Commission, October 29, 2018, No M.9019
EUROPEAN COMMISSION
Decision
MARS / ANICURA
Subject: Case M.9019 – Mars/AniCura
Commission decision pursuant to Article 6(1)(b) in conjunction with Article 6(2) of Council Regulation No 139/2004 (1)
Dear Sir or Madam,
(1) On 10 September 2018, the European Commission (the "Commission") received notification of a proposed concentration pursuant to Article 4 of the Merger Regulation by which Mars, Incorporated ("Mars" or the "Notifying Party", USA) intends to acquire, within the meaning of Article 3(1)(b) of the Merger Regulation, sole control of AniCura TC AB and its subsidiaries ("AniCura", Sweden), (the "Transaction"). (2) Mars and AniCura are further collectively referred to as the "Parties", whist the undertaking that would result from the Transaction is referred to as "the merged entity".
1. THE PARTIES
(2) Mars is a global privately held company active in various consumer product sectors. Through its business division Mars Petcare, Mars is a pet food and veterinary care provider, active in the dietetic pet food segment via its Royal Canin brand, which it markets worldwide, including in the EU. Mars Petcare also operates veterinary health businesses in North America and in the UK and is the world's biggest veterinary health group.
(3) AniCura operates a leading chain of veterinary clinics and hospitals throughout northern Europe, with significant operations (in order of importance in terms of turnover generated) in Sweden, Germany, Norway, the Netherlands and Denmark, and smaller operations in Austria, Italy, France and Spain.
(4) AniCura also operates VetFamily, a veterinary franchise operation described by the Parties as a "partnership network for independent veterinary clinics". VetFamily currently has over 1 000 independent members across Sweden, Norway, Denmark, Germany and the Netherlands. AniCura owns 17 of those members, all located in Denmark. AniCura is responsible for the procurement of products for these franchise veterinary clinics, including the procurement of dietetic pet food. VetFamily also offers a number of training events to its members.
(5) AniCura operates a similar veterinary franchise operation, called Sterkliniek, in the Netherlands which currently has 75 members (of which 27 are owned by AniCura).
2. THE OPERATION AND CONCENTRATION
(6) Pursuant to a sale purchase agreement entered into on 7 June 2018 between Mars (as buyer) and Nordic Capital, Fidelio Capital and other minority shareholders of AniCura (as sellers), Mars will acquire 100% of the issued and to be issued share capital in AniCura.
(7) As a result of the Transaction, Mars will obtain sole control over AniCura.
(8) Therefore, the Transaction constitutes a concentration within the meaning of Article 3(1)(b) of the Merger Regulation.
3. UNION DIMENSION
(9) The Parties have a combined aggregate world-wide turnover of more than EUR 5 000 million (3) (EUR: […]). Each of them has an Union-wide turnover in excess of EUR 250 million (Mars: EUR […]; AniCura: EUR […]), but each does not achieve more than two-thirds of its aggregate Union-wide turnover within one and the same Member State.
(10) The notified operation therefore has a Union dimension pursuant to Article 1(2) of the Merger Regulation.
4. APPLICABILITY OF THE EEA AGREEMENT
(11) Article 8 of the EEA Agreement (4) provides that, unless otherwise specified, the provisions of the EEA Agreement shall apply only to:
(a) products falling within Chapters 25 to 97 of the Harmonized Commodity Description and Coding System ("HS Nomenclature"), excluding the products listed in Protocol 2 to the EEA Agreement (5);
(b) products specified in Protocol 3 to the EEA Agreement (6), subject to the specific arrangements set out in that Protocol.
(12) The HS Nomenclature of dog or cat food, put up for retail sale, is 2309.10 and 2309.90 for other preparations of a kind used in animal feeding. Pet food therefore falls within Chapter 23, and not within Chapters 25 to 97, of the HS Nomenclature. Pet food is not covered in Protocol 3 to the EEA Agreement. Therefore, the EEA Agreement does not apply to pet food products and the assessment of the impact of the Transaction for pet food hence falls outside Article 57 of the EEA Agreement on merger control.
(13) The Notifying Party argues (7) that the Commission has exclusive jurisdiction to review the Transaction within the EEA, since the Transaction concerns the acquisition of a company active in the provision of veterinary services, which fall within the scope of the EEA Agreement.
(14) The Commission notes that the relevant downstream market in this case concerns not the provision of veterinary services, but the retail of pet food. The fact that the target company is also active in the provision of veterinary services is not sufficient to conclude that the downstream market is the provision of such services and that the Commission therefore has exclusive jurisdiction in the EEA.
(15) The assessment of the impact of the Transaction for pet food in the EFTA States hence falls outside the jurisdiction of the Commission. Consequently, the present Decision will analyse the effects of the Transaction on the EU market for pet food products and will not assess possible anticompetitive effects in Norway, Iceland and Lichtenstein.
5. RELEVANT MARKETS
5.1. Introduction
(16) The Transaction concerns two companies operating mostly at different levels of the supply chain. Mars is a manufacturer of dietetic pet food, which it supplies to veterinary clinics and animal hospitals. AniCura is the owner of a chain of veterinary clinics and animal hospitals (as well as of the VetFamily and Sterkliniek purchasing agreement networks of independent clinics), where dietetic pet food is sold to pet owners.
(17) "Dietetic pet food" (also referred to in the industry as "therapeutic" or "veterinary diet" pet food) is pet food that is intended to be formulated to meet the specific dietary requirements of a pet that is suffering from one or more specific health or dietary issues, and is typically recommended by a veterinarian. (8)
(18) Whilst AniCura also supplies ranges of private label dietetic pet food made available in its clinics in Denmark and in Sterkliniek clinics in the Netherlands, the horizontal overlaps with the supplies of dietetic pet food by Mars are very limited. (9)
(19) Mars owns and operates veterinary clinics in the UK, but given that AniCura is not present in the UK, no horizontal overlaps in veterinary services arise as a result of the Transaction.
(20) In order to assess the Transaction's impact on competition, the Commission has assessed the definition of the relevant upstream and downstream markets.
5.2. The upstream market – manufacture and supply of dietetic pet food
5.2.1. Relevant product market definition
5.2.1.1. The Notifying Party's arguments
(21) The Notifying Party claims that the relevant product market upstream concerns industrial (or prepared) pet food for dogs and for cats, (10) which could be sub- segmented into (i) dry dog food, (ii) wet dog food, (iii) dry cat food, and (iv) wet cat food products. (11) In particular, the Notifying Party does not believe that dietetic pet food forms a distinct product market. (12)
(22) The Notifying Party claims that there is a high level of demand-side substitutability between dietetic pet food and other pet food. (13) For example, in the Notifying Party's view, substitution between dietetic and 'conventional' (or mainstream, non-dietetic) pet food can occur where the pet owner supplements conventional pet food with over-the-counter medication or dietary supplements to manage the pet's health condition. (14)
(23) The Notifying Party also argues that there is a high level of supply-side substitutability between dietetic pet food and other pet food, since (i) many of Mars' competitors offer dietetic as well as non-dietetic pet food; (ii) the equipment processes required to manufacture dietetic and non-dietetic pet food are essentially similar and normally not regarded as a key determinant of differentiation (although the manufacturing processes are sometimes adjusted to reflect specific product requirements); and (iii) the investment into additional research and development, and product and brand awareness (including with professionals such as veterinarians), as well as specific product labelling and wider marketing, can be easily replicated by new entrants. (15)
(24) Finally, the Notifying Party also claims that […]. At the same time, the Notifying Party acknowledges that dietetic products have a more complex formulae, including a wide range of different (higher quality) ingredients (or specifically excluded ingredients) and vitamin and mineral additives, which manufacturers tailor to support their claims of assisting pets' fight against certain medical conditions. (16)
5.2.1.2. The Commission's assessment
(25) In its previous decisions relating to pet food, the Commission found that: (i) industrial dog and cat food is a distinct market, separate from home prepared pet food; (17) (ii) dog food and cat food are not part of the same relevant product market; (18) (iii) dry dog food, wet dog food, dry cat food, and wet cat food products each constitute a separate product market; (19) and (iv) it cannot be excluded that specialist (non-grocery) stores are a separate relevant product market from grocery stores. (20)
(26) The Commission in the present case particularly assessed the potential distinction of dietetic pet food and other types of pet foods. Based on the evidence available and on the results of the market investigation, the Commission finds that dietetic pet food constitutes a distinct relevant product market based on the following reasons.
(27) Demand-side substitutability between dietetic pet food and other pet food was shown to be very limited. None of the competitors and veterinarians that responded to the Commission's market investigation considered that non-dietetic pet food could be substituted for dietetic pet food from the view point of the consumer. (21) It was noted that dietetic pet food fulfils a particular purpose, being formulated to address the nutritional requirements of specific health conditions in pets. (22) For example, one market participant explained that "[f]rom a veterinary point of view, the use of dietetic feeds to support nutrition-related diseases […] is absolutely necessary and cannot be replaced by conventional feed." (23) It was further noted that dietetic pet food was not fit for healthy animals and that feeding healthy animals a dietetic pet food diet over a prolonged period of time may lead to malnutrition or other health problems. (24) Therefore, as was stressed by several market participants, a diagnosis and a medical indication from a veterinarian are required before a pet can be fed dietetic pet food. (25)
(28) A very small number of responding veterinarians stated that non-dietetic pet food could be substituted for dietetic pet food, but only if the former is modified by means of prescription or over-the-counter medicine and/or nutritional supplements. One veterinarian noted that "[s]ubstitution with feed prepared by oneself in accordance with a recipe is possible, but for most customers not practicable". (26) Another veterinarian explained that substitution is problematic for some specifically formulated dietetic pet food products, for example those designed to feed pets with allergies, or with kidney or urinary stones, given that those types of feed contain large reductions of certain raw materials (such as proteins and minerals). (27)
(29) There is very little evidence of switching between dietetic and non-dietetic (conventional) pet food once dietetic pet food has been recommended. For example, switching from dietetic to conventional pet food would occur only when the dietetic pet food product is no longer considered necessary, or if the pet does not find it palatable (refusing to eat it), in which case the recommended dietetic pet food is replaced by an alternative dietetic pet food product, or an alternative solution recommended by a veterinarian. (28) Market participants have explained that if a pet (especially with a long-term health condition) is switched to conventional pet food, the pet owner would generally switch back to giving the pet the dietetic pet food product once they notice that the health issue recurs and/or once the veterinarian at a follow-up visit draws their attention to the need to follow the recommended diet. (29)
(30) The results of the market investigation also show that dietetic pet food tends to be more expensive than non-dietetic pet food: the average prices for dietetic pet food products were estimated to be between 10-30% and up to 3 times higher than for non-dietetic pet food products. (30)
(31) As regards supply-side substitutability, the Notifying Party's arguments as to the high degree of substitutability were similarly not supported by the results of the market investigation, which revealed a very limited degree of supply-side substitutability. Most conventional pet food manufacturers do not manufacture dietetic pet food, (31) and specialised dietetic pet food manufacturers do not manufacture conventional pet food. (32)
(32) The key characteristic of dietetic pet food is the very specific nutritional product profile, (33) meaning that these products either contain or lack specific ingredients, substances, vitamins or minerals. Dietetic pet food therefore requires very different recipes from conventional pet food. One competitor explained that dietetic pet food is "characterised by a more rigorous attention to achieving exact nutrient levels – both at the design and ongoing manufacturing stages." (34) Another competitor explained that the formulation of dietetic pet food requires specialist knowledge and know-how, to ensure that it can be manufactured consistently and in particular, that "advanced nutritional mastery is required – either an experienced Animal Nutritionist well-versed in Clinical Conditions who understands the clinical condition, how to manage it from a nutritional standpoint and is well-versed in interpreting and applying PARNUTS, or a Veterinary Clinical Nutritionist." (35) Whilst competitors agreed that a conventional pet food manufacturer could achieve the specific nutritional product profile with the requisite attention to nutrient levels required for dietetic pet food, it would have to invest into R&D and gain expertise before it could start manufacturing dietetic pet food. (36)
(33) Dietetic pet food product development incurs significant research and development spending. All of the competitors that responded to the market investigation stated that the development of dietetic pet food products is more resource-intensive than the development of conventional pet food, since it requires more scientific research and takes significantly more time. (37) Mars itself admits that dietetic pet food requires a higher level of investment than conventional pet food, explaining that "[i]n addition to increased spend on research and development for certain health conditions, a key investment involves brand education and development, including with professionals in the pet industry, particularly veterinarians and breeders. […] such investment relates to awareness and education of certain conditions, effective treatments, and product performance." (38) Competitors have also referred to the need for the dietetic pet food manufacturer to provide a high level of clinical proof of efficacy in order to satisfy the high product quality standard demanded by veterinarians. (39)
(34) Dietetic pet food products must comply with the requirements of EU Directive 2008/38/EC (40) on animal feed for particular nutritional purposes (the so-called "PARNUTs" legislation), which is deemed by competitors to be "far more stringent than legislation for conventional pet food" (41). Directive 2008/38/EC prescribes the essential nutritional characteristics, the recommended length of time for the use of, and the specific labelling declarations that must be made with respect to the nutritional content of different types of, dietetic pet food. (42) Directive 2008/38/EC also requires specific wording to be indicated on the packaging of the dietetic pet food product: "It is recommended that a veterinarian's opinion be sought before use [or before extending the period of use]" (depending on the particular nutritional purpose in question).
(35) Further regulatory requirements are imposed by Article 13 of Regulation 767/2009, (43) which prescribes the conditions for the appearance of claims on pet food packaging and labelling. Thus, claims as to the absence or presence of a substance, or any specific nutritional characteristic or process, must be objective, verifiable by competent authorities, understandable by the user of the feed and supported by scientific substantiation when the feed is placed on the market. This means that dietetic pet food that is more likely to make such claims must comply with more stringent regulatory requirements than conventional pet food, which is less likely to make such claims. Furthermore, claims that the feed will prevent, treat or cure a disease are not allowed, nor are claims that the feed has a particular nutritional purpose, unless that purpose is one listed in Directive 2008/38/EC. In other words, dietetic pet food may display claims that are not allowed for non- dietetic pet food (and in turn is subjected to a heavier regulatory burden).
(36) Whilst the general plant and equipment used for the manufacture of dietetic pet food is similar or essentially the same as that used for the manufacture of conventional pet food, (44) special manufacturing processes may be required for dietetic pet food. For example, it is absolutely necessary to ensure consistency of nutritional and ingredient profiles between production batches. (45) Furthermore, certain types of dietetic pet food require specific quality controls or equipment adjustment. Mars explains that manufacturing processes are sometimes adjusted to reflect any specific product requirements (such as […]). (46) […]. (47)
(37) Finally, dietetic pet food is sold almost exclusively via the veterinary channel. Market participants estimate that the veterinary channel accounts for over 90% of dietetic pet food sales, especially in certain markets, like the Nordics. (48)
(38) In light of the foregoing and taking the results of the market investigation and all the evidence available to it into account, the Commission considers that the manufacture and supply of dietetic pet food constitutes a distinct relevant product market, separate from the market for the manufacture and supply of non-dietetic pet food.
5.2.2. Relevant geographic market definition
5.2.2.1. The Notifying Party's arguments
(39) The Notifying Party argues that the market definition can be left open but should not be sub-segmented below the national level, (49) noting also that the markets for (i) dry dog food, (ii) wet dog food, (iii) dry cat food, and (iv) wet cat food products have previously been defined as national in scope. (50)
5.2.2.2. The Commission's assessment
(40) In Nestle/Ralson Purina, the Commission considered that the relevant geographic market for industrial pet food is national in scope, given that the purchasing pattern even of internationally active retailers was predominantly national, and that significant price differences existed among Member States. (51)
(41) The market investigation in the present case has similarly shown that the relevant geographic market for the manufacture and supply of dietetic pet food is national in geographic scope, given that: (i) suppliers tend to have dedicated local presence, personnel and distributors in various countries, (52) (ii) the majority of competitors have indicated that they have different routes to market in different countries, (53) and (iii) there is evidence of varying brand presence in national markets, with regional and national actors present in certain national markets but not others. (54)
5.2.3. Conclusion on the relevant market for the manufacture and supply of dietetic pet food
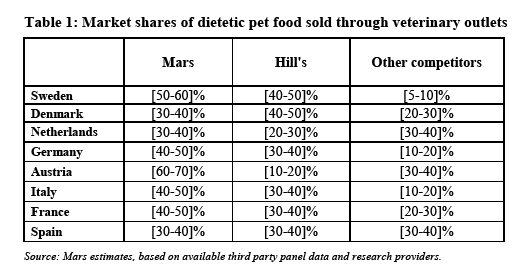
(42) In light of the above considerations and taking the outcome of the market investigation and all the evidence available to it into account, the Commission considers that the relevant product market upstream is the manufacture and supply of dietetic pet food, which constitutes a distinct relevant product market, separate from the market for the manufacture and supply of non-dietetic pet food.
(43) The market for the manufacture and supply of dietetic pet food is national in geographic scope.
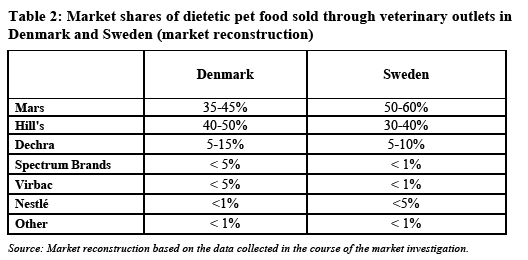
5.3. The downstream market - retail of dietetic pet food through the veterinary channel
5.3.1. Relevant product market definition
5.3.1.1. The Notifying Party's arguments
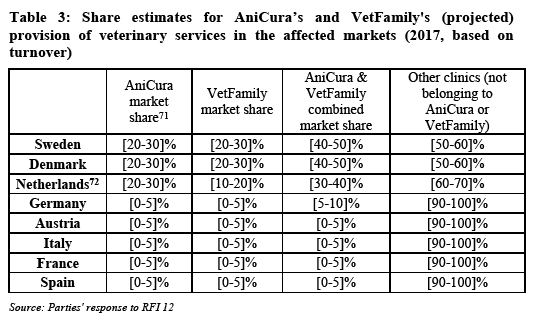
(44) The Notifying Party submits that the relevant product market is the provision of veterinary services through veterinary practices. (55) The Notifying Party considers that in the absence of any horizontal overlap in this sector, there is no need to consider potential market definitions in any detail, although it also acknowledges veterinary services' ancillary activity as a distribution channel for pet food. (56)
5.3.1.2. The Commission's assessment
(45) The Transaction concerns the supply and sale of dietetic pet food through veterinary clinics, and not the provision of veterinary services.
(46) In Nestlé/Dalgety, the Commission found that it cannot be excluded that specialist (non-grocery) stores are a separate relevant product market for the sale of pet food from grocery stores. (57)
(47) The results of the market investigation in this case are firmly conclusive of the fact that veterinary clinics constitute a separate relevant product market for the sale of dietetic pet food, because: (i) dietetic pet food is sold almost exclusively through veterinary clinics; (ii) dietetic pet food sold through veterinary clinics tends to be more expensive than dietetic pet food sold via other channels (e.g. online) and (iii) suppliers tend to market dietetic pet food at a clinic-per-clinic level. (58)
5.3.2. Relevant geographic market definition
5.3.2.1. Notifying Party arguments
(48) The Notifying Party submits that the market for veterinary services, to the extent that these are relevant in the present case in light of the vertical relationship of their ancillary activity as a distribution channel for pet food, have to be considered at national level. (59)
5.3.2.2. The Commission's assessment
(49) In Nestlé/Ralston Purina the Commission concluded that, at the retail level, the prepared pet food markets are national in scope. The market investigation in that case revealed, inter alia, that the purchasing pattern even of internationally active retailers is still predominantly national, that significant price differences and market structures exist between Member States and that specialty shops purchase their pet food requirements almost exclusively at national level. (60) This conclusion was largely confirmed by the Commission's findings in Masterfoods/Royal Canin, where the Commission found that for the majority of respondents, purchasing patterns of demand were still essentially national as concerns the main grocery retailers, whilst they were nearly always national or even sub-national as concerns the specialty retailers. (61)
(50) The market investigation in the present case confirms that the retail of dietetic pet food via the veterinary channel is local in geographic scope, given that: (i) veterinary clinics tend to attract customers from their vicinity, and (ii) the majority of respondent market participants indicated that the maximum distance travelled by pet owners to veterinary clinics is less than 90 km. (62)
5.3.3. Conclusion on the relevant market for the retail of dietetic pet food via the veterinary channel
(51) The retail of dietetic pet food via the veterinary channel constitutes a distinct relevant product market, separate from the retail of dietetic pet food via other channels. This market is local in geographic scope.
(52) Notwithstanding the above, and given the significant presence of AniCura and VetFamily across the territory of Denmark and Sweden, for the purposes of this decision, the effects of the Transaction were considered having regard to the entirety of the territories of the countries concerned (i.e. national).
6. COMPETITIVE ASSESSMENT
(53) Mars is active in the supply of dietetic pet food throughout the EU, whereas AniCura is currently present in Denmark, Sweden, the Netherlands, Germany, Austria, Italy, France and Spain.
(54) The Transaction gives rise to vertically affected markets only where AniCura is also present, i.e. in Denmark, Sweden, the Netherlands, Germany, Austria, Italy, France and Spain.
(55) There is a also a very limited horizontal overlap in Denmark and the Netherlands, due to AniCura's supply of dietetic pet food under the VetPro brand in AniCura clinics in Denmark and Sterkliniek private-label dietetic pet food in AniCura- owned Sterkliniek clinics in the Netherlands. (63)
6.1. Legal framework for competitive assessment of vertical mergers
(56) Vertical mergers involve companies operating at different levels of the same supply chain. Pursuant to the Commission Guidelines on the assessment of non- horizontal mergers under the Council Regulation on the control of concentrations between undertakings (the “Non-Horizontal Merger Guidelines”) (64), vertical mergers do not entail the loss of direct competition between merging firms in the same relevant market and provide scope for efficiencies.
(57) However, there are circumstances in which vertical mergers may significantly impede effective competition. This is in particular the case if they give rise to foreclosure (65).
(58) The Non-Horizontal Merger Guidelines distinguish between two forms of foreclosure: input foreclosure, where the merger is likely to raise costs of downstream rivals by restricting their access to an important input, and customer foreclosure, where the merger is likely to foreclose upstream rivals by restricting their access to a sufficient customer base (66).
(59) Pursuant to the Non-Horizontal Merger Guidelines, input foreclosure arises where, post-merger, the new entity would be likely to restrict access to its actual or potential rival in the downstream market to the products or services that it would have otherwise supplied absent the merger, thereby raising its downstream rivals' costs by making it harder for them to obtain supplies of the input under similar prices and conditions as absent the merger (67).
(60) For input foreclosure to be a concern, the merged entity should have a significant degree of market power in the upstream market. Only when the merged entity has such a significant degree of market power, can it be expected that it will significantly influence the conditions of competition in the upstream market and thus, possibly, the prices and supply conditions in the downstream market (68).
(61) Pursuant to the Non-Horizontal Merger Guidelines, customer foreclosure may occur when a supplier integrates with an important customer in the downstream market and because of this downstream presence, the merged entity may foreclose access to a sufficient customer base to its actual or potential rivals in the upstream market (the input market) and reduce their ability or incentive to compete which in turn, may raise downstream rivals' costs by making it harder for them to obtain supplies of the input under similar prices and conditions as absent the merger. This may allow the merged entity profitably to establish higher prices on the downstream market (69)
(62) For customer foreclosure to be a concern, a vertical merger must involve a company which is an important customer with a significant degree of market power in the downstream market. If, on the contrary, there is a sufficiently large customer base, at present or in the future, that is likely to turn to independent suppliers, the Commission is unlikely to raise competition concerns on that ground (70).
6.2. Market shares
6.2.1. Upstream market – manufacture and supply of dietetic pet food
(63) Mars is the market leader in the manufacture and supply of dietetic pet food in the EU. The second-largest supplier of dietetic pet food is Hill's.
(64) In the upstream market, Mars' shares in the supply of dietetic pet food are significant. Mars provided the following estimated market shares for dietetic pet food sold in 2017 via the veterinary channel in those Member States where AniCura is also present.
(65) According to Mars, this information constitutes its best estimate for the sales value of dietetic pet food products, taking into account its estimates of the proportion of non-dietetic pet food sold via the veterinary channel in each of the Member States concerned.
(66) On the basis of the responses received in the course of the market investigation, the Commission was able to reconstruct an approximation of the market shares of the other competitors active on the dietetic pet food market in Denmark and Sweden.
6.2.2. Downstream market – retail of dietetic pet food via the veterinary channel
(67) When calculating the shares of the Parties in the downstream markets, it is necessary to distinguish the shares of AniCura, which sources and resells dietetic pet food, from the shares of VetFamily member clinics. As explained above at paragraph (4), while VetFamily clinics are independent, they sign up to a purchasing framework controlled by AniCura through which they source a number of products for their practices, including dietetic pet food. The reasons why VetFamily shares are considered as shares controlled by the merged entity, in the competitive assessment, are assessed below at Section 6.3.2.4.
(68) While the geographic market definition is local, the Notifying Party was only able to provide complete market shares on the basis of turnover at national level. The narrowest geographic market share data that the Notifying Party was able to provide was at regional level (i.e. broader than local). The regional market shares are set out in the competitive assessment at country level at Sections 6.4, 6.5 and 6.6.
(69) In the downstream market, AniCura's and VetFamily member clinics' market shares in the provision of veterinary services, which were used as a proxy in the absence of reliable data on the shares in the retail sale of dietetic pet food, are also significant. These market shares are based on the veterinary clinics' turnover. The Notifying Party also provided market shares based on numbers of veterinary clinics and on the numbers of veterinarians. The Commission considers that shares based on turnover are likely to be closest in terms of judging the economic importance of the downstream clinics and consequently of their sales of dietetic pet food.
(70) As illustrated in Table 3 below, AniCura accounts for between [20-30]% and [20- 30]% of the market in Sweden, Denmark and the Netherlands, and when AniCura's market shares are considered together with VetFamily's, these increase to [40-50]% in Sweden, [40-50]% in Denmark and [30-40]% in the Netherlands.
(71) Finally, it must be noted that VetFamily has undergone a rapid expansion and is projected to continue to grow at a fast rate. Since AniCura purchased VetFamily in 2014, VetFamily has increased its membership from 120 clinics to over 1000 clinics. (73) By way of illustration of this rapid growth, between the date of notification and the date of this Decision, the number of VetFamily member clinics has increased by over [100-200] in total (by […]). (74)
6.3. General features of the markets
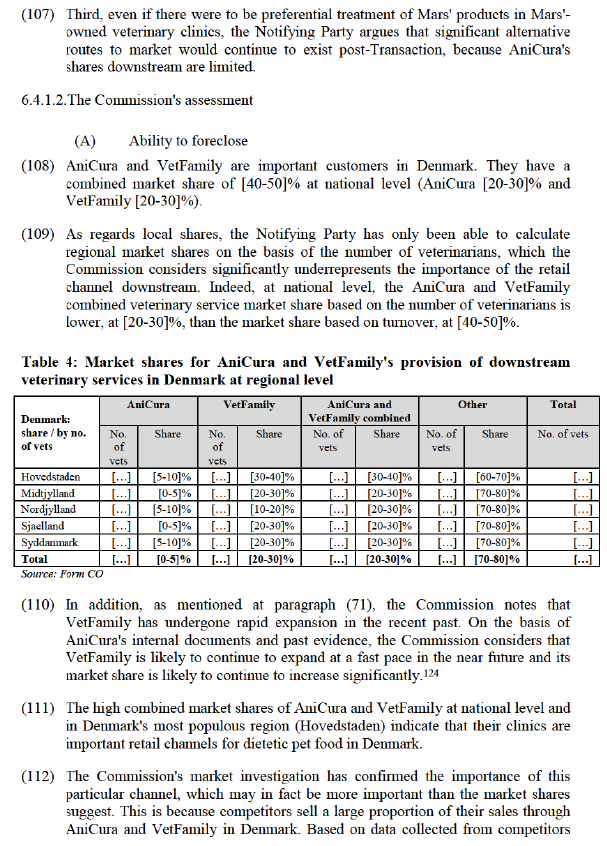
6.3.1. Notifying Party arguments
(72) The Notifying Party argues that the veterinary channel is of limited importance to the distribution of pet food.
(73) First, the Notifying Party estimates that no more than [20-30]% of overall pet food sold is distributed via veterinary practices (comprised almost entirely of dietetic pet food). (75) Royal Canin's internal strategy documents suggest that in 2016, the veterinary dog and cat food channel represented less than [0-20]% of the global veterinary market, and that although veterinarians are highly trusted by pet owners on the topic of pet food, only [0-20]% of pet owners buy pet food at a veterinary clinic. (76)
(74) Second, the Notifying Party also claims that even for those types of pet food that rely more heavily on veterinary distribution recommendation (i.e. dietetic pet food), there are ethical rules and behaviour driving the veterinary profession that prevent a veterinarian from pursuing commercial ends and instead ensure that the veterinary professional continues to recommend the best type of dietetic pet food for the pet in question. (77)
(75) Third, according to the Notifying Party, the existence of VetFamily and Sterkliniek are not relevant for the assessment of the transaction because clinics are free to procure pet food outside of the framework agreements concluded by and for the benefit of these purchasing organisations. (78)
(76) Fourth, the Notifying Party claims that the online channel is increasingly important in the distribution of pet food (79) and that there will remain significant alternative routes to market following the Transaction. (80)
6.3.2. Commission assessment
(77) The results of the market investigation in this case paint a different picture of the dietetic pet food market.
6.3.2.1. Veterinary channel as an essential distribution outlet for dietetic pet food
(78) Based on the results of the market investigation, the Commission finds that the veterinary channel is an essential distribution outlet for dietetic pet food, as it is indispensable to reach customers through a prescribing veterinarian.
(79) First, the veterinary channel is deemed by market participants to be the most important channel for the distribution of dietetic pet food. According to market participants' estimates, the veterinary channel accounts for over 90% of dietetic pet food sales, especially in certain markets, like the Nordics . (81)
(80) Second, the veterinary channel is important not simply as a retail outlet for physical sale of dietetic pet food, but also as an indirect promoter of dietetic pet food products, since without a recommendation by a veterinarian, pet owners are not likely to set out to purchase a specific dietetic pet food product by themselves. (82) For instance, on the importance of a veterinarian’s recommendations for the sale of dietetic pet food, a competitor explained that: "[v]eterinarians are already the main influencers on the worldwide global pet food market and from far, the main distribution channel for “therapeutic” pet food." (83)
(81) Third, dietetic pet food suppliers see access to veterinarians as a means of promoting their products to them as essential. (84) They invest heavily in promoting their dietetic pet food products to veterinarians by attending individual clinics, giving training and educational seminars to veterinarians in clinics as well as at conferences for veterinary professionals, and providing written product information and promotional materials to be displayed in veterinary clinics. (85) As one competitor explains, "[h]aving access to the staff in vet clinics is critical for pet food manufacturers because after attending training seminars in the clinics, vets are likely to have understood better the mode of action of the technology in question and the benefits of the products and are therefore more likely to recommend that specific brand for a pet who needs this type of diet." (86)
(82) Fourth, veterinarians have control over what dietetic pet food they recommend, this recommendation being typically trusted by pet owners. "People put their trust in a vet", (87) explained one competitor. Another competitor explained that "veterinarians play a key role in determining the appropriate food and their customers (pet owners) heavily rely on their veterinarian's recommendations before baking a purchase." (88) […]. (89) The results of the market investigation reveal that pet owners follow the veterinarian's recommendation as to the type, as well as the brand of dietetic pet food: an overwhelming majority of veterinarians stated that pet owners typically follow their recommendations, (90) with the majority also agreeing that pet owners tend to stay loyal to the brand originally recommended, (91) with one veterinarian stating that " *if the need for the diet has been understood, [pet owners] stick to the once-prescribed product". (92)
(83) As another veterinarian stated, "[m]ost pet owners follow our recommendations, as it is the best for their pet", (93) and yet another explained that "especialy [sic] in cases with Food allergies, owners tend to stick with a diet that reduces the animal's medical problems." (94) Veterinarians based in Denmark explained that owners "dont [sic] look for other options" if the dietetic pet food "works for their pet, and the pet likes it", (95) with another explaining that "[o]wners tend to stick" to the dietetic pet food prescribed by the veterinarian "as they consider it to be part of the treatment plan for a specific disease or as a preventive [sic] measure against developement [sic] of a more specific condition". (96)
(84) Fifth, similar views were expressed by the competitors, with all but one agreeing that pet owners typically follow vet recommendations. (97) One competitor remarked that pet owners who are prescribed a dietetic pet food product by their veterinarian "are usually loyal to the brand and stay with these products", (98) and another explained that in general, "a very high percentage […] of consumers will follow a recommendation of their vet which food to give their pets. An equally high amount of consumers will be loyal to that recommended brand throughout their pet's live [sic]". (99)
(85) Sixth, Mars itself recognises in internal documents the key importance of veterinary visits and prescription as a key opportunity of driving sales of its products. For example, in internal documents […] (100) […]. (101)
6.3.2.2. Veterinary prescriptions are affected by competition and commercial strategies of pet food manufacturers
(86) Based on the results of the market investigation, the Commission finds that competition among pet food manufacturers, and their commercial strategy, affect the choices made by veterinarians as to the prescribed dietetic pet food.
(87) First, given that a veterinarian's recommendation is generally required to start purchasing a dietetic pet food product, and that consumers tend to follow the veterinarian's recommendation, the choice as to the type and brand of dietetic product a consumer ends up buying is made by the veterinarian, and not by the consumer. As one veterinarian in Sweden explains: "Typically that is the available [dietetic pet] food at the local vet so they buy that. They do not have many choices. I have 85% royal canine and 15% Hills on my shelves." (102) The results of the market investigation also show that pet owners are not very likely to request generic or alternative dietetic pet food products to the ones recommended by their veterinarians, with only a minority of veterinarians reporting the occurrence of such requests. (103) This means that the choice of a local veterinary clinic to stock particular types and brand(s) of dietetic pet food is very likely to limit the choice that could otherwise be available to consumers.
(88) Second, whilst veterinarians may be guided by their ethical obligations and seek to have on offer a range of dietetic pet food that best suits the needs of the pets they treat, (104) the choice of brands to stock on the veterinary clinic's shelves (and subsequently to recommend) may be influenced by economic considerations (such as ease of procurement, rebates or other special pricing arrangements with a supplier). (105)
(89) Third, many market participants consider that the Transaction may have an impact on the market because of the economic or commercial considerations that influence a veterinarian’s choice of dietetic pet food brands in veterinary clinics. For example, one veterinarian's view is that "allowing Mars to acquire Anicura is a very bad idea. Veterinary practice needs to be independent to be able to remain objective and advise quality instead of only the products that the boss wants/produces" and that "health care should be about health and not about what is commercially attractive." (106) A competitor opined that "the free medical decision of the veterinarian who runs an Anicura Clinic as to which diet feed he recommends will be strongly influenced by the acquisition and the sales opportunities for us and other competitors will be severely impeded." (107)
(90) Fourth, the consumer's choice of veterinarian tends to be guided by factors other than the availability and price of dietetic pet food sold in-store. The choice of veterinarian is usually made on the basis of the type, quality and price of veterinary services, as well as the geographic location of the clinic. The sale of dietetic pet food is only an ancillary activity for veterinarians. This means that pet owners are not likely to switch veterinarians simply because their preferred brand of dietetic pet food is not available in-store.
(91) Fifth, there is evidence that exclusive purchasing arrangements between a chain of veterinary clinics and a supplier lead to a significant reduction in the sale of competing brands of dietetic pet food products. (108)
6.3.2.3. Online sales are not an alternative channel to veterinary prescribed dietetic food
(92) Based on the results of the market investigation, the Commission finds that online sales of dietetic food products are limited, and do not affect the primary importance of the veterinary channel in the retail of dietetic pet food.
(93) First, the market investigation confirmed that the online channel serves as a replenishment channel only, with consumers turning to buying dietetic pet food online only after the initial recommendation for a specific type (and often brand) of dietetic pet food has been made and the first purchase(s) of the product have taken place in the veterinary clinic. Indeed, as a competitor explained, "the online channel is not a substitute to the vet channel to enter or expand in the market for [dietetic] pet food." (109) This appears to be corroborated by Mars' internal documents. (110)
(94) Second, in any event, the online market accounted for a limited proportion of sales of dietetic pet food. The Notifying Party estimates that [10-20]% and [10- 20]% of sales of dietetic pet food in Sweden and Denmark respectively are made online. (111)
(95) This is broadly in line with estimates by competitors. Competitors estimate that less than 10% of their sales of dietetic pet food take place online in Denmark and no more than 20% (and often significantly less) in Sweden. (112) Whilst online sales are growing, they appear to be more important in countries in Europe other than Sweden and Denmark. (113)
6.3.2.4. VetFamily affects the procurement choices of its members.
(96) Based on the available evidence and on the results of the market investigation, the Commission finds that, despite the fact that VetFamily clinics are independent, their membership in the programme affects their procurement choices.
(97) First, even if there is no contractual requirement for VetFamily member clinics to purchase dietetic pet food under the VetFamily framework agreements (and no restrictions on purchasing outside those agreements), such purchases are strongly encouraged through contractual mechanisms.
(98) For example, a VetFamily contract for Denmark states that […]. (114)
(99) Second, […].
(100) Third, internal documents show that compliance of member clinics and that compliance in general already tends to be very high, between […]. Furthermore, compliance by VetFamily member clinics is directly rewarded through rebates from suppliers to the central VetFamily entity for purchases made under the framework agreements, and which account for […] of VetFamily's revenues. (115) This in turn reinforces further compliance with the framework agreements as the central VetFamily entity is directly incentivised to encourage independent clinics to purchase through the framework contracts, while the independent VetFamily clinics are able to benefit from lower prices.
(101) Fourth, in light of the particular features of the dietetic pet food market outlined above, and in particular of the fact that (i) recommendations by veterinarians drive sales and (ii) the assortment of dietetic pet food stocked by veterinary clinics limits and to a great extent determines consumer choice, it is competition that takes place on the upstream market that matters the most. For these reasons, purchasing alliances or buying groups (of which VetFamily is the largest) (116) play a very important role in driving product choice, by effectively incentivising veterinary clinics to stock a particular range of brands. Given the limited shelf space in veterinary clinics, preference is therefore more likely to be given to products procured at centrally-negotiated rates than to products for which a veterinary clinic would otherwise have to negotiate independently.
(102) Fifth, VetFamily arranges meetings for members once or twice per year, in order to discuss topics relevant for veterinary practice and to provide practice managers and opportunity to share their experiences and discuss best practices. (117) VetFamily also provides veterinary and leadership training for its members (118) and in Denmark acts as a forum where professionals from its member clinics discuss best practices and carry out training. (119) These activities can influence VetFamily members' choice of dietetic pet food to stock in veterinary clinics and/or recommend to pet owners.
(103) Sixth, VetFamily provides a range of other benefits to incentivise its members. For example, in Denmark, VetFamily provides marketing support to its members, primarily by helping clinics build websites, organising social media campaigns, and coordinating promotional campaigns with suppliers. (120) In Denmark, VetFamily also provides the VetPro range of own private label pet food, which consumers can buy from VetFamily members that choose to stock it, as well as in the VetFamily webshop. (121) In other countries, VetFamily also occasionally distributes to its members marketing or campaign materials on behalf of selected suppliers. (122) VetFamily has also developed a preventative care plan for pets, which is offered to customers on a subscription fee basis. VetPlan is managed with a specially developed IT system […]. (123)
(104) In conclusion, VetFamily affects the procurement choices of member clinics, and as VetFamily is controlled by AniCura, the downstream shares will be assessed as accruing to the merged entity in the context of the competitive assessment of the transaction.
6.4. Denmark
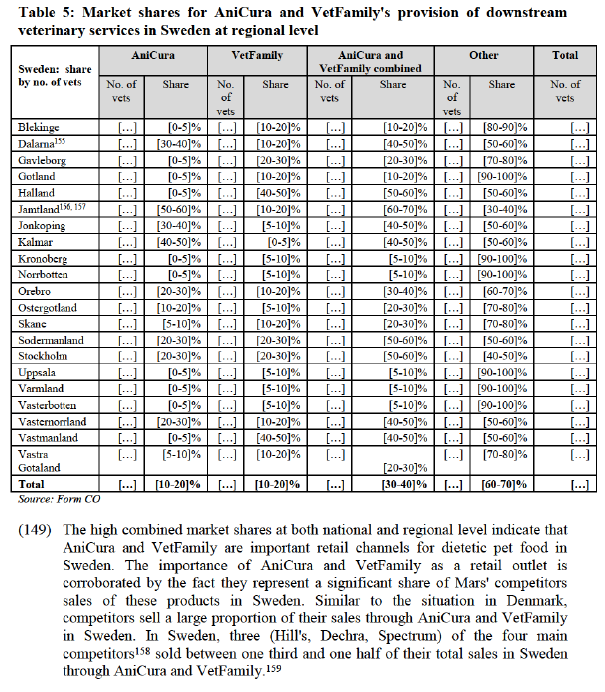
6.4.1. Customer foreclosure
6.4.1.1. The Notifying Party's arguments
(105) The Notifying Party argues that no competition concerns arise as a result of the Transaction, and in particular that there is no prospect of customer foreclosure, or input foreclosure, for a number of reasons. First, Mars' shares upstream and AniCura's shares downstream are not significant. Although Mars' shares may be above [30-40]% in a number of markets, significant competitors exist and continue to exert competitive pressure. Similarly, AniCura's shares in veterinary services are limited, representing less than [10-20]% of practices, less than [20- 30]% of veterinary service revenues and less than [10-20]% of veterinarians at national level.
(106) Second, the Notifying Party refers to an analogous situation in the United States, in order to argue that no competition concerns should arise. In particular, Mars notes that it already owns veterinary clinics in the United States where […]. Further, sales data indicates that […]. Mars argues that it intends to apply the same strategy in Europe, through its acquisition of AniCura.
during the course of the market investigation, the Commission notes that in Denmark, the main four competitors (Hill's, Dechra, Spectrum and Virbac) sold between one third and two thirds of their total sales in Denmark through AniCura and VetFamily. (125)
(113) When assessing the ability to foreclose, the Commission takes into account whether there are sufficient alternative outlets downstream for upstream competitors to sell their output. (126)
(114) In the present case, the Commission considers that in Denmark there are limited alternative outlets. Together, AniCura and VetFamily represent the largest retail outlet in Denmark, with a combined downstream market share of veterinary services of almost [40-50]% (see Table 4 above). The importance of AniCura and VetFamily as a retail outlet is corroborated by the fact that they represent a significant share of Mars' competitors sales of these products in Denmark. Other retail outlets are much more fragmented and sales through the online channel are low (on average 5-10% of sales are made online). Further, as noted at paragraph (93) the online channel merely serves as a replenishment channel.
(B) Incentive to foreclose
(115) Pet food purchases by veterinary clinics account for a limited proportion of their total procurement spend for veterinary items. For example, VetFamily members spent approximately [20-30]% of their purchases on dietetic pet food; this was significantly outweighed by other categories of spending, such as […]. (127) In addition, the sale of pet food by veterinary clinics is ancillary to their main activity of providing veterinary services to sick pets. For these reasons, the Commission considers that, if prices of dietetic pet food rise, veterinary clinics are unlikely to forego the convenience of purchasing through the VetFamily framework agreements in order to negotiate new contracts with other dietetic pet food suppliers. Rather, they are likely to pass those price increases to their customers.
(116) In addition, as noted at Section 6.3.2, upstream competition in this market is particularly important, while downstream switching is low. The purchase of dietetic pet food by customers visiting veterinary clinics is ancillary to the main purpose of the visit, which is to obtain veterinary services for their sick pet. Consequently, the Commission is of the view that the customer visiting a veterinary clinic is unlikely to switch away from a vet clinic if prices of dietetic pet food were to rise, or if the choice of dietetic pet food was limited to Mars' Royal Canin brand. This is particularly the case because Royal Canin is the leading brand of dietetic pet food in the EU and has the most extensive range of dietetic pet food. It is consequently likely to be an adequate substitute for any other brands that the veterinarian may have been stocking previously.
(117) In addition, the Commission notes that Mars' incentive to foreclose is increased in particular for the VetFamily member clinics. This is because VetFamily members are independent veterinary clinics and would not become part of the merged entity. Consequently, a reduction of choice or an increase in prices of dietetic pet food may not be offset by any decrease in customers for veterinary clinics, thus reducing the likelihood of a loss of profits for the merged entity in the downstream market.
(118) Further, the Commission notes that a merged entity's incentive to pursue a customer foreclosure strategy is stronger, where the upstream division of the merged entity can benefit from possibly higher price levels resulting from foreclosure of upstream rivals. (128) Mars' shares are significant upstream: in the market for manufacture and supply of dietetic pet food on the basis of its own estimates, Mars has a market share of [30-40]% in Denmark. However, the Commission considers that this may underestimate Mars' position and, as mentioned in paragraph (66), on the basis of the Commission's market reconstruction, Mars has a slightly higher market share within a range of 35-45%. The significant market position of Mars upstream would allow it to benefit from higher price levels upstream following the foreclosure strategy.
(B) Overall impact of customer foreclosure
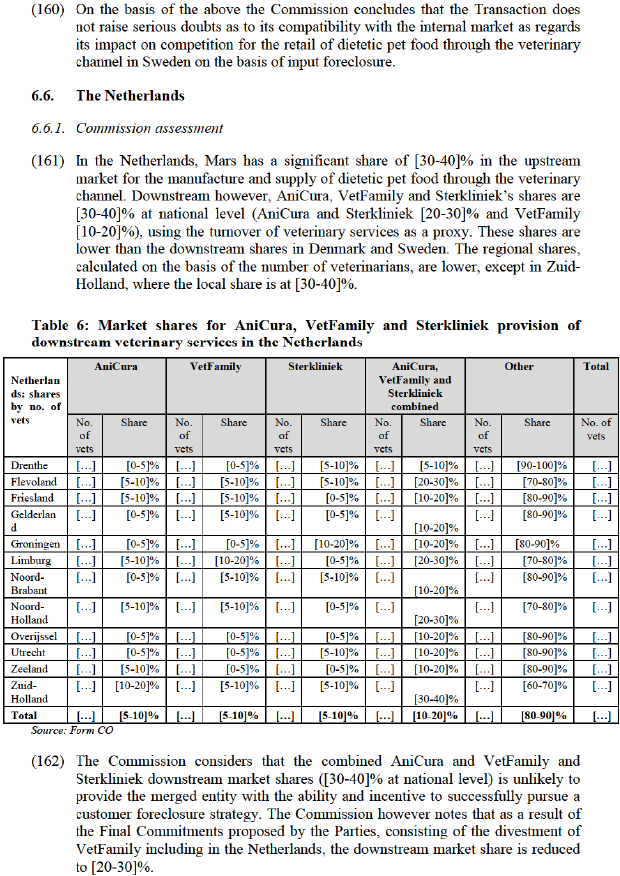
(119) The Commission considers that the merged entity may foreclose access to a significant customer base to its actual or potential rivals in the upstream market and reduce their ability or incentive to compete in Denmark. (129) As a result, also rival veterinary clinics in the downstream market are likely to be put at a competitive disadvantage, in particular due to raised costs of dietetic pet food. This in turn may allow the merged entity to profitably raise prices also in the downstream market.
(120) A number of market participants raised concerns that Mars could pursue a strategy to foreclose upstream competitors from the downstream retail channel. For example, one competitor noted that post-Transaction, Mars would likely give preferential treatment to its own brands in its downstream retail outlets (AniCura and VetFamily), resulting in reduced access to a key distribution channel and directly impacting their sales: "If this were to go ahead, Mars would have a dominant position in the Veterinary channel because they would own both the distribution channel and Food brands. They would most likely sell their own brands as a priority and block other brands including our own from this channel. This could potentially cut out a distribution channel/ key customer for our brands and reduce our selling potential." (130)
(121) Another competitor noted that Mars would, post-Transaction, sell its own products in the AniCura clinics, reducing the available retail outlets for its competitors: "With the acquisition of AniCura by Mars we expect that a number of clinics will take on Mars/Royal Canin’s pet food products, thereby reducing opportunities for [COMPETITOR] and other pet food competitors. Mars will most probably influence which pet foods the vet clinics in AniCura will use and/or sell." (131) Similar concerns were raised by veterinarians contacted during the market investigation, for example: “It is quite clear that Mars would push their own food lines into “their” clinics, so competition in the market would be reduced.” Another one mentioned, “AniCura-member clinics may sell only Royal Canin products and by this strengthens the brand.” (132)
(122) AniCura and VetFamily's combined downstream share of veterinary services is high, at [40-50]%. This means that almost half of the downstream retail outlets for dietetic pet food would effectively controlled by Mars, post-Transaction and represent channels from which Mars' competitors may be foreclosed. Further, the Commission notes that a sufficiently large fraction of upstream output appears to be affected by revenue decreases, due to the high percentage of competitor sales that are currently sold through AniCura and VetFamily, as mentioned at paragraph (112).
(123) The Commission considers that by restricting access to a significant customer base, the merger may put competitors at a competitive disadvantage, by increasing their costs to access the remaining customers. These increased costs could have an impact on their revenue streams and their ability to recoup costs. (133)
(124) In particular, for the competitors of Mars in dietetic pet food, the costs of establishing and maintaining presence in a country are high. As noted inSection 6.3.2 the importance of accessing the veterinary channel is crucial in order to sell dietetic products. Consequently, there are a number of important costs linked to local presence and developing relationships with veterinary clinics. The costs of entering a market (other than R&D spend and time it takes to develop a range of therapeutic diet) include distribution, marketing, brand awareness building (e.g. at veterinarian conferences and trade fairs), relationship building with veterinarians (visits and training in clinics, dedicated staff), staff training. (134) These costs are estimated, on average, at a minimum of EUR 0.5 million up to several million (EUR 5 million) depending on market size, characteristics, entry strategy and level of market presence. (135)
(125) If Mars' competitors' revenue streams are reduced upstream, the Commission considers that the merger may reduce their ability and incentive to invest in cost reduction, R&D and product quality. (136) This is particularly the case for the smaller competitors such as Dechra, Spectrum and Virbac, whose sales are significantly lower than Mars or Hill's. The Commission considers that the weakening of these competitors or their exit from the market may result in a loss of choice and of price competition in the upstream market, thereby enabling the merged entity to profitably raise prices in the downstream market. (137)
(126) Evidence that the merged entity may raise barriers to entry to potential competitors, making entry upstream by potential entrants unattractive by significantly reducing their revenue prospects (138) was also provided during the market investigation. In particular, competitors not already active in the Nordics, felt that entry, which is already difficult, would become even more so as a result of the Transaction. (139)
(127) The Commission considers that as a result of any potential Mars' customer foreclosure strategy, there is a risk that prices for dietetic pet food sold in the veterinary channel could increase. This risk of increased prices for dietetic pet food sold through the veterinary channel was highlighted by market participants, for example one veterinarian noted that, “AniCura destroys the market, if they fuse, they will be market leader and dictate prices.” (140)
(128) The market investigation also highlighted concerns regarding reduction of choice. In particular, market participants were concerned that as a result of the merger, veterinary clinics owned by Mars would be obliged to sells Mars' brand of dietetic food (Royal Canin) and that this could lead to a reduction in choice for the customer. For example, one competitor noted: "We do expect the acquisition to impact our business. Obviously all clinics will carry the RC [Royal Canin] brand as preferred/recommended brand. And since RC has by far the largest number of SKUs (141) of any therapeutic pet food company – this will effect and influence space management and limit competitors opportunities and restrict their number. Few clinics have the space and capacity to handle more than 3 brands." (142)
(129) In addition, a number of veterinarians expressed concerns that as a result of the merger, veterinary clinics belonging to Mars may no longer offer impartial advice, but would rather follow commercial imperatives to recommend Royal Canin pet food rather than being allowed to choose freely. For example, one veterinarian stated: "we think it could be problematic if animal hospitals are owned by a pet Food company. This might change the pet food market in an unfair manner. Furthermore, medical decisions could be influenced by economic interests." (143) A competitor raised similar concerns, noting that: "Veterinarians are already the main influencers…and… the main distribution channel for “therapeutic” pet food. If veterinarians were to lose their impartiality whilst recommending or prescribing such or such "therapeutic" pet food brand, it could lead to create / reinforce Mars dominant position in many countries." (144) Another competitor added: "as a consequence Mars as a pet food producer owns at the same time vet clinics where the products are used and sold. This might create a conflict between the commercial interest and the request for transparency in the vet profession." (145)
(130) The Notifying Party refers to the market situation in the United States, where Mars owns veterinary clinic chains, to support its argument that Mars would not foreclose customers in markets such as Denmark.
(131) However, the Commission notes that during the course of the merger proceedings, [AniCura future plans]. (146) […]. (147)
(132) The Commission considers it likely that […] significantly reduce the number of suppliers of dietetic pet food able to access the retail channels of AniCura and VetFamily. Further, the Commission considers it likely, […] that Mars, post- Transaction would have been selected […].
(133) The Commission also notes that as a result of the Transaction, Mars may face a reduction of countervailing buyer power. Mars' own internal documents note that […] (148) Post-Transaction in Denmark, Mars would no longer be subject to the same competitive pressure in pricing negotiations, due to the fact that it would then control a significant downstream purchaser (AniCura) that also operates a purchasing alliance.
(134) As explained above at Section (115) to (116), in view of the limited switching capability of downstream end customers, upstream competition has a prominent role in these markets, thereby making it likely that reduced upstream competition will result in higher downstream prices.
6.4.2. Other non-coordinated effects: access to confidential information of competitors
(135) Market participants also raised concerns that post-Transaction, Mars, as the owner of a downstream veterinary clinics, would gain an insight into the pricing of its competitors, to the extent that AniCura would continue to purchase dietetic pet food from these competitors. Examples of competitively sensitive information include: prices, quantities, level and timing of price increases, promotional activity grids, special pricing conditions, information about innovation and new product launches.
(136) The Commission notes that post-Transaction, through its ownership of VetFamily, Mars would obtain knowledge about its competitors' pricing (and other competitively sensitive information) to VetFamily clinics i.e. Mars' downstream competitors. As a result, and in line with what is described above in relation to the customer foreclosure concerns, Mars could subsequently adapt its commercial strategy to undermine any attempt by competitors to sell to [40-50]% of the market that would be controlled by Mars post-Transaction (i.e. Anicura's and VetFamily clinics). This is likely to put competitors at a competitive disadvantage, thereby dissuading them to enter or expand in the market. (149)
(137) For example, one competitor noted that the acquisition "will allow Mars to have access to significant sensitive and confidential information regarding [COMPETITOR] as well as other pet food suppliers." (150) Another competitor raised similar concerns, noting that: "As [Mars] are worldwide and European co- leaders in the "therapeutic" pet food business, we believe that it might impact negatively the relationship of the other players with those clinics (risk of leak of confidential information, lack of trust, partial choice of brand or even dereferencing of brand….)." (151)
(138) On the basis of the considerations in paragraphs (108) to (138) and in light of the results of the market investigation and of all the evidence available to it, the Commission concludes that the Transaction raises serious doubts as to its compatibility with the internal market as regards its impact on competition for the retail of dietetic pet food through the veterinary channel in Denmark, on the basis of (a) customer foreclosure resulting in increased prices for dietetic pet food sold through the veterinary channel, reduced choice, quality and innovation in dietetic pet food; and (b) the access to confidential information of competitors.
6.4.3. Input foreclosure
6.4.3.1. The Commission's assessment
(139) While the Commission notes that Mars has a strong position upstream (although it is the number 2 player in Denmark), and consequently may have the ability to restrict downstream customers from access to its dietary pet food products, the Commission considers that Mars would not have the incentive to foreclose. In its assessment, the Commission looks at whether the foreclosure would be profitable for the merged entity and the extent to which customers can be diverted away from downstream rivals. The effect is greater where the input, in this case dietetic pet food, represents a critical component of downstream rivals’ costs. (152)
(140) In this case, because the sale of dietetic pet food is not a key input to veterinary clinics, but is rather ancillary to the provision of veterinary services, the Commission considers that Mars would be unlikely to successfully divert customers away from AniCura’s downstream rivals, by restricting its supply of dietetic pet food to them. Rather, the Commission is of the view that Mars would face a greater risk of losing dietetic pet food sales, if it attempted an input foreclosure strategy regarding the supply of dietetic pet food. For these reasons, the Commission considers that Mars has the incentive to sell its dietetic pet food in as many retail outlets as possible.
(141) On the basis of the above, the Commission concludes that the Transaction does not raise serious doubts as to its compatibility with the internal market as regards its impact on competition for the retail of dietetic pet food through the veterinary channel in Denmark on the basis of input foreclosure.
6.4.4. Horizontally affected markets
(142) The Notifying Party notes that there is a minimal horizontal overlap between the Parties’ activities in the supply of pet food in Denmark, which gives rise to a horizontally affected market. This is because AniCura sells limited quantities of a private label dietetic pet food product, VetPro, through its clinics and the VetFamily network and a webshop. These sales represent [0-5]% of pet food sold through the veterinary channel in Denmark. (153)
(143) The Notifying Party argues that this overlap will not cause a significant impediment to effective competition on any relevant market in Denmark, because: (i) the share increment attributable to AniCura’s activities is negligible and will not affect Mars’ competitive position; (ii) there is no overlap in the manufacture of pet food (since VetPro is manufactured by a third-party pet food supplier), so the Transaction will not remove production capacity from the market; (iii) the overlap that exists is very limited, since it relates solely to the supply of branded pet food in VetFamily and AniCura clinics; and (iv) the Danish pet food market has many suppliers that will act as a competitive constraint to the merged entity. (154)
(144) As regards the Commission's assessment, the Commission notes that while the merged entity would have a significant combined share upstream, the horizontal market share increment arising from the merger is extremely limited ([0-5]% at national level). In addition, the Transaction does not give rise to any increase in the manufacturing of dietetic pet food, since VetPro is manufactured by a third- party supplier. Finally, the Commission notes that this horizontal overlap will be removed as a result of the Commitments offered by the Parties, as described in Section 7.
(145) On the basis of the above, the Commission therefore concludes that the Transaction does not raise serious doubts as to its compatibility with the internal market as regards its impact on competition for the retail of dietetic pet food through the veterinary channel in Denmark on the basis of horizontal overlaps.
6.5. Sweden
(146) The Notifying Party argues that no competition concerns arise in Sweden as a result of customer foreclosure or input foreclosure, for the reasons set out at Section 6.4.1.1.
6.5.1. Customer foreclosure
6.5.1.1. The Commission's assessment
(147) On the basis of the assessment set out below and the arguments also referred to in Section 6.4.1.2.A, the Commission considers that the Parties would have the ability to foreclose competitors in the market for the manufacture and supply of dietetic pet food in Sweden from downstream customers.
(148) In particular, AniCura and VetFamily are important customers in Sweden. They have a combined market share of [40-50]% at national level (AniCura [20-30]% and VetFamily [20-30]%). As regards regional shares, AniCura and VetFamily's combined shares are even higher, for example [50-60]% in Stockholm. Further, the Commission notes that this figure is likely to significantly underrepresent the importance of AniCura and VetFamily in the retail channel downstream, because it is calculated only on the basis of the number of veterinarians, rather than on the basis of turnover. Indeed, at national level, the AniCura and VetFamily combined veterinary service market share based on the number of veterinarians is significantly lower, at [30-40]%, than the market share based on turnover, at [40- 50]%.
(150) On the basis of the assessment set out below and the arguments also referred to in Section 6.4.1.2.B, the Commission considers that the Parties would have the incentive to foreclose competitors in Sweden from access to downstream customers.
(151) As regards Sweden, the Commission notes that, on the basis of Mars' own estimates, Mars' market share is [50-60]% in the manufacture and supply of dietetic pet food sold through the veterinary channel, which may in itself be evidence of Mars' dominant position. (160) On the basis of the Commission's market reconstruction, this may slightly underestimate Mars' position in Sweden, which calculated the higher range of [50-60%] instead. The Commission notes that a merged entity's incentive to pursue a customer foreclosure strategy is stronger, where the upstream division of the merged entity can benefit from possibly higher price levels resulting from foreclosure of upstream rivals. (161)
(152) Such customer foreclosure strategy would raise barriers to entry to potential competitors, making their entry upstream unattractive by significantly reducing their revenue prospects. (162)
(153) The foreclosure of upstream competitors would translate into negative effects in the downstream retail market. This risk of increased prices and reduced choice for dietetic pet food sold through the veterinary channel was highlighted by market participants in Sweden, for example a Swedish veterinarian noted: "For sure it would impact my business. Their dog food is already overpriced and there are not many alternatives. I have the feeling that with this merger their dominance will just further increase while they have a quite good source of medical information and distribution channel if the merger will happen. It is a very good entrance strategy from Mars." (163)
(154) As explained above at Section (115) to (116), in view of the limited switching capability of downstream end customers, upstream competition has a prominent role in these markets, thereby making it likely that reduced upstream competition will result in higher downstream prices.
6.5.2. Other non-coordinated effects: access to confidential information of competitors
(155) Market participants also raised concerns that post-Transaction, Mars, as the owner of downstream veterinary clinics, would gain an insight into the pricing of its competitors, to the extent that AniCura continued to purchase dietetic pet food from these competitors. Examples of competitively sensitive information include: prices, quantities, level and timing of price increases, promotional activity grids, special pricing conditions, information about innovation and new product launches.
(156) The Commission notes that post-Transaction, through its ownership of VetFamily, Mars would obtain knowledge about its competitors' pricing (and other competitively sensitive information also) to VetFamily clinics i.e. Mars' downstream competitors. As a result, and in line with what is described above in relation to the customer foreclosure concerns, Mars could subsequently adapt its commercial strategy to undermine any attempt by competitors to sell to [40-50]% of the market that would be controlled by Mars post-Transaction (i.e. Anicura's and VetFamily clinics). This is likely to put competitors at a competitive disadvantage, thereby dissuading them to enter or expand in the market. (164).
(157) On the basis of the considerations in paragraphs (147) and (156) and also the arguments referred to in Section 6.4.1.2.C, the Commission considers that the merged entity may foreclose access to a significant customer base to its actual or potential rivals in the upstream market and reduce their ability or incentive to compete in Sweden. (165) The Commission therefore concludes that the Transaction raises serious doubts as to its compatibility with the internal market as regards its impact on competition for the retail of dietetic pet food through the veterinary channel in Sweden, on the basis of: (a) customer foreclosure resulting in increased prices for dietetic pet food sold through the veterinary channel, reduced choice, quality and innovation in dietetic pet food; and (b) the access to confidential information of competitors.
6.5.3. Input foreclosure
6.5.3.1. The Commission's assessment
(158) While the Commission notes that Mars may have upstream market power, and consequently may have the ability to restrict downstream customers from access to its dietary pet food products, the Commission considers that Mars would not have the incentive to foreclose. In its assessment, the Commission looks at whether the foreclosure would be profitable for the merged entity and the extent to which customers can be diverted away from downstream rivals. The effect is greater where the input, in this case dietetic pet food, represents a critical component of downstream rivals’ costs. (166)
(159) In this case, because the sale of dietetic pet food is not a key input to veterinary clinics, but is rather ancillary to the provision of veterinary services, the Commission considers that Mars would be unlikely to successfully divert customers away from AniCura’s downstream rivals, by restricting its supply of dietetic pet food to them. Rather, the Commission is of the view that Mars would face a greater risk of losing dietetic pet food sales, if it attempted an input foreclosure strategy regarding the supply of dietetic pet food. For these reasons, the Commission considers that Mars has the incentive to sell its dietetic pet food in as many retail outlets as possible.
(163) For similar reasons to those set out at Section 6.4.3 and Section 6.5.3, the Commission also considers that the merged entity would not have the incentive to pursue an input foreclosure strategy in the Netherlands. In particular, although Mars may have upstream market power, and consequently may have the ability to restrict downstream customers from access to its dietary pet food products, the Commission considers that Mars would not have the incentive to foreclose.
(164) In this case, because the sale of dietetic pet food is not a key input to veterinary clinics, but is rather ancillary to the provision of veterinary services, the Commission considers that Mars would be unlikely to successfully divert customers away from AniCura’s downstream rivals, by restricting its supply of dietetic pet food to them. Rather, the Commission is of the view that Mars would face a greater risk of losing dietetic pet food sales, if it attempted an input foreclosure strategy regarding the supply of dietetic pet food. For these reasons, the Commission considers that Mars has the incentive to sell its dietetic pet food in as many retail outlets as possible.
(165) There is also a horizontally affected market in the retail of dietetic pet food in the Netherlands, due to the limited quantities of the supply of private label Sterkliniek dietetic pet food through Sterkliniek clinics (27 of which are owned by AniCura). As regards the Commission's assessment, the Commission notes that while the merged entity would have a significant combined share upstream, the horizontal market share increment arising from the merger is extremely limited (less than [0- 5]% at national level). (167) In addition, the Transaction does not give rise to any increase in the manufacturing of dietetic pet food, since the Sterkliniek dietetic pet food is manufactured by a third-party supplier.
(166) On the basis of the considerations in paragraphs (161) to (165) the Commission therefore concludes that the Transaction does not raise serious doubts as to its compatibility with the internal market as regards its impact on competition for the retail of dietetic pet food through the veterinary channel in the Netherlands on the basis of vertical effects, or on the basis of horizontal overlaps.
6.7. Other markets
(167) The transaction also gives rise to vertically-affected markets in Germany, Austria, Italy, France and Spain.
(168) The Commission notes that AniCura’s presence in these countries is limited. The combined AniCura and VetFamily shares are as follows: Germany ([5-10]%), Austria ([0-5]%), Italy ([0-5]%), France ([0-5]%) and Spain ([0-5]%). Due to the low market shares downstream, the Commission considers that the merged entity would not have the ability to pursue a customer foreclosure strategy. For similar reasons to those set out at Section 6.4.3 and Section 6.5.3,the Commission also considers that the merged entity would not have the incentive to pursue an input foreclosure strategy.
(169) On the basis of the above, the Commission therefore concludes that the Transaction does not raise serious doubts as to its compatibility with the internal market as regards its impact on competition for the retail of dietetic pet food through the veterinary channel in Germany, Austria, Italy, France and Spain on the basis of vertical effects.
7. PROPOSED REMEDIES
7.1. Framework for the assessment of the commitments
(170) Where, as in this case, a notified concentration raises serious doubts as to its compatibility with the internal market, the parties may modify the notified concentration so as to remove the grounds for the serious doubts identified by the Commission with a view to having it declared compatible with the internal market pursuant to Article 6(1)(b) in conjunction with Article 6(2) of the Merger Regulation.
(171) As set out in the Commission Notice on Remedies, (168) commitments have to eliminate the Commission's serious doubts entirely, they have to be comprehensive and effective from all points of view. In Phase I, commitments offered by the parties can only be accepted where the competition problem is readily identifiable and can easily be remedied. The competition problem therefore needs to be so straightforward and the remedies so clear-cut that it is not necessary to enter into an in-depth investigation and that the commitments are sufficient to clearly rule out serious doubts within the meaning of Article 6(1)(c) of the Merger Regulation.
(172) In assessing whether or not the commitments proposed by the parties would restore effective competition, the Commission considers all relevant factors, including inter alia the type, scale and scope of the proposed commitments, judged by reference to the structure and particular characteristics of the market in which the Commission has identified serious doubts as to the compatibility of the notified concentration with the internal market, including the position of the Parties and other participants on the market. (169)
(173) In order for the commitments to comply with those principles, they must be capable of being implemented effectively within a short period of time. The Commission must determine with the requisite degree of certainty, at the time of its decision, that they will be fully implemented and that they are likely to maintain effective competition in the market.
(174) As regards the form of acceptable commitments, the Merger Regulation leaves discretion to the Commission as long as the commitments meet the requisite standard.
(175) Divestiture commitments are often the most effective way to eliminate competition concerns. The intended effects of a divestiture will only be achieved if and once the business to divest is transferred to a suitable purchaser.
(176) In order to ensure that the business is divested to a suitable purchaser, the commitments have to include criteria to define its suitability which will allow the Commission to conclude that the divestiture of the business to such purchaser will likely remove the competition concerns identified
7.2. Commitments submitted by the Parties
(177) In order to remove the serious doubts raised by the Transaction in the markets for the retail of dietetic pet food through the veterinary channel in Denmark and Sweden, with a view to rendering the concentration compatible with the internal market, the Parties have modified the notified concentration by submitting commitments to the Commission pursuant to Article 6(2) of the Merger Regulation.
(178) The Parties submitted two sets of commitments. Notably, the Parties formally submitted a remedy proposal on 8 October 2018 (the 'Initial Commitments'). After the Commission gathered the views of market participants on the Initial Commitments (the 'market test'), and informed the Parties of the remaining serious doubts raised by the Transaction, the Parties submitted a revised remedy proposal on 19 October 2018 (the 'Final Commitments').
7.2.1. The Initial Commitments
7.2.1.1. Description of the Initial Commitments
(179) The Initial Commitments proposed by Mars and AniCura consisted of the divestment of the entire VetFamily business, currently wholly-owned by AniCura and which is responsible for the negotiation of framework contracts for VetFamily members, including for the purchase of dietetic pet food ("Divestment Business"). The Divestment Business included:
(a) the VetFamily legal entities.
(b) all membership contracts with VetFamily members.
(c) all framework supply agreements for all products and services which have been entered into for the benefit of VetFamily members, which will continue to be operated under their existing terms with all benefits (e.g. rebates) accruing to VetFamily assigned to VetFamily. AniCura will also continue to use the shared framework supply agreements (i.e. which apply to both VetFamily and AniCura clinics) for a limited period.
(d) all dedicated VetFamily personnel (22 employees) and one additional AniCura Group Retail Manager in order to provide procurement expertise for pet food i.e. 23 employees in total.
(e) 8 employees as Key Personnel, including the Group VetFamily Director, the Group VetFamily Manager, the Group Retail Manager and Country Managers for Denmark, Sweden, Norway, the Netherlands and Germany.
(f) intangible assets including all intellectual property rights needed to operate the VetFamily business including sales and marketing assets, all VetFamily trademarks and brand names including the brand VetPro, a fully paid up perpetual licence from AniCura to use an IT system through which VetPlan is administered and a CRM system.
(g) tangible assets including office equipment, all business and financial records held by VetFamily, including data held: on VetFamily members; purchases made by VetFamily members under the framework agreements; sales and customer data relating to VetPlan, data on suppliers. They do not include office premises, except in Denmark, where the lease will be transferred to the purchaser.
(h) transitional arrangements for IT support (including software licenses) and in Sweden and Germany only, services relating to back-office functions (accounting and payroll) to be provided by AniCura to the Divestment Business for a period of up to […], at cost and at the option of the purchaser.
(180) Moreover, the Parties committed not to establish, operate or enter into a joint purchasing group for or with any independent veterinary practices in Sweden, Denmark and Norway for a period of […] post-Closing. The Parties also committed not to acquire an ownership interest in any of the VetFamily Members, identified as of the date of the Commission decision, in Sweden, Denmark or Norway for the same […].
(181) In addition, if required by the Commission, the Parties committed to terminate the VetFamily membership contracts of the 17 AniCura clinics which are part of VetFamily in Denmark.
(182) Finally, the Parties entered into related commitments, inter alia regarding the separation of the divested businesses from their retained businesses, the preservation of the viability, marketability and competitiveness of the divested businesses, including the appointment of a monitoring trustee and, if necessary, a divestiture trustee.
7.2.1.2. Results of the market test
(183) The Commission launched a market test of the Initial Commitments on 10 October 2018, which was addressed to upstream competitors of Mars, VetFamily member clinics and potential purchasers.
(184) Despite being generally positive, the view of the respondents to the market test was that a number of elements of the Initial Commitments needed to be modified in order for them to be able to remedy the serious doubts identified by the Commission in the markets for the sale of dietetic pet food through the veterinary channel in Denmark and Sweden. (170).
(185) In particular, some respondents highlighted the need for the Divestment Business to have the ability to provide continuous education and training to VetFamily member clinics. (171)
(186) While the majority of respondents considered that the scope and duration of the transitional arrangements proposed in the Initial Commitments was sufficient to ensure the viability and competitiveness of the Divestment Business, a few stated that a longer duration of such arrangements may be necessary, in particular given the critical nature of some of those transitional services for the Divestment Business, such as IT. (172)
(187) In addition, the majority of respondents considered the proposed commitments of the Parties not to engage in joint purchasing groups and not to acquire VetFamily Members in Sweden, Denmark and Norway for the period of […] post-Closing (paragraph (180)) to be necessary and adequate to ensure the viability and competitiveness of the Divestment Business. Nevertheless, several respondents suggested that the duration should be longer than […]. (173)
(188) In other respects, the respondents generally considered that the Divested Business includes all necessary assets and would be able to compete effectively with the Parties after the Transaction. (174)
(189) Finally, the majority of respondents considered that the Divestment Business was sufficiently interesting to attract suitable purchasers. Several respondents indicated that would be potentially interested in purchasing the Divestment Business, subject to the above-mentioned improvements and those respondents' closer due diligence of the Divestment Business. (175)
7.2.2. The Final Commitments
7.2.2.1. Description of the Final Commitments
(190) In view of the results of the market test and following the feedback provided by the Commission, the Parties submitted the Final Commitments on 19 October 2018. The full text of the Final Commitments is attached as an Annex to this Decision. The Final Commitments address the shortcomings identified above and include other modifications to strengthen their effectiveness, as set out below:
(a) The scope of the Divestment Business was clarified to include the capability to provide all training and other on-boarding support services that are currently offered to VetFamily Members in connection with their membership of the VetFamily network.
(b) The possibility of extension of the transitional services agreements for a further period of […], at the request of the Purchaser, subject to the opinion of the Monitoring Trustee, was introduced.
(c) For the remaining term of the respective Shared Framework Agreements (or […]), all benefits accruing under those agreements (i.e. rebates, discounts, and other bonuses) will be fully transferred to VetFamily, with the only exception of benefits relating to volumes purchased by AniCura clinics.
(d) AniCura committed not to take any action on the tender for new suppliers of VetFamily Members prior to closing of the divestiture, except as deemed necessary by the Hold Separate Manager for the continued viability of the Divestment Business, subject to the opinion of the Monitoring Trustee.
(e) The First Divestiture Period, during which the Parties commit to find a purchaser and to enter into a final binding sale and purchase agreement for the sale of the Divestment Business, was shortened to three months, with the possibility of a one month extension by the Commission.
(f) During the […] period limiting the Parties ability to engage in joint purchasing groups and to acquire any of the VetFamily Members in Sweden, Denmark and Norway (paragraph (180)), the Parties committed also not to solicit veterinary practices for such purposes.
(191) The Final Commitments also clarify some language to ensure that all necessary rights and assets transfer to the Purchaser.
7.2.2.2. The Commission’s assessment of the Final Commitments
(192) For the reasons explained below, the Commission considers that the Final Commitments remove all serious doubts raised by the Transaction.
(193) The implementation of the Final Commitments will take VetFamily out of the control of the merged entity and will thus eliminate the influence of the merged entity on a significant number of veterinary clinics in Denmark and Sweden post- Transaction. The divestment of VetFamily will allow an independently-owned buying group to continue to operate in the relevant markets. The share of the downstream market of the merged entity at national level will be reduced from [40-50]% to [20-30]% in Sweden and from [40-50]% to [20-30]% in Denmark. The Commission considers that this structural change will ensure that the upstream competitors of the merged entity have access to a sufficient number of downstream veterinary clinics to remain viable and competitive in Sweden and Denmark.
(194) To maintain their structural effect, the Final Commitments contain a standard non-reacquisition clause preventing the merged entity from reacquiring VetFamily for the period of 10 years. In addition to that, the Final Commitments include an obligation on the merged entity not to engage in joint purchasing groups and not to acquire ownership in VetFamily Members in Denmark and Sweden for the period of […] following the sale of VetFamily. The Commission considers that such clause, even if it goes beyond the standard terms of a non- reacquisition clause as it concerns the acquisition of third parties, is necessary in the markets where serious doubts were identified to ensure that the Final Commitments are not circumvented by the merged entity acquiring influence downstream over the clinics through an alternative joint purchasing scheme or by acquiring current VetFamily Members. This is because the acquisition of influence over the clinics could enable the reacquisition of market power in those markets where that market power has been found to be problematic and is aimed to be removed through the divestiture remedy. Since the Commission has not identified serious doubts in Member States other than Denmark and Sweden, it is not necessary to extend the anti-circumvention clause limiting the Parties' conduct to other Member States, in particular considering that the viability and competitiveness of the Divestment Business are not affected (see paragraph (198)).
(195) Furthermore, the post-market test extension of the above-mentioned prohibition on joint purchasing schemes and acquisition of VetFamily Members to cover also non-solicitation further strengthens the Final Commitments since it prevents the Parties from approaching veterinary clinics, and hence potentially influencing their behaviour, already during the […] period. The Commission also considers the duration of […] to be sufficient to allow VetFamily to develop and establish itself in the hands of a new owner, in particular given the nature of VetFamily’s business and its growth to date.
(196) Moreover, in light of the results of the market test, the Commission considers the scope and scale of the Divestment Business to be sufficient to ensure its continued viability and competitiveness. In particular, the Divestment Business includes all tangible and intangible assets, benefits from framework agreements, personnel (including Key Personnel), and transitional services agreements which are necessary for its viability and competitiveness.
(197) While the Final Commitments submitted by the Parties provide for the full divestiture of VetFamily including in the countries where the Transaction does not raise serious doubts (that is, other than Sweden and Denmark), the Commission considers that the inclusion of VetFamily's operations in those countries is necessary and proportionate to preserve the viability and competitiveness of the Divestment Business. Indeed, the number of VetFamily member clinics in Sweden and Denmark comprise only around [20-30]% of the total number of VetFamily members. Carving out and divesting VetFamily's activities only in Sweden and Denmark would risk undermining its operations as a standalone business.
(198) On the other hand, the non-application of the […] prohibition on joint purchasing and acquisition of VetFamily Members (paragraph (180)) to Germany and the Netherlands, where VetFamily is also currently active, does not affect the viability and competitiveness of the Divestment Business in light of its scope and scale. In particular, VetFamily's operations in Germany and the Netherlands are still in the start-up stage (launched in the last two years) (176) generating EUR 0 membership fees in 2017 (177) and VetFamily has functioned as a viable business already since 2000. In addition, personnel are of key importance for the operation of VetFamily. The Divestment Business includes (key) personnel for all VetFamily countries including also for Germany and the Netherlands.
(199) Nevertheless, the Parties have proposed that the […] prohibition on joint purchasing and acquisition of VetFamily Members covers also Norway, one of the core markets of VetFamily. The Commission considers this commitment to further strengthen the viability of the Divestment Business.
(200) Furthermore, the Commission believes that VetFamily will be viable in the hands of a suitable purchaser even without the involvement of AniCura. In particular, VetFamily was established in 2000 and successfully operated and grew also before 2014 when it was acquired by AniCura. Also, VetFamily is rapidly expanding, as evidenced, for example, by the increase in the number of VetFamily member clinics by over 200 only during the Commission merger proceedings (paragraph (71)). (178)
(201) In addition, the Commission is of the view that short divestiture periods contribute largely to the success of a divestiture because they limit the period of uncertainty. (179) In the present case, the Parties reduced the First Divestiture Period in the Final Commitments to three months, subject to a possible extension by the Commission of one month. The Commission believes such a condensed time table for divestiture will facilitate the successful transfer of the Divestment Business to a new owner and will eliminate any possibility of anti-competitive effects arising in the meantime.
(202) Finally, the Commission notes that the Final Commitments remove serious doubts regarding Mars gaining access to competitively sensitive information in the downstream market (for example, on prices, quantities, product characteristics, etc.) of its upstream competitors. The Final Commitments ensure that VetFamily will be operated, and commercial terms will be negotiated, by an independent entity and hence Mars will not obtain access to the commercial terms that its upstream competitors offer to VetFamily clinics, Thus, Mars' upstream competitors will be able to offer commercial terms to non-AniCura clinics, which comprise around three quarters of the downstream market in each Sweden and Denmark, without making them known to Mars. At the same time, the terms offered by Mars' upstream competitors to AniCura clinics and to VetFamily clinics will not necessarily be the same post-Transaction, thus preserving uncertainty and conditions for competition.
(203) In light of the above considerations and taking into account the results of the market test and other information available to it, the Commission considers that the Final Commitments are sufficient to eliminate the serious doubts as to the compatibility of the Transaction with the internal market with respect to the markets for the retail of dietetic pet food through the veterinary channel in Denmark and Sweden. Moreover, the Final Commitments are comprehensive and effective from all points of view, and are capable of being implemented effectively within a short period of time.
8. CONDITIONS AND OBLIGATIONS
(204) Under the first sentence of the second subparagraph of Article 6(2) of the Merger Regulation, the Commission may attach to its decision conditions and obligations intended to ensure that the undertakings concerned comply with the commitments they have entered vis-à-vis the Commission with a view to rendering the concentration compatible with the internal market
(205) -The fulfilment of the measures that gives rise to the structural change of the market is a condition, whereas the implementing steps that are necessary to achieve this result are generally obligations on the Parties. Where a condition is not fulfilled, the Commission's decision declaring the concentration compatible with the internal market is no longer applicable. Where the undertakings concerned commit a breach of an obligation, the Commission may revoke the clearance decision in accordance with Article 6(3) of the Merger Regulation. The undertakings concerned may also be subject to fines and periodic penalty payments under Articles 14(2) and 15(1) of the Merger Regulation.
(206) In accordance with the basic distinction between conditions and obligations described in the preceding paragraph, the commitments in Section B as well as the Schedule of the Final Commitments set out in the Annex constitute conditions attached to this Decision, as only through their full compliance can the structural changes in the relevant markets be achieved. The other commitments set out in the Annex constitute obligations, as they concern the implementing steps that are necessary to achieve the modifications sought in a manner compatible with the internal market.
(207) The full text of the Final Commitments is attached to this Decision as the Annex and forms an integral part of this Decision.
9. CONCLUSION
(208) For the above reasons, the Commission has decided not to oppose the notified operation as modified by the commitments and to declare it compatible with the internal market, subject to full compliance with the conditions in Section B as well as the Schedule of the Final Commitments annexed to the present decision and with the obligations contained in the other sections of the said commitments. This Decision is adopted in application of Article 6(1)(b) in conjunction with Article 6(2) of the Merger Regulation.
19 October 2018
Case M.9019 – MARS / ANICURA
COMMITMENTS TO THE EUROPEAN COMMISSION
Pursuant to Article 6(2) of Council Regulation (EC) No 139/2004 (the Merger Regulation), Mars, Incorporated (Mars) hereby enters into the following Commitments (the Commitments) vis-à-vis the European Commission (the Commission) with a view to rendering its proposed acquisition of AniCura TC AB (AniCura) (the Concentration) compatible with the internal market and the functioning of the EEA Agreement.
This text shall be interpreted in light of the Commission’s decision pursuant to Article 6(1)(b) of the Merger Regulation to declare the Concentration compatible with the internal market and the functioning of the EEA Agreement (the Decision), in the general framework of European Union law, in particular in light of the Merger Regulation, and by reference to the Commission Notice on remedies acceptable under Council Regulation (EC) No 139/2004 and under Commission Regulation (EC) No 802/2004 (the Remedies Notice).
Section A. Definitions
1. For the purpose of the Commitments, the following terms shall have the following meaning:
Affiliated Undertakings: undertakings controlled by the Parties and/or by the ultimate parents of the Parties, whereby the notion of control shall be interpreted pursuant to Article 3 of the Merger Regulation and in light of the Commission Consolidated Jurisdictional Notice under Council Regulation (EC) No 139/2004 on the control of concentrations between undertakings (the Consolidated Jurisdictional Notice).
AniCura: a privately held provider of veterinary care for companion animals. AniCura is held by AniCura TC AB, incorporated under the laws of Sweden and has its registered office at Vendevägen 89, 182 32 Danderyd, Sweden, registered under number 556972-6689.
AniCura Clinics: veterinary practices in which AniCura has an ownership interest.
AniPlan: a monthly subscription-based preventative pet care service that is offered to pet owners through AniCura Clinics. AniPlan and VetPlan are administered via the AniCura IT System.
AniPlan IT System: an in-house, […] IT system developed and owned by AniCura to administer the AniPlan and VetPlan preventive care plans, managed by VetFamily. […].
Assets: the assets that contribute to the current operation or are necessary to ensure the viability and competitiveness of the Divestment Business as indicated in Section B and described more in detail in the Schedule.
Closing: the transfer of the legal title to the Divestment Business to the Purchaser.
Closing Period: the period of three months from the approval of the Purchaser and the terms of sale by the Commission.
Confidential Information: any business secrets, know-how, commercial information, or any other information of a proprietary nature that is not in the public domain.
Conflict of Interest: any conflict of interest that impairs the Monitoring Trustee's objectivity and independence in discharging its duties under the Commitments.
Divestment Business: the business or businesses as defined in Section B and in the Schedule which Mars commits to divest.
Divestiture Trustee: one or more natural or legal person(s) who is/are approved by the Commission and appointed by Mars and who has/have received from Mars the exclusive Trustee Mandate to sell the Divestment Business to a Purchaser at no minimum price.
Effective Date: the date of adoption of the Decision.
First Divestiture Period: the period of three months from the Effective Date, with the possibility of an extension of one additional month, such extension to be governed by the procedure set out in paragraph 42.
Framework Agreements: framework supply agreements for a range of veterinary supplies (e.g. pharmaceuticals, pet food, laboratory supplies) as described in the Schedule.
Hold Separate Manager: the person appointed by Mars for the Divestment Business to manage the day-to-day business under the supervision of the Monitoring Trustee.
Key Personnel: all personnel necessary to maintain the viability and competitiveness of the Divestment Business, as listed in the Schedule, including the Hold Separate Manager.
Mars: Mars, Incorporated, a privately held company incorporated under the laws of Delaware with its registered office at 6885 Elm Street, McLean, VA 22101-3883, USA.
Membership Contract: a membership contract entered into between VetFamily and an independent veterinary practice whereby the latter becomes a VetFamily Member.
Monitoring Trustee: one or more natural or legal person(s) who is/are approved by the Commission and appointed by Mars, and who has/have the duty to monitor Mars compliance with the conditions and obligations attached to the Decision.
Parties: Mars and AniCura.
Personnel: all staff currently employed by the Divestment Business, including staff seconded to the Divestment Business, shared personnel as well as the additional personnel listed in the Schedule.
Purchaser: the entity approved by the Commission as acquirer of the Divestment Business in accordance with the criteria set out in Section D.
Purchaser Criteria: the criteria laid down in paragraph 16 of these Commitments that the Purchaser must fulfil in order to be approved by the Commission.
Schedule: the schedule to these Commitments describing in more detail the Divestment Business
Trustee(s): the Monitoring Trustee and/or the Divestiture Trustee as the case may be.
Trustee Divestiture Period: the period of three months from the end of the First Divestiture Period.
VetFamily: a joint purchasing group owned by AniCura which enables VetFamily Members to procure veterinary supplies (e.g. pharmaceutical products, pet food, laboratory supplies etc.) on favourable terms under the Framework Agreements with selected suppliers. In Denmark only, VetFamily also provides its members with additional services, such as back-office support, website design, and the creation of marketing campaigns with key suppliers. VetFamily is currently active in Sweden, Denmark, Norway, the Netherlands, and Germany and is in the process of setting up operations in […].
VetFamily Legal Entities: the legal entities through which the VetFamily business operates, namely: VetFamily Holding AB (Sweden), VetFamily AB (Sweden), VetFamily AS (Norway), VetFamily ApS (Denmark), VetFamily GmbH (Germany), VetFamily B.V. (Netherlands), and VetFamily GmbH (Austria), as well as any other VetFamily legal entities which are incorporated before Closing.
VetFamily Members: veterinary practices that are members of VetFamily.
VetPlan: a monthly subscription-based preventative pet care service offered by VetFamily to pet owners, sold through VetFamily Member clinics. VetPlan and AniPlan are administered via the AniCura IT System.
VetPlan Contracts: customer contracts for VetPlan.
Section B. The Commitment to divest and the Divestment Business
Commitment to divest
2. In order to maintain effective competition, the Parties commit to divest, or procure the divestiture of the Divestment Business by the end of the Trustee Divestiture Period as a going concern to a purchaser and on terms of sale approved by the Commission in accordance with the procedure described in paragraph 17 of these Commitments. To carry out the divestiture, the Parties commit to find a purchaser and to enter into a final binding sale and purchase agreement for the sale of the Divestment Business within the First Divestiture Period. If Mars has not entered into such an agreement at the end of the First Divestiture Period, Mars shall grant the Divestiture Trustee an exclusive mandate to sell the Divestment Business in accordance with the procedure described in paragraph 29 in the Trustee Divestiture Period.
3. The Parties shall be deemed to have complied with this commitment if:
(a) by the end of the Trustee Divestiture Period, Mars or the Divestiture Trustee has entered into a final binding sale and purchase agreement and the Commission approves the proposed purchaser and the terms of sale as being consistent with the Commitments in accordance with the procedure described in paragraph 17; and
(b) the Closing of the sale of the Divestment Business to the Purchaser takes place within the Closing Period.
4. In order to maintain the structural effect of the Commitments, Mars shall, for a period of ten years after Closing, not acquire, whether directly or indirectly, the possibility of exercising influence (as defined in paragraph 43 of the Remedies Notice, footnote 3) over the whole or part of the Divestment Business, unless, following the submission of a reasoned request from Mars showing good cause and accompanied by a report from the Monitoring Trustee (as provided in paragraph 43 of these Commitments), the Commission finds that the structure of the market has changed to such an extent that the absence of influence over the Divestment Business is no longer necessary to render the proposed Concentration compatible with the internal market.
5. In addition:
(a) Subject to any transitional arrangements agreed with the Purchaser under the terms of these Commitments, the Parties commit that AniCura will withdraw from joint purchasing activities with the Divestiture Business for a period of ten years after Closing. By way of exception, this will not prevent the AniCura-owned veterinary practices which are currently VetFamily Members in Denmark from continuing to be VetFamily Members unless requested by the Commission.
(b) For a period of […] after Closing, the Parties commit that neither Mars, AniCura nor their Affiliated Undertakings shall:
(i) establish, operate, or enter into a joint procurement or purchasing group for or with any independent veterinary practices in Sweden, Denmark, or Norway, or solicit any such independent veterinary practice to do so; or
(ii) acquire an ownership interest in any veterinary practices in Sweden, Denmark or Norway which are VetFamily Members as at the Effective Date, or solicit any such VetFamily Member to be acquired.
For the avoidance of doubt, the obligations in this paragraph 5(b) shall not prevent Mars or AniCura or their Affiliated Undertakings from conducting any procurement or purchasing activity for the AniCura Clinics or their other Affiliated Undertakings.
Structure and definition of the Divestment Business
6. The Divestment Business consists of the entire VetFamily business as at Closing, as carried out through the VetFamily Legal Entities and the assets comprised within them. The legal and functional structure of the Divestment Business as operated to date is described in the Schedule to these Commitments. As described in more detail in the Schedule, the Divestment Business includes all assets, contracts, licences, and personnel that contribute to the current operation or are necessary to ensure the viability and competitiveness of the Divestment Business (including any assets or resources which are not otherwise covered in the Schedule but which are both used in the Divestment Business for the continued viability and competitiveness of the Divestment Business, or an adequate substitute for any such asset or resource), in particular:
(a) all tangible and intangible assets (including intellectual property rights);
(b) all contracts and licences (including but not limited to the Framework Agreements, the Membership Contracts, and the VetPlan Contracts); and
(c) the Personnel.
7. In addition, the Divestment Business includes the benefit, for a transitional period of up to […] after Closing (and at the request of the Purchaser, with a possible extension for a further period of […], subject to the opinion of the Monitoring Trustee) and on terms and conditions equivalent to those at present afforded to the Divestment Business, of all current arrangements under which AniCura or its Affiliated Undertakings supply services to the Divestment Business, as detailed in the Schedule, unless otherwise agreed with the Purchaser. Strict firewall procedures will be adopted so as to ensure that any competitively sensitive information related to, or arising from such supply arrangements will not be shared with, or passed on to, anyone outside AniCura’s operations.
Section C. Related commitments
Preservation of viability, marketability and competitiveness
8. From the Effective Date until Closing, the Parties shall preserve or procure the preservation of the economic viability, marketability and competitiveness of the Divestment Business, in accordance with good business practice, and shall minimise as far as possible any risk of loss of competitive potential of the Divestment Business. In particular the Parties undertake:
(a) not to carry out any action that might have a significant adverse impact on the value, management or competitiveness of the Divestment Business or that might alter the nature and scope of activity, or the industrial or commercial strategy or the investment policy of the Divestment Business;
(b) to make available, or procure to make available, sufficient resources for the development of the Divestment Business, on the basis and continuation of the existing business plans;
(c) to take all reasonable steps, or procure that all reasonable steps are being taken, including appropriate incentive schemes (based on industry practice), to encourage all Key Personnel to remain with the Divestment Business, and not to solicit or move any Personnel to the Parties’ remaining business. Where, nevertheless, individual members of the Key Personnel exceptionally leave the Divestment Business, the Parties shall provide a reasoned proposal to replace the person or persons concerned to the Commission and the Monitoring Trustee. The Parties must be able to demonstrate to the Commission that the replacement is well suited to carry out the functions exercised by those individual members of the Key Personnel. The replacement shall take place under the supervision of the Monitoring Trustee, who shall report to the Commission.
Hold-separate obligations
9. The Parties commit, from the Effective Date until Closing, to keep the Divestment Business separate from the business it is retaining and to ensure that unless explicitly permitted under these Commitments: (i) management and staff of the business retained by the Parties have no involvement in the Divestment Business; (ii) the Key Personnel and Personnel of the Divestment Business have no involvement in any business retained by the Parties and do not report to any individual outside the Divestment Business.
10. Until Closing, the Parties shall assist the Monitoring Trustee in ensuring that the Divestment Business is managed as a distinct and saleable entity separate from the business which Mars is retaining. Immediately after the adoption of the Decision, Mars shall appoint a Hold Separate Manager. The Hold Separate Manager, who shall be part of the Key Personnel, shall manage the Divestment Business independently and in the best interest of the business with a view to ensuring its continued economic viability, marketability and competitiveness and its independence from the businesses retained by Mars. The Hold Separate Manager shall closely cooperate with and report to the Monitoring Trustee and, if applicable, the Divestiture Trustee. Any replacement of the Hold Separate Manager shall be subject to the procedure laid down in paragraph 8(c) of these Commitments. The Commission may, after having heard Mars, require Mars to replace the Hold Separate Manager.
Ring-fencing
11. The Parties shall implement, or procure to implement, all necessary measures to ensure that it does not, after the Effective Date, obtain any Confidential Information relating to the Divestment Business and that any such Confidential Information obtained by the Parties before the Effective Date will be eliminated and not be used by the Parties. This includes measures vis-à-vis the Parties’ appointees on the supervisory board and/or board of directors of the Divestment Business. In particular, the participation of the Divestment Business in any central information technology network shall be severed to the extent possible, without compromising the viability of the Divestment Business. The Parties may obtain or keep information relating to the Divestment Business which is reasonably necessary for the divestiture of the Divestment Business or the disclosure of which to the Parties is required by law.
Non-solicitation clause
12. The Parties undertake, subject to customary limitations, not to solicit, and to procure that Affiliated Undertakings do not solicit, the Key Personnel transferred with the Divestment Business for a period of […] after Closing.
Due diligence
13. In order to enable potential purchasers to carry out a reasonable due diligence of the Divestment Business, the Parties shall, subject to customary confidentiality assurances and dependent on the stage of the divestiture process:
(a) provide to potential purchasers sufficient information as regards the Divestment Business;
(b) provide to potential purchasers sufficient information relating to the Personnel and allow them reasonable access to the Personnel.
Reporting
14. Mars shall submit written reports in English on potential purchasers of the Divestment Business and developments in the negotiations with such potential purchasers to the Commission and the Monitoring Trustee no later than 10 days after the end of every month following the Effective Date (or otherwise at the Commission’s request). Mars shall submit a list of all potential purchasers having expressed interest in acquiring the Divestment Business to the Commission at each and every stage of the divestiture process, as well as a copy of all the offers made by potential purchasers within five days of their receipt.
15. Mars shall inform the Commission and the Monitoring Trustee on the preparation of the data room documentation and the due diligence procedure and shall submit a copy of any information memorandum to the Commission and the Monitoring Trustee before sending the memorandum out to potential purchasers.
Section D. The Purchaser
16. In order to be approved by the Commission, the Purchaser must fulfil the following criteria:
(a) The Purchaser shall be independent of and unconnected to Mars and its Affiliated Undertakings (this being assessed having regard to the situation following the divestiture).
(b) The Purchaser shall have the financial resources, proven expertise and incentive to maintain and develop the Divestment Business as a viable and active competitive force in competition with the Parties and other competitors;
(c) The acquisition of the Divestment Business by the Purchaser must neither be likely to create, in light of the information available to the Commission, prima facie competition concerns nor give rise to a risk that the implementation of the Commitments will be delayed. In particular, the Purchaser must reasonably be expected to obtain all necessary approvals from the relevant regulatory authorities for the acquisition of the Divestment Business.
17. The final binding sale and purchase agreement (as well as ancillary agreements) relating to the divestment of the Divestment Business shall be conditional on the Commission’s approval. When Mars has reached an agreement with a purchaser, it shall submit a fully documented and reasoned proposal, including a copy of the final agreement(s), within one week to the Commission and the Monitoring Trustee. Mars must be able to demonstrate to the Commission that the purchaser fulfils the Purchaser Criteria and that the Divestment Business is being sold in a manner consistent with the Commission’s Decision and the Commitments. For the approval, the Commission shall verify that the purchaser fulfils the Purchaser Criteria and that the Divestment Business is being sold in a manner consistent with the Commitments including their objective to bring about a lasting structural change in the market. At Mars’ request, the Commission may approve the sale of the Divestment Business without one or more Assets or parts of the Personnel, or by substituting one or more Assets or parts of the Personnel with one or more different assets or different personnel, if this does not affect the viability and competitiveness of the Divestment Business after the sale, taking account of the proposed purchaser.
Section D. Monitoring Trustee
I. Appointment procedure
18. Mars shall appoint a Monitoring Trustee to carry out the functions specified in these Commitments for a Monitoring Trustee. Mars commits not to close the Concentration before appointment of a Monitoring Trustee.
19. If Mars has not entered into a binding sale and purchase agreement regarding the Divestment Business two weeks before the end of the First Divestiture Period or if the Commission has rejected a purchaser proposed by Mars at that time or thereafter, Mars shall appoint a Divestiture Trustee. The appointment of the Divestiture Trustee shall take effect upon the commencement of the Trustee Divestiture Period.
20. The Monitoring Trustee shall:
(i) at the time of appointment, be independent of Mars, AniCura and their Affiliated Undertakings;
(ii) possess the necessary qualifications to carry out its mandate, for example have sufficient relevant experience as an investment banker or consultant or auditor; and
(iii) neither have nor become exposed to a Conflict of Interest.
21. The Monitoring Trustee shall be remunerated by Mars in a way that does not impede the independent and effective fulfilment of its mandate. In particular, where the remuneration package of a Divestiture Trustee includes a success premium linked to the final sale value of the Divestment Business, such success premium may only be earned if the divestiture takes place within the Trustee Divestiture Period.
Proposal by Mars
22. No later than ten working days after the Effective Date, Mars shall submit the name or names of one or more natural or legal persons whom Mars proposes to appoint as the Monitoring Trustee to the Commission for approval. No later than one month before the end of the First Divestiture Period or on request by the Commission, Mars shall submit a list of one or more persons who Mars proposes to appoint as Divestiture Trustee to the Commission for approval. The proposal shall contain sufficient information for the Commission to verify that the person or persons proposed as Trustee fulfil the requirements set out in paragraph 19 and shall include:
(i) the full terms of the proposed mandate, which shall include all provisions necessary to enable the Trustee to fulfil its duties under these Commitments;
(ii) the outline of a work plan which describes how the Trustee intends to carry out its assigned tasks; and
(iii) an indication of whether the proposed Trustee is to act as both Monitoring Trustee and Divestiture Trustee or whether different trustees are proposed for the two functions.
Approval or rejection by the Commission
23. The Commission shall have the discretion to approve or reject the proposed Trustee(s) and to approve the proposed mandate subject to any modifications it deems necessary for the Trustee to fulfil its obligations. If only one name is approved, Mars shall appoint or cause to be appointed the person or persons concerned as Trustee, in accordance with the mandate approved by the Commission. If more than one name is approved, Mars shall be free to choose the Trustee to be appointed from among the names approved. The Trustee shall be appointed within one week of the Commission’s approval, in accordance with the mandate approved by the Commission (the Trustee Mandate).
New proposal by Mars
24. If all the proposed Trustees are rejected, Mars shall submit the names of at least two more natural or legal persons within one week of being informed of the rejection, in accordance with paragraphs 18 and 23 of these Commitments.
Monitoring Trustee nominated by the Commission
25. If all further proposed Monitoring Trustees are rejected by the Commission, the Commission shall nominate a Monitoring Trustee, whom Mars shall appoint, or cause to be appointed, in accordance with a trustee mandate approved by the Commission.
II. Functions of the Trustee
26. The Trustee shall assume its specified duties and obligations in order to ensure compliance with these Commitments. The Commission may, on its own initiative or at the request of the Trustee or Mars, give any orders or instructions to the Trustee in order to ensure compliance with the conditions and obligations attached to the Decision.
Duties and obligations of the Monitoring Trustee
27. The Monitoring Trustee shall:
(i) propose in its first report to the Commission a detailed work plan describing how it intends to monitor compliance with the obligations and conditions attached to the Decision.
(ii) oversee, in close co-operation with the Hold Separate Manager, the on-going management of the Divestment Business with a view to ensuring its continued economic viability, marketability and competitiveness and monitor compliance by the Parties with the conditions and obligations attached to the Decision. To that end the Monitoring Trustee shall:
(a) monitor the preservation of the economic viability, marketability and competitiveness of the Divestment Business, and the keeping separate of the Divestment Business from the business retained by the Parties, in accordance with paragraphs 8 and 9 of these Commitments;
(b) supervise the management of the Divestment Business as a distinct and saleable entity, in accordance with paragraph 10 of these Commitments;
(c) with respect to Confidential Information:
- determine all necessary measures to ensure that the Parties do not after the Effective Date obtain any Confidential Information relating to the Divestment Business,
- in particular strive for the severing of the Divestment Business’ participation in a central information technology network to the extent possible, without compromising the viability of the Divestment Business,
- make sure that any Confidential Information relating to the Divestment Business obtained by the Parties before the Effective Date is eliminated and will not be used by the Parties; and
- decide whether such information may be disclosed to or kept by the Parties as the disclosure is reasonably necessary to allow the Parties to carry out the divestiture or as the disclosure is required by law;
(d) monitor the splitting of assets and the allocation of Personnel between the Divestment Business and Mars or Affiliated Undertakings;
(iii) propose to the Parties such measures as the Monitoring Trustee considers necessary to ensure the Parties’ compliance with the conditions and obligations attached to the Decision, in particular the maintenance of the full economic viability, marketability or competitiveness of the Divestment Business, the holding separate of the Divestment Business and the non-disclosure of competitively sensitive information;
(iv) review and assess potential purchasers as well as the progress of the divestiture process and verify that, dependent on the stage of the divestiture process:
(a) potential purchasers receive sufficient and correct information relating to the Divestment Business and the Personnel in particular by reviewing, if available, the data room documentation, the information memorandum and the due diligence process, and
(b) potential purchasers are granted reasonable access to the Personnel;
(v) act as a contact point for any requests by third parties, in particular potential purchasers, in relation to the Commitments;
(vi) provide to the Commission, sending Mars a non-confidential copy at the same time, a written report within 15 days after the end of every month that shall cover the operation and management of the Divestment Business as well as the splitting of assets and the allocation of Personnel so that the Commission can assess whether the business is held in a manner consistent with the Commitments and the progress of the divestiture process as well as potential purchasers;
(vii) promptly report in writing to the Commission, sending Mars a non-confidential copy at the same time, if it concludes on reasonable grounds that Mars is failing to comply with these Commitments;
(viii) within one week after receipt of the documented proposal referred to in paragraph 17 of these Commitments, submit to the Commission, sending Mars a non-confidential copy at the same time, a reasoned opinion as to the suitability and independence of the proposed purchaser and the viability of the Divestment Business after the Sale and as to whether the Divestment Business is sold in a manner consistent with the conditions and obligations attached to the Decision, in particular, if relevant, whether the Sale of the Divestment Business without one or more Assets or not all of the Personnel affects the viability of the Divestment Business after the sale, taking account of the proposed purchaser;
(ix) assume the other functions assigned to the Monitoring Trustee under the conditions and obligations attached to the Decision.
28. If the Monitoring and Divestiture Trustee are not the same legal or natural persons, the Monitoring Trustee and the Divestiture Trustee shall cooperate closely with each other during and for the purpose of the preparation of the Trustee Divestiture Period in order to facilitate each other's tasks.
Duties and obligations of the Divestiture Trustee
29. Within the Trustee Divestiture Period, the Divestiture Trustee shall sell at no minimum price the Divestment Business to a purchaser, provided that the Commission has approved both the purchaser and the final binding sale and purchase agreement (and ancillary agreements) as in line with the Commission's Decision and the Commitments in accordance with paragraphs 16 and 17 of these Commitments. The Divestiture Trustee shall include in the sale and purchase agreement (as well as in any ancillary agreements) such terms and conditions as it considers appropriate for an expedient sale in the Trustee Divestiture Period. In particular, the Divestiture Trustee may include in the sale and purchase agreement such customary representations and warranties and indemnities as are reasonably required to effect the sale. The Divestiture Trustee shall protect the legitimate financial interests of Mars, subject to Mars’ unconditional obligation to divest at no minimum price in the Trustee Divestiture Period.
30. In the Trustee Divestiture Period (or otherwise at the Commission’s request), the Divestiture Trustee shall provide the Commission with a comprehensive monthly report written in English on the progress of the divestiture process. Such reports shall be submitted within 15 days after the end of every month with a simultaneous copy to the Monitoring Trustee and a non-confidential copy to Mars.
III. Duties and obligations of the Parties
31. The Parties shall provide and shall cause their advisors to provide the Monitoring Trustee with all such co-operation, assistance and information as the Monitoring Trustee may reasonably require to perform its tasks. The Monitoring Trustee shall have full and complete access to any books, records, documents, management or other personnel, facilities, sites and technical information necessary for fulfilling its duties under the Commitments, and the Parties shall provide the Monitoring Trustee upon request with copies of any document. If requested by the Monitoring Trustee, Mars shall make available to the Monitoring Trustee one or more offices on their premises and shall be available for meetings in order to provide the Monitoring Trustee with all information necessary for the performance of its tasks.
32. Mars shall provide the Monitoring Trustee with all managerial and administrative support that it may reasonably request on behalf of the management of the Divestment Business. This shall include all administrative support functions relating to the Divestment Business which are currently carried out at headquarters level. The Parties shall provide and shall cause their advisors to provide the Monitoring Trustee, on request, with the information submitted to potential purchasers, in particular give the Monitoring Trustee access to the data room documentation and all other information granted to potential purchasers in the due diligence procedure. Mars shall inform the Monitoring Trustee on possible purchasers, submit lists of potential purchasers at each stage of the selection process, including the offers made by potential purchasers at those stages, and keep the Monitoring Trustee informed of all developments in the divestiture process.
33. Mars shall grant or procure Affiliated Undertakings to grant comprehensive powers of attorney, duly executed, to the Divestiture Trustee to effect the sale (including ancillary agreements), the Closing and all actions and declarations which the Divestiture Trustee considers necessary or appropriate to achieve the sale and the Closing, including the appointment of advisors to assist with the sale process. Upon request of the Divestiture Trustee, Mars shall cause the documents required for effecting the sale and the Closing to be duly executed.
34. Mars shall indemnify the Trustee and its employees and agents (each an “Indemnified Party”) and hold each Indemnified Party harmless against, and hereby agrees that an Indemnified Party shall have no liability to Mars for, any liabilities arising out of the performance of the Trustee’s duties under the Commitments, except to the extent that such liabilities result from the wilful default, recklessness, gross negligence or bad faith of the Trustee, its employees, agents or advisors.
35. At the expense of Mars, the Trustee may appoint advisors (in particular for corporate finance or legal advice), subject to Mars’ approval (this approval not to be unreasonably withheld or delayed) if the Trustee considers the appointment of such advisors necessary or appropriate for the performance of its duties and obligations under the Mandate, provided that any fees and other expenses incurred by the Trustee are reasonable. Should Mars refuse to approve the advisors proposed by the Trustee the Commission may approve the appointment of such advisors instead, after having heard Mars’ reasons for its refusal. Only the Trustee shall be entitled to issue instructions to the advisors. Paragraph 34 of these Commitments shall apply mutatis mutandis.
36. The Parties agree that the Commission may share Confidential Information proprietary to the Parties with the Monitoring Trustee. The Monitoring Trustee shall not disclose such information and the principles contained in Article 17 (1) and (2) of the Merger Regulation apply mutatis mutandis.
37. Mars agrees that the contact details of the Monitoring Trustee are published on the website of the Commission's Directorate-General for Competition and they shall inform interested third parties, in particular any potential purchasers, of the identity and the tasks of the Monitoring Trustee.
38. For a period of ten years from the Effective Date the Commission may request all information from Mars that is reasonably necessary to monitor the effective implementation of these Commitments.
IV. Replacement, discharge and reappointment of the Trustee
39. If the Trustee ceases to perform its functions under the Commitments or for any other good cause, including the exposure of the Trustee to a Conflict of Interest:
(i) the Commission may, after hearing the Trustee and Mars, require Mars to replace the Trustee; or
(ii) Mars may, with the prior approval of the Commission, replace the Trustee.
40. If the Trustee is removed according to paragraph 39 of these Commitments, the Trustee may be required to continue in its function until a new Trustee is in place to whom the Trustee has effected a full hand over of all relevant information. The new Trustee shall be appointed in accordance with the procedure referred to in Part I of this Section D of these Commitments.
41. Unless removed according to paragraph 39 of these Commitments, the Trustee shall cease to act as Trustee only after the Commission has discharged it from its duties after all the Commitments with which the Trustee has been entrusted have been implemented. However, the Commission may at any time require the reappointment of the Trustee if it subsequently appears that the relevant remedies might not have been fully and properly implemented.
Section G. The review clause
42. The Commission may extend the time periods foreseen in the Commitments in response to a request from Mars or, in appropriate cases, on its own initiative. Where Mars requests an extension of a time period, it shall submit a reasoned request to the Commission no later than one month before the expiry of that period (subject to the exception that, in relation to a request by Mars to extend the First Divestiture Period by one additional month, Mars may submit a reasoned request to the Commission no later than two weeks before the expiry of the initial three-month period), showing good cause. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time send a non-confidential copy of the report to Mars. Only in exceptional circumstances shall Mars be entitled to request an extension within the last month of any period.
43. The Commission may further, in response to a reasoned request from Mars showing good cause waive, modify or substitute, in exceptional circumstances, one or more of the undertakings in these Commitments. This request shall be accompanied by a report from the Monitoring Trustee, who shall, at the same time send a non-confidential copy of the report to Mars. The request shall not have the effect of suspending the application of the undertaking and, in particular, of suspending the expiry of any time period in which the undertaking has to be complied with.
Section H. Entry into force
44. The Commitments shall take effect upon the date of adoption of the Decision.
…………………………………. duly authorised for and on behalf of Mars, Incorporated
…………………………………… duly authorised for and on behalf of AniCura TC AB
SCHEDULE
1. The Divestment Business consists of the entire VetFamily business as at Closing, including all legal entities through which VetFamily operates (the VetFamily Legal Entities) and the assets comprised within them. Further details on what is contained within the Divestment Business are set out in the remainder of this Schedule.
2. The Divestment Business includes, but is not limited to, the following:
(i) Legal entities
3. The VetFamily Legal Entities are:
a. VetFamily Holding AB (Sweden);
b. VetFamily AB (Sweden);
c. VetFamily AS (Norway);
d. VetFamily ApS (Denmark);
e. VetFamily GmbH (Germany);
f. VetFamily B.V. (Netherlands); and
g. VetFamily GmbH (Austria).
4. […] is a newly formed and incorporated holding company, into which the entities listed at paragraph 3.b) to 3.g) will be transferred prior to Closing. […] is a dormant entity, as VetFamily is not active in […].
5. VetFamily is in the process of incorporating new VetFamily entities in […]. These entities will be part of the Divestment Business to the extent that incorporation is complete at Closing, which is expected. All relevant documents, including any plans, records or other preparatory materials, relating to the incorporation of these entities will transfer to the Purchaser on Closing, including in the event that incorporation of these entities has not been finalised on Closing.
6. A structure chart showing the current positions of the VetFamily Legal Entities within the wider AniCura group is provided in Annex 1 for reference.
(ii) Tangible assets
7. The Divestment Business includes all tangible assets owned by the VetFamily Legal Entities, as well as tangible asset used exclusively by VetFamily, even if not owned by the VetFamily Legal Entities, including:
a. Office equipment used for the VetFamily business, e.g. laptops, mobile phones, and limited office furniture.
b. All business records, books of account, and financial records to the extent pertaining to VetFamily, including all data and records held on (i) VetFamily Members (previous and current) and VetFamily Membership Contracts;
(ii) purchases by VetFamily Members under the Framework Agreements;
(iii) sales and customer data relating to VetPlan; and (iv) suppliers with whom VetFamily has Framework Agreements, including those shared with AniCura (Shared Framework Agreements), as well as product and pricing information, account histories and commercial data relating to VetFamily.
c. Any other tangible assets not reflected in paragraph 7(a)-(b) above.
(iii) Intangible assets
8. The Divestment Business includes all the intellectual property rights needed to run the VetFamily business (almost all of which are currently registered and owned by […] or other AniCura entities), including but not limited to:
a. all sales and marketing assets, including marketing and distribution plans, VetFamily websites and domains, including the ‘VetFamily’, ‘VetPlan’, and ‘VetPro’ domain names (listed in Annex 3), as well as social media sites and the VetFamily webshop in Denmark, www.netdyredoktor.dk and www.netdyredoktor.com (which domain names are owned by VetFamily);
b. all VetFamily trademarks and brand names, including ‘VetFamily’, ‘VetPlan’, and ‘VetPro’ (see full list in Annex 4);
c. all documents relating to plans for future VetFamily Member benefits; and
d. a licence to use the IT system through which VetPlan is administered (the AniPlan IT System), to be granted on a perpetual, fully paid-up basis; and
e. ‘[…]’, a CRM system licensed to AniCura from […]. Prior to Closing, AniCura will take a copy of any information on the existing ‘[…]’ system which is needed for the ongoing operation of the AniCura Clinics.
9. The Divestment Business includes any additional assets, contracts, or licences which relate to the commercialisation of VetPro products in Denmark by VetFamily, including VetFamily webshop in Denmark (www.netdyredoktor.dk). Should AniCura Clinics which are also VetFamily Members in Denmark remain VetFamily Members, they may continue to supply VetPro products under agreement with VetFamily.
(iv) Contracts
10. The Divestment Business comprises all contracts that contribute to the current operation or are necessary to ensure the viability and competitiveness of VetFamily, including in particular:
a. all the membership contracts with VetFamily Members that are in place at Closing (the Membership Contracts).
b. all framework supply agreements for all products and services which have been entered into for the benefit of VetFamily Members, whether negotiated by VetFamily or AniCura, listed in Annex 5 (Framework Agreements).
c. all relevant customer contracts for VetPlan sold by VetFamily Members (VetPlan Contracts).
d. the lease agreement on VetFamily’s office premises in Denmark.
11. Shared framework agreements, which apply to both VetFamily Members and AniCura Clinics (Shared Framework Agreements), will continue to be operated according to their existing terms and conditions until the Purchaser takes control of VetFamily. The principles governing the handling of the Shared Framework Agreements until the Purchaser takes control of VetFamily are as follows:
a. AniCura will continue pooling its purchase volumes under the relevant agreements with VetFamily for […] (or up to […] post-Closing where such contracts have no termination date or have an automatic renewal provision).
b. AniCura will assign to VetFamily all benefits accruing under the Shared Framework Agreements (i.e. rebates, discounts, and other bonuses), except to the extent that such benefits relate to orders or purchases by AniCura Clinics under the relevant agreements, in which case these benefits are attributable and payable to AniCura as under the current arrangements.
c. To the extent necessary to avoid the expiry of Shared Framework Agreements prior to Closing and to allow the Purchaser a reasonable opportunity to negotiate standalone agreements, AniCura will roll-over or extend such Shared Framework Agreements for a period of […] post- Closing. Negotiations with suppliers relating to the roll-over or extension of Shared Framework Agreements will be conducted jointly by AniCura and VetFamily/the Hold Separate Manager and subject to the oversight and agreement of the Monitoring Trustee.
d. As regards Shared Framework Agreements that expire or are terminated after Closing, AniCura and the Divestment Business will each be responsible for negotiating and entering into separate agreements with suppliers without the continued pooling of purchase volumes.

a. System development for the AniPlan IT System, which is used to administer VetPlan and AniPlan, and which is owned by […] but managed by VetFamily; and
b. Training and servicing support for AniCura Clinics in relation to the roll-out of AniPlan.
(vii) Other
17. If there is any asset, personnel or service which is not otherwise covered in this Schedule but which is both used (exclusively or not) in the Divestment Business and necessary for the continued viability and competitiveness of the Divestment Business, that asset, personnel or service, or an adequate substitute, will be offered to potential purchasers.
18. For the avoidance of doubt, the Divestment Business includes the capability to provide all training and other on-boarding support services that are currently offered to VetFamily Members in connection with their membership of the VetFamily network, to the extent not otherwise covered in this Schedule.
19. For the avoidance of doubt, the Divestment Business does not include: a. […];
b. accounting and payroll support services provided by AniCura to VetFamily in Sweden and Germany, which will be terminated after Closing, subject to the transitional arrangements set out in paragraph 16.b) above; and
c. Personnel employed by AniCura who perform shared group functions but are not included in the Personnel listed in paragraph 12.a) above. The following AniCura employees, which provide a very limited amount of support to the VetFamily business amounting to less than 1% of their time, will not transfer with the Divestment Business:
i. […]
ii. […]
iii. […]
iv. […]
v. […]
20. If required by the Commission, AniCura will terminate the VetFamily Membership Contracts of the […] AniCura Clinics which are part of VetFamily in Denmark on Closing.
***
ANNEX 1: PRE-ACQUISITION VETFAMILY CORPORATE STRUCTURE (SIMPLIFIED)
[…]
ANNEX 2: VETFAMILY ORGANIGRAM
[…]
ANNEX 3: VETFAMILY DOMAIN NAMES
[…]
ANNEX 4: TRADEMARKS
[…]
ANNEX 5: FRAMEWORK AGREEMENTS
[…]
1 OJ L 24, 29.1.2004, p. 1 (the 'Merger Regulation'). With effect from 1 December 2009, the Treaty on the Functioning of the European Union ('TFEU') has introduced certain changes, such as the replacement of 'Community' by 'Union' and 'common market' by 'internal market'. The terminology of the TFEU will be used throughout this decision.
2 Publication in the Official Journal of the European Union No C 327, 17.9.2018, p.16.
3 Turnover calculated in accordance with Article 5(1) of the Merger Regulation and the Commission Consolidated Jurisdictional Notice (OJ C95, 16.4.2008, p. 1).
4 Agreement on the European Economic Area, OJ No L 1, 3.1.1994, p.3.
5 EEA Agreement, Protocol 2 on products excluded from the scope of the Agreement in accordance with article 8(3)(A) of the Agreement.
6 EEA Agreement, Protocol 3 concerning products referred to in Article 8(3)(B) of the Agreement.
7 Paper on the European Commission's jurisdiction over the affected markets in Norway, 12 October 2018.
8 Form CO, paragraphs 102(a), 104. Examples of medical conditions for which dietetic pet food is available include gastrointestinal, dermatological, obesity/weight management, allergies, joint/mobility (arthritis), urinary, renal, cardiac, and diabetes.
9 Form CO, paragraphs 24 - 26
10 Form CO, paragraph 91.
11 Form CO, paragraph 14.
12 Form CO, paragraph 103; Parties' response to RFI 1, question 2.
13 Form CO, paragraphs 103, 113, 116-117, 119-124, 127; Parties' supplemental submission on market definition and substitution, 22 October 2018.
14 Form CO, paragraph 124(a)-(e).
15 Form CO, paragraphs 145-146, 150-151.
16 Form CO, paragraphs 107, 118; Parties' response to RFI 1, question 2, paragraph 2.18.
17 Case IV/M.554 - Dalgety/The Quaker Oats Company, paragraph 12; confirmed in Case IV/M.1127 - Nestlé/Dalgety, paragraph 8 (this question was left open in that case, as the assessment of the competitive impact of the case did not change whether grocery and non-grocery stores were considered together or separately).
18 Case IV/M.1127 - Nestlé/Dalgety, paragraph 13; Case M.2337 – Nestlé/Ralston Purina, paragraph 10.
19 Case M.2337 – Nestlé/Ralston Purina, paragraph 12; Case M.2544 – Masterfoods/Royal Canin, paragraphs 9-11.
20 Case M.1127 - Nestlé/Dalgety, paragraph 8; Case M.2337 – Nestlé/Ralston Purina, paragraph 14.
21 Responses to Q1 - Questionnaire to Veterinarians, question 3; Responses RFI to Competitors, question 5.
22 Responses to Q1 - Questionnaire to Veterinarians, question 3; Responses to RFI to Competitors, question 5.
23 Response to RFI to Competitors, question 5.
24 Responses to RFI to Competitors, question 5.
25 Responses to RFI to Competitors, question 5; Responses to Q1 - Questionnaire to Veterinarians, question 3.1.
26 Response to Q1 - Questionnaire to Veterinarians, question 3.
27 Response to Q1 - Questionnaire to Veterinarians, question 3.
28 The majority of competitors agree – Responses to RFI to Competitors, question 8; Responses to Q1 - Questionnaire to Veterinarians, question 11, question 11.1 and question 12.1.
29 Responses to RFIs to Competitors, question 8; Responses to Q1 - Questionnaire to Veterinarians, question 11.1.
30 Responses to RFIs to Competitors, question 6.
31 Mars (with its Royal Canin brand of dietetic pet food) and Nestle (with its Purina brand of dietetic pet food) are the exceptions.
32 Specialised manufacturers that manufacture pet food sold via the veterinary channel (including dietetic pet food) do not manufacture conventional pet food that is normally found in grocery stores include Hill's, Dechra, Vet Concept and Virbac.
33 Responses to RFI to Competitors, question 5.
34 Response RFI to Competitors, question 7.
35 Response to RFI to Competitors, question 7.
36 Responses to RFI to Competitors, question 7.
37 Responses to RFI to Competitors, question 13; Responses to RFI to Competitors, question 7.
38 Form CO, paragraph 151.
39 Response to RFI to Competitors, question 7.
40 Commission Directive 2008/38/EC of 5 March 2008 establishing a list of intended uses of animal feedingstuffs for particular nutritional purposes (OJ L 202, 31.7.2008, p.48).
41 Response to RFI to Competitors, question 5.
42 The types of particular nutritional purposes for cats and dogs regulated by Directive 2008/38/EC are: (i) support of renal function in case of chronic renal insufficiency (ii) dissolution of struvite stones, (iii) reduction of struvite stone recurrence, (iv) reduction of urate stone formation, (iv) reduction of oxalate stones formation, (v) reduction of cysteine stones formation, (vi) reduction of ingredient and nutrient intolerances, (vii) reduction of acute intestinal absorptive disorders, (viii) compensation for maldigestion, (ix) support of heart function in the case of chronic cardiac insufficiency, (x) regulation of glucose supply (Diebetes mellitus), (xi) support of liver function in the case of chronic liver insufficiency, (xii) regulation of lipid metabolism in the case of hyperlipidaemia, (xiii) reduction of copper in the liver, (xiv) reduction of excessive body weight, (xv) nutritional restoration, convalescence, (xvi) support of skin function in the case of dermatosis and excessive loss of hair.
43 Regulation (EC) No. 767/2009 of the European Parliament and of the Council on the placing on the market and use of feed (OJ L 229, 1.9.2009, p.1).
44 Form CO, paragraph 146; Responses to RFI to Competitors, question 7; Minutes of a call with a competitor, 13 September 2018, 10.00 CET.
45 Response to RFI to Competitors, question 7.
46 Form CO, paragraph 146 and footnote 103; Parties' response to RFI 1, question 2, paragraph 2.17 and footnote 65; Form CO, paragraph 149.
47 Form CO, paragraph 149; Parties' response to RFI 2, question 4, paragraph 4.3.
48 Responses to RFI to Competitors, question 12.
49 Form CO, paragraph 162.
50 Form CO, paragraph 14.
51 Case M.2337 – Nestle/Ralston Purina, paragraph 21.
52 Responses to RFI to Competitors, question 15, question 16 and question 18.
53 Responses to RFI to Competitors, question 15, question 16 and question 17.
54 Responses to RFI to Competitors, question 3.
55 Form CO, paragraph 14.
56 Form CO, paragraph 14.
57 Case M.1127 - Nestlé/Dalgety, paragraph 8; Case M.2337 – Nestlé/Ralston Purina, paragraph 14.
58 Responses to RFI to Competitors, question 17; Minutes of a call with a competitor, 1 August 2018,
14.00 CET; Responses to Q1 – Questionnaire to Veterinarians, question 13.1, question 14 and question 14.1.
59 Form CO, paragraph 14.
60 Case M.2337 – Nestlé/Ralston Purina, paragraphs 21-23
61 Case M.2544 – Masterfoods/Royal Canin, paragraph 27.
62 Responses to Q1 - Questionnaire to Veterinarians, question 5 and question 5.1.
63 Form CO, paragraphs 24 - 26
64 OJ C 265, 18.10.2008, p. 6.
65 Non-Horizontal Merger Guidelines, paragraph 18.
66 Non-Horizontal Merger Guidelines, paragraph 30.
67 Non-Horizontal Merger Guidelines, paragraph 31.
68 Non-Horizontal Merger Guidelines, paragraph 35.
69 Non-Horizontal Merger Guidelines, paragraph 58.
70 Non-Horizontal Merger Guidelines, paragraph 61.
71 The AniCura market share column includes projected share of 'pipeline' clinics, taking into account any additional clinics for which an SPA or LOI has been signed (but completion has not occurred as at the day these data were provided by the Parties).
72 The AniCura market share in the Netherlands includes a [0-5]% market share attributed by the Parties to Sterkliniek, AniCura's partnership network of independent clinics that operates only in the Netherlands.
73 Parties' response to RFI 5, Annex 7.1.
74 Parties' responses to RFI 12, question 4, and RFI 9, question 1.
75 Form CO, paragraph 202.
76 Form CO, paragraph 202.
77 Form CO, paragraphs 203-206.
78 Parties' Submission in relation to the VetFamily arrangements, 1 October 2018.
79 Form CO, paragraph 199.
80 Form CO, paragraphs 213-214
81 Responses to RFI to Competitors, question 12.
82 Minutes of a call with a competitor, 1 August 2018, 14:00 CET.
83 Response to RFI to Competitors, question 21.
84 Minutes of a call with a competitor, 1 August 2018, 14.00 CET.
85 Response to RFI to Competitors, question 17, question 19; Minutes of a call with a competitor, 1 August 2018, 14.00 CET.
86 Minutes of a call with a competitor, 1 August 2018, 14.00 CET.
87 Minutes of a call with a competitor, 13 September 2018, 10.00 CET.
88 Response to RFI to Competitors, question 4.
89 Form CO, paragraph 202.
90 Responses to Q1 - Questionnaire to Veterinarians, question 14.
91 Responses to Q1 - Questionnaire to Veterinarians, question 12.
92 Responses to Q1 - Questionnaire to Veterinarians, question 12.1, *courtesy translation from original German "wenn die Notwendigkeit der Diät verstanden wurde, bleiben sie bei dem einmal verordneten Produkt".
93 Responses to Q1 - Questionnaire to Veterinarians, question 11.1.
94 Response to Q1 - Questionnaire to Veterinarians, question 11.1.
95 Response to Q1 - Questionnaire to Veterinarians, question 12.1.
96 Response to Q1 - Questionnaire to Veterinarians, question 12.1.
97 Responses to RFI to Competitors, question 8; one competitor stated that "owners may choose not to follow veterinary advice and continue or revert to feeding conventional foods." See also Minutes of a call with a competitor, 1 August 2018, 14:00 CET.
98 Response to RFI to Competitors, question 8.
99 Response to RFI to Competitors, question 21.
100 Parties' response to RFI 1, Annex 12.4.7.
101 Parties' response to RFI 1, Annex 12.1.7.
102 Response to Q1 - Questionnaire to Veterinarians, question 12.
103 Response to Q1 - Questionnaire to Veterinarians, question 9 and question 9.1.
104 Responses to Q1 - Questionnaire to Veterinarians, question 8.1, question 11.1.
105 Responses to Q1 - Questionnaire to Veterinarians, question 17.1.
106 Response to Q1 - Questionnaire to Veterinarians, question 17.
107 Response to RFI to Competitors, question 4.
108 Minutes of a call with a competitor, 1 August 2018, 14.00 CET.
109 Minutes of a call with a competitor, 1 August 2018, 14.00 CET.
110 Mars' internal documents […].
111 Form CO, paragraphs 255 and 309
112 Responses to RFI to Competitors, question 12.
113 Form CO, paragraphs 255 and 309; Responses to RFI to Competitors, question 12.
114 Parties' response to RFI 5, question 3.
115 Parties' response to RFI 5, question 5; Parties' response to RFI 5, Annex 7.12
116 Parties' response to RFI 10, question 15.
117 Parties' response to RFI 7, to question 3(b).
118 Submission in relation to the VetFamily arrangements, page 4.
119 Form CO, paragraph 297.
120 Form CO, paragraph 29; Parties' response to RFI 7, question 3(b).
121 Form CO, paragraph 300.
122 Parties' response to RFI 7, question 3(b).
123 Parties' response to RFI 7, question 3(b).
124 Non-horizontal Merger Guidelines, paragraph 26. Rapid expansion is one of the factors that the Commission will take into account when deciding to focus on the investigation of vertical mergers.
125 Response to RFI to Competitors, question 12.
126 Non-Horizontal Merger Guidelines, paragraph 61.
127 Parties' responses to RFI 7, question 3.
128 Non-Horizontal Merger Guidelines, paragraph 70.
129 Non-Horizontal Merger Guidelines, paragraph 72.
130 Response to RFI to Competitors, question 4.
131 Response to RFI to Competitors, question 4.
132 Responses to Q1 - Questionnaire to Veterinarians, question 17.
133 Non-Horizontal Merger Guidelines, paragraph 72.
134 Response to RFI to Competitors, questions 19 and 20.
135 Response to RFI to Competitors, questions 19 and 20.
136 Non-Horizontal Merger Guidelines, paragraph 65.
137 Non-Horizontal Merger Guidelines, paragraph 72.
138 Non-Horizontal Merger Guidelines, paragraph 75.
139 Response to RFI to Competitors, question 3.
140 Responses to Q1 - Questionnaire to Veterinarians, question 17.
141 Stock keeping unit (SKU) refers to a distinct product for sale and has its own unique identifier or code.
142 Response to RFI to Competitors, question 11.
143 Response to Q1 - Questionnaire to Veterinarians, question 17.
144 Response to RFI to Competitors, question 21.
145 Response to RFI to Competitors, question 21.
146 Parties' response to RFI 8, question 1.
147 Parties' response to RFI 8, questions 1-4.
148 Parties' response to RFI 5, Annex 12.4.6.
149 Non-Horizontal Merger Guidelines, paragraph 78.
150 Response to RFI to Competitors, question 4.
151 Response to RFI to Competitors, question 4.
152 Non-Horizontal Merger Guidelines, paragraphs 40-42.
153 Form CO, paragraphs 329-330.
154 Form CO, paragraphs 330-332.
155 AniCura notes that the methodology used to calculate this share overstates the actual market share, which is approximately [20-30]% based on internal calculations using FTEs.
156 The Notifying Party argues that this share overstates the actual market share (which is difficult to calculate with any accuracy).
157 The Notifying Party explains that this figure overstates the actual market share (which is difficult to calculate with any accuracy).
158 The fourth competitor is Nestlé.
159 Response to RFI to Competitors, question 12
160 Horizontal Merger Guidelines, paragraph 17.
161 Non-Horizontal Merger Guidelines, paragraph 70.
162 Non-Horizontal Merger Guidelines, paragraph 75.
163 Responses to Q1 - Questionnaire to Veterinarians, question 17.
164 Non-Horizontal Merger Guidelines, paragraph 78.
165 Non-Horizontal Merger Guidelines, paragraph 72.
166 Non-Horizontal Merger Guidelines, paragraphs 40 - 42
167 Form CO, paragraphs 433 - 435
168 Commission Notice on remedies acceptable under Council Regulation (EC) No 139/2004 and under Commission Regulation (EC) No 802/2004 (2008/C 267/01), (the "Commission Notice on Remedies").
169 Commission Notice on Remedies, paragraph 12.
170 Replies to Market Test Questionnaire on commitments offered by Mars and AniCura of 10 October 2018.
171 Ibid
172 Ibid.
173 Ibid.
174 Ibid.
175 Ibid
176 Form RM, paragraph 47.
177 Form CO, paragraph 85.
178 Parties' responses to RFI 12, question 4; Parties' responses to RFI 9, question 1.
179 Commission Remedies Notice, paragraph 98.