ clinical trials are […].
3. In accordance with Section B of these Additional Commitments, subject to third party consent where relevant and to the extent in Elanco’s or BAH’s ownership, control or possession at Closing, the ESP Divestment Business includes, but is not limited to, the following on a worldwide basis:
(i) All rights, title and interests in the Early Stage Pipeline Products (including the right to conduct the ongoing clinical trials (listed inter alia in Annex 1.1) and to develop, improve, manufacture and commercialise) for use in the Field.
(ii) All Contracts exclusively or primarily related to the Early Stage Pipeline Products, and the benefit of the portion of Contracts which are material or necessary to, but not exclusively or primarily used in, the ESP Divestment Business or the manufacture of the Early Stage Pipeline Products, including, but not limited to, the Contracts listed in Annex 1.2. The Parties undertake to use Best Efforts to obtain all necessary third-party consents where applicable. To the extent any such third-party consent could not be obtained, or any contract could not be otherwise transferred, the Parties will, as appropriate, either: (i) assist the Purchaser and the relevant third party to put in place arrangements to transfer any work product and work in progress, and support the Purchaser to put in place alternative arrangements; or (ii) enter into back-toback agreements with the Purchaser under the same terms and conditions as the relevant contract for a transitional period of at least […] and up to the duration of the existing relevant contract (if longer than […]).
(iii) All inventory of (to the extent that it has not been sold to a third party), including, but not limited to, the inventory listed at Annex 1.3:
a) Early Stage Pipeline Products in finished form (together with any product packaging materials and any samples) labelled and held for use by the ESP Divestment Business; and
b) bulk API, work-in-progress, excipients, labelling materials and packaging materials used or held for use exclusively or primarily in the manufacture of Early Stage Pipeline Products for use by the ESP Divestment Business.
(iv) All licences, permits, authorisations and registrations (“Regulatory Approvals”) for the Early Stage Pipeline Products, and all other product registrations or regulatory approvals to the extent exclusively or primarily relating to the Early Stage Pipeline Products for use in the Field.
(v) All documentation comprising any Regulatory Approvals, and to the extent exclusively or primarily related to the Early Stage Pipeline Products for use in the Field:
a) correspondence and reports submitted to or received from relevant regulatory authorities relating to the research, development, manufacture, testing, storage, import, export, use, labelling, distribution, sale, offer for sale, commercialisation, licensing, advertising, marketing and promotion (“exploitation”) of the Early Stage Pipeline Products;
b) other dossiers or compilations necessary to obtain or maintain any of the product registrations and Regulatory Approvals;
c) literature safety reports and documents relating to good manufacturing practices or issues, animal clinical trials, animal research, including laboratory and target animal research and all veterinary master files contained or referenced to in the product registrations and Regulatory Approvals;
d) environmental and safety documentation; and
e) data that resulted from any of BAH’s research or development activities related to the Early Stage Pipeline Products, or data referenced in any documentation and materials referred to above, but in all cases except where explicitly excluded and excluding all intellectual property rights of any third party unless they are necessary for the functioning of the ESP Divestment Business (in which instance, Elanco shall use reasonable best efforts to work with the Purchaser to reach an agreement with the entity that is able to grant a licence).
(vi) All relevant reports, databases and analysis (including all technical, clinical and marketing files, protocols, clinical data and studies, reports, plans, books and records) relating exclusively or primarily to the ESP Divestment Business in such form as maintained by BAH (except where explicitly excluded).
(vii) All existing marketing materials, plans and forecasts which are specific to the Early Stage Pipeline Products in such form as maintained by BAH to the extent they are exclusively or primarily used or held for exclusive or primary use in the ESP Divestment Business (except where explicitly excluded).
(viii) All intellectual property rights to the extent exclusively or primarily relating to the Early Stage Pipeline Products for use in the Field, including, but not limited to, the intellectual property rights listed at Annex 1.4 which, broadly, comprise:
a) […], which BAH licenses from […] this under the Licensor Agreement;
b) the patent families relating exclusively or primarily to the development of the Early Stage Pipeline Products for their use in the Field which include (inter alia):
(I) […];
(II) […];
(III) […]
(IV) […].
c) product-specific know-how that is identified or identifiable in tangible form that is or has been used for or held for use by or on behalf of BAH exclusively or primarily for or exclusively or primarily in connection with the development, improvement, manufacturing and commercialisation of the Early Stage Pipeline Products for use in the Field;
d) any copyrights or know-how owned by BAH that comprise or are contained or embodied in any of the marketing materials, plans and forecasts being transferred; and
e) an exclusive, perpetual, irrevocable, royalty-free sub-licensable and transferable licence with respect to necessary unregistered intellectual property and know-how used or held for use by or on behalf of BAH for or in connection with the conduct of the ESP Divestment Business or the development, improvement, manufacture or commercialisation of the Early Stage Pipeline Products.
(ix) The Key Personnel listed at Annex 1.5.
(x) The benefit of transitional arrangements to ensure the smooth transfer of the ESP Divestment Business and to enable the continuity of the clinical trials, including an R&D and regulatory transitional service agreement, transitional manufacturing and supply services, and a technology transfer (including knowhow) to the Purchaser, to ensure the viability and competitiveness of the ESP Divestment Business for a transitional period of […] following Closing, […].
4. All the Assets, which are necessary to, but not exclusively or primarily related to, the viability and competitiveness of the ESP Divestment Business and also relate to products/businesses retained by the Parties, shall also be transferred to the Purchaser but only to the extent they relate to the ESP Divestment Business (and at the Purchaser’s option). In the event that materials to be transferred to the Purchaser pursuant to the Additional Commitments contain information that is confidential to the Elanco/BAH’s retained businesses and not relevant to the ESP Divestment Business, the information shall be redacted as appropriate.
5. In the event that materials to be transferred contain information that is confidential to the Parties’ retained businesses and not relevant to the ESP Divestment Business, the information shall be redacted as appropriate.
6. The ESP Divestment Business shall not include any right, title or interest in or to any of the assets of the Parties or their Affiliated Undertakings other than those specified in paragraph 6 of the Additional Commitments and paragraph 3 of this Schedule and, for the avoidance of doubt, will not include, (inter alia):
(i) Any right to develop, manufacture, market or sell any other product of the Parties other than the Early Stage Pipeline Products for use in the Field.
(ii) Any asset that is not an Asset, and any asset that does not relate to the development, manufacture, marketing and sale of the Early Stage Pipeline Products.
(iii) The Parties’ names (specifically, Elanco, Bayer or Bayer Animal Health), or any trading name of the Parties, together with all variations thereof and all trademarks, service marks, domain names, trade names, corporate names, logos and other identifiers of source containing, incorporating or associated with any of the foregoing.
(iv) Subject to the Additional Commitments with regard to Personnel and Key Personnel in paragraphs 6 and 9, employees, real property and tangible personal property of the Parties.
(v) Any shared Contracts retained by the Parties.
(vi) Trade Secrets unrelated to the ESP Divestment Business.
(vii) Human resources and any other employee books and records (subject to paragraphs 6 and 9 of these Additional Commitments).
(viii) Items to the extent applicable law prohibit their transfer.
7. If there is any asset or personnel which is not covered by paragraph 3 of this Schedule but which is both used (exclusively or not) in the ESP Divestment Business and necessary for the continued viability and competitiveness of the ESP Divestment Business, that asset or an adequate substitute will be offered to the Purchaser.
Annex 1 .1
List of Scientific Studies
[…]
Annex 1.2
List of Contracts
[…]
Annex 1.3
List of Inventory
[…]
Annex 1.4
List of IPR
[…]
Annex 1.5
List of Key Personnel
[…]
SCHEDULE 2: The Alternative Divestment Business
1. The Alternative Divestment Business consists of:
(i) the EEA/UK-wide rights, title and interests in (including the right to develop, improve, manufacture and commercialise in the EEA/UK) Elanco’s lotilaner oral ectoparasiticide (“Credelio”), lotilaner being an ectoparasiticide in the isoxazoline chemical class; and
(ii) a licence for the EEA/UK rights to improve, manufacture and market the following Elanco […] pipeline products upon completion of clinical development, which will continue to be developed by Elanco until completion of clinical development (together with […] as set out in paragraph 3.1.3.a) below, the “Elanco Pipeline Products”):
a) […];
b) […];
c) […]; and
d) […].
(iii) a licence for the EEA/UK rights to improve, manufacture and market the following Elanco […] pipeline product:
a) […].
2. The clinical development of the Elanco Pipeline Products will be conducted and completed under the supervision of the Purchaser and the Monitoring Trustee (to the extent necessary for the exploitation of Elanco Pipeline Products in the Field under the license in the EEA/UK) in the following manner:
(i) Elanco will devote commercially reasonable efforts (including sufficient resources), in particular in terms of budget and personnel, to conclude the clinical development of the Elanco Pipeline Products without unnecessary delays;
(ii) Purchaser will, acting reasonably and in consultation with Elanco, have the final say on all decisions related to the clinical development of the Elanco Pipeline Products which could materially influence the development and/or market entry of the Elanco Pipeline Products in the EEA/UK; and
(iii) Any disputes between Elanco and Purchaser in relation to the development of the Elanco Pipeline Products shall be handled in accordance with the Fast- Track Dispute Resolution Procedure as set out in Annex 2.7.
3. The Alternative Divestment Business as operated to date is not a stand-alone business, rather it is integrated into a wider operational and commercial organisation; it will therefore be separated from Elanco’s current operations. As regards its legal and functional structure:
(i) The Alternative Divestment Business forms part of Elanco’s companion animal disease prevention segment which encompasses parasiticides and vaccine products for canines and felines, with the Elanco Pipeline Products forming part of Elanco’s R&D function. Please refer to the structure chart provided as Annex 2.1 for further detail in relation to Credelio.
(ii) Credelio has been marketed in the EEA since 2018 and has an established production process. The Elanco Pipeline Products’ clinical trials (listed inter alia in Annex 2.2) are conducted.
(iii) The EEA/UK (a) sales, technical and marketing and (b) manufacturing and quality Personnel for the Alternative Divestment Business are located in […].
4. In accordance with Section B, paragraph 6 of these Additional Commitments, subject to third party consent where relevant, and to the extent in Elanco’s ownership, control or possession at Closing, the Alternative Divestment Business includes, but is not limited to, the following on an EEA/UK-wide basis:
(i) All rights, title and interest in Credelio (including the right to develop, improve, manufacture and commercialise) for use in the Field on an EEA/UK wide basis, and a perpetual, irrevocable, royalty-free exclusive licence for the Purchaser to improve, market and manufacture the Elanco Pipeline Products (including the right to sub-license third parties) in the EEA/UK for use in the Field upon completion of clinical development.
(ii) All Contracts exclusively or primarily related to Credelio and, upon completion of clinical development, the Elanco Pipeline Products, and the benefit of the portion of Contracts which are material or necessary to, but not exclusively or primarily used in, the Alternative Divestment Business, including, but not limited to, the Contracts listed in Annex 2.3. Elanco undertakes to use Best Efforts to obtain all necessary third-party consents where applicable. To the extent any such third-party consent could not be obtained, or any contract could not be otherwise transferred, Elanco will, as appropriate, either: (i) assist the Purchaser and the relevant third party to put in place arrangements to transfer any work product and work in progress, and support the Purchaser to put in place alternative arrangements; or (ii) enter into back-to-back agreements with the Purchaser under the same terms and conditions as the relevant contract for a transitional period of up to […] and up to the duration of the existing relevant contract ([…]).
(iii) Inventory (to the extent that it has not been sold to a third party) including but not limited to the inventory listed in Annex 2.4 of:
a) Credelio in finished form (together with any product packaging materials and any samples) labelled and held for use by the Alternative Divestment Business; and
b) bulk API, work-in-progress, excipients, labelling materials and packaging materials used or held for use exclusively or primarily in the manufacture of Credelio for use by the Alternative Divestment Business.
(iv) All licences, permits, authorisations and registrations (“Regulatory Approvals”) for Credelio and, upon completion of clinical development, the Elanco Pipeline Products (including but not limited to the ones listed at Annex 5), and all other product registrations or regulatory approvals to the extent exclusively or primarily relating to Credelio for use in the Field or, upon completion of clinical development, the Elanco Pipeline Products (as far as is necessary or useful for the improvement, manufacture and marketing of the Elanco Pipeline Products in the Field).
(v) All documentation comprising the Regulatory Approvals (including but limited to the ones identified in Annex 2.5), and to the extent exclusively or primarily related to Credelio for use in the Field and, upon completion of clinical development, the Elanco Pipeline Products (as far as is necessary or useful for the improvement, manufacture and marketing of the Elanco Pipeline Products in the Field):
a) correspondence and reports submitted to or received from relevant regulatory authorities relating to the research, development, manufacture, testing, storage, import, export, use, labelling, distribution, sale, offer for sale, commercialisation, licensing, advertising, marketing and promotion (“exploitation”) of Credelio or the Elanco Pipeline Products;
b) other dossiers or compilations necessary to obtain or maintain any of the product registrations and regulatory approvals in Annex 2.5;
c) literature safety reports and documents relating to good manufacturing practices or issues, animal clinical trials, animal research, including laboratory and target animal research and all veterinary master files contained or referenced to in the product registrations and regulatory approvals in Annex 2.5;
d) environmental and safety documentation; and
e) data that resulted from any of Elanco’s research or development activities related to Credelio or the Elanco Pipeline Products, or data referenced in any documentation and materials referred to above, but in all cases except where explicitly excluded and excluding all intellectual property rights of any third party unless they are necessary for the functioning of the Alternative Divestment Business (in which instance, Elanco shall use reasonable best efforts to worh with the Purchaser to reach an agreement with the entity that is able to grant a licence).
(vi) All relevant reports, databases and analysis (including all technical, clinical and marketing files, protocols, clinical data and studies, reports, plans, books and records) relating exclusively or primarily to Credelio in such form as maintained by Elanco (except where explicitly excluded) and, upon completion of clinical development, the Elanco Pipeline Products (as far as is necessary or useful for the improvement, manufacture and marketing of the Elanco Pipeline Products in the Field).
(vii) All existing marketing materials, plans and forecasts which are specific to Credelio and, upon completion of clinical development, the Elanco Pipeline Products (as far as is necessary or useful for the improvement, manufacture and marketing of the Elanco Pipeline Products in the Field) in such form as maintained by Elanco to the extent they are exclusively or primarily used or held for exclusive or primary use in the Alternative Divestment Business (except where explicitly excluded).
(viii) All intellectual property rights exclusively or primarily relating to Credelio and the Elanco Pipeline Products for use in the Field (including but not limited to the intellectual property rights listed at Annex 2.6) which, broadly, comprise:
a) patents or pending patent applications;
b) domain names;
c) trademarks;
d) product-specific know-how that is identified or identifiable in tangible form that is or has been used for or held for use by or on behalf of Elanco exclusively for or exclusively in connection with the improvement, manufacturing and commercialisation of Credelio for use in the Field or, upon completion of clinical development, the Elanco Pipeline Products (as far as is necessary or useful for the improvement, manufacture and marketing of the Elanco Pipeline Products in the Field);
e) any copyrights or know-how owned by Elanco that comprise or are contained or embodied in any of the marketing materials, plans and forecasts being transferred; and
f) an exclusive, perpetual, irrevocable, royalty-free sub-licensable and transferable licence with respect to necessary unregistered intellectual property and know-how used or held for use by or on behalf of Elanco for or in connection with the conduct of the Divestment Business or the development, improvement, manufacture or commercialisation of Credelio or, upon completion of clinical development, the Elanco Pipeline Products (as far as is necessary or useful for the improvement, manufacture and marketing of the Elanco Pipeline Products in the Field) as applicable).
(ix) the benefit of transitional arrangements to ensure the smooth transfer of the Alternative Divestment Business and to enable the continuity of the clinical trials, including an R&D and regulatory transitional service agreement, transitional manufacturing and supply services, and a technology transfer (including know-how), a transitional agreement for the manufacture and supply of finished products to the Purchaser, to ensure the viability and competitiveness of the Alternative Divestment Business for a transitional period of […] following Closing […].
5. All the Assets, which are necessary to, but not exclusively or primarily related to, the viability and competitiveness of the Alternative Divestment Business and also relate to products/businesses retained by the Elanco, shall also be transferred to the Purchaser but only to the extent they relate to the Alternative Divestment Business (at the Purchaser’s option). In the event that materials to be transferred to the Purchaser pursuant to the Additional Commitments contain information that is confidential to the Elanco/BAH’s retained businesses and not relevant to the Alternative Divestment Business, the information shall be redacted as appropriate.
6. In the event that materials to be transferred contain information that is confidential to the Parties’ retained businesses and not relevant to the Alternative Divestment Business, the information shall be redacted as appropriate.
7. The Alternative Divestment Business shall not include any right, title or interest in or to any of the assets of the Parties or their Affiliated Undertakings other than those specified at paragraph 14 of the Additional Commitments and in paragraph 3 of this Schedule and, for the avoidance of doubt, will not include (inter alia):
(i) Any right to develop, manufacture, market or sell (as applicable) any other product of the Parties other than Credelio or the Elanco Pipeline Products for use in the Field in the EEA/UK.
(ii) Any asset that is not an Asset, and any asset that does not relate to the development, manufacture, marketing and sale of Credelio or the Elanco Pipeline Products.
(iii) The Parties’ names (specifically, Elanco, Bayer or Bayer Animal Health), or any trading name of the Parties, together with all variations thereof and all trademarks, service marks, domain names, trade names, corporate names, logos and other identifiers of source containing, incorporating or associated with any of the foregoing.
(iv) Subject to the Additional Commitments with regard to Personnel (to the extent required under paragraph 18 of these Additional Commitments), employees, real property and tangible personal property of Elanco.
(v) Any shared Contracts retained by Elanco.
(vi) Trade secrets unrelated to the Alternative Divestment Business.
(vii) Human resources and any other employee books and records (subject to paragraph 18 of these Additional Commitments).
(viii) Items to the extent applicable law prohibits their transfer.
8. If there is any asset or personnel which is not covered by paragraph 3 of this Schedule but which is both used (exclusively or not) in the Alternative Divestment Business and necessary for the continued viability and competitiveness of the Alternative Divestment Business, that asset or an adequate substitute will be offered to the Purchaser.
Annex 2.1
Structure Chart for Credelio
[…]
Annex 2.2
List of Scientific Studies
[…]
Annex 2.3
List of Contracts
[…]
Annex 2.4
List of Inventory
[…]
Annex 2.5
List of Regulatory Approvals
[…]
Annex 2.6
List of IPR
[…]
Annex 2.7
Fast-Track Dispute Resolution Procedure
1. Should the Purchaser wish to avail itself of the fast track dispute resolution procedure, it shall inform Elanco and the Monitoring Trustee in writing, setting out in detail the reasons leading it to believe that Elanco is failing to comply with the requirements of the Commitments (the “Request”). Elanco and the Purchaser will use their Best Efforts to resolve all differences of opinion and to settle all disputes that may arise through cooperation and consultation within a reasonable period of time not exceeding fifteen working days (such period being extendable by mutual consent of Elanco and the Purchaser) after receipt of the Request (the “Consultation Phase”).
2. Following receipt of the Request, the Monitoring Trustee shall present its own proposal (the “Trustee Proposal”) for resolving the dispute within eight working days, specifying in writing the action, if any, to be taken by Elanco in order to ensure compliance with the Commitments and be prepared, if requested, to facilitate the settlement of the dispute.
3. Should Elanco and the Purchaser fail to resolve their differences of opinion in the Consultation Phase, the Purchaser may serve a notice (the “Notice”), in the sense of a request for arbitration, to the International Chamber of Commerce (the “Arbitral Institution”), with a copy of such Notice and request for arbitration to Elanco and to the Monitoring Trustee.
4. The Notice shall set out in detail the dispute, difference or claim (the “Dispute”) and shall contain, inter alia, all issues of both fact and law, including any suggestions as to the procedure, and all documents relied upon shall be attached (e.g. documents, agreements, expert reports and witness statements). The Notice shall also contain a detailed description of the suggested action to be undertaken by Elanco and the Trustee Proposal, including a comment as to its appropriateness.
5. Elanco shall, within ten working days from receipt of the Notice, submit its answer (the “Answer”), which shall provide detailed reasons for its conduct and set out, inter alia, all issues of both fact and law, including any suggestions as to the procedure, and all documents relied upon (e.g. documents, agreements, expert reports and witness statements). The Answer shall, if appropriate, also contain a detailed description of the action Elanco proposed to take and the Trustee Proposal (if not already submitted), including a comment as to its appropriateness.
Appointment of the Arbitrators
6. The Arbitral Tribunal shall consist of three persons. The Purchaser and Elanco (the “Parties”) shall each nominate an arbitrator in the Notice and the Answer respectively. The arbitrators nominated by the Parties shall, within five working days of the nomination of the arbitrator in the Answer, nominate the chairman arbitrator, making such nomination known to the Parties and the Arbitral Institution which shall forthwith confirm the appointment of all three arbitrators.
7. Should a Party fail to nominate an arbitrator, or if the two arbitrators fail to agree on the chairman arbitrator, the default appointment(s) shall be made by the Arbitral Institution.
8. The three-person arbitral tribunal are herein referred to as the “Arbitral Tribunal”. Arbitration Procedure
9. The Dispute shall be finally resolved by arbitration under the ICC Rules of Arbitration, with such modifications or adaptions as foreseen herein or necessary under the circumstances (the “Rules”). The arbitration shall be conducted in London (United Kingdom) in the English language.
10. The procedure shall be a fast-track procedure. For this purpose, the Arbitral Tribunal shall shorten all applicable procedural time limits under the Rules as far as admissible and appropriate in the circumstances. The Parties shall consent to the use of e-mail for the exchange of documents.
11. The Arbitral Tribunal shall, as soon as practical after the confirmation of the Arbitral Tribunal, hold an organisational conference to discuss any procedural issues with the Parties. Terms of reference shall be drawn up and signed by the Parties and the Arbitral Tribunal at the organisational meeting or immediately thereafter and a procedural timetable shall be established by the Arbitral Tribunal. An oral hearing shall, as a rule, be established within a month of the confirmation of the Arbitral Tribunal.
12. In order to enable the Arbitral Tribunal to reach a decision, it shall be entitled to request any relevant information from the Parties, to appoint experts and to examine them at the hearing, and to establish facts by all appropriate means. The Arbitral Tribunal is also entitled to ask for assistance by the Monitoring Trustee in all stages of the procedure if the Parties agree.
13. The Arbitral Tribunal shall not disclose confidential information and shall apply the standards attributable to confidential information under the Merger Regulation. The Arbitral Tribunal may take the measures necessary for protecting confidential information in particular by restricting access to confidential information to the Arbitral Tribunal, the Monitoring Trustee, and outside counsel and experts of the Parties.
14. Each Party shall have the burden of proving the facts relied on to support its claim or defence. Involvement of the Commission
15. The Commission shall be allowed and enabled to participate in all stages of the procedure by:
i. Receiving all written submissions made by the Parties;
ii. Receiving all orders and other documents exchanged by the Arbitral Tribunal with the Parties (including Terms of Reference and procedural timetable);
iii. Giving the Commission the opportunity to file amicus curiae briefs; and
iv. Being present at the hearing(s) and being allowed to ask questions to the Parties, witnesses and experts.
16. The Arbitral Tribunal shall forward, or order the Parties to the Arbitration to forward, the documents mentioned to the Commission without delay.
17. In the event of disagreement between the Parties regarding the interpretation of the Commitments, the Arbitral Tribunal may seek the Commission’s interpretation of the Commitment before finding in favour of either Party and shall be bound by the interpretation.
Decisions of the Arbitral Tribunal
18. The Arbitral Tribunal shall decide the Dispute on the basis of the Commitments and the Decision. Issues not covered by the Commitments and the Decision shall be decided (in the order as stated) by reference to the Merger Regulation, EU law and general principles of law common to the legal orders of the Member States without a requirement to apply a particular national system. The Arbitral Tribunal shall take all decisions by majority vote.
19. Upon request of either Party, the Arbitral Tribunal may make a preliminary ruling on the Dispute. The preliminary ruling shall be rendered within one month after confirmation of the Arbitral Tribunal, shall be applicable immediately and, as a rule, remain in force until a final decision is rendered.
20. The Arbitral Tribunal shall, in the preliminary ruling as well as in the final decision, specify the action, if any, to be taken by Elanco or an Affiliated Undertaking in order to comply with the Commitments. The final decision shall be final and binding on the Parties and shall resolve the Dispute submitted to the Arbitral Tribunal. The final decision shall also determine the reimbursement of the costs of the successful Party and the allocation of the arbitration costs.
21. The final decision shall, as a rule, be rendered within three months after the confirmation of the Arbitral Tribunal. The timeframe shall, in any case, be extended by the time the Commission takes to submit an interpretation of the Commitments if asked by the Arbitral Tribunal.
22. The Parties shall propose a non-confidential version of the final decision, without business secrets. The Commission may publish the non-confidential version of the decision.
23. Nothing in the arbitration procedure shall affect the power of the Commission to take decisions in relation to the Commitments in accordance with its powers under the Merger Regulation.
FOOTNOTES :
1 For the avoidance of doubts, the Anticoccidials Divestment Business includes (a) all products in any dosage, strength or formulation containing diclazuril as the sole active pharmaceutical ingredient to the extent being sold by or on behalf of Elanco for the treatment or prevention of coccidial infections in lambs, calves and other ruminants and (b) pipeline products containing diclazuril as the sole active pharmaceutical ingredient to the extent such product has been developed or is being developed by Elanco for the treatment or prevention of coccidial infections in lambs, calves and other ruminants.
2 In the case of: (i) Osurnia, the treatment of otitis externa complicated by susceptible strains of yeast in dogs; […], together being “the relevant fields” for the Otitis Divestment Business.
3 Including the following products: (i) Drontal Tablet Cat 4kg; and (ii) Drontal Tablet Cat 6kg.
4 Including the following products: (i) Drontal Plus Tasty Tablet Dog 10kg; (ii) Drontal Plus Tablet Dog 10kg; and (iii) Drontal Plus Flavor Tablet Dog 10kg; and (iv) Drontal Plus Tasty Tablet Dog 35kg; (v) Drontal Plus Tablet Dog 35kg; and (vi) Drontal Plus Flavour Tablet 35kg.
5 Including the following products: (i) Profender Spot-on 0.35ml Cat > 0.5-2.5kg; (ii) Profender Spoton 0.7ml Cat >2.5-5kg (iii) Profender Spot-on 1.12ml Cat > 5.0-8.0kg; and (iv) Profender Spot-on 14.0ml Cat.
6 Including the following products: Profender Tablet Dog 3kg; and (ii) Profender Tablet Dog 10kg; and (iii) Profender Tablet Dog 30kg.
7 […].
8 This includes Drontal, Drontal Plus, Droncit and Drontal Puppy/Welpan. Drontal and Drontal Plus are also sold as second brands under the brand name Mansonil (in the Netherlands).
9 This includes Profender Tablet, Profender Spot-on, Dronspot and Procox.
10 […].