Commission, March 10, 2016, No M.7746
EUROPEAN COMMISSION
Decision
TEVA / ALLERGAN GENERICS
Dear Sirs,
To the notifying parties:
Subject: Case M.7746 – TEVA / ALLERGAN GENERICS
Commission decision pursuant to Article 6(1)(b) in conjunction with Article 6(2) of Council Regulation No 139/20041 and Article 57 of the Agreement on the European Economic Area2
(1) On 21 January 2016, the European Commission received notification of a proposed concentration pursuant to Article 4 of the Merger Regulation by which the undertaking Teva Pharmaceuticals Industries Limited ("Teva", Israel) intends to acquire within the meaning of Article 3(1)(b) of the Merger Regulation control of the global generic pharmaceuticals business ("Allergan Generics") of Allergan plc ("Allergan", Ireland), by way of purchase of shares and assets (the "Transaction").3 Teva and Allergan Generics are designated hereinafter as the "Parties".
I. THE PARTIES
(2) Teva is a global pharmaceutical company with its corporate headquarters in Israel, involved in the development, production and marketing of generic and proprietary pharmaceutical products, as well as biopharmaceuticals and active pharmaceutical ingredients.
(3) Allergan Generics includes the global generic pharmaceuticals business of Allergan (formerly known as Actavis), an international pharmaceutical company headquartered in Ireland, including the U.S. and international generic commercial units, third-party supplier Medis, global generic manufacturing operations, the global generic R&D unit, the international over-the-counter commercial unit (excluding eye care products) and some established international brands.
II. THE OPERATION
(4) On 26 July 2015, the Parties entered into a Master Purchase Agreement, pursuant to which Teva will acquire the shares and assets constituting Allergan Generics from Allergan.
(5) Teva will therefore acquire sole control over Allergan Generics within the meaning of Article 3(1)(b) of the Merger Regulation.
III. UNION DIMENSION
(6) The undertakings concerned have a combined aggregate world-wide turnover of more than EUR 5 000 million.4 Each of them has an EU-wide turnover in excess of EUR 250 million, but each does not achieve more than two-thirds of its aggregate EU-wide turnover within one and the same Member State. The Transaction therefore has a Union dimension.
IV. ASSESSMENT
IV.1. Introduction
(7) Following a trend of consolidation amongst generic players, the Transaction would combine two of the four largest generic players globally (with Sandoz/Novartis and Mylan), which are followed by a long tail of smaller suppliers.
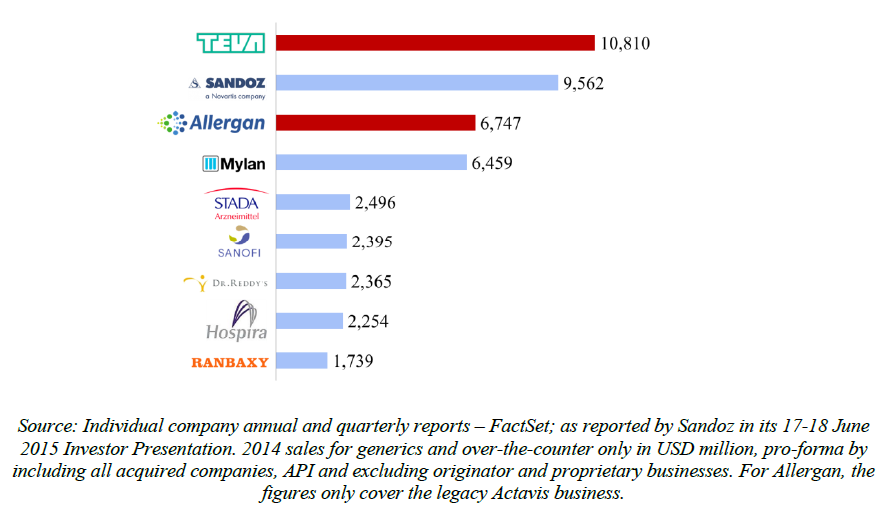
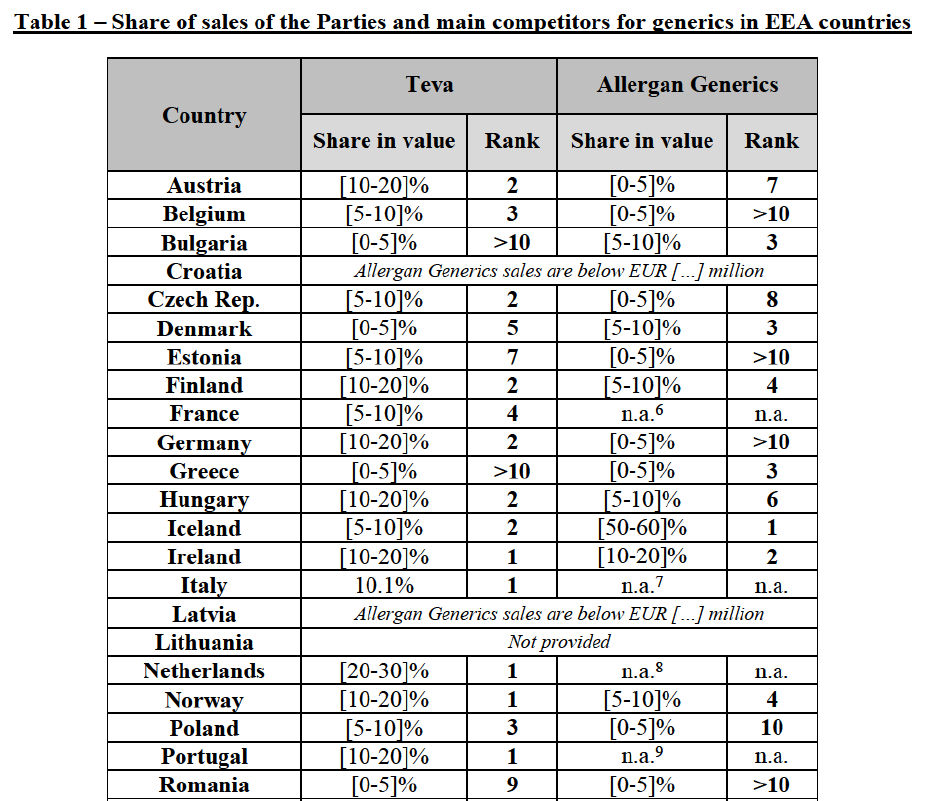
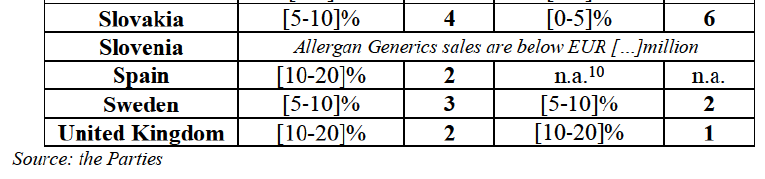
(8) The market share in value and rank of the Parties regarding the sales of generics is presented below for each EEA country in 2014.5
(9) The Parties activities overlap in relation to a vast number of affected markets in relation
to marketed and pipeline finished dose pharmaceuticals (hereafter "FDPs" or "pharmaceuticals"), see section IV.2. A vast number of vertical relationships also arise in relation to the out-licensing and contract manufacturing of FDPs to third parties, see section IV.3, as well as to the supply of active pharmaceutical ingredients ("API")11 used to manufacture FDPs, see section IV.4.
IV.2. Finished dose pharmaceuticals
IV.2.1. Market definition
IV.2.1.1. Marketing of pharmaceuticals
IV.2.1.1.a. Marketed generic pharmaceuticals
Product market definition
(10) Through the assessment of the relevant market definition in past decisions dealing with the marketing of generic pharmaceuticals,12 the Commission has established a number of principles. In those decisions it noted that medicines may be subdivided into therapeutic classes by reference to the Anatomical Therapeutic Classification ("ATC"), devised by the European Pharmaceutical Marketing Research Association ("EphMRA")13 and maintained by EphMRA and Intercontinental Medical Statistics ("IMS").
(11) In the EphMRA ATC system, medicines are classified into groups at four different levels. In the first and broadest level (ATC1), medicinal products are divided into the 16 main anatomical groups. The second level (ATC2) represents either a pharmacological or therapeutic group. The third level (ATC3) further groups medicinal products by their specific therapeutic indications, i.e. their intended use. The ATC4 level is the most detailed one (not available for all ATC3) and refers for instance to the mode of action (e.g. distinction of some ATC3 classes into topical and systemic depending on their way of action) or any other subdivision of the group. Finally, the level of the chemical substance is the so-called molecule level.
(12) In its past merger decisions, the Commission has referred to the ATC3 level as the starting point for defining the relevant product market. However, in a number of cases, the Commission found that the ATC3 level classification did not yield the appropriate market definition within the meaning of the Commission Notice on the Definition of the Relevant Market.14 Indeed, the overlap in therapeutic uses does not necessarily imply any particular economic substitution patterns between products. As a result, where appropriate and based on the factual evidence collected during the market investigation, the Commission defined the relevant product market at the level of the molecule.15
(13) In the present case, given the characteristics of the products involved (typically mature genericised medicines), the Commission takes the molecule level as the most plausible starting point for the product market definition, considering that a generic molecule is the closest substitute to the generic medicinal product based on the same molecule or API.16 Neither the Notifying Party nor the market investigation provided any indications that the Commission should depart from this approach.
(14) Furthermore, as the Commission has previously acknowledged, medicines are differentiated not only by their API(s), but also, in particular, as recognized by the European regulatory framework for medicines for human use,17 by their dosage, pharmaceutical form and route of administration, which may limit their substitutability.
(15) For the purposes of this decision, and in accordance with past practice,18 the Commission has looked whenever appropriate at the pharmaceutical form with reference to the first letter of the typology of form codes (the so-called "New Form Code" or "NFC") used by IMS / EphMRA. In general, the first letter ("NFC-1") differentiates between forms for systemic and topical effect, site of application (e.g. oral, nasal, parenteral or rectal), and long-acting and ordinary forms:
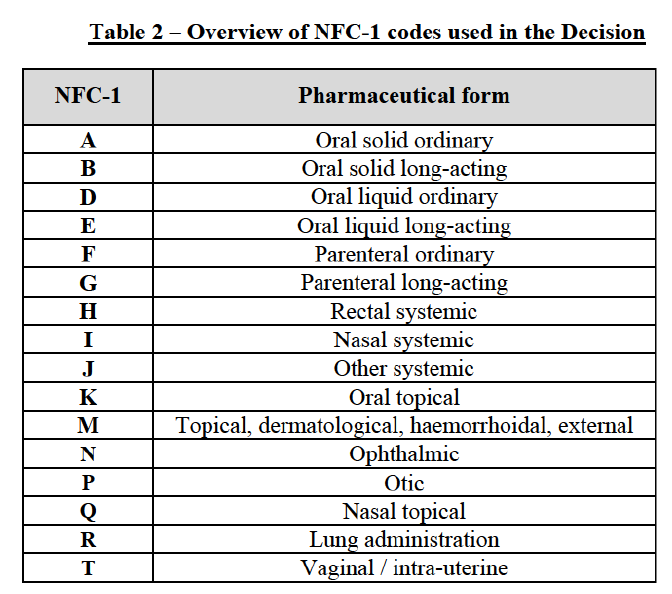
(16) The question whether the molecules analysed in the present decision need to be segmented by pharmaceutical form can be left open for the purposes of the competitive assessment of the Transaction since the Commission analysed all plausible market definitions (molecule and pharmaceutical form levels) where appropriate (i.e. in all cases where the competitive situation was different).
(17) Finally, in certain cases, pharmaceutical products may be further subdivided into various segments on the basis of a variety of criteria, and in particular demand-related criteria. The Commission has, in past decisions,19 defined separate markets for medicines which can be issued only on prescription and those, which can be sold over-the-counter ("OTC", that is non-prescription medicinal products). Medical indications, side effects, legal framework, distribution and marketing tend to differ between these drug categories, even if the active ingredients may be identical.
(18) The question whether the molecules analysed in the present decision need to be segmented between prescription (Rx) drugs and OTC drugs can be left open for the purposes of the competitive assessment of the Transaction since the Commission analysed all plausible market definitions (molecule and Rx/OTC levels) where appropriate (i.e. in all cases where the competitive situation was different).
Geographic market definition
(19) The Notifying Party has, in line with the Commission's prior decisions, submitted an overview of its activities on a country-by-country basis. The Commission has consistently considered that the markets for finished dose pharmaceutical products are national in scope, in particular in view of the national regulatory and reimbursement schemes and the fact that competition between pharmaceutical firms still predominantly takes place at a national level.20 Neither the Notifying Party nor the market investigation provided any indications that the Commission should depart from this approach.
IV.2.1.1.b. Pipeline generic pharmaceuticals
Product market definition
(20) Following the same approach as marketed generic pharmaceuticals and in line with past decisions,21 the Commission takes the molecule level as the most plausible relevant market for pipeline generic pharmaceuticals, considering that a pipeline generic molecule is typically the closest substitute to the other generic medicinal product based on the same molecule. Neither the Notifying Party nor the market investigation provided any indications that the Commission should depart from this approach.
(21) The question whether the pipeline molecules analysed in the present decision need to be segmented by pharmaceutical form or between prescription drugs and OTC drugs can be left open for the purposes of the competitive assessment of the Transaction since the Commission analysed all plausible market definitions where appropriate.
Geographic market definition
(22) For pipeline products, the Commission previously considered that the geographic scope of the relevant market is at least EEA-wide.22 Neither the Notifying Party nor the market investigation provided any indications that the Commission should depart from this approach.
IV.2.1.1.c. Originator pharmaceuticals
(23) The Parties identified two instances where one party (Allergan Generics) is planning to launch in the EEA the generic equivalent of the other party's (Teva) originator drug.23 This concerns the launch of the generic versions of glatiramer acetate (sold by Teva under the brand name "Copaxone") and rasagiline (sold by Teva under the brand name "Azilect").24
Product market definition
(24) In previous decisions,25 the Commission found that generic companies are usually developing a number of pipeline generic drugs which are intended to compete with originators which lose exclusivity (after expiry of patent and/or data exclusivity protections). The Commission concluded that the potential for these products to enter into competition with other products which are either on the market or at the development stage should be assessed by reference to their characteristics, intended therapeutic use, and expected therapeutic and economic substitutability.
(25) The exact scope of the product market (including or not other molecules with the same therapeutic indications) can be left open for the purposes of the competitive assessment of the Transaction since the competitive assessment will not change under any plausible product market segmentation.
Geographic market definition
(26) The approach laid out in section IV.2.1.1.a and section IV.2.1.1.b regarding marketed and pipeline generic products applies mutatis mutandis to glatiramer acetate and rasagiline.
(27) Therefore, the geographic market for pipeline generics should be defined as at least EEA-wide, while the market for marketed products should be defined at national level.
IV.2.1.2. Wholesale of pharmaceuticals
IV.2.1.2.a. Product market definition
(28) The wholesale of pharmaceuticals consists in selling a range of FDPs to customers, including pharmacies, dispensing doctors and hospitals.
(29) The Commission has previously defined a market for the wholesale of pharmaceuticals in some EEA countries.26 In previous decisions,27 the Commission has also considered a market for pre-wholesale services (including 3PLs),28 that is separate of the market for wholesale of pharmaceuticals.
(30) As to wholesale of pharmaceuticals, the Commission has previously considered three possible segmentations, based on the following categories:29
a. Categories of products (depending on whether the medicine may be sold with prescription or OTC; whether it is an originator, generic or parallel import medicine; and whether the medicine may be sold in retail pharmacies under the supervision of a pharmacist only, or also in other outlets such as supermarkets);
b. Categories of wholesalers (broad-line wholesalers30 and short-line wholesalers);31
c. Categories of customers (retail pharmacies, dispensing doctors32 and hospitals) due
to different purchasing and delivery patterns.33
(31) As acknowledged in previous Commission decisions,34 generic manufacturers can sell their products (i) indirectly through wholesalers or, (ii) directly to pharmacies and institutional customers (such as hospitals). In the latter case, pharmacies place orders to the generic manufacturer. Pharmacists may ask that the generics be provided through their wholesaler of choice, in which case the latter acts as a mere logistics provider (3PL).35 Given that the commercial terms are negotiated directly between the generic manufacturer and the pharmacy, such orders are considered as Direct-to-Pharmacy ("DTP") orders.
(32) In the present case, in some EEA countries, Teva and Allergan Generics are active as manufacturers selling directly generic pharmaceuticals to customers, through the DTP model.
(33) The market features however differ depending on the EEA country. For instance:
a. in Iceland, Allergan Generics is the only generic manufacturer selling generics directly to customers and it competes with wholesalers selling generics of other manufacturers (such as Lyfis for Teva; Teva does not run a DTP model);
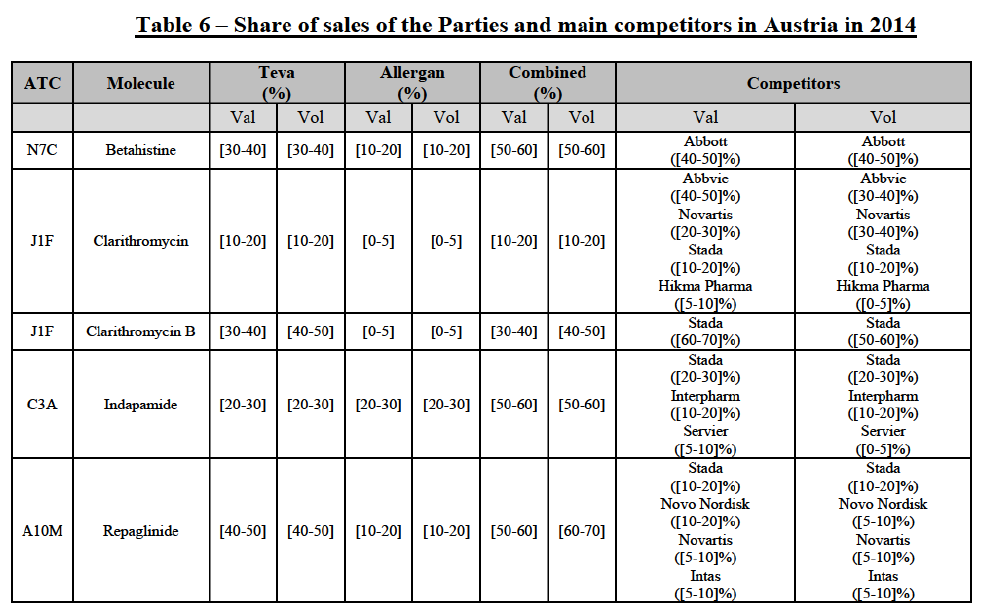
b. in Ireland, large generic manufacturers sell their products directly to customers (that is, they all use the DTP model), using wholesalers or 3PL as logistic providers). This appears to be a change following a major legislative change in 2013 impacting prices of generics in Ireland;36
c. in the United Kingdom, Teva and Allergan Generics are the only manufacturers with a DTP model, using wholesalers as logistic providers. They compete against wholesalers for the sale of FDP. At the same time, they also supply their products to wholesalers (just as other generic manufacturers do), while wholesalers purchase (and take title to) FDPs from generics manufacturers.
The Notifying Party's views
(34) The Notifying Party did not submit any views as to the market for the wholesale of pharmaceuticals and its possible segmentation(s) in general.
(35) However, the Notifying Party submitted that, in Ireland, since all manufacturers are supplying pharmacies directly, using wholesalers as distributors, it remains unclear how competition can occur simultaneously on a product-per-product basis37 and on a portfolio basis.38
The Commission's assessment
Distinction by category of product
(36) Pharmaceuticals include originator and generic products. While a given originator medicinal product typically has a single source of supply (typically the patent holder or its designee if the product is patented),39 a given generic medicinal product can often be sourced from several suppliers which offer medicinal products based on the same molecule. The number of generic suppliers typically depends on the product and on the country, and can be limited for instance for products that are difficult to manufacture or in case their manufacturing technology is protected by process patents.
(37) The market investigation indicated that the price regulation, purchasing patterns and competitive landscape differ between the sale of originator pharmaceuticals that benefit from exclusivity, on the one hand, and of originator pharmaceuticals that have lost exclusivity and generic pharmaceuticals, on the other hand.40 Indeed, there is direct competition on prices between all versions of a given off-patent molecule (including generics and the off-patent originator product).41 This competition can take place, depending on the country, in a free pricing environment, below a price ceiling set by regulatory authorities or through public tenders. This direct competition on prices, which has an impact on the purchasing patterns of customers (through comparison of price lists, tenders, etc.), does not exist for an originator pharmaceutical (except with parallel imports).
(38) In addition to competition through prices for each product separately, the market investigation indicated that some purchasing practices are specific to the purchase of generics. For instance, pharmacies tend to procure their generics through a limited number of suppliers.42 In some EEA countries, manufacturers and wholesalers selling a sufficiently large range of different generics have developed schemes with discounts to pharmacies based on a customer's overall generics spend.43 Furthermore, there are also elements of non-price competition. For instance, customers also adopt so-called cascade mechanisms for their generics purchases, establishing an ordered list of alternative generic suppliers in case of shortages (naturally, this mechanism cannot be replicated for originator pharmaceuticals, given that typically there is only a single source of supply).44
(39) As to the competitive landscape, the majority of pharmaceutical companies are either specialised in generic or originator pharmaceuticals. While certain players own both an originator business and a generics business (e.g. Teva itself, Novartis, Sanofi, Pfizer), they typically operate as very distinct business units. Teva for instance distinguishes between its Global Specialty Medicines business, for originator products, and Global Generic Medicines business, for generics. Finally, certain wholesalers (in particular short-line) can be specialised in generics.45
(40) For the purpose of the competitive assessment of the Transaction, the Commission considers that there is a separate market for the wholesale of generic pharmaceuticals.
Distinction by type of suppliers
General approach
(41) In previous cases,46 the Commission distinguished broad-line wholesalers from short-line wholesalers. The Commission noted that short-line wholesalers focus on a limited range of products, generally high volume products, and supply pharmacies with a less frequent delivery schedule.
(42) When generic manufacturers develop DTP sales channels, they engage in direct competition with wholesalers and can be considered as vertically integrated companies, competing with wholesalers on the market for the wholesale of generic pharmaceuticals to pharmacies, and, if they also sell generics to wholesalers, with other suppliers in the market for the marketing of generic pharmaceuticals where wholesalers are the customers.
(43) The market investigation further indicated that broad-line wholesalers and manufacturers selling a sufficiently broad range of generics and offering the same level of services would address pharmacies' needs in a similar way and thus could be part of the same relevant market for the wholesale of generic pharmaceuticals. Conversely, manufacturers selling a smaller range of generics or distributing generics on a less frequent basis would not belong to that market.
Specific situation of Ireland
(44) As indicated by the Parties, in Ireland, currently the wholesale of generic pharmaceuticals to pharmacies appears to be mostly done by manufacturers selling directly a wide range of generics to pharmacies. Therefore, the question arises as to whether the Parties can be considered as being active both in the wholesale market for generic pharmaceuticals in Ireland (without wholesalers being present) and in the markets for the marketing of individual molecules.
(45) In accordance with the previous practice of the Commission discussed above, the Irish Competition Authority, notably in its 2013 decision Uniphar/CMR,47 defined a market for the wholesale of pharmaceuticals in Ireland, where broad line wholesalers, short line wholesalers and manufacturers selling directly to pharmacies (DTP sales)48 would be active and would each be part of a separate segment due to the different range of products and services they would offer.
(46) For the wholesale of generics, the market investigation provided indications that, following a legislative change in 2013 impacting prices of generics in Ireland,49 wholesalers would now rather act as logistic providers for generics manufacturers, the latter selling their products directly to pharmacies.50 In particular, in this context, broad-line wholesalers have recently adopted a fee-per-pack model.51 Therefore, wholesalers no longer seem to materially influence commercial offers to pharmacies. Instead, generics manufacturers, such as Teva and Allergan Generics, sell directly to pharmacies and have developed their own sales and loyalty schemes, but would not compete with wholesalers.
(47) However, contrary to the Party's views, it is not inconsistent to conclude that the Parties compete both on the marketing of individual molecules and on the wholesale of generic pharmaceuticals in Ireland. Indeed, as evidenced by the market investigation and further detailed in the competitive assessment, pharmacies would typically source part of their generic pharmaceutical needs as a purchase of a broad portfolio of molecules from preferred suppliers, and complete these purchases with smaller scale, molecule-by-molecule type of purchases. Generic manufacturers having a broad portfolio of molecules, such as the Parties, compete on the market for wholesale of a wide range of generic molecules to pharmacies against other generic manufacturers of similar scale and offering similar level of services. Meanwhile, as regards the market for marketing of individual generic molecules all generic manufacturers offering the molecule are active, irrespective of their size.52
Conclusion
(48) For the purpose of the competitive assessment of the Transaction, the Commission considers that the segmentation of the market for the wholesale of generic pharmaceuticals by type of suppliers can be left open since the conclusion of the Commission as to whether serious doubts arise or not in the market for the wholesale of generic pharmaceuticals will not change under any plausible product market definition.
Distinction by category of customers
(49) Finished dose pharmaceuticals can be sold to individual pharmacies and pharmacy chains, dispensing doctors and hospitals.
(50) In previous cases,53 the Commission envisaged a segmentation of the wholesale market of finished dose pharmaceuticals by category of customers, such as retail pharmacies, doctors and hospitals, due to different purchasing and delivery patterns.
(51) The market investigation confirmed that purchasing patterns may differ depending on the category of customers. For instance, pharmacy chains which are vertically integrated to a wholesaler would generally purchase generics from the wholesaling arm of the group.54 In a number of EEA countries, hospitals would more frequently use tendering procedures to select its supplier of one specific generic.55 Independent pharmacies and pharmacy chains may or may not be part of purchasing groups depending on the country.56
(52) The exact scope of the product market for the wholesale of generic FDPs can be left open for the purposes of the competitive assessment of the Transaction since the conclusion of the Commission as to whether serious doubts arise or not in the market for the wholesale of generic finished dose pharmaceuticals will not change under any plausible product market definition.
IV.2.1.2.b. Geographic market definition
(53) In previous decisions,57 the Commission considered that pharmaceutical wholesaling is either national or regional (sub-national) in scope, due to the emphasis placed by customers on the frequency and speed of delivery of medical products.
(54) The market investigation in this case confirmed that the market for the wholesale of generic pharmaceuticals is not larger than national, in view of the existence of national regulatory and reimbursement schemes and purchasing practices and of the fact that competition between pharmaceutical companies still predominantly takes place at a national level.58
(55) The exact scope of the geographic market for the wholesale of generic pharmaceuticals can be left open for the purposes of the competitive assessment of the Transaction since the competitive assessment will not change under any plausible geographic market segmentation.
IV.2.2. Competitive assessment
IV.2.2.1. Introduction
Marketed generic pharmaceuticals
(56) In this case, affected markets were identified under all plausible market definitions, at the level of the molecule (ATC5) and at the level of the pharmaceutical form (NFC-1), with a distinction between prescribed and OTC sales, where relevant.
(57) Given the large number of affected markets in relation to marketed FDPs, and in accordance with past practice,59 the Commission has applied a system of filters aimed at determining the group of markets where concerns are most likely and on which it focused its analysis.
(58) Based on these filters, affected markets in relation to marketed FDPs are analysed according to four categories:
* Group 1: the Parties' combined market share exceeds 35% and the increment exceeds 1%.
* Group 1+: either (a) the Parties' combined market share is below 35%, but only one other competitor remains on the market, or (b) the Parties' combined market share exceeds 35% and the increment is below 1% but the Party with the small increment is a recent entrant.
* Group 2: the Parties' combined market share exceeds 35% but the increment is less than 1% (and the Party with the small increment is not a recent entrant).
* Group 3: the market is horizontally affected but the Parties' combined market share is less than 35% (and more than one competitor remains on the market).
(59) In light in particular of the combined market shares of the Parties and their competitors over the last three years, the date of patent expiry, the recent evolution of prices, the level of complexity of the Parties' products,60 the Parties' pipeline products, as well as replies to the market investigation, the Commission considers that the Transaction does not raise serious doubts as to its compatibility with the internal market with respect to all Group 3 markets, as well as the following Group 1/1+/2 markets, under all plausible product market definitions:
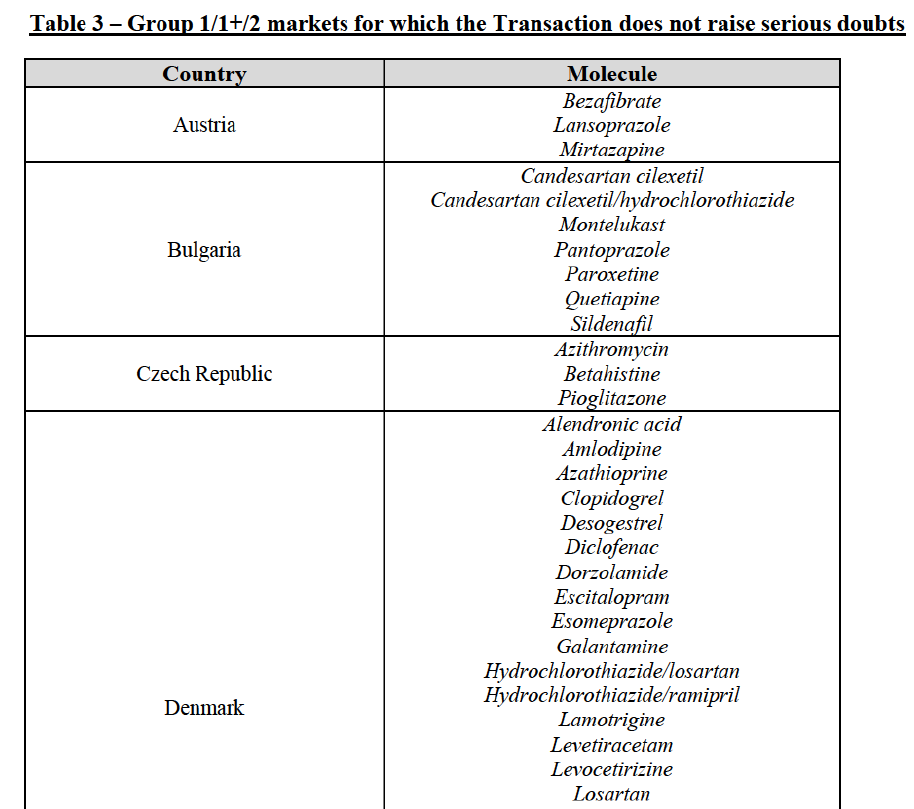
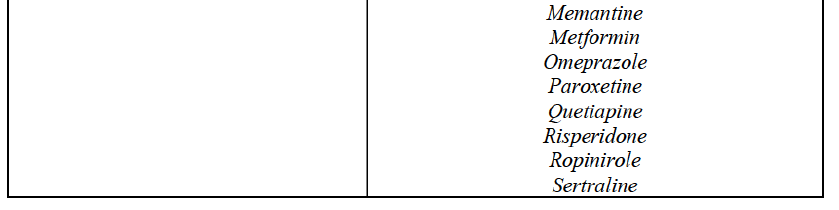
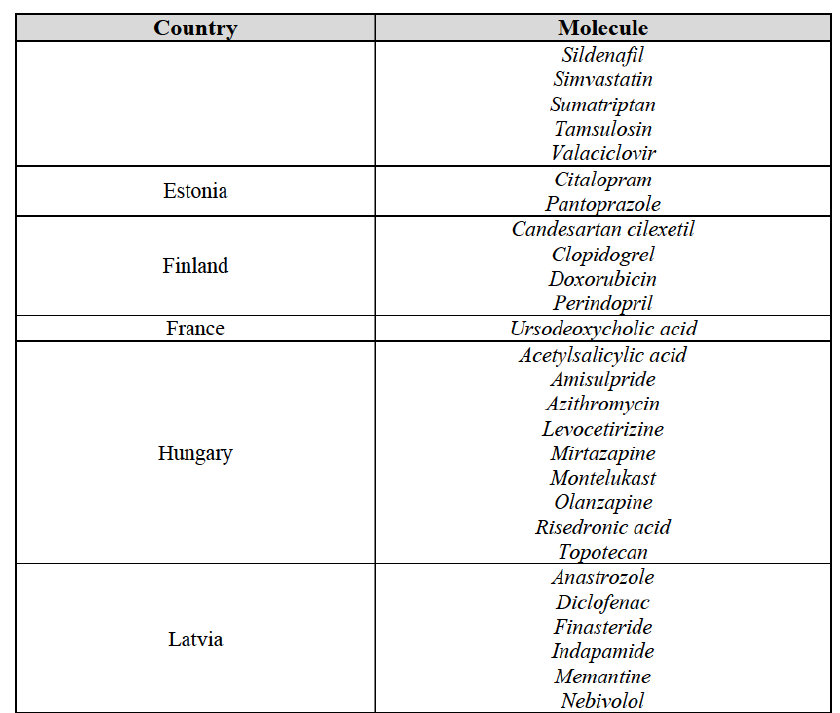
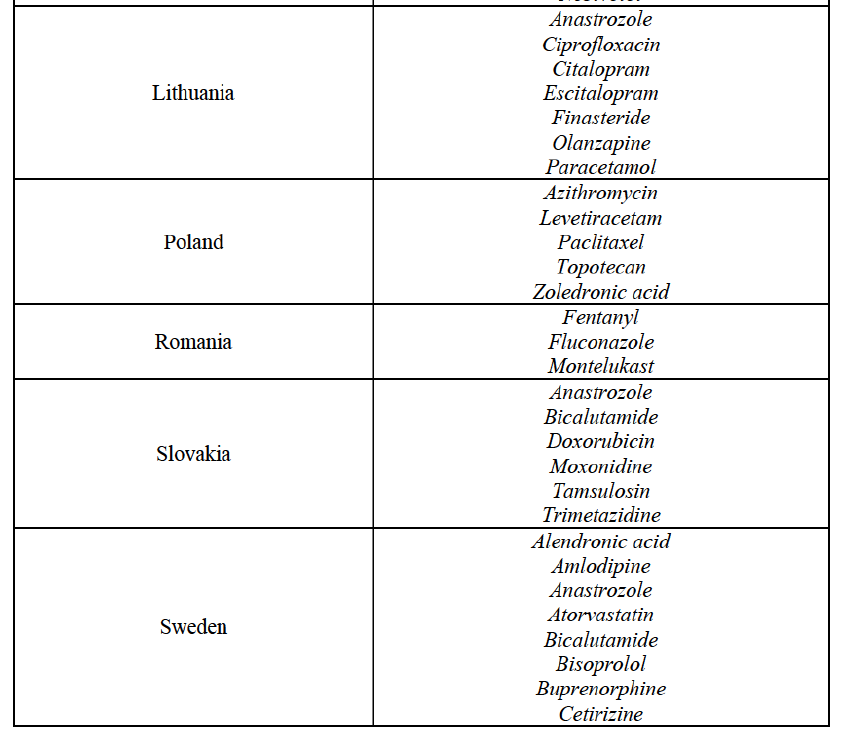
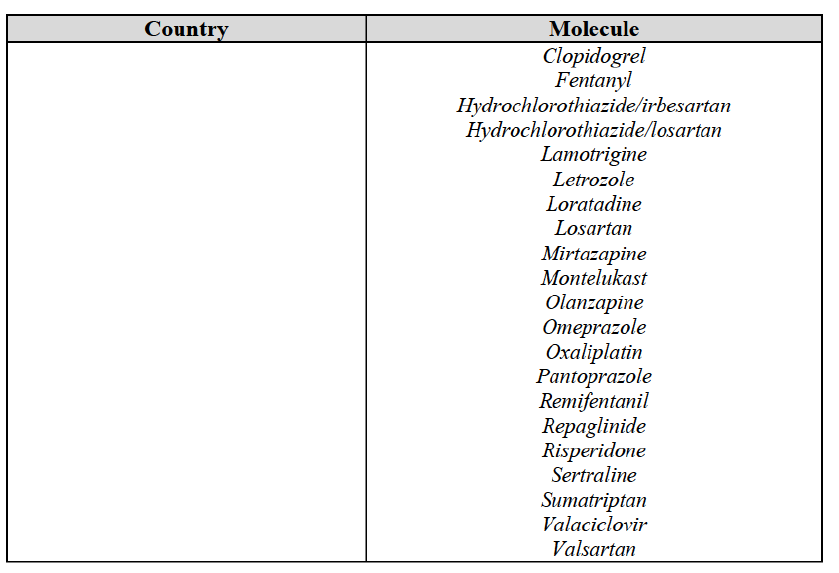
(60) The markets with respect to which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market in relation to marketed FDPs are further detailed below for each EEA country under the section "Marketed generic pharmaceuticals".
Pipeline generic pharmaceuticals
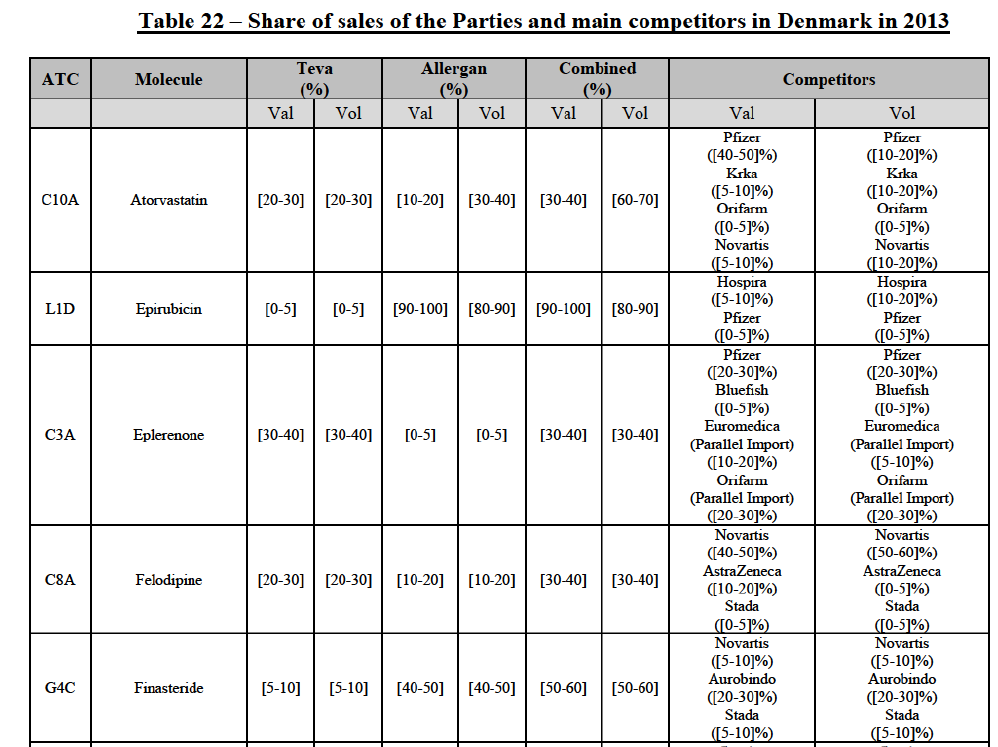
(61) Given the large number of markets in relation to pipeline FDPs, and in accordance with past practice,61 the Commission has applied a system of filters aimed at determining the group of markets where concerns are most likely and on which it focused its analysis.
(62) Based on these filters, markets in relation to pipeline FDPs are analysed according to four categories:
* Pipeline in affected markets: One Party is planning to launch a generic pipeline in a market where both Parties already hold a combined market share in excess of 35% (under all plausible market definitions, including distinction by pharmaceutical form and between Rx and OTC where relevant). For example, in E...], both Parties are active for [...] which is a Group 1 affected market and Allergan Generics has a pipeline for [...J.
* Pipeline-to-marketed affected markets: One Party is planning to launch a generic pipeline in a market where the other Party is the originator or holds a market share in excess of 35% (under all plausible market definitions, including distinction by pharmaceutical form and between Rx and OTC where relevant).
* Pipeline-to-pipeline affected markets: Both Parties are planning to launch a generic pipeline in a market where there is only one or two competitors (including the originator company, this is the case for instance in relation to molecules close to patent expiry).
* Originator-to-generic pipeline overlaps: One Party is planning to launch a generic version of a product for which the other Party is the originator. This is the case of Teva's Copaxone (glatiramer acetate) and Azilect (rasagiline), for which Allergan Generics is developing a generic version.
(63) In light, in particular, of the market shares of the in-market Party (pipeline-to-marketed affected markets) and the timing of the entry of pipeline products from competitors (pipeline-to-pipeline affected markets), the Commission considers that the Transaction does not raise serious doubts as to its compatibility with the internal market with respect to the following markets under all plausible product market definitions:
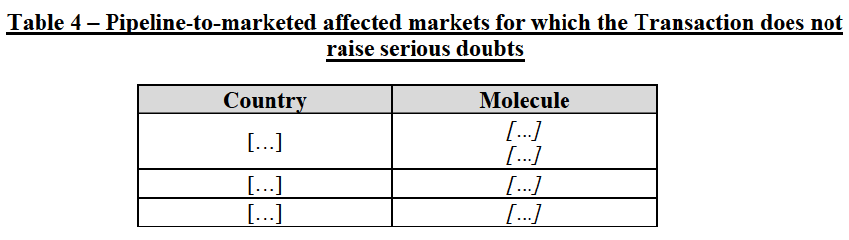
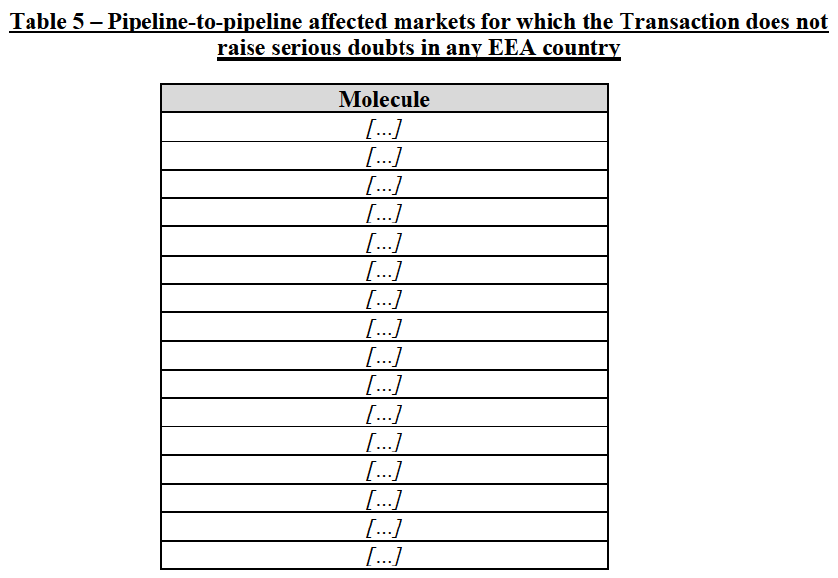
(64) The markets with respect to which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market in relation to pipeline FDPs are further detailed below:
* Pipeline in affected markets: For each EEA country, under the section "Marketed generic pharmaceuticals".
* Pipeline-to-marketed affected markets: For each EEA country, under the section "Pipeline generic pharmaceuticals".
* Pipeline-to-pipeline affected markets: Under section IV.2.2.29, "Pipeline-to-pipeline overlaps ").
* Originator-to-generic pipeline overlaps: Under section IV.2.2.30, "Originator-to-generic pipeline overlaps").
Wholesale of generic pharmaceuticals
(65) The Commission also considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the markets for the wholesale of generic pharmaceuticals in Iceland, Ireland and the United Kingdom. The competitive assessment of the impact of the Transaction on these markets is included in the relevant country sections.
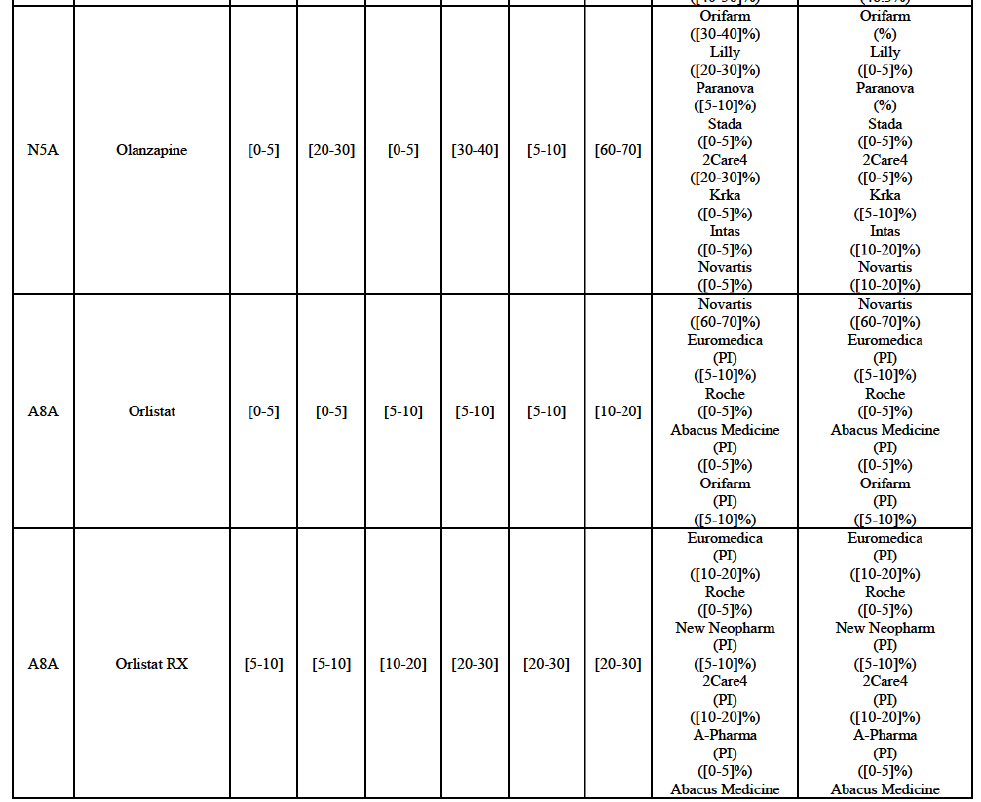
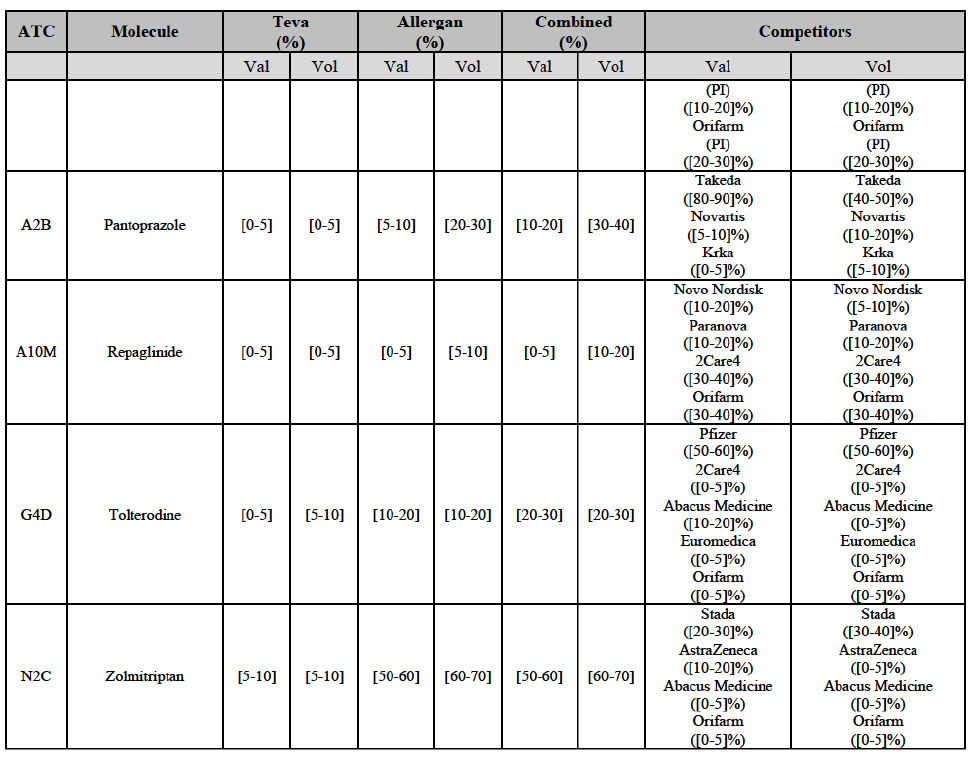
IV.2.2.2. Austria
IV.2.2.2.a. Marketed generic pharmaceuticals
(66) The following tables present the market shares of the Parties and their main competitors in 2012, 2013 and 2014 for the Group 1/1+/2 markets for which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market.
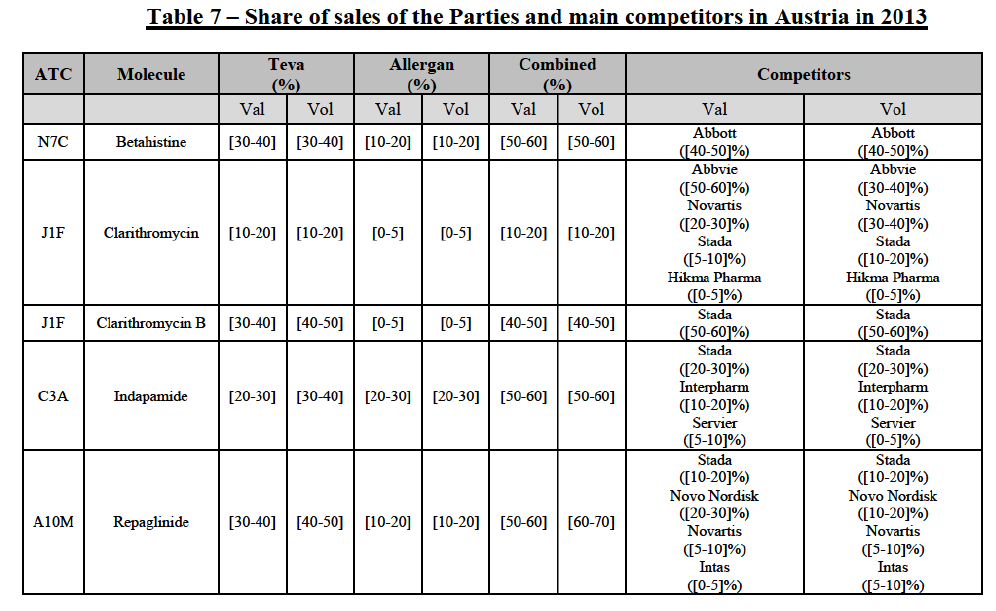
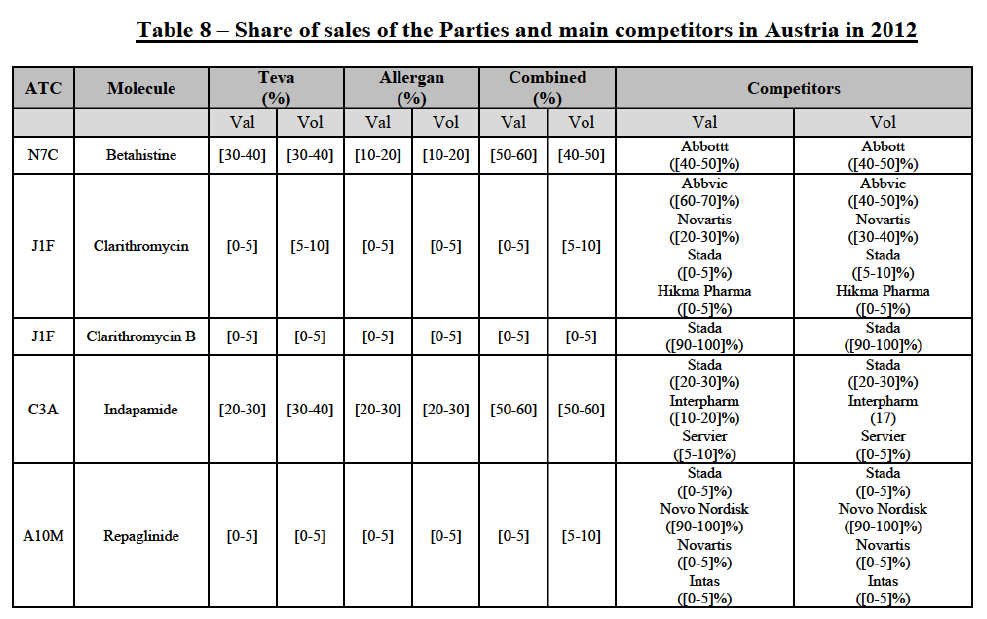
(67) The Commission presents below the competitive analysis on these markets, at molecule level, indicating also the ATC3 class to which it belongs, with a possible distinction by pharmaceutical form (at NFC1 level) and between prescribed and OTC sales where relevant.
Betahistine (N7C)
(68) For betahistine, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [50-60]% in value and in volume, at the molecule and pharmaceutical form level, with a significant increment (above [10-201%). Only one other competitor with a market share above 5% would remain. Furthermore, the market investigation indicated that there would be a negative impact of the Transaction on this molecule.62
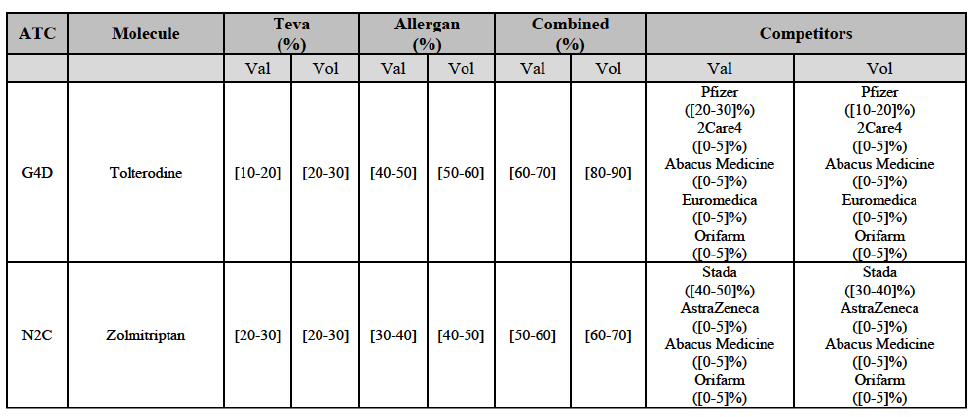
Clarithromycin (J1F)
(69) For clarithromycin, the Transaction gives rise to a Group 1 market at the level of the pharmaceutical form B in 2013. Allergan Generics recently entered the market (in 2012), and its market share fluctuated over the last three years (up to [5-10]% in 2013). In 2014, the combined market share of the Parties remained above [40-50]%. Only one other competitor would remain active on the market. Furthermore, the market investigation indicated that there would be a negative impact of the Transaction on this molecule.63
Indapamide (C3A)
(70) For indapamide, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [50-60]% in the last three years in value and in volume, with an increment always above [20-30]%. Only two competitors that have a market share above 5% would remain on the market. Furthermore, the market investigation indicated that there would be a negative impact of the Transaction on this molecule."
Repaglinide (A1011I)
(71) For repaglidine, the Transaction gives rise to a Group 1 market at molecule level. The Parties recently entered and achieved in two years a combined market share above 50% ([50-601% in value and [60-70]% in volume), with a significant increment ([10-20]% in value and [10-20]% in volume). The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.65
Conclusion
(72) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the markets for the marketing of betahistine, clarithromycin, indapamide and repaglinide in Austria.
IV.2.2.2.b. Pipeline generic pharmaceuticals
(73) The Transaction does not raise serious doubts as to its compatibility with the internal market with respect to pipeline generic pharmaceuticals in Austria.
IV.2.2.3. Belgium
IV.2.2.3.a. Marketed generic pharmaceuticals
(74) The following tables present the market shares of the Parties and their main competitors in 2012, 2013 and 2014 for the Group 1/1+/2 market for which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market.
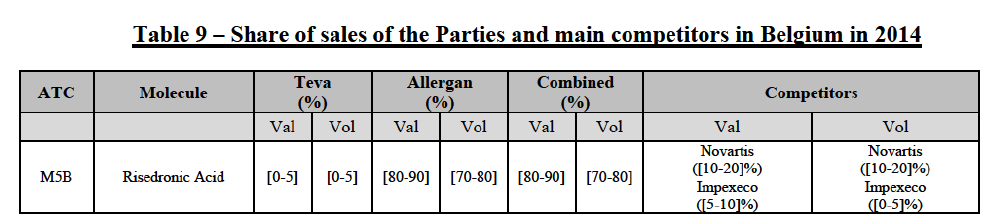
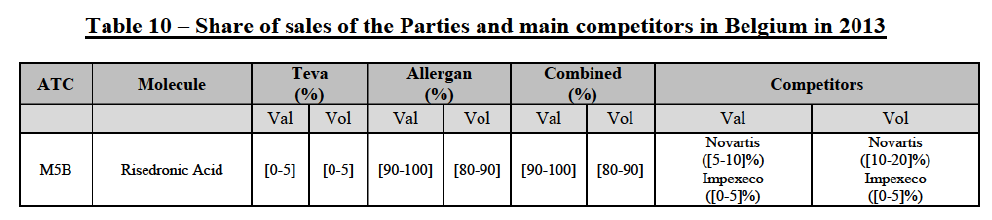
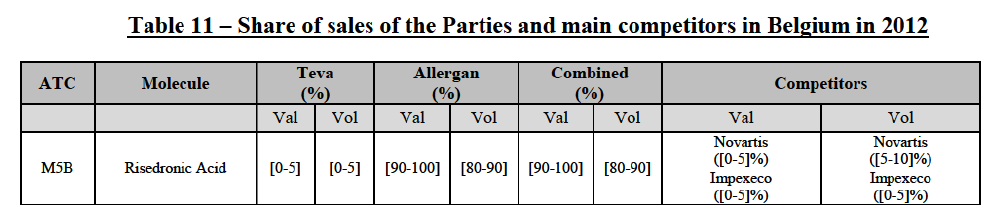
(75) The Commission presents below the competitive analysis on this market, at molecule level, indicating also the ATC3 class to which it belongs, with a possible distinction by pharmaceutical form (at NFC1 level) and between prescribed and OTC sales where relevant.
Risedronic Acid (M5B)
(76) For risedronic acid, the Transaction gives rise to a Group 1 market at molecule level. Allergan Generics is the originator of this molecule. The combined market share of the Parties is very high, above [80-90]% in volume and [90-100]% in value since 2013. Only one competitor with a market share above 5% would remain on the market. Furthermore, the market investigation confirmed the strong position of the Parties.66
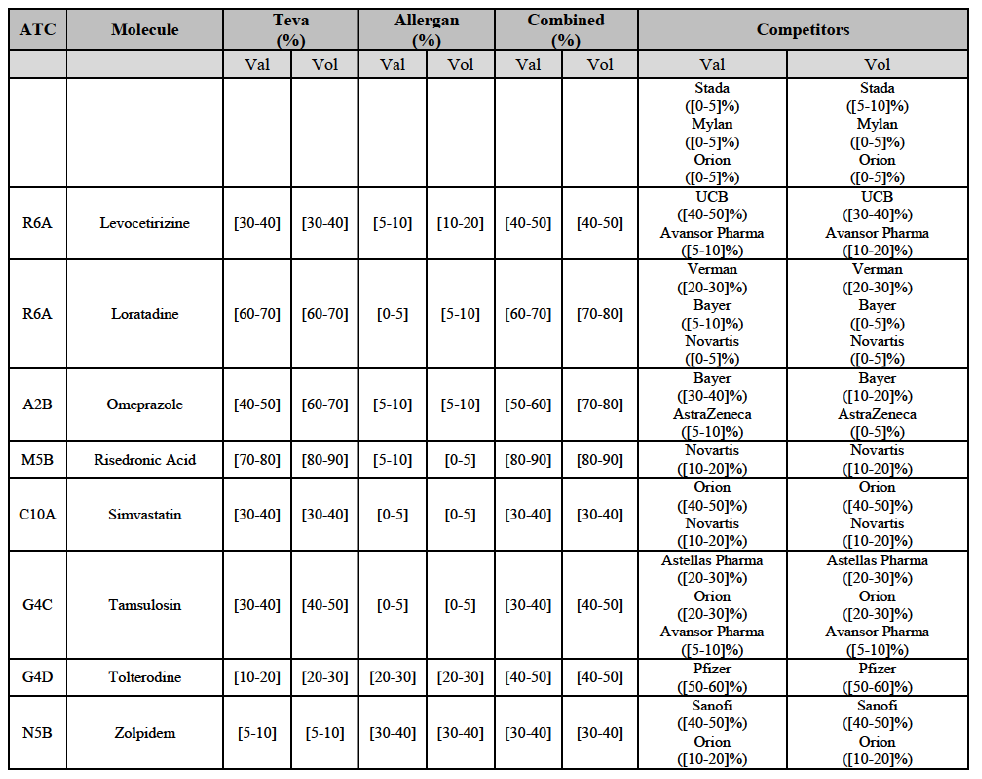
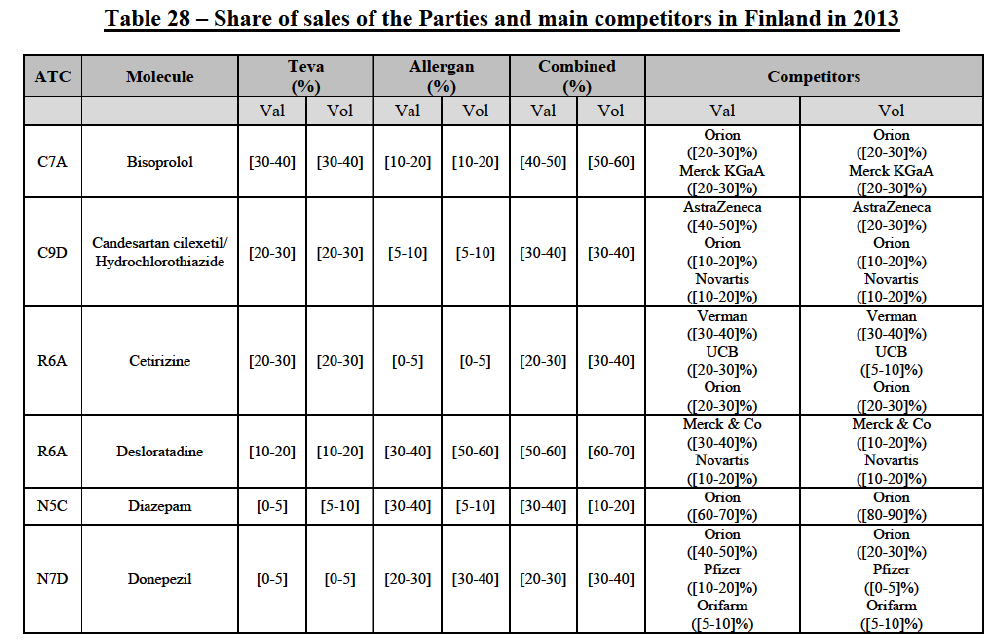
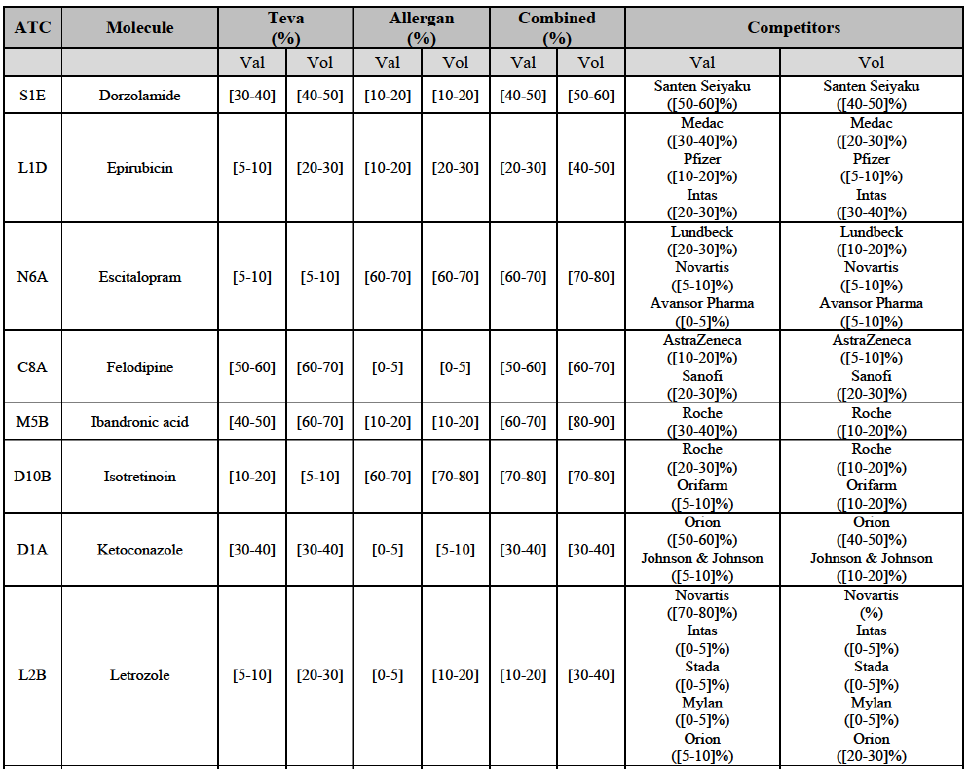
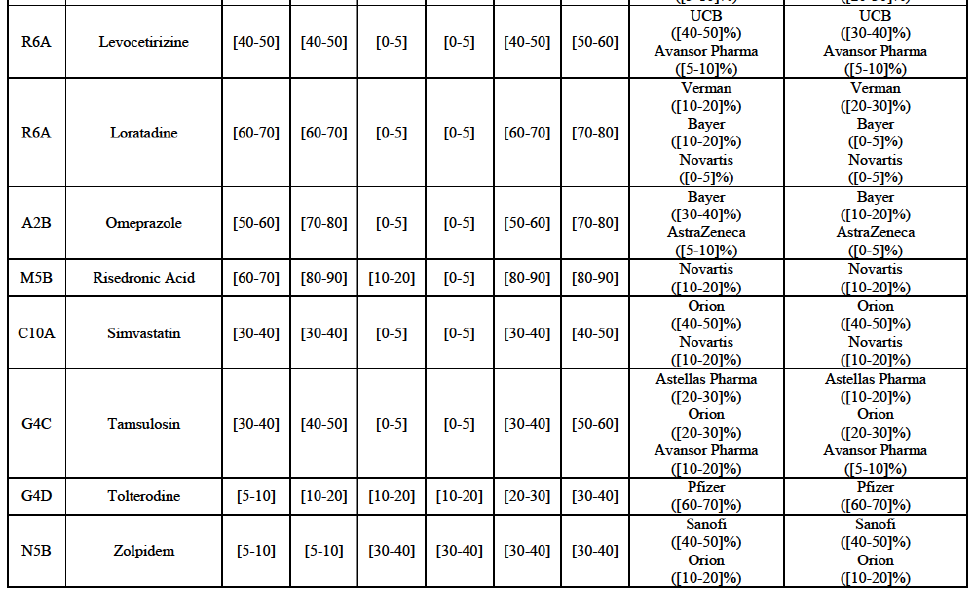
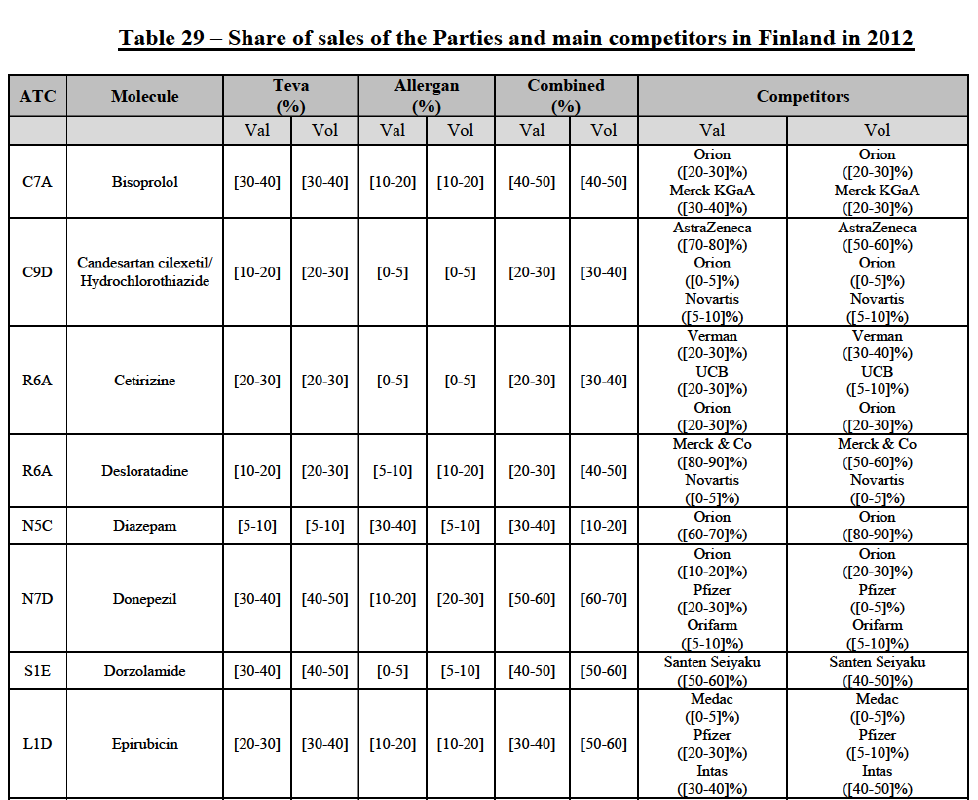
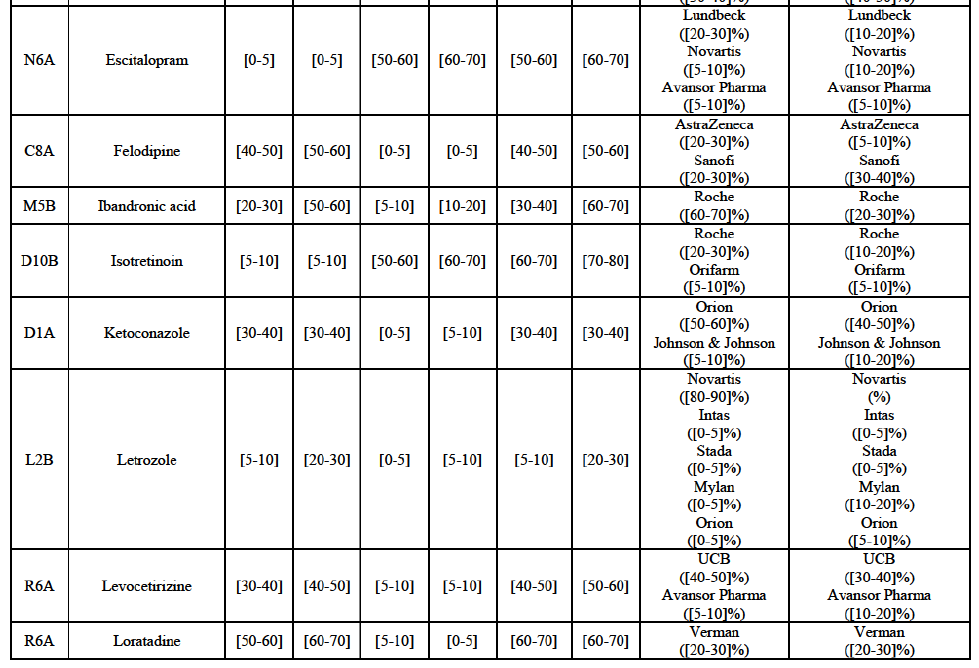
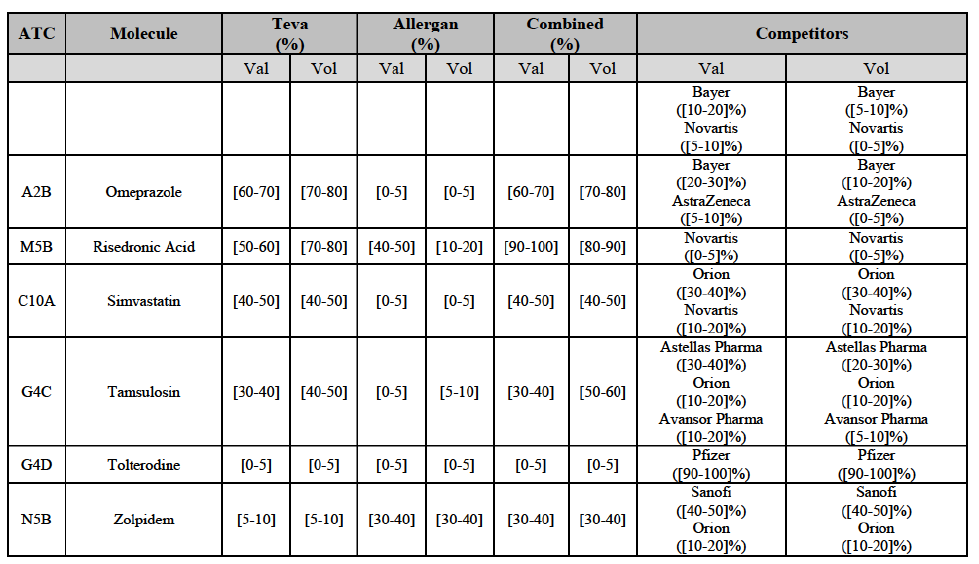
(77) The Notifying Party submits that the molecule risedronic acid has been delisted by Teva prior to the announcement of the Transaction. However, Teva's marketing authorisation is still valid, and the Parties acknowledge that it would therefore take up to six months only to come back to the market.67 Therefore, the Commission considers that the Parties' activities overlap irrespective of Teva's decision to delist the product.
Conclusion
(78) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the marketing of risedronic acid in Belgium.
IV.2.2.3.b. Pipeline generic pharmaceuticals
(79) Allergan Generics is planning to launch a generic [...] (pharmaceutical form [...]), for which Teva had a[40-50]% market share in value ([10-201% in volume) in 2014 on the same pharmaceutical form, and only one other competitor was active on the market in 2012-2014.
Conclusion
(80) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the market for the marketing of [...] in Belgium.
IV.2.2.4. Bulgaria
IV.2.2.4.a. Marketed generic pharmaceuticals
(81) The following tables present the market shares of the Parties and their main competitors in 2012, 2013 and 2014 for the Group 1/1+/2 markets for which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market.
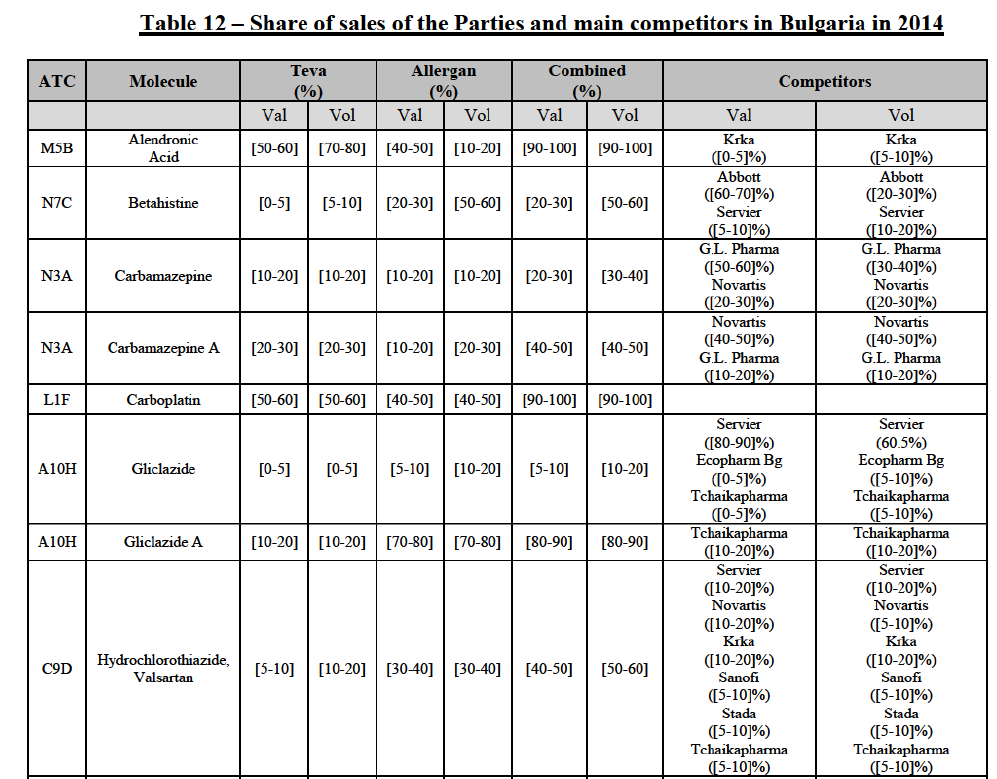
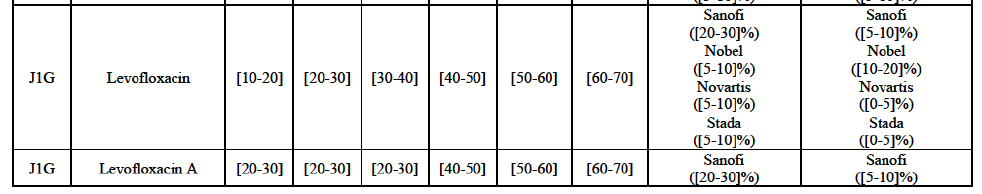
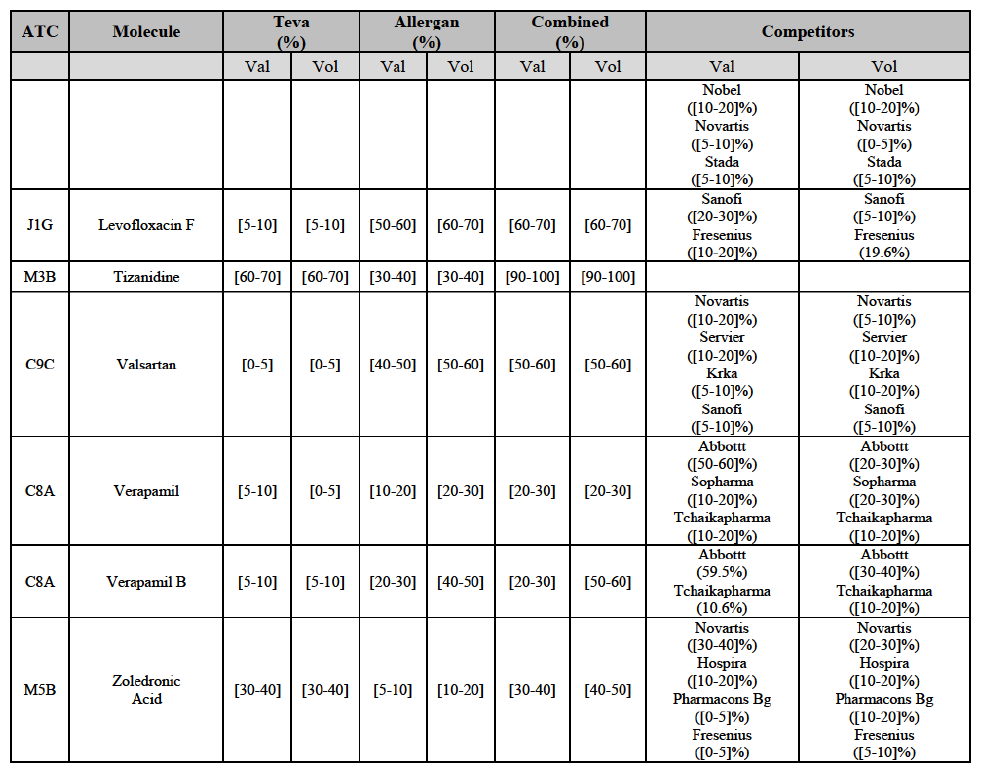
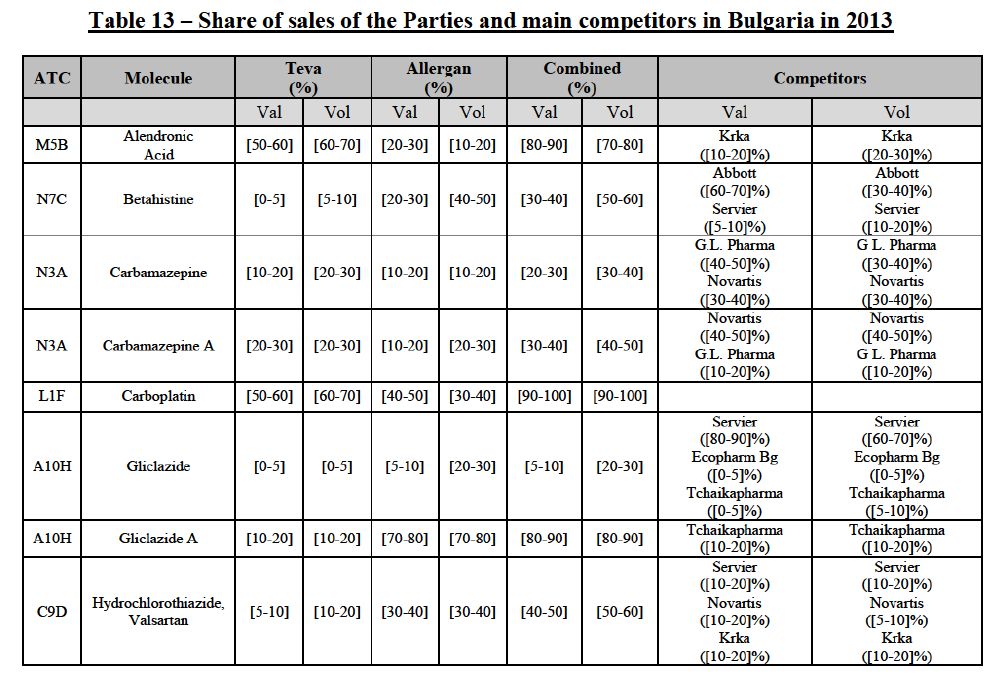
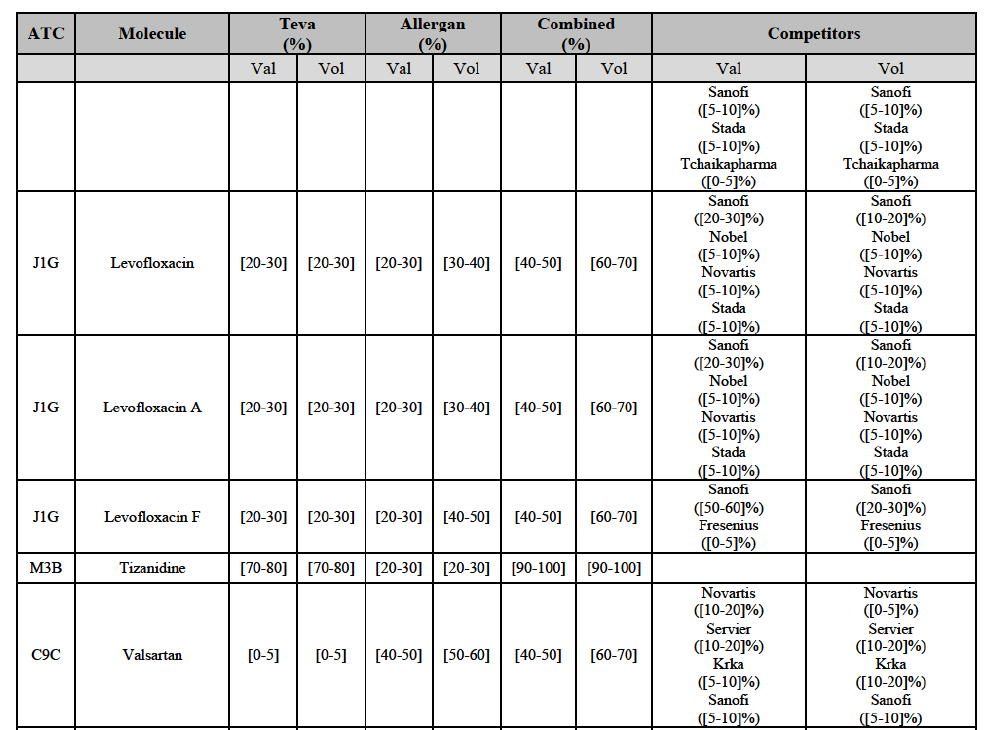
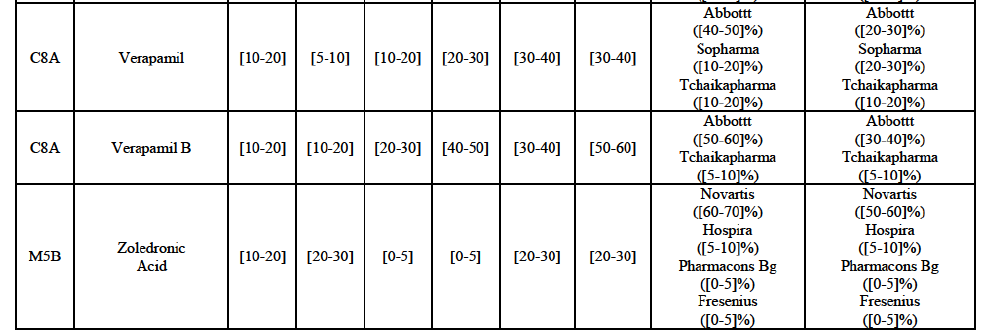
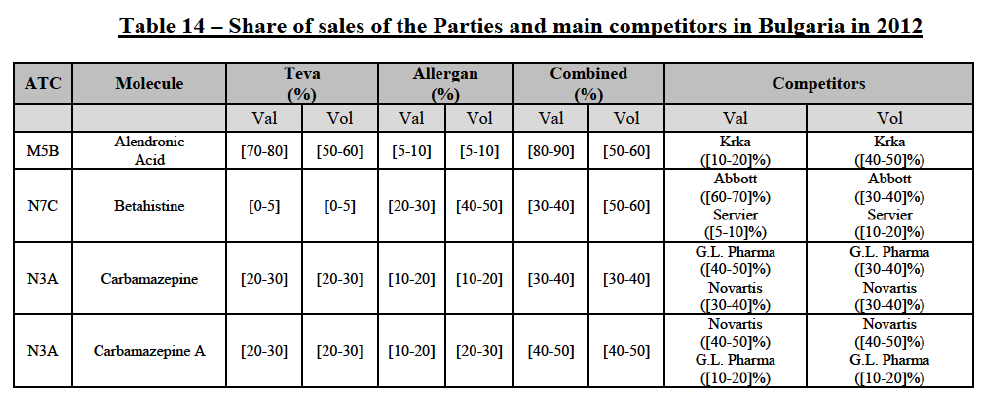
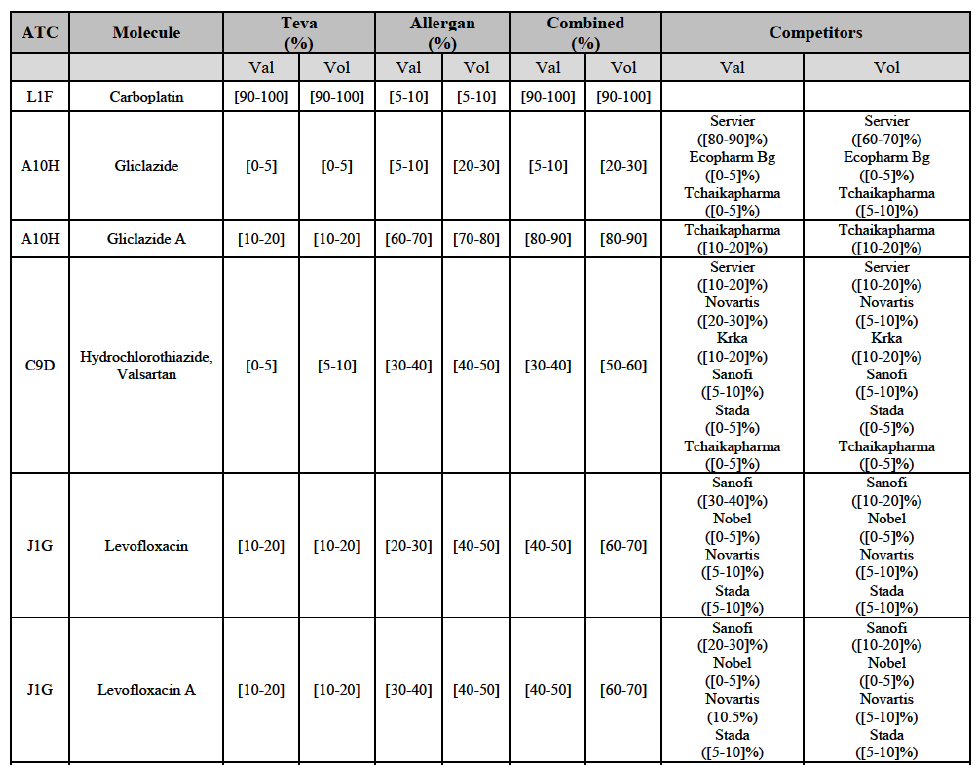
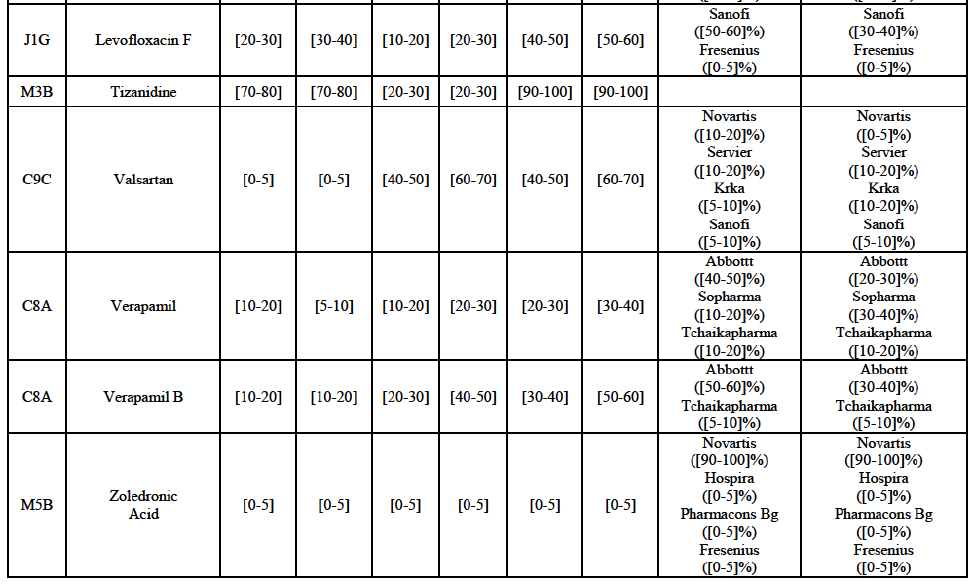
(82) The Commission presents below the competitive analysis on these markets, at molecule level, indicating also the ATC3 class to which it belongs, with a possible distinction by pharmaceutical form (at NFC1 level) and between prescribed and OTC sales where relevant.
Alendronic Acid (M5B)
(83) For alendronic acid, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above[90-100]% in value and in volume in 2014, with a significant increment (respectively [40-50]% and [10-20]% in 2014). There would be no remaining competitor with a market share above 5% in value (and only one in volume). Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market and that it would be difficult to switch to alternative suppliers.68
Betahistine (N7C)
(84) For betahistine, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [50-60]% in volume in the last three years. Only two competitors, including the originator, would remain with a market share above 5%. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.69
Carbamazepine (N3A)
(85) For carbamazepine, the Transaction gives rise to a Group 1 market at the level of the pharmaceutical level A. The Parties' combined market share was high over the last three years in value and in volume, between [30-40]% and [40-50]%. Only two competitors, including the originator, would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.70
Carboplatin (L1F)
(86) For carboplatin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was >[90-100]% in value and volume in 2013 and 2014, with a significant increment (above [40-50]%). No alternative competitors would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.71
(87) The Notifying Party submits that the molecule carboplatin has been delisted by Teva prior to the announcement of the Transaction. However, Teva's marketing authorisation is still valid, and the Parties acknowledge that it would therefore take up to six months only to come back to the market.72 Therefore, the Commission considers that the Parties' activities overlap irrespective of Teva's decision to delist the product.
Gliclazide (A10H)
(88) For gliclazide, the Transaction gives rise to a Group 1 market at the level of the pharmaceutical form A. The Parties' combined market share was above [80-90]% both in value and volume over the last three years, with a significant market share increment. Only one competitor with a market share above 5% would remain on the market at the given pharmaceutical form level. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market and that it would be difficult for customers to switch to alternative suppliers.73
(89) The Notifying Party submits that the molecule gliclazide has been delisted by Teva prior to the announcement of the Transaction. However, Teva's marketing authorisation is still valid, and the Parties acknowledge that it would therefore take up to six months only to come back to the market.74 Therefore, the Commission considers that the Parties' activities overlap irrespective of Teva's decision to delist the product.
Hydrochlorothiazide/valsartan (C9D)
(90) For hydrochlorothiazide/valsartan, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined volume market share was high, above [50-60]% over the last three years. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.75
Levofloxacin (J1G)
(91) For levofloxacin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined value and volume market share was high, in particular up to [60-70]% for pharmaceutical form A and [70-80]% for pharmaceutical form F in volume. For both pharmaceutical forms, only two competitors with a market share above 5% in 2014 would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.76
Tizanidine (M3B)
(92) For tizanidine, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was >[90-100]% in value and volume, with a significant increment (above [30-40]%). Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.77
Valsartan (C9C)
(93) For valsartan, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was high in value and volume over the last three years, ranging between [40-50]% and [60-70]%. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.78
Verapamil (C8A)
(94) For verapamil, the Transaction gives rise to a Group 1 market at the level of the pharmaceutical form B. The combined market share of the Parties was above [50-60]% in volume over the last three years. Only two competitors, including the originator, would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market and that it would be difficult for customers to switch to alternative suppliers.79
Zoledronic Acid (M5B)
(95) For zoledronic acid, the Transaction gives rise to a Group 1 market at molecule level. The Parties recently entered and achieved in two years a combined market share of [3040]% in value and [40-50]% in volume, with a significant increment ([5-10]% in value and [10-20]% in volume). Only two competitors with a market share above 5% would remain on the market, one of them being the originator. Finally, the market investigation indicated that the Transaction would have a negative impact in the market and that it would be difficult to switch to alternative suppliers.80
(96) The Notifying Party submits that the molecule zoledronic acid has been delisted by Teva prior to the announcement of the Transaction. However, Teva's marketing authorisation is still valid, and the Parties acknowledge that it would therefore take up to six months only to come back to the market.81 Therefore, the Commission considers that the Parties' activities overlap irrespective of Teva's decision to delist the product.
Conclusion
(97) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the marketing of alendronic acid, betahistine, carbamazepine, carboplatin, gliclazide, hydrochlorothiazide/valsartan, levofloxacin, tizanidine, valsartan, verapamil and zoledronic acid in Bulgaria.
IV.2.2.4.b. Pipeline generic pharmaceuticals
(98) The Transaction does not raise serious doubts as to its compatibility with the internal market with respect to pipeline generic pharmaceuticals in Bulgaria.
IV.2.2.5. Croatia
IV.2.2.5.a. Marketed generic pharmaceuticals
(99) The following tables present the market shares of the Parties and their main competitors in 2012, 2013 and 2014 for the Group 1/1+/2 markets for which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market.
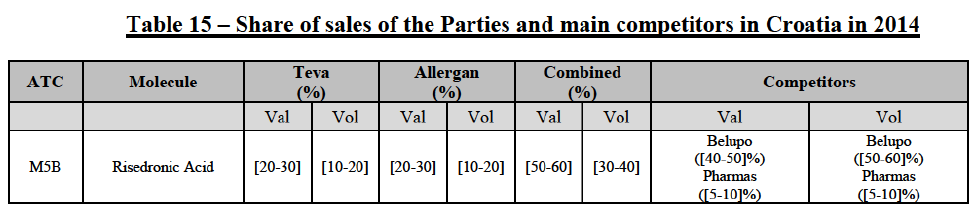
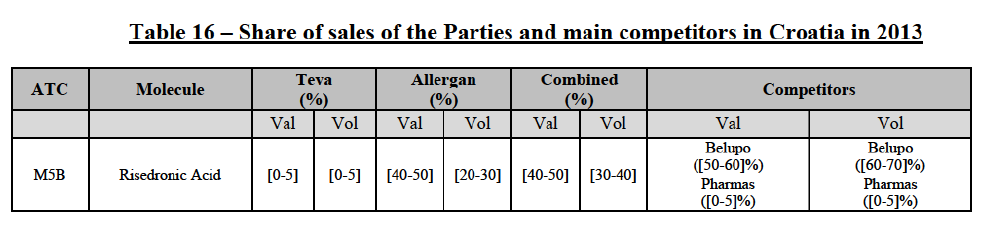
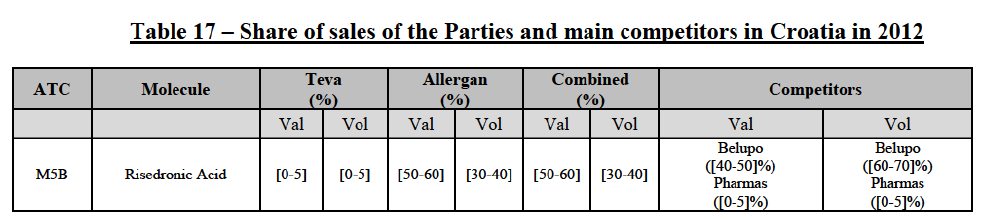
Risedronic Acid (M5B)
(100) For risedronic acid, the Transaction gives rise to a Group 1 market at molecule level. Allergan Generics is the originator of this molecule. The Parties' combined market share was high, above[50-60]% in value in 2014, and only two competitors with a market share above 5% would remain on the market. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position in value.82
Conclusion
(101) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the marketing of risedronic acid in Croatia.
IV.2.2.5.b. Pipeline generic pharmaceuticals
[...]
(102) Allergan Generics is planning to launch a generic [...] (pharmaceutical form [...]), for which Teva had a >[90-100]% market share in volume ([20-30]% in value) in 2014.
[...]
(103) Allergan Generics is planning to launch a generic [...] (pharmaceutical form [...]), for which Teva had [90-100]% of the market in 2014 on the same pharmaceutical form.
Conclusion
(104) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the markets for the marketing of [...] and [...]in Croatia.
IV.2.2.6. Czech Republic
IV.2.2.6.a. Marketed generic pharmaceuticals
(105) The following tables present the market shares of the Parties and their main competitors in 2012, 2013 and 2014 for the Group 1/1+/2 markets for which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market.
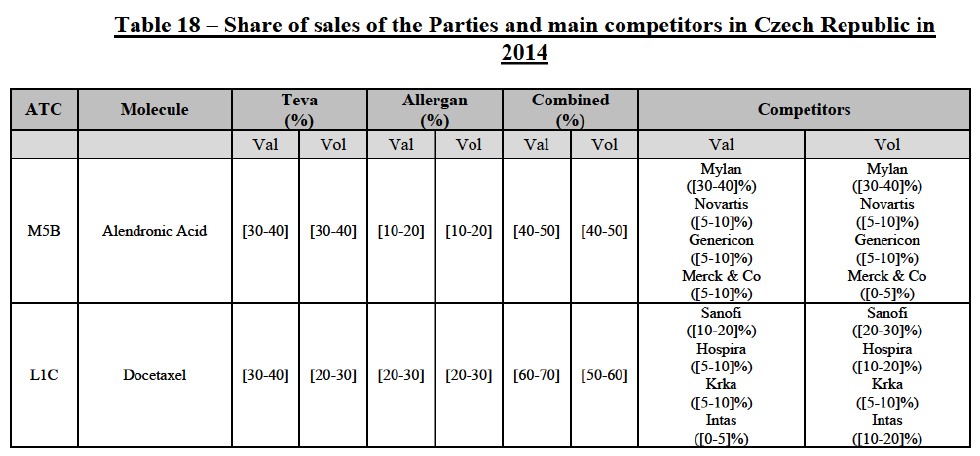
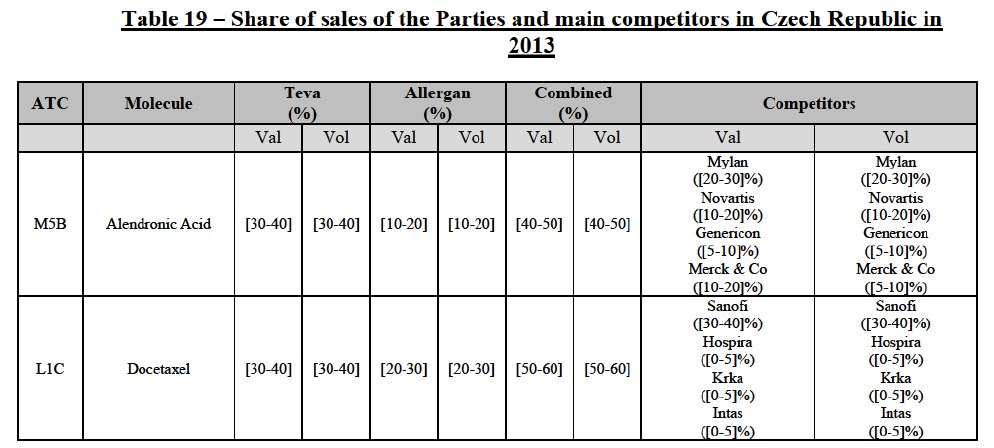
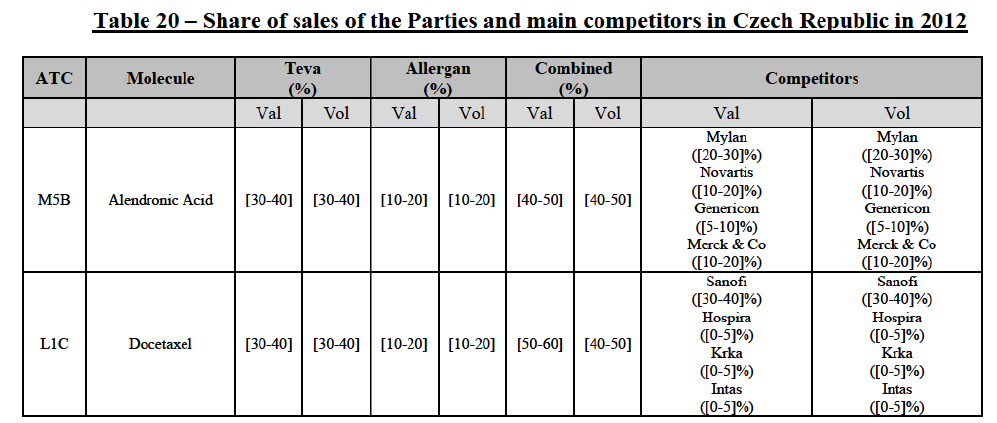
(106) The Commission presents below the competitive analysis on these markets, at molecule level, indicating also the ATC3 class to which it belongs, with a possible distinction by pharmaceutical form (at NFC1 level) and between prescribed and OTC sales where relevant.
Alendronic Acid (M5B)
(107) For alendronic acid, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market shares in value and volume were high in the last three years, ranging from [40-50]% to [40-50]%, with a significant increment (>[10-20]%). Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market and that it would be difficult for customers to switch to alternative suppliers.83
Docetaxel (L1C)
(108) For docetaxel, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was high over the last three years in value and in volume, ranging from [40-50]% to [60-70]%. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.84
Conclusion
(109) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction does raise serious doubts as to its compatibility with the internal market with respect to the marketing of alendronic acid and docetaxel in Czech Republic.
IV.2.2.6.b. Pipeline generic pharmaceuticals
[...]
(110) Allergan Generics is planning to launch a generic [...] (pharmaceutical form [...]), for which Teva had a [60-70]% market share in value and [70-80]% in volume in 2014, and only one other competitor was active on the market in 2012-2014.
Conclusion
(111) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the markets for the marketing of [...] in Czech Republic.
IV.2.2.7. Denmark
IV.2.2.7.a. Marketed generic pharmaceuticals
(112) The following tables present the market shares of the Parties and their main competitors in 2012, 2013 and 2014 for the Group 1/1+/2 markets for which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market.
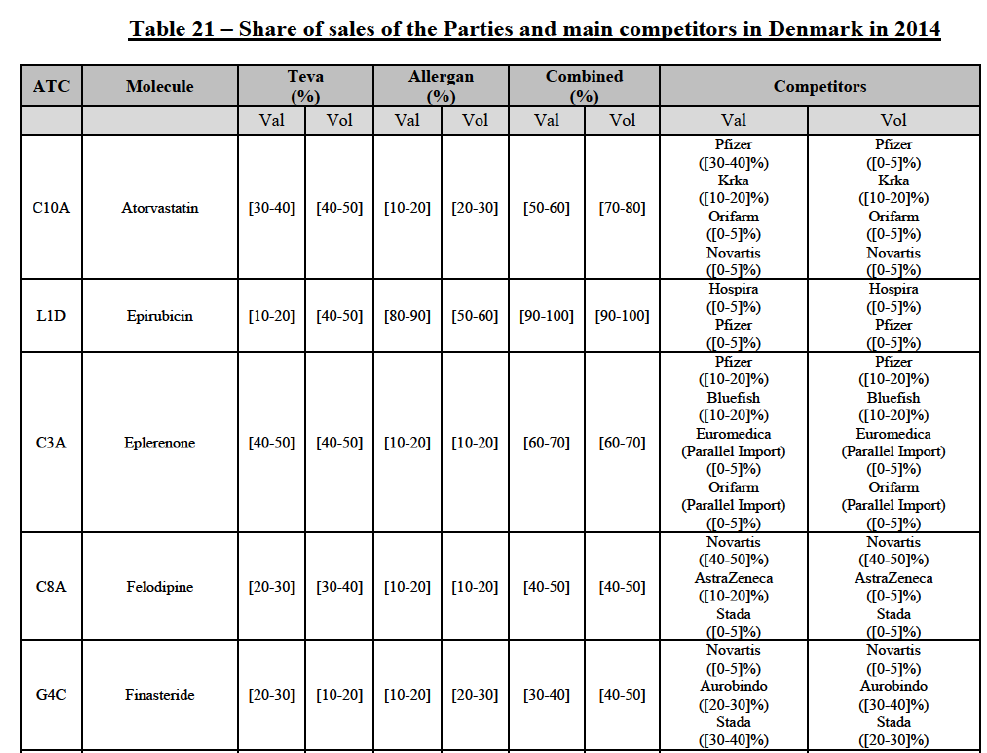
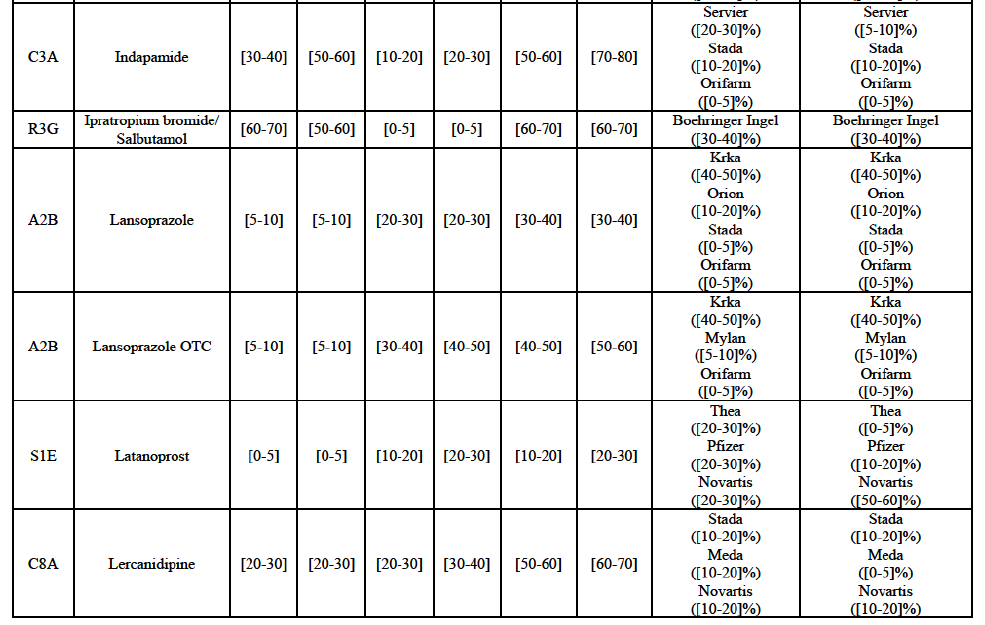
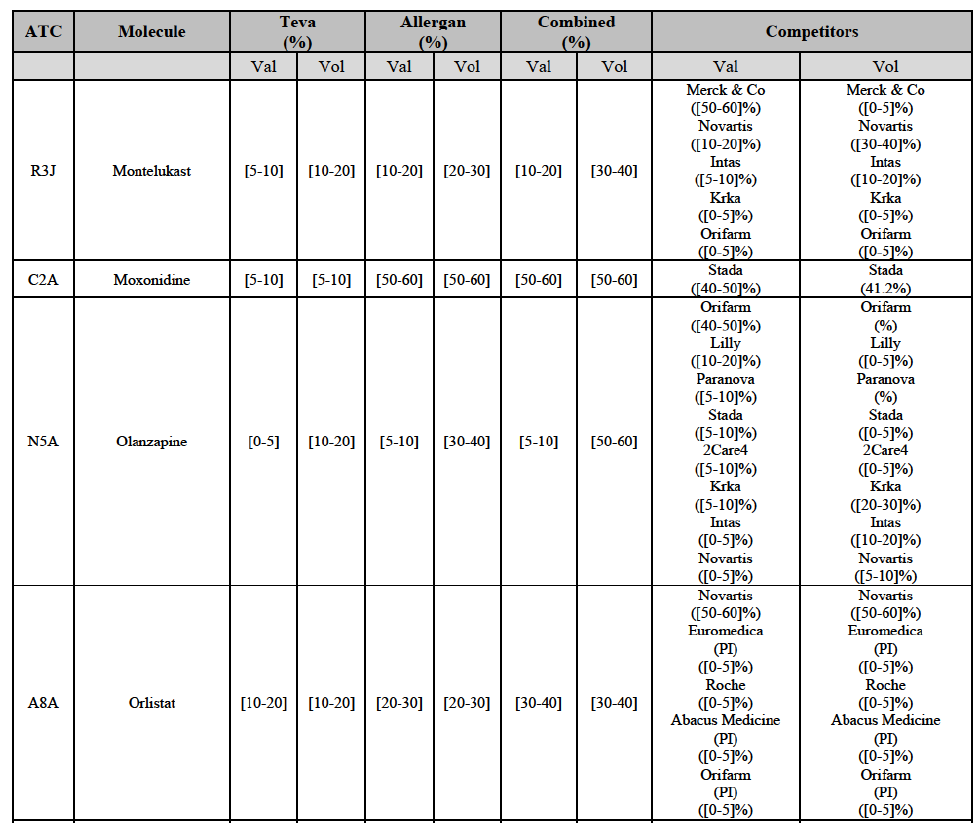
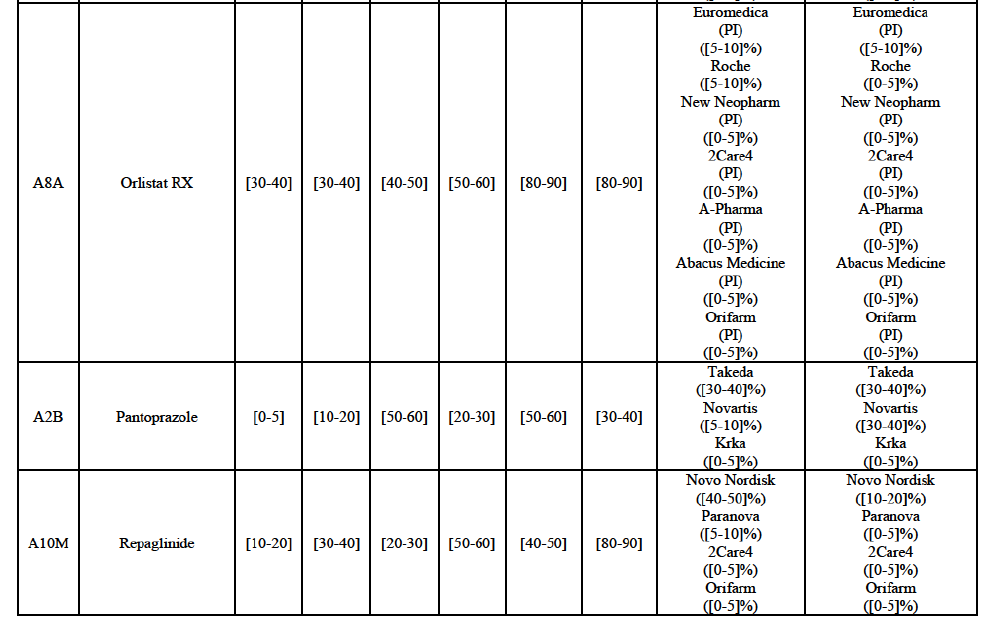
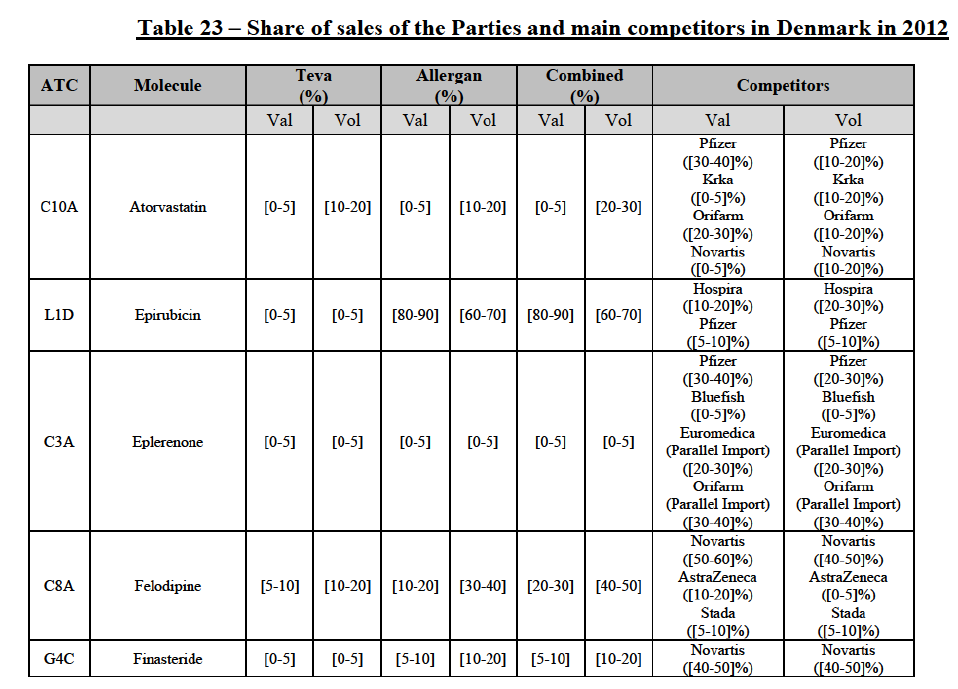
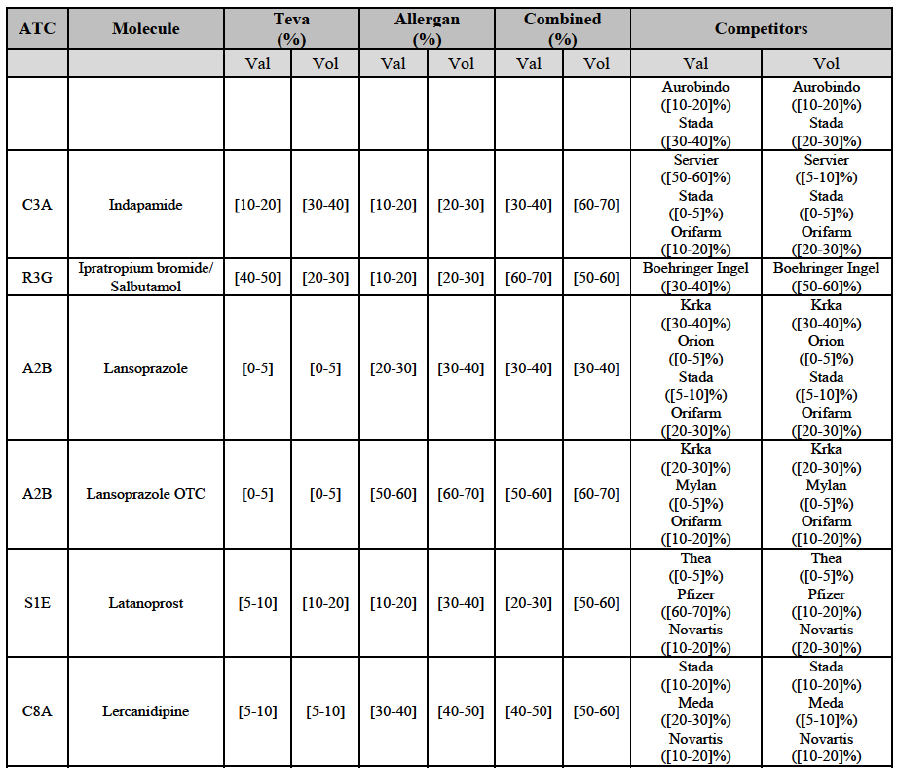
(113) The Commission presents below the competitive analysis on these markets, at molecule level, indicating also the ATC3 class to which it belongs, with a possible distinction by pharmaceutical form (at NFC 1 level) and between prescribed and OTC sales where relevant.
Atorvastatin (C10A)
(114) For atorvastatin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market shares increased over the last three years, up to [50-70]% in value and [70-801% volume in 2014, with a significant increment from Allergan Generics' market share. Only one competitor with a market share above 5% in value and volume in 2014 would remain on the market. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.85
Epirubicin (L1D)
(115) For epirubicin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was >[90-100]% in value and volume in 2014, with a significant increment from Teva's market share ([10-20]% in value and [40-50]% in volume). There would be no remaining competitor with a market share above 5%. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.86
Eplerenone (C3A)
(116) For eplerenone, the Transaction gives rise to a Group 1 market at molecule level. The Parties recently entered (in 2013) and achieved a combined market shares of [60-70]% in value and [60-70]% in volume in 2014, with a significant increment from Allergan Generics' market share. Only two competitors with market share above 5% would remain on the market. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.87
Felodipine (C8A)
(117) For felodipine, the Transaction gives rise to a Group 1 market at molecule level. The Parties’ combined market shares increased over the last three years, up to [40-50]% in value (which would lead to the merged entity being number 1) and [40-50]% in volume in 2014, with a significant increment from Allergan Generics' market share. Only one competitor with a market share above 5% in volume would remain on the market. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.88
Finasteride (G4C)
(118) For finasteride, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market shares fluctuate over the years, up to [50-60]% in value and [50-60]% in volume in 2013. In 2014, the merged entity would still be the number 1 player, with only two remaining competitors with market share above 5%. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.89
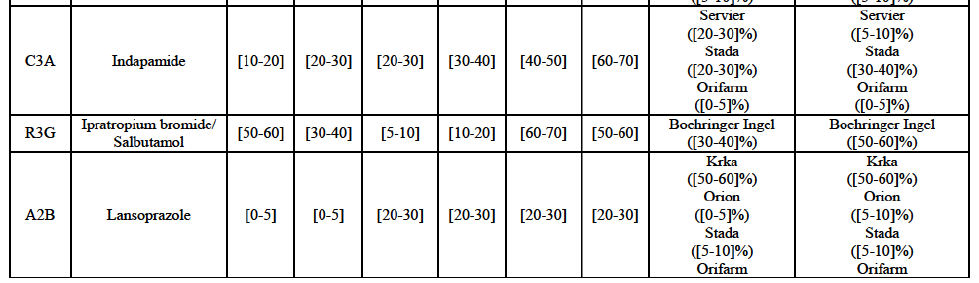
Indapamide (C3A)
(119) For indapamide, the Transaction gives rise to a Group 1 market at molecule level. The Parties’ combined market shares increased over the last three years, up to [50-60]% in value, [70-80]% in volume in 2014, with a significant increment from Allergan Generics' market share. Only one competitor with a market share above 5% in volume would remain on the market. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.90
Ipratropium bromide/Salbutamol (R3G)
(120) For ipratropium bromide/salbutamol, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was [60-70]% in the last three years in value and in 2014 in volume. It is a respiratory product for which Teva enjoys a very strong market position (market share between [20-30]% and [60-70]% in the last three years) and for which Allergan Generics also has a significant position (up to [20-30]% in volume in 2012). Only one competitor, the originator Boehringer Ingelheim, would remain on the market. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.91
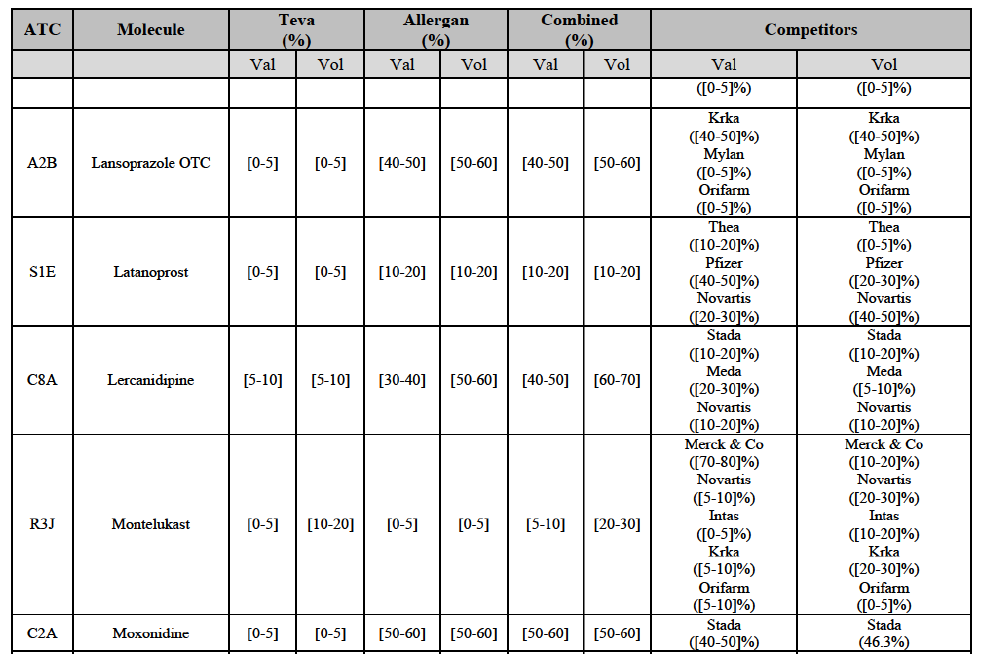
Lansoprazole (A2B)
(121) For lansoprazole, the Transaction gives rise to a Group 1 market at molecule level. The Parties’ combined market share was high, in particular in the OTC segment where it ranged from [40-50] to [60-70]% over the last three years. In all possible segments (Rx, OTC or Rx and OTC), only two competitors with a market share above 5% would remain on the market. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.92
Latanoprost (S1E)
(122) For latanoprost, the Transaction gives rise to a Group 1 market at the level of the pharmaceutical form N. The Parties entered in 2012 and their market shares have fluctuated over the last three years. The combined entity was number 1 player with [5060]% of combined market share based on 2012 data in volume. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.93
Lercanidipine (C8A)
(123) For lercanidipine, the Transaction gives rise to a Group 1 market at molecule level. The Parties’ combined market shares increased over the last three years, up to [50-60]% in value and [60-70]% in volume in 2014. The merged entity would by far lead the market, with only two remaining competitors, including the originator Meda, with a market share above 5% in both value and volume. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.94
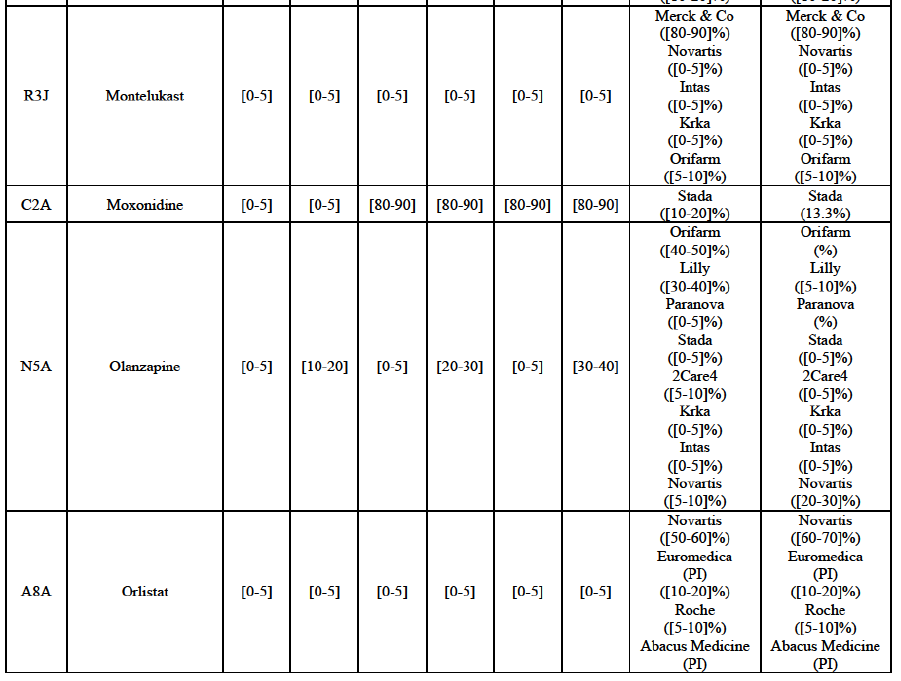
Montelukast (R3J)
(124) For montelukast, the Transaction gives rise to a Group 1 market at molecule level. The Parties recently entered (in 2013) and achieved a combined market share of [30-40]% in volume in 2014, with a significant increment from Teva's market share at [10-20]%. [...]. Based on 2014 data, only two competitors with a market share above 5% in volume would remain on the market. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.95
Moxonidine (C2A)
(125) For moxonidine, the Transaction gives rise to a Group 1 market at molecule level. The Parties’ combined market share was above [50-60]% in value and volume in the last three years. Only one competitor would remain on the market. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.96
Olanzapine (N5A)
(126) For olanzapine, the Transaction gives rise to a Group 1 market at molecule level. Olanzapine became off-patent in September 2011. The Parties' combined market share was above [60-70]% in volume in 2013 and [50-60]% in volume in 2014. The average prices reported by IMS for this molecule would have increased between 2012 and 2014 by [130-140]%. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.97
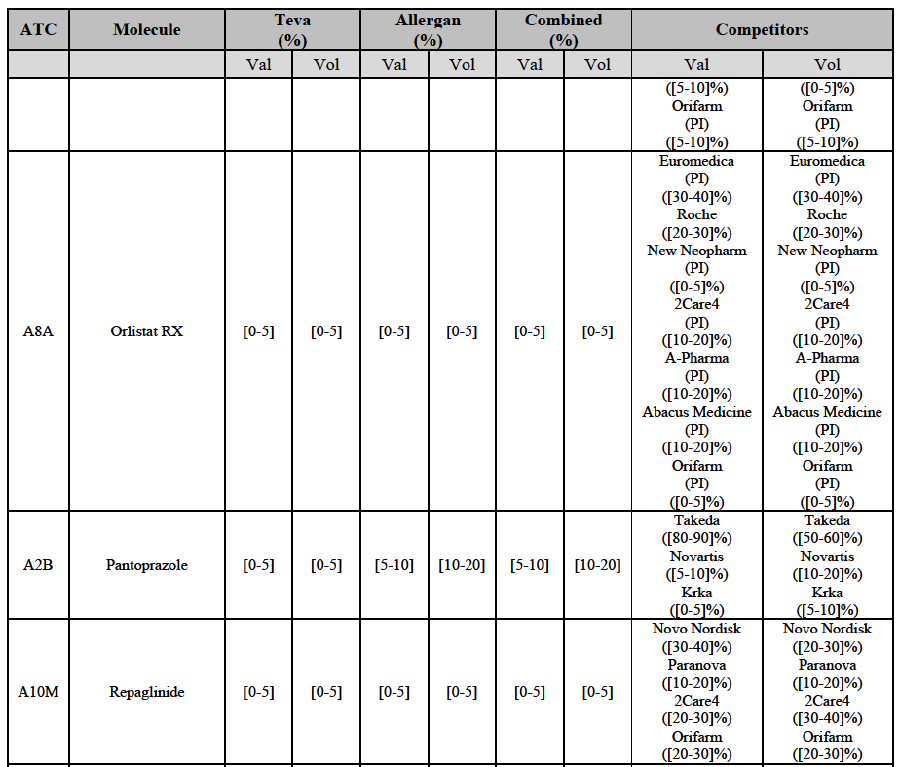
Orlistat (A8A)
(127) For orlistat, the Transaction gives rise to a Group 1 market at molecule level. The Parties recently entered the market (in 2012) and had a combined market share of [3040]% in value and volume in 2014, with a significant increment. In the segment for Rx, the Parties' combined market was [80-90] and [80-90]% in value and volume in 2014. Only one competitor with a market share above 5% would remain. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.98
(128) The Notifying Party submits that the molecule orlistat has been delisted by Teva prior to the announcement of the Transaction. However, Teva's marketing authorisation is still valid, and the Parties acknowledge that it would therefore take up to six months only to come back to the market.99 The Commission considers that the Parties' activities overlap irrespective of Teva's decision to delist the product.
Pantoprazole (A2B)
(129) For pantoprazole, the Transaction gives rise to a Group 1 market at molecule level. The Parties entered recently the market and acquired high combined market shares in two years, ranging from [5-10]% to [50-60]% in value. Only two competitors with a market share above 5%, including the originator Takeda, would remain on the market. In the segment for OTC only, the Parties are the only two generics with significant shares challenging the originator. The market investigation did not bring elements to dispel the strong competitive position of the Parties combined.100
Repaglinide (A10M)
(130) For repaglinide, the Transaction gives rise to a Group 1 market at molecule level. The Parties recently entered (in 2013) and achieved a combined market share of [40-50]% in value and [80-90]% in volume in 2014, with a significant increment from Allergan Generics' market share. Only one remaining competitor (Novo Nordisk, the originator) has a market share above 5% in value and volume. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.101
(131) The Notifying Party submits that the molecule repaglidine has been delisted by Allergan Generics prior to the announcement of the Transaction. However, Allergan Generics' marketing authorisation is still valid, and the Parties acknowledge that it would therefore take up to six months only to come back to the market.102 Therefore, the Commission considers that the Parties' activities overlap irrespective of Allergan Generics' decision to delist the product.
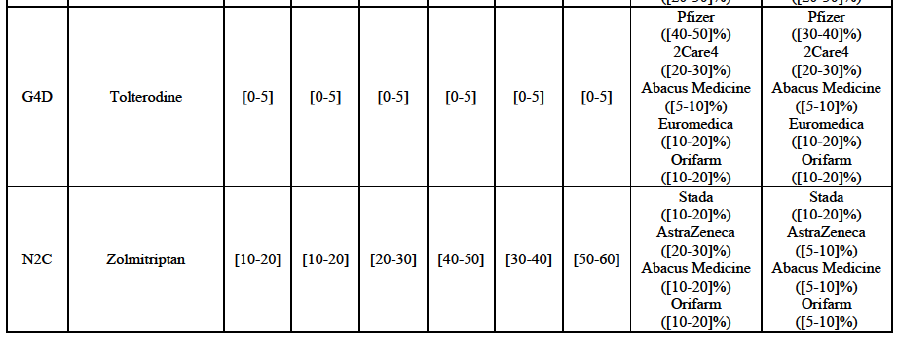
Tolterodine (G4D)
(132) For tolterodine, the Transaction gives rise to a Group 1 market at molecule level. The Parties recently entered (in 2013) and achieved a combined market shares up to 65.2% in value and 83.8% in volume, with a significant increment from Teva's market share. Only one competitor with a market share above 5% would remain on the market. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.103
(133) The Notifying Party submits that the molecule tolterodine has been delisted by Allergan Generics prior to the announcement of the Transaction. However, Allergan Generics' marketing authorisation is still valid, and the Parties acknowledge that it would therefore take up to six months only to come back to the market. 104 Therefore, the Commission considers that the Parties' activities overlap irrespective of Allergan Generics' decision to delist the product.
Zolmitriptan (N2C)
(134) For zolmitriptan, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above 60% in volume in 2013 and 2014, with a significant increment from Teva's market share. Only one competitor with a market share above 5% would remain on the market. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.105
Conclusion
(135) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raise serious doubts as to its compatibility with the internal market with respect to the marketing of atorvastatin, epirubicin, eplerenone, felodipine, finasteride, indapamide, ipratropium bromide/salbutamol, lansoprazole, latanoprost, lercanidipine, montelukast, moxonidine, olanzapine, orlistat, pantoprazole, repaglinide, tolterodine and zolmitriptan in Denmark.
IV.2.2.7.b. Pipeline generic pharmaceuticals
[...]
(136) Teva is planning to launch a generic [...] (pharmaceutical form [...]), for which Allergan Generics had a [50-60]% market share in volume ([30-40]% in value) in 2014 on the same pharmaceutical form, and only two other competitors with >5% share in volume were active on the market in 2012-2014.
Conclusion
(137) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the markets for the marketing of [...] in Denmark.
IV.2.2.8. Estonia
IV.2.2.8.a. Marketed generic pharmaceuticals
(138) The following tables present the market shares of the Parties and their main competitors in 2012, 2013 and 2014 for the Group 1/1+/2 markets for which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market.
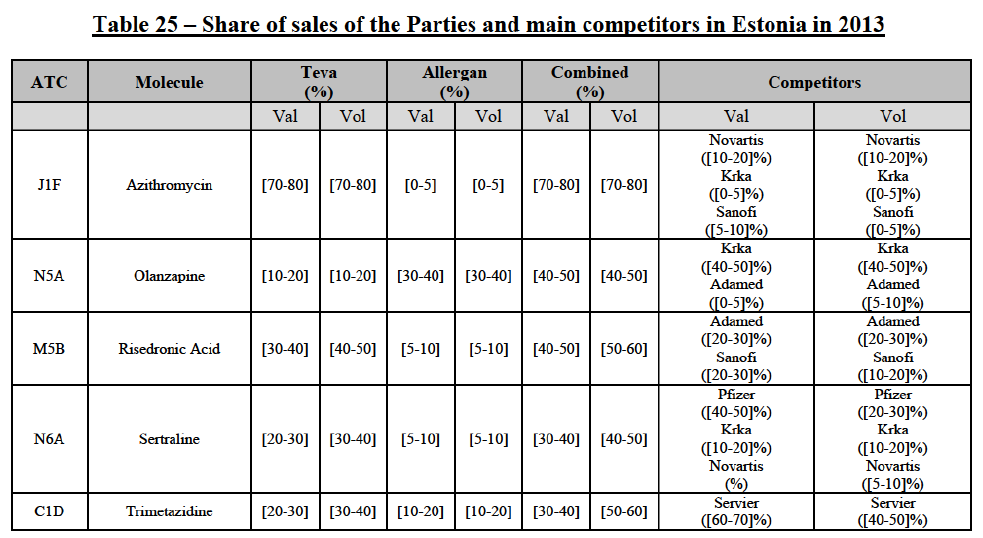
(139) The Commission presents below the competitive analysis on these markets, at molecule level, indicating also the ATC3 class to which it belongs, with a possible distinction by pharmaceutical form (at NFC1 level) and between prescribed and OTC sales where relevant.
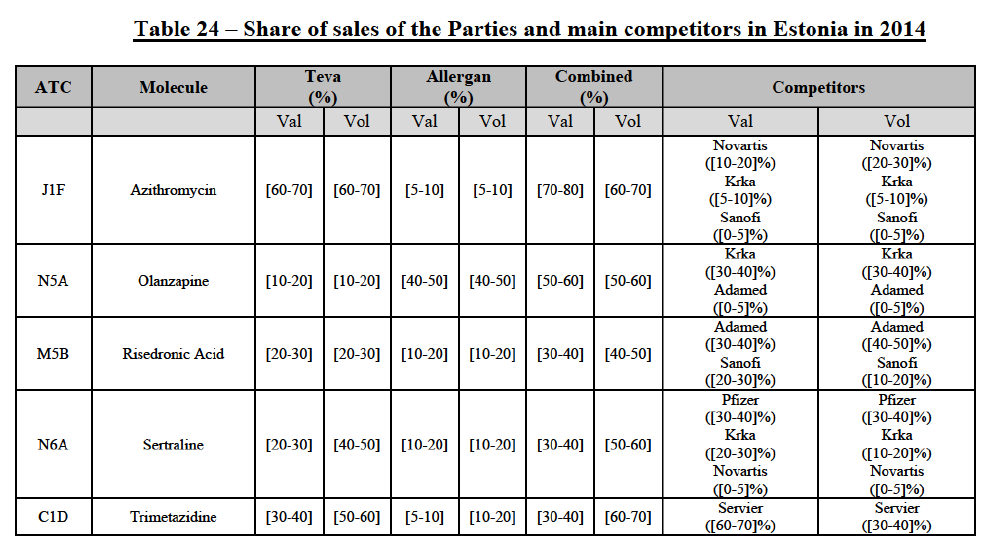
Azithromycin (J1F)
(140) For azithromycin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was at least [60-70]% in the last three years in value and volume. In 2014, the combined market share of the Parties was [70-80]% in value and [60-70]% in volume, with an increment from Allergen Generics' market share of more than [5-10]%. [...]. Only two competitors with a market share of 5% or above would remain on the market. Furthermore, the market investigation indicated that there would be a negative impact of the Transaction on this molecule.106
Olanzapine (N5A)
(141) For olanzapine, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above 50% in 2014 in value (58.6%) and volume ([50-60]%). [...]. Only one competitor with a market share above 5% in value and volume would remain on the market. Furthermore, the market investigation indicated that there would be a negative impact of the Transaction on this molecule.107
Rssedronic Acid (M5B)
(142) For risedronic acid, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [40-50]% in volume in the last three years, with a significant increment from Allergan Generics' market share. The combined entity was the number 1 player based on 2012-2014 data. Only two competitors with a market share above 5% would remain on the market. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.108
Sertraline (N6A)
(143) For sertraline, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above 50% in volume in 2014, with a significant increment from Allergan Generics' market share (more than 10%). Only two competitors with a market share above 5%, including the originator (Pfizer), would remain on the market. Furthermore, the market investigation indicated that that there would be a negative impact of the Transaction on this molecule.109
Trimetazidine (C1D)
(144) For trimetazidine, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market shares increased over the last three years, up to [60-70]% in volume in 2014. Only one competitor (Servier, the originator) would remain on the market with a market share above 5%. Furthermore, the market investigation indicated that there would be a negative impact of the Transaction on this molecule.110
Conclusion
(145) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the marketing of azithromycin, olanzapine, risedronic acid, sertraline and trimetazidine in Estonia.
IV.2.2.8.b. Pipeline generic pharmaceuticals
[...]
(146) Allergan Generics is planning to launch a generic [...] (pharmaceutical form [...]), for which Teva had a [70-80]% market share in value and [70-80]% in volume in 2014 on the same pharmaceutical form, and only one other competitor with >5% share in volume was active on the market in 2012-2014.
Conclusion
(147) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the market for the marketing of [...]in Estonia.
IV.2.2.9. Finland
IV.2.2.9.a. Marketed generic pharmaceuticals
(148) The following tables present the market shares of the Parties and their main competitors in 2012, 2013 and 2014 for the Group 1/1+/2 markets for which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market.
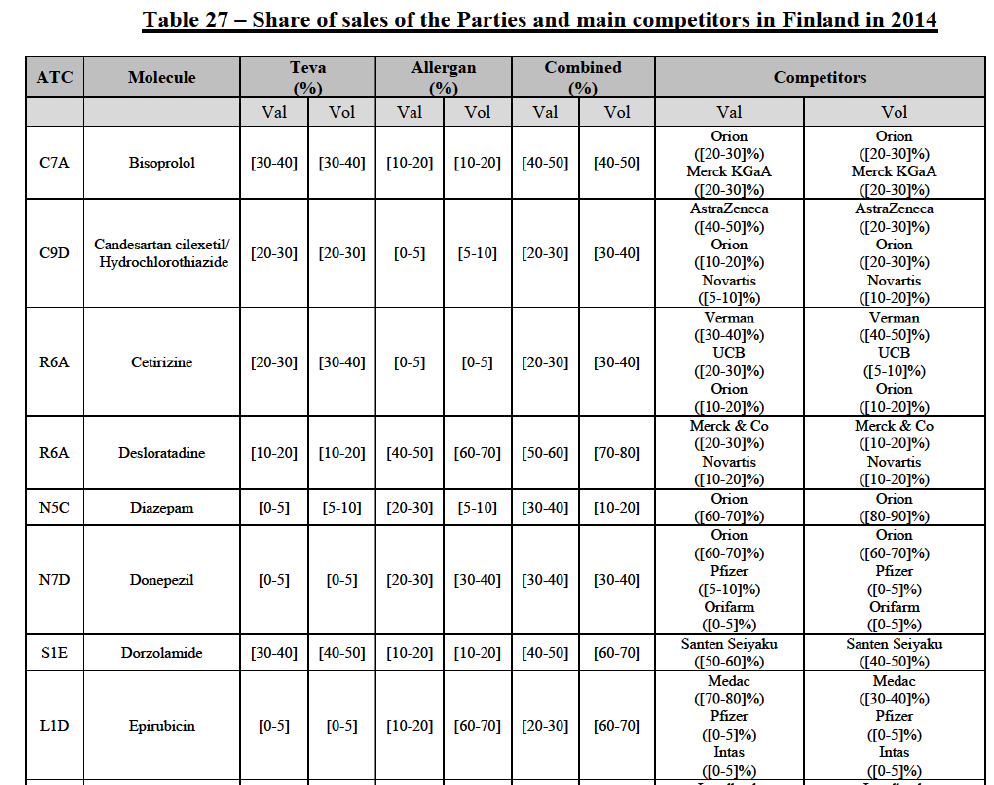
(149) The Commission presents below the competitive analysis on these markets, at molecule level, indicating also the ATC3 class to which it belongs, with a possible distinction by pharmaceutical form (at NFC1 level) and between prescribed and OTC sales where relevant.
Bisoprolol (C7A)
(150) For bisoprolol, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was between [40-50]% in value and [50-60]% in volume in the last three years, with a significant increment from Allergan Generics market share ([10-20]%). Only two competitors with a market share above 5%, including the originator (Merck KGaA), would remain on the market. Furthermore, the market investigation confirmed the Parties' leading position in this market.
Candesartan cilexetil/Hydrochlorothiazide (C9D)
(151) For candesartan cilexetil/ hydrochlorothiazide, the Transaction gives rise to a Group 1 market at molecule level. The Parties are among a few generic competitors which entered the market following patent expiry in 2012 and had a market share of more than 5% in volume in 2014 (together with Orion and Novartis). Teva was already pre-Transaction the market leader in volume, with a market share of more than [20-30]%. Furthermore, the market investigation confirmed the Parties' leading position in this market.
Cetirizine (R6A)
(152) For cetirizine, the Transaction gives rise to a Group 1 market at molecule level for cetirizine Rx. The Parties' combined market share would be slightly above [30-40]% in 2014. However, only two competitors with a market share above 5% would remain on the market. Furthermore, the market investigation indicated that it would be difficult for customers to switch to alternative suppliers.
Desloratadine (R6A)
(153) For desloratadine, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market shares increased in the last three years, up to [50-60]% in value and [70-80]% in volume, with a significant increment from Teva's market share ([10-20]%). [...]. Only two competitors with a market share above 5%, including the originator (Merck & Co), would remain. In the segment for desloratadine in the pharmaceutical form D, the Parties are the only two generic companies active in this market, competing with the originator. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.
Diazepam (N5C)
(154) For diazepam, the Transaction gives rise to a Group 1 market at molecule level in 2012 and 2013. The Parties' combined market share was [30-40]% in value and [10-20]% in volume. Although the combined market share seems moderate, only one competitor, the originator Orion, would remain on the market with a market share above 5%. The market investigation did not bring elements to dispel the strong competitive position of the Parties combined.
Donepezil (N7D)
(155) For donepezil, the Transaction gives rise to a Group 1 market at molecule level in 2012 and 2013. The Parties' combined market shares ranged from [30-40]% to [50-60]% in value and [30-40]% to [60-70]% in volume in the last three years. In 2014, only one competitor had a market share above 5% in value and volume. The market investigation did not bring elements to dispel the strong competitive position of the Parties combined.
Dorzolamide (S1E)
(156) For dorzolamide, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market shares was [40-50]% in value and [60-70]% in volume in 2014, with a significant increment (more than [10-20]%). Only one competitor with a market share above 1%, the originator, would remain in the market. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.
Epirubicin (L1D)
(157) For epirubicin, the Transaction gives rise to a Group 1 market at molecule level in 2012 and 2013. The Parties' combined market share was, in volume, 50% in 2012 (with an increment of [10-20]%), [40-50]% in 2013 (with an increment of [20-30]%) and [6070]% in 2014 (with an increment of less than [0-5]%). The fluctuation of market shares can be due to the hospitals' tendering system since epirubicin is an oncology product sold to hospitals. Over the last three years, only two competitors with a market share above 5% were active on this market. The market investigation did not bring elements to dispel the strong competitive position of the Parties combined.
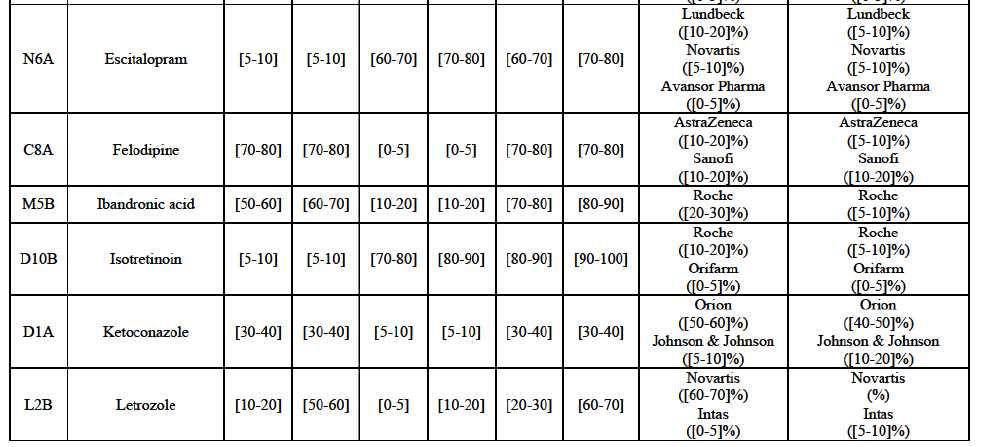
Escitalopram (N6A)
(158) For escitalopram, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share increased over the last three years, up to [60-70]% in value and [70-80]% in volume in 2014. Only two competitors having a market share above 5% in 2014 would remain on the market. Furthermore, the market investigation indicated that the combined share of the Parties would be higher than the Notifying Party's estimate, possibly above [80-90]%, and that there would be a negative impact of the Transaction on this molecule.
Felodipine (C8A)
(159) For felodipine, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [70-80]% in 2014 ([70-80]% in value and [70-80]% in volume). Only two competitors, being the originators (AstraZeneca and Sanofi), have a market share above 5%. The market investigation did not bring elements to dispel the strong competitive position of the Parties combined.
Ibandronic Acid (M5B)
(160) For ibandronic acid, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was [70-80]% in value and [80-90]% in volume in 2014 with a significant increment from Allergan Generics ([10-20]%). Only one competitor (the originator) with a market share above 5% would remain on the market. The market investigation did not bring elements to dispel the strong competitive position of the Parties combined.
Isotretinoin (D10B)
(161) For isotretinoin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was [80-90]% in value and [90-100]% in volume in 2014, with an increment of [5-10]% in value and [5-10]% in volume. Only one competitor, the originator Roche, would remain in the market with a market share above 5%. Furthermore, the market investigation indicated that there would be a negative impact of the Transaction on this molecule.
Ketoconazole (D1A)
(162) For ketoconazole, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was [30-40]% in value and [30-40]% in volume in 2014. The Parties are the only two generic companies active in this market, the only remaining competitor being the originator, Johnson & Johnson. The market investigation did not bring elements to dispel the strong competitive position of the Parties combined.
Letrozole (L2B)
(163) For letrozole, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share increased in the last three years, up to [60-70]% in volume, with an increment of more than [10-20]%. Only one competitor, the originator (Novartis), would have a market share above 5% in value. Furthermore, the market investigation the market investigation confirmed the Parties' leading position in this market (above 60%) and the possible negative impact of the Transaction.
Levocetirizine (R6A)
(164) For levocetirizine, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was between [40-50]% and [40-50]% in value and between [40-50]% and [50-60]% in volume, with a significant increment (more than [510]% in 2014). Only two competitors with a market share above 5%, including the originator (UCB), would remain in the market, and only one, the originator, for levocetirizine sold OTC. Furthermore, the market investigation indicated that the combined share of the Parties would be higher than the Notifying Party's estimate, possibly above 70%, and that there would be a negative impact of the Transaction on this molecule.
Loratadine (R6A)
(165) For loratadine, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was [60-70]% in value and [70-80]% in volume in 2014. Only one competitor with a market share above 5% in value and volume would remain in the market. Furthermore, the market investigation confirmed the strong combined position of the Parties and indicated that it would be difficult for customers to switch to alternative suppliers.
Omeprazole (A2B)
(166) For omeprazole, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [50-60]% in value and [70-80]% in volume in the last three years, with an increment from Allergan Generics growing over the years. Only one competitor, Bayer (one of the two originators), would remain with a market share above 5% in value and volume. The Parties are both active in the Rx and OTC segments. The combined market share of the Parties was even higher for omeprazole Rx, with [80-90]% in value and [90-100]% in volume and only one competitor, the originator, would remain post-Transaction for omeprazole OTC. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market and that it would be difficult to switch to alternative suppliers.
Risedronic Acid (M5B)
(167) For risedronic acid, the Transaction gives rise to a Group 1 market at molecule level. Allergan Generics is the originator of this molecule. The Parties' combined market shares was [80-90]% in value and [80-90]% in volume in 2014. Only one competitor with a market share above 5% would remain in the market. Furthermore, the market investigation the market investigation confirmed the Parties' leading position in this market (above 90%) and the possible negative impact of the Transaction.
Simvastatin (C10A)
(168) For simvastatin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share ranged between [30-40]% to [40-50]% in the last three years in value and volume. [...]. Only two competitors with a market share above 5% would remain on the market. The average prices reported by IMS for simvastatin increased by 86% between 2012 and 2014. Furthermore, the market investigation indicated that the Transaction would have a negative impact in this market and that it would be difficult to switch to alternative suppliers.
Tamsulosin (G4C)
(169) For tamsulosin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share in the last three years was between [30-40]%-[40-50]% in value and [40-50]%-[50-60]% in volume, with Teva being the market leader. Furthermore, the market investigation confirmed the Parties' leading position in this market and the possible negative impact of the Transaction.
Tolterodine (G4D)
(170) In tolterodine, the Transaction gives rise to a Group 1 market at molecule level. The Parties recently entered (in 2013) and achieved a combined market share of [40-50]% value and [40-50]% in volume at molecule level and [40-50]% value and [40-50]% in volume at the level of the pharmaceutical form B. For all possible segments, the increment of market share is significant (around [20-30]%). In addition, there will be only one remaining competitor with a market share above 5%, which is the originator Pfizer. Finally, the market investigation confirmed the Parties' leading position in this market (possibly above 50%) and the possible negative impact of the Transaction.
Zolpidem (N5B)
(171) For zolpidem, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market shares was [30-40]% in value and [30-40]% in volume in 2014. Only two competitors, including the originator, have a market share above 5%. Allergan Generics was the leading generic supplier over the last three years. Furthermore, the market investigation indicated that the combined share of the Parties would be higher than the Notifying Party's estimate, possibly more than [70-80]%, and that there would be a negative impact of the Transaction on this molecule.
Conclusion
(172) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to bisoprolol, candesartan cilexetil/ hydrochlorothiazide, cetirizine, desloratadine, diazepam, donepezil, dorzolamide, epirubicin, escitalopram, felodipine, ibandronic acid, isotretinoin, ketoconazole, letrozole, loratadine, omeprazole, risedronic acid, simvastatin, tamsulosin, tolterodine and zolpidem in Finland.
IV.2.2.9.b. Pipeline generic pharmaceuticals
(173) The Transaction does not raise serious doubts as to its compatibility with the internal market with respect to pipeline generic pharmaceuticals in Finland.
IV.2.2.10. France
IV.2.2.10.a. Marketed generic pharmaceuticals
(174) The following tables present the market shares of the Parties and their main competitors in 2012, 2013 and 2014 for the Group 1/1+/2 markets for which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market.
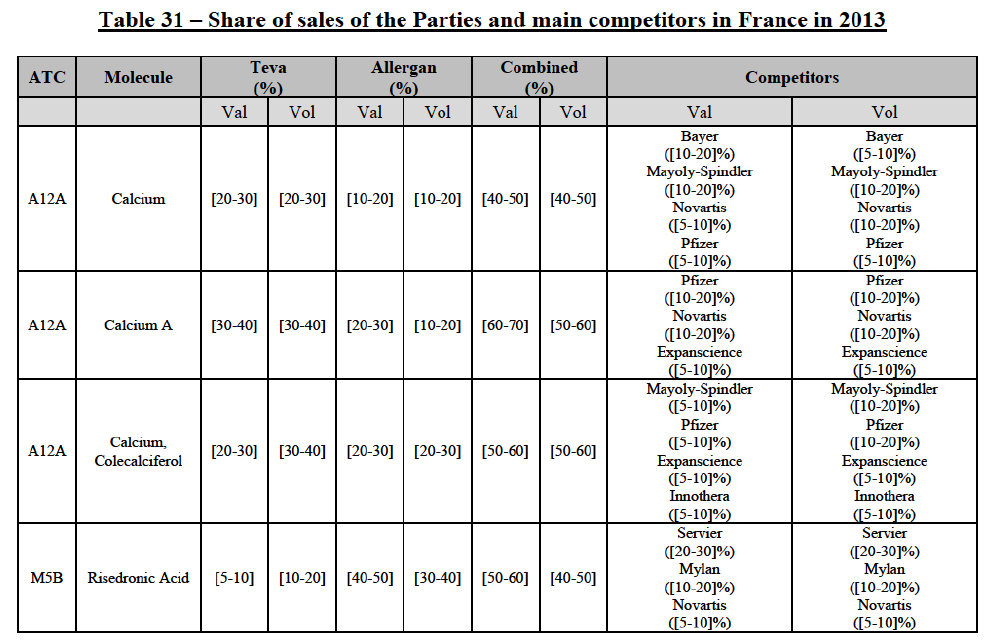
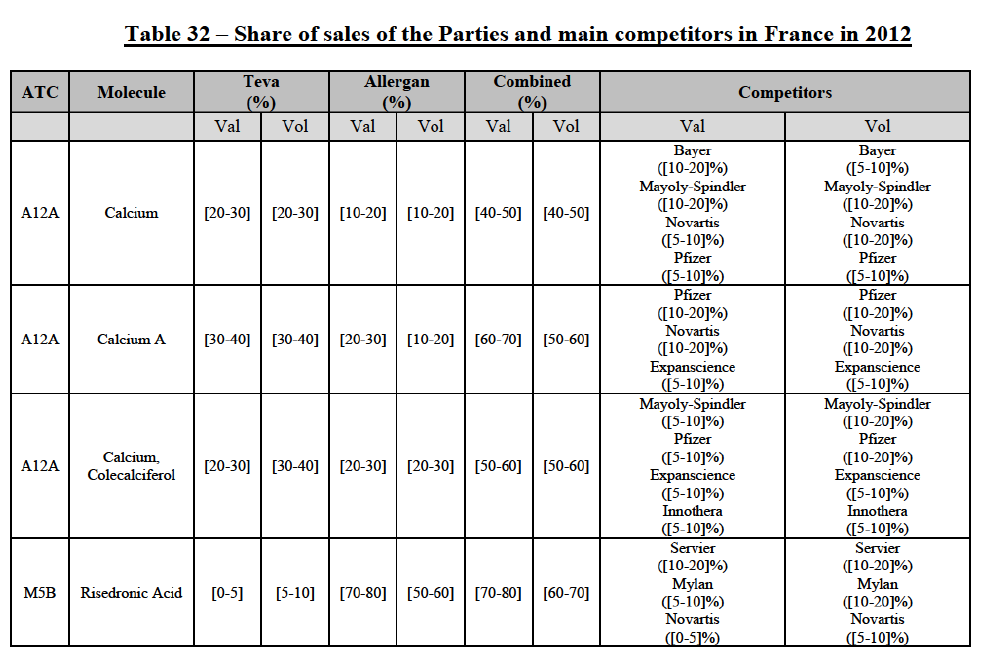
(175) The Commission presents below the competitive analysis on these markets, at molecule level, indicating also the ATC3 class to which it belongs, with a possible distinction by pharmaceutical form (at NFC1 level) and between prescribed and OTC sales where relevant.
Calcium (Al2A)
(176) For calcium, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market shares was above [40-50]% at molecule level and above [5060]% at the level of the pharmaceutical form A in the last three years in value and volume. For the pharmaceutical form A, only two competitors with a market share above 5% in value and volume would remain. Furthermore, the market investigation indicated that there would be a negative impact of the Transaction on this molecule."
Calcium/ Colecalciferol (Al2A)
(177) For calcium/colecalciferol, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [50-60]% in the last three years in value and volume, with a significant increment (more than [20-301%). [...]. Furthermore, the market investigation confirmed the leading position of merged entity (above 50%) and indicated that there would be a negative impact of the Transaction on this molecule.n2
Risedronic Acid (M5B)
(178) For risedronic acid, the Transaction gives rise to a Group 1 market at molecule level. Allergan Generics is the originator of this molecule. The Parties' combined market share was above [40-50]% ([40-50]% in value and [40-50]% in volume) in 2014. Furthermore, the market investigation indicated that the merged entity would have a higher combined market share than the Notifying Party's estimate, above [60-70]%, and indicated that there would be a negative impact of the Transaction on this molecule.113
Conclusion
(179) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the marketing of calcium, calcium/ colecalciferol and risedronic acid in France.
IV.2.2.10.b. Pipeline generic pharmaceuticals
(180) The Transaction does not raise serious doubts as to its compatibility with the internal market with respect to pipeline generic pharmaceuticals in France.
IV.2.2.11. Germany
IV.2.2.11.a. Marketed generic pharmaceuticals
(181) The following tables present the market shares of the Parties and their main competitors in 2012, 2013 and 2014 for the Group 1/1+/2 market for which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market.
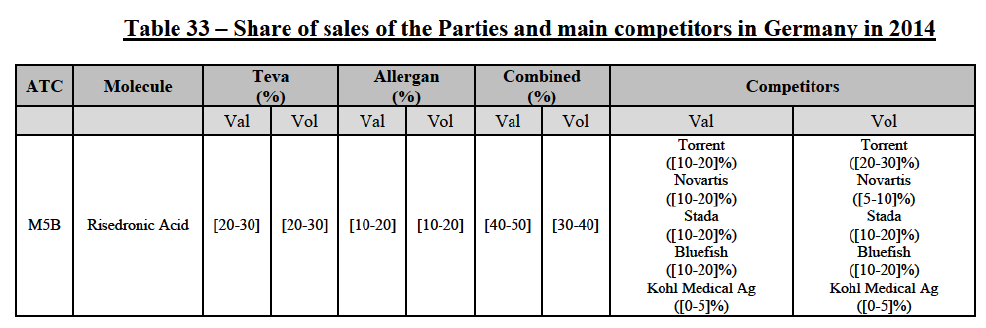
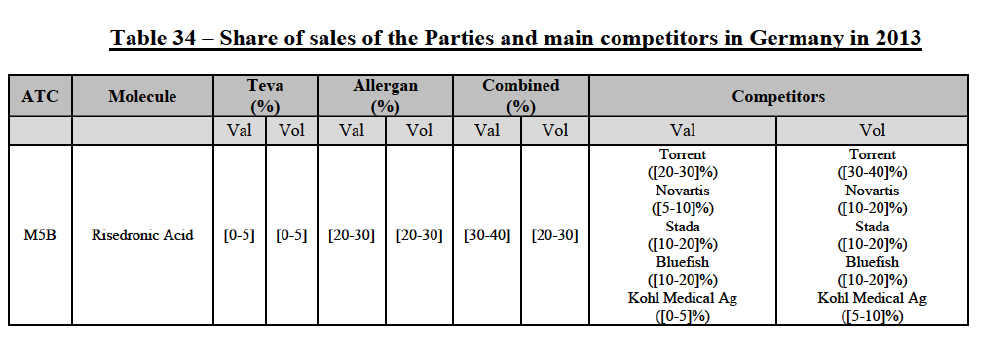
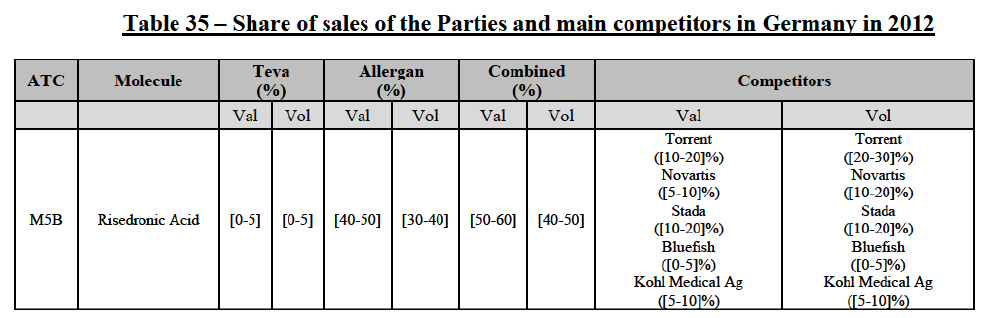
(182) The Commission presents below the competitive analysis on this market, at molecule level, indicating also the ATC3 class to which it belongs, with a possible distinction by pharmaceutical form (at NFC1 level) and between prescribed and OTC sales where relevant.
Ris edronic Acid (M5B)
(183) For risedronic acid, the Transaction gives rise to a Group 1 market at molecule level. Allergan Generics is the originator of this molecule. The Parties' combined market share was [30-40]% in value and [40-50]% in volume in 2014, the Parties being the number 1 and number 2 players. Teva's market shares have been growing over the years, up to more than [20-30]% in 2014. Furthermore, the market investigation indicated that there would be a negative impact of the Transaction on this molecule and that it will be difficult for customers to switch to alternative suppliers.n4
Conclusion
(184) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raise serious doubts as to its compatibility with the internal market with respect to the marketing of risedronic acid in Germany.
IV.2.2.11.b. Pipeline generic pharmaceuticals
(185) The Transaction does not raise serious doubts as to its compatibility with the internal market with respect to pipeline generic pharmaceuticals in Germany.
IV.2.2.12. Greece
IV.2.2.12.a. Marketed generic pharmaceuticals
(186) The following tables present the market shares of the Parties and their main competitors in 2012, 2013 and 2014 for the Group 1/1+/2 market for which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market.
(187) The Commission presents below the competitive analysis on this market, at molecule level, indicating also the ATC3 class to which it belongs, with a possible distinction by pharmaceutical form (at NFC 1 level) and between prescribed and OTC sales where relevant.
Granisetron (A4A)
(188) For granisetron, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market shares was [90-100]% in value and [90-100]% in volume in 2014, with a significant increment ([20-30]% in value and [10-20]% in volume). No competitor would remain on the market. Indeed, only Roche, the originator, was active in 2014, but its marketing authorizations have now been cancelled. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.115
(189) The Notifying Party submits that the molecule granisetron has been delisted by Teva prior to the announcement of the Transaction. However, Teva's marketing authorisation is still valid, and the Parties acknowledge that it would therefore take up to six months only to come back to the market.116 Therefore, the Commission considers that the Parties' activities overlap irrespective of Teva's decision to delist the product.
Conclusion
(190) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raise serious doubts as to its compatibility with the internal market with respect to the marketing of granisetron in Greece.
IV.2.2.12.b. Pipeline generic pharmaceuticals
[...]
(191) Allergan Generics is planning to launch a generic [...] (pharmaceutical form[...]), for which Teva had a >[90-100]% market share in volume ([50-60]% in value) in 2014 on the same pharmaceutical form, and only one other competitor was active on the market in 2012-2014.
[...]
(192) Teva is planning to launch a generic [...] (pharmaceutical form [...]), for which Allergan Generics had a [60-70]% market share in value and [50-60]% in volume in 2014. Since Allergan Generics is active with [...], the merged entity would have little incentive to pursue the development and launch of Teva's pipeline generic [...].
Conclusion
(193) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the market for the marketing of [...] and [...] in Greece.
IV.2.2.13. Hungary
IV.2.2.13.a. Marketed generic pharmaceuticals
(194) The following tables present the market shares of the Parties and their main competitors in 2012, 2013 and 2014 for the Group 1/1+/2 markets for which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market.
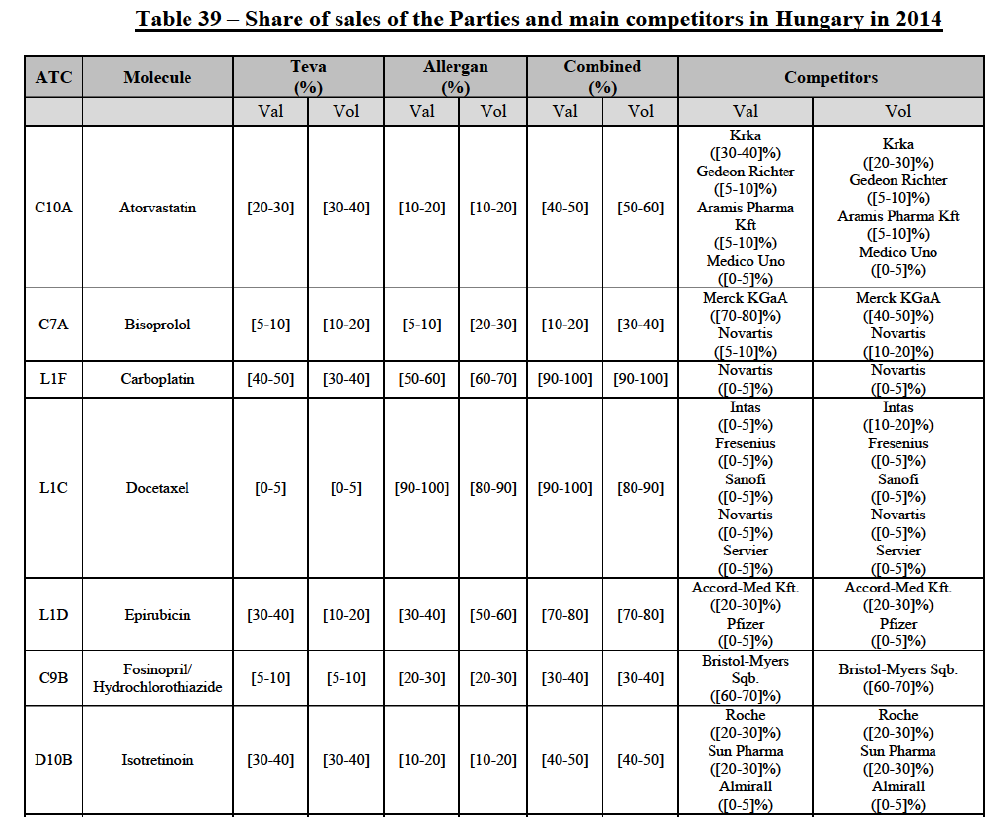
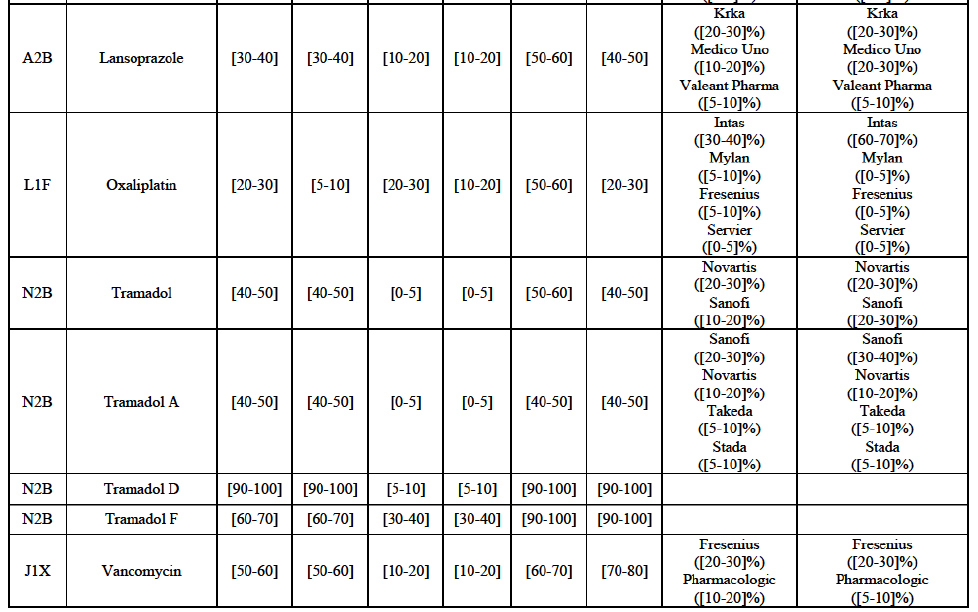
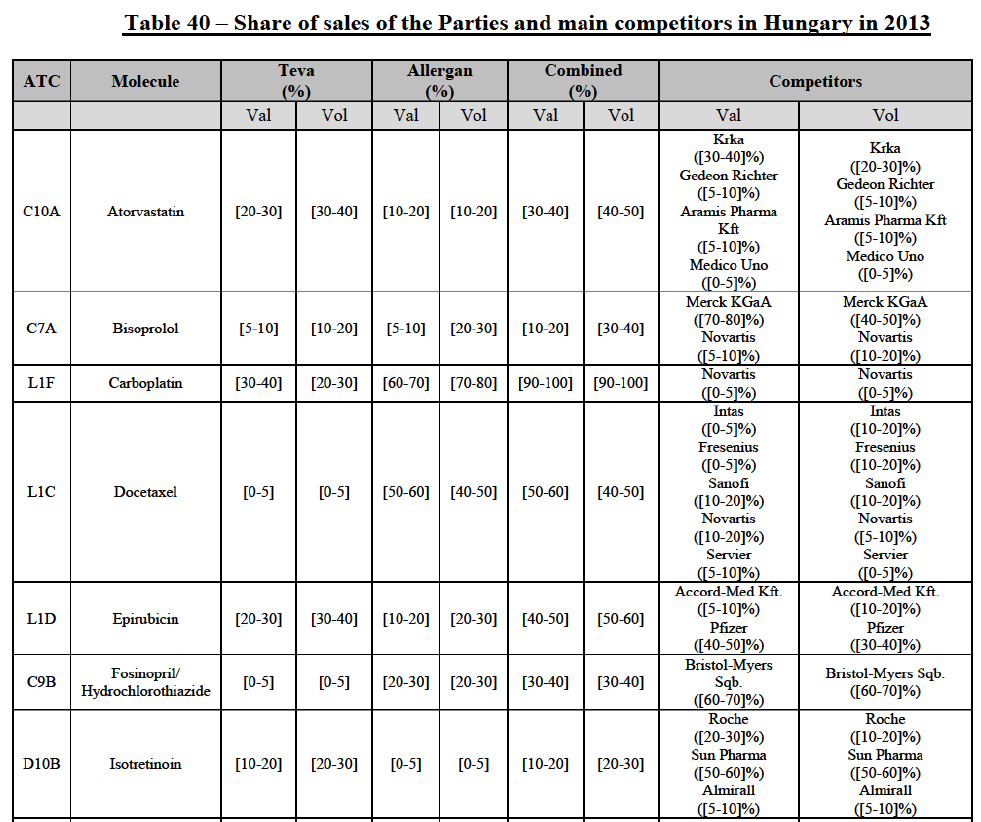
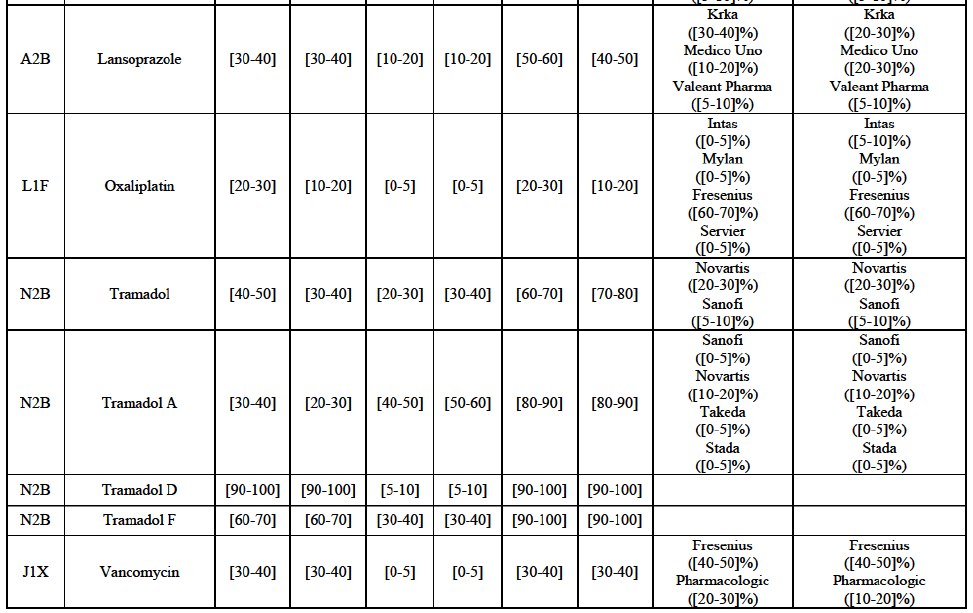
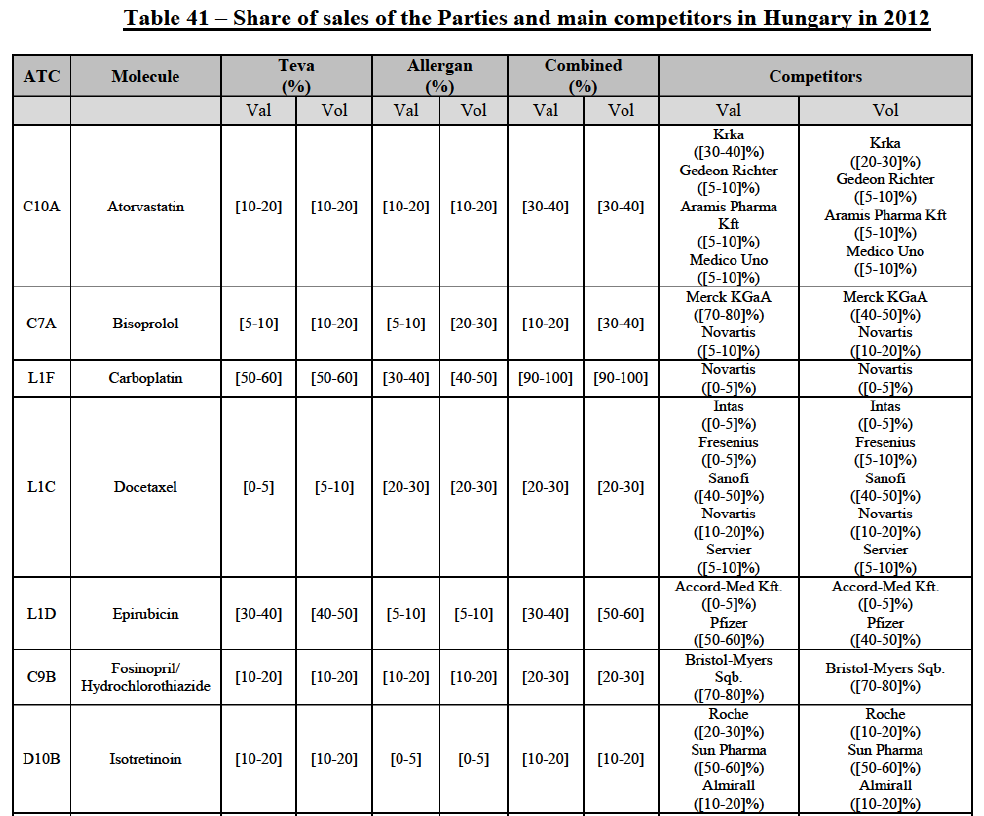
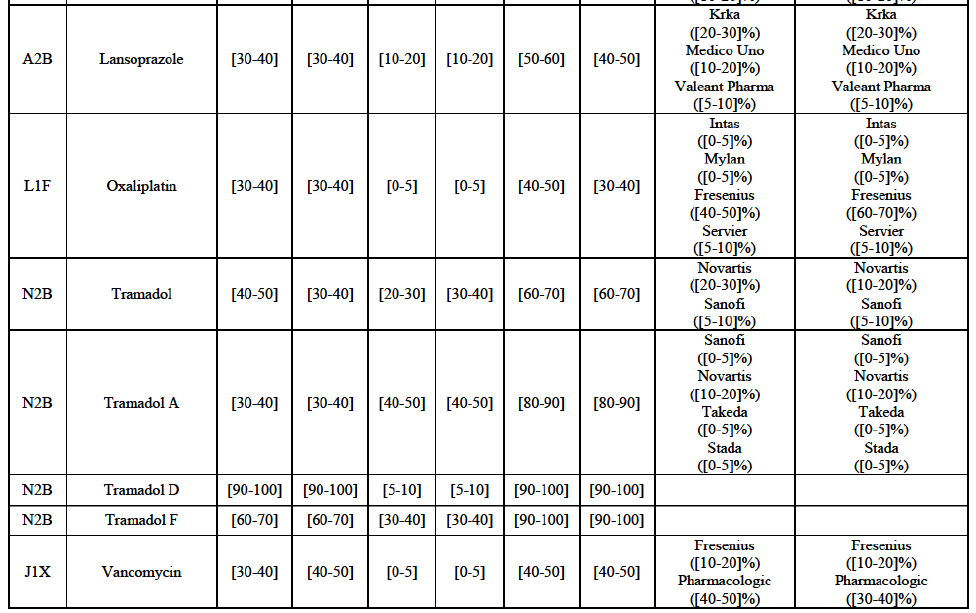
(195) The Commission presents below the competitive analysis on these markets, at molecule level, indicating also the ATC3 class to which it belongs, with a possible distinction by pharmaceutical form (at NFC1 level) and between prescribed and OTC sales where relevant.
Acetylsalicylic acid (B1C)
(196) For acetylsalicylic acid, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was [30-40]% in volume in 2014. Only two competitors having a market share above 5% would remain, one of them is the originator Bayer. [...]. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market and that it would be difficult for customers to switch to alternative suppliers.117
Atorvastatin (C10A)
(197) For atorvastatin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market shares increased in the last three years, up to more than [4050]% in value and more than [50-60]% in volume in 2014, with a significant increment (more than [10-20]%). Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.118
Bisoprolol (C7A)
(198) For bisoprolol, the Transaction gives rise to a Group 1 market at molecule level. The combined market share of the Parties was [30-40]% in volume in 2014. Only two competitors with a market share above 5%, including the originator, would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.119
Carboplatin (L1F)
(199) For carboplatin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was[90-100]% in value and [90-100]% in volume in 2012 and >[90-100]% in 2013 and 2014 in value and volume, with a significant increment (in 2014, [40-50]% in value and [30-40]% in volume). There would be only one remaining competitor Novartis with market share <0.1%. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market and that it would be difficult for customers to switch to alternative suppliers.120
Docetaxel (L1C)
(200) For docetaxel, the Transaction gives rise to a Group 1 market at molecule level in 2013. In 2014, Teva was active with a market share of [90-100]% in value and [80-90]% in volume, but Allergan Generics did not record any sales. However, in 2012, its market share was above 5% in volume. The fluctuation of market shares can be due to the hospitals' tendering system since docetaxel is an oncology product sold to hospitals. Furthermore, the market investigation confirmed the strong market shares of the Parties and indicated that the Transaction would have a negative impact in the market.121
(201) The Notifying Party submits that the molecule docetaxel has been delisted by Teva prior to the announcement of the Transaction. However, Teva's marketing authorisation is still valid, and the Parties acknowledge that it would therefore take up to six months only to come back to the market.122 Therefore, the Commission considers that the Parties' activities overlap irrespective of Teva's decision to delist the product.
Epirubicin (L1D)
(202) For epirubicin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was [70-80]% in value and [70-80]% in volume, with a significant increment ([30-40]% in value and [10-20]% in volume). Based on 2014 data, only one competitor had a market share above 5%. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market and that it would be difficult for customers to switch to alternative suppliers.123
Fosinopril/ Hydrochlorothiazide (C9B)
(203) For fosinopril/ hydrochlorothiazide, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was slightly above [30-40]% in value and volume. However, only one competitor, the originator, would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.124
Isotretinoin (D10B)
(204) For isotretinoin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market shares increased over the last three years, up to [40-50]% in value and [40-50]% in volume in 2014. Only two competitors with a market share above 5% would remain, one of them being the originator Roche. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.125
Lansoprazole (A2B)
(205) For lansoprazole, the Transaction gives rise to a Group 1 market at molecule level. The Parties’ combined market shares was [50-60]% in value and [40-50]% in volume in 2014, with a significant increment of market share ([10-20]%). Only two competitors with a market share above 5% in value and volume would remain. Furthermore, the market investigation confirmed that the merged entity would have a dominant position. The market investigation also indicated that the Transaction would have a negative impact in the market and that it would be difficult for customers to switch to alternative suppliers.126
Oxaliplatin (L1F)
(206) For oxaliplatin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was [50-60]% in value and [20-30]% in volume in 2014, with an increment of [20-30]% and [5-10]%. Based on 2014 data, only one competitor with a market share above 5% in value and volume would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.127
Tramadol (N2B)
(207) For tramadol, the Transaction gives rise to a Group 1 market at molecule level. Teva is the originator of this molecule. The Parties' combined market share ranged from [6070]% (2012) to [50-60]% (2014) in value and [60-70]% (2012) and [40-50]% (2014), with an increment of more than [20-30]% in 2012 and 2013. In 2014, only two competitors with a market above 5% remained in the market. At the level of the pharmaceutical forms D and F, post-Transaction, the combined entity would have [90100]% of the market (Allergan Generics having a market share between [5-10]% to [510]% for pharmaceutical form D and [30-40]% for pharmaceutical form F). Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market and that it would be difficult for customers to switch to alternative suppliers.128
Vancomycin (J1X)
(208) For vancomycin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market shares fluctuated over the years, up to [60-70]% in value and [70-80]% in volume in 2014, with a significant increment (more than [10-20]%). [...]. Only two competitors, including the originator, would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market and that it would be difficult to switch to alternative suppliers.129
Conclusion
(209) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raise serious doubts as to its compatibility with the internal market with respect to the marketing of acetylsalicylic acid,
atorvastatine, bisoprolol, carboplatin, docetaxel, epirubicin,
fosinopril/hydrochlorothiazide, lansoprazole, oxaliplatin, tramadol and vancomycin in Hungary
IV.2.2.13.b. Pipeline generic pharmaceuticals
[...]
(210) Allergan Generics is planning to launch a generic [...] (pharmaceutical forms [...] and [...]), for which Teva had a [90-100]% market share in value and volume in 2014 on the same pharmaceutical form, and no other competitor with >5% share in volume was active on the market in 2012-2014.
[...]
(211) Allergan Generics is planning to launch a generic [...] (pharmaceutical form [...]), for which Teva had a [90-100]% market share (both in value and volume) on the same pharmaceutical form in 2012-2014.
[...]
(212) Allergan Generics is planning to launch a generic [...] (pharmaceutical form [...]), for which Teva had a [60-70]% market share in volume ([5-10]% in value) and [70-80]% in volume in 2014, and only two other competitor with >5% share in volume were active on the market in 2012-2014.
Conclusion
(213) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the market for the marketing of [...],[...]and [...]in Hungary.
IV.2.2.14. Iceland
IV.2.2.14.a. Marketed generic pharmaceuticals
(214) The following tables present the market shares of the Parties and their main competitors in 2012, 2013 and 2014 for the Group 1/1+/2 markets for which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market.
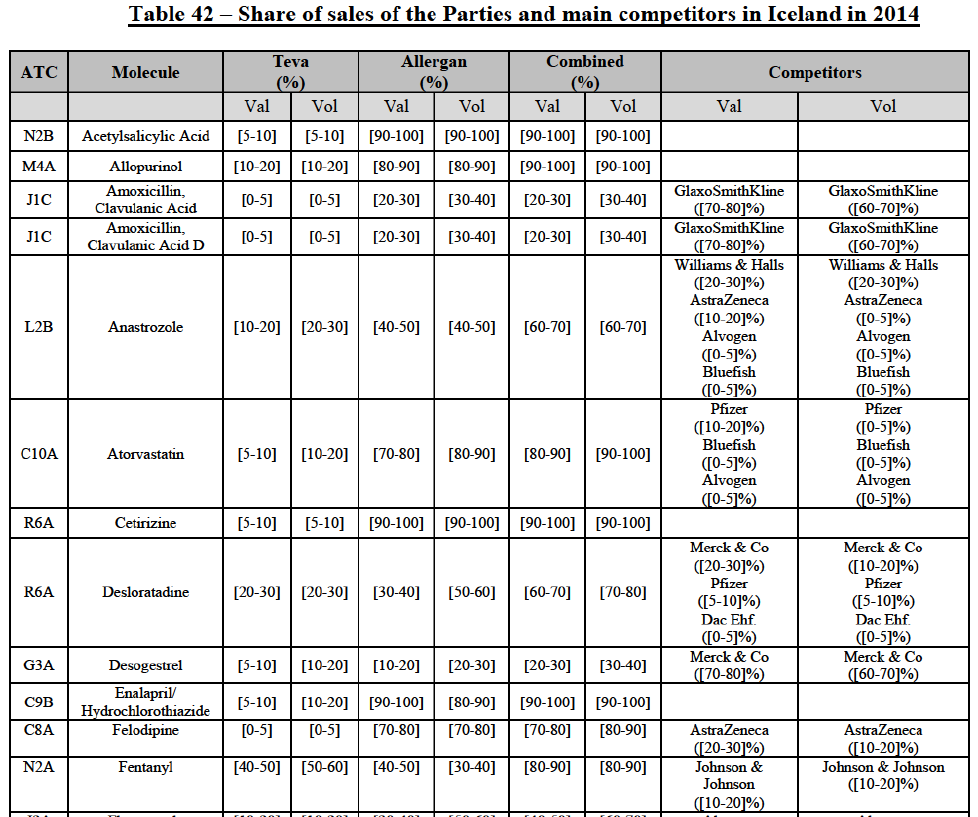
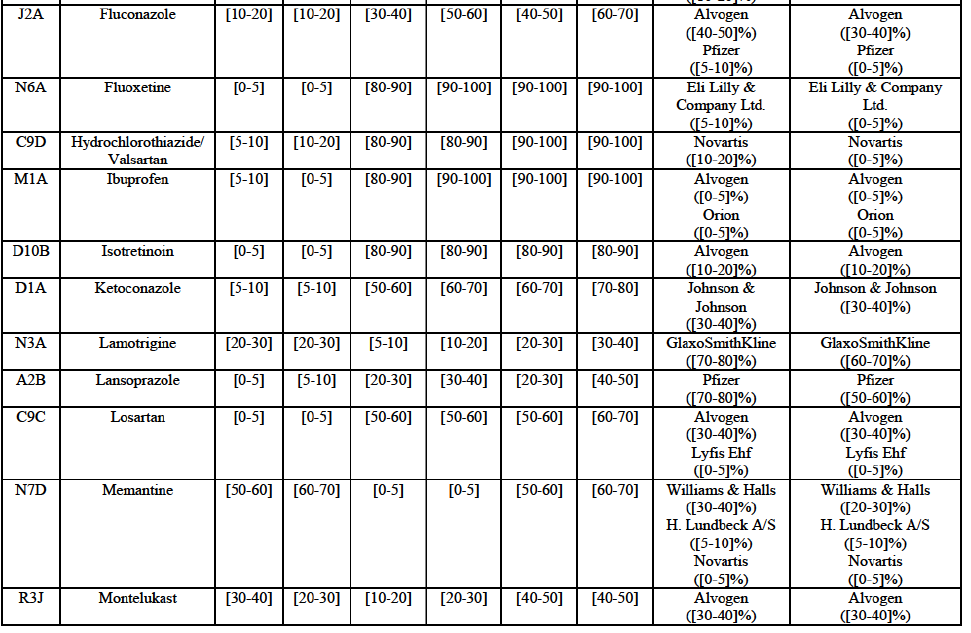
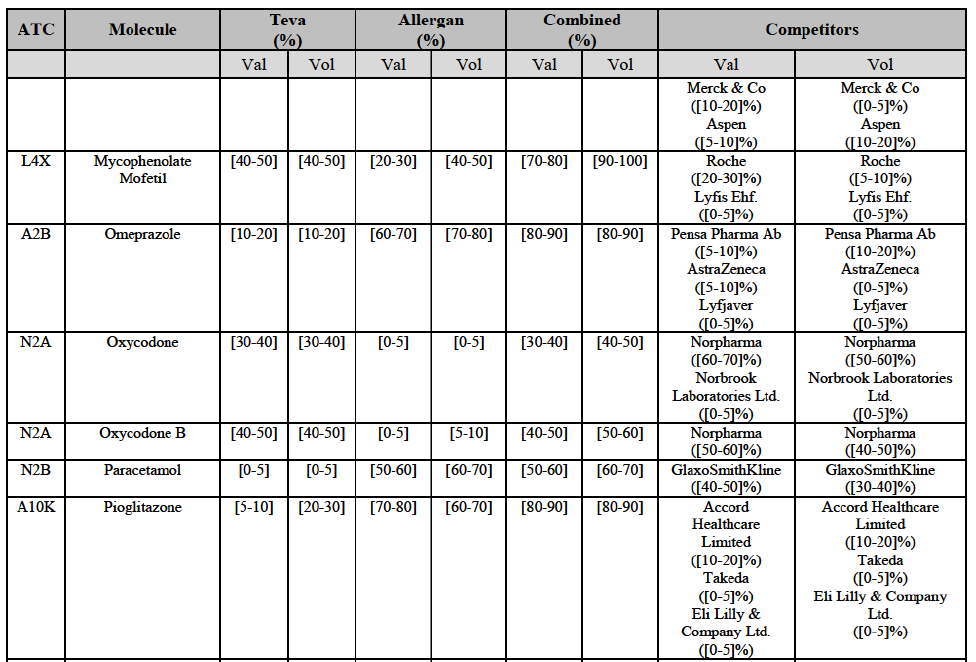
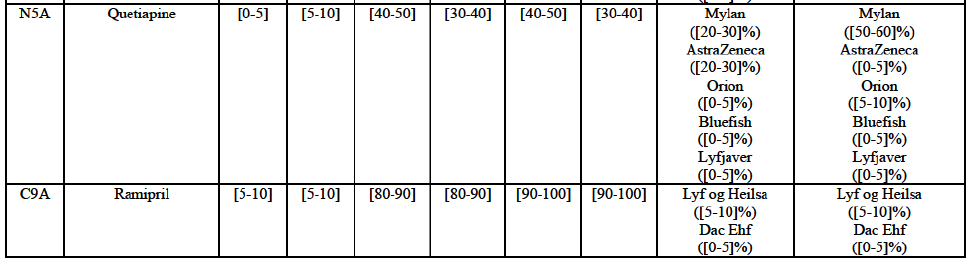
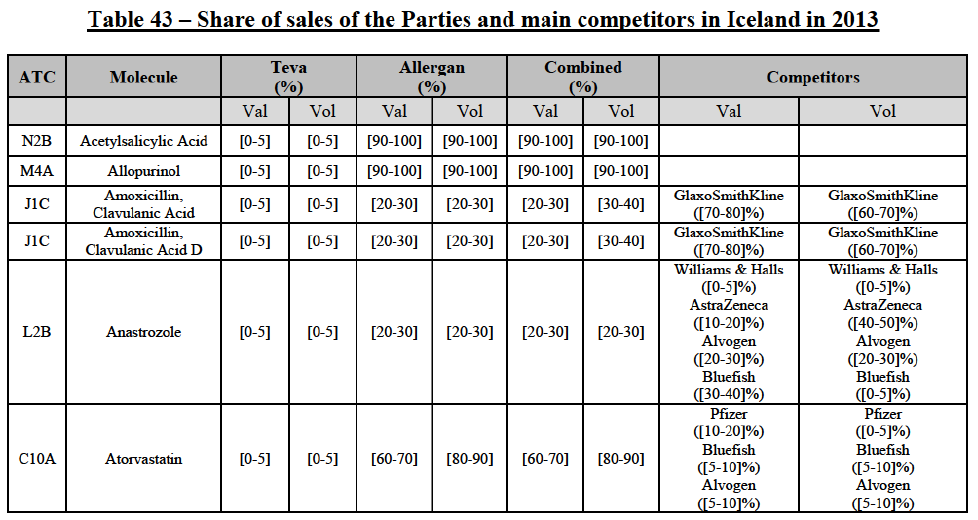
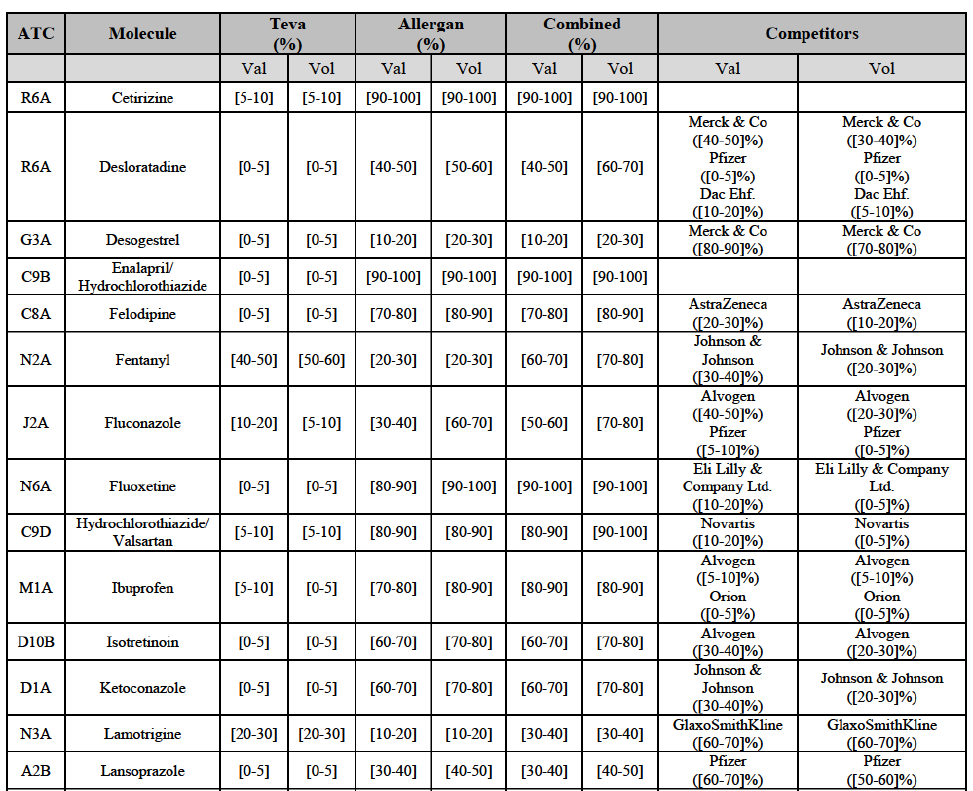
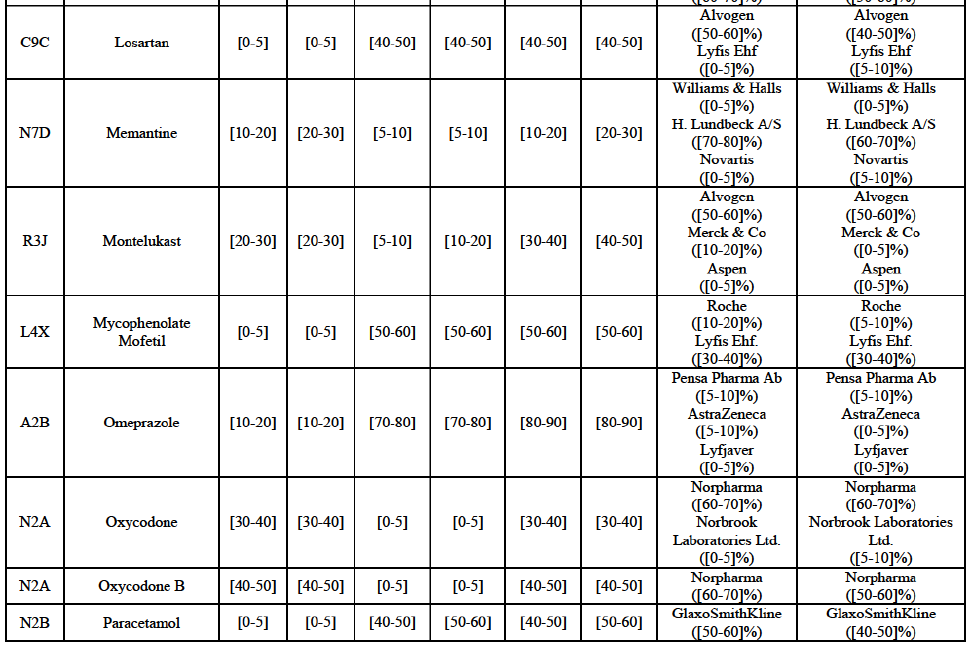
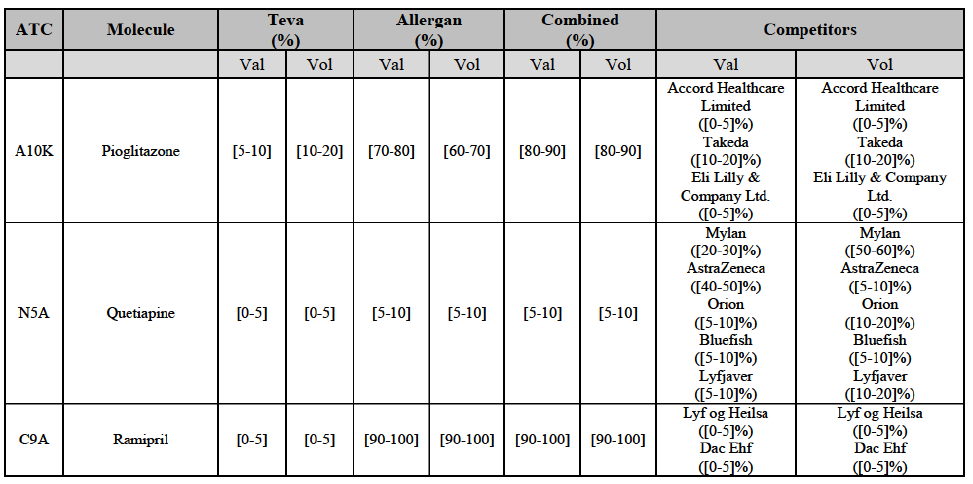
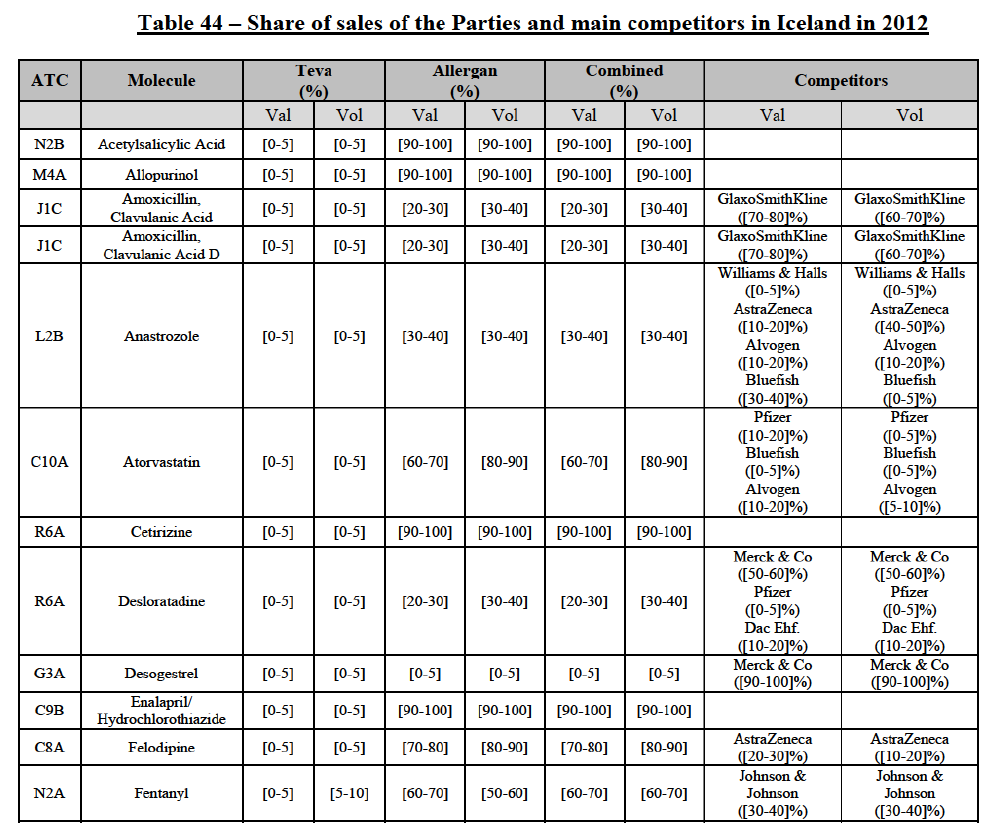
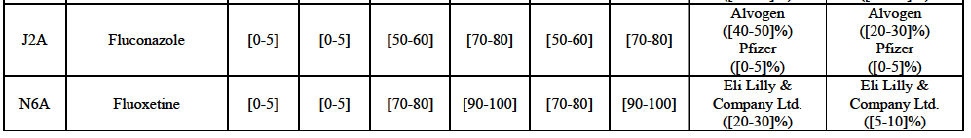
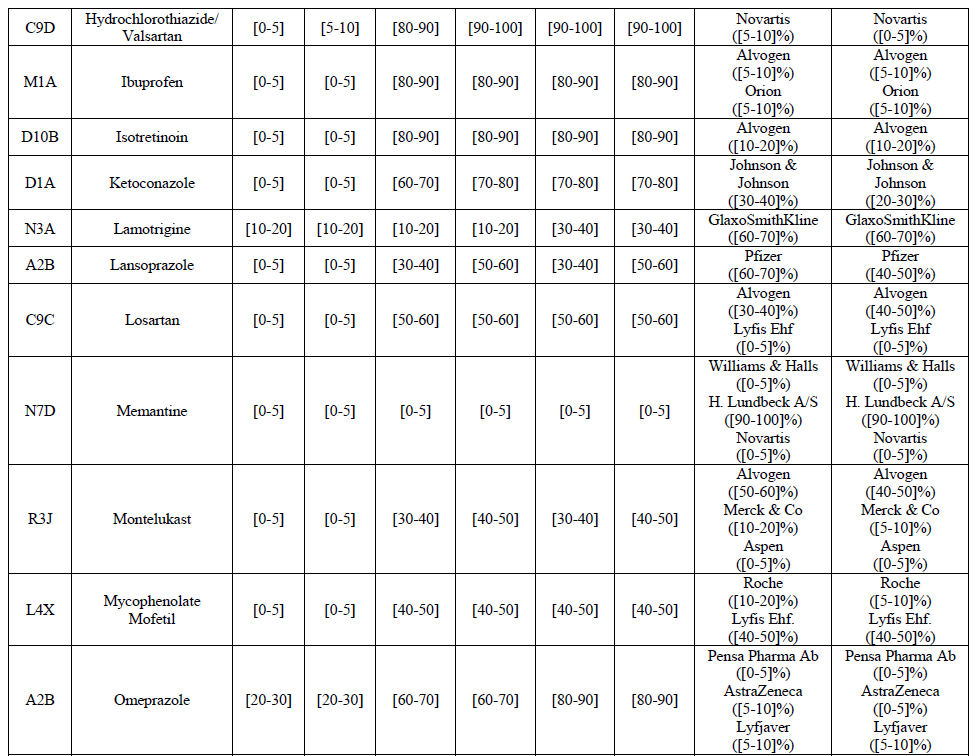
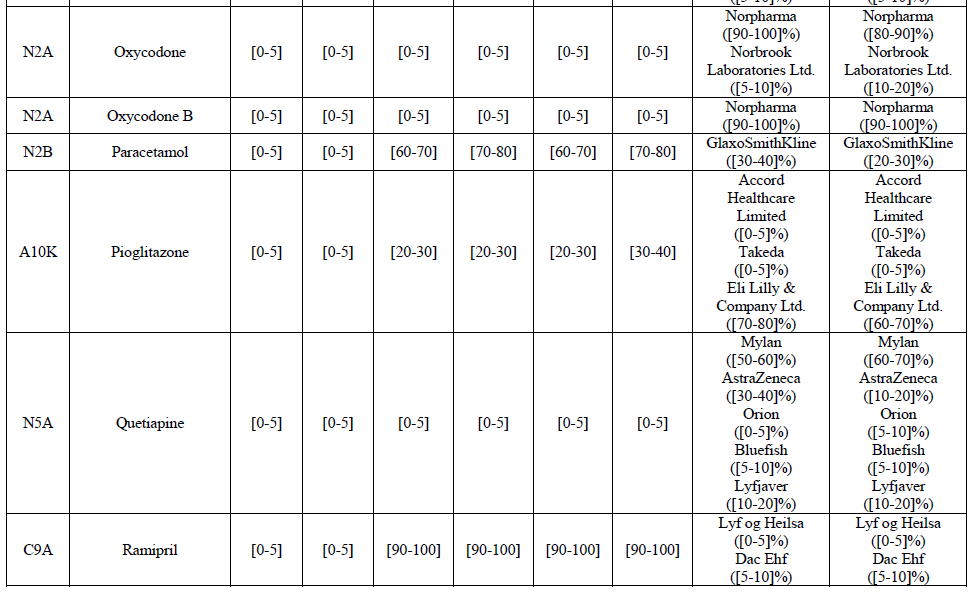
(215) The Commission presents below the competitive analysis on these markets, at molecule level, indicating also the ATC3 class to which it belongs, with a possible distinction by pharmaceutical form (at NFC1 level) and between prescribed and OTC sales where relevant.
Acetylsalicylic acid (N2B)
(216) For acetylsalicylic acid, the Transaction gives rise to a Group 1 market at molecule level. Since the Parties' combined market share was at [90-100]% over the last three years, the Transaction will result in a merger-to-monopoly for this market.
Allopurinol (M4A)
(217) For allopurinol, the Transaction gives rise to a Group 1 market at molecule level. Since the Parties' combined market share was at [90-100]% over the last three years, the Transaction will result in a merger-to-monopoly for this market.
Amoxicillin/clavulanic acid (J1C)
(218) For amoxicillin/clavulanic acid, the Transaction gives rise to a Group 1 market at the level of the pharmaceutical form D. While the Parties' combined market shares were moderate (up to [30-40]% at pharmaceutical form level in volume in 2014), only one competitor, the originator, would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.130
Anastrozole (L2B)
(219) For anastrozole, the Transaction gives rise to a Group 1 market at molecule level. Over the last three years, the Parties' combined market shares increased up to [60-70]% in value and [60-70]% in volume in 2014. Based on 2014 figures, only one competitor with a market share above 5% in value and volume would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.131
Atorvastatin (C10A)
(220) For atorvastatin, the Transaction gives rise to a Group 1 market at molecule level. Over the last three years, the Parties' combined market shares increased up to [80-90]% in value and [90-100]% in volume in 2014. Based on 2014 figures, no competitor with a market share above 5% in value and volume would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.132
Cetirizine (R6A)
(221) For cetirizine, the Transaction gives rise to a Group 1 market at molecule level. Since the Parties' combined market share was at [90-100]% over the last three years, the Transaction will result in a merger-to-monopoly for this market.
Desloratadine (R6A)
(222) For desloratadine, the Transaction gives rise to a Group 1 market at molecule level. Over the last three years, the Parties' combined market shares increased up to [60-70]% in value and [70-80]% in volume in 2014. Based on 2014 figures, only two competitors with a market share above 5% would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.133
Desogestrel (G3A)
(223) For desogestrel, the Transaction gives rise to a Group 1+ market at molecule level. While the Parties' combined market share was moderate over the last three years (up to [30-40]% in volume in 2014), the Parties are the only two generics and only one competitor (the originator) would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.134
Enalapril/hydrochlorothiazide (C9B)
(224) For enalapril/hydrochlorothiazide, the Transaction gives rise to a Group 1 market at molecule level. Since the Parties' combined market share was at [90-100]% over the last three years, the Transaction will result in a merger-to-monopoly for this market.
Felodipine (C8A)
(225) For felodipine, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [70-80]% in value and volume over the last three years. The Parties are the only two generics and only one competitor (the originator) would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market.135
Fentanyl (N2A)
(226) For fentanyl, the Transaction gives rise to affected Group 1 market at molecule level. The Parties' combined market shares increased over the last three years, up to [80-90]% in value and [80-90]% in volume. The Parties are the only two generics and only one competitor (the originator) would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.136
Fluconazole (J2A)
(227) For fluconazole, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [60-70]% in volume over the last three years. Only one competitor with a market share above 5% in value and volume would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.137
Fluoxetine (N6A)
(228) For fluoxetine, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market shares increased over the last three years, up to [90-100]% in value and [90-100]% in value in 2014. The Parties are the only two generics and only one competitor (the originator) would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.138
Hydrochlorothiazide/valsartan (C9D)
(229) For hydrochlorothiazide/valsartan, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [80-90]% in value and volume over the last three years. The Parties are the only two generics and only one competitor (the originator) would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.139
Ibuprofen (M1A)
(230) For ibuprofen, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [80-90]% in value and volume over the last three years. Based on 2014 figures, no competitor with a market share above 5% would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.140
Isotretinoin (D10B)
(231) For isotretinoin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [60-70]% in value and volume over the last three years (up to [80-90]% in value and [80-90]% in volume in 2014). Only one competitor with a market share above 5% would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.141
Ketoconazole (D1A)
(232) For ketonazole, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [60-70]% in value and volume over the last three years. The Parties are the only two generics and only one competitor (the originator) would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.142
Lamotrigine (N3A)
(233) For lamotrigine, the Transaction gives rise to a Group 1 market at molecule level. While the Parties' combined market share was moderate over the last three years (up to [3040]% in volume in 2014), the Parties are the only two generics and only one competitor (the originator) would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.143
Lansoprazole (A2B)
(234) For lansoprazole, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was between [40-50]% and [50-60]% in volume over the last three years. The Parties are the only two generics and only one competitor (the originator) would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market.144
Losartan (C9C)
(235) For losartan, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was between [40-50]% and [60-70]% in volume over the last three years and only one competitor with a market share above 5% in value and volume would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.145
Memantine (N7D)
(236) For memantine, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market shares increased up to [50-60]% in value and [60-70]% in volume over the last three years, and only two competitors with a market share above 5% would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.146
Montelukast (R3J)
(237) For montelukast, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was between [40-50]% and [50-60]% in volume over the last three years and only two competitors with a market share above 5% in value and volume would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.147
Mycophenolate mofetil (L4X)
(238) For mycophenolate mofetil, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market shares increased up to [70-80]% in value and [90100]% in volume over the last three years, and only one competitor with a market share above 5% would remain on the market based on 2014 figures. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.148
Omeprazole (A2B)
(239) For omeprazole, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was between [80-90]% and [90-100]% in value and volume over the last three years and only one competitor with a market share above 5% in value and volume would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market.149
Oxycodone (N2A)
(240) For oxycodone, the Transaction gives rise to a Group 1 market at molecule level. At pharmaceutical form level (B), the Parties' combined market shares increased up to [4050]% in value and [50-60]% in volume over the last three years. The Parties are the only two generics and only one competitor (the originator) would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.150
Paracetamol (N2B)
(241) For paracetamol, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market shares increased up to [50-60]% in value and [60-70]% in volume over the last three years, and only one competitor would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.151
Pioglitazone (A10K)
(242) For pioglitazone, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market shares increased up to [80-90]% in value and [80-90]% in volume over the last three years, and only one competitor with a market share above 5% would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.152
Quetiapine (N5A)
(243) For quetiapine, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market shares increased up to [40-50]% in value and [30-40]% in volume over the last three years. Only one competitor with a market share above 5% in value and volume would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.153
Ramipril (C9A)
(244) For ramipril, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [90-100]% in value and volume over the last three years and only one competitor with a market share above 5% would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.154
Valsartan (C9C)
(245) For valsartan, Teva launched a generic product in 2015. At molecule level in 20122014, Allergan Generics had market shares above [80-90]% in value and volume ([8090]% in value and [90-100]% in volume in 2014). The Transaction would therefore lead to the loss of a recent entrant in a market where Allergan Generics has already a dominant position. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.155
Conclusion
(246) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raise serious doubts as to its compatibility with the internal market with respect to the marketing of acetylsalicylic acid, allopurinol, amoxicillin/clavulanic acid, anastrozole, atorvastatin, cetirizine, desloratadine, desogestrel, enalapril/hydrochlorothiazide, felodipine, fentanyl, fluconazole, fluoxetine, hydrochlorothiazide/valsartan, ibuprofen, isotretinoin, ketoconazole, lamotrigine, lansoprazole, losartan, memantine, montelukast, mycophenolate mofetil, omeprazole, oxycodone, paracetamol, pioglitazone, quetiapine, ramipril and valsartan in Iceland.
IV.2.2.14.b. Pipeline generic pharmaceuticals
[...]
(247) Teva is planning to launch a generic [...] (pharmaceutical form [...]), for which Allergan Generics had a [50-60]% market share in value and [60-70]% in volume in 2014, and only two other competitors with >5% share in volume were active on the market in 2012-2014.
Conclusion
(248) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the market for the marketing of [...] in Iceland.
IV.2.2.14.c. Wholesale of generic pharmaceuticals
(249) Allergan Generics is historically an Icelandic company and it still owns a manufacturing plant in Iceland. Allergan Generics is currently the only generic manufacturer selling generics directly to customers in Iceland. All other manufacturers, including Teva, sell their products to wholesalers. In Iceland, two wholesalers are specialised in generics sales (Lyfis and Alvogen).
(250) Teva is supplying its generics to Lyfis. Lyfis is responsible for setting prices of Teva's products to customers, and is in charge of marketing and regulatory affairs. Teva is Lyfis' main supplier, but Lyfis also supplies products from other generic manufacturers such as Krka.
Notifying Party's views
(251) The Notifying Party submits that the impact of the Transaction would be limited to individual molecules where Allergan Generics and Teva hold an important combined market share as manufacturers.
(252) Teva did not submit any views on the impact of the transaction on the wholesale of generic pharmaceuticals in Iceland.
Commission's assessment
(253) The Commission identifies a risk of input foreclosure, of generic pharmaceuticals, for the wholesale market for generic pharmaceuticals in Iceland.
(254) According to the Commission's guidelines on non-horizontal mergers,156 input foreclosure arises where, post-merger, the merged entity is likely to restrict access to the products that it would have otherwise supplied absent the merger. In assessing the likelihood of such scenario, the Commission examines, first, whether the merged entity would have, post-merger, the ability to substantially foreclose access to inputs, second, whether it would have the incentive to do so, and third, whether a foreclosure strategy would have a significant detrimental effect on competition downstream.157
The ability of the merged entity to foreclose
(255) For input foreclosure to be a concern, the vertically integrated firm resulting from the merger must have a significant degree of market power upstream.158 This is the case in Iceland where Allergan Generics is by far the leading generics supplier and Teva's market shares have been growing over the last few years (in 2014, Teva became the second largest generics supplier in Iceland).
(256) The Notifying Party provided the following shares of the Parties and their main competitors for the marketing of generics in Iceland, at the level of manufacturers.
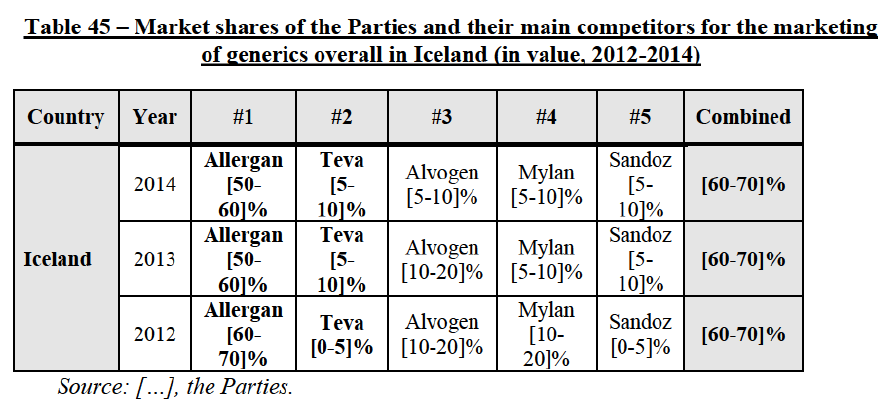
(257) Based on an internal document of Allergan Generics,159 the combined share of the Parties for last twelve months ending August 2015 was even higher at [60-70]% (Allergan Generics: [50-60]%; Teva: [5-10]%).
(258) The Parties, and in particular Teva, also have a strong position in R&D for generics, allowing them to be more efficient and faster in launching new generic products in Iceland. Indeed, out of the 90 new generic launches in Iceland in 2012-2015, Teva was present with its generic version in [40-50]% of cases, Allergan Generics in [60-70]% of cases. This is to be compared with the performance of Alvogen ([10-20]% of cases). Furthermore, the Parties launch their products on average quicker than their main competitors: Teva's launch was in the first 3 launches in [20-30]% of cases, Allergan Generics in [40-50]% of cases (versus Alvogen in [10-20]% of cases).
(259) Furthermore, the market investigation indicated that Allergan Generics would benefit from a strong brand premium due to its historical presence.160 Patients would frequently co-pay their generic pharmaceuticals when they come from Allergan Generics (meaning they would pay themselves the difference between the reimbursement price and the higher price of the Allergan Generics' product).161 Market participants also indicated that doctors would still often prescribe the Actavis brand from Allergan Generics.162
(260) The market investigation also indicated that, even if pharmacies are obliged to offer to patients the cheapest generic available163 based on a monthly price list, the cheapest generic would not necessarily be the most supplied in a given month. In that respect, one competitor indicated that "if Actavis wins a tender for a given month it will obtain up to 95% of the market, where as another generic company succeeding to Actavis may end up supplying only 10, 15 or 20% (the pharmacists using, for the rest, the stocks piled-up the month before)".164 This brand premium was also confirmed by the data provided by the Notifying Party on Allergan Generics' sales and market shares for 2014. Indeed, for a number of products,165 its market share was significant although its product was more than 5% more expensive than the cheapest product on offer (in some cases, from 50% to 100% more expensive).
(261) Furthermore, the market investigation indicated that Lyfis, through its partnership with Teva, has been challenging Allergan Generics' dominant position in the wholesale of generics since 2010. More specifically, according to market participants, Lyfis' ability to challenge Allergan Generics' position is derived from Teva's portfolio of generics, as well as its competitive pricing and ability to cover shortages.166 By way of example, one market participant indicated that "[Teva] has improved competition with lower prices and new products".167
(262) This was also confirmed by Lyfis itself indicating that "[Teva's] aggressive position, low prices and better services enabled Lyfis to gain market shares over the years. Lyfis could also build on Actavis' shortages, which happen quite often, to cover the supply gap and increase awareness of its products".168
(263) In its assessment, the Commission has considered, on the basis of the information available, whether there are effective and timely counter-strategies that Lyfis could deploy.169 The market investigation indicated that, post-Transaction, Lyfis would not be able to find an alternative manufacturer, with a similar portfolio, competitive prices, and the ability and incentive to serve the Icelandic market without significant costs and time.
(264) Indeed, it took Lyfis significant time and investment to build its generics wholesale business around Teva's (and, to a lesser extent, Krka's) products. In that respect, it should be noted that Iceland is a small generics market of a total size of approximately EUR 23 million, where Teva generated only EUR [...] million sales in 2014. The market investigation confirmed that this market is generally not attractive for manufacturers, and is considered a complement of sales generally generated in other Nordic countries.170 This is confirmed by the fact that Teva managed its commercial relationships with Lyfis from its Finnish subsidiary, that almost [30-40]% of its packs sold in Iceland are shared packs,171 and that [...], in order to incentivise Teva to serve the Icelandic market.172 This point was also confirmed by manufacturers, one of them indicating that "Iceland is just 350.000 people, which is not enough to support a pharma product in itself".173
(265) Lyfis summarised the situation by indicating that "it took five years (2005-2010) for Lyfis to establish and develop its wholesaling business in Iceland. Lyfis entered the market as a maverick and engaged in fierce price competition with Actavis, in particular thanks to Teva's products [...] [the] "stickiness" of well-known Actavis products made this process cumbersome and lengthy".174
(266) Post-Transaction, Krka would not be able to replace Teva in a timely manner. Indeed, although it recently entered, Krka is still a smaller competitor, with a market share of [0-5]% in 2014 in the marketing of generics in Iceland. Furthermore, its range of products would still be limited: 17 generic molecules in 2015 according to the Notifying Party, compared to 55 molecules for Teva and 136 molecules for Allergan Generics.
(267) The Commission considers that the merged entity will hold a dominant position in a number of upstream FDP markets (including in relation to the FDP markets identified in section IV.2.2.14.a above and the ones for which Teva alone holds a dominant position). Furthermore, Teva currently uses a competitor of Allergan Generics in order to supply its products in Iceland, and such competitor needs a comprehensive portfolio of molecules to be competitive in the downstream wholesale market and is unlikely to be able to deploy effective and timely-counter strategies should the merged entity enter into an input foreclosure strategy. In these circumstances, the Commission considers that the merged entity would have the ability to exercise input foreclosure in the wholesale market for generic pharmaceuticals in Iceland by not supplying Teva's products to Lyfis any longer.
The incentive of the merged entity to foreclose
(268) The incentive to foreclose depends on the degree to which foreclosure would be profitable. Essentially, the merged entity faces a trade-off between the profit lost in the upstream market due to a reduction of input sales to (actual or potential) rivals and the profit gain, in the short or longer term, from expanding sales downstream or, as the case may be, being able to raise prices to consumers.175
(269) The Guidelines on non-horizontal mergers point out in particular that the higher the downstream margins, the higher the profit gain from increasing market share downstream at the expense of foreclosed rivals.176 In the case at hand, given Allergan Generics' aforementioned brand premium, it is likely that it can extract higher profit margins than its competitors on the downstream market.
(270) Furthermore, the incentive to foreclose actual or potential rivals may also depend on the extent to which the downstream division of the integrated firm can be expected to benefit from higher price levels downstream as a result of a strategy to raise rivals' costs. The greater the market shares of the merged entity downstream, the greater the base of sales on which to enjoy increased margins.177
(271) Given Allergan Generics' dominant position on the downstream market (since it supplies all its generics directly, its market share would be at least [50-60]% in the wholesale of generics), it will benefit from a sizeable base of sales on which to enjoy increased margin. This increased margin is likely to compensate the margin loss by foreclosing Lyfis from Teva's generic products. In these circumstances, the Commission considers that the merged entity may have the incentive to exercise input foreclosure in the wholesale market for generic pharmaceuticals in Iceland.
The detrimental impact on the market
(272) Significant harm to effective competition normally requires that the foreclosed firms play a sufficiently important role in the competitive process on the downstream market. Despite a relatively small market share compared to other players, a specific firm may play a significant competitive role compared to other players, for instance because it is a close competitor of the vertically integrated firm or because it is a particularly aggressive competitor.178
(273) This is the case of Lyfis, which despite its small market share plays the role of a particularly aggressive competitor and challenger to Allergan Generics' dominant position, as evidenced above.
(274) In the absence of alternative supplier as efficient as Teva pre-Transaction for the wholesale market in Iceland, the Commission identifies a risk of price increase and higher amount of shortages. This concern was raised by market participants, including customers, during the market investigation.179
(275) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to wholesale of generics pharmaceuticals in Iceland stemming from vertical effects.
IV.2.2.15. Ireland
IV.2.2.15.a. Marketed generic pharmaceuticals
(276) The following tables present the market shares of the Parties and their main competitors in 2012, 2013 and 2014 for the Group 1/1+/2 markets for which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market.
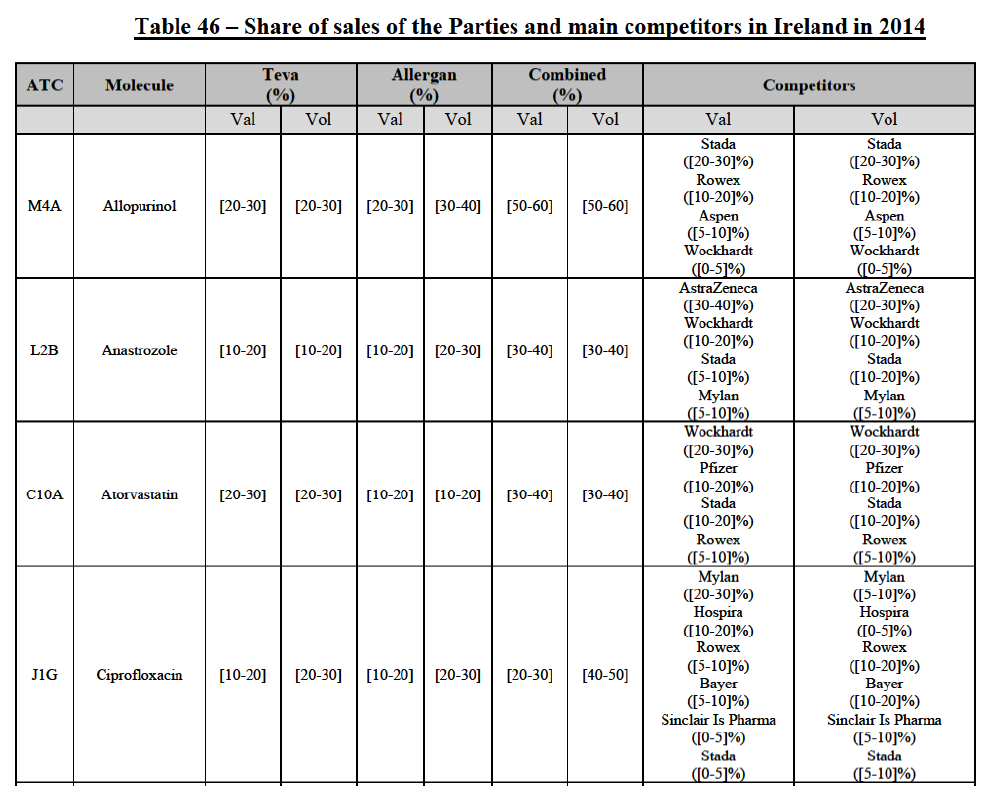
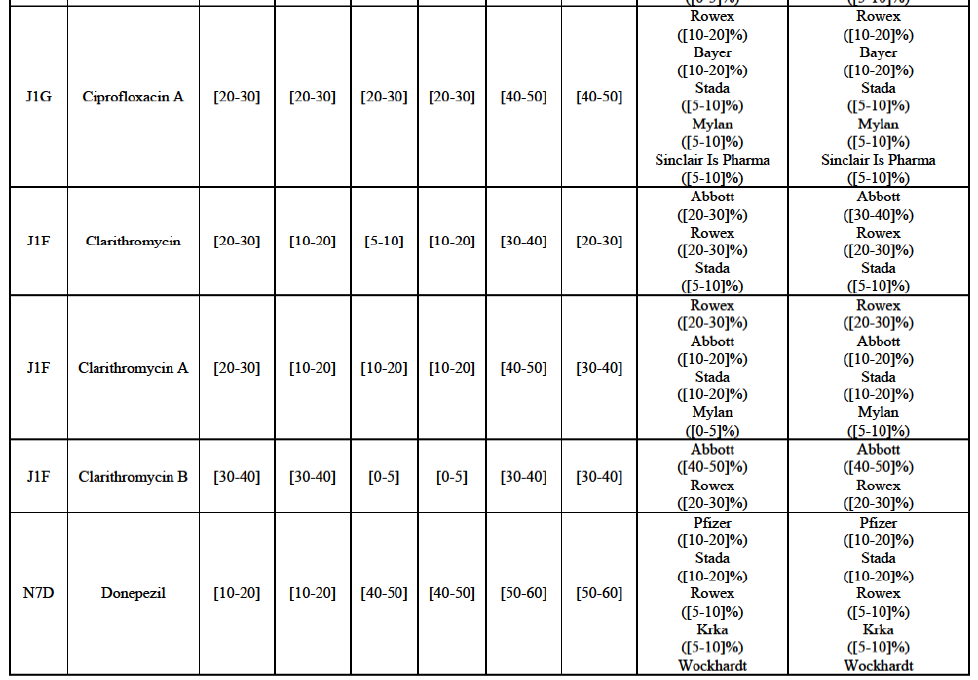
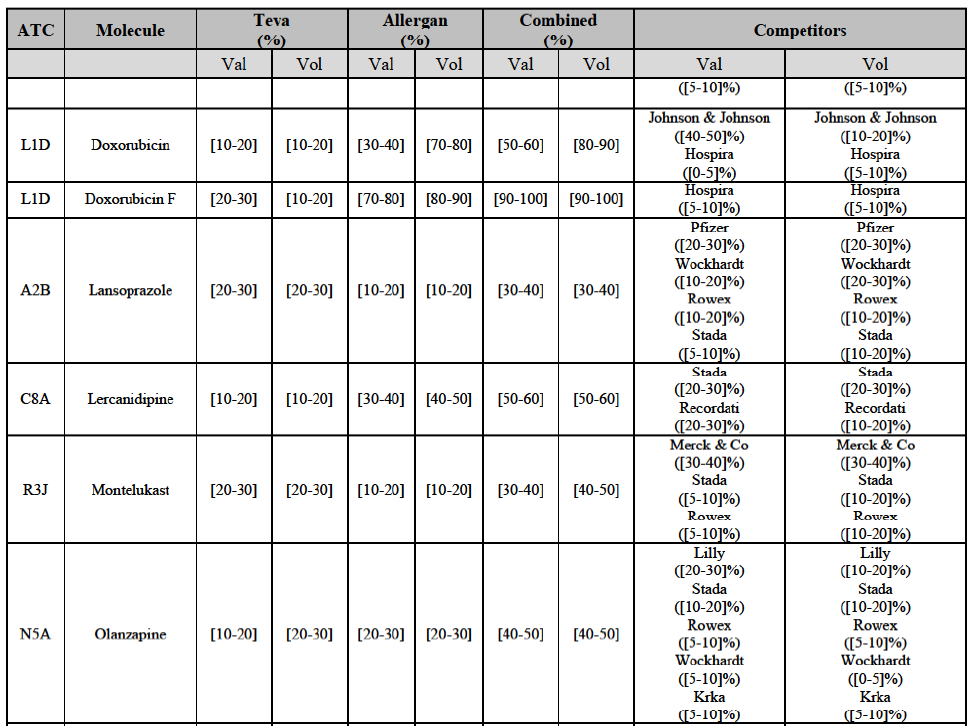
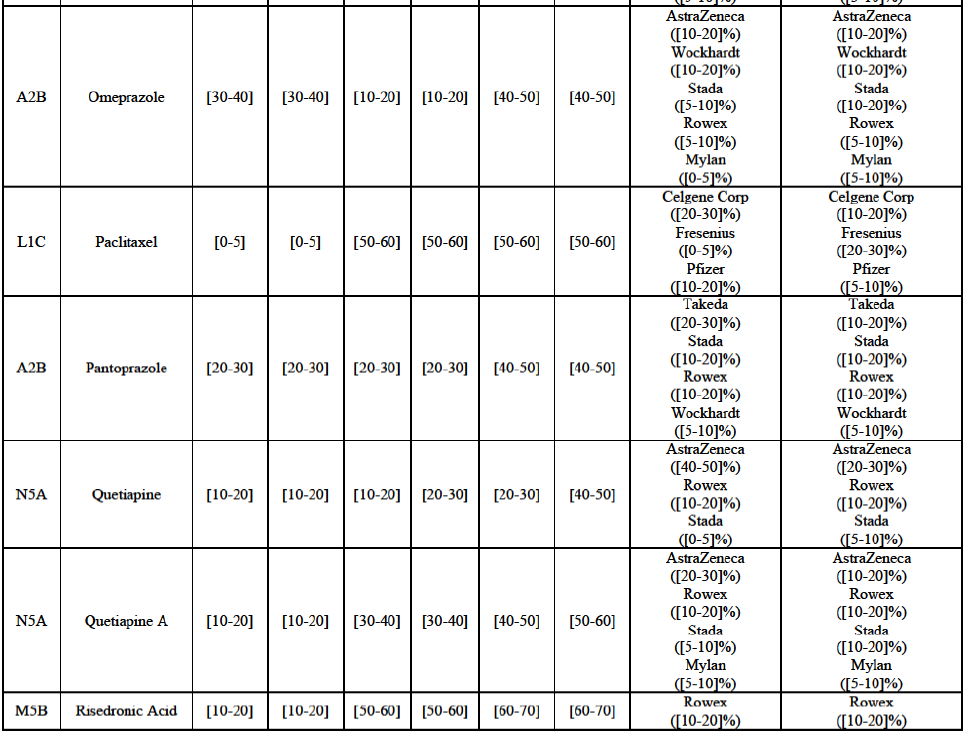
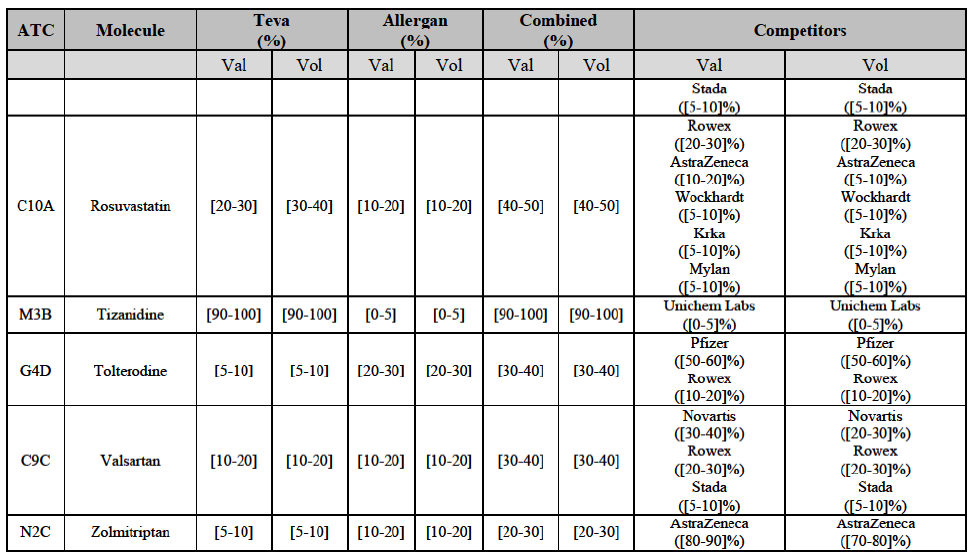
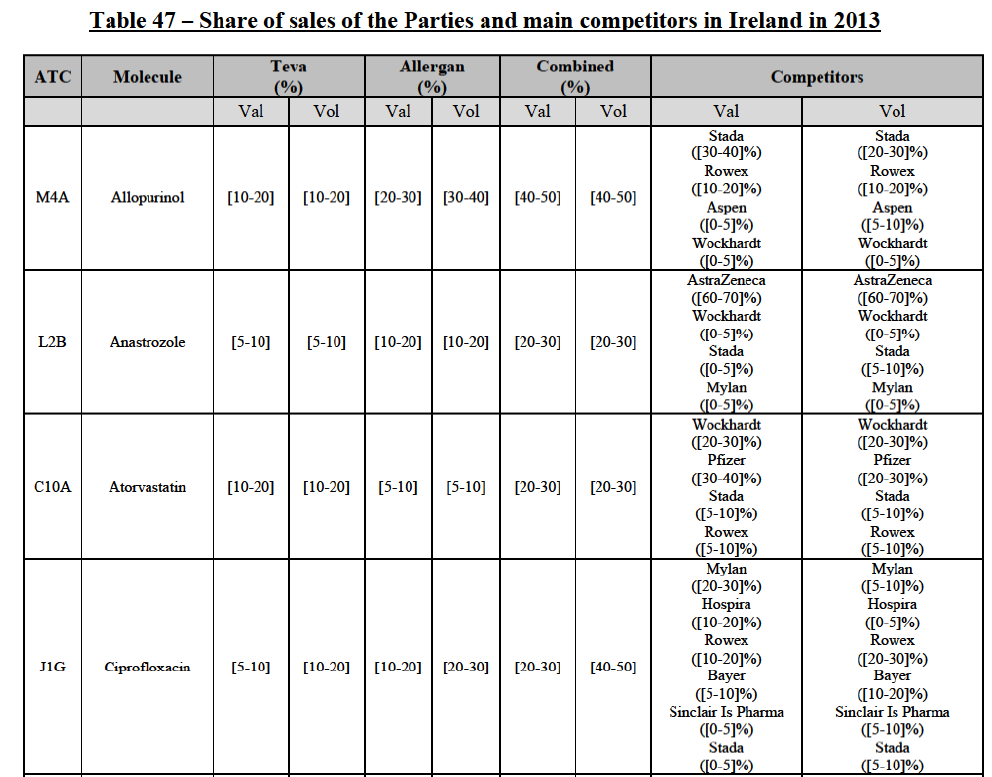

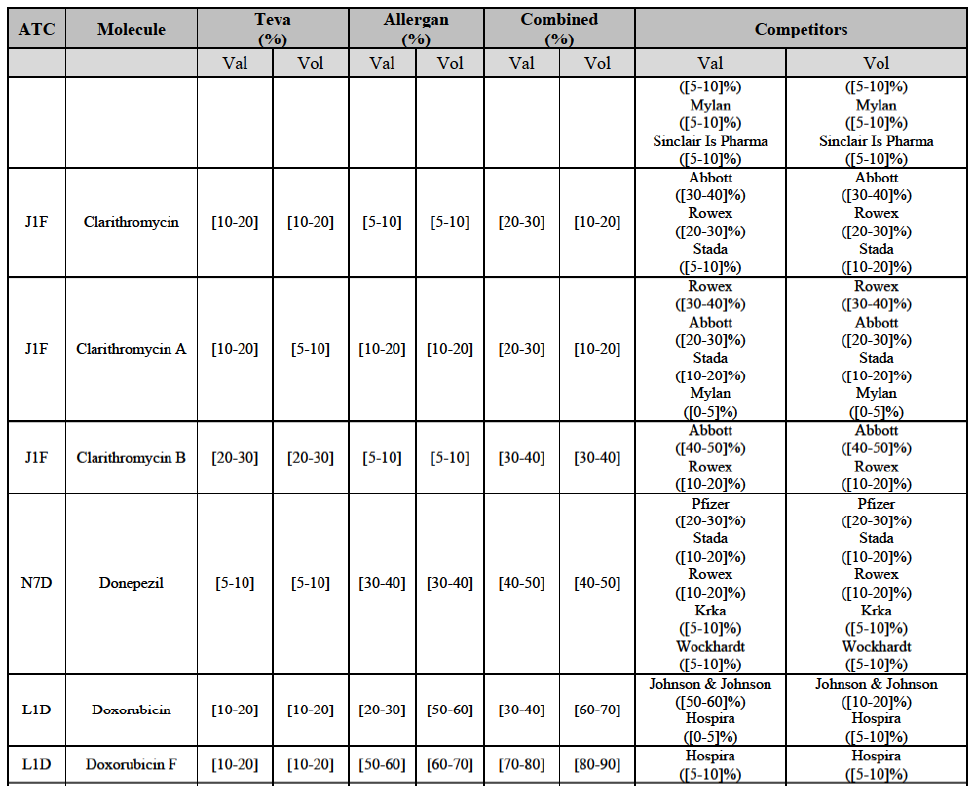
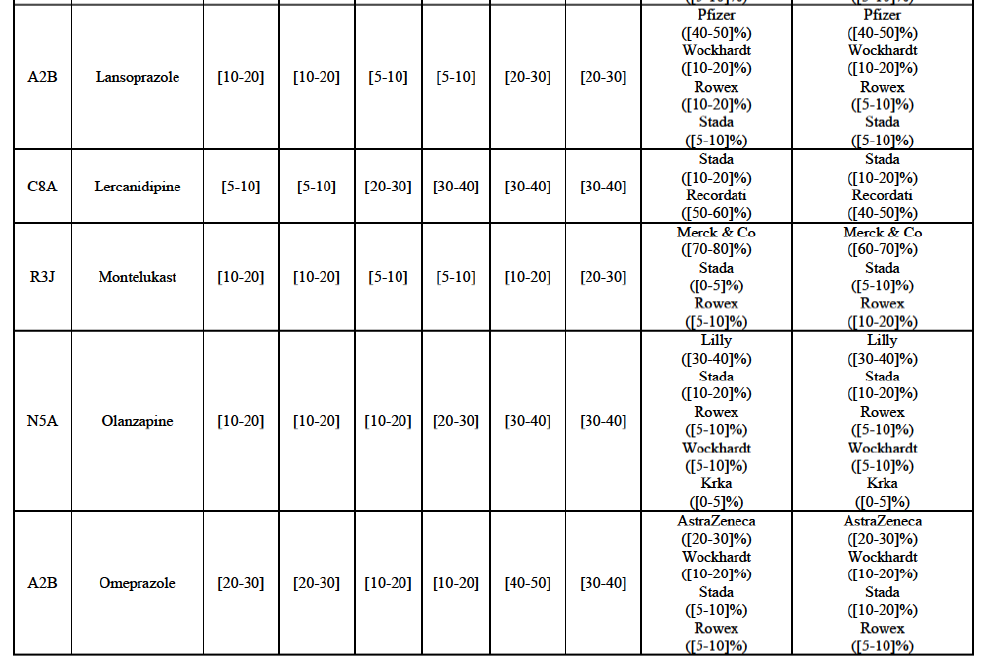
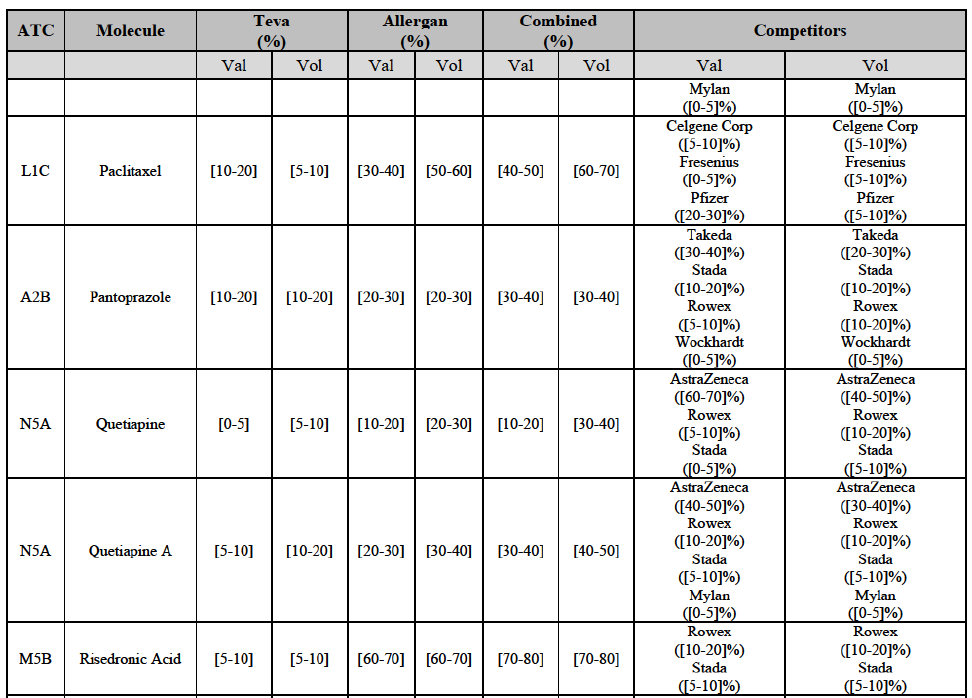
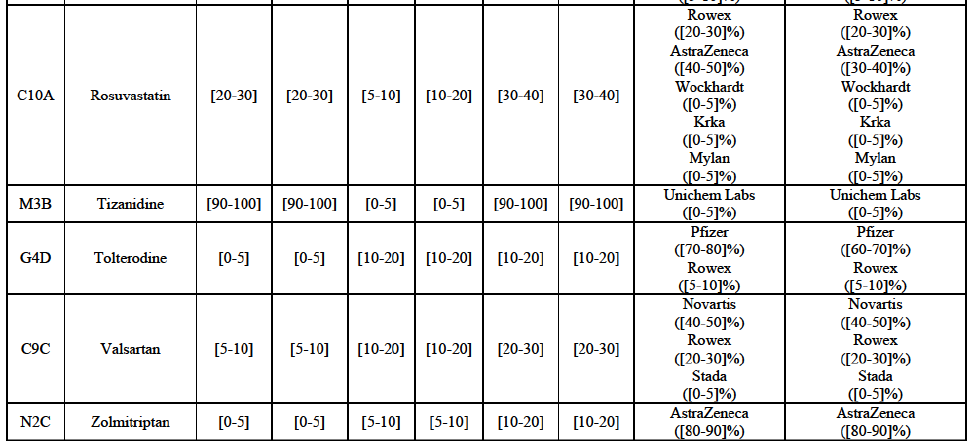
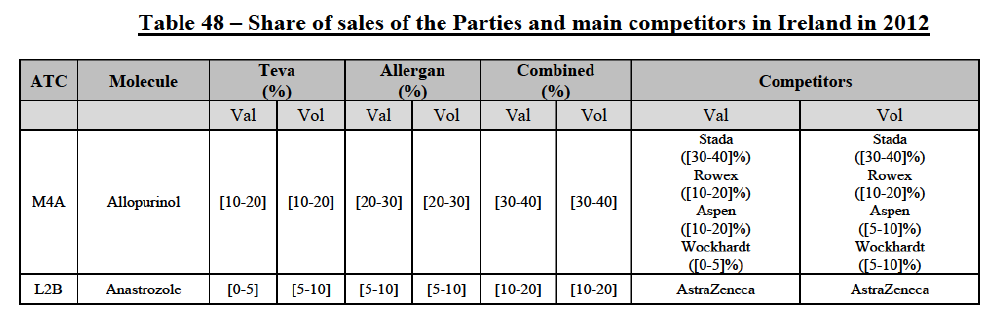
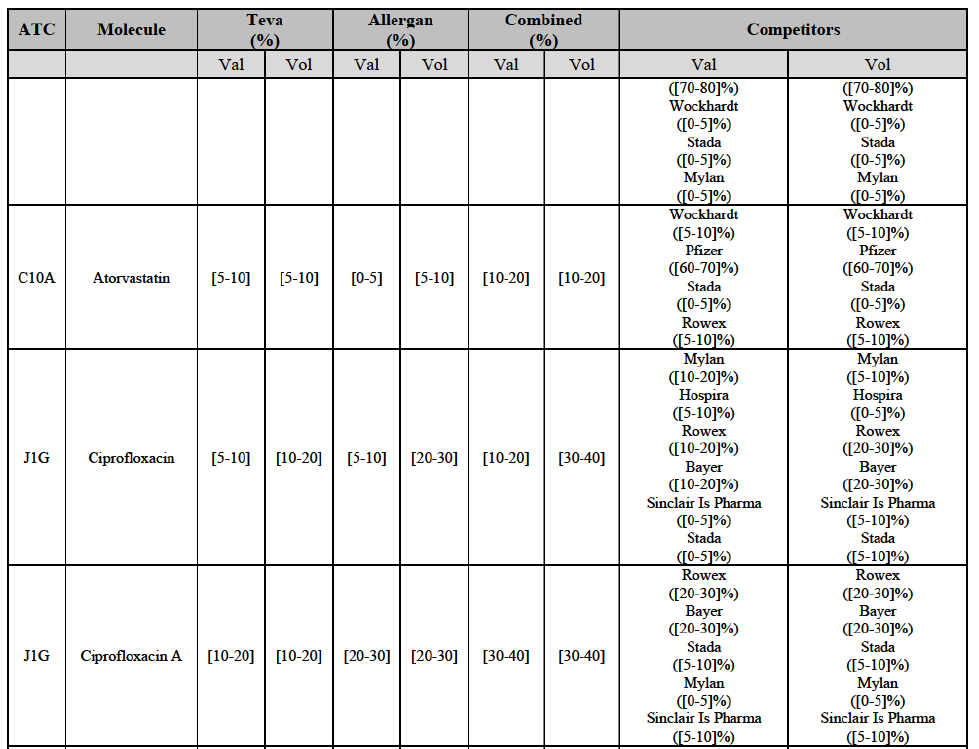
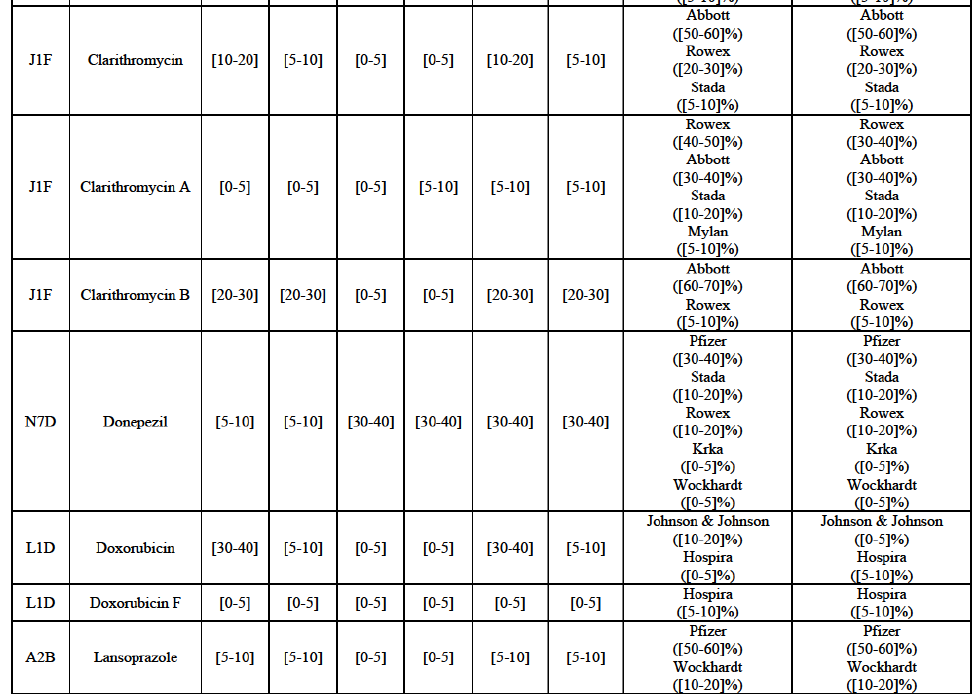
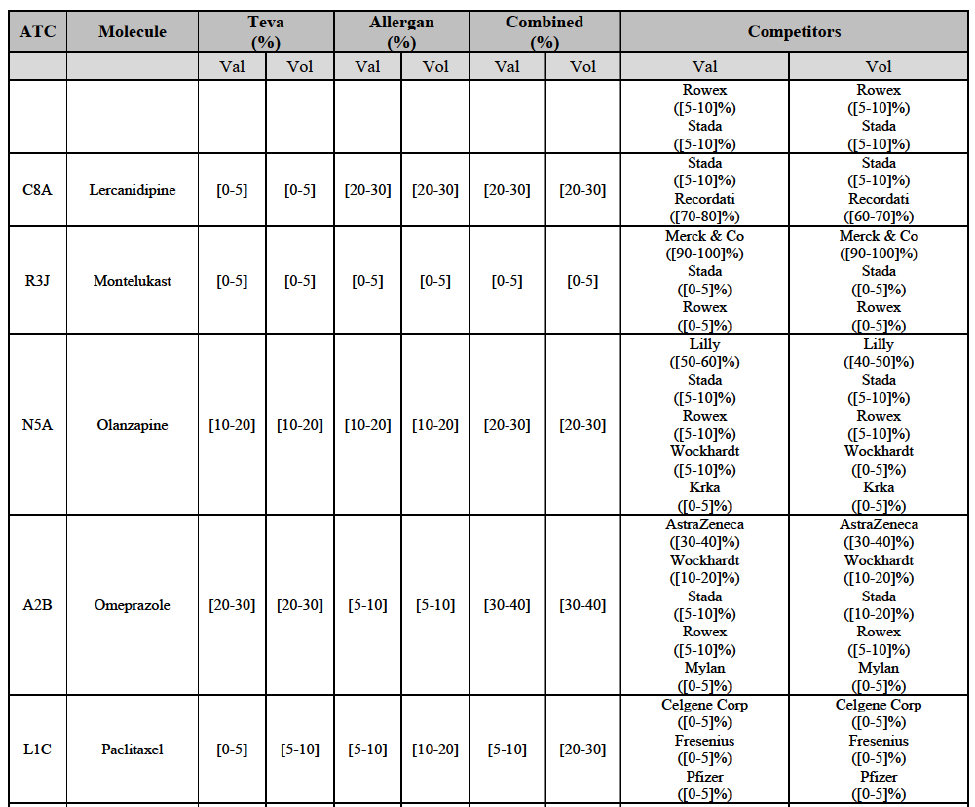
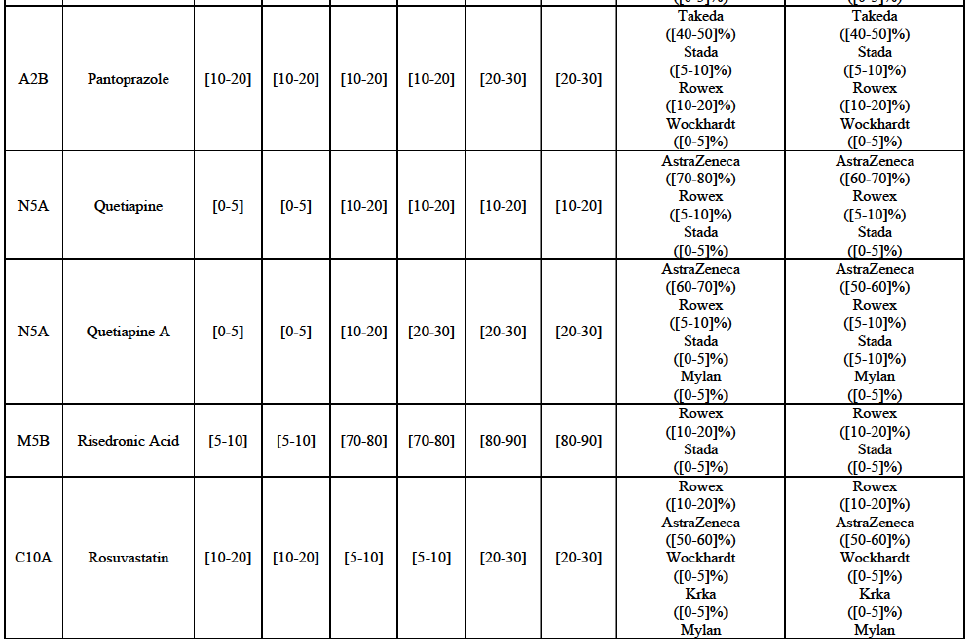
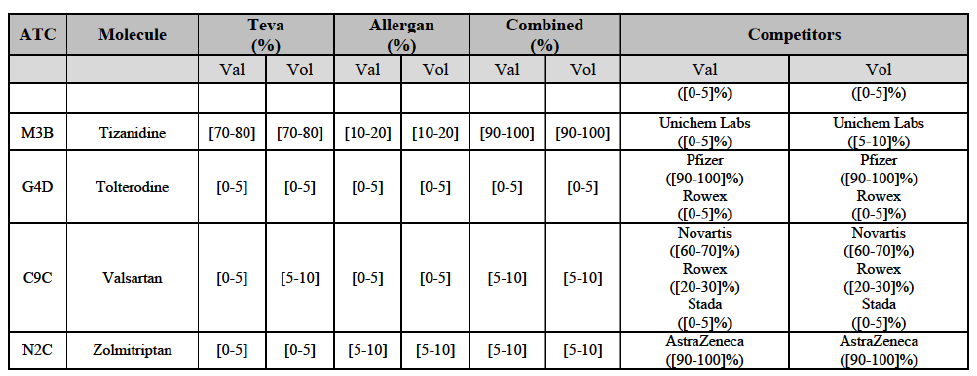
(277) The Commission presents below the competitive analysis on these markets, at molecule level, indicating also the ATC3 class to which it belongs, with a possible distinction by pharmaceutical form (at NFC1 level) and between prescribed and OTC sales where relevant.
Allopurinol (M4A)
(278) For allopurinol, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was increasing over the last three years, up to [50-60]% in value and [50-60]% in volume in 2014 (with a significant increment of more than [20-301%). Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market and that it would be difficult for customers to find alternative suppliers.'80
Anastrozole (L2B)
(279) For anastrozole, the Transaction gives rise to a Group 1 market at molecule level. Anastrozole became off-patent in February 2011. The Parties' combined market share increased over the last three years, up to [30-40]% in value and [30-40]% in volume in 2014, with a significant increment ([10-20]%). In 2015, the Parties' combined market share would reach [40-50]% in value (increment of [20-301%) and [50-60]% in volume (increment of [20-301%). Furthermore, the market investigation indicated that the Parties' combined market share would be higher than the Notifying Party's estimate (possibly 60%) and that the Transaction would have a negative impact in the market, in particular on prices.'$'
Atorvastatin (C10A)
(280) For atorvastatin, the Transaction gives rise to a Group 1 market at molecule level. The Parties recently entered the market following patent expiry in 2012. The Parties' combined market share increased over the last three years, up to [30-40]% in value and [30-40]% in volume (with an increment above [10-201%). Furthermore, the market investigation indicated that the Parties' combined market share would be higher than the Notifying Party's estimate (above 50%) and that the Transaction would have a negative impact in the market, in particular as to risk of shortages.182
Ciprofloxacin (J1G)
(281) For ciprofloxacin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share increased over the last three years, and above [40-50]% in volume in 2014 (with an increment of more than [20-30]%). The Parties combined market share was above [40-50]% in value and volume in 2014 for pharmaceutical form A. Furthermore, the market investigation indicated that the Parties' combined market share would be higher than the Notifying Party's estimate (possibly 60%) and that the Transaction would have a negative impact in the market, in particular on prices.183
Clarithromycin (J1F)
(282) For clarithromycin, the Transaction gives rise to a Group 1 market at pharmaceutical form A level in 2014 and pharmaceutical form B level in 2012 and 2013. For pharmaceutical form A, the Parties' combined market share increased over the last three years, up to [40-50]% in value and [30-40]% in volume in 2014 (with an increment of more than [10-20]%). In 2015, the Parties' combined market share would reach [5060]% in value (increment of [20-30]%) and [40-50]% in volume (increment of [2030]%). For pharmaceutical form B, the Parties' combined market share increased over the last three years, up to [30-40]% in value and [30-40]% in volume. Only two competitors, including the originator, would remain on this pharmaceutical form. The market investigation indicated that the reference price for this molecule would be set soon (in March 2015) and that it could have a negative impact on the number of suppliers active. The market investigation indicated that the Transaction would have a negative impact in the market.184
Donepezil (N7D)
(283) For donepezil, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share increased over the last three years, up to [50-60]% in value and [50-60]% in volume in 2014, with an increment of more than 10%. In 2015, the Parties' combined market share would reach [50-60]% in value (increment of [1020]%) and [50-60]% in volume (increment of [10-20]%). The market investigation indicated that the Transaction would have a negative impact in the market, in particular as to risk of shortages. 185
Doxorubicin (L1D)
(284) For doxorubicin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was increasing over the last three years, up to [50-60]% in value and [80-90]% in volume in 2014, with a significant increment (above [1020]%). Only one competitor with a market share above 5% in value and volume would remain. At the level of pharmaceutical form F, the Parties' combined market share reached [90-100]% in value and [90-100]% in volume in 2014 with only one competitor having a market share above 1% remaining. The market investigation indicated that the Transaction would have a negative impact in the market. 186
Lansoprazole (A2B)
(285) For lansoprazole, the Transaction gives rise to a Group 1 market at molecule level. The Parties recently entered the market, in 2012 for Allergan Generics. The Parties' combined market share was increasing over the last three years, up to [30-40]% in value and [30-40]% in volume. Furthermore, the market investigation indicated that the Parties' combined market share would be higher than the Notifying Party's estimate (possibly 60%) and that the Transaction would have a negative impact on the market, in particular as to risk of shortages. 187
Lercanidipine (C8A)
(286) For lercanidipine, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share increased over the last three years, up to [50-60]% in value and [50-60]% in volume with a significant increment ([10-20]%). Only two competitors with a market share above 5%, including the originator, would remain. Furthermore, the market investigation indicated that the Parties' combined market share would be higher than the Notifying Party's estimate (possibly 70%) and that the Transaction would have a negative impact on the market, in particular as to risk of shortages. 188
Montelukast (R3J)
(287) For montelukast, the Transaction gives rise to a Group 1 market at molecule level. The Parties recently entered the market (in 2013). Their combined market share increased over the last three years, up to [30-40]% in value and [40-50]% in volume in 2014 (with an increment of more than 13%). [...]. Furthermore, the market investigation indicated that the Parties' combined market share would be higher than the Notifying Party's estimate (possibly 50%) and that the Transaction would have a negative impact on the market. 189
Olanzapine (N5A)
(288) For olanzapine, the Transaction gives rise to a Group 1 market at molecule level. This molecule became recently off-patent (in 2011). The Parties' combined market share increased over the last three years, up to [40-50]% in value and [40-50]% in volume, with a significant increment ([10-20]% in value and [20-30]% in volume). In 2015, the Parties' combined market share would reach [40-50]% in value (increment of [20-30]%) and [50-60]% in volume (increment of [20-30]%). Furthermore, the market investigation indicated that the Parties' combined market share would be higher than the Notifying Party's estimate (possibly 60%) and that the Transaction would have a negative impact on the market. 190
Omeprazole (A2B)
(289) For omeprazole, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share increased over the last three years, up to [40-50]% in value and volume in 2014, with an increment of [10-20]%. Furthermore, the market investigation indicated that the Parties' combined market share would be higher than the Notifying Party's estimate (possibly above 60%) and that the Transaction would have a negative impact on the market, in particular as to risk of shortages. 191
Paclitaxel (L1C)
(290) For paclitaxel, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was [40-50]% in value and [60-70]% in volume in 2013 (with an increment of [10-20]% and [5-10]%) and [50-60]% in value and [50-60]% in volume in 2014. Only two competitors reached a market share above 5% in 2014. Furthermore, the market investigation indicated that the Transaction would have a negative impact due to the limited number of alternative suppliers.192
Pantoprazole (A2B)
(291) For pantoprazole, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share increased over the last three years, up to [40-50]% in value and [40-50]% in volume in 2014. In 2015, the Parties' combined market share would reach [40-50]% in value (increment of [20-30]%) and [50-60]% in volume (increment of [20-30]%). Furthermore, the market investigation indicated that the Parties' combined market share would be higher than the Notifying Party's estimate (possibly 60%) and that the Transaction would have a negative impact on the market, in particular as to risk of shortages.193
Quetiapine (N5A)
(292) For quetiapine, the Transaction gives rise to a Group 1 market at molecule level. This molecule became recently off-patent (in 2012). The Parties' combined market share increased over the last three years, up to [20-30]% in value and [40-50]% in volume in 2014. At the level of the pharmaceutical form A, the Parties' combined market share was [40-50]% in value and [50-60]% in volume, with a significant increment ([10-20]%). Furthermore, the market investigation indicated that the Parties' combined market share would be higher than the Notifying Party's estimate (possibly 60%) and that the Transaction would have a negative impact on the market.194
Risedronic acid (M5B)
(293) For risedronic acid, the Transaction gives rise to a Group 1 market at molecule level. Allergan Generics is the originator if this molecule and is in charge of its commercialisation in Ireland since 2015.195 The Parties' combined market share196 was [60-70]% in value and [60-70]% in volume in 2014, with a significant increment from Teva's market share ([10-20]% in value and [10-20]% in volume). Only two competitors with a market share above 5% would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.197
Rosuvastatin (C10A)
(294) For rosuvastatin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was increasing over the last three years, up to [40-50]% in value and [40-50]% in volume in 2014. [...]. Furthermore, the market investigation indicated that the Parties' combined market share would be higher than the Notifying Party's estimate (possibly above 50%) and that the Transaction would have a negative impact on the market, in particular as to risk of shortages.198
Tizanidine (M3B)
(295) For tizanidine, the Transaction gives rise to a Group 1 market at molecule level. Teva is the originator of this molecule. The Parties' combined market share increased from [90100]% in value and [90-100]% in volume in 2012 to [90-100]% in value and volume in 2014. Based on 2014 data, no competitor with a market share above 0.2 would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.199
Tolterodine (G4D)
(296) For tolterodine, the Transaction gives rise to a Group 1 market at molecule level. The Parties recently entered (in 2014) and their combined market share increased over the last three years, up to [30-40]% in value and [30-40]% in volume. Only two competitors with a market share above 5%, including the originator, would remain. Furthermore, the market investigation indicated that the Parties' combined market share would be higher than the Notifying Party's estimate (possibly more than 70%) and that the Transaction would have a negative impact on the market.200
Valsartan (C9C)
(297) For valsartan, the Transaction gives rise to a Group 1 market at molecule level. This molecule became off-patent recently in 2011. The Parties' combined market share was increasing over the last years, up to [30-40]% in value and [30-40]% in volume in 2014. In 2015, the Parties' combined market share would reach [30-40]% in value (increment of [20-30]%) and [40-50]% in volume (increment of [20-30]%). Furthermore, the market investigation indicated that the Parties' combined market share would be higher than the Notifying Party's estimate (possibly 50%) and that the Transaction would have a negative impact on the market, in particular as to risk of shortages.201
Zolmitriptan (N2C)
(298) For zolmitriptan, the Transaction gives rise to a Group 1+ market at molecule level. Indeed, the Parties' combined market share was moderate, up to [20-30]% in value and [20-30]% in volume (with an increment of [5-10]% in value and [5-10]% in volume). However, only one competitor, the originator, would remain in the market. Furthermore, the market investigation confirmed the monopoly situation of the Parties as generic suppliers and indicated that the Transaction would have a negative impact on the market.202
Conclusion
(299) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the marketing of allopurinol, anastrozole, atorvastatin, ciprofloxacin, clarithromycin, donepezil, doxorubicin, lansoprazole, lercanidipine, montelukast, olanzapine, omeprazole, paclitaxel, pantoprazole, quetiapine, risedronic acid, rosuvastatin, tizanidine, tolterodine, valsartan and zolmitriptan in Ireland.
IV.2.2.15.b. Pipeline generic pharmaceuticals
[...]
(300) Allergan Generics is planning to launch a generic [...] (pharmaceutical form [...]), for which Teva had a [70-80]% market share in value and in volume in 2014 on the same pharmaceutical form, and only one other competitor with >5% share in volume was active on the market in 2012-2014.
Conclusion
(301) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the market for the marketing of [...]in Ireland.
IV.2.2.15.c. Wholesale of generic pharmaceuticals
(302) In Ireland, the Parties are selling the generic medicines they manufacture under a DTP model, using broad-line wholesalers as mere logistic providers, which are remunerated on a fee-per-pack basis.
The Parties' views
(303) The Parties' submit that, given the market features in Ireland (manufacturers selling directly to retail pharmacies and using wholesalers as distributors), the Commission cannot raise serious doubts as to the compatibility of the Transaction both at the level of the marketing of individual molecules and at the level of the wholesale of generics.
(304) The Parties further submit that, even if the Commission were to focus on the wholesale of generics, the Parties will continue to face competition from at least four strong competitors which would have a wide market coverage, namely Rowex (a Sandoz / Rowa Wagner joint venture), Clonmel (belonging to Stada), Pinewood (belonging to Wockhardt) and Mylan.
(305) Moreover, the Parties consider that Teva and Allergan Generics cannot be considered as each other's closest competitors. Indeed, even if they both entered recently and consistently grew over the years, another recent entrant, Krka, would also be similarly growing in Ireland.
(306) Finally, the Parties indicate that the fact that some competitors (such as Stada and Wockhardt) do not distribute all their generics through broad-line wholesalers (but also have their own network of 3PLs) would not be a differentiating factor at their disadvantage. The Parties consider further that, even if it were to be a differentiating factor, it would on the contrary confer an advantage to Stada and Wockhardt offering an additional choice to customers.
The Commission's assessment
The Parties' market presence
(307) The Parties entered the Irish generics market recently: Allergan Generics in February 2008 and Teva in January 2006. It is only in 2009 that Teva had secured sufficient marketing authorisations to allow its Irish subsidiary to compete effectively in the market for wholesale of generics. Both Parties have been very successful: since 2014, Teva and Allergan are the two largest generics suppliers with market shares which have grown every year:
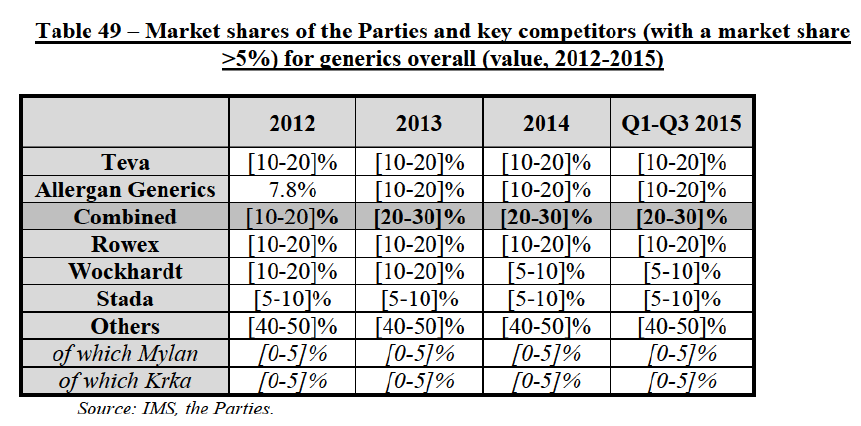
(308) The Parties are offering DTP loyalty schemes to customers, the "Accumulator" scheme for Allergan Generics203 and "Teva One / +pluspoints" for Teva.204 The market investigation confirmed that pharmacies are negotiating prices of generics with manufacturers based on the volume of their purchases of individual molecules, but also based on the range of products purchased and their overall spend.205
(309) The market investigation also provided elements showing that the above mentioned market shares of the Parties may be underestimated. A number of market participants estimated the Parties' combined market share to be above 40% in 2015.206 An internal document of Allergan Generics estimates the Parties' market shares for generics overall (for the twelve months ending August 2015) to be [20-30]% for Teva and [10-20]% for Allergan Generics, resulting in a combined market share of [40-50]% in value.207
(310) Only three competitors, Rowex, Wockhardt and Stada, have a market share above 5% in the market for generics' overall. Contrary to the Parties' view, Mylan has a more limited market position for generics overall, with a market share of less than 5% in value over the last three years. The market investigation, while acknowledging Mylan's price competitiveness, further highlighted the following weaknesses: "product range [...] availability [...] poor track record regarding continuity of supply" 208 As to Krka, it is a recent entrant and still a small player, with less than 2% of market share during the last three years. While a number of retail pharmacies responding to the market investigation acknowledged Krka's price competitiveness, the majority identified the limited range of products offered as its main weakness.209
Segmentation by type of customers
(311) As to the possible segmentation by type of customers, the Parties provided an estimate of their market shares based on a possible distinction between independent pharmacies, independent pharmacy chains and hospitals, which raises methodological questions. In particular, the Parties do not record their sales data on the same basis as IMS (both on value and volume terms), leading to the necessity to make a number of adjustments. 210 This explains in particular why the Parties have been unable to provide a similar split for their competitors.
(312) Notwithstanding the above, based on the Parties' estimates, the individual and combined market shares of Teva and Allergan Generics would not substantially differ depending on the customer segment: their combined market share would be [20-30]% by value in 2014 for independent pharmacy chains; [30-40]% by value in 2014 for independent pharmacies that do not belong to a pharmacy chain; and [20-30]% by value in 2014 for hospitals.
Segmentation by type of suppliers
(313) As to a possible segmentation by type of suppliers, the Parties, Rowex, Wockhardt and Stada are most consistently reported by market participants as offering a wide range of generics.211 This is confirmed by the data provided by the Notifying Party: in 2015, Rowex had 114 molecules in its portfolio, Stada 95 and Wockhardt was a little behind with 77 molecules, while Teva and Allergan Generics had respectively 104 and 95 molecules.
(314) Furthermore, these companies appear to offer a similar level of services. They distribute fully (Teva, Allergan Generics and Rowex) or partly (Wockhardt and Clonmel) their generics using broad-line wholesalers as logistics providers. The market investigation indicated that distributing through broad-line wholesalers would constitute a competitive advantage. Indeed, pharmacies indicated that they value short lead times and that the wholesaler distribution channel is "faster and [the] most efficient". Allergan Generics itself puts this element forward when promoting its DTP scheme: "[it] will make it transparent, easy and profitable to purchase generics through full-line wholesalers".212 Furthermore, respondents from the supply side largely confirmed this element, indicating for instance that "the reason why it is more difficult with a smaller portfolio to sell through broad line wholesalers is because a small company will pay greater costs to the wholesaler in terms of a fee per pack. Therefore, from a competitive point of view, they will not be able to offer as good deal to pharmacies".213 Other respondents indicated that "wholesale supply provides simple logistics for the manufacturer and higher service level for retail pharmacy supply but for the manufacturer there is a per pack service cost. Depending on the value of the portfolio the portfolio therefore drives the overall competitiveness" and that "holders of broad portfolios command loyalty from larger pharmacy customers and are therefore more attractive for broad-line wholesalers to carry. [...] The bigger the volume shipped to customers at one time the lower the cost per pack of logistics."214
(315) The ability of manufacturers having a wide range of products to offer loyalty schemes appears to be a key competitive advantage vis-à-vis smaller manufacturers. Market participants indicate for instance that "the manufacturing schemes therefore can apply discounts across the portfolio making it hard for individual product by product pricing" and that "broad portfolios increase customer loyalty and lessen the need for customers to meet with and trade with other providers".215 Teva and Allergan Generics seem to be pioneers in offering formal loyalty schemes in Ireland.216 As further evidenced below, this pricing innovation appears to have led other manufacturers to follow and develop their own loyalty and discount schemes, but without resulting in the same success so far.
(316) In a potential market comprised only of those generic manufacturers with a sizeable portfolio offering (allowing them to compete, through loyalty schemes, on discounts across a wide range of generics and to provide a comparably high level of services) that is constituted, conservatively, by Teva, Allergan Generics, Rowex, Wockhardt and Stada), the combined market share of the Parties has increased from 37% (increment: [10-20]%) in 2012 to [50-60]% (increment: [20-30]%) in Q1-Q3 2015. The market share of the next competitor, Rowex, has decreased from [20-30]% in 2012 to [10-20]% in Q1-Q3 2015.
(317) In addition to the leading position of the Parties and a high combined market share under a number of plausible market segmentations, the market investigation indicated that the Parties would be each other's closest competitors in the sales of generics to end-customers, in view in particular of their wide portfolio, pricing strategy and ability to launch new pipeline products on the market.217 As to their portfolio positioning, it should be noted that the Parties have a similar portfolio composition, with the majority of their sales occuring on overlapping molecules. As to their pricing behaviour, the Parties are consistently perceived as having the most aggressive pricing policies, while other manufacturers are generally considered to be price followers.218
(318) For instance, according to one pharmacy, "Teva and Actavis would be the market leaders and have the most aggressive pricing policy. They are very much pushing the market forward; the other companies just follow their lead. These two companies would offer the best range and price. They would be responsible for driving down the price of generics in Ireland [...] Teva and Actavis would have grown substantially. Traditional generic suppliers such as Clonmel and Pinewood would have suffered as they cannot compete with prices and do not have sufficient range and are slow to bring new products on the market".219 Another market participant indicated that Rowex is "operating off old commercial model and lacks innovative pricing, e.g. loyalty schemes".220 A customer also indicated that Teva and Allergan Generics would offer deeper discounts than Rowex.221
(319) In view of the above, the Commission concludes that Teva and Allergan Generics are each other's closest competitors in Ireland.
Impact on the market
(320) Indeed, in Ireland, generic manufacturers compete to offer prices below the reference price. The specific competitive pressure on prices (and discounts) and services exerted on each other due to their closeness of competition contributes to bringing down prices in Ireland.
(321) The Transaction is likely to reduce the intensity of competition in Ireland. This has been confirmed by the Irish national reimbursement agency for pharmaceuticals (part of the Health Service Executive), which has indicated that "a loss of a major supplier may bring with it a reduction in competition, competition which has been essential to the garnering of reduced prices via the processes underpinned by the Health (Pricing and Supply of Medical Goods) Act 2013. Savings from those reduced prices now underpin the delivery of many essential services".222
(322) This concern was also expressed by market participants, including customers. By way of example, one customer indicated that "combining the two most progressive and competitive suppliers in Ireland will ultimately lead to an increase in prices and reduction in availability of products for the consumer" while another one stated that "one would expect that there will be less competition in the market as Teva and Actavis competing against each other drove a lot competition and thus price reduction in the market".223
(323) Moreover, it is unlikely that, post-Transaction, the remaining competitors will be able to exert on the combined entity significant competitive pressure, that could make up for the loss of the current intensity of competition, which the Transaction will bring about.
Conclusion
(324) In view of the aforementioned market features, the Parties' overall presence and their closeness of competition, which is driven in particular by their pricing strategy, similarity of portfolio composition and range, the Transaction raises serious doubts as to its compatibility with the internal market with respect to the market for the wholesale of generic pharmaceuticals in Ireland.
IV.2.2.16. Italy
IV.2.2.16.a. Marketed generic pharmaceuticals
(325) The following tables present the market shares of the Parties and their main competitors in 2012, 2013 and 2014 for the Group 1/1+/2 market for which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market.
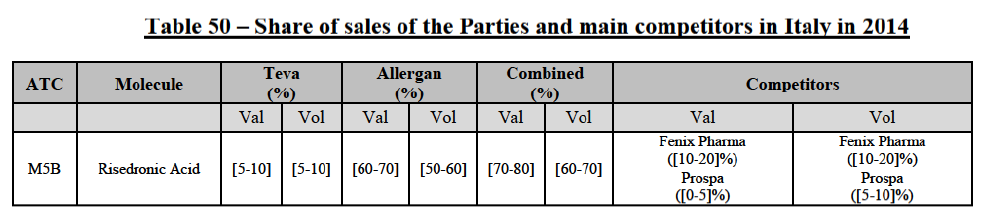
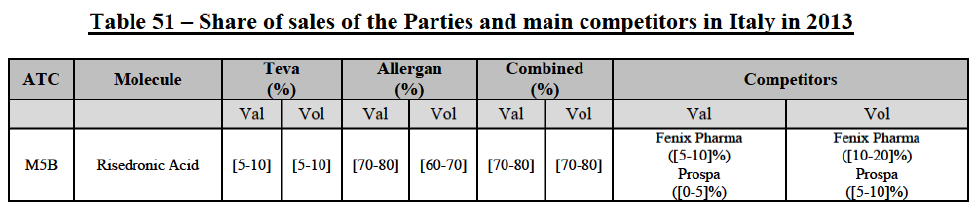
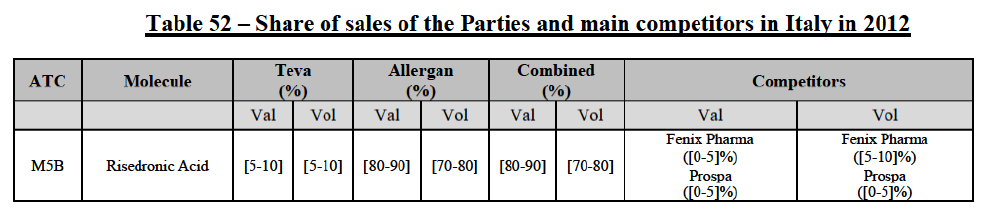
(326) The Commission presents below the competitive analysis on this market, at molecule level, indicating also the ATC3 class to which it belongs, with a possible distinction by pharmaceutical form (at NFC1 level) and between prescribed and OTC sales where relevant.
Risedronic Acid (M5B)
(327) For risedronic acid, the Transaction gives rise to a Group 1 market at molecule level. Allergan Generics is the originator of this molecule. The Parties' combined market share was high in the last three years in value and volume and above [70-80]% in value. Only one competitor with a market share above 5% in value would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.224
(328) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the marketing of risedronic acid in Italy.
IV.2.2.16.b. Pipeline generic pharmaceuticals
(329) The Transaction does not raise serious doubts as to its compatibility with the internal market with respect to pipeline generic pharmaceuticals in Italy.
IV.2.2.17. Latvia
IV.2.2.17.a. Marketed generic pharmaceuticals
(330) The following tables present the market shares of the Parties and their main competitors in 2012, 2013 and 2014 for the Group 1/1+/2 markets for which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market.
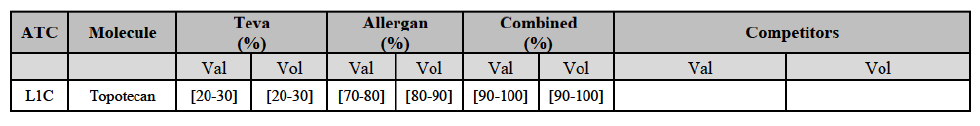
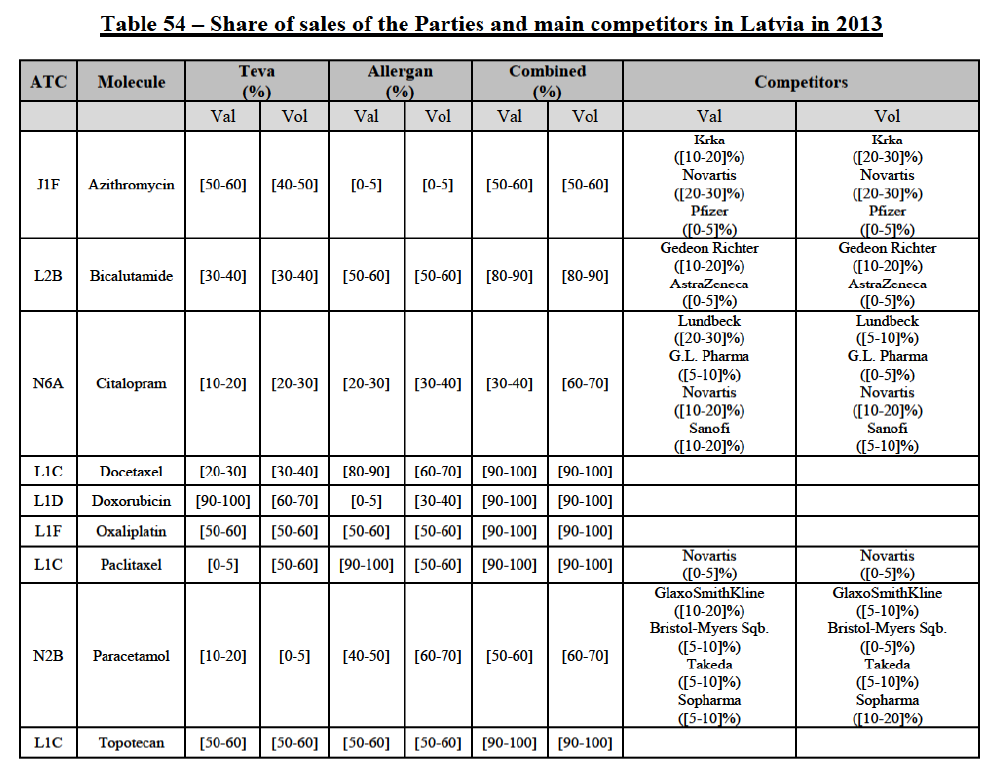
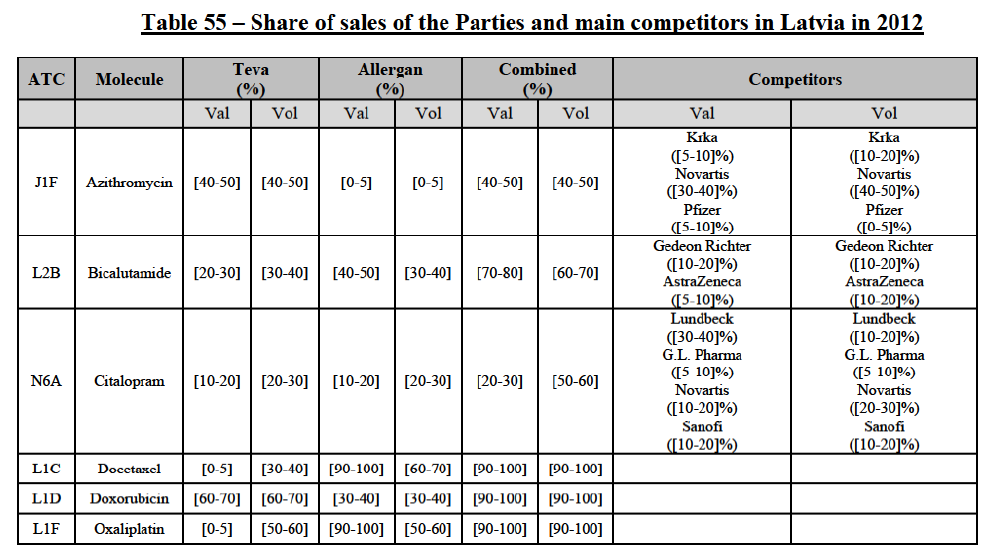
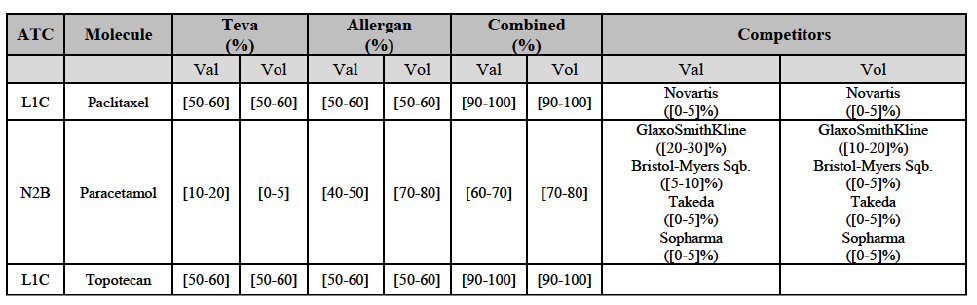
(331) The Commission presents below the competitive analysis on these markets, at molecule level, indicating also the ATC3 class to which it belongs, with a possible distinction by pharmaceutical form (at NFC1 level) and between prescribed and OTC sales where relevant.
Azithromycin (J1F)
(332) For azithromycin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [50-60]% in 2013 and 2014 in value and volume at molecule level. Only two competitors with a market share above 5% would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.225
Bicalutamide (L2B)
(333) For bicalutamide, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [60-70]% in value and volume over the last three years, up to [90-100]% in value and [90-100]% in volume in 2014. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.226
Citalopram (N6A)
(334) For citalopram, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market shares increased over the last three years, up to [50-60]% in value and [80-90]% in volume in 2014, with a significant increment ([10-201% in value and [...] in volume). Only two competitors with a market share above 5% would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.227
Docetaxel (L1 C)
(335) For docetaxel, the Transaction gives rise to affected Group 1 market at molecule level in 2013. The Parties' combined market share was >[90-100]% in the last three years in value and volume, with an increment of [20-30]% in 2013. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.228
Doxorubicin (L1D)
(336) For doxorubicin, the Transaction gives rise to a Group 1 market at molecule level. There would be a quasi-monopoly in this market post-merger, with a combined market share >[90-100]% in value and volume in the last three years. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.229
Oxaliplatin (L1D)
(337) For oxaliplatin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was [90-100]% in value and volume in the last three years. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.230
Paclitaxel (L1C)
(338) For paclitaxel, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was very high in the last three years in value and volume, above [70-80]% in 2014 and [90-100]% in 2012 and 2013, with a significant increment (up to [50-60]%). Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.231
Paracetamol (N2B)
(339) For paracetamol, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was in the last three years above [50-60]% in value and volume, and more than [70-80]% in volume in 2014. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.232
Topotecan (L1C)
(340) For topotecan, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market shares was [90-100]% in the last three years value and volume, with a significant increment (above [20-100]%). Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.233
Conclusion
(341) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the marketing of azithromycin, bicalutamide, citalopram, docetaxel, doxorubicin, oxaliplatin, paclitaxel, paracetamol and topotecan in Latvia.
IV.2.2.17.b. Pipeline generic pharmaceuticals
[...]
(342) Allergan Generics is planning to launch a generic [...] (pharmaceutical form [...]), for which Teva had a [70-80]% market share in value and [80-90]% in volume in 2014 on the same pharmaceutical form, and only two other competitors with >5% share in volume was active on the market in 2012-2014.
Conclusion
(343) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the market for the marketing of [...] in Latvia.
IV.2.2.18. Lithuania
IV.2.2.18.a. Marketed generic pharmaceuticals
(344) The following tables present the market shares of the Parties and their main competitors in 2012, 2013 and 2014 for the Group 1/1+/2 markets for which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market.
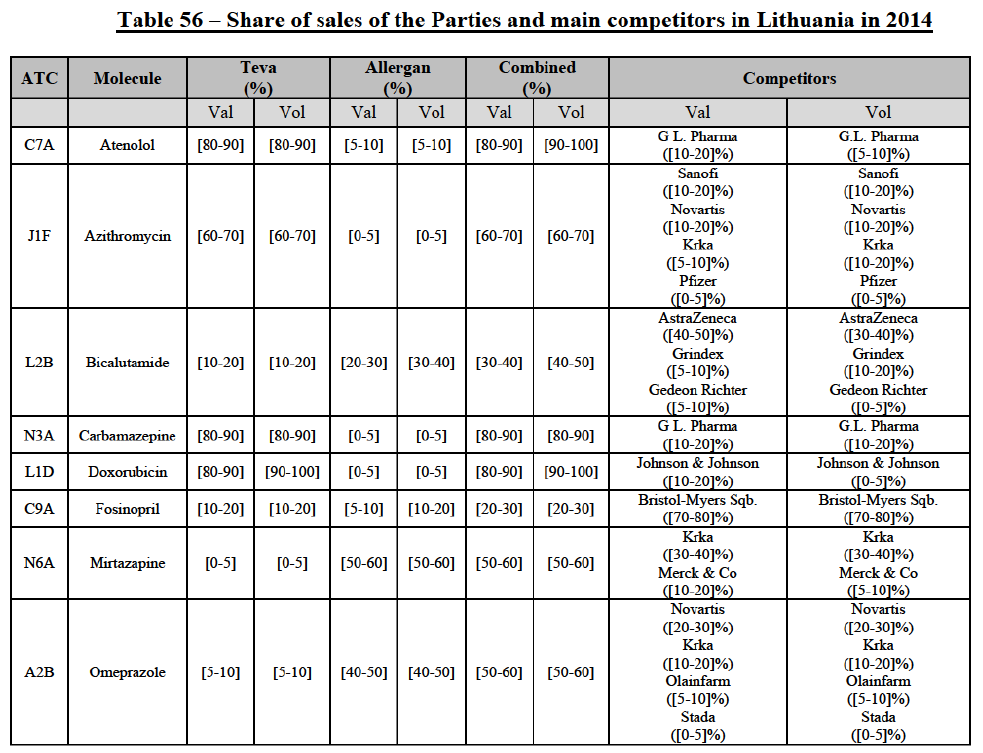
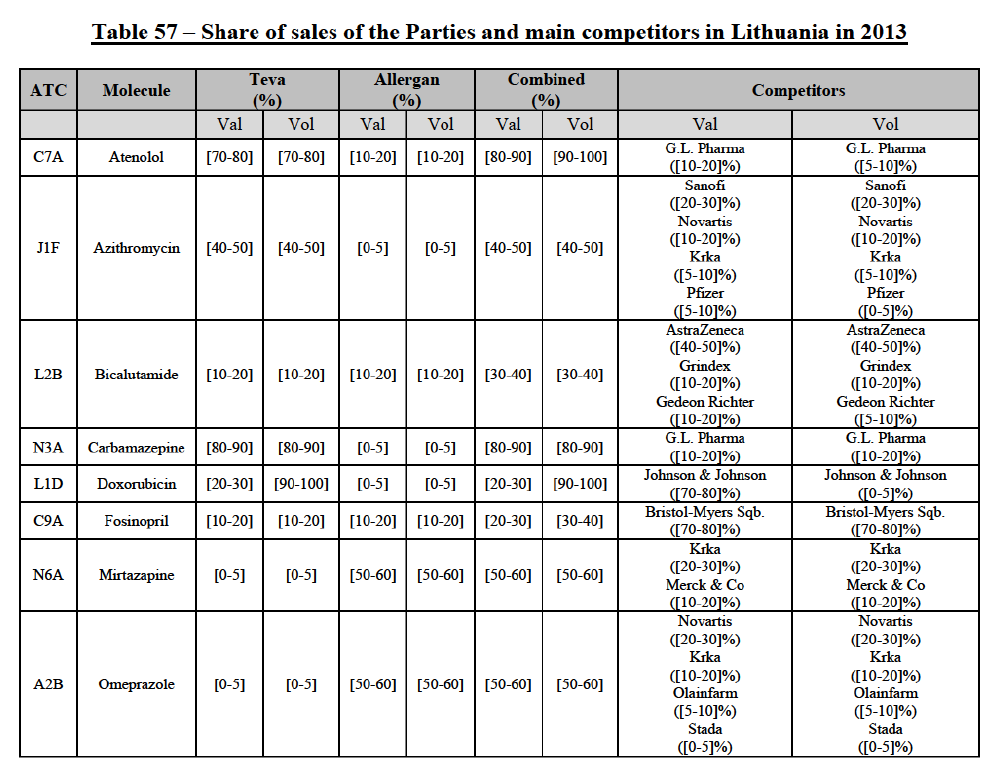
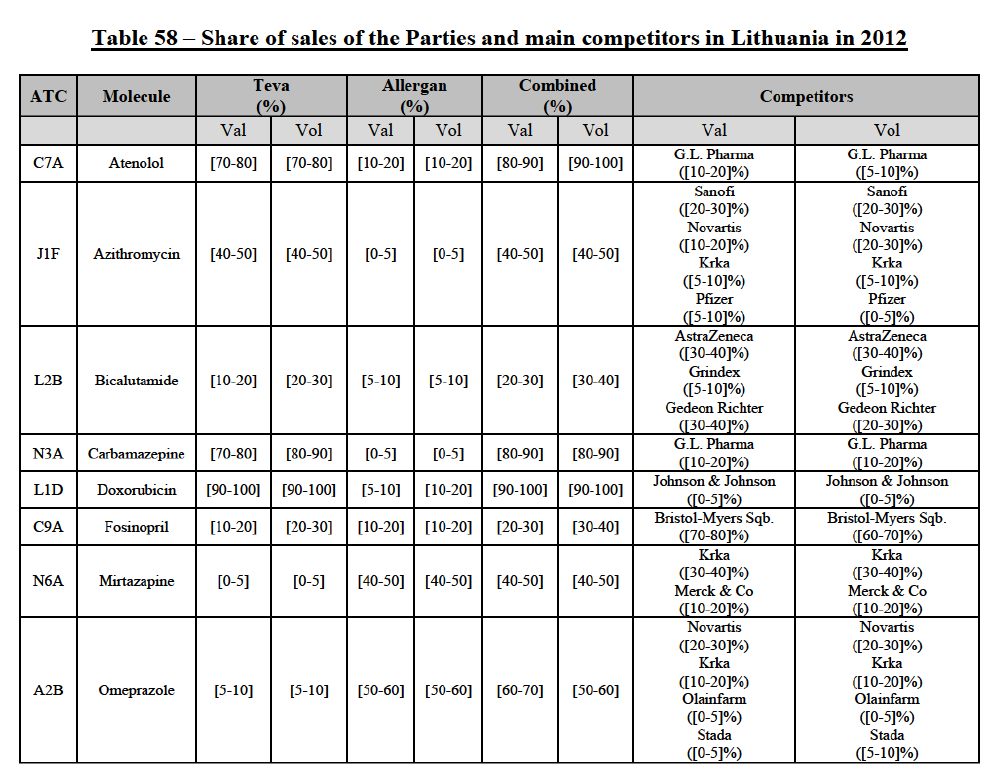
(345) The Commission presents below the competitive analysis on these markets, at molecule level, indicating also the ATC3 class to which it belongs, with a possible distinction by pharmaceutical form (at NFC 1 level) and between prescribed and OTC sales where relevant.
Atenolol (C7A)
(346) For atenolol, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [80-90]% in the last three years in value and volume. In 2014, the Parties' combined market share was [80-90]% in value and [90100]% in volume, with an increment of [5-10]-[10-20]%. Only one competitor would remain in the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market and that it would be difficult for customers to switch to alternative suppliers.234
Azithromycin (G4C)
(347) For a.ithromvcin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was increasing over the last three years, up to [60-70]% in value and [60-70]% in volume. Allergan Generics entered recently (in 2013) and Teva was leading the market. [...]. The market investigation confirmed the strong combined position of the Parties (above 60%) and indicated that the Transaction would have a negative impact in the market.235
Bicalutamide (L2B)
(348) For bicalutamide, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market shares increased over the last three years in value and volume, up to [40-50]% in volume (with an increment of [10-20]%). [...]. Only two competitors with a market share above 5% in volume and value in 2014, including the originator, would remain. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.236
Carbamazepine (N3A)
(349) For carbamazepine, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [80-90]% in the last three years in value and volume. Only one competitor with a market share above 5% would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market due to a risk of price increase.237
Doxorubicin (L1D)
(350) For doxorubicin, the Transaction gives rise to a Group 1 market at molecule level in 2012. In 2012, the Parties' combined market share was [90-100]%, with an increment of [5-10] % in value and [10-20]% in volume. In 2013 and 2014, Teva had a market share of [90-100]% in volume, while Allergan Generics did not generate sales. The fluctuation of market shares can be due to the hospitals' tendering system since doxorubicin is an oncology product sold to hospitals. Over the last three years, only one competitor generated sales, Johnson & Johnson. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.238
Fosinopril (C9A)
(351) For fosinopril, the Transaction gives rise to affected Group 1+ market at molecule level. Indeed, apart from the Parties, only one competitor, the originator Bristol-Myers, is active on this market. Both Parties hold a significant market share, [10-20]% or above in the last three years in value and volume. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market and that it would be difficult for customers to switch to alternative suppliers.239
Mirtazapine (N6A)
(352) For mirtazapine, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market shares increased over the last three years, up to [50-60]% in value and [50-60]% in volume. Only two competitors with a market share above 1% in 2014, including the originator, would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.240
Omeprazole (A2B)
(353) For omeprazole, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [50-60]% in the last three years in value and volume. In 2014, the Parties' combined market share was [50-60]% in value and [5060]% in volume, with an increment of more than 5%. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.241
Conclusion
(354) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raise serious doubts as to its compatibility with the internal market with respect to the marketing of atenolol, azithromycin, bicalutamide, carbamazepine, doxorubicin, fosinopril, mirtazapine and omeprazole in Lithuania.
IV.2.2.18.b. Pipeline generic pharmaceuticals
[...]
(355) Allergan Generics is planning to launch a generic [...](pharmaceutical form [...]), for which Teva had a [70-80]% market share in value and [70-80]% in volume in 2014 on the same pharmaceutical form, and only two other competitors with >5% share in volume were active on the market in 2012-2014.
Conclusion
(356) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the market for the marketing of [...] in Lithuania.
IV.2.2.19. Netherlands
IV.2.2.19.a. Marketed generic pharmaceuticals
(357) The following tables present the market shares of the Parties and their main competitors in 2012, 2013 and 2014 for the Group 1/1+/2 markets for which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market.
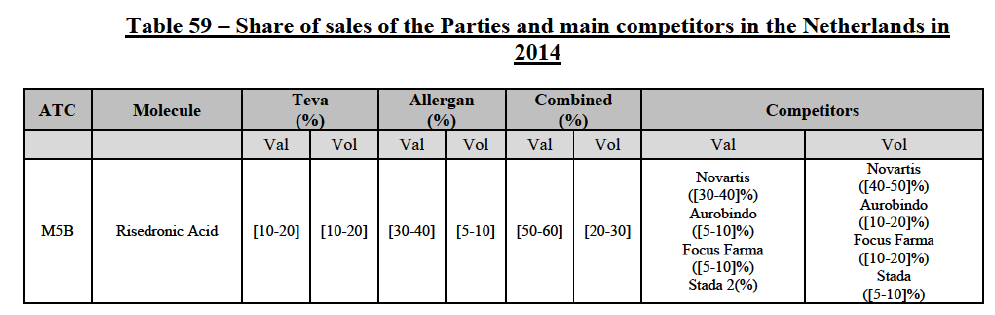
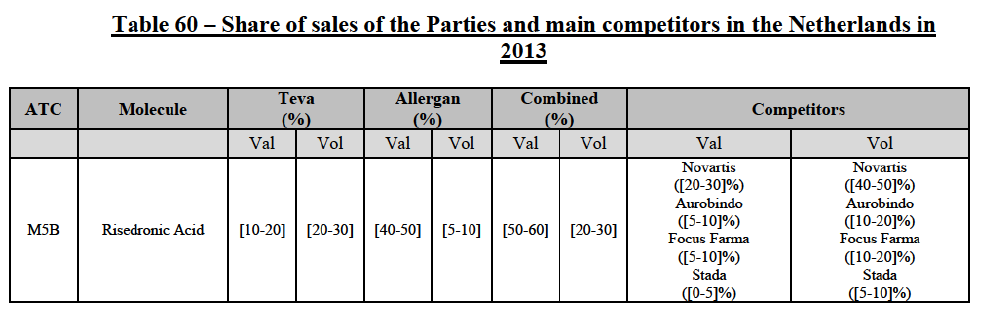
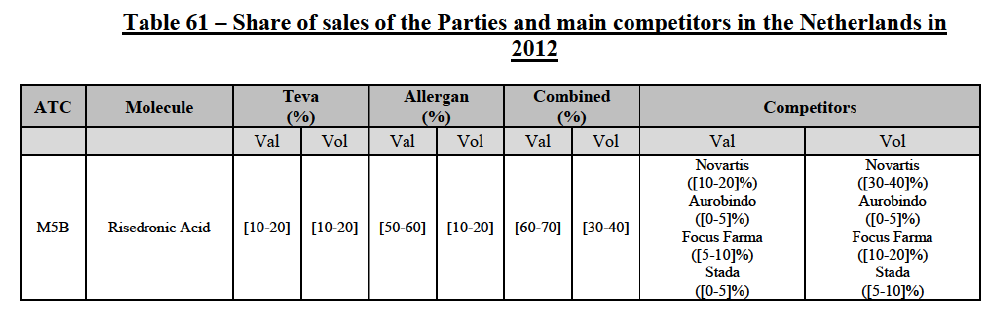
(358) The Commission presents below the competitive analysis on these markets, at molecule level, indicating also the ATC3 class to which it belongs, with a possible distinction by pharmaceutical form (at NFC 1 level) and between prescribed and OTC sales where relevant.
Risedronic Acid (M5B)
(359) For risedronic acid, the Transaction gives rise to a Group 1 market at molecule level. Allergan Generics is the originator of this molecule. The Parties' combined market share was above [50-60]% in value over the last three years. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.242
Conclusion
(360) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raise serious doubts as to its compatibility with the internal market with respect to the marketing of risedronic acid in the Netherlands.
IV.2.2.19.b. Pipeline generic pharmaceuticals
(361) Allergan Generics is planning to launch a generic [...] (pharmaceutical form [...]), which will be used for two different indications falling under ATC3 classes [...]. For the indication falling under [...], Teva had a [90-100]% market share in value and [90100]% in volume in 2014 on the same pharmaceutical form, and no other competitor with >5% share in volume was active on the market in 2012-2014. For the indication falling under [...], Teva had a [60-70]% market share in value and [60-70]% in volume in 2014, and only two competitors with >5% share in volume were active on the market in 2012-2014.
Conclusion
(362) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the market for the marketing of [...] in the Netherlands.
IV.2.2.20. Norway
IV.2.2.20. a. Marketed generic pharmaceuticals
(363) The following tables present the market shares of the Parties and their main competitors in 2012, 2013 and 2014 for the Group 1/1+/2 markets for which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market.
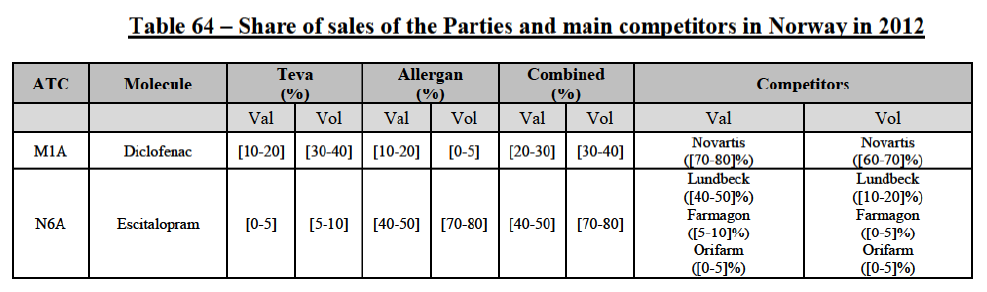
(364) The Commission presents below the competitive analysis on these markets, at molecule level, indicating also the ATC3 class to which it belongs, with a possible distinction by pharmaceutical form (at NFC1 level) and between prescribed and OTC sales where relevant.
Diclofenac (M1A)
(365) For diclofenac, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share in 2014 was [40-50]%, with only one other competitor, the originator, remaining on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market due to limited alternative suppliers.243
Escitalopram (N6A)
(366) For escitalopram the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market shares increased over the last three years, up to [40-50]% in value and [80-90]% in volume in 2014. Only one competitor with a market share above 5% in volume, the originator, would remain on the market. The average prices reported by IMS for this molecule would have increased by [90-100]% between 2012 and 2014. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market due to limited alternative suppliers.244
Conclusion
(367) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raise serious doubts as to its compatibility with the internal market with respect to the marketing of diclofenac and escitalopram in Norway.
IV.2.2.20.b. Pipeline generic pharmaceuticals
(368) Allergan Generics is planning to launch a generic [...] (pharmaceutical form [...]), for which Teva had a [90-100]% market share in value and [90-100]% in volume in 2014, and only one other competitor with >5% share in volume was active on the market in 2012-2014.
Conclusion
(369) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the market for the marketing of [...] in Norway.
IV.2.2.21. Poland
IV.2.2.21.a. Marketed generic pharmaceuticals
(370) The following tables present the market shares of the Parties and their main competitors in 2012, 2013 and 2014 for the Group 1/1+/2 markets for which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market.
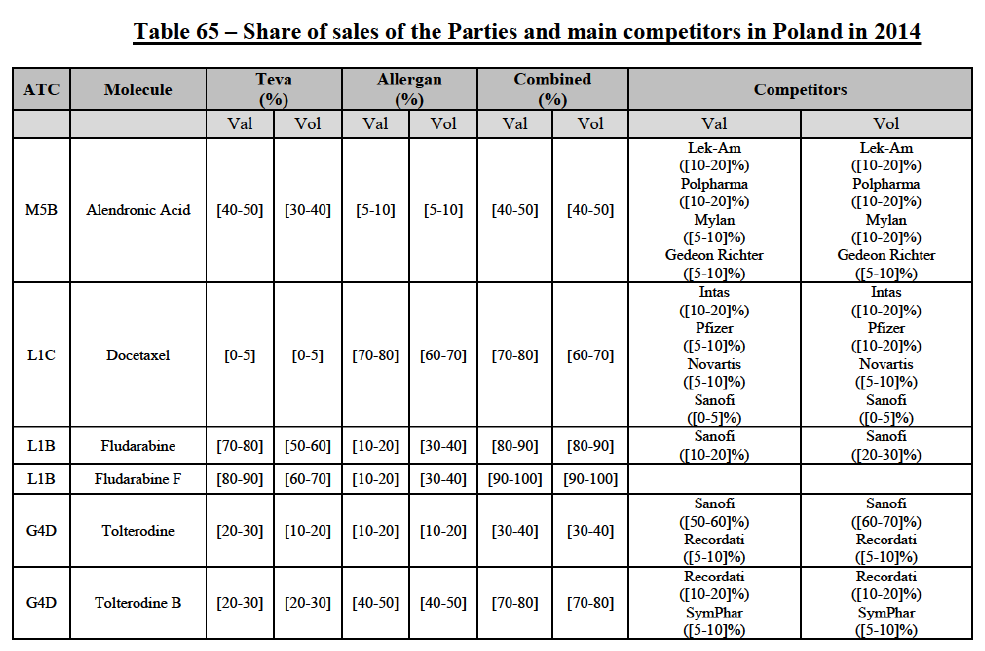
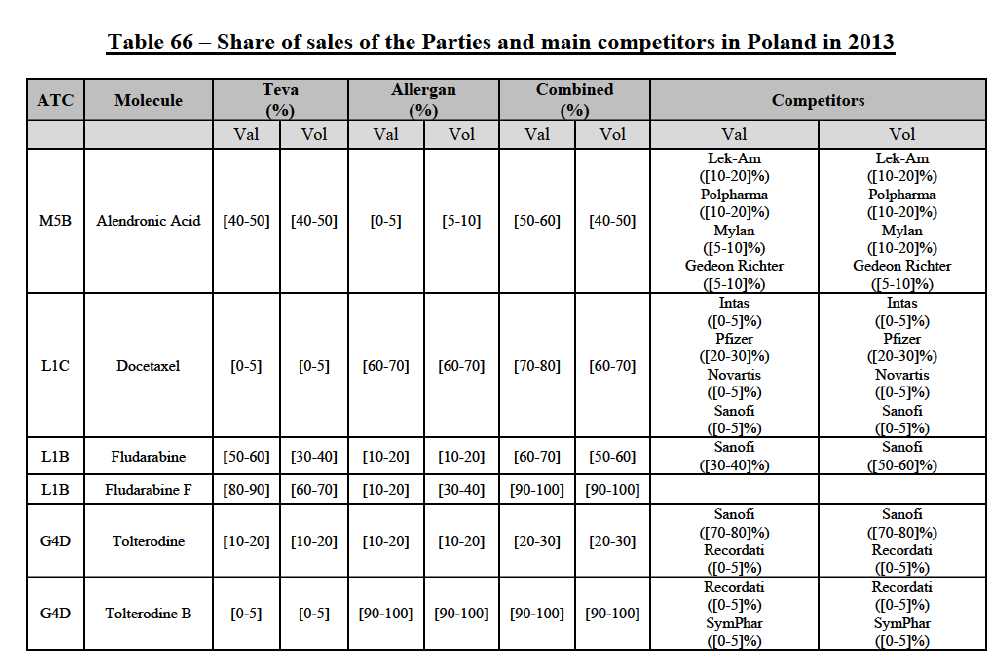
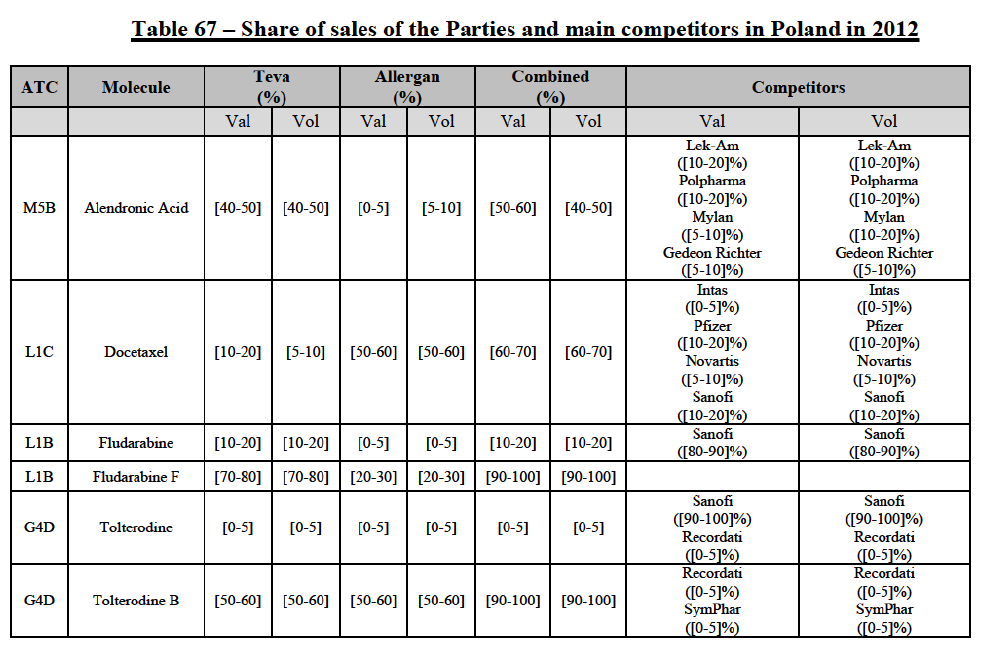
(371) The Commission presents below the competitive analysis on these markets, at molecule level, indicating also the ATC3 class to which it belongs, with a possible distinction by pharmaceutical form (at NFC 1 level) and between prescribed and OTC sales where relevant.
Alendronic Acid (M5B)
(372) For alendronic acid, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [50-60]% in value in 2012 and 2013 and [40-50]% in 2014, with an increment above 5%. Furthermore, the market investigation confirmed the strong combined position of the Parties (above [50-60]%) and indicated that the Transaction would have a negative impact in the market.245
Docetaxel (L1C)
(373) For docetaxel, the Transaction gives rise to a Group 1 market in 2012 and 2013. In 2014, Allergan Generics had a market share of [70-80]% in value and [60-70]% in volume, while Teva did not generate any sales. However, in 2012 and 2013, the Parties' combined market share was above [60-70]% in value and volume, with an increment from Teva's market share up to [10-20]%. The fluctuation of market shares may be the result of the hospitals' tendering system since docetaxel is an oncology product sold to hospitals. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.246
Fludarabine (L1B)
(374) For fludarabine, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share increased over the last three years, up to [80-90]% in value and 80% in volume, with a significant increment ([10-20]% in value and [3040]% in volume). Only one competitor with a market share above 1% would remain on the market. At the level of pharmaceutical form F, the Parties' would have a combined market share of [90-100]% (with an increment of [10-20]% in value and [30-40]% in volume). The average prices reported by IMS for fludarabine in pharmaceutical form F would have increased by more than 140% between 2012 and 2014. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.247
Tolterodine (G4D)
(375) For tolterodine, the Transaction gives rise to a Group 1 market at molecule level. Allergan Generics recently entered the market (in 2013). The Parties' combined market share increased over the last years, up to [30-40]% in value and [30-40]% in volume, with an increment of more than [10-20]%. Only two competitors would remain with a market share above 5%. At the level of pharmaceutical form B, the Parties' combined market share was >[90-100]% in 2012 and 2013 and more than [70-80]% ([70-80]% in value and [70-80]% in volume) with a significant increment ([20-30]% in value and [20-30]% in volume) in 2014, while only one competitor with a market share above 5% would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.248
Conclusion
(376) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raise serious doubts as to its compatibility with the internal market with respect to the marketing of alendronic acid, docetaxel, fludarabine and tolterodine in Poland.
IV.2.2.21.b. Pipeline generic pharmaceuticals
(377) Allergan Generics is planning to launch a generic [...] (pharmaceutical form [...]), for which Teva had a [50-60]% market share in value and [70-80]% in volume in 2014, and only two other competitors with >5% share in volume were active on the market in 2012-2014.
Conclusion
(378) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the market for the marketing of [...] in Poland.
IV.2.2.22. Portugal
IV.2.2.22.a. Marketed generic pharmaceuticals
(379) The following tables present the market shares of the Parties and their main competitors in 2012, 2013 and 2014 for the Group 1/1+/2 markets for which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market.
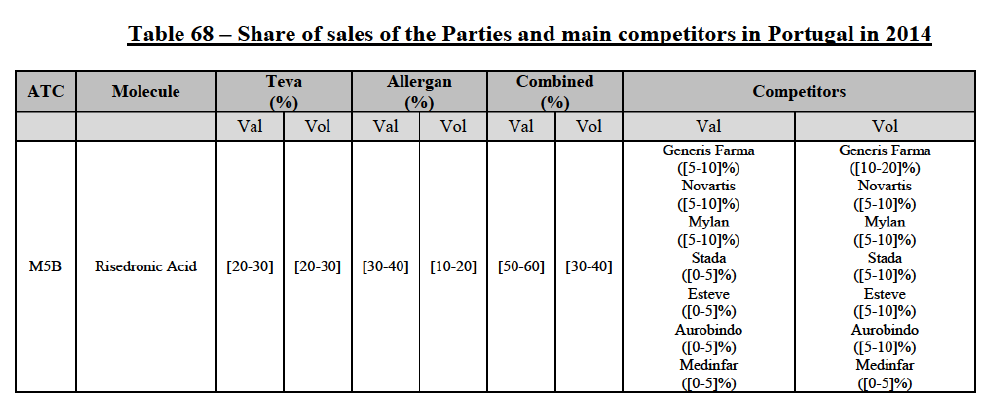
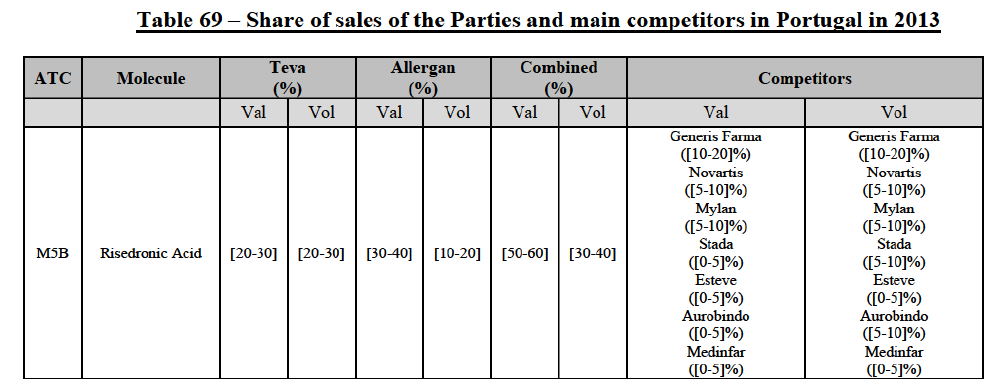
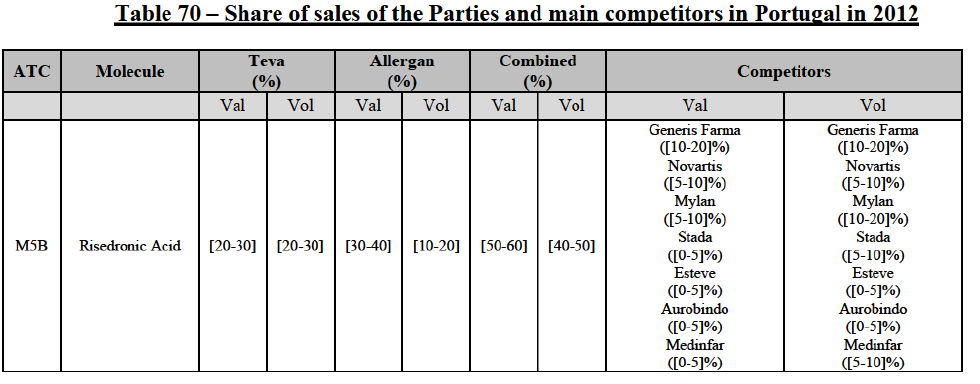
(380) The Commission presents below the competitive analysis on these markets, at molecule level, indicating also the ATC3 class to which it belongs, with a possible distinction by pharmaceutical form (at NFC1 level) and between prescribed and OTC sales where relevant.
Ris edronic Acid (M5B)
(381) For risedronic acid, the Transaction gives rise to Group 1 market at molecule level. Allergan Generics is the originator of this molecule. The Parties' combined market share was above [50-601% in value over the last three years, and all other competitors had a market share below [10-201% in value in 2014. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position 249
Conclusion
(382) In view of the above and of ail the evidence available to the Commission, the Commission considers that the Transaction raise serious doubts as to its compatibility with the internal market with respect to the marketing of risedronic acid in Portugal.
IV.2.2.22.b. Pipeline generic pharmaceuticals
(383) Allergan Generics is planning to launch a generic [...] (pharmaceutical form [...]), for which Teva had a [30-40]% market share in value and [90-100]% in volume in 2014 on the same pharmaceutical form, and only one other competitor with >5% share in volume was active on the market in 2012-2014.
Conclusion
(384) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the market for the marketing of [...] in Portugal.
IV.2.2.23. Romania
IV.2.2.23.a. Marketed generic pharmaceuticals
(385) The following tables present the market shares of the Parties and their main competitors in 2012, 2013 and 2014 for the Group 1/1+/2 markets for which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market.
![]()
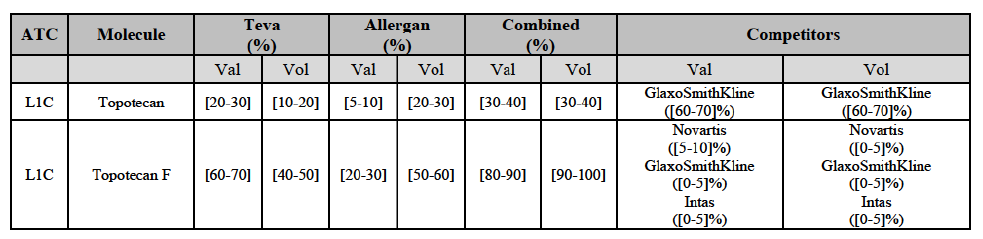
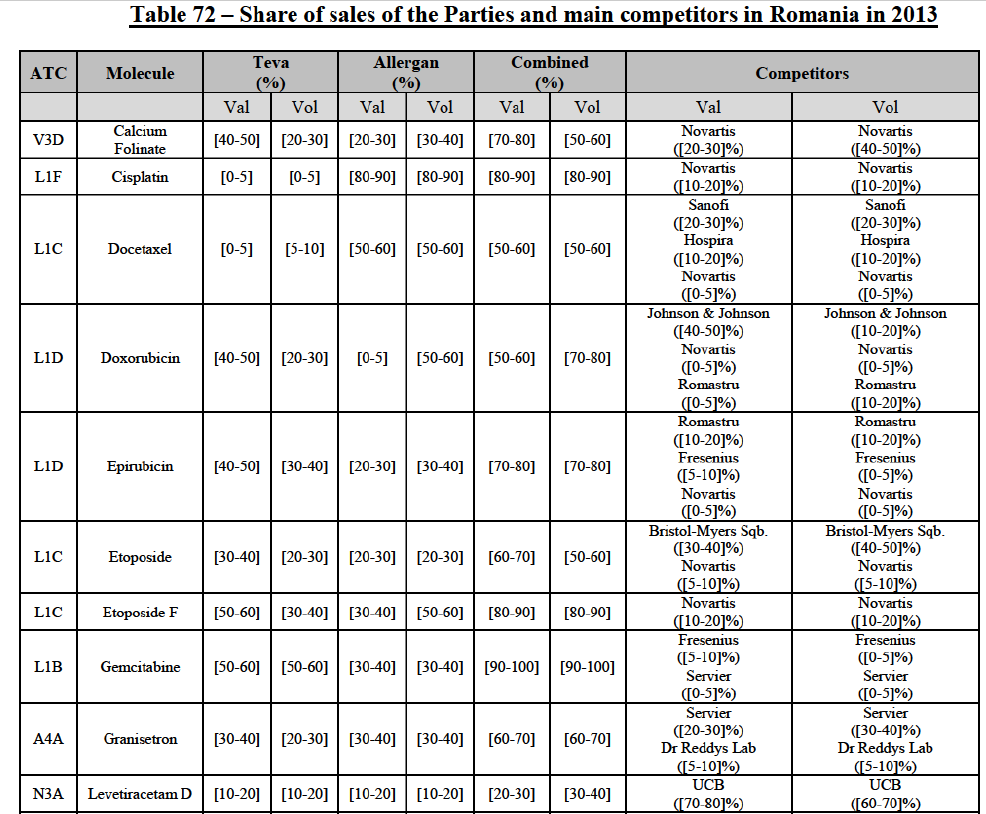
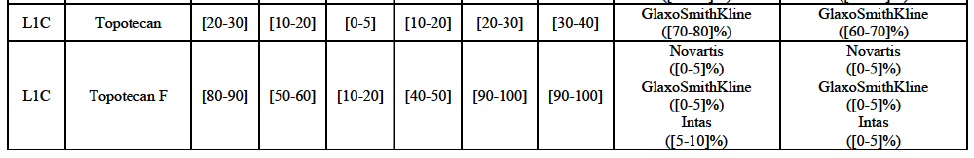
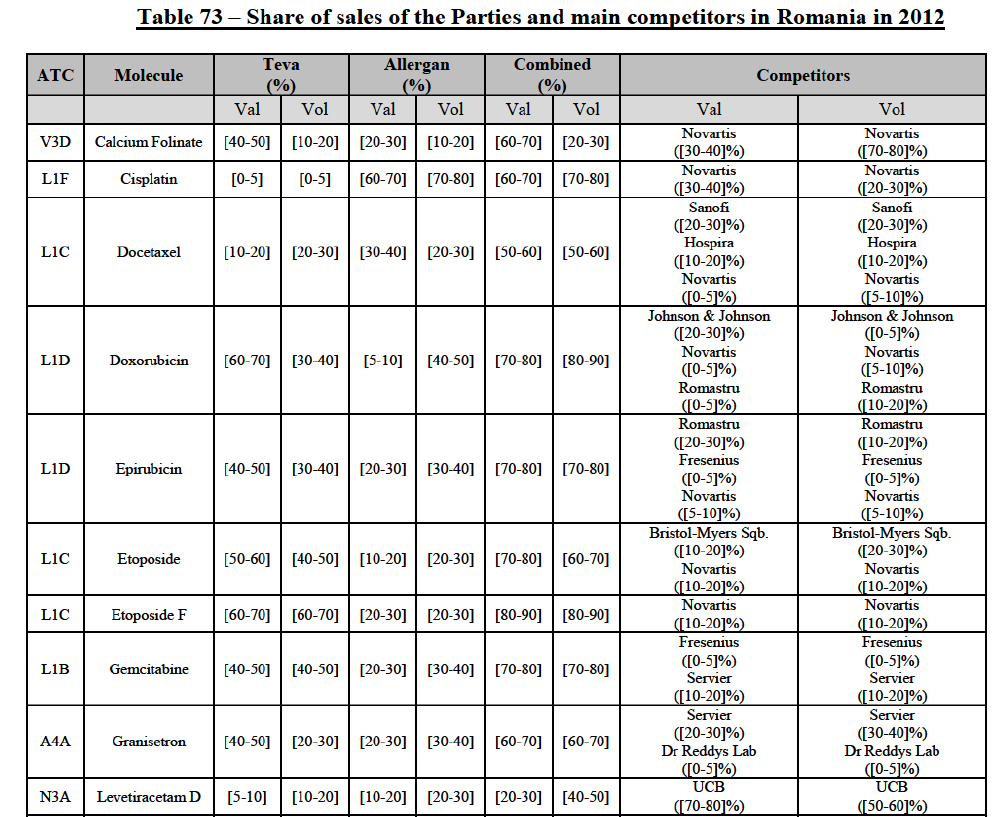
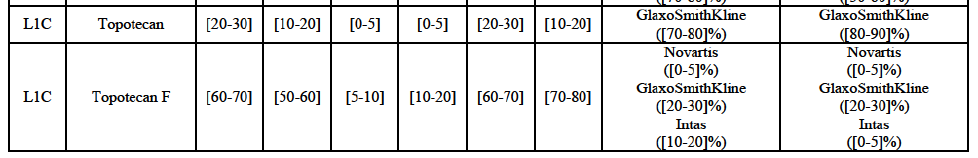
(386) The Commission presents below the competitive analysis on these markets, at molecule level, indicating also the ATC3 class to which it belongs, with a possible distinction by pharmaceutical form (at NFC 1 level) and between prescribed and OTC sales where relevant.
Calcium folinate (V3D)
(387) For calcium folinate, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [50-60]% in the last three years in value (up to [70-80]% in 2013), and only one competitor with a market share above 5% would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.250
Cisplatin (L1F)
(388) For cisplatin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market shares increased over the last three years, up to [90-100]% in value and [90-100]% in volume in 2014, and only one competitor with a market share above 5% would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.251
Docetaxel (L1C)
(389) For docetaxel, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market shares increased over the last three years, up to [80-90]% in value and [70-80]% in volume in 2014, and only two competitors with a market share above 5% in value and volume would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.252
Doxorubicin (L1D)
(390) For doxorubicin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [50-60]% in voume over the last three years and only two competitors with a market share above 5% would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.253
Epirubicin (L1D)
(391) For epirubicin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [70-80]% in value and volume over the last three years and only two competitors with a market share above 5% would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.254
Etoposide (L1C)
(392) For etoposide, the Transaction gives rise to a Group 1 market at molecule level. Over the last three years, the Parties' combined market share was above [70-80]% in value and volume at molecule level, and above [80-90]% in value and volume at the level of the pharmaceutical form F, while only one competitor with a market share above 5% would remain on the market. Furthermore, the market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.255
Gemcitabine (L1B)
(393) For gemcitabine, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market shares increased over the last three years, up to [90-100]% in value and [90-100]% in volume in 2014, and only one competitor with a market share above 5% in value and volume would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.256
Granisetron (A4A)
(394) For granisetron, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market shares increased over the last three years, up to [70-80]% in value and [70-80]% in volume in 2014, and only two competitors with a market share above 5% in value and volume would remain on the market. The market investigation indicated that the Transaction would have a negative impact in the market.257
Levetiracetam (N3A)
(395) For levetiracetam, the Transaction gives rise to a Group 2 market at the level of the pharmaceutical form D. While the Parties' combined market share was moderate over the last three years (up to [30-40]% in volume in 2014), the Parties are the only two generics and only one competitor (the originator) would remain on the market. Furthermore, the market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.258
Topotecan (L1C)
(396) For topotecan, the Transaction gives rise to affected Group 1 market at molecule level. At the level of the pharmaceutical form F, the Parties' combined market share was >[90100]% in volume in 2013 and 2014. Furthermore, the market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.259
Conclusion
(397) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raise serious doubts as to its compatibility with the internal market with respect to the marketing of calcium folinate, cisplatin, docetaxel, doxorubicin, epirubicin, etoposide, gemcitabine, granisetron, levetiracetam and topotecan in Romania.
IV.2.2.23.b. Pipeline generic pharmaceuticals
(398) Allergan Generics is planning to launch a generic [...] (pharmaceutical form [...]), for which Teva had a [30-40]% market share in value and [40-50]% in volume in 2014 on the same pharmaceutical form, but only one other competitor was active on the market in 2012-2014.
(399) Allergan Generics is planning to launch a generic [...] (pharmaceutical form [...]), for which Teva had a >[90-100]% market share in volume ([70-801% in value) in 2014, and only one other competitor was active on the market in 2012-2014.
Conclusion
(400) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the market for the marketing of [...] and [...] in Romania.
IV.2.2.24. Slovakia
IV.2.2.24.a. Marketed generic pharmaceuticals
(401) The following tables present the market shares of the Parties and their main competitors in 2012, 2013 and 2014 for the Group 1/1+/2 markets for which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market.
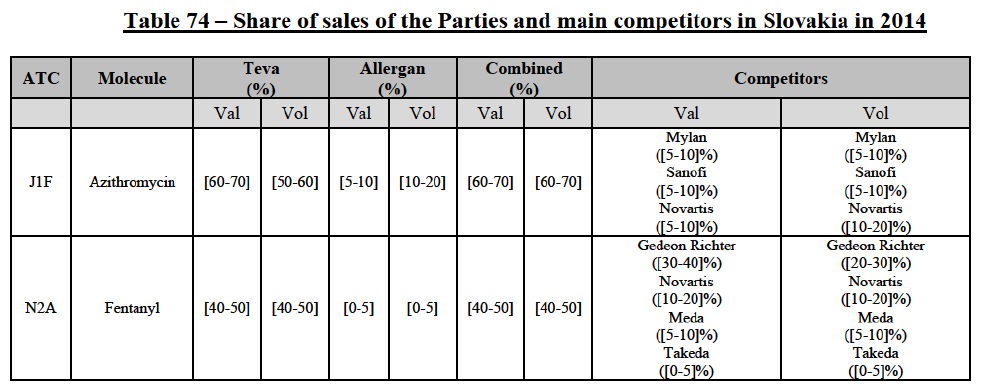
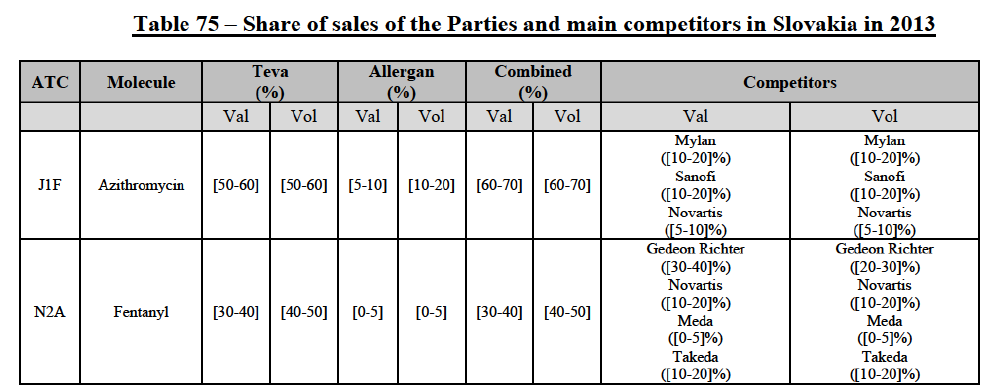
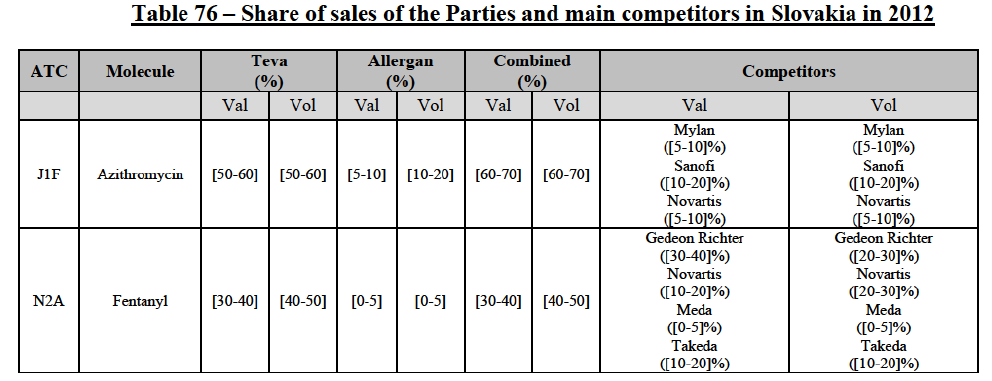
(402) The Commission presents below the competitive analysis on these markets, at molecule level, indicating also the ATC3 class to which it belongs, with a possible distinction by pharmaceutical form (at NFC1 level) and between prescribed and OTC sales where relevant.
Azithromycin (J1F)
(403) For azithromycin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [60-70]% in value and volume over the last three years (up to [70-80]% in value and [60-70]% in volume in 2014). Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.260
Fentanyl (N2A)
(404) For fentanyl, the Transaction gives rise to a Group 1 market at molecule level. Over the last three years, the Parties' combined market shares increased up to [40-50]% in value and [50-60]% in volume in 2014. Only three competitors with a combined market share above 5% would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the rnarket.261
(405) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the marketing of a.ithromvcin and fentanvl in Slovakia.
IV.2.2.24.b. Pipeline generic pharmaceuticals
(406) The Transaction does not raise serious doubts as to its compatibility with the internal market with respect to pipeline generic pharmaceuticals in Slovakia.
IV.2.2.25. Slovenia
IV.2.2.25.a. Marketed generic pharmaceuticals
(407) The following tables present the market shares of the Parties and their main competitors in 2012, 2013 and 2014 for the Group 1/1+/2 markets for which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market.
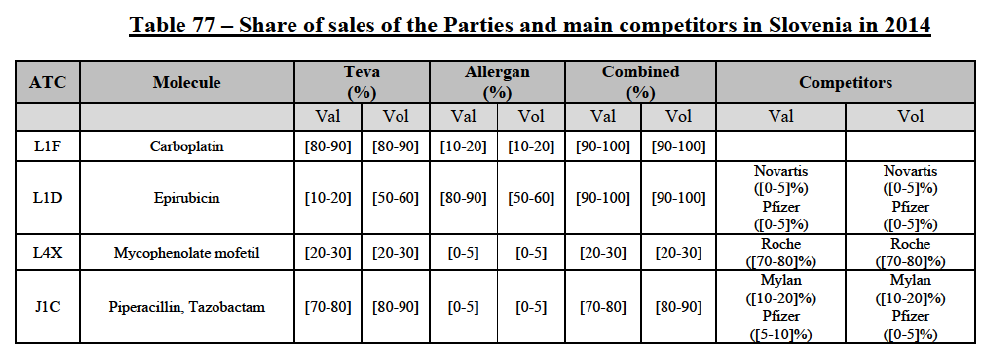
(408) The Commission presents below the competitive analysis on these markets, at molecule level, indicating also the ATC3 class to which it belongs, with a possible distinction by pharmaceutical form (at NFC1 level) and between prescribed and OTC sales where relevant.
Carboplatin (L1F)
(409) For carboplatin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was [90-1001% in value and volume over the last three years. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.262
Epiriibicin (L1D)
(410) For epirubicin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was [90-1001% in value in 2014. No competitor with a market share above 5% would remain on the market (based on 2013-2014 figures). Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market 263
Mycophenolate mofetil (L4À)
(411) For mycophenolate mofetil, the Transaction gives rise to a Group 1+ market at molecule level. The Parties' products are the only generics on the market, and only one other competitor, the originator, would remain on the market. Furthermore, the market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties are the only generics on the market.264
Piperacillin/tazobactam (11 C)
(412) For piperacillin/tazobactam, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market shares increased over the last three years up to [70-80]% in value and [80-90]% in volume in 2014. Only one competitor with a market share above 5% in value and volume would remain on the market (based on 2014 figures). Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.265
Conclusion
(413) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the marketing of carboplatin, epirubicin, mycoplrenolate nrofetil and piperacillin/ta:obactam in Slovenia.
IV.2.2.25.b. Pipeline generic pharmaceuticals
(414) The Transaction does not raise serious doubts as to its compatibility with the internal market with respect to pipeline generic pharmaceuticals in Slovenia.
IV.2.2.26. Spain
IV.2.2.26.a. Marketed generic pharmaceuticals
(415) The following tables present the market shares of the Parties and their main competitors in 2012, 2013 and 2014 for the Group 1/1+/2 markets for which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market.
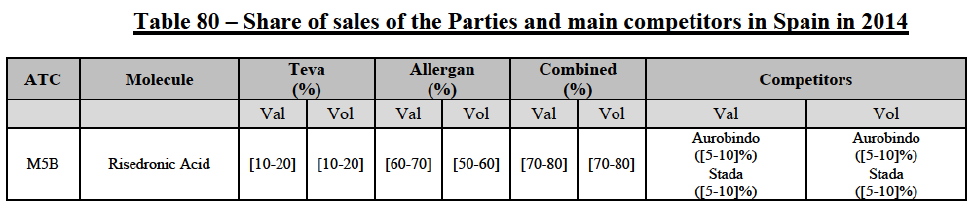
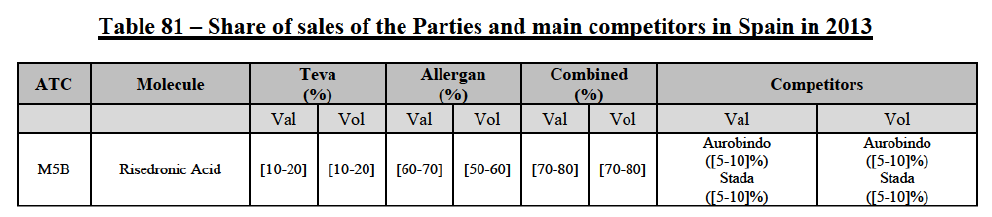
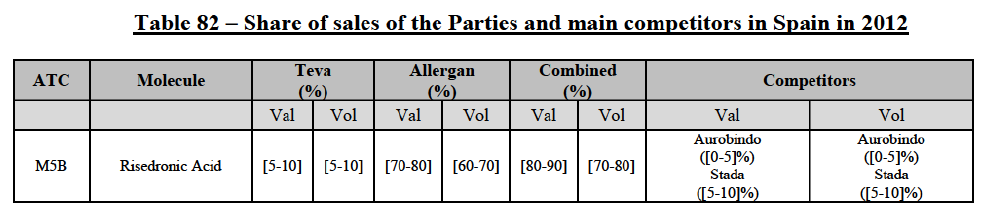
(416) The Commission presents below the competitive analysis on these markets, at molecule level, indicating also the ATC3 class to which it belongs, with a possible distinction by pharmaceutical form (at NFC1 level) and between prescribed and OTC sales where relevant.
Rssedronic Acid (M5B)
(417) For risedronic acid, the Transaction gives rise to a Group 1 market at molecule level. Allergan Generics is the originator of the molecule. The Parties' combined market share was above [70-80]% in value and volume over the last three years and only two competitors with a market share above 5% would remain on the market. Furthermore, the market investigation indicated that there would be a negative impact of the Transaction in the market.266
Conclusion
(418) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raise serious doubts as to its compatibility with the internal market with respect to the marketing of risedronic acid in Spain.
IV.2.2.26.b. Pipeline generic pharmaceuticals
(419) The Transaction does not raise serious doubts as to its compatibility with the internal market with respect to pipeline generic pharmaceuticals in Spain.
IV.2.2.27. Sweden
IV.2.2.27.a. Marketed generic pharmaceuticals
(420) The following tables present the market shares of the Parties and their main competitors in 2012, 2013 and 2014 for the Group 1/1+/2 markets for which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market.
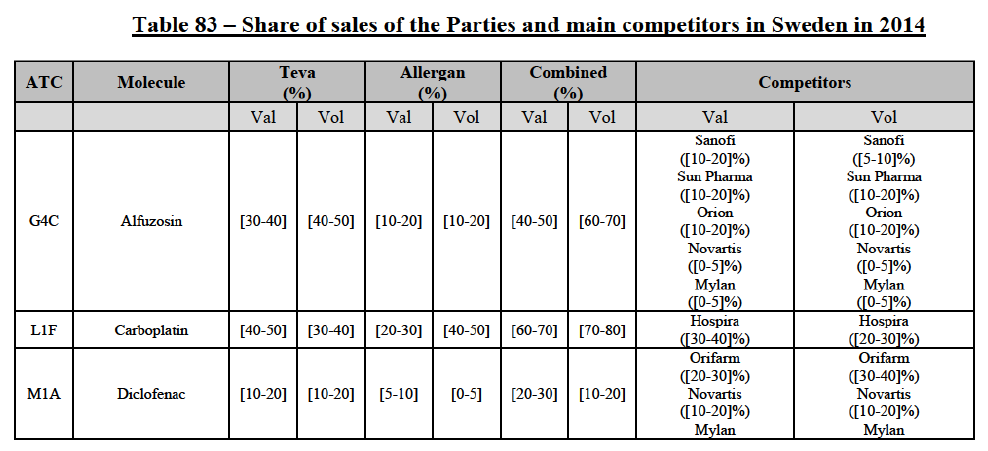
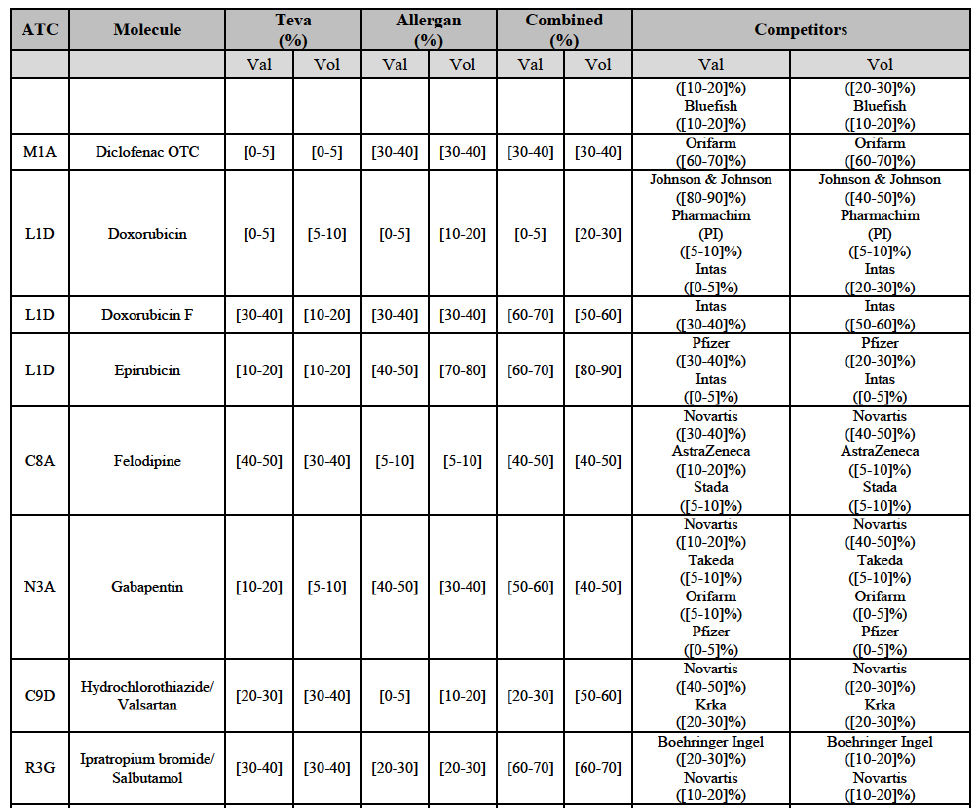
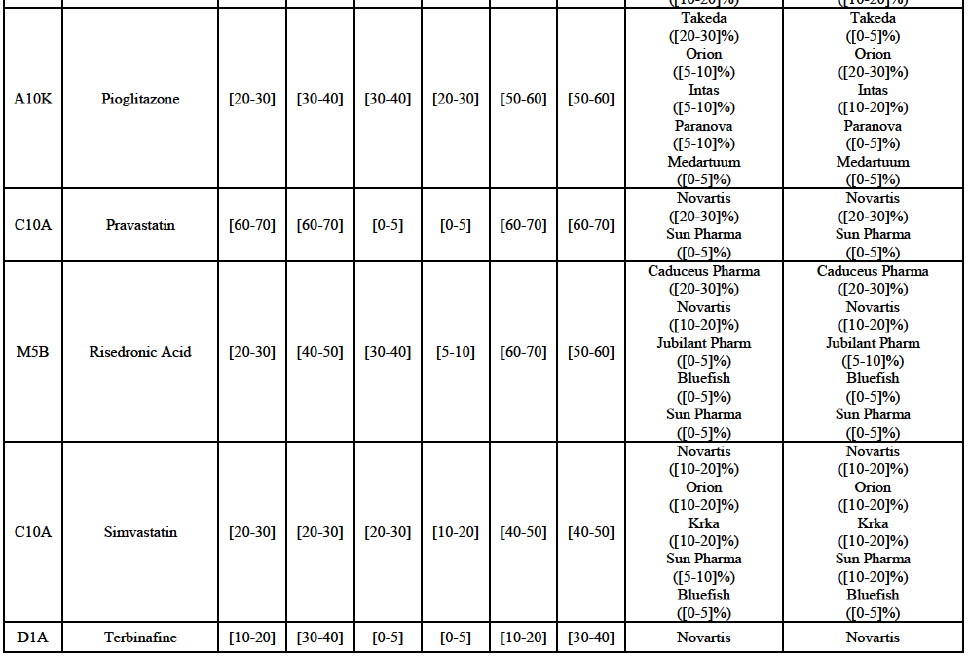
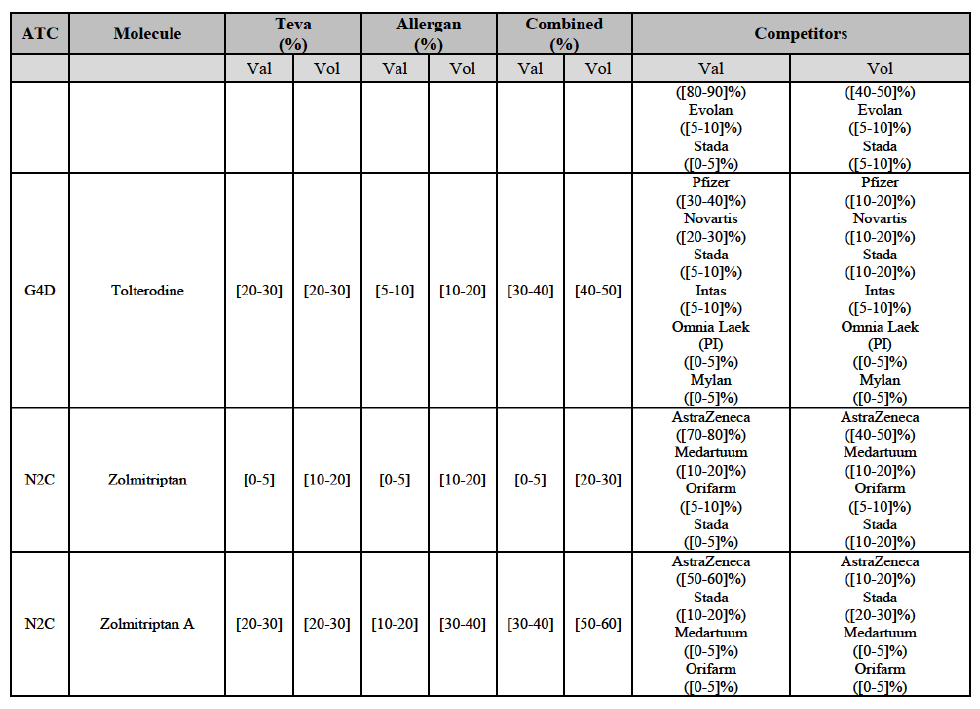
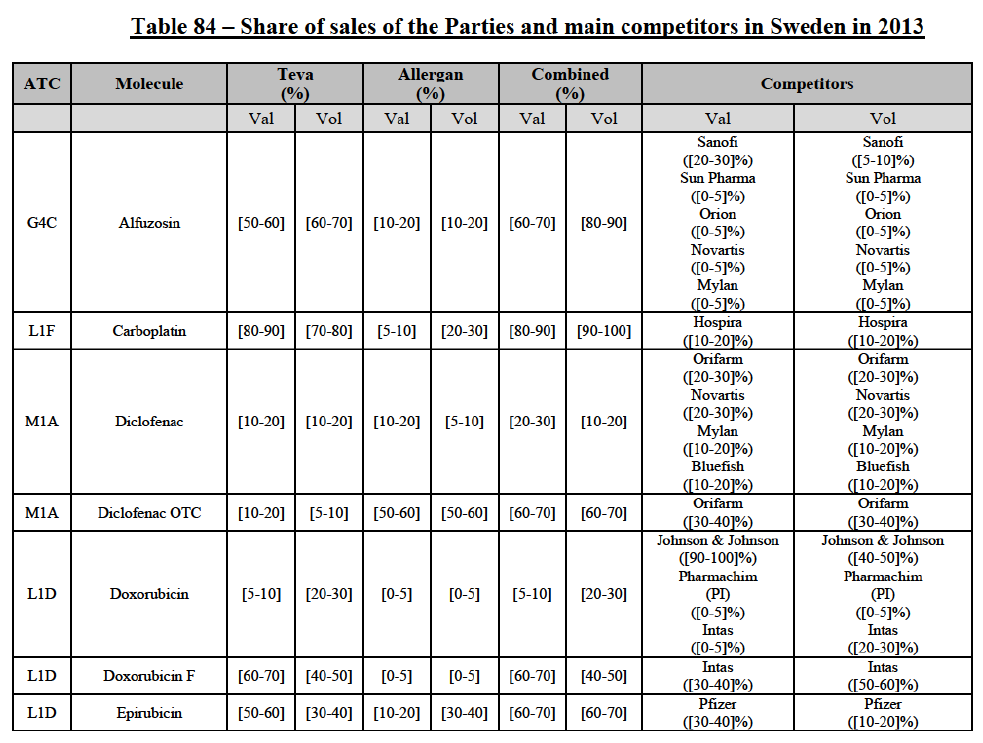
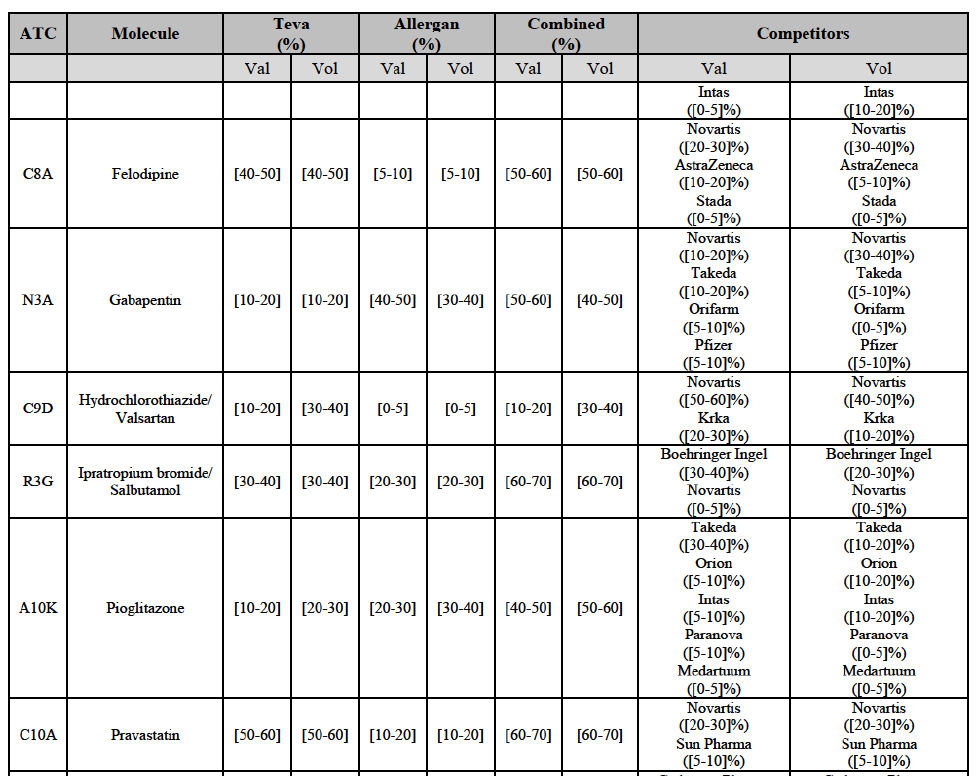
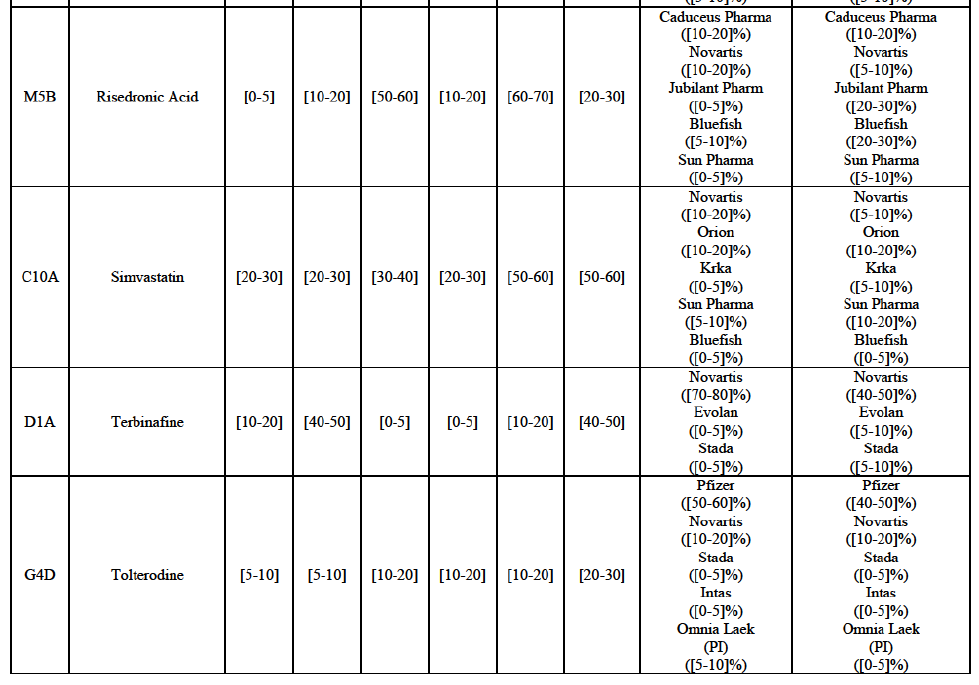
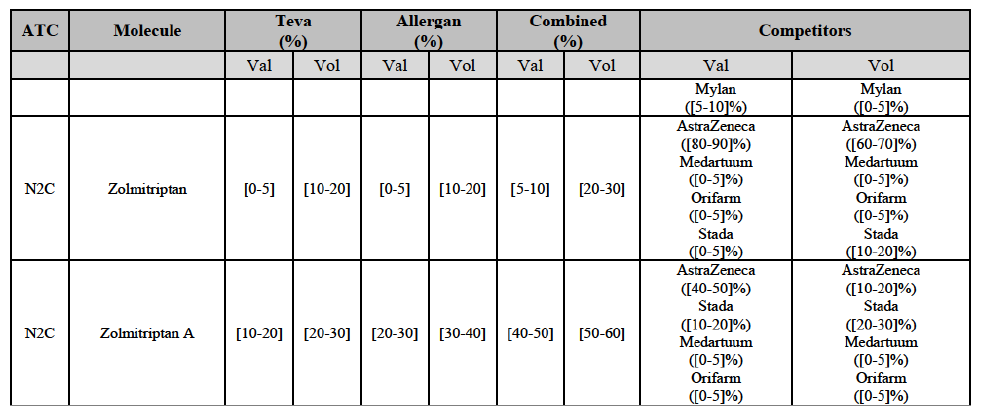
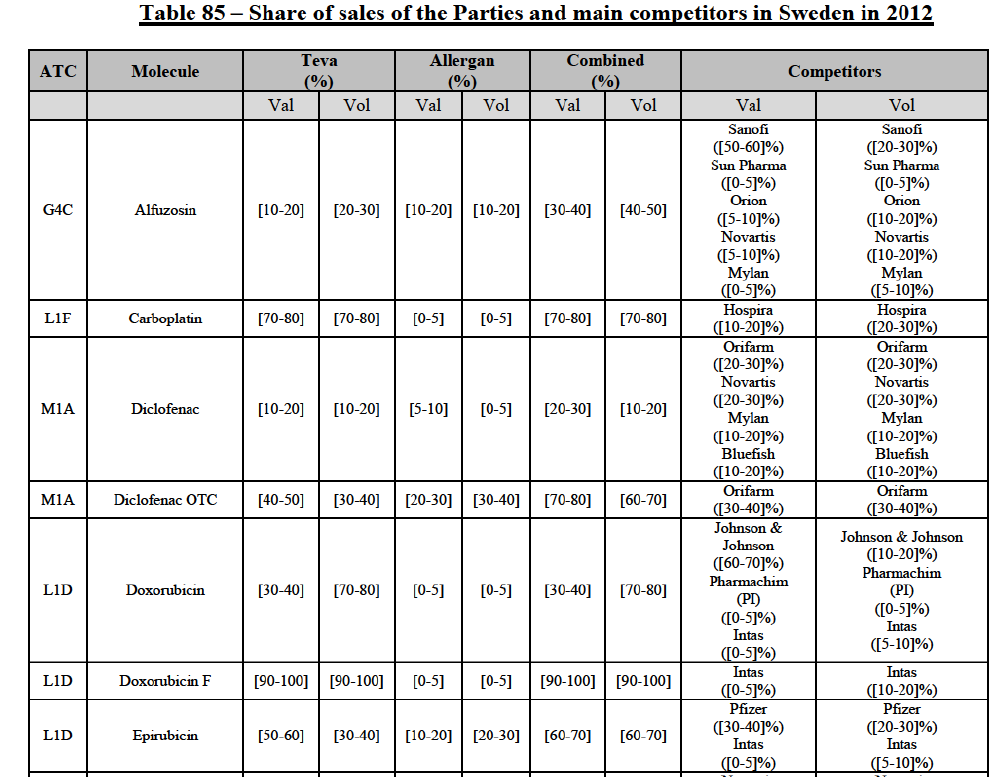
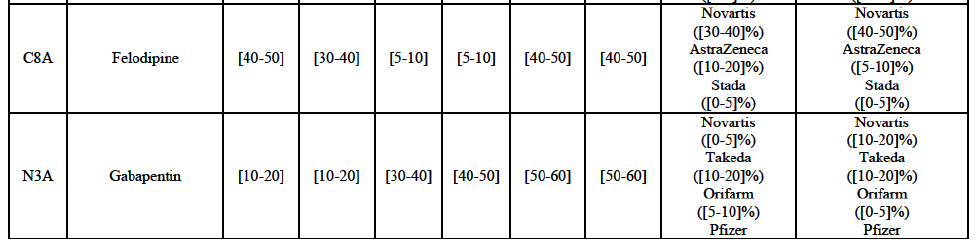
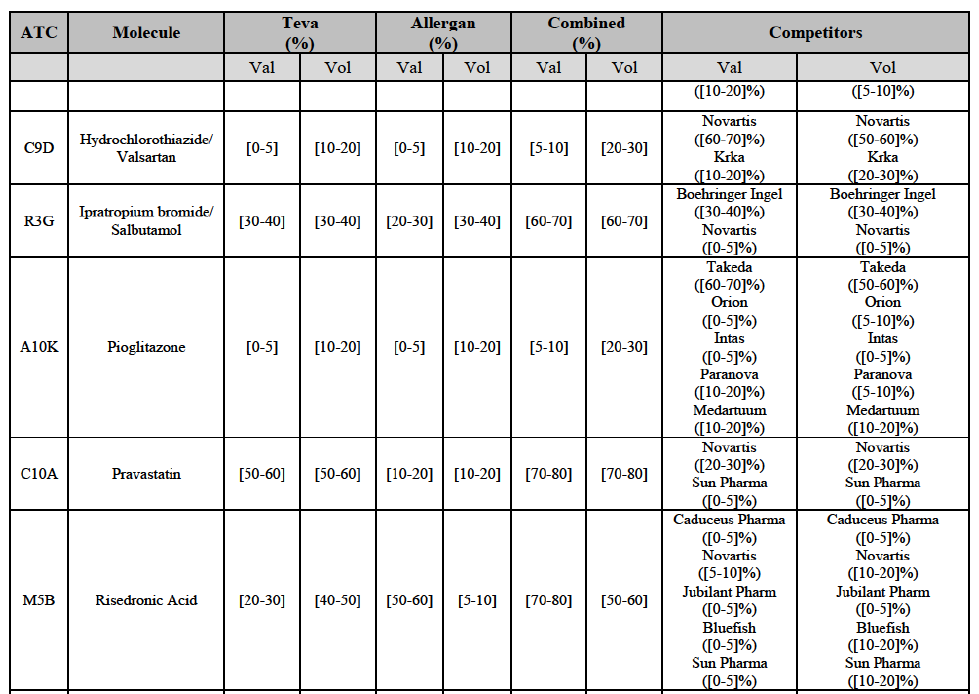
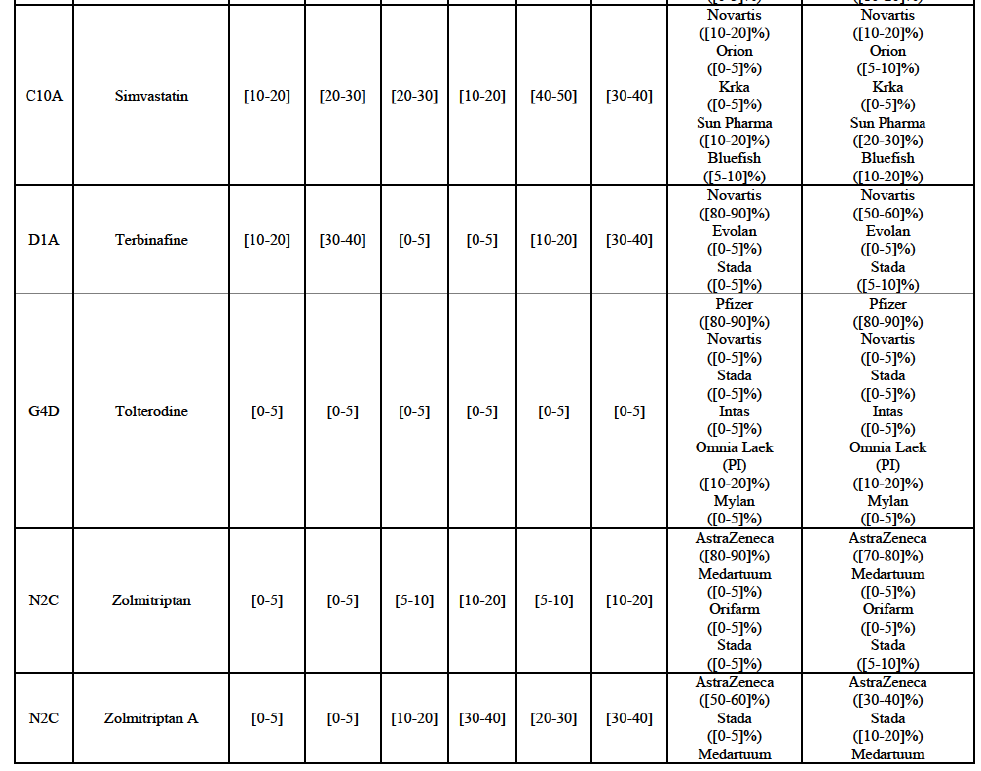
(421) The Commission presents below the competitive analysis on these markets, at molecule level, indicating also the ATC3 class to which it belongs, with a possible distinction by pharmaceutical form (at NFC1 level) and between prescribed and OTC sales where relevant.
Alfiizosin (G4C)
(422) For alfuzosin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [60-701% both in value and volume in 2013 and 2014. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.267
Carboplatin (L1F)
(423) For carboplatin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [70-801% in value over the last three years. Only one competitor with a market share above 5% would remain on the market. Furthermore, the market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.268
Diclofenac (M1A)
(424) For diclofenac, the Transaction gives rise to a Group 1 market at molecule level. Only one competitor with a market share above 5% would remain on the OTC segment of the market, and the market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.269
Doxorribicin (L1D)
(425) For doxorubicin, the Transaction gives rise to a Group 1 market at the level of the pharmaceutical form F. The Parties' combined market share was above [50-601% in volume and value over the last three years and only one competitor with a market share above 5% would remain on the market. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.270
Epirubicin (L1D)
(426) For epirubicin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [60-70]% in value and volume, and only one competitor with a market share above 5% would remain on the market. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.271
Felodipine (C8A)
(427) For felodipine, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [50-60]% in value and volume in 2013. Only two competitors with a market share above 5% were on the market in 2012-2013. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.272
Gabapentin (N3A)
(428) For gabapentin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [50-60]% in value over the last three years. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.273
Hydrochlorothiazide/valsartan (C9D)
(429) For hydrochlorothiazide/valsartan, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was [50-60]% in volume in 2014, and only two competitors with a market share above 5% would remain on the market. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.274
Ipratropium bromide/salbutamol (R3G)
(430) For ipratropium bromide/salbutamol, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [60-70]% in value and volume over the last three years and only two competitors with a market share above 5% would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market.275
Pioglitazone (A10K)
(431) For pioglitazone, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market shares increased over the last three years, up to [50-60]% in value and [50-60]% in volume. The market investigation did not provide any éléments to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.276
Pravastatin (C10A)
(432) For pravastatin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [60-70]% in value and volume over the last three years and only one competitor with a market share above 5% (two in 2013) would remain on the market. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.277
Risedronic Acid (M5B)
(433) For risedronic acid, the Transaction gives rise to a Group 1 market at molecule level. Allergan Generics is the originator of this molecule. The Parties' combined market share was above [60-70]% in value over the last three years. Only two competitors with a market share above 5% in value (three in 2013) would remain on the market. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.278
Simvastatin (C10A)
(434) For simvastatin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [50-60]% in value and volume in 2013. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.279
Terbinafine (D1A)
(435) For terbinafine, the Transaction gives rise to a Group 1+ market at molecule level, with Allergan Generics being a recent entrant in the market. Only two competitors with a market share above 5% in value would remain on the market (including the originator, which was the only competitor with a market share above 5% in value in 2012-2013). The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.280
Tolterodine (G4D)
(436) For tolterodine, the Transaction gives rise to a Group 1 market at molecule level. The Parties recently entered and achieved in two years a combined market share of [3040]% in value and [40-50]% in volume. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.281
Zolmitriptan (N2C)
(437) For zolmitriptan, the Transaction gives rise to a Group 1 market at the level of the pharmaceutical form A. The Parties' combined market share was [50-60]% in volume in 2014, and only two competitors with a market share above 5% would remain on the market. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.282
Conclusion
(438) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raise serious doubts as to its compatibility with the internal market with respect to the marketing of alfuzosin, carboplatin,
diclofenac, doxorubicin, epirubicin, felodipine, gabapentin,
hydrochlorothiazide/valsartan, ipratropium bromide/salbutamol, pioglitazone, pravastatin, risedronic acid, simvastatin, terbinafine, tolterodine and zolmitriptan in Sweden.
IV.2.2.27.b. Pipeline generic pharmaceuticals
[...]
(439) Teva is planning to launch a generic [...] (pharmaceutical form [...]), for which Allergan Generics had a [50-60]% market share in value and [50-60]% in volume in 2014, and only two other competitors were active on the market in 2012-2014.
Conclusion
(440) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raise serious doubts as to its compatibility with the internal market with respect to the market for the marketing of [...] in Sweden.
IV.2.2.28. United Kingdom
(441) In relation to the United Kingdom, the Notifying Party provided estimates of the market shares of the Parties and their competitors using a combination of the IMS and British Generic Manufacturers Association ("BGMA") datasets.
(442) An important limitation of market share data as reported by IMS is that, according to the Parties, IMS does not use actual gross or net sales from generic manufacturers, but instead multiplies volume sales by the drug tariff price (the reimbursement value that a pharmacist receives from the government when they dispense a product), and removes the standard wholesaler margin. This does not lead to obtaining net sales figures for manufacturers, because it does not remove the pharmacist's margin,283 which is likely to depend on the product and on the customer.
(443) Furthermore, in the United Kingdom, IMS data does not enable to reconstruct fully the markets, in particular because an important part of generics sales is not broken down by company and is aggregated as "Lab Unknown". Since a number of generic companies in the United Kingdom (such as the Parties, Ranbaxy, Sandoz, Wockhardt and Sanofi) report their monthly volume sales of company branded generics to BGMA, the Parties supplemented the IMS sales data with the data reported by certain members of BGMA.284 Since a number of generic manufacturers (such as Mylan) do not report their sales to BGMA, for many markets, a significant share is still attributed to "Others", which the Notifying Party was not able to split by company/ies. In light of the absence of available data, the Commission has taken a conservative approach by assuming that the competitive constraint posed by "Others" was limited (and has not included them in the below market share tables).
(444) The market shares provided below should be assessed in light of these methodological limitations.
IV.2.2.28.a. Marketed generic pharmaceuticals
(445) The following tables present the market shares of the Parties and their main competitors in 2012, 2013 and 2014 for the Group 1/1+/2 markets for which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market.
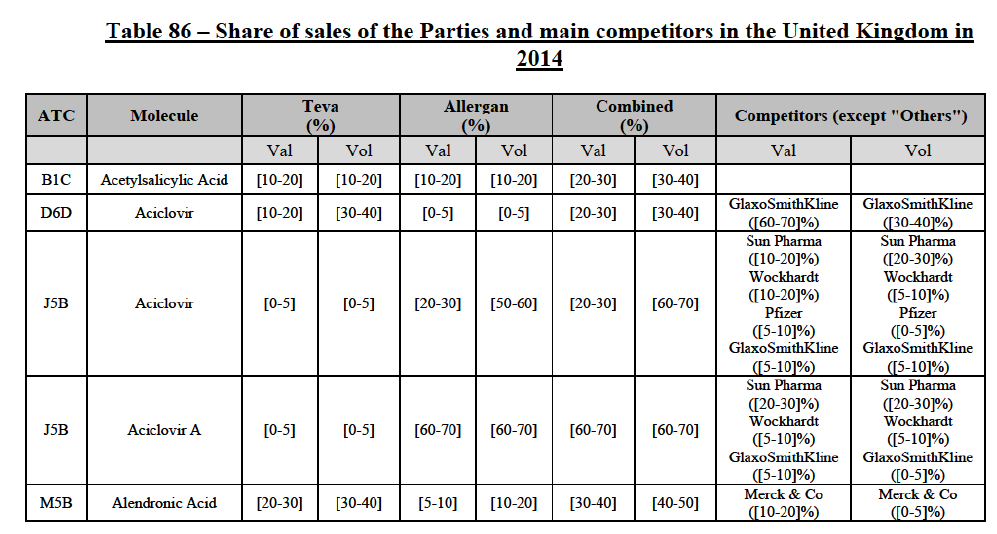
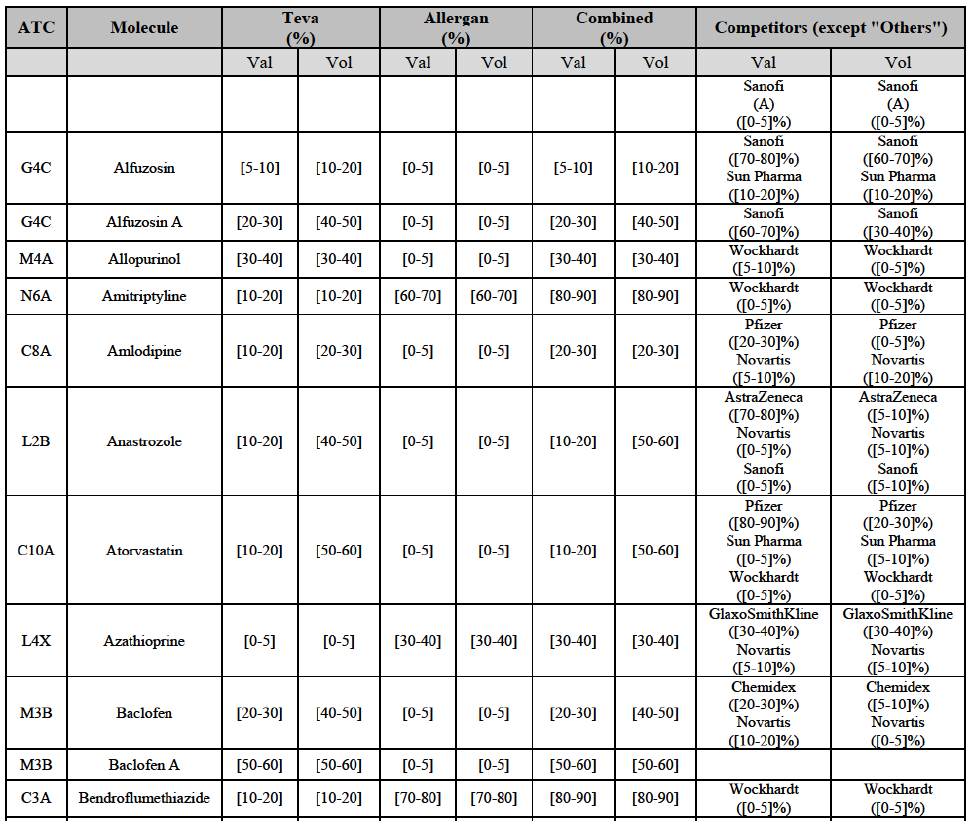
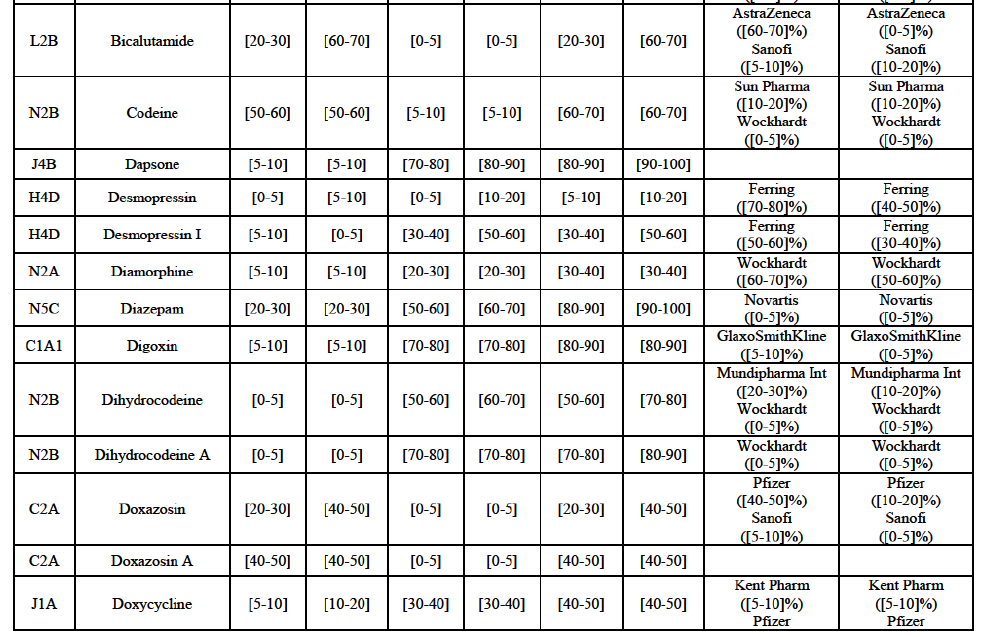
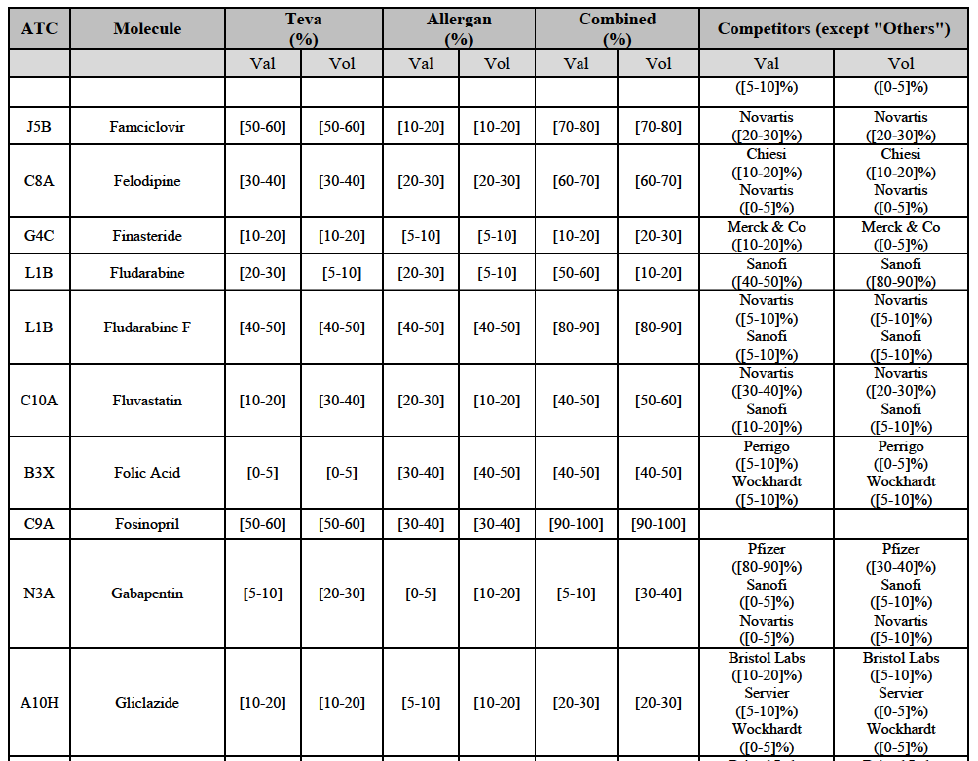
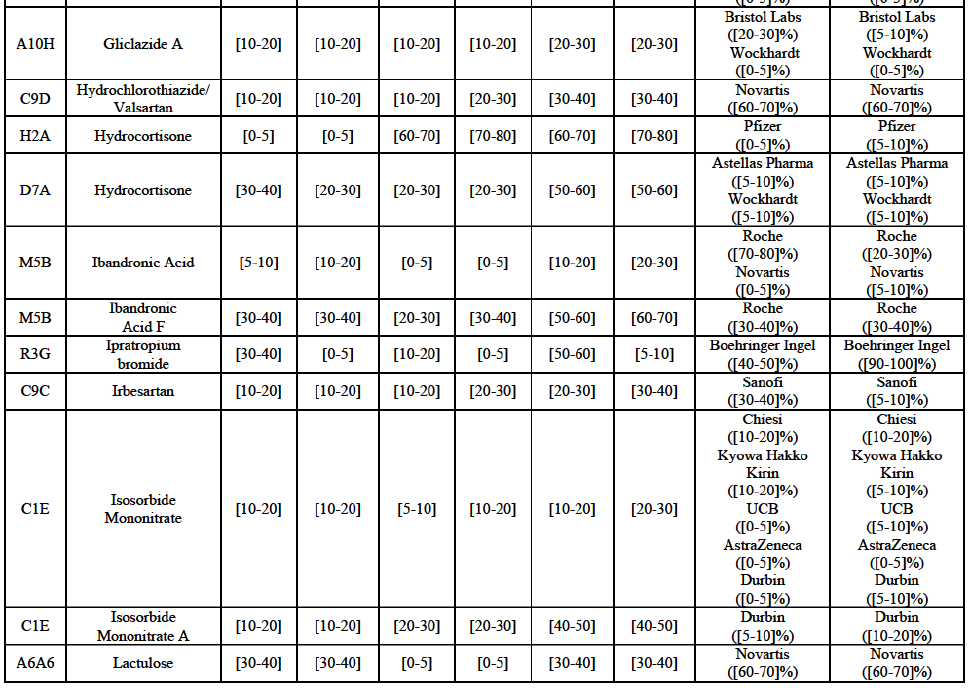
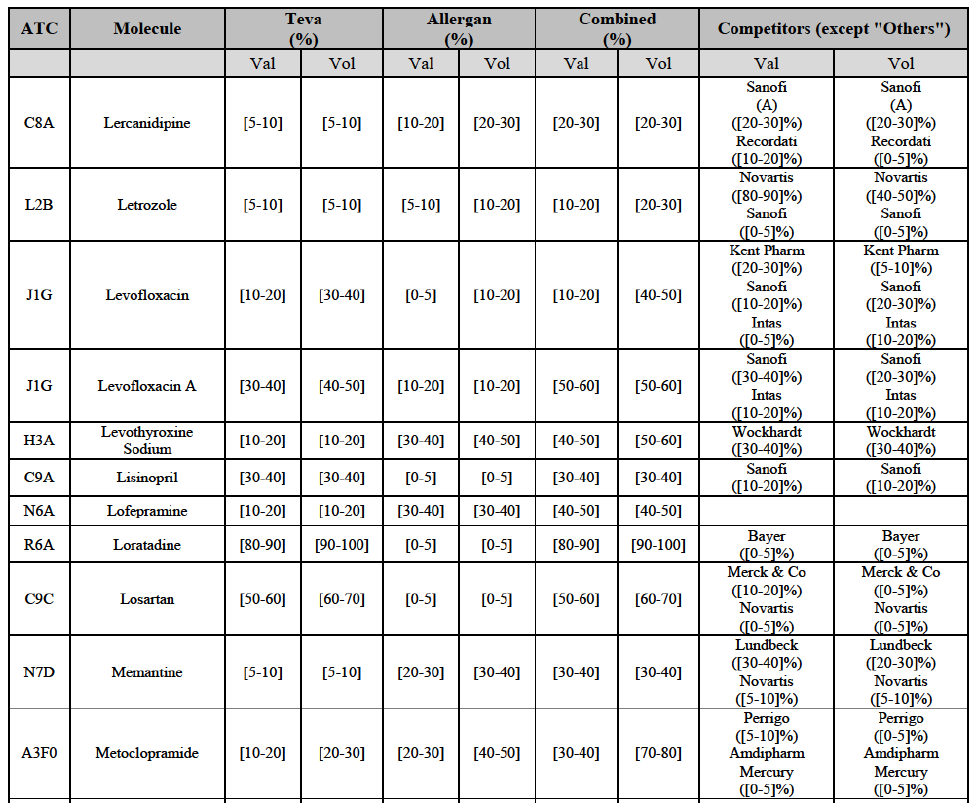
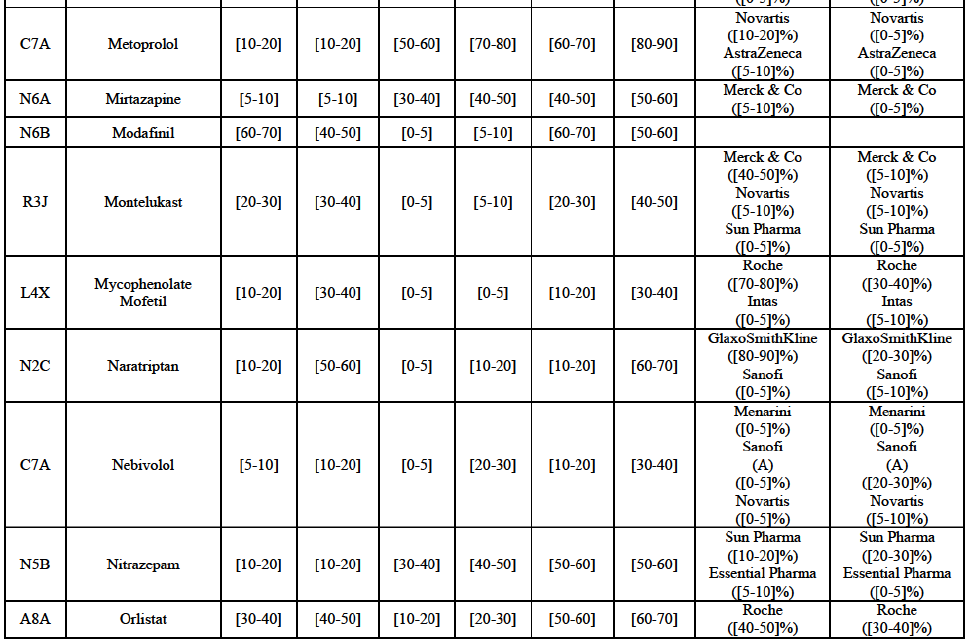
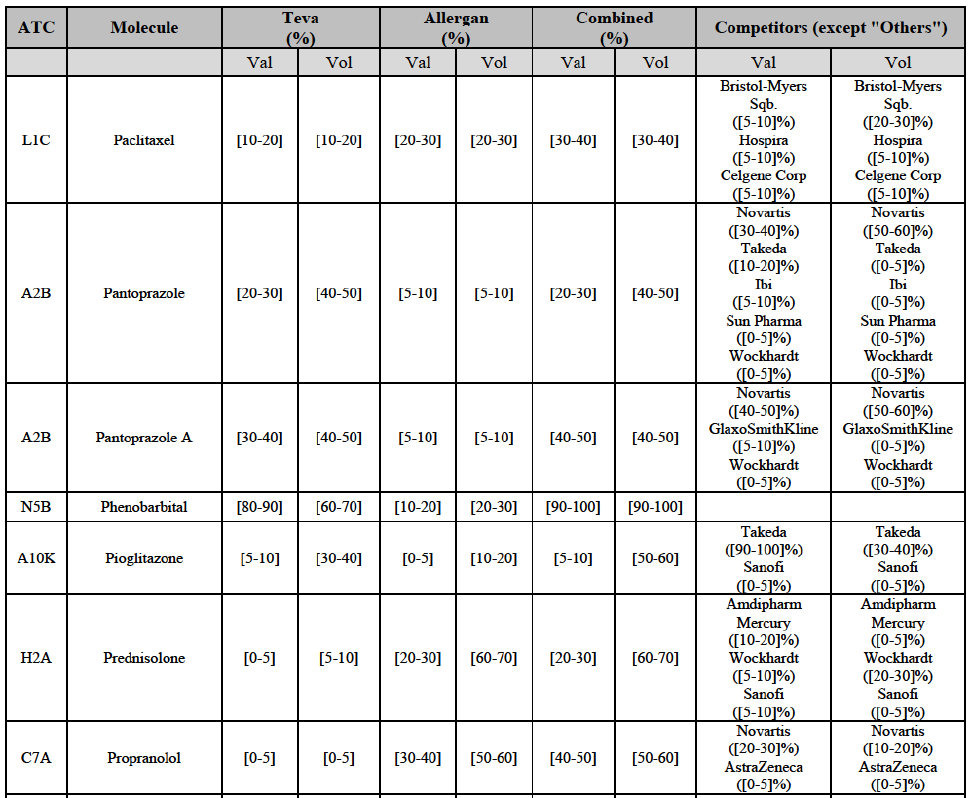
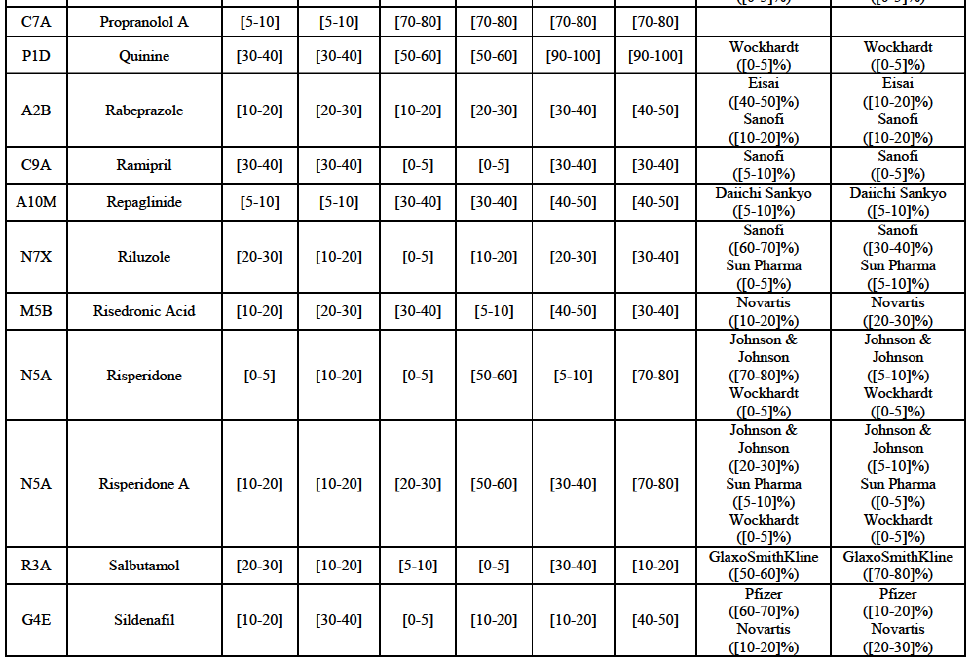
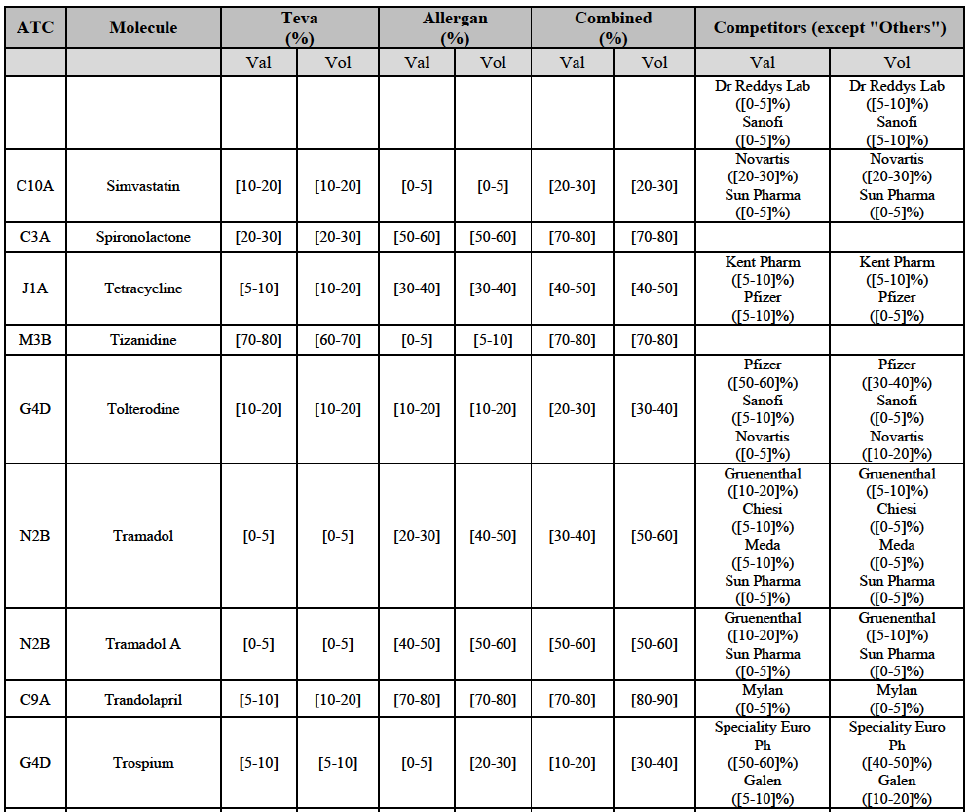
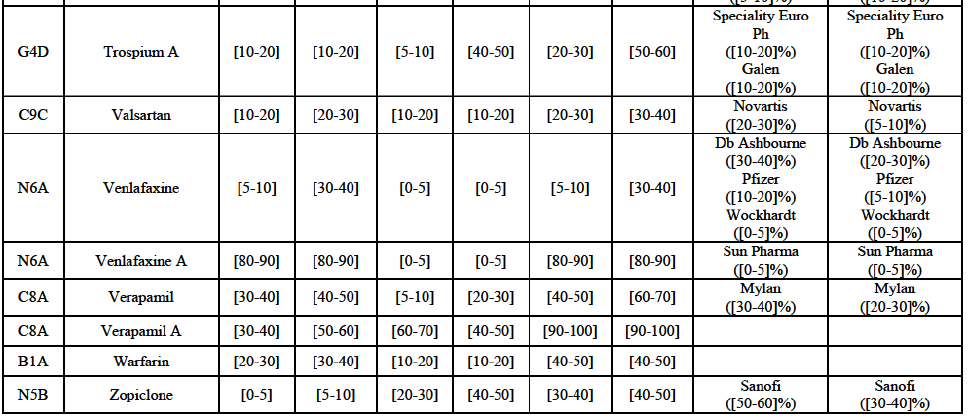
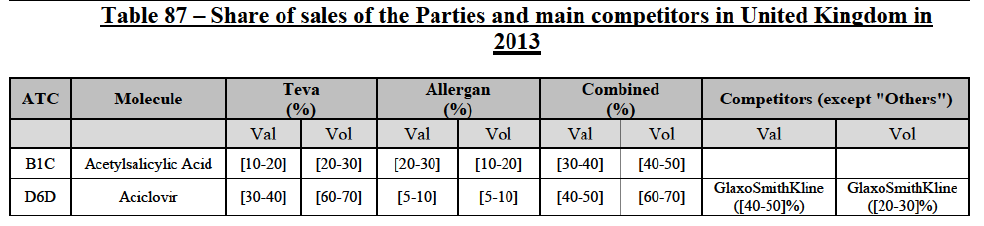
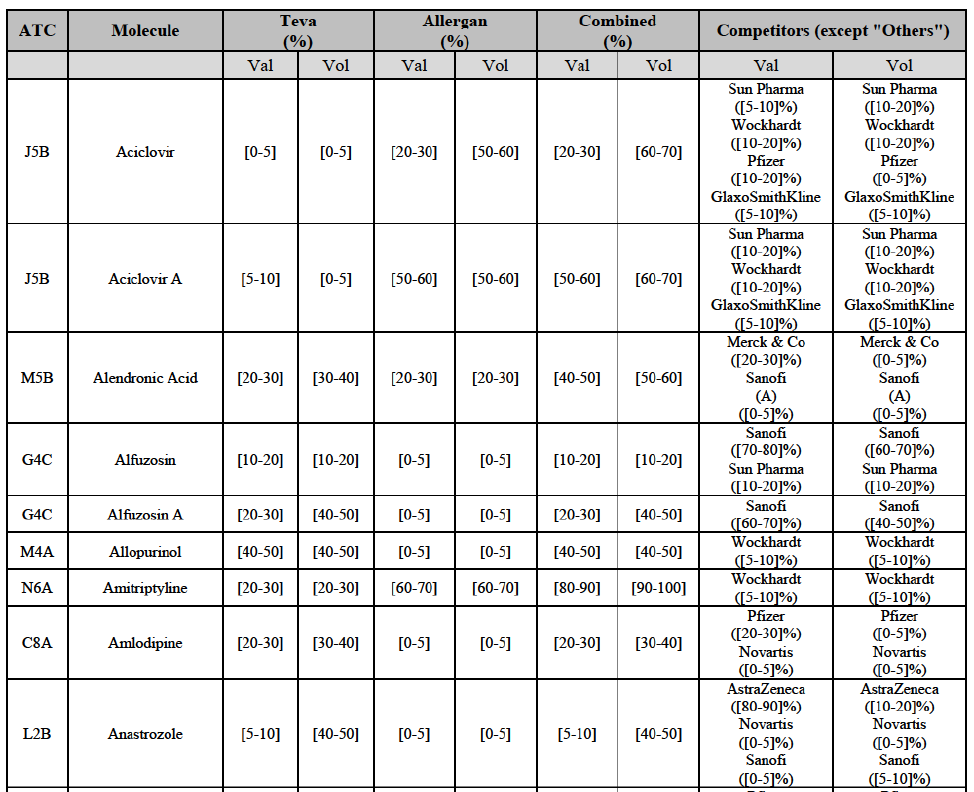
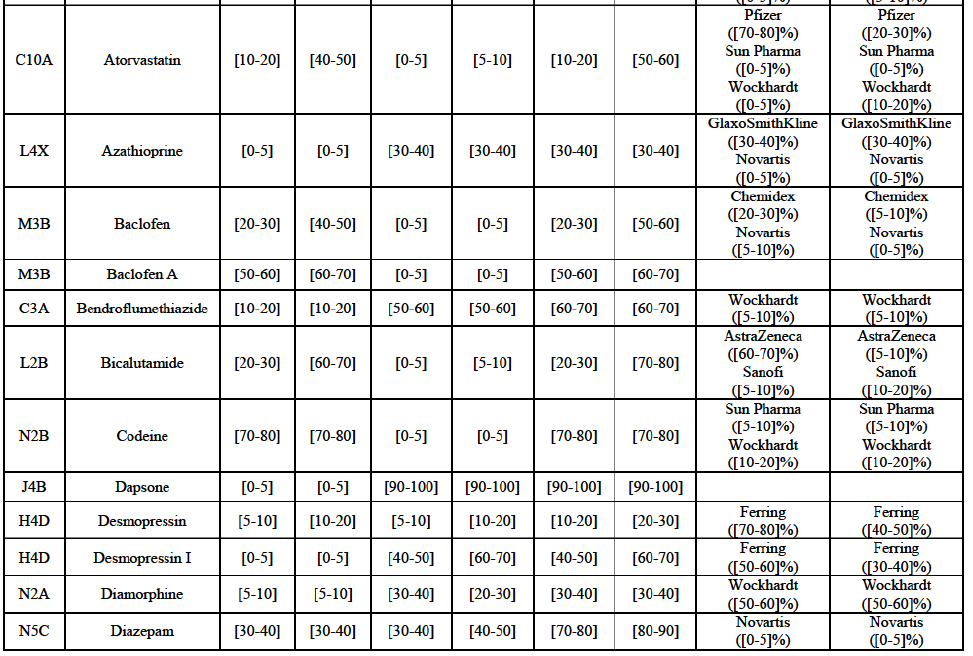
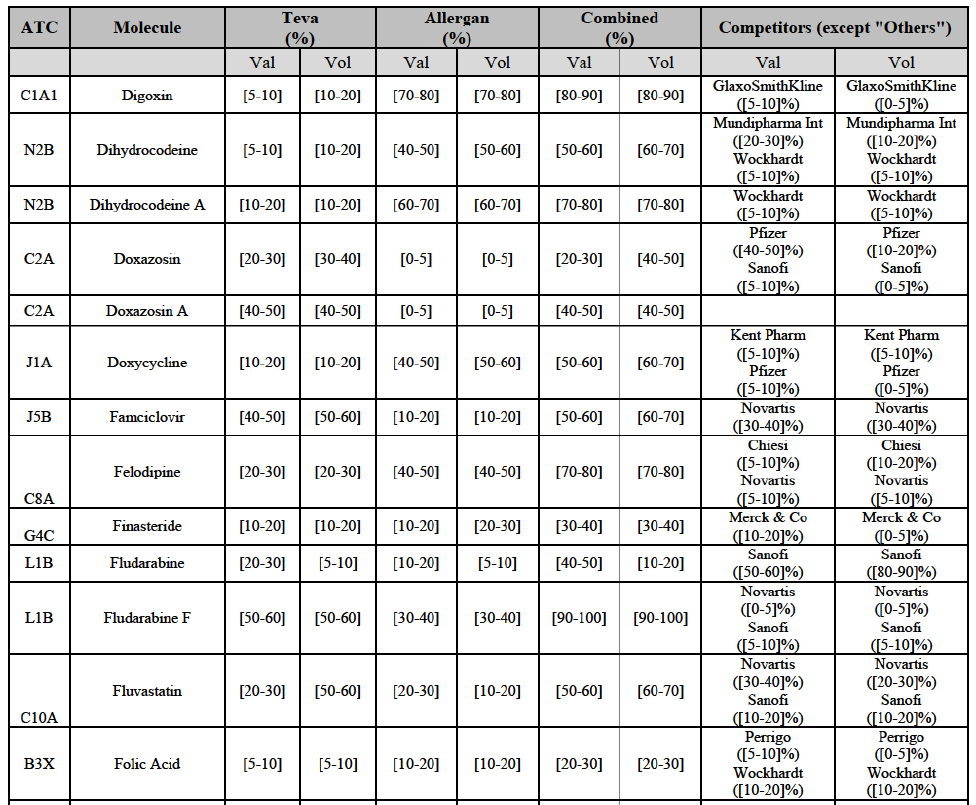
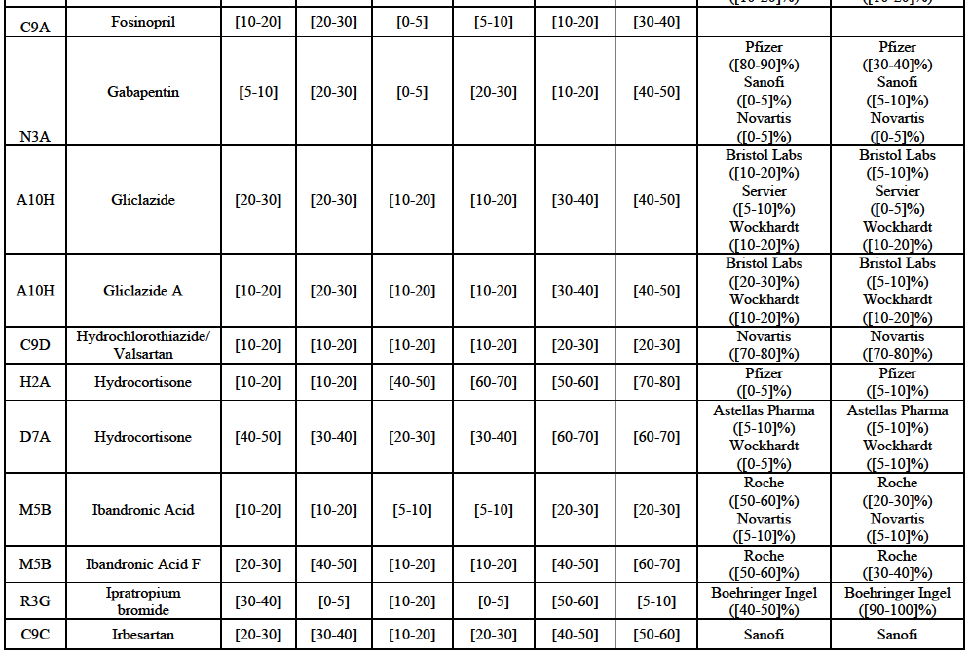
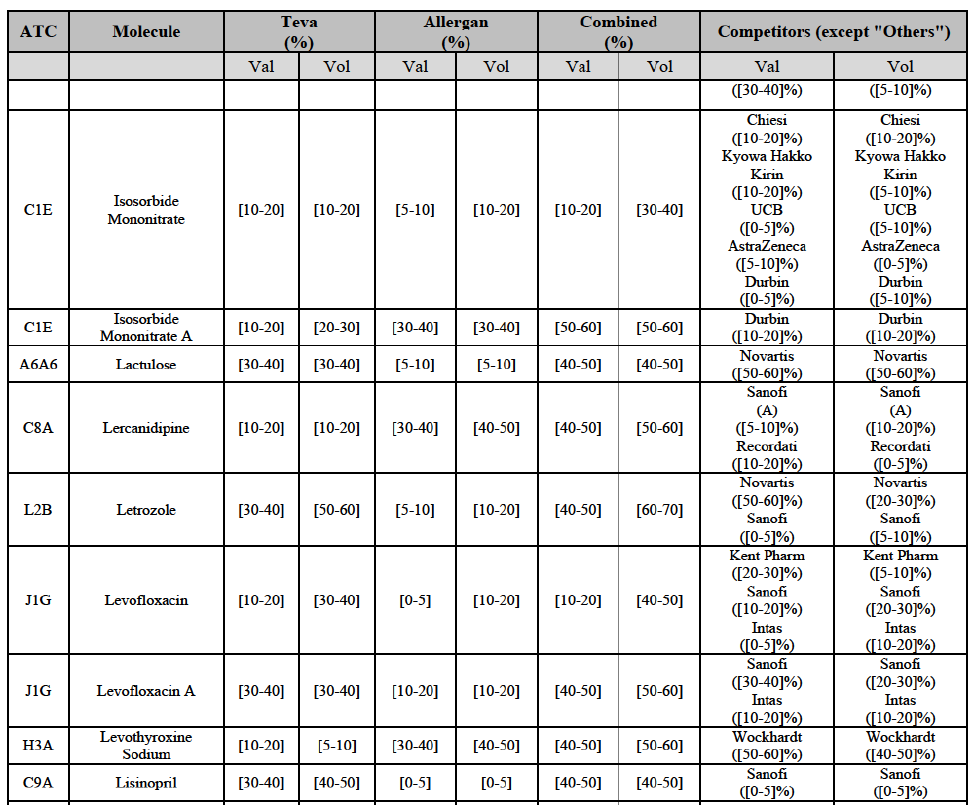
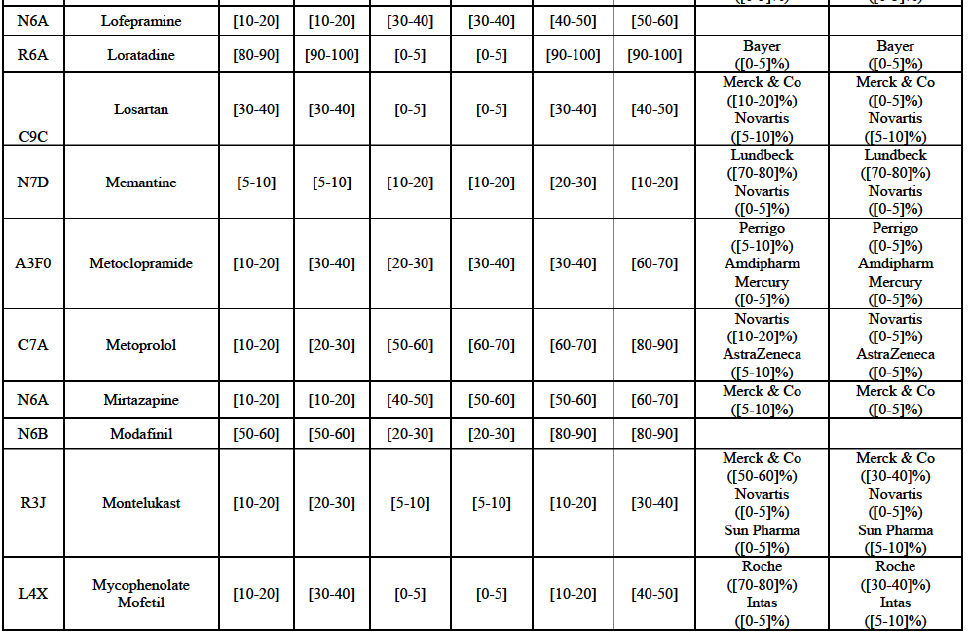
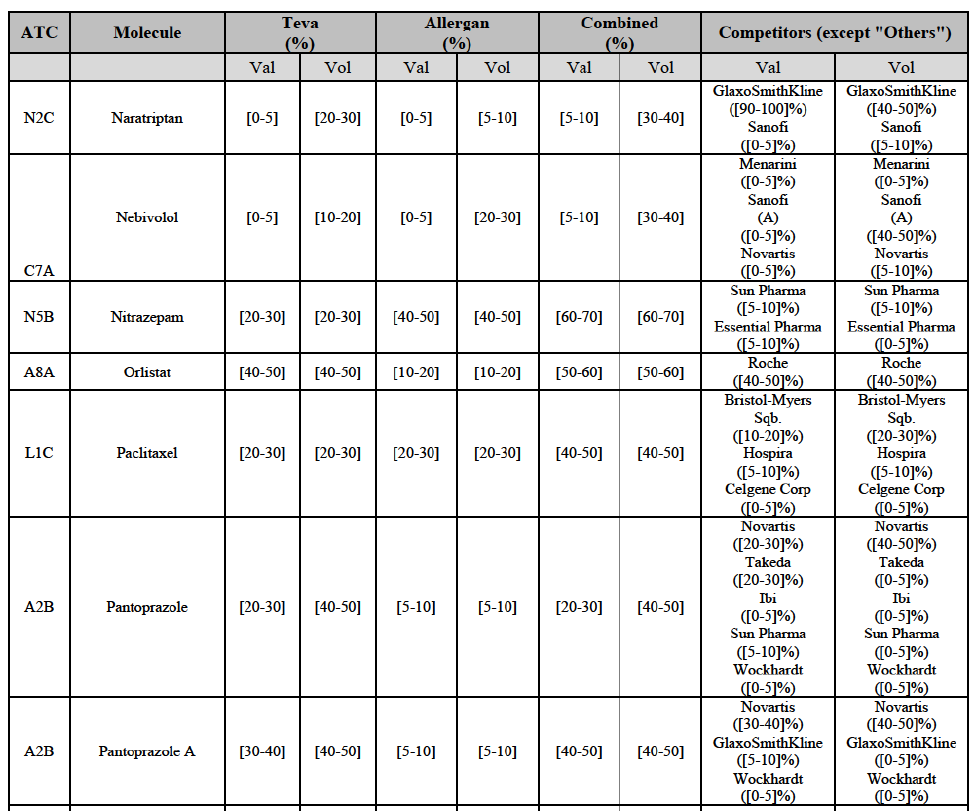
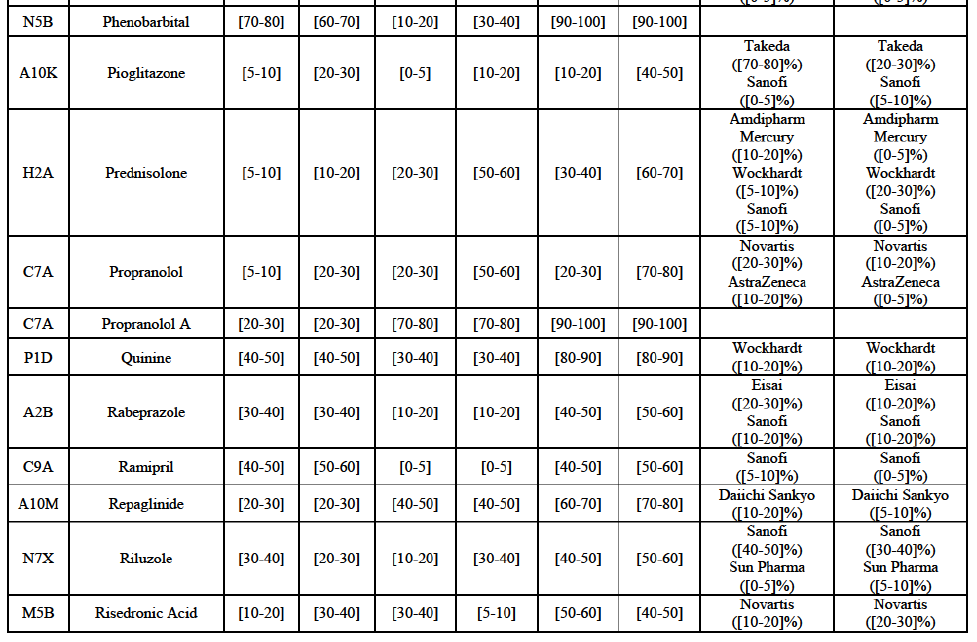
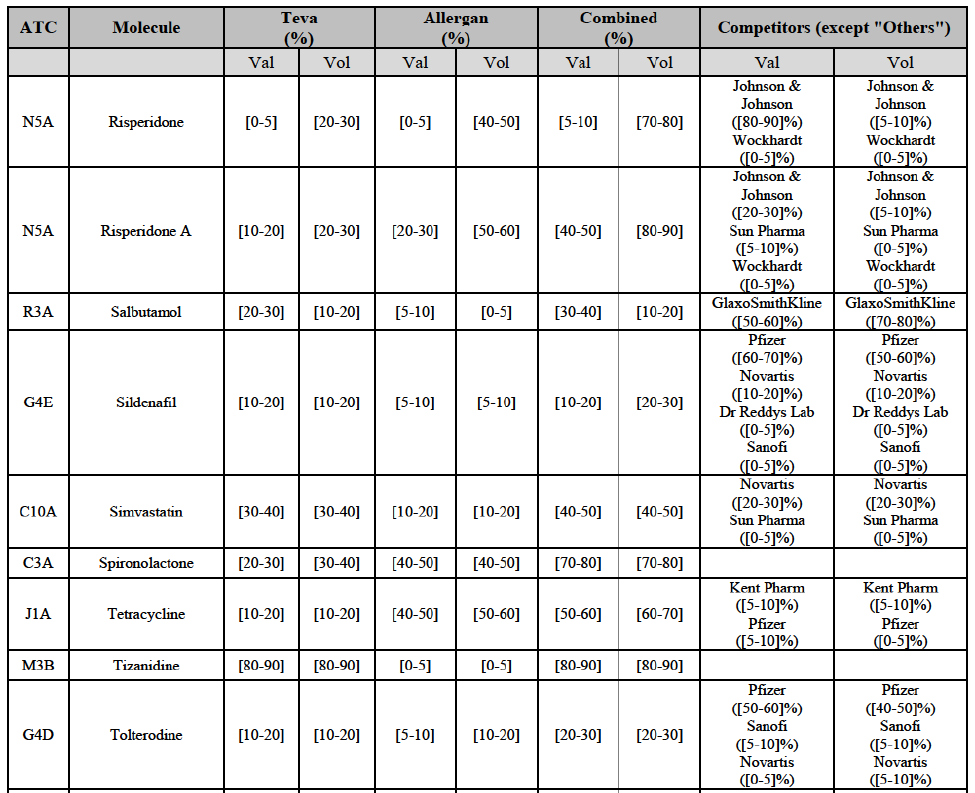
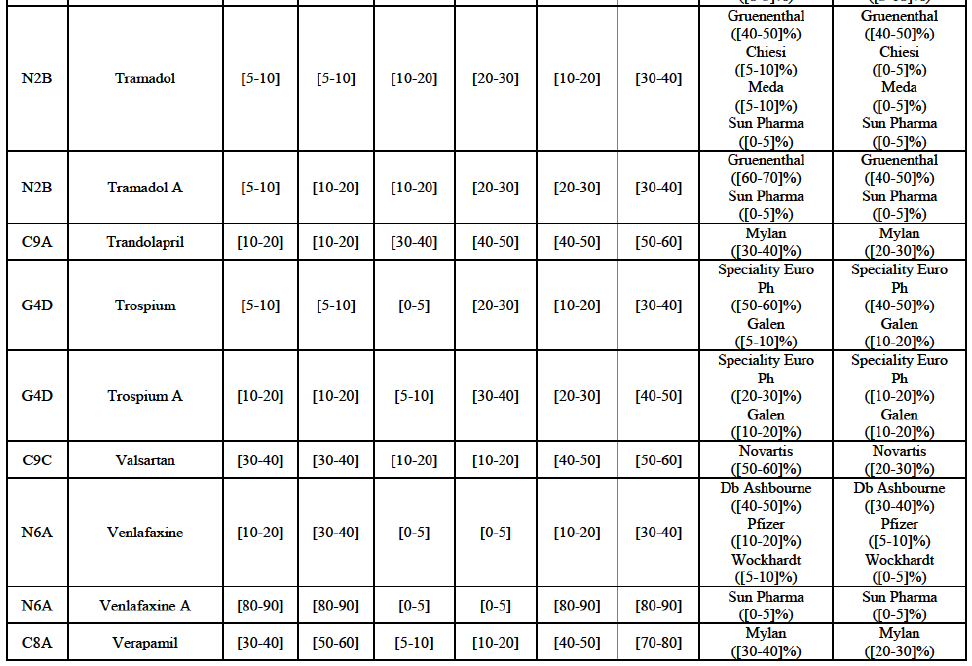
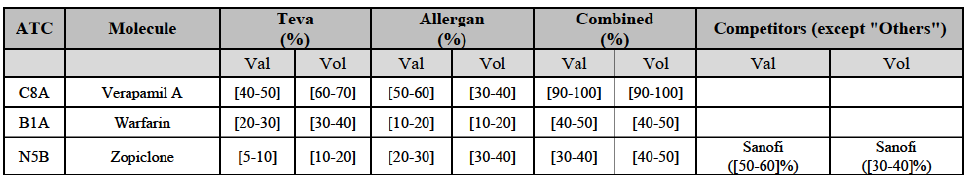
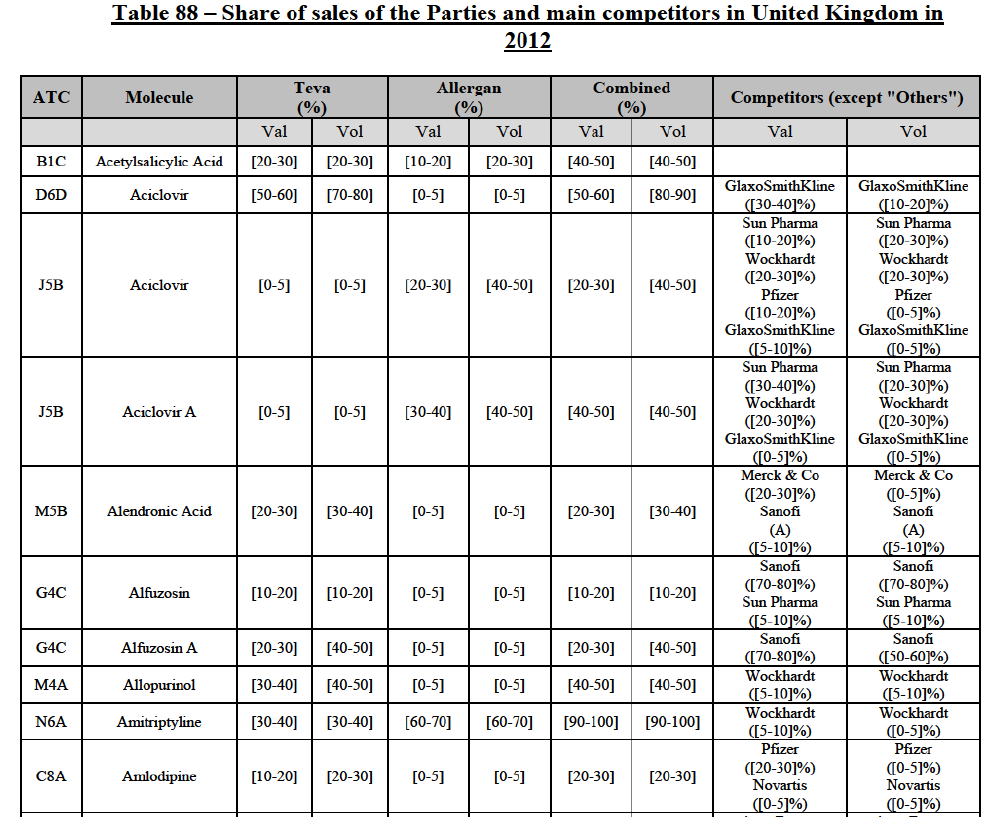
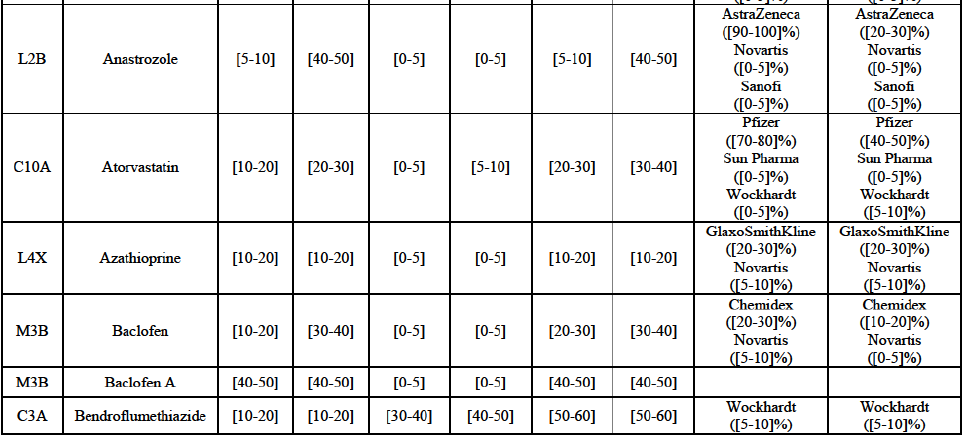
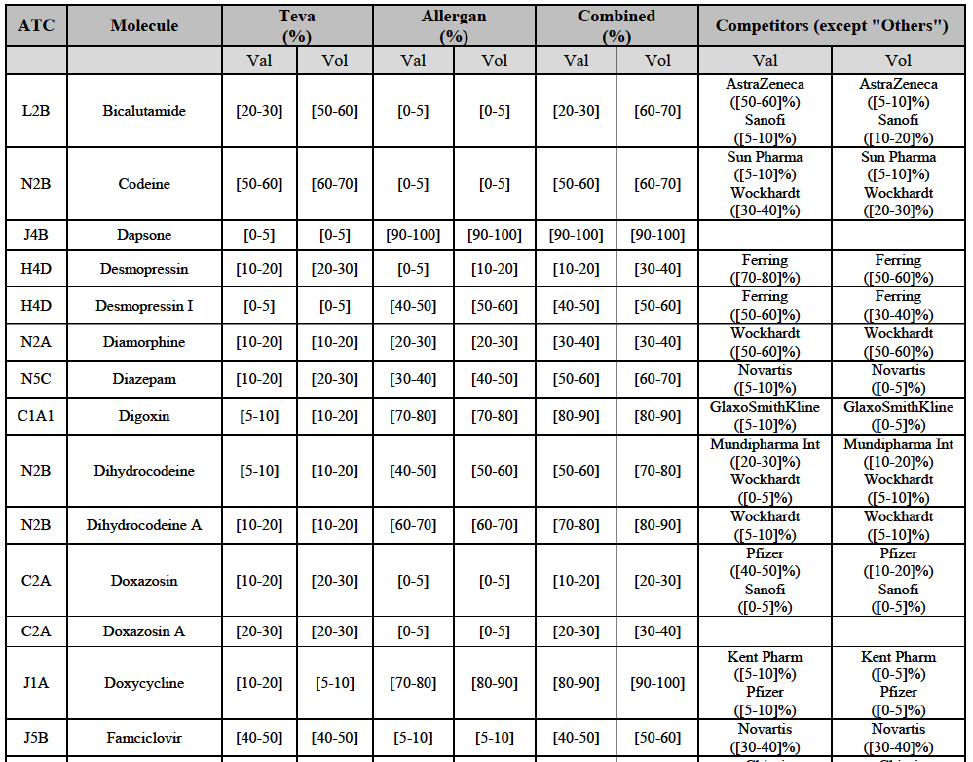
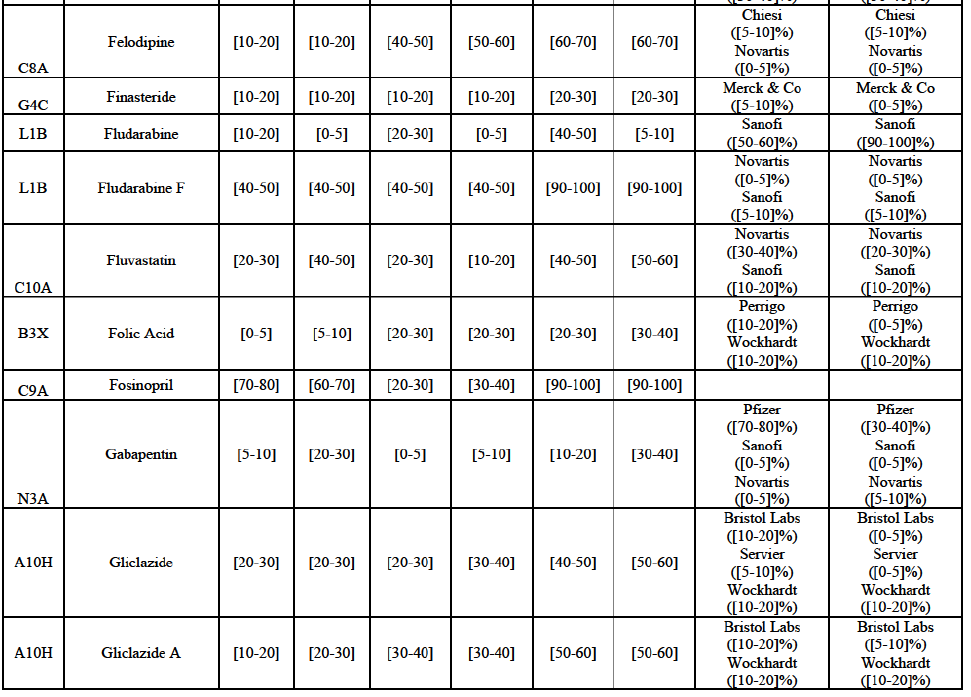
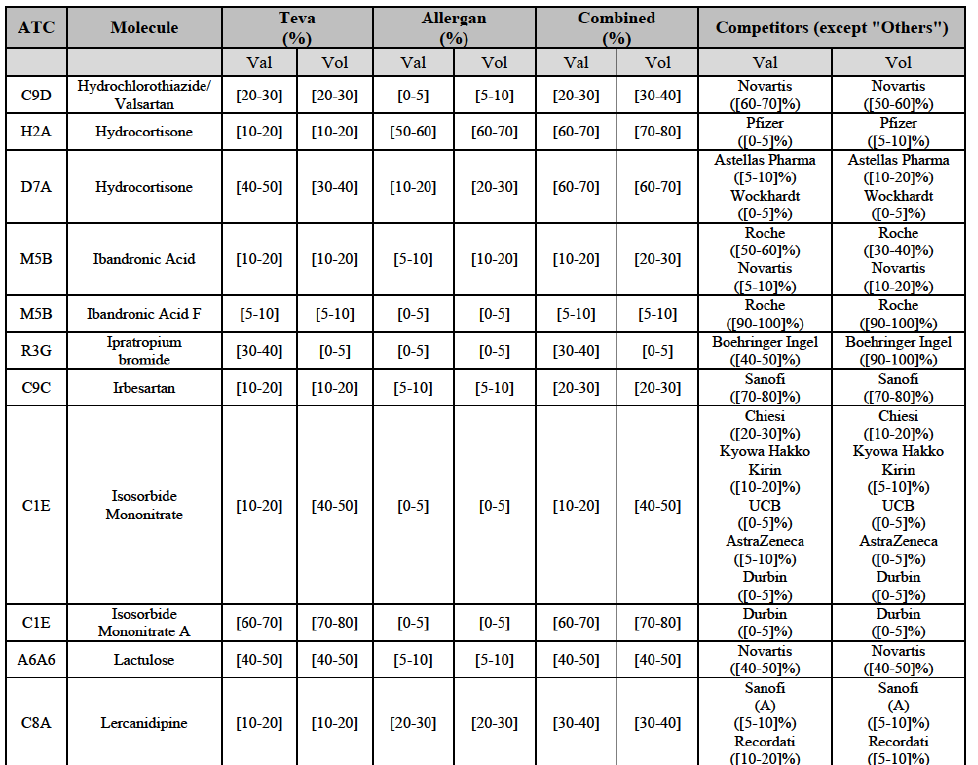
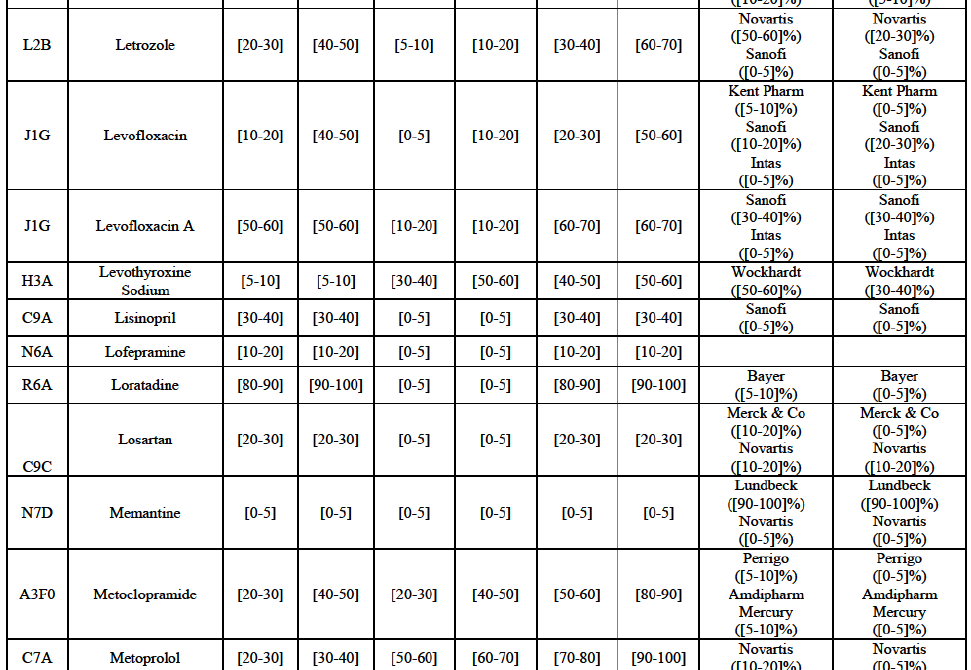
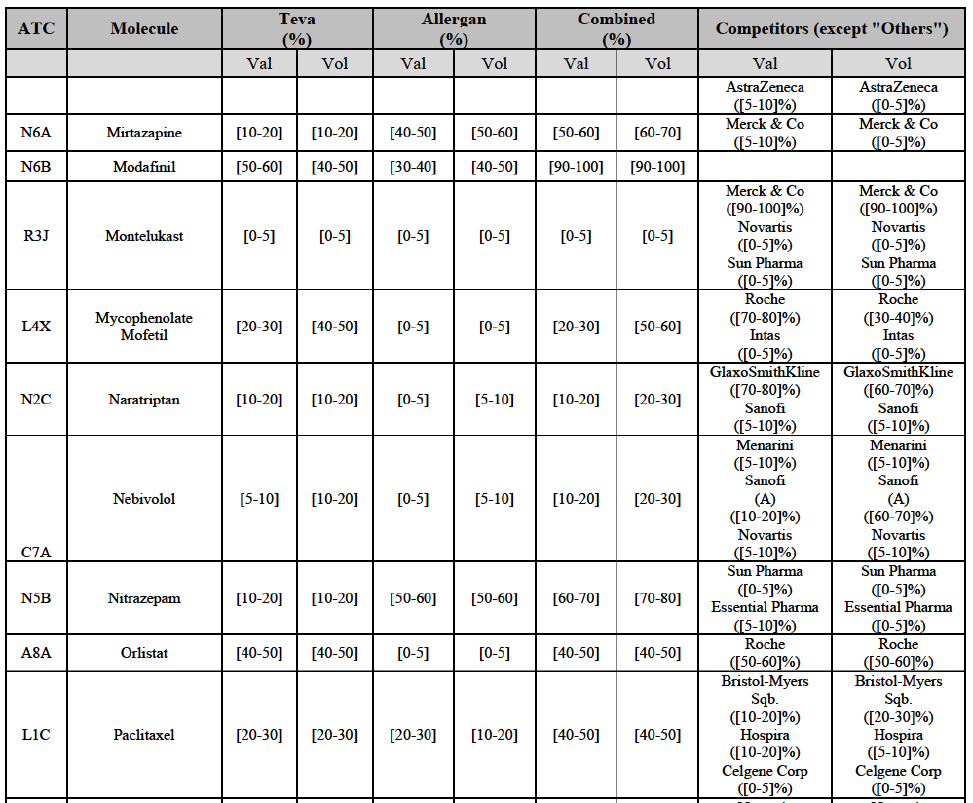
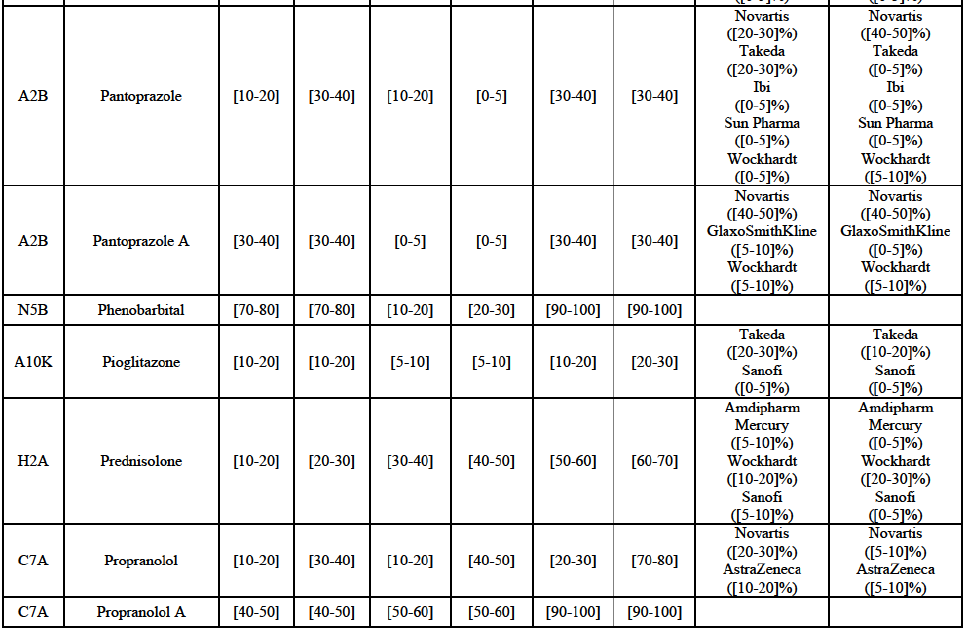
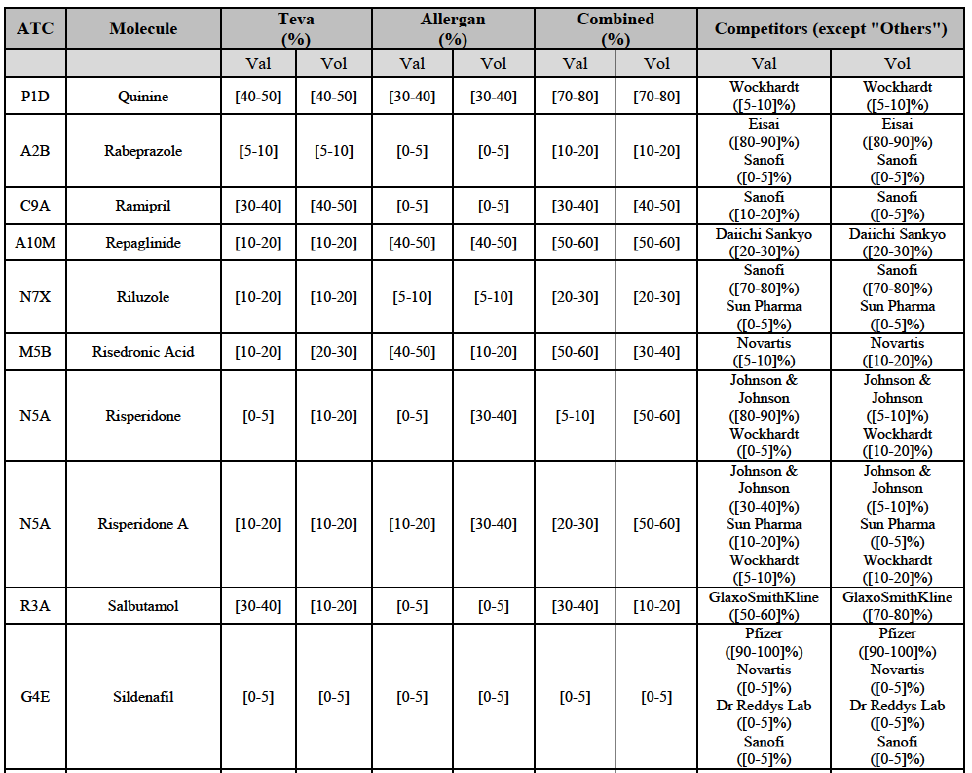
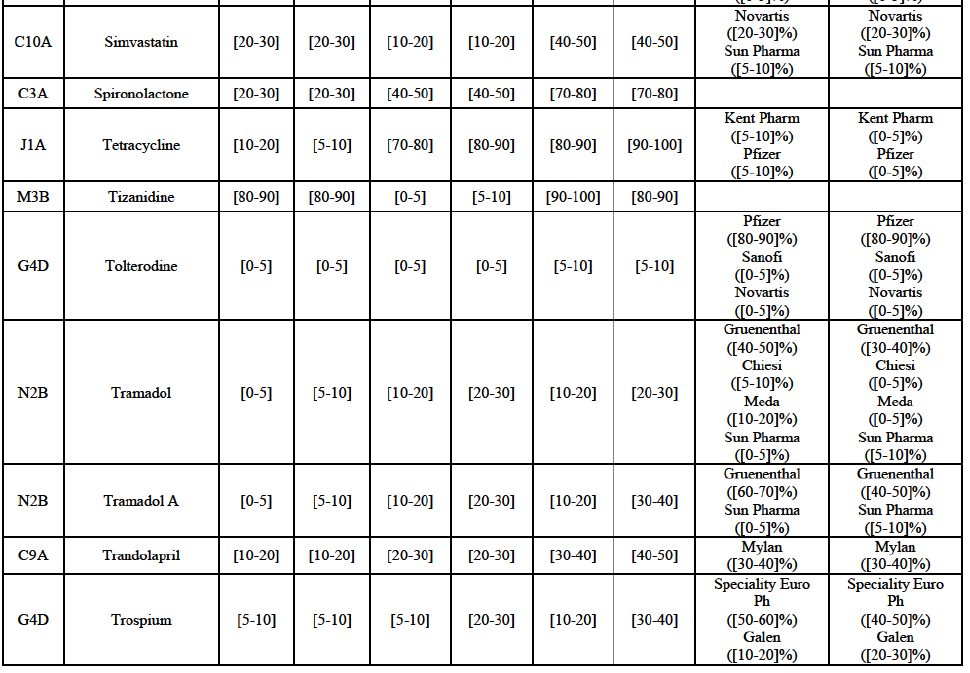
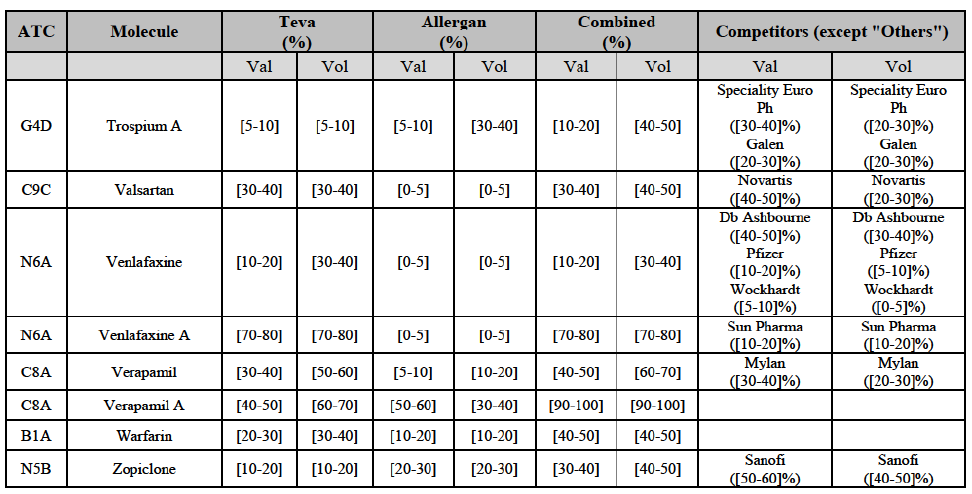
(446) The Commission presents below the competitive analysis on these markets, at molecule level, indicating also the ATC3 class to which it belongs, with a possible distinction by pharmaceutical form (at NFC1 level) and between prescribed and OTC sales where relevant.
Acetylsalicylic acid (BI C)
(447) For acetylsalicylic acid, the Transaction gives rise to a Group 1 market at molecule level in 2012 and 2013. Excluding on a conservative basis the constraints by competitors listed under "Others", the merged entity would lead the market, with a combined market share in 2014 of [20-30]% in value ([70-80]% is assigned to "Others") and [30-40]% in volume ([60-701% is assigned to "Others"). Apart from the unknown competitor(s) listed in "Others", no other competitor has a market share above 1%. Furthermore, in 2012, the Parties' combined market share was above [40-50]% ([40501% in value and [40-50]% in volume) with [40-50]-[50-60]% of market assigned to "Others". Furthermore, the market investigation indicated that the Transaction would have a negative impact on supply continuity and price, in particular due to the limited alternative suppliers and the fact that the merged entity's market share would be above 50%.285
Aciclovir (D6D)
(448) For aciclovir sold under D6D, the Transaction gives rise to a Group 1 market at molecule level 286 The Parties' combined market share was [30-40]% in volume in 2014 ([20-30]% is assigned to "Others"). Apart from the unknown competitor(s) listed in "Others", only one competitor with a market share above 5%, the originator (GlaxoSmithKline), would remain on the market. Furthermore, in 2012, where all the market was assigned to companies, the Parties' combined market share was [50-60]% in value and [80-90]% in volume. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers.287
Aciclovir (J5B)
(449) For aciclovir sold under J5B, the Transaction gives rise to a Group 1 market at molecule level. The merged entity would lead the market, with a combined market of [60-70]% in volume in 2014. For the pharmaceutical form A, the Parties' combined market share was above [60-70]% in value and volume ([60-70]% in value and [60-70]% in volume) in 2014, with only two competitors having a market share above 5%. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers.288
Alendronic acid (M5B)
(450) For alendronic acid, the Transaction gives rise to a Group 1 market at molecule level. Excluding on a conservative basis the constraints by competitors listed under "Others", the merged entity would lead the market, with a combined market share in 2014 of [3040]% in value ([40-50]% assigned to "Others") and [40-50]% in volume ([50-60]% is assigned to "Others"). In 2013, the Parties' combined market share was higher, [4050]% in value and [50-60]% in volume. Apart from the unknown competitor(s) listed in "Others", no competitor with a market share above 5% in value and volume would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact on supply continuity and price, in particular due to the limited alternative suppliers and the fact that the merged entity's market share would be above 50%.289
Alfuzosin (G4C)
(451) For alfuzosin, the Transaction gives rise to a Group 1 market at pharmaceutical form level (A) in 2012 and 2013. At molecule level, only two competitors with a market share above 1%, including the originator, would remain. At the pharmaceutical form A level, The Parties' combined market share was [40-50]% in volume, with [10-20]% assigned to "Others". Apart from the unknown competitor(s) listed in "Others", only one competitor having a market share above 5%, the originator, would remain on the market, all the other having a market share of 0.1% or below. The market investigation indicated that the Parties' combined market share could be higher than the Notifying Party's estimate (above 50%) and that there would be high barriers to entry since this product would be complicated to manufacture. 290 The market investigation also indicated that the Transaction would have a negative impact on supply continuity and price, in particular due to limited alternative suppliers.291
Allopurinol (M4A)
(452) For allopurinol, the Transaction gives rise to a Group 1 market at molecule level in 2012 and 2013. Excluding on a conservative basis the constraints by competitors listed under "Others", the merged entity would lead the market, with a combined market share in 2014 of [30-40]% in volume ([60-70]% is assigned to "Others"). Apart from the unknown competitor(s) listed in "Others", no competitor with a market share above 2% would remain on the market. The market investigation indicated that the Parties' combined market share could be higher than the Notifying Party's estimate (above 50%).292 Furthermore, the market investigation indicated that the Transaction would have a negative impact on supply continuity and price, in particular due to the history of shortages.293
Amitriptyline (N6A)
(453) For amitriptyline, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was [80-90]% in value and [80-90]% in volume (with an increment of [10-20]% and [10-20]%) in 2014. Apart from the unknown competitor(s) listed in "Others" ([10-20]% of the market), no competitor with a market share above 5% would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact on supply continuity and price, in particular due to the limited alternative suppliers and the fact that the merged entity's market share is above 50%.294
Amlodipine (C7A)
(454) For amlodipine, the Transaction gives rise to a Group 1 market at molecule level in 2013. The Parties' combined market share was [20-30]% in value and [20-30]% in volume in 2014 ([10-20]% and [50-60]% being assigned to "Others"). Apart from the unknown competitor(s) listed in "Others", only two competitors with a market share above 5% in value, including the originator, would remain on the market. In volume, only one competitor would have a market share above 5% in 2014. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market, in particular on price.295
Anastrozole (L2B)
(455) For anastrozole, the Transaction gives rise to a Group 1 market at molecule level. The merged entity would lead the market in volume, with a combined market share in 2014 of [50-60]% ([20-30]% is assigned to "Others"). In value, the Parties' combined market share was [10-20]% in 2014, with only one competitor, the originator, having a market share above 5%. Furthermore, the market investigation indicated that the Transaction would have a negative impact on price, in particular due the limited alternative suppliers.296
Atorvastatin (C10A)
(456) For atorvastatin, the Transaction gives rise to a Group 1 market at molecule level. This molecule became off-patent recently (in 2012). The Parties' combined market share in 2014 was [50-60]% in volume ([10-20]% is assigned to "Others"). Apart from the unknown competitor(s) listed in "Others", only two competitors, including the originator, would remain on the market with a market share above 5% in volume, the originator being the only competitor with a market share above 5% in value. Furthermore, the market investigation indicated that the Transaction would have a negative impact on price.297
Azathioprine (L4X)
(457) For azathioprine, the Transaction gives rise to a Group 1 market at molecule level. The merged entity would lead the market in volume, with a combined market share in 2014 of [30-40]% ([20-30]% is assigned to "Others"). Apart from the unknown competitor(s) listed in "Others", only two competitors, including the originator (GlaxoSmithKline), would remain on the market with a market share above 5%. Furthermore, the market investigation indicated that the Transaction would have a negative impact on price, in particular due to the fact that the merged entity's market share would be above 50% and that there would be additional barriers to entry related to complex manufacturing.298
Baclofen (M3B)
(458) For baclofen, the Transaction gives rise to a Group 1 market at molecule level. The merged entity would lead the market in volume, with a combined market share in 2014 of [40-50]% ([40-50]% is assigned to "Others"). Apart from the unknown competitor(s) listed in "Others", only one competitor would remain on the market with a market share above 5% in volume. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market, in particular due to the history of shortages.299
Bendroflumethiazide (C3A)
(459) For bendroflumethiazide, the Transaction gives rise to a Group 1 market at molecule level. The merged entity would lead the market with a combined market share in 2014 of [80-90]% in volume (with an increment of [10-20]% and [10-20]% being assigned to "Others"). Apart from the unknown competitor(s) listed in "Others", no other competitors would remain on the market with a market share above 2%. Furthermore, the market investigation indicated that the Transaction would have a negative impact on supply continuity and price, in particular due to the limited alternative suppliers and the history of shortages.300
Bicalutamide (L2B)
(460) For bicalutamide, the Transaction gives rise to a Group 1 market at molecule level. The merged entity would lead the market in volume, with a combined market share in 2014 of [60-70]%. Apart from the unknown competitor(s) listed in "Others" ([5-10]% in volume), the originator (AstraZeneca) would remain on the market as the only competitor with a market share above 5%. Furthermore, the market investigation indicated that the Transaction would have a negative impact on supply continuity and price, in particular due to the limited alternative suppliers.301
Codeine (N2B)
(461) For codeine, the Transaction gives rise to a Group 1 market at molecule level. The merged entity would lead the market, with a combined market share in 2014 of [6070]% in value ([10-20]% is assigned to "Others") and [60-70]% in volume ([10-20]% is assigned to "Others"), with an increment of almost [10-20]% in value and volume. In 2013, where all the market was assigned to companies, the Parties' combined market share was higher, [70-80]% in value and [70-80]% in volume. In 2014, apart from the unknown competitor(s) listed in "Others", only one competitor would remain on the market with a market share above 5%. Furthermore, the market investigation indicated that the Transaction would have a negative impact on price, in particular due to the limited alternative suppliers.302
Dapsone (J4B)
(462) For dapsone, the Transaction gives rise to affected Group 1 market at molecule level. Teva recently entered (in 2013). In 2014, the Parties' combined market share was [8090]% in value (with an increment of [5-10]% and [10-20]% being assigned to "Others") and [90-100]% in volume (with an increment of [5-10]% and [0-5]% being assigned to "Others"). Apart from the unknown competitor(s) listed as "Others", no competitor would remain on the market with a market share above 0.1%. Furthermore, the market investigation indicated that the Transaction would have a negative impact on supply continuity and price, in particular due to the limited alternative suppliers.303
Desmopressin (H4D)
(463) For desmopressin, the Transaction gives rise to a Group 1 market at the pharmaceutical form level (I). The merged entity would lead the market in volume, with a combined market share in 2014 of [50-60]% ([10-20]% is assigned to "Others"). In 2013, where all the market was assigned to companies, the Parties' combined market share was higher, [60-70]% in volume. Apart from the unknown competitor(s) listed in "Others", only one competitor (the originator) would remain on the market with a market share above 5%. Furthermore, the market investigation indicated that the Transaction would have a negative impact for this molecule on price, in particular due to limited alternative suppliers.304
Diamorphine (N2A)
(464) For diamorphine, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was [30-40]% in value and [30-40]% in volume in 2014 (with an increment of [5-10]%). Apart from the unknown competitor(s) listed in "Others" ([10-20]% in volume), only one competitor (the originator) with a market share above 1% would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact on supply continuity and price, in particular due to the limited alternative suppliers and the fact that there would be high barriers to entry because of import restrictions and complex manufacturing.305
Diazepam (N5C)
(465) For diazepam, the Transaction gives rise to a Group 1 market at molecule level. The merged entity would lead the market with a combined market share in 2014 of [8090]% in value (with an increment of [20-30]% and [10-20]% being assigned to "Others") and [90-100]% in volume (with an increment of [20-30]%). Apart from the unknown competitor(s) listed in "Others", no other competitor would remain on the market with a market share above 0.1%. Furthermore, the market investigation indicated that the Transaction would have a negative impact on price, in particular due to limited alternative suppliers.306
Digoxin (C1A)
(466) For digoxin, the Transaction gives rise to a Group 1 market at molecule level. The merged entity would lead the market with a combined market share in 2014 of [8090]% in value (with an increment of [5-10]% and [5-10]% being assigned to "Others") and [80-90]% in volume (with an increment of [5-10]% and [5-10]% being assigned to "Others"). Only one competitor, the originator, would remain on the market with a market share slightly above 5% in value. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market, in particular on price.307
Dihydrocodeine (N2B)
(467) For dihydrocodeine, the Transaction gives rise to a Group 1 market at molecule level. The merged entity would lead the market with a combined market share in 2014 of [5060]% in value ([10-20]% is assigned to "Others") and [70-80]% in volume ([10-20]% is assigned to "Others"). Apart from the unknown competitor(s) listed in "Others", only one competitor would remain on the market with a market share above 5%. Furthermore, the market investigation indicated that the Transaction would have a negative impact in the market, in particular on price.308
Doxazosin (C2A)
(468) For doxazosin, the Transaction gives rise to a Group 1 market at molecule level. The merged entity would lead the market in volume with a combined market share in 2014 of [40-50]% ([40-50]% is assigned to "Others"). Apart from the unknown competitor(s) listed in "Others", only one competitor, the originator, would remain on the market with a market share above 5%. Furthermore, the market investigation indicated that the Transaction would have a negative impact on price, in particular due to limited alternative suppliers.309
Doxycycline (J1A)
(469) For doxycycline, the Transaction gives rise to a Group 1 market at molecule level. The merged entity would lead the market with a combined market share in 2014 of [4050]% in value (with an increment of [5-0]% and [30-40]% being assigned to "Others") and [40-50]% in volume (with an increment of [10-20]% and [40-50]% being assigned to "Others"). In 2012, where all the market was assigned to companies, the Parties' combined market share was higher, [80-90]% in value and [90-100]% in volume. In 2014, apart from the unknown competitor(s) listed in "Others", only one competitor would remain on the market with a market share above 5%. Furthermore, the market investigation indicated that the Transaction would have a negative impact on supply continuity and price, in particular due to the limited alternative suppliers and the fact that the merged entity's market share would be above 50%.310
Famciclovir (J5B)
(470) For famciclovir, the Transaction gives rise to a Group 1 market at molecule level. The merged entity would lead the market with a combined market share in 2014 of [70-80]% in value (with an increment of [10-20]%) and [70-80]% in volume (with an increment of [10-20]%). Only one competitor, the originator, would remain on the market with a market share above 5%. Furthermore, the market investigation indicated that the Transaction would have a negative impact on supply continuity and price, in particular due to the limited alternative suppliers.311
Felodipine (C8A)
(471) For felodipine, the Transaction gives rise to a Group 1 market at molecule level. The merged entity would lead the market with a combined market share in 2014 of [6070]% in value (with an increment of [20-30]% and [20-30]% being assigned to "Others") and [60-70]% in volume (with an increment of [20-30]% and [20-30]% being assigned to "Others"). Only one competitor would remain on the market with a market share above 5%. Furthermore, the market investigation indicated that the Transaction would have a negative impact on supply continuity and price, in particular due to the limited alternative suppliers, the history of shortages, and the fact that there would be additional barriers to entry related to complex manufacturing.312
Finasteride (G4G)
(472) For finasteride, the Transaction gives rise to a Group 1 market at molecule level in 2013. Excluding on a conservative basis the constraints by competitors listed under "Others", the merged entity would lead the market with a combined market share in 2014 of [10-20]% in value (with an increment of [5-10]% and [60-70]% being assigned to "Others") and [20-30]% in volume (with an increment of [5-10]% and [70-80]% being assigned to "Others"). Apart from the unknown competitor(s) listed in "Others", only one competitor, the originator, would remain on the market with a market share above 5% in value only. Furthermore, the market investigation indicated that the Transaction would have a negative impact, in particular on price. 313
Fludarabine (L1B)
(473) For fludarabine, the Transaction gives rise to a Group 1 market at molecule level. The merged entity would lead the market in value with a combined market share in 2014 of [50-60]% (with an increment of [20-30]%). In value and volume, only one competitor, the originator (Sanofi), would remain on the market with a market share above 5%. The market investigation also indicated that the Transaction would have a negative impact on supply continuity and price, in particular due to limited alternative suppliers and the fact that there would be additional barriers to entry related to complex manufacturing.314
Fluvastatin (C10A)
(474) For fluvastatin, the Transaction gives rise to a Group 1 market at molecule level. The merged entity would lead the market with a combined market share in 2014 of [4050]% in value (with an increment of [10-20]% and [5-10]% being assigned to "Others") and [50-60]% in volume (with an increment of [10-20]% and [10-20]% being assigned to "Others"). Apart from the unknown competitor(s) listed in "Others", only two competitors, including the originator (Novartis), would remain on the market with a market share above 5%. Furthermore, the market investigation indicated that the Transaction would have a negative impact on supply continuity and price, in particular due to limited alternative suppliers.315
Folic acid (B3X)
(475) For folic acid, the Transaction gives rise to a Group 1 market at molecule level. The merged entity would lead the market with a combined market share in 2014 of [4050]% in value ([40-50]% is assigned to "Others") and [40-50]% in volume ([40-50]% is assigned to "Others"). Apart from the unknown competitor(s) listed in "Others", only one competitor would remain on the market with a market share above 5% in volume. Furthermore, the market investigation indicated that the Transaction would have a negative impact on price.316
Fosinopril (C9A)
(476) For fosinopril, the Transaction gives rise to a Group 1 market at molecule level. The merged entity would lead the market with a combined market share in 2014 of [90100]% in value ([5-10]% is assigned to "Others") and [90-100]% in volume ([5-10]% is assigned to "Others"). Apart from the unknown competitor(s) listed in "Others", no other competitor with a market share above 2% would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact on supply continuity and price.317
Gabapentin (N3A)
(477) For gabapentin, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was [30-40]% in volume in 2014, with an increment of [10-20]% ([10-20]% is assigned to "Others"). In value, Teva and Allergan Generics are the number 2 and number 3 generic manufacturers, after the originator Pfizer. The latter would be the only remaining competitor on the market having a market share above 5% in value. The market investigation indicated that the Parties' combined market share could be higher than the Notifying Party's estimate (possibly above 50%). The market investigation also indicated that the Transaction would have a negative impact on supply continuity and price, in particular due to limited alternative suppliers.318
Gliclazide (A10H)
(478) For gliclazide, the Transaction gives rise to a Group 1 market at molecule level. Excluding on a conservative basis the constraints by competitors listed under "Others", the merged entity would lead the market with a combined market share in 2014 of [2030]% in value (with an increment of [5-10]% and [50-60]% being assigned to "Others") and [20-30]% in volume (with an incrementof [10-20]% and [60-70]% being assigned to "Others"). Apart from the unknown competitor(s) listed in "Others", only one competitor would remain with a market share above 5% in value and volume. [...]. The market investigation indicated that the Parties' combined market share could be higher than the Notifying Party's estimate (above 50%) and that there would be additional barriers to entry since this product would be complicated to manufacture. The market investigation also indicated that the Transaction would have a negative impact on price.319
Hydrochlorothiazide/Valsartan (C9D)
(479) For hydrochlorothiazide, valsartan, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share in 2014 was 31.9% in value (with an increment of 14.6%) and 39.5% in volume (with an increment of 17.1%). Only one competitor, the originator, with a market share above 5% would remain on the market, the other competitors having a market share of 0.1% or below. The market investigation indicated that the Parties' combined market share could be higher than the Notifying Party's estimate (above 50%) and that there would be additional barriers to entry since this product would be complicated to manufacture. The market investigation also indicated that the Transaction would have a negative impact on supply continuity and price, in particular due to limited alternative suppliers.320
Hydrocortisone (D7A)
(480) For hydrocortisone sold under D7A, the Transaction gives rise to a Group 1 market at molecule level.321 The Parties' combined market share was [50-60]% in value (with an increment of [20-30]% and [20-30]% being assigned to "Others") and [50-60]% in volume in 2014 (with an increment of [20-30]% and [20-30]% being assigned to "Others"). Apart from the unknown competitor(s) listed in "Others", only two competitors would remain on the market with a market share above 5%, one of them being the originator (Astellas Pharma). Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers.322
Hydrocortisone (H2A)
(481) For hydrocortisone sold under H2A, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share in 2014 was [60-70]% in value ([30-40]% is assigned to "Others") and [70-80]% in volume ([10-20]% is assigned to "Others"). [...]. Apart from the unknown competitor(s) listed in "Others", no competitor with a market share above 5% in value would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers.323
(482) The Notifying Party indicated that Teva decided to discontinue its product since 2014 and removed this product from its price list in March 2015. However, the Notifying Party did not provide any elements indicating that Teva's marketing authorization would have been cancelled. Therefore, the Commission considers that the Parties' activities overlap irrespective of Teva's decision to discontinue this product.
Ibandronic acid (M5B)
(483) For ibandronic acid, the Transaction gives rise to a Group 1 market at the pharmaceutical form level (F). This molecule became off-patent recently (in 2011). The Parties' combined market share for ibandronic acid F increased over the last three years, up to [50-60]% in value (with an increment of [20-30]% and [5-10]% being assigned to "Others") and [60-70]% in volume (with an increment of [30-40]% and [5-10]% being assigned to "Others"). Apart from the unknown competitor(s) listed in "Others", only one competitor, the originator, would remain in the market. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.324
Ipratropium bromide (R3G)
(484) For ipratropium bromide, the Transaction gives rise to a Group 1 market at molecule level. The merged entity would lead the market in value, with a combined market share of [50-60]% (and an increment of [10-20]%) in 2014. In value and volume, only one competitor, the originator, would have a market share above 5%, the other competitors having a market share of 1.5% or below. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.325
Irbesartan (C9C)
(485) For irbesartan, the Transaction gives rise to a Group 1 market at molecule level. This molecule became off-patent recently (in 2012). The Parties' combined market share was [20-30]% in value (with an increment of [10-20]% and [30-40]% being assigned to "Others") and [30-40]% in volume (with an increment of [10-20]% and [50-60]% being assigned to "Others"). Only one competitor, the originator, would have a market share of more than 5%, the other competitors having a market share of 0.2% or below. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers.326
Isosorbide mononitrate (C1E)
(486) For isosorbide mononitrate, the Transaction gives rise to a Group 1 market at pharmaceutical form level (A). The merged entity would lead the market, with a combined market share of [40-50]% in value (with an increment of [10-20]% and [4050]% being assigned to "Others") and [40-50]% in volume (with an increment of [1020]% and [40-50]% being assigned to "Others") in 2014. Only one competitor with a market share above 5% would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact on supply continuity and price, in particular due to the limited alternative suppliers and the history of shortages.327
Lactulose (A6A)
(487) For lactulose, the Transaction gives rise to a Group 1 market at molecule level. Over the last three years (2012-2014), the Parties' combined market share was between [30-40]% and [50-60]% in value and [30-40]% and [40-50]% in volume. During the same period, only one competitor had a market share above 5%. Furthermore, the market investigation indicated that the Transaction would have a negative impact in terms of price and risk of shortage, due to the limited alternative suppliers.328
Lercanidipine (C8A)
(488) For lercanidipine, the Transaction gives rise to a Group 1 market at molecule level. Excluding on a conservative basis the constraints by competitors listed under "Others", the merged entity would lead the market with a combined market share of [20-30]% in value ([40-50]% is assigned to "Others") and [20-30]% ([30-40]% is assigned to "Others") in 2014. In 2013, the Parties' combined market share was [40-50]% in value and [50-60]% in volume, with an increment above [10-20]% and more than [30-40]% assigned to "Others". In 2013 and 2014, only one competitor had a market share above 5% in volume. Furthermore, the market investigation indicated that the Transaction would have a negative impact in terms of price and risk of shortage, due to the limited alternative suppliers.329
Letrozole (L2B)
(489) For letrozole, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [60-70]% in volume in 2012 and 2013. In 2014, apart from the unknown competitor(s) listed in "Others" ([30-40]% in volume) and the originator, no competitor had a market above 5%. Furthermore, the market investigation confirmed the Parties' strong position in the market (above 50%) and indicated that the Transaction would have a negative impact in terms of price, due to the limited alternative suppliers.330
Levofloxacin (J1G)
(490) For levofloxacin, the Transaction gives rise to a Group 1 market at molecule level. At the pharmaceutical form level (A), the Parties' combined market share ranged between [50-60]% and [70-80]% in volume over the last three years, and, apart from the originator (Sanofi), only one competitor with a market share above 5% in volume would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers.331
Levothyroxine sodium (H3A)
(491) For levothyroxine sodium, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share ranged between [50-60]% and [60-70]% in volume over the last three years. Apart from the unknown competitor(s) listed in "Others", only one competitor with a market share above 5% would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers.332
Lisinopril (C9A)
(492) For lisinopril, the Transaction gives rise to a Group 1 market at molecule level. Excluding on a conservative basis the constraints by competitors listed under "Others", the merged entity would lead the market with a combined market share in 2014 of [3040]% in value ([50-60]% is assigned to "Others") and [30-40]% in volume ([50-60]% is assigned to "Others"). Apart from the unknown competitor(s) listed in "Others", only one competitor with a market share above 5% would remain. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.333
Lofepramine (N6A)
(493) For lofepramine, the Transaction gives rise to a Group 1 market at molecule level. Excluding on a conservative basis the constraints by competitors listed under "Others", the merged entity would lead the market with a combined market share in 2014 of [4050]% in value ([50-60]% is assigned to "Others") and [40-50]% in volume ([50-60]% is assigned to "Others"). Apart from the unknown competitor(s) listed in "Others", no other competitor with a market share above 5% would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact on supply continuity.334
Loratadine (R6A)
(494) For loratadine, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share in value and volume was above [80-90]% over the last three years. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers and the fact that there would be additional barriers to entry related to complex manufacturing.335
Losartan (C9C)
(495) For losartan, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share in volume increased over the last three years from [2030]% in 2012 to [60-70]% in 2014. Apart from the unknown competitor(s) listed in "Others" and the originator (Merck), no other competitor with a market share above 5% would remain based on 2014 figures. Furthermore, the market investigation indicated that the Transaction would have a negative impact on price.336
Memantine (N7D)
(496) For memantine, the Transaction gives rise to a Group 1 market at molecule level. Excluding on a conservative basis the constraints by competitors listed under "Others", the merged entity would lead the market in volume with a combined market share in 2014 of [30-40]% ([20-30]% is assigned to "Others"). Apart from the unknown competitor(s) listed in "Others" and the originator (Lundbeck), only one competitor with a market share above 5% would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers and the fact that there would be additional barriers to entry related to complex manufacturing.337
Metoclopramide (A3F)
(497) For metoclopramide, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share in volume was between [60-70]% and [90-100]% over the last three years. Apart from the unknown competitor(s) listed in "Others", no other competitor with a market share above 5% in volume would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers.338
Metoprolol (C7A)
(498) For metoprolol, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share in volume was above [80-90]% over the last three years. Furthermore, the market investigation indicated that the Transaction would have a negative impact on price.339
Mirtazapine (N6A)
(499) For mirtazapine, the Transaction gives rise to a Group 1 market at molecule level. Excluding on a conservative basis the constraints by competitors listed under "Others", the merged entity would lead the market with a combined market share in 2014 of [4050]% in value ([40-50]% is assigned to "Others") and [50-060]% in volume ([40-50]% is assigned to "Others"). Apart from the unknown competitor(s) listed in "Others" and the originator (Merck), no other competitor with a market share above 2% would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers and the fact that there would be additional barriers to entry related to complex manufacturing.340
Modafinil (N6B)
(500) For modafinil, the Transaction gives rise to a Group 1 market at molecule level. Teva is the originator of this molecule. Apart from the unknown competitor(s) listed in "Others" ([30-40]% in value and [40-50]% in volume in 2014) no other competitor with a market share above 2% would remain based on 2014 figures. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers.341
Montelukast (R3J)
(501) For montelukast, the Transaction gives rise to a Group 1 market at molecule level. Excluding on a conservative basis the constraints by competitors listed under "Others", the merged entity would lead the market with a combined market share of [40-50]% in volume in 2014 ([40-50]% is assigned to "Others"), which increased from [0-5]% in 2012. Apart from the unknown competitor(s) listed in "Others" and the originator (Merck), only one competitor with a market share above 5% would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers and the fact that there would be additional barriers to entry related to complex manufacturing.342
Mycophenolate mofetil (L4X)
(502) For mycophenolate mofetil, the Transaction gives rise to a Group 1 market at molecule level. Apart from the unknown competitor(s) listed in "Others" and the originator (Roche), no other competitor with a market share above 5% in value would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers.343
Naratriptan (N2C)
(503) For naratriptan, the Transaction gives rise to a Group 1 market at molecule level. Excluding on a conservative basis the constraints by competitors listed under "Others", the merged entity would lead the market with a combined market share in 2014 of [1020]% in value ([80-90]% is assigned to "Others") and [30-40]% in volume ([40-50]% is assigned to "Others"). Apart from the unknown competitor(s) listed in "Others", no other competitor with a market share above 5% in value would remain (the originator, Menarini, had a market share of 2% in value in 2014). The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.344
Nebivolol (C7A)
(504) For nebivolol, the Transaction gives rise to a Group 1 market at molecule level. Excluding on a conservative basis the constraints by competitors listed under "Others", the merged entity would lead the market with a combined market share in 2014 of [1020]% in value ([80-90]% is assigned to "Others") and [30-40]% in volume ([40-50]% is assigned to "Others"). Apart from the unknown competitor(s) listed in "Others", no other competitor with a market share above 5% in value would remain (the originator, Menarini, had a market share of 2% in value in 2014). The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.345
Nitrazepam (N5B)
(505) For nitrazepam, the Transaction gives rise to a Group 1 market at molecule level. Excluding on a conservative basis the constraints by competitors listed under "Others", the merged entity would lead the market with a combined market share in 2014 of [5060]% in value ([10-20]% is assigned to "Others") and [50-60]% in volume ([10-20]% is assigned to "Others"). Apart from the unknown competitor(s) listed in "Others", only one competitor with a market share above 5% in volume would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers.346
Orlistat (A8A)
(506) For orlistat, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share increased over the last three years, from [40-50]% in value and [40-50]% in volume in 2012 to [50-60]% in value and [60-70]% in volume in 2014. Apart from the originator (Roche), no other competitor with a market share above 5% would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers.347
Paclitaxel (L1C)
(507) For paclitaxel, the Transaction gives rise to a Group 1 market at molecule level. Excluding on a conservative basis the constraints by competitors listed under "Others", the merged entity would lead the market with a combined market share in 2014 of [3040]% in value ([30-40]% is assigned to "Others") and [30-40]% in volume ([30-40]% is assigned to "Others"). Apart from the unknown competitor(s) listed in "Others" and the originator (Celgene), only one competitor with a market share above 5% in volume would remain based on 2014 figures. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.348
Pantoprazole (A2B)
(508) For pantoprazole, the Transaction gives rise to a Group 1 market at molecule level. In volume, the Parties' combined market share increased from [30-40]% in 2012 to [4050]% in 2014. Only one competitor with a market share above 6% in volume would remain on the market. Furthermore, the market investigation indicated that the Transaction would have a negative impact on price.349
Phenobarbital (N5B)
(509) For phenobarbital, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was above [90-100]% in value and volume over the last three years. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers.350
Pioglitazone (A10K)
(510) For pioglitazone, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share in volume increased over the last three years, from [2030]% in 2012 to [50-60]% in 2014. Apart from the unknown competitor(s) listed in "Others" ([10-20]% in volume in 2014) and the originator (Takeda), no other competitor with a market share above 5% in volume would remain based on 2014 figures. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers.351
Prednisolone (H2A)
(511) For prednisolone, the Transaction gives rise to a Group 1 market at molecule level. In volume, the Parties' combined market share was above [60-70]% over the last three years, and only one other competitor with a market share above 5% would remain apart from the unknown competitor(s) listed in "Others". Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers and the fact that there would be additional barriers to entry related to complex manufacturing.352
Propranolol (C7A)
(512) For propanolol, the Transaction gives rise to a Group 1 market at molecule level. At the pharmaceutical form level (A), the Parties' combined market share was above 75% in value and volume over the last three years, and no other competitor with a market share above 5% would remain apart from the unknown competitor(s) listed in "Others". Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers.353
Quinine (P1D)
(513) For quinine, the Transaction gives rise to a Group 1 market at molecule level. Over the last three years, the Parties' combined market shares increased up to [90-100]% in value and [90-100]% in volume in 2014. Apart from the unknown competitor(s) listed in "Others", only one competitor with a market share above 5% would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers.354
Rabeprazole (A2B)
(514) For rabeprazole, the Transaction gives rise to a Group 1 market at molecule level. Excluding on a conservative basis the constraints by competitors listed under "Others", the merged entity would lead the market in volume with a combined market share in 2014 of [40-50]% ([20-30]% is assigned to "Others"). Apart from the unknown competitor(s) listed in "Others" and the originator (Eisai), only one competitor with a market share above 5% would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact on price.355
Ramipril (C9A)
(515) For ramipril, the Transaction gives rise to a Group 1 market at molecule level. Excluding on a conservative basis the constraints by competitors listed under "Others", the merged entity would lead the market with a combined market share in 2014 of [3040]% in value ([50-60]% is assigned to "Others") and [30-40]% in volume ([50-60]% is assigned to "Others"). Apart from the unknown competitor(s) listed in "Others" and the originator (Sanofi), no competitor with a market share above 5% would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact on price.356
Repaglinide (A10M)
(516) For repaglinide, the Transaction gives rise to a Group 1 market at molecule level. Excluding on a conservative basis the constraints by competitors listed under "Others", the merged entity would lead the market with a combined market share in 2014 of [4050]% in value ([40-50]% is assigned to "Others") and [40-50]% in volume ([40-50]% is assigned to "Others"). The Parties' combined market share was >[70-80]% value and volume in 2013. Apart from the unknown competitor(s) listed in "Others" and the originator (Daiichi Sankyo), no competitor with a market share above 2% would remain. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position.357
Riluzole (N7X)
(517) For riluzole, the Transaction gives rise to a Group 1 market at molecule level in 2012 and 2013. The Parties entered the market in October 2012. The merged entity would lead the market as generic, after the originator (Sanofi), with a combined market share in 2014 of [20-30]% in value ([5-10]% is assigned to "Others") and [30-40]% in volume ([30-40]% is assigned to "Others"). The Parties' combined market share was [50-60]% in volume in 2013. Apart from the unknown competitor(s) listed in "Others" and the originator, no competitor with a market share above 5% value and volume would remain. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position. 358
Risedronic Acid (M5B)
(518) For risedronic acid, the Transaction gives rise to a Group 1 market at molecule level. Allergan Generics is the originator of this molecule. Excluding on a conservative basis the constraints by competitors listed under "Others", the merged entity would lead the market with a combined market share in 2014 of [40-50]% in value ([30-40]% is assigned to "Others") and [30-40]% in volume ([40-50]% is assigned to "Others"). Apart from the unknown competitor(s) listed in "Others", only one competitor would remain with a market share above 5%. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position. 359
Risperidone (N5A)
(519) For risperidone, the Transaction gives rise to a Group 1 market at molecule level. The merged entity would lead the market with a combined market share of [70-80]% in value ([10-20]% is assigned to "Others") in 2014. Apart from the unknown competitor(s) listed in "Others" and the originator (Johnson & Johnson), no competitor with a market share above 5% would remain. The market investigation indicated that there would be high barriers to entry since this product would be complicated to manufacture. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers.360
Salbutamol (R3A)
(520) For salbutamol, the Transaction gives rise to a Group 1 market at molecule level. The merged entity would lead the market as generic, after the originator (GlaxoSmithKline), with a combined market share of [30-40]% in value ([5-10]% is assigned to "Others") in 2014. Apart from the unknown competitor(s) listed in "Others" and the originator, no competitor with a market share above 2% would remain. The market investigation indicated that the Parties' combined market share could be higher than the Notifying Party's estimate (above 50%) and that there would be high barriers to entry since this product would be complicated to manufacture. Furthermore, the market investigation indicated that the Transaction would have a negative impact on price.361
Sildenafil (G4E)
(521) For sildenafil, the Transaction gives rise to a Group 1 market at molecule level. The merged entity would lead the market as generic, after the originator (Pfizer), with a combined market share of [10-20]% in value and [40-50]% in volume in 2014. Apart from the originator, only one competitor would remain with a market share above 5% in value and volume. Furthermore, the market investigation indicated that the Transaction would have a negative impact on supply continuity, in particular due to the limited alternative suppliers.362
Simvastatin (C10A)
(522) For simvastatin, the Transaction gives rise to a Group 1 market at molecule level in 2012 and 2013. The merged entity had a combined market share in 2014 of [20-30]% in value ([50-60]% is assigned to "Others") and [20-30]% in volume ([50-60]% is assigned to "Others"). The Parties' combined market share was >[40-50]% in volume in 2012 and 2013. Apart from the unknown competitor(s) listed in "Others", only one competitor would remain with a market share above 5%. Furthermore, the market investigation indicated that the combined market share of the Parties would be higher than the Notifying Party's estimate in 2014 (above 50%) and that the Transaction would have a negative impact on price, in particular due to the limited alternative suppliers and the history of shortages.363
Spironolactone (C3A)
(523) For spironolactone, the Transaction gives rise to a Group 1 market at molecule level. The merged entity would lead the market with a combined market share of [70-80]% in value in 2014 ([20-30]% is assigned to "Others"), with a significant increment of [2030]% from Teva. The Parties' combined market share was >[70-80]% in volume over the last three years. Apart from the unknown competitor(s) listed in "Others", no other competitor with a market share above 5% would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers and the history of shortages.364
Tetracycline (J1A)
(524) For tetracycline, the Transaction gives rise to a Group 1 market at molecule level. The merged entity would lead the market with a combined market share of [60-70]% in value ([40-50]% is assigned to "Others") and [60-70]% in volume ([30-40]% is assigned to "Others") in 2014. Apart from the unknown competitor(s) listed in "Others", no other competitor with a market share above 1% would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers.365
Tizanidine (M3B)
(525) For tizanidine, the Transaction gives rise to a Group 1 market at molecule level. The merged entity would lead the market with a combined market share of [70-80]% in value ([20-30]% is assigned to "Others") and [70-80]% in volume ([20-30]% is assigned to "Others") in 2014. Apart from the unknown competitor(s) listed in "Others", no other competitor with a market share above 1% would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers.366
Tolterodine (G4D)
(526) For tolterodine, the Transaction gives rise to a Group 1 market at molecule level. The Parties’ combined market share increased over the last three years, up to 29% in value ([5-10]% is assigned to "Others") and [30-40]% in volume ([5-10]% is assigned to "Others") in 2014, with an increment above [10-20]%. Apart from the unknown competitor(s) listed in "Others" and the originator (Sanofi), only one competitor would remain with a market share above 5%. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers.367
Tramadol (N2B)
(527) For tramadol, the Transaction gives rise to a Group 1 market at molecule level. The merged entity would lead the market with a combined market share in 2014 of [3040]% in value ([20-30]% is assigned to "Others") and [50-60]% in volume ([30-40]% is assigned to "Others"). At the level of pharmaceutical form A, the Parties' combined market share was [50-60]% in value and [50-60]% in volume in 2014. Apart from the unknown competitor(s) listed in "Others" and the originators (Gruenenthal and Meda), only one competitor would remain with a market share above 5% only in value. The market investigation did not provide any elements to dispel the serious doubts arising from the fact that the Parties have a strong combined competitive position. 368
Trandolapril (C9A)
(528) For trandolapril, the Transaction gives rise to a Group 1 market at molecule level. The Parties’ combined market shares increased over the last three years, up to [70-80]% in value ([10-20]% is assigned to "Others") and [80-90]% in volume ([10-20]% is assigned to "Others") in 2014, with a significant increment of [5-10]% value and [10-20]% volume from Teva. Apart from the unknown competitor(s) listed in "Others", no competitor with a market share above 5% would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers and the history of shortages.369
Trospium (G4D)
(529) For trospium, the Transaction gives rise to a Group 1 market at molecule level. The Parties recently entered the market (in 2011 and 2012), with a combined market share of up to [10-20]% in value and [30-40]% in volume in 2014. At the level of pharmaceutical form A, the Parties' combined market share was [50-60]% in volume in 2014 (with an increment of [10-20]%). Apart from the unknown competitor(s) listed in "Others" and the originator (Speciality Euro Ph), only one competitor would remain with a market share above 5%. Furthermore, the market investigation indicated that the Transaction would have a negative impact on price, in particular due to the limited alternative suppliers and the history of shortages.370
Valsartan (C9C)
(530) For valsartan, the Transaction gives rise to a Group 1 market at molecule level in 2012 and 2013. Excluding on a conservative basis the constraints by competitors listed under "Others", the merged entity would lead the market with a combined market share in 2014 of [20-30]% in value ([50-60]% is assigned to "Others") and [30-40]% in volume ([60-70]% is assigned to "Others"). In 2013 the Parties' combined market share was even higher, [40-50]% in value and [50-60]% in volume. Apart from the unknown competitor(s) listed in "Others" and the originator (Novartis), no competitor with a market share above 5% would remain. Furthermore, the market investigation indicated that the combined market share of the Parties would be higher than the Notifying Party's estimate in 2014 (above 50%) and that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers.371
Venlafaxine (N6A)
(531) For venlafaxine, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share in 2014 was [10-20]% in value ([30-40]% is assigned to "Others") and [30-40]% in volume ([20-30]% is assigned to "Others"). At the level of pharmaceutical form A, the Parties' combined market share was >[80-90]% value and volume in 2013 and 2014. Apart from the unknown competitor(s) listed in "Others", only two competitors, including the originator (Pfizer), would remain with a market share above 5%. Furthermore, the market investigation indicated that the combined market share of the Parties would be higher than the Notifying Party's estimate in 2014 (above 50%) and that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers and the history of shortages.372
Verapamil (C8A)
(532) For verapamil, the Transaction gives rise to a Group 1 market at molecule level. The merged entity would lead the market with a combined market share in 2014 of [4050]% in value ([5-10]% is assigned to "Others") and [60-70]% in volume ([5-10]% is assigned to "Others"). Apart from the unknown competitor(s) listed in "Others" and the originator (Mylan), no competitor with a market share above 5% would remain. At the level of pharmaceutical form A, the Parties' combined market share was >[90-100]% in value and volume over the last three years. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity and price, in particular due to the limited alternative suppliers and the history of shortages.373
Warfarin (B1A)
(533) For warfarin, the Transaction gives rise to a Group 1 market at molecule level. Excluding on a conservative basis the constraints by competitors listed under "Others", the merged entity would lead by far the market with a combined market share in 2014 of [40-50]% in value ([50-60]% is assigned to "Others") and [40-50]% in volume ([5060]% is assigned to "Others"). Apart from the unknown competitor(s) listed in "Others", no other competitor with a market share above 1% would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity, in particular due to the limited alternative suppliers.374
Zopiclone (N5B)
(534) For zopiclone, the Transaction gives rise to a Group 1 market at molecule level. The Parties' combined market share was in 2014 [30-40]% in value ([10-20]% is assigned to "Others") and [40-50]% in volume (16% is assigned to "Others"). Apart from the unknown competitor(s) listed in "Others" and the originator, no competitor with a market share above 2% would remain. Furthermore, the market investigation indicated that the Transaction would have a negative impact on the market in terms of supply continuity, in particular due to the limited alternative suppliers.375
Conclusion
(535) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raise serious doubts as to its compatibility with the internal market with respect to the marketing of acetylsalicylic acid, aciclovir, alendronic acid, alfuzosin, allopurinol, amitriptyline, amlodipine, anastrozole, atorvastatin, azathioprine, baclofen, bendroflumethiazide, bicalutamide, codeine, dapsone, desmopressin, diamorphine, diazepam, digoxin, dihydrocodeine, doxazosin, doxycycline, famciclovir, felodipine, finasteride, fludarabine, fluvastatin, folic acid, fosinopril, gabapentin, gliclazide, hydrochlorothiazide/valsartan, hydrocortisone, ibandronic acid, ipratropium bromide, irbesartan, isosorbide mononitrate, lactulose, lercanidipine, letrozole, levofloxacin, levothyroxine sodium, lisinopril, lofepramine, loratadine, losartan, memantine, metoclopramide, metoprolol, mirtazapine, modafinil, montelukast, mycophenolate mofetil, naratriptan, nebivolol, nitrazepam, orlistat, paclitaxel, pantoprazole, phenobarbital, pioglitazone, prednisolone, propranolol, quinine, rabeprazole, ramipril, repaglinide, riluzole, risedronic acid, risperidone, salbutamol, sildenafil, simvastatin, spironolactone, tetracycline, tizanidine, tolterodine, tramadol, trandolapril, trospium, valsartan, venlafaxine, verapamil, warfarin and zopiclone in the United Kingdom.
IV.2.2.28.b. Pipeline generic pharmaceuticals
[...]
(536) Allergan Generics is planning to launch a generic [...] (pharmaceutical form [...]), for which Teva had a [70-80]% market share in value and [70-80]% in volume in 2014, and only one other competitor with >5% share in volume was active on the market in 20122014.
[...]
(537) Allergan Generics is planning to launch a generic [...] (pharmaceutical form [...]), for which Teva had a [60-70]% market share in value and [70-80]% in volume in 2014, and only one other competitor with >5% share in volume was active on the market in 20122014.
[...]
(538) Teva is planning to launch a generic [...] (pharmaceutical form [...]), for which Allergan Generics had a [40-50]% market share in value and in volume in 2014, and only two other competitors with >5% share in volume were active on the market in 2012-2014.
Conclusion
(539) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the market for the marketing of [...l, [...land [...lin the United Kingdom.
IV.2.2.28.c. Wholesale of generic pharmaceuticals
(540) In the United Kingdom, the Parties are marketing generic medicines under the following two channels: (a) indirectly through wholesalers (which take title over the medicines and set prices for end-customers) and (b) directly to end-customers (pharmacies, dispensing doctors and hospitals), using wholesalers as logistics providers.376 The latter channel represented [70-80]% of Teva's and [50-60]% of Allergan Generics' net sales in 2014.
The Parties' views
(541) To the extent there is a relevant market for the wholesale of generic pharmaceuticals where the Parties would be active, the Parties submit that they face important competition from wholesalers, whose fundamental pupose is precisely to offer pharmacies a portfolio of manufacturers' products as a single offer.
(542) Furthermore, according to the Parties, there is no reason in theory to expect that the Parties would be better able to compete to supply independent pharmacies than the wholesalers, since the Parties are less effective competitors than wholesalers on both price and non-price factors, given in particular that, based on the economic evidence submitted by the Parties, the Parties' prices would be on a like-for-like basis higher than the wholesalers (Allergan Generics' schemes in particular would be significantly less price competitive than some broad-line and short-line wholesalers' own offerings), and that, even after the Transaction, the Parties' combined portfolio would still not be larger than that of its wholesaler competitors.
(543) In any event, the Parties argue that there is no basis for serious doubts in the context of the Parties' extremely limited combined market share on such a market definition.
(544) Finally, the Parties note that there is ample evidence that pharmacies do buy on a product-by-product basis, given that, in particular, computing and ordering systems such as PharmAssist and Cambrian's eCASS allow pharmacies to order from the cheapest price available across all manufacturers and multiple wholesalers' offers (akin to price comparison websites).
The Commission's assessment
The Parties' market presence
(545) The Parties are the two largest generic manufacturers in the United Kingdom, with the merged entity being five times the size of its next competitor (Wockhardt) in value in 2014.
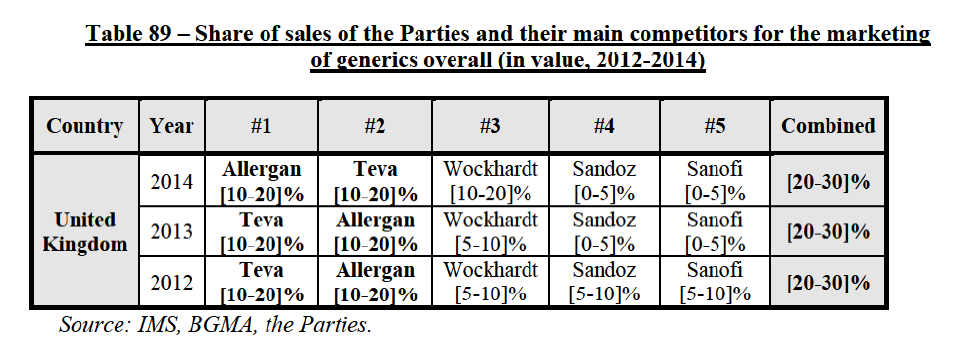
(546) According to an internal document of Allergan Generics, the combined market share of the Parties is even higher for the twelve months ending August 2015 (Teva: [10-20]%; Allergan Generics: [10-20]%; combined: [30-40]%).377
(547) As evidenced in the table below, the Parties are also by far the generic manufacturers with the largest offering in the United Kingdom, Teva holding 320 molecules and Allergan Generics 321 molecules in 2015. The merged entity would have a portfolio twice the size of its next competitor in terms of portfolio size, Mylan, and 3.5 times the size of its next competitor in terms of market share, Wockhardt.
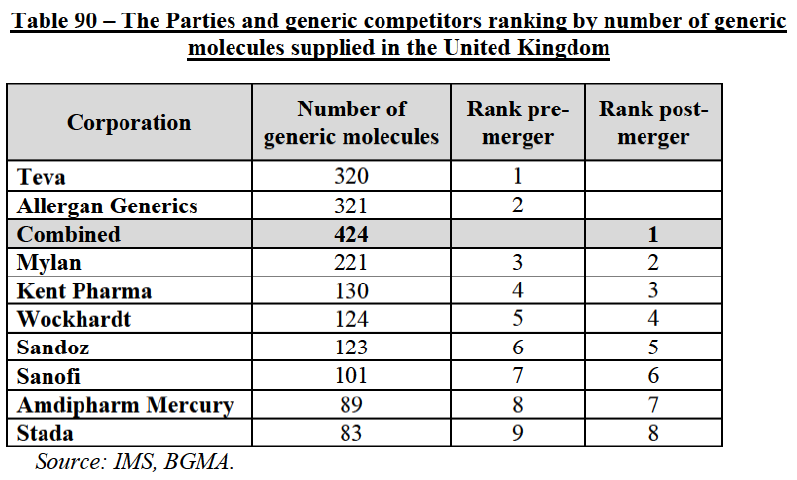
(548) The breadth of the Parties' portfolio is explained in part by their strong position in R&D for generics, allowing them to be more efficient and faster in launching new generic products in the United Kingdom than their respective market shares would suggest. Indeed, out of the 140 new generic launches in the United Kingdom in 2012-2015, Teva was present with its generic version in 70.7% of cases, Allergan Generics in 72.1% of cases. This is to be compared with the performance of Sandoz (47.1% of cases), Sanofi (44.3% of cases) and Mylan (19.3% of cases). Furthermore, the Parties launch their products on average quicker than their main competitors: Teva's launch was in the first 3 launches in 27.1% of cases, Allergan Generics in 22.1% of cases (versus Sandoz in 12.9% of cases, Sanofi in 5.7% of cases, Mylan in 19.3% of cases).
(549) The position of wholesalers is assessed further below. It should be noted from the outset that, in the United Kingdom, there are three national broad-line wholesalers (McKesson, Walgreens Boots Alliance and Phoenix), which each own a pharmacy chain (Lloyds Pharmacy, Boots and Rowlands Pharmacy, respectively), as well as a number of regional broad-line wholesalers and short-line wholesalers.
Segmentation by type of customers
(550) As to the possible segmentation by type of customers, the Parties provided estimates of their market shares based on a possible distinction between pharmacy chains that belong to a wholesaler, independent pharmacy chains,378 independent pharmacies that do not belong to a pharmacy chain, dispensing doctors and hospitals.
(551) The Commission considers that the Parties' estimates have a number of methodological limitations. First, the Parties provided their value sales as net sales, while the market size has been based on the so-called Value-at-Tariff, which is for prescription drugs the reimbursement price paid back by the National Health Service, and for non-prescription drugs the average retail price (i.e. the sales revenue from a pharmacy's perspective, not from the Parties' perspective). Therefore, the Parties made assumptions to apply a prorata conversion rate to their internal sales to obtain a figure comparable to the Value-at-Tariff data, which are not precisely detailed in their submission. Second, the Parties do not record their own sales per customer in Standard Units,379 and therefore have instead used the number of packs sold to pharmacies as a measure of the total market size in volume. However, the number of packs is unlikely to be a measure as reliable as Standard Units to aggregate volumes from different pharmaceuticals and pharmaceutical forms.380 Third, the Parties were unable to provide estimates of the share of supply of their wholesale competitors following the segmentation by type of customer. Finally, there seem to be inconsistencies between the Parties' estimates and data provided by the Parties in other submissions.381
(552) Based on the Parties' estimates, the Parties would have a significant combined market share in the segment of independent pharmacy chains: [50-60]% in 2012 and 2013 and [40-50]% in 2014 and 2015 in volume, [50-60]% in 2012, [40-50]% in 2013, [30-40]% in 2014 and 2015 in value. Their combined market share would be lower for independent pharmacies and dispensing doctors: ranging from [5-10]% to [10-20]% in volume and value over the last four years. However, due to the abovementioned methodological limitations, it cannot be excluded that the Parties' shares may be underestimated. By way of illustration, the Parties had a combined market share of [2030]% in value in 2014 for the supply of generics, which translates in a combined market share of [10-20]% in value for the wholesale of generics to all categories of customers (which they achieve through their DTP sales). This suggests that their proportion of DTP sales is [50-60]% on average, a number which could not be reconciled with the Parties' submission showing that DTP represented [70-80]% of Teva's and [50-60]% of Allergan Generics' net sales in 2014.
(553) Furthermore, as evidenced below, the Parties' are each other's closest competitors in the wholesale market of generics in the United Kingdom.
Segmentation by type of suppliers - Short-line vs. broad-line wholesalers and the Parties
(554) The Parties are the only generic manufacturers having sizeable DTP activities in the United Kingdom. Their competitors on the market are three national broad-line wholesalers (McKesson, Walgreens Boots Alliance and Phoenix) as well as the regional broad-line wholesalers and the short-line wholesalers.
(555) As discussed above, the Parties have not been able to provide a breakdown of the position of their competitors on the market. The only available data the Parties have in relation to the wholesalers' market shares are (a) not segmented by customers and (b) include in the wholesalers' sales the sales that they deliver on behalf of the manufacturers for their DTP schemes. According to this estimate, for the twelve months ending November 2015, the short-line wholesalers' generic activities would represent [60-70]% in value and [70-80]% in volume of the broad-line wholesalers' generic activities overall.382
(556) The Parties submit that short-line wholesalers should not be considered as belonging to a different market segment since (i) based on economic evidence, some short-line wholesalers (e.g. Trident, Waymade) would be as price competitive as the Parties and (ii) some short-line wholesalers would have a better market coverage than some broad-line wholesalers and the Parties (e.g. Lexon).
(557) The Commission considers, first, that the Parties did not provide information as to whether short-line wholesalers' offering significantly overlap or not with the Parties' and broad-line wholesalers' offering in terms of products. Second, the Parties' analysis does not take into account the level of services offered by short-line wholesalers, which was an element retained by the Commission in its previous practice to consider that they would not belong to the same market as broad-line wholesalers.383
(558) The market investigation also provided elements showing that the Parties and the broad-line wholesalers would be more closely competing than vis-à-vis short-line wholesalers.
(559) Indeed, pharmacies would generally receive the price lists (usually with monthly updates) of one broad-line wholesaler and the two Parties, so as to benefit from schemes.384 As indicated by one market participant, "pharmacies (chains as well as individual pharmacies) have a preference for concentrating their purchasing of generic medicines with a limited number of generic suppliers, frequently in return for and with the aspiration of getting discounts/rebates across the portfolio".385 By way of example, one pharmacy indicated "we are on Teva One scheme which gives us a competitive price, and save us time from comparing prices from other suppliers."386 In that respect, it should be noted that, contrary to the Parties' submission, during the market investigation, pharmacies never mentioned the importance of price comparison platforms in their purchase of generic pharmaceuticals.
(560) The importance of the Parties and the two broad-line wholesalers, McKesson and Walgreens Boots Alliance, is also highlighted by the fact that they are the only four suppliers on the basis of which the reimbursement price of generic products belonging to the so-called category A is defined by the United Kingdom Department of Health.387 The Department of Health calculates the actual reimbursement price based on a weighted average of the list price from the four suppliers, with McKesson and Walgreens Boots Alliance having a weighting of two, and each Party having a weighting of one.
(561) In light of the limited data available, the Commission has assumed that the split between short-line wholesalers and national/regional broad-line wholesalers does not vary depending on the category of customers. This allows for the calculation of market share estimates for the plausible market segmentation between the Parties and (national/regional) broad-line wholesalers on the one hand, and short-line wholesalers on the other hand. Based on this estimate, in the segment of independent pharmacy chains (excluding the short-line wholesalers), the Parties' combined market share would be: [50-60]% in 2014 and [50-60]% in 2015 in volume and [40-50]% in 2014 and 2015 in value.
Closeness of competition between the Parties
(562) The market investigation highlighted that the Parties would exert a unique competitive constraint on each other, distinct from the one exerted by broad-line wholesalers, and based on both price and non-price elements.
(563) Prices were consistently identified by pharmacists as an advantage of direct purchases from manufacturers (or a disadvantage of purchases from a wholesaler).388 This finding could be explained by the Parties' having a more efficient cost structure (in particular, they do not bear the search costs of the wholesalers sourcing their offering from a network of generic manufacturers).
(564) The Parties provided an economic submission to support the fact that they would not be more price competitive than wholesalers at least for the molecules present in both Parties' schemes.389
(565) The Commission considers that this analysis does not take into account the fact that price competitiveness should be assessed overall on a basket of products (including overlapping and non-overlapping molecules offered under the Parties' schemes). Indeed, as noted by one market participant, the Parties "can place extremely competitive pricing on some molecules whilst leveraging higher prices on others in order for contractors to obtain maximum rebates across the range as a whole."390 The result of the Parties' analysis can also be put in perspective of the fact that, according to their calculations, Walgreens Boots Alliance would be the least price competitive of the sample analysed, with less than [0-5]% of its portfolio being the lowest priced product. This contrasts with Walgreens Boots Alliance's position on the market (it is one of the two main broad-line wholesalers) and is therefore a likely indication of methodological shortcomings in the analysis.
(566) Teva also provided switching data is collected in its ordinary course of business. Indeed, it recorded over [...] pharmacies in 2015 that indicated to Teva sales staff that they will no longer use Teva as their preferred supplier of generics products. According to Teva, while some losses are due to pharmacy closures ([20-30]%), [60-70]% of pharmacies indicated that they had switched to alternative suppliers offering a more competitive pricing (Allergan Generics representing only [0-5]% of Teva's estimated lost spend). However, a major limitation of such analysis comes from the representativeness of the sample, in particular due to its declarative nature (some pharmacies may choose not to communicate the right reason for switching away from Teva, or the right alternative supplier) and the short time span covered (only one year). Furthermore, the Parties were unable to provide a similar analysis for Allergan Generics.
(567) As to non-price elements, respondents to the market investigation consistently indicated that the Parties offer differentiated services in comparison to broad-line wholesalers. For instance, pharmacies indicated that purchasing directly from manufacturers would ensure a better continuity of supply and consistency of packaging than purchasing through wholesalers.391 By way of example, one pharmacy indicated that "we purchase direct because we get stock with long expire date, can get the quantity we order, and keep the same packaging".392 Indeed, when purchasing a given product from wholesalers, pharmacies can receive packs from different manufacturers over time, as wholesalers generally include in their price list the cheapest generic they have in stock, without even specifying the brand to the customer.393 This switching of packages across manufacturers can have a negative impact on certain categories of patients: "we are very particular about packaging for our elderly patients, who could be easily confused with different packaging, from different generic suppliers".394 This is recognised by Teva itself which in one of its submissions395 indicates that "pack design plays a role in improving accuracy of the dispensing process, in improving pharmacist and patient medicine recognition through the use of unique colour combinations, and in driving medicine optimisation and patient adherence". These competitive features represent an important advantage of manufacturers selling directly compared to wholesalers which provide offerings of different manufacturers over time, and therefore inconsistent packaging.
(568) Furthermore, the reliability of supply and control over the supply chain is also considered as an advantage of direct purchase from manufacturers.396 Indeed, in case of a shortage, the customer can contact the manufacturer directly and obtain a better update on the situation as the sales representative will have a direct overview of the supply situation (contrary to wholesalers which have to contact their generic suppliers).
(569) For instance, questioned on why they would prefer purchasing through manufacturers' schemes rather than from wholesalers, one pharmacy indicated that "where we do operate direct we tend to see better trading performance as we are able to engage, get quicker feedback, build brand activation plans and get better category insights;397 and another one that "you have direct link to manufacturer and have conversations not on price but on supply issues arising from production delays/machinery breakdowns".398
(570) The closeness of competition between the Parties is also reflected by the fact that they have a similar portfolio composition, with the overwhelming majority of their sales ([70-80]% for Teva and [70-80]% for Allergan Generics) occuring on overlapping molecules.
(571) Finally, the Parties are typically part of a so-called generics "cascade mechanism", where customers can specify to their wholesaler of choice a list of generic manufacturers/wholesalers to go to in case the preferred supplier is subject to a shortage.399 For instance, for a customer "the AAH Generics Scheme cascades to Teva followed by Actavis where the AAH Generics Scheme is out of stock. This cascade is managed by AAH".
(572) All such factors explain why almost all pharmacies indicated that Teva and Allergan Generics are each other's closest competitors in the United Kingdom.400
(573) Based on the abovementioned elements, the Commission concludes that Teva and Allergan Generics are each other's closest competitors in the market for the wholesale of generics in the United Kingdom.
Impact on the market
(574) The United Kingdom is a free pricing market for generics. The Commission's investigation has established that the schemes of Teva and Allergan Generics contribute to bringing down prices in the United Kingdom, through the competitive pressure they exert on each other.
(575) By way of example, one market participant indicated in particular that "unlike some European markets the generic marketplace is predominantly commodity based, that is the price is defined by the number of competitors and the availability of product, which dictates market pricing. The United Kingdom has two manufacturers, Teva and Actavis that promote schemes, [...] giving rebates to customers against sales and portfolio ordered".401
(576) Post-Transaction, this unique competition between Teva and Allergan Generics' schemes on prices (and level of discounts) would not exist any longer. This concern was expressed by market participants, including customers. By way of example, one pharmacy indicated that, following the transaction, "competition will be reduced leading to a poorer service and higher prices as already seen with a lot of the DTP schemes whereby competition has been eroded between the various wholesalers" while another one concludes that "prices will rise, ultimately either costing the NHS more or reducing the accessibility of pharmaceutical services".402
(577) Finally, as evidenced above, the Transaction will lead to the loss of one of the only two generic manufacturers with sizeable DTP activities in the United Kingdom, which offers a differentiated service compared to wholesalers, with a quality and consistency of supply that is highly valued by pharmacists.
Conclusion
(578) In view of these market features, the Parties' overall presence and their closeness of competition, as well as the likely impact on prices and quality of supply of generic medicines, based on all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the wholesale market for generic pharmaceuticals in the United Kingdom.
IV.2.2.29. Pipeline-to-pipeline overlaps
(579) In relation to pipeline-to-pipeline affected markets (both Parties are developing a pipeline for a pharmaceutical where there is no or a single generic alternative on the market, i.e. one or two competitors including the originator), the Commission has identified whether a sufficient number of credible competitors would also be developing a pipeline for the same pharmaceutical, entering under a similar timeframe as the Parties. In particular, the Commission has asked competitors of the Parties whether there would be planning to enter with their own generic product, under which timeframe, and in which EEA countries.403
(580) For a number of markets for which generic competition is currently non-existent or limited (zero or one generic product available) and both Parties are planning to enter with their own generic product, the Commission found that an insufficient number of credible competitors would enter with a generic alternative under a similar timeframe as the Parties. Given that the merged entity would likely have little incentive to keep and invest in the development of two competing pipelines for the same generic molecule, the loss of one generic alternative stemming from the Transaction would reduce the (already limited) generic competition on these markets, leading to higher prices for patients and payers in the relevant EEA countries compared to the counterfactual where the two generic products of the Parties would likely compete on price once approved.
(581) Accordingly, the following table lists the pipeline FDPs for which the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market in relation to a number of EEA countries where both Parties are planning to enter and, based on the market investigation, an insufficient number of credible competitors are planning to enter:
(582) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility with the internal market with respect to the supply of the molecules listed in the above table.
(583) It should be noted that, for a number of these molecules, either (a) at least one Party already has a marketed product in certain EEA countries or (b) the Parties are planning to enter together in a limited number of EEA countries. These two facts are taken into account when assessing the proposed remedies in relation to pipeline-to-pipeline overlaps.
IV.2.2.30. Originator-to-generic pipeline overlaps
IV.2.2.30.a. Copaxone (glatiramer acetate)
(584) Copaxone is Teva's flagship blockbuster pharmaceutical, accounting for about half of Teva's profit and a fifth of its global sales.404 It is used for the treatment of multiple sclerosis. Copaxone's main patent expired in May 2015.
(585) An originator / generic overlap arises as Allergan Generics has partnered with a third party, [...], to market a generic version of glatiramer acetate for launch in the EEA. [...] [...].405
(586) [...]406[...].
(587) [...] has demonstrated to the Commission its ability and incentive to market its generic glatiramer acetate in the EEA (itself or in partnership with third parties). However, it indicated that it expects Allergan Generics to support [...] with the transfer of the marketing authorization application to [...], and fully cooperate with regulatory authorities as requested.407 The market investigation indicated that the merged entity may have the ability to delay, and possibly frustrate, the regulatory process leading to the granting of the marketing authorisation within the terms of the existing contractual arrangements. As such behaviour would result in a delayed entry of [...] generic glatiramer acetate on the market, it would extend the period in which Copaxone does not face competition. Given the importance of Copaxone in terms of sales and profits, it is clear that the merged entity would have the incentive to pursue such a strategy, which would lead to higher prices for patients and payers for a prolonged period of time.
(588) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raise serious doubts as to its compatibility with the internal market with respect to glatiramer acetate.
IV.2.2.30.b. Azilect (rasagiline)
(589) Teva's innovative portfolio includes Azilect, which is indicated for the treatment of the signs and symptoms of Parkinson’s disease.
(590) Allergan Generics has in its pipeline a generic version of Azilect. Allergan Generics has not launched its product in any EEA country, with the exception of the United Kingdom where it launched a generic product in August 2015. In September 2015, Teva obtained a preliminary injunction preventing the sale of Allergan Generics' product until the final determination of the litigation, on the ground that such product infringed one of Teva's formulation patents expiring in 2030. In October 2015, the Parties settled the United Kingdom litigation related to rasagiline. [...]. Therefore, Allergan Generics is [...]408 [...] enter the United Kingdom rasagiline market before 1 October 2016.
(591) [...]. Furthermore, Allergan Generics has already been granted a number of marketing authorisations in the countries where it was planning to launch its generic rasagiline.
(592) Given the importance of Azilect in terms of sales (it is Teva's fourth best-selling product overall after Copaxone, Treanda and ProAir), it is clear that the merged entity would have the incentive to discontinue or otherwise significantly delay Allergan Generics' generic rasagiline, which would lead to decreased generic competition and higher prices for patients and payers, compared to the counterfactual where Allergan Generics' product would likely compete on price with the other generic products once approved.
(593) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raise serious doubts as to its compatibility with the internal market with respect to rasagiline in [...] and [...].
IV.3. Out-licensing
(594) In out-licensing, typically, the out-licensor licenses to the out-licensee rights to use a dossier (upstream market) to obtain a marketing authorisation in one or several countries and subsequently commercialize the FDP in the relevant countries (downstream market) under its own name.
(595) Out-licensing agreements are generally accompanied by a supply agreement by which the out-licensor supplies the licensee with the FDP in question, which is produced either by the licensor, or by a third party (the latter case being called "contract manufacturing").409
IV.3.1. Market definition
IV.3.1.1. Product market definition
(596) In its previous decisions,410 the Commission considered out-licensing as a separate market which is upstream of the markets for the supply of FDPs and assessed the out-licensing relationship for each individual molecule (or API) as potentially constituting a relevant market.
(597) Neither the Notifying Party nor the market investigation provided any indications that the Commission should depart from this approach.
(598) The exact scope of the product market can be left open in the present case, because the competitive assessment would not change under any potential product market definition.
IV.3.1.2. Geographic market definition
(599) As regards the geographic scope of the market for out-licensing, the Commission previously considered the market to be at least EEA-wide.411
(600) Neither the Notifying Party nor the market investigation provided any indications that the Commission should depart from this approach.
(601) The exact scope of the geographic market can be left open in the present case, because the competitive assessment would not change under any potential geographical market definition.
IV.3.2. Competitive assessment
(602) In relation to out-licensing, in 2014, Teva generated worldwide sales of EUR [...] million, most of which was derived from sales in the EEA,412 and Allergan Generics (through its subsidiary Medis) generated worldwide sales of EUR [...] million, of which EUR [...] million was derived from sales in the EEA. Allergan Generics, through Medis, out-licenses to other companies the right to use Allergan Generics' dossiers and market the corresponding FDPs downstream, possibly in competition with Allergan Generics and/or Teva.
(603) Although the Parties were not able to estimate the exact position of Medis in the EEA out-licensing area, Medis would be one of the most important out-licensor in the EEA, competing with companies such as Krka, Synthon, Cipla, Intas and Sun Phanna.
(604) Medis' top 10 customers in the EEA over the last three years are provided in the table below.
(605) Aurobindo is [...], following its acquisition of Allergan Generics' businesses in France, Italy, Spain, Portugal, Belgium, Gennany and the Netherlands (the "Allergan/Aurobindo Transaction").413 Medis and Aurobindo concluded a [...]-year License and Supply agreement pursuant to which Medis supplies Aurobindo with the products covered by the Allergan/Aurobindo Transaction which Aurobindo does not yet manufacture itself.
IV.3.2.1. Methodology
(606) Due to the lack of reliable market share data at the level of the upstream out-licensing markets, the Notifying Party identified affected markets at the downstream level using the market shares of the out-licensee(s) as a proxy.
Vertically affected markets
(607) In line with the Commission's decisional practice,414 the Notifying Party identified vertically affected markets where (a) one party is active on a downstream FDP market and (b) the other party is active upstream as an out-licensor of a downstream competitor and (c) the combined market share of the Parties and the licensee(s) is in excess of 25%.
(608) The Notifying Party further identified Group 1 vertically affected markets where the combined market share of the Parties and the licensee(s) is in excess of 35% and the increment is over 1%.
(609) Based on this methodology, the Notifying Party identified 293 vertically affected markets (of which 177 are Group 1 vertically affected markets).
Horizontally affected markets
(610) The Notifying Party identified horizontally affected markets where (a) both Parties out-license a dossier for the same molecule (API) to competitors which are active downstream on the same FDP market and (b) the share of sales of the out-licensees in the FDP market downstream is above 20%. The Notifying Party further identified Group 1 horizontally affected markets where the share of sales of the out-licensees in the FDP market downstream is above 35%.
(611) Based on this methodology, the Notifying Party identified 6 horizontally affected markets (of which 1 is a Group 1 horizontally affected market).
IV.3.2.2. The Notifying Party's views
Vertically affected markets
(612) The Notifying Party considers that the merged entity will not have the ability and incentive to engage in an input foreclosure strategy or a customer foreclosure strategy post-Transaction in relation to all the affected markets.
(613) The Notifying Party argues that such strategies are unlikely because of:
a. the nature of the out-licensing arrangements, and in particular the merged entity inability to terminate out-licensing agreements with its customers;
b. the existence of numerous alternative source of supply for FDPs and marketing authorizations, the low barriers to entry for out-licensing and the ease for customers to switch;
c. the co-existence of out-licensing and direct supply for the same molecule by one and the same pharmaceutical company to maximise the company's overall revenue.
Horizontally affected markets
(614) The Notifying Party considers that the Transaction does not raise competition concerns, in particular since, for the only Group 1 horizontally affected market, sufficient alternative out-licensors would remain post-Transaction and barriers to entry for new out-licensors would be low.
IV.3.2.3. The Commission's assessment
(615) The Commission focused its analysis on all Group 1 affected markets.
Horizontally affected markets
(616) The Transaction does not raise serious doubts as to its compatibility with the internal market with respect to horizontally affected markets regarding out-licensing.
Vertically affected markets
(617) In light in particular of the combined market shares of the Parties, their out-licensee(s) and their competitors in the downstream markets, the Commission concluded that the Transaction would not have a significant effect on competition and thus excluded competitive concerns in a number of Group 1 affected markets.
(618) For the other Group 1 affected markets, the Commission's concerns relate to a risk of input foreclosure strategy, i.e. the merged entity deciding not to out-license and supply a number of molecules to customers with which it is competing downstream.415
(619) According to the Commission's guidelines on non-horizontal mergers,416 input foreclosure arises where, post-merger, the merged entity is likely to restrict access to the products that it would have otherwise supplied absent the merger. In assessing the likelihood of such scenario, the Commission examines, first, whether the merged entity would have, post-merger, the ability to substantially foreclose access to inputs, second, whether it would have the incentive to do so, and third, whether a foreclosure strategy would have a significant detrimental effect on competition downstream.417
Ability of the merged entity to foreclose
(620) In the case at hand, the ability to foreclose access to inputs requires that the merged entity is in a position to terminate its relationships with its out-licensees and that the latter cannot without significant costs and time turn to a new out-licensor.418
(621) On the merged entity's ability to terminate its relationships, contrary to the Parties' views, the market investigation indicated that the out-licensor generally has the ability to terminate its supply relationships with its out-licensee. Indeed, while Medis' out-licensing agreements (license on the IP rights) are concluded for an indefinite duration,419 the contract pursuant to which Medis supplies the products (the manufacturing contract) is concluded for a fixed term and, with the exception of Medis' agreements during the supply exclusivity period, can be terminated without penalties.420
(622) The market investigation indicated that the most critical aspect of an out-licensing relationship is the manufacturing contract, both for the out-licensor421 and for the out-licensee.
(623) As to the out-licensees, the market investigation higlighted that, in most cases, they would obtain the product from the out-licensor422 and have very rarely changed out-licensor following the exclusivity period.423 The Parties corroborated this finding, indicating that [70-80]-[80-90]% of their out-licensees would continue the supply agreement after the exclusivity period.
(624) In addition, the market investigation suggested that the technological transfer would not be an option for many out-licensees: one noted that "it is expensive to have a dossier from one company and to supply from another",424 while another argued that "[to supply from the out-licensor] is the most effective procedure at least in the course of the launching phase".425 Another respondent "suppl[ies] a product from a licensor due to lower costs linked with technological transfer, time saving linked with launching the product, advantages in negotiating the price and the licence fee",426 while another mentioned that "it is easier for us [to supply from the out-licensor] and we do not have the structure to manufacture ourselves".427
(625) Lastly, some of the respondents to the market investigation suggested that the process of technological transfer to a third party supplier is a costly and time-consuming procedure depending on the complexity of the project, and requiring between 6-12 months and EUR 25,000 to 100,000.428 To change out-licensor is even more demanding and requires according to the market investigation 1.5 to 3 years and around EUR 200,000 EUR.429 In addition, almost all current out-licensees of the Parties who responded to the market investigation were unable to identify an alternative out-licensor for the FDPs provided.430
(626) The Notifying Party did not provide an analysis of its ability to foreclose per molecule individually.
(627) In view of the above and of all the evidence available to the Commission, the Commission considers that the merged entity may have an ability to foreclose its out-licensees.
Incentive of the merged entity to foreclose
(628) Contrary to the Parties' view, the fact that a pharmaceutical company, like Allergan Generics, may be active on the same FDP market both directly and indirectly (through outlicensees), does not preclude the merged entity having the incentive to terminate its out-licensing relationships post-Transaction.
(629) The market investigation indicated that in some cases companies would indeed prefer not to out-license a product in view of their presence downstream, and that for instance Medis would refuse out-licensing certain products due to Allergan Generics' direct market presence on the same markets.431
(630) A number of out-licensees expressed concerns on the impact of the Transaction on the out-licensing upstream markets due to the merged entity's presence downstream: one indicated that "clients are also worried [of] continuity of supplies in cases of scarcity of a product, where most likely Medis/Teva will favour supplying to its own subsidiaries instead of to Medis’ out-licensing clients",432 another argue that "if [its] activity is restricted, this could impact availability of new products and supply of current products",433 and a third respondent mentioned that "both companies have their own field forces in EEA, and there is a possibility that they will compete with their licence-out partners and in case of supply, they will prefer their own affiliates".434
(631) In line with the Commission's guidelines,435 the incentive to foreclose depends on the degree to which the foreclosure would be profitable, i.e. is a trade-off between the profit lost in the upstream market due to a reduction of input sales to competitors downstream and the profit gain from expanding sales downstream. In addition, the greater the market share of the merged entity downstream, the greater the base of sales on which to enjoy increased margins.
(632) The Notifying Party did not provide any economic analysis on the incentive of the merged entity (or lack thereof) to foreclose the affected markets.
(633) The Commission carried out its own analysis on the basis of data requested from the Parties, i.e. the gross margin upstream and downstream regarding affected markets, together with the market shares of the Parties, the out-licensees and their competitors downstream.
(634) Based on the available data on Group 1 affected markets, the Commission assessed whether an input foreclosure scenario would be profitable under the assumption of the merged entity gaining the market share of the out-licensee(s) downstream and possibly increasing its margins. 436
(635) As a result, the Commission identifies 103 vertically affected Group 1 markets (out of the 177 Group 1 vertically affected markets identified by the Parties), for which the Parties may have the ability and incentive to foreclose the out-licensees.
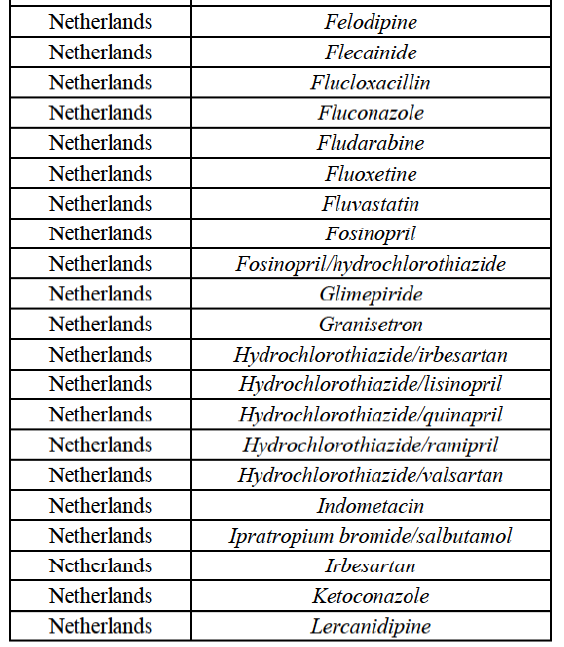
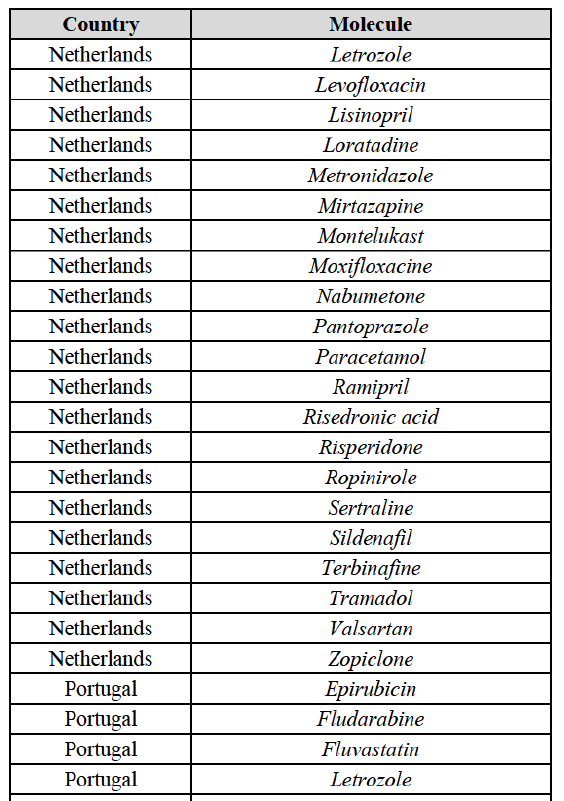
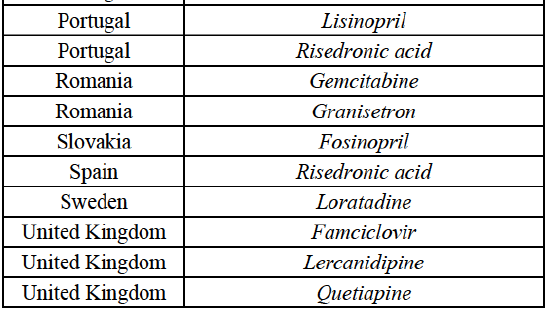
Detrimental effect on competition
(636) In line with the Commission guidelines,437 a merger will raise competitive concerns because of input foreclosure when it would lead to increased prices in the downstream market.
(637) In this case, by foreclosing access of one important competitive force (in view of the out-licensee's and the merged entity's market share downstream) to a key input (molecules/APIs) to compete downstream, there would be a risk of price increase downstream for all the above listed molecules.
(638) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction raises serious doubts as to its compatibility of the Transaction with the internal market in relation to the markets identified in the table above.
IV.4. Active pharmaceutical ingredients
(639) The API is the substance in a FDP that is pharmaceutically active and is suspended in excipients (that is, inert substances taking the form, for instance, of a tablet or a solution), for the purpose of administration.
(640) Teva has been an active supplier of APIs for many decades, including to internal Teva business units, through its subsidiary Teva Active Pharmaceutical Ingredients ("TAPI"). It has around [...] APIs (commercially available or under development) in its portfolio.
(641) Allergan Generics only has 6 APIs in its portfolio.438
(642) No horizontally affected markets arise as the Parties sell different APIs to third parties.
(643) However, as a result of the Transaction, a number of vertical relationships arise between Teva's TAPI activities, upstream, and Allergan Generics activities at the level of marketing of FDPs, downstream.
IV.4.1. Market definition
IV.4.1.1. Product market definition
(644) In the past, the Commission considered that APIs constitute separate markets that are upstream to the markets for marketing FDPs and that each individual API potentially constitutes a separate product market, whilst noting that it was not excluded that certain APIs may be substitutable with each other for all, or for a range of, applications.439
(645) In view of the fact that the Transaction does not raise serious doubts as to its compatibility with the internal market in relation to the API markets under any plausible market definition, the exact scope of the product market can be left open for the purposes of the competitive assessment of the Transaction.
IV.4.1.2. Geographic market definition
(646) In the past, the Commission considered that API markets are, from a geographic perspective, at least EEA-wide and possibly global in scope.440
(647) In view of the fact that the Transaction does not raise serious doubts as to its compatibility with the internal market in relation to the API markets under any plausible market definition, the exact scope of the geographic market can be left open for the purposes of the competitive assessment of the Transaction.
IV.4.2. Competitive assessment
(648) The Parties provided market share data at a worldwide level. Due to lack of data, the Parties were not able to provide the relevant market shares at the EEA level. However, they submitted that their worldwide market shares are representative of their position in the EEA, and, if anything, overestimate their EEA market shares.
(649) In line with the Commission's previous practice,441 the Notifying Party identified:
a. 136 upstream vertically affected markets, for which Allergan Generics holds a market share in excess of 30% on a downstream FDP market defined at the molecule level and, at the upstream level, Teva holds more than 5% of a corresponding API market;
b. 33 downstream vertically affected markets, for which Teva holds a market share in excess of 30% on an API market and Allergan Generics holds more than 5% on the corresponding downstream FDP market defined at the molecule level.
(650) In the instances where Allergan Generics has a significant market share in the downstream FDP market (upstream vertically affected markets), the API volumes sold for the purpose of manufacturing the FDP in the limited number of relevant country(ies) are very small compared to the corresponding total worldwide volume for the API. Since suppliers of APIs are active globally, a potential customer foreclosure strategy by the merged entity of competing API suppliers would have a negligible effect on the API markets.
(651) Furthermore, in the instances where Teva has a significant market share in the upstream API market (downstream vertically affected markets), an input foreclosure strategy is highly unlikely. Indeed, the API volumes needed by the competitors of Allergan Generics on the downstream markets are very limited compared to the overall demand, and could be easily covered by the remaining API competitors upstream.442 Furthermore, some of downstream competitors of Allergan Generics are vertically-integrated with respect to APIs, and therefore not affected by any potential input foreclosure strategy.
(652) Finally, respondents to the market investigation did not express concerns in relation to APIs.
(653) In view of the above and of all the evidence available to the Commission, the Commission considers that the Transaction does not raise serious doubts as to its compatibility with the internal market with respect to APIs.
V. COMMITMENTS
V.1. Framework for the assessment of the Commitments
(654) Where a concentration raises serious doubts as regards its compatibility with the internal market, the Parties may undertake to modify the concentration so as to remove the grounds for the serious doubts identified by the Commission. Pursuant to article 6(2) of the Merger Regulation, where the Commission finds that, following modification by the undertakings concerned, a notified concentration no longer raises serious doubts, it shall declare the concentration compatible with the common market pursuant to article 6(1)(b) of the Merger Regulation.
(655) As set out in the Commission’s Remedies Notice,443 the commitments have to eliminate the competition concerns entirely, and have to be comprehensive and effective from all points of view.444
(656) In assessing whether commitments will maintain effective competition, the Commission considers all relevant factors, including the type, scale and scope of the proposed commitments, with reference to the structure and particular characteristics of the market in which the Transaction is likely to significantly impede effective competition, including the position of the Parties and other participants on the market.445
(657) In order for the commitments to comply with those principles, they must be capable of being implemented effectively within a short period of time. Concerning the form of acceptable commitments, the Merger Regulation gives discretion to the Commission as long as the commitments meet the requisite standard. Structural commitments will meet the conditions set out above only in so far as the Commission is able to conclude with the requisite degree of certainty, at the time of its Decision, that it will be possible to implement them and that it will be likely that the new commercial structures resulting from them will be sufficiently workable and lasting to ensure that effective competition will be maintained.446 Divestiture commitments are normally the best way to eliminate competition concerns resulting from horizontal overlaps.
(658) It is against this background that the Commission analysed the proposed Commitments in this case.
V.2. Commitments submitted by the Parties
(659) In order to ensure that effective competition will be maintained, the Parties submitted a set of commitments under Article 6(2) of the Merger Regulation on 18 February 2016 (the "Initial Commitments"). The Commission market tested the Initial Commitments in order to assess whether they are sufficient and suitable to remedy the serious doubts identified above. Following the feedback received during the market test, the Initial Commitments were refined and improved, and amended commitments were submitted on 4 March 2016 (the "Final Commitments"). These Final Commitments are annexed to this Decision and form an integral part thereof.
V.2.1. Initial Commitments
V.2.1.1. Marketed pharmaceuticals (except in the United Kingdom, Ireland and Iceland)
(660) In order to dispel the serious doubts identified in relation to the marketed generic pharmaceuticals in Austria, Belgium, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Italy, Latvia, Lithuania, the Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain and Sweden, the Parties submitted commitments consisting of the rights, titles and interests of one Party in 159 molecule/country pairs447 (the "Other Countries On-Market Divestment Businesses").
V.2.1.2. Pipeline pharmaceuticals (except in the United Kingdom, Ireland and Iceland)
(661) In order to dispel the serious doubts identified in relation to the marketed-to-pipeline overlaps in Belgium, Croatia, Czech Republic, Denmark, Estonia, Greece, Hungary, Latvia, Lithuania, the Netherlands, Norway, Poland, Portugal, Romania and Sweden, the Parties submitted commitments consisting of the rights, titles and interests of one Party448 in 20 molecule/country pairs (the "Other Countries Marketed-to-Pipeline Divestment Businesses").
(662) In order to dispel the serious doubts identified in relation to the pipeline-to-pipeline overlaps in Austria, Cyprus, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Malta, Poland, Portugal and Romania, the Parties submitted commitments consisting of the rights, titles and interests of one Party in 32 molecule/country pairs (the "Other Countries Pipeline-to-Pipeline Divestment Businesses").
(663) In order to dispel the serious doubts identified in relation to the pipeline-to-pipeline overlaps EEA-wide,449 the Parties submitted commitments consisting of the rights, titles and interests of one Party in the relevant molecules in 14 molecules (the "EEA Pipeline-to-Pipeline Divestment Businesses").
V.2.1.3. United Kingdom and Ireland
(664) In order to dispel the serious doubts arising in relation to their activities in the United Kingdom and Ireland, the Parties submitted commitments consisting in particular in the following assets from Allergan Generics (the "UK-IE Divestment Business"):
a. 274 marketed and 62 pipeline generic pharmaceuticals for the United Kingdom, covering at least all molecules marketed by Allergan Generics which overlapped with Teva's products in 2015;
b. 81 marketed and 42 pipeline generic pharmaceuticals for Ireland, covering at least all molecules marketed by Allergan Generics which overlapped with Teva's products in 2015;
c. a manufacturing site located in Barnstaple, United Kingdom, where most of the generic pharmaceuticals of the UK-IE Divestment Business are manufactured;
d. 653 employees in the United Kingdom (of which 441 employees for the operations of the Barnstaple site) and 21 employees in Ireland, covering in particular the sales & marketing, regulatory & medical and general & administrative functions;
e. the Actavis brand in the United Kingdom and Ireland.
(665) The Initial Commitments provide that the purchaser should enter with Teva into a supply and services agreement at cost, for three years (renewable for two additional years), for all the products manufactured and/or packed at the Barnstaple site that will be retained by Teva.
V.2.1.4. Iceland
(666) In order to dispel the serious doubts arising in relation to their activities in Iceland, the Parties submitted commitments consisting in particular in the following assets from Teva (the "IS Divestment Business"):
a. 55 marketed and 37 pipeline generic pharmaceuticals, covering all marketed and pipeline molecules of Teva in 2015 in Iceland;
b. the Ratiopharm brand in Iceland.
(667) For each marketed or pipeline generic pharmaceutical (as applicable), the IS Divestment Business includes the same assets and transitory arrangements as for the other countries.
V.2.1.5. Copaxone (glatiramer acetate) and Azilect (rasagiline)
(668) In order to dispel the serious doubts arising in relation to Copaxone (glatiramer acetate), Teva and Allergan Generics commit that they will comply with all the steps agreed upon with [...] to terminate their licence of [...] marketing authorization application for its generic glatiramer acetate.
(669) In order to dispel the serious doubts arising in relation to Azilect (rasagiline), Teva commits to divest Allergan Generics' pipeline generic rasagiline [...]. Finally, for the United Kingdom, Allergan Generics' marketed generic rasagiline is included in the UK-IE Divestment Business.
V.2.1.6. Out-licensing
(670) In order to dispel the serious doubts arising in relation to out-licensing, the Parties' commitments are twofold.
(671) First, for 39 molecule/country pairs which are out-licensed to Aurobindo, the Parties commit that Medis will maintain its relationship with Aurobindo on terms equivalent to those that currently apply to those molecules for a duration of four years as from the date of adoption of the decision (the "Aurobindo Products Commitments").
(672) Second, for another 71 molecule/country pairs, Teva commit to divest either (i) the out-licensing business conducted by either Medis or Teva for the relevant product (the upstream business) or (ii) the marketed generic pharmaceutical of either Teva or Allergan Generics in the relevant country (the downstream business).
V.2.1.7. Purchaser criteria
(673) In addition to the Commission's standard text, the Initial Commitments provided that:
a. The UK-IE Divestment Business shall be sold to a single purchaser;
b. The Purchaser(s) shall be an established pharmaceutical company having the incentive and ability to become independent of the Notifying Party with respect to the manufacturing of the divested products;
c. With respect to the IS Divestment Business, the Purchaser shall have the incentive and ability to serve the Iceland market from another EEA country, and shall have an efficient channel to market the divested products into Iceland;
d. With respect to the Other Countries On-Market Divestment Businesses, the Purchaser(s) shall have the incentive and the ability to maintain and develop each of the divested products.
V.2.2. Results of the market test
(674) The market test was launched on 19 February 2016 and sought to assess mainly the scope and effectiveness of the Initial Commitments, their viability and competitiveness, the attractiveness of the Divestment Businesses as well as the suitability of the purchaser criteria.
(675) Generally, the market test yielded positive results, in particular regarding the scope, viability and competitiveness of the Divestment Businesses.450
(676) In relation to the UK-IE Divestment Business, the following key points were raised:
a. the majority of respondents indicated that the UK-IE Divestment Business needs the manufacturing facility included in the Initial Commitments,451 with a number of interested purchasers indicating that they would need it; 452
b. the majority of respondents indicated that acquiring the Actavis brand was not a necessary asset for the purchaser to effectively compete for the sale of generics in the United Kingdom and Ireland; 453
c. a number of assets such as the intellectual property rights, marketing materials, databases (including historical and forecasted pricing databases) and IT infrastructures, including websites, necessary for the purchaser to be able to replicate the Parties' DTP schemes were identified;454
d. finally, a number of contracts, such as the agreements with wholesalers, the contracts
for the supply of API or excipients relating to the manufacturing of the products, the private label agreement between Allergan and Almus in the United Kingdom, and Allergan's United Kingdom Emergency Medicines Buffer Stock Tender Agreement, were also identified.
(677) As to the suitable purchaser of the IS Divestment Business, the Other Countries On-Market Divestment Business and the Out-licensing Divestment Business (if Teva elects to divest the downstream activities of one Party), market participants indicated that the purchaser should be a generics company already active in the EEA. Indeed, respondents mentioned for instance that "the knowledge of the different country environment (legal, pricing...) is important in order to be successful" or that "in most markets, it would be difficult to start a business with only two or three molecules [...] if the purchaser is not already present in these markets, he will need time to establish such an organization plus all the relevant people (e.g. qualified persons) and structures required by law". Another respondent highlighted that "in case the purchaser is already present in the generic markets it would a) make the divestment process easier and faster to implement b) it would impact less the present market and cause less market turbulence".455
(678) As to the suitable purchaser of the Out-licensing Divestment Business (if Teva elects to divest the upstream activities of one Party), market participants indicated that the purchaser shall already have out-licensing activities in the EEA. One respondent highlighted in particular that "if the purchaser is an experienced oulicensor, the purchaser can easily consolidate it with its existing business. If not yet active in the outlicensing business it will take purchaser too long to set up such business".456
(679) Furthermore, respondents identified the need for a number of minor adjustments, which are addressed in the Final Commitments.
V.2.3. Final Commitments
(680) The Parties were informed of the shortcomings identified during the market test and submitted a final text of the Commitments addressing the issues on 4 March 2016.
(681) The full description of the assets and obligations of the Final Commitments is contained in particular in the Schedules thereof.
V.2.3.1. Marketed pharmaceuticals (except in the United Kingdom, Ireland and Iceland)
(682) In the Final Commitments, the Other Countries On-Market Divestment Businesses is composed of the following 139 molecule/country pairs457 in Austria, Belgium, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Italy, Latvia, Lithuania, the Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain and Sweden:
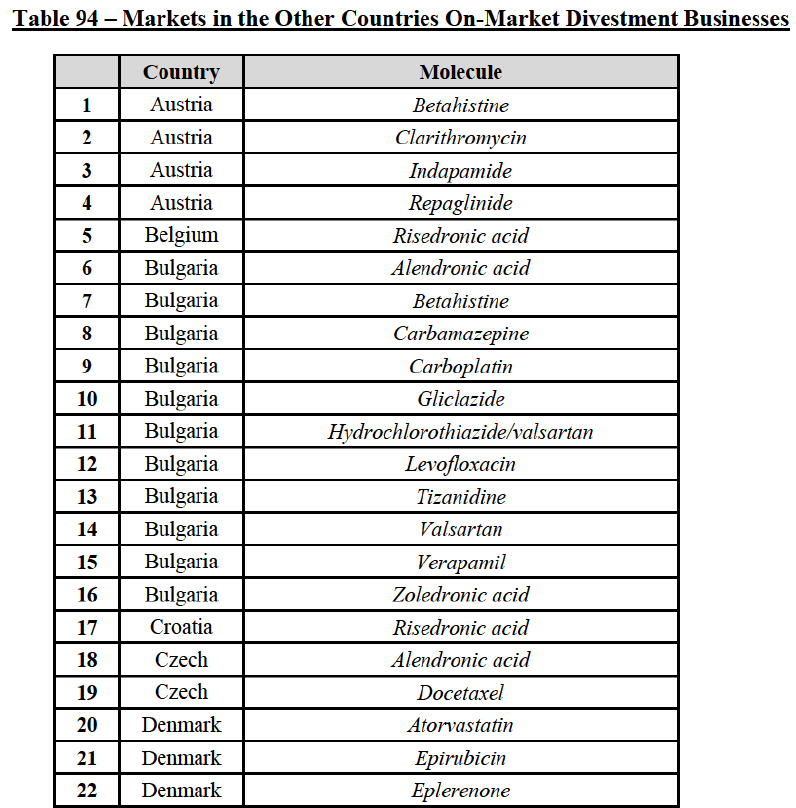
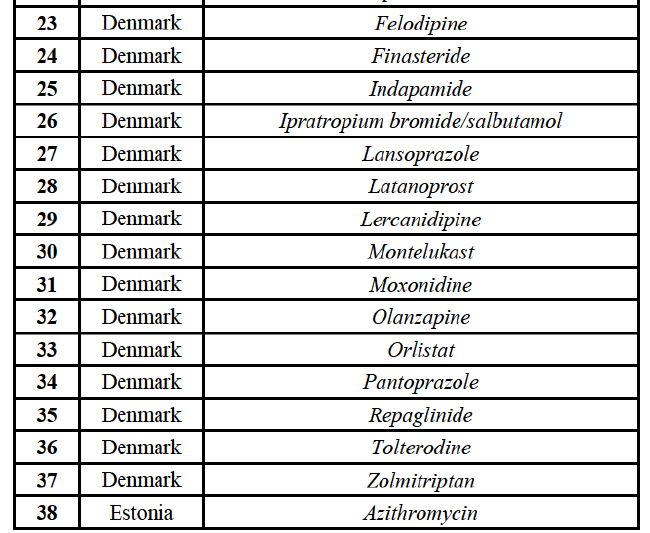
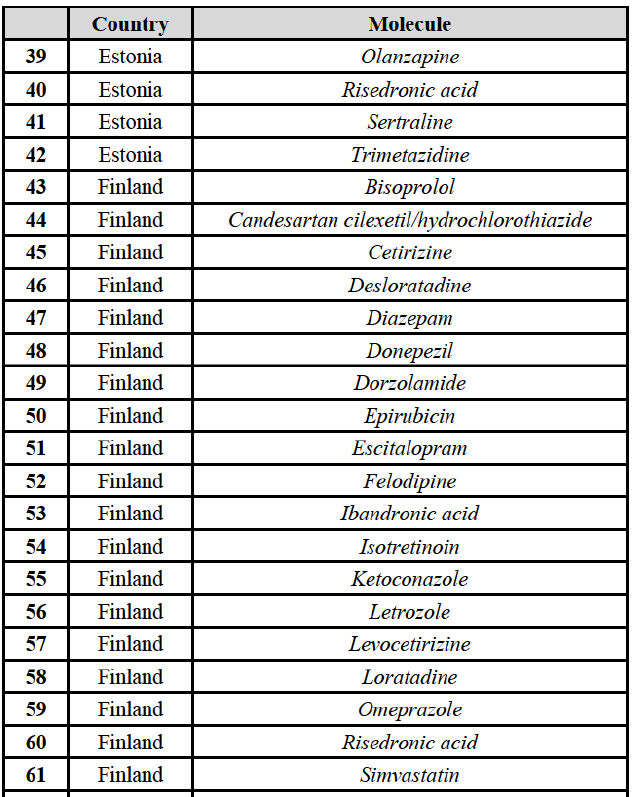
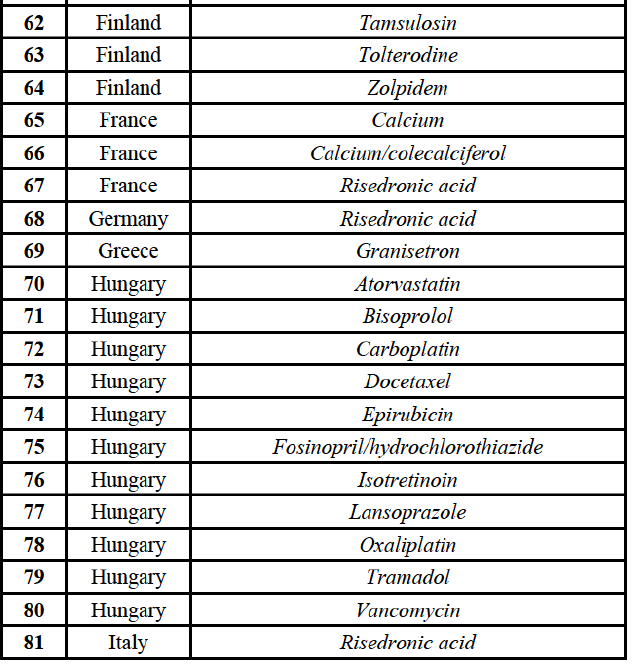
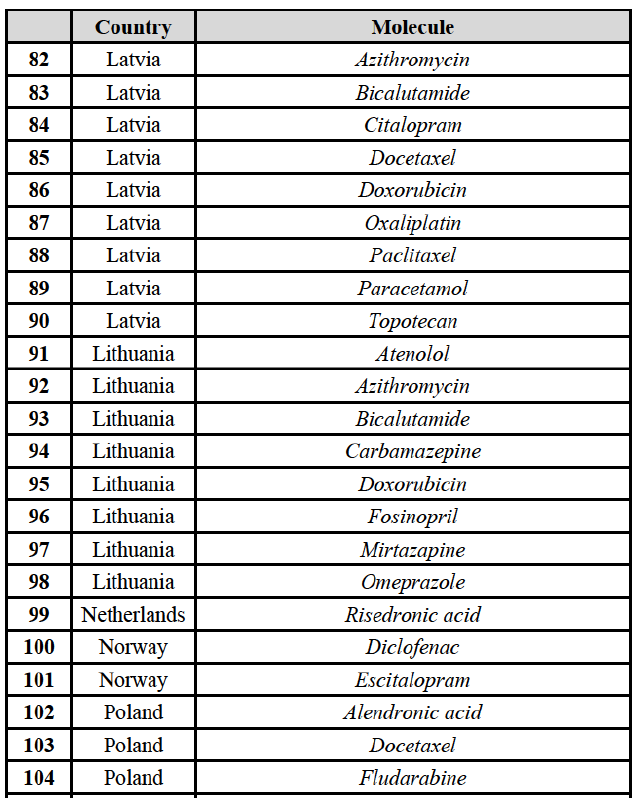
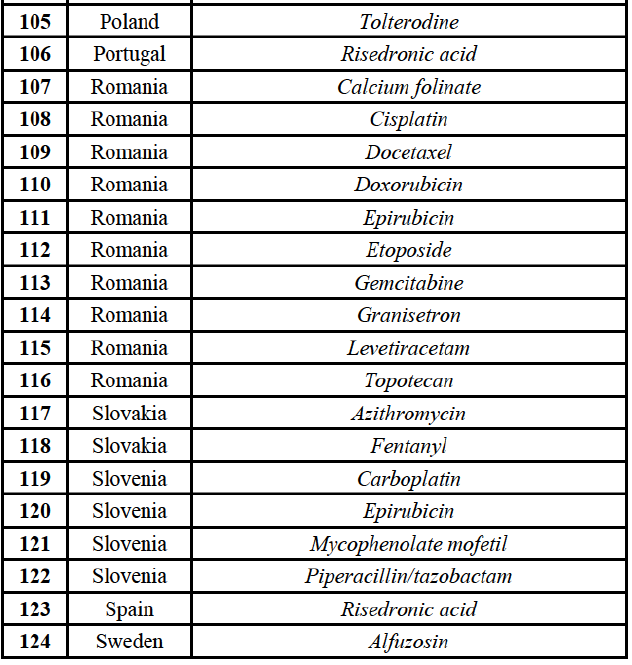
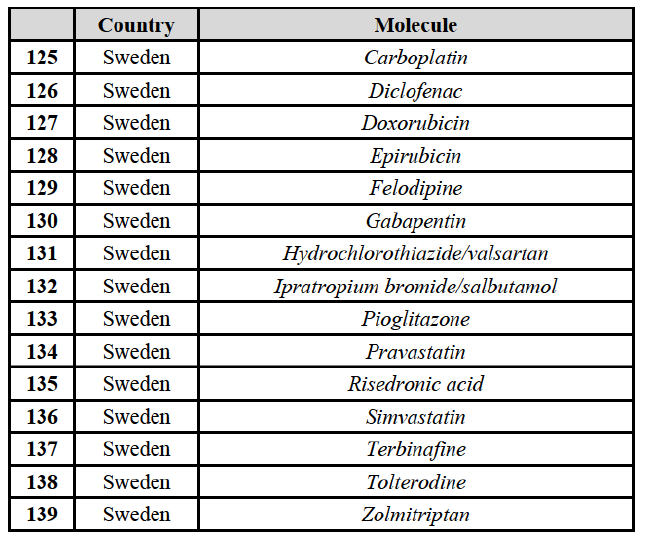
(683) The Other Countries On-Market Divestment Businesses include in particular the following assets for each marketed generic product:
a. the marketing authorizations458 for the relevant country and relevant registration dossier;
b. all contracts, leases, commitments and customer orders, all customer credit records, invoices, contact details and other records;
c. the sale of existing product inventory, sales, and promotional material in the relevant
countries, as far as available;
d. all historical information relationship with the API supplier(s) and a best effort obligation to obtain consent to assign the relevant API supply agreements;
e. all trademarks and generic brands associated with the product;
f. an option to hire one or more employees who would reasonable be considered necessary to maintain the viability, marketability, and competitiveness of the business.
(684) In case the marketing authorisation cannot be transferred for certain reasons outside the Parties' control, the activities of the other Party would be divested.
(685) At the option of the purchaser, the Other Countries On-Market Divestment Businesses also include in particular the following transitory agreements:
a. if the product is currently manufactured in-house, a supply arrangement for the relevant product for a period of three years (renewable for two additional years), at full manufacturing cost, ex-works;
b. if the API is currently manufactured in-house, a supply arrangement for the relevant API for a period of three years (renewable for two additional years), under commercially reasonable terms;
c. necessary technical assistance from Teva for the duration of the transitional supply agreement, for the purchaser to assume responsibility for (i) the manufacture and the procurement of raw materials necessary for the manufacturing of the product and (ii) the sale and marketing of the product, for such period as is required by the purchaser to establish it as a viable independent business.
(686) Finally, if the product is currently manufactured by a third party, the Other Countries On-Market Divestment Businesses include a best effort obligation to obtain consent to assign the contract manufacturing agreements entered with such third party and a supply arrangement for a period of three years (renewable for two additional years). In case the contract manufacturing agreements cannot be assigned for a reason outside the Parties' control, the activities of the other Party would be divested.
V.2.3.2. Pipeline pharmaceuticals (except in the United Kingdom, Ireland and Iceland)
(687) In the Final Commitments, the Other Countries Marketed-to-Pipeline Divestment Businesses is composed of the following 20 molecule/country pairs in Belgium, Croatia, Czech Republic, Denmark, Estonia, Greece, Hungary, Latvia, Lithuania, the Netherlands, Norway, Poland, Portugal, Romania and Sweden:
(688) In the Final Commitments, the Other Countries Pipeline-to-Pipeline Divestment Businesses is composed of the following 32 molecule/country pairs in Austria, Cyprus, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Malta, Poland, Portugal, Romania, Slovenia and Sweden:
(689) In the Final Commitments, the Other Countries Pipeline-to-Pipeline Divestment Businesses is composed of the following 14 molecules (EEA-wide):
(690) The Other Countries Marketed-to-Pipeline Divestment Businesses (in case the Parties elect to divest the pipeline product), the Other Countries Pipeline-to-Pipeline Divestment Businesses and the EEA Pipeline-to-Pipeline Divestment Businesses include in particular the following assets for each pipeline generic product:
a. all materials already developed to allow the purchaser to continue the development of the product;
b. all relevant data generated during the development project, including all material technical, preclinical, clinical and marketing files, clinical data and studies, reports, plans, know-how and records;
c. copies of all clinical data and studies relating to the development of the product;
d. all correspondence pertaining to regulatory filings and approvals;
e. to the extent necessary for a timely launch of the pipeline product, the benefit of a contract manufacturing agreement for a transitory period until the purchaser secures a supply source.
(691) The Parties further commit to complete the development of the pipeline to the extent necessary for the purchaser to launch and market the product in the relevant countries.
(692) Finally, Teva commits to provide expeditiously and at its cost the necessary technical assistance to assume responsibility for the transfer of the manufacturing process.
V.2.3.3. United Kingdom and Ireland
(693) In the Final Commitments, the UK-1E Divestment Business is composed in particular of the following assets:
e. 270 marketed459 and 62 pipeline generic pharmaceuticals for the United Kingdom, covering at least all molecules marketed by Allergan Generics which overlapped with Teva's products in 2015;
f. 80 marketed460 and 42 pipeline generic pharmaceuticals for Ireland, covering at least all molecules marketed by Allergan Generics which overlapped with Teva's products in 2015;
g. the manufacturing site located in Barnstaple, United Kingdom;
h. 653 employees in the United Kingdom (of which 441 employees for the operations of the Barnstaple site) and 21 employees in Ireland, covering in particular the sales & marketing, regulatory & medical and general & administrative functions;
i. out of the abovementioned employees, the following 8 employees constitute Key Personnel: (a) Senior VP, President United Kingdom & Ireland (Hold Separate Manager); (b) Managing Director United Kingdom; (c) Managing Director Ireland;
(d) Managing Director Operations; (e) Director Supply Chain; (f) Director Regulatory Affairs; (h) Director Human Resources; (i) Finance & IT;
j. the Allergan Generics' livery (pack design and artwork) in the United Kingdom and Ireland, as well as a licensing agreement, for a period of three years, on the Actavis brand for the products included in the UK-IE Divestment Business;
k. the Allergan Generics' Accumulator scheme in Ireland and the Accumulator Scheme, Partner Pricing scheme, and Allergan Buying Group Scheme in the United Kingdom, together with all the intellectual property rights, marketing materials, databases (including historical and forecasted pricing databases) and IT infrastructures, including websites, dedicated exclusively to Allergan Generics' discount schemes;
l. the agreements between Allergan and wholesalers and logistics companies in the United Kingdom and Ireland; the contracts for the supply of API or excipients relating to the manufacturing of the products included in the UK-IE Divestment Business; the private label agreement between Allergan and Almus in the United Kingdom; and Allergan's United Kingdom Emergency Medicines Buffer Stock Tender Agreement.
(694) For each marketed or pipeline generic pharmaceutical (as applicable), the UK-IE Divestment Business includes the same assets and transitory arrangements as for the other countries.
(695) The Final Commitments provide that the purchaser should enter with Teva into a supply and services agreement, for three years (renewable for two additional years), for the products manufactured and/or packed at the Barnstaple site that will be retained by Teva (at cost if they are not included in the UK-IE Divestment Business, under commercially reasonable terms otherwise).
V.2.3.4. Iceland
(696) In the Final Commitments, the IS Divestment Business is composed in particular of the following assets:
c. 54 marketed461 and 37 pipeline generic pharmaceuticals, covering all marketed and pipeline molecules of Teva in 2015 in Iceland;
d. a licensing agreement, for a period of three years, on the Ratiopharm brand for the products included in the IS Divestment Business,
e. at the option of the purchaser, a best effort obligation to transfer its commercial relationships with Lyfïs
(697) For each marketed or pipeline generic pharmaceutical (as applicable), the IS Divestment Business includes the same assets and transitory arrangements as for the other countries.
V.2.3.5. Copaxone (glatiramer acetate) and Azilect (rasagiline)
(698) In relation to Copaxone and Azilect, the Final Commitments remain unchanged.
V.2.3.6. Out-licensing
(699) In the Final Commitments, the Aurobindo Products Commitments are covering the following 50 molecule/country pairs for which Aurobindo is either the only out-licensee of the Parties or the other out-licensees have a de minimis market position:
(700) Separately, in the Final Commitments, the Out-licensing Divestment Businesses are composed of the following 53 molecule/country pairs which are not covered by the Aurobindo Products Commitments:
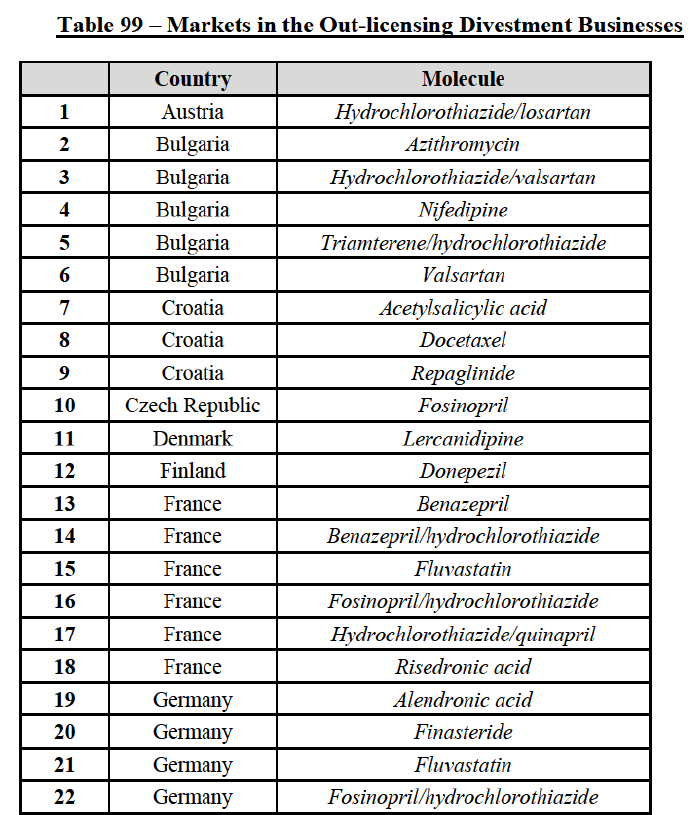
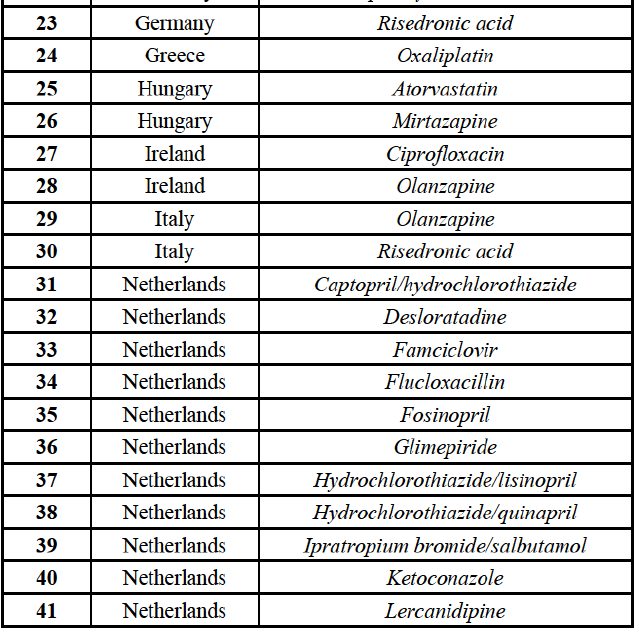
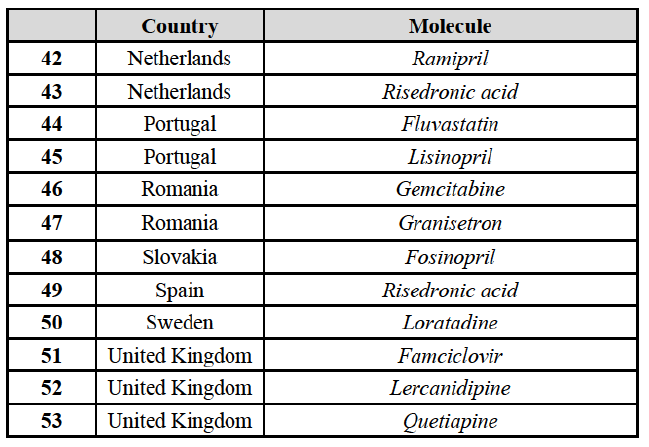
(701) If Teva elects to divest the downstream business, for each marketed generic pharmaceutical, the Out-licensing Divestment Business includes the same assets and transitory arrangements as for the Other Countries On-Market Divestment Businesses.
(702) If Teva elects to divest the upstream business, the Out-licensing Divestment Business include in particular the following assets for each product:
a. the dossier on which the out-licensing agreements are based;
b. all customer information and records and a best efforts obligation to transfer the out-licensing and contract manufacturing agreements, including all countries covered by the dossier;
c. all historical information concerning the relationship with the API supplier(s), and a best effort obligation to obtain consent to assign the relevant API supply agreements;
d. a supply arrangement for the relevant product for a period of three years (renewable for two additional years), at full manufacturing cost, ex-works.
(703) At the option of the purchaser, the Out-licensing Divestment Businesses also includes the necessary technical and regulatory support for the transfer of the manufacturing process.
(704) The Final Commitments provide that the purchaser should enter with Teva into a license back at full manufacturing cost, ex-works, on the dossier for: (i) any of the downstream finished dose products currently marketed by Teva itself based on the dossier, or (ii) any of the downstream finished dose products currently outlicensed to Aurobindo to ensure the continuation of its relationship with the merged entity.
(705) In case the dossier cannot be transferred for a reason outside the Parties' control, the downstream activities of the other Party would be divested.
V.2.3.7. Purchaser criteria
(706) In addition to the provisions of the Initial Commitments, the Final Commitments indicate that:
a. in relation to the IS Divestment Business, the Other Countries On-Market Divestment Business and the Out-licensing Divestment Business (if Teva elects to divest the downstream activities of one Party), the purchaser shall be a generics company already active in the EEA;
b. in relation to the Out-licensing Divestment Business (if Teva elects to divest the upstream activities of one Party), the purchaser shall already have out-licensing activities in the EEA;
c. if the Commission finds that, for a given product to be divested, the purchaser does not have the ability and incentive to maintain and develop each of the divested products, the activities of the other Party would be divested to the extent it confers such ability and incentive to the purchaser.
(707) Such additions address the shortcomings of the Initial Commitments as identified by the market test.
V.2.3.8. Conclusion
(708) On this basis the Commission considers that the Final Commitments are sufficient in scope and suitable to remove the competition concerns identified.
V.2.4. Overall assessment of the Final Commitments
(709) Outside of Iceland, Ireland and the United Kingdom (including the Other Countries On-Market Divestment Businesses, the Other Countries Marketed-to-Pipeline Divestment Businesses, the Other Countries Pipeline-to-Pipeline Divestment Businesses, the Out-licensing Divestment Businesses outside the Aurobindo Products Commitments, and in relation to Copaxone and Azilect), the Final Commitments remove the entire overlap between the Parties in relation to the markets for which the Commission raised serious doubts.
(710) In relation to the markets covered by the Aurobindo Products Commitments, for the products still manufactured by Medis, Aurobindo indicated that it "wants to be able to move the production in-house", which is achieved either by (i) switching to its own existing marketing authorisation or dossier if available, (ii) perform a technology transfer to rely on the existing dossier or (iii) build a new dossier to replace the current marketing authorisation.462 Any of these steps can be achieved within the four-year timeframe during which Aurobindo will benefit from the same contractual terms with Medis under the Aurobindo Products Commitments.
(711) In light of the specific situation of the markets above, and in particular given that (i) the only sizeable licensee of the Parties (Aurobindo) intends to manufacture the products in-house after a transitional period and (ii) Aurobindo became a licensee of Medis not under a normal course of business, but in the context of the sale of Allergan Generics' operations in France, Italy, Portugal, the Netherlands, Belgium, Spain and Germany to Aurobindo, the Commission concludes that the Aurobindo Products Commitments are sufficient in scope and suitable to remove the competition concerns identified.
(712) In relation to Iceland, the IS Divestment Business include all marketed and pipeline generic pharmaceuticals for which serious doubts were identified in section IV.2.2.14.a in the markets for the marketing of pharmaceuticals. The additional marketed and generic pharmaceuticals included in the divestment package further ensure the competitiveness of the remedy by aiming at replicating the economies of scale and scope existing pre-Transaction. Finally, these additional assets also remove the vertical relationship between Teva's manufacturing activities and Allergan Generics' activities in the wholesale of generic pharmaceuticals.
(713) In relation to the United Kingdom and Ireland, the UK-IE Divestment Business represents the vast majority of Allergan Generics' activities (more than [80-90]% of its Q1-Q3 2015 turnover).
(714) First, all marketed and pipeline generic pharmaceuticals for which serious doubts were identified in sections IV.2.2.15.a (for Ireland) and IV.2.2.28.a (for the United Kingdom) are included in the UK-IE Divestment Business.
(715) Second, the UK-1E Divestment Business include a wide product portfolio of marketed and pipeline generic pharmaceuticals, with a coverage463 comparable to each Party pre-merger:
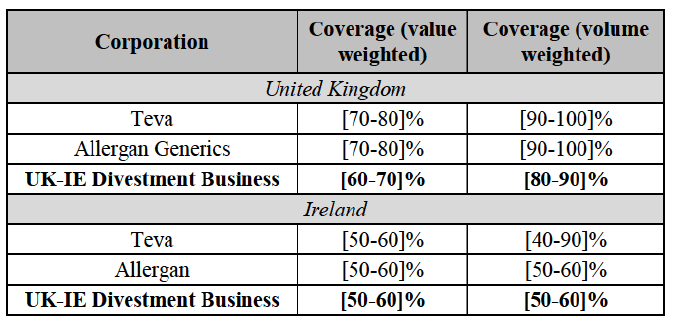
(716) The UK-IE Divestment Business includes all necessary assets from its pre-transaction operation. Specifically, it contains manufacturing assets which cover a sizeable part of the demand of the two countries (the majority in case of the United Kingdom),4M and solutions for bringing the product to the market. Indeed, even if the Purchaser were not to have any pre-existing sales capability, the Final Commitments would allow it to be immediately present in the market with a sales force and necessary materials to offer discount schemes.
(717) The assets included in the remedy package therefore cover all molecules for which serious doubts were identified in the markets for the marketing of FDPs. The additional assets will likely enable the purchaser to replicate the DTP model of the Parties in the United Kingdom and Ireland, as well as to benefit from similar economies of scale and scope as each Party pre-merger, thereby ensuring the competitiveness of the UK-IE Divestment Business. In addition, the remedy package removes the serious doubts identified as regards the Parties' activities in the wholesale of generic pharmaceuticals in the United Kingdom and Ireland. As the UK-IE Divestment Business will have a portfolio coverage that is comparable to each Party pre-merger, it will likely be able to compete in a similar fashion as each Party pre-Transaction in the wholesale of generics
(718) The attractiveness of the Divestment Businesses was evidenced by the number of potentially interested purchasers for the UK-IE Divestment Business,465 the IS Divestment Business,466 and the other Divestment Businesses.467
(719) As regards the timing, it is committed that the UK-IE Divestment Business will be sold within [...] months and the other Divestment Businesses within [...] months from the date of this decision. If unsuccessful, the Divestiture Trustee will get the mandate to sell the relevant Divestment Businesses within the following [...] months.
(720) Finally, the Final Commitments envisage the appointment of the Monitoring Trustee to oversee Teva and Allergan's compliance with the Commitments, and if necessary, of the Divestiture Trustee. This ensures that the Final Commitments will be implemented effectively and within a short period of time.
(721) On this basis, and in particular in view of a number of interested purchasers, the Commission considers that all Divestment Businesses included in the Final Commitments are attractive and likely to be acquired by suitable purchasers.
(722) For the reasons outlined above, and in view of the results of the market test and the ensuing improvements to the Commitments, the Commission considers the Final Commitments to be sufficient in scope and suitable to eliminate the serious doubts as to the compatibility of the Transaction with the internal market in relation to all markets for which the Commission raised serious doubts in section IV.2.2, given the purpose of Article 6(2) of the Merger Regulation.
V.3. Conditions and obligations
(723) Under the first sentence of the second subparagraph of Article 6(2) of the Merger Regulation, the Commission may attach to its Decision conditions and obligations intended to ensure that the undertakings concerned comply with the commitments they have entered into vis-à-vis the Commission with a view to rendering a notified concentration compatible with the internal market.
(724) The achievement of the measure that gives rise to the structural change of the market is a condition, whereas the implementing steps which are necessary to achieve this result are generally obligations on the Parties. Where a condition is not fulfilled, the Commission's decision declaring the concentration compatible with the internal market no longer stands. Where the undertakings concerned commit a breach of an obligation, the Commission may revoke the clearance decision in accordance with Article 6(3) of the Merger Regulation. The undertakings concerned may also be subject to fines and periodic penalty payments under Articles 14(2) and 15(1) of the Merger Regulation.
(725) In accordance with the distinction described above, the Decision in this case is conditioned on the full compliance with the requirements set out in Sections B and C (including Schedules A to I) of the Final Commitments (conditions), whereas the other sections of the Final Commitments constitute obligations on Teva and Allergan.
(726) The detailed text of the Final Commitments is annexed to the present Decision. The full text of the final Commitments forms an integral part to this Decision.
VI. CONCLUSION
(727) For the above reasons, the Commission has decided not to oppose the notified operation as modified by the commitments and to declare it compatible with the internal market and with the functioning of the EEA Agreement, subject to full compliance with the conditions in Sections B and C (including Schedules A to I) of the commitments annexed to the present Decision and with the obligations contained in the other sections of the said commitments. This decision is adopted in application of Article 6(1)(b) in conjunction with Article 6(2) of the Merger Regulation and Article 57 of the EEA Agreement.
FOOTNOTES :
1 OJ L 24, 29.1.2004, p. 1 (the "Merger Regulation"). With effect from 1 December 2009, the Treaty on the Functioning of the European Union ("TFEU") has introduced certain changes, such as the replacement of "Community" by "Union" and "common market" by "internal market". The terminology of the TFEU will be used throughout this decision.
2 OJ L 1, 3.1.1994, p.3 (the "EEA Agreement").
3 Publication in the Official Journal of the European Union No C 34, 29 January 2016, p.16.
4 Turnover calculated in accordance with Article 5(1) of the Merger Regulation and the Commission Consolidated Jurisdictional Notice (OJ C95, 16.04.2008, p1).
5 For Cyprus, Liechtenstein, Luxembourg and Malta, the Parties were not able to provide a breakdown of the market.
6 Allergan Generics is only present with off-patent originator products.
7 Allergan Generics is only present with off-patent originator products.
8 Allergan Generics is only present with off-patent originator products.
9 Allergan Generics is only present with off-patent originator products.
10 Allergan Generics is only present with off-patent originator products.
11 According to the European Directive 2001/83/EC on the Community code relating to medicinal products for human use (OJ L311, 28.11.2001, p.67, the "Directive 2001/83/EC"), an API or active substance is defined as "any substance or mixture of substances intended to be used in the manufacture of a medicinal product and that, when used in its production, becomes an active ingredient of that product intended to exert a pharmacological, immunological or metabolic action with a view to restoring, correcting or modifying physiological functions or to make a medical diagnosis".
12 See M.7559 – Pfizer/ Hospira; M.7379 – Mylan/ Abbott EPD-DM; M.6613 – Watson/Actavis.
13 Both the World Health Organization (WHO) and EphMRA maintain classification systems. WHO mainly classifies substances according to the therapeutic or pharmaceutical aspects and in one class only (particular formulations or strengths can be given separate codes, e.g. clonidine in C02A as antihypertensive agent, N02C as anti-migraine product and S01E as ophthalmic product). EphMRA classifies products, mainly according to their indications and use. Therefore, it is possible to find the same compound in several classes, depending on the product, (e.g. Naproxen tablets can be classified in M1A (antirheumatic), N2B (analgesic) and G2C if indicated for gynaecological conditions only).
The purposes of classification are also different. The main purpose of the WHO classification is for international drug utilisation research and for adverse drug reaction monitoring. This classification is recommended by the WHO for use in international drug utilisation research. The EphMRA classification has a primary objective to satisfy the marketing needs of the pharmaceutical companies (which explain why the Parties opted for the EphMRA classification). Therefore, a direct comparison is sometimes difficult due to the different nature and purpose of the two systems.
See for instance http://www.ephmra.org/user uploads/who-atc%202013%20final.pdf
EphMRA classification: http://www.ephmra.org/Anatomical-Classification
WHO classification: http://www.whocc no/atc/structure and principles
14 OJ C 372, 9.12.1997, p. 5.
15 See M.7559 – Pfizer / Hospira and M.5253 – Sanofi-Aventis / Zentiva.
16 See M.7559 – Pfizer/ Hospira; M.7379 – Mylan/ Abbott EPD-DM; M.6613 – Watson/Actavis.
17 See Directive 2001/83/EC.
18 See M.7645 – Mylan / Perrigo.
19 See M.7559 — Pfizer / Hospira; M.6969 — Valeant Pharmaceuticals International / Bausch&Lomb Holdings; M.5778 — Novartis/Alcoa.
20 See M.7559 – Pfizer / Hospira and M.5253 – Sanofi-Aventis / Zentiva.
21 See M.7645 – Mylan / Perrigo; M.7559 – Pfizer / Hospira; M.7379 - Mylan/ Abbott EPD-DM.
22 See M.7645 – Mylan / Perrigo; M.7559 – Pfizer / Hospira; M.7379 - Mylan/ Abbott EPD-DM.
23 “Originator” is defined as a novel drug that was under patent protection when launched in the market.
24 The Parties also identified Teva's risedronic acid product, which is the generic equivalent of Allergan Generics' originator drug sold under the brand name "Actonel". However, given that Teva's risedronic acid product is already marketed in a number of EEA countries, this overlap is treated under the framework of section IV.2.1.1 above. Teva's risedronic acid is a pipeline product only for [...], and as such it is treated under the framework of section IV.2.1.2 above.
25 See M.7480 – Actavis/Allergan; M.7275 – Novartis/GlaxoSmithKline Oncology Business; M.6258 – Teva/Cephalon.
26 See M.7494 – Brocacef/Mediq Netherlands for the Netherlands; M.7323 – Nordic Capital/GHD Verwaltung and M.6044 – Alliance Boots/Andreae-Noris Zahn in Germany and M.4301 – Alliance Boots/Cardinal Health in the United Kingdom.
27 See, e.g. M.6044 – Alliance Boots/Andreae-Noris Zahn.
28 A 3PL is a third party logistics supplier licensed to store and transport pharmaceuticals. Pre-wholesalers are 3PLs that receive bulk pallets of pharmaceuticals from manufacturers to a single warehouse site and then deliver smaller volumes to the regional warehouses of wholesalers and directly to large volume purchasers (such as some hospitals).
29 See M.7494 – Brocacef/Mediq Netherlands; M.7323 – Nordic Capital/GHD Verwaltung; M.6044 – Alliance Boots/Andreae-Noris Zahn and M.4301 – Alliance Boots/Cardinal Health.
30 A full-line wholesaler undertakes to supply the full range of prescription pharmaceuticals and provide a very frequent (often twice-daily) delivery service to customers. A number of full-line wholesalers still operate in the EEA but none exist in some countries, such as the United Kingdom or Ireland, due to the existence of exclusive arrangements for branded pharmaceuticals. In these countries, wholesalers undertake to supply the widest possible range of prescription pharmaceuticals are often referred to as broad-line wholesalers.
31 A short-line wholesaler does not carry the full range of prescription pharmaceuticals. It often concentrates on a more limited range of high volume products and tends to provide a once daily (or less frequent) delivery service to its customers.
32 A dispensing doctor is licensed to dispense pharmaceuticals to patients who live in areas with few or no retail pharmacies.
33 See M.4301 – Alliance Boots/Cardinal Health; M.7323 – Nordic Capital/GHD Verwaltung; M.7494 - Brocacef/Mediq Netherlands.
34 See M.6044 – Alliance Boots/Andreae Noris Zahn; M.6613 – Watson/Actavis; M.2573 – A&C/Grossfarma. See also decisions of the CNC (Spanish Competition Authority) in case S/0437/12 – ESPECIALIDADES FARMACÉUTICAS GENÉRICAS and of the Competition and Consumer Protection Commission (Irish Competition Authority) M/04/020 - Uniphar/Whelehan.
35 These orders are known as "transfer orders".
36 See paragraphs (45) to (46) below.
37 As it would be the case for the markets for the marketing of pharmaceuticals defined above, which includes for instance the supply of pharmaceuticals by manufacturers to wholesalers in countries such as the United Kingdom.
38 As it would be the case in the market for the wholesale of pharmaceuticals to pharmacies.
39 A second source of supply can come from parallel importers. Parallel imports are typically originator pharmaceuticals identical to the one manufactured for a given country, but purchased from abroad and imported by a third party.
40 See minutes of conference call with [regulatory authority] on 04.12.2015, with [regulatory authority] on 12.01.2016, with [customer] on 27.01.2016.
41 The intensity of the competitive pressure exerted by the off-patent originator product on its generic alternatives varies across EEA countries and molecules.
42 See replies to questions 12, 13 and 14 of Q2 – Retail pharmacies UK, questions 4 and 5 of Q4 – Retail pharmacies Ireland, to questions 11 and 37 of Q1 – Competitors. See also minutes of conference calls with [customers] on 13.01.2016. This tendency of pharmacies to purchase as much as they can from the same generic supplier can also be illustrated by Teva collecting in its ordinary course of business switching data on pharmacies that do not longer use Teva as "preferred supplier of generics products" in the United Kingdom.
43 This market trend (generic manufacturers offering "range discounts") was already highlighted by the Commission in the case M.5865 – Teva / Ratiopharm. See replies to questions 8 and 9 of Q2 – Retail pharmacies UK and replies to question 17.2 of Q1 – Competitors.
44 See replies to question 15 of Q2 – Retail pharmacies UK, minutes of conference call with [customer] on 19.01.2016.
45 In Iceland for instance, wholesalers are specialised in selling either generics (e.g. Lyfis and Alvogen) or originator products (e.g. Vistor). As an example, Teva supplies its generic products to Lyfis and its patented product Copaxone to Vistor.
46 See M.7494 – Brocacef/Mediq Netherlands; M.6044 – Alliance Boots/Andreae Noris Zahn; M.4301 Alliance Boots/Cardinal Health; M.1243 Alliance Unichem plc/Safa Galenica SA.
47 See case M/12/027 – Uniphar/CMR.
48 The Irish Competition Authority defined DTP as follows: "a pharmaceutical manufacturer uses a LSP, which may be a full-line wholesaler, to distribute its products directly to a pharmacy. The LSP does not take title of the human pharmaceutical drugs since the pharmaceutical manufacturer deals directly with the pharmacy. The pharmaceutical manufacturer sets the price and other terms of supply (e.g., the frequency of delivery) to the pharmacy and pays the LSP (or full-line wholesaler) a fee for delivering the product." The Irish Competition Authority also indicated that DTP have grown significantly in recent years, up to 12.8% of the total wholesale supply of pharmaceuticals in 2012.
49 Under the Irish legislation enacted in 2013, generics are progressively included on "interchangeable lists" and a "reference price" is set which determines the level of patients' reimbursement. Molecules which have been designated as interchangeable would represent 80% of total off-patent market volume and 60% of its value.
50 See replies to questions 30-40 of Q1 – Competitors. Minutes of conference calls with [wholesalers] on 7 January 2016 and 8 January 2016.
51 See minutes of conference calls with [wholesalers] on 7 January 2016 and 8 January 2016: "the level of fee is negotiated individually with manufacturers and depends on the overall activity and type of product".
52 The Commission used the molecule level market shares in the IMS data (provided by the Parties) as an approximation for the market shares on the market for marketing of individual generic molecules.
53 See M.7494 – Brocacef/Mediq Netherlands; M.4301 Alliance Boots/Cardinal Health.
54 See replies to question 6 of Q2 – Retail pharmacies UK and question 17.3 of Q1 - Competitors.
55 See replies to question 3 of Q4 – Hospital pharmacies UK and minutes of conference call with [tendering authority] on 12 January 2016 and [hospital] on 11 December 2015.
56 See minutes of conference call with [a pharmacy purchasing group] on 14 January 2016.
57 See M.7494 – Brocacef/Mediq Netherlands; M.7721 – Celesio/Sainsbury's UK pharmacy business; M.7323 – Nordic Capital/GHD Verwaltung; M.4301 Alliance Boots/Cardinal Health; M.2573 A&C/Grossfarma.
58 See minutes of conference calls with [wholesalers] on 24 November 2015 and 9 December 2015.
59 See M.7645 - Mylan/ Perrigo; M.7379 – Mylan/Abbott EPD-DM.
60 Both Parties define complex generics as products which require comparably higher investments before they may be launched on the market, due to the fact that they are more difficult to manufacture or formulate, or require a drug/device combination.
61 See e.g. M.7559 — Pfizer/Hospira; M.6258 — Teva/Cephalon; M.5778 — Novartis/Alcon.
62 See replies to question 80 of Q1 — Competitors and question 3 of Q8 — Customers — Other countries.
63 See replies to question 80 of Q1 — Competitors and question 3 of Q8 — Customers — Other countries.
64 See replies to question 80 of Q1 — Competitors and question 3 of Q8 — Customers — Other countries.
65 See replies to question 80 of Q1 — Competitors and question 3 of Q8 — Customers — Other countries.
66 See replies to questions 80 to 83 of Q1 — Competitors and questions 8 to 11 of Q8 — Customers — Other countries.
67 According to the Parties, registering the product takes only two weeks; updating the patient information leaflet, placing the orders, manufacturing the batches, and having the products in stock requires several months.
68 See replies to questions 80 to 83 of Q1 – Competitors and questions 12 & 13 of Q8 – Customers – Other countries.
69 See replies to questions 80 to 83 of Q1 – Competitors and questions 12 & 13 of Q8 – Customers – Other countries.
70 See replies to questions 80 to 83 of Q1 – Competitors and questions 12 & 13 of Q8 – Customers – Other countries.
71 See replies to questions 80 to 83 of Q1 – Competitors and questions 12 & 13 of Q8 – Customers – Other countries.
72 According to the Parties, registering the product takes only two weeks; updating the patient information leaflet, placing the orders, manufacturing the batches, and having the products in stock requires several months.
73 See replies to questions 80 to 83 of Q1 – Competitors and questions 12 & 13 of Q8 – Customers – Other countries.
74 According to the Parties, registering the product takes only two weeks; updating the patient information leaflet, placing the orders, manufacturing the batches, and having the products in stock requires several months.
75 See replies to questions 80 to 83 of Q1 – Competitors and questions 12 & 13 of Q8 – Customers – Other countries.
76 See replies to questions 80 to 83 of Q1 – Competitors and questions 12 & 13 of Q8 – Customers – Other countries.
77 See replies to questions 80 to 83 of Q1 – Competitors and questions 12 & 13 of Q8 – Customers – Other countries.
78 See replies to questions 80 to 83 of Q1 – Competitors and questions 12 & 13 of Q8 – Customers – Other countries.
79 See replies to questions 80 to 83 of Q1 – Competitors and questions 12 & 13 of Q8 – Customers – Other countries.
80 See replies to questions 80 to 83 of Q1 – Competitors and questions 12 & 13 of Q8 – Customers – Other countries.
81 According to the Parties, registering the product takes only two weeks; updating the patient information leaflet, placing the orders, manufacturing the batches, and having the products in stock requires several months.
82 See replies to questions 80 to 83 of Q1 – Competitors and to question 4 of Questionnaire to pharmacies – Croatia and Portugal.
83 See replies to questions 80 to 83 of Q1 – Competitors and questions 18 to 23 of Q8 – Customers – Other countries.
84 See replies to questions 80 to 83 of Q1 – Competitors and questions 18 to 23 of Q8 – Customers – Other countries.
85 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
86 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
87 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
88 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
89 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
90 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
91 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
92 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
93 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
94 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
95 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
96 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
97 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
98 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
99 According to the Parties, registering the product takes only two weeks; updating the patient information leaflet, placing the orders, manufacturing the batches, and having the products in stock requires several months.
100 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
101 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
102 According to the Parties, registering the product takes only two weeks; updating the patient information leaflet, placing the orders, manufacturing the batches, and having the products in stock requires several months.
103 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
104 According to the Parties, registering the product takes only two weeks; updating the patient information leaflet, placing the orders, manufacturing the batches, and having the products in stock requires several months.
105 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
106 See replies to questions 80 to 83 of QI — Competitors and questions 24 to 28 of Q8 — Customers — Other countries.
107 See replies to questions 80 to 83 of QI — Competitors and questions 24 to 28 of Q8 — Customers — Other countries.
108 See replies to questions 80 to 83 of Q1 – Competitors and questions 24 to 28 of Q8 – Customers – Other countries.
109 See replies to questions 80 to 83 of Q1 – Competitors and questions 24 to 28 of Q8 – Customers – Other countries.
110 See replies to questions 80 to 83 of Q1 – Competitors and questions 24 to 28 of Q8 – Customers – Other countries.
111 See replies to questions 80 to 83 of Q1 — Competitors and questions 41 to 45 of Q8 — Customers — Other countries.
112 See replies to questions 80 to 83 of Q1 — Competitors and questions 41 to 45 of Q8 — Customers — Other countries.
113 See replies to questions 80 to 83 of Q1 — Competitors and questions 41 to 45 of Q8 — Customers — Other countries.
114 See replies to questions 80 to 83 of Ql — Competitors and questions 46 to 49 of Q8 — Customers — Other countries.
115 See replies to Q8 — Customers — Other countries, questions 50 to 54; Q1 — Competitors, questions 80 to 83.
116 According to the Parties, registering the product takes only two weeks; updating the patient information leaflet, placing the orders, manufacturing the batches, and having the products in stock requires several months.
117 See replies to question 80 to 83 of Q1 – Competitors and question 55 to 61 of Q8 – Customers – Other countries.
118 See replies to question 80 to 83 of Q1 – Competitors and question 55 to 61 of Q8 – Customers – Other countries.
119 See replies to question 80 to 83 of Q1 – Competitors and question 55 to 61 of Q8 – Customers – Other countries.
120 See replies to question 80 to 83 of Q1 – Competitors and question 55 to 61 of Q8 – Customers – Other countries.
121 See replies to questions 80 to 83 of Q1 – Competitors and questions 55 to 61 of Q8 – Customers – Other countries.
122 According to the Parties, registering the product takes only two weeks; updating the patient information leaflet, placing the orders, manufacturing the batches, and having the products in stock requires several months.
123 See replies to questions 80 to 83 of Q1 – Competitors and questions 55 to 61 of Q8 – Customers – Other countries.
124 See replies to questions 80 to 83 of Q1 – Competitors and questions 55 to 61 of Q8 – Customers – Other countries.
125 See replies to questions 80 to 83 of Q1 – Competitors and questions 55 to 61 of Q8 – Customers – Other countries.
126 See replies to questions 80 to 83 of Q1 – Competitors and questions 55 to 61 of Q8 – Customers – Other countries.
127 See replies to questions 80 to 83 of Q1 – Competitors and question 55 of Q8 – Customers – Other countries.
128 See replies to questions 80 to 83 of Q1 – Competitors and question 55 of Q8 – Customers – Other countries.
129 See replies to questions 80 to 83 of Q1 – Competitors and questions 55 to 61 of Q8 – Customers – Other countries.
130 See replies to questions 64 to 66 of Q1 – Competitors and questions 17 to 23 of Q6 – Customers – Iceland.
131 See replies to questions 64 to 66 of Q1 – Competitors and questions 17 to 23 of Q6 – Customers – Iceland.
132 See replies to questions 64 to 66 of Q1 – Competitors and questions 17 to 23 of Q6 – Customers – Iceland.
133 See replies to questions 64 to 66 of Q1 – Competitors and questions 17 to 23 of Q6 – Customers – Iceland.
134 See replies to questions 64 to 66 of Q1 – Competitors and questions 17 to 23 of Q6 – Customers – Iceland.
135 See replies to questions 64 to 66 of Q1 – Competitors and questions 17 to 23 of Q6 – Customers – Iceland.
136 See replies to questions 64 to 66 of Q1 – Competitors and questions 17 to 23 of Q6 – Customers – Iceland.
137 See replies to questions 64 to 66 of Q1 – Competitors and questions 17 to 23 of Q6 – Customers – Iceland.
138 See replies to questions 64 to 66 of Q1 – Competitors and questions 17 to 23 of Q6 – Customers – Iceland.
139 See replies to questions 64 to 66 of Q1 – Competitors and questions 17 to 23 of Q6 – Customers – Iceland.
140 See replies to questions 64 to 66 of Q1 – Competitors and questions 17 to 23 of Q6 – Customers – Iceland.
141 See replies to questions 64 to 66 of Q1 – Competitors and questions 17 to 23 of Q6 – Customers – Iceland.
142 See replies to questions 64 to 66 of Q1 – Competitors and questions 17 to 23 of Q6 – Customers – Iceland.
143 See replies to questions 64 to 66 of Q1 – Competitors and questions 17 to 23 of Q6 – Customers – Iceland.
144 See replies to questions 64 to 66 of Q1 – Competitors and questions 17 to 23 of Q6 – Customers – Iceland.
145 See replies to questions 64 to 66 of Q1 – Competitors and questions 17 to 23 of Q6 – Customers – Iceland.
146 See replies to questions 64 to 66 of Q1 – Competitors and questions 17 to 23 of Q6 – Customers – Iceland.
147 See replies to questions 64 to 66 of Q1 – Competitors and questions 17 to 23 of Q6 – Customers – Iceland.
148 See replies to questions 64 to 66 of Q1 – Competitors and questions 17 to 23 of Q6 – Customers – Iceland.
149 See replies to questions 64 to 66 of Q1 – Competitors and questions 17 to 23 of Q6 – Customers – Iceland.
150 See replies to questions 64 to 66 of Q1 – Competitors and questions 17 to 23 of Q6 – Customers – Iceland.
151 See replies to questions 64 to 66 of Q1 – Competitors and questions 17 to 23 of Q6 – Customers – Iceland.
152 See replies to questions 64 to 66 of Q1 – Competitors and questions 17 to 23 of Q6 – Customers – Iceland.
153 See replies to questions 64 to 66 of Q1 – Competitors and questions 17 to 23 of Q6 – Customers – Iceland.
154 See replies to questions 64 to 66 of Q1 – Competitors and questions 17 to 23 of Q6 – Customers – Iceland.
155 See replies to questions 64 to 66 of Q1 – Competitors and questions 17 to 23 of Q6 – Customers – Iceland.
156 Guidelines on the assessment of non-horizontal mergers under the Council Regulation on the control of concentrations between undertakings, 2008/C 265/07, para. 31.
157 Guidelines on non-horizontal mergers, para. 32.
158 Guidelines on non-horizontal mergers, para. 35.
159 See Allergan Generics' internal document, "Actavis and Leading Competitors in Europe", September 2015.
160 Minutes of the conference call with [manufacturer] on 4 December 2015 — See replies to question 11 of 06 — Customers Iceland and replies to questions 56, 62-63 of 01— Competitors.
161 See replies to question 6 of 06 — Customers Iceland.
162 Minutes of the conference calls with [manufacturer] on 4 December 2015 and [wholesaler] on 26 January 2016. See also replies to question 59 of 01— Competitors.
163 A price difference smaller than 5% is accepted.
164 Minutes of the conference call with [manufacturer] on 4 December 2015.
165 The Parties provided price data at SKU level and market share data at molecule level so the comparison exercise could be made only for cases where only one SKU was sold per molecule.
166 See replies to questions 15-16 of Q6 – Customers Iceland and question 61 of Q1 – Competitors. See also minutes of the conference calls with [customer] on 20 November 2015 and [wholesaler] on 26 January 2016.
167 See replies to question 61 of Q1 – Competitors.
168 Minutes of conference call with Lyfis on [date].
169 Guidelines on non-horizontal mergers, para. 39.
170 See e.g. replies to question 28 of R1 – Market test of the Commitments.
171 The rest would be relabelled by Lyfis.
172 Minutes of conference call with Lyfis on [date].
173 See e.g. replies to question 28 of R1 – Market test of the Commitments.
174 Minutes of conference call with Lyfis on [date].
175 Guidelines on non-horizontal mergers, para. 40.
176 Guidelines on non-horizontal mergers, para. 41.
177 Guidelines on non-horizontal mergers, para. 43.
178 Guidelines on non-horizontal mergers, para. 48.
179 See replies to question 25 of Q6 – Customers Iceland and questions 65-66 of Q1 – Competitors: "The Transaction will amount to taking a huge step back to the old situation when Actavis' quasi-monopoly was synonym of high prices and lack of competition"; "If these companies merge, there will be much less competition in the Icelandic drug market. The consequence is a danger of higher prices of generic drugs and /or withdrawal of generic drugs from our small market" (reply to question 6 of Q6 – Customers Iceland).
180 See replies to questions 46 to 48 of Q1 — Competitors and questions 19 to 20 of Q4 — Retail pharmacies Ireland.
181 See replies to questions 46 to 48 of Q1 — Competitors and questions 19 to 20 of Q4 — Retail pharmacies — Ireland.
182 See replies to questions 46 to 48 of Q1 – Competitors and questions 19 to 20 of Q4 – Retail pharmacies – Ireland.
183 See replies to questions 46 to 48 of Q1 – Competitors and questions 19 to 20 of Q4 – Retail pharmacies – Ireland.
184 See replies to questions 46 to 48 of Q1 – Competitors and questions 19 to 20 of Q4 – Retail pharmacies – Ireland.
185 See replies to questions 46 to 48 of Q1 – Competitors and questions 19 to 20 of Q4 – Retail pharmacies – Ireland.
186 See replies to questions 46 to 48 of Q1 – Competitors and questions 19 to 20 of Q4 – Retail pharmacies – Ireland.
187 See replies to questions 46 to 48 of Q1 – Competitors and questions 19 to 20 of Q4 – Retail pharmacies – Ireland.
188 See replies to questions 46 to 48 of Q1 – Competitors and questions 19 to 20 of Q4 – Retail pharmacies – Ireland.
189 See replies to questions 46 to 48 of Q1 – Competitors and questions 19 to 20 of Q4 – Retail pharmacies – Ireland.
190 See replies to questions 46 to 48 of Q1 – Competitors and questions 19 to 20 of Q4 – Retail pharmacies – Ireland.
191 See replies to questions 46 to 48 of Q1 – Competitors and questions 19 to 20 of Q4 – Retail pharmacies – Ireland.
192 See replies to questions 46 to 48 of Q1 – Competitors and questions 19 to 20 of Q4 – Retail pharmacies – Ireland.
193 See replies to questions 46 to 48 of Q1 – Competitors and questions 19 to 20 of Q4 – Retail pharmacies – Ireland.
194 See replies to questions 46 to 48 of Q1 – Competitors and questions 19 to 20 of Q4 – Retail pharmacies – Ireland.
195 Before 2015, Sanofi was commercializing this molecule in Ireland.
196 These estimates assume that Sanofi's market share in 2012-2014 can be allocated to Allergan Generics which commercializes the molecule since 2015.
197 See replies to questions 46 to 48 of Q1 – Competitors and questions 19 to 20 of Q4 – Retail pharmacies – Ireland.
198 See replies to questions 46 to 48 of Q1 – Competitors and questions 19 to 20 of Q4 – Retail pharmacies – Ireland.
199 See replies to questions 46 to 48 of Q1 – Competitors and questions 19 to 20 of Q4 – Retail pharmacies – Ireland.
200 See replies to questions 46 to 48 of Q1 – Competitors and questions 19 to 20 of Q4 – Retail pharmacies – Ireland.
201 See replies to questions 46 to 48 of Q1 – Competitors and questions 19 to 20 of Q4 – Retail pharmacies – Ireland.
202 See replies to questions 46 to 48 of Q1 – Competitors and questions 19 to 20 of Q4 – Retail pharmacies – Ireland.
203 See http://www.actavis.ie/en/news/Accumulator.htm ("the best deal in the market on generics, simple prices, full portfolio inclusion and rewards for valued partners").
204 See https://www.teva.ie/teva/pluspoints/customer access ("our +pluspoints reward scheme allows you to collect points every time you order, and convert those points to credit off your next quarterly statement, providing real and meaningful savings to your business").
205 See replies to questions 8 and 10 of Q4 — Retail pharmacy Ireland. See also Minutes of conference call with [wholesaler] on 7 January 2016.
206 See replies to questions 20-21, 43 and 47 of QI — Competitors.
207 See Allergan Generics' internal document, "Actavis and Leading Competitors in Europe", September 2015.
208 See replies to question 13 of Q4 — Retail pharmacies Ireland.
209 See replies to question 13 of Q4 – Retail pharmacies Ireland.
210 In particular, the Parties do not record sales on either Standard Units basis or a gross price basis, as does IMS. Furthermore, the Parties record sales at the moment they leave the factory, instead of the moment when they are sold at the retail level, as does IMS. To ensure pharmacy sales are comparable, Teva applied the internal split of its sales by value between independent pharmacies and chains to the total sales value and volume of its retail products according to IMS. Allergan Generics has applied the internal split of its net sales and volumes (in number of packs) between hospitals, independent pharmacies and chains to the IMS value and volume figures of its total sales in each year. Finally, to obtain the split between independent pharmacies and pharmacy chains, the Parties have assumed that about 20% of total Retail sales were done to pharmacy chains in line with the Parties estimates of the number of pharmacy belonging to chains in the country.
211 Minutes of the conference call with [pharmacy] on 14 January 2016. See replies to question 3 of Q4 – Retail pharmacies Ireland.
212 See http://www.actavis.ie/en/news/Accumulator.htm
See replies to questions 5 and 6 of Q4 – Retail pharmacies Ireland.
213 See replies to questions 33 and 37 of Q1 – Competitors.
214 See replies to questions 33 and 37 of Q1 – Competitors.
215 See replies to question 37 of Q1 – Competitors.
216 Teva's TevaOne scheme would have been launched in 2010 (http://teva.ie/services/retail-portfolio/teva-one html) and Allergan Generics' Accumulator scheme would have been launched in Ireland in 2014 (http://www.actavis.ie/en/news/Accumulator htm).
217 See replies to questions 14-18 of Q4 – Retail pharmacies Ireland and 43 and 44 of Q1 – Competitors. See also minutes of conference call with [pharmacy] on 24 November 2015.
218 See replies to questions 14-18 of Q4 – Retail pharmacies Ireland and 43 and 44 of Q1 – Competitors.
219 See replies of [pharmacy] to questions 14 and 15 of Q4 – Retail pharmacies Ireland.
220 See reply of [wholesaler] to question 43 of Q1 – Competitors.
221 Minutes of the conference call with [pharmacy] on 14 January 2016.
222 See replies to question 22 of Q4 – Retail pharmacies Ireland.
223 See replies to question 21 of Q4 – Retail pharmacies Ireland. See also replies to question 48 of Q1 – Competitors.
224 See replies to questions 80 to 83 of Q1 — Competitors and questions 62 to 65 of Q8 — Customers — Other countries.
225 See replies to questions 80 to 83 of Ql — Competitors and questions 66 to 73 of Q8 — Customers — Other countries.
226 See replies to questions 80 to 83 of Ql — Competitors and questions 66 to 73 of Q8 — Customers — Other countries.
227 See replies to questions 80 to 83 of Ql — Competitors and questions 66 to 73 of Q8 — Customers — Other countries.
228 See replies to questions 80 to 83 of Q1 – Competitors and questions 66 to 73 of Q8 – Customers – Other countries.
229 See replies to questions 80 to 83 of Q1 – Competitors and questions 66 to 73 of Q8 – Customers – Other countries.
230 See replies to questions 80 to 83 of Q1 – Competitors and questions 66 to 73 of Q8 – Customers – Other countries.
231 See replies to questions 80 to 83 of Q1 – Competitors and questions 66 to 73 of Q8 – Customers – Other countries.
232 See replies to questions 80 to 83 of Q1 – Competitors and questions 66 to 73 of Q8 – Customers – Other countries.
233 See replies to questions 80 to 83 of Q1 – Competitors and questions 66 to 73 of Q8 – Customers – Other countries.
234 See replies to questions 80 to 83 of 01 — Competitors and questions 74 to 81 of Q8 — Customers — Other countries.
235 See replies to questions 80 to 83 of Q1 – Competitors and questions 74 to 81 of Q8 – Customers – Other countries.
236 See replies to questions 80 to 83 of Q1 – Competitors and questions 74 to 81 of Q8 – Customers – Other countries.
237 See replies to questions 80 to 83 of Q1 – Competitors and questions 74 to 81 of Q8 – Customers – Other countries.
238 See replies to questions 80 to 83 of Q1 – Competitors and questions 74 to 81 of Q8 – Customers – Other countries.
239 See replies to questions 80 to 83 of Q1 – Competitors and questions 74 to 81 of Q8 – Customers – Other countries.
240 See replies to questions 80 to 83 of Q1 – Competitors and questions 74 to 81 of Q8 – Customers – Other countries.
241 See replies to questions 80 to 83 of Q1 – Competitors and questions 74 to 81 of Q8 – Customers – Other countries.
242 See replies to questions 80 to 83 of QI — Competitors and questions 122 to 125 of Q8 — Customers — Other countries.
243 See replies to questions 80 to 83 of QI — Competitors and questions 82 to 88 of Q8 — Customers — Other countries.
244 See replies to questions 80 to 83 of QI — Competitors and questions 82 to 88 of Q8 — Customers — Other countries.
245 See replies to questions 80 to 83 of Q1 – Competitors and question 89 of Q8 – Customers – Other countries.
246 See replies to questions 80 to 83 of Q1 – Competitors and question 89 of Q8 – Customers – Other countries.
247 See replies to questions 80 to 83 of Q1 – Competitors and question 89 of Q8 – Customers – Other countries.
248 See replies to questions 80 to 83 of Q1 – Competitors and question 89 of Q8 – Customers – Other countries.
249 See replies to questions 80 to 83 of QI — Competitors and questions Questionnaire to pharmacies – Croatia and Portugal.
250 See replies to questions 80 to 83 of 01 — Competitors and questions 96 to 106 of 08 — Customers — Other countries.
251 See replies to questions 80 to 83 of Q1 – Competitors and questions 96 to 106 of Q8 – Customers – Other countries.
252 See replies to questions 80 to 83 of Q1 – Competitors and questions 96 to 106 of Q8 – Customers – Other countries.
253 See replies to questions 80 to 83 of Q1 – Competitors and questions 96 to 106 of Q8 – Customers – Other countries.
254 See replies to questions 80 to 83 of Q1 – Competitors and questions 96 to 106 of Q8 – Customers – Other countries.
255 See replies to questions 80 to 83 of Q1 – Competitors and questions 96 to 106 of Q8 – Customers – Other countries.
256 See replies to questions 80 to 83 of Q1 – Competitors and questions 96 to 106 of Q8 – Customers – Other countries.
257 See replies to questions 80 to 83 of Q1 – Competitors and questions 96 to 106 of Q8 – Customers – Other countries.
258 See replies to questions 80 to 83 of Q1 – Competitors and questions 96 to 106 of Q8 – Customers – Other countries.
259 See replies to questions 80 to 83 of Q1 – Competitors and questions 96 to 106 of Q8 – Customers – Other countries.
260 See replies to questions 80 to 83 of OI - Competitors and questions 107 to 111 of Q8 — Customers — Other countries.
261 See replies to questions 80 to 83 of OI — Competitors and questions 107 to 111 of Q8 — Customers — Other countries.
262 See replies to questions 80 to 83 of QI — Competitors and questions 112 to 117 of Q8 — Customers — Other countries.
263 See replies to questions 80 to 83 of QI — Competitors and questions 112 to 117 of Q8 — Customers — Other countries.
264 See replies to questions 80 to 83 of QI — Competitors and questions 112 to 117 of Q8 — Customers — Other countries.
265 See replies to questions 80 to 83 of OI — Competitors and questions 112 to 117 of 08 - Customers – Other countries.
266 See replies to questions 80 to 83 of QI — Competitors and questions 118 to 121 of Q8 — Customers — Other countries.

271 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
272 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
273 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
274 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
275 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
276 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
277 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
278 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
279 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
280 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
281 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
282 See replies to question 67 of Q1 – Competitors and question 13 to 16 of Q7 – Customers Denmark / Sweden.
283 The pharmacist margin is defined such that manufacturer's net sales + pharmacist margin + wholesaler margin = drug tariff price.
284 An additional limitation comes from the IMS and BGMA datasets being reported on a different basis. For BGMA, manufacturers report their monthly volume sales, and sales in value are calculated by applying the drug tariff price for generics. IMS, on the other hand, uses the drug tariff price but removes the standard wholesaler margin to obtain a proxy of the ex-manufacturer selling price. As a consequence, the Parties have multiplied BGMA revenue figures by 0.858 to ensure that they are comparable to IMS revenues.
285 See replies to question 26 of QI — Competitors, question 23 of Q2 — Retail pharmacies United Kingdom (UK) and question 14 of Q3 — Hospital pharmacies UK.
286 Aciclovir (D6D) is used for the treatment of topical viral infection and is generally sold under the pharmaceutical form M. Aciclovir (J5B) mentioned below is a systemic antiviral product generally sold under the pharmaceutical form A.
287 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
288 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
289 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
290 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
291 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
292 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
293 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
294 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
295 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
296 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
297 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
298 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
299 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
300 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
301 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
302 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
303 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
304 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
305 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
306 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
307 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
308 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
309 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
310 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
311 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
312 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
313 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
314 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
315 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
316 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
317 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
318 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
319 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
320 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
321 Hydrocortisone (D7A) for topical use is sold under the pharmaceutical form M. Hydrocortisone (H2A) mentioned below is a systemic product generally sold under the pharmaceutical form A.
322 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
323 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
324 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
325 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
326 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
327 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
328 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
329 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
330 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
331 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
332 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
333 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
334 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
335 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
336 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
337 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
338 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
339 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
340 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
341 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
342 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
343 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
344 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
345 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
346 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
347 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
348 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
349 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
350 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
351 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
352 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
353 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
354 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
355 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
356 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
357 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
358 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
359 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
360 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
361 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
362 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
363 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
364 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
365 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
366 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
367 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
368 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
369 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
370 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
371 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
372 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
373 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
374 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
375 See replies to question 26 of Q1 – Competitors, question 23 of Q2 – Retail pharmacies UK and question 14 of Q3 – Hospital pharmacies UK.
376 The Parties offer loyalty schemes to end customers, more specifically "TevaOne" for Teva and "Accumulator" and "Partner Pricing" schemes for Allergan, targeted at individual pharmacies and dispensing doctors.
377 See Allergan Generics' internal document, "Actavis and Leading Competitors in Europe", September 2015.
378 Pharmacy chains were defined by the Parties as all pharmacy chains with at least five pharmacies, including supermarkets and large national and regional groups.
379 According to EphMRA's Guide to Useful pharmaceutical market research terms and definitions, a Standard Unit is the "smallest common dose of a product formulation (e.g. 1 tablet or capsule, 5 millimetres of syrup, 1 ampoule etc.)".
380 EphMRA notes that "Standard Units are more appropriate than counting units when comparing sales and prices of products with different formulations".
381 By way of example:
In a submission dated 9 February 2016, Allergan Generics schemes' share in the market/segment for independent pharmacies and dispensing doctors was [5-10]% in 2014, while, according to the estimates of the Parties' share per customers segment (submission dated 24 February 2016), Allergan Generics' share in the market for independent pharmacies and dispensing doctors was [0-5]% in 2014.
The proportion of Allergan Generics' revenue derived from vertically integrated pharmacy chains differs significantly between the net sales provided in a submission dated 29 January 2016, where it represented [2030]% of Allergan Generics direct sales revenue, and the gross sales based on which the Parties made the estimates of their shares per customers segment (submission dated 24 February 2016), where it represented [40-50]% of Allergan Generics direct sales revenue.
382 Including national and regional broad-line wholesalers.
383 See case M. 4301 – Alliance Boots/Cardinal Health on the frequency of deliveries.
384 See replies to questions 5, 8 and 9 of Q2 – Retail pharmacies UK.
385 See replies to question 11 of Q1 – Competitors.
386 See replies to question 11 of Q2 – Retail pharmacies UK.
387 See e.g. minutes of conference call with the United Kingdom Department of Health on 19 January 2016 and with the Pharmaceutical Services Negotiating Committee on 27 January 2016. Category A products contains "less commonly prescribed but readily available generics".
388 See replies to question 7 of Q2 – Retail pharmacies UK.
389 The Parties compared the prices of Teva, Allergan Generics and the main wholesalers for all SKUs sold by both Parties under their schemes in January 2016 and provided the proportion represented by the lowest priced SKU in each company's portfolio (e.g. more than [30-40]% of its portfolio for McKesson, approx. [10-20]% for Teva, [0-5]% for Phoenix, [0-5]% for Allergan Generics and less than [0-5]% for Walgreens Boots Alliance).
390 See replies to question 14 – Q1 Competitors.
391 See replies to question 7 of Q2 – Retail pharmacies UK.
392 See replies to question 6 of Q2 – Retail pharmacies UK.
393 See replies to question 8 of Q2 – Retail pharmacies UK.
394 See replies to question 7 of Q2 – Retail pharmacies UK.
395 Teva's submission, Parties' views on brand related concerns in the UK, 18 February 2016. Teva further considers that "the Teva 360 pack design [...] is a significant and valuable identity asset that is highly recognised and valued by pharmacists and patients alike".
396 See replies to question 6 of Q2 – Retail pharmacies UK.
397 See replies to question 6 of Q2 – Retail pharmacies UK.
398 See replies to question 7 of Q2 – Retail pharmacies UK.
399 This cascade mechanism would generally apply to all generics. See replies to question 15 of Q2 – Retail pharmacies UK and reply of [wholesaler] on 10 February 2016: "this cascade is then applied to all of their purchases and cannot be specified by line or molecule".
400 See replies to questions 21-22 of Q2 – Retail pharmacies UK.
401 See replies to question 12 of Q1 – Competitors.
402 See replies to question 28 of Q1 – Competitors and questions 24-25 of Q2 – Retail pharmacies UK.
403 See replies to question 87 of Q1 - Competitors.
404 See: http://mobile reuters.com/article/mergersNews/idUSL5N10A3VA20150730
405 [...]
406 Under the Decentralised Procedure, with the reference Member State being the Netherlands.
407 See minutes of conference call with [a competitor] on 18 December 2015.
408 [...].
409 When the production is done by a third party, the manufacturer which provides contract manufacturing services does not own the IP rights for marketing the products concerned, but only acts as a sub-contractor. The contract manufacturing process may or may not include the provision of the final packaging of the product. As to contract manufacturing outside out-licensing agreements, the activities of the Parties are rather limited and do not overlap. Furthermore, the Parties do not contract manufacture for each other.
410 See e.g. M.6613 – Watson / Actavis; M.5865 – Teva/Ratiopharm; and M.6258 – Teva/Cephalon.
411 See e.g. M.6613 — Watson /Actavis.
412 Some products are delivered by Teva in Europe but are then marketed outside Europe by its customers.
413 See "Actavis Announces Agreement with Aurobindo Pharma Limited to Divest Commercial Operations in Seven Western European Countries":
http://www.allergan.com/NEWS/News/Thomson-Reuters/Actavis-Announces-Agreement-with-Aurobindo-Pharma
414 See e.g. M.7379 – Mylan/Abbott EPD-DM; M.6258 – Teva/Cephalon.
415 In view of the limited size of the affected downstream markets in the EEA demand for each molecule, the Commission concluded that the risk of customer foreclosure strategies is highly unlikely.
416 Guidelines on the assessment of non-horizontal mergers under the Council Regulation on the control of concentrations between undertakings, 2008/C 265/07, para. 31.
417 Guidelines on non-horizontal mergers, para. 32.
418 Guidelines on non-horizontal mergers, para. 33-39.
419 Unless the license agreements are terminated due to some circumstance (e.g. breach, insolvency). For Teva, intellectual properties are generally held by the licensee only for mutually agreed period of time.
420 [...].
421 The manufacturing contract is the one from which most of the out-licensor's revenue is derived. See replies to question 97 of Q1 – Competitors.
422 See replies to question 97 of Q1 – Competitors.
423 See replies to question 98 of Q1 – Competitors.
424 See replies to question 97 of Q1 – Competitors.
425 See replies to question 97 of Q1 – Competitors.
426 See replies to question 97 of Q1 – Competitors.
427 See replies to question 97 of Q1 – Competitors.
428 See replies to question 98 of Q1 – Competitors.
429 See replies to question 98 of Q1 – Competitors.
430 See replies to question 99 and 100 of Q1 – Competitors.
431 See replies to question 95 of Q1 – Competitors.
432 See replies to question 93 of Q1 – Competitors.
433 See replies to question 101 of Q1 – Competitors.
434 See replies to question 101 of Q1 – Competitors.
435 Guidelines on non horizontal mergers, para. 40-46.
436 In case that both Teva and Allergan are active downstream, the Commission took into consideration the sales of the Party with higher margins.
437 Guidelines on non horizontal mergers. para. 47-57.
438 These are adapalene, imiquimod, nabumetone, nitrofurantoin, prilocaine and urea.
439 See M.7645 – Mylan/Perrigo; M.6258 – Teva/Cephalon.
440 See M.7645 – Mylan/Perrigo; M.6258 – Teva/Cephalon.
441 See M.7645 – Mylan/Perrigo.
442 More specifically, for most of the affected markets, Teva holds a market share for the relevant APIs smaller or equal to 50% globally. For the other APIs, including the ones for which the Parties were not able to provide reliable market share estimates, namely caffeine/ergotamine, diltiazem, etoposide, imipramine, lovastatin and nicergoline, the demand represented by Allergan Generics competitors in the downstream markets is less than 10% of the global demand.
443 Commission Notice on remedies acceptable under Council Regulation (EC) No 139/2004 and under Commission Regulation (EC) No 802/2004 (OJ C 267, 22.10.2008, p. 1-27), the "Remedies Notice".
444 Remedies Notice, paragraphs 9 and 61.
445 Remedies Notice, paragraph 12.
446 Remedies Notice, paragraph 10.
447 A molecule/country pair divestment consists in the divestment of one generic molecule in one EEA country.
448 Either the on-market product of one Party, or the pipeline product of the other Party.
449 The pipeline-to-pipeline overlaps EEA-wide cover cases where (i) none of the Parties already market the molecule in an EEA country or (ii) the pipeline was already marketed by at least one of the Parties, but in a limited number of countries in comparison to the number of EEA countries where the launch was planned.
450 See replies to questions 1, 4, 14, 20, 24, 30, 33, 38, 45 and 48 of R1 – Market test of the Commitments.
451 See replies to question 7 of R1 – Market test of the Commitments.
452 See replies to question 22 of R1 – Market test of the Commitments.
453 See replies to question 7 of R1 – Market test of the Commitments.
454 See replies to questions 28, 29, 41, 42 and 43 of R1 – Market test of the Commitments.
455 See replies to questions 51 and 52 of R1 – Market test of the Commitments.
456 See replies to questions 10 and 11 of R1 – Market test of the Commitments.
457 Between the submission of the Initial Commitments and the Final Commitments, serious doubts were dispelled on 20 molecule/country pairs on the basis of additional information provided by the Parties. They were therefore removed from the Other Countries On-Market Divestment Businesses.
458 In particular, Teva commits to undertake, at its own expense and unless the costs involved are manifestly excessive for the product concerned, all regulatory changes that would be required as a result of such transfer in particular regarding marketing authorization variations and dossier improvements. With respect to dossier improvements, Teva commits to a reasonable efforts obligation to effectuate such improvements, to the extent they are required to operate the marketing authorization transfer.
459 Between the submission of the Initial Commitments and the Final Commitments, the Parties removed 4 non-overlapping molecules in the United Kingdom from the UK-IE Divestment Business: eucalyptus, lidocaine, lymecycline and nifedipine.
460 Between the submission of the Initial Commitments and the Final Commitments, the Parties removed 1 molecule in Ireland from the UK-IE Divestment Business: progesterone (which is not actually sold by Allergan Generics in Ireland).
461 Between the submission of the Initial Commitments and the Final Commitments, the Parties removed 1 molecule in Iceland from the IS Divestment Business: cetiri_ine dih drochlouide (which is actually the same molecule as cetiri_ine, already part of the IS Divesmtent Business).
462 See minutes of conference call with Aurobindo on [date].
463 For the purposes of this analysis, the coverage is the number of generic molecules (segmented by pharmaceutical form) provided by a corporation over the total number of generic molecules sold in the country. This is an imperfect metric of actual coverage given that certain molecules are overrepresented in value or volume. Therefore, value-weighted coverage (the weight on a molecule is the total 2015 value in the country) and volume-weighted coverage (the weight on a molecule is the total 2015 volume in the country) are used.
464 More specifically, in 2014, the Barnstaple site represented [30-40]% of the share of the demand in Ireland and [70-801% in the United Kingdom (excluding the demand served by third-party manufacturing). Furthermore, in 2014, more than [80-901% of its capacity served the United Kingdom.
465 See replies to question 22 of R1 – Market test of the Commitments.
466 See replies to question 32 of R1 – Market test of the Commitments.
467 See replies to question 44 of R1 – Market test of the Commitments.
Case M. 7746 – Teva/ Allergan Generics
COMMITMENTS TO THE EUROPEAN COMMISSION
Pursuant to Article 6(2), of Council Regulation (EC) No. 139/2004 (the “Merger Regulation”), Teva Pharmaceuticals Europe (“Teva” or the “Notifying Party”) and Allergan plc (“Allergan”, together with Teva, the “Parties”) hereby enter into the following Commitments (the “Commitments”) vis-à-vis the European Commission (the “Commission”) with a view to rendering the acquisition by Teva of sole control of the global generic pharmaceuticals business of Allergan, including the U.S. and international generic commercial units, third-party supplier Medis, global generic manufacturing operations, the global generic R&D unit, the international over-the-counter commercial unit (excluding over-the counter eye care products) and some established international brands (the “Concentration”), compatible with the internal market and the functioning of the EEA Agreement.
This text shall be interpreted in light of the Commission’s decision pursuant to Article 6(1)(b) of the Merger Regulation to declare the Concentration compatible with the internal market and the functioning of the EEA Agreement (the “Decision”), in the general framework of European Union law, in particular in light of the Merger Regulation, and by reference to the Commission Notice on remedies acceptable under Council Regulation (EC) No 139/2004 and under Commission Regulation (EC) No 802/2004 (the “Remedies Notice”).
SECTION A. DEFINITIONS
For the purpose of the Commitments, the following terms shall have the following meaning:
Affiliated Undertakings: undertakings controlled by either of the Parties and/or by the ultimate parent of either Party, whereby the notion of control shall be interpreted pursuant to Article 3 of the Merger Regulation and in light of the Commission Consolidated Jurisdictional Notice under Council Regulation (EC) No 139/2004 on the control of concentrations between undertakings (the "Consolidated Jurisdictional Notice").
Allergan: Allergan plc, a public limited liability company incorporated under the laws of Ireland, with its registered office at Clonshaugh Business & Technology Park, Coolock, Dublin, Ireland, and registered with the Irish Companies Registration Office under number 527629.
Allergan Generics Business: Allergan’s global generics business including Allergan’s U.S. and international generic commercial units, third-party supplier Medis, Allergan’s global generics R&D unit and global generic manufacturing operations, Allergan’s international over-the-counter commercial unit (excluding over-the-counter eye care products) and certain established international pharmaceutical brands as defined in the Master Purchase Agreement between Allergan and Teva dated July 26, 2015.
Assets: the assets that contribute to the current operation or are necessary to ensure the viability and competitiveness of the Divestment Businesses as indicated in Section B and Schedules G-I, G-II(1) and G-II(2).
Azilect Divestment Businesses: Allergan Generics Business’ pipeline products for Rasagiline as defined in Section B.
Closing: the transfer of the legal title to the Divestment Businesses to the Purchaser(s). Completion: the closing of the proposed Concentration.
Closing Period: the period of [...] months from the approval of the Purchaser(s) and the terms of sale by the Commission.
Confidential Information: any business secrets, know-how, commercially sensitive information, or any other information of a proprietary nature that is not in the public domain.
Conflict of Interest: any conflict of interest that impairs the Trustee's objectivity and independence in discharging its duties under the Commitments.
Divesting Party: either Teva or the Allergan Generics Business if currently operating the respective Divestment Businesses.
Divested Products: the products marketed or in development (pipeline) of the respective Divestment Businesses.
Divestiture Trustee: one or more natural or legal person(s) who is(are) approved by the Commission and appointed by Teva, and who has(have) received from Teva the exclusive Trustee Mandate to sell the relevant Divestment Businesses to Purchaser(s) at no minimum price.
Divestment Businesses: collectively the Other Countries On-Market Overlaps Divestment Businesses, On-Market-to-Pipeline Divestment Businesses, National Pipeline-to-Pipeline Divestment Businesses, EEA Pipeline-to-Pipeline Divestment Businesses, the Outlicensing Divestment Businesses, the IS Divestment Businesses, and IE-UK Divestment Businesses. For the avoidance of doubt, Divestment Businesses as described also in the Schedules and divested by Allergan are limited to businesses included in the Allergan Generics Business.
EEA Pipeline-to-Pipeline Divestment Businesses: Teva’s or Allergan Generics Business’ pipeline molecules in the EEA as defined in Section B paragraph 12 and Schedule B-III which Teva commits to divest.
Effective Date: the date of adoption of the Decision.
First Divestiture Period: the period of [...] months from the Effective Date for the IE-UK Divestment Businesses, and [...] months for all Divestment Businesses other than the IE-UK Divestment Businesses.
Hold Separate Managers: collectively the person(s) appointed by the Parties for the Other Countries On-Market Overlaps Divestment Businesses, IS Divestment Businesses, IE-UK Divestment Businesses to manage the day-to-day business under the supervision of the Monitoring Trustee
IE-UK Divestment Businesses: Allergan Generics Business’ business in Ireland and the United Kingdom as defined in Section B paragraphs 21 to 28 and Schedules E-I to E-II and F-I to F-II which Teva commits to divest.
The IE-UK Hold Separate Manager: the person(s) appointed by the Parties for the IE-UK Divestment Businesses to manage the day-to-day business under the supervision of the Monitoring Trustee.
The IE-UK Key Personnel: all personnel necessary to maintain the viability and competitiveness of the IE-UK Divestment Businesses, as listed in the Schedule E-III, including the IE-UK Hold Separate Manager.
IE-UK Personnel: all personnel listed in Schedule E-III.
IS Divestment Businesses: Teva’s business in Iceland as defined in Section B paragraphs 16 to 20 and Schedules D-I to D-II which Teva commits to divest.
IS Hold Separate Manager: the person(s) appointed by Teva for the IS Divestment Businesses to manage the day-to-day business under the supervision of the Monitoring Trustee.
Monitoring Trustee: one or more natural or legal person(s) who is(are) approved by the Commission and appointed by Teva, and who has(have) the duty to monitor Teva’s compliance with the conditions and obligations attached to the Decision.
National Pipeline-to-Pipeline Divestment Businesses: Allergan Generics Business’ or Teva’s pipeline molecule for a specific country as defined in Section B paragraph 11 and Schedule B-II which Teva commits to divest
On-Market-to-Pipeline Divestment Businesses: Allergan Generics Business’ or Teva’s pipeline or on-market businesses as defined in Section B paragraph 10 and Schedule B-I which Teva commits to divest.
Other Countries Hold Separate Manager: the person(s) appointed by Teva for the Other Countries On-Market Overlaps Divestment Businesses to manage the day-to-day business under the supervision of the Monitoring Trustee.
Other Countries On-Market Overlaps Divestment Businesses: Allergan Generics Business’ or Teva’s on-market businesses in all countries except Iceland, Ireland and the UK as defined in Section B paragraphs 8 to 9 and Schedule A which Teva commits to divest.
Outlicensing Divestment Businesses: Teva’s or Allergan Generics Business’ upstream or on-market businesses as defined in paragraph 14 and Schedules G-I and G-III which Teva commits to divest.
Parties: Teva and Allergan.
Personnel: all staff currently employed by the IE-UK Divestment Businesses listed in Schedule E-III.
Purchaser(s): the entity or entities approved by the Commission as acquirer(s) of the Divestment Businesses in accordance with the criteria set out in Section E.
Purchaser Criteria: the criteria laid down in paragraph 45 of these Commitments that the Purchaser(s) must fulfil in order to be approved by the Commission.
Teva: Teva Pharmaceutical Industries Ltd., a limited liability company incorporated under the laws of Israel, with its registered office at 5 Basel Street Peach Tikva 49131, Israel and registered with the Israeli Company Register under number 520013954.
Trustee(s): the Monitoring Trustee and/or the Divestiture Trustee as the case may be.
Trustee Divestiture Period: the period of [...] months from the end of the First Divestiture Period.
SECTION B. THE COMMITMENT TO DIVEST AND THE DIVESTMENT BUSINESSES Commitment to divest
1. The Commitments shall take effect upon the Effective Date. Allergan shall be bound only by paragraphs 30 to 41, 72 and 73 of the Commitments and only up until Completion.
2. In order to maintain effective competition, Teva commits to divest, or procure the divestiture of the Divestment Businesses by the end of the Trustee Divestiture Period as a going concern to Purchaser(s) and on terms of sale approved by the Commission in accordance with the procedure described in paragraph 46 of these Commitments.
3. To carry out the divestiture, Teva commits to find one or more Purchaser(s) and to enter into one or more final binding sale and purchase agreements for the sale of the Divestment Businesses within the First Divestiture Period. If Teva has not entered into such one or more agreement(s) at the end of the First Divestiture Period, Teva shall grant the Divestiture Trustee an exclusive mandate to sell the Divestment Businesses, or those parts of the Divestment Businesses that are not disposed of, in accordance with the procedure described in paragraph 59 in the Trustee Divestiture Period and subject to Completion.
4. Teva shall be deemed to have complied with this commitment if:
(a) by the end of the Trustee Divestiture Period, Teva or the Divestiture Trustee has entered into one or more final binding sale and purchase agreements and the Commission approves the proposed purchaser(s) and the terms of sale as being consistent with the Commitments in accordance with the procedure described in paragraph 46;
(b) the Closing of the sale of the Divestment Businesses to the Purchaser(s) takes place within the Closing Period; and
(c) Teva has complied with all its obligations under the Commitments, including in particular the transfer of assets and personnel and the assistance to be provided to the Purchaser(s),.
5. In order to maintain the structural effect of the Commitments, the Notifying Party shall, for a period of 10 years after Closing, not acquire, whether directly or indirectly, the possibility of exercising influence (as defined in paragraph 43 of the Remedies Notice, footnote 3) over the whole or part of the Divestment Businesses, unless, following the submission of a reasoned request from the Notifying Party showing good cause and accompanied by a report from the Monitoring Trustee (as provided in paragraph 73 of these Commitments), the Commission finds that the structure of the market has changed to such an extent that the absence of influence over the Divestment Businesses is no longer necessary to render the proposed concentration compatible with the internal market.
6. The Schedules attached form an integral part of the Commitments.
Structure and definition of the Divestment Businesses
7. The Divestment Businesses are described in detail below.
On Market Overlaps outside Iceland, Ireland and the UK
8. Teva commits to divest either Teva’s or Allergan Generics Business’ generic products listed in Schedule A, together with, for each of those products, the assets listed in Schedule G-I (collectively the “Other Countries On-Market Overlaps Divestment Businesses”). For the avoidance of doubt, all galenic forms and ATC classifications of the Divested Products are included.
9. The Other Countries On-Market Overlaps Divestment Businesses also include any pipeline products that the Divesting Party may have for the relevant molecule in the relevant country, together with, for each of those pipeline products, the assets listed in Schedule G-II(1).
Pipelines
On-market to pipeline overlaps
10. For all the products listed in Schedule B-I Teva commits to divest either (i) the on-market product of one of either Allergan Generics Business or Teva, together with the assets listed in Schedule G-I or (ii) the pipeline product of the other of Allergan Generics Business or Teva in the relevant country together with the assets listed in Schedule G-II(1) (collectively the “On-market-to-Pipeline Divestment Businesses”).
Pipeline to pipeline overlaps
11. For all the molecules listed in Schedule B-II, Teva commits to divest either (i) the on-market product of either Allergan Generics Business or Teva, together with the assets listed in Schedule G-I or (ii) the pipeline product of one of either Allergan Generics Business or Teva in the relevant country, together with the assets listed in Schedule G-II(1) (collectively the “National Pipeline-to-Pipeline Divestment Businesses”).
12. For all the molecules listed in Schedule B-III, the Teva commits to divest either Teva’s or Allergan Generics Business’ pipeline product in the EEA, together with the assets listed in Schedule G-II(2) (collectively the “EEA Pipeline-to-Pipeline Divestment Businesses”).
Azilect
13. Teva commits to divest Allergan Generics Business’ pipeline for Rasagiline for the following countries: [...], together with the assets listed in Schedule G-II(1) (collectively the “Azilect Divestment Businesses”). For the avoidance of doubt, with respect specifically to Rasagiline, the assets do not comprise any licence on the IP rights owned by Teva.
Outlicensing
14. For all the molecules listed in Schedule C-I (together “the Non-Aurobindo Products”), Teva commits to divest either (i) the outlicensing business conducted by either Medis or Teva for the relevant product, together with the assets listed in Schedule G-III (the “Upstream Outlicensing Divestment Businesses”), or (ii) the on-market business of either Teva or Allergan Generics Business in the relevant country together with the assets listed in Schedule G-I (the “Downstream Outlicensing Divestment Businesses”) (collectively the “Outlicensing Divestment Businesses”).
15. Teva will have the ability to require from the Purchaser(s) a license back at cost on the Dossier for any of its downstream finished dose products currently marketed based on the Dossier or the downstream finished dose products currently outlicensed to Aurobindo to ensure the continuation of its relationship with the merged entity, provided such licence does not concern markets for which the Commission identified competition concerns.
Iceland
16. Teva commits to divest to a single Purchaser the existing Teva business in Iceland (the “IS Divestment Businesses”), which comprises all the Teva products listed in Schedule D-I, together with, for each of those products, the assets described in Schedule G-I. For the avoidance of doubt, Schedule G-I covers at least all molecules marketed by Teva in 2015 in Iceland.
17. The IS Divestment Businesses also includes all Teva’s pipelines products listed in Schedule D-II, and, for each of those pipeline products, the assets described in Schedule G-II(1). For the avoidance of doubt, Schedule G-II covers all pipeline molecules of Teva in Iceland, as well as Allergan Generics Business’ pipeline product for [...] in Iceland.
18. The IS Divestment Businesses finally include the Ratiopharm livery (pack design and artwork) in Iceland.
19. For a period of three years after Closing and under the supervision of the Monitoring Trustee, Teva commits to grant to the Purchaser(s) a licensing agreement on the Ratiopharm brand for the products included in the IS Divestment Businesses.
20. At the option of the Purchaser, Teva commits to a best effort obligation to obtain the transfer of its existing distribution agreement with Lyfis.
United Kingdom & Ireland
21. Teva commits to divest to a single Purchaser a substantial part of Allergan Generics Business’ generic business in the United Kingdom and Ireland (the “IE-UK Divestment Businesses”), as further described below.
22. The IE-UK Divestment Businesses include :
(a) all Allergan Generics Business’ products listed in Schedule E-I (the “IE Marketed Divested Products”) together with, for each of those products, the assets listed in Schedule G-I. For the avoidance of doubt, Schedule E-I covers at least all molecules marketed by Allergan Generics Business which overlap with Teva’s products in 2015 in Ireland;
(b) all Allergan Generics Business’ pipelines products listed in Schedule E-II (the “IE Pipeline Divested Products”), together with, for each of those pipeline products, the assets listed in Schedule G-II(1). For the avoidance of doubt, Schedule E-II covers all pipelines molecules of Allergan Generics Business which overlap with Teva’s pipeline and marketed molecules in 2015 in Ireland;
(c) all Allergan Generics Business’ products listed in Schedule F-I (the “UK Marketed Divested Products”), together with, for each of those products, the assets listed in Schedule G-I. For the avoidance of doubt, Schedule F-I covers at least all molecules marketed by Allergan Generics Business which overlap with Teva’s marketed and pipelines molecules in 2015 in the UK;
(d) all Allergan Generics Business’ pipeline products listed in Schedule F-II (the “UK Pipeline Divested Products”), together with, for each of those pipeline products, the assets described in Schedule G-II(1). For the avoidance of doubt, Schedule F-II covers all pipeline molecules of Allergan Generics Business which overlap with Teva’s pipeline and on-market molecules in 2015 in the UK.
23. The IE-UK Divestment Businesses include the Personnel of Allergan Generics Business listed in Schedule E-III and Schedule F-III (the “IE-UK Personnel”).
24. The IE-UK Divestment Businesses include the Allergan livery (pack design and artwork) in the UK and Ireland.
25. Teva commits to grant to the Purchaser a licensing agreement, for a period of three years after Closing, on the Actavis brand for the products included in the IE-UK Divestment Businesses. For the avoidance of doubt, Teva shall retain the right to use the Actavis brand for all products which are not part of the IE-UK Divestment Businesses.
26. Teva commits to a best effort obligation to obtain transfer of the following contracts to the extent that they cover the IE-UK Divested Products:
(a) the agreements between Allergan and wholesalers and logistics companies such as [...] in the UK and Ireland;
(b) the contracts for the supply of API or excipients relating to the manufacturing of the IE-UK Divested Products;
(c) the Private label agreement between Allergan and Almus in the UK;
(d) Allergan’s UK Emergency Medicines Buffer Stock Tender Agreement.
27. The IE-UK Divestment Businesses also include the Allergan Generics Business’ Accumulator scheme in Ireland and the Accumulator Scheme, Partner Pricing scheme, and Allergan Buying Group Scheme in the UK (including intellectual property rights).
28. More specifically, the IE-UK Divestment Businesses include all the intellectual property rights, marketing materials, databases (including historical and forecasted pricing database), and IT infrastructures, including websites, dedicated exclusively to Allergan Generics Business’ discount schemes in the UK and Ireland, as well as an arrangement with the Purchaser to access the relevant IT infrastructure which would not be exclusively dedicated to Allergan Generic Business’ discount schemes in the UK and Ireland, until such date when the Purchaser is able to set up its own infrastructure.
29. The IE-UK Divestment Businesses further include Allergan Generics Business’ manufacturing site located in Barnstaple (the “Barnstaple Manufacturing Facility”), described in more detail in Schedule H. Teva will require from the Purchaser that it obtain the benefit of a supply and services agreement (the “Barnstaple Supply and Services Agreement”), for three years (renewable for two additional years under the supervision of the Monitoring Trustee), for the products manufactured and/or packed at Barnstaple that will be retained by Teva. For the avoidance of doubts, this shall include at least all molecules listed in Schedule I. For the products which are included in the IE-UK Divestment Businesses, supply will be made at commercially reasonable terms. For the products which are not included in the IE-UK Divestment Business, supply will be made at cost.
SECTION C. OTHER COMMITMENTS
Copaxone
30. Teva and Allergan commit that Allergan until Completion and Teva after Completion will comply with all the steps agreed upon with [...] to terminate their licence of [...] marketing authorization application for Glatiramer Acetate.
31. In the event that [...] believes that Allergan or Teva do not comply with this commitment, it will be entitled to address a reasoned request setting out its concerns to the Monitoring Trustee.
32. Teva and Allergan commit that they will inform [...] of the commitment described above and of its ability to send reasoned requests to the Monitoring Trustee.
Aurobindo Products
33. For the molecules listed in Schedule C-II, Teva and Allergan commit that Medis will maintain its relationship with Aurobindo on terms equivalent to those that currently apply to those molecules for a duration of 4 years as of the Effective Date and assist Aurobindo for the corresponding technology transfers (the “Aurobindo Products Commitment”).
34. In the event that Aurobindo believes that Allergan or Teva do not comply with this commitment, it will be entitled to address a reasoned request setting out its concerns to the Monitoring Trustee.
35. Teva and Allergan commit that they will inform Aurobindo of the commitment described above and of its ability to send reasoned requests to the Monitoring Trustee.
SECTION D. RELATED COMMITMENTS
Preservation of viability, marketability and competitiveness
36. Teva shall from the Effective Date until Closing, and Allergan shall from the Effective Date until Completion, preserve or procure the preservation of the economic viability, marketability and competitiveness of the Divestment Businesses, in accordance with good business practice, and shall minimise as far as possible any risk of loss of competitive potential of the Divestment Businesses. In particular, the Parties undertake:
(a) not to carry out any action that might have a significant adverse impact on the value, management or competitiveness of the Divestment Businesses or that might alter the nature and scope of activity, or the industrial or commercial strategy or the investment policy of the Divestment Businesses;
(b) to make available, or procure to make available, sufficient resources for the development of the Divestment Businesses, on the basis and continuation of the existing business plans;
(c) to take all reasonable steps, or procure that all reasonable steps are being taken, including appropriate incentive schemes (based on industry practice), to encourage all IE-UK Key Personnel to remain with the IE-UK Divestment Businesses, and not to solicit or move any Personnel to the Parties’ remaining business. Where, nevertheless, individual members of the IE-UK Key Personnel exceptionally leave the IE-UK Divestment Businesses, the Parties shall provide a reasoned proposal to replace the person or persons concerned to the Commission and the Monitoring Trustee. The Parties must be able to demonstrate to the Commission that the replacement is well suited to carry out the functions exercised by those individual members of the IE- UK Key Personnel. The replacement shall take place under the supervision of the Monitoring Trustee, who shall report to the Commission.
Hold-separate obligations
37. Teva commits, from the Effective Date until Closing, and Allergan commits from the Effective date until Completion, to keep the IE-UK Divestment Businesses separate from the business(es) it is retaining and to ensure that unless explicitly permitted under these Commitments: (i) management and staff of the business(es) retained by the Parties have no involvement in the IE-UK Divestment Businesses; (ii) the Key Personnel of the IE-UK Divestment Businesses have no involvement in any business retained by the Parties and do not report to any individual outside the IE-UK Divestment Businesses.
38. Teva shall until Closing, and Allergan shall until Completion, assist the Monitoring Trustee in ensuring that each part of the IE-UK Divestment Businesses is managed as a distinct and saleable entity or entities separate from the businesses which the Parties are retaining. Immediately after the Effective Date, the Parties shall appoint the IE-UK Hold Separate Manager. The IE-UK Hold Separate Manager shall manage the Divestment Businesses independently and in the best interest of the business with a view to ensuring its continued economic viability, marketability and competitiveness and its independence from the businesses retained by the Parties. The IE-UK Hold Separate Manager shall closely cooperate with and report to the Monitoring Trustee and, if applicable, the Divestiture Trustee. The Commission may, after having heard the Parties, require the Parties to replace the Hold Separate Manager.
39. Immediately after the Effective Date, the Parties, as applicable, shall also appoint a Hold Separate Manager for the IS and Other Countries On-Market Overlap Divestment Businesses who shall be the contact point for the Monitoring Trustee.
Ring-fencing
40. The Parties shall implement, or procure to implement, all necessary measures to ensure that they do not, after the Effective Date, obtain any Confidential Information relating to the Divestment Businesses and that any such Confidential Information obtained by them before the Effective Date will be eliminated and not be used. This includes measures vis-à-vis the Parties’ appointees on the supervisory board and/or board of directors of the Divestment Businesses. In particular, the participation of the Divestment Businesses in any central information technology network shall be severed to the extent possible, without compromising the viability of the Divestment Businesses. The Parties may obtain or keep information relating to the Divestment Businesses which is reasonably necessary for the divestiture of the Divestment Businesses or the disclosure of which to the Parties is required by law.
Non-solicitation clause
41. The Parties undertake, subject to customary limitations, not to solicit, and to procure that Affiliated Undertakings do not solicit, the Key Personnel transferred with the Divestment Businesses for a period of 12 months after Closing.
Due diligence
42. In order to enable potential purchasers to carry out a reasonable due diligence of the Divestment Businesses, Teva shall, subject to customary confidentiality assurances and dependent on the stage of the divestiture process:
(a) provide to potential purchasers sufficient information as regards the Divestment Businesses;
(b) provide to potential purchasers sufficient information relating to the Personnel and allow them reasonable access to the Personnel.
Reporting
43. Teva shall submit written reports in English on potential purchasers of the Divestment Businesses and developments in the negotiations with such potential purchasers to the Commission and the Monitoring Trustee no later than 10 days after the end of every month following the Effective Date (or otherwise at the Commission’s request) including, if applicable, on potential purchasers of the Divestment Businesses and developments in the negotiations with such potential purchasers, and on the status of the Divestment. Teva shall submit a list of all potential purchasers having expressed interest in acquiring the Divestment Businesses to the Commission at each and every stage of the divestiture process, as well as a copy of all the offers made by potential purchasers within five days of their receipt.
44. Teva shall inform the Commission and the Monitoring Trustee on the preparation of the divestiture process, in particular, on the data room documentation and the due diligence procedure and shall submit a copy of any information memorandum to the Commission and the Monitoring Trustee before sending the memorandum out to potential purchasers.
SECTION E. THE PURCHASER(S)
45. In order to be approved by the Commission, the Purchaser(s) must fulfil the following criteria:
(a) The Purchaser(s) shall be independent of and unconnected to the Notifying Party and its Affiliated Undertakings (this being assessed having regard to the situation following the divestiture).
(b) The Purchaser(s) shall have the financial resources, proven expertise, ability and incentive to maintain and develop the Divestment Businesses as a viable and active competitive force in competition with Teva and other competitors;
(c) The acquisition of the Divestment Businesses by the Purchaser(s) must neither be likely to create, in light of the information available to the Commission, prima facie competition concerns nor give rise to a risk that the implementation of the Commitments will be delayed. In particular, the Purchaser(s) must reasonably be expected to obtain all necessary approvals from the relevant regulatory authorities for the acquisition of the Divestment Businesses.
(d) The Purchaser(s) shall be an established pharmaceutical company having the incentive and ability to become independent of the Notifying Party with respect to the manufacturing of the Divested Products;
(e) With respect to the IS Business, the Purchaser(s) shall be a generic company already active in the EEA, and shall have the incentive and ability to serve the Iceland market from another EEA country, and shall have an efficient channel to market the divested products into Iceland;
(f) With respect to the Other Countries On-Market Overlap Divestment Businesses and the Downstream Outlicensing Divestment Businesses, the Purchaser(s) shall be a generic company already active in the EEA, and shall have the incentive and the ability to maintain and develop each of the Divested Products comprising the relevant Other Country Divestment Businesses; with respect to the Other Countries On-Market Overlaps Divestment Businesses, if the Commission finds that, for a given Divested Product, the Purchaser does not have such ability and incentive, Teva commits to divest the product of the non-Divesting Party between either Allergan Generics Business or Teva to the extent it confers such ability and incentive to the Purchaser;
(g) With respect to the Upstream Outlicensing Divestment businesses, the Purchaser(s) shall be a company that already has outlicensing activities in the EEA.
46. The final binding sale and purchase agreement (or agreements) (as well as ancillary agreements) relating to the divestment of the Divestment Businesses shall be conditional on the Commission’s approval. When Teva has reached an agreement (or agreements) with purchaser(s), it shall submit a fully documented and reasoned proposal, including a copy of the final agreement(s), within one week to the Commission and the Monitoring Trustee. Teva must be able to demonstrate to the Commission that the purchaser(s) fulfil(s) the Purchaser Criteria and that the Divestment Businesses are being sold in a manner consistent with the Commission's Decision and the Commitments. For the approval, the Commission shall verify that the purchaser(s) fulfil(s) the Purchaser Criteria and that the Divestment Businesses are being sold in a manner consistent with the Commitments including their objective to bring about a lasting structural change in the market. The Commission may approve the sale of the Divestment Businesses without one or more Assets or parts of the Personnel, or by substituting one or more Assets or parts of the Personnel with one or more different assets or different personnel, if this does not affect the viability and competitiveness of the Divestment Businesses after the sale, taking account of the proposed purchaser(s).
SECTION F. TRUSTEE
I. Appointment procedure
47. Teva shall appoint a Monitoring Trustee to carry out the functions specified in these Commitments for a Monitoring Trustee. The Notifying Party commits not to close the Concentration before the appointment of a Monitoring Trustee.
48. If Teva has not entered into a binding sale and purchase agreement regarding the Divestment Businesses one month before the end of the First Divestiture Period or if the Commission has rejected a purchaser proposed by Teva at that time or thereafter, Teva shall appoint a Divestiture Trustee. The appointment of the Divestiture Trustee shall take effect upon the commencement of the Trustee Divestiture Period.
49. The Trustee shall:
(i) at the time of appointment, be independent of the Notifying Party and its Affiliated Undertakings;
(ii) possess the necessary qualifications to carry out its mandate, for example have sufficient relevant experience as an investment banker or consultant or auditor; and
(iii) neither have nor become exposed to a Conflict of Interest.
50. The Trustee shall be remunerated by the Notifying Party in a way that does not impede the independent and effective fulfilment of its mandate. In particular, where the remuneration package of a Divestiture Trustee includes a success premium linked to the final sale value of the Divestment Businesses, such success premium may only be earned if the divestiture takes place within the Trustee Divestiture Period.
Proposal by Teva
51. No later than two weeks after the Effective Date, Teva shall submit the name or names of one or more natural or legal persons whom Teva proposes to appoint as the Monitoring Trustee to the Commission for approval.
52. No later than one month before the end of the First Divestiture Period or on request by the Commission, Teva shall submit a list of one or more persons whom Teva proposes to appoint as Divestiture Trustee to the Commission for approval. The proposal shall contain sufficient information for the Commission to verify that the person or persons proposed as Trustee fulfil the requirements set out in paragraph 49 and shall include:
(a) the full terms of the proposed mandate, which shall include all provisions necessary to enable the Trustee to fulfil its duties under these Commitments;
(b) the outline of a work plan which describes how the Trustee intends to carry out its assigned tasks;
(c) an indication whether the proposed Trustee is to act as both Monitoring Trustee and Divestiture Trustee or whether different trustees are proposed for the two functions.
Approval or rejection by the Commission
53. The Commission shall have the discretion to approve or reject the proposed Trustee(s) and to approve the proposed mandate subject to any modifications it deems necessary for the Trustee to fulfil its obligations. If only one name is approved, Teva shall appoint or cause to be appointed the person or persons concerned as Trustee, in accordance with the mandate approved by the Commission. If more than one name is approved, Teva shall be free to choose the Trustee to be appointed from among the names approved. The Trustee shall be appointed within one week of the Commission’s approval, in accordance with the mandate approved by the Commission.
New proposal by Teva
54. If all the proposed Trustees are rejected, Teva shall submit the names of at least two more natural or legal persons within one week of being informed of the rejection, in accordance with paragraphs 47 and 53 of these Commitments.
Trustee nominated by the Commission
55. If all further proposed Trustees are rejected by the Commission, the Commission shall nominate a Trustee, whom Teva shall appoint, or cause to be appointed, in accordance with a trustee mandate approved by the Commission.
II. Functions of the Trustee
56. The Trustee shall assume its specified duties and obligations in order to ensure compliance with the Commitments. The Commission may, on its own initiative or at the request of the Trustee or Teva, give any orders or instructions to the Trustee in order to ensure compliance with the conditions and obligations attached to the Decision.
Duties and obligations of the Monitoring Trustee
57. The Monitoring Trustee shall:
(i) propose in its first report to the Commission a detailed work plan describing how it intends to monitor compliance with the obligations and conditions attached to the Decision.
(ii) oversee, in close co-operation with the Hold Separate Manager, the on-going management of the Divestment Businesses with a view to ensuring its continued economic viability, marketability and competitiveness and monitor compliance by Teva with the conditions and obligations attached to the Decision. To that end the Monitoring Trustee shall:
(a) monitor the preservation of the economic viability, marketability and competitiveness of the Divestment Businesses, and the keeping separate of the Divestment Businesses from the business retained by the Notifying Party, in accordance with paragraphs 37 to 38 of these Commitments;
(b) supervise the management of the Divestment Businesses as a distinct and saleable entity, in accordance with paragraph 38 of these Commitments;
(c) with respect to Confidential Information:
- determine all necessary measures to ensure that Teva does not after the Effective Date obtain any Confidential Information relating to the Divestment Businesses,
- in particular strive for the severing of the Divestment Businesses’ participation in a central information technology network to the extent possible, without compromising the viability of the Divestment Businesses,
- make sure that any Confidential Information relating to the Divestment Businesses obtained by Teva before the Effective Date is eliminated and will not be used by Teva and
- decide whether such information may be disclosed to or kept by Teva as the
disclosure is reasonably necessary to allow Teva to carry out the divestiture or as the disclosure is required by law;
(d) monitor the splitting of assets and the allocation of Personnel between the Divestment Businesses and Teva or its Affiliated Undertakings;
(iii) propose to Teva such measures as the Monitoring Trustee considers necessary to ensure Teva’s compliance with the conditions and obligations attached to the Decision, in particular the maintenance of the full economic viability, marketability or competitiveness of the Divestment Businesses, the holding separate of the Divestment Businesses and the non-disclosure of competitively sensitive information;
(iv) mediate and settle any dispute that may arise between [...] and the Parties on the
application of the paragraphs 30 to 32of the Commitments;
(v) mediate and settle any dispute that may arise between Aurobindo and the Parties on the application of the paragraphs 33 to 35 of the Commitments;
(vi) review and assess potential purchasers as well as the progress of the divestiture process and verify that, dependent on the stage of the divestiture process:
(a) potential purchasers receive sufficient and correct information relating to the Divestment Businesses and the Personnel in particular by reviewing, if available, the data room documentation, the information memorandum and the due diligence process, and
(b) potential purchasers are granted reasonable access to the Personnel;
(vii) act as a contact point for any requests by third parties, in particular potential purchasers,
[...] and Aurobindo, in relation to the Commitments;
(viii) provide to the Commission, sending Teva a non-confidential copy at the same time, a written report within 15 days after the end of every month that shall cover the operation and management of the Divestment Businesses as well as the splitting of assets and the allocation of Personnel so that the Commission can assess whether the business is held in a manner consistent with the Commitments and the progress of the divestiture process as well as potential purchasers;
(ix) promptly report in writing to the Commission, sending Teva a non-confidential copy at the same time, if it concludes on reasonable grounds that Teva is failing to comply with these Commitments;
(x) within one week after receipt of the documented proposal referred to in paragraph 46 of these Commitments, submit to the Commission, sending Teva a non-confidential copy at the same time, a reasoned opinion as to the suitability and independence of the proposed purchaser and the viability of the Divestment Businesses after the Sale and as to whether the Divestment Businesses are sold in a manner consistent with the conditions and obligations attached to the Decision, in particular, if relevant, whether the Sale of the Divestment Businesses without one or more Assets or not all of the Personnel affects the viability of the Divestment Businesses after the sale, taking account of the proposed purchaser;
(xi) assume the other functions assigned to the Monitoring Trustee under the conditions and obligations attached to the Decision.
58. If the Monitoring and Divestiture Trustee are not the same legal or natural persons, the Monitoring Trustee and the Divestiture Trustee shall cooperate closely with each other during and for the purpose of the preparation of the Trustee Divestiture Period in order to facilitate each other's tasks.
Duties and obligations of the Divestiture Trustee
59. Within the Trustee Divestiture Period, the Divestiture Trustee shall sell at no minimum price the Divestment Businesses to Purchaser(s), provided that the Commission has approved both the Purchaser(s) and the final binding sale and purchase agreement (or agreements) (and ancillary agreements) as in line with the Commission’s Decision and the Commitments in accordance with paragraphs 45 and 46 of these Commitments. The Divestiture Trustee shall include in the sale and purchase agreement (or agreements) (as well as in any ancillary agreements) such terms and conditions as it considers appropriate for an expedient sale in the Trustee Divestiture Period. In particular, the Divestiture Trustee may include in the sale and purchase agreement (or agreements) such customary representations and warranties and indemnities as are reasonably required to effect the sale. The Divestiture Trustee shall protect the legitimate financial interests of Teva, subject to the Teva’s unconditional obligation to divest at no minimum price in the Trustee Divestiture Period.
60. In the Trustee Divestiture Period (or otherwise at the Commission’s request), the Divestiture Trustee shall provide the Commission with a comprehensive monthly report written in English on the progress of the divestiture process. Such reports shall be submitted within 15 days after the end of every month with a simultaneous copy to the Monitoring Trustee and a non-confidential copy to the Notifying Party.
III. Duties and obligations of Teva
61. Teva shall provide and shall cause its advisors to provide the Trustee with all such co-operation, assistance and information as the Trustee may reasonably require to perform its tasks. The Trustee shall have full and complete access to any of Teva’s or the Divestment Businesses’ books, records, documents, management or other personnel, facilities, sites and technical information necessary for fulfilling its duties under the Commitments and Teva and the Divestment Businesses shall provide the Trustee upon request with copies of any document. Teva and the Divestment Businesses shall make available to the Trustee one or more offices on their premises and shall be available for meetings in order to provide the Trustee with all information necessary for the performance of its tasks.
62. Teva shall provide the Monitoring Trustee with all managerial and administrative support that it may reasonably request on behalf of the management of the Divestment Businesses. This shall include all administrative support functions relating to the Divestment Businesses which are currently carried out at headquarters level. Teva shall provide and shall cause its advisors to provide the Monitoring Trustee, on request, with the information submitted to potential purchasers, in particular give the Monitoring Trustee access to the data room documentation and all other information granted to potential purchasers in the due diligence procedure. Teva shall inform the Monitoring Trustee on possible purchasers, submit lists of potential purchasers at each stage of the selection process, including the offers made by potential purchasers at those stages, and keep the Monitoring Trustee informed of all developments in the divestiture process.
63. Teva shall grant or procure its Affiliated Undertakings to grant comprehensive powers of attorney, duly executed, to the Divestiture Trustee to effect the sale (including ancillary agreements), the Closing and all actions and declarations which the Divestiture Trustee considers necessary or appropriate to achieve the sale and the Closing, including the appointment of advisors to assist with the sale process. Upon request of the Divestiture Trustee, Teva shall cause the documents required for effecting the sale and the Closing to be duly executed.
64. Teva shall indemnify the Trustee and its employees and agents (each an “Indemnified Party”) and hold each Indemnified Party harmless against, and hereby agrees that an Indemnified Party shall have no liability to Teva for, any liabilities arising out of the performance of the Trustee’s duties under the Commitments, except to the extent that such liabilities result from the wilful default, recklessness, gross negligence or bad faith of the Trustee, its employees, agents or advisors.
65. At the expense of Teva, the Trustee may appoint advisors (in particular for corporate finance, legal advice or sectorial expertise), subject to Teva’s approval (this approval not to be unreasonably withheld or delayed) if the Trustee considers the appointment of such advisors necessary or appropriate for the performance of its duties and obligations under the Mandate, provided that any fees and other expenses incurred by the Trustee are reasonable. Should Teva refuse to approve the advisors proposed by the Trustee the Commission may approve the appointment of such advisors instead, after having heard Teva. Only the Trustee shall be entitled to issue instructions to the advisors. Paragraph 64 of these Commitments shall apply mutatis mutandis. In the Trustee Divestiture Period, the Divestiture Trustee may use advisors who served Teva during the Divestiture Period if the Divestiture Trustee considers this in the best interest of an expedient sale.
66. Teva agrees that the Commission may share Confidential Information proprietary to Teva with the Trustee to the extent it relates to the Divestment Businesses. The Trustee shall not disclose such information and the principles contained in Article 17 (1) and (2) of the Merger Regulation apply mutatis mutandis.
67. The Parties agree that the contact details of the Monitoring Trustee are published on the website of the Commission's Directorate-General for Competition and they shall inform interested third parties, in particular any potential purchasers, of the identity and the tasks of the Monitoring Trustee.
68. For a period of 10 years from the Effective Date the Commission may request all information from Teva that is reasonably necessary to monitor the effective implementation of these Commitments.
IV. Replacement, discharge and reappointment of the Trustee
69. If the Trustee ceases to perform its functions under the Commitments or for any other good cause, including the exposure of the Trustee to a Conflict of Interest:
(a) the Commission may, after hearing the Trustee and Teva, require Teva to replace the Trustee; or
(b) Teva may, with the prior approval of the Commission, replace the Trustee.
70. If the Trustee is removed according to paragraph 69 of these Commitments, the Trustee may be required to continue in its function until a new Trustee is in place to whom the Trustee has effected a full hand over of all relevant information. The new Trustee shall be appointed in accordance with the procedure referred to in paragraphs 47 to 55 of these Commitments.
71. Unless removed according to paragraph 69 of these Commitments, the Trustee shall cease to act as Trustee only after the Commission has discharged it from its duties after all the Commitments with which the Trustee has been entrusted have been implemented. However, the Commission may at any time require the reappointment of the Monitoring Trustee if it subsequently appears that the relevant remedies might not have been fully and properly implemented.
SECTION F. THE REVIEW CLAUSE
72. The Commission may extend the time periods foreseen in the Commitments in response to a request from the Parties or, in appropriate cases, on its own initiative. Where the Parties request an extension of a time period, it shall submit a reasoned request to the Commission no later than one month before the expiry of that period, showing good cause. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time send a non-confidential copy of the report to the Notifying Party. Only in exceptional circumstances shall the Parties be entitled to request an extension within the last month of any period.
73. The Commission may further, in response to a reasoned request from the Parties showing good cause waive, modify or substitute, in exceptional circumstances, one or more of the undertakings in these Commitments. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time send a non-confidential copy of the report to the Parties. The request shall not have the effect of suspending the application of the undertaking and, in particular, of suspending the expiry of any time period in which the undertaking has to be complied with.
SECTION G. ENTRY INTO FORCE
The Commitments shall take effect upon the date of adoption of the Decision.
Signed on 4 March 2016 in Teva Pharmaceuticals Europe,
Rob Koremans
President & CEO Global Specialty Medicines
Duly authorised for and on behalf of Teva Pharmaceutical Europe.
Gianfranco Nazzi
SVP Europe Specialty Medicine
Duly authorised for and on behalf of Teva Pharmaceutical Europe.
SECTION G - ALLERGAN ENTRY INTO FORCE
The Commitments shall take effect upon the date of adoption of the Decision. Allergan will be bound only as specified by paragraph 1 of the Commitments and only up until Completion.
Signed on 4 March 2016 by Allergan plc,
A. Robert D. Bailey
EVP, Chief Legal Officer and Corporate
Secretary
Duly authorised for and on behalf of Allergan
plc.
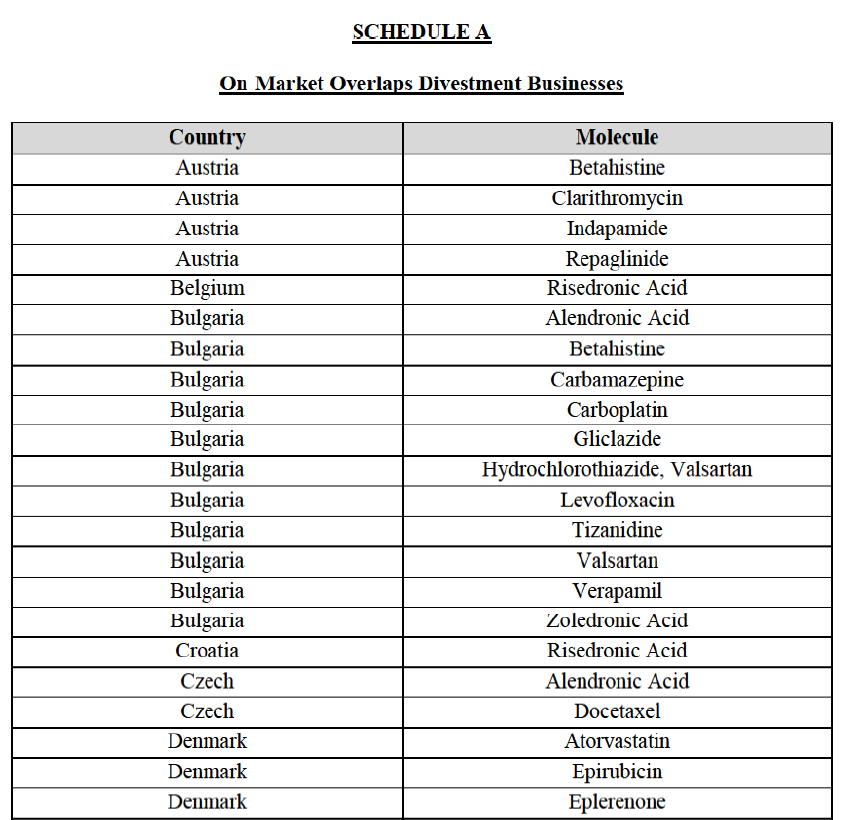
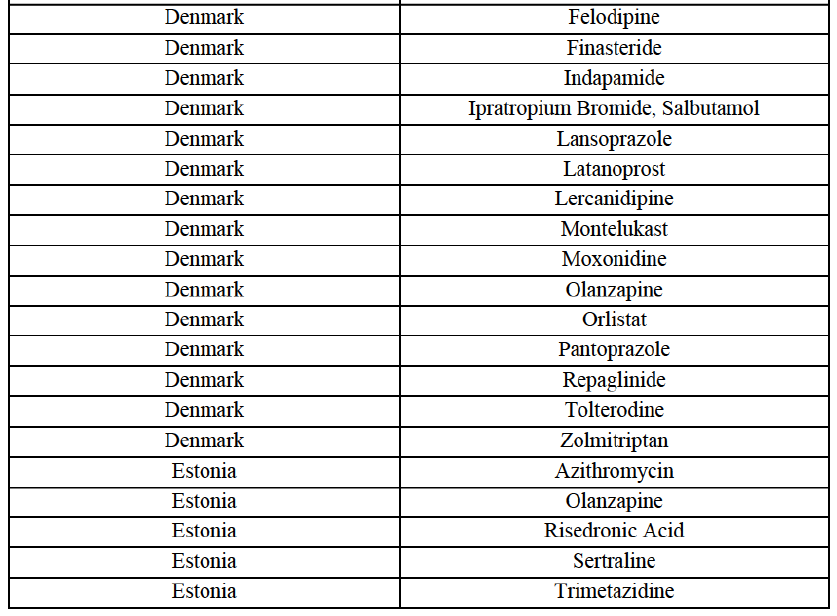
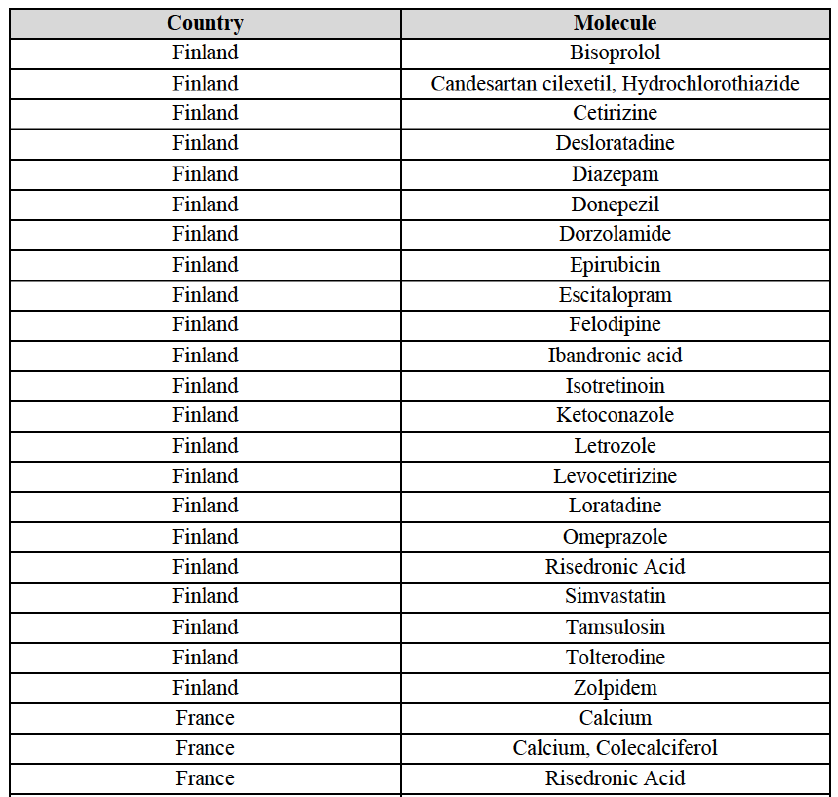
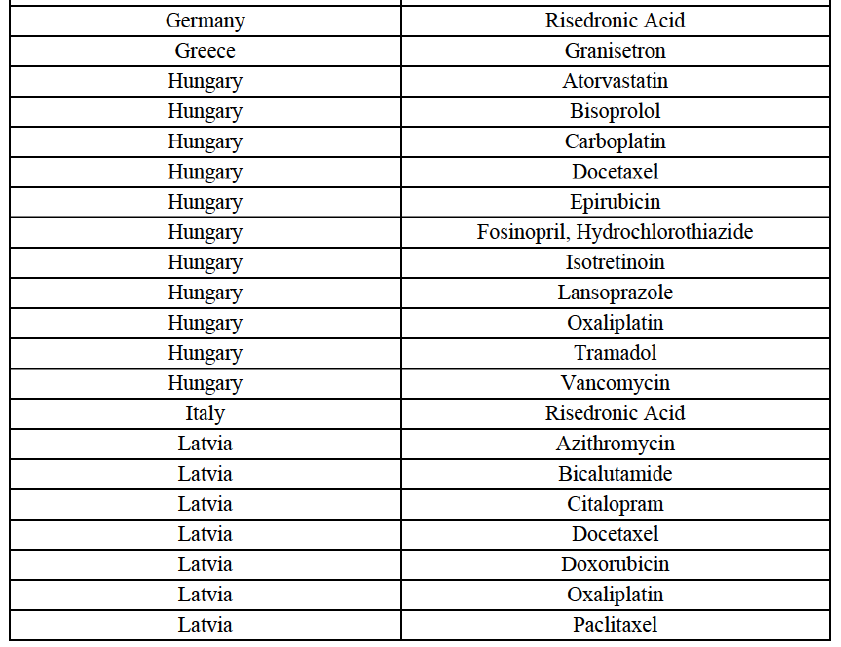
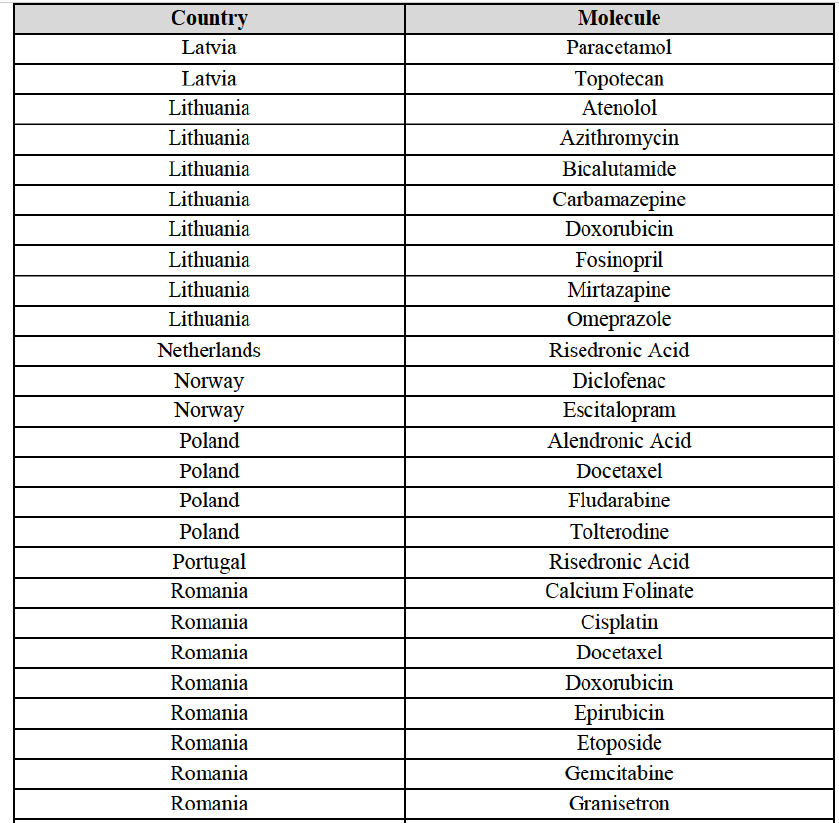
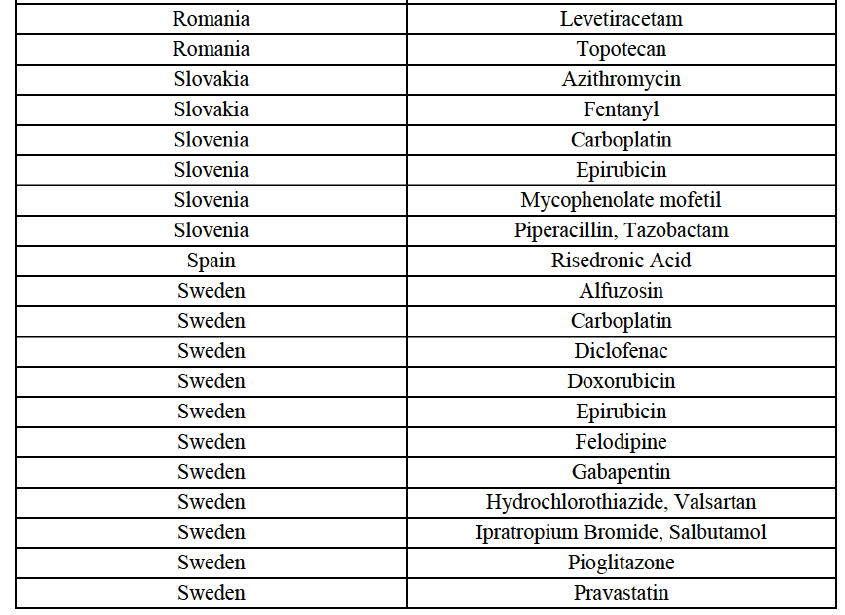
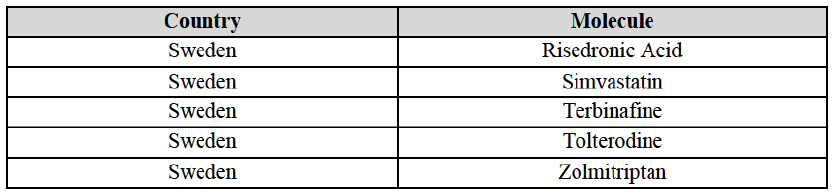
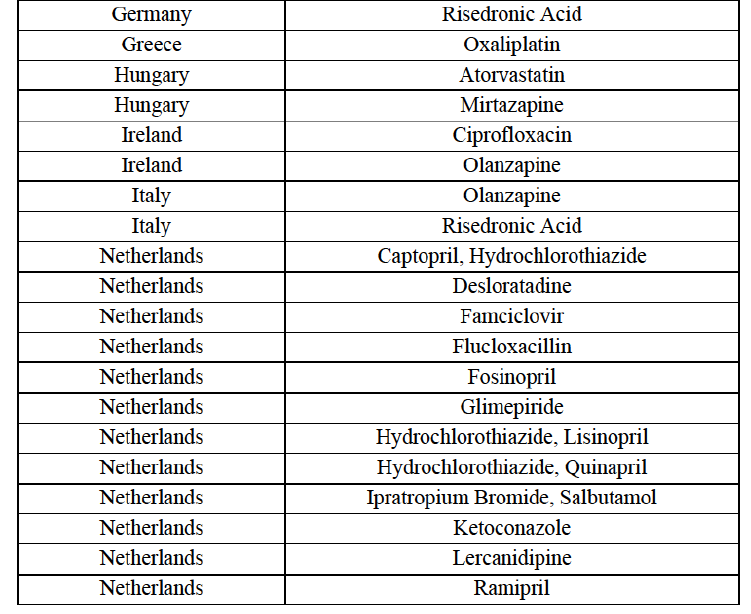
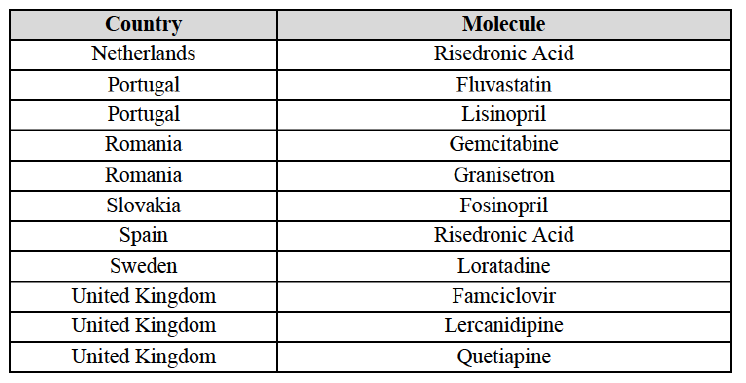
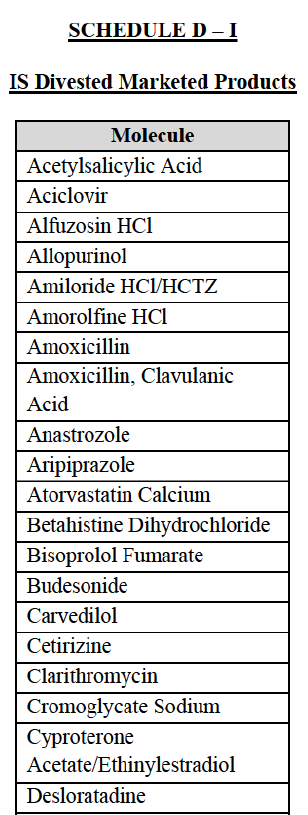
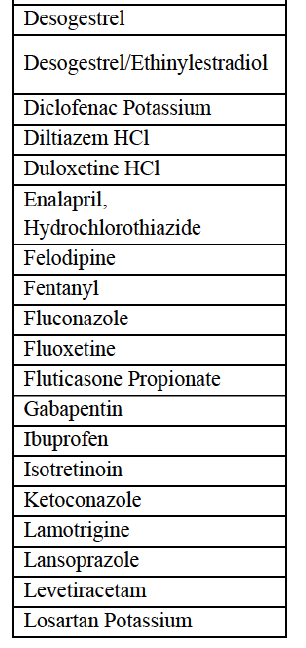
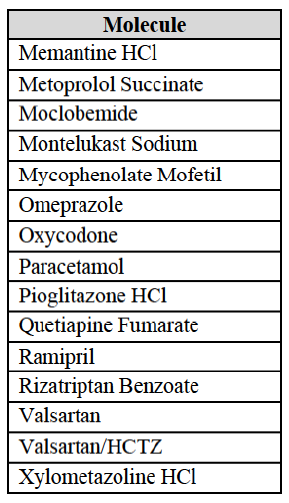
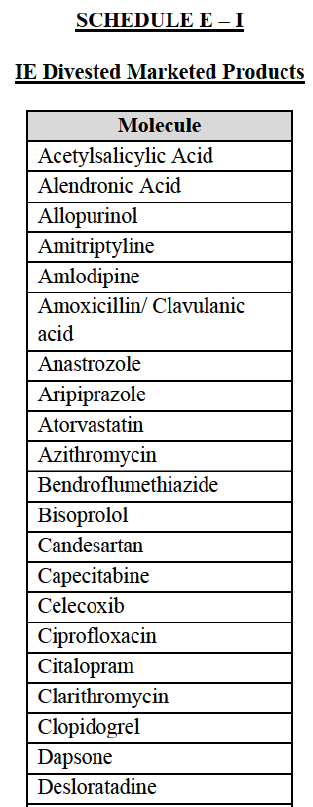
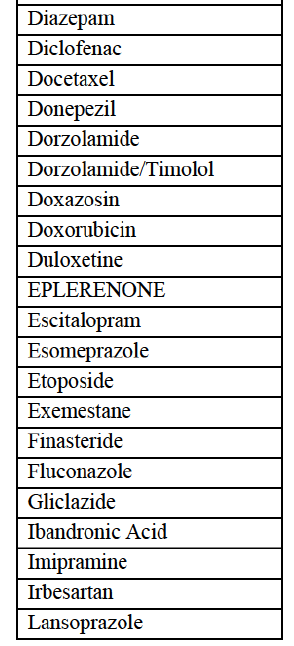
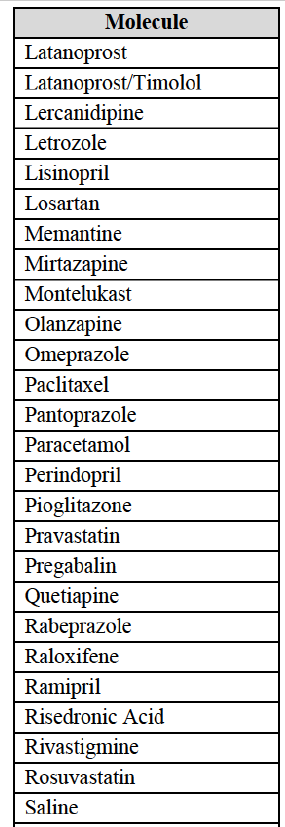
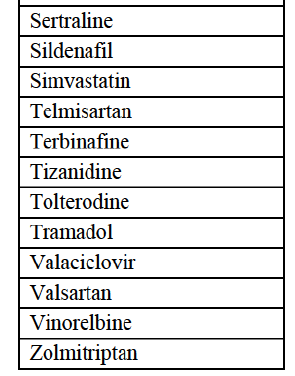
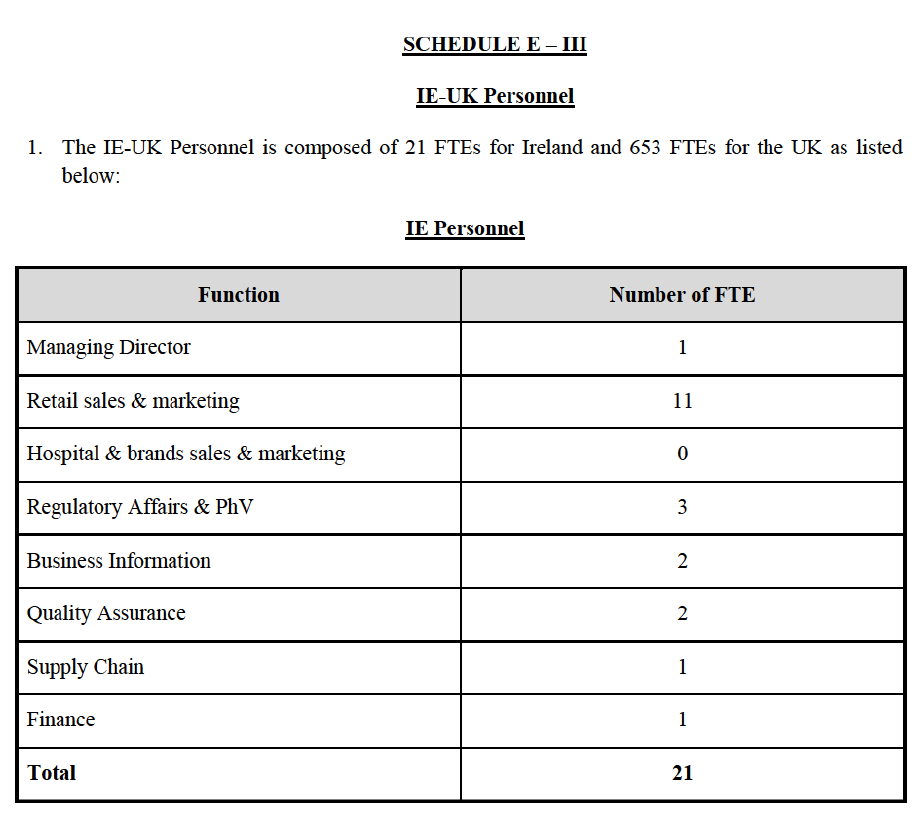
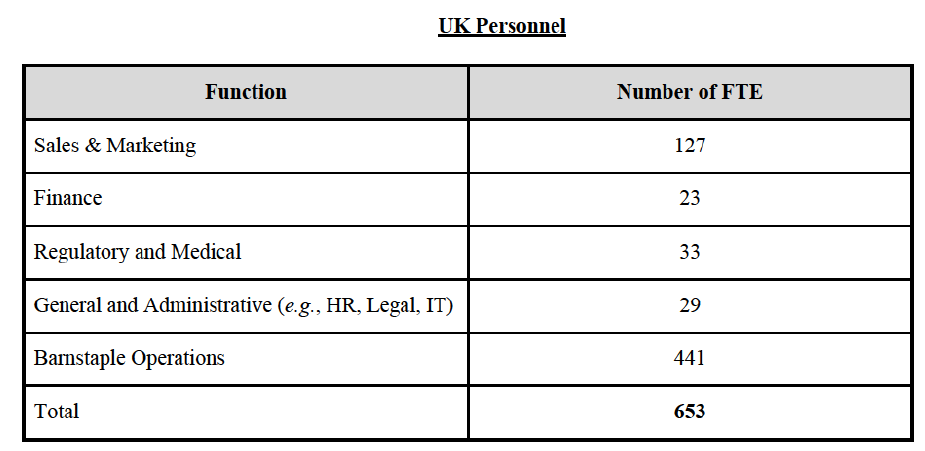
2. The Key Personnel necessary to maintain the viability and competitiveness of the IE-UK Divestment Businesses is listed below:
* [...] (Senior VP, President UK & Ireland) who will be appointed Hold Separate Manager
* [...] (Managing Director UK)
* [...] (Managing Director Ireland)
* [...] (Managing Director Operations)
* [...] (Director Supply Chain)
* [...] (Director –Regulatory Affairs)
* [...] (Director Human Ressources)
* [...] (Finance & IT)
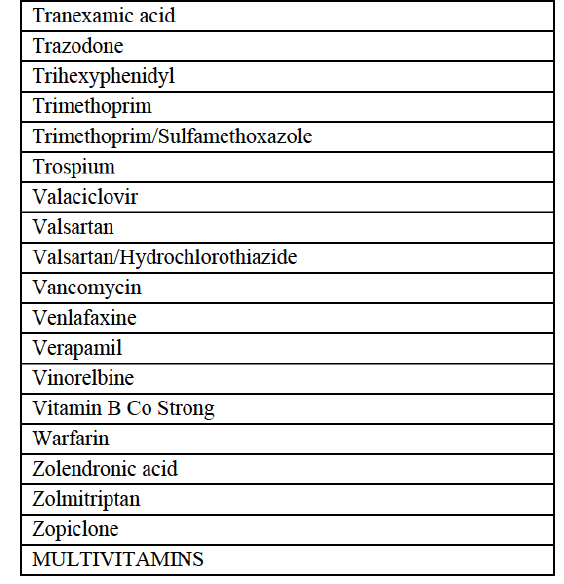
SCHEDULE G – I
On-Market Products Assets
1. The On-Market Products Assets consist of the Divesting Party’s rights, title, and interests in the relevant molecules in the relevant countries including the right to develop, manufacture and use such molecules with a view to its sale and marketing in any form and for any indication whatsoever in the relevant countries.
2. The On-Market Products Assets comprise in particular all tangible and intangible assets (including intellectual property rights), which contribute to the current operation or are necessary to ensure the viability and competitiveness of the Divestment Businesses, as of Closing, including:
(a) in compliance with current regulatory practice, a transfer of marketing authorizations for the relevant country and relevant registration dossier, which contain in particular the following modules:
* module 1: Administrative information about the marketing authorizations holder, release site, mock-ups of packaging, etc.
* module 2: High level summaries (the Quality Overall Summary, the Nonclinical Overview/Summaries and the Clinical Overview/Summaries);
* module 3: Quality documentation (chemical, pharmaceutical, biological and manufacturing documentation);
* module 4: Nonclinical Study Reports;
* module 5: Clinical Study Reports.
(b) a best effort obligation to obtain transfer of all contracts, leases, commitments;
(c) the transfer of customers information and orders, all customer credit records, invoices, contact details and other records of the Divestment Businesses;
(d) the sale of existing product inventory, sales, and promotional material in the relevant countries, as far as available,
(e) if applicable, all trademarks and generics brands associated with the Divestment Businesses;
(f) all licenses, permits and authorizations issued by any governmental organization for the benefit of the Divestment Businesses;
(g) all proprietary information associated with the production process of the Divestment Businesses, for use only within the corresponding countries in which the Divestment Businesses are located;
(h) an irrevocable, assignable, sub-licensable, and royalty-free license for all relevant intellectual property rights, data, books, records and effective arrangements for the transfer of all know-how to the extent that these are related to the development, manufacture, use of the Divestment Businesses, for use only within the corresponding countries in which the Divestment Businesses are located;
3. For the avoidance of doubt, no IP right would be transferred in case the divestiture concerns a third-party product.
4. For all Divestment Businesses, if Teva demonstrates that it is not possible to transfer the marketing authorisation held by the Divesting Party in the relevant country, either because the marketing authorisation was obtained through a centralised procedure or due to the Certificate of Pharmaceutical Product required for this product’s supply outside of the EEA, Teva commits to divest the product of the non-Divesting Party. If Teva further demonstrates that it is not possible to transfer the marketing authorisation held by the non-Divesting Party in the relevant country for one of the two reasons above, Teva shall send a reasoned request pursuant to paragraph 73 of the Commitments.
5. Teva commits to make its best efforts to cooperate with the Purchaser(s) to effectuate the transfer of the Divestment Businesses. In particular, Teva commits to undertake, at its own expense and unless the costs involved are manifestly excessive for the product concerned (subject to the assessment of the Monitoring Trustee), all regulatory changes that would be required as a result of such transfer in particular regarding marketing authorization variations and dossier improvements. With respect to dossier improvements Teva commits to a reasonable efforts obligation to effectuate such improvements, to the extent they are required to operate the MA transfer.
6. For all Divested Products which the Divesting Party manufactures itself, and at the option of the Purchaser(s), for a period of three years (renewable for two additional years under the supervision of the Monitoring Trustee), on a fully loaded cost basis to be agreed with the Purchaser(s), the On-Market Product Assets include:
(a) the benefit, for a period of three years (renewable for two additional years under the supervision of the Monitoring Trustee), at full manufacturing cost, ex-works, at current quality and quantity levels or quantities otherwise agreed between Teva and the Purchaser(s) that reflects changes in customer demand, all as overseen by the Monitoring Trustee, of a supply arrangement for the relevant product. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by the Divesting Party to the Purchaser(s). It shall not contain provisions requiring the delivery of minimum supply volumes or batches;
(b) all historical information (orders, prices, etc) concerning the Divesting Party’s relationship with its API supplier(s) in accordance with applicable law. Teva commits to a best effort obligation to obtain consent to assign the relevant API supply agreements for the relevant products.
7. For all Divested Products manufactured in-house for which the Divesting Party manufactures the API itself, and at the option of the Purchaser(s), the On-Market Product Assets include the benefit of a supply arrangement for a period of three years (renewable for two additional years under the supervision of the Monitoring Trustee), under commercially reasonable terms to be agreed between Teva and the Purchaser(s).
8. For all Divested Products which the Divesting Party does not manufacture itself, Teva commits to:
(a) a best effort obligation to obtain consent to assign the contract manufacturing agreements entered into between the Divesting Party and its supplier for the relevant product (the “Other Countries Manufacturer”);
(b) supply the relevant products for a period of three years (renewable for two additional years under the supervision of the Monitoring Trustee), for instance through Teva concluding with the Purchaser(s) a “back-to-back” supply agreement whereby Teva would continue to procure the relevant products from the Other Countries Manufacturer and supply these products to the Purchaser(s) at cost; and
(c) divest the product of the non-Divesting Party between either Allergan Generics Business or Teva in the event that Teva cannot, for a reason outside its control, obtain the consent of the Other Countries Manufacturer to items (a) and (b) above.
9. Teva commits to provide expeditiously and at Teva’s cost, at the option of the Purchaser(s), the necessary technical assistance at the option for the Purchaser(s), for the duration of the transitional supply agreement, to assume responsibility for:
(a) the manufacture and the procurement of raw materials necessary for the manufacturing of the products of Divestment Businesses;
(a) the sale and marketing of the Divestment Businesses, for such period as is required by the Purchaser(s) to establish the Divestment Businesses as a viable independent business.
10. The Purchaser(s) will be given an option to hire one or more Personnel, subject to applicable local employment legislation, who would reasonable be considered necessary to maintain the viability, marketability, and competitiveness of these Divestment Businesses to be supervised by the Monitoring Trustee.
11. For the avoidance of doubt, the Divestment Businesses shall, inter alia, not include (except when otherwise indicated in the Commitments):
(a) any manufacturing facilities of the Parties;
(b) any raw materials;
(c) any research and development, clinical data and studies or intellectual property relating to the Divestment Businesses after Closing;
(d) the Teva, Ratiopharm, Actavis or Allergan Generics Business brand, names or logos in any form;
(e) books and records required to be retained pursuant to any statute, rule, regulation or ordinance, provided that the Purchaser(s) shall obtain a copy of the same and shall be permitted access to the original of such books and records upon reasonable request during normal business hours;
(f) general books of account and books of original entry that comprise the Parties’ or an Affiliated Undertaking’s permanent accounting or tax records; and
(g) payments to be made to the Parties by the customers with respect to the purchase of the Divested Products prior to divestiture.
12. If there is any asset which is not covered by this Schedule but which is both used (exclusively or not) in the Divestment Businesses and necessary for the continued viability and competitiveness of the Divestment Businesses, that asset or adequate substitute will be offered to the Purchaser(s).
SCHEDULE G – II
Pipeline Assets
Schedule G-II(1) National pipeline divestitures
1. The Pipeline assets for national Pipeline divestitures shall include:
(a) all materials already developed by Allergan Generics Business or Teva, whoever is divesting the pipeline to allow the Purchaser(s) to continue the development the Market-to Pipeline Divestment Products;
(b) all relevant data generated during the development project, including all material technical, preclinical, clinical and marketing files, clinical data and studies, reports, plans, know-how and records in the possession of Allergan Generics Business or Teva, whoever is divesting the pipeline product;
(c) if applicable, copies of all clinical data and studies relating to the development of Market-to Pipeline Divestment Products, to the extent necessary for the manufacture, sale and marketing in the relevant country(ies) prior to closing of the Transaction;
(d) all correspondence pertaining to regulatory filings and approvals (if any) relating to the marketing of the Market-to Pipeline Divestment Products in the relevant country(ies);
(e) the intellectual property rights where available and necessary to give a Purchaser(s) the exclusive right to continue to develop the Market-to Pipeline Divestment Products for sale in the country(ies) by way of a perpetual, irrevocable licence. These intellectual property rights include product formulations, manufacturing know-how and other secret know-how, packaging specifications, rights to the trade dress, and all related copyright.
(f) copies of all relevant data, books, records, and other documents exclusively related to or necessary for the development and marketing of the Market-to Pipeline Divestment Products in the country(ies) provided that the Parties may redact from such copies any information that does not relate to the Market-to Pipeline Divestment Products.
(g) all proprietary information associated with the production process of the Pipeline Assets, for use only within the relevant country.
(h) to the extent necessary for a timely launch of the pipeline product, the benefit of a contract manufacturing agreement for a transitory period until the Purchaser(s) secures a supply source.
2. Teva commits to complete the development of the pipeline to the extent necessary for the Purchaser(s) to launch and market the product in the relevant countries with the assets listed in this Schedule.
3. Teva commits to provide expeditiously and at Teva’s cost, at the option of the Purchaser(s), the necessary technical assistance to assume responsibility for the transfer of the manufacturing process.
4. If there is any asset which is not covered by this Schedule but which is both used (exclusively or not) in the Divestment Businesses and necessary for the continued viability and competitiveness of the Divestment Businesses, that asset or adequate substitute will be offered to the Purchaser(s).
Schedule G-II(2) EEA pipeline divestitures
5. The Pipeline assets for EEA-wide Pipeline divestitures shall include:
(a) All materials already developed by the Allergan Generics Business or Teva, whoever is divesting the pipeline to allow the Purchaser(s) to continue the development the Market-to Pipeline Divestment Products;
(b) All relevant data generated during the development project, including all material technical, preclinical, clinical and marketing files, clinical data and studies, reports, plans, know-how and records in the possession of Allergan Generics Business or Teva, whoever is divesting the pipeline product;
(c) If applicable, copies of all clinical data and studies relating to the development of Market-to Pipeline Divestment Products existing in all EEA countries to the extent necessary for the manufacture, sale and marketing in the EEA prior to closing of the Transaction;
(d) All correspondence pertaining to regulatory filings and approvals (if any) relating to the marketing of the Market-to Pipeline Divestment Products in the EEA;
(e) The intellectual property rights where available and necessary to give a Purchaser(s) the exclusive right to continue to develop the Market-to Pipeline Divestment Products for sale in the EEA by way of a perpetual, irrevocable licence. These intellectual property rights include product formulations, manufacturing know-how and other secret know-how, packaging specifications, rights to the trade dress, and all related copyright.
(f) Copies of all relevant data, books, records, and other documents exclusively related to or necessary for the development and marketing of the Market-to Pipeline Divestment Products in the EEA provided that the Parties may redact from such copies any information that does not relate to the Market-to Pipeline Divestment Products.
(g) All proprietary information associated with the production process of the Pipeline Assets, for use only within the EEA.
(h) To the extent necessary for a timely launch of the pipeline product, the benefit of a contract manufacturing agreement for a transitory period until the Purchaser(s) secures a supply source.
6. Teva commits to provide expeditiously and at Teva’s cost, at the option of the Purchaser(s), the necessary technical assistance to assume responsibility for the transfer of the manufacturing process.
7. If there is any asset which is not covered by this Schedule but which is both used (exclusively or not) in the Divestment Businesses and necessary for the continued viability and competitiveness of the Divestment Businesses, that asset or adequate substitute will be offered to the Purchaser(s).
SCHEDULE G – III
Upstream Outlicensing Assets
1. The Upstream Outlicensing Assets include:
(a) the Dossier on which the outlicensing agreements entered into by Teva/Medis for the relevant products are based;
For the avoidance of doubt, Teva commits to divest the relevant on-market downstream business in the event that Teva cannot, for a reason outside its control, transfer the Dossier.
(b) a best efforts obligation to transfer the outlicensing and contract manufacturing agreements that Teva/Medis entered into on the basis of the Dossier. For the avoidance of doubt, this should include all countries covered by the Dossier;
(c) all customer information and records pertaining to the outlicensing and manufacturing agreements referred to in paragraph 1(b) above;
(d) all historical information (orders, prices, etc) concerning Teva/Medis relationship with its API supplier(s) in accordance with applicable law. Teva commits to a best effort obligation to obtain consent to assign the relevant API supply agreements for the relevant products.
(e) all proprietary information and know-how associated with the production process of the Upstream Outlicensing Businesses;
(f) the benefit, for a period of three years (renewable for two additional years under the supervision of the Monitoring Trustee), at full manufacturing cost, ex-works, at current quality and quantity levels or quantities otherwise agreed between Teva and the Purchaser(s) that reflects changes in customer demand, all as overseen by the Monitoring Trustee, of a supply arrangement for the relevant product. Such transitory arrangement shall include appropriate provisions designed to ensure the continued supply by the Divesting Party to the Purchaser(s).
2. Teva commits to provide expeditiously and at Teva’s cost, at the option of the Purchaser(s), the
necessary technical and regulatory support for the transfer of the manufacturing process.
3. Teva will have the ability to require from the Purchaser(s) a license back at full manufacturing cost, ex-works, on the Dossier for: (i) any of the downstream finished dose products currently marketed by Teva itself based on the Dossier, or (ii) any of the downstream finished dose products currently outlicensed to Aurobindo to ensure the continuation of its relationship with the merged entity.
SCHEDULE H
Description of the Barnstaple Manufacturing Facility
1. At the option of Purchaser(s), Teva commits to divest Allergan Generics Business’ Barnstaple manufacturing plant located at Whiddon Valley, Barnstaple, Devon EX32 8NS, United Kingdom. The divestment of Allergan Generics Business’ Barnstaple plant comprises the 55,000 m2 site and the 19,000 m2 facility housing the plant’s entire operations:
(a) Manufacturing and Packaging Tablets and Capsules;
(b) Cyclogest Pessary Production;
(c) Supply Chain Management;
(d) Warehouse Receiving, Sampling and Despatch;
(e) Quality Control;
(f) Quality Assurance;
(g) Quality Management for 3rd party suppliers;
(h) Pharm Tech Production Support;
(i) Transfers and Development;
(j) Site Engineering and Project Management; and
(k) Environment, Health, and Safety.
2. The divestment of Allergan Generics Business’ Barnstaple plant also covers the following business units:
(a) Commercial;
(b) Finance and IT;
(c) Human Resources; and
(d) Regulatory, Pharmacovigilance and Compliance.