Commission, August 4, 2015, No M.7559
EUROPEAN COMMISSION
Decision
PFIZER/ HOSPIRA
To the notifying party:
Dear Madam(s) and/or Sir(s),
Subject: Case M.7559 - PFIZER/ HOSPIRA
Commission decision pursuant to Article 6(1)(b) in conjunction with Article 6(2) of Council Regulation No 139/20041 and Article 57 of the Agreement on the European Economic Area2
(1) On 15 June 2015, the European Commission received notification of a proposed concentration pursuant to Article 4 of the Merger Regulation by which the undertaking Pfizer Inc. of the United States (“Pfizer” or “the Notifying Party”) acquires within the meaning of Article 3(1)(b) of the Merger Regulation control of the whole of the undertaking Hospira Inc. (“Hospira”), of the United States.
(2) Pfizer and Hospira are collectively referred to as “the Parties”.
I. THE PARTIES AND THE OPERATION
(3) Pfizer is a global research based biomedical and pharmaceutical company active in discovering, developing, manufacturing, marketing, and selling innovative medicines for humans.
(4) Hospira is a global provider of injectable drugs and infusion technologies, with a broad portfolio of generic, branded and biosimilar medicines for humans.
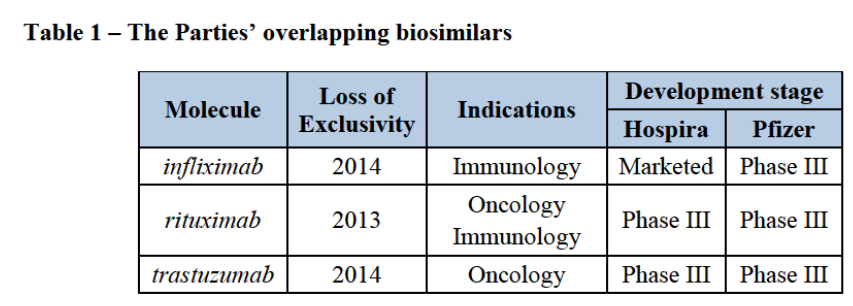
(5) On 5 February 2015, Pfizer and Hospira entered into an Agreement by which Hospira will become a wholly owned subsidiary of Pfizer. Pfizer will therefore acquire sole control over Hospira within the meaning of Article 3(1)(b) of the Merger Regulation.
II. UNION DIMENSION
(6) The undertakings concerned have a combined aggregate world-wide turnover of more than EUR 5 000 million3 (Pfizer: EUR 37 339 million, Hospira EUR 3 360 million). Each of them has an EU-wide turnover in excess of EUR 250 million (Pfizer: EUR [5000-10000] million, Hospira: EUR [250-500] million), and they do not achieve more than two-thirds of their aggregate EU-wide turnover within one and the same Member State. The notified operation therefore has Union dimension within the meaning of Article 1(2) of the Merger Regulation.
III. RELEVANT MARKETS AND COMPETITIVE ASSESSMENT
(7) The Parties’ activities overlap with respect to human health pharmaceuticals, in two main areas: (i) biosimilars and (ii) speciality injectable pharmaceuticals (“sterile injectables”).
(8) The Parties both supply Active Pharmaceutical Ingredients ("API") to third parties and undertake contract manufacturing for third parties. However, there is no vertical relationship between the Parties where the downstream share (finished dose-level) is above 30%, irrespective of the upstream share (API-level), and vice-versa.
III.1. Biosimilars
III.1.1. Introduction
(9) Biological medicines have an active substance made by or derived from living organisms. Biosimilars aim to have the same therapeutic mechanism as original patented medicines, but, unlike small molecule generics, are not exact copies of the originator drugs. According to the guidelines of the European Medicines Agency (“EMA”), in order to obtain a marketing authorisation for a biosimilar, its manufacturer needs to demonstrate similarity (in terms of quality, safety and efficacy) to a reference biological product. The clinical trials may be performed for only one indication for which the originator drug had been approved, and on that basis the approval may be granted for all the indications of the originator drug (the “extrapolation principle”).
(10) The biosimilar segment of the pharmaceutical sector is relatively new. The first-generation biosimilars launched in Europe since 2006 have been relative simple proteins such as erythropoietin. The second-generation biosimilars, which are at the core of this transaction, are more complex monoclonal antibodies (“mAb”).4 The first mAb biosimilar, a copy of J&J's Remicade (infliximab), was approved in Europe in 2013.
(11) Given that biological drugs are also some of the most expensive therapies available, the entry of biosimilars is expected to allow wider access by patients to biological drugs. Furthermore, as the entry of the first biosimilar products have led to price decreases compared to the originator product, there are significant expectations across the EEA that biosimilars will be an important factor in relieving the financial pressure on healthcare systems.
(12) The Notifying Party submits that the development of a biosimilar can be divided in a number of steps:
a. Analysis and characterisation of the reference biologic product – Such an analysis focuses on both the structural attributes and the functions of the reference biological product and is carried out on several samples from multiple lots of the reference product over the lifetime of that product. This analysis should lead to a comprehensive physicochemical and biological characterisation of the reference biological product.
b. Non-clinical studies and development of the manufacturing process – In this phase, manufacturers carry out in-vitro studies to assess any difference between the biosimilar product being developed and the reference biological product. In addition, a manufacturing process that guarantees consistent quality of the biosimilar molecule is developed.
c. Phase I (pharmacokinetics-PK/pharmacodynamics-PD) clinical trials – PK studies appraise the way the body affects the biosimilar product, e.g. in terms of absorption, while PD studies appraise the way the product affects the body, e.g. through its mechanism of action. The purpose is to show comparability with the reference biological product, in particular as regards safety. Phase I trials are normally carried out in healthy volunteers.
d. Phase III (efficacy) clinical trials – As phase II clinical trials are not required for biosimilars, the final step of the development of a biosimilar consist of the demonstration of comparable clinical efficacy of the biosimilar and the reference biological product. Phase III trials are carried out for one approved therapeutic indication of the reference product, as data can be extrapolated to other indications if non-clinical and PK/PD studies demonstrate biosimilarity.
(13) The Parties’ activities overlap in the development of mAb biosimilars, for which they have three common molecules marketed or in clinical trials (phase I or phase III).
(14) While Pfizer develops and markets its mAb biosimilars in-house, Hospira markets (or will market, once new biosimilars have been developed) biosimilars developed by Celltrion of South Korea. This cooperation envisages [...].
III.1.2. Market definition
III.1.2.1. Product market
(15) When defining relevant markets in past decisions dealing with pharmaceutical products, the Commission has established a number of principles.5 In those decisions it noted that medicines may be subdivided into therapeutic classes by reference to the "Anatomical Therapeutic Classification" (ATC), devised by the European Pharmaceutical Marketing Research Association (EphMRA) and maintained by EphMRA and Intercontinental Medical Statistics (IMS).6
(16) The ATC system is a hierarchical and coded four-level system which classifies medicinal products according to their indication, therapeutic use, composition and mode of action. In the first and broadest level (ATC1), medicinal products are divided into the 16 anatomical main groups. The second level (ATC2) is either a pharmacological or therapeutic group. The third level (ATC3) further groups medicinal products by their specific therapeutic indications, i.e. their intended use. Finally, the ATC4 level is the most detailed one (not available for all ATC3) and refers for instance to the mode of action (e.g. distinction of some ATC3 classes into topical and systemic depending on their way of action) or any other subdivision of the group.
(17) In its past merger decisions in the pharmaceutical sector, the Commission has referred to the third level (ATC3) as the starting point for defining the relevant product market. However, in a number of cases, the Commission found that the ATC3 level classification did not yield the appropriate market definition within the meaning of the Commission Notice on the Definition of the Relevant Market. As a result, where appropriate and based on the factual evidence collected during the market investigation, the Commission has defined the relevant product market at the ATC4 level or at a level of molecule or a group of molecules that are considered interchangeable so as to exercise competitive pressure on one another.7 The overlap in therapeutic uses does not necessarily imply any particular economic substitution patterns between products.
(18) In its previous decisions, the Commission recognised that the market for biosimilars should be treated differently than the market for small molecule generics.8 However, the Commission did not previously assess the markets for infliximab, rituximab and trastuzumab biosimilars.
III.1.2.1.a. infliximab
(19) infliximab is an anti-TNF (anti-tumor necrosis factor) agent used in autoimmune diseases (such as rheumatoid arthritis). The originator product, Remicade, was developed by Johnson & Johnson9 and is marketed by MSD (Merck, Sharp & Dohme, hereinafter referred to as “Merck”) in Europe. Its annual 2014 sales exceeded USD 10 billion globally (#3 best-selling pharmaceutical). infliximab is currently the only mAb for which a biosimilar version (by Hospira and Celltrion) has been approved by the European Commission based on the opinion of EMA (in 2013), and which has already been prescribed to patients for example in Norway, the UK, Hungary and Finland.
(20) There are currently a number of anti-TNF agents approved by the EMA, including monoclonal antibodies such as adalimumab (Humira), certolizumab pegol (Cimzia), infliximab (Remicade) and golimumab (Simponi), as well as etanercept (Enbrel), a fusion protein. The Notifying Party submits that some anti-TNF agents may substitute for others in certain indications, while different ones, such as infliximab, may not.
(21) As to the substitutability between infliximab and other anti-TNF agents, the majority of respondents to the market investigation from the demand side indicated that they purchase infliximab pharmaceuticals (Remicade and the biosimilars) through competitive tenders,10 which are typically organised per molecule (for instance infliximab).11 The majority of leading medical professionals in the field (i.e. Key Opinion Leaders) that responded to the Commission's investigation confirmed that none of their patients on other anti-TNF agents (such as adalimumab or etanercept) had been switched to a biosimilar version of infliximab in the past 12 months, while for the rest of Key Opinion Leaders only a very small minority of such patients were switched to a biosimilar version of infliximab (the maximum amount being 5%).12
(22) The Notifying Party's views are further confirmed by one key competitor noting in particular that it "observed only little impact of infliximab biosimilars on other TNF-inhibitors [anti-TNF agents]". Indeed, as opposed to other anti-TNF agents which have a subcutaneous formulation, infliximab has to be administered intravenously and "normally patients used to subcutaneous medicines would not switch to an intravenous one".13 Accordingly, only a minority of respondents from the supply side expect the introduction of a biosimilar anti-TNF agent to exert downward pressure on prices of the originator products of other molecules sharing the same indications with the reference product.14
(23) In light of the above, the Commission takes the view that infliximab belongs to a separate product market from other anti-TNF agents.
(24) As to the substitutability between Remicade and infliximab biosimilars, it should be noted that both are approved by the EMA for the same indications (by virtue of the principle of extrapolation), and that they compete for the same tenders related to infliximab. In practice, in a number of situations (more than half of the analysed tenders),15 Remicade is co-awarded the tender along with one infliximab biosimilar.
(25) In light of the above, for the purpose of the present Decision, as regards the molecule concerned the relevant product market should comprise infliximab pharmaceuticals, including both the originator infliximab (Remicade) and infliximab biosimilars.
III.1.2.1.b. rituximab
(26) rituximab is a humanised monoclonal antibody that binds to the CD20 protein, used in autoimmune diseases (such as rheumatoid arthritis) and oncology (such as for certain types of leukaemia and lymphoma). The originator product, MabThera, was developed by Roche. Its annual 2014 sales were USD 7.4 billion globally (#6 best-selling pharmaceutical).
(27) For the purpose of the present Decision, it is not necessary to delineate the precise product market definition in relation to rituximab, as no competitive concerns arise on a hypothetical market for rituximab pharmaceuticals, as well as under any wider market definition.
III.1.2.1.c. trastuzumab
(28) trastuzumab is a humanised monoclonal antibody that binds to the HER2 protein, used primarily for treatment of early and metastatic breast cancer. The originator product, Herceptin, was developed by Roche. Its annual 2014 sales were USD 6.7 billion globally (#9 best-selling pharmaceutical).
(29) For the purpose of the present Decision, it is not necessary to delineate the precise product market definition in relation to trastuzumab, as no competitive concerns arise on a hypothetical market for trastuzumab pharmaceuticals, as well as under any wider market definition.
III.1.2.2. Geographic market
(30) The Commission has consistently considered that the markets for finished dose pharmaceutical products are national.16 For pipeline products, the Commission previously considered that the geographic scope of the relevant market is at least EEA-wide.17
(31) There appears to be no reason to depart from this conclusion in the present case. Since all overlaps related to biosimilars concern pipeline products, the relevant geographic scope for the assessment of biosimilars in the present Transaction is at least EEA-wide.
III.1.3. Competitive assessment
III.1.3.1. The competitive dynamics of biosimilar markets differ from those of generics
(32) As part of its analysis in the present case, the Commission considered, on the basis of the evidence of this case, the differences between competition in markets for biologic drugs on the one hand and competition in markets for small-molecule drugs and generics versions of such drugs on the other hand. In particular, the Commission considered (as part of its analysis of the transaction) the differences between generics and biosimilars in terms of product differentiation, interchangeability, regulatory framework, cost structure and barriers to entry, all of which are key for the competitive assessment of the proposed transaction.
(33) Small-molecule originator products and generic products based on the same active principle can generally be considered homogeneous products that compete mainly on price, especially in the case of hospital drugs procured through competitive tenders. While manufacturers, especially originators, may try to differentiate their product as a strategy to soften the intensity of price competition, measures have been taken in European countries to constrain their ability to do so. Such measures include for example incentives for physicians to write generic prescriptions (i.e. financial incentives based on targets of generic prescriptions), generic substitution by the pharmacist regardless of the brand name used by the prescriber, incentives for pharmacists to dispense the cheapest available versions of a given medicine (e.g. regressive margins, obligation to stock and dispense the cheapest generic), and incentives for patients to ask for the cheapest available versions of their medicines (i.e. differentiated patient co-payments based on relative prices). These measures are designed to encourage generic uptake by making prescribers, pharmacists and patients more sensitive to price differences. Evidence shows that they can be effective at fostering price competition.18
(34) Biological products are intrinsically differentiated due to their complex molecular structure. As discussed above, no biosimilar product is identical either to the original biologic product on which it is based, or to any other biosimilar product. Despite their similarity stemming from pre-clinical bioequivalence studies, regulatory authorities consider their differences sufficiently significant to request clinical trials to prove the clinical equivalence between every new biosimilar and the original biologic product on which it is based, for at least one major indication. In particular, the EMA establishes for every family of biological medicines19 a specific set of clinical evidence required for the regulatory approval of new biosimilars. As a consequence, not only the regulatory approval and the clinical evidence available for biosimilar products differ from that of generic products, but such clinical evidence also differs between different families of biological medicines.
(35) Originator biological products and biosimilar products are therefore not identical in terms of molecular structure, and moreover they are distinct in terms of clinical evidence available on their efficacy and safety. According to the EMA, "it is for each national (health) authority to decide on the interchangeability and substitution of the originator and the biosimilar based on the scientific evidence submitted to the EMA and other available data and information (...) the EMA is not involved in this second decision-making process at national level and there is no common European legal framework regarding interchangeability".20 Accordingly, physicians and pharmacists do not necessarily consider originator and biosimilars based on the same biologic molecule to be fully interchangeable. This applies equally to the interchangeability amongst biosimilars based on the same molecule, which are also not identical in their chemical structure and clinical evidence. In practice, the situation varies from Member State to Member State and from indication to indication, depending in particular on the perception of the clinical risks for the patient associated to product switches. Competition between original biologic products and biosimilar products, as well as between any pair of biosimilar products, is therefore characterised by the limited degree of substitutability for patients already undergoing treatment. Hence biosimilars differ from small-molecule generics, which being chemically identical show a much higher degree of substitutability for all patients. Biosimilars of the same molecule, by contrast, show lower degree of substitutability for patients already in treatment, while still showing a high degree of substitutability for new patients.
(36) This lower degree of substitutability for a segment of patients has an impact both on commercial strategies and market outcomes. Original biologic products have the chance of building a stock of potentially locked-in patients during the period of market exclusivity, especially for chronic treatments (e.g. for immunological disorders). Upon loss of market exclusivity, if the perceived clinical risks of switching are not negligible, new biosimilar entrants for a given monoclonal antibody are less likely to attract patients that have already initiated treatment with the original product. In this case, biosimilar competition takes place mainly for newly diagnosed patients that are about to initiate treatment so have not received a therapeutic drug yet. The commercial strategy of new biosimilar entrants can consist both of price undercutting and product differentiation, for instance through investment on the development of superior clinical evidence. The originator firm may have an incentive to exploit its stock of locked-in patients through price premiums, while still competing for treatment-naive patients via product differentiation, leveraging on brand and product recognition acquired during the period of market exclusivity.
(37) This results in the segmentation of patients by product and implies that not the entire market is contestable at any point in time. In terms of market outcomes, it results in slower market penetration by biosimilar products, compared to what is typically observed in markets for small-molecule generics, even though price discounts offered by biosimilar manufacturers can be of similar magnitude. The market investigation has in fact shown that payers typically need to procure both the original product and biosimilar products, to guarantee treatment continuity to all their patients. Hence, price differentials do not automatically translate into shifts in market shares in favour of biosimilars.
(38) To the extent that new biosimilar entrants manage to attract treatment-naive patients and build their own stock of locked-in patients, they face the trade-off between continuing to price low to attract additional patients and increasing prices to exploit their stock of locked-in patients. Given their inability to price discriminate between new and locked-in patients, this trade-off weakens their incentives to aggressively compete in price for new patients. Therefore, while biosimilar competitors have an incentive to price low at entry, such incentive diminishes as they establish their position in the market, resulting in less intense price competition.
III.1.3.2. infliximab
(39) The competitive landscape in relation to infliximab pharmaceuticals in the EEA is as follows:
a. the originator drug Remicade (off-patent since 2014) is exclusively marketed by Merck in the EEA;
b. there is one approved biosimilar, marketed co-exclusively between Celltrion (the developer of the product, marketing it through distributors under the brand name Remsima) and Hospira (marketing it under the brand name Inflectra) based on a duplicate marketing authorisation;
c. two companies have infliximab biosimilars in phase III clinical trials: Pfizer and Samsung Bioepis of South Korea; and
d. a number of other companies (such as Epirus, Amgen/Actavis and Dr. Reddy's) are at earlier stages in the development of infliximab biosimilars.
(40) The Notifying Party submits that the Transaction will not have a significant impact on competition with respect to infliximab. Post-transaction Remicade would remain on the market as an active competitor, there would be two infliximab biosimilars on the market (one from Celltrion, and one from the merged entity), and at least one short term potential entrant, who already is seeking approval (Samsung Bioepis). According to the Notifying Party, Samsung Bioepis appears as a credible potential competitor that will be the third to enter the market, well before the next potential entrant (Epirus or Pfizer). Given that Pfizer is not the next entrant on the market (and in fact is over [...] years away from possibly entering the market) and that the originator biologic remains a strong competitor, the Notifying Party submits that it is not the only credible competitor to Hospira and Celltrion. In addition, existing competition between Celltrion and Hospira, as well as potential competition from the next entrants, would preclude the emergence of any anti-competitive effects on the infliximab market. According to the Notifying Party, by the time that Pfizer's product will be ready for market launch there will be four if not five infliximab on the market from Merck (Remicade), Hospira, Celltrion, Samsung Bioepis and potentially Epirus.
Nature of competition in the market
(41) The market for infliximab is characterised by three types of competitive interactions, each of a different nature: (i) the competition between the originator biologic and biosimilars; (ii) the actual price competition between Hospira and Celltrion, which both market the same biosimilar in the EEA, and (iii) the future competition between three differentiated biosimilar products (Hospira/Celltrion’s, Samsung Bioepis’ and Pfizer’s).
(42) Regarding competition between the originator biologic and biosimilars, this competition manifests itself mainly in a one-way price constraint form biosimilars on the originator drug. Indeed, the market entry of Inflectra and Remsima at a lower price than Remicade led to Merck reducing the price of Remicade in order to mitigate the loss of market share.21 However, given that it is still early days for biosimilars and given that biosimilars are only "similar" to the originator, the price levels of Remicade generally remain higher than those of Inflectra and Remsima.22 This is consistent with Merck having an incentive to maintain a relatively higher price to benefit from locked-in patients. The fact that Remicade is co-awarded the tender along with one biosimilar in a number of situations illustrates that it has differentiating features that still allow Merck to charge a premium compared to the biosimilar suppliers and that biosimilars are not fully replacing the originator. This is consistent with the need of guaranteeing treatment continuity to patients already initiated on Remicade, avoiding any clinical risks associated to changing the prescribed product. Customers and Key Opinion Leaders confirmed that not all infliximab patients, in particular patients already on Remicade, are prescribed Remsima or Inflectra,23 which highlights the absence of full interchangeability of biosimilars, as a result of which the hospitals continue purchasing Remicade in spite of higher prices. The availability of Remicade is also of importance for patients with indications for which biosimilars were not tested in clinical trials (in relation to which physicians tend to be more sceptical). One competitor indeed highlighted that it "has noticed a certain reluctance of gastroenterologists to prescribe biosimilars and is aware of an ECCO [European Crohn´s and Colitis Organisation] position paper published 18 months ago which raises doubts on extrapolation of biosimilars in gastroenterological diseases without significant clinical trials",24 while a Key Opinion Leader confirmed that "once studies on switching patients to biosimilars have been published, also gastroenterologists' reluctance will be countered. Current reluctance mainly exists due to biosimilar infliximab having been tested only for RA [Rheumatoid Arthritis], while the application for the gastroenterological indications was only extrapolated from the efficacy in RA".25 This highlights that the competition between infliximab biosimilars and the originator may be weaker for extrapolated indications such as gastroenterological diseases.
(43) Therefore, Remicade can be considered a distant competitor to infliximab biosimilars.
(44) Regarding actual competition between Hospira and Celltrion, respondents to the market investigation indicated that they are aware that Inflectra and Remsima are the same product, and that they generally select only one of the two (typically the cheapest).26 This is confirmed by the analysis of tender data across the EEA which shows that Inflectra and Remsima almost never win the same tender (whereas, as explained above, Remicade and one biosimilar often do).27 This is a unique feature of the market, specific to infliximab, where two commercially distinct biosimilar products are in fact identical in their molecular structure and clinical evidence. They compete as homogeneous products, with brand differentiation being consciously disregarded by customers, leading to intense downward pricing pressure in infliximab tenders since the entry of Hospira and Celltrion's biosimilars in 2013.
(45) Finally, the importance of future competition between differentiated biosimilar products (Hospira/Celltrion’s, Samsung Bioepis’ and Pfizer’s) also stems from the lack of full interchangeability between Remicade and infliximab biosimilars, as well as between the infliximab biosimilars. Provided that they all reach the market, each of them will show some degree of differentiation from each other, with its own clinical evidence being evaluated by the EMA for the purpose of regulatory approval. Indeed, the potential for market success of each of these biosimilars will depend on the degree of prescribers’ acceptance across therapeutic indications, which itself depends in particular on the robustness of the clinical data (as well as real-world experience) provided by each manufacturer. Therefore, contrary to generics, there is room for differentiation strategies and non-price competition between these three distinct biosimilars, with the likely result of less intense price competition than what has been observed so far between Hospira and Celltrion. In such circumstances, it is less likely that few biosimilar competitors can deliver significant price reductions than typically observed for small-molecule generics. The importance of the number of differentiated biosimilars for price competition is illustrated by the internal pricing forecasts of the Parties’, [...].28
(46) Given the limited experience so far with biosimilar products in general, it is difficult to assess at this stage the potential commercial success of each of these products (Hospira/Celltrion's, Samsung Bioepis', Pfizer's and the next entrants). However, the market investigation provided insights on the expectations of market participants (including the Parties).
Pfizer and Hospira/Celltrion are considered to be strong players in the field of biosimilars
(47) The majority of physicians and Key Opinion Leaders confirmed that the reputation of an individual biosimilar supplier plays a role (half of them "to some extent", the other half "to a large extent").29 In this context, Pfizer and Hospira are generally considered in the market to be very strong players in the field of biosimilars. Accordingly, they are often quoted by competitors, customers, physicians and Key Opinion Leaders alike as two of the five most important manufacturers/developers of biosimilars).30
(48) In particular, despite limited information available so far about the clinical evidence of Pfizer's biosimilars such as infliximab (none of which having being approved yet), customers perceive Pfizer's products as a strong potential competitor with the following strengths "brand name", "experience in biosimilars", "great manufacturer of biologicals", "experienced in rheumatology" and "marketing infrastructure".31 Such advantages seem consistent with Pfizer's internal documents showing [...].32
(49) Celltrion, on the other hand, is still working, in particular through partnerships, to gain market presence and reputation in the EEA. Leveraging on Hospira’s expertise and reputation in the EEA was indeed a key rationale for Celltrion to enter into a co-exclusive marketing agreement with Hospira for infliximab (and a number of other biosimilars). As evidenced by Celltrion, "for distributing its biosimilars, Celltrion Healthcare pursues a "dual channel" strategy, whereby each European country has two appointed distributors. For a number of biosimilars, including infliximab, Celltrion Healthcare manages one channel (and uses local distributors), and the other channel is Hospira".33 This lack of reputation is evidenced by customers which rarely identify Celltrion as one of the five most important manufacturers/developers of biosimilars.34
A future entrant still facing challenges: Samsung Bioepis
(50) Apart from Pfizer, Samsung Bioepis is the only other company with an infliximab biosimilar in phase III clinical trials. It submitted a marketing authorisation application to the EMA in March 2015.35 While it cannot be excluded that Samsung Bioepis' infliximab biosimilar will be marketed in Europe before Pfizer's, internal documents from the Parties suggest that [...].36 Furthermore, Samsung Bioepis does not have a marketing presence in the EEA and is partnering with Merck and Biogen Idec for the commercialisation of its biosimilars. Samsung Bioepis has a partnership with Merck to market its biosimilars in a number of countries (in particular in Europe). However, for infliximab, because Merck is marketing the originator drug Remicade in Europe, it was necessary for Samsung Bioepis to find another partner (it entered into a partnership with Biogen Idec in December 2013). [...].37
The other competitors are not expected to become a competitive constraint in the EEA in the foreseeable future
(51) Finally, the other competitors identified by the Notifying Party such as Epirus, Amgen/Actavis and Dr. Reddy's, do not have an infliximab biosimilar in advanced stages of development, and are therefore not expected to become a competitive constraint in the EEA in the foreseeable future.
(52) While Epirus has an infliximab biosimilar approved in India,38 it cannot readily use the same data to seek a marketing authorisation in the EEA. Indeed, it "plans to initiate a global [phase III] clinical program in late 2015/early 2016".39 This is confirmed by the EMA, which indicated that "side-by-side analysis of the biosimilar product (from commercial scale and site) with the EEA authorised reference product must be conducted". A comparative trial with a non-EEA authorised reference medicinal product would suppose that such product be approved by "a regulatory authority with similar scientific and regulatory standards as EMA (e.g. ICH countries)". 40,41
(53) Finally, Dr. Reddy's did not indicate having an infliximab biosimilar in its portfolio,42 and the Notifying Party highlights in its recent internal documents that [...].43
Barriers to entry
(54) Clinical trials required to provide the necessary evidence for regulatory approval are costly both in terms of financial resources and time, and require also certain R&D capabilities. Consequently, barriers to entry for biosimilars are typically higher than for generics and the pool of potential entrants upon patent expiry is typically smaller for biological drugs than for small-molecule chemical drugs. According to the Notifying Party, the development of a new biosimilar product takes on average between six and eight years of development, to which should be added on average one and a half year for regulatory approval.44 Small-molecule generic products, on the contrary, can be prepared for launch much faster.
(55) This has at least two consequences for the assessment of potential competition in markets of biologic drugs. On the one hand, the higher barriers to entry are likely to result in fewer successful entrants in the market. The larger sunk costs an entrant needs to incur (which range from USD [100-200] million to USD [300-400] million according to the Notifying Party) weaken the competitive constraints imposed by potential entrants. On the other hand, the length of the clinical development implies that the set of potential entrants can be easily identified, as the stage of development of each potential entrant can be observed early on.
Conclusion on the competitive landscape
(56) In light of the above, the Commission takes the view that the competitive landscape for the infliximab market in the foreseeable future is composed of one biosimilar co-marketed by Hospira and Celltrion (which are subject to intense price competition from each other), and two future differentiated biosimilar competitors from Samsung Bioepis and Pfizer, while the originator infliximab is a distant competitor to its biosimilars.
Effects of the proposed Transaction
(57) The proposed Transaction will therefore bring two infliximab biosimilars under the same ownership (Inflectra and Pfizer’s pipeline biosimilar). This situation will reduce
the Parties’ pre-merger incentives to compete in one of the two alternatives ways:
a) Pfizer will either delay or discontinue its pipeline biosimilar in order to focus on Inflectra, leading to the net loss of one of only three differentiated biosimilars marketed or in advanced stages of development. Pfizer’s internal documents [...];45 or
b) [Analysis of elements that could lead Pfizer to],46 hand back Hospira’s Inflectra rights to Celltrion, leading to the loss of price competition between Hospira and Celltrion.
(58) On the one hand, the reduced incentives to continue developing Pfizer's biosimilar translate into a lessening of innovation competition. Indeed, the delay or even cancellation of Pfizer's development program would deprive patients from timely access to a differentiated product that is currently assessed positively by market participants on the basis of available clinical evidence. It would lessen price competition for new patients in a market where, due to the presence of switching costs, every new entrant has an incentive to behave as an important competitive force , pricing low to gain market share.
(59) On the other hand, the return of Hospira's commercial rights to Inflectra to Celltrion would eliminate the particularly intense price competition currently observed between Hospira and Celltrion, which is a specific feature of these two biosimilar products due to the fact that they are the very same compound, and are perceived as homogeneous products fully interchangeable in clinical practice. The likely result of this would be higher prices at least for Hospira's and Celltrion's customers.
(60) The reduction of the Parties' pre-merger incentives to compete on the infliximab market was confirmed by competitors responding to the market investigation which indicated that a biosimilar company does not have any incentive to pursue the development of a pipeline biosimilar if it already markets a biosimilar for the same molecule.47 Customers and Key Opinion Leaders expressed concerns regarding the Transaction's effects on infliximab, leading either to “reduced price cuts” or to “Pfizer’s infliximab, which is a very good molecule, not becoming available”.48
(61) Therefore, based on all available evidence, the Commission concludes that the analysis of the proposed Transaction indicates that it is likely to significantly impede effective competition by either eliminating an important future competitive constrain (three-to-two differentiated biosimilars) or reducing the competitive pressure on the remaining competitors (loss of price competition), and thus raises serious doubts as to its compatibility with the internal market in relation to infliximab.
III.1.3.3. rituximab
(62) The Notifying Party submits that the competitive landscape in relation to rituximab pharmaceuticals in the EEA is as follows:
a. the originator drug MabThera is marketed by Roche in the EEA;
b. there is no approved biosimilar;
c. seven companies have differentiated rituximab biosimilars in phase III clinical trials: each of the Parties (Hospira in partnership with Celltrion), Sandoz (Novartis), Boehringer Ingelheim, Mabion, Amgen/Actavis and Merck Serono/Dr Reddy's; and
d. a number of other companies (such as Merck) are at earlier stages in the development of rituximab biosimilars.
(63) The market investigation broadly confirmed the competitive landscape in relation to rituximab biosimilars.49
(64) Furthermore, Pfizer's internal documents highlighted its intention to [...].50 Contrary to infliximab, which is already marketed by Hospira and Celltrion's distributors, Celltrion's rituximab biosimilar is still in development and estimated to enter the EEA in [...], and it is more likely than not that Celltrion would be able to find an alternative distributor in Europe (or distribute the product itself) should it obtain marketing authorisation.
(65) Excluding Mabion,51 none of the six companies appear to have a significant competitive advantage with respect to the development of their differentiated rituximab biosimilar. Internal documents from Pfizer highlight that [...].52 In any event, a number of future competitors of the merged entity will remain after the Transaction.
(66) Therefore, based on all available evidence, the Commission concludes that the proposed Transaction does not raise serious doubts as to its compatibility with the internal market in relation to rituximab.
III.1.3.4. trastuzumab
(67) The Notifying Party submits that the competitive landscape in relation to rituximab pharmaceuticals in the EEA is as follows:
a. the originator drug Herceptin is marketed by Roche in the EEA;
b. there is no approved biosimilar;
c. five companies have differentiated trastuzumab biosimilars in phase III clinical trials: each of the Parties (Hospira in partnership with Celltrion), Amgen/Actavis, Mylan/Biocon and Samsung Bioepis; and
d. a number of other companies (such as BioXpress and Nippon Kayaku) are at earlier stages in the development of trastuzumab biosimilars.
(68) The market investigation broadly confirmed the competitive landscape in relation to trastuzumab biosimilars.53
(69) Furthermore, Pfizer's internal documents highlighted [...].54 Contrary to infliximab, which is already marketed by Hospira and Celltrion's distributors, Celltrion's trastuzumab biosimilar is still in development and estimated to enter the EEA in [...], and it is more likely than not that Celltrion would be able to find an alternative distributor in Europe (or distribute the product itself) should it obtain marketing authorisation.
(70) None of the five companies appear to have a significant competitive advantage with respect to the development of their differentiated trastuzumab biosimilar. Internal documents from Pfizer highlight that [...].55 In any event, a number of future competitors of the merged entity will remain after the Transaction.
(71) Therefore, based on all available evidence, the Commission concludes that the proposed Transaction does not raise serious doubts as to its compatibility with the internal market in relation to trastuzumab.
III.2. Sterile injectables
III.2.1. General characteristics of sterile injectables markets
(72) The Parties are both active in the supply of generic sterile injectables. Even though Pfizer focuses its business activities on the development of new drugs, and is thus mainly active in originator drugs, it also markets generic drugs. Upon patent expiry (or shortly before), Pfizer's products are transferred to its Global Established Pharmaceuticals business ("GEP"). GEP is responsible for the commercialisation of the products that have lost or are about to lose patent protection and of generics. Approximately [...]% of Hospira's total sales in the EEA are accounted for by the sale of its generic sterile injectables.
(73) The term "sterile injectables" refers to a large group of medicines that are administered by the same route, i.e. with a hollow needle which is pierced through the skin. As such, sterile injectables do not correspond to a single relevant market within the meaning of the EU competition rules, but encompass a heterogeneous set of entirely different molecules which are not substitutable to one another. However, some characteristics relevant for the competitive assessment of the present merger apply to all the sterile injectables markets. These characteristics are described below.
Barriers to entry and to expansion
(74) The Notifying Party submits that the markets for sterile injectables within the EEA are fragmented with a significant number of suppliers present across different geographic areas. According to the Notifying Party there are no suppliers that hold a strong position in a particular molecule across different EEA countries. In that regard, the Notifying Party notes that geographic barriers to entry are low, and that a supplier active in one country could easily enter another geographic market (and even more so if it already provides other products in that geographic market) should prices increase post-Transaction.
(75) As regards the barriers to entry in sterile injectables, the Commission retained that the average duration to develop a generic sterile injectable is of four to six years, before the product reached the market. This implies two stages: development and the regulatory approval. The development of a generic drug typically takes from ten months (for companies specialising in generics) up to four and a half years depending on the complexity and sophistication of the originator drug, as well as on the process, formulation and method of use patents held by the originator. Each presentation will need to be developed, tested and (new) release methods or (new) analytics methods might have to be developed. Production batches would need to be made in order to see if the product under normal production capacity still tests out and meets specifications.
(76) Once the development of the drug has been finalised, a certain time is required for the administrative procedure. Development and administrative procedure may add up (depending on the product at hand) to more than one year and it might in individual cases take several years depending on the product. In addition to that, the Notifying Party submits that in general, API manufacturing leading times are of the order of [...] months, and fill and finish times are approximately [...] months. They are followed by freight and European re-testing (where appropriate) and release. This adds another [...] months to the time from the decision to manufacture a product to putting it on the market.
(77) As regards the barriers to enter into a geographically neighbouring market, the market investigation has revealed that once a supplier already markets a generic sterile injectable in certain countries, entry is only possible if a marketing authorisation has been granted. The supplier would also need a commercial organisation in that particular country.56 Even in case a marketing authorisation has already been obtained for a country where the supplier previously was not active, suppliers would still need to have a commercial organisation in order to be able to start selling and become a credible competitor in a new country.57
(78) In addition to the existence of a marketing authorisation and a local commercial organisation, suppliers take into consideration other factors as well when deciding to enter a new country, such as potential market size, prices of their competitors, tendering system, number of competitors.58
(79) In sum, entry can play a role in exerting competitive pressure for the supply of sterile injectables. However, barriers to entry cannot be qualified as low in an abstract and general way. The competitive constraint that entry can exert must be assessed on a case by case basis, taking into account in particular whether potential entrants supply the product in question in neighbouring countries, can scale up production to expand to a new geographic market, possess a marketing authorisation and a commercial organisation in the country concerned, and whether an hypothetical price increase would provide the financial incentive to enter such market.
Criteria for the selection of suppliers
(80) The Notifying Party submits that for generic sterile injectables, competition in most markets primarily takes place through tenders (with few exceptions), organised by individual hospitals, groups of hospitals or public authorities, depending on the country. Whilst in some countries tenders are organized for a number of products, suppliers are selected separately for each molecule (and sometimes even separately for particular presentations). Other criteria are qualitative and include, inter alia, type of packaging, packaging differentiation, shelf life, and ability to supply during the weekend or in the evenings.
(81) Price is an important criterion for the allocation of tenders. Each hospital chooses the exact importance attributed to price, but it usually accounts for an important part of the decision in the tender, which are in the vast majority of cases organised by the hospitals or purchasing groups.59 The replies to the market investigation have shown that indeed price is the main criterion taken into account by customers when choosing their sterile injectable suppliers, together with security of supply and range of strengths/concentrations.60 Other criteria mentioned as important, but not essential, were range of vials, capacity and long shelf life.
Switching
(82) For all the markets analysed in this case, the market investigation showed it is relatively easy for customers to change their supplier at the end of the contract.61 Moreover, the contracts with suppliers do not usually contain commitments to purchase minimum quantities of a given product.62 There are thus no legal barriers to switch.
(83) As regards the duration of the contracts, customers mentioned that in most of the cases these are between one and two years, sometimes also up to one year,63 therefore showing that in a relative short period of time they could have the possibility of switching their sterile injectable suppliers.
III.2.1.1. General approach to the product market definition
Therapeutic classes versus molecule approach
(84) As is explained above in paragraph 15 the Commission has in its previous decisions in the pharmaceutical sector referred to the third level (ATC3) as the starting point for defining the relevant product market.64 However, in a number of cases, the Commission found that the ATC3 level classification did not yield the appropriate market definition within the meaning of the Commission Notice on the Definition of the Relevant Market. As a result, where appropriate and based on the factual evidence collected during the market investigation, the Commission has defined the relevant product market at the ATC4 level or at a level of molecule or a group of molecules that are considered interchangeable so as to exercise competitive pressure on one another
Galenic form
(85) As the Commission has acknowledged in its previous decisions65 medicines are differentiated not only by their active ingredient(s), but also, in particular, as recognized by the European regulatory framework for medicines for human use, by their dosage, pharmaceutical form and route of administration and this may limit their substitutability.66
(86) For the purpose of the present decision the question of whether the relevant markets would comprise also other galenic forms than injectables can be left open, as the competitive assessment of individual markets would not change irrespective of galenic form concerned. Alternative candidate product market definitions would lead to fewer overlaps and less affected markets. Consequently, the Commission has focused its assessment of the competitive effects for injectable products at the molecule level.
Generics versus originator drugs
(87) Generics are in general less expensive, bioequivalent versions of originator drugs. In regulatory approval procedures, a generic drug manufacturer has to demonstrate that the generic version of the originator drug has the same qualitative and quantitative composition in terms of active substance and the same pharmaceutical form and is bioequivalent to the originator drug.
(88) In previous cases, the market investigation has often suggested that there may be differences in the demand for originator versus generic drugs, even when they are bioequivalent. This is the case more particularly in countries where the penetration of generics is lower and the importance of the brand is higher. On the other hand, the growing trend of regulatory pushes in some countries in favour of generics, such as for instance, mandatory substitution at the pharmacy level, mandatory INN prescription etc. increases the generic substitution. Finally, generic versions of originator medicines are specifically designed to compete with those medicines and normally represent the closest substitute to them.
(89) Also during the present market investigation, the large majority of respondents expressed that they thought of generics as a full alternative to originator products.
(90) Therefore, in line with the precedents and for the purposes of the present decision, the Commission considers that in relation to the overlapping molecules the product market includes both generic and originator versions.
Prescribed drugs versus over the counter ("OTC") drugs
(91) In certain cases, pharmaceutical products may be further subdivided into various segments on the basis of a variety of criteria, and in particular demand-related criteria. The Commission has in the past67 defined separate markets for medicines which can be issued only on prescription and those, which can be sold over the counter (OTC). Medical indications, side effects, legal framework, distribution and marketing tend to differ between these drug categories, even if the active ingredients are sometimes identical.
(92) This case only involves prescription drugs. Therefore the question as to whether separate relevant product markets for prescribed drugs and OTC drugs should be defined for the purpose of the present decision can be left open.
III.2.1.2. Relevant geographic market
(93) The Notifying Party has, in line with the Commissions prior decisions, submitted an overview of its activities on a country-by-country basis. The Commission has consistently considered that the markets for finished dose pharmaceutical products68 were national in scope. The market investigation in this case did not provide any indications that such market definition should be revisited, in particular in view of the purchasing practices, the national regulatory and reimbursement schemes and the fact that competition between pharmaceutical firms still predominantly takes place at a national level
(94) Therefore, for the purpose of this decision the Commission concludes that the scope of the geographic markets in relation to all assessed sterile injectable markets is national.
III.2.1.3. General approach to the competitive assessment
(95) In line with the past decisions,69 given a large number of affected markets in pharmaceutical mergers (numerous product and geographic markets), the Commission has applied a system of filters aimed at determining the group of markets where concerns are most likely and on which its focused its analysis.
(96) Based on this filter, pharmaceutical markets are analysed according to three categories:
* Group 1: where the Parties’ combined market share exceeds 35% and the increment exceeds 1%;
* Group 2: where the Parties’ combined market share exceeds 35% but the increment is less than 1%;
* Group 3: The Parties’ combined market share is between 20% and 35%.
(97) The Notifying Party has based its analysis mainly on the IMS data, measured by Standard Units (e.g., 1 vial), submitting that market shares by volume are the most relevant in this case. However, the Commission observed that these products are sold in different dosages (vials), which are all equally calculated as one unit, no matter the number or quantity that they comprise. Therefore, the Commission considers that the market shares in value are the most relevant in this case and show a better image of the market positioning of the different competitors.
(98) Based on this methodology, the Commission has identified all Group 1 markets under the narrowest plausible market definition, i.e. at the molecule level, in the following marketed molecules: carboplatin, cisplatin, cytarabine, epirubicin, fluorouracil, irinotecan, nitroglycerin, piperacillin/tazobactam, vancomycin and vincristine, a total number of 30 overlaps. Besides these ones, a total of 13 Group 2 and Group 3 affected markets were also examined.
(99) The markets which fall within Group 2 because the Parties' combined market shares exceeded 35% and the increment does not exceed 1% are the following: docetaxel in Spain, fluorouracil in Denmark, irinotecan in Spain, methotrexate in Italy and piperacillin/tazobactam in Belgium. Given the small increment, the fact that no concerns have been raised by customers or competitors in the course of the market investigation, and that respondents did not highlight any particular advantage of the Parties on these markets, the Commission concludes that the Transaction does not raise serious doubts as to its compatibility with the internal market in respect of these possible markets.
(100) The markets which fall within Group 3 because the Parties' combined market shares were between 20% and 35% are the following: calcium folinate in Norway, cisatracurium besilate in Spain, cisplatin in Italy, methotrexate in Portugal, piperacillin/tazobactam in Finland and the Netherlands, and vancomycin in the Netherlands and Spain. Given the moderate combined market shares in these markets, the fact that the combined entity will still face significant competition from a number of other competitors such as Teva, Intas, Fresenius, Mylan, Aurobindo or Novartis, and that no substantiated concerns have been raised by customers or competitors in the course of the market investigation, the Commission concludes that the Transaction does not raise serious doubts as to its compatibility with the internal market in respect of these possible markets.
(101) The remainder of the decision deals with the markets that fall within Group 1.
III.2.1.4. Product specific assessment
III.2.1.4.a. Carboplatin
Product market definition
(102) Carboplatin is a platinum compound used as a chemotherapy drug in the treatment of a number of different cancers (ovarian cancer, small cell lung cancer, head and neck cancer). The first platinum based drug that was developed was cisplatin. Carboplatin is a second generation drug that is administered intravenously as a short term infusion. Generic versions of carboplatin became available from 2004 onwards. Carboplatin belongs to the ATC3 class of platinum antineoplastics.
(103) The Commission, in its decision Teva/Barr, defined the relevant product market for carboplatin at the molecule level as the indications were only partially overlapping with other molecules in the same ATC class.70 Hospitals indicated that switching between molecules would be limited during the treatment of the serious illnesses that these medicines are aimed for. In another decision, Teva/Ratiopharm, the Commission left the market definition open, since under any alternative product market definition (ATC 3 class, ATC4 class or defined on the level of molecule) the transaction would not give rise to competition concerns.71
(104) The Notifying Party submits that in the present case the assessment should be carried out at the molecule level, since the substitutability between different types of platinum antineoplastics is limited and tendering procedures are very often organised on the level of the molecule only. The Notifying Party also submits that the product market definition could be ultimately left open, since the transaction would not significantly impede effective competition regardless of the product market definition.
(105) In the market investigation, the limited substitutability for carboplatin was confirmed. The indications for which carboplatin is prescribed only partially overlap with those of other molecules in the same ATC class. Moreover purchasing takes place on the level of the molecule, predominantly through molecule-specific tenders.
(106) On the basis of the above, the Commission concludes that for the purpose of the present decision the relevant product market should comprise sterile injectable carboplatin.
Competitive assessment
(107) In carboplatin, the Transaction give raise to two affected Group 1 markets in Belgium and Italy.
Belgium
(108) According to the Notifying Party, the market size of the carboplatin in Belgium was EUR [1-5] million in 2014. The combined market shares of the Parties reached [5060]% in value in 2014, with an increment of [10-20]% brought by Pfizer.
(109) The Notifying Party submits that on the Belgian market there are a number of other competitors, the main ones being Teva and Accord/Intas. The Notifying Party considers Teva as the strongest competitor with a market share of [30-40]%. In addition, Accord/Intas has entered the market and achieved a market share of almost [0-5]% in 2014, according to the Notifying Party.72 The Notifying Party expects that Accord/Intas' market share will grow, because the Notifying Party expects a growing number of tenders in the future due to a regulatory change in Belgium.73
(110) The Notifying Party submits that Hospira's market share has been significantly higher mainly due to the fact that it was the first generic on the market. However, between 2012 and 2014 its market share declined (in volume). The Notifying Party also submits that Pfizer's market share is relatively low, due to the fact that it does not have the [...] vials in its portfolio. According to the Notifying Party this weakens its competitive position, because hospitals typically choose one supplier for the whole range of dosages. The Notifying Party considers that request for this particular dosage will increase in the future and argues that Pfizer's competitive force on the market may therefore be decreasing.
(111) An analysis of the market share evolution over the last three years shows a relatively stable market size. As regards the positioning of the Parties, market shares in value which, as explained above provide a more appropriate picture of the situation in this case, show a slight increase in Pfizer's and a slight decrease in Hospira's position.
(112) The Commission also investigated whether there are enough alternative suppliers to the merged entity, taking into account that switching suppliers in this market appears to be relatively easy. The replies received during the market investigation have confirmed Teva as being the main challenger.74 However, even though the other competitor Accord/Intas confirmed its presence, it is considering withdrawal due to the fact that "the procurement decisions are opaque with a high degree of protectionism, local distribution is expensive and the unpredictable claw back tax regime".75 No other competitor amongst the respondent ones has confirmed presence, or intention to enter this market in near future.76 77
(113) The range of vials sold in Belgium is: 5ml, 15 ml, 45ml and 60ml, with a concentration of 10mg/ml.78 Competitors answering to questions posed during the market investigation did not identify the range of vials/products offered as a particular advantage of either Pfizer or Hospira. Nor did the market investigation confirm the decreasing importance of Pfizer for the Belgium market for carboplatin as a result of the current inability to provide the [...] vials. Moreover, an overview of the sales for the last five years did not reveal a decrease in volume, which would have been a plausible consequence of the preference of hospitals to buy bigger vials, as the Notifying Party claims. There are no other indications to show that Pfizer's lack of the [...] vial in Belgium represents a competitive disadvantage.
(114) Some customers in Belgium responding to the market investigation mentioned that they would expect lesser competition and price increases as a result of the transaction. Suppliers mentioned that Pfizer and Hospira's current main competitive advantage in carboplatin in Belgium is their pricing.79
(115) Customers in Belgium also pointed out that they feared that supply security would become an increasingly worrying issue. One important customer explained that with such a small number of credible players on the market it would be more difficult to negotiate guarantees on security of supply. The transaction would thus, according to those customers affect competition negatively.80
(116) According to well-established case law, very large market shares — 50 % or more — may in themselves be evidence of the existence of a dominant market position.81 It has been pointed out that in Belgium tendering procedures are not yet commonly adopted for sterile injectables.82 Therefore the market shares in Belgium reflect a strong market position that would stem from the transaction. The transaction would remove a competitive restraint from the market, in particular as Teva appears to be the only credible competitor to the merged entity. Moreover, a majority of Belgian based customers that responded to the questions posed to them during the market investigation expressed their substantiated concerns over the change in market structure as to the security of supply and pricing in the market concerned.
(117) Therefore, the analysis of the proposed concentration suggests that it would likely significantly impede effective competition, in the Belgian market for carboplatin, in particular as a result of the creation or strengthening of a dominant position. The Commission concludes that the transaction therefore raises serious doubts as to its compatibility with the internal market in relation to carboplatin in Belgium.
Italy
(118) According to the Notifying Party, its combined market shares only reached the level of a Group 1 market in 2012, with a share of [40-50]%. Since then, both Pfizer's and Hospira's market shares have been constantly decreasing to [30-40]% combined market share in 2013.. The combined market shares of the Parties reached only [10-20]% in value in 2014, with an increment of [5-10]% brought by Hospira. The market size of carboplatin in Italy was EUR [10-20] million in 2014.
(119) The other competitors on the market are Teva, Accord/Intas and Sun Pharma. The positioning on the market for carboplatin in Italy of all these suppliers has been strengthened since 2012. Teva increased its market share form [50-60]% in 2012 to [6070]% in 2014, confirming its market leader position and Intas from [0-5]% in 2012 to [5-10]% in 2014. Finally, Sun Pharma, a new entrant in 2013, reached [10-20]% market share in the next year. Post Transaction, the merged entity is expected to continue facing strong competition from a range first and foremost Teva, but also from Sun Pharma and Accord/Intas. These competitors are likely to increase their supply substantially should prices of the merged entity increase after the Transaction.83
(120) The range of vials sold in Italy is: 5ml, 15 ml, 45ml and 60ml, with a concentration of 10mg/ml.84 Pfizer is offering 5, 15 and 45ml vials and Hospira the whole range. Both Accord and Teva are offering the full range of vial as well.
(121) The Commission, taking into consideration all of the above, including the results of the market investigation, concludes that the transaction does not give rise to serious doubts as to its compatibility with the internal market in relation to carboplatin in Italy, because the proposed concentration would not significantly impede effective competition, in the Italian market for carboplatin.
III.2.1.4.b. Cisplatin
Product market definition
(122) Cisplatin is the predecessor of carboplatin. It is used for the treatment of cancers including sarcomas, a number of carcinomas, lymphomas, bladder cancer, cervical cancer and germ cell tumours.
(123) The Commission previously investigated cisplatin in the same cases as carboplatin. The Commission did not need to conclude on the market definition in one case and defined the relevant product market at the molecule level in the other case.85
(124) The Notifying Party tables the same arguments to assess the cisplatin market on the molecule level, but suggests leaving open the relevant product market definition.86
(125) In the market investigation the limited substitutability between cisplatin and carboplatin was confirmed. The indications for which cisplatin is prescribed are only partially overlapping with those for other molecules in the same ATC class, including carboplatin. Moreover purchasing takes place at the level of the molecule, predominantly through molecule-specific tenders.
(126) On the basis of the above, the Commission concludes that for the purpose of the present decision the relevant product market should comprise sterile injectable cisplatin.
Competitive assessment
(127) In cisplatin, the Transaction give raise to two affected Group 1 markets in Finland and Greece.
(128) In Finland, the market size of the cisplatin was only EUR [0-1] million in 2014 and, according to the Notifying Party, has constantly decreased since 2012. The combined market shares of the Parties reached [40-50]% in value in 2014, with a small increment of only [0-5]% brought by Pfizer. Accord/Intas is also present in Finland and has reinforced its market position over the last two years from only [10-20]% in 2013 to over [50-60]% in 2014.87
(129) Pfizer's market share has been declining since 2012, when it had a market share of [2030]%. Pfizer offers cisplatin in vials of 100, 10 and 50ml. Hospira has a narrower range of only 100 and 50 ml vials. The main challenger Accord/Intas seems to have the broadest range of vials, comprising 10, 25, 50 and 100 ml. All suppliers offer the same concentration of 1mg/ml.
(130) In Greece, the market size of cisplatin was only EUR [0-1] million in 2014 according to the Notifying Party. The combined market shares of the Parties reached [50-60]% in value in 2014, with a small increment of [0-5]% brought by Pfizer. The other competitors are Teva ([40-50]%) and Medicus (less than [0-5]%). Novartis was also present until 2013, with a market share of [10-20]% in 2012 and [0-5]% in 2013.
(131) The Notifying Party submits that they base their submission on IMS data, which for Greece only tracks sales to pharmacies. Hospira [...]88 [...] sells cisplatin in Greece in a 100 mg vial. Hospira is unable to participate in tenders where the two most widely used vials (10mg and 50mg) are required. Thus, in a recent national tender, initiated in December 2014, Hospira was not able to submit an offer as the specifications included 10mg and 50mg vials only. Equally, in a tender organised in January 2015, Hospira was unable to submit any offer as it requested 50mg vials only.
(132) Nevertheless, Hospira's share has increased from [10-20]% to [50-60]% over the same period, showing significant variability in market shares. Pfizer's market share has drastically decreased since 2012 from [50-60]% to only [0-05]% in 2014. The fluctuations in market shares reflect the fact that Greece is a tendering market with a very small market size. In such a market variations in market shares occur rather often and market shares may overstate the actual market power.
(133) During the market investigation customers did not express concerns regarding the competitive effects of the proposed transaction. The competitors to the merged entity such as Teva, Medicus and Novartis are likely to increase their supply substantially should prices of the merged entity increase after the Transaction.89These competitors would constrain the merged entity sufficiently, should the merged entity try to increase its prices.
(134) In view of the above, the Commission concludes that the transaction does not give rise to serious doubts as to its compatibility with the internal market in relation to cisplatin in Finland and Greece, because the proposed concentration would not significantly impede effective competition, in the Finnish and Greek markets for cisplatin.
III.2.1.4.c. Cytarabine
Product market definition
(135) Cytarabine belongs to the ATC3 class of antimetabolites, including agents which prevent cells from multiplying. Cytarabine is a chemotherapy agent used for different types of cancer treatment affecting blood cells (leukaemia) and is also used to treat meningeal leukemia and lymphoma. Its patent protection ended in the 1980s.
(136) There are no prior Commission decisions involving cytarabine.
(137) The Notifying Party submits that the relevant product market should be defined at the molecule level, mainly because of the limited substitutability with other agents that prevent cells from multiplying for cancer treatment and because of the fact that tendering procedures are very often geared towards cytarabine only. The Notifying Party argues that the relevant product market definition can in any event be left open as no competition concerns would raise as a result of the transaction.
(138) In the present market investigation the limited substitutability for cytarabine was confirmed. The indications for which cytarabine is prescribed are only partially overlapping with those of other molecules in the same ATC class. Moreover purchasing takes place on the level of the molecule, predominantly through molecule-specific tenders.
(139) On the basis of the above, the Commission concludes that for the purpose of the present decision the relevant product market should comprise sterile injectable cytarabine.
Competitive assessment
(140) Cytarabine was originally launched by Upjohn (i.e. Pfizer) in the 1970s, under the brand name Cytostar. Pfizer acquired cytarabine following the Pfizer/Pharmacia deal in 2003.90 From 2005 onwards, generic versions of the drug became available, first one being Hospira's (Cytarabine Hospira).
(141) The Transaction gives rise to five affected Group 1 markets for cytarabine in Belgium, Denmark, Italy, Portugal and Sweden.
Belgium, Italy, Portugal and Sweden
(142) In Belgium, the market size of the cytarabine was EUR [0-1] million in 2014, according to the Notifying Party. The combined market shares of the Parties reached [60-70]% in value in 2014, with an increment of [10-20]% brought by Hospira.
(143) The only other competitor on the market is Mundipharma International, an UK based innovator drugs developer, with a market share of [30-40]% in 2014. The Notifying Party submits that IMS data is incomplete as, according to their own competitive intelligence, other competitors are present on the market, namely Accord/Intas and Fresenius. As Accord/Intas won recent tenders, the Parties' combined market share is – according to the Notifying party – likely to be significantly lower. Moreover, the Notifying Party estimates that, the number of tenders in the future will increase in 2015, therefore the level of competition will increase as well. In addition, the Parties note that Strides holds a marketing authorisation in Belgium. While it is currently not active, the Parties believe that it could start supplying cytarabine effectively and immediately, should it take a business decision to do so.
(144) The market investigation did not confirm the market structure advocated by the parties, revealing that Mundipharma is the only established competitor. Recently Accord/Intas also entered the market. Furthermore, the offers of those competitors do not seem to equal Pfizer's range of products, especially as regards the concentration offered.
(145) As regards the products offered, both Pfizer and Hospira offer the 100mg/ml concentration in 10ml and 20ml vials. On top of this Pfizer also has a concentration of 20mg/ml (which it offers in 5ml and 25ml vials). According to the Notifying Party, hospitals typically require a full range of products from the same supplier, and therefore Hospira would be at a competitive disadvantage. Accord/Intas only recently started supplying the 100mg/ml strength in vials of 1, 5, 10, 20, 40 and 50, while Mundipharma offers only 50 mg suspension for injection 10mg/ml in 5 ml vials.
(146) According to well-established case law, very large market shares — 50% or more — may themselves be evidence of the existence of a dominant market position.91 The market shares are rather stable and consistently high. The market investigation did not confirm the presence of a number of other credible competitors that are likely to increase their supply substantially, should prices of the merged entity increase after the Transaction.92
(147) Therefore, the Commission considers that the analysis of the proposed transaction would likely significantly impede effective competition, in the market for cytarabine in Belgium. The Commission concludes therefore that the transaction raises serious doubts as to its compatibility with the internal market for cytarabine in Belgium.
(148) In Italy, the market size for cytarabine was EUR [1-5] million in 2014, according to the Notifying Party. The combined market shares of the Parties were [40-50]%, with an increment of [5-10]% brought about by Hospira. In 2012 the combined market share was [50-60]% and in 2013 it was [70-80]%. The main competitor is Mundipharma International with a market share of [40-50]% in 2014.93 The other competitors are Fresenius and new entrant Accord/Intas, both with [0-5]% market share in 2014. Fresenius has not registered sales since 2013, when it had a market share lower than [05]%.
(149) Apart from the merged entity, Mundipharma International seems to offer as well a suspension cytarabine presentation in a concentration of 10mg/ml94 and they seem to have a well-established positioning on the Italian market, therefore proving that the dry presentation form is not at all a disadvantage in this market. However, MundiPharma International had problems with the supply, due to product recall caused by quality issues at third party manufacturer plant identified by the regulatory authorities.95
(150) As regards the presence of the other competitors, the market investigation did not confirm their positioning according to IMS data. Mundipharma International remains the main competitor of the merged entity, but with a much weaker position that the one indicated by the Notifying Party. The absence of a number of other credible competitors that may be likely to increase their supply substantially, should prices of the merged entity increase after the Transaction, together with the high combined market shares would lead the Commission to conclude that the proposed transaction raises serious doubts as to its compatibility with the internal market in relation to cytarabine in Italy.
(151) In Portugal, the market size for cytarabine was EUR [0-1] million in 2014, according to the Notifying Party. The combined market shares of the Parties reached almost [90100]% in 2014, with an increment of [5-10]% brought about by Pfizer. The Notifying Party only mentions Lab Unknown96 as being present with a market share of less than [0-5]% in 2014.
(152) The Notifying Party submits that, [...].97 The Notifying party argued that [...]However, Pfizer failed to submit credible evidence [...].
(153) Because of the very high combined market share customers would have no alternative to turn to.98 The market investigation did not confirm any potential entrants from neighbouring markets, nor indicated that such entry has occurred in the recent past. This would enable the merging parties to increase prices to the detriment of the customers in Portugal.
(154) In view of the above the Commission concludes that the proposed transaction would significantly impede effective competition, in the market for cytarabine in Portugal. The Commission concludes therefore that the transaction raises serious doubts as to its compatibility with the internal market.
(155) In Sweden, the size of the market for cytarabine was EUR [0-1] million in 2014, according to the Notifying Party. The combined market shares of the Parties reached almost [60-70]% in 2014, with an increment of [20-30]%. The other competitors present are SkyePharma, a generic drug delivery specialist, with a market share of [20-30]% in 2014, Fresenius with [10-20]% and new entrant Accord/Intas with [0-05]%.
(156) The Notifying Party submits that in addition, two other companies submitted offers in recent tenders, namely Farmaplus and PharmaCoDane, thereby increasing the level of competition, despite the fact that they did not win any contracts. The market investigation did however not confirm the existence of any other additional competitor except for Accord/Intas, and to a lesser extent Fresenius and SkyePharma. Accord/Intas has had a small market share and SkyePharma has consistently had a very minor share for the last 5 years, in spite of the fact that the Swedish market can be characterised as a tendering market. In the markets described above, the proposed transaction would lead to very high market shares. The market investigation showed that some competitors were very small and remained very small throughout the years, thereby indicating that they have not been able to effectively win tenders. In these cases the Commission cannot dispel serious doubts purely based on the fact that the markets involved have some tendering characteristics. On the contrary, the fact that the proposed transaction would result in high and sometimes very high market shares would illustrate the strengthening of a dominant position and the loss of competitive pressure. In this situation, the competitors that are consistently small would not be able to constrain the merged entity.
(157) Moreover, a majority of customers in these markets that responded to the market investigation questionnaires expressed their concerns about the proposed transaction, fearing in particular the loss of competition to the detriment of their ability to negotiate the supply of cyterabine. Customers also pointed out that too few (credible) competitors in a tender would lead to suboptimal outcomes of the tendering procedure. Some of these customers described that the prices of cytarabine may increase as a result of the proposed transaction.99
(158) Taking into consideration all of the above, the Commission considers that the proposed concentration would significantly impede effective competition, in the market for cytarabine in Sweden, in particular as a result of the creation or strengthening of a dominant position. The Commission concludes therefore that on this market, the transaction raises serious doubts as to its compatibility with the internal market.
Denmark
(159) According to the Notifying Party, the market size of the cytarabine in Denmark was only EUR [0-1] million in 2014. Denmark was a Group 1 market in 2012 and 2013, where the combined market share of the Parties reached [60-70]% and [60-70]% respectively. In 2014, there was a significant drop in both Parties' position, therefore reaching only [10-20]% in value in 2014, with an increment of [5-10]% brought by Pfizer.
(160) The other competitors on the market are Pacira Pharmaceuticals, a US-based generic company, with a constant strong presence during the last three years of more than [3040]% of the market. The other main challenger proves to be Fresenius. Since its entry in 2013, it immediately succeeded to gain a [5-10]% market share in the same year and a much stronger one in 2014. Therefore, after merger, there will be enough alternative suppliers that could
(161) The Notifying Party submits that in Denmark there is one national tender covering all the public hospitals in the country which is held on a yearly basis organised by Amgros I/S (the hospital purchasing agency). The Notifying Party estimates that the Parties combined market share will [...].
(162) All competitors, including the Parties, offer the 100mg/ml concentration in 10ml and 20ml dosages. In addition, Pfizer offers a 20mg/ml in 5ml dosage which other suppliers do not offer. However, the Notifying Party submits that this presentation was not included in the tender specifications and is sold solely outside the tender processes.
(163) An analysis of the market share fluctuations shows that in 2014 the drop in sales of both Parties was compensated by the increase in sales of both of their rivals, mainly to the recent entry of Fresenius. Accord/Intas has also confirmed its recent entry. However, they have not recorded any sales in 2014. Given the recent drop in the Parties' combined market share due to successful entrance of further competitors, the Commission considers that, post-merger, there will be enough alternative suppliers that could constraint the merged entity.
(164) The Commission, taking into consideration all of the above, including the results of the market investigation, concludes that the transaction does not give rise to serious doubts as to its compatibility with the internal market in relation to cytarabine in Denmark, because the proposed concentration would not significantly impede effective competition.
III.2.1.4.d. Epirubicin
Product market definition
(165) Epirubicin is a medicine used for the treatment of various cancers, such as ovarian cancer, stomach cancer, lung cancer, bowel cancer and myeloma. It is also used to treat some types of lymphoma and leukemia. It belongs to the ATC3 class of antineoplastic antibiotics and has been off patent since 2007.
(166) The Commission has investigated a merger involving epirubicin in one case, Novartis/Ebewe, without needing to conclude on the product market definition.100
(167) The Notifying Party submits that the market investigation in the present case should be carried out at the level of the molecule, but that the relevant product market definition ultimately can be left open.
(168) The market investigation showed however limited substitutability for epirubicin. Moreover purchasing takes place on the level of the molecule, predominantly through molecule-specific tenders.
(169) On the basis of the above, the Commission concludes that for the purpose of the present decision the relevant product market should comprise sterile injectable epirubicin.
Competitive assessment
(170) In the market for epirubicin, the Transaction give raise to five affected Group 1 markets in Austria, Belgium, Italy, the Netherlands and Spain.
(171) In Austria, the market size of the epirubicin was EUR [5-10] million in 2014, according to the Notifying Party. The combined market shares of the Parties reached [50-60]% in 2014, with an increment of [5-10]% brought by Hospira. In 2012 and 2013 the combined market shares were [50-60]% and [70-80]% respectively. The other present competitors are Actavis, with a market share of [20-30]% in 2014, Novartis with [1020]% and a new entrant Accord/Intas with [5-10]% in 2014.
(172) In Belgium, the market size of epirubicin was EUR [1-5] million in 2014. The combined market shares of the Parties reached [70-80]% in 2014, with an increment of [0-5]% brought by Hospira. In 2012 and 2013 the combined market shares were [70-80]% and [70-80]% respectively. The other present competitors are Teva, with a market share of [10-20]%, Aurobindo with [10-20]% and a new entrant Accord/Intas with [0-05]% in 2014. The Notifying Party submits that Novartis is also present with a market share of less than [0-5]%.
(173) In Italy, the market size of the epirubicin was EUR [10-15] million in 2014. In 2012 and 2013 the combined market shares were [80-90]% and [80-90]% respectively. The combined market shares of the Parties reached [60-70]% in 2014, with an increment of [20-30]% brought by Hospira. The other present competitors are Teva, with a market share of [20-30]% and the new entrant Accord/Intas with [10-20]% in 2014. The Notifying Party submits that Novartis is also present with a market share of less than [05]%.
(174) In Netherlands the market size of the epirubicin was EUR [1-5] million in 2014. The combined market shares of the Parties reached [40-50]% in 2014, with an increment of [10-20]% brought by Hospira. In 2012 and 2013 the combined market shares were [6070]% and [40-50]% respectively. The other present competitors are the new entrant Accord/Intas with [50-60]% and Teva with a smaller market share of[5-10]% in 2014. The Notifying Party submits that Novartis was also present in 2013with a market share of less than [0-5]%.
(175) In Spain the market size of the epirubicin was EUR [1-5] million in 2014. The combined market shares of the Parties reached [40-50]% in 2014, with an increment of [10-20]% brought by Pfizer. In 2012 and 2013 the combined market shares were [7080]% and [70-80]% respectively. The other present competitors are Teva with [3040]%, Accord/Intas with [10-20]% market share, Aurobindo with [5-10]% and Ferrer with less than [0-5]% in 2014.
(176) However, in all these markets, the market investigation did not confirm the positioning or presence of all these alternative suppliers, revealing a weaker positioning of some of them. In addition, there seem to be capacity constraints for some of the competitors101 in a market that security of supply is seen as a very important aspect by customers.102 Moreover, no other supplier has confirmed the intention to enter any of these markets.103 Therefore, after the merger, there will be only a reduced number of alternative suppliers.
(177) During the market investigation a minority of customers in the markets concerned expressed concerns about the proposed transaction, fearing a loss of competition. These customers indicated that the possibility to negotiate good prices would significantly decrease as a result of the transaction.104
(178) The Notifying Party submits that [...].105 The Notifying Party argued that [...] However, Pfizer failed to submit credible evidence [...].
(179) The Commission, taking into consideration the concerns expressed by customers, the high combined market shares and the lack of competitive constraints by competitors placed on them and especially the fact that the Notifying Party was not able to proof [...], concludes that the proposed concentration would significantly impede effective competition, in the markets for epirubicin in Austria, Belgium, Italy, the Netherlands and Spain, in particular as a result of the creation or strengthening of a dominant position. Therefore, the Commission concludes that on these markets the transaction gives rise to serious doubts as to its compatibility with the internal market.
III.2.1.4.e. Fluorouracil
Product market definition
(180) Fluorouracil is also an agent that prevents cells from multiplying and is thus used for the treatment of various cancers such as colorectal cancer and breast cancer.
(181) The Commission has previously considered that fluorouracil should be considered a separate relevant product market because it only partially overlaps with other agents within the same class and because of the buying patterns of the hospitals.106
(182) The Notifying Party proposes that the assessment should be at the level of the molecule, but argue that the relevant product market definition could be left open, since according to the Parties the transaction would not lead to competitive concerns.
(183) The market investigation yielded some evidence pointing towards a relevant product market definition at the molecule level, for the same reasons as the other sterile injectable products that were investigated. However, in the present case the relevant product market definition can be left open as the transaction would not raise serious doubts as to a significant lessening of competition, regardless of the exact relevant market delineation.
Competitive assessment
(184) In fluoruracil, the Transaction give raise only to one affected Group 1 market in Norway.
(185) According to the Notifying Party, the market size of the fluoruracil in Norway was only EUR [0-1] million in 2014. The combined market shares of the Parties reached [6070]% in 2014, with an increment of [5-10]% brought by Hospira. Accord/Intas, a new entrant, is also present in this market with a considerable market share of [30-40]%.
(186) Hospira's market share has been declining since 2012 from [20-30]% to [5-10]% in 2014. The Notifying Party argues that [...].107
(187) Pfizer is offering 50mg/ml strength in 10 and 20ml vials, while Hospira is offering the same strength in 10, 50 and 100ml vials. Accord/Intas, the main challenger, is offering a much wider range of vials, covering vials from 5 to 100ml. Therefore, it was very easy for Accord/Intas to gain rapidly market share and a good position on the market because of its wide range of products. Also, from the analysis of the market share evolution over the last three years it can be easily observed that the loss of sale of both Parties has been reflected in the increase in the market position of Accord/Intas. The latter, together with the size of this market, suggest that the competitive dynamics between the existent participants were strong enough to give raise to a very different and changing positioning of the suppliers in this market.
(188) The market investigation showed that there will be sufficient competitive pressure from potential entrants in the future to constrain higher pricing by the merged entity. The market investigation indicated that this is indeed the case. 108 Fluoruracil is produced by a number of competitors who have marketing authorisations in other countries which would have no capacity constraint to serve the Norwegian market and which offer other drugs in Norway, thus have sales organisation there. For fluoruracil in Norway the market investigation hence did confirm the existence of a number of credible competitors that are likely to increase their supply substantially, should prices of the merged entity increase after the Transaction.109
(189) The Commission, taking into consideration all of the above, including the results of the market investigation, concludes that the transaction does not give rise to serious doubts as to its compatibility with the internal market. because the proposed concentration would not significantly impede effective competition, in the market for fluorouracil in Norway.
III.2.1.4.f. Irinotecan
Product market definition
(190) Irinotecan is an alkaloid molecule derived from plants. It interferes with the enzymes that control the manipulation of DNA structure that is necessary for cell replication. Blocking these enzymes leads to breaks in the DNA structure which in turn leads to cell death. It is primarily used for colorectal cancer. Irinotecan has been off patent since 2009.
(191) In a previous decision, Sanofi-Synthelabo/Aventis, the Commission concluded that there was a separate relevant product market for pharmaceutical products for the treatment of colorectal cancer.110 However, this decision did not specifically refer to irinotecan.
(192) For the purpose of the present case the Notifying Party argues that the assessment should take place at the level of the molecule, but that the market can be left open because do transaction would not give rise to anti-competitive effects.
(193) The market investigation showed limited substitutability for irinotecan Moreover purchasing takes place at the level of the molecule, predominantly through molecule-specific tenders.
(194) On the basis of the above, the Commission concludes that for the purpose of the present decision the relevant product market should comprise sterile injectable irinotecan.
Competitive assessment
(195) In irinotecan, the Transaction give raise to six affected Group 1 markets in Belgium, the Czech Republic, Finland, Greece, Italy and Spain.
Belgium, the Czech Republic and Italy
(196) In Belgium, the market size for irinotecan was EUR [5-10] million in 2014. The combined market shares of the Parties reached [70-80]% in 2014, with an increment of [5-10]% brought by Hospira. The Notifying Party submits that there are also other suppliers active on this market like Fresenius ([10-20]%), Aurobindo ([5-10]%), Teva ([0-5]%) and Mylan ([0-5]%).
(197) In the Czech Republic, the market size of the irinotecan was EUR [1-5] million in 2014. The combined market shares of the Parties reached [50-60]% in 2014, with an increment of [10-20]% brought by Hospira. The Notifying Party submits that there are also other suppliers active on this market like Mylan ([30-40]%) and Teva ([5-10]%), and some smaller ones like Servier, Fresenius and Accord/Intas, all with a market share of less than [0-5]%.
(198) In Italy, the market size of the irinotecan was EUR [20-25] million in 2014. The combined market shares of the Parties reached [50-60]% in 2014, with an increment of [10-20]% brought by Pfizer. The Notifying Party submits that there are also other suppliers active on this market like Fresenius ([30-40]%), Molteni ([10-20]%), a pharmaceutical company that focuses on pain therapy and drug addiction, Mylan ([510]%), Teva and Novartis, both with a market share of less than [0-5]% in 2014.
(199) However, in all these markets, the market investigation did not entirely confirm the positioning or presence of all these alternative suppliers, revealing a weaker positioning for some competitors with respect to what represented by the Parties. In addition, there seem to be capacity constraints for some of the competitors111 in a market where security of supply is seen as a very important aspect by customers;112 additionally, one competitor is exiting the market. Moreover, no other supplier has confirmed the intention to enter any of these markets. 113 Therefore, after the merger, there will be only a reduced number of alternative suppliers.
(200) A majority of customers responding to the Commissions market queries in these markets expressed their concerns with regard to the proposed transaction during the market investigation. A number of those customers described that the prices of irinotecan may go up as a result of the proposed transaction. A number of customers had voiced concerns over the security of supply as a result of the fewer alternative suppliers stemming from the proposed transaction.114
(201) In light of the high market shares and the limited number of credible alternative supply options that the customers would have post-transaction, the fact that the market investigation did not entirely confirm the presence of all alternative suppliers, showed capacity constraints of competitors and revealed concerns by a majority of customers, the Commission concludes that the proposed concentration would significantly impede effective competition, in the markets for irinotecan in Belgium, the Czech Republic and Italy in particular as a result of the creation or strengthening of a dominant position. Therefore, the Commission concludes that on those markets the transaction gives rise to serious doubts as to its compatibility with the internal market.
Finland and Spain
(202) In Finland, the market size of the irinotecan was EUR [0-1] million in 2014. The combined market shares of the Parties reached [90-100]% in 2014, but there was no overlap in 2014 (as in 2012), because Pfizer did not realise any sales. However, in the previous year 2013, the Parties reached [60-70]% combined market share, with an almost equal share of each Party. The Notifying Party submits that in 2013, there were also other competitors active like Novartis ([10-20]%), Fresenius ([5-10]%) and Teva ([5-10]%). Therefore, Finland was a Group 1 country exceptionally only in 2013.
(203) The fluctuations in market shares reflect the fact that Finland is a tendering market with a small market size. In such a market variations in market shares occur rather often and market shares may overstate the actual market power.
(204) During the market investigation customers did not express concerns as to negative competitive effects of the proposed transaction. The competitors to the merged entity such as Teva, Fresenius and Novartis are likely to increase their supply substantially should prices of the merged entity increase after the Transaction. These competitors would constrain the merged entity sufficiently, should the merged entity try to increase its prices.
(205) In Spain, the market size of the irinotecan was EUR [5-10] million in 2014. The combined market shares of the Parties reached [40-50]% in 2014, but the increment was less than [0-5]% brought by Pfizer. However, Spain was Group 1 market in 2012, when the combined market shares reached [60-70]% and the increment of [0-5]% of Pfizer. The Notifying Party submits that in 2012 there are also other suppliers active on this market like Accord/Intas ([10-20]%), GP Pharm ([10-20]%), a local pharmaceutical company, Fresenius ([5-10]%), Teva ([0-5]%), Aurobino ([0-5]%), Mylan and Novartis, both with a market share of less than [0-5]%.
(206) The Notifying Party further submits that in 2014, it started the official process of withdrawing the marketing authorisation in Finland. Equally, Pfizer submitted evidence that in Spain the official withdrawal, approved by the Medicines Agency, took place on 23 September 2014.115
(207) During the course of the investigation, Pfizer submitted documents showing that in Finland, it is not able to effectively compete on the market and therefore Pfizer initiated the marketing authorisation withdrawal procedure.116 Equally for Spain, the Notifying Party submitted documents concerning the withdrawal of marketing authorisation for irinotecan in Spain.
(208) The Commission, taking into consideration all of the above, including the documents submitted showing clear exiting of Pfizer from Spain and Finland, concludes that the transaction does not give rise to serious doubts as to its compatibility with the internal market in relation to irinotecan in Finland and Spain. because the proposed concentration would not significantly impede effective competition, in the markets for irinotecan in Finland and Spain.
Greece
(209) In Greece, the market size of the irinotecan was only EUR [0-1] million in 2014. The combined market shares of the Parties reached the level of a Group 1 market only in 2012, with a combined market share of [30-40]% and an increment of [5-10]% brought by Hospira. The Notifying Party submits that in 2012, there were also other competitors active like Mylan ([40-50]%) and Teva ([10-20]%).
(210) Mylan was clearly the market leader for the last three years, reaching to [50-60]% in 2014. Hospira did not record any sales in 2013 and 2014.
(211) The Notifying Party submits that the IMS data is incomplete. They estimate the total value of the market (public and private) in 2014 at approximately EUR [1-5]million. This estimate is based on the last national tender for public hospitals, which covers almost 95% of government hospitals. This last tender has been won by Mylan (Generics Pharma) and has been executed form spring 2014 to spring 2015 at a total value of EUR [0-1] million. Based on the Parties' actual sales, their estimated individual market shares would be [10-20]% for Pfizer, and [5-10]% for Hospira in 2015.
(212) The Commission, taking into consideration all of the above, including the results of the market investigation, concludes that the transaction does not give rise to serious doubts as to its compatibility with the internal market in relation to irinotecan in Greece, because the proposed concentration would not significantly impede effective competition, in the markets for irinotecan in Greece.
III.2.1.4.g. Methotrexate
Product market definition
(213) Methotrexate is an antimetabolite and antifolate drug which acts by inhibiting the metabolism of folic acid. It is used in treatment of cancer, autoimmune diseases, ectopic pregnancy, and for the induction of medical abortions.
(214) The market investigation yielded some evidence pointing towards a relevant product market definition at the molecule level, for the same reasons as the other sterile injectable products that were investigated. However, in the present case the relevant product market definition can be left open as the transaction would not raise serious doubts as to a significant lessening of effective competition, regardless of the exact relevant market delineation.
Competitive assessment
(215) In methotrexate, the Transaction give raise to two affected Group 1 markets in Italy and Portugal.
(216) In Italy the market size for methotrexate was EUR [30-40] million in 2014. The combined market shares of the Parties reached the level of a Group 1 market only in 2012, with [60-70]% and an increment of only [0-5]% brought by Hospira. The Notifying Party submits that in 2012, there were also other competitors active like Alpha Wassermann ([30-40]%), an Italian based pharmaceutical group, and Teva ([05]%).
(217) Due mainly to decreasing sales of both Parties in 2013 and 2014, Italy was only a Group 2 market in these two years. Hospira's share decreased from [0-5]% in 2012 to a mere [0-5]% in 2014. Pfizer's market share dropped from [60-70]% in 2012 to [50-60]% in 2014.
(218) An analysis of the market shares evolution during the last three years, shows that the main winner of the Parties' loss of sales was the main rival Alpha Wassermann, constantly improving its share up to [40-50]% of the market in 2014.
(219) In Portugal, the market size of the methotrexate was EUR [1-5] million in 2014. The combined market shares of the Parties reached the level of a Group 1 market only in 2012, with [30-40]% and an increment of [10-20]% brought by Hospira. The Notifying Party submits that in 2012, there were also other competitors active like Medac ([6070]%) and Teva ([5-10]%). However, due to decreasing sales of both Parties, Portugal was only a Group 3 market in 2013 and 2014.
(220) The market leader has been Medac, consolidating its position from [60-70]% in 2012 to [60-70]% in 2014, taking advantage of the Parties' loss in sales. Hospira's share decreased from [10-20]% in 2012 to [5-10]% in 2014. Pfizer's positioning was rather constant, with a market share around [20-30]% during the 2012-2014 period.
(221) The Commission, taking into consideration all of the above, including the results of the market investigation, concludes that the transaction does not give rise to serious doubts as to its compatibility with the internal market, because the proposed concentration would not significantly impede effective competition, in the markets for methothrexate in Italy and Portugal.
III.2.1.4.h. Nitroglycerin
Product market definition
(222) Nitroglycerin belongs to a group of drugs called nitrates, which includes many other nitrates like isosorbide dinitrate (Isordil) and isosorbide mononitrate (Imdur, Ismo, Monoket). These agents all exert their effect by being converted into nitric oxide, a potent natural vasodilator, in the body.
(223) In medicine, nitroglycerin is used as a medicine for angina pectoris, a symptom of ischemic heart disease caused by inadequate flow of blood and oxygen to the heart. Nitroglycerin corrects the imbalance between the flow of oxygen and blood to the heart. It is also a potent antihypertensive agent. In cardiac treatment, the lowering of pressure in the arteries reduces the pressure against which the heart must pump.
(224) Irrespective of the exact product market definition the transaction would not raise serious doubts as to a significant lessening of effective competition.
Competitive assessment
(225) Regarding nitroglycerin, the Transaction gives rise to only one affected Group 1 market, namely in Portugal.
(226) Here, the market size was EUR [1-5] million in 2014. The combined market shares of the Parties reached [40-50]% in 2014, but the increment was only [0-5]% brought by Hospira. The Notifying Party submits that in 2014 there were also other competitors present like Medac ([20-30]%), Merk&CO ([5-10]%), Novartis ([5-10]%), UCB ([510]%), a global biopharmaceutical company with headquarters in Belgium, Quilaban ([5-10]%), a local distributor and finally Faes Farma ([5-10]%), a Spanish distributor.
(227) Hospira's sales have been decreasing during the last three years from [0-05]% in 2012 to [0-5]% in 2014. The same stands valid for the market size, which seems to be shrinking from 2012 onwards from EUR [1-5] million in 2012 to EUR [1-5] million in 2014. The market position of the Parties' competitors proved relatively stable during the period analysed, with only very small variations, showing that there will be several active suppliers post-merger that will continue to compete with the merged entity exerting competitive constraint over it.
(228) The Commission, taking into consideration all of the above, concludes that the transaction does not give rise to serious doubts as to its compatibility with the internal market, because the proposed concentration would not significantly impede effective competition, in the market for nitroglycerin in Portugal.
III.2.1.4.i. Piperacillin/Tazobactam
Product market definition
(229) Piperacillin/Tazobactam is an extended-spectrum beta-lactam antibiotic of the ureidopenicillin class. It is normally used together with a beta-lactamase inhibitor, notably in the combination piperacillin/tazobactam. It is used for preventing infections that are proven or strongly suspected to be caused by bacteria.
(230) The market investigation yielded some evidence pointing towards a relevant product market definition at the molecule level, for the same reasons as the other sterile injectable products that were investigated. However, in the present case the relevant product market definition can be left open as the transaction would not raise serious doubts as to a significant lessening of competition, regardless of the exact relevant market delineation.
Competitive assessment
(231) In piperacillin/tazobactam, the Transaction gives raise to three affected Group 1 market in Ireland, Italy, the Netherlands.
(232) In Ireland the market size of the piperacillin/tazobactam was EUR [10-15] million in 2014. The combined market shares of the Parties reached the level of a Group 1 market only in 2013, with [40-50]% and an increment of only [0-5]% brought by Pfizer. The Notifying Party submits that in 2012, there were also other competitors active like Wockhardt ([10-20]%), a global generic pharmaceutical company and an API producer, Teva ([10-20]%), Stada ([10-20]%), Fresenius ([10-20]%) and Mylan ([5-10]%).
(233) The market share data shows that since 2012 Pfizer's sales have been drastically decreasing from [5-10]% to less than [0-5]% in 2014. Hospira's market share in 2012 was of [10-20]% and [10-20]% in 2014. In addition, there are several suppliers that will remain on the market post-transaction and that will impose competitive constraints on the merged entity.
(234) In Italy, the market size of the piperacillin/tazobactam was EUR [70-80] million in 2014. The combined market shares of the Parties reached the level of a Group 1 market only in 2012, with [60-70]% and an increment of just [0-5]% brought by Hospira. The Notifying Party submits that in 2012, there were also other competitors active like Magis Farmaceutici ([10-20]%), an Italian distributor, Teva ([5-10]%), Fresenius ([510]%), and several others like IBI, a local pharmaceutical producer, Bioton Group, Mylan and several small other ones.
(235) Both Parties' sales have drastically decreased since 2012. Pfizer was the market leader in 2012 with a market share of [60-70]%, whereas in 2014 it accounted for only [510]% of the market. Equally, Hospira's market share dropped from [0-5]% in 2012 to [0-5]% in 2014, remaining an insignificant player on this market.
(236) In the Netherlands, the market size of the piperacillin/tazobactam was EUR [1-5] million in 2014. The combined market shares of the Parties reached the level of a Group 1 market only in 2012, with [50-60]% and an increment of [20-30]% brought by Hospira and in 2013 with a combined market share of [30-40]% and a much smaller increment of Hospira's of [0-5]%. The Notifying Party submits that in 2013, there were also other competitors active like Fresenius ([40-50]%), Teva ([10-20]%), Mylan ([05]%) and Novartis (less than [0-5]%).
(237) The Notifying Party submits that [...] In addition to that, one competitor mentioned that during a supply disruption of one of the main suppliers on this market, the other participants were able to supply sufficient volume to cove this shortage.117
(238) The Commission, taking into consideration all of the above, including the results of the market investigation, concludes that the transaction does not give rise to serious doubts as to its compatibility with the internal market in relation to piperacillin/tazobactam in Ireland, Italy and the Netherlands.
III.2.1.4.j. Vancomycin
Product market definition
(239) Vancomycin is classified in an ATC3 catch all class, 'other antibacterials'. Such a class does not group interchangeable molecules together. Vancomycin is a natural occurring antibiotic made by a bacterium in the soil. It has been off patent since the early 1980's. Vancomycin is primarily used for the first line of treatment for MRSA (Methicillin-resistant Staphylococcus aureus).
(240) The Commission dealt with vancomycin in its Pfizer/Wyeth decision118, where it considered that generic vancomycin was not a close substitute for two other branded MRSA antibiotics, because those were reserve antibiotics. Vancomycin was considered to be the leading, first line treatment.
(241) For this reason and because tenders are often organised per molecule, the Notifying Party argues that the assessment should take place at the level of the molecule. The Parties submit that the relevant product market definition ultimately can be left open.
(242) In the present market investigation the limited substitutability for vancomycin was confirmed. The indications for which carboplatin is prescribed are only partially overlapping with those of other molecules in the same ATC class. Moreover purchasing takes place predominantly through molecule-specific tenders.
(243) On the basis of the above, the Commission concludes that for the purpose of the present decision the relevant product market definition can be left open as the transaction would not raise serious doubts as to a significant lessening of competition, regardless of the exact relevant market delineation.
Competitive assessment
(244) In vancomycin, the Transaction give raise to two affected Group 1 markets in Ireland and Norway.
Ireland
(245) In Ireland, the market size of vancomycin was EUR [1-5] million in 2014. The combined market shares of the Parties reached [50-6]% in 2014, with an increment of [0-5]% brought by Pfizer. The Notifying Party notes that there would be another competitor present in the market, with a share of [5-10]%. The Parties have submitted that the market share accounted for under the title of "Lab Unknown" is in reality compounded vancomycin, sold directly to hospitals from Fannin and Baxter. The other suppliers are Flynn Pharma ([10-20]%) and Actavis ([5-10]%).
(246) The Notifying Party submits that Pfizer launched vancomycin in Ireland in 2011, but its sales were first recorded by IMS in 2014. Thus, while Pfizer does not have estimates of its market share for the period between 2011 and 2014, it believes that its market share has risen gradually since 2011.
(247) In addition, while Pfizer is a new entrant in this market, Hospira's market share has been increasing too since 2012 onwards, from only [10-20]% in 2012 to [50-60]% in 2014, taking market share mainly from Lab Unknown. Therefore, the Transaction would even further enforce Hospira's recently consolidated leading position in this market.
(248) Moreover, no other supplier has confirmed its intention to enter this market in the near future.119 Therefore, after the merger, there will be only a reduced number of alternative suppliers.
(249) The Commission, taking into consideration all of the above, including the results of the market investigation, concludes that the transaction gives rise to serious doubts as to its compatibility with the internal market, because the proposed concentration would not significantly impede effective competition, in the market for vancomycin in Ireland.
Norway
(250) In Norway, the market size for vancomycin was only EUR [0-1] million in 2014. The combined market shares of the Parties reached [90-100]% in 2014, with an increment of [40-50]% brought by Hospira. The other suppliers are Mip Group ([0-5]%) and Fresenius ([0-5]%). The Notifying Party submits that Xellia, a primarily generic company which has its headquarters in Denmark, has a market share of less than 1% in 2014.
(251) The Notifying Party estimates the Parties' combined market share to be significantly lower in 2015, as [...]. Pfizer estimates that in 2015 [...] will have a market share of [40-50]%, [...] a market share of [30-40]% and that its own market share will be [1020]%.
(252) The Parties consider that their high market share results from the low prices they offer. Should Pfizer try to increase prices post-Transaction, it would lose market share to other competitors present on the market. This could be easily observed by the evolution of the bid prices [...] its market share:
(253) This proves that although the combined market shares of the Parties are high, in a small value market with a lumpy demand, it does not give Pfizer a degree of market power that would allow it to impose higher prices after the Transaction. As is the case with vincristine (see below), there are a number of potential suppliers constraining Pfizer and Hospira already now and they will continue to do so.
(254) The Commission, taking into consideration all of the above, including the results of the market investigation, concludes that the transaction does not give rise to serious doubts as to its compatibility with the internal market, because the proposed concentration would not significantly impede effective competition, in the market for vancomycin in Norway.
III.2.1.4.k. Vincristine
Product market definition
(255) Vincristine is an alkaloid microtubule agent derived from the periwinkle plant. Microtubules are parts of human cells that are ensuring the replication of the cells. Inhibiting the microtubule cells leads to cell death and is therefore used as a treatment for various forms of cancer, such as head and neck cancers, brain tumours, soft tissue sarcoma and many others. Vincristine has been off-patent for a number of years.
(256) There has not been a prior case with the Commission where it has looked at vincristine.
(257) For the purpose of the present case the Parties argue that the assessment should take place at the molecule level, but that in any even the market definition can be left open because the transaction would not give rise to anti-competitive effects under any plausible market definition.
(258) The market investigation yielded some evidence pointing towards a relevant product market definition at the molecule level, for the same reasons as the other sterile injectable products that were investigated. However, in the present case the relevant product market definition can be left open as the transaction would not raise serious doubts as to a significant lessening of competition, regardless of the exact relevant market delineation.
Competitive assessment
(259) For vincristine, the Transaction give raise to only one affected Group 1 market in Norway.
(260) Here, the combined market shares of the Parties reached [90-100]% in 2014, with an increment of [20-30]% brought by Pfizer. In 2013 and 2012, Pfizer accounted for the totality of the market. The size of the market in value accounted to only EUR [0-1 million] in 2014, and to similar amounts in previous years.
(261) The Notifying Party submits that Vincristine is purchased in Norway exclusively by hospitals through tenders held every three years (instead of the more common yearly tenders) because of the extremely small amounts involved. Until 2014, Pfizer [...]. Hospira entered the market in [...] and immediately gained significant market share by winning tenders on the basis of a lower price than Pfizer.
(262) The Notifying Party submits that it will have no incentives to increase prices post Transaction, as gains would be necessary very small in monetary terms, while such behaviour would seriously damage its reputation with Norwegian hospitals as a reliable supplier of a vast array of drugs.
(263) The Commission finds no merit in the reputational argument proposed by the Notifying Party. As tenders are organised by molecule, the price of each molecule is set individually. Hospitals rely on competition between bidders to obtain favourable price, while there is no evidence that reputational factors play a role in the determination of price.
(264) The relevant question is therefore whether there will be sufficient competitive pressure from potential entrants in the future to constrain higher pricing by the merged entity. The market investigation indicated that this is indeed the case120. First, the limited amount of suppliers for vincristine in Norway is due to the small market size and not to other factors. Vincristine indeed is produced by a number of competitors, including e,g. Stada, Teva and Novartis, which would have no capacity constraint to serve the Norwegian market. Whether to enter or not the market will be based on economic considerations of its attractiveness. Were the merged entity to raise prices post Transaction, it would provide the necessary incentives to new entrants. Second, entry is certainly feasible, as demonstrated by the fact that Hospira gained a large portion of the market within one year of entry. This strategy can be replicated by other companies (for example, Stada sells vincristine in neighbouring Denmark, Finland and Sweden). Finally, the market investigation did not evidence concerns on the side of customers for the procurement of vincristine post Transaction.
(265) The Commission, taking into consideration all of the above, including the results of the market investigation, concludes that the transaction does not give rise to serious doubts as to its compatibility with the internal market, because the proposed concentration would not significantly impede effective competition, in the market for vincristine in Norway.
III.2.2. Pipeline products
Product market definition
(266) The Parties also have overlapping pipeline activities for voriconazole (commercial name Vfend) and linezolid (Zyvox), where Pfizer is the originator company and Hospira is developing a generic alternative.121
(267) Voriconazole is generally used to treat serious, invasive fungal infections in immunocompromised patients. While there is some substitution at the individual level between molecules sharing the same indications, competition primarily takes place at the level of the molecule. According to the Notifying Party hospitals typically tender or purchase voriconazole at the molecule level. The Commission considers moreover that the fact that voriconazole is a broad spectrum antifungal agent sets it apart from other more narrow antifungal agents with a narrower spectrum. Therefore the Commission considers that for the purpose of the present assessment the competitive assessment should take place at the molecule level.
(268) Linezolid is an antibiotic used for the treatment of serious infections. Although for linezolid there is a number of partial alternatives (depending on the exact indication and the patient's reaction to the antibiotic), purchasing typically takes place at the molecule level. Moreover for serious infection it is understood that there is a need to maintain the highest number of available product options to address potential resistance. Even if alternatives exist, it was reported that linezolid is a reserve antibiotic that would be purchased separately. Linezolid is also much more expensive than other antibiotics used for the same indications. As such linozelid is not a close substitute to other antibiotics that are used for the same indications. For these reasons the Commission considers that for the purpose of the present assessment the competitive assessment should take place on a molecule level.
Competitive assessment
(269) Generic companies usually develop a number of pipeline generic drugs which are intended to compete with originators which go off-patent. In assessing pipeline competition, the Commission has previously focused on instances where one party is planning to enter a market with a new product within a period of two years and the other party (or the parties combined) has a market share of 35% or more on any possible market definition where the pipeline products and existing products overlap.122
III.2.2.1. Linezolid
(270) Pfizer is the originator in this market. It markets linezolid under the brand name Zyvox (or Zyvoxid). According to the Notifying Party it will lose patent protection in the EU in January 2016. Pfizer's Zyvox (linezolid) is marketed in a dextrose formulation and an oral formulation. Pfizer [...].
(271) Hospira is developing a generic equivalent to Zyvox. Hospira has developed two presentations of linezolid: one in dextrose solution (which matches the Pfizer product), and a second in saline solution.
(272) The dextrose and saline formulations are largely substitutable except for the small population of patients (approximately 1% of patients) with specific conditions that make one or the other preferable (e.g., saline is not preferred for patients with heart condition, or dextrose not preferred for a patient with diabetes). The Notifying Party submits that pricing of the two formulations is [...].
(273) Hospira [...] and was expecting to enter the market in [...]. An internal document123 of Hospira shows that [...]. Another internal document of Hospira shown that [...]. 124
(274) According to the Notifying Party, Hospira is currently exploring the options of [...]. Further on, the Parties state that they are aware of numerous players that are currently developing generic linezolid with launch in the EU expected around patent expiry. These players include major generic companies such as Teva, Sandoz, Stada and Fresenius, but also many other players including Alvogen, Helm, Hameln, Hetero, Polpharma, and Synthon. They also submit that Teva, Sandoz and Fresenius have received approval for their generic products in the EU through a decentralised procedure.
(275) Internal documents of the Parties show that [...].125 Teva has already launched its generic version of linezolid in the US.
(276) In any case, the market investigation replies have confirmed that a sufficient number of competitors intend to enter into EEA markets with a generic version of linezolid, in either an IV form or oral form. Therefore, at least four strong competing pharmaceutical companies will remain post-merger for linezolid, even more in some countries. The Proposed Transaction will therefore not lead to the removal of an important competitive constraint on Pfizer. First and foremost because [...]. But also, and more importantly, because a large number of equally suitable competitors such as Teva that has its generic version of linezolid already on the market in the US, will constrain the merged entity.
(277) Taking into consideration all of the above, the Commission concludes that the transaction does not give rise to serious doubts as to its compatibility with the internal market, because the proposed concentration would not significantly impede effective competition, in relation to linezolid.
III.2.2.2. Voriconazole
(278) Pfizer is the originator in this market. It markets voriconazole under the brand name Vfend. According to the Notifying Party, voriconazole will go off patent in the EU in between January and July 2016 (varying by country), but the intravenous (IV) formulations only in June 2018. Vfend is available in the EU as tablets, as an oral suspension and as a powder to be made up into a solution for infusion. Each of the galenic forms can be used for all approved indications. Choice of formulation is a decision made by the practitioner and generally based on severity of disease and of patient's age (for example oral solution is useful especially for children).
(279) Hospira has just developed a generic voriconazole product. It only developed it as powder for infusion formulation. According to the Notifying Party, on 26 March 2015, the Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion, recommending the granting of a marketing authorisation to Hospira for its medicinal product Voriconazole Hospira. Hospira planned to launch its product in [...] for the patent free markets only, e.g., CEE. On 27 May 2015, Hospira was granted the marketing authorisation valid throughout the EU.126
(280) Internal documents from the file have revealed that Hospira intended to [...].
(281) Pfizer submitted that [...].
(282) The Transaction will eliminate any such incentive and therefore Pfizer will lose one patent challenger, resulting in a likely delayed entry of generic versions for voriconazole. This is also shown in an internal document showing that [...].127
(283) In addition to that, Pfizer holds a patent as regards the process of making an excipient present in an IV formulation, which will expire in [...]. According to the Notifying Party, voriconazole has some complexities given that the injectable formulation contains a specialized solubilizer ([...]) for which pharmaceutical grade is not readily available and is expected to limit competition. Pfizer sources this excipient ([...]) from [...]. Hospira also executed an agreement in [...] with [...] for a co-exclusive right to obtain [...]. Therefore, following the merger, Pfizer will have exclusive right to an important input for the IV formulation of voriconazole, which according to its own declaration would limit competition.
(284) The above elements indicate that Hospira was in a privileged position to successfully enter the EEA markets for voriconazole. The market investigation did not provide indications that other companies took comparable steps to Hospira, in terms of securing of necessary ancillary inputs ([...]) that would allow them to exert a similar competitive pressure on Pfizer before or upon loss of exclusivity for voriconazole. The Transaction will therefore eliminate Hospira as a uniquely placed new entrant in the EEA markets for voriconazole.
(285) Consequently, the Commission concludes that the transaction gives rise to serious doubts as to its compatibility with the internal market in relation to voriconazole in the EEA.
III.3. Conclusion
(286) The transaction gives rise to serious doubts as to its compatibility with the internal market in relation to:
a. with respect to biosimilars, the market for infliximab in the EEA (or wider);
b. with respect to sterile injectables, the markets for carboplatin in Belgium; cytarabine in Belgium, Italy, Portugal and Sweden; epirubicin in Austria, Belgium, Italy, the Netherlands and Spain; irinotecan in Belgium, the Czech Republic and Italy; vancomycin in Ireland and voriconazole in the EEA.
IV. COMMITMENTS
IV.1. Framework for the assessment of the Commitments
(287) Where a concentration raises serious doubts as regards its compatibility with the internal market, the Parties may undertake to modify the concentration so as to remove the grounds for the serious doubts identified by the Commission. Pursuant to article 6(2) of the Merger Regulation, where the Commission finds that, following modification by the undertakings concerned, a notified concentration no longer raises serious doubts, it shall declare the concentration compatible with the common market pursuant to article 6(1)(b) of the Merger Regulation.
(288) As set out in the Commission’s Remedies Notice,128 the commitments have to eliminate the competition concerns entirely, and have to be comprehensive and effective from all points of view.129
(289) In assessing whether commitments will maintain effective competition, the Commission considers all relevant factors, including the type, scale and scope of the proposed commitments, with reference to the structure and particular characteristics of the market in which the Transaction is likely to significantly impede effective competition, including the position of the Parties and other participants on the market.130
(290) In order for the commitments to comply with those principles, they must be capable of being implemented effectively within a short period of time. Concerning the form of acceptable commitments, the Merger Regulation gives discretion to the Commission as long as the commitments meet the requisite standard. Structural commitments will meet the conditions set out above only in so far as the Commission is able to conclude with the requisite degree of certainty, at the time of its Decision, that it will be possible to implement them and that it will be likely that the new commercial structures resulting from them will be sufficiently workable and lasting to ensure that effective competition will be maintained.131 Divestiture commitments are normally the best way to eliminate competition concerns resulting from horizontal overlaps.
IV.2. Commitments submitted by the Parties
(291) In order to ensure that effective competition will be maintained, the Parties submitted a set of commitments under Article 6(2) of the Merger Regulation on 13 July 2015 ("Initial Commitments"). The Commission market tested the Initial Commitments in order to assess whether they are sufficient and suitable to remedy serious doubts identified in the markets for infliximab in the EEA (or wider); carboplatin in Belgium; cytarabine in Belgium, Italy, Portugal and Sweden; epirubicin in Austria, Belgium, Italy, the Netherlands and Spain; irinotecan in Belgium, the Czech Republic and Italy; vancomycin in Ireland and voriconazole in the EEA. Following the feedback received during the market test, the Initial Commitments were refined and improved, and amended commitments were submitted on 28 July 2015 ("Final Commitments"). These Final Commitments are annexed to this Decision and form an integral part thereof.
IV.2.1. Initial Commitments
IV.2.1.1. infliximab
(292) In order to dispel the serious doubts arising in relation to infliximab, Pfizer submitted commitments consisting of a full divestiture of the development, manufacturing and marketing rights of its infliximab pipeline biosimilar (the "Product"), with a reverse carve-out of ex-EEA marketing rights back to Pfizer (together, the "infliximab Divestment Business").
(293) Pfizer notes that the Product is now undergoing a phase III clinical trial, which is conducted [...] on a global basis. It further notes that the manufacturing of the Product is outsourced to [...] and that the manufacturing process incorporates certain intellectual property rights of [...].
(294) The commitments package includes in particular appropriate books and records, marketing plans and forecasts, contracts with third parties (such as correspondence with regulators), and personnel in relation to the Product.
(295) The personnel provided consists of all Pfizer's employees which provide substantial support to the infliximab Divestment Business and which is necessary to continue to develop, manufacture, have manufactured and market the Product, including the following Key Personnel:
a. Global infliximab Asset Lead
b. Global infliximab Medical Affairs Lead
c. Global infliximab Clinical Lead
d. Global infliximab Project Manager
e. Global infliximab Study Clinician
f. Global infliximab Commercial Lead
g. Global infliximab Regulatory Lead
(296) It further includes patents, copyrights, data and know-how relating exclusively to the clinical development, manufacture of sale of the Product, as well as a royalty-free, perpetual, irrevocable and sub-licensable license to patents, copyrights, data and know-how necessary for the development, manufacturing or sale of the Product in the EEA.
(297) This package is subject to the purchaser licensing-back to Pfizer the exclusive (including as to the purchaser) right to conduct non-EEA country specific development activities, as well as to manufacture and market the Product outside the EEA. To preserve Pfizer's legitimate interests in the Product, it will establish a Joint Development Committee (the "JDC") which would have authority over a certain number of key decisions. Such decisions, such as changes or delays in the clinical trial protocol and any clinical trial matter that is likely to adversely impact the development or future commercialisation of the Product in the Pfizer (ex-EEA) markets, would be decided by unanimity.
(298) Finally, the Initial Commitments include a transitional agreement between Pfizer and the Purchaser for a period of up to [...], including clinical development assistance for a period of up to [...].
(299) In terms of Purchaser requirements, besides the standard requirements, the Initial Commitments provided that the Purchaser be in a position to, in a timely manner, take over the existing agreements or conclude direct agreements on commercially reasonable terms with [...], [...] and [...], to the extent these relate to the infliximab Divestment Business.
IV.2.1.2. Sterile injectables
(300) Moreover, in order to dispel the serious doubts identified in relation to sterile injectables, Pfizer submitted commitments consisting of the rights, title and interests of one Party in the relevant molecules in the relevant countries covering all markets for which serious doubts were raised (the “sterile injectables Divestment Businesses”), namely:
a. Pfizer's rights to carboplatin in Belgium and vancomycin in Ireland;
b. Hospira's rights to cytarabine in Belgium, Italy, Portugal and Sweden; to epirubicin in Austria, Belgium, Italy, the Netherlands and Spain; to irinotecan in Belgium, the Czech Republic and Italy; and to voriconazole in the EEA.
(301) The sterile injectables Divestment Businesses fully remove the overlap in all markets were serious doubts were identified.
(302) The Divestment Businesses are structured as asset carve-outs; no legal entities are to be divested. Specifically, the businesses to be divested include the following assets:
a. The existing product inventories, sales and promotional materials at the time of the divestment;
b. Related contracts, commitments and/or records including but not limited to customers credit records, customer invoices, purchase orders, tender information and contact for the last three years and including tenders won;
c. Current and pending marketing authorisations including all relevant dossiers and the information contained in the full registration dossiers;
d. Licenses (perpetual, irrevocable, royalty free) for all relevant intellectual property rights, data, books, records, and effective arrangements for the transfer of all know-how related to the development and manufacture of the relevant products;
e. Trademarks relevant to the products and markets concerned;
f. At the option of the purchaser transitory supply agreements and a transitory distribution agreement designed to ensure a continuous supply of the Divestment businesses and distribution by the Divestment Businesses.
(303) In addition the undertakings concerned have entered into related commitments, inter alia regarding the separation of the divested businesses from their retained businesses, the preservation of the viability, marketability and competitiveness of the divested businesses, including the appointment of a monitoring trustee and, if necessary, a divestiture trustee.
IV.2.2. Results of the market test and assessment of the Initial Commitments
(304) The market test was launched on 13 July 2015 and sought to assess mainly the scope and effectiveness of the Initial Commitments, their viability, the attractiveness of the Divestment Businesses as well as the suitability of the Purchaser criteria.
(305) While generally the market test yielded positive results, in particular regarding the scope and viability of the Divestment Businesses,132 respondents identified certain shortcomings.
(306) In relation to infliximab, specifically, the following key issues were raised:
a. While the JDC decision-making process and scope of action is deemed to provide a suitable purchaser with sufficient ability and incentive to develop the infliximab biosimilar and, if successful, market it in the EEA, respondents from the supply side highlighted a risk to delay decisions in the absence of "specific binding timelines on decision making and escalation processes to ensure timely decisions", as well as a risk of Pfizer effectively having full control over the clinical trial by its ability of blocking key decisions.133
b. With respect to the clinical trial, the handover of the clinical trial from Pfizer to the purchaser is considered by respondents from the supply side as a potential cause of delay, and therefore an implementation risk.134
c. Finally, respondents from the supply side highlighted that registration of biosimilars is not granted based solely on the clinical trial results, but on the totality of the evidence available, with analytical, functional and non-clinical data being essential to success. Respondents also submitted that consistent manufacturing is essential, as comparability to the previous process version as well as similarity to the reference product have to be maintained.135
(307) As to the suitable Purchaser of the infliximab Divestment Business, respondents to the market test indicated that [...].136
(308) As to the suitable Purchaser of the sterile injectables Divestment Business, respondents to the market test indicated that it should be already marketing a portfolio of sterile injectables in the relevant EEA countries.137
IV.2.3. Final Commitments
(309) The Parties were informed of the shortcomings identified during the market test and submitted a final text of Commitments addressing the issues on 28 July 2015.
(310) Specifically, the Final Commitments submitted by the Parties provide in particular for the following additional improvements compared to the Initial Commitments with respect to the infliximab Divestment Business:
a. the key decisions of the JDC which must be adopted by unanimity must not give Pfizer joint control over the clinical trial and over the development of the Product exclusively in the EEA;
b. in order to mitigate the implementation risk in relation to the clinical trial and avoid any delay, until it is transferred to the purchaser, any deviation from the clinical trial timeline must be agreed between the Purchaser and Pfizer, as overseen by the Monitoring Trustee and the Commission; [...];
c. finally, to address the concerns regarding analytical, functional and non-
clinical data, Pfizer (or an affiliated undertaking) will provide reasonable support to the Purchaser in relation to the market approvals and post-authorisation procedures for the Product in the EEA, including, but not limited to, demonstration of bioequivalence to the reference product based on pre-clinical data, for a period of up to [...].
(311) Furthermore, [...], the Final Commitments include:
a. a transitory non-exclusive manufacturing or supply agreement for the infliximab DP for up to [...];
b. at the purchaser's request, the technology transfer of the infliximab DP manufacturing to a facility of the Purchaser’s choice.
(312) The Final Commitments also require the Purchaser of the infliximab Divestment Business [...].
(313) Finally, the Commitments now include specific Purchaser criteria with regards to the sterile injectables Divestment Businesses, [...].
(314) The full description of the assets and obligations of the Final Commitments is contained in the Schedules thereof.
IV.2.4. Overall assessment of the Final Commitments
(315) The Final Commitments remove the entire overlap between the Parties in relation to the markets for which the Commission raised serious doubts: infliximab in the EEA (or wider); carboplatin in Belgium; cytarabine in Belgium, Italy, Portugal and Sweden; epirubicin in Austria, Belgium, Italy, the Netherlands and Spain; irinotecan in Belgium, the Czech Republic and Italy; vancomycin in Ireland and voriconazole in the EEA.
(316) In particular, in relation to infliximab, the Final Commitments include the tangible and intangible assets necessary to conduct and complete the global phase III clinical trial for the Product, and, if successful, obtain a marketing authorisation and bring the product to the EEA markets
(317) Furthermore, the Final Commitments address the shortcomings of the Initial Commitments as identified by the market test.
(318) The attractiveness of the Divestment Businesses was evidenced by the number of potentially interested purchasers, including in particular large players in the area of biosimilars for the infliximab Divestment Business,138 and sterile injectables suppliers for the sterile injectables Divestment Businesses.139
(319) The Notifying Party commits to sell [...] Divestment Businesses within [...] months from the date of this decision. If unsuccessful, the Divestiture Trustee will receive a mandate to sell the two Divestment Businesses within the following [...].
(320) Finally, the Final Commitments envisage the appointment of a Monitoring Trustee to ensure that the Final Committments will be implemented effectively and within a short period of time.
(321) On this basis, and in particular in view of a number of interested Purchasers, the Commission considers that the infliximab Divestment Business and the sterile injectables Divestment Businesses are attractive and likely to be acquired by suitable Purchasers.
(322) For the reasons outlined above, and in view of the results of the market test and the ensuing improvements to the Commitments, the Commission considers the Final Commitments to be sufficient in scope and suitable to eliminate the serious doubts as to the compatibility of the Transaction with the internal market in relation to infliximab in the EEA (or wider); carboplatin in Belgium; cytarabine in Belgium, Italy, Portugal and Sweden; epirubicin in Austria, Belgium, Italy, the Netherlands and Spain; irinotecan in Belgium, the Czech Republic and Italy; vancomycin in Ireland and voriconazole in the EEA given the purpose of Article 6(2) of the Merger Regulation.
IV.3. Conditions and obligations
(323) Under the first sentence of the second subparagraph of Article 6(2) of the Merger Regulation, the Commission may attach to its Decision conditions and obligations intended to ensure that the undertakings concerned comply with the commitments they have entered into vis-à-vis the Commission with a view to rendering a notified concentration compatible with the internal market.
(324) The achievement of the measure that gives rise to the structural change of the market is a condition, whereas the implementing steps which are necessary to achieve this result are generally obligations on the Parties. Where a condition is not fulfilled, the Commission's decision declaring the concentration compatible with the internal market no longer stands. Where the undertakings concerned commit a breach of an obligation, the Commission may revoke the clearance decision in accordance with Article 8(6) of the Merger Regulation. The undertakings concerned may also be subject to fines and periodic penalty payments under Articles 14(2) and 15(1) of the Merger Regulation.
(325) In accordance with the distinction described above, the Decision in this case is conditioned on the full compliance with the requirements set out in section B (including Schedules 1 to 7) of the Final Commitments (conditions), whereas the other sections of the Final Commitments constitute obligations on Pfizer.
(326) The detailed text of the Final Commitments is annexed to the present Decision. The full text of the final Commitments forms an integral part to this Decision.
V. CONCLUSION
(327) For the above reasons, the Commission has decided not to oppose the notified operation as modified by the commitments and to declare it compatible with the internal market and with the functioning of the EEA Agreement, subject to full compliance with the conditions in section B (including Schedules 1 to 7) of the commitments annexed to the present Decision and with the obligations contained in the other sections of the said commitments. This decision is adopted in application of Article 6(1)(b) in conjunction with Article 6(2) of the Merger Regulation and Article 57 of the EEA Agreement.
FOOTNOTES :
1 OJ L 24, 29.1.2004, p. 1 (the "Merger Regulation"). With effect from 1 December 2009, the Treaty on the Functioning of the European Union ("TFEU") has introduced certain changes, such as the replacement of 'Community' by 'Union' and 'common market' by 'internal market'. The terminology of the TFEU will be used throughout this decision.
2 OJ L 1, 3.1.1994, p.3 ("the EEA Agreement").
3 Turnover calculated in accordance with Article 5 of the Merger Regulation and the Commission Consolidated Jurisdictional Notice (OJ C95, 16.04.2008, p1).
4 Monoclonal antibodies are used for the treatment of various cancers (e.g. breast cancer) and autoimmune diseases (e.g. rheumatoid arthritis). mAbs developed for oncology differ from more conventional chemotherapy compounds as they target a unique and specific component of the disease mechanism or of the tumour cell.
5 See cases M.6969 — Valeant Pharmaceuticals International/Bausch & Lomb Holdings. M.5778 — Novartis/Alcon, and M.5865 — Teva/Ratiopharm.
6 See cases M.6969 — Valeant Pharmaceuticals International/Bausch & Lomb Holdings, M.5865 —Teva/Ratiopharm and M.5295 — Teva/Barr.
7 See cases M.6969 – Valeant Pharmaceuticals International/Bausch & Lomb Holdings, M.6705 – Procter & Gamble/Teva Pharmaceuticals OTC II, M.6613 – Watson/Actavis, and M.5865 – Teva/Ratiopharm.
8 See cases M.5865 – Teva / Ratiopharm and M.5479 – Lonza / Teva / JV.
9 The marketing authorisation holder in the EU is Janssen Biologics B.V (company controlled by Johnson & Johnson).
10 See replies to question 10 of questionnaire Q2 – Biosimilars Customers.
11 See replies to question 11 of questionnaire Q2 – Biosimilars Customers.
12 See replies to question 13 of questionnaire Q3 – Biosimilars Physicians and Key Opinion Leaders.
13 See non-confidential minutes of a conference call with a competitor, 2 June 2015.
14 See replies to question 13 of questionnaire Q1 – Biosimilars Competitors.
15 Remicade was awarded [...] infliximab tenders analysed, typically alongside Inflectra or Remsima. Source: tender data provided by the Parties.
16 See e.g. cases M.7379 – Mylan / Abbott EPD-DM, M.7276 – GlaxoSmithKline / Novartis Vaccines Business (excl. Influenza) / Novartis Consumer Health Business, M.7275 – Novartis / GlaxoSmithKline Oncology Business and M.5253 – Sanofi-Aventis/Zentiva.
17 See e.g. cases M.7480 – Actavis / Allergan and M.7275 – Novartis / GlaxoSmithKline Oncology Business.
18 The Final Report of the Commission's Pharmaceutical Sector Inquiry presents econometric analysis showing the effectiveness of such regulations in facilitating faster generic entry upon loss of exclusivity, fostering generic penetration and delivering lower prices. See Annex to Chapter B, Part II, of the Final Report of the Pharmaceutical Sector Inquiry adopted on 8 July 2009.
19 For instance, specific EMA guidance documents exist for the manufacture, characterisation and control of the drug substance regarding monoclonal antibodies, immunotherapy medicinal products for the treatment of cancer, biological active substances produced by transgene expression in animals, etc.
20 See non-confidential minutes of a conference call with the EMA, 28 April 2015.
21 See replies to question 22 of questionnaire Q2 – Biosimilars Customers.
22 See replies to question 23 of questionnaire Q2 – Biosimilars Customers.
23 See replies to question 25 of questionnaire Q2 – Biosimilars Customers and replies to question 13 of questionnaire Q3 – Physicians and Key Opinion Leaders.
24 See non-confidential minutes of a conference call with a competitor, 2 June 2015.
25 See non-confidential minutes of a conference call with a Key Opinion Leader, 28 May 2015.
26 See non-confidential minutes of a conference call with a customer, 28 May 2015.
27 Inflectra and Remsima were co-awarded only [...] infliximab tenders analysed. Source: tender data provided by the Parties.
28 See in particular Pfizer's internal documents, [...].
29 See replies to question 8 of questionnaire Q3 – Biosimilars Physicians and Key Opinion Leaders.
30 See replies to question 17 of questionnaire Q1 – Biosimilars Competitors, question 29 of questionnaire Q2 – Biosimilars Customers and question 8 of questionnaire Q3 – Biosimilars Physicians and Key Opinion Leaders.
31 See replies to question 31 of questionnaire Q2 – Biosimilars Customers.
32 See Pfizer's internal document, [...].
33 See non-confidential minutes of a conference call with Celltrion, 13 May 2015.
34 See replies to question 29 of questionnaire Q2 – Biosimilars Customers.
35 See http://www.prnewswire.com/news-releases/samsung-bioepis-submits-marketing-authorization-application-for-sb2-a-remicade-infliximab-biosimilar-candidate-to-the-european-medicines-agency-300048841.html
36 See Pfizer's internal document, [...].
37 See Pfizer's internal document, [...].
38 See replies to question 4 of questionnaire Q1 – Biosimilars Competitors.
39 See replies to question 2 of questionnaire Q1 – Biosimilars Competitors.
40 It is noted that India is not an ICH member. ICH is the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, which includes the regulatory authorities of Europe, Japan and the United States.
41 See email of the EMA to the case team, 10 July 2015.
42 See replies to question 4 of questionnaire Q1 – Biosimilars Competitors.
43 See Pfizer's internal document, [...].
44 See non-confidential minutes of a conference call with the EMA, 28 April 2015.
45 Appendix II to Pfizer's Memorandum on Infliximab, 7 July 2015.
46 [...].
47 See replies to question 20 of questionnaire Q1 – Biosimilars Competitors.
48 See replies to question 22 of questionnaire Q2 – Biosimilars Customers and question 22 of questionnaire Q2 – Biosimilars Physicians and Key Opinion Leaders.
49 See replies to question 4 of questionnaire Q1 – Biosimilars Competitors.
50 See Pfizer's internal document, [...].
51 Mabion appears to be focusing on Easter Europe. [...].
52 See Pfizer's internal document, [...].
53 See replies to question 4 of questionnaire Q1 – Biosimilars Competitors.
54 See Pfizer's internal document, [...].
55 See Pfizer's internal document, [...].
56 See replies to question 9 of questionnaire Q4 – Sterile injectables Competitors,
57 See replies to question 11 of questionnaire Q4 – Sterile injectables Competitors,
58 See replies to question 10 of questionnaire Q4 – Sterile injectables Competitors,
59 See replies to question 16 of questionnaire Q5- Sterile injectables Customers,.
60 See replies to question 18 of questionnaire Q5- Sterile injectables Customers, and to question 5 of questionnaire Q4- Sterile injectables Competitors.,.
61 See replies to question 10 of questionnaire Q5- Sterile injectables Customers. .
62 See replies to question 9 of questionnaire Q5 – Sterile injectables Customers,.
63 See replies to question 7 of questionnaire -Q5 – Sterile injectables Customers.,.
64 See for example cases M.5778 – Novartis/Alcon, M.5865 – Teva/Ratiopharm, and M.5253 – Sanofi-Aventis/Zentiva.
65 See for example cases M.5778 – Novartis/Alcon, M.5865 – Teva/Ratiopharm, and M.5253 – Sanofi-Aventis/Zentiva.
66 See Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use (OJ L311, 28.11.2001, p.67), as amended by various subsequent acts.
67 See for example cases M.6969 – Valeant Pharmaceuticals International/Bausch&Lomb Holdings, M.5778 – Novartis/Alcon, M.5865 – Teva/Ratiopharm and M.5295 – Teva/Barr.
68 Finished dose pharmaceuticals are essentially pharmaceutical products in the form in which they are marketed for use, typically involving a mixture of active drug components and nondrug components (excipients), along with other non-reusable material that may not be considered either ingredient or packaging (such as a capsule shell, for example). All products in the present case concern finished dose pharmaceuticals.
69 See case M.5476 – Pfizer/Wyeth; M.4691 – Schering Plough/Organon Biosciences; M.2922 – Pfizer/Pharmacia,; and M.1681 – Akzo Nobel/Hoechst.
70 See case M.5295 – Teva/Barr.
71 See case M.5865 – Teva/Ratiopharm.
72 Market shares may not add up to 100% because of rounding of the percentages.
73 The Notifying Party estimates that [above 70]% of purchases were based on contract bilaterial negociation and only the remaining [below 30]% on tender.
74 See replies to question 22 of questionnaire Q4- Sterile injectables Competitors..
75 See replies to question 15 of questionnaire Q4- Sterile injectables Competitors.
76 See replies to questions 16 and 20 of questionnaire Q4- Sterile injectables Competitors.
77 Bristol-Myers SQB was also mentioned to be present, although in 2012 only in which year it achieved insignificant sales.
78 See replies to question 16 of questionnaire Q4- Sterile injectables Competitors.
79 See replies to question 23 of questionnaire Q4- Sterile injectables Competitors.
80 See replies to question 20, 21, 22 and 24 of questionnaire 5 Sterile injectables Customers.
81 Guidelines on the assessment of the horizontal mergers under the Council Regulation on the control of concentrations between undertakings, 2004/C31/03), para.17.
82 The Notifying Party submits that in 2014 [...] tenders were organized and Parties estimate a further increase to [...] tenders.
83 Guidelines on the assessment of the horizontal mergers under the Council Regulation on the control of concentrations between undertakings, 2004/C31/03), para.32.
84 See replies to question 16 of questionnaire Q4 – Sterile injectables Competitors.
85 See cases M.5295 – Teva/Barr and M.5865 – Teva/Ratiopharm.
86 Under a wider product market definition the market shares would not qualify for a Group 1 market.
87 The fluctuations in market shares reflect the fact that Finland is a true tendering market where market shares of only one year may overstate the actual market power.
88 [...].
89 Guidelines on the assessment of the horizontal mergers under the Council Regulation on the control of concentrations between undertakings, 2004/C31/03), para. 32.
90 See case M.2922 – Pfizer / Pharmacia.
91 Guidelines on the assessment of the horizontal mergers under the Council Regulation on the control of concentrations between undertakings, 2004/C31/03), para.17.
92 Guidelines on the assessment of the horizontal mergers under the Council Regulation on the control of concentrations between undertakings, 2004/C31/03), para.32.
93 The Notifying Party submits that Pfizer currently only offers a lyophilised (dry powder) presentation, which is much more expensive than the solution presentation, offered by its competitors.
94 See replies to question 42 of questionnaire Q4 – Sterile injectables Competitors.
95 See replies to question 51 of questionnaire Q4 – Sterile injectables Competitors.
96 "Lab unknown" refers to the molecule used/"manufactured" directly by the hospitals in the NHS.
97 Annexes to the Form CO RFI-I 3.1 – [...].
98 Guidelines on the assessment of the horizontal mergers under the Council Regulation on the control of concentrations between undertakings, 2004/C31/03), para. 17.
99 See replies to question 20, 21, 22 and 24 of questionnaire 5 Sterile injectables Customers.
100 See case M.5555 – Novartis / Ebewe.
101 See replies to question 63 of questionnaire Q4 – Sterile Injectable Competitors.
102 See replies to question 18 of questionnaire Q5 – Sterile Injectable Customers.
103 See replies to questions 54 and 59 of questionnaire Q4- Sterile injectables Competitors.
104 See replies to question 20, 21, 22 and 24 of questionnaire 5 Sterile injectables Customers.
105 See Hospira's internal documents, [...].
106 See case M.5295 – Teva/Barr.
107 Moreover the Notifying Party argues that Hospira does not have the 20ml dosage in its portfolio.
108 See in particular replies to question 20, 21, 22 and 24 of questionnaire Q5 – Sterile injectables Customers (Norway).
109 Guidelines on the assessment of the horizontal mergers under the Council Regulation on the control of concentrations between undertakings, 2004/C31/03), para. 32.
110 See case M.3354 – Sanofi-Synthelabo/Aventis.
111 See replies to question 89 of questionnaire Q4 – Sterile Injectable Competitors.
112 See replies to question 18 of questionnaire Q5 – Sterile Injectable Customers.
113 See replies to questions 54 and 59 of questionnaire Q4 – Sterile injectables Competitors.
114 See replies to question 20, 21, 22 and 24 of questionnaire Q5 – Sterile injectables Customers.
115 See Pfizer's internal documents, [...].
116 See Pfizer's internal document [...].
117 See replies to question 116 of questionnaire Q4 – Sterile injectables Competitors.
118 See case M.5476 – Pfizer/Wyeth.
119 See replies to questions 119 and 124 of questionnaire Q4 – Sterile injectables Competitors.
120 See in particular replies to question 20, 21, 22 and 24 of questionnaire Q5 – Sterile injectables Customers (Norway).
121 There are other [...] products that both Parties have in their pipeline: [...]. While Hospira is already developing a new product, Pfizer is still in the concept phase. [...] these [...] overlaps will not be further analysed.
122 See cases M.6258 – Teva/Cephalon and M.6613 – Watson/Actavis.
123 See Hospira's internal document – [...].
124 See Hospira's internal document, [...].
125 See Hospira's internal document, [...].
126 See:
http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003737/human med 001866.
jsp&mid=WC0b01ac058001d124
127 See Hospira's internal document [...].
128 Commission Notice on remedies acceptable under Council Regulation (EC) No 139/2004 and under Commission Regulation (EC) No 802/2004 (OJ C 267, 22.10.2008, p. 1-27).
129 Remedies Notice, paragraphs 9 and 61.
130 Remedies Notice, paragraph 12.
131 Remedies Notice, paragraph 10.
132 See replies to questions 3, 5, 25 and 26 of questionnaire R1 – Market Test of the Commitments – Competitors, question 1 of questionnaire R2 – Market Test of the Commitments – Customers biosimilars and questions 1 and 2 of questionnaire R2 – Market Test of the Commitments – Customers sterile injectables.
133 See replies to question 12 of questionnaire R1 – Market Test of the Commitments – Competitors.
134 See replies to questions 19-22 of questionnaire R1 – Market Test of the Commitments – Competitors.
135 See replies to question 6 of questionnaire R1 – Market Test of the Commitments – Competitors.
136 [...].
137 See replies to questions 28 and 29 of questionnaire R1 – Market Test of the Commitments – Competitors, and questions 4-8 of questionnaire R3 – Market Test of the Commitments – Customers sterile injectables.
138 See replies to question 15 of questionnaire R1 – Market Test of the Commitments – Competitors.
139 See replies to question 27 of questionnaire R1 – Market Test of the Commitments – Competitors.
Case M.7559 – PFIZER / HOSPIRA
COMMITMENTS TO THE EUROPEAN COMMISSION
Pursuant to Article 6(2) of Council Regulation (EC) No 139/2004 (the "Merger Regulation"), Pfizer Inc. (the "Notifying Party" or "Pfizer") hereby enter into the following Commitments (the "Commitments") vis-à-vis the European Commission (the "Commission") with a view to rendering the acquisition of Hospira, Inc. ("Hospira") (the "Concentration") compatible with the internal market and the functioning of the EEA Agreement.
This text shall be interpreted in light of the Commission's decision pursuant to Article 6(1)(b),of the Merger Regulation to declare the Concentration compatible with the internal market and the functioning of the EEA Agreement (the "Decision"), in the general framework of European Union law, in particular in light of the Merger Regulation, and by reference to the Commission Notice on remedies acceptable under Council Regulation (EC) No 139/2004 and under Commission Regulation (EC) No 802/2004 (the "Remedies Notice").
Section A. Definitions
1. For the purpose of the Commitments, the following terms shall have the following meaning:
Affiliated Undertakings: undertakings controlled by the Parties and/or by the ultimate parents of the Parties, whereby the notion of control shall be interpreted pursuant to Article 3 of the Merger Regulation and in light of the Commission Consolidated Jurisdictional Notice under Council Regulation (EC) No 139/2004 on the control of concentrations between undertakings (the "Consolidated Jurisdictional Notice").
Assets: the assets that contribute to the current operation or are necessary to ensure the viability and competitiveness of the Divestment Business as indicated in Section B, paragraph 5 (a), (b) and (c) and described more in detail in the Schedules.
Closing: the transfer of the legal title to the Divestment Business to the Purchaser.
Closing Period: the period of 3 months from the approval of the Purchaser and the terms of sale by the Commission.
Confidential Information: any business secrets, know-how, commercial information, or any other information of a proprietary nature that is not in the public domain, other than, as far as the Infliximab Divestment Business is concerned, such information as is or will be licensed to Pfizer pursuant to Schedule 1.
Conflict of Interest: any conflict of interest that impairs the Trustee's objectivity and independence in discharging its duties under the Commitments.
Divestment Businesses: Infliximab Divestment Business, Carboplatin Divestment Business, Cytarabine Divestment Business, Epirubicin Divestment Business, Irinotecan Divestment Business, Vancomycin Divestment Business and Voriconazole Divestment Business as defined in Section B and the Schedules which the Notifying Party commits to divest (each individual business is referred to as a "Divestment Business").
Divestiture Trustee: one or more natural or legal person(s) who is/are approved by the Commission and appointed by the Notifying Party and who has/have received from the Notifying Party the exclusive Trustee Mandate to sell the Divestment Business to a Purchaser at no minimum price.
Effective Date: the date of adoption of the Decision.
First Divestiture Period: the period of [...] from the Effective Date.
Hold Separate Manager: the person appointed by the Notifying Party for the Divestment Business to manage the day-to-day business under the supervision of the Monitoring Trustee. It is understood that separate Hold Separate Managers may be appointed, to the extent needed, for different Divestment Businesses.
Hospira: Hospira, Inc. and Affiliated Undertakings.
Key Personnel: all Key Personnel (if any) listed in the Schedule(s), including the Hold Separate Manager(s).
Monitoring Trustee: one or more natural or legal person(s) who is/are approved by the Commission and appointed by the Notifying Party, and who has/have the duty to monitor the Notifying Party's compliance with the conditions and obligations attached to the Decision.
Parties: Pfizer and Hospira.
Personnel: all personnel necessary to maintain the viability and competitiveness of the Divestment Business as described in the Schedule(s).
Pfizer: Pfizer Inc. and Affiliated Undertakings.
Purchaser: the entity approved by the Commission as acquirer of the Divestment Business in accordance with the criteria set out in Section D.
Purchaser Criteria: the criteria laid down in paragraph 18 of these Commitments that the Purchaser must fulfil in order to be approved by the Commission.
Schedules: the schedules to these Commitments describing more in detail the Divestment Businesses.
Trustee(s): the Monitoring Trustee and/or the Divestiture Trustee as the case may be.
Trustee Divestiture Period: the period of [...] from the end of the First Divestiture Period.
Section B. The commitment to divest and the Divestment Business Commitment to divest
2. In order to maintain effective competition, Pfizer commits to divest, or procure the divestiture of, the Divestment Businesses by the end of the Trustee Divestiture Period as a going concern to one or more Purchasers and on terms of sale approved by the Commission in accordance with the procedure described in paragraph 19 of these Commitments. To carry out the divestiture, Pfizer commits to find one or more Purchasers and to enter into a final binding sale and purchase agreement for the sale of each of the Divestment Businesses within the First Divestiture Period. If Pfizer has not entered into such an agreement at the end of the First Divestiture Period, Pfizer shall grant the Divestiture Trustee an exclusive mandate to sell the Divestment Business in accordance with the procedure described in paragraph 31 in the Trustee Divestiture Period.
3. Pfizer shall be deemed to have complied with this commitment if:
(a) by the end of the Trustee Divestiture Period, Pfizer or the Divestiture Trustee has entered into a final binding sale and purchase agreement and the Commission approves the proposed Purchaser and the terms of sale as being consistent with the Commitments in accordance with the procedure described in paragraph 19 for each of the Divestment Businesses;
(b) the Closing of the sale of the Divestment Businesses to the Purchaser takes place within the Closing Period; and
(c) the transfer of the development of the Product and the Clinical Trial have been effected as described in Schedule 1.
4. In order to maintain the structural effect of the Commitments, Pfizer shall, for a period
of 10 years after Closing, not acquire, whether directly or indirectly, the possibility of exercising influence (as defined in paragraph 43 of the Remedies Notice, footnote 3) over the whole or part of the Divestment Business, unless, following the submission of a reasoned request from the Notifying Party showing good cause and accompanied by a report from the Monitoring Trustee (as provided in paragraph 45 of these Commitments), the Commission finds that the structure of the market has changed to such an extent that the absence of influence over the Divestment Business is no longer necessary to render the proposed concentration compatible with the internal market.
Structure and definition of the Divestment Business
5. The Divestment Businesses consist of the Infliximab Divestment Business,
Carboplatin Divestment Business, Cytarabine Divestment Business, Epirubicin Divestment Business, Irinotecan Divestment Business, Vancomycin Divestment Business and Voriconazole Divestment Business. The legal and functional structure of each of the Divestment Business as operated to date is described in the corresponding Schedule. The Divestment Businesses, described in more detail in the Schedules, include all assets that contribute to the current operation or are necessary to ensure the viability and competitiveness of each of the Divestment Businesses, in particular:
(a) all tangible and intangible assets (including intellectual property rights);
(b) all licences, permits and authorisations issued by any governmental organisation for the benefit of the Divestment Business, as described in the Schedules;
(c) all contracts, leases, commitments and customer orders of the Divestment Business; all customer, credit and other records of the Divestment Business; and
(d) the Personnel.
6. For the avoidance of doubt, the Schedules form an integral part of these Commitments.
7. In addition, the Divestment Businesses include the benefit, for a transitional period of up to [...] after Closing and on terms and conditions equivalent to those at present afforded to each of the Divestment Business, of all current arrangements under which Pfizer or its Affiliated Undertakings supply products or services to the Divestment Business, as detailed in the Schedules, unless otherwise agreed with the Purchaser.
8. Strict firewall procedures will be adopted so as to ensure that any competitively sensitive information related to, or arising from such supply arrangements (for example, product roadmaps) will not be shared with, or passed on to, anyone other than for the purpose of implementation of these Commitments.
Section C. Related commitments
Preservation of viability, marketability and competitiveness
9. From the Effective Date until Closing, Pfizer shall preserve or procure the preservation of the economic viability, marketability and competitiveness of the Divestment Business, in accordance with good business practice, and shall minimise as far as possible any risk of loss of competitive potential of the Divestment Business. In particular Pfizer undertakes:
(a) not to carry out any action that might have a significant adverse impact on the value, management or competitiveness of the Divestment Business or that might alter the nature and scope of activity, or the industrial or commercial strategy or the investment policy of the Divestment Business;
(b) to make available, or procure to make available, sufficient resources for the development of the Divestment Business, on the basis and continuation of the existing business plans; and
(c) to take all reasonable steps, or procure that all reasonable steps are being taken, including appropriate incentive schemes (based on industry practice), to encourage all Key Personnel to remain with the Divestment Business, and not to solicit or move any Personnel to Pfizer's remaining business. Where, nevertheless, individual members of the Key Personnel exceptionally leave the Divestment Business, Pfizer shall provide a reasoned proposal to replace the person or persons concerned to the Commission and the Monitoring Trustee. Pfizer must be able to demonstrate to the Commission that the replacement is well suited to carry out the functions exercised by those individual members of the Key Personnel. The replacement shall take place under the supervision of the Monitoring Trustee, who shall report to the Commission.
Hold-separate obligations
10. Pfizer commits, from the Effective Date until Closing, to the extent reasonably
practical, to keep the Divestment Business separate from the business(es) it is retaining and to ensure that unless explicitly permitted under these Commitments: (i) management and staff of the business(es) retained by Pfizer have no involvement in the Divestment Business; (ii) the Key Personnel and Personnel of the Divestment Business have no involvement in any business retained by Pfizer and do not report to any individual outside the Divestment Business.
11. Until Closing, Pfizer shall assist the Monitoring Trustee in ensuring that the
Divestment Business is managed as a distinct and saleable entity separate from the business(es) which Pfizer is retaining and in accordance with provision 10 above. Immediately after the adoption of the Decision:
(a) Pfizer shall appoint a specific Hold Separate Manager for the Infliximab Divestment Business, upon consultation with the Monitoring Trustee and the Commission;
(b) By way of a derogation from the provision 11 above, for the Cytarabine Divestment Business, Epirubicin Divestment Business, Irinotecan Divestment Business and Voriconazole Divestment Business, Pfizer shall appoint one or more Hold Separate Manager(s) immediately after the closing date of the acquisition of Hospira by Pfizer;
(c) For the avoidance of doubt, a Hold Separate Manager(s) for the Carboplatin Divestment Business and the Vancomycin Divestment Business will be appointed by Pfizer immediately after the adoption of the Decision.
12. The Hold Separate Manager(s), who shall be part of the Key Personnel, shall manage the Divestment Business independently and in the best interest of the business with a view to ensuring its continued economic viability, marketability and competitiveness and its independence from the businesses retained by Pfizer. The Hold Separate Manager shall closely cooperate with, and report to, the Monitoring Trustee and, if applicable, the Divestiture Trustee. Any replacement of the Hold Separate Manager shall be subject to the procedure laid down in paragraph 9(c) of these Commitments. The Commission may, after having heard Pfizer, require Pfizer to replace the Hold Separate Manager. To preserve Pfizer's legitimate interest in the Infliximab Divestment Business post Closing date, Pfizer shall continue to have authority to decide together with the Hold Separate Manager, under the supervision of the Monitoring Trustee, on the Key Decisions listed in Schedule 1.
Ring-fencing
13. Pfizer shall implement, or procure to implement, all necessary measures to ensure that
it does not, after the Effective Date, obtain any Confidential Information relating to the Divestment Business and that any such Confidential Information obtained by Pfizer before the Effective Date will be eliminated and not be used by Pfizer. This includes measures vis-à-vis Pfizer's appointees on the supervisory board and/or board of directors of the Divestment Business. In particular, the participation of the Divestment Business in any central information technology network shall be severed to the extent possible, without compromising the viability of the Divestment Business. Pfizer may obtain or keep information relating to the Divestment Business which is reasonably necessary for the divestiture of the Divestment Business, or the disclosure of which to Pfizer is required by law.
Non-solicitation clause
14. The Parties undertake, subject to customary limitations, not to solicit, and to procure
that Affiliated Undertakings do not solicit, the Key Personnel transferred with the Divestment Business for a period of [...] after Closing.
Due diligence
15. In order to enable potential purchasers to carry out a reasonable due diligence of the
Divestment Business, Pfizer shall, subject to customary confidentiality assurances and dependent on the stage of the divestiture process:
(a) provide to potential purchasers sufficient information as regards the Divestment Business; and
(b) provide to potential purchasers sufficient information relating to the Personnel and allow them reasonable access to the Personnel.
Reporting
16. Pfizer shall submit written reports in English on potential purchasers of the
Divestment Business and developments in the negotiations with such potential purchasers to the Commission and the Monitoring Trustee no later than 10 working days after the end of every month following the Effective Date (or otherwise at the Commission's request). Pfizer shall submit a list of all potential purchasers having expressed interest in acquiring the Divestment Business to the Commission at each and every stage of the divestiture process, as well as a copy of all the offers made by potential purchasers within five working days of their receipt.
17. Pfizer shall inform the Commission and the Monitoring Trustee on the preparation of
the data room documentation and the due diligence procedure and shall submit a copy of any information memorandum to the Commission and the Monitoring Trustee before sending the memorandum out to potential purchasers.
Section D. The Purchaser
18. In order to be approved by the Commission, the Purchaser must fulfil the following criteria:
(i) For each of the Divestment Businesses,
(a) The Purchaser shall be independent of, and unconnected to, the Notifying Party and its Affiliated Undertakings (this being assessed having regard to the situation following the divestiture);
(b) The Purchaser shall have the financial resources, proven expertise and incentive to maintain and develop the Divestment Business as a viable and active competitive force in competition with the Parties and other competitors; and
(c) The acquisition of the Divestment Business by the Purchaser must neither be likely to create, in light of the information available to the Commission, prima facie competition concerns nor give rise to a risk that the implementation of the Commitments will be delayed. In particular, the Purchaser must reasonably be expected to obtain all necessary approvals from the relevant regulatory authorities for the acquisition of the Divestment Business.
(ii) As far as the Infliximab Divestment Business is concerned, the Purchaser, [...].
(iii) The Purchaser should, to the extent related to, respectively, the Carboplatin Divestment Business, Cytarabine Divestment Business, Epirubicin Divestment Business, Irinotecan Divestment Business and Vancomycin Divestment Business, [...].
19. The final binding sale and purchase agreement (as well as ancillary agreements) relating to the divestment of each of the Divestment Businesses shall be conditional on the Commission's approval. When Pfizer has reached an agreement with a purchaser, it shall submit a fully documented and reasoned proposal, including a copy of the final agreement(s), within one week to the Commission and the Monitoring Trustee. Pfizer must be able to demonstrate to the Commission that the purchaser fulfils the Purchaser Criteria and that each of the Divestment Businesses is being sold in a manner consistent with the Commission's Decision and the Commitments. For the approval, the Commission shall verify that the purchaser fulfils the Purchaser Criteria and that each of the Divestment Businesses is being sold in a manner consistent with the Commitments including their objective to bring about a lasting structural change in the market. The Commission may approve the sale of each the Divestment Businesses without one or more Assets or parts of the Personnel, or by substituting one or more Assets or parts of the Personnel with one or more different assets or different personnel, if this does not affect the viability and competitiveness of the Divestment Business after the sale, taking account of the proposed purchaser.
Section E. Trustee
I. Appointment procedure
20. Pfizer shall appoint a Monitoring Trustee to carry out the functions specified in these Commitments for a Monitoring Trustee. Pfizer commits not to close the Concentration before the appointment of a Monitoring Trustee.
21. If Pfizer has not entered into a binding sale and purchase agreement regarding the Divestment Business one month before the end of the First Divestiture Period or if the Commission has rejected a purchaser proposed by Pfizer at that time or thereafter, Pfizer shall appoint a Divestiture Trustee. The appointment of the Divestiture Trustee shall take effect upon the commencement of the Trustee Divestiture Period.
22. The Trustee shall:
(i) at the time of appointment, be independent of Pfizer and its Affiliated Undertakings;
(ii) possess the necessary qualifications to carry out its mandate, for example have sufficient relevant experience as an investment banker or consultant or auditor; and
(iii) neither have nor become exposed to a Conflict of Interest.
23. The Trustee shall be remunerated by the Pfizer in a way that does not impede the independent and effective fulfilment of its mandate. In particular, where the remuneration package of a Divestiture Trustee includes a success premium linked to the final sale value of the Divestment Business, such success premium may only be earned if the divestiture takes place within the Trustee Divestiture Period.
Proposal by Pfizer
24. No later than two weeks after the Effective Date, Pfizer shall submit the name or names of one or more natural or legal persons whom Pfizer proposes to appoint as the Monitoring Trustee to the Commission for approval. No later than one month before the end of the First Divestiture Period or on request by the Commission, Pfizer shall submit a list of one or more persons whom Pfizer proposes to appoint as Divestiture Trustee to the Commission for approval. The proposal shall contain sufficient information for the Commission to verify that the person or persons proposed as Trustee fulfil the requirements set out in paragraph 22 and shall include:
(a) the full terms of the proposed mandate, which shall include all provisions necessary to enable the Trustee to fulfil its duties under these Commitments;
(b) the outline of a work plan which describes how the Trustee intends to carry out its assigned tasks; and
(c) an indication whether the proposed Trustee is to act as both Monitoring Trustee and Divestiture Trustee or whether different trustees are proposed for the two functions.
Approval or rejection by the Commission
25. The Commission shall have the discretion to approve or reject the proposed Trustee(s) and to approve the proposed mandate subject to any modifications it deems necessary for the Trustee to fulfil its obligations. The Commission shall approve or reject the proposed Trustee(s) within one week of the proposal by Pfizer. If only one name is approved, Pfizer shall appoint or cause to be appointed the person or persons concerned as Trustee, in accordance with the mandate approved by the Commission. If more than one name is approved, Pfizer shall be free to choose the Trustee to be appointed from among the names approved. The Trustee shall be appointed within one week of the Commission's approval, in accordance with the mandate approved by the Commission.
New proposal by Pfizer
26. If all the proposed Trustees are rejected, Pfizer shall submit the names of at least two more natural or legal persons within one week of being informed of the rejection, in accordance with paragraphs 20 and 25 of these Commitments.
Trustee nominated by the Commission
27. If all further proposed Trustees are rejected by the Commission, the Commission shall nominate a Trustee, whom Pfizer shall appoint, or cause to be appointed, in accordance with a trustee mandate approved by the Commission.
II. Functions of the Trustee
28. The Trustee shall assume its specified duties and obligations in order to ensure compliance with the Commitments. The Commission may, on its own initiative or at the request of the Trustee or Pfizer, give any orders or instructions to the Trustee in order to ensure compliance with the conditions and obligations attached to the Decision.
Duties and obligations of the Monitoring Trustee
29. The Monitoring Trustee shall:
(i) propose in its first report to the Commission a detailed work plan describing how it intends to monitor compliance with the obligations and conditions attached to the Decision.
(ii) oversee, in close co-operation with the Hold Separate Manager, the on-going management of the Divestment Business with a view to ensuring its continued economic viability, marketability and competitiveness and monitor compliance by Pfizer with the conditions and obligations attached to the Decision. To that end the Monitoring Trustee shall:
(a) monitor the preservation of the economic viability, marketability and competitiveness of the Divestment Business, and the keeping separate of the Divestment Business from the business retained by the Parties, in accordance with paragraphs 9 and 10 of these Commitments;
(b) supervise the management of the Divestment Business as a distinct and saleable entity, in accordance with paragraph 11 of these Commitments;
(c) with respect to Confidential Information, in consultation with Pfizer and the Hold Separate Manager(s):
- determine all necessary measures to ensure that Pfizer does not after the Effective Date obtain any Confidential Information relating to the Divestment Business, in accordance with paragraph 13 of these Commitments,
- in particular strive for the severing of the Divestment Business' participation in a central information technology network to the extent possible, without compromising the viability of the Divestment Business,
- make sure that any Confidential Information relating to the Divestment Business obtained by Pfizer before the Effective Date is eliminated and will not be used by Pfizer, and
- decide whether such information may be disclosed to or kept by Pfizer as the disclosure is reasonably necessary to allow Pfizer to carry out the divestiture or as the disclosure is required by law;
(d) monitor the splitting of assets and the allocation of Personnel between the Divestment Business and Pfizer or Affiliated Undertakings;
(iii) propose to Pfizer such measures as the Monitoring Trustee considers necessary to ensure Pfizer's compliance with the conditions and obligations attached to the Decision, in particular the maintenance of the full economic viability, marketability or competitiveness of the Divestment Business, the holding separate of the Divestment Business and the non-disclosure of competitively sensitive information;
(iv) review and assess potential purchasers as well as the progress of the divestiture process and verify that, dependent on the stage of the divestiture process:
(a) potential purchasers receive sufficient and correct information relating to the Divestment Business and the Personnel in particular by reviewing, if available, the data room documentation, the information memorandum and the due diligence process, and
(b) potential purchasers are granted reasonable access to the Personnel.
(v) act as a contact point for any requests by third parties, in particular potential purchasers, in relation to the Commitments;
(vi) provide to the Commission, sending Pfizer a non-confidential copy at the same time, a written report within 15 days after the end of every month that shall cover the operation and management of the Divestment Business as well as the splitting of assets and the allocation of Personnel so that the Commission can assess whether the business is held in a manner consistent with the Commitments and the progress of the divestiture process as well as potential purchasers;
(vii) promptly report in writing to the Commission, sending Pfizer a non-confidential copy at the same time, if it concludes on reasonable grounds that Pfizer is failing to comply with these Commitments;
(viii) within one week after receipt of the documented proposal referred to in paragraph 19 of these Commitments, submit to the Commission, sending Pfizer a non-confidential copy at the same time, a reasoned opinion as to the suitability and independence of the proposed purchaser and the viability of the Divestment Business after the Sale and as to whether the Divestment Business is sold in a manner consistent with the conditions and obligations attached to the Decision, in particular, if relevant, whether the Sale of the Divestment Business without one or more Assets or not all of the Personnel affects the viability of the Divestment Business after the sale, taking account of the proposed purchaser;
(ix) assume the other functions assigned to the Monitoring Trustee under the Schedules; and
(x) assume the other functions assigned to the Monitoring Trustee under the conditions and obligations attached to the Decision.
30. If the Monitoring and Divestiture Trustee are not the same legal or natural persons, the Monitoring Trustee and the Divestiture Trustee shall cooperate closely with each other during and for the purpose of the preparation of the Trustee Divestiture Period in order to facilitate each other's tasks.
Duties and obligations of the Divestiture Trustee
31. Within the Trustee Divestiture Period, the Divestiture Trustee shall sell at no minimum price the Divestment Business to a purchaser, provided that the Commission has approved both the purchaser and the final binding sale and purchase agreement (and ancillary agreements) as in line with the Commission's Decision and the Commitments in accordance with paragraphs 18 and 19 of these Commitments. The Divestiture Trustee shall include in the sale and purchase agreement (as well as in any ancillary agreements) such terms and conditions as it considers appropriate for an expedient sale in the Trustee Divestiture Period. In particular, the Divestiture Trustee may include in the sale and purchase agreement such customary representations and warranties and indemnities as are reasonably required to effect the sale. The Divestiture Trustee shall protect the legitimate financial interests of Pfizer, subject to Pfizer's unconditional obligation to divest at no minimum price in the Trustee Divestiture Period.
32. In the Trustee Divestiture Period (or otherwise at the Commission's request), the Divestiture Trustee shall provide the Commission with a comprehensive monthly report written in English on the progress of the divestiture process. Such reports shall be submitted within 15 days after the end of every month with a simultaneous copy to the Monitoring Trustee and a non-confidential copy to Pfizer.
III. Duties and obligations of the Parties
33. Pfizer shall provide and shall cause its advisors to provide the Trustee with all such co-operation, assistance and information as the Trustee may reasonably require to perform its tasks. The Trustee shall have full and complete access to any of Pfizer's or the Divestment Business' books, records, documents, management or other personnel, facilities, sites and technical information necessary for fulfilling its duties under the Commitments and Pfizer and the Divestment Business shall provide the Trustee upon request with copies of any document. Pfizer and the Divestment Business shall make available to the Trustee one or more offices on their premises and shall be available for meetings in order to provide the Trustee with all information necessary for the performance of its tasks.
34. Pfizer shall provide the Monitoring Trustee with all managerial and administrative support that it may reasonably request on behalf of the management of the Divestment Business. This shall include all administrative support functions relating to the Divestment Business which are currently carried out at headquarters level. Pfizer shall provide and shall cause its advisors to provide the Monitoring Trustee, on request, with the information submitted to potential purchasers, in particular give the Monitoring Trustee access to the data room documentation and all other information granted to potential purchasers in the due diligence procedure. Pfizer shall inform the Monitoring Trustee on possible purchasers, submit lists of potential purchasers at each stage of the selection process, including the offers made by potential purchasers at those stages, and keep the Monitoring Trustee informed of all developments in the divestiture process.
35. Pfizer shall grant or procure Affiliated Undertakings to grant comprehensive powers of attorney, duly executed, to the Divestiture Trustee to effect the sale (including ancillary agreements), the Closing and all actions and declarations which the Divestiture Trustee considers necessary or appropriate to achieve the sale and the Closing, including the appointment of advisors to assist with the sale process. Upon request of the Divestiture Trustee, Pfizer shall cause the documents required for effecting the sale and the Closing to be duly executed.
36. Pfizer shall indemnify the Trustee and its employees and agents (each an "Indemnified Party") and hold each Indemnified Party harmless against, and hereby agrees that an Indemnified Party shall have no liability to Pfizer for, any liabilities arising out of the performance of the Trustee's duties under the Commitments, except to the extent that such liabilities result from the wilful default, recklessness, gross negligence or bad faith of the Trustee, its employees, agents or advisors.
37. At the expense of Pfizer, the Trustee may appoint advisors (in particular for corporate finance or legal advice), subject to Pfizer's approval (this approval not to be unreasonably withheld or delayed) if the Trustee considers the appointment of such advisors necessary or appropriate for the performance of its duties and obligations under the Mandate, provided that any fees and other expenses incurred by the Trustee are reasonable. Should Pfizer refuse to approve the advisors proposed by the Trustee the Commission may approve the appointment of such advisors instead, after having heard Pfizer. Only the Trustee shall be entitled to issue instructions to the advisors. Paragraph 33 of these Commitments shall apply mutatis mutandis. In the Trustee Divestiture Period, the Divestiture Trustee may use advisors who served Pfizer during the Divestiture Period if the Divestiture Trustee considers this in the best interest of an expedient sale.
38. Pfizer agrees that the Commission may share Confidential Information proprietary to Hospira with the Trustee. The Trustee shall not disclose such information and the principles contained in Article 17 (1) and (2) of the Merger Regulation apply mutatis mutandis.
39. Pfizer agrees that the contact details of the Monitoring Trustee are published on the website of the Commission's Directorate-General for Competition and they shall inform interested third parties, in particular any potential purchasers, of the identity and the tasks of the Monitoring Trustee.
40. For a period of 10 years from the Effective Date the Commission may request all information from the Parties that is reasonably necessary to monitor the effective implementation of these Commitments.
IV. Replacement, discharge and reappointment of the Trustee
41. If the Trustee ceases to perform its functions under the Commitments or for any other good cause, including the exposure of the Trustee to a Conflict of Interest:
(a) the Commission may, after hearing the Trustee and Pfizer, require Pfizer to replace the Trustee; or
(b) Pfizer may, with the prior approval of the Commission, replace the Trustee.
42. If the Trustee is removed according to paragraph 38 of these Commitments, the Trustee may be required to continue in its function until a new Trustee is in place to whom the Trustee has effected a full hand over of all relevant information. The new Trustee shall be appointed in accordance with the procedure referred to in paragraphs 20-27 of these Commitments.
43. Unless removed according to paragraph 41 of these Commitments, the Trustee shall cease to act as Trustee only after the Commission has discharged it from its duties after all the Commitments with which the Trustee has been entrusted have been implemented. However, the Commission may at any time require the reappointment of the Monitoring Trustee if it subsequently appears that the relevant remedies might not have been fully and properly implemented.
Section F. The review clause
44. The Commission may extend the time periods foreseen in the Commitments in response to a request from Pfizer or, in appropriate cases, on its own initiative. Where Pfizer requests an extension of a time period, it shall submit a reasoned request to the Commission no later than one month before the expiry of that period, showing good cause. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time send a non-confidential copy of the report to the Notifying Party. Only in exceptional circumstances shall Pfizer be entitled to request an extension within the last month of any period.
45. The Commission may further, in response to a reasoned request from Pfizer showing good cause waive, modify or substitute, in exceptional circumstances, one or more of the undertakings in these Commitments. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time send a non-confidential copy of the report to the Pfizer. The request shall not have the effect of suspending the application of the undertaking and, in particular, of suspending the expiry of any time period in which the undertaking has to be complied with.
Section G. Entry into force
46. The Commitments shall take effect upon the date of adoption of the Decision.
duly authorised for and on behalf of
Pfizer Inc.
SCHEDULE 1
INFLIXIMAB
1. The Divestment Business as operated to date has the following legal and functional
structure:
(a) The infliximab biosimilar pipeline product (the "Product") is under development by Pfizer and is now undergoing a Phase 3 clinical trial (the "Clinical Trial");
(b) The manufacturing process of the Product incorporates certain intellectual property rights of [...]; Pfizer has obtained a license from [...] to use [...] system to manufacture and sell the Product and multiple other products;
(c) The manufacturing of the Product was outsourced by Pfizer to [...];
(d) The Clinical Trial is conducted for Pfizer by [...] as a single clinical trial on a global basis;
(e) Since the Product is not approved for marketing, there are no sales or marketing teams for the Product and there is only limited stock of the product;
(e) The development of the Product is part of Pfizer's broader biosimilars development program and is currently supported within Pfizer's organisation by [...].
2. In accordance with Section B paragraph 5 of these Commitments, the Divestment
Business includes, but is not limited to:
(a) the following main tangible assets (together with the intangible assets listed below the "Transferred Assets"):
(i) All relevant Clinical Trial reports
(ii) Inventories of Product for use in the ongoing Clinical Trial of the Product
(iii) As far as the EEA regulatory files for the Product are concerned: the current investigator brochure, the investigational medicinal product dossier (the IMPD) and the protocol for the Clinical Trial; the Statistical Analysis Plan (SAP); minutes and correspondence regarding interactions with the EEA regulators regarding the Product, including the scientific advice from EEA regulators;
(iv) As far as the regulatory files outside the EEA for the Products are concerned: an option for the Purchaser to obtain a transfer of the investigator brochure, the application documents and minutes and correspondence regarding interactions with the non-EEA regulators regarding the Products, including scientific advice from the non-EEA regulators, subject to the Purchaser granting (i) continuing control over all interactions with non-EEA regulators to Pfizer and (ii) an exclusive (including as to Purchaser), royalty-free, perpetual, irrevocable and sub-licensable license to Pfizer to use the non-EEA regulatory files for any regulatory approvals for the Product for any non-EEA markets (it being understood that the Purchaser shall be able to use the non-EEA regulatory files for any regulatory approvals for the Product for the EEA markets);
(v) All relevant books and records relating exclusively to the Product, save for the books and records which relate exclusively to the clinical development or manufacture of the Product in or for any non-EEA markets; the books and records relating to the Product that also relate to other products developed or to be developed by Pfizer or its affiliates, shall only be transferred to the extent that they relate to the Product, it being understood that the other sections shall be redacted prior to the transfer to the Purchaser;
(vi) An option for the Purchaser to obtain a transfer of the books and records which relate exclusively to the clinical development or manufacture of the Product in or for any non-EEA market, subject to the Purchaser granting an exclusive (including as to Purchaser), royalty-free, perpetual, irrevocable and sub-licensable license to Pfizer to use the non-EEA books and records to conduct non-EEA country specific development activities, manufacture, have manufactured or market the Product in or for any countries other than the EEA countries (it being understood that the Purchaser shall be able to use the non-EEA books and records for EEA country specific development activities, and to manufacture, have manufactured or market the Product in or for any EEA countries);
(vii) Marketing plans and forecasts which are specific for the Product; the marketing plans and forecasts that also relate to other products developed or to be developed by Pfizer or its affiliates, shall only be transferred to the extent that they relate to the Product, it being understood that the other sections shall be redacted prior to the transfer to the Purchaser;
(viii) Any other assets identified by the Purchaser and Pfizer in the asset purchase agreement as overseen by the Monitoring Trustee.
(b) the following main intangible assets:
(i) Right to conduct the Clinical Trial and rights to develop the Product globally, subject to an exclusive (including as to Purchaser), royalty-free, perpetual, irrevocable and sub-licensable license to Pfizer to conduct non-EEA country specific development activities (it being understood that the Purchaser and Pfizer shall (i) provide mutual support to each other in relation to the market approvals for the Product in relation to their respective markets and (ii) exchange information in relation to any country specific development activities for the Product which are relevant for the other party's market);
(ii) Right to manufacture, have manufactured and market the Product globally, subject to an exclusive (including as to Purchaser), royalty-free, perpetual, irrevocable and sub-licensable license to Pfizer to manufacture, have manufactured and market the Product in or for any countries other than the EEA member states;
(iii) Sponsorship of any and all current Clinical Trial Authorisations or other regulatory filings for the Product in the EEA;
(iv) Patents, copyrights, data and know-how existing as of the Closing date and relating exclusively to the clinical development, manufacture or sale of the Product (the "In-Scope IP"), as set out in further detail in these Commitments; for the avoidance of doubt, the In-Scope IP does not include any patents, copyrights, data and know-how that also relates to other products developed or to be developed by Pfizer or its affiliates;
(v) A royalty-free, perpetual, irrevocable and sub-licensable license to patents, copyrights, data and know-how existing as of the Closing date necessary for the development, manufacturing or sale of the Product in the EEA other than In-scope IP, to be used solely by the Purchaser to perform development activities in relation to the Product and to seek and maintain regulatory approval for, manufacture, have manufactured and sell the Product in the EEA and in a manner which ensures that Pfizer's proprietary rights in relation to these patents, copyrights, data and know-how are adequately protected as overseen by the Monitoring Trustee; furthermore, Pfizer will, at the request of the Purchaser, assist the Purchaser in transferring technical information and know-how to a manufacturer selected after consultation among Purchaser, Pfizer and the Monitoring Trustee (during which consultation the reasonable comments and concerns of all three parties will be considered and addressed in good faith), in a manner which ensures that Pfizer's proprietary rights in relation to the manufacturing Process of the Product are adequately protected as overseen by the Monitoring Trustee;
(vi) Subject to having obtained all required regulatory and data protection consents, the Clinical Trial database, which will be transferred at a date to be mutually agreed by the Purchaser and Pfizer, as overseen by the Monitoring Trustee.
(c) the following main licences, permits and authorisations:
(i) To the extent that Pfizer has obtained rights to any of the Transferred Assets through a license or other agreement with a third party, Pfizer will assign its rights there under or grant a sublicense there under, as the case may be, to the extent Pfizer is permitted to do so in accordance with the terms of such license agreements, and if consent of any third party licensor is needed, Pfizer will use its best efforts to obtain any required consents;
(ii) All licences, permits and authorisations issued by any governmental organisation for the benefit of the Divestment Business, unless excluded under these Commitments. Since the Product is still in the clinical trial phase, no permits or authorisation have been granted yet in relation to the Product, other than the authorizations with respect to the Clinical Trial referred to in Section 2(a) above and in the Section 4 below.
(d) the following main contracts, agreements, leases, commitments and understandings
Contracts with third parties existing as of the Closing date to the extent relating exclusively to the clinical development of the Product. If any such contracts cannot be assigned to the Purchaser pursuant to their terms or because the scope of the contract includes products or services not related to the Product (as is the case for the contract with [...], with [...] and with [...]), Pfizer will use its best efforts to assist Purchaser in putting in place appropriate alternative arrangements with the relevant third parties or obtaining consents or waivers from such third parties as appropriate.
(e) the following customer, credit and other records:
(i) Since the Product is still in clinical trial phase, it is not yet being supplied for commercial use to customers;
(ii) Pfizer will provide the full list of Key Opinion Leaders for the Product on the date of Closing to the Purchaser.
(f) the following Personnel:
All personnel of Pfizer which at the time of Closing provides substantial support to the Infliximab Divestment Business and which is necessary to continue to develop, manufacture, have manufactured and market the Product as determined by the Purchaser and Pfizer overseen by the Monitoring Trustee, taking into account (i) the identity of the Purchaser, (ii) the transitional services provided by Pfizer to the Purchaser referred to under section 2(h) of this Schedule; and (iii) the rights granted to Pfizer for non-EEA countries in accordance with this Schedule. For the avoidance of doubt, the Purchaser's requests in relation to Personnel shall remain reasonable at all times.
(g) the following Key Personnel:
Key Personnel are the persons holding the functions below:
i) Global Infliximab Asset Lead (US based)
ii) Global Infliximab Medical Affairs Lead (US based)
iii) Global Infliximab Clinical Lead (US based)
iv) Global Infliximab Project Manager (US based)
v) Global Infliximab Study Clinician (US based)
vi) Global Infliximab Commercial Lead (US based)
vii) Global Infliximab Regulatory Lead (US based)
(h) the arrangements for the supply with the following products or services by Pfizer or Affiliated Undertakings:
(i) At the option of the Purchaser, Pfizer (or an affiliated undertaking) will provide:
* reasonable clinical development assistance to the Purchaser in connection with the completion of the Clinical Trial, and
* reasonable support to the Purchaser in relation to the market approvals and post-authorisation procedures for the Product in the EEA, including, but not limited to, demonstration of bioequivalence to the reference product based on pre-clinical data
such assistance to be provided for up to a period of [...] unless an extension has been mutually agreed between Pfizer and Purchaser, at a reasonable negotiated rate to be agreed with the Purchaser as overseen by the Monitoring Trustee.
(ii) At the option of the Purchaser, Pfizer shall enter into a transitory nonexclusive manufacturing and/or supply agreement for the infliximab drug product (DP) for up to [...]. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Pfizer to the Purchaser. It shall not contain provisions requiring the delivery of minimum or maximum supply volumes or batches.
(iii) In addition, at the request of the Purchaser, Pfizer will undertake the technology transfer of infliximab DP manufacturing to a facility of the Purchaser's choice in order to enable the Purchaser to manufacture the infliximab DP or have it manufactured at launch of the Product at the latest.
(iv) The Purchaser will grant to Pfizer an exclusive (including as to Purchaser), royalty-free, perpetual, irrevocable and sublicensable license to use the Transferred Assets to perform development activities in relation to the Product and to seek and maintain regulatory approval for, manufacture, have manufactured and sell the Product in or for the non EEA markets.
(v) Pfizer and the Purchaser may, under the supervision of the Monitoring Trustee and the Commission, enter into the necessary arrangements to ensure that the Infliximab Divestment Business is viable and competitive. [...]
3. Preservation of legitimate rights of Pfizer
(a) The parties will put in place appropriate arrangements so that both parties will have access to the clinical database such that any data from the Clinical Trial will be readily accessible to both Parties at all times, and both Parties will be permitted to use Clinical Trial data to seek, obtain and maintain regulatory approvals for the Product in their respective markets and to further develop the Product or other products. The parties will put in place a specific Pharmacovigilance agreement to ensure fulfilment of safety reporting responsibilities.
(b) The Purchaser will assume responsibility and authority for completion of the Clinical Trial in accordance with the existing clinical trial design, protocol, timeline, budget and in accordance with the clinical trial term agreed upon with [...]. The timeline of the Clinical Trial is attached to this Schedule. Until the Clinical Trial is transferred to the Purchaser, any deviation from this timeline must be agreed between the Purchaser and Pfizer, as overseen by the Monitoring Trustee and the Commission. The Parties acknowledge that the Clinical Trial design and the protocol have already been approved by the EMA and the FDA. The Purchaser will have sole authority to conduct the Clinical Trial, provided Pfizer's legitimate interests in the Product are preserved. To that end, Pfizer and the Purchaser will establish a joint development committee ("JDC"). The JDC would be made up of three representatives appointed by Purchaser and two representatives appointed by Pfizer. The Purchaser will be responsible for the marketing approvals for the Product for the EEA countries; Pfizer will be responsible for the marketing approvals for the Product for the non EEA countries.
(c) The JDC shall have authority for "Key Decisions" in relation to the Clinical Trial. The list of Key Decisions will be mutually agreed upon between the Purchaser and Pfizer in the asset transfer agreement for the Divestment Business as overseen by the Monitoring Trustee so as to preserve Pfizer's legitimate interests in the Product for the non-EEA markets. In particular, Pfizer will have a veto right over all decisions that could negatively affect the development or future commercialisation of the Product in the non-EEA markets including the rights as set forth below provided these rights do not give Pfizer joint control over the clinical trial and over the development and marketing of the Product exclusively in the EEA:
* Orderly completion of the Clinical Trial;
* Continuity of the Clinical Trial / (early) termination of the Clinical Trial
* Global consistency of regulatory strategies and adoption of the statistical analysis plan (after consultation with regulatory agencies);
* Changes to the Clinical Trial protocol;
* Delays in the Clinical Trial timeline or temporary halt in the Clinical Trial process;
* Changes to the Clinical Trial budget;
* Any Clinical Trial matter that is likely to adversely impact the development or future commercialization of the Product in the Pfizer markets;
* Responses to regulatory queries that are likely to negatively impact regulatory submissions outside EEA, including communications on any significant safety issue arising in the Clinical Trial;
* Any other decision mutually agreed upon between the Purchaser and Pfizer in the asset transfer agreement for the Divestment Business as overseen by the Monitoring Trustee.
(d) The Key Decisions shall be adopted by unanimity. Pfizer's or the Purchaser's consent should not be unreasonably withheld.
(e) If the JDC cannot reach a unanimous decision on a Key Decision within the time period to be mutually agreed by Pfizer and the Purchaser before it, the matter will be submitted to a senior executive of the Purchaser and a senior executive of Pfizer for resolution. If such senior executives are not able to resolve such matter within the time period to be mutually agreed by Pfizer and the Purchaser, the matter shall be referred to the dispute resolution mechanism agreed upon by the Purchaser and Pfizer in the asset transfer agreement for the Divestment Business.
4. The Divestment Business shall not include:
(a) Any manufacturing facility, physical property (other than the specific Product inventories described above) or equipment;
(b) Any right to manufacture, market or sell any product other than the Product or any license to use any asset of Pfizer in connection with any product other than the Product;
(c) Any asset that is not a Transferred Asset and any asset that does not relate to the clinical development, manufacture or sale of the Product;
(d) Marketing plans and forecasts for any territories outside the EEA;
(e) Sponsorship of any and all Clinical Trial Authorisations or other regulatory filings for the Product outside the EEA, such as the US IND;
(f) Any trade names or trademarks used or intended to be used by Pfizer in relation to the Product; and
(g) Any cash, accounts receivable or other similar current assets.
5. If there is any asset or personnel which is not covered by paragraph 2 of this Schedule, but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or adequate substitute will be transferred to potential purchasers by transfer or license, as appropriate as overseen the Monitoring Trustee.
SCHEDULE 2
CARBOPLATIN
Product: Pfizer's carboplatin products Territory: Belgium
1. The Divestment Business consists of Pfizer's rights, title and interests in carboplatin in Belgium (currently marketed under the name Carboplatinum Pfizer) including the right to develop, manufacture and use carboplatin with a view to its sale and marketing in any form and for any indication whatsoever in Belgium. Carboplatin is used to treat various types of cancers, including ovarian cancer, small cell lung cancer, head and neck cancer. For the avoidance of doubt, this Divestment Business does not include any rights to sell carboplatin outside of Belgium.
2. The Divestment Business includes:
(a) the sale of existing carboplatin product inventory, sales and promotional material in Belgium, at the time of the divestment and as far as available;
(b) the transfer of all carboplatin-related contracts, commitments and customer contracts and/or records including but not limited to customers credit records, customer invoices, purchase orders, tender information and contact for the last three years preceding Closing, whilst only the information related to carboplatin specifically will be provided;
(c) the full transfer of all current and pending marketing authorisations for carboplatin in Belgium including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorisations available to Pfizer;
(d) an irrevocable, assignable, sub-licensable, perpetual and royalty free license for all relevant intellectual property rights, data, books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of Divestment Business with a view to its sale in Belgium, including in particular the information contained in the registration dossier; and
(e) full transfer of all national trademarks of Pfizer specifically related to carboplatin in Belgium (if any) or, in the case of a wider than national specific carboplatin trademark, an irrevocable, assignable, sub-licensable, perpetual and royalty free license to use that trademark for the Divestment Business.
(items referred to under (a)-(e) hereinafter collectively referred to as "Assets of the Divestment Business").
3. If and to the extent that the know-how listed in paragraph 2 (d) above of this Schedule is not exclusively related to, and not exclusively used in respect of, the manufacture, use and sale of carboplatin in Belgium, Pfizer shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of carboplatin in Belgium.
4. At the option of the Purchaser, Pfizer shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of product in Belgium for up to [...]. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Pfizer to the Purchaser. To the extent a supply disruption would occur, Pfizer commits to, for as long as the supply disruption continues to occur, treat Purchaser equally to other parties (including Pfizer's own businesses) it supplies at that time. It shall not contain provisions requiring the delivery of minimum or maximum supply volumes or batches.
5. At the option of the Purchaser, and to the extent required by law in Belgium or necessary with a view to assigning or transferring the relevant contracts with the customers in Belgium pertaining to carboplatin to the Purchaser, Pfizer will enter into a transitional distribution arrangement related to the Divestment Business lasting until the relevant marketing authorisation is transferred into the name of the Purchaser on a reasonable cost-plus basis which determination is overseen by the Monitoring Trustee. Pfizer commits to make its best efforts to ensure that no supply disruption will occur or any other supply issue that might lead to the termination of the contract with the relevant customers.
6. If Pfizer were to win any tenders pertaining to carboplatin before Closing, Pfizer commits to make its best efforts to facilitate the assignment of the relationship or the contract as the case may be with the relevant customers to the Purchaser in line with the provisions contained in this Schedule concerning existing contracts with the relevant customers.
7. Pfizer will transfer all historical information (orders; price; etc.) concerning its relationship regarding carboplatin in Belgium with API supplier [...] to the Purchaser in accordance with applicable law. Pfizer commits to make its best efforts to ensure that the Purchaser can continue the existing relationship with [...] with respect to carboplatin.
8. Pfizer will transfer all historical information (orders; price; etc.) concerning its relationship with [...] regarding the finishing, filling and packaging of carboplatin in Belgium to the Purchaser in accordance with applicable law. Pfizer commits to make its best efforts to ensure that the Purchaser can continue the existing relationship with [...] with respect to the finishing, filling and packaging of carboplatin.
9. At the option of the Purchaser, Pfizer will transfer all historical information (orders; price; etc.) concerning its relationship with [...] regarding the shipping of carboplatin in Belgium to the Purchaser in accordance with applicable law. Pfizer commits to make its best efforts to ensure that the Purchaser can continue the existing relationship with [...] for Belgium with respect to the shipping of carboplatin to Belgium.
10. Pfizer commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer.
11. At the option of the Purchaser, Pfizer shall provide reasonable technical assistance to the Purchaser to assume responsibility for the manufacture, sale and marketing of carboplatin in Belgium for a period of up to [...] to be agreed with the Purchaser and which determination is overseen by the Monitoring Trustee. The transitional technical assistance agreement shall include appropriate provisions to ensure that Pfizer provides technical assistance to the Purchaser expeditiously.
12. The Divestment Business shall not include:
(a) Any manufacturing facility;
(b) Raw materials, other than the raw materials in stock used to produce the carboplatin in Belgium;
(c) Any research and development, clinical data and studies or intellectual property relating to carboplatin after Closing;
(d) All marketing authorizations currently held by the Parties outside of Belgium for carboplatin;
(e) Any other asset not part of the Carboplatin Divestment Business or which is used in relation to a business of the Parties other than the Carboplatin Divestment Business;
(f) The "Pfizer" name or the name of any Pfizer subsidiaries;
(g) Monies owed to the Parties by customers for the purchase of carboplatin, and monies owed by the Parties to suppliers for materials used in the production of carboplatin.
13. If there is any asset or personnel which is not be covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or adequate substitute will be offered to the Purchaser.
SCHEDULE 3
CYTARABINE
Product: Hospira's cytarabine products Territory: Belgium, Italy, Portugal and Sweden
1. The Divestment Business consists of Hospira's rights, title and interests in cytarabine in Belgium, Italy, Portugal and Sweden (currently marketed under the name Cytosar, Alexan, Aracytin and/or Cytarabine Hospira) including the right to develop, manufacture and use cytarabine with a view to its sale and marketing in any form and for any indication whatsoever in Belgium, Italy, Portugal and Sweden. Cytarabine is a chemotherapy agent used for the treatment of different types of cancer affecting white blood cells (leukaemia), including acute and chronic myelogenous and acute lymphocytic leukaemia. It is also used to treat meningeal leukemia and lymphoma (cancers found in the lining of the brain and spinal cord). For the avoidance of doubt, this Divestment Business does not include any rights to sell cytarabine outside of Belgium, Italy, Portugal and Sweden.
2. The Divestment Business includes:
(a) the sale of existing cytarabine product inventory, sales and promotional material in Belgium, Italy, Portugal and Sweden, at the time of the divestment and as far as available;
(b) the transfer of all cytarabine-related contracts, commitments and customer contracts and/or records including but not limited to customers credit records, customer invoices, purchase orders, tender information and contact details for the last three years preceding Closing, whilst only the information related to cytarabine specifically will be provided;
(c) the full transfer of all current and pending marketing authorisations for cytarabine in Belgium, Italy, Portugal and Sweden including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s) and all relevant clinical reports relating to the Divestment Business existing prior to Closing, relating to the current and/or pending marketing authorisations available to Hospira;
(d) an irrevocable, assignable, sub-licensable, perpetual and royalty free license for all relevant intellectual property rights, data, books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of Divestment Business with a view to its sale in Belgium, Italy, Portugal and Sweden, including in particular the information contained in the registration dossier; and
(e) full transfer of all national trademarks of Hospira specifically related to cytarabine in Belgium, Italy, Portugal and Sweden (if any) or, in the case of a wider than national specific cytarabine trademark, an irrevocable, assignable, sub-licensable, perpetual and royalty free license to use that trademark for the Divestment Business.
(items referred to under (a)-(e) hereinafter collectively referred to as "Assets of the Divestment Business").
3. If and to the extent that the know-how listed in paragraph 2 (d) above of this Schedule is not exclusively related to, and not exclusively used in respect of, the manufacture, use and sale of cytarabine in Belgium, Italy, Portugal and Sweden, Hospira shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of cytarabine in Belgium, Italy, Portugal and Sweden.
4. At the option of the Purchaser, Hospira shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of product in Belgium, Italy, Portugal and Sweden for up to [...]. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Hospira to the Purchaser. To the extent a supply disruption would occur, Pfizer commits to, for as long as the supply disruption continues to occur, treat Purchaser equally to other parties (including Pfizer's own businesses) it supplies at that time. It shall not contain provisions requiring the delivery of minimum or maximum supply volumes or batches.
5. At the option of the Purchaser, and to the extent required by law in Belgium, Italy, Portugal and Sweden or necessary with a view to assigning or transferring the relevant contracts with the customers in Belgium, Italy, Portugal and Sweden pertaining to cytarabine to the Purchaser, Hospira will enter into a transitional distribution arrangement related to the Divestment Business lasting until the relevant marketing authorisation is transferred into the name of the Purchaser on a reasonable cost-plus basis which determination is overseen by the Monitoring Trustee. Hospira commits to make its best efforts to ensure that no supply disruption will occur or any other supply issue that might lead to the termination of the contract with the relevant customers.
6. If Hospira were to win any tenders pertaining to cytarabine before Closing, Hospira commits to make its best efforts to facilitate the assignment of the relationship or the contract as the case may be with the relevant customers to the Purchaser in line with the provisions contained in this Schedule concerning existing contracts with the relevant customers.
7. Hospira will transfer all historical information (orders; price; etc.) concerning its relationship regarding cytarabine in Belgium, Italy, Portugal and Sweden with API supplier [...] to the Purchaser in accordance with applicable law. Hospira commits to make its best efforts to ensure that the Purchaser can continue the existing relationship with [...] with respect to cytarabine.
8. Hospira will transfer all historical information (orders; price; etc.) concerning its relationship with [...] regarding the finishing, filling and packaging of cytarabine in Belgium, Italy, Portugal and Sweden to the Purchaser in accordance with applicable law. Hospira commits to make its best efforts to ensure that the Purchaser can continue the existing relationship with [...] with respect to the finishing, filling and packaging of cytarabine.
9. At the option of the Purchaser, Hospira will transfer all historical information (orders; price; etc.) concerning its relationship with a distributor ([...] for Belgium (Hospira considers them a customer), [...] for Sweden (Hospira considers them a customer), [...] for Italy, [...] and [...] for Portugal) regarding the shipping of cytarabine in Belgium, Italy, Portugal and Sweden to the Purchaser in accordance with applicable law. Hospira commits to make its best efforts to ensure that the Purchaser can continue the existing relationship with a distributor ([...]) for Belgium, Italy, Portugal and Sweden with respect to the shipping of cytarabine to Belgium, Italy, Portugal and Sweden.
10. Hospira commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer.
11. At the option of the Purchaser, Hospira shall provide reasonable technical assistance to the Purchaser to assume responsibility for the manufacture, sale and marketing of cytarabine in Belgium, Italy, Portugal and Sweden for a period of up to [...] to be agreed with the Purchaser and which determination is overseen by the Monitoring Trustee. The transitional technical assistance agreement shall include appropriate provisions to ensure that Hospira provides technical assistance to the Purchaser expeditiously.
12. The Divestment Business shall not include:
(a) Any manufacturing facility;
(b) Raw materials, other than the raw materials in stock used to produce the cytarabine in Belgium, Italy, Portugal and Sweden;
(c) Any research and development, clinical data and studies or intellectual property relating to cytarabine after Closing;
(d) All marketing authorizations currently held by the Parties outside of Belgium, Italy, Portugal and Sweden for cytarabine;
(e) The "Hospira" name or the name of any Hospira subsidiaries;
(f) Monies owed to the Parties by customers for the purchase of cytarabine, and monies owed by the Parties to suppliers for materials used in the production of cytarabine.
13. If there is any asset or personnel which is not be covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or adequate substitute will be offered to the Purchaser.
SCHEDULE 4
EPIRUBICIN
Product: Hospira's epirubicin products
Territory: Austria, Belgium, Italy, the Netherlands and Spain
1. The Divestment Business consists of Hospira's rights, title and interests in epirubicin in Austria, Belgium, Italy, the Netherlands and Spain including the right to develop, manufacture and use epirubicin with a view to its sale and marketing in any form and for any indication whatsoever in Austria, Belgium, Italy, the Netherlands and Spain. Epirubicin is used for the treatment of breast cancer and other types of cancer including ovarian cancer, stomach cancer, lung cancer, bowel cancer and myeloma. It is also used to treat some types of lymphoma and leukaemia. For the avoidance of doubt, this Divestment Business does not include any rights to sell epirubicin outside of Austria, Belgium, Italy, the Netherlands and Spain.
2. The Divestment Business includes:
(a) the sale of existing epirubicin product inventory, sales and promotional material in Austria, Belgium, Italy, the Netherlands and Spain, at the time of the divestment and as far as available;
(b) the transfer of all epirubicin-related contracts, commitments and customer contracts and/or records including but not limited to customers credit records, customer invoices, purchase orders, tender information and contact for the last three years preceding Closing, whilst only the information related to epirubicin specifically will be provided;
(c) the full transfer of all current and pending marketing authorisations for epirubicin in Austria, Belgium, Italy, the Netherlands and Spain including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorisations available to Hospira;
(d) an irrevocable, assignable, sub-licensable, perpetual and royalty free license for all relevant intellectual property rights, data, books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of Divestment Business with a view to its sale in Austria, Belgium, Italy, the Netherlands and Spain, including in particular the information contained in the registration dossier; and
(e) full transfer of all national trademarks of Hospira specifically related to epirubicin in Austria, Belgium, Italy, the Netherlands and Spain (if any) or, in the case of a wider than national specific epirubicin trademark, an irrevocable, assignable, sub-licensable, perpetual and royalty free license to use that trademark for the Divestment Business.
(items referred to under (a)-(e) hereinafter collectively referred to as "Assets of the Divestment Business").
3. If and to the extent that the know-how listed in paragraph 2 (d) above of this Schedule is not exclusively related to, and not exclusively used in respect of, the manufacture, use and sale of epirubicin in Austria, Belgium, Italy, the Netherlands and Spain, Hospira shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of epirubicin in Austria, Belgium, Italy, the Netherlands and Spain.
4. At the option of the Purchaser, Hospira shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of product in Austria, Belgium, Italy, the Netherlands and Spain for up to [...]. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Hospira to the Purchaser. To the extent a supply disruption would occur, Pfizer commits to, for as long as the supply disruption continues to occur, treat Purchaser equally to other parties (including Pfizer's own businesses) it supplies at that time. It shall not contain provisions requiring the delivery of minimum or maximum supply volumes or batches.
5. At the option of the Purchaser, and to the extent required by law in Austria, Belgium, Italy, the Netherlands and Spain or necessary with a view to assigning or transferring the relevant contracts with the customers in Austria, Belgium, Italy, the Netherlands and Spain pertaining to epirubicin to the Purchaser, Hospira will enter into a transitional distribution arrangement related to the Divestment Business lasting until the relevant marketing authorisation is transferred into the name of the Purchaser on a reasonable cost-plus basis which determination is overseen by the Monitoring Trustee. Hospira commits to make its best efforts to ensure that no supply disruption will occur or any other supply issue that might lead to the termination of the contract with the relevant customers.
6. If Hospira were to win any tenders pertaining to epirubicin before Closing, Hospira commits to make its best efforts to facilitate the assignment of the relationship or the contract as the case may be with the relevant customers to the Purchaser in line with the provisions contained in this Schedule concerning existing contracts with the relevant customers.
7. Hospira will transfer all historical information (orders; price; etc.) concerning its relationship regarding epirubicin in Austria, Belgium, Italy, the Netherlands and Spain with API supplier [...] to the Purchaser in accordance with applicable law. Pfizer commits to make its best efforts to ensure that the Purchaser can continue the existing relationship with [...] with respect to epirubicin.
8. Pfizer will transfer all historical information (orders; price; etc.) concerning its relationship with [...] regarding the finishing, filling and packaging of epirubicin in Austria, Belgium, Italy, the Netherlands and Spain to the Purchaser in accordance with applicable law. Pfizer commits to make its best efforts to ensure that the Purchaser can continue the existing relationship with [...] with respect to the finishing, filling and packaging of epirubicin.
9. At the option of the Purchaser, Pfizer will transfer all historical information (orders; price; etc.) concerning its relationship with a distributor ([...] for Austria ([...]) and Belgium ([...]), [...] for Spain ([...]) and Netherlands) regarding the shipping of epirubicin in Austria, Belgium, Italy, the Netherlands and Spain to the Purchaser in accordance with applicable law. Pfizer commits to make its best efforts to ensure that the Purchaser can continue the existing relationship with the distributors mentioned above for Austria, Belgium, Italy, the Netherlands and Spain with respect to the shipping of epirubicin to Austria, Belgium, Italy, the Netherlands and Spain.
10. Hospira commits to use its best efforts to assist the Purchaser with obtaining the consent, waiver or alternative arrangements, as the case may, be in connection with the replacement of Hospira with the Purchaser for the purposes of the joint venture agreement with [...] as of Closing to the extent such agreement sees upon the manufacture of epirubicin products for Austria, Belgium, Italy, the Netherlands and Spain.
11. Pfizer commits to use its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer.
12. At the option of the Purchaser, Pfizer shall provide reasonable technical assistance to the Purchaser to assume responsibility for the manufacture, sale and marketing of epirubicin in Austria, Belgium, Italy, the Netherlands and Spain for a period of up to [...] to be agreed with the Purchaser and which determination is overseen by the Monitoring Trustee. The transitional technical assistance agreement shall include appropriate provisions to ensure that Pfizer provides technical assistance to the Purchaser expeditiously.
13. The Divestment Business shall not include:
(a) Any manufacturing facility;
(b) Raw materials, other than the raw materials in stock used to produce the epirubicin in Austria, Belgium, Italy, the Netherlands and Spain;
(c) Any research and development, clinical data and studies or intellectual property relating to epirubicin after Closing;
(d) All marketing authorizations currently held by the Parties outside of Austria, Belgium, Italy, the Netherlands and Spain for epirubicin;
(e) The "Hospira" name or the name of any Hospira subsidiaries;
(f) Monies owed to the Parties by customers for the purchase of epirubicin, and monies owed by the Parties to suppliers for materials used in the production of epirubicin.
14. If there is any asset or personnel which is not be covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or adequate substitute will be offered to the Purchaser.
SCHEDULE 5
IRINOTECAN
Product: Hospira's irinotecan products Territory: Belgium, the Czech Republic and Italy
1. The Divestment Business consists of Hospira's rights, title and interests in irinotecan in Belgium, the Czech Republic and Italy including the right to develop, manufacture and use irinotecan with a view to its sale and marketing in any form and for any indication whatsoever in Belgium, the Czech Republic and Italy. Irinotecan is used primarily for the treatment of colorectal cancer and, in particular, in combination with other chemotherapy agents. For the avoidance of doubt, this Divestment Business does not include any rights to sell irinotecan outside of Belgium, the Czech Republic and Italy.
2. The Divestment Business includes:
(a) the sale of existing irinotecan product inventory, sales and promotional material in Belgium, the Czech Republic and Italy, at the time of the divestment and as far as available;
(b) the transfer of all irinotecan-related contracts, commitments and customer contracts and/or records including but not limited to customers credit records, customer invoices, purchase orders, tender information and contact details for the last three years preceding Closing, whilst only the information related to irinotecan specifically will be provided;
(c) the full transfer of all current and pending marketing authorisations for irinotecan in Belgium, the Czech Republic and Italy including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorisations available to Hospira;
(d) an irrevocable, assignable, sub-licensable, perpetual and royalty free license for all relevant intellectual property rights, data, books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of Divestment Business with a view to its sale in Belgium, the Czech Republic and Italy, including in particular the information contained in the registration dossier; and
(e) full transfer of all national trademarks of Hospira specifically related to epirubicin in Belgium, the Czech Republic and Italy (if any) or, in the case of a wider than national specific irinotecan trademark, an irrevocable, assignable, sub-licensable, perpetual and royalty free license to use that trademark for the Divestment Business.
(items referred to under (a)-(e) hereinafter collectively referred to as "Assets of the Divestment Business").
3. If and to the extent that the know-how listed in paragraph 2 (d) above of this Schedule is not exclusively related to, and not exclusively used in respect of, the manufacture, use and sale of irinotecan in Belgium, the Czech Republic and Italy, Hospira shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of irinotecan in Belgium, the Czech Republic and Italy.
4. At the option of the Purchaser, Hospira shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of product in Belgium, the Czech Republic and Italy for up to [...]. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Hospira to the Purchaser. To the extent a supply disruption would occur, Pfizer commits to, for as long as the supply disruption continues to occur, treat Purchaser equally to other parties (including Pfizer's own businesses) it supplies at that time. It shall not contain provisions requiring the delivery of minimum or maximum supply volumes or batches.
5. At the option of the Purchaser, and to the extent required by law in Belgium, the Czech Republic and Italy or necessary with a view to assigning or transferring the relevant contracts with the customers in Belgium, the Czech Republic and Italy pertaining to irinotecan to the Purchaser, Hospira will enter into a transitional distribution arrangement related to the Divestment Business lasting until the relevant marketing authorisation is transferred into the name of the Purchaser on a reasonable cost-plus basis which determination is overseen by the Monitoring Trustee. Hospira commits to make its best efforts to ensure that no supply disruption will occur or any other supply issue that might lead to the termination of the contract with the relevant customers.
6. If Hospira were to win any tenders pertaining to irinotecan before Closing, Hospira commits to make its best efforts to facilitate the assignment of the relationship or the contract as the case may be with the relevant customers to the Purchaser in line with the provisions contained in this Schedule concerning existing contracts with the relevant customers.
7. Hospira will transfer all historical information (orders; price; etc.) concerning its relationship regarding irinotecan in Belgium, the Czech Republic and Italy with API supplier [...] to the Purchaser in accordance with applicable law. Hospira commits to make its best efforts to ensure that the Purchaser can continue the existing relationship with [...] with respect to irinotecan.
8. Hospira will transfer all historical information (orders; price; etc.) concerning its relationship with [...] regarding the finishing, filling and packaging of irinotecan in Belgium, the Czech Republic and Italy to the Purchaser in accordance with applicable law. Hospira commits to make its best efforts to ensure that the Purchaser can continue the existing relationship with [...] with respect to the finishing, filling and packaging of irinotecan.
9. At the option of the Purchaser, Hospira will transfer all historical information (orders; price; etc.) concerning its relationship with [...] for Belgium (Hospira considers them a customer), [...] for the Czech Republic and [...] for Italy regarding the shipping of irinotecan in Belgium, the Czech Republic and Italy to the Purchaser in accordance with applicable law. Hospira commits to make its best efforts to ensure that the Purchaser can continue the existing relationship with [...] for Belgium, [...] for the Czech Republic and [...] for Italy with respect to the shipping of irinotecan to Belgium, the Czech Republic and Italy.
10. Hospira commits to use its best efforts to assist the Purchaser with obtaining the consent, waiver or alternative arrangements, as the case may, be in connection with the replacement of Hospira with the Purchaser for the purposes of the joint venture agreement with [...] as of Closing to the extent such agreement sees upon the manufacture of irinotecan products for Belgium, the Czech Republic and Italy.
11. Hospira commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer.
12. At the option of the Purchaser, Hospira shall provide reasonable technical assistance to the Purchaser to assume responsibility for the manufacture, sale and marketing of irinotecan in Belgium, the Czech Republic and Italy for a period of up to [...] to be agreed with the Purchaser and which determination is overseen by the Monitoring Trustee. The transitional technical assistance agreement shall include appropriate provisions to ensure that Hospira provides technical assistance to the Purchaser expeditiously.
13. The Purchaser will be given an option (to be exercised within one year after signing the relevant Transfer Agreement) to request Hospira – whose acceptance thereof would not be unreasonably withheld – to make available one or more Personnel, subject to applicable local employment legislation, who would reasonably be considered necessary to maintain the viability, marketability and competitiveness of this Divestment Business to be supervised by the Monitoring Trustee.
14. The Divestment Business shall not include:
(a) Any manufacturing facility;
(b) Raw materials, other than the raw materials in stock used to produce the irinotecan in Belgium, the Czech Republic and Italy;
(c) Any research and development, clinical data and studies or intellectual property relating to irinotecan after Closing;
(d) All marketing authorizations currently held by the Parties outside of Belgium, the Czech Republic and Italy for irinotecan;
(e) The "Hospira" name or the name of any Hospira subsidiaries;
(f) Monies owed to the Parties by customers for the purchase of irinotecan, and monies owed by the Parties to suppliers for materials used in the production of irinotecan.
15. If there is any asset or personnel which is not be covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment
Business, that asset or adequate substitute will be offered to the Purchaser.
SCHEDULE 6
VANCOMYCIN
Product: Pfizer's vancomycin products Territory: Ireland
1. The Divestment Business consists of Pfizer's rights, title and interests in vancomycin in Ireland including the right to develop, manufacture and use vancomycin with a view to its sale and marketing in any form and for any indication whatsoever in Ireland. Vancomycin is used to treat various types of cancers, including ovarian cancer, small cell lung cancer, head and neck cancer. For the avoidance of doubt, this Divestment Business does not include any rights to sell vancomycin outside of Ireland.
2. The Divestment Business includes:
(a) the sale of existing vancomycin product inventory, sales and promotional material in Ireland, at the time of the divestment and as far as available;
(b) the transfer of all vancomycin-related contracts, commitments and customer contracts and/or records including but not limited to customers credit records, customer invoices, purchase orders, tender information and contact details for the last three years preceding Closing, whilst only the information related to vancomycin specifically will be provided;
(c) the full transfer of all current and pending marketing authorisations for vancomycin in Ireland including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s), relating to the current and/or pending marketing authorisations available to Pfizer;
(d) an irrevocable, assignable, sub-licensable, perpetual and royalty free license for all relevant intellectual property rights, data, books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of Divestment Business with a view to its sale in Ireland, including in particular the information contained in the registration dossier; and
(e) full transfer of all national trademarks of Hospira specifically related to vancomycin in Ireland (if any) or, in the case of a wider than national specific vancomycin trademark, an irrevocable, assignable, sub-licensable, perpetual and royalty free license to use that trademark for the Divestment Business.
(items referred to under (a)-(e) hereinafter collectively referred to as "Assets of the Divestment Business").
3. If and to the extent that the know-how listed in paragraph 2 (d) above of this Schedule is not exclusively related to, and not exclusively used in respect of, the manufacture, use and sale of vancomycin in Ireland, Pfizer shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of vancomycin in Ireland.
4. At the option of the Purchaser, Pfizer shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of product in Ireland for up to [...]. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Pfizer to the Purchaser. To the extent a supply disruption would occur, Pfizer commits to, for as long as the supply disruption continues to occur, treat Purchaser equally to other parties (including Pfizer's own businesses) it supplies at that time. It shall not contain provisions requiring the delivery of minimum or maximum supply volumes or batches.
5. At the option of the Purchaser, and to the extent required by law in Ireland or necessary with a view to assigning or transferring the relevant contracts with the customers in Ireland pertaining to vancomycin to the Purchaser, Pfizer will enter into a transitional distribution arrangement related to the Divestment Business lasting until the relevant marketing authorisation is transferred into the name of the Purchaser on a reasonable cost-plus basis which determination is overseen by the Monitoring Trustee. Pfizer commits to make its best efforts to ensure that no supply disruption will occur or any other supply issue that might lead to the termination of the contract with the relevant customers.
6. If Pfizer were to win any tenders pertaining to vancomycin before Closing, Pfizer commits to make its best efforts to facilitate the assignment of the relationship or the contract as the case may be with the relevant customers to the Purchaser in line with the provisions contained in this Schedule concerning existing contracts with the relevant customers.
7. Pfizer will transfer all historical information (orders; price; etc.) concerning its relationship regarding vancomycin in Ireland with API supplier [...] to the Purchaser in accordance with applicable law. Pfizer commits to make its best efforts to ensure that the Purchaser can continue the existing relationship with [...] with respect to vancomycin.
8. Pfizer will transfer all historical information (orders; price; etc.) concerning its relationship with [...] regarding the finishing, filling and packaging of vancomycin in Ireland to the Purchaser in accordance with applicable law. Pfizer commits to make its best efforts to ensure that the Purchaser can continue the existing relationship with [...] with respect to the finishing, filling and packaging of vancomycin.
9. At the option of the Purchaser, Pfizer will transfer all historical information (orders; price; etc.) concerning its relationship with [...] regarding the shipping of vancomycin in Ireland to the Purchaser in accordance with applicable law. Pfizer commits to make its best efforts to ensure that the Purchaser can continue the existing relationship with [...] for Ireland with respect to the shipping of vancomycin to Ireland.
10. Pfizer commits to use its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer.
11. At the option of the Purchaser, Pfizer shall provide reasonable technical assistance to the Purchaser to assume responsibility for the manufacture, sale and marketing of vancomycin in Ireland for a period of up to [...] to be agreed with the Purchaser and which determination is overseen by the Monitoring Trustee. The transitional technical assistance agreement shall include appropriate provisions to ensure that Pfizer provides technical assistance to the Purchaser expeditiously.
12. The Divestment Business shall not include:
(a) Any manufacturing facility;
(b) Raw materials, other than the raw materials in stock used to produce the vancomycin in Ireland;
(c) Any research and development, clinical data and studies or intellectual property relating to vancomycin after Closing;
(d) All marketing authorizations currently held by the Parties outside of Ireland for vancomycin;
(e) The "Pfizer" name or the name of any Pfizer subsidiaries;
(f) Monies owed to the Parties by customers for the purchase of vancomycin, and monies owed by the Parties to suppliers for materials used in the production of vancomycin.
If there is any asset or personnel which is not be covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or adequate substitute will be offered to the Purchaser.
SCHEDULE 7
VORICONAZOLE
Product: Hospira's voriconazole products Territory: EEA
1. The Divestment Business consists of Hospira's rights, title and interests in generic voriconazole in the EEA (currently not marketed) including the right to develop, manufacture and use voriconazole with a view to its sale and marketing in any form and for any indication whatsoever in the EEA. Voriconazole a triazole antifungal medication that is generally used to treat serious, invasive fungal infections. Pfizer holds the originator product (Vfend), which is close to losing patent protection as it will go off patent in the EEA in 2016. For the avoidance of doubt, this Divestment Business does not include any rights to sell voriconazole outside of the EEA.
2. The Divestment Business includes:
(a) the sale of existing voriconazole product inventory, sales and promotional material in the EEA, at the time of the divestment and as far as available;
(b) the transfer of all voriconazole-related contracts, commitments and customer contracts and/or records including but not limited to customers credit records, customer invoices, purchase orders, tender information and contact details for the last three years preceding Closing, whilst only the information related to voriconazole specifically will be provided, provided that the Parties may redact from such copies any information that does not relate to the Divestment Business;
(c) the full transfer of all current and pending marketing authorisations for voriconazole in the EEA including all relevant dossiers, as well as the information contained in the relevant full registration dossier(s) and all relevant clinical reports relating to the Divestment Business existing prior to Closing, relating to the current and/or pending marketing authorisations available to Hospira;
(d) an irrevocable, assignable, sub-licensable, perpetual and royalty free license for all relevant intellectual property rights, data, books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of Divestment Business with a view to its sale in the EEA, including in particular the information contained in the registration dossier;
(e) full transfer of all assets related to a patent litigation concerning voriconazole in any EEA Member State, including plans, strategies, and filings (if any);
(f) full transfer of all trademarks of Hospira related to voriconazole in the EEA (if any).
3. (items referred to under (a)-(f) hereinafter collectively referred to as "Assets of the Divestment Business").
4. If and to the extent that the know-how listed in paragraph 2 (d) above of this Schedule is not exclusively related to, and not exclusively used in respect of, the manufacture, use and sale of voriconazole in the EEA, Hospira shall have the right to retain the ownership of such asset and shall grant to the Purchaser at no additional charge an exclusive and perpetual right to use such asset for the manufacture, use and sale of voriconazole in the EEA.
5. At the option of the Purchaser, Hospira shall enter into a transitory non-exclusive manufacturing and/or supply agreement relating to the existing forms of product in the EEA for up to [...]. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by Hospira to the Purchaser. To the extent a supply disruption would occur, Pfizer commits to, for as long as the supply disruption continues to occur, treat Purchaser equally to other parties (including Pfizer's own businesses) it supplies at that time. It shall not contain provisions requiring the delivery of minimum or maximum supply volumes or batches.
6. At the option of the Purchaser, and to the extent required by law in the EEA member states or necessary with a view to assigning or transferring the relevant contracts with the customers in EEA pertaining to voriconazole to the Purchaser, Hospira will enter into a transitional distribution arrangement related to the Divestment Business lasting until the relevant marketing authorisation is transferred into the name of the Purchaser on a reasonable cost-plus basis which determination is overseen by the Monitoring Trustee. Hospira commits to make its best efforts to ensure that no supply disruption will occur or any other supply issue that might lead to the termination of the contract with the relevant customers.
7. If Hospira were to win any tenders pertaining to voriconazole before Closing, Hospira commits to make its best efforts to facilitate the assignment of the relationship or the contract as the case may be with the relevant customers to the Purchaser in line with the provisions contained in this Schedule concerning existing contracts with the relevant customers.
8. Hospira will transfer all historical information (orders; price; etc.) concerning its relationship regarding voriconazole in the EEA with the contract manufacturer [...] to the Purchaser in accordance with applicable law. Hospira commits to make its best efforts to ensure that the Purchaser can continue the existing relationship with [...] with respect to voriconazole.
9. Hospira will transfer all historical information (orders; price; etc.) concerning its relationship regarding [...] in the EEA with [...] in accordance with applicable law. Pfizer commits to make its best efforts to ensure that the Purchaser can continue the existing relationship with [...] with respect to voriconazole.
10. Hospira commits to make its best efforts to cooperate with the Purchaser to effectuate the transfer of the Divestment Business and to undertake all regulatory changes that would be required as a result of such transfer.
11. At the option of the Purchaser, Hospira shall provide reasonable technical assistance to the Purchaser to assume responsibility for the manufacture, sale and marketing of voriconazole in the EEA for a period of up to [...] to be agreed with the Purchaser and which determination is overseen by the Monitoring Trustee. The transitional technical assistance agreement shall include appropriate provisions to ensure that Hospira provides technical assistance to the Purchaser expeditiously.
12. The Divestment Business shall not include:
(a) Any manufacturing facility;
(b) Raw materials, other than the raw materials in stock used to produce voriconazole in the EEA;
(c) Any research and development, clinical data and studies or intellectual property relating to voriconazole after Closing;
(d) All marketing authorizations currently held by the Parties outside of the EEA;
(e) The "Hospira" name or the name of any Hospira subsidiaries;
(f) Monies owed to the Parties by customers for the purchase of voriconazole, and monies owed by the Parties to suppliers for materials used in the production of voriconazole.
13. If there is any asset or personnel which is not be covered by paragraph 2 of this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset or adequate substitute will be offered to the Purchaser.